Unicompartmental Knee Arthroplasty
II – Knee > Part C – Operative Treatment Methods > 24 –
Unicompartmental Knee Arthroplasty
a resurgence of interest in the past decade. For a selected subgroup of
patients with isolated advanced degenerative arthritis involving
primarily the medial or lateral compartment of the knee, UKA may be the
best surgical treatment option. Comparisons of the early outcomes of
UKA with those of total knee arthroplasty (TKA) typically reveal faster
recovery after UKA. In addition, often there is greater patient
satisfaction with UKA because the knee feels more like a normal knee,
possibly because of the preservation of both cruciate ligaments after
UKA (Table 24-1). Comparisons of the early
outcomes of UKA to those of osteotomy typically reveal faster recovery,
more predictable pain relief, and fewer surgical complications after
UKA. Progression of degenerative arthritis in the unresurfaced portions
of the joint after UKA remains a mode of failure that is not faced
after TKA. Controversy exists about the ability to predict, through
physical exam or radiographs, those patients at risk for developing
arthritis in other compartments after UKA. For that reason some
surgeons remain reluctant to use UKA and instead rely on TKA for those
patients who progress to require a knee arthroplasty. In large cohorts
of patients, however, it is fair to conclude that the survivorship of
modern UKA and modern TKA are essentially equivalent over the first
decade after implantation.
compartment of the knee is the most common indication for
unicompartmental knee arthroplasty. The pathogenesis of isolated medial
compartment disease is well recognized. Progressive loss of articular
cartilage leads to varus malalignment of the limb, which then further
overloads the articular cartilage and causes additional loss of
articular cartilage over time. In most patients with an intact anterior
cruciate ligament (ACL), the area of maximal articular cartilage loss
is the anteromedial portion of the tibia. When the ACL is intact, most
patients will have preservation of full-thickness articular cartilage
on the posteromedial portion of the tibia. On the femoral side, almost
all of the articular cartilage loss is from the distal femur, with the
posterior femoral cartilage relatively well preserved. In patients
without an ACL, the knee kinematics are altered substantially and the
pattern of arthritis is markedly less predictable. In many, but not
all, ACL-deficient patients, sufficient lateral compartment disease or
patellofemoral compartment disease will be present such that a UKA is
not appropriate.
compartment of the knee is decidedly less common than medial-sided
disease. Most TKA studies suggest a 10-to-1 predominance of medial over
lateral compartment disease, and most UKA studies suggest closer to
20-to-1 predominance of medial UKA versus lateral UKA. In many
surgeons’ experience, the patient with valgus deformity and lateral
compartment disease often presents with concomitant anterior knee pain
or patellofemoral radiographic findings that make UKA less appealing.
Even in those patients with isolated lateral compartment disease, the
pattern of degenerative change is less predictable than in patients
with isolated medial disease. This likely reflects the more complex
kinematics of the lateral compartment of the knee, which includes
greater amounts of gliding and rolling than the medial side.
the medial (or lateral) joint line as the source of pain that prevents
him or her from carrying out activities of daily living (Fig. 24-1).
Those patients who have diffuse knee pain or who clearly identify
anterior knee pain as substantially limiting likely will be served
better with TKA. Specific anterior knee pain symptoms when squatting or
standing from a seated position also would suggest TKA rather than UKA.
As with any knee problem, care should be taken to exclude
hip
disease or a neurologic cause for the pain. Patients with inflammatory
arthritis are better suited to TKA than UKA. Considerable debate exists
on how to factor age and body weight into the decision to proceed with
UKA. Interestingly, at this time the available evidence suggests that
weight does not affect early outcome or survivorship through the first
decade. This may be because many obese patients are relatively
sedentary. In distinction there is evidence from the Swedish Joint
Registry that younger age is adversely correlated with survivorship;
however, that applies to not just UKA but also TKA.
