AMPUTATIONS OF THE LOWER EXTREMITY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Amputations > CHAPTER 120 – AMPUTATIONS OF THE LOWER EXTREMITY
the United States annually has remained fairly steadily between 30,000
and 40,000 over the last 15 years (3). Most of
the amputees are older people with ischemic disease of the lower
extremities or diabetes of long standing. Amputations are a major
social and economic burden to society. Moreover, the amputation of one
lower extremity in the elderly all too frequently is followed by the
amputation of the contralateral limb; this occurs in 15% to 28% of
cases within 3 years. Only 50% of elderly amputees survive the first 3
years following an amputation. In spite of the advances in medicine
these statistics have not improved significantly for the dysvascular
amputee (9,12,14,59,63).
failure of treatment. Frequently, it is the treatment of choice for a
devastating injury to the lower extremity where reconstruction may be a
long and costly undertaking that leads to the preservation of a
functionally unsatisfactory extremity (33,34 and 35,65,72). It is important that amputation be done well and with adequate preoperative planning (4),
so that the outcome results in a residual limb that can wear a
prosthesis comfortably. With a prosthesis that fits well, the patient
is most likely to become an active member of society and independent in
his life-style.
includes the primary care physicians, the surgeons, a physical therapist, a prosthetist, and a social worker (53).
The patient must be involved and committed to a successful outcome. In
the case of patients with vascular disease, other organ systems are
usually involved. An internist who is familiar with the patient’s
condition must be involved and should counsel the patient (5,13,52).
requiring amputation and usually occurs in the geriatric patient. Since
preservation of a functioning knee greatly improves the chances of
rehabilitation of the amputee, consider reconstruction of occluded
major proximal arteries, which may save the limb or at least allow
amputation below the knee (1,25,58,67,71,72).
These patients have the added problems of peripheral neuropathy.
Therefore, they are subject to trophic ulcers and Charcot
osteoarthropathy. Indeed, frequently the amputation in patients with
diabetes is performed because of osteomyelitis caused by a trophic
ulcer that has exposed bones of the foot. Instability of the foot or
ankle because of the osteoarthropathy may also require amputation (8,44,63).
usually because of an irreparable acute vascular injury. Treatment of
lacerations of the popliteal artery has improved: During World War II,
amputation occurred in 72% of these cases (15), whereas 68% were successfully treated with vascular reconstruction in both the Korean War (39) and the Vietnam War. This rate of success has been reflected in the civilian experience (13,15,39,66).
with an open fracture, a vascular injury requiring repair, or
transection of the posterior tibial nerve is challenging, requiring a
long and expensive course of treatment with numerous surgical
procedures. Even if the limb can be saved, it is often painful and not
as useful as a prosthesis. This, together with a protracted course of
treatment, places an undue psychological burden on the patient (38,40,63). See Chapter 12 and Chapter 24 for a thorough discussion of these issues.
tumors of the extremities, combined with adjuvant therapy, has reduced
the incidence of amputations for primary malignancies of the lower
extremities (19,20,41,50,57,70,75). This is discussed in detail in Chapter 128 and Chapter 129.
wellvascularized subcutaneous tissue and skin that will withstand
pressure in weight-bearing areas, and friction in areas covered by the
prosthesis. Stumps that allow end bearing are particularly desirable.
Usually, end-bearing stumps retain a part of the sole pad or have a
bony surface of sufficient size that it can bear the body weight for
varying periods of time. These conditions are common in partial foot
amputations.
or knee disarticulation. Disarticulation is particularly desirable in
growing children and in the elderly. In children, overgrowth, which is
seen often in diaphyseal amputations, is avoided by retaining the
epiphysis (26,45). In the elderly, an end-bearing stump decreases problems with fitting and improves the chances for rehabilitation (37,64). The creation of a tibiofibular synostosis as described by Ertl (24) can create end-bearing conditions somewhat comparable to those of the disarticulation.
important for the healing of the amputation wound, several methods have
been developed to predict at which level of the lower extremity the
circulation in the skin is adequate for primary wound healing. One of
these is the xenon-133 (133Xe) clearance. After the ambient
conditions have been regulated, radionuclide is injected intradermally.
A gamma camera monitors the 133Xe activity for 10 minutes. The faster the 133Xe is cleared from the site of injection, the better the perfusion of the skin (34,46).
major amputations, the most accurate is probably the transcutaneous
measurement of oxygen tension. The oxygen sensor is calibrated for
temperature and atmospheric pressure and applied to the skin. The skin
is heated to 45°C. The sensor stabilizes after approximately 20
minutes. The minimal desirable tension varies between 30 and 50 mm of
mercury (7,47).
the affected extremity is entirely reliable for predicting success in
healing of the amputation wound. Other conditions, such as nutrition,
state of health, age, collateral circulation, and infection, play a
role in the success or failure of the amputation.
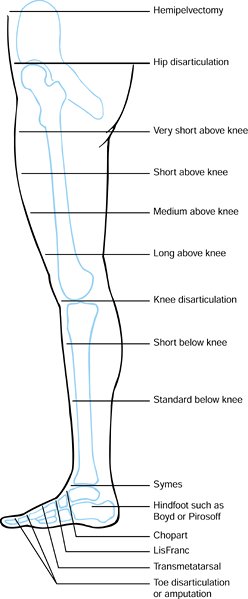 |
|
Figure 120.1. Levels of lower extremity amputation.
|
the amputation wound. It is therefore important that it be handled with
care. The use of skin hooks in particular is desirable in patients with
dysvascular extremities.
not entirely contraindicated in the lower extremity. As long as a
split-thickness graft is over soft tissue and not adherent to bone or
thick scars, it is likely to withstand the prosthetic wear. Pedicle
grafts do even better as long as they are not in the immediate
weight-bearing area of the residual extremity (74). Skin grafts are more successful in children than in adults.
soft-tissue mantle for the residual extremity. This can be achieved
best through myodesis (suturing the transected muscle end to drill
holes in the bone) or myoplasty (suturing the cut ends of the
antagonistic muscle groups and their fascias together). Under normal
circumstances, the cut muscle ends have the greatest amount of
capillary bleeding. Therefore, the suction drain should be in this
layer of the amputation stump.
stumps, such as in partial foot amputations or disarticulations, bony
prominence under this skin should be removed. Consider the possibility
of an osteoplastic treatment of the bones, such as a tibiofibular
synostosis (24).
without exception to the formation of neuromas. In the vast majority,
these neuromas are painless. However, where the cut end of the nerve is
within the weight-bearing area of the residual extremity, the neuroma
can become painful. Therefore, during the amputation each nerve should
be identified, dissected free to a level well above the amputation, and
sharply transected. Where a concomitant vessel is expected, ligate the
nerve before cutting it under minimal tension.
ligate them before transection. It is advisable not to catch
neighboring veins and arteries in the same ligature, since this might
lead to the development of aneurysms.
overwhelming infections, particularly infection by gas-producing
bacteria, an open amputation (43,66,69)
may be indicated. The aim of this surgery is to eradicate the diseased
area without creating a harbor for renewed infection. Although a
straight guillotine transverse amputation is the simplest, we prefer to
develop flaps as the secondary revision, and closure is then usually
easier.
-
Prepare the skin flaps that are necessary
for the secondary closure of the amputation wound. You may want to make
the initial skin flaps somewhat longer than usual, as some retraction
will occur. -
Transect the soft tissues and bone in the usual fashion.
-
Carry out hemostasis and transection of the nerves as described previously.
-
To prevent the skin flaps from
retracting, invert and suture the free skin edges to the subcutaneous
fascia at the base of the flaps with nonabsorbable sutures to be
unrolled at the time of secondary closure (Figure 120.2.). Only in patients with excellent circulation should you use skin traction to prevent skin retraction.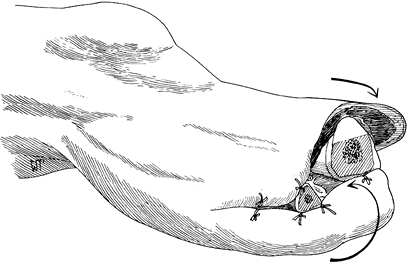 Figure 120.2. Open amputation with preservation of flap length using inversion technique. See text for details. (From Bohne WHO.Atlas of Amputation Surgery. New York: Thieme Medical, 1987:36, Fig. 8-9, with permission.)
Figure 120.2. Open amputation with preservation of flap length using inversion technique. See text for details. (From Bohne WHO.Atlas of Amputation Surgery. New York: Thieme Medical, 1987:36, Fig. 8-9, with permission.)
