Semiconstrained Total Elbow Replacement
replacement has been well established since the first edition of this
text. A major value is to broaden the indications beyond rheumatoid
arthritis. Improved stability with the anticipation of minimizing
stress to the bone cement interface is realized by the laxity or “play”
that occurs at the ulnohumeral articulation (13).
Although several designs are available, I will describe in detail the
surgical procedure for the insertion of a Mayo modified Coonrad total
elbow arthroplasty (Coonrad-Morrey), which has been used almost
exclusively at our institution since 1981.
to those of other joints, the most common being pain that significantly
alters activities of daily living. This is typically seen in the
patient with rheumatoid arthritis or certain traumatic conditions.
replacement is dysfunctional instability. This presentation is seen in
the very severe grade IV type of rheumatoid arthritic elbows as well as
with posttraumatic arthrosis resulting from distal humeral nonunion or
resection.
replacement is that of the ankylosed elbow; this may be seen in several
circumstances, including juvenile rheumatoid arthritis, some forms of
adult-onset rheumatoid arthritis, posttraumatic arthritis, and other
inflammatory conditions that involve this joint.
subacute infection and (b) neuromuscular deficiency of the elbow joint
flexors.
contraindication. Replacement may proceed if the absence of an active
infection can be demonstrated.
such a situation can usually be converted to an acceptable setting with
a soft-tissue surgical procedure that is performed before or
occasionally concurrent with joint replacement.
markedly decreases the effectiveness of elbow extension and the ability
to work overhead. However, in some instances, active flexion and relief
of pain justify joint replacement in this setting.
all the imaging that is necessary for this procedure. The most
important factors in this process are (a) to assess humeral bow and
medullary canal size in the lateral projection and (b) to note size and
angulation of the ulnar medullary canal in both projections. In
patients with juvenile rheumatoid arthritis, the medullary canal may be
extremely small. In such cases the special small ulnar implant should
be used.
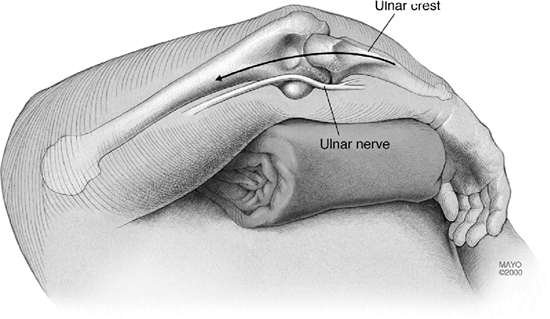
patient in a supine position with a sandbag under the scapula. The arm
is draped free and the table is rotated approximately 10 degrees away
from the operated extremities to further elevate the elbow and
extremity (Fig. 18-1). A general anesthesia is most often used.
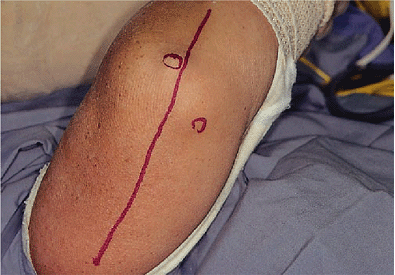
A straight incision is made just medial to the tip of the olecranon
between the medial epicondyle. The incision extends approximately 5 cm
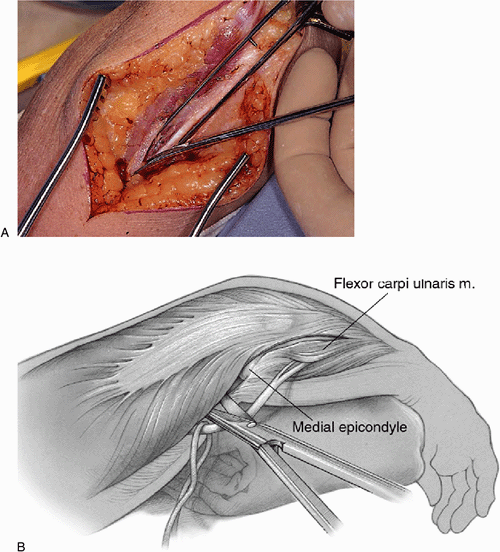
distal and 7 cm proximal to the tip of the olecranon (Fig. 18-2). As the subcutaneous tissue to the medial aspect of
the triceps is released, exposing the medial margin of the triceps and
the ulnar nerve, I always identify and translocate the ulnar nerve that
has not been previously moved. The nerve is isolated at the medial
margin of the triceps proximally. The dissection is carried distally to
the cubital tunnel retinaculum, which is split, and carried further
distally to the first motor branch to the flexor carpi ulnaris (Fig. 18-3). If there are adhesions to the capsule,
as sometimes occurs with rheumatoid arthritis or scarring from a
traumatic cause, the use of loops may be effective. Bipolar cautery is
used.
