Cervical Decompression
in the cervical spine occurs in two main locations—centrally, where the
spinal cord or exiting nerve root (or both) can be impinged, or in the
foramen, where the exiting root can be impinged. Depending on whether
the involved structure is the spinal cord or the nerve root, clinical
symptoms consistent with myelopathy, radiculopathy, or both can result.
Surgical management of these problems requires skill at identifying and
decompressing the neural elements in these two regions. This chapter
reviews the indications and techniques—anterior and posterior—for
achieving cervical spine decompression.
cervical nerve root and manifests as a radiating pain from the neck
into the upper extremity in the distribution of the affected root. The
exact location and pattern of pain can vary widely, and a classic
distribution of pain frequently is absent. Associated sensory, motor,
or reflex disturbances may or may not be present.
-
First, soft disc herniations, depending on the location, may impinge the exiting nerve root (Fig. 26-1A) as it leaves the spinal cord or as it traverses the neuroforamen (Fig. 26-1B).
-
Second,
chronic disc degeneration can result in the formation of degenerative
osteophytes. Most commonly, these osteophytes arise from the uncinate
regions of the posterolateral vertebral body (uncovertebral
osteophytes) and compress the exiting nerve root as it enters the
neuroforamen (Fig. 26-1C). -
Third,
chronic disc degeneration can lead to disc height loss, which may
result in loss of foraminal height. The loss of height, combined with
superior migration of the superior facet joint, can lead to foraminal
compression.Fourth, hypertrophy of the
facet joints can cause foraminal encroachment, more commonly in the
upper rather than the lower cervical spine.
nerve root compression, including the anatomic locations in which
compression occurs, is crucial to performing safe and effective nerve
root decompression.
from anterior structures, such as disc herniations and uncovertebral
osteophytes. As a result, the anterior approach is preferred in most
cases of cervical radiculopathy. The decision must be tempered,
however, by an understanding of the advantages and disadvantages of
each approach. A major advantage of anterior decompression is that it
provides for direct access and removal of uncovertebral osteophytes and
herniated discs without the need for neural retraction. In contrast,
posterior cervical discectomy, with potentially good results in certain
situations, requires manipulation of the nerve root and potentially the
spinal cord. Although posterior foraminotomy can enlarge the foramen
through partial resection of the facet joint, accessing and removing an
uncovertebral spur from the posterior route can be difficult. It also
is difficult to restore foraminal or disc height from a posterior
approach, whereas an anterior bone graft can be placed readily to
restore height.
extremely low rates of infection or wound breakdown. If the incision is
placed transversely in the creases (Langer’s lines) of the neck, the
incision heals with a virtually imperceptible scar. The anterior
approach also requires little muscle dissection and tends to be
associated with less perioperative incisional pain than the posterior
approach, which requires subperiosteal dissection.
performed with an associated fusion. Doing the anterior fusion has
several potential advantages. Fusion may help relieve associated neck
pain, improve overall alignment of the neck, address instability, and
protect the decompression from recurrent disease. Placement of
structural bone graft in the
anterior
disc space also can restore foraminal height, providing indirect
decompression of the nerve root. Regaining disc height additionally
reduces infolding of the posterior longitudinal ligament and ligamentum
flavum, which can relieve root compression.
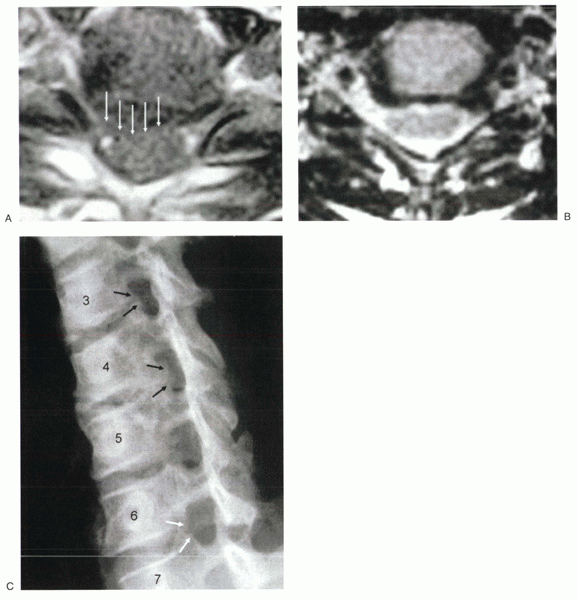 |
|
Figure 26-1 (A)
Large posterolateral disc herniation. The disc compresses the thecal sac and the exiting nerve root. A disc in this location would be difficult to access safely from a posterior approach. (B) Foraminal disc herniation, left side. (C) Uncinate hypertrophy. The uncinate spurs arise from the superior and the inferior vertebral bodies at C3-4 and C4-5, leading to foraminal narrowing (black arrows). This is a common mechanism for foraminal stenosis in the cervical spine. At C6-7 (white arrows), there is no foraminal stenosis and no uncovertebral osteophytes. |
anterior decompression come at a cost, including the potential for
accelerated adjacent segment degeneration. Pseudarthrosis also remains
a problem, particularly when multiple levels are performed. Other
potential disadvantages to the anterior approach include speech and
swallowing disturbances, which are encountered temporarily in virtually
every patient postoperatively and encountered permanently in a small
percentage. Airway obstruction also can occur, particularly after
multilevel anterior decompressions for myelopathy.
