Tibia and Fibula Fractures
understanding of the current best evidence. Evidence for the management
of tibial shaft fractures has expanded substantially with an
ever-increasing body of information. A Google internet search with the
term “tibial fracture” returns 281,000 Web sites currently providing
information about tibial fractures. Using a focused search engine such
as PubMed and the search terms tibia (or tibial) and fracture returns
14,385 published articles. Of these, 1513 were published since the last
edition of this textbook. Only 83 (5%) were clinical trials, while 123
(8%) were reviews. Not all of the information is equally valid or
reliable. However, the quantity of Web sites and articles serves to
illustrate the interest and continuing research tibial shaft fractures.
grades of recommendation associated with treatment, prognosis, and
diagnosis in this chapter, and we have used the Levels of Evidence as
used and defined in the Journal of Bone and Joint Surgery.296 We have placed higher weight on best evidence.
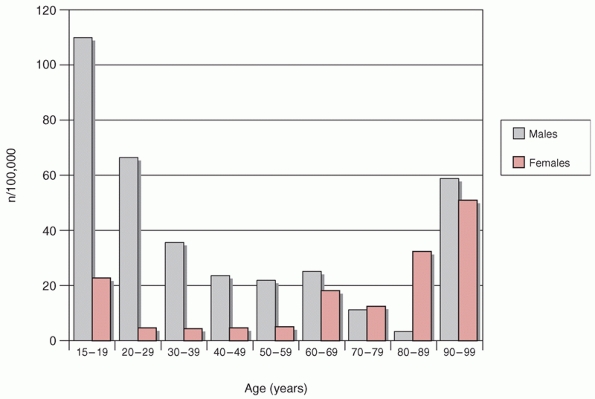
and occur at a frequency of about 26 fractures per 100,000 population
per year.65,73 They are approximately three times more
frequent in males than in females and the average age of patients is about 37 years65,73
In males, tibial fractures are common in the adolescent age group and
are generally attributed to higherenergy trauma such as motor vehicle
accidents.65 The frequency of their
occurrence increases again later in life with the development of
osteopenia and osteoporosis. This is the bimodal distribution as
described by Court-Brown (Fig. 55-1).65,67
In women, the highest incidence of tibial fractures occurs in later
life, over the age of 80, and is attributed to osteoporosis.67,73 However, this increase in the elderly was not seen by Grutter et al.,109 who reviewed 4889 fractures from the AO documentation center.109
They found that the majority of fractures occurred in those aged less
than 40 years, but they did agree that males were more commonly
affected than females in a ratio of approximately 2:1.109,110 Emami et al.88
also identified the increased incidence of tibial shaft fractures in
young males, but they noted that there was a decrease in the incidence
of tibial shaft fractures in Sweden in the late 1980s as compared to
the 1970s. They attributed this decrease to changing legislation
regarding seat belt laws and a decrease in the number of motor vehicle
accidents.88 With an average
societal cost of treating tibial shaft fractures of between 12,000 and
17,000 U.S. dollars, one can see that the burden on society is not
insignificant.54
potentially serve a number of purposes. They generally facilitate
communication between physicians and also assist in documentation and
research. They should also be of prognostic value for patients and
assist surgeons in planning the management of fractures. A number of
classification systems have been developed for both open and closed
tibial shaft fractures. However, the most comprehensive classification
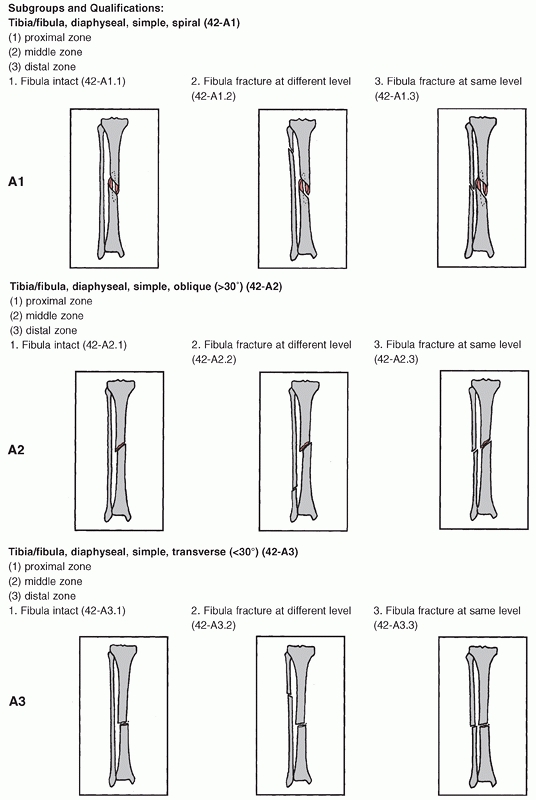
for tibial shaft fractures is the Orthopaedic Trauma Association (OTA)
classification, which is based on the classification of long bones put
forward by the AO group.180 This is a radiographic classification based on anteroposterior and lateral radiographs and is shown in Figure 55-2.
It consists of three fracture types subdivided into three groups, with
each group being further subdivided into three subgroups as detailed in
Table 55-1. Type A fractures are unifocal and
the subdivision into groups is based on the orientation of the fracture
line and the presence or absence of a fibula fracture. Type B fractures
are wedge fractures and are subdivided into spiral bending or
fragmented wedges. Type C fractures are more complex fractures and
include complex spiral fractures, comminuted fractures, and segmental
fractures.
 |
|
FIGURE 55-1
Age and gender distribution of tibial diaphyseal fractures. (Data from Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br 1995;77(3):417-421.) |
This classification is based on the degree of soft tissue injury and
progresses from Type I to Type III as the soft tissue injury increases.
Type III fractures are further subdivided into those with significant
periosteal stripping and soft tissue loss and the need for vascular
repair.113,115
Closed tibial shaft fractures can be classified according to the
Tscherne classification, which is based on the extent of soft tissue
damage, radiologic features of the fracture, rupture of major vessels,
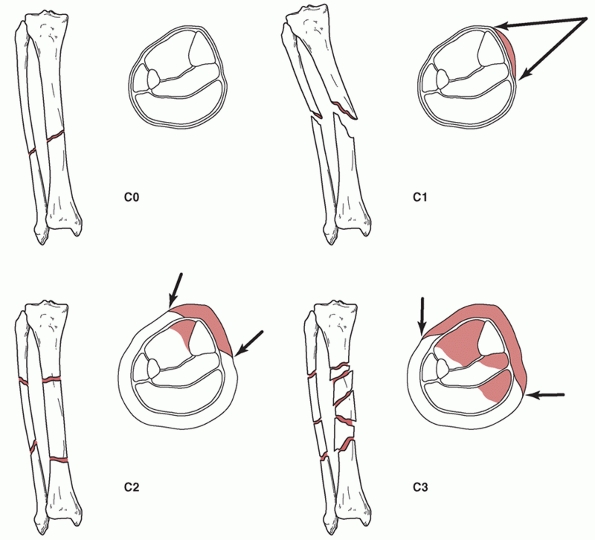
and the presence of closed degloving injuries or compartment syndrome.65,213 The four categories are shown in Figure 55-3.
There is progression in terms of soft tissue damage from the C0 class
of fracture, which has little or no soft tissue damage, to the C3
fracture, which is associated with extensive contusion or crushing of
the skin or muscle destruction.
 |
|
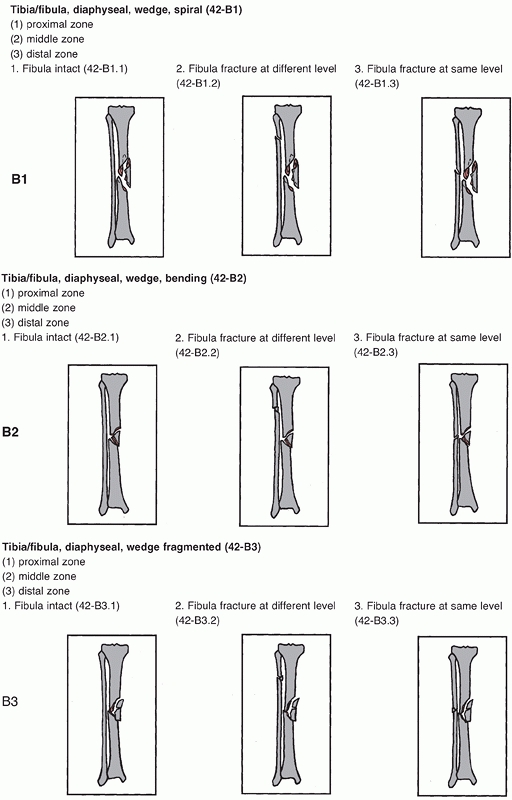
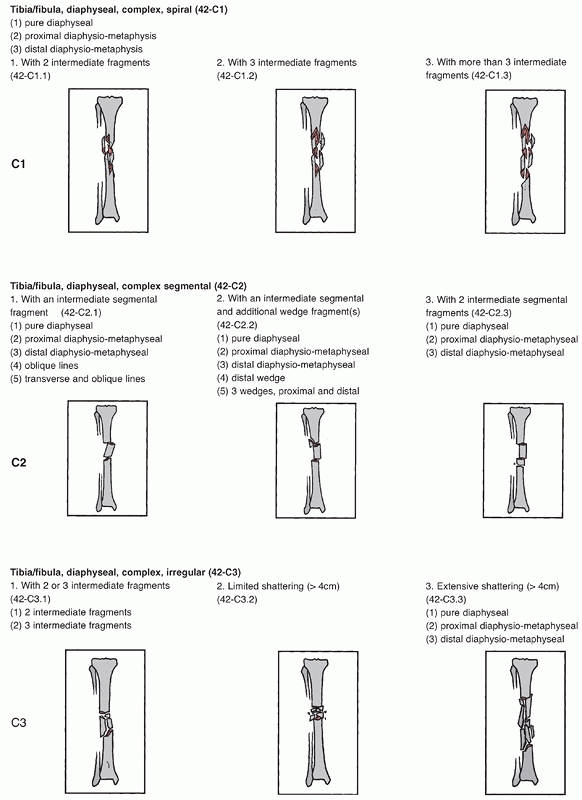
FIGURE 55-2
The Orthopaedic Trauma Association/AO classification of tibial diaphyseal fractures. For an explanation of the different types, groups, and subgroups see Table 55-1. (continues) |
 |
|
FIGURE 55-2 (continued)
|
 |
|
FIGURE 55-2 (continued)
|
|
TABLE 55-1 Orthopaedic Trauma Association AO Classification of Tibia Diaphyseal Fractures
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
assessed 200 patients with isolated lower extremity fractures. They
wanted to determine if there was a correlation between the severity of
AO/OTA classified injuries and functional performance, health status,
and scores of functional impairment.265
They found that while overall functional impairment was higher at 6
months in those patients who had a C type injury, there was variability
in this correlation, with the C type injuries being worse than those
patients with a B type injury but not necessarily worse than those
patients with an A type injury. They suggest that, “This classification
may not be a good predictor of 6- and 12-month functional performance
and impairment for patients with isolated unilateral lower extremity
fractures.”265
|
TABLE 55-2 Gustilo Classification of Open Fractures
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
also carried out a prospective study to investigate the predictive
value of classifications of diaphyseal fractures of the tibia. When
assessing the AO/OTA type classification, they found that classifying
the fractures into types A, B, and C potentially helped with an
indication of ability to undertake full weight-bearing but was not
predictive of other outcome measures, although the Tscherne grading
system did potentially help predict time to union.99 With respect to the Gustilo classification, Gaston et al.99
found that the presence of an open wound did predict time to union as
well as potentially predicting the incidence of nonunion, malunion, and
infection but was not significantly predictive of functional outcome.
They found that the strongest correlation with functional outcome was
associated with the Tscherne classification of closed fractures.99
This classification was predictive of the time to return to prolonged
walking and running as well as the patient’s time to return to walking
over difficult ground, jumping, climbing ladders, and returning to
normal sporting activities. After evaluating all of these
classification systems, however, they suggested that, “The
interpretation of the radiographs by an experienced surgeon is at least
as prognostic as any classification system in current use.”99
prognosis and also for the management of patients is that in many cases
the classification is used before any validation of the classification
system is carried out, and all of the above studies were undertaken
after the classification systems were in widespread use by orthopaedic
surgeons. As has been illustrated, subsequent reliability studies are
often conducted, which suggests varying degrees of reliability of the
fracture classification system.16,113,265
Classification systems that are put into place without significant
validity and reliability testing prior to their use may have potential
serious consequences. For example, an aggressive treatment may be given
to a patient that has been incorrectly classified into a more severe
fracture category.16 Conversely,
an incorrect classification into a less severe category may lead to treatment that is not aggressive enough.
 |
|
FIGURE 55-3
The Tscherne classification of closed fractures: C0, simple fracture configuration with little or no soft tissue injury; C1, superficial abrasion, mild to moderately severe fracture configuration; C2, deep contamination with local skin or muscle contusion, moderately severe fracture configuration; C3, extensive contusion or crushing of skin or destruction of muscle, severe fracture. |
development should be adhered to when classifications are being
proposed. Indeed, Audige et al.16
have suggested a three-phase protocol for the development of
classifications. This is based on clinical epidemiologic methodology
that a classification be valid, reliable, and accurate. Validity refers
to the classification measuring what it intends to measure and takes
many forms: face validity, content validity, and construct validity, to
name a few. Reliability refers to how the classification performs on
repeated measures and is related to both inter- and intrarater
repeatability. Lastly, the classification should be accurate. The
classification should represent the correct fracture morphology or
agree with a criterion standard. In constructing a classification, all
of these need to be taken into account and the algorithm proposed by
Audige et al.15 is as follows: Phase
one is development or revision of classification systems with clinical
experts. Phase two is a pragmatic multicenter agreement study in
clinical practice.15 Phase three is a prospective clinical observational study.15
the mechanisms of injury can be grouped into those of high-energy and
low-energy. It is important to make this distinction because with
increasing energy of injury, there is an associated increase in the
number of both bone and soft tissue complications. High-energy injuries
are typically caused by motor vehicle accidents, falls from height,
direct blows, and gunshots, both civilian and military. Lower-energy
injuries are usually caused by sporting injuries, falls from standing
height, and twisting injuries. They may also be associated with
pathologic conditions of bone.
undertook an in depth study into the epidemiology of tibial shaft
fractures. They reviewed 523 tibial diaphyseal fractures excluding
those extra-articular fractures within 5 cm of the proximal or distal
end of the bone. Table 55-3 shows the
distribution of the causes of tibial shaft fractures in their series
with the concomitant OTA classification of each. Four hundred (76.5%)
of these fractures were closed and 123 (23.5%) were open. In a further
review of 1106 tibial shaft fractures, 849 (76.8%) were closed injuries
and 247 (22.3%) were open.76
with motorcycle accidents. Unfortunately, those tibial fractures
associated with motorcycles tend to be significantly more severe with a
higher rate of type III open injuries and even amputation (Fig. 55-4).65,303 This higher-injury pattern is also noted to exist in pedestrians hit by an automobile, as shown in Table 55-4. Burgess et al.52
reviewed 130 tibia fractures in 102 adult patients, all of whom had
been pedestrians. They noted that the vast majority of these injuries
showed a high-energy injury pattern and that 65% were Gustilo type III
open injuries. They also observed a high prevalence (30%) of bilateral
injuries.52
|
TABLE 55-3 The Causes of Tibial Diaphyseal Fracture, Their Prevalence, and Their OTA Classification
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
vehicle accidents, it may be that such safety devices as airbags may
not entirely prevent lower extremity injuries. Burgess et al.51
conducted crash site and clinical investigations into 10 patients
involved in a motor vehicle accident with a deployed airbag. They
identified a significant number of lower-extremity injuries, as well as
abdominal and thoracic injuries in these patients including six tibial
fractures.51
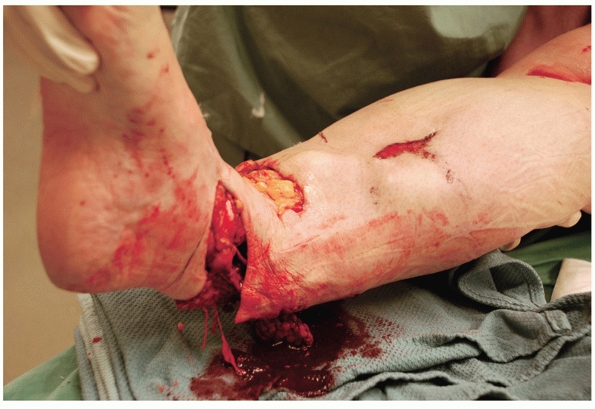 |
|
FIGURE 55-4
A motorcyclist sustained a Gustilo type IIIB fracture after being struck by oncoming traffic while turning a corner at low speed. This injury was completely circumferential with gross disruption of all of the medial sided tendons and the Achilles tendon. The zone of injury extended from the foot to the proximal third of the tibia with degloved skin, devitalized muscle, and a complete loss of bone from the midtibia to 1 cm above the plafond. This injury eventually resulted in a below knee amputation. |
|
TABLE
55-4 Descriptive Indices of Fractures Occurring in Motor Vehicle Accidents Comparing Pedestrians, Vehicle Occupants, and Motorcyclists |
||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||
direct blows or assaults accounted for 4.5% of all tibial fractures and
occurred in younger patients. These fractures have either a highor
low-energy morphology. Levy et al.172
reviewed 47 patients who had been assaulted by a baseball bat. They
found a significant number of complications including a ninefold higher
prevalence of compartment syndrome compared to the rest of their
population with tibial fractures.172
the tibia. Data from firearm-related deaths suggests that the incidence
of firearm use is higher in high and upper middle income countries in
the Americas as compared to those in Europe, Asia, and Oceania with the
highest incidence being in the United States.160
These can be both civilian and military, although military injuries can
also be blast type injuries from explosives such as land mines.65
Low-velocity injuries associated with a muzzle velocity of less than
2000 feet per second are more commonly seen in civilian practice. These
generally result in less soft tissue damage than is seen with
higher-velocity arms.284 Some authorities argue that all high-velocity injuries should be classified as Gustilo type III injuries.284 With regard to civilian arms, injuries Leffers and Chandler167
found that 54.3% of tibial diaphyseal fractures were comminuted OTA C
type injuries, 20% were type A, 8.6% were type B, and 17.1% only
involved one cortex.65
have identified soccer-related injuries as the largest contributor to
sports-related tibial shaft fractures, accounting for 80.1% of
sports-related tibial diaphyseal fractures.254 These fractures were typically lower-energy and
were usually Tscherne grade 0 or 1 injuries occurring in young men.254
These fractures can however have a higher-energy injury pattern when
they occur from a direct blow from a soccer tackle. The other commonly
reported sports-related tibial fracture is that seen with skiing.
Grutter et al.109 series reported a
high incidence of skiing-related tibial fractures, many of which were
torsional lower-energy injuries. These injuries were more common in the
early 1980s as compared to the late 1980s, and this is attributed to a
change in bindings with the newer bindings allowing for release of the
ski more readily.109
examination when assessing someone who is suspected of having a tibial
shaft fracture. Details surrounding the mechanism of injury, the timing
of the injury, any previous pain syndromes, or constitutional symptoms
should also be elicited. It is important to establish if the mechanism
of injury is high- or low-energy, as high-energy injuries may also have
significant soft tissue injuries associated with them. Tibial shaft
fractures caused by low-energy injuries have the potential to be
associated with pathologic or osteopenic conditions. It is important to
ask questions regarding the location and severity of pain in the entire
lower leg including the hip, knee, and ankle. Careful assessment of the
patient for any associated injuries is important. On physical
examination, one usually finds pain at the site of the fracture with
associated soft tissue swelling.
injury, there may be significant soft tissue damage and a thorough
examination of the skin should be done. Attention should be paid to
signs of soft tissue damage, including blistering or open wounds.
High-energy injuries are often open and may be associated with
compartment syndrome, especially in younger adult males.192
There may be deformity present, and the status of the neurovascular
structures throughout the entire leg should be assessed in detail. The
vascular and neurologic status should be recorded in the patient’s
chart at the time of examination. A feeling of fluctuance at the
fracture site may indicate an internal degloving injury. If there are
any open injuries identified, a record of their size and extent should
be made. If there is an open wound, any obvious debris at the site can
be removed and the wound gently irrigated with normal saline. It may be
prudent to take a digital picture of the wound for later documentation,
and the wound should be covered with a sterile dressing that should not
be disturbed until the patient reaches the operating room. Further
information about the initial management of open fractures can be
obtained in Chapter 10.
leg should be realigned and splinted with an above knee posterior back
slab or splint, avoiding overtightening with any circumferential
dressings, under appropriate analgesia. If there is bone protruding
from the skin, it is reasonable to reduce it and document the
protrusion. It is also imperative to avoid desiccation of the bone. If
the bone cannot be reduced, it should be covered with moist saline
gauze dressings and appropriate splinting should be undertaken. It is
also important to obtain a thorough social history of the patient to
include occupation and social living arrangements, as these may have a
bearing on the posttreatment rehabilitation protocols.
above should be looked for and consideration should be given to
monitoring the patient for compartment syndrome with either continuous
or intermittent pressure measurements.189,191
In the unconscious patient, a high index of suspicion of compartment
syndrome should be maintained for at least 24 to 48 hours after the
fracture, and compartment monitoring should be used where possible.
Further information about the diagnosis of compartment syndrome will be
found in Chapter 27. Any relevant information
about the mechanism of injury should be obtained. If the injury has
occurred as a result of a motor vehicle accident or is associated with
polytrauma, an advanced trauma life support (ATLS) protocol, as
outlined in the ATLS manual, should be completed.18 A thorough assessment of any other injuries should be undertaken.
lateral, as well as full-length tibial studies should be obtained to
assess the bone as well as the soft tissues. It is essential that the
knee and ankle are included in these radiographs. There are particular
associations within some fracture patterns. For example, distal third
tibial shaft fractures are associated with intra-articular fracture
lines and extensions.44 This may
also hold true with proximal tibial diametaphyseal fractures. If there
is any suspicion that these fractures extend into the adjacent joint,
then orthogonal views of the knee and ankle should be obtained. Further
imaging of any intra-articular extension can be done using computed
tomography (CT) examination, although CT examination is generally not
indicated for purely diaphyseal fractures. In assessing the plain
radiographs, it is essential to note the location and extent of the
fracture and whether there is significant comminution within the
fracture. It is also important to examine whether there is extension
proximally into the proximal tibial articulation or distally into the
ankle joint and to see if there is a concomitant fracture of the fibula
or dislocation of either the proximal or distal tibio-fibular
articulation.263 Assessment of the
canal size to determine the suitability for intramedullary nailing can
also be done. The overall quality of the bone should be assessed with
particular reference to generalized osteopenia, any other pathologic
processes, such as tumors or infection, or the presence of previous
fractures. The soft tissues on the plain radiographs should also be
reviewed. If there is any air, this may indicate an open injury or
possibly the presence of gas producing infective organisms, depending
on the patient’s presentation.
necessary in tibial diaphyseal fractures with or without an
intra-articular extension. However, there have been reported cases of
both knee ligament injury and meniscal damage in association with
tibial shaft fractures, and therefore an MRI examination of the knee
may be warranted if there is a clinical suspicion of knee injury.133
MRI and bone scan imaging may also be necessary if it is required to
exclude a stress fracture in the presence of pain but with no obvious
evidence of a fracture on plain radiographs. If there is evidence of
any vascular compromise, referral to a vascular surgeon is necessary
and arteriography may be required.
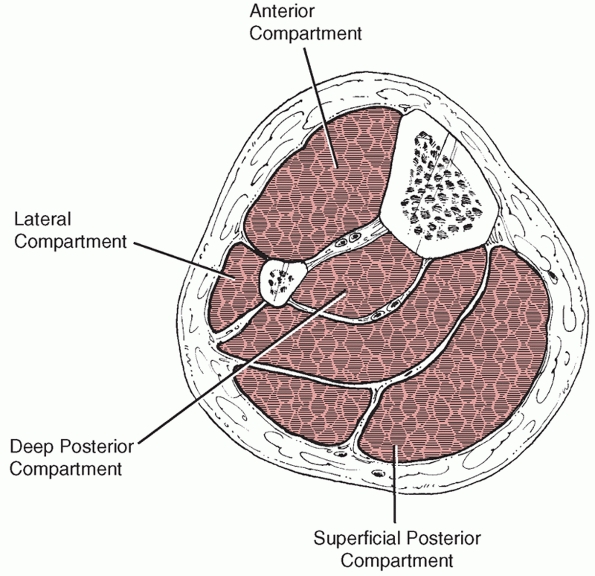
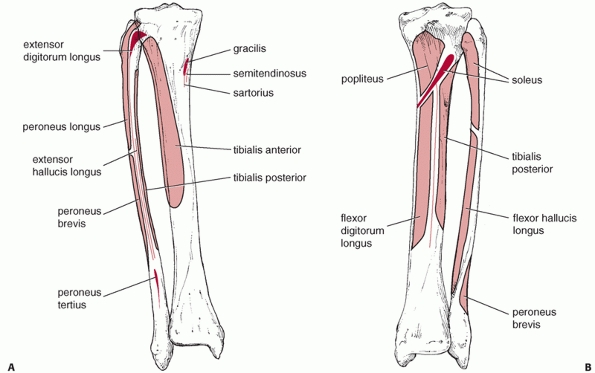
anterior and the extensor digitorum longus and extensor hallucis muscles (Fig. 55-5). These originate from the proximal lateral aspect of the tibia as well as from the anterior border of the fibula, as shown in Figure 55-6.
Within this compartment lies the anterior neurovascular bundle
including the deep peroneal nerve and anterior tibial artery. The
lateral compartment is composed of peroneus longus and brevis muscles
which arise from the lateral and posterior borders of the fibula.
Within this compartment, just posterior to the intermuscular septal
plane, lies the superficial peroneal nerve, which exits from the deep
fascia about 10 to 15 cm proximal to the ankle joint. The superficial
peroneal nerve is at risk when using minimally invasive approaches to
the distal tibia and also when releasing the anterior and lateral
compartments during fasciotomy. The deep peroneal nerve may be at risk
with placement of cross screws in the proximal tibia.
 |
|
FIGURE 55-5 The four compartments of the leg.
|
 |
|
FIGURE 55-6 The (A) anterior and (B) posterior anatomy of the leg, illustrating the origins and insertions of the muscles.
|
posterior compartment and the deep posterior compartment. Between these
two compartments run the posterior neurovascular structures, including
the peroneal and posterior tibial arteries and the posterior tibial
nerve. The superficial posterior compartment contains the
gastrocnemius, soleus, and plantaris muscles. The deep posterior
compartment contains the tibialis posterior, flexor digitorum longus,
and flexor hallucis longus muscles.
longitudinal. A longitudinal incision is made 1 cm lateral to the crest
of the tibia. The entire anterior aspect of the tibia can be visualized
through this incision as well as the anterolateral or anteromedial
aspects of the tibia. Minimally invasive approaches to the tibia can
also be used. A direct medial approach to the distal tibia just
overlying the medial malleolus will permit medial minimally invasive
plate osteosynthesis of the distal tibia. This incision can be placed
medially, anteromedially or posteromedially, and it may also curve
anteriorly or posteriorly at the distal end of the medial malleolus.
For minimally invasive plate osteosynthesis of the proximal tibia, an
anterolateral approach is used and will be described later in the
chapter. Traditionally, a posterolateral approach to the tibia has been
used for bone grafting when the soft tissues overlying the anterior
aspect of the tibia are not of good quality, due either to the presence
of open wounds or
previous surgical exposures.120
This approach is undertaken along the posterior border of the fibula
and over the posterior aspect of the interosseous septum. The interval
is developed between soleus and flexor hallucis longus and the peroneal
muscles. Tibialis posterior is dissected from its origin on the
interosseous membrane and retracted medially.120 The neurovascular structures are protected by the muscles of the deep posterior compartment during the dissection.
presence of compromised soft tissues, multiple scars or recurrent
infection, with or without a draining sinus, we have been using an
anterior approach with excision of these soft tissues and free flap
soft tissue reconstruction techniques. This technique will be discussed
further in the section dealing with surgical techniques in tibial
nonunion.
tibial shaft fractures, and the goal of each method is to stabilize the
bone in an acceptable alignment. The first method is nonoperative
management, which may include long leg casting, patellar tendon bearing
casting, or functional bracing. The second method is plate fixation,
which includes compression plating and bridge plating. Both traditional
nonlocking and locking screws may be used and both open and minimally
invasive submuscular approaches may be employed. The third technique is
intramedullary nailing, undertaken with or without reaming, and either
statically or dynamically locking the intramedullary nail. The fourth
method of treating tibial fractures is external fixation using either a
uniplanar, multiplanar, or circular tensioned fine wire fixator. All of
these techniques have their advocates and critics, and are associated
with specific advantages and disadvantages that will be discussed in
this chapter.
management of fractures of the tibial shaft, 577 surgeons were surveyed
with a good response rate of 77%.26
For closed fractures of the tibial shaft, the overwhelming majority of
surgeons preferred the use of an intramedullary nail for both
low-energy (96.3 %) and high-energy (96%) fractures of the tibia as
shown in Table 55-5. The majority (80.4%) also
used intramedullary nailing for closed fractures of the tibia that were
associated with compartment syndrome. Plate fixation was the least
popular technique of choice with 3.2% using plate fixation in closed
low-energy tibial shaft fractures, 2.1% in high-energy closed tibial
shaft fractures, and 7.4% in closed tibial shaft fractures associated
with compartment syndrome. It was also found that the type of nailing
technique varied between surgeons with surgeons preferring to use a
reamed intramedullary nail for low-energy fractures. This technique was
less popular for the treatment of high-energy fractures and in those
fractures associated with a compartment syndrome. In those cases,
surgeons more commonly chose to use a nonreamed nail. In open
fractures, there was a decline in the number of surgeons who opted to
use an intramedullary nail as the severity of the open fracture
increased from a type I to a type III with 95.5% of surgeons choosing
to use an intramedullary nail for type I open fractures and 68.4% and
48.4% choosing to use an intramedullary nail in type IIIA and IIIB
fractures respectively. Few surgeons used plate fixation for open
fractures with preference rates ranging from 0.8% to 1.1%. Most
surgeons (50.5%) were more likely to use external fixation for Gustilo
IIIB open fractures. Interestingly, the geographic location of the
surgeons seemed to influence their choice of implant, with North
American surgeons being significantly more likely to insert reamed
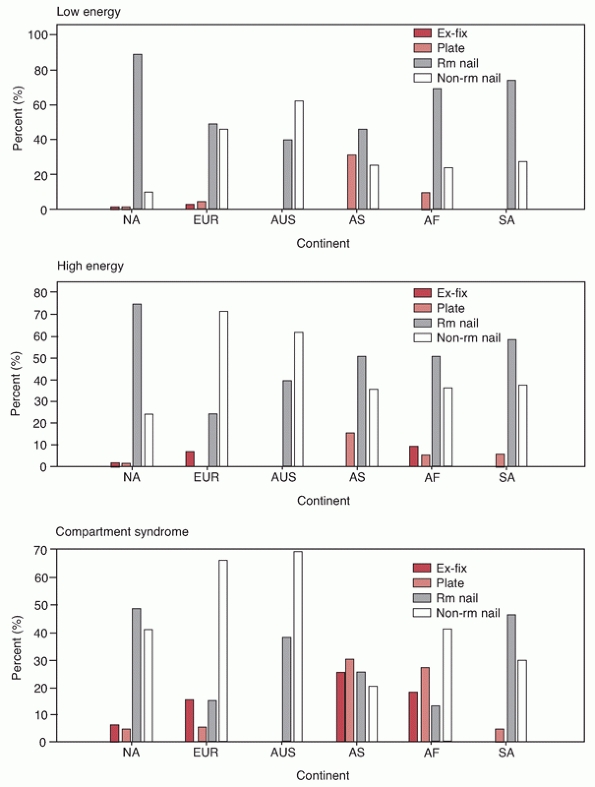
nails for both open and closed tibial shaft fractures (Fig. 55-7).26
|
TABLE 55-5 Surgeons’ Preferences for the Operative Treatment of Fractures of the Tibial Shaft
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
tibial shaft fractures in the 1960s and 1970s. There was considerable
debate about what constituted an unacceptable level of displacement
or
malalignment. Many surgeons accepted <1 cm of shortening, 5 degrees
of valgus but no varus malalignment, 10 degrees of malalignment in the
anteroposterior plane, and 5 to 10 degrees of external rotation but no
internal rotation deformity. There is no good evidence about what
degree of malalignment or shortening is associated with functional
impairment, and it seems likely that the definitions of acceptable
malalignment arose because of the significant difficulty in achieving
an anatomic reduction with nonoperative treatment. With increasing use
of intramedullary nailing, fewer surgeons now use nonoperative
treatment for tibial diaphyseal fractures and we only advocate its use
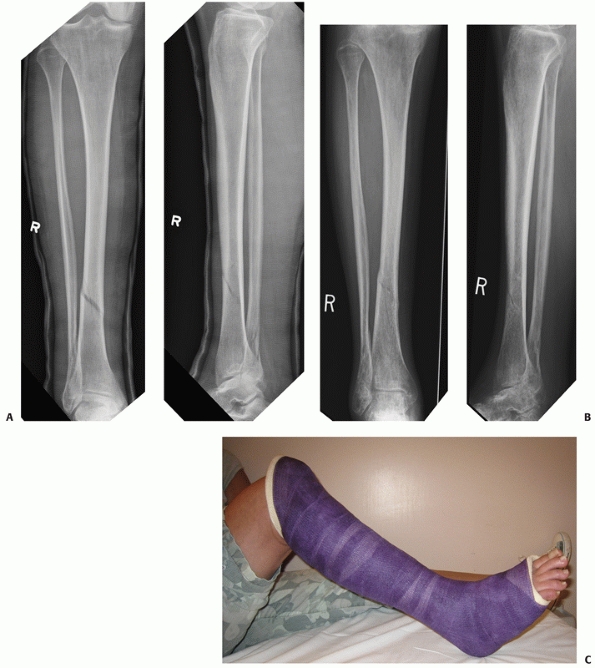
in low-energy undisplaced fractures as shown in Figure 55-8. In 1964, Nicoll208
described a series of 705 cases of tibial shaft fractures treated
nonoperatively in a long leg cast (level of evidence: 4). He documented
a union time of approximately 15.9 weeks and a malunion rate of 8.6%.
