BRACHIAL PLEXUS INJURIES
Professor Emeritus, University of Vienna Medical School; Vienna Private
Hospital; and Ludwig-Boltzmann Institute for Experimental Plastic
Surgery, Vienna, Austria.
Other traffic accidents or sports accidents are less frequently the
cause of a brachial plexus injury, and the resultant lesions are
generally less severe. Open injuries to the brachial plexus may result
from stabbing, but these lesions are rather rare. Gunshot wounds
usually lead to only partial lesions.
 |
|
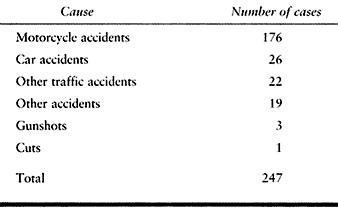
Table 60.1. Causes of Brachial Plexus Injuries
|
the shoulder with fixation of the brachial plexus between the clavicle
and the first rib. In such cases, the patient’s head continues to move
away from the shoulder, and a traction lesion results. The position of
the arm at the time of injury influences which roots are most exposed
to traction. The plexus may be compressed. If the humerus is fractured,
the plexus is exposed to uncontrolled traction.
brachial plexus by the fragments of the clavicle is possible.
Repositioning and osteosynthesis are indicated if there is an
ipsilateral plexus injury. A fracture of the transverse processes of
the cervical vertebrae may also cause external compression of parts of
the brachial plexus. Operative decompression is indicated.
subclavian vein, a huge hematoma forms that compresses the brachial
plexus. Vascular surgery to repair the artery or the vein is indicated
as an emergency procedure. The question arises whether immediate repair
of the brachial plexus should be performed. Usually, these patients
have suffered severe trauma and severe loss of blood. Because a surgeon
with experience in brachial plexus surgery might not be available,
vascular repair should be performed, and the vascular surgeon should
use an incision that will allow for later brachial plexus repair. Do
not attempt to identify the individual parts of the brachial plexus
other than what is necessary for the vascular repair, because this will
increase the fibrosis and make the secondary operation more difficult.
brachial plexus lesion by a competent brachial plexus surgeon has been
undertaken. I have had the opportunity to see the results of some of
these cases. My impression is that the repair performed under these
acute circumstances tends to be less fastidious than early elective
secondary repair and, consequently, the results are not as good.
a closed injury; however, they may also have suffered craniocerebral
trauma or other injuries of the extremities that require more immediate
attention. Although there is no indication for emergency surgery for a
brachial plexus injury, the question arises as to whether early
exploration is indicated (3,4).
An advantage of early surgery is that the lack of fibrous tissue makes
exploration easier. On the other hand, the extent of the damage is much
more difficult to assess because the damaged parts have not yet
developed the inevitable fibrosis, making it more difficult to identify
those lesions that may have a chance for spontaneous recovery.
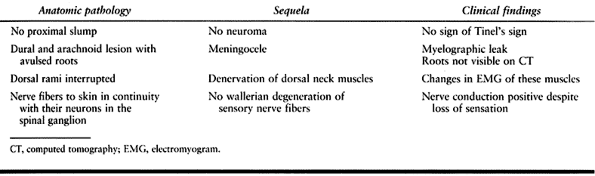
the spinal ganglion and the medulla (a supraganglionic lesion). There
is no proximal stump and no neuroma formation. The characteristics of
these lesions are summarized in Table 60.2.
 |
|
Table 60.2. Characteristics of Supraganglionic Brachial Plexus Injury with Avulsion of Roots
|
intervertebral canal and is situated outside, then the avulsion is
recognized easily during surgical exploration. If, after a root
avulsion, the spinal nerve remains within the intervertebral canal, the
situation is not as clear. If such a spinal nerve is transected, part
of the cross section may show fibrosis and degeneration but with the
sensory fibers still intact. Measurement of evoked potentials can
establish whether conduction to the central nervous system is possible.
roots remain intact (an infraganglionic lesion). There is a proximal
stump with neuroma formation. The characteristics of such lesions are
summarized in Table 60.3.
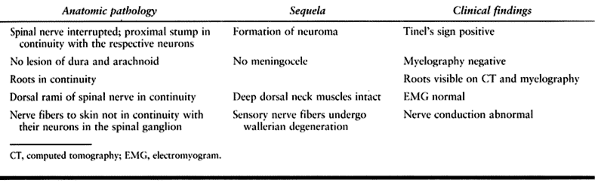 |
|
Table 60.3. Characteristics of Rupture of Spinal Nerves with Roots Intact
|
The long thoracic nerve, which exits the individual spinal nerves at a
very proximal level, remains intact in these cases. The suprascapular
nerve is usually involved. The pectoral nerves may or may not be
damaged, depending on the level of injury (Table 60.4).
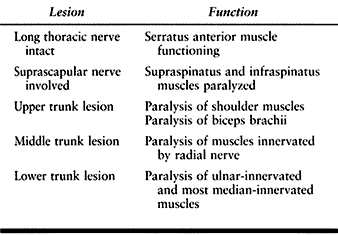 |
|
Table 60.4. Characteristics of Trunk Lesions in Brachial Plexus Injuries
|
pectoral nerves are intact (Table 60.5). A cord lesion may be suspected if a Tinel Hoffmann sign is located in the infraclavicular fossa.
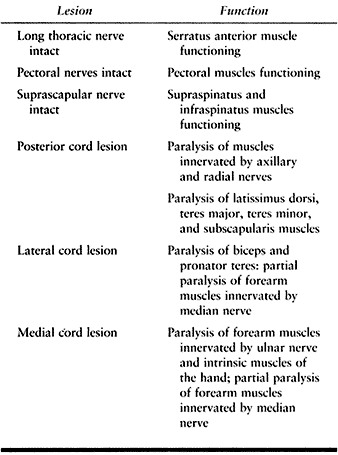 |
|
Table 60.5. Characteristics of Cord Lesions in Brachial Plexus Injuries
|
specific lesion. For example, a single spinal nerve may have both a
supraganglionic lesion and an infraganglionic lesion. One nerve root
may have suffered an avulsion, and a neighboring one may have suffered
a rupture. A common occurrence is the combination of a trunk lesion
with a cord lesion. Such combinations lead to difficulties in
diagnosis. Both false-positive and false-negative results occur with
myelography and computed tomography.
roots (C-5, C-6, C-7, C-8, and T-1). The brachial plexus may receive a
major additional source from C-4 or T-2. In the case of a prefixed
plexus, the brachial plexus consists of roots C-4 to C-8; in the case
of a postfixed plexus, it includes roots C-6 to T-2 (Table 60.6).
