The Adult Knee
increasing both joint congruity and contact area as well as preventing
focal contractions of stress. When a load is transmitted across the
knee joint, the circumferentially oriented collagen fibers within the
menisci generate a hoop stress, which resists extrusion of the menisci
from between the femoral condyle and tibial plateau. Menisci transmit
approximately 50% of weight-bearing forces across the knee joint in
extension and 85% at 90° of knee flexion. In the medial compartment,
the medial meniscus bears 50% of the load; whereas in the lateral
compartment, the lateral meniscus carries approximately 70% of the load
transmitted across the lateral compartment.
Removal
of the medial meniscus results in 50 to 70% reduction in femoral
condyle contact area and in a 100% increase in contact stress. Total
lateral meniscectomy causes a 40 to 50% decrease in contact area and
increases contact stress in the lateral compartment to 200 to 300% of
normal. Along with biomechanical changes that can occur with
meniscectomy, the improved joint congruity that occurs through the
meniscus contact is thought to play a role in joint lubrication and
cell nutrition.
stability. Medial meniscectomy in the ACL intact knee has little effect
on anteroposterior motion, but in an ACL deficient knee, it results in
an increase in anterior tibial translation of up to 58% at 90° of
flexion. The posterior horn of the medial meniscus provides the most
significant contribution to resisting anterior tibial displacement.
Although the inner two-thirds of the meniscus is important in
maximizing joint contact area and increasing shock absorption, the
integrity of the peripheral one-third is essential for both load
transmission and stability. The meniscus also plays a role in shock
absorption. Compression studies have demonstrated that meniscal tissue
is approximately one-half as stiff as articular cartilage. Shock
absorption capacity was reduced by 20% after meniscectomy.
presence of type I and II nerve endings in the menisci, that they
function in providing proprioceptive feedback for joint position sense.
C-shaped or semicircular fibrocartilaginous structures with bony
attachment at anterior and posterior tibial plateau. The medial
meniscus is C-shaped, with a posterior horn larger than the anterior
horn in the anteroposterior dimension. The anterior horn of medial
meniscus has the largest insertion site surface area (61.4 mm2) and the posterior horn of lateral meniscus, the smallest (28.5 mm2).
The capsular attachment of medial meniscus on the tibial side is
referred to as the coronary ligament. A thickening of the capsular
attachment in the midportion spans from the tibia to femur and is
referred to as the deep medial collateral ligament. The lateral
meniscus is also anchored anteriorly and posteriorly through bony
attachments and has an almost semicircular configuration. It covers a
larger portion of the tibial articular surface than does medial
meniscus. Discoid variants have been reported with an incidence of 3.5
to 5%, most being an incomplete type.
varied architecture of coarse collagen bundles. Scanning electron
microscopy has revealed the orientation of collagen fibers to be mainly
circumferential, with some radial fibers at the surface and within the
midsubstance. This orientation allows compressive loads to be dispersed
by the circumferential fibers while the radial fibers act as tie fibers
to resist longitudinal tearing. At the surface of the meniscus, fiber
orientation is more of a mesh network or random configuration, thought
to be important in distribution of sheer stress. Collagen is 60 to 70%
of the dry weight of meniscus. The majority of collagen (90%) is type
I, with types II, III, V, and VI present in smaller amounts. At birth
the entire meniscus is vascular; by age 9 months, the inner one-third
has become avascular. This decrease in vascularity continues by age 10
years, when the meniscus closely resembles the adult meniscus. In
adults, only 10 to 25% of the lateral meniscus and 10 to 30% of the
medial meniscus is vascular. This vascularity arises from superior and
inferior branches of the medial and lateral genicular arteries, which
form a perimeniscal capillary plexus. Because of the avascular nature
of the inner two-thirds of the meniscus, cell nutrition is believed to
occur mainly through diffusion or mechanical pumping.
female ratio ranges from 2.5:1 to 4:1. The peak incidence is in men 21
to 30 years old and in girls and women 11 to 20 years old. One-third of
tears in this age group are associated with an ACL injury. Degenerative
types of meniscal tears commonly occur in men in their fourth, fifth,
and sixth decades. In patients with acute ACL injury, lateral meniscus
tears occur more frequently than do medial meniscal tears. In patients
with chronic ACL deficient knees, however, medial meniscal tears are
more prevalent. Meniscal injury is also frequently associated with
tibial plateau fracture and femoral shaft fractures. Diagnosis of
meniscal injury is based primarily on a thorough history and physical
examination. In athletic populations, the patient typically describes a
twisting injury or hyperflexion as inciting event. Often with
degenerative tears, there is no one inciting event. Complaints of
locking or catching may be present but also may be secondary
to
other pathology, such as chondral injury or patellofemoral chondrosis.
Loss of motion with a mechanical block to extension is commonly the
result of a displaced bucket handle meniscal tear and usually requires
acute surgical treatment.
quadriceps hypotrophy, and any joint line swelling that may occur with
a perimeniscal cyst. Range of motion must be assessed to determine
whether a meniscal block to extension or loss of flexion is present.
Joint line tenderness, pain with squatting, a positive flexion McMurray
test, and positive Apley compression and distraction tests are all
indicative of meniscal injury. In one study, joint line tenderness was
the best clinical sign of a meniscal tear, with 74% sensitivity and 50%
positive predictive value.
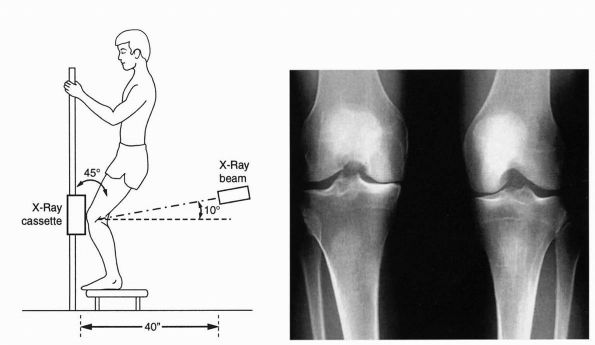
and include standard views, a 45° posteroanterior weight-bearing view
(also known as Rosenberg view), and lateral and axial (Merchant or
sunrise) views. Although these radiographs cannot confirm the diagnosis
of the meniscal tear, they are extremely important in defining bone
pathology and evaluating knee for joint space narrowing. Because
articular cartilage wear is often more advanced in the posterior aspect
of femoral condyles, the 30 or 45° posteroanterior flexion
weight-bearing view is more sensitive than standard standing views (Figure 19-1).
Magnetic resonance imaging (MRI) is noninvasive and has the ability to
assess the knee in multiple planes and other structures within the
joint. With improved technology and increased experience, the accuracy
of detecting the meniscal tear is now considered to be approximately
95% or better.
 |
|
FIGURE 19-1.
The Rosenberg view. PA weight-bearing view at 45° of flexion can facilitate the diagnosis if standard standing AP view is not sensitive enough. |
uniformly low signal. Meniscal tears are represented by a high signal
in the meniscus, which contacts the superior or inferior articular
surface. Areas of increased signal within the meniscus occur in
children and increase with age in adults. These intrasubstance changes
are seen frequently and are a common cause of overreading meniscal
tears on MRI scans. Extension of an abnormal signal to an articular
surface on only one image is often shown to be normal at arthroscopy.
transverse ligament with the anterior horn of the lateral meniscus can
mimic a meniscal tear. At the posterior horn of the lateral meniscus,
the popliteus tendon sheath may be mistaken for grade III signal. The
insertion of meniscofemoral ligament can mimic the appearance of
vertical tear in the posterior horn of the lateral meniscus. Separate
portions of the posterior horn can be mistaken for a bucket handle tear
as the most posterior coronal images traverse both the body and the
posterior horn, especially images of the lateral meniscus.
tears and are more likely to be degenerative in origin. These cysts are
more common laterally in
the
weak area between lateral collateral ligament (LCL) and iliotibial
band. Medial meniscal cysts are usually found posteriorly in the weak
area immediately posterior to the medial collateral ligament (MCL).
symptoms, chronicity, age, activity, and location and length of tear.
Options include no intervention, partial meniscectomy, and meniscal
repair. In the setting of ACL injury, the surgical treatment of
meniscal pathology is most often done concurrently with ACL
reconstruction. Surgical timing is most often dictated by issues
related to the ACL surgery, such as range of motion, swelling,
quadriceps function, and associated ligament injuries. The final
decision is made during arthroscopic examination. Thorough systematic
arthroscopic inspection allows the surgeon to delineate the type of
tear present. Use of 70° arthroscope allows for optimal visualization
of the posterior compartment, for defining the location of tear with
respect to the meniscofemoral junction, and for preparation of the
meniscus for any repair. Prior to arthroscopy, the patient must consent
to any possible procedure and all necessary equipment must be available
during initial arthroscopy.
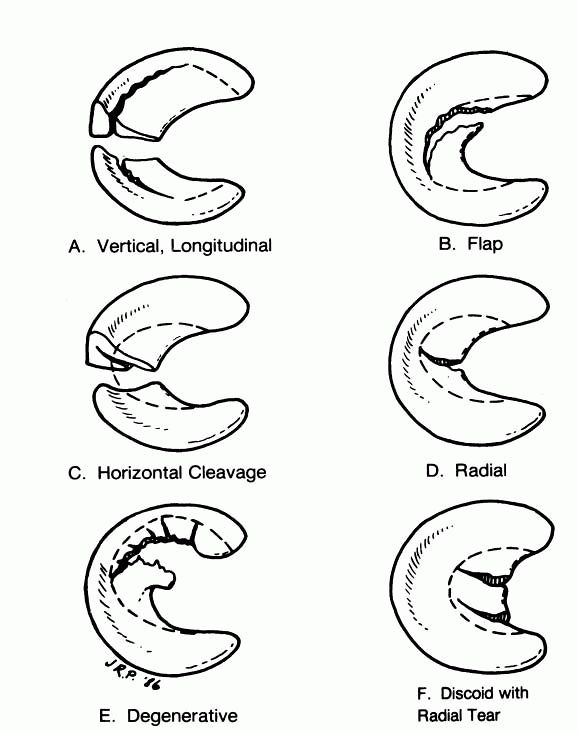
vertical longitudinal, oblique (flap), radial, horizontal, and complex
tears (Figure 19-2). With increasing age,
degenerative tears are more frequently seen, with most pathology in the
posterior horns. Individuals with degenerative tears may frequently
have an associated radiographic finding of joint narrowing, squaring of
condyles, and osteophyte ridges. Treatment decisions regarding
degenerative meniscus must be made with caution, as it can be difficult
to differentiate between symptoms of degenerative meniscus and
degenerative articular pathology.
(bucket handle) or incomplete. Incomplete tears usually occur in
younger individuals and occur either in superior or inferior surface of
meniscus. These tears have predilection for occurring in posterior
horns and are frequently seen in conjunction with ACL injuries. Stable
tears are those noted to be less than 1 to 1.5 cm in length and can be
abraded with arthroscopic rasps that can stimulate vascular ingrowth.
Larger incomplete tears greater than 1.5 cm in length or tears that
show instability with probing should be treated with meniscal repair or
partial meniscectomy, as the likelihood of tear propagation and
secondary cartilage damage is increased. Bucket handle tears are large
vertical longitudinal tears, typically occurring in young active
patients often with tears of the ACL. Treatment by partial meniscectomy
will most likely result in excision of large percentage of involved
meniscus; therefore, more recently many surgeons are advocating repair
of bucket handle tears, which can be congruently reduced. Chronically
displaced tears can be permanently deformed, and congruent reduction
cannot be obtained.
 |
|
FIGURE 19-2. Types of meniscal tears with typical area of resection.
|
they are typically located at the junction of middle and posterior
thirds of the medial meniscus or near the posterior attachment of the
lateral meniscus. Because radial tears, as well as the traditional
treatment that includes subtotal or total meniscectomy, can result in
accelerated degenerative changes in the knee, some surgeons have
advocated repair of large radial tears and have often used fibrin clot
at the time of meniscal repair citing good results. These repairs of
large radial or oblique tears can often be tenuous, and patients must
be prepared for the increased surgical morbidity and possible need for
further surgery.
margin of meniscus and extend toward the capsule. They may occur in all
age groups but may increase in frequency with age. They are commonly
seen in the lateral menisci of runners. Meniscal cysts are often
associated with horizontal tears and can be symptomatic because of
localized swelling. Treatment of these tears involves partial
meniscectomy with resection of flap peripherally until a stable rim is
obtained.
resection of meniscus is advocated when repair is not feasible. General
guidelines to arthroscopic resection that apply to most respectable
meniscal lesions include removal of all mobile fragments that can be
pulled past the inner margin of meniscus into the center of the joint.
Although a perfectly smooth rim is not necessary, the remaining rim
should be smoothed to remove any sudden changes in the contour. The
meniscocapsular junction and the peripheral meniscal rim should be
protected; in uncertain situations, more rather than less meniscal rim
should be left to avoid segmental resection, which essentially results
in a total meniscectomy (Figure 19-2).
meniscus as much as possible, especially when there is an associated
ACL injury and need for reconstruction. Many factors need to be
considered before making a decision. These factors include the
patient’s age, preinjury activity level and postinjury expectations,
chronicity, type, location and size of tear, and associated ligament
injuries. Based on vascularity, the meniscus has a red zone at the
periphery indicating the presence of vascularity and white zone with
the central two-thirds of the meniscus indicating avascularity. The
red-white zone is the transition zone between vascular and avascular
portion. The red-red tears have blood supply on both central and
capsular sides and have excellent healing potential. Red-white tears
have relatively good healing potential following adequate repair.
White-white tears have the worst potential of healing, as they are in
avascular zone. However repair of white-white tears with utilization of
fibrin clot has shown somewhat better results. The
ideal candidate for meniscal repair is the young, active individual who
has an acute longitudinal tear in peripheral vascularized meniscus
measuring 1 to 2 cm; the ideal situation is when performed at the same
time as ACL reconstruction. Beyond this ideal situation, the treatment must be individualized.
repair, all inside repair, inside-out repair, and outside-in repair.
All share the basic principles of adequate rim preparation and stable
fixation. Open repair is most useful in peripheral tears. In setting of
the open collateral ligament repair or reconstruction, open repair is
often necessary. Direct suturing of peripheral tear may be the most
effective means of treating these injuries. The success rate is high
because of acuteness of injury, peripheral tear, and associated
hemarthrosis.
is indicated for unstable vertical longitudinal tears of the peripheral
posterior horns of meniscus; tears of the anterior to posterior
one-third are not amenable to this technique. This technique is
performed entirely under arthroscopic control with an intra-articular
method of suturing the meniscus. The vertically oriented sutures oppose
the components of meniscal tear only without incorporating the joint
capsule. This technique necessitates specialized setup and equipment,
including 70° arthroscope and posterolateral and posteromedial portals.
Advantages of this technique include the ability to place vertically
placed sutures, and to achieve coaptation of the tear components
without entrapping the posterior capsule and any vital structures
contained within it. Disadvantages include technical difficulty in
passing the 70° arthroscope anterior to posterior through the
intercondylar notch as well as placing posterior operative cannula.
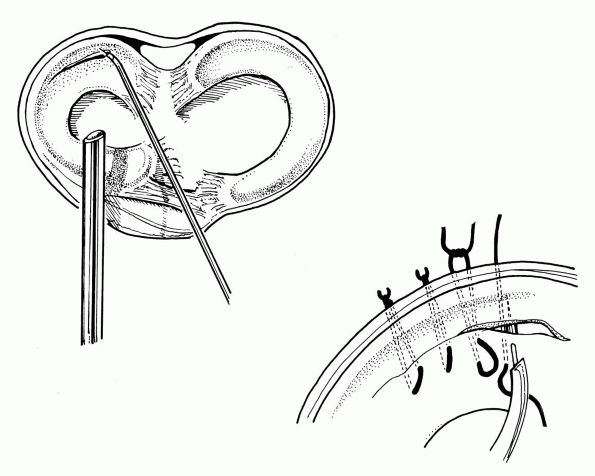
most common type of repair performed. This technique uses double-armed
sutures with long flexible needles positioned with arthroscopically
directed cannulas. Either a single or double-barreled cannula can be
used (Figure 19-3). The single barrel cannula
has the advantage of allowing for vertically oriented sutures, which
provide better coaptation at the tear site. A medial or lateral
incision is required to retrieve the needle as it exits the knee joint.
Proper positioning of the incision and appropriate dissection down to
the capsule are necessary to minimize the risk of neurovascular injury.