radiculopathy possess advantages to anterior decompression in selected
circumstances. The keyhole foraminotomy procedure can be used to
decompress the nerve root without significantly destabilizing the spine
and can be considered in cases of foraminal disc herniation or
stenosis. The key is to identify pathology on preoperative imaging
studies (e.g., magnetic resonance imaging [MRI] or computed
tomography-myelography) that is lateral enough to be accessible
posteriorly without necessitating spinal cord retraction and that can
be accessed through a laminoforaminotomy (see Fig. 26-1). Posterior discectomy can be performed after
laminoforaminotomy for foraminal disc herniations and potentially can
avoid the need for fusion. Posterior foraminotomy also can be effective
for treating high cervical radiculopathies (e.g., C2-3 or C3-4)
because, in contrast to the lower levels of the cervical spine,
foraminal stenosis at these upper levels more commonly arises from
facet overgrowth rather than uncovertebral hypertrophy. A major
advantage of the posterior foraminotomy is that it can be performed
with minimal patient morbidity. Because the procedure does not attempt
to restore disc height at the diseased level, however, a disadvantage
is the potential for deterioration of results with time if the
degenerative process continues at that level. Although fusion is not
routinely necessary with a posterior foraminotomy, if more than 50% of
both facet joints are resected to decompress the nerve root adequately,
posterior fusion with lateral mass plates or spinous process cables
should be considered.
decompressing the nerve root anteriorly or posteriorly. If a patient
has had prior surgery from one approach, it may be advantageous to
perform surgery from the opposite approach to avoid working through
scar tissue. A common salvage procedure for patients with persistent
radiculopathy after anterior cervical discectomy and fusion is
posterior foraminotomy. Alternatively a revision anterior procedure can
be performed with excellent results.
and spinal cord decompression, regardless of whether an anterior or
posterior approach is used. A high-speed bur is useful for removing
uncovertebral osteophytes and decorticating the end plates. A
self-retaining cervical retractor set allows anterior surgery to be
performed with minimal assistance, although care must be taken to
ensure proper placement of retractors and avoidance of prolonged
compression of vital neck structures, such as the esophagus, airway,
and carotid vessels. Some form of disc space distraction also is
helpful, such as vertebral body distraction pins or a small
self-retaining vertebral body spreader. Microcurets are mandatory to
remove osteophytes and disc herniations. Ideally, they should have a
thin footplate to avoid intrusion into the canal. Finally, to perform a
safe but complete and adequate neural decompression, high-quality
illumination and magnification are essential. I prefer to use an
operating microscope versus a headlight and loupes; the illumination
and visualization are superior, and the view obtained by the assistant
is equal to that of the operating surgeon.
gently. A head halter or Gardner-Wells tong is optional but not
routinely necessary. A doughnut pad is placed behind the occiput to
prevent pressure necrosis. The shoulders are taped down gently to
facilitate intraoperative radiographic visualization. Excessive force
should be avoided when taping to prevent brachial plexus injury. If a
patient has a short neck and a stocky body habitus, it may not be
possible to visualize the lower cervical segments radiographically. In
these situations, rather than taping the shoulders with excessive force
throughout the entire case, it is advisable to tape the shoulders more
loosely, then scrub out or have unscrubbed assistants briefly pull
longitudinally on the arms while radiographs are being taken. The
amount of preoperative cervical extension tolerated by the patient
determines the limit of initial intraoperative positioning. In
particular, excessive extension during positioning must be avoided in
patients with myelopathy.
text, but several points merit mentioning when performing anterior
cervical decompressions. The skin incision can be placed obliquely
anterior to the sternocleidomastoid or transversely in a major neck
crease. An oblique incision can be extensile and may be used for
multilevel surgery. In general, a transverse incision is preferred
because it heals much more cosmetically and still provides access to
multilevel pathology. A large incision placed in a crease heals more
cosmetically than a small incision placed outside of a crease. The
incision may extend from the anterior two thirds of the
sternocleidomastoid to beyond the midline. Because the skin in the
anterior neck tends to be highly mobile, multilevel access is possible
with a transverse incision if the tissue planes beneath the platysma
and in the interval medial to the sternocleidomastoid are undermined
and spread. The omohyoid, if it impedes access, can be divided with
minimal adverse consequences. Any crossing structures should be
preserved if possible so that inadvertent injury to the superior or
recurrent laryngeal nerves can be avoided. Crossing vessels can be
ligated, but the surgeon must be certain that the structure in question
is not a laryngeal nerve. After the appropriate levels have been
localized, the longus colli should be subperiosteally dissected
laterally such that the uncinates are visualized clearly. Exposure from
uncinate to uncinate is necessary because the lateral limits of the
decompression are determined relative to the position of the uncinates (Fig. 26-2).
The retractor blades are placed beneath the elevated longus colli. It
is preferable to place a toothed retractor blade under the longus colli
on the side ipsilateral to the carotid sheath (lateral). A smooth blade
is placed under the longus colli on the side epsilateral to the trachea
and esophagus (medial).
removed with a combination of curets and pituitary rongeurs.
Intervertebral body distraction pins can be placed to distract the disc
space gently and allow for better posterior visualization. A key
maneuver to facilitate disc space visualization and subsequent neural
decompression is to remove the anterior portion of the inferior end
plate of the superior vertebral body, particularly if the disc is
severely degenerated and the disc space is narrow (Fig. 26-3A).
