CONGENITAL HAND MALFORMATIONS
III – THE HAND > Reconstructive Procedures > CHAPTER 69 –
CONGENITAL HAND MALFORMATIONS
Associate Clinical Professor, Department of Orthopaedic Surgery,
University of California–Davis, Shriners Hospital for Children,
Northern California, Sacramento, California, 95817.
child’s use of the hand enables the development of intelligence, and
thereby language and culture (229). Remarkably,
the child whose hands are malformed or even absent but whose brain is
normal develops almost normally. As Southwood wrote in 1926 regarding
congenital deficiency of the ulna, “From the functional viewpoint… the
deformed limb is much more useful than its anatomical condition would
have led one to expect” (190, p. 349). Few surgical operations have
been conclusively proven to improve the function of the child’s
malformed hand, and surgeons tend to take credit for improvement that
occurs because of growth and development.
with a malformed hand is the psychological adjustment to the hand’s
appearance (17). The hand surgeon is an
educator and counselor, and when the family and surgeon share the goals
of treatment, everyone is more satisfied with the outcome.
days after fertilization. During the next 25 days, the upper limb is
completely differentiated (59,237).
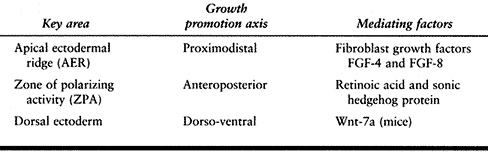
development determines the fate of that cell. Growth occurs along three
axes, and each growth axis is controlled by a key area of the upper
limb: the apical ectodermal ridge (AER) promotes growth along the
proximodistal axis; the zone of polarizing activity (ZPA) promotes
growth along the anteroposterior axis, which is radioulnar in the
embryo; and the dorsal ectoderm promotes growth along the dorsoventral
axis (175,237).
activity of these key areas. These include fibroblast growth factors
(FGF) expressed in the AER; FGF-8 may induce AER formation, and FGF-4
and FGF-8 together can replace AER function. Retinoic acid and sonic
hedgehog protein together mediate the activity of the ZPA. Mice lacking
Wnt-7a, a signaling molecule encoded by the Wnt gene family (and
expressed in the dorsal ectoderm), show alterations in dorsoventral
polarity (Table 69.1) (175,237).
These chemical signals interact with each other infeedback loops: Sonic
hedgehog induces the formation of an FGF, thereby influencing the AER,
and sonic hedgehog expression depends on Wnt-7a (175,237).
Chemical signals from the AER and ZPA also regulate the expression of
homeobox (Hox) genes, which control the expression of other genes,
confer positional information on different cell types, and determine
limb patterns such as digit identity (118,175).
 |
|
Table 69.1. Key Areas Controlling Upper Growth in the Embryo (175,237)
|
the AER, ZPA or dorsal ectoderm, or faulty genetic control of these
three essential control areas. In humans, researchers have localized
the genes responsible for some congenital hand malformations, including
preaxial polydactyly (237); in mice, this same
malformation has been shown to be due to an additional ZPA on the limb
bud, and the mutant genes responsible for ZPA duplication have been
well mapped (134). Genetically programmed hand
malformations may eventually become preventable, once their mechanisms
are understood well enough to be manipulated.
congenital anomalies of the upper extremity varies between 3.4 and 16
cases per 10,000 live births (58,68,108,204).
Most congenital malformations are more common in boys. Anomalies may be
caused by genetic or environmental factors, or some combination of
these. Genetic malformations are caused by chromosomal abnormalities,
or single (mendelian) or multiple (polygenic) gene disorders. The
genetic addresses of several hand malformations (two types of
polydactyly, cleft hand and foot, and brachydactyly) are known (237).
The next step after genetic localization is identification of the
nucleotide sequence mutation; the genetic mutations causing several
different orthopaedic conditions have already been identified (40).
hand malformation team. He or she keeps up with the rapid accumulation
of new information in the field of molecular genetics, helps diagnose
anomalies outside the musculoskeletal system (which are associated with
more than 80% of heritable limb deficiencies [230]) and provides genetic counseling to families (32). Many hand anomalies are visible on prenatal ultrasound (18,39,77,170); genetic counseling is especially useful in this event.
etiology, but little is known about the etiology of many congenital
hand deformities. We know enough to understand that the same underlying
cause can have many different effects, and the same effect can have a
myriad of causes. Classification schemes also help the surgeon
determine the prognosis and treatment, provide common nomenclature to
describe observed conditions, and allow surgeons to communicate.
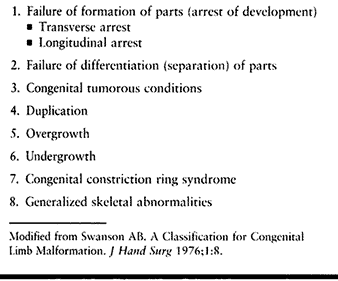
congenital upper limb malformations was originally proposed in 1968 by
Swanson, Barsky, and Entin (196), and adopted
in a modified form by the American Society for Surgery of the Hand and
the International Federation of Societies for Surgery of the Hand
(ASSH/IFSSH) in 1983 (Table 69.2) (195). Although this system has been used in at least two large studies of congenital hand anomalies (38,108), it is complex, many anomalies fit into more than one category, and some anomalies defy classification.
 |
|
Table 69.2. ASSH/IFSSH Classification of Congenital Upper Limb Malformations (195)
|
Malformation is an interruption of normal morphogenesis, following
which the affected structures fail to revert to normal form; most of
the conditions described in this chapter are malformations. Deformation
is the alteration in shape of structure that has differentiated
normally. Disruptions or disruption sequences are structural defects
resulting from destruction of a part that has differentiated normally,
such as constriction ring syndrome. Dysplasia is the abnormal growth or
differentiation of a structure.
parents and older children are extremely concerned about the appearance
of the malformed hand. They may hope that surgery can make the hand
appear normal, but normal appearance is possible only with certain
types of malformations. Surgery to alter appearance is elective. If the
outcome will be the same regardless of age, surgery should be postponed
until the child is older than 2 years of age, when general anesthesia
is safer (78). Surgery performed before the
child starts school may reduce teasing by other children and prevent
school absence. The parent and older child should share the surgeon’s
goals, and understand the risks and likely outcome of surgery.
and have a better outcome if they are performed early. For example,
thumb-index syndactyly release should be performed by the age of 6
months (65) and centralization for longitudinal deficiency of the radius by the age of 1 year (60).
In these cases, the increased risk of anesthesia is outweighed by the
benefits of surgery, if an experienced pediatric anesthesiologist is
available (78) and if the infant does not have
a bleeding disorder, heart anomalies, or other condition that further
increases anesthetic risk.
milestones in order to assess the functional abilities of a child with
a malformed hand. Palmar grasp with the thumb out of the palm begins at
around 5 months of age, with the wrist held in flexion. By 9 months,
most infants extend the wrist with grasp, and by 10 months they are
able to oppose thumb tip to index tip precisely and to control
voluntary release (59). When evaluating
functional results of an operation performed on the hand of a small
child, the surgeon should attribute to surgery only those functional
improvements that are beyond what would be expected for a child of the
same age with the same disability.
notice a hand difference in themselves, and early motor development is
unaffected by most upper extremity malformations. Infants with
transverse or longitudinal deficiencies use both upper limbs or
unilateral brachiothoracic grasp for prehension and may scoot instead
of crawling, but these alternative developmental pathways are not
deficiencies. Children in this age group adapt remarkably well to hand
malformations, and parents of older infants and toddlers with hand
malformations usually marvel at their child’s abilities.
transverse and longitudinal deficiencies may have difficulty performing
advanced dressing and hygiene activities (see the section entitled Occupational Therapy and Therapeutic Recreation).
They are in the process of developing a self-image. Surgery that
significantly changes the appearance of a malformed hand will be easier
psychologically for children younger than age 4 or older than age 8
years, because between these ages, they are old enough to understand
what is happening but not old enough to understand why. Assuming normal
intellectual development, after age 8 years children begin to gain the
ability to reason, make
stable
choices, and give assent to elective surgery. Giving assent refers to
the capacity of the child with developing decision-making skills to
understand the proposed operation and agree to proceed. Obtain the
assent of children older than age 8 years before proceeding with any
elective operation.
malformations is contraindicated, because postoperative bilateral
immobilization is frustrating for the younger child and disabling for
the older child. If the operations are small and do not require cast
immobilization, or if the child is very young for whom anesthesia poses
a high risk, the inconvenience of bilateral immobilization may be
outweighed by the benefit of simultaneous bilateral surgery. Long arm
(above-elbow) casts are used for the postoperative immobilization of
the hands of infants and younger children, who tend to wiggle out of
short arm casts.
operations used to reconstruct congenital hand malformations. The
surgeon should take care to avoid injuring the physis of a growing bone
unless the intent of the operation is to retard growth. Arthrodesis of
digital joints can be performed in children without damaging the physis
(105). Children with congenital hand
malformations should be reexamined periodically until they reach
skeletal maturity, to identify progressive deformities or recurrence of
surgically corrected abnormalities (109).
most physicians. Complex malformations are most effectively treated by
an experienced hand surgeon with the support of a congenital hand
malformation team. The hand surgeon directs the child’s care, with the
assistance and support of the nursing and social services staff, who
help educate the family and coordinate care. Other team members include
the occupational therapist (see later), the orthotist and prosthetist,
child life and therapeutic recreation specialists, a geneticist, a
pediatric anesthesiologist, and a medical librarian.
often feel bewildered and guilty, and underestimate their child’s
future abilities. The orthopaedic surgeon may be the first person
parents encounter who is knowledgeable about their child’s condition.
The surgeon can be very helpful to the parents if he or she listens to
them and answers their questions carefully and thoroughly. The first
visit with parents of an infant with a hand deformity cannot be rushed.
difficulty accepting their child’s malformation. Individual differences
in parental adjustment do not relate to the severity or type of
malformation, but rather to the amount of family support available (17)
and other factors. Many parents benefit from meeting an older child
with a similar malformation and his or her family. Parents and older
children who are planning to proceed with an operation that changes the
appearance of the hand, such as pollicization, may also benefit from
meeting a child who has undergone the same procedure. The congenital
hand malformation team can maintain a database of families willing to
be peer contacts, to facilitate matching parents and children with an
appropriate peer, and a list of family support groups for different
syndromes, internet addresses for relevant websites, and handouts
including Children with Hand Differences: A Guide for Families,* and Superkids newsletter.† The social worker can help refer families for psychological counseling when necessary.
functional abilities benefit from consultation with an occupational
therapist at around age 3 years and again when they are ready to start
school. On-going occupational therapy is indicated when the child is
not meeting developmental milestones or is unable to perform
age-appropriate activities of daily living. The child life therapist or
therapeutic recreation specialist can suggest play activities intended
to support the child’s self-esteem and help the family and child adapt
toys and recreational activities to the child’s abilities.
surgery. Supplement general anesthesia with local anesthetic, and
administer intravenous narcotic pain medication postoperatively until
the child can take oral pain medication. Most children require pain
medication for only 48 to 72 hours after surgery. Use a long arm
fiberglass cast shell to cover the postoperative bulky compressive
dressing
(Fig. 69.1).
If the cast does not turn a corner (the flexed elbow), the younger
child will wriggle out of it. Release the tourniquet at least 10
minutes before dressing and cast application, to confirm the
vascularity of the operated digit or digits and to allow for swelling.
Apply the cast without stretching the fiberglass casting tape; the
patient may be too young to complain of a too-tight dressing or cast.
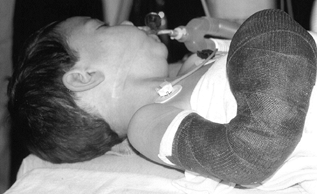 |
|
Figure 69.1. Long arm, flexed elbow fiberglass mitten cast shell, covering a bulky compressive hand dressing.
|
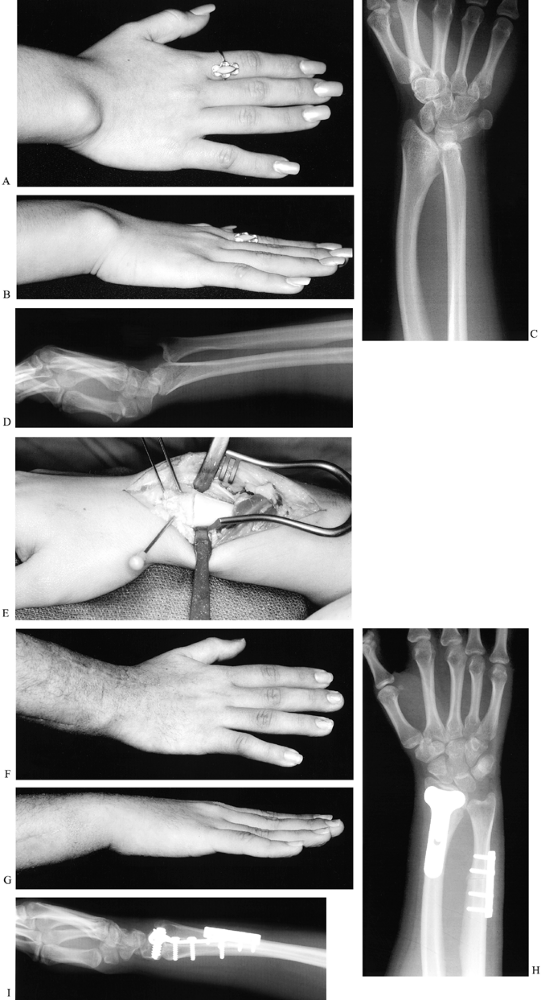
common congenital hand malformation (after syndactyly), and the thumb
is the most common duplicated or split digit (52). In China, thumb polydactyly is the most common congenital hand anomaly (86).
Although the incidence of thumb polydactyly is most often sporadic,
when it is associated with syndactyly, or when one or both of the bifid
thumbs is triphalangeal, it is more likely to be caused by a genetic
mutation; in these cases, it has been mapped to a specific gene (82,181). Polydactyly is caused experimentally by early disruption of the ZPA (237).
carpometacarpal joint (CMCJ), metacarpal, metacarpophalangeal joint
(MPJ), proximal phalanx, interphalangeal joint (IPJ), or distal
phalanx. Split thumbs are thinner and shorter than normal, and have
stiff joints, hypoplastic tendons with anomalous interconnections, and
abnormal vascular anatomy; thus, neither has completely normal function
(57). The ulnar thumb is usually larger and more functional than the radial thumb (57,158,179,222), but when the two “halves” are equal in size, or when both are triphalangeal, both are likely to be severely hypoplastic (85).
may have a wider than normal distal phalanx, with the remainder of the
thumb entirely normal; this condition does not require surgical
reconstruction. Wassel type I thumbs with duplication of the distal
phalanx, and Wassel type II thumbs may be treated with a combination
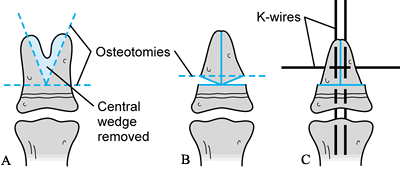
procedure, as described by Bilhaut (14).*
In this procedure, the surgeon removes a central wedge of tissue
(including bone, joint, physis, and nail bed and skin), and joins the
two remaining parts together. No large series of Bilhaut procedures has
been published. This procedure always causes a ridged nail, and if
performed in a more proximal duplication, joint stiffness and growth
arrest may occur (57,69,85,148,206).
Most authors agree that this operation should be reserved for distally
duplicated thumbs (Wassel type I or II) that are approximately equal in
size.
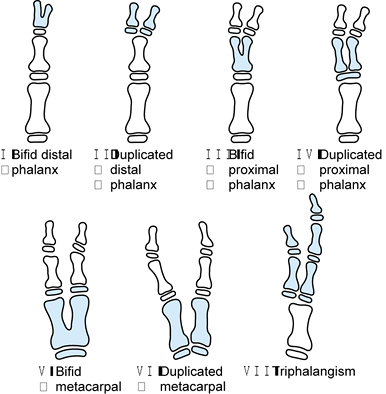 |
|
Figure 69.2. Wassel classification of thumb polydactyly.
|
split thumb is inadequate. Elements of the excised thumb, including
collateral ligament, skin, and tendons, are used to augment the
preserved thumb (29,57,85,122,158,206), to reduce the risk of angulation and joint instability (50,69,117,133,148,179,222).
Reconstruction includes collateral ligament reconstruction, tendon
realignment, and osteotomy, with the goals of stable and well-aligned
joints, with the physes perpendicular to the long axis of the thumb.
Even the carefully reconstructed thumb may
angulate
or develop joint instability with growth. Schedule follow-up visits for
the child until he or she is skeletally mature, because more surgery,
including osteotomy or an MPJ arthrodesis, may be necessary (96,98,105,122,148,158).
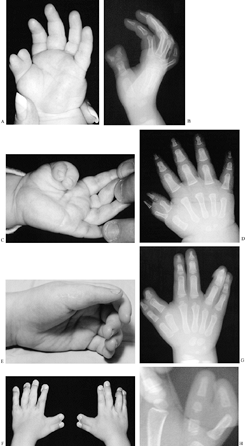 |
|
Figure 69.3. Thumb polydactyly. A: Type I. B: Type II (although the two distal phalanges are joined by cartilage at the base). C: Type IV, preoperative. D: Type IV. E: Type IV, postoperative. F: Type VI. G: Type VI. H: Type VII.
|
Light has also modified the classification described by Wassel,
focusing on clinically apparent differences; he points out that
Wassel’s illustration of type IV thumbs is inconsistent with the
remainder of the classification, because two proximal phalanges share a
single epiphysis (Fig. 69.2) (110).
 |
|
Figure 69.4. Horii et al.’s subdivisions of Wassel type IV thumb polydactyly into four types.
|
will always be smaller than the contralateral normal thumb, and that
further reconstructive surgery may be required. Describe the thumb as
split rather than as duplicated, to help parents understand why the
preserved thumb will not be normal (57).
there is only anecdotal evidence that the benefits of early surgery
outweigh the increased risk of general anesthesia in infants younger
than 1 year of age. If both thumbs are equal in size and the child uses
them both, postpone surgery until the child is old enough to undergo
functional testing to determine which thumb is the more functional. The
extrinsic tendon anomalies found at surgery usually cannot be
specifically diagnosed preoperatively, so be prepared to transfer
tendons and correct angulation by osteotomy.
abnormal. In most cases, each thumb has only one digital artery,
located on its ulnar side (102).
-
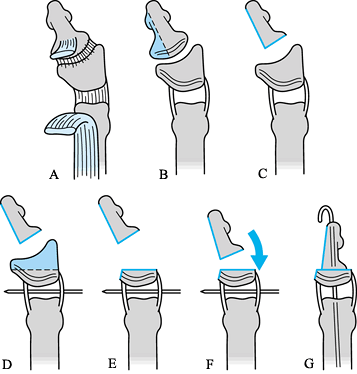
Exploration, ablation of the smaller thumb, reconstruction of the retained thumb: types I and II when one thumb is smaller (Fig. 69.5B).
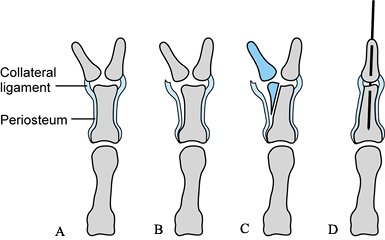 Figure 69.5. Ligamentous periosteal flap for the treatment of Wassel type II or IV thumb polydactyly See text for details.
Figure 69.5. Ligamentous periosteal flap for the treatment of Wassel type II or IV thumb polydactyly See text for details.-
Use loupe magnification, and perform the
operation with the patient under general anesthesia and tourniquet.
