Cervical Spondylosis and Stenosis
person ages and manifests as degenerative changes in the cervical
spine, including disc degeneration, facet arthropathy, osteophyte
formation, ligamentous thickening, and straightening of cervical
lordosis. When the spondylotic changes progress, they may cause
narrowing of the cervical canal, leading to spinal stenosis. This
condition can cause symptoms and signs consistent with radiculopathy,
myelopathy, or a combination of both. Spinal stenosis
is defined as a decreased space for the spinal cord. Spondylosis often
is thought of as a progressive and dynamic process, with most patients
with radiographic findings compatible with spondylosis remaining
asymptomatic.
associated clinical complaints of neck pain, referred scapular pain,
and possibly nerve root or cord compression or both. Radiographic
changes are seen in 95% of patients older than age 65 years. Men are
affected more than women, and the changes that occur in men often are
more severe. There are identifiable risk factors, including frequent
lifting, cigarette smoking, driving, and a congenitally narrow spinal
canal. Congenital spinal stenosis predisposes a patient to myelopathy
when spondylosis develops.
but a few salient points are reviewed here. The cervical spine
comprises seven cervical vertebrae, of which C3, C4, C5, and C6 are
considered typical vertebrae. The typical sagittal alignment of the
cervical spine is 20 degrees of lordosis. Vertebral discs compose 22%
of the height of the cervical spine, allow motion between segments, and
distribute weight evenly over the surface of the vertebral bodies.
as an intervertebral disc and the vertebrae above and below. Each
motion segment has five articulations. These articulations include the
intervertebral disc anteriorly, two facet joints posteriorly, and two
neurocentral (uncovertebral) joints that lie along the posterolateral
border of each typical vertebral body. With aging, these joints become
arthritic and hypertrophied. Disc degeneration often is the instigating
factor in spondylosis. With aging, the water content of the nucleus
decreases, as does the concentration of glycosaminoglycans, and there
is a resultant loss of disc height. There is a marked increase in the
glycosaminoglycan keratin sulfate and decrease in chondroitin sulfate
in the nucleus. Loss of intervertebral height and possible segmental
instability interfere with the normal hydrostatic load-bearing function
of the disc.
nucleus becomes less well defined. Desiccation of the disc results from
increased strain on the anulus, which fibrillates and weakens,
contributing to decreased disc height. Segmental instability may occur
at either a micro or a macro level. With the loss of disc height, the
spine may lose its lordotic posture, and kyphosis may result. Kyphosis
due to spondylotic changes leads to an abnormal strain on the posterior
facet joints, also called the zygapophyseal joints.
These true diarthrodial joints, with synovial membranes and fibrous
capsules, may form osteophytes, which decrease the space available for
the spinal cord. In addition, the neurocentral joints (the joints of
Luschka) hypertrophy and may encroach on the spinal canal. Spondylytic
change also affects the foramina, resulting in restricted motion and
possible nerve root compression. Osteophytes also may form at the
anterior and posterior aspects of the vertebral bodies. The posterior
osteophytes may impinge further on the neural space.
neurologic compression secondary to a mechanical etiology. Spondylosis
is most common at C5-6 followed by C6-7; these are the individual
motion segments with the most mobility in the lower cervical spine.
Disc function is the first to be affected, often by the 20s. When discs
degenerate, discogenic pain may be the result. This symptom complex
leads
to
axial neck and referred pain. Men present commonly with shoulder and
lower neck pain, and women present with midline pain. Typically, axial
pain increases with activity and decreases with rest. The pain often is
insidious, without a clear inciting event, and may wax and wane over
time. The exact anatomic location of the pain generator is difficult to
identify.
are defined as the osteophytes or bone spurs that result from
spondylosis. Lower motor neuron signs predominate in patients with
radiculopathy and include the following:
-
Numbness
-
Paresthesia
-
Weakness
-
Decreased deep tendon reflexes
the exiting nerve root (i.e. at C3-C4, the C4 nerve root). Herniations
of the nucleus most commonly are posterolateral, however, between the
posterior edge of the uncinate process and the lateral border of the
posterior longitudinal ligament. Here the exiting nerve root is
compressed along with the spinal cord if the herniation is large. When
the cord is compressed, myelopathic syndromes can develop. Central disc
herniations compress the cord directly and can manifest as myelopathy
or myeloradiculopathy.
-
Central stenosis
-
Lateral recess stenosis
-
Foraminal stenosis
-
Congenital stenosis
-
Acquired (degenerative) stenosis
process and becomes significant if the patient is symptomatic.