Importantly, however, he described a 25% prevalence of residual joint
stiffness that increased to 70% in those who had a nonunion associated
with an open fracture. He also observed a 15.3% prevalence of infection
and a 60% prevalence of delayed union or nonunion. In the entire
series, about 5% of fractures had not united by 1 year.208 During the same period, Dehne79
found that long leg casting resulted in shortening of at least half an
inch in approximately one third of patients in his series. Other
authors have described the prevalence of malunion as anywhere from
approximately 9% to 50% with the use of a long leg cast with joint
stiffness ranging from 7% to 42%.162,165,268,282 Unfortunately, these retrospective, historical studies do not make the distinction between displaced and undisplaced fractures.
 |
|
FIGURE 55-7 Surgeon preference by continent for implants for closed low-energy fractures (top panel), closed high-energy fractures (middle panel), and closed fractures associated with compartment syndrome (bottom panel).
AF, Africa; AS, Asia; AUS, Australia; EUR, Europe; Ex-Fix, external fixation; NA, North America; Rm Nail, nailing with reaming; Non-rm Nail, nailing without reaming; SA, South America. (Redrawn from Bhandari M, Guyatt GH, Swiontkowski MF, et al. Surgeons’ preferences for the operative treatment of fractures of the tibial shaft. An international survey. J Bone Joint Surg Am 2001;83-A(11):1746-1752.) |
 |
|
FIGURE 55-8 A. An OTA A1.3 undisplaced stable distal tibial diaphyseal fracture that was treated nonoperatively. B. Weight bearing was started at 4 weeks and was full at 6 weeks. Union was uneventful. C. A long leg cast was used for 4 weeks followed by a patellar-tendon bearing cast as shown here.
|
nailing gives better results than cast management. In a randomized
trial of displaced tibial shaft fractures, Hooper et al.130
randomized 62 patients to receive either an intramedullary nail (29
patients) or cast treatment (33 patients) (level of evidence: 1). They
found a significantly decreased time to union, decreased malunion rate,
and less time off work in the intramedullary nail group and concluded
that displaced tibial fractures should be treated with an
intramedullary nail.130 Karladani et al.144
enrolled 53 patients with displaced closed or Gustilo type 1 open
tibial shaft fractures into a trial and randomized 27 patients to
treatment with an intramedullary nail and 26 patients to treatment with
a plaster cast (level of evidence: 1). Fourteen fractures in the
plaster cast group showed redisplacement after reduction and required
further treatment. These 14 patients were treated with either cerclage
wires or screws and then continued cast treatment. The union time was
25 weeks for the cast managed group as compared to 19 weeks for the
intramedullary nail group.144
Using the Nottingham health profile as an outcome measure, scores of
physical ability and work ability were better in the intramedullary
nail group than in the cast managed group at 3 months. In those treated
with a cast, delayed union and malunion were more common.144 Bone et al.,37
in a retrospective cohort study of displaced tibial shaft fractures,
treated 47 patients with a closed intramedullary nail and 52 patients
with closed reduction and casting (level of evidence: 3). They found a
shorter time to union and improved functional scores in the
intramedullary nail group using both the Short Form-36 (SF-36) as well
as the Iowa Knee Evaluation and Ankle Evaluation rating system after
approximately 4 years of follow-up.37
identified 145 fractures treated with a long leg cast from the combined
literature. They reported a 13.1% incidence of delayed union, a 4.1%
incidence of nonunion, and a 31.7% incidence of malunion.62 These rates were higher than those seen with either plate or intramedullary nail fixation.62
method of managing tibial shaft fractures nonoperatively. Functional
bracing, as introduced by Sarmiento, is applied after the soft tissue
swelling has resolved. In 1995, Sarmiento et al.247
reviewed 1000 closed tibial diaphyseal fractures that were treated with
prefabricated functional braces (level of evidence: 4). They found that
the mean final shortening was 4.28 mm, which compared to an initial
mean shortening of 4.25 mm. They suggested that final shortening does
not increase beyond the initial observed shortening.247
They also observed that 90% of their patients had final angular
deformity in any plane ≤6 degrees. However, they found that the
presence of an intact fibula was more likely to result in an angulatory
deformity and suggested that this pattern may not be appropriate for
functional bracing. They reported an overall incidence of nonunion of
1.1%.247
outcome scores to assess patients in the study by Sarmiento. The good
results obtained by Sarmiento have not been found by other authors. Den
Outer et al.81 documented a 40%
prevalence of nonunion and noted that one third of patients took longer
than 20 weeks to unite (level of evidence: 4). Alho et al.5
suggested that there were fewer complications with intramedullary
nailing which had a higher rate of excellent and good results as
compared to functional brace management (level of evidence: 4). In a
review of 103 tibial shaft fractures treated with functional bracing by
Digby et al.,83 11% had restricted ankle motion and 45% had reduced subtalar motion (level of evidence: 4).
significant attention to detail and frequent follow-up to check
fracture alignment. Interestingly, a recent economic analysis of
management strategies for closed and Type 1 open tibial shaft fractures
by Busse et al.54 suggests that
reamed intramedullary nailing is the treatment of choice and
economically has less of a financial burden to society while casting
alone resulted in significantly higher financial costs.
surgeons, it can be seen that intramedullary nailing can be considered
the implant of choice for tibial shaft fractures (Fig. 55-9).26 Indeed, Court-Brown19
reviewed 1106 tibial shaft fractures managed by reamed intramedullary
nailing, this being the largest series to date (level of evidence: 4).
The overall infection rate following intramedullary nailing of closed
tibial shaft fractures was 1.9% and the aseptic nonunion rate was 4.4%.19 For open fractures, infection rates increased from 6.9% for Gustilo type I injuries to 16.9% for Gustilo type IIIB injuries.66
The nonunion rates seen in those with open fractures also increased
from 12.1% for Gustilo type I injuries to 49.2% in patients with
Gustilo type IIIB injuries.66 There
is, however, a continued debate regarding the use of reaming in
intramedullary nailing. Indeed, as seen from the international survey
of surgical preferences, there was significant decline in the use of
reamed intramedullary nailing as the severity of the open fractures
increased.26
undertaken to assess the role of reaming in the biology of bone
following fracture and osteotomy. In a standardized sheep tibia
fracture model, Schemitsch et al.248
found that reaming nailing affected bone blood flow more than
nonreaming nailing and that revascularization of the bone took about 6
weeks in the unreamed group compared with 12 weeks in the reamed group.
However, they also showed that in the same fracture model, reaming had
no deleterious effect on the strength of the callus or union of the
fracture.249 Using a canine fracture model, Klein et al.150
found that reaming decreased the cortical blood flow to two thirds of
the area of the cortex, and in some places this was almost full
thickness. In another canine fracture model, Hupel et al.136
found that there was no significant difference between bone formation
and mineral apposition in those tibias that were treated with no
reaming, limited reaming, or standard reaming, although the least
damage to cortical bone was caused by limited reaming. In this model,
the unreamed nail was inserted with a tight fit. Hupel134
also examined the effects of both a looseand tight-fitting unreamed
nail on cortical vascularity and found that a loose-fitting nail spared
cortical perfusion when compared to a tight fitting nail. At 11 weeks,
the perfusion of the cortex had increased in both groups, although the
perfusion was higher in the loose fitting nail group. Hupel et al.135
also found that reaming increased the vascularity and perfusion of the
surrounding muscles, and they argued that reaming may increase the
extraperiosteal blood supply. The basic science work carried out by
Hupel et al.134,135,136 and Schemitsch et al.248,249
suggests that the ideal implant is a small diameter nail with limited
reaming because it results in better cortical vascularity, improved
cortical porosity, the same strength of union, and the best stimulation
of the extraosseous circulation.
effects on the bone but also systemic effects on the patient. There is
an argument that reaming of the tibia may potentiate the risk of fat
embolism and pulmonary complications, especially if more than one long
bone is reamed as in multiple fractures.65,103,222 However, this argument is more relevant to femoral reaming, and experimental work by Christie et al.58
has shown that most clinical problems associated with fat embolism were
confined to those who received femoral reaming as opposed to tibial
reaming, even though transesophageal echocardiography identified emboli
in 92% of reaming procedures.
 |
|
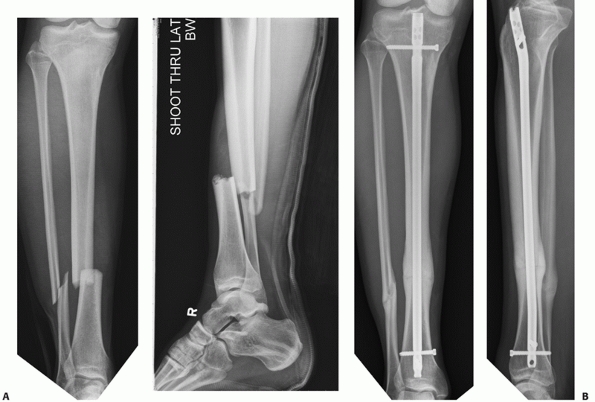
FIGURE 55-9 A. An unstable OTA A3.3 fracture. B. It was treated successfully with a locked reamed intramedullary nail.
|
undertook a meta-analysis of reamed versus nonreamed intramedullary
nailing and its use in lower extremity long bone fractures and found
from the published randomized trials that were included in the
meta-analysis, the pooled estimate of the effect of reaming on nonunion
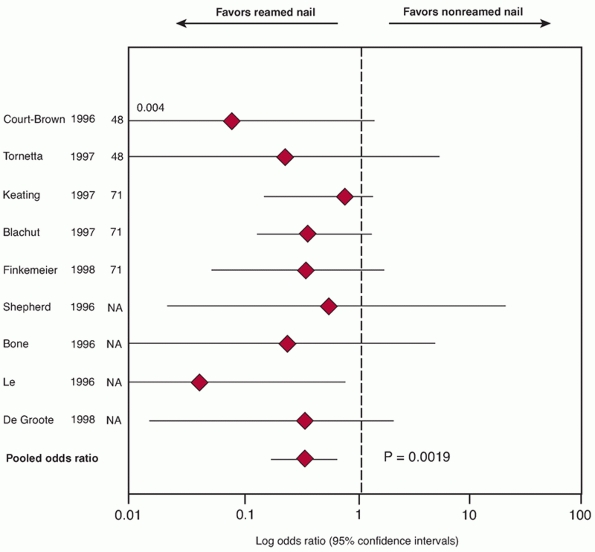
was an odds ratio of 0.32 (95% CI 0.17 to 0.67; p = 0.0019) (level of evidence: 1) (Fig. 55-10).
This suggests that reamed nailing would potentially eliminate two
thirds of nonunions that occur with nonreamed nailing and that,
depending on the baseline risk of patients to develop nonunion, the
number of patients that required to be treated with reamed nailing to
prevent one nonunion could potentially be anywhere from 5 to 25. The
study also found a higher rate of implant failure with nonreamed nails
than with reamed nails.28 One caveat
to consider when extrapolating this data to tibial shaft fractures is
that this meta-analysis also included fractures of the femoral shaft. A
second meta-analysis conducted by Coles and Gross62
also suggests that reamed intramedullary tibial nailing tends to show a
trend to a lower incidence of nonunion (8%) as compared to 16.7% with
unreamed nailing. A third systematic review and meta-analysis by
Forster et al.94 looked at
prospective randomized studies comparing reaming and nonreaming of
tibial nailing in adults (level of evidence: 1). They identified seven
studies that were eligible for inclusion, with 291 tibial shaft
fractures being combined from the included studies. They also
identified an increased rate of nonunion with nonreamed intramedullary
nails as compared to reamed intramedullary nails.94 Their pooled estimate of effect for nonunion was an odds ratio of 2.83 (95% CI 1.16 to 6.88; p = 0.02) for nonreamed nails.94
That is to say, a nonreamed intramedullary nail would increase the
chances of nonunion by approximately threefold. They also found an
increased incidence of implant failure with the use of unreamed nails
compared to reamed nails, but they found no differences in malunion or
the risk of compartment syndrome using either technique.94 This meta-analysis was limited by study heterogeneity and small sample sizes.
randomized 78 patients with tibial shaft fractures to treatment with
external fixation or a locked intramedullary nail (level of evidence:
1). They found that the time to radiographic union was not
significantly different between the groups, but there was a higher
reoperation rate in the external fixation group. They found that after
1 year there were no significant differences in knee motion, ankle
motion, or fracture site pain.48
intramedullary nails in tibial fractures (SPRINT) was a multicenter,
randomized trial comparing reamed versus nonreamed intramedullary
tibial nail insertion in 29 clinical sites in Canada, the United
States, and the Netherlands.30
SPRINT aimed to enroll 1200 skeletally mature patients with open
(Gustilo types I to IIIB) or closed fractures (Tscherne Types 0 to 3)
of the tibial shaft amenable to surgical treatment with an
intramedullary nail.30 Patients,
outcome assessors, and data analysts were blinded to treatment
allocation. Perioperative care was standardized, and reoperations
before
6 months were proscribed. Patients were assessed at discharge, 2 weeks
postdischarge, and at 6 weeks, 3, 6, 9, and 12 months postsurgery. A
committee blinded to allocation adjudicated all outcomes. The primary
composite outcome of reoperation included bone grafts, implant
exchanges, and dynamizations, as well as autodynamization, in patients
with fracture gaps less than 1cm postintramedullary nail insertion.
Outcome events also included operations for infection and fasciotomies
irrespective of the fracture gap.
 |
|
FIGURE 55-10
Pooled odds ratio (with 95% CI) for nonunion rates from nine studies. The pooled odds ratio for the studies is 0.32 (95%CI, 0.17 to 0.67) and favors reamed intramedullary nails. (Redrawn from Bhandari M, Guyatt GH, Tong D, et al. Reamed versus nonreamed intramedullary nailing of lower extremity long bone fractures: a systematic overview and meta-analysis. J Orthop Trauma 2000;14(1):2-9.) |
(93%). Reoperation rates in SPRINT were far lower than previously
reported with 57 (4.6%) requiring implant exchange or bone grafting for
nonunion in comparison to 12.4% in 5 prior randomized trials. Analysis
showed that 105 (16.9%) of the reamed group and 114 (18.9%) of the
nonreamed group experienced a primary outcome event (RR = 0.90, 95% CI
0.71 to 1.15). The events appeared to differ in closed and open tibial
fractures (interaction p = 0.01). In
patients with closed fractures, 45 of 416 (11%) in the reamed group and
68 of 410 (17%) in the unreamed group experienced a primary event (RR =
0.65, 95% CI 0.46 to 0.93; p = 0.03). This
difference was largely due to differential rates of dynamization. In
patients with open fractures, 60 of 206 (29%) in the reamed group and
46 of 194 (24%) in the unreamed group experienced a primary event (RR =
1.27, 95% CI:0.91 to 1.78; p = 0.16).
nail insertion in patients with closed tibial shaft fractures largely
due to dynamization. Optimization of perioperative care and delaying
reoperation for nonunion for at least 6 months may substantially
decrease the need for reoperation in tibial fracture patients.
nailing, of proximal tibial fractures has been associated with some
difficulties.24,210,273
This has been due to many factors such as the proximity of the fracture
line to the joint, maintaining and obtaining a reduction while nailing,
and difficulties with the passage of an intramedullary nail through the
proximal fracture
fragment.
This has resulted in many surgeons turning to plate fixation of
fractures that are either too proximal or too distal to nail easily.
However, it was rarely used in the early 1900s because of the
significant risk of sepsis and lack of good anaesthesia. It was not
until the introduction of antibiotics, good anaesthesia, and modern
surgical techniques that plating tibial shaft fractures became more
feasible.65 This was in the middle of the twentieth century. In 1976, Ruedi, Webb, and Allgower243
published a large series of 418 acute fractures treated with the
AO/ASIF dynamic compression plate. They reported very good or good
functional results in 98.1% of closed fractures and 88.4% of open
fractures, with a nonunion rate of 1%, an infection rate of 1% in their
closed tibial shaft fractures, a nonunion rate of 5.3%, and an
infection rate of 11.6% in their open tibial shaft fractures.243 In 1982, Christensen et al.57
also reviewed their series of 96 displaced fractures of the tibial
shaft that were treated by AO/ASIF plating techniques with rigid
internal fixation. In this series, 40% were open fractures and,
interestingly, the infection rate following surgery was 5.3% in closed
fractures and 0% in open fractures. They recorded that more than 90% of
their patients returned to work 6 months after the injury, and there
were no cases of nonunion or refracture at final review at 36 months.57 They suggested that rigid internal fixation facilitated secondary soft tissue reconstruction.
evaluated the treatment of displaced tibial shaft fractures by
comparing plating with nonoperative management. They found that there
were more complications in the plating group, but healing time and
anatomic results were better when compared to nonoperatively managed
patients.282 They suggested,
however, that open fractures were not suitable for plate fixation. They
found the most malalignment in the nonoperative group occurred at the
distal end of the tibia.282
rigid fixation with a dynamic compression plate to allow the bone to
heal by primary direct bone healing. This is much more easily
accomplished in simple fracture patterns where direct compression with
the plate can be obtained. However, this necessitates a significant
amount of soft tissue stripping of the fracture in addition to
periosteal stripping, both of which are avoided with the use of an
intramedullary nail for diaphyseal fractures. There is also concern
that full contact plates rigidly applied to the bone may result in some
bone resorption beneath the plate. This was initially thought to be the
result of stress shielding, but it is now thought to be due to
localized dysvascularity from plate compression of the periosteum.50,64,149,225,226,295 Because of this problem, plates were developed that had less contact with the bone.92,141,154
Since plating comminuted fractures with standard compression plating
techniques is technically challenging and results in significant
stripping of the soft tissues, biologic plating techniques were
developed whereby the plate spans the zone of injury with fixation
proximally and distally. To facilitate this indirect reduction
techniques including the use of fluoroscopy, bone distractors or
external fixators for reduction and ball-spike pushers and sharp bone
holding forceps have been developed.153,171
These changes in surgical technique have taken into account the amount
of soft tissue damage associated with open fracture surgery. Further
surgical changes include supraperiosteal plating techniques as well as
submuscular and minimally invasive plating techniques. Oh et al.214
have reported on the use of percutaneous plating techniques in unstable
tibial shaft fractures They found that of 24 unstable tibial fractures,
22 united without secondary surgery, and there was only one significant
angular malalignment with no infection being seen.
recently been applied to fractures of the proximal distal tibia and
have been used in conjunction with newer designs of locked plates.
Locked plating means that the screw head is locked to the plate by
either a threading mechanism or other type of device. This effectively
creates a fixed angle between the screw and the plate. There is a
significant risk of malunion in the treatment of proximal and distal
tibial diametaphyseal fractures with all treatment methods including
intramedullary nailing and nonoperative management. Recently, the use
of locked plate fixation utilizing minimally invasive techniques has
been put forward as one way of maintaining alignment in proximal and
distal tibial fractures.40,214
Locking the screw to the plate creates multiple fixed angle points of
fixation whose mode of failure differs from conventional plating.285
Conventional plate/screw constructs allow for some toggle at the screw
plate junction. Stability is provided by the compression of the plate
to the bone, the fixation of the screws into bone, as well as
compression at the fracture.285 The screws can fail sequentially, allowing the plate to come off the bone.
construct and the locked screws must fail together with the screws
pulling out of the bone at the same time.285
This type of construct has been known for a number of years to increase
the pull-out strength of the screws and indeed the Schuli nut was
initially developed to lock the screw to the plate and thus increase
pullout strength. It therefore represented an early form of low
contact, locked screw/plate fixation.151
This increase in pullout strength is desirable in weakened bone such as
may be encountered with osteopenia or osteoporosis. Also, the fixed
angle of the screws into the metaphyseal portion of the bone provides
bicolumnar support, transferring load from both sides of the proximal
or distal tibia to the shaft. While intramedullary nailing is advocated
as the treatment of choice for diaphyseal fractures, not all proximal
or distal fractures permit this technique, and locked plating may be a
useful technique for these fractures.
intra-articular proximal tibial fractures as well as intra-articular
distal tibial fractures, their use in OTA 41-A fractures is promising
as they not only help to provide bicolumnar support to the joint, but
they can be placed using minimally invasive techniques and they can
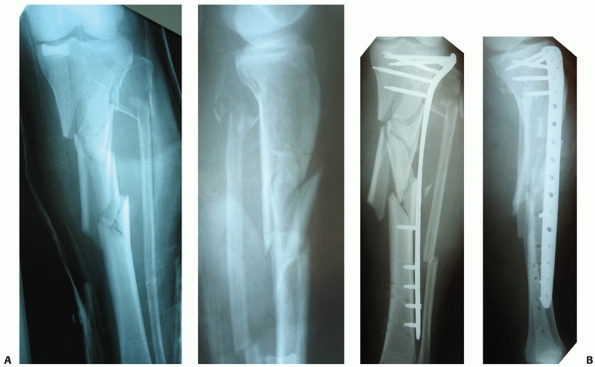
span large areas of comminution as shown in Figure 55-11. Ricci et al.236
have documented a series of 38 patients with proximal tibia fractures,
18 of which were OTA type A fractures. They were treated using a
minimally invasive approach.236 All of the fractures united in satisfactory alignment.
published the results of a series of 37 proximal tibial shaft fractures
treated with an intramedullary nail (level of evidence: 4). The average
distance from the articular surface was 67.8 mm. They found acceptable
alignment in 34 out of 37 (91.9%) of their fractures, with three
patients showing angular
malalignment.
They suggested that multiple techniques were needed to obtain and
maintain reduction. These techniques included the use of unicortical
plates as well as a femoral distractor.210
In a similar prospective study of 45 patients with proximal third
tibial shaft fractures treated with an intramedullary nail, Vidyadhara
and Sharath283 reported seven
(15.6%) cases of malunion. Bonegrafting procedures were done in three
cases to obtain union. They reported good functional outcomes in 96% of
patients using the lower extremity functional score. Their
recommendations include using an intramedullary nail with a high
proximal bend and static interlocking of proximal screws (level of
evidence: 4).283 Krettek et al.156,157
have described the technique of using blocking screws in 23 proximal
and distal fractures. They found a mean loss of reduction of 0.5
degrees in the frontal plane and 0.4 degrees in the sagittal plane.156,157
Blocking screws are placed so as to effectively reduce the size of the
tibial canal either proximally or distally and thereby guide both the
guide wire and the nail into an acceptable position. They can be placed
in any plane but are usually placed in either the sagittal or coronal
planes. Techniques for preventing or minimizing malalignment when using
an intramedullary nail for extra-articular proximal diaphyseal
fractures will be discussed in the authors’ preferred method of
treatment section.
 |
|
FIGURE 55-11 A. Anteroposterior and lateral radiographs of a comminuted proximal tibia fracture with an intra-articular extension. B. The fracture was successfully treated with a long locked plate and united uneventfully.
|
conducted a systematic review of the literature. At the time of their
review, there were three prospective case series evaluating
intramedullary nailing, three prospective case series evaluating
plates, and 14 retrospective case series evaluating plates, nails, or
external fixation. Based on these studies, there was weak evidence
(Grade C) to help guide treatment. Intramedullary nailing showed a
trend towards lower infection rates at 2.5% (95% CI, 0.1% to 3%)
compared to plating at 14% (95% CI, 8% to 23%) and external fixation at
8% (95% CI, 4% to 15%) (Table 55-6).24
The rates of nonunion and malunion were similar in all groups, and all
groups had overlapping confidence limits. Nonunion rates for
intramedullary nailing were 3.5% (95% CI, 1.7% to 7%) compared to 2%
(95% CI, 0.3% to 8%) for plating and 8% (95% CI, 4% to 15%) for
external fixation.24 Malunion rates
for intramedullary nailing were 20% (95% CI, 1.5% to 26% as compared to
10% (95% CI, 5% to 18%) for plating and 4% (95% CI, 1.5% to 10%) for
external fixation.24
fractures often presents the surgeon with some technical difficulties.
This is due to the anatomy of the distal metaphyseal flare, the
proximity of the fracture to the joint, and the technical difficulties
associated with distal nail fixation. There is also a known association
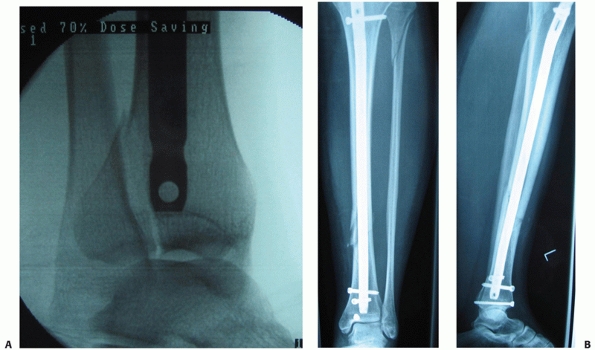
of posterior malleolar fractures with distal tibial spiral fractures (Fig. 55-12).39,161 This was initially described by Bostman44 who noted a 0.6% prevalence of this fracture. However, this association has been reported to vary from 25% to 48%.87,88,89
reported on a randomized controlled trial of 64 consecutive distal
diametaphyseal fractures treated with either an intramedullary nail or
plate fixation and found that union rates were similar between groups
(mean 18 weeks for intramedullary nail and 20 weeks for plate fixation)
and that the Olerud and Molander functional ankle scores were very
similar between the groups at 2 years. However, they did find that the
intramedullary nail group had increased ankle dorsiflexion and the
plate fixation group had six superficial infections and one deep
infection as compared to one superficial infection in the
intramedullary nail group (p = 0.03) (level of evidence: 1).137 More recently, Nork et al.209 reviewed their series of 36
tibial fractures, involving the distal 5 cm of the tibia, treated with
a reamed intramedullary nail (level of evidence: 4). They assessed
functional outcomes at 1 to 2 years and showed that there is continuing
improvement up to 2 years after the operation, but patients were still
worse by approximately one standard deviation as compared to population
norms when using the Musculoskeletal Functional Assessment Score.209
The advent of newer nail designs with very distal cross locks and
orthogonal cross locking screws, including two lateral and one
anteroposterior screws, help with distal fixation. In addition, close
attention to nailing technique including accurate guide wire placement
in the center of the plafond and close scrutiny of the ankle joint for
intra-articular fracture lines may help prevent previous problems seen
with intramedullary nailing of distal metaphyseal fractures. Recently,
Egol et al.86 have suggested that
adjunctive fibular plating in distal tibial fractures treated with an
intramedullary nail may maintain fracture alignment better than those
treated with only an intramedullary nail (level of evidence: 3).
Biomechanically, the addition of a fibular plate has been shown to
increase the resistance to torsional forces.199
|
TABLE 55-6 Pooled Estimates of Infection, Nonunion, Malunion, Compartment Syndrome, and Implant Failure*
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
FIGURE 55-12 A. A lateral fluoroscopic image showing a posterior malleolar fracture in association with a distal spiral tibial fracture. B.
