The Adult Hip
problems, whether pain, deformity, gait disturbance, or radiological
abnormality in the absence of clinical symptoms and signs, has altered
significantly in the last 50 years. Before that time, treatment of most
hip conditions arose as a sequel of infection leading to stiffness, or
posttraumatic deformity. Options for treating arthritic conditions were
limited. The advent of total hip joint arthroplasty has dramatically
increased treatment options and represents the greatest orthopaedic
advance of this century. Indications for total hip arthroplasty (THA)
are continually widening to include a multitude of other conditions in
progressively younger patients. This chapter gives a clinically
oriented view of the assessment and treatment of adult hip conditions.
Emphasis has been placed not only on the practicalities of a particular
surgical procedure but its indications. It commences with a review of
relevant anatomy and embryology.
greatly in the proper placement of skin incisions during hip surgery.
Identifiable landmarks include the anterosuperior spine, crest of
ilium, greater trochanter, and shaft of femur. The tip of the greater
trochanter is a reliable marker to the center of the femoral head and
acetabulum (Figure 17-1). Soft tissue
identifiable beneath the skin includes the tensor fascia lata muscle,
which originates from the external lip of the iliac crest below the
anterior iliac spine and inserts into the iliotibial tract, and the
gluteus maximus, which originates from the posterior half of the ilium
behind the posterior gluteal line and inserts three-quarters of its
fibers into the iliotibial tract and one-quarter into the posterior
shaft of the femur below the greater trochanter. These two muscles are
often termed the deltoid of the hip joint, and most surgical approaches
to the hip joint split them in some way.
 |
|
FIGURE 17-1.
Surface anatomy of the hip and thigh as patient lies on operating table. Important landmarks include anteroposterior spine, crest of ilium, greater trochanter, and iliotibial track. There is a prominent tensor fascia lata, and a depression between gluteus medius and the tensor fascia lata muscle. These surface landmarks assist in proper placement of skin incisions at the time of surgery. (Eftekhar NS. Total Hip Arthroplasty, vol 1. St. Louis: Mosby-Year Book, 1993) |
different planes from the side of the pelvis, with medius lying
superficial to minimus. What is not well illustrated in anatomy
drawings is that their tendons converge into a single layer that is
inserted into the greater trochanter. The original Hardinge approach
for total hip arthroplasty split these two muscles in one layer, but
separate identification of each and suturing of the split in gluteus
minimus
as
a separate repair will reduce potential dead space, which may decrease
the subsequent risk of postoperative dislocation. Care should be taken
during the lateral approach to protect the superior gluteal nerve that
lies between gluteus medius and minimus. Dissection should not be taken
beyond 5 cm from the tip of the greater trochanter to avoid damage to
this structure. A small bursa often lies under the most anterior fibers
of gluteus medius tendon. This bursa can often be confused with hip
capsule during the direct lateral surgical approach. During this
exposure, a reliable indicator of the outer surface of hip capsule is
the insertion of the anterior fibers of vastus intermedius. The
undersurface of the gluteus minimus may be adherent to the hip capsule
in osteoarthritis and must be dissected free from it prior to a
capsulectomy being performed.
underlying disease or previous surgery, and care should be taken to
avoid its fracture. The femoral neck may not be easily identified from
the femoral head if there is extensive osteophyte formation. During
THA, orientation of the femoral neck osteotomy in the correct
anteversion should always be assessed after hip dislocation by
reference to the shaft of the femur with the knee flexed at 90°,
because there is a wide variation in anteversion of the femoral neck.
If the femoral neck osteotomy is made with reference to femoral neck
anteversion, an inaccurate cut may result, and subsequent
malpositioning of the prosthesis may occur.
form a spherical receptacle for the femoral head. The ischium, which
makes up the posterior column, is the most substantial wall. The ilium
contributes to the superior wall or dome, and a thin contribution from
the pubis completes the anterior column. Medially, the floor of the
acetabulum receives contributions from all three bones. Screws placed
through cementless acetabular cups to aid fixation should be placed
between the 10 and 2 o’clock positions when the acetabulum is viewed
from the side, to avoid damage to the internal iliac vessels
anteriorly, and the sciatic nerve posteriorly. Revision surgery of the
acetabulum often involves bony defects in one or more of the columns,
putting neurovascular structures even more at risk.
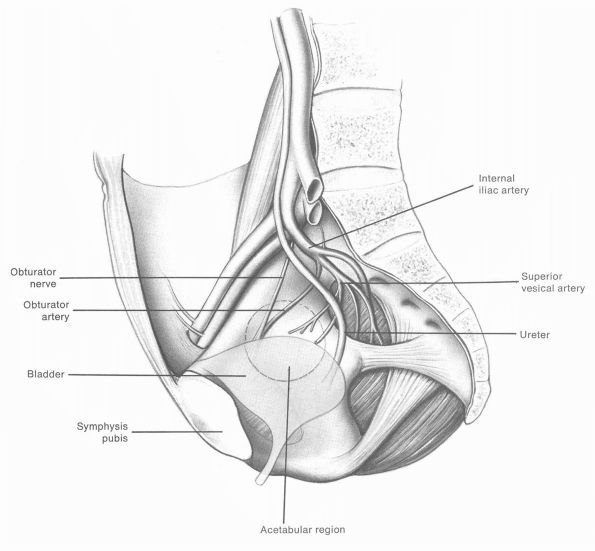
pseudomembrane, which overlies the iliacus muscle. Directly medial to
this lies the obturator nerve and artery, the ureter, and the bladder.
Great care should be taken when preparing the acetabulum to avoid
damage to any of these structures, which may have catastrophic
consequences (Figure 17-2).
(embryonic connective tissue), which undergoes chondrification to form
hyaline cartilage models of the bones. The limb buds appear toward the
end of the fourth week as slight elevations of the venterolateral body
wall. The apical ectodermal ridge, a thickening of ectoderm at the
distal end of the limb bud, exerts an inductive influence on the
mesenchyme in the limb buds that promotes growth and development of the
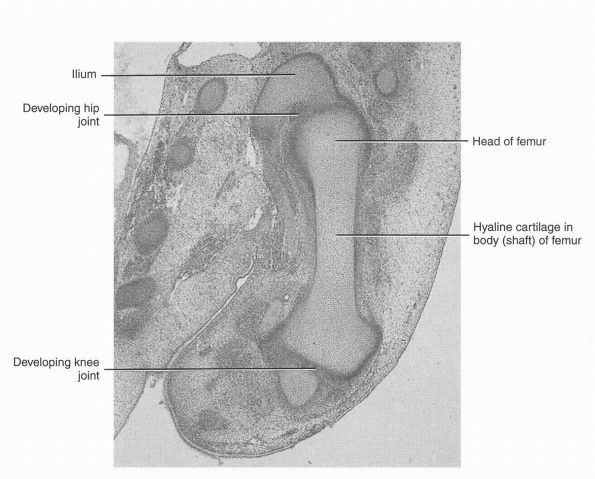
limbs. Nerves grow into the limb buds during the fifth week (Figure 17-3). The upper and lower limbs rotate in opposite directions and to different degrees.
period of limb development, formation of synovial mesenchyme occurs as
the synovium differentiates and is responsible for the development of
the synovial lining, the joint capsule, and the intracapsular ligaments
of the future hip joint. Cavitation begins in the central part of these
areas, with small multiple spaces that eventually coalesce to form the
joint cavity. At about the same time, a synovial membrane develops that
undergoes vascular invasion with accompanying macrophages and other
cell types. This sequence of differentiation suggests that development
of congenital hip dislocation must occur after the hip joint has formed.
ischium, and pubis as they join at the triradiate cartilage. The
proximal femur consists of the femoral head and neck and the greater
and lesser trochanters. Secondary ossification centers form in the
femoral head and greater trochanter. The triradiate cartilage fuses in
boys on average at 15 years of age and in girls on average at 13 years
of age. The femoral head growth plate fuses on average at 17 years of
age in boys and at 14 years of age in girls. The greater trochanteric
physis fuses on average at 16 years of age in boys and at 14 years of
age in girls.
 |
|
FIGURE 17-2.
Site of origin of gluteal muscles on the ilium is indicated. Relationship of the sciatic nerve to the acetabulum, acetabular branch of the obturator artery, femoral artery and vein, femoral nerve, and iliopsoas in relation to the acetabulum should be noted. The acetabular floor is marked by a horseshoe articulating zone and acetabular fossa. The ligamentum teres and its synovial folds are supplied by the acetabular branch of the obturator artery. (Eftekhar NS. Total Hip Arthroplasty, vol 1. St. Louis: Mosby-Year Book, 1993) |
condition being treated, the surgeon’s experience, and underlying
pathology. Surgeons should have a sound working knowledge of the
appropriate anatomy and be aware of the pitfalls of each approach when
applied to specific patients. There are four commonly used approaches
to the hip: the anterior or Smith-Peterson; the anterolateral or
Watson-Jones; the direct lateral or Hardinge; and the posterior or
Southern approach. There are many descriptions of these approaches,
most with a variation to meet a certain need. The principles, however,
are the same.
cases of suspected septic arthritis. The patient is placed supine on
the operating table. The skin incision runs along the anterior aspect
of the iliac crest, and as the anterior-superior iliac spine is
reached,
extends distally for approximately 10 to 15 cm. The incision extends
through the skin and subcutaneous tissue to the tensor fascia lata
muscle. Care is taken to identify and protect the lateral cutaneous
nerve of the thigh as it penetrates the fascia just below and medial to
the anterior superior iliac spine. The medial edge of tensor fascia
lata is easily seen laterally and the fascia is incised just medial to
this edge. The interval between tensor fascia lata and sartorious is
developed directly down to the tendinous portion of the rectus femoris
muscle.
 |
|
FIGURE 17-3.
Longitudinal section of the lower limb of an embryo at about 48 days. Chondrofication has begun in the bone and occurs in a proximodistal sequence. (Moore KL, Persaud TV, Shiota K. Color Atlas of Clinical Embryology, 2nd Ed. Philadelphia:W. B. Saunders, 2000) |
elevated off the edge of the pelvis to allow visualization of the hip
joint capsule. The hip capsule can then be incised over the front of
the femoral neck. This approach allows drainage of the hip in cases of
suspected infection. If a wider exposure is required, then the tensor
fascia lata muscle, and a varying amount of gluteus medius muscle, can
be reflected off the outer aspect of the ilium, and the interval
between tensor fascia lata and sartorious developed further distally.
This distal extension often results in disruption of the lateral
cutaneous nerve of the thigh and extensive dissection proximally may be
followed by the development of heterotopic ossification in the gluteus
medius.
arthroplasty. The approach’s major disadvantage is the potential for
damage to the anterior edge of gluteus medius while exposing the
femoral shaft. It should be used with caution in obese patients, as
access to the femur is very restricted. Also, as the approach places
considerable torsional stress on the femur, it should be used with
caution in patients with osteoporosis or other diseases likely to
weaken the femur. This approach does have the advantage of minimal
muscle disruption if performed well and maximum stability of the joint
postoperatively.
table, with the buttock elevated on a bolster, or they may be placed in
the lateral position. The skin incision begins at the iliac crest 5 cm
posterior to the anterior superior iliac spine, runs obliquely down to
the tip of the greater trochanter, and extends posteriorly from the tip
for 3 cm. It then curves anteriorly and distally for a further 10 to 15
cm. The incision is deepened to the fascia lata, and the anterior edge
of gluteus medius is identified beneath the fascia. The fascia is
incised longitudinally just distal to the anterior edge of gluteus
medius, and over the greater trochanter follows the skin incision. This
incision allows the interval between tensor fascia lata muscle and
gluteus medius to be developed. Be aware that the terminal branch of
the superior gluteal nerve crosses this interval in its proximal third
and is susceptible to injury in this area. Clear the fibrofatty tissue
off the front of the hip joint capsule and identify the reflected head
of rectus femoris crossing the capsule proximally. Place a Holman
retractor around the medial side of the capsule, one around the lateral
side, and one under the reflected head of rectus femoris.
visible. The capsule can be opened and the medial and lateral Holman
retractors placed around the neck of the femur within the joint. The
femoral neck can be transected and the head removed. Access to the
acetabulum is excellent, with the femur retracted posteriorly by a
Holman retractor placed immediately behind the acetabulum. Access to
the femoral canal is obtained by adduction and external rotation of the
leg, and a Holman retractor placed deep to the tip of the greater
trochanter. If the lower end of gluteus medius is attached more
distally on the anterior aspect of the greater trochanter, it may need
to be detached to improve access to the proximal femur.
the acetabulum and proximal femur. It has the potential disadvantage
that it may weaken the abductors with a subsequent limp, and it may not
be extensile enough to allow surgeons complete visualization of the
anterior and posterior acetabular columns during revision hip surgery.
Its major advantage is that dislocation following total hip replacement
is uncommon.
in the lateral decubitus position. If the lateral decubitus position is
used, care should be taken that the pelvis is correctly aligned to
ensure that component positioning is not compromised. The skin incision
begins in the midlateral line approximately 6 cm proximal to the tip of
the greater trochanter and runs distally for 10 cm. The incision is
deepened to the fascia lata, which is incised along the line of the
skin incision. A large self-retaining retractor is placed under the
fascia lata anteriorly and posteriorly, exposing the gluteus medius
proximally and vastus lateralis distally.
landmark where it inserts into the most distal aspect of the greater
trochanter. The tip of the greater trochanter is palpated, and
beginning proximally the gluteus medius is split along the line of its
fibers for approximately 3 to 4 cm, toward the anterior superior iliac
spine. This incision is then curved distally over the anterior third of
the greater trochanter, and beyond the trochanter into the vastus
lateralis muscle (Figure 17-4). Avoid splitting
the gluteus medius more than 5 cm proximal to the tip of the greater
trochanter to avoid damage to the superior gluteal nerve. A small
amount of sharp dissection (cutting diathermy) proximal to the greater
trochanter allows the gluteus muscle to be split along the line of its
fibers. Blunt dissection can be used to sweep the fat deep to gluteus
medius superiorly, thus protecting the superior gluteal neurovascular
bundle. The exposed gluteus minimus muscle can then be split along the
same line, giving access to hip capsule. Dissection superiorly up this
line allows placement of a Holman retractor deep to the reflected head
of rectus femoris, thus exposing the capsule up to the anterior
acetabular edge. Hip capsule is most easily identified at the inferior
extent of the greater trochanter, where sharp dissection combined with
external rotation of the leg will eventually expose muscle fibers that
are the capsular insertion of vastus intermedius. The bursa under
gluteus medius is often mistaken for hip capsule and is a much less
reliable indicator.
the acetabular margin, proximally under the free edge of the previously
split gluteus minimus tendon (which is often adherent to, and requires
dissection off, the superior hip capsule), and distally under the
vastus intermedius muscle (often a vascular area), allows good
visualization of the femoral neck and head. The remainder of the
exposure is similar to the anterolateral approach. The lateral approach
may be
combined
with trochanteric osteotomy, which allows wide exposure of the
acetabulum and improves access to the proximal femur. Many techniques
of osteotomy have been described, and when using this technique
surgeons should be aware that nonunion of the greater trochanter can be
a particular problem in total hip replacement.
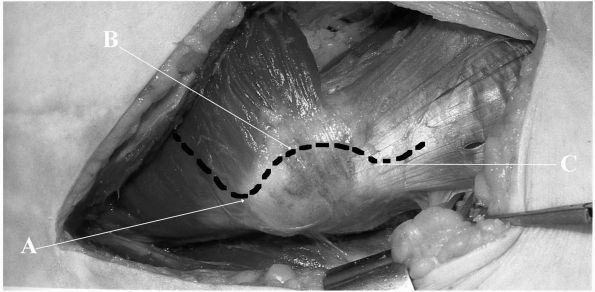 |
|
FIGURE 17-4.
Modification of the Hardinge direct lateral approach to the right hip. The fascia lata has been split along the line of the skin incision, allowing good exposure of the lateral deeper structures. (A) Tip of greater trochanter forms the initial landmark. (B) Incision is curved over anterior third of greater trochanter approximately 1 mm posterior to musculotendinous junction of gluteus minimus muscle. (C) Incision is extended for 2 to 4 cm into vastus lateralis muscle. Note the well-defined anterior edge of gluteus medius muscle inserting into the most distal extent of the greater trochanter. |
proximal femur and acetabulum, is extensile for revision surgery and
involves minimal muscle disruption. However, when used for
hemiarthroplasty or total hip replacement, dislocation is more common.
Careful attention to closure of the deep layers of the incision is
essential to minimize this risk.
with the pelvis appropriately positioned for correct orientation of the
components. The incision begins 3 cm distal to the tip of the greater
trochanter and is centered over it. The incision passes posteriorly and
proximally at 45° to the long axis of the femur for 8 to 10 cm. The
incision is deepened to the fascia lata, and beginning distally the
fascia is incised over the trochanter and then proximally over gluteus
maximus along the line of the skin incision. The gluteus maximus muscle
is split along the line of its fibers. A large self-retaining retractor
retracts the gluteus maximus. The leg should then be internally rotated
20° so as to better expose the fatty layer over the external rotators.
This fat is swept posteriorly off the short rotators by blunt
dissection, allowing visualization of the muscles and the sciatic
nerve. The tendon of piriformis is identified and an incision is made
along the proximal edge of piriformis distally, to the posterior margin
of the greater trochanter. It then turns distally along the posterior
margin of the greater trochanter as far as the proximal margin of
quadratus femoris. This incision extends through the capsule and thus
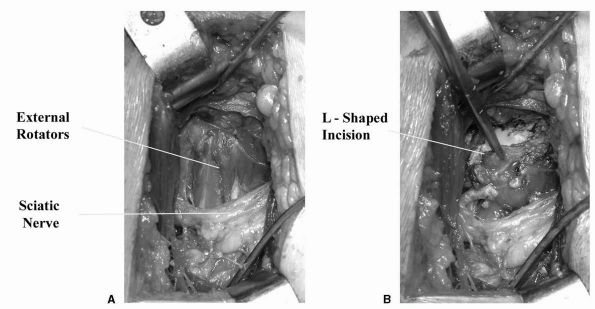
forms an L-shaped flap that is retracted to protect the sciatic nerve (Figure 17-5).
The hip can then be dislocated. Proximal and distal extension of the
exposure is possible to improve access to the acetabulum and femur as
necessary. The relatively high rate of postoperative dislocation (when
compared to other recognized surgical approaches for THA) has been
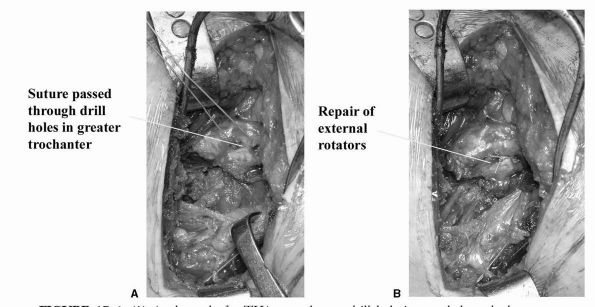
dramatically improved by repair of the external rotators (Figure 17-6).
affecting other parts of the body, and doctors taking a history and
examining patients whose main
complaint
is “hip” pain must not forget to examine other regions to exclude
pathology in those areas. Disorders of the spine and abdomen often
present with pain in the hip or proximal thigh and a thorough
assessment of these areas is essential if diagnostic errors are to be
avoided.
 |
|
FIGURE 17-5. (A)
Posterior approach to the hip joint for THA. Gluteus maximus has been split along the line of its fibers, exposing piriformis and the external rotators. (B) An L-shaped incision in the external rotators allows good exposure of the hip joint while the soft tissue flap is reflected posteriorly to protect the sciatic nerve. |
abnormalities such as a short leg, Trendelenburg, or antalgic should be
easily identified. More complex patterns such as those seen in patients
with ankylosed hips or hips that have had multiple surgical procedures
can be difficult to analyze, but each abnormality identified should be
documented. An assessment of leg length is performed by assessing the
level of the iliac crests with the patient standing evenly on both feet
with the knees straight. During
this
maneuver, a Trendelenburg sign is sought. The range of spinal movement
should be recorded, with particular attention to lateral flexion, as
this can be adversely influenced by long-standing hip disease or leg
length inequality. Patients with long-standing hip disease may also
have a marked increase in lumbar lordosis.
 |
|
FIGURE 17-6. (A) At the end of a THA procedure, a drill hole is passed through the greater trochanter. (B)
Strong suture material (5 Ticron) is used to repair the defect in the external rotator (which in the past has led to posterior dislocation). |
length should be assessed. Particular care is necessary in patients who
have had surgery that might influence pelvic geometry, and in these
cases leg length assessment using computerized tomography scanning may
be appropriate. If the patient has long-standing hip disease such as
dysplasia or frank dislocation, there may be secondary changes in the
lumbar spine. If this is suspected leg lengths should be equalized
using blocks and then the range of lumbar spine movement, particularly
lateral flexion, noted. The hip and pelvis are checked for surgical
scars and if any are present these should be noted as they may
influence future surgery and also may be an indicator of underlying
muscle damage that will influence the outcome of further surgery.
Muscle wasting around the hip is assessed. This is often seen in the
quadriceps and less commonly the buttock. Palpation of the hip area for
local tenderness can be helpful in identifying disorders such as
gluteus medius tendinosis, but as the hip itself is a deep joint,
palpation is of limited value.
carried out passively rather than actively. The presence of a fixed
flexion deformity is tested with the Thomas test. The maximum flexion,
abduction, and adduction should be measured taking care to immobilize
the pelvis to prevent pelvic motion giving a false sense of motion at
the hip. Rotation of the hip should be assessed with the patient prone
and supine. Assessment with the patient prone allows more accurate
measurement of rotation in the position of function. In young patients
an impingement test, done by flexing the hip to 90° and then adducting
and internally rotating the leg, may illicit pain if there is labral
pathology.
is performed to exclude disorders such as meralgia paresthetica that
may masquerade as hip disease. The remaining joints of the affected
extremity should be assessed, checking for any fixed or paralytic
deformity that may influence the outcome of any proposed hip surgery.
