SLIPPED CAPITAL FEMORAL EPIPHYSIS
It is defined as a posterior and inferior slippage of the proximal
femoral epiphysis relative to the metaphysis; it occurs through the
hypertrophic physeal zone. In actuality, the relationship of the
epiphysis and its articular surface relative to the acetabulum does not
change, and the slippage is better defined as an anterior and superior
slippage of the proximal femoral metaphysis (neck) relative to the
epiphysis. Familiarity with this concept makes the surgical techniques
much easier to visualize.
Because it occurs during the adolescent growth spurt, a subtle
endocrine influence is likely. Physeal shear strength decreases during
that period (17), probably reflecting the
increased physeal width in response to growth hormone. However,
circulating hormone levels are usually normal when standard assays are
used. The majority of children with SCFE are obese, typically above the
95th percentile for body weight for age (49).
In an adolescent child who is above the 95th percentile for body
weight, biomechanical studies have shown that the shear stress across
the proximal femoral physis from simple running is enough to create an
SCFE (17,68). This
stress is further increased with femoral retroversion, an associated
finding in obese children and in the contralateral “normal” hips of
children with SCFE (28,29). In addition, children with SCFE demonstrate a more vertical physis, further increasing the susceptibility to slip (61).
slipping until physeal closure; (b) avoid complications, primarily
those of avascular necrosis (AVN) and chondrolysis; and (c) maintain
adequate hip function. Four main treatments are described: (a) internal
fixation, (b) epiphysiodesis, (c) proximal femoral osteotomy, and (d)
spica cast immobilization.
An acute SCFE is one with a symptom duration of less than 3 weeks; a
chronic SCFE, greater than 3 weeks; and an acute-on-chronic SCFE is one
with chronic symptoms for more than 3 weeks but with a sudden
exacerbation of symptoms for less than 3 weeks. This classification
scheme is unreliable because many children and parents cannot remember
the exact duration of symptoms. It also gives no information regarding
hip prognosis.
A child with a stable SCFE is able to walk, with or without crutches; a
child with an unstable SCFE is unable to walk, with or without
crutches. The prognosis for a child with a stable SCFE is very good,
with an incidence of AVN approaching zero. The prognosis for a child
with an unstable SCFE is guarded because of the increased risk of AVN,
which may be up to 50%. The vast majority (>95%) of SCFEs are stable.
limp for several weeks to months that may or may not be associated with
thigh, knee, or groin pain. Hip pain is variably present, often
resulting in diagnostic delay. Physical examination demonstrates loss
of internal rotation and spontaneous external rotation with hip
flexion. Abduction and flexion are usually decreased, especially in the
more severe cases. In longstanding cases, shortening of the lower
extremity with varying degrees of thigh atrophy is noted; the parents
usually also describe a gradually increasing external rotation gait and
limb-length discrepancy.
severe pain; there is often a history of a minor fall, such as tripping
off a curb. The child lies perfectly still with the lower extremity in
a position of flexion, abduction, and external rotation. The hip is
extremely irritable, and any attempts toward active or passive hip
motion are resisted. These SCFEs are analogous to an acute Salter
Harris I fracture, which explains their painful nature and high AVN
rate.
anteroposterior (AP) and lateral pelvis radiographs; both views are
needed because an early SCFE often is seen only on the lateral view.
Always view both hips because the incidence of simultaneous bilaterally
may approach 20%. Either frog-lateral or cross-table lateral
radiographs may be used. Proponents of the cross-table lateral view
argue that the variability with the frog positioning resulting from
limitation of hip motion inaccurately represents the SCFE, and that the
frog view can also theoretically convert a stable SCFE to an unstable
SCFE. Proponents of the frog-lateral view argue that the lateral
epiphyseal–shaft angle, a commonly used method to assess slip
magnitude, is measured on the frog-lateral view. It is also the view
many of the preoperative osteotomy plans depend on.
Comparisons with the literature findings are also possible with this view because of its common use.
obtained, and the diagnosis is readily made. A cross-table lateral
radiograph can be attempted, but clearly a frog-lateral radiograph
should not be attempted, because of both the severe discomfort for the
child and the risk of further slippage. A frog-lateral view of the
opposite hip should be obtained; it is easy to forget this in the
excitement of assessing the unstable side.
The first involves the amount of epiphyseal displacement relative to
the metaphysis (16) (Fig. 172.1A).
A mild SCFE is one with less than 33% displacement; moderate, 33% to
50%; and severe, greater than 50%. This can be measured on both the AP
and the lateral radiographs. In the case of a stable SCFE of many
months’ duration, remodeling of the femoral neck makes this measurement
less reliable, underestimating the true magnitude of slip.
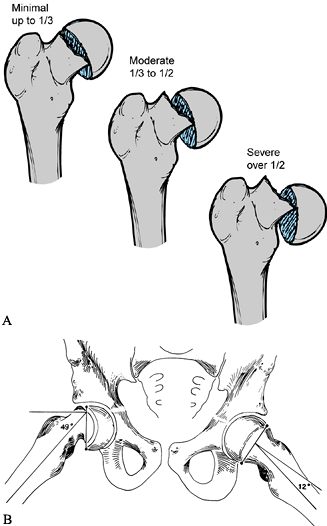 |
|
Figure 172.1. Two common methods of magnitude measurement for slipped capital femoral epiphysis (SCFE). A:
Measurement of the amount of displacement of the epiphysis relative to the metaphyseal width. The SCFE is considered mild if the measured tip is less than 33%, moderate if it is 33% to 50%, and severe if it is more than 50%. B: The head–shaft angle is measured on the frog-lateral pelvis radiograph of the pelvis to determine the degree of the slip, which is calculated by subtraction of the angle on the normal side from the angle of the affected hip: 49° – 12° = 37°. (From Aronson DD, Carlson WE. Slipped Capital Femoral Epiphysis: A Prospective Study of Fixation with a Single Screw. J Bone Joint Surg Am 1992;74:810, with permission.) |
the epiphyseal–shaft angle, which more accurately reflects the true
slip magnitude, is used (78) (Fig. 172.1B). This angle is measured on the frog-lateral pelvis radiograph by the following method.
-
Draw a line between the anterior and posterior tips of the epiphyseal at the physeal level.
-
Then draw a line perpendicular to this epiphyseal line.
-
Finally, draw a line along the mid axis of the femoral shaft.
-
The epiphyseal–shaft angle is the angle formed by the intersection of the perpendicular line and the femoral shaft line.
-
Measure this angle for both hips; the
magnitude of slip displacement is the angle of the involved hip minus
the angle of the contralateral normal hip. -
By using this angle, SCFEs can be classified as mild (<30°), moderate (30° to 50°), or severe (>50°).
-
In the case of bilateral SCFEs, 10° to 12° is used as the normal hip angle.
tomography (CT) scans are useful when doubt exists regarding the status
of physeal closure or when the postoperative screw position is not
adequately determined with plain radiographs. Bone scans are helpful in
the rare occasion when AVN or chondrolysis is suspected but not yet
visualized on plain radiographs. Magnetic resonance imaging is
unnecessary in either the diagnosis or treatment of SCFE.
without stabilization, progression is inevitable. Most authors advocate
an in situ technique (either internal
fixa- tion or epiphysiodesis) for any mild or moderate SCFE. The
treatment to use for severe SCFE is more controversial. Primary
osteotomy has been advocated to improve joint mechanics, motion, and
hip function. However, the incidence of complications is much higher
with osteotomy than in situ fixation, and thus most surgeons recommend in situ fixation as the primary treatment of a severe SCFE. In situ fixation allows the synovitis to subside, which will in itself result in improved motion (77).
After complete physeal closure (usually 1 or 2 years later), the
child’s functional limitations, gait pattern, and pain can be more
leisurely assessed (36,44).
A decision regarding the need for osteotomy can then be made after a
thorough discussion of the risks and benefits with both child and
parents.
treatment in the absence of severe degenerative changes is proximal
femoral osteotomy. Indications are functional limitations, unacceptable
gait, or cosmetic deformity. Here again, a thorough discussion of the
risks and benefits is needed before performing the procedure.
than the natural history of the disease. The natural history of SCFE is
one of gradual degenerative arthritis of the hip (16). The more severe the SCFE at diagnosis, the sooner the degenerative changes appear.
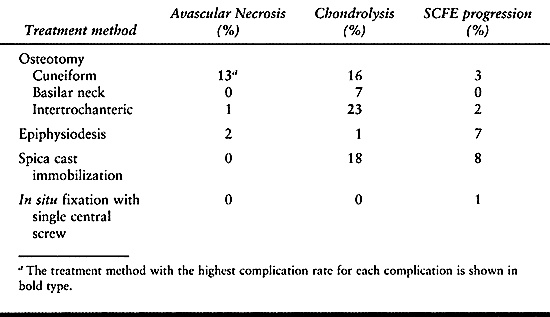
Although some of the literature demonstrates low complication rates
with osteotomy, the majority document a higher complication rate than
with in situ stabilization. The
complications, primarily AVN (13% with cuneiform osteotomy) and
chondrolysis (23% with intertrochanteric osteotomy, 16% with cuneiform
osteotomy, 7% with basilar neck osteotomy), result in poor long-term
outcomes. No long-term study has demonstrated an improved outcome in
severe SCFEs treated by osteotomy compared to in situ fixation (16,72).