Degenerative changes consisting of loss of disc height (with buckling
of the ligamentum flavum posteriorly), osteophytic change in the
uncovertebral joints and facets, and bulging or herniated discs all
contribute to stenosis. Lateral recess stenosis is a result of
osteophyte formation on the superior facet and ligamentum flavum
hypertrophy. Foraminal stenosis is a narrowing of the foramen secondary
to spondylosis of the superior facet. Congenital stenosis is primarily
responsible for causing central stenosis and may exist primarily or in
combination with degenerative stenosis. Congenital stenosis often
predisposes the patient to myelopathy, leaving less space available for
the cord to compensate for spondylotic changes.
(OPLL) is a hereditary disease found primarily in Asians. OPLL is a
pathologic condition found in 2% of the Asian population. OPLL stenosis
is a dynamic condition, wherein the compression of the cord becomes
more profound in extension of the neck. It has been shown in
biomechanical studies that the volume of the neural canal decreases in
extension and increases in flexion. Additionally the diameter of the
cord increases in flexion. OPLL may present with insidious progression
of myelopathy or myeloradiculopathy, with the ossified ligament often
compressing several levels of the spinal cord within the cervical canal.
history. There have been several retrospective studies with varying
outlooks on this condition. Epstein et al reported a series of
nonoperatively treated patients; 36% of patients improved, and 63% did
not. Of the group that did not improve, 26% deteriorated, with
decreasing neurologic function over time. Clark, in a similar study,
showed that 75% of patients did not improve, with 67% of these
deteriorating, some more rapidly than others. Symon published a series
in which 67% of patients displayed relentless, linear progression of
symptoms. There is a proportion of patients who do improve without
operative intervention, but currently there are no clinical or
radiographic signs that allow the physician to determine who would
benefit from conservative therapy and who would not. It is necessary to
follow these patients closely clinically and radiographically. Patients
who do deteriorate may decompensate steadily or may follow a more rapid
course. The best results from operative decompression are in patients
who are not yet severely myelopathic, because their spinal cord has not
yet been altered irreversibly pathologically by the compressing lesion.
Congenital stenosis often predisposes the patient to myelopathy,
leaving less residual space available for the cord to compensate for
spondylotic change. Most cases of myelopathy develop slowly, in a
stepwise fashion; however, with vascular insufficiency, the onset is
acute and the results are devastating, with irreversible ischemic
changes occurring within the cord.
stenosis and spondylosis. These syndromes require a thorough history
and physical examination; they often occur in elderly patients with
preexisting spondylosis or congenital stenosis or both. Older patients
are especially susceptible to traumatic injury to the cord, especially
hyperextension injuries. These syndromes are by definition incomplete,
with some neurologic function below the level of the lesion.
sclerosis with localized dysfunction of the corticospinal tract. The
corticospinal tract contains efferent nerve impulses that control
voluntary motor function. These fibers decussate at the level of the
medulla to the contralateral corticospinal fascicles. Anterior cord
syndrome results from compression of the anterior spinal artery, which
provides blood flow to the corticospinal tract. Motor function is
affected
severely,
with paralysis below the level of the lesion. Pain, temperature, and
light touch also are impaired with the involvement of the spinothalamic
tract. Partial sensory function is intact, however, with the posterior
columns (tactile discrimination, proprioception, and vibration)
remaining viable. In anterior cord syndrome, the upper and lower
extremities are affected equally. This syndrome has a poor prognosis
for functional recovery.
the corticospinal fasciculus, where the tracts for the upper
extremities are medial to the tracts for the lower extremities;
therefore, motor function often is retained in the lower extremities.
In the upper extremities, there is decreased motor and sensory
function, often with profound hand weakness. Central cord syndrome also
is characterized by sacral sparing. The mechanism of injury is usually
a hyperextension cervical injury that occurs in older patients with
preexisting cervical spondylosis. Some functional recovery can be
anticipated in 75% of these patients, in a specific pattern: first
recovery of the lower extremities, then bowel and bladder control, then
finally, and least predictably, hand function.
by injury to the lateral half of the spinal cord, with ipsilateral
motor and proprioception loss and contralateral pain and temperature
loss. This presentation is due to the spinothalamic tract decussating
two to three levels above a lesion. The prognosis for recovery is good
for these patients, with greater than 90% regaining some function.
The duration of symptoms before clinical diagnosis ranges widely.
