Lumbar Stenosis
lumbar spinal stenosis increasingly is being recognized as a cause of
low back pain and radiculopathy in elderly patients. Surgical treatment
for spinal stenosis increased in the United States by eightfold from
1979 to 1992, from 7.8 to 61 procedures per 100,000 persons 65 years
old and older. There has been no long-term prospective, randomized,
controlled study, however, to establish the superiority of surgical
treatment over the natural history of this disease.
as any condition involving narrowing of the spinal canal or neural
foramen. There are many forms of lumbar spinal stenosis. The most
common is degenerative stenosis, which occurs in virtually the entire adult population as a result of the natural process of aging.
-
Congenital/developmental stenosis
-
Idiopathic (hereditary)
-
Achondroplastic
-
-
Acquired stenosis
-
Degenerative
-
Combined congenital and degenerative stenosis
-
Sponylolytic/spondylolisthetic
-
Iatrogenic—postlaminectomy, postfusion
-
Posttraumatic
-
Metabolic—Paget’s disease, fluorosis
-
the spinal canal or intervertebral foramina or both caused by bone or
ligament hypertrophy (or both) in local, segmental, or generalized
regions. The narrowing results in compression of spinal nerves and
nerve roots, causing a constellation of symptoms, including lower back
pain, neurogenic claudication, and lower extremity pain.
usually presents at an early age, often between 30 and 40 years old.
Acquired lumbar spinal stenosis is more common and generally develops
when patients are 60 years old or older. The incidence of degenerative
lumbar stenosis is not influenced by sex, race, or ethnicity, and it is
not associated with any particular occupation or body habitus.
with progressively increasing leg pain. They usually have associated
low back pain. As always, low back pain from sources other than the
spine needs to be considered, such as abdominal aortic aneurysm, pelvic
tumors, and hip osteoarthrosis. Posture and gait should be noted.
Frequently, stenotic patients stand with hip hyperextension and knee
flexion to compensate for increased lumbar flexion. Gait may be
antalgic, broad-based with instability, or show a Trendelenburg lurch.
There is usually no demonstrable neurologic deficit at rest; however,
subtle findings may appear after exercise.
back or buttocks, which radiates into one or both thighs and legs (60%
bilateral pain). The pain, numbness, weakness, or paresthesia involving
the lower extremities typically develops with walking or other
activities. This condition is known as neurogenic claudication.
vascular claudication. In vascular claudication, peripheral pulses are
diminished or absent, calf/leg pain is not relieved by leaning forward,
and pain relief is slower after resting. Vascular claudicant calf pain
often occurs at night, awakening the patient from sleep and prompting
“dangling” the legs from the side of the bed to gain relief. In
contrast, neurogenic claudication pain typically is relieved by
stooping forward, and relief usually is quicker after stopping than
with vascular disease. Vascular claudication often produces burning
calf pain, whereas neurogenic claudication may produce pain associated
with tingling, numbness, and weakness. Although walking uphill produces
vascular claudicant pain quickly, patients with lumbar stenosis often
report that walking uphill is less painful than walking downhill.
Activities that encourage a flexed lumbar posture are tolerated better
by stenotic patients. Patients often feel relief by holding onto a
shopping cart while ambulating (the “shopping cart” sign). Similarly,
exercise endurance while pedaling a stationary bicycle can be nearly
normal in patients with lumbar stenosis, whereas patients with vascular
claudication quickly experience typical lower extremity symptoms.
understood. One component may be ischemia of the nerve roots induced by
exercise demands. Mechanical neural impingement is another important
factor. This impingement can occur centrally, in the lateral recesses,
or in the neural foramina. Facet joint hypertrophy and ligamentum
flavum infolding caused by loss of disc space height frequently are
present. Asymmetric collapse, rotatory and lateral listhesis,
anterolisthesis, or retrolisthesis can compromise further the
dimensions of the spinal canal and foramina.
clinical course of the disorder in untreated lumbar spinal stenosis
patients makes it difficult to gain a clear picture of the natural
history of lumbar stenosis. Although some patients experience a rapid
decline in physical function and a rapid increase in symptom severity,
for most progression is slow.
