Thoracolumbar Decompression: Anterior and Posterior
relieve compression of the neural elements without causing further
iatrogenic neurologic injury. A secondary consideration to
decompression is avoiding unnecessary destabilization of the spine.
There are numerous thoracolumbar spine pathologies that may result in
neural compression, including the following:
-
Trauma
-
Tumor
-
Infection
-
Deformity
-
Degenerative conditions (e.g., spinal stenosis or herniated disc)
chapter, each with unique advantages and disadvantages. In general, the
choice of decompressive procedure and surgical approach is dictated by
the location of the neural compressive pathology. Factors such as
spinal stability and alignment, medical comorbidities, history of prior
surgeries, and surgeon expertise all may play a role, however, in the
selection of a particular approach to decompression.
must have an intimate knowledge of spinal anatomy and biomechanics. The
surgeon should minimize the removal of uninvolved structures during
decompression that unnecessarily might compromise spinal stability. In
situations in which decompression renders the spine unstable, the
surgeon must be prepared to proceed to a stabilization procedure.
the vertebral body and adjacent intervertebral discs to effect
decompression of the neural elements. To perform a corpectomy, the
spine is approached through the thoracic or abdominal cavity or via the
retroperitoneal space, depending on the level of the offending
pathology.
is prescribed for the treatment of pathology located in the ventral
spinal canal, most commonly the result of burst fracture, tumor, or
infection. Corpectomy facilitates direct decompression of the anterior
neural elements and avoids manipulation and retraction of neural
tissue, as might be necessary to remove anterior compressive pathology
through a posterior approach. Particularly above the level of the cauda
equina, any manipulation of the spinal cord should be avoided to
prevent iatrogenic injury. When considering corpectomy for
decompression, the surgeon must consider the feasibility of
postcorpectomy reconstruction of the spine to ensure stability.
compression is caused by the posterior structures of the spine (i.e.,
from the lamina, facet joints, ligamentum flavum, or posterior abscess
or tumor). The ability to perform an anterior approach and corpectomy
also may be limited by prior abdominal or thoracic surgery. Resultant
scarring can make dissection and exposure difficult. In the lower
lumbar spine, anterior corpectomy and subsequent reconstruction is
technically challenging because of the intimate anatomic relationships
of the major vessels to the vertebral bodies.
underlying pathology necessitating the corpectomy. The decision to
proceed with an anterior corpectomy for decompression is usually the
result of identifying neural compression arising anterior to the neural
elements. A careful history and thorough neurologic examination are
essential to define the
patient’s
neurologic status. Laboratory studies may be helpful when considering a
diagnosis of infection or tumor. In general, plain films are useful to
determine spinal alignment, identify and classify vertebral fractures,
and understand the extent of vertebral body destruction. The health of
adjacent vertebrae, which may have implications for reconstruction
strategies, also should be assessed. Computed tomography
(CT)-myelography is valuable for identifying the extent of neural
compressio n and determining whether bone or soft tissue is the
offending pathology. The excellent bone visualization afforded by CT
also allows for evaluation of the extent of bone disruption or
destruction. Magnetic resonance imaging (MRI) allows for excellent soft
tissue visualization and is helpful in evaluating marrow replacement
processes, disc pathology, and visualization of the neural elements.
be medically fit to undergo a transthoracic, transabdominal, or
retroperitoneal surgical approach. If an approach through the thoracic
cavity is planned, the patient’s pulmonary function should be evaluated
preoperatively. If corpectomy is being performed to address a tumor,
preoperative tumor embolization may help reduce surgical blood loss.