Have a surgical microscope available; it may be necessary to separate
the digital nerves of the two thumbs. -
Plan incisions to avoid a longitudinal incision along the radial border of the reconstructed thumb.
-
Through a dorsal zigzag incision, explore
the thumbs for tendon abnormalities. Detach the extensor tendon from
the smaller thumb. -
For type II thumbs, dissect the IPJ radial collateral ligament (RCL) off the distal phalanx of the thumb to be ablated (Fig. 69.5B). Preserve the IPJ-RCL attachment to the proximal phalanx; if necessary, extend it using a periosteal flap (Fig. 69.5C) (122).
-
If the nails are connected, remove a
portion of the nail bed, retaining an appropriate-sized nail for the
underlying distal phalanx. -
Remove the bone from the smaller thumb after detaching the flexor tendon at its insertion.
-
Separate the digital nerves to the two thumbs, and transect the nerve to the smaller thumb as far proximally as possible.
-
For type II thumbs, shave the distal
radial portion of the proximal phalanx. If the joint surface is
angulated, perform a closing wedge osteotomy of the proximal phalanx,
and fix it with a small longitudinal Kirschner wire crossing the IPJ (Fig. 69.5D). -
For type II thumbs, if the pull of the
retained extensor or flexor tendon angulates the thumb at the IPJ,
reinsert it or transfer the tendon from the ablated thumb to correct
the angle of pull. Otherwise, transect the tendon that was previously
attached to the ablated thumb -
For type II thumbs, reattach the IPJ-RCL using a nonabsorbable pullout suture.
-
Close the skin with 5-0 chromic suture, augmenting the reconstructed thumb with eponychium and nail bed from the ablated thumb.
-
Apply a bulky compressive dressing covered with a long-arm, flexed-elbow fiberglass cast shell.
-
-
Combination procedure: types I and II when the thumbs are the same size (Fig. 69.6 and Fig. 69.7).
![]() Figure 69.6.
Figure 69.6.
Modification of combination (Bilhaut) procedure, which avoids damage to
the physis and joint. See text for details. (Personal communication: H.
Relton McCarroll Jr., M.D.)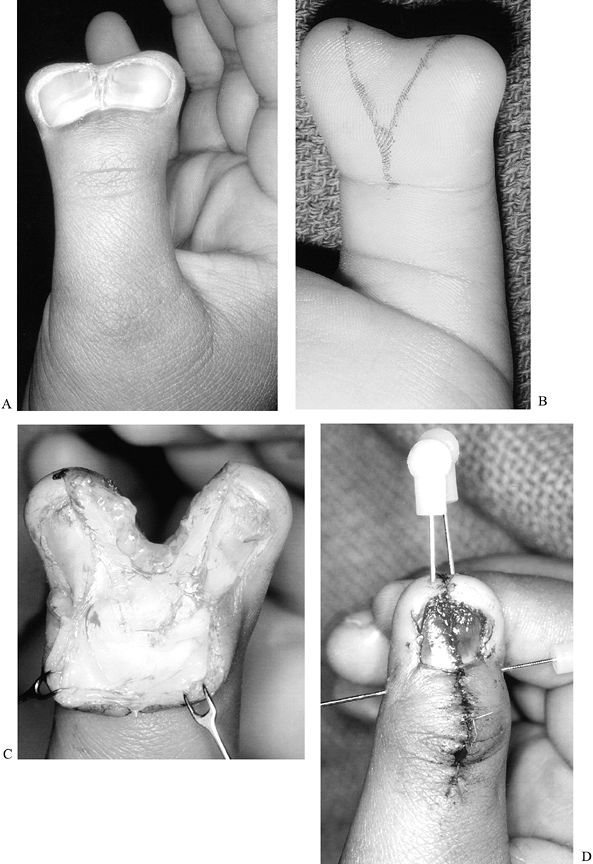 Figure 69.7. Modified combination (Bilhaut) procedure for type I thumb polydactyly. A: Preoperative dorsal view. B: Preoperative palmar view, with incision marked. C: Dorsal view, after central wedge resection. D: Postoperative dorsal view.P.1879P.1880
Figure 69.7. Modified combination (Bilhaut) procedure for type I thumb polydactyly. A: Preoperative dorsal view. B: Preoperative palmar view, with incision marked. C: Dorsal view, after central wedge resection. D: Postoperative dorsal view.P.1879P.1880-
Use loupe magnification, and perform the operation with the patient under general anesthesia and tourniquet.
-
Remove the nail plates. Resect a central
wedge of dorsal nail matrix, eponychium, and skin, and a similar-sized
wedge of palmar skin. Leave enough skin and soft tissue on either side
of the wedge so that when the two sides are approximated, the thumb is
nearly normal size (Fig. 69.6A). -
Using a small oscillating saw, resect the
central portions of the bone of the two thumbs, leaving enough bone so
that when approximated, the distal phalanx is nearly normal size. Do
not extend these osteotomies into the physis or joint. Instead, make a
transverse osteotomy just distal to the physis, to avoid the risk of
growth arrest and joint stiffness (Fig. 69.6B). -
Using 0.028 Kirschner wires, pin the two
pieces of distal phalanx together, then pin them both longitudinally to
the preserved base of the distal phalanx. Extend the longitudinal pins
across the IPJ (Fig. 69.6C). -
Approximate the nail bed and eponychium
using small absorbable suture. Replace the nail plate with
antibiotic-impregnated gauze if the original nail plate does not fit. -
Close the skin with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing, and
cover it with a long-arm, flexed-elbow fiberglass cast shell that
covers the tip of the thumb and the Kirschner wires.
-
-
Exploration, ablation of the smaller thumb, reconstruction of the retained thumb: type III and IV thumbs.
-
Proceed as described earlier for type I
and II thumbs when one is smaller, including tendon realignment and
soft-tissue combination. -
Do not resect the base of the proximal
phalanx for type III thumbs; preserve the physis and joint surface and
the MPJ-RCL of the radial thumb. -
For type IV thumbs, detach the MPJ-RCL
from the ablated thumb and reconstruct it, as described earlier for the
IPJ-RCL of type II thumbs.
-
-
MPJ arthrodesis: type II and IV thumbs,
if ligament reconstruction fails, resulting in painful thumb IPJ or MPJ
instability before skeletal maturity (105).-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Approach the joint from the dorsal aspect, if possible, or use incisions from previous operations.
-
Use a sharp scalpel to remove articular
cartilage sequentially from both joint surfaces. Expose the bony
ossification center of the epiphysis of the proximal phalanx, but do
not proceed beyond the ossific nucleus into the physeal plate. Expose
the cancellous subchondral bone of the distal end of the metacarpal. -
Oppose the two cancellous articular
surfaces with the joint in neutral position, and hold them together
with two crossed Kirschner wires. -
Close the skin with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing over the fingers and thumb, and cover it with a fiberglass cast shell.
-
radiographs. Remove buttons and pullout sutures, and remove Kirschner
wires after checking for bony union on radiograph (usually 6 to 8
weeks). Postoperative therapy is not necessary. Provide a custom-made
splint when the IPJ or MPJ-RCL has been reconstructed for the child to
wear at night until 3 months after surgery.
the principles described earlier, angulation and instability will occur
with growth. These complications also occur when the retained thumb is
especially hypoplastic, even if it is carefully reconstructed.
it is difficult to find room for two pullout sutures and a Kirschner
wire in a tiny thumb. The operation is easier when the child is at
least 1 year old, when the hand is considerably bigger.
function of the reconstructed thumb if they can accept that it is not
expected to be quite normal.
radial deficiency, which may involve the thumb alone, or the entire
radial hand, wrist, and forearm. This condition is rare, (1:30,000 to
1:100,000 live births) (60), frequently
bilateral, and often associated with congenital anomalies of the lower
extremities, spine and other organ systems (including the
cardiopulmonary, gastrointestinal, and genitourinary systems) (92). Several syndromes are
characterized by hypoplastic or absent thumbs, usually combined with radius deficiency, including the VACTERL association (Vertebral, Anal, Cardiac, Tracheo-Esophageal, Renal or Radial, Lung ), Holt-Oram syndrome and thrombocytopenia-absent radius (TAR) syndrome (see the section entitled Radius Deficiency) (92).
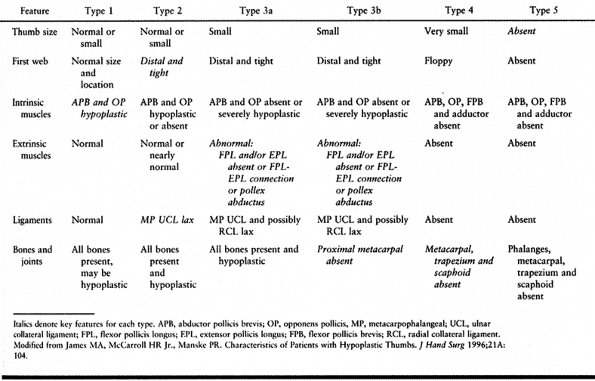
presence or absence of thenar intrinsic muscles, MPJ—ulnar collateral
ligament (UCL) stability, extrinsic muscles (flexor and extensor
pollicis longus), and CMCJ stability. There are six types of thumb
deficiency (Table 69.3).
 |
|
Table 69.3. Modified Blauth Classification (121)
|
reconstruction. The function of type 2 thumbs is enhanced by first web
deepening, MPJ-UCL reconstruction, and opponensplasty (63,103).
The abductor digiti minimi (ADM) muscle or the middle or ring flexor
digitorum superficialis (FDS) can be transferred to provide opposition (63,128,194).
Type 3a thumbs benefit from extrinsic thumb muscle realignment,
transfer or reconstruction if passive IPJ motion is present, in
addition to the procedures recommended for type 2 thumbs (75,130).
After reconstruction, type 2 and 3 thumbs function well, although they
are always smaller and weaker than a normal thumb. The child with a
type 2 or 3a deficiency recognizes the thumb and is able to use it to a
limited extent. Surgical reconstruction can be postponed until the
child is able to use the enhanced ability to manipulate small objects,
around 2 to 4 years of age; older children also benefit. The MPJ of
type 3a thumb is globally unstable and may eventually require
arthrodesis.
only in cultures in which the retention of a five-digit hand is of
primary importance (130,151), because it requires multiple operations (including vascularized [151] or nonvascularized [207] bone graft from the patient’s foot), and the final functional result is inferior to thumb ablation and index pollicization (23,103).
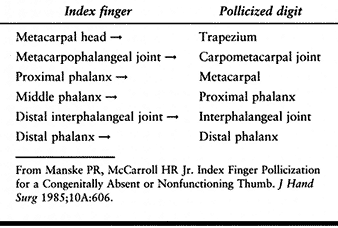
Index pollicization improves this pinch. The index finger is shortened
by removal of the index metacarpal and rotated so that it can function
as a thumb, and the hypoplastic thumb is removed (Table 69.4).
This procedure requires the surgical rearrangement of the skin,
skeleton, muscles, nerves, and blood vessels. Carefully plan and
mobilize
skin flaps to create a first web of appropriate depth, extending from
the MPJ of the new thumb to the MPJ of the long finger. A pollicized
index finger functions like a thumb (124), but the base is less mobile and stable; the appearance is usually quite satisfactory (Fig. 69.8 and Fig. 69.9).
Increasing severity of thumb hypoplasia is associated with increased
stiffness of the more radial fingers, especially the index finger;
pollicization of the stiff index finger has a less satisfactory outcome
than the same procedure performed on a more flexible finger.
 |
|
Table 69.4. Pollicization—Index Finger (129)
|
 |
|
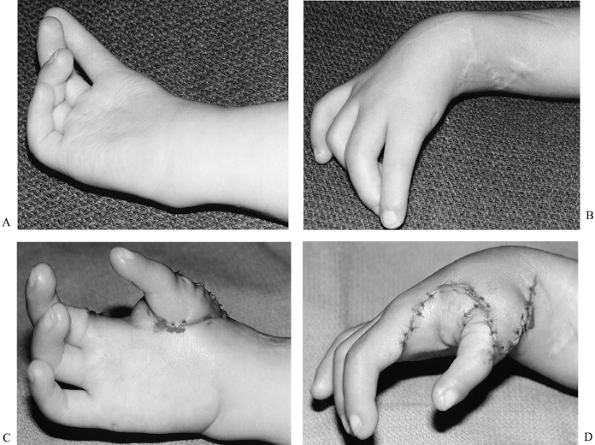
Figure 69.8. Index pollicization (this patient has already undergone centralization of the carpus on the ulna; see Fig. 69.48). A: Preoperative palmar view. B: Preoperative dorsal view. C: Postoperative palmar view. D: Postoperative palmar view.
|
 |
|
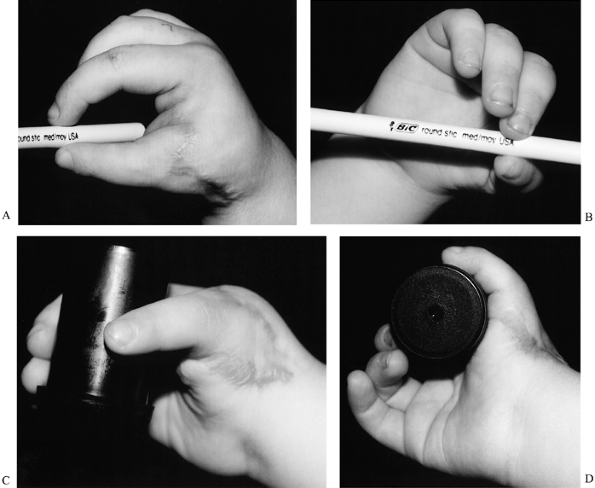
Figure 69.9. Index pollicization (different patient from the one shown in Fig. 69.8). A: Tip pinch using new thumb. B: Tip pinch showing pronation of new thumb. C and D: Grasp using new thumb.
|
argue for pollicization in the second 6 months of life, to allow the
child to recognize the pollicized digit as a true thumb, and to give
the former index finger the maximum amount of growth and development
possible in its new position. Manske (124)
has shown, however, that enhanced hand function following pollicization
is not age dependent. Although pollicization is performed primarily to
enhance function, it significantly changes the appearance of the hand;
the most significant benefit of early surgery may be that this change
occurs before the child has a fully developed self-image.
mental retardation or developmental delay. This is a relative, but not
absolute, contraindication to thumb reconstruction or pollicization. If
the surgeon, occupational therapist, and parent agree that the thumb
deficiency limits the child’s function, the operation appropriate for
the type of thumb hypoplasia (reconstruction or pollicization) is
indicated.
modification of the Blauth scheme, which helps determine prognosis and
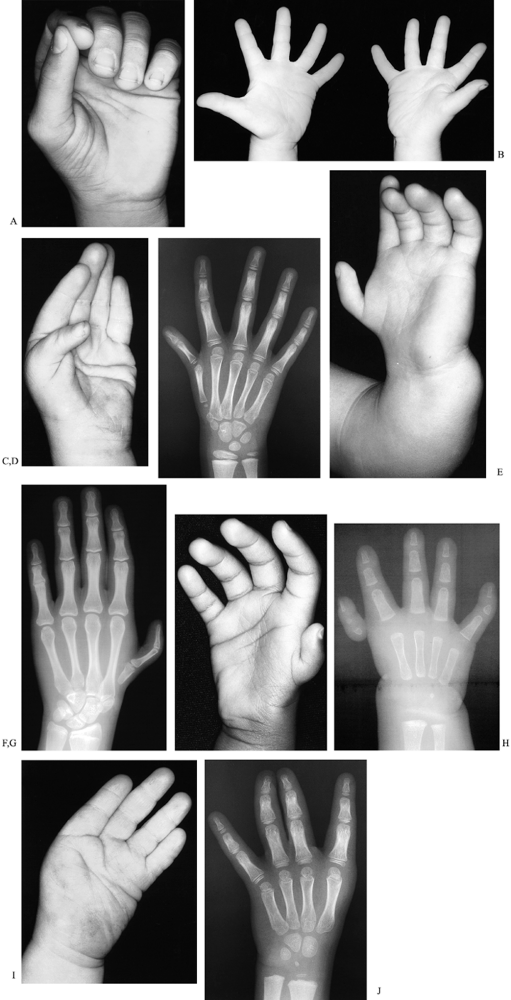
treatment. (Table 69.3 and Fig. 69.10) (16,63,92,103,130). The thumbs of children with TAR syndrome do not fit this classification scheme (91). This classification can be used in combination with a modification of the Bayne scheme for radius deficiency (Table 69.11) (91).
 |
|
Figure 69.10. Thumb deficiency: modified Blauth classification. A: Type 1, with mild flattening of thenar eminence. B: Type 2 (right hand). C: Type 3a, with lack of flexion at distal interphalangeal joint. D: Type 3a, with base of the thumb metacarpal present. E: Type 3b. F: Type 3b, with base of thumb metacarpal absent. G: Type 4. H: Type 4. I: Type 5. J: Type 5.
|
may increase the risk of general anesthesia; postpone surgery until
general anesthesia is safe.
centralization, perform centralization before index pollicization (see
the section entitled Radius Deficiency). Hands
with thumb hypoplasia amenable to reconstruction (types 2 and 3a)
usually do not have radius deficiency requiring centralization (91).
or 3a thumb may benefit from the application of a soft short opponens
splint to preposition the thumb. If the child refuses to use the
splint, it is probably not helpful and can be discarded.
using side-to-side pinch between the index and long or ring and small
fingers. The development of this type of pinch is associated with more
severe thumb hypoplasia and is an indication that the thumb is probably
not amenable to reconstruction; the child would benefit from thumb
ablation and index pollicization (23).
-
Reconstruction of the type 2 hypoplastic thumb: fourfold Z-plasty, MPJ-UCL reconstruction and ADM opponensplasty (Fig. 69.11, Fig. 69.12 and Fig. 69.13)
![]() Figure 69.11. Fourfold Z-plasty.
Figure 69.11. Fourfold Z-plasty.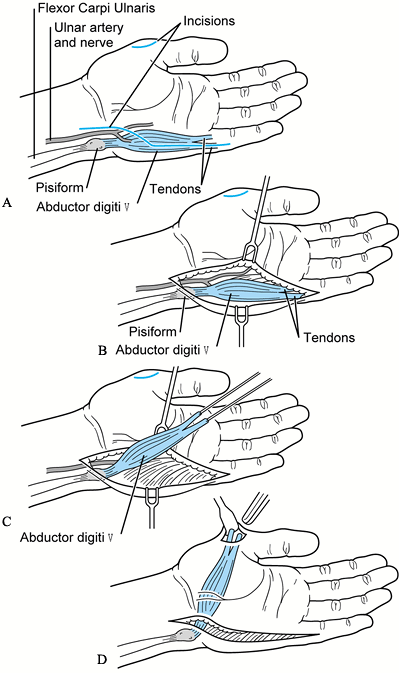 Figure 69.12. ADM opponensplasty.
Figure 69.12. ADM opponensplasty.![]() Figure 69.13. ADM opponensplasty. A: ADM muscle (bottom of photo, with suture attached). Attachment site is at base of the thumb. B: ADM muscle showing path of transfer.
Figure 69.13. ADM opponensplasty. A: ADM muscle (bottom of photo, with suture attached). Attachment site is at base of the thumb. B: ADM muscle showing path of transfer.-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Deepen the first web using a fourfold Z-plasty (Fig. 69.11). The angle of each flap tip should be 45° (63,233).
