Knee Dislocations and Fracture-Dislocations
unique understanding of anatomy, biomechanics, function, and principles
of the management of high-energy soft tissue injuries that involves
areas of expertise in both sports medicine and orthopaedic trauma. The
diagnosis of knee dislocation has changed remarkably as it has been
recognized that most patients present with the knee spontaneously
reduced following a dislocation. One recent study reported that 67% of
their patients with knee dislocation had the knee spontaneously reduced
on presentation.128 Careful
examination of the knee following high-energy trauma will reveal much
more frequent dislocations compared with what was reported in the
literature in past decades.42,63,85,101
Treatment options have also advanced in recent years with a
concentration on anatomic reconstructions using allografts. This
chapter will build on the preceding editions in an attempt to advance
the scope and review the current knowledge base and accepted treatment
practices for knee dislocations and fracture dislocations.
should include a careful history and physical examination, as well as an evaluation of function.83
Simple tests to help evaluate function include observing the patients
gait and evaluating the competence of the extensor mechanism using the
straight-leg raise test. With multiple trauma patients, many aspects of
this evaluation are more difficult to perform. Obtaining a good history
may be impossible or very limited if the patient is intubated or
requires sedation and analgesics as a result of the injuries.
Similarly, it is very difficult to examine the knee of a patient with
an ipsilateral pelvic or lower extremity fracture. Finally, many
functional tests are impossible to perform in the setting of acute
trauma. These limitations require the surgeon to improvise and combine
data from radiographs and magnetic resonance imaging (MRI) scans with
information obtained from the injury history and the often-limited
physical examination to arrive at the diagnosis of knee dislocation. A
high index of suspicion is required to avoid missing the diagnosis,
particularly in the patient whose knee dislocation has spontaneously
reduced. Finally, a careful examination of the knee under anesthesia
following stabilization of ipsilateral fractures is critical to making
the diagnosis and fully understanding the extent of the injury. MRI
does not take the place of a good examination under anesthesia and
should only be used as an important adjunctive test supplementing the
examination under anesthesia.
considered when treating patients following a knee dislocation.
Successful outcomes with these challenging injuries require combining
complex knee reconstruction techniques from sports medicine with a firm
understanding of the management of high-energy soft tissue injuries
from trauma. Many patients who sustain an isolated ligament injury
associated with an athletic event are relatively young, physically fit,
and motivated to resume their active lifestyle. Because the majority of
patients who experience a knee dislocation are involved in high-energy
trauma, their age, body habitus, and preexisting medical problems vary
significantly from patient to patient. The appropriate treatment for a
young patient who is physically active may be different than the
treatment for a patient in their 60s with osteoarthritis or an obese
40-year-old. The surgeon may also need to adjust treatment timing or
strategies if the patient has an open knee dislocation, a peroneal
nerve injury, or a popliteal artery injury.
associated conditions that can develop following knee dislocations.
Treatment decisions are based on the goal of obtaining a stable knee
and avoiding these other two outcomes. Arthritis in young or
middle-aged adults is extremely difficult to manage, particularly if
osteotomy is not a good option due to multicompartment involvement. The
use of total knee arthroplasty in the treatment of arthritis in a young
active patient carries significant risk of failure or infection and has
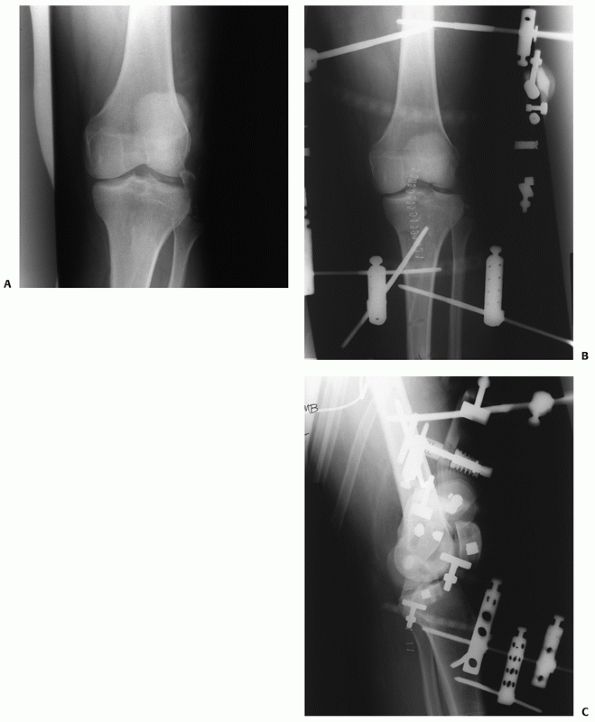
a very limited role in patients under 40 years of age (Fig. 54-1).
The best treatment strategy is to try to obtain stability through
anatomic reconstructions and to avoid arthritis. If a young patient
goes on to develop significant tricompartmental arthritis, the best
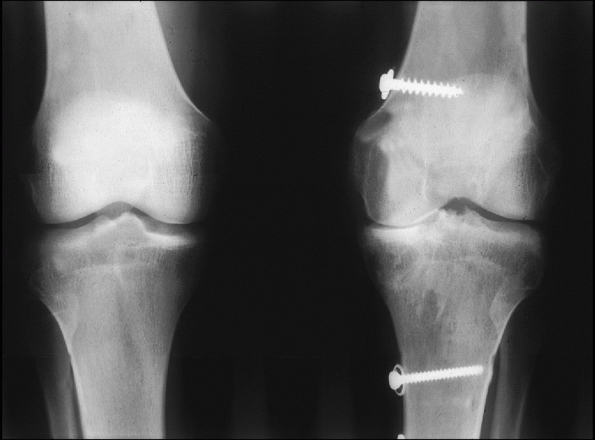
salvage procedure may be arthrodesis of the knee (Fig. 54-2).
associated with it represent the other major condition that can
complicate a knee dislocation. Again, prevention is the best treatment
for this difficult complication that often becomes permanent if not
managed early and aggressively. Although instability due to ligamentous
failure continues to be a problem following knee dislocations, there
are good revision options for recurrent instability with the use of
allograft tissues. Arthrofibrosis is more difficult to treat and is
often more functionally limiting. Avoidance of knee stiffness is
extremely important in the successful treatment of high-energy knee
dislocations.
 |
|
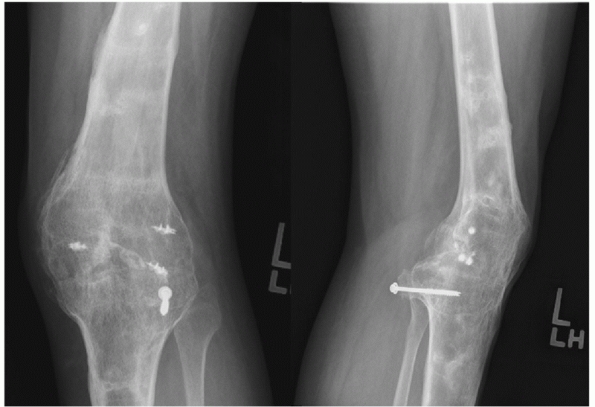
FIGURE 54-1
Medial compartment arthritis evidenced by medial joint space narrowing in a 24-year-old man, 5 years after a complete knee dislocation with popliteal artery injury (KD IIIM). |
variety of injuries that fall under the heading of knee dislocation. We
will discuss the knee and treatment options in terms of four major
ligament groups: anterior cruciate ligament (ACL); posterior cruciate
ligament (PCL); medial (posteromedial corner [PMC]); and lateral
(posterolateral corner [PLC]). We will also discuss major associated
injuries and their impact on treatment strategies and outcomes. Next we
will present some of the current treatment options, followed by the
preferred techniques of one of the authors (J.P.S.). Finally, we will
discuss complications and outcomes, as well as future directions and
controversies in the treatment of knee dislocations.
such as motor vehicle collisions or pedestrian versus motor vehicle accidents.120,121
In one large series of 126 patients, 115 (91%) of the dislocations
occurred as a result of a high-energy mechanism. The most common causes
of knee dislocation in that series were motor vehicle collisions (52%),
motorcycle collisions (17%), and motor vehicle versus pedestrian
accidents (16%).121 The diagnosis of
a knee dislocation in a high-energy multiple-trauma patient is often
difficult, particularly if the knee has spontaneously reduced. There
are frequently other obvious and life-threatening injuries present that
draw the attention of the physicians treating the patient.
Life-threatening injuries to the head, chest, or abdomen are reported
in 27%, associated fractures in 50% to 60%, and multiple fractures in
41% of knee dislocations.137
Additionally, ipsilateral skeletal injuries that draw the attention of
the orthopaedic trauma team make examination of the knee very
difficult. In the large series already cited, 56% of the patients had
ipsilateral fractures, with tibial plateau,38 acetabular,19 and femur19 fractures occurring most commonly.121
All of these associated fractures make examination of the knee
extremely difficult. The frequency and severity of associated injuries
are a major reason knee dislocations have often been overlooked in the
past. It is very important to carefully evaluate patients for knee
dislocation in the trauma room, since missing this diagnosis can be
associated with limb threatening vascular injuries.90,142
 |
|
FIGURE 54-2
Anteroposterior and lateral radiograph of a knee arthrodesis performed as a salvage procedure for severe tricompartmental arthritis following a knee dislocation. |
occur. Injuries that occur during athletic competition occasionally
will lead to a knee dislocation. Football and equestrian injuries are
two of the most common athletic events associated with knee
dislocations.121 Additionally,
low-energy knee dislocations have been noted to occur in obese
patients. These injuries often result from everyday activities such as
stepping down from a curb and are very challenging to treat. They are
associated with a high incidence of neurologic and vascular injuries,
despite the low-energy mechanism.110
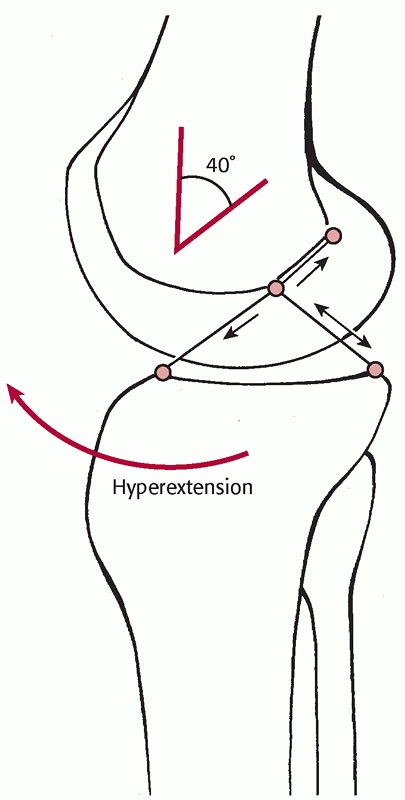
most common cause of knee dislocation. Kennedy conducted a study on
cadaver specimens and demonstrated that exaggerated hyperextension will
produce a knee dislocation. In his study on 10 cadaver specimens, the
ACL tore first, followed by the PCL and posterior capsule at 30 degrees
of hyperextension. The popliteal artery tore at approximately 50
degrees of hyperextension.56,57
A varus or valgus force combined with hyperextension can produce
variability in the pattern of collateral ligament injuries. Knee
dislocations have been reported to have a high incidence of avulsion
injuries to both ligamentous and tendinous insertions81 (Fig. 54-3). Sisto and Warren114 and Frassica et al.36 have both reported a high incidence of avulsion injuries to the cruciate ligaments (Table 54-1).
Both series involved a higher percentage of low-energy mechanisms of
injury than is seen in most trauma centers. These findings are in
direct contrast to the high frequency of midsubstance tears that are
seen in isolated low-energy (sports) injuries to the ACL. Their
findings are also in direct contrast to the experience of all three
authors of this chapter. The majority of high-energy knee dislocations
do not involve a bony avulsion. However, it is important to recognize
avulsion injuries when they occur, because it will directly impact
treatment options and an avulsion injury frequently simplifies the
treatment plan.
 |
|
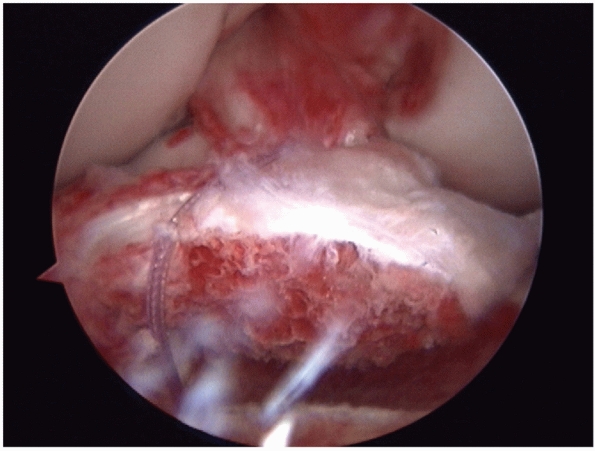
FIGURE 54-3
Arthroscopic view of an avulsion of the anterior cruciate ligament associated with a large bone block. The avulsion was repaired back into the bony bed with good results. |
They found the primary mode of failure was tibial avulsion at slow
rates and mid-substance ligament failure at faster rates. Kennedy et al.57
performed a similar study and found the primary location of failure was
mid-substance at both slow and faster strain rates. While these
laboratory studies termed their varying rates as slow and fast, both
were slow from a clinical perspective. Schenck et al. evaluated the
impact of much higher strain rates (approximately 5400%/sec) on the
PCL. They noted that the high strain rates produced a stripping lesion
of the femoral attachment of the PCL that correlates with the “peel
off” lesion that is frequently encountered following high-energy trauma106 (Fig. 54-4).
In their cadaveric study, the “peel off” occurred in an avulsion
pattern with very small bony fragments, with the primary injury
occurring through Sharpey’s fibers. When they used a low-velocity
model, they found the PCL tore more consistently in a midsubstance
pattern. They noted that the ACL was more variable in its injury
pattern, with midsubstance tears commonly occurring with both high- and
low-velocity injury rates. The authors hypothesized that the difference
between the ACL and PCL was in part created by the femoral notch. The
ACL is classically torn by the notch transverse to its fibers, whereas
the PCL
is torn by forces parallel to the ligament. This would make the PCL more sensitive to strain rate than the ACL (Fig. 54-5).
It is controversial whether “peel off” lesions can be treated like
avulsions and repaired with good results, rather than reconstructing
them. High-energy trauma exposes the knee to exceptionally high
velocity and strain rates. It is the experience of the lead author that
most ACL tears are midsubstance, while PCL tears can be either
midsubstance or a “peel off” lesion. In the vast majority of cases, we
reconstruct the ligament with either injury pattern.
|
TABLE 54-1 Presence of Cruciate Ligament Avulsions in Knee Dislocations
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
 |
|
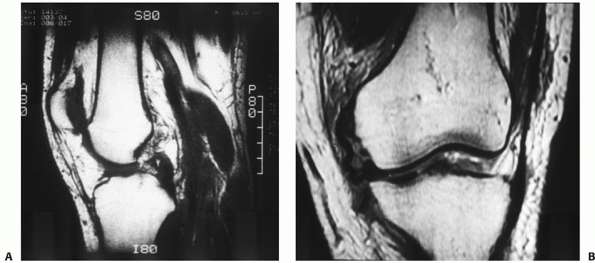
FIGURE 54-4
Magnetic resonance images of a dislocated knee with complete tears of the medial collateral ligament (MCL), posterior cruciate ligament (PCL), and anterior cruciate ligament with a “peel-off” lesion of the PCL (A) and a tibial avulsion of the MCL on the coronal T1-weighted magnetic resonance image (B). |
 |
|
FIGURE 54-5
The strain-rate sensitivity of posterior cruciate ligament (PCL) rupture may be in part related to the direction of forces from the notch in hyperextension. Anterior cruciate ligament tearing occurs with forces crossing the ligament, whereas PCL tearing forces occur along the ligament, in effect making the PCL more strain rate sensitive. (Redrawn from Schenck RC, Kovach, IS, Agarwal, A, et al. Cruciate injury patterns in knee hyperextension: a cadaveric model. Arthroscopy 1999;15:489-495, with permission.) |
explains the susceptibility of the popliteal artery to injury during a
knee dislocation. The artery is relatively tethered in space where it
emerges from the fibrous tunnel of the adductor hiatus or Hunter’s
canal. The artery then crosses the popliteal space giving off five
geniculate branches and exits through the fibrous arch formed by the
soleus muscle. Again, it is relatively tethered in space at the soleus
arch. Since the vascular structures are securely fixed proximally at
the adductor hiatus and distally at the soleus arch, the popliteal
artery and vein can be torn or stretched with the exaggerated
tibiofemoral displacement that occurs during knee dislocation.29,42
Most recent studies report an incidence between 7% and 15%. There are a
couple of explanations for the wide disparity in the reported incidence
of vascular injuries in the literature. One is that spontaneously
reduced knee dislocations are recognized and diagnosed much more
frequently than in the past. Since most dysvascular limbs were already
being diagnosed, even with a spontaneous reduction, the additional knee
dislocations being diagnosed are primarily those with normal
vascularity. A second reason is the decreasing use of arteriography
following knee dislocation. Hollis and Daley48
published a study that demonstrates the impact of arteriography on the
diagnosis of popliteal artery injury. In their study, there was a 10%
incidence of popliteal artery injury requiring surgical treatment.
However, there was a 49% incidence of abnormal arteriograms. Protocols
that use physical examination or ankle brachial index (ABI) to
determine the presence of popliteal artery
injury
will report a much smaller number than studies that report
arteriography findings. Most older studies are primarily based on
arteriography findings, leading to a much higher reported incidence of
popliteal artery injury.
limb-threatening condition. Two recent studies reported amputation
rates of more than 20% in patients with popliteal artery injuries and
knee dislocations, even when the injury was promptly recognized.90,142
Clearly, the diagnosis and treatment of a significant popliteal artery
injury are critical to the survival of the limb. In the past, many
authors have advocated routine arteriography for all patients with knee
dislocations.* However, a growing body of evidence has been
produced that suggests that routine arteriography is not necessary.
Applebaum et al.6 reported that the
most predictive physical finding for a vascular injury was a pulse
deficit on examination of the pedal pulses. There have been at least
eight retrospective and two prospective studies that evaluated the use
of physical examination to determine the need for arteriography.†
This process of using physical examination as the initial screening
examination is called selective arteriography. When the results of
these studies are combined, 545 patients have been evaluated using
selective arteriography. One hundred twenty-one (22%) patients had
abnormal physical examinations, and 73 (60%) of those had popliteal
artery injuries that required surgical treatment. Four hundred
twenty-four (78%) patients had normal physical examinations in these
studies. There have been no cases of significant vascular injuries in
patients with a normal physical examination (Table 54-2).
Selective arteriography represents a safe protocol, but it must be used
as described in many of these studies. Patients must undergo repeat
physical examinations by qualified personnel over the initial 24 to 72
hours following their injury. The protocol in the study by Stannard et
al.121 included vascular
examinations by physicians at the time of admission, 4 to 6 hours
later, and at 24 and 48 hours following admission. We have now used
this protocol on more than 300 knee dislocations without a single
missed significant vascular injury. We have had one patient who had
normal vascular examinations at admission and 4 hours later, who
developed an abnormal examination at approximately 24 hours and had a
significant vascular injury. That patient underwent vascular surgery
and had no complications following interpositional graft bypass.
