Distal Humerus Fractures
challenging injuries to manage. They are commonly multifragmented,
occur in osteopenic bone, and have complex anatomy with limited options
for internal fixation. Treatment outcomes are often associated with
elbow stiffness, weakness and pain. A painless, stable, and mobile
elbow joint is desired as it allows the hand to conduct the activities
of daily living, most notably personal hygiene and feeding. Therefore,
starting with a highly traumatized distal humerus and finishing with a
stable, mobile, and pain-free joint requires a systematic approach.
Thought is required in determining the operative indications, managing
the soft tissues, selecting a surgical approach, obtaining an anatomic
articular reduction, and creating a fixation construct that is rigid
enough to tolerate early range of motion.
of conservative management for distal humerus fractures and advocated
an aggressive approach that consisted of open reduction and internal
fixation.100 He described the
principles of osteosynthesis and believed anatomic restoration of
anatomy correlated with a better return to function. Unfortunately,
surgical outcomes in that era were plagued with a high risk of
infection and hardware failure. In 1937, Eastwood described the
technique of closed reduction under a general anesthetic and brief
immobilization in a collar and cuff.40
He reviewed 14 patients treated with this technique and reported that
12 returned to their original occupation. He stated “a perfect
anatomical reduction is not necessary in order to obtain a good
result.” Evans in 1953, termed this mode of treatment “bag of bones”
and believed that although it may be appropriate for the elderly
patient, it was not ideal for the young active patient.44
The conflict between operative and nonoperative management continued
for decades to follow. Riseborough and Radin, in 1969, reported that
operative treatment was unpredictable and often associated with poor
outcomes; therefore, they recommended
nonsurgical management.168
Similarly, Brown and Morgan in 1971 reported satisfactory results with
nonoperative management of 10 patients with distal humerus fractures.22 Their patients were managed with early active motion and at final follow-up had an average arc of motion of 98 degrees.
 |
|
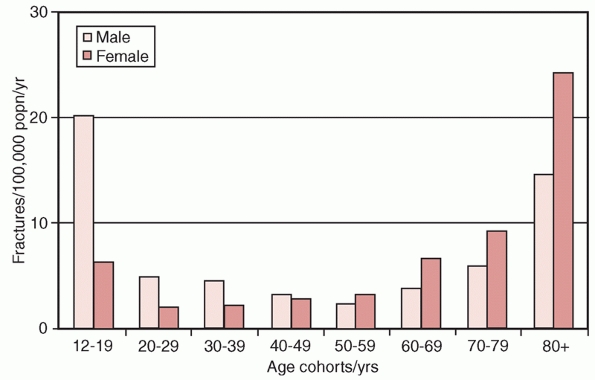
FIGURE 33-1
The age and gender related incidence of distal humerus fractures. (Data from Robinson CM, Hill RM, Jacobs N, et al. Adult distal humeral metaphyseal fractures: epidemiology and results of treatment. J Orthop Trauma 2003; 17(1):38-47.) |
reported with surgery for distal humerus fractures. The principles set
out by the Arbeitsgemeinschaft für Osteosynthesefragen (Association for
the Study of Internal Fixation, AO-ASIF) group, including anatomic
articular reduction and rigid internal fixation, allow for healing and
early postoperative range of motion.* The last decade has
seen advances in the understanding of elbow anatomy, improvements in
surgical approaches, new innovative fixation devices and an evolution
of postoperative rehabilitation protocols.
fixation of distal humerus fractures using modern fixation principles
is considered the gold standard. In elderly patients, restoration of
the anatomy and obtaining rigid internal fixation may be difficult
because of poor bone quality and comminution of the articular surface
and metaphysis. In cases in which rigid internal fixation cannot be
achieved to allow early range of motion, resultant prolonged
immobilization often leads to poor outcomes.4
Other complications associated with potentially poor outcomes include
malunion, nonunion, contracture, avascular necrosis, heterotopic
ossification, hardware failure, and symptomatic prominent hardware. In
the elderly patient, the prolonged rehabilitation, propensity for
stiffness, and increased reoperation rate associated with open
reduction and internal fixation may convert a previously independent
individual into a role of dependence.171
viable treatment option for elderly patients with articular
fragmentation, comminution, and osteopenic bone.** Most
recently, there has been a renewed interest in distal humerus
hemiarthroplasty for the treatment of distal humerus fractures,3,13,73,150 including fractures of the capitellum and trochlea.
with peak incidences occurring between the ages of 12 to 19 years,
usually in males, and those aged 80 years and older, characteristically
in females (Figure 33-1). In young adults, the
fractures are typically caused by high-energy injures, such as motor
vehicular collisions, falls from height, sports, industrial accidents,
and firearms. In contrast, greater than 60% of distal humerus fractures
in the elderly occur from low energy injuries, such as a fall from a
standing height.147,172
reviewed a consecutive series of 320 patients with distal humerus
fractures over a 10-year period. They calculated an overall incidence
in adults of 5.7 cases per 100,000 in the population per year with a
nearly equivalent male to female ratio. The most common mechanism of
injury was a simple fall from a standing height (Table 33-1)
and the most common fracture pattern was an extra-articular fracture
accounting for just under 40% of all fractures. Bicolumn or complete
intra-articular fractures were the second most common, accounting for
37%.
increasing, mimicking the increasing incidence in hip, proximal humerus
and wrist fractures.80,90,91 Palvanen et al.146
studied trends in osteoporotic distal humerus fractures in Finish
women. They reported a twofold increase in the age-adjusted incidence
of distal humerus fractures from 1970 (12/100,000) to 1995 (28/
100,000), and predicted an additional threefold increase by 2030. An
aging population with increasing life expectancy combined with the fact
that most of these fractures require surgical treatment is likely to
result in increased health care expenditures. The identification and
implementation of preventative strategies may help offset some of the
economic impact of this injury. The mainstay of current fracture
prevention strategy is to screen for osteopenia and osteoporosis with
bone mineral density measurements and then to treat with medication
therapy.80 Other authors argue that
a more important prevention strategy is to decrease the risk of
falling. Falling is the greatest single risk factor for fracture,90,91 and can be predicted based
on clinical risk factors, such as age, weight, smoking, previous fracture and mother’s hip fracture.19
|
TABLE 33-1 Mechanism of Injury in 320 Distal Humeral Fractures
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In general, 70% of patients that sustain an elbow fracture fall
directly on to the elbow because they are unable to break their fall
with an outstretched arm.147
High-energy injuries are the cause of most distal humerus fractures in
younger adults. Motor vehicle collisions, sports, falls from heights,
and industrial accidents predominate. These mechanisms are also
associated with a higher likelihood of accompanying injuries, such as,
open fractures, soft tissue injuries, other fractures in 16% of cases55 and polytrauma (Table 33-2).
the energy level, and the time since injury. In patients with
high-energy injuries, vigilance is required in identifying systemic
injures and associated fractures. The pain from polytrauma and other
concurrent issues such as inebriation and drug use may make
identification of all injuries difficult; patients and their families
should be pre-emptively counseled on the possibility of delayed
identification of occult injuries.
|
TABLE 33-2 The Relationship between Injury Mechanism and Soft-Tissue Injury
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
with distal humerus fractures, should be evaluated for the precipitants
of the characteristic fall, as they may have undiagnosed cardiac
arrhythmias, cerebrovascular disease, polypharmacy, or alcohol
dependence. Special attention is directed toward identifying
comorbidities and reversible illnesses that may impact upon the
treatment recommendations and perioperative risk. Mental status, the
ability to cooperate with rehabilitation, ambulatory status, and the
requirement of walking aides should be assessed. Additionally, the
preinjury functional abilities, demands, any limitations related to the
upper extremities as well as the patient handedness may each affect
treatment decision making.
all cases, particularly with high-energy trauma to identify systemic
injuries and associated fractures. The injured extremity should be
circumferentially examined for abrasions, bruising, swelling, fracture
blisters, skin tenting, and open wounds. Open distal humerus fractures
are not uncommon,118,172
and should be treated with a standard open fracture protocol involving
removal of gross contamination, covering of the wound with a sterile
dressing, splinting, antibiotics, tetanus, possible wound culture, and
early surgical irrigation and débridement.
documented preoperatively and postoperatively. Gofton et al. reported
that 26% of patients with distal humerus fractures had an associated
incomplete ulnar neuropathy at the time of presentation.55
Vascular injuries, although rare in distal humerus fractures, should be
assessed by examining the distal pulses, skin turgor, capillary refill,
and color. Pulse diminution or other positive findings should be
further examined with a brachial-brachial Doppler pressure index, which
has been shown to be as specific and sensitive as arteriography in
detecting brachial artery injuries.43,122,170 The normal brachial-brachial Doppler pressure index is approximately 0.95 and it rarely falls below 0.85.43,122,170 Patients with abnormal studies should be referred
for vascular surgery consultation. Patients with excessive pain after
high energy trauma should be examined for compartment syndrome of the
forearm. Compartment pressures should be conducted when the clinical
examination is inconclusive.20 If compartment syndrome is diagnosed clinically or by pressure measurement, urgent surgical fasciotomies are required.121
arthroplasty the contraindications must be addressed. Absolute
contraindications to elbow arthroplasty include active infection and
inadequate soft tissue coverage. The patient history requires probing
questions to rule out common infections, such as urinary tract
infections and active diabetic ulcers. Open wounds in low energy distal
humerus fractures are not an absolute contraindication to elbow
arthroplasty, as they are typically small and clean. Such wounds,
therefore, may undergo irrigation and débridement followed by staged
elbow arthroplasty.
elbow are usually sufficient for diagnosis, classification, and
surgical templating. However, initial radiographs obtained in plaster
or a splint may obscure the fracture pattern and should be repeated. In
some cases in which fracture shortening, rotation, and angulation
distort the images, gentle traction views with appropriate analgesia or
conscious sedation may improve the yield of the radiographs.
reconstructions greatly improves the identification and visualization
of fracture patterns. Although CT is not required for all cases, it is
recommended for certain situations. In patients in whom a less invasive
approach for open reduction and internal fixation is contemplated, such
as a paratricipital approach rather than an olecranon osteotomy, a CT
scan can assist with decision making and in identifying the locations
of fracture fragments intraoperatively. In elderly patients with highly
comminuted fractures, a CT scan may be useful in deciding whether an
attempt should be made at ORIF versus proceeding directly to
arthroplasty. When considering hemiarthroplasty for distal humerus
fractures, a CT will confirm the articular fragmentation and the
characteristics of the condylar fractures.
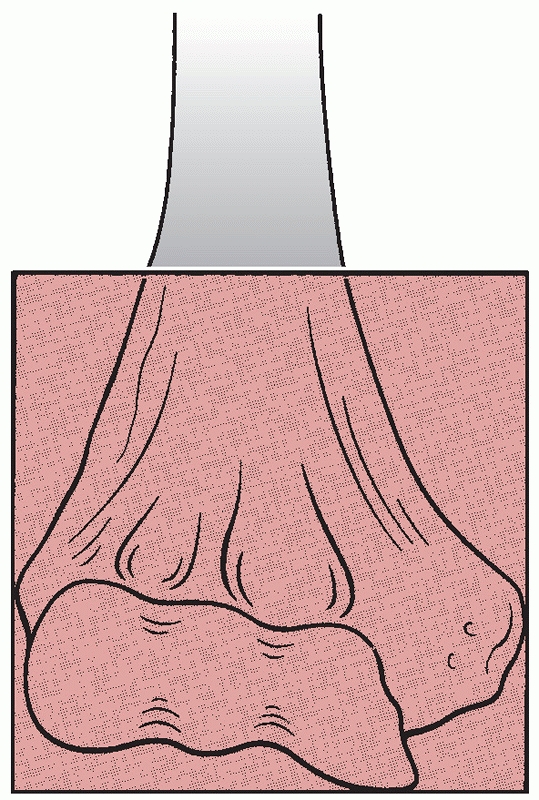
humerus were based on the anatomic location of the fracture and its
appearance, using terms such as supracondylar, intracondylar,
epicondylar, Y-type and T-type. In 1990, Müller defined the anatomic
boundaries of a distal humerus fracture as one with an epicenter that
occurs within a square whose base is the distance between the medial
and lateral epicondyles on an anteroposterior radiograph (Figure 33-2).134
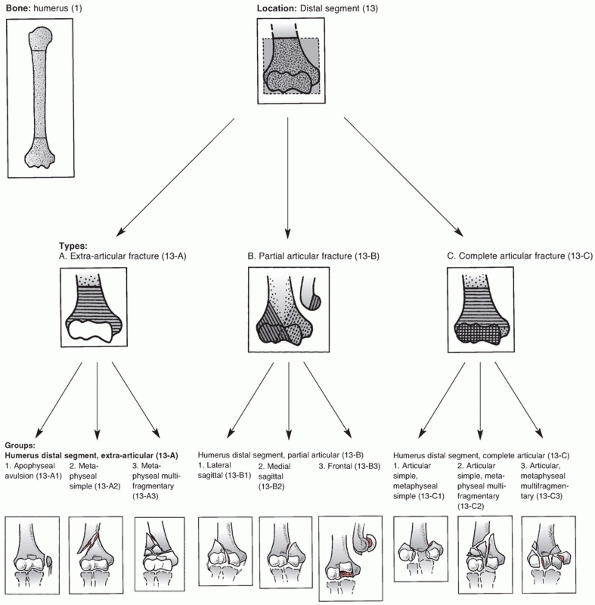
The AO group devised the first comprehensive classification of distal
humerus fractures, which was then adopted by the Orthopaedic Trauma
Association (OTA) in 1996.1 In 2007,
the AO Classification Supervisory Committee and the OTA Classification,
Database and Outcomes Committee updated the compendium to its present
form.110
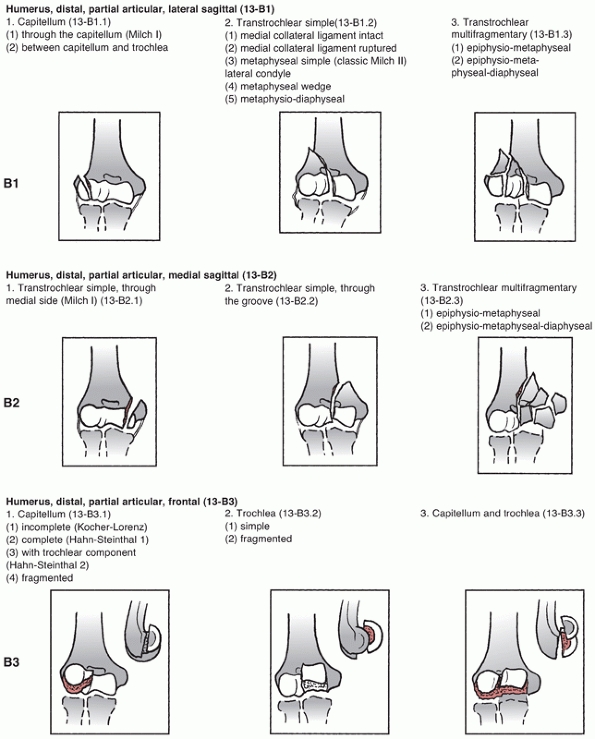
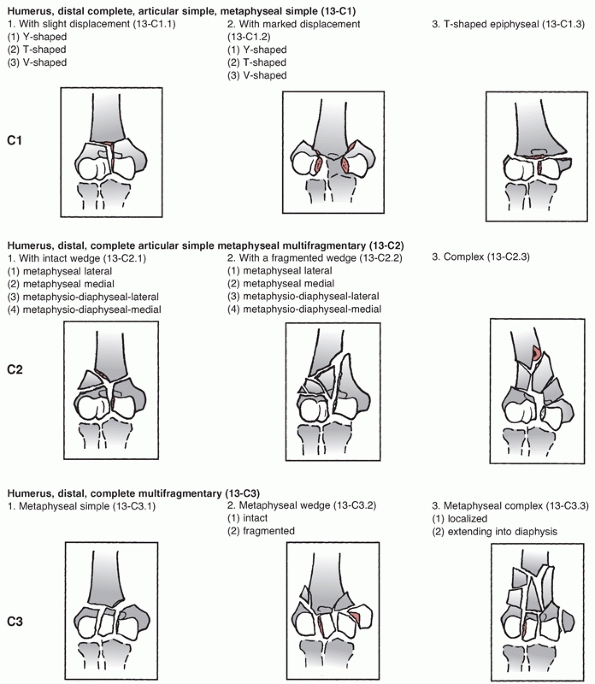
assigns the first two digits of 13 to distal humerus fractures and
classifies them based on location and degree of articular involvement (Figure 33-3).
The system then further subclassifies fractures based on fracture line
orientation, displacement direction and degree of fragmentation.110
Type A fractures are extra-articular and may involve the epicondyles or
occur at the distal humerus metaphyseal level. Although these fractures
receive less attention in the literature than the more complex
intra-articular type C fractures, they do account for one fourth of all
distal humerus fractures.172
 |
|
FIGURE 33-2
A distal humerus fracture is defined as a fracture with an epicenter that is located within a square whose base is the distance between the epicondyles on an anteroposterior radiograph. |
remains some continuity between the humeral shaft and the articular
segment. Type B fractures include unicondylar fractures and sagittal
plane or shear fractures of the articular surface involving the
capitellum, trochlea, or both.
lateral column, are intra-articular, and account for approximately 15%
of all distal humerus fractures.85,98,172 These fractures may also be classified by the Milch system,124
which is based on whether the lateral portion of the trochlea remains
attached to the humeral shaft. In a Milch type I fracture, the medial
or lateral column can be fractured, but the lateral eminence of the
trochlea remains attached to the humeral shaft. In a Milch type II
fracture, the lateral eminence of the trochlea is apart of the column
fracture.
described that occurs predominantly in younger patients who are
predisposed to this injury because of a septal aperture (fenestration)
in the olecranon fossa.54,98
This fracture pattern is theorized to occur after an axial load is
applied to the olecranon, which is then driven into the trochlea. A
fracture occurs that splits the trochlea and propagates proximally
between the columns to eventually exit either medially or laterally
creating a “high” single column fracture.
there is no continuity between the articular segments and the humeral
shaft. Type C fractures have historically been called intracondylar
fractures and the AO/OTA system further subclassifies them into simple
(C1), simple articular with metaphyseal fragmentation (C2), and
fragmentation of the articular surface and metaphyseal zone (C3). This
system is widely used in the literature and trauma databases, and helps
to standardize research protocols and treatment outcomes.
Unfortunately, the classification system does have weaknesses as it
does not account for factors such as the distal fragment height and
amount of displacement, both of which may influence treatment.67,164
The classification also does little to assist with the decisionmaking
process between ORIF and arthroplasty, and finally it has been
criticized as being overly complex.
 |
|
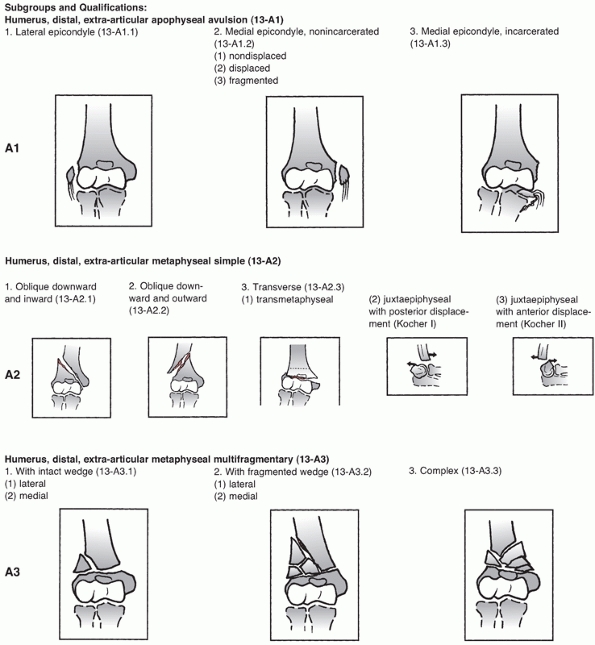
FIGURE 33-3
The AO/OTA Classification of Distal Humerus Fractures. (Redrawn from Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database, and outcomes committee. J Orthop Trauma 2007;21(10 Suppl):S1-133, with permission.) (continues) |
 |
|
FIGURE 33-3 (continued)
|
 |
|
FIGURE 33-3 (continued)
|
 |
|
FIGURE 33-3 (continued)
|
 |
|
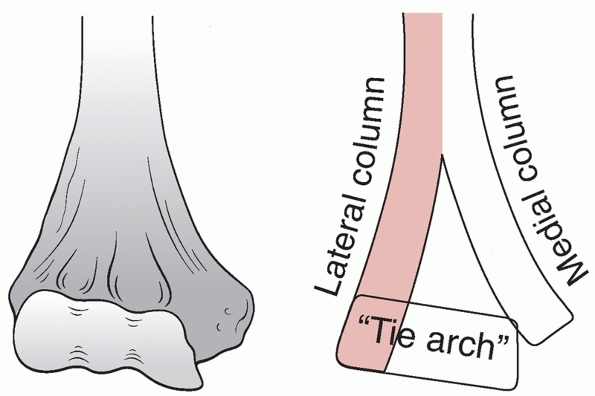
FIGURE 33-4
The medial and lateral columns support the articular segment. The distal most part of the lateral column is the capitellum and the distalmost part of the medial column is the nonarticular medial epicondyle. The trochlea is the medial part of the articular segment and is intermediate in position between the capitellum and medial epicondyle. The articular segment functions architecturally as a tie arch. |
which is similar to the AO concept of condyles. The classification has
three main categories: intra-articular, extra-articular intracapsular,
and extracapsular. The intra-articular group is further subdivided into
bicolumn, single column, and articular fractures. The extra-articular
intracapsular group consists of high and low transcolumn fractures and
the extracapsular group has medial and lateral epicondyle fractures (Figure 33-5).
This classification system has the same criticisms as the AO/OTA system
with high complexity and poor intra-rater and inter-rater reliability.
The classification also does not consider the specific types of
articular fracture and the degree of fragment displacement. It is the
author’s opinion that the AO/OTA classification is preferred because it
is more intuitive, it is ubiquitous and because it is the official
classification of the OTA.
