AMPUTATIONS OF THE UPPER EXTREMITY
Editors: Chapman, Michael W.
Title: Chapman’s Orthopaedic Surgery, 3rd Edition
Copyright ©2001 Lippincott Williams & Wilkins
> Table of Contents > SECTION
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Amputations > CHAPTER 121 – AMPUTATIONS OF THE UPPER EXTREMITY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Amputations > CHAPTER 121 – AMPUTATIONS OF THE UPPER EXTREMITY
CHAPTER 121
AMPUTATIONS OF THE UPPER EXTREMITY
Edward A. Athanasian
E. A. Athanasian:
Assistant Attending Orthopaedic Surgeon, Hospital for Special Surgery,
Memorial Sloan-Kettering Cancer Center, New York, New York 10021.
Assistant Attending Orthopaedic Surgeon, Hospital for Special Surgery,
Memorial Sloan-Kettering Cancer Center, New York, New York 10021.
INTRODUCTION
Upper-extremity amputations, excluding finger
amputation, account for 15% to 20% of major extremity amputations. More
than 90% of all upper-extremity amputations are a result of trauma, and
most of these occur in men between the ages of 20 and 40 years (1).
Limb-sparing surgery for primary bone and soft-tissue sarcoma of the
upper extremity is possible in the majority of patients, although
amputation may still be necessary for local control or palliation in 5%
to 10% of patients with malignant tumors (18).
Less common causes of upper-extremity amputation are peripheral
vascular disease, congenital malformations, neurologic disorders, and
severe infections.
amputation, account for 15% to 20% of major extremity amputations. More
than 90% of all upper-extremity amputations are a result of trauma, and
most of these occur in men between the ages of 20 and 40 years (1).
Limb-sparing surgery for primary bone and soft-tissue sarcoma of the
upper extremity is possible in the majority of patients, although
amputation may still be necessary for local control or palliation in 5%
to 10% of patients with malignant tumors (18).
Less common causes of upper-extremity amputation are peripheral
vascular disease, congenital malformations, neurologic disorders, and
severe infections.
The loss of an upper extremity has more devastating
consequences than the loss of a lower extremity. Upper-extremity
amputations are most common in young male trauma victims, in whom the
loss profoundly affects function and self-image. Despite improvements
in materials and design, long-term use of prosthetics by
upper-extremity amputees is only about 50%. Prosthetic use is reduced
in patients with higher levels of amputation, those with brachial
plexus injury, and when initiation of prosthetic limb rehabilitation is
delayed (4,12,13,23).
consequences than the loss of a lower extremity. Upper-extremity
amputations are most common in young male trauma victims, in whom the
loss profoundly affects function and self-image. Despite improvements
in materials and design, long-term use of prosthetics by
upper-extremity amputees is only about 50%. Prosthetic use is reduced
in patients with higher levels of amputation, those with brachial
plexus injury, and when initiation of prosthetic limb rehabilitation is
delayed (4,12,13,23).
INDICATIONS
Trauma is the most common indication for upper-extremity
amputation. Acutely severed, crushed, or mangled limbs may require
immediate amputation (19). Irreversible
brachial plexus injuries resulting in a flail, useless limb may come to
amputation following the acute injury period. Malignant bone or
soft-tissue tumors that contaminate or encase major nerves or vessels
may not be amenable to limb salvage. Tumors with extensive involvement
of the carpal tunnel or antecubital fossa may require amputation to
eradicate the tumor locally. Peripheral vascular disease that cannot be
corrected or reconstructed may require amputation, particularly in the
case of diabetes (10).
amputation. Acutely severed, crushed, or mangled limbs may require
immediate amputation (19). Irreversible
brachial plexus injuries resulting in a flail, useless limb may come to
amputation following the acute injury period. Malignant bone or
soft-tissue tumors that contaminate or encase major nerves or vessels
may not be amenable to limb salvage. Tumors with extensive involvement
of the carpal tunnel or antecubital fossa may require amputation to
eradicate the tumor locally. Peripheral vascular disease that cannot be
corrected or reconstructed may require amputation, particularly in the
case of diabetes (10).