Selective arteriography, when correctly employed, represents the
accepted standard of care for patients following knee dislocation.
|
TABLE 54-2 Published Cases Using Physical Examination to Determine Need for Arteriography
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
examination as the initial screening test to determine the need for
arteriography or immediate vascular surgery. The protocol involves a
careful physical examination of both the dorsalis pedis and posterior
tibial arteries, noting both the presence and intensity of the pulse.
The normal limb, if there is one, serves as the control for comparison.
The physician should also make a gross evaluation of the color and
temperature of the foot. As noted above, if the vascular examination is
normal, the patient should be admitted for observation and serial
examinations. If there is any asymmetry or abnormality between the two
lower extremities, a vascular surgery consultation should be obtained
immediately. Patients with an obviously dysvascular leg should be taken
emergently to the operating room and have an arteriogram performed in
the operating room. All other patients should have a formal arteriogram
performed to determine the need for a vascular surgical procedure (Fig. 54-6).
emergency. In most patients, collateral blood flow is inadequate and
popliteal artery blood flow should be restored within 6 to 8 hours to
maximize the likelihood of limb salvage. Debakey and Simeone published
their World War II experience with popliteal artery injuries. They
demonstrated the inadequacy of the collateral circulation when they
published an 80% amputation rate for soldiers who sustained a popliteal
artery injury and did not undergo revascularization.24 Green and Allen published a study
in 1977 that demonstrated that the time to revascularization is
critical. They observed that 90% of patients with popliteal artery
injuries whose limb was not revascularized within 6 to 8 hours required
amputations.42
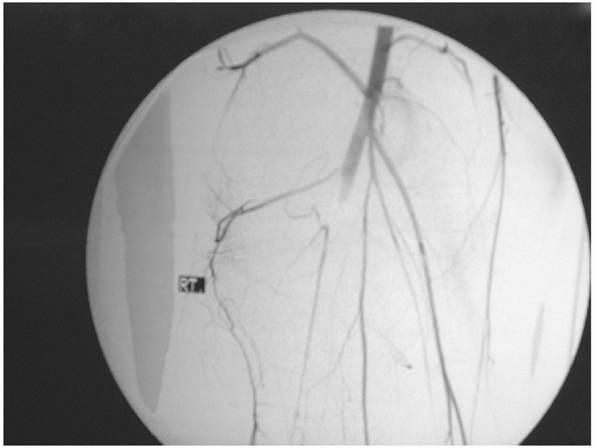 |
|
FIGURE 54-6
An arteriogram demonstrating disruption of the popliteal artery following a knee dislocation. (From Stannard JP, Schenck RC Jr. Knee dislocations and ligamentous injuries. In: Stannard JP, Schmidt AH, Kregor PJ, eds. Surgical Treatment in Orthopaedic Trauma. New York: Thieme, 2007:687-712, with permission.) |
index (ABI) as a noninvasive method to evaluate patients for the
presence of a significant popliteal artery injury.6,66,79 Both Mills79 and Lynch and Johansen66
used 0.90 as their cutoff for an abnormal study and reported excellent
sensitivity, specificity, positive predictive value, and negative
predictive value. The index is easy to obtain, and certainly can
provide additional objective evidence of the condition of the popliteal
artery. However, based on the data presented above, obtaining the ABI
is not required in order to assess the vascular status of a patient
following knee dislocation.
injury associated with knee dislocations. The incidence is reported to
range from 14% to 35%.47,86,92,99,129 Niall et al.86
report that the incidence of peroneal nerve injury in all of their
dislocations was 25%, but it was 41% in those patients who sustained a
bicruciate injury combined with a PLC injury. The prognosis for
patients with complete nerve injuries is poor, with no more than 50%
recovering useful function of the nerve with nonoperative treatment.*
Niall et al. reported that 3 of 14 patients with partial lesions
involving less than 7 cm of the nerve had complete recovery within 6 to
18 months. Partial recovery of useful motor function occurred in
another four patients, while no useful motor or sensory recovery
occurred in the remaining seven patients. The indications for peroneal
neurolysis or cable grafting are controversial. Patients who are
undergoing PLC repair or reconstruction and have a peroneal nerve
injury should be treated with at least a peroneal neurolysis.27,40
Tibial nerve injuries are far less common than peroneal nerve injuries
but carry an even less favorable prognosis for functional recovery.27,137
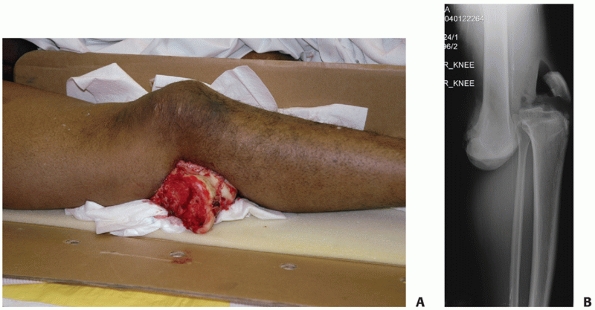 |
|
FIGURE 54-7 Clinical presentation (A) and radiograph (B) of a patient with an open knee dislocation following a high-energy coal mining accident.
|
Most patients have no idea of exactly what position their knee was in
during a motor vehicle collision. Occasionally, the history of a
hyperextension injury can be obtained. A history of a dashboard injury
to a flexed knee should increase the suspicion of a knee dislocation.
patient who has sustained a knee dislocation vary widely, ranging from
an irreducible knee dislocation to a spontaneously reduced dislocation
with minimal physical findings. While most patients present with knee
swelling, patients with extensive capsular tears may not demonstrate a
large hematoma following a knee dislocation. The physical findings are
generally obvious in patients who present with the knee dislocated, due
to the gross deformity. It is important to remember that this
represents a minority of the patients who have sustained a knee
dislocation.128 Subtle signs of injury such as an abrasion, minimal swelling, or complaints of pain may be the only signs of a knee
injury. An examination of the knee will generally reveal gross ligamentous instability.
and an ipsilateral fracture are the most challenging diagnostic
dilemma. Frequently, orthopaedic surgeons are concentrating on the
obvious skeletal injuries and ignoring the subtle signs of a knee
injury. A critical but often overlooked diagnostic step is to perform a
good examination under anesthesia after skeletal stabilization. This
will often unmask significant ligamentous knee instability that will
otherwise go undiagnosed. Data we have collected have demonstrated that
26% of tibial plateau fracture patients who sustained their injury as a
result of high-energy trauma have a concomitant bicruciate ligament
injury.118 We have also found a high association of knee dislocations with ipsilateral femur and acetabulum fractures.121 Accurately diagnosing knee dislocation in multiple trauma patients requires vigilance and a high index of suspicion.
clues of injury in patients with a knee dislocation. Possible findings
include small avulsion fragments, joint space asymmetry between the
medial and lateral joint spaces, or minimal subluxation of the joint.
Stress radiographs under anesthesia in varus or valgus with the knee in
extension can help document collateral ligament disruption.
MRI. The scan does not take the place of physical examination but
provides the surgeon with helpful data. MRI is particularly helpful in
patients with ipsilateral extremity fractures and subtle signs of knee
injury. Skeletal examination is virtually impossible prior to skeletal
stabilization and may be difficult even following stabilization.
Additionally, it is beneficial to identify patients with spontaneously
reduced knee dislocations prior to performing skeletal stabilization of
fractures. The knowledge of a multiple ligament knee injury will alert
the surgeon to the risk of vascular injury and also may lead to
decreased use of a tourniquet during skeletal stabilization procedures
to avoid clot formation adjacent to small intimal tears. To obtain
useful images, it is important to obtain the MRI scan prior to skeletal
stabilization with metallic implants, as metal artifact will often
yield MRI that is virtually useless. The use of MRI on patients with
tibial plateau fractures has been the subject of a number of
manuscripts. Five studies on the subject of MRI findings associated
with tibial plateau fractures have demonstrated a number of significant
findings. These studies report on a total of 197 tibial plateau
fractures, and demonstrate the following injuries: 54 (27%) medial
meniscus tears; 74 (38%) lateral meniscus tears; 63 (32%) ACL tears; 45
(23%) PCL tears; 30 (15%) PMC tears; and 54 (27%) PLC tears.9,49,59,111,124
Additionally, Yacoubian et al. found that adding MRI to CT and plain
radiographs in 52 patients led to a change in the fracture
classification in 21% and a change in the treatment plan in 23% of
cases.141 Similarly, Holt et al.
reported that MRI led to a change in the fracture classification in 48%
of their tibial plateau fractures, and a change in the treatment plan
in 19% of their patients.49
Additionally, they reported a 48% incidence of previously unrecognized
soft tissue injuries in the knee, including two spontaneously reduced
knee dislocations. Stannard et al. have reported on the incidence of
soft tissue injuries in 103 consecutive patients evaluated with MRIs
following tibial plateau fractures.118
Patients in this series were diagnosed with 25 medial meniscus tears,
35 lateral meniscus tears, 45 ACL tears, 41 PCL tears, 16 PMC tears,
and 46 PLC tears. Twenty-six patients were diagnosed with bicruciate
ligament injuries. Published studies have documented between 48% and
90% of patients with high-energy tibial plateau fractures also have
significant associated soft tissue injuries.9,10,16,49,59,111,141
Due to the high incidence of associated soft tissue injuries, obtaining
MRI appears to be justified following high-energy tibial plateau
fractures.
 |
|
FIGURE 54-8
Classification of knee dislocations based on displacement of the tibia on the femur. (Reproduced with permission from Schenck RC. The dislocated knee. AAOS Instruc Course Lect 1994:127-136.) |
This system described the position of the tibia with respect to the
femur, and categorized dislocations into five primary types: anterior,
posterior, lateral, medial, and rotatory (Fig. 54-8).
The rotatory dislocations had four subclassifications: anteromedial,
anterolateral, posteromedial, and posterolateral. The posterolateral
rotatory dislocation was described as the most frequently encountered
rotatory dislocation.2,68,116 A hallmark of posterolateral dislocations is that they may be irreducible (Table 54-3). This occurs
when the medial femoral condyle buttonholes through the medial capsule
and the medial collateral ligament (MCL) invaginates into the knee
joint, preventing reduction.47,92
The sine qua non of this condition is a transverse furrow seen on the
medial side of the knee. Peroneal nerve palsy is frequently associated
as a result of a traction injury of the nerve over the lateral femoral
condyle. Skin necrosis also frequently occurs as a result of pressure
from the medially displaced femur47 (Fig. 54-9).
|
TABLE 54-3 Position Classification of Knee Dislocations
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
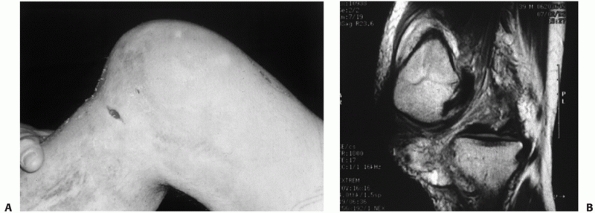
FIGURE 54-9
Posterolateral dislocation of the knee presenting 3 weeks postinjury with grossly positive tibial dropback and medial furrowing with early soft tissue necrosis (A). Magnetic resonance view of the same knee in the dislocated position (B). |
the surgeon regarding the potential reduction maneuvers needed to
reduce the dislocation. It also may help alert the surgeon to
associated complications or injuries with specific patterns. However,
since more than half of knee dislocations present following spontaneous
reduction, many cannot be classified by this system. Additionally, the
position classification system only suggests which structures may be
torn. It does not specifically guide surgical decision making. For
example, patients can sustain an anterior dislocation and have either a
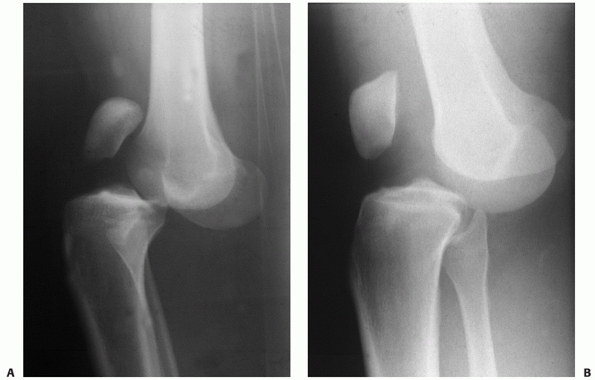
bicruciate ligament injury or a PCL intact dislocation (Fig. 54-10). The position classification system does not help differentiate between these two patterns.
 |
|
FIGURE 54-10 Lateral radiographs comparing a posterior cruciate ligament (PCL)-intact knee dislocation (A) and a complete bicruciate ligament knee dislocation (B)
in two different patients. Note the parallel alignment of the patella with the femur in the complete bicruciate injury Please check placement of “A” and “B.” and (B) the close proximity of the femur and tibia in the PCL-intact knee dislocation (A). |
what structures are torn and is very useful in guiding the surgeon
regarding treatment options. The anatomic classification was initially
described by Schenck and has been modified over the years. It is based
primarily on the findings from physical examination, particularly the
examination under anesthesia.63,104,134
MRI findings can be added to the physical examination, but the primary
tool used to classify these fractures is the physical examination.
on which ligaments are torn. As a general rule, the higher the number,
the higher is the energy of the dislocation and the greater is the
severity of the injury to the knee. The system considers the knee to be
made up of four ligament groups: ACL, PCL, PMC, and PLC. The following
five major categories are KD I, any knee dislocation with one cruciate
ligament intact; KD II, a bicruciate knee dislocation; KD III, a
bicruciate ligament dislocation with one of the corners torn as well;
KD IV, a bicruciate ligament dislocation with both corners torn; and KD
V, any fracture dislocation. Additional modifiers can be added to
indicate an arterial injury (the letter C) or a neurologic injury (the
letter N). Stannard et al. modified the classification of the fracture
dislocations to identify which structures were torn when there was an
associated fracture121 (Table 54-4).
the anatomic classification system to classify their injuries and
direct treatment.26,121,131,132
They noted that KD III was the most common pattern seen. Some of the
authors also noted that KD IIIL patients fared worse than KD IIIM
patients with regard to arthrofibrosis, instability, disability, and
outcome measures. KD IV injuries have been noted to be higher energy
injuries and to be associated with an increased risk of popliteal
artery injury compared with other dislocations.121
The anatomic system is very useful because if forces the surgeon to
focus on what structures are torn. It can then be used to direct
surgical treatment. It also allows for an accurate discussion of knee
dislocations between clinicians, as well as allowing accurate
comparison of similar injuries in the literature. We strongly advocate
the anatomic classification system to describe knee dislocations.
|
TABLE 54-4 Anatomic Classification of Knee Dislocation (KD)
|
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||
injury based on the structure(s) torn. Within the diagnosis of a knee
dislocation, multiple structures are frequently injured, with specific
patterns of injury described. As noted earlier in this chapter, the
classification of knee dislocations is most accurately determined by
the ligaments torn (the anatomic system); hence, knowledge of the
structure and function of the knee ligaments is very important in
defining the injury pattern. Furthermore, the associated disruption of
musculotendinous structures (patellar tendon, patellofemoral
dislocation, iliotibial band, biceps femoris, gastrocnemius, and
popliteus) and the importance of associated injuries adds to the
variability of the pathology involved in knee dislocations. Last, the
bony architecture is important, as joint surface fractures can occur
with a knee dislocation and are frequently underreported. Depending
upon the size of fracture fragments, the knee injury can be classified
as a fracture-dislocation when associated with a large condyle fracture
rather than a pure ligamentous injury as is implied with the term knee
dislocation.
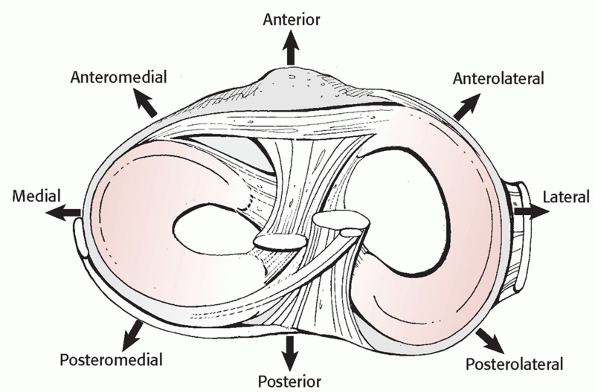
four major ligamentous structures (ACL, PCL, PMC/MCL, and PLC) is very
useful. The ACL and PCL can be torn in tandem (a bicruciate injury),
separately (ACL or PCL intact knee dislocations), or rarely not torn at
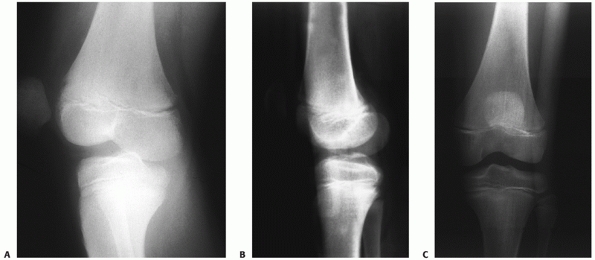
all (a cruciate-intact knee dislocation) (Fig. 54-11).
The injuries to the collaterals are frequently complete, with
involvement of at least one of the ligamentous corners. The anatomic
arrangement of the popliteus, the popliteofibular
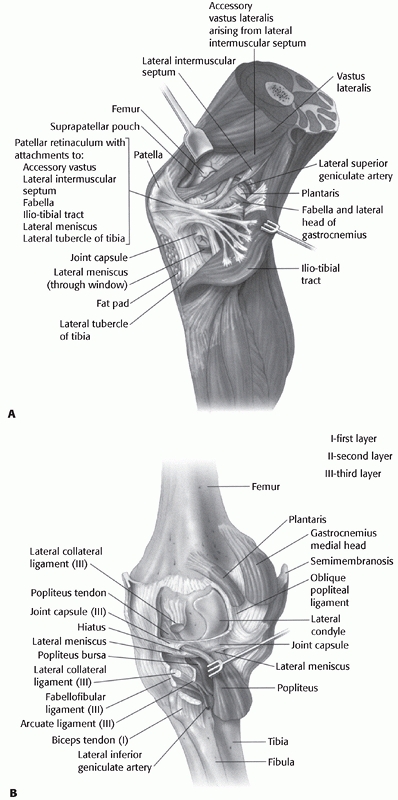
ligament, and the biceps femoris posterolaterally (Fig. 54-12),
and the semimembranosus/posterior oblique ligament complex
posteromedially allows for injury of those structures in conjunction
with the major ligament injury.
 |
|
FIGURE 54-11 Knee dislocations present with varying ligament injury patterns. A.
Radiograph of a knee in an adolescent with a cruciate ligament-intact rotary dislocation of the tibiofemoral joint (note the anteroposterior view of the femur and lateral view of the tibia). B,C. Reduction anteroposterior and lateral radiographs of the injured knee. An examination under anesthesia and an magnetic resonance image revealed integrity of both the cruciate and collateral ligaments. |
of the joint, synovectomy, fracture fixation, and repair of the
extensor mechanism. The patient is placed in the supine position and a
small linen roll is placed under the ipsilateral buttock. A tourniquet
is applied to the proximal thigh. A straight incision beginning
proximal to the superior pole of the patella is carried distally,
directly over the patellar tendon and medial to the tibial tubercle.
