Supracondylar Fractures of the Distal Humerus
children has been the subject of much discussion and dispute for many
years. Historically, these fractures were associated with complications
such as malunion that resulted in cosmetically and functionally
inferior results. Results have been improved and the frequency of these
complications dramatically decreased with more modern techniques of
treatment.67,110,122,127,182 With the advent of the image intensifier, which facillitates accurate pin placement, Blount’s28
caution against operative management is now of only historic interest.
Controversies about the treatment of supracondylar humeral fractures in
children, however, still exist: how long after injury can operative
treatment be done safely and effectively, is a crossed-pin
configuration better than a lateral-entry configuration, should type II
supracondylar fractures be treated operatively or nonoperatively, and
when does a pulseless hand require emergent treatment? In some areas of
North America, the treatment of supracondylar fractures in children is
shifting to pediatric subspecialists. In New England in 1991, 37% of
patients were treated by pediatric orthopaedic specialists; by 1999,
this figure rose to 68%.104
Although the incidence of these fractures generally has been reported
to be higher in boys, more recent reports indicate that the frequencies
of supracondylar humeral fractures in girls and boys seem to be
equalizing, and some series actually have reported higher rates in
girls.62,92 The left or nondominant side is most frequently injured in almost all studies (Table 14-1).41,62,92,195
and flexion types, depending on the direction of displacement of the
distal fragment. Extension type fractures are the most common,
accounting for approximately 97% to 99% of supracondylar humeral
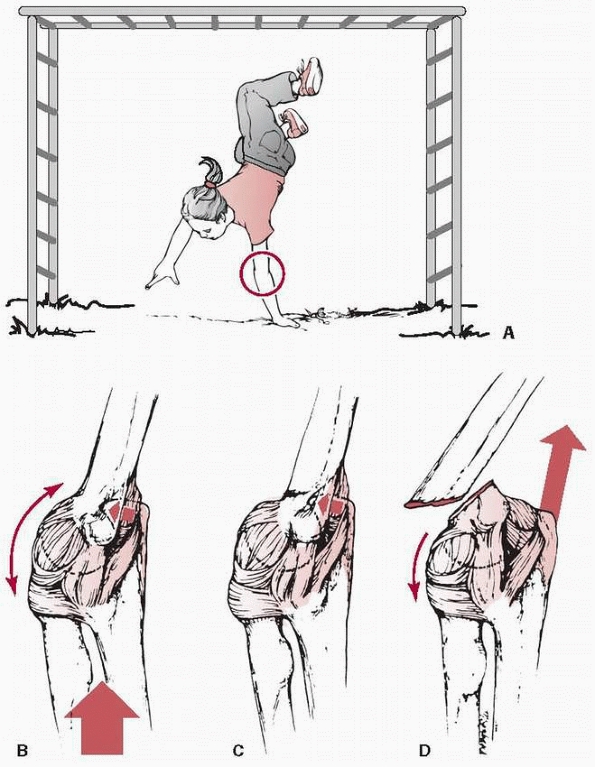
fractures.128 They usually are caused by a fall onto the outstretched hand with the elbow in full extension (Fig. 14-1). Most of this chapter discusses extension-type
fractures; flexion-type fractures are discussed separately at the end of the chapter.
|
TABLE 14-1 Overview of Supracondylar Fractures
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
FIGURE 14-1 Mechanism of injury—elbow hyperextension. A. Most children attempt to break their falls with the arm extended. With hyperextension, the elbow falls into hyperextension. B. The linear applied force (large arrow) leads to an anterior tension force. Posteriorly, the olecranon is forced into the depths of the olecranon fossa (small arrow). C. As the bending force continues, the distal humerus fails anteriorly in the thin supracondylar area. D.
When the fracture is complete, the proximal fragment can continue moving anteriorly and distally, potentially harming adjacent soft tissue structures such as the brachialis muscle, brachial artery, and median nerve. |
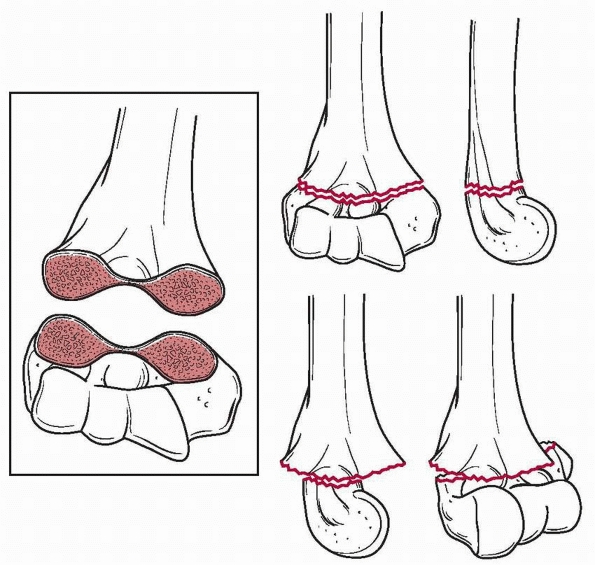
fossa anteriorly, the medial and lateral columns of the distal humerus
are connected by a thin segment of bone, which makes this area
especially vulnerable to fracture (Fig. 14-2). Normal anatomic variants include absence of the olecranon fossa (Fig. 14-3) and presence of a supracondylar process, identified to some extent in about 1.5% of adult cadavers56; this should not be mistaken for pathology.
the olecranon fossa and acts as a fulcrum through which the extension
force can propogate a fracture across the medial and lateral columns.
With the anterior capsule simultaneously providing a tensile force on
the distal humerus proximal to its insertion, an extension-type
supracondylar humeral fracture is created. It has been postulated that
ligamentous laxity with resulting elbow hyperextension may predispose
to a supracondylar humeral fracture,147 but this association is unclear.136
treatment. With extension-type injuries, although the anterior
periosteum is torn, the intact posterior periosteal hinge provides
stability and makes reduction easier. Abraham et al.3
described periosteal changes with extension-type supracondylar humeral
fractures in immature monkeys. With minimally angulated (<40
degrees) fractures, the periosteum was detached from the anterior
humeral surface by up to 3 cm, and with more
angulation
(>40 degrees), the detached anterior periosteum was pulled distally
and partially torn by the proximal fragment; in both of these
situations, stable reduction was easily obtained by flexing the elbow
to 90 degrees and pushing the distal fragment forward. They determined
that the most stable position of the reduced fracture was maximal elbow
flexion and forearm pronation, regardless of whether the distal
fragment was displaced medially or laterally; the most unstable
position was full elbow extension with forearm supination. Other
authors also have described using pronation to assist in reduction, but
this maneuver may not be appropriate for all fractures. The integrity
of the medial and lateral periosteum often can be determined by the
direction of fracture displacement. In a typical posteromedially
displaced fracture, the medial periosteum usually is intact. By placing
the medial periosteum on tension, pronation closes the hinge and
corrects varus malalignment (Fig. 14-4).
In fractures with posterolateral displacement, however, the medial
periosteum often is torn, making pronation counterproductive. If the
lateral periosteum is intact, as usually is the case, supination may be
better. Disruption of the posterior periosteal hinge makes the fracture
unstable in both flexion and extension. Leitch et al.125
described this fracture as a multidirectionally unstable, modified
Gartland type IV fracture because it is less stable than a Gartland
type III fracture.
 |
|
FIGURE 14-2
Supracondylar fractures occur through the thinist portion of the distal humerus in the AP plane. The thin bone makes the fracture unstable. |
 |
|
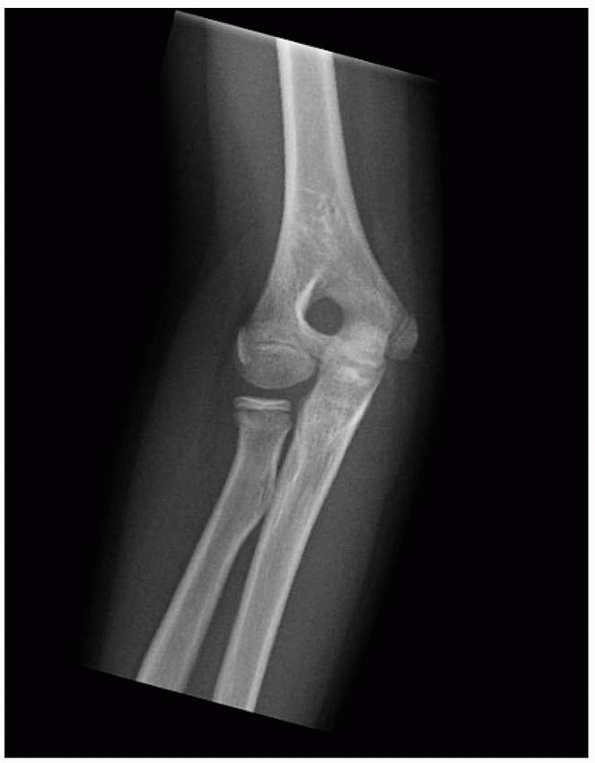
FIGURE 14-3
Normal anatomic variant in which there is no bone in the olecranon fossa. Note the minimally displaced radial neck fracture. (Reproduced with permission of Childrens Orthopaedic Center, Los Angeles, CA.) |
 |
|
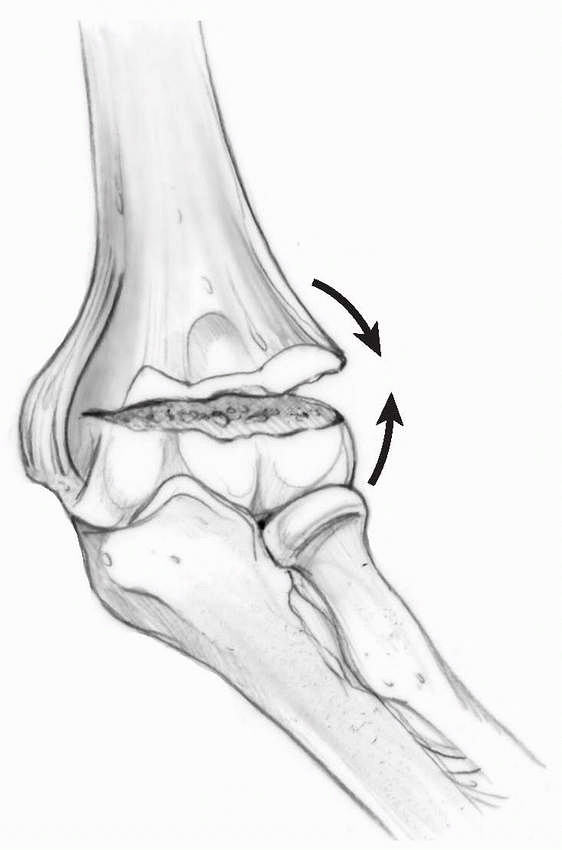
FIGURE 14-4
Laterally torn periosteum in a posteromedially displaced supracondylar humerus fracture. (From Skaggs DL. Closed reduction and pinning of supracondylar humerus fractures. In: Tolo VT, Skaggs DL, eds. Masters Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission) |
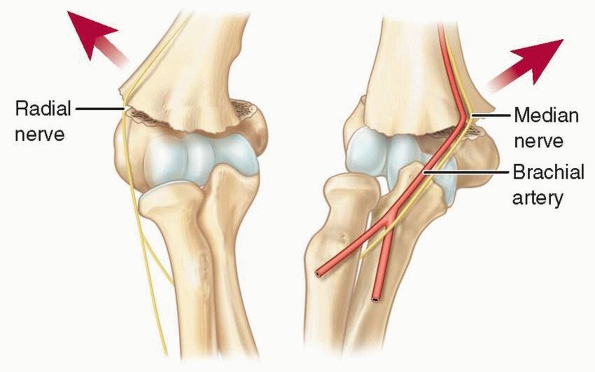
more common than lateral displacement, occurring in approximately 75%
of patients in most series. Whether the displacement is medial or
lateral is important because it determines which soft tissue structures
are at risk from the penetrating injury of the proximal metaphyseal
fragment. Medial displacement of the distal
fragment
places the radial nerve at risk, and lateral displacement of the distal
fragment places the median nerve and brachial artery at risk (Fig. 14-5).
The brachial artery and median nerve may become entrapped in the
fracture site with lateral displacement, but they are highly unlikely
to become entrapped with the distal fragment displaced medially. The
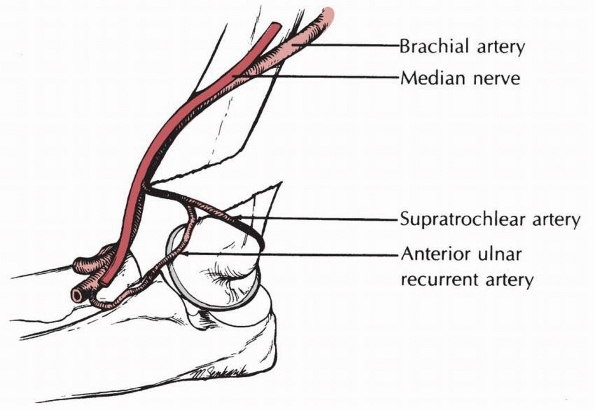
brachial artery is placed further at risk by the ulnar-sided tether of
the supratrochlear artery (Fig. 14-6).171
 |
|
FIGURE 14-5
Relationship to neurovascular structures. The proximal metaphyseal spike penetrates laterally with posteromedially displaced fractures and places the radial nerve at risk; with posterolaterally displaced fractures, the spike penetrates medially and places the median nerve and brachial artery at risk. |
found that this classification had higher kappa values for intra- and
interobserver variability than those reported for several other
fracture classification systems.
 |
|
FIGURE 14-6
Arterial pathology. The supratrochlear branch that arises from the anterior ulnar recurrent artery may bind the main trunk of the brachial artery against the sharp end of the proximal fragment. (From Rowell PJW. Arterial occlusion in juvenile humeral supracondylar fracture. Injury 1974;6:254-256, with permission.) |
instability, to the original classification seems appropriate because
of its treatment implications, but larger studies are needed to confirm
its usefulness.
loss of normal alignment in fractures with comminution and collapse of
the medial column (Fig. 14-7). Medial collapse
signifies malrotation in the frontal plane (which defines the injury as
at least a type III fracture) and is associated with a loss of the
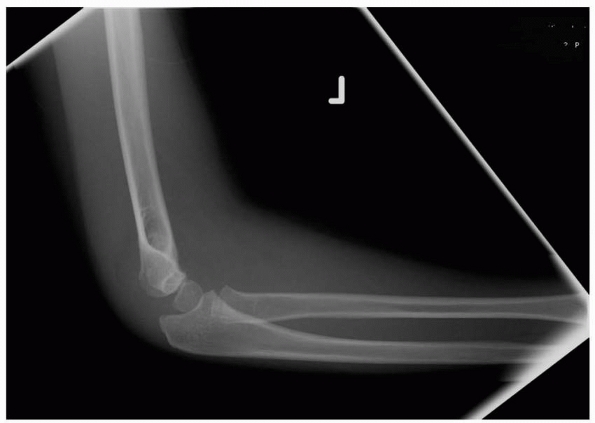
Baumann angle and varus malalignment. The lateral view (Fig. 14-8)
may show reasonable alignment, which may lead to an underappreciation
of the seriousness of this fracture, which requires operative
reduction. Bahk et al.16 reported
that fractures with more than 10 degrees of obliquity in the coronal
plane or 20 degrees in the sagittal plane were more likely than
fractures with less obliquity to result in malunion.
child with elbow pain or a child who fails to use the upper extremity
after a fall. Unless there is clearly localized tenderness with the
remainder of the arm nontender, initial radiographs should include the
entire extremity because multiple fractures may be present even with an
injury that seems to be minor trauma. In children with elbow pain and
failure to use the upper extremity, the differential diagnosis should
include occult fracture, nursemaid’s elbow, and infection. With a clear
history of a “pulling type” of injury, manipulation for a nursemaid’s
elbow can be done before a radiograph is obtained. In general, if the
history is not clear or if there is any question of a fall onto an
outstretched hand as the mechanism of injury, a radiograph should be
obtained before elbow manipulation. Point tenderness over the medial
and lateral columns suggests a supracondylar fracture, as opposed to
tenderness on only one side of the elbow, which suggests another type
of injury.
humeral tenderness and restriction of motion, particularly lack of full
extension. Radiographs may be negative except for a posterior fat pad
sign. In type III fractures, gross displacement of the elbow is evident
(Fig. 14-9).
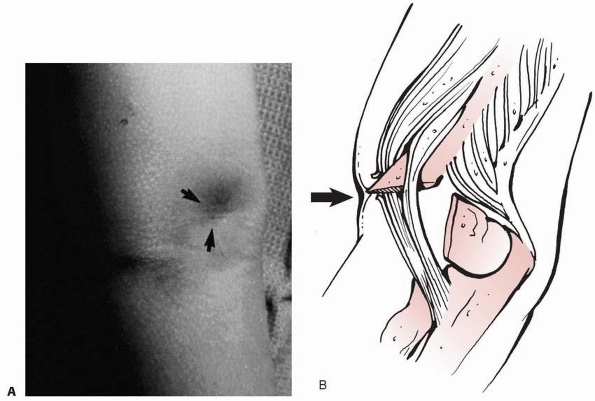
fragment has penetrated the brachialis and the anterior fascia of the
elbow (Fig. 14-10). Skin puckering results from
the proximal segment piercing the brachialis muscle and engaging the
deep dermis. This is a sign of considerable soft tissue damage. If any
bleeding from a punctate wound is present, this should be considered an
open fracture. When the proximal fragment is disengaged from its pucker
in the skin during reduction, there is sometimes bleeding, which is a
sign of a grade I open fracture.
be performed in all patients; this may be quite difficult in a young
child but should be attempted. Sensation should be tested in discrete
sensory areas of the radial nerve (dorsal first web space), medial
nerve (palmar index finger), and ulnar nerve (palmar little finger). If
a child is not cooperative or has altered mental status, a wet cloth
can be wrapped around the hand to check for wrinkling of the skin.
Motor examination should include finger, wrist, and thumb extension
(radial nerve), index
distal
interphalangeal flexion and thumb interphalangeal flexion (anterior
interosseous nerve), thenar strength (median nerve), and interossei
(ulnar nerve) muscle function. In young children, the interosseous
nerve can be tested by asking the child to pinch something with his or
her thumb and first finger while palpating the first dorsal
interosseous for muscle contracture.
|
TABLE 14-2 Modified Gartland Classification of Supracondylar Fractures
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
presence of pulse, as well as warmth, capillary refill, and color of
the hand. Assessment of the vascular status is essential, as series
report up to 20% of displaced fractures present with vascular
compromise.35,157,177 The vascular status can be classified into one of three categories:
 |
|
FIGURE 14-7
Medial comminution is a subtle radiographic finding and indicates a more unstable variant that may collapse into varus if not treated appropriately. (From Tolo VT, Skaggs DL, eds. Masters Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007: 1-15, with permission.) |
-
Hand well-perfused (warm and red), radial pulse present
-
Hand well-perfused, radial pulse absent
-
Hand poorly perfused (cool and blue or blanched), radial pulse absent
suspicion is needed to recognize signs of a developing forearm
compartment syndrome, such as considerable swelling or ecchymosis,
anterior skin puckering, and an absent pulse. Tenseness of the volar
compartment should be evaluated, and the amount of swelling about the
elbow should be noted. Passive finger extension and flexion should be
tested, and the findings should be accurately recorded. In the initial
examination of a child with a severe supracondylar fracture with high
parental and patient anxiety, it is easy to overlook vital information.
Because further decision making depends on an accurate initial
assessment, care should always be taken to obtain all of the previously
mentioned information as accurately as possible. When the elbow injury
is obvious, it is essential to evaluate the entire extremity because
associated forearm fractures can occur with supracondylar fractures and
substantially increase the risk of compartment syndrome (Fig. 14-11).
 |
|
FIGURE 14-8
Note the lateral view does not show significant displacement. This view alone would suggest nonoperative treatment may be sufficient. (Reproduced with permission of Children’s Orthopaedic Center, Los Angeles,CA.) |
 |
|
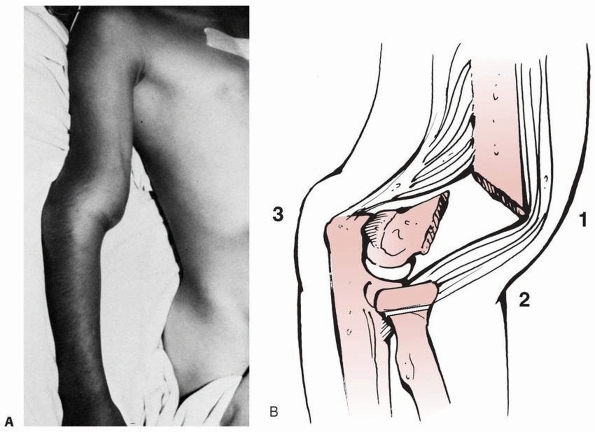
FIGURE 14-9 A. Clinical appearance. B.
The S-shaped configuration is created by the anterior prominence of the proximal fragment’s spike and extension of the distal fragment. |
 |
|
FIGURE 14-10 The pucker sign. This patient had penetration of the proximal fragment’s spike into the subcutaneous tissue. A. In the AP view, there is a large puckering or defect in the skin where the distal fragment has pulled the skin inward. B. Laterally, there is puckering of the skin (arrow) in the area where the spike has penetrated into the subcutaneous tissue.
|
 |
|
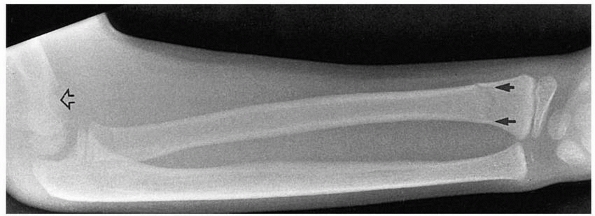
FIGURE 14-11 Occult ipsilateral fracture. Type II supracondylar fracture (open arrow) with an occult distal radial fracture (solid arrows).
|
outstretched hand as well as pain and inability to use the extremity
should have a thorough radiographic evaluation, generally including
anteroposterior (AP) and lateral views of the entire upper extremity.