Release the first dorsal interosseous and adductor pollicis fascia;
release of the muscles is not usually necessary in the younger child,
but if contractures of these muscles have developed, they can be
partially released near their insertions (103). -
Sharply dissect free a proximally based
flap of the MPJ-UCL, joint capsule and adductor pollicis insertion
through the incision created by raising the skin flaps for the
Z-plasty. Pull the flap tight, and secure it with nonabsorbable suture
by passing the suture through a hole drilled distal to the proximal
phalanx physis and tying it over a button on the radial side of the
proximal phalanx (Fig. 69.11). Pin the thumb MPJ in 0° extension and neutral radioulnar deviation. -
Transfer the ADM to provide opposition (Fig. 69.12 and Fig. 69.13). Make a straight incision along
P.1887
the ulnar border of the palm, and curve it toward the palm, ending over
the pisiform. Detach the ADM distally from the base of the small finger
metacarpal, and elevate it proximally. Leave the muscle origin attached
to the pisiform, to provide a firm proximal anchor and maintain the
blood supply to the muscle (47,63).
Create a generous subcutaneous tunnel across the palm, and pass ADM
muscle through it, opening it like a book at its origin. Split the ADM
tendon, and weave one slip of tendon into the radial capsule of the
MPJ, and the other into the extensor pollicis longus (EPL) and
tightened MPJ-UCL (63,128). Suture it in place with nonabsorbable 4-0 or 5-0 suture. -
Close the skin with 5-0 chromic suture.
-
Apply a bulky compressive dressing over the thumb and fingers, and cover it with by an above-elbow fiberglass cast shell.
-
-
Reconstruction of the type 3a hypoplastic thumb.
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Deepen the first web, reconstruct the
MPJ-UCL, and transfer the ADM as for the type 2 thumb, with one
modification. Because the MPJ RCL is lax in type 3a thumbs, instead of
inserting one slip into the radial capsule and the other into the EPL
and ulnar capsule, attach one slip to the radial side of the base of
the proximal phalanx and the other to the EPL (63,128). -
Reconstruct the extrinsic tendons if
passive IPJ motion is present. Tailor the reconstruction of the
extrinsic tendons (EPL and flexor pollicis longus [FPL]) to the
individual anomaly (75,130). The EPL may be hypoplastic or absent, the FPL aberrant or absent, or the two tendons may be interconnected. If both EPL and FPL are to be reconstructed, perform these two procedures in 2 stages.P.1888If EPL is absent, transfer the extensor indicis proprius
(EIP) to the extensor surface of the base of the distal phalanx, using
a pullout technique to attach the transfer.If the FPL is absent, transfer the ring FDS to the
flexor surface of the base of the distal phalanx using a similar
attachment technique; if pulley reconstruction is necessary, use local
tissue or a toe extensor tendon (see Chapter 47 and Chapter 48 [flexor pulley reconstruction]).If the EPL and FPL are interconnected radially, release
the connection and reroute the aberrant tendons, or if length is
insufficient, transfer the EIP or FDS as described earlier. -
Close the skin with 5-0 chromic suture.
-
Apply a bulky compressive dressing over the thumb and fingers, and cover it with an above-elbow fiberglass cast shell.
-
-
If collateral ligament reconstruction fails, resulting in painful thumb MP joint instability, perform MPJ arthrodesis (105) (see the section entitled Polydactyly for a description of this technique).
-
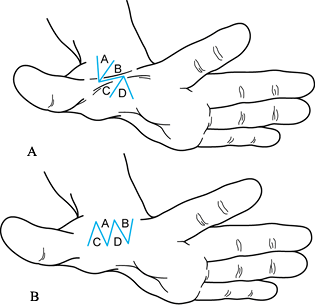
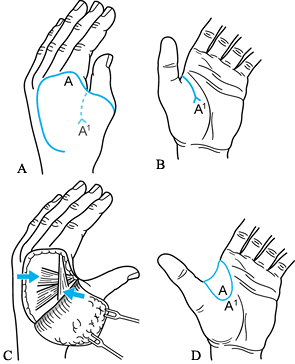
Pollicization of the index (type 3b, 4 or 5 hypoplastic thumb) (Fig. 69.14 and Fig. 69.15).
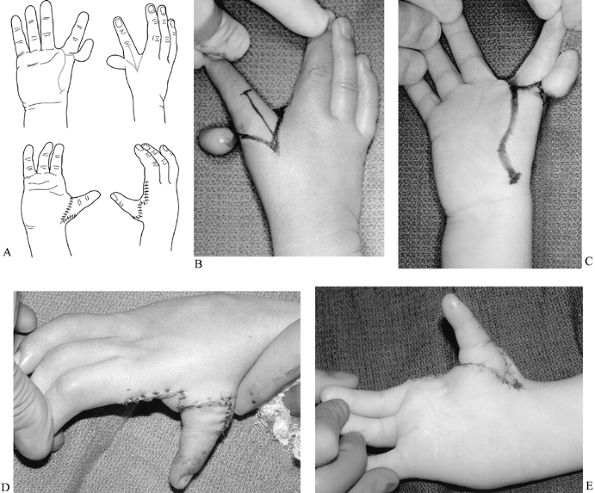 Figure 69.14. Pollicization of index finger. A: Incisions when thumb is absent. B: Incisions for very hypoplastic thumb, dorsal view. C: Palmar view. D: Postoperative dorsal view. E: Postoperative palmar view. (A from Buck-Gramcko D. Pollicization of the Index Finger. J Bone Joint Surg 1971;53-A:1605.)
Figure 69.14. Pollicization of index finger. A: Incisions when thumb is absent. B: Incisions for very hypoplastic thumb, dorsal view. C: Palmar view. D: Postoperative dorsal view. E: Postoperative palmar view. (A from Buck-Gramcko D. Pollicization of the Index Finger. J Bone Joint Surg 1971;53-A:1605.)![]() Figure 69.15. Pollicization of index finger, type 3B thumb. A: Incisions (21) B: Incisions, dorsal view. C: Incisions, palmar view. D: Postoperative radial view. E: Postoperative palmar view.P.1889P.1890
Figure 69.15. Pollicization of index finger, type 3B thumb. A: Incisions (21) B: Incisions, dorsal view. C: Incisions, palmar view. D: Postoperative radial view. E: Postoperative palmar view.P.1889P.1890-
Buck-Gramcko’s technique is recommended (20), with a few modifications (62,129). This technique is based on the work of Riordan (171), Zancolli (236), Littler (115), Malek (120), and others.
-
Use loupe magnification; have an
operating microscope available. Perform the operation with the patient
under general anesthesia and tourniquet. -
Carefully plan and mark the skin incision before inflation of the tourniquet (Fig. 69.14 and Fig. 69.15).
Curve around the index finger at the level of the proximal digital
flexion crease on the palmar aspect. Extend the incision to the base of
the long finger so that the index-long web skin is transposed with the
index finger, preventing a dog ear at the radial base of the long
finger. On the dorsum, angle the incision to a point at the MP joint.
Extend the incision obliquely from halfway between the tip of the
dorsal incision and the index-long web to the dorsum of the proximal
IPJ (PIPJ). On the palm, extend the incision from the palmar radial
circumferential incision to the base of the index metacarpal. Distally,
curve this incision convex radially, and proximally, convex ulnarly.
Avoid placing this incision too far ulnarly or the palmar wound will be
difficult to close. -
If a hypoplastic thumb is present, incorporate it into the distal aspect of the palmar incision (Fig. 69.15).
-
Inflate the tourniquet after
exsanguination of the arm. Raise the dorsal and palmar flaps. Mobilize
the dorsal flap superficial to the dorsal veins draining the index
finger. Mobilize the radial and ulnar flaps on the dorsum of the index.
If a hypoplastic thumb is present, remove it after mobilizing the flaps
overlying the index metacarpal. Carefully preserve the dorsal veins to
the index finger. They are of equal importance to the transposed
finger’s survival as are the digital arteries. -
Detach the insertion of the first dorsal
interosseous muscle, and tag it with a nonabsorbable 4-0 suture.
Mobilize the muscle proximally to expose the entire index metacarpal,
but leave the muscle origin intact. Find the distal physis of the
metacarpal with the tip of a #15 scalpel, and sharply transect the
metacarpal through the physis. Remove the index metacarpal proximal to
the physis. -
Detach the insertion of the palmar
interosseous muscle from the dorsoulnar aspect of the base of the index
finger and tag it with a nonabsorbable 4-0 suture. Transect the
transverse intermetacarpal ligament between the index and long fingers. -
Isolate and tie off the radial digital
artery to the long finger, if necessary. To place the index finger in
its new position, sometimes you must split the common digital nerve to
the index and long fingers. -
Release the index finger A1 pulley. It is not necessary to shorten the extrinsic flexor and extensor tendons to the index finger (129).
-
Place the new thumb in the bed created by
removal of the index metacarpal, rotated 150° to 180°, and in 40°
palmar abduction. Preposition the index MPJ (the new thumb CMCJ) in
hyperextension, to place the available range of motion in a
functionally useful arc. Suture the periosteum of the index metacarpal
head to the capsule of the index CMCJ with at least three nonabsorbable
4-0 Dacron sutures. -
Gently and bluntly create tunnels for the
first dorsal and palmar interosseous muscles under the radial and ulnar
flaps on the dorsum of the new thumb, protecting the dorsal veins.
Attach the insertions of these muscles to the extensor aponeurosis of
the new thumb, using nonabsorbable 4-0 Dacron suture. Release the
tourniquet. Observe that the digit is well vascularized and that there
is no venous congestion. -
Close the skin incisions with 5-0
chromic, after rotating the palmar flap into the first web space, the
tip of radial flap on the dorsum of the new thumb into the proximal end
of the palmar incision, and the dorsal flap into the split between the
radial and ulnar flaps on the dorsum of the new thumb. Trim excess skin
as necessary but avoid tight closures. Close the palmar incision first. -
Apply a bulky compressive dressing over the thumb, fingers, and hand, and cover it with an above-elbow fiberglass cast shell.
-
reconstruction) after 6 weeks. For the reconstructed thumb, prescribe a
custom-made soft neoprene short opponens splint for comfort and while
sleeping for approximately 6 weeks. Therapy is not always necessary for
thumb reconstruction or pollicization, but it may be helpful and
reassuring for both the child and the parents.
new thumb by 1 year after pollicization, ADM opponensplasty is
indicated. Supplemental opponensplasty is most commonly needed when the
first dorsal interosseous is hypoplastic (129). The surgeon can prepare the family for this possibility following the pollicization.
transfer is too tight, or if the origin of the muscle is kinked, the
transfer will not function well. If the reconstructed MPJ-UCL is too
tight in the thumb with RCL laxity (type
3a) the thumb will deviate in an ulnar direction at the MPJ.
common pitfalls of pollicization, and the best ways to avoid them.
Necrosis of the new thumb is extremely rare (Buck-Gramcko reports one
case of necrosis, in a patient with index finger arterial anomalies, in
a series of 460 pollicizations [23]).
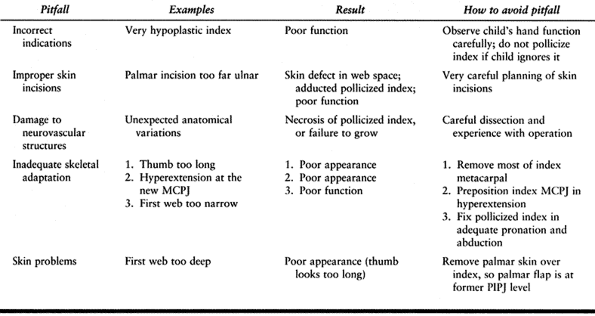 |
|
Table 69.5. Pitfalls of Pollicization (23)
|
thumbs appreciate the opportunity to visit and talk with another child
who has already undergone pollicization (preferably away from the
surgeon, so the families can discuss advantages and disadvantages
freely), and the opportunity to view preoperative and postoperative
photos of a pollicized index finger.
by an experienced hand surgeon. The wounds are easier to close and
their appearance is better if the operation is completed in less than 2
hours without releasing the tourniquet. Careful wound closure, with
meticulous trimming of excess skin, also improves the appearance of the
new thumb.
Trigger thumb in children is probably acquired, not congenital; recent
reports indicate that experienced examiners found no trigger thumbs in
two large series of newborns (174,186).
In theory, the normal infant thumb position (tightly clenched in the
palm until about 2 months of age, with a strong grasp reflex [59])
may contribute to the development of trigger thumb by pulling the
flexor tendon tight against the opening of the proximal pulley (174), causing tendon inflammation and edema at the theca.
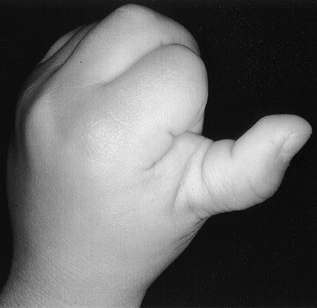
the adult’s trigger digit), but instead usually presents as a fixed
flexion contracture at the IPJ (average 35° [186]) (Fig. 69.16),
or rarely as a fixed extension contracture (when the enlarged portion
of the flexor pollicis longus [FPL] tendon gets trapped at the distal
margin of the proximal pulley) (45,51,70,174,185,186,193).
A palpable nodule in the FPL at the MPJ flexion crease of the thumb is
almost always present; this nodule is probably caused by the bunching
up and swelling of the FPL, which are attributable to chronic pressure
from the proximal pulley (135,153,174). Differential diagnosis includes congenital clasped thumb and thumb hypoplasia.
 |
|
Figure 69.16. Trigger thumb.
|
condition reported 12% spontaneous resolution for 131 trigger thumbs in
107 children observed for 6 months (average age at diagnosis was 2
years); for trigger thumbs noted at birth, the spontaneous resolution
rate was 31% by 1 year of age. In this study, children who underwent
surgery within 3 years of diagnosis did not have a residual flexion
contracture of the IPJ, but children older than 4 years of age at
surgery had a high rate of residual IPJ flexion contracture (41). Other authors have found a lower incidence of spontaneous correction (70,193) and no residual flexion contracture in children whose trigger thumbs were released after 3 years of age (185).
Trigger fingers may also occur in children, but they are much rarer
than trigger thumbs and more likely to resolve spontaneously (193).
is the treatment of choice. This is a simple and very successful
operation; of 402 reported releases of trigger digits, 401 were
successfully released (51,70,163,174,185,186,193,232), with only one recurrence (163) and no other significant complications. Do not use steroid injections for this condition in children (135).
 |
|
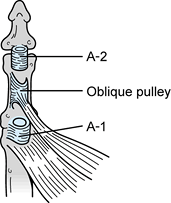
Figure 69.17. Anatomy of thumb pulleys (45).
|
thumb in an infant before the age of 1 year. The trigger thumbs of
children older than 3 years of age at the time of presentation are very
unlikely to resolve spontaneously, and should be released to enhance
the child’s hand function and prevent a permanent flexion contracture
of the IPJ.
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
The radial digital nerve to the thumb may
be stretched over the nodular enlargement of the FPL tendon, in the
region of the surgical incision. Make a transverse incision through
dermis only, at the proximal digital flexion crease of the thumb, and
do not hyperextend the thumb MPJ. -
Use blunt dissection to expose the flexor sheath. Locate and retract the ulnar and radial digital neurovascular bundles.
-
Find the proximal margin of the A1
pulley, and sharply incise it from proximal to distal. Excision of the
pulley is not necessary; it will fall open as it is incised. Move the
IPJ through its new passive range, and observe that the tendon nodule
no longer impinges. If it continues to impinge when the joint is
hyperextended, release a small amount of additional pulley. Do not
debride the nodule (unless the patient has a mucopolysaccharide storage
disease, in which case debridement may be necessary [215]). -
Pull on the FPL tendon with a Ragnell retractor to demonstrate that there are no other impediments to its excursion.
-
Close the skin incision with interrupted 5-0 chromic suture.
-
Apply a light compressive dressing, and cover it with Coban. A cast is not necessary.
-
Remove the dressing after 7 to 10 days. No postoperative rehabilitation is necessary.
in surgical technique. Do not release the oblique pulley, or the FPL
tendon may bowstring; this result is cosmetically unappealing and
reduces FPL efficiency. Take great care with the skin incision, because
the radial digital nerve to the thumb is immediately subcutaneous,
especially when tented over the FPL nodule.
the less experienced surgeon, but the radial digital nerve, which runs
very close to the midline, can also be damaged with this incision, and
the appearance of a healed transverse incision is much more
satisfactory.
year of age, observe the child until after the first birthday, when the
risk of general anesthesia is lower and the trigger thumb is unlikely
to resolve spontaneously. Trigger thumb in children should not be
confused with trigger digit in adults, because the pathophysiology,
natural history, and treatment differ (see Chapter 50: Trigger Digits).
isolation or as a feature of syndromes such as Freeman-Sheldon syndrome
or arthrogryposis (135,208).
It is diagnosed after age 2 months, when the infant’s normal palmar
grasp pattern has diminished but the thumb remains in the palm. There
is a genetic predisposition toward this condition, which is usually
bilateral and occurs more often in males than in females (208).
casting or splinting in abduction and extension usually restores normal
thumb position. This type of clasped thumb is due to hypoplastic
extensor tendons overpowered by thumb flexor tendons, and positioning
the thumb in extension for several months allows the extrinsic tendons
to recover their balance (135).
associated thumb anomalies are commonly seen, including MPJ-UCL laxity,
thenar muscle hypoplasia, and thumb CMCJ adduction contracture (135).
Splinting may reduce the thumb MPJ flexion contracture but will not
affect the other anomalies. Surgical correction should be tailored to
the particular deformities and may include MPJ contracture release, FPL
lengthening, ADM opponensplasty, tendon transfer to the EPL, and skin
Z-plasty or thick split-thickness skin graft (135,139).
Attempt splinting and stretching until the child is 2 to 3 years of age
before resorting to surgery. Reconstruction may improve the position of
the thumb, but does not restore normal function.
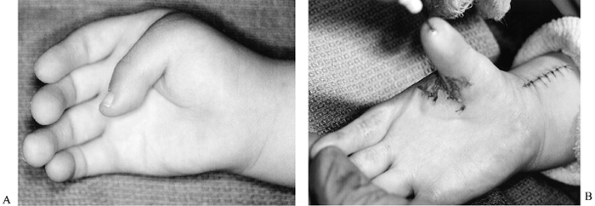
complex. Supple thumbs have good passive range of motion. Complex
clasped thumbs have passive contractures, and may be associated with
arthrogryposis and Freeman-Sheldon syndrome (Fig. 69.18A) (135,136). Other, more elaborate schemes have been described (208,226,227), but they do not assist the surgeon with the prognosis and treatment more than McCarroll’s simple scheme.
 |
|
Figure 69.18. Complex clasped thumb. A: Preoperative view. B: Postoperative view (following release; dorsal rotation flap was not necessary).
|
and stretching exercises. Supple thumbs will probably correct
themselves, and complex clasped thumbs may improve, making surgery
unnecessary or at least simpler.
for coverage. Graft harvested from just below the anterior iliac crest
leaves a scar in an inconspicuous location and does not bear hair when
the child enters puberty (see the section entitled Syndactyly.)
-
Tendon transfer (when supple thumbs that cannot be actively extended and splinting has failed).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Explore the dorsum of the thumb through a
zigzag incision. Determine the best attachment point for the
transferred tendon: either the hypoplastic EPL or extensor pollicis
brevis (if it pulls through) or extensor hood tissue on the dorsal base
of the proximal phalanx. -
Through a separate transverse incision at
the level of the distal end of the dorsal extensor retinaculum of the
wrist, explore the fourth dorsal compartment for the EIP tendon. This
is the preferred tendon to transfer to the thumb extensor mechanism,
but it is usually absent in patients with clasped thumbs (139).
If it is present, transect it as far distal as possible, create a blunt
subcutaneous tunnel between the wrist and thumb incisions, pass the EIP
tendon through the tunnel, and weave it through the chosen recipient
tissue, suturing it in place with nonabsorbable 4-0 suture. If the EIP
is absent, transfer either the extensor carpi radialis longus (ECRL)
(extended with a tendon graft from palmaris longus) or the
P.1894
ring FDS tendon (112,135,139,208).