The length of incision depends on the pathology being treated. Exposure
of the extensor mechanism involves incision of the arciform layer, the
condensation of layer I anteriorly, just deep to the subcutaneous fat.
Subcutaneous dissection is usually not be made superficial to this
fascial layer as devitalization of the overlying skin can occur,
especially if a concurrent lateral retinacular release is performed.
However, in knee dislocations, there is frequently subcutaneous
stripping from layer one, such that the knee dislocates within the
subcutaneous sleeve. If joint exposure is required, the quadriceps
tendon is incised in its midportion, followed by a medial capsulotomy
carried distally and medially to the tibial tubercle. A cuff of capsule
at least 5 mm wide should be preserved on the medial edge of the
patella for closure. Inadequate longitudinal release of the quadriceps
tendon can result in avulsion of the tendon from the tibial tuberosity
when the patella is displaced laterally. This incision allows exposure
of the anterior knee, distal femur, and proximal tibia. The tibial
tubercle and patellar tendon limit exposure of the lateral knee joint.
Frequently, when open exposure of the intercondylar notch is needed for
cruciate reattachment, an incision from the medial edge of the pole of
the patella can be carried down medially to the anterior tibial plateau
to achieve adequate exposure. Viewing the contents deep within the
notch frequently requires use of a headlamp in such open approaches102 and is much more difficult than with a purely arthroscopic approach.
internal fixation of distal femoral condyle fractures (femoral-sided
fracture-dislocations of the knee), tibial plateau fractures, and
reattachment of knee ligaments and lateral tendons. The patient is
positioned supine with a small bolster under the ipsilateral hip. The
incision is placed over the lateral side of the lateral femoral
condyle, anterior to the iliotibial band, and is carried distally over
Gerdy tubercle. The fascia lata is opened parallel to the skin
incision, anterior to the iliotibial band. If proximal extension is
necessary, the vastus lateralis is elevated off the lateral
intermuscular septum and any perforating vessels are ligated.
Retractors are placed subperiosteally over the anterior aspect of the
femur, exposing the entire distal aspect of the lateral femur. The
patellofemoral joint can be exposed by incising the lateral transverse
patellar ligament. Splitting the vastus lateralis muscle through its
belly should be avoided as it can cause excessive bleeding with loss of
control of the perforating vessels. Posterior exposure with this
approach is limited by the fibular collateral ligament and common
peroneal nerve.102
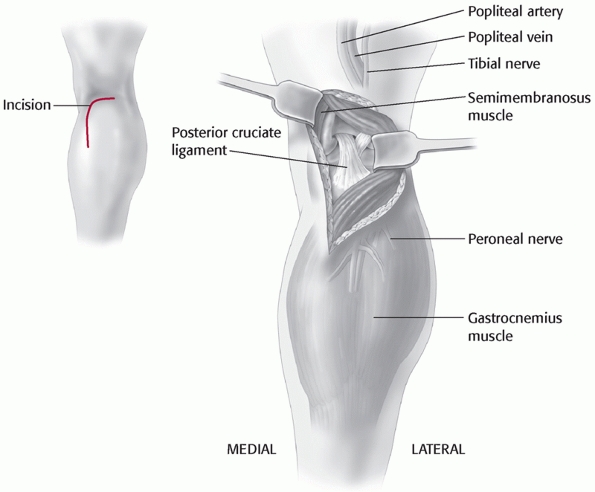
to these vessels. Variations of this approach have been used by Burks and Schaffer18 (Fig. 54-13) and Berg11 to approach the tibial attachment of the PCL when using the inlay PCL reconstruction technique (Fig. 54-14). Additionally, the cruciates and the PMC can be accessed via this approach. It has also been described by Muscat et al.85
for simultaneous repair of vascular injuries and ligaments when
associated with a traumatic knee dislocation. The approaches to the PCL
described separately by Burks and Schaffer and Berg involve a posterior
incision into the flexion crease of the knee combined with a similar
deepplane dissection as described next.
 |
|
FIGURE 54-12 A. The ligamentous structures of the lateral knee after reflection of the iliotibial band. B. The deep ligamentous structures of the lateral knee and the corresponding capsular (layer III) ligaments.
|
 |
|
FIGURE 54-13
The posteromedial approach to the tibial attachment of the posterior cruciate ligament using an incision in the knee flexion crease and partial detachment/release of the gastrocnemius tendon as described by Burks and Schaffer.18 |
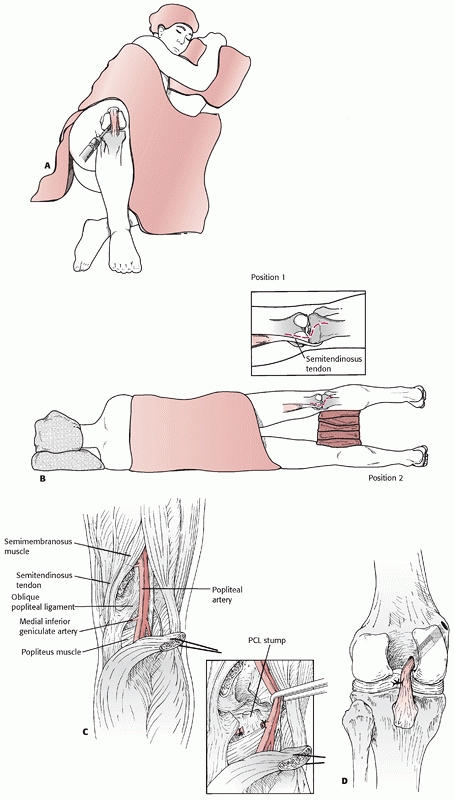
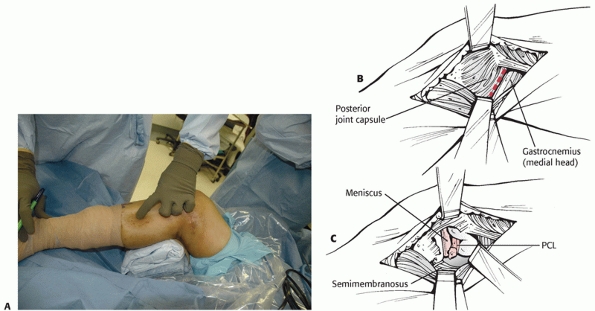
the supine position with the knee flexed and the hip externally rotated
in the figure-four position (Fig. 54-15). The
incision is placed along the posterior aspect of the medial femoral
epicondyle and courses distally along the posterior edge of the
proximal medial tibia. The saphenous vein is identified and should be
protected if possible. The saphenous nerve should be retracted
posteriorly with the skin flap and the saphenous vein to avoid neuroma
formation. For vascular access, the tendons of the pes anserinus are
usually transected 2 cm proximal to their tibial insertion and tagged
for subsequent repair at closure. The proximal portion of the pes is
reflected with the posterior flap, and the distal portion of the pes is
reflected anteriorly. This allows exposure of the medial head of the
gastrocnemius. When using this approach just to repair the PCL, the
medial meniscus, or the PMC, the pes is mobilized but left intact. The
medial head of the gastrocnemius may be detached (partially or
completely) from the medial femur to allow exposure of the popliteal
artery from Hunter canal to its trifurcation. With an approach to the
PCL or the medial knee ligaments, the tendon of the medial head of the
gastrocnemius should be left intact. Exposure requires development of
the plane between the posteromedial knee joint capsule anteriorly and
the medial gastrocnemius posteriorly. All retractors must remain
anterior to the medial head of the gastrocnemius to protect the
popliteal vessels. This approach is complex, and ideally it should be
performed on a cadaver specimen or with an experienced surgeon to
clearly understand the soft tissue planes. It is easy to inadvertently
dissect posterior to the medial gastrocnemius belly or anteriorly into
the joint capsule. Keeping the knee flexed to 70 degrees relaxes the
posterior muscles, simplifying the identification of the plane anterior
to the gastrocnemius. Damage to the saphenous nerve can produce
persistent medial knee pain from a traumatic neuroma and should be
avoided. Furthermore, the incision should be placed posteriorly over
the muscle bellies in the leg, since incisions directly over the tibia
can result in skin necrosis. In the presence of a knee dislocation with
evidence of MCL insufficiency the capsular structures are usually
completely disrupted, allowing exposure of the knee joint itself.
Access to the lateral side of the knee is limited with this approach.
Skin edges should be managed carefully to avoid delayed healing or
wound edge necrosis.
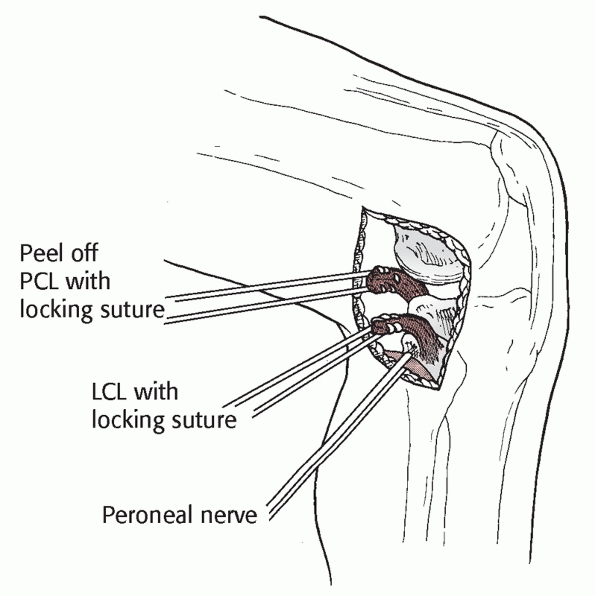
mobilize the lateral gastrocnemius muscle for use as a muscle pedicle
flap, and to explore and repair the common peroneal nerve. It is
frequently useful in knee dislocations for reattachment or repair of
the cruciate ligaments and especially the PLC. The peroneal nerve is
always isolated prior to deep joint exposure to prevent inadvertent
injury. In dislocations with complete tears of both cruciates, the
ligaments are frequently disrupted circumferentially in a half-circle
starting at the patellar tendon anteriorly, with disruption of the ACL
and the PLC, and with enough force
to
injure the PCL. With such injury, exposure of the tibiofemoral joint
can be facilitated by following the tissue planes created by the
dislocation.133
The patient is placed in the supine position with a tourniquet on the
thigh and a small bump under the ipsilateral buttock. The lateral knee
is most safely exposed with the knee flexed to 90 degrees. This relaxes
the peroneal nerve and allows for better protection of the nerve
throughout the procedure. The skin incision is placed in line with the
fibular head and carried in a straight line proximally, then curved
onto the lateral thigh. With proximal extension, the incision should
curve between the iliotibial band and the biceps femoris tendon. The
incision is carried down to the deep fascia, which is then opened
carefully with scissors. At this point the nerve must be identified (Fig. 54-16).
It can usually be palpated subfascially as it courses from the biceps
femoris through its perineural fat to the fibular neck. This approach
should always include exposure of the peroneal nerve prior to deep
dissection. The peroneal nerve is isolated by palpating its location
and carefully dissecting using fine scissors and forceps without teeth.
Once identified, the nerve is protected with a small vessel loop. It is
important not to clamp any instruments such as a hemostat onto the
vessel loop, as the traction from the instrument can cause an injury to
the peroneal nerve. The nerve can sustain injury with simple exposure,
and the patient should be counseled in this regard preoperatively. Once
the nerve is identified and protected, exposure of the posterolateral
structures is carried out by making two additional fascial incisions.
The first develops the interval between the biceps femoris tendon and
the iliotibial band, giving access to the distal attachments of the
lateral collateral ligament (LCL), the popliteofibular ligament, and
the popliteus tendon. The lateral gastrocnemius is mobilized
posteriorly, exposing the posterolateral capsule. The second fascial
incision splits the iliotibial tract over the lateral epicondyle. This
provides access to the proximal attachments of the LCL and
popliteofibular ligament as well as the femoral attachments of the
posterolateral capsule. In the exposure of combined ligament injuries
of the posterolateral knee, the dissection planes are usually already
formed secondary to the displacement occurring with the knee injury.
Straying posterior to the lateral gastrocnemius places the popliteal
neurovascular structures at risk. The artery is often slightly lateral
of midline at the level of the joint, so great care must be exercised
in this area. This approach provides minimal access to the medial side
of the knee. If the PLC and LCL are intact, the PCL cannot be exposed
from this approach. Furthermore, if the approach is performed for a
complete injury of the PCL and the PLC, an intact ACL will prevent
access medially to the PCL insertion on the tibia. In such a scenario,
a posteromedial incision will be necessary to access the PCL insertion
site133 (Fig. 54-17).
 |
|
FIGURE 54-14 Posterior cruciate ligament (PCL) reconstruction as described by Berg. A. Lateral decubitus position and arthroscopic preparation of the PCL femoral origin. B. Posterior approach to the tibial insertion for the inlay technique. C. Deep dissection of the posterior knee with detachment of the medial head of the gastrocnemius. D.
Final reconstruction with a tibial inlay, arthroscopic femoral PCL reconstruction technique. (Redrawn from Berg EE. Posterior cruciate ligament tibial inlay reconstruction. Arthroscopy 1995;11:70-73, with permission.) |
 |
|
FIGURE 54-15
Posteromedial approach to the tibial attachment of the posterior cruciate ligament (PCL). The patient is placed in a supine position with the knee flexed and the leg and hip externally rotated in the figure-of-four position. A. A skin incision is placed at the back edge of the medial tibia, coursing proximally to the posterior edge of the medial epicondyle. Superficial dissection is made through the sartorius fascia along the line of the skin incision. B. Deep dissection is made between the posterior knee joint capsule and the gastrocnemius. Partial detachment of the semimembranosus is required to access the interval. C. Exposure of the proximal tibia and capsulotomy allow identification of the PCL. Reattachment of avulsion fractures of the PCL and exposure for tibial inlay PCL reconstructions can be made through this approach, avoiding prone positioning of the patient. Externally rotating the tibia on the femur in conjunction with knee flexion allows access to the PCL. (Redrawn from Schenck RC. PCL surgery. AAOS videotape No. 10116,100, with permission.) |
 |
|
FIGURE 54-16
Posterolateral approach to the knee. The skin incision is curved from the lower edge of the iliotibial band proximally to the fibular head distally. This approach requires release of the peroneal nerve. |
the posterior surface of the femoral condyles, the posterior knee
capsule, and the posterior aspect of the distal tibia. It is useful for
resection of tumors from the popliteal fossa or posterior knee, nerve
or blood vessel exploration, reattachment of PCL avulsions from the
tibia, and reconstruction of the PCL.
 |
|
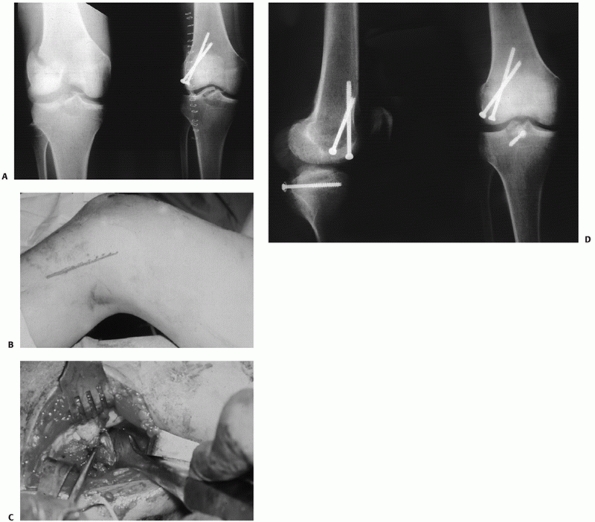
FIGURE 54-17 Sequential images of screw fixation of tibial avulsion of the posterior cruciate ligament (PCL). A.
Lateral femoral condyle fracture and bony avulsion of the PCL with an initial open reduction and internal fixation of the lateral femoral condyle. B,C. Then, through a posteromedial approach, open reduction and internal fixation of the PCL avulsion using a 4.0-mm cannulated A-O screw was achieved. D. Final radiograph. |
is placed on the thigh. The skin incision begins posteromedially over
the semitendinous and semimembranosus tendons, curves transversely
across the knee flexion crease, and then proceeds laterally over the
fibular head and fibula. The sural nerve and short saphenous vein are
identified and the vein is followed proximally to the trunk of the
popliteal vein. The short saphenous vein and sural nerve pierce the
fascia of the posterior knee in the area of the distal flexion crease.
The medial and lateral gastrocnemius muscle heads are identified. The
popliteal nerve, vein, and artery are exposed with ligation of the
genicular branches as required. It is best to approach the vessels from
the medial, proximal end of the fossa and work distally. The vessels
and nerve may be retracted together or, alternatively, the vessels may
be retracted medially and the nerve laterally.
Exposure
of the femoral and tibial surfaces is performed through the
neurovascular interval, with care taken to protect these structures.
Distally, the popliteus muscle is found on the posterior aspect of the
tibia and can be retracted to allow exposure of the proximal tibia (Fig. 54-18).
Blunt dissection is safest for access to the proximal tibia. This is
best performed with a finger or blunt scissors to avoid injury to the
neurovascular bundle. Release of the medial head of the gastrocnemius
can be performed to allow better exposure of the proximal tibia.133
 |
|
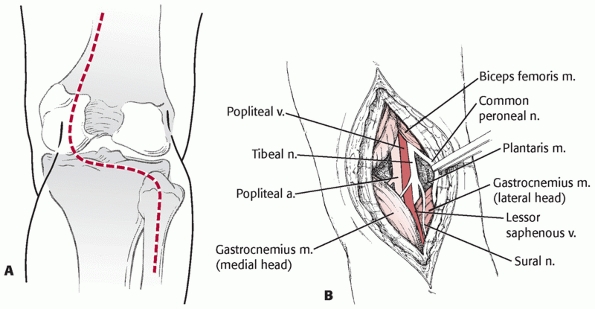
FIGURE 54-18 A.
Exposure of the posterior aspect of the knee can be done by placing the patient in the prone position and making an S-shaped incision crossing the flexion crease. B. Deep dissection is carried between the gastrocnemius heads and requires careful isolation and retraction of the popliteal neurovascular structures. |
neurovascular structures of the popliteal fossa that are directly
exposed in this approach. Because the patient is in a prone position,
access to the anterior knee is extremely difficult, making PCL
reconstruction impossible without turning the patient supine during a
portion of the procedure (requiring repeat prepping and draping). To
approach the tibial attachment of the PCL, the posteromedial approach
is preferred as it does not require a prone position (and the
associated higher pulmonary and cardiovascular risk with a trauma
patient) followed by reprepping and draping for the supine portion, and
does not require direct exposure of the complicated anatomy of the
popliteal neurovascular structures.
important historically and occasionally is an important option today
when circumstances dictate. Historically, knee dislocations were
reduced and treated in a cylinder cast. In the 1960s and 1970s, there
were studies supporting both nonoperative treatment and operative
repair of the torn structures, but there was no clear consensus.*
More recently, with improved surgical techniques including
arthroscopically assisted ligament reconstruction, and increased
understanding of the ligamentous anatomy and biomechanics around the
knee, there has been evidence supporting surgical reconstruction.30,31,32,33,108 These studies demonstrate improved ligamentous stability of the knee as well as improved function postoperatively.
warrant nonoperative treatment. Critically ill patients unable to
tolerate a surgical procedure and patients with grossly contaminated
wounds and/or significant soft tissue injuries around the prospective
surgical site may be candidates for nonoperative management. Also, in
the elderly sedentary person, it may be best to treat the knee
dislocation nonoperatively.109
long leg or cylinder cast. A long leg knee brace locked in extension
may also be used. The brace may be particularly helpful if there are
significant wounds about the knee that a cast would conceal. The knee
brace is often helpful in the setting of a critically ill patient. The
brace allows easy access and evaluation of the injured extremity. If
the cast or brace does not afford enough stability to maintain the knee
in a reduced position, a knee-spanning external fixator may be
necessary. The external fixator also provides better access to soft
tissue injuries if it can be positioned away from contaminated wounds.
