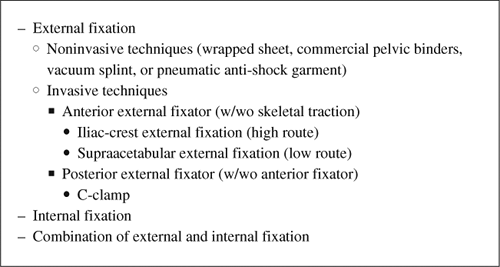
Pelvic Fractures: External Fixation and C-Clamp
fractures as the result of low-energy forces to life-threatening
injuries with hemodynamic instability. The incidence of pelvic
fractures in Sweden is approximately 37 per 100,000 patients per year (1).
Pelvic fractures account for 3% to 8% of all fractures seen in the
emergency room but are present in up to 25% of multiply injured
patients. Mortality rates in patients with complex pelvic fractures and
associated visceral injuries can be as high as 20% to 25%, and for
patients with open pelvic fractures, the mortality rate ranges from 15%
to 50% (1,2,3,4,5,6).
fractures, we favor the Tile classification, which is used to predict
instability in the injured pelvic ring. Tile A injuries are stable
fractures that can be managed nonoperatively. Tile B injuries are
rotationally unstable but vertically stable. Tile C injuries are both
rotationally and vertically unstable.
management of patients with hemodynamic instability following pelvic
fractures. Specific indications for anterior external fixation include
selected, rotationally unstable, Tile B1 and B2 pelvic fractures. We
strongly favor external fixation following fracture when the local
conditions in and around the pelvis or abdomen are contaminated, such
as with open fractures, diverting colostemies or a suprapubic urinary
catheter must be placed. In addition, patients with concomitant
visceral injuries, especially those whose injuries would be exacerbated
when the abdomen is open, are often treated with pelvic external
fixation. Occasionally, anterior-ring external fixation is used to
augment posterior ring fixation.
fixation is in a critically ill, unstable patient with a
translationally unstable pelvic injury (Tile C). A resuscitative frame
with ipsilateral supracondylar traction is used when a C-clamp is
unavailable or not applicable.
pelvic-ring injuries (Tile A) and patients with associated fractures of
the iliac wing in which stable pin placement would be difficult or
impossible. External fixation should be avoided when internal fixation
can be performed on a stable patient in a timely fashion.
workers and paramedics trained in advanced trauma life support (ATLS)
often play a critical role. In patients with hemodynamic instability
and a potentially unstable pelvic-ring injury, a single attempt at
reduction should be considered. The responder applies traction to the
unbroken lower extremity on the shortened or deformed side of the
pelvis with manual lateral compression on the iliac wings or greater
trochanters. The knees and ankles should be slightly flexed and
internally rotated and taped together before a noninvasive external
fixation device (wrapped sheet or pelvic binder) is applied (Fig. 37.1).
When done at the scene of an accident, emergent transfer to an
institution capable of treating pelvic trauma may be life saving (7,8).
injuries require prompt evaluation and simultaneous aggressive
resuscitation. Initial measures include airway control and aggressive
fluid resuscitation through two 14- to 16-gauge intravenous catheters
in the upper extremities. Physical examination should be directed for
signs of possible mechanical instability of the pelvis. Tell-tale signs
include a history of high-energy trauma from motor vehicle or
motorcycle collisions, falls from a height, or rollover motor-vehicle
collisions. On clinical examination, a shortened and malrotated
extremity, asymmetric iliac spines, swelling or blood on genitals and
perineum, contusion or ecchymosis in the lower abdomen or pelvis
suggest pelvic instability. Gentle, manual, iliac-wing compression from
lateral toward the midline may reveal a mobile hemipelvis and
mechanical instability. A careful neurological examination to assess
for leg sensation and motor function is critical. Vaginal and rectal
digital exams with guaiac test for occult blood and with speculums
(time permitting) help rule out an occult open fracture. If overlooked
and not treated, a fracture hematoma that comes in contact with a
contaminated environment may cause a life-threatening pelvic infection.
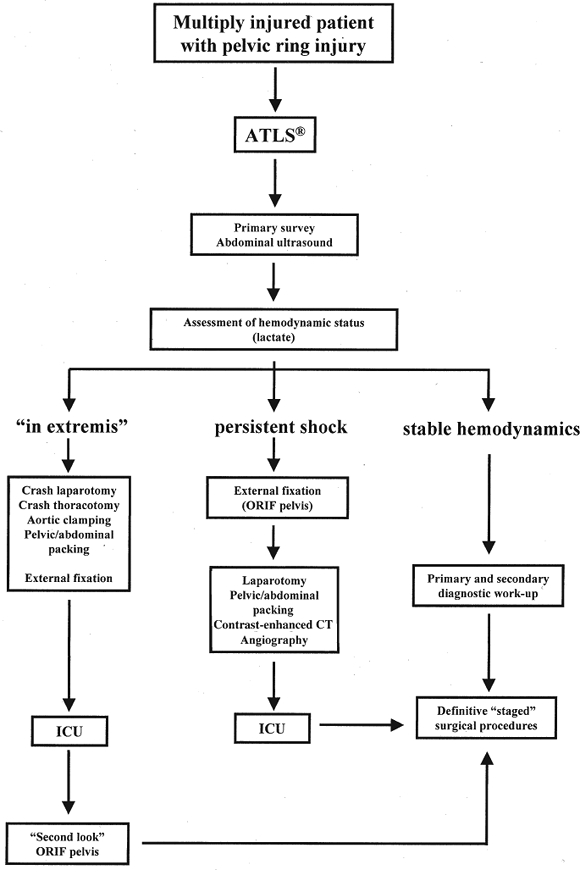
displaced pelvic fracture is the responsibility of a multidisciplinary
trauma team that includes a general surgeon, anesthesiologist,
orthopedic surgeon, and invasive radiologist. Trauma protocols are
helpful in evaluating and treating critically ill patients,
establishing priorities, and guiding treatment. Using the algorithm (Fig. 37.2), Ertel et al (9)
were able to save 15 of 20 patients (75%) who presented with multiple
injuries that included unstable pelvic injuries with an average Injury
Severity Score (ISS) of 41.2 ± 15.3. Fifteen patients had massive
hemorrhage (14 were in extremis), and five were in hemorrhagic shock.