 |
|
Figure 18-1. The patient is placed supine on the operating table. The arm is draped free and brought across the chest.
|
 |
|
Figure 18-2. A straight incision is made between the medial epicondyle and the tip of the olecranon.
|
 |
|
Figure 18-3. A,B: The ulnar nerve is identified at the medial margins of the triceps.
|
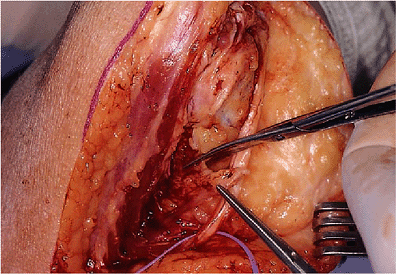
and a subcutaneous pocket is developed distally over the flexor
pronator fascia and proximally anterior to the triceps. An incision is
then made just to the medial aspect of the crest of the ulna, which
releases the forearm fascia and the periosteum over the ulna (Fig. 18-5).
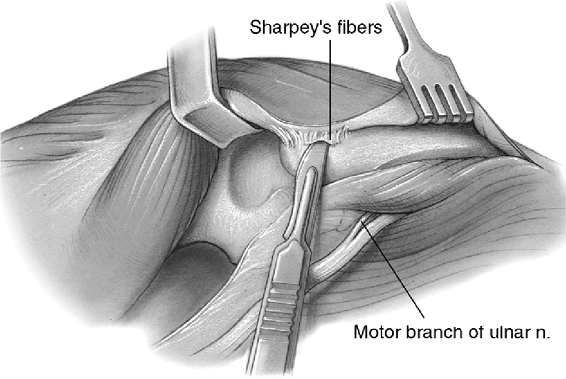
The medial aspect of the triceps is then elevated from the posterior
aspect of the humerus and from the posterior capsule. Retractors exist
proximally and distally to the triceps insertion. The discrete
insertion of the triceps by way of Sharpey’s fibers to the tip of the
olecranon is identified and released by a sharp dissection, allowing a
flap of tissue to be raised, including the triceps, forearm fascia, and
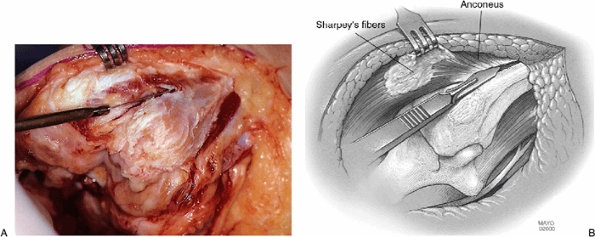
ulnar periosteum. As the extensor mechanism is further reflected
laterally, the anconeus is identified to the lateral aspect of the
proximal ulna. This is elevated from its bed (Fig. 18-6). Continued release of the extensor mechanism from the lateral
epicondyle allows complete exposure of the posterior aspect of the
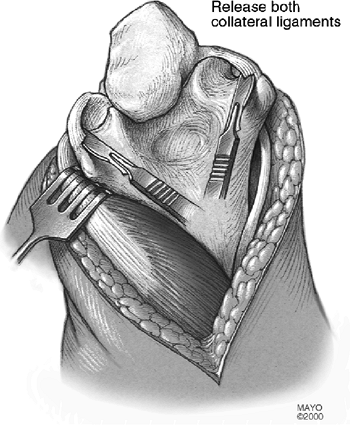
joint. The lateral and medial collateral ligaments are then released
from its humeral attachment and the elbow is fully flexed, providing
excellent exposure of the distal humerus and proximal ulna (Fig. 18-7). The ulnar nerve is carefully protected during this release.
 |
|
Figure 18-4. Intermuscular septum is removed. A deep subcutaneous pocket is created to receive the ulnar nerve.
|
 |
|
Figure 18-5.
The soft tissue is elevated from the subcutaneous border of the ulna and the medial margin of the triceps from the posterior aspect of the humerus. |
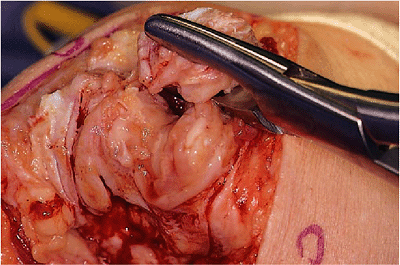
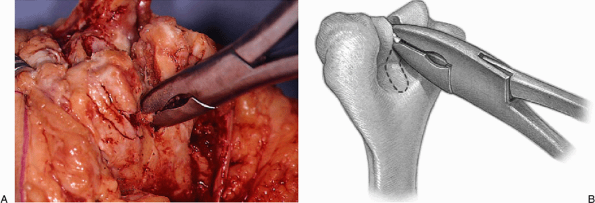
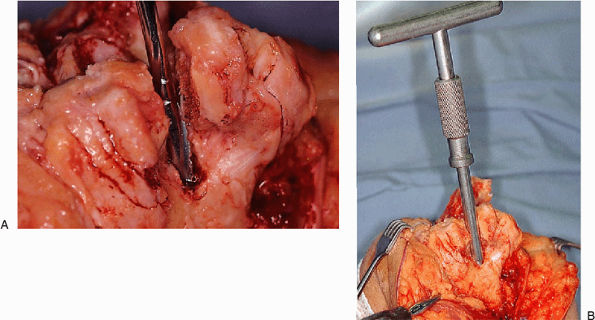
of the trochlea is removed with a rongeur or a saw, depending upon the quality of the bone (Fig. 18-10).