|
TABLE 24-1 Advantages and Disadvantages of Unicompartmental Knee Arthroplasty Versus Total Knee Arthroplasty
|
|||||
|---|---|---|---|---|---|
|
have no more than a 10- to 15-degree flexion contracture. More
substantial flexion contractures typically can be corrected only
partially with UKA. Varus or valgus deformity of >10 degrees is
typically accompanied by degenerative changes in the other compartments
of the knee that make UKA less predictable. Furthermore, varus/valgus
deformity of >10 to 15 degrees often requires collateral ligament
release at the time of surgery, which most authors have advised against
during UKA. The stability of the ACL must be assessed preoperatively. A
deficient ACL is a contraindication to the use of a mobile-bearing UKA
design because the risk of bearing dislocation is substantial. Some
authors suggest that a deficient ACL in a low-demand patient who has
not experienced giving way episodes is not a contraindication to a
fixed-bearing UKA. When UKA is selected for those low-demand ACL
deficient patients, care should be taken not to increase the posterior
slope of the tibial component. For active, high-demand patients and for
those who have experienced symptomatic giving way episodes, an isolated
UKA is contraindicated in the face of ACL deficiency. Some authors have
described concomitant or sequential ACL reconstruction and UKA, but the
data on that combination is limited.
hip-knee-ankle on a 3-foot film is useful. With that film the
mechanical axis and anatomic axis can be calculated and the presence or
absence of extra-articular bone deformity can be confirmed. On a
standing anteroposterior (AP) view of the knee, the contralateral
tibiofemoral compartment is examined for evidence of joint space
narrowing or osteophyte formation. Some surgeons, particularly those in
Europe, routinely obtain stress views of the knee in varus and valgus
as part of the evaluation for UKA. These stress views can confirm the
integrity of the opposite compartment and determine if adequate
correction of the varus-valgus alignment can be obtained without
collateral ligament release. On the lateral radiograph, superior and
inferior pole patellar osteophytes can be observed. Axial views of the
patella are used to grossly assess the patellofemoral articulation for
evidence of subluxation or loss of articular cartilage. In the absence
of symptoms, some surgeons will ignore the status of the patellofemoral
joint; however most surgeons would regard the presence of bone-on-bone
changes at the patellofemoral joint as a contraindication to UKA. The
presence of diffuse chondrocalcinosis on x-ray films (particularly when
accompanied by history or physical findings of recurrent effusion) is a
contraindication to UKA.
physical exam are sufficient to allow a definitive decision about
whether UKA is appropriate. In rare circumstances MRI might be helpful
in determining the status of the contralateral compartment or the ACL.
MRI, however is helpful in patients with avascular necrosis for whom
UKA is contemplated. Some surgeons make a distinction between patients
with so-called spontaneous avascular necrosis (AVN) and patients with
AVN secondary to corticosteroid use. Patients with spontaneous
osteonecrosis typically have small areas of necrotic bone confined to
the subchondral region, and those patients are often good candidates
for UKA. Some patients with AVN secondary to steroid use have large,
geographic avascular bone lesions that could compromise the fixation of
the femoral or tibial component after UKA. MRI can be helpful in
determining the depth and extent of that necrotic change. If it appears
that after the predicted bone cuts a substantial portion of the UKA
implant will rest on necrotic bone, then TKA may be a better choice (Fig. 24-2).
postoperative limb alignment should be after UKA. Most, but not all,
surgeons currently recommend that the limb remain slightly
undercorrected after UKA. For the typical varus knee undergoing medial
compartment UKA, this means leaving the limb with a mechanical axis
that passes through the medial compartment just medial to the tibial
spines. For most patients the postoperative anatomic femorotibial axis
would thus measure 2 to 4 degrees of valgus as opposed to the normal 6
degrees of valgus. The rationale for slightly undercorrecting the
mechanical axis is to avoid overloading the articular cartilage in the
opposite compartment. Markedly undercorrecting the knee, however, is
also inappropriate as
that
will place excessive load on the UKA bearing and lead to failure owing
to polyethylene wear. In both full extension and at 90 degrees of
flexion, the femoral and tibial components should be parallel such that
edge loading of the polyethylene does not occur. The knee should be
balanced to incorporate 2 mm of laxity in both flexion and extension.
