The Diagnosis and Management of Musculoskeletal Trauma
-
Epidemiology of orthopaedic trauma.
Musculoskeletal trauma has gained significant and increased attention
over the past 10 years for a number of reasons. Such reasons include
the realization of its societal impact
from health care costs and lost workdays in the labor force. These
statistics are coupled with an increased realization that the
orthopaedic surgeon and health care team can positively influence such
statistics, both through excellent intervention as well as education on
injury prevention. Leadership from key
organizations [Orthopaedic Trauma Association (OTA), American Academy
of Orthopaedic Surgeons (AAOS), American College of Surgeons (ACS),
American Orthopaedic Association (AOA), and many others] has played a
major role in lobbying for proactive trauma-related health care policy and implementation of public education programs for injury prevention.Whereas it is likely that certain of these educational
measures such as seatbelt safety, aggressive standards for highway
safety, and lower blood alcohol limits for drivers helps to lower
accident related injury rates, other forces seem to counter such
progress such as the pervasive trend toward faster cars, the burgeoning
enthusiasm for extreme sports, and increased numbers of trauma
survivors with significant musculoskeletal injuries due to airbags.An even greater awareness is emerging regarding the aging baby boomers
who will account for massive demands on the health care system. The
baby boomers will be hitting the 65-year-old age mark in approximately
10 years, and it is estimated that by the year 2040 there will be
35,000,000 more people over the age of 55 than there are now and that
the number of hip fractures alone will increase from 250,000 to 500,000
on an annual basis (1). The estimated uptick in geriatric musculoskeletal trauma
over the next 30 years is due to the vulnerability of the skeletal
system from the natural process of relative bone mineral loss
manifesting in the condition of osteoporosis.
Compounding the number of injuries in this group is the increasingly
active lifestyle of this aging population. To put it in perspective, it
is estimated that one-third of all women reaching the age of 90 will
sustain at least one hip fracture (2). -
Definition of musculoskeletal trauma. Musculoskeletal trauma includes any injury to bone, joint (including ligaments), or muscle (including tendons).
Nearly always, such injuries occur in combination, as the energy
imparted to breaking a bone or tearing a ligament is also dissipated to
impact structures nearby or even distant from the most obvious site.
With greater experience, such combinations of injuries become more
apparent to the diagnostician, which allows for swifter and more
accurate detection of injury characterization. -
Multiple injuries.
The energy it takes to render trauma to the musculoskeletal system can
also dissipate to injure other organs. This is particularly common with
the high energy mechanisms that are responsible for pelvic, spine, or
long bone fractures. Due to the greater density and strength of bone in
younger individuals, there is even greater energy required to create
fractures in this population. Therefore, it is incumbent upon the
trauma team to remain vigilant to the likelihood of injuries to other
bones and other organ systems. Often, the dramatic and salient injuries
during the initial patient evaluation will attract all the diagnostic
and therapeutic attention, while occult and sometimes equally grave
injuries remain initially undetected.P.2For example, it is estimated that only 7% of the
patients who die from life- threatening high-energy pelvic fractures
actually die from arterial exsanguination related to the pelvic
fracture itself (3), while the rest succumb due
to injury involving other organ systems. Forty percent of patients with
femur fractures have other associated fractures (4), and 90% of patients with scapula fractures have other associated injuries (5).
These impressive associated injury statistics demand most heightened
awareness when working up the trauma patient to keep the missed injury
rate to a minimum. -
Missed injury rate. The missed injury rate in the context of polytrauma has been reported to be 4% to 18% (6).
These statistics may be lowered with appropriate protocols and
underscore the importance of a most vigilant secondary survey, as well
as a re-review of the patient’s physical examination each ensuing day
after injury. A secondary survey is a head-to-toe review by a physician that occurs after the initial primary survey,
which is defined as the evaluation of three screening trauma films
(lateral cervical spine, anteroposterior chest, and pelvic x-ray) and,
most importantly, the patient’s airway, breathing, and circulation (the
ABCs).It is valuable to understand the main reasons cited for
missing injuries: significant multisystem trauma with another more
apparent orthopaedic injury, trauma victim too unstable for a full
orthopaedic evaluation, altered sensorium, hastily applied initial
splints obscuring other injuries, and poor radiographs (6). -
Multiple patients.
It is not uncommon, particularly at a Level I trauma center, to require
simultaneous evaluation of multiple patients, such as with motor
vehicle collisions in which multiple victims are involved. Doctors who
have had some training on the fundamentals of trauma surgery and, in
particular, Advanced Trauma Life Support (ATLS),
which includes strategies for triaging patients and resources during a
mass casualty situation, must be available in order to effectively “captain the ship.”
ATLS courses have been developed, refined, and sponsored by the
American College of Surgeons and have an excellent educational track
record. Typically (but not exclusively), in the United States, it is a
general surgery trauma surgeon who is running the trauma room. It is
beyond the scope of this orthopaedic text to delve into the specifics
of ATLS management, however; we will focus on certain of the
fundamentals and cover the triage process of multiple orthopaedic
injuries that may present during such circumstances. To further master
the details of ATLS management, please refer to the ATLS Manual (7th edition) published in 2004 (7).It is imperative to understand what is an orthopaedic emergency and what is orthopaedically urgent. A review of the “orthopaedic emergencies”
in a subsequent section of this chapter will help to understand how
these injuries need to be prioritized for treatment. Furthermore, it is
important to understand what measures can be taken to stage orthopaedic treatment. Not all broken bones need definitive treatment right away, and the practitioner must understand how to titrate the proposed treatment to the physiologic presentation
of the patient. For example, a patient with limited physiologic
reserve, due to a great physiologic challenge from hemorrhagic shock
and compromised ventilation from a hemothorax, should not spend 10
hours in the operating room getting several fractured bones fixed. In
such a case, it may be wiser to place an external fixator across a
broken femur rather than to immediately nail the femur and place a
plaster splint on a displaced ankle fracture rather than to fix it
right away. These measures save a lot of time, blood loss, anesthesia,
and fluid challenge during a potentially critical stage in postinjury
physiologic evolution.There are many ways to stage the treatment of injuries,
which also gives the orthopaedist more time to solicit expertise, get
to know the patient and family, plan the details of an operation, and
understand the comorbidities and the likelihood of patient compliance.
All these different factors may, in fact, impact the ultimate treatment
that the orthopaedic surgeon chooses to render and will most certainly
influence positive outcomes.
accident scene should receive a much different type of workup than
would be called for by a scheduled history and
physical
examination, though certainly history and physical examination are part
and parcel of the process. The difference in this setting is that the
sequence and algorithms in workup, diagnosis, and treatment are very
different than that for a patient presenting in a nonemergent setting.
of the ship,” typically an ATLS-trained general surgeon, who will have
the clearest overview of the patient and who will be delegating many
simultaneous responsibilities. The person taking primary responsibility
for the orthopaedic injuries must heed the captain’s call and clearly
communicate diagnostic or treatment priorities for the orthopaedic
conditions and ultimately fit into the context of overall priorities.
survey, secondary survey, and definitive management. The primary survey
occurs even before, or at least at the same time as, the history, so it
will be discussed here first. Meanwhile other members of the team are
simultaneously obtaining the trauma series of x-rays to be readily
available for interpretation, drawing blood, or inserting a urinary
catheter.
-
Primary survey. The primary survey is concerned with the preservation of life. The first steps in managing the trauma patient follow the ABCs.
It is important to correct each of these problems in sequence. Another
way to think of it is that a competent airway must be established if
life is to continue through the rest of the evaluation. These initial
steps generally have been performed by the paramedic team, but the
surgeon in charge should follow the established ABC sequence.-
Airway. The
most common cause of preventable death in accidents is airway
obstruction, so the trauma leader must immediately check that the
patient’s airway is adequate and patent. Any obstruction (e.g.,
vomitus, tongue, blood, dentures) must be removed and the airway
secured by a jaw thrust maneuver or tracheal intubation. -
Breathing.
After airway obstruction has been ruled out or controlled (i.e.,
intubation), the patient’s ventilation should be assessed. The major
life-threatening problems are tension pneumothorax, massive hemothorax,
and flail chest. Again, this aspect of the physical exam requires the
examiner to inspect, touch, and auscultate the patient as this is
typically done before roentgenographic diagnosis is available. -
Circulation.