This surface almost always is concave, and the anterior portion
overhangs the disc space, preventing direct visualization into the
posterior disc space if not removed. Removal can be done with either a
Kerrison
rongeur or a high-speed bur. Removing this portion often dramatically
improves access to the posterior disc space and provides a line of
sight that is parallel to the disc space (Fig. 26-3B);
this is crucial to performing the decompression, especially when using
a microscope. If a parallel view is not achieved, the surgeon may
become disoriented and stray away from the disc space into the body of
a vertebra. One way of maintaining a direct line of site is to fashion
the end plates such that they are parallel to each other. In effect,
the goal is to create a rectangular disc space by removing the
concavities naturally present in each vertebral body. Doing so also
facilitates optimal fit and contact between the end plate and graft or
cage. A posterior lip can be left in place to prevent posterior
impaction of the graft intraoperatively or subsequent posterior
displacement, if it does not impede adequate decompression.
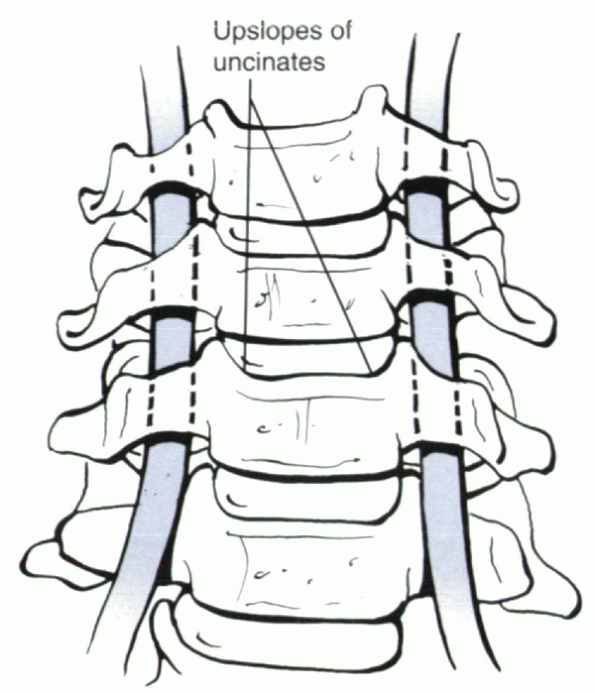 |
|
Figure 26-2
The longus colli should be elevated subperiosteally from uncinate to uncinate such that the upslope of each uncinate is identified clearly. Bipolar cautery is useful in doing this. The uncinates are the key anatomic landmarks for decompression. The vertebral artery is several millimeters lateral to the lateral border of the uncinate. If extreme lateral exposure is necessary, one can identify the lateral border of the uncinate by carefully placing a No. 4 Penfield dissector superiosteally around the lateral edge of the joint, protecting the vertebral artery. |
cartilaginous material and decorticated to reveal bleeding bony
surfaces. Alternating use of the high-speed bur, curets, and pituitary
rongeur allows the surgeon to reach the posterior disc space and the
posterior longitudinal ligament. Often, in an acute disc herniation,
there is a rent in the posterior longitudinal ligament readily seen
under the microscope. By carefully probing the posterior longitudinal
ligament with a microcuret, the extruded fragment can be gently “fished
out” of the spinal canal. Depending on the location of the disc
herniation, the exiting nerve root or spinal cord or both is visible.
Gentle probing posterior to the posterior longitudinal ligament can be
done until all loose fragments have been removed. The portion of the
posterior longitudinal ligament contralateral to the side of the disc
herniation does not routinely need to be removed. It is advisable,
however, to take down enough posterior longitudinal ligament carefully
on the side of the radiculopathy to confirm that the exiting nerve root
is free.
foraminotomy is necessary to treat foraminal stenosis. Direct anterior
foraminotomies have been considered unnecessary because of reports
indicating resorption of uncinate spurs after fusion, with subsequent
resolution of radiculopathy. I believe, however, that an anterior
foraminal decompression is important in consistently relieving
foraminal stenosis. Although foraminal height restoration is possible
with anterior grafting, this does not increase the anterior-posterior
dimension of the foramen, which often is narrowed by a posteriorly
protruding uncinate osteophyte (see Fig. 26-1C).
Although uncinate osteophytes may resorb over time, this process may
take months and is completely dependent on achieving a solid
arthrodesis, which does not happen in every case. Gratifying, immediate
relief of radicular symptoms can be achieved with direct anterior
foraminotomy, which is independent of achieving arthrodesis.
-
First,
orientation to the uncinates is essential because they represent the
lateral borders of a safe zone for working in the disc space without
injuring the vertebral artery (see Fig. 26-2). -
Second,
exposure and resection of the medial aspect of the uncinates is
important because osteophytes responsible for radiculopathy most
commonly arise from this region.
