Atypical Spine Infections
however, there are a wide variety of other offending agents.
Tuberculosis is of particular concern owing to its high worldwide
prevalence, with approximately one third of the world’s population
infected. Immigration, drug resistance, and immunoincompetency have
resulted in a reemergence of tuberculosis within the United States,
necessitating a new focus on its complications and treatments.
Immunosuppression and immunodeficiency resulting from various
conditions also have led to an increased incidence of nontuberculous
granulomatous infections.
so that much of the causal discussion becomes skewed by its particular
population dynamics. An overall increase in incidence of all atypical
infections within the United States can be attributed to
immunocompromised states. Iatrogenic immunosuppression and human
immunodeficiency virus (HIV)-related immunodeficiency are the primary
contributors to this immune system dysfunction. In particular regard to
M. tuberculosis, the resurgence is due to
several other factors as well. There is an ever-increasing immigration
of infected individuals into the United States from Asia and Central
America. In addition, poor compliance to antimicrobial treatment
regimens has generated multidrug resistance within strains of the
organism. Finally, elderly and homeless populations continue to grow,
with an accompanying increase in nursing home and shelter inhabitants.
owing to increased immunosuppression, but it has been associated with
greater use of hyperalimentation and chronic disease states.
Inoculation of the spine is almost universally through hematogenous or
lymphatic spread with atypical infections, generally after infestation
through other portals to the body (e.g., respiratory, genitourinary,
indwelling blood vessel catheters).
and approximately 10% to 15% of infections disseminate to
extrapulmonary sites. Only about 5% of these patients have spinal
involvement (approximately 0.5% of the world’s population, or 30
million people). Approximately 50% of extrapulmonary bone involvement
is found in the spine. In the United States, the incidence was at 17
per 100,000 in 1991, with that level trending upward over the last
several decades.
have a predilection for elderly patients. Immigrants, homeless
individuals, alcoholics, intravenous drug abusers, and other
immunocompromised individuals (iatrogenic or HIVrelated) compose the
bulk of people at highest risk in the United States. Children are the
most commonly affected in underdeveloped nations. Approximately one
third of patients with active tuberculosis of the spine develop
neurologic impairment. Although most cases of tuberculosis are caused
by M. tuberculosis, M. africanum and M. bovis infection also may result in “tuberculosis,” as we know it.
ubiquitous in the environment and rarely cause serious disease in
healthy individuals. Other atypical organisms have particular
geographic proclivities. Brucellosis is found more commonly in areas
that do not pasteurize milk. Mycobacterium avium-intracellulare
also is associated with poorly processed milk. Blastomycosis has a
regional distribution in the southeastern and midwestern United States,
whereas coccidiodomycosis exists in the southwestern United States,
Central America, and South America. Histoplasmosis has a large endemic
focus within the central eastern United States. Cryptococcus neoformans is found in the excreta of birds, such as pigeons and chickens.
diagnosis should be considered when taking the history and ordering laboratory studies.
|
TABLE 9-1 ORGANISMS CAUSING ATYPICAL SPINE INFECTIONS
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
through inhalation; however, other avenues are possible. Most atypical
spine pathogens infect the vertebral column through hematogenous
spread. Another less common conduit is the lymphatic system, which
frequently can be accessed by mycobacteria via lung or pleural
drainage. Spinal involvement rarely occurs via direct spread.
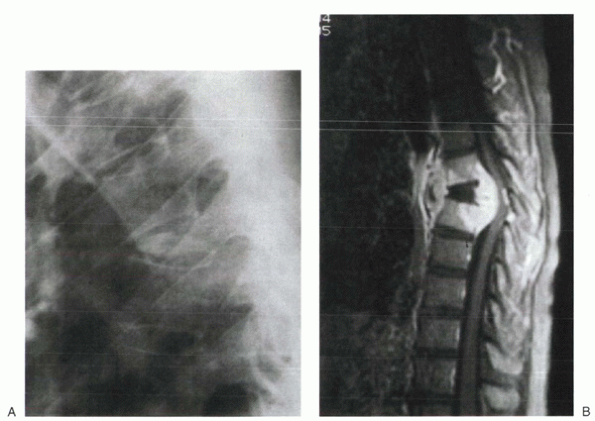 |
|
Figure 9-1 Plain radiograph (A) and T1-weighted MRI (B) of a patient with tuberculous infection of the thoracic spine. Note the kyphotic gibbous deformity at the site of collapse.
|
contagious through inhalation of aerosolized organisms. After primary
pulmonary involvement, many individuals develop secondary or
disseminated disease, in which extrapulmonary metastasis occurs.
