Lateral Epicondylitis (Tendinosis)
Sports Medicine Clinic/Virginia Hospital Center Sports Medicine
Fellowship Program, Arlington, Virginia. Associate Clinical Professor,
Georgetown University Medical School Washington, D.C.
more commonly on the lateral than on the medial aspect of the joint.
The selection factors to determine the candidates for surgery are
similar for each process, yet there are some distinct features with
regard to the surgical technique. Thus medial epicondylitis is
discussed separately in Chapter 13.
epicondylitis. There are three broad indications and a fourth feature
to consider (7).
-
Pain is of significant intensity as to limit function and interferes with daily activity or occupation.
-
Localization is precisely at the lateral epicondyle and origin of the ECRB and EDC to the epicondyle.
-
A legitimate period of nonoperative
management has been attempted. This typically includes at least 6
months of activity modification, forearm band, antiinflammatory agents,
and a quality rehabilitation program; -
Failure of cortisone injections is no
longer considered an absolute necessity before offering surgical
intervention. However, if injections have been used and the patient is
no longer benefiting or has not benefited from them, then the patient
is a candidate for a surgical procedure.
and patients who have demonstrated lack of compliance with the
recommendations, particularly that of activity modification.
Individuals on worker’s compensation disability should be assessed on
several occasions to ensure that the preceding indications have been
met.
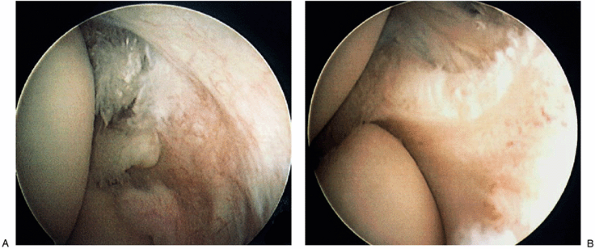 |
|
Figure 12-1. Arthroscopic identification of intraarticular pathology (A) was treated arthroscopically with relief of lateral epicondylitis symptoms (B).
|
over the tendon origin at the epicondyle. Provocative tests of pain
with resisted wrist extension for lateral involvement are invariably
positive, especially with the elbow in full extension (5).
In some cases the symptoms may be aggravated by performing the test in
elbow extension. If forearm pain is a component, examine for posterior
interosseous nerve irritation. The most sensitive is pain on resistive
supination (13).
process, and the principles of the surgical intervention should be
reviewed before undertaking the surgical procedure (7,8,9,11).
include precise identification of the pathologic tissue, resection of
all involved pathology, maintenance of normal tissue attachments,
protection of normal tissue, enhancement of vascular supply, firm
repair of the operative site, and quality postoperative rehabilitation.
extensor communis (anterior edge) are the tissues most commonly
involved laterally (100% and 35%, respectively) (1,2,3,4,9).
Histologically, the pathologic tissue is devoid of inflammatory cells
but has a characteristic pattern of fibroblasts and vascular elements (6,12). Recent electron microscopic evidence reveals lack of extracellular cross-linkage (6).
Furthermore, approximately 20% of surgical cases have been noted to
have some form of bony exostosis at the lateral epicondyle. Less common
pathologic changes include calcification in the soft-tissue elements
(extensor communis and radial collateral ligament). Intraarticular
changes, including synovitis and orbicular ligament abnormality, are
being recognized with increasing frequency with the advent of elbow
arthroscopy as a therapeutic tool (Fig. 12-1).
large majority of cases. It should be noted, however, that individual
variations can and do occur. In these instances, the pathologic
variations should be addressed as identified.
this time to draw a conclusion or to recommend this treatment. The
topic is addressed in Chapter 2.
