Inflammatory Spondylo-Arthropathy
expression of a new synovial antigen triggers the body to produce
rheumatoid factor (RF), an IgM molecule directed against the autologous
IgG antibody of the expressed aberrant synovial antigen. Approximately
80% of patients with active rheumatoid disease are positive for IgM
anti-IgG; however, the presence of RF alone in the synovial fluid does
not produce rheumatoid arthritis. Several other autoantibodies have
been identified in rheumatoid arthritis with specificity greater than
90%, including antikeratin, anticitrullinated peptides, anti-RA33,
anti-Sa, and anti-p68. T cell-mediated mechanisms also have been
implicated. Granulation tissue formed within the reactive synovium by
proliferating fibroblasts and inflammatory cells is called the pannus, which invades and destroys local articular structures.
spine, but uncommonly may involve the thoracic, lumbar, and sacroiliac
regions. Depending on the applied diagnostic criteria, cervical
involvement in rheumatoid arthritis ranges from 25% to 95%.
Approximately 10% of rheumatoid patients present with spondylitis as
the initial disease manifestation. Cervical spine subluxations are
observed in 43% to 86% of patients, more commonly in men despite
greater general disease incidence in women.
process, and the subsequent severity is correlated closely with the
extent and severity of the peripheral disease activity. Neurologic
impairment caused by cervical instability reportedly ranges from 11% to
58%. A postmortem study of rheumatoid patients identified unrecognized
medullary compression as the cause of death in 10% of patients.
and progressive erosion of adjacent cartilage and bone. Erosion of bone
results in cyst formation and osteoporosis. Extension of the pannus
into the adjacent disc space results in spondylodiscitis.
Considerable evidence has accumulated suggesting that rheumatoid
synovial fibroblasts are transformed cells that have acquired
proliferative and invasive properties and are susceptible to
significant up-regulation by interleukin-1 or tumor necrosis factor-α.
These synoviocytes have been identified as producing the important
degradative enzymes collagenase, stromelysin, and cathepsins involved
in articular destruction.
characterized by significant increased expression of the
proinflammatory cytokines interleukin-1 and tumor necrosis factor-α.
Although they may participate directly in articular matrix degradation,
their suggested predominant role may be in amplifying the pathogenic
cascade via cytokine-mediated up-regulation of synoviocyte proteolytic
enzyme production and resultant articular destruction.
disc and ligament structures by the rheumatoid inflammatory process may
result in segmental instability and neurologic compromise.
Additionally, proliferation of the pannus into the epidural space may
result in direct neural element compression compounded by the presence
of proinflammatory factors.
affected in rheumatoid arthritis, owing to the predominance of synovial
articulations. The axial plane orientation of the C1-2 facets, without
bone interlocking, further predisposes the rheumatoid-compromised
motion segment to instability.
-
Atlantoaxial subluxation (AAS)
-
Vertical subluxation of the odontoid (VS)
-
Subaxial subluxation (SAS)
49% of patients. The subluxation is usually anterior, although lateral
subluxation (>2 mm) occurs in 20% of patients, and posterior
subluxation occurs in 7%.
destructive erosion of the occipitoatlantal and atlantoaxial joint
complexes leading to vertical translation of the odontoid and settling
of the occiput. Direct odontoid compression on the brainstem or
excessive kyphosis (flexion) of the cervicomedullary junction can lead
to significant neurologic compromise or death.
patients. SAS occurs at multiple levels, most commonly at C2-4,
resulting in kyphosis and a “stepladder” appearance. Neurologic
compromise occurs directly from facet pannus extension or indirectly
from segmental subluxation. Progressive SAS after upper cervical fusion
has been reported in 36% of rheumatoid patients undergoing
occipitocervical fusion and 5.5% of patients undergoing atlantoaxial
fusion.
