Legg-Calvé-Perthes Disease
young children that causes collapse of the femoral head. It closely
resembles avascular necrosis in its radiographic appearance. It is a
very common cause of hip, knee, and thigh pain in children ages 3 to 10
years, and is a frequent cause of limp in this age group. Although it
has been recognized as a distinct entity for almost 100 years, its
exact etiology is not known, and the proper methods of treatment remain
controversial.
most current theories center on disruption of blood flow to the femoral
head. In the age group that is affected by Perthes disease, the blood
flow to the femoral head comes from a ring of vessels formed by the
medial and lateral circumflex arteries. The medial circumflex artery is
the primary source of blood flow to the posterior ring, and the
branches that come from the posterior ring give the majority of the
blood flow to the femoral head. The lateral ascending cervical vessel,
a termination of the medial femoral circumflex artery, is the most
important contributor of flow to the femoral head.
result of a single vascular occlusion, but multiple infarctions over
time. Single vascular insults in animal models have failed to produce
typical Perthes changes, but repeat vascular insults can produce the
changes. This has led investigators to question if patients with this
disorder are thrombophilic (i.e., experiencing thrombosis in the
vessels of the blood supply to the femoral head). The results have been
mixed. Studies by Eldridge and Glueck found a high percentage of
children with Perthes disease who had abnormal coagulation profiles.
Similar studies by other authors have found little tendency toward
thrombophilia in their patients with Perthes disease. Recent studies
have also implicated secondhand smoking as a risk factor for Perthes
disease.
ratio of 4 or 5 to 1. The peak incidence of the disease is between the
ages 4 to 8 years, although it presents as early as age 2 and may
present in children older than 10. Bilaterality is about 10%, but the
heads are usually in different stages of collapse. The overall
incidence in the United States is about 1 in 1,200 children.
their peers and some reports have found them to have a delay in their
bone age. There is a higher incidence of Perthes disease in children
who have a positive family history, but there is no current evidence
that it is an inherited condition.
with Perthes disease. The epiphysis shows areas of bone with avascular
necrosis, but there are also strands of cartilage that form clefts that
connect the articular surface to the physeal plate. This supports the
idea that this entity is different from avascular necrosis seen in the
adult population.
is a system that correlates radiographic appearance of the femoral head
and acetabulum in the healed stage to the long-term risk for
degenerative joint disease. It is based on the sphericity of the
femoral head and acetabulum. Higher Stulberg classes carry the worst
long-term results, with very early degeneration of class 5 hips.
Stulberg 1 and 2 hips generally have a good long-term prognosis.
widely used radiographic classification system for helping to determine
treatment and prognosis during the active
stage of the disease. It has replaced the Catterall method because it
is easier to apply, has better inter- and intraobserver reliability,
and has been shown to be a strong predictor of final clinical and
radiographic outcome.
space widening, increased density of the femoral epiphysis, and the
appearance of a subchondral fracture (the crescent sign).
the reossification phase, the femoral head and neck are left with
residual deformity, such as flattening of the femoral head or coxa
magna.
|
TABLE 6-1 STULBERG CLASSIFICATION OF RADIOGRAPHIC FINDINGS IN PERTHES DISEASE
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
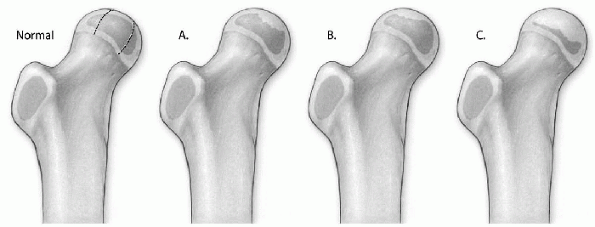
Figure 6-1
The Herring lateral pillar classification of Perthes disease. Normal: In the diagram of the normal hip, the femoral head is divided into three pillars. The lateral pillar occupies the lateral 15% to 30% of the femoral head. The central pillar represents about 50% of the head width, and the medial pillar occupies 20% to 35% of the head width. (A) In group A, the lateral pillar is radiographically normal, even though there is collapse in the central or medial pillars. (B) In group B, there is some collapse of the lateral pillar, but it maintains 50% to 100% of the height of the normal lateral pillar. (C) In group C, there is more collapse of the lateral pillar, and the height is less than 50% of the height of the normal lateral pillar. |
|
TABLE 6-2 LATERAL PILLAR CLASSIFICATION
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
15% to 30% of the femoral head width on an anteroposterior radiograph
of the hip. Classification is based on several radiographs taken during
the early fragmentation stage. The radiograph with the greatest
involvement of the lateral pillar is used for classification (Fig. 6-1 and Table 6-2).