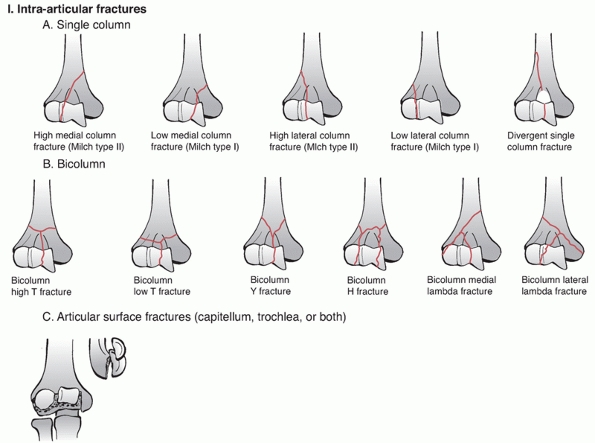 |
|
FIGURE 33-5 The Mehne and Matta classification of distal humerus fractures. (continues)
|
meaning that it has trochoid (rotatory) motion through the
radiocapitellar and proximal radioulnar joints and ginglymoid
(hinge-like) motion through the ulnohumeral joint. An understanding of
the complex bony anatomy of the elbow, soft tissue stabilizers, and
adjacent neurovascular structures is imperative when surgically treating distal humerus fractures.
 |
|
FIGURE 33-5 (continued)
|
section, with its apex directed anterior. As the shaft approaches the
distal humerus it bifurcates into two divergent cortical columns,
termed the medial and lateral columns. The medial column diverges
approximately 45 degrees from the humeral shaft in the coronal plane
and terminates as the medial epicondyle. The lateral column, in the
coronal plane, diverges at approximately 20 degrees from the shaft and
as it extends distally it curves anteriorly creating approximately a
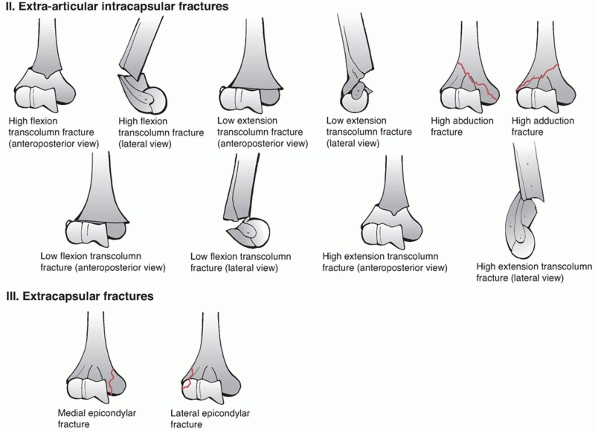
35- to 40-degree angle with the shaft in the sagittal plane (Figure 33-6).
In the coronal plane, the trochlea is more distal than the capitellum
resulting in a valgus alignment of 4 to 8 degrees. Overall, when
including the ulna, the elbow has a valgus angle in extension of 10 to
17 degrees, termed the carrying angle. Axially, the distal humerus
articular surface is internally rotated 3 to 8 degrees; therefore, as
the elbow flexes it also internally rotates resulting in slight varus
alignment.
flat and wide, well suited for application of a posterolateral plate.
The lateral column terminates in the capitellum anteriorly. The
articular surface of the capitellum starts at the most distal aspect of
the lateral column and encompasses an arc of approximately 180 degrees
in the sagittal plane. Posterior fixation can be applied distally on
the lateral column because of the absence of cartilage; however,
lengths of screws directed anteriorly into the capitellum must be
carefully scrutinized to prevent perforation into the radiocapitellar
joint.
intervening segment of bone between the terminal ends of the medial and
lateral columns that articulates with the greater sigmoid notch of the
ulna. It is covered by articular cartilage anteriorly, inferiorly and
posteriorly, creating an arc of almost 270 degrees. The trochlea is
shaped like a spool with a central sulcus, which articulates with the
central ridge of the greater sigmoid notch of the proximal ulna.
lateral columns lies the olecranon fossa posteriorly and the coronoid
fossa anteriorly. These fossae lie adjacent to each other and are
separated by a thin bony septum. Occasionally this septum is absent and
a septal aperture exists. The olecranon fossa is matched to the
olecranon and accepts it during extension; similarly, the coronoid
fossa is matched to the coronoid and accepts it during flexion. The
tolerances of the fossae to accommodate their respective bony processes
are narrow; therefore, screw placement through the fossae should be
avoided as it may lead to impingement and decreased elbow range of
motion. In distal humerus fractures with excessive metaphyseal
comminution requiring supracondylar shortening, recreation of the
fossae with a burr will improve range of motion.
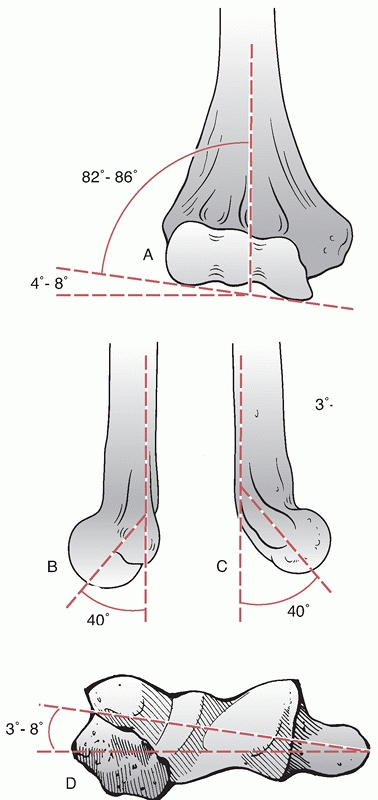
important soft tissue structures that require consideration when
treating distal humerus fractures. The lateral collateral ligament
(LCL)
complex consists of the radial collateral ligament, the lateral ulnar
collateral ligament and the annular ligament. The annular ligament
attaches to the anterior and posterior margins of the lesser sigmoid
notch, whereas the radial collateral ligament originates from an
isometric point on the lateral epicondyle and fans out to attach to the
annular ligament (Figure 33-7).
The lateral ulnar collateral ligament also arises from the isometric
point on the lateral epicondyle and attaches to the crista supinatoris
of the proximal ulna. The LCL complex functions as an important
restraint to varus and posterolateral rotatory instability.39,78
The LCL complex is vulnerable to injury during application of a direct
lateral plate; therefore, exposure of the lateral aspect of the distal
lateral column should not extend past the equator of the capitellum.
 |
|
FIGURE 33-6 The distal humerus articular surface is aligned in 4 to 8 degrees of valgus relative to the shaft (A)
and is angulated 35 to 40 degrees anteriorly in the sagittal plane. The medial epicondyle is the termination of the medial column and remains on the axis of the shaft in the sagittal view (B), whereas the lateral epicondyle follows the capitellum into flexion (C). Axially, the entire distal humerus articular surface is internally rotated 3 to 8 degrees (D). |
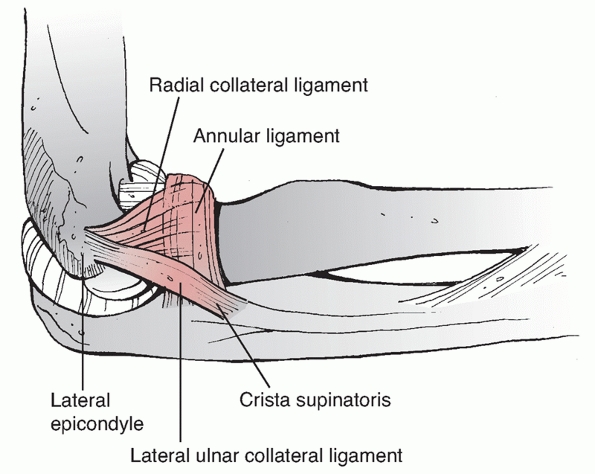
anterior bundle, posterior bundle and transverse ligament. The anterior
bundle is of prime importance in elbow stability (Figure 33-8).
It originates from the anteroinferior aspect of the medial epicondyle,
inferior to the axis of rotation, and inserts on the sublime tubercle
of the coronoid. The MCL functions as an important restraint to valgus
and posteromedial rotatory instability.9,139
It is susceptible to injury at its origin during placement of a medial
plate that curves around the medial epicondyle to lie on the ulnar
aspect of the trochlea.
 |
|
FIGURE 33-7
The lateral collateral ligament complex is an important restraint to varus and posterolateral rotatory instability and consists of the radial collateral ligament, the lateral ulnar collateral ligament, and the annular ligament. The annular ligament attaches to the anterior and posterior margins of the lesser sigmoid notch, whereas the radial collateral ligament originates from an isometric point on the lateral epicondyle and fans out to attach to the annular ligament. The lateral ulnar collateral ligament also arises from the isometric point on the lateral epicondyle and attaches to the crista supinatoris of the proximal ulna. |
knowledge of their precise locations is required to safely manage
distal humerus fractures (Figure 33-9). The
ulnar nerve pierces the medial intermuscular septum in the middle third
of the arm to travel along side the medial head of triceps. The arcade
of Struthers, a musculofascial band present in 70% of the population,186
is a potential area of nerve compression located approximately 8 cm
proximal to the medial epicondyle. As the nerve approaches the elbow it
travels behind the medial epicondyle to enter the cubital tunnel, a
fibro-osseous groove bordered by the medial epicondyle superiorly,
olecranon laterally and Osborne’s ligament medially. When the nerve
exits the cubital tunnel it travels between the two heads of the flexor
carpi ulnaris muscle.
 |
|
FIGURE 33-8
The medial collateral ligament functions as an important restraint to valgus and posteromedial rotatory instability. It consists of an anterior bundle, posterior bundle, and transverse ligament. The anterior bundle is of prime importance in elbow stability and it originates from the anteroinferior aspect of the medial epicondyle, and inserts on the sublime tubercle of the coronoid. |
 |
|
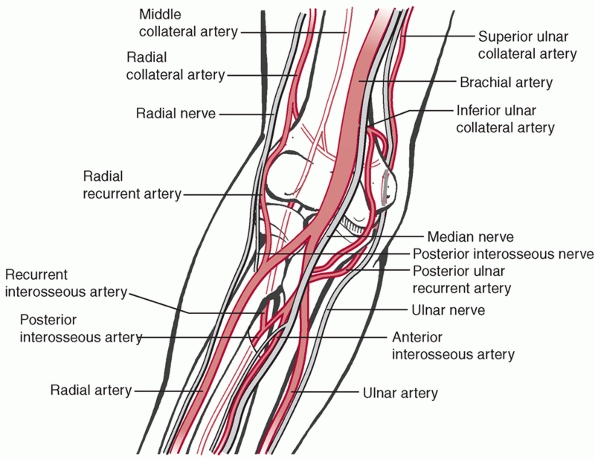
FIGURE 33-9 Three peripheral nerves, the median, ulnar, and radial, cross the elbow joint along with a robust collateral blood supply.
|
the mid-humeral shaft in the spiral groove. On average, the nerve
enters the spiral groove 20 cm proximal to the medial epicondyle (74%
of the length of the humerus) and exits approximately 14 cm proximal to
the lateral epicondyle (51% of the length of the humerus).53
Along the lateral aspect of the humerus, two branches come off the
nerve (nerve to the medial head of triceps and anconeus, and the
lateral brachial cutaneous nerve) before it pierces the lateral
intermuscular septum approximately 10 cm (36% of the length of the
humerus) proximal to the lateral epicondyle.53
The nerve then lies between brachialis and brachioradialis, where it
bifurcates to the posterior interosseous nerve and the radial sensory
nerve. The radial nerve is vulnerable to injury during exposure of
distal humerus fractures with proximal shaft extension and during
application of long posterolateral or direct lateral plates.
between the biceps and brachialis muscles in the anteromedial aspect of
the arm. The nerve passes under the bicipital aponeurosis to enter the
medial antecubital fossa, medial to the biceps tendon and brachial
artery. The nerve then passes between the heads of pronator teres.
During fixation of distal humerus fractures, the median nerve is
relatively protected from direct injury by the robust brachialis muscle.
which can be organized into three vascular arcades: medial, lateral,
and posterior.207 The lateral arcade
is formed by the interosseous recurrent, radial recurrent, and radial
collateral arteries and supplies the capitellum, radial head, lateral
epicondyle, and lateral aspect of the trochlea. The medial arcade is
formed by the superior and inferior ulnar collaterals and the anterior
and posterior ulnar recurrent arteries and supplies the medial
epicondyle and the medial aspect of the trochlea. The posterior arcade
is formed by the medial collateral artery and contributions from the
medial and lateral arcades and supplies the olecranon fossa and
supracondylar area.
exposure and fixation of distal humerus fractures. They can be
classified based on direction; posterior, lateral, medial, and
anterior, and then further subclassified based on their specific
anatomic intervals (Table 33-3). The ideal
approach to a specific fracture pattern should provide sufficient
exposure to allow anatomic reconstruction of the fracture and the
application of the required internal fixation with minimal soft tissue
or bony disruption to allow early mobilization. The selection of a
surgical approach depends on multiple factors, including facture
pattern, extent of articular involvement, associated soft tissue
injury, rehabilitation protocols, and surgeon preference.153
lateral, medial, or anterior depending on the surgical approach
selected. Most posterior approaches benefit from a posterior
longitudinal skin incision, which involves the elevation of
full-thickness fasciocutaneous medial and lateral flaps.36
The posterior skin incision can be straight or curve around the
olecranon, medially or laterally, depending on surgeon preference. It
is the author preference to conduct a relatively straight posterior
skin incision that curves gently around the medial aspect of the
olecranon (Figure 33-10A). The lateral
approaches can be accessed via a direct lateral skin incision or by a
posterior longitudinal skin incision with elevation of a lateral
fasciocutaneous flap. Similarly, the medial approaches can be accessed
by a direct medial skin incision or a posterior longitudinal skin
incision with elevation of a medial fasciocutaneous flap. There are
several advantages to a direct midline posterior longitudinal skin
incision, including access to both medial and lateral deep approaches
and a decreased risk of cutaneous nerve injury.36
The disadvantage of selecting a posterior longitudinal skin incision
for isolated medial or lateral approaches is the increased risk of flap
complications such as seromas and rarely necrosis.
|
TABLE 33-3 Surgical Approaches to the Distal Humerus
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
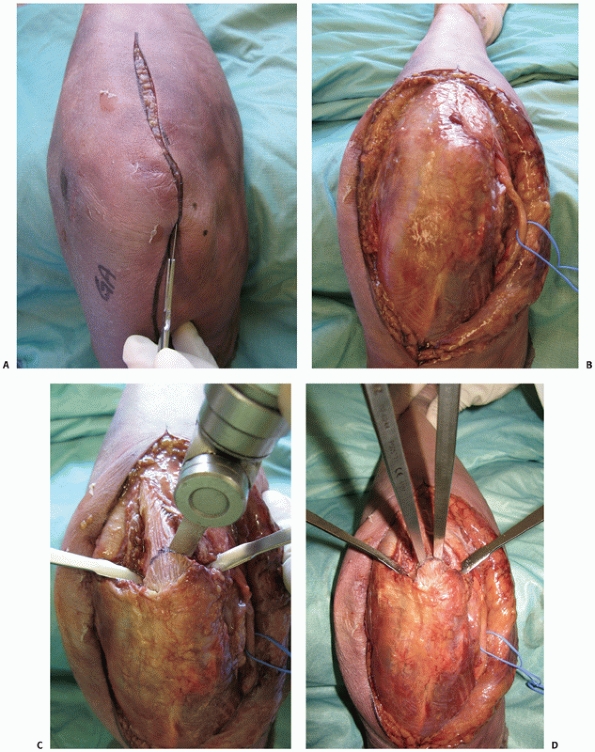 |
|
FIGURE 33-10 An olecranon osteotomy is approached via a longitudinal posterior skin incision (A). The ulnar nerve is exposed and may be prepared for anterior subcutaneous transposition (B).
The subcutaneous border of the proximal ulna is exposed and the nonarticular portion of the greater sigmoid notch (the bare area) between the olecranon articular facet and the coronoid articular facet is clearly identified. This is accomplished by dissection along the medial and lateral sides of the olecranon to enter into the ulnohumeral joint. Medial and lateral retractors are then placed into the ulnohumeral joint and an apex distal chevron osteotomy entering into the bare area is marked on the subcutaneous border of the ulna. A microsagittal saw is used to complete two-thirds of the osteotomy (C) and two osteotomes, placed into each arm of the chevron, apply controlled leverage to fracture the remaining third (D). (continues) |
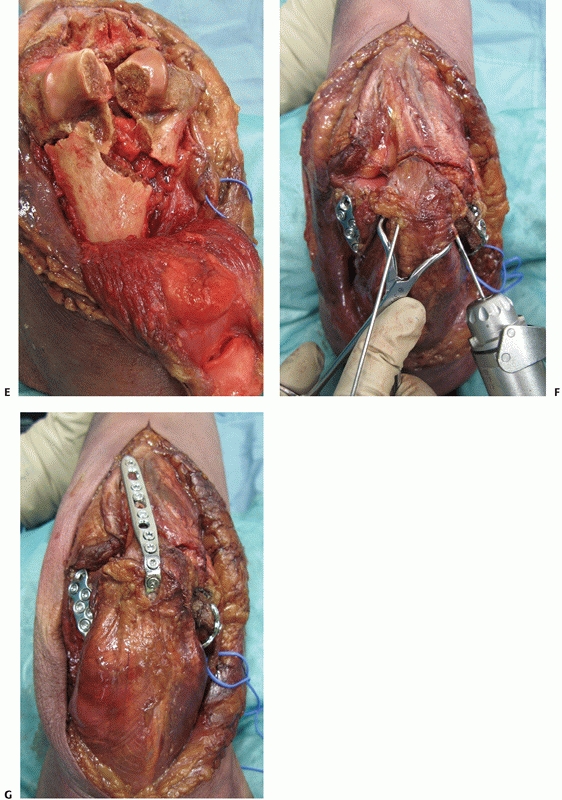 |
|
FIGURE 33-10 (continued)
Once conducted, the olecranon fragment along with the triceps tendon and musculature can be bluntly dissected off the posterior aspect of the distal humerus (E). At the completion of the case, provisional fixation of the olecranon fragment is done with crossing K-wires (F) followed by definitive compression plating (G). |
classified into five general types: olecranon osteotomy, paratricipital
(triceps-on), triceps splitting, triceps reflecting, and triceps
dividing. The selection of a particular type of posterior approach
depends on several factors, including: the degree of articular
visualization required for anatomic reduction and internal fixation;
the appropriateness of primary arthroplasty; patient factors
(elderly,
low demand); fracture characteristics (articular comminution); any
associated injuries (i.e., triceps laceration or olecranon fracture)
that may make one approach more favorable.
When compared with other posterior approaches, osteotomy of the
olecranon provides the best visualization of the distal humerus
articular surface,204 which is its
main advantage. The main disadvantages of the approach are the
complications associated with an osteotomy, including nonunion,
malunion, and hardware irritation. Olecranon osteotomies are most
commonly used for AO/OTA type C fractures, which require superior
visualization of the articular fragments for anatomic reduction and
internal fixation. An osteotomy can also be used for partial articular
fractures (AO/OTA type B), especially if they are comminuted. Relative
contraindications to an osteotomy are very anterior articular fractures
(AO/OTA type B3), which can be difficult to visualize through an
osteotomy and if a total elbow arthroplasty is planned as it may lead
to problems with implant stability and osteotomy healing and fixation.
requires identification and protection to avoid iatrogenic nerve injury
during fracture manipulation and fixation (Figure 33-10B).
It remains unclear whether the ulnar nerve should be transposed or
replaced in the cubital tunnel at the conclusion of the procedure.
exposed, the nonarticular portion of the greater sigmoid notch (the
“bare area”) between the olecranon articular facet and the coronoid
articular facet should be clearly identified. This is done by
subperiosteally dissecting along the medial and lateral sides of the
olecranon to enter into the ulnohumeral joint. Dissection should not
proceed distally as it places the collateral ligament insertions at
risk. Medial and lateral retractors are then placed into the
ulnohumeral joint to protect the soft tissues and to allow direct
visualization of the “bare area.” An apex distal chevron osteotomy
entering into the bare area is then marked on the subcutaneous border
of the ulna (Figure 33-10C). A microsagittal
saw is used to complete two thirds of the osteotomy. To avoid
unpredictable propagation of the osteotomy, multiple perforations are
carefully created through the remaining third using a Kirchner wire
(K-wire). Two osteotomes, placed into each arm of the chevron, apply
controlled leverage of the olecranon fragment causing fracture of the
remaining third (Figure 33-10D). The fractured
surface of the olecranon improves fragment interdigitation and
facilitates anatomic reduction and stability during the repair. A
chevron-shaped osteotomy provides rotation stability, increased surface
area for healing, and protects the collateral ligament insertions.151 A transverse olecranon osteotomy is also an option as it is technically simpler and can be performed more rapidly.49,70
Following the osteotomy, the olecranon fragment along with the triceps
tendon and musculature can be bluntly dissected off the posterior
aspect of the distal humerus (Figure 33-10E). Typically, the anconeus muscle must be divided to reflect the triceps posteriorly which causes its denervation.138
Anconeus muscle denervation can be avoided by reflecting the anconeus
muscle posteriorly along with the olecranon fragment and triceps.15 Once the osteotomy (Figure 33-10F,G) is conducted, flexion of the elbow is used to maximize visualization of distal humerus articular surface.
When using this method the plate is prefixed to the olecranon and then
removed before conducting the osteotomy. This facilitates osteotomy
reduction at the completion of the operative procedure. A 6.5- or
7.3-mm intramedullary compression screw may also be used for osteotomy
fixation; however, care should be taken during screw insertion as
malreduction is possible when the distal screw threads deflect into the
normal varus bow of the ulna.57
(bilaterotricipital, triceps sparing, or triceps-on) approach was first
reported by Alonso-Llames in 1972 for the management of pediatric
supracondylar fractures.7 The
approach involves the creation of surgical windows along the medial and
lateral sides of the triceps muscle and tendon without disrupting its
insertion on the olecranon.179
incision and mobilization of the ulnar nerve. Along the medial side of
the triceps, the interval between the triceps muscle and the medial
intermuscular septum is developed (Figure 33-11A) and the triceps is elevated off the posterior aspect of the humerus (Figure 33-11B).