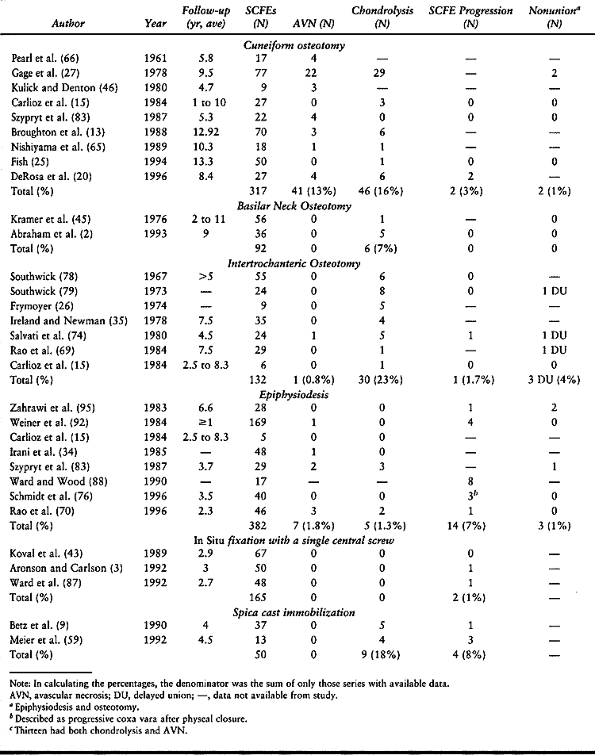 |
|
Table 172.1. Results of Various Treatment Methods for Stable Slipped Capital Femoral Epiphysis (SCFE)
|
 |
|
Table 172.2. Synopsis of Complication Rates with Different Treatments for Stable Slipped Capital Femoral Epiphysis (SCFE)
|
typical body habitus of a child with SCFE, and it has an unacceptably
high rate of chondrolysis (18%) and slip progression (8%) (9,59). Difficulties in mobility are encountered in these very large children in spica casts and with prolonged bed rest.
fixation with a single central screw using today’s intraoperative
imaging technology are uniformly excellent (0% AVN and chondrolysis, 1%
slip progression), as long as attention to technical details is
maintained. The use of two screws does not double the biomechanical
strength of the physeal–screw construct,
and it increases the risk of complications (e.g., joint penetration) (38,41). In situ
epiphysiodesis does not give comparable results (2% AVN, 1%
chondrolysis, 7% slip progression, 1% nonunion) and is also plagued
with increased morbidity [e.g., blood loss, wound problems
(hematoma/seroma, infection), longer operative time, larger incisions,
failure of fixation, and further slippage]. For all these reasons, most
surgeons presently recommend in situ fixation with a single central screw for all stable SCFEs (30).
bear weight on the involved limb. The ideal treatment is immediate
hospital admission, enforced bed rest, and next-day surgery. There is
no need or role for preoperative traction. The goal is to obtain
epiphyseal fixation before the SCFE can become unstable. However, it is
often difficult to convince the family, and especially insurance
companies, that this is a medical urgency. If such immediate procedures
cannot be done, keep the child strictly non-weight-bearing; strongly
counsel regarding the concerns of walking, running, or falls that might
create an unstable SCFE; and rapidly schedule stabilization in the next
few days. Waiting several weeks for an opening on an elective surgical
schedule is inappropriate management.
If an unstable SCFE resembles a displaced femoral neck fracture in a
young adult in terms of concerns about AVN, then an immediate anatomic
reduction should be obtained (closed, or open if necessary), and the
hip joint decompressed to relieve intracapsular pressure, all in the
hopes of reducing the risk of AVN. However, the present data are
inadequate to either support or refute this approach. My approach is to
admit the child and schedule surgery within the next 24 hours. Some
advocate keeping the child at bed rest for 1 or 2 weeks; this allows
the joint to quiet down, early healing to occur, and a more stable
situation to develop (5). Here again, the data are lacking to either support or refute this approach.
The proponents of gentle preoperative traction argue that it allows a
gradual reduction in the hopes of reducing the risk of AVN. Again, the
data are inadequate to answer the question. If traction is used, the
hip should be flexed. Extension decreases intracapsular volume, makes
the child more uncomfortable, and theoretically increases the risk of
AVN. My approach is to
keep the child comfortably positioned in bed with pillows and other supports until surgery.
ensure that a contralateral SCFE is not missed. This is especially
important when in situ fixation with a single screw is the selected treatment, because both hips should be treated under one anesthetic.
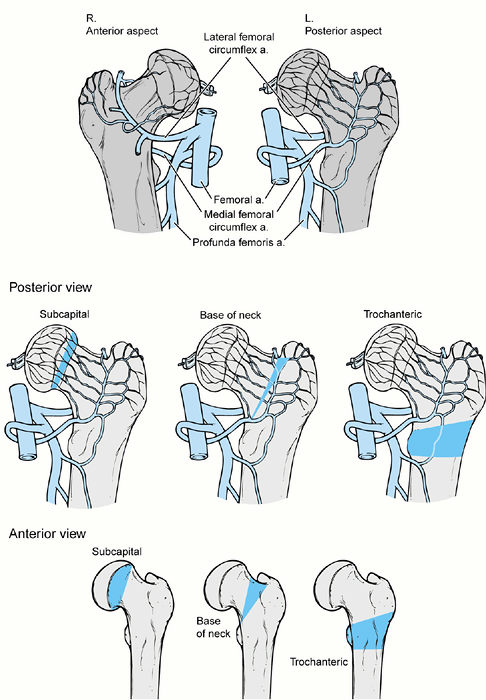
locations: (a) physis (Fish or Dunn cuneiform osteotomy); (b) basilar
neck (e.g., Abraham); and (c) intertrochanteric/subtrochanteric level
(Southwick, Müller osteotomy) (Fig. 172.2.). The physeal osteotomy is at the level of pathology and allows for maximal correction (20,25,65).
Its serious disadvantage is a high rate of AVN. The few studies with
low AVN rates indicate that its success depends on the individual
surgeon (15,25). The
object of the basilar neck osteotomy is to reduce the risk of AVN by
operating immediately distal to the entry site of the epiphyseal blood
supply, yet close enough to the deformity for adequate correction (2,45). The intertrochanteric/subtrochanteric osteotomies are compensatory and introduce a distal reverse deformity (69,78).
Their advantage is a low risk of AVN. However, they do not allow as
much correction and are complicated by chondrolysis and fixation
problems. Later joint replacement arthroplasty is more difficult
because of the distorted proximal femoral anatomy (19).
 |
|
Figure 172.2.
The three osteotomy locations for proximal femoral osteotomy for slipped capital femoral epiphysis as shown in both anterior and posterior views. These locations are subcapital, basilar neck, and trochanteric. The wedges of bone necessary for removal are different in shape anteriorly and posteriorly. On the posterior view it is noted that the vascular supply to the proximal femoral epiphysis enters the femur just proximal to the basilar neck osteotomy, whereas with the subcapital osteotomy the entry point of the vascularity is distal to the osteotomy which increases the risk of avascular necrosis with that osteotomy. The trochanteric osteotomy is safely distal to the entry point of the vessels into the femoral neck. |
treatment, accurate preoperative planning is mandatory. Use paper
tracing cut-outs and implant templates to ensure that the osteotomy is
properly performed and that the necessary internal fixation is
available. In certain circumstances, the fixation device can be
preoperatively bent and only fine adjustments need to be made
intraoperatively.
-
Use a standard technique to obtain AP and
frog-lateral radiographs of the pelvis incorporating the proximal
femurs. Maintain the pelvis flat on the x-ray table and center the beam
midline between the hips. For the AP film, maintain the hips in as
neutral a position as possible by keeping the patellae pointing as
straight up as possible. For the frog-lateral film, place the hips in
maximal abduction and external rotation, with the knees flexed, the
plantar surface of the feet facing each other, and their lateral
surfaces resting on the table. Determine the osteotomy angles by
marking the angular relationships of the femoral head to the shaft on
the radiographs; use the opposite normal side for comparison (78). -
The AP measurement determines the amount
of varus deformity, and the frog-lateral measurement determines the
amount of posterior epiphyseal tilting (Fig. 172.3A, Fig. 172.3B).
The difference in the epiphyseal–shaft angle on the AP view determines
the anterior osteotomy. (If there is bilateral involvement, use 145° as
the normal angle.) The difference in the epiphyseal–shaft angle on the
frog-lateral view determines the lateral osteotomy. (If there is
bilateral involvement, use 10° as the normal angle of retroversion.)
Mark the wedges of bone to be removed on both radiographs, and
fabricate templates for intraoperative use (Fig. 172.3C, Fig. 172.3D).
Southwick initially used tin for the templates, but any malleable
material that can be safely sterilized can be used; I have found
metallic suture wrappers to be helpful (Fig. 172.3E).
The template outlines the size and shape of the bone wedge to be
removed. Typical angles are 20° to 30° anteriorly and 45° laterally. A
wedge of 25° typically cuts through two thirds of the femoral shaft,
and a wedge of 50° typically cuts through one half of the femoral
shaft. Never exceed 45° anterior and 60° lateral.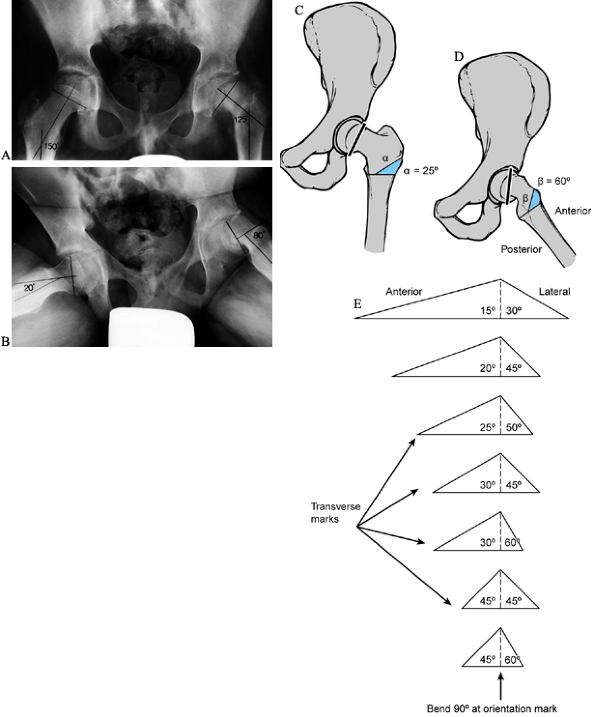 Figure 172.3.
Figure 172.3.
Preoperative planning for the Southwick intertrochanteric osteotomy. AP
and frog-pelvis radiographs of a 14-year-old boy with a left SCFE. A:
The epiphyseal–shaft angle on the AP view is 150° on the normal side
and 125° on the affected side; thus a 25° wedge is needed. B:
The epiphyseal–shaft angle on the frog-lateral view is 20° on the
normal side and 80° on the affected side; thus a 60° wedge is needed. C: The AP wedge is marked on the radiograph. The lateral wedge is similarly marked. D: The proposed osteotomy is made by using paper cut-outs from the radiographic markings. E:
After the angles that give the desired correction have been determined,
life-size paper templates are obtained from the original Southwick
manuscript and traced onto a malleable, autoclavable material (e.g.,
tin or a metallic suture wrapper). This template is then sterilized at
the time of surgery. -
Next, plan the internal fixation. After
obtaining paper tracings of the proximal femurs in both AP and lateral
projections, draw the intended osteotomy on paper. Use overlay
templates of the selected internal fixation device(s) to plan their
appropriate position and length (e.g., length and angle of side plate
and lag screw if using a hip compression screw system, or the length
and angle of the blade plate if using a blade-plate system). Take care
to ensure that the tips of the lag screw or blade plate do not violate
the posterior retinacular blood supply or penetrate the articular
surface. The length of the lag screw or blade plate is often much
shorter than expected; preoperative planning is imperative to ensure
that the appropriate selection of devices is available at surgery. Note
that some sizes of lag screws or blade plates may be special orders.