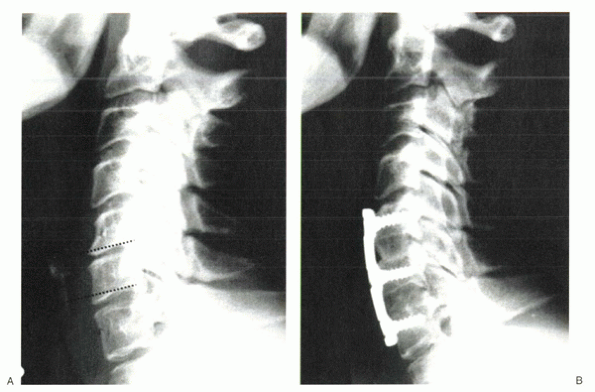 |
|
Figure 26-3 (A)
Spinal images of a 54-year-old woman with radiculopathy arising from C5-6 and C6-7 spondylosis. Severe disc degeneration at C5-6 impedes access to the posterior disc space, unless the inferior lip of C5 is resected (dotted line). A similar procedure should be considered at C6 to gain access to the posterior structures at C6-7 (dotted line). (B) Status-post two-level anterior cervical discectomy and fusion. The inferior lip at each level was resected, resulting in parallel disc spaces with parallel end plates. Doing so allowed visualization posteriorly for uncovertebral osteophyte resection and complete resolution of radiculopathy. The rectangular disc space created allowed intimate contact of the end plates with the bone graft. Because this patient did not have any central stenosis, it was not necessary to decompress the spinal cord. The posterior lips were left alone centrally at each level to protect against graft intrusion. |
discectomy has been performed to the level of the posterior
longitudinal ligament, and exposure of the uncinate has been achieved.
The medial aspect of the posterior uncinate is thinned under direct
visualization with a high-speed bur. Constant irrigation is performed
to prevent thermal injury and to clear away bone debris. If
visualization is adequate, continued thinning of the osteophyte can
progress until only a thin shell of bone is left. A microcuret is used
to resect the thinned osteophytes and the posterior longitudinal
ligament medial to the uncinate. This move allows visualization of the
exiting nerve root and the lateral edge of the dural sac and provides
access to the foramen. Using microcurets and the bur, the foramen can
be carved out gently and progressively to increase its
anterior-posterior diameter and to resect the posteriorly protruding
uncinate osteophytes. When entering the foramen, the curet should hug
the posterior aspect of the uncinate to avoid nerve root injury. In
addition, the exiting nerve root should be visualized during the
maneuver. Blind placement of a curet into the foramen is dangerous and
should be avoided. Foraminotomy is complete when a micro-nerve hook or
microcuret can be passed easily into the foramen anterior to the
exiting root without resistance. Bone grafting and plating are
performed if indicated.
necessary, it can be done by undercutting within the posterior one
third of the disc space, which usually is dorsal to the location of the
vertebral artery. In this manner, sufficient foraminal decompression
can be achieved safely laterally at the level of the disc space while
reducing potential risks of vertebral artery laceration. Because the
vertebral artery typically lies in the anterior two thirds of the disc
space, undercutting within the posterior one third usually does not put
the artery at risk (Fig. 26-4). Going out
further laterally in the anterior two thirds of the disc space can be
dangerous, however. When curetting disc material in this area,
vertebral artery laceration might occur if the curet strays lateral to
the lateral border of the uncinate. This event is more likely to occur
when the disc space is under distraction because
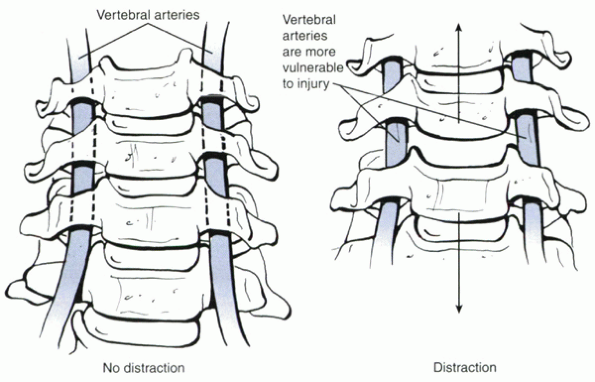
this reduces the protective overhang between adjacent level uncinates (Fig. 26-5).
In all cases, to minimize the incidence of vertebral artery
complications, one should scrutinize preoperative MRI or computed
tomography to rule out the presence of an anomalous vertebral artery
coursing medial to its usual position within the intertransverse
foramen and lateral to the lateral border of the uncinate. If anomalies
are suspected, angiography should be considered preoperatively.
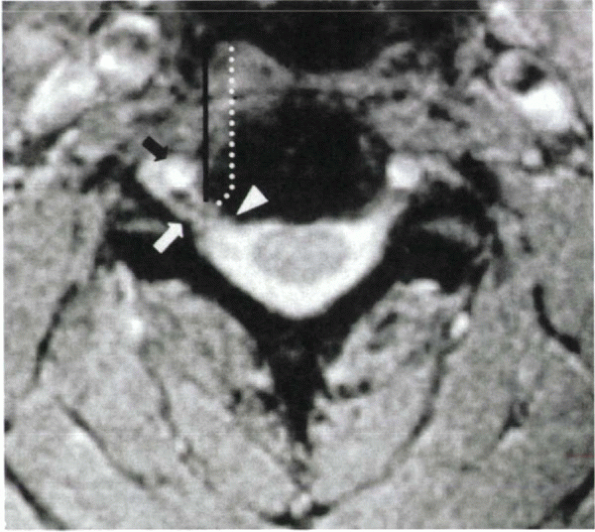 |
|
Figure 26-4 The vertebral artery (black arrow)
typically lies in the anterior two thirds of the disc space. It is ventral to the usual location of nerve root compression (i.e., at the level of the posterior uncinate [white arrowhead]). The lateral border of the uncinate marks the lateral safe zone (black line). To decompress the nerve root laterally in the foramen (white arrow), while avoiding injury to the vertebral artery, the trajectory of decompression should course laterally in the posterior aspect of the disc space (dotted line). |
 |
|
Figure 26-5
When the disc space is distracted, the protective overhang of the uncinates on the vertebral artery is reduced, making the artery more susceptible to laceration by a stray curet, bur, or other instrument. It is crucial to control carefully all instruments passed into the disc space during anterior cervical surgery. |
the same neurologic and spinal cord monitoring considerations apply
when positioning patients for posterior surgery. The amount of
preoperative extension tolerated should be assessed, and consideration
should be given to using awake fiberoptic intubation and spinal cord
monitoring for patients with concomitant myelopathy. In addition to
potential neurologic sequelae, excessive extension may make posterior
foraminotomy technically more difficult to perform because of the
increased overlap between adjacent laminae, which results with relative
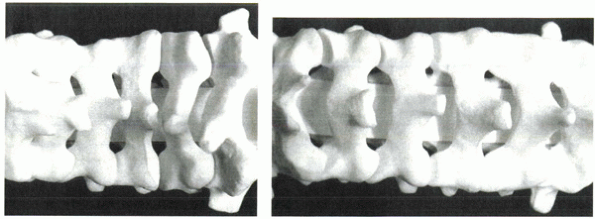
neck extension versus flexion (Fig. 26-6).