Where appropriate, the contralateral leg and the upper extremities
should be assessed. This is particularly important in patients with
multijoint disease such as rheumatoid arthritis. The vascular supply of
the affected leg should also be assessed. Functional assessment of the
hip joint and the patient as a whole may be appropriate in patients who
have had multiple surgical procedures or who have polyarthritis.
stiffness. However, the pattern of symptoms varies with the pathology
and the pathology varies with age. Thus, an elderly patient most often
presents with primary or secondary osteoarthritis whereas a young
patient may have acetabular dysplasia or labral pathology. Thus, it is
logical to divide patients presenting with hip symptoms into three age
bands although, of course, there may be considerable overlap.
synovitis, and loose bodies may present in the young adult. Dysplastic
hips are associated with intermittent postexercise pain, which becomes
more frequent with time. The pain is usually felt in the groin or
proximal thigh, but as with all patients with hip pain it may be felt
in the knee and occasionally even further down the leg. Stiffness is
rarely an early complaint, but as the condition progresses, reduction
in abduction and rotation is common.
causes pain and this may be positional, such that there is pain
associated with extreme flexion and adduction but not with other
positions. The patient may have a feeling of a “catch” in the hip with
certain movements. There is often a history of minor trauma preceding
the onset of pain. Loose bodies cause pain and occasional “catching” in
the hip, although frank locking of the hip is unusual.
into early osteoarthritis. Pain, which may have been a relatively mild
problem in early adulthood, is now a much greater problem. Stiffness
and the development of a leg length discrepancy are not uncommon,
particularly if the acetabular dysplasia is
severe.
Other causes of secondary osteoarthritis, such as gout and pseudogout
may also present at this age. Ankylosing spondylitis and avascular
necrosis of the femoral head commonly present with hip pain in middle
age. In the case of the former there may be a history of back stiffness
but this is not always the case. Patients with avascular necrosis may
have some identifiable risk factors such as alcohol abuse or
corticosteroid ingestion but in many cases no risk factors are evident.
Transient osteoporosis of the femoral head is a recently recognized
condition whose onset is sudden, with rapidly developing pain and
apparently normal radiographs. The diagnosis can only be confirmed on
MRI.
aging population. Hip, thigh, and knee pain are common and stiffness
influences simple activities such as cutting toenails and putting on
underwear. The history and physical findings are usually diagnostic,
but other diseases such as spinal stenosis and metastatic neoplasm
should always be considered.
development of the acetabulum, proximal femur, or both. Symptoms of
dysplasia vary in both site and severity, but commonly appear in
patients of 20 to 30 years.
prior treatment of the hip, especially surgery, for this may alter the
already abnormal anatomy. Special aspects of examination include the
Trendelenburg sign, the presence or absence of a fixed flexion
deformity, and the range of internal and external rotation measured
with the patient prone. Signs of labral pathology should be sought with
the impingement test.
(AP) pelvic radiograph, a lateral of the hip, a false profile view to
assess anterior acetabular coverage, and AP views with the hip in
abduction and adduction, and internal and external rotation. From the
radiographs, a number of measurements to determine the type and
severity of the dysplasia can be made. The center-edge angle, the
acetabular index, the teardrop to head distance, and the anterior head
coverage as determined from the false profile view allow accurate
assessment of the pelvic contribution to hip dysplasia. The
abduction-adduction and internal-external rotation views, together with
measurement of the neck shaft angle allow the surgeon to assess the
contribution by the femur to hip dysplasia. The position of the greater
trochanter relative to the center of the femoral head should be
assessed. Occasionally CT scanning of the hip is advisable if there has
been prior surgery as the anatomy of the acetabulum, in particular, may
be hard to define with standard radiographs.
to 6-month program of nonoperative treatment including analgesia,
anti-inflammatory medication, and physiotherapy, then surgery should be
considered. Surgery can take several forms from acetabular redirection
to acetabular supplementation and femoral osteotomy.
surgery for hip dysplasia. The shape of the femoral head and acetabulum
need to be considered. If the femoral head is nonspherical, a
repositioning osteotomy of the femur or pelvis is generally
contraindicated. If the patient has a fixed deformity of the hip,
however, a repositioning osteotomy of femur may improve hip function
and reduce pain. If the hip joint is congruous, then the site of the
“maximum” dysplasia needs to be identified. In many cases dysplasia is
most marked on one side of the hip and correction of that abnormality
is sufficient to alleviate symptoms.
view of reduced anterior femoral head coverage, the acetabular index is
at least 15°, and there are no or minimal degenerative changes, then an
acetabular redirecting osteotomy is indicated. Many redirection
osteotomies have been described but ones in contemporary use include
the Ganz, Tonnis, and Ninomiya. The type chosen depends on the
experience and philosophy of the surgeon. The Ganz osteotomy is
performed through a single Smith-Peterson type incision and is a
periacetabular
osteotomy,
which preserves the posterior column of the pelvis allows correction of
deficient anterior coverage, and may allow alteration in the position
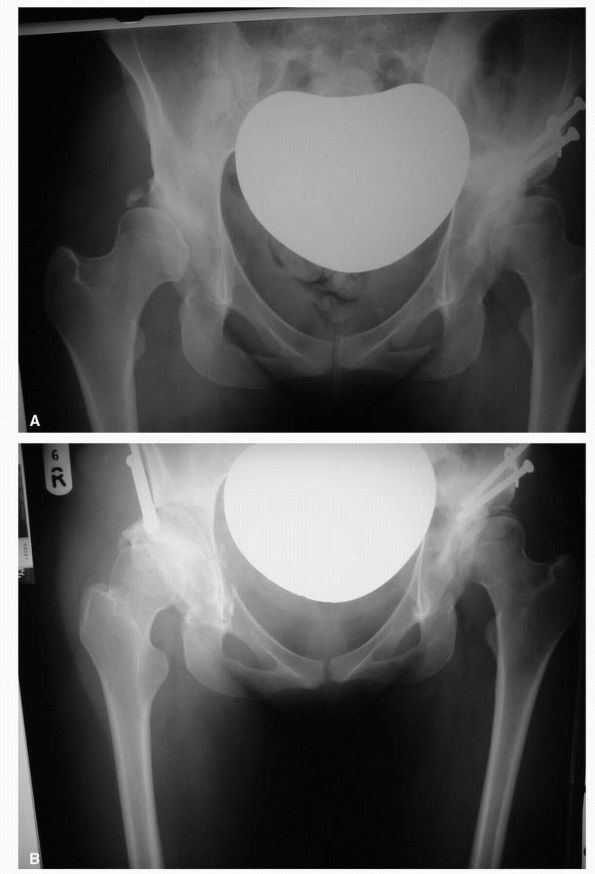
of the center of rotation of the hip (Figure 17-7).
The Tonnis osteotomy uses two incisions and divides the pelvis into
two, achieving a similar degree of correction to that of the Ganz. Both
osteotomies have a number of potentially serious complications,
including nerve palsies, nonunion, heterotopic ossification, and
fractures extending into the joint. If these are avoided, however, the
short- to medium-term results are excellent. If there are advanced
degenerative changes then the results tend to be less satisfactory. The
long-term results of periacetabular osteotomies of the Ganz and Tonnis
types have yet to be determined. The long-term (10-23 years) results of
a different type of periacetabular osteotomy, described by Ninomiya,
have been reported as very good, providing that there was little
evidence of osteoarthritis at the time of surgery. If the radiographs
show evidence of advanced degenerative change, then a Chiari
supra-acetabular osteotomy may be considered as a pain relieving
procedure.
 |
|
FIGURE 17-7. (A) Radiograph of a 27-year-old female with severe acetabular dysplasia of the right hip. (B) Postoperative radiograph after undergoing a right periacetabular osteotomy of the Ganz type.
|
a more straightforward procedure, which is indicated if the radiographs
show that proximal femoral dysplasia is the predominant dysplasia.
Another requirement for proximal femoral osteotomy to be considered is
whether the femoral head can be better covered, if the head can be
better centered in the acetabulum, and if the hip is either abducted,
or rotated or both. Careful preoperative planning is necessary to
provide the optimal femoral head coverage. The degree of varus
angulation seldom exceeds 15°. If greater than 15° is planned, then
advancement of the greater trochanter is necessary to avoid a
Trendelenburg gait. Varus femoral osteotomy has the advantage that the
recovery is reasonably short and the risk of serious complications is
low. All varus femoral osteotomies, however, have the potential to
shorten the leg, and this should be explained to the patient
preoperatively. The results in appropriately selected patients are
excellent, although pain over the internal fixation device often
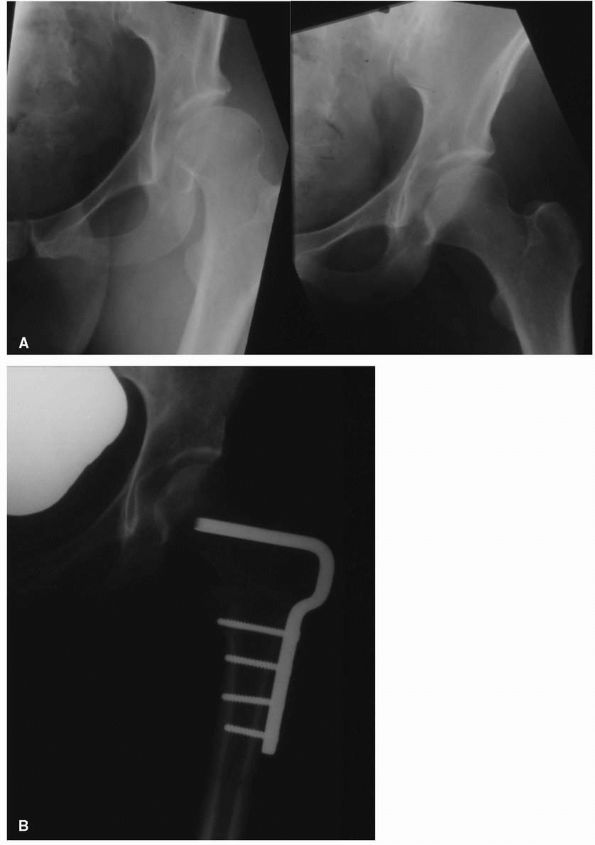
necessitates its removal (Figure 17-8).
 |
|
FIGURE 17-8. (A)
Adduction and abduction radiographs of a 29-year-old female with moderate left hip dysplasia. Her pain was severe enough that surgery was considered necessary. The adduction view shows that there is good coverage of the femoral head when the femur is placed in that position. (B) A varus intertrochanteric osteotomy gave her good pain relief without subjecting her to the risks of THA at a young age. |
osteoarthritis in other joints strongly suggests that even in ideal
circumstances, osteotomies for the treatment of hip dysplasia can only
be expected to give satisfactory results for 10 to 12 years before pain
becomes a significant problem again.
dysplastic hip, the two components of the hip, the acetabulum and the
femur, should be considered individually and then together. Dealing
first with the acetabulum, it may be present in its normal position, in
a more proximal position, or absent (with or without a false
acetabulum). When it is present in its normal position, it may be
relatively small—in which case the surgeon requires small diameter
acetabular components. Depending on the degree of femoral dysplasia,
the anterior, or less commonly posterior, wall of the acetabulum may be
dysplastic or absent, compromising acetabular fixation. If the surgeon
has any doubts about the geometry of the acetabulum, then preoperative
Judet views or a CT scan should be obtained. If the acetabulum is
present but has migrated proximally, the shape of the ilium causes it
to become progressively more shallow. Also, as previously indicated,
the anterior or posterior walls may be deficient, and the roof may
slope. In these circumstances, a CT scan of the pelvis with appropriate
reconstructions allows the surgeon to plan the surgery.
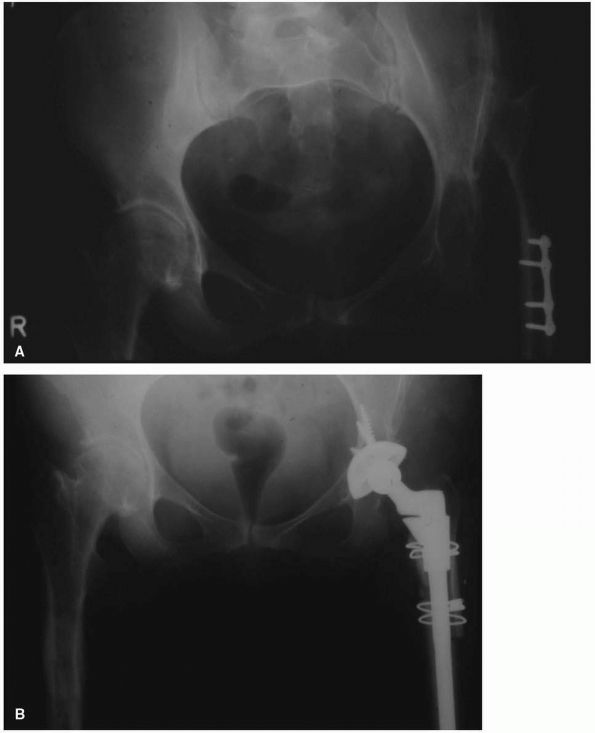 |
|
FIGURE 17-9. (A)
Radiograph of a 57-year-old woman with high-riding congenital dislocation of the left hip. Her major symptom was back pain and fatigue with exercise, not hip pain. (B) Careful preoperative planning was required to restore her center of rotation to the anatomic position. Use of a small diameter (46 mm) acetabular shell prevented a structural graft being required. A subtrochanteric femoral shortening was required to prevent lengthening the leg more than 5 cm. |
is little or no anterior or posterior columns to aid with cup fixation.
Restoration of the center of the rotation to the anatomical position in
this situation not only improves the biomechanics of the THA but also
improves the amount of host bone available into which an acetabular cup
can be placed (Figure 17-9). Location of the
true acetabular position may be difficult in a high-riding DDH, but can
be achieved in one of three ways: (1) an intact ligamentum teres that
runs from the femoral head down into the
acetabular
fossa; (2) identification of the greater sciatic notch posteriorly and
the anterior column, which runs down and converges at the teardrop; (3)
if neither of these anatomic landmarks is easily identified, an
intraoperative AP radiograph may be performed. Most surgeons prefer
cementless fixation of the acetabular implant with supplemental screw
fixation.
surgery, and the femoral anatomy need to be taken into consideration.
When the roof is sloping, the femoral head may be used as a graft to
support the acetabular component (Figure 17-10).
When there is a high dislocation, there is at best a false acetabulum,
which generally plays no part in the reconstruction. Many of these hips
do not cause pain until later life, and THR is not indicated. If
surgery is considered desirable, then a CT scan of the true acetabulum
usually reveals a small acetabulum with relatively osteoporotic bone.
This situation requires small diameter acetabular components, and
because of the osteoporosis, great care should be taken to prevent
reaming through the floor.
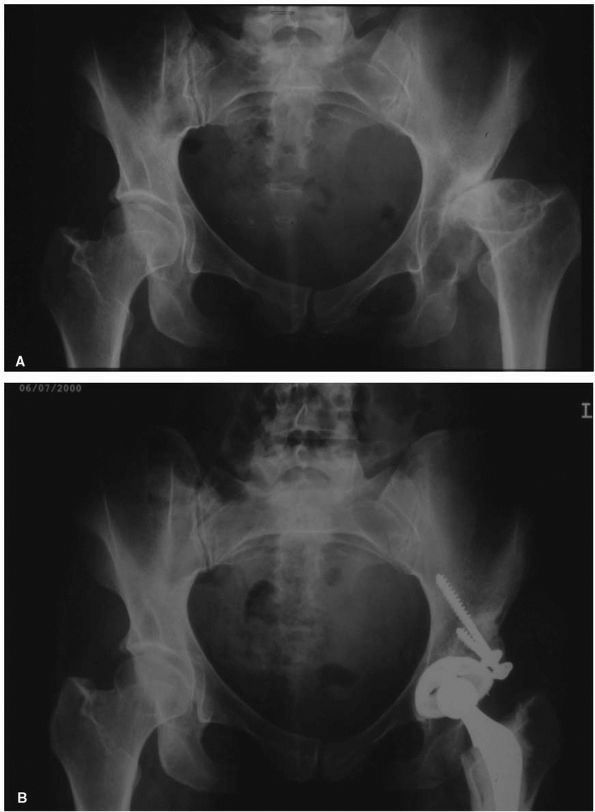 |
|
FIGURE 17-10. (A)
This 38-year-old woman with severe acetabular dysplasia of the left hip, developed severe pain from secondary osteoarthritis of the femoral head articulating against a false acetabulum. (B) The patient’s own femoral head was required as a structural bone graft to support the acetabular cup. Note that screws used to hold the graft should be directed superiorly into the pelvis to prevent collapse of the graft before bone ingrowth of the acetabular cup has occurred. |
femoral canal should be considered, as it is often small or distorted.
It will be necessary to have available a number of small implants,
including micro implants for some patients. Choice of femoral fixation
is still controversial, but surgeons should be aware that cemented
femoral components in the small femora may give an inadequate cement
mantle. In cases of femoral dysplasia there is frequently a marked
increase in the femoral anteversion. This possibility should be
assessed preoperatively with CT scanning. If the anteversion is greater
than 15°, then there are several different strategies available. A
small femoral component may be used in association with a low neck cut
to correct the anteversion. If using uncemented components of a tapered
design, then cutting out a piece of the back of the femoral neck allows
the component to be inserted in the correct degree of anteversion. The
anterior neck may have to be trimmed to prevent impingement. If the
anteversion is severe, then a modular prosthesis that allows the
femoral component to be rotated within a proximal metaphyseal sleeve
may be used. If a femoral shortening needs to be performed, then the
proximal fragment can be rotated back to its correct position.
If the leg is short, do not consider lengthening of over 5 cm. However,
to work within this limit and to achieve the appropriate acetabular and
femoral component positioning, it may be necessary to shorten the femur
through a subtrochanteric osteotomy. This requires special prostheses,
and should not be undertaken without appropriate training. If there is
bilateral high riding DDH, but only one side is symptomatic and
requires THA, consideration has to be given to resulting leg length
inequality and the functional disturbance to gait this might cause. In
this situation, the contralateral hip occasionally requires THA to
maintain function (Figure 17-11).
always makes hip replacement more difficult. The old incisions may make
access difficult. There may be extensive scarring on the lateral side
of the pelvis and femur, making dissection, access, and restoring leg
length more difficult. The bony anatomy may be quite abnormal, and
preoperative CT scanning is very useful to delineate the features of
the pelvis and proximal femur. The surgical approach adopted for THR in
dysplasia depends on the experience of the surgeon, but as a general
rule the posterior approach is more extensile and allows better
visualization of the acetabulum than the direct lateral. Also dealing
with severe femoral abnormalities may be facilitated through the
posterior approach.
disorder whose incidence shows great international variation, and thus
its origin varies from region to region. Generally trauma is the major
cause of AVN. Avascular necrosis has also been associated with steroid
use, alcohol abuse, Gaucher’s disease, hemoglobinopathies, and in
patients subject to severe changes in barometric pressure, such as deep
sea divers. The common pathway in the latter conditions is likely to be
alteration in the fat content or composition of the bone marrow, with a
consequent increase in the intraosseous pressure and a reduction in
blood flow to bone trabeculae. The exception to this pathway is in
trauma, where the blood supply to the femoral head is disrupted when
the retinacular vessels crossing the surface of the femoral neck are
torn as a result of displacement of the femoral head following a
fracture, or stretched when the femoral head dislocates from the
acetabulum. Thus, AVN is common following femoral neck fractures in the
elderly, and its incidence is related to the degree of head
displacement.
the patient. In the elderly who have sustained a subcapital fracture,
AVN is often heralded by increasing pain and a radiograph that shows
fixation failure, collapse of the femoral head, or both. In younger
patients with idiopathic AVN, pain is the most common presenting
feature. An associated feature is synovitis of the hip, which manifests
clinically with a decrease in the range of movement, and the hip is
irritable (there is pain throughout the range of motion). If the
patient presents late in the evolution of the disease, then the
symptoms and signs will be indistinguishable from advanced
osteoarthritis.
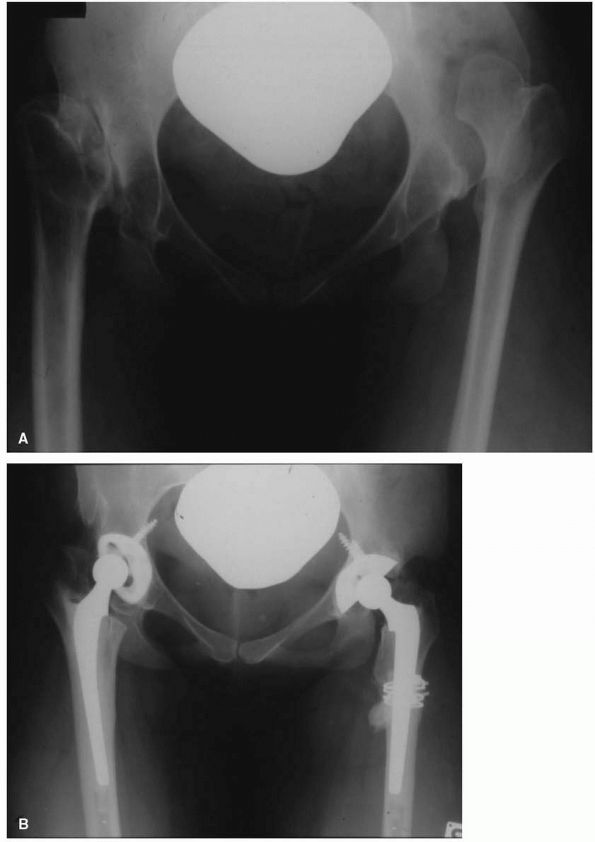 |
|
FIGURE 17-11. (A)
Twenty-nine-year-old female who used a motor scooter outside the house for mobility, and was unable to get up or down stairs. She developed pain in her right hip that threatened her independence. (B) A right THA was performed. Despite no complications and good relief of her pain, her functional status and mobility were not improved. Seven months later a left THA with femoral shortening was performed. She now lives independently, does not require walking aids, and can manage three flights of stairs. Her leg lengths are equal. |
anteroposterior and lateral radiographs of the hip. Early in the
disease, radiographs may be normal, but as the disease progresses it
goes through several stages. A number of authors have classified the
stages, the most commonly used classification being that described by
Ficat. Ficat’s classes divide the disease into four stages. Stage 1
refers to a hip that on plain radiographs appears normal. The diagnosis
is made by measuring a raised intraosseous pressure, histologic
examination of biopsy specimens, or an abnormal magnetic resonance
imaging (MRI) examination. Stage 2 avascular necrosis may show patchy
osteoporosis but an otherwise normal radiographic examination.
