Microinnervation: Pain Generators
physiology of nociceptive spinal anatomy. Many parts of the spinal
column are pain sensitive, are richly innervated, and are potential
pain generators. These parts of the spinal column include the following:
-
Anterior and posterior longitudinal ligaments
-
Vertebral body
-
Synovium of the articular facets
-
Nerve roots
-
Muscle
-
Supporting soft tissues
-
Presence and degree of myelination (e.g., unmyelinated C fiber versus myelinated A fiber)
-
Type of stimulation that provokes nerve fiber response
-
Type of response
structure must have nociceptive innervation. In general, the skin,
mucous membranes, and periosteum may be considered the most
“peripherally” innervated tissues. Innervation is concentrated within
these “coverings.” There are various receptors in these structures.
Some are specific for a single stimulus type (e.g., heat or pressure),
whereas others are polymodal.
usually suggests a nociceptive function for that fiber. Two nerve fiber
types in particular seem to be important for pain transmission:
nonmyelinated C fibers and a specific subset of myelinated A fibers
known as thinly myelinated A delta fibers.
The relative amounts of myelin influence the speed of nociceptive pain
transmission. The cell bodies of these afferent fibers are located
within the dorsal root ganglion of each spinal nerve root or within the
gasserian ganglia of the trigeminal nerves (centrally, cranial nerves).
The neurons themselves are bipolar, with axons extending from the cell
bodies to the innervated structure (afferent) and another extending to
the spinal cord or brainstem.
the central fibers of the primary nociceptive afferents (A delta and C)
terminate in the superficial regions of the dorsal aspect of the spinal
cord. Pain information first is “integrated” within the dorsal root
entry zone. The dorsal root entry zone is
defined as the proximal aspect of the dorsal nerve root and its
corresponding superficial layers within the dorsal horn of the spinal
cord. From there, secondary fibers proceed to the thalamus via the
spinothalamic tract. Physiologically the A delta fibers are faster
conducting (because they are partially myelinated) and transmit sharp,
localized pain; the unmyelinated C fibers are slower and transmit
poorly localized, dull, burning, or aching sensations.
mechanical/heat nociceptors are present in high density within the skin
of the hand. Mechanically insensitive afferent fibers have a high
mechanical threshold and are more prevalent within highly mobile
diarthrodial joints with synovial capsules, such as the knee.
character of the pain that is transmitted. Deeper structures, such as
muscle, ligament, and the intervertebral disc, produce pain sensations
that are diffuse and poorly localized, whereas cutaneous nociception
usually is sharp and easily localized. Deep pain also can be associated
with autonomic nervous responses. These often are produced by stimuli
that are not tissue damaging.
covering the spinal cord and nerve roots has copious unmyelinated fiber
nociceptive innervation. Little is known about the physiology of the
primary afferent fibers innervating the meninges (Fig. 30-2).
Janig and Koltzenburg suggested that pain fibers in the ventral root
may serve as the primary afferent fibers that innervate the root or
sheath itself. These fibers have no resting neural discharge and seem
to be maximally activated by noxious stimuli.
radiculopathy from a herniated disc. Assuming that these fibers are
relatively mechanically insensitive, compression of a noninflamed,
normal nerve may not produce radicular pain, whereas it causes neural
deficit. Compression of an inflamed nerve root is more likely, however,
to produce typical referred neuropathic pain, in addition to
corresponding dermatomal anesthesia or motor deficit. This phenomenon
has been shown elegantly in animal studies.
