Bone and Joint Infections in Children
different forms of acute and subacute osteomyelitis and septic
arthritis. The treatment of these bone and joint infections has
evolved. We currently recommend a short course of IV antibiotics for
almost all types of bone and joint infections followed by oral therapy.
Surgical debridement is necessary for infections with documented
abscesses and where there has been bone destruction. The debridement of
the infected bone enhances antibiotic penetration and thereby shortens
the course of IV antibiotics. Antibiotic therapy alone is sufficient if
the bone infection is diagnosed early, and there is no abscess found or
bone destruction seen radiographically. The need for arthrotomy for the
drainage of acute septic arthritis of the hip has become controversial.
Nevertheless, most authors and pediatric orthopaedic surgeons feel that
anterior arthrotomy of the hip is the safest and most secure means of
drainage of the infected hip. Other forms of bone and joint infection
are discussed thoroughly.
has continued to evolve since the development of antibiotics in the
early 1940s. We have witnessed the development of penicillin resistance
by staphylococci and the subsequent development of the semisynthetic
penicillins and cephalosporins, which eradicate these
penicillin-resistant staphylococci. Because of the risk of chronic bone
infection, acute hematogenous osteomyelitis was traditionally treated
with 6 weeks of intravenous (IV) antibiotics in the hospital. However,
a short course (5 to 10 days) of IV antibiotics in the hospital
followed by a longer course of oral therapy has been shown to be
effective in eradicating these infections. The incidence of acute bone
and joint infections in children has declined. The reason for the
decline is unclear; however, numerous articles in the literature have
documented this reduced incidence of these infections.
ways. The age of the child at the onset of the infection determines the
type of infection that develops. Acute hematogenous osteomyelitis
behaves differently in neonates from the way it does in children.
Because of the existence of blood vessels that cross the growth plate
in neonates and infants younger than age 18 months, the bone infection
that develops in that age group will likely cross the physis; however,
in older children, acute infections rarely cross the growth plate (Figure 5-1).
severity of the infection and the rapidity with which it develops.
Acute hematogenous osteomyelitis has a rapid onset, and children with
this illness are usually seen within one to several days from the onset
of the infection. Another form of bone infection is subacute
hematogenous osteomyelitis, which resembles the acute form; however,
the children are less ill, and the infection causes fewer systemic
findings (Table 5-1). Chronic osteomyelitis is
usually present for months before either detection or treatment. It may
also result from inadequate treatment of an acute bone infection. It
more commonly is the result of an infection that is secondary to an
open fracture of a long bone.
infecting organism, which is the most common means of production of
osteomyelitis in the child. Bone may also become infected secondarily
from the spread of a contiguous area of infection, although this is
uncommon. A bone infection may also result from direct inoculation of
bacteria. If infection occurs, it is usually the result of an open
fracture of a long bone or penetration of a bone such as is seen after
nail punctures of the foot. Lastly, bone infections may be classified
according to the type of infectious agent. Both pyogenic and
granulomatous organisms may infect bone. We discuss only pyogenic
infections in this chapter.
osteomyelitis begins. The nutrient artery of the long bone divides
within the medullary canal of the bone, ending in small arterioles that
ascend toward the physis. Just beneath the physis, these arterioles
bend away from the physis and empty into venous lakes that drain into
the medullary cavity. It is here, in the bend of these arterioles, that
the infection begins. Bacteria injected into the osseous circulation
are phagocytosed in the medullary cavity of the bone; phagocytosis,
however, seems to be less active in the metaphysis. This differential
in phagocytosis may explain the predilection of the metaphysis for the
development of acute hematogenous osteomyelitis. A lack of
reticuloendothelial cells in the metaphysis often exists, so bacteria
that lodge there are more likely to multiply and establish an
infection. The bacteria may also lodge in the metaphysis because of a
decrease in the rate of circulation at the bend of the terminal
arterioles before they empty into the venous lakes. Nade has shown with
the use of electron microscopy that the new metaphyseal vessels that
are growing as the physis itself grows have a lack of an endothelial
lining. Therefore, the blood that circulates in these vessels is in
direct contact with the recently ossified metaphyseal bone. Not only
would the red blood cells be in direct contact with the osteocytes, but
any circulating bacteria would also directly contact the metaphyseal
bone. This fact is the most likely reason for the nearly universal
localization of acute hematogenous osteomyelitis in the metaphysis of
long bones.
 |
|
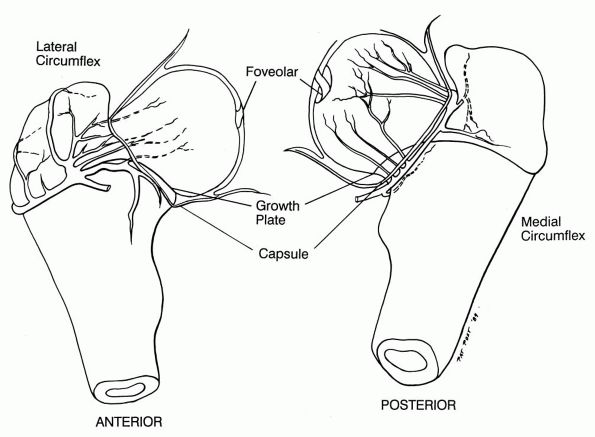
FIGURE 5-1.
Views of the hip in a neonate. The intraosseous circulation in the femoral head of a neonate is different from that in a child older than 12 to 18 months. Blood vessels are seen crossing the growth plate in the femoral neck and head of a neonate. |
bone infection in a certain location. Children with acute hematogenous
osteomyelitis frequently complain of trauma as an inciting incident. It
is well known that the trauma may simply bring the child’s attention to
a preexisting lesion. It has been shown, however, that rabbits in whom
a bone is traumatized develop an infection more frequently in the
traumatized bone than in nontraumatized areas after the production of a
bacteremia. Clinical evaluations of patients with acute hematogenous
osteomyelitis frequently reveal a history of blunt trauma to the
affected bone. This may well be an accurate cause and effect, but the
association of trauma is always considered by anyone who has a painful
extremity whether or not trauma is the cause.
|
TABLE 5-1. Comparison of Acute and Subacute Hematogenous Ostemyelitis
|
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
if the infection remains untreated, pus is produced. The fluid formed
seeks the path of least resistance for egress from the metaphysis. The
fluid can spread in one of three ways. It can spread from the
metaphysis into the diaphysis; it can spread into the epiphysis across
the physis; or it can exit
the
cortex of the bone, producing a collection of subperiosteal pus.
Although an infection that commences in the metaphysis of a long bone
may occasionally spread into the diaphysis, it rarely does so. Instead,
the infection generally spreads through the cortex of the metaphysis of
the bone of a child. It is important to this discussion to understand
the anatomy of a human bone at different ages to appreciate how the
same infection behaves differently in patients of various ages. For
example, the presence of transphyseal vessels in the neonate allows an
infection that begins in the metaphysis of a long bone to easily spread
into the epiphysis through these vessels (see Figure 5-1).
osteomyelitis that begins in the metaphysis of a long bone usually
spreads through the cortex of the metaphysis. The metaphyseal cortex of
the infant is porous, thereby providing easy access for the egress of
an exudate or pus. As the fluid exits the bone cortex, it elevates the
periosteum, which in the child is loosely adherent to the cortex of the
bone. The periosteum is, however, thick and therefore not easily
penetrated, so the pus remains subperiosteal until there is enough
periosteal destruction to allow the development of a soft tissue
abscess. The periosteum of the adult is more thickly adherent to the
bone cortex, but it is also much thinner and easily torn.
that begins in the metaphysis produces loss of the endosteal blood
supply of the involved bone because of thrombosis of the venous and
arterial blood supply. Once pus escapes through the metaphyseal cortex,
it elevates the periosteum, thereby depriving the bone of its remaining
vascular supply. The portion of the bone that has become avascular is
termed a sequestrum. The elevated
periosteum remains viable because its blood supply, which is derived
from the overlying muscle, is undisturbed. The cambium layer of the
periosteum continues to produce bone; however, this bone is produced at
a distance from the bone cortex because the periosteum has been
elevated. This new periosteal bone is termed the involucrum.
“cellulitis” phase in which no obvious pus has been produced. The
patient will exhibit at the signs of a bone infection, but there will
be no obvious pus formation. If this infection is left unchecked, an
abscess will form. The pus then escapes through the cortex of the
metaphysis of the long bone, producing a subperiosteal abscess. This
concept of an initial cellulitis of bone is important because it is
during this stage that medical treatment alone usually results in cure
of the infection.
organism that causes the most cases of acute hematogenous
osteomyelitis—is responsible for more than 90% of cases in otherwise
normal children. Other organisms may produce acute hematogenous
osteomyelitis, such as bacteroides, pneumococcus, Kingella kingae, and Haemophilus influenzae. In neonates, Staphylococcus is still common, as are group B Streptococcus and Gram-negative organisms. In patients with sickle cell disease, both Staphylococcus and Salmonella are known to cause acute hematogenous osteomyelitis. Group-A β-hemolytic streptococcus
is the most common organism in patients with bone, joint, and soft
tissue infections (necrosing fasciitis), which occur as a complication
of varicella. This organism may also be seen in children who have not
been infected with varicella. They tend to be very young (preschool
age), have a very high temperature, and have a high leucocytosis.
depends on a high index of suspicion. Children with acute bone pain and
systemic signs of sepsis should be considered to have acute
hematogenous osteomyelitis until proved otherwise. Unfortunately, not
all children with osteomyelitis have the characteristic findings
typically associate with this disease, so the diagnosis is not always
easily made. It is important, however, to make the diagnosis of acute
hematogenous osteomyelitis early in the course of the disease, because
both the course of the disease and its ultimate prognosis depend on the
rapidity and adequacy of treatment.
not been established, the diagnosis of acute hematogenous osteomyelitis
may be established if a patient fulfills two of the following criteria:
(1) bone aspiration yields pus; (2) bacterial culture of bone or blood
is positive; (3) presence of the classical signs and symptoms of acute
osteomyelitis exists; and (4) radiographic changes typical for
osteomyelitis occur. It is important to have diagnostic criteria for
acute hematogenous osteomyelitis; these criteria should include the
typical patient history and physical findings because occasionally the
cultures of bone may be negative. Even with negative cultures one may
make the presumptive diagnosis of acute hematogenous osteomyelitis
if
other criteria have been established. Most of the time, however, the
diagnosis of acute hematogenous osteomyelitis may result from the
typical history and physical findings combined with positive bone and
blood cultures. Not all patients with acute hematogenous osteomyelitis
will have positive bone or blood cultures; therefore, the other above
criteria may have to be used to confirm the diagnosis. Interestingly,
it has been shown that culture negative patients have a less severe
disease than do culture positive patients with acute hematogenous
osteomyelitis. They are less likely to have antecedent trauma,
overlying skin changes, and less duration of pain.
present with a history of bone pain of one to several days’ duration.
The pain may be well localized if the child is old enough to cooperate.