-
Select the level of amputation depending
on the pathology being treated. Preservation of some part of the toe
helps prevent migration of the other toes into the void created by the
missing toe. -
Develop equivalent-length dorsal and
plantar full-thickness, fishmouth-type skin flaps, favoring the tougher
plantar skin for the end of the stump. -
Transect the extensor tendon and let it retract.
-
Work from dorsal to plantar. Disarticulate the joint or transect the bone at the appropriate level and smooth the end.
-
Identify the neurovascular bundles. Cut the digital nerves back sharply and ligate the arteries and veins.
-
Then transect the flexor tendons and let them retract.
-
Close the skin flaps with interrupted
absorbable sutures in the subcutaneous fat, and interrupted 5-0 nylon
sutures in the skin. Do not resect “dog-ears,” as they will retract and
assume a smooth contour.
perforating ulcer under one of the metatarsal heads that has not healed
in spite of prolonged care, and when osteomyelitis involves the
metatarsal.
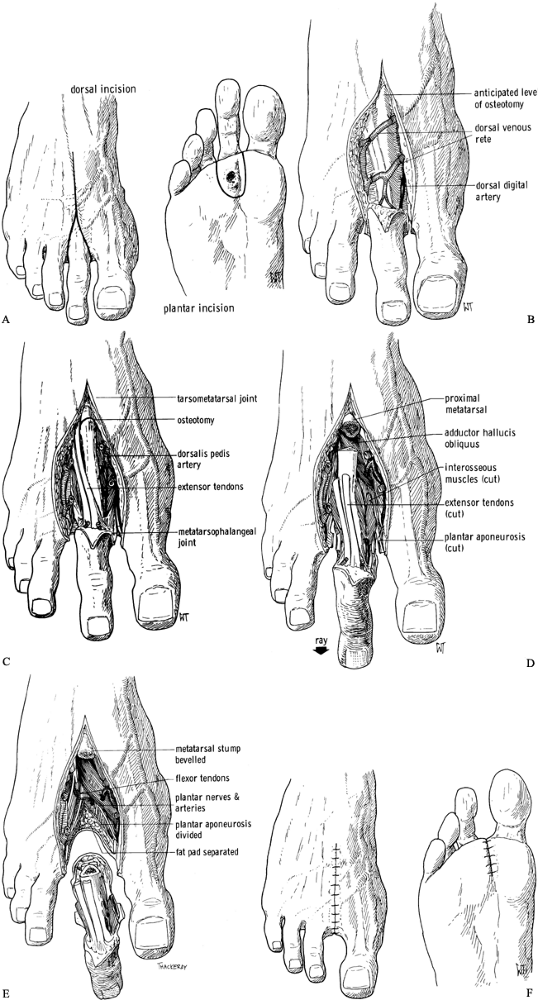
-
Make a racket-type incision
circumferentially around the toe, with a single limb over the dorsum of
the involved metatarsal, and including the area of the ulcer on the
plantar surface (Figure 120.3.A).![]() Figure 120.3. Amputation of the second ray of the foot. See text for details. A: Skin incision. B: Dorsal soft-tissue dissection to expose the metatarsal. C: Osteotomy at the base of the metatarsal. D: Release of other soft tissues. E: The metatarsal and toe are now free. F: Skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:40–42, Fig. 10-1, Fig. 10-2, Fig. 10-3, Fig. 10-4,Fig. 10-5 and Fig. 10-6 with permission.)
Figure 120.3. Amputation of the second ray of the foot. See text for details. A: Skin incision. B: Dorsal soft-tissue dissection to expose the metatarsal. C: Osteotomy at the base of the metatarsal. D: Release of other soft tissues. E: The metatarsal and toe are now free. F: Skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:40–42, Fig. 10-1, Fig. 10-2, Fig. 10-3, Fig. 10-4,Fig. 10-5 and Fig. 10-6 with permission.) -
Dissect open the skin on the dorsum of
the foot like a double door. Follow the intermetatarsal space without
incising the periosteum except at the very base of the metatarsal (Figure 120.3.B).
Identify and transect the digital nerves to the amputated toe. Preserve
the branches to the adjacent toes. Identify and ligate the digital
arteries and veins. -
Transect the base of the metatarsal (Figure 120.3.C),
lift up the distal end, and continue the dissection on the plantar
surface, including the part of the plantar fascia belonging to the
diseased ray. You can then lift the ulcer, the toe, and the surrounding
skin out of the wound. Leave the surrounding intrinsic muscles attached
to the metatarsal (Figure 120.3.D, Figure 120.3.E). -
Obtain hemostasis. Either leave the wound
open until secondary closure, or close the intermetatarsal space with
interrupted absorbable sutures and the skin with interrupted 5-0 nylon
sutures (Figure 120.3.F) (15).
leads to a transfer lesion on the second metatarsal. Therefore, in
these cases a transmetatarsal amputation may be the better choice.
toes and the metatarsal heads and necks. It requires a long plantar
flap to cover the anterior part of the amputation wound. In general, it
creates an excellent weight-bearing foot remnant. However, you must
consider that the fifth metatarsal is more horizontal than the first
metatarsal, so the shoe insert that is required after the
rehabilitation has to provide sufficient medial support to distribute
weight bearing evenly.
-
This procedure requires transection of
the dorsal skin slightly distal to the level of the bony amputation.
The plantar flap must be long enough to cover the ensuing amputation
wound. It must avoid any ulcerations that may be present on the plantar
surface of the forefoot (Figure 120.4.A).
Expose the metatarsals dorsally. Transect the extensor tendons, ligate
the dorsal veins, and cut back sharply the cutaneous nerves (Figure 120.4.B).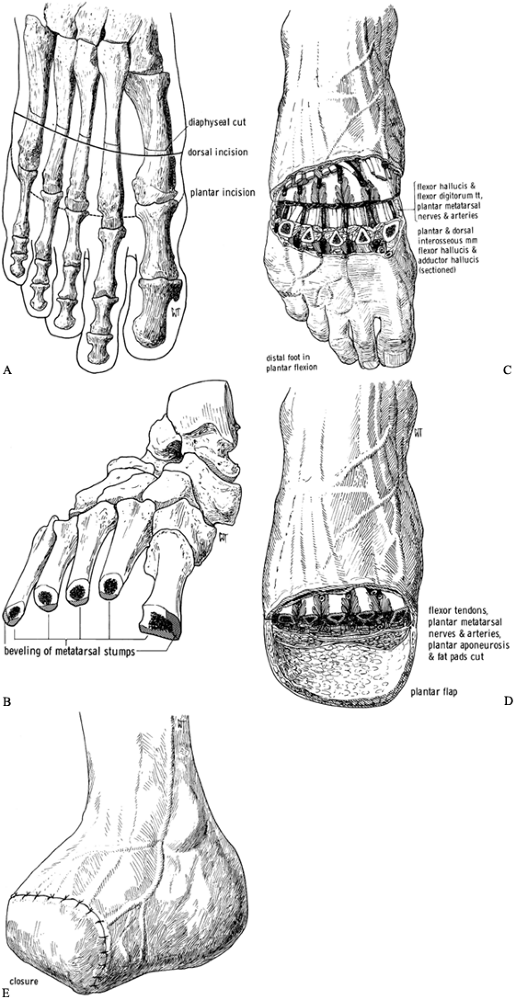 Figure 120.4. Transmetatarsal amputation. See text for details. A: Dorsal and plantar skin incisions relative to level of transection of the metatarsals. B:
Figure 120.4. Transmetatarsal amputation. See text for details. A: Dorsal and plantar skin incisions relative to level of transection of the metatarsals. B:
Transection of the metatarsals produces a cascade in which the fifth
metatarsal remnant is slightly shorter than the first. Bevel the
prominent plantar edges of the metatarsal remnants as well as the
lateral aspect of the fifth and medial aspect of the first. C: Plantar-flex the forefoot after transection of the metatarsals to gain access to the plantar structures. D: Completed amputation. E: Skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:44–47, Fig. 11-2, Fig. 11-5, Fig. 11-6, Fig. 11-7 and Fig. 11-8, with permission.) -
Carry out bony transection from the
dorsum of the foot. There should be a slight cascade, with the fifth
metatarsal remnant being minimally shorter than the first one. -
Transect the metatarsals with a small
oscillating saw. Deliver the distal ends of the metatarsal into the
wound and expose the flexor tendons and plantar neurovascular bundles (Figure 120.4.C). Divide and ligate these in an orderly fashion with the techniques described previously, preserving the plantar flap (Figure 120.4.D). -
Where possible, tack down the plantar pad
to the periosteum on the dorsum of the metatarsal remnants and
approximate the edges of the skin of the plantar and dorsal aspects (Figure 120.4.E).
tarsometatarsal joints, whereas the Chopart amputation is a
disarticulation through the talonavicular and calcaneocuboid joints.