spinal stenosis in 32 patients observed over 49 months. Based on a
visual analogue pain scale, 15% improved, 70% remained unchanged, and
15% deteriorated at 4 years. Based on clinical examination, however,
41% improved, 41% remain unchanged, and 18% were worse. The authors
concluded that observation seems to be an important alternative to
surgical treatment because severe progression is unlikely.
and the possibility of spontaneous improvement, nonoperative treatment
is an important option. Activity modification is the mainstay of
nonsurgical treatment. Flexion exercises and aerobic conditioning
should follow a short period of rest for symptom flare-ups. Stationary
bicycle riding, aquatic exercises, and partially unloaded treadmill
exercise are helpful. A lumbar corset may decrease motion and reduce
pain; however, prolonged bracing may lead to paraspinal muscle weakness
and should be avoided.
antiinflammatory drugs, are often helpful, but are not without
associated risks of gastrointestinal ulceration and renal impairment,
especially in elderly patients. The selective cyclooxygenase-2
inhibitors may be safer in regards to gastrointestinal complications.
Use of narcotics should be limited to control of acute flare-ups to
minimize constipation, drug dependence, and mental function impairment.
Antidepressants in low doses occasionally are helpful as an adjuvant to
pain medication, particularly in controlling neuropathic pain.
Calcitonin treatment in lumbar spinal stenosis has been established to
be beneficial in a randomized, placebo-controlled, double-blind,
crossover study with 1-year follow-up.
in a few cases. Epidural steroid injections are used more frequently,
but they are controversial. Rosen et al reported temporary relief of
radicular pain in 50% of patients. In contrast, in a prospective,
randomized, double-blind study, Cuckler et al failed to establish any
efficacy of injecting methylprednisolone acetate over physiologic
saline. The caudal route of epidural injection in elderly patients with
spinal stenosis is technically easier than injecting through arthritic
posterior elements of the spine, but it has the disadvantage of failure
of the drug to reach beyond a level of tight stenosis.
manipulation, acupuncture, stress reduction, ultrasound, transcutaneous
electrical nerve stimulation, thermal modalities, and traction. These
modalities, although anecdotally beneficial in a few patients, cannot
be recommended based on the available data.
study of 68 patients treated nonoperatively. Twenty patients eventually
underwent surgery because of early deterioration between 3 and 27
months (median 3.5 months). Of the remaining 48 cases, good outcome was
observed in more than 70%. The authors could not identify any predictor
of successful outcome after conservative treatment. They found that
delayed surgical intervention in patients who failed a trial of
conservative care still could be expected to have a good result.
stenosis treated conservatively for 16 to 55 months. Treatment included
exercise, analgesics, and epidural steroid injections. Of patients, 18%
underwent surgery, 14% worsened, 24% remain unchanged, and nearly 50%
had mild or sustained improvement. The authors concluded that
aggressive nonoperative treatment remains a reasonable option.
treatment regimen that included an intensive 1-month inpatient
rehabilitation program, calcitonin injections, oral calcium
supplementation, heat, ultrasound therapy, and active exercises in 145
patients. The authors reported 91% of patients became pain-free,
whereas 5% failed to have satisfactory improvement. Only two patients
went on to surgical treatment. Duration of follow-up and long-term
results were not reported.
prospective observational study of a cohort of 67 surgically treated
and 52 conservatively treated patients with 4-year follow-up.
Surgically treated patients had more severe symptoms. Despite this
difference, patient satisfaction at 4 years was higher with surgical
treatment (63%) compared with nonsurgical treatment (42%), even after
adjustment for other independent predictors of outcome. The relative
benefit of surgery declined over time, however, whereas the outcomes
for nonsurgically treated patients who improved were relatively stable
over 4 years.
intractable pain, who have failed an appropriate nonoperative course,
and who have spinal stenosis as the cause of their symptoms.
