The Pediatric Hip
dislocation is difficult to make in a newborn. The diagnosis is based
on the subtleties of the physical examination. The consequences of not
making the diagnosis may be disastrous to the patient. A confusing area
in the literature is the terminology used to discuss this condition.
Various authors use the terms instability, dysplasia, subluxation, and dislocation interchangeably. We prefer to use the term developmental hip dysplasia
(or developmental dysplasia of the hip, DDH) to refer to any hip in
which the normal relation between the femoral head and the acetabulum
is altered.
components—the femoral head and acetabulum—develop from the same
primitive mesenchymal cells. In the seventh week of gestation, a cleft
develops in these precartilaginous cells, defining the femoral head and
acetabulum. By 11 weeks of gestation, the hip joint is fully formed.
Although rare, this is theoretically the earliest point in development
that a hip dislocation could occur. At birth, the femoral head is
deeply seated in the acetabulum and held there by the surface tension
of the synovial fluid. A normal infant’s hip is extremely difficult to
dislocate even after division of the hip joint capsule. In hips with
dysplasia, however, this tight fit between the femoral head and the
acetabulum is lost, and the head can be easily displaced from the
acetabulum. The femoral head displacement is usually in a
posterosuperior direction. Pathologic specimens of this condition show
varying degrees of hip joint malformation from mild capsular laxity to
severe acetabular, femoral head, and neck malformations. Therefore, developmental hip dysplasia probably appropriately refers to the many stages of this complex deformity.
clinically for any hip that may be provoked to subluxate (partial
contact between the femoral head and the acetabulum) or dislocate (no
contact between the femoral head and acetabulum) or for any hip in
which the femoral head is either subluxated or dislocated in relation
to the acetabulum but that can be reduced into the acetabulum. We
prefer to use the term developmental hip dislocation
when there is no contact between the femoral head and the acetabulum
and the femoral head is not reducible. True dislocations in the newborn
are rare and are usually associated with generalized conditions or
anomalies such as arthrogryposis or myelodysplasia. These antenatal
teratologic dislocations are at the extreme end of the DDH pathologic
spectrum and account for only 2% of the cases seen in most series. The
diagnosis and prognosis of these two separate conditions (dysplasia
versus dislocation) are quite different.
influenced by geographic and ethnic factors as well as the diagnostic
criteria used, the acumen of the examiner, and the age of the patient
at diagnosis. The results of newborn screening programs estimate that 1
in 100 newborns have some evidence of hip instability but that the true
incidence of dislocation is between 1 and 1.5 per 1,000 live births.
factors no doubt play a key role. The incidence of DDH has been
reported to be as high as 25 to 50 cases per 1,000 live births in Lapps
and North American Indians and to be almost nonexistent among Chinese
and blacks. Up to one-third of patients may give a positive family
history for DDH. The genetic effects of the condition may be manifest
primarily by acetabular dysplasia, joint laxity, or a combination of
both. The role of excessive femoral neck anteversion or acetabular
anteversion in the development of DDH remains controversial.
Intrauterine mechanical and neuromuscular mechanisms can profoundly
affect the intrauterine development of the hip.
in firstborn children. It has been postulated that the prima gravida
uterus and abdominal muscles are unstretched and subject the fetus to
prolonged periods of abnormal positioning. This positioning tends to
force the fetus against the mother’s spine, limiting motions of the
hip, particularly hip abduction. This “crowding phenomenon” may also be
manifested by the association of other abnormalities thought to be due
to the intrauterine compression, such as torticollis (up to 20% of
patients with torticollis may have associated DDH) and metatarsus
adductus. DDH is also manifested in patients with oligohydramnios,
another condition that causes limited fetal mobility. The left hip is
more frequently involved; in the uterus, it is the left side that is
most often forced into the adducted position against the mother’s
sacrum.
feature. About 60% of children with DDH are firstborn children.
Firstborn children have a high association of breech presentation.
About 30 to 50% of patients with DDH are delivered in the breech
presentation. About 60% of breech presentations are in firstborn
children, and most breech-born infants have leg-folding mechanism
arrests. Children born frank breech (knees in the extended position)
are at an even greater risk of developing hip instability. This is
evidenced by the higher incidence of DDH in children born with
congenital recurvatum or dislocation of the knee. Eighty percent of the
cases of DDH occur in girls. A contributory factor is that twice as
many girls are born breech as boys. The extrauterine environment may
also have a profound effect on the development of DDH. Societies in
which swaddling is used postnatally (hips kept extended and adducted),
for example, in many native North American tribes, the incidence of DDH
is considerably higher than expected.
secondary to maternal estrogens and those hormones necessary for pelvic
relaxation at delivery, may have an effect on the development of DDH.
These hormones have been thought to cause temporary laxity of the hip
joint capsule in the newborn, particularly the newborn girl. Hip joint
laxity, however, is seen often in newborn infants. This may allow for
some instability in the absence of a positive Ortolani sign. DDH is
extremely rare in conditions characterized by excessive laxity such as
Down, Ehlers-Danlos, and Marfan syndromes.
newborn screening programs, some cases are missed. The diagnostic test
for DDH is caused by the femoral head gliding in and out of the
acetabulum over a ridge of abnormal acetabular cartilage. This test was
originally described by LeDamany. He referred to the sensation palpated
as signe de ressaut. The Italian
pediatrician, Ortolani, in 1936 described the pathogenesis of this
diagnostic sign and referred to the sensation palpated as the segno dello scotto. This palpable sensation has been likened to the femoral head gliding in and out of the acetabulum over a ridge. This ridge
of hypertrophied acetabular cartilage (Figure 15-1) was called the neolimbus
by Ortolani. Unfortunately, inadequate translation of both LeDamany’s
and Ortolani’s work into English has resulted into the use of the term click
to describe this diagnostic sign. Experienced evaluators of hips in
newborns realize that many high-pitched soft tissue clicks are often
elicited in the hip examination of newborns that have no diagnostic
significance. Unfortunately, this poor understanding of the pathology
of the diagnostic sign in DDH has led to the misdiagnosis and
overtreatment of infants. This diagnostic maneuver must be done gently.
In the newborn period, such findings as asymmetry of the gluteal,
thigh, or labial folds (asymmetric thigh and skin folds occur in a
significant percentage of normal infants); limitation of abduction; or
asymmetry of range of motion may make the physician suspect the
presence of hip dysplasia, but the most reliable diagnostic sign is the
Ortolani sign. The Ortolani test is performed with the infant in the
supine position and the hips and knees flexed at 90°. The middle finger
is placed over the greater trochanter, while the thumb is placed on the
lesser trochanter bilaterally. The hips are then slowly abducted with
pressure over the greater trochanter. A palpable sensation indicates
reduction of a dislocated or subluxated hip. Also with the legs in
mid-abduction-adduction, posterior pressure can be applied to the
lesser trochanters with the thumbs, and a similar sensation can be
palpated, indicating whether the hip is subluxating or dislocating.
(The provocation portion of the diagnostic test is often referred to as
the Barlow maneuver.) It is essential that this test be performed with
the infant relaxed.
 |
|
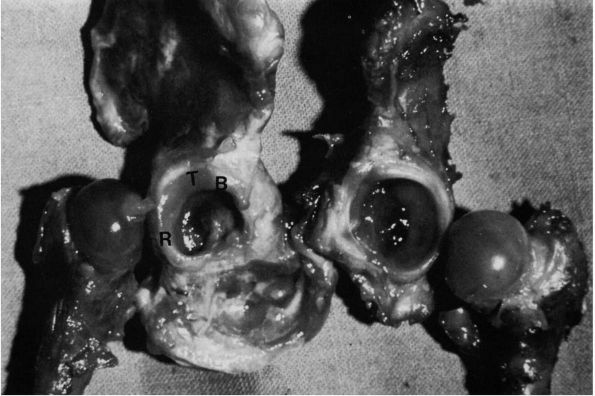
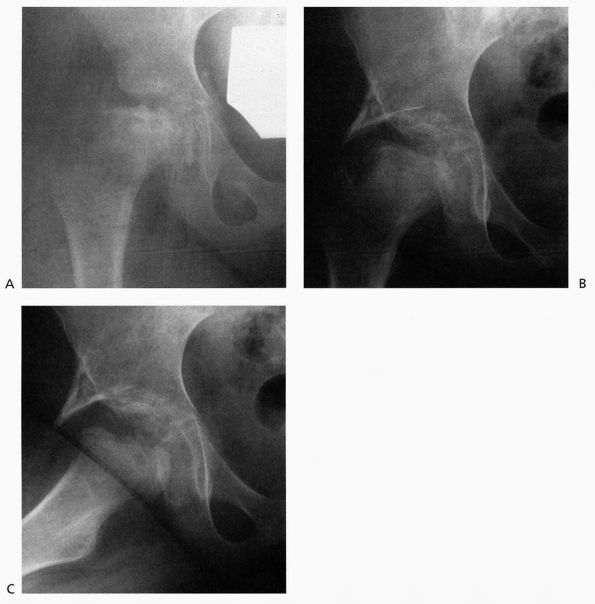
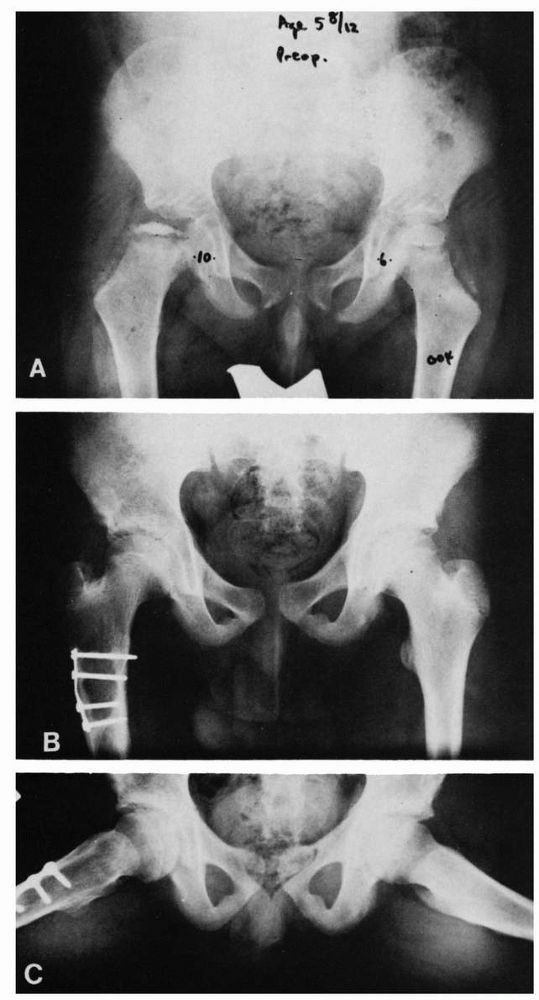
FIGURE 15-1.
In this full-term female infant with fairly severe dysplasia of the right hip, the acetabulum and femoral head are smaller on the right than on the left. Extending along the posterosuperior margin of the articular surface of the right dysplastic acetabulum is a shallow trough (T). At the anterior end of this trough is a bulge (B), and extending posteriorly along the inferior and anterior margin of the trough down to the inferior margin of the acetabulum is a ridge (R) that separates the primary acetabulum inferiorly and anteriorly from the trough and the rest of the secondary acetabulum superiorly and posteriorly. (Ponseti IV. Morphology of the acetabulum in congenital dislocation of the hip: gross, histological and roentgenographic studies. J Bone Joint Surg 1978;60A:586-599) |
DDH may occur late. It is therefore extremely important to continually
look for this condition after the newborn period when the disorder is
manifested by the secondary adaptive signs. It is especially important
to look for DDH in high-risk infants. The high-risk group of infants
includes those who have a combination of any of the following risk
factors: breech position, female, positive family history, lower limb
deformity, torticollis, metatarsus adductus, significant persistent
asymmetric thigh folds, excessive ligamentous laxity, any other
significant musculoskeletal abnormality, and ethnic background
associated with an increased incidence of DDH.
likelihood of the patient exhibiting physical findings secondary to
adaptive changes. With persistent subluxation or dislocation, the
patient develops secondary contractures of the adductor muscles on the
involved side. This leads to limited abduction (Figure 15-2),
the key late diagnostic finding in DDH. In addition, after the newborn
period, the incidence of a positive Ortolani sign decreases markedly,
particularly after 1 to 2 months of age. The disturbed relation between
the proximal femur and the acetabulum may, in addition to limitation of
abduction, lead to the presence of asymmetry of the gluteal, thigh,
buttock, or labial folds. The patient may manifest apparent shortening
of the femur (Allis sign) in comparison to the opposite side (Figure 15-3)
or “pistoning” or “telescoping” of the involved extremity, depending on
the laxity of the hip joint capsule. In a child of walking age with
unilateral DDH, the apparent limb length inequality may result in a
limp secondary to the apparent shortening of the extremity. This also
may lead to a secondary equinus deformity of the ankle. Clinically,
bilateral dislocations are much more difficult to detect because the
physical findings may be symmetric. Also in bilateral DDH, the child
may walk with a waddling gait and hyperlordosis of the lumbar spine.
Any gait abnormality in a child should not be dismissed without a
careful clinical and radiographic evaluation of the hips.
one. The femoral ossific nucleus is not present in the newborn, and a
great portion of the pelvis of an infant is cartilaginous. Thus, normal
relations are difficult to interpret radiographically, and all
treatment decisions in the newborn nursery should be based on the
clinical examination. Routine radiographs are generally unnecessary. A
normal-appearing radiograph does not rule out the presence of DDH.
Complete dislocations may be missed, and mild degrees of dysplasia are
not easily detected. Ultrasonography, while routinely used as a
screening tool for DDH in Europe, has not been shown to be cost
effective in the United States as a screening device. It is very
operator and position dependent and has resulted in the overdiagnosis
and overtreatment of infants. With increasing age and lack of the
normal relation between the proximal femur and the acetabulum, the
anatomic changes of this abnormal relation become increasingly evident.
The femoral ossific nucleus, which normally appears between 4 and 7
months of age, may be delayed in its appearance and its general overall
development stunted. The proximal femur is seen to lie laterally with
varying degrees of proximal migration compared with the ilium. The
Shenton line is disrupted. The acetabulum fails to develop, as
manifested by an increase in the slope of the acetabular roof (Figure 15-4).
Most important in assessing radiographic measurements is the accurate
positioning of the child for the radiograph. The lower extremities must
be aligned and in neutral rotation. Unless radiographic positioning is
standardized, measurement differences between patient visits may not be
reliable.