On the lateral side, peroneal nerve is at greatest risk;
on the medial side, the most commonly injured structure is one of the branches of saphenous nerve.
 |
|
FIGURE 19-3. Inside-out technique for meniscal repair. Vertical sutures provide the strongest repair.
|
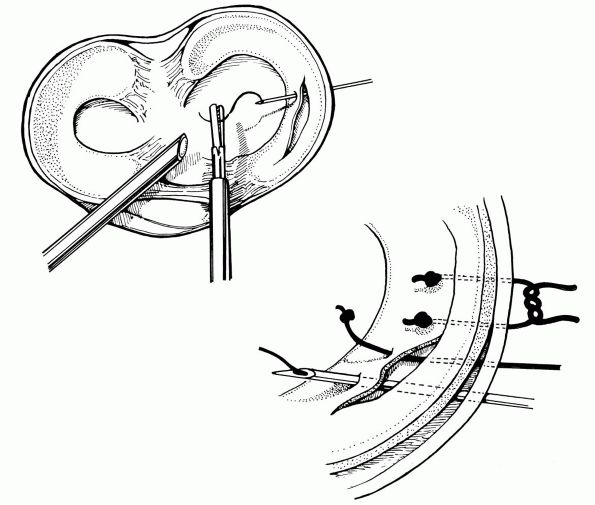
involves the passage of an 18-gauge needle across the tear from outside
to inside the joint where it is grasped and brought out the anterior
portal. The suture is then tied into a mulberry knot and withdrawn back
abutting the meniscus. A series of sutures 3 to 4 mm apart are placed
in this manner and tied to one another over the capsule, after the soft
tissue is bluntly dissected to avoid possible entrapment of important
neurovascular structures (Figure 19-4). This technique is most applicable for tears involving anterior and middle thirds of the meniscus.
involves sutureless meniscus fixation devices, which obviate the need
for additional incisions. The meniscus arrow (Bionx Implants, Bluebell,
PA) is made of poly-L-lactic acid, and its barbed design allows for
compression of vertical tears. Numerous other sutureless implants have
been designed for all inside fixation of meniscal tears. Although
initial studies have shown efficacy, further studies are necessary.
transplant have demonstrated improved contact areas and decreased
contact pressures after allograft placement in cadaveric models,
provided both the anterior and posterior horns of meniscus are secured.
Indications of meniscal transplantation continue to change as clinical
experience increases. At present, the ideal
indication is the patient who has previously undergone a total or near
total meniscectomy and has joint line pain, early chondral changes,
normal anatomical alignment, and a stable knee. In this setting,
meniscal transplantation may decrease pain and possibly prevent
progressive degeneration of articular cartilage. In addition, in
patients who have complete rupture of ACL and a completely destroyed
medial meniscus, it is felt that medial meniscus transplantation will
provide additional joint stabilization and help protect the
reconstructed ACL. The role of meniscal transplantation in asymptomatic
patients who have undergone total meniscectomy is controversial.
 |
|
FIGURE 19-4. Outside-in technique for meniscal repair. The knot captures the meniscus being repaired.
|
technique. In general, menisci do not elicit an immune response.
However, the primary concern is the transmission of diseases. Fresh
frozen, and cryopreserved grafts are commonly used. Fixation of the
meniscal graft has been described with soft tissue fixation alone, or
in conjunction with bone plug or bone bridge fixation between the
anterior and posterior horns placed into a bony trough to provide
secure fixation and recreate hoop stress within the meniscus when
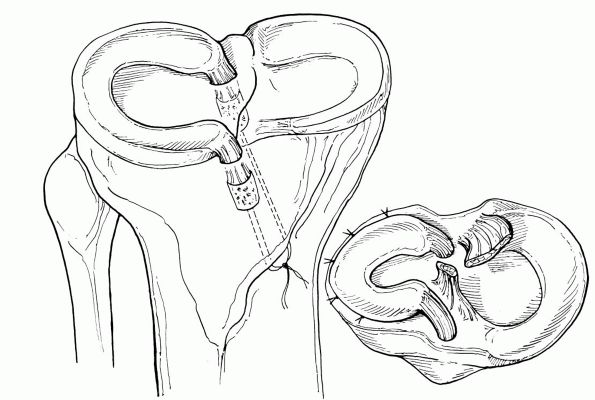
loaded and prevent meniscus extrusion (Figure 19-5).
damage to other structures because the extreme joint displacement
required to disrupt a ligament completely must produce at least some
disruption in the other supporting structures. Single collagen fibers
are not extensible and begin to fail at 7 to 8% elongation. The number
of disrupted collagen fibers in the ligament determines whether it is
functionally or morphologically disrupted. Complete disruption with
loss of continuity requires extreme joint displacement. Although
methods of treating ligamentous injuries have seen substantial
improvement in recent years, there remain many questions about
enhancing the rate, quality, and completeness of ligament healing.
histologic and morphologic appearance of healed ligaments is different
from that of noninjured ligaments. When tissue is viewed using electron
microscopy after 2 years of healing, the number of collagen fibrils is
increased compared with the noninjured ligament, but their diameter and
masses are actually smaller. Additionally, crimping patterns within the
healing ligament remain abnormal for up
to
a year and collagen fiber alignment remains poor. The number of mature
collagen cross-links is only 45% of normal after one year. If the joint is immobilized during the healing process, significant changes in the collagen synthesis and degradation can occur.
Immobilization results in disorganization of collagen fibrils,
decreases the structural properties of boneligament-bone complex, and
resorption of bone at ligament insertion sites. Intermittent passive
motion has been reported to improve the longitudinal alignment of cells
and collagen at 6 weeks, as well as matrix organization and collagen
concentration and ultimate load.
 |
|
FIGURE 19-5. Meniscal allograft with bone plugs placed into osseous tunnels.
|
ultimate load and healing of the MCL, are debated for ACL and PCL
injuries. It is generally agreed that early rehabilitation is critical
to prevent arthrofibrosis and restore knee function; some have
supported less aggressive rehabilitation to allow vascularization and
incorporation of graft.
activities such as American football. Other sports like skiing, ice
hockey, and gymnastics can also produce enough stress to disrupt knee
ligaments. Motor vehicle accidents, especially those involving
motorcycles, are common causes of knee ligament disruptions. Sudden
severe loading without a fall or contact, like deceleration of a
running athlete can also cause ligament disruption. By far the most
common mechanism is abduction, flexion, and internal rotation of femur
on tibia. The medial structures MCL and medial capsular ligament are
first to fail, followed by ACL tear, if the force is of sufficient
magnitude. The medial meniscus may be trapped between condyles and have
a peripheral tear, thus producing unhappy triad of O’Donoghue.
The mechanism of adduction, flexion, and external rotation is less
common and produces primary lateral disruption. Hyperextension force
usually injures the ACL. If the force is severe, stretching and
disruption of posterior capsule and PCL can occur. Anteroposterior
forces, such as a tibia striking a dashboard can cause injuries to
either ACL or PCL, depending on the direction of tibial displacement.
the position of the knee, weight-bearing status, direct or indirect
force, and previous injury
are
also important. The patient’s description of knee buckling, jumping,
audible pop, location of pain, ability to bear weight after injury, and
rapidity of knee swelling may be valuable. Intra-articular swelling
within first 2 hours of trauma suggests hemarthrosis, whereas swelling
that occurs over-night usually is an indication of acute traumatic
synovitis that may be caused by degenerative meniscus or a chronic
process. Hemarthrosis suggests rupture of cruciate ligament, an
osteochondral fracture, or peripheral meniscal tear. Absence
of hemarthrosis does not necessarily indicate a less severe ligament
injury because in severe disruptions blood escapes into soft tissues of
popliteal space, rather than distending the joint. Palpation of
the collateral ligament and its attachment, joint line, patellofemoral
compartment should be systematically performed. Neurovascular
examination should be accurate and complete. Stability of the knee is
determined by stress testing. Both knees should be examined for
comparison.
extremity first for later comparison. The involved knee is flexed to
30°, and a gentle valgus stress is applied to the knee with one hand
placed on the lateral aspect of the thigh and the other hand grasping
the foot and ankle. It tests for medial ligamentous laxity. The varus
stress test is similar to the valgus stress test and is carried out
with the knee both in full extension and in 30° of flexion. The
integrity of the lateral ligamentous structures is tested by this
maneuver. Flexing the knee 30° removes the lateral stabilizing effect
of the iliotibial band so that the lateral collateral ligament can be
isolated for examination. Testing in extension that reveals significant
varus and valgus instability suggests cruciate ligament disruption in
addition to collateral ligament disruption.
opposite knee indicates torn ACL. Examiner must make sure that tibia is
not sagging posteriorly from a lax PCL and returning to neutral
starting position indicating PCL deficiency. Small degrees of anterior
translation of tibia on femur may be better detected in more extended
position, to avoid doorstop effect of posterior horn of medial meniscus
(Figure 19-6).
 |
|
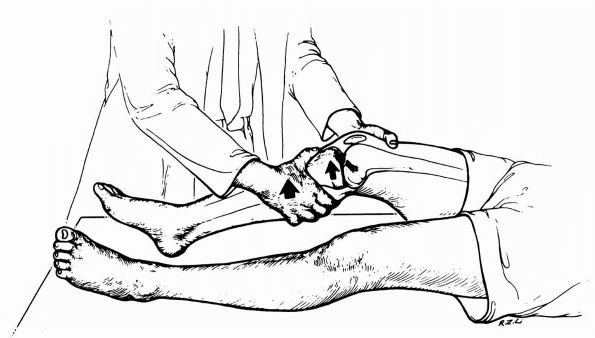
FIGURE 19-6. Anterior drawer test is performed with the patient supine and the affected knee bent 90°. The examiner applies anterior force.
|
stiffness with the knee in about 20° of flexion and applying an
anterior drawer to the proximal calf. Endpoint stiffness is assessed. A
soft endpoint signifies a torn anterior cruciate ligament, whereas a
firm endpoint demonstrates an intact structure (Figure 19-7).
prevents misinterpretation. Loss of normal 1 cm step-off of the medial
tibial plateau with respect to the medial femoral condyle indicates a
torn PCL (Figure 19-8). Examining the patient with hips and
knees flexed to 90° with the heel supported by examiner’s hands,
posterior sag can be visible when examined from the side in patients
with posterior instability.
 |
|
FIGURE 19-7. The Lachman test is performed with the knee flexed between 15 and 30°. The examiner applies anterior force.
|
quadriceps muscle in a knee with PCL deficiency will result in an
anterior shift of tibia of 2 mm or more.
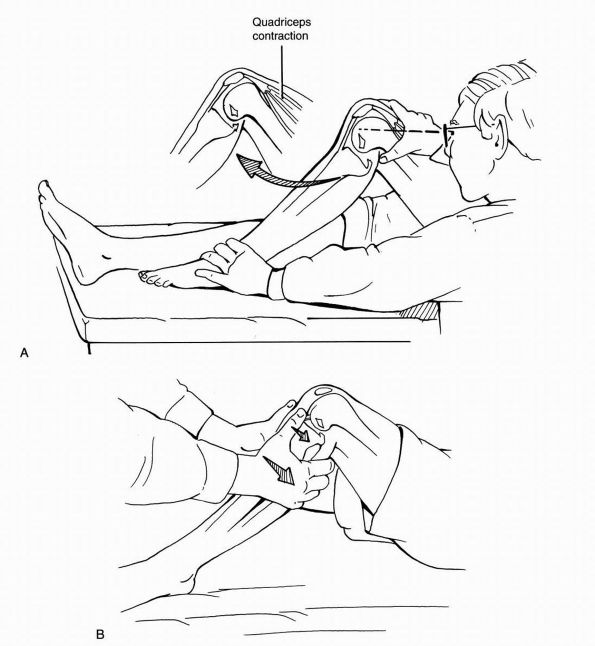 |
|
FIGURE 19-8. Tests for posterior cruciate laxity. (A)
With knee at 90°, note the posterior sag of tibial tubercle relative to normal knee (posterior sag sign). In the PCL deficient knee, the pull of quadriceps translates tibial tubercle anteriorly toward its preinjury position. (B) The posterior drawer test is done with the knee flexed 90°. A posteriorly directed force is applied to the tibia. |
internally rotated, and a valgus stress is applied to the lateral side
of the leg. The knee is flexed slowly while valgus and internal
rotation is maintained. In ACL deficient knee, the tibia is subluxed
anteriorly, as the knee is flexed past 30°, and the iliotibial band on
lateral femoral condyle passes posterior to the center of rotation of
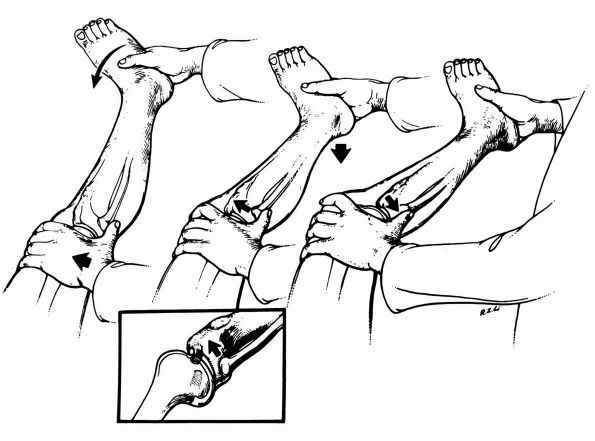
knee and reduces the lateral tibial plateau on lateral femoral condyle (Figure 19-9).
 |
|
FIGURE 19-9. Pivot shift test is performed with the knee in full extension. A valgus and internal rotation stress is applied.
|
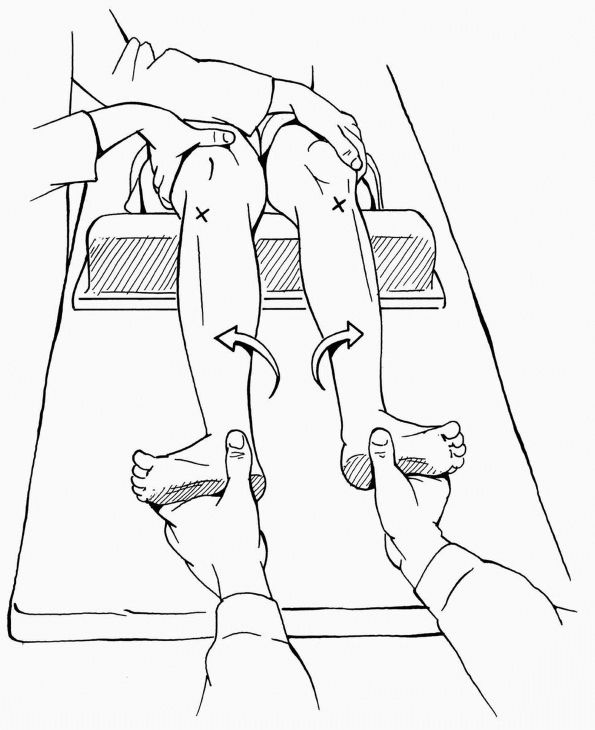
External rotation of tibia on femur at 30 and 90° of flexion is
measured and compared to the normal side. A 10° difference is
pathological. More than 10° difference in external rotation at 30° of
knee flexion but not at 90° indicates an isolated posterolateral corner
injury, while increase of more than 10° external rotation at both 30
and 90° indicate injury of both PCL and posterolateral corner (Figure 19-10).
injuries to the posterolateral ligament complex. The clinician supports
the limb with a hand under the heel and puts the knee in full extension
and neutral rotation. A valgus stress is applied, and the knee is
flexed. In a positive test, at about 20 to 30° of flexion, the tibia
externally rotates, and the lateral tibial plateau subluxates
posteriorly and remains in this position during further flexion. When
the knee is extended, the tibia reduces (Figure 19-11A).
simultaneously and lifts the lower off the table. Positive findings
indicative of posterolateral injury and instability include recurvatum
of the knee, external rotation of the tibia, and increased varus
deformity of the knee (Figure 19-11B).
by a tear of the medial ligaments combined with a tear of the anterior
cruciate ligament. In full extension, the knee joint opens on the
medial side with a valgus stress test with the knee in a fully extended
position. This instability indicates disruption
of
the medial collateral ligament, the medial capsular ligament, the
anterior cruciate ligament, the posterior oblique ligament, and the
medial portion of the posterior capsule.
 |
|
FIGURE 19-10.
External rotation test or dial test. An increase in external rotation of greater than 10° relative to normal knee indicates tearing of posterolateral complex. |
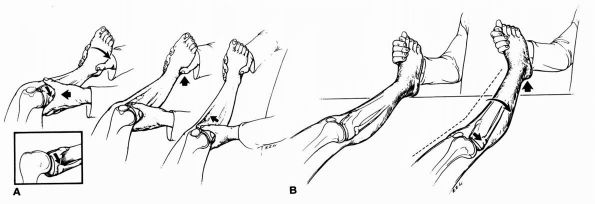 |
|
FIGURE 19-11. (A) Reverse pivot shift test. (B) External rotation recurvatum test.
|
from a tear of lateral structures and the posterior cruciate ligament.