This was successfully treated with an anteroposterior compression screw. We advocate two compression screws for this fracture, but in this case the anteroposterior locking screw provided supplementary fixation. |
reported on percutaneous plating techniques in unstable tibial shaft
fractures and found that of 24 unstable tibial fractures, 22 united
without secondary surgery (level of evidence: 4). They used a narrow
limited contact dynamic compression plate and had one significant
rotational malalignment of more than 10 degrees and no angular
deformity of more than 5 degrees of varus or valgus.214 Maffulli et al.178
also looked at 20 of their patients in whom a percutaneous plating
technique was used (level of evidence: 4). They found that with
traditional nonlocking plates, the majority of patients had excellent
and good results, although seven patients reported stiffness around the
ankle. They also reported few soft tissue complications.178
They identified only retrospective observational studies, and of those
that were included, they recorded a nonunion rate in those treated with
an intramedullary nail of 5.5% (95% CI, 3.78% to 8.1%) whereas in those
patients treated with a plate, the rate was 5.2% (95% CI, 2.4% to
10.9%). In the nonoperatively managed group, the nonunion rate was 1.3%
(95% CI, 0.7% to 2.7%) (Table 55-7). The
malunion rate was similar in all three groups, ranging from 13.1% (95%
CI, 8% to 20.8%) in those treated with a plate to 16.2% in the
intramedullary nail group (95% CI, 16.0% to 20%). They suggest that the
inferences that can be made from these observational studies are
limited due to patient numbers. Further large scale randomized
controlled trials would be necessary to help ascertain which technique
is preferred. Indeed these trials are currently ongoing.
|
TABLE 55-7 Pooled Estimates of Nonunion, Infection, Malunion, and Secondary Surgical Procedures*
|
||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||
the operative treatment of closed tibial shaft fractures, there is
still a significant proportion of surgeons who opt to use external
fixation in the management of the severely traumatized limb. This is
usually in open tibial shaft fractures, fractures associated with
compartment syndrome, or in the polytraumatized patient who is deemed
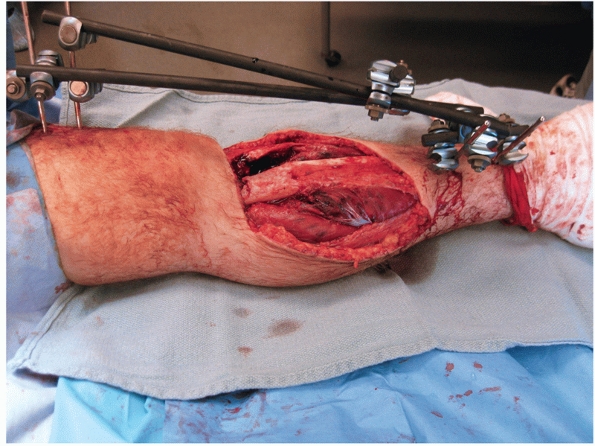
too hemodynamically unstable to undergo definitive fixation (Fig. 55-13).
External fixation frames in tibial shaft fractures can take the form of
uniplanar monoaxial frames that use Schanz pins above and below the
fracture site, multiplanar external frames that use configurations that
place the Schanz pins in different planes, which are usually
orthogonal, and the use of circular tensioned fine wire external
fixation frames, or a combination of both tensioned fine wire circular
frames and half pin fixation. This is often referred to as hybrid
external fixation.
multiplanar, and Ilizarov type ring fixators. Many factors such as
number of pins proximal and distal to the fracture site, the distance
of the pins from the fracture site, the use of double stacking cross
bars and multiplanar cross bars, and the inclusion of one, two, or
three rings proximal and distal to the fracture site all have a bearing
on fracture stability when assessed biomechanically.70,76,100,155,304
However, it is not known which configuration provides the optimal
stability for fracture healing. Arguably, the most important detail in
this regard is the quality of fracture reduction and amount of cortical
apposition irrespective of the fixator used.
have conducted a systematic review and meta-analysis of the literature
regarding the management of open fractures. They identified comparative
studies which had evaluated clinical outcomes following external
fixation, plating and external fixation, and unreamed intramedullary
nailing of open tibial shaft fractures. One quasirandomized trial by
Bach and Hansen20 compared plate fixation with external fixation. They found a statistically significant reduction in the rates of
reoperation with external fixation compared to plate fixation (6.7% compared to 50%).20
However, all of the fractures healed in their study and the
meta-analysis by Bhandari et al. suggested that external fixation did
not significantly alter the risk of nonunion (RR = 0.52, 95% CI,
0.21%-1.28%), deep infection (RR = 0.39, 95% CI, 0.13%-1.11%), failure
of fixation (RR = 0.58, 95% CI, 0.1%-3.2%), or malunion (RR = 2.6, 95%
CI, 0.29%-23.5%). The trend, however, was for increased nonunion, deep
infection or fixation failure in the plate group, and a trend toward
increased malunion in the external fixation group.20,27
In a comparison of unreamed nailing and external fixation, the pooled
estimate of effect favored the use of unreamed nails over external
fixators in the management of open fractures, with the use of unreamed
nails significantly reducing the risk of reoperation (RR = 0.51, 95%
CI, 0.37%-0.69%) (Table 55-8). The use of an
unreamed nail reduced the risk of reoperation by 49% when compared with
external fixation. This means that in low-risk patients, an orthopaedic
surgeon would need to treat 41 patients with an unreamed nail instead
of an external fixator to avoid a single reoperation. In high-risk
patients, surgeons would have to treat three patients with an unreamed
nail instead of an external fixator to avoid a single reoperation.27
This provides strong evidence that unreamed nails provide benefit in
the treatment of open tibial shaft fractures when compared with
external fixation.
 |
|
FIGURE 55-13 A spanning external fixation frame used for the acute management of a type IIIB open proximal tibial shaft fracture.
|
|
TABLE
55-8 Results of Statistical Pooling of Studies Assessing Unreamed Nailing and External Fixation as Well as Reamed Nailing and Unreamed Nailing on the Outcomes of Reoperation, Nonunion, Deep Infection, Superficial Infection, Malunion, and Implant Failure |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
open fractures of the tibial shaft, patients were randomized to receive
either an Ilizarov external fixator (32 patients) or an unreamed tibial
nail (29 patients).138 This was a
pseudorandomized study that had no concealment of allocation and for
this reason would be considered level 2 evidence. They noted a
statistically significant shorter time to healing in the Ilizarov
external fixation group (19 weeks) as compared to the unreamed tibial
nail group (21 weeks) (p = 0.039). They
found a higher need for reoperation to obtain bony union in the
unreamed tibial nail group but also noted a significant incidence of
pin tract infections and joint contracture with the Ilizarov fixator.138
There were no differences in the rates of malunion. They suggest that
the use of either of these methods of fixation should be made on a case
by case basis.138
suggested that the use of reamed intramedullary nails did not
significantly reduce the risk of reoperation for nonunion, delayed
union, or infection when compared to unreamed nails in the treatment of
open tibial fractures (RR = 0.75, 95% CI, 0.43%-1.32%). While the
number of patients with severe open fractures was small, a similar
trend was seen in Gustilo IIIB fractures.27
However, there was a significant increased rate of implant failure
associated with the use of an unreamed nail compared with a reamed nail
(23.4% and 7.5%, respectively).
series of 1106 tibial shaft fractures treated with an intramedullary
nail, infection rates ranged from 6.9% to 16.4% as Gustilo open
fracture types went from type I to type IIIB. The rates of aseptic
nonunion also increased according to open fracture grade, ranging from
12.1% for type I open fractures to 49% for type IIIB open fractures.
This reflects problems secondary to an increasing severity of injury
and soft tissue damage rather than to the use of intramedullary nails.66
Open fracture data from the recently completed SPRINT trial comparing
reamed and unreamed tibial nailing suggests a trend towards decreased
rates of reoperation with an unreamed nail (RR = 1.27, 95% CI,
0.91%-1.78%, p = 0.16].62 This difference, however, did not meet conventional levels of statistical significance.30
identified 417 citations with their search strategy and 11 of these met
their inclusion criteria. These 11 studies reported on 492 tibial shaft
fractures. All except for one were retrospective case series, the one
exception being a quasirandomized trial. They found that the union rate
ranged from 62% to 95%, the infection rate ranged from 4% to 35%, and
the reoperation rate ranged from 8% to 69%.104
The majority of the studies used standard 4.5 mm dynamic compression
plates, and one study assessed the use of biologic plate fixation. They
suggest that plating may be a viable option in the treatment of open
fractures, although this would have to be tested in a large randomized
controlled trial.
as temporary stabilization for a severely traumatized limb or as a
quick stabilization procedure for a severely traumatized patient.223
When using external fixation for temporary stabilization, it is prudent
to adhere to three principles that differ from those used when applying
external fixation for definitive stabilization. These principles
include keeping any pin sites outside of the zone of injury, which may
necessitate spanning the joint if the fracture is either distal or
proximal in the tibia. Positioning the external fixation pins far away
from the fracture decreases fracture stability, and thus stability may
need to be obtained in other ways such as the use of double stacking
rods or multiplanar external fixation techniques. The second principle
is to use radiolucent bars and to keep any metal joints away from the
fracture site so as not to cause any difficulties with further imaging.
The third principle is to use either a uniplanar or multiplanar
external fixation frame with two or three Schanz pins proximal or
distal to the fracture to allow for rapid application of the frame in
the severely polytraumatized patient.
the preference for intramedullary nailing of open tibial shaft
fractures (specifically type IIIA, B, and C) decreases, and external
fixation is more frequently used for primary treatment in the presence
of significant segmental bone loss and contamination. In damage control
surgery in the severely injured patient, external fixation is often the
treatment of choice because of its rapid application.128,220
intramedullary nailing following external fixation has reported on 9
studies (n = 268 patients) where there was a planned conversion from an
initial external fixator to an intramedullary nail and 12 studies (n =
236 patients) where they reported on the use of intramedullary nailing
as a reconstructive procedure. The protocols in these studies usually
suggested removing the external fixation with curettage, débridement,
and irrigation of pin sites and adjunctive antibiotic coverage.
of external fixation frame results in an 83% reduction in the risk of
infection (p < 0.001) when compared a period of external fixation that exceeds 28 days (Table 55-9).
However, union rates with subsequent intramedullary nailing are up to
90% (95% CI, 88%-93%). Casting following external fixation was found
not to significantly reduce the risk of infection, but it significantly
increased the rate of nonunion.33
While the overall incidence of fractures associated with bone loss is
generally low, the literature suggests that tibial fractures can
account for up to 68% to 79% of these fractures.147,299 While cases of reimplantation of large segments of bone have been described in adolescents,183 this is not advocated in the majority of open fractures.
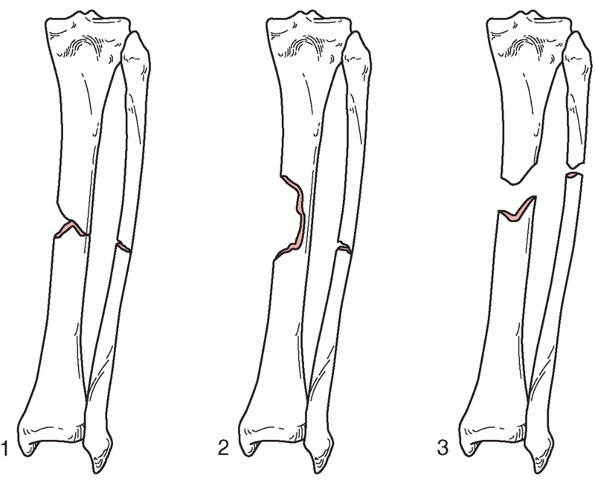
Type 1 bone loss involves less than 50% of the bone diameter, type 2
involves more than 50% of the bone circumference, and type 3 involves
segmental loss.65 Bone loss is not uncommon. It can occur in 11% to 12% of open fractures, and most commonly occurs in the tibia.147
Published guidelines exist for the various reconstructive options.
However, due to the prevalence of tibial fractures associated with
segmental bone loss, the evidence supporting these guidelines is
retrospective and is in most instances is considered level 4.147,238,299 However, Robinson et al.238
suggest that critical size defects less than 6 cm are amenable to bone
grafting techniques, and those more than 6 cm require other
reconstructive options. Thus, treatment for segmental bone loss must
necessarily be individualized and be based on patient’s values and
preferences as well as on surgical resources and technical issues. We
will discuss further reconstructive options in the section on
nonunions, however we use critical cut-off values for defects of 1 to 6
cm and defects more than 6 cm. Defects up to 6 cm are treated with an
intramedullary nail, if possible, and a later bone graft procedure is
undertaken after first ensuring adequate soft tissue coverage. With
defects more than 6 cm, careful planning is needed, the options being
nailing, with lengthening over the
nail,
immediate fine-wire tensioned frame application with later bone
transport, or immediate shortening and subsequent lengthening or
external fixation with planned free fibula transfer. Patient factors to
be considered in the decision-making process are the patient’s age,
preinjury functional status, comorbid medical conditions such as
diabetes and peripheral vascular disease, smoking status, and
concomitant injuries. Patient values and preferences include the
ability to psychologically deal with potentially multiple operations
including both soft tissue and bony reconstruction, or potentially
coping with months in an external fixation device depending on which
treatment is undertaken. Surgical issues include the condition of both
soft tissue and bone donor sites and the availability of surgical
expertise in using microvascular tissue transfer techniques or complex
fine-wire external fixation frames.
|
TABLE
55-9 The Crude and Weighted Infection Rates for Different Durations of External Fixation and for Different Durations of Conversion from External Fixation to Intramedullary Nailing* |
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||
 |
|
FIGURE 55-14 The OTA classification of bone loss.
|
crucial first step in the management of open tibial wounds.
Unfortunately, the débridement may result in increasing the amount of
segmental bone loss as devitalized bone must be removed as part of the
procedure. Intramedullary nailing has been put forward as the technique
of choice, when technically possible, for the treatment of tibial
fractures associated with bone loss. The use of unreamed statically
locked nails is supported to an extent by the recent SPRINT randomized
controlled trial.30 Those patients
who had an open fracture treated with an unreamed nail showed a trend
toward fewer outcome events than those who received a reamed
intramedullary nail (RR = 1.27,95% CI, 0.91%-1.78%; p
= 0.16) (level of evidence: 1). Intramedullary nailing confers the
benefits of minimally invasive stabilization with soft tissue
dissection which is essentially limited to the débridement. It also
provides stable internal fixation, allows access to the soft tissues
for wound management and possible free tissue transfer if deemed
necessary, and allows for potential lengthening over the nail with
external fixation if desired.147
However, the problems associated with initial fixation include
obtaining the correct alignment in the face of significant bone loss.
It may be necessary to template length and rotation from the
contralateral limb or potentially the ipsilateral fibula if there is
enough of it. The use of intramedullary nailing, however, is limited to
mainly
diaphyseal
fractures or metadiaphyseal fractures that allow for placement of
proximal and distal cross-locking screws and usually for defects less
than 6 cm. In the face of large proximal or distal defects that
preclude intramedullary nailing, temporary or definitive external
fixation may become necessary. External fixation in the form of
tensioned fine-wire frames confers the advantage of allowing bone
transport at a later date or facilitating immediate shortening with
later lengthening. However, it increases the complexity of wound
management and potential free tissue transport. There is the risk of
pin site infection and the problems associated with a prolonged period
of frame application that may not be desirable in some patients.
Definitive plate fixation after the initial acute management, while
providing stability to the bone, may necessitate further soft tissue
dissection and may therefore preclude later bone transport and limit
bony reconstruction to bone grafting or free bone tissue transport
techniques.65,74,147
we use a long leg cast for 4 to 6 weeks and switch to a patellar tendon
bearing cast as shown in Figure 55-13. We
consider a fracture to be unstable when there is a high-energy fracture
pattern, fracture comminution, or the fibula and tibial fractures are
at the same level. We maintain non-weight-bearing status for 4 to 6
weeks and undertake weekly radiographic examination for the first 3 to
8 weeks to ensure that the fracture does not displace. If it does,
reamed intramedullary nailing is carried out. If the fracture remains
undisplaced and a patellar tendon bearing cast is applied, knee
mobilization is encouraged. Casting is continued until there is
bridging callus in 3 out of the 4 cortices, and there is no pain on
palpation or with weight bearing.
manner as tibia and fibular fractures, as the degree of varus
malalignment seen with nonoperative management ranges from 25% to 60%,
depending on the fracture pattern with oblique fractures having the
highest incidence of varus malalignment.65
We also use a reamed intramedullary nail for both proximal and distal
metadiaphyseal fractures that are far enough away from either the knee
or ankle joint to allow two cross screws to be inserted through the
intramedullary nail. Newer nail designs have a number of features to
allow for the management of proximal and distal fracture patterns which
include a higher and shorter Herzog bend at the top of the nail and
cross screw placement as little as 2 to 3 mm from the distal end of the
nail and 5 mm from the proximal end of the nail. Newer nail designs
also have oblique cross screw configurations at the proximal end of the
nail, which has been shown biomechanically to help increase stability.164
deemed too proximal or too distal to use an intramedullary nail,
minimally invasive plate osteosynthesis techniques are used to insert a
proximal or distal periarticular locking plate. If an intramedullary
nail is used for proximal third metadiaphyseal fractures, we routinely
use blocking screws and a semiextended knee position. These techniques
will be discussed later in this chapter. If there are undisplaced
proximal or distal fracture extensions into the knee or ankle joints,
but the main fracture allows for intramedullary nailing with placement
of at least two proximal cross screws into the metaphyseal component,
we routinely use interfragmentary screw fixation of the intra-articular
extension and subsequent reamed intramedullary nailing.
are also treated by intramedullary nailing with careful débridement and
irrigation of the soft tissues. In polytraumatized patients or in
heavily contaminated IIIB or IIIC injuries, we will use a unilateral
temporary spanning external fixator usually with the addition of an
antibiotic bead pouch as described by Keating et al.,145 with definitive fixation being done less than 14 days after the initial surgery.33
In open proximal and distal diametaphyseal fractures where plate
fixation is required, Gustilo type I fractures are treated with primary
plating if the wound is completely clean following débridement and we
are able to adequately cover the plate with soft tissue. If the
fractures are Gustilo type II or III in severity, we treat the patient
with primary external fixation with definitive plate fixation being
done when the patient’s condition and the state of the soft tissues
allows secondary surgery. Primary plating of these fractures has been
shown to be associated with a raised incidence of complications,
including osteomyelitis.20 The early management of open fractures is described in detail in Chapter 10.
statically locked reamed intramedullary nail for defects which are less
than 6 cm in length, with secondary bone grafting being undertaken
after 6 to 8 weeks. For larger segmental defects, we will either use an
Ilizarov fine-wire tensioned frame with later bone transport techniques
or a free-fibular vascularized graft.
Alternatively, the patient can be positioned supine on a radiolucent
table. We routinely use a radiolucent adjustable “A” frame underneath
the leg with the knee bent at 90 degrees and the leg draped free, as
shown in Figure 55-15, as the set-up time is
faster and multiple procedures may be undertaken in polytrauma patients
without reprepping and draping and without compromising the quality of
the reduction.185 Intraoperative
fluoroscopy is brought in from the opposite side with the beam being
directed from below to the image intensifier above as shown in Figure 55-16.
Care should be taken to ensure that the entire tibia can be visualized
radiographically, with true anteroposterior and lateral views of the
knee, tibial shaft, and ankle being obtainable. A number of different
incisions can be used, with most surgeons favoring longitudinal
incisions that are either anterior or medial to the patellar tendon.
However, some surgeons use a transverse incision placed halfway between
the joint line and the tibial tubercle.65
 |
|
FIGURE 55-15 The patient is positioned supine, with the leg draped free at 90 degrees over an adjustable radiolucent “A” frame.
|
 |
|
FIGURE 55-16 The image intensifier is brought in from the opposite side.
|
for most intramedullary nails, but we may use a lateral parapatellar
approach for high proximal tibia fractures as discussed later in this
section. For both medial and lateral parapatellar approaches, the plane
between the subcutaneous tissue and fascia is used to undermine the
skin which then allows great mobility of the incision over the
underlying fascia. Flaps of skin and subcutaneous tissue are then
elevated. We then use a medial parapatellar approach to gain access to
the tibia. The medial parapatellar approach should be made from the
patella to just below the superoanterior aspect of the tibia. The
extra-articular high starting point can then be identified by palpation
behind the patellar tendon, and the anatomic safe zone as described by
McConnell et al.184 is then identified (Fig. 55-18). A curved awl is then introduced behind the patellar tendon as shown in Figures 55-19 and 55-20.
By raising the foot up into a semiextended position, we are able to
move the patella more laterally to allow the awl to find the starting
point, and we use this position routinely for tibial nailing.270
 |
|
FIGURE 55-17 We use a high medial incision just medial to the edge of the patella. L, lateral; M, medial; P, patella; TT, tibial tubercle.
|
tibia. Confirmation of the starting point can then be done with image
intensification. The starting point should be just medial to the
lateral tibial spine high on the superior-anterior tibia.250
Correct radiographic rotation of the proximal tibia is required as
external rotation will affect the radiographic view of the starting
point and lead to a starting point that is too medial. We routinely use
the lateral edge of the tibia as a radiographic landmark for rotation
and attempt to bisect the fibular head with the lateral edge of the
tibia radiographically to help determine appropriate radiographic
rotation for start site identification (Figure 55-21).287
done, one needs to push posteriorly on the awl handle to direct the awl
tip anteriorly within the bone. Care must be taken to not scrape down
the front of the tibia, which may damage the patellar tendon insertion.
Also, the patella tends to push the awl medially which then directs the
point of the awl laterally. This must be corrected so that the awl
progresses in line with the tibial shaft. Once the awl has been
inserted and has created a starting hole, a T-handled reamer with a
slight
distal anterior curve is introduced into the tibia. Again, the reamer
is placed in line with the tibial crest and force on the distal reamer
tip is directed anteriorly to maintain the position of the reamer in
the canal. One must take care not to force the T-handled reamer tip
laterally or posteriorly as the cortex can be penetrated, putting the
soft tissues at risk.
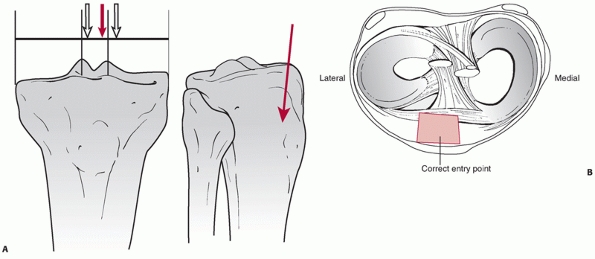 |
|
FIGURE 55-18 A. Diagram showing the high tibial extra-articular start point. B. Proper tibial nail start point.
|
inserted into the tibial diaphysis. Appropriate manual traction and
manipulation is then undertaken to align the tibia and allow the guide
wire to pass across the fracture site and into the distal tibia. Care
must be taken to place the ball tip into the center of the plafond on
both the anteroposterior and lateral fluoroscopic views to maintain
proper alignment of the tibia while reaming. If a solid unreamed nail
is being used, the advantage of guide wire placement is lost and both
reduction and placement of the nail must be done while the nail is
being inserted. This can potentially lead to distraction at the
fracture site as well as loss of alignment. For these reasons, we use a
guide wire and a reamed intramedullary nail.
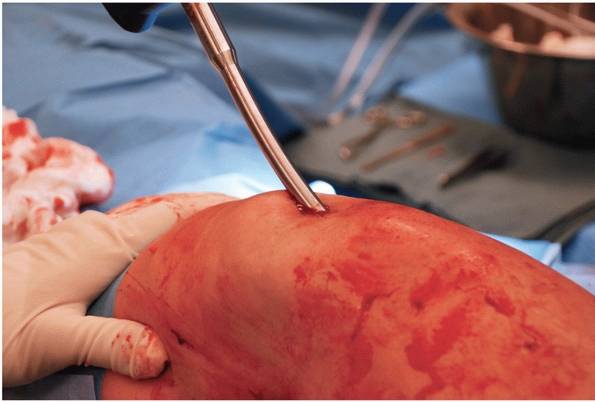 |
|
FIGURE 55-19
A curved awl is inserted behind the patellar tendon and is inserted into the tibia at the correct starting point as visualized on the image intensifier. As it is inserted, care is taken to keep the awl tip anterior in the tibia and in line with the canal. |
position, this position is checked fluoroscopically with
anteroposterior and lateral views above and below the fracture site,
ensuring that (a) the position of the distal tip is correct, (b) that
the guide wire is within the tibia on two orthogonal views, and (c) to
ensure reduction prior to reaming. Sequential reaming is then done in
0.5 mm increments. It is important to start with a small, end cutting
reamer head and, in many sets, this is the smallest reamer. The next
reamers in the set are side cutting reamers, and reaming is done to 1
to 1.5 mm past cortical chatter. We use a reamer with wide deep cutting
flutes and a narrow bore shaft, and the reamer is advanced slowly at
the full speed setting. We tend to ream 1 to 1.5 mm beyond the diameter
of the nail that will be inserted. Most tibial shafts will allow
passage of a 9 to 11 mm diameter nail. However, it is important to
template the canal diameter and length of the tibia preoperatively. In
general, we use a limited reaming technique whereby the nail diameter
is 2 mm less than the measured diameter of the tibia on anteroposterior
and lateral imaging.
ensure that the appropriate nail size is present in the operating room
but the definitive nail length is measured from the guide wire. The
nail is then mounted on a handler and introduced into the tibia. Most
nail systems come with a proximal cross lock screw insertion jig that
can be applied to the insertion handle of the nail. It is important to
check prior to inserting the nail that the holes on the jig line up
with the cross screw holes within the nail. The nail is then introduced
over the guide wire. The surgeon should be careful that the nail does
not rotate as it progresses into the tibia. To ensure this, the
proximal Herzog bend of the nail should be kept in the sagittal plane.
Once the nail is inserted, it is important to check the overall
alignment of the fracture and the position of the nail
fluoroscopically. Once this is done the cross screws can be inserted.
to remove the guide wire and to ensure that the rotation, length, and
alignment of the tibia are correct. This may be facilitated by
comparing the leg with the contralateral side. We routinely use two
oblique proximal cross screws in bone that is osteopenic or
osteoporotic. If the bone is not osteopenic, we use two medial to
lateral proximal cross screws. These are inserted through percutaneous
incisions using the proximal cross screw locking jig, as shown in Figure 55-22.
Distal cross screws are then inserted with the image intensifier using
a freehand technique. This can be done with a radiolucent or regular
drill. The radiolucent drill has the advantages of both providing a
radiolucent targeting device within the drill handle as well as
generally providing a longer handle so as to keep the surgeon’s hands
away from the x-ray beam. When placing cross screws using the freehand
technique, it is imperative that one obtains perfect circles of the
cross screw holes with the image intensifier. A percutaneous incision
is then made, and the bone is exposed by blunt spreading dissection of
the subcutaneous tissues. The drill is then placed directly on the bone
in the center of the circle. Alternatively, a Steinmann pin can be used
to locate the center of the circle (Fig. 55-23).
The Steinmann pin is tapped with a mallet, and it makes a divot in the
appropriate place to allow for drill placement. The drill is then
aligned with the image intensifier beam and a hole is made in the near
cortex and into the nail. Prior to penetration of the far cortex, it is
important to check that the drill is indeed in the hole of the nail. If
it is not, and is either anterior or posterior, the drill is then
backed out and redirected into and through the nail. Once the drill
abuts on the distal cortex, some systems allow for measurement from the
drill bit itself using a small measuring device. If this is not
possible, the distal cortex is penetrated and a depth gauge is used to
assess cross screw length. The screw is then inserted into the tibia
and the image intensifier is used to check that it is appropriately
placed.
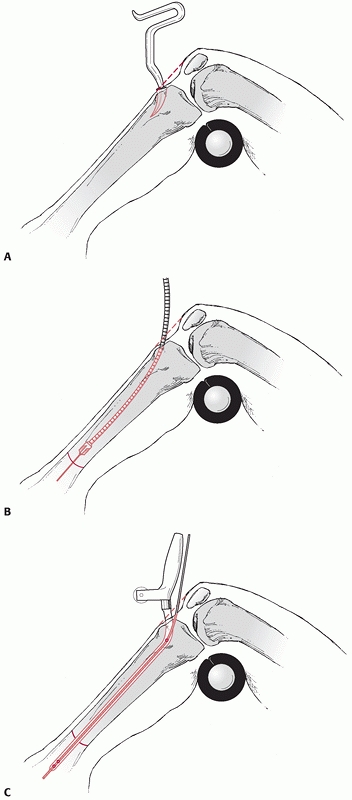 |
|
FIGURE 55-20 A. The use of a bone awl to penetrate the cortex. B. A guide wire is passed over the fracture site followed by a reamer if a reamed nail is to be used. C. The nail is passed into the tibia.
|
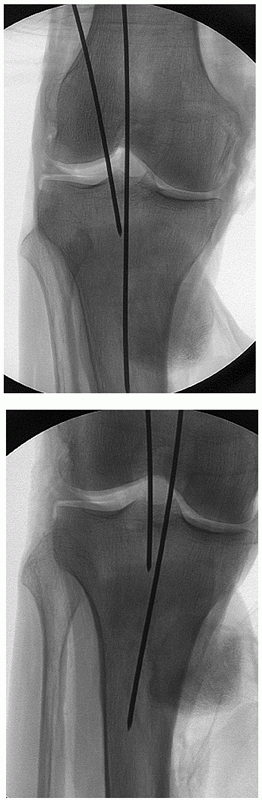 |
|
FIGURE 55-21
The laterally located K-wire is inserted into the correct starting point. However, one can see with only 10 degrees of external rotation, the medial K-wire looks radiographically to be in-line with the tibia but it is in fact too medial. Note how the lateral edge of the tibia bisects the fibula once the correct orientation is established. This is seen on the right. |
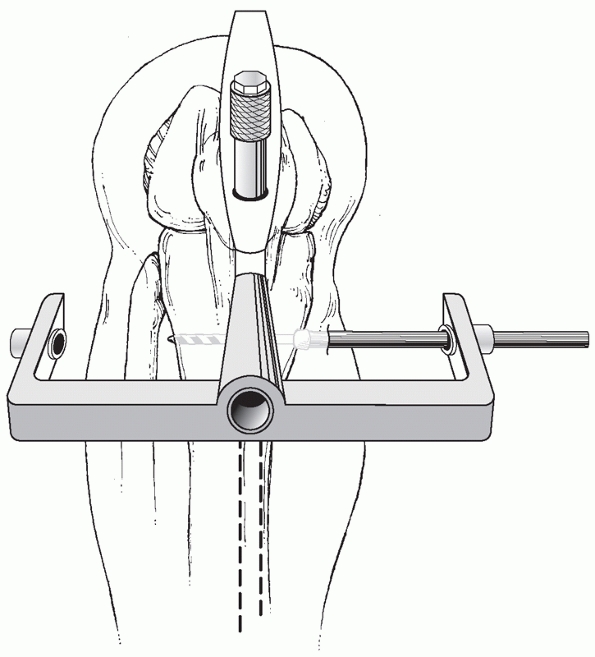 |
|
FIGURE 55-22 Insertion of proximal cross screws is facilitated by the use of a proximal insertion guide.
|
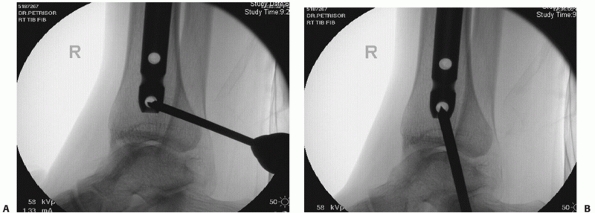 |
|
FIGURE 55-23 Distal cross screws are inserted using fluoroscopy and either a radiolucent or regular drill as shown in (A) or by first finding the hole and indenting the cortex with a Steinmann pin as shown in (B).
|
cross screws can be inserted first and then used to “back slap” the
distal fragment to the proximal fragment. However, newer nail designs
allow for more controlled compression by the use of a compression screw
placed inside the nail. With the distal cross screws in place and one
proximal cross screw placed in an oblong hole in the dynamic position,
the compression screw will push on the proximal cross screw and draw
the distal tibia proximally.
routinely use three cross screws, two medial to lateral, and one
anterior to posterior. Medial to lateral screw placement allows for
easier removal should this be necessary and may confer a greater
resistance to bending. However, this is achieved at the risk of placing
the screws slightly closer to the posterior tibial neurovascular bundle
and superficial peroneal nerve.237
Care is taken to protect the neurovascular structures and, if anterior
to posterior screws are being inserted, the tibialis anterior tendon
and neurovascular bundle should be protected by using a drill sleeve.