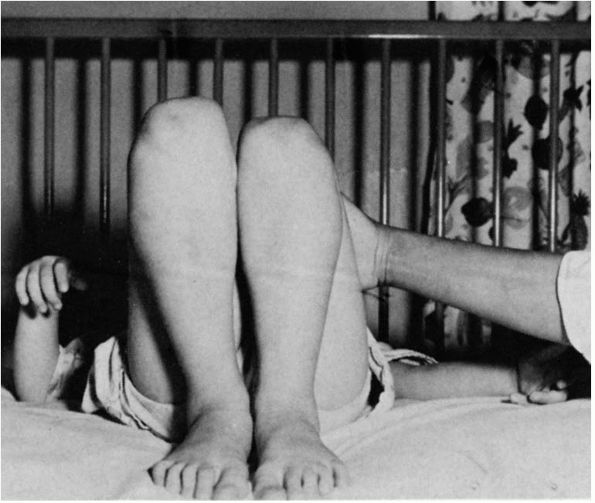
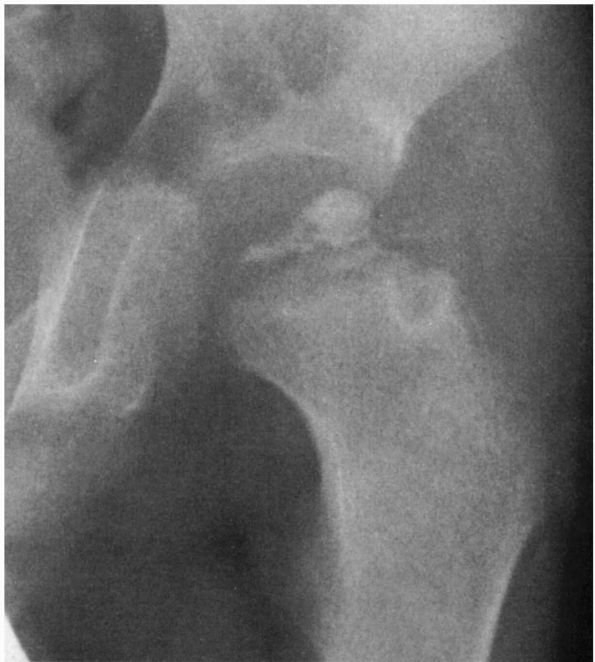
 |
|
FIGURE 15-2. Eighteen-month-old girl with left congenital hip dysplasia. Note the limited abduction of left hip compared with the right.
|
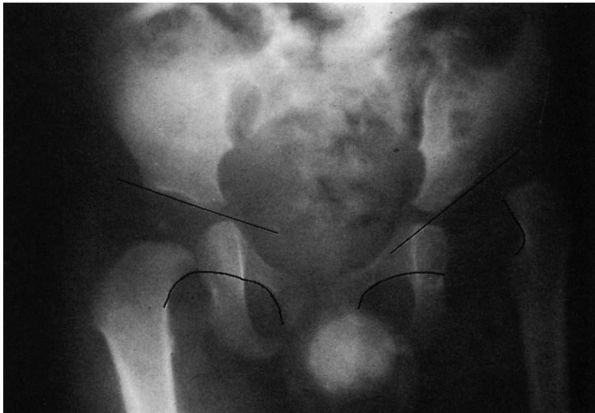
 |
|
FIGURE 15-3. The Allis or Galeazzi sign. The knee is lower on the dislocated side.
|
varying degrees of capsular laxity and the thickening of the acetabular
cartilage in the superior, posterior, and inferior aspects of the
acetabulum. This thickening in the cartilage was called neolimbus by Ortolani (see Figure 15-1). It is the
sensation of the femoral head gliding in and out of the acetabulum over
this thickened ridge that produces the Ortolani sign. Without
treatment, this ridge of hypertrophied acetabular cartilage may become
more prominent, and within a few weeks or months after birth, the
femoral head may remain dislocated into a secondary acetabulum. The
child manifests the secondary adaptive physical findings mentioned
previously. Pathologically, the anatomic obstacles to reduction change
and become more difficult to overcome. The extra-articular and
intra-articular pathologic changes may prevent concentric reduction.
Extra-articular obstacles may include contraction of the adductor
longus and the iliopsoas muscles as a consequence of the dislocation.
The most common secondary intra-articular change is varying degrees of
anteromedial capsular constriction. The ligamentum teres may become
thickened and hypertrophied or elongated, and in some cases, its sheer
bulk precludes reduction. In the crawling or walking child, the
constant pull of ligamentum teres on its attachment at the base of the
acetabulum may cause hypertrophy of the transverse acetabular ligament,
which secondarily decreases the diameter of the acetabulum. A true
inverted labrum or limbus (hypertrophied labrum) may also be an
obstacle reduction in the late diagnosed DDH. This, however, is a rare
finding and is seen only in teratologic dislocations (2%) and in
previously failed closed reductions, in which case it is an iatrogenic
condition.
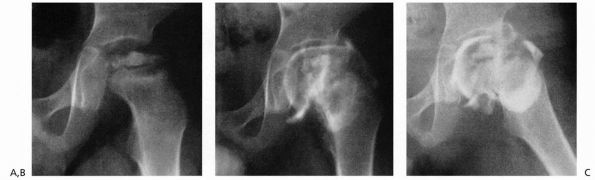
 |
|
FIGURE 15-4.
Eleven-month-old boy with left congenital hip dysplasia. Note the delayed appearance of left femoral ossific nucleus, disruption of the Shenton line with proximal migration of the femur, and lack of development of the acetabulum manifested by an increased slope of the acetabular roof. |
is important to appreciate that the normal concave shape of the
acetabulum develops in response to the presence of a spherical femoral
head. Experimental studies in animals as well as observations in humans
with unreduced congenital hip dislocations show that the acetabulum
does not develop its normal concave shape. Instead, with a complete
dislocation, the triradiate cartilage grows normally, and hence the
innominate bone reaches its normal length (Figure 15-5);
but the acetabular cartilage atrophies and degenerates, and the
acetabulum appears flattened. The depth of the acetabulum increases
normally as a result of continued interstitial growth within the
acetabular cartilage, oppositional growth at the periphery of this
cartilage, and periosteal new bone growth at the edge of the acetabulum
along the ilium. The depth of the acetabulum is further increased at
puberty by the development of secondary centers of ossification in the
three pelvic bones. For this normal growth and development to occur, a
concentric relation must be maintained between the femoral head and the
acetabulum throughout growth.
certain percentage of untreated hips, however, go on to subluxation
(partial contact with the acetabulum) or dislocation (no contact
between the femoral head and acetabulum), and some hips may remain
located but retain dysplastic features. Unfortunately, the means to
determine which of the unstable hips will attain spontaneous stability
are
not
available, and hence all unstable hips in the newborn period must be
treated to ensure the proper environment for hip joint development.
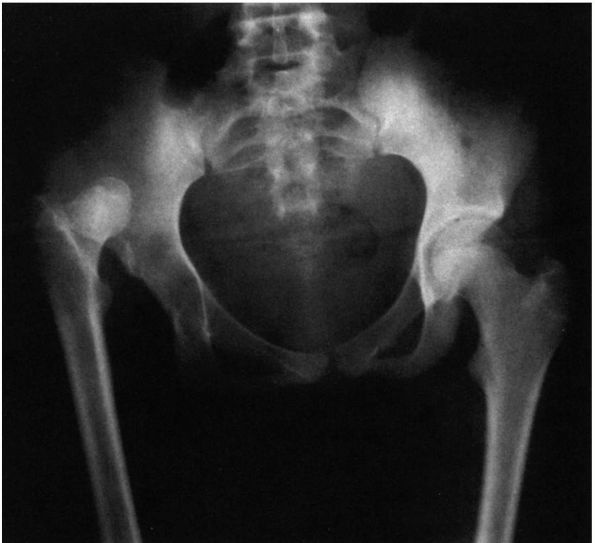 |
|
FIGURE 15-5.
Untreated right congenital hip dysplasia in an adult. Note the lack of development of acetabular shape and depth. No secondary acetabulum exists. The left hip is normal. |
institute treatment so that normal development may occur. If the hip
remains completely dislocated, its natural history depends on two
factors: the presence or absence of a false acetabulum and
bilateralness.
complete dislocations do well, maintaining a good range of motion and
little functional disability. Completely dislocated hips with
well-developed false acetabuli, however, are more likely to develop
degenerative joint disease in the false acetabulum and have a poor
clinical result (Figure 15-6). Degenerative
joint disease in the false acetabulum usually occurs in the fourth and
fifth decades of life. In bilateral complete dislocations, lower-back
pain may occur. This may be secondary to the hyperlordosis of the
lumbar spine associated with the hip flexion adduction deformities
caused by the dislocations.
is affected by the secondary problems of limb length inequality,
ipsilateral knee deformity, pain (usually on the lateral side of the
knee), secondary scoliosis, and gait disturbances. In these patients,
the same factors concerning the development of secondary degenerative
changes in any false acetabulum that may occur are also applicable.
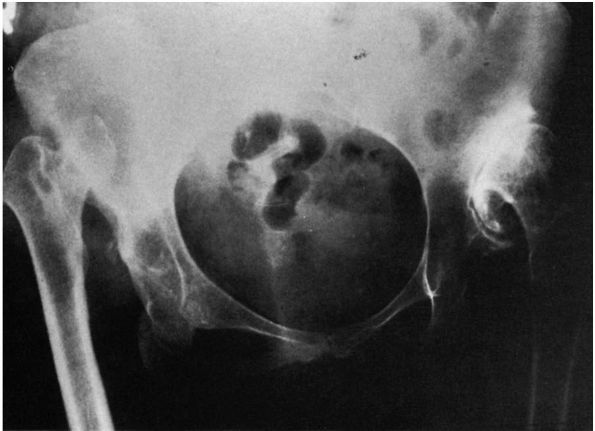 |
|
FIGURE 15-6.
Radiograph of a 43-year-old woman with complete dislocation of both hips. She has no symptoms on the right but has disabling symptoms from the left hip. She has no false acetabulum on the right but has a well-developed false acetabulum on the left with secondary degenerative changes present. (Weinstein SL. Natural history of congenital hip dislocation [DDH] and hip dysplasia. Clin Orthop 1987;225:62-76) |
refers to inadequate development of the acetabulum, femoral head, or
both. All subluxated hips are by definition dysplastic.
Radiographically, however, the major difference between dysplasia and
subluxation is the intactness of the Shenton line (Figure 15-7).
In subluxation, the Shenton line is disrupted, and the femoral head is
superiorly or laterally displaced from the medial wall of the
acetabulum. In dysplasia, the normal Shenton line relation is intact.
Unfortunately, in the DDH natural history literature, these two
radiographic and clinical entities are often not separated. In
addition, the development of secondary degenerative arthritis in the
dysplastic hip may convert it to a subluxated hip. Because the physical
signs of hip dysplasia are usually lacking, cases are often diagnosed
only incidentally on radiographs taken for other reasons or not until
the patient develops symptoms.
that this condition leads to the development of radiographic
degenerative joint disease and clinical disability. The more severe the
subluxation, the earlier is the symptom onset. Those patients with the
most severe subluxations usually develop symptoms of degenerative joint
disease during the second decade of life. The symptoms of degenerative
joint disease and hip subluxation and dysplasia often predate
radiographic changes of degenerative joint disease (decreased joint
space, cyst formation, double acetabular floor, inferomedial femoral
head osteophyte) by as much as 10 years. Often, the only
radiographic
feature present at symptom onset may be increased sclerosis in the
weight-bearing area. In the absence of subluxation, the natural history
of dysplasia cannot accurately be predicted, but hip dysplasia is
definitely associated with radiographic degenerative joint disease,
especially in female patients.
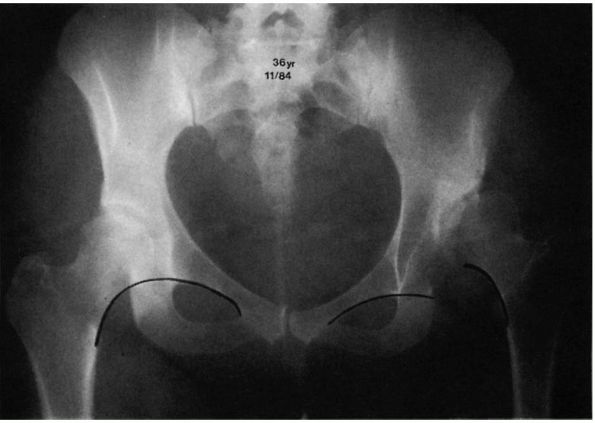 |
|
FIGURE 15-7.
Radiographically, the major difference between dysplasia and subluxation is the intactness of the Shenton line. The right hip is dysplastic (Shenton line intact). The left hip is subluxated (Shenton line disrupted). All subluxated hips are, by definition, dysplastic. (Weinstein SL. Natural history of congenital hip dislocation [DDH] and hip dysplasia. Clin Orthop 1987;225:62-76) |
initiated immediately. The use of triple diapers in the treatment of
DDH in the newborn should be condemned. It is ineffective and gives the
family a false sense of security. Pathologic changes seen in the
newborn with DDH are reversible in 95% of cases with simple,
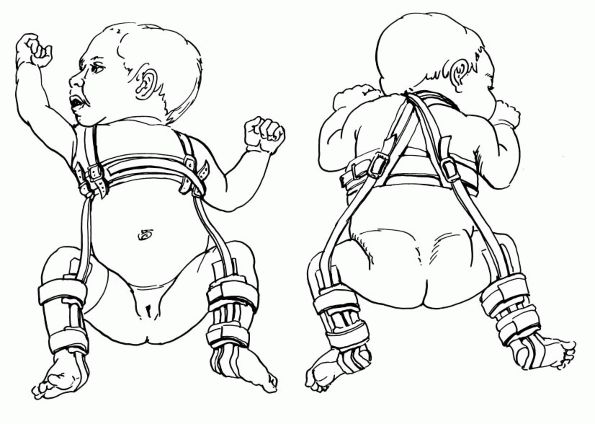
appropriately applied treatment methods. The most widely used device in
North America is the Pavlik harness (Figure 15-8).