Adjust the tension of the transfer so that the thumb tip touches the
index finger in a key pinch position when the wrist is passively
extended and the thumb MPJ position is 0° when the wrist is in neutral
position. -
Close the thumb incision with interrupted
5-0 chromic suture, and close the wrist incision with a running
absorbable subcuticular 4-0 suture and Steri-Strips. -
Apply a bulky compressive dressing over the thumb, fingers, and hand, and cover it with an above-elbow fiberglass cast shell.
-
-
Thumb flexion-adduction contracture release and reconstruction (complex clasped thumbs).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Explore the dorsal thumb through a
longitudinal incision centered over the MPJ. Release the dorsal capsule
of the MJP if it is adherent to the metacarpal head, because it can
block MPJ extension (135). -
Explore the first web and palmar MPJ structures through a fourfold Z-plasty (Fig. 69.10) or, if necessary, plan and raise a large, radially based dorsal rotation-advancement flap (Fig. 69.19) (67,136).
Release the palmar plate of the MPJ and, if necessary, the RCL. Release
the first dorsal interosseous fascia; the muscle does not usually
require release.![]() Figure 69.19. Dorsal rotation flap. (From McCarroll HR Jr, Manske PR. The Windblown Hand: Correction of the Complex Clasped Thumb Deformity. Hand Clin 1992;17A:34.)
Figure 69.19. Dorsal rotation flap. (From McCarroll HR Jr, Manske PR. The Windblown Hand: Correction of the Complex Clasped Thumb Deformity. Hand Clin 1992;17A:34.) -
If the adductor is tight, release the
origin of the transverse head from the third metacarpal through a
separate longitudinal midpalmar incision. -
If necessary, release the thumb CMCJ.
-
Z-lengthen the FPL through a separate wrist incision.
-
Reconstruct the MPJ-UCL with a
pants-over-vest repair using available tissue. Pin the joint in neutral
position place using a small Kirschner wire (Fig. 68.18B). -
If skin coverage is inadequate, harvest a thick split-thickness graft from the groin (see the section entitled
P.1895
Syndactyly). Shift local flaps from the
fourfold Z-plasty so that the graft is situated over thenar muscle or
palmar fascia, not in the web space. If a dorsal rotation flap is used,
harvest a graft for the dorsum of the hand. -
Close hand wounds with interrupted 5-0 chromic suture and wrist wounds with running 4-0 absorbable subcuticular suture.
-
Apply a bulky compressive dressing over the thumb, fingers, and hand, and cover it with an above-elbow fiberglass cast shell.
-
-
ADM Opponensplasty (may be performed at
the same time as release and reconstruction, discussed earlier, or as a
second stage for the complex clasped thumb that does not develop
opposition after reconstruction).-
See the section entitled Hypoplasia.
-
-
MPJ arthrodesis (for a painful or unstable MPJ in the older child).
-
See the section entitled Polydactyly.
-
Prescribe a custom-made soft neoprene short opponens splint to be worn
for comfort and while sleeping for approximately 6 weeks. Therapy is
not usually necessary.
limited benefit and the extensive surgical scars following complex
clasped thumb reconstruction.
is a complex undertaking. Sometimes, this type of thumb is best left
untreated until the child is older, when the MPJ can be fused.
function in spite of very restricted joint motion and limited strength.
Avoid operating on these patients unless the planned operation is
likely to improve functional deficits perceived by the child or careful
observers (parent, surgeon, and therapist).
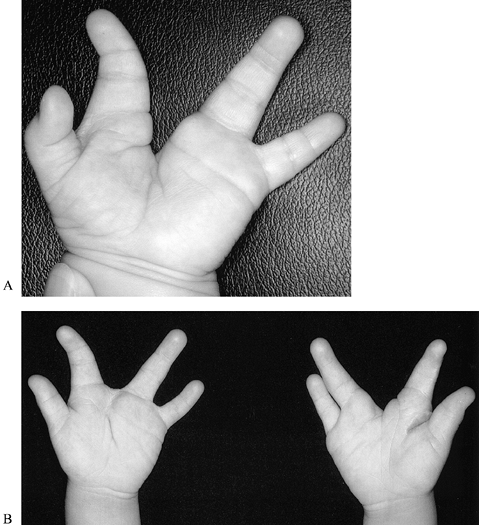
The gene for triphalangeal thumb has been mapped to chromosome 7q; this
was the first human gene involved in pathologic morphogenesis of the
hand to be localized (80).
probably because the excessive length of the thumb makes opposition
difficult. The distal phalanx is frequently partially duplicated, and
many parents and older children do not like the appearance of this
long, broad thumb (Fig. 69.20).
 |
|
Figure 69.20. Bilateral triphalangeal thumbs.
|
The infant with this anomaly may be treated with excision of the
accessory phalanx; as long as this operation is performed early, the
thumb IPJ usually retains stability and
motion (57,157).
For the older child, a reduction osteotomy may be performed, in which
the proximal portion of the distal phalanx and the distal portion of
the middle phalanx are removed; this operation also preserves motion
and stability (94).
appearance, the physes of the thumb ray can be ablated by
epiphysiodesis when the child’s thumb reaches the same length as the
thumb of the same sex parent. This operation is the least invasive, but
the extra segment is retained. If the triphalangeal thumb is
nonopposable, it must be shortened and rotated by pollicization,
followed by opponensplasty if necessary. None of these operations
provide normal function, but shortening the thumb and providing
opposition improve function.
the first type, the thumb is opposable and has an adequate first web
space, a normal thumb CMCJ and a proximal metacarpal epiphysis. In the
second type, the thumb is nonopposable; thenar muscles are absent. It
has a narrow first web space and a distal metacarpal epiphysis; this
type has also been called five-fingered hand (57).
-
Removal of accessory phalanx (hypoplastic middle phalanx; opposable triphalangeal thumb) (157).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Approach the accessory phalanx through a midlateral incision on the convex side of the thumb.
-
Incise the collateral ligament and the adjacent periosteum longitudinally, and remove the accessory phalanx.
-
Repair the collateral ligament and use a Kirschner wire to fix the IPJ in neutral position.
-
Close the skin with 5-0 chromic suture.
-
Apply a bulky compressive dressing over the thumb and hand, and cover it with an above-elbow fiberglass cast shell.
-
-
Reduction osteotomy (opposable triphalangeal thumb) (Fig. 69.21) (94).
![]() Figure 69.21.
Figure 69.21.
Reduction osteotomy for triphalangeal thumb. See text for details.
(From Jennings JF, Peimer CA, Sherwin FS. Reduction Osteotomy for
Triphalangeal Thumb: An 11 Year Review. J Hand Surg 1992;17A:8.)-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Approach the middle phalanx and the distal IPJ (DIPJ) through a dorsal incision, detaching the extensor tendon (Fig. 69.21A).
-
If necessary, narrow the nail and distal phalanx (Fig. 69.21B, Fig. 69.21C).
-
Pass a fine Kirschner wire through the proximal IPJ (PIPJ), to orient transverse osteotomies (Fig. 69.21D).
-
Excise the epiphysis of the distal phalanx, using a scalpel to transect the physis (Fig. 69.21E).
-
Cut the middle phalanx perpendicular to
the PIPJ, retaining the PIPJ collateral ligaments. This closing wedge
shortens and realigns the thumb (Fig. 69.21F). -
Fix the osteotomies with longitudinal Kirschner wire or wires (Fig. 69.21G).
-
Repair the extensor tendon with nonabsorbable nylon suture.
-
Close the skin with 5-0 chromic suture.
-
Apply a bulky compressive dressing over the thumb, fingers, and hand, and cover it with an above-elbow fiberglass cast shell.
-
-
Epiphysiodesis (opposable triphalangeal thumb).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Through midlateral incisions on each side
of the thumb, approach the physes of the three phalanges. The
metacarpal physis is approached from a dorsal incision. -
Using Fluoroscan guidance, use small curets (size 0000) to ablate the physeal cartilage.
-
Close the skin with 5-0 chromic suture.
-
Apply a soft bulky compressive dressing over the thumb and hand, and cover it with Coban.
P.1897 -
-
Pollicization and opponensplasty (staged; nonopposable triphalangeal thumb) (see the section entitled Hypoplasia and Absence).
radiographic evidence of healing of osteotomies. Allow the child to use
the hand as tolerated; therapy and splint immobilization are usually
unnecessary.
series of patients have been reported. Postoperative instability and
stiffness have not been reported, but undercorrection and
overcorrection and recurrence of the deformity have occurred (94,157).
early. Treatment of the well-developed triphalangeal thumb is more
complex. If the thumb functions well, and the parents are not bothered
by the appearance, defer treatment until the child is older, when
epiphysiodesis is an option.
malformation; in the United States, it is slightly less common and more
often associated with a syndrome, than is thumb polydactyly. Finger
polydactyly may be associated with chromosomal abnormalities, eye and
orofacial abnormalities, bone dysplasias, and mental retardation (56).
The small finger is the most commonly duplicated finger, especially in
blacks (1 in 143 to 300 live births, compared with 1 in 1339 live
births of whites [56,223]).
Duplication of the index, long, or ring finger is quite rare and is
usually associated with other hand anomalies, including cleft hand.
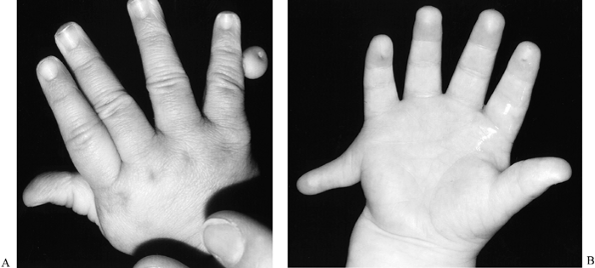
ligated by a suture soon after birth. Remove duplicated digits with
more substantial pedicles or those connected to other digits by webs (Fig. 69.22)
when the child is older, because a large pedicle may bleed with suture
ligation. The functional result of removal of a duplicated small finger
is usually quite good, although suture ligation often leaves a bump.
The outcome of removal of a duplicated index,
long,
or ring finger depends on the associated anomalies. Retained fingers
are less likely to require reconstruction than are retained duplicated
thumbs.
 |
|
Figure 69.22. Postaxial polydactyly. A: Small pedicle, but not small enough for suture ligation. B: Well-developed postaxial polydactyly, also not suitable for suture ligation.
|
described as central or axial, and duplication of the small finger is
postaxial. The duplicated digit may articulate with a broad metacarpal
head, or, less commonly, the metacarpal may also be duplicated (56).
Temtamy and McKusick divided postaxial polydactyly into type A (fully
developed extra digit) and type B (rudimentary or pedunculated extra
digit) (201).
size, wait until the child is older than 1 year of age, and observe his
or her use of the fingers to plan treatment. Determine which finger
functions best and preserve it. Delay surgery until age 3 years of age
in most cases. Ligate the type B finger in the newborn; inform the
parents that the digit will turn black and fall off.
-
Suture ligation (type B postaxial duplication).
-
If the pedicle is broad, do not use
suture ligation. Instead, perform a formal amputation under general
anesthesia, through a zigzag incision. -
If the pedicle is small, ligate the pedunculated digit at its base with undyed 2-0 Vicryl suture.
-
Apply a bulky compressive hand dressing and cover with Coban.
-
-
Ablation of a type A postaxial duplication (56).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
If the ulnarmost finger is to be removed,
use an elliptical incision along the midaxial line. Detach and preserve
the insertions of the ADM and the (MPJ-UCL). If the metacarpal is
duplicated, extend the incision to remove it. -
If the retained metacarpal head has an ulnar bulge, shave it.
-
Reattach the ADM and UCL to the retained finger.
-
Close the skin with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing over the fingers and hand, and cover it with an above-elbow fiberglass cast shell.
-
-
Reconstruction of the duplicated index, long or ring finger.
-
In the case of duplicated index, long,
and ring finger, each of these hands has a unique set of anomalies,
which may include stiff joints, a longitudinal epiphyseal bracket,
complex syndactyly, transverse phalanx, rotational or angulatory
malalignment, and cleft hand. See the section entitled “Cleft Hand” and follow these basic principles; tailor the operation to the malformations:-
Establish as normal a skeleton as possible. Combine parts from two fingers, if necessary.
-
Avoid damaging epiphyses and physes.
-
Correct bony alignment with phalangeal osteotomy.
-
Separate syndactyly, and plan flaps incorporating skin from the ablated digit so that no additional skin graft is needed.
-
Close the skin with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing, and cover it with an above-elbow fiberglass cast shell.
-
-
and bony healing, as appropriate. Splinting and therapy are not usually
necessary.
which is distressing to some parents. Sometimes the necrotic digit does
not fall off, and requires surgical removal. The residual bump left by
suture ligation can be unsightly (223).
performed by an experienced hand surgeon. Residual deformity is common
even when the hand is reconstructed by an expert. Sometimes, when both
duplicated fingers are inadequate, removal of both (leaving a
three-fingered hand) may be preferable.
form normally; differentiate them from fingers that are deformed
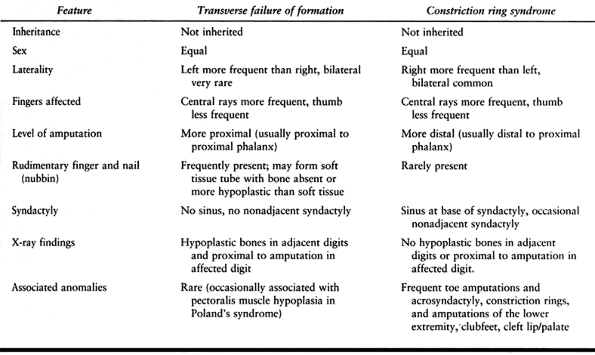
prenatally by constriction ring syndrome (see the section entitled Constriction Ring Syndrome, and Table 69.6). Transverse failure of formation, or terminal deficiency,
occurs in about 1.5 in 10,000 births (this estimate includes failure of formation at levels proximal to the fingers) (108), and 98% are unilateral (234). Short digits are often incompletely separated.
 |
|
Table
69.6. The Differential Diagnosis of Short and Absent Fingers: Transverse Failure of Formation vs. Congenital Constriction Ring Syndrome (90,142,162) |
short fingers, and surgeons have devised numerous ways to augment
length. No single procedure, however, is vastly superior to any other,
normal appearance is usually unattainable, and lengthening the digits
of a child with a normal hand on the contralateral side does not
improve function. Children with one normal hand perform most functional
activities at about the same developmental age as children with two
normal hands. Indications for reconstructing the unilateral aphalangic
hand are based on the following principles:
-
Children will put almost any sensate reconstructed digit to good use.
-
More digits usually function better than fewer, as long as one does not get in the way of the others.
-
Increased length usually enhances function, although a stiff digit should be shorter than flexible ones (73).
-
When short digits are incompletely separated, web deepening enhances function and appearance.
that have failed to develop metacarpals, with the exception of the
thumb (see the section entitled Hypoplasia and Absence—Pollicization).
ablation of nubbins, “on-top-plasty,” in which one short digit is
transplanted onto the top of another (42,49,61,166); web deepening (49,61);
and single-stage osteotomy, distraction, and insertion of intercalary
bone graft. Also included are distraction lengthening with or without
second-stage bone grafting (81,101,164,166,188); nonvascularized toe phalanx transfers (22,61,73,90,169); and microvascular toe-to-hand transfer (71,99,100,113,114,220).
“On-top-plasty” is technically difficult and diminishes the number of
digits present. Web deepening is simple and useful, either by itself or
when combined with another operation, but if webs are made deeper than
normal, the metacarpals are “phalangized” and the appearance of the
hand is usually aesthetically displeasing. Distraction lengthening of
finger phalanges is not usually indicated, because stiff fingers should
be short, or they will interfere with the function
of more flexible fingers. Distraction lengthening of short metacarpals may enhance function and appearance.
a soft-tissue tube large enough to hold bone graft has developed distal
to the metacarpal; toe phalanges are selected as a source of graft
because terminally placed bone graft is resorbed unless it is
cortically perimetered (61). The indications
for free toe-to-hand transfer for transverse defects and
symbrachydactyly are controversial, and the arterial supply, venous
drainage, and nerves and muscles in a malformed hand are always
abnormal and sometimes nonexistent. This operation is sometimes
technically feasible, however, and it seems to preserve growth in many
cases (99,220).
digits and even more different names for them. Brachydactyly is the
term most commonly, consistently, and accurately used to describe short
fingers. Ectrodactyly is also used as a general descriptive term, but
different authors define it differently; most commonly, it connotes
digital absence. Some terms are used to describe specific types of
short fingers, such as aphalangia (phalanges failed to develop, some
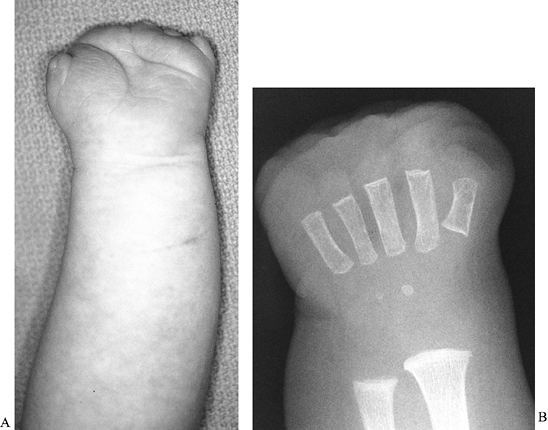
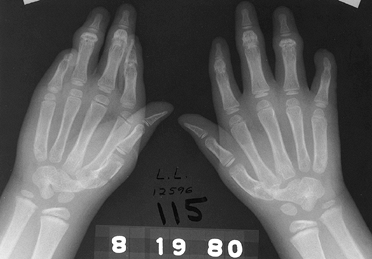
soft-tissue structures may be present; Fig. 69.23), symbrachydactyly (short, webbed digits, more deficient centrally, previously called atypical cleft hand, Fig. 69.24), and symphalangia (absence of the proximal interphalangeal joints, Fig. 69.25), among many others.
 |
|
Figure 69.23. A and B: Aphalangia.
|
 |
|
Figure 69.24. Symbrachydactyly.
|
 |
|
Figure 69.25. Symphalangia.
|
as Bell’s classification of brachydactyly, to describe and categorize
short fingers, because some types of short fingers have a dominant
inheritance pattern. These systems do not help the surgeon predict
function or plan treatment.
a child with aphalangia, especially when the deformity was not
diagnosed prenatally. They may want to donate their own fingers or toes
to their child, or obtain a cosmetic prosthesis immediately. Counsel
parents early on that surgery cannot bring about normal appearance in
most cases of aphalangia and that enhancing function will be the
primary goal, at least until the child is old enough
to participate in decision making, when enhancing appearance may take precedence. Peer contacts can be very valuable.
shown that the transferred toe phalanx physis is more likely to remain
open and grow in younger patients (22,73,90,169).
Growth of a transferred toe phalanx is negligible in many cases,
however, and never normal, so surgery may be postponed until the child
is older and the phalanx larger. Before performing toe proximal phalanx
transfers, obtain radiographs with 10 cm markers of the affected hand
or hands and feet; these radiographs provide an estimate of
magnification, enabling accurate measurement of the toe phalanx. The
transferred toe phalanx can be attached either to the end of the
metacarpal (in which case it is effectively a one half joint
transplant) or to a hypoplastic proximal phalanx.
-
Nubbin ablation.
-
Web deepening (see the section entitled Syndactyly).
-
“On-top plasty”: this operation is rarely indicated. See Dobyns (42) for details.
-
Metacarpal distraction lengthening (see Chapter 32 and Chapter 71) (101,161,164,188)
-
Nonvascularized toe proximal phalanx transfer (Fig. 69.26).