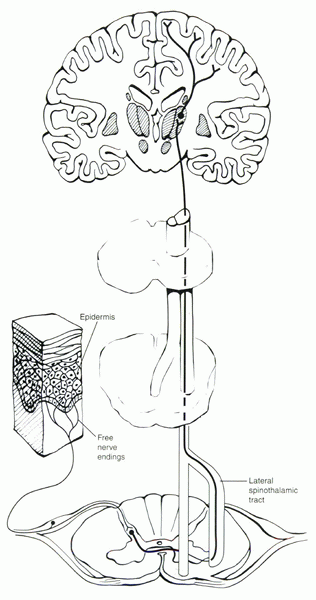 |
|
Figure 30-1
Pathways for nociception, which begin with the pain receptor (free nerve ending) and end in the brain, where pain actually is perceived. |
is associated with referral along the peripheral distribution of fibers
in the root. Although dermatomal maps are accurate and clinically
useful, the exact cutaneous afferent distributions of spinal nerves are
not defined precisely in humans, with considerable variability within
and among individuals. This has been referred to as normal pain,
which is thought to be mediated via the nociceptive innervation of the
nerve root sheath resulting in the perception of sciatica.
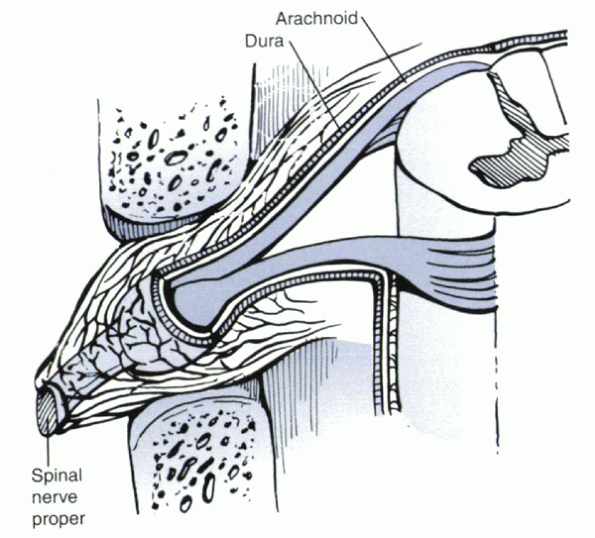 |
|
Figure 30-2
The dura, becoming confluent with the perineurium of the spinal nerve proper within the neural foramen, is richly innervated and may be a source of pain itself. |
This pain classically is related to nerve root or spinal cord injury.
Clinically, neuropathic pain may result in different sensations. It can
be spontaneous and not associated with tissue damage. Neuropathic pain
may result in allodynia, in which a normally benign stimulation produces pain; hyperalgesia, in which an exaggerated painful sensation follows a noxious stimulation; or hyperpathia,
which is characterized by abnormal pain from an area where fibers have
reset to a lower threshold for detection of any sensation. Referral of
pain and allodynic tenderness with skin stimulation without deep tissue
damage is relatively common.
mechanism in which successive painful stimuli lead to greater than
usual pain sensation. This mechanism is physiologically mediated by C
fiber nociceptors. If peripherally activated by stimuli that are no
more than 3 seconds apart, the pain intensity increases with each
successive stimulus. This increase in intensity is thought to be due to
an increased response of spinal dorsal horn neurons to repeated C fiber
input.
in patients with autonomic dysfunction. In these patients, the
sensation of mechanical allodynia is mediated by activation of tactile
A beta low-threshold mechanoreceptors (not C fibers), whose activation
normally is followed by tactile sensations. Repeated A beta stimuli
produce burning pain sensations of increasing intensity when stimuli
are presented at intervals of 3 seconds or less. This situation clearly
is pathologic because the lower threshold mechanoreceptors are
providing direct input to the central mechanism via an underlying
wind-up mechanism, which normally is mediated by C fiber nociceptor
input.
is important to realize that acute and chronic pain may coexist. Normal
pain due to a reversible source, such as inflammation or injury, may
coexist with neuropathic pain. A classic example is persistent
radiculopathy after lumbar disc excision. Although residual compression
to a nerve root should be ruled out as a source of the failed surgery,
coexistent nerve damage may be producing symptoms of allodynia or
hyperpathia, which may not respond to revision decompression. Allodynia
and hyperpathia should be differentiated from normal motion segment
pain from activation of periosteal nociceptors in the facets and normal
sciatic pain due to activation of nociceptors within the affected
neural sheath. Normal pain is that which is produced through
nonpathologic nociceptive mechanisms.