The pain may be poorly localized if the child is young or if the area
of involvement produces confusing findings, such as might be seen in
patients with osteomyelitis of the pelvis. The pain usually is severe
enough to seriously limit or completely restrict the use of the
involved extremity. The child usually is febrile and relates a history
of generalized malaise consistent with the generalized sepsis. Some
children, however, present without generalized sepsis and therefore do
not exhibit all of these complaints. Thus, do not exclude the diagnosis
of acute hematogenous osteomyelitis simply on the basis of a lack of
sepsis, because this disease may be more or less virulent depending on
the organism involved and the host resistance.
important to establish the correct diagnosis. The examination may be
difficult to perform because these children are frightened and
experience considerable pain. Approach the child slowly and carefully,
gaining the child’s confidence before beginning the examination. This
usually takes a few extra minutes, but the time is well spent. First
attempt to establish which limb is involved before the examination
begins and also have an idea where in the limb the pain is localized.
The examiner begins by palpating the uninvolved areas of the extremity
after the rest of the child has been examined. The final portion of the
examination focuses on the area of involvement.
have swelling of the involved extremity. The swelling is localized to
the area of the infection unless the infection has spread to involve
much of the soft tissues of the extremity. Early in the course of the
disease, the swelling is localized to the metaphysis of the involved
long bone, which is warm. The overlying skin, however, is not red
unless the bone involved is subcutaneous or unless the infection has
spread and a subcutaneous abscess has developed.
child suspected of having osteomyelitis; however, acute bone and joint
infections are diagnosed by clinical means, and laboratory studies are
used only as confirmatory evidence and never to make a diagnosis.
rate should be obtained, both of which are usually elevated. In
addition, the differential count of the white blood cells frequently
shift left. Although these blood studies are usually abnormal in
children with acute hematogenous osteomyelitis, never dismiss the
diagnosis of acute hematogenous osteomyelitis simply because the white
blood cell (WBC) count or the sedimentation rate is normal. Neonates
frequently have no signs of infection; which makes the diagnosis of
osteomyelitis more difficult in them.
obtained; however, the bone changes that are characteristic of
osteomyelitis are not seen for at least 10 to 14 days after the onset
of the infection. Soft tissue swelling with loss of the normal soft
tissue planes is seen before bone changes become apparent. The
radiographic finding of soft tissue swelling, however, simply confirms
the findings of a good physical exam that has established the existence
of the swelling and determined its location (Figure 5-2).
Magnetic resonance imaging (MRI) has not been shown to be of greater
benefit for the evaluation of suspected osteomyelitis than have other
more conventional modalities, but in some instances MRI may be useful
in the evaluation. Gadolinium-enhanced MRI has the advantage of
excellent delineation of fluid collections and soft tissue. It is,
therefore, helpful in identifying medullary edema
consistent
with osteomyelitis in cases where the diagnosis is unclear and in
identifying subperiosteal or adjacent soft tissue abscess that may
require drainage. Additionally, MRI has been used to differentiate
osteomyelitis from acute medullary bone infarct in patients with sickle
cell disease and systemic lupus erythematosus. Cost and need for
sedation in young children are the main disadvantages of MRI in
comparison to other imaging techniques.
 |
|
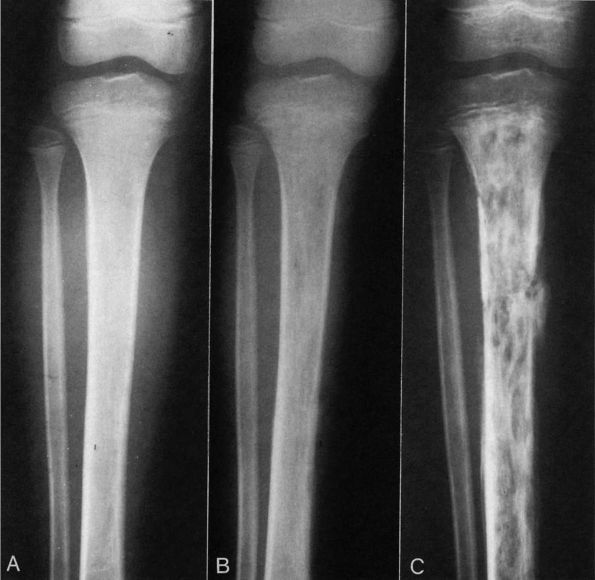
FIGURE 5-2. Radiographs of a child with progressive radiographic changes as the result of acute hematogenous osteomyelitis of the tibia. (A) AP radiographs of the tibia that demonstrate soft tissue swelling with loss of the normal tissue planes. (B)
Radiographs of the same leg taken 2 weeks after the one in A. Note the early mottled appearance of the tibia with a small amount of periosteal new bone seen on the medial cortex of the tibia. (C) Five weeks after presentation this AP radiograph of the tibia shows progressive destruction of the entire diaphysis and proximal metaphysis of the tibia. (Courtesy of Dr. R.H. Hensinger) |
evaluation of children with suspected osteomyelitis. With the advent of
technetium-99m (99mTc) bone scanning, the evaluation of the
abnormal bone in the child became possible. The low radiation dose of
this radioisotope and its affinity for bone make it ideal for the
evaluation of the skeleton. The 99mTc is taken up in areas
of rapid bone formation or in areas of increased blood flow. Thus, one
would expect that there would be increased uptake of 99mTc
in areas of acute hematogenous osteomyelitis, but this is not always
the case. The bone scan has been shown to have an accuracy rate as low
as 80% in patients with acute hematogenous osteomyelitis. In some
children, especially those in whom the infection is fulminant, the
involved bone may have become avascular by the time of the scan,
producing a cold scan. The reason for the cold scan is the loss of the
endosteal circulation as a result of
occlusion
of the nutrient artery along with the loss of the periosteal
circulation resulting from the elevation of the periosteum by a
subperiosteal abscess. This cold scan should alert one that the
infection of the bone demands immediate drainage.
of osteomyelitis much as one would use the sedimentation rate or the
WBC count; however, treatment must never be delayed while awaiting the
results of a bone scan. It is important to note that needle aspiration
of a bone does not alter the bone scan. Thus, bone aspiration itself
will not cause a bone scan to be positive.
of an acute infection is in doubt, such as in infections of the spine
or the pelvis. Bone scanning may be of use in neonates, in whom
multiple sites of infection are common, and it may also be helpful in
the case of bone pain in children with sickle cell disease, for whom
the differential diagnosis of acute hematogenous osteomyelitis from
acute bone infarction is difficult.
because radioactive gallium localizes in white blood cells and would
seem to be more specific for osteomyelitis than is 99mTc. Gallium scanning may be more specific than technetium scanning, but it is not more sensitive. If a 99mTc
scan is negative, a gallium scan is likely to be negative also. In
addition, the gallium scan requires at least 24 to 36 hours for an
adequate study. Some authors have advocated the use of gallium scanning
for the differentiation of bone infarction from osteomyelitis in
patients with sickle cell disease.
tissue abscess, especially if there is an inadequate response to
antibiotic therapy.
osteomyelitis is established, a bacteriologic diagnosis is made by
culturing the involved bone. Bone aspiration is mandatory not only to
establish an accurate bacteriologic diagnosis but also to determine
whether an abscess is present. Aspiration of the bone should be
performed immediately after completion of the physical evaluation so
that treatment may be started.
swelling and pain, which is usually at the metaphyseal end of a long
bone. Use a large bore needle such as a 16- or 18-gauge spinal needle
with an inner stylet. The stylet is necessary to prevent plugging the
end of the needle with bone. The needle is inserted just to the outer
cortex of the bone, and the subperiosteal space is aspirated. If an
abscess is encountered, the pus is cultured and a Gram’s stain is
performed. In this instance, the diagnosis has been firmly established,
and the need for drainage of the abscess has been determined. If no
abscess is encountered, the needle is advanced into the bone through
the cortex, which can be accomplished with ease in the metaphysis. The
needle is gently twisted as it is advanced into the bone. Once the
needle is through the cortex and is in the medullary cavity of the
bone, the marrow is aspirated. Usually one obtains only marrow, but
this must be cultured because almost invariably the cultures are
positive. If no pus is found, the infection is in an early cellulitis
stage (i.e., before an abscess has developed). If an abscess is
encountered, it should be cultured and surgically drained.
obtain cultures of any and all lesions that could potentially have been
the source of a bacteremia. Blood cultures should be obtained, too, but
they should not be relied on to make a bacterial diagnosis because only
50% of patients with acute hematogenous osteomyelitis have positive
blood cultures. We have studied a large group of patients with
confirmed acute hematogenous osteomyelitis to evaluate the rate and
location of bacterial recovery. We found that there were about 50% of
blood cultures positive as has previously been reported. In addition,
about 60% of the bone aspirations were positive for bacteria. When we
combined both bone and blood culture the bacterial recovery rate was
70%, therefore, the likelihood of obtaining a bacteriologic diagnosis
is enhanced if cultures of all possible sources plus the blood have
been obtained. Isolating an etiologic organism for susceptibility
testing has become even more important in recent years as rates of
colonization and infection with methicillin-resistant S. aureus
(MRSA) continue to rise in pediatric patients. MRSA is found not only
in patients with identifiable risk factors (i.e., health care
acquired), but also increasingly in those lacking traditional risk
factors (i.e., community acquired).
sufficient antibiotic must be delivered to the site of the infection
for an adequate period to
sterilize
the bone and eradicate the infection. Controversy occasionally arises
between pediatrician and orthopaedist concerning the appropriate form
of treatment. The pediatrician may recommend only parenteral antibiotic
therapy, whereas the orthopaedic surgeon may recommend both surgical
drainage and antibiotic treatment. This controversy will not arise if
acute hematogenous osteomyelitis is considered an infectious disease
rather than either a surgical or a medical disease. Principles of the
treatment of infection then become evident. It is well established that
sequestered abscesses require surgical drainage, but areas of simple
inflammation without abscess formation respond to antibiotics alone.
Therefore, bone aspiration is important in determining the future
course of therapy for the child with an osteomyelitis. If an abscess is
encountered either under the periosteum or within the bone itself,
surgical drainage of the abscess is required. If no abscess is found,
antibiotics alone should suffice in eradicating the infection, because
treatment begins during the cellulitis stage of the infection, before
the formation of an abscess.
patient does not respond to appropriate antibiotic therapy after a
negative bone aspiration. In that instance, an abscess may have
developed that requires drainage. If a child with acute hematogenous
osteomyelitis does not show symptomatic improvement with decrease in
swelling and tenderness after 36 to 48 hours of appropriate antibiotic
treatment, the bone should be aspirated again and surgical drainage
considered. The fever should also begin to decline, although it may
remain elevated for several more days.
should be approached directly over the area of involvement. A
subperiosteal abscess should be thoroughly drained and debrided.