Clinically, however, the hip is painful, and there may be a reduced
range of motion. Stage 3 shows some change in the contour of the
femoral head, and there may be a subchondral fracture. Stage 4 shows a
loss of joint space and collapse of the femoral head. Radiographs may
be indistinguishable from advanced osteoarthritis of the hip joint.
early in the evolution of the disease. Care must be taken, however, not
to misdiagnose transient osteoporosis of the hip as AVN. The former
condition involves the whole of the femoral head and lacks the focal
changes seen in AVN. Transient osteoporosis is a self-limiting
condition with symptoms and MRI findings clearing in 6 to 8 months.
size of the lesion, and stage of the disease. In the elderly who
develop AVN following a hip fracture, total hip arthroplasty is the
treatment of choice. In younger patients with idiopathic AVN,
determining the size of the lesion is important as this has a direct
influence on the prognosis. The size is best determined by MRI. Small
lesions (less than 15% of the femoral head) may resolve without
treatment. Large lesions (greater than 50% of the femoral head) tend to
progress to collapse of the head and secondary osteoarthritis, despite
treatment. For intermediate-sized lesions, a number of treatment
options have been proposed. A classification based on the location of
the lesion also has prognostic significance. If the lesion is medial in
the head, then progression is rare; if it is lateral in the head the
prognosis is worst.
conclusively that any one treatment is superior. Treatments range from
simple decompression of the femoral head to lower the intraosseous
pressure, to bone grafting of the involved area either directly after
dislocating the hip and elevating the involved segment, or by using a
vascularized fibular graft placed up the center of the femoral neck.
There have been a number of osteotomies described whose purpose is to
remove the affected area of the femoral head from the weight-bearing
axis of the hip. These include varus and valgus osteotomies and more
complex rotational osteotomies. The choice of osteotomy depends on the
location of the lesion and the ability of the proposed procedure to
remove the affected segment from the weight-bearing axis of the hip.
Osteotomies are generally contraindicated for those patients who remain
on corticosteroids or have untreated metabolic bone disease. They are
generally only effective when a small area of the femoral head is
involved.
head and prevent progression of the disease to degenerative arthritis,
surgeons should be aware that, ultimately, conversion to THA is common
following these procedures and the risks and potential benefits of such
surgery should be very carefully weighed in each case. Hemiarthroplasty
or bipolar prostheses should be avoided in AVN of the femoral head, as
histologic assessment of the acetabular cartilage shows abnormalities
in the majority of cases, which may adversely influence the outcome of
such surgery. Idiopathic AVN is frequently bilateral with a reported
incidence of 50 to 80%, and thus MRI should be performed in all cases.
inflammatory disorder with a predilection for articular cartilage. The
hip joint is less frequently afflicted in the adult form of rheumatoid
arthritis than other joints; however, its involvement may result in
disabling symptoms and significant diminishment of function. Likewise,
juvenile rheumatoid arthritis also more frequently involves larger,
more rapidly growing joints, including the knees, wrists, elbows, and
ankles and less commonly affects the hip joints. It has been estimated
that about 1% of the U.S. adult population has rheumatoid arthritis.
Therefore, it is reasonable to estimate that between 4 and 6 million
cases of rheumatoid arthritis are found in the United States.
female-male ratio of between 2:1 and 4:1. The disease affects all ages
but generally increases in incidence with advancing age. In women, the
peak incidence is between the fourth and sixth decades.
cause of rheumatoid arthritis remains obscure. Regardless of its cause,
it may be described as an inflammatory process that somehow is
triggered and centers in the joints with articular cartilage. The
inflammation manifests as a stimulus for synovium to hypertrophy and
becomes increasingly hyperplastic
and
hypervascular with increasing cellularity. This hypertrophic synovial
tissue invades and degrades articular cartilage. The actual destruction
of articular cartilage is done in large part by rheumatoid pannus, a
fibrovascular granulation tissue that protrudes from the inflamed
synovium into articular cartilage. It contains fibroblasts, small
vessels, and multiple inflammatory cells that are responsible for the
destruction of articular cartilage and its underlying bone.
varying degrees of articular cartilage loss secondary to the
inflammatory process. This loss usually involves the entire femoral
head, resulting in concentric loss of cartilage and subsequent
concentric joint space narrowing. There may also be varying amounts of
bone loss and even femoral head collapse. Varying degrees of cyst
formation occur, and in about 5% of patients, significant protrusio
acetabuli develops.
performed in someone presenting for consideration of surgical treatment
of a rheumatoid hip. Special attention should be taken of what
medications the patient is taking. Prior to any joint replacement, a
review with a rheumatologist to minimize or stop steroids or
antimetabolic drugs should occur, because these medications compromise
fixation of the implant and predispose the patient to an increased risk
of infection. Careful attention to the state of the cervical spine and
jaw for ease of anesthesia, and the state of the upper limbs and
contralateral leg for ease of postoperative mobilization, should be
made.
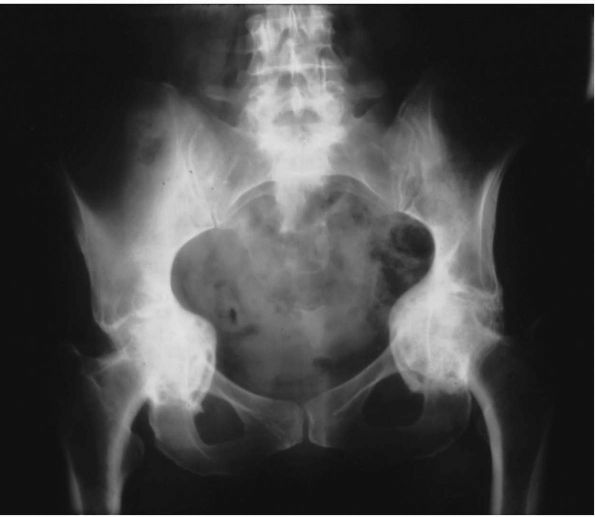 |
|
FIGURE 17-12.
Severe protrusio of both hips in a 35-year-old female with juvenile rheumatoid arthritis. At the time of THA, an in situ femoral neck osteotomy prior to dislocation of the femoral head. |
osteopenia and loss of joint space. The loss of joint space, in
contrast to that seen in osteoarthritis, is generally concentric, and
varying degrees of protrusio acetabuli may be present (Figure 17-12).
Osteophyte formation is rare, and subchondral sclerosis is not a
prominent feature. Cysts may develop within both the femoral head and
the acetabulum. These changes may culminate in varying degrees of
femoral head collapse, and in severely advanced cases, spontaneous bony
fusion may occur with no range of motion.
rheumatologist and include analgesics, nonsteroidal
anti-inflammatories, steroids, and antimetabolite drugs such as
methotrexate.
hip. In mature adults with rheumatoid arthritis, care should be taken
during dislocation to prevent fracture in osteoporotic bone, and
reaming of soft acetabular bone. Large cysts should be bone grafted
with reamings from the femoral head or acetabulum.
array of technical complexity because of the small size of the patient
and deficiencies in the femoral and acetabular bone stock. These
problems may require customized or miniature components. Additionally,
although the early result of cemented total hip arthroplasty may be
good, these patients are young, and the total hip replacement must
withstand many years of function.
commonly than that of the knee in patients with severe hemophilia A
(less than 1% of factor VIII levels). Management of the hemophilic
patient uses a dedicated multidisciplinary clinic, involving
hematologists, physiotherapists, occupational therapists, and
orthopaedic surgeons.
hemophiliac arthropathy of the hip should be severe, disabling pain
with activity and at rest that is unresponsive to nonoperative
treatment. Careful preoperative planning to overcome deformities of the
proximal femur, resulting from growth arrests from multiple childhood
bleeds, is often needed. Osteoporosis is common and care should be
taken to avoid fracture of the femur. Full factor VIII replacement is
required not only during the surgery but until the stitches have been
removed. A serious problem affecting the complication rate is a high
prevalence of seropositivity for HIV and the eventual development of
AIDS.
patients, at a mean follow-up of 8 years, 3 patients (11%) had
developed a deep infection necessitating prosthesis removal, 7 hips
(21%) had required revision for aseptic loosening, and 9 patients (33%)
had died. Almost all the deaths were related to AIDS.
problems surgeons face when performing THA in young immunocompromised
patients.
complaints in young patients presenting with hip problems, but other
clinical complaints can include locking, crepitus, loss of muscle
strength, instability, and feeling of a mass lesion. Recent
advancements in both hip arthroscopy and MRI have elucidated several
sources of intra-articular abnormalities that result in chronic and
disabling hip symptoms. Many of these conditions were previously
unrecognized and, thus, left untreated.
significantly in the past few years. Referred pain and common hip
pathologies, such as stress fractures of the femoral neck of pelvis
avascular necrosis and early osteoarthritis, can usually be diagnosed
by a combination of careful history and physical examination, followed
up by AP and lateral radiographs and possibly a Technetium-99 isotope
bone scan. If a diagnosis has still not been obtained, an MRI can be
requested.
labral tears, hip capsule laxity and instability, chondral lesions,
osteochondritis dissecans, ligamentum teres injuries, snapping hip
syndrome, iliopsoas bursitis, loose bodies (for example, synovial
chondromatosis), bony impingement, synovial abnormalities, crystalline
hip arthropathy (gout and pseudogout), infection, and posttraumatic
intra-articular debris. Occasionally, MRI arthrography can be a
particularly useful technique for dedicated assessment of hip joint
internal derangements.
presence of symptomatic acetabular labral tears, hip capsule laxity and
instability, chondral lesions, osteochondritis dissecans, ligamentum
teres injuries, snapping hip syndrome, iliopsoas bursitis, and loose
bodies (for example, synovial chondromatosis). Less common indications
include management of osteonecrosis of the femoral head, bony
impingement, synovial abnormalities, crystalline hip arthropathy
(gout
and pseudogout), infection, and posttraumatic intra-articular debris.
In rare cases, hip arthroscopy can be used to temporize the symptoms of
mild-to-moderate hip osteoarthritis with associated mechanical symptoms.
distraction of the joint, usually with a dedicated fracture table, and
image intensification to ensure the portal placement is accurate. The
patient may be positioned supine or in the lateral decubitus position.
Anterior and peritrochanteric portals are most commonly used, giving
good access and working space, to the lateral and superior part of the
hip joint (Figure 17-13).
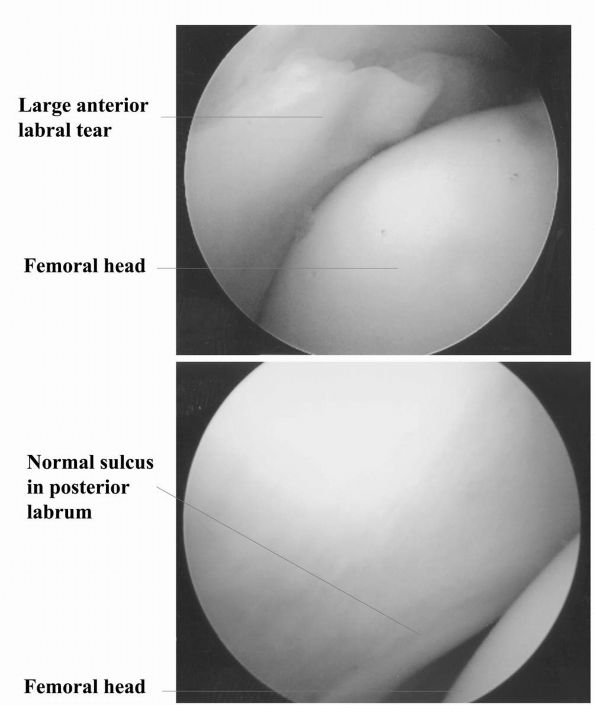 |
|
FIGURE 17-13.
Intraoperative photographs made during arthroscopy of the hip. Distraction of the femoral head out of the acetabulum is important to prevent inadvertent damage to the articular cartilage by intra-articular surgical instruments. |
new technique for surgical dislocation of the hip, based on detailed
anatomical studies of the blood supply. It combines aspects of
approaches that have been reported previously and consists of an
anterior dislocation through a posterior approach with a “trochanteric
flip” osteotomy. The external rotator muscles are not divided and the
medial femoral circumflex artery is protected by the intact obturator
externus. He reported his experience using this approach in 213 hips
over a period of 7 years. Perfusion of the femoral head was verified
intraoperatively and none subsequently developed avascular necrosis. It
allows the treatment of a variety of conditions, which may not respond
well to other methods, including arthroscopy. The most common
conditions treated with this technique include impingement of the
femoral neck on the bony acetabulum, and debridement of osteophytes and
osteochondral lesions in patients with early osteoarthritis of the hip
who are not suitable candidates for THA.
surrounding total hip arthroplasty have evolved to their current form,
it is important to review the history of hip arthroplasty. This history
can be broken down into three distinct eras based around the
innovations of the man considered to be the father of total hip
arthroplasty, Sir John Charnley.
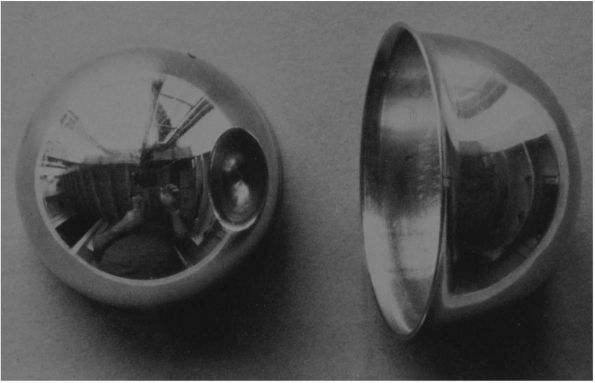 |
|
FIGURE 17-14. Smith-Peterson Vitallium mold arthroplasty.
|
represent this century’s most significant developments in orthopaedic
surgery.
hip joint generated many innovative solutions to restore movement in
the 1700 and 1800s. The first osteotomy of the femur below a stiff hip
was credited to John Rhea Barton in 1826, who then manipulated the
osteotomy site for 20 days following surgery to maintain motion. The
patient was said to have enjoyed a pain-free functional “joint,” until
his death 10 years later of pulmonary tuberculosis.
material between the two bone ends forming a joint, so-called
interposition arthroplasty. As well as tissue from the patient, such as
the tensor fascia lata muscle and skin, a variety of foreign material
was used, including gold foil, pigs’ bladder, silver plates, wooden
blocks, and rubber sheets. In 1923, Smith-Peterson placed a glass mould
in a patient’s hip. It turned out to be too fragile, but in 1938, at
the suggestion of his dentist, Smith-Peterson used Vitallium, a
cobalt-chrome alloy, as an interpositioning material (Figure 17-14). This method was probably the first clinically successful precursor to the modern THA and proved that the acetabulum
could tolerate a foreign body performing a weight-bearing function.
with the first publication of resection of the femoral head for
ankylosis. This procedure was made popular in 1928 by an Oxford
surgeon, Girdlestone, and the procedure still bears his name today. In
1940, Bohlman and Moore removed a tumor from the upper end of a femur
and inserted the first metallic prosthesis. They performed this case in
South Carolina through a posterior surgical approach, and because they
came from the southern United States, their approach became known as
the Southern approach, a name it still bears today. In 1948, the Judet
brothers in France replaced the femoral head with a plastic (methyl
methacrylate) prosthesis, but breakage and loosening caused early
failure and the procedure fell into disrepute (Figure 17-15).
were introduced. The short-stem type was replaced by the intramedullary
long-stem type, which gave more stability, and the nonmetallic type was
replaced by the metallic type, which provided greater durability. Most
shared similar design features to those prostheses developed by F. R.
Thompson in 1950 and Moore in 1952. These procedures never became
popular for osteoarthritis because of ongoing pain from movement of the
prosthesis within the femur, as well as ongoing disease in the
acetabulum.
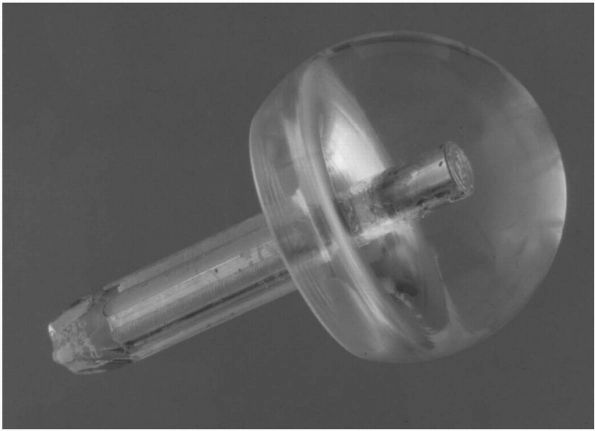 |
|
FIGURE 17-15.
Judet glass mold of a patient’s hip. This was secured to the patient by a pin inserted down the stump of the femoral neck. It never gained widespread acceptance due to premature failure of the base material. |
phenomenon of lubrication that produces low friction in normal joints.
He had previously observed that squeaking, which occurs in artificial
joints, does not occur in normal joints. A previous investigator had
suggested this was due to hydrodynamic lubrication (fluid enters the
zone of contact and lubricates it) by synovial fluid. Charnley
speculated this could not occur because of the unique situation in the
hip joint of weight bearing, which would prevent synovial fluid from
performing this function.
smooth, even after having been being wiped clean (boundary
lubrication), he concluded that boundary lubrication was responsible
for the low frictional resistance of the hip joint. He then assumed
that a material such as polytetrafluoroethylene (Teflon), which was
self-lubricating, would be an appropriate substitute for the damaged
cartilage, and pursued its use with spectacular (early) results.
which replaced the damaged articular cartilage. After 12 months,
however, there was evidence of mechanical loosening and failure when
the stump of the femoral neck lost its blood supply and became
necrotic, and the two Teflon surfaces lost their frictional properties
and became bound together, thereby causing movement and wear between
the plastic socket and the native bone of the acetabulum.
within an artificial joint, torque transmitted from a metal femoral
head to a plastic socket can be minimized by reduction of femoral head
size. Likewise, for a plastic socket to be stable within an acetabulum,
torque should be maximized, achievable by maximizing the outer diameter
of the acetabular cup. These principles led Charnley to begin work with
a smaller diameter metal femoral head and method of bonding implants to
bone. He began by rejecting the premise of using small amounts of
acrylic cement as an adjuvant to a tight mechanical fit, instead using
cement as a grout (rather than an adhesive) with the components
achieving a loose mechanical fit. Results with Teflon had been
disappointing, and an alternative was required. Although initially
rejected in favor of Teflon’s self-lubricating properties, high-density
polyethylene had been tested in Charnley’s laboratory, and proved to be
remarkably wear resistant. The first high-density acetabular prosthesis
was inserted into a human hip joint in November 1962.
to continue the procedure, but he continued to search for the cause of
the 10% failure. Chemical rejection of the cement was initially
suspected, but this was reduced to 5% by introduction of the clean air
enclosure, suggesting infection was the major culprit (Figure 17-16).
 |
|
FIGURE 17-16.
Radiograph of a left Charnley THA. This patient had his original operation in 1978. The hip was still functioning well 22 years postoperatively. Many of the original surgical principles, as promoted by Charnley, are evident: all-polyethylene acetabular cup with wire marker (showing evidence of loosening and wear), well-fixed monoblock femoral stem with 22.225 mm diameter femoral head, and absence of distal plug. |
principles, was an astonishing success for sufferers of hip arthritis,
its use was limited to surgeons who had been trained directly by
Charnley himself. Other surgeons throughout the world also developed
total hip arthroplasty prostheses. Maurice Muller from Switzerland
developed a plastic acetabular cup with a 32 mm diameter
chromium-cobaltmolybdenum femoral head, which he used extensively
between 1966 and the early 1980s (Figure 17-17).
Peter Ring began using metal-to-metal components without cement in
1964, but its use never became popular because poor tolerances between
the components led to binding of the articular surfaces and eventual
failure.
the early 1970s, but with rigorous follow-up of patients, a problem
with radiological loosening of the component at the bone cement
interface was identified. Continuing follow-up showed this was a
progressive problem, with massive resorption of bone around the
prosthesis, limiting options for treatment. This loss of bone was
initially attributed to the acrylic cement and became known as cement
disease.
researchers to the concept of cementless fixation of the prosthesis to
bone. Prostheses were designed allowing solid initial fixation to bone
during the
operation.
A porous-coated surface finish of the prosthesis then allowed bone to
grow onto the prosthesis, and in some cases, into it. Initially, two
types of porous coating became popular, a titanium fiber-metal
composite wire mesh developed by Harris, Galante, and coworkers, and
cobalt-chrome beads developed by Engh, Bobyn, Hungerford, and coworkers
(Figure 17-18). Later, plasma-sprayed titanium was used.
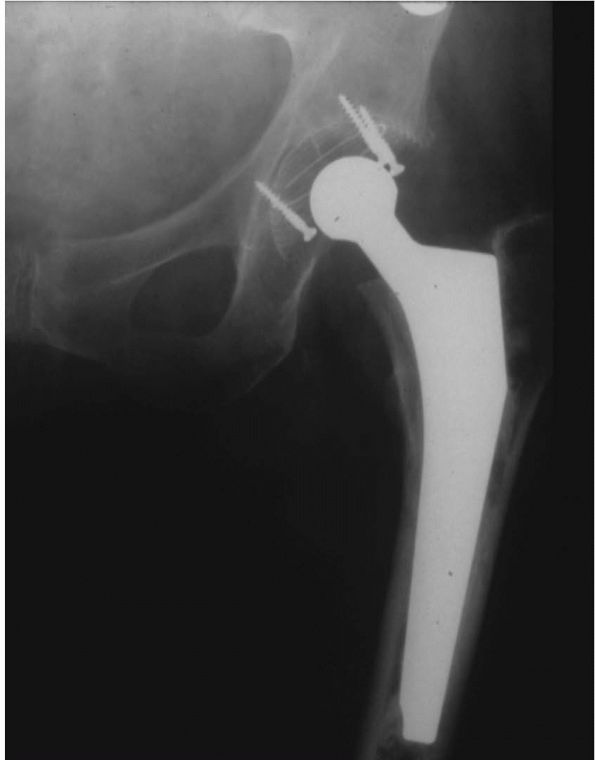 |
|
FIGURE 17-17. The SLS femoral stem and the Morscher cup. This combination of design features is very commonly used in Europe.
|
 |
|
FIGURE 17-18.