The knee opens on the lateral side with a varus stress test with the
knee in the fully extended position. It indicates disruption of the
lateral capsular ligament, the lateral collateral ligament, and
commonly, the posterior cruciate ligament.
develops after disruption of the posterior cruciate ligament, the
arcuate ligament complex, and the posterior oblique ligament complex.
manifest in tibial abduction, external tibial rotation, and anterior
tibial translation and causes the medial tibial plateau to translate or
subluxate anteriorly in relation to the femur. This implies disruption
of the medial capsular ligament, medial collateral ligament, posterior
oblique ligament, and anterior cruciate ligament. An intact medial
meniscus may provide added stability in this instability.
shown by excessive internal rotation of the tibia on the femur with the
knee at 90° of flexion. This implies disruption of the lateral capsular
ligament, the arcuate complex, and the anterior cruciate ligament.
is a result of the lateral tibial plateau rotating posteriorly in
relation to the femur with lateral opening of the joint. This implies
disruption of the popliteus tendon, the arcuate complex, and the
lateral capsular ligament, and at times injury to the posterior
cruciate ligament. This results in an external rotatory subluxation in
which the tibia rotates around an axis in the intact posterior cruciate
ligament.
manifest by medial tibial plateau rotation posteriorly in reference to
the femur with medial opening of the joint. This implies a disruption
of the medial collateral ligament, the medial capsular ligament, the
posterior oblique ligament, the anterior cruciate ligament, and the
medial portion of the posterior capsule.
for 85% of the resistance to the anterior drawer test when the knee is
at 90° flexion and neutral rotation. The ACL is 31 to 35 mm in length
and 31.3 mm2 in cross-section. The primary blood supply to
the ligament is from middle geniculate artery. The anteromedial band is
tight in flexion, and the bulky posterolateral band is tight in
extension. The posterolateral band provides the principal resistance to
hyperextension. Tension in the ACL is least at 30 to 40° of knee
flexion. In addition to excessive anterior
translation,
the ACL also resists tibial rotation and varus valgus angulation. The
muscle forces around the knee can introduce large changes in the forces
experienced by the knee. In general, quadriceps muscle forces induce
increased tibial translation, while the hamstring has the potential of
negating the increased strains in the ACL caused by quadriceps
contracture and may indicate the usefulness of closed chain
(cocontraction) kinetic exercises during rehabilitation following ACL
reconstruction.
valgus and external rotation, hyperextension, deceleration, and
rotational knee movements. Often the history is of noncontact
deceleration, jumping, or cutting action. Athletic shoes and artificial
turf may play a role in ACL injury. The patient often describes the
knee as having been hyperextended or popping out of joint with an
audible pop. Frequently there is swelling within the next few hours of
injury indicating hemarthrosis. Injury to ACL has been shown to occur
in 70 to 75% of all cases of acute hemarthrosis. The Lachman test is
most sensitive. The pivot shift test requires a relaxed patient and
intact MCL. A side-to-side difference of more than 3 mm or maximum
translation of 10 mm is highly suggestive of ACL insufficiency. MRI is
very helpful; the reported accuracy of detecting tears is 70 to 100%.
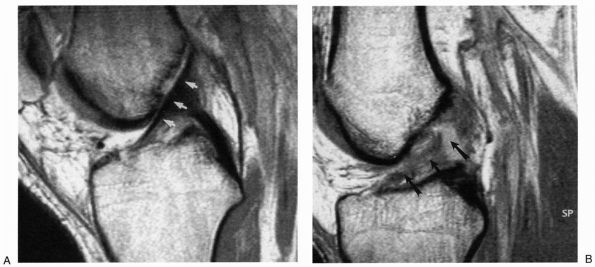
Because ACL crosses the knee joint at a slightly oblique angle, MRI in
an orthogonal plane that is obtained by externally rotating the knee
approximately 15° will show the entire ACL in one frame (Figure 19-12). Often there is evidence of bone bruising and edema in the lateral femoral condyle associated with ACL tear.
 |
|
FIGURE 19-12. (A) Normal ACL signal. (B) Acute ACL tear.
|
ACL rupture and resultant abnormal force distribution can lead to
progressive knee deterioration. Current research also focuses on the
biochemical environment of the knee after ACL injury. In chronic ACL
injury, proinflammatory cytokine such as interleukin-1 and tumor
necrosis factor-α are elevated whereas protective anti-inflammatory
proteins such as interleukin receptor antagonist protein are
significantly decreased. Recent studies have implicated gender and
intercondylar notch index as factors contributing to ACL injury. The notch index
is the ratio of width of intercondylar notch to the width of distal
femur. The normal ratio is 0.231 +/- 0.044. Athletes sustaining
noncontact ACL tears have statistically significant notch stenosis.
Data from the National College Athletic Association Injury Surveillance
system shows significantly higher ACL injury in female soccer and
basketball players than in male players. Female soccer players have ACL
injury rate of more than double and women’s basketball players have ACL
injury rate of more than four
times
as compared to their male counterparts. Possible causative factors may
be hormonal influences, limb alignment, notch dimensions, ligament
size, skill level, muscle strength, and body movement.
with the ACL attached. The avulsed bony fragment often can be replaced
and fixed with transosseous sutures or screws. ACL avulsion is usually
at the tibial insertion. Avulsion of femoral attachment has been
reported with low velocity ski injuries. ACL tears can be treated
nonoperatively with lifestyle changes—avoiding activities that cause
recurrent instability—and an aggressive rehabilitation program. The use
of a knee brace is controversial and has not been shown to reduce the
incidence of reinjury significantly if a patient returns to high
activity sports. Persistent instability and significant reinjury to the
knee are potential problems of nonoperative management. There is
evidence in the literature that ACL reconstruction may prevent
secondary injury to the meniscus.
correlate with need for surgery are younger age, amount of anterior
instability as measured by KT-1000 arthrometer, and preinjury hours of
participation in sports. Repair of ACL is no longer advocated.
Reconstruction with bone patellar tendon bone and hamstring autograft
are the two most popular techniques (Figure 19-13).
Timing of surgery is important. It is recommended that surgery be
delayed until the swelling, pain, inflammation, and stiffness subside,
and full range of motion and muscle function, especially quadriceps
have returned. Waiting reduces the incidence of postoperative
stiffness. The operative details are beyond the scope of this chapter;
however we will discuss key issues of graft selection, placement,
tension, pitfalls, and complications.
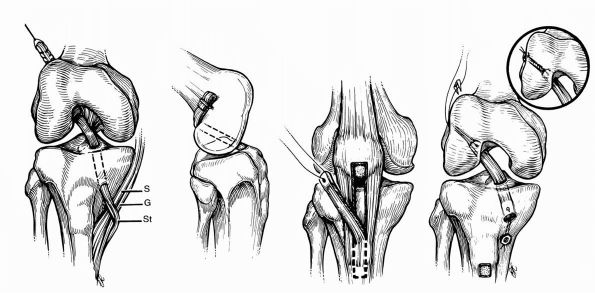 |
|
FIGURE 19-13. (A)
Anterior cruciate ligament reconstruction using the transfer of the semitendinosis tendon secured proximally with a staple. The gracilis tendon may also be used along with the semitendinosis tendons to add strength to the overall surgical construct. (B) Anterior cruciate ligament reconstruction using the bone-patellar tendon-bone preparation. The middle third of the patellar tendon is used. Direct fixation devices include interference screws (most common), staples, washers, and cross pins. |
graft and quadrupled hamstring tendon graft. Bone patellar tendon bone
graft has high tensile strength, and adjacent patella and tibial bone
plug provide possibility of rigid fixation. The quadruple stranded
tendon graft also has high tensile strength, provides multiple bundle
replacement, and has minimum donor site morbidity. Recently quadriceps
graft with or without patella bone plug has attracted interest.
tibial side because of closer proximity to the center of axis of knee
motion. A femoral tunnel that is too anterior will produce lengthening
of intra-articular distance between tunnel with knee flexion, resulting
in loss of flexion or stretching and failure of graft. Posterior
placement of femoral tunnel produces a graft that is taut in extension
and lax in flexion, with acceptable clinical result as ACL deficiency
occurs in full extension. However if this position is chosen, graft
should be secured in full extension, as securing graft in flexion may
result in loss of extension.
the roof of intercondylar eminence. The desired tension in the graft
should be sufficient to obliterate the instability. Too much tension
results in difficulty with regaining motion, or it may lead to
articular degeneration from altered joint mechanics.
point of fixation. Fixation of replacement grafts can be classified
into direct and indirect methods. Direct fixation devices include
interference screws, staples, washers, and cross pins. Indirect
fixation devices include polyester tape-titanium button and
suture-post. Bioabsorbable screws recently have been introduced. With
improvements made in the material properties as well as in screw
design, the pullout strength of bioabsorbable screws is comparable to
their metal counterparts. Soft tissue grafts can be secured to bone
with soft tissue interference screws, screws and spiked washers, screws
and fixation plates, or staples.
is composed of two parts, a large anterior portion, that forms the bulk
of the ligament and a smaller posterior portion that runs obliquely to
the back of tibia. It has a broad origin that forms a semicircle on the
lateral aspect of the medial femoral condyle, and it inserts in a
depression on fovea 1 cm inferior to the articular surface on the
posterolateral aspect of the knee between medial and lateral tibial
plateaus. Most authors believe that it is stronger and larger than ACL.
The anterolateral portion, which is 95% of the
total PCL substance, is taut with knee flexion and lax with knee
extension. The posteromedial portion is lax with knee flexion and taut
in extension. Either the anterior or posterior meniscofemoral
ligament is present in approximately 70% of all knees. The posterior
meniscofemoral or ligament of Wisberg is more common and its femoral
origin merges with that of PCL.
(1,627 +/- 491N and 1,725+/- 660N). PCL is more vertical than obliquely
oriented, and it appears to guide the screw-home mechanism on internal
rotation of femur during terminal extension of knee. The PCL accounts
for 90% of resistance to posterior translation and checks
hyperextension only after the ACL is ruptured. Selected cutting of the
PCL demonstrates that it is important in flexion. Rotational stability
is unchanged in extension but altered in flexion after the PCL is cut.
whether the PCL deficient knee is at risk for the development of
degenerative changes is not clear at this time. Despite the lack of
prospective studies, it appears that progressive degenerative changes
may occur in some PCL deficient knees. In theory, compartment
degeneration could result from acute chondral injury associated with
PCL injury or from increased joint forces created by the absence of the
PCL. In cadaver models, increased medial and patellofemoral compartment
pressures have been demonstrated after sectioning of the PCL. In
chronically PCL-deficient knees, moderate to severe medial compartment
changes have been reported in knees that underwent PCL reconstruction
more than 4 years after original injury.
include a posterior drawer of less than 10 mm with tibia in neutral
rotation, rotatory laxity of less than 5°, and no significant varus
valgus laxity. It is clear that not all knees treated conservatively do
well; more recent longer-term studies show that knee function tends to
deteriorate with time and that most patients are eventually affected by
some degree of disability.
tibial insertion surgically, with open reduction and internal fixation.
In case of midsubstance tears of the PCL, there is considerable debate.
For grade I with loss of anterior step-off, but with proximal
tibial eminence remaining anterior to the distal femur, PCL is stretched with less than 5 mm laxity. In grade II
with proximal tibial eminence flushed with the distal femur on
posterior drawer and if the PCL is torn with 5 to 9 mm of laxity, there
is agreement on conservative management consisting of brief
mobilization and early quadriceps strengthening program. For grade III
tears with proximal tibial eminence posterior to the distal femur PCL
and meniscofemoral ligament torn with more than 10 mm laxity,
reconstruction of PCL is recommended in young, active patients with
greater than 10 mm of posterior drawer. For grade IV or combined ligamentous injuries, surgical reconstruction is recommended (Figure 19-14).
Reconstruction can be performed with open or arthroscopic-assisted
techniques. If an arthroscopically assisted technique is chosen,
fluoroscopic control and a posteromedial portal to assist tibial
preparation is helpful. This procedure is technically demanding.
Because the graft is passed at a sharp angle from the tibia to femur,
it may create fraying of the patella tendon graft and subsequent
laxity. If the tibia is of poor bone quality, the patellar tendon graft
may erode through proximal tibia, creating laxity. The
arthroscopic-assisted technique requires patella tendon of 40 mm or
more to maintain bone blocks within their tunnel (Figure 19-15).
To circumvent the problems associated with the graft making an acute
turn at tibial tunnel exit with potential for graft abrasion and
difficulties with graft tensioning, posterior open approaches to tibial
attachment of the PCL with tibial inlay reconstruction have been
described with encouraging results.
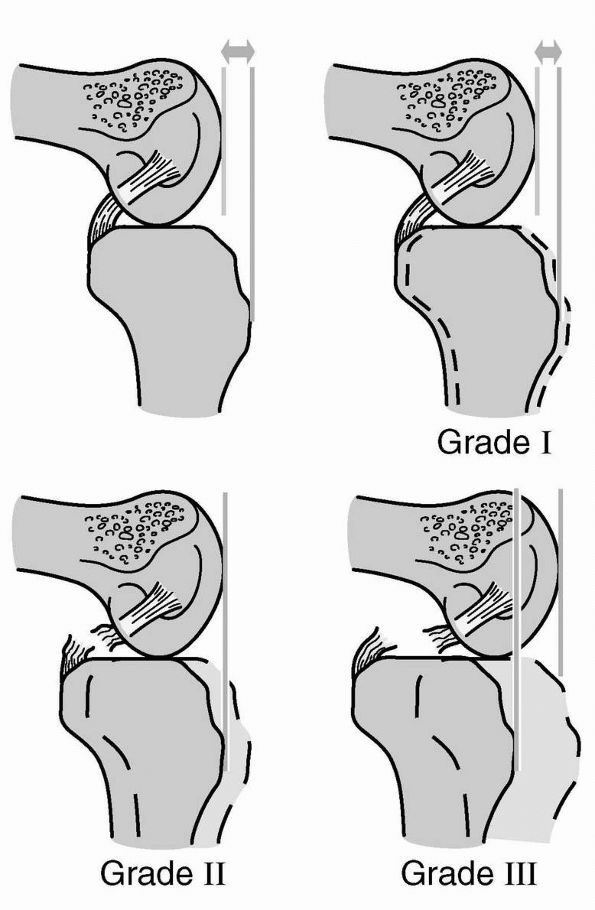 |
|
FIGURE 19-14. Grades of PCL laxity.
|
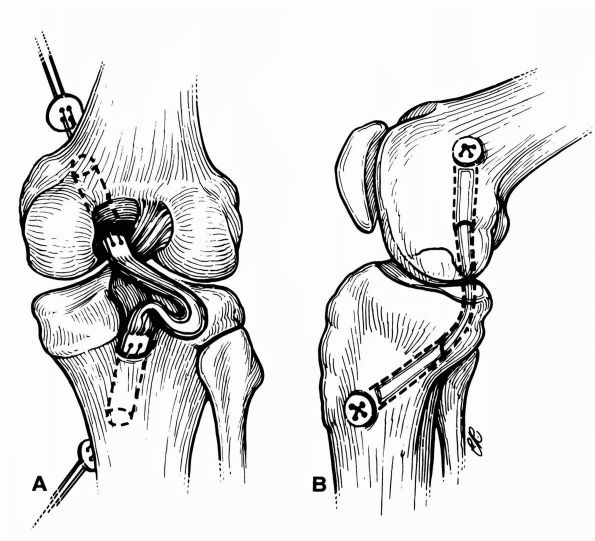 |
|
FIGURE 19-15.
The Clancy technique of posterior cruciate reconstruction uses the same bone-patellar tendon-bone preparation that is seen in the anterior cruciate ligament reconstructions. However, the placement of bone tunnels is different. Note the sharp angle (“killer turn”) at posterior edge of tibia. |
loss of motion. Flexion loss is more common than extension loss, most
likely caused by improper graft placement or inadequate rehabilitation.
The position of femoral tunnel is more critical than the tibial tunnel.
Femoral attachment anterior and distal to the isometric region results
in increased graft tension with flexion loss. Femoral tunnel placement
posterior and proximal to the most isometric region results in
decreased graft tension in flexion and hence laxity. Loss of extension
or flexion contracture is caused by prolonged immobilization in
flexion. Graft laxity with inability to prevent posterior sag is
another complication resulting in failure to obtain objective
stability. Selection of improper graft material with insufficient
strength like the iliotibial band or hamstring may result in failure.
Improper tunnel placement can result in graft abrasion and failure.
contributes 78% to the restraining valgus force on the medial aspect of
the knee. Because of its parallel collagen arrangement, only 5 to 8 mm
of increased opening indicates a complete failure of the ligament. The
MCL is attached proximally to the medial femoral condyle and distally
to the metaphyseal area of the tibia, 4 to 5 cm distal to the medial
joint line, beneath the pes anserinus insertion. Immediately deep to
the MCL is the medial capsular ligament. Posterior to the MCL is a
thickening of the capsular ligament referred to as posterior oblique
ligament. The MCL complex also resists abnormal external tibial
rotation and prevents an increase in anterior tibial translation in the
ACL deficient knee.
It is important to apply this force through the foot and ankle rather
than the distal tibia as applying force to distal tibia constrains the
knee. In addition to joint opening, joint line crepitations or clunk
would be suspicious of the medial meniscus tear, chondral injuries, or
baseline medial compartment arthritis. Amount of medial opening is
graded according to American Medical Association guidelines; grade I
injuries would be less than 5 mm; grade II, 5 to 10 mm; grade III or
complete tear, more than 10 mm. It is important to assess side-to-side
difference. In an adolescent with open physis, it
is important to verify with stress radiograph that the injury is
ligamentous and not a Salter-Harris fracture.
of combined MCL and posterior oblique ligament injury, and possible
cruciate ligament injury. If the knee is stable, in full extension,
there is no significant damage to posterior oblique ligament. MRI can
be helpful to highlight the location and extent of ligamentous damage,
as well as damage to other structures.
temporary immobilization, use of crutches for pain control, range of
motion exercises performed within the first 24 to 48 hours, and an
attempt to regain full range of motion as soon as possible. Isometric,
isotonic, and eventually isokinetic progressive resistive exercises are
begun within a few days of subsidence of pain and swelling. Complete
MCL tears without structural damage to other ligaments can be treated
in similar fashion; if the knee is not too painful, a hinged brace is
used, and quadriceps strengthening exercises and straight leg raises
are encouraged immediately.
are less common but can be disabling due to both instability and
articular cartilage degeneration. A coupled relationship exists between
posterolateral structures and the cruciate ligaments. As a result, a
high incidence of combined injury is clinically observed. Lateral
structures of the knee have been described in three layers. Layer I, or
the superficial layer, consists of iliotibial band with its anterior
expansion and superficial portion of biceps femoris with its expansion
posteriorly. The middle layer or layer II, consists of the quadriceps
retinaculum anteriorly and patellofemoral ligament posteriorly. Layer
III, or deep layer, is composed of the lateral joint capsule and
coronary ligaments, the popliteus tendon, the lateral collateral
ligament (LCL), and the fabellofibular and arcuate ligaments. The
popliteofibular ligament is the part of deep layer.
stress of the knee and also provides resistance to external rotation.
