Pathophysiology of Muscle, Tendon, and Ligament Injuries
development and consist of a core of mesenchyme covered by the apical
ectodermal ridge. During limb bud growth, the proliferating mesenchyme
gives rise to all of the skeletal rudiments. Myotome cells from the
adjacent somites advance into the limb buds and give rise to the
skeletal muscles. By the seventh week, distinct muscle formation has
reached to the level of the hand and foot. The lower five cervical and
first thoracic myotomes lie opposite the upper limb bud, whereas the
second through fifth lumbar and upper three sacral myotomes lie
opposite the lower limb bud. Branches of the spinal nerves that supply
these myotomes reach the base of the limb bud and, as the bud elongates
to form a true limb, the nerves grow into it.
neurocontacts with the developing skeletal muscle fibers will occur.
This is critical for muscular development, with complete
differentiation and function of the muscle fibers. Large motor neurons
contact the developing motor fibers of the growing muscles and
establish formation of the neuromuscular junctions. Voluntary control
of skeletal muscle contraction is completed when myelination of the
nerve fibers of the corticospinal tract is complete. Each muscle fiber
is innervated by one nerve ending.
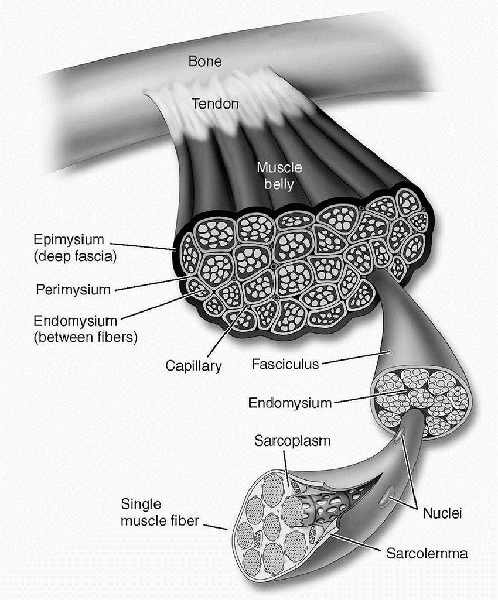
of skeletal muscle. Skeletal muscle is a highly organized structure
that is surrounded by well-defined fascial layers (Fig. 1-1).
The individual muscle is surrounded completely by a fascial layer
called the epimysium. From the epimysium, extensions of the surrounding
fascia (perimysium) divide the muscle belly itself into multiple
fascicles. Finally, each fascicle is further subdivided into individual
muscle fibers by the endomysium. The muscle fiber is the basic
structural element of skeletal muscle. Skeletal muscle fibers range in
size from 10 to 80 µm in diameter. Each muscle fiber is surrounded by a
plasma membrane known as the sarcolemma. Immediately beneath the
sarcolemma, along the periphery of the muscle fiber, are numerous cell
nuclei. There can be several hundred nuclei for each centimeter of
fiber length. Satellite cells lie along the surface of the muscle and
are thought to be stem cells capable of regenerating muscle tissue in
the event of injury. Individual fibers are made up of smaller subunits
called myofibrils running the length of the muscle. At the end of the
muscle fiber cell, membranes and collagen tissue collect into bundles
to form muscle tendons. Muscle fibers are arranged either parallel or
oblique to the long axis of the muscle. Oblique arrangements are
commonly described as pennate, bipennate, multipennate, or fusiform.
thousand longitudinally oriented myofibrils. Myofibrils are composed of
thick and thin protein filaments. The thick filaments (myosin) and the
thin filaments (actin) provide the mechanical force during a muscle
contraction by sliding past one another. In addition to myosin, the
thick filaments are also made up of C-protein, M-protein, and titin.
The thin filaments are anchored at one end by a protein structure
called the Z-band, which is oriented at right angles to the filaments.
These Z-bands occur at regular intervals along the length of the
myofibrils and give skeletal muscle its striated appearance.
Therefore, myofibrils are constructed of many sarcomeres linked end to
end. Understanding the structure and function of the sarcomere is
important because it is the basic unit of contraction. The sarcomere
can be further divided into an A-band, which is a subunit containing
the critical interdigitation of actin and
myosin
filaments. In the middle of this A-band is the M-band, representing the
middle portion of the thick filaments only. Another section, called the
I-band, is made up of only actin; it does not interdigitate with the
myosin molecules and therefore overlaps two successive sarcomeres where
the actin molecules are anchored to the Z-bands. In the normal resting
state, the thin filaments of the sarcomere are attached at either end
to the Z-band and point toward one another. However, they do not touch
or overlap. This creates a region in the middle of the sarcomere where
the thick filaments are not overlapped by the thin filaments. This is
called the H-zone.
 |
|
Figure 1-1
Macroscopic structure of skeletal muscle. (From McArdle WD, Katch FI, Katch VL. Essentials of Exercise Physiology, 2nd ed. Baltimore: Lippincott Williams & Wilkins, 2000.) |
 |
|
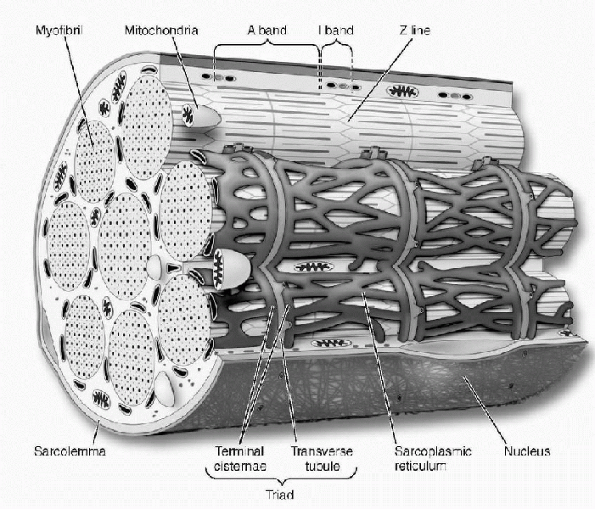
Figure 1-2
Microscopic structure of a skeletal muscle fiber. (From McArdle WD, Katch FI, Katch VL. Essentials of Exercise Physiology, 2nd ed. Baltimore: Lippincott Williams & Wilkins, 2000.) |
proteins called myosin. On electron microscopic examination, these
molecules look like long rods with two paddles attached at one end.
These paddles are critical in forming the cross bridges between the
thick and thin filaments. In a relaxed muscle, the paddles point toward
the Z-bands. The actin in its normal state resides in the form of a
double helix. Along the notches between the strands of actin are
molecules of troponin and tropomyosin. These proteins enable calcium to
regulate the contraction-relaxation cycle.
muscle fibers and their associated tendons. Sarcomere length remains
fixed throughout development. Additional sarcomeres are added to the
muscle fibers to achieve longitudinal growth in the region of the
musculotendinous junction.
point. From there, the nerve cell axon branches many times. Skeletal
muscle fibers are innervated by neurons entering the
muscle
in a region called the endplate zone. The cell bodies of these neurons
are located in the anterior horn of the spinal cord. Each motor neuron
branches several times within the muscle and innervates a variable
number of muscle fibers. However, each muscle fiber can be innervated
by only one motor neuron. The motor unit is composed of a single motor
neuron and all the muscle fibers it innervates. Muscle fiber type is
related to its interaction with the singular motor nerve, as all muscle
fibers in a single motor unit have the same metabolic and contractile
properties (Fig. 1-3).
The strength of a muscle contraction depends on the number of muscle
fibers that are activated at the same time. As each motor neuron
branches several times and innervates many muscle fibers, the central
nervous system cannot activate a single muscle fiber, but most work
through individual motor neurons in the activation of multiple muscle
fibers comprising the motor unit. Therefore, the degree of control that
is exerted on the strength of the muscle contraction in part depends on
the number of muscle fibers comprising each motor unit that are
activated. Powerful extremity muscles may contain more than 1,000
muscle fibers in each motor unit, whereas when fine control is
required, the motor units may contain only a few muscle fibers. Smaller
motor neurons usually innervate fewer muscle fibers and therefore have
smaller motor units. Often, these neurons are activated first. If more
power is needed, larger motor units are progressively recruited. This
has been referred to as the size principle of motor control.
metabolic, and functional variations—have been identified. Different
classification schemes have been proposed. Muscles are able to function
for a short time without oxygen by using the glycolytic pathway to
generate adenosine triphosphate (ATP). Muscle fibers that generate high
power over a short time have been called “fast-twitch,” “white,” or
type II and make extensive use of this pathway. These muscle fibers
release energy rapidly from ATP but regenerate energy stores slowly.
Therefore, these muscles become easily fatigued. Fast-twitch motor
units are generally larger and generate more strength. They have higher
enzymatic activity for the phosphagen and glycolytic systems and are
used predominantly during activities dependent on anaerobic energy.
Type II motor units can be further subdivided. Most commonly, two main
subgroups are considered. Type IIB motor units (or fast glycolytic
motor units) have the fastest contraction time and are the least
resistant to fatigue. This motor unit has the largest number of muscle
fibers, the largest axon, and the largest cell body. Type IIA motor
units are considered to be in between type I and type IIB groups.