Porous-coated anatomic (PCA) THA. The first major cementless femoral and acetabular components. Many are still functioning well today, nearly 20 years after their introduction. They did, however, introduce new problems of thigh pain and osteolysis not commonly seen with cemented THA. |
developed. The femoral component now came as two distinct parts,
assembled by the surgeon during the operation. A femoral stem could be
individually sized to a patients femoral canal, to which was attached a
separate femoral head using a morse taper. This gave the surgeon
flexibility to alter femoral head diameter to match the chosen
acetabular cup, and femoral neck length to restore correct offset and
length of the patient’s leg. The acetabular component also came as two
parts—a metal shell, which was fixed to the patient’s acetabular bone
(often supplemented with spikes, lugs, or screws), and a high-density
polyethylene liner, which was fixed to the shell using some form of
locking mechanism. Even the original Charnley prosthesis adopted this
trend with the development of the Charnley Elite prosthesis, which had
a modular femoral head.
size of the femoral head from the 22.225 mm of Charnley’s original
work, to 32 mm, in order to increase the range of hip movement and
decrease the rate of dislocation. This increase in head size coupled
with modularity of the acetabular cup was to have a dramatic effect
that was not foreseen.
in the United States, were the porous-coated anatomic prosthesis from
Howmedica (associated with Hungerford and Headley), the AML prosthesis
from DePuy (associated with Engh), and the Harris-Galante prosthesis
from Zimmer. These were the so-called first generation cementless THA.
By the mid 1980s, it became apparent using these components to
eliminate cement did not eliminate cement disease. Indeed, the massive
resorption of bone around these components continued to occur at an
even more alarming incidence and volume than had occurred with the
original Charnley components.
acetabular cups during that period led to the concept of the so-called
hybrid hip, which incorporated cementless acetabular fixation and a
cemented femoral stem. Some cementless femoral stems had introduced a
new problem, thigh pain, which did not seem to occur with cemented
prostheses. This pain was attributed to the tip of a stiff femoral stem
rubbing on the femoral cortex and generated some dissatisfaction among
surgeons with cementless femoral stems.
bone loss from both physiologic and histologic perspectives. It rapidly
became apparent that this so called cement disease was actually
particle disease. Any foreign material, if broken into small enough
particles, when introduced into the prosthesis bone interface, caused
resorption of bone by the body, leading to the radiological findings of
bone loss and clinical signs of pain caused by loosening. This
particulate material could be generated from cement or prosthesis
substrate, but the most common source was high-density polyethylene.
surface was identified as the major cause of loosening and late failure
of total hip replacement. Decrease of polyethylene thickness below a
critical level was found to increase polyethylene wear debris, and many
first generation prostheses had inadvertently contributed to this by
increasing femoral head size, and decreasing liner thickness to
accommodate for the metal shell and locking mechanism.
major contributor to prosthetic loosening and clinical failure,
researchers in the 1990s turned to identification of alternate bearing
surfaces to reduce or eliminate generation of particulate debris.
Improved machining-processes and prosthesis-manufacturing techniques
have seen a reemergence of metal-on-metal articulations. Although there
have been concerns raised about metal ion levels in blood and tissues,
to date there has been no recorded incidence of problems in patients
from these metals.
property of self-polishing, which diminishes surface disparities caused
by third body wear (damage to the bearing surface caused by a material
getting “caught” between the two articulations). Ceramics can
articulate with either themselves or with high-density polyethylene.
The major concern with ceramic use is the risk of fracture. This was
illustrated in a 2001 worldwide recall of all zirconia ceramic femoral
heads, after a defect was found in the manufacturing process.
with laboratory testing to be promising, clinical results were
disappointing. Poly-Two was an attempt to strengthen high-density
polyethylene with carbon fiber but was a spectacular clinical failure.
Hylamer was an attempt to stiffen polyethylene, but clinical results
suggest a 15% increase in wear, not a 15% decrease as first believed.
Methods of sterilization and the shelf life after manufacture are also
known to have an effect on wear, but to date have not been used to
successfully improve wear.
polyethylene to improve its wear is cross-linking the polyethylene by
subjecting it to ionizing radiation during the manufacturing process.
This process produces cross-linked polyethylene, which is currently in
extensive use worldwide since its approval in 2000. Laboratory
simulator testing shows a 90 to 99% reduction in wear, but clinical
results in patients are not yet available.
osteoarthritis, combined with a decreasing patient acceptance of
disability, is leading to increasing strain on a the health care
budget, accounting for up to 1 to 2.5% of a county’s gross national
product. In the last 15 years, surgeons have been asked to evaluate the
success of a THA not only for prosthesis longevity, but also cost
effectiveness. Health economists can now create models for comparing
the cost effectiveness of a THA with coronary bypass and renal
transplantation. These models measure cost-benefit analysis using a
Health Related Quality of Life Index, and for patients in their fifth
and sixth decade of life, THA comes out significantly ahead of these
other more complex procedures. Governments use these economic
indicators and other outcome measures to allocate health budgets more
appropriately.
analysis (CBA) of THA is the age of the patient. Despite an increase of
cost over benefit with increasing age, THA has been shown to be
beneficial in the octogenarian population. The benefit of THA can now
be accurately measured, and none would argue that it is a huge success,
leading politicians and health providers to look more closely at cost
containment. With the advent of new design, materials and surface
finishes over the last 20 years, nowhere is this more applicable than
implant costs. The original Charnley cemented stem and cemented cup
costs approximately 30% of a cementless porous-coated femoral and
acetabular component. In young active total hip recipients, a new
prosthetic design, which offered a 90% improvement in survivorship over
15 years and a 15% reduction in the cost of revision surgery, could be
sold at a price of 2 to 2½ times that of conventional cemented
components such as the Charnley prosthesis and still remain cost
effective. Using more likely estimates of the improved performance of
new technology, however, the upper limit of cost effectiveness is an
increase of 1½ to 1. Only a very small increase in the cost of a
prosthesis could ever be justified for older patients of either sex.
concept of implant matching, where cementless porous-coated prostheses
are reserved for younger more active patients, and cemented THA
reserved for elderly more sedentary patients. Another area that is
being evaluated for cost savings is length of hospitalization. The time
a patient spends in hospital contributes the largest proportion to the
overall cost of THA. Day of surgery admission, and improved surgical
technique, rehabilitation, and home services all allow patient
discharge on day 3 or 4.
of stay is the newly emerging miniincision surgery (MIS) techniques.
Although promising from a surgical perspective, length of
hospitalization is probably more influenced by age, patient
co-morbidities, patient expectations, and home circumstances. The
potential benefits of MIS have yet to be proven and careful analysis of
the long-term results will be needed.
the clinical results following primary THA. Most studies attempt to
measure the outcomes of technique and procedure, and do not assess the
effect on general function and the satisfaction of the patient.
Measurement of outcome in THA is usually achieved using two different
systems, one that measures health-related quality of life (HRQOL), and
the second group that contain joint-specific tools. Only validated
outcome measures should be used for assessment.
Status Survey is widely used for measuring the HRQOL. The patient
responds to 36 questions regarding their physical and social
functioning and mental health, with no physician input to bias the
results. It has the sensitivity to document improvement in HRQOL
following surgery and to reveal differences in THA.
Osteoarthritis Index is a tested questionnaire to assess symptoms and
physical functional disability in patients with osteoarthritis of the
hip and knee. The three domains assessed include pain, stiffness, and
function.
able to distinguish between symptoms and functional impairment produced
by the index joint, as compared with other joints and conditions. The
Merle d’Aubigne and Harris hip scores are widely used to grade
improvement after THA.
and evaluate the relationships of expectations and outcome to patient
satisfaction. Patients’ different expectations can be grouped into five
categories reflecting improvement in pain, walking, psychological
state, essential activities, and nonessential activities. Approximately
90% of patients will be satisfied with the results of surgery. Lower
rates of satisfaction can be expected in patients who have a better
preoperative condition.
small percentage of acetabular components implanted in the United
States, but continue
to
be popular in many parts of Europe. Long-term data on cemented THA
continues to show that acetabular component loosening is generally more
of a problem than femoral stem loosening beyond 10 years. For a group
of patients whose prosthesis remains in place at 25 years
postoperatively, the prevalence of acetabular revision is approximately
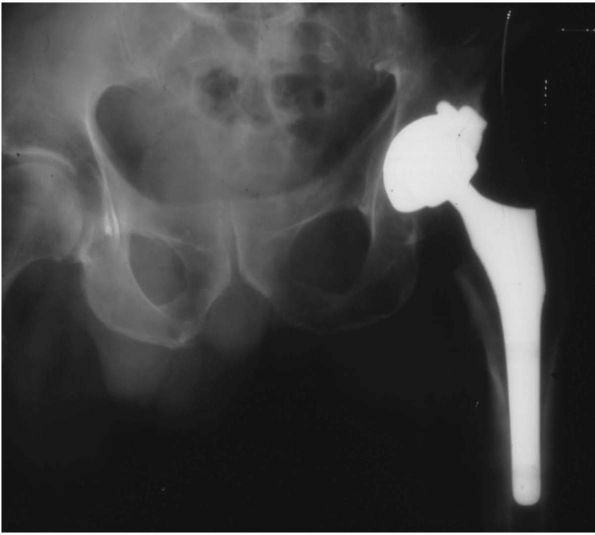
15%, compared with a prevalence of 7% for revision of the femoral stem.
These figures are magnified when patients receive a THA under the age
of 50 years. In a group of patients under the age of 50 with an average
18-year follow-up, 50% of the acetabular components were radiologically
or clinically loose, compared with only 8% of the femoral stems. Modern
consensus is that failure of a cemented femoral component is nontime
dependent, reaching a failure rate of approximately 8% by 15 years, and
maintaining this rate beyond that time. Conversely, rate of failure of
a cemented acetabular component is time dependent and may increase in a
nonlinear manner after 10 years.
practice to the use of porous-coated cementless acetabular components
during the early 1980s. The concept of the hybrid hip—a cemented
femoral stem articulating with a press-fit porous-coated modular
acetabular component—became increasingly popular during this period.
acetabular cups, metal-backing was introduced during the mid 1980s.
This backing was thought to distribute stress more evenly through the
cement mantle, leading to improved longevity. Follow-up of these
implants has revealed the converse situation, with a higher incidence
of radiological lucent lines at the cement bone interface, and a higher
incidence of clinical failure than was documented for all-polyethylene
acetabular cups. These poorer results were attributed to reduced
polyethylene thickness and the introduction of another interface for
ingress of wear debris.
cup implantation has occurred not with cup design but with cementing
technique. Since 1962, cement has been hand-mixed and then finger
packed into the femur. This is now termed first generation cement technique,
and has been superceded by mixing of cement in a gun that is used to
deliver cement, under pressure, into the femoral canal in a retrograde
fashion. A distal plug below the tip of the prosthesis prevents cement
from being pushed too far down the canal. These alterations in cement
delivery are now called second generation cement technique. Although developed mainly for the femoral component, similar principles are applied to cementing of the acetabular component.
implanted, surgical technique is very important in achieving a good
postoperative radiograph appearance. Preparation of the acetabular
bone, clearance of soft tissue from the acetabular margins, and careful
drying of the acetabular bone bed are all essential to eliminating the
appearance of lucent lines at the cement bone interface. When the early
postoperative radiograph shows radiolucency in the lateral margin of
acetabulum, the incidence of subsequent acetabular loosening increases
dramatically. In a recent long-term follow-up study, additional
drilling of peripheral holes around the acetabular margin for anchorage
of the cement was shown to increase longevity of the acetabular
component.
technique has been largely developed for the femoral component, making
its impact on the cemented all-polyethylene acetabular component
difficult to assess. Femoral components implanted with the use of
second-generation cementing techniques appear to have fared much better
than acetabular components inserted with second generation cement
techniques in most series. Recent matched-pair studies comparing
cemented acetabular components to cementless porous-coated components,
have shown a lower revision rate and lower incidence of radiographic
loosening for porous-coated components compared with cemented
components.
continues to be defined. They seem to function best over the long term
in older, less active patients. A recent study of 132 THA in 112
patients over 75 years of age showed that, at a mean 14.6 year
follow-up, no acetabular component in those patients still living had
required revision for aseptic loosening.
porous-coated cementless acetabular components with a minimum 10-year
follow-up have now been reported by a number of centers, independent of
their developers. From a clinical and radiologic perspective, reliable
fixation to the acetabular socket is reported in the range 95 to 99% of
cases. During the development of titanium fiber mesh, plasma spray, or
sintered cobalt-chrome beads as a surface finish
applied
to the outer diameter of metal shells, animal models suggested that
only 20 to 40% of the roughened surface achieved histologic bony
ingrowth (Figure 17-19).
Time has proven that this figure seems to give satisfactory stability
for prolonged loading, and the results do not appear to deteriorate
over time.
however, produced a new problem. Access of particulate debris,
especially polyethylene from the bearing surface to these noningrown
areas, gives rise to large areas of bone resorption that we now term osteolysis.
Although this phenomena of bone resorption was seen with cemented
acetabular cups and femoral stems, it was present at a much lower rate,
size of the lesions were much less, and perhaps most importantly, their
increase in size over time was much less than is seen with cementless
metal-backed acetabular components.
design alterations in the porous-coated acetabular components.
Increased thickness of the metal shell to increase its stiffness, and
increase in femoral head size to 32 mm to improve range of hip movement
and joint stability led to a large decrease in thickness in the
polyethylene insert. In many cases, thickness of the insert fell below
the critical level of 6 mm, which is now known to cause a dramatic
increase in polyethylene wear at the bearing surface. Two other factors
played a part in this early problem: introduction of a secondary
bearing surface between the polyethylene liner and the inner surface of
the metal shell, and poorly designed locking mechanisms.
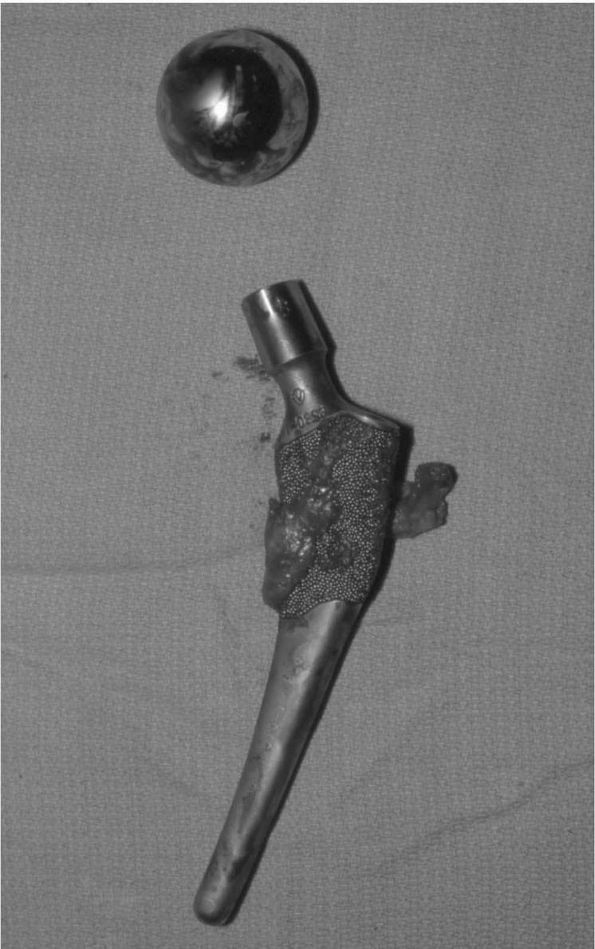 |
|
FIGURE 17-19.
This PCA femoral component was removed for intractable thigh pain in a 49-year-old male. It had been implanted 2½ years previously. Radiological appearances suggested no evidence of loosening, and at the time of revision it was found to be quite stable. Despite this, after explantation, only 25% of the proximally coated porous surface was found to be bony ingrown. |
congruity between the inner surface of the metal shell and the outer
surface of the polyethylene liner, and in some cases, there was up to 2
mm of gap between the two surfaces. Subsequent loading of the liner by
the femoral head transmitted the force through the polyethylene in a
nonuniform manner, leading to stress concentration, liner deformation,
and high wear not only at the primary bearing surface but also on the
outer surface of the liner, so-called back-side wear. An additional
problem with this lack of conformity also occurred. Any fluid in the
gap between the shell and liner is hydraulically forced out by the
bottoming out of the liner during weight bearing. This fluid, laden
with polyethylene particles, follows the path of least resistance
through screw holes in the shell, and so gains access to the
prosthesis-bone interface and leads to osteolysis (Figure 17-20).
Fluid can also be forced, under this hydraulic pressure, into other
areas of the prosthesis bone interface of both the femoral and
acetabular component where bone ingrowth has not occurred, again
causing osteolysis. This process has led to the concept of the
“effective joint space,” where surfaces of the prosthesis that are not
bony ingrown can potentially be bathed in joint fluid rich in debris
particles that cause osteolysis.
polyethylene liner involve the use of peripheral mechanisms around the
opening of the shell that capture the polyethylene liner. Incorporation
of this locking mechanism often involved additional sacrifice of
polyethylene thickness in an area that often was subjected to the high
stress and subsequent polyethylene wear. With time, these locking
mechanisms often lost their rigid grip on the liner, allowing movement
that further aggravated back-side polyethylene wear.
anatomic (PCA) THA (Howmedica, Rutherford, NJ) with a mean age of 58
years. At 15-year follow-up, 17% (17 hips) of the entire cohort and 23%
(15 hips) of the living cohort (64 hips in 55 patients) had undergone
revision due to loosening of the acetabular component or osteolysis.
These results compare favorably with a study of cemented acetabular
components with a 25-year follow-up reported by the same author.
Patients still alive 25 years after surgery had a mean age of 56 years
at the time of operation. These patients received a cemented Charnley
THA with first generation cementing techniques. There was a 15%
revision rate for acetabular loosening, and a further 36% that
demonstrated definite or probable radiographic loosening.
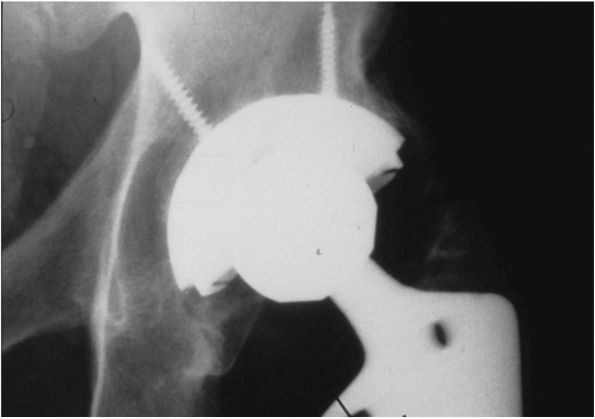 |
|
FIGURE 17-20.
The Harris-Galante I titanium fiber mesh acetabular component has proved to be the most durable first generation acetabular component. Thin polyethylene and a suboptimal locking mechanism, however, led to the concept of the “effective joint space.” Particle-laden joint fluid is forced through the screw holes into the areas of nonbone ingrowth, leading to screw osteolysis, as seen in this radiograph. The component has been implanted for 4½ years, but required revision because of this appearance. |
the Harris-Galante I (Zimmer, Inc, Warsaw, IN). This component
comprises a titanium fiber mesh coating that was usually implanted with
line-to-line reaming (last acetabular reamer used is exactly the same
diameter as prosthesis to be implanted) and screw fixation. A 9- to
14-year follow-up study of 71 hips in 56 patients less than 50 years
old at the time of surgery, revealed no revision or radiological
loosening of the components, but a 23% incidence of pelvic osteolysis.
the number of screw holes through the acetabular metal shell to prevent
fluid gaining access to the prosthesis bone interface. The central
hole, present to allow the surgeon to ensure the cup is fully seated in
the acetabular bed after cup insertion, can now be blocked with a plug.
The locking mechanism has been redesigned to ensure the polyethylene
shell is solidly fixed to the metal shell, congruity between the inner
surface of the metal shell and outer surface of the polyethylene liner
has improved, and that the inner surface of the shell is smooth to
reduce abrasive wear against the liner. The minimum thickness of the
polyethylene (even with incorporation of the locking mechanism) is 8
mm. This has necessitated a return to smaller femoral head size when
smaller cups are used. The use of 32 mm metal femoral heads
articulating against conventional polyethylene has disappeared.
fixation. The first involves implantation of the component using a
technique that ensures initial
stability
of the metal shell against the acetabular bone. Supplemental fixation,
such as screws through the cup, spikes, lugs, or fins in the periphery,
may be used to achieve stability. Another method is to under-ream the
acetabular bone bed by 1 to 2 mm, and then use the roughness of the
outer surface of the metal shell to achieve a “scratch fit.” Other
initial fixation modes include an expansion cup, where the cup diameter
is reduced with a special instrument, the cup is implanted, and then
allowed to return to its initial diameter; and screw in designs, where
the cup has a thread incorporated into its outer surface, and is
screwed into the acetabular bone.
to occur, that of bony ingrowth onto and into the metal shell.
Different surface finishes of the cup, including titanium fiber mesh,
cobalt-chromium beads, titanium plasma spray, tantalinium fiber metal,
and grit-blasting of the surface, are currently used by manufacturers
to obtain bony ingrowth. Hydroxyapatite may be used as supplemental
fixation and to improve initial stability, but unless used in
conjunction with a porous ingrowth surface, it does not appear to
maintain long-term fixation on its own.
shell and polyethylene liner assembled at the time of surgery) was
introduced to reduce surgical inventory, increase surgical options, and
allow future liner exchanges, a disadvantage is that it introduces a
further interface from which wear debris can be generated. This has
lead surgeons back toward using a single piece acetabular cup, where
the bony ingrowth surface and polyethylene come as one piece.