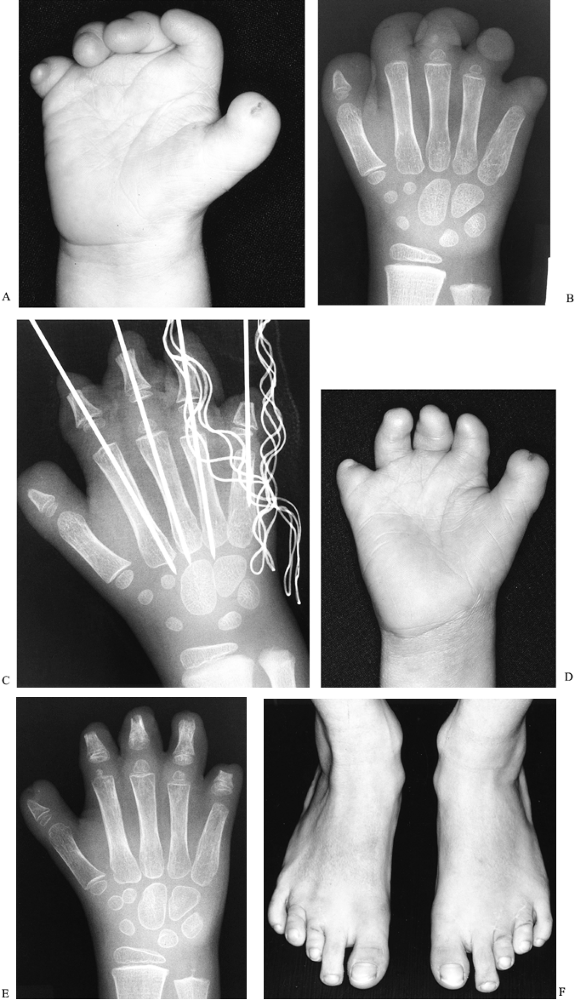 Figure 69.26. Nonvascularized toe proximal phalanx transfers. A: Preoperative palmar view. B: Preoperative radiograph view, with soft-tissue tubes distal to metacarpals. C: Postoperative radiograph, following toe proximal phalanx transfers to index, long, ring, and small fingers. D: Palmar view, 2 years after phalanx transfer. E:
Figure 69.26. Nonvascularized toe proximal phalanx transfers. A: Preoperative palmar view. B: Preoperative radiograph view, with soft-tissue tubes distal to metacarpals. C: Postoperative radiograph, following toe proximal phalanx transfers to index, long, ring, and small fingers. D: Palmar view, 2 years after phalanx transfer. E:
Radiograph, 2 years after phalanx transfer. Note the open physis of the
ring phalanx, and the resorption of the tips of the long and small
phalanges. F: Feet, 4 years after phalanges were harvested from third and fourth toes of both feet.-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquets.
-
Drape free the affected hand and selected
foot or feet. If four toe phalanges are needed, select the third and
fourth toes of each foot. -
Explore the digits chosen to undergo toe
phalanx transfer through dorsal chevron incisions. Split the extensor
tendon, which inserts into subcutaneous tissue distally. -
Create a pocket in each digital tube, but
take care not to remove too much subcutaneous tissue distally. Release
the arm tourniquet while you harvest the toe proximal phalanges. -
Approach the selected toe or toes through a dorsal chevron incision. Split the extensor tendon.
-
Dissect the toe proximal phalanx from
distal to proximal. Stay subperiosteal distally, but leave the
periosteum with the phalanx proximally. Remove the metatarsophalangeal
collateral ligaments and part of the plantar plate with the phalanx;
leave the flexor tendon intact. -
Suture the flexor and extensor tendons
together.Close the wound with 5-0 chromic. After harvesting all of the
toe phalanges needed, deflate the leg tourniquet or tourniquets. -
Reexsanguinate the hand and arm, and reinflate the arm tourniquet.
-
Skewer the toe phalanx on a 0.035 or
0.045 in. Kirschner wire. Pass the wire antegrade by hand out of the
tip of the previously prepared digital tube, then drive it retrograde
into the finger hypoplastic proximal phalanx or metacarpal or both. If
the fit seems tight, remove the phalanx, and trim the distal end before
reinserting it. -
After all the phalanges have been
inserted, deflate the tourniquet. If the tip of a digit is not
vascularized, remove the phalanx, trim it, and reinsert it. -
If there is no proximal phalanx remnant
present, attach the collateral ligaments to the metacarpal periosteum
with absorbable suture. -
Check the Kirschner wire placement radiographically.
-
Close the skin with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing over the fingers and hand, and cover it with an above-elbow fiberglass cast shell.
-
Dress the foot or feet and apply a short-leg walking cast or casts.
P.1904 -
-
Free toe-to-hand transfer (see Chapter 35 and Chapter 36).
remove the leg cast or casts in 3 weeks. Change the hand cast under
sedation in 6 weeks, and remove the second hand cast and Kirschner
wires in the clinic 12 weeks after surgery. Obtain radiographs of the
hand every 6 months, with markers placed 10 cm apart so magnification
can be calculated, and the growth of the phalanx can be accurately
measured. At a later date, deepen web spaces and lengthen transferred
phalanges and metacarpals as indicated.
transfers are tip necrosis, physeal arrest, and resorption. Avoid early
tip necrosis by taking care to trim the transferred phalanx to fit the
digital tube. Late tip necrosis can occur if growth of the phalanx is
especially vigorous, and may require a second operation to trim the tip
of the phalanx. Toes from which proximal phalanges have been harvested
are short, but toe complaints are rare following this operation. Do not
fail to deflate the leg tourniquet after you have harvested all the toe
phalanges needed.
physeal arrest seems less likely to occur in younger children
(especially those younger than 18 months of age at surgery) (22,73,169),
but even the phalanges achieving the most growth do not grow as much or
for as long as they would if they had been left in the toe (90). Waiting until the child is older and the phalanx is bigger seems sensible.
expect from surgery. Often, they believe it can make short fingers
normal. Peer contact with other children and families who have adjusted
well to similar malformations is especially useful for children with
short fingers. Sometimes, no matter how carefully they are counseled,
parents and children are disappointed with the results of any operation.
separating short, webbed fingers. This procedure “phalangizes” the
metacarpals, making the hand appear aesthetically displeasing. In
addition, it requires release of the intermetacarpal ligament, which
may make the digit unstable, and ligation of one of the digital
arteries, which puts the digit at unnecessary risk of vascular
insufficiency.
unilateral malformation, because this operation does not restore normal
appearance, and children with one normal hand are able to perform most
developmental tasks.
malformation (1:2500 births); it may occur as an isolated anomaly
(usually inherited in an autosomal dominant pattern) or as part of a
syndrome (65). Syndromes commonly associated
with syndactyly include Poland’s syndrome (sporadic occurrence of
symbrachydactyly associated with variable deficiency of the pectoralis
major muscle), probably part of the subclavian artery supply disruption
sequence (10,89,203) (Fig. 69.27);
and acrosyndactyly, which occurs with congenital constriction band
syndrome. Apert’s syndrome is a complete and complicated form of
syndactyly of multiple digits that is associated with craniosynostosis (Fig. 69.28) (2,203,210). Pfeiffer’s syndrome is a mild syndactyly associated with acrocephaly (5,203).
Other associated syndromes have been described (203).
 |
|
Figure 69.27. Poland’s syndrome.
|
 |
|
Figure 69.28. Apert’s syndrome.
|
ring fingers, followed by ring-small, index-long, and least commonly,
thumb-index (65) (see the section entitled Cleft Hand
in this chapter for a discussion of thumb-index syndactyly). The normal
level of a finger web is halfway between the MPJ and PIPJ; for the
thumb-index configuration, the normal web extends from the index MPJ to
the thumb MPJ. If the distal web ends proximal to the PIPJ and extends
no farther distal to normal level than the palm is deep, it can be
adequately deepened with a butterfly flap without using skin graft. If
the web extends distal to the PIPJ, release requires full-thickness
skin graft. Harvest the graft from an inconspicuous location such as
the groin (from an area that will not bear pubic hair at a later date,
or the graft will bear hair in its new location).
clinical and radiographic findings, by both the degree of webbing and
the presence of bony fusion or other bony anomalies (43,65,74).
Syndactyly is described as incomplete when the web does not extend to
the fingertips, and complete when the web extends to the tips; in
complete syndactyly, the fingers may share a common nail (synonychia).
The term simple is used to describe syndactyly that involves only soft
tissues, complex when synostosis is present, and complicated when the
webbed digits conceal polydactyly, dislocations, longitudinal
epiphyseal brackets, and other bony malformations.
McKusick propose five types based on localization of syndactyly in the
hands and feet; these phenotypes seem to be correlated with genotype
for isolated syndactyly (202).
and the common digital arteries may branch distally, with one branch
requiring ligation at the time of release. (A distally branching common
digital nerve can be split into fascicles supplying each finger). Thus,
when the syndactyly involves more than two digits, stage the releases
to avoid operating on both sides of the same digit in the same
procedure. At the second stage, refer to the report from the previous
operation to ascertain whether a digital artery was ligated; if so, do
not ligate the other artery to the same finger.
the degree and complexity of the syndactyly. Release border digits
early (usually around 6 to 12 months of age), because their length
discrepancy may cause the longer digit to develop a flexion contracture
and angulate toward the shorter one with growth. Although there is no
such urgency to release long-ring or index-long syndactyly, parents may
prefer early surgery. As long as anesthetic risk is not increased by
airway, pulmonary, or cardiac anomalies, the experienced hand surgeon
can honor this preference without compromising the long-term result.
duplicated toe), schedule this operation for the same anesthetic as the
syndactyly release, and harvest skin from the amputated part, because
the color of foot skin better matches the hand than does groin skin.
-
Butterfly flap (incomplete syndactyly release; Fig. 69.29).
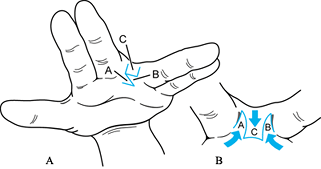 Figure 69.29. Butterfly flap.
Figure 69.29. Butterfly flap.-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Draw the flaps, aligning the proximal
margin of the palmar Z with the normal level proximal digital flexion
creases of the other digits. Each limb of the palmar Z and each limb of
the dorsal rectangle should be the same length. The dorsal rectangle
originates several millimeters proximal to the palmar proximal digital
flexion creases, in order to create the normal dorsal-palmar slope of
the web. -
Raise the dorsal rectangle at the subdermal level, taking a thin layer of subdermal fat with the flap.
-
Raise palmar Z flaps of the same thickness.
-
Transect distal fascial bands, protecting the digital neurovascular bundles.
-
Interdigitate the dorsal and palmar
flaps, attaching the corners of the dorsal rectangle to the palmar side
at the normal level of the proximal digital flexion crease and wrapping
a palmar flaps around the base of each digit. -
Close with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing,
covered with either Coban (for the older child who can keep the
dressing clean and dry) or an above-elbow fiberglass cast shell.
P.1906 -
-
Complete syndactyly release (for syndactyly extending distal to the PIPJ; Fig. 69.30, Fig. 69.31 and Fig. 69.32).
![]() Figure 69.30. Incisions for syndactyly release.
Figure 69.30. Incisions for syndactyly release.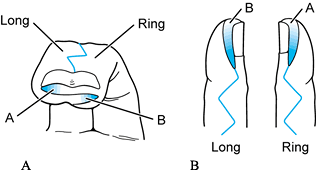 Figure 69.31. Syndactyly tip release.
Figure 69.31. Syndactyly tip release.![]() Figure 69.32. Syndactyly release. A: Dorsal incisions. B: Palmar incisions. C: Tip incisions. D: Fingers spread open after release. E: Web reconstruction. F: After closing incisions and skin grafting. G: Immediately after cast removal.
Figure 69.32. Syndactyly release. A: Dorsal incisions. B: Palmar incisions. C: Tip incisions. D: Fingers spread open after release. E: Web reconstruction. F: After closing incisions and skin grafting. G: Immediately after cast removal.-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Prepare and drape the full-thickness graft donor site.
-
Carefully plan and mark all flaps before inflating the tourniquet.
-
Place the base of the palmar V at the
normal level of the proximal digital flexion crease. Place the base of
the dorsal rectangle several millimeters proximal to the V, to create
the normal dorsal-palmar slope of the web. The palmar V and the dorsal
rectangle should be slightly longer than the depth of the palm. The
sides of the dorsal rectangle should not extend beyond the midline of
either digit (Fig. 69.30 and Fig. 69.31). -
If the syndactyly is complicated, and
there is no redundant soft tissue between the digits, consider using
crossed triangles instead of the dorsal rectangle-palmar V technique
described here. -
Plan the palmar and dorsal triangular flaps on the fingers to interdigitate with each other after the fingers are separated.
-
-
Elevate all flaps with a thin layer of subdermal fat.
-
Separate the synonychia, if present (Fig. 69.31 and Fig. 69.32).
Plan two long narrow flaps across the fingertips, one based on each
fingertip. Each flap is the length of the nail. Raise these flaps
carefully, and divide the nail, the nail bed, and any synostosis of the
distal phalanges. Fold each flap down alongside the divided side of the
nail to create the nail wall. -
Separate the digits carefully, searching for the neurovascular bundles.
-
If the common digital artery and nerve
bifurcate distal to the new web margin, separate the fascicles of the
common digital nerve into the two proper digital nerves. Ligate the
smaller of the two digital arteries, or the artery that does not supply
a border digit (for example, preserve the radial digital artery to the
small finger, since the ulnar digital artery to the small finger is
often hypoplastic). -
Secure the flaps in place with 5-0
chromic suture. Split the dorsal rectangular flap longitudinally,
almost to its base. Wrap half around each finger, suturing the corner
closest to the midline of the flap in the triangular defect adjacent to
the palmar triangular flap. -
Grafts are always needed on the proximal
dorsal aspect of each finger. Usually one finger can be closed without
any additional graft, and the remaining finger needs 3 to 5 grafts to
fill in defects not covered by flaps. -
Make patterns of all defects requiring grafts.
-
Deflate the tourniquet.
-
Transfer the patterns to the groin.
Harvest thick split-thickness graft in the shape of the patterns.
Stretch the skin while transferring the patterns and harvesting the
grafts, or the grafts will be too big. Remove the remaining dermis from
the groin wound and close in layers as a full-thickness defect. -
Attach the grafts to the hand using interrupted 5-0 chromic suture.
-
Dress hand wounds with xeroform gauze and
cotton balls soaked in normal saline. Apply a bulky compressive
dressing, and cover it with an above-elbow fiberglass cast shell.
P.1907P.1908 -
-
Separation of symbrachydactyly (see the sections entitled Transverse Failure of Formation [Hypoplasia and Absence] and Cleft Hand).
-
Follow the procedure described earlier.
Avoid the temptation to make the webs deeper than normal in order to
make the fingers appear longer.
-
-
Separation of thumb-index syndactyly (see the section entitled Cleft Hand).
-
Separation of syndactyly in Apert’s
syndrome: Syndactyly associated with Apert syndrome can be classified
into several different types (210). All are
complex, and involve at least one border digit. Because multiple
operations will be necessary to separate the digits, operate as early
as the pediatric anesthesiologist deems safe. If possible, coordinate
syndactyly release to be performed at the same anesthetic as the
craniofacial surgery these infants require. Reconstruct three fingers
instead of four if bony and soft tissue are inadequate. The details of
the treatment of this condition are too complex to cover in this
chapter. See Upton (210) and Van Heest (216) for details.
wet, especially within the first week after surgery, the cast must be
removed and the dressing changed, or the graft may fail. Remove the
cast and dressing after 4 weeks; the child may then use the hand
without restrictions. No splinting or therapy is necessary.
margin that ends up more distal than the surgeon intended) are most
commonly caused by too much tension in the web space closure. Plan
pedicle flaps carefully so they cover the base of the web, because
graft at the base will contract and narrow the web. Use graft
generously, but avoid making each individual graft too large for its
space, or the contour will not be smooth.
hand operations, if it is not done well the first time, it is very
difficult to redo well.
which is used to describe a flexion contracture of the PIPJ, usually of
the small finger. The incidence of camptodactyly is unknown.
Malformation of several different anatomic structures has been cited as
the underlying cause of this deformity: anomalies of musculotendinous
structures such as the lumbrical (87,141), the FDS (84,160,189), both of these (137), and the extensor mechanism (104,105).
splinting and stretching of the flexion contracture. The older child
and adolescent should wear a static splint at night and a dynamic
splint for 30- to 60-minute intervals during the day. The infant uses
the static night splint only. Splinting usually improves the
contracture (72,145,183), especially for isolated infantile camptodactyly (13), but many patients and parents find splinting and stretching too burdensome.
patient to use a splint is not an indication for surgery, inasmuch as
postoperative management includes therapy and splinting. Surgery should
be considered only when the contracture worsens in spite of splinting
and interferes with function. The results of surgical release are often
poor (less than 50% of fingers with postoperative increased range of
motion) (48,183) and tend to deteriorate with follow-up (13).
when initially seen, and whether the condition is associated with a
syndrome (13). Type I, the most common type, is
an isolated anomaly affecting the small finger. It is present in the
infant and almost always improves with splinting and stretching. Type
II is similar to type I, except that the patient with type II
camptodactyly is first seen in adolescence (Fig. 69.33). This type also improves with splinting, and even if the deformity is severe, it rarely interferes with function (55).
In type III camptodactyly, multiple digits are involved, the
contractures are more severe than types I and II, and the child has an
associated syndrome. Type III also improves with splinting, but is the
most likely of the three types to require surgery.
 |
|
Figure 69.33. Adolescent camptodactyly.
|
occupational therapist; for infants, the splint is forearm based, and
for older children, the splint is hand or finger based. Finger-based
dynamic splints are commercially available. Always first try splinting
and stretching before surgical release.
change in these ranges with wrist position, in order to help
differentiate intrinsic from extrinsic causes. Obtain a lateral
radiographic view of the involved digit; if the palmar aspect of the
distal end of the proximal phalanx is flattened, the deformity is less
likely to respond to surgical release than if it is not (55).
-
Corrective soft-tissue release and tendon transfer (small finger, less than 40° PIPJ flexion contracture) (55).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Plan the incision to allow skin coverage
for the straightened finger (midline palmar incision, which can be
converted to Z-plasties; obtain consent for full-thickness skin graft)
and to allow exploration of the palm. -
Explore the full length of the fourth lumbrical; free any adhesions. Inspect the fourth palmar interosseous insertion.
-
Divide the small FDS just proximal to the
vincula longa, through a window in the flexor sheath. Withdraw it into
the palm. If possible, separate it from any adhesions to the ring FDS,
pass it dorsal to the transverse metacarpal ligament, and transfer it
to the radial lateral band of the extensor expansion. If the small FDS
cannot be separated from the ring FDS, excise the small FDS and
transfer the ring FDS instead. -
If the PIPJ contracture is still greater than 30°, release as described in the next section.
-
-
Release of type III camptodactyly (7,104).
-
Use loupe magnification and perform the operation under general anesthesia and tourniquet.
-
Approach the PIPJ from the dorsum.
Release the subluxated lateral band by transecting the transverse
retinacular ligament; plicate the central slip. -
Transfer the FDS to the radial lateral
band of the extensor expansion, either through the dorsal incision, or
through a separate palmar incision. -
If the PIPJ flexion contracture is still greater than 30°, release as described in the next section.
-
-
PIPJ flexion contracture release.
-
Through a window in the flexor tendon
sheath, release the proximal origin of the palmar plate and the
check-rein ligaments. Stop here if the PIPJ can be straightened. -
If not, release the proximal origins of the collateral ligaments. Stop here if the PIPJ can be straightened.
