Osteonecrosis of the Femoral Head
– HIP > Part B – Evaluation and Treatment of Hip Disorders > 4 –
Osteonecrosis of the Femoral Head
disease that if left untreated often results in subchondral fracture,
collapse of the femoral head, and debilitating arthrosis. The precise
pathophysiology of ON remains unclear; however, it appears to be the
final common pathway of either traumatic or atraumatic factors that
compromise the tenuous circulation of the femoral head. The disease
typically affects young patients, thereby significantly impacting both
work and leisure activity. Accordingly, early diagnosis and treatment
are crucial to limit the progression of ON and the subsequent need for
total hip arthroplasty. In many cases, however, diagnosis is made in
later stages of the disease, when femoral head–preserving treatments
are no longer effective. This chapter discusses the natural history of
ON, the current diagnostic and treatment options for both early and
late stages of the disease, and the limitations of these existing
therapies.
dislocations, with increased risk associated with prolonged duration of
dislocation. 1,2
The incidence of traumatic ON of the femoral head following
nondisplaced femoral neck fractures is approximately 10%, whereas the
incidence following displaced fractures ranges from 15% to 50% and
generally correlates with the degree of displacement, time until
reduction, and accuracy of reduction. 3,4
The true incidence of atraumatic ON is unknown; however, some studies
in Western populations show that about 10% of all total hip
arthroplasties are performed for ON, leading to estimates that there
are at least 20,000 to 30,000 new cases per year in the United States. 5
The disease affects men four times more frequently than women and
generally presents in the third to fifth decades of life. Atraumatic ON
is bilateral in 30% to 70% of patients at the initial time of
presentation; however, the stage of disease typically presents
asymmetrically. 6
associated with the development of ON of the femoral head. Mechanical
interruption of the blood supply to the femoral head has been
identified as the causative factor of ON following femoral neck
fracture or hip dislocation. 7
Conversely, the precise genesis of atraumatic ON is unclear, but it has
been associated with numerous risk factors and underlying clinical
conditions (Table 4-1). It is hypothesized that
these etiologic factors either result in a compromise of the blood
supply of the subchondral region of the femoral head or have direct
toxic effect on cells, resulting in cellular necrosis and impaired
remodeling potential of the subchondral bone with eventual collapse of
the compromised region.
hyperlipidemia, renal osteodystrophy, sickle cell anemia, caisson
disease, and other systemic disorders have all been associated with ON
of the femoral head. Of these recognized risk factors, corticosteroid
use and alcohol abuse are the most commonly implicated, representing
90% of new cases of ON. 8 High-dose oral steroid regimens have a stronger association with ON as compared with low-dose therapy. 7,9
However, in a study of liver transplant patients receiving
immunosuppressive corticosteroids, no association was noted between
steroid dose and the development of ON. 10
It appears that transplant patients who develop ON demonstrate an
idiosyncratic response to the drug secondary to an underlying
hypercoagulability or hypofibrinolysis. With some diseases such as
liver and renal failure, it is difficult to separate the effects of
corticosteroids on bone from those of the underlying disease. Defining
the quantity of alcohol intake that increases the risk of ON has been
problematic. One prospective study suggested that patients who consume
over 400 mL of alcohol per week were 9.8 times likely to develop ON
versus nondrinkers. 11
Additionally, an increasing number of reports document a relationship
between human immunodeficiency virus (HIV) infection and ON of the hip.
12 The causal relationship is
difficult to establish because many of these patients have numerous
concomitant risk factors; however, there is some evidence implicating
antiviral therapy as a causative agent.13
|
TABLE 4-1 Risk Factors Associated with Osteonecrosis of the Femoral Head
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
considered, it is important to recognize that the vast majority of
patients with the aforementioned risk factors do not develop ON, and in
other patients, no risk factor is identified, underscoring the
multifactorial genesis of this disease.
atraumatic ON of the femoral head are not clearly defined; however,
several theories have implicated both intravascular and extravascular
factors that may contribute to this pathologic process. Each of these
phenomena shares the final outcome of ischemia, cellular necrosis, and
failure of remodeling of the subchondral bone. Osteocyte death has been
attributed to alterations in blood flow that may be the result of local
or systemic factors. Following fracture or dislocation, disruption of
the lateral retinacular arteries may compromise the primary blood
supply of the femoral head. This precarious arterial blood supply may
also be altered by intravascular microemboli that are generated by
systemic diseases including the thrombophilias, sickle cell disease,
fat emboli resulting from hyperlipidemia, or air embolization secondary
to dysbaric phenomena. 7,8,12
Local hyperlipidemia and intravascular lipid deposits have also been
noted in patients with corticosteroid and alcohol use, suggesting a
causal role for these agents.
predispose the region to extravascular compression and local ischemia.