Both amputations sacrifice the insertions of the muscles that aid in
the extension of the ankle joint; therefore, the anterior tibial
tendon, the toe extensors, and the peroneal tendons must be reinserted
into the tarsal bones. Even so, dorsiflexion of the ankle joints often
is inadequate, requiring a transcutaneous Achilles tenotomy. These
levels of amputations are less desirable than a transmetatarsal
amputation, as the stump has a tendency to develop an equinus posture
over time. Increased weight-bearing pressures over the plantar aspect
of the end of the
stump
may lead to pain and/or ulceration. On the other hand, when one of
these amputations is successful, its advantages are that it is end
bearing, does not sacrifice leg length, and requires only a filler in a
regular shoe in most cases.
-
Transect the dorsal skin at the level of
the disarticulation. The plantar skin flap must be long enough to cover
the anterior aspect of the remaining limb. -
Transect the extensor tendons somewhat distal to the skin incision to allow their insertion into the tarsal bones.
-
Transect the ligaments and joint capsules
of the tarsometatarsal joints, in the case of the LisFranc amputation,
and of the talonavicular and calcaneocuboid joints, in the case of the
Chopart amputation. Sharply plantar-flex the forefoot. The capsule and
plantar ligaments can be transected as well as the flexor tendons. Take
care not to injure the medial or lateral plantar artery and nerves. -
Dissect the part of the foot to be
amputated off the plantar flap, which should not be debrided to allow
full-thickness coverage of the anterior part of the stump. -
Leave the cartilage of the tarsal bones
and the remaining limb intact. This cartilage presents an effective
barrier against infections of the bone, and it decreases blood loss
from bone bleeding. Nevertheless, introduce a drain into the amputation
wound. -
Close the plantar flap to the dorsal
incision, securing the deep fascia to the periosteum and deep fascia of
the stump with absorbable sutures. Close the skin with interrupted 4-0
or 5-0 nylon or similar sutures.
amputation of the foot that allows the possibility of fitting with a
shoe and shoe filler; however, frequently the patient prefers a formal
prosthesis because the shoe is unstable (10).
surface of the heel by creating an arthrodesis between the distal tibia
and the tuber of the calcaneus (45). Compared
to a Syme’s amputation, it provides more length and better preserves
the weight-bearing function of the heel pad. Its increased complexity
and morbidity have made it less used now than the Syme’s amputation.
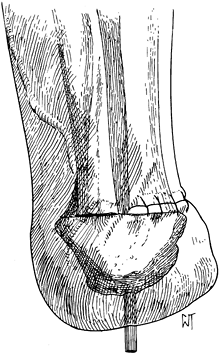
The Pirogoff amputation removes the anterior two thirds of the
calcaneus (Figure 120.5.) but has no advantage over the Boyd amputation, which is described later.
 |
|
Figure 120.5. Pirogoff amputation with fixation of the tuberosity of the calcaneus to the tibia.(From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:53, Fig. 12-7, with permission.)
|
-
Make the dorsal skin incision from the medial to the lateral malleolus across the anterior aspect of the ankle
P.3157
joint. Connect this to a plantar incision through the plantar fat pad
at the midtarsal joint region. Take care not to injure the tibial
neurovascular bundle. Plantar-flex the foot. Place a bone hook in the
posterior talus and pull it forward. Excise the talus. The plantar pad
remains attached to the undersurface of the calcaneus. Remove the
portion of the foot to be amputated (Figure 120.6.A).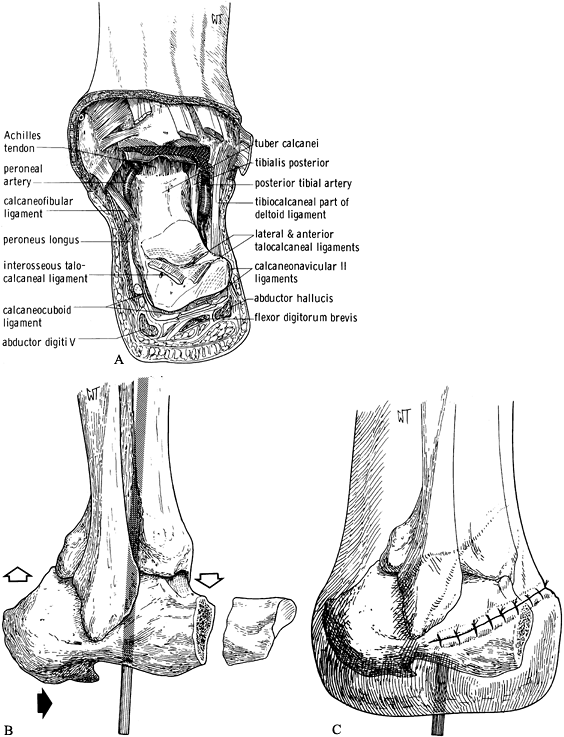 Figure 120.6. Boyd amputation. See text for details. A: Anterior–dorsal skin incision and exposure with excision of the talus. B:
Figure 120.6. Boyd amputation. See text for details. A: Anterior–dorsal skin incision and exposure with excision of the talus. B:
Excise the anterior process of the calcaneus and remove cartilage from
the articular facets. Fit the calcaneus into the ankle mortise,
shifting it anteriorly. Secure it with a Steinmann pin. C: Completed amputation with skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:56–57, Fig. 12-11, Fig. 12-13, Fig. 12-14, with permission.) -
Remove the anterior process of the
calcaneus. Fashion the ankle mortise in such a way that the
decorticated superior part of the tuber calcanei can be fitted into the
ankle mortise with good bone apposition. Slide the calcaneus somewhat
anteriorly. -
Fix the tuber calcanei into the ankle mortise with either a Steinmann pin or a partially threaded cancellous screw (Figure 120.6.B).
-
Pull the plantar skin flap over the
anterior aspect of the calcaneal remanent, and close it in layers at
the anterior aspect of the ankle joint (Fig. 120.6C). Postoperative casting may be necessary to provide secure arthrodesis between the ankle mortise and the calcaneal remanent.
in many circumstances allows ambulation without a prosthesis over short
distances. It is an excellent amputation for children, in whom it
preserves the physes at the distal end of the tibia and fibula (26).
assuming that the heel flap has been spared from the trauma. In the
past, it has had a high failure rate in ischemic limbs because of
failure of wound healing. Today, the success of amputation at this
level has increased because local tissue perfusion is preoperatively
determined with Doppler ultrasound measurement of blood pressures, with
radioactive 133Xe clearance tests, and with transcutaneous measurement of oxygenation.
two-stage amputation, where the wound is left open initially and the
viability of the heel flap is established prior to definitive wound
closure, has also increased success rates. The two most common problems
with the Syme’s amputation are skin slough due to trimming of the
dog-ears of the skin flaps, which interferes with the blood supply to
the heel pad, or late migration of the heel pad due to instability. The
risk of both of these problems can be minimized by careful attention to
surgical technique.
that a below-knee prosthesis must be worn, which is rather bulky around
the ankle because of the need to accommodate the flair of the distal
tibial metaphysis. The prothesis consists of a molded plastic socket
with a removable medial window through which the stump is inserted.
Usually a solid-ankle, cushion-heel (SACH) foot is attached. For this
reason, the Syme’s amputation has not often been recommended for women,
but active athletic women probably would prefer this level of
amputation because it is much more functional for sports activities
than a standard below-knee amputation.