After osteotomy, the femoral head should appear erect in the acetabulum
in the AP view, and at a right angle to the long axis of the femoral
shaft in the lateral view.
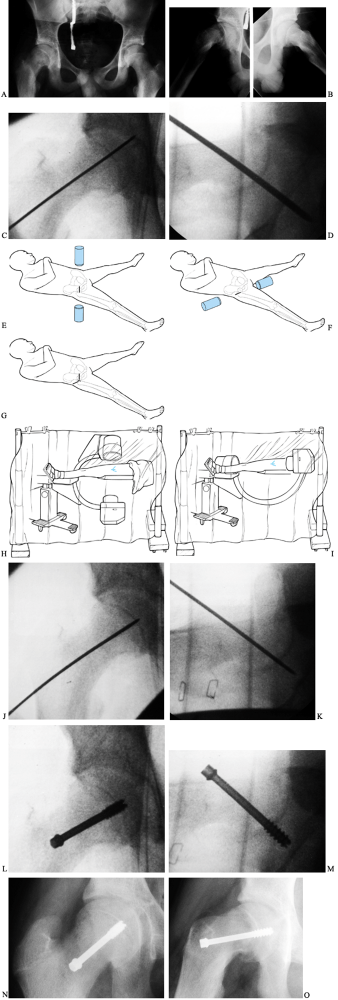 |
|
Figure 172.4. Technique of in situ single-screw fixation for SCFE. Preoperative AP (A) and frog-lateral (B)
radiographs of both hips in a 14-year-2-month-old boy. There is an SCFE of 40° on the right; the left hip is normal. A guide pin is placed onto the skin overlying the hip so that the pin is positioned in the center of the epiphysis and is perpendicular to the physis in both AP (C) and lateral (D) images. Skin lines are drawn to record the position of the guide pin on both the AP (E) and lateral (F) projections. The incision is marked at the intersection of the skin lines (G). After draping, multiple Kocher clamps are placed on the base of the drape to act as weights, which allows for movement of the image between AP (H) and lateral (I) images without violating surgical field sterility. The guide pin is advanced onto the anterolateral cortex of the femur so that the pin will enter the center of the epiphysis perpendicular to the physis. The guide pin is then advanced across the physis, and its tip is advanced no deeper than 5 mm from the subchondral bone [AP (J) and lateral (K) views]. The appropriate depth of the pin tip is checked using the lateral image. A cannulated screw is inserted in routine fashion after drilling and tapping the hole (L,M). Postoperative radiographs demonstrate ideal screw position in the center of the epiphysis perpendicular to the physis in both AP (N) and lateral (O) projections. |
-
Position the patient supine on a fracture
table, moving the image intensifier rather than the lower extremity.
Take care when transporting the patient onto the fracture table; no
reduction maneuvers are performed and forceful traction is not applied
to the lower extremity. I use the fracture table only as a positioning
device, allowing the involved limb to lie comfortably in its natural
position of rotation. -
Place the opposite limb into abduction
with the hip extended, and move the image intensifier into position
between the two lower extremities. -
Prior to surgical draping, confirm the ability to obtain adequate AP and cross-table lateral images.
-
Place a guide pin onto the skin overlying the proximal femur and obtain an AP image (48).
Position the pin in the center of the epiphysis and perpendicular to
the physis. Draw a line on the skin to record this guide pin position
in the AP projection. -
Draw a similar skin line for the lateral
image, again positioning the pin so that it is in the center of the
epiphysis and perpendicular to the physis.
relative to the femoral neck, and the guide pin in the lateral
projection angles from anterior to posterior. This is the opposite of
femoral neck fractures, where it angles from posterior to anterior.
Thus the two skin lines intersect on the anterolateral aspect of the
thigh, and as the slip becomes more severe, the intersection point
becomes more anterior. Because of the retroversion in the posteriorly
displaced epiphysis in SCFE, the osseous entry point of the guide pin
is on the anterior aspect of the femur. In mild SCFEs, it is often at
the anterior intertrochanteric line; in severe SCFEs, it moves up onto
the anterior femoral neck.
-
Prepare and drape the anterolateral
portion of the thigh. I prefer to use a transparent shower-curtain-type
of isolation drape with multiple Kocher clamps on the base of the drape
as weights; this allows movement of the image intensifier in both AP (Fig. 172.4H) and lateral (Fig. 172.4I) projections without violating surgical field sterility. -
Introduce the guide pin through the skin
at the intersection of the skin lines; it may be introduced through
either a stab wound or a small, 1–2 cm incision. -
Advance the guide pin onto the
anterolateral cortex of the femur, keeping the drill and guide pin
aligned according to the skin lines. Once the guide pin contacts the
femoral cortex, its point of entry and angular direction is confirmed
in both AP and lateral projections. -
When you are satisfied that the entry
point and direction of the guide pin are correct, carefully advance the
guide pin into the femoral neck, frequently checking the angle of entry
on both AP and lateral images. Ideally, there should only be one entry
point into the femoral cortex; extra holes act as stress risers and
increase the risk of postoperative fracture. Do not advance the guide
pin across the physis until you are certain that the pin will enter the
center of the epiphysis perpendicular to the physis in both AP and
lateral projections. -
After the pin has crossed the physis,
advance the tip to the proper depth (no closer than 5 mm from the
subchondral bone; any permanent pin position less than 5 mm from the
subchondral bone increases the risk of joint penetration). This depth
is determined on the lateral projection. Take care to ensure that the
pin is not in the superior quadrant of the femoral head, because this
position may jeopardize the epiphyseal blood supply. -
When the pin is in the appropriate
position, determine screw length by placing another guide wire of
identical length along the intraosseous guide wire and measuring the
difference. -
Insert a cannulated screw in routine fashion after drilling and tapping. The screw should be at least 6.5 mm in diameter.
-
While drilling and tapping, closely
monitor the guide pin to ensure that it (a) does not break, (b) does
not penetrate the joint and enter the abdominal cavity, or (c) does not
withdraw from the femoral neck. -
After inserting the screw, remove the
guide pin and confirm that the screw tip does not penetrate the joint.
This can be done by one of several techniques: (a) move the limb in
multiple directions in both AP and lateral views to confirm that it
does not penetrate the joint, (b) use the approach-withdraw phenomenon,
or (c) use intraoperative arthrography through the cannulated screw. My
approach is a variation of the first technique. I obtain images of the
hip every 10° to 15° while moving from a lateral to an AP projection,
ensuring that the screw tip is no closer than 5 mm to the subchondral
bone of the epiphysis. If it is closer than 5 mm, use a shorter screw. -
After confirmation of appropriate screw position and depth, close the incision.
The advantages of this approach compared to the anterior approach are
shorter operating time, less blood loss, easier instrument insertion,
avoidance of lateral femoral cutaneous nerve injury, and fewer wound
complications.
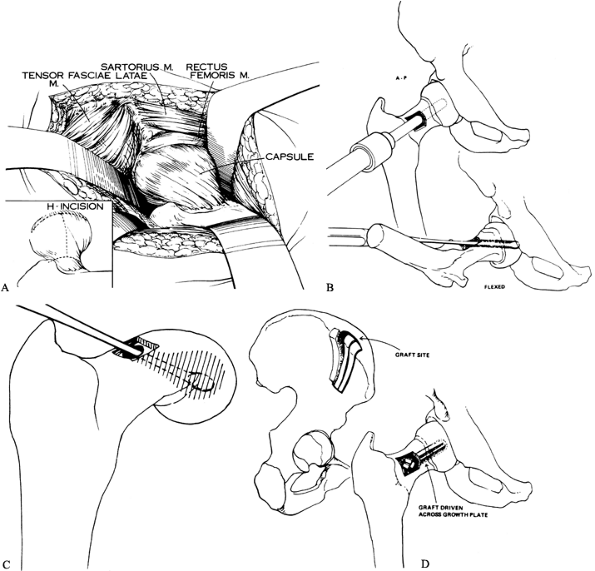 |
|
Figure 172.5. Technique of open autograft epiphysiodesis. A: The exposure of the capsule and the H-shaped capsulotomy. B: The hollow mill drill used to create a tunnel across the physis. C: A curet is used to further enlarge the opening in the physis. D:
Iliac crest bone graft is made into a sandwich and driven across the physis. (Parts A,C from Melby A, Hoyt WA Jr, Weiner DS. Treatment of Chronic Slipped Capital Femoral Epiphysis by Bone-Graft Epiphysiodesis. J Bone Joint Surg Am 1980;62:119, with permission. Parts B,D from Weiner DS, Weiner S, Melby A, Hoyt WA Jr. A 30-Year Experience with Bone Graft Epiphysiodesis in the Treatment of Slipped Capital Femoral Epiphysiodesis. J Pediatr Orthop 1984;4:145, with permission.) |
-
Position the patient supine on a radiolucent table.
-
Bump up the affected hip, and be sure you can obtain adequate AP and lateral images prior to surgical draping.
-
Prepare and drape free the entire lower extremity, hip, and iliac crest, as for a total hip arthroplasty.
-
Make a mid-lateral incision starting 4
inches below the level of the greater trochanter in the lateral midline
of the upper thigh, continue the incision proximally to the greater
trochanter, and then angle it obliquely to the anterior superior iliac
spine. -
Split the tensor fascia femoris proximally to the level of the anterior superior iliac spine.
-
Retract the tensor anteriorly and posteriorly, exposing the underlying gluteal musculature.
-
Retract the anteriormost fibers of the gluteus medius posteriorly to view the capsule.
-
Perform an H capsulotomy (Fig. 172.5A) and use retractors (large Cobra type) to expose the femoral head and neck; the area of slipping is now directly in view.
-
Make a rectangular or square window in
the femoral neck and insert a large hollow mill drill through this
window. Under image control, drill the hollow mill across the physis
into the epiphysis (Fig. 172.5B). -
Remove a cylindrical core consisting of metaphyseal bone, physis, and epiphyseal bone.
-
Enlarge the cylindrical tunnel further by curettage, removing more of the physis (Fig. 172.5C).
-
Expose the outer table of the ilium,
removing sections of corticocancellous bone, which are packaged
together in sandwich fashion and driven into the epiphysis across the
physis as a composite peg (Fig. 172.5D). -
Perform routine closure.
-
In unstable cases, apply a bilateral hip spica cast with the epiphysis in the reduced position.
-
Position the patient supine on a fracture table with the involved hip in the neutral position and the knee extended.
-
Determine stability of the SCFE radiographically before sterile draping.