Laterally, the triceps is elevated off the lateral intermuscular septum
and the posterior humerus in conjunction with the anconeus muscle (Figure 33-11).7,179
Distally, the paratricipital approach allows visualization of the
medial and lateral columns, the olecranon fossa, and the posterior
aspect of the trochlea. A modification of the paratricipital approach
involves the creation of a third surgical window in Boyd’s interval
between the anconeus and lateral olecranon.13 The third surgical window allows improved visualization of the distal humerus articular surface.
including, avoidance of an olecranon osteotomy, therefore the risks of
nonunion and symptomatic olecranon hardware are avoided. Additionally,
the triceps tendon insertion is not disrupted, allowing early active
range of motion. This approach also preserves the innervation and blood
supply of the anconeus muscle,179
which provides dynamic posterolateral stability to the elbow. Finally,
if further articular exposure is required, the paratricipital approach
can be converted into an olecranon osteotomy. If further proximal
exposure is required for associated fractures of the humeral shaft, the
lateral side of the paratricipital approach can be converted into the
Gerwin et al.53 approach. This
approach involves reflection of the triceps muscle unit from lateral to
medial to expose 95% of the posterior humeral shaft and the radial
nerve.
limited visualization of the articular surface of the distal humerus;
therefore, the approach is usually inadequate for fixation of type C3
fractures. The several advantages of this approach certainly indicate
its use for AO/OTA types A2, A3, B1, B2, and possibly C1 and C2
fractures.111,154,179
which the intent is to proceed directly to total elbow arthroplasty,
the paratricipital approach is preferred because it avoids the problems
associated with osteotomies and extensor mechanism healing in triceps
detaching approaches. The approach is also useful in cases in which an
initial attempt at ORIF is planned and there is a possibility of an
intraoperative convertion to total elbow arthroplasty should fixation
be deemed unsuccessful.
Triceps
Splitting Approach. The triceps splitting approach described by
Campbell involves a midline split through the triceps tendon.25 The medial and lateral columns are exposed with subperiosteal dissection starting from the midline and moving outwards (Figure 33-12).
Visualization of the articular surface of the distal humerus is
challenging and can be improved by partial excision of the olecranon
tip and flexion of the elbow. This approach can be extended proximally
to the level of the radial nerve as it crosses the humeral shaft in the
spiral groove. To expand the approach distally, the split can be
extended through the triceps insertion to the subcutaneous border of
the ulna. The triceps insertion is split midline, with release of
Sharpey fibers creating medial and lateral fasciotendinous sleeves. At
the conclusion of the procedure, the triceps tendon is repaired to the
olecranon via transosseous nonabsorbable braided sutures.
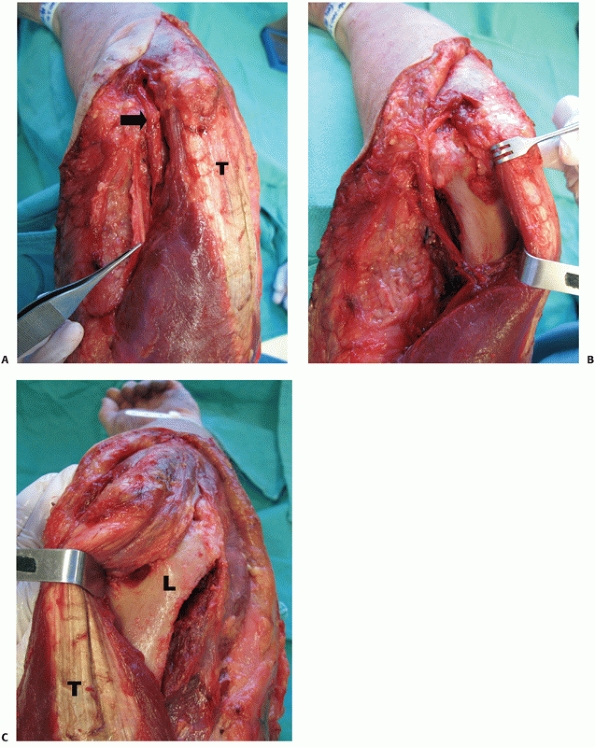 |
|
FIGURE 33-11 The paratricipital approach7 is done through a longitudinal posterior skin incision. Medially (A), the ulnar nerve (black arrow)
is identified. The medial intermuscular septum (forceps) is excised and the triceps muscle is elevated off the posterior aspect of the distal humerus (B). Laterally, the triceps muscle is elevated off the posterolateral aspect of the distal humerus allowing exposure of the lateral column, olecranon fossa and posterior aspect of the trochlea (C). (L, lateral column; T, triceps.) |
relative technical ease and the ability to convert from open reduction
and internal fixation to total elbow arthroplasty with few
consequences. The disadvantages of the approach include limited
visibility of the articular surface, disruption of the extensor
mechanism requiring postoperative protection and the risk of triceps
dehiscence. In order to improve triceps healing, Gschwend et al.59 modified the approach to incorporate a flake
of olecranon bone. McKee et al.118
compared the extensor mechanism strength of patients treated with an
olecranon osteotomy versus a triceps splitting approach and found no
statistical significant difference, concluding that both approaches are
effective.
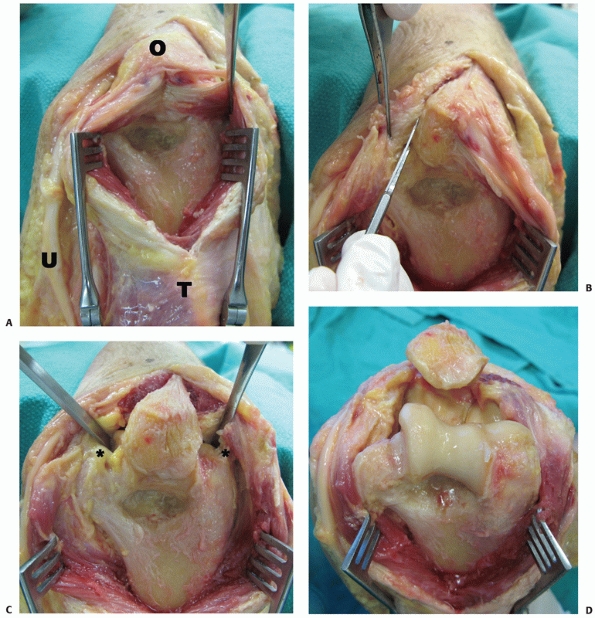 |
|
FIGURE 33-12 The triceps split approach described by Campbell25 involves a midline split through the triceps tendon and medial head (A).
The approach can be extended distally by splitting the triceps insertion on the olecranon and raising medial and lateral full-thickness fasciotendinous flaps (B,C). To gain further exposure of the posterior trochlea, the elbow is flexed and the olecranon tip may be excised. For ORIF, the medial and lateral collateral ligaments are preserved (asterisk); however, to obtain further exposure for TEA, they may be released (D). (O, olecranon; T, triceps; U, ulnar nerve.) |
is commonly used for total elbow arthroplasty. The approach can be used
for ORIF of distal humerus fractures; however, exposure of the lateral
column for the application of fixation is limited. The approach has
been termed “triceps-sparing” which has led to confusion. The approach
does not “spare” the triceps, but rather detaches the triceps tendon in
continuity with the ulnar periosteum and anconeus creating a large
reflection or sleeve.
then the triceps insertion and the ulnar periosteum are sharply
reflected off the proximal ulna in a medial to lateral direction (Figure 33-13). The sleeve of tissue created incorporates the anconeus muscle. As with the triceps splitting approach, careful
and solid repair of the triceps tendon is required via transosseous
sutures. This approach is best suited for unrepairable distal humerus
fractures in which primary elbow arthroplasty is planned.
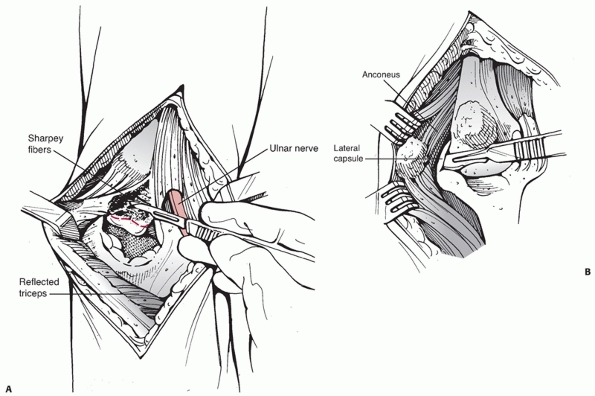 |
|
FIGURE 33-13 The Bryan-Morrey23
approach is commonly used for total elbow arthroplasty. A posterior longitudinal skin incision is used and the ulnar nerve is identified and protected. The ulnar periosteum, triceps insertion and anconeus muscle are sharply reflected off the proximal ulna in a medial (A) to lateral (B) direction. To access the articular surfaces for arthroplasty, the collateral ligaments are released. |
The extended Kocher approach may be used for total elbow arthroplasty
and is seldom used for ORIF of distal humerus fractures. The approach
is analogous to the Bryan-Morrey in that the triceps is reflected;
however, the direction of reflection is lateral to medial.
The triceps reflecting anconeus pedicle approach (TRAP) involves
completely detaching the triceps from the proximal ulna with the
anconeus muscle.138 The approach is
done through a longitudinal posterior skin incision after
identification of the ulnar nerve. Kocher’s interval is used to elevate
the anconeus muscle and develop the distal lateral portion of the flap (Figure 33-14A).
The medial portion of the flap is created by subperiosteal dissection
from the subcutaneous border of the ulna. The anconeus flap is then
reflected proximally to expose the triceps insertion, which is also
sharply released (Figure 33-14B). The entire
triceps-anconeus flap is then reflected proximally releasing the
triceps muscle from the posterior aspect of the distal humerus (Figure 33-14C).
This approach provides good exposure to the posterior elbow joint while
protecting the neurovascular supply to the anconeus muscle. The TRAP
approach also avoids the complications of an olecranon osteotomy and
allows the use of the trochlear sulcus as a template to assist with
articular reduction of the distal humerus. The major disadvantage of
this approach is that the triceps is completely released from its
insertion, therefore, there is a risk of triceps dehiscence and
extensor weakness.
The approach is most commonly used for total elbow arthroplasty and
rarely for ORIF of distal humerus fractures. Transection of the triceps
is done in the shape of a V, so that a V to Y plasty can be done if
lengthening of the extensor mechanism is required. As the triceps is
completely divided in this approach, it has the same risks as the TRAP
approach. This approach is indicated for ORIF of distal humerus
fractures when there is an associated complete or high grade partial
triceps tendon laceration.
direct lateral skin incision or by a posterior longitudinal skin
incision with elevation of a lateral fasciocutaneous flap. The
approaches that will be discussed are the Kocher, Kaplan, and the
extensor digitorum communis (EDC) split. Access to the radiocapitellar
joint can also be obtained through a lateral epicondylar osteotomy or
via a concurrent fracture of the lateral epicondyle.
treat capitellar and radial head fractures. Proximal extension of these
approaches can be used to access the lateral column, to treat partial
articular lateral column fractures and some transcolumn fractures.
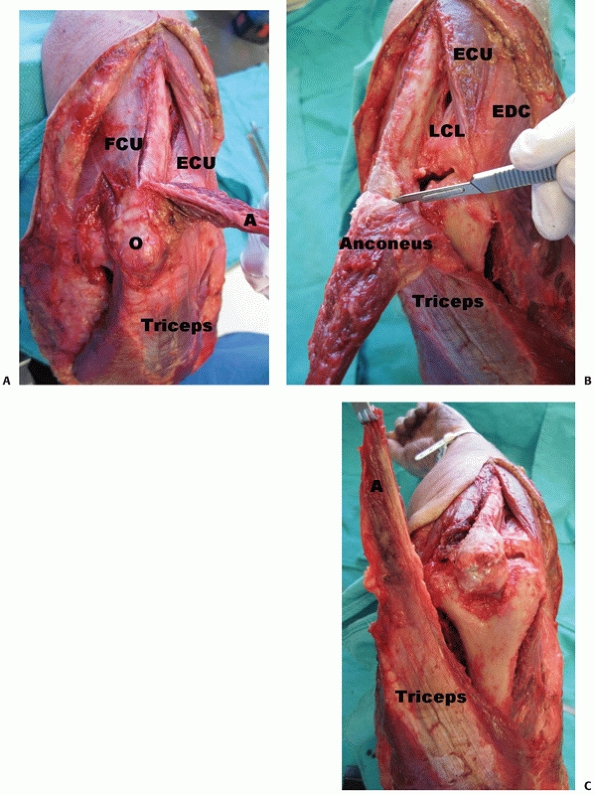 |
|
FIGURE 33-14 The triceps reflecting anconeus pedicle138
(TRAP) approach is done through a longitudinal posterior skin incision after identification of the ulnar nerve. The interval between anconeus and extensor carpi ulnaris is used to elevate the anconeus muscle and develop the distal lateral portion of the flap. The medial portion of the flap is created by subperiosteal dissection from the subcutaneous border of the ulna. The anconeus flap is then reflected proximally (A) to expose the triceps insertion, which is also sharply released (B). The entire tricepsanconeus flap is then reflected proximally releasing the triceps muscle from the posterior aspect of the distal humerus (C). (A, anconeus; ECU, extensor carpi ulnaris; FCU, flexor carpi ulnaris; LCL, lateral collateral ligament; O, olecranon.) |
This interval can be identified by a thin fat stripe or by the
perforating branches of the recurrent posterior interosseous artery (Figure 33-15A).
The interval is developed by bluntly undermining the anconeus muscle,
which will allow identification of the elbow joint capsule and the
capsular thickening that is the lateral ulnar collateral ligament
(LUCL) (Figure 33-15B,C). Some of the common
extensor tendon origin will have to be elevated off the LUCL to allow
an arthrotomy to be made anterior to the ligament (Figure 33-15D).
The forearm is pronated during the approach, which moves the posterior
interosseous nerve more anterior and distal. The radial neck is exposed
by incising the annular ligament. This approach can be extended
proximally by releasing the extensor carpi radialis longus (ECRL) and
the brachioradialis off the anterolateral supracondylar ridge. To
expose the posterolateral elbow joint and posterior aspect of the
lateral column, another arthrotomy is made posterior to the LUCL and
the triceps is elevated off the posterior lateral column.
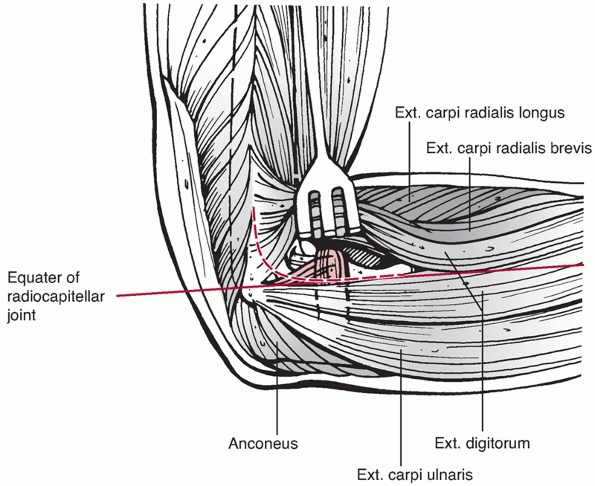
radiocapitellar joint is the extensor digitorum communis (EDC) split.
This approach involves creation of a lateral elbow arthrotomy at the
equator of the radiocapitellar joint (Figure 33-16). The site of the arthrotomy is chosen by palpating the capitellum
and radial head to determine the midequator. The structures below the
equator include the LUCL and the posterolateral joint capsule, which
should not be incised as they are important elbow stabilizers. The
arthrotomy, therefore, is made in-line with the tendon fibers of EDC at
the equator of the radiocapitellar joint and may be extended proximally
along the anterolateral aspect of the lateral column. Dissection below
the midequator is avoided, as it may disrupt the LUCL.
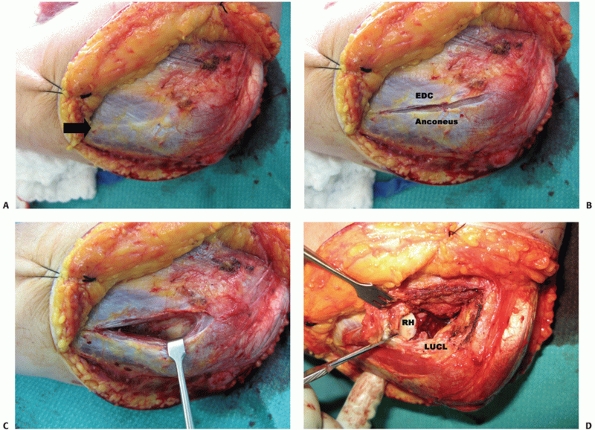 |
|
FIGURE 33-15 Kocher’s approach96 to the anterolateral elbow joint uses the interval between extensor carpi ulnaris (ECU) and anconeus (A). This interval can be identified by a thin fat stripe (black arrow).
The interval is developed by bluntly undermining the anconeus muscle, which allows identification of the elbow joint capsule and capsular thickening that is the lateral ulnar collateral ligament (LUCL) (B,C). The posterior portion of the common extensor tendon origin has to be elevated off the LUCL to allow an arthrotomy to be made anterior to the ligament (D). (RH, radial head.) |
The approach provides good exposure of the radial head and capitellum
and remains anterior to the lateral collateral ligament insertion. The
forearm should be pronated during distal extension of the approach to
maximize the distance to the posterior interosseous nerve.32
direct medial skin incision or a posterior longitudinal skin incision
with elevation of a medial fasciocutaneous flap. When using a direct
medial skin incision, care should be taken in identifying and
protecting the branches of the medial antebrachial cutaneous nerve. The
medial approaches can be used to treat isolated partial articular
medial column fractures, trochlear fractures, coronoid fractures and
fractures of the medial epicondyle.
The medial supracondylar ridge is identified and the flexor-pronator
origin is release off the ridge to the level of the medial epicondyle.
At the medial epicondyle, the flexor origin is split distally in-line
with its fibers. Dissection directly inferior to the medial epicondyle
is avoided, as it may disrupt the anterior bundle of the medial
collateral ligament.
collateral ligament (MCL) and the posteromedial ulnohumeral joint can
be accessed through an approach that starts at the floor of the cubital
tunnel. The humeral head of the flexor carpi ulnaris, palmaris longus,
flexor carpi radialis, and pronator teres are bluntly elevated off the
anterior bundle of the MCL and joint capsule in a posterior to anterior
direction. Once exposed, an arthrotomy is made anterior to the anterior
bundle of the MCL
to
enter the anterior aspect of the ulnohumeral joint. The posteromedial
aspect of the ulnohumeral joint is accessed by dividing the posterior
and transverse bundles of the MCL. Taylor and Scham195
described a similar approach with the only difference being that the
ulnar head of FCU is elevated anteriorly with the other flexors.
 |
|
FIGURE 33-16
The extensor digitorum communis (EDC) split approach. The EDC tendon is split anterior to the midequator of the radiocapitellar joint to avoid injury to the lateral ulnar collateral ligament. |
because it provides little access to the medial and lateral columns for
the application of internal fixation. This approach may be used if
access to the brachial artery or median nerve is required for repair or
in release of posttraumatic elbow contractures.
managed with surgical intervention; however, circumstances exist where
nonoperative management may be most appropriate. Nonoperative
management techniques include above-elbow casting, olecranon traction,
and collar and cuff treatment, the so-called “bag of bones” method.
Traction is applied for 3 to 4 weeks, until there is sufficient early
callous to allow cast bracing. The major disadvantages of this method
are the complications associated with prolonged bed rest. Patients that
are typically treated nonoperatively, the frail elderly, have
significant medical comorbidities that put them at high risk of bed
rest related complications, such as deep venous thrombosis, pulmonary
embolism, and decubitus ulcers. The technique is largely of historical
significance and has little use in modern distal humerus fracture care.
before it was first reported in modern medical literature in 1937 by
Eastwood.40 He described a closed
reduction followed by application of a collar and cuff with the elbow
between 90 and 120 degrees of flexion. The elbow is hung freely to
allow gravity assisted reduction via a ligamentotaxis-type effect.
Shoulder motion and active elbow flexion are initiated at 2 weeks and
progressed.
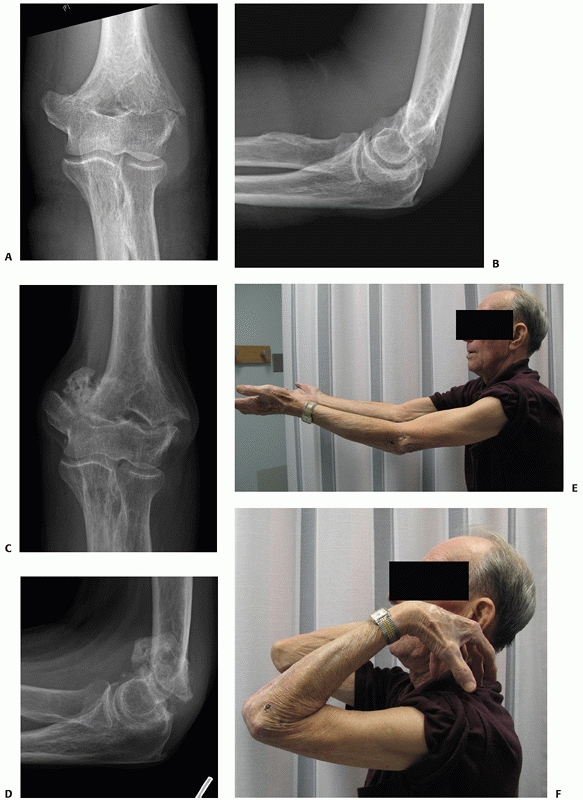
young patients is rarely recommended and it is generally reserved for
patients deemed medically unfit to undergo surgery (Figure 33-17).
Other circumstances are elderly patients with unrepairable distal
humerus fractures in which arthroplasty is the most reasonable option,
however, is contraindicated because of soft tissue compromise, such as
skin loss. Once the soft tissue issues have been dealt with, delayed
arthroplasty can be done if patients are sufficiently symptomatic.
with a trial of nonoperative management. These patients should be
followed for the first 3 to 4 weeks with weekly serial radiographs to
ensure displacement or angulation does not occur. Surgical fixation of
these fractures, however, enhances stability, allows immediate motion,
and obviously decreases the risk of delayed fracture displacement.
compared operative with nonoperative management in 29 patients with
intra-articular distal humerus fractures. They reported better range of
motion and less pain with nonoperative management, consisting of
skeletal traction or manipulation and casting. The surgically treated
group was plagued with early fracture displacement because of hardware
failure from nonrigid fixation constructs. Brown and Morgan22
in 1971 reported their results with nonoperative management of
intra-articular distal humerus fractures in 10 patients at a mean
follow-up of 2.5 years (range, 9 months to 4 years).