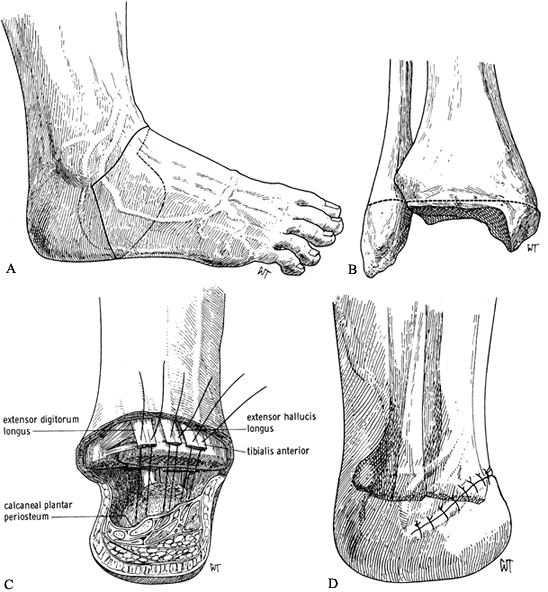
-
Make a dorsal skin incision from the
medial to the lateral malleolus over the anterior aspect of the ankle
joint. Continue the plantar incision around the anterior process of the
calcaneus (Figure 120.7.A). Transect the extensor tendons and cut back sharply the cutaneous nerves. Ligate the dorsalis pedis artery and vein.![]() Figure 120.7. Syme’s amputation. A: Skin incisions. B: Level of bone transection in adults. C: Begin closure of the wound by attaching the extensor tendons to the calcaneal periosteum to help stabilize the heel pad. D: Skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:58–62, Fig. 12-15, Fig. 12-21, Fig. 12-22 and Fig. 12-23, with permission.)
Figure 120.7. Syme’s amputation. A: Skin incisions. B: Level of bone transection in adults. C: Begin closure of the wound by attaching the extensor tendons to the calcaneal periosteum to help stabilize the heel pad. D: Skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:58–62, Fig. 12-15, Fig. 12-21, Fig. 12-22 and Fig. 12-23, with permission.) -
Disarticulate the talus from the ankle
mortise by transecting the medial and lateral collateral ligaments.
Take care not to injure the tibial neurovascular bundle. Strip the
calcaneus subperiosteally and remove it from the heel pad. This
dissection can be difficult and is aided by placing a bone hook into
the posterior aspect of the talus and pulling it and the calcaneus
anteriorly. Then use a sharp scalpel to meticulously dissect the
calcaneus free from the heel pad, at a subperiosteal level. -
When the calcaneus is free, transect the
plantar tendons and neurovascular bundles, taking care to protect the
vascular supply and sensory nerves to the heel pad. -
In a standard Syme’s amputation in adults, remove the malleoli at the level shown in Figure 120.7.B. In children and for first-stage amputations in diabetics, leave the malleoli intact.
-
Start closure of the wound by suturing
the anterior tibial tendon as well as the extensor digitorum longus
tendon into the anterior part of the heel pad. This prevents, at least
to some extent, posterior migration of the heel pad (Figure 120.7.C). Complete a layered closure as described previously. -
Since the removal of the calcaneus from
the heel pad creates a large empty space, insert a suction drain to
prevent the formation of a large hematoma. There is a mismatch between
the thin skin at the anterior aspect of the ankle joint and the thick
plantar skin. However, the ridge that is created by the skin closure
flattens out eventually. Large dog-ears are usually created by this
closure. Do not trim these. They eventually flatten out.
good soft-tissue coverage of the bony stump end is possible. The
minimal length of the bony stump for good function is about 12 cm, as
measured from the medial tibial plateau distally, and the maximum
length is 17 cm, depending on the height of the patient. Longer stumps
require less energy consumption during gait and can be fitted with
sockets without special suspension. Very short stumps often
require knee hinges and a thigh lacer and are much less functional.
the second provides equal skin flaps posteriorly nand anteriorly; the
third and most common approach provides a long posterior flap. The
latter is most desirable in dysvascular patients and leaves the patient
with a scar on the anterior aspect of the residual limb. However, other
methods of skin closure have been successful (Figure 120.8.) (32,42,68).
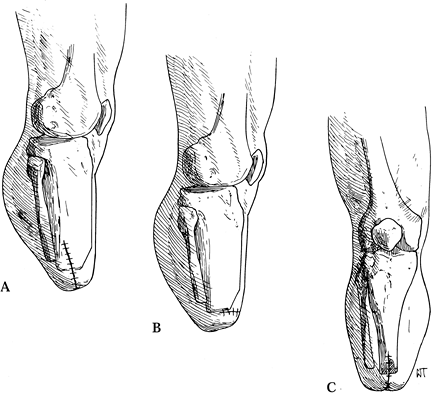 |
|
Figure 120.8. Suture line in various types of below-knee amputations. A: In the nonischemic limb. B: Amputation of the ischemic limb in the coronal plane. C: Amputation of the ischemic limb in the sagittal plane. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:69, Fig. 13-8, with permission.)
|
-
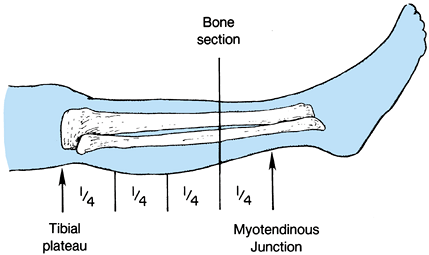
Determine the level of transection of the tibia by direct measurement, or using the method depicted in Figure 120.9.
![]() Figure 120.9.
Figure 120.9.
Use a sterile marking pen to divide the leg into four equal quadrants
from the tibial plateau to the myotendinous junction of the
gastrocsoleus muscle. In most cases, transect the bone at the distal
portion of the third quarter. -
In the long posterior flap method, make
the skin incision at the anterior aspect of the tibia, 1.3 cm distal to
the level of the bone transection. The posterior flap must be long
enough to provide soft-tissue coverage without undue tension (Figure 120.10.). Extend the incision down to the deep fascia and periosteum of the tibia veering medially and laterally to point B on Figure 120.10.,
which is two thirds of the anteroposterior (AP) diameter of the calf.
Then curve the incision directly distal for a distance of 1.3 cm before
sloping posteriorly to create the posterior flap. The length of the
posterior flap must be more than two thirds of the AP diameter of the
calf at the level of the bone transection.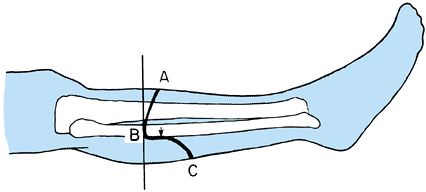 Figure 120.10. Skin incision for a below-knee amputation as described by Mooney.
Figure 120.10. Skin incision for a below-knee amputation as described by Mooney. -
Raise the periosteum of the tibia and
transect it with an oscillating saw. Bevel the anterior aspect of the
tibia. Transect the fibula with an oscillating saw 1–2 cm proximal to
the level of the tibial transection. -
Working from anterior to posterior, transect the muscles
P.3161
and neurovascular bundles. Pulling the distal tibia anteriorly places
the soft tissues under tension and facilitates dissection. Transect the
muscles of the anterior, lateral, and deep posterior compartments just
distal to the end of the tibia, so that when they retract they are even
with the end of the tibia. Identify each neurovascular bundle. Cut the
nerves back sharply and doubly ligate the arteries and veins
independently. -
Bevel the posterior flap to produce
adequate coverage for the end of the amputation stump. At the level of
the skin, it should consist only of fascia. -
Carry out a myoplastic closure, suturing
the fascia of the posterior flap to the anterior compartment and the
periosteum of the tibia. Lead a drain out through the skin laterally.
Particularly in the dysvascular patient, avoid any tension in the skin
closure (Figure 120.11.).![]() Figure 120.11.
Figure 120.11.
Below-knee amputation. Myoplastic closure with approximation of the
fascia of the posterior flap to the fascia of the anterior compartment
and periosteum of the tibia, and subsequent closure of the subcutaneous
tissues and skin over a drain placed at the level of the bone. (From
Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:68, Fig. 13-7, with permission.)
The main advantage is the creation of an endbearing stump and
preservation of the distal femoral physes, which is particularly
desirable in children. Another advantage is the maintenance of a long
active lever arm for control of the prosthesis, with excellent muscle
attachments. The bulbous distal stump enhances suspension of the
prosthesis. In elderly dysvascular patients, the longer stump helps
prevent hip flexion contractures and it provides better balance for
wheelchair activities. Knee disarticulation is most useful in young
athletic amputees in whom a below-knee amputation is not feasible.
Several modifications have been introduced by Mazet and Hennessy (60) and Burgess (6), and they are incorporated here.
-
Equal anteroposterior or mediolateral skin flaps using a fishmouth type of incision are acceptable.
-
Start the anterior skin incision
approximately 2 cm below the tibial tubercle. The posterior incision is
usually at the level of the distal popliteal flexion crease. -
Raise a skin and subcutaneous fat flap and transect the patellar ligament as far distally as possible.
-
Open the anterior capsule and transect
the collateral ligaments. Transect the cruciate ligaments at their
insertion on the tibial plateau. -
Open the posterior capsule and doubly
ligate the popliteal vessels distally to the level of the superior
geniculate artery. Sharply transect the nerves well proximally to the
skin incision. -
Transect the tendons of the knee flexors as far distally as possible.