-
Gently mobilize the hip through internal
rotation under the image intensifier. If the epiphysis moves relative
to the metaphysis, the slip is considered to be unstable. -
At this point, gently position the
involved hip in 10° to 15° of abduction and with internal rotation such
that the femoral neck is parallel to the floor. This position allows
for a true anterior and lateral view with the image intensifier. A
forced reduction maneuver is never performed. -
After prepping and draping using an
isolation drape, determine the guide pin entry point by the
intersection of two skin lines using the same technique as in situ single-screw fixation. -
Introduce the guide pin through a stab
incision at the intersection of the skin lines, and advance it onto the
anterolateral cortex of the femur. The ideal bone entry position for
the guide pin is just below the greater trochanteric physis and above
the thick cortical bone of the femoral shaft to avoid creating a stress
riser in the subtrochanteric region. -
Confirm the point of entry and angular direction of the guide pin in both AP and lateral views.
-
Carefully advance the guide pin into the
femoral neck; it should not be advanced across the physis until
confirmation that it will enter the center of the epiphysis
perpendicular to the physis in both AP and lateral projections. Avoid
placement of the pin into the anterolateral region of the epiphysis or
traversing the posterior or inferior cortex of the femoral neck; either
of these may injure the vascular supply of the proximal epiphysis and
result in AVN. -
After the pin has crossed the physis,
advance the tip to the proper depth, stopping within 2 mm of the
subchondral bone but not penetrating the joint. This depth is
determined on the lateral projection. -
Now place a second guide pin parallel to
the first. This second guide pin secures the femoral epiphysis during
subsequent reaming. Place it in a position that does not interfere with
reaming, usually inferior to the first pin. A series of movements of
the lower extremity in combination with movements of the image
intensifier through its full arc of motion ensures no aberrantly placed
pins. -
Measure the centrally placed guide pin, and place a 10 mm cannulated reamer over it.
-
Drill the femoral neck over the guide pin
to a depth of about 10 mm beyond the physis, but no closer than 2 mm to
the subchondral bone. -
Set the drill on reaming speed to avoid
thermal necrosis. Drills more than 10 mm in diameter are not
recommended; a 10 mm diameter is adequate to incite physeal closure. -
Fashion a piece of freeze-dried
irradiated cortical strut allograft to the appropriate dimensions using
a high-speed burr. The approximate size of prepared allograft is
10×5×85 mm. This strut graft should pass through the tissue protector
apparatus of the 10 mm drill with some resistance. -
Remove the guide pin, and pass the allograft through the hole and across the physis at least 1 cm.
-
Trim any allograft protruding beyond the drill hole in the femoral cortex flush with the cortex.
-
Remove the second guide pin stabilizing the epiphysis, and close the wound in routine fashion.
-
If the SCFE is deemed to be unstable, apply a spica cast.
osteotomies, but two procedures are included here for the sake of
completeness. With either the open reduction procedure of Dunn or the
cuneiform osteotomy of Fish, a complete anatomic reduction can be
achieved, although coxa breva will occur. Note that there is a high
risk of AVN with these procedures.
severe SCFE, is popular in the United Kingdom and other countries with
a British orthopaedic influence (13,22).
An absolute prerequisite for this procedure is an open physis. The
indications for this procedure in Dunn’s original paper were a chronic
SCFE of more than one-third the diameter of the physis, or an
acute-on-chronic SCFE radiographically demonstrated by the presence of
new bone along the posterior aspect of the metaphysis.
-
Place the patient in the lateral decubitus position with the involved extremity up.
-
Prepare and drape free the entire lower extremity, hip, and iliac crest area as for a total hip arthroplasty.
-
Make an incision over the proximal part of the lateral femoral shaft, across the greater trochanter, and into the buttock.
-
Incise the fascia lata and gluteus maximus in line with the skin incision.
-
Define the anterior margin of the gluteus medius and drive a Jones bone spike between the glutei and the hip capsule.
-
Similarly, define the posterior margin of the gluteus medius and drive a Jones bone spike between the abductors and the capsule.
-
Divide the vastus lateralis 1.5 cm distal to its origin and elevate the greater trochanter through the trochanteric physis.
-
Use sharp dissection to separate the muscles from the hip capsule.
-
Retract the abductor muscles and attached greater trochanter proximally.
-
Incise the hip capsule in the long axis of the neck and extend it around the anterior and posterior edges of the acetabulum (Fig. 172.6).
The anterior capsular flap may be mobilized distally, but this must not
be done with the posterior flap because the base of the posterior flap
carries the blood supply to the femoral epiphysis.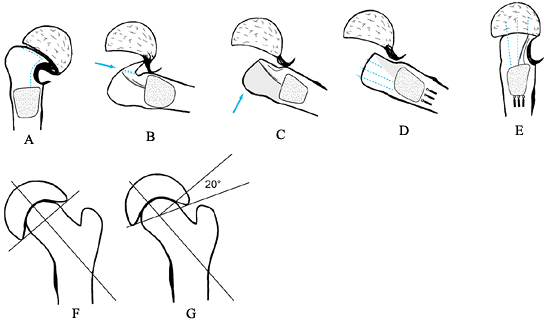 Figure 172.6.
Figure 172.6.
Dunn procedure. See text above for a description of the technique.
(From Dunn DM, Angel JC. Replacement of the Femoral Head by Open
Operation in Severe Adolescent Slipping of the Upper Femoral Epiphysis.
J Bone Joint Surg Br 1978;60:394, with permission.) -
At this point, the subluxated femoral
head is seen, as is the posterior surface of the femoral neck, which
has a red, velvety appearance. The anterior femoral neck is pale and
avascular (Fig. 172.6A). -
Incise the synovial membrane on the neck just anterior to the vascular area and around the anterior margin of the head.
-
Strip off the posterior covering to the
margin of the head down to the base of the neck. This dissection must
be done sharply and without electrocautery. -
Slip a wide gouge into the plane between
the physis and the femoral metaphysis. With gentle motion it will cut
the physis, making it possible to lever the epiphysis off the neck.
Once the epiphysis is detached from the metaphysis, it spontaneously
reduces from its subluxated position and disappears back into the
acetabulum (Fig. 172.6B). -
Now make two osteotomies. The first is in
the long axis of the neck to remove the bony beak. The second is to
shorten the neck a few millimeters (Fig. 172.6C). -
Make the second osteotomy with a slightly
curved sweep, transverse to the top of the neck. This osteotomy removes
the remains of the physis from the neck (Fig. 172.6D). -
Draw the femoral neck to one side and use a wide gouge to curet the remains of the physis from the epiphysis.
-
At this point, drill three threaded pins
up the metaphysis, so that when the epiphysis is reduced they will
engage the epiphysis in different parts (Fig. 172.6E). -
Now reduce the epiphysis and advance the
pins into the epiphysis. After reduction, the epiphysis should sit
squarely on the neck in the lateral view, and in 20° of valgus in the
AP view (Fig. 172.6F, Fig. 172.6G). -
After radiographic confirmation of
reduction and internal fixation, lightly suture the synovial membrane
over the femoral neck and close the capsule. -
Reattach the greater trochanter with a screw and close the wound in a routine manner.
-
Position the patient on a radiolucent table in the supine position with a small bump under the involved hemipelvis.
-
Drape the entire limb, hip, and iliac crest area as for a total hip arthroplasty.
-
Approach the hip through an anterolateral
exposure. Dissect between the sartorius and tensor fascia femoris
muscles, exposing the anterior capsule. The capsule must be generously
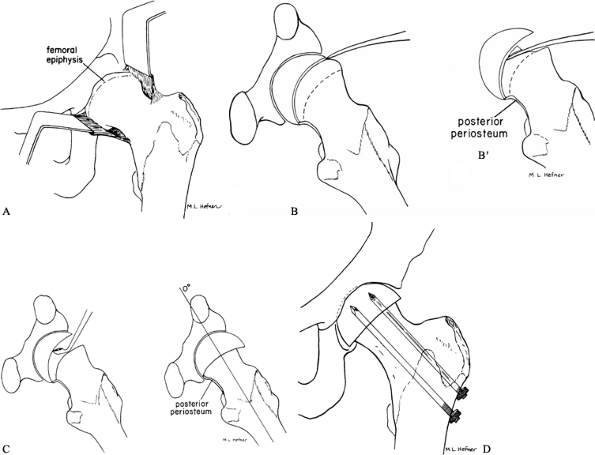
exposed proximal to the acetabular rim for adequate visualization (Fig. 172.7A).![]() Figure 172.7. Cuneiform osteotomy of Fish. A: Exposure of the femoral metaphysis. Note the minimal amount of the epiphysis that is initially seen. B: Location of the physis using a small curved osteotome. B’:
Figure 172.7. Cuneiform osteotomy of Fish. A: Exposure of the femoral metaphysis. Note the minimal amount of the epiphysis that is initially seen. B: Location of the physis using a small curved osteotome. B’:
The osteotomy is made by removing small pieces of bone with a sharp
osteotome and mallet. The fragments are wiped away while being removed
to ensure continuous identification of the physis. C:
Further removal of the physeal cartilage with a curet, and then
reduction of the epiphysis on the metaphysis. The diameter of the head
is larger than that of the neck after removal of the bone wedge; thus
the epiphysis overlaps the neck. D: After
reduction of the epiphysis, it is fixed with three or four threaded
pins. Note how much more of the articular surface is now visible
compared to the preoperative situation (A). (From Fish JB. Cuneiform Osteotomy of the Femoral Neck in the Treatment of Slipped Capital Femoral Epiphysis. J Bone Joint Surg Am 1984;66:1153, with permission.) -
Make a longitudinal incision in the
anterior capsule, and extend it in an H fashion both proximally and
distally. Carefully retract the capsule; no retractors should be placed
around the femoral neck either medially or laterally. -
Identify the proximal femoral epiphysis;
it usually is barely visible at the acetabular rim. The anterior
projection of the metaphysis is quite obvious and can be mistaken for
the capital femoral epiphysis. In a very severe SCFE, it may be
necessary to remove a portion of the metaphysis to visualize the
epiphysis. -
Next, identify the location of the physis and determine its plane by gentle probing with a Keith needle.
-
Determine the size of wedge to be removed by the degree of the slip and position of the epiphysis (Fig. 172.7B).
-
Remove enough bone to allow an effortless
anatomic reduction of the epiphysis on the metaphysis. A larger wedge
is needed in a more severe SCFE. The base of the wedge must be in the
plane of anticipated correction of the epiphysis, and the curved
contour of the physis should match the corresponding curved metaphyseal
neck. -
Gently remove the wedge in small pieces with an osteotome and mallet. Maintain continuous identification of the physis.
-
Use extreme caution when approaching the
posterior aspect of the neck. The posterior periosteum must be
protected and preserved to avoid vascular damage. -
Remove the posterior bone (a curet is usually used), and use a large curet to remove any remaining physis (Fig. 172.7C).