At
follow-up, the mean flexion was 128 degrees, the mean extension was 30
degrees, and the mean arc of motion was 100 degrees. Seven patients
described no symptoms, whereas three complained of elbow aches in cold
and damp weather.
 |
|
FIGURE 33-17
Radiographs of an 88-year-old man with a transcolumn fracture (AO/OTA type A2) deemed medically unfit for surgery because of severe congestive heart failure and inoperable coronary artery disease (A,B). The patient was treated with a collar and cuff and early range of motion. Radiographs at 1-year follow-up (C,D). The patient has no pain with a functional range of motion (E,F). |
considered the gold standard for most displaced intra-articular distal
humerus fractures (AO/OTA types B and C). Rigid internal fixation
allows fracture healing to occur anatomically while permitting early
range of motion to maximize functional recovery. The traumatized elbow
is particularly prone to stiffness; therefore, early motion is vital,
but not at the expense of fracture displacement. In cases in which
sufficient fracture stability cannot be obtained to allow early motion,
anatomic reconstruction of the articular surface and overall elbow
alignment take precedence. An anatomically aligned stiff elbow with a
healed articular surface can be subsequently managed with contracture
release, but a fracture with hardware failure and articular nonunion or
fragmentation may be difficult to manage with revision surgery.
angulated extra-articular fractures (transcolumn) of the distal humerus
(AO/OTA Type A2 and A3). Closed reduction and percutaneous K-wire
fixation has been described for treatment of these injuries in adults.82
The technique in adults is similar to the technique used pediatric
supracondylar fractures with crossing K-wires inserted medially and
laterally. In adults, this technique may be modified to exchange the
K-wires for 3.5- or 4.5-mm cannulated screws. Closed reduction and
percutaneous fixation has several disadvantages when used in adults.
The fixation is semi-rigid and therefore requires supplementary
splinting for up to 6 weeks, which may lead to elbow stiffness. K-wires
are also inadequate for elderly patients with osteopenic bone. In
general, the crossing K-wire or cannulated screw technique is not
recommended for adult patients with AO/OTA Type A2 or A3 fractures.
fixation technique for these fractures (Transcolumn, AO/OTA Types A2
and A3). These fractures can be exposed through a paratricipital
approach or a limited triceps split. Exposure of the articular surface,
as obtained from an olecranon osteotomy, is not required for these
extra-articular fractures. Bicolumnar fixation is recommended with
orthogonal or parallel plating techniques. When the transcolumn
fracture line is just proximal to the articular segment, the pattern
can be referred to as a “low” transcolumn fracture. Low transcolumn
fractures have limited bone available for distal fixation; therefore,
bicolumn plating is necessary with plates applied as distal as possible
with as many screws as possible in the distal fragment. Commercially
available precontoured plates have extra screw holes distally to allow
highdensity screw insertion into the distal articular segment. In
certain low transcolumn fractures in elderly patients with severe
osteopenia or pre-existing arthritis, total elbow arthroplasty may be
the most appropriate form of treatment. Elbow arthroplasty is discussed
later in this chapter.
for fracture displacement, fracture angulation, or the severity of the
soft tissue injury. These factors should be considered when deciding
upon surgical management. In general, medically fit patients with
distal humerus fractures with displacement or angulation meet the
indications for surgical intervention.
preoperative planning, specialized implants, instruments, and surgical
expertise. Medically fit and stabilized patients with uncompromised
soft tissues may be best managed with early surgery within 48 to 72
hours.76 Early surgery may lead to
decreases complications such as heterotopic ossification and stiffness.
Polytrauma patients that are unstable or those with identified
modifiable risk factors should be medically optimized preoperatively.
In cases with injured soft tissues, such as excessive swelling,
bruising, fracture blisters, or abrasions, delay of surgery may be most
appropriate. Generally, patients admitted to the intensive care unit
can be managed with a well-padded splint that is checked daily and
removed every 2 to 3 days to examine the soft tissues for compromise
and pressure points. In some cases, prolonged secondary surgical
procedures may be contraindicated for several weeks because of medical
issues. In these patients, static external fixation may be of benefit
to stabilize the extremity for pain control, transfers, hygiene, and
wound care. Ideally, external fixator pins should be placed as far away
as possible from planned internal fixation implants to decrease the
likelihood of infection. Although no literature exists to define a
suitable delay, surgery should be conducted within 2 or 3 weeks. Delay
beyond this time interval is possible; however, ORIF is made more
difficult with increased surgical time, difficult fracture reductions
owing to partial healing and callous, increased bleeding, and the
increased risk of heterotopic ossification.
fractures are similar to those used for any periarticular fracture. The
objectives are to obtain anatomic restoration of the articular surface
and recreation of joint alignment with rigid internal fixation, stable
enough to allow early range of motion.
the selection of an appropriate surgical approach. The chosen approach
should be accommodating to intraoperative findings, which may alter the
surgical procedure. For example, a paratricipital approach may be used
to initially access a noncomminuted intra-articular fracture (AO/OTA
type C1 or C2); however, if the fracture proves difficult to reduce or
if more comminution is present than expected, the approach can be
converted to an olecranon osteotomy. Similarly, an olecranon osteotomy
should not be the index approach for an elderly patient with a highly
comminuted distal humerus fracture, which may be intraoperatively
deemed unrepairable, necessitating total elbow arthroplasty. AO/OTA
type B1 (lateral column) fractures can be surgically approached by
Kocher’s interval with proximal extension to expose the lateral column.
AO/OTA type B2 (medial column) fractures can be approached via a
Hotchkiss approach with proximal extension to expose the medial column.
Single column fractures (medial and lateral) may also be exposed by the
paratricipital approach that allows visualization
of
the posterior aspects of both columns and the posterior aspect of the
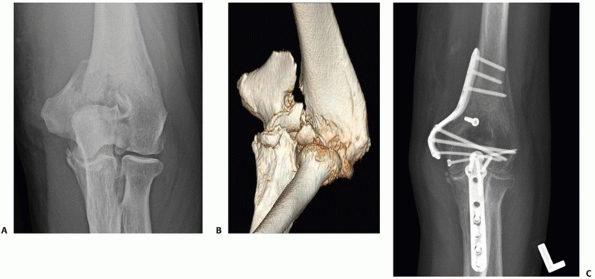
articular surface. In cases in which there is extensive articular
comminution (AO/OTA types B1.3 and B2.3) an olecranon osteotomy may be
required for improved visualization of the fracture and improved access
for fixation (Figure 33-18).
 |
|
FIGURE 33-18
A 73-year-old woman with a comminuted intra-articular fracture of the medial column (AO/OTA type B1.3) treated with ORIF via an olecranon osteotomy (A-C). |
For AO/OTA type C fractures, once the distal humerus articular surface
is adequately exposed, the fracture hematoma is evacuated and the raw
fracture surfaces are cleaned of loose debris. The origins of the
common flexor and extensor tendons are preserved on the epicondyles, as
are the collateral ligament origins. The fracture fragments can be
manipulated manually or with small-diameter K-wires used as joy sticks.
Once the fractured articular fragments are reduced and interdigitated,
a large tenaculum can be used to hold the reduction until provisional
transfixion with K-wires. Definitive fixation of the articular segment
can be done with one or two centrally placed screws along the
capitellar-trochlear axis (Figure 33-19) or by screws placed through plates that are applied in a parallel fashion (Figure 33-20).
Ideally, intrafragmentary compression is best; however, not at the
expense of shortening the trochlea in the medial-lateral plane. The
trochlea is particularly susceptible to shortening when central
comminution exists and lag screw fixation is used. In these instances,
fully threaded (non-overdrilled) position screws rather than lag screws
should be used to stabilize the articular segment.
incorporated into the greater fixation construct should be
independently fixated. Supplementary implants should be available to
address these small osteochondral fragments, such as minifragment
plates, headless compression screws, countersunk small diameter screws,
threaded K-wires, and bioabsorbable pins. These supplementary implants
require strategic placement such that they do not interfere with
trochlear fixation and bicolumnar plate application that will link the
articular segment to the diaphysis.
OTA Type C fractures after articular fixation), requires rigid
attachment to the medial and lateral columns or distal humerus shaft.
This can be accomplished by orthogonal,67,84,181 parallel,13b,174,175 or triple55,111 plating. No clinical superiority of either method has been reported when comparing orthogonal with parallel plating techniques.
Usually, the lateral plate is placed as distal as possible along the
posterior aspect of the lateral column. The lateral plate should be
contoured with a bend that matches the posterior curvature of the
lateral column. To achieve maximum distal fixation, the end of the
plate should lie just proximal to the posterior articular surface of
the capitellum. Placement of the plate further distal may lead to
impingement of the radial head on the plate in extension, resulting in
pain and limited range of motion. Ideally, the lateral plate should be
a 3.5-mm dynamic compression plate or equivalent. The medial plate is
usually applied on the medial supracondylar ridge with contouring to
curve around the medial epicondyle. The plate is typically a 3.5-mm
reconstruction plate to allow easier bending; however, a 3.5-mm dynamic
compression plate or a newer fracture-specific precontoured plate may
be used.
plates are placed parallel to each other on their respective
supracondylar ridges (Figure 33-22). Screws
into the articular segment are preferentially placed through the plates
to link the articular segment to the humeral shaft. Ideally, the
longest possible screws should be inserted through the plate, to
capture as many articular fragments as possible and engage fragments
that are secured to the opposite column.13b,136,137,174
This technique may be difficult to achieve and not always possible to
perform. For example, longer screws can deflect and bend as they pass
one another, causing displacement of tenuously stabilized osteochondral
fragments.
through a range of motion to ensure there is no hardware impingement.
Also, an attempt should be made to dynamically compress the
supracondylar level fracture with eccentrically placed screws through
the medial and lateral plates.136,137
If possible, the plates should end at different levels on the humeral
shaft to minimize the stress riser effect and each plate should have at
least three bicortical screws proximal to the metaphyseal comminution.111,137
 |
|
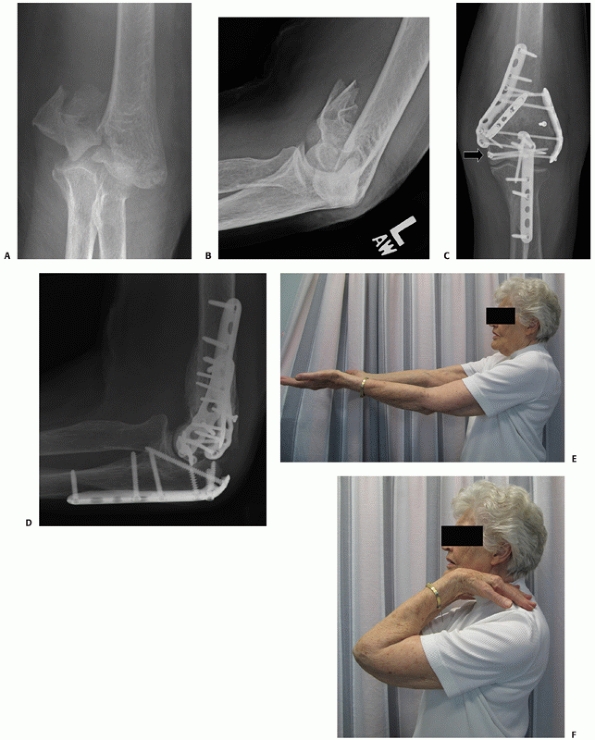
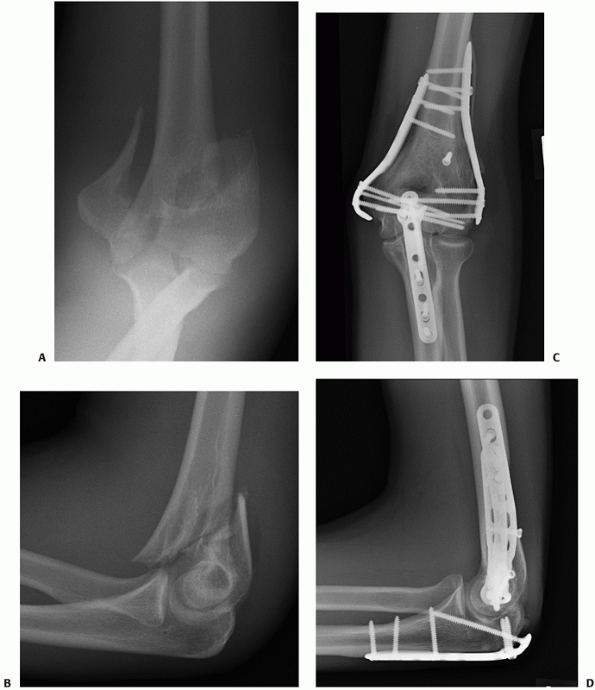
FIGURE 33-19 Anteroposterior and lateral radiographs (A,B)
of a comminuted intra-articular distal humerus fracture (AO/OTA type C3) in an active 85-year-old woman. The articular fragments were first fixated with two (black arrow) centrally placed screws along the capitellar-trochlear axis (C,D). The reduced articular segment was then fixated to the shaft with triple plating. At 12 months follow-up, the fractures have healed and the patient has functional range of motion (E,F). |
 |
|
FIGURE 33-20 A bicolumn (AO/OTA type C1) fracture (A,B)
treated with ORIF via an olecranon osteotomy. The distal humerus articular segment is fixated with three medial and three lateral screws placed through parallel plates (C,D). |
comminuted distal humerus fractures. This bone loss can be addressed
with supracondylar shortening or bridge plating with autologous bone
graft or allograft. Supracondylar shortening involves removing the
comminuted fragments of metaphyseal bone and compressing the
reconstructed articular segment to the distal humeral shaft. Typically,
the distal end of the shaft will require reshaping to increase the
contact area between it and the articular segment.140
If absolute rigid fixation cannot be achieved to allow early range of
motion, triple plating should be considered as recommended by Gofton55 and Jupiter.83 Triple plating can also be useful for fixation of coronal plane fractures (Figure 33-19).
for fixation of distal humerus fractures. Although these plates are
marketed as precontoured, they generally still require some contouring
to match distal humeral anatomy. Newer precontoured locking plates are
also available and are of two types, fixed angle locking and variable
angle locking. These plates may offer enhanced fixation in osteopenic
bone; however, this has not
yet
been shown to be clinically superior. The disadvantages of the fixed
angle locking plates are the screws have predetermined trajectories,
which may not accommodate all fracture patterns in all patients. In
some plate designs, the predetermined screw trajectory aims toward the
articular surface, which may predispose to joint penetration if screws
are placed too long.
 |
|
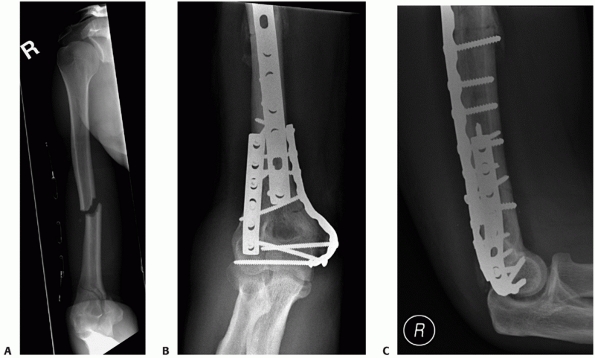
FIGURE 33-21 An AP injury radiograph (A)
demonstrating a displaced intra-articular distal humerus fracture in association with an ipsilateral humeral shaft fracture. The fractures were exposed via a paratricipital approach extended proximally into a Gerwin et al.53 approach. The patient’s distal humerus fracture was fixated with orthogonal 3.5-mm dynamic compression plates (B,C) that were intra-operatively contoured. This technique has been popularized by the AO group and involves the placement of plates at 90-degree angles to each other. Usually, the lateral plate is placed as distal as possible along the posterior aspect of the lateral column. The medial plate is placed over the medial supracondylar ridge and curved around the medial epicondyle. |
In general, the fixation principles and techniques used for AO/OTA type
C (bicolumn) fractures are applicable to type B1 and B2 (single column)
fractures. These fractures may be fixed with multiple screws or with
single column plating.85 Single
column plating has the advantage of providing an antiglide construct at
the proximal fracture line between the column and humeral shaft (Figure 33-18).
In certain highly comminuted partial-articular fractures in elderly
patients with osteopenia, total elbow arthroplasty may also be an
appropriate treatment option. Elbow arthroplasty is discussed later in
this chapter.
configurations confer the greatest amount of stability when treating
distal humerus fractures. Jacobson et al.79
tested five different distal humerus plating constructs in cadaveric
specimens. They reported that a medially applied 3.5-mm reconstruction
plate along with an orthogonally applied posterolateral 3.5-mm dynamic
compression plate provided the greatest sagittal plane stiffness, and
equivalent frontal plane and torsion stiffness, when compared with
other constructs which included parallel and triple plating.
also found that orthogonal plating provided greater rigidity and
fatigue resistance when compared with a single Y plate or crossed
screws.
found that parallel plating with a medial 3.5-mm reconstruction plate
and a lateral J plate had the greatest construct rigidity when compared
to four other plate configurations, including orthogonal plating with
3.5-mm reconstruction plates. Self et al.181
found that parallel plating trended towards having greater rigidity and
load to failure than orthogonal plating, however, the differences did
not reach statistical significance. Arnander et al.,11
however, found that two 3.5-mm reconstruction plates applied in a
parallel fashion did have statistically significant increased stiffness
and strength in the sagittal plane when compared with two 3.5-mm
reconstruction plates applied orthogonally.
demonstrated that locking 3.5-mm reconstruction plates applied
orthogonally had superior cyclic failure properties when compared with
conventional nonlocked plates applied in a similar fashion in cadavers
with low bone mineral density. Stoffel et al.190
compared the mechanical stability of two different commercially
available precontoured locking distal humeral plating systems. They
reported significantly higher stability in compression, external
rotation, and a greater ability to resist axial plastic deformation in
the parallel plate system versus the orthogonal plate system. It should
be noted that no clinical difference has yet be demonstrated between
parallel and orthogonal plating, and more likely than not, both are
acceptable as long as the principles of rigid internal fixation are met.
strength and are susceptible to breakage.70,136,137,196 These plates should not be used in the primary two-plate construct; however, they may be used as a supplementary third plate.
 |
|
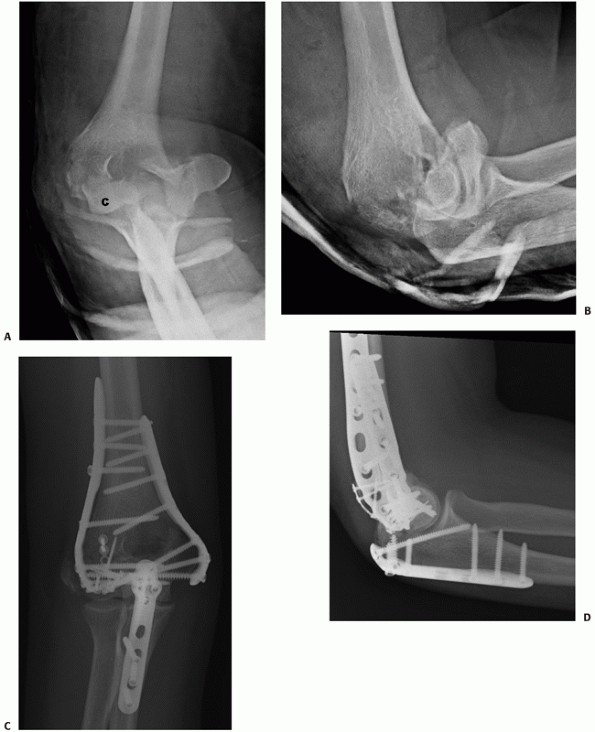
FIGURE 33-22
A 21-year-old man sustained an intra-articular distal humerus fracture associated with a coronal shear fracture of the capitellum (A,B). The capitellar fracture was fixated with a minifragment plate applied posteriorly and a headless compression screw. The articular segment was then rigidly linked to the humeral shaft with a parallel plating technique (C,D). (C, capitellum.) |
surface, bicolumn plating, and rigid internal fixation to allow early
range of motion are employed, good outcomes can be expected for
patients with intra-articular distal humerus fractures* When averaging the outcomes of 17 series published between 2002 and 2009 (Table 33-4), 85% of patients experienced good to excellent outcomes at a mean follow-up of 50 months. Doornberg et al.35
have shown that the rate of good to excellent outcomes is durable in
the long-term (12 to 30 years). Patients that sustain isolated
intra-articular fractures of the distal humerus can expect some loss of
elbow range of motion, although, functional range of motion (30 to 130
degrees) is usually attained. As would be expected, patients who
sustain distal humerus fractures in association with polytrauma or
severe soft tissue injuries can anticipate worse outcomes.
|
TABLE 33-4 Summary of Outcomes of AO/OTA Type C (Intra-articular) Distal Humerus Fractures
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
reported the patient-rated outcomes and physical impairments after
orthogonal plating of AO/OTA Type C distal humerus fractures in 23
patients. The SF-36 scores of patients at final follow-up compared with
age- and sex-matched controls showed no significant differences.
Patients rated their overall satisfaction at 93% and on functional
assessment indicated a 10% subjective loss of function when comparing
the affected with unaffected limb. The mean score on the Disabilities
of the Arm, Shoulder and Hand (DASH) questionnaire was 12, which is
very close to the overall normative score of 10.1.74
The isometric strength of the affected elbow was significantly reduced
in all ranges, although, grip and pinch strength were not statistically
different between affected and unaffected limbs. McKee and colleagues120
also found decreased strength in the affected elbows and rated it at
approximately 75% of normal. The mean DASH score was 20 points,
indicating a mild residual impairment. Two of the eight parameters of
the SF-36, physical function and role-physical, demonstrated small but
significant differences among age-matched controls.