P.3176
TRAUMA
Before the development of optical magnification and
microsurgical technique, proximal arm replantation was accomplished
with limited success (14). Application of
microsurgical vascular and nerve repair has allowed successful
replantation of more distally severed arms and fingers. Preservation of
the arm or hand even with limited sensation or function is often
superior to an insensate prosthetic limb. Specific indications for
digit replantation have been developed as limitations in function of
the replanted part have become better appreciated (6,8,24). Absolute and relative contraindications for limb replantation are listed in Table 121.1.
Limb replantation at the transhumeral level is valuable in recovering
function of the elbow in most patients; however, more distal recovery
is less predictable. Replantation at this level may permit conversion
of an above-elbow amputation to a below-elbow amputation, thereby
improving rehabilitation potential and success of prosthetic use (22).
Short residual limbs following traumatic amputation in selected
individuals can be lengthened using bone distraction techniques (20).
microsurgical technique, proximal arm replantation was accomplished
with limited success (14). Application of
microsurgical vascular and nerve repair has allowed successful
replantation of more distally severed arms and fingers. Preservation of
the arm or hand even with limited sensation or function is often
superior to an insensate prosthetic limb. Specific indications for
digit replantation have been developed as limitations in function of
the replanted part have become better appreciated (6,8,24). Absolute and relative contraindications for limb replantation are listed in Table 121.1.
Limb replantation at the transhumeral level is valuable in recovering
function of the elbow in most patients; however, more distal recovery
is less predictable. Replantation at this level may permit conversion
of an above-elbow amputation to a below-elbow amputation, thereby
improving rehabilitation potential and success of prosthetic use (22).
Short residual limbs following traumatic amputation in selected
individuals can be lengthened using bone distraction techniques (20).
 |
|
Table 121.1. Contraindications to Replantation
|
BRACHIAL PLEXUS INJURY
Brachial plexus injury most commonly occurs in young men
following motor vehicle accidents or industrial or farming injuries.
The prognosis for recovery depends the location of the injury and its
extent. Nerve root avulsions are not reparable but may be reconstructed
with distal neurotization and muscle transfer, provided sufficient
donors are available. Extensive, multilevel nerve root avulsions may
result in a flail limb with little potential for recovery. Computed
tomography contrast myelography, axon response to intradermal
histamine, electromyography, somatosensory evoked potentials, and
magnetic resonance imaging may be useful means to identify nerve injury
location (11,15). Early
repair and intraoperative nerve conduction and stimulation have been
advocated to improve results of brachial plexus repair (9,15).
following motor vehicle accidents or industrial or farming injuries.
The prognosis for recovery depends the location of the injury and its
extent. Nerve root avulsions are not reparable but may be reconstructed
with distal neurotization and muscle transfer, provided sufficient
donors are available. Extensive, multilevel nerve root avulsions may
result in a flail limb with little potential for recovery. Computed
tomography contrast myelography, axon response to intradermal
histamine, electromyography, somatosensory evoked potentials, and
magnetic resonance imaging may be useful means to identify nerve injury
location (11,15). Early
repair and intraoperative nerve conduction and stimulation have been
advocated to improve results of brachial plexus repair (9,15).
Signs of irreversible complete brachial plexus injuries include (17)
-
Absence of any clinical return of function after 1 year
-
Three or more pseudomeningoceles on myelography
-
Absence of voluntary action potentials in the area of C-5 to T-1 on repeated electromyographic examinations
-
Positive histamine tests in the area of C-5 to T-1
If an irreversible injury occurs, consider early
above-elbow amputation and prosthetic rehabilitation. Within 3 to 6
months after a complete brachial plexus injury the patient becomes a
“one-handed” person, transferring most activities from the injured limb
to the normal limb. More effective rehabilitation and better prosthetic
compliance have been achieved with early above-elbow amputation and
prosthetic training, avoiding the development of single-hand function
patterns (13,16,17).
Upper-extremity amputation for intractable pain following brachial
plexus injury is not successful in the majority of patients (3) (see Chapter 60).
above-elbow amputation and prosthetic rehabilitation. Within 3 to 6
months after a complete brachial plexus injury the patient becomes a
“one-handed” person, transferring most activities from the injured limb
to the normal limb. More effective rehabilitation and better prosthetic
compliance have been achieved with early above-elbow amputation and
prosthetic training, avoiding the development of single-hand function
patterns (13,16,17).
Upper-extremity amputation for intractable pain following brachial
plexus injury is not successful in the majority of patients (3) (see Chapter 60).