Once sufficient posterior bone has been removed, the epiphysis will
effortlessly reduce with flexion, abduction, and internal rotation of
the limb. If inadequate posterior bone has been removed, undue tension
will be placed on the posterior periosteum, potentially compromising
epiphyseal vascularity. -
Once an anatomic reduction has been
obtained, achieve fixation with three or four threaded pins directed
toward the center of the femoral head and only deep enough to obtain
firm epiphyseal fixation (Fig. 172.7D). -
Confirm the position of the pins and epiphysis radiographically and perform routine closure.
at the physis because it is performed just distal to the entry of the
posterior retinacular vessels. It may be either intracapsular (the
Kramer technique) (45) or extracapsular (the Barmada/Abraham technique) (2,6). The maximum amount of correction is less than with a physeal osteotomy, usually no more than 55°.
-
Approach the hip laterally with an
incision starting 2 cm distal and lateral to the anterosuperior iliac
spine, curving distally and posteriorly over the greater trochanter and
lateral femoral shaft to a point 10 cm distal to the base of the
greater trochanter. -
Incise the fascia lata longitudinally and
develop the interval between the gluteus medius and the tensor fascia
lata. This dissection should be carried proximally to the inferior
branch of the superior gluteal nerve, which innervates the tensor
fascia lata. -
Open the hip capsule anteriorly along the
anterosuperior surface of the femoral neck, and widely release it along
the anterior intertrochanteric line. -
Reflect the vastus lateralis distally, exposing the base of the greater trochanter and the proximal femoral shaft.
-
The margin between the articular
cartilage of the femoral epiphysis and the callus, as well as the
junction of the callus with the normal cortex of the femoral neck, can
now be seen. Compare the distance between these two junctions with the
amount calculated preoperatively from the radiographs. The widest part
of the wedge will be in line with the widest portion of the slipped
epiphysis, in the anterior and superior aspects
P.4404P.4405
of
the neck. The most common mistake is to make the superior portion of
the wedge too small, resulting in incomplete correction of the varus;
if the anterior wedge is too wide, overcorrection of retroversion
occurs. -
Make the distal osteotomy first,
perpendicular to the femoral neck and following the anterior
intertrochanteric line from proximal to distal. The cut should reach
the posterior cortex but leave it intact. -
Direct the second osteotomy obliquely so
that the cutting edge of the osteotome remains distal to the posterior
retinacular blood supply. Anteriorly the capsule reaches the
intertrochanteric line, but posteriorly the lateral third of the
femoral neck is extracapsular; thus an osteotomy done at this level
does not violate the posterior capsule and its retinacular supply. -
Before completing the osteotomy, drill
one or two 5 mm, threaded Steinmann pins into the proximal fragment to
ensure control of the osteotomy. The osteotomy is completed without
penetrating the posterior cortex. -
Remove the bone wedge, and “greenstick” the posterior cortex, closing the osteotomy.
-
Insert several 5-mm-diameter Steinmann
pins from the outer cortex of the femoral shaft through the neck,
across the osteotomy and into the epiphysis. -
Cut the pins to the appropriate length after radiographic confirmation of the osteotomy position and fixation.
-
Close the wound in routine fashion.
-
Position the patient supine on the fracture table.
-
Rotate the involved limb maximally internally by gently positioning the foot plate; abduct it approximately 5°.
-
Widely abduct the contralateral limb, and place the image intensifier between the two lower extremities.
-
Outline the patella with a marking pen
and estimate the degree of fixed external rotation; most patients with
a moderate or severe SCFE lack at least 15° of internal rotation. -
Prepare and drape the entire hip, thigh, and knee area.
-
Make an anterolateral approach to the hip (Fig. 172.8A).
Begin the incision at the anterosuperior iliac spine, and extend it
distally and posteriorly to the anterior aspect of the greater
trochanter and then distally along the proximal lateral femoral shaft.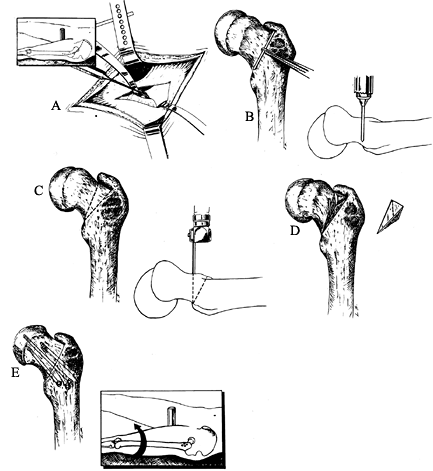 Figure 172.8. Extracapsular basilar neck osteotomy of Barmada and Abraham. A: A periosteal elevator is used to elevate the anterior iliofemoral ligament. Inset: The incision of the anterolateral exposure. B:
Figure 172.8. Extracapsular basilar neck osteotomy of Barmada and Abraham. A: A periosteal elevator is used to elevate the anterior iliofemoral ligament. Inset: The incision of the anterolateral exposure. B:
The proximal osteotomy cut is determined by placing a 3 cm K-wire along
the base of the neck. The correct site is scored with an osteotome
after fluoroscopic confirmation. A vertically placed K-wire is drilled
vertical to the femoral neck at the scored line. C:
The distal osteotomy starts at the base of the neck inferiorly and
extends obliquely along the intertrochanteric line to the greater
trochanter. The proximal osteotomy starts at the same position distally
and extends proximally so that a proximally based triangle is formed. D: The bone wedge is removed. E:
The lower extremity is internally rotated and abducted to close the
osteotomy site. Fix with three to four screws. (From Abraham E, Garst
J, Barmada R. Treatment of Moderate to Severe Slipped Capital Femoral
Epiphysis with Extracapsular Base of Neck Osteotomy. J Pediatr Orthop 1993;13:294, with permission.) -
Longitudinally incise the fascia lata and develop the interval between the gluteus medius and tensor fascia lata.
-
Locate the anterior joint capsule at the intertrochanteric line between the gluteus medius and the vastus lateralis.
-
Using a periosteal elevator, elevate the anterior iliofemoral ligament from the anterior aspect of the femoral cortex (Fig. 172.8A).
-
Gently place a narrow-tip Hohmann
retractor around the superior aspect of the femoral neck superior and
deep to the iliofemoral ligament; place another deep to the iliofemoral
ligament proximal to the lesser trochanter. -
Plan the two-plane wedge osteotomy on the
anterior surface by delineating a triangle based anteriorly and
superiorly. The triangle base is usually 15–20 mm wide. -
Locate the proximal osteotomy by placing
a 3-cm-long Kirschner wire (K-wire) on the anterior femoral surface
from the lesser trochanter to the greater trochanter at the base of the
neck and along the edge of the hip capsule (Fig. 172.8B). -
Confirm the position radiographically and mark the bone along the wire edge with an osteotome.
-
After externally rotating the limb, drill
a second K-wire just distal to the first wire in the AP plane, vertical
to the femoral neck. -
Then internally rotate the limb and take a lateral image to confirm proper wire placement.
-
The second, distal osteotomy line again
starts from the lesser trochanter and goes to the physis of the greater
trochanter. The angle this line makes with the first varies according
to the amount of correction needed. A 15-mm-wide superiorly based wedge
is usually needed. -
Make the osteotomy cuts with an oscillating saw (Fig. 172.8C), converging posteriorly to a single cortical cut.
-
For maximal correction, remove the entire wedge of bone (Fig. 172.8D), especially superiorly.
-
Internally rotate and abduct the lower
extremity to close the osteotomy; maintain traction to prevent proximal
femoral migration. Adequate correction is achieved when the patella can
be internally rotated 15°. If the correction is inadequate, further
bone may be removed from the metaphyseal side, but 20 mm of width
anterosuperiorly is the maximum that should be removed. -
Now fix the osteotomy with three or four
cannulated screws; only one screw need cross the physis to stabilize
the slipped epiphysis (Fig. 172.8E). Avoid the superolateral quadrant of the femoral head when placing the transphyseal screw. -
Obtain permanent radiographs to confirm correction and internal fixation and then close the wound routinely.
-
Reattach the iliofemoral ligament and capsule only if excessively elevated from the bone.
regard to the risk of AVN, but it has an increased risk of chondrolysis
in some studies (26,35,74,78,79).
It is a compensatory osteotomy because of its distance from the level
of pathology. This makes future total hip arthroplasty more
difficult (19). Because it is in the inter- and subtrochanteric areas, the risk of delayed union may be increased (69,74,79); internal fixation is more difficult because of the zigzag deformity that is present after osteotomy. According to Southwick (79), the maximal amount of correction that can be achieved is 70°.
Southwick is an SCFE greater than 30°. This osteotomy corrects all
three planes of deformity (varus, rotation, and flexion). The biplane
osteotomy corrects the varus and adduction; rotation of the distal
fragment relative to the proximal corrects the rotational deformity.
Meticulous preoperative planning is essential (78,79).
-
Position the patient supine on the fracture table with the affected limb draped free.
-
Make a lateral incision 15–20 cm long along the posterior border of the greater trochanter.
-
Incise the tensor fascia lata and vastus lateralis, and expose the femoral shaft subperiosteally.
-
Identify the lesser trochanter; it can be brought into prominence by abduction and external rotation of the hip.
-
Detach the psoas insertion from the lesser trochanter, taking care not to injure the nearby vessels or sciatic nerve.
-
Identify the junction between the flat
anterior surface and the slightly curved lateral surface of the femur;
make a longitudinal mark along this junction using a sharp osteotome or
oscillating saw. This mark identifies the anterolateral edge of the
femur. It corresponds to the lateral (AP radiograph) and anterior edge
of the femur (frog-lateral radiograph) used to make the intraoperative
template. -
Next, make a transverse mark at the level of the lesser trochanter (Fig. 172.9A).