The medial and lateral columns are identified, and the roof of the
olecranon is entered using either a bur or a rongeur, again depending
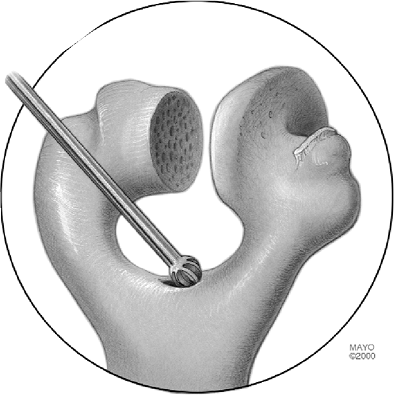
on the bone quality (Fig. 18-11). The medullary
canal of the humerus is then identified with a long twist reamer, which
also serves as the alignment stem. The humeral canal is typically very
spacious, allowing easy access of this instrument (Fig. 18-12).
 |
|
Figure 18-6. A,B: The anconeus is released and maintains the continuity of the extensor mechanism.
|
 |
|
Figure 18-7. Release of the medial-lateral collateral ligaments.
|
 |
|
Figure 18-8. The tip of the olecranon is removed with a rongeur or oscillating saw if the bone is dense.
|
 |
|
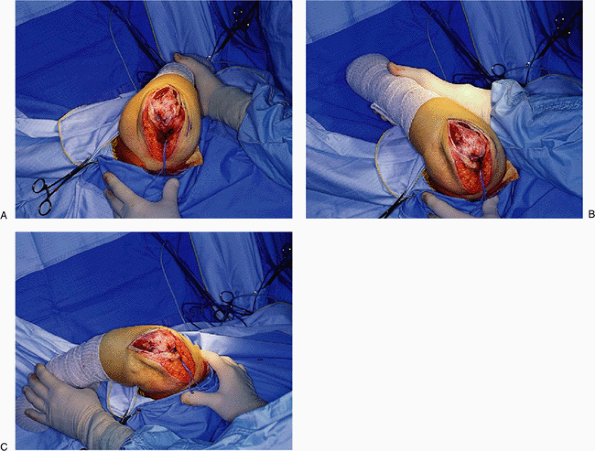
Figure 18-9. A-C:
External rotation of the humerus is termed “the maneuver.” Externally rotating the humerus and flexing the elbow allows ready access to the entire humerus and ulna, especially after the ligaments are released. |
the humeral canal accurately centers the distal cut. The handle is
removed and a cutting block is attached, which allows accurate
dimensions and a template for removal of the articular surface of the
distal humerus (Fig. 18-13). The side arm,
which rests on the capitellum, is interchangeable and the same device
can be used for the left or right elbow. The flat of the template is
oriented to the plane of the posterior columns to ensure accurate
rotatory alignment (Fig. 18-14).
With an oscillating saw the trochlea is removed (Fig. 18-15).
We prefer not to cut tightly on the cutting template so as to avoid too
narrow a resection that could place excessive force on the medial or
lateral column with the introduction of the component. For the
transverse cut, the oscillating saw blade is not angled from posterior
to anterior but rather is oriented obliquely to lessen the likelihood
of a cross-hatch at the junction of the column and the olecranon fossa
that can serve as a stress-riser, allowing the column to fracture (Fig. 18-16).
A small, thin rasp is initially used to again identify the canal and
ensure that the rasp is centered on the completed cut of the humerus.