is reported in 40% to 88% of patients and frequently is associated with
occipital headaches due to greater occipital nerve (C2) irritation or
referred pain from the posterior ramus of C1 nerve root. Ear pain or
facial pain may be present, owing to C2 sensory contributions to the
greater auricular nerve or nucleus of the spinal trigeminal tract.
weakness, loss of endurance, loss of dexterity, and gait imbalance, may
be subtle and should be reviewed in context relative to historical
patient function. Neck motion may result in electric shock-like
sensations of the torso or extremities (Lhermitte’s sign). Urinary
retention or incontinence may be the first indication of myelopathy in
the advanced rheumatoid patient. Vertebrobasilar insufficiency or
cervicomedullary compression may present with visual disturbances,
vertigo, tinnitus, or dysphagia.
myelopathy may be difficult to assess secondary to rheumatoid
peripheral extremity involvement. Hyperreflexia, Hoffmann’s sign,
Babinski’s sign, and clonus sign may be difficult to elicit secondary
to peripheral joint mutilation. Presence of a scapulohumeral reflex
(Shimuzu’s sign) may indicate compression proximal to C4.
according to the Ranawat classification of rheumatoid myelopathy, which
has proved useful in planning treatment and prognosticating outcome:
-
Grade I—no neural deficit
-
Grade II—subjective weakness, hyperreflexia
-
Grade IIIA—objective weakness, long-tract signs, ambulatory
-
Grade IIIB—objective weakness, long-tract signs, nonambulatory
classified according to the Japanese Orthopaedic Association scale with
modification to Western parameters.
erythrocyte sedimentation rate (ESR), RF, and antinuclear antibody. RF
is positive in 80% of patients with active disease; antinuclear
antibody is positive in 20% to 60% of patients. ESR typically is
greater than 30 mm, depending on disease activity and degree of anemia.
flexion/extension lateral radiographs must be obtained at some point in
the historical evaluation of the rheumatoid patient. Radiographs are
required on any patient requiring endotracheal intubation or cervical
manipulation. Fifty percent of patients with radiographic abnormalities
are asymptomatic at initial presentation. Of patients undergoing
peripheral extremity procedures (arthroplasty), 61% show radiographic
abnormalities on preoperative cervical screening radiographs.
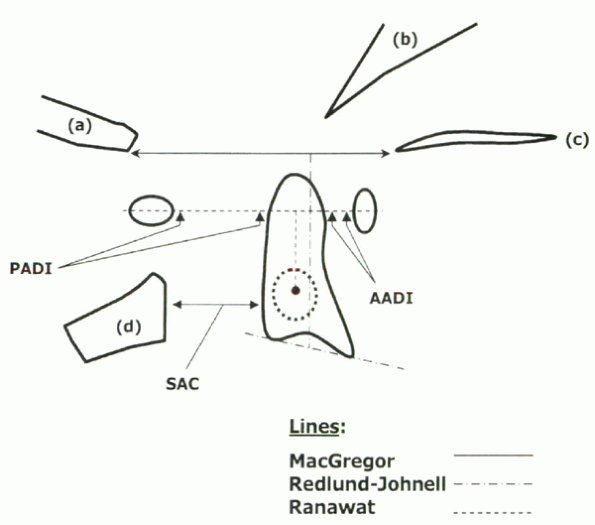
-
Anterior atlantodental interval (AADI) and posterior atlantodental interval (PADI) for AAS
-
McGregor’s line, Ranawat index, and Redlund-Johnell measurement for VS
-
Subaxial canal diameter for SAS
odontoid to the posterior surface of the anterior C1 ring; the PADI is
measured from the posterior odontoid to the anterior surface of the
posterior C1 ring. Normal AADI in adults is less than 3 mm; greater
than 10 mm indicates instability and risk of neurologic compromise.