Isolated injuries of the LCL are uncommon and usually occur in
conjunction with injuries to other ligamentous structures. Popliteus
plays a major role in both dynamic and static stabilization of the
lateral tibia on the femur including restriction of posterior tibial
translation, restriction of external and varus rotation of tibia, and
dynamic internal rotation of tibia. The popliteofibular ligament
represents a direct static attachment of popliteus tendon from the
posterior aspect of the fibular head to the anterior aspect of the
lateral femoral epicondyle. It provides a significant share of overall
resistance to posterior tibial translation, external rotation, and
varus rotation. The arcuate ligament reinforces the posterolateral
capsule and spans from lateral femoral condyle to fibular styloid. The
biceps femoris in conjunction with iliotibial band is a strong external
rotator, as well as dynamic lateral stabilizer of the knee; it is
frequently injured in posterolateral injuries.
resist posterior translation as well as external and varus rotation of
tibia; they act with the PCL to provide overall stability. Selective
ligament sectioning in cadaveric models has demonstrated the importance
of posterolateral structures. Sectioning the posterolateral structures
alone results in an increase in posterior translation of the lateral
tibial plateau primarily at 30° of flexion, with a minimum increase at
90° of flexion. However when both the PCL and posterolateral structures
are sectioned, increases in posterior translation are observed at both
30 and 90° of flexion. Isolated sectioning of posterolateral complex,
primarily the LCL, results in increased varus rotation from 0 to 30° of
flexion, with maximum increase observed at 30°. Combined sectioning of
the PCL and posterolateral structures results in increased varus
rotation of the knee at all angles of flexion, with maximal increase
observed at 60°. Thus, the posterolateral structures appear to provide
resistance to posterior translation, restraint to varus rotation, and
tibial external rotation at lesser degrees of flexion. Sectioning of
the PCL and posterolateral structures also results in increased medial
and lateral compartment pressures and increased patellofemoral
pressures secondary to a “Reverse Maquet” effect.
are secondary to trauma, with approximately 40% occurring as a result
of sports injuries. The usual mechanism is hyperextension with a varus
moment combined with twisting force. Other mechanisms can be noncontact
hyperextension and external rotation, sudden deceleration, with a fixed
lower leg. Presenting features in addition to history of trauma can be
pain, weakness, numbness, and paresthesias associated with peroneal
nerve injury. Chronic PLRI patients may describe pain localized to
joint line and may also report instability primarily with knee in
extension, such as knee buckling in toe-off. These patients typically
exhibit gait abnormalities characterized by varus thrust at the knee
coupled with a knee hyperextension in the stance phase. Patients often
maintain the knee in internal rotation as the knee is more unstable in
external rotation. In addition, patients may exhibit overall varus
alignment with increased adduction moment. In acute situations, the
examiner should have high suspicion for knee dislocations in cases of
multiple ligamentous injuries. A careful neurological examination must
be performed.
posterolateral injuries are the prone external rotation test at 30 and
90° of flexion and varus stress test at 0 and 30° of flexion. Other
tests like reverse pivot shift test and external rotation recurvatum
tests can be used to supplement the clinical impression.
joint space, avulsion of proximal tip of fibular head, avulsion of
Gerdy’s tubercle, or a Segond fracture (lateral capsular sign), which
is avulsion of lateral aspect of capsule from tibial plateau. Although
Segond fracture is usually associated with ACL injury, it can also
occur with isolated posterolateral injury (Figure 19-16).
Chronic posterolateral injury patients may have degenerative changes in
the tibiofemoral and patellofemoral compartment. The lateral
compartment is usually more involved. Varus stress radiographs may be
helpful. In addition full-length weight-bearing radiographs of both
lower extremities may be helpful in determining overall alignment. MRI
is very helpful in evaluating posterolateral injuries. A bone contusion
of the posteromedial femoral condyle is frequently observed. MRI can
provide visualization of individual posterolateral structures. Coronal
oblique T2 images provide better visualization than standard coronal
and sagittal images.
delineated; nonoperative treatment of complete tears involving the
posterolateral corner has generally led to poor results. It is believed
that there is an increased degree of disability with combined injury
pattern and predisposition to early degenerative joint changes. In
general nonoperative treatment should be prescribed for patients with
mild instability and without significant symptoms or limitations.
Patients with chronic PLRI often have quadriceps atrophy and gait
abnormalities, and programs consisting of gait training and muscle
rehabilitation may be beneficial.
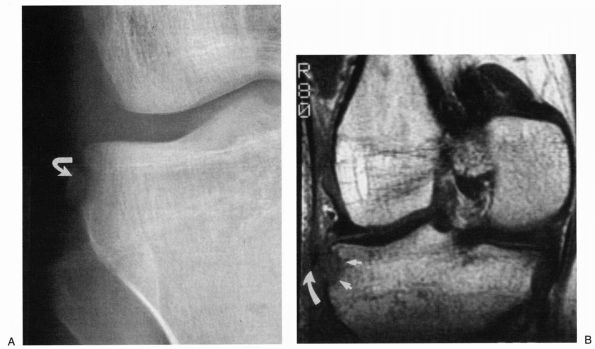 |
|
FIGURE 19-16. Segond fracture. (A) AP radiograph demonstrates the capsular avulsion fragment. (B) MRI demonstrates the defect within the lateral tibia and small adjacent avulsion fracture.
|
PLRI include symptomatic instability with functional limitations as
confirmed by significant objective physical findings. Operative
treatment of acute injuries is usually more successful than surgery for
chronic injury. In general, surgical repair is recommended within the
first 2 to 3 weeks. It is important to simultaneously evaluate and
treat other injuries. In one study, the most common identifiable cause
of ACL reconstruction failure was unrecognized and untreated
posterolateral corner injuries. In cases of concomitant injuries,
reconstruction of the ACL or PCL should be performed either prior or
with the reconstruction of posterolateral structures. In chronic cases,
it is also important to correct any varus knee alignment, and valgus
osteotomy with distal advancement of iliotibial band with bone block
may be performed.
initially. Major structures that should be evaluated include the
iliotibial tract, biceps femoris, peroneal nerve, LCL, popliteus
muscle, and tendon and popliteofemoral ligament. Treatment of
posterolateral injuries should proceed from deep to superficial, with
repair of structures by direct repair, suture by drill holes through
bone, or suture anchors as appropriate. In acute situations where the
severity of injury precludes direct repair, involved structures can be
augmented with hamstring tendons, biceps tendon, iliotibial band, or
allograft.
the best technique of operative treatment for chronic injury. Proximal
advancement of arcuate complex (lateral head of gastrocnemius, LCL,
popliteus tendon, and arcuate ligament) in line with the LCL into a
trough in distal femur can be performed with good results. Tensioning
is performed with the knee in 30° flexion and the tibia in neutral
rotation. The disadvantage of the procedure is that the insertion sites
of the LCL and popliteus are drifted anterior to the center of
rotation, which may lead to attenuation and eventual failure.
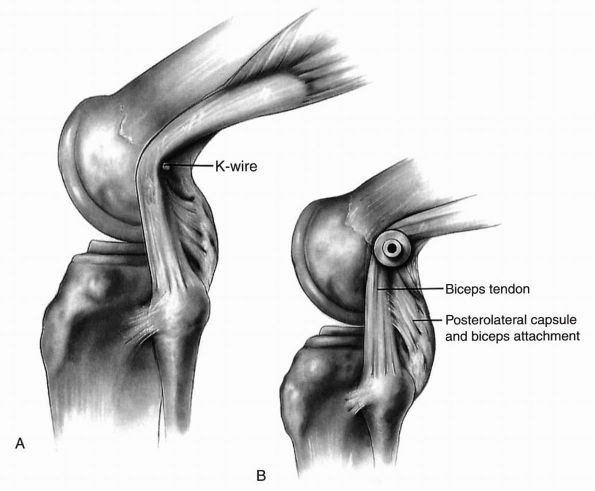
arcuate complex is tightened. The entire biceps tendon is transferred
anteriorly to the lateral femoral epicondyle leaving the distal
insertion intact (Figure 19-17).
However, the disadvantage is that the popliteus and popliteofemoral
ligaments are not reproduced, and a fixation point other than 1 cm
anterior to the LCL femoral origin results in nonisometric graft
position that does not reduce external rotation or varus stress at any
degree of flexion. Salvage posterolateral reconstruction after failed
biceps tenodesis may be quite difficult.
allograft for reconstructing the popliteus and popliteofemoral
ligament, an extracapsular sling procedures reconstructing LCL by using
bone tendon bone allograft secured with interference screws in the
fibular head, and lateral femoral condyle have been described with
favorable results. Surgical complications of these procedures include
peroneal nerve palsy, failure of reconstruction, knee stiffness,
hamstring weakness (especially in biceps tenodesis), infection, and
hardware irritation.
 |
|
FIGURE 19-17. Biceps tenodesis.
|
affected by osteonecrosis. Osteonecrosis of knee occurs in
approximately 10% of patients with osteonecrosis of hip. Ahlbäck and
associates first described the disease in 1968. Osteonecrosis
of knee represents two distinct entities—spontaneous osteonecrosis of
the knee and steroidassociated osteonecrosis.
weight-bearing portion of the femoral condyle or tibial plateau with
associated subchondral fracture and collapse. In the typical case, the
patient, who is usually older than 60 years of age, presents with an
acute onset or exacerbation of pain on the medial side of the knee with
or without associated minor trauma or increased activity. Patients
often have a history of insidious knee pain, commonly experienced in
early and mild osteoarthritis. Mechanical symptoms, such as locking,
catching, and buckling, are not widespread. However, during the acute
phase, the knee can appear locked because of pain, effusion, and muscle
contracture. Although it
is
most common in the medial femoral condyle, osteonecrosis may also occur
in the lateral femoral condyle and tibial plateau. The tibial lesions
occur on the medial side. Radiographs may be normal, but bone
scintigraphy is markedly abnormal. MRI also shows this lesion during
the acute phase before it is clearly shown on plain radiographs. After
2 or 3 months, radiographs typically show flattening and radiolucency
of the subchondral bone of the medial femoral condyle with a sclerotic
line of demarcation around the lesion. Degenerative changes may also be
present at this time if a large weight-bearing area of the femoral
condyle is involved.
spontaneous tear of the medial meniscus and should be differentiated
from it in a patient who is older than 60 years of age. These patients
often erroneously undergo arthroscopic surgery for presumed meniscal
tear, based on changes observed on MRI studies that are so common in
this age group, without symptomatic relief.
traumatic theories also have been proposed, with the vascular theory
being the most widely accepted. Fat embolism has been suggested as a
possible mechanism. Bone microcirculation is contained within an
expandable compartment, and an increase in bone marrow pressure can
cause bone ischemia. Elevated bone marrow pressure is found in patients
on steroid therapy, but it is also found in osteoarthritis of the knee.
In stage 1, the radiographs are normal, but MRI is abnormal. Slight
flattening of the condyle is seen in stage 2. An area of radiolucency
with a distal sclerosis and a faint halo of bony reaction are found in
stage 3. Stage 4 shows a calcified plate with radiolucency surrounded
by a definite sclerotic halo. Stage 5 represents narrowing of the joint
space with subchondral sclerosis and osteophyte formation typical of
osteoarthritis.
dissecans, osteoarthritis, meniscal tears, pes anserinus bursitis, and
insufficiency fracture. The area of the lesion is important in
predicting which knee will develop osteoarthritis. Knees with lesions
smaller than 5 cm2 have a better clinical and radiographic prognosis.
treatment of osteonecrosis: (1) conservative care, (2) core
decompression of the distal femur, (3) arthroscopic debridement, (4)
proximal tibial osteotomy, and (5) total or unicompartmental
arthroplasty.
protected weight bearing, and activities to tolerance. Excellent to
good results can be obtained with conservative means if lesions are
relatively small, that is, less than 40% of the width of condyle. If
the disease progresses, a few cases can be treated by arthrotomy, or
arthroscopy and drilling of the lesion. Arthroscopy may be effective in
debriding unstable or delaminating chondral fragments, particularly to
resolve mechanical symptoms like catching and locking. It is more
advisable to drill in an antegrade nonarticular direction, rather than
retrograde through overlying intact cartilage, to stimulate
revascularization of the osteonecrotic fragment. Core decompression by
extra-articular drilling of the femoral condyle can relieve the initial
acute pain that occurs with the onset of spontaneous osteonecrosis of
the knee. The best results are reported in stage 1 lesions. If femoral
flattening is already apparent, the progression cannot always be
avoided. Core decompression is a really effective treatment for
steroid-induced osteonecrosis because steroid-induced osteonecrosis is
an entirely different entity with different anatomic involvement in the
distal femur. The central factor is that steroid-induced osteonecrosis
does not necessarily involve the subchondral plate. The principal
involvement is metaphyseal; therefore it has different structural and
mechanical ramifications for the joint surface.
and total knee replacement are reserved for knees in which advanced
osteoarthritis has developed. The indication of high tibial osteotomy
is in stage 3 lesions where less than 50% of the condyle is involved in
active patients younger than 65 years of age. There is a role of
unicompartment replacement of spontaneous osteonecrosis of one condyle
or plateau; however the possibility of subsequent osteonecrosis of the
opposite compartment is a critical concern. Any change involving the
epiphysis of metaphyseal bone on the preoperative MRI should be
considered as compromised osseous structures that may predispose the
subsidence and compromise long-term survivorship. Total knee
arthroplasty (TKA) has provided good results in well over 90% of
patients; however careful review of the literature of these results for
spontaneous osteonecrosis suggests that while satisfactory results are
obtainable, there is need to remain guarded in the final results.
Inferior results are reported in TKA for spontaneous and
steroid-induced osteonecrosis when compared with matched group of
patients with osteoarthritis.
healing. In adults it possesses neither a blood supply nor lymphatic
drainage, and is sheltered from immunologic recognition by the
surrounding extracellular matrix. Although the cells continue to
produce the extracellular matrix, they are ineffective in responding to
injury. Wounds that are limited to cartilage itself without injuring
the subchondral bone stimulate only a slight reaction in the adjacent
chondrocytes. The natural history of isolated chondral defects is not
known; it is assumed that these chondral and osteochondral defects may
progressively enlarge with time and play a role in the development of
more generalized osteoarthritic changes. Trauma to articular cartilage
beyond a critical level causes a reduction in viscoelasticity and
stiffness of cartilage. As a result, more force is transmitted to the
subchondral bone, with consequent thickening and eventual stiffening of
the subchondral plate. The increased stiffness of subchondral bone
allows more impact stresses to be transmitted to the cartilage,
creating a vicious circle of cartilage degeneration and stiffening.
Treatment of full thickness articular surface lesions in young and
middle-aged individuals is a challenge. These lesions may be small and
asymptomatic at discovery or may increase in size and may become
painful. In a review of more than 31,000 arthroscopies, chondral
lesions were reported in more than 60% of patients. It is proposed that
5 to 10% of all patients who present with acute hemarthrosis of the
knee after work- or sport-related injury have full thickness chondral
injury.
articular cartilage lesion is a loose body. It may be associated with
an acute injury with large effusion or may have insidious onset with no
effusion. Some patients may have joint line pain and mechanical
symptoms of locking. Injuries associated with full thickness articular
surface injuries include patellar dislocation with lateral femoral
condyle and medial patella facet lesions, dashboard injuries, and
ligament injuries. The physical examination usually does not elicit a
distinct consistent finding other than localized pain with or without
an effusion. The presence of loose body is considered predictive of
articular surface injury.
flexion views, may show compartment joint space narrowing or an
osteochondritis dissecans type of defect, with or without loose body.
With full thickness articular cartilage lesions, plain radiographs may
not reveal any changes, and MRI may be helpful. Defects in articular
cartilage appear as focal areas of cartilaginous thinning in which the
defect is filled with synovial fluid, which demonstrates a
characteristic bright signal of T2 images. An abnormal signal in the
underlying bone often aids diagnosis. On follow-up MRI or arthroscopy,
the contusions in the subchondral bone are associated with high
incidence of osteochondral abnormalities, such as thinning, or loss of
articular cartilage and subchondral sclerosis. Proton density imaging
and T2 imaging with fat saturation sequences optimize resolution of the
articular chondral surface. The sensitivity of MRI in consistently
analyzing changes in the articular surface has been reported to be 40
to 70%. Arthroscopy is a more accurate technique for diagnosing
articular surface lesions, documenting the location, size, shape, or
depth of the lesion.
articular cartilage changes do not cause symptoms of any significant
disability. However, some patients may present with complaints of pain,
swelling, giving way, and mechanical symptoms of locking, catching, or
crepitus. The goal of nonoperative treatment is to reduce symptoms
related to articular cartilage lesion. Treatment modalities include
patient education, activity modification, and physical therapy for
muscle strengthening, a nonaggravating fitness program.
anti-inflammatory drugs such as cyclooxygenase-2 (cox-2) inhibitors;
local corticosteroid injections; and chondroprotective agents, such as
oral glucosamine and chondroitin sulfate and injectable hyaluronic acid
for viscosupplementation. In symptomatic patients for whom these
treatment modalities are unsuccessful, surgical interventions can be
considered. The appropriate treatment of an asymptomatic patient with
an incidental finding of a full thickness articular cartilage lesion is
an enigma. The natural history of this lesion is not well known.