The appropriate rasp is then used, depending upon the size of the canal
(standard or small) (Fig. 18-17). The most typically employed is the 4-inch in the rheumatoid elbow, as there may be shoulder pathology.
 |
|
Figure 18-10. A,B: A rongeur for soft bone or oscillating saw for more sclerotic bone is used to remove the midportion of the trochlea.
|
 |
|
Figure 18-11. The roof of the olecranon is entered with a rongeur or bur and expanded to receive the twist reamer.
|
 |
|
Figure 18-12. The canal is entered with a twist reamer (A) that serves as an alignment guide with a removable handle (B).
|
 |
|
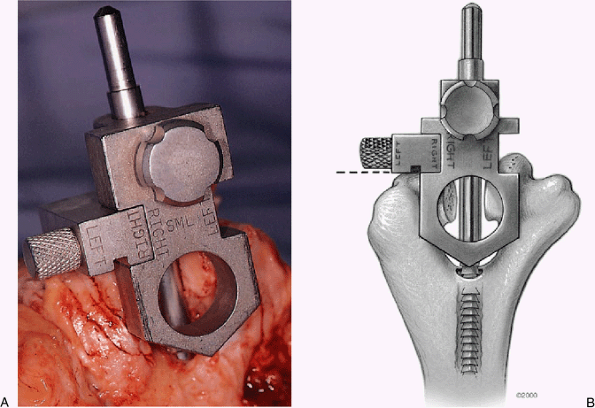
Figure 18-13. A,B: The handle is removed from the alignment device and the humeral cutting jig is in place.
|
 |
|
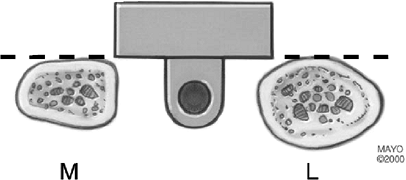
Figure 18-14.
The humeral cutting block is oriented coplanar with the plane of the posterior aspect of the medial and lateral humeral columns. |
 |
|
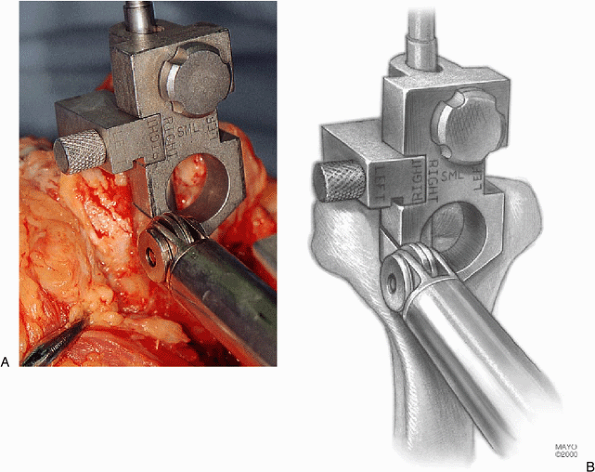
Figure 18-15. A,B: Accurate removal of bone with the oscillating saw.
|
brachialis muscle is released from the anterior cortex of the humerus
with a curved elevator to accommodate the flange (Fig. 18-18).
The articulation of the trial humeral component is placed within the
resected trochlea. If there has been adequate bone removed to
accommodate the width of the implant articulation, it is then inserted
down the canal (Fig. 18-19).
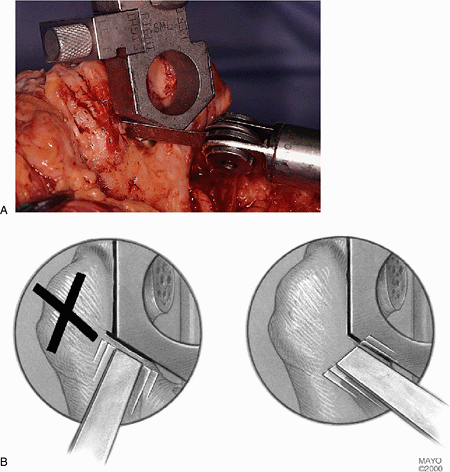 |
|
Figure 18-16. A,B: By placing the oscillating saw at an oblique angle one can avoid cross-hatching the supracondylar columns.
|
 |
|
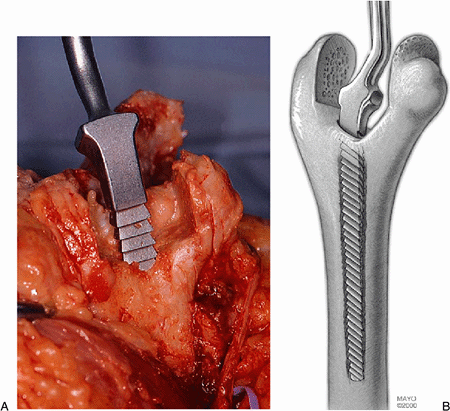
Figure 18-17. A,B: The humeral canal is rasped to the appropriate size.
|
 |
|
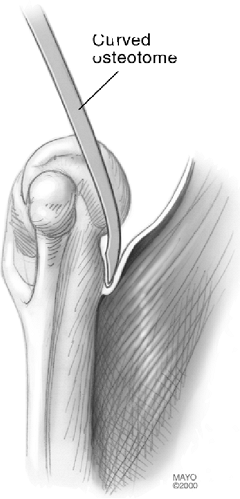
Figure 18-18. The anterior capsule and brachialis muscle insertion are released from the anterior humeral cortex with a curved osteotome.