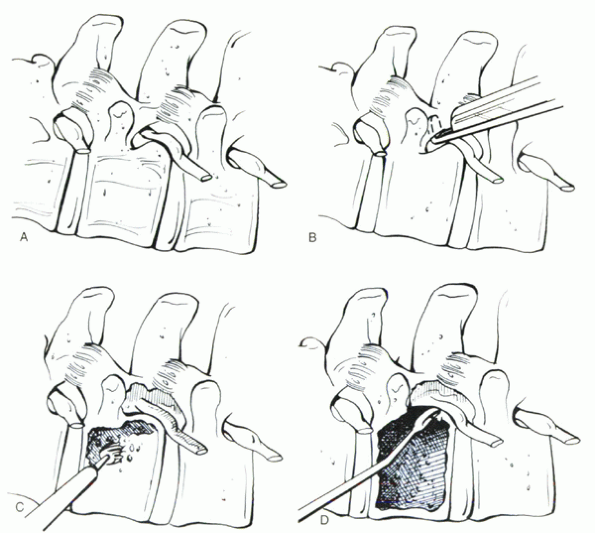 |
|
Figure 27-1 Technique of anterior thoracolumbar corpectomy. (A)
Exposure of the involved vertebral level. The pedicle and associated exiting nerve root and foraminal vessels are identified. In the thoracic spine, resection of the rib head facilitates visualization of the pedicle. (B) The caudal portion of the pedicle is removed with a Kerrison rongeur. This allows for visualization of the neural elements and floor of the spinal canal. (C) After removal of the intervertebral discs cephalad and caudad to the involved vertebra, the vertebral body is removed with a Leksell rongeur and high-speed bur. The posterior vertebral body cortex is maintained to protect the neural elements until the final stage of decompression. (D) Using a reverse-angle curet, the remaining shell of the posterior vertebral body cortex is collapsed into the vertebral body defect (arrow), decompressing the neural elements. |
In general, anterior access to the T4-10 vertebral bodies requires a
thoracotomy, whereas exposure of T11-S1 levels typically is
accomplished through a retroperitoneal approach. Access to the lower
lumbar spine also may be achieved via a transperitoneal anterior
approach. Intraoperative radiographs are essential to identify the
appropriate vertebral level. When the vertebral body is exposed, the
segmental vessels at the level of the pathologic vertebra are ligated.
The pedicle of the involved vertebra serves as an important landmark
for orientation
with
regard to the location of the spinal canal and should be identified. In
the thoracic spine, the rib head may be excised to facilitate
visualization of the underlying pedicle of the involved vertebra.
Resection of the pedicle or part of the pedicle with a Kerrison rongeur
allows visualization of the neural elements and identification of the
spinal canal. During resection of the pedicle, the exiting nerve root
and foraminal vessels that hug the caudal aspect of the pedicle should
be protected. A discectomy at the disc above and below the involved
vertebra is performed. This procedure may be facilitated by
subperiosteal elevation of the disc from the end plates. An attempt
should be made to perform as complete a discectomy as possible,
including removal of contralateral (far-side) disc material, which
helps facilitate complete corpectomy. During discectomy, care should be
taken to preserve the integrity of the vertebral end plates.
or rongeurs, which allow for collection of corpectomy bone that may be
used for later bone grafting (e.g., with a burst fracture) or culture
and biopsy (e.g., tumor or infection). During vertebral body removal,
the posterior vertebral body cortex is maintained to protect the neural
elements until the final stages. The corpectomy is completed from
ventral to dorsal with a high-speed bur until a thin shelf of posterior
vertebral cortex remains. The remaining thin cortical shelf can be
collapsed into the corpectomy defect using reverseangle curets with
minimal pressure exerted on the adjacent neural elements. The
decompression should be continued across the entire width of the
vertebral body until the far-side pedicle can be palpated, to ensure
complete decompression. Unless a total corpectomy is indicated, such as
for a primary bone malignancy, the far-side lateral vertebral body wall
may be maintained.
with reconstruction of the anterior column. This reconstruction usually
involves placement of a strut spanning the corpectomy defect, which may
be stabilized by spinal instrumentation.
48 hours or until drains are removed. If the anterior approach was
accomplished through a thoracotomy, a chest tube often is placed, and
this needs to be managed postoperatively. Attention should be directed
toward pulmonary toilet after thoracotomy. Patients may sit and
ambulate at a graduated pace as comfort and strength permit after
surgery. Based on the stability of the postcorpectomy reconstruction, a
brace may be required. In cancer patients, adjuvant radiation therapy
should be delayed at least 3 weeks to allow soft tissue healing and
initial fusion healing.
decompressing the neural elements. In most cases in which degenerative
pathology gives rise to neural compression, the posterior approach
provides effective decompression. When indicated, posterior
decompression can be combined with posterior instrumentation.