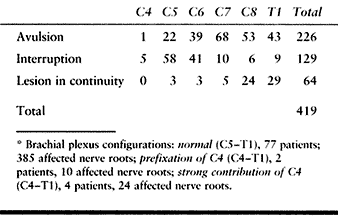 |
|
Table 60.6. Involvement of Individual Nerve Roots in 83 Patients with Complete Brachial Plexus Palsies*
|
lesions involve all roots but to different degrees. Initially there
might be a complete brachial plexus lesion with partial recovery.
Regard recovered muscles as weaker than normal muscles; this may be
important if palliative surgery is being considered. In type 2 lesions,
some roots are damaged whereas others are completely intact. In this
case, perform palliative surgery according to the usual indications.
-
Upper brachial plexus lesion (C-5, C-6)
-
Extended upper brachial plexus lesion (C-5, C-6, C-7)
-
C-7 lesion
-
Lower brachial plexus lesion (C-8, T-1)
-
Peripheral lesion involving the
suprascapular, the axillary, and the musculocutaneous nerves; this
lesion simulates an upper brachial plexus lesion.
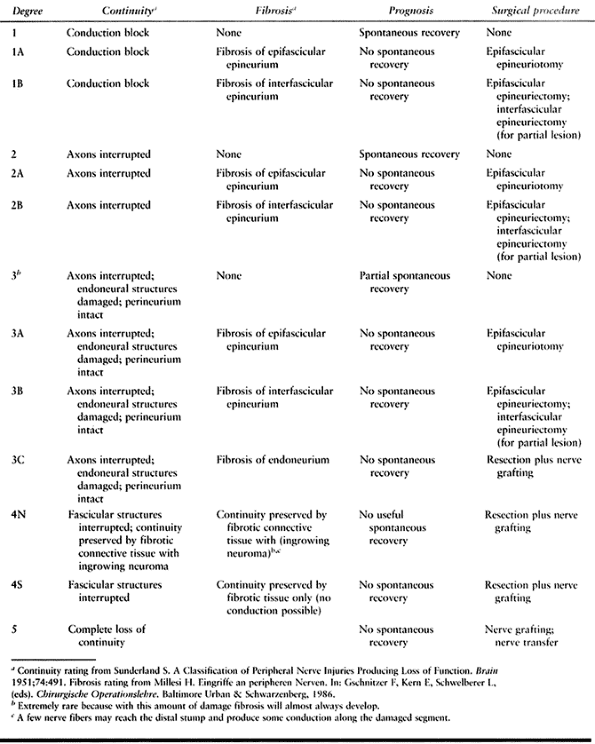
-
First-degree lesions
involve a conduction block without morphologic changes, which can be
recognized by electrophysiologic examination. If after several days the
conductivity of motor nerve fibers distal to the lesion remains intact
despite paralysis, assume a first-degree lesion. Complete spontaneous
recovery is possible. -
Second-degree lesions
are typified by a loss of continuity of axons with other structures
intact. This lesion cannot be recognized as easily as a first-degree
lesion. Complete spontaneous recovery is still possible. There is no
indication for surgery, but the occurrence of external compression and
the development of fibrosis may hinder spontaneous recovery. -
The amount of fibrosis, not considered in Sunderland’s original scheme, may be classified as follows (19,21):Type A fibrosis, in which the epifascicular epineurium
has become fibrotic and the whole nerve is compressed like a tight
stocking;Type B fibrosis, in which the interfascicular epineurium has also become fibrotic;Type C fibrosis, in which the content of the fascicles
of the endoneurium has become completely fibrotic. If type A or type B
fibrosis is combined with a first- or second-degree lesion, spontaneous
recovery will be impeded and neurolysis is indicated. -
Third-degree lesions
are marked by a loss of continuity of axons and endoneural structures
with intact perineurium. Type A or B fibrosis is possible; the content
of the fascicles may have become completely fibrotic (type C) if the
lesion is very severe or if a long time has elapsed since the injury.
With type A or B fibrosis, neurolysis is
P.1707
indicated.
With type C fibrosis, neurolysis cannot effect regeneration, and
resection with replacement of the involved fascicles by nerve grafts is
indicated. -
In fourth-degree lesions,
continuity is preserved only by connective tissue. This connective
tissue can become completely fibrotic, in which case there is no chance
for nerve fibers to grow beyond the lesion (type 4S; S = scar). In
other cases, a neuroma may grow into the connective tissue that still
unites the two stumps; in such instances, some nerve fibers may reach
the distal stump, and therefore some conduction may be elicited during
intraoperative stimulation (type 4N; N = neuroma). However, there is no
chance for functional recovery. All fourth-degree lesions necessitate
resection and repair by nerve grafts. -
In fifth-degree lesions, there is a complete loss of continuity.
combined lesions. The differentiation between these degrees is made
during intraoperative dissection with or without nerve stimulation (Table 60.7).
 |
|
Table 60.7. Combined Evaluation System for Extent of Brachial Plexus Injuries
|
have good chances for spontaneous recovery, and no surgery is
indicated. However, first- or second-degree lesions with epifascicular
fibrosis (type 1A or 2A, respectively) usually have initial signs of
recovery with subsequent failure to improve, or even some
deterioration. Exploration is indicated, and after neurolysis a
complete return of function is possible. The same is true for first- or
second-degree lesions with interfascicular fibrosis (type 1B or 2B,
respectively).
recovery, but this takes longer and the recovery will always be less
than complete. Pure third-degree lesions without fibrosis are rare;
usually there is a type A or B fibrosis. Such lesions can be converted
into pure third-degree lesions by internal neurolysis, after which
recovery may occur. If the endoneural space has become completely
fibrotic (type 3C), however, there is no chance of spontaneous
recovery. In such cases, resect the fascicles and repair continuity by
nerve grafts.
fibers may get into the distal stump. Usually, regeneration takes a
long time and the muscles may have already atrophied and fibrosed
before regeneration can occur. Therefore, spontaneous recovery cannot
be expected in these lesions, and surgical repair is indicated. This is
also the case for fourth-degree lesions without neuroma formation (type
4S). Likewise, if there is a loss of continuity (type 5), there is no
chance for spontaneous recovery.