Comparison views rarely are required by an experienced physician, but
occasionally may be needed to evaluate an ossifying epiphysis. A true
AP view of the distal humerus, rather than of the elbow, allows more
accurate evaluation of the distal humerus and decreases the error in
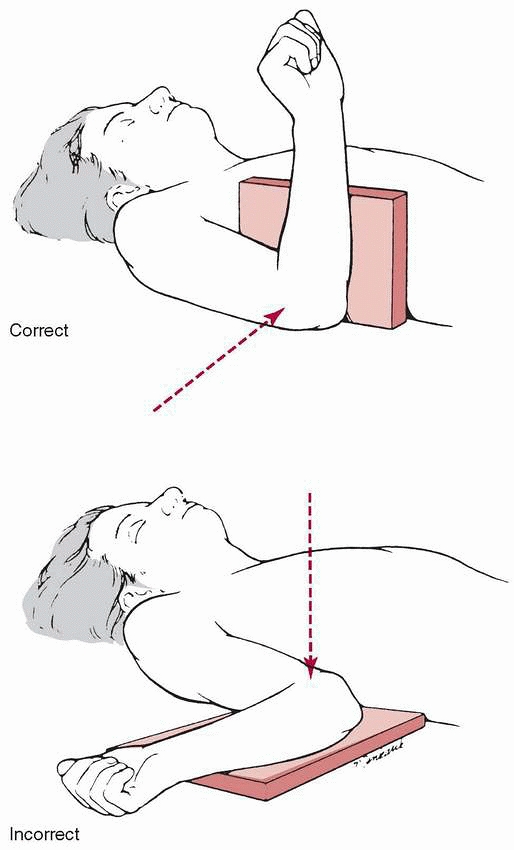
determining angular malalignment in the distal humerus. The lateral
film should be taken as a true lateral with the humerus held in the
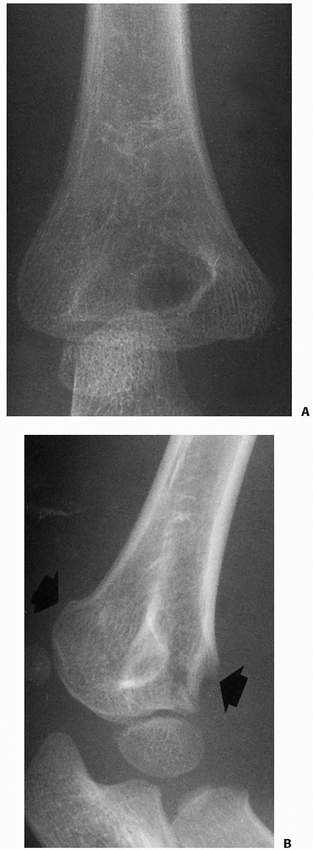
anatomic position and not externally rotated (Fig. 14-12). Oblique views of the distal humerus (Fig. 14-13)
occasionally may be helpful when a supracondylar fracture or occult
condylar fracture is suspected but not seen on standard AP and lateral
views, but should not be routinely ordered to evaluate an elbow injury.
of 34 patients with traumatic elbow pain and a posterior fat pad sign
but no visible fracture found that 18 (53%) had a supracondylar humeral
fracture, 9 (26%) had a fracture of the proximal ulna, 4 (12%) had a
fracture of the lateral condyle, and 3 (9%) had a fracture of the
radial neck.
 |
|
FIGURE 14-12
Radiograph positioning for taking a lateral view is with the upper extremity directed anteriorly rather than externally rotated. |
 |
|
FIGURE 14-13 Oblique views. Often, the fracture line is not visualized on any of the lateral or anteroposterior views (A). B. An oblique view of the distal humerus may demonstrate the extent of the fracture line (arrows).
|
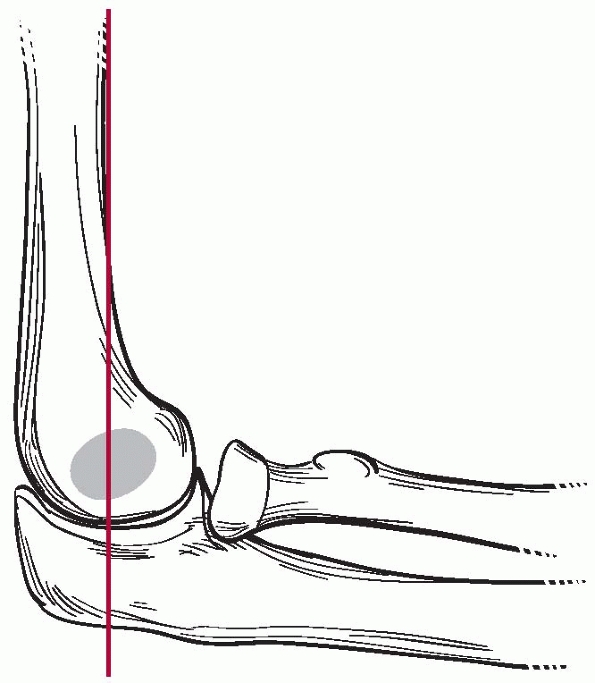
for the presence of a supracondylar fracture. The anterior humeral line
should cross the capitellum through the middle third on a true lateral
of the elbow (Fig. 14-14). In an extension-type
supracondylar fracture, the capitellum is posterior to this line. The
Baumann angle, also referred to as the humeral capitellar angle, is the
angle between the long axis of the humeral shaft and the physeal line
of the lateral condyle (normal range, about
9 to 26 degrees) (Fig. 14-15). A rule of thumb is that a Baumann angle of at least 10 degrees is acceptable; a decrease in the Baumann angle is a sign that a fracture is in varus angulation.
 |
|
FIGURE 14-14
Anterior humeral line should cross the capitellum on a true lateral of the elbow. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007: 1-15, with permission.) |
 |
|
FIGURE 14-15
The Baumann angle is between the line perpendicular to the long axis of the humeral shaft and the physeal line of the lateral condyle. A decrease in the Baumann angle may indicate medial comminution. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
III supracondylar fracture but do not show full detail of the distal
humeral fragment, we usually obtain further x-ray evaluation in the
operating room with the patient anesthetized. Repeat trips for
radiographs evaluation in the emergency setting generally result in
increased pain without significant improvement in radiographic quality.
Detailed radiographs need to be obtained at some point, however, to
define the fracture anatomy with particular emphasis on impaction of
the medial column, supracondylar comminution, and vertical split of the
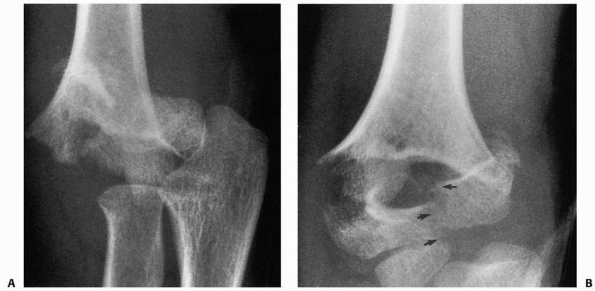
epiphyseal fragment. T-condylar fractures (Fig. 14-16)
can initially appear to be supracondylar fractures, but these generally
occur in children over 10 years of age in whom supracondylar fractures
are less likely.
can mimic an elbow dislocation. In an epiphyseal separation, the
fracture propagates through the physis without a large metaphyseal
fragment. This fracture occurs in very young children with primarily
chondral epiphyses. On physical examination, the patient appears to
have a supracondylar fracture with gross swelling about the elbow and
marked discomfort. The key to making the diagnosis and differentiating
this injury from an elbow dislocation is the alignment of the
capitellum with the radial head. Sometimes, a supracondylar fracture
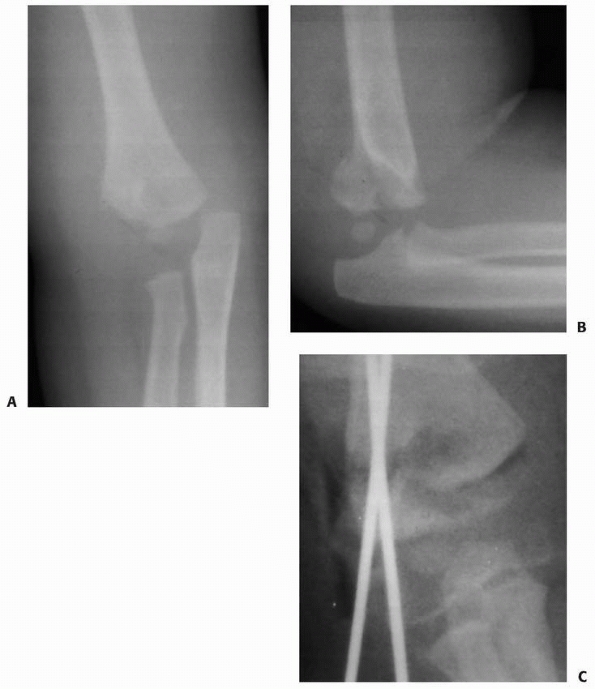
with a small metaphyseal fragment can mimic a lateral condylar fracture
(Fig. 14-17). In such cases, more data are
required to initiate treatment. An arthrogram may be helpful to
determine the extent of the elbow injury. In selected patients,
magnetic resonance imaging (MRI) or ultrasonography214 may also aid in evaluating the injury to the unossified epiphysis.
 |
|
FIGURE 14-16 Occult T-condylar fracture. A. Original radiographs appear to show a type III posteromedial supracondylar fracture. B. After manipulation, the vertical intercondylar fracture line (arrows) was visualized.
|
 |
|
FIGURE 14-17 This 1.2-year-old girl sustained a fracture that on the anteroposterior view (A) appears like an elbow dislocation and on the lateral view (B) has the appearance of a lateral condyle fracture. C.
Arthrography showed the outline of the entire cartilaginous epiphysis. This is an epiphyseal separation with a metaphyseal fragment (Salter-Harris type II). |
|
Pro |
Con |
|
|
Casting in-situ |
Good for type I fractures |
Not for displaced fractures |
|
Closed reduction and casting |
No surgery |
Cannot reliably hold reduction Risks compartment syndrome if elbow flexed to hold reduction Radiographs difficult to interpret |
|
Closed reduction and pinning |
Predictable good outcome Few complications |
Can be technically challenging to the inexperienced |
|
Open reduction and pinning |
Allows exposure and repair of neurovascular structures |
Makes fracture less stable Scarring Possible stiffness |
|
Removes impediments to reduction |
||
|
Traction |
Salvage for rare severely comminuted fractures |
Prolonged hospitalization Malunion |
initial splinting with the elbow in approximately 20 to 40 degrees of
flexion provides comfort and allows further evaluation. Tight bandaging
or splinting should be avoided, as should excessive flexion or
extension, which may compromise the vascularity of the limb and
increase compartment pressure.19,129
The arm should then be gently elevated. A careful examination of the
neurologic and vascular status is vital in all patients with a
supracondylar fracture, as well as an assessment of the potential for
compartment syndrome. The remainder of the limb should be assessed for
other injuries, and radiographs should include any area that is tender,
swollen, or lacks motion.
treated with closed reduction and pinning. Unless the fracture is open,
a closed reduction should be initially attempted in all fractures,
including type III fractures. With the patient supine and the child’s
arm on a radiolucent arm board, the fracture is first reduced in the
frontal plane and reduction is checked with fluoroscopy. The elbow is
then flexed while the olecranon is pushed anteriorly to correct the
sagittal deformity. Restoration of the Baumann angle (which is
generally >10 degrees) on the AP view, intact medial and lateral
columns on oblique views, and the anterior humeral line passing through
the middle third of the capitellum on the lateral view are indications
of a successful reduction. Because of the amount of rotation present at
the shoulder, some rotational malalignment in the axial plane can be
tolerated at the fracture site, but any rotational malalignment can
compromise fracture stability, so, if present, stability of the
reduction should be carefully evaluated, and a third pin probably
should be used.
(Kwires), as discussed later in this chapter, and the elbow is
immobilized in 50 to 60 degrees of flexion, depending on the amount of
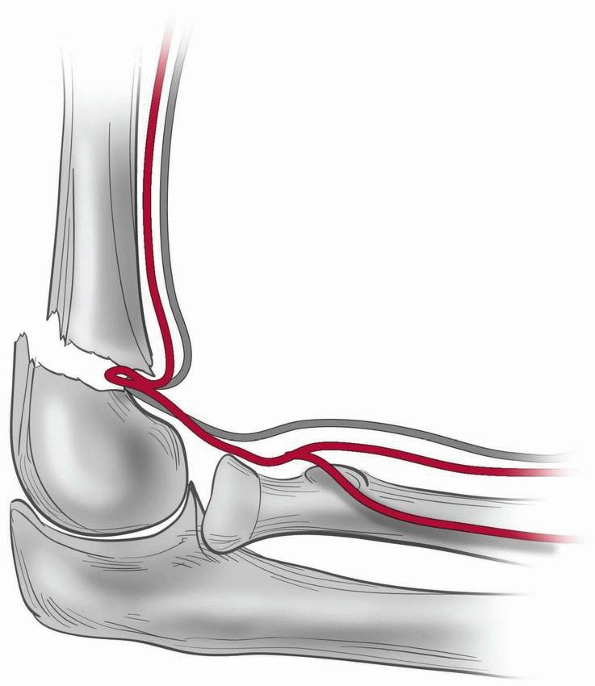
swelling and vascular status. A considerable gap in the fracture site
or an irreducible fracture with a rubbery feeling on attempted
reduction may be signs that the median nerve and/or brachial artery is
trapped in the fracture site and open reduction is indicated (Fig. 14-18).
large number of patients have been reported with both pinning
techniques. Although many of these reports are case series, two
prospective randomized clinical trials have been reported. Kocher et al.110
showed no statistically significant difference between the two
treatment groups in any radiograph or clinical outcome measures in
their prospective randomized clinical trial comparing lateral and
crossed-pinning techniques in the treatment of 52 type III
supracondylar humeral fractures. Another prospective randomized study
by Blanco et al.26 compared 57
patients with type III supracondylar fractures treated with
lateral-entry pinning to 47 treated with crossed-pinning and found no
statistically significant difference in the radiographic outcomes.
 |
|
FIGURE 14-18
Brachial artery and median nerve may be trapped at the fracture site. If a reduction feels rubbery, and a gap at the fracture site is seen on imaging, entrapment is possible, especially in the setting of vascular compromise or median nerve or anterior interosseous nerve injury. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
with use of crossed pins has been reported to be as low as 0%; however,
the two largest series of supracondylar fractures have shown the
prevalence to be 5% (17 of 345) and 6% (19 of 331).33,41,94,127,165,169,183,211 Others have reported that these iatrogenic injuries are more frequent.169,206 In 1977, Arino et al.12
recommended the use of two lateral pins to avoid injury to the ulnar
nerve, and a recent meta-analysis seems to support this recommendation:
iatrogenic ulnar nerve injury occurred in 40 of 1171 (3.4%) patients
with medial and lateral crossed pins and in only 5 of 738 (0.7%) of
those with lateral-entry pins. Although iatrogenic ulnar nerve injuries
usually resolve, several permanent iatrogenic ulnar nerve injuries have
been described.161,165,183
identified a group of children who may be at particular risk of ulnar
nerve injury during fracture reduction: those whose ulnar nerves sublux
onto the medial epicondyler and especially those whose nerves dislocate
anterior to the medial epicondyle. In 61% of children younger than 5
years of age, the ulnar nerve migrated over, or even anterior to, the
medial epicondyle when the elbow was flexed more than 90 degrees.211 Wind et al.,206 however, identified ulnar nerve subluxation in fewer than 2% of 22 children with supracondylar fractures. Flynn et al.65
recommended palpating the point of the medial epicondyle and inserting
the pin anterior to avoid the nerve; however, Wind et al.206
found that the location of the ulnar nerve was not determined
accurately enough by palpation to allow blind medial pinning. They
found an average difference of almost 2 mm between the predicted and
the actual locations of the nerve. Making an incision over the medial
epicondyle to make certain the ulnar nerve is not directly injured by a
pin may not ensure protection of the nerve,183 but Weiland et al.200
reported no iatrogenic ulnar nerve injuries in 52 patients with crossed
pins in whom a small medial incision was used. Green et al.80
reported only one iatrogenic nerve injury (1.5%) in 65 patients treated
with two lateral pins and one medial pin inserted with a mini-open
technique. In their systematic literature review, Brauer et al.31
found the probability of iatrogenic ulnar nerve injury to be five times
higher with crossed pins than with lateral-entry pins; however, when
only the two prospective studies were included there was no significant
difference.
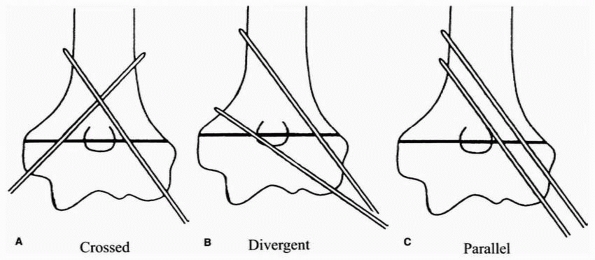 |
|
FIGURE 14-19 Three pinning techniques in study by Lee et al.123 A. Crossed: one medial and one lateral pin. B. Divergent: two divergent lateral pins. C. Parallel: two parallel lateral pins.
|
described iatrogenic ulnar nerve injuries in 6 patients in whom early
exploration found direct penetration of the nerve by the pin in 2,
constriction of the cubital tunnel in 3, and fixation of the nerve
anterior to the medial epicondyle in 1. In a series of 345
supracondylar humeral fractures treated by percutaneous pinning, Skaggs
et al.183 found that ulnar nerve
injury occurred in 4% of patients with medial pins placed without
hyperflexion and in 15% of those in whom the medial pin was placed
while the elbow was hyperflexed. No iatrogenic ulnar nerve injuries
occurred in the 125 fractures treated with lateral-entry pins alone.
These studies indicate that if a medial pin is used, the lateral pin(s)
should be placed first, then the elbow should be extended and the
medial pin placed without hyperflexion of the elbow. Avoidance of a
medial pin is the simplest way to avoid iatrogenic ulnar nerve injury:
no iatrogenic nerve injuries occurred in a series of 124 consecutive
fractures stabilized with lateral-entry pins, regardless of
displacement or fracture stability.182
configuration is stability, which remains somewhat unclear because some
previous biomechanical studies of stability of various pin
configurations have not used currently recommended configurations. For
example, two studies that evaluated the torsional strength of pin
configurations and found crossed-pins to be stronger than two lateral
pins,151,213
tested two lateral pins placed immediately adjacent to each other and
not separated at the fracture site as is currently recommended.174,182 Lee et al.,123
on the other hand, found that two divergent lateral pins separated at
the fracture site were stronger than crossed pins in extension loading
and varus but the configurations were equal in valgus (Fig. 14-19).123
They attributed the greater strength with lateral pins to the location
of the intersection of the two pins and greater divergence between the
two pins, which allowed some purchase in the medial column as well as
the lateral column (see Fig. 14-6).
pin fixation has been advocated. In a study of 21 children with type
III fractures, two lateral-entry pins were inserted after reduction and
stability was assessed by comparing lateral fluoroscopic
images in internal and external rotation.28
If the fracture remained rotationally unstable, a third lateral-entry
pin was inserted and images were obtained. A medial pin was added only
if instability was demonstrated after the insertion of three lateral
pins. Rotational stability was achieved with two lateral-entry pins in
6 patients, with three lateral-entry pins in 10 patients, and with an
additional medial pin in 5. No patient required a reoperation using
this protocol. The authors concluded that supracondylar fractures that
are rotationally stable intraoperatively after pin fixation are
unlikely to displace postoperatively. It is notable that they found
only a small proportion (26%) of these type III fractures to be
rotationally stable after fixation with two lateral-entry wires.28
reduction in none of 849 fractures treated with crossed pins and in 4
of 606 (0.7%) of fractures treated with lateral-entry pins.31
Deformity, defined as a carrying angle loss of more than 10 degrees or
cubitus varus of more than 5 degrees, occurred in 29 (3.4%) of those
with crossed pinning and 36 (5.9%) of those with lateral-entry pins.
However, this article did not include the largest series in the
literature that favored lateral-entry pins.31
recommended consideration of a three-pin pattern, either three
laterally divergent pins or two lateral pins and one medial pin, for
supracondylar humeral fractures with a less than complete anatomic
reduction. In their biomechanical analysis, three lateral divergent
pins were as strong as crossed-pinning and both were stronger than two
lateral divergent pins.27 Another
biomechanic study of simulated fractures with medial comminution
determined that three lateral divergent pins provided torsional
stability equal to that of standard medial and lateral crossed-pinning.120
These biomechanical studies support clinical recommendations that three
lateral-entry pins provide the most stable fixation of type III
fractures.174,182
found no malunions or loss of fixation. From this successful series,
combined with a failure analysis of eight fractures outside of this
series, they concluded that the important technical points for fixation
with lateral-entry pins are: (i) maximizing separation of the pins at
the fracture site, (ii) engaging the medial and lateral columns
proximal to the fracture, (iii) engaging sufficient bone in both the
proximal segment and the distal fragment, (iv) maintaining a low
threshold for use of a third lateral-entry pin if there is concern
about fracture stability or the location of the first two pins, and (v)
using three pins for type III fractures.182 Gordon et al.78
also recommended adding a third pin for type III fractures when
intraoperative stress indicated lack of stability with 2 pins. Lee et
al.122 reported 92% excellent
clinical results in 61 consecutive types II and II fractures treated
with three lateral pins. None of their patients had loss of reduction,
cubitus varus, hyperextension, loss of motion, or iatrogenic nerve
injury; none required additional surgery, and only one patient had a
minor pin track infection.122
studied eight supracondylar humeral fractures in which reduction was
lost and determined that loss of fixation in all was due to technical
errors that could have been identified on the intraoperative
fluoroscopic images and could have been prevented with proper
technique. Three types of pin-fixation errors were identified: (i)
failure to engage both fragments with two pins or more, (ii) failure to
achieve bicortical fixation with two pins or more, and (iii) failure to
achieve adequate pin separation (>2 mm) at the fracture site.174
fractures in which closed reduction fails, and fractures associated
with a dysvascular limb. Although earlier authors expressed concerns
about elbow stiffness, myositis ossificans, ugly scarring, and
iatrogenic neurovascular injury with open reduction, several more
recent reports have shown low rates of complications with open
reduction. Weiland et al.200
reported that, in 52 displaced fractures treated with open reduction
through a lateral approach, 10% had a moderate loss of motion but none
had infection, nonunion, or myositis ossificans. Fleuriau-Chateau et
al.,63 in a series of 34 patients
treated with open reduction through an anterior approach, reported a 6%
(2 of 34) unsatisfactory loss of motion but no infection, myositis
ossificans, malunion, or Volkmann contracture. Reitman et al.167
reported that 78% (51) of 65 patients treated with open reduction
(through either a medial or a lateral approach) had excellent or good
results according to the criteria of Flynn et al.65; 4 patients had loss of motion. Ay et al.14 found no loss of motion or clinical deformity in 61 patients treated with open reduction. Kaewpornsawan99
compared closed reduction and percutaneous pin fixation with open
reduction (through a lateral approach) in 28 children and found no
differences in the frequency of cubitus varus, neurovascular injury, or
infection; range of motion, union rate, and results according to the
criteria of Flynn et al.65 also were not significantly different.
are more familiar with lateral and medial approaches, we prefer a
direct anterior approach, especially in those with neurovascular
compromise. An advantage of the anterior approach is that it allows
direct visualization of the brachial artery and median nerve as well as
the fracture fragments. The relatively small (5 cm) transverse incision
along the cubital fossa produces a scar that is much more cosmetically
acceptable than that of the lateral approach, and scar contraction
limiting elbow extension is not an issue. In addition, this approach
does not disrupt the posterior periosteal hinge, which is useful for
fracture reduction. A series of 26 patients treated with the anterior
approach showed results equivalent to those of the traditional lateral
or combined lateral and medial approach in terms of malunion, the
criteria of Flynn et al.,65 and range of motion.
loss of motion and osteonecrosis caused by disruption of the posterior
end arterial supply to the trochlea of the humerus,32,209 and it generally is not recommended for open reduction of supracondylar humeral fractures in children (Fig. 14-20).
been increasingly accepted because there are relatively few
complications with this method. Surgical experience11,13,38,48,63,69,75,112,150,167,209 has dispelled the fears of infection, myositis ossificans, and neurovascular injury.73,176,186,199 In a combined series of 470 fractures treated by open reduction, the incidence of infection was 2.5%, all of which resolved.7,22,36,52,74,77,81,86,106,114,146,157,163,178,198,200 The incidence of neurovascular complications from the procedure itself was essentially zero.
Four patients with myositis ossificans (1.4%) were reported, all in a single series.77
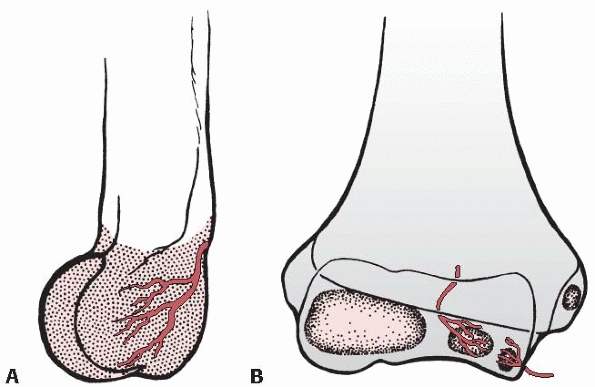 |
|
FIGURE 14-20 Intraosseous blood supply of the distal humerus. A.