Two of them required subdiaphragmatic clamping of the aorta to
stabilize a disastrous hemodynamic situation.
sonogram for trauma (FAST) or computed tomography (CT) scan can be used
to determine the presence of free fluid in intraperitoneal,
retroperitoneal,
or pericardial cavities. Arterial blood gas with blood lactate levels
and/or base deficit analyses are good indicators of the hemorrhagic
state and tissue oxygenation (9,10,11).
 |
|
Figure 37.1. Methods of pelvic fracture stabilization.
|
 |
|
Figure 37.2. An orthopedic damage-control algorithm for patients with pelvic fracture. (From
Ertel W. General assessment and management of the polytrauma patient. In: Tile M, Helfet D, Kellam J, eds. Fractures of the pelvis and acetabulum. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2003:71
with permission.) |
because it can be used to rule out a pneumothorax, hemopneumothorax,
tension pneumothorax, or flail chest. A single
anteroposterior
(AP) radiograph of the pelvis can be used to diagnose a pelvic fracture
correctly in approximately 90% of cases. X-ray signs of instability are
(a) >5-mm displacement of the sacroiliac (SI) joint in any plane
(inlet and outlet views will improve accuracy if circumstances allow),
(b) posterior fracture gap, and (c) avulsions of the transverse process
of fifth lumbar vertebra or avulsion fractures of the sacrospinous
ligament. By definition, a stable pelvic injury can withstand normal
physiologic forces without abnormal deformation. The surgeon should not
routinely manual test for pelvic ring stability because he/she could
dislodge blood clots in a hemodynamically unstable patient. One-time
manual testing, to exclude a situation in which the pelvis had been
unstable but has since been reduced, may be permissible by an
experienced surgeon in cases where the x-rays do not clearly
demonstrate an unstable injury (12,13).
multiple injuries, exsanguination and closed head injury are the main
causes of mortality within the first 24 hours after injury. Damage
control surgeries, for hemorrhage control, decompression of body
cavities such as in the chest and head, and contamination control of
abdominal perforations, as well as devitalized tissue debridement in
the extremities, improve survival. Determination of the patient’s
hemodynamic status and initial response to resuscitation measures allow
placement of the patient in one of three protocol categories that
dictate treatment (see Fig. 37.2).
extremis (i.e., without measurable vital signs). Most of these patients
need a crash laparotomy, thoracotomy, and/or pelvic/abdominal packing
(with or without temporary aortic cross-clamping) to survive (Fig. 37.3).
While awaiting the response of the patient to aggressive resuscitation
therapy, a C-clamp or an anterior, pelvic, external fixator should be
applied. Subsequent, repeated, pelvic/abdominal packing against a firm
stabilized pelvis will be much more effective in tamponading the
bleeding.
adequate fluid replacement, blood transfusions, and medications over 2
hours, an external fixator or C-clamp should replace a pelvic sheet or
binder. Numerous studies have shown that in patients with unstable
pelvic injuries in which ligaments and fascial planes that support the
pelvic floor have been disrupted, self-tamponade rarely occurs (14). Huittinen and Slätis (15)
have estimated that 80% to 90 % of bleeding following fracture and/or
dislocation comes from disruption of the lumbosacral venous plexus and
fracture bone surfaces and that only 10% have an arterial origin. The
most common technique for stopping the diffuse bleeding (venous or
arterial small vessels) is by tamponade. In patients who require
abdominal exploration, laparotomy
will make the pelvis more unstable because the muscle forces pulling on the iliac wings are diminished (16).
A correctly applied pelvic frame will improve pelvic stability yet not
impede the surgeon’s ability to perform either a supraumbilical
laparotomy (from xiphoid process to symphysis for positive
intra-abdominal fluid) (Fig. 37.4) or
infraumbilical (absent intraperitoneal injury). If a patient with an
unstable pelvic fracture requires a laparotomy for intraperitoneal
injuries, exploration and packing to control retroperitoneal bleeding
may be accomplished as well. Disruption of the soft tissues in the
pelvic floor allows easy, direct access to both sides of sacrum and
bladder for packing (17). Major arteries and
veins (external iliac and femoral) that are damaged also require direct
access and surgical hemostasis. In massive retroperitoneal bleeding
caused by blunt trauma, Ertel et al (9)
recommended that the hematoma in the central zone be explored. This
method also allows assessment of the posterior reduction by direct
manual palpation from inside the pelvis. Bleeding is better controlled
when the bone surfaces or joint are directly opposed (interdigitating)
and compressed. If significant displacement exists, loosening and
adjusting the external fixator, previously applied to improve the
fracture reduction, can be performed. To achieve firm local compression
against the reduced posterior pelvis, the surgeon may need to repeat
the tamponade and look quickly again for any major bleeding sources. If
hemodynamic instability persists despite all measures (including
partially closed distal-abdominal fascia for better support of
packing), then the patient should be transferred for a
contrast-enhanced CT of the abdomen. The study is fast and highly
accurate in determining the presence or absence of ongoing pelvic
hemorrhage (18). Patients with visualized
contrast extravasation, as shown on CT scans, are candidates for
angiography and embolization. Following a similar protocol, the
Hannover Trauma Center was able to reduce mortality rate from 46% to
25% (8,10,15,17,19).
 |
|
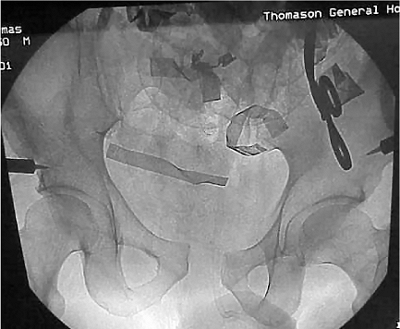
Figure 37.3.