Normal PADI is greater than 14 mm; less than 14 mm is associated with
increased risk of neurologic deficit. The PADI seems to be a better
predictor of paralysis than the AADI (Fig. 22-2).
 |
|
Figure 22-1 Radiographic parameters of cervical rheumatoid arthritis: (a) occiput, (b) clivus, (c) hard palate, (d) C2 arch.
|
 |
|
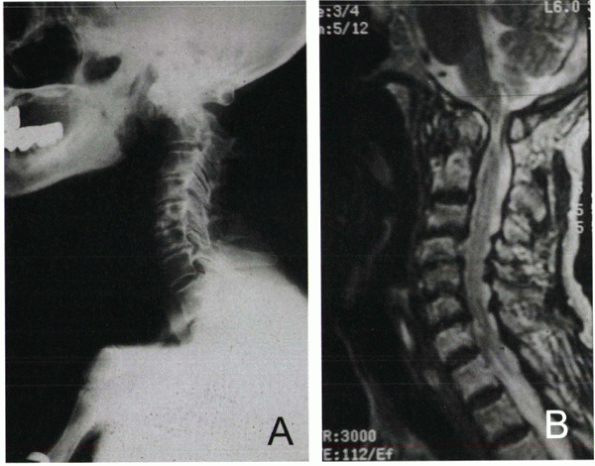
Figure 22-2 Rheumatoid arthritis of the cervical spine. (A) Lateral radiograph demonstrating atlantoaxial subluxation (AAS) instability pattern. (B) Sagittal MRI, T2-weighted image. Note C1-C2 leval canal compromise and pannus formation.
|
vertebral morphology in rheumatoid patients. MacGregor’s line is a line
drawn on lateral view from the hard palate to the base of the occiput.
VS is defined as migration of the superior odontoid greater than 4.5 mm
above MacGregor’s line. The Redlund-Johnell measurement is useful in
cases of odontoid mutilation and is determined by measuring the
distance between the midpoint of the inferior margin of the body of the
axis (C2) to MacGregor’s line. A value of less than 34 mm in men and 29
mm in women is considered abnormal. The Ranawat index is derived on the
lateral view as the distance between the center of the C2 pedicle and a
line intersecting the anterior and posterior arches of C1. A distance
of less than 15 mm for men and 13 mm for women is regarded as
significant for VS.
views. Subluxation of greater than 20% of the lateral vertebral width
or 4 mm of listhesis is considered significant. Additionally, patients
with subaxial canal diameters, as measured from the posterior vertebral
cortex to ventral laminar surface, of less than 13 mm are at increased
risk for neurologic deficit.
reconstruction, is useful in delineating rheumatoidaltered bone anatomy
and in assessing rotatory or lateral subluxation. Additionally, clear
visualization of the vertebral artery foramen assists in preoperative
planning about the C1-2 complex.
visualization of soft tissue and neurologic structures, including
spinal cord parenchyma, rheumatoid pannus, and epidural space
compromise (space available for the cord [SAC]). Minimal SAC is 13 mm
at C1-2 and 12 mm at the subaxial regions. Upper cervical pannus
thickness greater than 3 mm has been correlated with neural
compression. MRI, particularly a supervised flexion view, is useful in
assessing VS and the spinal cord configuration. Patients with a
cervicomedullary angle of less than 135 degrees, as measured by
intersecting lines of the anterior cervical spinal cord and medulla
surfaces, show significant VS and probable neurologic compromise.
treatment. The goals of treatment in rheumatoid involvement of the
cervical spine are:
-
To avoid development of irreversible neurologic deficit
-
To prevent sudden death caused by unrecognized neural compression
-
To avoid unnecessary surgery, given that 50% of patients with radiographic evidence of instability remain asymptomatic
treatment. The clinical course of spinal involvement is correlated
directly to the severity of the systemic disease presentation.