3C, 4N, or 4S lesions, continuity must be restored. Because of the
complexity of the structure of the brachial plexus and the long
distance between the site of the lesion and the target organs, expect
only partial recovery. Except in infants, the return of intrinsic hand
function has not been observed.
flexion. If this occurs, the result can be regarded as satisfactory. In
addition, some control of the shoulder joint, with the correction of
subluxation, and the return of some protective sensation can be
expected. If some forearm muscles become active, the result can be
regarded as good. It is the experience of surgeons involved with
brachial plexus surgery that the development of pain syndromes is less
common in patients treated operatively than in those who are not
treated. In some instances, pain is relieved by surgery.
based in part on the historical development of brachial plexus surgery
since the 1960s. In 1966, a roundtable discussion on brachial plexus
surgery was held on the occasion of the tenth Sociéaate Internationale
de Chirugie Orthopédique et Traumatologie (SICOT) congress in Paris.
All roundtable members agreed that surgery has nothing to offer in the
case of panplexus root lesions, except to verify the
diagnosis.
With the diagnosis of a root lesion, their recommendation was to not
waste time with physiotherapy, and to perform early above-elbow
amputation with arthrodesis of the shoulder joint, provided that there
is a strong serratus anterior and trapezius muscle (31).
have performed an intercostal nerve transfer linking the third and
fourth intercostal nerves with the musculocutaneous nerve via a nerve
graft. This experience was furthered by Tsuyama et al. (35).
The percentage of good functional recovery of the biceps muscle has
constantly improved and has reached a very high standard in recent
years (29,34). In the
early 1960s, Millesi developed the combined approach to brachial plexus
surgery based on the new, reliable technique of interfascicular nerve
grafting. In recent years, two new trends have developed: the
reimplantation of avulsed roots by Carlstedt et al. (9) and immediate free muscle grafting by Doi (13). These new trends are listed in more detail in the following paragraphs.
recommended intercostal nerve transfer only “if there is no shadow of
doubt that the plexus is completely destroyed,” Tsuyama et al. (35)
applied this technique in all cases that showed signs of root avulsion
(e.g., one or two meningocelias in the myelogram), without convincing
himself that really all roots were avulsed. The advantages of this
strategy are that it is simple and the brachial plexus need not be
explored. The disadvantage is that it neglects other possibilities to
gain additional functions for the patient.
the combined approach to treatment was immediately applied to brachial
plexus lesions. This approach proved to be very helpful because
brachial plexus lesions usually involve very long defects that cannot
be treated by end-to-end coaptation. From the beginning, the goal was
to restore the continuity of as many structures as possible in
peripheral lesions, and to exploit not only the intercostal nerve
transfer but also all other available nerve transfers, such as
accessory nerve, cervical plexus, and so on. In these concepts,
reconstructive procedures at a later stage are included (26). Early results of this approach were presented in February 1969 at a meeting in Lausanne, Switzerland (20).
avulsed roots, and considerable experimental successes have been
presented by Carlstedt et al. (9). He applied this technique also in human cases with some success.
dorsal laminectomy to prove avulsion. After clarifying the diagnosis,
the wound would be closed and the brachial plexus explored from an
anterior approach. Information about the continuity for the dorsal
roots but not for the ventral roots can be obtained more easily through
intraoperative studies of evoked potentials.
With stimulation of the precentral gyrus of the cerebrum, action
potentials reach all the peripheral nerves. Lack of a peripherally
recorded potential indicates that the rootlets are avulsed. This is a
reliable technique and it makes surgical exploration to verify root
avulsion obsolete.
brachial plexus lesions by immediate free muscle grafting. In a first
stage, the gracilis muscle was connected to the accessory nerve and
used to activate the extensor digitorum communis tendons. At the same
time, sensory fibers from the cervical plexus were transferred to the
median nerve. In a second stage, a free muscle graft of the
contralateral gracilis muscle was used to activate the flexor muscles
of the fingers and also to produce flexion of the elbow joint. This
muscle was connected to intercostal nerves II, III, and IV, for motor
function. The sensory components of the intercostal nerves were
transferred to the ulnar nerve. At the same stage, the motor components
of intercostal nerves V and VI were transferred to the triceps muscle
to achieve elbow extension. With this technique, elbow flexion and
extension as well as finger flexion and extension were achieved.
cases in which the original muscles of the arm are atrophic, but it may
lead to a waste of muscle and nerves in cases less than 4 to 6 months
old if no attempt is made to reinnervate them. In addition, nothing is
done for shoulder abduction and external rotation. I feel that function
of the shoulder joint is key to positioning the forearm and hand for
function.
with the patient in a beach-chair position. This has the advantage that
the surgeon sees all anatomic structures as they are depicted in the
textbooks. The primary disadvantage, however, is that the clavicle is
an obstacle for the exposure, and it is understandable that many
surgeons do an osteotomy of the clavicle to facilitate the exposure.
After the brachial plexus surgery, the clavicle is fixed with plate and
screws (27,28).
-
I prefer the supine position and an
anterior approach for exploration. Place the patient supine with some
support beneath the scapula to elevate the shoulder joint slightly.
Turn the head to the contralateral side, prep the arm, and drape it
free. -
For exploration of the supra- and
infraclavicular fossas, sit between the head of the patient and the
abducted arm. For exploration of the axilla and the upper arm, move
into the angle between the trunk and the upper arm.
infraclavicular fossae are approached from the cranial direction. The
clavicle can be lifted and does not form an obstacle, and an osteotomy
of the clavicle is never necessary. Also, the roots can be visualized
from both the dorsal and anterior aspects. This is further facilitated
by a sagittal incision, as outlined below. The only disadvantage is
that the anatomy is seen upside down in relation to the usual anatomic
illustrations.
-
Make a sagittal incision directly across the supraclavicular fossa, crossing the clavicle at an oblique angle (Fig. 60.1).
This incision has the advantage that it can be extended dorsally to the
border of the trapezius muscle, and the supraclavicular fossa can be
explored very dorsally. This also provides a good approach to the
spinal nerves from a dorsal aspect (Fig. 60.2 and Fig. 60.3).![]() Figure 60.1.
Figure 60.1.
Approach to the brachial plexus by a sagittal incision. The figure
shows the patient on his back. His head is on the right, the shoulder
on the left side in the position of surgery. Figure 60.2.
Figure 60.2.
The exploration of the upper, middle, and lower trunks through a
sagittal incision in a patient with a lesion in continuity. The lesion
is on the right side. Left is proximal and right distal.![]() Figure 60.3. The remaining scar of the patient from Figure 60.1.