Changes in the patient’s activities may be subtle and not readily
associated by the patient with an ongoing problem. Neck and arm pain
are common presenting symptoms. A combined radicular and myelopathic
syndrome is the most common presentation of cervical spondylotic
myelopathy. Patients typically have compression of one or more roots,
leading to lower motor neuron findings of radiculopathy in the upper
extremity. Below the lesion, the spinal cord is compressed, manifesting
as upper motor neuron involvement. Upper motor neuron signs predominate
in myelopathy. Below the offending lesion, hyperreflexia (87% according
to Lundsford), spasticity, and clonus in lower extremity are typical.
Large central disc herniations also are implicated in myelopathy.
Radicular pain with or without neurologic deficit, bowel or bladder
dysfunction, gait disturbances, and long tract signs often are found
together at presentation, creating a myeloradiculopathy. In central
disc herniations, the corticospinal tracts are compressed anteriorly
and are the first tracts to be affected, with patients presenting with
diffuse lower extremity weakness. The posterior column is affected in
more severe stenosis. An impairment in proprioception leads to a
wide-based gait and ataxia.
radiculopathy, also may be found at the level of the lesion and include
areflexia, atrophy, and fasciculations. Bilateral or unilateral upper
extremity (lower motor neuron compression) and bilateral lower
extremities (spinal cord compression and upper motor neuron
compression) may be affected. Weakness, clumsiness, and fatigability
often are present. Most commonly, there is a decreased ability to
ambulate, with a jerky, broad-based gait and an uneven cadence.
Patients may report an unsteady feeling on their feet, difficulty
walking, and an inability to maintain their balance. There is often a
loss of fine motor function in the upper extremities, leading to
difficulty holding a pen, combing one’s hair, or opening bottles or
jars, secondary to diminished dexterity.
patient’s gait to identify the telltale broad-based, jerky gait. This
gait may be the presenting symptom and mimics a parkinsonian gait. The
neck should be palpated for painful muscle spasms. Range of motion
should be assessed, noting any decreased motion and noting maneuvers
that may exacerbate the patient’s symptoms. Extension maneuvers, which
effectively decrease the amount of space available for the cord in the
spinal canal, often elicit an increase in symptoms. A complete,
root-specific neuromuscular exam follows. Pain and paresthesia in a
radicular pattern are common presenting symptoms (34% to 39%), with
variable presentations due to the involvement of multiple roots. Lower
extremity weakness and spasticity (58%) are early presenting symptoms.
Patients often notice decreased dexterity in their hands, often noting
that they are becoming clumsy. A “myelopathy hand” can be caused by a
lesion at or above C6-7 and manifests as a loss of power grip and
subtle loss of proprioception and vibratory sense in the hand.
Spasticity and hyperreflexia (50%) are found in the lower extremities
and are signs of an upper motor neuron lesion. Bladder abnormalities
present as retention and incontinence, with retention (50%) being more
common. In extreme cases, the anal sphincter loses its tone, resulting
in cases of fecal incontinence.
signs that are associated with myelopathy. Lhermitte’s sign is a
shocklike feeling that progresses down the spine to the extremities
when the neck is flexed and compressed. The scapulohumeral reflex,
occurring with C1-3 myelopathy, consists of tapping on the acromion,
with resultant abduction of the humerus and elevation of the scapula.
Hoffmann’s sign consists of flicking the nail of the long finger of a
relaxed hand with resultant thumb or index finger flexion at the distal
interphalangeal joint (13%). A myelopathic hand also can present with
the “finger escape sign”: The small finger cannot be adducted back to
the palm, signaling an upper motor nerve lesion to nerves supplying the
intrinsic musculature of the hand (C8 and T1). The inverted radial
reflex is elicited with a reflex hammer tap to the brachioradialis,
resulting in a diminished brachioradialis reflex accompanied by finger
flexion. Clonus, which often is present in the lower extremities, is a
rhythmic, repetitive oscillation of the foot at the ankle in response
to dorsiflexion of the foot and stretch of the Achilles tendon.