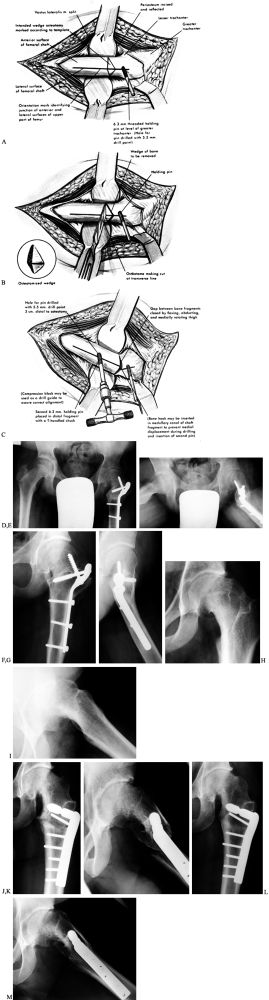
![]() Figure 172.9. Operative technique of the Southwick intertrochanteric osteotomy. A: The osteotomy site is marked as shown. B: The osteotomy is made and the bone wedge removed. C:
Figure 172.9. Operative technique of the Southwick intertrochanteric osteotomy. A: The osteotomy site is marked as shown. B: The osteotomy is made and the bone wedge removed. C:
The remainder of the medial and posterior transverse cut is made and,
while controlling the proximal fragment with the pin attached to a
T-handle chuck, the distal fragment is abducted and flexed, bringing
the osteotomy surfaces together. The osteotomy is then internally
fixed. Postoperative radiographs (D,E) of the child in Figure 172.2 demonstrate fixation with a Southwick plate. The AP radiograph (D) demonstrates equal epiphyseal–shaft angles of 147°; the lateral radiograph (E) demonstrates a residual epiphysis–shaft angle on the left of 17° (34° – 17°).Radiographs at last follow-up (F,G)
(child now 16 years old) demonstrate union of the osteotomy; note the
remodeling at the osteotomy seen on the lateral views. Fixation of a
Southwick osteotomy with the more customary intertrochanteric
lag/compression hip screw system (H–M). This 20-year-old man presented
with a longstanding SCFE as shown on the AP (H) and lateral (I)
views. The old SCFE deformity measured 82° using the lateral
epiphysis–shaft angles (94° – 12°). Immediate postoperative radiographs
[AP (J) and lateral (K)
views] demonstrate fixation with a hip screw system using a 95° dynamic
compression plate. Note the short lag screw: It does not penetrate into
the femoral head but rather into the proximal femoral metaphysis and
neck, stopping just short of the calcar. The amount of correction
achieved was 34°, with a final lateral epiphysis–shaft angle of 48°.
The radiographs at last follow-up, at age 22.7 years, demonstrate
osteotomy union [AP (L) and lateral (M) views]; again note the remodeling of the osteotomy in the lateral view. (Parts A–C from Tachdjian MO. Pediatric Orthopaedics, vol. 2, 2nd ed. Philadelphia: Saunders, 1990:1057, plate 37, with permission.) -
Wrap the template around the anterior and lateral surfaces of the femur and the outlines marked (Fig. 172.9B).
-
Drill a 5.6 mm Haynes pin (or Schanz
screw) into the greater trochanter parallel to the hypotenuse of the
anterior triangle, starting about 6 mm proximal to the hypotenuse and
directed toward the lesser trochanter. This pin is attached to a
T-handled chuck and used to control the proximal fragment. -
Make the osteotomy following the template outline.
-
Remove the wedge of bone, which consists
of the lateral and anterior femoral cortices. The proximal oblique
surface is flat and angled by the amounts previously calculated in both
sagittal and frontal planes (Fig. 172.9C). -
Make the remaining transverse cut of the
osteotomy through the posterior and medial cortices at the level of the
lesser trochanter. -
While stabilizing the proximal fragment
with the pin, abduct the distal fragment and flex it to place the
proximal oblique surface in contact with the transverse surface of the
distal fragment (Fig. 172.9D). The orientation
marks on both fragments should meet. As the osteotomy is closed, the
two halves of the lesser trochanter separate, adding length to the
limb. The combination
P.4409
of
posterior tilting and external rotation are related, so that internal
rotation of the shaft relative to the proximal fragments is rarely
necessary except in a very severe deformity. If the posterior SCFE is
greater than 60°, then the distal fragment may need some additional
internal rotation. -
Now fix the osteotomy with the special side plate discussed by Southwick (79) (Fig. 172.9E), or with more typical intertrochanteric fixation (57,93) (either blade plate, compression hip screw, or dynamic compression plate) (Fig. 172.9F).
Whatever method is selected, it must be appropriately templated out
preoperatively using paper tracings and implant drawings. The operating
room is not the place to first consider the method of fixation. -
If the Southwick plate is used, apply it
first to the posterolateral aspect of the greater trochanter using
cancellous bone screws at least 5 cm long. The calcar should be engaged
with at least one of the screws, and another one should be placed up
into the femoral neck. -
Next, attach the distal portion of the plate.
-
Apply compression at the osteotomy; use
of a temporary compression device with pins while attaching the plate
is often necessary. -
Take final radiographs to confirm correction and fixation, and close the wound in routine fashion.
-
Use the standard exposure; make the
transverse mark at the level of the lesser trochanter and the
longitudinal mark at the junction of the anterior and lateral femoral
cortices. -
Mark the wedge to be cut using length measurements (18).
Measure 15 mm proximal from the transverse mark along the longitudinal
orientation mark and draw a line across the anterior shaft; this
represents the anterosuperior wedge of bone to be removed (Fig. 172.10A).
On the lateral surface of the shaft, measure 13 mm posteriorly along
the transverse mark and draw a line from this point to the superior
proximal line (Fig. 172.10B). This completed
wedge is nearly the entire anterior surface and between a half to two
thirds of the lateral surface of the shaft along the transverse line.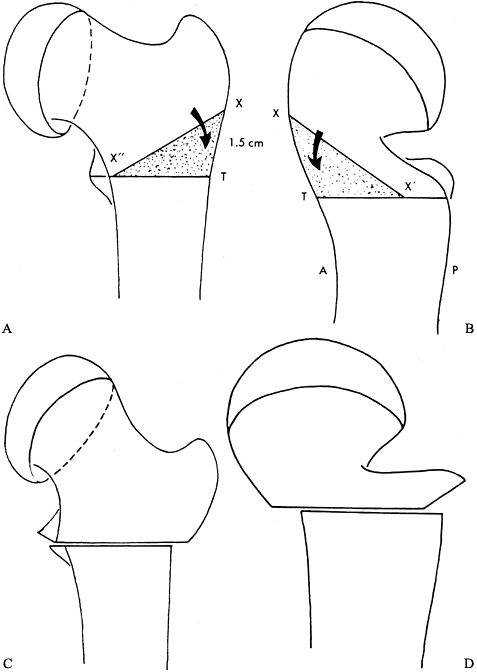 Figure 172.10. Clark modification of the Southwick intertrochanteric osteotomy. A: The transverse line is inscribed on the anterior and lateral surfaces of the femur at the level of the lesser trochanter (line X” – T). Then a point X is measured 15 mm proximal from the transverse line along the longitudinal orientation mark. The line from X” to X is then drawn, and the triangle X”XT denotes the anterior wedge to be removed. B: Next, the point X‘ is measured 13 mm posterior to the longitudinal mark at the level of the transverse mark. The line from X to X‘ is drawn. The triangle X‘XT denotes the posterior wedge to be removed. The osteotomy is then made in standard Southwick fashion and the osteotomy closed. C: AP view after osteotomy. D:
Figure 172.10. Clark modification of the Southwick intertrochanteric osteotomy. A: The transverse line is inscribed on the anterior and lateral surfaces of the femur at the level of the lesser trochanter (line X” – T). Then a point X is measured 15 mm proximal from the transverse line along the longitudinal orientation mark. The line from X” to X is then drawn, and the triangle X”XT denotes the anterior wedge to be removed. B: Next, the point X‘ is measured 13 mm posterior to the longitudinal mark at the level of the transverse mark. The line from X to X‘ is drawn. The triangle X‘XT denotes the posterior wedge to be removed. The osteotomy is then made in standard Southwick fashion and the osteotomy closed. C: AP view after osteotomy. D:
Lateral view after osteotomy. (From Clark CR, Southwick WO, Ogden JA.
Anatomic Aspects of Slipped Capital Femoral Epiphysis and Correction by
Biplane Osteotomy. Instr Course Lect 1980;29:90, with permission.) -
Then continue with the osteotomy in
standard Southwick fashion, first making the oblique osteotomy, then
the transverse cut, removing the wedge of bone, and then completing the
transverse cut. The bone wedge that is removed should be large enough
so that the upper oblique surface squarely fits on the lower transverse
surface. -
Close the osteotomy (Fig. 172.10C, Fig. 172.10D) and perform internal fixation as previously described.
-
Gently transfer the child from the bed to
the fracture table after anesthesia induction. The induction of
anesthesia removes the child’s muscle spasm and guarding. This often
results in a spontaneous, unintentional reduction of the slip with
simple positioning of the child on the fracture table. I do not employ
any intentional reduction maneuver unless the deformity is so severe
that adequate internal fixation is not possible because of inadequate
osseous contact between the epiphysis and metaphysis. -
Then proceed with cannulated screw
fixation in the usual fashion. Place the first screw just as you would
for a stable SCFE. The use of a second screw is controversial. Some
authors advocate a second screw to control rotation and increase
stability. Biomechanical studies do not show a two-fold increase in
strength, and any screw off center axis has a much higher chance of
joint penetration (38,41).
Therefore, if a second screw is used, place it inferior to the first
screw and with the final tip position at least 1 cm from the
subchondral bone to reduce the risk of intraarticular penetration.
will undertake their procedures in an acute-on-chronic SCFE as long as
the other operative indications are met. There is no difference in the
operative technique from that for a stable SCFE.
In children with underlying endocrine or metabolic disorders (e.g.,
renal failure), prophylactic fixation of the uninvolved hip should be
strongly considered (51,53). However, these types of SCFE are infrequent compared to the idiopathic SCFE.
with simultaneous presentation of bilaterality of 10% to 20%. Thus, if
all children with unilateral SCFE have the opposite hip
prophylactically fixed, 65% to 80% of these fixations will be
unnecessary. This high rate of unnecessary surgery makes it difficult
to recommend prophylactic fixation. There might be justification for
prophylactic fixation to prevent an asymptomatic SCFE when patient
follow-up is unreliable. The true incidence of asymptomatic SCFEs is
unknown, as is the potential for the development of degenerative
arthritis later in life. Also, it is not known if prophylactic fixation
of these asymptomatic SCFEs will prevent the development of
degenerative arthritis. Until this information is known, prophylactic
fixation in the idiopathic situation should be approached with caution.
Discharge is often the same evening for a morning surgery, or the next
day for an afternoon surgery. I recommend toe-touch weight bearing for
4–6 weeks; however, many children have no postoperative discomfort and
it is not uncommon to see them return for their first postoperative
visit 1–2 weeks later carrying their crutches! At the first
postoperative visit, check the incision and obtain radiographs to
ensure no change in fixation. The next visit is 4–6 weeks after
surgery; obtain radiographs then also. Obtain both AP and frog-lateral
radiographs of the pelvis so as to follow the opposite hip. After this
time, allow normal activities except for running, jumping, and contact
sports.
return visits occur every 3 or 4 months, with repeat AP and
frog-lateral radiographs of the pelvis, until complete physeal closure (50).