The Pavlik harness prevents adduction and extension while allowing
further flexion, abduction, and rotation. This position allows for
gentle spontaneous reduction of dislocated hips. Stretching of tight
adductors is also achieved with the Pavlik harness. The device is worn
full time until hip stability is achieved. The physician must provide
extensive parent education in addition to the use of the Pavlik
harness. Patient noncompliance is the main cause of failure of this
device. Appropriate application of the device is essential. Careful
follow-up at weekly intervals is extremely important. Adjustments in
the flexion and abduction straps of the Pavlik harness must be made to
accommodate the hip stability assessed by physical examination of the
patient. Clinical hip stability is usually obtained within 2 to 4 weeks
of treatment by this method. Most physicians use the harness for a
period of 6 to 12 weeks on a full-time basis. Initial radiographs or
sonograms should be obtained in the harness to document adequate
flexion and redirection of the femoral shaft toward the triradiate
cartilage in the harness. Once clinical stability is obtained, a
radiograph is not indicated until about 3 months of age to determine
acetabular development. The Pavlik harness may be used in dysplasia and
subluxation up to 6 months of age. Once the child begins to crawl, use
of the Pavlik harness is extremely difficult, and the success rate with
the harness decreases to less than 50%.
 |
|
FIGURE 15-8. Pavlik harness. The posterior strap acts as a check rein against adduction to prevent redislocation.
|
mentioned time frame, treatment with the Pavlik harness should be
discontinued and alternative methods of treatment employed. The Pavlik
harness is contraindicated in the patient who has DDH in association
with conditions of muscle imbalance (e.g., upper-level
meningomyelocele), joint stiffness (e.g., arthrogryposis), or excess
ligamentous laxity, (e.g., Ehlers-Danlos syndrome). Applied correctly
and used for the appropriate indications, the Pavlik harness may
achieve 95% successful results in treatment of DDH. Inappropriately
applied and poorly monitored use of the harness is associated with
problems such as inferior hip dislocations from prolonged excess
flexion of the hip in the harness. This hyperflexion may also be
associated with femoral nerve palsies, which are usually transient.
Brachial plexus palsies from pressure of the shoulder straps have also
been reported. The parent must pay attention to skin care in the groin
folds and the popliteal fossa area to prevent skin maceration and
breakdown. The most devastating complication of the Pavlik harness is
aseptic necrosis of the femoral head. Reported incidence of this
complication ranges from 9 to 15%. This is generally produced by excess
of
tightening of the abduction strap. It has been well documented that the
hyperabduction position of the hip compromises the vascular supply to
the proximal femur.
than 6 months of age, a fixed-abduction orthosis may be used to achieve
hip stability and allow for growth and development of the hip joint. It
can be used only if the hip is well reduced on a radiograph taken in
the orthoses. The complications of fixed-abduction orthoses include
skin problems and aseptic necrosis. It is important in positioning the
fixed-abduction orthoses that the hip not be placed in extreme
positions of abduction to avoid aseptic necrosis.
case that fails treatment with a Pavlik harness and is not amenable to
a fixed-abduction orthosis, the obstacles to reduction are different,
treatment has greater risks, and the results are less predictable. The
general goals of treatment in the late diagnosed case are to obtain and
maintain a reduction, to allow for femoral head and acetabular
development, and to avoid a development of aseptic necrosis.
harness treatment, closed reduction is indicated. Closed reduction is
generally preceded by a 1- to 2-week period of traction (Figure 15-9).
Although the use of prereduction traction is somewhat controversial,
the purpose of the traction is, in theory, to allow gradual stretching
of the soft tissue structures impeding reduction as well as of the
neurovascular bundle. The primary purpose of traction is the avoidance
of aseptic necrosis, the most devastating complication of the treatment
of DDH. The traction can be applied in the hospital or at home.
Generally, 1 to 2 weeks is sufficient. Skin traction is usually
adequate; skeletal traction is rarely necessary. The skin tapes should
be applied above the knee to distribute the traction over a large area.
Complications of traction include skin loss and ischemia of the lower
extremities due to inappropriate application. Neurocirculatory checks
must be done frequently and traction applied in a carefully supervised
fashion. Home traction has become popular because of the decreased cost
and convenience. Home traction should follow a 24-hour hospitalization
to familiarize the parents with application of the traction and how to
look for problems. It can only be used with cooperative, informed
parents.
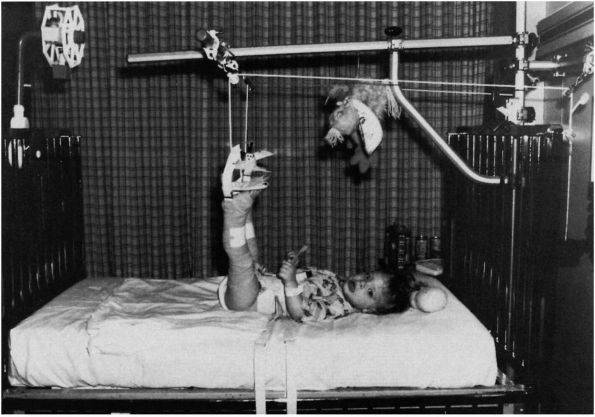 |
|
FIGURE 15-9.
Preliminary traction. Bryant traction was used before attempted closed reduction to stretch soft tissue structures about the hip. |
room setting. Under general anesthesia, the hip is gently manipulated
into the acetabulum. Arthrography is extremely helpful in assessing the
adequacy of reduction (Figure 15-10). Because a
large portion of the acetabulum is cartilaginous, the relations of the
femoral head and acetabulum are nicely visualized on arthrography. The
use of arthrography can help to assess any obstacles to reduction and
also the quality of reduction. Reduction is then maintained by a
well-molded cast (Figure 15-11) for a variable amount of time (range, 6 weeks to 4 months), depending on the child’s age.
The so-called human position of hyperflexion and limited abduction
should be used in closed reductions. Extreme positions of abduction, as
well as abduction and internal rotation, should be avoided because of
their association with the development of aseptic necrosis. After
removal of the cast, a fixed-abduction orthosis is applied and worn at
night and during napping hours until acetabular development has
returned to normal. The postoperative reduction can be confirmed by the
use of computed tomographic (CT) scanning or ultrasound.
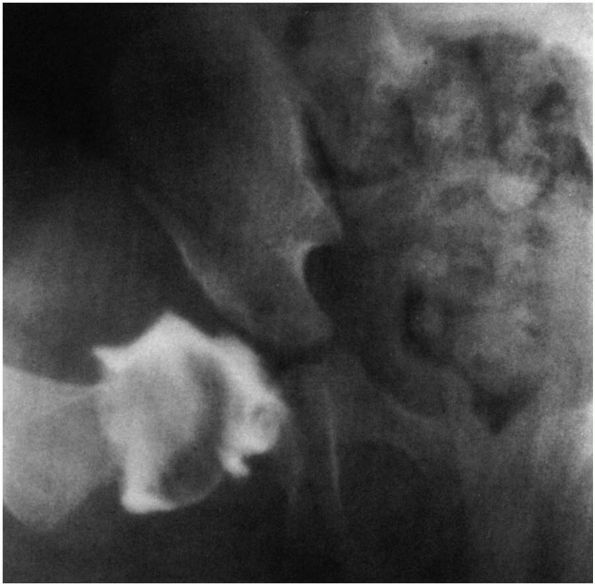 |
|
FIGURE 15-10. Attempted closed reduction under arthrographic control. Note pooling of dye medially. The hip cannot be reduced.
|
closed reduction, failure to maintain a closed reduction, or an
unstable reduction. If an open reduction is necessary, it can be done
through a variety of surgical approaches. During the open reduction,
each obstacle to reduction must be addressed. The most common obstacle
is the tight anteromedial joint capsule, which must be released. The
transverse acetabular ligament often requires sectioning, and the
ligamentum teres may need to be removed. A true inverted labrum or
limbus should never be excised but only radially incised because
excision of this tissue may interfere with the normal growth and
development of the acetabulum.
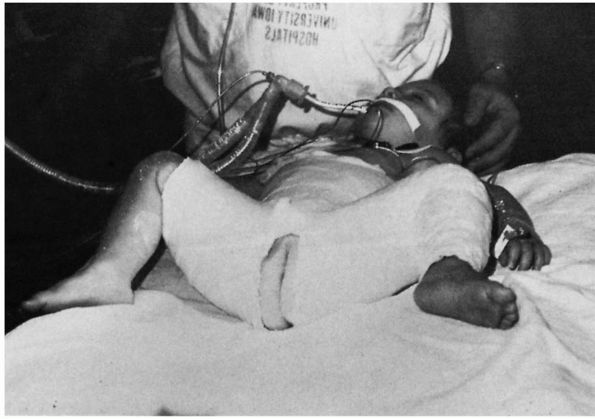 |
|
FIGURE 15-11. Reduction of left congenital hip dysplasia is maintained by a well-molded 1½ hip spica cast.
|
a patient’s requiring an open reduction to obtain and maintain a
reduction. By the age of 3 years, preliminary traction should not be
used, but open reduction should be accompanied by a femoral shortening
(removal of a section of the proximal femur) to decrease the incidence
of aseptic necrosis. Between the ages of 2 and 4 years, the question of
whether to use femoral shortening versus traction before open reduction
remains unanswered. The trend today however for pediatric orthopaedic
surgeons treating the over 2-year-of-age group is to accompany open
reduction by a femoral shortening procedure.
once a closed or open reduction has been obtained. If, however, the
acetabulum does not make adequate progress toward normal development
after a closed or open reduction, one of several types of innominate
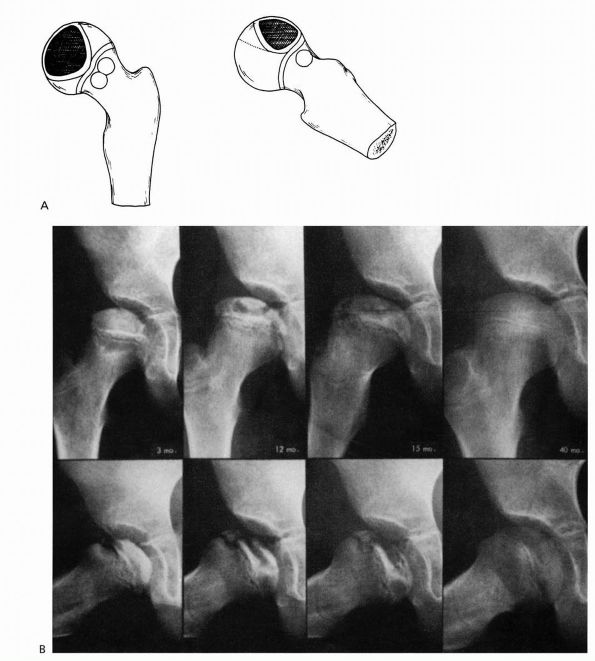
osteotomy should be performed to increase femoral head coverage (Figure 15-12).
anteversion. In general, children reduced before they are 2 years of
age rarely require derotation osteotomies to correct the anteversion.
Anteversion usually corrects once the reduction is obtained. Aseptic
necrosis is the most devastating complication associated with the
treatment of DDH. Aseptic necrosis may be caused by many errors in
treatment, as mentioned previously. In the newborn, excessive use of
abduction or the abduction internal rotation position can cause aseptic
necrosis. In the older child, aseptic necrosis may be caused by
insufficient use of prereduction traction, failure to perform an
adductor tenotomy, injuries to the blood vessels during surgery,
failure to do femoral shortening, or persistence of closed techniques
in the face of obstacles to reduction.
deformity may be present in the femoral head, acetabulum, or both. In
these cases, normal anatomy
and relation must be restored. In many cases, osteotomies are necessary on both the femoral and pelvic sides of the hip joint.
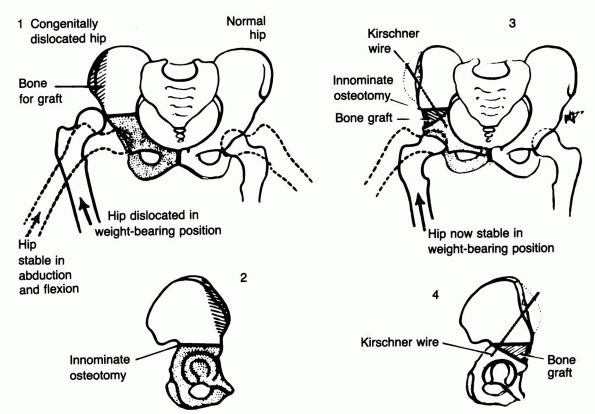 |
|
FIGURE 15-12.
Technique of Salter innominate osteotomy. Diagram of principle involved. (Salter RB. Innominate osteotomy in the treatment of congenital dislocation and subluxation of the hip. J Bone Joint Surg 1961;43B:518. |
hip in young children. The disease is characterized by varying degrees
of necrosis of the femoral ossific nucleus. It is most common in the
age range of 4 to 8 years, but has been reported in children as young
as 2 years of age and also in the late teenage years. It is more common
in boys than girls by a ratio of 4:1, and the incidence of
bilateralness is about 10 to 12%.
Legg-Calvé-Perthes disease reveal an incidence of a positive family
history of about 10%. There is a high association of abnormal birth
presentation, such as breech or transverse lie, in affected patients.
There are also racial and ethnic factors, with LCPD being more common
in Japanese, Eskimos, and Central Europeans and uncommon in native
Australians, Polynesians, American Indians, and blacks.
and delay in skeletal maturation as evidenced by retarded bone age.
Affected children are shorter than nonaffected children. Anthropometric
studies have confirmed this growth delay, with affected children being
smaller in all dimensions except head circumference and with the distal
portion of the extremities affected more than the proximal. The short
stature of the patients affected with the disorder at a young age tends
to correct during adolescence, while those affected at an older age
tend to be small throughout life. An abnormality of growth
hormone-dependent somatomedin in males with LCPD has recently been
demonstrated.
children, particularly the third to the sixth child, and in lower
socioeconomic groups.
particularly in urban rather than rural communities. Parental age of
affected patients is higher than in the general population. Affected
children have an increased association of genital urinary tract
abnormalities, inguinal hernia, and minor congenital abnormalities.
to be an inflammatory disease, secondary to trauma or a developmental
disorder. Toxic synovitis is thought by some authors to be a
precursor
to LCPD; however, a literature review of patients with toxic synovitis
revealed that only about 3% subsequently develop LCPD. The most widely
accepted etiologic theories are those involving interruption of the
vascular supply to the femoral head. It has been well demonstrated in
animal studies and confirmed by human pathologic material that LCPD is
caused by repetitive episodes of infarction. Recent studies have
postulated that the cause of the vascular embarrassment may be
disturbed venous drainage, intraosseous venous hypertension, or
increased blood viscosity leading to decreased blood flow.
disorder of epiphyseal hyaline cartilage and thus should be called
Legg-Calvé-Perthes syndrome. This may account for the delayed skeletal
maturation and for the disease’s manifestation in the hip because of
the unusual and precarious blood supply of the proximal femur, which
makes the femoral head especially vulnerable.
irregularities of ossification in other epiphyses and abnormalities in
the contralateral, so-called unaffected capital epiphysis compared with
matched controls.
