the Elbow Region: General Concepts in the Pediatric Patient
echoed the opinion of that era about elbow injuries: “The difficulties
experienced by surgeons in making an accurate diagnosis; the facility
with which serious blunders can be made in prognosis and treatment; and
the fear shared by so many of the subsequent limitation of function,
serve to render injuries in the neighborhood of the elbow less
attractive than they might otherwise have proved.” These concerns are
applicable even today. In other bones, good results can often be
obtained with minimal treatment, but in the elbow, more aggressive
treatment is often required to avoid complications. An understanding of
the basic anatomy and radiographic landmarks of the elbow is essential
in choosing appropriate treatment.
outstretched arms when they fall, upper-extremity fractures account for
65% to 75% of all fractures in children. The most common area of the
upper extremity injured is the distal forearm5,33; 7% to 9% of upper-extremity fractures involve the elbow.
fractures about the elbow region. Supracondylar fractures are the most
frequent elbow injuries in children, reported to occur in 55% to 75% of
patients with elbow fractures. Lateral condylar fractures are the
second most common, followed by medial epicondylar fractures. Fractures
of the olecranon, radial head, and neck and medial epicondyle and
T-condylar fractures are much less common.
reported that the average age of 355 children with elbow fractures was
7.9 years (7.2 years in boys and 8.5 years in girls). Contrary to most
reports, these investigators found elbow fractures more frequent in
girls (54%) than in boys. In a study of 450 supracondylar humeral
fractures, Cheng et al.13 found a
median age of 6 years (6.6 years in boys and 5 years in girls) and a predominance of injuries (63%) in boys.
older children between the ages of 10 and 13; however, the peak age for
injuries to the distal humeral physes is 4 to 5 years in girls and 5 to
8 years in boys. In most physeal injuries, the increased incidence with
advanced age is believed to be due to weakening of the perichondrial
ring as it matures (see Chapter 5). Thus, some
different biomechanic forces and conditions must exist about the elbow
to make the physis more vulnerable to injuries at an earlier age. (For
more data on the relationship of fractures about the elbow to all types
of fractures, see Chapter 1).
individual joints contained within a common articular cavity. Several
anatomic concepts are unique to the growing elbow.
the center of the long bones and progresses distally. The ossification
process begins in the diaphyses of the humerus, radius, and ulna at the
same time. By term, ossification of the humerus has extended distally
to the condyles. In the ulna, it extends to more than half the distance
between the coronoid process and the tip of the olecranon. The radius
is ossified proximally to the level of the neck. The bicipital
tuberosity remains largely unossified (Table 13-1).20 Brodeur et al.8
compiled a complete atlas of ossification of the structures about the
elbow, and their work is an excellent reference source for finer
details of the ossification process about the elbow.
predictable rate. In general, the rate of ossification in girls exceeds
that of boys.18,19,22
In some areas, such as the olecranon and lateral epicondyle, the
difference between boys and girls in ossification age may be as great
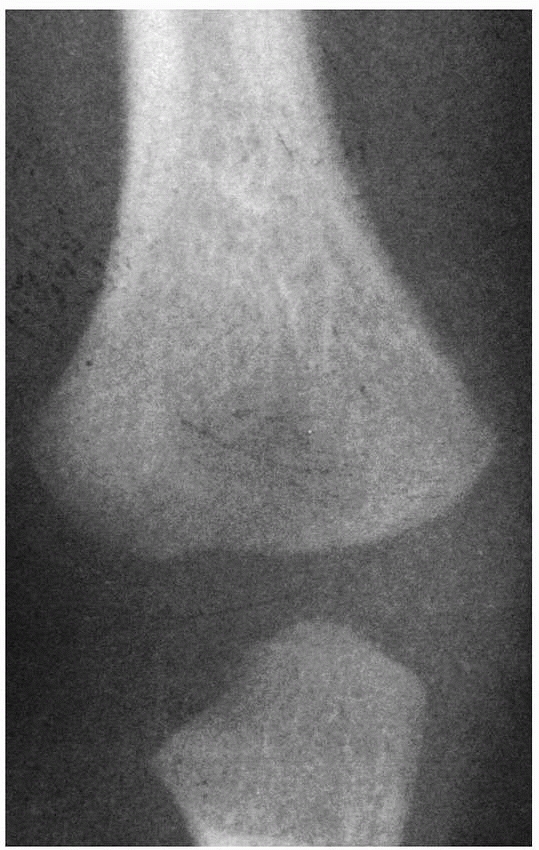
as 2 years.19 During the first 6 months, the ossification border of the distal humerus is symmetric (Fig. 13-1).
|
TABLE 13-1 Sequence and Timing of TABLE 13-1 Ossification in the Elbow
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
 |
|
FIGURE 13-1 During the first 6 months, the advancing ossifying border of the distal humerus is symmetric.