Flexion of the neck is helpful if an isolated foraminotomy without
fusion is being performed. If an associated fusion is necessary,
however, excessive flexion during positioning may lead to undesirable
kyphosis over the instrumented segments. In these situations,
relative—although not excessive—extension is preferred, or the neck can
be repositioned into lordosis after decompression.
positioning also can lead to excessive bleeding. The abdomen must be
free of compression. Putting the patient into reverse Trendelenburg’s
position reduces venous pressure in the neck and bleeding. Although
bleeding from posterior cervical operations is rarely of the magnitude
to cause hemodynamic compromise, undue bleeding may hinder
visualization
during
foraminotomies. Usually, if the patient is positioned properly,
foraminal bleeding can be controlled readily with the brief application
of absorbable gelatin sponge (Gelfoam) and cottonoids. In contrast, if
the patient is not positioned properly and venous pressure is unduly
high, foraminal bleeding may obscure safe visualization during
foraminotomy despite attempts at hemostasis.
 |
|
Figure 26-6 When the neck is in relative extension (A), there is increased overlap of the facets and laminae, leading to shingling of the laminae. In relative flexion (B), the facets and laminae overlap less.
|
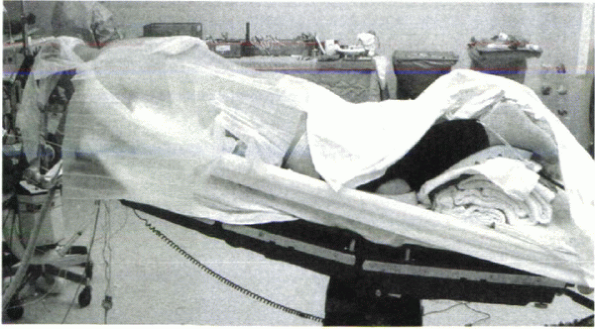
for prone cervical surgery. A horseshoe headrest is not recommended
because of the possibility for pressure necrosis on the eyes and face.
Two longitudinal bolsters are placed (one on each side of the body)
spanning the chest and abdomen to allow the abdomen to hang free. The
shoulders are taped down, with care being taken to avoid excessive
traction. When positioning a patient into reverse Trendelenburg’s
position, care also must be taken to avoid the inadvertent application
of traction on the neck and spinal cord. This traction can occur with
the use of tongs (especially Mayfield) because the head is relatively
fixed, whereas the body may slide caudally owing to gravity. This
problem can be avoided by flexing the table at the knees (Fig. 26-7).
A sling placed behind the buttocks with an upward-directed vector also
can be beneficial in this regard. Alternatively, seated, awake
positioning for surgery has been described for performing posterior
foraminotomies. Air embolism is a potential risk, however.
 |
|
Figure 26-7 A properly positioned patient for posterior cervical surgery.
|
invasive fashion for nerve root decompression without fusion.
Alternatively, it can be done in conjunction with a fusion,
laminectomy, or laminoplasty. The amount of exposure necessary depends
on the procedure being performed. Laminoforaminotomy alone can be done
with unilateral muscle dissection and a small incision. Fusion,
laminoplasty, or laminectomy requires bilateral exposure and a larger
incision. The surgical approach is described elsewhere in this text. To
limit muscle bleeding and perioperative neck pain, it is important to
stay in the midline raphe during the approach to the spinous process,
then maintain a strict subperiosteal plane as dissection proceeds
laterally. It also is necessary to avoid injury to the facet capsules
of uninvolved levels.
facet joint can be removed without risking iatrogenic instability when
performing foraminotomies. Because the first priority should be to
achieve satisfactory nerve root decompression, consideration should be
given to concomitant fusion if more facet resection is necessary. In
most cases, adequate neural decompression is possible, however, without
sacrificing stability.