Progressive neurologic deficit in radicular distribution and presence
of bladder and bowel dysfunction are relatively uncommon in spinal
stenosis. Except in these two situations, surgery for spinal stenosis
is an elective procedure. Delayed surgery does not seem to worsen the
outcome. Predominant low back pain is not alleviated reliably with
surgical decompression because isolated back pain is not a good
indication for surgery. Most patients with lumbar spinal stenosis
present because of pain and activity limitations. Treatment decision
making should be driven by the patient’s assessment of how these
factors are affecting his or her daily life.
the key to a successful outcome after surgery. The decision for the
extent of surgery, unilateral or bilateral decompression, number of
levels, and need for fusion depends on proper identification of the
location of stenosis and instability.
spinal stenosis, but signs of disc degeneration, disc height loss,
osteophytes, hypertrophic facet arthropathy, and degenerative
spondylolisthesis should be noted. The radiographic study should
include standing anteroposterior and lateral views, which may show
associated scoliosis, and flexion/extension lateral views, which may
show segment instability. Congenital stenosis is noted by short
pedicles in lateral views and a narrow interpedicular distance on
anteroposterior views.
degree and location of the stenosis. CT has several limitations,
however. Viewing of soft tissues generally is inadequate. The
morphology of the canal may be different in recumbent compared with
standing or sitting posture. In the presence of deformity, the CT scan
may be difficult to interpret.
intrathecal contrast myelography. Central canal stenosis may be
diagnosed correctly by direct measurement of the canal diameter in only
20% cases without contrast enhancement versus 83% after contrast
images. Often in elderly patients with a pacemaker or other metal
implants contraindicating magnetic resonance imaging (MRI),
CT-myelography is a reliable preoperative imaging study. High-grade
stenosis may limit visualization of the distal regions; however, this
often may be overcome by flexion of the spine for a few minutes before
imaging.
noninvasive, permits visualization of the soft tissues, and allows
surveillance of the entire spine. The bone canal is imaged better with
CT, however. MRI and contrast-enhanced CT are comparable in their
ability to show spinal stenosis, but MRI is more sensitive in showing
disc degeneration.
as mild, moderate, or severe. There is no consensus on criteria for
these definitions. Speciale et al reported only a fair level of
interobserver reliability (average κ score 0.26) in grading the
severity of spinal stenosis on the basis of MRI. It cannot be
overemphasized that radiologic severity of stenosis does not correlate
with clinical severity. Surgical treatment is indicated only when the
patient has symptoms not responding to conservative treatment and has
concordant radiologically demonstrable stenosis.
predominantly radicular symptoms, it may be difficult to identify the
level generating symptoms. Selective nerve root block may help in
identifying the pain source and may provide at least temporary pain
relief when combined with steroid.
and more sensitive than electromyography to identify the level of nerve
root compression to implicate one level over another. Somatosensory
evoked potentials have a significant rate of false-positive readings
and should be considered in conjunction with imaging studies.
evaluations are essential, particularly in elderly patients with
multiple comorbidities. For extensive surgical procedures, it may be
preferable to donate 2 to 3 U of autologous blood before surgery. Use
of cell saver to avoid homologous transfusion may not be cost-effective.
postoperative pulmonary complications, and it is adequate for routine
decompression of one or two levels that requires less than 2 hours of
operating time. If the patient coughs during surgery, it may cause
major nerve root prolapse even through a small dural tear, making
repair more difficult.
prophylaxis, sequential compression pump devices (calf “squeezers”),
and antiembolic stockings to help prevent deep vein thrombosis.
Patients should be positioned on the table with adequate padding of the
pressure points. The abdomen should be hanging freely to reduce
intraabdominal pressure. The head of the table should be elevated
during prolonged surgery to prevent facial edema. Pressure to the eyes
must be avoided. Use of loupes, a headlight, or a microscope depends on
the surgeon’s preference. Adequate illumination and magnification are
essential.
stenosis without radiographic evidence of instability (i.e., less than
grade I degenerative spondylolisthesis, less than 20 degrees of
degenerative scoliosis) and no history of previous lumbar surgery.