Figure 60.3. The remaining scar of the patient from Figure 60.1. -
Always use a second curved incision,
starting at the coracoid process and following the lines of tension to
reach the anterior axillary fold. Where it turns to follow the anterior
axillary fold, it can be continued to the middle aspect of the medial
surface of the upper arm. -
Elevate the skin between these two
incisions to unite the two fields. The only drawback to this approach
is that the pedicled skin flap between these two incisions has to be
moved in a medial or lateral direction to provide a proper field of
vision. -
If spinal nerves C-5 and C-6 have to be explored, make a third incision in the transverse direction, again following the tension lines in the caudal third of the neck.
-
In very rare instances, a fourth
transverse incision far more cranially on the neck is necessary to
provide access to the cervical plexus and the accessory nerve. -
Begin the exploration of the brachial
plexus in the deltopectoral groove, sparing the cephalic vein. Transect
the fascia beneath the muscle and find the lateral cord. Cranially, the
posterior cord is seen; inferiorly, the artery is encountered. Medial
to the artery, expose the medial cord. Proceeding in a lateral
direction, the division of the lateral cord into the musculocutaneous
nerve and the lateral root of the median nerve is seen. Follow the
posterior cord, dorsal to the artery, to explore the division
P.1711
into
the radial, axillary, and thoracodorsal nerves. Follow the medial cord
distally to expose the ulnar nerve and the medial origin of the median
nerve as well as the medial antebrachial cutaneous nerve. Usually, this
area is free of scar and the dissection is easy. -
If this part of the brachial plexus is
damaged, it will be fibrotic. If it is scarred in, I do not dissect at
this level. Lengthen the incision to the medial aspect of the upper arm
and explore the nerves where they are normal, following them proximally
into the area of injury. -
Having defined the aforementioned structures in the deltopectoral sulcus, dissect in a medial direction.
-
Isolate and retract the pectoralis minor muscle to expose the plexus along its medial border.
-
Continue the dissection to the area beneath the clavicle. This level is usually fibrotic.
-
Next, enter the supraclavicular fossa by
transecting the superficial cervical fascia. The external jugular vein
is seen and spared, and the omohyoid muscle is identified. -
Dissect bluntly from both sides beneath
the clavicle. Partially detach the clavicular origin of the pectoralis
major muscle and isolate the subclavius muscle to facilitate this
procedure. -
Follow the cords to define the divisions
and to isolate the upper, middle, and lower trunks. Follow them up to
the spinal nerves. -
The brachial plexus is now exposed in its full length and access is available through several windows:
-
The supraclavicular fossa cranial to the omohyoideus muscle.
-
The space between the omohyoid muscle and the clavicle.
-
The space between the clavicle and the subclavian muscle.
-
The space between subclavian muscle and the pectoralis major muscle proximal to the pectoralis minor muscle.
-
The space between the pectoralis major and the deltoid muscle distal to the pectoralis minor muscle.
-
The axillary groove and the medial aspect of the arm.
-
brachial plexus are defined as well. This includes the suprascapular
nerve in the supraclavicular fossa, originating from the superior trunk
at its division into the anterior and posterior division, the medial
and the lateral pectoral nerves, going to the major pectoralis muscles.
The long thoracic nerve and the dorsal scapular nerve are met where
they emerge from the scalenus medius muscle. The phrenic nerve is seen
on the anterior aspect of the scalenus anterior muscle.
-
Using the third transverse incision in
the lower part of the neck just described to approach the roots of C-5
and C-6, explore the plexus and the accessory nerve. In exceptional
cases, make another more cranially located transverse incision to
facilitate the exposure, again undermining the skin between the two
incisions. -
On the dorsal border of the
sternocleidomastoid, define the nerves ascending around the border of
this muscle. Here are found the transversus colli nerve, the major
auricular nerve, and the minor occipital nerve. Follow these nerves in
a central direction to reach the roots of C-4 and C-3. The C-4 root can
also be reached by following the phrenic nerve after its exposure on
the anterior surface of the scalenus anterior muscle. The different
branches of the supraclavicular nerves, followed in a central
direction, lead to C-4. Use electric stimulation to recognize motor
branches. -
Following the technique of Brunelli and Monini (8), expose the anterior motor branches of the cervical plexus.
-
Slightly lateral and deep to the punctum
nervosum, a group of lymph nodes is encountered. Deeper to these
structures is the accessory nerve, emerging from a layer beneath the
sternocleidomastoid muscle. Farther distally, the accessory nerve
becomes more superficial. Before it enters the trapezius muscle, it
gives off one branch to the muscle, which is able to maintain the
function of the muscle even if the accessory nerve is transected
distally to it. In this way, the function of the trapezius muscle can
be preserved. Frequently, the trapezius muscle gets an additional
innervation by a branch from the cervical plexus.
component that innervates in its dorsal segment the serratus posterior
superior and serratus posterior inferior muscles. Along their course,
they give off branches to the intercostal muscles, and finally they
innervate the transversus thoracis muscle. The sensory component leaves
the nerve at the anterior border of the serratus anterior muscle, as
lateral cutaneous rami, which reach the subcutaneous tissue and divide
into a ventral and a dorsal branch. Close to the sternum, the anterior
cutaneous branches leave the nerves and again divide into a ventral and
a lateral branch. It is therefore obvious that these nerves contain a
decreasing number of motor fibers as they progress distally. In the
proximal segment before the lateral cutaneous branches leave the nerve,
they contain more sensory fibers than after the departure of these
nerves. Intercostal nerves VII to XII contain more motor fibers because
they innervate the muscles of the abdominal wall.
-
Make an incision that runs parallel to
the ribs. The nerves can be transected far distally, isolated to a more
proximal level, turned in the direction of the nerve to be neurotized
(mainly the musculocutaneous nerve), and connected directly with this
nerve. The advantage of this procedure is that only one coaptation is
necessary. The disadvantage is that the incision has to be performed
far in an anterior direction and the number of motor fibers is lower
than in the midaxillary line, for example.An alternative to this technique is the following: -
Perform a longitudinal incision in the lateral thoracic wall between the pectoralis major and the latissimus dorsi muscle.
-
Split the fibers of the serratus anterior longitudinally along each rib; expose the ribs for a distance of 8–10 cm.