After physeal closure, all physical activities are allowed. Screw
removal is controversial. The morbidity and complications (incision,
operative time, blood loss, fracture risk) for screw removal are much
greater than for insertion.
epiphysiodesis surgery and keep the child non-weight-bearing until
physeal closure ensues. Full weight bearing is usually achieved by the
tenth postoperative week (70,76,91).
recommended 4 weeks of postoperative skin traction, with active range
of motion exercises starting on the first postoperative day. Hip
flexion to 90° should be achieved by 4 weeks. Then mobilize the patient
with crutches; do not allow full weight bearing until radiographic
union of the osteotomy, usually 2–3 months after surgery.
Permit full weight bearing when there is radiographic evidence of
osteotomy union, at an average of 5 months. Fish recommends pin
removal; allow full activity 2 months after pin removal.
recommend partial weight bearing with crutches starting in the eighth
postoperative week. Progression to full weight bearing varies according
to the patient’s weight, reliability, and generalized hip osteopenia.
partial weight bearing with crutches for 6 to 8 weeks. Full weight
bearing is allowed at 8 weeks. In the case of bilateral osteotomies,
permit weight bearing as tolerated.
bed rest with the limb in balanced suspension for 2 to 4 weeks. Keep
the hip at 30° flexion. On the second or third postoperative day, allow
the patient to sit at the bedside with support. Encourage mild active
flexion of the hip and knee; when this flexion is comfortable and the
wound is healed, allow non-weight-bearing with crutches.
patient to get out of bed on the first postoperative day and to walk
with crutches without bearing weight on the third postoperative day.
Start active range of motion exercises of the hip and knee on the
seventh postoperative day. Permit full weight bearing when there is
radiographic evidence of osteotomy union; physeal closure is not
necessary for full weight bearing.
bed rest for the first postoperative week. The limbs are held in
abduction and internal rotation with the aid of boots and bars. On the
tenth postoperative day, allow crutch walking with minimal weight
bearing. Allow full weight bearing after 6 months.
If there is any question regarding compliance or stability of fixation,
I recommend the use of a wheelchair for the first 6 weeks. Maintain
non-weight-bearing until early callus is seen at the slip. Once there
is evidence of early healing, allow gradual and progressive weight
bearing. This typically begins at 8–12 weeks. Progression to full
weight bearing is usually achieved by 3–4 months after fixation. These
children must be closely observed for the development of AVN; it will
usually occur within the first 12 months after the slip. The remainder
of the postoperative rehabilitation is no different from that of a
stable SCFE.
6–8 weeks. After cast removal, recommend non-weight-bearing until there
is physeal closure. Full weight bearing is usually achieved 10 weeks
after surgery (76,91,92).
AVN has not been reported in an untreated chronic stable SCFE. Its
iatrogenic occurrence may be due to either realignment procedures
(either closed reduction or proximal femoral osteotomy) or intraosseous
vascular injury from internal fixation. Fixation that posteriorly exits
the neck and reenters the epiphysis may damage
the posterior retinacular vessels (Fig. 172.11) (71).
Also, the superior weight-bearing quadrant of the femoral head is
supplied by an artery that can be potentially injured by fixation
devices (12). This may explain the high incidence of AVN when the fixation device is in this portion of the femoral head (Fig. 172.12). In the unstable SCFE, AVN is common and a result of the disease itself.
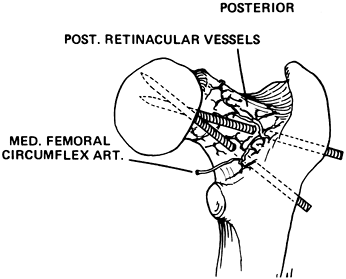 |
|
Figure 172.11.
Diagram illustrating the potential for vascular injury when internal fixation is placed posteriorly through the neck in an SCFE. (From Riley PM, Weiner DS, Gillespie R, Werner SD. Hazards of Internal Fixation in the Treatment of Slipped Capital Femoral Epiphysis. J Bone Joint Surg Am 1990;72:1500, with permission.) |
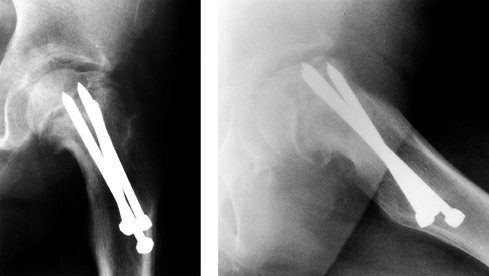 |
|
Figure 172.12.
The radiographs of a 12-year-old girl with avascular necrosis after pinning of a left SCFE. Note cluster of pins placed anteriorly. |
many patients do reasonably well for some time. Degenerative changes
gradually develop, but reconstructive surgery can usually be delayed
until adulthood (44). In one series of 24 hips
with AVN after an SCFE at 31 year follow-up, reconstructive surgery was
required in four hips during adolescence and in five during adulthood.
The remainder had not required reconstructive surgery but did show
degenerative changes on recent radiographs. AVN from an acute unstable
SCFE appears to be worse than that from a stable SCFE.
Treatment is difficult and there is no perfect solution. The first
goals are to maintain joint motion and, as much as possible, to prevent
further collapse. Relief from weight bearing is initially recommended.
Unfortunately, healing of the necrotic areas may require a prolonged
time, and most adolescents will not be compliant with prolonged
non-weight-bearing.
If the physis is still open, the epiphysis needs to be restabilized
with appropriately redirected internal fixation. Further progression of
a slip with concomitant AVN after hardware removal is a difficult
problem that should be avoided.
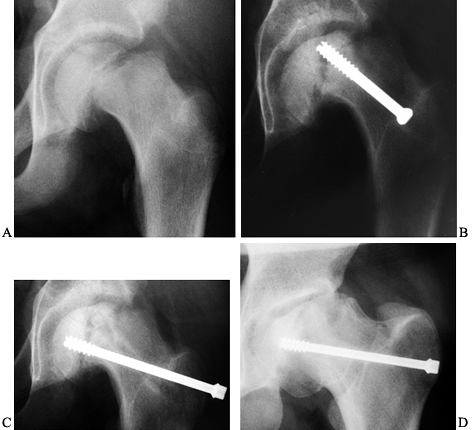 |
|
Figure 172.13. A: AP radiograph of the pelvis of a 14-year-old boy with an unstable left SCFE. B:
Four months after fixation, the onset of avascular necrosis is apparent, and there is a concern for intraarticular screw penetration. C: The screw was removed and redirected in a different position. D: The last follow-up, 4 years after the initial SCFE, demonstrates physeal closure, partial joint incongruity, and a healed necrotic segment. |
reasonably good if the AVN is segmental with no gross joint deformity
present. With more extensive involvement, poor motion and pain may
persist. In this case, there are multiple options to consider when
medical therapy fails [e.g., nonsteroidal anti-inflammatory drugs
(NSAIDs),
range-of-motion
exercises, activity modification]. If hip motion can be improved by
redirecting a noninvolved area of the femoral head to a more congruent
weight-bearing position, then proximal femoral osteotomy may be
considered. Another alternative is bone grafting the collapsed area to
improve joint congruity (42,75)
and to provide further containment by femoral and/or pelvic osteotomy.
Arthrodesis or total joint arthroplasty are considered if the hip is
not salvageable. It must be remembered that these patients are young
and heavy, which is a concern regarding the longevity of a total hip
arthroplasty.
Unlike AVN, chondrolysis can occur in the untreated stable SCFE. It may
be aggravated by persistent intraarticular fixation or with spica
casts. In the past, it was thought that black children and those of
Hawaiian ancestry were more susceptible to its development (58,85); recent reports question this view (4,40,80).
The exact etiology is unknown, although it may be an autoimmune process
aggravated by the persistent pin penetration and secondary mechanical
joint damage (63). Chondrolysis does not occur
in all joints with pin penetration; however, its incidence is higher as
the number of pins increases or they become closer to the subchondral
bone (10). Transient intraoperative pin penetration that is corrected at surgery does not increase the risk of chondrolysis (96).
diagnosis of the SCFE. Its clinical hallmark is severe loss of motion
and pain in relation to slip magnitude. It is radiographically defined
as a loss of more than 50% of the width of the weight-bearing portion
of the articular space in children with unilateral SCFE (Fig. 172.14),
or less than 3 mm width of the articular space in children with
bilateral SCFE. When the diagnosis is suspected but there is no plain
radiographic evidence, a technetium-99 methylene-diphosphonate bone
scan may be helpful (57). Marked periarticular
uptake and premature closure of the greater trochanteric physis are
highly predictive of future chondrolysis.
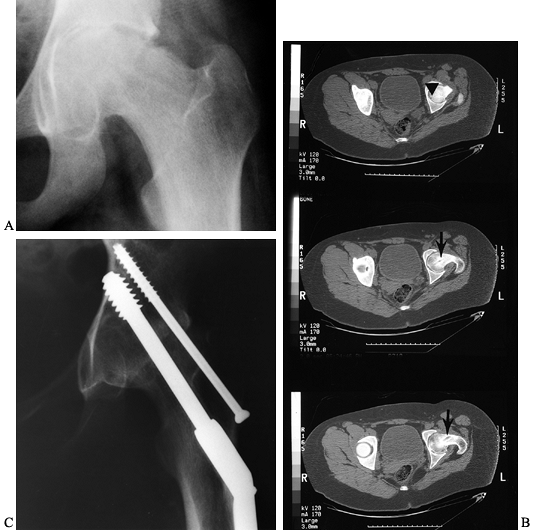 |
|
Figure 172.14. A: AP radiograph of the pelvis of a 13-year-old girl, 13 months after in situ
fixation of a left SCFE. The radiographs with the fixation present were not available, and the single screw had since been removed. B: CT scan demonstrates the old screw track (arrow), with the tip being extremely close to the cartilage surface (arrowhead). The hip was painful and in an unacceptable position due to a marked flexion contracture. Conservative therapy was not beneficial and an arthrodesis was performed at age 14 years. C: Last follow-up at age 16 years demonstrated no pain, excellent ambulation ability, and a solid arthrodesis. |
If there is, the fixation must be removed and repositioned if the
physis is still open. Occult sepsis must also be ruled out. The initial
treatment for chondrolysis is rest, NSAIDs, and maintenance of joint
motion by physiotherapy, traction, and protected weight bearing. In the
refractory case, aggressive capsulotomy has been advocated (73).