Contraction times and fatigue resistance profiles are between type I
and type IIB, as both the oxidative and glycolytic pathways are well
developed. Motor unit size is also intermediate. In contrast, muscle
fibers that are active over a long period of time are considered
“slow-twitch,” “red,” or type I. These fibers are rich in mitochondria
and have a greater oxidative aerobic capacity. Type I motor units are
resistant to fatigue. These motor units are often small and used in
fine manipulations. In addition, they are the first fibers activated
when lower levels of power are required.
 |
|
Figure 1-3
Structure of a skeletal muscle and a motor unit. The aggregate of a motor neuron axon and all muscle fibers innervated by it constitute a motor unit. (From Moore KL, Agur A. Essential Clinical Anatomy, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2002.) |
distribution of motor unit types. Variations in muscle fiber type
between individuals are common. A preponderance of one type over
another may lead to a greater chance of success in a given sport. For
example, power athletes or sprinters should benefit from higher
concentrations of fast-twitch fibers within their muscles, whereas
distance runners would benefit from having predominantly type I fibers.
It is believed that the distribution of muscle fibers in any one
individual is determined genetically. However, there is evidence for
interconversion between type IIA and type IIB fibers. Certainly, during
training, there is selective recruitment of the appropriate fibers that
are best suited for specific athletic demands.
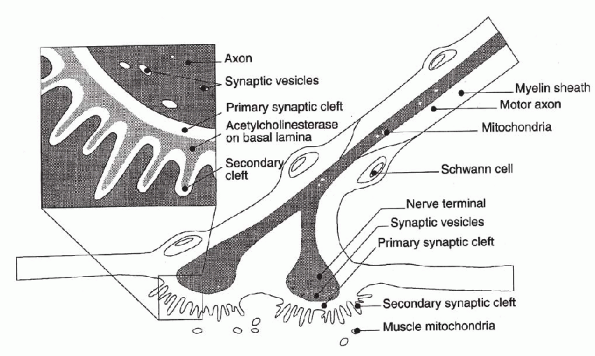
myelinated fibers. The nerve forms a synapse with the muscle at a
specialized region known as the motor endplate (Fig. 1-4).
Transmission of the impulse is not achieved by direct electrical
transmission but requires a chemical transmission at the motor
endplate. At the end of the neuron, there are fingerlike projections
found between the membranes of the nerve and the muscle. These primary
synaptic folds act to increase the area of membrane interaction between
the nerve and muscle. The nerve terminal is rich in mitochondria and
contains many synaptic vesicles that contain the neurotransmitter
acetylcholine. The presynaptic and postsynaptic membranes are separated
by a small (50 nm) synaptic cleft. In the muscle membrane are
junctional folds containing acetylcholine receptors that mediate the
action of the neurotransmitter and acetylcholinesterase, which acts to
destroy the neurotransmitter.
are propagated along the axon toward the neuromuscular junction. When
the action potential arrives at the motor unit, the depolarization
opens up calcium channels in the
axon
terminal. This results in calcium becoming concentrated in the
presynaptic nerve terminal. The sudden increase in the calcium
concentration causes the vesicles to fuse with the terminal axon
membrane and results in the release of acetylcholine into the synaptic
cleft. This acetylcholine passes across this cleft and binds to a
receptor molecule on the postsynaptic membrane. This results in the
opening of channels to permit the influx of sodium ions and the efflux
of potassium ions. The net effect is the depolarization of the muscle
membrane and triggering of the muscle action potential. The
acetylcholine is then rapidly hydrolyzed and deactivated by the enzyme
acetylcholinesterase into choline and acetate. Breakdown products are
then reabsorbed into the terminal axon to be used in the resynthesis of
a new transmitter.
 |
|
Figure 1-4 The motor endplate. (From Woo
SL-Y, An KN, Frank CB, et al. Anatomy, biology, and biomechanics of tendon and ligament. In Buckwalter JA, Einhorn TA, Simon SR, eds. Orthopaedic Basic Science, 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:581-616.) |
is possible. Severe muscle weakness in the disease myasthenia gravis is
the result of a shortage of acetylcholine receptors. Inhibition of the
acetylcholinesterase enzyme with neostigmine and edrophonium can allow
the acetylcholine molecules to have a longer life and a better chance
to interact with receptors before they are broken down. Impulse
transmission can be blocked by Curare, which binds to the acetylcholine
receptors. Succinylcholine produces muscle relaxation by keeping the
acetylcholine channels open for too long a period of time. This keeps
the muscle membrane depolarized and refractory to further impulse
initiation.
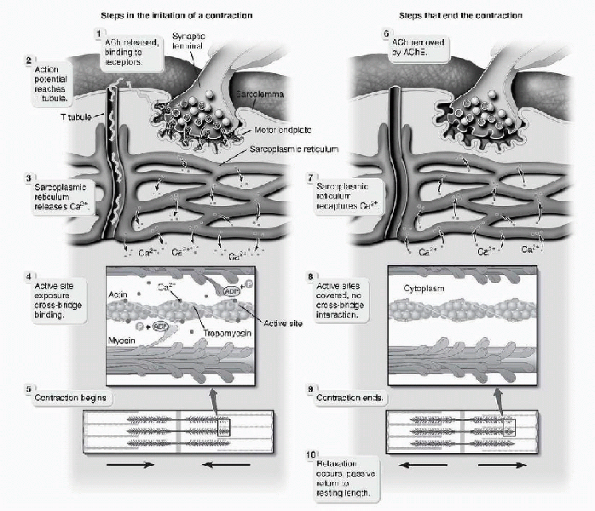
entire length of the muscle fiber. Between the adjacent myofibrils are
elements of the conducting pathway, called the sarcoplasmic
reticulum-transverse tubule system. The T-tubules are internal
extensions of the cell membrane, oriented perpendicular to the long
axis of the cell and bring the action potential into the interior of
the muscle fiber. These often lie at the level of the A- and I-band
junctions. Calcium ions are held in high concentrations in the
sarcoplasmic reticulum. When the adjacent T-tubule system is excited by
a muscle action potential, calcium ions are released into the muscle
cytoplasm and diffuse to the nearby myofibrils. There, they bind
strongly to troponin. This results in structural changes that allow
actin to bind to the myosin cross bridges. This binding elicits a
muscle contraction as ATP is hydrolyzed by myosin and the thick
filaments slide past the thin filaments. The cross-bridging cycle
occurs many times as the thick filaments release from the actin on the
thin filament, return to their original configuration, create another
cross bridge with conformational changes, and further shorten the
muscle fiber (Fig. 1-5). This process may occur
in rapid succession. The thick filaments pull toward each other and
toward the center of the A-band, and the sarcomeres shorten or resist
stretch. During a muscle contraction, the angle between the cross
bridges and the rod portion of the myosin becomes more acute. This has
been described as the sliding filamentswinging cross-bridges theory of
muscle contraction. The sarcoplasmic reticulum contains a calcium pump
that removes the calcium from the myofibrils at the end of a single
contraction. When calcium is no longer available, tropomyosin undergoes
a conformational change, thus preventing further cross-bridge formation.
stimulus is called a muscle twitch. If a second contraction is elicited
before the first one has relaxed, a stronger contraction results. As
the stimulation frequency increases, the tension in the muscle also
increases. When the frequency of activation is high enough, a
continuous contraction (tetanus) will result. Involving more motor
units can also increase the force of muscle contraction. This has been
termed “recruitment.” To increase the overall strength of muscle
contraction, both the frequency of activation and the recruitment of
more motor units are required.
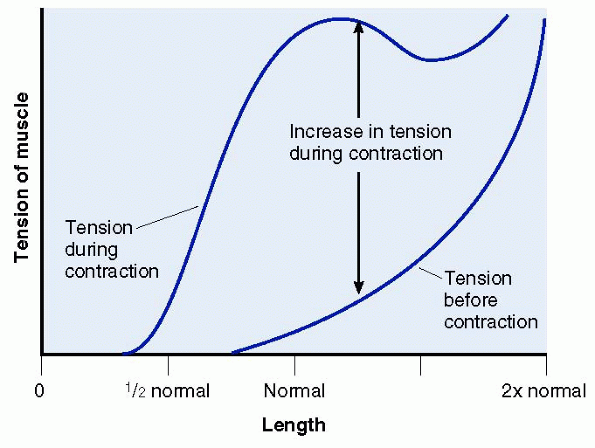
the length of that muscle when the contraction begins. The Blix curve
describes this important muscle length-tension relationship (Fig. 1-6). When a muscle is at its normal resting
state and length, there is maximal overlap of the thick and thin
filaments. This maximal overlap allows for the creation of maximum
cross-bridging tension to be developed. Once a muscle is in a
contracted position, the thin actin filaments impede one another. This
interferes with cross bridging and effectively reduces the maximum
tension that can be created. Conversely, stretching the muscle out to a
point where the filaments have minimal cross-bridging contact also
results in a weak muscle contraction. The maximum amount of force
produced by a muscle is proportional to its cross-sectional area. Also,
the total amount and speed of muscle shortening is proportional to the
individual muscle fiber length.
 |
|
Figure 1-5
Excitation-contraction coupling. (From Premkumar K. The Massage Connection, Anatomy and Physiology, 2nd ed. Baltimore: Lippincott Williams & Wilkins, 2004.) |
different ways. An isometric contraction (same length) occurs when the
muscle length is held constant and the resultant force is measured. In
an isotonic contraction (same load), the muscle is activated to shorten
against a constant load while muscle length changes with time are
measured. Muscle can also be evaluated under isokinetic activation
(same speed), in which the load accommodates to maintain a constant
velocity of shortening or lengthening. When a muscle is activated,
shortening of the sarcomeres results in force generation. The muscle
will shorten (concentric action) if the resisting load is less than the
force generated by the muscle. Conversely, the muscle will lengthen
(eccentric action) if the resisting force is greater than that
generated by the muscle. Muscles that are stimulated eccentrically can
produce more work than muscles that are activated concentrically. No
motion will occur if the forces are equal.
 |
|
Figure 1-6
Blix curve length-tension diagram for a single sarcomere. (After Guyton AC. Textbook of Medical Physiology,7th ed. Philadelphia: WB Saunders,1986:128.) |
the myotendinous junction before integration into the distal tendon.