The vessels supplying the lateral condylar epiphysis enter on the posterior aspect and course for a considerable distance before reaching the ossific nucleus. B. Two definite vessels supply the ossification center of the medial crista of the trochlea. The lateral vessel enters by crossing the physis. The medial one enters by way of the nonarticular edge of the medial crista. (From Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of the intraosseous vasculature in the distal humerus. Acta Orthop Scand 1959;38(Suppl: 1-232, with permission.) |
appears to be a loss of range of motion. One of the reasons given in
the past for loss of motion was the use of a posterior approach. It has
been stated that approaching the fracture through the relatively
uninvolved posterior tissues induces added scarring, leading to
stiffness. In earlier reported series using a posterior approach, loss
of range of motion was significant. Use of the anterior approach has
resulted in lower stiffness rates and complications similar to those of
closed treatment. Residual cubitus varus occurred in as many as 33% of
patients in some of the earlier series,8,51,77,200
most due to inadequate surgical reduction. When good reduction was
obtained, the incidence of cubitus varus deformity was low. Surgical
intervention alone does not guarantee an anatomic reduction; the
quality of the reduction achieved at the time of surgery is important.
reported that delayed open reduction, 11 to 17 days after injury, did
not increase the frequency of myositis ossificans. If a supracondylar
fracture is unreduced or poorly reduced, delayed open reduction and pin
fixation appear to be justified. Ağş4 showed that delayed open reduction and pinning can be safely accomplished after skeletal traction and malreduction.
puncture wound where the metaphyseal spike penetrates the skin. Even if
the open wound is only a small puncture in the center of an anterior
pucker, open irrigation and débridement are indicated. The anterior
approach, using a transverse incision with medial or lateral extension
as needed, is recommended. The neurovascular bundle is directly under
the skin and tented over the metaphyseal fragment, so care should be
taken even as the skin is incised. The skin incision can be extended
medially proximally and laterally distally. Often, only the transverse
portion of the incision is required, which gives a better cosmetic
result. The brachialis muscle usually is transected because its muscle
belly inserts on the coronoid attachment and is highly vulnerable to
trauma from the proximal metaphyseal fragment. The fracture surfaces
are examined and washed, and a curette is used to remove any dirt or
entrapped soft tissue. Once the débridement and washing are complete,
the fracture is stabilized with K-wires. All patients with open
fractures also are treated with antibiotics, generally, cephalothin for
Gustilo types I, II, and IIIA injuries, with the addition of an
aminoglycoside for types IIIB and IIIC fractures.
supracondylar fractures in children in most modern centers. Indications
for traction may include severe comminution, lack of anesthesia,
medical conditions prohibiting anesthesia, lack of an experienced
surgeon, or temporary traction to allow swelling to decrease. Devnani58
reported using traction in the gradual reduction of 28 fractures with
late presentation (mean of 5.6 days), although 18% of these children
required a corrective osteotomy for malunion. Cubitus varus has been
reported after traction treatment in 9% to 33% of patients in some
series,91,160 while others have reported excellent results.51,71,187
Because of the excellent results obtained with closed reduction and
pinning and the brief hospital stay required after this procedure
(usually no more than one night), it is difficult to justify the 14 to
22 days of hospitalization required for traction treatment. Advocates
of traction in the treatment of supracondylar fractures have reported
that the use of overhead traction with use of an olecranon wing nut15,155,208 gives superior results to sidearm traction (Fig. 14-21).
fracture, the periosteum is intact with significant inherent stability
of the fracture. A type I fracture may become apparent only with repeat
radiographs at 1- to 2-week follow-up after presentation with elbow
pain and initial radiographic findings limited to a posterior fat pad
sign. Periosteal reaction in the distal humerus may be all that is
visible on radiograph.
60 to 90 degrees of elbow flexion with side supports is preferred by
some.39,203
We prefer a carefully constructed, nonconstrictive, circumferential
cast to avoid skin problems, blistering, and the tourniquet effect of
elastic wraps left in place for a few weeks. The arm always looks
better when it comes out of an adequatelypadded well-made cast than
when it comes out of splint with an elastic wrap. If there is no
significant swelling about the elbow, circumferential casts can be
used, but the parents must be educated about elevation and the signs
and symptoms of compartment syndrome. The elbow should not be flexed
more than 90 degrees. Using Doppler examination of the brachial artery
after supracondylar fractures, Mapes and Hennrikus129
found that flow was decreased in the brachial artery in positions of
pronation and increased flexion. Before the splint is applied, it
should be confirmed that the pulse is intact and that there is good
capillary refill with the amount of elbow flexion intended during
immobilization. A sling helps decrease torsional forces about the
fracture.
document lack of displacement. If there is any evidence of distal
fragment extension, as judged by lack of intersection of the
anterior humeral line with the capitellum, the fracture has changed into a type II fracture and should be treated as such.
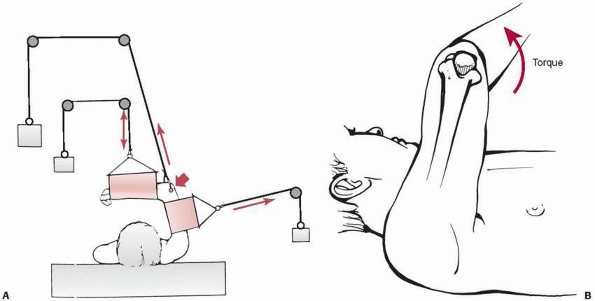 |
|
FIGURE 14-21 Overhead olecranon wing nut traction. The arm is suspended by a threaded wing nut through the olecranon (short arrow). The forces maintaining the reduction (long arrows) are exerted upward (A) through the pin and sideways through a counter-sling against the arm. The forearm is supported with a small sling (double arrow).
By placing the traction rope eccentric to the axis of the screw, a torque can be created to correct varus or valgus alignment (B). |
humeral line transecting the capitellum on the lateral radiograph, and
a Baumann angle of more than 10 degrees or equal to the other side. The
duration of immobilization for supracondylar fractures is 3 weeks,
whether type I, II, or III. In general, no physical therapy is required
after this injury. Patients may be seen 4 weeks after immobilization is
removed to ensure that range of motion and strength are returning
normally. Because the outcomes of type I fractures are predictably
excellent if alignment is maintained at the time of early healing,
follow-up visits after cast removal are optional depending on family
and medical circumstances.
actual injury that may involve soft tissue disruption much greater than
might be expected from the minimal bony abnormality. Excessive
swelling, nerve or vascular disruption, and excessive pain are
indicative of a more significant injury than a type I fracture, in
which case periosteal disruption may render this fracture inherently
unstable.
Cortex in Continuity). This fracture category encompasses a broad array
of soft tissue injuries. Careful assessment of the soft tissue injury
is critical in treatment decision-making. Because the posterior cortex
is in continuity, good stability should be obtained with closed
reduction. Significant swelling, obliteration of the pulse with
flexion, neurovascular injuries, excessive angulation, and other
injuries in the same extremity are indications for pin stabilization of
most type II fractures.
is operative stabilization rather than cast immobilization. The distal
humerus provides only 20% of the growth of the humerus and has little
remodeling potential. The upper limb grows approximately 10 cm during
the first year of life, 6 cm during the second year, 5 cm during the
third year, 3.5 cm during the fourth year, and 3 cm during the fifth
year of life.59 In toddlers (<3
years of age), some remodeling potential is present so nonoperative
treatment may be appropriate for a type II fracture in which the
capitellum abuts the anterior humeral line but does not cross it. In a
child who is 8 to 10 years old, however, only 10% of growth of the
distal humerus remains, so adequate reduction is essential to prevent
malunion.
noted that if pinning of all type II fractures in their series had been
done, 77% (37 of 48) of patients would have undergone an unnecessary
operative procedure; however, 23% (11 of 48) of the patients required
later operative reduction because they lost reduction after closed
reduction; 14% (2 of 14) who were followed had poor outcomes by the
Flynn criteria.64 Parikh et al.,64
in a retrospective review of 25 elbows treated with closed reduction
and casting, found similar results: 28% (7 of 25) loss of reduction,
20% (5 of 25) delayed surgery, and 2% (2 of 25) unsatisfactory outcomes
according to the Flynn criteria.
with type II fractures treated with closed reduction and pinning,
Skaggs et al.182 found no
radiographic or clinical loss of reduction, no cubitus varus, no
hyperextension, and no loss of motion. No patient had an iatrogenic
nerve palsy, and none required additional surgery.182
In a review of 191 consecutive type II fractures treated with closed
reduction and percutaneous pinning, there were four (2%) pin track
infections, of which three were treated successfully with oral
antibiotics and pin removal and one required operative irrigation and
débridement
for
a wound infection not involving the joint. No nerve or vascular
injuries, no loss of reduction, and no delayed unions or malunions were
reported.185
choosing operative treatment of these injuries. The amount of
hyperflexion needed to maintain reduction of unpinned type II fractures
predisposes these patients to increased compartment pressures.19 Mapes et al.129
used Doppler examination to determine that pronation and increased
flexion caused decreased flow in the brachial artery, leading them to
recommend a position of flexion and supination for “vascular safety.”
Pinning of these fractures avoids immobilization with the elbow
markedly flexed. In general, for any fracture that would require elbow
flexion of more than 90 degrees to hold reduction, pins should be used
to hold the reduction, and the elbow should be immobilized in less
flexion (usually about 45 to 70 degrees). If pinning is chosen, two
lateral-entry pins174,181,183,195
through the distal humeral fragment, engaging the opposite cortex of
the proximal fragment, generally are sufficient to maintain fracture
alignment (Figs. 14-22 and 14-23), although three pins can be used for added stability.182
Because the posterior cortex and the intact periosteum provide some
degree of inherent stability, crossed-pinning of type II fractures
generally is not needed. The techniques for crossed and lateral pinning
are described later in this chapter. If pin stabilization is used, the
pins are left protruding through the skin and are removed 3 to 4 weeks
after fixation, generally without the need for sedation or anesthesia.
emergency room with the elbow in either extreme flexion or extension,
the arm is carefully placed in 30 degrees of flexion to minimize
vascular insult and compartment pressure. In a type III fracture, the
periosteum is torn, there is no cortical contact between the fragments,
and soft tissue injury may accompany the fracture. Careful preoperative
evaluation is mandatory. If circulatory compromise is indicated by
absent pulse and a pale hand or if compartment syndrome is suspected,
urgent reduction and skeletal stabilization are mandatory. Most type
III fractures require operative reduction and pinning.
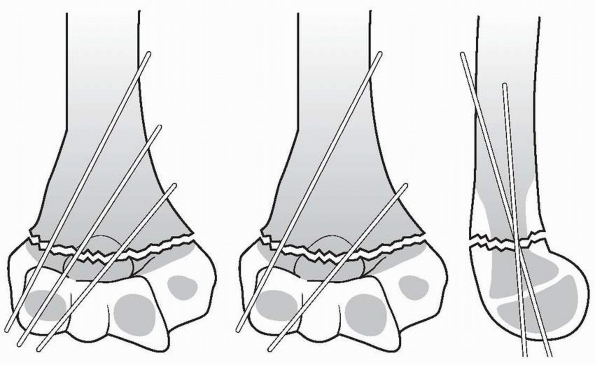 |
|
FIGURE 14-22
Properly placed divergent lateral entry pins. On the AP view, there should be maximal pin separation at the fracture site, the pins should engage both medial and lateral columns just proximal to the fracture site, and they should engage an adequate amount of bone proximal and distal to the fragments. On the lateral view, pins should incline slightly in the anterior to posterior direction in accordance with normal anatomy. (From Skaggs DL, Cluck MW, Mostofi A, et al. Lateral-entry pin fixation in the management of supracondylar fractures in children. J Bone Joint Surg Am 2004;86(4):702-707, with permission.) |
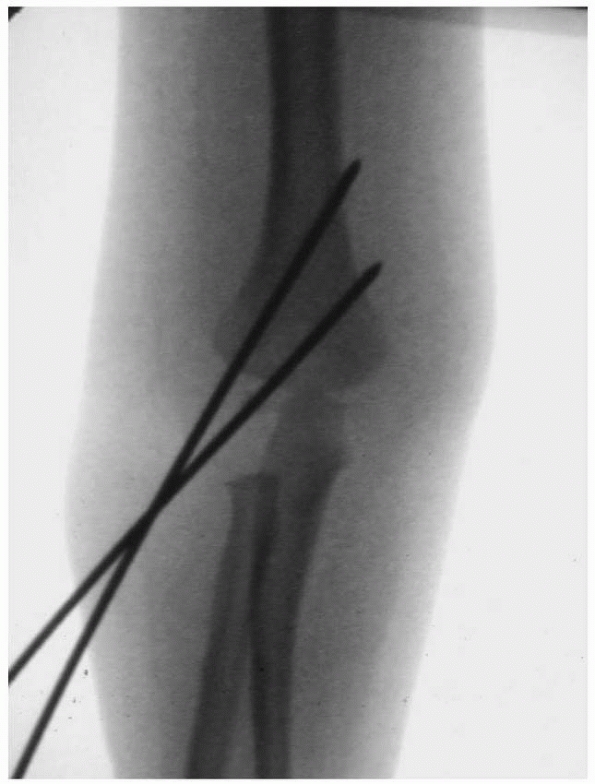 |
|
FIGURE 14-23
Intraoperative fluoroscopy of two lateral entry pins placed for a type II fracture. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
well. Because type III fractures are inherently unstable, the elbow
must be held in extreme flexion to prevent the distal fragment from
rotating; these fractures tend to rotate with flexion of less than 120
degrees.141 A figure-of-eight cast (Fig. 14-24)
can be used to maintain flexion of at least 120 degrees. If the arm can
be flexed to 120 degrees with an intact pulse, casting can be
used
as primary treatment. Usually, however, severe swelling prevents the
elbow from being kept in hyperflexion or compartment syndrome could
result. Alburger et al.6
reported that using skin traction initially until swelling decreased
allowed successful casting without pinning. In most series,48,115,158,198
the results of type III fractures treated with closed reduction and
cast immobilization are not as good as the results of pinning. Hadlow
et al.,84 however, suggested that
selective use of casting is beneficial, reporting that in their series,
61% of type III and 77% of type II fractures were successfully treated
without pinning.
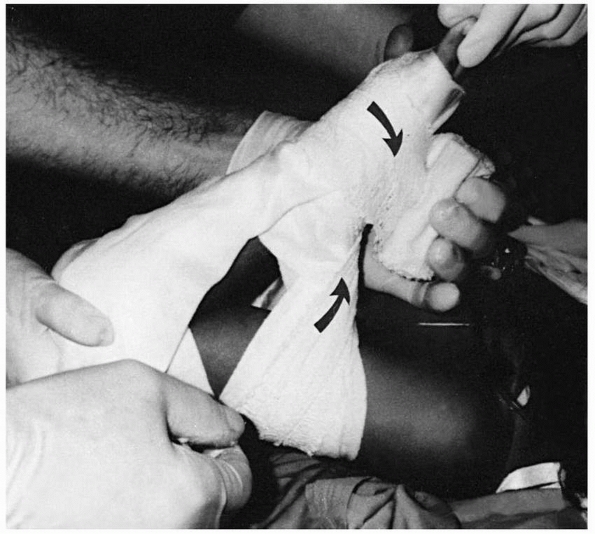 |
|
FIGURE 14-24 Figure-of-eight wrap. In the figure-of-eight cast, both the padding and the plaster are wrapped in a figure-of-eight manner (arrows).
Flexion of a swollen elbow with a supracondylar fracture beyond 90 degrees increases the risk of compartment syndrome and is generally not recommended if operative treatment is available. (From Wilkins KE. The management of severely displaced supracondylar fractures of the humerus. Techniques Orthop 1989;4:5-24, with permission.) |
worn for 3 to 4 weeks. Although a number of historic series used
casting as primary treatment, most recent reports favor pinning of this
fracture because of concerns about vascular compromise, compartment
syndrome, and malunion. It must be emphasized that flexion of the elbow
of 90 degrees or more with a type III supracondylar fracture
significantly increases the risk of compartment syndrome and should
rarely, if ever, be done if modern operative facilities and an
experienced surgeon are available.19 Traction may be a safer alternative.
Although not as markedly displaced as most type III fractures,
fractures with medial comminution usually require operative reduction
because collapse of the medial column will lead to varus deformity in
an otherwise minimally displaced supracondylar fracture (see Fig. 14-7).55 De Boeck et al.55
recommended closed reduction with percutaneous pinning for fractures
with medial comminution even with minimal displacement to prevent
cubitus varus. In their retrospective review of 13 patients with medial
comminution, none of the 6 who had operative fixation had cubitus
varus, while 4 of 7 treated nonoperatively developed cubitus varus.
described closed reduction in 9 patients. Their technique involves
placing two K-wires into the distal fragment, reducing the fracture in
the AP plane, and verifying the reduction with fluoroscopy. Then the
fluoroscopy unit, rather than the arm, is rotated to obtain a lateral
view (Fig. 14-25). The fracture is reduced in
the sagittal plane, and the K-wires are driven across the fracture
site. All fractures treated with this technique united with no cubitus
varus, malunion, loss of motion, or additional operative treatment.
Because of the limited number of these multidirectionally unstable
fractures in their series, neither the need for open reduction nor the
true complication rates can be determined.
swelling, overnight observation is standard. The caregivers should be
instructed about elevation and cast care and warning signs of infection
and compartment syndrome. The first postoperative visit usually is 1
week after surgery, though this is not evidence-based. Ponce et al.159
compared 52 patients with closed reduction and pinning of supracondylar
fractures who had follow-up appointments 10 days or less after surgery
to 52 who had follow-up visits later than 10 days. The overall
complication rate was 7.7%, including four infections, three pin
migrations requiring surgery, and no loss of reduction. Six
complications occurred in those with earlier follow-up and two in those
with later follow-up. The authors concluded, and we agree, that early
follow-up with radiographs after routine percutaneous pinning provides
no added benefits to patient care and may unnecessarily tax the
resources of the surgeon, clinic, and patient. They suggested that
clinical and radiographic evaluation can be safely delayed until pin
removal in most patients; however, if there is any concern about the
stability of the pin configuration, we recommend early follow-up with
radiographs in 5 days or less, because rereductions become increasingly
more difficult beyond 1 week.159
 |
|
FIGURE 14-25
In very unstable fractures, rotation of the shoulder into external rotation in order to obtain a lateral image of the elbow may lead to a loss of reduction. In these rare instances, rotation of the c-arm, rather than the elbow, is a useful trick. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007, with permission.) |
approximately 60 to 90 degrees of elbow flexion for approximately 3
weeks. Follow-up radiographic evaluation at 1 week is recommended for
assessment of fracture position.
supracondylar fractures. Two lateral pins are used for initial fixation
in nearly all cases. If two lateral pins fail to provide acceptable
fixation, we do not hesitate to place a third lateral pin. We believe
it is safer to hold a type II fracture reduced with pins than to flex
the elbow more than 90 degrees (Fig. 14-26).
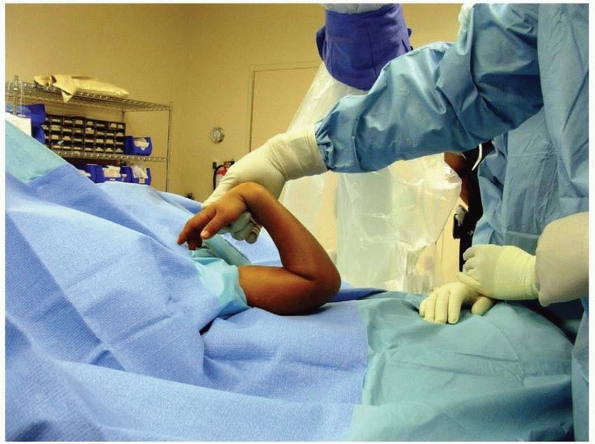
general anesthetic and prophylactic antibiotics. We prefer to have the
fluoroscopy monitor opposite the surgeon for ease of viewing (Fig. 14-27).
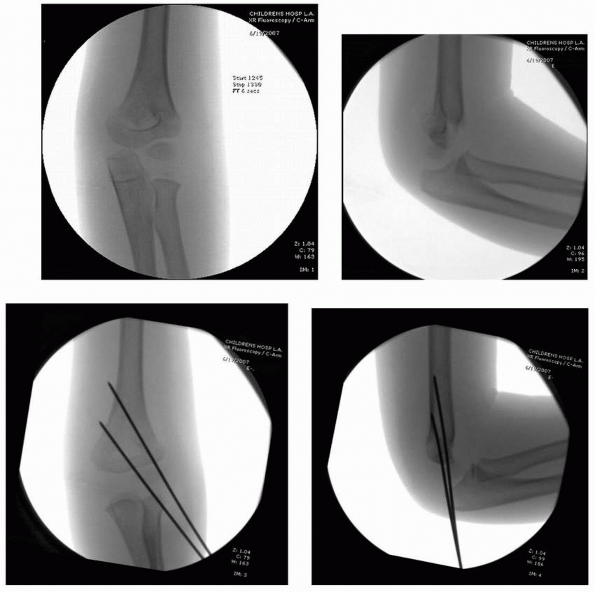 |
|
FIGURE 14-26
These intraoperative images are of a 4-year-old with a type II supracondylar humeral fracture that was closed reduced and pinned with two lateral entry pins according to the authors’ preferred technique. (Reproduced with permission of Children’s Orthopaedic Center, Los Angeles, CA.) |
with the fractured elbow on a short radiolucent arm board. Some
surgeons use the wide end of the fluoroscopy unit as the table, but
this does not allow rotation of the fluoroscopy unit for lateral images
of the elbow in cases of unusual instability in which rotation of the
arm leads to loss of reduction. It is essential that the child’s arm is
far enough onto the arm board that the elbow can be well visualized
with fluoroscopy. In very small children, this may mean having the
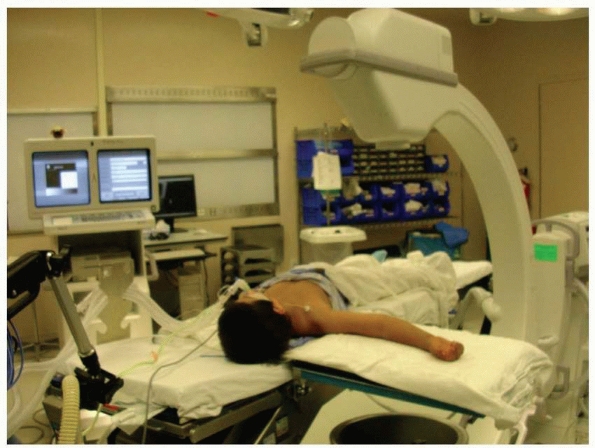
child’s shoulder and head on the arm board (Fig. 14-28).
 |
|
FIGURE 14-27
Positioning the fluoroscopy monitor on the opposite side of the bed allows the surgeon to easily see the images while operating. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
traction is applied with the elbow flexed about 20 degrees to avoid
tethering the neurovascular structures over an anteriorly displaced
proximal fragment. For badly displaced fractures, significant traction
is held for 60 seconds to allow soft tissue realignment with the
surgeon grasping the forearm with both hands, and the assistant
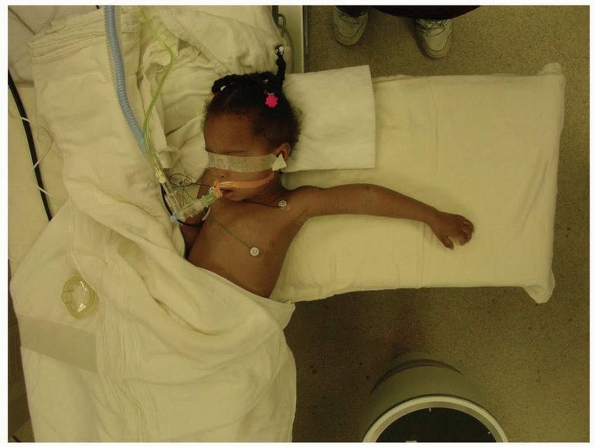
providing countertraction in the axilla (Fig. 14-29).
 |
|
FIGURE 14-28
In small children, imaging of the elbow may be difficult if the arm is not long enough to reach the center of the fluoroscopy unit. By placing the child’s head in the crack between the operating room table and the armboard, the elbow is more easily imaged, and the child’s head is unlikely to be inadvertently pulled off the side of the bed during the procedure. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007: 1-15, with permission.) |
 |
|
FIGURE 14-29 Reduction maneuver: traction with elbow flexed 20 to 30 degrees. Assistant provides countertraction against patient’s axilla (white arrow)
to allow for significant traction to be applied. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
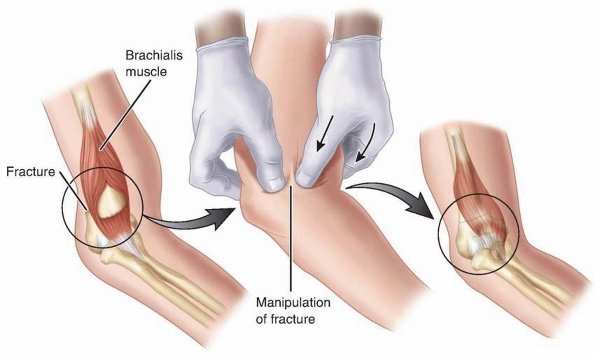
brachialis muscle, the “milking maneuver” is used: the biceps are
forcibly “milked” in a proximal to distal direction past the proximal
fragment, often culminating in a palpable release of the humerus
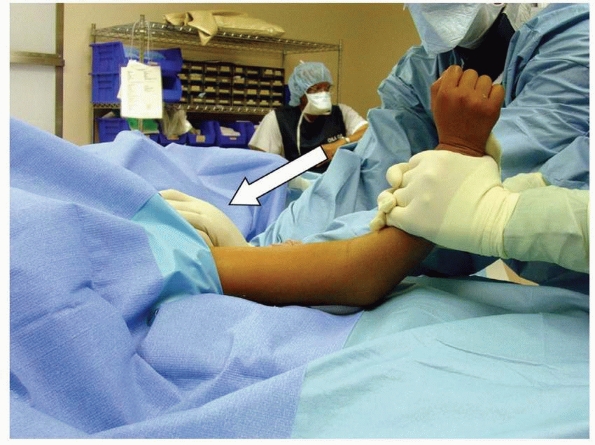
posteriorly through the brachialis (Fig. 14-30).156
movement of the forearm. Medial and lateral fracture translation is
corrected with direct movement of the distal fragment by the surgeon’s
thumb(s) with image confirmation. The elbow is then slowly flexed while
anterior pressure is applied to the olecranon with the surgeon’s
thumb(s) (Fig. 14-31).
sufficiently flex so that the fingers touch the shoulder. If not, the
fracture likely is still not reduced and is in extension (Fig. 14-32).
stay reduced and a “rubbery” feeling is encountered instead of the
desired “bone on bone” feeling, the median nerve or brachial artery may
be trapped within the fracture site (see Fig. 14-18). If this occurs, open reduction generally is necessary to remove the neurovascular structures from the fracture site.
 |
|
FIGURE 14-30
Brachialis muscle interposition is indicated on the left. The “milking maneuver” frees the brachialis muscle from its location in the fracture, allowing a closed reduction. |
 |
|
FIGURE 14-31
Reduction maneuver: flex elbow while pushing anteriorly on olecranon with the thumb(s). (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15 with permission.) |
not be done routinely. Most supracondylar humeral fractures are
posterior-medially displaced with an intact medial periosteum, and
pronation may aid reduction by placing tension on the medial periosteum
and closing down the otherwise open lateral column (see Fig. 14-4).