For proximal third tibial shaft fractures, two oblique proximal cross
screws are usually enough to ensure stability of the proximal fragment,
although
we occasionally add a third medial to lateral screw depending on bone quality.
immediately postoperatively. We also allow weight bearing as tolerated
postoperatively if good cortical contact has been obtained. Otherwise,
we will progress from partial weight bearing. In cases where there is
an intra-articular fracture that was internally fixed, the patient is
asked to remain nonweight bearing for approximately 6 to 8 weeks. This
routine is similar for type I and type II open tibial shaft fractures
where flap cover is not necessary. In type III open fractures, the
extent of weight bearing and range of motion that is allowed will also
be determined by the views of the plastic surgeon.
need to be observed when using an intramedullary nail to fix proximal
tibial diametaphyseal fractures. If the proximal fracture fragment
allows for the insertion of two cross screws, we commonly use an
intramedullary nail for these fractures. If, however, we are unable to
insert two proximal cross screws, then we proceed to minimally invasive
plating with a proximal tibial locking plate.
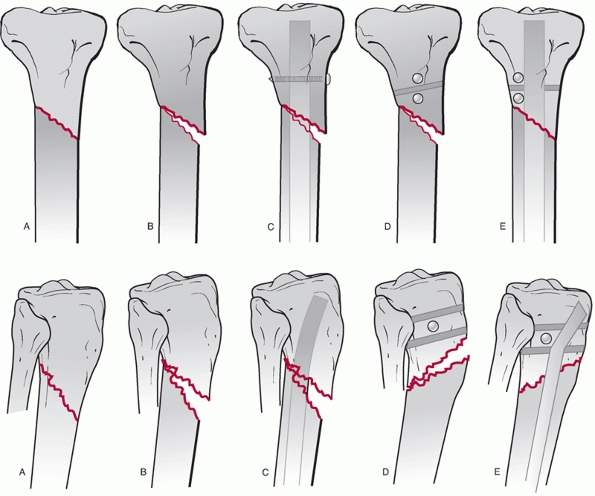 |
|
FIGURE 55-24 Diagram illustrating blocking screw technique. We commonly use 3.5- or 4.5-mm cortical screws.
|
We use either 3.5-mm or 4.5-mm cortical screws placed just off the
midline opposite to the apex of the deformity. Thus, if the deformity
is valgus, the blocking screw is placed just lateral to the midline in
the anteroposterior plane. If there is a flexion deformity, the
blocking screw is placed just posterior to the midline in the lateral
image intensifier projection. The blocking screw is also placed
posteriorly if there is posterior translation of the distal fragment.
The blocking screws help to obtain alignment but also increase the
construct stability.157
this technique using 3/16th smooth Steinmann pins placed in the same manner as blocking screws (Fig. 55-26).
One downside to this technique is that the Steinman pins are removed,
so good fixation with the locking screws is necessary to avoid loss of
reduction. Another method of obtaining the reduction is to use a small
unicortical plate as in Figure 55-27. This
necessitates an incision, and we do not routinely use this technique
for closed injuries unless we are unable to obtain an acceptable
reduction by the other means that have been described. We do, however,
routinely use this technique if we are treating an open proximal shaft
fracture with an intramedullary nail as it is essential that the
fracture is reduced prior to reaming and nail insertion.
 |
|
FIGURE 55-25 Blocking screw used to guide the nail anteriorly into the canal and aid in reduction of the proximal fragment.
|
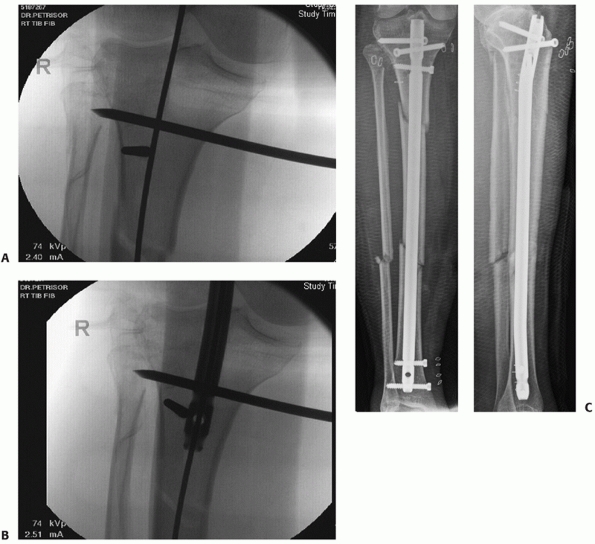 |
|
FIGURE 55-26 A segmental tibial diaphyseal fracture treated with an intramedullary nail. A. Two 3/16 Steinmann pins were used as blocking aids for the proximal fracture. B. The fracture is reduced with the passage of the nail. C. Anteroposterior and lateral radiographs showing that a good reduction was achieved.
|
This allows for manual reduction of a proximal fracture and also allows
permits more lateral movement of the patella such that the awl and
reamer can be kept close to the anterior cortex. We also use a nail
with a high short proximal Herzog bend which further facilitates this.
It is important to use image intensification to confirm the starting
point when nailing high proximal diametaphyseal fractures. It has been
suggested that a slightly lateralized starting point may be necessary
if the fracture is in valgus. This may be facilitated by either
performing a patellar tendon splitting approach or using a lateral
parapatellar approach to gain access to the proximal tibia. It is also
important to be aware of any intra-articular extension of the fracture.
If an undisplaced intraarticular fracture line is present, this can be
fixed with compression screws prior to inserting the nail. If the
compression
screws are placed in the appropriate position, these can also be used
as blocking screws for guide wire and nail insertion. It is, however,
important that the screw should not be dislodged when passing the nail,
as this may alter the reduction of the intra-articular component of the
fracture.
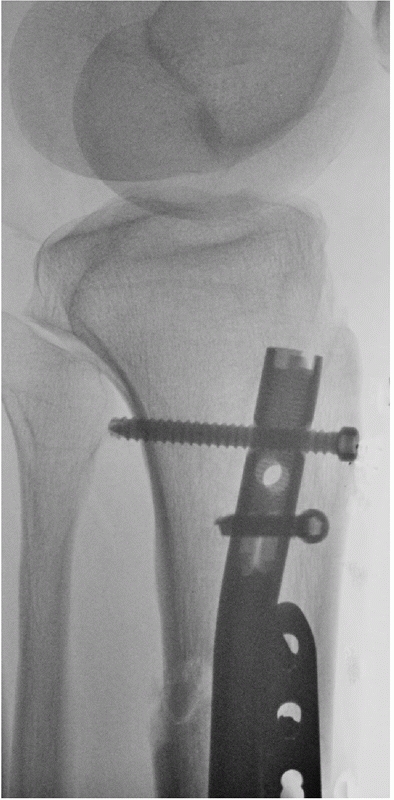 |
|
FIGURE 55-27
A small four-hole plate was used in this open proximal tibial fracture. In this case, the fracture was open on the lateral aspect so the plate was applied to the lateral side. A medial plate is usually used and removed after the reduction has been achieved and the fracture stabilized with an intramedullary nail. |
fractures, it is important to be able to obtain at least two cross
screws in the distal fracture fragment. It is imperative that the guide
wire be placed in the center of the tibial plafond, and it is important
to follow the principle of “ream where you wish the nail to go.” If the
guide wire is placed slightly off center and reaming progresses over
top of the guide wire, the fracture will be reduced in an angulated
position. If it is difficult to place the guide wire in the center of
the tibial plafond, we use anterior to posterior or medial to lateral
blocking screws to facilitate guide wire placement. As with proximal
fractures, if the fracture is in varus, we place the blocking screws
medial to the center line or in the concavity of the deformity. We use
a similar approach with anteroposterior deformities. In distal third
diametaphyseal fractures, there may be an associated posterior
malleolar fracture, and it is important to check for this using
intraoperative fluoroscopy. We fix undisplaced, posterior malleolar
fractures with anterior to posterior lag compression screws, prior to
inserting the nail, as nail insertion may disrupt the articular
fracture fragment. We also use two parallel medial to lateral distal
cross screws as well as a third anteroposterior cross screw as this
aids distal stability.86 We also fix
associated fibular fractures within 6 to 8 cm of the ankle joint using
the standard fixation techniques that are outlined in Chapter 57. This has been shown to increase rotational stability and decrease postoperative loss of reduction.86,199
the operation involves making an incision on the anterolateral aspect
of the tibia in order to place the plate on the lateral aspect of the
tibia as shown in Figure 55-28. An
extraperiosteal plating technique is undertaken using a 4.5 broad or
narrow low-contact dynamic compression plate depending on the size of
the tibia. If the fracture is transverse, a compression plating
technique is used. If the fracture is slightly more comminuted, a
bridge plating technique is employed to preserve the vascularity of the
fracture fragments. If bridge plating is done, we routinely use a plate
with a locking option that may be useful in osteopenic bone. However,
one must remember that the reduction needs to be obtained prior to the
application of the locking plate, as will be described further in the
next section. In addition, the contouring of locking plates may distort
the locking mechanism of the screw-plate interface. The plate may be
applied to the lateral or medial border of the tibia.
those tibial fractures that are too proximal to treat with an
intramedullary nail. We do this using a minimally invasive plate
osteosynthesis technique. An anterolateral incision is made at the
proximal end of the tibia, curving posteriorly toward the lateral
aspect of the femur (Fig. 55-29). The
iliotibial band is split as it inserts into Gerdy’s tubercle. The
fascia over tibialis anterior is incised at its insertion on the
anterolateral aspect of the tibia, and the anterior compartment
musculature is dissected free from the proximal tibia with a periosteal
elevator until the tibiofibular joint is reached. A periosteal elevator
is then slid underneath tibialis anterior muscle, so that it is
submuscular but supraperiosteal, with care taken to not disrupt the
anterior neurovascular bundle that lies close to the interosseous
membrane deep to the anterior musculature. The fracture is then reduced
using indirect reduction techniques with the help of intraoperative
fluoroscopy. A proximal locking plate is then slid down the tibia
underneath tibialis anterior and the extensor musculature and placed in
an appropriate position on the proximal aspect of the tibia. It is then
important to check distal placement of the plate underneath the muscle.
Using the image intensifier, the end of the plate is localized and a
small incision is made from the distal end of the plate extending
proximally about 2 to 3 cm. It is important to dissect down to bone
carefully to identify and protect the superficial peroneal nerve in the
lateral compartment and the anterior neurovascular bundle in the
anterior compartment. If a less invasive stabilization system (LISS)
plate is used, the superficial peroneal nerve is most at risk around
holes 11 to 13, and the anterior tibial artery is at risk around holes
9 to 12 (Figs. 55-30 and 55-31).61
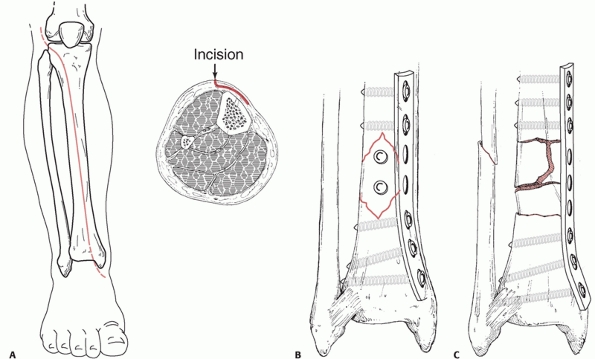 |
|
FIGURE 55-28 A. The anterolateral approach to the leg. B. The use of interfragmentary screws and a neutralization plate to stabilize a distal tibial diaphyseal fracture. C.
The use of a bridging plate. The comminuted fragments have not been mobilized and the soft tissues remain undisturbed. The plate bridges the fracture. |
 |
|
FIGURE 55-29
An anterolateral incision used for minimally invasive plating of proximal tibia fractures. L, lateral; M, medial; P, patella; TT, tibial tuberosity. |
distal third of the tibia. The incision is placed somewhat more
laterally over the anterior tibial compartment once the fascia is
released and the musculature retracted posteriorly. Alternatively, a
muscle splitting approach may be used with care being taken to protect
and avoid the anterior neurovascular bundle. The end of the plate is
then identified and fixed in the center of the lateral tibial cortex
with a K-wire placed in the most distal hole. It is important to then
place holding wires in the proximal end of the plate as well as in the
middle of the plate to ensure that the overall alignment is maintained
and that the plate is well centered on the bone. To aid reduction,
nonlocking screws can be placed through the plate into the diaphysis
which can draw the fracture into alignment without necessarily
compressing the bone to the plate. Once fracture reduction is obtained
and the plate is in a suitable position, locking screws can then be
inserted starting proximally with one screw and moving distally with
the next screw then centrally with the third screw. The overall
alignment is then checked fluoroscopically (Fig. 55-32).
Once the appropriate position of the fracture, plate, and the initial
three screws is confirmed, the rest of the locking screws are placed
from proximal to distal.
shaft; however, we place the screws using the “near-far” technique with
a minimum of two screws close to the fracture site and two more screws
at the distal end of the plate. More screws may be added depending on
bone quality and the desired rigidity of the construct. It is important
to reduce the fracture prior to applying the plate and not use the
plate
as
a reduction tool when using locked screws. If the fracture pattern is
“simple,” we will obtain compression either through the plate or with a
lag screw technique. This must be done prior to the insertion of any
locked screws. The fine tuning of shaft reduction may be made once the
plate has been fixed to the bone with K-wires and before the locking
screws are placed, by using either a cortical screw in the shaft which
will then bring the shaft closer to the plate thus aligning the
fracture or by using a compression device.
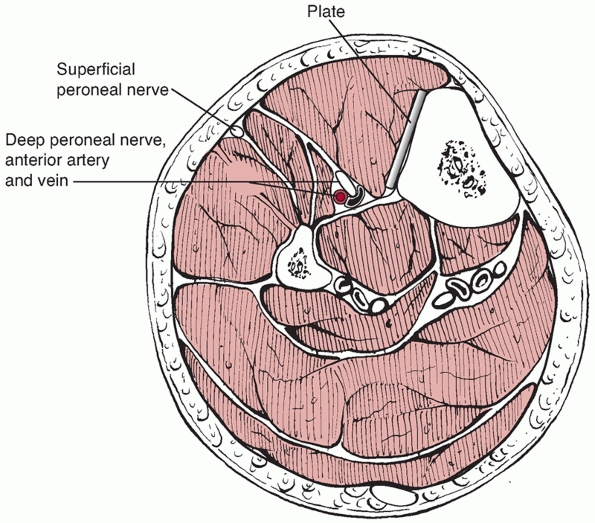 |
|
FIGURE 55-30 Cross section of the distal third of the leg showing the proximity of the neurovascular structures to the plate.
|
using a medially based periarticular locked plate. We use minimally
invasive plate osteosynthesis techniques with a longitudinal incision
of 3 to 4 cm over the medial malleolus (Fig. 55-33).
Supraperiosteal dissection is carried out with a periosteal elevator up
the medial aspect of the tibia. A plate is then inserted subcutaneously
along the medial aspect of the tibia. Diametaphyseal fractures of the
distal third of the tibia are usually spiral in nature, and a large
towel clip or reduction forceps can be used through percutaneous stab
incisions to help align the fracture site once the plate is alongside
the bone. Alternatively, a reduction clamp can be used and provisional
K-wire stabilization of the fracture can then be done with the wires
being placed from lateral to medial. The medial locking plate is then
placed alongside the bone. Once this is done and the position of the
plate is confirmed, we find the proximal aspect of the plate using the
image intensifier and make an incision from the most proximal aspect of
the plate which runs distally for 3 to 4 cm, with care being taken to
protect the greater saphenous vein and saphenous nerve. We ensure that
the plate is centered on the bone proximally and held there with a
K-wire. We then generally place one cortical screw at the distal end of
the tibial shaft, just proximal to the fracture line to allow for
fixation of the plate to bone, if the contour of the plate allows for
good anatomic reduction. A cancellous or cortical screw can then be
placed distal to the fracture in the tibial metaphysis, which will draw
the distal fragment to the plate. Distal and proximal locking screws
are then inserted. It is important to note that any articular reduction
must be done prior to locking screw placement. If the fracture pattern
is simple, we obtain compression of the fracture through the plate
prior to inserting the locking screws. If the fracture is comminuted,
we using a spanning locked plate construct avoiding dissection through
the zone of injury.
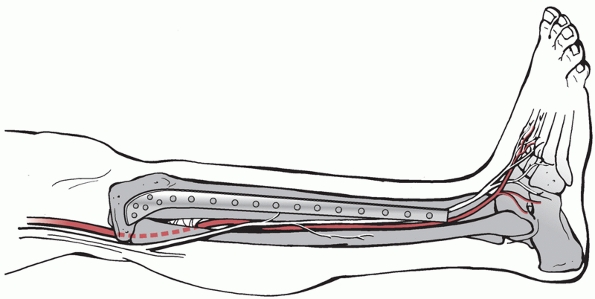 |
|
FIGURE 55-31 Longitudinal anatomy of neurovascular structures and their proximity to the distal holes.
|
 |
|
FIGURE 55-32
It is important to initially insert K-wires in the near, far, and central positions, as shown here. The overall check alignment is then checked with fluoroscopy, locking screws inserted in the same order. The plate must be centered distally and the reduction obtained prior to insertion of locking screws. |
maintain nonweight bearing for 6 weeks. However, we advocate the use of
physiotherapy to encourage active and passive range of motion of both
the knee and ankle. In Gustilo type I open fractures, the soft tissues
are closed primarily if, and only if, the wound is completely clean.
Type II and III injuries require a second look procedure and may also
require the use of an antibiotic bead pouch after the initial procedure
(Fig. 55-34). If free flap cover is necessary
after the acute management, we provide definitive fixation at that
time. A free flap is done in conjunction with our plastic surgical team.
shaft fractures when the condition of the patient or the extent of the
injury does not permit definitive fixation, as in Figure 55-35.
We use a medially based uniplanar external fixation frame with two
proximal and two distal bicortical Schanz pins. There are two important
principles that should be followed when placing external fixation
frames. For rigidity, the “near-far” technique, with two pins placed
close to either side of the fracture and two pins placed within the
bone as far away from the fracture as possible, allows for a more solid
frame construct particularly if the bars are placed close to the skin
and double stacked. Orthogonal pin placement and parallel bars may also
be used. However, we usually use the external fixator temporarily in
either severely open, heavily contaminated fractures or if there are
significant fracture blisters in proximal or distal third tibial shaft
fractures that are not amenable to immediate definitive fixation. When
plating techniques are going to be used for definitive fixation, we
keep the Schanz pins out of the zone of injury. For proximal tibia
fractures, we routinely use anterior or anterolateral distal femoral
pins and anteromedial or anterior distal tibial pins joined centrally
by double stacked bars being careful to use radiolucent carbon fiber
rods and to keep any metal joints away from the fracture site as
further imaging will probably be necessary. Keeping the Schanz pins
away from our definitive plating area does weaken the strength of the
construct, and we use this construct for temporary spanning fixation
only.
that are not amenable to intramedullary nailing, we use a medially
based spanning frame with two proximal tibial pins away from our area
of definitive plate fixation and a Schanz pin in the calcaneus and
either talar neck or first metatarsal. The pins are joined with a
medially based carbon fiber rod. Alternatively, a Delta frame
configuration may be used with a 3/16th threaded Steinmann pin or
centrally threaded Denham pin placed in the calcaneus 2 cm anterior and
2 cm proximal to the posterodistal aspect of the heel and 2 Schanz pins
placed in the proximal tibia. A further pin may be placed in the first
metatarsal or talar neck to control foot dorsiflexion and
plantarflexion.
be placed through a stab incision in the skin, being careful to protect
or the greater saphenous vein and saphenous nerve (Fig. 55-36).
Blunt dissection is then carried out down to the tibia. We generally
use 5-mm Schanz pins for the tibia, and predrill with a 3.5-mm drill
bit. Newer Schanz pin designs allow for self-drilling and self-tapping
of the Schanz pin. When these Schanz pins are used in the tibia, it is
usually necessary to predrill the cortices even though the Schanz pins
themselves are self-drilling and self-tapping. In hard young bone, the
self drilling component of the Schanz pin cuts through the first
cortex; however, the threaded portion
of
the Schanz pin bites on the first cortex and pushes the drill bit into
the second cortex. If the bone is hard, the first cortex may have
dulled the bit, and there is a risk of stripping the threads of the
near cortex as the drill is being pushed into the second cortex.
Therefore, we always predrill our Schanz pin holes. If the bone is
osteopenic, it may be necessary to augment the frame fixation with
further Schanz pins.
 |
|
FIGURE 55-33 A. Anteroposterior and lateral radiographs showing an OTA B1.3 distal tibial fracture. B. The fracture was treated with a medially based locked plate applied using minimally invasive techniques. C. Good union was achieved.
|
-
Classifications should be based on a
systematic approach using a multiphase technique. Currently, most
tibial fracture classification systems do not meet these criteria. -
The most common cause of tibial shaft
fractures is motor vehicle accidents. Motorcycle accidents have a
higher incidence of open fractures. -
Statically locked intramedullary nails
are the most popular choice of implant for all closed and open
fractures. Reamed intramedullary nail insertion is preferred for all
closed and open fractures up to Gustilo IIIA in severity. For more
severe open fractures, nonreamed nails and external fixation are more
popular. -
Nonoperative immobilization is advocated
mainly for low-energy undisplaced tibial shaft fractures or in those
patients with significant medical comorbidities that may preclude
operative intervention. -
The best available evidence suggests that
intramedullary nail fixation or minimally invasive locked plate
fixation provide equivalent results in the treatment of proximal or
distal diametaphyseal tibial
P.1902fractures. Large randomized controlled trials are necessary to clearly differentiate between these two treatment techniques.
-
Intramedullary nailing, if technically
possible, is the treatment of choice for closed and open tibial shaft
fractures, including Gustilo IIIB fractures.
 |
|
FIGURE 55-34
Antibiotic bead chain made with tobramycin/gentamycin impregnated polymethylmethacrylate. We usually use surgical steel wire. These chains may also be purchased premade. |
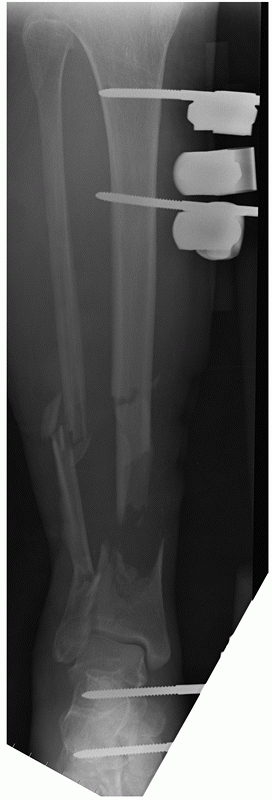 |
|
FIGURE 55-35 Temporary spanning external fixation frame for a distal third Gustilo IIIB open tibial fracture.
|
involve the bone or the soft tissues. Soft tissue complications can be
either acute, such as those associated with open fractures, compartment
syndrome, or neurovascular injury, or delayed, such as infection. Bony
complications include septic or aseptic nonunion, malunion, or
infection. Bony complications may occur together with soft tissue
complications or they may occur in isolation. In general terms, as the
energy of the mechanism of injury increases, so does the potential risk
of both bony and soft tissue complications. In this section, we will
discuss complications of intramedullary nailing as it is currently the
recommended treatment of tibial shaft fractures, soft tissue
complications, which includes open fractures, compartment syndrome, and
the mangled extremity. We will discuss nonunion and malunion.
have reported on a series of 169 patients treated with an
intramedullary nail. They found that anterior knee pain was present in
56.2% of patients and seemed to affect younger more than older patients
(level of evidence: 4).69 A
significant number of the patients with anterior knee pain had pain
with kneeling (91.8%) and pain at rest (33.7%). They found also found
that the pain resolved or improved with nail removal.69
also reported on the prevalence of knee pain following intramedullary
nailing, and in a consecutive series of 110 tibial shaft fractures they
found that 57% of patients developed anterior knee pain (level of
evidence: 4). They also found no significant correlation between
proximal protrusion of the nail and knee pain, but they suggested that
there might be an association with a patellar tendon splitting approach
as compared to a parapatellar insertion with a higher number of
patients (77%) having pain with the patellar tendon splitting approach
as compared to the paratendinous approach (50%). They found that 80% of
their patients required nail removal and that the majority had their
pain either completely or partially relieved.146
randomized 50 patients to receive an intramedullary nail through either
a paratendinous or transtendinous approach. They then followed these
patients for an average of 3 years (level of evidence: 1).269
They found no significant difference in anterior knee pain between the
transtendinous to the paratendinous approach (RR = 1.06, 95% CI,
0.81%-1.39%); however, they reported a high incidence of anterior knee
pain with both techniques (67% with the transtendinous approach and 71%
with the paratendonous approach).269
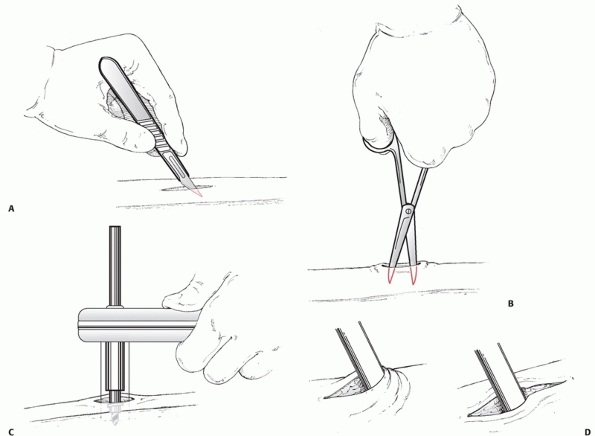 |
|
FIGURE 55-36 A. A 1- to 1.5-cm skin incision is made with a pointed scalpel blade. B. The soft tissues are mobilized down to the level of the periosteum. C. The bone is predrilled if required, and a pin is inserted. D. Care must be taken to release the skin around the pins.
|
compared those with anterior knee pain and those without. They found no
significant differences in the ultrasonographic appearance of the
tendon and specifically found no differences in patellar tendon blood
circulation, calcification of the patellar tendon, or granulation
tissue.281 This suggests that the etiology of anterior knee pain following intramedullary nailing is still unclear.281
A follow-up study by the same authors found that anterior knee pain
symptoms in a significant number of patients may disappear between 3
and 8 years.279 Interestingly, those
patients in whom anterior knee pain was still present at 8 years had a
lower functional knee score and quadriceps weakness (level of evidence:
4).280 Bhattacharyya et al.34
assessed the effect of nail prominence on anterior knee pain and
suggested that anterior nail prominence was associated with pain at
rest, while superior nail prominence was associated with pain while
kneeling and walking (level of evidence: 4). They suggested placing the
nail just deep to the bone.34
intramedullary nailing is as yet unclear; however, it is seen to
decrease in the majority of patients up to 8 years following the
procedure.
documented the prevalence of neurologic injury to be approximately 30%
in a retrospective review of 60 patients treated with a reamed
intramedullary nail, but they stated that the majority were minor
sensory neurapraxias with 89% of these being transient and resolving
within 3 to 6 months. However, 2 patients in their series continued to
have a nerve deficit at 1-year following the procedure (level of
evidence: 4).152
shaft fracture treated with a reamed intramedullary nail, 5.3% were
seen to develop peroneal nerve dysfunction postoperatively.239
The mean age of patients in whom this developed was 25.6 years and most
of the patients had had a closed fracture caused by a varus force with
no evidence of compartment syndrome.239
The majority showed good recovery after 3 to 4 months, but 3 of 11
patients still had residual symptoms resulting in weakness of their
extensor hallucis longus.239 Williams294
also found that 19% of patients with tibial fractures treated with a
reamed intramedullary nail had peroneal nerve lesions. It has also been
suggested
that oblique proximal cross screws can damage the peroneal nerve.84
inserting oblique proximal cross screws and to avoid placing the drill
and screws too far through the distal cortex. Fluoroscopic imaging can
be used to check that the screws are not placed close to the fibular
neck.84
described thermal necrosis in 3 patients, all of whom had narrow
intramedullary canals (level of evidence: 4). They found that after
reamed intramedullary nailing, these patients developed pretibial
blistering and further went on to develop osteomyelitis. They
attributed this to thermal necrosis from tight reaming of the canal.
Thermal necrosis has also been implicated in the development of
postoperative infection following intramedullary nailing in another
series of reamed nails.227
have assessed thermal necrosis in vivo in adult subjects. They used
temperature probes while reaming and found that in canals that were
either 9, 10, or 11 mm in diameter, the amount of reaming did not
produce a clinical problem. However, they did note a significant rise
in temperature in those canals that were 8 mm in diameter once they
were reamed to at least 10 mm.102 To
minimize the risk of thermal necrosis, it is suggested that a
tourniquet should not be used and reaming should not be forceful.
but open tibial fractures are relatively common and there are a number
of issues that need to be discussed. Infection is a significant problem
with rates of infection following severe open tibial fractures ranging
from 5% to 50%, and both infection and delayed fracture healing often
result in multiple operative procedures.8,27,66,75,131,227,228
Most surgeons concur that a meticulous débridement of contaminated soft
tissues and bone with fluid irrigation is the most important initial
step in the surgical management of open fractures (Figure 55-37).8,9
Since open fractures are usually associated with a higher energy of
injury, it is vital to undertake a thorough physical examination of the
affected limb including an assessment of the degree of soft tissue and
neurovascular damage. The leg should also be examined for other
ipsilateral injuries, and the overall condition of the patient should
be monitored. While much has been written on open fracture management,
a number of issues remain unresolved. With regard to initial wound
management, these include the amount and type of irrigating fluid and
the degree of pressure used in delivering the irrigating fluid.