compression of the neural elements originating from the posterior
elements of the spine. Specific indications for decompressive
laminectomy include:
-
Spinal stenosis (degenerative or congenital)
-
Tumors involving the posterior bone elements
-
Fractures of the posterior bone elements
-
Large herniated lumbar discs, particularly when centrally located
-
Epidural hematoma or abscess
compressive pathology is located anterior to the neural elements,
laminectomy is less successful in effecting decompression than direct
anterior decompression. In the presence of kyphosis, posterior
decompression (laminectomy) also may be less effective. Laminectomy has
not been shown to be effective in the treatment of axial low back pain.
of disc degeneration, and spinal alignment and stability. In addition,
if short pedicles are seen, congenital spinal stenosis might be
suspected. Radiographs remain the primary imaging modality on which a
diagnosis of spinal instability or deformity necessitating arthrodesis
surgery is based. When considering surgery, CT-myelography and MRI may
be considered complementary studies. CT-myelography provides detailed
bone anatomy and delineates the extent and location of neural
compressive pathologies. MRI with its excellent soft tissue detail
allows direct visualization of the neural elements and further
assessment of neural compression.
in a prone position. A kneeling position (hips and knees flexed) helps
increase the interlaminar spaces, which is helpful during lumbar
decompression. This position reduces lumbar lordosis, however, which
should be considered during instrumented fusions. The abdomen should be
free of compression to reduce epidural bleeding. All bone prominences
should be well padded. Standard posterior midline exposure of the spine
is performed. The facet joints and pars interarticularis should be
visualized clearly so that these structures can be preserved to
maintain spinal stability. Before bone decompression, the anatomic
level should be confirmed with radiographs. The spinous process and
interspinous ligaments are removed as necessary. The laminae are
removed in piecemeal fashion with a 45-degree Kerrison rongeur,
starting in the midline, where a space beneath the lamina typically
exists even in the presence of severe spinal stenosis. In areas of
thick bone where large instruments
may
cause injury to the underlying neural structures, the lamina may be
thinned using a Leksell rongeur or a high-speed bur before bone
removal. If severe stenosis exists, working from less severely involved
levels toward the most severely stenotic areas reduces the likelihood
of iatrogenic neural injury.
extent of decompression. Identifying the pedicle from within the spinal
canal helps facilitate locating the neural foramina and the takeoff and
path of the exiting nerve roots. Bone ledges adjacent to the pedicles
should be removed to decompress the lateral recess. When the shoulder
of the nerve root is decompressed adequately, the neural foramen must
be inspected meticulously for stenosis. When performing foraminotomy,
the exiting nerve root should be visualized and protected. To minimize
the risk of cutting across the nerve root, the Kerrison rongeur should
be advanced into the foramen parallel to the exiting nerve root. The
foramen is decompressed by undercutting the facet joint. Adequacy of
the foraminal decompression may be assessed visually and by palpation
of the foramen with an angled instrument, such as a Penfield elevator.
At least 50% of the facet joint and the pars interarticularis should be
preserved to avoid creating iatrogenic instability (particularly if not
performing a concomitant arthrodesis). Discectomy in the presence of a
laminectomy may result in instability. If decompression renders the
spine unstable, the surgeon should be prepared to proceed with
arthrodesis.
antibiotics are administered. Early postoperative ambulation and
resumption of usual activities are encouraged. If concomitant
arthrodesis is performed, postoperative rehabilitation and activity
level usually are dictated more strongly by the type and extent of
arthrodesis procedure performed. Back strengthening and rehabilitative
exercises may be recommended postoperatively.
the treatment of radiculopathy caused by lumbar disc herniation.
Indications for discectomy include radiculopathy not responsive to a
reasonable period of nonoperative care or profound or progressive motor
deficit with concordant disc pathology identified on imaging studies.
Laminotomy with foraminotomy may be used to treat isolated foraminal
bone stenosis. Multilevel laminotomies have been described to treat
degenerative spinal stenosis.
modality of choice because it details the extent and location of the
disc herniation. Gadolinium enhancement may be useful in helping to
differentiate scar tissue from a recurrent or retained herniated disc
in a patient who has had previous spinal surgery. In cases in which
isolated lateral recess stenosis or hard disc (ossification or
calcification of a prolapsed disc) is the suspected pathology,
CT-myelography may offer more information with regard to the bone
architecture.