Disadvantages of this design, however, are that visualization of the
medial acetabular margin during implantation is not possible (ensuring
the cup is fully seated), and also that few options exist for
additional fixation with screws through the metal shell if initial
stability is not achieved with a scratch fit.
of cementless acetabular components is a major concern. Surgeon
selection of a porous ingrowth acetabular cup should balance wear
issues with concerns of initial stability (i.e., unusual acetabular
anatomy requiring additional fixation) and bony ingrowth (previously
damaged bone from irradiation or fracture seems to reduce the amount of
bony ingrowth) for each patient.
acetabular components over 10 years suggests that problems with
component fixation are rare. Periprosthetic pelvic and acetabular
osteolysis caused by polyethylene wear at a linear rate greater than
0.2 mm/year, has led to a search for alternative bearing surfaces to
articulate on both the femoral and acetabular side. A metal femoral
head articulating with conventional polyethylene has been intensively
studied and has by far the longest follow-up, but the accelerated wear
seen in a small number of patients with porous-coated cementless
acetabular components means that there will always be a limited
longevity of the prosthesis with this bearing surface.
activities, including sport and heavy manual labor after THA, means an
alternative must be found. Articulations can be broken into two broad
groups: conventional bearing surfaces, where the acetabular side of the
joint consists of conventional or cross-linked polyethylene; and
hard-on-hard bearing surfaces, where both sides of the joint are made
up of either alumina oxide ceramic or metal.
recently oxinium, have been used as femoral head bearing surfaces
against conventional polyethylene. Alumina oxide has also been used in
a ceramic-on-ceramic (hard-on-hard) bearing surface. Early hip
simulator studies have suggested a five- to tenfold reduction in
polyethylene wear rate compared to metal femoral heads, but more recent
simulator studies are variable with some showing a similar rate to
metal on polyethylene, depending on variables such as temperature of
the polyethylene, type of lubrication, and introduction of third body
debris. In clinical studies of polyethylene wear rates made from
clinical radiographs, there are conflicting results, with some showing
that ceramic femoral heads confer an advantage and an equal number
showing no difference.
over their metal counterparts is their self-polishing ability, making
them resistant to scratches. Generation of particulate debris is a
timedependent process, meaning that the advantage of
ceramics
only becomes evident after a given time period (claimed to be 5 to 7
years). This phenomena might explain the variability of the clinical
studies, where only those with a follow-up greater than 5 to 7 years
show a difference, but this has yet to be proven.
potential for fracture and the cost. With improved manufacturing
techniques, the risk of ceramic femoral head fracture was lowered to
approximately 1 in 50,000 implants. In 1999, a defect in the
manufacturing process of the supplier of 80% of the world’s zirconia
femoral heads led to a significant number of zirconia femoral head
fractures, which prompted a worldwide recall of all zirconia femoral
heads. Use of zirconia femoral heads, because of this defect and recall
has been limited.
were approximately three times as costly as metal femoral heads, and
although these costs have declined, the clinical studies to date make
it difficult for surgeons to justify their use on the basis of improved
prosthesis longevity.
years as a hard-on-hard bearing surface. Wear rates with this
articulation have been reported as 10 times less than the lowest
conventional polyethylene wear rates. An identified drawback of
ceramic-on-ceramic is the limited head and neck sizes available. There
is also concern about the state of the bearing surface and the rim of
the acetabular component if a dislocation occurs. The elimination of
polyethylene does not eliminate the problem of wear debris and
osteolysis, as at least one alumina-on-alumina hip of early design had
a high incidence of wear and osteolysis.
metal-on-polyethylene THA, but results from early designs were poor
because of poor manufacturing tolerances between the acetabular cup and
femoral head, leading to binding of the surface and eventual loosening
of the prosthesis. This result was compounded by the early prostheses
not being fixed to the bone, but even the introduction of acrylic
cement could not improve the results sufficiently to compete with the
results of metal-on-polyethylene THA. Improved manufacturing techniques
and research into lubrication methods at the interface have led to a
resurgence of interest in metal-on-metal THA. There is no doubt that
there is a dramatic reduction in wear rate to negligible levels, using
both radiographic wear measurement techniques and retrieval specimens,
but there remains major concern regarding the production of cobalt and
chromium metallic debris, and its elimination from the body.
metal-on-metal with metal-on-polyethylene had to be abandoned when very
high levels of erythrocyte and urine cobalt-chromium were detected.
Metallic wear particles have also been isolated in lymph nodes, livers
and spleens of patients with THA, raising concerns about the long-term
effects these materials might produce.
characteristics of polyethylene by altering its material properties.
Highly crystalline polyethylene (Hylamer) and Poly-Two (carbon
fiber-reinforced polyethylene) were developed to improve the wear
performance of conventional polyethylene, but clinical results have
been disappointing and their use has been discontinued. The most
promising current area for improving the wear performance of
polyethylene is by increasing the amount of cross-linking that occurs
between the individual polyethylene molecules.
long-term results of this cross-linked polyethylene using cemented
nonmetal backed all-polyethylene cups. A report on 62 ultra high
molecular components gamma irradiated in air with 100 megarads of
ionizing radiation and a control group of 10 nonirradiated polyethylene
cups implanted between 1971 and 1978. Twenty-eight hips (45%) were
available for follow-up at a mean 17.3 years after operation. At 2
years, the cross-linked group had a linear wear rate of 0.15 mm/year
and the control group a linear wear rate of 0.39 mm/year. This high
initial wear rate was attributed to a highly oxidized surface layer.
After 2 years, the rates decreased to a steady state of 0.06 mm/year
for the cross-liked group and 0.29 mm/year for the nonirradiated cups.
These figures suggest a 79%
reduction in linear wear when using cross-linked polyethylene.
all-polyethylene acetabular cups articulating with a 22 mm diameter
zirconia femoral head. There were four non-cross-linked
all-polyethylene cemented acetabular cups with a 22 mm diameter
zirconia femoral head used as a control group. Follow-up ranged between
10 and 11.3 years. Rate of linear wear in the cross-linked polyethylene
group was 0.04 mm/year, while rate of wear in the control group was
0.16 mm/year—a 75% reduction in wear rate using cross-linked
polyethylene. Historical studies of THJR polyethylene wear using the
same implants, similar follow-up, and same wear measurement technique
gave a wear rate of 0.07 mm/year, demonstrating a 45% reduction in wear
when cross-linked polyethylene is used.
interpreted with caution, however, because the method of cross-linking
was radically different from that currently used by manufacturers,
resulting in a material so brittle that a locking mechanism for use in
modular acetabular components could not be machined. Careful attention
to heating and cooling temperatures as well as cooling times by
manufacturers, has reduced this problem, and the material stiffness is
now much closer to traditional polyethylene.
of carbon atoms in adjacent molecular chains, achieved by replacing
carbon-hydrogen bonds with carbon-carbon bonds. An unwanted side effect
of this process is the generation of free hydrogen atoms, charged
particles known as free radicals. Free radicals are known to cause
surface oxidation, which in turn increases polyethylene wear, and so
their reduction is critical during the manufacturing process.
its own proprietary process for cross-linked polyethylene manufacture.
This process involves four steps, and each surgeon who uses a
particular brand of cross-linked polyethylene should be aware of the
steps used in its manufacture. There are choices available at each step
in the manufacture of cross-linked polyethylene.
-
Consolidation—either compression molding or ram extrusion
-
Cross-linking—either electron beam or gamma irradiation
-
Free radical reduction—either heat anneal or remelt in oven
-
Sterilization—either ethylene oxide or gas plasma or gamma in nitrogen
result in differences in the wear performance and material properties
of cross-linked polyethylene from the different manufacturers. To date,
all wear testing of cross-linked polyethylene has been performed using
multidirectional wear simulators. Crossing path wear tracts are used in
an attempt to reproduce in vivo THJR conditions. Depending on the
degree of cross-linking (determined mainly by the dosage of radiation
of strength of chemical cross-linking), a 70 to 90% reduction in
polyethylene wear using a hip simulator can be achieved.
approved a number of highly cross-linked polyethylene components for
clinical use. The 2-year results of many clinical studies comparing
conventional and highly cross-linked polyethylene are expected in the
near future, but it will be some years before the true benefits of
cross-linked polyethylene (if any!) are known.
extensively documented results in the literature, including several
published studies with 25-year follow-up. The femoral stem, as
developed by Charnley, was designed to overcome mechanical failures
resulting from the use of earlier prostheses. The original flat-back
design derived from the Austin Moore and Thompson prosthesis was
modified to a round-back design, and then further altered with an
additional flange design (Cobra) to enhance load transfer to the cement.
had adopted a straight tapered configuration for the stem. Larger
caliber prostheses were introduced to reduce the incidence of stem
fracture, but this problem was virtually eliminated by improved
metallurgical processes and a better choice of metals accompanied by
improvements in design. The various types of Charnley prosthesis have
been developed over a period of 30 years and have been based on
clinical experience demonstrating the need for modifications. Whether
design modifications will be an improvement over the original has not
been proven, because so many other variables such as improved surgical
technique and cement
technique
also play a role. All of the modifications have retained the original
monoblock Charnley principle of even stress distribution to the cement
mantle, but have included increases in prosthesis diameter and length,
modularity of the femoral head using a morse taper, and increases in
offset that may be selected by the surgeon. The current system is
called the Charnley Elite Plus.
several dedicated hip centers. The most widely quoted is that from the
Iowa group and were from Charnley THAs implanted during the early
1970s. Of 330 Charnley THAs performed between 1970 and 1972, 62 hips
(51 patients) were available for follow-up. Mean age at surgery of
these 51 patients was 56 years. Fourteen of these hips (23%) had been
revised at a minimum 25-year follow-up, and the prevalence of revision
due to aseptic loosening of the femoral component was only 7% (4 hips).
This demonstrates the durability of the Charnley femoral component when
implanted by a single dedicated surgeon.
were evaluated across an entire health region rather than from a single
dedicated total joint center. Results were dramatically different from
those of a dedicated hip center, with a known rate of aseptic loosening
of 2.3% at 5 years, infection rate of 1.4%, dislocation rate of 5%,
revision rate of 3.2%, and a radiographic impending failure rate of
5.2%. These results suggest an overall failure rate of 10% within 5
years and may be more representative of the true situation.
initiated in 1979 by Dr. Peter Herberts et al., has given invaluable
information on the comparison of different prostheses and surgical
techniques across an entire population. Although the Charnley cemented
stem is frequently referred to as the gold standard, the Swedish Hip
Register has reported higher stem survival rates with other cemented
stems. Across the entire country during a similar time period (which
assumes the use of similar cementing technique), the Charnley cemented
hip had a 92% 10-year survival rate compared to a Stanmore at 91.9%,
Exeter at 94.9%, and Spectron at 98.4%.
the original Charnley stem has come with the manufacturing of
prostheses with different surface finishes. These surface finishes are
described in terms of “roughness,” as measured by a profilometer. The
average roughness (Ra) is the average distance between peaks
and valleys across the surface of the prosthesis, and can vary by a
factor of 40 from the polished Exeter femoral stem (Ra < 2 µ inch) to the Precoat femoral stem (Ra = 80 µ inch). The smoother implants used initially, including the Charnley stem (Ra
= 2 µ inch), had lower cement-metal interface fixation strength,
whereas rougher surfaces have greater fixation strength. This greater
fixation strength has a lower probability of interface motion, but at
the same time, due to its greater abrasiveness, a higher debris
generation consequence if motion does occur.
Robin Ling, actively discourages fixation between the metal surface and
the cement. The consequence of this is distal migration under loading
conditions, but the double wedge taper design of the prosthesis
transfers only compression stress to the cement mantle when this
occurs. Cement is strongest under compression and the Exeter femoral
stem ues this principle to obtain outstanding long-term results.
stems due to high rates of wear and osteolysis from the debris products
generated by abrasive wear of some femoral stem designs employing a
rough surface finish. Rough surfaces, in themselves, are not
necessarily associated with a high incidence of osteolysis or
loosening. The Spectron (Smith & Nephew, Memphis, TN) femoral stem
has a grit-blast surface in its proximal third, yet enjoys the highest
10-year survivorship of any prosthesis (98.4%) reported by the Swedish
National Arthroplasty Register. Excellent results with the Spectron are
attributed mainly to stem geometry being a rectangular cross-section to
enhance rotational stability and smoothing of the rectangular edges to
decrease stress concentration within the cement mantle.
such as occurred with the Exeter, Iowa and T-28 stems, has a higher
incidence of loosening and osteolysis. Other considerations such as
length, substrate material, cross-sectional shape, and incorporation of
a collar, all contribute to the successful long-term results of a
cemented femoral stem.
results of cemented femoral stems has occurred through improvement in
surgical technique, specifically at the time of cement and prosthesis
insertion
into the femur. Cementing technique has been broken into three distinct
generations by many authors, but the evolution of cementing technique
essentially represents a continuum over time, with various components
of each generation being introduced at different times.
1962, involved limited sizes of femoral stem, finger packing of the
cement in an antegrade fashion, no femoral canal preparation, no
intramedullary cement restrictor, and no pressurization of the cement
with a gun. Second generation cementing technique, first used as a term
in 1975 (although cement guns had been available since 1971!), involved
a greater number of femoral stem sizes, broaching the canal to obtain a
uniform thickness of cement around the femoral stem, insertion of an
intramedullary cement restrictor, pulsatile lavage followed by brushing
and drying of the canal, use of a venting tube to remove fluid, and
retrograde filling of the femoral canal using cement pressurized into
the canal with a cement gun.
1985, involved porosity reduction of the cement using vacuum mixing and
a centralizer on the tip of the femoral component to ensure it remained
in the center of the cement during insertion. Each of the generations
have been described using specific femoral component designs (e.g.,
third generation is usually associated with roughened femoral stems),
but strictly speaking, each of the three techniques has been applied to
all femoral stem designs during the time of their use.
cementing technique improves implant survival when compared to first
generation cementing technique comes from the Swedish National
Arthroplasty Register. Cemented implants used during the period
1979-1989 (first generation cementing technique) have a 91.5% 10-year
survival with index diagnosis osteoarthritis and endpoint revision due
to aseptic loosening. The same implants used during the period
1990-2000 (second generation cementing technique) had a 94.8% 10-year
survival. Evidence of third generation cementing technique further
improving prosthesis survival is not (yet!) available.
is a popular approach. Most early failures of cementless components
occurred on the acetabular side associated with accelerated
polyethylene wear and osteolysis. Porous-coated femoral stems have been
relatively successful in achieving constant fixation with a minimum of
unwanted problems. Assuming good initial fixation and a porous-surface
that encouraged bony ingrowth, the major problems have been with thigh
pain and stress shielding. Clinical results with the first generation
cementless stems are now out to 15 years, and assuming a good initial
femoral stem design is used with a good acetabular component,
porous-coated stems do not appear to show the deteriorating results
with time that have been reported with cemented components.
femoral stems, the Harris-Galante I, was that titanium fiber-metal pads
were attached to the proximal ¾ sides of the implant in a
noncircumferential fashion. This design left areas of smooth titanium
proximally with no bony ingrowth, allowing a channel through which
polyethylene wear debris could gain access from the articulating
surface to the prosthesis-bone interface distally, leading to
osteolysis around the femoral stem. The problem has since been
addressed and all current cementless femoral stems have circumferential
porous coating, but there is still considerable controversy regarding
the length of proximal porous coating required to give durable
long-term clinical results.
initial stability through a tight fit in the isthmus of the femoral
canal, use porous coating at least 5/8 of the proximal length down the
prosthesis. These components are fabricated from cobalt-chrome because
notch sensitivity of titanium (weakening of material in areas of high
stress concentration, such as where porous coating is applied) does not
allow extensively coated femoral stems at diameters used in primary
THJR. Results of the anatomic medullary locking stem (AML, DePuy,
Warsaw, IN) have reported a 97% stem survival at 12 years.
Approximately 9% of patients with this stem, however, have pain that
limits activity, but only 3% localized this to the thigh. A recent
study reported that a proximally coated stem had twice the incidence of
thigh pain compared to a fully porous-coated stem. The incidence of
thigh pain with a fully coated stem is similar to that reported
using
a cemented femoral stem. In another study of 174 hips followed for more
than 2 years, 26% showed radiological evidence of stress shielding.
This same group, however, when followed to 5 years, showed no
progression of stress shielding. The long-term consequences of stress
shielding are not known.
stems is their difficulty of removal at revision surgery for reasons
other than aseptic loosening, such as infection or dislocation.
Techniques for removing an extensively porous-coated femoral component
that is solidly fixed include an extended femoral osteotomy, use of a
Gigli saw to free the prosthesis from calcar bone, cutting the
prosthesis transversely with a diamond saw, and trephining the
remaining stem with a dedicated mill slightly larger than the diameter
of the prosthesis.
femoral stems have led many surgeons to only use these components in a
revision situation when there is poor quality proximal bone.
Howmedica, Rutherford, NJ), which has a cobalt-chrome, proximally
circumferentially porous-coated femoral stem, reported that of 64 hips
in 55 patients still alive at 15 years, only 4 femoral stems (4.0%) had
been revised for isolated loosening (without osteolysis, which is
attributed to accelerated polyethylene wear from factors unrelated to
the stem). In another proximally coated implant system, the Omnifit
cementless total hip (Osteonics Corporation, Allendale, NJ), with an
average 10-year follow-up, there was a 2.6% incidence of femoral stem
revision. The incidence of thigh pain in this series was 4.5%.
first generation porous-coated femoral prostheses have led to
modifications of design. The PCA E stem is modified by widening of the
proximal flare as well as modification of the curve of the tip to
reduce the incidence of stem tip abutment on the anterior cortex of the
femur, which was thought to be responsible for thigh pain with the
original PCA prosthesis. A study comparing the PCA E series with a
matched group of patients who received the original PCA component,
showed significant improvement in all areas, with 98% versus 81% good
or excellent clinical results, 2% versus 19% revision rate, and 19%
versus 50% incidence of femoral radiolucencies.
involved use of a stem with a tapered geometry to achieve mechanical
stability in the proximal portion of the femur, achieved by wedging the
component into place rather than using a cylindrical reamer. This
design, with a porous coating or a roughened titanium surface, has been
popular in Europe for many years. In one series of 100 hips, using a
plasma spray proximally coated tapered titanium stem (Taperloc, Biomet,
Warsaw, IN), with a 10-year follow-up, there were no instances of
femoral loosening and only seven cases of femoral osteolysis. Similar
results have been obtained with a cobalt-chrome hip (Trilock, DePuy,
Warsaw, IN) where only 1 in 66 femoral components were revised at 10
years, and no cases of distal osteolysis were reported.
a titanium tapered stem, using the same type of acetabular component
and polyethylene. Although clinical results were equivalent in both
groups, the linear wear rate was significantly higher for the titanium
stems with a plasma spray surface (0.22 mm/year) compared with the
cobalt-chrome stems with a sintered bead surface (0.07 mm/year).
Prevalence of osteolysis was 16% in the titanium group and 0% in the
cobalt-chrome group. One potential cause for the higher wear rate and
higher incidence of osteolysis is accelerated polyethylene wear
secondary to third body particulates from either the modular head-neck
junction or the titanium plasma spray surface.
calcium phosphate coating used in total hip arthroplasty. It was
originally used as the sole coating on the surface of a prosthesis to
obtain initial stability. Although early clinical results were good,
over time it appears to resorb leading to loosening and failure
necessitating revision. In one study comparing the same cup design with
either hydroxyapatite coating or porous coating, at a minimum follow-up
of 5 years, the aseptic loosening rate was 11% (21 in 188) for the
hydroxyapatite component versus 2% (2 in 109) for the porous-coated
component.
polyethylene wear and increase the incidence of osteolysis. This is not
shown, however, in one study of 314 hips (274 patients) in which a
tapered
hydroxyapatite-coated titanium femoral stem (ABG, Howmedica,
Rutherford, NJ) was assessed at a minimum 10-year follow-up. No stems
showed aseptic loosening and there were no cases of distal endosteal
femoral osteolysis. In another study of the same prosthesis, a mean
6-year follow-up (4-10 years) of 97 patients showed that although there
were very good clinical results and 100% survivorship of the femoral
stem, there was an alarmingly high rate of polyethylene wear, 0.24
mm/year (range, 0.05-0.76 mm/year).
acetabulum with a thin layer of bearing surface, and replacement of
only the femoral head (not neck) with a metal ball. Historical failure
of surface replacement has been due to the production of wear debris
with subsequent bone resorption, loosening, and failure. Recently, to
avoid these problems a surface replacement using a metal-on-metal
bearing, allowing thin components and femoral design and
instrumentation to avoid varus alignment, has been designed. McMinn,
the designer of this prosthesis, reported on his experience with 235
joints over a 5-year period. There were no femoral neck fractures and
no dislocations. Over that time, problems with premature failure of
component fixation necessitated changes in fixation technique from
press-fit, to cemented, to the current system that uses a peripherally
expanded hydroxyapatite coated acetabular cup and a cemented metal
head. Potential advantages of this system over conventional THA include
conservation of bone for later revision surgery if necessary,
improvement in range of motion, and reduction in dislocation rate. The
major concern is early femoral neck fracture, which seems to occur more
commonly in females where osteoporosis might be a factor.
in performing THA through very small incisions by both the public and
implant companies, there are several reports in the literature of using
small incisions 15 years or more ago. Interest in this as a technique
for performing THA has probably followed from a resurgence of interest
in performing unicompartment total knee replacement approximately 5 to
7 years ago.
single-incision technique and the two-incision technique. Almost all
THA done in this manner are press-fit using porous-coated femoral and
acetabular components because of difficulty cementing through a small
incision. The single-incision technique can be performed as a limited
anterior approach as described by Hardinge, where a very limited
splitting of gluteus medius occurs through a 5 cm incision, or a
posterior approach through a 5 cm incision based over the femoral neck
posteriorly.
greater than the femoral head diameter, based over the femoral neck
anteriorly. Through this, the hip is dislocated anteriorly and a
femoral neck osteotomy performed. Acetabular preparation is performed
with the aid of an image intensifier, which is also used to ensure
correct positioning of a press-fit acetabular cup. A separate 4 cm
incision is made over the tip of the greater trochanter, and femoral
canal preparation and stem insertion are again aided by an image
intensifier.
dissection include quicker postoperative mobilization and a shorter
hospital stay. Studies that have attempted to demonstrate an
improvement in these outcome measures have found that patient
comorbidities and domestic circumstances have a greater impact on these
than the length of the surgical incision. Long-term results of
prosthesis survival using current minimally invasive surgical
techniques are not available.
categories: intraoperative, in-hospital but postoperative, 30-day
rates, and 90-day rates. Intraoperative rates in one study from the
Mayo Clinic database on 38,488 THAs in 29,431 patients between 1969 and
1997 showed 23 deaths during surgery. Predisposing factors included
elderly patients with preexisting cardiovascular conditions with acute
hip fractures or pathological fractures (17 of
23).
The strongest predictor of sudden death was use of cement for fixation
in all 23 cases. The authors recommend avoidance of cement if possible
in predisposed patients with acute fracture or malignancy.
THA is in the range 0.1 to 0.8%, 30-day mortality between 0.15 to
1.42%, and 90-day mortality between 0.2 to 0.74%. Ninety-day mortality
after revision surgery is approximately the same as for primary THA
(0.6 to 0.9%).
1%) but concerning outcome for both the surgeon and the patient.
Informed consent should include this complication as a possible
outcome. Predisposing factors include female gender, revision surgery,
lengthening of the limb, and bleeding tendencies. The most common nerve
injured is the sciatic (78%), followed by the femoral (13.2%) and the
obturator (1.6%).
(medial) and common peroneal (lateral) divisions that usually (90%) are
united as a single nerve down to the lower border of the thigh.