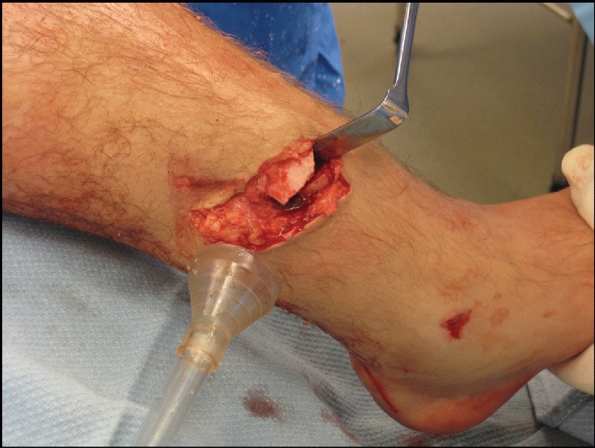 |
|
FIGURE 55-37 The use of jet lavage in the irrigation of an open tibial fracture.
|
surveyed 1764 surgeons, 984 of whom responded. About 70% of surgeons
favoured irrigation with normal saline, whereas bacitracin was
routinely added to the saline by 16.8% of surgeons. The majority of
surgeons (71%) used low pressure when delivering irrigation solutions
to the wound, but there was, in fact, variation in surgeon opinions as
to what constituted low or high pressure.
enrolled 400 patients with 458 open fractures and randomized them to
either receive a Castile soap solution or normal saline with
bacitracin. He found no significant difference between these irrigation
solutions, noting that the infection risk with soap was 13% compared
with 18% for bacitracin solution (p = 0.2).8
The confidence intervals were wide, but the point estimate suggested a
trend toward soap solution being better in reducing the risk of
infection (RR reduction of 28%) (level of evidence: 1).8
suggests that high pressure lavage is effective at removing debris and
bacteria from contaminated open wounds. However, this may be achieved
at the expense of potentially further damaging the bone ends, as well
as displacing bacteria further into the intramedullary canal.22,23,166
There is also some experimental data to suggest that there are effects
at the cellular level from high pressure lavage. Callous strength in a
rat femur fracture model is less in those treated with high pressure
lavage. In addition, high pressure promotes stem cell differentiation
towards an adipocyte cell type rather than osteoblast cell type.31
controlled trial (fluid lavage in open fracture wounds [FLOW])
assessing open fracture irrigation techniques is underway. It is
comparing normal saline solution with Castile soap as well as comparing
high pressure lavage, low pressure lavage, and bulb syringe pressure in
a 3 × 2 factorial design.
meta-analysis of the use of antibiotics for preventing infection in
open limb fractures has been done by the Cochrane group.105
They reviewed seven studies that provided data on 913 patients. They
found a reduction in the rates of early infection with the use of
antibiotics (RR = 0.41, 95% CI, 0.27%-0.63%). This translates to an
absolute risk reduction of 0.08 (95% CI, 0.04%-0.12%) and a number
needed to treat of 13 (95% CI, 8%-25%). This means that for every 13
patients treated with antibiotics, 1 infection can be prevented. The
number could be as low as one for every 8 patients or as high as one
for every 25 patients. Even though these studies were of low quality
and lacked the power to find significant differences, this represents
the highest level of evidence available. Unfortunately, there was no
clear consensus as
to which type of antibiotic to use, which route to use, or for how long.
delivery using antibiotic impregnated cement beads has been developed
as an adjunct to systemic antibiotic therapy. Experimental data
suggests that antibiotic beads impart high local levels of antibiotic
to the bone and surrounding tissues without significantly affecting
systemic antibiotic levels.21,127,286 Henry et al.127
retrospectively reviewed 404 open fractures in 339 patients who
presented with all types of open injuries, with 30.7% being Gustilo
type III. They found a 2.7% incidence of acute wound infection with
antibiotic beads and systemic antibiotics compared with 11.4% with
systemic antibiotics alone (level of evidence: 4).127 A further study by Ostermann et al.216
compared 157 open fractures treated with prophylactic antibiotics to
547 open fractures treated with antibiotic bead pouches in addition to
prophylactic systemic antibiotics (level of evidence: 4). They found a
4.2% prevalence of acute or chronic infection compared to 17% with
prophylactic antibiotics alone.216 Keating et al.145
reviewed their series of 81 open tibial fractures treated with
intramedullary nails (level of evidence: 4). Their protocol did not
include the use of antibiotic bead pouches as they were introduced
later in their study. The found a 4% prevalence of deep infection after
this protocol change compared with 16% before it.145
usually use a systemic first generation cephalosporin for type I and
clindamycin if there is a penicillin allergy. For type II or IIIA open
wounds, we add either tobramycin or gentamicin, and for type IIIB or C
injuries or severely contaminated wounds, we add penicillin or other
anaerobic cover. We start this immediately after ATLS resuscitation and
continue intravenous treatment for 3 to 5 days following definitive
closure of the wound. For local antibiotic delivery, commercially
available antibiotic-polymethylmethacrylate bead pouches can be used.
However, we usually make our own (see Fig. 55-43) as the elution from both types has been shown to be equivalent.251
We typically use one package of polymethylmethacrylate with the
addition of 2.4 g of Tobramycin and 1 g of Vancomycin. Small 1 to 1.5
cm diameter beads are then made and threaded with surgical steel wire.
We then place these into the wound bed (counting the number of beads
inserted) and cover this with an operative site dressing. This creates
a sterile and antibiotic laden environment. We change this dressing at
the next débridement after 48 to 72 hours with no dressing changes
being carried out on the ward.
for the closure of open tibial fracture wounds. Immediate closure of
open tibial wounds has been shown to result in a higher incidence of
infection and possibly nonunion.245 Russell et al.245
reviewed all types of open tibial injuries (41% type 1, 39% type 2, and
20% type 3) and found the deep infection rate to be 20% after primary
closure and 3% after delayed closure (level of evidence: 4). However,
it may be that some conditions may allow for primary closure as
discussed by Rajasekaran.234 These
include an adequate débridement performed within 12 hours, no
significant skin loss due to either injury or débridement, skin closure
without tension, no farmyard contamination, and no vascular
insufficiency. This approach has been substantiated by DeLong et al.,80 and more recently by Hohmann et al.,129
who suggest that primary wound closure may be safe in selected cases
which include low-energy uncomplicated open fracture wounds. The
adequacy of the débridement and skin perfusion and the importance
tensionless skin apposition cannot be overstated when considering a
wound for primary closure. Advantages of this approach include possible
prevention of nosocomial infection and decreased length of hospital
stay.129
international survey of orthopaedic surgeons suggested that 94% of
surgeons believed that open fracture wounds should be closed in less
than 7days.29 Breugem et al.49
have conducted a systematic review of the literature in an effort to
determine if there are evidence based guidelines for the timing of
wound closure. They identified 10 studies for inclusion, each with
methodologic flaws, with a mixture of retrospective and prospective
studies and because of this were unable to pool any data. They suggest
that if the reviewed literature indicates that early aggressive
débridement and closure of the open fracture wound between 3 to 5 days
reduces the risk of osteomyelitis and delayed union. They did not,
however, present an estimate of this risk because of nonpooling of the
data. They also say that a lack of clearly defined parameters regarding
wound grading and outcomes limits the inferences that can be made from
these studies.
increasing in the management of acute traumatic wounds. Basic science
data presents biologic rationale for the use of negative pressure wound
therapy in that it has been shown to increase granulation tissue,
enhance wound blood flow, and aid in the clearance of some bacterial
species.13,14,200,201,202
Clinical data on negative pressure wound therapy in acute wound
management is increasing; however, the majority of randomized
controlled trials have been conducted on chronic wounds. A recent
systematic review and meta-analysis of randomized controlled trials has
been carried out by Gregor et al.108
It identified 17 published studies for inclusion as well as 19
unpublished trials, of which 5 were stopped early. Of the 17 included
studies in the review, 7 were randomized controlled trials, which
included 1 study on acute open wounds, and 10 were nonrandomized
trials. Of the 19 ongoing trials, 2 were related to high-risk fractures
and open fractures.108,260,261
Their analysis showed that the pooled estimate of effect on changes in
wound size favored negative pressure wound therapy (standardized mean
difference of -1.40; 95% CI, -2.07 to -0.054).108
Since most of these trials were for chronic wound closure, some
extrapolation to the acute setting is necessary. However, given that
initial basic science data provides a biologic rationale and clinical
evidence suggests a benefit as regards wound size, the continued use of
negative pressure wound therapy is promising. Further randomized trials
in the setting of acute trauma, measuring both wound closure and
functional outcome, are necessary to help delineate the indications for
its use. However, we have observed a decrease
in
the use of free flaps for open wounds in their institution secondary to
the use of negative pressure wound therapy. Once an appropriate
granulation bed of tissue has been created, less aggressive plastic
surgical techniques, such as split thickness skin grafting or full
thickness grafting, may be employed.
increasing, there are still wounds where it is inappropriate to use
this technique. These include those with exposed bone and tendon; these
being most commonly seen at the distal aspect of the tibia where
rotational flap muscle coverage is not generally feasible. Demographic
data from the Lower Extremity Assessment Project (LEAP) concerning
those patients who required soft tissue showed that the patients were
commonly young, healthy males who were involved in a motor vehicle or
motorcycle accident.232 In this
cohort, free flaps were more commonly used for distal tibial fractures.
The authors suggested that the use of a free flap as compared to a
rotational flap was less likely to result in wound complications that
may require further operative intervention.232
Free muscle flaps have been used in the past for reconstruction,
although, recently fasciocutaneous flaps have become more popular.
Yazar et al.300 found no significant
difference in functional outcomes between the use of free muscle flaps
or fasciocutaneous flaps for reconstructing open distal third tibial
shaft fractures.
is often difficult, but not infrequently a surgeon can be faced with
this decision when treating severe lower leg trauma. To help guide the
surgeon in making the decision to amputate or salvage a severely
injured lower extremity, different extremity severity scores have been
developed such as the Mangled Extremity Severity Score,126 the Limb Salvage Index,246 or the Predictive Salvage Index.132
However, one of the largest prospectively collected cohort studies done
by the LEAP study group found that these scores were not valid in their
use as predictors of amputation.41,176 They did, however, find that low scores had high specificity and could possibly be used to predict limb salvage potential.41 Similarly, Dagum et al.78
found that amputation index scores only predicted an amputation in 32%
of patients and did not correlate with physical outcome scores (SF-36
and Western Ontario and McMaster Osteoarthritis Index). Further work by
the LEAP study group has suggested that the severity of the soft tissue
injury had the greatest impact on clinical decision-making as regards
limb salvage or amputation. Interestingly, using the Sickness Impact
Profile Outcome Score, the same group found that after 2 years, there
was no significant difference in scores between those who had an
amputation and those who had a reconstruction (p = 0.53).42 They also noted that 53% of the amputation group and 49.4% of the reconstruction group had returned to work at 2 years.42
considered a significant factor in predicting those who would
potentially benefit the most from an amputation. The lack of plantar
sensation in the face of severe lower extremity trauma was a relative
indication for amputation of the extremity. The LEAP study group
assessed 55 patients who had an insensate extremity and were able to
divide these groups into two cohorts: those who had an insensate foot
and underwent amputation and those who had an insensate foot but
underwent salvage surgery. They also compared these groups to control
patients who had no sensory loss and a salvaged foot. Interestingly,
those patients whose legs were salvaged but who had an insensate foot
reported similar outcomes, at both the 1-year and 2-year mark, to those
patients who had a sensate foot and underwent salvage surgery.43
It was also noted that there was a similar proportion in both groups
who had normal plantar sensation at 2 years. They suggest that the
initial plantar sensation is not necessarily prognostic of long-term
plantar sensation or functional outcomes 2 years after an injury.43
health care costs of those who have undergone an amputation for a
mangled or severely traumatized lower limb and found that the projected
lifetime costs associated with an amputation were in fact potentially
up to three times higher than those whose limbs were salvaged.175,177
This is mainly due to the significant costs of lifetime prosthetic
wear. Interestingly, 2 years following the injury, the costs for those
who had an amputation and those whose limbs were salvaged were similar.177
While this is prospective cohort data and would therefore be considered
level 2 evidence, the inability to randomize patients between an
amputation or limb salvage would rate this as the highest level of
evidence for this particular problem (level of evidence: 2). Indeed,
the LEAP study group has the largest prospective cohort of patients
dealing with this issue.42
showed that in 164 patients with acute compartment syndrome presenting
to the Edinburgh Trauma Unit, 69% of these were associated with a
fracture, and in half of those, the fracture was in the tibial
diaphysis. Compartment syndrome was more common in younger men under
the age of 35, and the highest incidence was observed in adolescents.192
They also found that the overall prevalence of compartment syndrome
following tibial shaft fractures was 4.3%.192 Further literature
suggests that the prevalence of compartment syndrome associated with a
tibial shaft fracture may range from 1% to 10%.47,192,274 Compartment syndrome is discussed in detail in Chapter 27.
compartment syndrome. Diagnostic criteria include clinical parameters
such as pain, pain with passive stretch of muscles within the
compartment, and paresthesiae, while other criteria are based on
measurements such as the actual compartment pressure or the ΔP value,
this being the diastolic pressure minus the compartment pressure. There
has been some discussion about the value of these diagnostic
parameters. Ulmer274 undertook a
systematic review of diagnostic studies and identified 104 articles
from the literature which addressed the diagnosis of compartment
syndrome. Because of insufficient data and retrospective study designs,
which have an inherent risk of introducing bias, only four studies were
eligible for inclusion, and these studies provided the data to
calculate positive and negative predictive
values.274 Ulmer274
found that the clinical signs of compartment syndrome, namely pain,
pain with passive stretch, paresthesiae, and paresis had a low
sensitivity and high specificity. That is if compartment syndrome was
not present, there was a significant chance that the signs were not
present. He went on to use likelihood ratios to determine the posttest
probabilities (using odds ratios) of having compartment syndrome with
one, two, three, or four of these clinical signs. Using a prevalence
rate of 5% for compartment syndrome associated with a tibial shaft
fracture, this being the median value from the collated study data, he
determined that with one clinical sign the probability of compartment
syndrome is approximately 25%, with two clinical signs it rises to 68%,
and with three and four clinical signs, the probability of compartment
syndrome rises to 93% and 98%, respectively.274
clinically in unconscious patients or in patients on ventilators. It
may also be difficult to make the diagnosis in alcoholics and drug
addicts.189 A good argument can be
made for monitoring those patients who are potentially at risk for
developing compartment syndrome or who are not able to respond
appropriately to pain or clinical examination.189,241
Monitoring of compartments can be done either continuously or as a
single measurement. There are commercially available products that
allow for single use compartment pressure monitoring with a digital
manometer and also allow for indwelling catheters to be placed. Other
manometers can be used including an arterial line transducer as well as
Whitesides’ technique, which uses a mercury manometer.291 Examples of pressure measurement devices are shown in Figure 55-38.
have assessed three needle configurations and three pressure measuring
devices. They suggest that slit catheters and needles with a side-port
are more accurate than a straight needle, and that an arterial line
transducer and the Stryker device are more accurate than the Whitesides
technique.38 The most accurate
device in their study was the arterial line manometer with a slit
catheter. They also found that straight needles tended to overestimate
the pressure measurements.38 These results are comparable to those found by Moed and Thorderson,198
who reported that straight needles overestimated compartment pressures
by 18 to 19 mm Hg when compared to slit catheters or side-ported
needles.
 |
|
FIGURE 55-38 A.
The Stryker compartment pressure monitor. This is a digital manometer that comes with disposable syringes, needles, and catheters. B. This arterial line transducer can also be used to assess compartment pressures using either a side-ported needle or a slit catheter. Note the components are a transducer, three-way stopcock, and tube to the patient. The entire assembly is filled with normal saline, and the needle or catheter is inserted into the patient’s muscle compartment, usually the anterior compartment. A small amount of saline is injected into the compartment to create a saline “column” and the pressure is then read from the arterial line tracing. The pressure is read close to the fracture and the transducer is kept at the same height as the leg. |
Experimental canine models have suggested that there is an increased
risk of muscle damage with absolute compartment pressure levels that
exceed 30 mm Hg.119 Some authors have recommended values as high as 45 mm Hg as the absolute pressure threshold for fasciotomy.203 However, Whitesides291
suggested that the pressure threshold for fasciotomy should be when the
pressure difference between the compartment pressure and the diastolic
pressure is less than 30 mm Hg, the ΔP. It has been recommended that
the ΔP be used because the use of the absolute measurement of 30 mm Hg
results in an excessively high number of fasciotomies.191,291
Indeed, in a prospective study of 116 patients with continuous
monitoring, it was found that in the first 12 hours of monitoring, 53
patients had absolute pressures over 30 mm Hg, 30 patients had
pressures over 40 mm Hg, but only 1 patient had a ΔP less than 30mm Hg,
and he had a fasciotomy (level of evidence: 4).191
Over the next 12 hours of the study, only 2 patients had a ΔP less than
30 mm Hg and had fasciotomies, whereas 35 patients had absolute
pressures over 30 mm Hg with 7 having pressures over 40 mm Hg. None of
the patients in this series developed any sequelae of compartment
syndrome.189,191 The authors suggested that the use of an absolute decompression threshold of
30 mm Hg would have resulted in a 43% rate of fasciotomy.191
This is not insignificant as a significant proportion of patients will
have long-term sequelae and morbidity from the fasciotomy including
pain, sensation changes around the incision, scar tethering, and muscle
herniation.93 In another study of 25
patients with tibial diaphyseal fractures complicated by acute
compartment syndrome, of which 13 of the patients had undergone
continuous monitoring of compartment pressures, the investigators found
that the delay from injury to subsequent fasciotomy in the monitored
group was 16 hours compared with 32 hours in the nonmonitored group
(level of evidence: 4).189,191 They recommend continuous compartment monitoring of all patients with tibial shaft fractures.
randomized 200 extra-articular tibial shaft fractures into monitored
and unmonitored groups for compartment pressure measurement. The
monitored group received continuous compartment pressure monitoring for
36 hours (level of evidence: 1).121
They had five cases of compartment syndrome in the nonmonitored group
and no cases in the monitored group of patients; however, within the
monitored group of patients, there were 18 patients with a ΔP of less
than 30 mm Hg. None of these patients developed compartment syndrome or
late sequelae. They found that in the awake and alert patient, they
were able to diagnose compartment syndrome in an appropriate time using
clinical signs.121 They suggest that in the awake and alert patient, continuous compartment pressure monitoring may not be necessary.
continuous compartment pressure monitoring in those patients who are of
significant risk of developing compartment syndrome, such as adolescent
males, and those who are unable to communicate pain. This includes
patients with head injury and ventilated patients as well as alcoholics
and drug addicts. One caveat is that the diastolic blood pressure in
patients under anaesthesia may be lower than in the same patients when
measured preoperatively and postoperatively. This was observed in a
prospective cohort study looking at a consecutive series of 242
patients with tibial shaft fractures undergoing intramedullary nailing
(level of evidence: 2).143,271
The authors state that the intraoperative ΔP may be low when compared
with values recorded when the patient is awake, and it is suggested
that the decision to perform a fasciotomy should be delayed until the
patient is awake and has had serial compartment pressure monitoring.143
One other factor to consider when monitoring patients is that the
highest pressures are obtained close to the fracture site, and the
recommendation is to use the highest recorded pressure.124
have been studied to determine their relative effectiveness. These
include MRI, pulsed phase-locked loop ultrasound, scintigraphy, laser
Doppler flowmetry, and near-infrared spectroscopy.3,11,252,293
These modalities all carry the benefits of noninvasive monitoring, but
they have mixed degrees of sensitivity and specificity due to the
traumatic nature of compartment syndrome associated with tibial shaft
fracture. Some techniques also require specialized equipment and
expertise which limits their effectiveness and overall
generalizability. Most of these have been used for chronic exertional
compartment syndrome and while they show promise, their routine use in
the traumatized limb is still to be determined.
However, clinical evidence suggests that the compartment pressure may
be raised by the reduction of the fracture and passage of the nail as
opposed to differences in reaming technique.191,205 McQueen et al.190
continuously monitored 67 patients who were undergoing intramedullary
tibial nailing for fracture. They found no significant correlation
between the severity of injury or delay to surgery and the
intraoperative compartment pressures. The overall incidence of
compartment syndrome was 1.5%.190 Nassif et al.205
conducted a randomized trial comparing the effect of reamed and
unreamed intramedullary nailing on intraoperative compartment
pressures. They found that highest pressures occurred during reaming in
the reamed group and during nail insertion in the unreamed group.
However, there was no significant difference in pressures between the
two groups.205 They also found that
even though ΔP values were greater than 30 mm Hg after nail insertion,
compartment syndrome did not occur in any patient.205 Tornetta and French271
have also documented that intracompartmental pressures are raised
during manual traction and nail insertion but that they decreased
postoperatively. They suggest that perioperative continuous monitoring
in this instance is not required.271
Data from the SPRINT trial showed no significant difference in the
rates of fasciotomy following reamed and unreamed tibial nailing.30
syndrome in those patients who are awake and otherwise alert. In this
patient population, we do not routinely perform invasive pressure
monitoring. However, in patients with a tibial shaft fracture who are
at a high risk of developing compartment syndrome, including young
males, and in those patients in whom clinical signs are inconsistent
such as intubated patients, obtunded patients, or those with analgesic
medications, alcohol, or other drugs affecting consciousness, we assess
compartment pressures with continuous compartment monitoring using a
slit catheter or side-port needle technique and a ΔP of 30 mm Hg to
determine the need for fasciotomy. When required, fasciotomy should be
done on an emergent basis.189,242,277,278,292 We recommend a two-incision fasciotomy. A one-incision technique has been described.181,255 This is less commonly used but both techniques are described in Chapter 27.
malunion in tibial diaphyseal fractures. Indeed, surgeons have defined
shortening as varying from less than 5 mm to greater than 15 mm and
angular malunion as varying from 5 degrees to 20 degrees.25
In literal terms, a malunion would be present when the tibia healed in
any nonanatomic position. However, surgeons generally accept that the
term malunion commonly describes a deformity that results in a
functional disability. Unfortunately, there is no definition as to what
degree of tibial malalignment actually causes functional disability,
and as we
have already indicated the definition of malunion varies considerably between surgeons.25
tibial diaphyseal malunion is difficult to define. The literature is
complicated by a lack of prospective reporting and variable follow-up
times which are both important when assessing issues of prognosis.
Also, the different techniques for measuring malunion have not been
standardized, and many scoring systems have been used to assess the
functional sequelae malunion.46 A
number of small observational case series have been conducted which
suggest that there may be no significant long-term functional detriment
from a malunited tibial shaft fracture. For instance, Merchant and Dietz194
found no major differences in outcome between patients with less than 5
degrees of angulation as compared to those with greater deformity.
However, they only assessed a small number of patients (level of
evidence: 4).194 Kristensen et al.158
examined 17 patients with an angular deformity of greater than 10
degrees after 20 years and using different nonvalidated functional
outcome and radiographic criteria, they found no significant evidence
of knee or ankle arthritis (level of evidence: 4). Milner et al.195
examined 167 patients after 36 years. They used the Western Ontario and
McMaster (WOMAC) functional outcome score to assess knee function
(level of evidence: 4) and found no significant association between the
development of knee or ankle arthritis and tibial malalignment.
However, they suggest that there might be a trend for the tibiae in
varus malalignment to have increased medial compartment knee arthritis
and for the tibiae in varus or valgus to be associated with subtalar
stiffness. However, this was not a statistically significant result.195
reviewed two groups of patients in a retrospective cohort designed
trial (level of evidence: 3). The first group had tibial shaft
fractures treated by community surgeons, and the second group had
tibial shaft fractures treated by orthopaedic trauma surgeons. Both
groups were treated by intramedullary nailing.212
Their follow-up time was less than 2 years, and they defined a malunion
as greater than 5 degrees of angulation in any plane, greater than 10
mm of shortening, and greater than 10 degrees of rotation. They used
the Musculoskeletal Outcomes Data Evaluation and Management System’s
(MODEMS) Pain/Disability instrument lower limb module for their
functional outcome measure.212 They
found that the community surgeon group had a higher rate of malunion as
compared to the orthopaedic trauma surgeon group. However, they found
no significant functional differences between the two patient groups,
save for one subset of the MODEMS score. They recorded a significantly
worse outcome in the malunion group for the bodily function subscale.
However, this result should be interpreted with caution as the numbers
of patients for multiple subgroup analyses was small and there was a
risk of a spuriously positive result.212
looked at malunion in a retrospective case series using a functional
outcome score (level of evidence: 4). They defined malunion as greater
than 10 degrees of rotation, 5 degrees of varus or valgus, and greater
than 10 degrees of antecurvatum or recurvatum.210
Patients were assessed using three functional outcome scores: the
WOMAC, the Lower Extremity Functional Scale, and the Assessment System
of Lower Extremity Function. They found no significant correlation
between the subject’s response to any of the three scores and tibial
malalignment in any plane at a follow-up time of 5.5 years; however,
their patient sample size was small.46
assessed 28 tibial shaft fractures and found that greater ankle
malalignment correlated with poorer clinical results. However, they did
not see the same correlation in more proximal fractures which caused
malalignment around the knee (level of evidence: 4).233 Kyro et al.163
looked at 17 patients treated with an intramedullary nail and found
that those with a malunion had an increased number of subjective
functional findings such as increased swelling of the limb, loss of
knee or ankle motion or subtalar motion, and squatting difficulties
(level of evidence: 4). They defined malunion as being greater than 5
degrees of angulation with any malrotation and greater than 10 mm of
shortening.163
regarding what constitutes a tibial malunion or how a malunited tibia
affects long-term outcome.
patients do present with symptomatic tibial malunion. We have seen
patients who presented with different degrees of varus or valgus, but
especially with rotational malalignment or shortening, that have had
difficulty with walking and that required modified shoe wear (Fig. 55-39).
In making the decision to potentially operate on a symptomatic
malunion, a number of factors must be considered. The surgeon should
investigate to see if there is any arthritis in the ankle, hindfoot, or
knee that may be causing the patient’s pain. If this is the case, it
may well need to be treated either at the time of or before the
osteotomy. It is also important to ensure that there is no nonunion
associated with a partially malunited fracture, no painful hardware or,
in cases of previously open fractures, no deep or occult infection that
may be causing pain.
has been made, preoperative planning studies should be undertaken.
Full-length tibial views and standing leg views should be obtained to
assess the deformity and the overall alignment of the limb. CT
examination to include the knee proximally and the ankle distally may
help assess the degree of angular and rotational deformity. There is no
good evidence as to the best method of operatively correcting a
malunion. The options of using an intramedullary nail, plate, or
external fixator should be individualized based mainly on the location
and severity of the malunion.
understand the nature of the malunion as well as a bone scan and white
blood cell scan to investigate the possibility of occult infection,
particularly if there has been previous surgery or an open injury.
Malunions of tibial shaft fractures are more common in the or proximal
diametaphyseal region and both closed or opening wedge tibial
osteotomies can be done. If the malunion is rotational, transverse
osteotomies may be necessary to allow for derotation of the tibia. If
shortening is present, transverse or step-cut osteotomies using a nail
and bone graft or lengthening over a nail or with an Ilizarov type
frame may be required. We prefer to correct malunions at the site of
the actual malunion rather than correct the angular deformity proximal
or distal to the malunion.
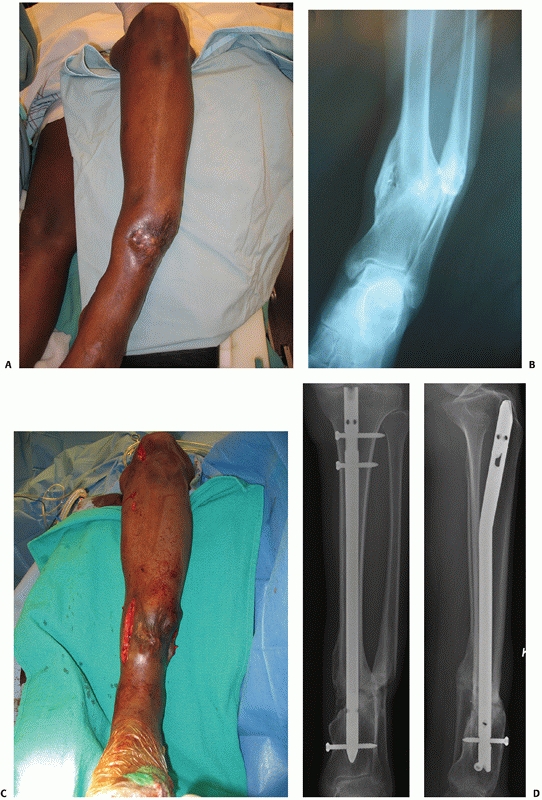 |
|
FIGURE 55-39 A.