These patients may be treated by decompression alone. Complex
spinal stenosis is defined as cases associated with degenerative
spondylolisthesis exceeding grade I, degenerative scoliosis with curves
exceeding 20 degrees, postoperative radiographic evidence of
instability, or postlaminectomy junctional stenosis. These cases often
benefit from decompression and fusion with or without instrumentation. Algorithm 16-1 is a systematic treatment alogrithm to aid in effective and appropriate surgical decision making.
-
Central canal stenosis often is associated with congenital short pedicle.
-
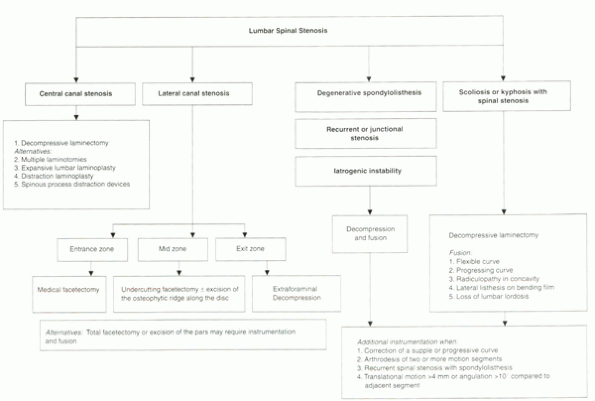
Lateral recess of the spinal canal is divided into three zones:
most cephalad part, located medial to or underneath the superior
articular process. This zone has only anterior and posterior walls. The
medial and lateral aspects are open. The anterior wall is the posterior
surface of the disc, and the posterior wall is the facet joint (Fig. 16-1).
 |
|
Algorithm 16-1
An algorithm for the surgical treatment of spinal stenosis. (From Sengupta DK, Herkowitz HN. Lumbar Spinal Stenosis: Treatment Strategies And Indications For Surgery. Orthop Clin North America 2003;34:281-295.) |
under the pars interarticularis part of the lamina and below the
pedicle. The anterior border of this zone is the posterior aspect of
the vertebral body. The posterior border is the pars interarticularis,
the lateral border is the pedicle, and the medial border is open to the
central spinal canal. The mid zone contains the dorsal root ganglion
(see Fig. 16-1).
surrounding the intervertebral foramen. The posterior border is the
lateral aspect of the facet joint of the lower level, and the anterior
border is the disc of the lower level (see Fig. 16-1). Stenosis may involve one or more of the three zones.
lumbar laminectomy at the stenotic segment. The decompression should
begin away from the area of maximal stenosis and progress from caudad
to cephalad. The lamina is removed
out
to the most medial portion of the articular facets. Care should be
taken to preserve the pars. At the end of the procedure, it is
important to check if adequate decompression was obtained by palpating
the nerve root canals with a “hockey-stick” or a ball-point probe. If
the nerve root path is tight, further decompression is indicated as
described subsequently.
 |
|
Figure 16-1 The lateral spinal canal in the lumbar spine showing the different zones. (A) Entrance zone. (B) Mid zone. (C) Exit zone. (Redrawn with modification from Lee
CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1988;13:313-320.) |
nerve root may be decompressed by laminotomy. The spine is approached
by midline incision, but only the symptomatic side is exposed. The
nature of the decompressive procedure depends on the location of the
stenosis.
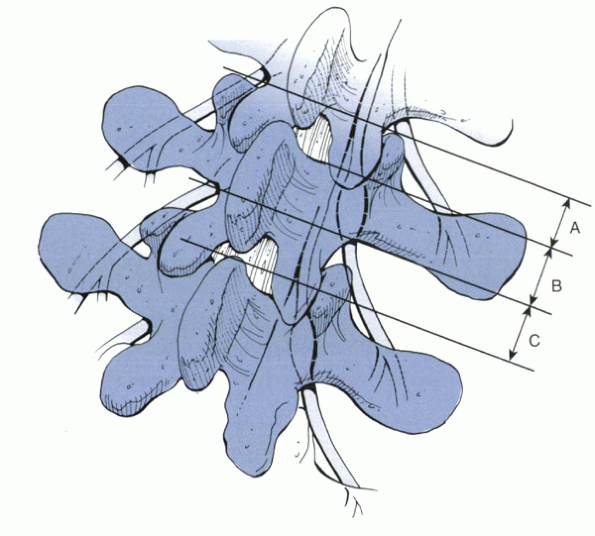
facetectomy. Partial excision of the medial margin of the superior
facet may be done with a Kerrison rongeur or with an osteotome. If the
ridge is too thick, it may be thinned with a bur. Without significantly
compromising stability, 50% of the facet joint can be removed
bilaterally. After satisfactory completion of the procedure, the nerve
root should be able to be displaced by 1 cm medially, the root canal
should allow free passage of a probe, and evoked potentials may show an
improvement (Fig. 16-2).