After breathing has been addressed, cardiovascular status must be
immediately evaluated and supported. Prompt determination of vital
signs is essential. Control of external bleeding
is accomplished by direct pressure and bandage. Simple elevation of the
lower extremities helps prevent venous bleeding from the limbs and
increases cardiac venous return and preload. The classic Trendelenburg
(head down) position is not used for more than a few minutes because it
can interfere with respiratory exchange. In the critically injured or
hemodynamically labile patient, venous blood samples should be taken
for type and cross matching.Until cross-matched blood is available, rapidly infuse 1 to 2 L of isotonic Ringer lactate or normal saline solution. If blood loss is minimal,
then blood pressure should return to normal and remain that way with
only a maintenance intravenous amount of balanced saline solution.In general, hypotension in a trauma patient should not
be assumed to come from a long bone fracture, and another source must
be sought. The following gross estimates of localized blood loss (units) from adult closed fractures can be useful in establishing baseline blood replacement requirements:Pelvis 1.5–4.5 Hip, femur 1.0–2.5 Humerus, knee, tibia 1.0–1.5 Elbow, forearm, ankle 0.5–1.0
-
-
Trauma x-ray series. Recall that the trauma x-ray series
was being taken in the trauma room while the primary survey was being
conducted. Now that the primary survey has been performed and the most
critical steps have been taken, even before a thorough history and
physical exam, this x-ray trauma series should be
P.4
reviewed;
the examiner is ruling in or out the next most critical clues to saving
life and limb. The trauma series consists of three x-rays: lateral cervical spine, an anteroposterior chest, and an anteroposterior pelvic
view. Any patient who is involved in high-energy trauma, has head
injuries, or is under chemical substance influence should have these
views.The cervical spine roentgenogram must show the inferior
endplate of cervical vertebrae 7 (C7), or it should be deemed
inadequate and repeated. Both odontoid and C7–C8 pathology are
frequently missed injuries even after the secondary survey. If a spine fracture is detected,
then a complete spinal series including anteroposterior, lateral and
odontoid cervical views, and thoracic plus lumbar spine view is
mandatory. Computed tomography (CT) may be required to rule out upper
cervical fractures. The documented incidence of multiple level spine
fractures is 7% to 12%. A full spine series should be obtained in the
unconscious trauma victim.All the x-rays should be taken with excellent technique
so as not to obscure the many potential clues to danger which exist on
the radiograph. Care must be taken not to be misled by overlying
backboards, over- and underpenetrated films, and equipment, clips, and
buckles which are frequently left on the x-ray field. Examples abound
of subtle femoral neck fractures that were obscured on the x-ray by a
belt buckle, a pneumothorax in the upper lobe that was cut out of view
due to positioning, or a critical sacral fracture masked by the opacity
of a backboard. -
History and physical examination. The history
should include a careful account of the accident, a description of the
mechanism of injury, and a statement of the degree of violence
involved. Concomitant medical disease, drug abuse, and alcoholism
should be considered as contributing factors. The transporting
paramedic team or member of the accompanying family should be
interviewed for these details if the patient cannot reliably give an
appropriate history. A useful mnemonic to guide the initial history is
the word AMPLE:-
A: AllergiesThe physician working up an orthopaedic patient should
be particularly aware that open fractures should be treated with
certain antibiotics to cover the spectrum of bacteria that are at risk
for certain types of wounds (see open fractures
below). Furthermore, every patient having an orthopaedic operation
should receive perioperative antibiotics, making the question of
allergies quite germane. A penicillin allergy is the most common. -
M: MedicationsMedications can influence surgical decision making. They
will also tip off the practitioner to important comorbidities and
perhaps imply the need for a general medicine consultation prior to
surgery. Patients on anticoagulants should have bleeding and clotting
parameters checked as it may be prudent to stop such meds or reverse a
coagulopathy prior to surgery. -
P: Past illnessDiabetes can influence outcomes of orthopaedic surgery,
and heart disease can increase surgical risk. Steroids and nicotine
(the use of tobacco products) increases orthopaedic surgical
complications as well as outcomes as measured by healing time and
healing rates. -
L: Last mealThis is important when considering whether or not the
patient needs to go to the operating room urgently, as the risk of
aspiration of food or vomitus is higher postprandial. Most
anesthesiologists opt to hold on administration of anesthesia within 6
to 8 hours of food intake. This concern should not, however, override
the emergent nature of certain life- or limb-threatening conditions
which will be discussed below. -
E: Events of accidentAccident circumstances such as direction of impact,
extrication time from vehicle, hours in the field, outside temperature,
being trapped under heavy objects, smoke inhalation, and many other
possibilities are warning flags to the experienced practitioner, which
clue in certain medical or orthopaedic conditions and injury patterns.
-
-
Secondary survey. The Secondary Survey is a complete physical examination
from head to toe. By this juncture, the potentially life-threatening
pathology of the ABCs has been addressed, and necessary resuscitation
is underway. The patient should be completely undressed for the
secondary survey for a most thorough exam.-
Neurologic mental status. The level of consciousness of the patient should first be noted. A brief “disability exam”
in an awake patient is a rapid, organized neurologic examination which
documents mental orientation, verbal response to questioning, and
response to stimuli. Furthermore, each extremity should be examined for
motor and sensory function as well; accurate documentation is crucial
since neurologic examinations can reveal progressive deficits. The
extremity neurologic exam can also be documented with the specific
physical exam of each extremity, but it is imperative that all four
extremities be included. It is good to develop a pattern of examination
and stick with that pattern each time for consistency.In the unconscious patient, a Glasgow coma score is rapidly conducted based on pupil response to light, motor activity, and withdrawal to painful stimuli (Table 1-1).
This information is initially obtained by the medics who perform the
initial in-the-field evaluation. The Glasgow score therefore is used as
the measure of neurologic progress or deterioration. The medics
generally also note the position of the patient at the scene of the
accident, especially the head, and whether all limbs were actively
moving. It is frustrating to the orthopaedic or neurosurgeon to be
asked to evaluate a patient who has been sedated and chemically
paralyzed in the trauma room, particularly when the initial neurologic
exam was not properly documented. In general, the use of maximal monitoring and minimal medication is a useful trauma room principle which avoids such frustration by the examiner who relies on accurate neurologic exams. -
Head and neck. Carefully palpate skull and facial bones and look for lacerations hidden in the hair. Cranial trauma should raise an immediate suspicion for cervical spine injury given the sudden and violent force it
P.6
takes to injure the face and cranium. Roentgenograms of facial bones
are difficult to interpret unless previous clinical examination
suggests the presence of trauma. The association between cervical spine and head injuries
must be emphasized. In a guided fashion with cervical immobility,
remove or loosen the C-collar to palpate the posterior cervical spine
looking for tenderness or spasm. In a conscious patient, any neck pain
or spasm is a cervical spine injury until proven otherwise. In the
unconscious patient, the neck must be protected with a hard C-collar
until bony injury is ruled out by cervical roentgenography and physical
exam. A benign physical exam by itself is unreliable if there are
distracting injuries or if the patient is intoxicated. If a cervical
spine injury is diagnosed, appropriate orthopaedic or neurosurgical
spine consultation should be obtained immediately, and the extremity
neurologic exam should be reported and documented.TABLE 1-1 Glasgow Coma ScaleEye opening (E) Spontaneous 4 To speech 3 To pain 2 None 1 Verbal response (V) Oriented 5 Confused conversation 4 Inappropriate words 3 Incomprehensible sounds 2 None 1 Motor response (M) Obeys command 6 Localizes 5 Withdraws to pain 4 Abnormal flexion 3 Extensor response 2 None 1 (E + M + V) = coma score between 3–15 -
Thorax and abdomen.
Though the thorax and abdomen are largely the domain of the general
surgeon, the examiner must inspect, palpate, and auscultate the abdomen
and thorax to determine possible underlying injury. Hemothorax and pneumothorax
often cause preventable death. Therefore, the chest should be examined
carefully and the examination repeated frequently. Furthermore, this
assessment helps the orthopaedist place musculoskeletal injuries in the
broader context of the patient. Abdominal injury
is also a common cause of preventable death. The imprint of clothes or
a contusion of the abdominal wall from the seat belt suggests
intraabdominal injury. Airbags have altered patterns of injury in
frontal collision (8). Appropriate diagnostic
studies should follow the suspicion of injury, and in many centers the
spiral “whole body” CT scan of the chest, abdomen, and pelvis has
supplanted selective CT scans, ultrasounds, and peritoneal lavage. -
Pelvis. Low
back pain, pubic tenderness, or pain with compression of the iliac
crest can indicate a pelvic ring injury. Sequential anterior to
posterior compression over the iliac wings can help to discriminate
gross pelvic motion. Pelvic fractures may cause severe internal
bleeding, and as stated earlier, a patient can easily lose four or more
units of blood after a displaced pelvic fracture.A rectal examination must be done in all patients with a spine or pelvic injury, both to check for bleeding as well as loss of sphincter tone indicative of neurologic injury. Furthermore, a high-riding prostate also indicates major urologic disruption common to high-energy pelvic fractures in men. An inspection of the penile meatus for hemorrhage should also be performed, and such a finding is further indication of a genitourinary system disruption. Bloody urine or the inability to void raises the suspicion of a urethral injury, so a retrograde urethrogram should be considered before a catheter is inserted (9).