We include spanning external fixation as a definitive form of
nonoperative management even though we understand it is applied in the
operating room. In this situation, it is being used the same way as the
brace or cast, but it is providing better stability and access to the
soft tissues. Regardless of the type of nonsurgical management, it is
critical to obtain high-quality radiographs to establish that the knee
is well reduced with no subluxation. If an adequate reduction has been
obtained, it must be followed by frequent radiographs to verify
continued reduction of the knee. Our protocol for treatment with a cast
or external fixator involves 7 to 8 weeks of immobilization, followed
by removal of the cast or external fixator and manipulation under
anesthesia. Rehabilitation then concentrates primarily on achieving
maximum knee motion.
assisted ACL/PCL reconstruction has become popular. Several
advancements have made these techniques possible: (a) better
procurement, sterilization, and storage of allograft tissue; (b)
improved arthroscopic surgical instrumentation; (c) better graft
fixation methods; (d) improved surgical techniques; and (e) improved
understanding of the ligamentous anatomy and biomechanics of the knee.
Few reports of combined ACL/PCL reconstruction are available in the
literature, but surgical reconstruction
appears
to afford at least comparable, if not better, results than direct
repair of the ligaments. Several studies have demonstrated excellent
results with return to near full function measured with the objective
parameters of physical examination, knee ligament rating scales,
arthrometer testing, and stress radiography.30,31,32,33,109
reconstruction in a complex knee injury depends on the timing of the
surgery, as well as the nature of the injury itself. In the case of an
open knee dislocation, irrigation, débridement, and repair of
repairable lesions may be indicated in the first few weeks.31,33
If a reconstruction or repair is done in the first few weeks, the
capsular tissue may be torn, and open techniques may have to be used to
avoid extravasation of fluid with the risk of creating a compartment
syndrome. Bony avulsions of ligaments are generally best treated open
and soon after injury. Under ideal circumstances, the preferred
treatment of knee dislocations consists of arthroscopic ACL/PCL
reconstruction and open repair/reconstruction of the posterior lateral
and/or the medial complexes.
an arthroscopic ACL/PCL reconstruction using the transtibial technique
for the ACL, with collateral/capsular ligament surgery as indicated.
Two of the authors prefer a tibial inlay for the PCL (Fig. 54-19), while one prefers the transtibial technique (Fig. 54-20).
Not all cases are amenable to the arthroscopic approach, and the
surgeon must assess each case individually. Surgical timing is
dependent upon vascular status, reduction stability, skin condition,
systemic injuries, traumatic knee wounds, meniscal and articular
surface injuries, other orthopaedic injuries, and the specific
collateral/capsular ligaments involved.
treatment of the MCL followed by arthroscopic combined ACL/PCL
reconstruction in 4 to 6 weeks after healing of the MCL. If this method
is being considered, it is important to differentiate between a torn
MCL and a torn PMC. The PMC will likely require surgical repair or
reconstruction if it is torn.
 |
|
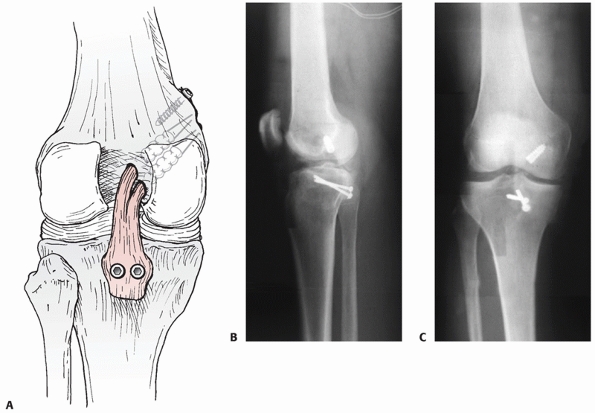
FIGURE 54-19 A. Drawing and B,C. radiographs of a tibial inlay posterior cruciate ligament reconstruction.
|
surgically as early as is safely possible. An ACL/PCL/PLC
repairreconstruction performed between 2 and 3 weeks postinjury allows
for healing of capsular tissues to permit an arthroscopic approach and
still permits primary repair of the injured posterolateral structures.
require staged procedures. The collateral/capsular structures are
repaired after thorough irrigation and débridement, and the combined
ACL/PCL reconstruction is performed at a later date after wound healing
has occurred. Care must be taken in all cases of delayed reconstruction
that the tibiofemoral joint reduction is maintained (Fig. 54-21).
considered in the context of the individual patient. Many patients with
multiple ligament injuries of the knee are severely injured multiple
trauma patients with multisystem injuries. Modifiers to the ideal
timing protocols outlined above include the vascular status of the
involved extremity, reduction stability, skin condition, open wounds,
and other orthopaedic and systemic injuries. These additional
considerations may cause the knee ligament surgery to be performed
earlier or later than desired. We have previously reported excellent
results with delayed reconstruction in the multiple ligament-injured
knee.30,31,32
is the Achilles tendon allograft for single-bundle PCL reconstructions
and an Achilles tendon and tibialis anterior allografts for
double-bundle PCL reconstructions. Another author prefers Achilles
tendon alone for double-bundle PCL reconstructions (Fig. 54-22).
We prefer Achilles tendon allograft or other allograft for the ACL
reconstruction. The preferred graft material for the PLC is allograft
tissue combined with a primary repair, or the posterolateral capsular
shift procedure. Cases requiring MCL and PMC surgery may undergo
primary repair, reconstruction, or a combination of both. Our preferred
method for MCL and posteromedial reconstructions is a primary repair
and/or posteromedial capsular advancement with allograft
supplementation as needed.
 |
|
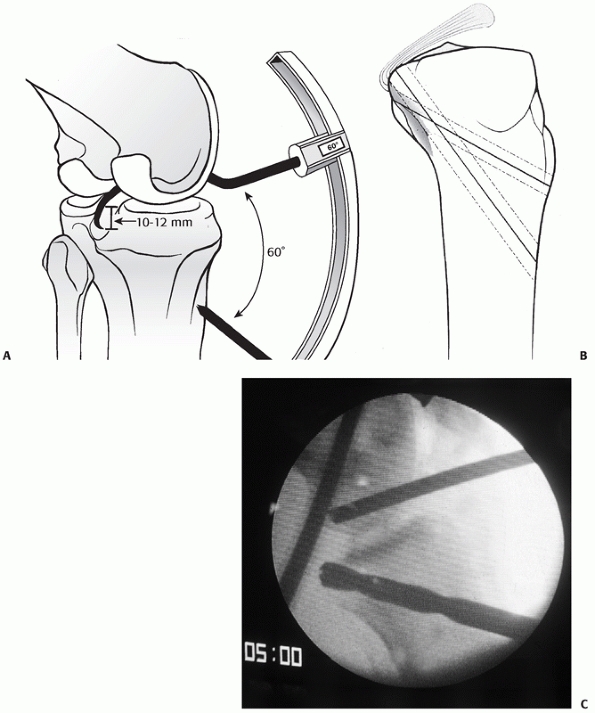
FIGURE 54-20 A. The transtibial arthroscopic tunnel created for posterior cruciate ligament reconstruction. B.
Creating the tunnel properly necessitates a posterior and relatively distal position on the tibia to recreate the posterior cruciate ligament (PCL) insertion site. C. This lateral radiograph of a failed PCL reconstruction using a transtibial tunnel approach shows the tunnel was placed too anteriorly. A,B. redrawn from and C from Scott WN, Insall JN. Video textbook. Philadelphia: JB Lippincott, with permission.) |
ligament-injured knee are to identify and treat all pathology, create
accurate tunnel placement and anatomic graft insertion sites, use
strong graft material, achieve secure graft fixation, and pursue a
deliberate postoperative rehabilitation program. The patient is
positioned supine on the operating room table. The surgical leg hangs
over the side of the operating table, and the well leg is supported by
the fully extended operating table. A lateral post is used for control
of the injured limb. We do not use a leg holder. The surgery is done
under tourniquet control as needed unless prior arterial or venous
repair contraindicates the use of a tourniquet. It is always wise to
minimize tourniquet time due to the risk of small, non-flow-limiting
vascular injuries, which could clot off with extensive tourniquet use.
Fluid inflow is exclusively by gravity for one of the authors and by
low pressure fluid pump for another.
instruments are placed with the inflow in the superior lateral portal,
the arthroscope in the inferior lateral patellar portal, and
instruments in the inferior medial patellar portal. An accessory
extracapsular extra-articular posteromedial safety incision is used to
protect the neurovascular structures and to confirm the accuracy of
tibial tunnel placement.
and PCL stump débridement, bone removal, and contouring of the medial
wall of the lateral femoral condyle and the intercondylar roof. This
allows visualization of the over-the-top position and prevents ACL
graft impingement throughout the full range of motion. Specially curved
Arthrotek (Arthrotek, Inc., Warsaw, IN) PCL instruments are used to
elevate the capsule from the posterior aspect of the tibia. This step
is very important with the transtibial PCL reconstruction technique.
The arm of the Arthrotek Fanelli PCL ACL Guide is inserted through the
inferior medial patellar portal to begin creation of the PCL tibial
tunnel. The tip of the guide is positioned at the inferior lateral
aspect of the PCL anatomic insertion site. This is below the tibial
ridge posteriorly and in the lateral aspect of the PCL anatomic
insertion site. The bullet portion of the guide contacts the
anteromedial surface of the proximal tibia at a point midway between
the posteromedial border of the tibia, and the tibial crest anterior
approximately 1 cm below the tibial tubercle. This will provide an
angle of graft orientation such that the graft will turn two very
smooth 45-degree angles on the posterior aspect of the tibia and will
not have an acute 90-degree angle turn, which may cause pressure
necrosis of the graft. The tip of the guide, in the posterior aspect of
the tibia, is confirmed with the surgeon’s finger through the
extracapsular extra-articular posteromedial safety incision.
Intraoperative AP and lateral x-rays may also be used. The arthroscope,
in the posterior medial portal, visualizes the tip of the guide wire.
The surgeon’s
finger
confirms the position of the guide wire through the posterior medial
safety incision. This maneuver provides a double safety check.
 |
|
FIGURE 54-21 A.
Knee dislocation in a multiple trauma patient who had an avulsed fibular collateral ligament and a disrupted extensor mechanism. B. Initially, the patient was treated with a spanning external fixator, which was then converted to a Compass Knee Hinge (Smith & Nephew, Memphis, TN) after repair of the fibular collateral ligament and repair of the extensor mechanism. The cruciates were not reconstructed at that time, but were reconstructed later. C. Lateral radiograph of the knee with the Compass Knee Hinge. (Copyright © Robert C. Schenck, Jr. Albuquerque, NM. Reprinted with permission.) |
 |
|
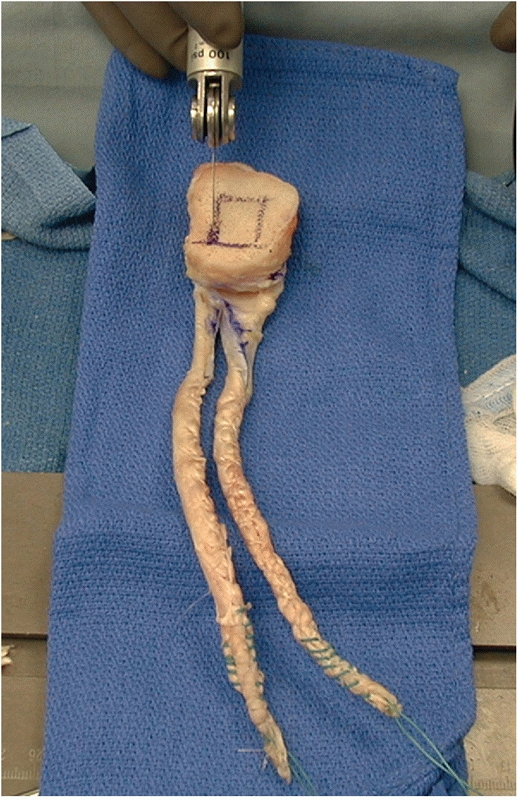
FIGURE 54-22
Achilles tendon allograft being prepared as a double-bundle posterior cruciate ligament reconstruction graft. It is critical to leave the bone block at least 10 mm thick. |
used to create the tibial tunnel. The curved PCL closed curette is
positioned to cup the tip of the guide wire. The arthroscope is
positioned in the posterior medial portal and is used to visualize the
guide wire being cupped, which protects the neurovascular structures.
The surgeon’s finger positioned in the extracapsular extra-articular
posteromedial incision is also monitoring the position of the guide
wire. The drill is advanced until it comes to the posterior cortex of
the tibia. The chuck is disengaged from the drill, and completion of
the tibial tunnel is performed by hand. This gives an additional margin
of safety for completion of the tibial tunnel. The tunnel edges are
then chamfered and rasped with the PCL/ACL system rasp.
can be made from inside out using double-bundle aimers. Inserting the
appropriately sized double-bundle aimer through a low anterior lateral
patellar arthroscopic portal creates the PCL anterior lateral bundle
femoral tunnel. The double-bundle aimer is positioned directly on the
footprint of the femoral anterior lateral bundle PCL insertion site.
The appropriately sized guide wire is drilled through the aimer,
through the bone, and out a small skin incision. Care is taken to
ensure there is no compromise of the articular surface. The
double-bundle aimer is removed, and an acorn reamer is used
endoscopically to drill from inside out the anterior lateral PCL
femoral tunnel. The tunnel edges are chamfered and rasped. The reaming
debris is evacuated with a synovial shaver to minimize the fat pad
inflammatory response with subsequent risk of arthrofibrosis. When the
surgeon chooses to perform a double-bundle double femoral tunnel PCL
reconstruction, the same process is repeated for the posterior medial
bundle of the PCL. Care must be taken to ensure that there will be an
adequate bone bridge (approximately 5 mm) between the two femoral
tunnels prior to drilling. This is accomplished using the calibrated
probe, and direct arthroscopic visualization.
technique. The tibial tunnel begins externally at a point 1 cm distal
to the tibial tubercle on the anteromedial surface of the proximal
tibia to emerge through the center of the stump of the ACL tibial
footprint. The femoral tunnel is positioned next to the over the top
position on the medial wall of the lateral femoral condyle near the ACL
anatomic insertion site. The tunnel is created to leave a 1- to 2-mm
posterior cortical wall so interference fixation can be used. The ACL
graft is positioned and anchored on the femoral side, followed by ACL
graft tensioning and tibial fixation.
treatment options for the acutely injured LCL or PLC are varied. Acute
injuries without pathologic laxity can be treated nonsurgically with
rehabilitation and careful observation. In patients with pathologic
motion, surgical treatment is the mainstay. The goal of surgery should
be to stabilize the knee by addressing all injured structures. Surgery
can involve primary repair of all injured ligaments, posterolateral
capsular advancement, and/or augmentation of the lateral and
posterolateral structures. Many surgical techniques have been described
to address PLC instability. One must also be diligent in identifying
concomitant injuries (ACL/PCL/menisci/articular cartilage) that may
affect treatment, rehabilitation, or outcomes.31,33,34
to lead to posterolateral rotatory instability. Some authors have
defined chronic PLC injuries to include those more than 3 weeks old,
because after this period, the quality of the tissues makes primary
repair difficult. Numerous surgical options have been described to
reconstruct the posterolateral structures. Local rotation of a strip of
iliotibial band on a distally based pedicle has been used to
reconstruct the popliteus, while a central slip of the biceps tendon
has been used to augment and reconstruct the popliteofibular ligament.
Arcuate complex and capsular advancement procedures have been used to
reapproximate anatomy and reduce capsular volume. Numerous autograft
and allograft reconstructions have been described in the treatment of
chronic posterolateral rotatory instability.21,35,91
In the setting of a varus mechanical axis, undue stress will be put on
lateral and posterolateral reconstructions, lending to early failure.
High tibial osteotomy prior to or at the time of the formal ligament
reconstruction can improve the results in this situation.91
is the free graft figure-of-eight technique utilizing semitendinosus
autograft or allograft, Achilles tendon allograft, or other soft tissue
allograft material. This technique, combined with capsular repair
and/or posterolateral capsular shift procedures, mimics the functions
of the popliteofibular ligament and lateral
collateral
ligament, tightens the posterolateral capsule, and provides a post of
strong autogenous tissue to reinforce the PLC. When there is a
disrupted proximal tibiofibular joint, or an hyperextension external
rotation recurvatum deformity, a two-tailed (fibular head, proximal
tibia) posterior lateral reconstruction is used.
figure-of-eight technique uses soft tissue allograft material. A
curvilinear incision is made in the lateral aspect of the knee
extending from the lateral femoral epicondyle to the interval between
Gerdy’s tubercle and the fibular head. The peroneal nerve is dissected
free and protected throughout the procedure. The fibular head is
exposed and a tunnel is created in an anterior-to-posterior direction
at the area of maximal fibular diameter. The tunnel is created by
passing a guide pin followed by a cannulated drill, usually 7 mm in
diameter. The peroneal nerve is protected during tunnel creation and
throughout the procedure. The free tendon graft is then passed through
the fibular head drill hole. An incision is then made in the iliotibial
band in line with the fibers directly overlying the lateral femoral
epicondyle. The graft material is passed medial to the iliotibial band,
and the limbs of the graft are crossed to form a figure-of-eight. A
longitudinal incision is made in the lateral capsule just posterior to
the fibular collateral ligament. The graft material is passed medial to
the iliotibial band, and secured to the lateral femoral epicondylar
region with a screw and spiked ligament washer with the allograft
insertion sites corresponding to the anatomic insertion sites of the
fibular collateral ligament and the popliteus tendon. The
posterolateral capsule that had been previously incised is then shifted
and sewn into the strut of figure-of-eight graft tissue material to
eliminate posterolateral capsular redundancy. The anterior and
posterior limbs of the figure-of-eight graft material are sewn to each
other to reinforce and tighten the construct. Final graft tensioning is
done in approximately 30 to 40 degrees of knee flexion. The iliotibial
band incision is closed.
injuries heal well with nonoperative management. Even isolated grade
III MCL injuries generally heal well with bracing and rehabilitation.