-
Using an oscillating saw, remove the femoral condyles 1.5 cm proximal to the knee joint.
-
Shell the patella out of the patellar
tendon. Suture the patellar tendon and the flexor tendons of the knee
to the cruciate ligaments (Fig. 120.12).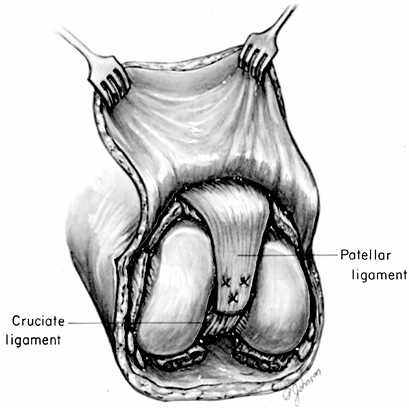 Figure 120.12.
Figure 120.12.
Knee disarticulation. Suturing of the patellar ligament to the cruciate
ligaments. Suturing of the hamstring tendons to the cruciate ligaments
and resection of the distal femoral condyles as described by Burgess (6) is not illustrated.
diaphyseal amputation through the femur becomes necessary. The
soft-tissue coverage of the above-knee stump is excellent and usually
provides good arterial perfusion to allow primary healing of the
amputation. It is the most commonly used amputation for vascular
disease because it heals reliably. The optional level for prosthetic
fitting is at the junction of the distal and middle thirds.
gluteal crease have been fitted successfully with prostheses, a longer
amputation stump makes prosthetic fitting, as well as rehabilitation,
easier and more successful. Equal anterior and posterior soft-tissue
flaps are used most commonly, but atypical soft-tissue coverage is well
tolerated in above-knee amputations.
-
Make a fishmouth skin incision to create equal anterior and posterior flaps (Figure 120.13.A).
![]() Figure 120.13. Above-knee transdiaphyseal amputation. A: Skin incisions relative to the level of transection of the femur. B: Myodesis closure of the stump over a drain. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:87–89, Fig. 14-4, 14-7, with permission.)
Figure 120.13. Above-knee transdiaphyseal amputation. A: Skin incisions relative to the level of transection of the femur. B: Myodesis closure of the stump over a drain. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:87–89, Fig. 14-4, 14-7, with permission.) -
After the skin incision, transect the
quadriceps musculature anteriorly; this will leave the muscle even with
the level of bone transection. -
Next, either cut the femur (as this
permits the transection of tissues from anterior to posterior) or
complete the soft-tissue work prior to cutting the bone. Cut the
adductors and hamstrings so that they also lie even with the cut bone. -
If you have not done so already, transect the bone and round off the sharp edges.
-
Obtain hemostasis and isolate and doubly
ligate the large vessels with at least one suture ligature. Place a
ligature on the sciatic nerve well above the level of amputation, prior
to sharply transecting the nerve. -
Fashion the muscle flaps and do a myoplasty by approximating the anterior to the posterior myofascial flap (Figure 120.13.B).
In younger active patients, place two drill holes in the bony stump end
for each compartment, and do a myodesis by pulling the muscles out to
length and securing them to the bone end with sutures. This improves
the power and control over the stump and keeps the bone centralized in
the stump. -
Place a drain and close the subcutaneous flap and skin loosely with interrupted sutures or staples.
malignancies. Prosthetic fitting is quite possible, although
rehabilitation is most successful in younger and more vigorous
individuals, since the prosthetic gait requires considerable
expenditure of energy. Very short above-knee amputations (Figure 120.1.) are fitted with a hip disarticulation prosthesis (Chapter 122), as is the short stump that cannot control an above-knee prosthesis even with a hip hinge and waist belt.
-
Place the patient in the lateral
decubitus position with the operated side uppermost, and prepare and
drape the limb free, including the hip and groin. -
Make a racket-type incision down to the
deep fascia, that starts at the anterior superior iliac spine, follows
the inguinal ligament to a point 5 cm below the pubic tubercle, and
curves around the medial and posterolateral aspects of the thigh
approximately 5 cm below the ischial tuberosity. The incision then
extends anteriorly to the greater trochanter and joins the beginning of
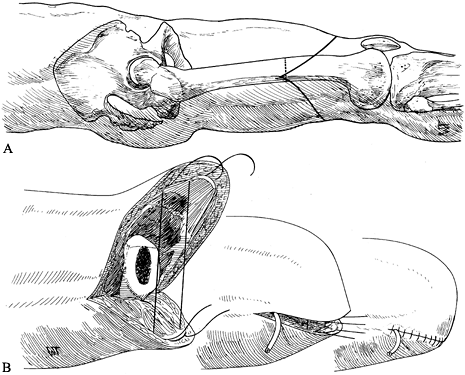
the incision at the anterior iliac spine (Figure 120.14.A).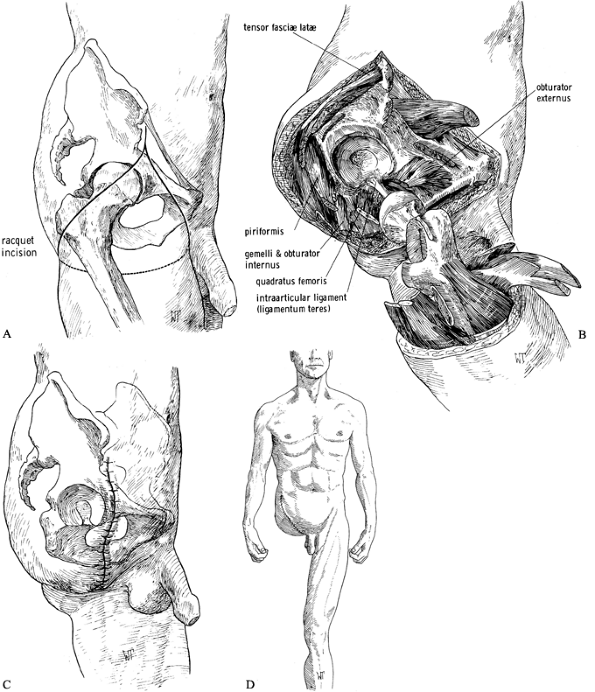 Figure 120.14. Disarticulation of the hip. See text for details. A: Racket-type skin incision. B: The hip disarticulation is completed except for cutting the ligamentum teres. C: Skin closure. D: Frontal view of a patient after hip disarticulation. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:106–111, Fig. 14-28, Fig. 14-31, Fig. 14-34, Fig. 14-35, with permission.)
Figure 120.14. Disarticulation of the hip. See text for details. A: Racket-type skin incision. B: The hip disarticulation is completed except for cutting the ligamentum teres. C: Skin closure. D: Frontal view of a patient after hip disarticulation. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:106–111, Fig. 14-28, Fig. 14-31, Fig. 14-34, Fig. 14-35, with permission.) -
Anteriorly expose the femoral artery and
vein. Isolate them and doubly ligate them. Dissect the femoral nerve
free, ligate it, and transect it sharply farther proximally. -
Transect the muscles originating from the
iliac spine and retract them distally. Transect the pectineus muscle a
finger’s breadth laterally to the pubic ramus. Externally rotate the
hip and transect the psoas tendon. Next, detach the muscles from the
pubic bone and the adductor magnus as it originates from the ischium. -
Between pectineus and the hip external
rotators, dissect the obturator artery free and ligate it. Transect the
obturator externus distal to the obturator foramen, because
P.3163
inadvertent
transection of the obturator artery leads to retraction of the vessel
into the inner pelvis, where it is difficult to recover for ligation. -
Then internally rotate the hip and transect the gluteus medius and minimus muscles at the greater trochanter.
-
Divide the fascia lata either above or below the insertion of the tensor fasciae latae muscle.
-
Next divide the lower fibers of the
gluteus maximus and detach its tendon from its insertion into the linea
aspera. Retract the gluteus maximus and identify and ligate the sciatic
nerve and divide it as far proximally as possible, but outside the
sciatic foramen. Then transect the external rotators of the hip and
divide the origins of the hamstring muscles at the ischial tuberosity. -
Complete the amputation by transecting the joint capsule and intra-articular ligament as close to the acetabulum as possible (Figure 120.14.B). Ensure good hemostasis.
-
Place large suction drains, one deep in the wound and one above the fascia.
-
Begin the closure of the wound by
suturing the tendons of the glutei medius and minimus to the transverse
acetabular ligament. Suture the gluteus maximus forward to the origin
of the pectineus, obturator externus, iliopsoas, and adductor muscles.