MALIGNANT MUSCULOSKELETAL TUMORS
The majority of bone and soft-tissue sarcomas in the
upper extremity, excluding the hand, can be resected with wide margins
that spare the limb without adversely affecting survival (18).
Bone and soft-tissue reconstruction using tendon transfers, nerve and
vein grafts, and free microvascular tissue transfer allow both
functional and cosmetic reconstruction in the majority of cases. Tumors
that cannot be resected without the sacrifice of multiple major nerves,
or without excessive risk of local recurrence, usually require
amputation. The level of amputation must provide margins that preclude
local recurrence regardless of the functional impairment that results
from a more proximal level of amputation. See Chapter 126 and Chapter 128.
upper extremity, excluding the hand, can be resected with wide margins
that spare the limb without adversely affecting survival (18).
Bone and soft-tissue reconstruction using tendon transfers, nerve and
vein grafts, and free microvascular tissue transfer allow both
functional and cosmetic reconstruction in the majority of cases. Tumors
that cannot be resected without the sacrifice of multiple major nerves,
or without excessive risk of local recurrence, usually require
amputation. The level of amputation must provide margins that preclude
local recurrence regardless of the functional impairment that results
from a more proximal level of amputation. See Chapter 126 and Chapter 128.
UPPER-EXTREMITY AMPUTATION
-
Perform upper-extremity amputations at
the most distal level compatible with uncomplicated wound healing.
Handle soft tissues atraumatically. -
A tourniquet may be used; however, exsanguination is contraindicated in limbs being amputated for infection or tumor.
BELOW-ELBOW AMPUTATION
If the vascular status of the limb is satisfactory,
amputation at the most distal level provides the optimal stump for
prosthetic use. If the vascular status of the limb is compromised,
healing at the distal third of the forearm may be impaired owing to the
lack of well-vascularized muscle deep to the subcutaneous tissue. A
tourniquet may be used.
amputation at the most distal level provides the optimal stump for
prosthetic use. If the vascular status of the limb is compromised,
healing at the distal third of the forearm may be impaired owing to the
lack of well-vascularized muscle deep to the subcutaneous tissue. A
tourniquet may be used.
-
Fashion equal anterior and posterior skin flaps (Fig. 121.1), and ligate the radial and ulnar arteries.
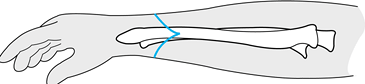 Figure 121.1. A below-elbow amputation, demonstrating equal dorsal and volar flaps.
Figure 121.1. A below-elbow amputation, demonstrating equal dorsal and volar flaps. -
Identify the major nerves (i.e., radial,
ulnar, median), sharply divide them as far proximally as possible, and
allow them to retract into the soft tissues. -
Section the radius and ulna proximal to
the most proximal portion of the skin incision, and smooth the rough
edges with a rasp or rongeur. -
Perform a myoplastic closure. If the
amputation level is proximal to the myotendinous junction of the flexor
and extensor tendons of the forearm, suture the palmar compartment
muscles over the end of the bone to the extensor compartment. -
The most proximal level compatible with
below-elbow prosthetic fitting is the level of the biceps tendon
insertion on the radius. If circumstances require amputation at this
level, releasing the distal 2.5 cm of the biceps tendon provides a
longer stump for prosthetic fitting. -
If the level of amputation is in the
distal one third of the forearm, bring the tendinous portion of the
flexor digitorum superficialis over the end of the bone and suture it
to the extensor compartment fascia. -
Obtain hemostatis. If necessary, use a drain. Close the wound without tension, and apply a bulky compressive dressing.
ELBOW DISARTICULATION
Amputation through the elbow has the same advantages as
a through-knee amputation in the lower extremity. The bulbous distal
humerus allows suspension of a prosthesis. The long lever arm allows
humeral rotation of the prosthesis, alleviating the need for the
mechanical turntable required in more proximal brachial amputations.
However, the soft tissue is thin at the elbow, and fitting of the
prosthesis must be exact.
a through-knee amputation in the lower extremity. The bulbous distal
humerus allows suspension of a prosthesis. The long lever arm allows
humeral rotation of the prosthesis, alleviating the need for the
mechanical turntable required in more proximal brachial amputations.
However, the soft tissue is thin at the elbow, and fitting of the
prosthesis must be exact.