spinal column, specifically the lumbar discs, facets, and supporting
ligaments.
degeneration per se does not cause discogenic pain. The pathophysiology
of discogenic pain is incompletely understood. The interplay of the
underlying anatomy and physiology of disc innervation and the
degenerative cascade appear to be key components (Fig. 30-3).
penetrate more deeply than the outer one third of the anulus, shown as
an association between ingrowth of nerves expressing substance P and
discal degeneration. The extent of this neoneuralization was greatest
at the intervertebral disc level where the patients experienced pain. Coppes et al
noted that disc degeneration is associated with centripetal growth of
nerve fibers into the disc. This finding provides a potential
morphologic basis for discogenic pain.
the outer anulus that he described based on anatomic organization
(e.g., simple versus loops). He also found encapsulated and partially
encapsulated nerve endings on the superficial anulus. These studies
agree with clinical work that identifies the disc as a source of back
pain. In these studies, stimulation of the posterior anulus produced
back pain in most subjects. One theory of disc degeneration
hypothesizes that peripheral tears of the anulus lead to acceleration
of dehydration and fraying of the nucleus. This theory has been tested
in a sheep model, wherein peripheral tears in the anulus were observed
to contain vascular ingrowth. Nociceptors may accompany this vascular
ingrowth and, in the degenerated disc, may account for the presence of
an afferent sensory nerve supply in the inner anulus. This does
not appear to be the case in the normal (i.e., undegenerated) disc (Fig. 30-4).
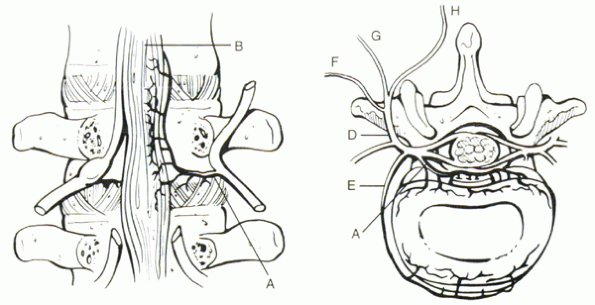 |
|
Figure 30-3 (Left) The innervation of the disc and facet joints is complex. Branches of the sinuvertebral nerve (A) innervate the posterior longitudinal ligament (B). Usually the nerve root overlies these branches. (Right) The sinuvertebral nerve (A) also innervates the dorsal portion of the disc. Branches from the ventral ramus (E)
supply the ventral disc and anterior longitudinal ligament. Only the outer 50% of the anulus is richly innervated in the normal disc. The dorsal ramus (D) divides into lateral (F), intermediate (G), and medial (H) branches. The medial branches innervate the facet joint and are the target of radiofrequency rhizotomy. |
to be a potential pain generator. Central stimulation of the posterior
longitudinal ligament produces central back pain with right or left
stimulation producing right-sided or left-sided pain. The posterior
longitudinal ligament is connected intimately with the posterior aspect
of the anulus fibrosus; the microinnervation is similar in this
structure. The main afferent pathway involves the nerve to the
vertebral body (sinuvertebral nerve). This nerve also may be implicated
in the pathophysiology of spinal compression fractures.