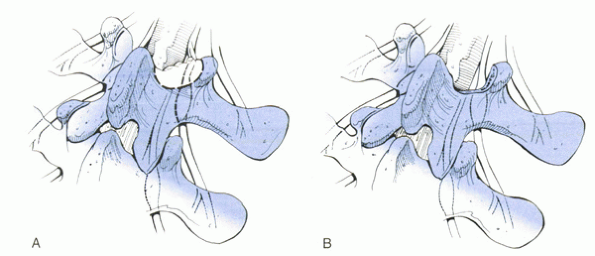
pressure-sensitive section of the nerve root, lies within the mid zone.
Total facetectomy and excision of the pars ensure complete
decompression but destabilize the segment. Partial decompression may be
achieved by removing the anterior half of the superior facet and the
lamina with an osteotome. In the presence of spondylolysis, abundant
soft tissue from the pseudocapsule of the pars defect usually causes
the compression and may be removed by a curet or rongeur (Fig. 16-3).
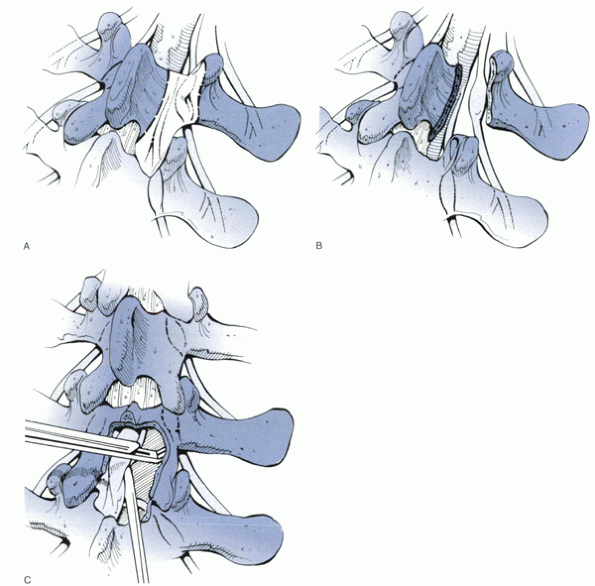
osteophytes from the facets or osteophytic ridge along the disc and
causes entrapment of the exiting nerve root. The L4 nerve root may be
entrapped by a hypertrophic superior articular facet of L5 or an
osteophytic ridge along the L4-5 disc. In the presence of degenerative
listhesis with an intact pars, the exiting nerve root may be entrapped
between the pedicle of the vertebra above and the superior margin of
the vertebra and disc below. A conservative approach to decompress the
nerve root in this zone may involve medial facetectomy, discectomy, or
impaction of the osteophytic ridge along the disc with a bone tamp.
Total facetectomy and removel of the pars ensure adequate decompression
but induce instability requiring stabilization and fusion.
 |
|
Figure 16-2
The extent of decompression for entrance zone stenosis. A partial excision of the medial margin of the superior facet and removal of osteophytes along the superior margin of the lamina usually are necessary. (A) Before decompression. (B) After decompression. (Redrawn with modification from Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1988;13:313-320.) |
may be approached best by the paraspinal muscle-splitting approach
described by Wiltse. The perforating posterior branch of the lumbar
artery is located immediately lateral to the facet joint and may be
ligated or controlled with bipolar cautery. The nerve root should be
decompressed by removing the transverse process, part of the pedicle,
and osteophytes from the facet joints (Fig. 16-4).
have multiple comorbidities, many less invasive procedures have been
developed.
alternative to laminectomy, to preserve the midline structures.