Whether or not the bone should be opened is subject to debate. Some
authors think that if a subperiosteal abscess is found, an intraosseous
abscess is not likely because it would have spontaneously drained
itself into the subperiosteal space through the porous metaphyseal
cortex. Conversely, if no abscess is found under the periosteum, the
intraosseous abscess will not have drained itself into the
subperiosteal space, and the bone must be opened. Although we and
others have not found pus under pressure within the bone cortex at the
time of drainage of a subperiosteal abscess, it is probably wise to
drill the metaphyseal cortex to be certain that no abscess is
sequestered within the bone. It is probably not necessary to widely
open the bone to curette it unless pus is discovered at the time of
drilling of the bone cortex.
tissue damage than has already been created by the infection itself. If
pus escapes through the metaphyseal cortex, the periosteum is elevated
and the periosteal blood supply is compromised, leaving the bone cortex
avascular. When draining an abscess, do not elevate the periosteum more
than it has already been elevated, so as to avoid creating further
sequestration of the bone.
be closed over a drain. It is not necessary nor recommended to leave
the wound open, unless dealing with a chronic, long-standing
osteomyelitis. A suction drain or a Penrose drain may be used and
removed in 2 to 4 days. Closed suction-irrigation is not necessary and
introduces a significant risk of superinfection with Gram-negative
organisms.
obtained, whether or not surgical drainage is necessary. The initial
choice of antibiotic is made on a best-guess basis. At least 90% of the
cases of acute hematogenous osteomyelitis in otherwise normal children
are caused by coagulase-positive staphylococci. Thus, the antibiotic
chosen should be one that effectively treats this organism. For
patients who are not allergic to penicillin, a semisynthetic penicillin
that is β-lactamase resistant should be chosen. The antibiotic of
choice is either oxacillin or nafcillin. Methicillin is also effective,
but this antibiotic carries a higher risk of interstitial nephritis
than do the others. These agents remain the most rapidly bactericidal
drugs for susceptible strains of staphylococci, have a desirably narrow
spectrum of activity, and demonstrate a proven track record in the
treatment of acute osteomyelitis. In choosing nafcillin, be careful
with peripheral needle sites for the administration of the drug IV,
because nafcillin may cause significant sloughing of the skin and
subcutaneous tissues if infiltration of the IV solution occurs (Table 5-2).
Cefazolin is an acceptable alternative, at a dosage of 150 mg/kg/24 hr,
in place of the semisynthetic penicillin. In patients with identifiable
risk factors for MRSA, empiric use of vancomycin should be considered
while awaiting culture and susceptibility data. In places where a
significant percentage of community-acquired staphylococcal isolates
are
methicillin
resistant, local susceptibility patterns of such isolates should guide
empiric antibiotic choice. Clindamycin has generally been effective in
treating community-acquired MRSA infections in children, including
acute osteomyelitis, and this is a particularly attractive option in
patients who are allergic to penicillin. Regardless of risk factors or
local rates of resistance, it is recommended that patients with
life-threatening infections likely to be staphylococcal be treated
empirically with both vancomycin and a semisynthetic penicillin such as
oxacillin or nafcillin.
|
TABLE 5-2. Doses of Antibiotics Used in Osteomyelitis
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
administered in divided doses over 24 hours. The appropriate length of
therapy has been a subject of debate for many years. In the past,
children with acute hematogenous osteomyelitis were treated for 6 weeks
with IV antibiotics in the hospital. It became apparent that this was
excessive, and a regimen of 3 weeks of IV antibiotics followed by 3
weeks of oral therapy was adopted. Because studies have shown that
adequate blood levels of antibiotic may be achieved with oral
administration, the current mode of therapy involves a shorter period
of initial IV therapy, given a good response by the patient, followed
by oral therapy.
accepted as the standard treatment for acute hematogenous
osteomyelitis. This mode of therapy is more complicated than the simple
treatment of the child with IV antibiotics for the entire course,
because it requires the complete cooperation of the family and the
child. In addition, the antibiotic must be adequately absorbed from the
gastrointestinal tract, providing sufficient blood levels of the drug.
with IV antibiotics. If the patient responds quickly to this form of
therapy, consider switching the child to oral antibiotics (Table 5-2). To do this, the patient must meet certain requirements (Table 5-3).
awaiting the culture results. Once the organism is identified, the
antibiotic is adjusted if necessary. It is important to retain the
bacteria so that the laboratory may test it against the antibiotic
being used to be certain that adequate blood levels can be obtained. If
the child responds quickly to the initial therapy with IV antibiotics,
consider beginning oral therapy. The IV antibiotics are continued for
at least 5 days, although some physicians prefer to treat with IV
antibiotics for a longer period before beginning oral therapy. Oral
therapy is begun 5 to 7 days after the initiation of IV therapy if the
child shows a good clinical response to the initial treatment.
compliance and patient tolerance of the drug. If the patient is
reliable, he or she may be discharged
from
the hospital and followed as an outpatient once all studies have been
completed. Treatment should continue for a total of 4 to 6 weeks, which
includes the time of IV and oral therapy combined (Table 5-3).
|
TABLE 5-3. Contraindications to Oral Antibiotic Therapy
|
|
|---|---|
|
dicloxacillin may be started at a dosage of 100 mg/kg/24 hr. As an
alternative, cephalexin may be administered at a dosage of 150 mg/kg/24
hr. The oral suspension form of dicloxacillin is not palatable, and
parents may find it difficult to persuade young children to swallow it.
For that reason, cephalexin may be preferred, as the oral suspension of
this antibiotic is more palatable. Cephalexin may also be used as the
IV drug, at a dosage of 150 mg/kg/24 hr, in place of the semisynthetic
penicillin. The oral dosage of clindamycin is 30 mg/kg/24 hr IV,
followed by 50 mg/kg/24 hr by mouth. This is particularly attractive in
patients who are allergic to penicillin. Clindamycin has excellent oral
bioavailability, but as with dicloxacillin, the oral suspension has an
unpleasant taste that may contribute to therapeutic noncompliance
(MRSA) has become a more common pathogen in bone and joint infections
in children. This organism used to be thought of as hospital acquired,
however, community-acquired MRSA is now seen very frequently. Accurate
culture of bone and joint infections is necessary to document the
organism because of the prevalence of MRSA. Any child who does not
respond appropriately to adequate therapy should be considered to have
infection caused by MRSA.
should use vancomycin, 50 mg/kg/24 hr IV, combined with rifampin, 15
mg/kg/24 hr orally as covered earlier.
that seen in children because of the variety of organisms involved, the
frequency of multiple sites of infection, and the presence of
transphyseal vessels until age 12 to 18 months, which leads to
infection on both sides of the physis. As a result, the infection
destroys the center of ossification of the epiphysis and the physis
itself, producing complete growth arrest (Figure 5-3).
This is most likely to occur in the proximal femur, where the result is
destruction of the head of the femur. The infection frequently spreads
out of the involved epiphysis into the joint, producing a septic
arthritis.
are also common; therefore, antibiotics that cover all of the organisms
must be given while awaiting the results of cultures. Neonates with
acute hematogenous osteomyelitis frequently have multiple sites of
involvement—as often as 40% of the time. Infants with multiple sites of
osteomyelitis are usually sick before the onset of
the
infection, and most have an umbilical catheter. Infants with single
sites of osteomyelitis have a milder disease and are generally less ill
than those with multiple sites of infection.
 |
|
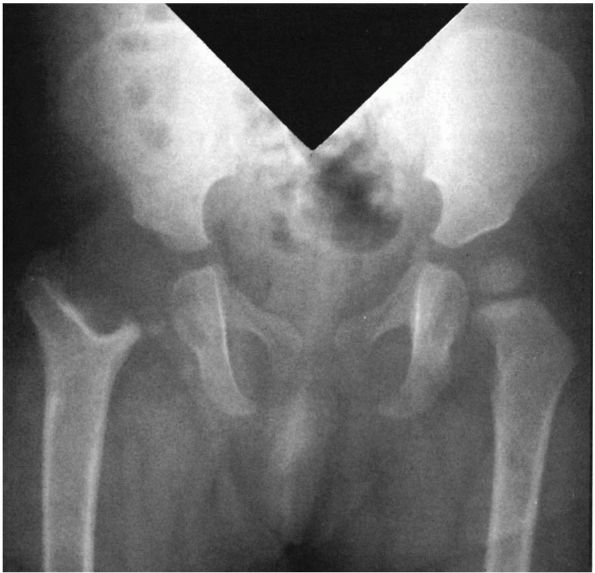
FIGURE 5-3.
AP radiograph of the pelvis of a 1-year-old girl who had an osteomyelitis of the proximal femur and a septic arthritis of the hip as a neonate. These infections resulted in destruction of the physis and the epiphysis. |
difficult to diagnose, requiring a high index of suspicion establish
the correct diagnosis. Children with acute infection of the pelvis
often are initially thought to have infection of the hip joint because
the pain is frequently intense and often limits motion of the hip
joint. The correct diagnosis can be established by performing a careful
examination. Carefully moving the hip joint usually demonstrates a
free, painless range of movement, whereas palpation of the pelvis
establishes the area of maximum tenderness. Septic arthritis of the
sacroiliac joint is also frequently confused with osteomyelitis of the
pelvis. In this disease, tests specific for pain in the sacroiliac
joint—such as the figure 4 test (one leg is
placed across on top of the other leg with the knee bent to 90°, as in
the number 4, and the pelvis is then rocked using the crossed leg as a
lever arm) and pelvic compression—are positive. Plain radiographs of
the pelvis are normal in the early stages of pelvic osteomyelitis and
in septic arthritis of the sacroiliac joint. Bone scintigraphy usually
is positive. As in acute osteomyelitis of other bones, however, a
certain percentage of these infections have false-negative bone scans.
Bacterial confirmation of the diagnosis is established by bone
aspiration.
hematogenous osteomyelitis of the pelvis. If this occurs, surgical
drainage of the abscess is necessary. A child with acute hematogenous
osteomyelitis of the pelvis should be evaluated in the same manner as
the child with an infection of a long bone, with an appropriate history
and physical examination. In addition, laboratory data should be
obtained. Bone aspiration should also be performed, and antibiotics
should be started once all cultures have been obtained. Because of the
possibility of developing an intrapelvic abscess that is not detectable
either on physical examination or through needle aspiration, CT or MRI
scanning of the pelvis should be performed. If an abscess is seen, it
should be drained through an appropriate surgical approach and the
child treated with antibiotics to sterilize the bone (Figure 5-4).
have been reported as a cause of vertebral osteomyelitis in children
without sickle cell disease. A much more common presentation of
infection of the spinal column is the disc space infection, which is
discussed in the subacute hematogenous osteomyelitis section. The mean
age of patients with vertebral osteomyelitis is significantly higher
than those with disc space infection. The latter is more commonly a
disease of school-aged children (mean of 7 to 8 years), whereas the
mean age for disc space infection is 2 to 3 years. True osteomyelitis
of the spine produces significant bone destruction. Neonates with
osteomyelitis of the spine develop abnormalities of the spine that
resemble congenital defects.
cell disease differs from osteomyelitis in otherwise normal patients.