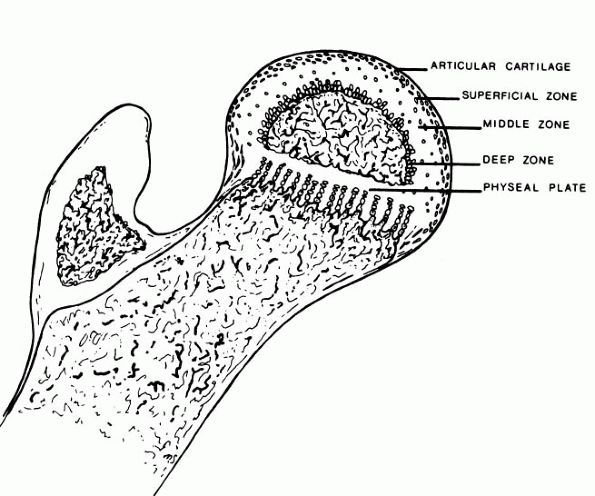
cartilage of patients with LCPD were described as early as 1913 by
Perthes. The superficial zone of the cartilage covering the affected
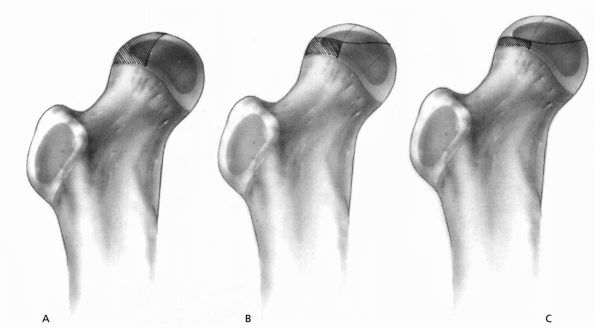
femoral head is normal but thickened (Figure 15-13).
In the middle layer of the epiphyseal cartilage, however, two types of
abnormalities are seen: areas of extreme hypercellularity, with the
cells varying in size and shape and often arranged in clusters; and in
other areas, a loose, fibrocartilaginous-like matrix. These abnormal
areas in the epiphyseal cartilage have different histochemical and
ultrastructural properties than normal cartilage or fibrocartilage.
Areas of small secondary ossification centers are evident, with bony
trabeculae of uneven thickness forming directly on the abnormal
cartilage matrix.
 |
|
FIGURE 15-13. Anatomic regions of the proximal femur in a growing child. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:962)
|
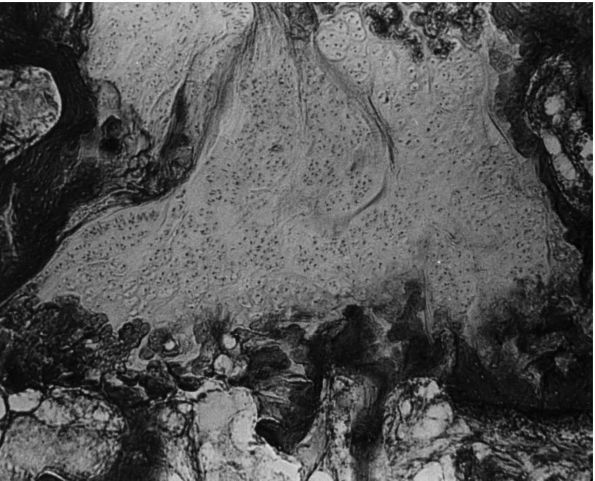
formation with amorphous debris and extravasation of blood. In the
metaphyseal region, enchondral ossification is normal in some areas;
but in other areas, the proliferating cells are separated by a
fibrillated cartilaginous matrix that does not calcify. The cells in
these areas do not degenerate but continue to proliferate without
enchondral ossification. This is evidenced by “tongues” of cartilage
extending into the metaphysis as bone growth proceeds in adjoining
areas (Figure 15-14).
sporadic calcification, and diminished evidence of ossification in the
deep zone of the articular cartilage of the unaffected hip. He also
demonstrated the physeal plate in these unaffected hips to be thinner
than normal, with irregular cell columns and cartilage masses remaining
unossified in the primary spongiosa. Similar histologic changes have
been seen in the acetabular cartilage and other epiphyses. Thus, the
epidemiologic, anthropometric, radiographic, and histologic data lend
support to the concept of the susceptible child. LCPD may thus
represent a localized manifestation of the generalized transient
disorder of the epiphyseal hyaline cartilage, clinically manifested in
the proximal femur because of its unusual and precarious blood supply.
The persistence of the abnormally soft cartilage through which blood
vessels have to penetrate into the femoral head could cause repeated
episodes of infarction and prolong the disease.
similar to that seen in the adult or child after a femoral neck
fracture or a traumatic dislocation of the hip. After a fracture at the
femoral neck or a traumatic dislocation of the hip in a child, the
vascular insult usually heals rapidly without going through the
prolonged stages of fragmentation and repair that are seen in LCPD.
of the insidious onset of a limp. Most patients do not complain of much
discomfort unless
specifically
questioned about this aspect. Pain when present is usually activity
related and relieved by rest. Because of its mild nature, most patients
do not present for medical attention until weeks or months after the
clinical onset of disease. The pain that patients experience is
generally localized to the groin or referred to the anteromedial thigh
or knee region. Failure to recognize that thigh or knee pain in the
child may be secondary to hip pathology may cause further delay in the
diagnosis. Some children present with more acute symptom onset. As with
most childhood musculoskeletal disorders, patients with LCPD usually
present with limited hip motion, particularly abduction and medial
rotation. Early in the course of the disease, the limited abduction is
secondary to muscle spasm of the adductor muscles; however, with time,
subsequent deformities may develop, and limitation of abduction may
become permanent. Occasionally, long-standing adductor spasm leads to
adductor contracture. The Trendelenburg test in patients with LCPD is
often positive. These children most commonly have evidence of thigh,
calf, and buttock atrophy from inactivity secondary to pain. This is
further evidence of the long-standing nature of the condition before
detection. Limb length should be measured; inequality is indicative of
significant head collapse and a poor prognosis. Evaluation of the
patient’s overall height, weight, and bone age may be helpful in the
differential diagnosis and may provide confirmatory evidence of the
disorder. Laboratory studies are generally not helpful in LCPD,
although they may be necessary to rule out other conditions.
 |
|
FIGURE 15-14.
Photomicrograph (×80) showing a large area of cartilage in between the bone trabeculae of the femoral neck (case 1). (Ponseti IV. Legg-Perthes disease: observations on pathological changes in two cases. J Bone Joint Surg 1956;38A:739) |
plain radiographs taken in the anteroposterior (AP) and frog-leg
lateral positions. These radiographs are generally sufficient for the
assessment of the patient and for subsequent follow-up evaluations.
From the plain radiographs, the physician can determine the stage of
the disease and the extent of epiphyseal involvement. Additional
radiographic or imaging studies may be helpful in the initial
assessment or follow-up of the condition.
collimation may be helpful in the early stages of the disease when the
diagnosis is in question, but this is rarely necessary. Some
investigators consider scanning helpful in determining the extent of
the epiphyseal involvement and hence prognosis.
detecting infarction, but as of yet cannot accurately portray the
stages of healing. Its role in the management of LCPD is yet to be
defined.
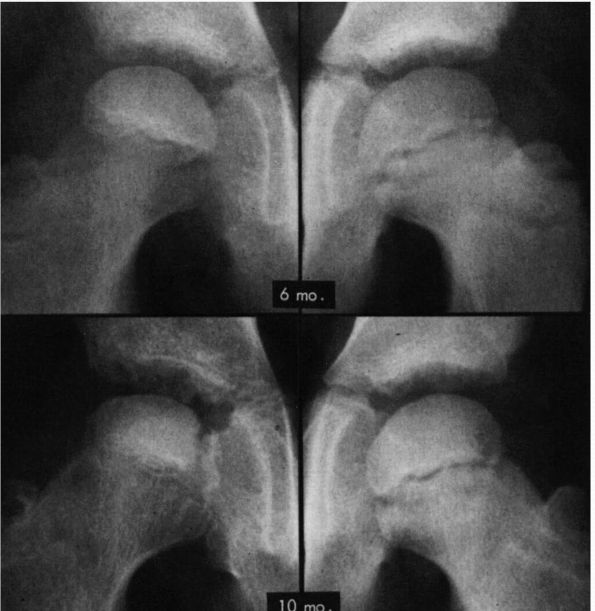
initial stage, the earliest radiographic signs of LCPD are failure of
the ossific nucleus to grow compared with the unaffected hip and
widening of the medial joint space caused by hypertrophy of the
articular cartilage of the femoral head (Figure 15-15).
The physician may also see a relative increase in radiodensity of the
femoral ossific nucleus in relation to the femoral neck. Radiolucencies
may be present in the metaphysis with thinning and irregularity of
the physeal plate (Figures 15-16 and 15-17).
A subchondral radiolucent zone (crescent sign) may also be present.
This radiolucent zone generally corresponds to the extent of the
necrotic portion of the ossific nucleus (Figure 15-18).
 |
|
FIGURE 15-15.
AP radiographs of the hip in a patient who developed Legg-Calvé-Perthes disease. On the initial film taken 6 months after onset of symptoms, the right ossific nucleus is smaller than the left, and the medial joint space is widened. Note also the retained density of the ossific nucleus compared with that of the normal hip and the relative osteopenia of the viable bone of the proximal femur and pelvis. Ten months after onset of symptoms, the evolution of the radiographic changes is seen. (Weinstein SL. Legg-Calvé-Perthes disease. Instr Course Lect 1983;32:272) |
phase. In this phase, the physician sees resorption of the necrotic
portion of the ossific nucleus (Figure 15-19).
This is followed by a reparative or reossification phase, in which the
physician sees return to normal radiodensities of the ossific nucleus
until the lesion is completely healed (see Figure 15-19).
result of the disease, the repair process, or premature physeal plate
closure. The actual deformity that develops is profoundly influenced by
the duration of the disease. This in turn is proportional to the extent
of the epiphyseal involvement, the age of disease onset, the remodeling
potential of the patient, and the stage of disease when treatment is
initiated. An additional factor may be the type of treatment.
long-term follow-ups. In the 20- to 40-year postsymptom-onset
follow-ups, most patients (70 to 90%) are active and free of pain. Most
patients maintain a good range of motion despite the fact that few
patients have normal-appearing radiographs. Clinical deterioration,
increasing pain, decreasing range of motion, and loss of function are
observed in only those patients with flattened irregular femoral heads
at the time of primary healing and in those with evidence of premature
physeal closure. The follow-up studies beyond 40 years, however,
demonstrate marked reduction in function, with most patients developing
degenerative joint disease by the sixth or seventh decade.
identify certain clinical and radiographic features that have
prognostic value. These interrelated factors include deformity of the
femoral head and hip joint incongruence, age of disease onset, extent
of epiphyseal involvement, growth disturbance secondary to premature
physeal closure, protracted disease course, acetabular and femoral
head remodeling potential, type of treatment, and stage during which treatment is initiated.
 |
|
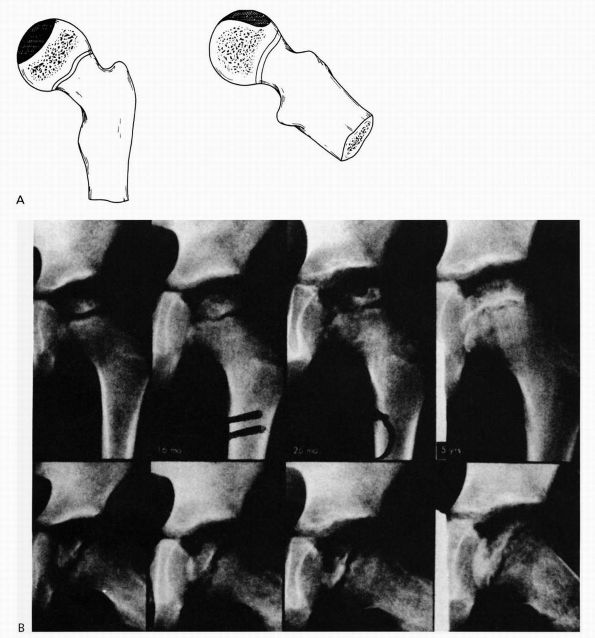
FIGURE 15-16. (A)
Catterall group 1 disease: anterior head involvement, with no evidence of sequestrum or of a subchondral fracture line or metaphyseal abnormalities. (B) Catterall group 1 disease 1 week to 5 years after onset of symptoms. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT and Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:964) |
favorable prognosis than whole femoral head involvement. Catterall
demonstrated the importance of the extent of epiphyseal involvement
relating to prognosis and proposed four groups based on the presence or
absence of seven radiographic signs in 97 untreated hips (Table 15-1; see Figures 15-16 and 15-19).
He reported that 90% of the good results in untreated patients were in
groups 1 and 2, while 90% of the poor results were in groups 3 and 4.
classification based on prognosis: group A had less than 50% femoral
head involvement (Catterall groups 1 and 2); and group B had more
than
50% femoral head involvement (Catterall groups 3 and 4). The major
determining factor between groups A and B is the presence or absence of
a viable lateral pillar of the epiphysis. This intact lateral column
(Catterall group 2, Salter-Thompson group A) may thus shield the
epiphysis from collapse and subsequent deformity (see Figures 15-17 and 15-18).
 |
|
FIGURE 15-17. (A)
Catterall group 2 disease: anterolateral involvement, sequestrum formation, and a clear junction between the involved and uninvolved areas. There are anterolateral metaphyseal lesions, and the subchondral fracture line is in the anterior half of the head. The lateral column is intact. (B) Catterall group 2 disease 3 to 40 months after onset of symptoms. Note the intact lateral pillar. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams and Wilkins, 2001:975) |
 |
|
FIGURE 15-18. (A)
Catterall group 3 disease: large sequestrum involving three-quarters of the head. The junction between the involved and uninvolved portions is sclerotic. Metaphyseal lesions are diffuse, particularly anterolaterally, and the subchondral fracture line extends to the posterior half of the epiphysis. The lateral column is involved. (B) Catterall group 3 disease 4 months to 6 years after onset of symptoms. Note involvement of the lateral pillar as well as the subchondral radiolucent zone on the radiograph taken 8 months after onset of symptoms. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:967-968) |
classification, depends on the radiographic appearance of the lateral
pillar (lateral 15 to 30% of the femoral head) on an AP radiograph (Figure 15-20 and Table 15-2). The more the lateral pillar height is maintained in the maximal fragmentation phase of the disease, the better the outcome.
epiphysis and metaphysis) and calcification lateral to the epiphysis.