The tibial component must not overhang medially where it can irritate
the medial collateral ligament. The femoral component must not extend
anteriorly beyond the edge of subchondral bone or it can impinge
against the patella.
 |
|
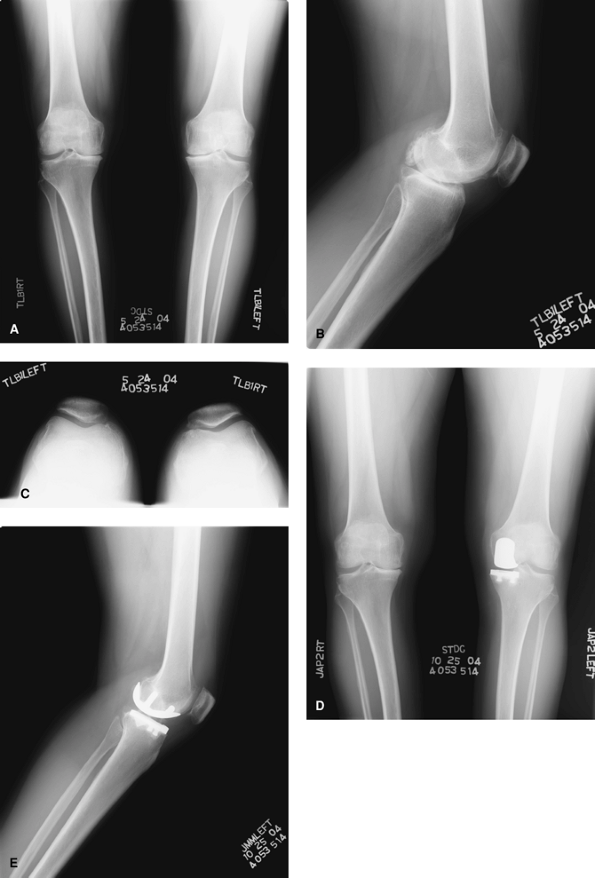
Figure 24-1
A 53-year-old female with advanced medial compartment degenerative arthritis. The symptoms are confined to the medial joint line with no anterior or lateral pain with activities or at rest. The anterior cruciate ligament is intact. A: The anteroposterior weight-bearing x-ray film reveals bone-on-bone changes in the medial compartment. The lateral compartment is well preserved. There is no translation of the femur on the tibia and no evidence of tibial spine impingement. B: The lateral x-ray film reveals mild degenerative spurs at the superior and inferior poles of the patella. C: The axial view of the patella shows a well-preserved patellofemoral joint space with some minimal degenerative changes involving the medial facet of the patella. D: The postoperative anteroposterior weight-bearing x-ray film shows a unicompartmental knee in good position. The overall limb alignment has been deliberately left slightly undercorrected, there has been a minimal resection of tibial bone, the femoral and tibial components are parallel in extension, and the femur is well centered over the tibial component. E: The postoperative lateral x-ray film reveals that the femoral component is well sized without anterior extension that would impinge on the patella, the tibial component fills the space from anterior to posterior without any overhang, and the posterior slope is not excessive. |
 |
|
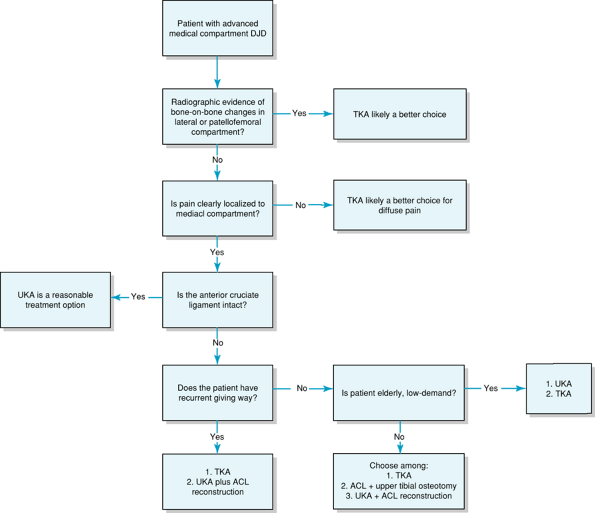
Figure 24-2
A treatment algorithm for the management of advanced medial compartment degenerative arthritis. ACL, anterior cruciate ligament; DJD, degenerative joint disease; TKA, total knee arthroplasty; UKA, unicompartmental knee arthroplasty. |
designs. Those techniques include noninstrumented, free-hand
preparation through intramedullary, extramedullary, and
computer-assisted instrumentation systems. Surgeons must understand the
rationale for a given instrumentation system before using the system
clinically. Contemporary UKA is often done through a so-called
minimally invasive surgical approach (MIS). The MIS technique typically
involves an 8- to 12-cm skin incision and a short medial arthrotomy
that stops at the superior pole of the patella. A short split into the
vastus medialis muscle can be made (mini midvastus approach) or
alternatively the subvastus interval can be exploited if more exposure
is needed. The patella does not need to be dislocated for UKA, and
leaving the patella reduced in the trochlea helps the surgeon avoid
some component orientation errors. When UKA is done using a traditional
TKA approach with the patella everted and the knee flexed, the tibia
tends to externally rotate and the medial flexion space tends to gap
open, and that can lead to
component
orientation problems. MIS techniques continue to be debated in the
realm of TKA, but in UKA contemporary instruments are well suited to
this approach. A portion of the retropatellar fat pad and the anterior
horn of the medial meniscus can be excised for visualization early in
the case. In midflexion the status of the ACL, the lateral compartment,
and the patellofemoral joint are noted. Any intercondylar osteophytes
can be removed from the notch to prevent impingement on the ACL, and
patellar osteophytes can be debrided. The sequence of bone cuts is
determined by the particular instrumentation system chosen by the
surgeon. Typically, on the tibial side the emphasis is on minimal bone
resection with at most 2 mm of bone removed from the most worn portion
of the tibia. This cut is generally made perpendicular to the long axis
of the tibia. The degree of posterior slope is most often 5 degrees but
can vary based on patient and implant selection factors. For patients
with ACL-deficient knees, less slope may be preferable. When an implant
is used that has substantial sagittal plane conformity, then matching
the posterior slope to the patient’s anatomy is appropriate.