|
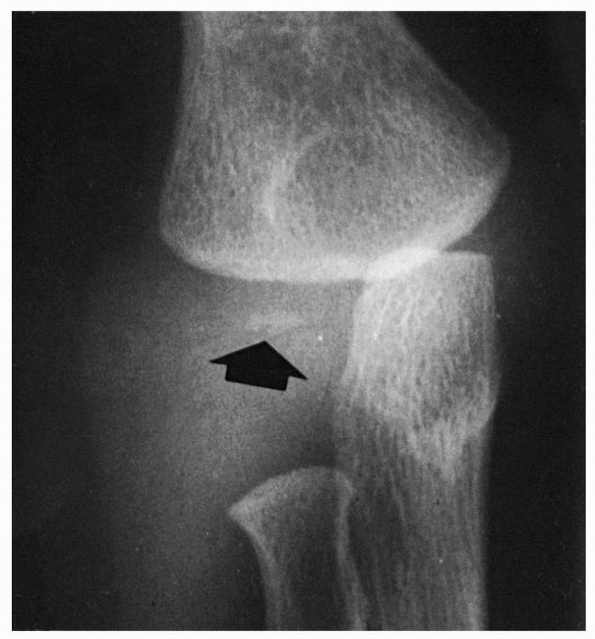
condyle appears just before 1 year of age but may be delayed as late as
18 to 24 months.10 When the nucleus
of the lateral condyle first appears, the distal humeral metaphyseal
border becomes asymmetric. The lateral border slants and becomes
straight to conform with the ossification center of the lateral condyle
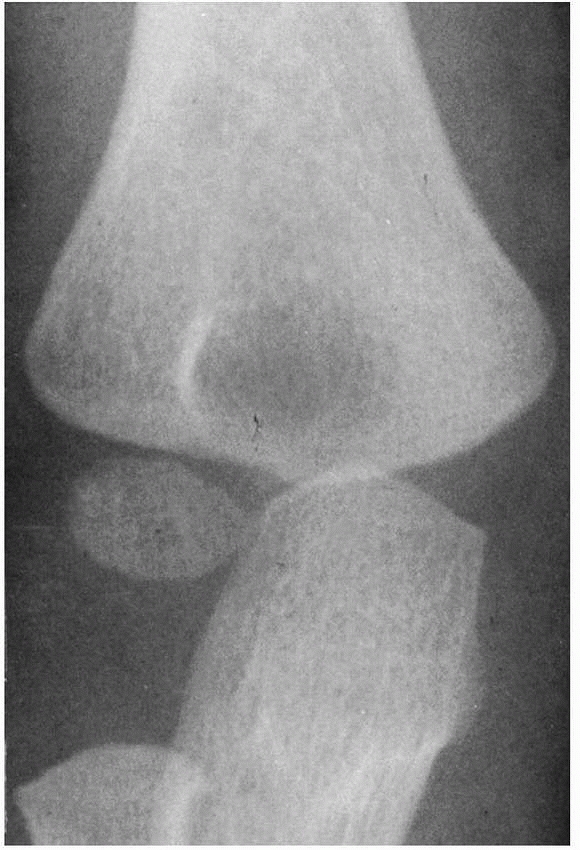
(Fig. 13-2). By the end of the second year, this border becomes well defined, possibly even slightly concave. This ossification center
is usually spherical when it first appears. It becomes more hemispherical as the distal humerus matures,9 and the ossific nucleus extends into the lateral ridge of the trochlea (Fig. 13-3).
On the lateral view, the physis of the capitellum is wider posteriorly.
This is a normal variation and should not be confused with a fracture.9
 |
|
FIGURE 13-2 Ossification at 12 months. As the ossification center of the lateral condyle develops (arrow), the lateral border of the metaphysis becomes straighter.
|
 |
|
FIGURE 13-3
At 24 months, the oval-shaped secondary ossification center of the lateral condyle extends into the lateral crista of the trochlea. The lateral border of the neck (metaphysis) of the radius is normally angulated both anteriorly and laterally. |
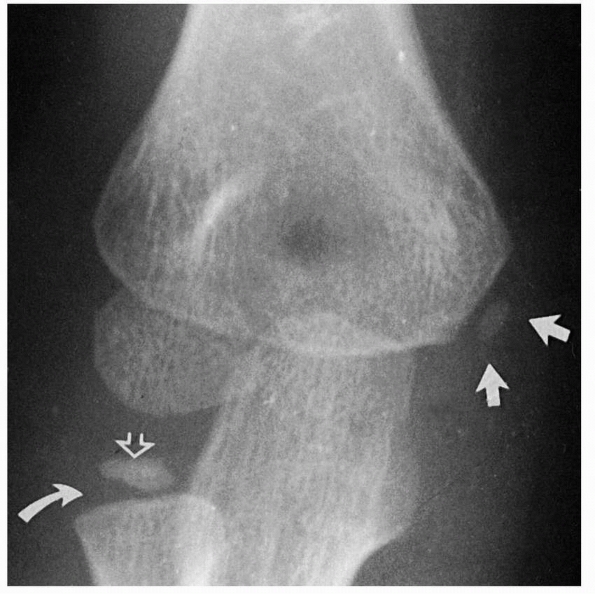
on the medial aspect of the metaphyseal ossification border. In this
area, a medial epicondyle begins to ossify (Fig. 13-4).
 |
|
FIGURE 13-4 At about 5 or 6 years of age, a secondary center develops in the medial epicondylar apophysis (white arrows). At this same time, the ossification center of the radial head also develops (open arrow). Note that the physis of the proximal radius is widened laterally (curved arrow).
|
 |
|
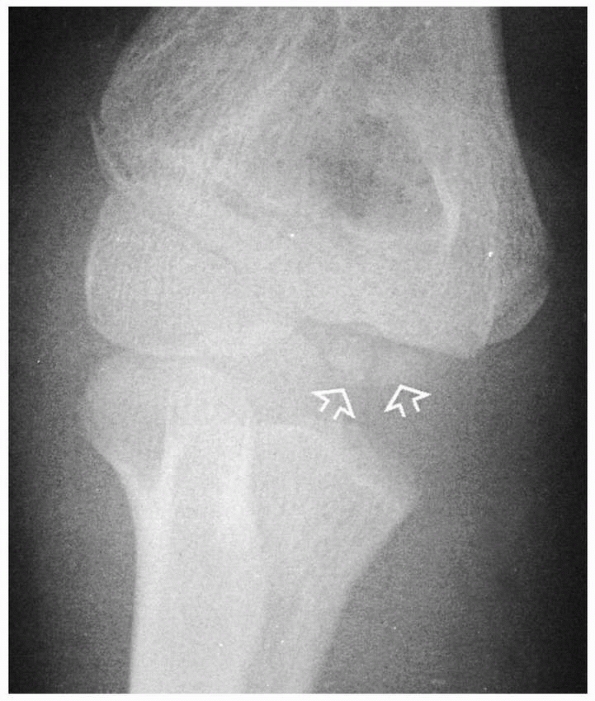
FIGURE 13-5 At about 9 years of age, the ossification of the medial crista of the trochlea may begin as two well-defined centers (arrows). These multiple centers can give the trochlea a fragmented appearance.
|
lateral epicondyle, and trochlea fuse to form one epiphyseal center.
Metaphyseal
bone separates the extra-articular medial epicondyle from this common humeral epiphyseal center (Fig. 13-7).
The common epiphyseal center ultimately fuses with the distal humeral
metaphysis. The medial epicondyle may not fuse with the metaphysis
until the late teens.