Aortic cross-clamping and C-clamp placement in a policeman, who was struck by an automobile while standing alongside a highway. On admission, he was in extremis, and crash laparotomy was conducted, and a C-clamp and pelvic packing were applied. Bleeding was controlled by temporary cross-clamping of the aorta. The patient survived the night but expired the following morning secondary to a severe closed-head injury. |
intra-abdominal free fluid is not found on ultrasound exam or
diagnostic peritoneal lavage (DPL) after noninvasive pelvis
stabilization, angiography and embolization of identifiable bleeding
arteries is usually the next step. Angiography does not address venous
bleeding, is time consuming (which is particularly problematic for a
patient in hemorrhagic shock), and may cause gluteal muscle necrosis.
In addition, only 3% to 5% of patients with unstable pelvis receive
major benefit from the procedure, which requires specialized personnel
and equipment (13,20,21,22).
High-energy pelvic injuries that are concomitant with a major vessel
injury (e.g., to the common external iliac or femoral artery), which
are characterized by lack of blood flow to the extremity, and a
profound neurologic deficit (and/or intraoperative finding of stretched
and ruptured nerves) cause such persistent hemodynamic instability that
reconstruction attempts are futile.
a diverting colostomy placed as far as possible from subsequent
surgical approaches to the pelvis, and immediate distal-colon washout.
In addition, a broad spectrum of prophylactic antibiotics should be
given (2,3,4,5,6).
 |
|
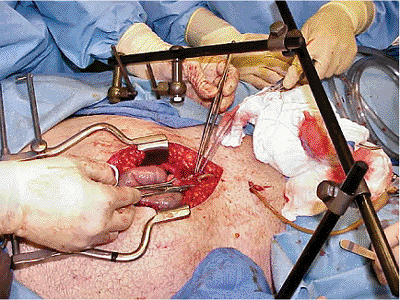
Figure 37.4. Laparotomy following application of an anterior external-fixation device. A C-clamp is covered by sheets.
|
or without blood on ureteral meatus) requires a retrograde urethrogram
before bladder catheterization. In our experience, a rectal exam cannot
reliably predict the position of the prostate and possible urethral
injury. A urethral injury in this setting is usually treated with a
suprapubic catheter inserted percutaneously or openly during
laparotomy. Patients with hematuria and intact urethra must undergo a
contrast study to rule out a bladder rupture. If no explanation for
hematuria is identified, an abdominal CT and intravenous pyeolography
is used to investigate the upper urogenital tract. In a polytrauma
patient, routine CT scans without contrast will show a ruptured kidney
perirenal hematoma; contrast may be contraindicated with kidney damage
or poor function. If an emergent invasive radiology procedure is
contemplated, contrast studies (urological and gastrointestinal) should
be done after angiography (13,20).
require early pelvic stabilization to improve fracture stability,
provide a tamponade effect, improve clotting, and reduce pain. In
patients with multiple injuries, including life-threatening conditions,
the speed and safety of pelvic stabilization is more important than the
initial accuracy of reduction or sophistication of frame constructs.
hand-tied knot placed around the pelvis and greater trochanter, with
the hips slightly flexed and internally rotated, can often provide
short-term tamponade and stabilization (2 to 3 hours) (Fig. 37.5).
This stabilization method is most effective when applied after a simple
reduction maneuver using traction and pressure over the iliac wings.
Unnecessary transfers should be avoided, and the patient should only be
turned once for a spinal exam.
However, these devices limit access to the abdomen and groin. Vacuum
splints and beanbag positioners, while bulky, can be helpful and allow
better abdominal and inguinal access while providing pelvic
immobilization (Fig. 37.7). It is unfortunate that they are not always available and do not work well in large patients.
 |
|
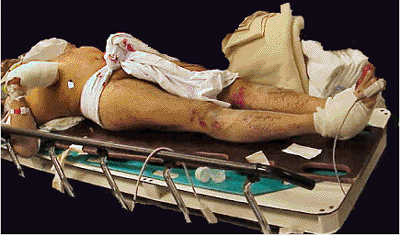
Figure 37.5.
External immobilization with a sheet as a pelvic binder in a patient with an unstable pelvic injury. Sheet wrapped around the iliac-crests, ankles and lower extremities fixed in internal rotation with a gauze-roll provide rapid temporary immobilization with ongoing fluid resuscitation. |
 |
|
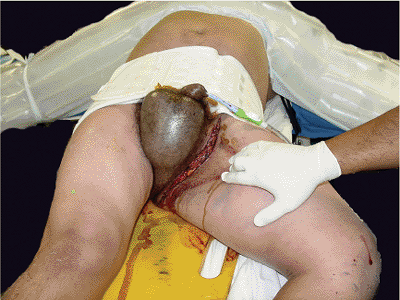
Figure 37.6.
The use of a pelvic binder in a mechanically unstable, open, pelvic fracture. It is easy to apply and adjust as needed. Access to the abdomen and inguinal regions may be limited, and binder repositioning to the trochanteric regions may be necessary. |
 |
|
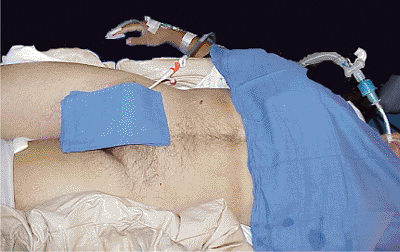
Figure 37.7.
Vacuum splints apply pressure over a large area and provide good temporary fixation. They are radiolucent and allow full access to the abdomen and inguinal areas. |
decrease in the mortality rate from 26% to 6% when external fixation
was introduced as a part of resuscitation protocol at their
institution. External fixation provides better bony stabilization than
noninvasive methods, and the technique is minimally invasive and
relatively easy and safe to apply. External fixation also helps control
pain and patient mobilization. However, an anterior frame does not
adequately stabilize translational, Tile C fracture patterns. Unlike
the posterior C-clamp, the frame may limit access to the abdomen. With
attempts to compress and immobilize the anterior pelvic ring, the
fracture gap posteriorly may be increased, which may lead to greater
instability and bleeding. Transfemoral skeletal traction of 25 to 30
lb, with the hip flexed, can improve the posterior fracture reduction.
primarily as a temporary resuscitation frame until the patient’s
general condition has improved and elective internal fixation can be
employed. When anterior frames were used as a definitive fixation
method for Tile C fractures, failure rates as high as 70% were
reported. While the optimal time to convert from external to internal
fixation is unknown, we prefer to wait at least 7 days to avoid “the
second hit” phenomena. Earlier fixation may be feasible in patients in
which percutaneously placed ilio-sacral screws can produce adequate
reductions in the supine position (see Fig. 37.33).