Multiple laminotomies may be associated with a lower incidence of
postoperative instability but are associated with a higher incidence of
neurologic sequelae and require longer operating time. Multiple
laminotomies may be indicated for mild-to-moderate degenerative
stenosis or low-grade degenerative spondylolisthesis. Total laminectomy
is preferred for patients with severe degenerative stenosis or higher
grade degenerative spondylolisthesis.
laminoplasty, a variant of a procedure initially developed for the
cervical spine. The purpose was to preserve stability, particularly in
younger active subjects, while achieving decompression. Matsui et al
reported results of 27 patients treated with open doortype expansive
lumbar laminoplasty. They observed 80% good or excellent results in an
average follow-up of 5.6 years. Only one case required additional
surgery, which involved discectomy at a caudal adjacent level.
of routine laminectomy that allows decompression of the lumbar canal
with maximal bone preservation. The technique involves the application
of a distraction force, in conjunction with an undercutting
laminoplasty. This maneuver allows removal of the medial 20% of the
facet joints and the inner one third of the lamina. Clinical outcomes
were not reported.
through a micro-discectomylike approach. In a prospective series of 54
consecutive cases, they reported good or excellent outcomes in 96% of
patients at 4 years. No progression of slip was reported, even in cases
with preoperative degenerative spondylolisthesis.
devised, including X-Stop (St. Francis Medical Technologies, Inc,
Concord, CA) and Wallis system, a polyetheretherketone-based system
described by Senegas and others. These devices distract the spinous
processes at the stenotic segment, essentially to hold the spine in
flexion, which is the most comfortable posture in patients with spinal
stenosis. The decompression is indirect. The hypertrophied ligamenta
flava are unfolded by intersegmental distraction. Implantation can be
performed under a short local anesthetic period. The procedures
currently are recommended for elderly patients with comorbid conditions
that may preclude conventional decompression surgery.
 |
|
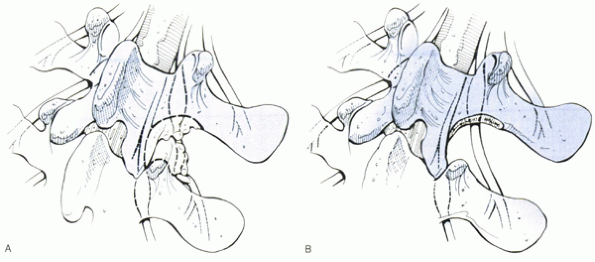
Figure 16-3 The extent of decompression for mid zone stenosis. (A and B)
Total laminectomy and total removal of the inferior articular facet ensure adequate decompression but may lead to a significant amount of instability of a motion segment. The osteophytes and hypertrophic ligamentum flavum under the pars interarticularis can be excised and curettaged without sacrificing the facet joint or an entire lamina. Careful undercutting of the facet and removal of the bone from the undersurface of the lamina overlying the root canal can be performed with a Kerrison rongeur (C). This can achieve neural decompression, while minimizing destabilization of the spine. (Redrawn with modification from Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1988;13:313-320.) |
 |
|
Figure 16-4
The extent of decompression for exit zone stenosis. This procedure is performed best using a lateral-to-medial approach, as shown here. (A) Before decompression. (B) After decompression. (Redrawn with modification from Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1988;13:313-320.) |
results after decompressive lumbar laminectomy, but the benefits seem
to decline over time. In a prospective study, Atlas et al reported 63%
of patients were satisfied at 4 years; the benefit of surgery declined
over time. Katz et al also reported
progressive deterioration of initially good results, with 23%
undergoing revision surgery at 7- to 10-year follow-up. In a subsequent
study, these authors found that patients’ own assessments of their
health and comorbidity were the most powerful preoperative predictors
of good outcome after surgery. Hansraj et al reported patient
satisfaction in 95% of the 103 cases treated with decompression alone
for typical lumbar stenosis. Only four patients went on to revision
surgery during the first year; however, no additional revision surgery
was performed at 2 to 5 years. In a metaanalysis, Niggemeyer et al
found that the least extensive surgical procedure could obtain the best
results if the correct diagnosis and determination of the symptomatic
levels were made and operation was done relatively early. Female
gender, compensation or litigation, negative preoperative diagnostic
nerve root block, previous surgery, obesity, and smoking have been
suggested to be predictors of poor outcome after surgery.