These two signs indicate early ossification in the enlarged epiphysis
and are therefore present only when the head is deformed but at a stage
when the changes are reversible. Another at-risk sign is metaphyseal
lesions. These radiolucencies may herald the potential for growth
disturbance of the physeal plate. The final two at-risk signs are
lateral subluxation and a horizontal growth plate. Lateral subluxation
indicates a widened femoral head. The horizontal growth plate indicates
a developing deformity that, if left untreated, leads to a fixed
deformity, hinge abduction, and further deformity. These radiographic
at-risk signs are manifested clinically by loss of motion and adduction
contracture. Catterall reported no poor results in patients not
manifesting two or more at risk signs.
 |
|
FIGURE 15-19. (A)
Catterall group 4 disease: whole head involvement with either diffuse or central metaphyseal lesions and posterior remodeling of the epiphysis. (B) Catterall group 4 disease 2 to 52 months after onset of symptoms. Note the stage: 14 months—fragmentation; 18 months—early reossification; 25 months—late reossification; 52 months—healed. Note also the growth arrest line and evidence of reactivation of the growth plate along the femoral neck. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:966-967) |
|
TABLE 15-1. Catterall Groups
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
FIGURE 15-20. Lateral pillar classification. See Table 15-2. (Weinstein
SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:976) |
|
TABLE 15-2. Lateral Pillar Classification
|
||||||
|---|---|---|---|---|---|---|
|
 |
|
FIGURE 15-21.
Shelf arthroplasty in Legg-Calvé-Perthes disease. A popular procedure currently that will have to await long-term results to know if it improves prognosis. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001—Fig. 24-27) |
 |
|
FIGURE 15-22. A 6-year, 5-month-old boy with Catterall group 4 disease and all the at-risk signs. (Weinstein
SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:978) |
duration of the disease. In general, the greater the extent of
epiphyseal involvement, the longer the duration and course of the
disease. End results are worse with prolonged disease duration.
to outcome. The younger the patient at disease onset, the better is the
prognosis. Eight years of age appears to be the watershed age in most
long-term series. Age at healing, however, is probably a more important
factor. Because of the overall skeletal maturation delay and the
knowledge that this is usually compensated for during the adolescent
growth spurt, patients affected at a younger age have an enhanced
potential for femoral head and acetabular remodeling. Femoral head
at-risk signs are also less likely to occur in younger patients,
particularly those younger than 5 years of age.
geometric pattern within it during growth, and because the acetabulum
continues to have significant development potential up to age 8 or 9
years of age, if a young patient does develop deformity, the
immature
acetabulum conforms to the altered femoral head shape. This leads to
aspheric congruency, which may be compatible with normal function for
many years.
radiographs are usually sufficient to make a diagnosis of LCPD.
Diagnosis early in the initial phase of the disease, however, must be
differentiated from conditions such as transient synovitis (Table 15-3)
and septic arthritis (primary or secondary to proximal femoral
osteomyelitis). A complete blood count, including white blood cell
differential, erythrocyte sedimentation rate, C-reactive protein, and
hip joint aspiration, and analysis of the fluid may be necessary to
rule out infection. All laboratory studies of LCPD are generally
normal, although the erythrocyte sedimentation rate and or the
C-reactive protein may be slightly elevated. In early cases, if all the
laboratory studies are normal, and doubt as to the diagnosis persists,
radionuclide scanning may be helpful.
disorders, such as multiple epiphyseal dysplasia and hypothyroidism,
must be considered. Patients with bilateral involvement, particularly
those with atypical radiographic features, must have a careful family
history obtained as well as a bone survey to rule out a metabolic or a
genetic condition. In children younger than 4 years of age, Meyer
dysplasia, a benign-resolving condition, must be considered.
Treatment modalities have evolved from the earliest treatments of
weight relief until the head was reossified to the present day
containment methods. The essence of containment is that, to prevent
deformities of the diseased epiphysis, the femoral head must be
contained within the depths of the acetabulum to equalize the pressure
on the head and subject it to the molding action of the acetabulum.
Containment is an attempt to reduce the forces through the hip joint by
establishing an actual or relative varus relation between the femoral
head and the acetabulum. Considering all methods of containment, the
physician must realize that the femoral head represents over
three-fourths of a sphere and the acetabulum only half of a sphere.
Therefore, no method of containment can provide a totally contained
femoral head within the acetabulum during all portions of the gait
cycle.
|
TABLE 15-3. Differential Diagnosis of Legg-Calvé-Perthes Disease
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
prevent deformity, alter growth disturbance, and prevent degenerative
joint disease. To attain these goals, the patient must be assessed
clinically and radiographically. Clinically, the patient is evaluated
for at-risk signs of pain and loss of motion. AP and lateral
radiographs are evaluated to determine the radiographic stage of the
disease, the extent of epiphyseal involvement, and the presence of any
at-risk signs. The optimal time for treatment is during the
radiographic initial or fragmentation stage of the disease. Once the
head is in the reossification stage, little further deformity occurs;
thus, to influence deformity, treatment must be initiated earlier. Some
difficulties may be encountered in determining the extent of epiphyseal
involvement, especially early in the disease process, and radionuclide
scanning or MRI may be helpful.
if the child demonstrates none of the clinical or radiographic at-risk
signs, if the patients has Catterall group 1 disease, lateral pillar A
(Salter-Thompson group A), or if the disease is already in the
reossification stage. A child who demonstrates clinical or radiographic
at-risk signs, regardless of the extent of epiphyseal involvement,
should receive treatment. Even patients with Catterall group 2 disease
or lateral pillar B who are at risk may end up with a poor result
without treatment.
motion. Motion enhances synovial nutrition and thus cartilage
nutrition. Restoration of motion can be accomplished by putting the
patient at rest with skin traction and progressive abduction to relieve
the adductor spasm. Occasionally, surgical release of the contracted
adductors may be necessary. Restoration of motion allows abduction of
the hip, which reduces the force on the hip joint and allows
positioning of the uncovered anterolateral aspect of the femoral
head
in the acetabulum (containment). Mobilization of the hip joint may also
be obtained by use of progressive abduction casts. Treatment appears to
give superior results in severely involved patients (Catterall groups 3
and 4, or Salter-Thompson group B, lateral pillar B, C) compared with
no treatment.
 |
|
FIGURE 15-23. A 4-year, 9-month-old boy with Catterall group 4 disease and at-risk status. (A) Plain film. (B)
Arthrogram in neutral abduction, adduction, and rotation. Note enlargement and flattening of the cartilaginous femoral head and how the lateral margin of the acetabulum is deformed by the femoral head. (C) Arthrogram in abduction and slight external rotation. Note how the femoral head hinges on the lateral edge of the acetabulum, further deforming the lateral acetabulum. Also note the slight pooling of dye medially. (Weinstein SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:966) |
the head actually can be contained and, if so, in what position this is
best accomplished. Arthrography demonstrates any flattening of the
femoral head that may not be seen on plain film. More important, it may
demonstrate the hinge abduction phenomenon, which is a contraindication
to any type of containment treatment. Once the femoral head becomes
deformed and is no longer containable within the acetabulum, the only
motion that is allowed is in the flexion and extension plane, with
abduction leading to hinging on the lateral edge of the acetabulum.
This hinge abduction causes acetabular and secondary femoral head
deformity (Figure 15-23).
treatment are femoral osteotomy and innominate osteotomy. Abduction
bracing, which was the most commonly used method of treatment for many
years, is now rarely used. Most abduction braces were modifications of
the Atlanta Scottish Rite orthosis (Figure 15-24). Several studies, however, questioned the efficacy of brace treatment in LCPD, and in recent years braces are rarely used.
containment offer the advantage of early mobilization and avoidance of
prolonged brace or cast treatment. Varus osteotomy with or without
rotation offers the advantage of deep seating of the femoral head and
positioning of the vulnerable anterolateral portion of the head away
from the deforming influences of the acetabular margin (Figure 15-25).
It has been reported that this procedure improves disturbed venous
drainage and relieves interosseous venous hypertension, thus
accelerating the healing process. This, however, has not been
conclusively confirmed. Prerequisites for the procedure include full
range of motion, congruency between the head and the acetabulum, and
the ability to seat the head in abduction and internal rotation. As
with all containment treatment modalities, to have any effect,
treatment must be instituted in the initial or fragmentation stage of
the disease. The negative aspects of this treatment include the
associated risks and costs of the surgical procedure in addition to a
second surgical procedure necessary for any hardware removal. The
affected limb is also shortened by the procedure. The varus angulation
normally decreases with growth, but if there has been physeal plate
damage by the disease, this remodeling potential may be lost, leaving
the patient with a permanent varus deformity and limb shortening.
provides for containment by redirection of the acetabular roof,
providing better coverage for the anterolateral portion of the head. It
places the head in relative flexion, abduction, and internal rotation
with respect to the acetabulum in the weight-bearing position. Any
shortening caused by the disease process is corrected. Prerequisites
for innominate osteotomy include a full range of hip joint motion,
joint congruency with the
ability
to seat the head in flexion, abduction, and internal rotation. The
procedure must be done in the initial or fragmentation stage of the
disease. The disadvantages of innominate osteotomy are the inherent
risks of the surgical procedure, the fact that the operation is being
performed on the normal side of the joint, and the suggestion that the
procedure may increase the forces on the femoral head by lateralizing
the acetabulum and increasing the lever arm of the abductors.
Satisfactory anatomic results have been reported for all these
containment methods in carefully selected patients.
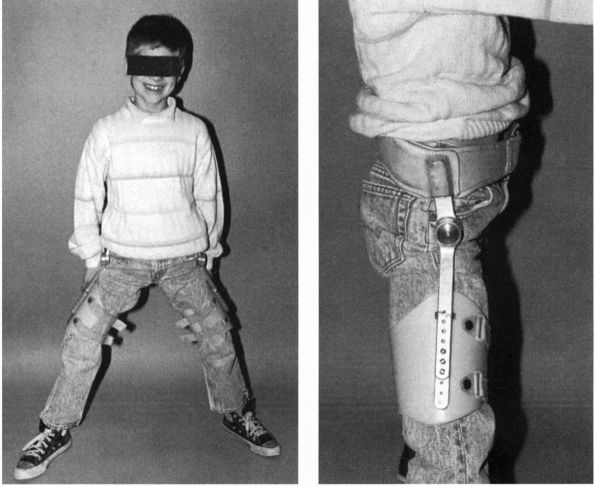 |
|
FIGURE 15-24. An abduction orthosis. (Weinstein
SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001:986) |
has been gaining in popularity in the patient with a poor prognosis
(Catterall 3, 4, lateral pillar B, C, children > 8 years of age).
This procedure is aimed at providing coverage for a femoral head that
is certain to enlarge because of the disease process. Long-term
outcomes of this procedure will determine its role in LCPD treatment.
chosen, any episode indicative of loss of containment (i.e., recurrent
pain and loss of range of motion) must be treated aggressively by rest
and traction or casting to restore lost motion.
demonstrate the hinge abduction phenomenon on arthrography, the
physician must consider other alternatives. These salvage procedures
include Chiari osteotomy, cheilectomy, abduction extension osteotomy,
and acetabular shelf procedures alone or in combination with femoral
osteotomies. These procedures in an already deformed head must be
viewed as salvage procedures with the limited aims of pain relief,
correction of limb length inequality, and improvement of movement and
abductor weakness. Cheilectomy removes the anterolateral portion of the
head that is impinging on the acetabulum in abduction. This procedure
must only be done after the physis is closed; otherwise, a slipped
capital femoral epiphysis (SCFE) may ensue. This procedure does not
correct any residual shortening or abductor weakness. The Chiari
osteotomy improves the lateral coverage of the deformed femoral head
but does not reduce the lateral impingement in abduction and may
exacerbate any existing abductor weakness. Its role in LCPD is yet to
be defined. Abduction extension osteotomy of the femur is indicated
when arthrography demonstrates joint congruency improved by the
extended, adducted position. Preliminary results indicate improvement
in limb length, decrease in limp, and improvement in function and range
of motion. This osteotomy is gaining many advocates because of its
early promising results. Long-term results will be necessary to
determine its role in the treatment of LCPD.
matched for age and degree of epiphyseal involvement are needed to
determine the
most
effective treatment of LCPD. As our fundamental understanding of LCPD
increases, so too will our understanding of how various treatment
modalities influence this complex growth disturbance.
 |
|
FIGURE 15-25.
Varus/derotation osteotomy of Axer. This embodies the principle of containment of the diseased femoral head in the treatment of Legg-Calvé-Perthes disease, which is achieved by surgical means. Postoperatively, the child is permitted to walk with no restrictions, and the range of motion is full, so that the molding effect of the acetabulum on the femoral head is attained. (A) Severe involvement of femoral epiphysis in a boy 5 years 8 months of age, 9 months after onset of limp and pain in the left hip. (B) and (C) Ten years after varus/derotation osteotomy, excellent development of the femoral head is seen. (Courtesy of Dr. A. Axer) |
pain in the young child. This condition is often referred to in the
literature by other terms, including irritable hip, toxic synovitis, observation hip, coxitis serosa, and coxal gia fugax, to name a few.
percentage of the children have a recent history of an upper
respiratory tract infection, a viral origin has been suspected, as has
an allergic reaction to an infectious agent. A history of trauma can
sometimes be associated with symptom onset, but no causal relation has
been established. Biopsy material from patients with transient
synovitis demonstrates nonspecific inflammatory changes and synovial
hypertrophy.
and limp in children under 10 years of age. Affected children range in
age from 3 to 12 years, with the average patient being between 5 and 6
years of age. Boys are affected two to three times as often as girls.
Right and left hips are affected equally. Ninety-five percent of cases
are unilateral.
with a history of hip pain or limp. The pain onset is acute in about
half of cases, with symptoms being present for 1 to 3 days before
presentation. In the other half of patients, the symptoms of limp or
pain are more chronic in nature, often being present for weeks to
months. The pain in most cases is mild, but in some children, it may be
severe enough to awaken the child at night. In some cases, the patient
may not admit to pain. When present, pain is usually localized to the
groin region but may be referred to the medial thigh or knee region.
Take a careful medical history, looking for any history of antecedent
infection, such as an upper respiratory infection, otitis media, strep
throat, trauma, or other precipitating factors.
rotation of the hip joint. Pain can usually be elicited at the extremes
of motion, especially abduction and medial rotation. In some children,
guarding may be evident by gently trying to roll the leg into internal
rotation while the hip is extended. Patients may have evidence of thigh
atrophy, depending on the duration of symptoms. There may also be
tenderness to palpation in the groin. The gait of an affected child is
usually antalgic. The child may walk with the hip in slight flexion,
external rotation, and abduction.
protein are usually normal but may be mildly elevated. The white blood
cell count is generally normal with a normal differential.
Bone density is normal in all cases; if alteration in normal densities
is present, another source of the hip pain should be sought. Loss of
the hip capsular shadow outline has been reported in cases of toxic
synovitis; this sign, however, is a radiologic artifact related to
holding the hip in abduction and external rotation. Bone scanning may
reveal normal or increased uptake in the proximal femoral epiphysis.
Ultrasonography may demonstrate the presence of a mild effusion.
important in that certain of these conditions can have devastating
consequences if not diagnosed. Septic arthritis must be ruled out.
Children with septic arthritis generally present with pain, elevated
temperature, elevated white blood cell count, and elevated
sedimentation rate and C-reactive protein. Septic arthritis of the hip
may be accompanied by osteomyelitis of the proximal femur (Chapter 5).