Combined posterior–internal fixation and anterior–external fixation
(when anterior internal fixation is not applicable) can provide
adequate stability for patient mobilization. However, discomfort, pin
tract problems, and loosening limit long-term application (13,25,26,27,28,29,30).
The stability of external fixation is determined by the (a) host (type
of pelvis mechanical instability, patient size, and quality of bone);
(b) frame characteristics (design and location, number and size of
pins), and (c) application (quality of reduction and pin placement).
position on a flat top radiolucent operating fracture table with
traction attachments available, and if time allows, c-arm control (Fig. 37.8).
Except in dire circumstances, a full prep and sterile technique is used
for pin placement in either an open or percutaneous approach. With the
open technique,
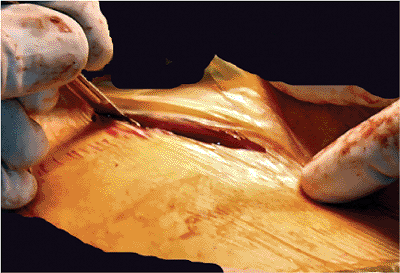
an
8-cm incision in the anterior third of the iliac crest is started 2- to
3-cm posterior to the anterior–superior iliac spine (ASIS) (Figs. 37.9 and 37.10)
to avoid damage to the lateral femoral cutaneous nerve. To decrease the
chance of skin stretching by the fixator pins, the incision should be
made after manual reduction and compression of iliac wings. This
incision provides appropriate orientation for the insertion point of
the pins, and it could be incorporated into an internal fixation
approach as part of a staged reconstruction. Stab incisions have also
been advocated if later surgical approaches might be necessary. In
cases where lasting external fixation is planned, 2-cm incisions
directed toward the umbilicus are less likely to cause soft-tissue
necrosis and are used for a percutaneous approach after iliac wing
reduction.
 |
|
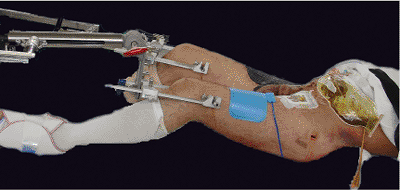
Figure 37.8.
A patient with a Tile C, unstable, open, pelvis fracture as well as an avulsed rectum, liver injuries, and a right leg amputation. The previously applied C-clamp has been removed. Staged reconstruction with percutaneous IS screws on the left side and an anterior external-fixator frame is planned. Both lower extremities are secured to the traction attachments to facilitate fracture reduction. |
 |
|
Figure 37.9.
An open approach to the right iliac crest. It is started 2 to 3 cm proximal to the ASIS (surgeon’s right index finger is on ASIS). |
 |
|
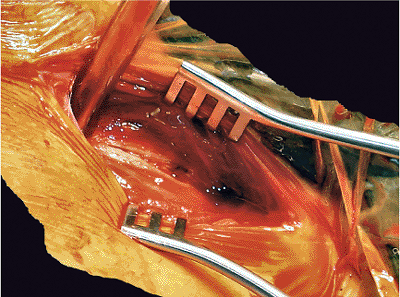
Figure 37.10.
The incision is deepened. Separating the insertions of abdominal and gluteal muscles and incising the periosteum allow for good orientation of the width of the iliac crest. If the iliacus muscle is not elevated from the inner table of iliac crest, the danger of entering the retroperitoneal hematoma can be minimized. |
to place the pins between the external and internal iliac-cortical
table, the insertion of pins should be initiated between the medial
third and half of iliac crest. Two pins should be placed in each ilium
for a emergently used (before laparotomy) resuscitation frame, or three
pins should be placed when adequate time is available. The iliac wings
have a slight curvature, and the pins may not align in a straight line.
The pins should be at least 1 cm apart. In the supine position, the
iliac crest angles approximately 45 degrees toward the operating table,
but the angle varies from patient to patient (Fig. 37.11).
The outer cortex is opened with an appropriate drill bit inclined from
cranial toward the greater trochanter; it is aimed for the bone stock
of anterior pillar above the acetabulum. The surgeon manually advances
5-mm-diameter pins through the opening hole while trying to feel and
not penetrate the internal or external tables. This procedure is not
always easy, especially in obese patients.
of pin placement. The surgeon can insert Kirschner (K) wires on both
sides of the iliac wing to serve as a guide. Some external fixation
sets come with a special guide where a long arm is rested on the inner
table. In another technique, the c-arm is used. Obturator oblique views
show the iliac wing profile and pin position (Fig. 37.12).
Of course, elevation of the gluteal and iliac muscles from both sides
of the pelvis allows direct visual control of the iliac wing for pin
placement. However, extensive soft-tissue dissection on the inside of
the iliac wings can enter the retroperitoneal hematoma and should be
avoided. The stability of the pins is assessed by a gentle trial of
in-line traction. When secure, the pins are captured by pin-to-bar
clamps and attached to a connecting bar. The same is done on the
opposite side, and both independent bars can be manipulated to improve
the fracture reduction.
alone will suffice. For bucket handle, lateral-compression injuries
(Tile B2), opening and external rotation of the compressed iliac wing
is required. Completely unstable, translational injuries (Tile C)
require anterior pins and some type of posterior fixation. The bars are
connected anteriorly with one or two transverse bars by bar-to-bar
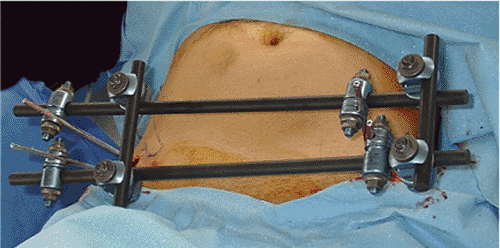
connectors that form a trapezoidal frame (Slätis type) (Fig. 37.13).