-
Release the FDS, if you have not already done so.
-
Hold the PIPJ as straight as possible
with a Kirschner wire. Release the tourniquet; if the finger is not
well vascularized, remove the Kirschner wire, allow the PIPJ to flex,
and pin it in more flexion, ascertaining that the finger is well
vascularized in the position of immobilization. -
Close the incision with Z-plasties of the
longitudinal incision, or if necessary, full-thickness skin graft,
using interrupted 5-0 chromic suture. -
Apply a bulky compressive dressing and cover with a fiberglass cast shell.
-
finger in extension full time (except during bath or flexion exercises,
which should be performed several times each day) for 4 to 6 more
weeks. Splint at night indefinitely.
It is usually caused by malformation of the distal end of the middle
phalanx but may also be due to a longitudinal epiphyseal bracket (LEB,
or delta phalanx) or to soft-tissue contracture (35).
Although it can occur in any finger, it is most common in the small
finger. This condition is quite common; in the United States, radial
deviation of the small finger at the DIPJ may occur in up to 1% of
normal children and 10% of children with other congenital anomalies (35); other series report up to 21% incidence in certain populations (200).
Familial clinodactyly shows autosomal dominant inheritance with reduced
penetrance and is not usually associated with other anomalies (200).
 |
|
Figure 69.34. Clinodactyly of bilateral small fingers.
|
Splinting does not affect the deformity. Surgical treatment is
indicated for worsening clinodactyly caused by an LEB, or when the
patient is bothered by crossing of the flexed fingers.
and extends longitudinally along the diaphysis and transversely across
the opposite end of the affected bone, forming a C shape (Fig. 69.35) (154,218,231). For this type of clinodactyly, simple division of the abnormal LEB is not reliably successful (55).
If the patient is skeletally immature, remove a portion of the midzone
or isthmus of the continuous epiphysis and replace it with fat graft
(physiolysis) (218). For clinodactyly of any etiology, regardless of skeletal maturity, an ulnar closing wedge osteotomy corrects the angulation (35,224).
If modest lengthening is desired in addition to straightening, in the
larger hand, a reversed-wedge osteotomy can be performed by removing a
wedge of bone from the convex (usually ulnar) side, reversing it, and
inserting it on the concave (usually radial) side (27,95).
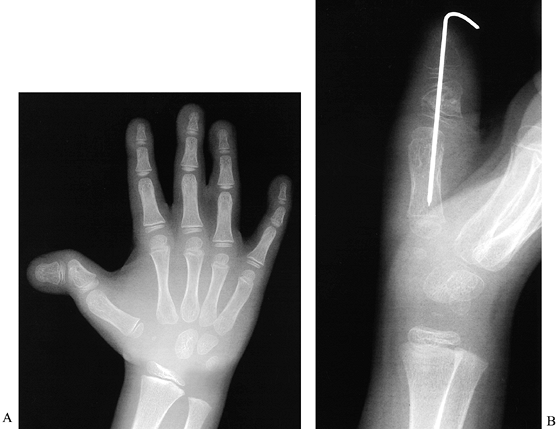 |
|
Figure 69.35. Longitudinal epiphyseal bracket. A: Preoperative radiograph. B: Following closing wedge osteotomy.
|
Hand surgeons classify clinodactyly by the tissue involved and
subclassify by severity of angulation and the presence of other
malformations (Table 69.7) (35).
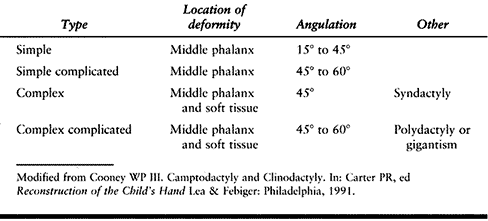 |
|
Table 69.7. Classification of Clinodactyly (35)
|
bracketed diaphysis, and C-shaped epiphysis. LEB has been classified
into five types by the shape of the bone, and the presence and shape of
the epiphysis (Table 69-8) (55).
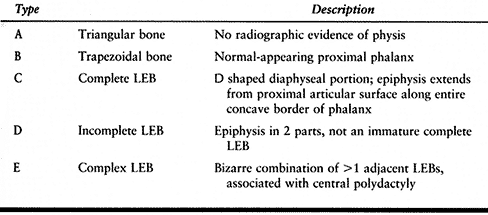 |
|
Table 69.8. Classification of Longitudinal Epiphyseal Bracket (LEB) (55)
|
to determine if the angulation is due to an LEB. Physiolysis requires
further growth of the phalanx for straightening
to
occur; 7 years is the earliest age at which this operation has been
reported, although the author estimates that the optimal age for this
operation would be about 3 years (218).
Osteotomy is difficult in very small phalanges; serial radiography
helps the surgeon determine if the deformity is progressive and whether
the phalanx is big enough for this operation.
-
Physiolysis (218).
-
Use loupe magnification and perform the
operation with the patient under general anesthesia and tourniquet.
Have radiography (Fluoroscan) available. -
Through a midlateral incision on the
radial (concave) side of the affected digit, reflect the periosteum to
expose the diaphysis. -
Use Kirschner wires or fine needles and radiography to locate the area to be removed.
-
Remove the isthmic region of the continuous epiphysis and physis with a fine rongeur.
-
Use a fine curet to remove bone from the
metaphysis, leaving the cartilage of the longitudinal physis more
prominent than the bone. -
Wash out this cavity and fill it with fat
graft harvested from the medial forearm (harvesting fat from the finger
may cause scarring, which could tether growth). -
Close the periosteum to hold the graft in place.
-
Close the wound with interrupted 5-0 chromic suture.
-
Immobilize by buddy-taping to the ring finger, or apply a bulky compressive dressing and cover it with a fiberglass cast shell.
-
-
Wedge osteotomy (closing or opening) (Fig. 69.36).
 Figure 69.36.
Figure 69.36.
Opening wedge osteotomy of longitudinal epiphyseal bracket. See text
for details. (From Wood VE. Congenital Triangular Bones in the Hand. J Hand Surg 1977;2:179.)-
Use loupe magnification and perform the
operation with the patient under general anesthesia and tourniquet.
Have radiography (Fluoroscan) available. -
If possible, approach the affected
phalanx from the dorsum. If this approach will damage the extensor
mechanism, approach from the convex side of the deformity. -
Insert a fine Kirschner wire or needle
across the joint proximal to the affected phalanx. Plan the proximal
cut of the wedge parallel to this wire. -
Plan the distal cut of the wedge at an angle to the proximal limb sufficient to correct the angulation of the phalanx.
-
Plan either closing or opening wedge osteotomy (opening is preferable if additional length is needed, but requires bone graft).
-
Make the osteotomies using either a power saw with a 4 mm blade, or the tip of a fine bone cutter.
-
After adequate correction, pass a fine
Kirschner wire antegrade through the osteotomy and out the tip of the
digit, the back across the osteotomy. Cross as few joints as possible.
If possible, pass another fine Kirschner wire to cross the osteotomy at
an angle. -
Close the wound with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing over the fingers and hand, and cover it with a fiberglass cast shell.
-
-
Reverse wedge osteotomy.
-
Proceed as described earlier, except for the following:
-
Use a dorsal approach.
-
Plan the second, distal cut of the osteotomy to slightly undercorrect the deformity.
-
Use the tip of a fine bone cutter to make
the osteotomies if at all possible, because the width of the saw blade
or osteotome creates further shortening. Nibble away at the bone
slowly, or it will splinter. -
After the wedge is removed, place a small
longitudinal Kirshner wire antegrade through the osteotomy, then
replace the reversed wedge, and transfix it with the wire by driving it
retrograde across the wedge and into the phalanx. Cross as few joints
as possible. There will not be room for a second wire.
P.1914 -
elbow, if necessary, to keep the cast in place) for 4 to 6 weeks. Then
remove the Kirschner wires if radiographic signs of healing are
present. Splints and therapy are not usually necessary.
The reverse wedge technique is especially challenging in a small
child’s finger. Adjacent structures, including the interphalangeal
joints, flexor and extensor tendons, and the neurovascular bundles are
easily damaged.
clinodactyly associated with an LEB. Clinodactyly associated with
deformity of the distal end of the middle phalanx is unlikely to
progress or cause functional limitations; wait until skeletal and
emotional maturity before proceeding with surgical correction of this
cosmetic deformity.
Anomalies similar to those seen in CRS have been reported to be
associated with maternal cocaine use and chorionic villus sampling (6,83).
Leading theories about causation include disruption during
blastogenesis; embryonic vascular disruption; and mechanical disruption
(ensnaring of fetal fingers in the chorion) (6). CRS should be differentiated from transverse deficiency (142,162) (Table 69.6).
Digits may be amputated, constricted by a partial or complete ring (a
tight band of tissue), or webbed. Webbed fingers are typically short
and fenestrated, with a sinus at the original web location
(acrosyndactyly); syndactyly may be nonadjacent (146). Nail deformities are common (54).
Surgical treatment includes Z-plasty of constriction rings and release
of acrosyndactyly, usually when the child is older than 1 year of age.
When the condition is severe with multiple-digit involvement, simple
finger separation in the first 10 days of life can prevent serious
later deformities. Deep circumferential rings may need to be released
earlier if lymphedema is severe, if the vascular supply of the digit or
extremity is compromised, or if the ring is causing a peripheral nerve
palsy (209,228).
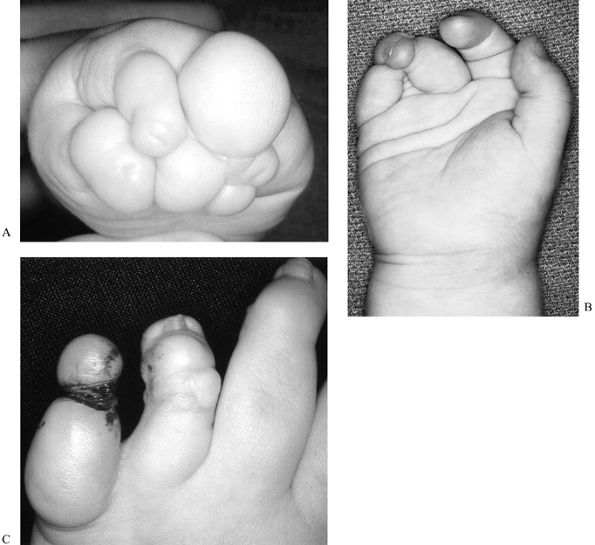 |
|
Figure 69.37. Congenital constriction ring syndrome. A: Acrosyndactyly. B: Nonadjacent syndactyly. C: Constriction band.
|
normal location of a digital flexion crease, do not require release;
the appearance will improve with growth and absorption of infant fat (54).
With growth, angulatory or rotational malalignment may occur and
require correction. Deformed nails may require ablation, if they become
bothersome. Distraction osteogenesis and free toe transfers may be
indicated; unlike finger hypoplasia, proximal neurovascular and
musculotendinous structures are probably normal.
including constriction bands, annular rings or bands, amniotic band
syndrome, and Streeter’s syndrome. Acrosyndactyly is the term used to
describe syndactyly associated with CRS: fusion of two or more digits
with epithelial lined sinuses at or distal to the normal location of
the
web margin, indicating that the digits fused together after developing a web (24). Constriction rings, acrosyndactyly, and nonadjacent syndactyly are pathognomonic for CRS.
-
Simple constriction rings.
-
Constriction rings with distal deformity, with or without lymphedema.
-
Constriction rings with distal fusion (acrosyndactyly).
-
Intrauterine amputation.
mild (three phalanges, two interphalangeal joints in affected digits);
moderate (two phalanges and only one interphalangeal joint); and severe
(one phalanx and no interphalangeal joints) (65). Mild involvement is the least common.
-
Z-plasty of constriction ring (Fig. 69.38).
![]() Figure 69.38. Z-plasty of congenital constriction ring (211).
Figure 69.38. Z-plasty of congenital constriction ring (211).-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Draw serial Z-plasties along the constriction ring. Release the entire circumference, if necessary (76).
If the ring occurs at a normal digital flexion crease, that portion of
the ring does not need to be released. The serial Z-plasty must start
and end with a Z, or the flaps will not interdigitate properly. -
Raise the flaps as marked, including a
thin layer of subdermal fat. Remove additional subcutaneous fat and
lymphedematous tissue, or redistribute as necessary to improve the
contour of the digit (Fig. 69-38) (211). -
Interdigitate the flaps, and suture in place with 5-0 chromic suture.
-
Apply a bulky compressive dressing, and cover it with an above-elbow fiberglass cast shell.
P.1916 -
-
Acrosyndactyly release.
-
Each of these conditions of the hands has a unique set of anomalies. Some resemble a bunch of grapes (54),
and it may be difficult to match the fingertip with the finger. Follow
these basic principles, and tailor the operation to the malformations:-
In especially severe cases, separate the
digits to the level of the epithelial sinus early, and deepen the webs
later when the child is older. -
Use the epithelial sinus as the base of
the web if it is found at the approximate normal web location. Excise
the entire sinus if its location or size is inappropriate. -
Plan local flaps to cover the web surface and, if necessary, the fingertips.
-
Defat to reduce the coverage needed and improve the contour; use a full-thickness skin graft as needed.
-
Defat and use skin from any amputated parts as graft.
-
If necessary, revise webs when the child is a teenager.
-
-
-
Nail ablation (see Chapter 38).
-
Distraction osteogenesis (see Chapter 32 and Chapter 171).
-
Free vascularized toe transfer (see Chapter 35 and Chapter 36).
release, remove the cast and dressing after soft-tissue healing has
occurred (about 3 weeks). Splinting and therapy are not usually
necessary.
the base of the web) or removed, an epidermal inclusion cyst will form,
and eventually require surgical incision.
performed by an experienced hand surgeon, because residual deformity is
common even when the hand is reconstructed by an expert. Sometimes,
when the skeleton is inadequate to support five digits, the surgeon may
elect to reconstruct a four-digit hand.
In the cleft hand, central bony elements are missing. The deficiency
varies from absent long finger phalanges, to absent index, long, and
ring finger rays (53), to monodactyly, or even absence of all digits (119). Wider clefts are associated with more adducted and deficient thumbs (53,127).
Children and adults with this malformation are able to function almost
normally, but they may hide their hands to avoid embarrassment.
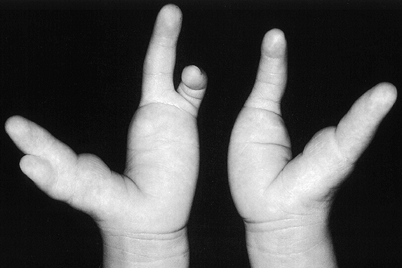 |
|
Figure 69.39. Cleft hands.
|
mapped to two different loci: 7q21.2-q21.3 (25) and 10q25. Fibroblast growth factors 2 and 8, and 2 different HOX genes have also been mapped to 10q25 (168).
and is frequently associated with other musculoskeletal anomalies
including central polydactyly, camptodactyly, longitudinal epiphyseal
bracket, and absence of the tibia, and anomalies of other organ systems
including cleft lip and palate (144,156,198,199). Cleft hand is also frequently associated with various syndromes (199). The true cleft hand should be differentiated from symbrachydactyly, formerly called atypical cleft hand (Table 69.9; see the section entitled Transverse Failure of Formation [Hypoplasia and Absence]) (125).
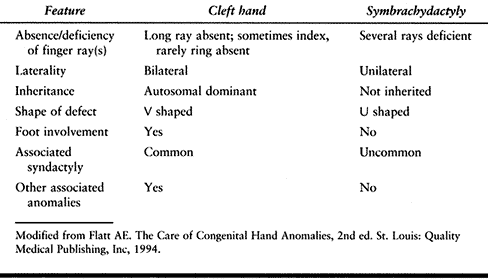 |
|
Table 69.9. Differential Diagnosis of Cleft Hand vs. Symbrachydactyly (53)
|
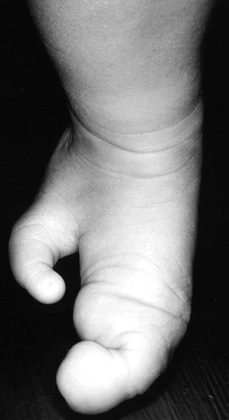 |
|
Figure 69.40. Cleft foot.
|
different combinations of anomalies are seen in different cleft hands.
Surgical treatment is not always indicated, and surgical planning is
complex, because various operations may be indicated in varying
combinations. Normal appearance is rarely attainable.
by releasing the adducted thumb or thumb-index syndactyly, to separate
other syndactylies, and to remove transverse bony blocks. Surgical
closure of the cleft, correction of camptodactyly, and rotational
osteotomies may be indicated, but these operations should be postponed
until the child is older and the surgeon can be more certain that they
will not interfere with function.
this term is no longer used. Ectrodactyly is sometimes used to describe
cleft hand, but this term, which usually means missing digits, is used
differently by different authors. Geneticists frequently call this
condition split hand. Until recently, cleft hand was divided into two
types—typical and atypical (8,121,176,198). Atypical cleft hand is now termed symbrachydactyly, and typical cleft hand is called cleft hand, or true cleft hand (Table 69.9) (53,125).
on which bones are missing; this complicated classification has 11 subtypes (152),
but these subtypes do not help determine prognosis or treatment of this
condition. Manske has classified cleft hand based on the
characteristics of the thumb web (Table 69.10) (127).
Types I and II are treated with thumb-web deepening and cleft closure;
type III with web deepening, often combined with index-to-long
metacarpal transposition or excision of bony remnants of the index;
types IV and V did not usually require surgical correction.
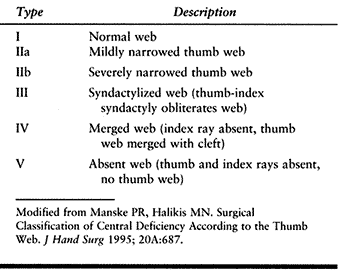 |
|
Table 69.10. Classification of Cleft Hand Based on the Thumb Web
|
geneticists have described two types of nonsyndromal cleft hand based
on two different inheritance patterns: in the first, cleft hand or foot
is inherited in an autosomal dominant pattern with high penetrance; in
the second, the gene is nonpenetrant, and expression may skip several
generations (240). The second pattern implies the existence of another gene, which controls the expression of the cleft hand and foot gene.
bones are absent or anomalous. If a transverse phalanx is present,
remove it as soon as possible because it will cause the cleft to widen
with growth. Thumb-web deepening can be combined with cleft closure, by
transposing the index to the base of the long metacarpal and shifting
the excess skin from cleft closure to the first web. If the thumb and
index are syndactylized, however, cleft closure should be postponed,
because the child may prefer prehension using the digits on either side
of the cleft instead of the thumb, and cleft closure would interfere
with this pattern.
wear shoes, schedule syndactyly release for the same anesthetic,
because skin removed in order to close the foot cleft can be used as
graft on the hand.
-
Deepening of thumb web.
-
Release of thumb-index syndactyly (Fig. 69.41)
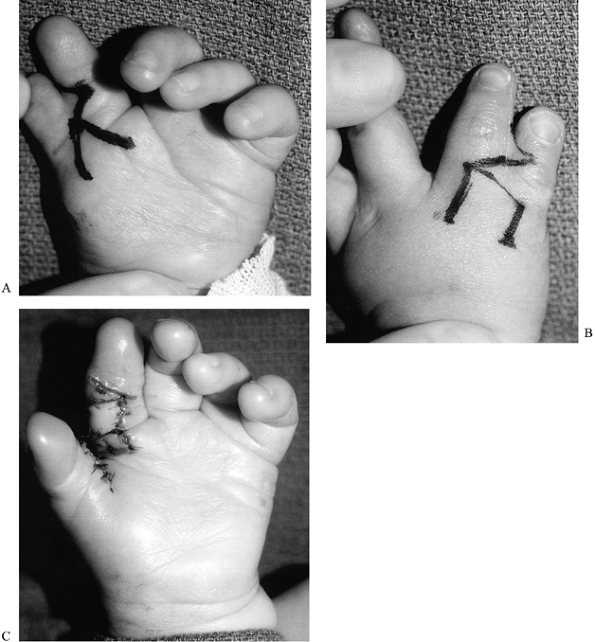 Figure 69.41. Thumb index syndactyly release. A: Palmar incisions. B: Dorsal incisions. C: Postoperative palmar view.