Aspiration of the joint must be done when the diagnosis is uncertain.
An arthrogram should be done at the same time as the aspiration to make
sure that the hip joint has been entered. Rheumatic fever must also be
excluded. The hip may be the first joint involved before the
development of migratory polyarthralgia. These patients usually give a
history of a β-hemolytic streptococcal
infection 1 to 3 weeks before the onset of hip pain. Other major or
minor manifestations of this disorder should be sought.
the same age range as transient synovitis but has a slightly greater
male predominance. Most LCPD patients have retardation of bone age.
Bone scans in the early stages of LCPD may show decreased uptake in the
femoral head. MRI scans may prove to be helpful to differentiate
transient synovitis from LCPD. Many studies in the literature suggest
that transient synovitis is a precursor to LCPD disease. The
literature, however, suggests that only 1 to 3% of cases of transient
synovitis are associated with the later development of LPCD.
 |
|
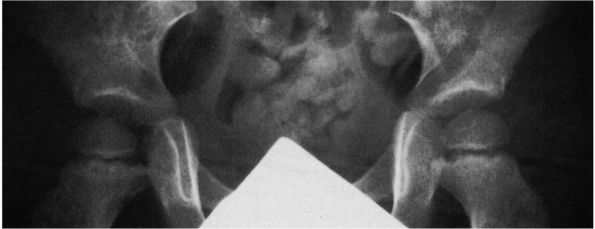
FIGURE 15-26.
AP radiograph of a child with transient synovitis of the right hip. Note the slightly widened medial joint space in the right hip. |
particularly osteoid osteoma of the proximal femur, must be included in
the differential diagnosis of any child with hip pain. Osteoid osteoma
usually is accompanied by a history of night pain relieved by aspirin.
SCFE is usually seen in the obese adolescent during a growth spurt and
has typical radiographic features.
synovitis reveal that many of the patients have secondary coxa magna
and a widened femoral neck as a residual of the condition. The question
of whether these patients will develop degenerative arthritis over the
long-term remains to be answered.
condition. When the diagnosis is in question or the patient is
particularly uncomfortable, hospitalization is often necessary. Light
skin traction may be applied for comfort. Anti-inflammatory agents may
be used for a short time to relieve pain. As symptoms resolve,
crutch-protected weight bearing may begin, with gradual resumption of
full weight bearing as symptoms abate. Most patients have resolution of
symptoms in 3 to 7 days, but in many patients, symptoms may persist for
weeks to months. The condition is self-limiting; most children have
only a single episode of hip pain, and recurrences are uncommon unless
the child is returned to full activity before symptoms resolve.
descriptive term referring to the angular relation between the femoral
head or neck, or both, and the femoral shaft, which is less than the
normal value for the patient’s age. This abnormal relation may be
congenital, developmental, or acquired. It is most important to
distinguish between these three etiologic groups because each has its
own natural history. This section deals only with developmental coxa
vara.
the cervical region of the proximal femur that are accompanied by a
widened and vertically oriented physeal plate (Figure 15-27).
The shaft of the femur is normal. Clinical and radiographic features
are not present at birth. Developmental coxa vara is an extremely rare
condition equally affecting boys and girls. About 30% of the cases are
bilateral. A familial tendency has been reported, but the exact mode of
inheritance is unknown. The cause of developmental coxa vara is
unknown, but many theories have been postulated, including an embryonic
vascular disturbance and regional dysplasia of the proximal femur.
limb length inequality or abnormal gait. Although the gait abnormality
may be evident when the child starts to walk, patients generally do not
seek medical attention until the child is 3 to 7 years of age. The limp
or waddling gait (in bilateral cases) is painless and usually
progressive. Older children may complain of easy fatigability. Limb
shortening is usually evident.
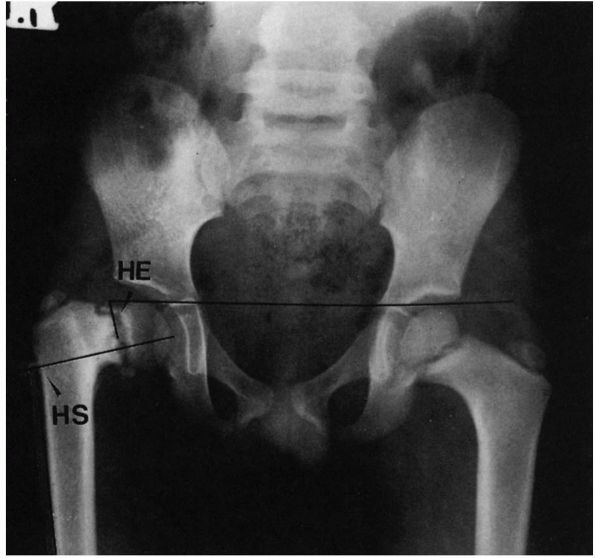 |
|
FIGURE 15-27. Coxa vara development. Note head-shaft angle (HS) and Hilgenreiner epiphyseal angle (HE).
|
Examination of the involved extremity reveals limited abduction and
internal rotation, a positive Trendelenburg test, limb shortening, and
trochanteric elevation. In bilateral cases, hyperlordosis of the lumbar
spine is present, and the patient may have genu valgum. Limb length
inequalities in developmental coxa vara rarely exceed 2 cm. In
bilateral cases, the amount of shortening may be asymmetric.
femurs and hips. The diagnosis is made by the presence of anatomic coxa
vara, widened vertically oriented physeal plate, shortened neck, normal
straight femoral shaft, and separate triangular ossification center on
the inferior part of the femoral neck. This triangular ossification
center may appear irregular and fragmented. A vertically oriented
physeal plate borders the triangular fragment medially, while lateral
to it is a vertical defect in the femoral neck. The femoral head is
spherical and the acetabulum generally normal, although mild dysplasia
may be apparent in comparison to the opposite, normal side.
These measurements include the head-shaft angle, the neck-shaft angle,
and the Hilgenreiner epiphyseal angle. The head-shaft angle has been
found best to follow progression of deformity in that the neck-shaft
angle remains fairly constant even in the face of progressive
deformity. The Hilgenreiner epiphyseal angle has been found to be a
method of evaluation and prognostication for patients with
developmental coxa vara.
congenital coxa vara and coxa vara acquired secondary to other
conditions. Congenital coxa vara is detectable at birth and is
accompanied by shortening of the proximal femur. Congenital coxa vara
with a short femur is part of the spectrum of proximal femoral focal
deficiency. The varus in this condition is generally in the
subtrochanteric region or in the upper femur, and varying degrees of
femoral shortening are seen. The head is abnormal in appearance, and
acetabular dysplasia is generally present. The varus relation in this
condition generally does not worsen with time and in general need not
to be addressed. Anatomic coxa vara can also be seen in patients with
metabolic bone disease such as
rickets,
fibrous dysplasia, osteogenesis imperfecta, Ollier’s disease, SCFE, and
sepsis. A radiographic appearance similar to developmental coxa vara is
seen in patients with coxa vara secondary to metaphyseal
chondrodysplasia and in patients with coxa vara and cleidocranial
dysostosis. Coxa vara associated with cleidocranial dysostosis is
usually present at birth, and patients have clavicular abnormalities,
wormian bones, and abnormal dentition. In metaphyseal chondrodysplasia,
there is generalized widening of the physeal plates. The hip
radiographic abnormalities are bilateral and symmetric, and the femoral
shafts are bowed. In bilateral cases of developmental coxa vara, the
deformity may not be symmetric.
promote ossification of the defect and to correct the varus deformity,
allowing restoration of the mechanical advantage of the hip abductors
to improve gait and to equalize limb lengths. In progressive coxa vara,
the natural history suggests increasing deformity (Figure 15-28),
decreasing function, and early degenerative joint disease. The general
indications for surgical treatment include increasing coxa vara and a
neck-shaft angle of less than 100°. In mild, nonprogressive cases with
a neck-shaft angle of greater than 100° and a Hilgenreiner epiphyseal
angle of less than 45°, resolution of the defect may occur, and
observation of the patient with serial follow-up radiographs is
indicated. In patients with a limp, progressive deformity, and a
Hilgenreiner epiphyseal angle of greater than 60°, intertrochanteric or
subtrochanteric abduction osteotomy is the treatment of choice (Figure 15-29).
In these patients, the neck-shaft angle should be restored to decrease
the shear stress across the vertical defect. With surgery, the defect
generally heals, but growth plate arrest may be seen in a significant
number of patients, leading to limb length inequality. Generally,
patients older than 5 years of age at the time of surgery maintain
their correction.
 |
|
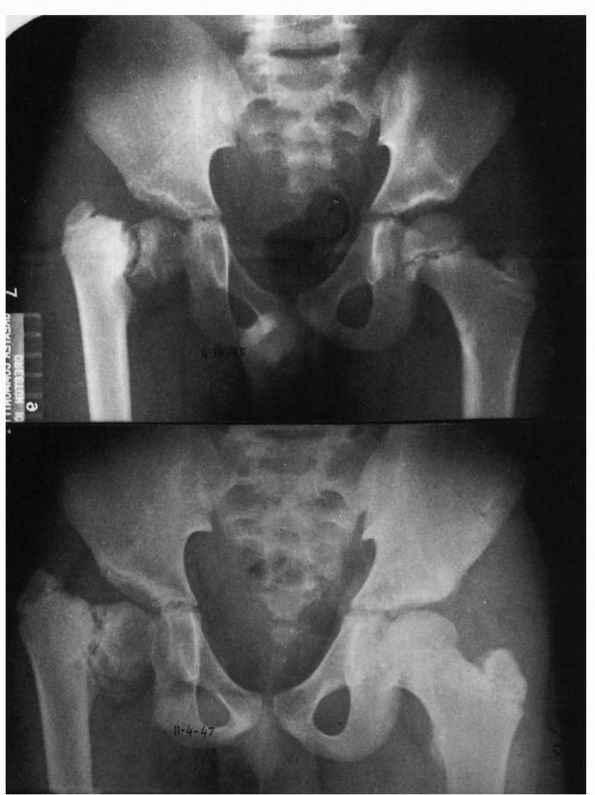
FIGURE 15-28. Coxa vara development. Note triangular fragment and worsening of condition over a 2-year period (bottom).
|
in which the capital femoral epiphysis is displaced from the metaphysis
through the physeal plate (Figure 15-30). The term slipped capital femoral epiphysis
is actually a misnomer in that the head is held in the acetabulum by
the ligamentum teres, and thus it is actually the neck that comes
upward and outward while the head remains posterior and downward in the
acetabulum (Figure 15-31). In most cases, a
varus relation exists between the head and neck, but occasionally the
slip is into valgus, with the head displaced superiorly and posteriorly
in relation to the neck.
is about 2 cases per 100,000. The incidence of SCFE is higher in all
blacks, but especially black girls. The disorder generally occurs in
the age range of 10 to 14 years in girls (mean, 11.5 years) and 10 to
16 years in boys (mean, 13.5 years). Seventy percent of affected
patients have delayed skeletal maturation. Skeletal age may lag behind
chronologic age by as much as 20 months. There is a male predominance
of 2.5:1. The left hip is twice as often affected as the right hip.
Other epidemiologic factors may include seasonal variations and social
class.
half of affected patients are above the 95th percentile in weight for
their age. Three-fourths of affected boys and half of affected girls
are
above
the 90th percentile in weight for their height. It has been disproved
that tall thin people are predisposed to this condition.
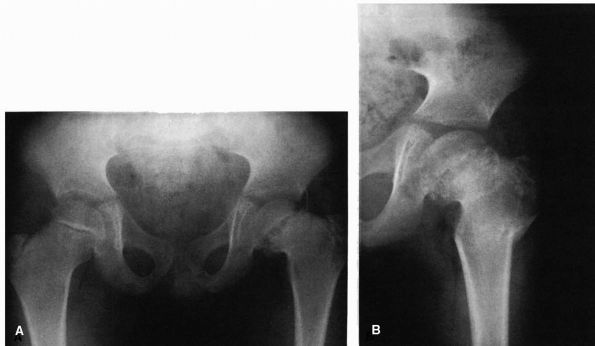 |
|
FIGURE 15-29. (A) Eight-year-old boy with coxa vara. (B) Eighteen months after abduction osteotomy.
|
accepted as being 25%. This figure may be low in that about half of
bilateral slips are asymptomatic. This factor becomes important when
considering the natural history of the disease.
generally exhibits changes characteristic of synovitis, with
hypertrophy and hyperplasia of the synovial cells, villus formation,
increased vascularity, and round cell infiltration. Light microscopic
studies reveal that the physis is widened and irregular, sometimes
reaching 12 mm in width (normal is 2.6 to 6 mm). Normally, the resting
zone accounts for 60 to 70% of the width of the physis, whereas the
hypertrophic zone accounts for only 15 to 30% of the width. In SCFE,
the hypertrophic zone may constitute up to 80% of the physis width.
Light microscopic studies also document that the actual slip takes
place through the zone of hypertrophy, with occasional extension into
the calcifying cartilage (Figure 15-32). On the
basis of histologic studies, it is apparent that the slip occurs
through the weakest structural area of the plate, the hypertrophic zone.
probably act either by altering the strength of the zone of hypertrophy
or by affecting the shear stress to which the plate is exposed.
Although trauma may be a contributing factor, it is certainly not the
sole cause; the pathology of SCFE differs from that seen in physeal
fractures.
implicated in the cause of SCFE. Although there have been no specific
endocrine abnormalities detected in patients with SCFE, there are
numerous reports of SCFE associated with specific endocrine
abnormalities, such as primary hypothyroidism, hypothyroidism secondary
to panhypopituitarism, intracranial tumors, hypogonadism, treatment
with chorionic gonadotropin, and treatment of hypothyroidism and growth
hormone deficiency with growth hormone. SCFE has also been reported in
patients undergoing radiation to the pelvis, patients with rickets, and
patients with renal osteodystrophy.
castrated rats leads to increased thickness of the zone of hypertrophy
and decreased shear strength. Estrogen, on the other hand, given to
otherwise normal rats, leads to thinning of the growth plate and
increased shear strength in similar experiments (Figure 15-33).
the slippage because 78% of slips occur during the adolescent growth
spurt, and in girls, slips occur before menarche. Hormonal theories
have been used to account for many of the epidemiologic factors. For
example, the age range of occurrence corresponds to the adolescent
growth spurt for boys and girls. The gender prevalence of boys
may
be accounted for by boys having a longer and more rapid growth spurt.
The high percentage of obesity (adiposogenital syndrome patients) has
been attributed to an abnormal relation between growth hormone and sex
hormone, giving a relative predominance to the growth effect. Thus,
hormonal imbalance may lead to a structural weakening of the growth
plate, leaving it more susceptible to slipping.