In male patients, blood at the penile meatus or a “high-riding”
prostate seen on rectal examination is a clear indication for obtaining
a retrograde urethrogram before bladder catheterization. If the
catheter does not pass easily, then it should not be forced and the
urologist should be consulted. If a bladder injury is suspected, then
it is essential to insert an indwelling catheter unless the patient is
voiding clear urine.A bimanual pelvic examination is appropriate in female patients to rule out open fractures which can penetrate the vaginal vault. Perineal inspection
for integument lacerations should be conducted and in the setting of
displaced pelvic fractures should be assumed to represent an open
pelvic fracture. -
Back and spine. Carefully log roll the patient and palpate the entire spine
to detect tenderness or defects of the interspinous ligaments. It is
very important that a log roll be conducted properly with three
assistants controlling simultaneous rotation of the entire body. A
fourth assistant should be controlling the cervical spine (while in a
hard collar) with gentle traction. An increase in the interspinous
distance accompanied by local swelling may signify injury.
Occasionally, ecchymosis or kyphosis can be recognized, and their
presence or absence should be documented. -
Upper and lower extremity examination. When gross deformity and crepitation
are present, further examination of the fracture site is not necessary.
Otherwise, all four limbs should be palpated thoroughly and each joint
placed through a passive range of motion. Look specifically for point
tenderness. Any obvious fractures or deformities are splinted, and any open wounds are covered
with sterile dressings. Dressings over open wounds, particularly over
fractures, should not be taken down multiple times by multiple
examiners. Such repeated exposures will only increase the rate of
infection with each exposure to the contaminated environment (10).
A more detailed description of fracture wound management is given later
in this chapter. Every diagnosed fracture should have properly centered
x-rays of the joint above and below. Carefully evaluate the circulation
of the limb distal to any fracture and record the presence of all
wounds after applying a sterile dressing.
P.7 -
classified as emergent or urgent and typically can be done on a
semielective basis. For example, an isolated, closed fracture which is not threatening local blood supply may wait days to weeks. There are many considerations, however, which go into the optimal timing of surgery, and immediate consultation with an orthopaedist clarifies the issue of timing of surgery.
The lack of circulation affects adequacy of tissue oxygenation, and
consequently limb or life is threatened. This may occur on a
macroscopic level, such as with a hemorrhaging pelvis in which a
person’s life is threatened, or on a microscopic basis, such as when
end-organ perfusion is cut off beginning with occlusion of the venules
in a muscle bed due to increased interstitial pressure exceeding
intravenous pressure during the condition of compartment syndrome.
Threatened blood supply to local tissues can be a more subtle
phenomenon that requires further understanding of the vasculature to
certain bones. For example, a relatively benign appearing x-ray of a
femoral neck fracture to the inexperienced eye may not gain much
attention, but the experienced clinician knows that even a nondisplaced
femoral neck fracture can threaten the hip joint forever through a
process called avascular necrosis. Certain other orthopaedic injuries
may not accurately be classified as emergent since life or limb is not
immediately at risk, but they still warrant heightened attention. Such
injuries may be classified as urgent since they need prompt action by
an orthopaedist and surgical timing in the range of 6 to 24 hours. In
the next two sections on emergent and urgent orthopaedic injuries, the
discussion will address these in descending order from most to least
acute.
-
Orthopaedic emergencies
-
Hemodynamically unstable patient with a pelvic fracture.
This is the one injury in which circulation can be compromised to the
extent that a life is immediately at risk and in which an orthopaedic
intervention can save such a life. The pelvic ring can be disrupted in
high-energy accidents (or low-energy falls in osteoporotic patients)
and most always is disrupted in at least two points around the ring.
The saying, “it is impossible to break a ring at a single point” nearly
always applies to the pelvis. Therefore, the examiner should look for a
lesion posteriorly in the sacrum or sacroiliac joint and anteriorly in
the pelvic ramii or pubic symphisis.When a pelvic fracture is
recognized on the anteroposterior x-ray view obtained with the initial
trauma series, two more radiographs should be obtained: a pelvic inlet
and pelvic outlet view. These are orthogonal views of the pelvis
which help to critically evaluate all the pelvic bony landmarks as well
as displacement of fractures. If there is significant displacement
(more than 5 mm) at any one pelvic fracture line, a pelvic CT scan
should be obtained. Many orthopaedists will prefer a CT scan with even
lesser displacements to more critically evaluate the injury or
preoperatively plan. If a fracture line enters the acetabulum, then Judet x-ray views should be obtained.
These are 45-degree angled x-ray views from the right and left side of
the patient centered on the pelvis, once again giving the examiner
orthogonal views to critically assess the
P.8
bony landmarks of each acetabulum. Note that it is wasteful to obtain “five views of the pelvis” for every pelvic fracture
as the Judet views are not needed unless the acetabulum is involved.
Likewise, it is not necessary to get five views of an acetabular
fracture, omitting the inlet and outlet pelvic x-rays when the
posterior pelvic ring (sacrum or sacroiliac joint) is not involved.The pelvis is like a cylinder or sphere of bone that
contains many critical soft tissue structures and organs such as the
bladder, the iliac vessels, prostate or vaginal vault, and the rectum.
All these organs are at risk, but the worrisome life-threatening
hemorrhage is what must be diagnosed promptly and addressed. Bleeding
typically continues until tamponade can occur and clotting factors take
control. It is highly recommended to tie a sheet
around the pelvis of a patient who is hemodynamically unstable until
the anteroposterior radiograph of the pelvis rules in or out a
displaced pelvic fracture. The sheet must be tied very snug, and
it is recommended to be applied at the level of the greater trochanters
for maximal effectiveness in closing down the volume of the broken and
separated sphere, thus leading to earlier tamponade of bleeding vessels
(11). There is little to lose if the patient
does not have such an injury, and the sheet is simply removed.
Commercially available pelvic slings, now with pressure calibration,
are becoming commonplace in trauma units for such a purpose. There is
essentially no role for the trauma room application of an external
fixator as this maneuver has been simplified by the more effective use
of a pelvic sling. -
Extremity arterial injury.
Probably the next most emergent condition which an orthopaedist faces
is the extremity that is at risk for limb loss. This can occur due to a
torn or lacerated artery or compartment syndrome. Arterial injury can
be caused by blunt or penetrating trauma. There are four “hard signs” of arterial injury which warrant immediate vascular exploration, and time should not be wasted ordering and performing a diagnostic arteriogram (12).
The rationale is that a vascular surgeon knows the proximity of the
injury based on the wound or the x-ray that demonstrates the pathology.
There is no sense in using precious minutes finding out what is already
known when irreversible ischemic damage to nerve and muscle tissue
occurs after 4 hours of warm ischemia time. A warm ischemia time
interval of less than 6 hours is the generally accepted time interval
within which arterial continuity must be restored in order to avoid
loss of limb (13).The Four “Hard Signs” of Arterial Injury:-
Pulsatile hemorrhage
-
Expanding hematoma
-
Audible bruit
-
Pulseless limb
The only time an arteriogram would be warranted in such
an acute circumstance is when there is multilevel injury (multiple
fractures or shotgun wound) in which the vascular surgeon cannot be
sure what level the arterial damage has occurred.The more difficult diagnostic problem occurs in the
majority of patients who present with more subtle clues to vascular
injury. Such “soft signs” might include a history of severe hemorrhage
at the accident scene, subjectively decreased pulses, a deficit of an
anatomically related nerve, or a nonpulsatile hematoma. Other soft
signs include the orthopaedic injury patterns that have been associated with a high incidence of arterial damage:-
Knee dislocations
-
Highly displaced tibia plateau fractures
-
Medial tibia plateau fractures
-
Ipsilateral fractures on either sides of a joint (floating joint)
-
Gunshot or knife wounds in proximity to neurovascular structures
-
The mangled extremity
The best screening exam for an arterial injury should be
quick, non-invasive, portable, and cost effective, as well as reliable.
Determination of the
P.9
arterial
pressure index (API) requires the use of a Doppler machine and a blood
pressure cuff. It has been investigated as a screening tool for
clinically significant arterial compromise (14).