The principles of open fracture treatment have remained unchanged for
the last three decades. The priorities are wound irrigation and
débridement, intravenous antibiotics, tetanus coverage, fracture
stabilization and appropriate soft tissue management.61,142 The common complications associated with open fractures include infection, nonunion, hardware failure, and wound problems.
injury. Most blunt mechanisms of injury lead to grade I punctures,
whereas blast or gun shots lead to grade III wounds.172
Typically, most grade I puncture wounds are located posteriorly or
posterolaterally on the elbow and are commonly associated with
lacerations of the triceps tendon or muscle.118,172
In cases of an intra-articular fracture with a triceps tendon
laceration, the Van Gorder (triceps tongue) or triceps splitting
approaches are preferred, as they prevent a second disruption of the
extensor mechanism with an olecranon osteotomy.
devascularized bone and soft tissues are excised. This rule, however,
does not hold absolutely true when dealing with large segments of
articular surface. The risk of infection with retaining the fragments
must be weighed against the risk of posttraumatic arthritis and
potential need for secondary bone grafting or allograft reconstruction
if the fragments are removed. Generally, an attempt should be made to
preserve all articular segments with thorough cleansing and meticulous
removal of all foreign material and contamination.
reviewed 26 patients who underwent open reduction and internal fixation
of open distal humerus fractures. According to the system of Gustilo
and Anderson, 50% were grade I, 35% were grade II, and 15% were grade
III. At follow-up, 15 patients (57%) had good to excellent outcomes
based on the Mayo Elbow Performance score, and the mean DASH score was
24, indicating minimal to moderate disability. The mean elbow arc of
flexion-extension motion was 97 degrees (range, 55 to 140 degrees). The
overall infection rate was 11% (three patients), with only one patient
sustaining a deep infection requiring operative débridement. Four
patients (15%) were diagnosed with delayed union (>16 weeks), with
two patients going on to require revision surgery with bone grafting.
radiographs of the elbow out of plaster are usually sufficient to
determine the fracture pattern. If the radiographs are difficult to
interpret or poorly demonstrate the articular fracture, I prefer a CT
scan, with three-dimensional reconstructions, over traction radiographs
requiring patient sedation. A CT scan can identify difficult fracture
patterns, such as coronal fractures of the capitellum or trochlea,
“low” fracture types, and segmental articular fractures (for example, a
fracture between the medial trochlea and the medial epicondyle,
producing a free medial trochlear fragment). In elderly patients with
comminuted fractures in whom ORIF may not be possible and elbow
arthroplasty is considered, a CT scan may assist with the preoperative
decision making.
well-padded elbow splint and are encouraged to elevate the arm, ice the
elbow, and maintain hand and finger range of motion. On the day of
surgery, the skin and soft tissues are re-examined and the neurologic
status is redocumented. Patients generally receive a general
anaesthetic with an upper extremity regional block for postoperative
pain control and therapy. Preoperatively, prophylactic antibiotics are
administered intravenously.
bolster placed under the ipsilateral scapula and the elbow is supported
by another bolster made of wrapped sterile sheet on the patient’s chest
(Figure 33-23A). The surgeon and assistant
stand on the side of the injury while the scrub nurse and instruments
are on the contralateral side, allowing the nurse to assist with arm
positioning as required. A sterile tourniquet is used and the iliac
crest is prepped and draped if bone grafting is anticipated. Portable
(mini) fluoroscopy is used for all cases and is positioned on the
operative side.
available, I prefer to position the patient in the lateral decubitus
fashion on a bean-bag with a small axillary bolster. The elbow is then
flexed over an elbow arthroscopy positioner (Figure 33-23B)
and the scrub nurse and instrumentation are positioned on the same
side. In the rare circumstance of bilateral fractures, when a second
surgical team is available, the patient may be positioned prone with
the elbows flexed over a positioner to allow simultaneous surgery.
with elevation of full-thickness medial and lateral fasciocutaneous
flaps. The ulnar nerve is exposed, tagged, and prepared for anterior
subcutaneous transposition, which will be done at the completion of the
procedure.
C3 fractures, I prefer a chevron-shaped osteotomy through the bare
area, which is then fixated with a precontoured olecranon plate (please see section on Olecranon Osteotomy). The plate is preapplied to the olecranon before the osteotomy; this facilitates olecranon reduction and plate application
at the end of the operative procedure. For simple articular fractures
(AO/OTA type C1 and C2) and extra-articular fractures (AO/OTA type A2
and A3), I prefer the paratricipital approach (Figure 33-11).7
This approach allows bicolumn exposure and plating with preservation of
the triceps mechanism. Simple intraarticular fractures can be reduced
indirectly by anatomic reduction of the supracondylar level fracture.
The articular reduction can be assessed with elbow flexion and direct
visualization of the posterior aspect of the trochlea or fluoroscopy.
The articular reduction may also be visualized directly by creation of
a third surgical window in the Boyd interval, between the anconeus
muscle and lateral olecranon.13
 |
|
FIGURE 33-23 The patient may be positioned supine with a bolster placed under the ipsilateral scapula (A) or lateral decubitus on a bean-bag with the elbow flexed over an arthroscopy positioner (B).
|
in which the reparability of the fracture will be determined
intraoperatively. If the fracture is deemed fixable, it may be carried
out through the paratricipital approach or the approach can be
converted to an olecranon osteotomy. In cases in which the fracture is
deemed irreparable, a total elbow arthroplasty may be done via the same
approach.
techniques, including parallel, orthogonal, and triple plating, as some
fractures will lend themselves to one technique over another.
Generally, I prefer the technique of parallel plating; however, it does
have its disadvantages. Thin and active patients may complain of
hardware irritation from a prominent lateral plate. Therefore, in cases
with a “high” lateral supracondylar level fracture, a posterolateral
plate may be preferable (Figure 33-21).
fragments are irrigated and débrided of hematoma and fibrous tissue.
The articular reduction proceeds first (Figure 33-24A-D).
I prefer the use of K-wires, to function as joysticks, for manipulation
of the fracture fragments. Typically, I place one K-wire through the
fractured surface of the medial trochlea, aiming toward the medial
epicondyle, running along the trochlear axis. This K-wire is then
pulled out through the medial epicondyle until its tip lies flush and
perpendicular to the fracture surface of the medial trochlea. A similar
K-wire is placed through the lateral articular fragment. These wires
are then used to individually manipulate the fracture fragments, to
reduce and interdigitate them. A large reduction tenaculum is used to
hold the reduction and to provide compression until the medial K-wire
can be drilled into the lateral fragment and the lateral K-wire drilled
into the medial fragment. This provides provisional fixation of the
articular segment. Next, I typically place a single fully threaded
standard screw (2.7, 3.0, or 3.5 mm) along the axis of the articular
segment to maintain the reduction (Figure 33-24E).
This screw is usually inserted medial to lateral with its starting
point located in the center of the medial trochlea. A small diameter
axis screw is used to minimize its effect on other screws that will
eventually be used to fixate the articular segment through plates to
the diaphysis. When using small-diameter screws, my preference is not
to use titanium as the resistance encountered during screw insertion in
good quality bone has been known to shear off the screw heads.
are addressed before screw fixation of the large medial and lateral
articular segments. These small articular fractures may be located
anteriorly and can be exposed by internally rotating the appropriate
column fragment. My preference is to fixate these small articular
fragments with countersunk small-diameter screws (1.0-, 1.3-, 1.5-, or
2.0-mm) or with any small-diameter headless compression screw.
Precountoured plates are then provisionally applied to the medial and
lateral columns with K-wires placed distally and serrated bone
reduction clamps proximally. Then, as many screws as possible are
inserted through the plates into the articular segment (Figure 33-24G),
ideally the screws should be as long as possible and engage as many
articular fragments as possible. Screws should not be placed through
the olecranon fossa as they may lead to impingement. The plates are
then fixated to the humeral shaft with the first diaphyseal screws
inserted in an eccentric fashion to provide supracondylar fracture
compression.136 Ideally, the plates
should end at different levels on the humeral shaft to minimize the stress riser effect (Figure 33-24H,I).
Once ORIF of the distal humerus fracture is complete, the elbow is
placed through a range of motion to ensure there is no impingement or
instability.
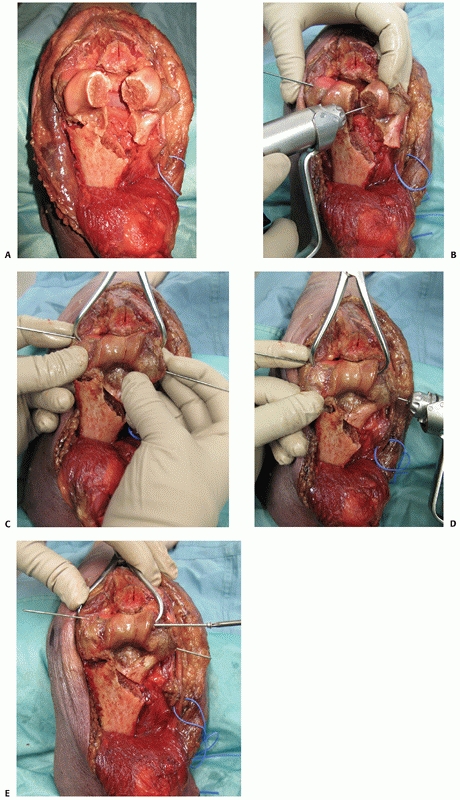 |
|
FIGURE 33-24 Open reduction and internal fixation of an intra-articular distal humerus fracture via an olecranon osteotomy (A). K-wires are used as joysticks to manipulate the fracture fragments into an anatomic reduction (B). A large tenaculum is used to stabilize the reduction (C) while the K-wires are drilled into the opposite articular fragment (D) to provisionally fixate the segment. A small diameter screw is then inserted from medial to lateral (E). (continues)
|
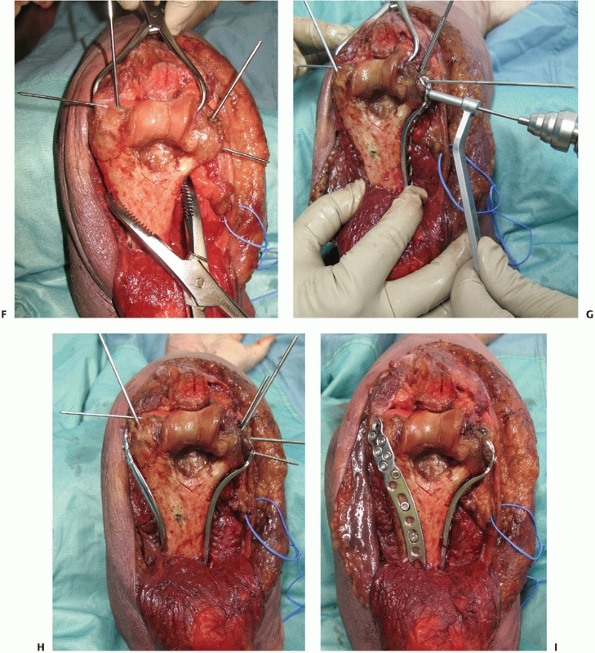 |
|
FIGURE 33-24 (continued)
After the articular segment is fixated, it is reduced to the shaft and provisionally stabilized with long bicortical K-wires inserted up each column (F). Definitive articular segment to shaft fixation is obtained with bicolumn plating in a parallel or orthogonal fashion (G-I). Ideally, as many screws as possible are inserted through the plates into the articular segment, the screws should be as long as possible and they should engage as many articular fragments as possible. Screws should not be placed through the olecranon fossa as they may lead to impingement and decreased range of motion. |
plaster extension splint applied anteriorly and are encouraged to keep
the arm elevated to minimize swelling. Active hand range of motion is
started immediately. Elbow range of motion is started between days 2
and 7 postoperatively, depending on the status of the incision.
Generally, active-assisted and active range of motion are encouraged
(flexion, extension, pronation, and supination) for patients with a
paratricipital approach or an olecranon osteotomy fixated with a plate.
Passive extension is reserved for patients that underwent an extensor
mechanism disrupting approach. Typically, a night extension splint is
used for the first 6 weeks. At 6 weeks postoperative, passive
stretching and
static
progressive splinting are used if required. Strengthening may begin at
12 weeks providing there is evidence of radiographic union (Table 33-5).
|
TABLE 33-5 Pearls and Pitfalls in the Management of Distal Humerus Fractures
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
most challenging fractures to manage. Nonoperative treatment, although
appropriate for some elderly patients, often leads to loss of motion
and unsatisfactory functional outcomes. Open reduction and rigid
internal fixation is considered gold standard; however, it may not be
attainable in elderly patients with osteopenia, comminution, and
articular fragmentation or in patients with pre-existing conditions of
the elbow such as rheumatoid arthritis (Figure 33-25).
In cases in which rigid internal fixation cannot be achieved to allow
early range of motion, resultant prolonged immobilization often leads
to poor outcomes.4 Total elbow arthroplasty for such factures is a reliable treatment option with good outcomes*
for distal humerus fractures include active infection and insufficient
soft tissue coverage. The most important relative contraindication to
elbow replacement for trauma is the younger active patient who is more
appropriate for open reduction and internal fixation. Elderly patients
with low-energy Gustilo and Anderson grade I open fractures are not an
absolute contraindication to elbow arthroplasty. Generally, the wounds
are punctures that
are
small and clean. However, if there has been a time delay until open
fracture management or the cleanliness of a wound is questioned, a
staged procedure with initial irrigation and débridement followed by
splinting and antibiotics until definitive surgery is deemed
appropriate.
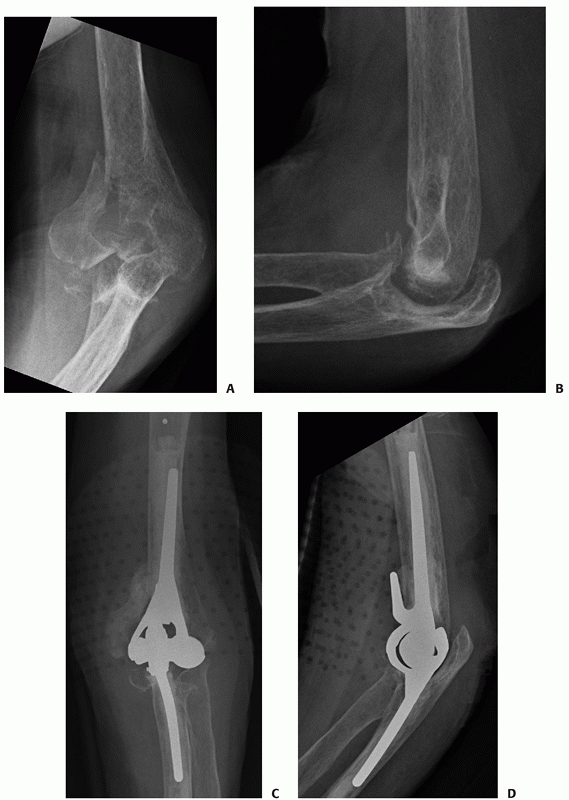 |
|
FIGURE 33-25
AP and lateral radiographs of a 79-year-old woman with rheumatoid arthritis and a displaced intra-articular medial column fracture (A,B) managed with a linked total elbow arthroplasty via a paratricipital approach (C,D). |
procedure and should be done by an experienced upper limb or trauma
surgeon. Radiographs should be templated preoperatively to ensure
implants of the appropriate size and lengths are available. The
surgical approach starts with an extensile posterior skin incision and
the elevation of medial and lateral fasciocutaneous flaps. The ulnar
nerve requires identification and protection during the surgical
procedure as it is vulnerable to direct injury or indirect traction
injury. Presently, the literature is unclear whether the ulnar nerve
should be anteriorly transposed or returned to the cubital tunnel at
the completion of the procedure.
exposure of distal humerus fractures in preparation for elbow
arthroplasty. Although all posterior approaches may be used for
arthroplasty, some have advantages over others. The paratricipital
approach has the advantage of maintaining complete integrity of the
extensor mechanisms, whereas its disadvantage is that it increases the
complexity of the procedure because it provides less visualization of
the elbow joint. The triceps splitting, triceps reflecting, and triceps
dividing approaches all provide good visualization of the elbow joint;
however, they all
disrupt
the triceps insertion in one way or another and therefore require
postoperative protection. Conducting a total elbow arthroplasty through
an olecranon osteotomy is possible, however, not recommended. Ulnar
component fixation may be compromised with certain implant designs.
There are also concerns with osteotomy healing after disruption of the
intramedullary blood supply by ulnar component cementation.
should be used in the setting of distal humerus fracture. Unlinked
designs may be used; however, great care must be taken in anatomically
reducing and rigidly fixing the medial and lateral columns. Anatomic
fixation of the columns allows appropriate tensioning of the medial and
lateral collateral ligaments that are required for unlinked implant
stability.
have shown that condylar resection during total elbow arthroplasty does
not affect strength or functional outcome; therefore, through the
medial arthrotomy while protecting the ulnar nerve, the medial
collateral ligament is released and the medial fracture fragments are
excised. Similarly, through the lateral arthrotomy the lateral
collateral ligament complex is released and the lateral fracture
fragments are excised. The distal humeral shaft with the remaining
metaphyseal bone can now be delivered from under the triceps and ulna.
The humerus is then prepared following the technical manual of the
implant being used. Usually, the metaphyseal cutting blocks are not
required as the fractured condyles have already been excised. The keys
to judging correct humeral component rotation are to examine the
trefoil shape of the distal humerus shaft, as the posterior cortex is
parallel to the axis of elbow joint rotation, and to examine the
insertions of the medial and lateral intermuscular septa, which are
also parallel to the axis. Reaming and broaching of the humeral canal
are done as described in the technical manual and the canal is sized
for a cement restrictor. The length of the humerus and the level of the
joint-line must also be recreated. This is done by placing the resected
condyles onto the remaining humeral shaft to measure the location of
the joint line. The tension in the soft tissues can also be used to
judge appropriate humeral component length, once trial components are
in place. Most humeral components are designed with an anterior flange
that accepts a bone graft, which can be prepared from resected bone
fragments.
|
TABLE 33-6 Summary of Outcomes of Total Elbow Arthroplasty for the Management of Distal Humerus Fractures171
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
requires strategic retractor and arm positioning. The proximal ulna is
delivered medial to the humeral shaft to avoid excessive tension on the
ulnar nerve. The forearm is then rotated 90 degrees, the elbow is
flexed, and a rake retractor is used to draw back the triceps insertion
to allow exposure of the greater sigmoid notch. The ulna is prepared as
per the manufacturer’s recommendations. As with the humerus, particular
attention should be taken to ensure correct ulnar component placement.
The correct rotation of the ulnar component can be determined by
ensuring that the axis of rotation bisects the radial head and that the
axis is parallel to the flat surface on the proximal dorsal ulna.38
A cement gun with a small diameter injector nozzle is used to ensure
complete filling of the intramedullary canal. The humeral and ulnar
components can be cemented together at the same time or separately.
humerus fractures at short-term and mid-term follow-up have been
reproducibly good (Table 33-6).* Most studies have used the Coonrad-Morrey implant (Zimmer, Warsaw, IN), which is
linked and described as semiconstrained because of its “sloppy” hinge.
reduction and internal fixation with elbow arthroplasty in 24 women
over the age of 65 years with AO/OTA type C distal humerus fractures.
They reported improved outcomes in the arthroplasty group at short-term
follow-up. The small sample size and selection bias, however, confounds
the interpretation of the results, as 8 of 12 patients in the
arthroplasty group had rheumatoid arthritis (RA) and none had RA in the
ORIF group. McKee and colleagues113
presented preliminary data from a randomized prospective study
comparing open reduction and internal fixation to elbow arthroplasty in
elderly patients with comminuted distal humerus fractures. Outcomes
were assessed with the Mayo Elbow Performance Score (MEPS) and
Disabilities of the Arm, Shoulder, and Hand (DASH) score. Twenty-one
patients were initially randomized into the two treatment groups;
however, five patients randomized to ORIF were intraoperatively
converted to arthroplasty. At 2 years follow-up, the MEPS was
significantly better in the total elbow arthroplasty group; however,
the DASH score was not significantly different between groups. The
reoperation rate between the arthroplasty and the open reduction and
internal fixation groups was also not significantly different.
studies comparing the outcomes and complications of open reduction and
internal fixation with total elbow arthroplasty for the treatment of
complex distal humerus fractures in elderly persons. It is probable
that the revision surgery rate would increase over time in patients
treated with elbow arthroplasty, when compared with patients undergoing
ORIF because of polyethylene wear, aseptic loosening, periprosthetic
fracture, and infection.
internal fixation, anteroposterior and lateral radiographs of the elbow
out of plaster are usually sufficient to determine the fracture
pattern. If the feasibility of ORIF is questioned in an elderly
patient, a CT scan may assist with preoperative decision making.
ensure implants of the appropriate size and lengths are available.
While awaiting surgery, patients are placed in a well-padded elbow
splint and are encouraged to elevate the arm, ice the elbow, and
maintain hand and finger range of motion. On the day of surgery, the
skin and soft tissues are re-examined and the neurologic status is
redocumented. Patients generally receive a general anaesthetic with an
upper extremity regional block for postoperative pain control and
therapy. Before starting the operative procedure and inflating the
tourniquet, prophylactic antibiotics are administered intravenously.
bolster placed under the ipsilateral scapula and the elbow is supported
by another bolster made of a wrapped sterile sheet on the patient’s
chest (Figure 33-23A). The surgeon and
assistant stand on the side of the injury while the scrub nurse and
arthroplasty instruments are on the contralateral side, allowing the
nurse to assist with arm positioning as required. A sterile tourniquet
is used.
unreparable preoperatively, in which the surgical plan is to proceed
directly to TEA is the paratricipital approach. This is also my
preferred approach if an attempt at ORIF is planned for less comminuted
articular fractures. In circumstances with high articular comminution
in the elderly, in which a complete attempt at ORIF is planned, with
the intraoperative bail-out being a TEA, I prefer the triceps split.
The triceps split approach affords the best visualization of the joint
for a complete attempt at ORIF, and still leaves the option open for a
TEA if rigid internal fixation cannot be achieved.
arthroplasty for trauma and posttraumatic conditions. The ulnar nerve
is identified, released, and prepared for anterior subcutaneous
transposition. The medial and lateral fracture fragments, including the
epicondyles, are excised and the distal end of the humeral shaft is
delivered out from under the ulna and triceps. The humerus is prepared
first with the appropriate rasps and broaches. Correct humeral
component rotation is obtained by ensuring the axis of rotation of the
component is parallel to the posterior cortex of the humeral shaft and
parallel to the insertions of the medial and lateral intermuscular
septa. The correct length of the humeral component is ensured by
replacing the resected medial column fracture fragment on to the distal
humerus. The location of the central axis of the humeral component
should be at the approximate level of the distal aspect of the medial
epicondyle.
arm positioning. The ulna is delivered medial to the distal humeral
shaft to limit tension on the ulnar nerve. The forearm is then rotated
90 degrees, the elbow is flexed, and a rake retractor is used to
retract the triceps insertion to allow exposure of the greater sigmoid
notch. The ulna is prepared using rasps and broaches as per the
implant’s technical manual. The tip of the olecranon may be excised to
improve visualization of the greater sigmoid notch. Correct rotation of
the ulnar component is obtained by ensuring the center axis of the
component bisects the radial head and is parallel to the flat spot of
the dorsal ulna. A test reduction is done with the trial implants and
the elbow is taken through a range of motion to ensure there is no
impingement.
cement restrictors in the humerus and ulna. Cement is inserted into the
humerus first with a small-diameter nozzle and then into the ulna. The
ulnar cement is manually pressurized and the component is inserted,
followed by pressurization of the humerus and humeral component
insertion. Excess bone cement is removed and the components are held
still until the cement has cured. Elbow implants can also be cemented
separately. Once cured, a wedge of bone graft fashioned from the
resected articular segment is placed underneath the anterior flange of
the humeral component. The components are
then linked and the elbow is taken through a range of motion to ensure there is no impingement.
elbow is splinted in extension with an anteriorly applied slab of
plaster. The arm is elevated for 24 hours and active hand range of
motion is started immediately. Elbow range of motion is started between
days 2 and 7 postoperatively, depending on the status of the soft
tissues. Generally, unrestricted active range of motion is encouraged
(flexion, extension, pronation, and supination) for patients with a
paratricipital approach, while patients with a triceps split approach
are restricted to gravity-assisted extension for 6 weeks to protect the
triceps repair.
unreconstructable distal humerus fractures. This procedure has been
described in the past,123,182,191 and has recently experienced a renewed interest.3,73,192
Two commercially available elbow arthroplasty systems have humerus
implants that replicate the distal humeral articular surface, the
Sorbie-Questor (Wright Medical Technology, Arlington, TN) and the
Latitude (Tornier, Stafford, TX), and therefore can be used for
hemiarthroplasty (Figure 33-26). The added
benefit of the Latitude hemiarthroplasty is that it can be converted to
a linked or unlinked total elbow arthroplasty. This is beneficial if
intraoperative hemiarthroplasty stability cannot be achieved
necessitating conversion to total elbow arthroplasty. Other systems
that have nonanatomic humeral components, such as the Kudo (Biomet,
Inc., Warsaw, IN),3 have also been used for hemiarthroplasty. The use of nonanatomic components, however, is not recommended.
virtually identical to total elbow arthroplasty. The theoretical
advantage of a hemiarthroplasty is the absence of polyethylene wear
debris and the associated osteolysis and aseptic loosening, which are
common modes of failure with total elbow arthroplasties.