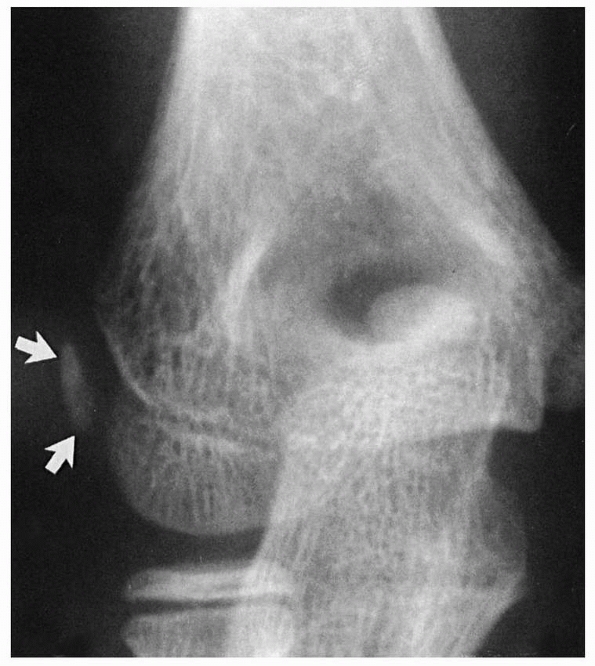 |
|
FIGURE 13-6 The apophysis of the lateral epicondyle ossifies as either an oblong or a triangular center (arrows). The wide separation of this center from the metaphyseal and epiphyseal borders of the lateral condyle is normal.
|
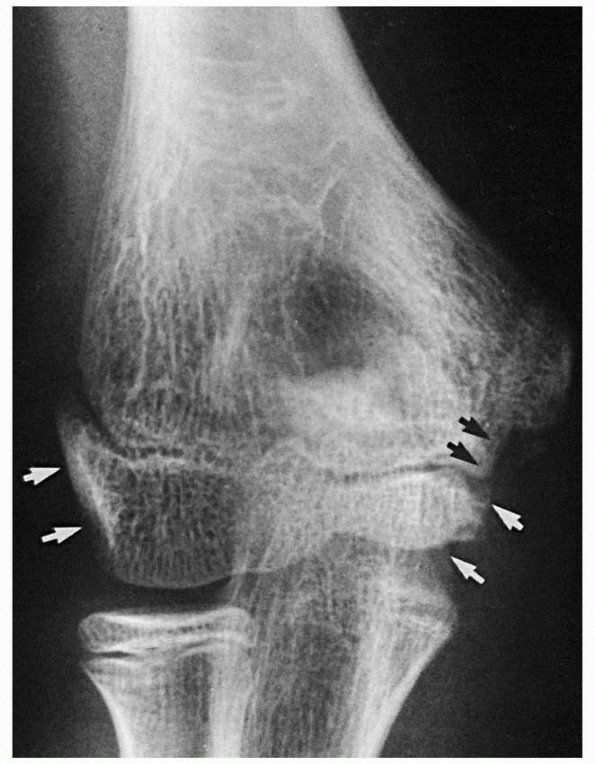 |
|
FIGURE 13-7
The secondary ossification centers of the lateral condyle, trochlea, and lateral epicondylar apophysis fuse to form one center (white arrows). This common center is separated from the medial epicondylar apophysis by advancing metaphyseal bone (black arrows). |
The ossification center is present in at least 50% of girls by 3.8
years of age, but may not be present in the same proportion of boys
until around 4.5 years.18 Initially,
the ossification center is elliptical, and the physis is widened
laterally due to the obliquity of the proximal metaphysis. The
ossification center flattens as it matures. At about age 12, it
develops a concavity opposite the capitellum.9
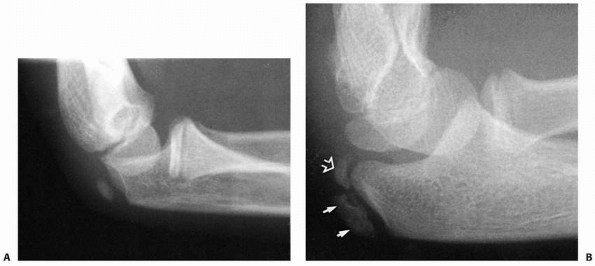 |
|
FIGURE 13-8 Ossification of the olecranon. A. Secondary ossification begins as an oblique oblong center at about 6 to 8 years of age. B. It may progress as two separate ossification centers: articular (open arrow) and traction (closed arrows).
|
produce an irregularity of the second center. These secondary or
irregular ossification centers should not be interpreted as fracture
fragments.
ulnar metaphysis. At birth, the ossification margin lies halfway
between the coronoid process and the tip of the olecranon. By about 6
or 7 years of age, it appears to envelop about 66% to 75% of the
capitellar surface. The final portion of the olecranon ossifies from a
secondary ossification center that appears around 6.8 years of age in
girls and 8.8 years in boys (Fig. 13-8A). Peterson38 described two separate centers: one articular and the other a traction type (Fig. 13-8B). This secondary ossification center of the olecranon may persist late into adult life.37
humerus fuse as one unit and then fuse later to the metaphysis. The
medial epicondyle is the last to fuse to the metaphysis. The ranges of
onset of the ossification of various centers and their fusion to other
centers or the metaphysis are summarized in Figure 13-9. Each center contributes to the overall architecture of the distal humerus (Fig. 13-9C).
centers with their respective metaphyses occurs at around the same time
that the common distal humeral epiphysis fuses with its metaphysis
(i.e., between 14 and 16 years of age).6,8,42
six secondary ossification centers around the elbow were mainly derived
from studies conducted more than 30 years ago, Cheng et al.13 evaluated elbow radiographs of 1577 Chinese children. They found that the sequence of ossification was the same in
boys and girls—capitellum, radial head, medial epicondyle, olecranon,
trochlea, and lateral epicondyle—but ossification was delayed by about
2 years in boys in all ossification centers except the capitellum (see Table 13-1).
 |
|
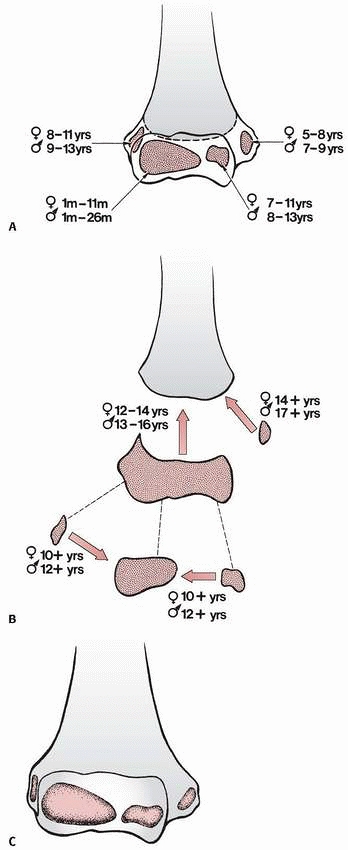
FIGURE 13-9 Ossification and fusion of the secondary centers of the distal humerus. A. The average ages for the onset of ossification of the various ossification centers are shown for both boys and girls. B.