The two major differences in the two forms of osteomyelitis are that
the infection in patients with sickle cell disease is usually located
in the diaphysis of long bones rather than in the metaphysis. In
addition, the organism responsible for the infection is frequently
salmonella, although S. aureus is also
common in patients with sickle cell disease. The salmonella bacteria
enter the blood stream through microinfarcts in the gut lining,
producing a bacteremia. The bacteria may then produce bone infection at
the site of an acute bone infarction.
present a difficult diagnostic problem, because acute bone infarcts are
painful and produce clinical findings often identical to those of
patients with osteomyelitis. Thus, it is frequently difficult to
differentiate between an area of acute bone infarction and one of acute
osteomyelitis. The infarction and the infection are usually located in
the diaphysis of a long bone, and both are associated with severe pain
and restricted use of the involved extremity. Patients with acute
osteomyelitis usually have a higher and more persistent fever than
those with infarction. The sedimentation rate and peripheral WBC count
may be elevated in both but are usually higher in patients with
infection.
 |
|
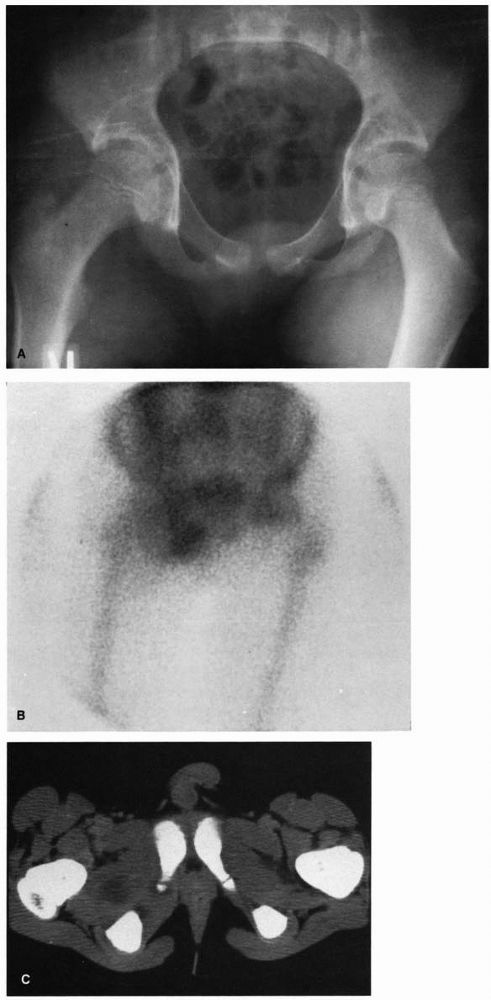
FIGURE 5-4. Eight-year-old boy with osteomyelitis of the pubis and a pelvic abscess. (A) AP radiograph of the pelvis of this child demonstrating no bony abnormalities. (B) Technetium-99m bone scan demonstrating increased uptake of the isotope in the region of the right pubis. (C) CT scan through the obturator region of the pelvis demonstrating an abscess in the obturator region of the right hemipelvis.
|
may be difficult. As mentioned earlier, children with infection exhibit
somewhat severer clinical findings. Bone scanning maybe helpful in
these patients. Skaggs et al. (2001) have
recently shown that one may be able to differentiate bone infarct from
osteomyelitis with the use of both bone marrow and bone scans. They
found that if the patient with bone pain has decreased uptake on a bone
marrow scan and abnormal bone scan at the site of pain, the diagnosis
of bone infarct could accurately be confirmed. If, on the other hand,
there is normal uptake on bone marrow scan and abnormal uptake on bone
scan, the diagnosis is suggestive of osteomyelitis. They also found
that if both the bone marrow and bone scans were normal, the diagnosis
was neither bone infarct nor osteomyelitis. One study with a relatively
small number of patients, found gadolinium-enhanced MRI useful in
distinguishing between bone infarct and osteomyelitis. Even with the
findings on scanning that suggest the diagnosis of osteomyelitis,
cultures of the patient’s stool culture of the bone by bone aspiration
should be performed to obtain bacteriological diagnosis.
osteomyelitis in the severity of the clinical signs. The systemic signs
seen in patients with subacute forms of the disease are either absent
or much less severe than those seen in patients with the acute form of
the disease. In addition, the location of the subacute form of the
disease may differ from that seen with acute osteomyelitis (see Table 5-1).
according to the location and radiographic appearance of the lesion.
This, however, does not consider the differences in clinical
presentation of these different forms of subacute osteomyelitis. The
classification based on radiographic appearance was first described by
Gledhill and subsequently modified by others. In this classification,
the type 1 lesion is a central metaphyseal lesion. The type 2 lesion is
also metaphyseal, but it is eccentrically placed with cortical erosion
present. The type 3 lesion is an abscess in the cortex of the
diaphysis, and the type 4 lesion is a medullary abscess in the
diaphysis without cortical destruction but with periosteal reaction
present. The type 5 lesion is primary epiphyseal osteomyelitis. The
type 6 lesion is a subacute infection that crosses the physis (Figure 5-5).
osteomyelitis that begins in the metaphysis and crosses the physis to
involve the epiphysis. Acute hematogenous osteomyelitis in the child
older than age 18 months rarely crosses the physis; however, subacute
osteomyelitis frequently does. The lesion may be primarily metaphyseal
with only a small portion of the physis and epiphysis involved (Figure 5-6). Conversely, much of the physis may be involved (Figure 5-7).
In the neonate with acute hematogenous osteomyelitis that crosses the
physis to involve the epiphysis, the physis is frequently destroyed as
is the growth center of the epiphysis (see Figure 5-3). Conversely, the subacute infection that crosses the physis rarely causes permanent damage to the growth plate.
rapidity of onset and severity of presenting symptoms. One type has a
fairly acute presentation, and children with this type of infection
usually present within a week or two of the onset of symptoms.
Radiographic changes may be present at the time of presentation;
however, the infection is usually diaphyseal. This type of infection
thus encompasses types 3 and 4 of the Gledhill classification system.
This diaphyseal infection could easily be confused with Ewing sarcoma,
and a biopsy may be necessary to exclude this diagnosis. Frequently,
however, the clinical and radiographic picture is characteristic enough
to make a presumptive diagnosis of infection. Children with this type
of subacute infection may have a fever, although it will not be as
elevated in acute hematogenous osteomyelitis. In addition, children
with this type of infection usually continue to walk even if the femur,
the most commonly involved bone, is infected. They will, however, limp
and complain of pain. The peripheral WBC count and the sedimentation
rate may be elevated, although the sedimentation rate is more commonly
elevated (Figure 5-8).
The second type of subacute infection encompasses types 1, 2, 5, and 6.
Children with this type of infection have minimal symptoms, but the
complaints are frequently of longer duration. There are no systemic
signs, and the peripheral WBC count and the sedimentation rate usually
are normal. Radiographic changes are present at the time of
presentation and may be described as a lucency located anywhere within
a bone.
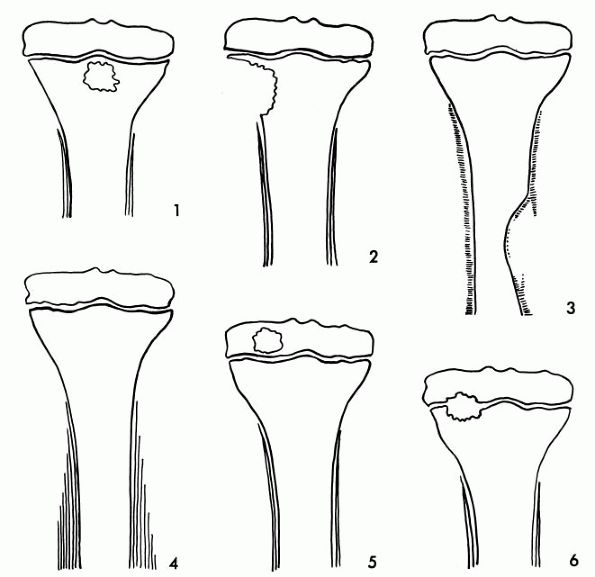 |
|
FIGURE 5-5.
Classification of subacute osteomyelitis type 1 is a central metaphyseal lesion. Type 2 is an eccentric metaphyseal lesion with erosion of cortex. Type 3 is a lesion of cortex of diaphysis. The type 4 lesion of the diaphysis demonstrates periosteal new bone formation but without a definite bone lesion. Type 5 is primary subacute epiphyseal osteomyelitis, and type 6 represents subacute osteomyelitis that crosses physis involving both the metaphysis and epiphysis. |
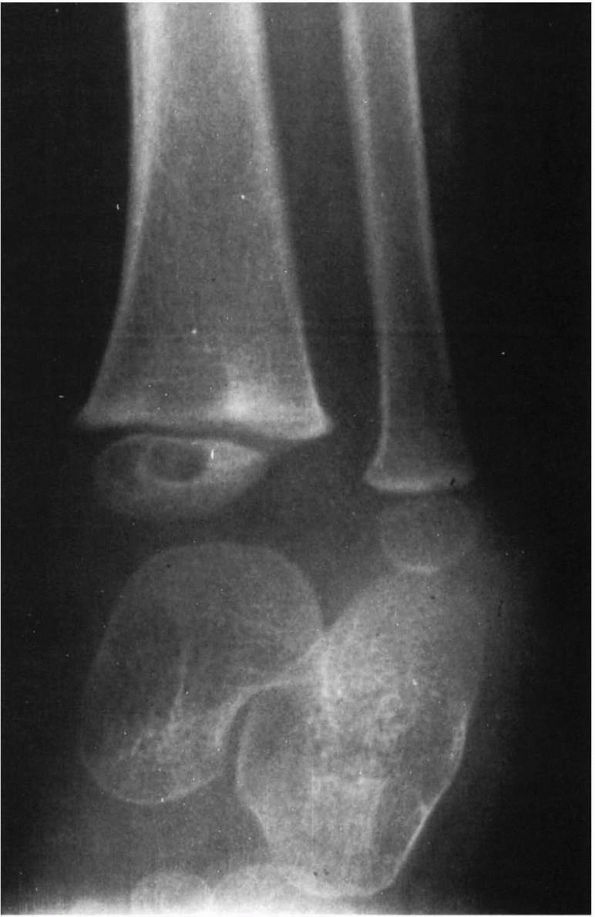 |
|
FIGURE 5-6.
AP radiograph of the distal tibia demonstrating an area of subacute osteomyelitis that involves the metaphysis, physis, and the epiphysis. |
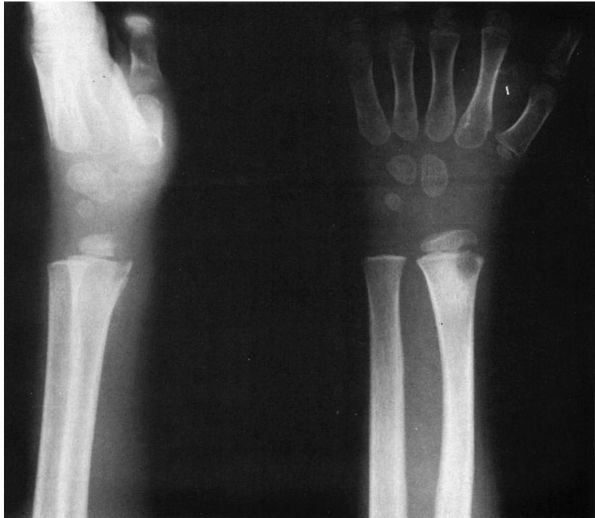 |
|
FIGURE 5-7.