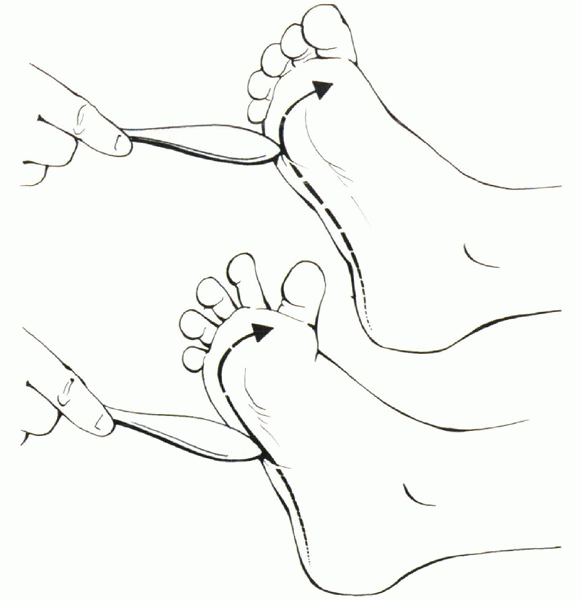
Babinski’s reflex often is seen in conjunction with hyperreflexia. A
positive Babinski’s (50%) reflex is manifested by up-going toes when
the sole of the foot is stimulated with the handle of a reflex hammer (Fig. 13-1).
 |
|
Figure 13-1 Babinski’s sign.
|
-
Grade 0—root signs and symptoms without cord involvement
-
Grade I—signs of cord involvement with a normal gait
-
Grade II—mild gait abnormality; patient remains employable
-
Grade III—gait abnormality prevents employment
-
Grade IV—able to ambulate only with assistance
-
Grade V—chair bound or bedridden.
clinical examination is prognostic of recovery, with lower grade
patients predictably responding more favorably to operative and
nonoperative treatment. Additional factors associated with an
optimistic prognosis for recovery include duration less than 1 year,
unilateral motor deficit, and younger age. The presence of Lhermitte’s
sign also has been shown to have a positive predictive value for
recovery.
causal relationship between the patient’s presenting symptoms and
structural changes of and around the spinal column. Radiographs are the
initial studies to be obtained for acute symptoms. Clinical correlation
is poor in patients older than 40 years old, with a high percentage of
asymptomatic patients showing radiographic evidence of spondylosis. It
is crucial to correlate the patient’s symptoms to the radiographic
findings and not to treat the radiograph reflexively.
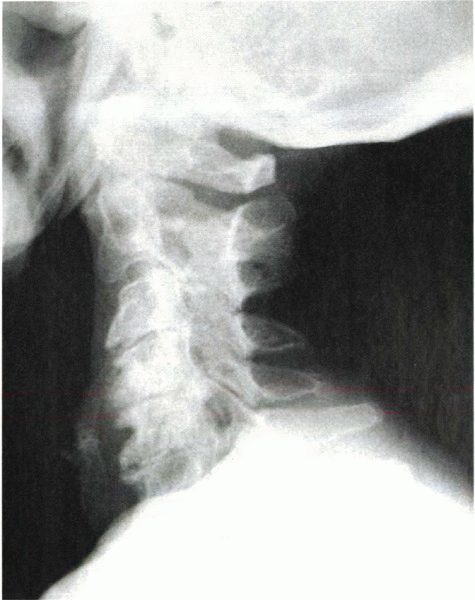
lateral, and oblique views. In advanced spondylosis, these studies
reveal narrowing of the intervertebral space, degenerative changes in
the facets and neurocentral joints, loss of cervical lordosis,
osteophyte formation, and foraminal encroachment (Fig. 13-2).
Instability of the cervical spine can be determined radiographically by
White’s criteria. An unstable segment is diagnosed by noting a
translation of one vertebra on another of 3.5 mm or an angulation of
the end plates of two adjacent vertebrae of 11 degrees more than a
normal adjacent segment.
which is not determined easily with radiographs. Measurements are
determined best with either computed tomography (CT) or magnetic
resonance imaging (MRI). CT is excellent for evaluating canal diameter,
as long as the gantry of the CT machine is aligned properly parallel to
the disc spaces. MRI has been shown to overestimate the amount of canal
stenosis by overstating the contribution of soft tissues. Each modality
provides enough information, however, to evaluate spinal canal
diameter. A normal canal diameter in the subaxial cervical spine is 17
mm. A measurement of less than 13 mm is defined as relative stenosis,
and a measurement of 10 mm is absolute stenosis. The proportion of
patients who present with myelopathy is related directly to
lower
measurements of canal diameter. Ratios also can be measured to
determine stenosis. The Torg ratio divides the canal diameter by the
length of the vertebral body as measured on a lateral radiograph. A
ratio of less than 0.8 is considered stenotic. The cord compression
ratio is the sagittal diameter of the cord divided by the transverse
diameter on MRI; a ratio of less than 0.40 represents a significant
amount of cord compression.