or recurrent stenosis after previous decompression has been described
as complex stenosis. Fusion and
instrumentation often are employed in these cases. Although there seems
to be a consensus on the role of decompression for lumbar spinal
stenosis, recommendations for fusion or stabilization are less clear.
The goals of fusion are relief of mechanical back pain from a
degenerated disc or elimination of instability. The goals of
stabilization are to promote fusion and to correct deformity.
recommended after laminectomy for lumbar stenosis in the following
situations:
-
Degenerative spondylolisthesis
-
Iatrogenic instability after decompression
-
Recurrent stenosis (at same or adjacent level of previous decompression)
-
Degenerative scoliosis or kyphosis
surgical treatment for degenerative spondylolisthesis with spinal
stenosis. More recently, improved clinical results have been documented
with the addition of posterolateral fusion.
Kurz reported 50 cases of spinal stenosis with degenerative
spondylolisthesis treated with either decompression alone or
decompression and posterolateral fusion. The study showed significantly
better outcomes in the fusion group. Although the pseudarthrosis rate
was 36%, the clinical results were good or excellent in all the
patients in this group. It has been postulated that even with a
nonunion, the clinical benefit of substantially decreasing motion
(albeit not complete) are significant.
Since this landmark study, subsequent studies have supported these findings.
and Cinotti retrospectively compared 16 cases of degenerative
spondylolisthesis treated with decompression alone versus 10 cases with
an added arthrodesis. The decompression group had more bone regrowth
and a significantly poorer outcome than the arthrodesis group. It has
been suggested that solid fusion may promote resorption of compressive
osteophytes that were not removed at the time of the initial
decompression.
prospective randomized study of 45 patients with spinal stenosis
without instability, Grob et al found no difference in outcomes between
decompression alone, decompression with selective fusion, or
decompression with fusion of all the segments. These authors concluded
that arthrodesis was not justified in the absence of radiographically
documented instability. Other authors have reported similar findings (Fig. 16-5).
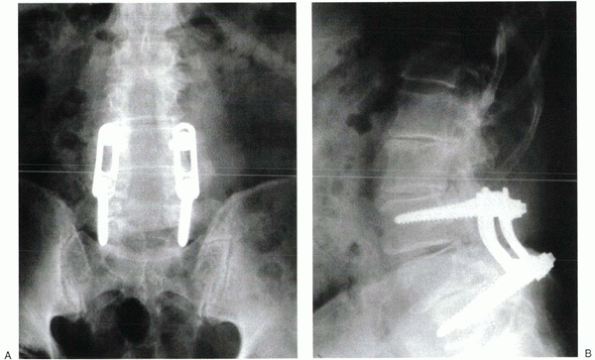 |
|
Figure 16-5 (A, B)
A 68-year-old man with multiple-level central and lateral canal stenosis and degenerative spondylolisthesis at L4-5 was treated surgically by decompressive laminectomy from L2-S1 levels. At operation, adequate decompression at L3-4 required substantial bilateral facet joint resection. A selective fusion of L3-5 with instrumentation was performed in addition to the decompression. (From Sengupta DK, Herkowitz HN. Lumbar Spinal Stenosis: Treatment Strategies And Indications For Surgery. Orthop Clin North America 2003;34:281-295.) |
-
Whether instrumentation improves fusion rate
-
If it does, whether clinical outcome also is improved
instrumentation in a prospective randomized study in 124 patients
(including 56 with degenerative or isthmic spondylolisthesis). Bridwell et al
compared instrumented versus uninstrumented fusion in 44 patients with
degenerative stenosis and spondylolisthesis. The instrumented group
showed a much higher fusion rate, better functional outcome, and
improved restoration of sagittal alignment compared with the
uninstrumented group (87% versus 30%). In an historical cohort study of
2684 patients with degenerative spondylolisthesis, Yuan et al observed
a significantly quicker and more reliable fusion in the instrumented
group and a better clinical outcome.