This is a region of highly folded membranes,which increases the contact
area and decreases stress. In this area, stresses are changed from
tensile to shear. A well-developed basement membrane allows the muscle
force generated to be linked to the collagen fibers of the tendon.
Fibronectin, laminin,and type IV collagen are among the proteins
present in the basement membrane.
contraction. Once ATP is broken down into adenosine diphosphate
(ADP)/adenosine monophosphate and inorganic phosphate(s) to release
energy,the body must re-create the ATP from one of three available
sources. The most readily available source is creatine phosphate (CP).
When enzymatically broken down,the energy released is used to form ATP
again:
high-intensity,short-duration activities such as sprinting,because the
amount of energy available is limited. CP cannot be used directly by
the muscle cells as a source of energy. Some athletes have used oral
creatine supplementation in an attempt to maximize CP levels that may
then promote greater muscle hypertrophy from resistance training.
is the second source of energy. Glucose is metabolized and releases
energy to convert ADP to ATP,as well as forming lactic acid as a
byproduct. Lactic acid buildup causes the symptoms of fatigue. This
inefficient system is a limited source of energy and is used by the
muscle when a lot of energy is needed for a relatively short period of
time.
are completely broken down to carbon dioxide and water in the presence
of oxygen. This process occurs in the mitochondria of the muscle cells.
It is responsible for releasing the large amounts of energy needed for
ATP resynthesis. A single molecule of glucose can yield 34 molecules of
ATP. This is an excellent energy source for prolonged endurance type of
activities. The amount of oxygen available to the cell is the limiting
factor. Athletes whose diets are rich in carbohydrate have higher
stores of glycogen and therefore may benefit from a higher energy
production (carbohydrate loading). For most individuals,fat storage
provides an abundant source of energy. Breakdown of free fatty acids
can provide sufficient energy to convert large amounts of ADP to ATP.
These fatty acids can be found within the muscle or mobilized from
adipose tissue. It is unlikely that availability is a limiting factor
in energy metabolism.
separate systems,most often, they occur simultaneously to provide
working muscles the energy required for the formation of ATP. The
different motor unit types have varying levels of enzymes required for
both aerobic and anaerobic capacity. The body will emphasize the
appropriate metabolic system based on the intensity and duration of
activity undertaken. This variation has been described as the energy
continuum. For example,brief high-intensity exercise relies on the CP
system. If intensity falls and length of exercise increases,the
anaerobic system is used more to replenish the required ATP. If the
exercise intensity falls further and the length of activity continues
to increase,then the aerobic system becomes most efficient for
supplying energy. The training an athlete performs can significantly
influence which pathways are chosen.
the body to return to its pre-exercise state after vigorous activity.
The lactic acid must be removed from the muscle, muscle glycogen must
be replenished,phosphagen and ATP must be restored,and the remaining
oxygen debt must be eliminated. Athletes benefit from warming down
after competition because light exercise hastens the removal of the
built-up lactic acid. Lost muscle glycogen can be resynthesized within
a couple of hours after moderate exercise,but it may take as long as 48
hours following prolonged endurance activity. Muscle phosphagen store
replacement occurs rather quickly. Oxygen debt recovery requires
replenishing myoglobin with oxygen in the aerobic recovery of
phosphagen stores,as well as recovery from the oxygen debt needed to
assist the conversion of lactic acid. Conditioning can shorten all
recovery times.
concern in the sporting community. Insulin is a hormone secreted by the
pancreas for the regulation of metabolism of food products. This
hormone is considered anabolic because it increases glucose entry into
the muscle cell and increases glycogen synthesis. Amino acid uptake is
increased, resulting in protein synthesis,and protein catabolism is
decreased. These effects are synergistic with growth hormone.
Glucocorticoids oppose these actions by accelerating protein
degradation and increasing the resulting amino acid release. Growth
hormone,a product of the pituitary gland,increases amino acid transport
into the muscle
cell
and stimulates protein synthesis, which results in increased muscle
synthesis. Growth hormone also reduces glucose and protein metabolism
by shifting metabolism toward the use of fatty acids. Synthetic human
growth hormone has led to its increasing use as an anabolic agent.
Testosterone has the main anabolic effect on muscle tissue. It
increases protein synthesis while decreasing the rate of protein
catabolism within the muscle. This results in an increase of the muscle
size, weight, and strength. Another effect of testosterone is the
expression of male sexual characteristics, including increased hair
growth, voice deepening, and genital enlargement. Anabolic steroids
were developed to decrease these “unwanted” characteristics. The
benefit of these compounds to athletes has been to increase strength
with an associated improvement in anaerobic performance. Although
controversy remains in the medical literature on the effectiveness and
safety of these compounds, competing athletes worldwide understand the
possible gains in strength that are possible when used in conjunction
with high-intensity exercise and an appropriate supporting diet.
-
Muscles have the capacity to respond significantly to both increasing and decreasing stimulation.
-
Atrophy may be quick and profound when appropriate stimulation of the muscle is removed.
-
Conversely, a progressively increasing
resistance training program can result in significant muscle
hypertrophy and strength gains. -
This hypertrophy is more commonly seen in type II fibers than in type I fibers.
-
At the present time, it is still unknown
whether this hypertrophy is the result of an increase in the size of
the muscle fibers or an increased number of muscle fibers. -
Either will result in an increasing amount of contractile proteins available.
-
-
Strength training also improves the capacity for motor unit recruitment.
-
Untrained individuals may have as little as 60% of their muscle fibers firing simultaneously.
-
With an aggressive muscle strengthening program, greater than 90% of the muscle fibers become active.
-
With an increased capacity for motor unit
recruitment and increased number of available contractile units, the
targeted muscle will show increased strength and work capacity.
-
-
A generalized recommendation for
improving strength is to stress a muscle against a high resistance so
that only several repetitions are possible before failure.-
These high-intensity exercises often last less than a minute and rely on the CP/anaerobic glycolysis systems for ATP formation.
-
Highly conditioned athletes may have increased levels of stored phosphagens.
-
Conversely, training for endurance would
require a lower muscle resistance so that many repetitions are required
before muscle failure. -
The critical point for endurance training is the appropriate supply of energy rather than hypertrophy of the muscle.
-
The cardiovascular system must supply
enough oxygen to the muscles to allow the aerobic metabolism system to
provide energy continually for the formulation of ATP. -
The density of mitochondria within the muscle increases.
-
As the time of training progresses past 2 hours, fatty acids replace glycogen as the main fuel source.
-
-
Delayed-onset muscle soreness (DOMS) is common within 2 to 3 days after new or increased levels of exercise.
-
This is often associated with eccentric exercise.
-
-
Clinically, athletes complain of
soreness, swelling, stiffness, and weakness within the affected muscles
after a particularly intense workout. -
Often, this will resolve itself within a
couple of days, but if the insult to the muscle is severe enough, the
soreness may last for 1 to 2 weeks. -
As the muscle adapts and responds to this level of stress, the occurrence of DOMS stops.
-
However, when the muscle is again pushed to new levels of stress, the DOMS may occur again.
-
-
It is believed that the soreness is the result of intramuscular damage to the structural elements of the muscle.
-
Histologic analysis reveals Z-band streaming, A-band disruption, and malalignment of the myofibrils.
-
Fast-twitch type IIB fibers are most at risk.
-
There is associated connective tissue breakdown.
-
Most important, this injury is reversible.
-
-
Some athletes will use this sense of DOMS as a guide to their training routine.
-
The theory is that the involved muscles become stronger and more resistant to damage from the initial inciting level of stress.
-
This adaptation allows for further muscle
strengthening as the fragile fibers are replaced by stronger fibers
that can resist further levels of stress. -
Once there is no more muscle soreness,
muscle growth slows, and a new level of stress is required to produce
further muscle hypertrophy.
-
-
Some athletes are at risk for muscle cramps, and others appear to be immune.
-
The exact reason is unknown.
-
-
The pathophysiology of muscle cramps is poorly understood.
-
It appears that the muscle cramps are initiated from the motor nerves once they enter the affected muscle.
-
The cramped muscle usually becomes symptomatic when it is in a shortened position.
-
Unexpectedly, there will be a powerful and painful active contraction of the muscle.
-
Usually, this can be interrupted by a forceful stretch of the affected muscle into an elongated resting position.
-
Afterward, however, the muscle may remain with altered excitability.
-
Athletes at risk often show fatigue from prolonged muscle use or dehydration.
-
Treatment includes aggressive hydration, electrolyte replacement, stretching, and acclimatization.
-
Muscle stretching is part of nearly every exercise program.
-
Proposed benefits of stretching include improved performance and reduced injury risk.
-
The diminished stiffness and increased
range of motion seen after stretching can be explained by the
viscoelastic properties of the muscle. -
The tension developed in a stretched muscle diminishes over time, resulting in stress relaxation.
-
Most stretching programs recommend a slow static stretching of the muscle.
-
Ballistic movements should be avoided.
-
This allows the electrical activity within the muscle to be quiescent and the stretching to be unopposed.
-
Muscle strains are among the most common injuries sustained by athletic individuals.
-
Rather than direct trauma, excessive
force along the muscle can result in an anatomic disruption in the
region of the musculotendinous junction.-
Most commonly, this is from a significant eccentric muscle contraction.