AP and lateral radiographs of a distal radius of a child with subacute osteomyelitis that crosses the physis. The AP radiograph demonstrates that the lesion involves both the metaphysis and the epiphysis. On the lateral radiograph, significant erosion of the epiphysis is seen. |
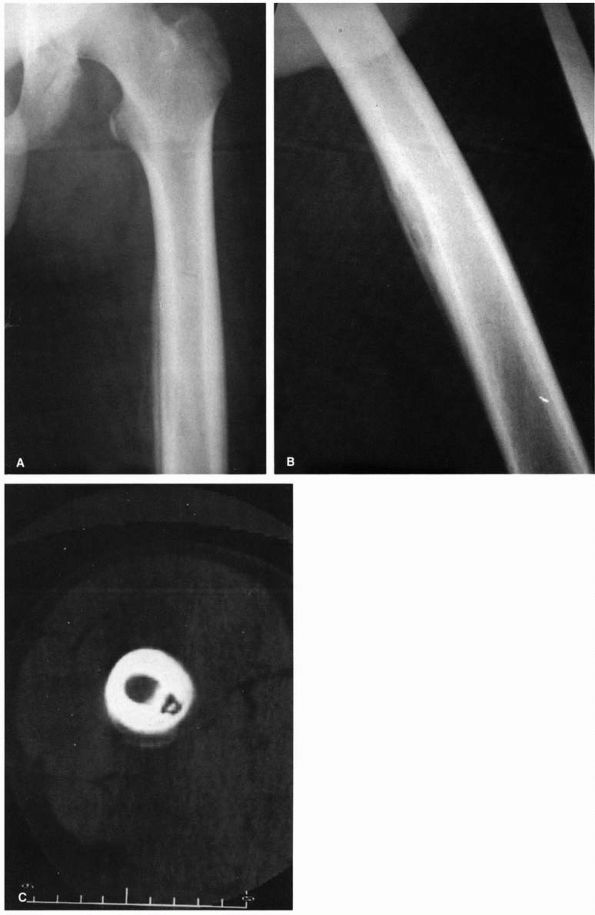 |
|
FIGURE 5-8.
Radiographs of 15-year-old boy with subacute osteomyelitis of the femur. He had a 1-month history of pain in the leg and a limp. (A) AP radiograph of femur showing bone destruction with periosteal reactive bone. (B) Lateral radiograph demonstrating bone destruction. (C) CT section demonstrating the destruction of the bone cortex with a sequestrum within the cavity. |
diaphysis of a long bone is confirmed by bone aspiration for bacterial
culture. The cultures usually are positive for S. aureus.
The treatment of this diaphyseal lesion depends on several factors. If
the diagnosis is in question, open biopsy may be required, at which
time debridement is carried out if infection is established. If a
sequestrum is present, sequestrectomy is required for eradication of
the infection. Most commonly, however, the patient presents with
minimal radiographic bone changes, and simple antibiotic therapy alone
usually results in eradication of the infection, because no abscess is
present.
epiphysis, can usually be diagnosed as subacute osteomyelitis
radiographically; however, in some instances the diagnosis is in
question. When the diagnosis is not clear radiographically, open biopsy
is required in making the diagnosis (Figure 5-9).
In addition, because there is a radiographic lesion, an abscess has
formed and debridement is usually required, although some authors have
reported healing without debridement. Frequently no pus is evident at
exploration; however, one may find granulation tissue within the cavity
that should be debrided. Cultures may be sterile, but Staphylococcus aureus and Staphylococcus epidermidis
are the most common organisms. Some have suggested that debriding these
lesions is not required. These lesions will heal with adequate
antibiotic treatment. It has been recommended that patients be treated
with antibiotics for at least 4 weeks or until significant healing is
seen radiographically (Cole 1982; Ezra et al. 2002).
Therefore, if one is comfortable with the radiographic diagnosis of
subacute osteomyelitis, then antibiotic treatment alone without biopsy
is appropriate. If there is inadequate response, as judged by the
clinical course and radiographic healing, then biopsy is required.
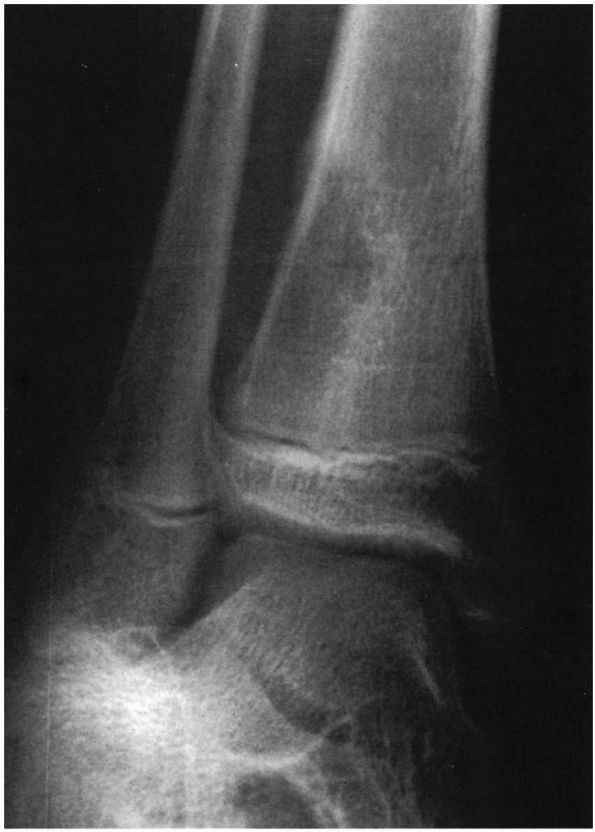 |
|
FIGURE 5-9.
Oblique radiograph of the distal tibia of an 11-year-old girl with a 1-month history of pain, swelling, and redness of the ankle. A metaphyseal lesion of the distal tibia resembles subacute osteomyelitis; however, the biopsy revealed that the lesion was an osteogenic sarcoma. |
similar to that of acute osteomyelitis. Begin with IV antibiotics and
switch to oral antibiotics if there are no contraindications. As
mentioned earlier, debridement is usually necessary for subacute
osteomyelitis with a radiographic lesion or if a sequestrum has formed
or if the patient has not responded adequately to antibiotics.
presenting with symptoms that have been present for months or longer.
Also included in the diagnosis of chronic osteomyelitis is any
recurrent osteomyelitis. This chapter does not deal with the chronic
osteomyelitis that results from a recurrence of a previously treated
infection or from an open wound such as an open fracture. The only
exception is the special circumstance of nail puncture wound infections
of the foot, which are covered in the next section.
at the present time, but it is presumed to be due to an infectious
agent. The disease produces vague bone pain in multiple sites.
Frequently the symptoms seem to be unilateral, despite the fact that
lesions occur bilaterally. Children with this disease usually do not
exhibit systemic signs of infection such as an elevated temperature.
Although the peripheral WBC count is normal, the sedimentation rate is
frequently elevated.
reaction and are generally located in the metaphyses of the long bones
are seen radiographically (Figure 5-10). The
medial end of the clavicle seems to be the bone that is most frequently
involved, followed by the distal tibia and then the distal femur.
infectious agent is thought to cause these lesions. The histology of
these lesions is typical of osteomyelitis; however, the agent
responsible has not as yet been identified with certainty. The
treatment is symptomatic. The natural history of this disease usually
involves spontaneous resolution of the lesions and the clinical signs
and symptoms, which may take anywhere from 1 to 15 years.
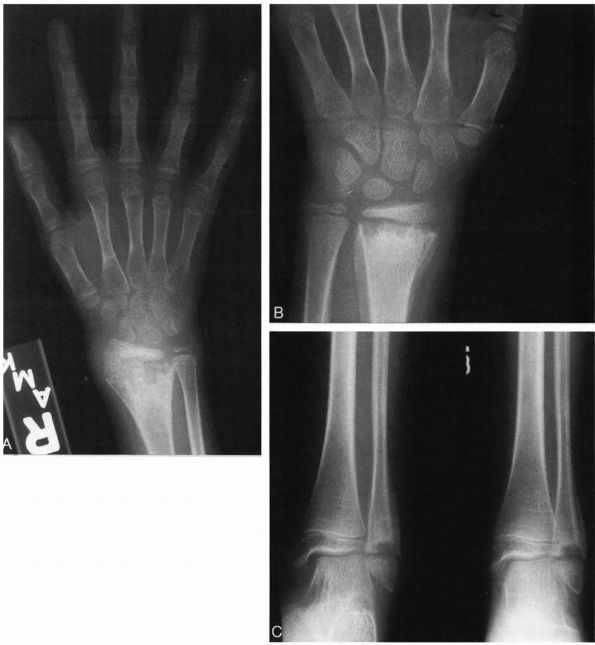 |
|
FIGURE 5-10.
Radiographs of an 8-year-old girl with chronic multifocal osteomyelitis who had a longer than 2-year history of multiple sites of bone pain. (A) AP radiograph of the right wrist demonstrating bone destruction of the metaphysis, physis, and epiphysis. (B) AP radiograph of the left wrist demonstrating bone destruction in the metaphysis with periosteal reactive bone. (C) Radiographs of the left ankle demonstrating metaphyseal and physeal destruction of the distal fibula with periosteal reactive bone. |
inflammatory disease that should be treated with nonsteroidal
inflammatory drugs (NSAID). We have cultured Mycoplasma from three patients with this
disease and have subsequently treated all of our patients with antibiotics that would be useful for Mycoplasma.
All of our patients responded dramatically with resolution of their
symptoms very shortly after beginning therapy. In addition, the
sedimentation rate has shown a rapid decline to normal in all patients.
As a result of these findings, we feel that this disease is infectious,
likely the result of Mycoplasma infection,
and believe that very long-term antibiotics such as doxicycline or
clindamycin is appropriate for the treatment of this disease.
This disease is usually regarded, however, as an osteomyelitis of the
vertebral endplates that secondarily invades the disc without producing
an acute osteomyelitis of the vertebral body. The organism that
produces the infection in children is usually S. aureus, although other organisms are common in older patients, especially drug abusers and in debilitated patients.
is most common in younger children, who may present with an inability
to walk as the primary presenting feature, although the most common
complaint is back pain. Unfortunately, in infants and toddlers
diagnosing back pain may be difficult. Some children present with
abdominal pain as the primary complaint. Frequently, the infant is
irritable without other definite complaints.
characteristic, and the diagnosis can usually be established on the
basis of the physical examination alone. Because the disc space
infection usually occurs in the lumbar spine, the child splints the
spine, refusing to flex it. Although the child may be able to bend at
the waist, no flexion occurs in the spine itself. Some children are
adept at compensating for the pain and may function relatively
normally. They will, however, exhibit complete restriction of motion of
the spine on examination.
spine in that there are usually few, if any, systemic signs in patients
with disc space infection. The patient’s body temperature is usually
normal as is the peripheral WBC count. The sedimentation rate is
frequently, although not invariably, elevated.
diagnosis. The disease characteristically produces narrowing of the
disc space with irregularity of the adjacent vertebral endplates. This
may be difficult to see radiographically, especially in a young child
early in the course of the disease. Lateral tomography of the involved
area of the spine is helpful in demonstrating the disc space and bone
abnormality. These radiographs help eliminate the overlying gas that
frequently obscures the spine in the lumbar region.
disc space infections. The bone scan usually demonstrates an area of
increased uptake in the infected disc space, but some scans will be
false-negative. Therefore, the diagnosis of disc space infection should
not be excluded because of a normal bone scan. MRI has been shown to be
able to accurately demonstrate an abnormal disc space, and we have
recently demonstrated that the MRI is abnormal before the bone scan is
positive and before radiographic changes are evident (Figure 5-11).
clinical grounds because of the characteristic physical findings. The
clinical diagnosis is confirmed with radiographs that show the
characteristic disc space narrowing and erosion of the vertebral
endplates. Normally with a bone infection, a tissue or bacteriologic
confirmation of the diagnosis would be necessary; however, because of
the morbidity of needle aspiration of the spine, this procedure is
usually not justified for the child exhibiting the characteristic
findings of a disc space infection. The infecting organism is usually S. aureus.