The ages at which these centers fuse with each other are shown for both boys and girls. (Modified and reprinted with permission from Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intraosseous vasculature in distal humerus. Acta Orthop Scand 1959;Suppl 38:1-232.) C. The contribution of each secondary center to the overall architecture of the distal humerus is represented by the stippled areas. |
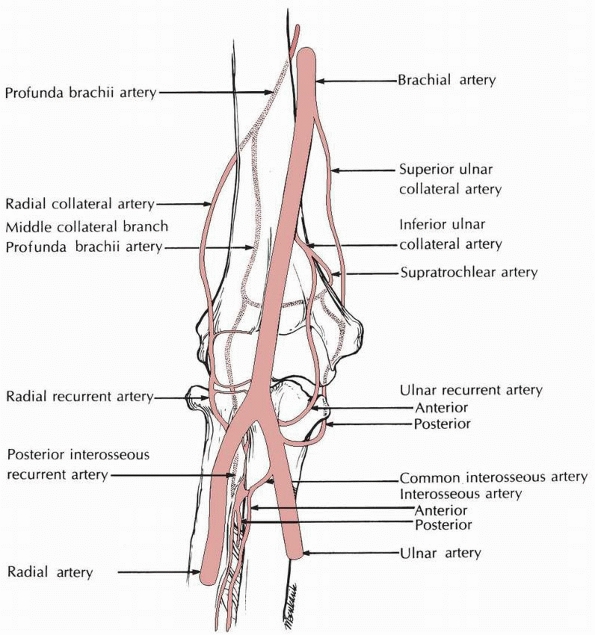
The major arterial trunk, the brachial artery, lies anteriorly in the
antecubital fossa. Most of the intraosseous blood supply of the distal
humerus comes from the anastomotic vessels that course posteriorly.
entrance of the vessels into the developing epiphysis. First, there is
no communication between the intraosseous metaphyseal vasculature and
the ossification centers. Second, vessels do not penetrate the
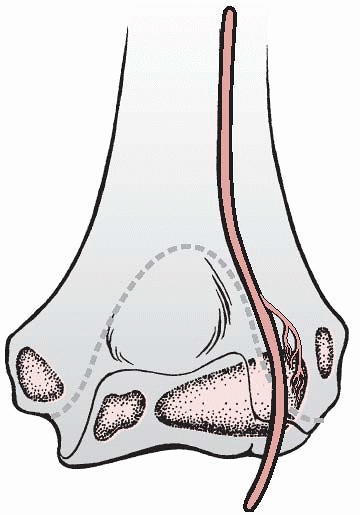
articular surfaces. The lateral condyle is nonarticular only at the
origin of the muscles and collateral ligaments. Third, the vessels do
not penetrate the articular capsule except at the interface with the
surface of the bone. Thus, only a small portion of the lateral condyle
posteriorly is both nonarticular and extracapsular (Fig. 13-11).23
who demonstrated that there are two types of vessels in the developing
lateral condyle. These vessels enter the posterior portion of the
condyle just lateral to the origin of the capsule and proximal to the
articular cartilage near the origin of the anconeus muscle. They
penetrate the nonossified cartilage and traverse it to the developing
ossific nucleus. In a young child, this is a relatively long course (Fig. 13-12A).
These vessels communicate with one another within the ossific nucleus
but do not communicate with vessels in either the metaphysis or
nonossified chondroepiphysis. Thus, for practical purposes, they are
end vessels.
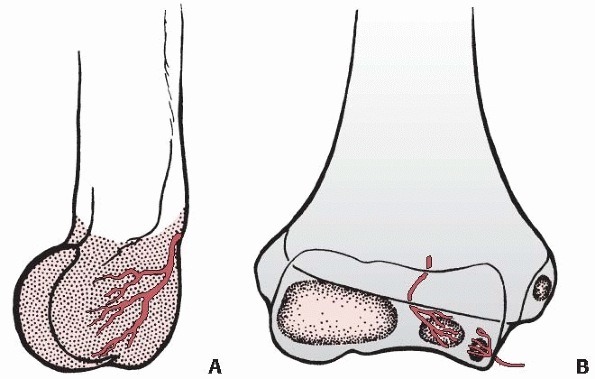
into the lateral portion of the trochlea. Thus, the lateral crista or
ridge of the trochlea derives its blood supply from these condylar
vessels. The medial ridge or crista remains unossified for a longer
period of time. The trochlea is covered entirely by articular cartilage
and lies totally within the confines of the articular capsule. The
vessels that supply the nucleus of the ossific centers of the trochlea
must therefore traverse the periphery of the physis to enter the
epiphysis.
The lateral vessel, on the posterior surface of the distal humeral
metaphysis, penetrates the periphery of the physis and terminates in
the trochlear nucleus. Because this vessel supplying the trochlea is an
end vessel, it is especially vulnerable to injury by a fracture that
courses through either the physis or the very distal portion of the
humeral metaphysis. Injury to this vessel can markedly decrease the
nourishment to the developing lateral ossific nucleus of the trochlea.