-
Isolate the intercostal nerves after
dissecting off the external intercostal muscles, elevating the
periosteum of the rib, and lifting the rib to gain access to the space
beneath the lower border of the rib. -
Define the intercostal nerves here. Sometimes there are separate motor and sensory components.
-
Use electric stimulation to identify the
motor component for transfer to muscles and eventually the sensory
component for transfer of sensory fibers.
cm of the nerve are isolated, the target nerve is not reached and a
nerve graft has to be used in all cases. The disadvantage of this
technique is that two lines of coaptation must be crossed by the nerve
fibers. The advantage is that the incision is less conspicuous and the
nerves contain relatively more motor fibers. It is obvious that from
intercostal nerve V onwards, all further intercostal nerve transfers
would need a graft because the distance between the thoracic wall and
the proximal arm is too long. Because the motor fibers in general are
small in number, three intercostal nerves are needed to neuroticize a
nerve of the caliber of the musculocutaneous nerve. Considering the
high success rate of Tetsuya et al. (34) and Ogino and Naito (29),
a transfer with end-to-end coaptation seems safer than a transfer by
nerve graft. I recommend using intercostal nerves III and IV as nerve
transfers by end-to-end repair; use intercostal nerves V, VI, and so
forth via nerve grafts.
-
Explore the intercostal nerves dorsally with the patient in a prone position.
-
Bring the intercostal nerves above the pleura to the anterior side.
-
Continue the operation after turning the patient on his or her back.
adhesions to the surrounding tissues. An epifascicular epineuriotomy
(single or multiple) is indicated to decompress the nerve trunk with
type A fibrosis. If there is a moderate degree of interfascicular
fibrosis (type B), epineuriotomy will not be sufficient. An
epifascicular epineuriectomy (dissecting the epifascicular tissue) is
then indicated. With more extensive interfascicular fibrosis (type C),
the interfascicular tissue must be partially excised to achieve
decompression (interfascicular epineuriectomy).
stumps is rarely possible with brachial plexus lesions. It is most
appropriate in cases of clean transection (e.g., stab injuries) and
follows the usual techniques.
denervated nerve brought with its cross section end-to-side in contact
with an innervated nerve may be neurotized from the innervated nerve.
The neurotization occurs across the perineurium if an
epineurial
window is created, and the neurotization is even better, of course,
when a perineurial window is also made. The possibility of end-to-side
coaptation offers many opportunities in brachial plexus surgery. I have
successfully performed an end-to-side coaptation between a functioning
dorsal scapular nerve and the denervated long thoracic nerve. Before
that, my colleagues and I had transected the dorsal scapular nerve and
sacrificed its function to achieve neurotization of a serratus anterior
muscle, which is extremely important. By using end-to-side coaptation,
we not only preserve the dorsal scapular function but also innervate
the serratus anterior muscle. In a similar way, we have used nerve
grafts that were coapted end-to-side to the phrenic nerve with its
proximal end, and end-to-end to the lateral and medial pectoral nerves.
In this way, the phrenic nerve could be used as an axon donor without
sacrificing its function. Our results with end-to-side coaptation have
been excellent in small nerves with one function. Whether it works in
major nerves with multiple functions is not yet known (see the
following discussion).
perineurial window does not seem to be necessary. It is obvious that
this method offers a vast amplification of the possibilities in
brachial plexus surgery. Its full value has yet to be shown in
sufficient clinical cases.
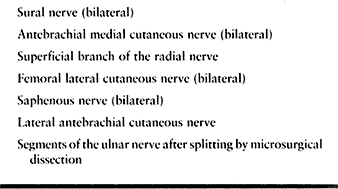
performed using the sural nerve, the medial antebrachial cutaneous
nerve, and occasionally the superficial branch of the radial nerve. The
use of cutaneous nerve segments for nerve grafting has the great
advantage that well-defined points of the proximal stump can be coapted
to well-defined points of the distal stump. Place the grafts
individually to increase the spontaneous revascularization and to avoid
central fibrosis. The grafts must be long enough to avoid longitudinal
tension. Very few stitches are necessary to approximate the graft to
the proximal and distal stumps. In recent years, the use of fibrin
glues has been recommended to shorten the operative time considerably (27,28).
If the grafts are long enough and the coaptations are absolutely
tensionless, only a few sutures between the stumps and the grafts are
necessary. In this case, the decrease of operative time by using tissue
glue is minimal (Table 60.8).
 |
|
Table 60.8. Donor Nerves for Free Nerve Grafts
|
have demonstrated that a free microvascular transfer is possible by
using the superior ulnar collateral artery as the nutritive vessel
without sacrificing the ulnar artery (Table 60.9).
 |
|
Table 60.9. Donor Nerves for Vascularized Nerve Grafts
|
ulnar nerve can be transferred as an island flap without interrupting
the blood supply. If the blood supply is not interrupted by a
complication, this type of nerve graft offers the advantage that it is
not dependent on the vascularity of the recipient bed. Achieving
connections of well-defined spots on the proximal and the distal stumps
is more difficult. Initial expectations that the qualitative result of
regeneration would be significantly improved by this method have not
been fulfilled. Most authors agree that the first signs of recovery
might occur somewhat earlier but the final result is the same as with
free nerve grafting with spontaneous revascularization (1,2,5,16,17,19).
graft after the epifascicular epineurium has been removed and the nerve
trunk has been split into minor units by interfascicular dissection (19).
the distal stump of a denervated nerve, nerve fibers can be transferred
to the denervated nerve and will neurotize this denervated nerve. After
reaching the target organ, functional recovery can be achieved;
however, the patient must learn to use the stimuli from the donor nerve
to perform a different movement with the newly reinnervated muscles. In
the vast majority of cases, patients learn this quite easily. A correct
term for this procedure would
be nerve-fiber transfer. The term neurotization
is widely used for this procedure, but this is incorrect because
neurotization means to bring nerve fibers into a denervated area.