Aspiration biopsy of the spine should be reserved for the child who
does not respond to initial treatment with antistaphylococcal
antibiotics. One should also perform a biopsy of the disc space when
the disease is unusual in any respect. If the infection involves an
older child such as a teenager, or if drug abuse is
suspected, a biopsy should be performed because of the possibility of a Gram-negative organism being the etiologic agent.
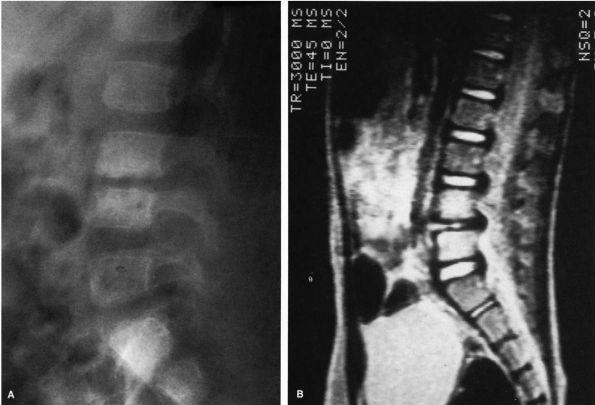 |
|
FIGURE 5-11.
MRI of the spine demonstrating an abnormal L4-L5 disc. Note the loss of height and the change in the density of the disc. The bone scan, the plain radiographs, and the lateral tomograms of this patient were normal. The lateral radiograph of the spine subsequently demonstrated the typical changes seen in disc space infections. |
spinal immobilization for children with disc space infection. They have
shown that many patients respond to immobilization alone and
consequently reserve antibiotics for the child who does not respond to
immobilization alone. Currently most authors, however, favor the use of
antibiotics as for any other bone infection. These children should,
therefore, be started on oxacillin or cephazolin in doses that would be
used for osteomyelitis. Treatment should be continued for 3 to 7 days
with IV antibiotics, followed by oral antibiotics for another 4 weeks.
The length of IV antibiotic therapy is determined by the patient’s
clinical response. One may also wish to use the C-reactive protein as a
guide for treatment, because it falls more quickly than does the
sedimentation rate during the treatment of osteomyelitis. We generally
treat these children with IV antibiotics for several days only and then
they are switched to oral antibiotics that are continued on an
outpatient basis for a minimum of a total of 4 weeks of total
antibiotic therapy.
treatment of disc space infection. However, most children with disc
space infection usually respond quickly to IV antibiotics. Therefore,
immobilization is used only in those patients who do not exhibit a
rapid and dramatic response to the IV antibiotic treatment and need
immobilization for comfort. One should also immobilize children with
osteomyelitis of the spine because of the bony destruction that occurs.
injuries. The exact number of infectious complications of these
injuries is not known, although rates up to 15% have been reported.
Although the majority of infections are limited to the soft tissue,
bone and joint infections occur in approximately 2% of puncture wounds
of the foot.
punctures is characteristic. Typically, the patient is a child with
tennis shoes who steps on a nail, sustaining a puncture of the foot
that invariably either enters a bone or joint or punctures the plantar
fascia. These children experience pain from the initial puncture wound,
but this pain usually diminishes quickly. Children who develop a
pseudomonal infection experience increasing pain in the foot 2 to 4
days after the initial trauma. These symptoms worsen so that within a
day or so the child is not able to bear weight.
area of the puncture wound and throughout the area of the bone or joint
infection, with signs of inflammation, including redness of the skin.
Careful examination of the foot reveals pain and tenderness on the
dorsum of the foot over the involved bone or joint.
frequently are no or few systemic signs of infection. The child’s
temperature usually is normal, as is the WBC count. Unfortunately,
these findings have prompted many to underestimate the seriousness of
this infection.
which has been grown from cultures taken from within the sole of tennis
shoes. This infection requires thorough surgical debridement and
antibiotic treatment. Antibiotic coverage alone does not eradicate the
infection and only allows the infection to destroy more tissue. Once
the diagnosis is made, a thorough debridement is performed. Prior
aspiration of the area of infection may be performed but is not
necessary because the signs and symptoms of this infection are so
typical that culture of the tissues at the time of debridement is
sufficient. However, do not give antibiotics until obtaining adequate
cultures of the area of infection. Superficial cultures from the area
of the puncture wound are not sufficient; it is necessary to obtain
cultures from the bone or joint involved. P. aeruginosa
rarely produces the thick pus typical of other infections. Rather, one
finds a thin, watery, serosanguineous fluid typical of early P. aeruginosa infections.
on the area of involvement. If one is dealing with a septic arthritis
of the metacarpophalangeal joint, the joint may be drained through a
dorsal incision rather than through the puncture wound itself. If
infection exists only within the sole of the foot under the plantar
fascia, a plantar incision is necessary. If one of the bones of the
foot is extensively infected, debridement may be required, using both
dorsal and plantar incisions.
empiric antibiotics should have antipseudomonal activity. Additionally,
the presence of cellulitis suggests infection with Gram-positive
organisms (often in addition to P. aeruginosa) and should prompt empiric coverage that includes S. aureus. Local resistance rates of P. aeruginosa and S. aureus
should dictate the empiric choice of antibiotics. For a bone infection
alone, cefepime, ceftazidime, piperacillin, ticarcillin, or tobramycin
are reasonable choices as single agents for empiric antipseudomonal
therapy. If cellulitis accompanies the osteochondritis, the addition of
an antistaphylococcal penicillin (i.e., nafcillin or oxacillin) to
ceftazidime or tobramycin or the addition of a β-lactamase inhibitor to
one of the antipseudomonal penicillins (i.e., piperacillin/tazobactam
or ticarcillin/clavulanate) is warranted on an empiric basis. Cefepime
has activity against both S. aureus and P. aeruginosa that is adequate for empiric therapy. If rates of methicillin resistance are high among local S. aureus
isolates, the use of vancomycin for staphylococcal coverage may be
warranted on an empiric basis. Antibiotic susceptibility data from the
cultures collected during debridement should guide definitive
antibiotic therapy.
response, the extent of the infection, and the thoroughness of the
debridement. The sedimentation rate may be used as a guide to the
length of therapy, with the recommendation that antibiotics be
discontinued when the sedimentation rate falls to normal. In general,
the more thorough the debridement, the
shorter
the length of antibiotic treatment necessary. However, the largest
available study in children suggests that an intravenous antibiotic
course of 7 days following thorough surgical debridement is effective.
In adult patients, a small study demonstrated the efficacy of oral
ciprofloxacin for 7 to 14 days after surgery for pseudomonal
osteomyelitis or septic arthritis of the foot. The use of
fluoroquinolones in pediatric patients remains controversial. Due to
cartilage damage in multiple juvenile animal models with therapeutic
doses, fluoroquinolones are generally contraindicated, according to
their FDA-approved labeling. There is not a single report of
ciprofloxacin causing arthropathy in children, and the American Academy
of Pediatrics Committee on Infectious Diseases states that the use of
oral ciprofloxacin can be justified in certain cases. One such
situation is when no other oral agent is available, necessitating an
alternative drug be given parenterally. After the risks and benefits
are explained to the parents, oral ciprofloxacin can be considered for
pseudomonal osteomyelitis when the patient is well enough to be
discharged from the hospital.
between the puncture wound and the onset of appropriate treatment. The
longer the delay before debridement of the foot and the commencement of
aminoglycoside therapy, the worse the sequelae. To minimize sequelae,
it is important to quickly establish the correct diagnosis and to
perform the surgical debridement.
It may be associated with acute osteomyelitis, especially in the
proximal femur, where bacteria escape the cortex of the metaphysis and
invade the adjacent joint, producing a joint infection. In other cases,
the joint infection is simply the result of hematogenous infection of
the synovium or synovial fluid without prior bone infection. This
isolated joint infection may be treated differently from the bone
infection, which requires longer antibiotic therapy because of the
possible presence of necrotic bone within the area of the infection.
With a pure septic arthritis, bone sequestration does not occur. In
addition, antibiotics are delivered across the synovium into the joint
in high concentrations.
was common, and in some series was the most common agent in septic
arthritis in this age group; however, we have shown that since the
advent of the H. Flu vaccine, the incidence of H. Flu septic arthritis
has declined to near zero. In the older child S. aureus is the most common organism. Recently joint infections caused by Kingella kingae
have been diagnosed relatively commonly, and in one recent report this
organism has been seen more frequently than staph. This organism is
present in the nasopharynx of normal children and may spread to produce
joint infections via the bloodstream. In the teenager, however, Neisseria gonorrhoeae
is common and may be the most common cause of septic arthritis. It is
certainly the most common cause of polyarthritis in that age group.
clinical signs of sepsis, with elevated temperature, malaise, and local
signs of inflammation. The exception is seen in children with acute
septic arthritis secondary to Kingella kingae
who frequently are not febrile at the time of diagnosis and may not
have all of the signs of hip sepsis. The onset of septic arthritis is
frequently more acute than is the onset of osteomyelitis. The child,
especially the neonate, may present with pseudoparalysis of the
extremity. The older child will protect the extremity, and if a joint
of the lower extremity is involved, the child will usually refuse to
walk.
a painful joint. Few other diseases produce such exquisite joint pain.
The differential diagnosis includes acute rheumatic fever or acute
juvenile arthritis, both of which may produce acute joint inflammation
that is as painful as that produced by septic arthritis. In both of
these diseases, the joint effusion also is significant, and the WBC
count in the synovial fluid may occasionally be as high as 100,000
cells. The diagnosis usually can be made by other laboratory means,
although the diagnosis
occasionally is made retrospectively after treating a child for an infection.
with an effusion. The child will resist movement, splinting the joint
in the position of greatest comfort. Laboratory data may be helpful.