Before skin incision, a localizing radiograph should be obtained to
allow for precise placement of a small incision, limiting soft tissue
dissection required to access the spine. Magnification provided by the
operating microscope or loupes is helpful when performing discectomy.
The paraspinal muscles are elevated from only the side of the spine on
which the disc herniation is present. The interlaminar window, bordered
superiorly by the inferior edge of the lamina of the cranial vertebra,
laterally by the facet joint and pars interarticularis of the caudal
vertebra, inferiorly by the leading edge of the lamina of the caudal
vertebra, and medially by the spinous process, should be identified
carefully (Fig. 27-2). The spinal canal is
entered through the interlaminar window after the ligamentum flavum is
elevated or removed, allowing for visualization of the underlying
neural elements. We prefer initially detaching the ligamentum flavum
from its attachment to the cranial edge of the caudal lamina. A small
amount of bone resection may be required to visualize the neural
structures and to access the intervertebral disc. The involved nerve
root is identified and retracted gently, allowing for visualization of
the underlying offending disc. Retraction on the nerve root should be
released frequently to prevent iatrogenic nerve root injury. If the
disc is contained,
the
anulus and posterior longitudinal ligament are incised with a sharp
knife directed away from the neural elements. The offending disc
fragment and any loose fragments within the disc space are removed with
a pituitary rongeur. After the disc fragment is removed, the nerve root
should be tension-free as it traverses into the neural foramina.
 |
|
Figure 27-2
Interlaminar window. The interlaminar window provides access to the spinal canal for performing a discectomy. The interlaminar window is bordered superiorly by the inferior edge of the lamina of the cranial vertebra, laterally by the facet joint and pars interarticularis of the caudal vertebra, inferiorly by the leading edge of the lamina of the caudal vertebra, and medially by the spinous process. |
far-lateral) disc herniation, the paramedian muscle splitting approach
advocated by Wiltse is favored by many spine surgeons. With this
approach, the erector spinae muscle is split bluntly longitudinally
approximately 5 cm lateral to the posterior midline. The appropriate
level transverse processes are identified. The intertransverse
ligaments and fascia are incised, and the intertransverse membrane is
opened bluntly so that the extraforaminal nerve root is visualized. The
nerve is traced medially at 45 degrees toward the foramen. The nerve
root may be retracted, and the underlying disc is identified. The
offending disc fragments may be removed safely.
outpatient procedure or with an overnight hospital stay. Patients are
encouraged to resume usual activities in the early postoperative
period, and patients may ambulate as soon as comfort permits after
surgery.
the spine to access pathology anterior to the neural elements, such as
may occur with tumors or burst fractures. With this approach, the
vertebral body or intervertebral disc or both are accessed lateral to
the spinal cord or cauda equina so that any neural retraction is
minimized. This approach is useful to relieve thoracic spinal cord
compression arising from involvement of the anterior vertebral elements
in patients who are unable to tolerate the risk or morbidity of a
thoracotomy. It also can be used in the lower lumbar region.
required for any other decompressive procedure. After transpedicular
decompression, spinal stability usually is compromised, so the surgeon
must be prepared to reconstruct the spinal column. The surgical team
must be prepared to manage substantial blood loss that can occur during
transpedicular decompression.
posterior midline incision is used to expose the spinous process,
lamina, and facet joints. After laminectomy, the pedicle is identified
at the junction of the transverse process and the superior articular
process. The pedicle may be entered with a rongeur or high-speed drill.
While initially preserving the inferior-medial pedicle wall to serve as
a protective barrier during the decompression, the remainder of the
pedicle is removed. Combinations of straight and angled curets are
placed through the base of the pedicle to remove cancellous bone from
the vertebral body to create room for subsequent fragment reduction.