This patient had a symptomatic varus malunion and limb length discrepancy resulting from an injury caused by an explosive device 13 years previously. B. Anteroposterior radiograph showing the significant deformity. C. A tibial opening wedge osteotomy and fibular osteotomy was performed to both correct the varus malalignment and gain some length. D. Autogenous bone graft was used at the time of the nail insertion and the patient went on to uneventful healing. |
have the benefit of providing good bony contact but they do potentially
shorten the limb. Opening wedge osteotomies have the benefit of either
preserving limb length or lengthening it to some degree, but they cause
an opening wedge which may need to bonegrafted. A fibular osteotomy
must be done to obtain tibial correction. This is usually undertaken as
an oblique osteotomy or by removing 1 to 2 cm of the fibula 7 cm above
the ankle syndesmosis.
nailing for distal or proximal diametaphyseal malunion fixation and
tibial nailing for diaphyseal malunion. It can, however, be difficult
to pass a nail through callus. As opening the malunion site is
necessary to osteotomize the tibia, a curved awl can be passed in a
retrograde manner into the shaft and through the osteotomy site to
allow for passage of the intramedullary nail. In distal tibial
diaphyseal malunions that have been initially treated with an
intramedullary nail, nail removal is required and a distal tibial
osteotomy is done. It is very difficult to generate a new reaming path
in the distal aspect of the tibia with an intramedullary nail, and we
routinely use plate fixation, as stable anatomic fracture fixation is
achieved.
treat tibial malunions. These have the advantage of allowing the use of
distraction osteogenesis techniques. However, malunion correction often
results in prolonged period of frame application with the inherent risk
of developing pin tract infections with multiple fine-wire fixation
points. Limb lengthening is not advocated unless the limb length
discrepancy is greater than 2 cm. It should be remembered that opening
wedge osteotomy and correction of deformity can also lengthen the limb
to some degree, and this needs to be taken into account in preoperative
planning.
basic types: atrophic, oligotrophic, and hypertrophic nonunions. The
full classification is shown in Figure 55-40.288
Classically hypertrophic or oligotrophic nonunions represent a failure
of stability while atrophic nonunions represent both a failure of
stability and/or biology. Nonunions may also be classified as septic or
aseptic. Nonunion is a recognized complication of tibial diaphyseal
fractures. However, it is difficult to define what constitutes a true
nonunion or a delayed union. It is also difficult to find agreement as
to when delayed union or nonunion has occurred and when treatment
should be undertaken in an established nonunion. In a cross sectional
survey of 577 orthopaedic surgeons, Bhandari et al.33
asked surgeons which variables should be used in the assessment of
fracture healing. The variables were callus size, cortical continuity,
progressive loss of the fracture line, pain with weight bearing, and
pain on palpation of the fracture site. Surgeons were also asked to
provide a time limit beyond which a delayed union becomes a nonunion.25
They had a survey response rate of 77%. Surgeon definitions of what
constituted a delayed union ranged from 1 to 8 months and definitions
of when delayed union became a nonunion ranged from 2 to 12 months.25
The average elapsed time prior to the establishment of a delayed union
was 3.5 +/- 1.4 months, and the average time for a fracture to be
labelled a nonunion was 6.3 +/- 2.1 months.25
Interestingly, a surgeon’s age, academic practice, fellowship training
in trauma, or trauma volume did not necessarily predict their
definition of delayed union or nonunion. Some surgeons have defined
delayed union as ranging from 3 to 4 months and nonunion as ranging
from 6 to 8 months.289 Others have
not used absolute times and have employed a more physiologic approach
such as the lack of continued healing as assessed on three follow-up
visits.87
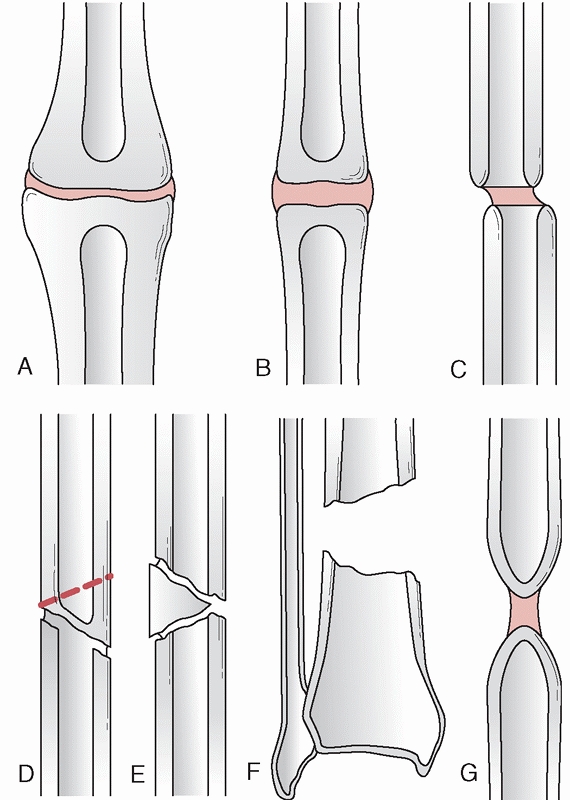 |
|
FIGURE 55-40 The Weber and Cech228 classification of nonunion. The top line (A,B,C) represents nonunions capable of biologic reaction. A. Elephant’s foot. B. Horse’s foot. C. Oligotrophic. The bottom line (D,E,F,G) represents nonunions incapable of biologic reaction. D. Dystrophic (torsional) wedge fracture. E. Necrotic (avascular segment). F. Bone defect. G. Atrophic.
|
attempted to provide estimates for healing time in 943 patients with
closed tibial shaft fractures. Union was defined as being present with
radiographic evidence of peripheral callus bridging the fracture site,
lack of pain with weight bearing, and no pain on palpation of the
fracture site.247 The healing times
ranged from 6.7 weeks to 75 weeks with a mean of 18.8 weeks.
Seventy-five percent of the fractures had healed by 21 weeks and 90%
were healed by 26 weeks.247 Oni et al.215
prospectively followed 100 patients with closed tibial shaft fractures
defining union as callus bridging the fracture site. They reported that
all but four fractures had healed by 30 weeks.215
reported that the incidence of nonunion for closed tibial shaft
fractures ranged from 0% to 3.5% depending on the Tscherne grading of
injury and that on average closed tibial fractures take 17 weeks to
unite. In open fractures, Court-Brown65 reported that nonunion rates ranged from 7.1% to 38.5% depending on the Gustilo fracture grade. Whelan et al.289
have examined the interobserver and intraobserver variation in the
assessment of healing of tibial fractures. Four surgeons (three
orthopaedic trauma surgeons and one orthopaedic trauma fellow) assessed
30 sets of anteroposterior and lateral radiographs of tibial shaft
fractures treated with an intramedullary nail in various stages of
healing.289 Each surgeon rated the
radiographs using the criteria proposed by Hammer et al.118 as well as their own general overall view of fracture healing (Table 55-10).
They also assessed the extent and quality of callus, the number of
cortices with bridging callus, and the number of cortices with a
visible fracture line. There was moderate overall agreement when using
Hammer’s criteria (k = 0.6, 95% CI, 0.52%-0.68%). The agreement of
their general impression had an overall weighted kappa of 0.89 (95% CI,
0.79%-0.99%). This suggests that the surgeons’ general impressions were
as reliable as the strict criteria suggested by Hammer.118
|
TABLE 55-10 Classification of Fracture Healing According to Hammer et al.118
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
prevalence of nonunion, how it should be diagnosed, or when it should
be treated. Whelan et al.289 have
recommend assessing the number of cortices bridged by callus on the
anteroposterior and lateral radiographs of the tibia at each follow-up
visit as the most reliable method for assessing the progression of
healing of fractures after intramedullary nailing, but it is important
that the orthopaedic community develops a consensus regarding the
diagnosis and timing of treatment of nonunions.
pain, motion, and swelling at the fracture site. An examination of the
overlying skin and soft tissues should be undertaken looking for any
draining sinuses or other clinical signs of infection as well to
ascertain how well the soft tissues may be able tolerate further
surgery. It is also important to assess the overall alignment of the
leg for angular and rotational malalignment and limb-length
discrepancies. A thorough examination of the knee, ankle, and hindfoot
joints should be carried out to assess range of motion and to look for
contractures. Radiologically, the nonunion may present as a persistent
fracture line or with a lack of bridging callus in of three out of the
four cortices on the anteroposterior and lateral radiographs.289 The radiographs may also show hardware breakage or failure, as shown in Figure 55-41.
Malalignment may also be present particularly if the fracture was
originally treated nonoperatively. Further imaging with CT scans or
bone scans may also be useful.
factors that help to predict nonunion. These are fracture morphology,
mechanism of injury, degree of soft tissue injury, diabetes, alcohol
consumption, smoking history, surgical delay, the use of steroids or
anti-inflammatory medication, vasculopathy, age, and gender.215,247,273 In an observational study to determine predictors of reoperation, Bhandari et al.32
identified 200 patients with tibial shaft fractures and assessed 20
possible prognostic factors. They used both univariate and multivariate
regression modeling techniques and suggested that three variables
predicted the risk of reoperation. These were the presence of an open
fracture wound (RR = 4.32, 95% CI, 1.76%-11.26%), lack of cortical
continuity between the fracture ends following fixation (RR = 8.33, 95%
CI, 3.03%-25%), and the presence of a transverse fracture (RR = 20.0,
95% CI, 4.34%-142.86%) (level of evidence: 3) (Table 55-11).
The authors suggest that using these three variables, one can
categorize patients into four different risk groups. These are low
risk, where there are no risk factors and a 4.4% prevalence of
nonunion, moderate risk, where there is one risk factor and an 18%
prevalence,
high risk, with two risk factors and a 47% prevalence, and very high
risk, with three risk factors and a 94% prevalence of requiring
reoperation to promote bone healing. The only modifiable risk factor
suggested in the multivariate analysis was cortical continuity.32
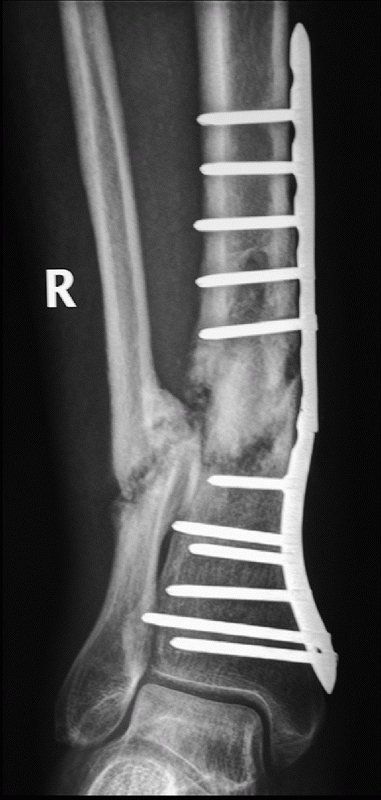 |
|
FIGURE 55-41 Distal tibia fracture treated with a medial locked plate with development of aseptic nonunion and hardware failure.
|
|
TABLE 55-11 Predictors of Reoperation for Tibial Shaft Fractures*
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Audige et al., who attempted to construct a path analysis of prognostic
factors that were associated with delayed healing or nonunion.17
Their path analysis and multivariate model of prognostic factors
determined that the most important factors were the extent of the skin
injury, the presence of a postoperative fracture gap, and a distal
location of the fracture in the shaft.222
Interestingly, they identified associations between some of the
prognostic variables. For instance, surgical treatment using either a
nail, external fixator, or plate was potentially influenced by the
fracture type as well as the skin injury.17
In this path analysis, the skin injury severity was shown to have the
most significant effect on the risk of delayed or nonunion with an open
skin injury of greater than 5 cm in length resulting in a RR of 5.7
(95% CI, 3.0%-10.9%) or approximately a sixfold increase in the risk of
delayed union or nonunion when compared with no skin injury with a RR
of 1.0. It is interesting to note that a closed skin injury resulted in
an approximately threefold increase in the risk of nonunion (RR = 2.8,
95% CI, 0.85%-8.9%). This is in keeping with previous work by Gaston et
al.,99 which suggests that the
Tscherne classification of closed fractures correlates with functional
outcome, and the presence of an open wound correlates with the time to
union. Thus, from path analysis work there would seem to be range of
predicted risk of nonunion from the lowest predictive risk which would
include no skin injury, a midshaft fracture, and no postoperative
fracture gap, to a 70% risk in patients with a distal tibial shaft
fracture, an open skin injury greater than 5 cm in length, and a
postoperative fracture gap.17
skin and soft tissue damage, a postoperative facture gap, and distal
fractures with a higher-energy fracture morphology.
Intramedullary nailing is advocated by many authors for the treatment
of aseptic hypertrophic but otherwise aligned nonunions. Megas et al.193
looked at 50 patients with aseptic tibial nonunion treated with a
reamed intramedullary nail (level of evidence: 4). The group consisted
of 36 patients initially treated with external fixation, 6 with a
plate, 1 with a previous intramedullary nail, and 7 with nonoperative
treatment. Sixteen of these patients required opening of the nonunion
site to remove hardware. All of the fractures united within 6 months of
the intramedullary nailing procedure.193
The intramedullary nailing in those who had previously been treated
with external fixation was performed 10 days after removal of the
external fixator.193 Sledge et al.257
also used reamed intramedullary nailing in 51 patients with nonunion
who were initially treated with a number of different procedures. Ten
patients required bone grafting and 33 were treated with a partial
fibulectomy. Ninety-six percent of their patients united in an average
of 7 months (level of evidence: 4).257 Alho et al.6
also analyzed 25 nonunions treated with a reamed intramedullary nail.
All of their nonunions united, but they did require to bone graft 1
patient (level of evidence: 4).
nonunions is slightly more demanding as passage of the nail past
through fibrous nonunion site may be difficult. The canal may need to
be opened at the nonunion site with an awl being passed proximally and
distally from the nonunion to allow for the subsequent passage of the
power reamers and the nail. It is important that the initial power
reamer is an end cutting reamer to facilitate passage through the
nonunion. If there is any tibial malalignment, a fibular osteotomy will
probably be required to permit anatomic alignment of the tibia. This is
especially true of distal tibial fractures (Fig. 55-42). It is also important to remove all previous hardware as this may impede placement of the nail.
found a nonunion rate of 4.4% in closed tibial shaft fractures. In open
tibial fractures, the nonunion rate varied with the Gustilo grade, but
it was noted to be as high as 38% in Gustilo IIIB fractures. Most of
the nonunions were treated by exchange nailing, which is the practice
of removing the previously placed intramedullary nail, overreaming the
canal, and placing a larger nail into the tibial shaft.66
Usually, the nail that is placed is 1 to 2 mm larger in diameter than
the previously placed intramedullary nail, depending on the size of the
original nail. Cross screws are rarely required as the fibrous union
provides stability, but if they are used, the nail is usually locked
dynamically with a screw being in the bone end of the nail closest to
the nonunion.
that exchange nailing was successful in 89.5% of nonunions in closed
tibial shaft fractures (level of evidence: 4). In a earlier study,
Court-Brown et al.71 had looked at
33 tibial nonunions treated with an exchange nail and found that
approximately 88% united after one exchange nail, and the rest united
after a second exchange nailing procedure (level of evidence: 4).
Complications after exchange nailing included a higher incidence of
wound infection (12.1%) when compared with primary nailing, with 1
patient developing deep infection.71 Templeman et al.266
reported an 11% infection rate in their series of 28 patients with
aseptic non-union treated with exchange nailing. Two of their three
infections occurred in patients who had had Gustilo IIIB fractures
(level of evidence: 4).266 However,
they had a 93% union rate after the exchange nail with the other two
fractures healing after a second bone graft procedure. Analysis of
these two studies shows that the contraindications to exchange nailing
include bone loss over 2 cm in length and involving more than 50% of
the circumference of the tibia and active infection.71,266
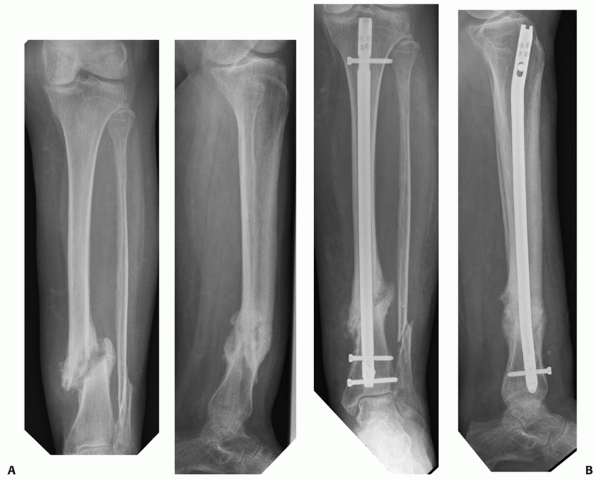 |
|
FIGURE 55-42 A.
Anteroposterior and lateral radiographs of a distal tibia fracture that was treated initially with a long leg cast. Note the translated, angulated hypertrophic nonunion. B. The patient was successfully treated with an intramedullary nail and fibular osteotomy and radiographs show successful union. |
which a statically locked intramedullary nail is converted to one that
is dynamic. This is done by either removing the proximal or distally
statically locked cross screws or by removing one statically locked
proximal cross screw, leaving the other proximal screw in the dynamic
slot in the proximal end of the nail. The principle is to increase
compression at the fracture site with weight bearing. It is a procedure
that is associated with low morbidity, and it is used to encourage
union. It is, however, limited in its scope and is not recommended in
unstable tibial
nonunions or if there is a segmental defect. Clinical evidence of its usefulness is very limited. Wu and Chen297
demonstrated a 54% success rate of union in tibial and femoral
fractures after dynamizing the intramedullary nail (level of evidence:
4) but Court-Brown and his colleagues65,68 have suggested that dynamization has little effect on fracture union.
These studies suggest that a 6 cm bone is the practical limit for the
use of autogenous bone graft techniques. However, it has been shown
that defects of less than 2 cm associated with less than 50%
circumferential bone may heal with exchange nailing.71
Broadly, we are in agreement with these view but we will bone graft
defects greater than 1 cm, and we reserve exchange nailing for those
defects less than 1 cm. Defects of less than 4 to 6 cm are amenable to
treatment with autogenous bone grafting after the wound has healed, and
there is no clinical, biochemical, or radiographic evidence of
infection.
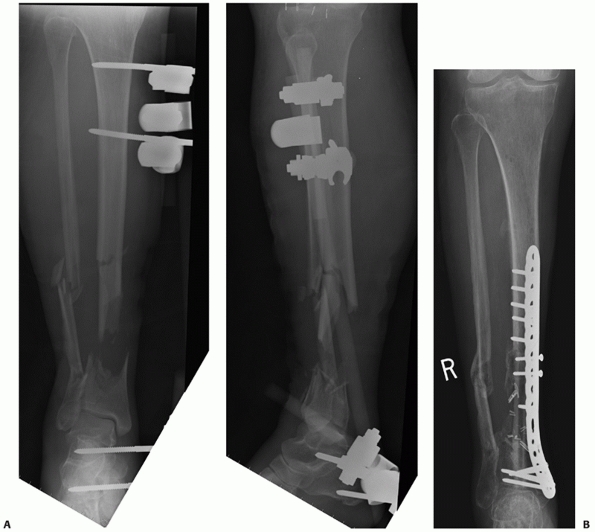 |
|
FIGURE 55-43 A.
Anteroposterior and lateral radiographs of Gustilo IIIB open fracture with segmental bone loss which was thought to be too distal for tibial nailing. B. The patient was treated initially with a spanning external fixator and then definitively with a free-fibular bone graft with a paddle of overlying skin. This fracture united and the patient is now weight bearing and undergoing rehabilitation. |
morphogenetic protein (BMP)-2 or BMP-7, suggests that there may be role
for their use as inductive agents. However, they require the use of a
conductive carrier, usually an allograft, and have in some instances
shown equivalency but not superiority to autograft.142 Jones et al.142
compared autogenous bone grafting and BMP-2/freeze-dried cancellous
allograft for tibial defects that averaged 4 cm in length. They
assessed 30 patients and found that 10 patients treated with autograft
and 13 patients treated with BMP-2/allograft healed without any further
interventions. They suggest that BMP-2/allograft composite is both safe
and effective for treating this size of tibial defect (level of
evidence: 2).142 However the cost-effectiveness of the use of BMPs in healing segmental tibial defects is still being debated.7
treatment plans are necessary. The main treatment choices include the
use of repeated bone graft or vascularized bone transport techniques
compared to techniques incorporating the Ilizarov or other subsequent
lengthening, or bone transport techniques either with the frame or over
a nail (Figs. 55-43 and 55-44).19,77,147,188,299
assessed 44 patients in a nonrandomized fashion who had tibial
segmental defects from 4 cm to 12 cm. Twenty-one patients were managed
with Ilizarov distraction, and 23 patients were managed with massive
cancellous grafts and tissue transfers. They found consolidation in 71%
of the Ilizarov group and 74% in the bone graft group; however, the
major complication rate in the bone graft group was 60% compared to 33%
in the Ilizarov group.59 They also
found that the Ilizarov technique was better for compromised hosts and
was less expensive. However, they comment that early patient
independence and the need for minimal patient cooperation makes
“conventional” treatment more appealing with defects less than 6 cm.59 Paley and Maar221
assessed their series of 19 patients, with a mean tibial defect of 10
cm, treated with Ilizarov bone transport techniques. Union was achieved
in all patients, but 10 patients required bone grafting or débridement
of the bone ends at the docking site and the average time in the
Ilizarov frame was 16 months.221 Similar results using the Ilizarov technique have been found by other authors.2,36
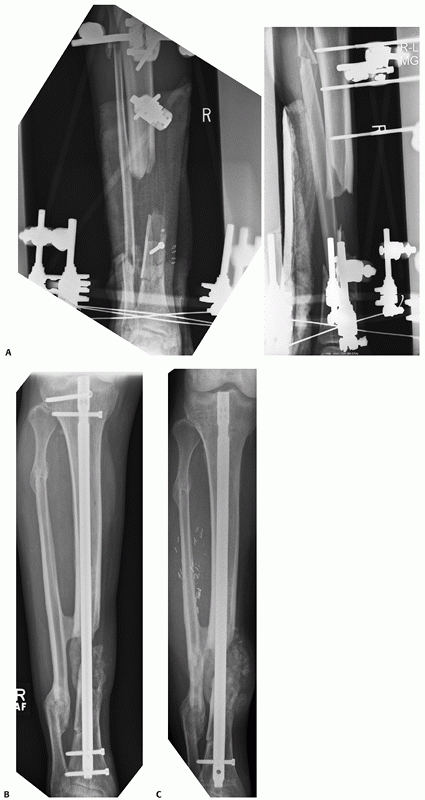 |
|
FIGURE 55-44 A.
Anteroposterior and lateral radiographs of a Gustilo IIIB open distal tibial fracture with segmental bone loss. The fracture was initially treated with a hybrid external fixator. B. Intramedullary nailing was subsequently performed together with autogenous bone grafting. C. The patient required a second bone graft operation. A free anterolateral thigh flap was performed because of compromised soft tissues. The fracture united. |
the patient and also provides a major challenge to the treating surgeon
as regards optimal treatment strategies. The treatment usually needs to
be individualized depending on a number of factors. These factors
include the extent and duration of infection, the timing of infection
following operative management, the presence of previously placed
hardware, the degree and nature of soft tissue compromise around the
fracture, as well as whether there is segmental tibial bone loss.
categorized them into two groups. Type A being infected nonunions of
the long bone with nondraining or quiescent infection with or without
an implant.140 Type B cases were an infected nonunion of the long bone with a draining or active infection.140
They then subdivided both these categories based on the length of the
fracture gap. Subtype 1 was a nonunion with a bone gap smaller than 4
cm and subtype 2 was a nonunion with a bone gap longer than 4 cm.140
reviewed 459 tibial shaft fractures treated by reamed nailing and
classified infection into three groups. Group 1 patients were those who
presented early with pain, erythema, and swelling at the fracture site
but no evidence of a pyogenic collection.72 Group 2 consisted of patients who presented early but had evidence of pyogenic collection.72 Group 3 included patients who presented late and had developed a pyogenic discharge.72 Zych and Hutson305
also separated patients with infection into three subgroups: acute,
subacute, and chronic phases. They defined the acute phase as being
within 2 weeks of injury. Patients presented with pain and fever, but
radiographs usually showed no changes. In the subacute group, the
patients presented more than 2 weeks after nailing, and they often
presented with a pyogenic collection. Radiologic changes were common.
In the chronic group, patients presented with chronic infection. Zych
and Hutson305 subdivided this group
into those who had a persistent nonunion and those in whom the nonunion
had united but the infection persisted.
clinical grounds. Patients often present with erythema either around
skin incisions or related to underlying hardware. There may also be
soft tissue fluctuance or a draining sinus. It is important to ask
about constitutional symptoms such as fevers, chills, sweats, and
general malaise. Current imaging, in the form of nuclear medicine
scanning, is usually undertaken. Two scans are often used: the
technetium labelled phosphate bone scan and the indium-111-labelled
white cell scan. Other imaging techniques that are useful in the
diagnosis of infection are discussed in Chapter 24.
methylene diphosphonate bone scans and indium-111-labelled white cell
scans have a high sensitivity and specificity for diagnosing infection
in the presence of fracture nonunion. Indeed, they have been shown to
range from sensitivities of 60% to 86% and specificities of 84% to 97%.148,207
Using these numbers, we can calculate the likelihood ratio, given a
positive test, as being approximately 2.5 to 7. However, there are
situations that may result in a false-positive test and these include
metaphyseal sites of fracture that are adjacent to a joint with
arthritis, instability in the delayed or nonunion site, recent surgery,
as well as conditions such as heterotopic ossification or myositis
ossificans.207,219
White cell-labelled imaging studies may also be combined with bone
marrow imaging. It has been suggested that this may increase test
accuracy up to 90%.216,217
This is due to the fact that fractures, hardware, recent surgery, or
neuropathic joints may lead to alterations in the bone marrow.217,218
Although these tests are not necessary in every case of infected tibial
nonunion, they may be valuable diagnostic aids when the clinical or
radiographic signs are not clear.
severity of soft tissue damage. Some of the potential risk factors for
developing infection following intramedullary nailing include
inappropriate fasciotomy closure, exchange nailing, and thermal
necrosis.66,227 Marginal necrosis of skin flaps after plastic surgical reconstruction of soft tissue defects may also cause bone infection.227 Jain and Sinha,140
based on their classification, recommend single stage débridement with
bone grafting and fracture stabilization for type A1 infected nonunions
and a staged protocol for type B1 infected nonunions. They suggest
distraction osteogenesis is the procedure of choice for both A2 and B2
nonunions.140
from their classification system, recommend treating Group 1 patients
with high dose intravenous antibiotics followed by oral antibiotics for
6 to 8 weeks. In Group 2 patients, treatment with exchange nailing with
reaming of the intramedullary canal was undertaken. Union occurred in
these patients, but was prolonged compared to the Group 1 patients. The
Group 3 patients required extensive débridement as well as
reconstruction of the bone and soft tissues.72 Zych and Hutson305
suggest that acute phase patients are treated with appropriate
antibiotics. Their subacute phase patients required surgical
débridement with exchange nailing, and their chronic phase patients
generally required bone and soft tissue débridement with subsequent
bone and soft tissue reconstruction. The most common pathogen in their
series was Staphylococcus aureus, which occurred in 64% of patients.305
16 case series (level of evidence: 4) that discussed a one stage
revision. Thirteen of these studies included fractures of the tibia.
They also identified 18 case series (level of evidence: 4) that
assessed the use of a two stage revision procedure for infected
nonunion, of which 14 case series discussed fractures of the tibia.262
The rest of the series and the review discussed fractures of the femur.
Interestingly, both protocols of management resulted in union rates and
persistent infection rates that were similar. With regard to one stage
surgery, there was low quality evidence from observational case series
suggesting a union rate of 70% to 100% and a persistent infection rate
of 0% to 55%. One subgroup of the one stage surgery group included
patients who were treated with débridement and bone transport using an
Ilizarov frame This technique was associated with union in 70% to 100%
and persistent infection in 0% to 55%.262
In the two stage revision technique, there was low quality evidence
(Grade C) from the observational case series that suggested union rates
of 66% to 100% with persistent infection rates of 0% to 60%. In the
subgroup of débridement and planned secondary bone grafting, union
rates were 75% to 100% with persistent infection from 0% to 60%. The
subgroup of those who received débridement, antibiotic beads, and
planned secondary fixation had union rates of 93% to 100% with
persistent infection in 0% to 18%.262
They concluded that there were significant limitations in all of the
case series as well as significant heterogeneity between studies which
made it difficult to suggest clear recommendations based on the current
literature.262
treating both the patient and the tibial nonunion. Early in the
treatment process, we attempt to optimize patient factors that affect
healing. Clinical determination of the patient’s general health status
including a full history and physical examination to ascertain whether
or not the infection is localized to the bone or whether the infection
is systemic is crucial. If the infection is systemic, treatment with
high dose intravenous antibiotics and supportive therapy based on the
patient’s condition is necessary. We obtain laboratory studies
including a white blood cell count, an erythrocyte sedimentation rate
(ESR), and C-reactive protein (CRP), as well as blood cultures. Full
anteroposterior and lateral radiographs are mandatory. We do not
routinely obtain a bone scan or white cell labelled scan if the
diagnosis of infection is obvious, but if there is any doubt about the
extent and depth of infection, we do use these investigative
modalities. Ultrasound examination may also prove useful in helping to
determine whether or not there is any underlying collection of fluid. A
CT examination may also help to identify sequestra and collections of
pyogenic material. We do not routinely use MRI examination, as most
patients have indwelling hardware that can obscure the images. We also
routinely counsel patients regarding substance abuse and smoking
cessation, but we do not preclude surgery based on these factors.
with wound infections but with no obvious underlying pyogenic
collections are treated with systemic or oral antibiotics depending on
the extent of the infection. The infection resolves in most cases. In
those patients who present late, we commonly use a two stage technique
for reconstruction. The first stage is an extensive and thorough
débridement. All necrotic and dysvascular bone is removed as well as
obviously necrotic and devitalized tissue. If an intramedullary nail
has been used, we routinely overream the intramedullary canal and
undertake a thorough lavage. We enlarge the locking screw holes to
ensure a satisfactory flow of lavage fluid through the medullary canal.
We place an antibiotic cement nail in the intramedullary canal as
described by Paley.220 We then place
a guide wire inside a 32F chest tube and fill the tube with antibiotic
laden cement. We use prepackaged cement with tobramycin but add 1 to 2
g of vancomycin. The antibiotics thicken the cement, so the tube must
be filled while the cement is still fluid. As the cement cures, the
chest tube is cut away from the cement tube, which is then placed in
the canal. Cement is then used to fill the bony defect. It is packed
into the defect gently to facilitate its later removal. The guide wire
is left slightly proud of the tibia to also facilitate later removal.
All retained hardware is removed at the time of the initial débridement
but deformity correction, if required, is undertaken at the second
stage of reconstruction.
compromised or there is a sinus and we feel, in conjunction with a
plastic surgeon, that free flap reconstruction is necessary, we will
undertake the free flap reconstruction at this stage. Our plastic
surgical team has been using an anterolateral thigh free flap for the
reconstruction of soft tissue defects.224,301
This flap is a fasciocutaneous flap based on the descending branch of
the lateral femoral circumflex artery. These vessels are found
proximally in the anterior aspect of the thigh in the layer between the
rectus and the vastus lateralis.91
The artery and its accompanying two vena comitantes are divided using
clips, and the fasciocutaneous flap is transferred to the lower limb
and most commonly anastomosed to the posterior tibial artery. However,
an end to side anastomosis with any patent artery in the lower limb can
be done. In the case of a one vessel leg, it can be attached as a flow
through flap. Preoperative flow CT angiography is recommended to assess
the patency of the three vessels in the lower limb when planning flap
reconstruction. This flap provides a significant amount of tissue for
coverage. It also brings in a good blood supply, and results in a good
cosmesis at the donor site with minimal donor site morbidity (Figs. 55-45 and 55-46).
stability, and if this is the case we do not use any internal fixation
initially but prefer to immobilize the patient in a functional brace or
cast leaving the knee free to allow mobilization. If, however,
stability is required, an indwelling cement filled intramedullary nail
and cement spacer often imparts adequate stability that can be
supplemented with a cast as necessary. If there is a segmental bone
defect following plate débridement, we routinely use external fixation
to stabilize the nonunion. We place this external fixator outside of
the zone of injury to allow for flap reconstruction, if necessary, and
also to facilitate further reconstructive procedures.
antibiotic laden cement spacers or antibiotic impregnated,
osteoconductive bioabsorbable bone substitutes as these have
both been shown to be effective in delivering local antibiotic to tissues and helping to eradicate infection.186,220,251
We routinely consult infectious diseases specialists and administer a
prolonged course of appropriate antibiotics. Intravenous treatment is
given for 7 days followed by oral treatment for 6 weeks, as described
by Swiontkowski et al.264
Postoperatively, we routinely assess both clinical and hematologic
information and obtain a technetium-labelled bone scan as well as an
indium-labelled white blood cell scan after the antibiotic course to
ensure that there is no residual occult infection prior to
reconstruction.