-
-
Muscle sarcomeres within a few
millimeters of this junction appear more stiff than their more proximal
counterparts and therefore at higher risk for failure. -
Incomplete tears are most commonly seen.
-
Muscles that cross two joints appear clinically to be more at risk for this injury.
-
These include the lower-extremity hamstring, rectus femoris, and gastrocnemius muscles.
-
These muscles appear to have an increased length of their musculotendinous junction.
-
-
Muscle healing has been described as occurring in three distinct phases.
-
Initially, there is inflammation with necrosis of the damaged muscle.
-
This allows for phagocytosis of the necrotic debris.
-
-
The second phase is characterized by protein synthesis from activation of the satellite cells that are myogenic precursors.
-
These cells differentiate into myotubules and muscle fibers.
-
-
In the last or final phase, there is
maturation or remodeling of the repair tissue with a gradual return of
the muscles’ functional properties.
-
-
Pharmacologic manipulation of this
healing process with either oral anti-inflammatory medication or
intramuscular corticosteroid injection has yielded conflicting results. -
Use of ultrasound as a therapeutic soft-tissue modality has also been tried.
-
Contusion is a common sports-related injury that can vary widely in its resulting symptomatology.
-
In response to a blunt injury, a localized hematoma occurs and an inflammatory reaction begins.
-
This repair process is regulated by growth factors and cytokines.
-
The magnitude of these events is directly related to the level of trauma.
-
Necrotic tissue is removed, and new muscle fibers are formed as myotubes fuse into mature muscle cells.
-
Both muscle regeneration and scar occur at the site of injury.
-
Although the injured athlete will place
the injured muscle in a shortened and relaxed position to decrease
pain, this may hamper recovery and delay rehabilitation because of the
resulting muscle shortening and stiffness. -
Gentle mobilization of the muscle may result in a faster recovery of tensile strength.
-
If the soft-tissue injury is severe
enough, or if there is a history of previous muscle contusions, bone
formation may occur in the muscle belly (myositis ossificans).-
This condition may mimic osteogenic sarcoma.
-
-
Laceration of the muscle belly perpendicular to the long axis of the muscle results in denervation of the distal segment.
-
Necrotic muscle at the injury site is removed by macrophages.
-
New muscle cells appear from surrounding satellite cells forming myoblasts and muscle fibers.
-
At the same time, there is a proliferation of connective tissue.
-
This connective tissue fills the void
left by the laceration and interferes with the ability of the muscle to
return to its normal anatomic state. -
Therefore, recovery from a laceration is dependent on the magnitude of injury and relative location from nerve innervation.
-
Immobilization results in muscle atrophy.
-
Initially, there is significant and rapid muscle wasting with atrophy of the muscle fibers.
-
The rate of protein synthesis in the muscle decreases within hours.
-
However, after this initial stage, the rate of loss lessens.
-
-
Less cross-sectional muscle mass equates with less strength.
-
Work capacity decreases and muscle fatigue increases with any applied stress.
-
These changes are related to the length at which the muscle is immobilized.
-
If a muscle is immobilized under some
tension, these atrophic changes will be less than if the muscle is
immobilized under no tension. -
This common clinical condition is seen in
the lower extremity where an injured knee is braced in extension,
placing the quadriceps mechanism under no tension and a hamstring
musculature under tension. -
Quadriceps atrophy is seen to be greater than hamstring atrophy.
-
Immobilized muscles also respond differently to passive stretch.
-
If a muscle is immobilized in a shortened position, it will develop more tension in response to passive stretch.
-
If the immobilized muscle is held in a lengthened position, it will develop less tension in response to passive stretch.
abnormality of muscle. This process must be understood by orthopaedic
surgeons because it most commonly develops after the administration of
general anesthesia, although it may be triggered by other stimuli.
During childhood, men and women appear to be equally at risk, but with
advancing age, men appear more at risk. The peak incidence occurs at
around 30 years of age. The pathophysiology of this disease appears to
involve an abnormality in calcium transport of the cell membranes of
the sarcoplasmic reticulum and mitochondria. A triggering event
precipitates a leak of calcium from the sarcoplasmic reticulum
resulting in a sustained actin-myosin combination that causes continued
contraction and muscle rigidity. This results in the production of
heat, metabolic acidosis, and carbon dioxide production with resulting
respiratory acidosis. Protein denaturation results in a coagulopathy.
If not appropriately treated, this process can result in death.
-
An accurate history of family or personal anesthetic problems is the best method for preventing this disease.
-
However, a malignant hyperthermia event does not necessarily occur with the first exposure to anesthesia.
-
-
Susceptible patients also tend to be healthy and athletic with large muscle masses.
-
A history of leg cramping at night and
exercise intolerance in hot weather may also provide insight into a
possible at-risk individual. -
Conclusive testing can be achieved by muscle biopsy.
-
Careful intraoperative monitoring is critical for the management of this disease.
-
Early warning signs are nonspecific but include tachycardia, possible ventricular arrhythmias, and an unstable blood pressure.
-
More worrisome is the finding of a combined respiratory and metabolic acidosis.
-
Increasing temperatures of 1°C or more requires further investigation.
-
If malignant hyperthermia is suspected, the surgical procedure and anesthetic are terminated as rapidly as possible.
-
Medical management includes dantrolene sodium, which inhibits calcium release from the sarcoplasmic reticulum.
-
Acidosis is corrected by hyperventilation with oxygen.
-
Sodium bicarbonate may be required.
-
Fluid management and a diuretic may be
required to maintain urine output, which is important to clear away the
products of muscle degradation. -
Accumulation of these degradation products could lead to renal damage.
-
Surface cooling with an ice bag and cold intravenous fluids may also be helpful.
muscle compartment defines a compartment syndrome. This rising pressure
results in a reduced muscle capillary blood flow that is required for
tissue viability. The local ischemia produced must be relieved by
surgical decompression of the muscle compartment to prevent permanent
muscle and nerve necrosis. Muscle microcirculation is compromised at
tissue pressures of approximately 30 to 40 mm Hg. Practitioners must
understand that the central arterial blood flow through the pathologic
compartment is typically normal and results in normal peripheral
pulses. In the right setting, one must be vigilant of the potential for
this process to occur. Compartment syndromes are commonly described as
occurring in two distinct clinical settings: the first with acute
trauma resulting in immediate and unrelenting compartment swelling with
the resulting compromise of capillary function and muscle/nerve
necrosis, and the second in overuse situations (such as found in
endurance athletes, where there are early symptoms related to
microcirculatory embarrassment without serious progression and
necrosis).
-
The first and most important symptom of
an acute impending compartment syndrome is pain that is greater than
would be expected from the primary problem. -
Pain with passive stretch of the muscles in the involved compartment is a common finding.
-
Palpation of the involved compartment will often show greater than expected swelling and tenseness.
-
Nerve ischemia manifests itself early on by an alteration of sensation.
-
Most commonly, the patient will complain of paresthesia in the nerve distribution of the involved compartment.
-
If untreated, this will be followed by a
decreased sensation and then anesthesia in the nerve distribution.
These are late findings. -
Even with a full-blown compartment
syndrome, distal pulses are almost always palpable and normal unless
there is a concomitant vascular injury.-
Normal distal pulses therefore do not rule out a compartment syndrome.
-
-
Many studies have been performed to help
identify an exact compartment pressure measurement in which a
compartment syndrome exists.P.10-
The basic underlying premise is that,
when compartment pressure measurements exceed 30 to 40 mm Hg, the
microcirculation to that compartment will be occluded and nutrition to
the soft-tissue structures will stop.
-
-
Given the sometimes inaccurate and
potentially poorly reproducible compartment pressure measurements, any
patient with an appropriate clinical history and physical examination
for a progressing compartment syndrome with impending permanent tissue
damage should be considered for surgical decompression.
-
Chronic or recurrent exertional
compartment syndromes may be more common than acute compartment
syndromes and are more difficult to accurately diagnose. -
Commonly, athletes will present with diffuse pain or aching over the anterior or lateral aspect of their lower leg.
-
This pain is usually related to prolonged exercise, and it may be severe enough to cause the athlete to stop or reduce exercise.
-
Symptoms may be unilateral or bilateral.
-
Subjective complaints should be validated with objective elevated compartment pressure measurements to make this diagnosis.
-
Sometimes these patients may have higher resting compartment pressures.
-
To establish this diagnosis, athletes
must have an abnormal pressure elevation during exercise and a slower
return to their resting value at the end of the exercise.-
Recommended pressure measurements for the
diagnosis of chronic exertional compartment syndrome include a resting
pressure higher than 12 mm Hg and a 1-minute recovery pressure above 30
mm Hg. -
Also, diagnostic is if the 5-minute postexercise pressures are elevated above 20 mm Hg.
-
-
Every effort should be made to pursue conservative management of this patient population.
-
Anti-inflammatory medication, cross
training, relative rest, rehabilitation, massage, soft-tissue release,
and orthotics should be considered. -
Surgery should be considered only if an extended period of conservative management does not improve the athlete’s symptoms.
-
The physician must make sure that there are no other causes for the patient’s subjective complaints.
-
Elective fasciotomy of the involved compartments should then be considered.
-
Appropriate realistic preoperative counseling is mandatory.