Nonoperative treatment is supportive with use of soft collars, physical
therapies, patient education, and close monitoring of neurologic status.
 |
|
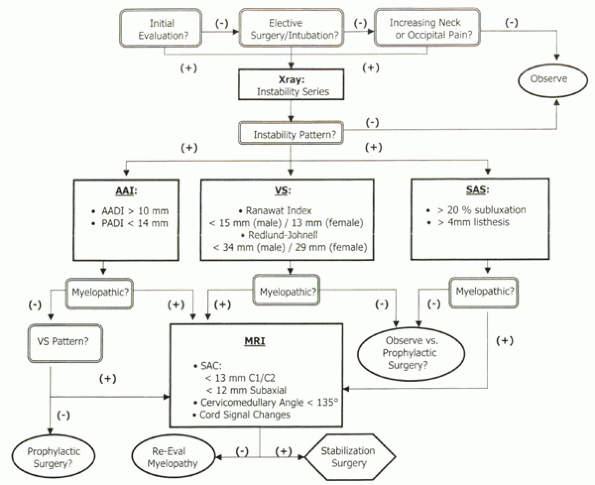
Algorithm 22-1
Ranawat classification of rheumatoid myelopathy. AADI, anterior atlantodental interval; AAS, atlantoaxial subluxation; PADI, posterior atlantodental interval; SAC, space available for the cord; SAS, subaxial subluxation; VS, vertical subluxation of the odontoid. |
presenting neurologic condition, with a poorer prognosis associated
with more significant neurologic deficits. Ambulation seems to be an
important prognostic hallmark, with some studies reporting at least one
grade of improvement in 58% of surgically treated Ranawat grade IIIA
patients versus 20% in Ranawat grade IIIB patients. Operative goals
include the following:
-
Stabilization of the C1-2 complex
-
Prevention of further deformity
-
Reduction of pain
-
Stabilization or reversal of myelopathy
neurologic deficit (Ranawat grade II) and radiographic evidence of
spinal instability (PADI < 14 mm, AADI > 10 mm, SAC <13 mm,
evidence of VS, or spinal cord diameter in flexion <6 mm). Relative
indications include intractable neck pain (typically cervicooccipital)
and radiographic instability.
patient (Ranawat grade I) with radiographic instability is
controversial. The natural history of radiographic instability is that
progression occurs in a predictable pattern of reducible AAS to
reducible combined AAS and VS to irreducible VS. Numerous authors
advocate early stabilization of AAS to prevent progression to
irreducible VS and the increased morbidity associated with such
advanced disease. The prognostic value of radiographic studies for
myelopathy development is variable. Several studies report no
conclusive correlation between AADI and neurologic status. PADI seems
more useful with a reported sensitivity of 97% for paralysis with PADI
less than 14 mm and a negative predictive value for paralysis of 94%
with PADI greater than 14 mm. MRI
findings
of subarachnoid encroachment are sensitive in that affected patients
with neurologic findings show a 12 times risk of progressive neurologic
deterioration.
reduction and posterior fusion using either Gallie or Brooks wiring
techniques, transarticular screw fixation, or a combination of screws
and wiring. Posterior wiring techniques require an intact posterior C 1
and C2 lamina. Safe sublaminar wire passage may be compromised by
inadequate subluxation reduction. Typically, supplemental postoperative
halo fixation is required. Reported fusion rates are 67% to 90% and are
increased with halo fixation. Higher fusion rates are reported with the
Brooks wiring technique. Several studies have shown spontaneous pannus
resorption subsequent to solid posterior fusion. Anterior transoral
decompression is reserved for patients with persistent neurologic
deficit and no evidence of pannus resorption on postoperative MRI.
occipitocervical fusion. As with AAS, improved reduction, neurologic
recovery, and higher rates of fusion are reported with plate and screw
techniques. Irreducible deformity may require C1 laminectomy or
odontoid resection to achieve adequate decompression. Surgical
treatment of SAS is achieved best with posterior lateral mass reduction
and screw and plate fixation. If the subluxation is not reduced
adequately, complementary anterior decompression and fusion is
indicated.
cervical involvement and myelopathy show progressive neurologic deficit
and paralysis if left untreated. One third experience sudden death due
to neurologic compromise, typically with VS.
reported in 27% to 100% of patients. Variably, 95% of patients improved
one Ranawat grade after treatment of AAS; 76%, after combined AAS and
VS; and 94%, after isolated SAS. Universally, ultimate neurologic
outcome is determined by the preoperative neurologic condition, with
only 20% of Ranawat grade IIIB patients showing one grade of
postoperative improvement versus 58% of Ranawat grade IIIA patients.