Although grossly united, the funicular bundles of the two are separate
and there is no exchange of bundles between them. In 10% of cases the
two divisions exit the pelvis as distinct nerves. In these situations
the tibial nerve always passes below the piriformis muscle while the
common peroneal component can pass above or through piriformis. The
common peroneal nerve is more prone to mechanical injury than the
tibial nerve because it has larger and more tightly packed funiculi
with less protective connective tissue, and because of its more lateral
position and tethering in the sciatic notch and head of fibula.
reasons: direct physical damage from dissection, poor retractor
placement, or entrapment during cerclage femoral wiring from excessive
traction or from compression usually by postoperative hematoma
associated with anticoagulation. During surgery attention should always
be given to sharp retractor placement over the anterior of posterior
columns. Formal dissection of the sciatic nerve is not usually
considered necessary, but its position should always be kept in mind
during surgery. If cerclage wiring of the femur is required, care
should be taken to split the posterior linea apsera as close to the
femoral shaft as possible. The wire should always be passed from
posterior and lateral to anterior and medial, and the leg should be
placed in the anatomic, not dislocated position during passing of the
wire.
attempt should be made to identify its origin. After checking that it
is not anesthesia related (patient received a partial spinal or
epidural anesthetic), the operative procedure should be reexamined. If
there is any chance of the nerve being trapped by a cerclage wire, or
the limb has been lengthened more than 3 cm, the patient should be
returned immediately to the operating room for either removal of the
wire or shortening of the leg (femoral head exchange if possible).
Hematoma compressing the nerve may give clinical signs of ongoing
bleeding and buttock pain. Discontinuation of anticoagulation may be
considered, but there is no absolute indication for reoperation to
evacuate a hematoma. Good outcomes have been reported with conservative
management.
possibility of the leg being lengthened more than 2 to 3 cm (such as
commonly occurs with reconstruction of a congenital hip dislocation), a
wake-up test can be discussed with the patient. This involves telling
the patient he or she will be asked to “wriggle their toes” on both
feet before extubation. The anesthetist must ensure no motor block or
muscle relaxant, and the ability to increase the patient’s level of
consciousness to allow understanding of commands. If the patient is
able to dorsiflex his or her toes on the contralateral foot but not the
toes on the operated leg, the patient is assumed to have a sciatic
nerve palsy, and consideration should be given to reoperation.
41% chance of complete recovery, a 44% chance of partial recovery, and
15% of patients will have a poor outcome. Prognosis is good if limited
motor function is present immediately after surgery or if some motor
function returns in the first 2 weeks postoperatively. Femoral nerve
injury generally has a better outcome than sciatic nerve injury.
primary THA is a leg-length inequality (approximately 25%). Most
studies of large groups of people show that approximately 30% of the
population have a discrepancy between 1 and 2 centimeters,
however,
and often the patients’ complaint of a longer leg after operation is
not borne out with clinical or radiologic measurement. This functional
leg-length difference is caused by contractures around the hip that
create a pelvic obliquity. Commonly, this is caused by tightness of the
gluteus medius muscle in a hip that has been short and has had a
decreased offset and then is corrected to the normal hip length and
offset. Over time, the feeling of a “long leg” will become less
obvious, but it is important to warn the patient of this preoperatively
so they are not worried that something was done incorrectly at the time
of surgery.
be avoided by accurate preoperative planning, anatomic component
geometry, and intra-operative assessment (including use of outrigger
jigs, center of head to lesser trochanter distance, and relationship of
knees and heels before and after reconstruction as well as
intraoperative radiographs if there is still uncertainty. Correct
restoration of offset and leg length in a diseased hip is always
balanced against risk of dislocation by making the soft tissues too
loose, but both goals can always be achieved with careful surgical
technique.
(excluding acute fractures) in the United States reported a 3.9%
dislocation rate during the first 6 months after surgery, and for
12,956 revision THAs, a 14.4% dislocation rate. These figures are much
higher than are generally quoted for single surgeon series of THA,
which are approximately 0.5 to 2%. Factors that influence rate of
dislocation include patient factors (including cerebral dysfunction and
alcoholism), surgical approach (traditional posterior approach has a
fourfold greater risk of dislocation compared to Hardinge approach),
surgical factors (correct positioning of acetabular and femoral
component, trimming of marginal osteophytes from the acetabulum), and
implant factors (head size, head-neck ratio, elevated rim acetabular
liner, choice of neck length, choice of offset).
capsule and external rotators to the hip have reduced dislocations with
the posterior approach to 0.5%. A large study from the Mayo Clinic
demonstrated no benefit in dislocation rates with a larger head, but a
definite benefit with use of an elevated rim liner (2.2% incidence if
elevated rim used, 3.85% if no rim). Laboratory data suggests decreased
impingement between the femoral neck and acetabular rim with a 28mm
femoral head compared to a 22 mm femoral head, but no additional
benefit is gained by using a 32 mm femoral head for this purpose.
dislocation rate when the acetabular cup diameter was 32 mm or greater
than the femoral head diameter. The authors postulated that site of
attachment of pseudocapsule is moved further from the femoral head,
potentially allowing greater laxity, and they recommended use of a 22
mm diameter femoral head for acetabular cups less than 50 mm diameter,
and a 32 mm femoral head for acetabular cups greater than 62 mm outer
diameter.
cause and the number of previous dislocations. Most first-time
dislocations where there is no obvious cause such as osteophyte
impingement or component malposition are treated by closed reduction
and gradual mobilization (Figure 17-21). There
is no evidence that prolonged immobilization or physiotherapy after
closed reduction decreases the likelihood of recurrence. If an obvious
cause is identified, then operative treatment is indicated. The late
dislocation (occurring at least 2 to 5 years after surgery) raises the
possibility of wear of the acetabular component, which may require
revision.
patient because they cannot be sure of the circumstances when they will
occur. Often these patients request surgery. If the anteroposterior and
lateral radiograph fail to show acetabular malposition that can be
corrected by revision surgery, a decision must be made as to the best
form of treatment. Most recurrent dislocations are posterior, and in
the majority of these cases the original THA was performed through a
posterior approach. There is little guidance in the literature as to
which surgical approach should be performed in this situation, but the
authors prefer a Hardinge type approach through nonscarred tissue.
tension by increasing the length (and offset) of the femoral neck and
performing a careful soft tissue repair. A further option is
augmentation of the cup with a custom-made wedge of polyethylene (often
a liner cut into thirds), held to the existing cup with screws, placed
in the position where the dislocation was occurring. Trochanteric
advancements have not been reported as useful in the literature.
Complete revision of both components is a drastic option if both are
well-fixed and there is no obvious component malposition.
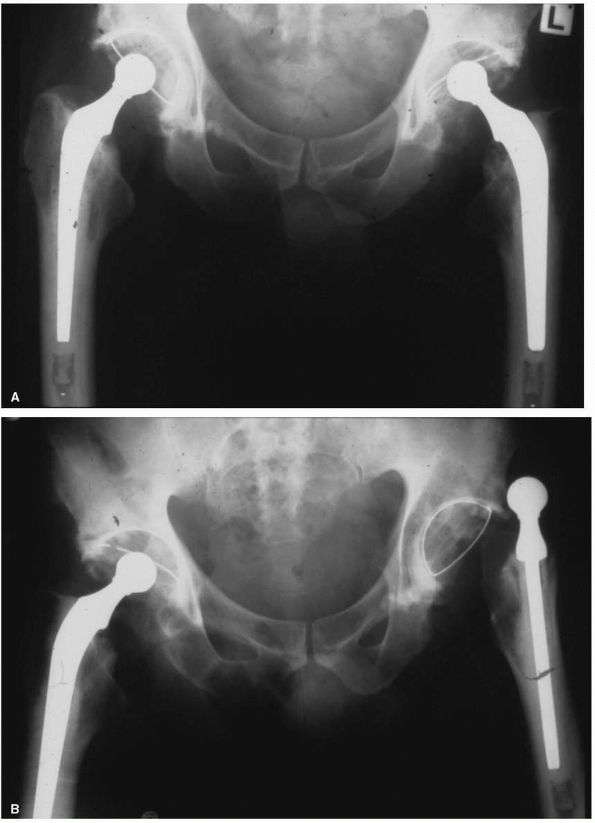 |
|
FIGURE 17-21. (A)
This 47-year-old male underwent bilateral simultaneous cemented THA through a direct lateral approach for severe idiopathic osteoarthritis. His postoperative radiographs appeared satisfactory and he was discharged from hospital 6 days after surgery. (B) He was readmitted with an anterior dislocation of the left hip 3 weeks after surgery. He claimed that he fell forward out of a chair, causing the dislocation. The hip was reduced with sedation, and he was discharged the following day. No splint was used. He made an uneventful recovery, and at latest follow-up (9 years) has not had any further instances of dislocation. |
dislocating hips using one of the above operations, showed only a 61%
success rate. An increasingly popular alternative is revision of the
acetabular cup with a constrained liner. A constrained liner is one
that “captures” the femoral head and prevents it from dissociating from
the acetabulum. A 5-year follow-up of 56 patients who were treated with
a constrained acetabular component showed a 97% success rate at
preventing further dislocation. The potential disadvantage of decreased
range of movement and component loosening (because the internal surface
of the polyethylene is greater than a hemisphere causing impingement
and greater stress transmission to the prosthesis-bone interface) has
not been seen in medium-term follow-up studies of this component. It
remains the most effective solution to date for this distressing and
very difficult problem.
the laying down of bone in tissues, should be identified preoperatively
and some form of prophylaxis instituted. Risk factors include
ankylosing spondylitis, previous acetabular or pelvic fracture, or
previous problems with heterotopic ossification during THA on the
contralateral hip.
pharmacological means or radiation. Aspirin has been shown to be not
effective in preventing HO, and most surgeons would now recommend
indomethacin, which is the most studied nonsteroidal anti-inflammatory
drug used for this purpose. One study showed that in 123 male patients
undergoing primary THA, given a 10-day course of 25 mg Indomethacin
three times daily, there was a 7.6% incidence of mild HO and no cases
of Brooker grade III or IV compared to the normal quoted prevalence of
3 to 10% of patients who experience functional impairment in the form
of diminished range of motion due to HO.
3.3 Gy of radiation with 150 mg of diclofenac for 3 weeks showed that
radiation was slightly more effective than nonsteroidal
anti-inflammatory tablets in reducing the incidence of HO, but that
both were very effective. The dose of radiation that is effective at
preventing HO has steadily decreased over the last 20 years, and now
0.7 Gy is shown to be effective.
decreased range of motion after maturity (as determined by isotopic
bone scan usually 6 months after surgery), the HO may be excised and
the patient treated with postoperative radiation. Often a preoperative
CT scan is helpful to document exactly where the HO is located, and an
appropriate surgical approach can be used. With this regime an average
increase of 45° flexion and 25° of abduction can be anticipated (Figure 17-22).
on the elapsed time since surgery. Acute infections occurring within 4
to 6 weeks of surgery, where the components are judged to be stable and
there is no reaction in the surrounding bone, can be treated with
thorough debridement of infected material, pulsatile lavage, and
prolonged IV antibiotics, yielding a very high prosthesis retention
rate (>95% depending on the infecting organism). The majority of
organisms in early infection are staphylococcus, and the antibiotics of
choice here are flucloxacillin combined with rifampicin.
weeks after surgery, there is a greater chance of infected material
having gained access to the prosthesis patient interface. This does not
always equate with prosthetic loosening, but almost always necessitates
removal of the prosthesis. Removal of the prosthetic components can be
performed as a one- or two-stage revision procedure. The two-stage
exchange remains the standard for treatment, with the first stage
consisting of removal of all components and foreign material such as
cement, cement restrictors and wires, meticulous extensive debridement,
and accurate identification of the infecting organism. Use of an
articulating spacer containing antibiotic to maintain tissue tension is
becoming more popular, but even if not used, some form of antibiotic
impregnated cement should be placed in the acetabulum and upper femoral
canal.
2 to 4 weeks, followed by 2 to 4 weeks of oral antibiotics. Debate
exists about whether the patient should undergo a period without
antibiotic cover before reimplantation, and the value of hip aspiration
to ensure no organisms are present prior to reimplantation. The
patient’s erythrocyte
sedimentation
rate and C-reactive protein should be close to normal before
reimplantation, and this usually occurs 6 to 10 weeks following
prosthesis removal. Reimplantation is usually performed with a cemented
femoral component to allow further direct antibiotic delivery to the
bone. Acetabular revision may be performed with a cemented or
cementless component. Success rates for this procedure with a
nonresistant organism exceed 95%.
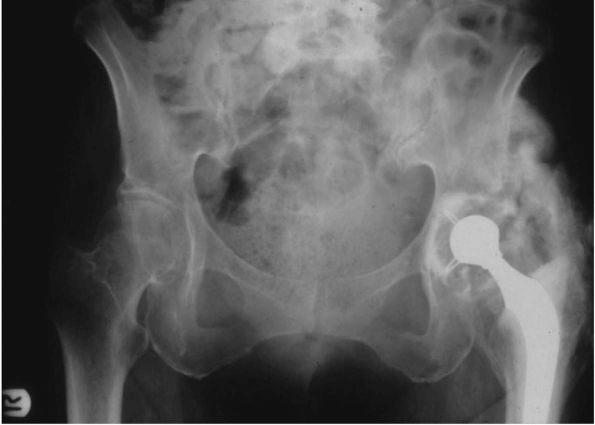 |
|
FIGURE 17-22.
Anteroposterior radiograph of a 52-year-old patient involved in a motor vehicle accident. He sustained a displaced left femoral neck fracture and a severe head injury. Although admitted in an unconscious state with a Glasgow Coma Scale of 12, a CT scan of his head revealed no intracerebral lesion. A THA was performed on the night of admission to hospital. He remained intubated and unconscious in the intensive care unit for 13 days. Despite making a full neurological recovery, he developed severe heterotopic ossification around the left THA. |
one-stage exchange, where the organism is considered of low virulence
and responsive to antibiotic therapy, an adequate debridement can be
achieved, and the patient’s medical condition contraindicates further
major surgery. Success rates in excess of 80% can be achieved when this
approach is used in carefully selected cases.
of a THA, both intraoperative and postoperative, has been increasing
over the last 20 years. Intraoperative fractures are generally caused
by the need to achieve good initial stability of porous-coated
components. They are usually stable, involving a split in the femoral
cortex, and can be treated by keeping the patient from weight bearing
in the early post operative period. If an intraoperative fracture is
identified as unstable, cerclage wires can be passed around the femur.
Postoperative fractures are occurring due to an increase in the number
of THAs, younger patients, increasing survival of the prosthesis in
patients who are simultaneously loosing bone mass, and fractures
through osteolytic lesions.
fractures is based on the level of the fracture and stability of the
femoral component. Type AG is a fracture of the greater trochanter. Type AL
is a fracture of the lesser trochanter. Type A fractures are treated
conservatively if stable and operatively if unstable. Type B fractures
occur around the lower stem or tip of the prosthesis. Type B1 includes
fractures where the femoral component is solidly fixed. They are
treated with plating or strut allografts. Type B2 includes fractures where the femoral component
is loose and are treated with revision of the loose femoral component
to a long-stemmed prostheses, usually cementless, to bypass the
fracture, and fixation of the fracture with wires or plates (Figure 17-23). Type B3 fractures occur when there is severe underlying bone stock loss, and the femoral component is almost always loose (Figure 17-24). They are treated in a similar fashion to Type B2
fractures, often with additional allograft. Type C fractures occur
below the tip of the femoral stem and are treated independently of the
prosthesis.
by revision to a long-stemmed porous-coated component, but treatment is
difficult with a high rate of complications and a poor functional
outcome.
femur, and usually occur during insertion of a porous-coated component
into an acetabulum that has been under-reamed by 1 to 2 mm to gain good
initial stability. Fractures occur more frequently in women with
osteopenic bone. If an intraoperative acetabular fracture is
recognized, assessment of stability of both the fracture and the
component is required. An unstable component requires removal. If the
medial wall has been breached, over-reaming and a larger component with
rim fit can achieve a good result. If a column fracture has occurred,
it will need to be exposed and plated, followed by implantation of an
acetabular cage or a larger diameter cup. If a fracture occurs but the
cup appears stable, the situation can be accepted, and the fracture
will usually heal, but the component has a higher chance of not
achieving bony ingrowth, and thus requiring revision at a later date.
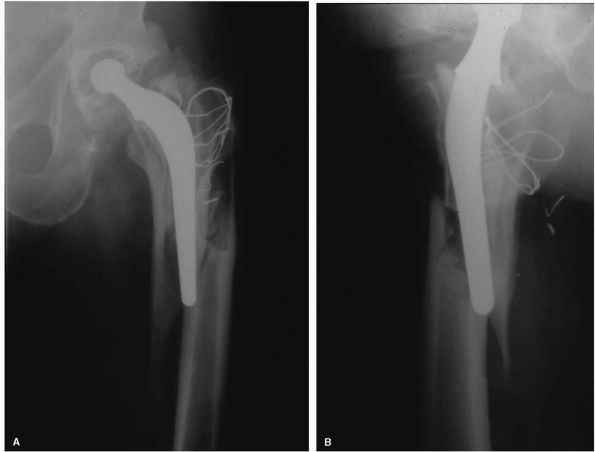 |
|
FIGURE 17-23. (A)
Anteroposterior radiograph of a 61-year-old male who fell in the shower. This Charnley type THA had been in place for 18 years. (B) Lateral radiograph of the same patient. A loose prosthesis, associated with extensive osteolysis required revision of both the femoral stem and acetabular cup. |
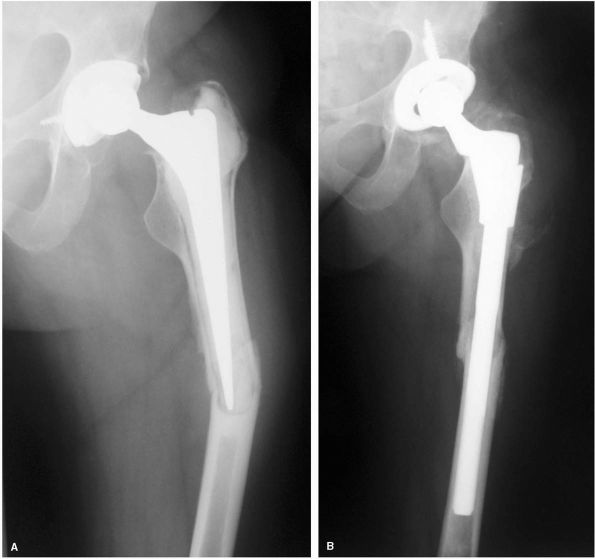 |
|
FIGURE 17-24. (A) Distal femoral fracture around a radiologically loose cemented THA that had been in place for 7 years. (B)
At the time of operation, the cement was able to be easily removed from the proximal femur without disturbing the fracture site with any soft tissue exposure. A modular cementless stem bypassed the fracture and led to radiological healing at 10 weeks postoperatively. |
failure in THA. Wear is the removal of material, with the generation of
wear particles that occurs as the result of relative motion between two
opposing surfaces under load. The mechanical consequences (mechanical
thinning) of polyethylene wear have been recognized since the early
1970s when the first wear studies on THA suggested a limited life span
of the polyethylene bearing. This concept was partly responsible for
the development of modular acetabular components, which allowed
exchange of the plastic liner without disturbance of the bone
prosthesis interface (Figure 17-25).
clinical consequences of wear—the release of excessive wear particles
into the biological environment—is the predominant cause of late
failure of THA, and this still remains the case today. When particles
within a certain size range are phagocytized in sufficient amounts by
macrophages, they enter a state of activated metabolism, with the
release of cytokines that result in periprosthetic bone
loss leading to eventual loosening and necessitating component revision (Figure 17-26).
The concept of the effective joint space includes all areas of the
joint that can be accessed by synovial fluid laden with wear particles.
This includes all areas of the bone and prosthesis (cementless
implants) and bone and cement (cemented implants) interfaces, which
often extend far beyond the areas of capsular attachment associated
with a nonreplaced hip. Synovial fluid can excite this response,
leading to areas of osteolysis (focal areas of progressive
periprosthetic bone loss) in locations removed from the primary
articular surface (Figure 17-27).
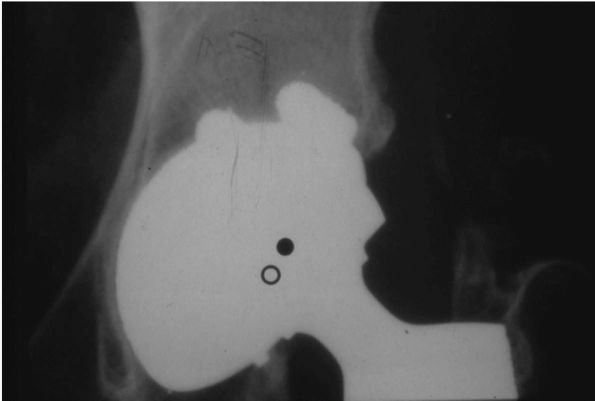 |
|
FIGURE 17-25.
A marker has been placed on this radiograph of a PCA total hip arthroplasty to indicate both the initial and final position of the center of the femoral head. The prosthesis had been implanted 4½ years previously. Most of this femoral head penetration was attributed to a small cup combined with a 32 mm femoral head leading to a polyethylene thickness of 7mm, and a suboptimal locking mechanism. |
(adhesion, abrasion, and fatigue) and wear modes (conditions under
which the prosthesis was functioning when the wear occurred). Adhesive
wear occurs when two surfaces are bonded together under load, and
material is pulled away from one or more surfaces when movement occurs
between them. Abrasive wear is a mechanical process wherein asperities
on the harder surface removes material form the softer surface. Fatigue
occurs when local stresses exceed the strength of the material, causing
it to fail, usually after a particular number of cycles. Bearing
surfaces are designated primary (intended to articulate) or secondary
(not intended to move against any other material, whether this is the
patient’s bone or another component of the THA prosthesis).
intended to occur) between two primary bearing surfaces. Mode 2 wear
occurs when a primary bearing surface moves against a secondary surface
(dislocation). Mode 3 wear takes place when a third substance gains
access between two primary bearing surfaces (often referred to as third
body wear). Mode 4 wear occurs when two secondary bearing surfaces rub
together, such as impingement of the femoral neck on the rim of the
acetabular cup, motion of the prosthesis in the bone, or motion between
modular connections of the femoral stem (morse taper fretting) or
acetabular cup (back-side wear).
implantation should evaluate all of these areas for that system
carefully, because the type and incidence of wear modes for that
prosthesis vary widely, a well as variation in when they occur over the
service life of that prosthesis. In most primary THA, the greatest
contribution to wear is from mode 1 wear—polyethylene particles
generated from the primary bearing surface (Figure 17-28). Two types of assessment for this are available: simulator studies and measurement from clinical radiographs.
 |
|
FIGURE 17-26.