Whether these lesions if left untreated become symptomatic in short
time or if the joint destruction can be prevented by treating
these
lesions—these questions at present remain unanswered. Also the natural
history of surgically treated symptomatic patients is still evolving;
until it is confirmed, surgical treatment of asymptomatic patients is
not recommended, though continual observation and follow-up monitoring
is warranted.
arthroscopic debridement, lavage, and repair stimulation. The direct
transplantation of cells or tissue into a defect and replacement of
defect with biological substitutes can restore articular surface in
selected patients.
debridement to remove loose flaps or edges can improve symptoms;
however, the effects are temporary and there is no potential for
healing. Attempts to enhance the intrinsic healing potential of
articular cartilage have been focused on recruiting pluripotential
cells from the bone marrow by penetrating the subchondral bone or
providing mechanical, electrical, laser, or other stimulus for healing.
The usual result of these penetrating techniques (drilling, abrasion
arthroplasty, or microfracture) is the partial filling of articular
defect with fibrocartilage that contains principally type I collagen. Unlike
the desired hyaline cartilage, this fibrocartilage has diminished
resilience and stiffness, poor wear characteristics, and predilection
for deterioration with time.
although they have some chance of helping the patient and are a
reasonable first step in the management of a previously untreated
cartilage defect. Because of the limited capacity of the cartilage to
heal, a more attractive approach is to transplant cells or tissue with
chondrogenic potential. These living cells or tissue may be directly
transplanted into an articular cartilage defect. Both autologous
committed chondrocytes and undifferentiated mesenchymal cells placed in
articular defects survive and are capable of producing new
cartilaginous matrix.
the defect with a substitute, either primarily or a series of small
osteochondral plugs (mosaicplasty). This procedure involves the
autogenous transplantation of at least one cylindrical osteochondral
plug from a relatively nonweight-bearing region of the knee into an
articular defect (Figure 19-18). The donor site
is usually the edge of the patella groove or the area just next to
intercondylar notch. The technique involves excising all injured or
unstable tissue, creating cylindrical holes in the base of defect and
underlying bone. These holes are filled with cylindrical plugs of
healthy cartilage and bone in mosaic fashion. The goal is to fill the
defect as completely as possible. Histologic evidence demonstrates that
hyaline cartilage on cylindrical graft has the ability to survive in
its new setting and maintain its structural integrity. Osteochondral
autografts are indicated for patients less than 45 years of age, with
sharply defined defect surrounded by healthy cartilage. Lesions should
be unipolar and no more than 2 to 2.5 cm2. The technique of fixation and continuous passive motion are reported to be important in obtaining optimal results.
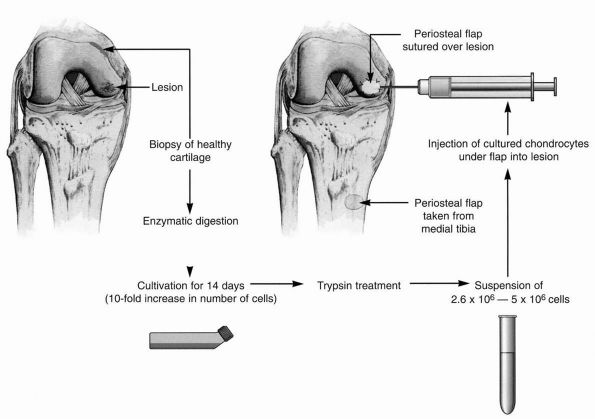
harvested, expanded in cell culture, and implanted into the defect.
This technique preserves the subchondral bone plate (Figure 19-19).
Early results indicate a good to excellent result in more than 80% of
patients. Follow-up arthroscopic examination showed good fill with
repair tissue, good adherence, and hardness close to that of adjacent
tissue. ACI is indicated for the younger (20 to 50 year old) active patient with an isolated traumatic femoral chondral defect greater
than 2 to 4 cm2. Accompanying
ligamentous, meniscal lesions, joint malalignment, and patellofemoral
instability must be corrected concurrently. Absence of meniscus,
bipolar lesions, osteoarthritis, and instability may preclude such
treatment.
 |
|
FIGURE 19-18.
Location of recommended donor sites for osteochondral grafts. Osteochondral plugs 15 to 25 mm long are harvested and implanted in the prepared base of defect. Coverage of 80 to 90% of defect is recommended. |
 |
|
FIGURE 19-19.
From the harvested cartilage slices the chondrocytes are isolated and cultured for 2 weeks before the implantation can take place. |
full thickness lesions after failure of one or two previous surgical
procedures. Fresh allografts provide the greatest likelihood of
chondrocyte survivability, but also carry higher risk of immunologic
and transmissible disease. Use of shell graft with less than 1 cm of
subchondral bone reduces immunogenecity of the graft. The technical
constraints of surgical implantation of fresh osteochondral graft are
demanding. Fresh tissue from a young (<30 years) donor must be
available and recipient and surgeon must be on call at all hours of day
and night.
osteochondritis dissecans. Concern related to preservation techniques,
disease transmission, tissue viability and availability, and graft host
reactions limit the use of this technique. Although fresh frozen
allografts have a decreased risk of immunogenic response and visual
transmission, there is concern regarding viability of chondrocytes
potentially decreasing the longevity of allograft.
cushion underlying exposed bone, reestablish a congruent articulating
surface, and provide physical and mechanical properties of articular
cartilage. Synthetic matrices can bridge the void of the osteochondral
defect and potentially delay or avoid major surgery. An example of one
of these polymers is flowable in situ curable polyurethane, which is a
cross-linked, segmented
polyurethane that exhibits high tensile strength and excellent fatigue resistance under physiologic loads.
the most important function of the patella is to improve the efficiency
of the quadriceps by increasing the lever arm of the extensor
mechanism. The thickness of the patella displaces the patellar tendon
away from the femorotibial contact point throughout knee range of
motion, thereby increasing the moment arm of the patellar tendon. It
has been shown that 3.3 times the body weight is generated across the
patellofemoral joint at 60° of knee flexion during stair climbing, and
up to 7.8 times the body weight at 130° during deep knee bends.
Patellofemoral contact pressures are uniformly spread over all contact
areas, but peak pressures are highest between 60 and 90° of flexion.
The symptoms of patellar dysfunction include anterior knee pain, giving
way, locking, and swelling. Occasionally, pain may be referred to the
joint lines, mimicking the symptoms associated with meniscal tears. In
addition to routine radiographs, patellofemoral radiographs in the form
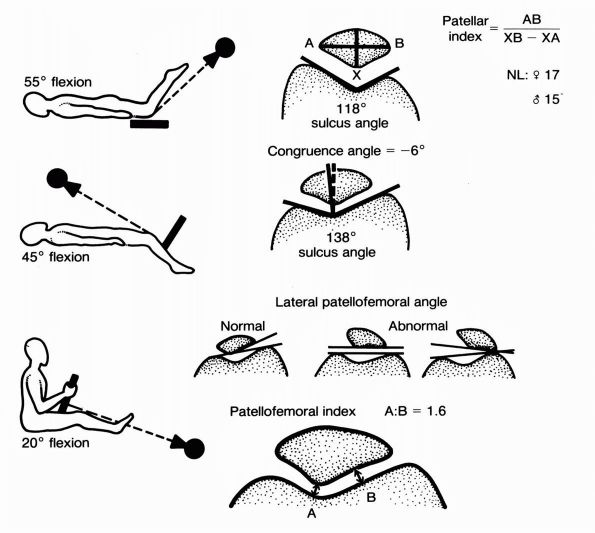
of axial views in different degrees of extension should be obtained (Figure 19-20).
There are several congenital anomalies of the patella, including
bipartite patella, congenital absence of the patella (patellar
aplasia), a small patella (patellar hypoplasia), and a large patella
(patella magna).
 |
|
FIGURE 19-20.
Schematic representations of the different radiographic evaluations of the patella: the Hughston (55°), Merchant (45°), and Laurin (20°) patella views. |
a slightly overexposed lateral radiograph. An axial (“skyline”)
radiographic view determines whether the lesion is in the medial or
lateral facet. In the patella it usually is associated with
chondromalacia that extends considerably beyond the peripheral margins
of the avascular bone. Residual disability after treatment usually is
proportional to the size of the chondromalacia area. Treatment options
include conservative management, excision of the fragment and curetting
the crater, excision of the lesion followed by curettage, and drilling
of the lesion. Arthroscopic treatment of a patellar osteochondritic
lesion using retrograde placement of a Herbert screw or absorbable
screws have also been reported. Rarely is chondromalacia so extensive
as to require patellectomy; the patella should almost always be
preserved.
incidentally on an anteroposterior or tunnel tangential radiograph.
When present, it occurs
bilaterally
in approximately 40% of patients. Three types have been described. Type
I, which accounts for 5%, occurs at the inferior pole and may be
associated with Sinding-Larsen-Johannson syndrome. Type II, which
accounts for 20%, occurs along the entire lateral border of the patella
and may be associated with a nonunion of a patellar fracture. Type III,
the most common type, occurs as an elliptical area in the superolateral
portion of the patella and accounts for 75% of cases. Pain is unusual
in bipartite patella, and when present it is caused by overuse. A
diagnostic radiographic test to determine if pain is caused by a
nonunion at the bipartite site is to obtain a normal skyline view
followed by a skyline view taken with the patient in a squatting
weight-bearing position. The test is considered positive if the
separation is greater in the squatting weight-bearing position than on
the normal skyline view. If pain occurs with a bipartite patella,
treatment should include limiting and restricting activity, correcting
the activity that is causing an overuse syndrome, and prescribing
nonsteroidals and a short arc exercise program. Immobilization for 3
weeks or more relieves the symptoms of repetitive microtrauma of the
synchondrosis. Rarely does conservative treatment fail, making
operative treatment unnecessary. Excision of the bipartite fragment,
especially in the superolateral quadrant can be successful in relieving
symptoms if conservative measures fail.
is characterized by pain. Pain is characteristically dull, poorly
localized, and increased by activities that overload the patellofemoral
joint. Symptoms may follow a trauma. A sense of the knee buckling may
occur, which makes this syndrome difficult to differentiate from
patients with true patellar instability. True episodes of patellar
instability, however, followed by considerable swelling that persists a
few days, are lacking. An apprehension test (whereby the patient
becomes apprehensive when the physician attempts to move the patella
medially and laterally with the knee extended, simulating the feeling
of instability) is negative, and only minor tracking abnormalities of
the patella are present.
easily appreciated. There may be a mild varus relation between the
tibia and the femur. A half-squat usually provokes pain. A quadriceps
angle of greater than 20° is abnormal. This angle is formed by a line
drawn through the center of the long axis of the thigh across the
midportion of the patella and a line drawn along the patellar tendon.
The lateral retinaculum is considered tight if the patella is unable to
be moved medially more than one-fourth of its width. Pain on
compression of the patella against the femoral sulcus is usually
present. Radiographically, lateral placement of the patella on an
axial, skyline (Merchant) view may be evident. Radiographic clues of
long-standing ELPS include lateral facet sclerosis, lateralization of
trabeculae, and lateral traction spurs (Figure 19-21).
The pain is thought to result from excessive lateral loading of the
patellar ridge. Excessive lateral ligamentous tension may also
contribute to the syndrome.
quadriceps training, anti-inflammatory medication, rehabilitation
program, and taping. Occasionally, surgical treatment is necessary and
includes lateral retinacular release done by open arthrotomy or
arthroscopic means.
secondary to damaged or softened articular cartilage of the
patellofemoral joint. The term chondromalacia
should be used only to describe the changes that occur in the articular
cartilage, if the disease has progressed to involve changes of the bone
(osteophyte formation, subchondral sclerosis, and cysts) and of the
synovium (synovitis), it should be classified as patellofemoral
arthritis.
commonly after the second decade and in almost all knees after the
fourth decade. Unfortunately, the term chondromalacia
has become synonymous with patellofemoral pain. Numerous other terms
have been proposed to describe the syndrome, such as patellofemoral
syndrome, patellofemoral arthralgia, extensor mechanism dysplasia,
anterior knee pain syndrome, and others, but these are not commonly
accepted. Some patients with
minimal
changes in the articular surface have marked patellofemoral joint
symptoms, and conversely, some patients with no patellofemoral joint
pain have marked changes in the articular surface of the patella. Chondromalacia of the patella should describe a pathologic condition of the cartilage and not a clinical syndrome.
In chondromalacia of the patella, the initial lesion is a change in the
ground substance and collagen fibers at the deep levels of the
cartilage. It is a disorder of the deep layers of the cartilage that
involves the surface layer only late in its development. In contrast,
in osteoarthritis the initial changes occur on the surface of the
cartilage, with loss of continuity of the transverse fibers followed by
fibrillation, which usually becomes grossly visible. Changes occur most
commonly at one of two sites in the deep layer of cartilage. The first
is an area about 1 cm in diameter astride the ridge that separates the
lateral facet from the medial facet; the second area straddles the
inferior part of the central ridge that separates the medial and
lateral facets.
 |
|
FIGURE 19-21. Radiographic clues of long-standing lateral compression syndrome.
|
cartilage erosion down to bone. The most comprehensive and specific
grading system for articular cartilage lesions was published by Noyes
and Stabler. They classified articular cartilage lesions by defining
the surface description, the extent of the articular cartilage
involvement, the diameter, location and the degree of flexion that will
put the lesion in contact with the weight-bearing area. Grade 1 lesion
is chondromalacia 1A: soft cartilage, 1B: softening with definite
indentation; Grade 2 are open lesions, 2A: fissures/fragmentation of
half thickness and 2B: full thickness; Grade 3A: bone exposed and 3B:
bone cavity.
however, result from lateral patellar compression syndrome, patellar
instability, trauma, previous anterior cruciate ligament surgery,
prolonged immobilization for fracture treatment, or synovial conditions
affecting the articular cartilage surface. Pain is associated with
increased patellar pressure and basal degeneration of the cartilage.
The signs and symptoms of chondromalacia of the patella are
nonspecific; there is no pathognomonic symptom. Most patients with
chondromalacia describe a dull, aching discomfort that is well
localized to the anterior part of the knee and that is most prominent
after sitting in one position for a long time. This has been called the
“movie sign.” Crepitation in the patellofemoral joint also varies. The
patient may describe a catching or giving way sensation with activity;
pain and giving way both tend to be much more prominent while
descending stairs. Puffiness or swelling may be noted, depending on the
degree of synovitis present.
therefore cannot be the direct source of pain. The synovium and
subchondral bone probably are the two areas producing pain in
chondromalacia of the patella. Many authors believe, however, that pain
in the patellofemoral syndrome, with or without chondromalacia,
originates from the subchondral bone. It is hypothesized that the
biomechanical failure of articular cartilage in chondromalacia of the
patella results in an alteration of load transfer to the subchondral
bone. Insall suggested that most young people with chondromalacia of
the patella have an extensor mechanism malalignment and this, rather
than the articular changes themselves, is responsible for the pain.
portions of patella articular surface, depending on the nature of
malalignment or trauma. When there is recurrent shear stress as in
patellar malalignment, the distal central portion may break down. The
lateral facet is the site of breakdown in excessive lateral pressure
syndrome (ELPS) with chronic patellar subluxation and tilt. The medial
facet is more commonly damaged at the time of patellar dislocation and
relocation. Another cause of medial facet articular cartilage breakdown
is previous medial tibial tubercle transfer with posterior placement of
tibial tubercle (Hauser procedure). The proximal patella is more
commonly damaged as a result of a dashboard or crush type of injury in
which the knee is flexed at the time of injury. This injury responds
poorly to tibial tubercle anteriorization procedures as these
procedures cause load shift onto more proximal patella. Diffuse
articular cartilage damage more frequently occurs as a result of
generalized arthritis or extensive damage following malalignment and
recurrent dislocations.
lateral joint line in most cases. Narrowing of the joint line, with
osteophytes on the lateral patellar border and trochlea, subchondral
sclerosis of the lateral facet, and possibly cyst formation, may be
present on axial radiographs. Most often, patellofemoral arthrosis
appears to arise de novo in a structurally normal joint for which no
obvious cause can be assigned. There is a frequently associated
femorotibial arthrosis.
exercises, knee braces, and anti-inflammatory medications. Surgical
treatment involves procedures that relieve stress on the patellofemoral
joint and that directly address the pathology of the articular
cartilage. Patellar cartilage shaving can be done open or
arthroscopically and yields the best results (83% satisfactory) in the
earlier stages of chondromalacia. Subchondral bone drilling with
cortical abrasion yields more unpredictable results (60% satisfactory),
as does lateral retinacular release. Elevation of the tibial tubercle,
patellar resurfacing, and patellectomy show limited success (70%
satisfactory) and are formidable procedures with high complication
rates.
the underlying cause of the articular surface changes and should be
directed to the cause rather than to the results. Most often, this
treatment involves nonsurgical measures, such as anti-inflammatory
medications, quadriceps exercises, and hamstring stretching. Prone
quadriceps stretching exercises are particularly helpful, they enable
the patient and physician to see the progress and reduce stiffness
around the anterior knee. Strengthening of the entire kinetic chain
will benefit the patient, although specific emphasis on vastus medialis
obliquus (VMO) will improve support to the patella. Some patients also
benefit from braces and activity modification.
treatment directed at malalignment and other abnormalities of the
extensor mechanism and patellofemoral joint and (2) treatment of the
diseased cartilage. Surgery is indicated for chronic patellofemoral
pain after all attempts at nonoperative management have failed.