controlled study comparing decompressive laminectomy and arthrodesis
with or without instrumentation for degenerative lumbar
spondylolisthesis. All patients underwent decompressive laminectomy,
with 35 having fusion with pedicle screw supplementation and 33
undergoing uninstrumented posterolateral fusion. After a minimum
24-month follow-up, Fischgrund et al found an 83% fusion rate in the
instrumented group versus a 45% fusion rate in the uninstrumented
group. There was no detectable significant
difference
in the clinical outcome. These authors concluded that instrumentation
improves fusion rate but does not improve the clinical outcome.
and improve fusion rate by decreasing tension on the posterior fusion
mass. The procedure is technically difficult, is associated with a
higher rate of neurologic complications, and often needs an additional
segment of fusion to achieve biomechanical stability. Montgomery and
Fischgrund prospectively evaluated a technique of passive reduction in
a series of patients undergoing decompressive laminectomy and fusion
for degenerative spondylolisthesis. They found an average decrease in
listhesis by 24% by comparing an intraoperative prone radiograph with a
preoperative flexion radiograph. This postural reduction may obviate
the need for fusion of an extra level (usually one extra cranial level)
and reduces the risk of complication associated with active reduction
maneuvers.
decompression before causing instability is uncertain, but it generally
is believed that less than half of both sides or all of one facet at a
given level may be tolerated without significant instability. In a
biomechanical study in cadaver spines, Abumi et al
showed that removal of greater than 50% of each facet joint led to
instability. When facet excision is greater than 50% in each side (or
>50% of the total facets—25% of one and 75% of the other), an
instrumented fusion may be indicated.
Hazlett and Kinnard reported that only 4 of 33 patients who underwent
unilateral or bilateral complete facet excisions in addition to disc
excision were unstable. White and Wiltse found only a 2% incidence of
postdecompression spondylolisthesis in 182 patients. Some authors
believe that the development of postoperative spondylolisthesis is
related to facet joint orientation and dimensions, rather than the
absolute amount of joint removed.
previously decompressed level often necessitates additional resection
of the pars interarticularis and facet joints. If stability is
compromised, an instrumented fusion is recommended.
incidence of adjacent segment stenosis is unknown. In a retrospective
study with long-term follow-up, Lehman et al observed adjacent segment
disease in 42% of cases. Recurrent stenosis may be produced by laminar
regrowth. Postacchini and Cinotti reported some degree of bone regrowth
in 88% of cases after laminectomy or laminotomy with or without fusion;
40% were symptomatic from either moderate or severe stenosis.
adjacent level stenosis treated with decompression and fusion. They
found an 80% pseudarthrosis rate with uninstrumented fusion compared
with only 17% with instrumentation.
level stenosis. They found that symptoms developed more frequently and
earlier if the initial surgery involved instrumentation compared with
uninstrumented fusion. Adjacent segment stenosis was found to be more
frequent in the proximal segment. Although all patients underwent a
revision laminectomy, 33 of the 42 had extension of the fusion to the
adjacent level.
K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar
spinal stability after graded facetectomies. Spine 1990; 15:1142-1147.
KH, Sedgewick TA, O’Brien MF, et al. The role of fusion and
instrumentation in the treatment of degenerative spondylolisthesis with
spinal stenosis. J Spinal Disord 1993;6:461-472.
AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for
degenerative lumbar stenosis. J Neurosurg 1992;77: 669-676.
JN, Lipson SJ, Chang LC, et al. Seven- to 10-year outcome of
decompressive surgery for degenerative lumbar spinal stenosis. Spine
1996;21:92-98.
CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis:
classification, pathologic anatomy and surgical decompression. Spine
1988;13:313-320.
SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis: a
meta-analysis of literature 1970-1993. Spine 1994;19: 2256S-2265S.