Low power microscopy (×250) of the tissue retrieved from an osteolytic granuloma at the time of revision surgery. The polyethylene particles in the tissues are easily identified under polarized light by their birefringence. |
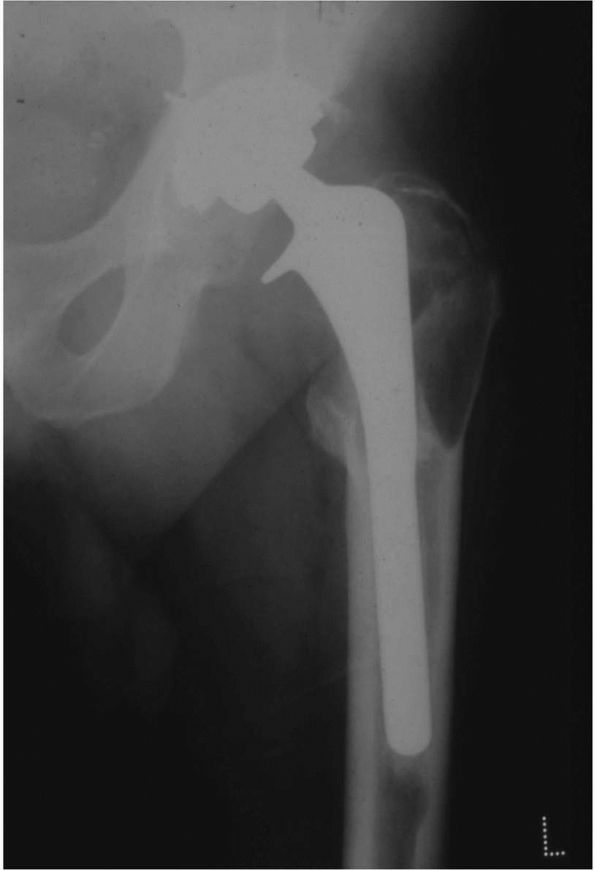 |
|
FIGURE 17-27.
Despite very little visible wear appearing in the articulating surface of this Harris-Galante I total hip arthroplasty which had been implanted 5 years previously, a massive osteolytic lesion is seen extending from the greater trochanter down the lateral side of the femur. At the time of surgery, there was an impingement of the titanium femoral neck on the acetabular cup, which had generated much of the wear debris causing the osteolysis. Another source of wear in this case was titanium debris generated from a loose femoral component. There was no visible wear of the acetabular liner. |
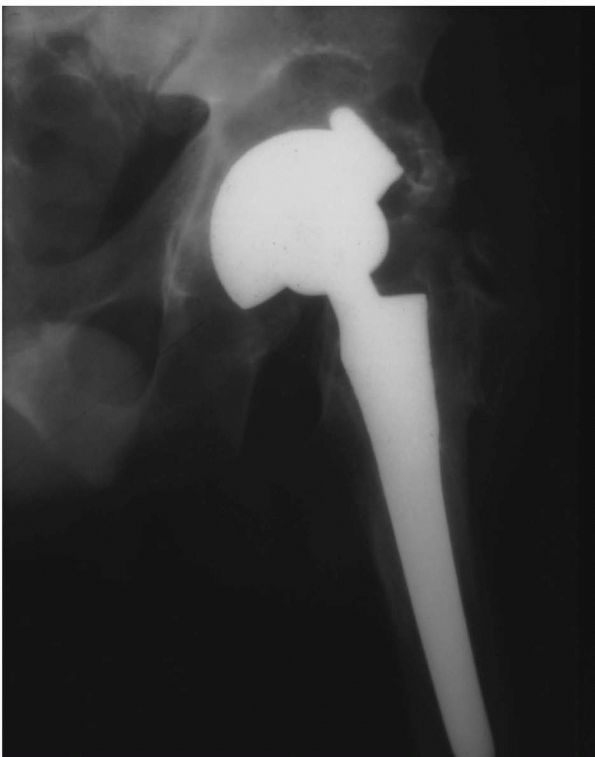 |
|
FIGURE 17-28.
Wear from the primary bearing surface of this PCA total hip arthroplasty has contributed to the large osteolytic lesion seen in the superior quadrant of the acetabular component. |
incorporate the critical fact that, in the hip as opposed to the knee,
the path that a point on the femoral head makes within the polyethylene
is multidirectional, and there are often crossing paths. Although this
allows comparison of two bearing surfaces within the same simulator,
many surgeons are skeptical that simulator wear studies can truly
reproduce the in vivo situation under which a THA functions. Variables
still to be fully understood include lubrication medium, presence of
third body particles, temperature of the primary bearing surface, and
loading (there is some recent radiologic evidence that the primary
bearing surfaces of a THA dissociate by up to 1 mm during a normal gait
cycle). Wear simulator results vary greatly but tend to show
polyethylene wearing at a rate of 0.06-0.10 mm/million cycles.
Historically one million cycles were taken to represent one year’s
wear, but a recent pedometer study shows variation between 0.4 to 4
million cycles/year among study patients.
change in displacement of the femoral head between two radiographs
taken at different times represents polyethylene wear. Calculation of
volumetric polyethylene wear is then made on the basis of femoral head
diameter and direction of displacement. Measurements are based on
locating the center of the femoral head, and either measuring the
shortest distance to the edge of the acetabular cup, or locating the
center of the cup and measuring the difference between head and cup
center. Radiostereometric analysis (RSA), originally developed for
measurement of component migration, can be used if beads are
prospectively implanted in the pelvis at the time of surgery, the
acetabular cup has beads implanted, and a dedicated RSA setup for
taking radiographs is available. It can only be used prospectively, and
expense usually constrains numbers of subjects that can be recruited.
be made by placing radiographs on a tablet and using a stylus to
digitize points (manual method) or by taking points directly from the
digital image of an radiograph (usually automated). Recent studies have
shown good correlation between radiologic measurements and retrieved
prostheses. Hips with linear wear rates of greater than 0.2 mm/year
have been associated with an increased prevalence of osteolysis, while
hips with linear wear less than 0.1 mm/year have infrequently been
associated with osteolysis.
acetabular and femoral components was by aseptic loosening associated
with mild or moderate osteolysis, and moderate or severe component
migration. With the advent of modularity, hybrid THA, multiple
revisions, porous-coated femoral and acetabular components, and
alternate problems (fractured ceramic femoral heads), different modes
of failure require different solutions to be applied. The primary
philosophy is to achieve a solidly fixed, stable component with minimal
bone loss (or bone restoration where necessary). The minimum surgery,
carrying with it the minimum risk of complications to achieve this aim,
is often the best solution.
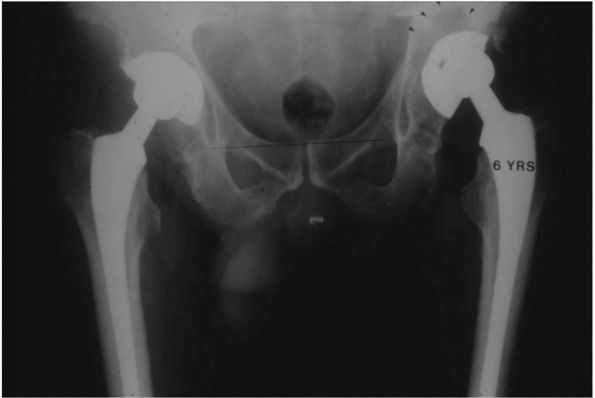 |
|
FIGURE 17-29.
A large osteolytic lesion in the superior acetabulum, around a titanium grit-blasted acetabular component, caused by excessive mode 1 wear from a titanium femoral head articulating against polyethylene. Despite this cup having a reasonable track record and being stable at revision, the locking mechanism was nonfunctional after removal of the polyethylene liner. An intraoperative decision was made to revise the acetabular component, because cementing an appropriate sized polyethylene liner into the metal shell would have given a polyethylene thickness less than 8 mm. |
record is found to be solidly fixed at the time of revision surgery,
and there is minimal evidence of wear of its primary bearing surface
(or it can be replaced, as with a modular femoral head or modular
acetabular cup), then consider retaining the component at the time of
revision. This decision must also take into account ease of exposure
(exposure of an acetabular defect around a solidly fixed femoral stem),
leg length and tissue tension issues (increased risk of postoperative
dislocation if neck length options are limited), and head size (a
monoblock femoral stem with a femoral head diameter that cannot be
matched to options available for the acetabular revision).
defects in the presence of well-fixed components often involves
grafting of the defect with allograft, removal of screws from the cup,
and downsizing the diameter of the primary bearing surface from 32 to
28 or less millimeters (to increase polyethylene thickness) by
replacing the polyethylene liner (Figure 17-29). Increasing the neck length,
to rectify poor tissue tension caused by the exposure (which can lead to an increased risk of dislocation), is often necessary.
good quality anteroposterior and lateral radiographs of the hip, as
well as proximal femur beyond the tip of the femoral prosthesis.
Defects are always more extensive than they appear on radiograph.
Acetabular defects often require additional imaging, such as Judet
views to assess the anterior and posterior columns, and occasionally CT
if a pelvic dissociation (loss of bony continuity through both anterior
and posterior columns) is suspected. If there is intrapelvic migration
of the acetabular component, pelvic angiography to document the
proximity of the iliac vessels is often required.
dictated by the experience, training and philosophy of the operating
surgeon, and tempered by the extent of any bony defect (or other major
structural consideration, such as pelvic dissociation) present. The
most commonly used grading system for acetabular bone loss emphasizes
the presence or absence of the acetabular rim, deficiencies of the
acetabular dome and walls, and integrity of the anterior and posterior
columns. Type I defects, with intact rims, walls, and columns, may be
dealt with using cementless components with or without supplemental
screw fixation, or cemented components using impaction grafting
techniques. Twelve-year results of the Harris-Galante titanium fiber
mesh socket used for revision of type I defects approach the 95% good
or excellent results they enjoy in primary THA.
intact anterior and posterior column, and may be treated with a
cementless acetabular component with supplementary screw fixation.
Defects are filled with compacted allograft bone chips. If the major
acetabular deficiency is superior, with minimal anterior or posterior
column bone loss, a slightly higher hip center may be accepted to
prevent over-reaming and sacrifice of good bone from the anterior and
posterior columns.
severely compromised, and the columns offer no support. Two choices are
present here—a very large (“jumbo”) acetabular component (outer
diameter greater than 66 mm) or an antiprotrusio cage. The guarded
long-term prognosis of structural acetabular allografts (failure rates
up to 50% at 10 years have been reported), and the difficulty and
expense of supporting a dedicated structural allograft bone bank, have
limited their use outside of dedicated hospital units. For most
patients (elderly with a multiply revised hip and component migration)
with a type III defect, the component of choice is either a jumbo or
bilobed cup. Jumbo cups are a good option when there is a large central
defect. The acetabular margin is reamed down to create a
horseshoe-shaped lateral acetabular margin, and an oversized acetabular
cup is then placed on this margin and held with multiple screws. The
central defect can be allografted. Alternatively, if an oval defect is
present and the surgeon wishes to restore the hip center to normal, a
bilobed cup may be used.
stock preservation and restoration must be maximized to allow future
procedures during that patient’s lifetime, compacted allograft bone
chips can be packed into defects (including structural allograft
supplementation to prevent bone chip migration). If there is likely to
be less than 50% contact between host bone and the acetabular
prosthesis, an antiprotrusio cage should be used. This is attached to
the ilium ischium and pubis, overcoming problems with column or medial
wall deficiency. Bypassing of the acetabulum allows load sharing to
occur with underlying allograft, encouraging its incorporation for
restoration of bone stock. A polyethylene acetabular socket is then
cemented into the cage. Care should be taken with component
orientation, because bony landmarks that can usually be identified will
no longer be available (Figure 17-30).
preoperatively, but where there are major column defects and acetabular
component migration, it should be suspected, and appropriate component
inventory for intraoperative treatment of discontinuity should be
available. Three choices are available:
-
Stabilization with a pelvic
reconstruction plate along the posterior column, followed by a
cementless acetabular component. This is most commonly used where a
posterior column fracture has occurred (often during
P.567
extraction of a well-fixed component), but there is sufficient posterior column bone to obtain stable fixation. -
Bypassing the discontinuity using an
antiprotrusio cage with stable fixation proximally into the ilium and
distally into the ischium. -
Use of a jumbo cup. If the metal shell of
the cup is thick enough in the acetabular system chosen, the screws
placed through the cup will only travel in one direction,
perpendicularly away from the cup center. This greatly enhances
fixation of the two mobile pelvic bones by the cup, and if careful
screw placement is selected, allows a very stable construct without a
supplemental posterior column plate (Figure 17-31).
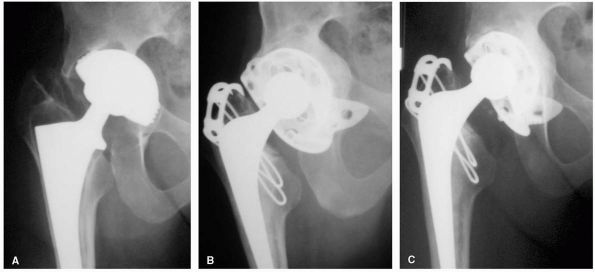 |
|
FIGURE 17-30. (A)
Radiograph of the right hip of a 31-year-old female patient with rheumatoid arthritis who had a THA with a threaded type acetabular component 6 years previously. She presented with increasing groin pain after a minor stumble. A pelvic dissociation was assumed. (B) At the time of revision, the radiological diagnosis of a pelvic dissociation was confirmed. When a young patient is being treated for revision THA, the emphasis is always on bone stock preservation. For this reason, an acetabular cage into which was cemented a polyethylene component was chosen as the implant of choice. This allowed a large amount of morselized bone allograft (two femoral heads) to be placed medial to the cage. (C) An 8-year postoperative radiograph shows incorporation of the medial morselized allograft. Currently the patient is pain free with a stable construct. If this reconstruction fails, a press-fit acetabular cup will be the procedure of first choice. |
should take into account any difficulties with potential femoral
fractures, cortical windows, or extended femoral osteotomies that might
be encountered during extraction of the existing implant. Well-fixed
cemented and cementless stems may be difficult to remove, and vigorous
extraction attempts that might lead to bony fracture should be avoided (Figure 17-32).
Cemented implants should be initially removed from the cement mantle. A
decision can then be made as to whether the cement can be removed from
the canal proximally, or whether a distal cortical window will be
required. An extended femoral osteotomy may be performed if there is
varus femoral remodeling. Most well-fixed cementless femoral implants
have to be removed using thin power instruments to divide the areas of
bone where ingrowth has occurred into the porous-coated surfaces of the
prosthesis.
between the cement and the bone for a mechanically durable interface.
The loss of cancellous bone seen at revision surgery compromises this
interdigitation. Although the 10-year results of cemented femoral
revisions have an 80 to 85% prosthesis survival, improved cementless
prosthesis design has decreased the role of cemented femoral revision.
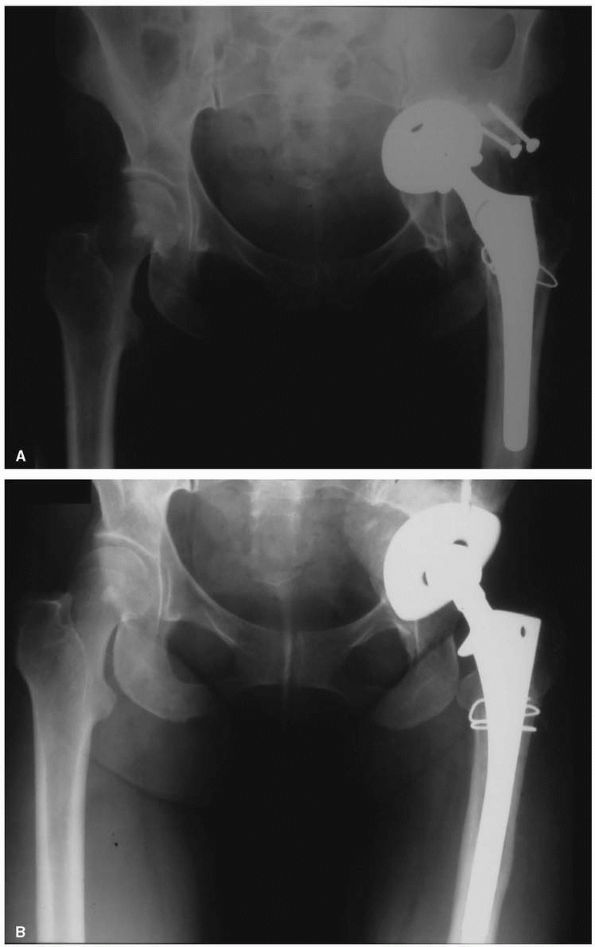 |
|
FIGURE 17-31. (A)
This loose uncemented acetabular component has eroded through into the pelvis, and caused disruption of both anterior and posterior acetabular columns. (B) Morselized allograft femoral head is used to fill the lesion. A 70 mm diameter acetabular cup with multiple screws (inferior screws placed into ischium are hidden by the bulk of the cup) was then used to stabilize the pelvic dissociation. |
well-fixed cemented femoral component due to unusual head size, or leg
length issues. If the cement mantle is radiologically intact, another
component may be cemented into the intact cement mantle without its
removal, with good medium-term (5 to 7 year) results.
popular as it provides a stable initial construct and has good
long-term results. Proximal porous-coated implants provide the
theoretical advantage of biologic fixation with a minimum of stress
shielding (loss of bone from the proximal femur when it is not loaded).
This benefit is outweighed by the
disadvantage
of obtaining initial stability and long-term fixation in the damaged
proximal femur, and their use in the revision setting is limited.
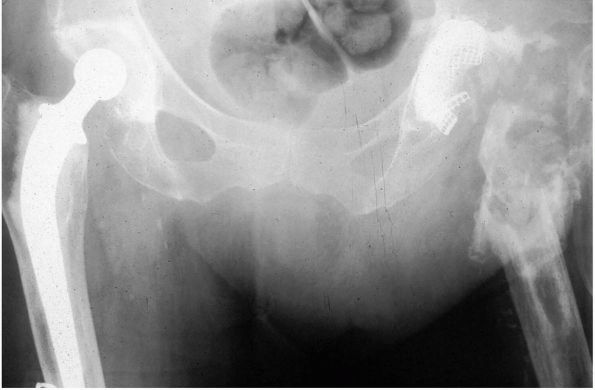 |
|
FIGURE 17-32.
Severe destruction of the proximal femur and pelvis, following removal of a cemented THA. The patient had undergone six previous THAs. |
bypassing the damaged proximal bone using diaphyseal fixation. Most
femoral stems designed for diaphyseal fixation are either extensively
porous-coated devices with a cylindrical distal shape, or grit-blasted
fluted devices with a conical distal shape. Titanium stems have a lower
modulus of elasticity to potentially reduce stress shielding, but
cannot be fully porous coated because notch sensitivity reduces their
strength, increasing the risk of stem fracture. The stress shielding
associated with diaphyseal fixation has not yet become a major clinical
problem, but concern remains about extraction of an extensively
porous-coated device.
approach to employ in revision surgery. The posterior approach makes an
extensile exposure of the femoral shaft easier, but, as in primary THA,
suffers from a higher dislocation rate. If an anterior approach has
been used previously, there is no contraindication to using a posterior
approach for revision, and the converse also applies.
exposure of an anterior or posterior column deficiency is required,
such as when an antiprotrusio cage is used. A recent advance in
revision surgery has been the extended greater trochanteric osteotomy,
where in the greater trochanter and the lateral third of the
circumference of the femur are raised as a flap, with their soft
tissues still attached, for approximately 10 cm down the femoral shaft (Figure 17-33).
As well as good acetabular exposure, it has the advantage of
facilitating removal of well-fixed cemented and cementless implants in
a bone sparring manner. Indications include:
-
Trochanteric overhang above the prosthesis, increasing the risk of fracture during device extraction.
-
Removal of a well-fixed large amount cement in the femoral canal.
-
Removal of extensively porous-coated solidly fixed cementless femoral stem.
-
Varus remodeling of the proximal femur
such that placement of a long straight stemmed femoral component would
predispose a fracture.
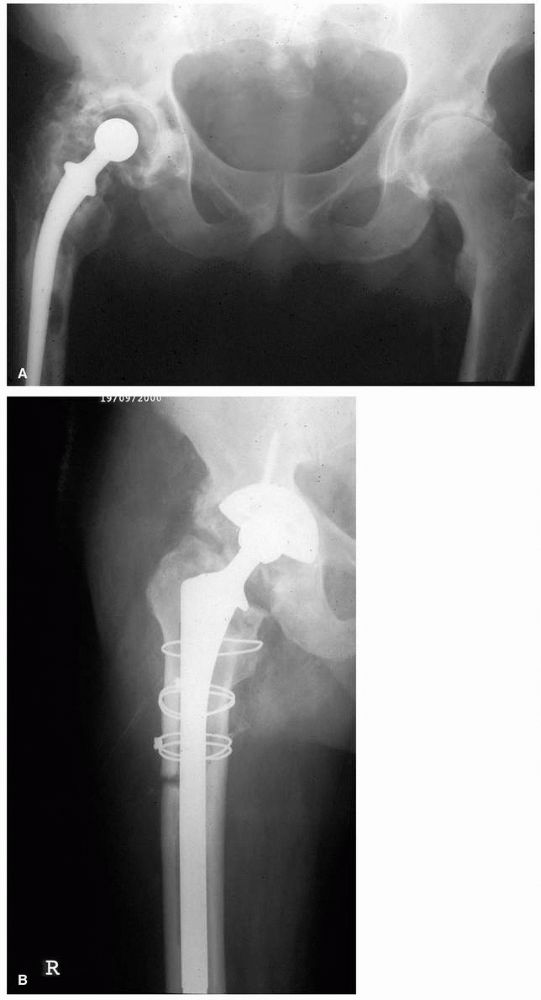 |
|
FIGURE 17-33. (A)
This Stanmore THA had been implanted 16 years previously. The patient presented with pain, severe stiffness, and a limp. He weighed 270 pounds. (B) An extended trochanteric osteotomy through a direct lateral approach gave excellent exposure of the acetabulum, and allowed easy cement removal from the shaft. It was repaired with three cables, and the patient kept touch weight bearing for 6 weeks postoperatively. |
currently use cementless fixation of both the acetabular and femoral
components. A technique popularized
by
the Exeter group has used an alternative for many years with good
medium-term clinical results—impaction grafting with cement. This
method seeks to restore cancellous structure to the femoral canal using
densely packing cancellous bone chips. These bone chips are packed
tightly enough to provide axial and rotational stability to a femoral
stem when it is cemented into them. Technical difficulty has limited
its use, however, with many centers reporting a high incidence of
femoral fracture and component subsidence. Ten-year results from the
center that initiated the technique have recently been published, but
until long-term results show a definite improvement over cementless
femoral revision with regards to restoration of bone stock, its use
will remain limited to a few dedicated revision centers.
segmental proximal femoral bone loss that is inadequate to provide
initial stability for conventional revision femoral stems. Allograft
prosthetic composites, where the prosthesis is cemented into the
allograft and has a porous-coated surface for bony ingrowth of host
bone where available, allows better soft tissue attachment than does an
all-metal tumor prosthesis. A tumor prosthesis is reserved for the
older low-demand patients who need a faster operation and earlier
weight-bearing mobilization.
term have been good, but there remains a high rate of dislocation and
infection. Healing of the allograft-host bone junction usually requires
3 to 6 months of protected weight bearing. Segmental allografts remain
largely avascular, with a zone of revascularization only extending for
1 to 2 cm into the allograft from the junction with host bone. Greater
trochanteric union to an allograft with a stable fibrous or bony
interface occurs in approximately 70% of cases.