Tetanus is characterized by generalized skeletal muscle rigidity and
convulsive spasms. It can occur after spores or vegetative bacteria
gain access to injured tissue and produce the toxin locally. Because C. tetani
is a noninvasive organism, the usual mode of entry is through a
puncture wound or cut on an extremity during an athletic event. It is
anticipated that extremity wounds are frequently contaminated with
these spores, but the clinical manifestations of tetanus rarely
develop. This is because the germination of spores occurs only when the
oxygen tension is much lower than that of normal tissue. Toxin
production in wounds is favored by necrotic tissue, foreign bodies, and
associated infections that establish a low oxidationreduction
potential. The toxin produced may be transported to the central nervous
system. There, the tetanus toxin attacks synaptic junctions to produce
disinhibition. This results in generalized muscle rigidity from
uninhibited afferent stimuli.
-
The time between injury and the appearance of clinical manifestations is usually 14 days or less.
-
Commonly, patients will present with complaints of pain and stiffness of the jaw, abdomen, or back.
-
Swallowing may be difficult.
-
Trismus (lockjaw) is the most common early manifestation of tetanus.
-
Sustained contractions of the facial muscles produced a characteristic expression, termed risus sardonicus.
-
As the disease progresses, minimal stimuli produce a more intense and longer lasting spasm.
-
Respiration may be impaired.
-
The diagnosis of tetanus is a clinical one.
-
It is not dependent on bacteriologic confirmation, as cultures are positive in less than 50% of patients.
-
Involved patients should be hospitalized
and treated with tetanus prophylaxis, antibiotics, surgical debridement
of the wounds, and administration of muscle relaxants, as well as
generalized supportive measures.
still considered to be in its infancy. Relatively little is known about
the embryogenesis of tendons, compared with other musculoskeletal
tissues such as muscle, cartilage, and bone. Tendons, like muscles,
originate from mesoderm. More specifically, they arise from the lateral
plate mesoderm. Although intimately functionally connected when mature,
muscle and tendon are able to develop autonomously. One of the earliest
steps in tendon embryogenesis is the formation of a mesenchymal lamina
along the ectodermal basement membrane. Precursor cells then condense
on the dorsal and ventral sides of this mesenchymal lamina to form the
anlage that will eventually become the flexor and extensor tendons of
the adjacent joints. Interestingly, although tendons are
developmentally autonomous, subsequent muscle-tendon interaction is
required to prevent tendon degeneration. Because tendons attain size
and strength relative to the muscle mass and distance from insertion
during growth and development, it is believed that tenocytes are
responsive to the magnitude and direction of the load. Although the
exact mechanism remains unknown, investigators have shown that isolated
tendon fibroblasts respond to mechanical strains placed upon them. The
role of specific
proteins and the required pattern of expression for normal tendon development are just beginning to be elucidated.
that joint motion is possible. They are generally cylindrical in shape
with slight widening and flattening at the musculotendinous junction
and their bony insertion. Notable exceptions are the rotator cuff
tendons and pectoralis muscle tendons, which are flat and platelike at
their insertions.
fusiform cells are responsible for the production and maintenance of
collagen and other proteins that confer the flexibility and tensile
strength of tendons. Collagen is by far the largest constituent of
tendons. Almost 90% of the dry weight is accounted for by collagen.
Tendons are primarily composed of type I collagen, but they also
contain small amounts of type III, type IV, type V, and type VI
collagen. The primary structure of collagen is a three-amino acid
residue-repeating pattern. Glycine is present every third amino acid on
average, whereas proline and hydroxyproline each make up 15% of the
molecule.
predominating, but biglycan, lumican, and fibromodulin have also been
detected. Decorin is a sulfate-rich proteoglycan. Studies have shown it
to bind collagen together along the length of fibrils, much in the way
a rubber band holds a bunch of pencils together. It is believed to aid
in the formation of the collagen fibrils. It has been postulated that
decorin regulates fibril diameter, halting further accumulation past a
certain point.
diameter, separate individual fiber bundles, and minimize the shear
stresses fibers experience as they move relative to each other during
normal function. Although tendons function under predominantly tensile
loads, they do experience compressive loads as they pass around
skeletal prominences and pulleys. Some of the pressures experienced in
these regions are substantial, with measurements being reported in the
range of 700 mm Hg. Glycosaminoglycan (specifically aggrecan) content
in these regions is elevated relative to the rest of the tendon. This
is probably a functional adaptation allowing for greater water content
and resultant structural resiliency under compressive conditions.
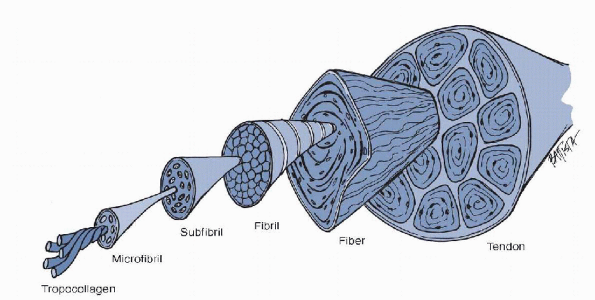 |
|
Figure 1-7
Structural composition of ligament and tendon. (From Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore: Lippincott Williams & Wilkins, 2004.) |
maintain and repair themselves is just beginning to be understood.
Although tenocytes initially appear to be spatially distinct within the
matrix of the tendon, like osteocytes, they possess very long processes
that form gap junctions to facilitate communications with other cells
in their locale.
Microfibrils make up subfibrils which, in turn, make up fibrils. There
are hundreds of these fibrils within each fascicle. It is these
fascicles that make up the tendon itself. These fascicles are separated
from one another by the endotenon. The endotenon is made of
longitudinally oriented adventitia that is cell-poor. On the surface,
and adherent to the tendon proper, is the epitenon. This diaphanous
layer is composed of fibroblasts one to two cell layers thick.
healing and maintenance. This supply is from three sources: the
perimysium, the periosteal insertion of the tendon, and the paratenon.
The paratenon is, in turn, supplied by the surrounding tissues. Flexor
tendons of the hand and wrist also have an additional blood supply.
This is the mesotenon,
which is condensed into the vincula on digital flexor tendons (Fig. 1-8).
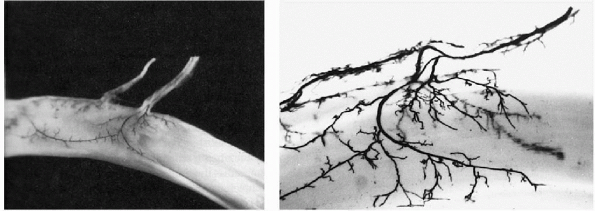 |
|
Figure 1-8 India ink preparation of vincula and blood supply to tendon. (From Garrett
WE, Best TM. Anatomy, physiology, and mechanics of skeletal muscle. In: Buckwalter JA, Einhorn TA, Simon SR, eds. Orthopaedic Basic Science, 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:683-716.) |
endotenon. The blood supply of the digital flexor tendons has been best
studied. Blood is supplied in a segmental manner from four sources:
-
Intrinsic longitudinal vessels that are continuous with those in the palmar region of the tendon
-
Synovial folds in the proximal reflection of the tendon sheath
-
Vincula
-
Osseous insertions of the tendons
refer to locations where tendon, ligament, and joint capsules attach to
bones. These are not just locations where these structures plug into
bones, but rather highly organized tissues that prevent local stress
concentration between two interfaces. Because tendon and bone have
differing elastic moduli, transmission of a certain level of force
across their junction would result in a stress concentration and
possible damage to either or both tissues. Entheses have developed to
dissipate stress away from these junctions.
increasing the surface area of tendon insertion. If the area of an
enthesis is too small, the stress generated by the tendon will be
concentrated in a small area with resultant avulsion of the tendon from
bone. To counteract this, many tendons fan out at their attachment
sites (e.g., tibialis posterior tendon, which has attachment sites on
all the bones of the tarsus, except the talus, and the proximal second,
third, and fourth metatarsals). In addition to their morphology,
entheses have unique compositions to aid in force dissipation at the
tendon-bone interface. Traditionally, entheses have been divided into
two groups according to the character of their tissue at the
tendon-bone interface. Generally, in the limbs, fibrous entheses are present at junctions located at the diaphyses of bone, whereas fibrocartilaginous entheses are typical of epiphyses or apophyses.
present in fibrous entheses that anchor tendons to bone. Fibrous
entheses can be classified into subgroups—periosteal and bony—according
to their method of insertion. Periosteal fibrous entheses may become
bony fibrous entheses with aging. This is necessitated by marked
thinning or disappearance of the periosteum after completion of bony
development. More research will be needed on fibrous entheses because
this is the type of junction initially formed when surgical
reattachment of tendon to bone is performed. This has obvious
implications not only for tendon transfers but also for procedures such
as rotator cuff, anterior cruciate ligament (ACL), and lateral ankle
soft-tissue reconstructions.
fibrocartilaginous than fibrous entheses because fibrocartilaginous
entheses are much more vulnerable to overuse injuries. These entheses
have no periosteum at the attachment site. In this form of enthesis,
there are four zones of tissue. From superficial to deep, these include
the following: dense fibrous connective tissue, uncalcified
fibrocartilage, calcified fibrocartilage, and bone. The fibrous layer
is a fanning out of the tendon. The uncalcified and calcified
fibrocartilage layers are avascular zones that are separated from one
another by a calcification front that is represented by a basophilic
line on stained sections. This is termed the tidemark.