Then the inferior-medial pedicle cortex can be removed. As the dura is
protected with a curved elevator, the retropulsed bone fragments or
tumor tissue either are pushed anteriorly with an impactor or
reverseangle curet or are removed carefully. In view of the
destabilizing nature of a transpedicular decompression, posterior
instrumentation is recommended.
particularly in revision cases in which peridural scarring may be
abundant. When noted intraoperatively, incidental durotomies should be
repaired primarily if possible. The repair may be reinforced with
fibrin sealant. Occasionally, subarachnoid drainage may be required to
help promote sealing of the tear. Clear drainage from the wound
postoperatively may indicate a dural tear. This tear may be confirmed
by the presence of β2-transferrin in the wound drainage or
by visualization of a cerebrospinal fluid collection on MRI. Treatment
options include epidural blood patches or placement of a subarachnoid
drain to promote sealing of the leak. If cerebrospinal fluid leakage
persists, surgical repair or “patching” of the dural tear may be
required. Unrecognized dural tears may result in pseudomeningoceles or
cerebrospinal fluid fistulas.
associated more commonly with discectomy (L4-L5 > L5-S1). Injury to
major abdominal vessels typically is the result of forceful use of a
pituitary rongeur with penetration through the anterior anulus
fibrosus. The mortality rate from arterial injury and venous injury has
been reported to be 78% and 89%, respectively. A vascular injury should
be suspected in any patient undergoing discectomy who develops
hypotension or abdominal distention. This possibility must be
surgically explored immediately and repaired. If a vascular injury is
unrecognized, the patient may develop an aneurysm or arteriovenous
fistula, which may manifest with high cardiac output and abdominal
bruit.
the major vessels must be well visualized and retracted to prevent
vascular injury. The segmental vessels at the corpectomy site must be
securely ligated.
reported to occur in less than 5% of patients, with a higher incidence
in patients with instrumentation. Irrigation, débridement, and
antibiotics are the mainstay of treatment for infection after
noninstrumented spinal surgery. For wound infection in the presence of
spinal instrumentation, a combination of serial irrigation,
débridement, systemic antibiotics, and antibiotic-impregnated beads
have been shown to be effective treatment. When instrumentation is
required to maintain spinal stability, the surgeon should attempt to
retain the instrumentation.
result of surgical interruption of the various motion segment
stabilizers is imprecise, but results of biomechanical studies may
provide some objectivity to this determination. The extent of bone
resection performed can assist in making an intraoperative decision as
to whether to proceed with arthrodesis after laminectomy. Experimental
division of the interspinous ligament alone does not increase sagittal
motion; however, removal of greater than 50% of the facets at a level
or complete unilateral facetectomy results in significant instability.
Destruction of the facet capsule and articular cartilage significantly
destabilizes the motion segment. In addition, the intact pars
interarticularis plays an important role in maintaining stability and
should not be disrupted inadvertently during decompression. If
iatrogenic instability is created, the surgeon should be prepared to
proceed with arthrodesis.
the result of excessive retraction, contusion, laceration, or
electrocauterization of the neural structures. The incidence of
neurologic complication after all lumbar spine surgery has been
estimated to be 0.2%. The risk is higher in complex cases in which
instrumentation is required and deformity correction is performed.
Excessive neural retraction can be avoided by ensuring adequate
exposure and by gentle and mindful handling of the nerve roots and
spinal cord. To minimize the risk of cutting across a nerve root,
decompression should be performed parallel rather than perpendicular to
the nerve root. In addition, the surgeon should visualize the nerve
root to avoid accidental nerve injury.
particular spinal anatomy is crucial in avoiding neural injury.
Preoperative imaging studies may reveal spina bifida occulta, a
laminectomy defect, anomalous nerve roots, and peridural scarring,
which, if unrecognized, may increase risk of neural injury. These
findings mandate a more cautious surgical exposure and decompression.
damage to the lumbar sympathetic plexus, causing retrograde ejaculation
(0 to 2%) and transient lower extremity sympathectomy (10%) To reduce
the risk of these complications, blunt dissections should be employed
anterior to L4, L5, S1, and bipolar electrocautery only should be used.
HH, Kirkpatrick JS. Anterior decompression for late pain and paralysis
after fractures of the thoracolumbar spine. Clin Orthop 1994;330:24-29.
KK, O’Leary PF, Cammisa FP, et al. Decompression, fusion, and
instrumentation surgery for complex lumbar spinal stenosis. Clin Orthop
2001;384:18-25.
LH III, Frassica DA, Kostuik JP, Frassica FJ. Metastatic disease to the
spine: diagnosis and treatment. Instr Course Lect 2000;49: 471-476.