The medial vessel penetrates the nonarticulating portion of the medial
crista of the trochlea. This multiple vascular source may account for
the development of multiple ossification centers in the maturing trochlea, giving it a fragmented appearance (see Fig. 13-5).
 |
|
FIGURE 13-10 The major arteries about the anterior elbow.
|
 |
|
FIGURE 13-11
The vessels supplying the lateral condylar epiphysis enter the posterior aspect of the condyle, which is extra-articular. (Modified and reprinted with permission from Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intraosseous vasculature in distal humerus. Acta Orthop Scand 1959;Suppl 38:1-232.) |
vessels anastomose freely. The blood supply from the central nutrient
vessel of the shaft reaches the epicondylar regions in the skeletally
mature distal humerus.31
capsule, but nonarticulating areas involving the coronoid and radial
fossae anteriorly and the olecranon fossa posteriorly are also within
the confines of the articular cavity.46 The capsule attaches just distal to the coronoid and olecranon processes. Thus, these processes are intra-articular.28
The entire radial head is intra-articular, with a recess or
diverticulum of the elbow’s articular cavity extending distally under
the margin of the orbicular ligament. The medial and lateral
epicondyles are extra-articular.
longitudinally directed fibers are very strong and become taut with the
elbow in extension. In hyperextension, the tight anterior bands of the
capsule force the ulna firmly into contact with the humerus. Thus, the
fulcrum of rotation becomes transmitted proximally into the tip of the
olecranon in the supracondylar area.
This is an important factor in the etiology of supracondylar fractures.
 |
|
FIGURE 13-12 Intraosseous blood supply of the distal humerus. A.
The vessels supplying the lateral condylar epiphysis enter on the posterior aspect and course for a considerable distance before reaching the ossific nucleus. B. Two definite vessels supply the ossification center of the medial crista of the trochlea. The lateral vessel enters by crossing the physis. The medial one enters by way of the nonarticular edge of the medial crista. (Modified and reprinted with permission from Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand 1959;Suppl 38:1-232) |
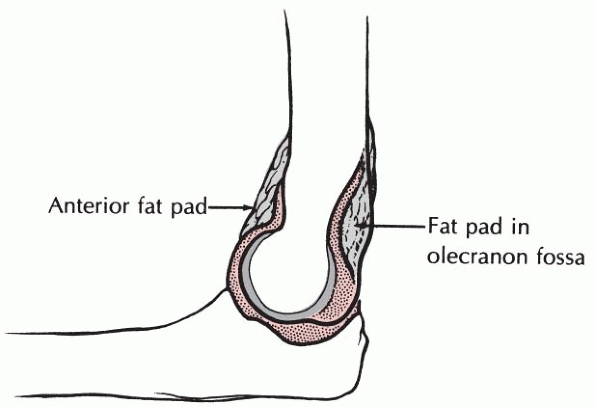
The posterior fat pad lies totally within the depths of the olecranon
fossa when the elbow is flexed. The anterior fat pad extends anteriorly
out of the margins of the coronoid fossa. The significance of these fat
pads in the interpretation of radiographs of the elbow is discussed
later.
orbicular and collateral ligaments is discussed in the sections on the
specific injuries involving the radial neck, medial epicondyle, and
elbow dislocations.
identification and delineation of fractures about the elbow in the
immature skeleton may be subject to misinterpretation. The variables of
ossification of the epiphyses should be well known to the orthopaedic
surgeon who treats these injuries.
 |
|
FIGURE 13-13
The elbow fat pads. Some of the coronoid fat pad lies anterior to the shallow coronoid fossa. The olecranon fat pad lies totally within the deeper olecranon fossa. |
normal range of elbow motion after elbow trauma do not require
immediate radiographic evaluation. In a large multicenter prospective
study, Appelboam et al.2 found that
of 780 children evaluated for elbow trauma, 289 were able to fully
extend their elbow; among these 12 (4%) fractures were identified, all
at their first evaluation. Among the 491 children who could not fully
extend their injured elbow, 210 (43%) had confirmed fractures. These
authors suggested that an elbow extension test can be used to rule out
the need for radiographs, provided the physician is confident that an
olecranon fracture is not present and that the patient can return for
re-evaluation if symptoms have not resolved in 7 to 10 days. Lennon et
al.,34 in a study involving 407
patients ranging in age from 2 to 96 years, proposed that patients aged
no more than 16 years with a range of motion equal to the unaffected
side do not require radiographic evaluation. Darracq et al.15
found that limitation of active range of motion was 100% sensitive for
fracture or effusion, while preservation of active range of motion was
97% specific for the absence of fracture. Other studies16,24,32
have confirmed a high sensitivity (91% to 97%) of an inability to
extend the elbow as a predictor of elbow fracture in both children and
adults.
landmarks and angles should be evaluated and measured, including any
displacement of the fat pads about the elbow. It is important to be
familiar with these landmarks and angles and to be aware of the
significance of any deviation from normal.
anteroposterior (AP) view with the elbow extended and a lateral view
with the elbow flexed to 90 degrees and the forearm neutral.
The distal humerus is normally difficult to interpret due to the
superimposed proximal radius and ulna. There is often a high index of
suspicion for a fracture, but none is visible on routine AP and lateral
radiographs. In this case, internal and external oblique views may be
helpful. This is especially true in identifying fractures of the radial
head and coronoid process.
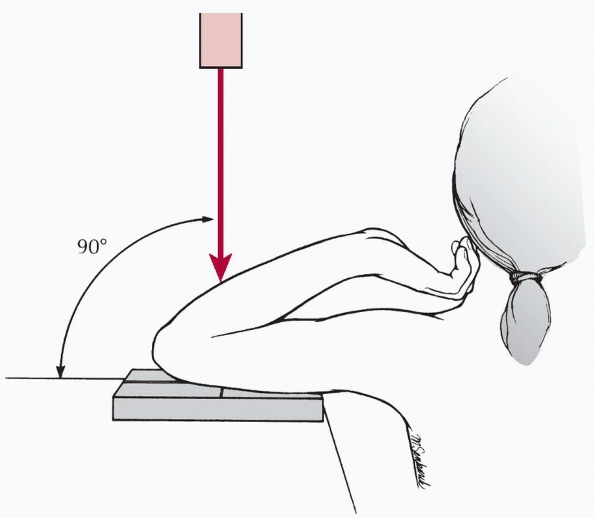 |
|
FIGURE 13-14 Jones axial radiographic view of the elbow.
|
angulation of the physeal line between the lateral condyle and the
distal humeral metaphysis. The ossification center of the lateral
condyle extends into the radial or lateral crista of the trochlea (see Fig. 13-9C).