The cancellous bone within the subchondral region of the femoral head
is enclosed within rigid cortical bone. This system is particularly
susceptible to increases in pressure, and the venous outflow can be
exquisitely sensitive to compression. Disorders in fat metabolism,
generated by corticosteroid or alcohol use, may cause both adipocytes
and osteocytes to hypertrophy, resulting in local microvascular
compression. 7,8,12
In Gaucher disease, macrophages enlarge as they accumulate
sphingolipids, resulting in a similar compressive phenomenon. The
direct cytotoxic effects of alcohol and corticosteroids have also been
implicated in osteocyte necrosis and may inhibit osteogenic
differentiation of mesenchymal stromal cells. 14,15
which may result in a more favorable outcome. A thorough history
focused on determining associated risk factors should be undertaken.
Patients may not have any specific complaints during the early stages
of the disease; however, with progression, patients will complain of
deep groin pain with ambulation or pain referred to the knee. The onset
of pain may be insidious or acute in nature and is typically described
as throbbing; night pain and morning stiffness are not uncommon. The
findings on physical examination are variable. Some patients have a
complete, pain-free, range of motion of the hip and walk without a
limp. Others have a limp and discomfort with active and passive range
of motion. Collapse of the femoral head is associated with painful
internal rotation and a limited range of motion. Individuals with
chronic symptoms may have a flexion contracture. It is of utmost
importance that the contralateral hip be examined, as bilateral disease
is common. Because some patients may develop ON without the existence
of any risk factors, an index of suspicion must be developed for young
patients with persistent groin pain that is unresponsive to rest and
activity modification.
anteroposterior and frog-leg lateral radiographs to determine the
status of the femoral head. The early stages of the disease may not be
visible on plain radiographs, but over time, a predictable pattern of
radiographic change becomes evident. This sequence begins with
radiolucencies and sclerosis in the femoral head, resulting from bone
resorption and new bone formation. Progressive microfractures may
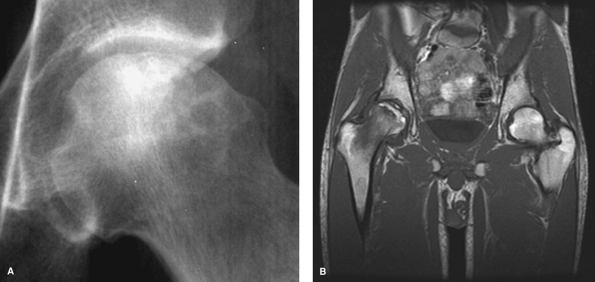
result in a pathognomonic crescent sign, most readily visible on
frog-leg lateral views (Fig. 4-1A). This
represents precollapse of the weakened necrotic subchondral bone. The
necrotic angle (measured referencing the center of the femoral head)
can be calculated from plain films to stage the size of the necrotic
region. This value is the sum of the angle of the necrotic segment as
measured on both the anteroposterior and lateral radiographs. Patients
with a necrotic angle >200 degrees
have less favorable results following certain femoral-head sparing procedures. 16
The end stage of the disease manifests as a complete collapse of the
femoral head and subsequent arthritic changes noted on both the femoral
head and acetabulum.
 |
|
Figure 4-1 Plain radiograph of the hip demonstrating adjacent sclerosis and lucency along with subchondral collapse or crescent sign (A). T1-weighted MRI illustrating low signal at the normal-ischemic bone interface (B).