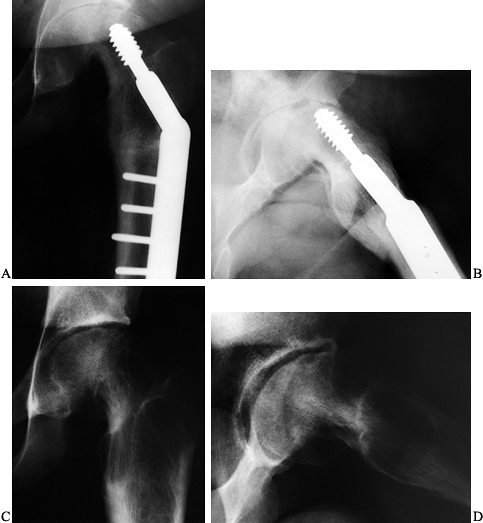 |
|
Figure 172.15. The AP (A) and lateral (B)
radiographs of a 15-year-old boy who had undergone an osteotomy for a left SCFE 5 months before. He presented with increasing pain and stiffness. Note the joint narrowing and proximity of the lag screw tip to the joint. A CT scan confirmed intraarticular penetration by the lag screw. The hardware was removed. At last follow-up at age 18, his pain was completely gone and joint motion had improved. Note partial reconstitution of the joint space on both AP (C) and lateral (D) radiographs. |
with at least partial reconstitution of the articular cartilage and
restoration of a clinically useful range of motion. In other cases,
spontaneous ankylosis may occur. If spontaneous ankylosis occurs in an
acceptable position, nothing further needs to be done. If spontaneous
ankylosis occurs in an unacceptable position, then a femoral osteotomy
below the ankylosis may be necessary to appropriately reposition the
lower extremity in space. A painful, malpositioned hip often requires a
formal hip arthrodesis (Fig. 172.14).
complications beside joint penetration, femoral fracture, nerve
palsy/injury, and nonunion or delayed union of an osteotomy or
epiphysiodesis (71).
cortical bone entry point, loosening of the screw due to a
“windshield-wiper effect” can occur (56). A false aneurysm has also been reported with retained prominent hardware (33).
Because of this, the screw head should not be more than 1.5 cm from the
cortical surface. Sciatic nerve injury and septic arthritis are rare
but described complications with internal fixation of SCFE.
smaller, multiple pins were used, especially when they entered the
femoral neck posteriorly and reentered the epiphysis (Fig. 172.16) (71).
It is less common with the single-cannulated screw and anterolateral
placement. The hardware may also strip or break during removal, making
complete removal impossible. This is much more common with titanium
cannulated screws; in the young child with SCFE and hard bone, no
titanium implants should be used (47,86).
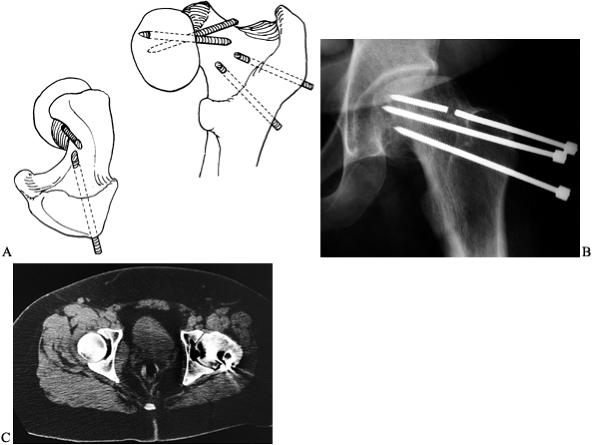 |
|
Figure 172.16. Broken pins in SCFE. A: Diagram showing how pins can break when they exit the femoral neck and then reenter the femoral head. B:
The AP radiograph of the pelvis of a 23-year-old man who had an SCFE treated as a teenager with multiple-threaded Steinmann pins. Note the broken pin. C: The CT scan demonstrates the pin exiting the femoral neck and then reentering the femoral head. (Part A from Riley PM, Weiner DS, Gillespie R, Werner SD. Hazards of Internal Fixation in the Treatment of Slipped Capital Femoral Epiphysis. J Bone Joint Surg Am 1990;72:1500, with permission.) |
intertrochanteric, or neck level. Holes after hardware removal act as a
stress riser and may lead to an intertrochanteric or subtrochanteric
fracture. Fracture may also occur immediately after internal fixation
if multiple starting points are made on the femur, even in the region
of the femoral neck (7).
This is not surprising when considering the low intertrochanteric
position of the osteotomy in these obese children. With bone graft
epiphysiodesis, there is a low but persistent incidence of
epiphysiodesis failure.
For the rare case in which a trap door or other bone grafting procedure
is needed for AVN, the reader is referred to the original manuscripts (42,75).
procedures peculiar to children with SCFE. The most important is their
young age and obesity. Union of an arthrodesis is more difficult
because of obesity; a postoperative hip spica cast is recommended, even
if rigid internal fixation is used. Arthrodesis after AVN is also more
difficult, because of the lack of blood supply. If possible, both an
intra- and an extraarticular arthrodesis should be performed.
makes arthroplasty more appealing. However, the risk of loosening and
need for revision arthroplasty is likely to be quite high in these
patients. Unfortunately, there are no published series specifically
addressing the outcomes of total hip arthroplasty as the treatment for
associated complications of SCFE.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
E, Garst J, Barmada R. Treatment of Moderate to Severe Slipped Capital
Femoral Epiphysis with Extracapsular Base of Neck Osteotomy. J Pediatr Orthop 1993;13:294.
R, Bruch RF, Gimbel JS, Ray RD. Base of the Neck Extracapsular
Osteotomy for Correction of Deformity in Slipped Capital Femoral
Epiphysis. Clin Orthop 1978;132:98.
TE, Richards BS, Haideri N, Smith C. Intermediate Follow-Up of a Simple
Method of Hip Arthrodesis in Adolescent Patients. J Pediatr Orthop 1996;16:30.
JS, Taylor B, Johnston CE II. Comparison of Single Pin vs Multiple Pin
Fixation in Treatment of Slipped Capital Femoral Epiphysis. J Pediatr Orthop 1992;12:384.
DM, Angel JC. Replacement of the Femoral Head by Open Operation in
Severe Adolescent Slipping of the Upper Femoral Epiphysis. J Bone Joint Surg Br 1978;60:394.
JR, Sundberg AB, Nolan DR, et al. Complications after Cuneiform
Osteotomy for Moderately or Severely Slipped Capital Femoral Epiphysis.
J Bone Joint Surg Am 1978;60:157.
WW, Johnson JT, Robertson WW Jr. Single Screw Fixation for Acute and
Acute-on-Chronic Slipped Capital Femoral Epiphysis. Clin Orthop 1996;322:86.
CH, Heyman CH, Bell DM. Treatment of Slipped Capital Femoral Epiphysis
by Epiphysiodesis and Osteoplasty of the Femoral Neck. A Report of
Further Experiences. J Bone Joint Surg Am 1963;45:999.
RN, Rosenzweig AH, Cotler HB, Schwentker EP. Epiphysiodesis in Slipped
Capital Femoral Epiphysis: A Comparison of Various Surgical Modalities.
J Pediatr Orthop 1985;5:661.
PE, Paterson DC, Foster BK, Lequesne GW. Classification in Slipped
Capital Femoral Epiphysis. Sonographic Assessment of Stability and
Remodeling. Clin Orthop 1993;294:196.
LA, Doane RM, Cornicelli SF, et al. Single versus Double Screw Fixation
for Treatment of Slipped Capital Femoral Epiphysis: A Biomechanical
Analysis. J Pediatr Orthop 1992;12:741.
JL, Keggi KJ, Soutwhick WO. The Incidence and Distribution of Slipped
Capital Femoral Epiphysis in Connecticut and Southwestern United
States. J Bone Joint Surg Am 1970;52:1203.
LJ, Doane RM, Karol LA, et al. Biomechanical Analysis of Single versus
Double Screw Fixation in Slipped Capital Femoral Epiphysis at
Physiological Load Levels. J Pediatr Orthop 1994;14:627.
TH, Canale ST, Beaty JC, et al. Long-term Follow-up of Patients with
Avascular Necrosis after Treatment of Slipped Capital Femoral
Epiphysis. J Pediatr Orthop 1993;13:154.
LM, Canale ST, Beaty JH, Warner WC. A Fluoroscopic Technique for
Determining the Incision Site for Percutaneous Fixation of Slipped
Capital Femoral Epiphysis. J Pediatr Orthop 1991;11:397.
RT, and 47 Coinvestigators from 33 Orthopaedic Centers and 6
Continents. The Demographics of Slipped Capital Femoral Epiphysis. Clin Orthop 1996;322:8.
GB, Bassett GS. Windshield-Wiper Loosening: A Complication of in Situ
Screw Fixation of Slipped Capital Femoral Epiphysis. J Pediatr Orthop 1993;13:607.
N, Weiner DS, Askew M. The Evolving Slope of the Proximal Femoral
Growth Plate Relationship to Slipped Capital Femoral Epiphysis. J Pediatr Orthop 1988;8:268.
K, Sakamaki T, Ishii Y. Follow-up Study of the Subcapital Wedge
Osteotomy for Severe Chronic Slipped Capital Femoral Epiphysis. J Pediatr Orthop 1989;9:412.
PM, Weiner DS, Gillespie R, Werner SD. Hazards of Internal Fixation in
the Treatment of Slipped Capital Femoral Epiphysis. J Bone Joint Surg Am 1990;72:1500.
EA, Robinson HJ Jr, O’Dowd TJ. Southwick Osteotomy for Severe Chronic
Slipped Capital Femoral Epiphysis: Results and Complications. J Bone Joint Surg Am 1980;62:561.
DB, Kasser JR, Sponseller P, Gelberman RH. Slipped Capital Femoral
Epiphysis: A Quantitative Analysis of Motion, Gait, and Femoral
Remodeling after in Situ Fixation. J Bone Joint Surg Am 1991;73:659.
WO. Compression Fixation after Biplane Intertrochanteric Osteotomy for
Slipped Capital Femoral Epiphysis. A Technical Improvement. J Bone Joint Surg Am 1973;55:1218.
EP, Clement DA, Colton CL. Open Reduction or Epiphysiodesis for Slipped
Upper Femoral Epiphysis. A Comparison of Dunn’s Operation and the
Heyman-Herndon Procedure. J Bone Joint Surg Br 1987;69:737.
DS, Weiner S, Melby A. Anterolateral Approach to the Hip for Bone Graft
Epiphysiodesis in the Treatment of Slipped Capital Femoral Epiphysis. J Pediatr Orthop 1988;8:349.
DS, Weiner S, Melby A, Hoyt WA Jr. A 30-Year Experience with Bone Graft
Epiphysiodesis in the Treatment of Slipped Capital Femoral
Epiphysiodesis. J Pediatr Orthop 1984;4:145.
LA, Schoenecker PL. Combined Valgus Derotation Osteotomy and Cervical
Osteoplasty for Severely Slipped Capital Femoral Epiphysis. Clin Orthop 1978;132:88.
LE. Transient Penetration of the Hip Joint During in Situ Cannulated
Screw Fixation of Slipped Capital Femoral Epiphysis. J Bone Joint Surg Am 1991;73:1054.