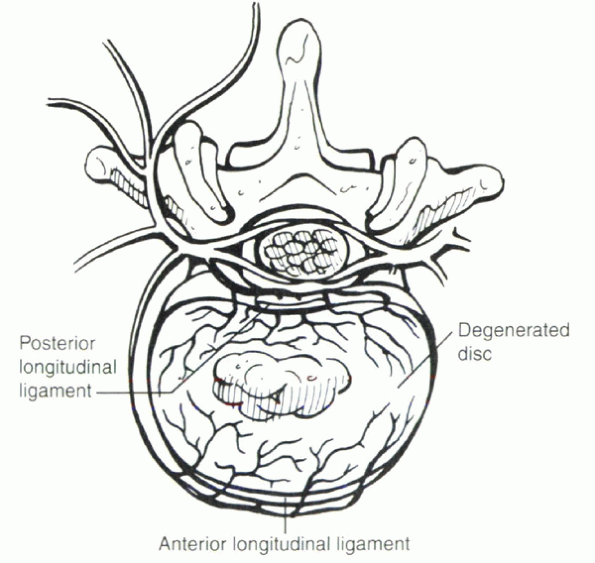 |
|
Figure 30-4
In the degenerated disc, nociceptive nerve fibers can penetrate more deeply (>50%). These fibers may be accompanied by buds of vascular ingrowth. In the degenerated disc, this may account for the presence of an afferent sensory nerve supply within the inner anulus. This does not appear to be the case in the “normal” (i.e., undegenerated) disc. |
formed by the ventral ramus and autonomic root from the gray-ramus
communicans. This nerve innervates the ventral aspect of the dural sac,
posterior longitudinal ligament, anulus, and blood vessels of the
vertebral body. The branches of the sinuvertebral nerve go only to
structures in the spinal canal. Transverse and descending branches
supply the posterior longitudinal ligament in intervertebral discs at
the level of nerve entry, with an ascending branch to the next rostral
level, overlapping the innervation of the sinuvertebral nerve at that
level. The anterior longitudinal ligament is supplied by the gray-rami
communicans or from the sympathetics.
in disc tissue from patients undergoing discectomy for sciatica versus
patients undergoing fusion for discogenic low back pain. There were
significant differences in the production of interleukin-6 and
interleukin-8 in the sciatica and in the low back pain groups, with
high levels of proinflammatory mediator found in disc tissue from
patients undergoing fusion. This finding suggests that production of
these mediators within the nucleus may be a factor in the development
of a painful disc. These mediators may stimulate the nociceptive
pathways that have penetrated the anulus as part of the degenerative
cascade.
As a rule, free nerve endings may be pain receptors or temperature
sensitive, whereas encapsulated nerve endings usually are pressure or
position sensitive. The medial branch of the posterior primary ramus is
the primary afferent pathway from the capsule. The capsule can undergo
extensive stretch during normal physiologic loads.
the presence in the capsule of substance P, calcitonin gene-related
peptide, and vasoactive intestinal peptides, which are known mediators
of inflammation. The facet joint can serve as a pain generator. Does it
serve as a clinically relevant pain generator? A question arises as to
the exact mechanism of pain generation: Does capsular deformation or
bone impingement result in pain? Classically the literature suggests
that synovial folds lack innervation. This has been shown not to be the
case, however, with several works reporting nerve endings on blood
vessels and fat cells in synovial folds in the human. Whether or not
these nerve endings are sensitive is questionable. In several studies,
human tissue did not show immunoreactivity to substance P. On the basis
of the relative neuropeptide immunoreactivity, it has been suggested
that the sensory nerves are involved in regulation of blood flow and
not nociception. Clinically the association of posterior element pain
with facet degeneration has been well described.
by injection of hypertonic saline into the facet joint capsules. The
clinical significance of this study is not clear, however. The most
reproducible diagnostic technique to identify so-called facet-mediated
pain remains the intracapsular extraradicular facet block. In a
prospective study of 109 patients with back pain, Lilius et al found no
difference in rates of pain relief in the group that received a
standard steroid/local anesthetic injection from the group that
received a saline injection. In a larger study (n = 454), Jackson et al attempted to identify the clinical characteristics of patients responsive to facet injections but could not do so.