chondromalacia of the patella. The degree of fibrillation and
fragmentation of the surface can be probed under direct vision, and
careful probing can identify areas of softness.
one of the most common causes of patellofemoral pain and chondromalacia
changes in the patellofemoral joint. Malalignment problems related to
bony abnormalities, such as excessive femoral anteversion, excessive
external tibial torsion, and severe genu valgum, may require osteotomy
for skeletal realignment.
arthroscopic technique is indicated for painful arthrosis with
radiographically documented incongruity, lateral tilting, or lateral
orientation of the patella in the patellofemoral joint. Surgery for chondromalacia of the patella has been unpredictable in patients with normal axial radiographic studies.
include (1) arthroscopic patellar shaving, (2) local excision of
defects with drilling of the subchondral bone, (3) facetectomy, (4)
mechanical decompression of the patellofemoral joint by elevating
anteriorly the tibial tuberosity (Maquet procedure), and (5)
patellectomy. Patellar resurfacing or patellofemoral replacements have
not been sufficiently successful to indicate their use for
unicompartmental patellofemoral arthritis or severe chondromalacia of
the patella.
the major benefit is probably from the lavage of the joint and removal
of articular surface debris.
elevation of the insertion of the patellar tendon onto the tibial
tuberosity to reduce the articular pressures in the patellofemoral
joint. This technique has been used more often for patellofemoral
arthritis than for chondromalacia of the patella. Patellectomy should
be used only in advanced chondromalacia and severe patellofemoral joint
degeneration. Fulkerson described slightly oblique osteotomy of the
tuberosity, (Figure 19-22) that transfers the
tuberosity anteriorly and medially. This procedure achieves both
unloading of the patella and improved alignment in one operation and
does not require bone graft or distraction of tibial tubercle.
biological events that destabilize the normal processes of degradation
and synthesis of articular cartilage chondrocytes, extracellular
matrix, and subchondral bone. These changes include increased water
content, decreased proteoglycan content, and altered collagen matrix,
all leading to the deterioration of articular cartilage. Arthritis of
the knee results from a wearing away of the articular cartilage of the
joint. Arthritis may be idiopathic, or it can result from trauma,
rheumatoid synovitis, pigmented villonodular synovitis, and
seronegative arthropathies such as gout, chondrocalcinosis,
osteonecrosis, and idiopathic disorders. Osteoarthritis is
predominantly a mechanical deterioration that may be associated
with
malalignment of the knee. Familial, genetic predisposition may exist,
however, and osteoarthritis may result from and be associated with
significant synovitis. Rheumatoid arthritis is associated with
significant synovitis, with 80% of patients having a positive
rheumatoid factor, indicating an autoimmune basis for cartilage
destruction.
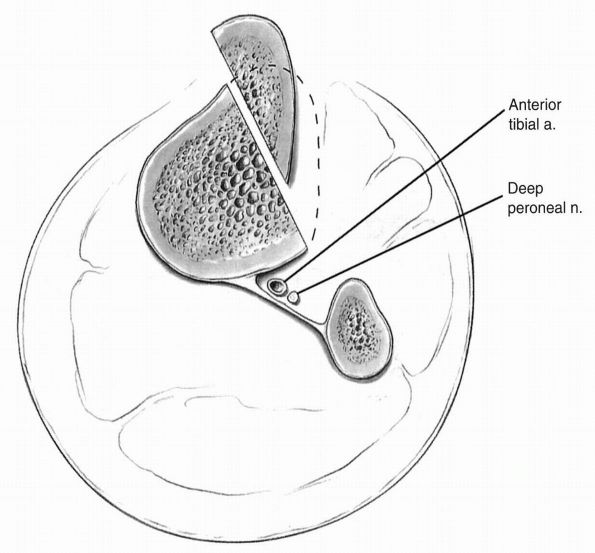 |
|
FIGURE 19-22.
Anteromedial tibial tubercle transfer as described by Fulkerson. Note the close relationship of deep peroneal nerve and anterior tibial vessel to the oblique osteotomy. |
osteoarthritis. Posttraumatic causes include torn menisci with previous
complete meniscectomy, fractures, patellar instability, and loose
bodies caused by chondromalacia or synovial chondromatosis. Mechanical
varus or valgus malalignment with obesity may cause abnormal loading of
the knee over time and produce premature degeneration of the joint
cartilage. Infection causes joint cartilage destruction owing to the
proteolytic enzymes released by the leukocytes that enter the knee to
combat infection where the enzymes are nonselective in their
destructive capabilities.
initially with stiffness and pain. Characteristically, the patient has
stiffness on initiation of gait, which gets better as the knee “warms
up.” There may be an antalgic or painful gait in which the stance phase
of the walking cycle is shortened. A knee with varus malalignment may
demonstrate a lateral thrust due to instability of the lateral
collateral ligament. A patient with valgus malalignment may show a
medial thrust due to an incompetent medial collateral ligament. The
knee may show swelling caused by an effusion and synovitis. Locking may
also occur as the bare bony surfaces grate against each other, causing
severe pain. Osteophytes may also be palpable on clinical examination.
obtained to give the appearance of the knee while it is under stress. A
weight-bearing view in full extension shows most of the clinical
destruction of the joint. Only the posterior condyles may be involved,
however, in which case a weight-bearing view with the knee flexed 45°
shows joint space narrowing, indicating significant cartilage
destruction. Osteoarthritis is characterized by joint space narrowing,
osteophytes, cortical sclerosis on the weight-bearing bony surfaces,
and subchondral cysts. Usually, the medial or lateral joint with
mechanical malalignment is seen, with or without patellofemoral
involvement, on the radiograph. Rheumatoid arthritis, on the other
hand, demonstrates a more symmetric joint
destruction appearance without osteophytes on the standing radiographs.
nonoperative and may include physical therapy, bracing, orthoses,
ambulatory aids, nonsteroidal anti-inflammatory medications,
intra-articular steroid injections, and analgesics. After the acute
pain and synovitis has calmed, gentle exercises and stretching of the
joint may begin. Exercises often slow the onset of permanent stiffness
and flexion contractures. Changes in daily, work, and recreational
activities also may be necessary. Obesity is a known risk factor for
osteoarthritis of the knee, and weight loss has been shown to slow the
progression of the disease. Because of the progressive nature of the
disease, many patients with osteoarthritis of the knee eventually
require operative treatment. A variety of procedures have been
described for treatment of the osteoarthritic knee, ranging from
arthroscopic lavage and debridement to total knee arthroplasty. The
choice of procedure depends on the patient’s age and activity
expectations, the severity of the disease, and the number of knee
compartments involved.
of a period of rest and avoidance of weight bearing if the knee is
painful. Occasionally, a splint or elastic bandage is needed to allow
the synovitis to calm down. Moist heat may be applied to decrease
stiffness. Ice should be used if the knee becomes acutely swollen and
painful. Nonsteroidal anti-inflammatory medications may be prescribed
to decrease the synovitis. Prudent use of intra-articular cortisone
injections may be used; however, the use of cortisone injections at
frequent intervals (e.g., every month) has been shown to accelerate
arthritic deterioration. After the acute pain and synovitis has calmed,
gentle exercises and stretching of the joint may begin. Exercises often
slow the onset of permanent stiffness and flexion contractures.
Surgical intervention is indicated if the patient has persistent pain
that is not relieved by rest, anti-inflammatory medications, and
cortisone injections.
treatment. A radical resection of the synovium and removal of
osteophytes with shaving of degenerated cartilage down to subchondral
bone through an open arthrotomy was used in the past. However,
arthroscopic debridement is now the procedure of choice. It can be
redone in the future if the patient feels successful long-term pain
relief was obtained. Debridement is less predictable if there is
mechanical deformity of the joint. Knees with loose bodies and
cartilage tears were shown to do well with arthroscopic intervention.
Arthroscopic debridement should be considered in active, older adults
with mild to moderate osteoarthritis of the knee after conservative
treatment has been exhausted. Response to treatment is unpredictable,
and patients should be informed of this. Patient selection should be
based on the history of symptoms, physical examination, and
radiographic findings. Age should not be the sole criterion for
selecting arthroscopic treatment. Patients with symptoms of short
duration and those with mechanical symptoms tend to do well. Patients
with radiographic malalignment, especially valgus deformities, tend to
have poor outcomes, as do patients with pending litigation or worker’s
compensation claims. Several authors have reported a “placebo effect”
after arthroscopy for osteoarthritis of the knee that occurs even when
no specific procedure is performed, but most suggested that this effect
was of short duration. None of these arthroscopic procedures can
significantly alter the natural progression of osteoarthritis. In a
recent study that retrospectively reviewed over 14,000 arthroscopic
debridement procedures performed for osteoarthritis, almost 20% had
total knee arthroplasty within 3 years of the surgery. At best,
arthroscopic techniques may delay the need for a more definitive
procedure, especially in younger, active patients with localized
degenerative arthritis that causes pain at rest without malalignment or
instability.
the surgeon. The surgical decision is based primarily on the age and
activity of the patient, the mechanical deformity, and the type of
arthritis.
active person who has premature severe destruction of the knee due to
arthritis. Most of the time, however, the patient refuses arthrodesis
owing to the postoperative appearance and the inability to bend the
knee after the procedure. The procedure provides permanent pain relief
and allows the patient to return to durable, active work.
years of age can be treated by upper tibial osteotomy. This procedure
alters the mechanical axis of the knee by the removal of a triangular
wedge of bone based laterally from the proximal aspect of the tibia
above the tibial tubercle (Figure 19-23).
Success remains durable in patients who have a postoperative alignment
of at least 8° of valgus and who are not obese. The correction is done
on the tibial side since most of the bone destruction is tibial. Women
tolerate the postoperative valgus alignment less well than men and
represent a relative contraindication for the procedure. The procedure
is not indicated in grossly obese patients, unstable knees, or knees
with less than 75° of motion, greater than a 15° flexion contracture,
and instability. Deformities of up to 10° of varus can be treated in
this manner. There are numerous techniques of fixation of the fragments
after osteotomy, including staples, plates, and cylinder casts. The
procedure allows the patient to remain active and delays the time when
total knee replacement becomes necessary. The procedure permits
participation in rigorous sports. Another technique described for high
tibial osteotomy is opening wedge osteotomy with a dynamic uniplanar
external fixator using hemicallotasis techniques. In this procedure,
the medial osteotomy is made below the tibial tuberosity. A dynamic
external fixator is applied, and beginning 7 days postoperatively the
fixator is distracted 0.25 mm four times a day until correction is
obtained. Most reports have shown approximately 80% satisfactory
results 5 years after osteotomy. However, these results also have been
shown to deteriorate over time. Nevertheless, high tibial osteotomy
still is a useful procedure for some patients. Reported complications
of proximal tibial osteotomy include recurrence of deformity (loss of
correction), peroneal nerve palsy, nonunion, infection, knee stiffness
or instability, intra-articular fracture, deep venous thrombosis,
compartment syndrome, patella infera, and avascular necrosis of the
proximal fragment.
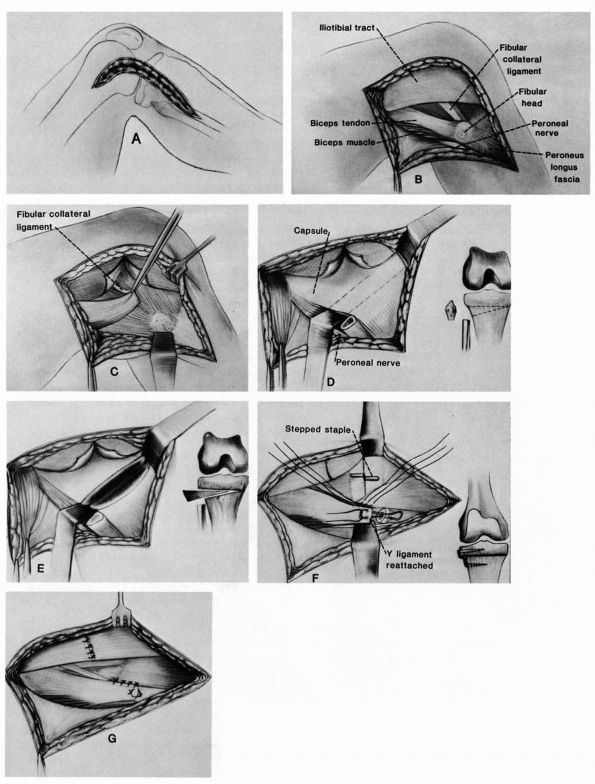 |
|
FIGURE 19-23.
Proximal tibial osteotomy is performed by removal of a laterally based wedge of bone from the proximal tibia, which in turn creates a valgus alignment to restore the normal mechanical axis to the leg. The tibiofibular joint must be released or the proximal fibular head should be resected to allow closure of the osteotomy. |
Arthritic knees with valgus malalignment typically have bone
destruction located on the lateral femoral condyle. Hence, correction
of the deformity should be on the femoral side. If the valgus deformity
at the knee is more than 12 to 15° or the plane of the knee joint
deviates from the horizontal by more than 10°, Coventry recommended a
distal femoral varus osteotomy rather than a proximal tibial varus
osteotomy. Reported success rates for distal femoral osteotomies
performed for osteoarthritis range from 71 to 86% good or excellent
results. Poor outcomes have been noted in patients with rheumatoid
arthritis or those with inadequate motion of the knee before distal
femoral osteotomy.
controversial since its introduction in the early 1970s. Early reports
on the success of the procedure were conflicting. However, recent
long-term reports have showed success rates for unicompartmental knee
arthroplasty approaching those of primary total knee arthroplasty in
properly selected patients. Early reports of unicompartmental
arthroplasty noted failure and revision rates of up to 40%, most
related to mechanical alignment, implant design, cemented fixation, and
debris from polyethylene wear. Newer implant designs that provide more
adequate surface replacement with less stress concentration, thickened
and metal-backed polyethylene tibial inserts, improved fixation, and
less bony resection have reduced the incidence of mechanical failure
dramatically. More recent reports have indicated that intermediate and
long-term results of unicompartmental arthroplasty are comparable to
those of total knee arthroplasty and high tibial osteotomy.
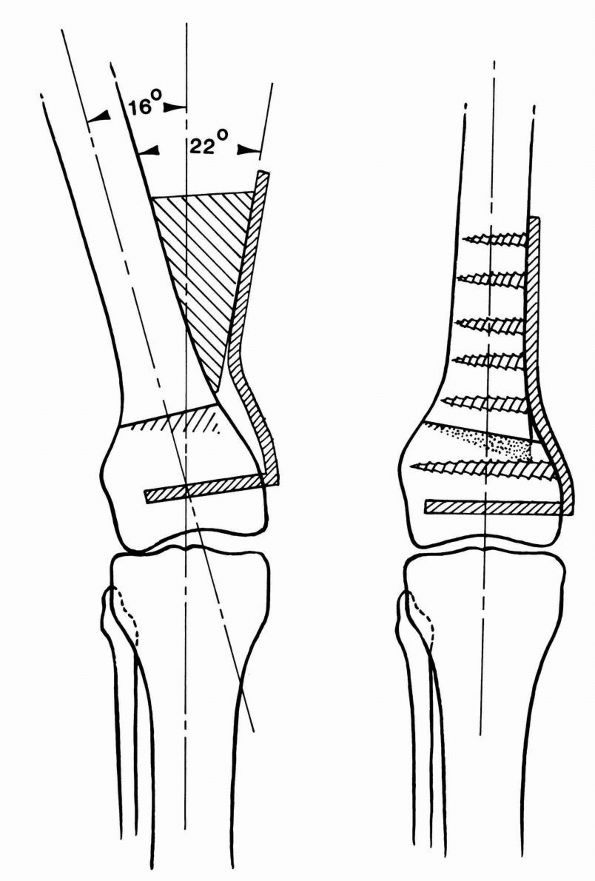 |
|
FIGURE 19-24. Distal femoral osteotomy for valgus knee.
|
narrow indications, difficult surgical technique, results inferior to
total knee arthroplasty, and complexity of revision similar to that of
tricompartmental replacement. With recent design improvements
unicompartmental arthroplasty is becoming a viable alternative for
patients in whom
osteoarthritis is limited to either the medial or lateral compartment. Ideal
candidates for unicompartmental arthroplasty are older than 60 years of
age, have low activity demands, weigh less than 180 pounds, experience
minimal pain at rest, and have preoperative arc of flexion of 90°, no
flexion contracture of more than 5°, and no angular deformity of more
than 15°. Although we prefer proximal tibial osteotomy and total
joint arthroplasty, unicompartmental arthroplasty may be appropriate in
carefully selected patients.
knee replacement is the preferred treatment. The indications for joint
replacement have been stretched for younger patients with
tricompartmental (medial and lateral tibiofemoral and patellofemoral)
disease owing to the predictable pain relief and durability of the
implants over time. Joint replacement for the young, active patient,
however, ensures the need for future revision surgery, thus the
operation should be delayed as long as possible for these patients. Age
is not a factor in recommending total knee replacement for rheumatoid
arthritic patients because the disease generally affects the patient’s
overall activity level significantly so that wear and loosening of the
implants are of less concern.
namely stability between the tibial and femoral components due to the
shape and design features of each. In considering different levels of
constraint, they can be appreciated by reviewing the evolution of
modern TKA. The first total knee
arthroplasties are the fixed hinges of Waldius and Shiers. These are
examples of maximal constraint. Flexion-extension motion is about a
fixed, single axis. There is no internal or external rotation. Early
loosening and other problems led to more anatomic designs, which
started with the Gunston and Geomedic knees. These bicondylar and
biplateau components are four ligament knees, generally preserving the
cruciates and the collaterals. While not operating about a fixed hinge,
the component shapes led to nearly single axis rotation, and the
interfaces between tibia and femur provided little rotation. Early
failure rates led to designs with less constraint. As well, the
discrete pieces, especially the four-piece nature of the polycentric
Gunston knee spurned designs that were relatively monolithic. Also, the
next generation of TKAs offered resurfacing of the patellofemoral
joint—patellar plastic buttons and coverage of the femoral trochlear
groove.