Hemiarthroplasty, therefore, may function as an “in between” in those
patients with unreconstructable distal humerus fractures who are deemed
too young or too active for total elbow arthroplasty. It should be
noted, however, that the believed benefits of hemiarthroplasty are
completely speculative and no literature exists to support its use over
total elbow arthroplasty.
 |
|
FIGURE 33-26 The Sorbie-Questor (Wright Medical Technology, Arlington, TN) (A) and the Latitude (Tornier, Stafford, TX) total elbow system (B)
are two commercially available arthroplasty systems that have humeral implants that replicate the distal humeral articular anatomy, and therefore can be used for hemiarthroplasty. |
articular segments of the trochlea and capitellum. For it to function
optimally to allow elbow stability and range of motion, it relies on
the integrity of the primary and secondary elbow stabilizers.73,135 Therefore, when considering hemiarthroplasty, the medial and lateral columns must be reconstructable (Figure 33-27),
the radial head and coronoid must be intact, and the medial and lateral
collateral ligaments must be repairable or intact on their respective
condyles.13,73
are similar to those for total elbow arthroplasty. Additional
contraindications include deficient medial or lateral column bone,
deficient medial or lateral collateral ligaments, or fractures of the
radial head or coronoid that cannot be rigidly stabilized. Chondral
damage to the greater sigmoid notch or radial head are also relative
contraindications, as patients may experience postoperative arthritic
pain and limited motion. In these circumstances with deficient bone or
soft tissue, linked total elbow arthroplasty should be considered.
technically demanding procedure and should only be conducted by
surgeons experience in upper limb arthroplasty or complex trauma.
Standard elbow radiographs are usually sufficient for assessing the
fracture pattern and implant templating. Computed tomography (CT) will
confirm the articular fragmentation, will identify occult fractures
(such as fractures of the coronoid and radial head) and may assist with
open reduction and internal fixation of the columns. Patients can be
positioned supine
or
in the lateral decubitus fashion. A sterile tourniquet is used and the
approach starts with a longitudinal posterior skin incision. The ulnar
nerve is identified, released, and prepared for anterior subcutaneous
transposition. The options available for exposure of the elbow for
hemiarthroplasty include: olecranon osteotomy, paratricipital approach,
triceps split, triceps reflection, and triceps dividing approaches
(please see section on surgical approaches). The most commonly used
approaches for hemiarthroplasty are the olecranon osteotomy and the
paratricipital approach. The olecranon osteotomy allows the best
visualization of the joint, however, has a higher rate of complications
if intraoperative conversion to a total elbow arthroplasty is required.
The paratricipital approach maintains integrity of the extensor
mechanism, however, affords less visualization of the articular
surfaces. For hemiarthroplasty, the paratricipital approach can be
modified by maintaining the collateral ligament attachments on the
epicondyles and working through the fracture interval.
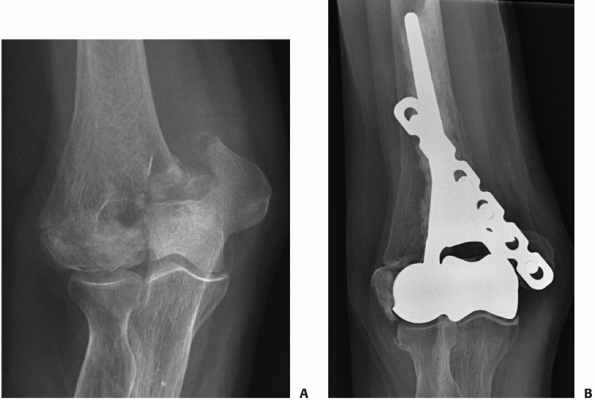 |
|
FIGURE 33-27 Distal humerus hemiarthroplasty with plate fixation of the medial column and suture fixation of the lateral epicondyle.
|
inspected to ensure open reduction and internal fixation is not
possible. If hemiarthroplasty is deemed appropriate, sizing of the
implant should be done next. The determination of correct humeral
component size can be done three ways, preoperatively templating of
contralateral elbow radiographs, piecing together the fractured
trochlea and capitellum and comparing with the available trial
implants, and placing trial implants into the greater sigmoid notch to
select which one best aligns with the coronoid and radial head. The
humeral canal is then entered by resecting the superior aspect of the
olecranon fossa. The canal is reamed and broached to accept the chosen
trial implant. The trial implant must be inserted to the correct depth
to recreate the joint line. Local landmarks, such as the collateral
ligament origins and condyles, are used to gauge correct implant
length. Provisional fixation of one or both of the fractured columns
with Kirschner wires may also assist with determination of the correct
implant length. Conservative bone cuts are then made using the
available cutting blocks. If use of the cutting blocks is not feasible,
conservative free-hand cuts are made and revised as necessary. The
trial implant is then inserted into the humerus and the elbow is
reduced and taken through a range of motion to ensure there is no
restriction or impingement.
trial implant have been determined, the next step involves cementation
of the true prosthesis and definitive fixation of the columnar
fractures. This step can be done in one of several different orders:
(i) the fractured columns can be definitively fixated in anatomic
position with contoured plates/screws, or with K-wires/tension bands
augmented with sutures (Figure 33-28) and then
the implant cemented; (ii) the implant can be cemented first in
anatomic position followed by columnar fracture fixation; (iii) the
less comminuted column is definitively fixated first to allow easier
fracture reduction and to assist with correct implant orientation and
length. The implant is inserted and once the cement has hardened, the
other column undergoes ORIF to the humeral shaft and stable implant.
When conducting this procedure through an olecranon osteotomy, all the
preceding methods are feasible; however, if using a paratricipital
approach only the latter two are possible.
restrictor is inserted and the canal is lavaged and dried. Antibiotic
cement is inserted via a thin-nozzled pressurization gun. All excess
cement is removed, especially around the medial and lateral column
fracture surfaces. Once the implant has been cemented and columnar ORIF
is complete, the elbow is placed through a range of motion and
stability is checked. Postoperatively, the elbow is splinted for 2 to 3
days and then early active assisted range of motion is initiated.
At a mean of 10 months follow-up (range, 3 to 14 months), the Mayo
Elbow Performance Scores were excellent in three patients and good in
one. The mean arc of elbow motion was 106 degrees and all patients
described having no pain.
Parsons et al.150
also reported on four patients undergoing hemiarthroplasty for acute
fractures using the anatomic Sorbie-Questor implant (Wright Medical
Technology, Arlington, TN). At early follow-up, they reported a mean
ASES score of 83.5 and a mean elbow flexion of 130 degrees and
extension of 16 degrees. Unfortunately, these two short-term follow-up
studies are all that is available in the literature; therefore, further
studies are required to determine if distal humeral hemiarthroplasty
for acute trauma is feasible in the long-term.
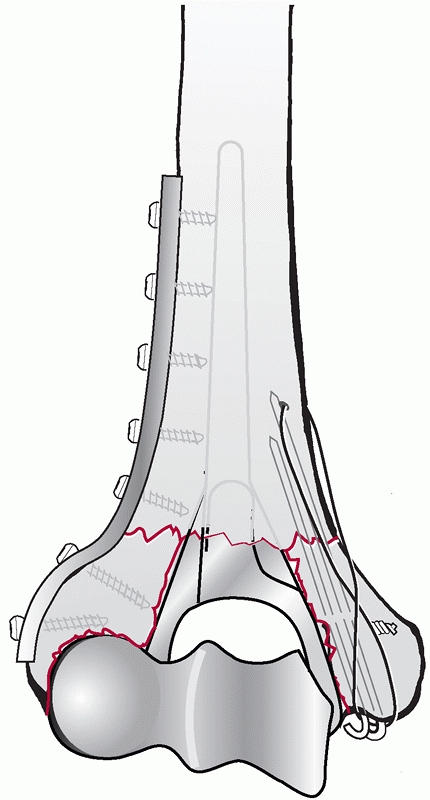 |
|
FIGURE 33-28
Medial and lateral column fixation in hemiarthroplasty for distal humerus fractures may be accomplished by plates and screws, sutures, and tension band constructs. |
Nonunions typically occur at the supracondylar level, are rarely
intra-articular and are usually related to inadequate fixation.5,66,112,148,163,183,184
Other risk factors for nonunion include “low” fracture types with
limited distal bone for screw purchase, extensive comminution, and
severe osteopenia. Patients typically present with pain, stiffness, and
functional limitation. If there is associated failure of fixation, the
patient may also present with abnormal motion owing to a mobile
nonunion.
distal humerus fractures, it is important to establish the cause of the
nonunion. All patients should undergo infection screening blood work
(complete blood count with differential, erythrocyte sedimentation
rate, and C-reactive protein levels). Injury and postoperative
radiographs should be examined critically to determine the initial
facture pattern and the adequacy of initial ORIF. Examining
postoperative radiographs in a serial fashion may reveal the cause of
failure.
splinting, revision open reduction, and internal fixation and elbow
arthroplasty. Splinting with externally applied bone stimulators, such
as ultrasound, may be effective if fixation failure has not occurred.
If surgical treatment is deemed necessary, a CT scan may be beneficial
in examining the quality and quantity of the remaining distal humerus
bone.
the procedure of choice in healthy active patients. Revision procedures
are technically demanding because of the altered anatomy, presence of
failed hardware, excessive scarring, and generally poor bone quality.
Because of these issues, an olecranon osteotomy is the preferred
approach to allow the best access to the joint.5,66,112,163
The goals of surgery are to obtain an anatomic articular reduction,
rigid bicolumn fixation, and stimulate bone healing with autologous
bone graft. Additional procedures that are usually required with
revision ORIF of distal humerus fractures are anterior and posterior
capsulectomy to address elbow stiffness and ulnar nerve neurolysis and
transposition.66,112,163 The outcomes of revision ORIF are generally satisfactory,5,66,112,163 with bony union occurring in greater than 90% of patients.
whether it is due to extensive bone loss or posttraumatic arthrosis. In
these cases, total elbow arthroplasty is a reliable treatment option.115,125,130
Patients that have a healed prior olecranon osteotomy can be approached
via a paratricipital approach, Bryan-Morrey (triceps reflecting)
approach, or by a triceps split. Patients with a nonunion of an
olecranon osteotomy are approached through the osteotomy site. After
elbow arthroplasty, the olecranon is fixated with a K-wire/tension band
construct, plate/screws, or excision of the fragment and triceps
advancement.109 Other treatment options for distal humerus nonunions include arthrodesis,159 resection arthroplasty, allograft distal humerus replacement,2,31,197,198 vascularized bone grafting,17 and Ilizarov methods.21
after open reduction and internal fixation of distal humerus fractures.
Risk factors for elbow stiffness and heterotopic ossification (HO) are
head injury, polytrauma, severe soft tissue injury, delay to surgical
intervention, prolonged postoperative immobilization, and open
fractures.**
The majority of patients with HO do not experience any significant
functional deficits; therefore, resection is not always necessary.
Heterotopic ossification about the elbow can be classified by the
system of Hastings and Graham (Table 33-7).63 The incidence of elbow stiffness and contracture is difficult to determine as
most all patients who undergo ORIF have some limitation in motion. The
distinction between an elbow contracture and a normal postoperative
outcome is dependent on several variables, including patient’s
expectations, activity level, age, and occupation. Morrey, in an effort
to identify the etiology of elbow contractures, has classified them as
intrinsic, extrinsic, and combined.127,128,129 Intrinsic causes involve the articular surface, whereas extrinsic causes include capsular contracture and HO.
|
TABLE 33-7 The Hastings Classification of Heterotopic Ossification
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
limit posttraumatic elbow contracture. For patients at high risk of HO,
such as those with head injures, postoperative indomethacin, and/or
radiation is recommended. For the treatment of elbow contractures,
initial management should be nonoperative with physiotherapy, splints,
and braces. Static progressive splinting under the direction of a
physiotherapist has been reported as an effective method of regaining
elbow range of motion.34
of motion, the elbow may be treated surgically using either open or
arthroscopic techniques. Generally, arthroscopy has a limited role in
the treatment of contractures after distal humerus ORIF because of the
often extensive internal fixation hardware that requires open removal.
Open contracture releases may be done via a medial “over-the-top”
exposure, a lateral column procedure or a combined approach.127,128,129,194
The preoperative assessment of patients includes identification of
prior surgical incisions, examination of the ulnar nerve, and clear
localization of the pathology to determine the most appropriate
surgical approach. The procedure typically involves ulnar nerve
release, capsulectomy, débridement of the olecranon, coronoid and
radial fossae, excision of symptomatic HO, and finally removal of
internal fixation hardware. Postoperatively, patients are managed with
continuous passive motion devices and static progressive splints. The
routine use of indomethacin for HO prophylaxis after contracture
release remains controversial.
delayed until its growth has ceased and it has become corticated.
Preoperative or early postoperative single-dose radiation treatment has
been recommended to decrease HO recurrence; however, there is little
literature to support its use. The excision of HO is associated with
significantly better gains in range of motion than release of
soft-tissue only contractures.149
ORIF of distal humerus fractures. Elbows should be examined to ensure
there are no deep fluid collections that may indicate infected
hematomas or seromas. The management of superficial infections consist
of oral antibiotics, dressing changes and close observation.
Management consists of surgical débridement and organism-specific
intravenous antibiotics. Intraoperatively, fracture fixation is
assessed to ensure it is stable. If stable, the patient is managed with
serial surgical débridements as required and intravenous antibiotics
until healing. If fracture stability is lost, staged revision ORIF is
required along with intravenous antibiotics until healing.
after ORIF of distal humerus fractures. This is managed with surgical
débridement to viable tissue. The remaining soft tissue defect is
assessed to determine whether primary closure is possible or coverage
is required. Coverage options depend on several variables, including
size and depth of the defect, exposed hardware or vital structures,
patient comorbidities, and potential donor site morbidity.27,81 Consultation with a plastic or soft tissue reconstructive surgeon is recommended.
patients with distal humerus fractures. Injury can occur at the time of
fracture, intraoperatively or postoperatively. At the time of fracture,
the nerve may be injured by a direct impact or indirectly by traction
owing to wide displacement of the fracture fragments. Intraoperatively,
injury may occur by traction, manipulation of the nerve, or injury to
its blood supply causing ischemia. Postoperatively, neuritis may occur
by nerve “kinking” in flexion or extension, exuberant scarring, or
irritation against fixation hardware.
ulnar nerve exploration during the surgical procedure. The nerve should
be decompressed and examined with loop magnification to ensure it is
intact. If partially or completely lacerated, the nerve should undergo
microsurgical repair. If the nerve is intact, a complete neurolysis
should be done. The decision whether or not to transpose the nerve
remains controversial.
transposed nerve, can be managed with initial observation. The
management of postoperative neuropathy in a nerve left in situ remains
controversial. Some recommend observation, whereas others recommend
acute decompression with anterior transposition. It is the author’s
practice to conduct a complete release and anterior subcutaneous
transposition in all surgically treated distal humerus fractures.
good. Patients have a high rate of satisfaction, good return of
intrinsic muscle strength, and a return of hand functionality.117 Although the prognosis is generally good after ulnar neuropathy, patients do not return to completely normal.16,117
the articular surface of the distal humerus, and therefore is a
valuable
approach
for comminuted articular fractures. An olecranon osteotomy should be
conducted in a systematic way to avoid complications, such as
inadvertent fracture propagation, incorrect osteotomy location, and
malreduction. Complications associated with olecranon osteotomies have
been reported to occur in 0% to 31% of cases.*
The theorized risk factors for nonunion include use of a tension band
technique, a transverse osteotomy, and single screw fixation, although,
the literature does not support this. Three recent studies30,68,162
on the outcomes of olecranon osteotomy looked at a total of 129
patients. All patients underwent an apex distal chevron osteotomy,
although the types of fixation varied (plates, single medullary screws,
and tension band constructs). There were no nonunions, one delayed
union, three patients had early hardware failure require revision ORIF,
and 18 patients (14%) had hardware removal specifically for irritation.
out infective causes and then revision plate ORIF with autologous bone
grafting. In some cases when revision ORIF is not feasible because of
the small size of the fragment or associated poor bone quality, the
fragment may be excised with advancement of the triceps insertion.109,200 Delayed unions of the olecranon are managed expectantly with consideration given to external bone stimulation devices.
of olecranon osteotomies. Patients may experience local pain,
tenderness, or an inability to rest the elbow hard surfaces. These
symptoms can be addressed by hardware removal after olecranon healing.
infection and wound healing, neuropathies, triceps insufficiency,
instability, osteolysis and loosening, mechanical failure,
periprosthetic fracture, and stiffness.
The rate of infection can be minimized by meticulous surgical
technique, use of perioperative antibiotics, sterile tourniquets, and
antibiotic-laden cement. The consequences of deep infection can be
devastating. The treatment often includes organism-specific intravenous
antibiotics and surgical débridements with possible staged
reconstruction. Organisms such as Staphylococcus epidermidis are particularly difficult to eradicate, and resection arthroplasty may be the consequence.
the elbow. The probability of persistent ulnar neuropathy after total
elbow arthroplasty for trauma is high, and is reported to occur in up
to 28% of patients, with permanent dysfunction in up to 10%.****
Ulnar nerve exposure, complete neurolysis, and anterior transposition
is recommended, although transposition also has risks such as
devascularization. The surgical approach used for elbow arthroplasty is
also influential, as the extended Kocher approach has a higher risk of
postoperative ulnar nerve palsy.69,104
elbow arthroplasty performed via an extensor mechanism disrupting
approach, and is reported in up to 11% of patients.131,152,158,160,203
Surgical exposures that use a triceps-on approach such as the
paratricipital approach, although more technically challenging, may
avoid this complication. When using a tricepsdisrupting approach, this
complication can be minimized by solid anatomic repair of the triceps
insertion and postoperative protection of the repair by avoiding active
extension for 6 weeks. Many patients, such as those who use ambulatory
aids or self-propelled wheelchairs, require a strong intact triceps
mechanism. These patients may be best suited for the paratricipital
approach. Patients who develop extensor mechanism insufficiency and
rely on active extension may benefit from extensor mechanism revision
repair or reconstruction with autograft or allograft.
associated with unlinked designs. These designs rely on correct implant
positioning, preserved bony architecture, and intact soft tissue
stabilizers. Typically, unlinked designs are not used for distal
humerus fracture because of disrupted bony and soft tissue stabilizers;
however, they may be used if these structures are anatomically
repaired. Newer unlinked designs have several advantages. They have
greater contact surface area in the ulnohumeral articulation providing
increased constraint; some have the option of a radial head
arthroplasty, which provides additional stability and others have the
ability to convert to a linked implant. If intraoperative instability
exists in an unlinked arthroplasty after repair of the bony structures
and soft tissues, then conversion to a linked prosthesis should be
performed.
inevitable. Many implants allow for change of the bearing surface
without revision of the components. Accelerated wear rates have been
found in younger patients, in patients with posttraumatic conditions,
and in cases with persistent postoperative malalignment or deformity.75,101,108,125
The problem with polyethylene wear is the host reaction that causes
osteolysis, which can lead to aseptic loosening and loss of bone stock.
Metal fatigue most commonly affects titanium implants because of their
notch sensitivity. Implants at risk are those with insufficient bony
support of their metaphyseal segments caused by fractured or resected
condyles or osteolysis. These at-risk implants experience high
cantilever bending forces at the junction of the poorly supported
metaphyseal segment and the well-fixed diaphyseal segment.
postoperatively. Risk factors for intraoperative fractures include
osteopenic bone, excessive diaphyseal bowing with use of long stem
implants, overly aggressive reaming, and revision cases. Fixation of
condylar fractures when using a linked system is not required; however,
shaft fractures require reduction and stabilization with some
combination of long stem components with cerclage wires, allograft
struts, or plate and screw fixation. Postoperative periprosthetic
fractures can occur secondary to trauma or through pathological bone
weakened by osteolysis. Periprosthetic fractures with unstable
components likely require
revision
arthroplasty in the medically fit patient. Periprosthetic fractures
with stable components may be managed with immobilization or open
reduction and internal fixation. Allograft strut grafts are useful
adjuncts in these situations, especially in those with bone loss.
group of fractures that involve the capitellum and/or trochlea with
variable involvement of other periarticular structures, such as the
epicondyles, radial head, medial collateral ligament, or lateral
collateral ligament complex. These injuries are distal and do not
extend proximal to the olecranon fossa to involve either column. They
are typically caused by coronal shear forces, which fracture the
anterior articular surfaces of the distal humerus. The capitellum is
thought to be particularly susceptible to shear forces because its
center of rotation is more anterior in reference to the humeral shaft.
The most common mechanism of injury is a simple fall on the
outstretched hand from a standing height.
The reported annual incidence of articular fractures of the distal
humerus is 1.5 per 100,000 population, with a marked female
predominance.202 In women, there is
a bimodal distribution with peaks under the age of 19 and above 80
years. The increased prevalence of this injury in women over the age of
60 years is believed to be a result of the increase elbow carrying
angle in women and osteoporosis.56,202
In men there is a unimodal distribution with a peak incidence under the
age of 19, with the mechanism of injury typically being high energy,
such as motor vehicle collisions or falls from heights. Other
associated injuries, such as ligament tears and radial head fractures,
occur in up to 20% of cases.37,167,171,201,202
conducted in all cases as outlined in the beginning of this chapter.