Combined ACL/MCL injuries can be treated with early (within 21 days)
reconstruction of the ACL only with results equal to ACL reconstruction
combined with MCL repair.43 With
initial management of the multiple ligament-injured knee, there is a
wide spectrum of healing of MCL injuries. When early surgery is
performed, MCL and posteromedial capsule repair is performed for Type I
and Type II injury patterns. MCL reconstruction is performed in Type
III injury patterns and in cases of chronic MCL laxity. Most MCL
reconstructions describe routing a soft tissue graft between the
attachment points of the superficial MCL, sometimes accompanied by a
posteromedial capsular reefing.11,12
Limited clinical data suggest that functional valgus stability can be
restored in the majority of patients. Complications of MCL surgery
include recurrent valgus laxity, stiffness, and saphenous nerve injury.
through a medial hockey stick incision. Care is taken to maintain
adequate skin bridges between incisions. The superficial MCL is
exposed, and a longitudinal incision is made just posterior to the
posterior border of the superficial MCL. Care is taken not to damage
the medial meniscus during the capsular incision. The interval between
the posteromedial capsule and the medial meniscus is developed. The
posteromedial capsule is shifted anterosuperiorly. The medial meniscus
is repaired to the new capsular position, and the shifted capsule is
sewn into the MCL. When superficial MCL reconstruction is indicated, it
is performed using allograft or autograft tissue. This graft material
is attached at the anatomic insertion sites of the superficial MCL on
the femur and tibia using a screw and spiked ligament washer, or suture
anchors. The posteromedial capsular advancement is performed, and sewn
into the newly reconstructed MCL. Final graft tensioning is
accomplished in approximately 30 to 40 degrees of knee flexion.
first followed by the anterior cruciate followed by the posterolateral
complex and/or the medial side. The Arthrotek tensioning boot
(Arthrotek, Inc., Warsaw, IN) is used for tensioning the anterior and
PCL reconstructions. Tension is placed on the PCL graft distally, and
the knee is cycled through a full range of motion to allow
pretensioning and settling of the graft. The knee is placed in 70 to 90
degrees of flexion, the Arthrotek tensioning boot is tensioned to 20
pounds to restore the normal tibial step-off, and fixation is achieved
on the tibial side of the PCL graft with a screw and spiked ligament
washer, and a bioabsorbable interference screw. The knee is maintained
at 70 to 90 degrees of flexion, the Arthrotek tensioning boot is
tensioned to 20 pounds with tension on the ACL graft, and final
fixation is achieved with a bioabsorbable interference screw and
ligament fixation button or spiked ligament washer backup fixation. The
knee is then placed in 30 degrees of flexion, the tibia is internally
rotated, a slight valgus force is applied to the knee, and final
tensioning and fixation of the PLC is achieved. Reconstruction and
tensioning of the MCL and PMC are performed after the ACL, PCL, and PLC
reconstructions, and are done in 30 degrees of knee flexion.
extension and a non-weight-bearing status is maintained for 6 weeks.
Progressive range of motion occurs after the third week. The brace is
unlocked at the end of 3 weeks and the crutches are discontinued after
progression to full weight bearing has been achieved. Alternatively,
one of the authors (J.P.S.) initiates motion immediately and allows
full weight bearing within 1 week of reconstruction. Progressive closed
kinetic chain strength training and continued motion exercises are
performed. The brace is discontinued after the tenth week. Return to
sports and heavy labor occurs after the ninth postoperative month when
sufficient strength, proprioceptive skills, and range of motion have
returned. It should be noted that a loss of 10 to 15 degrees of
terminal flexion might be expected in these complex knee ligament
reconstructions. This usually does not cause a functional problem for
these patients and is not a cause for alarm.
(J.P.S.) is a combination of the tibial inlay and two femoral tunnel
techniques. These two techniques combined create an excellent
reconstruction of the normal anatomy of the
PCL.
An Achilles tendon allograft is divided into a larger anterolateral and
a smaller posteromedial bundle. The bone block is trimmed into a
rectangle that should be no less than 15 mm long by 10 mm wide by 10 mm
thick (Fig. 54-22).
It is particularly critical to maintain a bone block thickness of at
least 10 mm, to minimize the risk of fracture of the bone block when
the fixation screw is tightened. A permanent number 2 suture is placed
into each bundle using locked stitches to assist passage into the
appropriate femoral tunnel.
entire procedure. Some authors advocate placing the patient in the
prone position and then moving them to either the supine or lateral
position after preparing the back of the tibia. I have found it
unnecessary to flip the patient during the middle of the procedure and
prefer to avoid that step. Initially, I make standard arthroscopy
portals after placing the involved leg on a bump of sheets and hanging
it off the side of the bed. The notch is debrided arthroscopically
using an aggressive shaver, and any meniscus pathology is addressed
simultaneously. It is very common to see peripheral meniscus tears
associated with knee dislocations, and it is important to carefully
examine both the medial and lateral meniscus for tears. When that is
complete, a guide wire is drilled from the outside in at a position
approximately 8 mm from the articular surface of the femoral condyle
within the footprint of the PCL at the top of the notch. A second guide
wire is also drilled from outside in and positioned immediately
inferior to the first guide wire. It is important to make certain to
space the two guide wires so that there will be at least a 4-mm bone
bridge between the two tunnels. The tunnel size is selected based on
the size of the Achilles tendon allograft, with a 9-mm anterolateral
tunnel and a 7-mm posteromedial tunnel being the most common sizes.
Drilling the guide wires and reaming the tunnels from the outside in
rather than endoscopically have a number of advantages. It allows
precise placement of the tunnels, divergence of the two tunnels, and an
angle that prevents the formation of a femoral “killer turn” (Fig. 54-23).
Once the tunnels are reamed, I tap them to the same size as what was
drilled and use an oscillating shaver to débride periosteum from the
external edge of the tunnel to make it easier to start the interference
screws used for graft fixation. The arthroscope is then removed from
the knee and we shift to the open portion of the case.
placed in a figure-of-four position with the knee flexed and the hip
externally rotated. A posteromedial approach is made along the
posteromedial border of the tibia. This approach is similar to that
described by Burks and Schaffer,18
except we do not curve the incision into the popliteal fossa.
Electrocautery is used to cut down to the posteromedial border of the
tibia just proximal to where the semitendinosus crosses the tibia. A
Cobb periosteal elevator is used to elevate the popliteus muscle from
the back of the tibia, and a blunt Hohman retractor is placed to keep
the popliteus between the surgeon and the neurovascular structures.
This approach allows the surgeon to visualize the posterior tibia. A
0.5-inch curved osteotome is used to cut a trough in the bone at the
site of the tibial insertion of the PCL. The Achilles allograft bone
block is then drilled with a 4.5-mm drill bit. Fixation is obtained
with a single 4.5-mm cannulated screw (Fig. 54-24)
and washer placed using a lag technique. The correct placement of the
screw is confirmed using fluoroscopy prior to proceeding with the case.
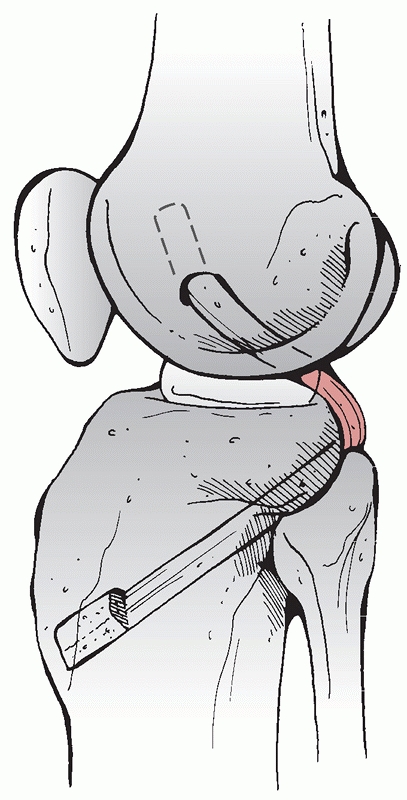 |
|
FIGURE 54-23
The “killer turn” that occurs following transtibial posterior cruciate ligament reconstruction. (Redrawn from Stannard JP, Schenck RC Jr. Knee dislocations and ligamentous injuries. In: Stannard JP, Schmidt AH, Kregor PJ, eds. Surgical Treatment in Orthopaedic Trauma. New York: Thieme, 2007:687-712, with permission.) |
capsule if one is not already present from the dislocation. A Hewson
suture passer is then placed in the anteromedial portal and through the
hole in the posterior capsule. The posteromedial bundle suture is
placed through the loop of the suture passer and pulled into the knee.
The suture and graft are then pulled into the posteromedial tunnel. The
process is then repeated with the anterolateral bundle, making
sure
it enters the posterior capsule lateral to the posteromedial bundle.
The anterolateral bundle is pulled into the anterolateral tunnel. The
grafts are then pretensioned by ranging the knee 20 times while
maintaining tension on the graft. The anterolateral bundle is tensioned
at approximately 70 to 80 degrees of flexion, with the posteromedial
bundle tensioned at approximately 15 to 20 degrees of flexion. Both
bundles are secured with absorbable interference screws that are the
same size as the tunnel.
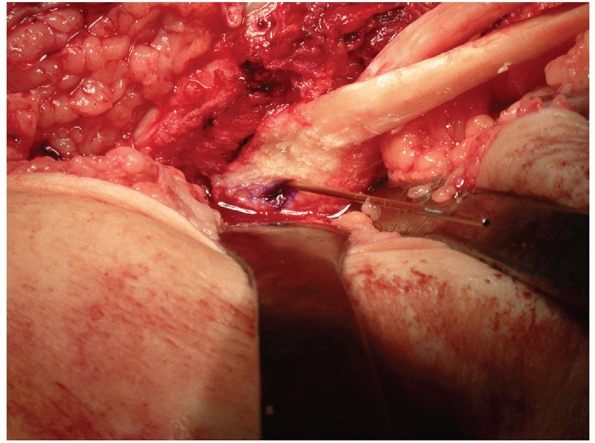 |
|
FIGURE 54-24
Inlay posterior cruciate ligament bone block in place with the guide wire for a 4.5-mm cannulated screw protruding through the drill hole. |
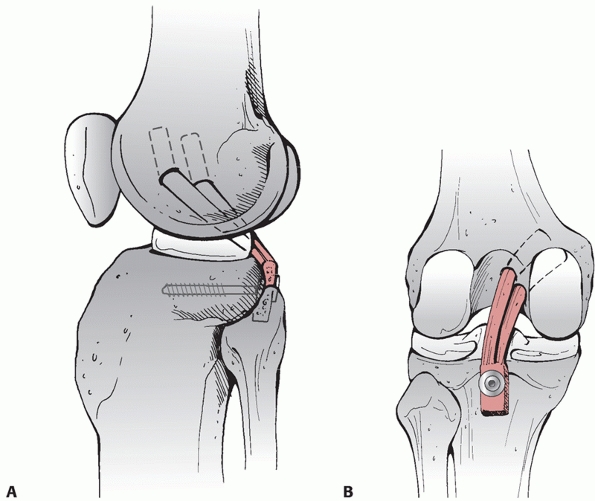 |
|
FIGURE 54-25 A,B. Anatomic posterior cruciate ligament reconstruction combining the tibial inlay technique with the double-bundle technique.
|
major advances in PCL reconstruction. The tibial inlay eliminates the
killer turn, which has been shown to be associated with graft stretch
and failure in cadaver studies.12 The double-bundle technique re-establishes a stable graft through the full range of motion (Fig. 54-25). Our initial experience with this reconstruction has been very positive, with only a 3% failure rate in our first 31 cases.119 I have now performed more than 300 PCL reconstructions using this technique, with excellent results.
(J.P.S.) is the modified two-tailed technique using either tibialis
anterior or tibialis posterior allograft. A minimum graft length of 27
cm is necessary to assure that the entire reconstruction can be
completed. However, to be on the safe side we prefer a graft that is 30
cm or longer if it can be obtained. When the PLC is reconstructed
following a knee dislocation, we reconstruct the PCL first. The PLC
reconstruction is then performed, but the final tensioning of both
grafts is delayed until all other ligament repairs and reconstructions
are accomplished. In most cases, I do not use a tourniquet during the
PLC reconstruction. This procedure anatomically reconstructs three
critical components of the deep layer of the PLC: the popliteus tendon,
the popliteofibular ligament, and the LCL (Fig. 54-26).
Regardless of what reconstruction technique is used for PLC
reconstruction, it is critical to reconstruct these three components.
LaPrade describes a similar technique using Achilles tendon allograft.
He uses the Achilles tendon to create two separate grafts to
reconstruct the critical components of the PLC.60
patient is placed in the supine position with a tourniquet on the thigh
but not inflated, and a small bump is placed under the ipsilateral
buttock. The PLC should be exposed with the knee flexed 90 degrees,
allowing relaxation and protection of the peroneal nerve. The skin
incision is placed
in
line with the fibular head and carried in a straight line proximally
and distally. The incision is carried down to the deep fascia, which is
then opened carefully with Metzenbaum scissors. At this point, the
peroneal nerve is identified and dissected, and a Penrose drain is
placed around it for gentle retraction. Never place clamps or other
surgical instruments on the Penrose drain, as the tension on the nerve
over the course of the operation can lead to a traction injury. Once
the nerve is identified and protected, exposure of the PLC and lateral
gastrocnemius is straightforward.
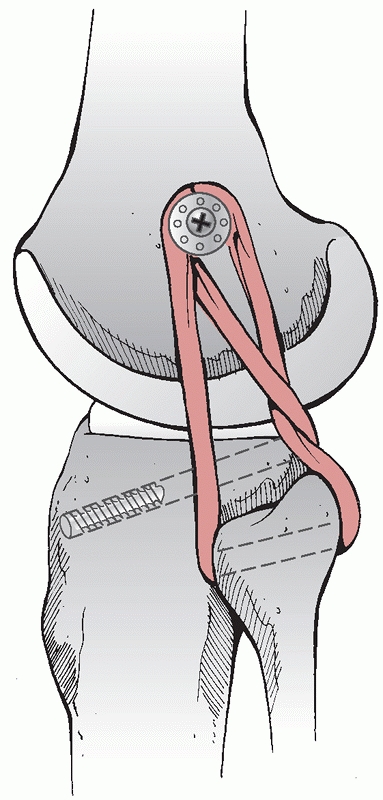 |
|
FIGURE 54-26
Reconstruction of the posterolateral corner using the modified two-tailed technique. (Redrawn from Stannard JP, Schenck RC Jr. Knee dislocations and ligamentous injuries. In: Stannard JP, Schmidt AH, Kregor PJ, eds. Surgical Treatment in Orthopaedic Trauma, New York: Thieme, 2007:687-712, with permission.) |
the lateral head of the gastrocnemius. In the exposure of combined
ligament injuries following a knee dislocation, the dissection planes
are usually already formed secondary to the translation occurring with
the knee injury. It is critical to never stray posterior to the
gastrocnemius as it places the popliteal neurovascular structures at
risk. The remnant of the LCL is identified at its point of insertion
onto the anterolateral portion of the head of the fibula. Proximal and
posterior dissection will lead to the femoral origin of the ligament,
which is posterior to both the isometric point and the popliteus. It is
often necessary to release a portion of the tensor fascia lata to
completely expose the origin of LCL. The popliteus tendon courses deep
to the LCL, lying in the popliteus hiatus on the femur. The
popliteofibular ligament traverses from the posterior aspect of the
head of the fibula to the popliteus tendon. Unfortunately, the normal
anatomy is frequently markedly disrupted in patients with knee
dislocations. At this point, we bluntly dissect around the head of the
fibula between the peroneal nerve and the biceps femoris to the
posterolateral aspect of the tibia. This technique involves drilling a
5-mm hole from anterior to posterior through the lateral tibia, exiting
where the popliteus tendon traverses the back of the tibia. I drill the
tibial tunnel using a free hand technique. The drill enters the tibia
directly inferior to the lateral arthroscopy portal, well below the
joint line. The free hand technique is accomplished by positioning the
index finger of the nondominant hand at the posterolateral edge of the
tibia through the interval described above, approximately 1 cm distal
to the joint line. The tibial tunnel is tapped with a 7-mm tap.
allograft is trimmed to a diameter of 5 mm and passed into the tunnel
from posterior to anterior using a Hewson suture passer. The graft is
secured in the tibial tunnel with a 7-mm bioabsorbable ligament screw.
A second 5-mm drill hole is made through the proximal fibula, aimed
from anterolateral to posteromedial. The isometric point on the lateral
femoral condyle is then located using fluoroscopy. A lateral view of
the knee is obtained, and the isometric point is located where a line
extended from the posterior femoral cortex intersects Blumensaat’s line
(Fig. 54-27). A 3.2-mm drill bit is then used
to drill a hole for a 4.5-mm-long bicortical screw going from lateral
to medial. It is important to aim approximately 30 degrees anterior and
30 degrees proximal to prevent this screw from crossing the tunnel that
will be needed for an ACL reconstruction. A spiked ligament washer is
used with the 4.5-mm screw. An osteotome is used to decorticate the
bone around the screw, allowing the allograft to heal to bone in the
anatomic locations of the LCL and Popliteus, respectively.
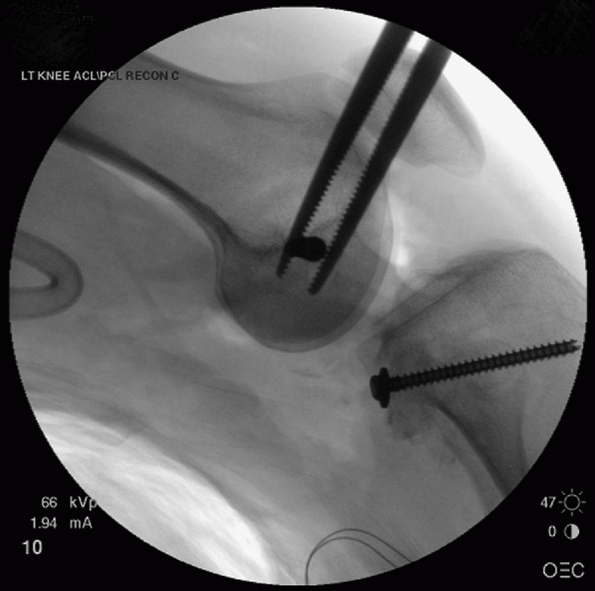 |
|
FIGURE 54-27
Guidewire placed at the isometric point of the lateral femoral condyle. This point is located where a line drawn from the posterior femoral cortex intersects with Blumensaat line. |
and around the screw and washer, back down to the fibular tunnel,
through the tunnel, and back up to the screw and washer. The graft is
tensioned with the foot slightly internally rotated and the knee flexed
approximately 40 degrees. This graft reconstructs the three key
components: the popliteus, popliteofibular ligament, and LCL. Results
of this reconstruction have been good, with failure rates below 10% in
patients following knee dislocations.115,117
motion beginning on the first postoperative day in all patients.