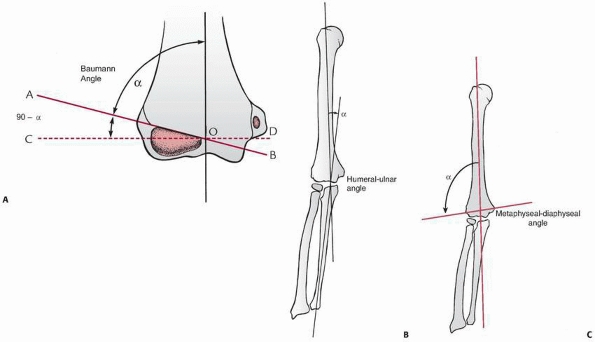
This physeal line forms an angle with the long axis of the humerus. The
angle formed by this physeal line and the long axis of the humerus is
the Baumann angle (Fig. 13-15A).3 The Baumann angle is not equal to the carrying angle of the elbow in older children.9
This is a consistent angle when both sides are compared, and the x-ray
beam is directed perpendicular to the long axis of the humerus. Acton
and McNally1 reviewed the
descriptions of the Baumann angle in a number of commonly used
textbooks and discovered three variations of measurement technique.
They recommended that the angle should always be measured between the
long axis of the humerus and the inclination of the capitellar physis,
as Baumann described, and that it should be called the “shaft-physeal”
angle to avoid confusion.
 |
|
FIGURE 13-15 AP radiographic angles of the elbow. A. Baumann angle. B. The humeral-ulnar angle. C.
The metaphyseal-diaphyseal angle. (Reprinted with permission from O’Brien WR, Eilert RE, Chang FM, et al. The metaphyseal-diaphyseal angle as a guide to treating supracondylar fractures of the humerus in children. Presented at 54th Annual Meeting of AAOS; San Francisco, CA; 1987.) |
left angulation of the tube by as much as 30 degrees changes the
Baumann angle by less than 5 degrees. If, however, the tube becomes
angulated in a cephalad-caudad direction by more than 20 degrees, the
angle is changed significantly and the measurement is inaccurate. In
their cadaver studies, Camp et al.11
found that rotation of the distal fragment or the entire reduced
humerus can also alter the projection of the Baumann angle. They found
that to be accurate, the humerus must be parallel to the x-ray plate,
with the beam directed perpendicular to the film as well. Thus, in the
routine AP radiographs of the distal humerus, including the Jones view,
the Baumann angle is a good measurement of any deviation of the
angulation of the distal humerus.14
used to determine the proper alignment of the distal humerus or
carrying angle. The humeral-ulnar angle is determined by lines
longitudinally bisecting the shaft of the humerus with the shaft of the
ulna (Fig. 13-15B).4,27,37 The metaphyseal-diaphyseal angle is determined by a line that longitudinally bisects the
shaft of the humerus with a line that connects the widest points of the metaphysis of the distal humerus (Fig. 13-15C).36
The humeral-ulnar angle is the most accurate in determining the true
carrying angle of the elbow. The Baumann angle also has a good
correlation with the clinical carrying angle, but it may be difficult
to measure in adolescents in whom the ossification center of the
lateral condyle is beginning to fuse with other centers. The
metaphyseal-diaphyseal angle is the least accurate of the three.44
The anterior dense line making up the teardrop represents the posterior
margin of the coronoid fossa. The posterior dense line represents the
anterior margin of the olecranon fossa. The inferior portion of the
teardrop is the ossification center of the capitellum. On a true
lateral projection, this teardrop should be well defined (Fig. 13-16A).
degrees between the long axis of the humerus and the long axis of the
lateral condyle (Fig. 13-16B). This can also be
measured by the flexion angle of the distal humerus, which is
calculated by measuring the angle of the lateral condylar physeal line
with the long axis of the shaft of the humerus.40
distal humeral shaft, it should pass through the middle third of the
ossification center of the capitellum. This is referred to as the anterior humeral line (Fig. 13-16C).
Passage of the anterior humeral line through the anterior portion of
the lateral condylar ossification center or anterior to it indicates
the presence of posterior angulation of the distal humerus. In a large
study of minimally displaced supracondylar fractures, Rogers et al.41 found that this anterior humeral line was the most reliable factor in detecting the presence or absence of occult fractures.
the coronoid process should barely touch the anterior portion of the
lateral condyle (Fig. 13-16D). Posterior displacement of the lateral condyle projects the ossification center posterior to this coronoid line.42
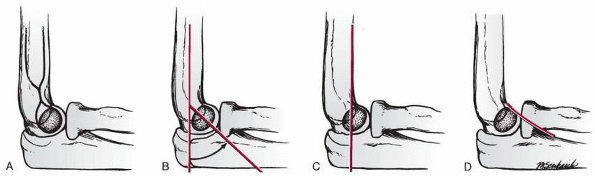 |
|
FIGURE 13-16 Lateral radiograph lines of the distal humerus. A. The teardrop of the distal humerus. B. The angulation of the lateral condyle with the shaft of the humerus. C. The anterior humeral line. D. The coronoid line.
|
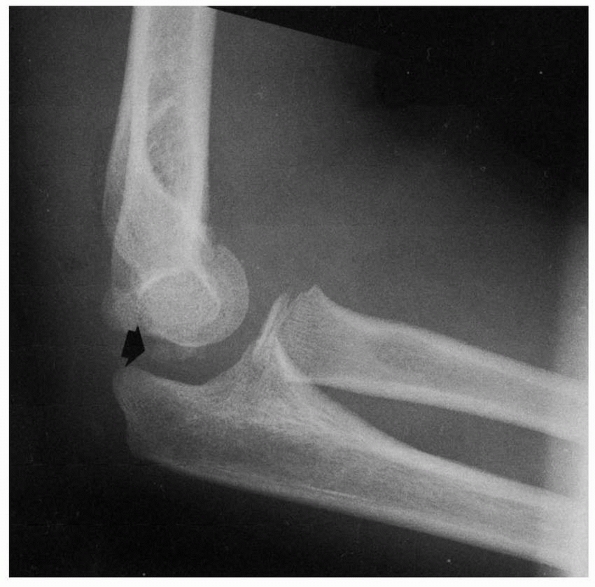 |
|
FIGURE 13-17
Pseudofracture of the elbow. The trochlea with its multiple ossification centers may be misinterpreted as fracture fragments lying between the joint surfaces (arrow). |
This fragmentation can be misinterpreted, especially if the distal
humerus is slightly oblique or tilted. These secondary ossification
centers may be mistaken for fracture fragments lying between the
semilunar notch and lateral condyle (Fig. 13-17).
lateral condyle and the distal humeral metaphysis is wider posteriorly.