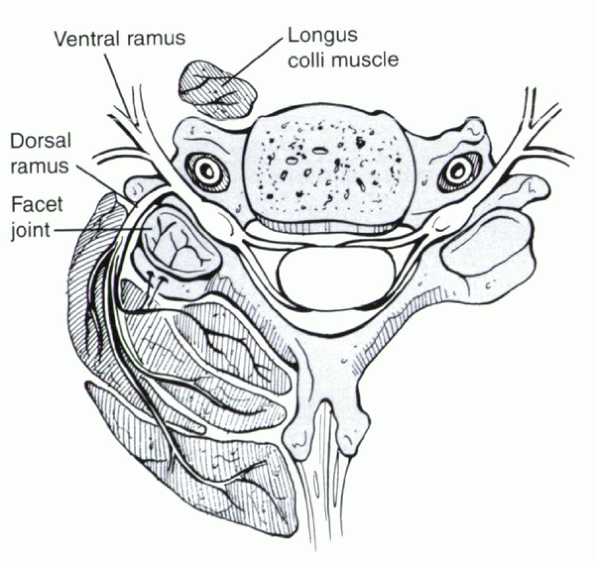 |
|
Figure 30-5
Branches from the dorsal ramus supply the muscles of the paraspinal muscles of the cervical spine and the facet joint. This may help explain the common occurrence of muscle soreness and pain in patients with facet degeneration, despite a lack of structural injury to the muscle itself. |
whiplash syndrome, facet-mediated pain may play a more readily
definable role. A percutaneous radiofrequency rhizotomy or neurotomy
has been described as a potential treatment for facet-mediated pain via
interruption of the medial branch of the posterior primary ramus. In a
prospective study, patients with chronic facet-mediated neck pain
secondary to whiplash syndrome identified by facet blockade were
randomized to one of two groups. One group was treated with
radiofrequency rhizotomy (which ablates the nociceptive innervation of
the facet) versus a sham procedure. Greater pain relief (statistically
significant) was observed in the experimental versus control group.
Pain relief was not permanent in the rhizotomy group, however.
“sprain or strain,” scientific evidence for a muscular origin for low
back pain is lacking. Whether or not there is primary persistent muscle
pain that occurs in the absence of an underlying structural disease is
not clear.
however, nociceptors are in the relative minority. Mence studied muscle
units in the triceps and the tendocalcaneus of cats, and found a
variety of fibers, including low-threshold, pressure-sensitive,
nociceptive, contraction-sensitive, and thermosensitive fibers.
Quantitatively the nociceptors accounted for approximately 38% of the
fibers.
Group I and group II are related to proprioception; group III and group
IV are afferents. Group III are A delta fibers signaling pain,
temperature, and touch, whereas group IV are C fibers signaling itching
as well.
bradykinin, prostaglandin E2, and serotonin. Authors have speculated
that an increased discharge rate on the basis of muscle inflammation
with consequent expression of these mediators could account for the
spontaneous pain in tissue inflammation. The clinical significance of
this speculation is unclear, however.
initiating or conducting painful impulses. Many of the neuropathic pain
impulses are mediated via the dorsal root ganglion. The large-diameter
cells in the ganglion give rise to large myelinated A beta fibers,
whereas small-diameter cells give rise to unmyelinated C fibers and
finely myelinated A delta fibers. The dorsal root ganglion also has
been shown to contain inflammatory peptides and may serve as a
modulator of disc-related nociception. Additionally, virtually all
of
the structural elements in the spine (intervertebral disc, supporting
spinal ligaments, especially the posterior longitudinal ligament, facet
capsules, and muscles) may be sources of pain generation. The interplay
of the various elements is complex, and the ability of various
structures (e.g., muscle) to cause pain in the absence of a more global
problem with the motion segment is unclear.
IK, Ashton BA, Gibson SJ, et al. Morphological basis for low back pain:
the demonstration of nerve fibers and neuropiptides in the lumbar facet
joint capsule and not in the ligamentum flavum. J Orthop Res
1992;10:72-78.
JG. Watson RW, McCormack D, et al. Intervertebral discs which cause low
back pain secrete high levels of proinflammatory mediators. J Bone
Joint Surg 2002;84B:196-201.
RP, Jacobs RR, Montesano PX. Facet joint injection in low back pain: a
prospective statistical study. Spine 1988;13:966-971.
G, Laasonen EM, Myllymen P, et al. Lumbar facet syndrome: a randomized
clinical trial. J Bone Joint Surg 1989;71B:681-684.
SM, Baensley L, Wallis BJ. Percutaneous radiofrequency neurotomy for
chronic cervical zygapophyseal joint pain. N Eng J Med
1996;335:1721-1726.