GM, High WA, Morrey BF. Dislocation after revision total hip
arthroplasty: an analysis of risk factors and treatment options. J Bone
Joint Surg 2002;84A(10):1788-1792. A database of
1,548 patients was evaluated for the risk factors leading to
instability after revision THA. The expected outcome of various
treatment strategies is discussed.
replacement is now much improved with instrumentation for
nontrochanteric osteotomy approaches and off-the-shelf components in 1
mm increments. For arthritic hips, a new era of surface replacement has
emerged. With metal-on-metal bearings, the volumetric wear has been
reduced 20-100 times from those with polyethylene, and there is no
penalty for the large ball size. The devices are now conservative on
the acetabular as well as femoral side. Hybrid or all-cementless
fixation is superior to earlier all-cemented devices.
U, Ozturk MH. MR imaging of the acetabular labrum: a comparative study
of both hips in 180 asymptomatic volunteers. Eur Radiol
2001;11(4)567-574. The aim of this study was to
determine the MR imaging characteristics of normal acetabular labra on
both hips. The most common labrum shape was triangular, whereas absence
of labrum was the least common condition. A difference of labral shapes
between both hips was present in approximately 15% of volunteers. A
size difference of over 25% between each labrum was noted in
approximately one-fourth of 180 volunteers.
YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip
worthwhile? a prospective review of 328 adults investigated for hip
pain. J Bone Joint Surg 1999;81B(4):600-603. Arthroscopy
altered the diagnosis in 176 hips (53%). The new primary diagnoses were
osteoarthritis (75 patients), osteochondral defects (34), torn labra
(23), synovitis (11), and loose bodies (9). In 172 hips (52%), an
operative procedure was undertaken.
RL, Paprosky W, Butler RA et al. Patients’ perception of pain after
total hip arthroplasty. J Arthroplasty 2000;15(5): 590-596. Type
of stem fixation was the only parameter statistically correlated with a
higher incidence of thigh pain. Patients with proximallycoated stems
were more than twice as likely to complain of pain than patients with
fully coated or cemented hips.
RA, Jacobs JJ, Quigley LR et al. Primary cementless acetabular
reconstruction in patients younger than 50 years old. 7- to 11-year
results. Clin Orthop 1997;344:216-226. During
this study, two stable acetabular reconstructions were revised during
femoral revision. Two excessively worn polyethylene liners were
exchanged and one acetabular osteolytic area was debrided and grafted;
these procedures retained the metal shell. At final follow-up, all 72
acetabular reconstructions were radiographically stable. Acetabular
osteolysis occurred in five cases (7.4%), from 84 to 104 months.
postoperative dislocations and first or second dislocations usually are
treated with closed reduction and a hip guide brace or hip spica cast,
but when dislocation becomes recurrent, surgical treatment usually is
needed. When possible, surgical treatment is based on identifying and
treating a specific problem leading to the dislocation,
DJ, Lewallen DG, Hanssen AD et al. Pelvic discontinuity in revision
total hip arthroplasty. J Bone Joint Surg 1999; 81A(12):1692-1702. Twenty-seven
patients (31 hips) with a pelvic discontinuity are described.
Twenty-seven were reconstructed in an attempt to obtain a stable
construct, often supplemental plate fixation of the posterior column.
Nine of the 27 reconstructions required a further operation, showing
just what a difficult problem this can be to treat.
JD, Jacobs JJ, Tanzer M et al. The susceptibility of smooth implant
surfaces to periimplant fibrosis and migration of polyethylene wear
debris. Clin Orthop 1995;311:21-39. A landmark
paper where an animal model of a femoral stem implant showed that non
circumferentially coated implants allow access of wear debris to the
distal femur, causing osteolysis.
RB, Rorabeck CH, Skutek M et al. The Harris Design-2 total hip
replacement fixed with so-called second-generation cementing
techniques: a ten to fifteen-year follow-up. J Bone Joint Surg
1998;80A(12):1775-1780. Excellent results were
achieved with second generation cementing technique, where 186 of 195
acetabular cups were still in situ at 12 years.
experimental study to determine if the slight lengthening (<2cm)
often associated with THA altered forces across a replaced hip. On the
side of the replaced hip, mean peak intersegmental resultant hip forces
were actually decreased by 6 to 12%.
JJ, Forest EE, Olejniczak JP et al. Charnley total hip arthroplasty in
patients less than fifty years old: a twenty to twenty-five-year
follow-up note. J Bone Joint Surg 1998; 80A(5):704-714. Seventy
of the 72 hips in the living patients were followed radiographically
for at least 20 years. Twenty-seven hips (29%) had a revision or a
resection of the prosthesis during the follow-up period. Eighteen
acetabular components (19%) and 5 femoral components (5%) were revised
because of aseptic loosening, and an additional 14 acetabular
components (15%) and 7 femoral components (8%) demonstrated definite or
probable radiographic loosening. This landmark study demonstrates the
long-term durability of total hip arthroplasty performed with cement in
an active population of patients, but demonstrated that long-term
fixation of a cemented acetabular prosthesis remains the weak link.
JC, Harris WH. The Harris-Galante porous-coated acetabular component
with screw fixation: an average ten-year follow-up study. J Bone Joint
Surg 1999;81A(1):66-73. One hundred and
ninety-six hips in 177 patients were followed up at a mean of 122
months (range, 84 to 155 months). The average age of these 177 patients
at the time of the operation was 59 years (range, 23 to 87 years).
Eight well-fixed acetabular shells (4%) were revised: 3 were revised
because of dissociation of the liner in association with fractures of
the tines, 3 were revised during revision of the femoral component, and
2 were revised because of retroacetabular osteolysis. In 8 other hips,
the acetabular liner was exchanged during revision of a loose femoral
component. These 10-year results of a cementless acetabular component
equal or better those of a cemented acetabular component.
JT, Harris WH. Postoperative mortality after total hip arthroplasty: an
analysis of deaths after two thousand seven hundred and thirty-six
procedures. J Bone Joint Surg 1998; 80A(9):1291-1294. A
retrospective review of mortality as many as 90 days after 2,736
primary and revision total hip arthroplasties performed in 2,002
patients by one surgeon at a teaching hospital. All but 71 of the
patients had received prophylaxis against venous thromboembolic
disease. There were no intra-operative deaths, and no events during the
operation could be linked directly to postoperative mortality. Eight
deaths (mortality rate, 0.3%) occurred within 90 days after the 2,736
procedures. Four deaths (mortality rate, 0.15%) occurred during the
initial hospitalization.
HA, Rorabeck CH, Bourne RB et al. Instability in primary total hip
arthroplasty with the direct lateral approach. Clin Orthop
2001;393:168-180. One thousand five hundred and
fifteen primary total hip arthroplasties done via a direct lateral
approach were reviewed. At the most recent examination, 11.6% of the
patients had a moderate or severe limp and 2.5% had severe heterotopic
ossification. Only six hips (0.4%) had a dislocation or episode of
instability. Three patients had more than one dislocation and required
revision surgery.
JE, Sychterz CJ, Young AM et al. Characterization of long-term
femoral-head-penetration rates: association with and prediction of
osteolysis. J Bone Joint Surg 2000;82A(8):1102-1107. Polyethylene
wear of 48 hips was measured. The true wear rate averaged 0.18 mm per
year (range, 0.01 to 0.44 mm per year). Osteolysis at 10 years was
strongly associated with increasing true wear rates (p < 0.001).
Osteolysis did not develop in any of the nine hips with a true wear
rate of less than 0.1 millimeter per year. However, osteolysis
developed in 9 (43%) of 21 hips with a rate between 0.1 and less than
0.2 millimeter per year, in 8 of 10 hips with a rate between 0.2 and
0.3 millimeter per year, and in all 8 hips with a rate of greater than
0.3 millimeter per year.
S, Hankemayer S, Muller ME. Nerve palsy after leg lengthening in total
replacement arthroplasty for developmental dysplasia of the hip. J Bone
Joint Surg 1999;81B(5):843-845. In 508 THA for
dysplasia, there were 8 nerve palsy’s (2 femoral, 6 sciatic), 2
complete and 6 incomplete. No statistical correlation between the
amount of lengthening and the incidence of nerve damage was found (p =
0.47), but in 7 of the 8 hips, the surgeon had rated the intervention
as difficult because of previous surgery, severe deformity, a defect of
the acetabular roof, or considerable flexion deformity.
CA, Hooten JP, Zettl-Schaffer KF et al. Evaluation of bone ingrowth in
proximally and extensively porous-coated anatomic medullary locking
prostheses retrieved at autopsy. J Bone Joint Surg 1995;77A(6):903-910.
Three proximally (40%) and 5 extensively (80%)
porous-coated anatomic medullary locking femoral components were
retrieved from 7 cadavers at autopsy. All 8 components had some bone
growth into the porous space. A mean of 35% of the surface of the
implants had bone ingrowth. In the areas where bone was present, 67% of
the available porous space on the extensively coated stems and 74% on
the proximally coated stems contained bone.
R, Gill TJ, Gautier E et al. Surgical dislocation of the adult hip a
technique with full access to the femoral head and acetabulum without
the risk of avascular necrosis. J Bone Joint Surg
2001;83B(8):1119-1124. A technique is described
for operative dislocation of the hip, based on detailed anatomical
studies of the blood supply. It combines aspects of approaches that
have been reported previously and consists of an anterior dislocation
through a posterior approach with a “trochanteric flip” osteotomy. The
external rotator muscles are not divided and the medial femoral
circumflex artery is protected by the intact obturator externus. It
allows the treatment of a variety of conditions, which may not respond
well to other methods including arthroscopy.
detailed discussion of acetabular defects and their treatment with
allografts. Surgical technique is evaluated, and the results of the
author’s vast clinical experience in this area is presented.
FS, Garbuz DS, Masri BA, et al. Structural proximal femoral allografts
for failed total hip replacements: a minimum review of five years. J
Bone Joint Surg 2000;82B(6):830-836. A series of
40 proximal femoral allografts performed for failed total hip
replacements with a minimum follow-up of 5 years is analyzed. In all
cases the stem was cemented into both the allograft and the host femur.
The proximal femur of the host was resected in 37 cases. There were
four early revisions (10%), two for infection, one for nonunion of the
allograft-host junction, and one for allograft resorption noted at the
time of revision of a failed acetabular reconstruction. Junctional
nonunion was seen in three patients (8%), two of whom were managed
successfully by bone grafting, and bone grafting and plating
respectively. Instability was observed in four (10%). Trochanteric
nonunion was seen in 18 patients (46%) and trochanteric escape in 10 of
these (27%). Although a high radiological complication rate, these
results are good when severity of the cases is considered.
JJ, Skipor AK, Patterson LM et al. M etal release in patients who have
had a primary total hip arthroplasty: a prospective, controlled,
longitudinal study. J Bone Joint Surg 1998;80A(10):1447-1458. This
study showed that, 36 months postoperatively, patients who have a well
functioning prosthesis with components containing titanium have as much
as a threefold increase in the concentration of titanium in the serum
and those who have a well functioning prosthesis with cobalt-alloy
components have as much as a fivefold and an
eightfold increase in the concentrations of chromium in the serum and
urine, respectively. The predominant source of the disseminated
chromium-degradation products is probably the modular head-neck
junction and may be a function of the geometry of the coupling. Passive
dissolution of extensively porous-coated cobalt-alloy stems was not
found to be a dominant mode of metal release.
RP, Callaghan JJ, Sullivan PM et al. Long-term results of revision
total hip arthroplasty with improved cementing technique. J Bone Joint
Surg1997;79B(2):322-326. Eighty one consecutive
cemented revision THA using improved cementing techniques were
analyzed. The average age of the patients at revision was 63.7 years.
At the final follow-up 18 hips (22%) had had a reoperation, two (2.5%)
for sepsis, three (4%) for dislocation and 13 (16%) for aseptic
loosening. The incidence of rerevision for aseptic femoral loosening
was 5.4% and for aseptic acetabular loosening 16%. These results
confirm that cemented femoral revision is a durable option in revision
hip surgery when improved cementing techniques are used, but that
cemented acetabular revision is unsatisfactory.
SS, Lachiewicz PF, Hickman JM et al. Relationship of femoral head and
acetabular size to the prevalence of dislocation. Clin Orthop
1998;355:163-170. Femoral head size (22 mm) and acetabular component outer diameter (≥56 mm) were found to increase the risk of dislocation.
of static and dynamic contractures around the hip provides significant
immediate benefits for the patient and accelerates postoperative
rehabilitation. Knee pain is decreased, groin pain is eliminated, range
of motion of the hip is increased, and functional leg length difference
is reduced. This article emphasizes the importance of techniques used
to ensure soft tissue balance.
WJ, Herzwurm P, Paprosky W et al. Treatment of pelvic osteolysis
associated with a stable acetabular component inserted without cement
as part of a total hip replacement. J Bone Joint Surg
1997;79A(11):1628-1634. Thirty-five patients who
had had a primary total hip replacement with a porous-coated acetabular
component inserted without cement had a revision procedure to treat
pelvic osteolysis. The metal shell was left in place and the acetabular
liner was exchanged in all 35 patients. The osteolytic lesions were
debrided, and 35 of the 46 lesions were filled with allograft bone
chips. Evaluation at a minimum of 2 years (range, 2 to 5 years; mean,
3.3 years) after the revision operation, showed all 35 sockets were
found to be radiographically stable.
WF, Harris WH Revision total hip arthroplasty with use of so-called
second-generation cementing techniques for aseptic loosening of the
femoral component: a fifteen-year-average follow-up study. J Bone Joint
Surg 1996;78A(3):325-330. Thirty-five hips had
revision of the femoral component with so-called second generation
cementing technique. At an average duration of follow-up of 15.1 years,
seven (20%) had a repeat revision of the femoral component because of
aseptic loosening. These results support the concept that so-called
second-generation cementing techniques have decreased the prevalence of
aseptic loosening after femoral revision.
S, Ninomiya S, Takatori Y et al. Long-term outcome of rotational
acetabular osteotomy: 145 hips followed for 10-23 years. Acta Orthop
Scand 1998;69(3):259-265. The long-term outcome
of rotational acetabular osteotomy in 145 dysplastic hips of 131
patients after an average follow-up of 13 (10 to 23) years is
presented. The long-term outcome of rotational acetabular osteotomy was
satisfactory for a dysplastic hip with little, if any, osteoarthrosis,
but was unsatisfactory for a hip with more advanced osteoarthrosis.
WG, Perona PG, Lawrence JM. Acetabular defect classification and
surgical reconstruction in revision arthroplasty: a 6-year follow-up
evaluation. J Arthroplasty 1994;9(1):33-44. One
hundred and forty-seven cemented acetabular components were revised
with cementless hemispheric press-fit components, with an average
follow-up period of 5.7 years (range, 3 to 9 years). Acetabular defects
were typed from 1 to 3 and reconstructed with a bulk or support
allograft. Classification of the defects allows appropriate surgical
treatment. The results of treatment of each defect type are presented.
J, Johnson BG, Rowland C, et al. Thirty-day mortality after elective
total hip arthroplasty. J Bone Joint Surg 2001; 83A(10):1524-1528. Factors
that are associated with an increased risk of mortality within 30 days
after elective hip arthroplasty include an older age, male gender, and
a history of cardiorespiratory disease. There has been a significant
decline in the 30-day mortality rate after elective hip arthroplasty in
the last decade (p < 0.0002).
PM, Bostrom M, Poss R. Posterior approach to total hip replacement
using enhanced posterior soft tissue repair. Clin Orthop
1998;355:224-228. The two senior authors
independently began using an identical enhanced posterior soft tissue
repair after total hip replacement through a posterior approach. In the
first author’s experience, a dislocation rate of 4% in 395 patients
before using the enhanced closure was reduced to 0% in 395 patients in
whom the enhanced closure was performed. In the second author’s
experience, 160 total hip replacements had a dislocation rate of 6.2%
before the enhanced closure whereas 124 total hip replacements had a
dislocation rate of 0.8% after the enhanced closure.
MA. The cemented acetabular component of a total hip replacement: all
polyethylene versus metal backing. Clin Orthop 1995;311:69-75. All-polyethylene
total hip replacements demonstrated statistically improved survival
rates as compared with the metal-backed acetabular components.
TP, Shepherd EF, Dorey FJ et al. The John Charnley Award: wear is a
function of use, not time. Clin Orthop 2000;381:36-46. Polyethylene
wear in 37 hip replacements was measured. Patient activity was assessed
with a pedometer, a step activity monitor, and a simple visual analog
scale. Joint use was related to wear at the 90% confidence level.
Without three recognized outliers, wear was highly correlated to use.
TP, Noordin S, Amstutz HC. Update on nerve palsy associated with total
hip replacement. Clin Orthop 1997;344: 188-206. Overall
prevalence of nerve palsy following THA is approximately 1%. The
sciatic nerve, or the peroneal division of the sciatic nerve, is
involved in nearly 80% of cases. The risk of nerve palsy in association
with total hip replacement is increased for female compared with male
patients, with a diagnosis of developmental dysplasia, and with
patients undergoing revision surgery.
TP, Callaghan JJ. Current concepts review wear in total hip and knee
replacements. J Bone Joint Surg 1999; 81A(1):115-136. This article is a very comprehensive review of the current literature concerning wear of the primary bearing surface in THA.
AS, Hasselman CT, Rubash HE. The John Charnley Award: inhibition of
wear debris mediated osteolysis in a canine total hip arthroplasty
model. Clin Orthop 1997;344:33-43. Oral
bisphosphonate therapy was found to significantly inhibit wear debris
mediated bone resorption when evaluated in a canine total hip
replacement model.
DG, Plakseychik AY, Rubash HE. Free vascularized fibula grafting for
the treatment of osteonecrosis of the femoral head. Clin Orthop Rel Res
1997;344:243-256. Eighty-eight hips that received
free vascularized fibula grafting for treatment of osteonecrosis of the
femoral head are described. The hip survival rate for subgroups at 5.5
years was 100% for stages IC and IIA, 94% for stage IIB, 50% for stage
IIC, 80% for stage IIIB, 58% for stage IIIC, 72% for stage IVA, and 58%
for stage IVB.
GM, Fitzgerald RHJ. Total hip arthroplasty in the ankylosed hip: a
ten-year follow-up. J Bone Joint Surg 1988;70A(7): 963-966. Failure
of the total hip arthroplasty performed for an ankylosed hip was more
common (p < 0.05) in the patients who had had a previous surgical
attempt at arthrodesis and in the patients who were 50 years old or
less at the time of the arthroplasty.
D, Heinecke, A. Current concepts review: acetabular and femoral
anteversion: relationship with osteoarthritis of the hip. J Bone Joint
Surg 1999;81A(12):1747-1770. A clinical and theoretical examination of why osteoarthritis occurs in hips of a certain geometry is presented.
RT, Ekkernkamp A, Ganz R et al. Periacetabular and intertrochanteric
osteotomy for the treatment of osteoarthrosis in dysplastic hips. J
Bone Joint Surg 1995;77A(1):73-85. Forty-two
patients who underwent a periacetabular with or without an
intertrochanteric femoral osteotomy for dysplastic hips. Mean age was
37 years (11 to 57 years). At mean follow-up of 4 years, six patients
had a subsequent total hip arthroplasty and three patients had an
additional intertrochanteric osteotomy. Five of the nine patients who
had a second major operation had had grade-3 osteoarthrosis before the
periacetabular osteotomy.
JR, Coogan PG, Gunneson EB et al. Treatment of osteonecrosis of the
femoral head with free vascularized fibular grafting: a long-term
follow-up study of one hundred and three hips. J Bone Joint Surg
1995;77A(5):681-694. The results for 103
consecutive hips (89 patients) that had been treated with free
vascularized fibular grafting because of symptomatic osteonecrosis of
the femoral head were reviewed in a prospective study. By the time of
the most recent follow-up evaluation (minimum 5 years), a total
arthroplasty had been performed in 31 hips.
BM, Siney PD, Fleming PA. Charnley low-frictional torque arthroplasty
in patients under the age of 51 years: follow-up to 33 years. J Bone
Joint Surg 2002;84B(4):540-543. A group of 1,092
patients under the age of 51 years at the time of surgery, underwent
1,434 primary Charnley low-frictional torque arthroplasties and were
followed up indefinitely. The mean follow-up was 15 years (11 to 33
years). The incidence of deep infection for the whole group was 1.67%.
The indication for revision was aseptic loosening of the cup (11.7%),
aseptic loosening of the stem (4.9%), a fractured stem (1.7%), deep
infection (1.5%), and dislocation (0.4%).
TI, Bradford MS, Magnus RE et al. Extended proximal femoral osteotomy:
a new technique for femoral revision arthroplasty. J Arthroplasty
1995;10(3):329-338. An osteotomy technique for
removal of distally fixed cemented and cementless femoral components is
described. The anterolateral proximal femur is cut for one-third of its
circumference, extended distally, and levered open on an anterolateral
hinge of periosteum and muscle. This creates an intact muscle-osseous
sleeve composed of the gluteus medius, greater trochanter,
anterolateral femoral diaphysis, and vastus lateralis, and exposes the
fixation surface as well as distal cement. The first 20 patients
treated with this technique are reviewed.