If the medial periosteum is torn, as it often is in a
posterior-laterally displaced fracture, pronation can be
counterproductive.
lateral, and oblique planes. Good reduction is indicated by (i) an
anterior humeral line that intersects the capitellum, (see Fig. 14-14), (ii) a Baumann angle of more than 10 degrees (see Fig. 14-15), and (iii) intact medial and lateral columns on oblique views (Figs. 14-33 and 14-34).
 |
|
FIGURE 14-32
If fingers cannot touch shoulder, flexion deformity may not be reduced. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007, with permission.) |
reduction when eternally rotating the shoulder for a lateral view of
the elbow, the C-arm can be moved instead of the patient’s arm.125
perhaps 25%) and a moderate amount of persistent rotational
malalignment, as long as the above criteria are met; the shoulder joint
has so much rotation that a small amount of rotational malalignment is
highly unlikely to cause a functional problem. Once reduction is
satisfactory, the elbow is taped in the reduced position of elbow
hyperflexion with elastic tape to prevent loss of reduction during
pinning (Fig. 14-35).
lateral humeral condyle is palpated. Most commonly, 0.062-inch smooth
K-wires are used (Zimmer, Warsaw, IN), although smaller or larger sizes
may be needed if the child is extremely small or large.
 |
|
FIGURE 14-33
Oblique fluoroscopic view of the elbow demonstrating continuity of the medial column following adequate fracture reduction. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
 |
|
FIGURE 14-34
Demonstration of lateral column continuity. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15 with permission.) |
pins at the fracture site to engage both the medial and lateral columns
(see Fig. 14-22). Whether the pins are
divergent or parallel and which pin is placed first is of little
importance. Care must be taken to ensure that there is sufficient bone
engaged in the proximal and distal fragments. It is acceptable to cross
the olecranon fossa, which adds two more cortices, to improve fixation,
but this means that the elbow cannot be fully extend until the pins are
removed. In the sagittal plane, to engage the most bone with the wire
in the distal fragment, the wire can be passed through the capitellum.
Because the reduced capitellum lies slightly anterior to the plane of
the fracture, the pin can be started a bit anterior to the plane of the
fracture and angulated about 10 to 15 degrees posteriorly to maximize
osseous purchase. A key element to ensure a correctly placed pin is to
feel the pin go through the proximal cortex. If this is not clearly
appreciated,
careful
fluoroscopic imaging often reveals that the pin did not engage the
proximal fragment. As a general rule, we recommend two pins for type II
fractures and three pins for type III fractures. Even though two
properly placed pins probably are sufficient, placing three pins
increases the odds of actually having two in proper position.
 |
|
FIGURE 14-35
Fracture redcution is maintained by taping elbow in hyperflexed position. The wire may be pushed through the skin and into the cartilage, using the cartilage of the distal lateral condyle as a pincushion that will hold the K-wire in place while carefully examining the AP and lateral images. (Reproduced with permission of Children’s Orthopaedic Center, Los Angeles, CA.) |
piercing the skin, and the position is checked with an AP fluoroscopic
image to assess the starting point. The K-wire is held free in the
surgeon’s hand at this point, not in the drill, to allow maximal
control. If the starting point and trajectory are correct, the wire can
be pushed through the skin and into the cartilage, using the cartilage
of the distal lateral condyle as a “pincushion” (see Fig. 14-35)
that will hold the K-wire in place while the AP and lateral images are
carefully examined. If imaging verifies correct pin placement, then the
pin is advanced with a wire driver. Precise pin placement is an
important part of the procedure that should not be rushed. We believe
incorrect pin placement is the cause of loss of reduction in most
fractures. Whether it is important if the pins are divergent or
parallel is debatable, but it is clearly important that the pins are
well separated at the fracture site.
Stress should be applied in varus and valgus under fluoroscopy to
ensure fracture stability, and lateral views should be obtained with
the elbow flexed and extended to assess movement of the capitellum
relative to the anterior humeral line. It is much better to identify
any instability in the operating room rather than a week later. If
there is instability, we add another lateral-entry pin.
 |
|
FIGURE 14-36
Assessment of sagittal alignment with lateral view. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
 |
|
FIGURE 14-37
Both oblique views are checked to assess reduction of medial and lateral columns. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
“worst,” particularly if some translational or rotational malreduction
is accepted, in order to have them for comparison during postoperative
visits to determine if movement of the
fracture
has occurred. Vascular status is assessed, and the wires are bent and
cut. The wires are left at least 1 to 2 cm off the skin to prevent
migration of the wires under the skin. A sterile felt square with a
slit cut into it is then placed around the wires to protect the skin (Fig. 14-39).
 |
|
FIGURE 14-38
If the lateral and oblique views show good reduction, the tape is removed and reduction and pin placement are checked, in the AP view with elbow in relative elbow extension. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
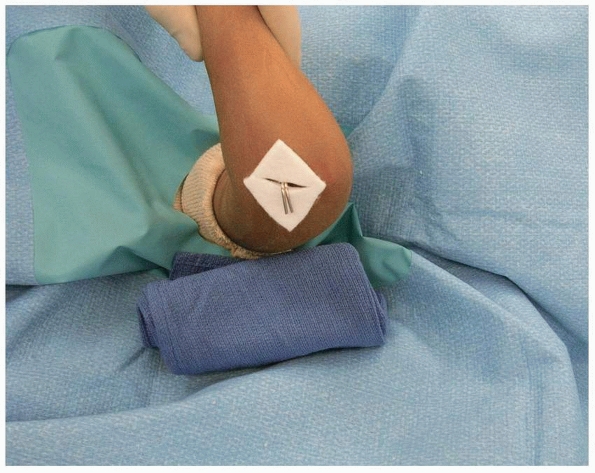 |
|
FIGURE 14-39
Skin is protected from pins with felt squares. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
techniques. One (David L. Skaggs) applies foam to the arm on the
anterior and posterior aspects of the elbow (Fig. 14-40)
before the cast is applied with the elbow flexed 45 to 70 degrees of
elbow flexion, as flexion to 90 degrees may needlessly increase the
risk of compartment syndrome (Fig. 14-41). The
other (John M. Flynn) applies a well-padded cast that has absolutely no
tightness to it, with a supracondylar mold to prevent arm rotation in
the cast, with the elbow flexed about 60 or 70 degrees. It is important
to remember that the pins, not the cast, are holding the fracture
reduction. We use fiberglass casting material for its strength, weight,
and radiolucency and believe that, when properly applied, fiberglass
does not lead to a tight cast.
 |
|
FIGURE 14-40
Sterile foam is placed directly on skin. If there is any circumferential dressing placed under the foam, it may be restricting. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007, with permission.) |
 |
|
FIGURE 14-41
Cast with elbow flexion no more than 70 degrees and less flexion for very swollen elbows. (From Tolo VT, Skaggs DL, eds. Master Techniques in Orthopaedic Surgery: Pediatric Orthopaedics. Philadelphia: Lippincott, 2007:1-15, with permission.) |
reduction in the operating room, the surgical preparation (betadine)
should be removed to evaluate the skin color. In addition, Doppler
evaluation can be used to assess pulse. If perfusion diminishes after
reduction, the artery or adjacent tissue must be assumed to be trapped
in the fracture site, and the pins should be immediately removed to
allow the fracture to return to its unreduced position. If there is no
pulse postoperatively in an arm that had no pulse preoperatively, but
the hand is warm and well-perfused, we prefer to observe the child in
the hospital for 48 hours with the arm mildly elevated. The rich
collateral circulation about the elbow generally is sufficient.
are believed to be at little risk for compartment syndrome are
discharged to home with appropriate postoperative instructions, but
otherwise children generally are admitted overnight for elevation and
observation. The patient customarily returns 5 to 7 days
postoperatively, at which time AP and lateral radiographss are
obtained. This allows rereduction if reduction has been lost; however,
this is rare and this return visit probably is not necessary for most
children. The cast generally is removed 3 weeks postoperatively, at
which time radiographs are obtained out of the cast and the pins are
removed. Range-of-motion exercises targeting gentle flexion and
extension are taught to the family and are started a few
days after cast removal. The child returns 6 weeks after surgery for a range-of-motion check, with no radiographs at that time.
valgus stress is applied with the elbow fully extended to reduce the
varus deformity, and this is confirmed with imaging. Then standard
reduction and pinning techniques are used. For type IV fractures, we
use the method described by Leitch et al.125 Neither of the authors has ever used traction or seen traction used in their centers over the last 10 years.
-
Aim to separate the pins as far as
possible at the fracture site—this is more important than whether the
pins are divergent or parallel. -
To optimize pin placement, think of the
cartilaginous distal humerus as a pincushion. With the K-wires in your
fingers (not the drill) push them into the cartilage in the exact
location and trajectory you want. Verify with imaging and then advance
the pin with a drill. -
In general, plan on a minimum of two pins for type II fractures and three pins for type III fractures.
-
If the first pin is in between the target
for two pins, just leave it and place a pin on either side of it for a
total of three pins. -
A small amount of translation or axial
rotational malalignment can be accepted rather than doing an open
reduction, but accept very little frontal plane or sagittal angular
malalignment. -
After reduction and fixation, stress the
fracture under live imaging to the point where there is confidence it
will not fall apart postoperatively. -
Cast the elbow in significantly less than
90 degrees of flexion to avoid compartment syndrome; the pins are
holding the reduction, not the cast. -
If placing a medial pin, extend the elbow when placing the pin to keep the ulnar nerve posterior and out of harm’s way.
 |
|
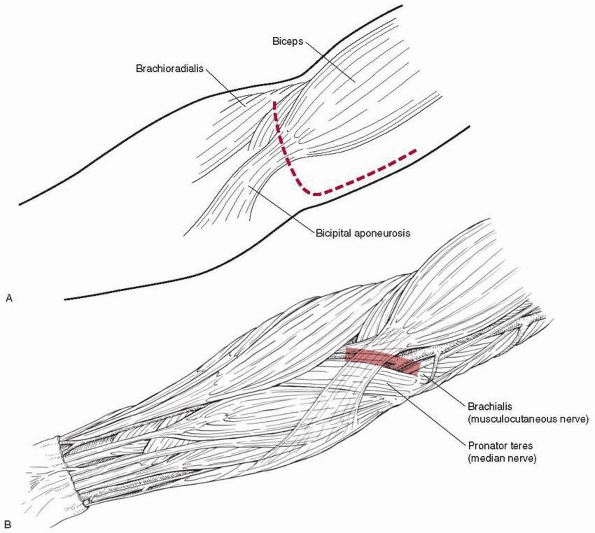
FIGURE 14-42
Anterior approach for open reduction and brachial artery and median nerve exploration. Most often, just the transverse part of the incision is needed for fracture reduction alone. |
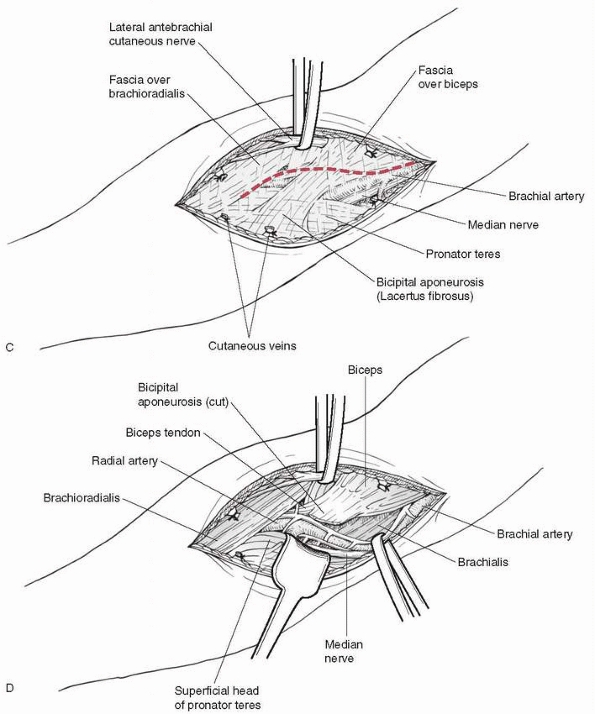
antecubital fossa about 4 to 5 cm long, which allows access to the
neurovascular structures and produces a better cosmetic result than a
lateral or medial incision (Fig.14-42). If more
visualization is needed, this incision can be extended medially or
laterally based on displacement. Care must be taken during dissection,
because the neurovascular bundle may be immediately superficial as it
is pushed against the skin by the proximal fragment (Fig. 14-43).
The first major structure to be incised is the bicipital aponeurosis
(lacertus fibrosus), which runs just superficial to the median nerve
and brachial artery; from the biceps tendon it runs medially to the
superficial flexors of the forearm. Just medial to the biceps tendon is
the brachial artery, with the median nerve just medial to the artery.
If the artery cannot be located in a patient with a pulseless, poorly
perfused hand, the lacerated ends of the artery may have retracted (see
Fig. 14-43).
 |
|
FIGURE 14-42 (continued).
|
identified, they should be retracted out of the fracture site.
Identifying the distal fragment can be the most challenging aspect of
the procedure. It is posterior and lateral and the periosteum is folded
over its surface. Reduction is obtained by reaching into the fracture
site with a hemostat and grasping the cut edge of the periosteum. This
cut edge is extended with scissors to increase the size of the
buttonhole and help free up the distal fragment. The fragment is then
brought anteriorly and reduced. Alternatively, the surgeon can hold his
thumb on the proximal fragment and push downwards while an assistant
applies traction to the forearm with the elbow flexed 90 degrees. A
periosteal elevator can be used as a lever to assist the reduction.
Because much of the periosteum is torn, fracture reduction may be less
stable after open reduction
than
it is after closed reduction. Once a reduction has been obtained, it
must be pinned to maintain it. This is accomplished in the same manner
as percutaneous pinning after closed reduction.
 |
|
FIGURE 14-43
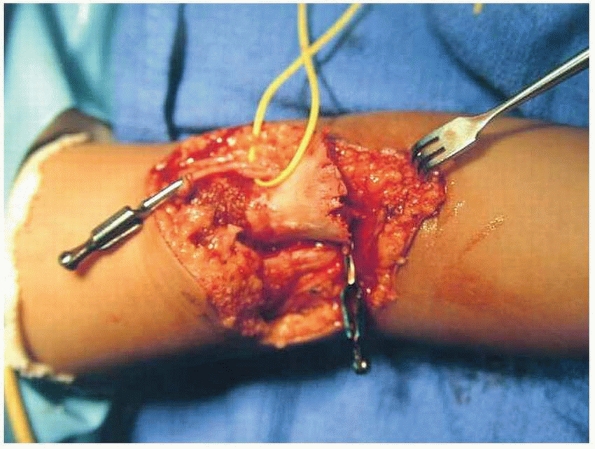
The brachial artery was lacerated by the proximal fragment. Bulldogs have been placed on each end of the artery to control bleeding, and the median nerve is within the vessel loop. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
The presence of a pulse and perfusion of the hand should be documented.
Perfusion is estimated by color, warmth, and capillary refill.
Capillary refill alone can be deceiving; after wrapping a rubber band
around a finger, there is instant venous capillary refill, so this must
be differentiated from arterial capillary refill.
demonstrated which patients are likely to need arterial repair or to
develop a compartment syndrome based on preoperative presentation. Of
1255 patients with operatively treated supracondylar humeral fractures,
33 presented with absent distal pulses. The key difference in the
outcome of these patients was whether the hand was well-perfused at
time of presentation. Of the 24 patients whose hands were well-perfused
(but pulseless) at presentation, none required vascular repair or
developed a compartment syndrome, and fracture reduction alone was
effective treatment. Of the 9 patients who presented with poor distal
perfusion, 4 required vascular repair, and compartment syndrome
developed in 2 during the postoperative period. In 5 of these 9
patients, fracture reduction alone was definitive treatment.
collateral circulation may keep the limb well-perfused, but timely
reduction with pinning in the operating room is preferable.172
A pulseless arm with signs of poor perfusion is an emergency. A patient
with a severely displaced supracondylar fracture and compromised
vascularity to the limb should have a splint applied with the elbow in
approximately 20 to 40 degrees of flexion rather than extremes of
flexion or extension which may compromise bloodflow.105
all had reduction and percutaneous pinning without preoperative
angiogram. In 3 of the 17 patients, bloodflow to the hand was not
restored after reduction and open exploration was required; in the
other 14, bloodflow to the hand was restored without complications. The
authors concluded that prereduction angiography would have added
nothing to the management of these injuries. Another study used
angiogram in 4 of 17 patients with supracondylar humeral fractures and
dysvascular extremities and found that the angiogram did not alter the
course of management in any.45 Choi et al.44
reported that of 25 patients presenting with a pulseless but
well-perfused hand, all did well clinically without arterial repair—11
(52%) had a palpable pulse following surgery and 10 (48%) remained
pulseless but well-perfused.
if anatomic reduction cannot be obtained by closed reduction in a
patient with an absent pulse; this allows evaluation of all vital
structures at risk for incarceration between the fracture fragments.13,63
Once the artery is freed from the fracture, arterial spasm may be
relieved by the application of lidocaine, warming, and 10 to 15 minutes
of observation. If the pulse does not return after fracture reduction
and the hand remains poorly perfused, vascular reconstruction (usually
by a vascular surgeon) is indicated.
perfusion of the hand should be evaluated. Most extension-type
supracondylar fractures are reduced and pinned with the elbow in
hyperflexion. With more than 120 degrees of elbow flexion, the radial
pulse generally is lost, even in patients with an initially intact
pulse. After pinning, when the arm is extended, the pulse frequently
does not return immediately. This is presumably secondary to arterial
spasm, aggravated by swelling about the artery and decreased peripheral
perfusion in the anesthetized, somewhat cool intraoperative patient.
Because of this phenomenon, 10 to 15 minutes should be allowed for
recovery of perfusion in the operating room before any decision is made
regarding the need for exploring the brachial artery and restoring flow
to the distal portion of the extremity. Because most patients without a
palpable pulse maintain adequate distal perfusion, the absence of a
palpable pulse alone is not an indication for exploring the brachial
artery.
well-perfused, the treatment is controversial. We prefer to admit the
child to the hospital for gentle elevation and observation for at least
48 hours. Loss of perfusion or impending compartment syndrome can occur
during this time and may require immediate treatment. Gillingham and
Rang76 recommended observing
patients with absent pulses, because most pulses returned within 10
days. Alternatively, vascular reconstruction can be performed. However,
Sabharwal et al.172 showed that
early repair of the brachial artery has a high rate of symptomatic
reocclusion and residual stenosis and recommended a period of close
observation with frequent neurovascular checks before more invasive
correction of this problem is contemplated. Immediate rereduction,
usually open reduction, is indicated in a patient in whom a pulse was
present preoperatively but is absent postoperatively, because the
artery or adjacent tissue is presumed to be entrapped in the fracture
site.
long-term sequel in children without palpable distal pulses, though
there is little literature on this subject. There is a single case
report of a child with minor cold intolerance and decreased systolic
arterial forearm blood pressure more than 1 year after a supracondyalr
fracture with rupture of the median nerve and ligation of the brachial
artery.130
based on objective criteria. Traditionally, the decision has been based
only on whether the hand was warm and pink. The following case
indicates the difficulty with these criteria. A 2-year-old girl was
injured in a fall from a couch (Fig. 14-44). A
type III supracondylar fracture and a pale, cool hand were documented
on presentation to a local emergency department. Two hours later, the
patient was brought to the operating room, where a cool, pale hand with
poor capillary refill and an absent pulse
was
noted. Immediate closed reduction and pinning was done with nearly
anatomic reduction. The hand felt warmer, and capillary refill was
present. The pulse was not palpable, but because of the improved state
of the patient’s hand, the brachial artery was was not explored. During
the next 4 hours, the patient was observed closely with increasing
fussiness, a nonpalpable pulse, and slow capillary refill. An
arteriogram showed brachial artery obstruction. Compartment pressures
were measured, and increased pressure was noted in the volar
compartment. A decision was made to return to the operating room for
exploration, repair of the brachial artery, and forearm fasciotomy.
Although the outcome in this case was satisfactory with no long-term
sequelae other than scarring, the question is whether or not the low
perfusion with subsequent ischemia and compartment syndrome could have
been identified at the time of the closed reduction and immediate
vascular repair done. What would have happened if no repair had been
done in this case? Had a repair been done immediately, could the
development of a compartment syndrome have been averted? What is the
relationship between compartment syndrome and perfusion? Is there a
more objective way to determine when flow is insufficient?
 |
|
FIGURE 14-44 A. This 2-year-old patient sustained a type III supracondylar fracture with vascular compromise. B. Pinning was performed in a nearly anatomic position. C,D.
Six hours postoperatively, increasing pain, a pale hand, and evolving compartment syndrome prompted arteriography, showing brachial artery occlusion. |
for vascular repair in addition to warmth and color in the patient with
absence of a palpable pulse.175,177 In the absence of a palpable pulse, a Doppler device can be used to measure lower flow states with small-pulse amplitude. Shaw177
found no falsepositive explorations when patients with pulses that
could not be palpated but were identified on Doppler evaluation were
observed, and exploration was done in those in whom no pulse was found
with either palpation or Doppler. The brachial artery was either
transected or entrapped in all patients with surgical exploration, and
none with “spasm” underwent surgery. Using the same criteria,
Schoenecker et al.175 identified 6
patients for exploration. Three had a damaged or transacted brachial
artery with no flow, and 3 had an artery kinked or trapped in the
fracture. At follow-up, all patients with vascular repair had a radial
pulse. One patient with more than 24 hours of vascular insufficiency
had an unsatisfactory outcome. Copley et al.45
reported that of 17 patients with type III supracondylar fractures and
no palpable pulse at presentation, 14 recovered pulse after reduction.
The three explorations identified significant vascular injury, and the
brachial artery was repaired. Most significant in their series was the
finding of increasing vascular insufficiency during postoperative
observation in some patients. If the hand is well-perfused despite not
having a palpable pulse, close follow-up in the hospital is mandatory.