free-hand cut from anterior to posterior, and this should be done as
close to the medial tibial spine as possible without damaging the ACL.
The surgeon should reference the tibial tubercle to avoid the tendency
to internally rotate that sagittal plane cut. Typically, the largest
tibial component that does not overhang should be selected. On the
femoral side, most instrumentation systems resect the same thickness of
bone (both distally and posteriorly) that will be replaced by the
femoral implant. If an intramedullary cutting guide is used, the knee
is brought to midflexion to facilitate access to the intramedullary
canal. Care is taken to protect the patellar ligament and skin during
this part of the procedure. The femoral component is sized from
anterior to posterior such that 1 mm of subchondral bone is left
exposed at the anterior edge of the component. That sizing will
eliminate impingement of the femoral component with the patella even if
the patient goes on to develop patellofemoral arthritis years later. In
the medial-lateral direction, the femur should be centered over the
tibial component without impingement into the notch and without
overhang medially. The femoral component should be rotated at 90
degrees of flexion such that the femur and tibia are parallel, thus
ensuring that edge loading of the femoral component will not occur.
With a trial insert in place, the knee should be balanced with
symmetric flexion and extension gaps of 2 mm. The overall mechanical
alignment of the leg should be assessed with a cautery cord or long
drop rod. If questions exist about component position or limb
alignment, an intraoperative x-ray film or fluoroscopy can be used.
of problems encountered with total knee arthroplasty including
infection, bleeding, nerve injury, prosthetic loosening, wear,
continued pain, thromboembolic disease, and bearing dislocation. The
prevalence of infection after UKA has historically been equal to or
slightly less than that after TKA. Substantial bleeding after
contemporary UKA is uncommon, and it is rare for a patient to require
blood transfusion after a single UKA. Injury to the peroneal or tibial
nerves is rare after UKA and is substantially lower than that reported
after upper tibial osteotomy. Prosthetic loosening, wear, or failure
that requires revision surgery can be estimated to occur at a rate of
1% to 1.5% per year over the first decade. Slightly higher rates of
failure have been observed in patients younger than 65 years of age
compared with those older than 65 years according to the Swedish Joint
Registry data and from the group in Oxford, England. Continued pain in
the early period after UKA typically is the result of improper patient
selection, although infection, early implant loosening, or tibial
plateau fracture should be excluded. Late onset of pain can occur from
progression of disease in the unresurfaced compartments of the knee,
implant loosening, or from polyethylene wear with associated synovitis.