-
A sterile tourniquet may be used. Fashion
equal anterior and posterior flaps, with the posterior flap extending
to a point 2.5 cm distal to the olecranon and the anterior flap
extending to the biceps insertion on the radius. -
Ligate the brachial artery. Sharply
divide the radial, ulnar, and median nerves proximally. Allow them to
retract into soft tissue. -
Disarticulate the elbow by dividing the
insertion of the biceps (i.e., radius) and the insertion of the
brachialis (i.e., ulna) anteriorly and by dividing the triceps tendon
at its olecranon insertion. Release the medial flexor and pronator mass
from the medial epicondyle, and divide the extensors from the lateral
humeral epicondyle. -
Perform an anterior capsulotomy, and remove the forearm, leaving the articular surface of the distal humerus intact.
-
Bring the triceps tendon anteriorly and
suture it to the tendons of the biceps and brachialis muscles over the
trochlea of the humerus. Place a drain and close the wound without
tension. Apply a bulky compressive dressing.
ABOVE-ELBOW AMPUTATION
To allow fitting of an above-elbow prosthesis, which
includes an elbow lock mechanism for flexion and extension and an elbow
turntable for rotation, perform above-elbow amputations 3.8 cm proximal
to the joint. Amputation at the level of the surgical neck of the
humerus functions as a shoulder disarticulation; however, this level
has the cosmetic advantage of preserving normal shoulder contour.
includes an elbow lock mechanism for flexion and extension and an elbow
turntable for rotation, perform above-elbow amputations 3.8 cm proximal
to the joint. Amputation at the level of the surgical neck of the
humerus functions as a shoulder disarticulation; however, this level
has the cosmetic advantage of preserving normal shoulder contour.
Midarm Amputation
-
For amputations through the midbrachium (Fig. 121.2), fashion equal anterior and posterior flaps.
![]() Figure 121.2. An above-elbow amputation using equal anterior and posterior flaps.
Figure 121.2. An above-elbow amputation using equal anterior and posterior flaps. -
Identify and ligate the brachial artery.
-
Divide the anterior compartment muscles
approximately 1.3 cm distal to the intended level of bone transection.
Divide the triceps 5 cm distal to the intended level of bone
transection. -
Section the humerus and smooth its rough edges with a rasp.
-
Perform a myoplastic closure, suturing
the anterior compartment muscles to the triceps. Close the wound
without tension over a drain. Apply a bulky compressive dressing.
P.3178
Proximal Third Amputation
-
For amputations through the surgical neck
of the humer us, make an incision anteriorly from the coracoid process
along the anterior border of the deltoid to the lateral insertion of
the deltoid on the humerus. Extend the incision posteriorly along the
posterior margin of the deltoid to the posterior axillary fold, and
connect the two incisions by an axillary incision. -
Ligate the cephalic vein. Release the
deltoid muscle from its humeral insertion, and reflect it proximally.
Release the pectoralis major muscle from its humeral insertion, and
reflect it medially (Fig. 121.3).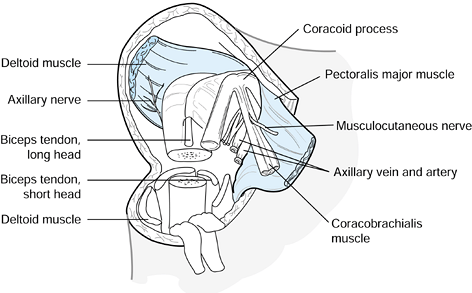 Figure 121.3. A proximal humeral amputation using the anterior approach.
Figure 121.3. A proximal humeral amputation using the anterior approach. -
Identify the neurovascular bundle, and
ligate the axillary artery. Sharply divide the musculocutaneous,
median, ulnar, and radial nerves, and allow them to retract proximally. -
Divide the teres minor and latissimus
dorsi muscles close to their humeral insertion. At a point
approximately 2 cm distal to the intended bone section, divide the
coracobrachialis and biceps muscles, and reflect them distally. -
Amputate the humerus at the surgical
neck. Suture the biceps and coracobrachialis muscles to the triceps
muscle over the stump of bone. Trim the deltoid laterally, and suture
it medially. Place appropriate drains, and close the skin without
tension.