Close the skin without tension.
hindquarter amputation, is the eradication of malignant tumors of the
soft tissues or bone of the hip or pelvis. Occasionally, overwhelming
infections such as gas gangrene or the consequences of trauma may
require this operation. Prosthetic fitting is difficult and relies on
weight bearing on the uninvolved side. Energy expenditure for
prosthetic gait is considerable. Depending on the location of the
tumor, an anterior-flap or a posterior-flap technique can be used. In
an attempt to improve the prosthetic fitting, consider a conservative
hemipelvectomy, which retains the ileum and its attachment to the
sacroiliac joint. Another possibility is the excision of the
hemipelvis, including the hip joint, while maintaining the lower
extremity
without its attachment to the pelvic girdle. Expect considerable blood loss during the operation.
and sometimes anteriography is usually necessary to plan for safe
margins for tumor resection and to determine what type of
hemipelvectomy is appropriate.
-
Insert a ureteral catheter for identification of the ureter and a Foley catheter to drain the bladder.
-
Close the anus temporarily with a purse-string suture.
-
Position the patient in the lateral
decubitus position but make the supports loose, so he can be shifted
from a more supine to a more prone position. Do a wide preparation and
drape of the involved extremity, the pelvis, and the lower thorax.
procedure with an anterior part, perineal portion, and posterior
component.
-
For the anterior portion, start the skin
incision at the pubic tubercle and extend it along the inguinal
ligament to the anterior superior iliac spine. From there, follow the
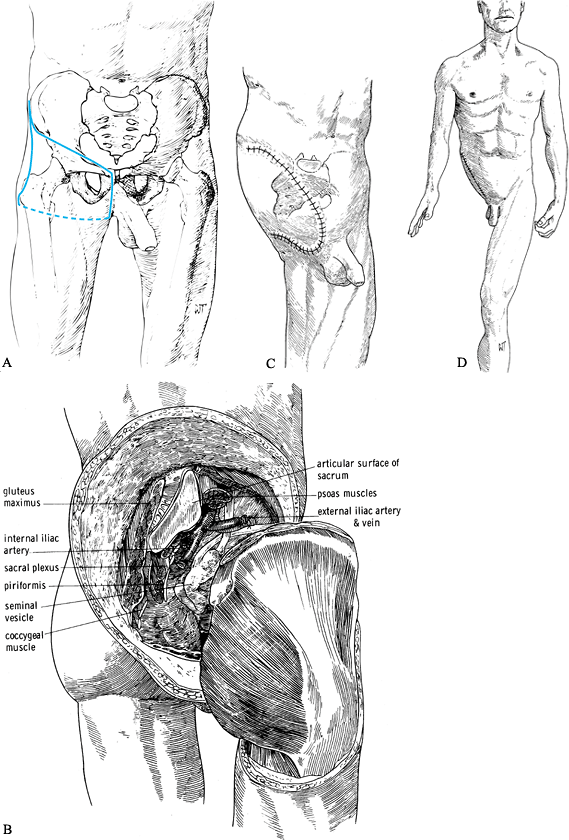
anterior part of the iliac crest eventually to the mid ileum (Figure 120.15.A).![]() Figure 120.15. Hemipelvectomy. See text for details. A:
Figure 120.15. Hemipelvectomy. See text for details. A:
Skin incision. Start the incision at the anterior superior iliac spine
(ASIS) and extend along the inguinal ligament to the pubic tubercle.
Then extend vertically into the perineum to the ischial tuberosity, to
the fold of the gluteus maximus. Follow the gluteal crease laterally to
the greater trochanter and with a gentle anterior curve return to the
ASIS. B: The hemipelvectomy has been completed and the hindquarter detached. C,D: Skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:118–119, Fig. 15-1, Fig. 15-7, Fig. 15-8, with permission.) -
Detach the inguinal ligament as well as
the abdominal muscles from the iliac crest, and develop the space
between the peritoneum and the iliacus muscle. -
Then detach the inguinal ligament and the
rectus abdominus tendon from the pubic bone. In men, retract the
spermatic cord medially. Now enter the space of Retzius and retract the
bladder into the pelvis. -
Doubly ligate and transect the external
iliac artery and veins and cut the femoral nerve back sharply. Now pack
the anterior wound to control hemorrhage and protect the soft tissues. -
For the perineal part, abduct the hip and
extend the incision from the pubic tubercle vertically through the
perineum to the ischial tuberosity. Expose the pubic and ischial rami,
and detach subperiosteally the adductor, hamstring, and perineal
muscles originating from them, as well as the corpus cavernosum. Then
divide the symphysis pubis. Take care not to injure the urethra. -
To complete the posterior part, roll the
patient somewhat forward, and while flexing and adducting the hip,
complete the skin incision by extending it from the ischial tuberosity
laterally along the gluteal crease to the greater trochanter, and from
there superiorly to join the anterior incision at the midiliac crest (Figure 120.15.A).
Expose the posteroinferior margins of the gluteus maximus, incise its
aponeurosis in line with the skin incision, detach its femoral
insertion, and reflect it and the overlying fat and skin as a composite
flap proximally. Identify the gluteus medius, the external rotators of
the hip, and the sciatic nerve. -
Identify, ligate, and sharply transect the sciatic nerve and allow it to retract into the pelvis.
-
Pass a Gigli saw through the greater
sciatic notch; divide the sacrotuberous and sacrospinous ligaments and
divide the ileum just lateral to the sacroiliac joint. This is easier
than disarticulating the sacroiliac joint. The joint can be
disarticulated or even a portion of the sacrum resected if required to
establish a surgical margin. -
Then externally rotate the innominate
bone, ligate and divide the obturator vessels and nerve, and transect
the psoas muscle at the level of the sacroiliac joint. Divide the hip
external rotator muscles close to the ischium. -
Transect the levator ani close to its origin on the ischium and the pubis. This will free the hindquarter (Figure 120.15.B).
-
Place a deep and superficial suction
drain and begin closure by bringing the posterior skin and muscle flap
forward and suturing it to the lateral border of the abdominal muscles
and the rectus abdominus muscle. Posteriorly,
P.3166P.3167
secure it to the psoas and quadratus lumborum muscles. Use #0 or #1 absorbable sutures. -
Then close the skin loosely (Figure 120.15.C, Figure 120.15.D).
-
Create the anterior flap by starting an
incision at the anterior superior iliac spine, and extend it along the
lateral aspect of the thigh to the level of the superior pole of the
patella. Then continue the incision transversely across the quadriceps
tendon and medially to the midline, and subsequently proximalward to
the groin (Figure 120.16.A).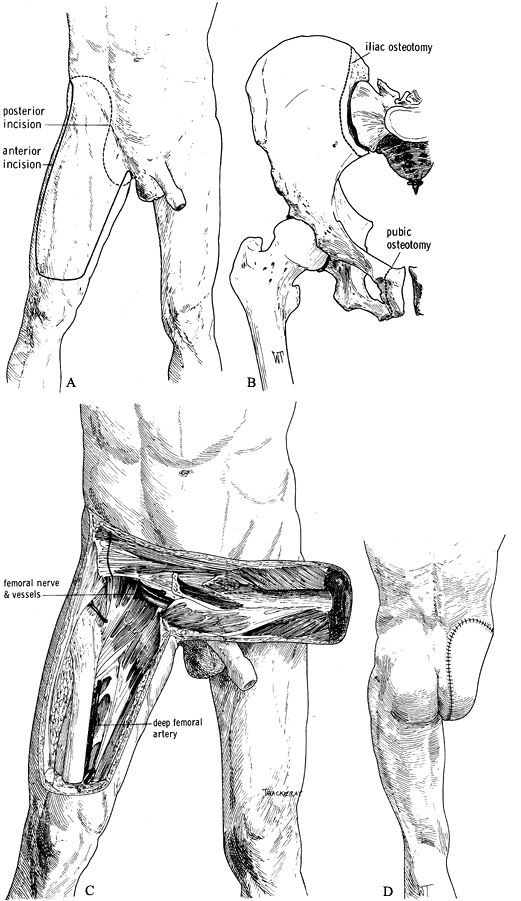 Figure 120.16. Larsen and Liang (51) modified hemipelvectomy using an anterior quadriceps flap. See text for details. A: Skin incision. B: Remove the innominate bone with osteotomies of the pubic rami and the ileum. C:
Figure 120.16. Larsen and Liang (51) modified hemipelvectomy using an anterior quadriceps flap. See text for details. A: Skin incision. B: Remove the innominate bone with osteotomies of the pubic rami and the ileum. C:
The anterior quadriceps musculocutaneous flap is supplied by the
superficial femoral artery and vein, which are carefully preserved. D: The skin closure. (From Bohne WHO. Atlas of Amputation Surgery. New York: Thieme Medical, 1987:120–124, Fig. 15-9, Fig. 15-10, Fig. 15-13, with permission.) -
Develop the musculocutaneous flap from
lateral to medial by incising the deep fascia and transecting the
quadriceps tendon horizontally. Elevate the quadriceps muscle from the
femur, developing the dissection along the vastus medialis. -
Find the adductor canal, transect the
sartorius muscle and ligate, and transect the femoral vessels as far
distally as possible so that their proximal ends remain with the
musculocutaneous flap. With further proximal dissection, take care not
to injure the femoral vessels. Only the profunda femoris artery is
ligated farther proximally (Figure 120.16.C). -
Then complete the hemipelvectomy as
described previously, but exclude the posterior flap, which is replaced
by the anterior flap. Removal of the innominate bone can be made easier
by osteotomy of the pubic rami rather than by disarticulation of the
pubic symphysis (Figure 120.16.B). -
Closure of the amputation is as described previously (Figure 120.16.D).