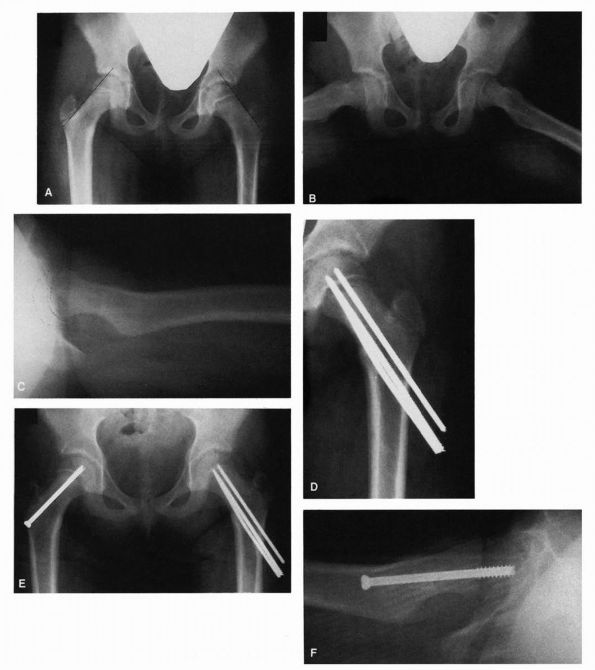 |
|
FIGURE 15-30. Ten-year-old girl with left hip pain for 3 months. (A) AP radiograph of pelvis. Note the Klein line. It should normally intersect at least 20% of the femoral head. (B) Lauenstein view demonstrates mild slip on left side. (C) True lateral view of left hip demonstrating mild slip. (D) The slip was treated with multiple threaded pins. (E)
One year later, the patient complained of pain in the right groin and had a minimal slip. The right-sided slip was treated with a single screw (AP view). (F) Lateral view of postoperative pinning. Note central position of screw in epiphysis. With the advent of cannulated screws, threaded pins are rarely used unless cannulated screws are unavailable. Threaded pins often cause soft tissue irritation and later removal. |
manifestation of a generalized systemic disorder, secondary to a
biochemical abnormality in cartilage collagen production or secondary
to a
mechanical
disturbance caused by shear forces applied to a retroverted proximal
femoral epiphysis. Genetic factors may contribute but have thus far not
been identified. SCFE is probably a multifactorial disorder, with the
slip representing the final manifestation of one of several
predisposing factors.
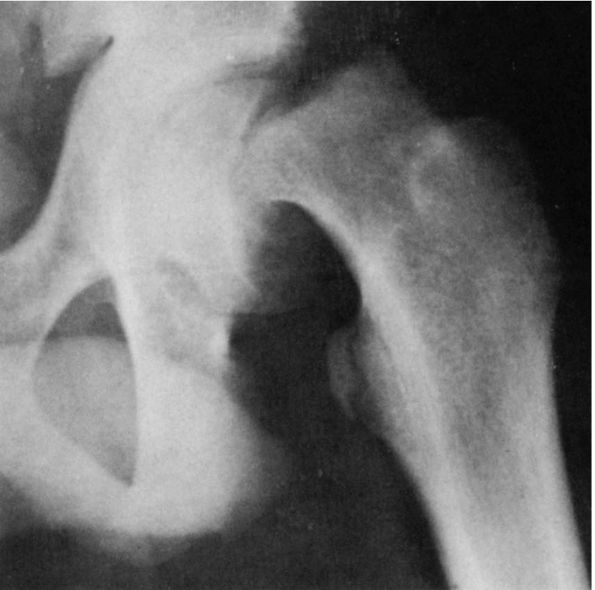 |
|
FIGURE 15-31. Complete slipped upper femoral epiphysis. Commonly termed acute slipped epiphysis, in reality, it is a form of fracture displacement that can also occur at other epiphyseal sites.
|
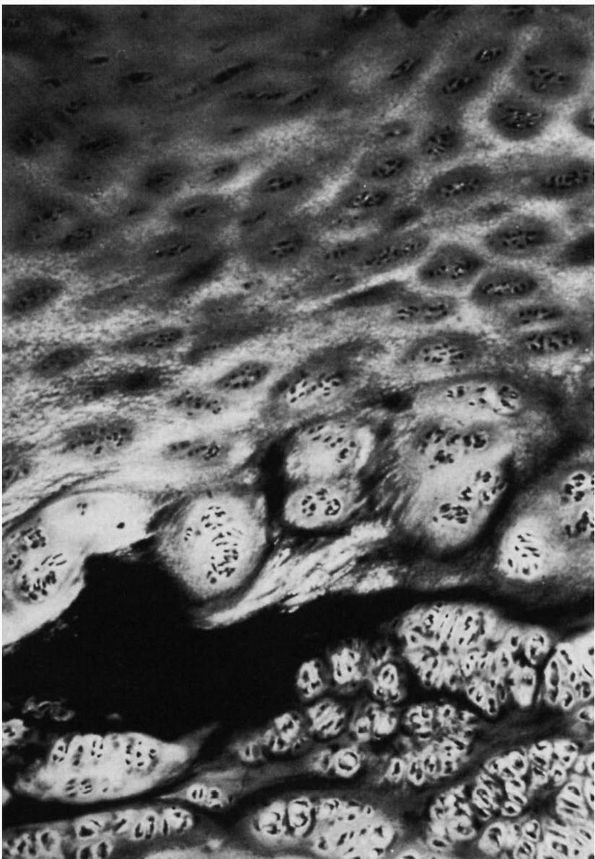 |
|
FIGURE 15-32.
Physeal plate from a patient with slipped capital femoral epiphysis. Note slip (cleft) in zone of hypertrophy. Also note the abnormal architecture of the physeal plate. The zone of hypertrophy is increased in width, and the cells are in clusters and clumps. |
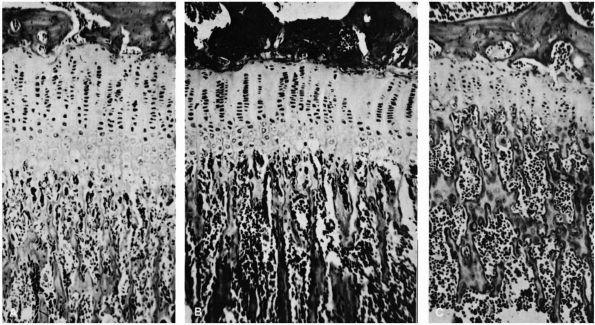 |
|
FIGURE 15-33. Effect of growth and sex hormone on the epiphyseal plate. (A)
Growth hormone treated. The plate is thickened mainly by marked proliferation and accumulation of maturing chondrocytes. The layer of hypertrophied chondrocytes is fragile because it is deficient in matrix. (B) Untreated control. The layer of maturing chondrocytes is narrow and contributes little to the thickness of the plate. (C) Treated with sex hormones. The entire plate is narrow, the chondrocytes lack the orderly columnar arrangement, mature cartilage cells are rare, and bony trabeculae are numerous and thick. Epiphyseal separation is unlikely. (Harris WR. The endocrine basis for slipping of the upper femoral epiphysis. J Bone Joint Surg 1950;32B:5) |
examination, and radiographs, SCFE can be classified into four
categories—preslip, acute, acute on chronic, and chronic. This
traditional classification is being superceded by a more clinically
relevant two group scheme (stable versus unstable), which is dependent
on stability of the hip and relates well to outcome.
weakness in the leg or limping on exertion; pain may occur in the
groin, adductor region, or knee with prolonged standing or walking. On
physical examination in this phase, the most consistent positive
finding is lack of medial rotation of the hip in extension. When the
affected leg is fixed, the thigh goes into abduction and external
rotation, a sign pathopneumonic for SCFE. Radiographically, there is
generalized bone atrophy and disuse osteopenia of the hemipelvis and
upper femur only in those patients who limped or limited their
activity. There is widening, irregularity, and blurring to the physeal
plate (Figure 15-34). The preslip may in
actuality be a minimal slip that is not seen on standard radiograph but
may possibly be seen on CT or MRI scans.
proximal epiphyseal cartilage plate in which there was a preexisting
epiphysiolysis. Acute slips account for about 10% of the slips in most
large reported series (see Figure 15-31). The
clinical criterion of having the acute onset of symptoms for less than
2 weeks is generally accepted as the clinical definition of an acute
slip. A review of the literature, however, reveals that 76% of patients
with acute slips give a history of mild prodromal symptoms for 1 to 3
months before their acute episode, thus indicating that they probably
had a preslip or mild slip preceding their acute symptom onset. These
prodromal symptoms of mild weakness, limp, and intermittent groin,
medial thigh, or knee pain are usually followed by some history of
minor trauma or of direct trauma, with immediate increase in pain and
inability to use the extremity. The pain is usually severe enough to
prevent weight bearing. If the patient can walk, it is with difficulty
and with a limp. SCFE in the patient with a history of mild prodromal
symptoms may better be classified as an acute-on-chronic slip.
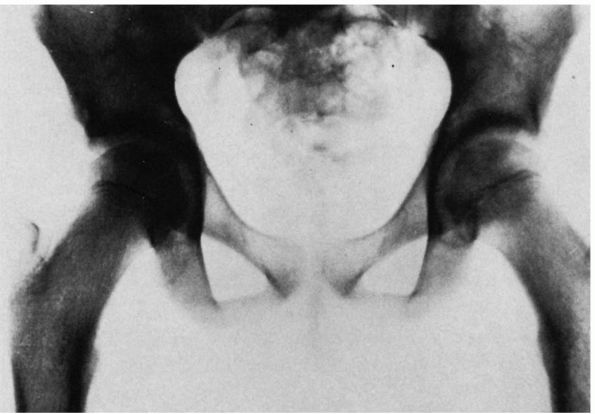 |
|
FIGURE 15-34. Before slip. Note widened, irregular, and blurry physeal plate on left hip. Left hemipelvis is relatively osteopenic.
|
an antalgic gait. These patients have an external rotation deformity,
shortening, and marked limitation of motion. Any attempted motion is
painful because of the marked spasm of the hip muscles. In general, the
greater the amount of slip, the greater is the restriction of motion.
The physical examination must be performed gently so as not to cause
further displacement. In the acute slip, because of pain, the physician
may not be able to elicit the classic sign of SCFE (thigh abduction and
external rotation as the thigh is flexed). Generally thigh or calf
atrophy is present, except in those few patients without any prior
symptoms.
groin or medial thigh pain for months to years. A high percentage have
knee or lower medial thigh pain as their initial symptom. They may give
a history of exacerbations and remissions of the pain or limp. On
physical examination, all these patients have limitation of motion
(particularly medial rotation) and shortening, and most have thigh or
calf atrophy.
presents much like an acute trauma patient with the inability to walk
even with crutches. This slip encompasses many patients who would fall
into the old classification as acute or acute on chronic. This unstable
group has a significant risk of developing aseptic necrosis of the
epiphysis, which generally leads to a poor long-term outcome. Stable slips
are all others that present with nonacute fracturelike symptoms—that
is, the patient is able to walk on the hip even with crutches. Outcomes
of treatment of this group are related to the amount of displacement
and the avoidance of complications of treatment.
lateral. Because of the unstable nature of the hip, the frog-leg
lateral view may accentuate the deformity in the unstable, acute, or
acute-on-chronic slip.
by drawing a line along the superior basal margin of the neck.
Normally, it should transect 20% or more of the epiphyses (see Figure 15-28).
Widening and irregularity of the physeal growth plate and decreased
height of the epiphyses in the central acetabulum are also seen, and
the medial junction of the epiphyses and the metaphyses fall outside
the acetabulum. The lateral view is probably the best to detect the
slip because the head is posterior in relation to the neck at first and
thus may be missed on the AP view. In minimal slips, the displacement
is initially posterior and may only be seen on the lateral radiograph.
anatomic displacement either on the AP or lateral radiograph. This
classification is important as long-term outcome in the absence of
surgical complications is totally related to the amount of
displacement. One classification of displacement is as follows:
-
Minimal slip: maximal displacement is less than one-third the diameter of the neck
-
Moderate slip: greater than 1 cm of displacement but less than half the diameter of the neck
-
Severe slip: displacement greater than half the diameter of the neck
predisposing factors such as retroversion or a widened physeal plate in
the radiographically normal hip to try and predict a propensity to
slip. The role of these modalities must await further investigation.
controversial because there are very few studies. There are few
long-term studies of patients with SCFE, and included in these are few
untreated patients. What is clear however is that SCFE should be
considered an orthopaedic emergency and patients treated immediately.
Any delay in management runs the risk of further displacement and worse
long-term outcome.
disease, the percentage of patients with known SCFE is small, averaging
about 5%. The actual incidence may be higher because controversy exists
about whether a higher percentage of patients with so-called primary
degenerative arthritis had unrecognized slips or whether these
radiographic features are merely secondary to primary degenerative
joint disease.
long-term prognosis, with degenerative joint disease and function being
related to the severity of deformity. Those with moderate slips retain
good function for many years, whereas those with severe slips have
early degenerative joint disease and poor function. Poor results are
occasionally seen even in minimal slips, usually as a result of a
complication of treatment. The radiographic changes, however, do not
always correlate with clinical symptoms over time.
further displacement of the epiphysis and to promote closure of the
physeal plate. Treatment of SCFE should be considered an emergency.
Once the diagnosis is made, the patient should not be allowed to bear
weight. Treatment should be initiated immediately. Delay in treatment
as mentioned above may result in further displacement of the femoral
epiphysis with compromise of the remaining intact blood supply to the
epiphysis. Further displacement also leads to increasing deformity and
secondary increased risk of degenerative joint disease over time.
Long-term goals of treatment include restoration of a functional range
of motion, freedom from pain, and avoidance of aseptic necrosis and
chondrolysis. Treatment considerations are based on the clinical
classification (preslip, acute, acute on chronic, chronic; stable
versus unstable) and the radiographic classification (mild, moderate,
severe).
acute slips and the acute component of acute-on-chronic slips if
displaced more than mildly, followed by stabilization of the epiphysis.