The API has also been referred to in the literature as the ABI (Ankle
Brachial Index) or AAI (Ankle Arm Index), and the terms are
interchangeable. To conduct an API examination, a blood pressure cuff
is placed just above the ankle or wrist in the injured limb so that a
systolic pressure can be determined with a Doppler probe at the
respective posterior tibial artery or radial artery. The dorsalis pedis
or ulnar arteries may logically be used as well, as long as the blood
pressure cuff is placed distal to the injury. The same measurement is
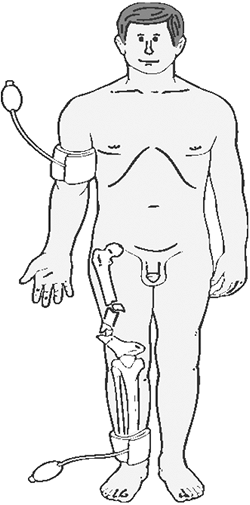
determined on an uninjured upper or lower extremity limb (Fig. 1-1). The
API is simply the calculation of the systolic pressure of the injured
limb divided by the systolic pressure of the uninjured limb: Since pulses have been reported to be palpable distal to major arterial lesions, including complete arterial disruption (15,16,17),
Since pulses have been reported to be palpable distal to major arterial lesions, including complete arterial disruption (15,16,17),
and perception of a pulse is subjective and impossible to quantify,
physical exam alone or the detection of a palpable pulse is not
appropriate for diagnosis.As it is impossible to spell out every clinical scenario
that may be associated with an arterial injury, it should be reiterated
that every case bears
P.10
individual
judgment, and given the absent morbidity of the API examination, a
conservative approach to testing and documentation is the most prudent
course. The clinician should approach the patient who has a high-risk
vascular injury with a clear diagnostic algorithm (Fig. 1-2).
Besides the patient with one of the four hard signs of vascular
arterial injury who warrants immediate surgical exploration, a
patient’s API should dictate the next step. If the API is greater than
0.9, then the patient may be followed clinically without any further
workup. If the API is less than 0.9, then they should proceed to the
next diagnostic step of either an arteriogram or duplex ultrasound, the
results of which will dictate the final plan of action.![]() Figure 1-1.
Figure 1-1.
The placement of the pressure cuff and the Doppler probe is
illustrated. One systolic pressure measurement is taken in an uninjured
limb, and the other systolic pressure measurement is taken on the
injured limb distal to the injury.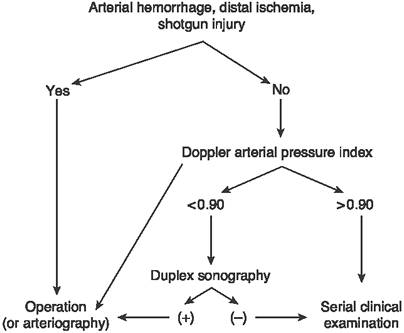 Figure 1-2. The diagnostic algorithm for a patient with a possible extremity arterial injury.
Figure 1-2. The diagnostic algorithm for a patient with a possible extremity arterial injury. -
-
Compartment syndrome.
Compartment syndrome is a condition in which there is increased
pressure within a closed soft tissue space, with the capacity to cause
damage or necrosis to such tissues. Therefore, it should be recognized
that the condition can occur in any muscular compartment of the body,
though it is most commonly encountered in the leg. It is perhaps the
most common orthopaedic emergency and often difficult to diagnose. In
the awake and alert patient, symptoms include-
Pain out of proportion to the injury (despite adequate narcotic analgesia).
-
Pain with passive stretch of the muscles within the compartment.
-
ParesthesiasFurthermore, these symptoms should occur in the setting of swollen tissues. Diminished
or absent pulse is specifically not listed, as it is such a late and
subjective sign, that its absence should never be relied upon to
exclude the diagnosis of compartment syndrome. These clinical
symptoms obviously cannot be used in the obtunded patient and should
not even be relied upon in a patient with altered sensorium due to
intoxication, for example. The clinician should have great suspicion
for compartment syndrome in the setting of high-energy trauma or
comminuted and displaced fractures, and if he or she encounters such a
patient with very swollen tissues (often characterized as “tense”),
then a pressure measurement should be taken of the suspected
compartments. It is important to note that compartment syndrome is well described in low energy mechanisms and does not have to be associated with a fracture. Chart
P.11
documentation should be rigorous
when tracking the possibility of compartment syndrome, and excellent
patient examination should occur at intervals no more than 3 hours
apart until compartment syndrome can be ruled out.Most emergency and operating rooms have readily
available pressure measuring devices such as the Stryker Quickstick,
which can be used to measure suspected compartments. An indwelling
catheter rigged with a mercury manometer (18) or an arterial line attached to a pressure transducer can also be used. If
there is ever any doubt as to whether a patient has compartment
syndrome, then such measurements must be taken to confirm or rule out
the diagnosis. Intracompartmental pressures exceeding the diastolic
pressure minus 30 mm Hg warrant emergent facsiotomies (19).
Fasciotomy incisions should extend to nearly the length of the
compartment to ensure complete decompression and adequate visualization
and assessment of tissues. A text should be reviewed prior to the
operation to review recommended incisions which address each and every
compartment of the suspected part of the extremity (thigh, leg, foot,
hand, antebrachium, brachium, buttock, and more).
-
-
Mangled extremity and traumatic amputations.
Another clinical entity that should warrant great concern for limb
viability is the so-called mangled extremity. The mangled extremity is
not clearly defined, and there is no objective criteria on which
clinicians agree on definition. Suffice it to say that it represents
the end of an injury spectrum that involves a magnitude of trauma which
destroys soft tissue to the extent that limb survival is in question.The principles of open fracture management as discussed
in the next section should be heeded, and the algorithm for a vascular
workup should be followed expeditiously as described in the former
section (III.A).
Most importantly, several services should come to bear in assessment,
workup, and coordination of care including trauma surgery, orthopaedic
surgery, plastic surgery, and, if necessary, vascular surgery.
Communication around treatment considerations and timing should be
open, clear, and decisive. In the same way, the patient and loved ones
should be included in the communication in order to understand the
gravity of the injury and that amputation is a real and sometimes
optimal solution (20).An accurate neurovascular exam should be performed and documented. If an adult patient has a severed tibial nerve, then amputation should be executed,
though absent tibial nerve sensation does not necessarily mean that
neurodiscontinuity has occurred. A patient with a mangled extremity
should be managed at a Level I Trauma Center where the appropriate
expertise and experience is available.There are several prognostic factors that influence
outcome and therefore should be weighed in the consideration for limb
salvage versus amputation. A number of scoring systems have been
developed to account for these variables, but none has proved reliable
in predicting limb viability. A simple and popular grading system is
the MESS (Mangled Extremity Severity Score), which helps to guide the
clinician. The MESS yields a score to the variables of injury energy, limb ischemia, shock or hypotension, and age (Table 1-2) (21). A score of more than seven was found to be predictive of the need for amputation. This scale is merely a guide.Early management includes skeletal stabilization versus
amputation, wide and aggressive debridement of all devitalized tissue,
abundant irrigation, reestablishing vascular continuity, and
reoperations every couple of days for wound management until definitive
coverage can be executed by a microvascular team if necessary. An
antibiotic bead pouch or perhaps a vacuum-assisted closure system for
open wounds are helpful in the interim between cases.For the complete traumatic
amputation of a finger or entire extremity, the team approach should
also be used to assess the possibility of a replantation. The proximal stump is first dressed with Ringer’s
P.12
lactate soaked dressing and pressure is applied. A tourniquet is to be avoided. The amputated
part is wrapped in a Ringer’s lactate moistened sterile sponge and
placed in a plastic bag. It should be cooled by placing it in a
container with ice, which delays autolysis and thus allows time for transport to a center with a replantation team. The part must not be frozen or placed in direct contact with ice.
If the travel distance by car is less than 2 hours, then this form of
transport can be used. If not, arrangements should be made for air
evacuation. Make no promises to the patient regarding whether replantation can be attempted or what the outcome will be. Absolute
indications for attempts at replantation include thumb amputations and
pediatric amputations, and border digits are a relative indication. Prognosis
is improved when an extremity has been amputated in a sharp cutting
mechanism as opposed to a crushing or traction mechanism.TABLE 1-2 MESS (Mangled Extremity Severity Score) VariablesComponent Points Skeletal and soft tissue injury Low energy (stab, simple fracture, “civilian gunshot wound”) 1 Medium energy (open or multiplex fractures, dislocation) 2 High energy (close-range shotgun or “military gunshot wound,” crush injury) 3 Very high energy (same as above plus gross contamination, soft tissue avulsion) 4 Limb ischemia (score is doubled for ischemia > 6 hours) Pulse reduced or absent but perfusion normal 1 Pulseless; parasthesias, diminished capillary refill 2 Cool, paralyzed, insensate, numb 3 Shock Systolic blood pressure always greater than 90 mm Hg 0 Hypotensive transiently 1 Persistent hypotension 2 Age (years) less than 30 0 30–50.1 1 greater than 50 2 Note: A MESS of greater than 7 warrants immediate transfer to a Level 1 Trauma Center.