Walker, and Townley are good examples of these less constrained knees.
They relied on tibial surface dishing to create anterior posterior
stability. Posterior and anterior cruciates were routinely excised.
Most initially, and nearly all ultimately, provided matching patellar
buttons as well as shaping of the femur component to model the
trochlear groove of the biologic knee.
and MAR Freeman began to emphasize ligament balancing or correction of
preoperative varus and valgus deformities. Limited ranges of motion due
to shapes, tightness in the collaterals, and absence of the cruciates
led to less conforming tibial surfaces while preserving the PCL. This
type may be called cruciate condylar
knees. An alternative developed was the posterior substituting design.
This is earliest exemplified by the Insall-Burstein prosthesis, which
uses an intercondylar tibial peg to articulate inside an intercondylar
femoral housing. The interaction of these two prevents posterior
displacement of the tibia in relation to the femur, especially during
flexion. Interaction of this post with the femoral housing also usually
provides a camming relationship so that the femur is induced into a
posterior roll as the knee is flexed. This kinematic pattern allows
better clearance of the posterior aspect of the distal femur in its
relationship to the posterior edge or the tibial surface. Various forms
of posterior cruciate retain (PCR) sparing knees (derivatives of the
cruciate condylars just mentioned) are, together with posterior
substituting (PS) knees, the most popular implants. An interminable
debate exists as to the relative merits of each, that is PS versus PCR
for the routine and even difficult knee cases.
indications for one versus the other. The choices are largely due to
surgeon training, experience, and taste. Most would probably agree that
the postpatellectomized knee and the cruciate deficient knee with
posterior tibial subluxation should be managed with PS components. A
variation, reintroduced to the array has been a deep-dished PCR
version. The rationale is to permit maintenance of the PCL and some of
its function while enhancing posterior stability by having a deeper
tibial dish, particularly at the anterior lip. This is different from
the original total condylar, which relied more on a posterior lip to
prevent anterior dislocation.
Anterior subluxation or dislocation seems not to have been a clinical problem.
They have been in use for more than 15 years and are based on a
rationale of providing complex motion with maximum contact surface
area. Their use has been increasing, but the value versus cost and
potential risks are not so clearly established. This is a violently
debated issue.
used now for tibial baseplates, and cobalt-chrome alloys for femoral
components, as well as tibial baseplates. The softer titanium proved to
be a bad choice for a bearing surface. Nearly all knees worldwide use
polyethylene tibial surfaces at the articulation. The poly, as it is
frequently termed, may constitute the entire baseplate so that the poly
is cemented to the bone, or may be an insert fitting on a metallic
baseplate. These latter are mostly modular designs supplied with
separate metal baseplates. Polyethylene has also been the most
consistent material for resurfacing the patella. Patellar design
features have related to component thickness, general size, and overall
surface shape. In the 1980s metallic features connected to thinner
polyethylene structures, were provided with porous surfaces to permit
bone ingrowth. The correspondingly thinner plastic frequently led to
wear-through and its associated problems of metal debris, scratching of
the femoral component and osteolysis. Metal-backed ingrowth patellar
components exist, but they are not very popular. The overall question
of whether even to use a patellar component is another hotly contested
one. Clearly the vast majority of U.S. orthopaedic surgeons do
resurface, while a moderate number of European and Asian surgeons do
not. True experts line up enthusiastically on both sides of the topic
and marshal convincing arguments for the two views. Indications for
leaving the patella unresurfaced are a primary diagnosis of
osteoarthritis, satisfactory patellar cartilage with no eburnated bone,
congruent patellofemoral tracking, a normal anatomical patellar shape,
and no evidence of crystalline or inflammatory arthropathy. Patient
weight also appears to be a factor, with lighter patients tending to do
well with unresurfaced patellae.
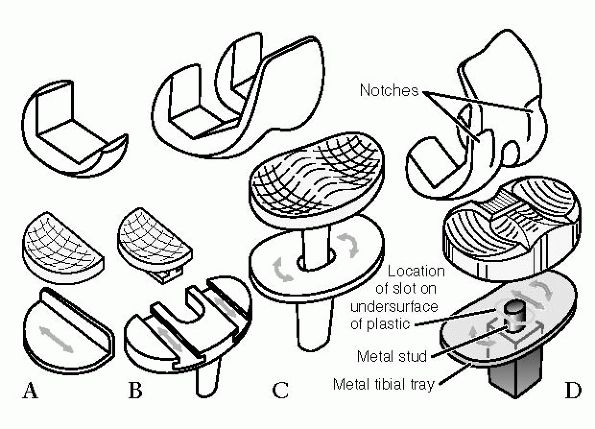 |
|
FIGURE 19-25. Schemes of mobile bearing knees.
|
size. In general, surgeons seek to place components that are as close
to equal in size as the bone removed and the aspect of bone that is
covered or capped. The finite size problem
leads to the necessity of compromise. A nearly continuous array of
patient sizes has to be fit with a fixed, limited selection of
prosthesis sizes. Thus, the component size may be just right, or the
component will be a bit large or a bit small. Undersizing can lead to
lack of maximal bone interface and therefore support. Oversize can lead
to tightness—tightness of gaps, the capsule, and so on—all of which can
lead to poor motion.
via methyl methacrylate cement. Bone ingrowth porous surfaces were
introduced in 1980. Other cobalt-chrome beads, titanium beads, fiber
metal, and pseudocancellous structures, including mesh-like constructs
have been used. Many of the clinical results with these materials were
excellent, but failure rates on balance have been higher than with
cemented implants. Surgeons in many countries remain dedicated to
uncemented fixation; however the majority moved back to the use of
cement in combination with many other improvements in cementation,
realignment, and so on. Uncemented fixation may reappear as there is
greater successful experience obtained with the use of
hydroxyl-apatite-coated surfaces.
the four deformities that are encountered. Varus, generally in
combination with mild flexion contracture, is the most common.
Particularly varus and valgus are seen on radiographs, and seen most
clearly
on long-standing views. Drawing a few simple lines displays the angle
of deformity on these radiographs. The accepted target for normal, that
is the surgeon’s goal, should be clear. A line drawn from the center of
the femoral head should pass through the center of the knee as it goes
on through the center of the ankle. The net anatomic tibiofemoral angle
equals the angle of offset between the anatomic shaft of the femur and
the mechanical line of the femur—the line from the center of the
femoral head to the center of the distal femur. That number is
generally between 5 and 6° (Figure 19-26).
Numerous studies have shown a correlation between long-term success of
total knee arthroplasty and restoration of near-normal limb alignment.
implicated in long-term difficulties, including tibiofemoral
instability, patellofemoral instability, patellar fracture, stiffness,
accelerated polyethylene wear, and implant loosening. Rotational
alignment of total knee components is difficult to discern
radiographically, making the assessment of rotation primarily is an
intra-operative determination. The rotation of the femoral component
has effects not only on the flexion space but also on the
patellofemoral tracking. Because the proximal tibial cut is made
perpendicular to the mechanical axis of the limb instead of in the
anatomically correct 3° of varus, rotation of the femoral component
also must be altered from its anatomical position to create a
symmetrical flexion space (Figure 19-27). To
create this rectangular flexion space, with equal tension on the medial
and lateral collateral ligaments, the femoral component usually is
externally rotated approximately 3° relative to the posterior condylar
axis. In a normal femur, this technique rotationally places the femoral
component with the posterior condylar surfaces parallel to the
epicondylar axis. This technique fails when the posterior aspect of
either the native femoral condyle has significant wear or when the
lateral femoral condyle is hypoplastic, as is frequently seen in knees
with valgus deformity.
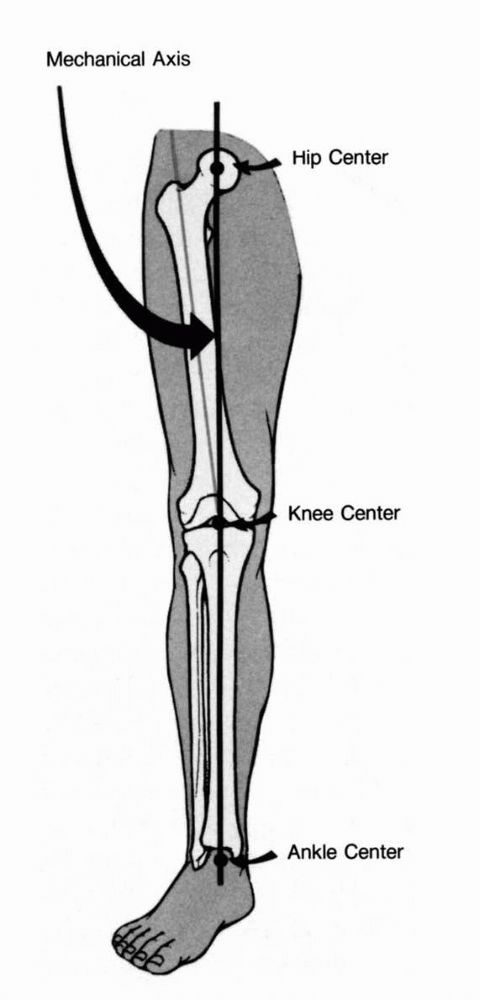 |
|
FIGURE 19-26. Mechanical and anatomical alignment.
|
anteromedial skin incision in combination with some form of medial
capsulotomy. The capsulotomy
may
be extended proximally within the quad tendon—the standard approach;
extended into the muscle close to the tendon; projected otherwise in
the mid-aspect of the vastus—a midvastus approach; or extended at the
medial margin of the vastus medialis—a subvastus approach. Other
variations include (1) the Muller lateral skin incision in combination
with a medial capsulotomy, and (2) a lateral incision—lateral
capsulotomy generally in combination with a tibial tubercle osteotomy,
and generally done for direct access to the lateral stabilizers when
managing valgus deformity.
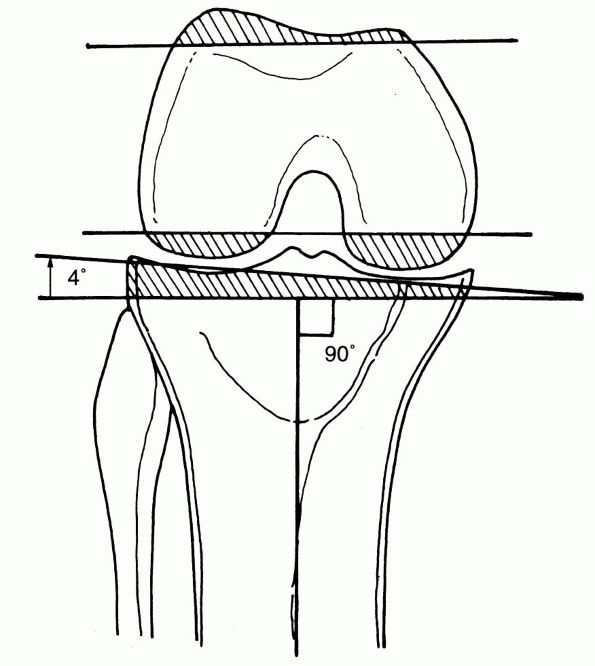 |
|
FIGURE 19-27.
Bone resection with classic arthroplasty alignment. The tibial plateau is cut perpendicular to its anatomic axis, resulting in more bone resection laterally than medially. To equalize the medial and lateral flexion space, more bone should be resected from the medial posterior femoral condyle. This is achieved by slight external rotation of femoral component. |
minimal incision, small exposure techniques, principally on the medial
aspect but also laterally. The term MIS, for minimal incision surgery,
has been introduced. An analogous approach from the lateral aspect is
being developed as well. The goal of all of this work is less soft
tissue trauma leading to less discomfort, better initial rehab, and
better overall results. It is not clear as of this date whether such
techniques are safe, are teachable or transferable, or even whether the
final result is any better.
difficult to expose. Achieving adequate access from the anterior aspect
can require extraordinary manipulation of the extensor mechanism. The
options include tibial tubercle osteotomy, quadriceps tendon snip, and
quite uncommonly today a quad-turndown—modified Coonse-Adams exposure.
Another approach is skeletonizing, basically elevating
capsular-ligamentous-tendinous tissue as far around and as far proximal
and distal as necessary to fold things back and get the knee bent and
exposed. The arguments for and against each are voluminous. We very
aggressively favor the tibial tubercle osteotomy. It gives the best
amount of exposure of the least soft tissue disruption and is
associated with the strongest repair. The problems originally noted
have largely been overcome.
essentially equal flexion and extension gaps. The distance from the
posterior femoral cut to the proximal tibia approximates the distance
of the distal femoral cut surface to the proximal femur when the tibia
is distracted away from the femur and assessed respectively at 90° and
0°. This gap equality is achieved to some degree either by specifically
measuring the distances and cutting the bones in the two positions to
achieve this, or by using measured resection. Measured resection is
accomplished by placing instruments on the distal and posterior femur
so that the amounts of bone removed from those aspects of the femoral
surface approximate the thickness of the corresponding femoral
component surfaces. Pure flexion contracture and pure recurvatum
deformity, meaning those without any varus or valgus components, may be
viewed as representing a priori inequalities in the flexion versus
extension gaps. Viewing the flexion contracture situation as one
predisposed to a smaller extension gap as the recurvatum predisposes to
a larger extension space.
of the soft tissue sleeve as a result of mostly varus and valgus
malalignment, but also the presence of flexion contracture. The most
common and basic approach is to release those ligamentous, capsular,
and tendinous structures that lie on the concave aspect of the
deformity, that is the medial side of the varus knee, posteromedial as
well if there is flexion contracture. Knees with severe varus deformity
need subperiosteal release of the medial collateral ligament from the
proximal portion of the tibia. This is required because the medial
collateral ligament becomes contracted as the varus alignment becomes
more severe. In valgus knees, the iliotibial band, popliteus tendon,
and lateral collateral ligament frequently need to be released from the
femur. Flexion contractures are corrected by removal of osteophytes
from the posterior femoral condyles and release of the posterior joint
capsule; occasionally, more distal femoral bone needs removal.
lax, convex side of a deformity. This technique may be indicated in
younger patients in whom moving to a higher order of prosthetic
constraint—varus or valgus constraint imparted by a highly conforming
intercondylar peg, may be undesirable.
variety of ways. Probably most common is the use of a tourniquet, until
components are completely in place. Then, the tourniquet is deflated as
the surgeon searches to control the more obvious bleeding points. Some
keep the tourniquet inflated until the dressing is wrapped into place.
Others let the tourniquet down after bone preparation, search for
bleeding points, then reinflate for cementation and closure, or
cementation alone. If the surgeon is going to look for bleeding points,
and it is our practice and recommendation to do that, there are at least four locations to check.
The most common moderate bleeding point is at the inferolateral
geniculate artery in the posterolateral corner of the knee. This vessel
courses between the lateral collateral ligament and the popliteus
tendon. Especially with removal of the posterior cruciate ligament for
implantation of a PS knee, surgeons are encouraged to inspect the
location of the intermediate geniculate at the posterior center. If
there has been any lateral patellar release, or significant dissection
at the anterolateral fat pad and parapatellar region, then careful
examination of the lateral patellar “gutter” is appropriate. A final
region is at the medial tibial metaphysis in the case of large medial
release for varus deformity. The surgeon can see significant bleeding
from injured branches of the inferior medial geniculate vessel.
the surgery and many subtasks and points involved. The surgeon must
direct attention to the following elements: (1) definite, adequate
pressurization into clean dry cancellous bone; (2) full impaction of
the associated component, checking that this is full by seeing that the
component is properly down, against the bone surfaces; and (3) clearing
of the overflown cement. To accomplish all of this, it is necessary to
cleanse and dry the cut surfaces using pulsatile lavage, suction, and
sponging. The cement must not be too liquid. Very liquid cement may be
injected into the bone, but it then runs away from anything other than
a horizontal surface. Also, as the component over the cement is
impacted there is less viscosity to create a pressurization at the time
of component seating. Similarly, do not use cement that has become too
completely cured and cannot intrude into trabecular interstices.
Antibiotic impregnated cement is controversial. We use it routinely,
and many others do. Most probably do not use it except for revision
after infection, for especially long, complicated cases, or because of
compromised defense mechanisms in the patient.
bone cuts and achieving initial prosthesis bone fixation. Cuts have to
be very accurate; alignment must be nearly perfect and initial
stability enhanced to some degree by stems, pegs, or maybe even screws
for some designs.
it is crucial. Accurate edge approximation at each level without
strangulation and ischemia of tissue is extremely important. The large
TKA implant may lie less than a centimeter away from the outer surface
of the skin. The chances for retrograde infection due to failure of
wound sealing and healing is great.
using computer assistance to oversee alignment and kinematic features
during performance of the total knee operation. This equipment uses
hightech position detection devices that observe using infrared lights
on bone and instrument markers. The relative movement of bones and
surfaces is immediately detected. Inputting to the computer the
locations of those bony features which form the bases of alignment and
rotation, the system indicates the relative positions and actual
alignment of the components and the respective bones—all at once and
essentially instantaneously. Navigation is not currently the standard,
but many predict that it will be by the next 5 to 10 years.
rehabilitation are as simple as achieving range of motion and
protective as well as functional strength. The real challenge is to get
and to keep the knee moving so that the anterior structures do not
contract and so the various tissue planes do not get fibrosed to each
other and to the components.
instability, fracture, loosening, poly wear, osteolysis, bone fracture,
patellar maltracking, component breakage, and other factors.