AMPUTATIONS ABOUT THE SHOULDER
Amputations about the shoulder, which include shoulder
disarticulation and the forequarter or scapulothoracic amputation, are
performed almost exclusively for tumors (2). A
functional prosthesis is not available for these amputations. The
Tikhor–Lindberg resection is performed for malignant tumors of the
shoulder girdle if there is no involvement or limited tumor involvement
of the neurovascular bundles. This resection resects the proximal
humerus, scapula, and clavicle but preserves the brachium and distal
arm (7). The anterior approach for forequarter
amputation has the advantage of allowing early control of the major
vessels under optimal visualization, whereas the posterior approach may
be advocated due to the progressively improved exposure obtained while
the weight of the arm acts to retract the major soft-tissue mass.
disarticulation and the forequarter or scapulothoracic amputation, are
performed almost exclusively for tumors (2). A
functional prosthesis is not available for these amputations. The
Tikhor–Lindberg resection is performed for malignant tumors of the
shoulder girdle if there is no involvement or limited tumor involvement
of the neurovascular bundles. This resection resects the proximal
humerus, scapula, and clavicle but preserves the brachium and distal
arm (7). The anterior approach for forequarter
amputation has the advantage of allowing early control of the major
vessels under optimal visualization, whereas the posterior approach may
be advocated due to the progressively improved exposure obtained while
the weight of the arm acts to retract the major soft-tissue mass.
SHOULDER DISARTICULATION
-
Begin an anterior incision at the
coracoid process, and proceed distally along the anterior margin of the
deltoid to its humeral insertion (5). Continue
posteriorly along the posterior margin of the deltoid, and connect the
anterior incision with a posterior incision across the axilla. -
Identify the neurovascular bundle in the
interval between the coracobrachialis and the short head of the biceps,
and ligate and divide the axillary artery and vein. Sharply divide the
median, ulnar, and musculocutaneous nerves, and allow them to retract
into soft tissue. -
Detach the deltoid from its humeral
insertion, and retract it along with its overlying skin proximally.
Release the coracobrachialis and short head of the biceps from their
origin from the coracoid, and release the humeral insertion of the
pectoralis major. -
Externally rotate the arm, and divide the
anterior joint capsule and subscapularis muscle. Internally rotate the
arm, and divide the short external rotators and the teres major. -
Divide the triceps and inferior capsule, and remove the arm.
-
Suture the muscle ends into the glenoid
to fill dead space. Bring the deltoid with its overlying skin
inferiorly, and suture it inferior to the glenoid to the margin of the
posterior axilla incision, completing the procedure.
P.3179
SCAPULOTHORACIC DISARTICULATION
-
Begin the incision lateral to the
clavicular insertion of the sternocleidomastoid muscle, and extend the
incision distally along the clavicle to the acromioclavicular joint
over the acromion to the spine of the scapula and posteriorly along the
vertebral border of the scapula (5,7). -
Begin the lower incision at the middle
third of the clavicle. Proceed distally to the deltopectoral groove and
cross the axilla horizontally, and join the first incision posteriorly
at the spine of the scapula. -
Release the pectoralis major from the
clavicle, and divide the clavicle lateral to the insertion of the
sternocleidomastoid. Excise the clavicle to the level of the
acromioclavicular joint. -
If necessary, ligate the external jugular
vein and release the pectoralis major and minor from their insertions,
exposing the neurovascular bundle. -
Ligate and divide the subclavian artery and vein. Section the components of the brachial plexus, and allow them to retract.
-
Release the latissimus dorsi and axillary
fascia from the humerus, allowing the limb to fall posteriorly. Hold
the arm across the chest, and from superiorly to inferiorly divide the
remaining muscles that fix the shoulder to the scapula. Divide the
muscles that hold the scapula to the thorax, starting with the
trapezius and continuing through the omohyoid, levator scapulae,
rhomboid major and minor, and serratus anterior. -
Remove the arm and scapula.
-
Suture the remaining muscle over the lateral chest wall, and close the skin flaps over suction drainage.
RESECTION REPLANTATION
-
Resection replantation has been described by Windhager et al. (21) and is analogous to a Van Ness rotation plasty of the lower limb.
-
Resect the tumor-bearing portion of the
arm as a cylindric segment, including prior biopsy sites and all
contaminated structures. -
Dissect uninvolved vessels or major
nerves from the tumor-bearing segment through longitudinal incisions,
if this can be done with wide margins (21). -
Accomplish reconstruction by limb
shortening, osteosynthesis, and vascular, nerve, and soft-tissue
repair. This is an effective procedure for malignant tumors that offers
an alternative to amputation in carefully selected patients.