From Johansen K, et al. J Trauma. 1990;30:568–573. -
Femoral neck fracture in the nonelderly.
A femoral neck fracture in a young patient is considered an emergency
because the blood supply to the femoral head is threatened. The lateral epiphyseal artery branch off the medial circumflex artery is the dominant blood supply to the femoral head (the artery of the ligamentum teres supplies 10%). There is an associated risk of avascular necrosis
(AVN) of the femoral head after a femoral neck fracture, which can lead
to femoral head collapse, which is a catastrophic complication in a
young active patient in whom a hip replacement or a hip fusion is a
dreaded salvage option.The risk of AVN exists even in nondisplaced fractures;
therefore all such femoral neck fractures (in a hip which needs to be
saved) are regarded as emergent. For the displaced variety, an open
reduction is indicated to establish anatomic alignment followed by
internal fixation, classically with three large
P.13
cannulated
screws. In the nondisplaced variety, percutaneous fixation may be
appropriate, but the hip capsule should still be surgically
decompressed.The possible mechanisms for arterial insufficiency include (22)-
Intracapsular tamponade from bleeding into a closed space
-
Kinking of vessels from tenting bone fragments
-
Arterial disruptionWhen considering these mechanisms, one can understand
that urgent decompression and stable realignment can be helpful in
restoring blood flow. In fact, studies indicate that prognosis is
related to the time it takes to get to the operating room (23).
-
-
Hip dislocation.
For the same reason as for the femoral neck fracture, a dislocated hip
is an emergent condition as prognosis and the rate of AVN are directly
related to the amount of time dislocated (24,25).
One or two attempts at a closed reduction in the emergency room is
indicated, but, if unsuccessful, a trip to the operating room for
complete anesthesia and muscular relaxation usually suffices. -
Threatened soft tissues.
Anytime a fracture or dislocated bone is tenting the skin, and the
injury cannot be reduced, the patient should go to the operating room
emergently so that a closed reduction with pharmacologic paralysis can
be attempted. If that fails, an open reduction should be performed.
This scenario commonly occurs with ankle and subtalar fractures or
dislocations, wrist fractures, and even fractures and dislocations
around the knee. Leaving any joint dislocated does not make sense when considering the tissues at risk (even if it is not compromising skin), venous obstruction, and pain.
-
-
Orthopaedic urgencies
-
Open Fractures
-
Emergency room management.
Early and careful treatment of wounds is necessary to decrease the
chance of infection. Simple limb realignment should be performed for
provisional splintage. Wounds, large or small,
should immediately be covered with a sterile dressing, and the
temptation for multiple examiners to reexpose the wound must be avoided
to decrease the likelihood of infection. Any laceration of the
integument in the vicinity of a fracture should be assumed to represent
an open fracture and, therefore, should be formally explored in the
operating room. Cover the wound with a simple saline-moistened dressing. Do
not probe or blindly use surgical hemostats in the wound. Do not push
or stuff extruded soft tissue or bone back into the wound.
Externalized material is contaminated and will contaminate deeper
recesses if replacement into the wound is attempted outside the
operating environment.Open fractures are generally classified using Gustilo system (Table 1-3). With increasing severity, the complications of deep infection, nonunion, and amputation increase. A
Type IIIB open fracture requires a muscle flap for wound closure. A
Type IIIC fracture is one which requires a vascular repair for limb
viability. The Gustilo classification is useful but because of
its subjective criteria, such as high energy, comminution, and
contamination, there is poor intraobserver reliability (26). -
Operating room
management. All large wounds, open fractures, nerve disruptions, and
most tendon lacerations should be debrided and repaired in the
operating room. Debridement means removal of all foreign matter
and devitalized tissue in or about a lesion. Irrigation with large
quantities of saline does not replace the need for proper surgical
debridement technique. Pulsating saline lavage is a useful adjunct to
good debridement.The wound should be debrided from the outside in.
The skin edges are sharply trimmed to viable margins. The debridement
is then continued into the depth of the wound until the entire damaged
area has been identified and
P.14
resected.
Muscle viability is evaluated based on the criteria described by Artz,
et al: capacity to bleed, color, contractility, and consistency (27). These are helpful descriptors in determining whether or not to resect muscle.TABLE 1-3 Gustilo and Anderson Classification of Open FracturesType I Skin opening of 1 cm or less,
quite clean; most likely inside out; minimal muscle contusion; simple
transverse or short oblique fractures.Type II Laceration more than 1 cm;
extensive soft tissue damage; minimal to moderate crushing component;
simple transverse or short oblique fractures with minimal comminution.Type IIIA Extensive soft tissue
laceration associated with muscle, skin, and/or neurovascular injury
but with adequate coverage of bone; typically segmental or comminuted
fractures.Type IIIB Extensive soft tissue injury with periosteal stripping and bone exposure; usually associated with severe contamination. Type IIIC High energy features of other type IIIs but with arterial injury requiring repair. Adapted from Gustilo RB,
Mendoza RM, Williams DM. Problems in management of type III open
fractures. A new classification of type III open fractures. J Trauma 1984;24:742.With an open fracture, all devitalized bone (bone without enough soft-tissue attachments to maintain adequate blood supply) should be removed.
The exception is pieces with articular cartilage attached that should
be saved to attempt to reconstruct a joint surface. Great care must be
taken not to devitalize the bone further. Initial internal fixation of
open fractures is preferable if rigid stabilization is provided without
significantly jeopardizing the blood supply (28). Again, the soft-tissue coverage should not create enough tension to cause any devitalization from a lack of blood supply. Use monofilament sutures for skin closure,
not braided wire or multifilament synthetic, cotton, or silk sutures.
Adequate control of bone bleeding may be difficult, so the surgeon
should use either a loose skin closure that allows exodus of some of
the hematoma or a skin closure combined with a suction drain.Open fractures should generally be treated by a delayed primary closure
(3–5 days). An exception may be made for uncomplicated hand fractures,
wounds that enter joints, or small wounds with no extensive hemorrhage.
The burden of proof rests with the surgeon electing to close the wound.
Cover all exposed tendons, nerves, and bone but not at the expense of
compromising blood supply to injured skin and subcutaneous tissues.
Acute consultation with an orthopaedic or plastic surgeon skilled in
myoplasty is indicated if adequate coverage over implants and bone
cannot be accomplished. During the interval between debridement and
definitive soft tissue coverage, it is wise to cover the wound with an
antibiotic bead pouch (29) or a vacuum-assisted wound closure technique (30,31). This aids in maintaining an aseptic environment during the waiting interval (28). -
Antibiotic management. It should be emphasized that the primary management of an open fracture is surgical and that antibiotics play a strictly adjunctive role.
Furthermore, all patients with an open wound should be up to date with
tetanus immunization or be treated with toxoid. Although there are
numerous recommended tetanus prophylaxis schedules, the authors
generally follow the recommendations of the American College of
Surgeons. Table 1-4 lists the current guidelines. With any open fracture or major wound, start parenteral bactericidal antibiotics immediately in the emergency department (28).P.15P.16Cephalosporins are the drug of choice for prophylaxis of a Gustilo type I and II injury.
Patients who are allergic to penicillin (excluding history of
anaphylaxis) usually may receive cephalosporins. A small test dose is
recommended before giving the entire dose. To obtain an adequate
concentration of antibiotics in the fracture hematoma, begin the
antibiotic therapy as soon as an open fracture is diagnosed. To avoid
many of the side effects of antibiotics, such as superinfections, limit
the duration of prophylactic antibiotic to 48 to 72 hours postoperative.An acceptable alternative is vancomycin 1 g intravenously (IV) daily (32).
Use this drug only if there is both penicillin and cephalosporin
allergy or a history of anaphylaxis since the isolates of resistant
strains of bacteria have increased in number recently, and this drug is
the mainstay of treatment for methicillin-resistant Staphylococcus aureus (MRSA).For type III open wounds with
marked contamination or large exposure, gram negative coverage should
be added to the antibiotic spectrum. An aminoglycoside is
typically used as an appropriate agent during this short-term period of
treatment for gram-negative organisms. If there is a risk for soil, or sewage contamination, an agent against that acts against clostridium bacterial species is important to add.
Though penicillin may be added to the regimen for this purpose, the
antibiotic Zosyn is a good choice offering broad coverage and
eliminates the need for administration of three different medications
for infection. -
Gunshot wounds. If possible, identify the caliber and type of weapon.