|
 |
|
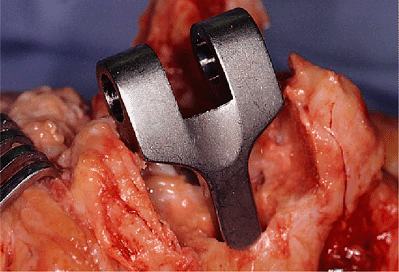
Figure 18-19. Trial reduction of the humeral component.
|
since the proximal ulna has been denuded. The canal is most easily
entered with a high-speed bur placed approximately at a 45-degree angle
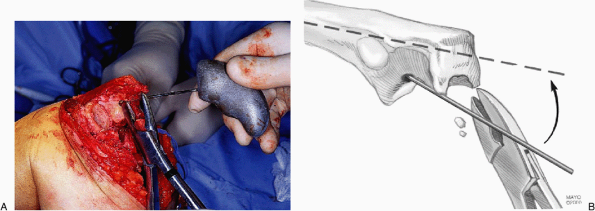
to the olecranon at the base of the coronoid (Fig. 18-20). The orientation of the canal is established with a small awl (Fig. 18-21). To ensure longitudinal access down the ulna the olecranon is notched in line with the awl (Fig. 18-22). A pilot rasp is used with a twisting motion to further identify and enlarge the canal (Fig. 18-23).
Next, the appropriate-sized ulnar rasp is inserted initially with a
twisting motion. Complete seating often requires the use of a mallet (Fig. 18-24), and care is taken to ensure that rotation is at a right
angle with the flat portion of the olecranon implanted (Fig. 18-25). In small bones the “trial rasp” is used. If the canal allows, the larger rasp is inserted.
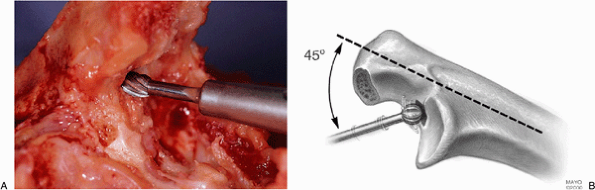 |
|
Figure 18-20. A,B: Medullary canal of the ulna is identified with a high-speed bur. If the bone is soft, a rongeur can be used.
|
 |
|
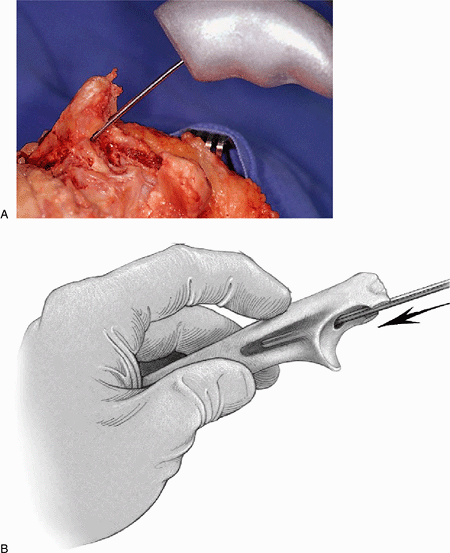
Figure 18-21. A,B: An awl identifies the medullary canal.
|
 |
|
Figure 18-22. A,B: To assist in proper alignment the olecranon is notched with a rongeur in line with the awl.
|
 |
|
Figure 18-23. The canal is enlarged with a pilot rasp using a twisting maneuver.
|
 |
|
Figure 18-24. A,B:
The appropriate-sized rasp is introduced down the canal. Occasionally, the orifice must be opened with a bur. This stage often requires the use of a mallet to adequately seat the rasp. |
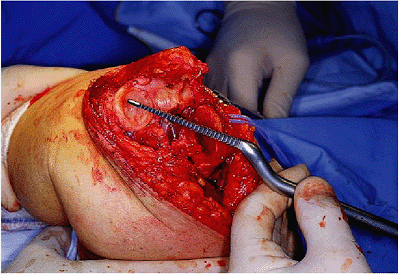
component is inserted down the canal. The depth of the insertion should
be such that the center of the olecranon component is coincident with
the center of curvature of the greater sigmoid fossa that is halfway
between the tip of the olecranon and the coronoid (Fig. 18-26).