This appearance may give a misinterpretation that the lateral condyle
is fractured and tilted.9
normally some lateral angulation to the radial border of the neck of
the radius that may give the appearance of subluxation (see Fig. 13-3).
The true position of the radial head can be confirmed by noting the
relationship of the proximal radius to the ossification center of the
lateral condyle on the lateral projection.41
major structures of the elbow. Displacement of any of the fat pads can
indicate an occult fracture. The first two areas are the fat pads that
overlie the capsule in the coronoid fossa anteriorly and the olecranon
fossa posteriorly. Displacement of either or both of these fat pads is
usually referred to as the classic elbow fat pad sign. A third accumulation of fat overlies the supinator muscle as it wraps around the proximal radius.
fossa is deep, the fat pad here is totally contained within the fossa.
It is not visible on a normal lateral radiograph of the elbow flexed to
90 degrees (Fig. 13-18A).
with an occult intra-articular fracture, a spontaneously reduced
dislocation, or even an infection, can cause the dorsal or olecranon
fat pad to be visible.48
Because the coronoid fossa is shallow, the fat pad in this area
projects anterior to the bony margins and can be seen normally as a
triangular radiolucency anterior to the distal humerus. Although
displacement of the classic elbow fat pads is a reliable indication of
an intra-articular effusion, there may be instances in which only one
of the fat pads is displaced. Brodeur et al.9 and Kohn31
have shown that the coronoid fat pad is more sensitive to small
effusions than the olecranon fat pad. It can be displaced without a
coexistent displacement of the olecranon fat pad (Fig. 13-18C).
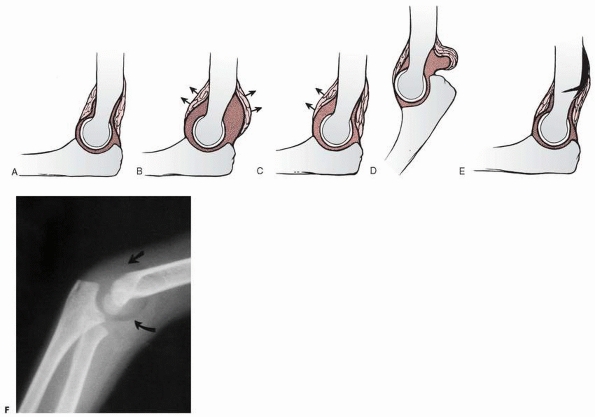 |
|
FIGURE 13-18 Radiographic variations of the elbow fat pads. A. Normal relationships of the two fat pads. B. Displacement of both fat pads (arrows) with an intra-articular effusion. C. In some cases, the effusion may displace only the anterior fat pad (arrows). D. In extension, the posterior fat pad is normally displaced by the olecranon. E. An extra-articular fracture may lift the distal periosteum and displace the proximal portion of the posterior fat pad. F. A radiograph showing displacement of both fat pads (arrows)
from an intra-articular effusion. (Modified and reprinted with permission from Murphy WA, Siegel MJ. Elbow fat pads with new signs and extended differential diagnosis. Radiology 1977; 124:656-659.) |
of the supinator muscle wraps around the proximal radius. This layer of
fat or fat pad may normally bow anteriorly to some degree. Brodeur et
al.9 stated that displacement may
indicate the presence of an occult fracture of the radial neck.
Displacement of the fat line or pad is often difficult to interpret; in
a review of fractures involving the proximal radius, Schunk et al.42 found it to be positive only 50% of the time.
the capsule must be intact. This can explain why there may be no
displacement of the fat pads with an elbow dislocation that has
spontaneously reduced due to capsule rupture. Murphy and Siegel36
described other variations of classic fat pad displacement. If the
elbow is extended, the fat pad is normally displaced from the olecranon
fossa by the olecranon (Fig. 13-18D). Distal
humeral fractures may cause subperiosteal bleeding and may lift the
proximal portion of the olecranon fat pad without the presence of an
effusion (Fig. 13-18E). These false-negative
and falsepositive findings must be kept in mind when interpreting the
presence or absence of a fat pad with an elbow injury.
elbow injuries indicated that if a displacement of the posterior fat
pad existed, a fracture was almost always present. Displacement of the
anterior fat pad alone, however,
could occur without a fracture. Corbett14 also determined that the degree of displacement bore no relation to the size of the fracture. Skaggs and Mirzayan43
reported that 34 of 45 children (76%) with a history of elbow trauma
and an elevated posterior fat pad had radiographic evidence of elbow
fractures at an average of 3 weeks after injury, although AP, lateral,
and oblique radiographs at the time of injury showed no other evidence
of fracture. They recommended that a child with a history of elbow
trauma and an elevated fat pad should be treated as if a nondisplaced
elbow fracture were present. Donnelly et al.,17
however, found evidence of fracture in only nine of 54 children (17%)
who had a history of trauma and elbow joint effusion but no
identifiable fracture on initial radiographs. They concluded that joint
effusion without a visible fracture on initial radiographs does not
correlate with the presence of occult fracture in most patients (83%).
Persistent effusion did correlate with occult fracture: 78% of those
with occult fractures had persistent effusions, compared with 16% of
those without fractures.
radiographs in a child with an injured elbow due to the difficulty
evaluating the irregularity of the ossification process, the
indications for ordering comparison radiographs are rare. Kissoon et al.30
found that using routine comparison radiographs in children with
injured elbows did not significantly increase the accuracy of
diagnosis, regardless of the interpreter’s training. Petit et al.39
reviewed 3128 radiographs of 2470 children admitted to a pediatric
emergency department for osteoarticular trauma and found that only 22%
of the radiographs revealed abnormal findings; 33.3% of elbow
radiographs revealed abnormalities. Fewer than half of clinically
suspected fractures were confirmed by radiograph.
used magnetic resonance imaging (MRI) to evaluate seven children who
had radiographs that showed effusion but no fractures; four of the
children had fractures identified by MRI. These investigators suggested
that an occult fracture is usually present when effusion occurs, even
if a fracture is not visible on radiograph. Griffith et al.21
reviewed the radiographs and MRI scans of 50 children with elbow
trauma. Radiographs identified effusions in 34% of the children and
fractures in 52%; MRI identified effusions in 96% and fractures in 74%.