Anteroposterior and lateral radiographs of the elbow are usually
sufficient to initially diagnose the injury; however, they may not be
able to fully characterize complex articular fracture patterns.
Computed tomography is strongly recommended if there is any suspicion
of articular comminution or involvement of the epicondyles, radial
head, posterior capitellum, or trochlea. It is the author’s practice to
routinely obtain CT scans for all articular fractures of the distal
humerus.
modified this classification and added type III fractures, which are
comminuted capitellar fractures. A fourth fracture pattern was added by
McKee et al.,116 which consisted of a type I fracture with medial extension to include the lateral half of the trochlea.
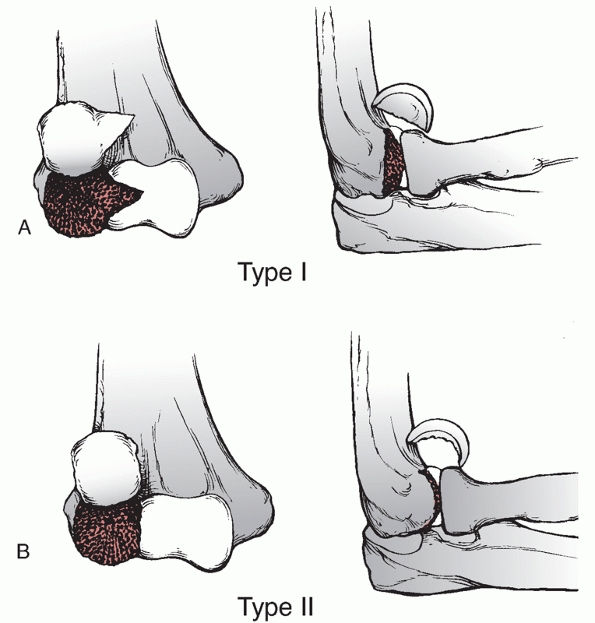 |
|
FIGURE 33-29
The Hahn-Steinthal (Type I) fracture of the capitellum involves the articular surface and a large portion of the subchondral bone (A). The Kocher-Lorenz (Type II) fracture involves the articular surface of the capitellum with a thin layer of subchondral bone (B). |
further examined articular shear fractures of the distal humerus and
described them as a spectrum of injury. They observed that apparent
isolated capitellar fractures on plain radiographs may turn out to be
much more complex injuries when further imaged with CT. The authors
identified five unique fracture patterns that progress in complexity (Figure 33-30).
recently reported another classification for capitellar and trochlear
fractures that was correlated to clinical outcome. The Dubberly et al.37
classification has three types with a modifier for distal
posterolateral column comminution. A type 1 fracture involves primarily
the capitellum with or without the lateral trochlear ridge. A type 2
fracture involves the capitellum and most of the trochlea as one piece,
whereas in the type 3 fracture the capitellum and trochlea are separate
pieces. The authors found that as the complexity of the articular
fractures increased, the outcomes worsened.
distal humerus in young patients is rarely recommended and it is
generally reserved for patients deemed medically unfit to undergo
surgery.
Nonoperative management techniques include above-elbow casting, and
collar and cuff treatment with early mobilization, the so-called “bag
of bones” method.
 |
|
FIGURE 33-30 The Ring et al.167
classification of distal humerus articular fractures has five patterns. A type 1 fracture involves the capitellum and the lateral portion of the trochlea. This fracture pattern has previously been described as a conventional type IV fracture. A type 2 fracture is described as a type 1 fracture that may be comminuted but includes a fracture of the lateral epicondyle. A type 3 fracture is a type 2 fracture that has comminution behind the capitellum with impaction of bone posteriorly. A type 4 fracture is a type 3 fracture with an additional fracture of the posterior trochlea. A type 5 fracture is a type 4 fracture that includes fracture of the medial epicondyle. |
The reduction maneuver involves placing the elbow into full extension
and forearm supination, which usually results in the capitellum
spontaneously reducing. If still displaced, manual pressure over the
capitellum and a slight varus force to the elbow may assist with the
reduction. If successful, the elbow is flexed so the radial head
captures the capitellar fragment and then fluoroscopy is used to
confirm the reduction. The elbow is immobilized in an above-elbow
plaster for 3 weeks with weekly radiographs to confirm maintenance of
the reduction. If this technique is used, the author recommends
postoperative CT imaging to confirm an anatomic reduction.
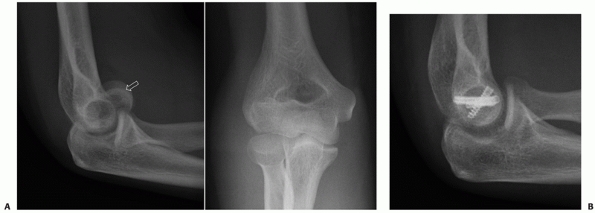 |
|
FIGURE 33-31 Fracture of the capitellum and the lateral ridge of the trochlea (A). The double arc sign116 is evident on the lateral radiograph (arrow).
One arc represents the subchondral bone of the capitellum and the other arc represents the lateral ridge of the trochlea. This patient underwent open reduction and internal fixation with three headless compression screws inserted anterior to posterior (B). |
considered the gold standard for most displaced articular fractures of
the distal humerus. Rigid internal fixation allows fracture healing to
occur anatomically while permitting early range of motion to maximize
function. In cases in which sufficient fracture stability cannot be
obtained to allow early motion, anatomic reconstruction of the
articular surface and overall elbow alignment take precedence. An
anatomically aligned stiff elbow with a healed articular surface can be
subsequently managed with contracture release, but a fracture with
hardware failure and articular nonunion or fragmentation may be
difficult to manage with revision surgery.
Type B3.1). Fractures of the capitellum with or without involvement of
the lateral ridge of the trochlea can be approached through an
extensile posterior skin incision or a direct lateral skin incision.
The advantages of a posterior longitudinal skin incision are that it
allows access both medially and laterally and decreases the risk of
cutaneous nerve injury.36 The deep lateral approach is via the Kocher interval96
between anconeus and extensor carpi ulnaris. The arthrotomy is made
anterior to the lateral ulnar collateral ligament and is extended
proximally along the anterior aspect of the lateral supracondylar
ridge, which then allows access to the fractured capitellum. The
fragment is typically anteriorly displaced and is reduced by elbow
extension, forearm supination, and application of a gentle varus force.
Once an anatomic reduction is obtained the fragment is provisionally
fixated with smooth small-diameter K-wires. Permanent rigid internal
fixation is obtained by countersunk screws placed anterior to posterior
through the articular surface (Figure 33-31), or by screws placed into the capitellum in a retrograde
fashion from the posterior aspect of the lateral column or by a combined method (Figure 33-32).
The placement of posterior to anterior screws has been shown to be
biomechanically more stable and has the added benefit of not violating
the articular surface.42 In cases in which rigid internal fixation is obtained, early active range of motion can be initiated.
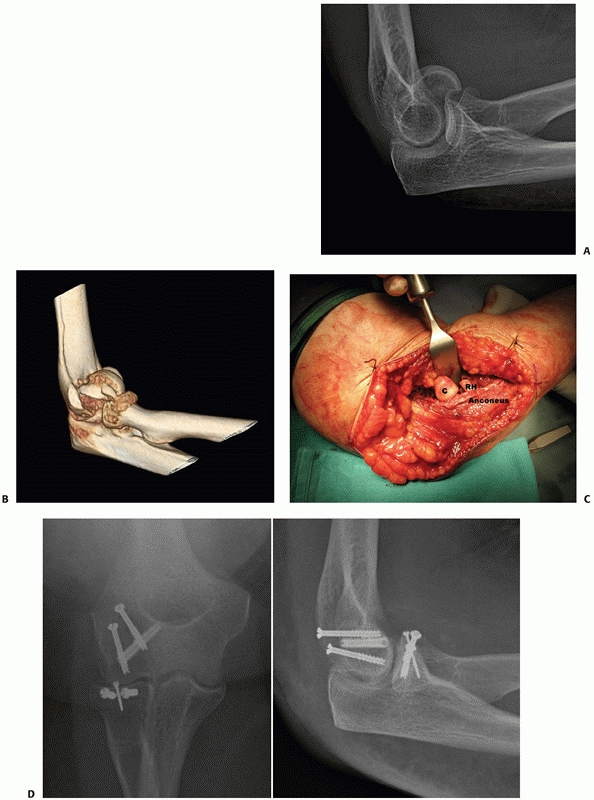 |
|
FIGURE 33-32 Fracture of the capitellum and lateral ridge of trochlea associated with a radial head fracture (A,B).
Through a posterior longitudinal skin incision, the Kocher interval was used to approach the fractures for open reduction and internal fixation (C,D). (C, capitellum; RH, radial head.) |
type B), it may prevent anatomic reduction of the anterior capitellar
fracture. These impaction fractures may require disimpaction and
possibly bone grafting to fill the bony defects. In case with severe
posterior comminution that may compromise
anterior
articular fixation, supplemental posterior lateral column plating may
be required. Capitellar fractures that involve the lateral epicondyle
(Ring et al. type 2) may be exposed by using the lateral epicondylar
fracture as an osteotomy, reflecting the epicondylar fragment with the
origin of the lateral collateral ligament distally (Figure 33-33).
After fixation of the capitellum, the lateral epicondyle fracture may
be fixated with screws or a plate, if large enough. If the fragment is
too small to fixate, it is treated as a lateral ligament tear with
repair through bone tunnels.
or conventional type II osteochondral fractures is difficult, as the
fragments are thin and may be comminuted. Treatment options for these
fractures include attempted fixation with bioabsorbable pins, excision
of the fragment, osteochondral grafts, or capitellar arthroplasty.
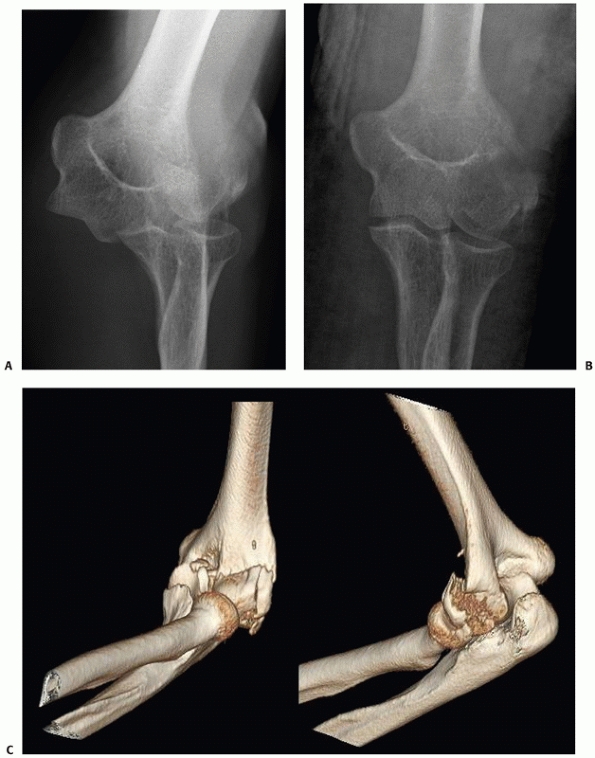 |
|
FIGURE 33-33
Posterolateral dislocation of the elbow associated with a capitellar fracture, lateral epicondyle fracture, and comminution and impaction of the posterior aspect of the lateral column (A-C). (continues) |
and should be treated with open reduction and internal fixation.
Fixation may be done antegrade through the cartilage or retrograde from
posterior through a medial deep approach.
also require anatomic reduction and rigid internal fixation. Generally,
these fractures require improved exposure of the medial trochlea and
several surgical approach options exist. The lateral collateral
ligament origin on the lateral epicondyle can be released, to allow the
elbow to be hinged open laterally. By releasing the anterior and
posterior joint capsules the distal humerus articular surface is booked
open on the intact medial collateral ligament. A similar approach uses
a lateral epicondylar fracture, which may be reflected distally to
hinge open the elbow joint. Medial joint exposure can also be obtained
by a separate medial approach, such as the flexor-pronator split or the
Hotchkiss medial “over-the-top” approach. Finally, an olecranon
osteotomy can be used to obtain optimum distal humerus articular
exposure.
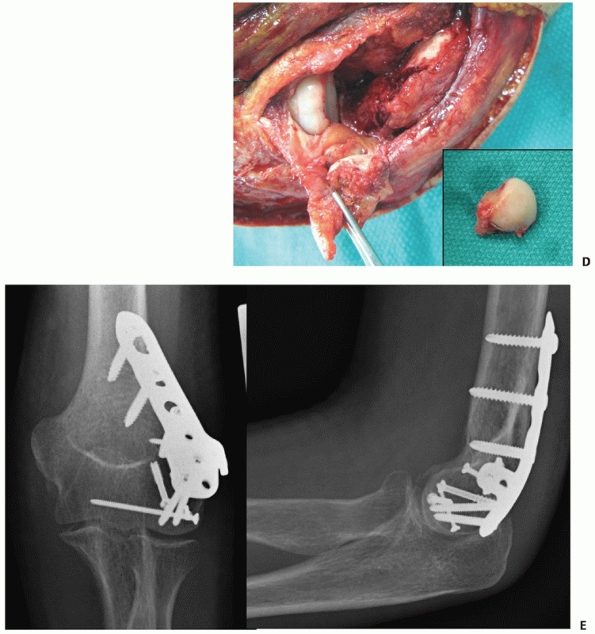 |
|
FIGURE 33-33 (continued) The lateral epicondyle fracture with the lateral collateral ligament (forceps) was reflected distally (D), allowing access to the free capitellar fragment (inset). Because of the posterior comminution, a posterolateral plate was required to support the articular segment (E).
|
small-diameter headless compression or standard countersunk screws
inserted antegrade, or with standard screws inserted retrograde.
Fractures that are comminuted or have epicondylar involvement may
benefit from additional plate application. Bone grafting may also be
required for fractures that are comminuted or impacted.
and osteopenia, or in patients with pre-existing conditions of the
elbow such as rheumatoid arthritis, open reduction and internal
fixation may not be feasible. In such cases where rigid internal
fixation cannot be achieved, elbow arthroplasty is a reliable treatment
option. Total elbow arthroplasty can be done through a paratricipital
approach using a linked implant. (Please see prior section on TEA
insertion technique.) Distal humerus hemiarthroplasty is another
surgical option for unreconstructable articular fractures (Figure 33-34).
In cases with severe articular destruction with preserved columns and
collateral ligaments, hemiarthroplasty presents an attractive option
that resurfaces the damaged articulation. The theoretical advantage of
a hemiarthroplasty is the absence of polyethylene wear debris and the
associated osteolysis; however, it is a technically demanding procedure
and no literature exists to support its use over total elbow
arthroplasty. Further studies are required to determine the role of
distal humerus hemiarthroplasty in elbow trauma.
of capitellar fractures with or without involvement of the lateral
trochlear
ridge have been shown to be predictably good.* More complex fracture patterns with involvement of the anterior trochlea also have a relatively good prognosis;37,116,167,188 however, Dubberley et al.37 have shown that the outcomes do deteriorate with increasing fracture complexity.
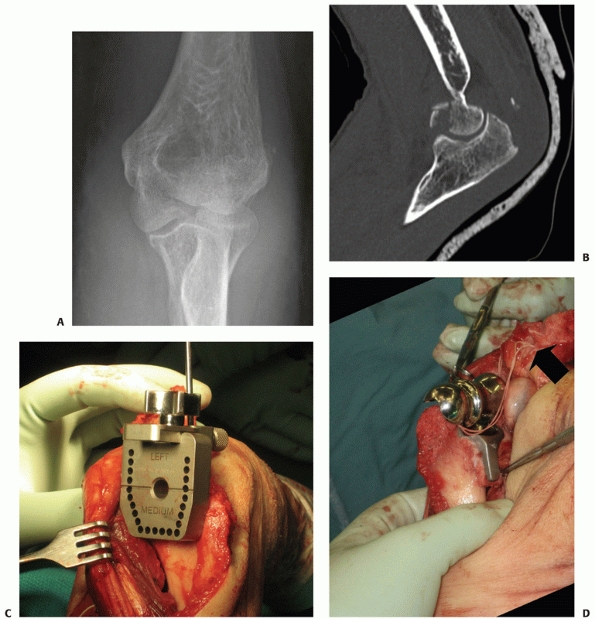 |
|
FIGURE 33-34 Fractures of the capitellum, trochlea and lateral epicondyle (A) with associated osteochondral fragmentation (B)
in a 78-year-old active woman. As the fracture was deemed unrepairable intraoperatively, hemiarthroplasty was done via an approach that hinged open the elbow on the intact medial collateral ligament (C,D). (continues) |
This is initially managed with nonoperative techniques, such as
physiotherapy and splints. In refractory cases, open and arthroscopic
contracture releases have proved successful. Other complications
include nonunion,166 malunion,18 ulnar neuritis,167 and avascular necrosis.37,103
the last few years. The use precontoured and locking plates has become
ubiquitous; however, no clinical advantages have been reported. Further
study is required to determine if their additional cost leads to
improved patient outcomes, especially in today’s fiscally responsible
health care environment.
predictably good outcomes in short to medium term follow-up; however,
as with all total joints, the survivorship decreases over time. The
role of hemiarthroplasty also requires further investigation to
determine if it effectively functions as an intermediate to total joint
replacement.
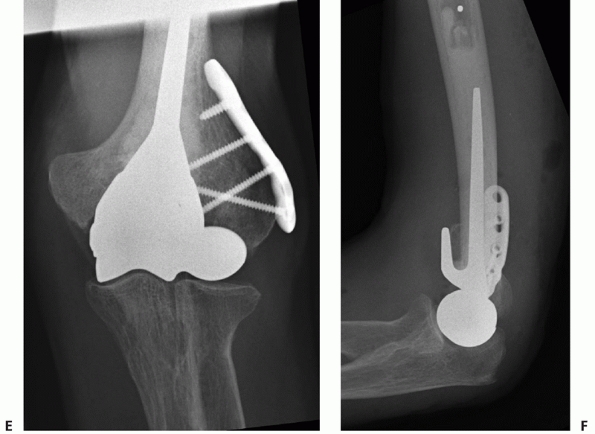 |
|
FIGURE 33-34 (continues The lateral epicondyle fracture was fixated with sutures through the axis of the implant (arrow) and with a precontoured unicortical plate (E,F).
|
C. Michael Robinson, Graham J.W. King, Kenneth J. Faber, Jennifer M.
Wolf, Samir Mehta, and Eric Benson for their assistance with the
preparation of this chapter.
and dislocation compendium. Orthopaedic Trauma Association Committee
for Coding and Classification. J Orthop Trauma 1996;10 Suppl 1:v-ix,
1-154.
L, Hammer R. Elbow hemiarthroplasty for acute reconstruction of
intraarticular distal humerus fractures: a preliminary report involving
four patients. Acta Orthop 2006;77(5):785-787.
A, Douglas H, Stanley D. Revision surgery for nonunion after early
failure of fixation of fractures of the distal humerus. J Bone Joint
Surg Br 2005;87(8):1107-1110.
CA, Allende BT, Allende BL, et al. Intercondylar distal humerus
fractures— surgical treatment and results. Chir Main 2004;23(2):85-95.
M. Bilaterotricipital approach to the elbow. Its application in the
osteosynthesis of supracondylar fractures of the humerus in children.
Acta Orthop Scand 1972;43(6):479-490.
AD, Dunning CE, Faber KJ, et al. Single-strand ligament reconstruction
of the medial collateral ligament restores valgus elbow stability. J
Shoulder Elbow Surg 2002;11(1):65-71.
MW, Reeves A, MacLeod IA, et al. A biomechanical comparison of plate
configuration in distal humerus fractures. J Orthop Trauma
2008;22(5):332-336.
N, Willett K. Functional outcome following internal fixation of
intraarticular fractures of the distal humerus (AO type C). Acta Orthop
Belg 2004;70(2):118-122.
GS, Goetz TJ, Pollock JW, et al. Prosthetic replacement for distal
humerus fractures. Orthop Clin North Am 2008;39(2):201-212, vi.
GS, Hoxie SC, Rispoli DM, et al. Precontoured parallel plate fixation
of AO/OTA type C distal humerus fractures. J Orthop Trauma
2009;23(8):578-580.
GS, Rispoli DM, Steinmann SP. The anconeus flap transolecranon approach
to the distal humerus. J Orthop Trauma 2006;20(4):282-285.
RH, Grotenhuis JA. Anterior submuscular transposition of the ulnar
nerve. For postoperative focal neuropathy at the elbow. J Bone Joint
Surg Br 2004;86(7): 998-1001.
PK, Hotchkiss RN, Athanasian EA, et al. Recalcitrant nonunion of the
distal humerus: treatment with free vascularized bone grafting. Clin
Orthop Relat Res 2005 Jun(435):134-9.
R, Kolundzic R, Anticevic D. Absorbable implants in surgical correction
of a capitellar malunion in an 11-year-old: a case report. J Orthop
Trauma 2006;20(1): 66-69.
DM, Steinbuch M, Palermo L, et al. An assessment tool for predicting
fracture risk in postmenopausal women. Osteoporos Int
2001;12(7):519-528.
AR, Wongworawat MD. Accuracy in the measurement of compartment
pressures: a comparison of three commonly used devices. J Bone Joint
Surg Am 2005; 87(11):2415-2422.
MR, O’Connor DP, Crouch CC, et al. Ilizarov treatment of infected
nonunions of the distal humerus after failure of internal fixation: an
outcomes study. J Orthop Trauma 2007;21(3):178-184.
RF, Morgan RG. Intercondylar T-shaped fractures of the humerus. Results
in ten cases treated by early mobilisation. J Bone Joint Surg Br
1971;53(3):425-428.
RS, Morrey BF. Extensive posterior exposure of the elbow. A
triceps-sparing approach. Clin Orthop Relat Res 1982 Jun(166):188-192.
RS, Morrey BF. Fractures of the distal humerus. In: Morrey BF, editor.
The Elbow and Its Disorders. Philadelphia: WB Saunders, 1985, pp.
302-339.
UH, Moran SL, Li S, Khan S. Soft-tissue coverage of the elbow: an
outcome analysis and reconstructive algorithm. Plast Reconstr Surg
2007;119(6):1852-1857.
TM, Jago ER, Sidhu DP, Markovic L. Fractures of the capitellum: a new
method of fixation using a maxillofacial plate. Clin Orthop Relat Res
2001 Mar(384):232-236.
TK, Morrey BF. Total elbow arthroplasty as primary treatment for distal
humeral fractures in elderly patients. J Bone Joint Surg Am
1997;79(6):826-832.
CP, Barei DP, Nork SE, et al. The olecranon osteotomy: a 6-year
experience in the treatment of intraarticular fractures of the distal
humerus. J Orthop Trauma 2006; 20(3):164-171.