It is wise then to insert the humeral component and perform a trial
reduction to ensure that there is no residual flexion contracture to
identify impingement and to ensure that the humeral component can
readily be articulated with the ulnar component (Fig. 18-27).
 |
|
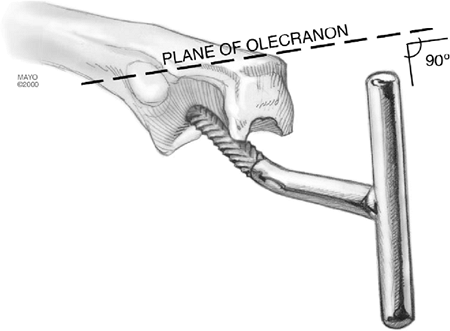
Figure 18-25. Proper orientation of the rasp is with the handle perpendicular to the flat of the proximal ulna.
|
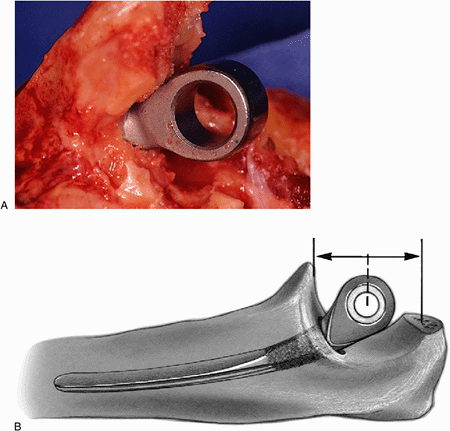 |
|
Figure 18-26. A,B:
A trial reduction of the ulna ensures axial rotation, and proper depth of insertion replicates the center of the normal articular contour. |
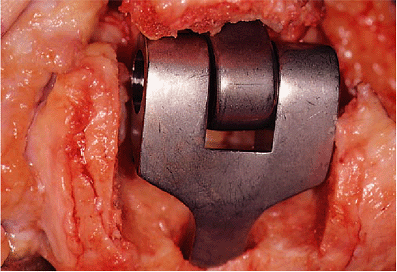 |
|
Figure 18-27.
A trial reduction of both components ensures that the flexion contracture has been released and an adequate arc of motion has been attained. |
pulsating lavage system and dried. For those less experienced with the
procedure it is safest to cement the implants separately. If this is
done, the ulnar component is first cemented using an injection system
that is cut to the length of the ulnar component (Fig. 18-28). The ulnar component is inserted into the cemented medullary canal and impacted into proper position as noted earlier.
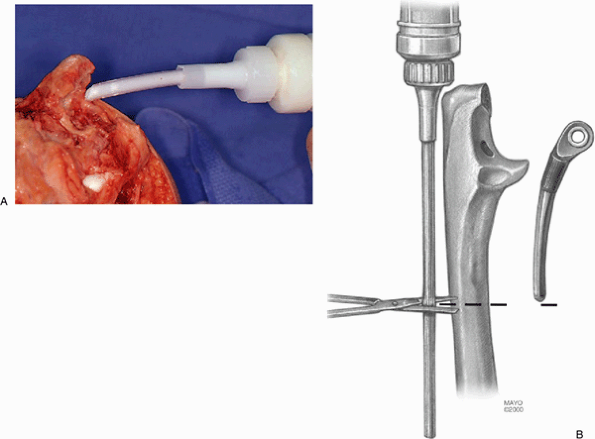 |
|
Figure 18-28. A,B: An injection system is used to reliably and reproducibly introduce cement down the medullary canal of the ulna.
|
exists about cement extending too far down the canal, cancellous bone
chips from the trochlea may be used. Otherwise, no attempt is made to
plug the canal and the injector nozzle is cut to the proper length and
placed down the medullary canal to deliver the cement to the
appropriate depth (Fig. 18-29).
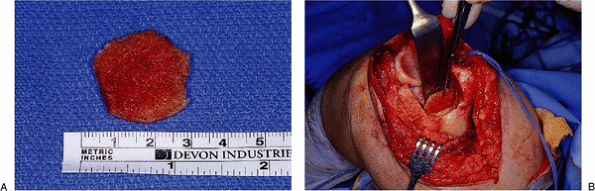
measuring approximately 2 з 2 cm and 2 to 4 mm in thickness. This is
placed at the anterior aspect of the humerus just proximal to the cut
in the distal humerus (Fig. 18-30). The humeral
component is then inserted down the canal to the point at which
articulation with the ulna is possible. The previously
placed bone graft engages the flange at the time of implant coupling (Fig. 18-31).
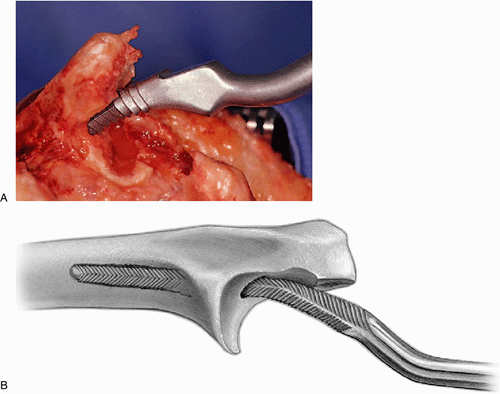
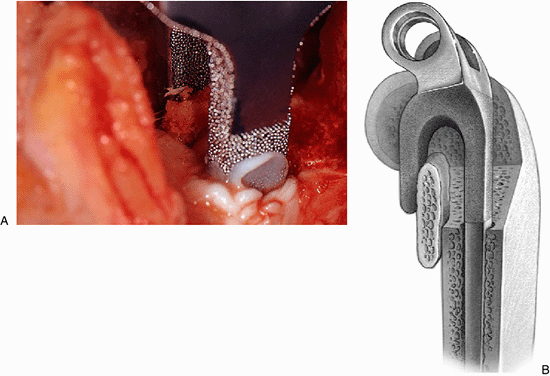
The ulnar component is then articulated by placing a pin across the
humerus through the ulna and is secured with the “pin-in-pin” axis
system (Fig. 18-32).