Regardless of what type of transfer I do—end-to-end, end-to-side
repair, or nerve grafting—I always neurotize a denervated nerve from an
innervated proximal stump.
nerve, especially after the first branch has left the nerve. The
superior part of the trapezius muscle remains innervated by this branch
and can even be used for a muscle transfer at a later stage. The
trapezius muscle frequently has an additional innervation from the
cervical plexus (ramus trapezius) and does not become totally
denervated, even if the whole accessory nerve has been transected.
donor. The consequence of transecting the nerve would be the loss of
function of the ipsilateral diaphragm. In a young patient, this might
not do much harm if the thoracic muscles are intact. It should,
however, never be combined with an intercostal nerve transfer. The
phrenic nerve, of course, may easily be used if there is an accessory
phrenic nerve available. Today, we use it in an end-to-side fashion.
source for the brachial plexus; however, this may cause some problems
with tongue function.
clean transection of C-7 does not create many adverse consequences, and
contralateral transfers can be done. Usually after transection of C-7,
the patient notes some weakness in the triceps as well as wrist and
finger extensors. This weakness, however, disappears within a few weeks
by compensatory hypertrophy of the remaining innervated fibers. The
patient experiences some minimal sensory loss in the thumb and index
finger, which does not create a long-term problem. Gu et al. (14) and Chuang et al. (11)
performed a series of C-7 transfers that provided additional useful
function. The only problem, however, is that the patient has to
consciously use the nonparalyzed side to have an effect on the contralateral paralyzed side.
loss of function caused by a variation in the distribution of the nerve
fibers. Intraoperative electric stimulation studies may avoid this, but
in my opinion they are not reliable enough. Therefore, before a C-7
transfer, I perform an exposure of the contralateral root C-7 and place
a ligature on the divisions. On the day after the operation, the real
loss of function can be estimated very well and the patient can decide
whether this functional loss, which decreases with time, is acceptable.
If the patient accepts this condition, the C-7 transfer is performed at
a second stage.
evaluating the functional result of the first three cases several years
thereafter, I started to do it as a routine procedure. A C-7 transfer
is appropriate in patients with avulsions of all five roots, or in
patients with avulsion of several roots who have had the usual
reconstructive procedures (restoration of continuity and ipsilateral
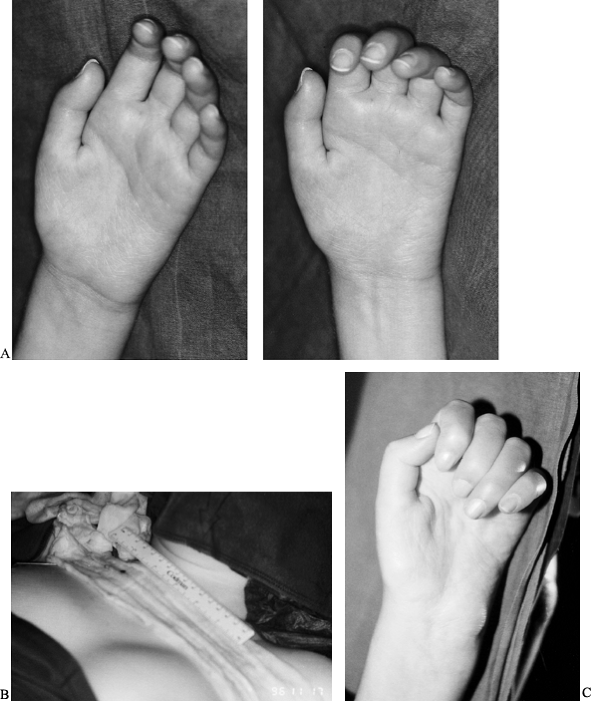
nerve transfers) and who desire additional strength (Fig. 60.4).
 |
|
Figure 60.4.
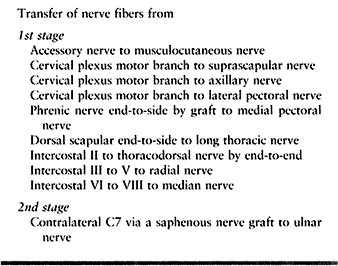
This 26-year-old patient suffered a complete brachial plexus lesion in a skiing accident. The first operation was performed in May 1996. There was a peripheral lesion of C-5 and C-6 and an avulsion of C-7, C-8, and T-1. At the first operation, nerve grafts were used to establish continuity from the accessory nerve to the musculocutaneous nerve. From a motor branch of the cervical plexus, the pectoralis nerve was neurotized. From C-5, nerve grafts were used to establish the continuity with the suprascapular, axillary, and radial nerves. C-6 was used to innervate the median nerve. A: From the neurotization of the median nerve, the patient achieved very little flexion activity of the finger flexors. B: The patient developed quite good shoulder control and external rotation, and good function of the triceps but weak function of the biceps muscle. A triceps transfer had to be performed to achieve sufficient elbow flexion. To improve the hand function, a C-7 transfer was performed in November 1996, connecting the dorsal division of C-7 by two saphenous nerve grafts with the ulnar nerve. C: This operation led to a significant improvement in the finger flexors with useful flexion of the fingers including the flexor pollicis longus, providing a key grip function between thumb and index finger (see Table 60.11). |
 |
|
Table 60.11. Avulsion of All Five Roots (1st Alternative)
|
perform nerve transfers to improve shoulder and elbow function. In a
second stage, do a C-7 transfer to provide additional functional
recovery in the forearm and finger flexors. With recovery of these
muscles, reconstruct a gripping function of the hand.
vascularized graft to connect C-7 with the median nerve. In a follow-up
series, we used the ulnar nerve as a vascularized graft to connect the
anterior division of C-7 with the median nerve, and free nerve grafts
to connect the posterior division of C-7 with the radial nerve distal
to the branches to the triceps muscle.
other donors, the anterior division was connected via a saphenous nerve
graft with the median nerve, and the posterior division via a saphenous
nerve graft with the ulnar nerve, attempting to restore the function of
both the median and the ulnar nerves. So far, the results are
encouraging, and late results will be available in a few years.
case, the biceps muscle became paralyzed after the ligature, in spite
of the fact that this was not recognized during intraoperative
electrostimulation. In this case, we opened the ligature and performed
the C-7 transfer as an end-to-side coaptation between the now-preserved
anterior and posterior division of C-7 and the grafts. This case is
especially
interesting and we presently are awaiting the final result. If this
end-to-side coaptation would work also with major nerve trunks, new
possibilities would be available.
 |
|
FIGURE 60-5.