As with articular cartilage, this line represents the boundary between
hard and soft tissues. If there is adjacent articular cartilage, the
tidemark of the enthesis is contiguous with the tidemark of the joint,
which is usually linear with minimal undulations. Although not
technically Sharpey’s fibers, there are fibers that continue from the
tendon through the uncalcified fibrocartilage, tidemark, and calcified
fibrocartilage. Unlike the tidemark, the junction between calcified
fibrocartilage and bone is irregular and undulating. Anatomically, this is where the tendon ends and the bone begins.
higher than the maximal forces that the muscles with which they are
contiguous can generate. In addition, they should be able to
accommodate cyclic loading, as well as static loads without diminution
of tensile properties, fatigue, or irreversible elongation. Tendons are
anisotropic structures that demonstrate viscoelastic properties. They
have the highest tensile strength of any soft tissue. There are two
reasons for this fact. Collagen is the strongest fibrous protein in the
body, and the linear arrangement of its fibrils is parallel to the
direction of tensile force, making these structures ideally suited to
resist tension. Strain rate-dependent lengthening is observed. As the
elongation rate is increased, tendons appear stiffer. Because tendons
contain a relatively larger proportion of collagen to ground substance
than other musculoskeletal tissues, they demonstrate less
viscoelasticity and more purely elastic properties than these tissues.
The ultimate tensile strength of human tendons is 50 to 105 MPa.
Elastic strain energy recovery upon unloading of tendons is 90% to 96%
per cycle. The stress strain curve observed for tendons is similar to
those of other soft tissues (Fig. 1-9).
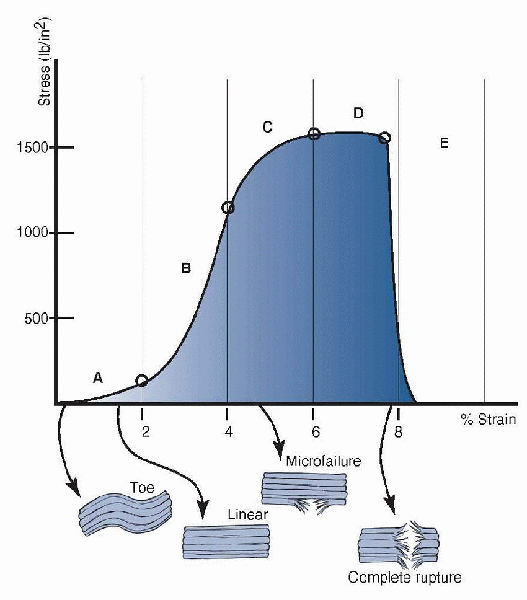 |
|
Figure 1-9 Stress-strain curve for a ruptured Achilles tendon. The five distinct regions are the toe region (A), the linear region (B), the progressive failure region (C), the major failure region (D), and the complete rupture (E). (From Hendrickson T. Massage for Orthopedic Conditions. Philadelphia: Lippincott Williams & Wilkins, 2003.)
|
crimps in the fibrillar structure. This requires relatively small
amounts of force, but because the fibers are already aligned in a
parallel fashion, the region is truncated relative to other
viscoelastic soft tissues. As with other substances, the slope of the
linear region of the curve represents the elastic modulus of the tendon.
biomechanical effect of exercise on tendons. Many of the results have
been conflicting. Ultrasonography of human tendon showed that the free
tendon of the vastus lateralis was significantly stiffer in
long-distance runners than in control subjects. A follow-up study
revealed that the knee extensor tendons became stiffer with isometric
training. The mechanism of this increased stiffness does not seem to be
increased collagen concentration, an increase in collagen cross-links,
or hypertrophy of the tendon itself. As alluded to later in this
section, decreased fatigability may be an adaptive response that serves
to prevent catastrophic damage associated with repetitive loading.
anatomical site and exercise. Tendons from young animals have
relatively small fibrils that fall within a unimodal distribution. As
animals age, fibril diameters increase and generally segregate in a
bimodal distribution. The stiffness and modulus continue to increase up
until maturity and then levels off. Collagen cross-linking increases
with age. The toe region of the stress-strain curve diminishes because
crimps in the tendon diminish.
manifestations of each, are diverse. The mechanisms and pathogenesis of
these injures are also quite different. Tendinopathies may involve the
tenosynovium, the peritenon, the tendon itself, or a combination of
these structures. The initial condition may be inflammation of the
peritenon secondary to overuse. If this inflammation becomes chronic,
the tendon proper may become inflamed or hypovascular as a result of
reduced perfusion through the peritenon. This induces degenerative
changes in the tendon. The mechanism of injury has a direct impact on
how these structures heal. Three main mechanisms of tendon injury are
laceration, contusion, and tensile overload. Tensile overload may
result in midsubstance tears, tears at the musculotendinous junction,
avulsion from bone, or avulsion fracture of the bone at the insertion
site.
by the acuity of their onset. Lacerations, contusions, tears, and
avulsion from bone are the most sudden in their acuity. Although the
treatment of these injuries may be similar, the reasons for their
occurrence are quite different. Because most tendons can handle tensile
forces greater than their accompanying muscles can generate, and
greater than the sheer forces able to be withstood by the bones into
which they anchor, midsubstance tears are uncommon. Musculotendinous
junction tears or avulsion fractures are more common than midsubstance
tendon injuries. Midsubstance tears of tendons require preexisting
tendinopathy at or near the site of the tear. Failure that occurs only
in collagenous material is due to the pulling apart of adjacent
collagen molecules, not the breakage of tropocollagen molecules.
Although within-the-hand and distal upper extremity lacerations
constitute the majority of tendon injuries, degenerative-type injuries
are more common in other anatomical locations.
and enthesopathies. Tendonitis most commonly occurs as a result of
overuse and is characterized by macroscopic or microscopic injury to
collagen fibrils, tendon matrix, and the supporting microvasculature
that results in inflammation and, secondarily, pain. Overuse tendon
injuries account for a significant percentage of sports injuries. The
ability to repair itself continually may be most important in
preventing tendon overuse injuries. In simulating an in vivo loading
pattern of the extensor digitorum longus tendon of the foot, the tendon
was loaded to 20% of its failure stress. After the equivalent of 4
months of normal walking (approximately 300,000 cycles), the tendon
failed. Because such tendon failure is not observed in vivo, repair and
remodeling must be central to physiological maintenance of the tendon.
It is enticing to speculate that overuse injuries of tendons may be due
to reparative mechanisms that are not able to counterbalance the
microtrauma associated with the inciting activity.
has been described as angiofibroblastic hyperplasia. The inflammatory
cells present are not characteristic of other acute inflammatory
conditions. Enthesopathies are defined as diseases that occur at the site of insertion of muscle tendons and ligaments into bone or joint capsules. Tendinoses are chronic degenerative conditions of tendons. More specific than chronic degeneration, tendinosis
refers to intrasubstance degeneration without histologic or clinical
signs of inflammation within the tendon. The morphologic changes
evident in tendinosis include proliferation of fibroblasts, appearance
of new capillary tufts, a decrease in collagen fibril diameter, and a
more wavy orientation of the collagen fibers. Matrix components also
show histologic changes. At the gross level, tendinosis shows
thickened, condensed, and desiccated-appearing regions.
interstitial microscopic failure of the tendon substance or central
tissue necrosis with mucoid degeneration. Because inflammation may or
may not be a part of this process, tendinosis may or may not be
associated with symptoms.
systemic disease, most cases result from overuse syndromes. These
overuse syndromes all have some component of chronic inflammatory
response that occurs in or around the tissue. A “tendinosis cycle” has
been described in which tendinosis results from changes in the load
experienced by tendons that are not compensated by adaptations of the
cell matrix. Microtears occur through pathologic tendinous tissue and
eventually result in tissue failure if there is not an adequate
healing, reparative, or hypertrophic response.
bacteriocidal and function through disruption of bacterial DNA gyrase.
Over the past decade, the increased use of fluoroquinolones in athletic
individuals as antimicrobial chemotherapeutic agents has resulted in
cases of fluoroquinoloneinduced tendinopathies being reported in the
literature. Frank ruptures have also been reported to occur. An
increased relative risk of Achilles tendon disorders with standard use
of these drugs has been epidemiologically demonstrated and is estimated
at 3.2 times that of a control population. It appears that this
increased risk is limited to those patients who are over 60 years of
age. Concomitant use of these antibiotics with corticosteroids in those
over 60 further increases the risk to 6.7 times that of a control
population. It appears that the risk of tendinopathy is increased in
those currently using the drugs and not those who have used them in the
past.
fluoroquinolone-induced Achilles tendonitis resulted in degenerative
alterations of the tenocytes. Electron microscopic findings include
multiple vacuoles and vesicles in the cytoplasm that
had
developed as a result of swellings and dilatations of cell organelles.
Cells not only lost normal cell-matrix interactions but also detached
from the extracellular matrix.
on tendons are not fully characterized, it is believed that the adverse
effects are the result of altered tendon fibroblast metabolism. Culture
of tendon fibroblasts with ciprofloxacin resulted in a 66% reduction in
cell proliferation relative to control cultures. Collagen synthesis was
also decreased by up to half of control values. Proteoglycan synthesis
was also diminished. These studies also suggested that fluoroquinolones
stimulate tendon matrix degradation by the upregulation of protease
activity. At this point, it seems likely that fluoroquinolones not only
decrease the synthesis of tendon structural components, but also
accelerate the degradation of these components. Caution should be used
when treating athletes with these medications.
process: the inflammatory phase, the fibroblastic phase, and the
remodeling phase. These phases have characteristic cellular, temporal,
and biomechanical patterns (Fig. 1-10).
-
The first phase has been labeled inflammatory phase and occurs in the first week following injury.
-
It starts with the migration of macrophages from tissues surrounding the injury.