Involvement of the vertebral body begins with deposition of bacilli in
the vertebral body, then accumulation of monocytes, epithelioid cells,
and Langerhans’ cells as part of a delayed-type hypersensitivity
reaction. The continued host immune response of the human body
generates the enlarging masses and subsequent damage to surrounding
tissues. Expansion occurs within the vertebral body and progresses
outward along the longitudinal ligaments or into the spinal canal, but
usually spares the intervertebral disc space (Fig. 9-1).
Granulomas of the vertebral body may originate in the metaphyseal area
near the subchondral end plates, anteriorly or centrally, and typically
cause collapse and deformity (Table 9-2).
Tuberculosis of the spine almost always occurs in the anterior
elements, although it has been identified rarely in the posterior
elements (vertebral arch) or primarily in neural tissue.
other atypical pathogens, but it is thought to be similar in most
cases. Granulomatous reactions are elicited by most of these organisms,
and indolent clinical courses tend to be the rule, rather than the
exception. Syphilis has shown an ability to involve the neural elements
directly, causing tabes dorsalis and bone structures by gumma
formation. Gummas
are granulomatous lesions with large central zones of acellular necrosis.
|
TABLE 9-2 TUBERCULOSIS: SITES OF VERTEBRAL ORIGIN
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||
presents late in the disease with atypical spine infections. There is
wide variability in initial clinical presentation due to the insidious
nature of these infections. A thorough history often elicits reports of
constitutional symptoms, such as weakness, night sweats, chills, weight
loss, and fever. In the presence of HIV or other chronic disease
states, it can be difficult to make immediate assumptions based on such
symptoms, however. Pain often begins with vertebral collapse or spinal
deformity and tends to occur more frequently with thoracic involvement.
Paraplegia is the most common neurologic deficit with tuberculosis of
the spine. Deficits usually are associated with cervical or thoracic
involvement, with a 40% incidence of cord compression when found in the
cervical spine. Physical examination sometimes may reveal draining
sinus tracts. Truncal rigidity and muscle spasm can be present.
-
Anemia
-
Albumin and total protein depletion
-
Lymphocytosis
-
Mild elevations in white blood cell count and erythrocyte sedimentation rate
(erythrocyte sedimentation rate and C-reactive protein) are less
elevated than with pyogenic infections.
anergy panel should be performed on all patients suspected of having
tuberculosis. Sputum samples or biopsy specimens should be sent for
acid-fast stains and cultures. Identification by culture can take 8
weeks, but polymerase chain reaction and other serologic testing can
accelerate the identification process.
D-arabinitol-to-L-arabinitol ratio, whereas Fontana-Masson stain should
be ordered if cryptococcosis is the suspected pathogen. Aspergillosis,
as with many other fungi, can be detected with potassium hydroxide
preparations of affected tissues.
for pyogenic infections. The insidious nature of atypical spine
infections causes plain film abnormalities to develop more slowly. Bone
changes often are advanced on initial radiographs owing to the delayed
presentation of many patients. These include the following:
-
Collapse of affected segments that may lead to kyphotic gibbous deformities (Fig. 9-2)
-
Paravertebral encroachment, which may be
seen more readily on x-rays of atypical infections, as the
granulomatous mass spreads outward, along the longitudinal ligaments
modality for confirming spinal involvement and can help differentiate
infection from metastatic disease. Although pyogenic infection can be
differentiated from neoplastic disease by the former involving
(crossing) the disc space, this distinction may not be useful in
evaluating tuberculosis of the spine. Tuberculous infections often
spare the disc space. With advanced disease, large anterior soft masses
eventually can involve two or more vertebral bodies, however,
“crossing” disc spaces. Use of gadolinium may help define the margins
of active disease when performing an MRI. Computed tomography scans can
be used for diagnostic imaging, if MRI is unavailable, and may serve as
an important tool for surgical decision making. Nuclear medicine
studies have roles similar to their roles in pyogenic infections.
because it is similar at the outset. Based on history of a more
indolent process, and a higher suspicion of atypical pathogens, unique
laboratory studies (e.g., serologic testing, PPD) may be warranted
initially. Public health agencies also may need to be contacted early
in the process.
when dealing with any atypical spine infection. In the United States,
tuberculosis infection confirmation activates a cascade
of
public health events. Pharmacotherapy is the primary modality of
treatment for patients with tuberculosis of the spine. Because of
progressively worsening drug resistance, treatment regimens almost
always consist of at least two medications. A standard empirical
regimen comprises isoniazid, rifampin, pyrazinamide, and streptomycin
(or ethambutol). Withdrawal of one or more medications may be prudent
when cultures and sensitivities are complete. Treatment duration
recommendations range from 6 to 12 months and can vary based on
clinical response. Vitamin B6 should be given with isoniazid to prevent
peripheral neuritis. Other medications used to treat tuberculosis
include aminosalicylic acid, capreomycin, cycloserine, ethionamide,
kanamycin, thioacetazone, and viomycin. These medications may be
employed in the case of multidrug resistance, immunodeficiency, or
inability to tolerate standard regimens.