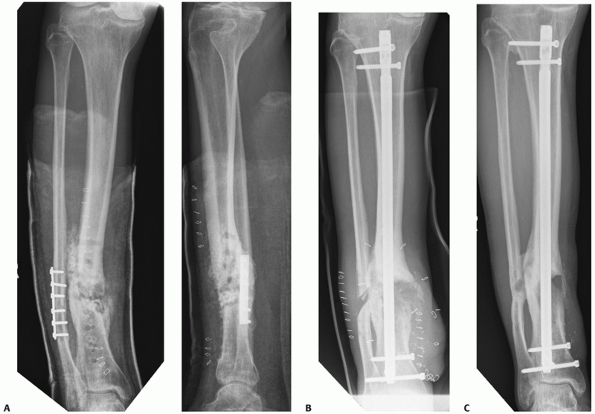 |
|
FIGURE 55-45 A.
Anteroposterior and lateral radiographs of a patient who developed an infected tibial nonunion after initial plate fixation of a distal diametaphyseal fracture. The plate was subsequently removed and a limited débridement undertaken. After transfer to our care, it was noted that there was still drainage on the medial aspect of the leg. A further débridement and sequestrectomy was performed. The patient then received antibiotic therapy. After the CRP and ESR had returned to normal and a bone scan and white blood cell scan showed a low probability of infection, definitive reconstruction was undertaken. B. The patient was treated with an anterolateral thigh free flap, autogenous bone grafting, and an intramedullary nail. C. While the medial cortex has still not completely filled in, the patient is pain-free and has returned to work. |
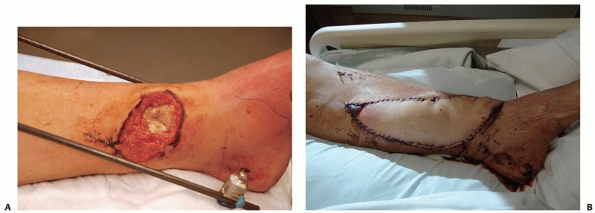 |
|
FIGURE 55-46 A. A patient was referred with exposed medial bone having had two previous irrigation and débridement procedures. B.
After a further wound toilet and débridement of devitalized bone, we undertook definitive fixation, and an anterolateral thigh flap which has healed. The patient awaits further bone grafting for a 2-cm bone defect. |
reconstruction. We remove the intramedullary cement spacer and place
another reamed intramedullary nail into the canal placing bone graft
into the fracture site as necessary. Many nonunions are distal in
location, and if we are unable to use an intramedullary nail, we
routinely use a medial locking plate as this provides significant
stability. It is easily placed percutaneously underneath a flap and
also allows for compression of the fracture site using a tensioning
device as necessary.
one stage reconstructive procedure utilizing the Ilizarov device. This
requires two surgical teams, composed of orthopaedic and plastic
surgeons. Any sinus tracts or open wounds are explored, irrigated, and
débrided of all necrotic skin, muscle, and bone, leaving healthy
tissue. Old hardware is removed. The surgical incision is extended to
facilitate exposure and the subsequent vascular anastomosis. With
traditional stabilization, the plastic surgical team raises and secures
a free flap for skin coverage, and the Ilizarov frame is assembled and
applied. Tension wires of 1.8 mm are inserted into the proximal and
distal segments of the tibia with the goal of obtaining two rings or
two or three points of fixation both proximally and distally. Care is
taken to stay outside of the zone of flap coverage and to not damage
the vascular pedicle. If there is less than 10 degrees of malalignment
or 1 cm of leg length discrepancy, bonegrafting is done, but if there
is greater malalignment or leg length discrepancy, then a corticotomy
and bone transport is undertaken. Postoperatively, distraction is
started at 14 days at 1 mm per day in four increments. The pins sites
are cleaned with 1:20 chlorine once a day and left open. Full weight
bearing is allowed at 4 weeks. The frame is removed when there is both
clinical and radiographic evidence of bony union as defined by bridging
bone on three of four cortices on anteroposterior and lateral
radiographs.
low-intensity pulsed ultrasound, electrical stimulation, and
extracorporeal shock wave therapy. These bone stimulation devices all
purport to accelerate fracture healing and to aid in the healing of
nonunions. Most of the literature relates to low intensity ultrasound
and electrical stimulation therapy. Shock wave therapy is currently
under investigation with early reports suggesting its efficacy in fresh
fractures and nonunions.
many authorities in the past considered fractures an absolute
contraindication to therapeutic ultrasound. More recent work, however,
has shown that the intensity of therapeutic ultrasound determines its
effect on healing bone. The high-intensity (1.0 W/cm2) continuous wave ultrasound applied in earlier animal studies appears to be harmful,235,272 whereas low intensity pulsed ultrasound (30 mW/cm2) accelerates healing.211 Positive findings in animal trials85,230 and uncontrolled human trials298
have provided further support for the inference that low intensity
ultrasound may be beneficial. The mechanisms for these beneficial
effects may include positive effects on signal transduction
(second-messenger activity of chondroblasts and osteoblasts), gene
expression, blood flow, tissue modeling and remodeling, and the
mechanical attributes of callus.12,85,231,235,272
and two further trials, which reported on fractures that had had prior
operative treatment with an intramedullary nail, appeared to report on
a shared data set.89,90
When pooled, the results from the five original studies found a
nonsignificant advantage in the use of low-intensity pulsed ultrasound
when compared with placebo for fracture healing (ES = -2.73; 95% CI,
-5.53%-0.06%). However, there was substantial heterogeneity between
trials (I2 = 98%). When the results
for the two trials that reported on similar populations were pooled,
the test of heterogeneity was 0% and a significant effect of large size
was found in favor of therapeutic ultrasound for reducing fracture
healing time versus placebo (-6.03; 95% CI, -6.87%–5.20%). Converting
this effect size to healing time results in a difference of 60 days
between ultrasound and control groups. This effect, however, was not
seen in the two studies that assessed ultrasound after intramedullary
nailing of tibial fractures, as they found no significant difference in
healing time.89,90
Recent studies suggest that electromagnetic stimulation has an impact
on many cellular pathways, including growth factor synthesis,1,111,174 proteoglycan and collagen regulation,60,125 and cytokine production.259
These pathways may enable bone to respond to changing environments,
ultimately stimulating the calcium/calmodulin pathway thus enhancing
bone healing.98,107
on fracture healing identified 10 randomized trials. Evidence from four
trials reporting on 106 delayed or nonunited fractures demonstrated
an overall pooled relative risk of 1.76 (95% CI, 0.8%-3.8%; I2 = 60.4%; Q = 7.577) in favor of electrical stimulation, but was not statistically significant.116
Single studies reported a positive benefit of electrical stimulation on
callus formation in femoral intertrochanteric osteotomies, a limited
benefit of electrical stimulation for conservatively managed Colles’
fracture or for lower limb-lengthening, and no benefit on limb length
imbalance or the need for reoperation in surgically managed
pseudarthrosis. Pain was significantly improved in one of the three
trials assessing this outcome.
trials that focus largely on radiographic fracture healing. Until well
conducted trials demonstrate efficacy of bone stimulators to improve
patient quality of life and function, their role in orthopaedic surgery
is uncertain.
they were isolated from demineralized bone matrix and found to promote
bone formation when implanted into muscle. There have been
approximately 15 BMP proteins isolated, and they can be divided into
subfamilies based on homogeneity between amino acid sequences.107
The BMPs function via transmembrane serine/threonine kinase proteins
that become phosphorylated upon ligand binding forming a “BMP-receptor
II-receptor I complex.”107 This
complex then phosphorylates and activates the transforming growth
factor beta transducers, or Smad proteins, which then act to regulate
eventual protein production via regulation of gene transcription in the
nucleus.107 This signal transduction
cascade then acts to potentiate the differentiation of mesenchymal stem
cells to osteoblasts. In vitro studies suggest that this conversion may
in part be dose dependent with higher concentrations resulting in
differentiation to osteoblast function.107 Currently, there are 2 BMPs available for use: BMP-2 and BMP-7.
the use of BMPs in fracture care as well as a cost-effectiveness
analysis has been done by Garrison et al.98
They identified nine randomized trials assessing BMPs used for the
management of nonunions or acute fractures associated with open
injuries. Eight of these assessed fractures of the tibia. The largest
of these trials and most methodologically rigorous was conducted by
Govender et al.,106 who assessed the
use of BMP-2 in addition to standard of care and standard of care alone
in the management of open fractures of the tibial shaft. They
randomized 450 patients to receive either standard of care, this being
an intramedullary nail with standard wound management, or standard of
care plus the addition of low- (0.75 mg/mL) or high-dose (1.5 mg/mL)
BMP-2.106 Overall, they found a 44%
reduction in secondary interventions for nonunion (RR = 0.56, 95% CI,
0.4-0.78). They suggested that BMP-2 is safe and demonstrated greater
efficacy than the standard of care in reducing the risk of secondary
interventions for nonunion. Two other studies included in the pooled
analysis assessed acute fractures and union rates.98,142
The pooled estimate of effect on the rates of union were in favor of
BMP treatment (OR 1.65; 95% CI, 1.12%-2.45%). With regards to
functional outcomes, the methodology precluded pooling of the results.98 Four trials were included that assessed the healing of tibial nonunions.95,98
The pooled estimate of effect in those patients with tibial nonunions
treated with an intramedullary nail and either autogenous bone graft or
BMP showed no significant difference between the groups (OR 0.82; 95%
CI, 0.25%-2.64%).98 These studies
were small and had methodologic weaknesses that may have limited their
power to detect the treatment effects of BMP.
acute fractures. However, much of this effect was driven by one large
randomized controlled trial which carried “95% of the weight.”98
The available evidence has not shown superiority but equivalency of BMP
compared to autogenous bone grafting in the treatment of nonunions.98
This suggests, however, that the use of BMP for nonunion treatment may
decrease the potential complications associated with harvesting iliac
crest bone.
significant fracture gap, where, together with the patient, we have
decided against further surgery, we will suggest the use of a bone
stimulator. We use ultrasound as it is an easily applied device with a
20 minute per day application, and we have found it to be
well-tolerated by patients compared with electrical stimulation, which
has a longer treatment time. We will continue this treatment over a
period of 2-3 months with serial radiographs to assess for further
fracture healing. Even if unsuccessful, we have found that patients are
appreciative of having “tried everything” prior to a further surgical
procedure.
patients with recalcitrant nonunions who have already had one further
surgical attempt to obtain fracture union after the initial procedure
or for those patients we think are at high risk of developing a
nonunion, such as those with diabetes, smokers using more than 1 to 1.5
packets a day, or patients on steroids.55,187 We will use them in conjunction with autogenous bone graft or allograft if there is an associated bone defect.
implanting fixation devices into tibiae for the stabilization of
fractures, hardware removal takes up considerable operating room
resources and surgical time.45
Reasons for implant removal include symptomatic hardware, hardware
breakage, loss of fixation, malunion, nonunion, and patient psychology.
Sidky and Buckley256 reviewed 134
tibial shaft fractures treated with an intramedullary nail. They
identified gender and litigation to be predictors of nail removal. They
had a removal rate of 24%, although 35.9% of females had nail removal
compared with 18.3% of males, and 72.2% of patients had an improvement
in their pain following removal. Removal of the nail was for pain in
60.7%, for exchange nailing in 28.6%, for infection in 7.1%, and
because of a periprosthetic fracture in 3.6%.256 They removed locking screws because of pain (85.7%) and breakage (14.3%).
hardwarerelated pain, infected hardware, and broken plates or nails
broken secondary to malunion or nonunion. We do not routinely remove
broken intramedullary nail cross screws unless we believe them to be
symptomatic or infected. We do not remove symptomatic tibial hardware
until union is complete, and then will remove it 12 to 18 months after
insertion.
have full-length tibial views to ensure all cross screws can be
located. If there are broken cross screws, we will remove the portion
with the intact head as usual and will often use a 3/16 Steinman pin
and a mallet to push the other piece through the bone until it is felt
on the other side. We then make a small incision on the opposite side
to remove the screw. It is necessary to have a universal nail removal
set if there is uncertainty as to the manufacturer of the nail. This
usually includes a conical self-tapping device that can screw into the
top part of the nail to facilitate removal with a slap hammer. It is
also useful to have long hooks that can go through the center of the
nail and hook the distal end if the proximal threads are broken. If the
nail is broken, these hooks are useful to attempt to retrieve the
distal portion of the nail. After routine intramedullary nail removal,
patients are allowed to weight-bear as tolerated, but restricted from
undertaking excessive torsional activities for 6 weeks.
screw removal set which usually includes trephines to go over broken
screws, reverse self-tapping conical devices, that can fit into
stripped screw heads, and reverse threading instruments to allow
removal of screws whose heads have been broken off. With the use of
locking plates, the screws can sometimes be very tightly fixed to the
plate which can result in stripping of the screw heads.101,117,197
This may necessitate removal with a reverse conical cutting broken
screw removal instrument, a Midas rex drill (Medtronic, Inc.,
Minneapolis, MN), or diamond cutting burr. After routine plate removal,
patients are kept partial weight bearing for 6 weeks with crutches and
instructed to avoid torsional forces on the tibia.
prolonged muscular action on bone that is structurally ill-prepared for
this type of injury. There are two types of stress fracture. The first
is a fatigue fracture caused by the application of abnormal muscular
stress to bone. The second type is an insufficiency fracture which
occurs when normal muscular activity causes a fracture in bone that is
deficient in mineral.65 Stress
fractures present as spontaneous leg pain that is exacerbated by
particular activities and relieved by rest. Fatigue fractures are most
commonly seen in young active patients such as military recruits and
athletes. Indeed, the prevalence of stress fractures in military
recruits can be as high as 56%.244 Ruohola et al.244
reviewed 154 military recruits who had had an MRI examination during a
5-year period for stress-related anterior lower leg pain. They found
that 57% of the fatigue fractures were located in the distal third of
the tibial shaft, 30% were in the middle third, 10% were in the
proximal third, and 3% were in the medial tibial plateau.244 In a cohort of 375 male recruits and 138 female recruits, Gam et al.97 found a significantly higher risk of fatigue fractures in females (RR = 2.13; p
<0.001) with most occurring in the tibia. The reported overall rates
of fatigue fractures in these recruits were much lower at 11.2% for
males and ~24% for females. Further work by Shaffer et al.253
suggests that predictors of fatigue fractures in female recruits
include low aerobic fitness and an absence of menses during the
previous year. Iwamoto et al.139
examined over 10,000 patients visiting their clinic within a 10-year
period and found that classical ballet, aerobics, tennis, volleyball,
basketball, and soccer were predominantly associated with stress
fractures of the tibial shaft with the average age of patients with
fatigue fractures being 20.1 years old (ranging from 10 to 46 years).139
postmenopausal women with osteoporosis. Other risk factors for this
include the use of corticosteroids, bisphosphonates, or sodium
fluoride, as well as other metabolic causes such as rickets,
osteogenesis imperfecta, rheumatoid arthritis, fibrous dysplasia, Paget
disease, and other pathologic conditions of bone.
fractures is shin splints; shin splints are defined as pain and
discomfort in the leg from repetitive running on hard surfaces or
excessive use of foot flexors. The diagnosis of shin splints is limited
to musculotendinous inflammation and excludes fractures and ischemic
disorders.258 Because these two
conditions may be treated differently at the outset, it is important to
differentiate between them. Indeed, stress fractures may require
cessation of sporting activity for anywhere from 4 to 8 weeks, while
shin splints may not need such a prolonged time.10
Plain radiography may be negative for stress fractures in the initial
phase However, radiographs may begin to show periosteal reaction after
about 4 to 6 weeks. Other studies, such as MRI scans or
technetium-labelled bone scanning, may help in making the diagnosis
sooner.
assessed the role of MRI imaging in stress fractures and shin splints.
They examined 22 athletes who had chronic pain in the middle or distal
portion of the medial aspect of their leg both during and after
sporting activity. They obtained radiographic and MRI studies at
initial examination and after 2 weeks, 4 weeks, and 8 weeks. The
criterion standard test for the diagnosis of tibial stress fracture was
local periosteal reaction, callus.10
In their study groups, eight patients who were diagnosed with stress
fractures all showed “an abnormally wide high signal in the localized
bone marrow” on T2-weighted fat-suppressed MRI scans. In the patients
who had shin splints, the coronal fat-suppressed MRI showed a linear
abnormally high signal along the medial posterior surface of the tibia.
They suggested that “those longitudinal linear high signals were
located just along the medial aspect of the tibial bone marrow and
never extended throughout the entire tibial bone marrow.”10
Interestingly, technetium-labelled bone scanning was also done in these
patients and revealed abnormal local uptake in those patients who had
stress fracture. In those patients with shin splints, bone scanning
revealed either uptake in a longitudinal linear spindle-shaped fashion
or no uptake in about 50% of cases. They suggest that fat-suppressed
MRI is useful for discriminating
between stress fractures and shin splints in the presence of normal radiographs.10
has been done. They included 16 trials in their systematic review, 13
of which were prevention trials involving military recruits.
Unfortunately, the methodologic quality of the included trials was
quoted as being poor. In 10 prevention trials, the effect of foot
inserts or footwear modification was assessed. Four studies showed a
trend towards a decreased number of stress fractures in intervention
groups. One study assessed running modification but showed no
significant difference in stress fracture. Another study assessed calf
stretching and showed no significant decrease in stress fractures
compared to no calf stretching (RR = 1.22, 95% CI, 0.82%-1.83%).240
modification with cessation of sporting activities and cast management
for 4 to 8 weeks. Chang and Harris have suggested that midanterior
stress fractures, which are unicortical fractures involving the
anterior cortex of the tibia, may progress to nonunion because of an
insufficient vascular supply of the tibia in this area.56
They have suggested intramedullary nailing for these fractures. Indeed,
they reported on five of their cases of intramedullary nailing for
recalcitrant stress fractures in army recruits who had failure of
nonoperative management for more than 1 year (level of evidence: 4).
They reported two excellent results and three good results; however,
they did note that the medullary canal was narrow and had a thickened
anterior cortex. This would suggest that intramedullary reaming and
subsequent nail passage may be difficult in these cases.
They usually result from direct injury, from either a blow to the side
of the leg or a fall. Patients who present with fibular fractures
require a thorough assessment of the lower limb looking for any
ligamentous instability in the knee as well as injury to the ankle
joint. OTA Type 44-C ankle fractures tend to present with a
suprasyndesmotic fibular fracture within 15 cm of the ankle joint or
with a Maisonneuve fracture of the fibular neck (see Chapter 57).
It is rare to see a fibular fracture associated with an ankle fracture
between these locations. However, the ankle should be carefully
examined and appropriate radiographs obtained.
generally symptomatic, although we tend to use a below knee walking
cast brace which may help with pain and subsequent mobility.
Complications related to fractures of the fibula include damage to the
peroneal nerve, although arterial and superficial peroneal nerve damage
have also been reported as has entrapment of the peroneal nerve by
callus formation.4,196,229
Symptomatic nonunions may occur and can be treated with a 3.5 mm
compression plate and autogenous bone grafting. We do not routinely
remove these plates, but if they cause discomfort we undertake plate
removal after 12 to 18 months.
RK, Boyan BD, Ciombor DM, et al. Stimulation of growth factor synthesis
by electric and electromagnetic fields. Clin Orthop 2004;419:30-37.
P, Leftheriotis G, Saumet JL. Laser Doppler flowmetry in the diagnosis
of chronic compartment syndrome. J Bone Joint Surg Br
1998;80(2):365-369.
Awami SM, Sadat-Ali M, Sankaran-Kutty M. Arterial injury complicating
fracture of the fibula: a case report. Injury 1987;18(3):214-215.
A, Benterud JG, Hogevold HE, et al. Comparison of functional bracing
and locked intramedullary nailing in the treatment of displaced tibial
shaft fractures. Clin Orthop Relat Res 1992;277:243-250.
A, Ekeland A, Stromsoe K, et al. Nonunion of tibial shaft fractures
treated with locked intramedullary nailing without bone grafting. J
Trauma 1993;34(1):62-67.
V, Heissel A. Economic considerations for the use of recombinant human
bone morphogenetic protein-2 in open tibial fractures in Europe: the
German model. Curr Med Res Opin 2006;22(Suppl 1):S19-S22.
JO. Comparison of soap and antibiotic solutions for irrigation of
lower-limb open fracture wounds. A prospective, randomized study. J
Bone Joint Surg Am 2005; 87(7):1415-1422.
Y, Yasuda K, Tohyama H, et al. Magnetic resonance imaging in stress
fractures and shin splints. Clin Orthop Relat Res 2004;421:260-267.
S, Brundage SI, Gentilello LM. Near-infrared spectroscopy: a potential
method for continuous, transcutaneous monitoring for compartmental
syndrome in critically injured patients. J Trauma 1999;47(5):829-833.
NI, Janes JM, Herrick JF. Ultrasonic energy and surgically produced
defects in bone. J Bone Joint Surg Am 1957;39-A(2):394-402.
LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound
control and treatment: clinical experience. Ann Plast Surg
1997;38(6):563-576.
LC, Morykwas MJ, Marks MW, et al. Vacuum-assisted closure: state of
clinic art. Plast Reconstr Surg 2006;117(7 Suppl):127S-142S.
L, Bhandari M, Hanson B, et al. A concept for the validation of
fracture classifications. J Orthop Trauma 2005;19(6):401-406.
L, Bhandari M, Kellam J. How reliable are reliability studies of
fracture classifications? A systematic review of their methodologies.
Acta Orthop Scand 2004;75(2): 184-194.
L, Griffin D, Bhandari M, et al. Path analysis of factors for delayed
healing and nonunion in 416 operatively treated tibial shaft fractures.
Clin Orthop Relat Res 2005; 438:221-232.
AW, Hansen ST Jr. Plates versus external fixation in severe open tibial
shaft fractures. A randomized trial. Clin Orthop Relat Res
1989;241:89-94.
R, Milner RD. The sustained release of antimicrobial drugs from bone
cement: an appraisal of laboratory investigations and their
significance. J Bone Joint Surg Br 1982;64:460-464.
M, Adili A, Lachowski RJ. High-pressure pulsatile lavage of
contaminated human tibiae: an in vitro study. J Orthop Trauma
1998;12(7):479-484.
M, Adili A, Schemitsch EH. The efficacy of low-pressure lavage with
different irrigating solutions to remove adherent bacteria from bone. J
Bone Joint Surg Am 2001; 83-A(3):412-419.
M, Audige L, Ellis T, et al. Operative treatment of extra-articular
proximal tibial fractures. J Orthop Trauma 2003;17(8):591-595.
M, Guyatt GH, Swiontkowski MF, et al. A lack of consensus in the
assessment of fracture healing among orthopaedic surgeons. J Orthop
Trauma 2002;16(8): 562-566.
M, Guyatt GH, Swiontkowski MF, et al. Surgeons’ preferences for the
operative treatment of fractures of the tibial shaft. An international
survey. J Bone Joint Surg Am 2001;83-A(11):1746-1752.
M, Guyatt GH, Swiontkowski MF, et al. Treatment of open fractures of
the shaft of the tibia. J Bone Joint Surg Br 2001;83(1):62-68.
M, Guyatt GH, Tong D, et al. Reamed versus nonreamed intramedullary
nailing of lower extremity long bone fractures: a systematic overview
and meta-analysis. J Orthop Trauma 2000;14(1):2-9.
M, Guyatt GH, Tornetta P III, et al. Current practice in the
intramedullary nailing of tibial shaft fractures: an international
survey. J Trauma 2002;53(4):725-732.
M, Guyatt G, Tornetta P III, et al. Randomized trial of reamed and
unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint
Surg Am 2008;90: 2567-2578.
M, Schemitsch EH. High-pressure irrigation increases adipocyte-like
cells at the expense of osteoblasts in vitro. J Bone Joint Surg Br
2002;84(7):1054-1061.
M, Tornetta P III, Sprague S, et al. Predictors of reoperation
following operative management of fractures of the tibial shaft. J
Orthop Trauma 2003;17(5):353-361.
M, Zlowodzki M, Tornetta P III, et al. Intramedullary nailing following
external fixation in femoral and tibial shaft fractures. J Orthop
Trauma 2005;19(2):140-144.
T, Seng K, Nassif NA, et al. Knee pain after tibial nailing: the role
of nail prominence. Clin Orthop Relat Res 2006;449:303-307.
A, Grass R, Holch M, et al. Intramedullary nail placement with
percutaneous Kirschner wires. Illustration of method and clinical
examples. Unfallchirurg 2002; 105(1):65-70.
GD, Gold S, Zinar D. Ten-year experience with use of Ilizarov bone
transport for tibial defects. Bull Hosp Jt Dis 2003;61(3-4):101-107.
LB, Sucato D, Stegemann PM, et al. Displaced isolated fractures of the
tibial shaft treated with either a cast or intramedullary nailing. An
outcome analysis of matched pairs of patients. J Bone Joint Surg Am
1997;79(9):1336-1341.
AR, Wongworawat MD. Accuracy in the measurement of compartment
pressures: a comparison of three commonly used devices. J Bone Joint
Surg Am 2005; 87(11):2415-2422.
S, Gardner MJ, Helfet DL, et al. High association of posterior
malleolus fractures with spiral distal tibial fractures. Clin Orthop
Relat Res 2008;466(7):1692-1698.
J Jr, Prickett W, Song E, et al. Extraosseous blood supply of the tibia
and the effects of different plating techniques: a human cadaveric
study. J Orthop Trauma 2002;16(10):691-695.
MJ, MacKenzie EJ, Kellam JF, et al. A prospective evaluation of the
clinical utility of the lower-extremity injury-severity scores. J Bone
Joint Surg Am 2001;83-A(1):3-14.
MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of
reconstruction or amputation after leg-threatening injuries. N Engl J
Med 2002;347(24):1924-1931.
MJ, McCarthy ML, Jones AL, et al. The insensate foot following severe
lower extremity trauma: an indication for amputation? J Bone Joint Surg
Am 2005;87(12): 2601-2608.
OM. Displaced malleolar fractures associated with spiral fractures of
the tibial shaft. Clin Orthop Relat Res 1988;228:202-207.
O, Pihlajamaki H. Routine implant removal after fracture surgery: a
potentially reducible consumer of hospital resources in trauma units. J
Trauma 1996;41(5): 846-849.
M, Leone J, Pierrynowski M, et al. Three-dimensional assessment of
tibial malunion after intramedullary nailing: a preliminary study. J
Orthop Trauma 2002; 16(7):473-483.
M, Helland P, Grontvedt T, et al. External fixation versus locked
intramedullary nailing in tibial shaft fractures: a prospective,
randomized study of 78 patients. Arch Orthop Trauma Surg
2005;125(1):21-26.
CC, Strackee SD. Is there evidence-based guidance for timing of soft
tissue coverage of grade III tibia fractures? Lower Extremity Wounds
2006;5(4):261-270.
AR, Dischinger PC, O’Quinn TD, et al. Lower extremity injuries in
drivers of airbag-equipped automobiles: clinical and crash
reconstruction correlations. J Trauma 1995;38(4):509-516.
JW, Bhandari M, Kulkarni AV, et al. The effect of low-intensity pulsed
ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ
2002;166(4):437-441.
JW, Bhandari M, Sprague S, et al. An economic analysis of management
strategies for closed and open grade I tibial shaft fractures. Acta
Orthop 2005;76(5):705-712.
RC, Bosse MJ, MacKenzie EJ, et al. Impact of smoking on fracture
healing and risk of complications in limb-threatening open tibia
fractures. J Orthop Trauma 2005; 19(3):151-157.
PS, Harris RM. Intramedullary nailing for chronic tibial stress
fractures. A review of five cases. Am J Sports Med 1996;24(5):688-692.
J, Greiff J, Rosendahl S. Fractures of the shaft of the tibia treated
with AO-compression osteosynthesis. Injury 1982;13(4):307-314.
J, Robinson CM, Pell AC, et al. Transcardiac echocardiography during
invasive intramedullary procedures. J Bone Joint Surg Br
1995;77(3):450-455.
G III, Zorn KE. Segmental tibial defects. Comparing conventional and
Ilizarov methodologies. Clin Orthop Relat Res 1994;301:118-123.
PA, Zlowodzki M, Kregor PJ. Less Invasive Stabilization System (LISS)
for fractures of the proximal tibia: indications, surgical technique,
and preliminary results of the UMC Clinical Trial. Injury 2003;34(Suppl
1):A16-A29.
CP, Gross M. Closed tibial shaft fractures: management and treatment
complications. A review of the prospective literature. Can J Surg
2000;43(4):256-262.
SD, Ryaby JP, McCabe J, et al. Acceleration of tibia and distal radius
fracture healing in patients who smoke. Clin Orthop Relat Res
1997;337:198-207.
J, Perren SM, Steinemann SG. Stress protection due to plates: myth or
reality? A parametric analysis made using the composite beam theory.
Injury 2000;31(Suppl 3):C1-13.
CM. Fractures of the Tibia and fibula. In: Bucholz R, Heckman J,
Court-Brown C, et al, eds. Rockwood and Green’s Fractures in Adults,
6th ed. Philadelphia, PA: Lippincott Williams and Wilkins,
2005:2079-2146.
CM, Christie J, McQueen MM. Closed intramedullary tibial nailing. Its
use in closed and type I open fractures. J Bone Joint Surg Br
1990;72(4):605-611.
CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing:
its incidence, etiology, and outcome. J Orthop Trauma
1997;11(2):103-105.
CM, Keating JF, Christie J, et al. Exchange intramedullary nailing. Its
use in aseptic tibial nonunion. J Bone Joint Surg Br 1995;77(3):407-411.
CM, Keating JF, McQueen MM. Infection after intramedullary nailing of
the tibia. Incidence and protocol for management. J Bone Joint Surg Br
1992;74(5): 770-774.
CM, McQueen MM. High success rate with exchange nailing to treat tibial
shaft aseptic nonunion. J Orthop Trauma 1999;13(4):274.
CM, Wheelwright EF, Christie J, et al. External fixation for type III
open tibial fractures. J Bone Joint Surg Br 1990;72(5):801-804.
F, Roukoz S. Compound tibial fractures with bone loss treated by the
Ilizarov technique. J Bone Joint Surg Br 1991;73(2):316-321.
AB, Best AK, Schemitsch EH, et al. Salvage after severe lower-extremity
trauma: are the outcomes worth the means? Plast Reconstr Surg
1999;103(4):1212-1220.
Outer AJ, Meeuwis JD, Hermans J, et al. Conservative versus operative
treatment of displaced noncomminuted tibial shaft fractures. A
retrospective comparative study. Clin Orthop Relat Res 1990;252:231-237.
Nunno R. L’azione degli ultrasuoni sulla formazione del callo osseo
(ricerche sperimentali). Ann Italiani di Chir 1952;29:211-220.