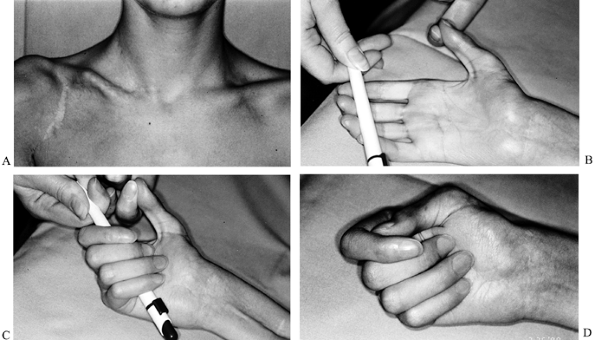
This 7-year-old boy suffered a skiing accident that caused a complete lesion of the right brachial plexus, along with a lesion of the subclavian artery and a fracture of the clavicle. The artery was reconstructed by a vein graft and the brachial plexus was operated on in the boy’s home town, but there was no recovery. I saw the patient for the first time 11 months after the accident. Avulsion of the C-7, C-8, and T-1 roots was diagnosed, and a reexploration with a C-7 transfer from the contralateral side was planned. The left root C-7 was ligated, and there was no
unexpected loss of function. Six days later, the right brachial plexus was explored, and the avulsion of C-7, C-8, and T-1 was confirmed. Only the right C-6 root could be used as a proximal stump. It was connected with the musculocutaneous nerve by a graft 18 cm long, with good success (biceps now M-4). Another graft was used to neurotize the axillary nerve (abduction with the shoulder joint now 30°), and two nerve grafts were interposed between C-6 and the radial nerve (with no recovery). A vascularized ulnar nerve segment 30 cm long was used to connect the contralateral C-7 and the ipsilateral median nerve. These images represent the patient’s hand at a follow-up examination 6 years after the initial surgery. A:
On the patient’s right side, note the scar of the sagittal incision and the somewhat broadened scar from the curved incision over the deltopectoral sulcus. On the patient’s left, note a sagittal scar from the exploration of the C-7 contralateral. B,C,D: There is good functional return in the flexor digitorum superficialis and profundus, the flexor pollicis longus, the flexor carpi radialis, and the palmaris longus. A tendon transfer for finger extension is scheduled.
|
the index finger was more than had been expected. In this case, the C-7
transfer was performed with only the dorsal division, and the anterior
division was preserved. The area of loss of sensibility was reduced
significantly.
intraneural neurolysis to define the degree of damage. If it becomes
clear that the fascicular structure remains intact and decompression
has been achieved, end the surgery. If not, the next step must be
carried out until you are convinced that the damage makes spontaneous
recovery impossible. In this case, resect the damaged part and restore
continuity by nerve grafting.
and distal stumps by resection or by interfascicular dissection until
normal tissue is found. Then restore continuity by nerve grafts. In
short defects (5–6 cm), direct end-to-end repair can be done. If,
however, the defect is very large and extends, for example, from the
trunk to the distal cord level, we prefer to do nerve grafts directly
to the peripheral nerves (e.g., from C-5 directly to the axillary
nerve, or from C-6 directly to the musculocutaneous nerve).
restoring continuity of all structures is usually impossible; you must
then decide which parts are to be repaired. The highest priority is to
restore elbow flexion. If possible, C-6 is united with distal
structures leading to that part of the lateral cord that forms the
musculocutaneous nerve, or in longer lesions we unite C-6 directly with
the musculocutaneous nerve. The next important function is the control
of the shoulder joint. Thus we try to neurotize the suprascapular nerve
with fibers coming from C-5 or even C-4. Nerve fibers of the dorsal
aspect of C-5, C-6, and C-7 are united with the posterior cord to
provide nerve fibers to the axillary and radial nerves or in long
defects with the nerves directly. C-8 and T-1 have a poor prognosis and
therefore are the last priority.
made for each patient. If the avulsion involves C-8 and T-1, the ulnar
nerve can be used as a nerve graft. No attempt is made to neurotize the
medial cord because of the low success rate (27), but this does not exclude neurotization of the ulnar nerve if a sufficient number of axon donors are available.
musculocutaneous nerve; in addition, it is connected with the scapular
nerve. If C-7 is avulsed, the accessory nerve may be the donor for
neurotization of the radial nerve.
neurotize the suprascapular, and the axillary nerve and intercostal
nerves are used for the musculocutaneous nerve. The musculocutaneous,
however, can also be neuroticized successfully by the accessory nerve (15).
A similar approach is used in avulsion of C-5, C-6, and C-7. If all
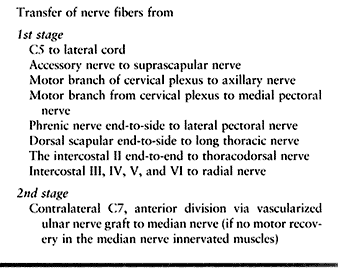
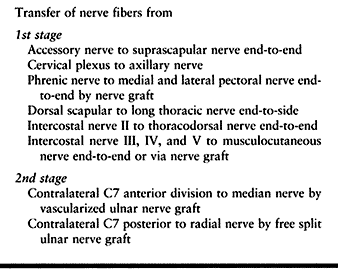
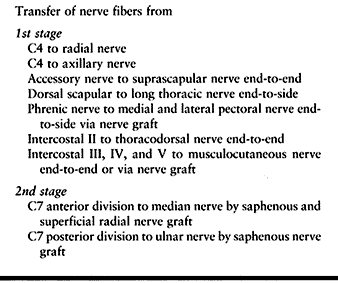
roots are avulsed, we prefer transfers at present, as suggested in Table 60.10, Table 60.11, Table 60.12 and Table 60.13.
 |
|
Table 60.10. Avulsion of Four Roots Except C5
|
 |
|
Table 60.12. Avulsion of All Five Roots (2nd Alternative)
|
 |
|
Table 60.13. Avulsion of All Five Roots (3rd Alternative) (Source from C4 to C5 Available)
|
in the intraoperative position by a plaster cast that includes the
head, the trunk, and the arm. After this time, initiate passive and
active mobilization for as wide a range of motion as possible.
Electrotherapy of the denervated muscles is useful. Splinting should
avoid overstretch of muscles, especially the biceps. There is still
some question as to whether the shoulder joint should be immobilized in
abduction to maintain full range of motion. In my experience, return of
active motion in the shoulder joint is always limited; therefore, it is
unnecessary to maintain a wide range of passive motion. The use of a
custom orthosis is recommended.
procedures to replace lost elbow flexion. Such procedures include the
following:
-
Transfer of the lateral segment of the pectoralis major muscle (12);
-
Transfer of the latissimus dorsi muscle (40);
-
Transfer of the common forearm flexor muscles from the medial epicondyle of the humerus to the shaft of the humerus (32).
available muscle force. To improve the functional result in a
regenerating biceps, a shortening of the biceps tendon some times
helps. The biceps tendon can also be transferred 1 or 2 cm distally to
provide a better lever arm and better flexion power by sacrificing
supination. Simultaneous innervation of the biceps and triceps occurs
quite often, and usually the triceps is the stronger muscle. In this
case, mobilize the triceps, transfer its tendon to the anterior aspect,
and unite it with the biceps tendon so that both muscles act as
flexors. Sometimes the triceps has successfully replaced a
nonfunctioning biceps, and in some cases we have initially restored the
function of the triceps by nerve transfer, planning a later muscle
transfer. The control of shoulder function can be achieved by
transferring the horizontal part of the trapezius muscle, including the
bony insertion at the acromion, to the humerus, as described by Saha (30).