This information helps determine whether the wound was caused by a
high- or low-velocity weapon. The majority of civilian injuries are low velocity and interestingly do not seem to be associated with higher infection rates, even when associated with a fracture (33,34,35). Decision making regarding the fracture proceeds as with a closed fracture. If the bullet enters a joint, formal lavage and debridement is indicated.The protocol for management of a low velocity gunshot is as follows:-
Tetanus prophylaxis
-
1 day of antibiotics (first-generation cephalosporin)
-
Local cleansing
-
Debridement of devitalized skin
-
Superficial irrigation
-
Sterile dressing
High-velocity weapons have a
muzzle velocity greater than 610 m/sec or an impact velocity of 2,000
to 2,500 ft/sec. These weapons cause severe cavitation within the
wound, which make debridement necessary. Big-game rifles, such as a
0.30 to .030 or a 0.30 to 0.06, can approach this high-velocity impact
energy, and wounds from these must be treated accordingly with
irrigation and debridement, possibly on a serial basis depending on the
cavitation injury. Gunshot wounds of high-impact energy cause marked
comminution of the fracture and leave a gaping exit. These are managed
like an open fracture and certainly require appropriate vascular
screening as previously discussed. -
-
-
Open joints. Open joint injuries are also at risk for septic complications. The surgeon should assume that lacerations over a joint extend into the joint until the contrary is proven in the operating room.
Air in the joint noted on roentgenography is a sign that a laceration
extends into the joint. It is a reasonable idea to inject the joint
with saline or a methylene blue-enhanced saline to check for
communication with the suspected laceration. A healthy volume of fluid
under pressure must be used to enhance sensitivity of this test—(for
the knee, no >50 mL). This method is not 100% sensitive and should
be used with judgment. An operative joint lavage, either open or
arthroscopic technique, should be performed if an open joint is
suspected. Certainly, if there are foreign bodies in the joint, such as
missile fragments from gunshot wounds, these must be evacuated. A
course of 48 hours of
P.17
gram-positive
and gram-negative coverage with parenteral antibiotics (as with a
Gustilo type IIIA fracture) is a reasonable and prudent adjunct to
surgery. -
Talus fractures.
A fracture of the talus is considered a relative operative emergency
due to its vulnerable blood supply. Though underpowered, and
inconclusive, a recent study seems to suggest that time to operative
fixation does not matter, while talar neck comminution and open
fractures do correlate with poorer outcomes and a higher rate of AVN (36).
Since the talus is at risk for AVN, collapse, and subsequent arthrosis,
and since it is a weight-bearing joint, most orthopaedic
traumatologists prefer to operate on this fracture urgently for the
same reasons described for the femoral neck fracture in a young
patient. Certainly the displaced variety of talus fracture, in which
soft tissue tenting occurs, is an emergency due to the eventuality of
full-thickness skin necrosis, which can lead to catastrophic
complications. -
Long bone fractures in the face of multisystem trauma.
Patients who present with a femur fracture and other injuries to
critical organs such as the lung, brain, or abdomen, or who have
extended periods of hypotension, are at risk for complications such as
acute respiratory distress syndrome, fat embolism syndrome, extended
internsive care unit (ICU) stays, and pneumonia. It is generally well
accepted that early stabilization of femur
fractures helps to decrease such complications and will allow for
earlier mobility and thus less consequent problems from extended
recumbent time periods on a ventilator in the ICU. Patients with femur fractures and head injury should generally have the femur fracture stabilized (37).
It is important that fluid management be well controlled by anesthesia
in such a setting. Aggressive stabilization for femur and pelvic
fractures has a favorable effect on pulmonary function following blunt
trauma (38). In general, fixation of femur fractures with an interlocking nail is indicated in the multiply injured patient (37,39,40). Evidence supports femur fixation within 24 hours of injury in the polytrauma patient (41,42,43).Timing and titration of orthopaedic procedures, however,
is very important and requires significant judgment. Damage control
orthopaedics is a recently espoused philosophy which favors femur
stabilization with an external fixator rather than an intramedullary
nail in order to prevent the “second hit” or second physiologic insult
which occurs from intramedullary reaming and manipulation (44).
It is thought that the intramedullary nailing should be delayed further
stabilization, if a patient is physiologically challenged from multiple
injuries; however, early (less than 24 hours) femur stabilization with
an external fixator is still prudent.
-
-
General principles of fracture care.
Pediatric orthopaedics is a separate discipline because in part there
are many nuances in diagnosis and management that are very different
than adult orthopaedic fracture care. Obtaining a history is more
difficult and takes significant patience, and often family help to
solicit appropriate information. Children have injury patterns that are
distinctive, and with an understanding of these recurring patterns,
effective management can be learned and applied with greater confidence.Reducing fractures is frequently more effective in a
setting where general anesthesia can be administered as it is otherwise
difficult to gain the cooperation of the child. The rule “one doctor, one manipulation”
should be observed in the emergency room setting. With all types of
injuries involving the epiphyseal plate, an accurate diagnosis as to
the type of injury is important. Minor residual deformity in
Salter-Harris I and II (see below) injuries correct themselves with
subsequent growth, so open reduction is not indicated because the
operation itself may cause more trauma. What the clinician can accept
for angular deformity is more liberal in children because of their
remarkable capacity to remodel deformity. For this reason, there are
many fewer indications for surgical stabilization with internal
fixation than in adults. Helpful treatment principles follow.P.18-
Up to 30 degrees of angulation in the plane of joint motion is acceptable in metaphyseal fractures in young children. The younger the patient, the greater
the angulation acceptable. The closer the fracture is to the dominant
growth plate, the more angular deformity, and so the greater the
capacity to remodel. The dominant growth plate is-
Femur–distal
-
Tibia–proximal
-
Humerus–proximal
-
Radius–distal
-
-
If a fracture deformity is obvious on inspection, it should be reduced.
-
Fractured femurs in the 3- to 8-year-old group can be allowed to have 1.0 to 1.5 cm of overlap due to the potential for overgrowth.
-
Children do not experience stiffness of otherwise normal joints.
-
-
Growth plate
injuries. The epiphyseal plate is weakest at the site of cell
degeneration and provisional calcification (growth plate zones of
calcification and hypertrophy). Children who have undergone a
rapid growth spurt, and in those who are excessively heavy for their
skeletal maturity, are particularly vulnerable to such growth plate
injuries. Salter classified traumatic epiphyseal separations into the following functional groups.-
Class 1. A
fracture through the zone of provisional calcification without fracture
of bone tissue. Such an injury does not involve a germinal layer unless
associated with severe trauma either from the initial injury or from
attempted reductions. Growth disturbances are rare but do occur. -
Class 2. An
epiphyseal plate fracture with an associated fracture through the bony
metaphysis. Growth disturbance (physeal arrest) is also rare in this
category of injury. -
Class 3. An
epiphyseal plate fracture associated with fractures through the
epiphysis. These fractures involve the articular surface.
Histologically, there is a fracture through the germinal layers.
Accurate reduction is essential to prevent subsequent growth
disturbance, but even so, alterations in growth are unpredictable. If
the articular surface has more than 1 mm of “step off,” then open
reduction is indicated. -
Class 4. A
fracture through the epiphysis, epiphyseal plate, and metaphysis. Such
an injury almost invariably results in significant growth disturbance
unless it is anatomically reduced. Open reduction and internal fixation
is indicated if there is any displacement. -
Class 5. This
is an impact or “smash” injury that destroys all or part of the
epiphyseal plate and results in growth arrest. Close monitoring for
remaining growth is essential. Surgical resection of the bone bridge
and fat interposition are necessary if growth arrest results.
-
-
Diagnostic and therapeutic pediatric pitfalls
-
Treating accessory ossicles as fractures
-
Missing an osteochondral fracture
-
Not following a child long enough to follow effect of growth arrest (valgus or varus)
-
Missing a stress fracture
-
Confusing an epiphyseal fracture for a ligament injury (“kids don’t sprain”)
-
Missing a tibial spine fracture
-
Overdiagnosing instability of C2–C3 (“pseudosubluxation”)
-
Overtreating an upper humeral fracture (tremendous remodeling capacity)
-
Failing to realize the instability of an apparently undisplaced lateral condylar fracture of the humerus
-
Overlooking radial head dislocation (should bisect the capitelum) for both the anteroposterior and lateral radiographs
-
Distal forearm fractures lose initial reduction frequently
-
Overlooking abdominal injury in a child with a thoracolumbar flexion injury
-
Always obtain an opposite limb (joint) x-ray to aid interpretation of a physeal injury, particularly in the injured elbow.
-
absolutely dependent upon excellent radiological execution and
interpretation. The clinician must, at the very
least, demand two good quality, orthogonal, appropriately penetrated,
x-ray views centered on the bone or joint of interest without overlying
objects obscuring detail. This basic principle is perhaps the
most violated orthopaedic axiom, which leads to mismanagement,
frustration, litigious outcomes, and compromised patient care.
fractures deserve a critical radiographic assessment typically with
oblique views in addition to an anteroposterior and lateral view.