involvement have slower reossification, which leads to more femoral
head flattening. Patients in group A typically have a uniformly good
outcome with mainly Stulberg 1 and 2 results. Patients in group B have
a good outcome when
they
are less than 9 years of age at onset of disease, and patients in group
C have a poor prognosis regardless of the age at onset.
-
Thigh pain
-
Knee pain
-
Limp, with Trendelenburg gait
-
Rare complaints of “hip pain”
-
Decreased hip abduction
-
Decreased hip internal rotation
-
Slight limb length discrepancy
-
Positive Trendelenburg test
-
Joint space widening
-
Increased density of femoral epiphysis
-
Subchondral fracture, or “crescent sign,” seen on lateral radiograph
-
Fragmentation and flattening of head (Fig. 6-2)
-
Widening of the physis
-
Femoral neck cysts
-
Extrusion of the femoral head
 |
|
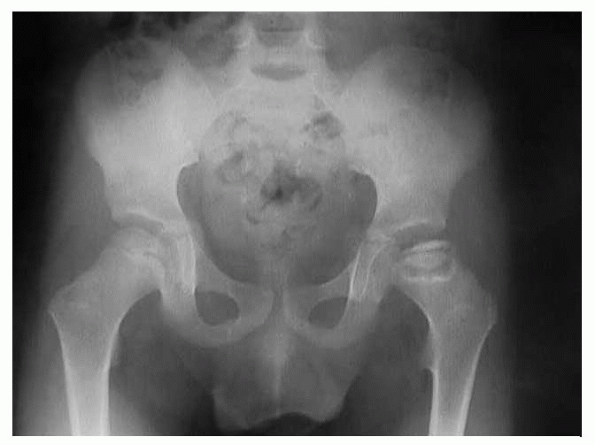
Figure 6-2 Flattening and sclerosis of the femoral head in Perthes disease.
|
-
Toxic synovitis
-
Multiple epiphyseal dysplasia
-
Spondyloepiphyseal dysplasia
-
Hypothyroidism
-
Gaucher disease
-
Sickle cell disease
-
Meyer dysplasia
-
Traumatic avascular necrosis
-
Coxa magna
-
High-riding trochanter
-
Flattened femoral head
-
Irregular articular surface
needed to make the diagnosis of Perthes disease. Although some authors
have proposed classification systems based on these studies, plain
radiographs remain the most important imaging studies for Perthes
disease at this time.
clinical presentation and radiographic appearance are typical, and the
diagnosis is not difficult. In the very early stages of disease, before
femoral head collapse, the disease may be mistaken for toxic synovitis (Box 6-2).
that both hips are in the exact same stages of collapse. Symmetric
Perthes changes should always raise the suspicion of a skeletal
dysplasia, or diffuse metabolic process. Multiple epiphyseal dysplasia
is a common cause of bilateral Perthes-like changes in the femoral
head. Meyer dysplasia is a poorly understood process that causes
collapse of the femoral head similar to Perthes disease, but the
disease runs its course quickly, and usually has a benign outcome.
treatment. Patients with lateral pillar group A disease tend to do well
without any formal treatment. Patients with group B disease who
maintain a good range of motion and do not show femoral head extrusion
may also need minimal intervention.
treatment, and patients in group B who present above the age of 8 years
or have significant pain and stiffness tend to benefit from active
treatment.