|
in diagnosing ON and should be obtained in all suspected cases in which
the plain radiographs are normal. In such cases, examination of both
hips should be performed because more than half of all cases are
bilateral. The changes noted on T1-weighted images typically include
subchondral signal changes located in the anterior superior quadrant of
the femoral head with a single-density line demarcating the
normal-ischemic bone interface (Fig. 4-1B). The
T2-weighted images may demonstrate a high-signal line inside a
low-signal region (double-line sign). As lesion size has been
associated with prognosis and response to therapy, MRI can be used to
determine lesion size or volume. 17
was proposed by Arlet and Ficat in the 1960s and has undergone
subsequent modification (Table 4-2). 18
This classification relies solely on plain radiographs, which are often
unrevealing early in the disease. Steinberg et al. have proposed a
radiographic classification that incorporates plain x-ray, bone scan,
and MRI findings to create a comprehensive and specific description
that may be more effective in characterizing the progression of the
disease (Table 4-2). 19
Moreover, this system considers volumetric assessment of femoral head
involvement that may have predictive value in the outcomes of specific
interventions.
history focused on delineating risk factors for the development of ON
should be obtained. However, other causes of hip pain should be
considered. An examination of the spine should be performed to rule out
lumbar pathology. In cases where infection is suspected, hip aspiration
may prove useful. Plain anteroposterior and frog-leg lateral
radiographs of both hips should be obtained to evaluate for sclerosis
or collapse of the femoral head in ON, but these studies may also
reveal other painful conditions including hip dysplasia or neoplasm.
When plain radiographs are normal, or sclerosis of the femoral head is
noted, MRI examination of the affected and the contralateral hip should
be undertaken. In pregnant females or males in the fifth decade of
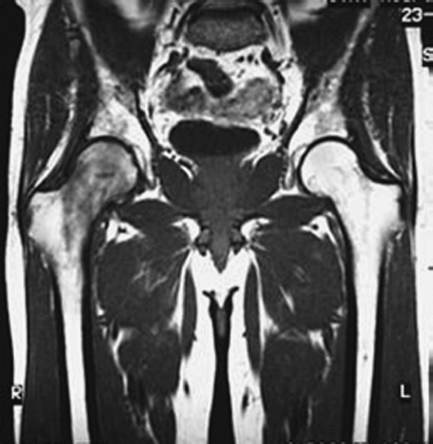
life, it is important to consider transient osteoporosis of the hip
(TOH), which, if diagnosed, is self-limited. Unlike the localized
changes found in ON, TOH demonstrates diffuse osteopenia on plain
radiographs, and the MRI often has a global decrease in T1-weighted
signal throughout the femoral head and neck metaphysis (Fig. 4-2)
and a global increase in the T2-weighted signal in the same regions.
Treatment includes protected weight bearing until the condition
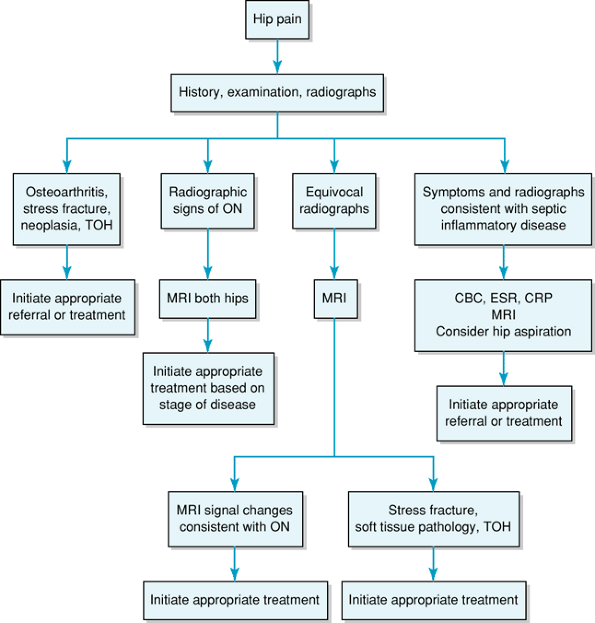
resolves, which may take up to 6 months. 9 Figure 4-3 presents a diagnostic workup algorithm.
findings to formulate a treatment plan. Young, healthy patients without
significant acetabular disease will generally be better served by
procedures that attempt to preserve the femoral head. Conversely,
arthroplasty may be an excellent option for patients with collapse of
the femoral head or acetabular involvement. It is important to
recognize that the indications for existing treatment regimens remain
controversial and are often dictated by the surgeon’s clinical
expertise and familiarity with available surgical options.
|
TABLE 4-2 Radiographic Classifications of Osteonecrosis of the Femoral Head
|
|||
|---|---|---|---|
|
ON of the femoral head remains limited. The prescription of protected
weight-bearing regimens in forestalling the progression of disease has
proven ineffective in most cases. 6,20
This approach may be reserved for those patients who are incapable of
tolerating a surgical intervention or are of limited life expectancy.