Stable slips or chronic slips and the chronic component of
acute-on-chronic slips should never be reduced but only stabilized. Any
attempt at reduction of chronic components (or of a stable slip) runs
the risk of aseptic necrosis (the most common cause of a poor result)
and long-term disability.
with the sudden acute displacement of the epiphysis by interruption of
blood supply to the capital femoral epiphysis. Reduction attempts of
the slip must be gentle. The use of skeletal traction to reduce the
acute or unstable slip remains controversial. Most pediatric
orthopaedic surgeons do not use traction, but treat the unstable slip
as an orthopaedic emergency; taking the patient to the operating room
as soon as is feasible. Skin traction may be used while waiting for the
patient to be ready to go to the operating room. In the operating room,
reduction is obtained of the moderate or
severely
displaced unstable slip by gentle traction on the fracture table with a
gentle internal rotation moment—the goal being to reduce a moderate or
severe displacement to a mild one so as to improve the long-term
prognosis. If displacement is mild, no attempt at reduction is done.
accomplished by epiphysis pinning. The most widely used method of
stabilization is pinning with large cannulated bone screws or multiple
large threaded pins (see Figure 15-30). These
techniques require the use of image intensification in the operating
room. Radiographic control intraoperatively must include the ability to
obtain images at 90° to each other to minimize the risk of pin
penetration, which is thought to be associated with chondrolysis. The
fixation device must enter the epiphysis perpendicular to the physeal
plate of the femoral head. The fixation device must cross the physis
into the head but must be well short of the subchondral cortex. The
pins or screws should be in the center of the epiphysis on the AP and
lateral views of the hip (see Figure 15-30).
Pins or screws should also avoid the superior quadrant of the femoral
head to avoid the lateral epiphyseal vessels. Pins in this quadrant are
associated with a higher incidence of aseptic necrosis. Although many
authors recommend two screws for unstable slips, a single, large
diameter centrally placed screw is generally adequate.
bearing until they are pain free and have a comfortable range of motion
(generally 4 to 6 weeks). Rapid advance to full weight bearing may then
begin.
plate closes. Loss of range of motion after treatment may be the first
sign of chondrolysis. Pain in the other hip (groin, medial thigh, or
knee) warrants careful investigation. Most bilateral slips are
diagnosed within 1 year of the diagnosis of the initial slip. Pins are
generally not removed, but if for some reason removal is considered, it
should be delayed until physeal closure.
reduction but should be treated by stabilization procedures primarily,
regardless of their degree of displacement. Stable slips pinned in situ
may rapidly advance postoperatively to full weight bearing. Long-term
results of mild and moderate slips are generally good concerning
function and range of motion. A certain amount of remodeling of the
proximal femur can be expected.
procedures primarily. Debate exists about whether these slips should be
treated by realignment procedures in addition to stabilization to
improve joint kinematics and hence long-term outcome. Long-term
follow-up studies indicate that the greatest risk to the long-term
outcome of patients with SCFE is the development of aseptic necrosis or
chondrolysis, not malalignment. The use of realignment procedures
(neck, intertrochanteric, or subtrochanteric osteotomy or manipulative
reductions in chronic slips) is associated with significantly higher
complication rates than pinning in situ alone. Realignment procedures
should therefore be reserved for those situations in which restricted
range of motion impairs function after plate physeal closure.
SCFE. It is most commonly associated—in acute slips and unstable
slips—with the abrupt displacement of the epiphysis disrupting
retinacular vessels. In chronic stable slips, aseptic necrosis can
occur as a result of treatment. As mentioned previously, attempts at
reduction of stable or chronic slips, over-reduction of unstable, acute
slips, improper pin placement, and femoral neck osteotomies are
associated with this complication.
with SCFE. Chondrolysis is manifest clinically by loss of range of
motion, pain, limp, and joint contracture. Radiographically, the
condition is manifest by loss of joint space, irregularity of the
subchondral bone of the femoral head and the acetabulum, and disuse
osteopenia. It can occur in untreated slips, but this is unusual (it
can also occur as an isolated disorder). The cause of this condition
remains unknown, but it is associated with prolonged immobilization,
unrecognized pin penetration, severe slips, and a long duration of
symptoms before treatment. Whether the condition represents an
autoimmune phenomenon or other source of interference with cartilage
nutrition remains to be proved. Treatment of chondrolysis is difficult.
Symptomatic treatment should begin immediately and should include
anti-inflammatory agents and bed rest in skeletal traction to relieve
pain and contractures. The role of continuous passive motion with or
without a surgical capsulectomy is yet to be defined.
and management by accepted techniques that have a high rate of success
with minimal risk of complications.
TA, Weinstein SL. Closed reduction for congenital dysplasia of the hip:
functional and radiographic results after an average of thirty years. J
Bone Joint Surg 1994;76A:1777. The functional and
radiographic results of closed reduction in 152 congenitally dislocated
hips of 119 patients who had been managed between 1938 and 1969 were
reviewed retrospectively. The average age of the patients at the time
of reduction was 21 months (range, 1 to 96 months. The average duration
of follow up was 30 years (range, 15 to 53 years). At the latest
follow-up evaluation, the Iowa hip rating averaged 91 points.
Sixty-five hips (43%) had radiographic evidence of degenerative joint
disease. Function tended to deteriorate with time, even in the absence
of disturbance of growth in the proximal end of the femur. Despite
generally good function at the latest follow-up evaluation, the
prognosis for those patients remained guarded.
SJ, Garfin S, Vance R et al. Pitfalls in the use of the Pavlik harness
for treatment of congenital dysplasia, subluxation and dislocation of
the hip. J Bone Joint Surg 1981; 63A:1239. The
authors review 18 cases of failure of management of DDH with the Pavlik
harness. They describe the pitfalls in the use of the most common
device employed in the management of DDH. The appropriate protocol for
use of the Pavlik harness is described.
IV. Growth and development of the acetabulum in the normal child:
anatomical, histological and roentgenographic studies. J Bone Joint
Surg 1978;60A:575. This classic article deals
with normal acetabular development based on anatomic, histologic, and
radiologic evaluation of the normal hip. Information in this article is
essential to the management of DDH.
IV. Morphology of the acetabulum in congenital dislocation of the hip:
gross, histological and roentgenographic studies. J Bone Joint Surg
1978;60A:586. The author describes acetabular
development in DDH in contrast to previous studies of normal
development. Alteration in ossification of acetabulum is described in
late, reduced DDH by the development of accessory ossification centers.
The pathology of the “neolimbus” is described.
SL. Developmental hip dislocation and dysplasia. Lovell and Winters’
Pediatric Orthopaedics, 5th Ed. Philadelphia: Lippincott Williams &
Wilkins, 2001:905. Textbook chapter reviewing in detail all aspects of DDH.
SL, Mubarak SJ, Wenger DR. Developmental hip dysplasia and dislocation:
Part I. J Bone Joint Surg 2003;85A: 1824-1832. Part I of an instructional course lecture from American Academy of Orthopaedic surgeons course on DDH.
classic article describes the radiographic extent of epiphyseal
involvement and prognosis based on natural history in a large group of
untreated patients.
is a superb monograph on the subject by the leading authority. The
author addresses all aspects of the disease. The monograph is
beautifully illustrated and well referenced.
JA, Neustadt JB, Williams JJ et al. The lateral pillar classification
of Legg-Calvé-Perthes disease. J Pediatr Orthop 1992;12:143. Description of the Lateral Pillar classification which is widely used in making treatment decisions.
AG, Weinstein SL, Dietz FR. The weight-bearing abduction brace for the
treatment of Legg-Perthes disease. J Bone Joint Surg 1992;74A:12. This
study reviewed 34 hips in 31 patients, who had severe LCPD (5 hips with
Catterall group 3 disease and 29 hips with Catterall group 4 disease).
These patients were treated with weight-bearing abduction orthoses. The
mean duration of follow-up was 7 years. The authors concluded that,
although containment is the most widely accepted principle of treatment
for patients who have LPCD, and the Atlanta Scottish Rite orthosis is
the most commonly used orthosis for this condition, there are few
clinical data supporting the effectiveness of this device. On the basis
of the results of their studies, the authors did not recommend the use
of a weight-bearing abduction brace for the treatment of severely
involved hips. These results were also confirmed by another study in
the same issue of the journal from the Atlanta Scottish Rite Hospital.
authors describe a longitudinal 48-year average follow-up of a group of
patients with Perthes disease. Marked deterioration of function was
seen between 36 and 48 years of follow-up. By an average age of 56
years, 40% of patients had undergone arthroplasty, and an additional
10% had disabling osteoarthritis.
article describes a long-term follow-up study of a large number of
patients from three hospitals. The authors describe five radiographic
classes of deformity at maturity and discuss the long-term prognosis of
each class.
SL. Legg-Calvé-Perthes disease. In: Morrissy RT, Weinstein SL, eds.
Lovell and Winter’s Pediatric Orthopaedics 5th Ed. Philadelphia:
Lippincott Williams & Wilkins, 2001:957. This textbook chapter covers all aspects of Legg-Calvé-Perthes disease in detail and has an extensive bibliography.
SL. Long-term follow up of pediatric orthopaedic conditions: natural
history and outcomes of treatment. J Bone Joint Surg 2000;82A:980. The
author summarized long-term follow up to maturity of patients with CPD
who have had either no treatment or brace treatment. Generally
favorable results are found at 20 to 40 years follow-up, unless the
femoral head is flattened and irregular, the neck is deformed, or the
trochanter is overgrown.
article describes a follow-up study of 23 patients with an average
21-year follow-up (range, 15 to 30 years). Twelve patients had evidence
of coxa magna, osteoarthritis, and widening of the femoral neck. The
author discusses possible mechanisms for causation of coxa magna.
DC, Weiner DS, Weiner SD. The characterization of “transient synovitis
of the hip” in children. J Pediatr Orthop 1986;6:11. This
is an excellent, well-referenced review of the topic and includes a
30-year retrospective review of 497 cases. The authors report a
detailed radiographic and clinical follow-up of 147 cases.
A, Scheller S. A clinical and radiological follow-up study of transient
synovitis of the hip. Acta Orthop Scand 1969;40:479. The
study described is a 20- or 22-year follow-up of 73 cases of transient
synovitis. Of the original pool, 6% (102 patients) subsequently
developed Perthes disease. There was a statistically significant
difference in the incidence of coxa magna, cysts, and radiodensity in
the femoral heads of affected patients, but these were of no clinical
significance. The paper documents the benign natural history of this
condition.
author reviews 101 children with irritable hip syndrome who were
followed an average of 8.2 years (range, 5 to 15 years). Most patients
had prompt resolution of symptoms (within 16 days). Only one subsequent
case of Perthes disease and one of coxa magna were seen, both in
patients who had prolonged symptoms and radiologic abnormalities on
presentation.
H, Egund N, Carlin NO et al. Intracapsular pressure in transient
synovitis of the hip. Acta Orthop Scand 1985; 56:204. The
authors evaluated 14 patients with sonography, scintigraphy, and
intracapsular pressure recording and aspiration. They found an effusion
in all cases and increased intracapsular pressure with the hip in
extension. The authors recommended treatment with rest with the hip
joint in about 45° flexion to reduce intracapsular pressure and
decrease the risk of ischemia to the femoral head from vascular
tamponade.
HC. Developmental (infantile) coxa vara—a distinct entity: report of 2
patients with previously normal roentgenograms. Clin Orthop
1970;72:242. The author distinguishes between
developmental coxa vara and proximal femoral focal deficiency. Two
patients are reported who had normal hip radiographs obtained
incidentally during the first year of life and later developed
developmental coxa vara. The author further refines a classification
previously presented.
HC, Wilson PD Jr. Dysgenesis of the proximal femur (coxa vara) and its
surgical management. J Bone Joint Surg 1962;44A:1. This
classic article includes classification of coxa vara into the
categories of congenital and acquired. The authors discuss in detail
the general characteristics and treatment of patients with congenital
short femur with coxa vara, congenital bowed femur with coxa vara, and
congenital coxa vara.
TL, Kalamchi A. The fate of the capital femoral physis and acetabular
development in developmental coxa vara. J Pediatr Orthop 1982;2:534. A
retrospective review of 22 hips in 15 children with developmental coxa
vara. The authors found that acetabular depth did not improve if the
neck-shaft angle was not corrected to at least 140 degrees. Premature
physeal plate closure was a frequent sequelae (89%) of valgus
osteotomy, leading to relatively greater trochanteric overgrowth and
the development of limb length inequality.
article describes a retrospective long-term review of 22 cases of
congenital coxa vara. This study presents the Hilgenreiner epiphyseal
angle and the natural history and surgical indications based on this
angle.
article describes a long-term follow-up study of 155 hips in 124
patients, with a mean follow-up of 41 years after onset of symptoms.
The authors determined that the natural history of the malunited slip
is mild deterioration related to the severity of the slip and
complications. Techniques of realignment are associated with a risk of
appreciable complications and adversely affect the natural history of
the disease. Regardless of the severity of the slip, pinning in situ
provides the best long-term function and delay of degenerative
arthritis, with a low risk of complications.
1915 to 1952, 31 hips in 28 patients with slipped capital femoral
epiphysis were observed without interventional treatment. The mean
duration of patient follow-up from the onset of symptoms was 41 years.
Untreated slipped capital femoral epiphysis can progress to a severe
degree. The natural history of chronic slipped capital femoral
epiphysis is favorable provided that displacement is minimal and
remains so.
R, Billing L, Hansson G et al. Bilaterality in slipped capital femoral
epiphysis: importance of a reliable radiographic method. J Pediatr
Orthop 1996;5B:80. This retrospective
radiographic study of 100 patients with SCFE assessed the incidence of
bilaterality. Evidence of bilateral SCFE was found in 59% of patients;
71% of these patients were asymptomatic. There was no evidence of a
second slip during adolescence in 18% of patients. A standard
radiographic view improved measurement.
this retrospective multicenter analysis of 1,630 children with SCFE,
the high prevalence of SCFE in Polynesian children and low prevalence
in Indo-Mediterranean children were identified. Age, gender, and
physical characteristics were similar throughout the world. More than
60% of children with SCFE were above the 90th percentile for weight.
MJ, Patton CM, Luhmann S et al. Knee pain as the initial symptom of
slipped capital femoral epiphysis: an analysis of initial presentation
and treatment. J Pediatr Orthop 1999;19: 455. This
retrospective review analyzed the presenting complaint of 106 patients
with SCFE. A primary report of knee pain, a longer delay to diagnosis,
and a more severe femoral deformity were seen in 15% of children. The
presence of knee pain alone may delay the diagnosis because the knee
might be investigated before the hip.
PA, Exner GU, Hansch O. Prevention of secondary coxarthrosis in slipped
capital femoral epiphysis: a long-term follow-up study after corrective
intertrochanteric osteotomy. J Pediatr Orthop 1996;5B:135. This
is a retrospective study of 51 hips treated with early
intertrochanteric osteotomy for severe SCFE. Good results at an average
follow up of 24 years were seen in 55% of patients; 45% of patients had
decreased motion and degenerative arthritis. The complication rate was
low. The average age at review was 37 years for women and 39 years for
men.
PJ, Sullivan CM, Phillips WA et al. Slipped capital femoral epiphysis:
prediction of contralateral involvement. J Bone Joint Surg
1996;78A:1149. In a retrospective review of 50
children with unilateral SCFE, the characteristics of those in whom a
contralateral slip developed were evaluated. The authors found that,
for boys, younger age at presentation of the first slip was predictive
of bilateral involvement.