 |
|
Figure 13-2
Lateral radiograph shows severe spondylosis. Note the loss of disc height and osteophyte formation at the anterior and posterior margins of the end plates. There also is a loss of physiologic lordosis. |
disc disease and is useful for identifying intradural lesions and
tumors. It is noninvasive, but a high-resolution machine is required
with a powerful magnet of at least 0.5 Tesla and preferably 1.5. This
high resolution affords a high degree of sensitivity of identification
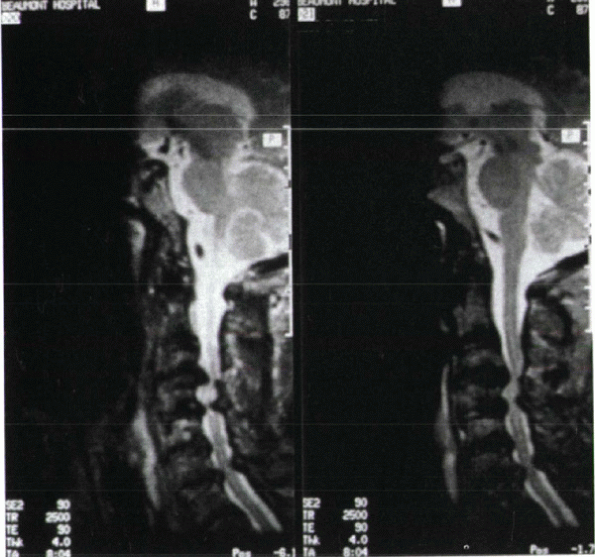
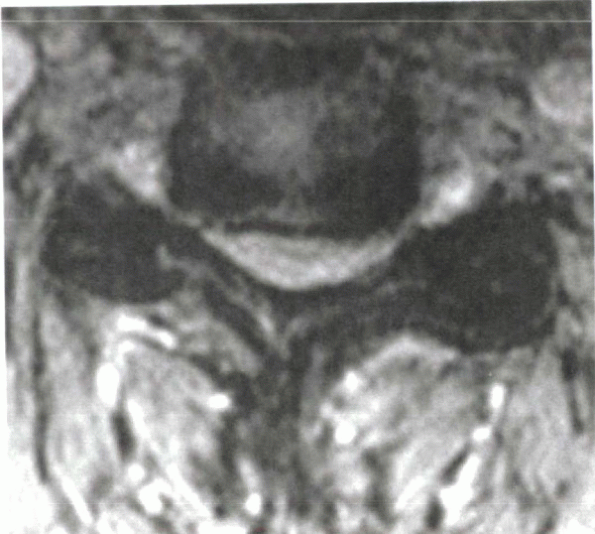
of compressive lesions of the cervical spine (Figs. 13-3 and 13-4).
There is a high incidence of abnormalities in asymptomatic patients,
with a low specificity due to a high false-positive rate. In one study,
MRI was interpreted as abnormal in 19% of asymptomatic patients. When
stratified by age, the false-positive rate was 28% in patients older
than 40 years old and 14% in patients younger than 40. The clinician
must be cautious in recommending operative treatment based on
diagnostic tests alone; findings must match clinical signs and symptoms.
study still has indications. CT-myelography is better than CT alone in
patients with spondylosis, allowing direct visualization of
compression. An ideal indication for this study would be a patient who
has undergone previous instrumentation. In this case, the artifact
associated with the ferromagnetic metal implants likely would not allow
an accurate reading of an MRI study. The myelogram may reveal
nonfilling of the proximal nerve root sleeves, flattening of the spinal
cord, obstruction of contrast flow, or multiple indentations anteriorly
(disc) or posteriorly (ligamentum flavum). CT-myelography allows direct
visualization of compression. Its disadvantages are the difficulty in
differentiating soft disc from hard disc protrusions, the difficulty in
determining the extent of intradural lesions, and the possibility that
distal pathology may be missed if a myelographic block is present.
 |
|
Figure 13-3
Multiple-level cervical spondylosis with loss of disc space, facet hypertrophy, buckling of the ligamentum flavum, and resultant cervical stenosis. |
 |
|
Figure 13-4
Axial cut T2-weighted MRI in a myelopathic patient. Note the central stenosis with decreased room available for the cord, facet hypertrophy, and foraminal stenosis. |
and radiologic examinations and are not useful by themselves. They are
not required for diagnosis of radiculopathy or myelopathy.