Partial weight bearing using crutches is encouraged for the first week,
with advancement to full weight bearing if other injuries permit. Knee
rehabilitation is dictated by the cruciate ligament injuries, with
concentration on early motion and closed chain exercises. Patients with
an isolated PLC injury do not begin aggressive motion work for 10 days
to 2 weeks.
dislocation may not require surgical reconstruction medially. However,
when the PMC structures (specifically, the posterior oblique ligament)
are also involved, reconstruction will often be necessary (Fig. 54-28). The following is a simple reconstruction that has worked well for one of the authors (J.P.S.).
the leg in the figure-of-four position, with the knee flexed and the
hip externally rotated. The skin incision is the same as that described
for the anatomic PCL reconstruction technique. Details of the approach
are given in the section on approaches at the beginning of this chapter.
trimmed to approximately 5 to 6 mm in diameter. Locking stitches are
then placed in each end of the graft to facilitate graft passage (Fig. 54-29). The isometric point on the knee is
identified using the same technique as was described for the PLC
reconstruction. Briefly, that involves obtaining a lateral fluoroscopy
view and identifying the point where the posterior femoral cortex
intersects with Blumensaat line. A 3.2-mm drill bit is place on that
point, and a hole is drilled across the femoral condyle. A 4.5-mm screw
with a spiked ligament washer is then placed in the hole. This is
generally a long screw to allow the surgeon to obtain bicortical
purchase. Periosteum is debrided from around the screw hole, and the
cortical bone is shingled with a 0.25-inch osteotome. A second hole is
drilled with the 4.5-mm drill bit through the tibia, starting at the
point of insertion of the semitendinosus. Again, a bicortical screw and
spiked ligament washer are placed (Fig. 54-30).
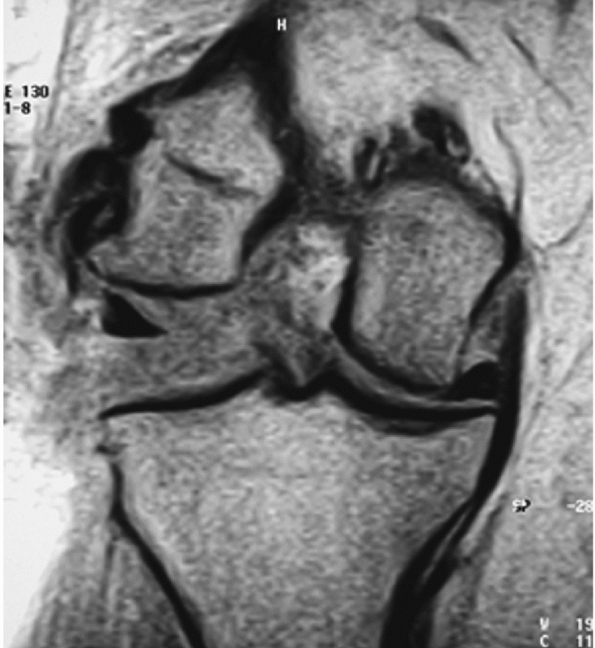 |
|
FIGURE 54-28 Coronal magnetic resonance image demonstrating a complete tear of the MCL in this patient with a multiligament knee injury.
|
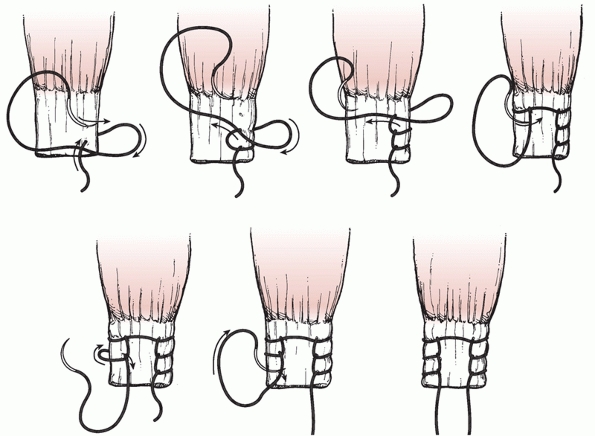 |
|
FIGURE 54-29
Sequential steps of placing Krakow locking loop ligament sutures for tendon or ligament repair. (Redrawn from Krackow KA, Thomas SC, Jones LC. A new stitch for ligament tendon fixation: brief note. J Bone Joint Surg Am 1980;68A:359, with permission.) |
one limb running down to the tibial screw in line with the deep MCL.
The second limb runs posteriorly under the semimembranosus, and then
turns the corner and is taken to the tibial screw and washer. The graft
is then tensioned in approximately 40 degrees of flexion. The femoral
screw is tightened initially, followed by the tibial screw and washer.
Additional stability can be attained by using permanent suture to draw
the two limbs together near the tibial screw. This maneuver will
tighten the poteromedial corner or posterior oblique portion of the
reconstruction123 (Fig. 54-31).
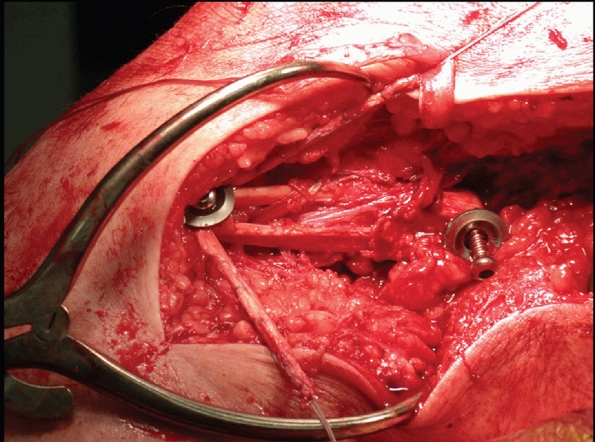 |
|
FIGURE 54-30
Posteromedial corner reconstruction using allograft tibialis anterior and screws with spiked ligament washers in both the tibia and the femur. |
Memphis, TN) is a circular external fixator with a hinge placed at the
center of rotation of the knee joint. It consists of two carbon fiber
5/8 rings bolted to multihole Rancho Cubes (Smith & Nephew) that
connect to 5- or 6-mm half-pins. The two rings are connected by
calibrated hinges that are placed at the center of knee rotation
medially and laterally. The placement of the whole apparatus is based
on a centering wire temporarily placed at the isometric point of the
femur. The hinges are centered by holes that accommodate this centering
wire. Once the femoral half-pins have been placed, the centering wire
is removed and the corner reconstructions can be completed. The
determination of the isometric point for this technique is identical to
the method described above in the PLC and PMC reconstruction
techniques. Working from a perfect lateral image of the femoral
condyles using fluoroscopy, the point where a line from the posterior
femoral cortex intersects with Blumensaat’s line corresponds with the
isometric point. The centering wire is driven into the femur at this
point perpendicular to the femoral shaft and parallel with the knee
joint.
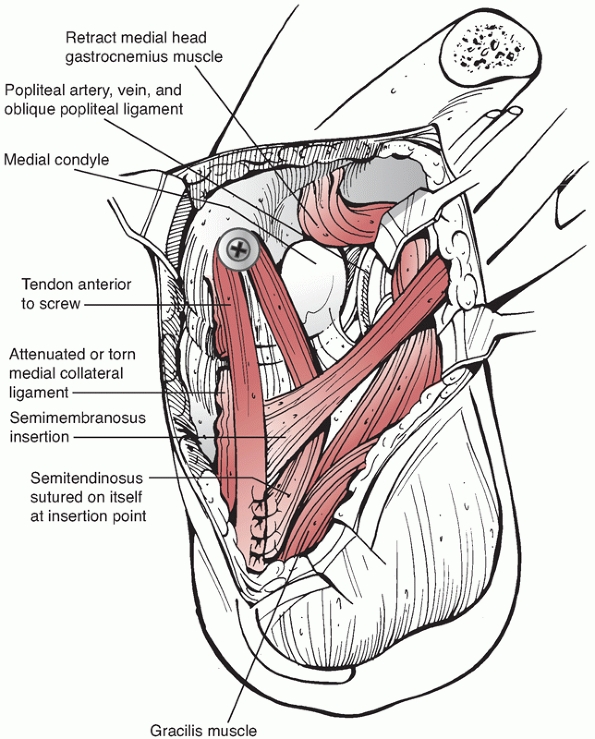 |
|
FIGURE 54-31
Posteromedial corner reconstruction using semitendinosis autograft around a screw and washer placed at the isometric point on the femur. (Redrawn from Stannard JP, Schenck RC Jr. Knee dislocations and ligamentous injuries. In: Stannard JP, Schmidt AH, Kregor PJ, eds. Surgical Treatment in Orthopaedic Trauma. New York: Thieme, 2007:687-712, with permission.) |
the wire and two 6-mm half-pins are placed into the femur. These are
placed from the most posterior point on the medial (Fig. 54-32)
and lateral sides of the 5/8 ring. The instrument set comes with a
triple trochar that is placed through the Rancho cube and a stab
incision and then centered on the shaft of the femur. After drilling
the holes through a onehole Rancho cube medially and a three-hole
Rancho cube
laterally,
two 6-mm half-pins are placed. It is important to hold the ring
perpendicular to the shaft of the femur while placing the half-pins.
Three 5-mm pins are normally placed into the tibia through Rancho cubes
hung from the distal ring.
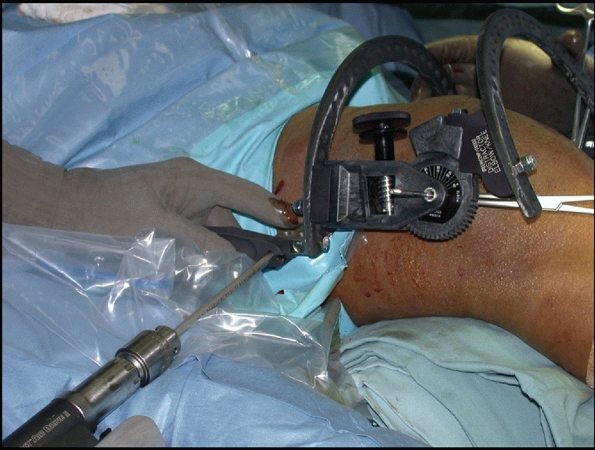 |
|
FIGURE 54-32 Drilling the posteromedial femoral pin for the Compass Knee Hinge (Smith & Nephew, Memphis, TN).
|
steps in the application on a patient with a multiligament knee injury.
If repair or reconstruction of either the posteromedial or PLC is part
of the planned surgical procedure, the centering wire should be placed
before the repair or reconstruction of the corner. This is important
because the wire is placed through the area of the corner repair, often
requiring a number of passes to get the position exactly right. The
risk of damaging a repair of the PLC is unacceptably high if the
centering pin is placed after repair. Also, the screw and spiked washer
we use in PLC reconstructions are placed in the same location as where
the centering pin must be placed. This precludes placement of the hinge
following PLC or PMC reconstruction. The solution is to place the
centering wire first, mount the hinge, and place the two femoral
external fixation pins through Rancho cubes. The CKH is then detached
from the pins, leaving them in place in the femur only. The CKH is
placed on the back table until the completion of the case. The
centering wire is removed and the remainder of the repair or
reconstruction is accomplished.
remounted on the two femoral pins through the same holes in the 5/8
ring, ensuring an isometric placement of the hinge on the knee. Three
5-mm tibial pins are then placed using the triple trochars through stab
incisions to complete application of the hinge. I normally use a
three-hole, a four-hole, and a five-hole Rancho cube hanging down from
the tibial 5/8 ring. The tibial pins should not be placed until the end
of the case because they are in the way during the ligamentous
reconstruction or repairs. It is also much easier to close all surgical
incisions prior to reattaching the hinge to the femoral pins and
placing the tibial pins.
This allows aggressive rehabilitation of the knee in flexion and
extension, while protecting the reconstructions from rotational forces
and varus/valgus stress. I have found it very helpful with remarkably
unstable KD III and KD IV dislocations. It is also extremely helpful
with fracture-dislocations. There have been two case reports98,113 and one small series122 published regarding the use of the CKH in knee dislocation patients.
 |
|
FIGURE 54-33 Knee flexion after placing Compass Knee Hinge (Smith & Nephew, Memphis, TN).
|
to 2 years. It is important to emphasize the time and energy commitment
to the patient at the beginning of the process. Postoperative range of
motion should be monitored carefully and the patient should be taken
back to the operating room for a manipulation under anesthesia with or
without an arthroscopic lysis of adhesions if the patient has not
achieved at least 90 degrees of flexion by 2 months following
reconstruction. In our experience, the risk of needing a manipulation
to treat stiffness is approximately 20%, and it is useful to counsel
the patient prior to the first procedure. The patient’s ability to
achieve full extension should be carefully monitored, and any flexion
contracture should be aggressively treated with physical therapy
hanging weights in extension and the use of dynamic splinting at night.
The patient should be told to avoid placing a pillow under the knee for
comfort during the first 6 weeks following surgery, as this habit can
lead to a flexion contracture.
treatment of the multiple ligament-injured (dislocated) knee result
from diagnostic, surgical, and postoperative deficiencies.
Complications resulting from diagnostic deficiencies include failure to
recognize and treat vascular injuries (both arterial and venous),
associated ligament injuries about the knee (posterolateral and
posteromedial instability), other knee structural injuries (meniscus,
articular surface), and lower extremity malalignment. Failure to
recognize these entities adversely affects the treatment plan,
resulting in a suboptimal outcome.
vascular injury, nerve injury (Fig. 54-34),
compartment syndrome, wound-healing problems, postoperative loss of
motion, persistent laxity, physeal injuries, osteonecrosis, and
fractures. Complications occurring in the postoperative period include
wound-healing problems, progressive laxity, arthrofibrosis, heterotopic
ossification, osteonecrosis of the femoral condyle, and fractures.
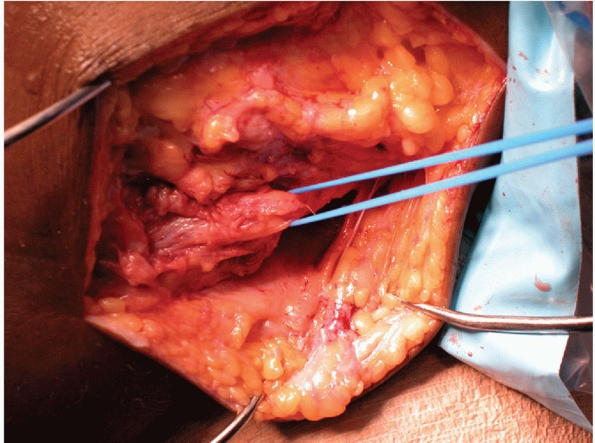 |
|
FIGURE 54-34 Segmental stretch injury of the peroneal nerve.
|
severe injuries that will frequently result in serious complications
even when the treating physician does everything correctly. Adherence
to the principles discussed in this chapter will improve the
probabilities of success; however, the possibility of significant
complications remains, even in the hands of the most experienced
surgeons.
a broad array of injuries, ranging from an open KD IV injury with an
ipsilateral fracture and popliteal artery injury to an isolated KD I
injury. This broad variability of injury among individual patients is
one factor that makes comparison of studies and determination of
outcomes difficult. Other factors that make assessing outcomes
difficult include the facts that most studies involve relatively small
patient cohorts and nearly all are retrospective studies. There have
been no prospective randomized studies published regarding knee
dislocations. An additional challenge in assessing outcomes is the lack
of uniformity in outcome scoring systems used in the literature for
knee dislocations. Despite these limitations, we have evaluated 25
published studies that report outcomes data in patients following knee
dislocations. By combining multiple studies, we were able to identify
trends that are not clear when evaluating individual studies with small
enrollments. We will look at a number of different categories of
outcomes in the following pages.
strong trend toward surgical reconstruction following knee
dislocations. At least nine studies have been published that
specifically compare operative and nonoperative treatment of patients
following knee dislocations.* All of these studies are
retrospective and feature a wide variety of surgical techniques and
rehabilitation protocols. A total of 172 patients were treated
surgically in these nine studies, compared with 84 patients treated
nonoperatively. All nine authors concluded that surgical treatment is
superior to nonoperative treatment for patients following knee
dislocations. Roman et al.100 did
note increased stiffness with patients treated surgically, but this
experience was not reported in the other studies. All of the authors
found marked improvements in stability in patients who had surgical
reconstruction of their knee compared with those who had nonoperative
treatment. Richter et al.97
published a large series of 77 knee dislocations. They reported that
patients treated surgically had better motion, stability, and return to
work and recreational activities. They also noted improved outcomes
using the Lysholm Knee Scale score (a score of 78 for surgical versus a
score of 65 for nonoperative treatment) and the International Knee
Document Committee (IKDC) score (41% were severely abnormal in the
nonoperative group) following surgical treatment. Wong et al.140
recently reported a 27% long-term incidence of instability following
reconstruction compared with a 91% incidence of instability following
nonoperative care. Rios et al.99 reported improved results using both the Lysholm and Meyers scores in patients following surgical care.
the ideal time to undertake reconstruction of a knee dislocation. There
are many variables that will always require this decision to be
individualized to each patient. These variables include associated
injuries, whether the dislocation is open or closed, the degree of
contamination if open, and the degree of associated soft tissue injury
if closed. Most surgeons consider surgery performed within the first 3
or 4 weeks to be an acute repair or reconstruction of the knee
dislocation, while anything delayed longer than a month would be a
chronic knee reconstruction. There are advantages to allowing the
patient to recover from the initial effects of the trauma and
performing the reconstructive surgery during the chronic phase. These
advantages include a better nutritional state after recovering from the
trauma, improved local soft tissue condition, and therefore potentially
a decreased risk of infection and wound dehiscence. There are different
advantages to reconstructing a knee dislocation during the acute injury
phase. These advantages include potentially decreased total trauma
recovery time because the patient is recovering from the knee
reconstruction at the same time as he or she is recovering from other
injuries sustained in the trauma, the ability to repair torn structures
if the tissue quality allows, and improved healing of the injury to the
capsule of the knee by taking advantage of the inflammatory reaction
associated with the initial injury.
to initiate the repair or reconstruction of the knee following a
dislocation. Harner et al.44
retrospectively evaluated 47 consecutive knee dislocations of patients
who presented to a single level 1 trauma center. Fourteen patients were
eliminated from consideration because of confounding injury variables
such as open dislocations or vascular injuries. The remaining 31 were
divided
into two groups. Nineteen were treated acutely, which the authors
defined as less than 3 weeks following their index injury. The
remaining 12 patients were treated during the chronic period which they
defined as more than 3 weeks following their dislocation. They noted no
significant difference in the final arc of motion obtained by patients
in the two groups, but better final knee stability was seen in the
acute treatment group. The difference was significant when they
evaluated their patients for posterior tibial translation, where 84% of
the patients in the acute group were stable compared with only 50% of
the patients in the chronic group. They also evaluated the patients
using multiple outcome scores. In all cases, patients in the acute
group attained better scores than patients in the chronic group. Using
the Lysholm Knee Scale score, acutely treated patients achieved a mean
score of 91 compared with 80 for the chronic group. On the Meyers
functional rating, 84% of the acute group were rated good or excellent
compared with only 58% of the chronic group. On the Knee Outcome Survey
Activities of Daily Living score, the acute group scored a mean of 91
compared with 84 for the chronic group. Finally, on the Knee Outcome
Survey Sports Activities Scale, patients in the acute group scored a
mean of 89 compared with a mean of 69 for patients in the chronic
group. They concluded that patients with knee dislocations treated
acutely had better functional outcomes than patients treated on a
delayed basis.
evaluated the time to treatment for patients following knee
dislocations. Their retrospective study evaluated 21 patients with 22
knee dislocations. They had two very distinct groups. Eight patients
had their knee dislocation treated within 2 weeks of the index injury,
while the remaining 14 knees were not treated until at least 6 months
following injury. Again, there was no significant difference between
the two groups in total arc of motion attained. They did note that the
only patient in their series who developed arthrofibrosis was in the
acute treatment group. The patients in the acute treatment group had a
mean Lysholm Knee Scale score of 87 compared with a score of 75 for the
chronic treatment group. The Tegner Activity Scale levels were similar,
with a mean score of 5.0 for the acute group and 4.4 for the chronic
group.
reconstruction following knee dislocations. Clearly, additional data
are needed before we can strongly recommend acute treatment.