under constant irrigation is used to outline the margins of the
foraminotomy. In most cases, the initial bur hole can be centered over
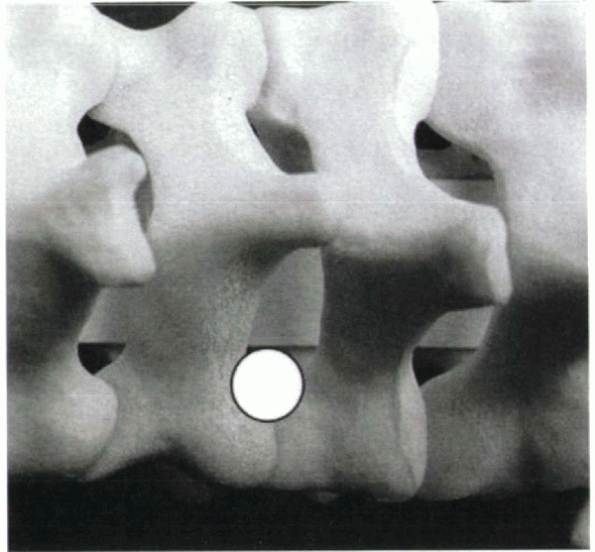
the lamina-lateral mass junction (Fig. 26-8).
The inferior facet of the level above (e.g., the C5 facet when
performing a C5-6 foraminotomy) is not the offending structure
narrowing the foramen but is an “obstacle” to access of the true
culprit—the superior facet of the level below (e.g., the C6 facet).
Because the inferior facet is “protected”
by
the more anteriorly positioned superior facet, initial burring through
the inferior facet can be quick and relatively aggressive. When the
superior facet is reached, the pace of burring should proceed more
slowly. Bleeding is common during foraminotomy, but can be controlled
with Gelfoam and pledgets. Burring should continue until the anterior
cortex of the superior facet has been reached. This landmark can be
seen under the microscope as the bone changes color and takes on the
gray-blue tinge of the nerve root and dura. When the superior facet has
been thinned in this manner, a microcuret can be introduced over the
nerve root to lift away the thinned bone. As the hole is enlarged, a
micro-Kerrison rongeur is introduced to decompress the nerve root
progressively. The bone at the lateral mass-lamina junction also should
be removed to visualize the takeoff of the exiting root from the spinal
cord. Decompression is judged complete when a nerve hook can be passed
into the foramen without resistance beyond the lateral border of the
pedicle. A fusion, laminectomy, or laminoplasty can be performed if
necessary.
 |
|
Figure 26-8
Approximate area of bone resection during laminoforaminotomy. The superior, not inferior, facet represents the posterior bone wall of the foramen. Because the nerve root in a sense is protected by the superior facet during drilling, removal of the inferior facet can be done with appropriate speed and aggressiveness. When the foramen has been entered by removing the circled portion of the facet joint, undercutting can be done laterally if necessary with a micro-Kerrison rongeur or curet. |
surgical approach for treating cervical myelopathy. Anterior and
posterior approaches can improve neurologic function, and each has its
advantages and disadvantages. Anterior decompression directly relieves
spinal cord compression due to the most common impinging structures,
such as herniated discs, spondylotic bars, and ossification of the
posterior longitudinal ligament. Stenosis with kyphosis, which in some
cases can contribute to compression because the cord is draped over the
posterior vertebral body osteophytes, is approached better anteriorly.
Posterior laminoplasty avoids fusion and its attendant graft-related
complications and complications related to the anterior approach. It
can be performed quickly and with minimal blood loss. Because the major
offending structures impinging on the spinal cord tend to arise
anteriorly, laminoplasty, in contrast to anterior decompression
techniques, relies primarily on an indirect decompression as the cord
floats away posteriorly. In most cases, the cervical spine should be
relatively lordotic or at least neutral for the spinal cord to be able
to drift posteriorly and the desired decompressive effect to be
obtained with laminoplasty.
principles in selecting the appropriate approach. If myelopathy is
arising from one or two levels, anterior decompression and fusion
typically is preferred. If pathology exists at three levels, either
anterior decompression or laminoplasty can be considered. If more than
three levels are involved, laminoplasty is preferred because of its
relative simplicity and lack of need for a long strut graft. In
patients who are medically frail or have poor potential for healing a
fusion (e.g., smokers, diabetics, steroid users), laminoplasty may be
favored. Laminoplasty also may be preferable in severe cases of
ossification of the posterior longitudinal ligament to avoid
complications related to dural deficiencies and dural tears with an
anterior approach. If a patient has a substantial amount of neck pain,
a fusion should be considered, either anteriorly or posteriorly, in
conjunction with laminectomy or laminoplasty.
because laminoplasty cannot undrape the cord over a kyphos. The absence
of lordosis is not an absolute contraindication to laminoplasty,
however. In patients showing compressive lesions the presence of
arising posteriorly, laminoplasty can achieve a direct decompressive
effect despite kyphosis (Fig. 26-9). Also, in
kyphotic patients with extremely tight cervical stenosis, laminoplasty
can be considered as a first-stage operation. If the clinical
circumstances indicate and postoperative imaging studies show
persistent compression after laminoplasty, a staged anterior
decompression and fusion of selected levels can be performed at a later
date. In these situations, even if the cord drifted only a few
millimeters posteriorly after laminoplasty and persistent anterior
compression exists, the anterior decompression now may be less risky
because of the amount of space gained. Fewer levels now may need
anterior decompression. One of the unsolved complications of
laminoplasty, however, remains segmental root level palsy (especially
C5), which may arise in 5% to 12% of cases.
the facet joints without fusion is not recommended because of the risk
of instability and postlaminectomy kyphosis. Neurologic function may
improve early on after laminectomy but can deteriorate over time if the
neck becomes kyphotic. For this reason, laminoplasty is preferred to
multilevel laminectomy. Studies have indicated superior outcomes
with laminoplasty over laminectomy, even with fusion.