The frame should be positioned to allow for laparotomy, additional
soft-tissue swelling, and the patient to maintain an upright position
in bed (Fig. 37.14). If any soft-tissue tension is caused by the pins (Fig. 37.15),
they should be released by small relaxing incisions and sutured without
tension to avoid pin-tract discomfort, necrosis, and infection (Fig. 37.16).
The fracture reduction and pin placement are checked with the c-arm,
and final permanent radiographs are obtained as condition permits (10,13,25).
 |
|
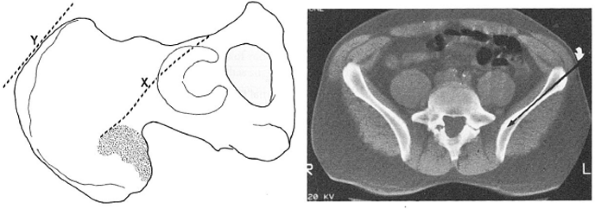
Figure 37.11.
A CT scan showing the orientation of the pelvis in the supine position. The ilium lies at an angle of approximately 45 degrees. The surgeon must consider this orientation when placing the iliac crest pins. (From Rommens
PM, Hesmann MH. External fixation for the injured pelvic ring. In: Tile M, Helfet DL, Kellam JF, eds. Fractures of the pelvis and acetabulum. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2003:208 with permission). |
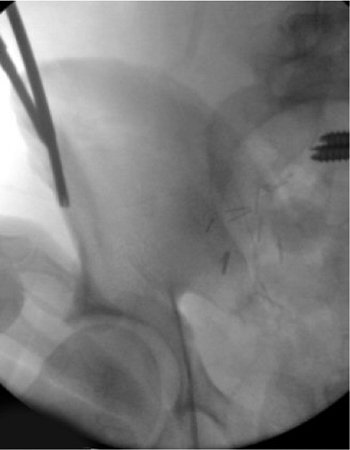 |
|
Figure 37.12. C-arm view following pin placement.
|
 |
|
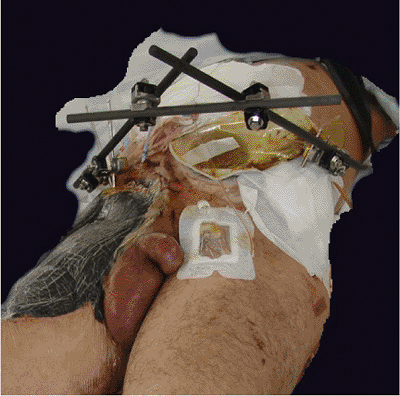
Figure 37.13.
An anterior–superior iliac crest, trapezoidal frame. This patient has a diverting colostomy, suprapubic catheter, and significant soft-tissue defect on right upper thigh. |
holds pins well. Biomechanical studies have been shown that pins and
frames in this “low route” provide better SI joint stability than
“upper route” (iliac crest) frames (31,32). Some authors (20)
feel that this route is ideal for emergent anterior-pelvic fixation
because of the ease of palpating this thick bone, quickly putting one
pin at each side, reducing the pelvis, and connecting the frame with a
single anterior-transverse bar. We and others (10,33)
feel that for those with limited experience with this technique, the
risk of penetrating the hip joint or damaging the neurovascular
structures in the greater sciatic notch, especially in the absence of
the c-arm, is high.
the anterior–inferior iliac spine (AIIS). Just lateral to the AIIS
projection, a 2-cm, transverse, skin incision is made. This will allow
less skin-pin interference when the patient is mobilized.
longitudinal fashion to avoid potential damage to the lateral, femoral,
cutaneous nerve. A protection sleeve with an inner trocar is advanced
with an oscillating motion. Through the trocar, the outer cortex is
opened with a drill. A 5- or 6-mm diameter pin with threads of 50 to 70
mm and length of at least 180 mm is advanced through the trocar (Fig. 37.17).
The entry point should be at the level of the AIIS, advanced toward the
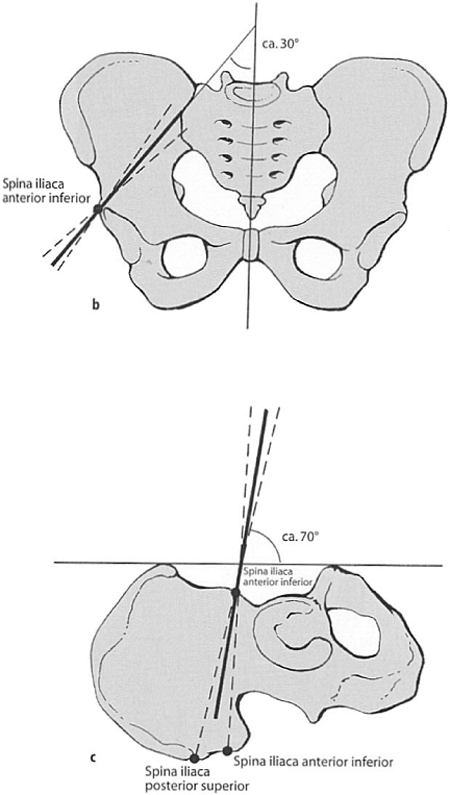
acetabular roof, and checked on an iliac, oblique, c-arm view (Fig. 37.18). To avoid the sciatic notch, the pin is directed toward the SI joint: 30 degrees
medial in the sagittal direction (Fig. 37.19) and 20 degrees less than perpendicular (about 70 degrees) to the caudo-cranial axis (Fig. 37.20).
Another pin is placed in the opposite side, and after additional
reduction maneuvers, both pins are connected by a single bar (Figures 37.21 and 37.22).