patients experience persistent residual extremity pain, swelling, a
sense of instability, and an inability to tolerate extended prosthetic
ambulation. The residual extremity undergoes atrophy as a result of
inactivity and becomes a passive participant in walking. This symptom
complex is referred to as the inactive residual extremity syndrome. The
Ertl osteomyoplastic lower extremity amputation reconstruction is
directed at treating difficult transtibial and transfemoral amputee
symptoms and reversing the inactive residual extremity syndrome (21,22,23 and 24).
Patients in our transtibial and transfemoral series were surgically
reconstructed after nonoperative and prosthetic modifications were
exhausted (22,23,24).
-
Utilize the previous amputation incision
for exposure. Isolate the neurovascular structures, and ligate the
arteries and veins separately. Distract the nerves and cut them well
above the bone level, allowing them to retract into a soft-tissue bed. -
Separate the anterior and posterior
muscle groups, exposing the distal tibia and fibula in preparation for
forming osteoperiosteal cortical island flaps. -
Elevate the osteoperiosteal cortical
island flaps with a 30° to 45° angled chisel from the medial and
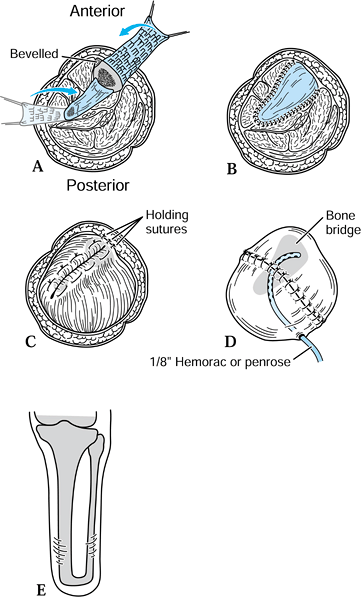
lateral borders of both the tibia and the fibula (Figure 120.17.A).![]() Figure 120.17. Ertl osteomyoplastic transtibial amputation reconstruction. A:
Figure 120.17. Ertl osteomyoplastic transtibial amputation reconstruction. A:
Transverse section of amputation level, with elevated osteoperiosteal
flaps from the medial and lateral aspects of the tibia and fibula. The
roof is created with the interior flaps. B:
The outer osteoperiosteal flaps are folded over the medullary canals
and sutured to one another as well as to the superior flap, forming a
closed tubelike structure (osteoperiosteal bridge) and closing the
medullary canal. C: The opposing muscle
groups are sutured together forming the myoplasty. Muscle rotation may
be necessary to completely cover the bridge and anterior tibia. D: The skin is contoured to the underlying myoplasty, avoiding dog-ear formation, creating a smooth surface and interface. E: Completed transtibial reconstruction with mature bridge and reestablishment of the medullary canal. -
Remove approximately 1.5–2 cm of the end
of the tibia, and suture the retained osteoperiosteal flaps together in
tubelike fashion, forming a bony bridge between the tibia and fibula (Figure 120.17.B). -
To complete the myoplasty, secure the
ends of the opposing muscle groups to each other over the end of the
bone and the underlying bridge, reestablishing some muscle tension (Figure 120.17.C, Figure 120.17.D and Figure 120.17.E). -
Contour the skin to the underlying myoplasty, forming a smooth, cylindrical residual extremity (Figure 120.17.D).
-
Elevate and suture together the
osteoperiosteal cortical island flaps, covering the open-ended
medullary canal. Remove minimal bone after flap elevation (Figure 120.18.A, Figure 120.18.B, Figure 120.18.C, Figure 120.18.D and Figure 120.18.E).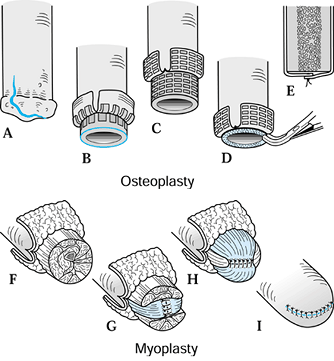 Figure 120.18. Ertl osteomyoplastic transfemoral amputation reconstruction. A: A periosteal incision is made. B: Osteoperiosteal flaps are created and elevated. C: The femur is transected below the osteoperiosteal flaps. D: The exposed distal bone surface is freshened with a rongeur. E: The osteoperiosteal flaps are sutured to each other, closing the medullary canal. F: The four major muscle groups are isolated. G: The adductors are sutured to the abductors, incorporating sutures into the periosteal sleeve or through bone holes. H: The extensors are sutured to the flexors, circumferentially covering the medial/lateral myoplasty. I: The skin is contoured to the underlying myoplasty, avoiding dog-ear formation.
Figure 120.18. Ertl osteomyoplastic transfemoral amputation reconstruction. A: A periosteal incision is made. B: Osteoperiosteal flaps are created and elevated. C: The femur is transected below the osteoperiosteal flaps. D: The exposed distal bone surface is freshened with a rongeur. E: The osteoperiosteal flaps are sutured to each other, closing the medullary canal. F: The four major muscle groups are isolated. G: The adductors are sutured to the abductors, incorporating sutures into the periosteal sleeve or through bone holes. H: The extensors are sutured to the flexors, circumferentially covering the medial/lateral myoplasty. I: The skin is contoured to the underlying myoplasty, avoiding dog-ear formation. -
Secure the opposing muscle groups to the
distal periosteum and to each other to reestablish muscle length and to
stabilize the femoral shaft within the muscle envelope (Figure 120.18.F, Figure 120.18.G and Figure 120.18.H).
a mean follow-up of 9 years and a mean time to reconstruction of 9.5
years. We also performed 74 transfemoral reconstructions, with a mean
follow-up of 9.8 years and a mean time to reconstruction of 13.3 years.
A 30-point clinical assessment score was used to rate postoperative
pain, function, stability, swelling, hours able to wear the prosthesis,
and radiographic evaluation. A score of over 25 points was graded as
excellent, 20–24 as good, 15–19 as fair, and less than 15 as poor. In
the transtibial group, there were 73.3% excellent, 18.7% good, 5.3%
fair, and 2.7% poor results, with an overall patient satisfaction of
97%. The transfemoral group had 70% excellent, 20%
good, 4% fair, and 6% poor, with an overall patient satisfaction of 94% (23,24).
has been shown to improve vascularity to the residual extremity and
return an elevated intramedullary pressure to normal (23,24,29,49,54,55).
In the transtibial population, medullary closure stabilizes the fibula
by forming a bony bridge, thus improving the rotational control of the
extremity within the prosthesis. The potential for tolerating end
bearing is also enhanced with medullary closure for both transtibial
and transfemoral patients. Myoplasty has been demonstrated to improve
the arterial supply and venous return of the residual extremity,
benefiting vascular amputees as well (21,22,28,30,31,62).
Additionally, the osteomyoplasty improves extremity control and
strength and can provide a greater surface area for prosthetic fitting.
Other authors have demonstrated a more powerful residual extremity that
is prosthetically more satisfactory (11,16,17 and 18,49,54,55).
directed at generating an active and functional residual extremity by
reestablishing as normal a physiologic environment as possible. It is
our feeling that the resultant residual extremity will provide the
patient with a more durable limb with improved stability and
proprioception. The residual limb is more powerful and prosthetically
provides better adherence to the socket. No unnecessary bone length is
removed to achieve the end result, but we do not hesitate to sacrifice
length when an increase in function can be achieved. We have
successfully applied this reconstruction to both primary and secondary
amputations.
amputation: soft dressings, soft dressings with pressure wrapping,
semirigid dressings (56), and rigid dressings.