Additionally, the surgeon will still need to individualize care to each
patient considering the associated injuries.
there has been a trend toward an improving arc of motion over time. The
mean arc of motion in the pre-1994 studies was 106 degrees, compared
with 123 degrees in the more contemporary studies. The reasons for the
improvement are most likely multifactorial, including improved
reconstruction techniques and the use of aggressive rehabilitation
protocols. The use of continuous passive motion machines was common in
many studies.‡ Despite these impressive final ranges of
motion, arthrofibrosis has remained a major problem for patients
following knee dislocations. The remarkable improvements seen in total
arc of motion have frequently required secondary surgical treatment in
the form of either manipulation under anesthesia or arthroscopic lysis
of adhesions. Reports of the need for surgical intervention to treat
arthrofibrosis have varied from a low of 5%62 to a high of 71%88
following acute dislocations. The mean reported incidence of
arthrofibrosis requiring surgical intervention was 29% of the patients
from 14 different studies.§ The incidence of arthrofibrosis
is improving with time. A similar analysis of the literature completed
5 years ago revealed an incidence of arthrofibrosis of 38%120
compared with a rate of 29% in the current analysis. In the final
analysis, motion has improved remarkably over the years for patients
following knee dislocations. However, while the incidence of
arthrofibrosis is improving, it remains a major problem. Great
vigilance by the treating surgeon regarding motion is required in the
postoperative period. Nearly one third of patients will require
surgical intervention to treat arthrofibrosis and maximize the final
arc of motion.
rather than instability are the primary problems for patients treated
surgically following knee dislocation. While published outcome studies
support that pain and loss of motion are major problems, the data
demonstrate that recurrent instability following reconstruction remains
a major problem as well. One difficulty in assessing the literature is
the wide range of methods used for reporting knee stability in the
literature. For the purposes of this chapter, instability has been
defined as grade 2 or 3 on a scale 0 to 3, KT 1000 or 2000 Ligament
Arthrometer (MEDmetric Corporation, San Diego, CA) data that are
greater than a 3-mm side-to-side difference for either anterior or
posterior translation or greater than 5 mm for total anteroposterior
translation, or groups C or D on the IKDC score.
In five of the six studies, the authors reported 100% of the patients
had residual instability in at least one direction following treatment.
In the remaining study,140 the authors reported 91% of their patients had residual instability as a final result using the criteria defined above.
outcome data following surgical repair or reconstruction of knee
dislocations.* All of the studies that report on all the
ligament groups torn in the dislocation list at least an 18%
instability rate. The range is from 18% to 100%, with a mean of 42% of
patients reported to have at least one ligament with chronic
instability following repair or reconstruction. In many cases, more
than one ligament in an individual patient meets the criteria for
chronic instability. Anterior and/or posterior instability is reported
more frequently than instability of the posteromedial
or
PLCs. It is interesting that while motion results have improved
slightly since our analysis 5 years ago, chronic knee instability has
gotten slightly worse.
knee dislocation, and it occurs to some degree in virtually every
patient. It can vary from occasional pain that has minimal impact on
daily activities to severe and disabling pain. There are a wide variety
of potential causes of knee pain following dislocation, including:
articular cartilage injury, chronic instability, arthrofibrosis, and
posttraumatic arthritis. Severe pain may cause a patient with a stable
knee and excellent motion to have a poor functional outcome. It is very
common for patients with arthrofibrosis to experience significant pain
at their limits of motion.
Two studies found that chronic pain was less prevalent in patients who
had their dislocation reconstructed acutely compared with those
patients who had reconstruction of a chronic dislocation.3,88
Two possible causes of increased pain in patients treated chronically
are simply the pain associated with chronic instability and subsequent
osteoarthritis.4 Additional outcome
studies that specifically address the issue of chronic pain are needed.
Patients who sustain knee dislocations must be prepared for the
possibility of significant long-term pain.
associated with multiple injuries. The vast majority of patients are
unable to return to work immediately following treatment for a knee
dislocation. Eight outcome studies address the issue of patient
employment following knee dislocations.* Most patients are
able to resume gainful employment, with many being able to resume
high-intensity occupations following contemporary reconstruction
techniques and extended rehabilitation. The combined results from the
eight studies show that 93% of patients were able to resume some type
of work following knee dislocation, with 31% having to accept a job
that was either light duty or less physically demanding than their
previous job. Again, there was a higher rate of return to employment
for patients who had treatment in the acute period compared with those
who had chronic reconstructions. Richter et al.97
reported that only 58% of their patients with a repaired chronic
dislocation returned to work compared with 85% of those undergoing an
acute repair. It is clear from these data that it is possible for
patients to resume work, including high physical demand fields,
following reconstruction of knee dislocations.
report on patient results following knee dislocations. One problem is
that there are a variety of scores used in the literature. The most
commonly used scores are the Meyers,77 Lysholm, and IKDC scores. Five authors reported their results using Meyers’ criteria.36,44,77,99,101
There were excellent results in 29%, good in 53%, fair in 11%, and poor
in 7% of those patients treated surgically. When evaluating
nonoperative treatment with the Meyers score, there were no excellent
results and 6% good, 16% fair, and 78% poor results. Clearly, the
outcomes reported using the Meyers score favor surgical treatment of
knee dislocations.
A score of 90 to 100 is considered excellent, 80 to 89 is good, 70 to
79 is fair, and below 70 is a poor result. The mean score for all of
these studies for patients treated with surgical reconstruction was 84,
with a range from 75 to 91. Three studies reported Lysholm scores on
patients treated nonoperatively.82,97,99
The mean Lysholm score for patients in these three studies was 64.
Clearly, surgical treatment yields better results than nonoperative
treatment using the Lysholm score. Two studies compared Lysholm scores
for patients treated surgically in the acute time period with those
treated during the chronic period.44,62
The mean Lysholm score for patients treated acutely was 89, while the
score for those treated chronically was 78. Again, acute reconstruction
appears to have better results as measured by the Lysholm score in
these two studies.
This system uses the following designations: Group A, normal knee;
Group B, nearly normal knee; Group C, abnormal knee; and Group D,
severely abnormal knee. Combining the seven studies, we get the
following results: Group A, 4%; Group B, 37%; Group C, 41%; and Group
D, 17%. The results, using this scoring system, are still
disappointing, with nearly 60% of patients falling into the abnormal or
severely abnormal categories. Richter et al.97 reported on IKDC results in nonoperatively treated patients and found that 94% were either abnormal or severely abnormal knees.
activities is difficult because of the variability of the definition of
return to activities among studies. Many patients with knee dislocation
are not involved in competitive athletics. The reported level of return
of patients to some recreational activity following knee dislocation
varies from 0%3 to 97%119
with a mean of 65%. Richter et al. reported that 56% of their patients
treated surgically returned to athletic activity compared with only 17%
of their patients treated conservatively. They reported that 49%
returned to their prior level of play, 40% decreased by one level of
play, and 10% decreased by two levels.97
Seven studies reporting on patients treated surgically noted that 76%
of patients returned to some form of athletic or recreational
activities. Only 39% of patients were able to return to their preinjury
level of activity.**
report outcome scores continues to increase. Despite the limitations of
small series and retrospective data already mentioned, combining the
data can yield valuable information. Surgical treatment using
contemporary techniques clearly yields results that are superior to
nonoperative treatment. There is also a small amount of data that
suggest that treatment in the acute time period may yield results that
are superior to the results obtained for patients treated on a delayed
basis in the chronic time period.
Motion,
pain, and instability are all major problems for patients following
knee dislocations. Motion is improving slowly over the years, possibly
as a result of aggressive rehabilitation protocols. However, chronic
instability remains a problem for up to 42% of patients following
reconstruction. This number has risen slightly over the past 5 years.
Most patients are able to achieve adequate stability to perform most
activities of daily living, but stability to resume athletic activities
and other high stress activities is much more elusive. The outcomes
regarding patient employment are very encouraging and suggest patients
can return to productive lives following knee dislocation. Finally,
outcome scores using the Meyer and Lysholm scores are frequently good
to excellent. The outcomes using the IKDC score are far less favorable.
attempt to recreate the functional anatomy of the PCL. The larger
bundle is the anterolateral bundle, which is tensioned with the knee
place in 70 degrees of flexion. The smaller posteromedial bundle is
tightened with the knee in relative extension (approximately 30 degrees
of flexion). The combination of these two bundles may improve the
stability of the PCL through the entire arc of motion. A recently
published study of 30 PCL reconstructions combining the inlay technique
and double-bundle technique reported excellent stability 2 to 3 years
following reconstruction.51 Apsingi et al.9
evaluated nine cadaver knees, comparing single- to double-bundle PCL
reconstruction in both posterior and posterolateral laxity. They found
double-bundle reconstruction to prevent posterior laxity better than
single-bundle reconstruction at full extension, the difference being
1.6 mm; there was no difference at all other flexion angles.
on the back of the tibia and inlaying a graft at the anatomic insertion
of the PCL, rather than drilling a transtibial tunnel. He described the
problem of the graft having to traverse around the “killer turn” when
the transtibial tunnel is used and that this might lead to graft
failure.11,20
Markolf confirmed in a biomechanical study that traversing the “killer
turn” caused one third of cadaver grafts to fail and the remainder to
have significantly more thinning and elongation than inlay grafts.93,125 Seon and Song108 and MacGillivray et al.67
both retrospectively compared the inlay to the transtibial technique
with a minimum 2-year follow-up. The former study cited good clinical
outcomes in both groups, while the latter demonstrated that neither
technique fully recreates anterior-posterior stability with a
single-bundle femoral attachment site.
involved the use of a hinged external fixator (Compass Knee Hinge,
Smith & Nephew, Memphis, TN) and early motion following surgical
reconstruction of the knee. A preliminary report indicated that less
than 10% of patients using the hinge had any ligament instability
following early motion rehabilitation, compared with approximately 40%
of patients treated with a conventional brace.122
The data are very preliminary but create possible treatment options for
patients with marked knee instability. Indications for the use of the
hinged external fixator combined with early motion following
reconstruction include KD III, KD IV, and KD V knee dislocation
patterns.
repaired if surgery is accomplished within 3 or 4 weeks of injury and
the tissue quality appears good. However, there were no published
series supporting that view. Stannard et al.115
recently presented a series that compared repair using suture anchors
to bone with a modified two-tailed reconstruction technique that
recreates the popliteus tendon, the popliteofibular ligament, and
lateral collateral ligament. Both groups underwent early motion
rehabilitation. The authors found a significant improvement in
stability of the PLC with reconstruction compared with repair. These
findings may not occur if early motion rehabilitation is not used or if
alternative repair techniques are used.
knee, attention must be given in particular to the normal anatomy of
the tibial and femoral condyles. The femoral condyles are asymmetric in
size and shape. The medial femoral condyle is approximately 1.7 cm
longer than the lateral condyle in its outer circumference. This
asymmetry in length produces axial rotation of the tibia on the femur
during flexion and extension.19 With
regard to width, the lateral condyle is slightly wider than the medial
when measured at the center of the intercondylar notch. In the sagittal
axis of the knee, the lateral femoral condyle is longer or more
anterior than the medial condyle. In the coronal or anterior-posterior
plane, the medial femoral condyle projects farther distally than does
the lateral condyle.5,41
When the femur is viewed along its anatomic axis, this appearance
becomes obvious. However, in normal weight-bearing alignment, the
condyles appear to be equal in length. The parallel condylar surfaces
are created by the mechanical axis configuration of the lower extremity.
tibial articulation involves the menisci to provide congruity with the
distal femoral condyles, and in reality the menisci should be
considered tibial extensions. The menisci function to create conformity
between the flat tibial and curved femoral surfaces. The medial condyle
of the tibia is nearly flat and has a larger surface area than the
lateral condyle. The lateral condylar surface is slightly concave. Both
condyles have a 10-degree posterior inclination to the tibial shaft in
the saggital plane. Bordering the notch are the tibial spines (or
tubercles), both medial and lateral, which function to stabilize the
condyles from side-to-side motion. The interspinous area is void of
hyaline cartilage and is the insertion site for the meniscal horns and
the cruciate ligaments.
The cruciates insert on the intertubercular sulcus and not on the spines themselves.4,39,41,78,84
Due to the asymmetry of the tibial condyles and the relative covering
of each respective meniscus, the medial tibial plateau does not
tolerate joint surface incongruity as well as does the lateral tibial
plateau. With the more extensive covering of the lateral meniscus, up
to 10 mm of lateral plateau displacement may be tolerated clinically,
depending on the exact configuration of the fracture. Although anatomic
reduction is considered the goal in fracture management, the clinician
should remember the differences specifically between the medial and
lateral tibial plateaus.
explains their susceptibility to injury. The popliteal artery and vein
course from the fibrous tunnel of the adductor hiatus through the
popliteal space (giving off the five geniculates) and exit through the
fibrous arch of the soleus muscle. Being securely fixed proximally at
the adductor hiatus and distally at the soleus arch, the popliteal
artery and vein can be torn or stretched with the exaggerated
tibiofemoral displacement or hyperextension that produces the
dislocation.
frequently involves the use of open approaches. Although the lateral
compression type of injuries with an associated contralateral ligament
injury can be treated using arthroscopic-assisted approaches, the
clinician is usually faced with a large condylar fragment, gross
capsular disruption, and the need for open reduction of the fracture
fragment. Furthermore, with acute injuries to both the condyle and
ligament, the risk of arthroscopic fluid extravasation is a concern, as
it can result in a compartment syndrome or vascular insufficiency.
Certainly delayed ligament reconstruction is best performed with
arthroscopic approaches; in contrast, early surgery involving fracture
fixation and frequently ligament repair or reconstruction requires an
open approach. Selection of the specific approach depends upon the
fracture configuration and the ligaments involved; treatment may
require the use of combined incisions. In such scenarios, the
importance of maintaining an adequate soft tissue bridge between
incisions must be kept in mind. The posteromedial, posterolateral, and
anterior approaches are commonly used in the treatment of these
fracture-dislocations.
can be treated through either a direct posterior or posteromedial
approach. Galla and Lobenhoffer described a direct posteromedial
approach that does not cross the popliteal crease and does not divide
the tendon of the medial head of the gastrocnemius.38 This report was introduced into the English language by Fakler et al.28
With the patient prone and a thigh tourniquet in use, a 6- to 8-cm skin
incision is made along the medial border of the medial head of the
gastrocnemius, its proximal extent being at the joint line. The
popliteal crease is not crossed. The small saphenous vein is identified
between the medial and lateral heads of the gastrocnemius. Medial to
the vein is the medial sural cutaneous nerve; the nerve is not
retracted, as dissection is carried medial to the medial head of the
gastrocnemius. The medial head is then retracted laterally, carefully
placing the retractor to avoid the major neurovascular structures. The
semimembranosus is also dissected and retracted medially; its insertion
on the posteromedial tibial plateau is not disrupted. The superior
border of the popliteus is elevated off the posterior tibia, exposing
the fracture fragment. If further distal or medial exposure is
required, the soleus origin or the semimembranosus insertion can be
elevated, respectively. Reduction of the posterior fracture fragment
can be accomplished by placing the knee in either flexion, to relax the
posterior capsule, or hyperextension.13,28
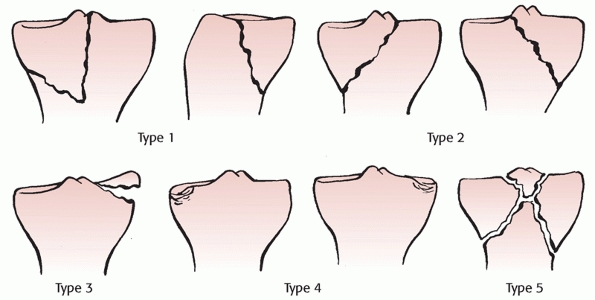 |
|
FIGURE 54-35
Moore classification of fracture dislocations of the knee. (Redrawn from Moore TM. Fracture-dislocation of the knee. Clin Orthop 1981;156:128-140, with permission.) |
presentation and severity, and for that reason they require an
individualized approach depending on the soft tissue envelop, the
fracture geometry, the ligaments involved, the vascular status, the
patient’s overall condition, and surgeon experience.
-
Use techniques of treatment with which you are familiar.
When forming a preoperative plan for a relatively rare injury type,
consult a more experienced surgeon to assist in the plan and even the
surgery itself. Obtain as much information as possible with an
examination under anesthesia and a secondary imaging study, either MRI
or CT, once a vascular injury has been ruled out and prior to
definitive reconstructive surgery. -
Protect the soft tissues.
Use short-term immobilization with a bulky Jones splint with frequent
vascular checks. Frequently reevaluate the leg compartments. -
Educate your patient.
The complexity of such an injury will not be obvious to the patient and
family. If the patient allows, involve the family in this education
progress. The family will remember
P.1864more clearly the details, especially if the patient has a complication you had previously discussed.
-
Get your vascular surgeon involved early and always.
Know your institution’s vascular protocol; if one is not present,
establish institutional guidelines. Such guidelines will protect you in
the middle of the night. Do not isolate yourself in the vascular
decision making; always obtain a consultation. Clearly communicate the
orthopaedic plan to your vascular surgeon, as he or she may have little
background information on the orthopaedic injury. Your insight and
direction will be invaluable to the vascular surgeon. Try to place the
external fixator before the vascular repair, but do not be surprised if
the vascular team wants you to wait until they have reestablished flow
to the leg. Assist the vascular team in the four-compartment
fasciotomy. As orthopaedic surgeons we have tremendous experience in
this area that will be appreciated by the vascular team. Carefully plan
with your vascular surgeon the timeline to mobilization of the knee
joint after arterial repair. -
Use only stable internal fixation.
Fracture-dislocations are highly unstable and will tend to displace,
especially with weight-bearing. Obtain intraoperative plain x-rays to
critically evaluate the reduction, fixation, and screw placement.
Always re-evaluate ligament injuries after fracture fixation to
determine the need for further ligament surgery. -
Reconstruct or
repair the collateral ligament complex at the time of fracture fixation
if varus or valgus stability persists once stable fracture fixation has
been achieved. Delay cruciate ligament reconstructions.
Simultaneous open surgery, cruciate reconstruction, and immobilization
are a recipe for severe permanent postoperative stiffness and
heterotopic ossification. -
Avoid trying something new in the middle of the night, if possible.
Delay a challenging reconstructive effort for the best time of day for
you and the operating room staff. Hence, use short-term spanning
external fixation to obtain initial stability, especially when there is
gross instability, an open wound, or a vascular injury. Occasionally
work is required at night due to open or vascular injuries. In such
scenarios, avoid any delay. Examine the ligaments under anesthesia and
do not rely solely on the MRI studies. -
Avoid transverse incisions.