Although MRI revealed a broad spectrum of bone and soft tissue injury
beyond that shown on radiographs (bone bruising, muscle and ligament
injuries, physeal injury, fracture), the additional information
provided by MRI had little influence on patient treatment and no value
in predicting clinical outcome. We have found MRI to be helpful in
evaluating articular fractures to identify fracture pattern and extent,
fragment position, and any interposed structure.
children with posttraumatic elbow effusions, but these can be painful
and invasive. The use of computed tomography (CT) in pediatric imaging
has been limited by the need for sedation, but the development of
multidetector CT (MDCT) technology allows examinations to be completed
in seconds, eliminating the need for sedation in most cases. MDCT
studies also can be reformatted and evaluated in multiple planes,
reducing the manipulation necessary for a series of radiographs.
Chapman et al.12 reported that, in a
series of 31 children with posttraumatic elbow effusion and normal
radiographs, MDCT depicted occult injuries in 52%. They cited as
additional advantages of MDCT the minimal manipulation required, making
it relatively easy and pain-free, the speed with which the image is
obtained, the lower radiation dose than conventional radiographs, and
its sensitivity (92%), specificity (79%), and high negative predictive
value (92%). A limitation of this method may be its high cost compared
to standard radiographic examination.
A, Reuben AD, Benger JR, et al. Elbow extension test to rule out elbow
fracture: multicentre, prospective validation, and observational study
of diagnostic accuracy in adults and children. Brit Med J
2008;337:a2428.
SP. The fat pad sign following elbow trauma: its usefulness and
reliability in suspecting “invisible” fractures. Clin Radiol
1970;21:90-94.
AE, Silberstein JJ, Graviss ER, et al. The basic tenets for appropriate
evaluation of the elbow in pediatrics. Current Prob Diag Radiol
1983;12(5):1-29.
J, Ishizue K, Gomez M, et al. Alteration of Baumann’s angle by humeral
position: implications for treatment of supracondylar humerus
fractures. J Pediatr Orthop 1993; 13:521-555.
V, Grottkau B, Albright M, et al. MDCT of the elbow in pediatric
patients with posttraumatic elbow effusions. Am J Roentgenol
2006;187:812-817.
JC, Wing-Man K, Shen WY, et al. A new look at the sequential
development of elbow-ossification centers in children. J Pediatr Orthop
1998;18:161-167.
MA, Vinson DR, Panacek EA. Preservation of active range of motion after
acute elbow trauma predicts absence of elbow fracture. Am J Emerg Med
2008;26:779-782.
MA, Schwab RA, John O. Can elbow extension be used a test of clinically
significant injury? South Med J 2002;95:539-541.
LF, Klostermeier TT, Klosterman LA. Traumatic elbow effusions in
pediatric patients: are occult fractures the rule? AJR 1998;171:243-245.
O. The normal development of the ossific centers during infancy and
childhood. Acta Paediatr Scand 1946;33(Suppl 1):1-79.
JF, Roebuck DJ, Cheng JC, et al. Acute elbow trauma in children:
spectrum of injury revealed by MR imaging not apparent on radiographs.
AJR Am J Roentgenol 2001; 176(1):53-60.
S. The intraosseous vasculature of the distal end of the humerus with
special reference to capitulum. Acta Orthop Scand 1957;27:81-93.
S. On osteochondrosis deformans juvenilis capituli humeri including
investigation of intraosseous vasculature in distal humerus. Acta
Orthop Scand 1959;Suppl 38:1-232.
CRE, Freeland P. Inability to fully extend the injured elbow: an
indicator of significant injury. Arch Emerg Med 1991;8:253-256.
S, Mehdi B, Larsen MS. The epidemiology of elbow fracture in children:
analysis of 355 fractures, with special reference to Supracondylar
humerus fractures. J Orthop Sci 2001;6(4):312-315.
E, Caterini R, Scola E. Supracondylar fractures of the humerus in
children. Analysis at maturity of 53 patients treated conservatively. J
Bone Joint Surg Am 1986; 68:333-344.
N, Galpin R, Gayle M, et al. Evaluation of the role of comparison
radiographs in the diagnosis of traumatic elbow injuries. J Pediatr
Orthop 1995;15:449-453.
A, Vlasis K, Siuampou E, et al. Can elbow-extension test be used as an
alternative to radiographs in primary care? Eur J Gen Pract
2007;13:221-224.
RI, Riayt MS, Hilliam R, et al. Can a normal range of elbow movement
predict a normal elbow x-ray? Emerg Med J 2007;24:86-88.
NM, Crawford ST. Elbow effusions in trauma in adults and children: is
there an occult fracture? AJR Am J Roentgenol 2002;178(2):413-418.
WR, Eilert RE, Chang FM, et al. The metaphyseal diaphyseal angle as a
guide to treating supracondylar fractures of the humerus in children.
Presented at 54th Annual Meeting of AAOS; San Francisco, CA; 1987.
P, Sapin C, Henry G, et al. Rate of abnormal osteoarticular
radiographic findings in pediatric patients. AJR Am J Roentgenol
2001;176(4):987-990.
E. Fracture of the lower end of the humerus in children: treatment and
end of the elbow in children. J Bone Joint Surg Am 1999;81:1429-1433.
DL, Mirzayan R. The posterior fat pad sign in association with occult
fracture of the elbow in children. J Bone Joint Surg Am
1999;81:1429-1433.
KE. Fractures and dislocations of the elbow region. In: Rockwood CA Jr,
Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia:
Lippincott-Raven, 1996: 653-904.
Z, Wang Y, Gilula LA, et al. Microcirculation of the distal humeral
epiphyseal cartilage: implications for posttraumatic growth
deformities. J Hand Surg Am 1998;23: 165-172.