Each has advantages and disadvantages. The rigid dressing leads to the
most successful early maturation of the stump. Indeed, in
well-vascularized amputation stumps, the rigid dressing can be combined
with an immediate postoperative fitting that will allow early weight
bearing (8). However, the rigid dressings do not allow easy access to the amputation wound.
(IPOP) can be considered for all levels of the lower extremity
amputation. Use the IPOP with caution in dysvascular amputees, as
excessive pressure may lead to wound necrosis. Potential advantages
include decreased postsurgical edema, a decreased incidence of
thromboembolic disease, less pain, protection of the stump wound, and
earlier mobilization of the patient and fitting of a pylon prosthesis,
which enhances rehabilitation and is psychologically good for the
patient.
the operating room. Cover the wound with a sterile dressing and a
sterile stump sock. Fill the area between the amputation wound and the
cast with either fluffed gauze or a premanufactured polyurethane foam
cup. Pad bony prominences with strips of felt, and over this apply an
elastic plaster-of-paris bandage to gently compress the area of the
amputation wound. In the below-knee amputation, apply the cast to above
the knee and keep the knee at approximately 10° to 20° of flexion.
Incorporate into the cast a suspension strap for a waist belt as well
as an attachment plate for the pylon that holds the prosthetic foot.
not only for the surgeon and prosthetist but also for the physical
therapist who has to supervise the early partial weight bearing (25).
Delay weight bearing in dysvascular amputees. Removable rigid dressings
have been devised. After 2 weeks at the most, remove the circular rigid
dressing for wound inspection and reapplication.
edema. This advantage is lost in soft dressing. If a soft dressing with
pressure wrapping is used, apply the elastic bandage with care to avoid
excessive compression and strangulation of the residual limb.
dressings as for the rigid dressing; however, the outer layer of this
dressing consists of Unna paste bandages, which have to be held in
place with a stockinet. This type of dressing has the same advantage of
preventing excessive postoperative edema as the rigid dressing but does
not allow any attachment of the provisional prosthesis.
occur at a higher incidence than after normal elective surgery when
amputations are performed in the absence of preexisting vascular
disease or infection in the extremity. The incidence of infection,
however, is much higher when amputation is performed in the presence of
active infection in the extremity, particularly when the level of
amputation is close to the site of infection. In dysvascular amputees,
occasionally too distal a level of amputation is selected in spite of
preoperative studies indicating that the level of amputation is
appropriate.
stump complications in amputations performed for open fractures of the
tibia secondary to high-velocity crush injuries,
when
the open fracture wound has gone on to progressive necrosis and
infection. These tend to occur in young active patients, so there is a
tendency to try to preserve a below-knee level, which may lead to
amputation through marginal tissues. The risks of stump wound breakdown
and infection in these cases can be minimized by careful preoperative
workup to ensure that the amputation is occurring through viable
tissue, and by performing staged amputations. The initial stage of the
amputation can be a simple guillotine-type amputation, followed by
definitive amputation with or without primary closure, or the initial
amputation can be with the usual flaps but be left open and then closed
secondarily when it is evident that the tissues are viable and
infection is not present.
infection, but necrosis of wound edges as well as of underlying muscle
can occur in amputation for peripheral vascular disease because of
inadequate vascularity at the level of amputation. Current techniques
for evaluating extremity perfusion (as discussed previously) have
lessened this risk, but in spite of the best of care breakdown still
occasionally occurs. Closure of the wound under tension predisposes to
breakdown. This can be avoided by always closing stumps loosely, since
this allows accommodation for postoperative swelling. If revision of
the level of amputation to a more proximal level may result in loss of
the knee joint or preclude the use of a prosthesis, then reconstructive
options such as free microvascular transfer of tissues and Ilizarov
bone transport methods may be indicated to preserve function in young
active individuals.
are only occasionally painful. Isolating all superficial and deep
nerves, gently applying traction to them, and cutting them with a
sharp, fresh blade to allow them to retract into soft tissue where the
prosthesis will not bear on the nerve end will avoid most problems with
neuromas. Treat neuromas initially nonoperatively, with adjustments of
the prosthesis and desensitizing techniques in physical therapy. If a
neuroma is refractory to nonoperative treatment, explore the nerve, and
transect it higher deep in soft tissues where it will not be a problem.
as well as a physical trauma. One of my patients told me on the day
after the operation, “Part of me has died. When is the rest to follow?”
To overcome this feeling of depression and disability, the amputation
should be undertaken with a view toward the postoperative life of the
patient: The patient must be the most important member of the team that
is undertaking the rehabilitation after the amputation.
end-bearing limb remnant in the very young and the very old is
important. In the growing individual, the reason for the end-bearing
stump is to prevent bony overgrowth, which can require numerous
revisions of the amputation. Also, in our experience, the
patellar-tendon-bearing prosthesis in the growing individual often
leads to the development of a patellar alta, which in turn, is
frequently the cause for recurrent dislocation of the patella. In the
very old, the end-bearing prosthesis makes rehabilitation considerably
easier, and ultimately the prosthesis is more comfortable than after
diaphyseal amputations.
The new prosthetic components have made the use of the below-knee
prosthesis easier and more satisfactory than in times gone by. Indeed,
many of our younger patients have returned to high-achievement sports.
In older below-knee amputees, energy consumption is much more favorable
than in above-knee amputees of similar age, allowing them to
participate in physical activities with greater ease.
much of the limb as possible as long as the disease process allows, is
highly desirable. In lower extremity amputations, this is particularly
obvious when comparing internal hemipelvectomy with hindquarter
amputation.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
SB, Henriksen BM, Holstein PE. Minor Amputaions on the Feet after
Revascularization for Gangrene. A Consecutive Series of 95 Limbs. Acta Orthop Scand 1997;68:291.
J. Ertl Osteomyoplastic Lower Extremity Amputation Reconstruction:
Technique and Long Term Results. Presented at the annual meeting of the
American Association of Orthopaedic Surgeons, San Francisco, CA, Feb.
17, 1997.
C. Muscle Blood Flow after Amputation with Special Reference to the
Influence of Osseous Plugging of the Medullary Cavity. Acta Orthop Scand 1976;47:613.
JM, Abdu WA, Mayor MB. Reconstructive Amputation after Grade IIIC Open
Tibial Fracture. One Method of Preserving residual Limb Length. J Orthop Trauma 1994;8:354.
D, Howery T, Sanders R, Johansesn K. Limb Salvage versus Amputation:
Preliminary Results of the Mangled Extremity Severity Score. Clin Orthop 1990;256:80.
R, Betz AM, Cometet JJ, Berger AC. Decision Making and Results in
Subtotal and Total Lower Leg Amputations: Reconstruction versus
Amputation. Microsurgery 1995;16:830.
A, Allen A, Luff R, McColl I. Rehabilitation after Lower Limb
Amputation: A Comparative Study of Above-Knee, Through-Knee, and
Gritti-Stokes Amputations. Br J Surg 1989;76:622.
H, Poole G, Hansen F, et al. Salvage of Lower Extremities Following
Combined Orthopedic and Vascular Trauma: A Predictive Salvage Index. Am J Surg 1987;53:205.
J, Wood D, Hornby R, et al. The Measurement of Skin Blood Flow in
Peripheral Vascular Disease by Epicutaneous Application of 133 Xenon. J Bone Joint Surg Am 1976;58:833.
F, Christiansen J, Ebskov B. Prevention and Treatment of Ulceration of
the Foot in Unilaterally Amputated Diabetic Patients. Acta Orthop Scand 1982;53:481.
J, Agardh CD, Apelqvist J, Stenstrom A. Clinical Characteristics in
Relation to Final Amputation Level in Diabetic Patients with Foot
Ulcers: A Prospective Study of Healing Below or Above the Ankle in 187
Patients. Foot Ankle Int 1995;16:69.
HE. Biological and Biomechanical Principles in Amputation Surgery.
Prosthetics International. Proceedings of the Second International
Prosthetics Course, Copenhagen, 1962.
M, Stahlgrew L. Evaluation of Factors which Influence Mortality and
Morbidity following Major Lower Extremity Amputation for
Atherosclerosis. Surg Gynecol Obstet 1965;120:1217.
K, Bernon G, Katz R, et al. Neoadjuvant Chemotherapy for Osteogenic
Sarcoma: Results of a Cooperative German/Austrian Study. J Clin Oncol 1984;2:617.