The patient should be observed for increasing narcotic requirements,
increasing pain, and decreased passive finger motion. A very low
threshold for returning to the operating room for exploration and
fasciotomy must be maintained rather than assuming that perfusion from
collaterals is sufficient.
reported that of eight patients presenting with a pulseless and poorly
perfused hand, two developed compartment syndrome after operative
reduction of the fracture. Perhaps the threshold for compartment
release at the time of the initial surgery should be lower in this
subset of patients presenting with a pulseless and poorly perfused hand.
for evaluating postreduction circulation. We have found pulse oximetry
useful for evaluating patients after vascular repair or pinning but not
for intraoperative decision making.
intact preoperative radial pulse after closed reduction and pinning is
a strong indication for brachial artery exploration only when
accompanied by evidence of impaired circulation to the hand. After 10
to 15 minutes is allowed for resolution of arterial spasm as a cause
for loss of pulse, the brachial artery should be explored if the hand
is not warm and pink. Either direct arterial entrapment at the fracture
or arterial compression by a fascial band pulling across the artery may
cause loss of pulse after fracture reduction. As described earlier, the
other indication for brachial artery exploration is persistent vascular
insufficiency after reduction and pinning.
together to manage this problem emergently. Often, the release of a
fascial band or an adventitial tether resolves the problem of
obstructed flow. This is a simple procedure done at the time of
exploration of the antecubital fossa and identification of the brachial
artery. In some patients, however, a formal vascular repair and vein
graft are required. The brachial artery should be approached through a
transverse incision across the antecubital fossa, with a medial
extension turning proximally at about the level of the medial
epicondyle as described for open reduction (see Fig. 14-42).
After reduction and pinning, care must be taken because the
neurovascular bundle may be difficult to identify when it is surrounded
by hematoma, and it may lie in a very superficial position. At the
level of the fracture, the artery may seem to disappear into the
fracture site, covered with shredded brachialis muscle. This occurs
when the artery is tethered by a fascial band or arterial adventitia
attached to the proximal metaphyseal spike pulling the artery in the
fracture site. Dissection should occur proximally to distally, along
the brachial artery, identifying both the artery and the median nerve.
Arterial injury generally is at the level of the supratrochlear artery
(see Fig. 14-6), which provides a tether,
making the artery vulnerable at this location. Arterial transection or
direct arterial injury can be identified at this level (see Fig. 14-43). Entrapment of the neurovascular bundle in the fracture is best identified by proximal to distal dissection.
repair a damaged artery or use a vein graft. If arterial spasm is the
cause of inadequate flow and collateral flow is not sufficient to
maintain the hand, methods to relieve the spasm can be tried. Both
stellate ganglion block and application of papaverine or local
anesthetic to the artery have been found to be beneficial in this
situation. If these techniques do not relieve the spasm and if
collateral flow is insufficient, the injured portion of the vessel is
excised and a vein graft is inserted. When flow is restored, the wound
is closed, and a splint is applied with the elbow flexed much less than
90 degrees and the forearm in neutral pronation and supination.
Postoperative monitoring should include temperature, pulse oximetry,
and frequent examinations for signs of compartment syndrome or
ischemia. Although injecting urokinase has been suggested to increase
flow,34 we have had no experience with this technique.
documented that 3.2% of patients with type III supracondylar fractures
have an absent pulse at presentation, for which they recommended
noninvasive monitoring. MR angiography and color-flow duplex Doppler
were helpful in
deciding whether or not to explore the brachial artery. Although repair is technically feasible, Sabharwal et al.172
cautioned that high rates of reocclusion and residual stenosis argued
against early revascularization if not absolutely necessary. Early
reocclusion, however, was not reported by Schoenecker et al.175 or Shaw et al.177
in patients in whom vascular compromise was treated within 12 hours.
The frequency of this complication increased steadily with repair
between 12 and 24 hours; after 24 hours of delay in treatment, outcomes
were uniformly poor. This series presents convincing evidence that
prompt exploration of arterial insufficiency markedly decreases the
incidence of Volkmann ischemic contracture. Note that brachial artery
obstruction and compartment syndrome, although related, are not
equivalent, and both are fortunately rare problems. Ischemia will lead
to a compartment syndrome, but the presence of a radial pulse does not
preclude it.
reported a 9% prevalence (3 of 33) of forearm compartment syndrome in
patients with a supracondylar fracture and an ipsilateral radial
fracture. In acute compartment syndrome,91,143
increased pressure in a closed fascial space causes muscle ischemia.
With untreated ischemia, muscle edema increases, further increasing
pressure, decreasing flow, and leading to muscle necrosis, fibrosis,
and death of involved muscles. A compartment syndrome of the forearm
may occur with or without brachial artery injury and in the presence or
absence of a radial pulse. The diagnosis of a compartment syndrome is
based on resistance to passive finger movement and dramatically
increasing pain after fracture. The classic five “Ps” for the diagnosis
of compartment syndrome—pain, pallor, pulselessness, paresthesias, and
paralysis—are poor criteria for the early detection of compartment
syndrome. Special attention must be paid to supracondylar fractures
with median nerve injuries because the patient will not feel pain in
the volar compartment.143
recommended forearm fasciotomy if clinical signs of compartment
syndrome are present or if intracompartmental pressure is greater than
30 mm Hg. Heppenstall et al.89
suggested that a difference of 30 mm Hg between diastolic blood
pressure and compartment pressure should be the threshold for release.
If pain is increasing and finger extension is decreasing, fasciotomy is
clearly indicated. Measuring compartment pressures in a terrified,
crying child is difficult, and if clinical signs of compartment
syndrome are present, a trip to the operating room for evaluation and
possible fasciotomy is often a better course of action than pressure
measurement and observation.
of compartment syndrome are direct muscle trauma at the time of injury,
swelling with intracompartmental fractures (associated forearm
fracture), decreased arterial inflow, restricted venous outflow, and
elbow position. If the mechanism of injury is high-energy, as evidenced
by crushing or associated fractures, there is an increased risk for
compartment syndrome. An associated forearm fracture or forearm crush
injury significantly increases the likelihood of compartment syndrome.25
An arterial injury in association with multiple injuries or crush
injury further diminishes blood flow to the forearm musculature and
increases the probability of a compartment syndrome. Interestingly,
Battaglia et al.19 documented the
relationship between increasing elbow flexion above 90 degrees and
increasing volar compartment pressure. In a patient with an evolving
postoperative compartment syndrome, not only should the dressings be
loosened, but the elbow should be extended to a position well below 90
degrees.
identified 11 children with supracondylar fractures who developed
compartment syndrome despite presenting with closed, low-energy
injuries and no associated fractures or vascular compromise. This
series is disturbing because it demonstrates that compartment syndrome
still occurs with modern treatment, even in children who may be thought
to be at low risk. Ramachandran et al.162
found that in 10 of 11 patients’ charts, excessive swelling was noted
at time of presentation, and the one child without severe swelling had
arterial entrapment after reduction, with subsequent compartment
syndrome requiring fasciotomy 25 hours later. The 10 children with
severe elbow swelling documented at presentation had a mean delay of 22
hours before surgery. This study suggests that excessive swelling
combined with delay in treatment is a risk factor for the development
of compartment syndrome.
Increased swelling over the compartment, increased pain, and decreased
finger mobility are cardinal signs of an evolving compartment syndrome.
Evaluation of possible compartment syndrome cannot be based on the
presence or absence of a radial pulse alone. If a compartment syndrome
does appear to be evolving, initial management includes removing all
circumferential dressings. The volar compartment should be palpated,
and the elbow should be extended. We believe that the fracture should
be immediately stabilized with K-wires to allow proper management of
the soft tissues.
compartment syndrome is warm ischemic time after injury. When bloodflow
is compromised and the hand is pale with no arterial flow, muscle
ischemia is possible, depending on the time of oxygen deprivation.
After fracture reduction and flow restoration, the warm ischemic time
should be noted. If this time is more than 6 hours, compartment
syndrome secondary to ischemic muscle injury is likely. Prophylactic
volar compartment fasciotomy can be done at the time of arterial
reconstruction. The exact indication for prophylactic fasciotomy in the
absence of an operative revascularization is uncertain. Even when the
diagnosis is delayed or the if compartment syndrome is chronic,
fasciotomy has been shown to be of some value.
recent systematic review of 55 reports of 1920 fasciatomies of the
upper and lower extremities found that, compared with fasciotomy before
6 hours, delayed fasciotomy beyond 12 hours was associated with a lower
rate of acceptable outcome (15% for more than 12 hours versus 88% for
<6 hours), a higher rate of amputation (14% versus 3.2%), and death
(4.3% versus 2.0%).87
or an ulnar approach (Fig. 14-45).
If the compartment syndrome is associated with brachial artery and
median nerve injuries, we generally use the Henry approach as an
extension of the vascular repair. The advantage of the ulnar approach,
as described by Willis and Rorabeck,204
is that it produces a more cosmetically pleasing scar. A volar
fasciotomy involves opening the volar compartment from the carpal
tunnel distally to the lacertus fibrosa and antecubital fascia
proximally. The fascia over the deep flexors is opened, as is the
superficial fascia, to decompress the deep volar compartment of the
forearm. Failure to release the deep volar fascia may cause contracture
of the deep finger flexors. Generally, only the volar compartment is
released, with an associated decrease in pressure in the dorsal or
extensor compartment. If the volar Henry approach is used, the interval
between the brachioradialis and the flexor carpi radialis is developed
and the radial artery is retracted ulnarward. The deep volar
compartment is exposed. The flexor digitorum profundus and flexor
pollicis longus are exposed along with the pronator teres proximally
and the pronator quadratus distally.
 |
|
FIGURE 14-45 Surgical approach for forearm fasciotomy. A. Ulnar approach, skin incision. B. Ulnar approach, intermuscular interval. (FDS, flexor digitorum sublimis; FCU, flexor carpi ulnaris.) C. Henry approach, skin incision. D.
Henry approach. (BR, brachioradialis; ECRB, extensor carpi radialis brevis; FCR, flexor carpi radialis interval.) (From Willis RB, Rorabeck CH. Treatment of compartment syndrome in children. Orthop Clin N Am 1990;21:407-408, with permission.) |
the release is done from the carpal canal to the antecubital fossa, as
with the Henry approach. The skin incision begins above the elbow
crease, medial to the biceps tendon (see Fig. 14-45);
it crosses the elbow crease and extends distally along the ulnar border
to the volar wrist, where it courses radially across the carpal canal.
The fascia over the flexor carpi ulnaris is incised, and the interval
between the flexor carpi ulnaris and the flexor digitorum sublimis is
identified. The ulnar nerve and artery are retracted, exposing the deep
flexor compartment of the forearm. The deep flexor fascia is incised.
The ulnar nerve and artery, as well as the carpal tunnel, are
decompressed distally.
effective way to manage the wound is with a criss-crossed rubber band
technique, securing the rubber bands in place with skin staples. An
alternative is to simply place a sterile dressing over the open wound,
but this makes wound closure difficult and probably increases the need
for skin grafting. Definitive closure or skin grafting generally is
done within 5 to 7 days. Skeletal stabilization of the supracondylar
and forearm fractures is important to properly manage compartment
syndrome.
The direction of fracture displacement determines the nerve most likely
to be injured. If the distal fragment is displaced posteromedially, the
radial nerve is more likely to be injured. Conversely, if the
displacement of the distal fragment is posterolateral, the
neurovascular bundle is stretched over the proximal fragment, injuring
the median nerve or anterior interosseous nerve or both. In a
flexion-type supracondylar fracture, which is rare, the ulnar nerve is
the most likely to be injured. Injury to the anterior interosseous
nerve causes paralysis of the long flexors of the thumb and index
finger without sensory changes. Complete median nerve injury can be
caused by contusion or transection of the nerve at the level of the
fracture and causes sensory loss in the median nerve distribution as
well as motor loss of all muscles innervated
by the median nerve.189,191 Nerve transections are rare and almost always involve the radial nerve.50,17,132,134
not necessarily indicated for nerve injury in a closed fracture.
Regardless of which nerve is injured, neural recovery generally occurs
in the first 2 to 3 months, but may take up to 6 months.33,105 Observation is indicated.
described eight injured nerves in five patients in whom spontaneous
recovery did not occur by 5 months after injury. Neurolysis was
successful in restoring nerve function in all but one patient. Nerve
grafting may be indicated for nerves not in continuity at the time of
exploration. Neurolysis for perineural fibrosis generally is successful
in restoring nerve function. There is no indication for early
electromyographic analysis or treatment other than observation for
nerve deficit until 4 to 6 months after fracture.
reported that, of 12 injuries that did not spontaneously recover within
6 months of injury, only one was associated with a supracondylar
fracture. Perineural fibrosis was present in four patients, three
nerves were entrapped in callus, and five were either partially or
totally transected.
to be the most common cause of prolonged nerve deficit. Although nerve
injury is related to fracture displacement, a neural deficit can exist
with even minimally displaced fractures. Sairyo et al.173
reported one patient in whom radial nerve palsy occurred with a
slightly angulated fracture that appeared to be a purely extension-type
fracture on initial radiographs. Even in patients with mild injuries, a
complete neurologic examination should be performed before treatment.
An irreducible fracture with nerve deficit is an indication for open
reduction of the fracture to ensure that there is no nerve entrapment.
Chronic nerve entrapment in healed callus can give the appearance of a
hole in the bone, the Metev sign.
In a large series of type III supracondylar fractures, the rate of
iatrogenic injury to the radial nerve was less than 1%. The course of
the ulnar nerve through the cubital tunnel, between the medial
epicondyle and the olecranon, makes it vulnerable when a medial pin is
placed. Rasool165 demonstrated with
operative exploration that the pin usually did not impale the ulnar
nerve, but more commonly constricted the nerve within the cubital
tunnel by tethering adjacent soft tissue. These findings were later
confirmed by an ultrasonographic study by Karakurt et al.103 Zaltz et al.211
reported that in children less than 5 years of age, when the elbow is
flexed more than 90 degrees, the ulnar nerve migrated over, or even
anterior to, the medial epicondyle in 61% (32/52) of children.
offer an evidence-based approach to an iatrogenic ulnar nerve injury
that occurs following medial pin placement. Lyons et al.127
reported full return of function in 17 patients with iatrogenic ulnar
nerve injuries presumably caused by a medial pin, although function did
not return until 4 months after surgery in several. Only 4 of the 17
(24%) had the medial pins removed, indicating that nerve function can
eventually return without pin removal. Brown and Zinar33
reported four ulnar nerve injuries associated with pinning of
supracondylar fractures, all of which resolved spontaneously 2 to 4
months after pinning. Rasool165
reported six patients with ulnar nerve injuries in whom early
exploration was performed. In two patients, the nerve was penetrated,
and in three, it was constricted by a retinaculum over the carpal
tunnel, aggravated by the pin. In 1 patient, the nerve was subluxed and
was fixed anterior to the cubital tunnel by the pin. Full recovery
occurred in three patients, partial recovery in two, and no recovery in
two. Royce et al.169 reported
spontaneous recovery of ulnar nerve function in three patients. One
nerve that was explored had direct penetration, and the pin was
replaced in the proper position. Two patients had late-onset ulnar
nerve palsies discovered during healing, and the medial pin was removed.
documented, we prefer to replace the pin in the proper position or
convert to a lateral pin construct, earlier rather than later. Routine
exploration of the ulnar nerve is not recommended.33,64,165,211
treating ulnar neuropathy. Because of the frequency of ulnar nerve
injury with crossed pinning, most surgeons prefer to use two or three
lateral pins if possible and no medial pin. Successful maintenance of
alignment of type III supracondylar fractures with lateral pins has
been reported in many series.41,110,182,183,195
Many believe that if crossed-pinning is to be used, nerve penetration
and indirect trauma to the nerve cannot be prevented by making an
incision over the medial epicondyle and avoiding the nerve while
placing the medial pin directly on the medial epicondyle. Skaggs et al.183
reported that the use of crossed pins with a medial cut-down did not
decrease the risk of iatrogenic ulnar nerve injuries. It is important
to remember that most iatrogenic ulnar nerve injuries do not result
from a pin directly penetrating the nerve, but from a pin going through
adjacent tissue, resulting in pinching of the nerve. Michael and
Stanislas140 described another
alternative for protecting the ulnar nerve; they attached a nerve
stimulator to a needle, which was used for localizing the ulnar nerve.
Once the ulnar nerve was identified, a standard pinning technique was
used, placing the medial pin 0.5 to 0.75 mm anterior to the nerve. We
have no experience with this technique. Some surgeons palpate the ulnar
nerve and push it posteriorly. Wind et al.206
however, showed this method to be unreliable. The only technique for
avoiding iatrogenic ulnar nerve injury appears to be to use
lateral-entry pins and avoid the use of crossed pins.
and found that fractures treated closed had an average loss of motion
of 4 degrees. In those treated with open reduction, the loss of flexion
was 6.5 degrees and the flexion contracture was 5 degrees. In a series
of 43 children with supracondylar fractures who had open reduction,
about half received no physical therapy.107
While the group with physical therapy had better motion at 12 and 18
weeks postoperatively, there was no difference in elbow motion after 1
year. The authors concluded that physical therapy is unnecessary. Loss
of motion has been reported with the posterior triceps splitting
incision for open reduction.77,81,131
While most children do not require formal physical therapy, we
generally teach the parents range-of-motion exercises to be done at
home after pin and cast removal at about 3 weeks. A follow-up
appointment to assess range of motion is scheduled about 4 to 6 weeks
later, and if motion is not nearly normal at that time, physical
therapy to improve elbow motion is begun.
 |
|
FIGURE 14-46 Distal fragment rotation. A. Posterior angulation only of the distal fragment. B. Pure horizontal rotation without angulation. C. Pure posterior translocation without rotation or angulation. D.
Horizontal rotation with coronal tilting, producing a cubitus varus deformity. There is a positive crescent sign. (From Marion J, LaGrange J, Faysse R, et al. Les fractures de l’extremite inferieure de l’humerus chez l’enfant. Rev Chir Orthop 1962;48:337-413, with permission.) |
loss of flexion can occur. This problem generally is caused by a lack
of anatomic fracture reduction: either posterior distal fragment
angulation, posterior translation of the distal fragment with anterior
impingement, or medial rotation of the distal fragment with a
protruding medial metaphyseal spike proximally (Fig. 14-46).
In young children with significant growth potential, there may be
significant remodeling of anterior impingement, and any corrective
surgery should be delayed at least 1 year. Although anterior
impingement can significantly remodel, there is little remodeling to
persistent posterior angulation or hyperextension.
fractures treated with percutaneous K-wire fixation varies from less
than 0% to 21%.20,95 The reported prevalence of pin track infections in supracondylar humeral fractures ranges from 0% to 6.6%.30,41,95,138,182 In a series of 202 pediatric fractures, 92.5% of which were upper extremity fractures, Battle et al.20
reported an 8% infection rate. Twelve of the 16 infections required
oral antibiotics and local pin care, one required intravenous
antibiotics, and three required operative incision and débridement. In
124 supracondylar fractures, Skaggs et al.182 noted only one pin track infection (less than 1%) which resolved with oral antibiotics and pin removal. Gupta et al.82
also reported only one (<1%) pin track infection in 150 patients,
which also resolved with oral antibiotics and pin removal. In a larger
series by Mehlman et al.138 (198
fractures), there were five (2.5%) pin track infections, which were
treated with oral antibiotics and resolved without sequlae. Iobst et al.95
reported no infections in 304 patients using a “semisterile” technique,
despite the fact that only 68% of their patients received perioperative
antibiotic. Their protocol involved unsterile reduction of the fracture
with an assistant holding the arm in the reduced position. The surgeon
then put on sterile gloves, placed sterile towels around the surgical
field, and prepared the elbow locally in the anticipated area of the
pin sites. There are no other reports on this technique.
and antibiotics. Fortunately, by the time a pin track infection
develops the fracture usually is stable enough to remove the pin
without loss of reduction. Although we have not seen this complication,
an untreated pin track infection could theoretically result in a septic
joint and should be treated as soon as it is recognized or suspected.
postoperative manipulation or physical therapy is believed to be the most commonly associated factor.113,157
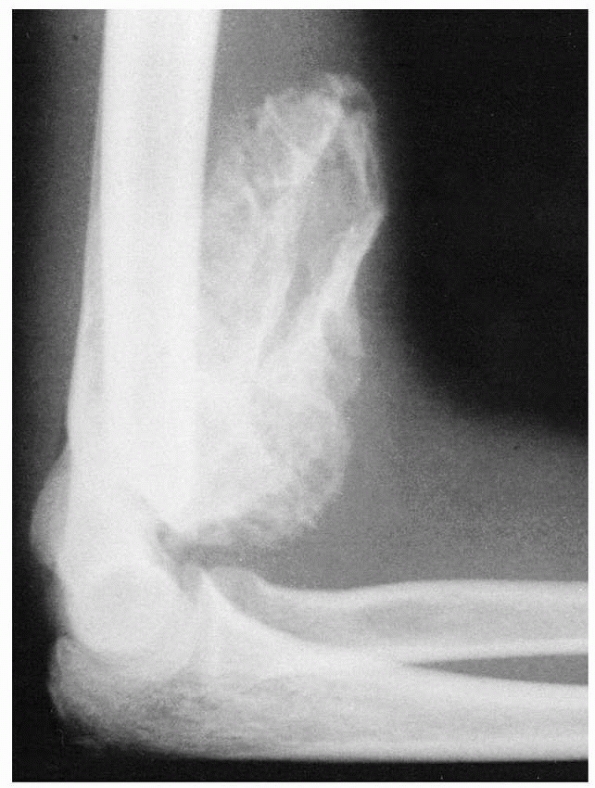 |
|
FIGURE 14-47
Myositis ossificans. Ossification of the brachialis muscle developed in this 8-year-old who had undergone multiple attempts at reduction. (Courtesy of John Schaeffer, MD.) |
noted that limitation of motion and calcification disappeared after 2
years. Postoperative myositis ossificans can be observed with the
expectation of spontaneous resolution of both restricted motion and the
myositis ossificans. There is no indication for early excision. Spinner190
reported a single case of myositis ossificans associated with sudden
onset of pain after trauma in which a 1-year-old lesion of myositis
ossificans was fractured. With excision, the pain was relieved, and
full range of motion returned.
area with remarkably rapid healing, and nonunion of a supracondylar
fracture is rare, with only a single case described by Wilkins and Beaty202
after open reduction. We have not seen nonunion of this fracture. With
infection, devascularization, and soft tissue loss, the risk of
nonunion would presumably increase.
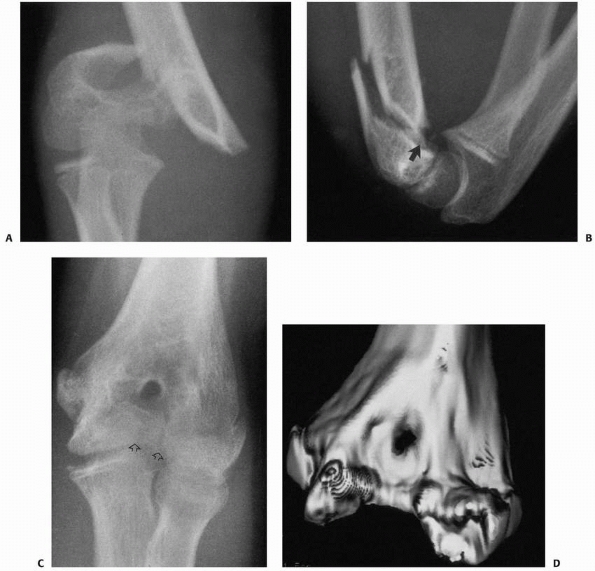 |
|
FIGURE 14-48 Osteonecrosis of the trochlea. A. AP injury film of an 8-year-old with a distal type III supracondylar fracture. B. The distal extension of the fracture (arrow)
is best appreciated on the lateral reduction film. Postfracture, the patient was asymptomatic until 2 years later, when elbow stiffness developed. C. Repeat radiographs at that time showed atrophy of the trochlea (arrows). D. Three-dimensional CT reconstruction demonstrates atrophy of the trochlea. |
fracture has been reported. The blood supply of the trochlea’s
ossification center is fragile, with two separate sources. One small
artery is lateral and courses directly through the physis of the medial
condyle. It provides blood to the medial crista of the trochlea. If the
fracture line is very distal, this artery can be injured, producing
osteonecrosis of the ossification center and resulting in a classic
fishtail deformity (Fig. 14-48). Kim109
identified 18 children with trochlear abnormalities after elbow
injuries, five of which were supracondylar fractures. MRI indicated
low-signal intensity on T2, indicative of cartilage necrosis. Cubitus
varus
deformity developed in all cases. Bronfen et al.32
described six patients with severe osteonecrosis of the trochlea, which
they termed “dissolution of the trochia” following closed reduction and
pin fixation, indicating that open reduction is not always the cause of
osteonecrosis. Five of their six patients complained of pain,
crackling, and stiffness.32
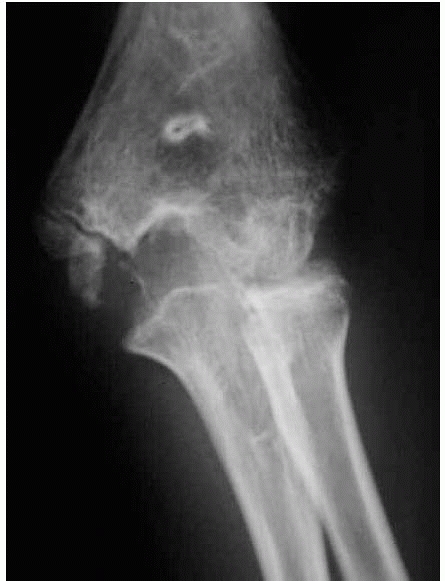 |
|
FIGURE 14-49
Osteonecrosis of the trochlea developed following open reduction through a posterior approach. The child had limited motion and symptoms of occasional catching. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
for months or years. Healing is normal, but mild pain and occasional
locking develop with characteristic radiographic findings and motion
may be limited depending on the extent of osteonecrosis. In our
experience, the most common cause of osteonecrosis of the trochlea is
open reduction of a supracondylar fracture through a posterior
approach, which presumably disrupts the blood supply of the trochlea (Fig. 14-49);
however, we are aware of several cases of osteonecrosis that occurred
after simple closed pinning. The artery to the trochlea may be
disrupted at the time of the injury.