Use longitudinal incisions with a large skin bridge (7 to 9 cm at a
minimum) between them. Plan for future reconstructive efforts with your
first surgical approach to the knee. -
Do not forget to warn your patient and, if the patient allows, the family as well.
Be detailed in your discussion of potential pitfalls. In our
experience, patients want to be educated. Clearly explain the negatives
of the injury to them. -
Do not delay vascular consultation or vascular treatment.
Injuries such as these present frequently at night in the worst of
clinical scenarios. Never rely on the concept of spasm as an
explanation for poor distal limb perfusion, and do not wait for
possible collateral flow to offset a vascular injury. If ischemia is
present, the arterial tree should be explored surgically and
revascularized. -
Do not immobilize the knee unless it is necessary to protect a vascular repair or an extensive soft tissue repair.
If fracture fixation remains unstable, immobilization carries with it a
high risk of arthrofibrosis. The combination of a closed head injury
and immobilization can frequently produce severe heterotopic
ossification. Despite achieving stable fixation and pursuing early
range of motion, we advise our patients that there will be a need for
manipulation in approximately 20% of cases. -
Do not rely on fluoroscopic images to make final judgments regarding your fixation efforts.
Intraoperative plain radiographs show the quality of reduction and
fixation best and allow the surgeon to make a more accurate assessment
of fixation and joint position. -
Do not reconstruct the cruciates early unless necessary.
Focus on the fracture and collateral and corner ligament management.
Delayed cruciate reconstruction can follow once motion is reestablished
and the fracture has healed.
L, Logan T. Results following treatment of traumatic dislocations of
the knee joint. Clin Orthop Relat Res 1992;284:203-207.
R, Yellin AE, Weaver FA, et al. Role of routine arteriography in blunt
lower-extremity trauma. Am J Surg 1990;160:221-225.
S, Nguyen T, Bull AM, et al. Control of laxity in knees with combined
posterior cruciate ligament and posterolateral corner deficiency:
comparison of single-bundle versus double-bundle posterior cruciate
ligament reconstruction combined with modified larson posterolateral
corner reconstruction. Am J Sports Med 2008;36:487-494.
FM. Evaluation and treatment of chronic medial collateral ligament
injuries of the knee. Sports Med Arthrosc Rev 2006;14:84-90.
JA, Graham SM, Parker RD, et al. A biomechanical comparison of
posterior cruciate ligament reconstructions using single- and
double-bundle tibial inlay techniques. Am J Sports Med 2005;33:976-981.
T, McCarty LP 3rd, Harris MB, et al. The posterior shearing tibial
plateau fracture: treatment and results via a posterior approach. J
Orthop Trauma 2005;19: 305-310.
SI, Nam TS. Surgical outcome of two-stage management of multiple knee
ligament injuries after knee dislocation. Arthroscopy 2007;23:1066-1072.
C, Fankhauser F, Hofer HP, et al. Three-year results of proximal tibia
fractures treated with the LISS. Clin Orthop Related Res
2006;445:222-229.
TS, Malone JM, Moody M, et al. Frequency of vascular injury with blunt
traumainduced extremity injury. Am J Surg 1990;160:226-228.
RT, Schaffer JJ. A simplified approach to the tibial attachment of the
posterior cruciate ligament. Clin Orthop 1990;254:216-219.
FS, Rokito AS, Pitman MI. Acute and chronic posterolateral rotatory
instability of the knee. J Am Acad Orthop Surg 2000;8:97-110.
WG, Shelbourne KD, Zoeller GB. Treatment of knee joint instability
secondary to rupture of the PCL. J Bone Joint Surg Am 1983;65A:310-322.
CH Jr, Braitman HE. Popliteal artery injury following fracture or
dislocation at the knee: diagnosis and management. Arch Surg
1977;112:969-973.
JW, Jagger C, Butcher JL, et al. Reassessing the role of arteriograms
in the management of posterior knee dislocations. J Trauma
1993;35:692-697.
RK, Schenck RC, Guarducci C. The dislocated knee: classification,
treatment, and outcome. US Army Med Dept J 1997 PB 8-97-11/12:1-9.
L, Benum P, Sundalsvoll S. Primary suture of the ACL: a 6-year
follow-up of 74 cases. Acta Orthop Scand 1989;60:561-564.
JKM, Ryzewicz M, Hartshorn C, et al. Optimizing the management of Moore
type I posteromedial split fracture dislocations of the tibial head:
description of the Lobenhoffer approach. J Orthop Trauma
2007;21:330-336.
GC. Complications of multiple ligamentous injuries. In: Schenck RC Jr,
ed. Multiple Ligamentous Injuries of the Knee in the Athlete. Rosemont,
IL: American Academy of Orthopaedic Surgeons, 2002:101-107.
GC, Edson CJ. Arthroscopically assisted combined ACL/PCL
reconstruction. Two- to ten-year follow-up. Arthroscopy 2002;18:703-714.
GC, Gianotti BF, Edson CJ. Arthroscopically assisted combined anterior
and posterior cruciate ligament reconstruction. Arthroscopy
1996;12:5-14.
GC, Orcutt DR, Edson CJ. The multiple-ligament injured knee:
evaluation, treatment, and results. Arthroscopy 2005;21:471-487.
GC, Tomaszewski DJ. Allograft use in the treatment of the multiple
ligament—injured knee. Sports Med Arthrosc 2007;15:139-148.
F, Sinco SM Surgical treatment of chronic posterolateral rotatory
instability of the knee using capsular procedures. Sports Med Arthrosc
2006;14:44-50.
DR, Allen JW, Richardson JD. Blunt popliteal artery injury: is physical
examination alone enough for evaluation? J Trauma 1997;43:541-544.
M, Lobenhoffer P. The direct, dorsal approach to the treatment of
unstable tibial posteromedial fracture-dislocations [in German].
Unfallchirurg 2003;106:241-247.
FG, Marshall JL, Monjem ARS. The cruciate ligaments of the knee joint:
anatomical, functional, and experimental analysis. Clin Orthop
1975;106:216-231.
J, Lindahl J, Hirvensalo E, et al. Operative and nonoperative
treatments of medial collateral ligament rupture with early anterior
cruciate ligament reconstruction: a prospective randomized study. Am J
Sports Med 2006;34:1134-1140.
MS, Richardson MW, Miller MD. Knee dislocation. Complications of
nonoperative and operative management. Clin Sports Med 2000;19:519-543.
TF, Klatt J, Bachus KN. Biomechanical analysis of bicondylar tibial
plateau fixation: how does lateral locking plate fixation compare to
dual plate fixation? J Orthop Trauma 2007;21:301-306.
JA, Rana NA. Complications of posterolateral dislocation of the knee:
case report and literature review. Clin Orthop 1981;154:212-215.
JC. The importance of the posterior oblique ligament in repairs of
acute tears of the medial ligaments in knees with and without an
associated rupture of the ACL—results of a long-term follow-up. J Bone
Joint Surg Am 1994;76A:1328.
DL, Urban WP, Caborn DN, et al. Articular cartilage changes seen with
magnetic resonance imaging-detected bone bruises associated with acute
anterior cruciate ligament rupture. Am J Sports Med 1998;26:409-414.
RW, Taylor DC, Salvian AJ, et al. The role of arteriography in
assessing vascular injuries associated with dislocations of the knee. J
Trauma 1993;35:875-878.
JC, Hawkins RJ, Willis RB, et al. Tension studies of human ligaments:
yield points, ultimate failure, and disruption of the cruciate and
tibial collateral ligaments. J Bone Joint Surg Am 1976;58A:350.
EO, Crites BM, Flinn WR, et al. The role of arteriography in assessing
popliteal artery injury in knee dislocations. J Trauma 2004;56:786-790.
L, Lieberman JM, Motta AO, et al. Evaluation of tibial plateau
fractures: efficacy of MR imaging compared with CT. AJR Am J Roentgenol
1994;163:141-147
RY, McNicholas MJ, Keating JR, et al. Ligament repair and
reconstruction in traumatic dislocation of the knee. J Bone Joint Surg
Br 2003;85B:845-851.
JH, Dupuy DE, Siliski JM. Comparison of magnetic resonance imaging with
operative findings in acute traumatic dislocations of the adult knee. J
Orthop Trauma 2000;14:183-186.
K, Johansen K. Can Doppler pressure measurement replace “exclusion”
arteriography in the diagnosis of occult extremity arterial trauma? Ann
Surg 1991;214:737-741.
JD, Stein BES, Park M, et al. Comparison of tibial inlay versus
transtibial techniques for isolated posterior ligament reconstruction:
minimum 2-year follow-up. Arthroscopy 2006;22:320-328.
S, Schlegal T, Gill T, et al. Incidence and location of bone bruises
after acute PCL injuries. Presented at American Orthopaedic Society for
Sports Medicine, 1999 Annual Meeting, Traverse City, MI.
P, Santoriello, Iannone S, et al. Comparison of surgical treatments for
knee dislocations. Am J Knee Surg 1999;12:214-221.
KL, Zemanovic JR, McAllister DR. Cyclic loading of posterior cruciate
ligament replacements fixed with tibial tunnel and tibial inlay
methods. J Bone Joint Surg Am 2002;84A:518-524.
V, Steinbacher G, Friederich NF, et al. Operative treatment of combined
anterior and posterior cruciate ligament injuries in complex knee
trauma. Am J Knee Surg 2000;13:74-82.
GF, Hannon DG, Barr RJ, et al. Vascular injury associated with
low-velocity dislocations of the knee. J Bone Joint Surg Br
1987;69B:285-287.
JD, Gillham NR. Injury to the popliteal artery associated with
dislocation of the knee: palpable distal pulses do not negate the
requirements for arteriography. Injury 1989;20:307-310.
M, Moore T, Harvey JP. Follow-up notes on articles previously published
in the journal: traumatic dislocation of the knee joint. J Bone Joint
Surg Am 1975;57A:430-433.
MD, Bergfeld JA, Fowler PJ, et al. The posterior cruciate ligament
injured knee: principles of evaluation and treatment. AAOS Instru
Course Lect 1999;48:199-207.
WJ, Barei DP, McNair P. The value of the ankle-brachial index for
diagnosing arterial injury after knee dislocation: a prospective study.
J Trauma 2004;56:1261-1265.
FE, Dennis JW, Veldenz HC, et al. Confirmation of the safety and
accuracy of physical examination in the evaluation of knee dislocation
for injury of the popliteal artery: a prospective study. J Trauma
2002;52:247-252.
T, Savioe F, White J, et al. Orthopedic management of knee
dislocations: comparison of surgical reconstruction of surgical
reconstruction and immobilization. Am J Knee Surg 1995;8:97-103.
JO, Rogers W, Cruz AB, et al. Arterial injuries in orthopaedics: the
posteromedial approach for vascular control about the knee. J Orthop
Trauma 1996;10:476-480.
DM, Nutton RW, Keating JF. Palsy of the common peroneal nerve after
traumatic dislocation of the knee. J Bone Joint Surg Br
2005;87B:664-667.
T, Hladki W, Mierniczek W. Knee dislocation treatment with temporary
tibio-patellar fixation (patellar olecranization). Chir Narzadow Ruchu
Ortop Pol 1999;64:209-213.
F, Barber-Westin S. Reconstruction of the anterior and posterior
cruciate ligaments after knee dislocation: use of early protected
postoperative motion to decrease arthrofibrosis. Am J Sports Med
1997;25:769-778.
BD, Neault M, Benson E, et al. Primary repair of knee dislocations:
results in 25 patients (28 knees) at a mean follow-up of 4 years. J
Orthop Trauma 2007;21:97.
BM, Agel J, Swiontkowski MF, et al. Knee dislocations with vascular
injury: outcomes in the Lower Extremity Assessment Project (LEAP)
study. J Trauma 2007;63:855-858.
P, Wolf BR, Amendola A. Role of high tibial and distal femoral
osteotomies in the treatment of lateral-posterolateral and medial
instabilities of the knee. Sports Med Arthrosc 2006;14:96-104.
AG, Sharrard WJW. Posterolateral dislocation of the knee with capsular
interposition. J Bone Joint Surg Br 1958;40B:660-663.
A, Amis AA. PCL reconstruction: in vitro biomechanical comparison of
“isometric” versus single and double bundled “anatomic” grafts. J Bone
Joint Surg Br 1998;80B:173-179.
JR, Werner FW, Green JK, et al. Medial buttress versus lateral locking
plate in a cadaver medial tibial plateau fracture model. J Orthop
Trauma 2007;21:444-448.
PK, Posteraro RH, Schenck RC Jr. The role of MRI in evaluation of the
cruciate ligaments in knee dislocations. Orthopedics 1996;19:166-170.
M, Bosch U, Wippermann B, et al. Comparison of surgical repair of the
cruciate ligaments versus nonsurgical treatment in patients with
traumatic knee dislocations. Am J Sports Med 2002;30:718-727.
M, Lobenhoffer P. Cronic posterior knee dislocation: treatment with
arthrolysis, posterior cruciate ligament reconstruction and hinged
external fixation device. Injury 1998;29:546-549.
A, Villa A, Fahandezh H, et al. Results after treatment of traumatic
knee dislocations: a report of 26 cases. J Trauma 2003;55:489-494.
PD, Hopson CN, Zenni EJ Jr. Traumatic dislocation of the knee: a report
of 30 cases and literature review. Orthop Rev 1987;16:917-924.
RC. Approaches to the knee. In: Williams RP, Heckman JD, eds.
Contemporary Extensile Approaches in Orthopaedic Surgery. Baltimore:
Williams & Williams, 1997:389.
RC, Kovach IS, Agarwal A, et al. Cruciate injury patterns in knee
hyperextension: a cadaveric model. J Arthroscopy 1999;15:489-495.
JK, Giffin JR, Harner CD. Posterior cruciate ligament injuries:
isolated and combined patterns. In: Schenck RC Jr, ed. Multiple
Ligamentous Injuries of the Knee in the Athlete. Rosemont, IL: AAOS,
2002:73-90.
JK, Song EK. Reconstruction of isolated posterior cruciate ligament
injuries: a clinical comparison of the transtibial and tibial inlay
techniques. Arthroscopy 2006;22:27-32.
MS and Freedman EL: Allograft reconstruction of the anterior and
posterior cruciate ligaments after traumatic knee dislocation. Am J
Sports Med 1995;23:580-587.
L, Abdollahi K, Lee J, et al. The prevalence of soft tissue injuries in
nonoperative tibial plateau fractures as determined by magnetic
resonance imaging. J Orthop Trauma 2002;16:628-631.
L, Mitral M, Cave EF. Complete dislocation of the knee: experience at
the Massachusetts General Hospital. J Trauma 1969;9:192-215.
PT, Wickiewicz TL, Hotchkiss RN, et al. Chronic knee dislocation:
reduction, reconstruction, and application of a skeletally fixed knee
hinge. Am J Sports Med 1998;26:591-596.
JP, Brown SL, Farris RC, et al. The posterolateral corner of the knee:
repair versus reconstruction. Am J Sports Med 2005;33:881-888.
JP, Brown SL, Lopez-Ben RR, et al. Soft tissue injury of the knee
following tibial plateau fractures. Podium Presentation, 23rd Annual
Meeting of the Arthroscopy Association of North America, Orlando, FL,
April 2004.
JP, Brown SL, Robinson JT, et al. Reconstruction of the posterolateral
corner of the knee. Arthroscopy 2005;21:1051-1059.
JP, Martin SL. Tibial plateau fractures. In: Stannard JP, Schmidt AH,
Kregor PJ, eds. Surgical Treatment in Orthopaedic Trauma. New York:
Thieme Publishers, 2007:713-741.
JP, Riley RS, Sheils TM, et al. Anatomic reconstruction of the
posterior cruciate ligament after multiligament knee injuries: a
combination of the tibial-inlay and two femoral-tunnel techniques. Am J
Sports Med 2003;31:196-202.
JP, Schenck R C Jr. Knee dislocations and ligamentous injuries. In:
Stannard JP, Schmidt AH, Kregor PJ, eds. Surgical Treatment in
Orthopaedic Trauma. New York: Thieme Publishers, 2007:687-712.
JP, Sheils TM, Lopez-Ben RR, et al. Vascular injuries in knee
dislocations: the role of physical examination in determining the need
for arteriography. J Bone Joint Surg Am 2004;86A:910-915.
JP, Sheils TM, McGwin G, et al. Use of a hinged external knee fixator
after surgery for knee dislocation. Arthroscopy 2003;19:626-631.
JP, Wilson TC, Sheils TM, et al. Heterotopic ossification associated
with knee dislocation. Arthroscopy 2002;18:835-839.
JP, Wilson TC, Volgas DA, et al. Fracture stabilization of proximal
tibial fractures with the proximal tibial LISS: early experience in
Birmingham, Alabama (USA). Injury 2003;34:A36-A42.
AR, Arden GP, Rainey HA. Traumatic dislocation of the knee: a report of
43 cases with special references to conservative treatment. J Bone
Joint Surg Br 1972;54B:96-102.
GS, Yellin AF, Weaver FA, et al. Examination of the patient with a knee
dislocation: the case for selective arteriography. Arch Surg
1992;127:1056-1063.
BC, Bidwell TA, Chapman JR. Knee dislocations: where are the lesions? A
prospective evaluation of surgical findings in 63 cases. J Orthop
Trauma 2003;173:198-202.
M, Diamantopoulos A, Xenakis T, et al. Surgical treatment of multiple
knee ligament injuries in 44 patients: 2 to 8 years follow-up results.
Knee Surg Sports Traumatol Arthrosc 2006;148:739-749.
D, Rogers W, Schenck RC. Immediate vascular and ligamentous repair in a
closed knee dislocation: a case report. J Trauma 1994;35:898-900.
LF, Marshall JL. The supporting structures and layers on the medial
side of the knee. J Bone Joint Surg Am 1979;61A:56-62.
DC. Bicruciate injuries. In: Schenck RC Jr, ed. Multiple Ligamentous
Injuries of the Knee in the Athlete. Rosemont, IL: AAOS, 2002:91-99.
DC, Becker JR, Dexer JG, et al. Reconstruction of the anterior and
posterior cruciate ligaments after knee dislocation: results using
fresh-frozen nonirradiated allografts. Am J Sports Med 1999;27:189-196.
DC, Dvirnak PC, DeCoster TA. Knee dislocation: initial assessment and
implications for treatment. J Orthop Trauma 1997;11:525-529.
RE, Kakkasseril J, Cranley JJ. Complete dislocations of the knee with
popliteal vascular injury. J Trauma 1981;21:450-453.
CH, Tan JL, Change HC, et al. Knee dislocations: a retrospective study
comparing operative versus closed immobilization treatment outcomes.
Knee Surg Sports Traumatol Arthrosc 2004;12:540-544.
SV, Nevins RT, Sallis JG, et al. Impact of MRI on treatment plan and
fracture classification of tibial plateau fractures. J Orthop Trauma
2002;16:632-637.
MM, Mwipatayi BP, Abbas M, et al. Popliteal artery injury: Royal Perth
experience and literature review. Aust N Z J Surg 2005;75:882-886.
S., Kuroda R, Mizuno K, et al. Medial collateral ligament
reconstruction using autogenous hamstring tendons: technique and
results in initial cases. Am J Sports Med 2005;33:1380-1385.