Active gain of abduction is limited, but usually subluxation can be
avoided and some control achieved. In some cases, after tenolysis and
mobilization of the supraspinatus muscle and tendon, the horizontal
part of the trapezius muscle was directly transferred to the
supraspinatus tendon, proximal or in other cases distal to the
acromion, passing beneath the acromion. There are cases in which the
clavicular and posterior heads of the deltoid muscle recover but the
acromial component does not. The clavicular head and the posterior
third then act as adductors. In such cases, we have transferred
successfully the clavicular and the posterior heads toward the median
line of the shoulder joint (medianization) to make both parts abductors.
patient cannot externally rotate the shoulder. External rotation is a
crucial function and must be considered from the beginning. We feel it
is extremely important to achieve
a
strong pectoralis major muscle. We transfer the pectoralis major muscle
around the humerus shaft to the dorsal side along with the latissimus
dorsi muscle, to act as an external rotator. If there is an internal
rotation contracture, perform an osteotomy of the humerus at the same
time to derotate the humerus by about 60°. The osteotomy is immobilized
by plate and screws, and the pectoralis and the latissimus dorsi, if
recovered, are transferred to the lateral aspect of the humeral shaft.
Some patients develop a supination contracture, which has to be treated
by mobilizing the radius and ulna with transection of the interosseus
membrane, ensuring that the radial tuberosity clears the ulna during
pronation. The biceps tendon can be inserted into the coronoid process
to avoid supination and pronation contracture.
flexion are still paralyzed, we perform an arthrodesis or a tenodesis
of the wrist joint. If one or two forearm muscles have returned, it is
possible to restore simple gripping function (key grip) by tendon
transfers. If all these procedures fail, and the local muscles are
degenerated, a free muscle graft can still replace one or two important
functions (13).
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JY, Oberlin C, Bellaicke H. Vascularized Ulnar Nerve Transfer in Total
Palsy of the Brachial Plexus. Presented at the joint meeting of the Groupe pour l’Avancement de la Microchirurgie and the Deutschsprachige Arbeitsgemeinschaft für Mikrochirurgie der peripheren Nerven und Gefäβe, Strasbourg, France, May 1984.
L, Mingione A, Landi A. New Technical Acquisitions in the Surgery of
Brachial Plexus Lesions. Nuove Acquisizioni di Tecnica Chirurgica Nelle
Lesioni della Plesso Brachiale. Indicazioni alla Neurolisi, Autoinneste
e Trapianti Nervosi. Atti XIX Congr della Soc Italiana di Ortopedia e Traumatologia, Cagliari, 1974.
ChD, Wei FC, Noordhoff MS. Cross-Chest C7 Nerve Grafting Followed by
Free Muscle Transplantation for the Treatment of Avulsed Brachial
Plexus Injuries. Plast Reconstr Surg 1993;92:717.
K. Double Free Muscle Transfer to Reconstruct Prehension Following
Complete Avulsion of Brachial Plexus. Video presentation at 11th Congress of the International Society of Plastic, Reconstructive and Aesthetic Surgery, Yokohama, Japan, April 16–22, 1995.
YD, Zang GM, Yan JG, et al. Seventh Cervical Root Transfer from the
Lateral Healthy Side for Treatment of the Brachial Plexus. J Hand Surg 1992;17B:518.
M. Neurotization of Brachial Plexus Lesions with the Spinal Accessory
Nerve—Functional Results. Presented at the 2nd annual meeting of the American Society of Reconstructive Microsurgery, New Orleans, United States, February 1986.
M, Lebreton E, Bour C, et al. Free Vascularized Nerve Transfer in
Brachial Plexus Injuries. Presented at joint meeting of the Groupe pour l’Avancement de la Microchirurgie and the Deutschsprachige Arbeitsgemeinschaft für Mikrochirurgie der peripheren Nerven und Gefäβe, Strasbourg, France, May 1984.
H. Résultats Tardifs de la Greffe Nerveuse Interfasciculaire: Chirurgie
Réparatrice de Lésions du Plexus Brachial. Presented at the Symposium at the Clinique Longeraie (Dir Prof Claude Verdan), Lausanne, Switzerland, February, 1969.
H, Ganglberger J, Berger A. Interfasciculäre Nerventransplantation mit
Hilfe der Mikrochirurgischen Technik. Transactions of the 43th Congress
of Plastic and Reconstructive Surgery, Rome, Italy, October 1967. Excerpta Medica Int Corp Ser 174,56.
T, Naito T. Intercostal Nerve Crossing to Restore Elbow Flexion and
Sensibility of the Hand for a Root Avulsion Type of Brachial Plexus
Surgery. Microsurgery 1995;16:571.
H, Nagano A, Yoshihisa A, et al. The Present and Future of the
Intercostal Nerve Crossing as a Treatment of Brachial Plexus Injuries.
In: Vastamäki M, ed. Curr Trends Hand Surg 1995;289.
N, Sagakuchi R, Hara T, et al. Reconstructive Surgery in Brachial
Plexus Injuries. In: Proceedings of the 11th annual meeting of the Japanese Society of the Hand. Hiroshima, Japan, 1968:39.
E, Millesi H, Turkof R, et al. Intraoperative Electroneurodiagnostics
(Transcranial Electrical Motor Evoked Potentials) to Evaluate the
Functional Status of Anterior Spinal Roots and Spinal Nerves during
Brachial Plexus Surgery. Plast Reconstruct Surg 1997;99:1632.
E, Monsivais J, Dechtyar I, et al. Motor Evoked Potential as a Reliable
Method to Verify the Conductivity of Anterior Spinal Roots in Brachial
Plexus Surgery: An Experimental Study on Goats. J Reconstr Microsurg 1995;11:357.