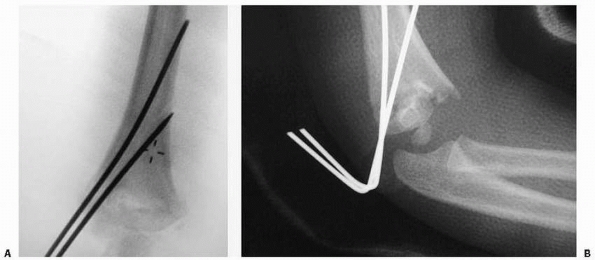 |
|
FIGURE 14-50
If the pins are placed too close together, they biomechanically function as one pin, and rotation around the pins is not surprising. A. Intraoperative radiograph. B. Postoperative loss of reduction in same patient. (From Skaggs DL, Cluck MW, Mostofi A, et al. Lateral-entry pin fixation in the management of supracondylar fractures in children. J Bone Joint Surg 2004;86-A(4):702-707, with permission.) |
supracondylar humeral fractures, including completely unstable
fractures, has taught us that lateral-entry pins, when properly placed,
are strong enough to maintain reduction of even the most unstable
supracondylar humeral fractures.182
In an informal collection of reports of loss of reduction of
supracondylar fractures from members of the Pediatric Orthopaedic
Society of North America, Skaggs et al.182
reported only eight fractures with loss of reduction after treatment
with lateral-entry pins. They identified technical points of pin
placement that may be associated with loss of fracture reduction. Two
lateral pins were used for all eight fractures. In five, the pins were
too close together and engaged only the lateral column at the level of
the fracture (Fig. 14-50). In each of these,
fracture reduction was lost when the distal fragment rotated around the
two pins. In another case, the two lateral pins crossed at the fracture
site (Fig. 14-51). In the remaining two cases, the two lateral pins did not engage the distal fragment (Figs. 52A,B). There were no reports of loss of fixation after the use of three lateral-entry pins.
a series of 322 factures, reported that 2.9% had postoperative loss of
fixation. All eight were type III fractures treated with two pins
(seven lateral-entry and one crossed-pin). In all eight, loss of
fixation was due to technical errors that were identifiable on the
intraoperative fluoroscopic images (Fig. 14-53)
and that could have been prevented with proper technique. They
identified three types of pin-fixation errors: (i) failure to engage
both fragments with two pins or more, (ii) failure to achieve
bicortical fixation with two pins or
more, and (iii) failure to achieve adequate pin separation (>2 mm) at the fracture site.174
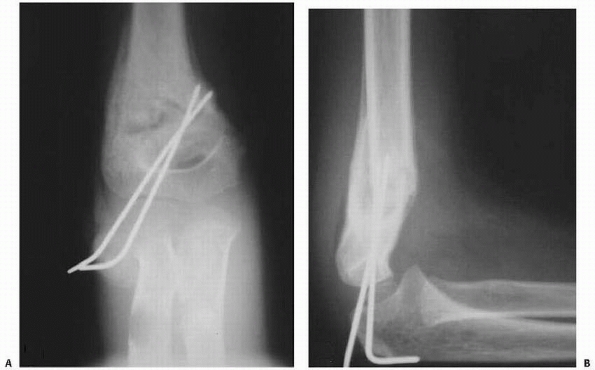 |
|
FIGURE 14-51 A,B.
In accordance with standard fracture principles, pins that cross at the fracture site do not provide stability. (From Skaggs DL, Cluck MW, Mostofi A, et al. Lateral-entry pin fixation in the management of supracondylar fractures in children. J Bone Joint Surg 2004;86-A(4):702-707, with permission.) |
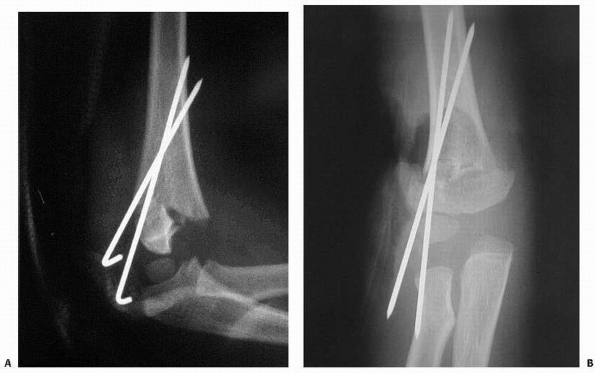 |
|
FIGURE 14-52 A,B.
Insufficient bone is engaged in the distal fragments, so loss of fixation may be expected. (From Skaggs DL, Cluck MW, Mostofi A, et al. Lateral-entry pin fixation in the management of supracondylar fractures in chilren. J Bone Joint Surg 2004;86-A(4):702-707, with permission.) |
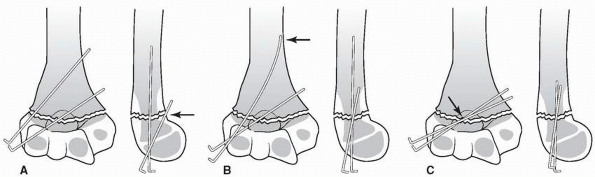 |
|
FIGURE 14-53 Illustrations depicting errors in pin-fixation technique. A. The black arrow demonstrates the anterior pin failing to transfix the proximal bone. B. The black arrow demonstrates one pin without bicortical purchase. C. The black arrow demonstrates pins too close together at the fracture site.
|
reported 22 patients near skeletal maturity with underreduction of the
extension component of the fracture. They found that 17 (77%) of the
patients had radiographic abnormalities of the humerocondylar angle (a
difference of 5 degrees or more compared with the uninjured side).
Eleven patients (50%) had limited elbow flexion, and 7 (31%) were aware
of this deficit. Patients who were left to heal with some degree of
extension developed limited end-elbow flexion and were aware of it.
Although only 3 patients perceived minor subjective functional
disability at the last follow-up, 10 patients had unsatisfactory
results according to the Flynn criteria for motion restriction.180
The malunion most commonly also includes hyperextension, which leads to
increased elbow extension and decreased elbow flexion (Figs. 14-55 and 14-56).
The appearance of cubitus varus deformity is distinctive on radiograph.
On the AP view, the angle of the physis of the lateral condyle (Baumann
angle) is more horizontal than normal (Fig. 14-57).
On the lateral view, hyperextension of the distal fragment posterior to
the anterior humeral line correlates with the clinical findings of
increased extenion and decreased flexion of the elbow (Fig. 14-58).
though this is unlikely because not enough growth remains in this area
to cause cubitus varus within the time it is recognized. A more likely
reason for most cubitus varus deformities in patients with
supracondylar fractures is malunion.12,35,51,64,200
Cubitus varus can be prevented by making certain that the Baumann angle
is intact at the time of reduction and remains so during healing, which
generally is best assured with pin fixation. In a series of 206
patients with supracondylar humeral fractures, with ages ranging from
1.5 to 14 years (mean of 6.4 years), Pirone et al.157
reported cubitus varus deformities in eight (7.9%) of 101 patients
treated with cast immobilization compared to two (1.9%) in 105 patients
treated with pin fixation. A decrease in the frequency of cubitus varus
deformity after the use of percutaneous pin fixation has been reflected
in other recent series.29,30,64,65,101,139,157,158 with one large retrospective182 and one prospective study111 reporting no cubitus varus deformities.
overall length of the humerus. In a 5-year-old, therefore, the amount
of distal humeral growth in 1 year is approximately 2 mm, making it
unlikely that growth asymmetry is a significant cause of varus
deformity that occurs within the first 6 to 12 months after fracture. A
case report of a 4-year-old girl with progressive cubitus varus caused
by a physeal bony bar demonstrated with MRI (Fig. 14-59) after cast treatment of a supracondylar fracture noted the slow progress of the deformity.56
The Baumann angle went from 88 degrees at 6 months following injury to
92 degrees at 18 months and 96 degrees at 3 years after injury. Thus,
over
3
years from injury, the Baumann angle changed by only 8 degrees, which
we suspect is not too different from measurement variability.
Osteonecrosis of the trochlea or medial portion of the distal humeral
fragment can result in progressive varus deformity, however. In a
series of 36 varus deformities reported by Voss et al.,197 only 4 patients had medial growth disturbance and distal humeral osteonecrosis as a cause of progressive varus deformity.
 |
|
FIGURE 14-54
A 5-year-old girl with cubitus varus of right elbow following a malunion of a supracondylar humerus fracture. (Reproduced with permission of Children’s Orthopaedic Center, Los Angeles, CA.) |
 |
|
FIGURE 14-55 Hyperextension of right elbow. (Reproduced with permission of Children’s Orthopaedic Center, Los Angeles, CA.)
|
considered a primarily cosmetic procedure, but several consequences of
cubitus varus such as an increased risk of lateral condylar fractures,
pain, and tardy posterolateral rotatory instability may be indications
for supracondylar humeral osteotomy.1,2,23,135,148,188 Our experience suggests that many patients have elbow discomfort with significant cubitus varus. O’Driscoll et al.148
reported 17 patients with cubitus vars deformities after a pediatric
distal humeral fracture who presented an average of 27 years following
injury. The average varus deformity was 15 degrees, and all patients
had tenderness over the lateral collateral
ligament
complex and posterolateral rotator instability. These authors concluded
that cubitus varus deformity secondary to supracondylar malunion may
not always be a benign condition and may have important long-term
clinical implications.148 An increased risk of fracture,53 especially of the lateral condyle, has been linked with cubitus varus deformity. Takahara et al.193
reported nine patients with distal humeral fractures who deveoped varus
deformities. Supracondylar fractures as well as epiphyseal separations
were included in these nine fractures.
and the triceps shifts a bit ulnarward. Investigators theorized that
this ulnar shift might compress the ulnar nerve against the medial
epicondyle, narrowing the cubital tunnel and resulting in chronic
neuropathy. In a recent report,2 a fibrous band running between the heads of the flexor carpi ulnaris was thought to cause ulnar nerve compression.
 |
|
FIGURE 14-56 Decreased flexion of right elbow. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.)
|
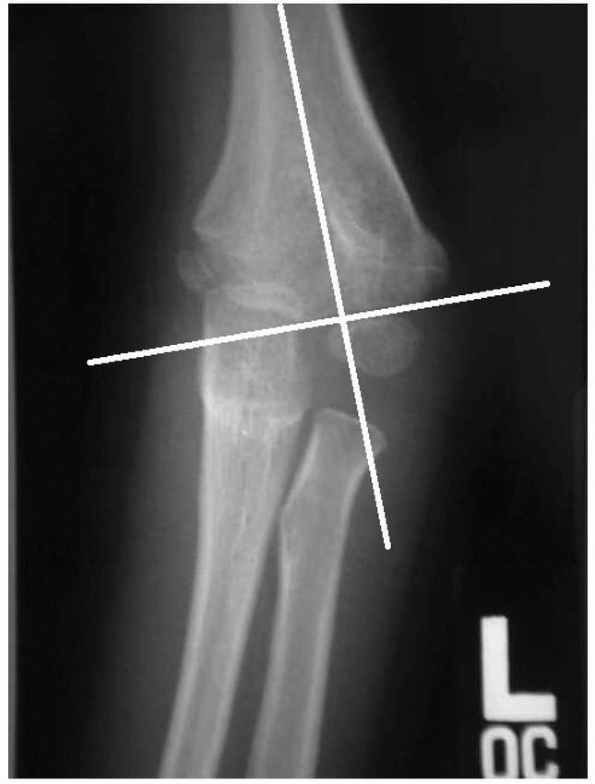 |
|
FIGURE 14-57
AP radiograph of girl in preceeding clinical photos. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
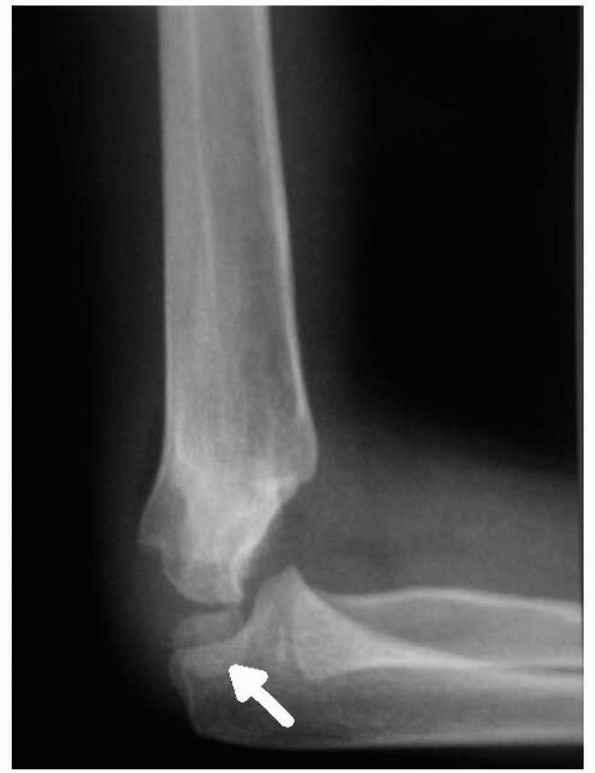 |
|
FIGURE 14-58 Lateral radiograph shows overlapping of the distal humerus with the olecranon (arrow)
producing the typical crescent sign. Note the anterior humeral line is anterior to the capitellum. (Reproduced with permission of Children’s Orthopaedic Center, Los Angeles, CA.) |
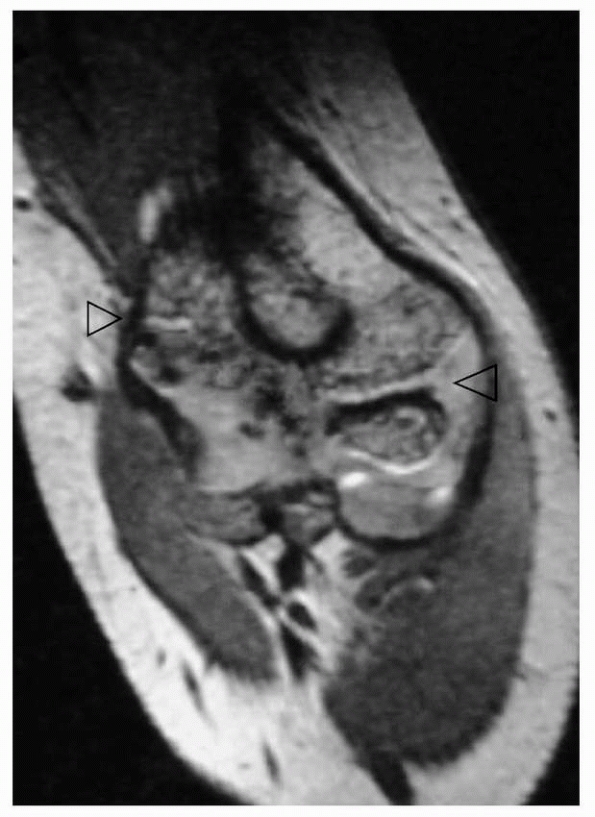 |
|
FIGURE 14-59
At 3 years postinjury, coronal dual echo steady state MRI (TR 27, TE 9) shows interruption of high signal from trochlear growth plate involving 35% of the physis. The medial epicondylar and capitellar growth plates (arrowheads) are preserved. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
 |
|
FIGURE 14-60 A hyperextension deformity in the distal humerus may remodel somewhat, whereas varus and valgus deformities do not. A. Hyperextension deformity in the distal humerus after fracture. B. Four years later, a more normal distal humeral anatomy is seen with remodeling of the hyperextension deformity; 2 years later (C), a normal distal humeral anatomy is reconstituted.
|
treatment of any posttraumatic malalignment, options include (i)
observation with expected remodeling, (ii) hemiepiphysiodesis and
growth alteration, and (iii) corrective osteotomy. Observation
generally is not appropriate because, although hyperextension may
remodel to some degree in a young child (Fig. 14-60), in an older child little remodeling occurs even in the plane of motion of the joint.
of value, but only to prevent cubitus varus deformity from developing
in a patient with clear medial growth arrest or trochlear
osteonecrosis. If untreated, medial growth disturbance will lead to
lateral overgrowth and progressive deformity. Lateral epiphysiodesis
will not correct the deformity, but will prevent it from increasing.
Voss et al.197 used
hemiepiphysiodesis with osteotomy in two patients with growth arrest
and varus deformity. The humerus varies in length by a few centimeters
from one individual to another, but in general, it is about 30 cm long
at skeletal maturity. Approximately 65% of the length of the humerus is
achieved by age 6 years. A 6-year-old child has approximately 10 cm of
growth left in the entire humerus, with
only
approximately 2 cm provided by the distal physis. Growth arrest, in the
absence of osteonecrosis or collapse, will be a very slowly evolving
phenomenon, and epiphysiodesis in a child older than 6 years will have
little effect on longitudinal growth. In general, prevention of
increasing deformity from medial growth arrest is the only role for
lateral epiphysiodesis. Because of the slow growth rate in the distal
humerus, we do not believe there is any role for lateral epiphysiodesis
in correcting a varus deformity in a child with otherwise normal physis.
|
TABLE 14-3 Results of Several Studies Using Various Osteotomies
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
cubitus varus deformity with a high probability of success. A variety
of corrective osteotomies have been described, almost all with
significant complications (Table 14-3). Stiffness, nerve injury,
and recurrent deformities are the most commonly reported complications.
An overall complication rate approaching 25% in many series has led to
some controversy about the value of a distal humeral corrective
osteotomy for cubitus varus deformity.
of the deformity must be determined. Because malunion is the cause of
most cubitus varus deformities, the angular deformity usually occurs at
the level of the fracture. Rotation and hypertension may contribute to
the deformity, but varus is the most significant factor.43 Hyperextension can produce a severe deformity in some patients. An oblique configuration (Fig. 14-61)
places the center of rotation of the osteotomy as close to the actual
level of the deformity as possible. On an AP radiograph of the humerus
with the forearm in full supination, the size of the wedge and the
angular correction needed are determined. An “incomplete” lateral
closing wedge osteotomy can be done, leaving a small medial hinge of
bone intact. The osteotomy usually is fixed with two K-wires placed
laterally. In the absence of an intact medial hinge, two lateral wires
probably are not sufficient to secure this osteotomy.197 Wilkins and Beaty202
recommended crossed wires in this situation. In general, a lateral
closing wedge osteotomy with a medial hinge will correct the varus
deformity, with minimal correction of hyperextension.10,21,46,68,70,79,98,100,116,192,197,207
but this osteotomy tends to leave a lateral bump with poor cosmesis.
Residual rotational deformity was not found to be a significant problem
in studies by Voss et al.197 and Oppenheim et al.,152 which is logical given the amount of rotation available from the shoulder. The French osteotomy68 aims to enable axial rotational correction as well, but does so at the expense of stability and we do not use this osteotomy.
described a dome osteotomy in which a curved osteotomy is made in the
supracondylar area. Proponents of this osteotomy suggest that
multiplane correction is possible without inducing translation in the
distal fragment and that rotation can be corrected. DeRosa and Graziano57
described a step-cut osteotomy in which the distal fragment is slotted
into the proximal fragment and the osteotomy is secured with a single
screw.
 |
|
FIGURE 14-61 A. By moving the apex of the closing wedge distally, the center of rotation of the osteotomy is moved closer to the deformity. B. Upon closing a distally based wedge osteotomy, there is less translational effect than in a more proximally based osteotomy.
|
preoperative functional deficit is nearly always minor in patients with
cubitus varus deformities. Complications of humeral osteotomy include
stiffness, nerve injury, and persistent deformity (see Table 14-3); however, with a properly performed osteotomy, complications are relatively few. Ippolito et al.96 reported long-term follow-up of patients with supracondylar osteotomies, 50% of whom had poor results. Weiss et al.201
reported a complication rate of 16% (nine of 57 patients) with various
types of osteotomies. It was notable that the authors found a
complication rate of 6% (two of 34) when the surgery was done by
full-time staff at a tertiary pediatric referral center, with no
patient requiring additional surgery and all having excellent results.
The complication rate among patients treated by non-full-time staff was
30% (seven of 23), including two transient ulnar nerve palsies, four
deformity recurrences or loss of fixation requiring reoperation, and
one deep infection requiring operative treatment. This can be a
challenging operation, and it is not surprising that surgeons with more
experience have fewer complications.
hyperextension deformities remodeled as much as 30 degrees in very
young children, but in older children, there was no significant
remodeling in the flexion-extension plane. If hyperextension appears to
be a major problem, osteotomy should also be directed at this deformity
rather than simple correction of the varus deformity; this situation
requires a multiplane osteotomy.
but with the complete cut as distal as possible to the superior edge of
the olecranon fossa to place the axis of rotation of the osteotomy as
close as possible to the deformity see (Fig. 14-61). A similar technique has been described by Yun et al.210
with good results in 22 children. Preoperative templating is used to
determine the angle of correction required for correction of the varus
and, if necessary, for correction of any extension deformity.
Templating is based on AP radiographs of both upper extremities
centered on the elbow, combined with clinical examination comparing one
arm to the other in terms of frontal plane appearance and arc of
motion. For example, if the affected arm has 20 degrees more extension
and 20 degrees less flexion than the contralateral arm, a 20-degree
wedge osteotomy is planned in the sagittal plane.
over the lateral distal humerus. The antebrachial cutaneous nerve and
its branches are identified and protected. The radial nerve generally
is proximal to the field of dissection. Dissection is carried out in
the interval between the brachioradialis and triceps. Subperiostial
dissection is performed to expose the distal humerus. There is
sometimes scarring from fracture healing about the area of fracture.
Posterior dissection is continued to the olecranon fossa as a landmark,
but not distal to it to prevent harming the blood supply to the
trochlea.
Chandler retractors are used for circumferential protection with
special care medially near the ulnar nerve. An osteotomy is made just
above the olecranon fossa perpendicular to the shaft of the humerus.
The proximal humerus is delivered out of the wound to allow the more
complex part of the osteotomy to be made with maximal visualization and
protection. With the second cut, the osteotomy is angled correctly to
account for sagittal malalignment. On average, an anterior closing
wedge osteotomy of about 20 degrees is used, but this is adjusted as
needed to make certain the postfixation image demonstrates the anterior
humeral line through the midthird of the capitellum. A small lateral
portion of the proximal fragment is left intact and a similarly shaped
area with a 90-degree angle is made in the lateral portion of the
distal fragment with a rongeur to allow the pieces to fit together for
added stability. This technique is adapted from the osteotomy described
by Wiltse205
and prevents excessive lateral translation of the distal fragment,
keeps the axis of rotation near the site of deformity, and has some
inherent stability if done correctly (Fig. 14-62).