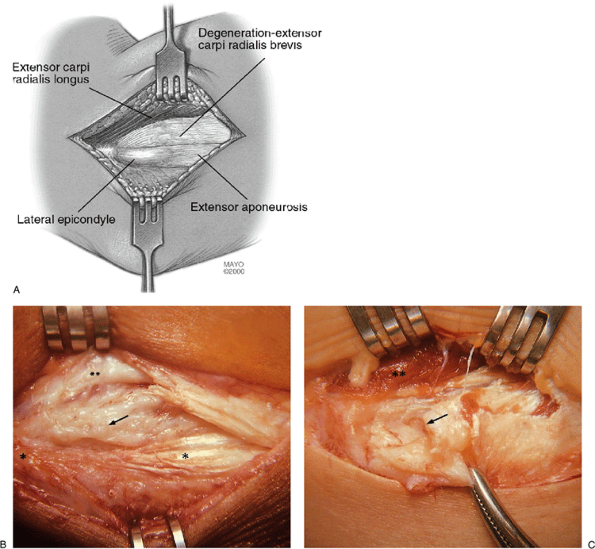
anteromedial to the lateral epicondyle just to the level of the joint
(i.e., 1 cm distal to the epicondyle) (Fig. 12-3). The subcutaneous
tissue and superficial fascia are incised and retracted, locating the
interface between the extensor longus muscle and the firm anterior edge
of the extensor aponeurosis. A palpable crevice is present at this
interface as the fascia over the extensor longus is thin and the
anterior edge of the aponeurosis is firm and thick (Fig. 12-4).
A splitting incision 2 to 3 mm in depth is made between the extensor
longus and the extensor aponeurosis in the identified interface
extending from 1 to 2 cm proximal to the lateral epicondyle distally to
the level of the joint line. The extensor longus is released by scalpel
dissection and retracted anteromedially to 2 to 3 cm. This retraction
brings the extensor brevis into direct view (Fig. 12-5).
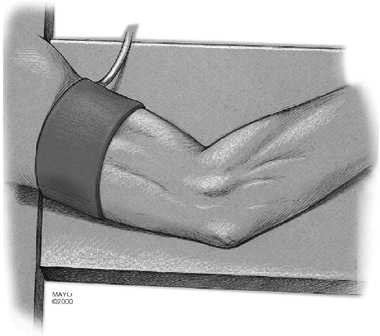 |
|
Figure 12-2. All illustrations are of a right elbow. The arm was draped with a nonsterile tourniquet and placed on an arm board.
|
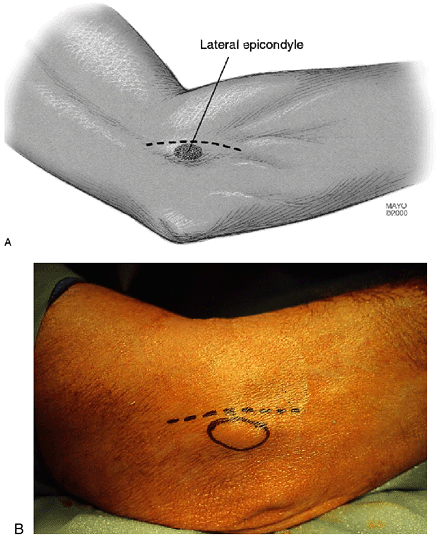 |
|
Figure 12-3.
The skin incision extends from 1 to 2 cm proximal and just anterior to the lateral epicondyle distally 1 cm just to the level of the elbow joint (A). Circled area identifies lateral epicondyle (B). |
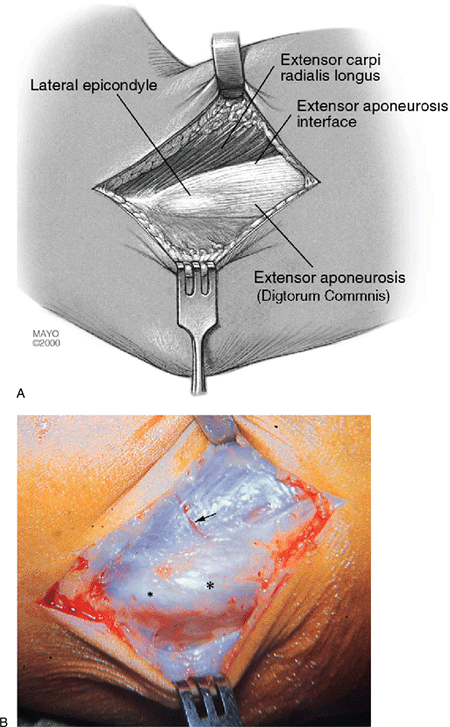 |
|
Figure 12-4. Identification of tendons. Exposure of the lateral epicondyle and interface between extensor longus and extensor aponeurosis (A). Note that the extensor brevis is not visible as it is hidden under the extensor longus (B). Small asterisk, lateral epicondyle; large asterisk, extensor aponeurosis; arrow, extensor longus.
|
error in the incision at the extensor longus interface is to penetrate
too deeply by vertical dissection. As noted, the extensor longus is
only 2 to 3 mm thick at this level. Once the 2- to 3-mm depth is
reached, the dissection is primarily horizontal progressing medially.