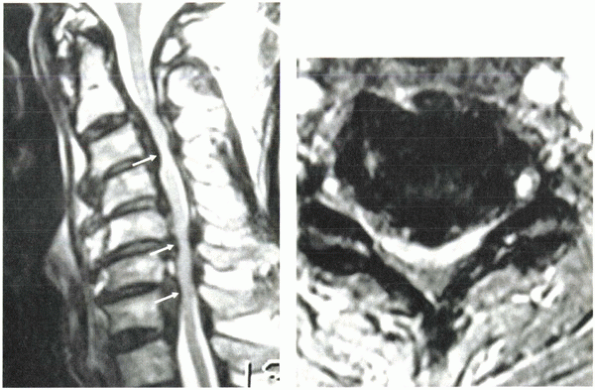 |
|
Figure 26-9 (A and B)
Spinal images of a 78-year-old man with severe insulin-dependent diabetes, chronic renal failure, stroke, and chronic obstructive pulmonary disease, who presented with a 1-year history of progressive myelopathy rendering him nonambulatory. Circumferential stenosis due to severe multilevel ossification of the posterior longitudinal ligament and congenital stenosis is noted at multiple levels (arrows) on the sagittal image (A). Cord signal change is evident at C3-4. Axial image (B) also shows circumferential stenosis. Despite his cervical kyphosis, a laminoplasty was performed because of his tenuous medical status and the presence of circumferential stenosis. Even if the cord fails to drift posteriorly completely, some degree of decompression is achieved by relieving the dorsal compression. A staged anterior decompression and fusion can be done after obtaining postoperative MRI, if necessary. |
on myelopathic patients, regardless of whether the surgery is performed
anteriorly or posteriorly. Excessive extension during positioning must
be avoided in these patients because extension diminishes the space
available in the spinal canal and can cause cord compression
intraoperatively. Awake, fiberoptic intubation should be considered to
avoid excessive extension and potential spinal cord injury during
intubation. Spinal cord monitoring is recommended when operating on
patients with myelopathy and is optional when operating on
nonmyelopathic patients. A baseline set of data should be obtained
after intubation, then compared after positioning to ensure that the
patient has been positioned safely.
discectomy. Placement of Gardner-Wells tongs with mild traction (5 to
10 lb) is recommended to stabilize the head and neck when doing
corpectomies. The corpectomy is performed after the anterior exposure,
discectomy, and foraminotomy (if necessary) as described earlier.
Completing the discectomies first allows the surgeon to estimate the
depth of the corpectomy. The width of the corpectomy required is the
width of the spinal cord and should be estimated based on preoperative
imaging studies. It rarely is necessary to extend lateral to the
uncinates, and the uncinates are again a key surgical landmark. It is
imperative to analyze carefully the course of the vertebral arteries on
preoperative imaging studies to ensure that they do not traverse medial
to their usual location.
a large Leksell rongeur can be used to remove large portions of the
vertebral body. This bone also is useful for subsequent grafting. Under
direct visualization, a high-speed bur is used to remove bone until a
thin shell of posterior cortex remains. Microcurets are used to flake
off the remaining bone. When choosing a location to enter the spinal
canal, it may be safer to find a plane laterally, where there is
usually more room, then proceed centrally. Extreme caution must be
observed when performing a corpectomy over regions with ossification
of
the posterior longitudinal ligament. If severe, the dura may be
deficient or absent, and the surgeon should be prepared to place a
dural patch and insert a lumbar drain. Instead of removing the entire
ossification of the posterior longitudinal ligament, an alternative
technique is the “floating method,” in which the adherent ossification
of the posterior longitudinal ligament fragment is detached laterally
where it is not adherent to the dura, then allowed to float away
anteriorly with the spinal cord. Fusion and plating are performed.
-
Open door
-
French door (T-saw)
-
Z-plasty
positioned in the same manner as for posterior cervical foraminotomy.
Overall neutral or slightly flexed alignment of the cervical spine
facilitates opening of the laminoplasty by diminishing the shingling
effect of the C2 lamina on the C3 lamina. If the neck is too extended,
the overlap of the C2 lamina onto C3 prevents opening of C3, unless the
inferior portion of C2 is removed. For a standard C3-7 laminoplasty,
exposure should be performed from the inferior edge of the C2 lamina to
the superior portion of T1. Some authors believe that the nuchal
attachments onto the C2 spinous process should be preserved or, if
necessary, removed with a piece of bone to facilitate later
reattachment and prevention of postoperative kyphosis. The facet joints
should be preserved—only the medial aspect of the joint needs to be
exposed.
side is created first using a high-speed bur. The hinge is located at
the junction of the lamina with the lateral mass. Only the posterior
cortex needs to be removed. To preserve the “springiness” of the hinge,
aggressive removal of bone from the hinge side should be avoided. The
thickest portion of the lamina is always at the cephalad end. Because
of the shingling effect, it is common for the cephalad portion of the
lamina to be covered by the caudal portion of the lamina above. If the
hinge fails to give, it almost always is due to inadequate posterior
cortical bone removal at the cephalad portion of the lamina.