The bar should be far enough from the skin to allow for additional
swelling. Correctly done, this low route will not obstruct a laparotomy
approach, and patients are usually able to sit without difficulties
with a frame in place (34).
 |
|
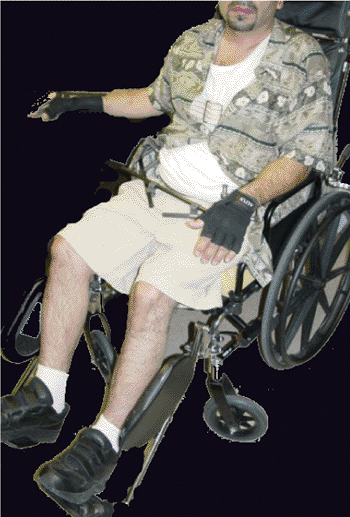
Figure 37.14. External fixation frames should be placed to allow eventual mobilization and an upright position.
|
 |
|
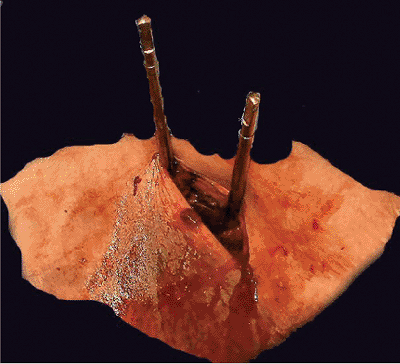
Figure 37.15.
An example of soft tissues under tension around the pins. If the incision is not made in reduced iliac-wing position and it is not perfectly above the medial half of the iliac crest, the soft tissue may be compromised after pin placement. Such an outcome may cause significant discomfort, tissue necrosis, and possible infection. |
 |
|
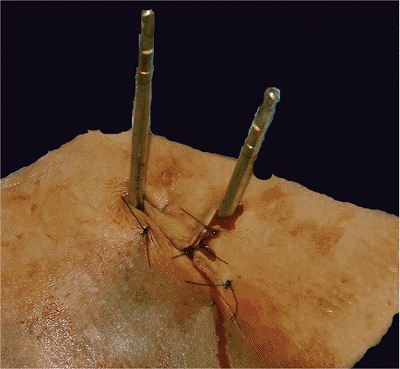
Figure 37.16.
A soft-tissue release by an incision perpendicular toward the direction of tension. The incision can be closed without any undue tissue stretching. |
 |
|
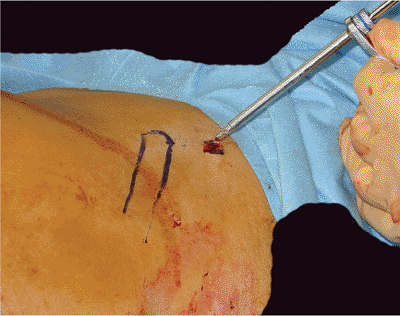
Figure 37.17.
Supra-acetabular external-fixation pin placement under c-arm control. A 2-cm incision is made, and the soft tissues are spread down to the bone. The external fixation pin with soft-tissue-protecting sleeve should be utilized. |
 |
|
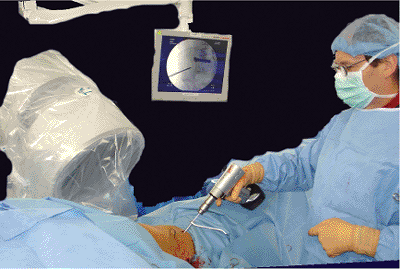
Figure 37.18.
Supra-acetabular pin placement. The iliac oblique view helps the surgeon ensure that the pin does not penetrate the hip joint nor pass through the greater sciatic notch. |
 |
|
Figure 37.19. Supra-acetabular pin placement. The obturator oblique view helps the surgeon guide the drill and pin toward the SI joint.
|
gauze sponges for light compression for a day or two, and they are
cleaned regularly to avoid crusting, fluid retention, and infection.
Skin tension around the pins should be identified and released under
local anesthesia. The frame construct should allow the patient to
maintain an upright position such that pulmonary function is
facilitated.
high-frame configuration or one pin in a low-frame configuration
provide enough stability for patients to bear weight. Selected,
oblique, unilateral, posterior, ilium or sacral fractures with an
anterior injury, if reduced well, are stabilized sufficiently with an
anterior fixator to permit weight bearing. After mobilization, pelvic
x-rays should be used to verify stability and assure that no reduction
has been lost. An anterior frame does not provide enough stability for
weight bearing on bilateral posterior injuries.
compression, several large diameter pins, a curved bars–bow fixator, a
combination of iliac crest and supra-acetabular frames, and application
of multiple techniques. Lateral compression injuries heal in 6 to 8
weeks; symphyseal diastasis heals in 6 to 10 weeks.
cannot be sufficiently controlled with an anterior frame alone. If a
C-clamp is unavailable or not applicable (posterior iliac fracture),
then in addition to an anterior frame, supracondylar femoral traction
with 25 lb can be used with the hip slightly flexed to improve
posterior and superior displacement (13,25,26).
in the German literature in 1964 and 1972, but the modern era reemerged
with the reports of Ganz et al in 1991 and
Buckle et al in 1995 (20,35,36).
Biomechanical testing has shown that the C-clamp provides better
fixation than any other type of pelvic external fixation in posterior,
unstable, pelvic injuries (26). It improves the
conditions for effective hemostasis by compressing fracture surfaces,
eliminating motion and dislodgement of formed clots, decreasing pelvic
volume. It also may enhance self-tamponade and provides firm support
for pelvic packing when needed. Many patients show an immediate
improvement of vital signs after frame application.
 |
|
Figure 37.20. Supra-acetabular pin placement angles. (Modified from
Pohlemann T, Lobenhoffer PH, Tscherne H. Therapie. In: Tscherne H, Pohleman T, eds. Becken and acetabulum. Berlin: Springer; 1998:143
with permission.) |
 |
|
Figure 37.21.