Other nonoperative modalities including electrical stimulation and
hyperbaric oxygen have been evaluated in the treatment of ON. These
modalities have demonstrated varying success in preventing collapse of
the femoral head. 21,22
More recently, the results of extracorporeal shock-wave therapy were
compared with those of core decompression and bone grafting. The
authors concluded that extracorporeal shock-wave treatment appeared to
be more effective than core decompression and nonvascularized fibular
grafting in patients with early-stage ON of the femoral head. 23
The role of pharmacologic therapies in the treatment of ON has not been
well defined and requires further investigation. Antihyperlipidemic,
antihypertensive, and anticoagulant medications all have been proposed
as candidate treatment agents. Most recently, the bisphosphonate
alendronate has been shown to be effective in delaying the progression
of femoral head collapse in a cohort of patients with early-stage
disease. 24 Again, long-term
evaluation is mandated to determine if this agent truly prevents,
rather than merely retards, collapse of the femoral head.
 |
|
Figure 4-2
T1-weighted MRI with low-intensity signal representing bone marrow edema in the femoral head, neck, and metaphysis consistent with transient osteoporosis of the hip. The left hip appears normal. |
attempts to prevent collapse, arthrosis, and the subsequent need for
arthroplasty. Currently, core decompression is the most commonly used
and most comprehensively studied treatment for early-stage ON of the
femoral head. Originally described by Ficat and Arlet as a diagnostic
intervention,
core
decompression was found to alleviate pain, presumably by reducing
femoral head pressure and restoring physiologic blood flow. 7
Eventually core decompression was adopted as a treatment modality. The
procedure involves creating a decompression tract from the lateral
cortex of the femur to the area of necrosis, the diameter of which can
range from 9 to 12 mm depending on the diameter of the femoral neck. A
biopsy is usually obtained at the time of surgery, and protected weight
bearing is advised for a minimum of 6 weeks following the procedure.
Although the success rates following the procedure are variable, for
small and medium-sized precollapse lesions, the results of core
decompression are generally 80% to 90% successful. 25 However, the results are poor in the presence of a crescent sign or definitive collapse of the femoral head. 26,27
 |
|
Figure 4-3
Diagnostic algorithm for osteonecrosis of the hip. CBC, complete blood count; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; MRI, magnetic resonance imaging; ON, osteonecrosis; TOH, transient osteoporosis of the hip. |
been advocated for treating early-stage disease. Vascularized fibular
grafts have the potential advantage of providing structural support,
osteoconductive factors, osteoinductive factors, and a vascular supply
to the necrotic region. However, this procedure requires a longer
operation and is associated with donor site morbidity, ankle
instability, peroneal nerve palsy, heterotopic ossification, and
subtrochanteric fracture. 28,29,30
Patients may not bear weight for a minimum of 6 weeks following the
procedure and may be only partially weight bearing for an additional 3
to 5 months. The relative benefit of vascularized fibular graft versus
nonvascularized graft or core decompression has yet to be conclusively
proven. However, reported results are satisfactory in hips that do not
have significant head depression. 28,29,30
bone matrix is an appealing option because it may enhance healing
without significantly altering the anatomy of the femoral neck if
arthroplasty is necessary. Biologic adjuvants including growth factors
(such as VEGF, BMP) and autologous bone marrow cells may also play a
role in treating osteonecrotic lesions and have prompted a great deal
of clinical interest. Lieberman et al. demonstrated that allograft bone
grafts in combination with BMP prevented radiographic progression of ON
in 14 of 17 hips at an average follow-up of 53 months. 31 Randomized trials evaluating the efficacy of these agents in preventing femoral head collapse are necessary.
option for carefully selected patients with ON of the femoral head. The
goal of osteotomy in these patients is to reposition the necrotic
segment away from the weight-bearing surface and bring normal articular
cartilage supported by healthy bone into the weight-bearing
area.