Pain relief has been reported in 80% to 100% of patients with solid
fusion.
nonunion, and adjacent segment subluxation. Morbidity and mortality are
higher in more neurologically compromised patients. Postoperative
mortality has been reported to be 13% in Ranawat grade IIIB patients.
Improved perioperative techniques of fiberoptic intubation, meticulous
soft tissue surgical technique, and biomechanically sound constructs
have reduced postoperative morbidity significantly. Mean fusion rates
of 83% have been reported, with the highest rates shown by plate and
screw techniques. Nonunion rates are increased in patients with
advanced mutilating disease and osteoporosis. Neurologic compromise
resulting from cervical spine surgery is uncommon and associated with
inadvertent subluxation during operative positioning or canal
compromise during sublaminar wire passage.
deposition of cellular infiltrates and immunoglobulins in skin and
joint lesions, supports an immunologic mechanism of disease origin.
Autoantibodies, leukotrienes, increased levels of complement activation
fragments, and T-cell activity imbalance all have been described as
possible causative factors, although the exact mechanism is unknown.
with typical presentation in the 20s or 30s. Variably, 6% to 40% of
patients with cutaneous lesions develop psoriatic arthritis; 15% of
patients develop arthritis manifestations before developing cutaneous
lesions. The male-to-female ratio is approximately 1:1. Ten percent of
patients with psoriatic arthritis show spinal involvement.
through a combination of proliferative synovial destruction and
inflammatory ankylosis. In contradistinction to ankylosis spondylitis,
discovertebral erosion and axial ankylosis occur in a patchy,
asymmetric involvement with mixed marginal and nonmarginal
syndesmophyte presentation. Proliferative synovial destruction of the
diarthrodial joints of the upper cervical spine can result in
instability patterns similar to rheumatoid arthritis. The major
predictor of disease involvement is the duration of disease at
presentation.
-
Asymmetric oligoarthritis
-
Distal arthritis
-
Arthritis mutilans
-
Symmetric polyarthritis
-
Spondyloarthropathy
show spondyloarthropathy. Variably, 35% to 75% of this subset show
principally cervical spine involvement.
-
Type I—inflammatory response with diarthrodial erosion or joint subluxation, or both, without evidence of ankylosis
-
Type II—vertebral ankylosis with apophyseal joint fusion and mixed marginal/nonmarginal syndesmophyte formation
include presence of cutaneous lesions and axial spine symptoms. Typical
age of onset is in the 20s or 30s. Physical manifestations of psoriatic
spondyloarthropathy include axial pain, stiffness, and diminished range
of motion. Laboratory findings include mildly elevated ESR and
C-reactive protein, particularly in cases of erosive disease. RF is
characteristically negative. Approximately 20% of patients with
psoriasis are HLA-B27 positive, whereas 60% to 80% of patients with
radiographic evidence of psoriatic spondyloarthropathy are HLA-B27
positive. Of patients, 10% to 20% have elevated serum uric acid levels.