The peripheral WBC count is usually elevated, as is the sedimentation
rate, although the diagnosis of septic arthritis should not be excluded
simply on the basis of normal values for these two studies. Radiographs
may demonstrate the joint swelling, although they are of little benefit
early in the course of the disease except to exclude other problems.
culture of the synovial fluid. The joint fluid should be inspected
visually. The fluid in patients with an infection of the joint varies
in color from cloudy yellow to creamy white or gray, especially if the
infection has been present for a period or if the organism is
particularly virulent. Thus, the earlier the infection is diagnosed and
the joint aspirated, the clearer the fluid. The fluid should be
analyzed for cell count with a differential count of the WBCs. In most
septic joints, the WBC count is greater than 50,000, and usually
greater than 100,000. The one exception is in gonococcal arthritis, in
which the WBC count is frequently lower than 50,000 cells. The
differential count of polymorphonuclear leukocytes demonstrates that
they constitute over 90%, and usually over 95%, of the total WBCs in
the fluid.
obviously important and provides the basis for the definitive diagnosis
of septic arthritis. The fluid should be transported immediately to the
laboratory and plated on the appropriate medium. Laboratory personnel
must be informed that the fluid is from a joint and should also know
what the physician suspects, which enables the technician to perform
the appropriate cultures. Some special circumstances require special
techniques for organism retrieval. H. influenzae is difficult to culture, and the plates must be incubated under a carbon dioxide environment. Kingella kingae
is fastidious organism, and its recovery in the microbiology laboratory
is increased significantly by direct inoculation of synovial fluid into
automated blood culture system bottles. Despite meticulous culture
techniques, a percentage of septic joints yield negative cultures. In
some series, the percentage of organism retrieval was only 70%.
Therefore, blood cultures should also be performed. Despite this, the
diagnosis of septic arthritis may have to be made on clinical grounds
in some patients because of negative bacterial cultures.
cultures, one should also perform glucose determination on the joint
fluid. In addition, lactic acid determination of the joint fluid and
counter immunoelectrophoresis may be helpful for the detection of H. influenzae.
differ from those of the treatment of infections in other areas of the
body. The infection should be considered an abscess that requires
drainage. In addition, the infection requires appropriate antibiotic
therapy to sterilize the joint. The joint infection differs from other
infections, in that it occurs in a closed space with easy access for
needle aspiration and irrigation. In addition, antibiotics readily
cross the synovial barrier and are concentrated in the synovial fluid.
completely eradicate the infection of the joint. In some instances,
eradication may be accomplished with aspiration and irrigation of the
joint without surgical debridement. Most reports of aspiration and
irrigation technique of joint debridement of infection have reported
good results. The requirements for this technique are specific. The
major contraindication to aspiration irrigation technique for the
treatment of joint infections is the hip joint being the site of
infection. This joint must always be surgically drained in the face of
an acute infection because the vascular supply of the hip joint is
intracapsular, and therefore these vessels are easily obliterated if
the pressure within the hip joint is elevated. In the case of acute
septic arthritis of the hip joint, the joint must be surgically drained
as an emergency. Recently, articles have been published that document
satisfactory medical treatment of septic arthritis of the hip without
surgical drainage. They state that the diagnosis must be made very
early in the course of the disease and that there should be immediate
response to treatment. They do make the diagnosis of septic arthritis
of the hip with joint aspiration and then begin antibiotic treatment.
It is probably safer to conservatively surgically drain the septic hip
until adequate literature confirms the efficacy and safety of
medical-only treatment.
joints requires that the joint be easily accessible for aspiration.
Because of its accessibility, the knee joint is most frequently treated
with this technique. The ankle
joint
is also relatively accessible. The other joints of the body are less
accessible and therefore more difficult to adequately debride through a
needle.
use of a large-bore needle. The fluid is fully drained from the joint
and sent for appropriate studies. Without removing the needle, the
joint is irrigated with sterile IV saline until the fluid that is
returned is clear. The joint should be splinted, and the patient
started on antibiotics while awaiting the culture results. If the WBC
count of the fluid is low (i.e., below 80,000 to 100,000), and there is
no particulate matter in the aspirate, this aspiration-irrigation may
be the only mechanical treatment needed. The joint should be inspected
the following day, and if the fluid has reaccumulated, a second
aspiration-irrigation should be performed. If the reaccumulation of
fluid is significant, and if the patient is still febrile, consider
performing surgical drainage. If, in addition, the WBC count of the
aspirated fluid is not significantly lower on the second day than that
seen in the initial aspirate, surgical debridement should be strongly
considered. If a second aspiration is performed and the fluid
reaccumulates significantly on the third day, surgical drainage should
be performed. Parenteral antibiotics enter the inflamed joint so
readily that there is no need for direct joint instillation of
antibiotics.
the fluid is thick (i.e., with a WBC count over 100,000), and when
particulate matter is seen in the aspirate. This particulate matter is
precipitated fibrin that must be removed to eradicate the infection.
The arthrotomy of the knee joint should be performed through a small
lateral parapatellar incision that allows inspection of the joint. A
medial parapatellar incision is not performed because release of the
medial retinaculum might lead to patellar subluxation. The joint may be
closed over a small drain such as a Penrose drain. Suction drainage may
be used; however, a suction-irrigation system should not be employed
because of the possibility of superinfection with Gram-negative
organisms. Arthroscopy has become a popular tool for the inspection of
the joint and has been proposed for the debridement of the infected
joint. Proponents state that one can effectively debride the joint and
that the fibrinous material may be removed with the debridement tools
available to the arthroscopist.
joint fluid and blood have been cultured and the other studies such as
Gram’s staining and WBC count have been performed. The diagnosis may
have to be confirmed on a presumptive basis; however, it is important
to begin treatment as long as the criteria for making the diagnosis of
septic arthritis have been met. In all age groups, most acute septic
arthritis is caused by S. aureus. In children 6 months to 5 years, H. influenzae is a common cause in unvaccinated children. In the neonate, group B Streptococcus
and Gram-negative organisms are common etiologic organisms. While
awaiting the culture results, appropriate antibiotics should be started
to cover the most likely organisms. Because S. aureus
is ubiquitous, all acute septic joints are treated initially with an
antistaphylococcal drug. The antibiotics are then modified when the
culture and sensitivity results are known.
patient with acute hematogenous osteomyelitis. The duration of
antibiotic treatment, however, is not as long as in osteomyelitis
because antibiotics reach the infected joint readily and in high
concentration. In addition, one does not have to deal with necrotic
bone in a septic arthritis as in a patient with osteomyelitis.
Treatment of the septic joint should be started with IV antibiotics for
3 to 5 days. The patient may then be switched to oral antibiotics if
the patient responded well to the treatment, which should include no
fever for 24 hours and excellent clinical response. The total length of
treatment is generally 2 to 4 weeks.
emergency because of the potential for the development of avascular
necrosis of the femoral head. The initial evaluation of the hip joint
is performed in the same manner used for any other joint. However,
because the hip joint is deep and may be difficult to aspirate
accurately, it must be aspirated using fluoroscopy. The needle may be
directed from the anterior or medial approach. The anterior approach is
used if the child is able to extend the hip. If, because of pain, the
hip is in a position of abduction, flexion, and external rotation, a
medial approach to the hip is easier. The exact needle entry point
through the skin is easily determined by placing the needle on the skin
and positioning the needle point using fluoroscopy. After skin
penetration, the needle is directed toward the femoral neck at about
the level of the junction of the head and the neck. If no fluid is
collected from the joint, contrast medium is injected into the joint
and an arthrogram obtained, which will reveal whether the hip joint has
been entered.
has been confirmed, the joint must be surgically drained as mentioned
earlier. However, recent articles have documented excellent response to
medical therapy alone in children with acute septic arthritis of the
hip. These reports require critical evaluating before making a decision
about the need for surgical drainage based on multiple factors. One
need not await culture reports before draining the infected hip. Strong
presumptive evidence is sufficient. Therefore, a positive Gram’s stain
or WBC count of the joint fluid of more than 90,000 to 100,000 cells is
sufficient evidence if seen in combination with the characteristic
history and physical findings.
and irrigation because of the danger of avascular necrosis developing
as a result of increased joint fluid pressure from the infection. The
hip may be drained from either an anterior or a posterior approach.
Each approach has its proponents. The posterior approach is generally
easier and less damaging to the muscles of the hip. In addition, it
allows for dependent drainage. If the femoral neck must be opened
because of an intraosseous abscess, this cannot be performed
posteriorly because the blood supply to the femoral head would be
damaged. Thus, if the femoral neck should be windowed, the hip should
be approached anteriorly. This approach allows one to drill the femoral
neck in every patient (Figure 5-12). It is wise
to always drain the infected hip joint from an anterior approach. This
method allows easy access to the femoral neck for drilling if necessary.
 |
|
FIGURE 5-12. A 14-year-old boy with septic arthritis of his left hip. (A)
AP radiograph of the left hip at the time of presentation. The hip was drained posteriorly, but the femoral neck was not drained. (B) AP radiograph of the left hip taken 6 weeks later demonstrating chondrolysis, avascular necrosis of the femoral head, and chronic osteomyelitis of the femoral neck with sequestrum within the femoral neck. |
allow for a decrease in the acute inflammation. The hip may be placed
in split Russell traction and the other joints splinted. Once the acute
inflammation has subsided, motion should be instituted. Continuous
passive motion has been advocated, and it may be used in children of
adequate size. Joint motion helps prevent fibrosis and assists in
cartilage nutrition.
unusual because of its symptomatology and treatment, and therefore
deserves separate discussion.
Sacroiliac
(SI) joint infection may be difficult to diagnose unless one is
familiar with its signs and symptoms and considers it in patients with
pain about the pelvis and hip. Frequently, children with this disease
are referred with abdominal or hip pain. Occasionally, they may be
thought to have back pain. A careful history and examination, however,
reveal that the pain is localized to the posterior pelvis. Pelvic
compression is positive and usually produces exquisite pain in the
region of the SI joint. The figure 4 test or Fabre test produces exquisite pain in the region of the SI joint.
infection. The initial radiographs of the pelvis are usually normal,
although tomograms of the SI joint may show some erosion of the margins
of the joint if the infection has remained untreated for a sufficiently
long period. Usually, however, all radiographs are normal. The 99mTc
bone scanning may be positive; however, false-negative bone scans are
seen in 25% or more of patients with acute infections. The diagnosis,
therefore, depends on a careful history and examination, combined with
confirmatory laboratory studies.
to determine the bacteriologic cause of the infection; however, the SI
joint is difficult to aspirate. Several descriptions of SI joint
aspiration technique have been published, but even with this assistance
the technique is demanding. Children with SI joint infection experience
considerable pain and are usually distraught, making aspiration even
more difficult. The SI joint is best aspirated using an approach with
CT assistance. This approach allows for direct access to the joint. In
our experience, little if any material is aspirated, however, whatever
is obtained is submitted for Gram’s stain and culture. Appropriate
antibiotics are immediately begun while awaiting culture results. The
infecting organism seen most commonly in non-drug-abusing children is S. aureus. Surgical drainage is not required unless the infection does not respond to IV antibiotics.
is probably the most common form of septic arthritis in the sexually
active population. The typical syndrome of disseminated gonococcal
infection frequently consists of three stages, although all patients
with gonococcal septic arthritis do not go through all of these stages.
The first stage is a septic stage that is similar to a septicemia
caused by any other bacteria. The second stage is a transition stage,
and the final stage is the septic joint stage. Eighty percent of
patients with gonococcal arthritis complain of a migratory
polyarthralgia, which is most commonly seen on the dorsum of the hands,
wrists, ankles, and feet. This history is so characteristic that the
diagnosis of gonococcal arthritis may be made on the basis of the
history of migratory polyarthralgia combined with the typical joint
findings.
more than one involved joint. The joints of the upper extremity,
especially those of the fingers and wrist, are more commonly involved
than are the joints of the lower extremity.
analysis. Typically, the WBC count of the fluid is much lower than one
would see in the fluid of a joint infected with another organism. In a
series of teenagers with gonococcal arthritis, the average WBC of the
synovial fluid was 48,000, with a range of 3,800 to 152,000. The
organism is difficult to culture in the laboratory, and joint fluid
cultures of septic joints are positive only half of the time.