-
During this phase, the macrophages remove
necrotic tissue and hematoma from the area of the injury, thereby
preparing the tissue bed for reconstruction. -
Collagenases and matrix
metalloproteinases play a key role in removing not only collagen
debris, but also matrix components from the site of injury.
-
-
The second phase of the healing response is the fibroblastic phase.
-
Fibroblasts proliferate and begin to
synthesize collagen and other proteins required for extracellular
matrix construction at this time. -
The fibroblastic phase is initiated
approximately 1 week after injury, but collagen synthesis reaches a
maximum 3 to 4 weeks after injury. -
It is believed that the fibroblasts that drive this phase originate from locally resident cells of the perivascular tissues.
-
Revascularization at the site of injury is also initiated during the fibroblastic phase.
-
-
The last phase is the remodeling phase.
-
The collagen fibers are originally oriented perpendicular to the long axis of the tendon.
-
At approximately 8 weeks after injury,
the recently laid down collagen fibers are brought into orientation
along the axis of the tendon. -
It is during this period that adhesions may become more numerous and tenacious.
-
Older individuals have a lower metabolic
activity within these structures that may be responsible for the
diminished age-related tendon healing capacity observed.
-
between collagen breakdown and synthesis. Initially, collagenase
activity and collagen breakdown predominate, but these levels diminish
and become equal to the rising synthesis levels by 4 to 6 weeks after
injury. After this time, synthesis and remodeling occur at a much
greater rate than breakdown.
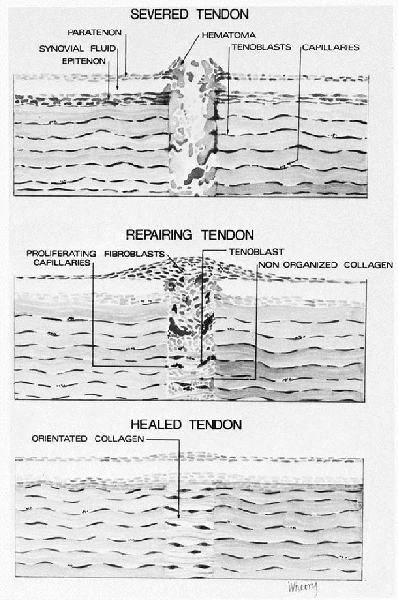 |
|
Figure 1-10
Sequence of events following tendon laceration. A hematoma forms between the tendon ends. Stimulated by chemotactic factors, inflammatory cells migrate into the hematoma, followed by blood vessels and fibroblasts. The fibroblasts synthesize a new matrix and then remodel the repair tissue to restore the structure and function of the tendon. Healing of the other dense fibrous tissues follows the same pattern. |
closely matches the histologic phases through which tendons pass during
healing. During the inflammatory phase, there is a decrease in the
tensile strength of the repair probably secondary to edema and tendon
degradation. In the fibroblastic phases, there is an increase in
strength that is furthered during the remodeling phase.
responses of these conditions are both reliant on, and severely
hampered by, the animal models used to study them. Most tendon,
ligament, and capsular injuries result from acute trauma to the tissues
or overuse syndromes. With the
exception
of lacerations, re-creating either acute or chronic soft-tissue
injuries in laboratory animals is difficult to perform and standardize.
Unlike the exquisite “knock-out” or “knock-in” recombinant techniques
that have resulted in transgenic animals to model single molecular
defect diseases, no elegant models for most tendon, ligament, and
capsular disease amenable to repair are available.
tendon injury was predominantly an intrinsic or extrinsic phenomenon.
The extrinsic mechanism depends on fibroblasts and inflammatory cells
entering from the periphery of the injury to effect repair of the
tendon. The intrinsic mechanism involves migration of fibroblasts and
inflammatory cells from within the tendon and epitenon. It is now
believed that tendon healing involves both intrinsic and extrinsic
mechanisms, with the latter predominating in the early phases and the
intrinsic predominating in a more delayed fashion. Some hypothesize
that an imbalance favoring the extrinsic mechanism leads to increased
collagen content at the repair sight, as well as a suboptimal level of
collagen organization. As a consequence, predominance of the extrinsic
mechanism may result in scar formation and adhesions between the tendon
and surrounding tissues.
the strength of repairs and excursion able to be obtained at the end of
healing. As little as 2 mm of passive excursion at low levels of force
is adequate to inhibit adhesion formation and promote healing.
Increases of force and excursion beyond this do not accelerate the
healing process. Repair of lacerated digital flexor tendons should be
done within a few days of injury to maximize final tendon excursion and
minimize the angular rotation of the repair.
not suffice to return the athlete to competition. In most cases, a
repaired tendon must maintain a substantial amount of its excursive
ability to maintain appropriate function. The ability to do this is
dependent on the ability to achieve a strong repair at the injury site,
as well as prevent adhesions from forming during the healing process.
Adhesions can severely restrict tendon gliding after tendon healing has
occurred. As previously described, it is believed that an overly
aggressive extrinsic healing response results in the formation of these
adhesions. Physical, pharmacologic, and biologic approaches have been
used to combat this problem.
effects by causing thymidine depletion and disrupting RNA processing.
The use of this pharmacologic agent to prevent tendon adhesions has
been examined. At the sites of tendon repair, there is a less vigorous
cellular response and a decrease in the local levels of transforming
growth factor-β (TGF-β) which is a known potentiator of the fibrotic response. Fewer adhesions are observed histologically at repair sites.
applied to the sites of suture-repaired tendons resulted in fewer
adhesions relative to control groups. Although further work needs to be
done to identify and isolate those factors responsible for the
inhibitory properties of human amniotic fluid, the latter treatment may
provide a currently available, cost-effective, and simply applied
physiochemical barrier by which peritendinous adhesions can be
minimized or prevented at repair sites.
tendon repair decreases the amount of peritendinous adhesions without
adverse affect on gliding or strength of the treated tendons. Heat
shock proteins limited the local inflammatory response and subsequent
adhesion formation.
musculoskeletal injuries and affect 5% to 10% of people up to age 65.
Anatomically, ligaments connect bone to bone across an articulation.
They function as joint stabilizers resisting forces applied to the
joint while allowing joint motion. Other structures (including bone,
cartilage, and tendons) contribute to joint stability by sharing the
load across the joint. When ligaments are injured, forces they normally
resist are shifted to the other structures. When the ligament does not
heal properly, these structures are at risk for degeneration and
failure. Thus, it is important to consider several biological,
mechanical, and surgical factors to optimize functional recovery of the
injured ligament.
aqueous components. Cell types are fibroblasts, endothelial cells, and
nerve cells. Fibroblasts are the primary cells in ligaments and are
responsible for synthesizing extracellular matrix components, including
collagen. The extracellular matrix is composed of 60% water and 40%
collagens, elastin, proteoglycans, and noncollagenous proteins.
Collagens are fibrillar proteins with high tensile strength composed of
three polypeptide chains arranged in a helical pattern (tropocollagen)
and covalently cross-linked together. Different combinations of
polypeptide chains yield different types of collagen and higher levels
of cross-linking between tropocollagen chains confers greater
mechanical strength to the collagen and ligament. Collagen constitutes
70% to 80% of ligament dry weight. More than 90% of collagen found in
ligaments is type I, approximately 10% is type III, and other types may
be present in smaller concentrations. Ligaments contain a lower
percentage of collagen than tendons. Elastin, a protein rich in glycine
and proline, comprises less than 5% of dry weight in most ligaments. It
is found in higher concentrations in flexible ligaments, such as the
ligamentum flavum. Proteoglycans are composed of polysaccharide chains
[glycosaminoglycans (GAGs)], which are connected to a protein core.
They are negatively charged molecules that bind water and other
positively charged ions. They form less than 1% of ligament dry weight.
Most ligaments have higher GAG content than tendons. Two types of
proteoglycans are found in ligaments: larger molecules with chondroitin
and keratin sulfate GAG chains, and smaller molecules with dermatan
sulfate chains. Proteoglycans serve to maintain ligament water content,
organize the
extracellular
matrix, and interact with cellular elements. Noncollagenous proteins
include fibronectin, a protein that allows cells to interact with the
extracellular matrix.
small blood vessels originating at the ligament insertion sites. Cells
contained within the ligament are maintained by this blood supply and
through diffusion from the local aqueous environment. Nerve fibers have
been noted in medial collateral ligament (MCL), and ACL specimens and
are postulated to have proprioceptive, mechanoreceptive, and
nociceptive functions.
sheet-like structures that insert into bone on either side of an
articulation. There are intra-articular and extra-articular ligaments.