-
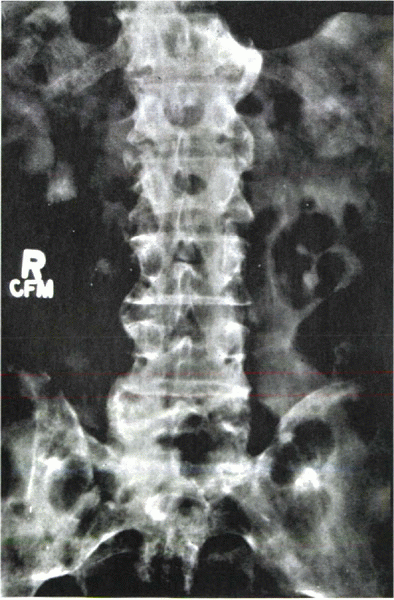
Type I presentation—erosive destruction of the spinal diarthrodial joints without overt segmental ankylosis (Fig. 22-3).
-
Type II presentation—similar to ankylosing spondylitis with spontaneous apophyseal joint fusion and syndesmophyte formation
is similar to that of rheumatoid arthritis. Refractory cases may
require adjunct drug therapies, including gold, penicillamine,
methotrexate, and cyclosporine. Steroids should be used judiciously,
given reports of an erythrodermic picture and significant cutaneous
lesion flares with dose reductions. Surgery is reserved for patients
with segmental instability or neurologic deficit or both.
 |
|
Figure 22-3
Psoriatic spondylitis of the lumbar spine. Note the patchy pattern of asymmetric non-marginal syndesmophytes. (Courtesy of B. Gehlman, MD) |
postinfective reactive arthritis in susceptible individuals. Commonly,
Reiter’s syndrome presents within 1 month of a defined infectious
event, commonly a urethritis or enteritis.
20s or 30s. Postvenereal disease seems to affect men more than women;
enteric forms affect men and women equally. Approximately 1% to 2% of
enteric-affected individuals develop Reiter’s syndrome. Approximately
50% of Reiter’s syndrome patients manifest symptom recurrence at
intervals 3 weeks to 20 years after initial presentation. Twenty
percent of patients exhibit progressive degenerative disease in the
peripheral joints and axial spine. HLA-B27 histocompatibility antigen
is present in 60% to 85% of patients and seems to correlate with the
severity and chronicity of Reiter’s syndrome.
seems to involve an inflammatory initiation by an “infection trigger,”
gastrointestinal in nature, in susceptible individuals.
-
Urethritis (80%)
-
Conjunctivitis
-
Unilateral polyarthritis
-
Mucocutaneous lesions
and stiffness in 50% of affected patients. The lumbar spine is involved
in 29% of cases; the cervical spine rarely is involved. Spinal pain is
characterized by morning stiffness, rest pain, and improvement with
exercise. Variably, 30% to 80% of patients develop asymmetric
sacroiliitis, especially early in the disease. HLA-B27-positive
patients are more likely to show radiographic changes.
General treatments include antiinflammatory agents and physical
therapies. Surgical treatment typically involves joint arthroplasty at
end-stage disease presentation. Spinal surgery is uncommon.
arthritis in patients with inflammatory bowel disease (i.e., ulcerative
colitis or Crohn’s disease). The reported incidence of HLA-B27 in
patients with inflammatory bowel disease and spondyloarthropathy is 50%
to 75%. The occurrence of ankylosing spondylitis is highest in patients
with HLA-B27 phenotype and is more prone to develop with greater
severity, at a younger age, in women with inflammatory bowel disease.
Two forms of axial arthropathy present with inflammatory bowel disease:
-
Ankylosing spondylitis indistinguishable from idiopathic variants
-
Asymptomatic sacroiliitis
spondylitis generally are HLA-B27 positive, and asymptomatic patients
generally are HLA-B27 negative. In symptomatic patients, clinical and
radiographic findings are identical to those of ankylosing spondylitis,
including low back pain, stiffness, spondylitis, and marginal
syndesmophyte formation. The peripheral arthritis is nondeforming
radiographically.