Supra-acetabular frame from the front. One pin on each side is enough for resuscitation purposes, and if longer use is planned or a higher degree of instability is present, then two pins and two connected bars will provide better stability. |
 |
|
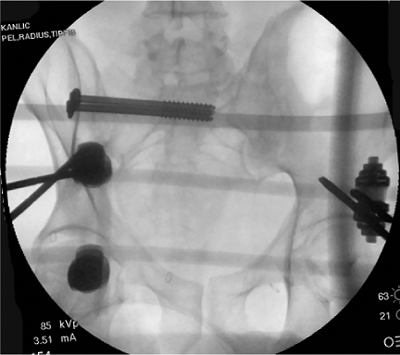
Figure 37.22.
AP radiograph with right SI screws and a supra-acetabular frame. This minimally invasive combination of fixation was chosen to treat a patient with multiple injuries that include a Tile C pelvic fracture. |
 |
|
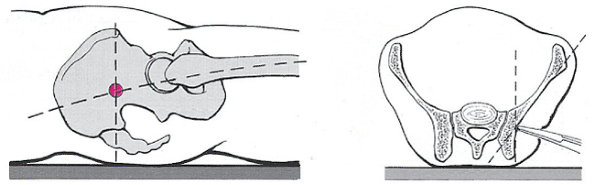
Figure 37.23.
The landmarks for placement of a pelvic C-clamp. In the reduced position, the intersection of a line in the femur longitudinal axis and one that is vertical from the ASIS is the correct entry point. Palpation to effect transition of the oblique portion of iliac wing from an anterior into more vertical posterior position is helpful. (Modified from Pohlemann T, Regel G, Bosch U, et al. Notfallbehandlung und komplextrauma. In: Tscherne H, Pohleman T, eds. Becken and acetabulum. Berlin: Springer; 1998: 98
with permission.) |
It is indicated only for a posteriorly unstable pelvic injury in a
hemodynamically unstable patient. Contraindications are hemodynamically
stable patients and those with posterior iliac-wing fractures.
semicircular tubes connected by a central ratcheting gear (Browner’s
pelvic stabilizer). Both have large pins with sharp tips, and outside
threads allow for connection with the frame and for additional dialed
compression. Sets should be sterilely packed and available in emergency
and operating rooms.
position of the pin insertion at the intersection of a line along the
long axis of the femur and with a vertical line angled down from the
ASIS. If landmarks are missing because of deformity and swelling, the
C-arm makes for a much safer procedure. The pin tip should be placed on
the outside surface of the ilium at the level of the reduced,
posterior, SI joint, where the bone is the thickest. The transition of
the oblique and vertical portion of the iliac wings’ lateral surfaces
is presented in Figure 37.23. With the patient
supine, the surgeon can palpate the bony landmark with a hemostat
through a 2- to 3-cm longitudinal incision on the side of injury (Figs. 37.24 and 37.25).
Compression pins are screwed into the threaded compression bolts and
attached to the arms connected with the central ratcheted gear. The
central gear is then released so the arms can be spread to accommodate
patient size, and the compression pin attached to the fixator arm is
“walked” on the lateral iliac wing until the vertical portion is felt (Fig. 37.26). The sharp tip is pushed into the bone and held there until the same procedure is done with other pin on opposite side.
fracture is reduced, the arms are compressed, and central gear is
tightened. Additional incremental compression is possible by screwing
the pins centrally through the compression bolts (Figs. 37.27, 37.28, 37.29, 37.30, 37.31, 37.32 and 37.33).
Overtightening should be avoided if in cases of transforaminal sacral
fracture, which can cause additional sacral-nerve damage. If the
patient’s condition does not improve despite aggressive resuscitation
efforts and pelvic stabilization, then the patient should be taken to
the operating room for laparotomy and pelvic packing (see Fig. 37.2).
Otherwise, the pins are sterilely covered, the frame supported by
towels, and pin care is assumed in day or two. Pelvic x-rays are
obtained when possible.
 |
|
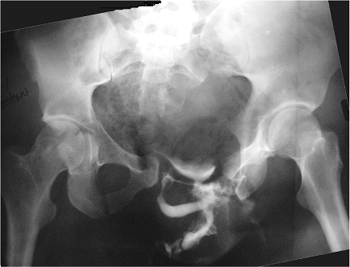
Figure 37.24.
AP pelvic x-ray shows a translationally unstable, open (ruptured rectum and perirectal tissue), left-sided pelvic-ring injury with extravasation of contrast after urethrogram and right T-type acetabular fracture. The patient also had a closed head injury, chest injury, right-open-forearm fracture, and left-proximal-humerus fracture. |
 |
|
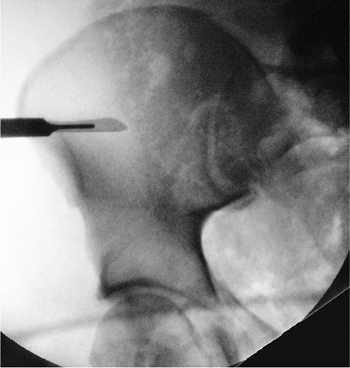
Figure 37.25.
The incision location for C-clamp pin placement. If the patient’s hemodynamic status allows and an operating room is available, c-arm control should be used because it will improve pin placement and the accuracy of reduction. |
 |
|
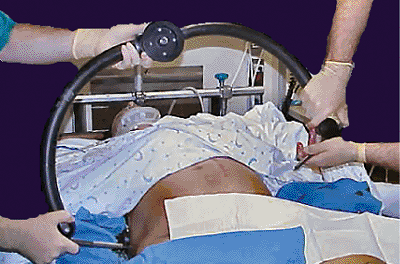
Figure 37.26.
C-clamp application. Compression pins are inside of the frame, and the first pin is “walked” on the lateral wall of iliac wing on the stable side. The pin is pushed hard into the bone and held in place until reduction and the opposite pin is placed. The clamp may be applied in the emergency room, intensive care unit (ICU), or operating room. |
 |
|
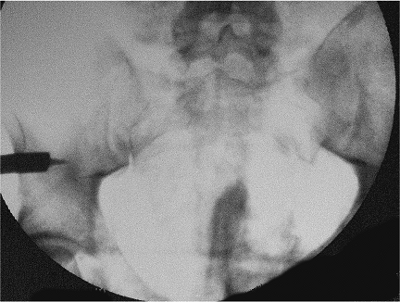
Figure 37.27. The c-arm view shows the first pin anchored on the intact side.
|
 |
|
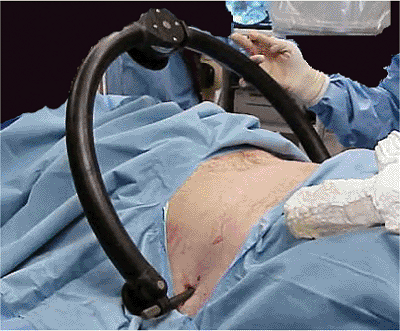
Figure 37.28.