This technical subtlety is important to avoid iatrogenic distortion as
well as to bypass the body of the extensor brevis tendon. Such
iatrogenic distortion can easily complicate the identification of the
pathologic regions.
 |
|
Figure 12-5.
An incision in the extensor longus/extensor aponeurosis interface with anteromedial retraction of extensor longus exposes the pathologic origin of the extensor brevis (A). A key technical point is not to incise too deeply but more medially as the extensor longus is only 2 to 3 mm in depth at this level. Variations in pathoanatomic damage include degeneration of the extensor brevis origin (B) and a full avulsion rupture of the extensor brevis origin, as shown in a world-class tennis player (C). Small asterisk, lateral epicondyle; large asterisk, extensor aponeurosis; double asterisks, extensor longus; arrow, brevis tendinosis. |
 |
|
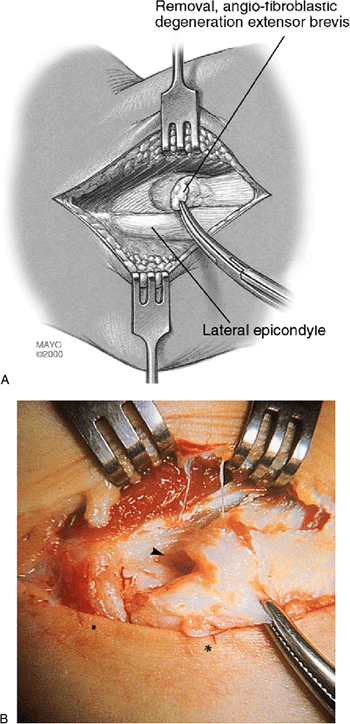
Figure 12-6. Resection of pathologic tissue. Resection of pathologic sections (histologically termed angiofibroblastic tendinosis) of extensor brevis origin. In this rendering, 100% of the origin is involved and a partial rupture is depicted (A).
In no circumstance is the extensor aponeurosis totally released from the epicondyle. Surgical photograph of resection of pathologic extensor brevis origin (B) shows major tendinosis with an underside rupture. Note a small strip of normal tendon at the edge of the extensor longus muscle. The remaining pathologic alteration has a dull-grayish edematous gross appearance typical of angiofibroblastic tendinosis. (Small asterisk, lateral epicondyle; below skin; large asterisk, extensor aponeurosis; arrow, tear.) |
 |
|
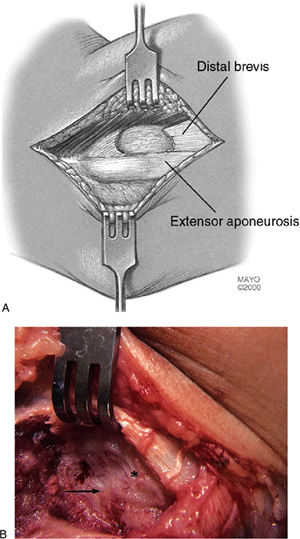
Figure 12-7. Completed resection of pathologic tissue. Resected pathologic section of the entire extensor brevis tendon (A). Surgical photograph depicting completed resection of the degenerated extensor brevis origin (B). Arrow, defect left after brevis resection; asterisk, healthy fascia over retracted longus.
|
entire origin of the extensor brevis will be easily identified. The
gross appearance of the pathologic change is dull-grayish tissue, often
edematous and friable, and, on occasion, ruptured (Fig. 12-5).
Normal tissue in contrast is shiny, is firm, and has a slightly
yellowish-white hue. The pathologic tissue often encompasses the entire
origin of the extensor brevis, and in our series the anterior 10% edge
of the extensor aponeurosis is abnormal in approximately 35% of cases.
origin is performed en bloc. This tissue block is somewhat triangular
in shape with the base distal (Fig. 12-6). The typical size of tissue is 2 з 1 cm (Fig. 12-6).