GS, Holliger EHT, Urbaniak JR. Elbow allograft for reconstruction of
the elbow with massive bone loss. Long-term results. Clin Orthop Relat
Res 1997 Aug(341): 12-22.
T, Botte MJ, Abrams RA. Anatomical considerations regarding the
posterior interosseous nerve during posterolateral approaches to the
proximal part of the radius. J Bone Joint Surg Am 2000;82(6):809-813.
J, Lindenhovius A, Kloen P, et al. Two- and three-dimensional computed
tomography for the classification and management of distal humeral
fractures. Evaluation of reliability and diagnostic accuracy. J Bone
Joint Surg Am 2006;88(8): 1795-1801.
JN, Ring D, Jupiter JB. Static progressive splinting for posttraumatic
elbow stiffness. J Orthop Trauma 2006;20(6):400-404.
JN, van Duijn PJ, Linzel D, et al. Surgical treatment of
intra-articular fractures of the distal part of the humerus. Functional
outcome after 12 to 30 years. J Bone Joint Surg Am 2007;89(7):1524-1532.
PA, Bain GI, King GJ, Patterson SD. The midline posterior elbow
incision. An anatomical appraisal. J Bone Joint Surg Br
1995;77(5):696-699.
JH, Faber KJ, Macdermid JC, et al. Outcome after open reduction and
internal fixation of capitellar and trochlear fractures. J Bone Joint
Surg Am 2006;88(1): 46-54.
N, Dunning CE, Johnson JA, et al. The flat spot of the proximal ulna: a
useful anatomic landmark in total elbow arthroplasty. J Shoulder Elbow
Surg 2004;13(2): 206-207.
CE, Zarzour ZD, Patterson SD, et al. Ligamentous stabilizers against
posterolateral rotatory instability of the elbow. J Bone Joint Surg Am
2001;83-A(12): 1823-1828.
ET, Goldwasser M, Bonomo AL. Functional outcome of complex
intercondylar fractures of the distal humerus treated through a
triceps-sparing approach. J Shoulder Elbow Surg 2008;17(3):441-446.
SJ, Polatsch DB, Egol KA, et al. Capitellum fractures: a biomechanical
evaluation of three fixation methods. J Orthop Trauma
2002;16(7):503-506.
KJ, Cordy ME, Milne AD, et al. Advanced cement technique improves
fixation in elbow arthroplasty. Clin Orthop Relat Res 1997
Jan(334):150-156.
MA, Herscovici D Jr, DiPasquale TG, et al. A comparison of open
reduction and internal fixation and primary total elbow arthroplasty in
the treatment of intraarticular distal humerus fractures in women older
than age 65. J Orthop Trauma 2003; 17(7):473-480.
BJ, Moussa F, Schott T. Healing rate of transverse osteotomies of the
olecranon used in reconstruction of distal humerus fractures. J South
Orthop Assoc 1995;4(4): 263-268.
R, Riand N, Stern R, et al. Total elbow replacement for complex
fractures of the distal humerus. An option for the elderly patient. J
Bone Joint Surg Br 2001; 83(7):974-978.
JA, Mykula R, Stanley D. Complex fractures of the distal humerus in the
elderly. The role of total elbow replacement as primary treatment. J
Bone Joint Surg Br. 2002; 84(6):812-816.
DE, O’Hollaren RM. Fractures and dislocations about the elbow in the
head-injured adult. Clin Orthop Relat Res 1982 Aug(168):38-41.
M, Hotchkiss RN, Weiland AJ. Alternative operative exposures of the
posterior aspect of the humeral diaphysis with reference to the radial
nerve. J Bone Joint Surg Am 1996;78(11):1690-1695.
EV. Perforation of the coronoid-olecranon septum. Humeroulnar
relationships in Netherlands and African populations. Am J Phys
Anthropol 1967;26(1):85-92.
WT, Macdermid JC, Patterson SD, et al. Functional outcome of AO type C
distal humeral fractures. J Hand Surg [Am] 2003;28(2):294-308.
W, Clement H, Pichler W, et al. The influence of lateral and anterior
angulation of the proximal ulna on the treatment of a Monteggia
fracture: an anatomical cadaver study. J Bone Joint Surg Br
2007;89(6):836-838.
S, Haas NP, Bail HJ. Outcome after open reduction and angular stable
internal fixation for supra-intercondylar fractures of the distal
humerus: preliminary results with the LCP distal humerus system. Arch
Orthop Trauma Surg 2008;128(7):723-729.
R, Khanchandani P. Intercondylar fractures of the distal humerus in
adults: a critical analysis of 55 cases. Injury 2002;33(6):511-515.
RB, Anderson JT. Prevention of infection in the treatment of one
thousand and twenty-five open fractures of long bones: retrospective
and prospective analyses. J Bone Joint Surg Am 1976;58(4):453-458.
H 2nd, Graham TJ. The classification and treatment of heterotopic
ossification about the elbow and forearm. Hand Clin 1994;10(3):417-437.
H 2nd, Theng CS. Total elbow replacement for distal humerus fractures
and traumatic deformity: results and complications of semiconstrained
implants and design rationale for the Discovery Elbow System. Am J
Orthop 2003;32(9 Suppl):20-28.
DL, Hotchkiss RN. Internal fixation of the distal humerus: a
biomechanical comparison of methods. J Orthop Trauma 1990;4(3):260-264.
DL, Kloen P, Anand N, et al. Open reduction and internal fixation of
delayed unions and nonunions of fractures of the distal part of the
humerus. J Bone Joint Surg Am 2003;85-A(1):33-40.
DL, Schmeling GJ. Bicondylar intraarticular fractures of the distal
humerus in adults. Clin Orthop Relat Res 1993 Jul(292):26-36.
SP, Parkinson RW, Noble J. Capitellocondylar total elbow replacement
for rheumatoid arthritis. J R Coll Surg Edinb 1991;36(2):133-135.
BJ, Mossad MM. Fractures of the adult distal humerus. Elbow function
after internal fixation. J Bone Joint Surg Br 1990;72(3):362-365.
RN. Elbow contractures. In: Green DP, Hotchkiss RN, Pederson WC, eds.
Green’s Operative Hand Surgery. Philadelphia: Churchill Livingstone,
1999, pp. 673-674.
TL, Chiu FY, Chuang TY, Chen TH. The results of open reduction and
internal fixation in elderly patients with severe fractures of the
distal humerus: a critical analysis of the results. J Trauma
2005;58(1):62-69.
JSPM. Distal humeral hemiarthroplasty. In: Yamaguchi GK, McKee MD,
O’Driscoll SW, eds. Advanced Reconstruction Elbow. Rosemont, NY:
American Academy of Orthopaedic Surgeons, 2006, pp. 219-228.
FG, Cioffi DA, Amadio PC, et al. The American Academy of Orthopaedic
Surgeons outcomes instruments: normative values from the general
population. J Bone Joint Surg Am 2002;84-A(2):208-215.
M, Belt EA, Kautiainen H, Lehto MU. Souter arthroplasty for elbows with
severe destruction. Clin Orthop Relat Res 2004 Apr(421):126-133.
J, Morito Y, Hashizume H, Inoue H. Internal fixation for coronal shear
fracture of the distal end of the humerus by the anterolateral
approach. J Shoulder Elbow Surg 2001;10(6):554-556.
J, Ogura T, Morito Y, et al. Anatomic and histologic studies of lateral
collateral ligament complex of the elbow joint. J Shoulder Elbow Surg
1999;8(6):625-627.
SR, Glisson RR, Urbaniak JR. Comparison of distal humerus fracture
fixation: a biomechanical study. J South Orthop Assoc 1997;6(4):241-249.
TL, Sievanen H, Khan KM, et al. Shifting the focus in fracture
prevention from osteoporosis to falls. BMJ 2008;336(7636):124-126.
JB, Neff U, Holzach P, Allgower M. Intercondylar fractures of the
humerus. An operative approach. J Bone Joint Surg Am 1985;67(2):226-239.
JB, Neff U, Regazzoni P, Allgower M. Unicondylar fractures of the
distal humerus: an operative approach. J Orthop Trauma
1988;2(2):102-109.
S, Sinopidis C, El Meligy M, et al. Unlinked elbow arthroplasty as
primary treatment for fractures of the distal humerus. J Shoulder Elbow
Surg 2008;17(2): 287-292.
S, Morrey BF. Distal humeral fractures treated with noncustom total
elbow replacement. J Bone Joint Surg Am 2004;86-A(5):940-947.
S, Morrey BF. Distal humeral fractures treated with noncustom total
elbow replacement. Surgical technique. J Bone Joint Surg Am 2005;87
Suppl 1(Pt 1):41-50.
P. Preventing osteoporosis, falls, and fractures among elderly people.
Promotion of lifelong physical activity is essential. BMJ
1999;318(7178):205-206.
P, Niemi S, Parkkari J, et al. Why is the age-standardized incidence of
lowtrauma fractures rising in many elderly populations? J Bone Miner
Res 2002;17(8): 1363-1367.
EB. Surgical approaches to the proximal end of the radius and its use
in fractures of the head and neck of the radius. J Bone Joint Surg Am
1941;23:86.
RP, Griffin TW. Open reduction of T-condylar fractures of the humerus
through an anterior approach. J Trauma 1969;9:901-914.
T. Beitrage zur kenntniss einger praktisch wishctiger fraktur formen.
Mitheil a Klin u Med Inst and Schweiz Basal, reihe. 1896:767.
J, Lill H, Muller LP, et al. Distal humerus fractures in elderly
patients: results after open reduction and internal fixation.
Osteoporos Int 2005;16(Suppl 2):S73-79.
JE, Louis DS, Loder RT. Divergent single-column fractures of the distal
part of the humerus. J Bone Joint Surg Am 1995;77(4):538-542.
K, Braun W, Wieberneit J, et al. Intraarticular distal humerus
fractures. Factors affecting functional outcome. Clin Orthop Relat Res
1996 Nov(332):200-208.
KT, Lai CH, Singh S. Results of total elbow arthroplasty in the
treatment of distal humerus fractures in elderly Asian patients. J
Trauma 2006;61(4):889-892.
N, Katz T, Howard CB, et al. Fixation of capitellar fractures with the
Herbert screw. Arch Orthop Trauma Surg 1991;110(3):155-157.
P, Jonsson K, Rydholm U. Short-term complications of the lateral
approach for non-constrained elbow replacement. Follow-up of 50
rheumatoid elbows. J Bone Joint Surg Br 1995;77(6):937-942.
M, Kiral A, Solakoglu C, et al. Treatment of fractures of the humeral
capitellum using herbert screws. J Hand Surg [Br] 2006;31(3):320-325.
P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed
and stiff elbows. J Bone Joint Surg Am 2000;82(9):1260-1268.
G, Morrey BF, Gallay SH, et al. Fracture and nonunion of the olecranon
in total elbow arthroplasty. J Shoulder Elbow Surg. 2006;15(4):486-494.
JL, Slongo TF, Agel J, et al. Fracture and dislocation classification
compendium, 2007: Orthopaedic Trauma Association classification,
database and outcomes committee. J Orthop Trauma 2007;21(10
Suppl):S1-133.
M, Jupiter J, Toh CL, et al. Reconstruction after malunion and nonunion
of intra-articular fractures of the distal humerus. Methods and results
in 13 adults. J Bone Joint Surg Br 1994;76(4):614-621.
MD. Randomized trial of ORIF versus total elbow arthroplasty for distal
humerus fractures. AAOS 2007 February 16, 2007; San Diego; 2007.
MD, Jupiter JB. A contemporary approach to the management of complex
fractures of the distal humerus and their sequelae. Hand Clin
1994;10(3):479-494.
MD, Jupiter JB, Bamberger HB. Coronal shear fractures of the distal end
of the humerus. J Bone Joint Surg Am 1996;78(1):49-54.
MD, Jupiter JB, Bosse G, Goodman L. Outcome of ulnar neurolysis during
posttraumatic reconstruction of the elbow. J Bone Joint Surg Br
1998;80(1):100-105.
MD, Kim J, Kebaish K, et al. Functional outcome after open
supracondylar fractures of the humerus. The effect of the surgical
approach. J Bone Joint Surg Br 2000;82(5):646-651.
MD, Pugh DM, Richards RR, et al. Effect of humeral condylar resection
on strength and functional outcome after semiconstrained total elbow
arthroplasty. J Bone Joint Surg Am 2003;85-A(5):802-807.
MD, Wilson TL, Winston L, et al. Functional outcome following surgical
treatment of intra-articular distal humeral fractures through a
posterior approach. J Bone Joint Surg Am 2000;82-A(12):1701-1707.
RHP. Arthroplasty of the elbow by replacement of the distal portion of
the humerus with an acrylic prosthesis. J Bone Joint Surg Am
1947;29:348-353.
JK, King GJ. Total elbow arthroplasty in the treatment of posttraumatic
conditions of the elbow. Clin Orthop Relat Res 2000 Jan(370):102-114.
BF. Posttraumatic contracture of the elbow. Operative treatment,
including distraction arthroplasty. J Bone Joint Surg Am
1990;72(4):601-618.
LP, Kamineni S, Rommens PM, et al. Primary total elbow replacement for
fractures of the distal humerus. Oper Orthop Traumatol
2005;17(2):119-142.
SW. The triceps-reflecting anconeus pedicle (TRAP) approach for distal
humeral fractures and nonunions. Orthop Clin North Am 2000;31(1):91-101.
SW, Morrey BF, Korinek S, An KN. Elbow subluxation and dislocation. A
spectrum of instability. Clin Orthop Relat Res 1992 Jul(280):186-197.
SW, Sanchez-Sotelo J, Torchia ME. Management of the smashed distal
humerus. Orthop Clin North Am 2002;33(1):19-33, vii.
SA, Rhorer AS. Orthopaedic trauma for the general orthopaedist:
avoiding problems and pitfalls in treatment. Clin Orthop Relat Res 2005
Apr(433):30-37.
H, Urguden M, Soyuncu Y, et al. [Long-term functional results of adult
intraarticular distal humeral fractures treated by open reduction and
plate osteosynthesis]. Acta Orthop Traumatol Turc 2002;36(4):328-335.
H, Solak S, Turanli S, et al. Intercondylar fractures of the distal
humerus treated with the triceps-reflecting anconeus pedicle approach.
Arch Orthop Trauma Surg 2005; 125(7):469-474.
J, Bjorkenheim JM. Operative treatment of type C intercondylar
fractures of the distal humerus: results after a mean follow-up of 2
years in a series of 18 patients. J Shoulder Elbow Surg
2002;11(1):48-52.
M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic
fractures of the distal humerus in elderly women. Eur J Epidemiol
1998;14(2):159-164.
M, Kannus P, Parkkari J, et al. The injury mechanisms of osteoporotic
upper extremity fractures among older adults: a controlled study of 287
consecutive patients and their 108 controls. Osteoporos Int
2000;11(10):822-831.
N, Babis GC, Kalavritinos J, et al. Operative treatment of type C
intraarticular fractures of the distal humerus: the role of stability
achieved at surgery on final outcome. Injury 1995;26(3):169-173.
M, O’Brien RJ, Hughes JS. Elbow hemiarthroplasty for acute and salvage
reconstruction of intra-articular distal humerus fractures. Techn
Shoulder Elbow Surg 2005; 6(2):87-97.
D, Claydon P, Stanley D. Total elbow replacement using the Kudo
prosthesis. Clinical and radiological review with 5- to 7-year
follow-up. J Bone Joint Surg Br 2003; 85(3):354-357.
N, Dent C. Outcome of total elbow replacement for distal humeral
fractures in the elderly: a comparison of primary surgery and surgery
after failed internal fixation or conservative treatment. J Bone Joint
Surg Br 2008;90(3):343-348.
PM, Weiland AJ. The triceps-preserving approach for semiconstrained
total elbow arthroplasty. J Shoulder Elbow Surg 2008;17(3):454-458.
PS, Kakarlapudi K, Rajsekhar C, et al. Total elbow arthroplasty as
primary treatment for distal humeral fractures in elderly patients.
Injury 2000;31(9):687-692.
RR, Khoury GW, Burke FD, et al. Internal fixation of capitellar
fractures using Herbert screws: a report of four cases. Can J Surg
1987;30(3):188-191.
D, Gulotta L, Chin K, Jupiter JB. Olecranon osteotomy for exposure of
fractures and nonunions of the distal humerus. J Orthop Trauma
2004;18(7):446-449.
D, Gulotta L, Jupiter JB. Unstable nonunions of the distal part of the
humerus. J Bone Joint Surg Am 2003;85-A(6):1040-1046.
D, Jupiter JB. Operative release of complete ankylosis of the elbow due
to heterotopic bone in patients without severe injury of the central
nervous system. J Bone Joint Surg Am 2003;85-A(5):849-857.
EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A
comparison of operative and nonoperative treatment in twenty-nine
cases. J Bone Joint Surg Am 1969;51(1):130-141.
DM, Athwal GS, Morrey BF. Neurolysis of the ulnar nerve for neuropathy
following total elbow replacement. J Bone Joint Surg Br
2008;90(10):1348-1351.
RM, String ST. Arterial injuries in extremity shotgun wounds: requisite
factors for successful management. Surgery 1984;96(5):902-908.
CM. Fractures of the distal humerus. In: Bucholz RWHJ, Court-Brown C,
Tornetta P, et al., eds. Rockwood and Green’s Fractures in Adults. 6th
ed. Philadelphia: Lippincott Williams and Wilkins, 2005, pp. 1051-116.
CM, Hill RM, Jacobs N, et al. Adult distal humeral metaphyseal
fractures: epidemiology and results of treatment. J Orthop Trauma
2003;17(1):38-47.
SH, Melton LJ 3rd, Morrey BF, et al. Epidemiologic features of humeral
fractures. Clin Orthop Relat Res 1982 Aug(168):24-30.
J, Torchia ME, O’Driscoll SW. Complex distal humeral fractures:
internal fixation with a principle-based parallel-plate technique. J
Bone Joint Surg Am 2007; 89(5):961-969.
J, Torchia ME, O’Driscoll SW. Complex distal humeral fractures:
internal fixation with a principle-based parallel-plate technique.
Surgical technique. J Bone Joint Surg Am 2008;90(Suppl 2):31-46.
RA, Raney EM, Pipkin S. Operative treatment of bicondylar
intra-articular fractures of the distal humerus. Orthopedics
1992;15(2):159-163.
EH, Tencer AF, Henley MB. Biomechanical evaluation of methods of
internal fixation of the distal humerus. J Orthop Trauma
1994;8(6):468-475.
TA, Nork SE, Mills WJ, Henley MB. Extensor mechanism-sparing
paratricipital posterior approach to the distal humerus. J Orthop
Trauma 2003;17(5):374-378.
I, Korner J, Arzdorf M, et al. Mechanical comparison in cadaver
specimens of three different 90-degree double-plate osteosyntheses for
simulated C2-type distal humerus fractures with varying bone densities.
J Orthop Trauma 2008;22(2):113-120.
J, Viegas SF, Buford WL Jr, et al. A comparison of double-plate
fixation methods for complex distal humerus fractures. J Shoulder Elbow
Surg 1995;4(1 Pt 1):10-16.
PG, Johnson DP. Elbow hemiarthroplasty with 20-year follow-up study. A
case report and literature review. Clin Orthop Relat Res 1990
May(254):128-33.
J, Sandelin J, Bostman O. Mechanical failures of internal fixation in T
and Y fractures of the distal humerus. J Trauma 1992;33(5):687-690.
J, Sandelin J, Bostman O. Postoperative complications of distal humeral
fractures. 27/96 adults followed up for 6 (2-10) years. Acta Orthop
Scand 1992;63(1): 85-89.
JL, Chan BK, Low CO. Surgical fixation of intra-articular fractures of
the distal humerus in adults. Injury 2004;35(1):44-54.
M, Kaplan EB. The relationship of the ulnar nerve to the medial
intermuscular septum in the arm and its clinical significance. Hand
1976;8(3):239-242.
RJ, Morgenlander JC, Nunley JA. Ulnar nerve function following total
elbow arthroplasty: a prospective study comparing preoperative and
postoperative clinical and electrophysiologic evaluation in patients
with rheumatoid arthritis. J Hand Surg [Am] 2000;25(2):360-364.
E, Paxinos O. The treatment and functional outcome of type IV coronal
shear fractures of the distal humerus: a retrospective review of five
cases. J Orthop Trauma 2003;17(4):279-284.
K, Cunneen S, Morgan R, et al. Comparative stability of perpendicular
versus parallel double-locking plating systems in osteoporotic
comminuted distal humerus fractures. J Orthop Res 2008;26(6):778-784.
DM, Stevens PS. A humeral replacement prosthesis for the elbow: results
in ten elbows. J Bone Joint Surg Am 1974;56(6):1147-1158.
B, Scott RD. Humeral hemiarthroplasty of the elbow joint in young
patients with rheumatoid arthritis: a report on 7 arthroplasties. J
Arthroplasty 1999;14(5): 553-559.
A, Nakamura H, Yoshioka T, et al. Postoperative results and
complications of total elbow arthroplasty in patients with rheumatoid
arthritis: three types of nonconstrained arthroplasty. Mod Rheumatol
2008 May 29.
V, Daluiski A, Simic P, Hotchkiss RN. Outcome of open release for
posttraumatic elbow stiffness. J Trauma 2006;61(3):673-678.
TK, Scham SM. A posteromedial approach to the proximal end of the ulna
for the internal fixation of olecranon fractures. J Trauma
1969;9(7):594-602.
M, Panagopoulos A, Papadopoulos AX, et al. Functional evaluation of
comminuted intra-articular fractures of the distal humerus (AO type C).
Long-term results in twenty-six patients. Acta Orthop Belg
2004;70(2):123-130.
Gorder GW. Surgical approach in supracondylar “T” fractures of the
humerus requiring open reduction. J Bone Joint Surg Am 1940;22:278.
AJ, Weiss AP, Wills RP, et al. Capitellocondylar total elbow
replacement. A long-term follow-up study. J Bone Joint Surg Am
1989;71(2):217-222.
JM, Stanley D. Posterior surgical approaches to the elbow: a
comparative anatomic study. J Shoulder Elbow Surg 2001;10(4):380-382.
K, Sweet FA, Bindra R, et al. The extraosseous and intraosseous
arterial anatomy of the adult elbow. J Bone Joint Surg Am
1997;79(11):1653-1662.
KH, Park HW, Park SJ, et al. Lateral J-plate fixation in comminuted
intercondylar fracture of the humerus. Arch Orthop Trauma Surg
2003;123(5):234-238.