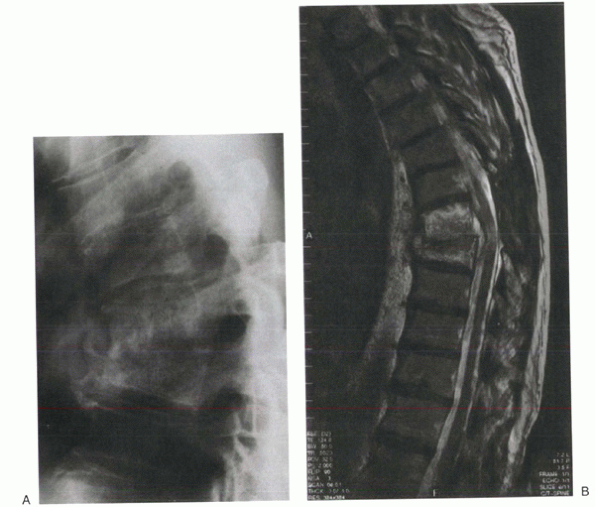 |
|
Figure 9-2 Thoracic brucellosis infection shows adjacent segment involvement and kyphotic collapse on plain film (A) and T1-weighted MRI (B).
|
5-fluocytosine, but advanced disease usually requires surgical
intervention. Aspergillosis most often can be treated with amphotericin
B and rifampin initially. Amphotericin B and ketoconazole combined are
effective for coccidioidomycosis and blastomycosis.
in pyogenic infections. Reduction of pain, stabilization for improved
healing of infection, and prevention or slowing of deformity all are
theoretical advantages.
-
Neurologic compromise
-
Failure of nonoperative treatment
-
Spinal instability
-
Progressive spinal deformity
-
Need for tissue biopsy
-
Advanced disease
-
Recurrence of infection
infections, urgent surgical intervention usually is required only in
the presence of progressive neurologic deficit. Surgical decompression
has shown significantly better results for neurologic recovery than
pharmacotherapy alone, even when done late in the disease course.
infection. Most commonly, the anterior spine is affected and requires
anterior débridement. Rarely, posterior elements are the focus of
infection, and in these unique circumstances, a posterior approach and
débridement can be employed. Although there have been reported series
of anterior débridements alone, progression of kyphosis has been
significant in nearly one fifth of these patients. For this reason,
current recommendations are for anterior reconstruction
via
strut graft. There are many different strut graft options, including
autograft and allograft bone, to be used for anterior reconstruction.
Anterior instrumentation can be added for reinforcement without
significant concern for persistent infection from hardware seeding, as
is the case with pyogenic infections. It also may reduce the chances of
progressive kyphotic deformity. Options for anterior instrumentation
are evolving, but currently they include plates, rods, and metal cages.
|
TABLE 9-3 SURGICAL INDICATIONS FOR ATYPICAL SPINE INFECTIONS
|
|||||||
|---|---|---|---|---|---|---|---|
|
unstable anterior constructs or when multilevel corpectomies are
performed. Because of the increased likelihood of growth-related
deformity, some authors have advocated posterior instrumentation in all
immature spines after anterior débridement. In addition, any posterior
débridements that destabilize the spinal column necessitate posterior
instrumentation for definitive treatment. Posterior fusion alone, in
the presence of anterior disease, does not protect adequately against
progressive kyphotic deformity. In the presence of advanced kyphosis,
posterior closing wedge or pedicle-subtraction osteotomy variants may
be considered for deformity correction. These are technically demanding
procedures with increased neurologic risks.
adequately with proper pharmacotherapy. External support with bracing
or other methods of immobilization should be chosen based on fixation
quality and surgeon preference.
with current treatments consisting of surgery and medications, the cure
rate is high. Fusion success with the recommended approaches exceeds
90%.
JB Jr, Farer LS, Hopewell PC, et al. Treatment of tuberculosis and
tuberculosis infection in adults and children. Am J Respir Crit Care
Med 1994;149:1359-1374.
BL, Eismont FJ. Infections of the spine. In: Herkowitz HN, Garfin SR,
Balderston RA, et al, eds. The spine, 4th ed. Philadelphia: WB
Saunders, 1999:1207-1258.
D, Swash M. Diagnosis and management of tuberculous paraplegia with
special reference to tuberculous radiculomyelitis. J Neurol Neurosurg
Psychiatry 1979;42:12-18.
AA, Rinsky LA, Fountain S, et al. Coccidiomycosis of the spine: unusual
roentgenographic presentations. Clin Orthop 1979; 140:78-79.
Research Council Working Party on Tuberculosis of the Spine. A 15-year
assessment of controlled trials of the management of tuberculosis of
the spine in Korea and Hong Kong. J Bone Joint Surg Br 1998;80:456-462.