Between 10 and 15 years after UKA, symptomatic patellofemoral arthritis
has been reported in ≤10% of UKA patients in some series. The
prevalence of deep venous thrombosis and pulmonary embolus has not been
studied as well after UKA as after TKA, but the available evidence
suggests lower prevalence of thromboembolic disease after UKA. For
mobile-bearing designs, dislocation of the tibial bearing can occur,
and the reported prevalence is 0.5% to 1.5%. Patients with a deficient
ACL are at particular risk for bearing dislocation after mobile-bearing
UKA. Fracture of the medial tibial plateau has been reported after UKA
and is associated with the use of multiple pins to fix tibial cutting
jigs to the proximal medial tibia. Similar fractures can occur after
excessively deep tibial resections as well.
than after TKA. Most studies reveal that the mean range of motion after
UKA is substantially better than that after TKA even when accounting
for differences in preoperative motion. One prospective randomized
trial of UKA versus TKA demonstrated more excellent results after UKA,
and those superior results were maintained at 5 years follow-up. Early
series of UKA reported survivorship of 85% to 88% at 10 years. More
recent series suggest 90% to 98% survivorship at 10 years, which may be
attributable to the combination of more appropriate patient selection
and improved instrumentation and technique. Most of these studies,
however, have been done on elderly patients with a predominance of
females over males, and that makes extrapolation of these data to the
younger active middle-aged patient difficult. Several recent studies in
younger, more active patients have been encouraging with 10 year
survivorship of 90% to 92%. Those UKAs that require revision typically
are divided equally between patients who fail because of disease
progression in the unresurfaced compartments and those who fail because
of loosening or wear of the UKA components. Early reports of conversion
of the failed UKA to TKA suggested that substantial bone loss was
encountered commonly and that these were difficult reoperations. In
contrast, many authors now suggest that conversion of
the
failed contemporary UKA to TKA is relatively straightforward. There are
data to suggest that revision of a UKA to TKA is more reliable than
revision of UKA to another UKA. Surgeons continue to disagree on
whether conversion of a failed UKA to TKA is more or less difficult
than conversion of a failed upper tibial osteotomy to TKA.
local anesthetic into the capsule and subcutaneous tissues prior to
closing the wound. Patients can typically begin weight bearing as
tolerated early after surgery and progress with activities as
tolerated. Although some surgeons will perform UKA as an outpatient
procedure, most patients are hospitalized for 1 to 3 days. Most
surgeons now use some form of rapid rehabilitation protocol such that
patients use ambulatory aids for a short period of time after surgery.
Just as in TKA, these patients should work diligently early after
surgery to regain maximal knee extension and flexion.
arthroplasty designs has had a lasting influence on surgeons and has
resulted in the continued limited use of patellofemoral arthroplasty.
Nonetheless, there likely is a small subgroup of patients for whom
contemporary patellofemoral arthroplasty is a good treatment option.
For older patients with advanced patellofemoral arthritis, TKA has
proved to be a reliable, reproducible, and durable procedure. For
patients younger than 55 years of age who have substantial primary or
posttraumatic patellofemoral degenerative arthritis without patellar
malalignment, patellofemoral arthroplasty may be considered.
Furthermore, patellofemoral arthroplasty can be considered in patients
with arthritis secondary to trochlea dysplasia. Because a
patellofemoral arthroplasty allows retention of both cruciate
ligaments, the knee kinematics are better preserved as compared with
TKA, and thus patients may perceive the knee to feel more normal.
Although contemporary implant designs do offer improvements over
historical designs, patellofemoral arthroplasty remains a technically
demanding operation. Implant malposition can result in prosthetic
impingement, pain, and extensor mechanism instability problems. Implant
loosening with contemporary cemented patellofemoral arthroplasty has
not proved to be common. With longer-term follow-up, however, a
substantial number of patients (25% at 15 years) will go on to develop
symptomatic degenerative arthritis in the tibiofemoral articulation.
Conversion of patellofemoral arthroplasty to TKA typically is not
particularly difficult.
JN, Komistek RD, Aubaniac JM, et al. In vivo determination of knee
kinematics for subjects implanted with a unicompartmental arthroplasty.
J Arthroplasty. 2002;17:1049–1053.
AJ, Pandit H, Price AJ, et al. Oxford medial unicompartmental
arthroplasty for focal spontaneous osteonecrosis of the knee. Acta Orthop. 2005;76:688–692.
JH, Ackroyd CE, Shah NA. Unicompartmental or total knee replacement?
Five year results of a prospective randomized trial of 102
osteoarthritic knees with unicompartmental arthritis. J Bone Joint Surg Br. 1998;80:862–865.
AJ, Dodd CA, Svard UG, et al. Oxford medial unicompartmental knee
arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87:1488–1492.
NP, Jahroni I, Lewis PL, et al. Patient-perceived outcomes and return
to sport and work: TKA versus mini-incision unicompartmental knee
arthroplasty. J Knee Surg. 2006;19:112–116.