An alternative to oblique x-rays is the CT scan, but x-rays should
never be omitted altogether. Just under the epiphysis is the
metaphysis, which is made up of the broad funnel-shaped area of bone
with thin cortices and dense trabecular bone. In between each
metaphysis is the area of bone called the diaphysis. In general, the
metaphyseal and diaphyseal fragments do not need to be reduced
anatomically during treatment and healing. The treatment principle in
these areas of bone is to restore length, alignment, and rotation of
the bone. Any diaphyseal fracture warrants
orthogonal x-rays of the joint above and below the injury to look for
associated fractures or luxations.
radiograph and go directly to a CT scan to interpret fractures. This
practice is wrong. Most of the time, radiographs suffice for common
orthopaedic injuries and, in fact, contain all the necessary detail the
clinician needs for appropriate treatment. The ubiquitous ordering of
CT scans is an extremely expensive and wasteful strategy and simply
bypasses appropriate diagnostic algorithms. Furthermore, x-rays yield
better information about the quality or density of bone and better
information about displacement and spatial context of related bones.
might be necessary in complex fractures, particularly those that enter
joints. It is also particularly useful for assessment of spine and
pelvic injuries. The CT scan helps to assess greater bony detail and
often provides a roadmap during preoperative planning. Critical CT
findings to look for in certain injuries include:
-
Spine-subluxation of vertebral elements
-
Pelvis-sacral fractures and sacroiliac involvement
-
Acetabulum-intraarticular fragments of the acetabulum or femoral head (which suggests necessary axial traction)
-
Impaction injury to the acetabulum
-
Distal femur-coronal plane (Hoffa) fractures
-
Tibia plateau and pilon-fracture vector and comminution
-
Talus-talar dome and lateral process injuries (often missed on x-ray)
-
Calcaneus comminution at subtalar joint
-
Too much motion
destroys the vascular budding into the fracture hematoma and interferes
with revascularization. Adequate stabilization of the fracture,
therefore, is mandatory (46). -
Distraction decreases the surrounding vascularity as well as increases the length of the bony bridge necessary to heal the fracture.
-
Patient factors include smoking, diabetes, steroid medications, and poor nutrition.
when subjected to unaccustomed use. This condition can range from a
stress fracture of a fibula in a runner who has recently increased his
or her training distance or in an older person who is being mobilized
after having been confined to a chair or bed. A history
of having done something out of one’s normal routine, followed by pain,
should raise the question of stress fracture in the mind of the
physician. Common sites of stress fracture include the metatarsals
after unusually long walks or running, the distal
fibula
in runners, the tibia in football players (frequently misdiagnosed as
shin splints), and the femoral neck in both young and older patients.
reveals tenderness to pressure on the bone at the site of the fracture.
Occasionally, swelling and erythema are present. Radiographic
examination usually is negative in the first 10 to 14 days, after which
a small, radiolucent line can usually be seen in association with
increasing adjacent bone sclerosis. A bone scan shows radioactive
uptake earlier and may be indicated in the competing athlete,
particularly if the suspected fracture is in the tibia or femoral neck,
both of which have a high incidence of complete fracture if the athlete
continues competition. Magnetic resonance imaging (MRI) can
definitively identify a stress fracture. Healing stress fractures have
been mistaken for bone tumors (47).
the relief of symptoms unless there is danger of complete fracture
under normal use. Under such circumstances, the injury should be
treated like any undisplaced fracture.
-
Tendon
-
Diagnosis (48)
-
First-degree strain (mild)
-
The etiology is trauma to a portion of the musculotendinous unit from excessive forcible stretch.
-
Symptoms include local pain that is aggravated by movement or by tension.
-
Signs of injury include mild spasm, swelling, ecchymosis, local tenderness, and minor loss of function and strength.
-
Complications include recurrence of the strain, tendonitis, and periostitis at the tendinous insertion site.
-
Pathologic changes cause a low-grade inflammation and some disruption of muscle-tendon fibers by no appreciable hemorrhage.
-
-
Second-degree strain (moderate) (48)
-
The etiology is trauma to a portion of the musculotendinous unit from violent contraction or excessive forcible stretch.
-
Symptoms and signs
include local pain that is aggravated by movement or tension of the
muscle, moderate spasm, swelling, ecchymosis, local tenderness, and
impaired muscle function. -
Complications include a recurrence of the strain.
-
The pathologic findings consist of hemorrhage and the tearing of muscle–tendon junction fibers without complete disruption.
-
-
Third-degree strain (severe) (48)
-
Symptoms and signs
include severe pain and disability, severe spasm, swelling, ecchymosis,
hematoma, tenderness, loss of muscle function, and usually a palpable
defect. An avulsion fracture at a tendinous insertion may mimic a
severe strain. -
A complication is prolonged disability.
-
Roentgenograms can demonstrate an avulsion fracture at the tendinous attachment as well as soft-tissue swelling.
-
The pathology
consists of a ruptured muscle or tendon with the resultant separation
of muscle from muscle, muscle from tendon, or tendon from bone.
-
-
-
Treatment.
Direct treatment toward immobilization, with the disrupted tissue ends
approximated. In some settings, this requires removal of devitalized
tissue and repair by the sites and type of suture that will not cause
further devitalization. When possible, sutures are placed in the
surrounding fascia and not in the muscle itself.
-
-
Tendon
-
Tendons are relatively avascular
structures and do not handle infection well. At sites where they course
along long synovial tunnels, blood supply is via the long axis of the tendon or vincula. Trauma or sheath infections can jeopardize nutrition of the tendon. -
As a general principle,
a lacerated or ruptured tendon should be repaired primarily with a
nonreactive material and a suture technique to ensure continued
approximation of the tendon ends. Even with prophylactic antibiotics,
primary repair of tendons in wounds more than 12 hours old carries
considerable risk. A nonreactive synthetic suture or braided wire is
the suture of choice. If the tendon is expected to glide subsequently,
then handling the tendon with sponges and forceps is avoided because
this causes further trauma and may be associated with dense adhesions. -
Any involved tendon sheath
should be opened in a longitudinal fashion so that the tendon is
unroofed for the entire excursion of a repaired laceration site to-
Prevent “triggering” of the enlarged sutured site.
-
Allow for revascularization of the tendon at the suture site.
-
Prevent fixation on the relatively immobile sheath.
-
-
Only those with special training in hand surgery should repair digital flexor tendons in the hand.
P.21 -
-
Ligaments
-
Types of injury
-
First-degree sprain (mild)
-
Signs include mild point tenderness, no abnormal motion, little or no swelling, minimal hemorrhage, and minimal functional loss.
-
Complications include a tendency toward recurrence.
-
The pathology consists of minor tearing of the ligamentous fibers.
-
-
Second-degree sprain (moderate)
-
Signs include point tenderness, moderate loss of function, slight-to-moderate abnormal motion, swelling, and localized hemorrhage.
-
Complications can include a tendency toward recurrence, persistent instability, and traumatic arthritis.
-
This pathology is a partial tear of a ligament.
-
-
Third-degree sprain (severe)
-
Signs include a loss of function, marked abnormal motion, possible deformity, tenderness, swelling, and hemorrhage.
-
Complications can involve persistent instability and traumatic arthritis.
-
Stress roentgenograms demonstrate abnormal motion when pain is adequately relieved.
-
The pathology is a complete tear of a ligament.
-
-
-
Diagnosis of
the extent of the ligamentous injury presents one of the major problems
in orthopaedics. Rupture may be suspected from the mechanism of injury
or from physical examination, which reveals tenderness over the
ligament. If the patient is seen shortly after injury, a gap may be
felt where the ligament is normally located. The injury might be fairly
painless, especially if the ligament is completely disrupted. Once
hemorrhage and swelling occur, this diagnostic possibility is
eliminated. Another diagnostic aid is a stress roentgenogram, but it
must be compared with the opposite and normal side. Such films should
be made when the pain is inhibited by regional or general anesthesia.
Arthroscopy or arthrography can provide pertinent information and a
diagnosis, but a skilled arthroscopist should first be consulted. An
MRI scan can be used to make the diagnosis. -
Treatment of
a complete ligamentous rupture is in essence the treatment of a
dislocated joint after the dislocation has been reduced. In general,
preserving motion is most important, and early mobilization is the
treatment of choice. Ligaments are relatively avascular, so healing is
slow. The larger ligaments must be protected until the scar matures
(8–16 weeks). -
There often is no clear answer when operative repair of ligaments is essential, but open repair is indicated in the following instances:
-
Whenever the joint cannot be anatomically reduced
-
When there is reasonable evidence of infolding or turning of the ligament on itself so that with any closed treatment the ends of the torn ligament would not be in close proximity
-
When ligaments are injured about a joint that has no internal stability (relies primarily on ligamentousvg structure for its stability)
P.22 -
-
-
Nerves
-
Nerve injuries are of three types: contusion or neurapraxia, crush or axonotmesis, and complete division or neurotmesis.