POSTOPERATIVE CARE AND REHABILITATION
After surgery, a patient with an upper-extremity
amputation may be treated with a rigid dressing and early prosthetic
fitting, as described by Burkhalter (4). Approximately 50% of upper-extremity adult amputees are rehabilitated with functional prosthetics (23).
Before initiating prosthetic fitting and training, consider the age,
extremity dominance, occupation, and psychosocial status of the patient
(12,13). Bilateral
upper-extremity adult amputees should be fitted with at least one
functional prosthesis, usually one that is externally powered.
amputation may be treated with a rigid dressing and early prosthetic
fitting, as described by Burkhalter (4). Approximately 50% of upper-extremity adult amputees are rehabilitated with functional prosthetics (23).
Before initiating prosthetic fitting and training, consider the age,
extremity dominance, occupation, and psychosocial status of the patient
(12,13). Bilateral
upper-extremity adult amputees should be fitted with at least one
functional prosthesis, usually one that is externally powered.
There are two major categories of upper-extremity
prostheses. The conventional body-powered prosthesis relies on proximal
shoulder girdle muscles to provide the force to power a terminal grasp
device through a system of harness and cables. Depending on the level
of amputation, the elbow and wrist “joints” must be positioned manually
by the contralateral normal limb. Myoelectric prostheses rely on
electrical potentials in active muscles in the stump to activate
electrodes in the prostheses, which switch electrical motors on and off
within the device. These prostheses are expensive, heavy, and require
frequent maintenance.
prostheses. The conventional body-powered prosthesis relies on proximal
shoulder girdle muscles to provide the force to power a terminal grasp
device through a system of harness and cables. Depending on the level
of amputation, the elbow and wrist “joints” must be positioned manually
by the contralateral normal limb. Myoelectric prostheses rely on
electrical potentials in active muscles in the stump to activate
electrodes in the prostheses, which switch electrical motors on and off
within the device. These prostheses are expensive, heavy, and require
frequent maintenance.
Regardless of the type of prosthesis used, the timing of
initial prosthetic fitting determines the ultimate success of
rehabilitation. Malone demonstrated in a series of upper-extremity
amputees that prosthetic fitting within 1 month of amputation resulted
in a 93% success rate of prosthetic rehabilitation (26 of 28 patients) (12,13).
Among upper-extremity amputees fitted later than 1 month after
amputation, only 42% (9 of 19) achieved prosthetic rehabilitation.
Although advocates of rigid postoperative dressings
initial prosthetic fitting determines the ultimate success of
rehabilitation. Malone demonstrated in a series of upper-extremity
amputees that prosthetic fitting within 1 month of amputation resulted
in a 93% success rate of prosthetic rehabilitation (26 of 28 patients) (12,13).
Among upper-extremity amputees fitted later than 1 month after
amputation, only 42% (9 of 19) achieved prosthetic rehabilitation.
Although advocates of rigid postoperative dressings
P.3180
note
higher rates of early prosthetic use, their use should be limited to
those patients with normal sensation and well-vascularized soft tissues
that have not been injured by trauma.
REFERENCES
Each reference is categorized according to the following
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
# 1. Baumgartner R. The Surgery of Arm and Forearm Amputation. Orthop Clin N Am 1981;12:805.
+ 2. Bhagia SM, Elek EM, Grimer RJ, et al. Forequarter Amputation for High-Grade Malignant Tumours of the Shoulder Girdle. J Bone and Joint Surg 1997;79B:924.
+ 3. Bruxelle J, Travers V, Thiebaut JB. Occurrence and Treatment of Pain After Brachial Plexus Injury. Clin Orthop 1988;237:87.
* 4. Burkhalter WE, Mayfield G, Carmona LS. The Upper-Extremity Amputee: Early and Immediate Post-surgical Prosthetic Fitting. J Bone Joint Surg 1976;58A:46.
# 5. Clippinger FW. Amputations and Limb Substitutions. In: Sabiston D, ed. Textbook of Surgery, 13th ed. Philadelphia: WB Saunders, 1986:1488.
# 6. Jager SH, Tsai TM, Kleinert HE. Upper Extremity Replantation in Children. Orthop Clin North Am 1981;12:897.
* 7. Janecki CJ, Nelson CL. En Bloc Resection of the Shoulder Girdle: Technique and Indications. J Bone Joint Surg 1972;54A:1754.