It should be noted that the brevis origin is released from the lateral
epicondyle and the anterior edge of the extensor aponeurosis. If the
anterior aponeurosis has pathologic alteration, the pathologic tissue
is also removed (but not normal tendon).
appearance and confirmed by the “Nirschl scratch test.” This makes use
of the friability of pathologic tissue, which easily peels off by
utilizing a scratching motion with the scalpel. When healthy tissue is
reached, it no longer peels off with the scratching motion. This
technique is especially helpful when removing the pathologic changes in
the anterior edge of the aponeurosis.
prominence of the lateral epicondyle, the proximal edge of the
aponeurosis is temporarily peeled off the epicondyle for adequate
exposure and the exostosis is removed by rongeur and smoothed by a rasp.
defect is present in the prior area of the extensor brevis tendon
origin. The more distal aspect of the extensor brevis is still attached
to the orbicular ligament, distal anterior aponeurosis, and underside
of the extensor longus (Fig. 12-7). The brevis
therefore does not retract distally to any appreciable degree, thereby
maintaining an essentially normal working length of the entire extensor
brevis muscle-tendon unit (i.e., from elbow to wrist). If a segment of
the anterior edge of the extensor aponeurosis or a bone exostosis is
present on the lateral epicondyle, these abnormalities are resected at
this time. Firm repair of the aponeurosis is always undertaken in these
circumstances. The goal of the operation is resection of all pathologic
tissue, not tendon release.
inspect the anterolateral joint compartment. Unless the patient
presents with clear intraarticular signs and symptoms preoperatively,
it is uncommon to find meaningful intraarticular changes.
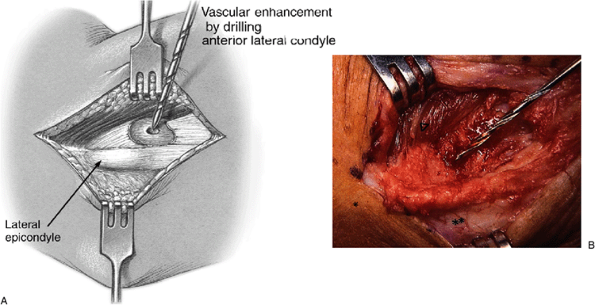
are drilled through cortical bone in the resected area. This technique
is theorized to encourage rapid replacement of this triangular tissue
void with healthy fibrotendinous tissue (Fig. 12-8).
 |
|
Figure 12-8. A,B:
Enhancement of revascularization by cortical drilling. The cortical bone is drilled with a 5/64-inch bit to enhance revascularization of the area. (Asterisk, lateral epicondyle.) Note: do not drill the tip area of the lateral epicondyle as bony disturbance here causes increased post-op pain. |
 |
|
Figure 12-9. A,B:
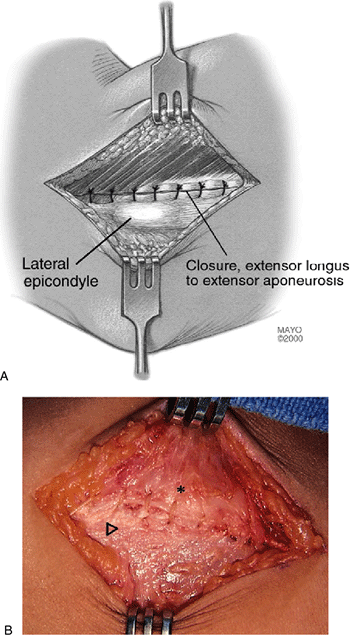
Repair of tendon interface. In all cases the interface between the extensor longus and the extensor aponeurosis is firmly closed. It is theorized that blood clot transformed to biologically healthy fibrous tissue (painless) replaces the proximal defect of the resected brevis area further reinforcing the security of the ultimate brevis origin. Arrow, lateral epicondyle; asterisk, extensor longus. |
longus and the remaining anterior edge of the extensor aponeurosis is
now firmly closed (Fig. 12-9). I use a simple
running stitch of No. 1 PDS. It is unnecessary to suture the distal
extensor brevis since a firm attachment is retained to the orbicular
ligament, distal aponeurosis, and underside of extensor longus
distally. The extensor aponeurosis is firmly repaired anteriorly, and
its proximal attachment is largely undisturbed; thus rapid mobilization
postoperatively is possible and encouraged. The skin is closed with 3-0
subcutaneous cat gut, subcuticular 3-0 Prolene and supporting
Steri-strips.
 |
|
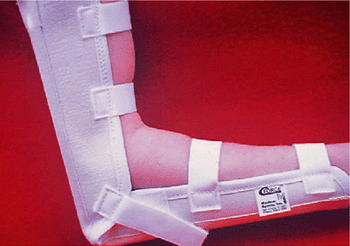
Figure 12-10.