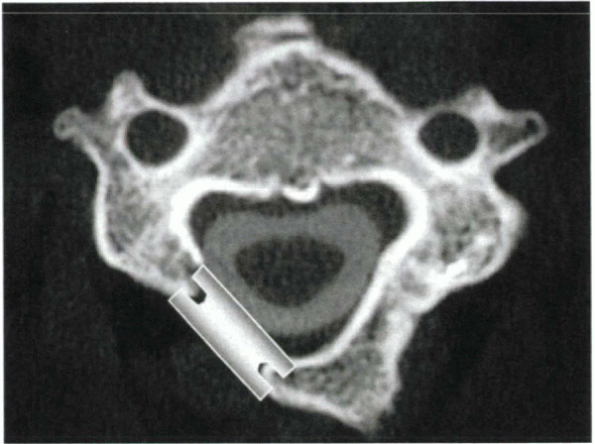 |
|
Figure 26-10 (A)
During initial burring of the posterior cortex, the bur can be oriented vertically. If this course continues, however, the bur tends to carve the lateral mass rather than enter the spinal canal. (B) To enter the spinal canal, the bur subsequently should be oriented perpendicular to the lamina, at approximately a 45-degree angle. |
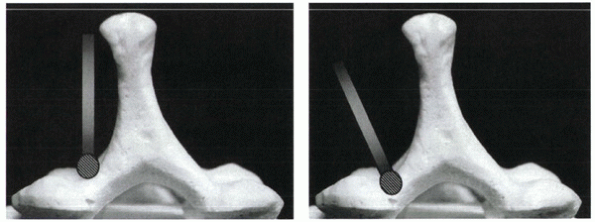 |
|
Figure 26-11
One method of maintaining an open-door laminoplasty. A clothespin-shaped graft is wedged between the lateral mass and the cut edge of the lamina. If the springiness of the hinge side has been maintained, excellent stability of the graft can be achieved, and additional fixation is not necessary. |
of greatest compression or clinical symptoms. A high-speed bur is used
to remove the posterior cortex at the junction of the lateral mass with
the lamina. If burring is performed too medially, there may be a
portion of the spinal cord that is not uncovered after opening the
hinge. If burring occurs too laterally, the surgeon enters the lateral
mass rather than the spinal canal and is not able to open the lamina.
During
the initial passes of the bur, it should be held vertically (Fig. 26-10).
Subsequent burring should be done at approximately a 45-degree angle,
however, so that the bur enters the canal rather than burrowing into
the lateral mass. The bur is used to thin the lamina to a flake of
anterior cortex. At this point, a microcuret or micro-Kerrison rongeur
can be used to remove the remaining bone. Extreme care must be
exercised to avoid spinal cord injury during this maneuver.
C7-T1 interspinous ligaments are resected. The surgeon then firmly, but
gently, pushes on the spinous processes from the open side, creating
greenstick fractures on the hinged side. After the laminoplasty is
opened, dural pulsations can often be seen. Epidural bleeding, if
encountered, can be controlled with gentle application of Gelfoam or
bipolar cautery. If the hinge does not yield and the laminoplasty does
not open, further burring may be necessary, usually of the cephalad
portion of the lamina. If overlap of C2 on C3 prevents opening of C3, a
dome osteotomy of the inferior portion of C2 may be necessary. A C2
dome osteotomy also may allow for greater posterior spinal cord drift
in patients with cervical kyphosis. Ideally, each lamina should open
with a “springy” sensation. Complete fractures on the hinge side should
be avoided if possible, especially if allograft rib struts are used to
keep the laminoplasty open, because the struts would not lock in as
firmly. The rib struts typically are 12 to 14 mm in height and are
fashioned with grooves that lock in to the lateral mass on one side and
the cut edge of the lamina on the other (Fig. 26-11).
The tension of the greenstick fracture keeps the struts in place, and
typically no supplemental fixation is necessary. Three struts placed at
C3, C5, and C7 are recommended. Alternatively, sutures, suture anchors,
or miniplates can be used to keep the laminoplasty open (Fig. 26-12).
Regardless of the method used, the objective is to keep the
laminoplasty open until the greenstick fracture heals on the hinge
side. Premature closure of the laminoplasty may result in inadequate
spinal cord decompression.
|
Figure 26-12
Status-post open-door laminoplasty with rib struts at C3, C5, and C7 and mini-laminoplasty plates at C4 and C6. This degree of door stabilization typically is not necessary. Note the cut edge of the laminae seen en face and the widening of the spinal canal resulting from this procedure. |
R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine
surgery: a prospective study. Spine 2000;27:2453-2458.
LJ, Mason HC, Bohlman HH, Yoo JU. Tortuous course of the vertebral
artery and anterior cervical decompression: a cadaveric and clinical
case study. Spine 2000;25:2860-2864.
CC, Heller JG, Murakami H. Corpectomy versus laminoplasty for
multilevel cervical myelopathy: an independent matched-cohort analysis.
Spine 2002;27:1168-1175.
JP, Kitchen ND, Moore AJ, Marsh HT. Results of posterior cervical
foraminotomy for treatment of cervical spondylitic radiculopathy. Br J
Neurosurg 2000;14:40-43.
I, Kurosa Y, Matuoka T, Shindo S. Anterior floating method for cervical
myelopathy caused by ossification of the posterior longitudinal
ligament. Clin Orthop 1999;359:27-34.
TA, Bohlman HH. Cervical kyphosis and myelopathy: treatment by anterior
corpectomy and strut-grafting. J Bone Joint Surg 1989;71A:170-182.