-
Restoration of range of motion.
-
“Containment” of the femoral head: The
concept of containment is to keep the femoral head within the limits of
the acetabulum as it reossifies. By keeping the head contained, it is
believed that the head will remodel with a more spherical contour.
Abduction braces such as the Scottish Rite brace have been used in the
past as a means of nonsurgical containment, but long-term studies have
failed to support their efficacy. Support can be found in the
literature for surgical containment by femoral, pelvic, or combined
osteotomies, with the use of the shelf for selected cases.
-
Nonsteroidal antiinflammatory drugs—to decrease synovitis in the hip
-
Physical therapy
-
Traction
-
Nighttime abduction splints
-
Petrie casts (long leg casts separated with an abduction bar and internally rotated)
-
Adductor tenotomy (often in conjunction with Petrie casts)
-
Varus (femoral) osteotomy
-
□ Reproducible results
-
□ Causes limb shortening
-
□ Residual abductor weakness
-
□ Second surgery required for hardware removal
-
-
Pelvic osteotomy
-
□ Usually a Salter osteotomy
-
□ Reproducible results
-
□ Lengthens limb
-
□ Technically more demanding
-
□ Requires good range of motion preoperatively
-
□ May cause loss of motion
-
-
Combined varus and pelvic osteotomy
-
□ Good for hips that need maximum containment
-
□ No significant shortening or lengthening
-
□ Increases operative time and blood loss
-
-
Shelf arthroplasty
-
□ Fewer clinical studies
-
□ Usually used in older patients (more than 8 years of age)
-
□ Good for hips that have large extrusion or hinge abduction that are not candidates for varus or pelvic osteotomy
-
T, Yamano K, Muraki M, et al. The blood supply of the lateral
epiphyseal arteries in Perthes disease. J Bone Joint Surg (Br)
2000;82:392.
CJ, Paterson JM, Woods KR, et al. Femoral osteotomy in Perthes’
disease. Results at maturity. J Bone Joint Surg (Br) 1990; 72:581.
J, Dilley A, Austin H, et al. The role of protein C, protein S, and
resistance to activated protein C in Legg-Perthes disease. Pediatrics
2001; 107:1329.
CJ, Crawford A, Roy D, et al. Association of antithrombotic factor
deficiencies and hypofibrinolysis with Legg-Perthes disease. J Bone
Joint Surg (Am) 1996;78:3.
S, Kenet G, Lubetsky A, et al. Does thrombophilia play an aetiological
role in Legg-Calve-Perthes disease? J Bone Joint Surg (Br) 1999;81:686.
JA. The treatment of Legg-Calve-Perthes disease. A critical review of
the literature. J Bone Joint Surg (Am) 1994;76:448.
JA, Neustadt JB, Williams JJ, et al. The lateral pillar classification
of Legg-Calve-Perthes disease. J Pediatr Orthop 1992;12:143.
RW, Guille JT, Bowen JR. Shelf arthroplasty in patients who have
Legg-Calve-Perthes disease. A study of long-term results. J Bone Joint
Surg (Am) 1991;73:1338.
PL, Angel D, Nelson JM. The Scottish Rite abduction orthosis for the
treatment of Legg-Perthes disease. A radiographic analysis. J Bone
Joint Surg (Am) 1992;74:2
BW, As her MA. Combined innominate and femoral osteotomy for the
treatment of severe Legg-Calve-Perthes disease. J Pediatr Orthop
1985;5:645.
PD, Desai SS, Millis MB. Comparison of femoral and innominate
osteotomies for the treatment of Legg-Calve-Perthes disease. J Bone
Joint Surg (Am) 1988;70:1131.
SL. Legg-Calve-Perthes syndrome. In Morrissy RT, Weinstein SL, eds. 4th
ed. Philadelphia: Lippincott-Raven, 1996:951-991.