A clinical picture of the C-clamp in place. Posterior stabilization is achieved, and the frame can be rotated up or down to make space for additional procedures. |
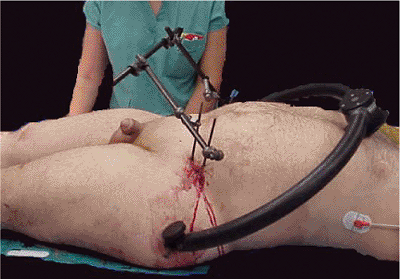 |
|
Figure 37.29.
Posterior C-clamp and anterior iliac-crest frame in place. The patient’s condition was stable, and enough time was available to apply an anterior frame before a laparotomy was conducted. |
-
vascular or nerve damage if pins slip into greater sciatic notch,
-
intrapelvic pin penetration and intestinal perforation,
-
loss of reduction,
-
pin loosening, and/or
-
displacement of unstable hemiplevis into true pelvis.
soon as patient condition allows, usually in 2 to 5 days. If internal
fixation is impossible, then the clamp should be removed, and anterior
fixation with supracondylar traction applied (9,35,36).
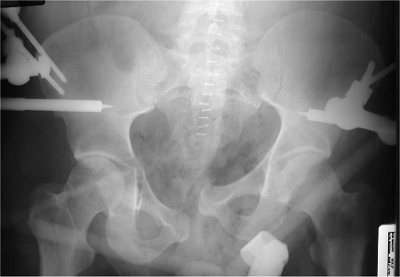 |
|
Figure 37.30.
AP pelvis x-ray shows the pelvic ring temporarily reduced and fixed with the posterior C-clamp and iliac-crest anterior fixator. |
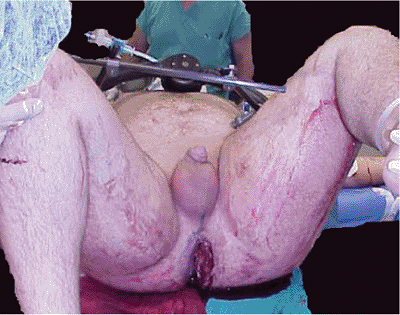 |
|
Figure 37.31. Pelvic fixation allows lithotomy position for surgery on the rectal injury.
|
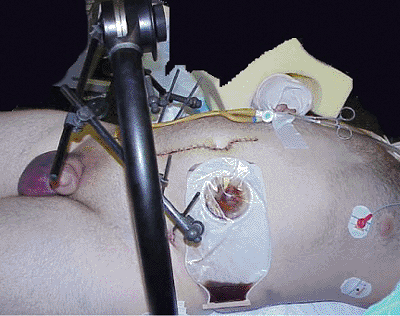 |
|
Figure 37.32.
Clinical picture after primary surgeries with stable, external, posterior and anterior fixation. Rectal injury was debrided and irrigated, and a suprapubic cystotomy and diverting left-sided colostomy with distal colon irrigation were performed as well. |
 |
|
Figure 37.33.
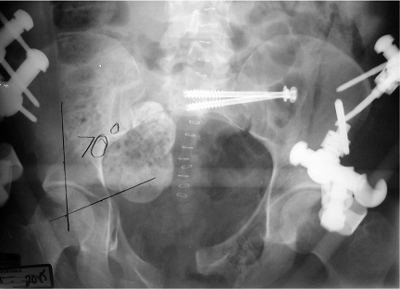
AP pelvic x-ray with anatomically reduced, left, SI joint and SI screws that were used to replace the C-clamp 2 days after it was employed. |
T, Tscherne H, Baumgärtel, et al. Beckenverletzungen: epidemiologie,
therapie und langzeitverlauf. Übersicht über die multizentrische studie
der arbeitsgruppe becken. Unfallchirurg 1996; 99:160–167.
W, Keel M, Eid K, et al. Control of severe hemorrhage using C-clamp and
pelvic packing in multiply injured patients with pelvic ring
disruption. J Orthop Trauma 2001;15:468–474.
AJ, Wilber JH, Lieberman JM, et al. The effect of laparotomy and
external fixator stabilization on pelvic volume in an unstable injury. J Trauma 1995;38(3):396–400.
SF, Shah K, Jaffe J, et al. Arterial embolization is a rapid and
effective technique for controlling pelvic fracture hemorrhage. J Trauma 1997;43(3):395–399.
N, Shindo M, Tanaka K, et al. Gluteal muscle necrosis following
transcatheter angiographic embolization for retroperitoneal hemorrhage
associated with pelvic fracture. Injury 2001;32(1):27–32.
BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with
injuries to the pelvic ring: the role of early patient mobilization and
external fixation. J Trauma 1993;35:671–677.
PM, Hessmann MH. External fixation for the injured pelvic ring. In:
Tile M, Helfet D, Kellam J, eds. Fractures of the pelvis and
acetabulum. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;
2003:203–216.
T, Krettek C, Hoffmann E, et al. Biomechanischer vergleich
verschiedener notfallstabilisierungmassnahmen am beckenring. Unfallchirurg 1994;97(10):503–510.
HJ, Draijer F, Haveman D, et al. Stabilisierung des beckenrings mit
fixateur externe–biomehanische untersuchungen und klinische
erfahrungen. Orthopäde 1992;21:363–372.
R, Browner B, Morandi M. Emergency reduction for pelvic ring
disruptions and control of associated hemorrhage using pelvic
stabilizer. Tech Ortho 1994;4(9):258–266.