Figure 69.41. Thumb index syndactyly release. A: Palmar incisions. B: Dorsal incisions. C: Postoperative palmar view.-
Syndactyly release: see the section entitled Syndactyly, with the following changes:
-
Plan the base of the dorsal rectangular
flap at the level of the thumb MPJ. Make this flap as wide as possible,
but do not extend the sides of the rectangle past the midline of the
thumb and index fingers. -
Release the first dorsal interosseous and adductor pollicis fascia (217).
-
-
-
Removal of transverse phalanx (Fig. 69.42).
![]() Figure 69.42. Transverse phalanx.
Figure 69.42. Transverse phalanx.-
Removal of a transverse phalanx is combined with other operations including thumb web release and cleft closure, as indicated.
-
Approach the transverse phalanx through a longitudinal zigzag dorsal incision.
-
Remove the entire phalanx, including the
periosteum. Removal is more difficult than it appears, because the
phalanx is larger than radiography indicates (because of incomplete
ossification) and is intimately connected to surrounding structures. -
If removal of the transverse phalanx destabilizes the MPJs, reconstruct collateral ligaments using available tissue.
-
Reconstruct the transverse intermetacarpal ligament with nonabsorbable suture (see the section entitled Closure of Cleft).
-
Close the skin incision with interrupted 5-0 chromic suture.
-
Apply a bulky compressive dressing over the fingers and hand, and cover it with an above-elbow fiberglass cast shell.
-
-
Closure of cleft (Fig. 69.43 and Fig. 69.44).
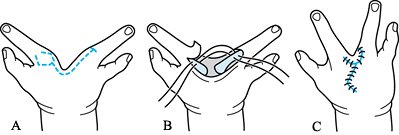 Figure 69.43. Cleft closure. See text for details. (8)
Figure 69.43. Cleft closure. See text for details. (8)![]() Figure 69.44. Cleft hand. A: Preoperative palmar view. B: Postoperative palmar view (left hand).
Figure 69.44. Cleft hand. A: Preoperative palmar view. B: Postoperative palmar view (left hand).-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Plan skin incisions to produce zigzag
interdigitations on both palmar and dorsal surfaces. Create a flap
along the side of one digit to form the new web. A diamond shape works
well for this (8) (Fig. 69.43A). -
Remove any bony or soft tissue holding the metacarpals apart.
-
Construct an intermetacarpal ligament out
of adjacent soft tissue. If no tissue is available, drill holes in the
metacarpals as far distal as possible, but avoid damaging the
epiphyses, and pass nonabsorbable suture through these holes to hold
the metacarpals together. -
Close the skin with interrupted 5-0
chromic suture, excising excess skin as necessary and contouring the
closure to create a normal web slope (Fig. 69.43B, Fig. 69.43C). -
Apply a bulky compressive dressing designed to support the cleft closure. Cover with an above-elbow fiberglass shell.
-
-
Transposition of the index finger (combined cleft closure and first web deepening) (Fig. 69.45)
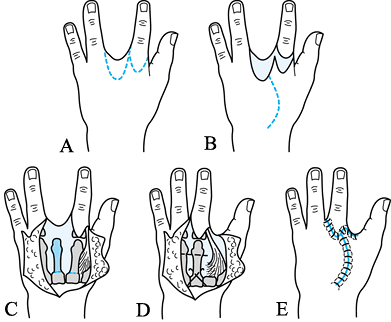 Figure 69.45.
Figure 69.45.
Combined cleft closure and first web deepening. See text for details.
(From Miura T, Komada T. Simple Method for Reconstruction of the Cleft
Hand with an abducted thumb. Plast Reconstr Surg 1979;64;65.)-
If the thumb and index are not
syndactylized (Manske type II), the index may be transposed, the cleft
closed, and the excess skin from cleft closure used to deepen the web
space, all at the same operation. This complex operation as originally
described by Snow and Littler, is detailed elsewhere (53). A simpler modification, by Miura and Komada, is described in the following section (143). -
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Approach the cleft and base of the index
through a curved dorsal incision, combined with other incisions
designed to transfer excess cleft skin to augment the first web. (Fig. 69.45A, Fig. 69.45B) -
Detach the index metacarpal at its base,
releasing the adductor pollicis and first dorsal interosseous fascia,
and possibly the thumb attachment of the first dorsal interosseous. -
Move the index to the stump of the long metacarpal or its space. Check rotation, and pin it in place using Kirschner wires (Fig. 69.45C, Fig. 69.45D).
-
Repair or create an intermetacarpal ligament (see the section entitled Closure of Cleft).
-
Close the skin with interrupted 5-0 chromic suture (Fig. 69.45E).
-
Apply a bulky compressive dressing over the fingers and hand, and cover it with an above-elbow fiberglass cast shell.
-
reconstruction of the transverse intermetacarpal ligament. Check bony
healing radiographically and remove the pin or pins at 6 weeks
postoperatively for index transposition. No splinting or therapy is
necessary.
close it until you are certain that thumb function can be restored. If
the cleft is closed without construction of a transverse
intermetacarpal ligament, it will widen (it may do so anyway). If the
cleft is closed too tightly, or if the surgical scar contracts, the
fingers may overlap, impairing function.
best treated by an experienced hand surgeon. The treatment must be
tailored to the individual cleft hand. Complete thumb-index syndactyly
cannot usually be released to the normal thumb web level. If the only
space available for prehension is the cleft (Fig. 69.39), surgery is not indicated, but peer contacts and counseling are often helpful.
that is nearly always associated with thumb and carpal deficiencies and
frequently associated with other upper extremity anomalies, anomalies
of other organ systems, and syndromes (60,79,91,107,126,184,198).
The newborn with radius deficiency should be carefully examined for
signs of the VACTERL association (not inheritable; it may be
accompanied by Vertebral, Anal, Cardiac, Tracheo-Esophageal, Renal or Radial, and Lung
anomalies), Holt-Oram syndrome (autosomal dominant inheritance of
cardiac septal defects associated with upper limb anomalies), and TAR
syndrome (autosomal dominant or recessive inheritance of completely
absent radius with a near-normal thumb and thrombocytopenia) (3,4,9,12,177,180,199).
Multiple other syndromes, many inheritable, are associated with radius
deficiency. Radius deficiency is usually bilateral, although the two
sides are frequently asymmetric; when the condition is unilateral, it
is more common on the right (91,107).
varying degrees. Flatt has described radial clubhand as “a profoundly
abnormal hand joined to a poor limb by a bad wrist” (60),
and he has meticulously documented multiple abnormalities of muscles,
nerves, and blood vessels found in anatomical dissections of upper
limbs with radius deficiency (184). The radial
wrist extensors and extrinsic thumb motors are usually absent or
aberrant. The radial nerve is usually absent below the elbow, and the
median nerve is always present and often the most prominent structure
on the radial side of the wrist. The radial artery is usually absent.
usually require stretching and splinting, and no surgical treatment.
Moderately severe type 2 is treated with radius lengthening; severe
type 2 with centralization of the carpus on the end of the ulna. The
wrist instability and radial deviation associated with types 3 and 4 (Fig. 69.46 and Table 69.11 and Table 69.12)
is treated with centralization of the carpus on the end of the ulna,
with the best results if aberrant radial wrist extensors are
transferred to help maintain the new position, and if the operation is
performed before the child is 1 year of age (60). Before centralization, serial casting or application of a distraction device (149,187) helps stretch the radial structures. Stabilization of the wrist enhances the appearance and function of the hand (60)
but is difficult to maintain throughout growth; radial deviation tends
to recur unless the wrist is quite stiff. Centralization is
contraindicated for the following patients:
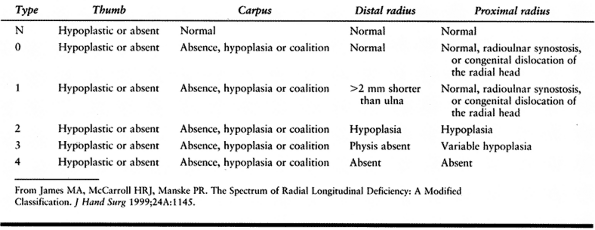 |
|
Table 69.11. Modified Classification of Radial Longitudinal Deficiency (91)
|
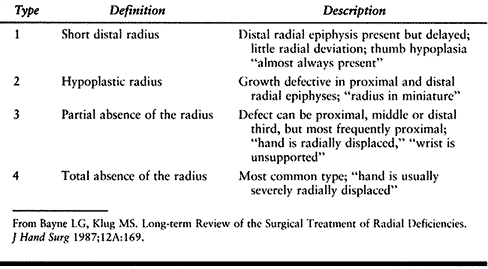 |
|
Table 69.12. Classification of Radial Longitudinal Deficiency (Bayne) (11)
|
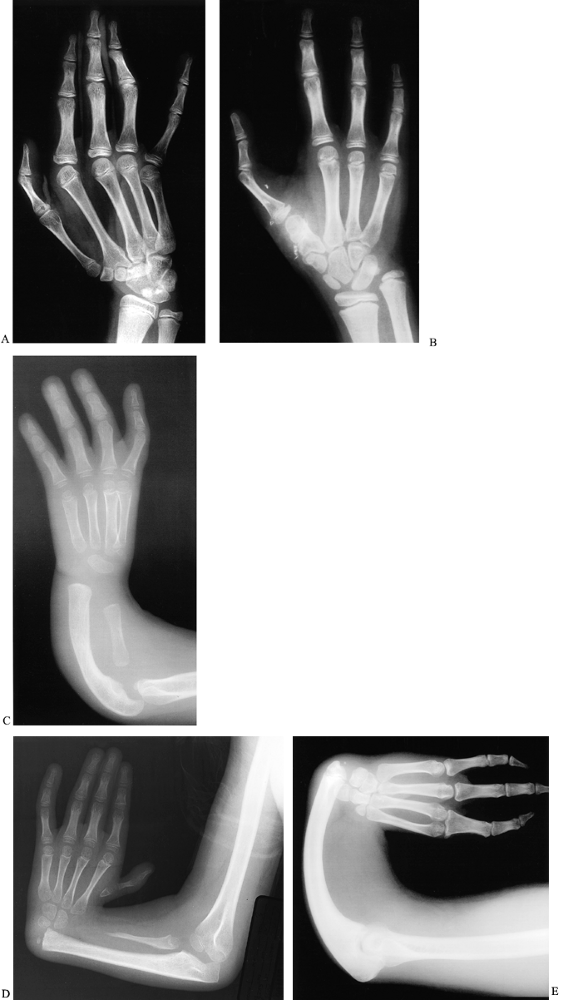 |
|
Figure 69.46. Radius deficiency. A: Type 0 (carpal anomalies; distal radius is normal). B: Type 1 (short distal radius). C: Type 2 (radius is deficient proximally and distally). D: Type 3 (distal radius is absent). E: Type 4 (the entire radius is absent).
|
-
Infants younger than 6 months of age for whom major organ defects may not yet have been diagnosed.
-
Children with severe associated anomalies that significantly decrease life expectancy.
-
Adults with firmly established functional patterns (see Fig. 69.47) (60).
![]() Figure 69.47. Untreated radius deficiency in adults. A: Photo. B: Radiograph (different patient from that shown in A).
Figure 69.47. Untreated radius deficiency in adults. A: Photo. B: Radiograph (different patient from that shown in A). -
Children without adequate elbow flexion for the hand to reach the mouth after the wrist is straightened.
the forearm may be only half as long as the unaffected side. Ulnar
lengthening using the distraction osteogenesis (such as with the
Ilizarov system) can successfully lengthen the forearm (1,28,97).
This technique is primarily indicated to improve the appearance of
unilateral radius deficiency, or to improve reach (for example, for
personal hygiene) in bilateral radius deficiency. Patients should be
carefully selected for this procedure, because the lengthening device
requires meticulous care and may be in place for several months.
described the combination of soft-tissue distraction and microvascular
epiphysis transfer for the treatment of type 4 radius deficiency.
Historically, attempts to treat longitudinal radius deficiency with
surgical transfer of nonvascularized growing bone have
failed, because the graft has not continued to grow (79,171,192). Vilkki’s early results are promising, although the technique he describes is technically demanding.
favor of longitudinal deficiency of the radius, or radius deficiency.
The term radius is used to refer to the radius bone; the term radial is
used to describe the radial structures of the forearm, wrist, and hand,
including the radius, radial carpus, and thumb.
deficiency, described by Bayne, includes four types based on increasing
radiographic severity of the radius deficiency (Table 69.12) (11).
In order to define Bayne type 1 better, and to include patients with
deficiency of the thumb or carpus in the presence of a normal-length
radius, James,
McCarroll, and Manske have modified Bayne’s classification scheme (Table 69.11 and Fig. 69.46) (91). This scheme is used in combination with the modified Blauth scheme for classifying thumb hypoplasia (Table 69.3).
deficiency as soon as possible after birth. Gently stretch the wrist as
close to neutral as possible, and apply an above-elbow flexed elbow
cast in the maximally corrected position with the elbow flexed. Change
the cast weekly, stretching the wrist further at each cast change. When
the wrist reachesneutral, apply a custom fabricated above-elbow
orthoplast splint and instruct the parents to stretch the wrist several
times each day. Continue with splinting and stretching until
centralization is performed.
-
Centralization (ulnar approach) (131) (Fig. 69.48).
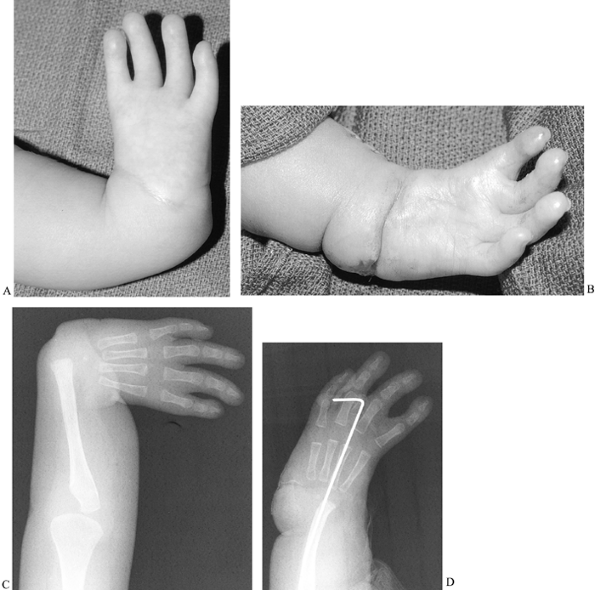 Figure 69.48. Centralization for radius deficiency (same patient as shown in Figure 69.8). A: Pre-operative palmar view. B: Postoperative palmar view. C: Preoperative radiograph. D: Postoperative radiograph.
Figure 69.48. Centralization for radius deficiency (same patient as shown in Figure 69.8). A: Pre-operative palmar view. B: Postoperative palmar view. C: Preoperative radiograph. D: Postoperative radiograph.-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Begin the incision at the level of the distal end of
P.1926P.1927
the ulna, near the dorsal wrist extension crease, just radial to the
midline. Extend it ulnarward, initially transverse, then forming an
ellipse in order to excise excess skin and subcutaneous tissue after
the carpus is reduced onto the ulna. -
Identify and preserve the dorsal ulnar sensory nerve.
-
Expose the extensor retinaculum.
-
Identify the extensor carpi ulnaris;
detach it from its insertion into the base of the fifth metacarpal, and
retract it proximally. -
Raise the extensor retinaculum as a radially based flap.
-
Identify and retract the finger extensor
tendons radially. If radial wrist extensors are present, detach them
and retract them proximally. -
Incise the wrist capsule transversely,
exposing the distal ulna. The carpal bones will be palmar and radial to
the distal ulna, and may be difficult to identify. -
Free the ulna of soft-tissue attachments distal to the physis. Detach the carpal bones from the palmar capsule.
-
Serially carve away central carpus and
shave distal ulna until the carpus fits on the end of the ulna without
tension. Do not injure the distal ulna physis or attached soft tissue.
Very tight wrists may require resection of the lunate and part of the
capitate before the wrist can be reduced. -
If the ulna is quite bowed, perform a closing wedge osteotomy through a separate proximal incision.
-
Pin the carpus on the distal ulna using a
long Kirschner wire. The wire can exit either through the distal end of
the long finger metacarpal or through the proximal ulna. If an ulnar
osteotomy has been performed, use the same Kirschner wire to fix it. -
Imbricate the wrist capsule.
-
Transfer radial wrist extensors into the
distal fragment of the extensor carpi ulnaris. Reattach the extensor
carpi ulnaris, advancing it to increase soft-tissue tension on the
ulnar side. -
Pass the retinacular flap underneath the finger extensors.
-
Excise excess skin and subcutaneous tissue.
-
Close the skin with interrupted 5-0 chromic suture.
-
Apply an above-elbow bulky compressive dressing, and cover with a fiberglass cast shell.
-
-
Ulnar lengthening (Fig. 69.49).
![]() Figure 69.49. Ulnar lengthening for radius deficiency. A: Preoperative radiograph. B: Postoperative radiograph. C: Ilizarov device in place during lengthening. D: Bone regenerating in gap created by lengthening.
Figure 69.49. Ulnar lengthening for radius deficiency. A: Preoperative radiograph. B: Postoperative radiograph. C: Ilizarov device in place during lengthening. D: Bone regenerating in gap created by lengthening.-
Follow the principles of Ilizarov lengthening of the forearm described in Chapter 32 and Chapter 171.
-
Use two half-pins and one transfixion
wire proximal to the osteotomy, and one half-pin and two transfixion
wires (one in the ulna and one in the metacarpals) distal to the
osteotomy. -
If the hand is radially deviated, correct the deviation at the same time as application of the fixator, using
P.1928P.1929
a closing wedge osteotomy through the carpus or distal ulna, and fixing
it with Kirschner wires. Otherwise, radial deviation will worsen when
the fixator is removed, because of increased tension placed on the
radial soft tissue by the lengthening.
-
to 12 weeks. Apply a custom-made above-elbow orthoplast splint to
maintain wrist position. Prescribe full-time wear for 3 to 6 months,
then nighttime wear indefinitely.
to lengthen the ulna, then proceed at a rate of approximately 1 mm per
day in three increments (the nuts in the pediatric Ilizarov set are six
sided, so the child can advance 1/3 turn three times per day). Check
progress on radiograph when lengthening begins and weekly thereafter,
adjusting the lengthening rate depending on regenerate formation. Teach
the child and family to perform the lengthening and pin care and to
maintain elbow and digit motion. Stop lengthening when the regenerate
appears thready or the elbow or digits start to develop flexion
contractures. The ulna can usually be lengthened by 30% to 50%. Leave
the fixator in place until four cortices are visible in the regenerated
bone (this usually takes three times as long as bone lengthening), then
remove the fixator under general anesthesia, and apply an above-elbow
fiberglass cast.
make reduction of the carpus on the distal ulna easier, but reduction
is nonetheless difficult to accomplish. The postcentralization cast
must be carefully molded and the elbow flexed to 90° or it will fall
off—or worse, it will slip partway off with the child’s wrist wedged in
the flexed portion of the cast. With long-term follow-up, centralized
wrists tend to be either stiff and straight, or flexible and deviated.
procedure acquires at least one pin tract infection. Treat these
infections with oral antibiotics unless the patient has a fever or
cellulitis, in which case intravenous antibiotics are necessary. If the
area is not carefully monitored, regenerated bone can heal too quickly
(requiring a second osteotomy) or too slowly (requiring bone grafting).