Therefore, it is necessary to culture all orifices in patients
suspected of having gonococcal arthritis. Additionally, it is
imperative that all patients with gonococcal infections be tested for
other sexually transmitted infections (syphilis, hepatitis B, HIV) and
treated presumptively for Chlamydia trachomatis.
required because the organism is so easily eradicated with appropriate
antibiotic therapy. In addition, the organism usually does not destroy
the involved joint until late in the course of an infection; therefore,
if an infection is treated promptly, no joint destruction should be
expected. Penicillin and tetracycline resistance among N. gonorrhoeae
isolates from the United States is common; therefore, penicillin is no
longer an appropriate empiric choice for suspected gonococcal
infection. In treating patients with gonococcal arthritis, one should
follow the Centers for Disease Control and Prevention recommendations,
which are to treat with ceftriaxone 50 mg/kg (up to 1g) as a single IV
or IM dose for 7 days. Options for the cephalosporin allergic patient
or in cases of proven cephalosporin resistant gonnococcal infection are
spectinomycin or a fluoroquinolone, such as ciprofloxacin. Consider
using a fluoroquinolone in patients under the age of 18 as discussed
above in the section “Pseudomonas: Infections of the Foot Following Puncture Wounds.” Presumptive treatment for
concomitant C. trachomatis
infection is a single oral dose of azithromycin 20 mg/kg (up to 1g).
Additionally, evaluation and treatment of the patient’s sexual partners
is important in preventing reinfection.
S, Louis JS, Barbier-Frebourg N, et al. Kingella kingae osteoarticular
infections in children. A report of a series of eight new cases. Arch
Pediatr 2000;7(9):927-932. Kingella kingae is a Gram-negative bacillus that belongs to the Neisseriaceae family,
which is usually present in the nasopharynx. It has been recognized as
the cause of bone and especially joint infections. It is difficult to
culture in the laboratory, and it is necessary to use blood culture
bottles for inoculation. This organism produces an infection that is
usually less severe than seen with staph. In this series only 75% of
patients had a fever at the time of diagnosis.
SG, Green NE, Mencio GA. Decline of bone and joint infections
attributable to haemophilus influenza type b. Clin Orthop
1997;341:128-133. The authors demonstrate that H.
Flu has virtually been eliminated as a cause of bone and joint
infections since the onset of widespread inoculation with the H. Flu
vaccine. Therefore, coverage for H. Flu is not needed in patients who
have been vaccinated.
MJ, Kincaid R, Craigen MA, et al. The changing epidemiology of acute
and subacute haematogenous osteomyelitis in children. J Bone Joint Surg
Br 2001;83(1):99-102. This article documents the
decreasing incidence of both acute and subacute osteomyelitis; however,
the decrease in the incidence of acute osteomyelitis was significantly
greater than in the subacute form.
ST, Harkness RM, Thomas PA, et al. Does aspiration of bones and joints
affect results of later bone scanning? J Pediatr Orthop
1985;5(1):23-26. The authors demonstrate that
bone aspiration with a needle does not alter the bone scan. They
emphasize that if bone scanning is to be done in acute hematogenous
osteomyelitis, it should not delay a bone aspiration and treatment.
Treatment should be carried out immediately, and if a bone scan is
necessary, it may be done without fear of altering the results after
needle aspiration.
study documents satisfactory results of septic arthritis of the hip
with arthrocentesis and antibiotic treatment without arthrotomy. They
did have 4 of 7 with poor results, and all of those were associated
with osteomyelitis of the proximal femur. One should not automatically
assume this method of treatment without long-term documentation of
larger series of patients with septic arthritis of the hip treated
without arthrotomy.
authors present a review of 55 children with acute hematogenous
osteomyelitis. Ninety-two percent were cured if diagnosed early, and
their cure was effected with a single course of antibiotics without
operation. The patients were kept in the hospital for less than 1 week.
The authors quickly switched to oral antibiotics as soon as the patient
had responded to the IV antibiotics with excellent results.
authors used the sedimentation rate for determining the course of
antibiotic treatment for pseudomonal osteomyelitis of the foot after
puncture wounds. They found that once the sedimentation rate had
returned to normal, the antibiotic therapy could be stopped.
review article demonstrating the etiology and appropriate treatment of
disc space infection in children. The authors confirm a bacteriological
etiology and advocate antibiotic therapy. Response to treatment is
rapid and permanent.
E, Cohen N, Segev E, et al. Primary subacute epiphyseal osteomyelitis:
role of conservative treatment. J Pediatr Orthop. 2002;22(3):333-337. The
authors present a series of patients with subacute epiphyseal and
combined metaphyseal-epiphyseal osteomyelitis that were successfully
treated with antibiotics alone. They recommend conservative,
nonoperative treatment of subacute osteomyelitis.
authors have studied children with culture negative osteomyelitis and
have found that they are less likely to have had antecedent trauma. In
addition, their symptoms and clinical presentation are less severe than
children with culture positive osteomyelitis.
WJ, Mayo KM. The management of acute hematogenous osteomyelitis in the
antibiotic era. J Bone Joint Surg Br 1981;63-B(1):126-131. The
authors review the treatment of acute hematogenous osteomyelitis seen
in children between 1947 and 1976. They had a high failure rate of 20%
and found that failure was more common in surgically treated patients.
Their results are worse than one would expect today. The organism
recovered was S. aureus in more than 90%, but they isolated the organism in only 55% of patients who were treated successfully.
author identifies the cause of pseudomonal infections of the foot after
puncture wounds. These require debridement in all cases and prolonged
antibiotic therapy.
authors identify lesions of the epiphysis as representing primary
subacute epiphyseal osteomyelitis. The lesions are characteristic with
well-circumscribed borders. The treatment is debridement and antibiotic
therapy.
authors outline the appropriate treatment for acute septic arthritis.
They emphasize that early treatment of the infection is mandatory and
that poor results are more likely to be seen in infants and young
children than in older children and that poor results are more common
in children with a delay in the diagnosis and treatment. They also
point out that associated osteomyelitis of the femoral neck is more
likely to result in a poor outcome.
is a good review of the diagnosis and treatment of osteomyelitis in
children. All forms of osteomyelitis are discussed, and the means of
diagnosis and treatment are outlined.
DP, Stott NS. Community-acquired methicillin-resistant Staphylococcus
aureus: a cause of musculoskeletal sepsis in children. J Pediatr Orthop
1999;19(3):413-416. Methicillin-resistant staph
aureus bone and joint infections have become more commonplace. They
documented a 20% incidence of MRSA in community-acquired bone and joint
infections in children. As a result one should be aware of and consider
MRSA as the potential cause of a bone or joint infection.
authors discuss the prevalence of GABHS in bone and joint infections,
especially in children who have concurrent varicella infection.
reviewed their patients with pseudomonas infections of the foot after
nail puncture. Of 77 patients, 70 were wearing tennis shoes. They
discovered that after aggressive surgical debridement a shorter course
of antibiotics (7 days) was effective in obtaining a cure for the
infection.
MA, Nelson JD. Etiology and medical management of acute suppurative
bone and joint infections in pediatric patients. J Pediatr Orthop
1982;2(3):313-323. The authors review the
etiology and management of acute bone and joint infections and outline
oral therapy of acute hematogenous osteomyelitis.
the primary cause for infections of the bones of the foot after nail
puncture wounds. These infections should be treated aggressively.
authors identify the syndrome of chronic multifocal osteomyelitis. This
is an unusual disease with no known cause as of yet. It seems to run a
self-contained course that may last as long as 15 years.
author presents a good review of the cause, diagnosis, and treatment of
bone and joint infections in children. He emphasizes the need for
aspiration of the bone and joint for diagnosis and treatment.
JD, Bucholz RW, Kusmiesz H, et al. Benefits and risks of sequential
parenteral-oral cephalosporin therapy for suppurative bone and joint
infections. J Pediatr Orthop 1982;2:255-262. The
authors outline the sequential IV-oral treatment of acute bone and
joint infections in children. They emphasize the need for high-dose
antibiotics both in the IV and oral routes. They stress that the oral
antibiotics should not be given unless the child has had an excellent
response to the IV antibiotics.
authors review their findings of the origin of bone and joint
infections and present good scientific evidence to demonstrate why
acute hematogenous osteomyelitis begins in the metaphysics of long
bones.
JM, Drummond DS, Breed AL, et al. Subacute hematogenous osteomyelitis
in children: A retrospective study. J Pediatr Orthop 1982;2:249-254. The
authors outline the diagnosis of subacute hematogenous osteomyelitis in
children. This disease does not produce systemic symptoms but does
produce local findings. Treatment is debridement and antibiotic therapy.
is a 6% incidence of bone, joint, and soft tissue infections in
patients with varicella, which included osteomyelitis, septic
arthritis, and necrotizing fasciitis. Eighty-four percent of those
infections were caused by group-A β-hemolytic streptococcus.
authors identify the syndrome of intervertebral diskitis in children.
This is an infection of the disc space that should be treated
aggressively.
DL, Kim SK, Greene NW, et al. Differentiation between bone infarction
and acute osteomyelitis in children with sickle-cell disease with the
use of sequential radionuclide bone marrow and bone scans. J Bone Joint
Surg Am 2001;83-A: 1810-1813. The authors point
out the efficacy of differentiating between bone infarction and
osteomyelitis in patients with sickle cell disease with the use of
sequential bone marrow and bone scans.
JA, Vasileff T, Leonard JC. An evaluation of nuclear scanning in
orthopaedic infections. J Pediatr Orthop 1981;1:73-79. The
authors demonstrate that bone scanning is less than 100% accurate in
patients with acute hematogenous osteomyelitis. As a matter of fact,
they find in their series only 80% of the children with acute
hematogenous osteomyelitis had a positive bone scan. Therefore, the
diagnosis of acute hematogenous osteomyelitis should not depend on bone
scanning.
J. The three types of acute hematogenous osteomyelitis: A clinical and
vascular study. J Bone Joint Surg 1959;41-B: 671-680. The
author describes the three types of osteomyelitis—neonatal, childhood,
and adult—and demonstrates the difference in blood supply that forms
the basis for this classification system.
H, Haramati N, Flusser G. The diagnostic role of gadolinium enhanced
MRI in distinguishing between acute medullary bone infarct and
osteomyelitis. Magn Reson Imaging 2000;18: 255-262. The
authors were able to distinguish between bone infarct and osteomyelitis
in patients with sickle cell disease with the use of
gadolinium-enhanced MRI. In acute infarcts, one saw thin linear rim
enhancements on MRI while in osteomyelitis there was a more geographic
and irregular marrow enhancement.
DR, Bobechko WP, Gilday DL. The spectrum of intervertebral disc-space
infection in children. J Bone Joint Surg Am 1978;60-A:100-108. The
authors elucidate the cause, diagnosis, and treatment of disc space
infection in children. They emphasize the infectious nature of this
disease.