GI, Stavropoulos NI, Kazakos KI. Peroneal nerve damage by oblique
proximal locking screw in tibial fracture nailing: a new emerging
complication? Arch Orthop Trauma Surg 2007;127(6):449-451.
KA, Weisz R, Hiebert R, et al. Does fibular plating improve alignment
after intramedullary nailing of distal metaphyseal tibia fractures? J
Orthop Trauma 2006;20(2): 94-103.
A, Mjoberg B, Ragnarsson B, et al. Changing epidemiology of tibial
shaft fractures. 513 cases compared between 1971 to 1975 and 1986 to
1990. Acta Orthop Scand 1996; 67(6):557-561.
A, Petren-Mallmin M, Larsson S. No effect of low-intensity ultrasound
on healing time of intramedullary fixed tibial fractures. J Orthop
Trauma 1999;13(4):252-257.
A, Larsson A, Petren-Mallmin M, et al. Serum bone markers after
intramedullary fixed tibial fractures. Clin Orthop Relat Res
1999;368:220-229.
M, Reza FH, Hatamipour E, et al. Anatomical study of lateral circumflex
femoral arterial system for the anterolateral thigh flap. Minerva Chir
2008;63(4):283-288.
JR, Edmonds-Wilson R, Stanley RM. An evaluation of interface contact
profiles in two low-contact bone plates. Injury 2004;35(6):551-556.
GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic
protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am
2001;83-A(Suppl 1 Pt 2):S151-S158.
A, Goldstein L, Karmon Y, et al. Comparison of stress fractures of male
and female recruits during basic training in the Israeli antiaircraft
forces. Mil Med 2005;170(8): 710-712.
KR, Donell S, Ryder J, et al. Clinical effectiveness and
cost-effectiveness of bone morphogenetic proteins in the nonhealing of
fractures and spinal fusion: a systematic review. Health Technol Assess
2007;11(30):1-150,iii-iv.
P, Will E, Elton RA, et al. Fractures of the tibia. Can their outcome
be predicted? J Bone Joint Surg Br 1999;81(1):71-76.
J, Tornetta P III, Tiburzi D, et al. Tension wire position for hybrid
external fixation of the proximal tibia. J Orthop Trauma
2000;14(7):502-504.
PV, Snowden S, Matthews SJ, et al. Temperature rise during reamed
tibial nailing. Clin Orthop Relat Res 2002;395:255-261.
PV, Papakostidis C, Kouvidis G, et al. The role of plating in the
operative treatment of severe open tibial fractures: a systematic
review. Int Orthop 2009;33(1): 19-26.
RA, Roberts I, Gillespie WJ. Antibiotics for preventing infection in
open limb fractures. Cochrane Database Syst Rev 2004;1:1-11.
S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic
protein-2 for treatment of open tibial fractures: a prospective,
controlled, randomized study of 450 patients. J Bone Joint Surg Am
2002;84-A(12):2123-2134.
JM, Oliveira RC, Bustos-Valenzuela JC, et al. Bone morphogenetic
proteins: from structure to clinical use. Braz J Med Biol Res
2005;38(10):1463-1473.
R, Cordey J, Wahl D, et al. A biomechanical enigma: why are tibial
fractures not more frequent in the elderly? Injury 2000;31(Suppl
3):C72-C77.
HH, Lohmann CH, Liu Y, et al. Pulsed electromagnetic fields increase
growth factor release by nonunion cells. Clin Orthop 2001;384:265-279.
RB. Interobserver agreement in the classification of open fractures of
the tibia. The results of a survey of 245 orthopaedic surgeons. J Bone
Joint Surg Am 1995;77(8): 1291-1292.
RB, Gruninger RP, Davis T. Classification of type III (severe) open
fractures relative to treatment and results. Orthopedics
1987;10(12):1781-1788.
RB, Mendoza RM, Williams DN. Problems in the management of type III
(severe) open fractures: a new classification of type III open
fractures. J Trauma 1984; 24(8):742-746.
JB, Obolensky AG, Shinnick P. The biologic effects and the therapeutic
mechanism of action of electric and electromagnetic field stimulation
on bone and cartilage: new findings and a review of earlier work. J
Altern Complement Med 2007;13(5): 485-490.
RR, Hammerby S, Lindholm B. Accuracy of radiologic assessment of tibial
shaft fracture union in humans. Clin Orthop Relat Res 1985;199:233-238.
AR, Akeson WH, Mubarak SJ, et al. Fluid balance within the canine
anterolateral compartment and its relationship to compartment
syndromes. J Bone Joint Surg Am 1978;60(4):499-505.
P. A simplified surgical approach to the posterior tibia for bone
grafting and fibular transference. J Bone Joint Surg Am
1945;27(3):496-498.
IA, Kadir A, Donald G. Continuous compartment pressure monitoring for
tibia fractures: does it influence outcome? J Trauma
2006;60(6):1330-1335.
E. On the causes and treatment of pseudarthrosis and especially the
form of it sometimes called supernumerary joint. Am J Med
1841;1:121-156.
JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing
by noninvasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am
1994;76(1):26-34.
MM, Whitesides TE Jr, Grewe SR, et al. Compartment pressure in
association with closed tibial fractures. The relationship between
tissue pressure, compartment, and the distance from the site of the
fracture. J Bone Joint Surg Am 1994;76(9):1285-1292.
K, Spanner M, Trager J, et al. Effects of extremely low-frequency
electromagnetic field (EMF) on collagen type I mRNA expression and
extracellular matrix synthesis of human osteoblastic cells.
Bioelectromagnetics 1998;19(4):222-231.
DL, Howey T, Sanders R, et al. Limb salvage versus amputation.
Preliminary results of the Mangled Extremity Severity Score. Clin
Orthop Relat Res 1990;256: 80-86.
SL, Osterman PAW, Seligson D. The prophylactic use of antibiotic
impregnated beads in open fractures. J Trauma 1990;30(10):1231-1238.
E, Tetsworth K, Radziejowski MJ, et al. Comparison of delayed and
primary wound closure in the treatment of open tibial fractures. Arch
Orthop Trauma Surg 2007;127(2):131-136.
GJ, Keddell RG, Penny ID. Conservative management or closed nailing for
tibial shaft fractures. A randomised prospective trial. J Bone Joint
Surg Br 1991;73(1): 83-85.
HR Jr, Poole GV Jr, Hansen KJ, et al. Salvage of lower extremities
following combined orthopedic and vascular trauma. A predictive salvage
index. Am Surg 1987; 53(4):205-208.
YH, Liu PC, Chien SH, et al. Isolated posterior cruciate ligament
injuries associated with closed tibial shaft fractures: a report of two
cases. Arch Orthop Trauma Surg 2009;129(7):895-899.
TM, Aksenov SA, Schemitsch EH. Cortical bone blood flow in loose and
tight fitting locked unreamed intramedullary nailing: a canine
segmental tibia fracture model. J Orthop Trauma 1998;12(2):127-135.
TM, Aksenov SA, Schemitsch EH. Muscle perfusion after intramedullary
nailing of the canine tibia. J Trauma 1998;45(2):256-262.
TM, Weinberg JA, Aksenov SA, et al. Effect of unreamed, limited reamed,
and standard reamed intramedullary nailing on cortical bone porosity
and new bone formation. J Orthop Trauma 2001;15(1):18-27.
GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective
randomized trial of closed reduction and intramedullary nail versus
open reduction and plate and screws fixation. J Trauma
2005;59(5):1219-1223.
M, Halici M, Ayan I, et al. Treatment of type IIIA open fractures of
tibial shaft with Ilizarov external fixator versus unreamed tibial
nailing. Arch Orthop Trauma Surg 2007;127(8):617-623.
R, Podworny N, Hearn T, et al. Effect of stainless steel and titanium
low-contact dynamic compression plate application on the vascularity
and mechanical properties of cortical bone after fracture. J Orthop
Trauma 1997;11(7):490-495.
AL, Bucholz RW, Bosse MJ, et al. Recombinant human BMP-2 and allograft
compared with autogenous bone graft for reconstruction of diaphyseal
tibial fractures with cortical defects. A randomized, controlled trial.
J Bone Joint Surg Am 2006;88(7): 1431-1441.
S, Firoozabadi R, McKean J, et al. Diastolic blood pressure in patients
with tibia fractures under anaesthesia: implications for the diagnosis
of compartment syndrome. J Orthop Trauma 2007;21(2):99-103.
AH, Granhed H, Edshage B, et al. Displaced tibial shaft fractures: a
prospective randomized study of closed intramedullary nailing versus
cast treatment in 53 patients. Acta Orthop Scand 2000;71(2):160-167.
JF, Blachut PA, O’Brien PJ, et al. Reamed nailing of open tibial
fractures: does the antibiotic bead pouch reduce the deep infection
rate? J Orthop Trauma 1996;10(5): 298-303.
EE, Pjura GA, Lowry PA, et al. Osteomyelitis complicating fracture:
pitfalls of 111 in leukocyte scintigraphy. AJR Am J Roentgenol
1987;148(5):927-930.
K, Fengels I, Perren SM. Long-term effects of plate osteosynthesis:
comparison of four different plates. Injury 2000;31(Suppl 2):S-B51-62.
MP, Rahn BA, Frigg R, et al. Reaming versus nonreaming in medullary
nailing: interference with cortical circulation of the canine tibia.
Arch Orthop Trauma Surg 1990;109(6):314-316.
KJ, Clapper MF, Brumback RJ, et al. Complications of reamed
intramedullary nailing of the tibia. J Orthop Trauma 1991;5(2):184-189.
KJ, Sanders R, Borrelli J, et al. Indirect reduction and percutaneous
screw fixation of displaced tibial plateau fractures. J Orthop Trauma
1992;6(3):340-346.
MJ, Schemitsch EH, Harrington RM, et al. A comparative biomechanical
evaluation of a noncontacting plate and currently used devices for
tibial fixation. J Trauma 1996;40(1):5-9.
M, Schemitsch EH, Harrington RM, et al. Comparative biomechanical
evaluation of different external fixation sidebars: stainless-steel
tubes versus carbon fiber rods. J Orthop Trauma 1996;10(7):470-475.
C, Stephan C, Schandelmaier P, et al. The use of Poller screws as
blocking screws in stabilising tibial fractures treated with small
diameter intramedullary nails. J Bone Joint Surg Br 1999;81(6):963-968.
C, Miclau T, Schandelmaier P, et al. The mechanical effect of blocking
screws (“Poller screws”) in stabilizing tibia fractures with short
proximal or distal fragments after insertion of small-diameter
intramedullary nails. J Orthop Trauma 1999;13(8): 550-553.
KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after
malaligned tibial-shaft fracture. Acta Orthop Scand 1989;60(2):208-209.
TK, Ryaby JP, McCabe J, et al. Accelerated healing of distal radial
fractures with the use of specific, low-intensity ultrasound. A
multicenter, prospective, randomized, double-blind, placebo-controlled
study. J Bone Joint Surg Am 1997;79(7): 961-973.
EG, Powell KE, Dahlberg LL. Firearm-related deaths in the United States
and 35 other high- and upper-middle-income countries. Int J Epidemiol
1998;27(2):214-221.
J, Heikkila JT, Kyyronen T, et al. Posterior malleolar fracture is
often associated with spiral tibial diaphyseal fracture: a
retrospective study. J Trauma 2006;60(5): 1058-1060.
A, Tunturi T, Soukka A. Conservative treatment of tibial fractures.
Results in a series of 163 patients. Ann Chir Gynaecol
1991;80(3):294-300.
GY, Heimlich D, Stephen D, et al. Proximal tibial fracture stability
with intramedullary nail fixation using oblique interlocking screws. J
Orthop Trauma 2003; 17(7):496-502.
EW, Dirschl DR, Duff G, et al. High-pressure pulsatile lavage
irrigation of fresh intraarticular fractures: effectiveness at removing
particulate matter from bone. J Orthop Trauma 2002;16(3):162-165.
KS, Lee WS, Tsui HF, et al. Complex tibial fracture outcomes following
treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol
2004;30(3):389-395.
M, Hertel R. Thermal necrosis after tibial reaming for intramedullary
nail fixation. A report of three cases. J Bone Joint Surg Br
1996;78(4):584-587.
M, Hertel R, Siebenrock KA, et al. The evolution of indirect reduction
techniques for the treatment of fractures. Clin Orthop Relat Res
2000;375:7-14.
CH, Wei FC, Chen HC, et al. Outcome comparison in traumatic
lower-extremity reconstruction by using various composite vascularized
bone transplantation. Plast Reconstr Surg 1999;104(4):984-992.
CH, Schwartz Z, Liu Y, et al. Pulsed electromagnetic fields affect
phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like
cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res
2003;21(2):326-334.
EJ, Bosse MJ. Factors influencing outcome following limb-threatening
lower limb trauma: lessons learned from the Lower Extremity Assessment
Project (LEAP). J Am Acad Orthop Surg 2006;14(10 Suppl):S205-S210.
EJ, Bosse MJ, Castillo RC, et al. Functional outcomes following
traumarelated lower-extremity amputation. J Bone Joint Surg Am
2004;86-A(8):1636-1645.
EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with
amputation or reconstruction of a limb-threatening injury. J Bone Joint
Surg Am 2007;89(8): 1685-1692.
G. Tierexperimentelle untersuchungen über die wirkung der
ultraschallwellen auf die knochenregeneration. Strahlentherapie
1950;82:631-638.
JL, Slongo TF, Agel J, et al. Fracture and dislocation classification
compendium—2007: Orthopaedic Trauma Association Classification,
Database, and Outcomes Committee. J Orthop Trauma 2007;21(10
Suppl):S1-133.
FA III, Winquist RA, Krugmire RB Jr. Diagnosis and management of
compartmental syndromes. J Bone Joint Surg Am 1980;62(2):286-291.
E, Rudzki MM, Rudzki M, et al. [Does low intensity, pulsed ultrasound
speed healing of scaphoid fractures?]. Handchir Mikrochir Plast Chir
2000;32(2):115-122.
MT, Pennington SE, Mills WJ. Successful reimplantation of a large
segment of femoral shaft in a type IIIA open femur fracture: a case
report. J Orthop Trauma 2003;17(4):295-299.
T, Tornetta P III, Tilzey J, et al. Tibial portal placement: the
radiographic correlate of the anatomic safe zone. J Orthop Trauma
2001;15(3):207-209.
MD, Schemitsch EH, Waddell JP, et al. A prospective, randomized
clinical trial comparing tibial nailing using fracture table traction
versus manual traction. J Orthop Trauma 1999;13(7):463-469.
MD, Wild LM, Schemitsch EH, et al. The use of an
antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute
in the treatment of infected long bone defects: early results of a
prospective trial. J Orthop Trauma 2002;16(9):622-627.
MD, DiPasquale DJ, Wild LM, et al. The effect of smoking on clinical
outcome and complication rates following Ilizarov reconstruction. J
Orthop Trauma 2003; 17(10):663-667.
MD, Yoo DJ, Zdero R, et al. Combined single-stage osseous and soft
tissue reconstruction of the tibia with the Ilizarov method and tissue
transfer. J Orthop Trauma 2008;22(3):183-189.
MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial
diaphyseal fractures. J Bone Joint Surg Br 1996;78(1):95-98.
MM, Christie J, Court-Brown CM. Compartment pressures after
intramedullary nailing of the tibia. J Bone Joint Surg Br
1990;72(3):395-397.
MM, Court-Brown CM. Compartment monitoring in tibial fractures. The
pressure threshold for decompression. J Bone Joint Surg Br
1996;78(1):99-104.
P, Panagiotopoulos E, Skriviliotakis S, et al. Intramedullary nailing
in the treatment of aseptic tibial nonunion. Injury 2001;32(3):233-239.
TC, Dietz FR. Long-term follow-up after fractures of the tibial and
fibular shafts. J Bone Joint Surg Am 1989;71(4):599-606.
SA, Davis TR, Muir KR, et al. Long-term outcome after tibial shaft
fracture: is malunion important? J Bone Joint Surg Am
2002;84-A(6):971-980.
BR, Strom DE. Compartment syndrome after closed intramedullary nailing
of the tibia: a canine model and report of two cases. J Orthop Trauma
1991;5(1):71-77.
BR, Thorderson PK. Measurement of intracompartmental pressure: a
comparison of the slit catheter, side-ported needle, and simple needle.
J Bone Joint Surg Am 1993; 75(2):231-235.
PM, Reindl R, Harvey EJ, et al. Fibular fixation as an adjuvant to
tibial intramedullary nailing in the treatment of combined distal third
tibia and fibula fractures: a biomechanical investigation. Can J Surg
2008;51(1):45-50.
MJ, Faler BJ, Pearce DJ, et al. Effects of varying levels of
subatmospheric pressure on the rate of granulation tissue formation in
experimental wounds in swine. Ann Plast Surg 2001;47(5):547-551.
MJ, Simpson J, Punger K, et al. Vacuum-assisted closure: state of basic
research and physiologic foundation. Plast Reconstr Surg 2006;117(7
Suppl): 121S-126S.
CM, Vos MC, van den Bemd GJ, et al. Bacterial load in relation to
vacuum-assisted closure wound therapy: a prospective randomized trial.
Wound Repair Regen 2004;12(1):11-17.
SJ, Owen CA, Hargens AR, et al. Acute compartment syndromes: diagnosis
and treatment with the aid of the wick catheter. J Bone Joint Surg Am
1978;60(8): 1091-1095.
JM, Gorczyca JT, Cole JK, et al. Effect of acute reamed versus unreamed
intramedullary nailing on compartment pressure when treating closed
tibial shaft fractures: a randomized prospective study. J Orthop Trauma
2000;14(8):554-558.
JV, Seabold JE, Marsh JL, et al. Diagnosis of infection in ununited
fractures. Combined imaging with indium-111-labeled leukocytes and
technetium-99m methylene diphosphonate. J Bone Joint Surg Am
1993;75(12):1816-1822.
SE, Schwartz AK, Agel J, et al. Intramedullary nailing of distal
metaphyseal tibial fractures. J Bone Joint Surg Am 2005;87(6):1213-1221.
SE, Barei DP, Schildhauer TA, et al. Intramedullary nailing of proximal
quarter tibial fractures. J Orthop Trauma 2006;20(8):523-528.
WT, Medina M. Comparison of intramedullary nailing of distal third
tibial shaft fractures: before and after traumatologists. Orthopedics
2004;27(11):1180-1184.
CW, Kyung HS, Park IH, et al. Distal tibia metaphyseal fractures
treated by percutaneous plate osteosynthesis. Clin Orthop Relat Res
2003;408:286-291.
OO, Hui A, Gregg PJ. The healing of closed tibial shaft fractures. The
natural history of union with closed treatment. J Bone Joint Surg Br
1988;70(5):787-790.
PAW, Henry SL, Seligson D. The role of local antibiotic therapy in the
management of compound fractures. Clin Orthop Relat Res
2008;295:102-111.
CJ, Love C. Radionuclide imaging of musculoskeletal infection:
conventional agents. Semin Musculoskelet Radiol 2007;11(4):335-352.
CJ, Love C, Tronco GG, et al. Combined labeled leukocyte and technetium
99m sulfur colloid bone marrow imaging for diagnosing musculoskeletal
infection. Radiographics 2006;26(3):859-870.
D, Herzenberg JE. Intramedullary infections treated with antibiotic
cement rods: preliminary results in nine cases. J Orthop Trauma
2002;16(10):723-729.
HC, Giannoudis P. The biological and physiological effects of
intramedullary reaming. J Bone Joint Surg Br 2007;89(11):1421-1426.
HC, Giannoudis P, Krettek C. The timing of fracture treatment in
polytrauma patients: relevance of damage control orthopedic surgery. Am
J Surg 2002;183(6): 622-629.
JE, Rodriguez ED, Bluebond-Langer R, et al. The anterolateral thigh
flap is highly effective for reconstruction of complex lower extremity
trauma. J Trauma 2007;62(1): 162-165.
SM, Allgower M, Cordey J, et al. Developments of compression plate
techniques for internal fixation of fractures. Prog Surg
1973;12:152-179.
SM, Cordey J, Rahn BA, et al. Early temporary porosis of bone induced
by internal fixation implants. A reaction to necrosis, not to stress
protection? Clin Orthop Relat Res 1988;232:139-151.
B, Anderson S, Court-Brown CM. Infection after reamed intramedullary
nailing of the tibia: a case series review. J Orthop Trauma
2005;19(7):437-441.
B, Jeray K, Schemitsch E, et al. Fluid lavage in patients with open
fracture wounds (FLOW): an international survey of 984 surgeons. BMC
Musculoskelet Disord 2008;9:7.
W, Clement H, Boldin C, et al. Primary transection of the superficial
peroneal nerve resulting from a distal fibula fracture. J Orthop Trauma
2007;21(3):212-214.
AA, Mont MA, Nasser PR, et al. Noninvasive low-intensity pulsed
ultrasound accelerates bone healing in the rabbit. J Orthop Trauma
1990;4(3):246-253.
AA, Figueiredo M, Nasser PR, et al. Noninvasive low-intensity pulsed
ultrasound intensity on accelerated bone repair in a rabbit model.
Trans Orthop Res Soc 1991; 37:452.
AN, McCarthy ML, Burgess AR. Short-term wound complications after
application of flaps for coverage of traumatic soft-tissue defects
about the tibia. The Lower Extremity Assessment Project (LEAP) study
group. J Bone Joint Surg Am 2000;82-A(12):1681-1691.
RM, Vaughan JJ, Stetten ML, et al. Long-term effects of tibial angular
malunion on the knee and ankle joints. J Orthop Trauma
1991;5(3):247-254.
P, Elbeshir E, Harvey W, et al. The stimulation of bone formation in
vitro by therapeutic ultrasound. Ultrasound Med Biol
1997;23(8):1251-1258.
WM, Rudzki JR, Borrelli J Jr. Treatment of complex proximal tibia
fractures with the less invasive skeletal stabilization system. J
Orthop Trauma 2004;18(8):521-527.
CS, King D, Wang M, et al. Should distal interlocking of tibial nails
be performed from a medial or a lateral direction? Anatomical and
biomechanical considerations. J Orthop Trauma 1999;13(1):27-32.
CM, McLauchlan G, Christie J, et al. Tibial fractures with bone loss
treated by primary reamed intramedullary nailing. J Bone Joint Surg Br
1995;77(6):906-913.
CM, O’Donnell J, Will E, et al. Dropped hallux after the intramedullary
nailing of tibial fractures. J Bone Joint Surg Br 1999;81(3):481-484.
K, Handoll HH, Ashford R. Interventions for preventing and treating
stress fractures and stress reactions of bone of the lower limbs in
young adults. Coch Data Syst Rev 2005;2:CD000450.
CH, Castle GS, Hardie R, et al. Compartmental pressure measurements: an
experimental investigation using the slit catheter. J Trauma
1981;21(6):446-449.
T, Webb JK, Allgower M. Experience with the dynamic compression plate
(DCP) in 418 recent fractures of the tibial shaft. Injury
1976;7(4):252-257.
JP, Kiuru MJ, Pihlajamaki HK. Fatigue bone injuries causing anterior
lower leg pain. Clin Orthop Relat Res 2006;444:216-223.
GG, Henderson R, Arnett G. Primary or delayed closure for open tibial
fractures. J Bone Joint Surg Br 1990;72(1):125-128.
WL, Sailors DM, Whittle TB, et al. Limb salvage versus traumatic
amputation. A decision based on a seven-part predictive index. Ann Surg
1991;213(5):473-480.
A, Sharpe FE, Ebramzadeh E, et al. Factors influencing the outcome of
closed tibial fractures treated with functional bracing. Clin Orthop
Relat Res 1995; 315:8-24.
EH, Kowalski MJ, Swiontkowski MF, et al. Comparison of the effect of
reamed and unreamed locked intramedullary nailing on blood flow in the
callus and strength of union following fracture of the sheep tibia. J
Orthop Res 1995;13(3): 382-389.
EH, Kowalski MJ, Swiontkowski MF, et al. Cortical bone blood flow in
reamed and unreamed locked intramedullary nailing: a fractured tibia
model in sheep. J Orthop Trauma 1994;8(5):373-382.
AH, Templeman DC, Tornetta P, et al. Anatomic assessment of the proper
insertion site for a tibial intramedullary nail. J Orthop Trauma
2003;17(1):75-76.
D, Popham GJ, Voos K, et al. Antibiotic-leaching from
polymethylmethacrylate beads. J Bone Joint Surg Am 1993;75:714-719.
B, Menon M, O’Brien PJ, et al. Diagnostic techniques in acute
compartment syndrome of the leg. J Orthop Trauma 2008;22(8):581-587.
RA, Rauh MJ, Brodine SK, et al. Predictors of stress fracture
susceptibility in young female recruits. Am J Sports Med
2006;34(1):108-115.
AD, Gustilo T, Court-Brown CM. Epidemiology and outcome of tibial
diaphyseal fractures in footballers. Injury 1997;28(5-6):365-367.
GW, Matsen FA III. Fasciotomy in the treatment of the acute compartment
syndrome. J Bone Joint Surg Am 1976;58(1):112-115.
SL, Johnson KD, Henley MB, et al. Intramedullary nailing with reaming
to treat non-union of the tibia. J Bone Joint Surg Am
1989;71(7):1004-1019.
J. Complex orthopaedic wounds: prevention and treatment with negative
pressure wound therapy. Orthop Nurs 2004;23(Suppl 1):3-10.
JP, Robinson JT, Anderson ER, et al. Negative pressure wound therapy to
treat hematomas and surgical incisions following high-energy trauma. J
Trauma 2006; 60(6):1301-1306.
MF, Hanel DP, Vedder NB, et al. A comparison of short- and long-term
intravenous antibiotic therapy in the postoperative management of adult
osteomyelitis. J Bone Joint Surg Br 1999;81(6):1046-1050.
MF, Agel J, McAndrew MP, et al. Outcome validation of the AO/OTA
fracture classification system. J Orthop Trauma 2000;14(8):534-541.
D, Thomas M, Varecka T, et al. Exchange reamed intramedullary nailing
for delayed union and nonunion of the tibia. Clin Orthop Relat Res
1995;315:169-175.
JA, Kyro A, Heiskanen T, et al. Which displaced spiral tibial shaft
fractures can be managed conservatively? Int Orthop 2000;24(3):151-154.
JA, Vaisto O, Kannus P, et al. Anterior knee pain after intramedullary
nailing of fractures of the tibial shaft. A prospective, randomized
study comparing two different nail-insertion techniques. J Bone Joint
Surg Am 2002;84-A(4):580-585.
P III, Collins E. Semiextended position of intramedullary nailing of
the proximal tibia. Clin Orthop Relat Res 1996;328:185-189.
P III, French BG. Compartment pressures during nonreamed tibial nailing
without traction. J Orthop Trauma 1997;11(1):24-27.
CL, Chang WH, Liu TK. Preliminary studies of duration and intensity of
ultrasonic treatments on fracture repair. Chin J Physiol
1992;35(1):21-26.
GM, Keating JF, Court-Brown CM. Extra-articular fractures of the
proximal tibial diaphysis: their epidemiology, management and outcome.
J R Coll Surg Edinb 1997;42(5):334-338.
T. The clinical diagnosis of compartment syndrome of the lower leg: are
clinical findings predictive of the disorder? J Orthop Trauma
2002;16(8):572-577.
C, Shrier I, Falk M, et al. Quantifying delays in the recognition and
management of acute compartment syndrome. CJEM 2001;3(1):26-30.
C, Shrier I, Vandal A, et al. Acute compartment syndrome: how long
before muscle necrosis occurs? CJEM 2004;6(3):147-154.
O, Toivanen J, Kannus P, et al. Anterior knee pain after intramedullary
nailing of fractures of the tibial shaft: an 8-year follow-up of a
prospective, randomized study comparing two different nail-insertion
techniques. J Trauma 2008;64(6):1511-1516.
O, Toivanen J, Kannus P, et al. Anterior knee pain and thigh muscle
strength after intramedullary nailing of a tibial shaft fracture: an
8-year follow-up of 28 consecutive cases. J Orthop Trauma
2007;21(3):165-171.
O, Toivanen J, Paakkala T, et al. Anterior knee pain after
intramedullary nailing of a tibial shaft fracture: an ultrasound study
of the patellar tendons of 36 patients. J Orthop Trauma
2005;19(5):311-316.
der Linden W, Larsson K. Plate fixation versus conservative treatment
of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am
1979;61(6A):873-878.
S, Sharath KR. Prospective study of the clinico-radiological outcome of
interlocked nailing in proximal third tibial shaft fractures. Injury
2006;37(6):536-542.
RW, McKee MM, Waddell JP, et al. Ideal tibial intramedullary nail
insertion point varies with tibial rotation. Orthopaedic Trauma
Association annual meeting: Scientific poster #74; 2006.
B, Cech O. Pseudoarthrosis: pathology, biomechanics, therapy, results.
Berne, Switzerland: Hans Huber Medical Publisher, 1976.
DB, Bhandari M, McKee MD, et al. Interobserver and intraobserver
variation in the assessment of the healing of tibial fractures after
intramedullary fixation. J Bone Joint Surg Br 2002;84(1):15-18.
TE Jr, Haney TC, Harada H, et al. A simple method for tissue pressure
determination. Arch Surg 1975;110(11):1311-1313.
TE Jr, Haney TC, Morimoto K, et al. Tissue pressure measurements as a
determinant for the need of fasciotomy. Clin Orthop Relat Res
1975;113:43-51.
TE Jr, Heckman MM. Acute compartment syndrome: update on diagnosis and
treatment. J Am Acad Orthop Surg 1996;4(4):209-218.
JM, Ueno T, Leek BT, et al. Noninvasive measurements of intramuscular
pressure using pulsed phase-locked loop ultrasound for detecting
compartment syndromes: a preliminary report. J Orthop Trauma
2006;20(7):458-463.
SL, Lothringer KS, Akeson WH, et al. Less rigid internal fixation
plates: historical perspectives and new concepts. J Orthop Res
1984;1(4):431-449.
S, Lin CH, Wei FC. One-stage reconstruction of composite bone and
soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg
2004;114(6):1457-1466.
S, Lin CH, Lin YT, et al. Outcome comparison between free muscle and
free fasciocutaneous flaps for reconstruction of distal third and ankle
traumatic open tibial fractures. Plast Reconstr Surg
2006;117(7):2468-2475.
S, Gideroglu K, Akoz T. Anterolateral thigh flap: ideal free flap
choice for lower extremity soft-tissue reconstruction. J Reconstr
Microsurg 2003;19(4):225-233.
BA, Bhandari M, Espiritu M, et al. Treatment of distal tibia fractures
without articular involvement: a systematic review of 1125 fractures. J
Orthop Trauma 2006; 20(1):76-79.
GA, Hutson JJ Jr. Diagnosis and management of infection after tibial
intramedullary nailing. Clin Orthop Relat Res 1995;315:153-162.