Once correction is achieved, bony contact is maximized by further cuts
if needed. Three 0.062-inch or 2-mm K-wires are then placed across the
osteotomy from lateral to medial. A goniometer is used to measure
alignment. Elbow flexion and extension are then checked to ensure that
the fingers can touch the ipsilateral shoulder and full extension is
achieved. The wound is irrigated, and a small amount of local bone
graft from the excised wedge is packed around the osteotomy site,
making certain bone graft is not in the olecranon fossa. After closure,
flexion and extension are checked under live imaging to ensure that
there is no motion at the osteotomy site. A long-arm cast is applied
with the elbow in 60 to 80 degrees of flexion and the arm in neutral
supination and pronation. The cast is removed when good callus is
demonstrated on radiographs, usually approximately 4 weeks
postoperatively (as opposed to 3 weeks for fractures), and the pins are
removed in clinic at that time. In eight osteotomies done by one of us
with this technique, there has been full frontal and sagittal plane
correction with no complications (unpublished data) (Figs. 14-63, 14-64, 14-65 and 14-66).
 |
|
FIGURE 14-62
This osteotomy can correct frontal and sagittal plane malalignment and offer some inherent bony stability, while not producing a lateral bump that occurs with simple closing wedge osteotomies. |
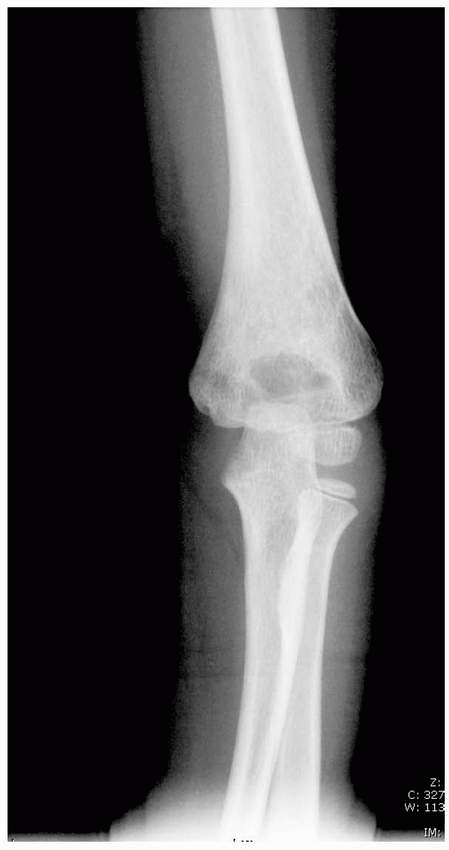 |
|
FIGURE 14-63
AP radiograph of elbow of 5-year-old girl in cubitus varus with Baumann angle about 0 degrees. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
(see pages 496 through 498), the effect of delaying operative treatment
remains a matter of controversy, with several studies concluding that a
delay of surgery of 8 to 21 hours has no deleterious effects on the
outcomes82,97,124,138,179;
however, these studies were all retrospective and their high percentage
of good results may have been due to the selection bias of experienced
pediatric orthopaedic surgeons who selected which fractures required
urgent treatment. Although there are no high-level studies to support
our opinion, we and other authorities believe162
that urgent operative treatment is indicated for patients with poor
perfusion, an associated displaced forearm fracture, firm compartments,
skin puckering, antecubital ecchymosis, or very considerable swelling.
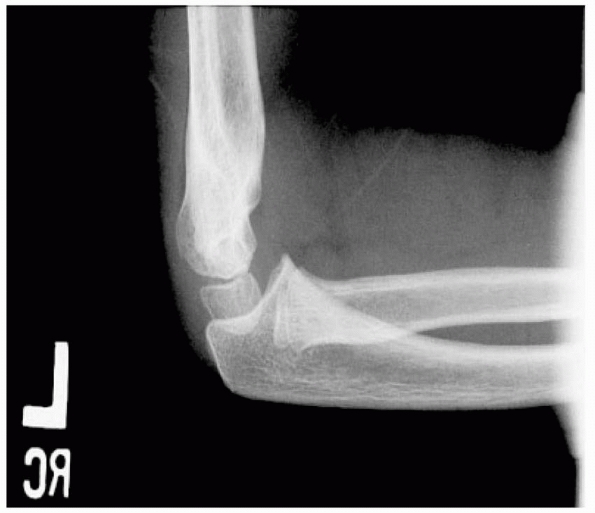 |
|
FIGURE 14-64
Lateral radiograph demonstrates capitellum posterior to the anterior humeral line. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
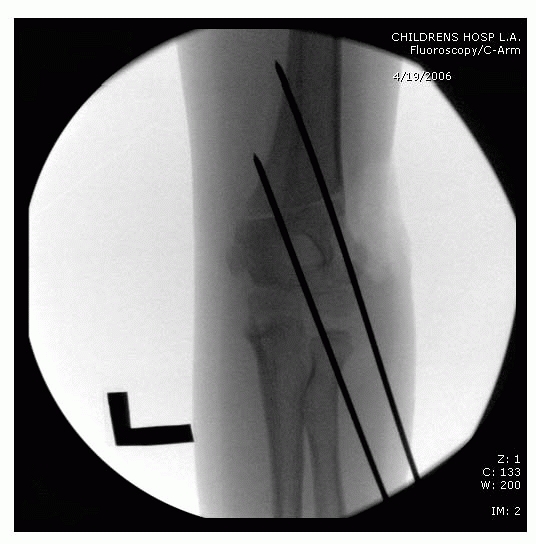 |
|
FIGURE 14-65
Intraoperative AP image demonstrates restoration of Baumann angle after Wiltse-type osteotomy. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
A flexion pattern of injury may not be recognized until reduction is
attempted because initial radiographs are inadequate. A key to
recognizing a flexion-type supracondylar fracture is that it is
unstable in flexion, whereas extension-type fractures generally are
stable in hyperflexion. A laterally displaced supracondylar fracture
may actually be a flexion-type injury.
 |
|
FIGURE 14-67
Flexion mechanism. Flexion-type fractures usually result from a blow to the posterior aspect of the elbow. The obliquity of the fracture line may be opposite that of an extension type. The large black arrows demonstrate the usual direction of fragment displacement. |
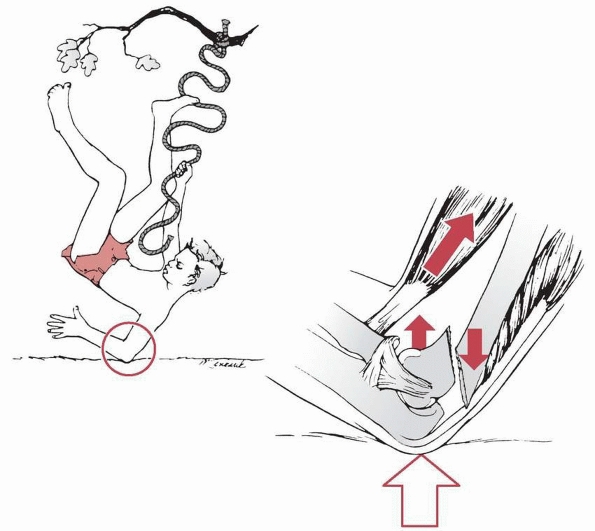 |
|
FIGURE 14-66
Lateral intraoperative image demonstrates anterior humeral line now intersecting the capitellum. Postoperatively a normal arc of elbow flexion and extension was restored. (Reproduced with permission from Children’s Orthopaedic Center, Los Angeles, CA.) |
fall directly onto the elbow rather than a fall onto the outstretched
hand with hyperextension of the elbow (Fig. 14-67).
The distal fragment is displaced anteriorly and may migrate proximally
in a totally displaced fracture. The ulnar nerve is vulnerable in this
fracture pattern,66,85,128,170 and it may be entrapped in the fracture or in the healing callus.118
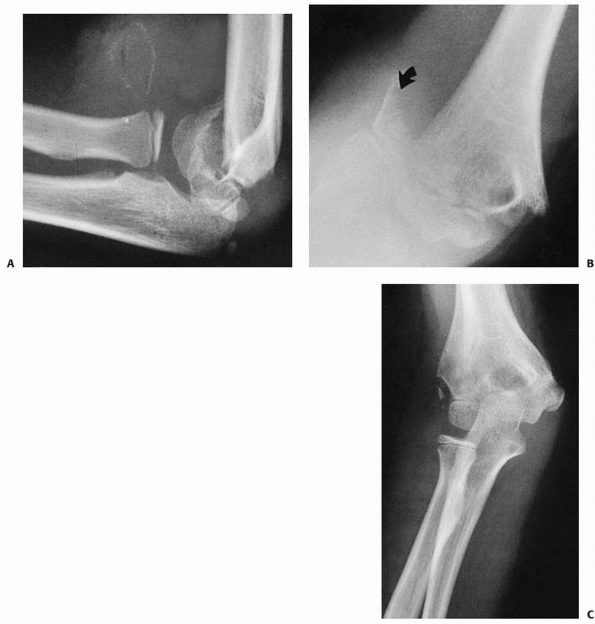 |
|
FIGURE 14-68 Flexion valgus deformity. Lateral (A) and AP (B) views of a flexion-type supracondylar fracture. The distal fragment (arrow) is laterally displaced in the coronal plane as well. C.
Despite aggressive treatment, there was mild residual cubitus valgus plus a flexion contracture when the fracture healed. (From Wilkins KE. Residuals of elbow trauma in children. Orthop Clin N Am 1990;21:291-314, with permission.) |
varies from mild angular deformity to complete anterior displacement.
Anterior displacement often is accompanied by medial or lateral
translation (Fig. 14-68). Associated fractures
of the proximal humerus and radius mandate full radiographic evaluation
of the upper extremity. Fracture classification is similar to
extension-type supracondylar fractures73:
type I, nondisplaced fracture; type II, minimally angulated with
cortical contact; and type III, totally unstable displaced distal
fracture fragment.
are stable nondisplaced fractures that can simply be protected in a
long-arm cast.54,61,145,164
If mild angulation, as in a type II fracture, requires some reduction
in extension, the arm can be immobilized with the elbow fully extended.
Radiographic evaluation with the elbow extended is easily obtained and
accurate in determining the adequacy of reduction. Reduction is
assessed by evaluating the Baumann angle, the anterior humeral line
intersecting the lateral condyle, and the integrity of the medial and
lateral columns at the olecranon fossa. If reduction cannot be
obtained, as is often the case, or if rotation persists, soft tissue
interposition, possibly the ulnar nerve, should be suspected. DeBoeck54
studied 22 flexion-type supracondylar fractures and found cast
treatment satisfactory for nondisplaced fractures. In the other 15
fractures, closed reduction and percutaneous pinning were successful in
most patients.
is that reduction is not easy to achieve and when achieved, the elbow
usually is in extension, making it technically challenging to stabilize
the distal fragment using pins.
generally are reduced if any angular displacement is seen on
fluoroscopic intraoperative evaluation. Type II fractures can be
immobilized in an extension cast with the elbow fully extended (see Fig. 14-70).
The cast is removed at 3 weeks. If closed reduction is done without
skeletal stabilization, follow-up radiographs usually are taken at 1
week and when the cast is removed at 3 weeks. True lateral radiographs
in a fully extended cast are
important and may require a few attempts or the use of live fluoroscopy.
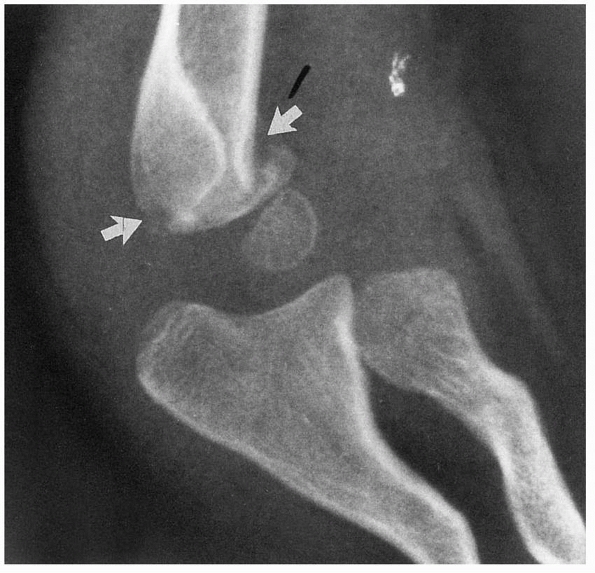 |
|
FIGURE 14-69 Type I flexion injury. A type I flexion supracondylar fracture pattern (arrows)
in a 6-year-old below-the-elbow amputee. There is only about a 10-degree increase in the shaft condylar angle. The patient was treated with a simple posterior splint. |
III flexion supracondylar fractures. The pinning technique de scribed
for extension-type supracondylar fractures is not appropriate for this
fracture because its instability in flexion precludes pinning with the
elbow hyperflexed. In a flexion-type supracondylar fracture, the
posterior periosteum is torn, so reduction can be obtained in
extension, placing tension across the intact anterior periosteum. In
general, a slightly less-than anatomic reduction can be accepted as
long as (a) there is no soft tissue interposition of tissue, (b) the
Baumann angle is close to that of the other side, and (c) neither
flexion nor extension is seen on the lateral view. Although rotating
the arm often is possible for a lateral view of an extension
supracondylar fracture, the C-arm must be moved to obtain satisfactory
radiographic results when pinning a flexion-type supracondylar
fracture, because they often are rotationally unstable even when
reduced (see Fig. 14-25).
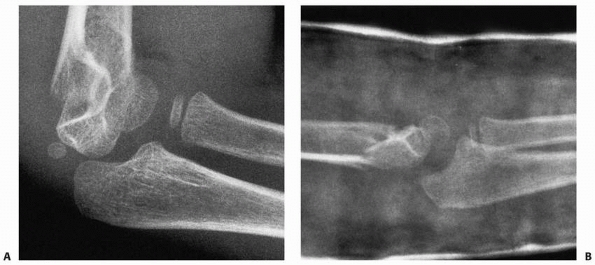 |
|
FIGURE 14-70 Closed reduction, extension cast. A. A 5-year-old girl sustained a type II flexion pattern. B. The fracture was manipulated into extension and found to be stable, and was maintained in a long-arm cast in extension.
|
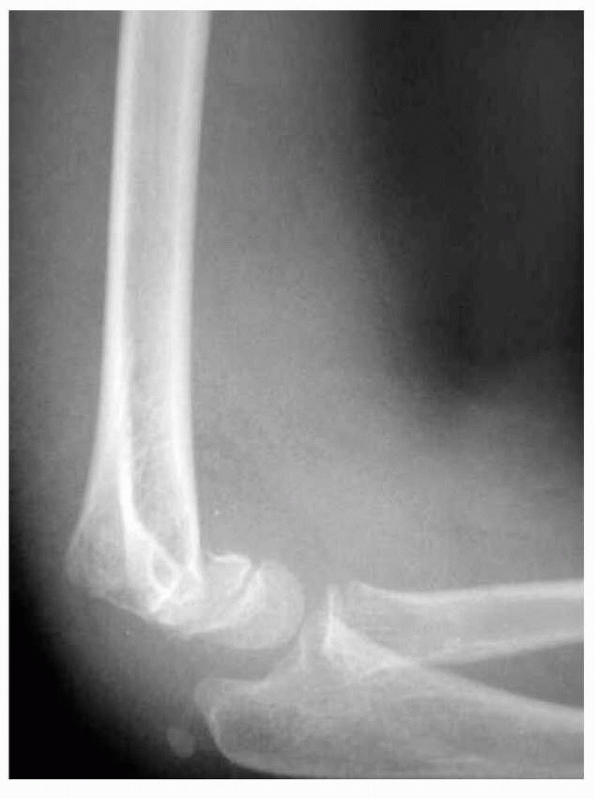 |
|
FIGURE 14-71 Lateral view of a flexion-type supracondylar fracture. The capitellum is in front of the anterior humeral line.
|
approximately 30 degrees of flexion, holding the elbow in a reduced
position. A roll of towels under the distal humerus at the fracture
site elevates the arm and often aids in fracture reduction. If closed
reduction can be obtained, pinning can be accomplished in this
position. Placing two lateral-entry pins in the distal fragment first
allows them to be used as a joystick to help manipulate the fracture
into a reduced position, at which time the pins can be driven across
the fracture site (Figs. 14-71, 14-72, 14-73 and 14-74).
a flexedarm cast can be used to provide better patient comfort, but a
cast with the elbow in almost full extension is acceptable.
supracondylar fractures and is best done through an anteromedial or
posterior approach, rather than an anterior approach as is used for
extension-type supracondylar fractures. With flexion-type fractures,
the brachialis remains intact and must be retracted to expose the
fracture, necessitating a medial extension to the anterior approach. To
ensure that the ulnar nerve is not entrapped in the fracture site,
exploring the ulnar nerve or at least identifying it is probably
advisable with this fracture, which is another reason for a medial
approach to open reduction.
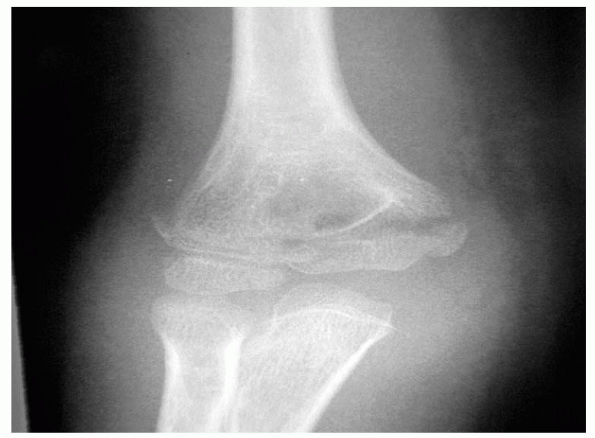 |
|
FIGURE 14-72
AP view of fracture often underestimates the amount of displacement if taken of a bent elbow rather than a true AP of the distal humerus. |
 |
|
FIGURE 14-73
Intraoperative view shows anatomy has been restored, with the anterior humeral line crossing the middle third of the capitellum. |
 |
|
FIGURE 14-74
AP view demonstrates three well-placed lateral pins with maximal separation at fracture site, with all pins engaging solid bone. |
fossa, curving proximally posterior to the neuromuscular bundle.
Dissection is carried down to the level of the superficial fascia of
the forearm and antecubital fossa. The neurovascular bundle is
identified and retracted medially. The brachialis and biceps tendons
are retracted laterally to expose the fracture site and facilitate
reduction. If there is medial soft tissue impingement or a question of
ulnar nerve entrapment within the fracture, the dissection should be
carried around posterior to the medial epicondyle, so the ulnar nerve
and fracture can be identified.
weeks until good callus formation is present. Pins generally are left
out through the skin and removed in the office without the need for
anesthetic. No formal rehabilitation is given, but the patient is
encouraged to begin gentle activities with the arm and to begin
regaining motion without a stressful exercise program.
fractures with a splint or cast with the elbow flexed for comfort.
Minimally displaced type II fractures that reduce in extension are
treated in an extension cast. Unstable types II and III fractures are
pinned. Open reduction through an anteromedial or posterior approach is
used if an anatomic closed reduction cannot be obtained.
M, Ishizu T, Morikawa J. Posterolateral rotatory instability of the
elbow after posttraumatic cubitus varus. J Shoulder Elbow Surg
1997;6:405-409.
E, Powers T, Witt P, et al. Experimental hyperextension supracondylar
fractures in monkeys. Clin Orthop Relat Res 1982;171:309-318.
H, Kalenderer O, Kayali C, et al. Skeletal traction and delayed
percutaneous fixation of complicated supracondylar humerus fractures
due to delayed or unsuccessful reductions and extensive swelling in
children. J Pediatr Orthop B 200;11(2):150-154.
WH, Bowden BW, Miller PR. Displaced supracondylar fractures of the
humerus in children: long-term follow-up of 69 patients. J Am Osteopath
Assoc 1997;76(12): 910-915.
M. Bilaterotricipital approach to the elbow. Its application in the
osteosynthesis of supracondylar fractures of the humerus in children.
Acta Orthop Scand 1972;43(6):479-490.
S, Barrios RH, Martínez-Peric R, et al. Surgical treatment of the
radial nerve lesions associated with fractures of the humerus. J Orthop
Trauma 1993;7(3):211-215.
JC, Messenbaugh JF Jr. Supracondylar osteotomy of the humerus for
correction of rotational and angular deformities of the elbow. South
Med J 1964;7:846-850.
DA, Roberts JA, Smith MG. Transarticular fixation for severely
displaced supracondylar fractures in children. J Bone Joint Surg Br
1991;73(1):147-149.
VL, Lluch EE, Ramirez AM, et al. Percutaneous fixation of supracondylar
fractures of the humerus in children. J Bone Joint Surg Am
1977;59(7):914-916.
DC, van Vollenhoven E, Meeuwis JD. K-wire fixation of supracondylar
humeral fractures in children: results of open reduction via a ventral
approach in comparison with closed treatment. Injury 1993;24(3):179-181.
S, Akinci M, Kamiloglu S, et al. Open reduction of displaced pediatric
supracondylar humeral fractures through the anterior cubital approach.
J Pediatr Orthop 2005; 25(2):149-153.
NP, Howard PW. Olecranon screw traction for displaced supracondylar
fractures of the humerus in children. Injury 1998;29(6):457-460.
A, Volz RG. Traumatic laceration of the radial nerve following
supracondylar fracture of the elbow. A case report. Clin Orthop Relat
Res 1984;184:150-152.
KL, Kaminsky CK, Green DW, et al. Reliability of a modified Gartland
classification of supracondylar humerus fractures. J Pediatr Orthop
2001;21(1):27-30.
TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment
pressures in children with supracondylar fractures of the humerus. J
Pediatr Orthop 2002;22(4):431-439.
J, Carmichael KD. Incidence of pin track infections in children’s
fractures treated with Kirschner wire fixation. J Pediatr Orthop
2007;27(2):154-157.
MC, Barrett IR, Middleton RW, et al. Supracondylar osteotomy of the
humerus for correction of cubitus varus. J Bone Joint Surg Br
1984;66(4):566-572.
J. Cubitus varus after supracondylar fracture of the humerus in
children: can this deformity be prevented? Reconstr Surg Traumatol
1979;17:100-106.
MJ, Reid JT, Schemitsch EH, et al. Effect of distal humeral varus
deformity on strain in the lateral ulnar collateral ligament and
ulnohumeral joint stability. J Bone Joint Surg Am 2004;86(10):2235-2242.
LC, Cooperman DR, Thompson GH, et al. Compartment syndrome in
ipsilateral humerus and forearm fractures in children. Clin Orthop
Relat Res 2000;376: 32-38.
JS, Gaston G, Cates T, et al. Lateral pin vs crossed pin fixation in
type 3 supracondylar (SC) humerus fractures: a randomized prospective
study. Presented at 2007 Annual Meeting of the Pediatric Orthopaedic
Society of North America, Hollywood, Floida, May 23-26, 2007.
T, Mahar A, Pring M, et al. Comparison of supracondylar humerus
fracture pinning when the fracture is not anatomically reduced.
Presented at the 34th Annual David H. Sutherland Pediatric Orthopedic
Visiting Professorship; May 17-18, 2007.
O, Mäkelä EA, Söderğrd J, et al. Absorbable polyglycolide pins in
internal fixation of fractures in children. J Pediatr Orthop
1993;13(2):242-245.
DW, Aronson DD. Supracondylar fractures of the humerus: a prospective
study of percutaneous pinning. J Pediatr Orthop 1992;12(6):789-794.
CA, Lee BM, Bae DS, et al. A systematic review of medial and lateral
entry pinning versus lateral entry pinning for supracondylar fractures
of the humerus. J Pediatr Orthop 2007;27(2):181-186.
CE, Geffard B, Mallet JF. Dissolution of the trochlea after
supracondylar fracture of the humerus in childhood: an analysis of six
cases. J Pediatr Orthop 2007; 27(5):547-550.
IC, Zinar DM. Traumatic and iatrogenic neurological complications after
supracondylar humerus fractures in children. J Pediatr Orthop
1995;15(4):440-443.
RA, MacKenzie WG, Culham JA. Urokinase treatment of forearm ischemia
complicating supracondylar fracture of the humerus in three children.
Pediatr Radiol 1993; 23(5):391-394.
CC, Waters PM, Emans JB, et al. Neurovascular injury and displacement
in type III supracondylar humerus fractures. J Pediatr Orthop
1995;15(1):47-52.
M, Bergoin M, Hornung H. Results of operative treatment of severe
supracondylar fractures of the elbow in children. J Pediatr Surg
1972;7(6):676-679.
O, Pestilci FI, Tuzuner M. Supracondylar fractures of the humerus in
children: analysis of the results in 142 patients. J Orthop Trauma
1990;4(3):265-269.