-
Understanding the true cause of failure in the problematic knee
-
Difficulties of exposure
-
Loss of bone stock, this leading to difficulty with fixation
-
Difficulty with fixation
-
Difficulty with balancing and stability
complication. Preventing infection is an integral part of performing
the procedure, while early and appropriate treatment of the established
infection is of utmost importance. In recent reports, deep infection
complicates 1.3 to 2.9% of total knee arthroplasties.
generally the result of contamination at the time of surgery. Late
infection may occur in a previously sterile joint that becomes septic
secondary to hematogenous seeding of the arthroplasty. Diabetes
mellitus; rheumatoid arthritis and its variant, corticosteroid use;
immunosuppressive medications; extreme old age; concurrent infections;
prior history of septic arthritis; obesity; and prior knee surgery have
all been shown to increase the risk of early and late sepsis of the
knee.
Together these microbes are identified in over half of all infected
arthroplasties in a wide range of studies. Gram-negative bacilli are
involved when arthroplasty infection results from acute wound
complications or late hematogenous seeding. Indolent infections
presenting 3 to 24 months after surgery are caused by avirulent
organisms such as negative staphylococci, viridans streptococci,
anaerobic Gram-positive cocci and corynebacteria. Candida organisms
that contaminated the wound perioperatively cause rare episodes of
indolent prosthetic joint infection. Hematogenous infections of
arthroplasties are caused by virulent organisms such as S. aureus, β-hemolytic streptococci, and Gram-negative bacilli.
period. The patient is seen during the first postoperative month, and
the diagnosis is evident on the basis of the medical history and
physical examination. Systemic signs of infection such as fever,
chills, and sweating may be present. Pain is usually continuous, on
local examination wound may be erythematous, swollen, fluctuant, and
tender. Wound drainage, if present, is usually purulent. Laboratory
tests such as white blood cell count (WBC) and erythrocyte
sedimentation rate (ESR) are of limited use, since moderate elevation
is expected in this time period. C-reactive protein (CRP) normalizes
within 3 weeks of surgery, much earlier than ESR and persistent high
level of CRP is suggestive but not diagnostic. Clinically, it is
difficult to distinguish between sterile and infected hematomas.
Culture of drainage fluid has not been reliable. Aspiration is helpful,
but if the patient has been on antibiotics within the preceding weeks,
a false negative may be obtained. Treatment of the wound is the primary
consideration. Tense infusions or hematomas are best surgically
evacuated, whether or not infection is present. Infection in this time
period is initially treated with surgical debridement, administration
of antibiotics, and attempted retention of the components.
time of the operation, but because of a small inoculum or the low
virulence of the organism the onset of symptoms is delayed. The patient
is seen between 6 and 24 months after the index procedure. The hallmark
of this type of infection is a gradual deterioration in function and an
increase in pain. Type III infections are the least common and are
caused by hematogenous spread to a previously asymptomatic
arthroplasty, usually 2 or more years after the arthroplasty. Generally
there is an acute febrile episode accompanied
by
sudden rapid deterioration in the function of the knee. The diagnosis
can usually be based on history and physical examination. Seeding can
occur at the site of a loose prosthesis, a solidly osseo-integrated
prosthesis, or a solidly fixed cemented prosthesis. These patients
typically have a significant pain-free interval. A type III infection
is likely to occur in patients who are immunosuppressed. Other factors
that may be associated are dental work, respiratory infection, remote
periprosthetic infection, open skin lesions, endoscopy, and
contamination of operative sites.
evaluating the painful total knee replacement even though they are
unremarkable in the majority of cases, particularly in the early
period. Occasionally, the patient will have diagnostic changes, such as
periostitis, rapidly progressive osteolysis, or endosteal scalloping.
The development of a complete radiolucency around a component over a
short time is highly suggestive of advanced infection. Subchondral bone
resorption and patchy osteoporosis are more subtle, earlier findings
present in some infected cases. Periprosthetic osteolysis is the most
common finding but is nonspecific. Periosteal new bone formation, with
or without loosening of a component, has been considered by some to be
pathognomonic of deep infection.
suspected total knee infection. In one series, only 28% of infected
knees had a WBC greater than 11,000. When a patient does have an
abnormal WBC count, the systemic infection is usually clinically
obvious.
investigations for the diagnosis of a potential infection following
total joint replacement. Average ESR of more than 50 has been reported
in different series of infected total knee arthroplasty but both false
positives and false negatives are seen. False positives can occur
because of other inflammatory conditions such as rheumatoid arthritis.
The ESR may remain elevated for months after an uncomplicated joint
arthroplasty and in early postoperative period. The CRP levels increase
from trace amounts to reach maximum values within 48 hours of surgery
and then returns to trace amounts in approximately 2 to 3 weeks. Some
care must be taken in interpreting the ESR or CRP level before a
revision surgery. The physician must determine whether any other
factors such as rheumatoid arthritis, a recent operation, neoplasia,
collagen vascular disease, infection, or an inflammatory condition are
present. If no such conditions are applicable, an
ESR of more than 30 or 35 mm per hour and a C-reactive protein level of
more than 10 mg per liter should be considered abnormal and should
warrant additional investigations to rule out infection.
potential for the diagnosis of infections following joint replacement.
Scintigraphy though is limited by cost of scans, time required, and
most importantly its inability to yield consistently acceptable levels
of sensitivity and specificity. Often scans are no more accurate than
other, less expensive serological investigations. Technetium-99m bone
scans are sensitive but not specific. Also persistent increased uptake
of Tc-99m adjacent to asymptomatic total knee arthroplasties
particularly around the tibial component is expected for up to a year.
TKA infection; its specificity is only 20%. Gallium-67 citrate is a
radioisotope that accumulates in areas of inflammation. Gallium scan
sensitivity is high and a negative scan can reliably rule out
infection; however the positive predictive value is 70 to 75% as
gallium may show increased uptake at uninfected sites of bone
remodeling. Indium-111-labeled white blood cells are useful for the
diagnosis of increased vascularity and white blood cell uptake, and
indium-111-labeled WBC scintigraphy has been used to study
periprosthetic infection. Other radiolabeled markers like radiolabeled
Immunoglobulin G have been investigated and though preliminary results
are encouraging, their routine use cannot be advocated till further
studies are available. “Radionuclide scans can be
specifically of benefit in equivocal situations in which serological
investigations may be falsely elevated and aspirate cultures are
unreliable because of the administration of antibiotics.”
determining whether there is deep joint infection.” It can also
identify the bacterial species and antibiotic sensitivity. A synovial
fluid WBC of greater than 25,000 or a differential with more than 75%
PMNs is suggestive of infection. Also, elevated protein and low glucose
are consistent with infections. The most common reason for false
negative is the administration of antibiotics before aspiration. If
there is any suspicion of infection and the first aspirate is negative,
we routinely do repeat weekly aspirations for 3 to 4 weeks.
septic knee arthroplasty. These options include chronic antibiotic
suppression, irrigation and debridement with prosthesis retention,
arthrodesis, amputation and debridement with reimplantation. A number
of factors have to be considered before the treatment option selection.
These factors include the time elapsed from index procedure; host
factors affecting treatment of infection; soft tissue condition;
implant status in terms of fixation, type of organism, and its
sensitivity to antibiotics; and, most importantly, the patient’s
expectations and functional requirements.
alone is difficult. Long-term suppression with antibiotics alone is
rarely indicated. In rare circumstances when medical condition
precludes surgery and removal of well-fixed component and the organism
has low virulence and is susceptible to an oral antibiotic, oral
suppression may be justified. Although this treatment method is rarely
indicated, it is commonly attempted and only prolongs the presence of infection and complicates subsequent treatment attempts.
infected material and retention of the prosthesis. Suggested treatment
criteria include short duration of infection (less than 2 to 3 weeks),
susceptible Gram-positive organisms, absence of prolonged drainage of
sinus tract, and no prosthetic.
done as first line of management; however it is a potentially
beneficial means of managing infected arthroplasties, with low risk of
reinfection and reliable pain relief and knee stability. Absence of
knee motion and its affect on activities of daily living counterbalance
some of its advantages. We consider an arthrodesis generally after
failed reimplantation in patients with high functional demand, single
joint disease extension mechanism disruption with poor soft tissue
envelope.
life-threatening systemic sepsis. Earlier attempts at arthrodesis when
there is adequate bone stock and a viable soft tissue envelope, rather
than repeated attempts at revision in the presence of infection and
deceased use of stemmed hinged implants, should lower the incidence of
amputation following failed total knee surgery.
prosthesis and cement with subsequent arthroplasty at a later date with
intervening period of antibiotics is the most commonly used protocol
for the treatment of infected total knee arthroplasty. One-stage
reimplantations have also been reported in literature; however, most
series have small numbers, and the success has not been seen to be
corroborated by other groups. We have no personal experience with
one-stage revision and believe like most, that two-stage exchanges
arthroplasty is still the treatment of choice for infected total knee
replacements. Two-stage reimplantation usually implies revision
arthroplasty more than 4 to 6 weeks following prosthetic removal. In
the first stage, a complete debridement and drainage, including removal
of the total-joint prosthesis and acrylic cement, is performed. A
6-week course of antibiotic therapy follows the first stage. At the
conclusion of antibiotic therapy, a new knee replacement is inserted as
the second stage. The use of modular and custom-designed implants may
be necessary to augment any bone loss that may have developed during
the active course of the infection.
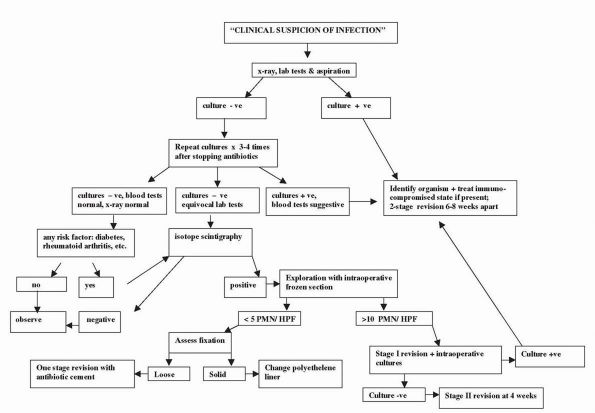 |
|
FIGURE 19-28. Algorithm for workup and treatment of suspected TKA infection.
|
P, Buzzi R, Zaccherotti G et al. Patellar tendon versus doubled
semitendinosus and gracilis tendons for anterior cruciate ligament
reconstruction. Am J Sports Med 1994;22:211-217. Article
compares 60 patients treated with patellar tendon versus hamstring
grafts. There was no difference between the two groups.
P, Insall J, Deschamps G et al. The results of treatment of idiopathic
osteonecrosis of the knee. J Bone Joint Surg 1983;65B:588. This article characterizes the clinical course of osteonecrosis based on radiographic area of involvement.
SP, Warren RF. The microvasculature of the meniscus and its response to
injury: an experimental study in the dog. Am J Sports Med
1983;11:131-141. This article forms the basis of meniscal repair.
RL, Wolfe MW, Waldman DA et al. Resurfacing of the patella in total
knee arthroplasty. J Bone Joint Surg 1997; 79A:1121-1131. A
prospective randomized double blind study of eighty nine patients
assigned to resurfacing or retention of patella. The prevalence of
anterior knee pain was not influenced by whether the patella was
resurfaced or not.
MD, Tietjens BR. Long-term follow up of the untreated isolated
posterior cruciate ligament-deficient knee. Am J Sports Med
1996;24:306-310. This study is a retrospective
evaluation of 38 patients with isolated PCL deficient knees at 13.4
years after injury. As time from injury increased, articular
degeneration on radiographs was seen.
JJ, Squire MW, Goetz DD et al. Cemented rotatingplatform total knee
replacement: a nine- to twelve-year follow-up study. J Bone Joint Surg
2000;82:705-711. In 119 consecutive TKAs with
rotating platform at 9- to 12-year follow-up, no reoperations were
required, and no periprosthetic osteolysis and loosening was noted.
review of indications and techniques of meniscus repair. A review of
indications, different techniques for meniscal reapir, patient
selection and outcomes.
surviorship analysis was used to compare the failure rate and overall
success of 2629 cemented primary total knee anthroplasties during a
22-year period.
CD, Olson E, Irrgang JJ, et al. Allograft versus autograft anterior
cruciate ligament reconstruction: 3- to 5-year outcome. Clin Orthop
1996;324:134-144. This study compares 64 patients
with autograft versus 26 patients with allograft. There was no
difference between the two graft sources.
JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of
infected total knee arthroplasty, J Bone Joint Surg 1983;65A:1087-1098.
This article describes the staged reimplantation of infected knee replacement.
TM, LaValley MP, Gulin JP et al. Glucosamine and chondroitin sulphate
for treatment of osteoarthritis: a systematic quality assessment and
meta-analysis. JAMA 2000;283: 1469-1475. This
meta-analysis showed that glucosamine and chondroitin sulfate
demonstrate moderate to large effects on osteoarthritic symptoms.
MD, Harner CD. The anatomic and surgical considerations for posterior
cruciate ligament reconstruction. In: Jackson DW, ed. Instr Course Lect
44. Rosemont, IL: American Academy of Orthopaedic Surgeons,
1995:431-440. The authors discuss anatomy, biomechanics, and current surgical techniques.
D, Bourne RB, Rorabeck CH et al. Survivorship of the high tibial valgus
osteotomy: a 10- to 22-year follow up study. Clin Orthop
1999;367:18-27. In this study 85 patients with
minimum 10-year follow up were evaluated. The percentage not requiring
TKA was 73% at 5 years and 39% at 15 years. Early failure was
associated with age older than 50 years, lateral thrust, previous
arthroscopic debridement, range of motion less than 120°,
undercorrection, and delayed or nonunion.
JH, Ackroyd CE, Shah NA. Unicompartmental or total knee replacement?
Five-year results of prospective, randomized trial of 102
osteoarthritic knees with unicompartmental arthritis. J Bone Joint Surg
Br 1998;80B:862-865. Knees deemed suitable for
unicompartmental replacement at the time of surgery were randomized to
total or unicompartmental knee replacement. Pain relief was equal in
both groups; unicompartmental knee replacement group had better range
of motion, less morbidity, and a faster recovery.
FR, Barber-Westin SD. Treatment of complex injuries involving the
posterior cruciate and posterolateral ligaments of the knee. Am J Knee
Surg 1996;9:200-214. The article reviews multiple studies on treatments for PCL and posterolateral injuries of the knee.
F, Mooar P, Matthews D et al. The symptomatic anterior crucial
deficient knee: part I. J Bone Joint Surg 1983;65A:154-162. This study presents the natural history of anterior cruciate-deficient knees.
DB. Arthroscopically assisted reconstruction of anterior cruciate
ligament: a prospective randomized analysis of three techniques. J Bone
Joint Surg 1996;78A:803-813. No significant difference noted between hamstring autograft, and one and two incision patellar tendon autograft.
RD, Volatile TB. Twelve years’ experience with posterior
cruciate-retaining total knee arthroplasty. Clin Orthop 1986; 205:100-.
Results of early posterior cruciate retaining
design presented with increased range of motion as compared to the
early substituting design.
MJ, Warren RF, Ortiz GJ et al. The effects of sectioning of the
posterior cruciate ligament and the posterolateral complex on the
articular contact pressures within the knee. J Bone Joint Surg
1993;75A:694-. Articular contact pressures in ten
cadaveric knees with intact ligaments were measured with the use of
film, the measurements were repeated after sequential sectioning of the
posterior cruciate ligament and posterolateral complex.
KD, Gray T. Anterior cruciate ligament reconstruct with autogenous
patellar tendon autograft followed by accelerated rehabilitation: a
two- to nine-year follow-up. Am J Sports Med 1997;25:786-795. This
study describes an accelerated rehabilitation program. The average
return to athletic competition was 6.2 months postoperatively.
KD, Patel DV. Management of combined injuries of the anterior cruciate
and medial collateral ligaments. J Bone Joint Surg 1995;77A:800-806. Results
of nonsurgical treatment of MCL complex with ACL injuries presented.
Authors found that it is possible to treat MCL nonsurgically. The
authors also recommended that surgical treatment of ACL should be
delayed for 4 to 6 weeks until full knee motion was regained.
anatomy of posterolateral corner is meticulously described. The article
contains excellent photographs of posterolateral corner of the knee.
DM, Deng XH, Torzilli PA et al. The role of the cruciate and
posterolateral ligaments in stability of knee: a biomechanical study.
Am J Sports Med 1995;23:436-443. Cadaveric study to assess the role of posterolateral and cruciate ligaments in restraining knee motion.
KG, Insall JN, Kelly MA. The total condylar prosthesis: 10-to 12-year
results of a cemented knee replacement. J Bone Joint Surg
1989;71B:793-797. The authors present a long-term
evaluation of the original total condylar prosthesis design that
sacrificed the posterior cruciate ligament.