Electrodiagnostic studies are used effectively to confirm a diagnosis
of myelopathy or to rule out neurologic disorders, such as amyotrophic
lateral sclerosis, multiple sclerosis, or peripheral nerve entrapment
syndromes. Electromyography reveals fibrillations and sharp waves in a
positive test done 3 to 6 weeks after radicular symptoms develop, and
nerve conduction velocity exams reveal decreased amplitudes and
velocities.
physical exam and is supported by radiographic studies. Radiographs
must correlate with clinical findings because more than 70% of patients
older than age 70 have degenerative changes on radiographs. The
differential diagnosis for myelopathy is vast. Patients with tumors or
infections can present with myelopathic symptoms. Nonmechanical pain;
night pain; and systemic signs, such as weight loss, night sweats,
fever, and chills, all should be red flags for the evaluating
physician. The pain associated with tumor and infection is often
constant in nature and may escalate over time, occasionally with a
rapid increase in pain. Multiple sclerosis may present with fatigue and
focal deficits similar to myelopathy. Multiple sclerosis is associated
with visual changes and upper motor neuron lesions that wax and wane
over time. MRI may reveal focal plaques within the brain or spinal cord
consistent with multiple sclerosis. Serum and cerebrospinal fluid with
increased oligoclonal bands and immunoglobulins help to confirm this
diagnosis. Amyotrophic
lateral
sclerosis often presents in people in their 50s through 70s with
painless weakness beginning about the shoulder girdle and progressing
to complete motor loss without sensory involvement. Peripheral
neuropathies can mimic cervical pathology and can be ruled out with a
thorough physical exam and electromyography or nerve conduction
velocity test.
discogenic and axial neck pain without accompanying myelopathy or
radiculopathy. Axial neck pain tends to resolve on its own, but less
frequently completely in patients with spondylosis (79% improved, 43%
pain-free). Treatment regimens include nonsteroidal antiinflammatory
drugs, moist heat, isometric and aerobic exercises, and occasionally a
soft cervical collar. Surgery for discogenic neck pain is less
rewarding than surgery for cases of radiculopathy or myelopathy.
arm symptoms, may respond to nonoperative treatment. Epidural
injections may be of short-term benefit to a patient with
radiculopathy, but there is no long-term research proving their
efficacy. Myelopathy rarely resolves completely.
pathophysiology of the disease by directly decompressing the cord.
Candidates for surgery include patients who have difficulty using their
arms, hands, or legs in activities of daily living; patients who rely
on ambulatory aids for walking; and patients who are confined to a bed
or chair. Radiculopathy commonly is present in myelopathic patients,
and radiculopathy with persistent or disabling pain is an indication
for surgery. The greatest predictor of postoperative recovery is the
extent of preoperative involvement, as outlined by Nurick’s
classification, with age, sex, and duration of symptoms having less of
an effect on outcome.
offending discs and osteophytes. The procedure can be used for
single-level and multilevel disease (Fig. 13-5).
An anterior cervical discectomy and fusion is the procedure of choice
for removing a single central disc herniation. A subtotal corpectomy
and strut graft is a successful procedure for multilevel disease,
providing 80% of patients with pain relief and 90% with some neurologic
improvement (Fig. 13-6). In this operation,
most of the vertebral body is removed, leaving only the lateral walls
behind. A 3-mm margin on each side of the corpectomy leaves a safety
zone between the surgeon’s bur (used for removing the vertebral body)
and the vertebral arteries, which course just lateral to the lateral
margins of the vertebral body. Either structural allograft or autograft
can be used to fill the corpectomy defect. If autograft is chosen, the
iliac crest and the fibula are frequent choices. Harvesting is
associated with some morbidity, including hematomas, infection, and
nerve damage. For these reasons, many surgeons prefer to use
allograftgraft taken from a cadaver donor. The graft is placed while
the anesthesiologist distracts the head. Plating has been shown to
improve the fusion rates.
including direct removal of compressive pathology, stabilization of the
cervical spine with an arthrodesis, ability to correct a kyphotic
deformity, and excellent relief of axial neck pain. The disadvantages
are that the procedure is technically demanding and may require
postoperative bracing. Additionally, graft extrusion or collapse is
worrisome owing to the proximity of the graft to the neural structures
posteriorly and the esophagus anteriorly. The graft should be well
placed in the midline between the vertebral end plates. The end plates
should be well prepared with a posterior lip to help prevent posterior
dislodgment.
treat cervical myelopathy. Laminoplasty is a canal-expanding procedure
that maintains cervical stability by leaving an intact hinge on the
posterior elements (see Fig. 13-6). This
procedure is most effective for unilateral radiculopathy at multiple
levels with accompanying stenosis, especially for patients with OPLL.
Laminoplasty is contraindicated in a patient who has lost physiologic
lordosis on the lateral radiograph,
or
has preoperative instability. Advantages of laminoplasty over wide
laminectomy include retention of the osseous protection of the spinal
cord and maintenance of soft tissue stabilizers.