JC, Lam TP, Maffulli N. Epidemiological features of supracondylar
fractures of the humerus in Chinese children. J Pediatr Orthop B
2001;10(1):63-67.
JC, Lam TP, Shen WY. Closed reduction and percutaneous pinning for type
III displaced supracondylar fractures of the humerus in children. J
Orthop Trauma 1995; 9(6):511-515.
JC, Ng BK, Ying SY, et al. A 10-year study of the changes in the
pattern and treatment of 6,493 fractures. J Pediatr Orthop
1999;19(3):344-350.
PD, Melikian R, Skaggs DL. Management of vascular injuries in pediatric
supracondylar humeral fractures. Presented at the Annual Meeting of
American Academy of Pediatrics, Section of Orthopaedics. San Francisco,
CA, 2008.
LA, Dormans JP, Davidson RS. Vascular injuries and their sequelae in
pediatric supracondylar humeral fractures: toward a goal of prevention.
J Pediatr Orthop 1996; 16(1):99-103.
KE, Devito DP, Green NE. Comparison of closed reduction and
percutaneous pinning versus open reduction and percutaneous pinning in
displaced supracondylar fractures of the humerus in children. J Orthop
Trauma 1992;6(4):407-412.
KE, Green NE, Devito DP. Incidence of anterior interosseous nerve palsy
in supracondylar humerus fractures in children. J Pediatr Orthop
1993;13(4):502-505.
RW, Osterman AL, Davidson RS, et al. Neural injuries associated with
supracondylar fractures of the humerus in children. J Bone Joint Surg
Am 1990;72(8): 1211-1215.
L, Pettersson H. Open reduction and pin fixation of severely displaced
supracondylar fractures of the humerus in children. Acta Orthop Scand
1980;51(2): 249-255.
JR, Maguire MF, Mubarak SJ, et al. Lateral condylar fracture of the
humerus following posttraumatic cubitus varus. J Pediatr Orthop
1994;14(4):466-470.
Boeck H, De Smet P, Penders W, et al. Supracondylar elbow fractures
with impaction of the medial condyle in children. J Pediatr Orthop
1995;15(4):444-448.
A. Growth in pediatric orthopaedics. In: Morrissy RT, Weinsten SL, eds.
Lovell and Winters’s Pediatric Orthopaedics. 6th ed. Philadelphia:
Lippincott Williams and Wilkins, 2006:35-65.
JP, Squillante R, Sharf H. Acute neurovascular complications with
supracondylar humerus fractures in children. J Hand Surg Am
1995;20(1):1-4.
MD. Supracondylar fractures of the humerus in children with a note on
the surgical correction of late cubitus varus. Injury 1974;6(1):45-56.
P, McIntyre W, Letts M. An analysis of open reduction of irreducible
supracondylar fractures of the humerus in children. Can J Surg
1998;41:112-118.
JC, Matthews JG, Benoit RL. Blind pinning of displaced supracondylar
fractures of the humerus in children. Sixteen years’ experience with
long-term follow-up. J Bone Joint Surg Am 1974;56(2):263-272.
JC, Zink WP. Fractures and dislocations of the elbow. In: MacEwan GD,
Kasser JR, Heinrich SD, eds. Pediatric Fractures: A Practical Approach
to Assessment and Treatment. Baltimore: Williams & Wilkins,
1993:133-164.
JV, Kassab MT. Displaced supracondylar fractures of the elbow in
children. A report on the fixation of extension and flexion fractures
by two lateral percutaneous pins. J Bone Joint Surg Br
1974;56(3):490-500.
J, Strong M. Deformity and function in supracondylar fractures of the
humerus in children variously treated by closed reduction and
splinting, traction, and percutaneous pinning. J Pediatr Orthop
1992;12(4):494-498.
M, Mark G, Ruedi T. Management of displaced supracondylar fractures of
the humerus in children. Injury 1991;22(4):259-262.
BC, Manske PR, Pruitt DL, et al. Distal humeral osteotomy for
correction of posttraumatic cubitus varus. J Pediatr Orthop
1994;14(2):214-219.
A, Hayhurst C, Maffulli N, et al. Elevated, straight-arm traction for
supracondylar fractures of the humerus in children. J Bone Joint Surg
Br 2005;87(1):82-87.
H, Gotzen L, Giannadakis, K et al. [Treatment and outcome of
supracondylar humeral fractures in childhood]. Unfallchirurg
1995;98(2):93-97.
DR, Leong JCY, Yau A. Open reduction and internal fixation of
supracondylar fractures of the humerus in children in Hong Kong:
long-term results. Abbot Proc 1978;9:30-34.
JE, Patton CM, Luhmann SJ, et al. Fracture stability after pinning of
displaced supracondylar distal humerus fractures in children. J Pediatr
Orthop 2001;21(3): 313-318.
B, Tredwell SJ, Beauchamp RD, et al. Supracondylar osteotomy of the
humerus for correction of cubitus varus. J Pediatr Orthop
1990;10(2):228-231.
DW, Widmann RF, Frank JS, et al. Low incidence of ulnar nerve injury
with crossed pin placement for pediatric supracondylar humerus
fractures using a mini-open technique. J Orthop Trauma
2005;19(3):158-163.
MA, Hudson OC. Supracondylar fracture of the humerus in childhood. End
result study of open reduction. J Bone Joint Surg Am 1964;46:1245-1252.
N, Kay RM, Leitch K, et al. Effect of surgical delay on perioperative
complications and need for open reduction in supracondylar humerus
fractures in children. J Pediatr Orthop 2004;24(3):245-248.
I, Bayrakci K, Tasbas B, et al. Posterior instability of the shoulder
after supracondylar fractures recovered with cubitus varus deformity. J
Pediatr Orthop 2002;22(2): 198-202.
AT, Devane P, Nicol RO. A selective treatment approach to supracondylar
fracture of the humerus in children. J Pediatr Orthop
1996;16(1):104-106.
R. Skin-traction-treatment of supracondylar fractures of the humerus in
children. A ten-year review. Acta Orthop Scand 1964;35:138-148.
GM, Wilson DW, Arden GP. The operative management of the difficult
supracondylar fracture of the humerus in the child. Injury
1977;9(1):30-34.
DJ, Aldington RA. Moore acute traumatic compartment syndrome: a
systematic review of results of fasciotomy. Trauma 2009;11(1):5-35.
B. Supracondylar fracture of the humerus in children. A late review of
end results with special reference to the cause of deformity,
disability, and complications. Acta Chir Scand 1966;369(Suppl):1-72.
RB, Sapega AA, Scott R, et al. The compartment syndrome. An
experimental and clinical study of muscular energy metabolism using
phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat
Res 1988;226:138-155.
T, Ikuta Y. The new operation method of the domedosteotomy for four
children with varus deformity of the elbow joint. J Jpn Orthop
1982;31:300-335.
S, Mehdi B, Larsen MS. The epidemiology of elbow fracture in children:
analysis of 355 fractures, with special reference to supracondylar
humerus fractures. J Orthop Sci 2001;6:312-315.
A. Treatment of supracondylar fracture of the humerus by skeletal
traction in an abduction splint. J Bone Joint Surg Am
1952;34(3):623-637.
MA. Ulnar nerve palsy: a complication following percutaneous fixation
of supracondylar fractures of the humerus in children. Injury
1996;27(5):303-305.
CA, Spurdle C, King WF, et al. Percutaneous pinning of pediatric
supracondylar humerus fractures with the semisterile technique: the
Miami experience. J Pediatr Orthop 2007;27(1):17-22.
E, Moneta MR, D’Arrigo C. Posttraumatic cubitus varus. Long-term
follow-up of corrective supracondylar humeral osteotomy in children. J
Bone Joint Surg Am 1990;72(5):757-765.
SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and
pinning of type III displaced supracondylar fractures of the humerus in
children: a comparative study. J Orthop Trauma 1999;13(1):51-55.
K. Comparison between closed reduction with percutaneous pinning and
open reduction with pinning in children with closed totally displaced
supracondylar humeral fractures: a randomized controlled trial. J
Pediatr Orthop B 2001;10(2): 131-137.
N, Herold HZ. Correction of axial deviations after supracondylar
fractures of the humerus in children. Int Surg 1973;58(10):735-737.
PE, Foster BK, Paterson DC. Difficult supracondylar elbow fractures in
children: analysis of percutaneous pinning technique. J Pediatr Orthop
1992;12(1):11-15.
L, Ozdemir H, Yilmaz E, et al. Morphology and dynamics of the ulnar
nerve in the cubital tunnel after percutaneous cross-pinning of
supracondylar fractures in children’s elbows: an ultrasonographic
study. J Pediatr Orthop B 2005;14(3):189-193.
JR, Beaty JH. Supracondylar fractures of the distal humerus. In: Beaty
JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th
ed. Philadelphia: Lippincott Williams and Wilkins, 2006:543-589.
M, Luoma R, Rikalainen H, et al. Operative reduction and fixation of a
difficult supracondylar extension fracture of the humerus. J Pediatr
Orthop 1984;4(1): 13-15.
P, Salem K, Schwarting B, et al. The effectiveness of physiotherapy
after operative treatment of supracondylar humeral fractures in
children. J Pediatr Orthop 2005; 25(3):314-316.
GN, Gautam VK, Kochkar VL, et al. Prevention of cubitus varus deformity
in supracondylar fractures of the humerus. Injury 1991;22(3):202-206.
HT, Song MB, Conjares JN, et al. Trochlear deformity occurring after
distal humeral fractures: magnetic resonance imaging and its natural
progression. J Pediatr Orthop 2002;22(2):188-193.
MS, Kasser JR, Waters PM, et al. Lateral entry compared with medial and
lateral entry pin fixation for completely displaced supracondylar
humeral fractures in children. A randomized clinical trail. J Bone
Joint Surg Am 2007;89(4):706-712.
MJ, De Ridder VA, De Lange S, et al. Pediatric supracondylar humerus
fractures: the anterior approach. J Orthop Trauma 2002;16(6):409-412.
M, Keller IL, Solgaard S. Displaced supracondylar fractures of the
humerus in children. Clin Orthop Relat Res 1987;221:215-220.
MH, Regan MW. Completely displaced supracondylar fracture of the
humerus in children. A review of 1708 comparable cases. Clin Orthop
Relat Res 1990;256: 205-214.
H, Bunnell WP, Duhaime M, et al. Cubitus varus deformity following
supracondylar fractures of the humerus in children. J Pediatr Orthop
1982;2(5):539-546.
T, Laurence WN. Entrapment of the ulnar nerve in the callus of a
supracondylar fracture of the humerus. Injury 1984;16(2):129-130.
A, Kivilaakso R. Varus and valgus deformity of the elbow following
supracondylar fractures of the humerus. Acta Orthop Scand
1967;38:313-320.
L, Firoozbakhsh K, Passarelli R, et al. Biomechanical analysis of
pinning techniques for pediatric supracondylar humerus fractures. J
Pediatr Orthop 2006;26(5): 573-578.
W, Mahaisavariya B, Kowsuwon W, et al. Pentalateral osteotomy for
cubitus varus. Clinic experience of a new technique. J Bone Joint Surg
Br 1989;71: 667-670.
YH, Lee SK, Kim BS, et al. Three lateral divergent or parallel pin
fixations for the treatment of displaced supracondylar humerus
fractures in children. J Pediatr Orthop 2008;28(4):417-422.
SS, Mahar AT, Miesen D, et al. Displaced pediatric supracondylar
humerus fractures: biomechanical analysis of percutaneous pinning
techniques. J Pediatr Orthop 2002;22(4):440-443.
AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3
supracondylar humerus fractures in children. J Pediatr Orthop
2002;22(2):203-207.
KK, Kay RM, Femino JD, et al. Treatment of multidirectionally unstable
supracondylar humeral fractures in children. A modified Gartland
type-IV fracture. J Bone Joint Surg Am 2006;88(5):980-985.
PR, Burleson RJ. Vascular and neural complications in supracondylar
fractures of the humerus in children. J Bone Joint Surg Am
1955;37(3):487-492.
JP, Ashley E, Hoffer MM. Ulnar nerve palsies after percutaneous
cross-pinning of supracondylar fractures in children’s elbows. J
Pediatr Orthop 1998;18(1):43-45.
ST, May CD, Kocher MS. Operative management of displaced flexion
supracondylar humerus fractures in children. J Pediatr Orthop
2007;27(5):551-556.
RC, Hennrikus WL. The effect of elbow position on the radial pulse
measured by Doppler ultrasounography after surgical treatment of
supracondylar elbow fractures in children. J Pediatr Orthop
1998;18(4):441-444.
KW, Kooiman AM, Binnendijk B. Brachial artery rupture following
supracondylar fracture of the humerus. Neth J Surg 1986;38(3):81-84.
J, LaGrange J, Faysse R, et al. Les fractures de l’extremite inferieure
de l’humerus chez enfant. Rev Chir Orthop 1962;48:337-413.
DF, Tolo VT, Sellers DS, et al. Radial nerve laceration and retraction
associated with a supracondylar fracture of the humerus. J Hand Surg
1989;14(3):542-545.
GF, Piggot J. Supracondylar osteotomy for cubitus varus. The value of
the straight arm position. J Bone Joint Surg Br 1988;70:283-286.
JJ, Akbarnia BK, Hanel DP, et al. Neurological complications resulting
from supracondylar fractures of the humerus in children. J Pediatr
Orthop 1986;6(6): 647-650.
MD. To the editor: progressive cubitus varus due to a bony physeal bar
in a 4-year-old girl following a supracondylar fracture. A case report.
J Orthop Trauma 2006;20(5):372.
GJ, Walker CR, Cowan B, et al. Extension of the elbow and supracondylar
fractures in children. J Bone Joint Surg Br 1999;81(3):402-405.
CT, Crawford AH, McMillion TL, et al. Operative treatment of
supracondylar fractures of the humerus in children: the Cincinnati
experience. Acta Orthop Belg 1996;62(Suppl 1):41-50.
CT, Strub WM, Roy DR, et al. The effect of surgical timing on the
perioperative complications of treatment of supracondylar humeral
fractures in children. J Bone Joint Surg Am 2001;83(3):323-327.
WL, Meehan PL. Treatment of the displaced supracondylar fracture of the
humerus (type III) with closed reduction and percutaneous cross-pin
fixation. J Pediatr Orthop 1991;11(6):705-711.
SP, Stanislas MJ. Localization of the ulnar nerve during percutaneous
wiring of supracondylar fractures in children. Injury
1996;27(5):301-302.
MB, Singer IJ, Hall JE. Supracondylar fracture of the humerus in
children. Further experience with a study in orthopaedic
decision-making. Clin Orthop Relat Res 1984; 188:90-97.
A, Munishige H, Ikuta Y, et al. Internal rotation deformity and tardy
ulnar nerve palsy after supracondylar humeral fracture. J Shoulder
Elbow Surg 1995;4(1 Pt 1):23-29.
JL, Ecker ML, Chung SM, et al. Supracondylar fractures of the humerus
in children treated by closed reduction and percutaneous pinning. Clin
Orthop Relat Res 1983;177:203-209.
A, Chater E. Open reduction and Kirschner wire fixation for
supracondylar fracture of the humerus. J Bone Joint Surg Br
1976;58:135-136.
SE, Hennrikus WL, Loncarich DP, et al. Relationship between ligamentous
laxity and the site of upper extremity fractures in children: extension
supracondylar fracture versus distal forearm fracture. J Pediatr Orthop
B 1999;8(2):90-92.
SW, Spinner RJ, McKee MD, et al. Tardy posterolateral rotatory
instability of the elbow due to cubitus varus. J Bone Joint Surg Am
2001;83(9):1358-1369.
CW, Park BC, Kim PT, et al. Completely displaced supracondylar humerus
fractures in children: results of open reduction versus closed
reduction. J Orthop Sci 2003;8(2): 137-141.
ON, Nwobi DG. Evaluation of the stability of pin configuration in
K-wire fixation of displaced supracondylar fractures in children. Int
Surg 1998;83:271-274.
WL, Clader TJ, Smith C, et al. Supracondylar humeral osteotomy for
traumatic childhood cubitus varus deformity. Clin Orthop Relat Res
1984;188:34-39.
EE, Niemann KK, Vesely D, et al. Supracondylar fractures of the humerus
in children. J Bone Joint Surg Am 1978;60(5):653-656.
CL, Scott SM, Stevens PM. Closed reduction and percutaneous pinning of
displaced supracondylar humerus fractures in children: description of a
new closed reduction technique for fractures with brachialis muscle
entrapment. J Orthop Trauma 1995; 9(5):430-434.
AM, Graham HK, Krajbich JI. Management of displaced extension-type
supracondylar fractures of the humerus in children. J Bone Joint Surg
Am 1988;70(5): 641-650.
AM, Krajbich JI, Graham HK. Management of displaced supracondylar
fractures of the humerus in children [letter]. J Bone Joint Surg Am
1989;71:313.
BA, Hedequist DJ, Zurakowski D, et al. Complications and timing of
follow-up after closed reduction and percutaneous pinning of
supracondylar humerus fractures: follow-up after percutaneous pinning
of supracondylar humerus fractures. J Pediatr Orthop 2004;24(6):610-614.
CA. Supracondylar fractures of the humerus. A comparative study of
Dunlop’s traction versus percutaneous pinning. J Bone Joint Surg Am
1979;61(3):425-428.
M, Birch R, Eastwood DM. Clinical outcome of nerve injuries associated
with supracondylar fractures of the humerus in children. The experience
of a specialist referral centre. J Bone Joint Surg Br 2006;88(1):90-94.
M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar
fractures in children: Has the pendulum swung too far? J Bone Joint
Surg Br 2008; 90(9):1228-1233.
RH, Griz J. Immediate open reduction and internal fixation of severely
displaced supracondylar fractures of the humerus in children. Clin
Orthop Relat Res 1973; 90:131-132.
MN. Ulnar nerve injury after K-wire fixation of supracondylar humerus
fractures in children. J Pediatr Orthop 1998;18(5):686-690.
SA, Ivory JP, Beavis JP. Use of pulse oximetry during manipulation of
supracondylar fractures of the humerus. Injury 1991;22(2):103-104.
RD, Waters P, Millis M. Open reduction and internal fixation for
supracondylar humerus fractures in children. J Pediatr Orthop
2001;21(2):157-161.
RO, Dutkowsky JP, Kasser JR, et al. Neurologic complications after
K-wire fixation of supracondylar humerus fractures in children. J
Pediatr Orthop 1991;11(2): 191-194.
S, Tredwell SJ, Beauchamp RD, et al. Management of pulseless pink hand
in pediatric supracondylar fractures of humerus. J Pediatr Orthop
1997;17(3):303-310.
K, Henmi T, Kanematsu Y, et al. Radial nerve palsy associated with
slightly angulated pediatric supracondylar humerus fracture. J Orthop
Trauma 1997;11(3): 227-229.
WN, Hebela NM, Skaggs DL, et al. Loss of pin fixation in displaced
supracondylar humeral fractures in children: causes and prevention. J
Bone Joint Surg Am 2007; 89(4):713-717.
PL, Delgado E, Rotman M, et al. Pulseless arm in association with
totally displaced supracondylar fracture. J Orthop Trauma
1996;10(6):410-415.
BA, Kasser JR, Emans JB, et al. Management of vascular injuries in
displaced supracondylar humerus fractures without arteriography. J
Orthop Trauma 1990;4(1): 25-29.
PG, Gehring HW, Iglesias LJ. Open reduction and internal fixation of
displaced supracondylar fractures of the humerus in children. Orthop
Clin N Am 1976;7(3): 573-581.
M, Sharma H, Bennet GC. Early versus delayed treatment of extension
type-3 supracondylar fractures of the humerus in children. J Bone Joint
Surg Br 2006;88(3): 380-381.
N, Lamdan R, Mosheiff R. Underreduced supracondylar fracture of the
humerus in children: clinical significance at skeletal maturity. J
Pediatr Orthop 2007; 27(7):733-738.
DL. Closed reduction and pinning of supracondylar humerus fractures. In
Tolo VT, Skaggs DL, eds. Masters Techniques in Orthopaedic Surgery:
Pediatric Orthopaedics. Philadelphia: Lippincott, 2007: 1-15.
DL, Cluck MW, Mostofi A, et al. Lateral-entry pin fixation in the
management of supracondylar fractures in children. J Bone Joint Surg Am
2004;86A(4):702-707.
DL, Hale JM, Bassett J, et al. Operative treatment of supracondylar
fractures of the humerus in children. The consequence of pin placement.
J Bone Joint Surg Am 2001;83(5):735-740.
DL, Mirzayan R. The posterior fat pad sign in association with occult
fracture of the elbow in children. J Bone Joint Surg Am
1999;81(10):1429-1433.
DL, Sankar WN, Albrektson J, et al. How safe is the operative treatment
of Gartland type 2 supracondylar humerus fractures in children? J
Pediatr Orthop 2008; 28(2):139-141.
RJ, O’Driscoll SW, Davids JR, et al. Cubitus varus associated with
dislocation of both the medial portion of the triceps and the ulnar
nerve. J Hand Surg Am 1999; 24(4):718-726.
M, Schreiber SN. Anterior interosseous-nerve paralysis as a
complication of supracondylar fractures of the humerus in children. J
Bone Joint Surg Am 1969;51(8): 1584-1590.
M, Sasaki I, Kimura T, et al. Second fracture of the distal humerus
after varus malunion of a supracondylar fracture in children. J Bone
Joint Surg Br 1998;80(5): 791-797.
B, Kapoor V, Fairhurst J, et al. Progressive cubitus varus due to a
bony physeal bar in a 4-year-old girl following a supracondylar
fracture: a case report. J Orthop Trauma 2005;19(9):669-672.
RE, Blanco JS, Davis TJ. Clinical evaluation of crossed-pin versus
lateral-pin fixation in displaced supracondylar humerus fractures. J
Pediatr Orthop 1995;15(4): 435-439.
FR, Kasser JR, Trepman E, et al. Uniplanar supracondylar humeral
osteotomy with preset Kirschner wires for posttraumatic cubitus varus.
J Pediatr Orthop 1994; 14(4):471-478.
A, Egund N, Eikelund L. Supracondylar fracture of the humerus in
children: review of closed and open reduction leading to a proposal for
treatment. Injury 1985; 16(5):296-299.
AJ, Meyer S, Tolo VT, et al. Surgical treatment of displaced
supracondylar fractures of the humerus in children. Analysis of
fifty-two cases followed for five to fifteen years. J Bone Joint Surg
Am 1978;60:657-661.
JM, Kay RM, Waters P. Distal humeral osteotomy for supracondylar
fracture malunion in children: a study of perioperative complications.
To be published in Am J Orthop Surg, January 2010.
DM, Cole WG. Treatment of selected extension supracondylar fractures of
the humerus by manipulation and strapping in flexion. Injury
1993;24(4):249-252.
LL. Valgus deformity of the ankle: a sequel to acquired or congenital
abnormalities of the fibula. J Bone Joint Surg Am 1972;54(3):595-606.
WM, Schwend RM, Armstrong DG. Predicting ulnar nerve location in
pinning of supracondylar humerus fractures. J Pediatr Orthop
2002;22(4):444-447.
HK, Balasubramaniam P. Humeral torsional deformity after supracondylar
osteotomy for cubitus varus: its influence on the postosteotomy
carrying angle. J Pediatr Orthop 1992;12(4):490-493.
PH, Colton C. Severely displaced supracondylar fractures of the humerus
in children: a simple method of treatment. J Pediatr Orthop
1987;7(1):49-53.
Z, Wang Y, Gilula LA, et al. Microcirculation of the distal humeral
epiphyseal cartilage: implications for posttraumatic growth
deformities. J Hand Surg Am 1998; 23(1):165-172.
YH, Shin SJ, Moon JG. Reverse V osteotomy of the distal humerus for the
correction of cubitus varus. J Bone Joint Surg Br 2007;89(4):527-531.
M, Ramachandran M, Milne B, et al. Intraoperative stability testing of
lateral-entry pin fixation of pediatric supracondylar humeral
fractures. J Pediatr Orthop 2007; 27(6):695-702.
LE, McKellop HA, Hathaway R. Torsional strength of pin configurations
used to fix supracondylar fractures of the humerus in children. J Bone
Joint Surg Am 1994; 76(2):253-256.
N, Litwin A, Katz K, et al. Definitive diagnosis of fracture-separation
of the distal humeral epiphysis in neonates by ultrasonography. Pediatr
Radiol 1996;26(7): 493-496.