Light elbow immobilizer with Velcro straps provides comfortable support in the immediate postoperative period. Motion exercises are usually started 48 hours postoperative but intermittent immobilizer protection is usually maintained for 5 to 6 days. (Courtesy of Medical Sports, Inc., Arlington, VA.) |
The joint is at 90 degrees flexion, the forearm is in neutral, and the
wrist and hand are free. I use full immobilization for 2 days. Motion
exercises are usually started within 48 hours following surgery (tendon
and/or tendon with nerve surgery when combined medial and lateral
surgery is done).
maintained for 5 to 6 days, at which time normal activities of daily
living are resumed. Counterforce support (forearm band) providing
protective function is utilized thereafter until full forearm strength
returns (usually 3 to 6 months). The brace is used at times of forearm
and shoulder rehabilitation exercise and more vigorous forearm
activities such as heavier household activities. A gradual return to
sports such as tennis and golf often is initiated at 8 to 10 weeks with
brace protection.
In the remainder, improvement is usually seen, but not adequate to
allow the person to return to all sport activity levels. In less than
5% no improvement is observed, and in rare instances the patient’s
condition has deteriorated. Thus less than 5% of patients are
considered failures.
elbow is residual pain of varying degrees. This is not common in our
experience and occurs in fewer than 10% of patients. When pain after
surgery is present, a logical analysis is conducted and the following
determinations must be considered (7,11):
-
Has there been sufficient time and/or proper rehabilitation to allow adequate healing?
-
Did the proper diagnosis exist before the surgical intervention?
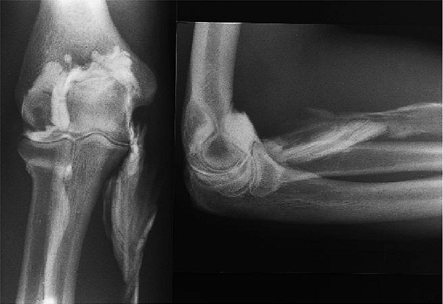 Figure 12-11. Arthrogram reveals extravasation of contrast material in forearm musculature after ill advised tennis elbow surgery technique.
Figure 12-11. Arthrogram reveals extravasation of contrast material in forearm musculature after ill advised tennis elbow surgery technique. -
Did something occur at the time of surgery to cause iatrogenic symptoms.
-
Was the true patho-anatomy adequately addressed?
the pathologic tendinosis tissue is implicated most commonly as the
cause of failure. In this case, a second surgical procedure should be
considered (7,11). Worker’s compensation may affect an individual’s motivation and should be considered during the rehabilitation phase.
lateral or medial epicondyle can result in a release of the collateral
ligament and resulting joint instability (7,11).
Occasionally, instability is manifested as residual pain and not as
laxity. This is diagnosed by stress view radiographs and occasionally
by an arthrogram (Fig. 12-11). The treatment is collateral ligament repair or reconstruction.
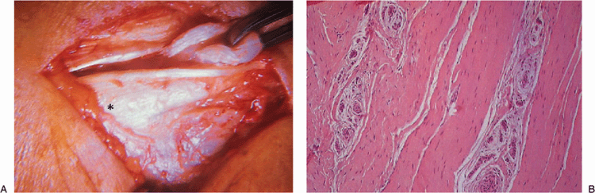
for 6 months. She had not responded to activity modification and
counterforce bracing. At surgery a complete rupture of the degenerated
brevis origin was identified and resected (Fig. 12-12).
Effectiveness was slightly altered but good enough to reach the
quarter-finals of the U.S. Open 9 months after surgery and to achieve a
ranking thereafter in the world’s top 10.
 |
|
Figure 12-12. Complete rupture of brevis tendon in a professional tennis player (A). Histologic examination confirmed this to be a degenerative, rather than inflammatory, process (B). Asterisk, lateral epicondyle.
|
B., Nirschl R.: Tendinosis of the Elbow (Tennis Elbow) Clinical
Features and Findings of Histological Immunohostochemical and Electron
Microscopy Studies, J Bone Joint Surg Vol. 81-A (2)1999:259–278.
RP. Sports and overuse injuries to the elbow. Muscle and tendon trauma;
medial and lateral tennis elbow. In: Morrey BF, ed. The elbow and its disorders, 3rd ed. Philadelphia: WB Saunders; 2000.