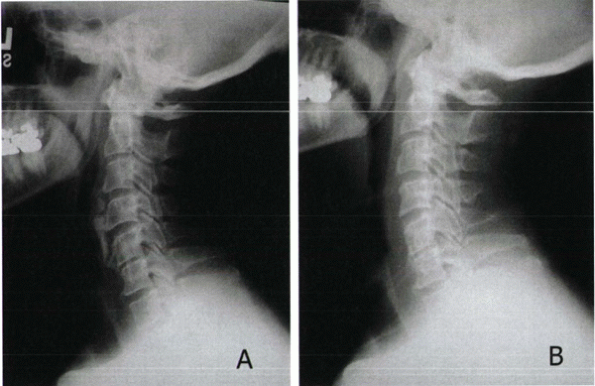 |
|
Figure 22-4
Diffuse idiopathic skeletal hyperostosis (DISH) of the cervical spine in a 55-year-old man with severe dysphagia. (a) Preoperative lateral radiograph. Note significant, noncontiguous osteophyte formation in the cervical region. (b) Postoperative lateral radiograph 6 months after osteophyte resection. |
manifestation. Peripheral arthritis responds to suppression of bowel
disease, including sulfasalazine and colectomy. The axial condition is
independent of the bowel disease course. Surgical treatment indications
are the same as described for ankylosing spondylitis.
systemic arthropathy of unknown etiology first described by Forestier
in 1950 as senile ankylosing hyperostosis of the spine.
enthesopathy affecting the insertion of soft tissue structures and
ligaments into bone (the enthesis) with resultant osteophyte formation.
The most commonly affected anatomic areas are the spine, shoulder,
elbow, knee, and calcaneus. In contrast to ankylosing spondylitis, DISH
affects middle-aged and older age groups, shows no HLA-B27 association,
and has no findings of apophyseal or sacroiliac joint ankylosis. In
differentiating DISH from ankylosing spondylitis or degenerative disc disease, classification criteria include the following:
-
Preservation of intervertebral disc space height
-
Ossification of at least four consecutive vertebral bodies
-
Absence of sacroiliac or apophyseal joint ankylosis
-
Absence of vacuum phenomenon or vertebral body marginal sclerosis
localization. Thoracic and lumbar involvement is characterized by
thoracolumbar stiffness or pain, although of no greater frequency than
in age-matched spondylosis patients. Laboratory values are generally
unremarkable. Prolific anterior osteophyte formation may evoke clinical
symptoms of dysphagia, aspiration, stridor, Horner’s syndrome, or
thoracic outlet syndrome. Dysphagia is reported in 17% to 28% of
cervical DISH patients. Symptoms do not always correlate with
osteophyte mass. Endotracheal intubation or endoscopic procedures may
be difficult. Concurrence with ossification of posterior longitudinal
ligament may show significant myelopathy (Fig. 22-4).
the anterior cervical osteophyte for dysphagia. DISH concurrence with
ossification of posterior longitudinal ligament and myelopathy may
require canal decompression. Fusion stabilization with posterior
approaches may be deferred secondary to anterior osteophyte stability.
As with ankylosing spondylitis, DISH patients with extensive cervical
involvement can incur catastrophic neurologic compromise from forceful
trauma subsequent to fracture instability of the compromised motion
segment. Surgical treatment requires fracture reduction and
multisegmental stabilization as with ankylosing spondylitis.
RH, Kaufman RL. Erosive and subluxing cervical spine disease in
patients with psoriatic arthritis. J Rheumatol 1987;14:111-117.
SD, Dodge LD, Bohlmann HH, Rechtine GR. Rheumatoid arthritis of the
cervical spine: A long-term analysis with predictors of paralysis and
recovery. J Bone Joint Surg 1993A;75:1282-1297.
Keyser F, Elewaut D, De Vos M, et al. Bowel inflammation and the
spondyloarthropathies. Rheum Dis Clin North Am 1998;24: 785-813.
S, Fortin PR, Fitzcharles MA, et al. A controlled study of diffuse
idiopathic skeletal hyperostosis: clinical features and functional
status. Medicine 1997;76:104-117.