The ideal patient for this procedure is a young adult possessing a
mobile hip with a small isolated lesion who does not require
corticosteroids or abuse alcohol. 20,32
The type of osteotomy will be contingent on the size and location of
the lesion and may include intertrochanteric, rotational
transtrochanteric, valgus flexion, or varus intertrochanteric
osteotomies. Outcomes following osteotomies are better in small or
medium-size lesions of early stage whereas these procedures are less
predictable following femoral head collapse. 20
These technically demanding procedures should be performed only by
experienced surgeons, and subsequent conversion to a total hip
arthroplasty may be difficult.
|
TABLE
4-3 Treatment Algorithm According to the University of Pennsylvania System of Classification and Staging (Radiographic Stage, Symptoms, and Procedure) |
||||||
|---|---|---|---|---|---|---|
|
arthrosis are indications for reconstructive procedures. Failure rates
for total hip arthroplasties (THA) and hemiarthroplasties in this
cohort are higher than failure rates for other diagnoses, which is most
likely attributable to the relative youth of the patients and the lack
of other factors limiting physical activity. 33
Accordingly, temporizing procedures have evolved to address this
difficult-to-treat group of patients. Joint resurfacing, or surface
arthroplasty, has been proposed as a means of providing pain relief to
patients who are deemed too young for conventional arthroplasty.
Hemiresurfacing uses a cemented hemispheric femoral head prosthesis
that is matched to the patient’s native acetabulum. This mode of
resurfacing may be considered for patients with little or no acetabular
disease. In the presence of significant articular cartilage
degeneration, a total resurfacing procedure (which incorporates a
prosthetic acetabular component in addition to the femoral resurfacing)
may be considered. Although these resurfacing procedures have
demonstrated clinical promise, the current short- and long-term results
for resurfacing procedures remain variable. 33,34,35
end-stage arthrosis, total hip replacement is indicated. Although early
studies evaluating THA in patients demonstrated high failure rates,
newer surgical techniques have yielded more favorable results. 36,37
With the advent of highly cross-linked polyethylene, metal-on-metal,
and ceramic-on-ceramic weight-bearing surfaces, patients with ON may
have more favorable success rates with total hip arthroplasty,
obviating the need for multiple surgeries. A treatment algorithm based
on radiographic stage and clinical symptom proposed by Lieberman et al.
is outlined in Table 4-3. 9
K, Hirohata T, Sugioka Y, et al. Influence of alcohol intake, cigarette
smoking, and occupational status on idiopathic osteonecrosis of the
femoral head. Clin Orthop Relat Res. 1988;234:115–123.
SA, Smith AM, Mashoof AA, et al. Osteonecrosis of the femoral head in
patients infected with HIV: a report of 4 cases and literature review. Am J Orthop. 2004;33:618–622.
P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool
in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81(2):349–355.
ND, Schwartz O, Militianu D, et al. Hyperbaric oxygen therapy as a
treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85(3):371–375.
CJ, Wang FS, Huang CC, et al. Treatment for osteonecrosis of the
femoral head: comparison of extracorporeal shock waves with core
decompression and bone-grafting. J Bone Joint Surg Am. 2005;87:2380–2387.
KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early
collapse of the femoral head in patients with nontraumatic
osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159.
SY, Kim YG, Kim PT, et al. Vascularized compared with nonvascularized
fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;87:2012–2018.
D, Furey C, Shaffer JW. Osteonecrosis of the femoral head. A study of
101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am. 2005;87:742–747.
JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head
with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–145.
YH, Oh SH, Kim JS, et al. Contemporary total hip arthroplasty with and
without cement in patients with osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85-A(4):675–681.
YH, Oh SH, Kim JS, et al. Contemporary total hip arthroplasty with and
without cement in patients with osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85-A:675–681.
SY, Kim TG, Kim PT, et al. Vascularized compared with nonvascularized
fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;87:2012–2018.