# 8. Kleinert HE, Jablon M, Tsai TM. An Overview of Replantation and Results of 347 Replants in 245 Patients. J Trauma 1980;20:390.
+ 9. Kline DG, Judice DJ. Operative Management of Selected Brachial Plexus Lesions. J Neurosurg 1983;58:631.
+ 10. Lagaard SW, McElfresh EC, Premer RF. Gangrene of the Upper Extremity in Diabetic Patients. J Bone Joint Surg 1989;71A:257.
# 11. Leffert R. Lesions of the Brachial Plexus Revisited. Instr Course Lect 1989;38:245.
+ 12. Malone J, Fleming L, Robertson J, et al. Immediate, Early, and Late Postsurgical Management of Upper-Limb Amputation. J Rehabil Res Dev 1984;21:33.
+ 13. Malone
JM, Leal, JM, Underwood CPJ, Childers SJ. Brachial Plexus Injury
Management Through Upper Extremity Amputation with Immediate
Postoperative Prostheses. Arch Phys Med Rehabil 1982;63:89.
JM, Leal, JM, Underwood CPJ, Childers SJ. Brachial Plexus Injury
Management Through Upper Extremity Amputation with Immediate
Postoperative Prostheses. Arch Phys Med Rehabil 1982;63:89.
* 14. Malt RA, McKhann CF. Replantation of Severed Arms. JAMA 1964;189:114.
* 15. Marshall RW, DeSilva RDD. Computerised Axial Tomography in Traction Injuries of the Brachial Plexus. J Bone Joint Surg 1986;68B:734.
+ 16. Ransford AO, Hughes SPF. Complete Brachial Plexus Lesions: A Ten Year Follow-up of Twenty Cases. J Bone Joint Surg 59B:417, 1977.
+ 17. Rorabeck CH. The Management of the Flail Upper Extremity in Brachial Plexus Injuries. J Trauma 1980;20:491.
* 18. Rosenberg
SA, Kent H, Costa J. Prospective Randomized Evaluations of the Role of
Limb-Sparing Surgery, Radiation Therapy and Adjuvant Chemoimmunotherapy
in the Treatment of Adult Soft Tissue Sarcomas. Surgery 1978;84:62.
SA, Kent H, Costa J. Prospective Randomized Evaluations of the Role of
Limb-Sparing Surgery, Radiation Therapy and Adjuvant Chemoimmunotherapy
in the Treatment of Adult Soft Tissue Sarcomas. Surgery 1978;84:62.
* 19. Slauterbeck
JR, Britton C, Moneim MS, Clevenger FW. Mangled Extremity Severity
Score: An Accurate Guide to Treatment of the Severely Injured Upper
Extremity. J Orthop Trauma 1994;8:282.
JR, Britton C, Moneim MS, Clevenger FW. Mangled Extremity Severity
Score: An Accurate Guide to Treatment of the Severely Injured Upper
Extremity. J Orthop Trauma 1994;8:282.
+ 20. Stricker SJ. Ilizarove Lengthening of a Posttraumatic Below Elbow Amputation Stump: A case report. Clin Orthop 1994;306:124.
* 21. Windhager
R, Millesi H, Kotz R. Resection-Replantation for Primary Malignant
Tumours of the Arm: An Alternative to Fore-quarter Amputation. J Bone Joint Surg 1995;77B:176.
R, Millesi H, Kotz R. Resection-Replantation for Primary Malignant
Tumours of the Arm: An Alternative to Fore-quarter Amputation. J Bone Joint Surg 1995;77B:176.
+ 22. Wood MB, Cooney WP. Above Elbow Limb Replantation. Functional Results. J Hand Surg 1986;11A:682.
+ 23. Wright TW, Hagen AD, Wood MB. Prosthetic Usage in Major Upper Extremity Amputations. J Hand Surg 1995;20A:619.
# 24. Zhong-Wei
C, Meyer VE, Kleinert HE, Beasley RW. Present Indications and
Contraindications for Replantation as Reflected by Long-Term Functional
Results. Orthop Clin North Am 1981;12:849.
C, Meyer VE, Kleinert HE, Beasley RW. Present Indications and
Contraindications for Replantation as Reflected by Long-Term Functional
Results. Orthop Clin North Am 1981;12:849.