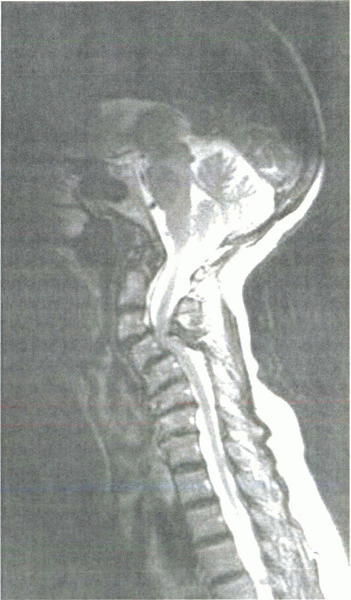 |
|
Figure 13-5
Sagittal T2-weighted MRI in a myelopathic patient. Note the decreased room available for the cord and loss of lordosis resulting from severe spondylosis. |
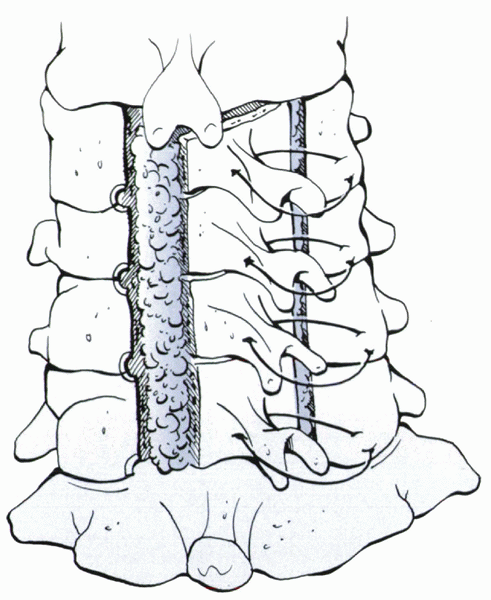 |
|
Figure 13-6 Laminoplasty: open door technique with sutures from the lateral mass to the spinous process to anchor the laminoplasty open.
|
compression. It is a less technically demanding procedure than either
the anterior approach or laminoplasty. The spine should be stable, with
preservation of lordosis. If excessive motion does exist (as defined on
flexion/extension radiographs), the laminectomy should be followed by
posterior instrumentation and fusion to avoid subsequent
postlaminectomy kyphosis. Laminectomies are used most commonly after
failed laminoplasties or in patients with bony ankylosis of the
anterior cervical spine. Advantages associated with the posterior
approach include a minimal loss of motion (compared with the anterior
approach) and a less technically demanding procedure. Disadvantages
include the understanding that this is an indirect decompression,
expanding the canal volume, but not directly addressing the anterior
pathology. Kyphosis resulting from instability is an often cited
complication of laminectomies in which an excessive amount of the
facets have been removed; posterior instrumentation and stabilization
may be recommended in patients with preoperative instability or
patients who require excessive facet resection for decompression (Fig. 13-7).
injury during surgery owing to the compromised state of the cord. There
is a higher incidence of neurologic injury with posterior procedures,
especially laminectomies. Canal stenosis creates an environment of cord
vulnerability. Postoperatively, 5.5% of myelopathic surgical patients
may experience neurologic deterioration. The postoperative deficit may
be secondary to surgical manipulation, such as a surgical instrument’s
being introduced into the spinal canal, but it is more commonly due to
late kyphotic deformity or hematoma formation. Nerve root irritation
most commonly is related to graft complications, either displacement or
collapse of the graft. The C5 nerve root most commonly is involved,
leading to deltoid weakness. Postoperative C5 nerve root dysfunction
may be due to the following:
-
C5 is often at the center of the
decompression in myelopathic patients and is subject to more stretch
from being tethered to the spinal cord. -
C5 root is shorter than other roots.
commonly cited and include pain, hematoma, infection, abdominal
herniation, and injury to the lateral femoral cutaneous nerve (meralgia
paresthetica, with anterior harvest) or the superior cluneal nerves
(with posterior harvest). Postoperative pain from iliac crest bone
harvest has been reported, with 12% to 14% of patients having severe,
but temporary pain. One study by DePalma noted 36% of patients had
severe persistent pain.