The proximal half-pins are the most troublesome; they tend to loosen if
the fixator needs to remain in place for more than 6 to 8 months.
two experienced people. Centralization is a technically demanding
operation that is best performed by the experienced hand surgeon.
realistic expectations and emotional stamina on the part of the
patient. It should be reserved for the mature older child or teenager
with good family support who can manage the care of the external
fixator. Preoperative peer counseling (by another patient with a
fixator in place) and preoperative psychological assessment by the
social worker are helpful. The support of an Ilizarov procedure team
(including a nurse, social worker, and occupational therapist) is very
helpful to the surgeon.
have hypoplasia of the entire upper extremity. The elbow is malformed
or absent (humeroradial synostosis) in the majority of cases (197). Deficiency of the ulna may be partial or complete, and a cartilaginous ulna “anlage” is often present (19,26,132,140,155).
All children with ulna deficiency have hand and carpal anomalies; about
90% of hands are missing digits, 30% have syndactyly, and 70% have
thumb abnormalities (33,64,123,197). Unilateral involvement is twice as common as bilateral involvement (197).
This condition is often associated with other musculoskeletal
anomalies, most commonly proximal femoral focal deficiency, fibula
deficiency, phocomelia, and scoliosis (65,197,199), but it is rarely associated with anomalies of other organ systems (123,197,199). In spite of their upper extremity malformation, children with ulna deficiency usually function well (15,64,190).
underwent thorough functional testing had no deficits in bimanual
function but performed one-handed tasks much more slowly with the
affected side. Patients with humeroradial synostosis and absent or
stiff fingers fared worst, and no correlation was found between
functional abilities and classification systems based on the elbow or
forearm (see the section entitled Classifications, later) (15).
series of patients with this condition are small, and authors disagree
about its natural history. Two sequelae of ulna deficiency are
especially controversial: progressive ulnar deviation at the wrist, and
forearm instability. Some authors report that ulnar deviation at the
wrist always worsens and is due to tethering by the ulna anlage; they
advocate early excision of the ulna anlage to prevent progression (26,155).
More recent reports disagree, and indicate that ulnar deviation usually
does not progress; they advocate anlage resection only if progression
is documented (64,132,140).
In order to treat forearm instability, some authors advocate surgical
construction of a one-bone forearm by cross-union between the radius
and ulnar remnant (26); others believe that loss of forearm rotation is not a good trade-off for forearm stability (64).
external rotation osteotomy of the humerus are well-accepted treatments
for malformations associated with ulna deficiency. First web deepening,
opponensplasty, thumb metacarpal rotational osteotomy, pollicization,
and syndactyly release should be performed when the child is old enough
to benefit from the operations, usually by age 1 to 4 years. The child
in whom the hand rests on the buttock or flank and cannot reach the
mouth or top of the head because of internal rotation of the arm and
forearm, combined with humeroradial synostosis, benefits from external
rotation osteotomy of the humerus (Fig. 69.50) (33,64,123,140).
Lengthening may improve the appearance of a short forearm but is
indicated only when the elbow is stable and has active elbow flexion.
 |
|
Figure 69.50. Ulnar deficiency.
|
preferred name for this condition is longitudinal deficiency of the
ulna, or ulna deficiency.
have used hand anomalies as the basis for classification. Cole and
Manske have described the only classification system for ulna
deficiency that correlates with treatment; their system is based on
thumb and first web deformities, and can be combined with one of the
elbow and forearm classifications (preferably that of Ogden, Bayne, or
Swanson, because these classifications address both forearm and elbow
anomalies) (Table 69.14).
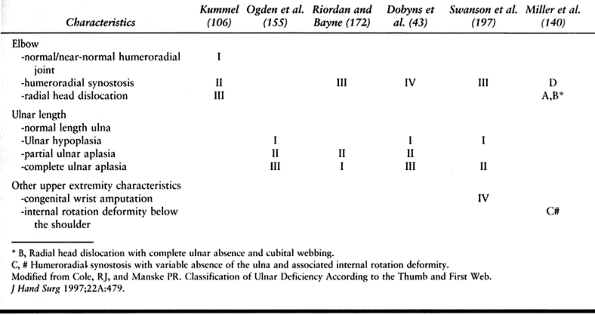 |
|
Table 69.13. Classification of Ulna Deficiency Based on Elbow and Forearm Anomalies (33)
|
 |
|
Table 69.14. Classification of Ulna Deficiency According to the Thumb and First Web (33)
|
-
Thumb reconstruction (first web deepening, opponensplasty, pollicization) (see the section entitled Hypoplasia and Absence).
-
Syndactyly release (see the section entitled Syndactyly).
-
Rotational osteotomy of the humerus (140).
-
Perform the operation with the patient
under general anesthesia, and if possible, tourniquet (a sterile
tourniquet may be necessary). -
Use a lateral incision to approach the
distal humerus subperiosteally. Score the bone longitudinally to mark
the preosteotomy alignment, or place parallel Kirschner wires above and
below the planned osteotomy site. -
Make a transverse osteotomy, and rotate
the distal fragment enough to place the hand in front of the trunk. If
the soft tissue is tight, shorten the bone to prevent traction injury
to neurovascular structures. -
Fix the bone with crossed Kirschner wires or a small A-O plate.
-
Close the subcutaneous tissue with absorbable suture, and the skin with running subcuticular absorbable suture.
-
Apply a well-fitted above-elbow cast, and attach it to the body with a velpeau-type dressing.
-
-
Creation of one-bone forearm. This operation is rarely indicated. See Lloyd-Roberts (116) or Spinner et al. (191) for details.
-
Forearm lengthening (Ilizarov technique). (See Chapter 32 and Chapter 171, and the section entitled Radius Deficiency.)
has a humeroradial synostosis in extension, the postoperative cast
cannot turn a corner and will tend to slip off, unless a Velpeau wrap
is applied.
reconstruction, syndactyly release, and rotational osteotomy improves
function. Some authors have advocated combining a closing wedge
osteotomy with the rotational osteotomy, in order to place the elbow in
flexion. This procedure is probably not necessary, because children
with humeroradial synostosis have a short upper limb and can usually
reach the mouth once rotation is corrected. Similarly, although the
forearm is usually supinated, forearm rotational osteotomy is rarely
necessary. Lengthening is not indicated unless the elbow is mobile and
stable.
angulation of the distal radius associated with an ulna-plus wrist,
caused by a growth disturbance of the palmar and ulnar portion of the
distal radial physis. This growth disturbance may be due to a
combination of a bony lesion in the ulnar portion of the distal radius
physis and an abnormal palmar ligament tethering the lunate to the
radius proximal to the physis (219). The growth
disturbance is the final common pathway for many different disorders,
including dysplasia, trauma, chromosomal abnormalities, infection, and
tumors. Girls are affected more often than boys, and the disorder is
usually bilateral, appearing most commonly between the ages of 6 and 13
years (34,37,43,44,205).
associated with Leéri-Weill syndrome (dyschondrosteosis), which is
inherited in an autosomal dominant fashion with 50% penetrance (119,182). It may also be associated with other syndromes, including nail-patella syndrome (onycho-osteodysplasia) (88). Repetitive loading of the wrist in the growing child may cause Madelung deformity in gymnasts (gymnast wrist) (36) or in javelin throwers (37).
necessary, although untreated Madelung deformity has been associated
with spontaneous extensor tendon rupture, probably due to the ulna-plus
deformity and disruption of the distal radioulnar joint (46,93).
If the deformity is painful, and the patient is not skeletally mature,
physiolysis may reduce pain and, with additional growth, improve the
deformity (219). In the skeletally mature
patient with wrist pain associated with Madelung deformity, correction
of the deformity may be helpful. Correction may be attained by Ilizarov
radial correction, radial closing wedge osteotomy, and ulnar
shortening; radial opening wedge osteotomy (147); radial osteotomy and distal ulna resection (213,225); or radial osteotomy and SauveéKapandji procedure (178).
Although osteotomy will also improve appearance, the magnitude of the
operation renders it unjustifiable for this indication alone.
physis, causing a reverse Madelung deformity, in which the distal
radius is angulated dorsally and radially (37,219).
This type of Madelung deformity has the same underlying etiologies. One
patient has been reported to have classic Madelung deformity on one
side and reverse Madelung deformity on the other side (219).
to estimate the size and shape of the wedge to be removed or inserted.
See wedge 4 determined by lines 1 and 2 in Fig. 69.51.
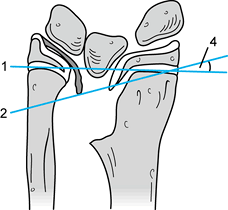 |
|
Figure 69.51.
Physiolysis for Madelung deformity. (From Vickers D, Nielsen G. Madelung deformity: surgical prophylaxis (physiolysis) during the late growth period by resection of the dyschondrosteosis lesion. J Hand Surg 1992;17B:401.) |
-
Physiolysis (Fig. 69.51).
-
Use loupe magnification and perform the operation with the patient under general anesthesia and tourniquet.
-
Use a palmar transverse incision 1.5 cm
proximal to the most proximal wrist flexion crease. Pass ulnar to the
palmaris longus and flexor carpi radialis tendons, and protect the
radial artery and median nerve. Locate and elevate the distal edge of
the pronator quadratus muscle. -
Raise a longitudinal flap of distal
radius, based ulnarward, approximately 5 mm from the distal radioulnar
joint. Take care not to injure the lunate. A sagittal section of the
distal radius is now visible. Remove fibrous tissue and bone until the
physis is identified; at first, it will be narrow and wavy. When
physeal cartilage is clearly defined, use a burr to remove bone from
the metaphyseal side so that the cartilage profile is proud from dorsal
to palmar periosteum. -
Release the tourniquet, and obtain hemostasis.
-
Reinflate the tourniquet, wash the
cavity, obtain fat from a separate incision in the proximal medial
forearm, and fill the cavity with fat. -
Allow soft tissue to fall together to hold the fat in place.
-
Close the skin with a running absorbable subcuticular suture. Release the tourniquet.
-
Apply a bulky compressive dressing and a removable wrist splint.
-
The same procedure can be done dorsally for reverse Madelung deformity.
-
-
Dorsal closing wedge osteotomy of the distal radius and ulnar shortening (Fig. 69.52)
![]() Figure 69.52. Radial osteotomy and ulnar shortening for Madelung Deformity. A: Preoperative dorsal view. B: Preoperative ulnar view. C: Preoperative AP radiograph. D: Preoperative lateral radiograph. E. Intraoperative view, with Kirschner wires in place to help plan osteotomy. F: Postoperative dorsal view (immediately after cast removal). G: Postoperative ulnar view. H: Postoperative AP radiograph. I: Postoperative lateral radiograph.
Figure 69.52. Radial osteotomy and ulnar shortening for Madelung Deformity. A: Preoperative dorsal view. B: Preoperative ulnar view. C: Preoperative AP radiograph. D: Preoperative lateral radiograph. E. Intraoperative view, with Kirschner wires in place to help plan osteotomy. F: Postoperative dorsal view (immediately after cast removal). G: Postoperative ulnar view. H: Postoperative AP radiograph. I: Postoperative lateral radiograph.-
Approach the ulna diaphysis (at
approximately the distal 1/4, proximal 3/4 junction) through a
longitudinal ulnar incision. Select a four-hole dynamic compression
plate (244 series); prepare the distal two holes, then make a
transverse osteotomy. Sharply dissect the interosseous membrane from
the proximal fragment so that the cut ends can overlap. -
Approach the distal radius through the
third and second dorsal compartments. Remove Lister’s tubercle. Score
the radius longitudinally across the planned osteotomy location to help
maintain correct rotation. Using fluoroscopy guidance, insert Kirschner
wires distally close to the radiocarpal joint, one parallel to radial
angulation and the other parallel to palmar angulation. Plan the distal
cut to reduce radial angulation to 20° and palmar angulation to 10°.
Make the proximal cut transverse. The radius osteotomy should be as
close to the radiocarpal joint as possible, leaving room for a T or L
plate for internal fixation. Provisionally fix the osteotomy with
Kirschner wires. -
Contour the selected plate. Apply to the dorsal distal radius. Remove the Kirschner wires.
-
Under fluoroscopic guidance, pull the
distal ulnar fragment proximally until the distal end of the ulna is
neutral or slightly ulna-minus. Resect the appropriate amount of ulna;
morsellize the resected portion and use for graft around the
osteotomies if desired (avoid placing graft between the radius and
ulna). Fix the two ends of the ulna with a four-hole plate, using the
distal holes drilled earlier, and compressing at the osteotomy site. -
Apply a bulky compressive dressing, and cover with an above-elbow plaster splint.
-
Sauveé-Kapandji procedure: see Chapter 43.
physiolysis. Therapy and splinting are not usually necessary. Following
osteotomy, immobilize it in an above-elbow splint for 2 to 3 weeks,
followed by a below-elbow cast for 3 more weeks. Remove hardware 1 year
after surgery.
not in intimate contact with the surgical defect, the physeal tether
may recur following physiolysis.
ulnar shortening restores distal radius angulation and the relationship
between the distal radius and ulna well, without requiring additional
bone graft. The Suaveé-Kapandji procedure is preferable to distal ulna
resection, but it should probably be used only as a salvage procedure
if radius osteotomy and ulnar shortening fail to relieve pain.
Hospital Northern California, for her assistance with references, and
Julia Serat, photographer, Shriners Hospital Northern California, for
her assistance with clinical photographs and radiographs. H. R.
McCarroll, Jr., M.D. was the technical consultant for the section
entitled Dorsal Closing Wedge.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JN, Weaver DD. Subclavian Artery Supply Disruption Sequence: Hypothesis
of a Vascular Etiology for Poland, Klippel-Feil, and Mobius Anomalies. Am J Med Genet 1986;23:903.
PA, Yu JS, Wiand W, et al. Madelung Deformity in Skeletally Immature
Patients: Morphologic Assessment Using Radiography, CT, and MRI. J Comput Assist Tomogr 1996;20:505.
Smet L, Matton G, Monstrey S, et al. Application of the
IFSSH(3)-classification for Congenital Anomalies of the Hand; Results
and Problems. Acta Orthop Belg 1997;63:182.
F, Teot L, Benningfield N, Humeau C. Ultrasonography of the Normal and
Abnormal Antenatal Development of the Upper Limb. Ann Chir Main Membr Super 1992;11:389.
JM, Meggitt BF. Trigger Thumbs in Children. A Review of the Natural
History and Indications for Treatment in 105 Patients. J Bone Joint Surg 1974;56B:153.
P, LeClercq C, Lisfranc R, Saffar P. Spontaneous ruptures of the
extensor tendons of the fingers in Madelung’s Deformity. J Hand Surg 1991;16B:329.
D, Lenoble E, Marin-Braun F, Foucher G. [Camptodactyly: Classification
and Results of Treatment Based on a Series of 50 Cases]. Ann Chir Main Membr Super 1994;13:20.
HVA. Aplasia and Hypoplasia of the Radius. Studies on 64 Cases and on
Epiphyseal Transplantation in Rabbits with the Imitated Defect. Acta Orthop Scand 1959;Supplementum no. 39:1.
P, Zguricas J, van Oosterhout L, et al. The Gene for Triphalangeal
Thumb Maps to the Subtelomeric Region of Chromosome 7q. Nat Genet 1994;6:287.
R, Wilhelm K, Brehl B. Distraction Lengthening of Metacarpal and
Phalangeal Stumps in Congenital Hand Deformities—Personal Experience
and Current Concept Review. Handchir Mikrochir Plast Chir 1998;30:196.
LB. Report of National Institute of Child Health and Human Development
Workshop on Chorionic Villus Sampling and Limb and Other Defects. Teratology 1992;48:7.
MM, Schuurman AH, Kon M. Camptodactyly Caused by an Anomalous Origin of
the Flexor Digitorum Superficialis Tendon. Case Report. Scand J Plast Reconstr Surg and Hand Surg 1996;30:71.
E, Nakamura R, Sakuma M, Miura T. Duplicated Thumb Bifurcation at the
Metacarpophalangeal Joint Level: Factors Affecting Surgical Outcome. J Hand Surg 1997;22A:671.
H, Masatomi T, Shimada K, et al. Treatment of Residual Instability and
Extensor Lag in Polydactyly of the Thumb [See Comments]. J Hand Surg 1993;18B:5.
H, Shibata T, Masatomi T, Yasui N. Residual Deformity in Congenital
Radial Club Hands after Previous Centralisation of the Wrist. Ulnar
Lengthening and Correction by the Ilizarov Method. J Bone Joint Surg 1998;80B:762.
LA, Toby EB, Poehling GG. Congenital Flexion Deformities of the
Proximal Interphalangeal Joint in Children: A Subgroup of
Camptodactyly. J Hand Surg 1990;15A:582.
Bioethics Center Task Force on Health Care Rights for Minors. Health
Care Treatment Decision-making Guidelines for Minors. Bioethics Forum 1995;11:A1.
T, Nakamura R, Tamura Y. Long-standing Extended Dynamic Splintage and
Release of an Abnormal Restraining Structure in Camptodactyly. J Hand Surg 1992;17B:665.
JA, Ganey TM, Light TR, et al. Ossification and Pseudoepiphysis
Formation in the “Nonepiphyseal” End of Bones of the Hands and Feet. Skeletal Radiol 1994;23:3.
A, Manouvrier S, Gonzales M, et al. Refined Mapping of a Gene for Split
Hand–Split Foot Malformation (SHFM3) on Chromosome 10q25. J Med Genet 1996;33:996.
RE, Foy PM, Mendiratta V, et al. Ease and Accuracy of Evaluation of
Fetal Hands During Obstetrical Ultrasonography: A Prospective Study. J Ultrasound Med 1995;14:813.
PL, Cohn AK, Sedgwick WG, et al. Dysplasia of the Knee Associated with
the Syndrome of Thrombocytopenia and Absent Radius. J Bone Joint Surg 1984;66A:421.
RJ, Kaplan EB. Camptodactyly and Similar Atraumatic Flexion Deformities
of the Proximal Interphalangeal Joints of the Fingers. J Bone Joint Surg 1968;50A:1187.
Heest AE, House J, Krivit W, Walker K. Surgical Treatment of Carpal
Tunnel Syndrome and Trigger Digits in Children with Mucopolysaccharide
Storage Disorders. J Hand Surg 1998;23A:236.
D, Nielsen G. Madelung Deformity: Surgical Prophylaxis (Physiolysis)
During the Late Growth Period by Resection of the Dyschondrosteosis
Lesion. J Hand Surg 1992;17B:401.
N, Barr A. Radial, Ulnar, and Median Nerve Palsies Caused by a
Congenital Constriction Band of the Arm: Single-stage Correction. Plast Reconstr Surg 1994;94:872.
R, Lamb DW. Congenital Upper Limb Anomalies: An Etiologic Grouping of
Clinical, Genetic, and Epidemiologic Data from 387 Patients with
“Absence” Defects, Constriction Bands, Polydactylies, and Syndactylies.
J Hand Surg 1985;10A:958.
J, De Raeymaecker DMJ, Snijders PJLM, et al. Psychomotor Development in
Children with Triphalangeal Thumbs: A Preliminary Study. J Hand Surg 1998;23B:526.
In most texts, this procedure is described as the Bilhaut-Cloquet
procedure. No treatise by Cloquet on the treatment of thumb polydactyly
has been found (50); thus Bilhaut should be given the credit.