Blunt injuries and those associated with fractures tend to be either
neurapraxia or axonotmesis. For this reason, the fracture should be
treated in its usual manner and the nerve injury observed. If it is
neurapraxia, recovery will be complete within 6 to 12 weeks. If it is
an axonotmesis, recovery from the trauma site to the next muscle to be
innervated should be followed, keeping in mind that the expected
recovery rate is 1 mm/day or 1 in./month. If reinnervation does not
occur on time, exploration is indicated. When the distance from the
site of trauma to the next innervated muscle that can be assessed
causes a 6-month delay, early exploration is indicated. An
electromyogram shows reinnervation approximately 1 month before it can
be detected clinically, but one is dependent on the skill of the
electromyographer for interpretation (see App. F).
A traction injury is usually a mixed lesion with a large element of
neurotmesis of individual axons at various places along the nerve. A
nerve injury associated with sharp trauma is usually neurotmesis, and
surgical repair is indicated. The brachial plexus
presents a special diagnostic and treatment problem. Injuries from
lacerations, especially in children, should be repaired primarily. Most
brachial plexus injuries, however, are caused by traction and are
either an avulsion of the root from the cord or the typical tearing of
the axons at multiple levels along the nerve. MRI is essential for
differentiation. If the lesion is an avulsion injury, no recovery is
possible, and the patient should be started early on rehabilitation. If
the lesion is the typical traction injury, the patient should be
followed up to document recovery. If no recovery appears at the
appropriate time intervals, exploration and possible suture or nerve
graft should be considered. -
As a general principle, secondary repair (3–6 weeks after injury) is preferable to primary repair for the following reasons:
-
The repair is done as an elective procedure by the first team. The surgeon is rested and prepared for the procedure.
-
There is less hesitancy in extending the incision for proper mobilization of the nerve.
-
It is easier to delineate the extent of damage along the nerve.
-
The epineurium has some degree of scarring and hence holds the suture better.
-
The distal axon tubules are open because wallerian degeneration has occurred, and regeneration has a chance to proceed.
-
-
There are many exceptions to the preference for secondary repair, such as suturing
-
A digital nerve
-
A nerve in the brachial plexus
-
An isolated nerve injury less than 8 to 12 hours old inflicted by a razor or sharp knife
-
-
Nerve surgery should not be performed at any time by a surgeon inexperienced in microscopic techniques.
-
-
Hematomas
-
Treatment of
large hematomas (large compared to the area of confinement) whether
subcutaneous or in muscle usually should consist of evacuation as an
elective procedure in the operating room. A hematoma is not absorbed
but undergoes organization, fibrosis, and scarring. Aspiration of a
clot is not possible, so a large hematoma is evacuated by open
drainage. Before considering this, the surgeon must be sure the
hematoma is not expanding or is the cause of shock. If it is, vascular
surgery consultation is mandatory to consider primary repair.
-
-
Classification. Frostbite is a pathologic entity that occurs on a spectrum of severity depending on temperature and duration of exposure. Frostnip results in pallor
P.23
and numbness but no tissue damage after rewarming. Chilblain typically involves the patient’s face, pretibial region, or dorsum of the hands, and results from repeated exposure. Trenchfoot occurs during water immersion of an extremity in subfreezing conditions.Frostbite occurs commonly in temperatures less than 2°C and includes the following degrees of severity:-
1° = hyperemia and edema
-
2° = hyperemia and vesicle formation with partial-thickness necrosis
-
3° = full-thickness skin necrosis
-
4° = full-thickness skin and underlying structure necrosis
-
-
Treatment
-
Prehospital care. Protection of the frostbitten part from mechanical trauma. Avoid rewarming until it can be done definitively.
-
Rewarming.
Hypothermia of the patient should be treated first. Stimulation of the
vagus or myocardium with Naso-gastric (NG) tubes, Swan-Ganz catheter,
or other methods should be avoided. If the patient is breathing,
intubation is not appropriate. The patient should be rewarmed in a
water bath with mild antibacterial soap at 40° to 42°C (104°–108°F). A
flushed appearance indicates reperfusion and the patient should be
removed from the bath. -
Definitive care.
The goals of definitive care are to preserve viable tissue and prevent
infection. If possible, a burn center should be contacted for
initiation of thrombolytic therapy to salvage threatened tissue and
limbs. The injured limb should be elevated and protected from even mild
trauma. Lambs wool should be placed between the toes. Analgesics and
ibuprofen 4 mg/kg should be administered 3 times a day. Tetanus
prophylaxis is appropriate. Any source of nicotine should be strictly
prohibited.
-
SR, Rubin SM, Black D. The future of hip fractures in the united
states: numbers, cost, and potential effects of postmenopausal
estrogen. Clin Orthop 1990;252:163–166.
GT, Siegel JH, Dischinger PC, et al. Airbag protection versus
compartmental intrusion effect determines the pattern of injuries in
multiple trauma motor vehicle crashes. J Trauma 1996;41:935–951.
K, Johansen K. Can Doppler pressure measurement replace “exclusion”
arteriography in the diagnosis of occult extremity trauma? Ann Surg 1991;214:737–741.
FA, Yellin AE, Bauer M, et al. Is arterial proximity a valid indication
for arteriography in penetrating extremity trauma? A prospective
analysis. Arch Surg 1990;125:1256–1260.
MF, Winquist RA, Hansen ST. Fractures of the femoral neck in patients
between the ages of twelve and forty-nine years. J Bone Joint Surg (Am) 1984;66:837–846.
JH, Aufranc OE. Avascular necrosis of the femoral head in the adult. A
review of its incidence in a variety of conditions. Clin Orthop 1977;86: 43–62.
E, Hamori CA, Bergman S, et al. A prospective randomized trial of
vacuum assisted closure versus standard therapy of chronic nonhealing
wounds. Wounds 2000;12:60–67.
H, Wiss DA, Moldawer TD. Gunshot wounds to the musculoskeletal system.
In: Browner BD, Jupiter JB, Levine AM, et al. eds. Skeletal trauma, 3rd ed., Vol. 1. Philadelphia, PA: WB Saunders, 2003:1884–1924.
MD, Schemitsch EH, Vincent LO, et al. The effect of a femoral fracture
on concomitant closed head injury in patients with multiple injuries. J Trauma 1997;42:1041–1045.
MI, Simonian PT, DeFalco AJ, et al. Internal fixation in pelvic
fractures and primary repairs of associated genitourinary disruptions:
a team approach. J Trauma 1996;40:784–790.
MJ, MacKenzie EJ, Reimer BL, et al. Adult respiratory distress
syndrome, pneumonia, and mortality following thoracic injury and a
femoral fracture treated either with intramedullary nailing with
reaming or with a plate: a comparative study. J Bone Joint Surg (Am) 1997;79:799–809.
PJ, Huckfeldt R, Mullins RJ, et al. The effects of femoral
intramedullary reaming on pulmonary function in a sheep lung model. J Bone Joint Surg (Am) 1997;79:194–202.
KD, Cadambi BA, Seibert GB. Incidence of adult respiratory distress
syndrome in patients with musculoskeletal injuies: effect of early
operative stabilization of fractures. J Trauma 1985;25:375–383.
EB, Bonsdorff HV, Hakkinen S, et al. Primary operative fixation of long
bone fractures in patients with multiple injuries. J Trauma 1977;17:111–121.
HC, Grimme K, Van Griensven M, et al. EPOFF study group. Impact of
intramedullary instrumentation versus damage control for femoral
fractures on immunoinflammatory parameters: prospective randomized
analysis by the EPOFF study group. J Trauma 2003;55:7–13.
PP, Esterhai JL Jr. Delayed union, nonunion and synovial
pseudarthrosis. In: Brighton CT, Friedlaender GE, Lane JM, eds. Bone formation and repair. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1994:505–527.
DC, Dalton JD Jr, Seaber AV, et al. Experimental muscle strain injury:
early functional and structural deficits and the increased risk for
reinjury. Am J Sports Med 1993;21:190–194.
RB, Mendoza RM, Williams DM. Problems in management of type III open
fractures. A new classification of type III open fractures. J Trauma 1984;24: 742–746.
SR, Hollingworth-Fridlund P, Cooper GF, et al. The effect of
regionalization upon the quality of trauma care as assessed by
concurrent audit before and after institution of a trauma system: a
preliminary report. J Trauma 1986;26:812–820.
PA, Young AB, Bivins BA, et al. Diagnosis of acute abdominal injuries
in patients with spinal shock: value of diagnostic peritoneal lavage. J Trauma 1980;20:55–57.