anteriorly (0.7% to 2.8%); this may be due to the abundant blood supply
of the neck and the relatively atraumatic tissue dissection involved in
the exposure. Prophylactic antibiotics, usually a first-generation
cephalosporin, given 1 hour before surgery, have proved to be
invaluable in preventing infection. Conditions associated with an
increased risk of infection include diabetes, malnutrition,
immunocompromised status, rheumatoid arthritis, malignancy, alcoholism,
and poor dentition.
in less than 2% of all anterior cervical procedures. Hoarseness may
develop postoperatively secondary to excessive traction or, more
seriously, transection of the recurrent laryngeal nerve. The recurrent
laryngeal nerve on the right side courses transversely across the field
at the C6-7 level, exposing it to injury with sharp dissection or
retraction. For this reason, many surgeons prefer the left-sided
approach. The recurrent laryngeal nerve courses in the
tracheoesophageal groove and is protected by the trachea and esophagus.
Transient sore throat and difficulty swallowing commonly are seen in
the immediate postoperative period. Esophageal laceration is a rare but
potentially lethal injury if not addressed intraoperatively.
Postoperative tears may be evident by saliva or food in the wound,
dysphagia, or neck pain; this can progress quickly to systemic sepsis.
Esophageal perforation can be diagnosed with an esophogram. A positive
exam shows water-soluble contrast material extravasating into the
operated region. Treatment includes placement of a nasogastric tube,
and the patient usually is taken back immediately to the operating
room. Late perforations are related to prominent hardware (i.e., plates
and screws) that are too proud on the anterior vertebral bodies.
Finally, the thoracic duct of the lymphatic system enters the
subclavian vein on
the
left side. Low, lateral approaches on the left potentially can injure
the duct. If the thoracic duct is damaged, it should be double ligated.
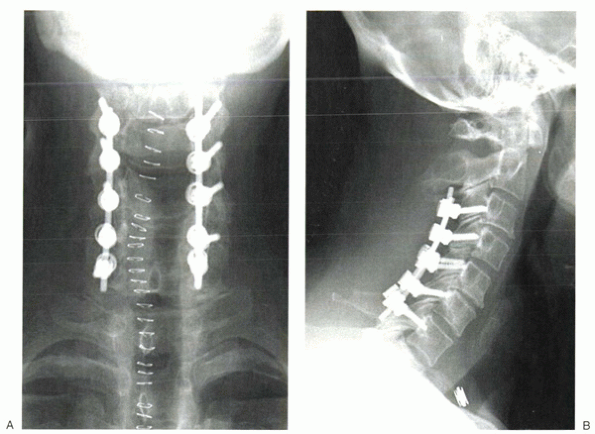 |
|
Figure 13-7 Postoperative anteroposterior (A) and lateral (B)
radiographs after wide decompressive laminectomy and the use of posterior instrumentation to prevent postoperative kyphosis from developing. |
avoid microfactures in the graft, which can occur when osteotomes are
employed in the harvest. The graft should be well placed in the midline
between the vertebral end plates. The end plates should be well
prepared with a posterior lip to help prevent posterior dislodgment.
Grafts that are too thick may lead to collapse, and grafts that are too
thin may predispose to pseudarthrosis.
graft, is not always clinically significant, but it is related to a
poorer clinical outcome. Pseudarthrosis is related directly to the
number of levels fused. One-level fusion has a pseudarthrosis rate of
5% to 10%; two-level, 10% to 15%; and three-level, 20% to 30%. Rates
also are increased with smoking and the use of allograft.
Pseudarthrosis may be diagnosed in a patient experiencing continued
mechanical cervical pain after 6 to 9 months. Motion on
flexion/extension lateral films confirms the diagnosis. An asymptomatic
pseudarthrosis can be observed. With graft resorption and resultant
kyphosis, a partial anterior corpectomy with bone graft and plating
would be the recommended revision surgery through an anterior approach.
In partial fusions in which lordosis is maintained, a posterior fusion
with foraminotomy (if radicular symptoms persist) stabilizes the
construct.
SD, McCowin PR, Davis DO, et al. Abnormal MRI scans of the cervical
spine in asymptomatic subjects: a prospective investigation. J Bone
Joint Surg 1990;72A:1178-1184.
JA, Carras R, Epstein BS, Lavine LS. Cervical myelopathy caused by
developmental stenosis of the spinal canal. J Neurosurg 1979;51:362.
I, Oh-Hama M, Shingu H, Yonago K. Cervical myelopathy treated by canal
expansive laminoplasty. J Bone Joint Surg 1984;66A:914-920.
S. The natural history and the results of surgical treatment of spinal
cord disorder associated with cervical spondylosis. Brain
1972;95:101-108.