 |
|
Figure 18-29. A,B:
The length of the injector system is determined by the anticipated length of the appropriate-sized humeral component to be used. |
 |
|
Figure 18-30. A,B: A bone graft is placed behind the anterior cortex of the distal humerus against which the flange of the implant articulates.
|
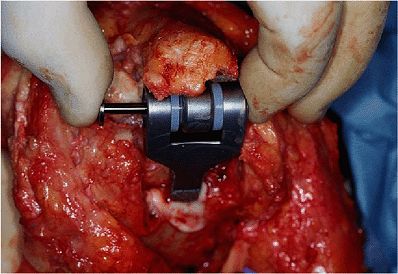
there is a slight bow to the humerus and a small canal. These
circumstances are recognized with the trial reductions, and a slight
bow measuring approximately 5 degrees is placed in the distal one-third
of the humeral component with the plate binder. This allows
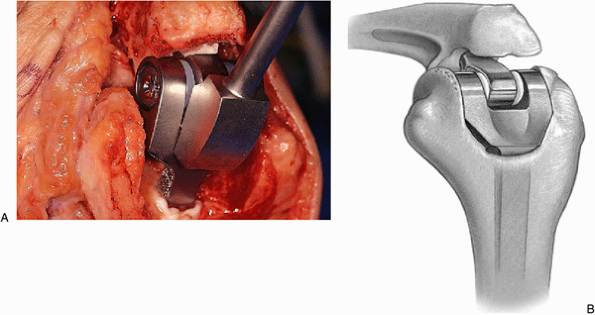
accommodation of the anterior bow of the humerus (Fig. 18-33).
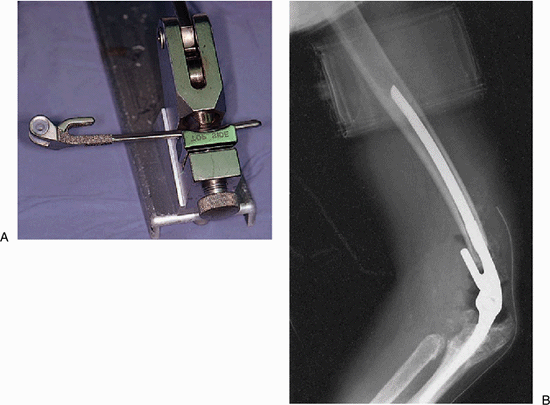
placed in a 90-degree angle and the humeral component is impacted down
the medullary canal (Fig. 18-34). The humeral
component is usually inserted such that the distal aspect of the
component is at the level of or slightly proximal (1 or 2 mm) to the
contour of the distal capitellum. The actual depth of insertion is
governed by the depth at which the flange articulates with the roof of
the olecranon. The bone graft is anterior to the distal humeral cortex
and posterior to the flange at
this point. After the prosthesis has been inserted, the tourniquet is deflated and hemostasis is obtained.
 |
|
Figure 18-31. A,B:
After the ulnar component has been rigidly fixed, the bone graft is partially elevated and the humeral component is inserted to the point where the graft is captured by the flange, and this allows the juxtaposition and articulation of the ulna with the humeral component. |
 |
|
Figure 18-32. The pin-within-the-pin articulating mechanism couples the device.
|
 |
|
Figure 18-33. A,B:
In those instances in which there is a small canal and an anterior bow of the humerus the humeral component is slightly bent with a plate bender. |
 |
|
Figure 18-34. A,B: The humeral component is placed to the proper depth by impaction with the elbow at 90 degrees of flexion.
|
full extension and flexion. Usually, an arc of 0 to 140 degrees is
obtainable at the time of surgery. The radial head need not be removed
for proper functioning of the device but should be excised if the
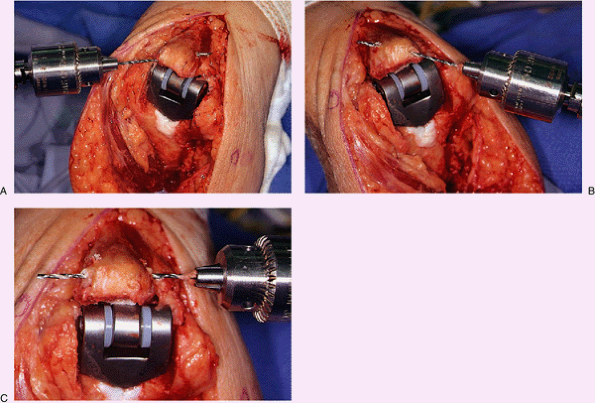
pathology dictates this. The proximal ulna is prepared for reattachment
of the triceps by placing drill holes obliquely both medially and
laterally in a cruciate fashion (Fig. 18-35A,B). A third transverse hole is placed across the proximal ulna (Fig. 18-35C).