Their insertions have been described as direct or indirect based how
the collagen fibers attach to bone. Direct insertions, such as the
femoral insertion of the MCL or tibial insertion of the ACL, have
ligament fibers passing directly into the cortex. This transition has
been observed occurring over four zones: ligament, fibrocartilage,
mineralized fibrocartilage, and bone. Indirect insertions, such as the
tibial insertion of the MCL, have a broader area of insertion,
superficial fibers that insert obliquely into the periosteum, and
deeper fibers inserting via Sharpey’s fibers forming the indirect
insertion. Indirect insertions may be elevated off the bone without
cutting the ligament itself, where direct inserting ligaments require
cutting the substance of the ligament to detach it.
in terms of the structural properties of the bone ligament bone complex
and mechanical properties of the ligament substance. The structural
properties of the bone ligament bone construct, which are influenced by
ligament composition and insertion type, are tested with tensile stress
and plotted on load-elongation curves. Stiffness is defined as the
resistance of a structure to deformation, or the slope of the
load-elongation curve. This curve has an initial low-stiffness toe
region followed by a higher-stiffness linear region. This increase in
stiffness has been attributed to the initial straightening of the
undulating crimp pattern and nonuniform recruitment of the individual
fibers as represented in the low-stiffness (and larger elongation) toe
region of the curve. As the fiber bundles straighten and more fibers
are recruited, stiffness increases. The ultimate load on the curve is
where construct failure occurs. The slope may decrease before failure
if individual fibers fail before the entire construct fails. Area under
the curve is energy absorbed before failure.
influenced by collagen composition, collagen fiber orientation, and
interaction with the extracellular matrix—are tested with tensile
stress and plotted on stress-strain curves. This slope of this curve is
the elastic modulus. The tensile strength is the maximum stress before
failure. The elastic modulus of the MCL is approximately twice as much
as the ACL. The MCL has more densely packed fiber bundles per unit area
with less crimp, or wave pattern, and greater fiber diameter than the
ACL. Ligaments also display viscoelastic properties that reflect
interactions of collagen and other extracellular matrix molecules:
creep, stress relaxation, and hysteresis. It has been shown that
preconditioning ACL grafts prior to reconstruction may reduce the
amount of stress relaxation by approximately 50%, compared with
ligament grafts with no preconditioning.
ligaments, including biochemistry, immobilization, and aging.
Stiffness, ultimate load, and energy absorbed at failure increase with
age and skeletal maturity, which may be attributable to further
tropocollagen chain cross-linking. As ligaments mature, the
bone-ligament-bone construct failure sites change. Rabbit MCL testing
has shown that younger rabbit ligaments fail by tibial avulsion and
mature ligaments fail by intrasubstance ligament tears. Ligament
immobilization causes intrasubstance and insertion site changes that
alter structural properties. Insertion sites reveal disruption of deep
fibers inserting into bone and osteoclastic activity that results in
subperiosteal bone resorption. Mobilization causes slow reversal of
these effects with ultimate load and energy absorbed at failure almost
reaching control levels.
Extra-articular ligament healing passes through stages similar to
general wound healing. An inflammatory phase is followed by a
proliferative repair phase and, ultimately, a remodeling phase.
-
The inflammatory phase, which usually
occurs within 24 hours of injury, is characterized by release of
inflammatory mediators, increased blood flow, and migration of
inflammatory cells.-
Immediately following ligament tear, the gap between the torn ligament ends is filled by hematoma.
-
Vasodilators and inflammatory mediators
are released into the local environment causing increased blood flow,
vascular permeability, and plasma fluid exudation, which are
responsible for local tissue edema. -
Factors released also stimulate inflammatory cell migration into the zone of injury.
-
Polymorphonuclear cells initially (and
later, monocytes) are present in the injured tissue and release enzymes
that breakdown necrotic tissue. -
Macrophages phagocytose the necrotic debris.
-
Within 3 or 4 days, capillary venules
form new vascular buds, as endothelial cells are attracted toward the
zone of injury by chemotactic factors. -
These cells begin to proliferate and
ultimately form new capillary vessels, establishing blood flow in the
forming granulation tissue. -
Toward the end of the inflammatory phase, fibroblast migration and proliferation begin.
-
These cells will produce collagen, proteoglycans, and other matrix molecules that form the initial scar.
P.18 -
-
The proliferative or repair phase is characterized by an increase in cells and matrix molecules.
-
Hematoma is replaced with highly cellular immature scar with limited tensile strength during this stage.
-
It starts within 48 to 72 hours of injury and continues over the next several weeks.
-
Fibroblasts proliferate in the granulation tissue filling the ligament gap.
-
New blood vessels are forming while fibroblasts synthesize matrix.
-
-
The remodeling and maturation phase of
ligament healing is characterized by a decrease in cellularity and
vascularity, whereas organization of collagen and matrix increases.-
This phase starts at several weeks after injury and may last longer than 12 months.
-
Cellular and vascular density also decrease to near-normal levels.
-
Ultimately, this phase results in a
mature healed ligament with a greater volume of ligamentous tissue but
with material properties that are less than normal uninjured ligament.
-
|
TABLE 1-1 GRADING OF LIGAMENT INJURIES
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
It has less tensile strength, usually 50% to 70% of normal. Mechanical
properties of healing intrasubstance MCL tears are inferior, but they
have a larger cross-sectional area than the intact MCL. Although the
healed ligament has lower tensile strength than an uninjured ligament,
it does not usually result in alteration of joint function. This may be
partially explained by the increased volume of the repaired tissue
compared with the uninjured ligament.
extent of injury, treatment type, immobilization, and systemic factors.
Intrasubstance ligament tears of extra-articular ligaments appear to
heal, resulting in a more functional ligament than in intra-articular,
intrasubstance tears. This may be influenced by the synovial
environment and decreased vascular ingrowth of the intra-articular
ligaments. The extent of injury and multiple ligament involvement also
impacts ligament healing. Combined ligament injuries have a worse
prognosis than single ligament sprains. The torn MCL has some support
from uninjured structures, including the ACL and joint capsule. When
the ACL is torn in addition to the MCL, the knee is less stable and MCL
healing quality is diminished.
the injury gap that would be filled with scar tissue and may increase
initial structural strength by 10% to 30%. Repair of collateral
ligaments in ACL-deficient knees did not improve structural strength.
This is likely caused by the instability of the ACL injury leading to
increased strain on the MCL. Suture repair has not been shown to
ultimately affect increases in ligament strength, laxity, or stiffness.
disorganization of collagen fibrils with decreased matrix remodeling,
decreased mechanical and structural properties, and bone resorption at
ligament insertion sites. Prolonged joint immobilization may also
result in damage to the articular surface, changes in the osseous
geometry, and inducement of joint adhesions.
injured ligaments results in improved scar stiffness and strength
without compromising scar length. Extreme loading results in repair
tissue disruption and may delay or prevent healing. Totally unloading a
ligament also diminishes healing and results in decreased collagen
fibril diameter and decreased ligament mechanical properties. The
positive effect of early motion is likely mediated by local mechanical
effects on fibroblasts, as well as effects on blood flow and
inflammation. Movement may be responsible for stimulation of collagen
synthesis and increased matrix remodeling, resulting in increased scar
mass with increased tensile strength. Ligament mobilization has been
reported to increase the ultimate load of the healed tissue. Increased
scar formation appears to occur when injured ligaments are mobilized
within weeks of injury. Later mobilization stimulates scar remodeling
but may not result in increased scar mass. Short-term immobilization
for pain control, followed by early mobilization, will provide optimal
results. Clinical studies suggest that controlled motion favorably
affects MCL healing and knee function. Current recommendations for
isolated MCL injuries include controlled early range of motion as soon
as pain subsides. The goal is to avoid aggressive movement that will
cause excessive disruptive force across healing ligament. Early
controlled motion is also important after ACL reconstruction because it
had been shown to minimize capsular contractions that lead to joint
stiffness and have a positive effect on articular cartilage.
environment. Endocrine abnormalities may lead to altered collagen and
proteoglycan synthesis causing decreased mechanical strength. Diabetes,
vascular disease, and infection may prolong the inflammatory stage.
and stem cell therapy may enhance quality of ligament healing. Growth
factors are small peptides that bind to cell surface receptors and
stimulate various cellular functions, including protein synthesis and
cellular feedback loops. Growth factors—such as TGF-β,
vascular endothelial growth factor, platelet-derived growth factor,
basic fibroblast growth factor, and insulinlike growth factor—have been
shown to be elevated at various stages of ligament and tendon healing.
These factors are involved with chemotaxis of fibroblasts and
inflammatory cells to the injury site; they stimulate cell
proliferation and angiogenesis, as well as matrix molecule synthesis.
Manipulation of these factors may lead to improved
ligament
healing. Gene therapy, transferring growth factor genes in viruses or
liposomes, is under investigation to improve delivery of growth factors
to injury sites. Mesenchymal stem, or progenitor, cells implanted into
injury sites may be a source for cell types necessary for the healing
process.
PC. Tendon and ligament. In: Lindblad WJ, ed. Wound Healing:
Biochemical & Clinical Aspects. Philadelphia: WB Saunders,
1992:384-395.
JA, Woo SL. Basic science and injury of muscle, tendon, and ligaments.
In: Drez D, ed. Orthopaedic Sports Medicine: Principles and Practice.
Philadelphia: WB Saunders, 2003:39-49.
K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment
strategies and performance factors. Sports Med 2003;33: 145-164.
RJ, Braun J, Khan MA. Entheses and enthesitis: a histopathologic review
and relevance to spondyloarthritides. Curr Opin Rheumatol
2001;13:255-264.
CB, Hart DA, Shrive NG. Molecular biology and biomechanics of normal
and healing ligaments—a review. Osteoarthritis Cartilage 1999;7:130-140.
WE Jr, Best TM. Anatomy, physiology, and mechanics of skeletal muscle.
In: Dimon S, ed. Orthopedic Basic Science. Rosemont, IL: American
Academy of Orthopaedic Surgeons, 1994:97-118.
RJ, Beynnon BD, Nichols CE, et al. The treatment of injuries of the
anterior cruciate ligament. J Bone Joint Surg Am 1992;74: 140-151.
B, Sathy MR, Talkington J, et al. Treatment of isolated medial
collateral ligament injuries in athletes with early functional
rehabilitation. A five-year follow-up study. Am J Sports Med
1994;22:470-477.
RJ III, Attia E, Wickiewicz TL, et al. The effect of ciprofloxacin on
tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med.
2000;28:364-369