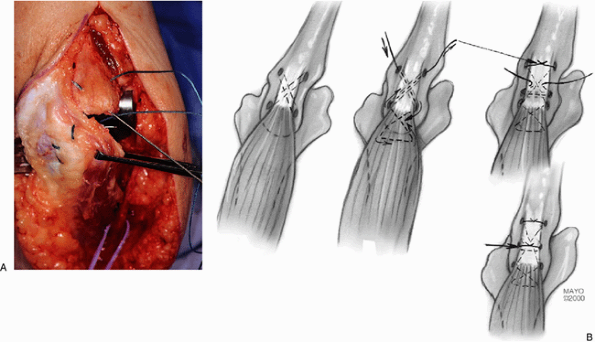
A heavy nonabsorbable (No. 5) suture is then brought through the distal
medial hole with a Keith needle. The elbow is placed in 90 degrees of
flexion and the triceps is reduced over the tip of the olecranon. The
suture is then inserted in the triceps at this point and a criss-cross
stitch is placed in the triceps tendon (Fig. 18-36).
The suture then penetrates from the tendon medially and is directed
obliquely through the second hole to emerge on the lateral aspect of
the ulna. The suture is then brought through the forearm and ulnar
periosteum in such a way as to meet the initial suture on the medial
aspect of the ulna and is tied off the subcutaneous border (Fig. 18-36). A transverse suture is then placed across the ulna and through the tendon to further stabilize the attachment.
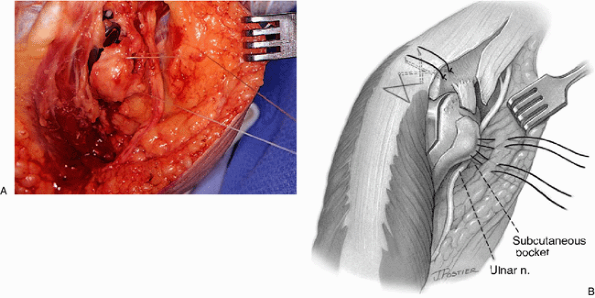
taken to move the ulnar nerve into the subcutaneous pocket. Stitches
are placed in the subcutaneous tissue to the soft tissue at the medial
column (Fig. 18-37). This keeps the ulnar nerve
from subluxing into the articulation. A drain is optional and the
remainder is closed in layers with absorbable sutures on the deep
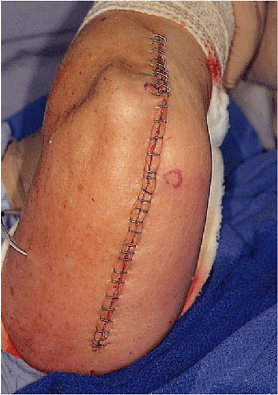
structures and stainless steel staples on the skin (Fig. 18-38). In patients with rheumatoid arthritis a 3-0 monofilament running suture is used if the skin is thin or atrophic.
 |
|
Figure 18-35. Cruciate (A,B) and transverse (C) drill holes are placed in the proximal ulna.
|
 |
|
Figure 18-36. A,B:
The sutures have been placed through the ulna and in a criss-cross fashion in the tendon. The elbow is at 90 degrees of flexion when these sutures are tied. |
 |
|
Figure 18-37. A,B:
The ulnar nerve is carefully brought into the subcutaneous pocket and secured with a 3-0 absorbable suture placed in the subcutaneous tissue to the medial epicondylar region. |
 |
|
Figure 18-38. Skin closure is routine. In this instance, dermal staples were used.
|
a significant flexion contracture, an anterior splint is used. The arm
is elevated overnight and for the second day. If drains are used they
are removed the following day. The patient is then allowed to move the
elbow as tolerated. A light-weight dressing is used during this period
of time. A collar and cuff is helpful to keep the patient comfortable.
No formal physical therapy is required or employed. Occupational
therapy may be helpful for very debilitated patients, but we have not
found this necessary in recent years.
after the procedure are not to lift 1 to 2 pounds on a repetitive basis
or more than 5 to 10 pounds in a single event. Occasionally, if a
flexion contracture of greater than 40 degrees is observed by the fifth
postoperative day, then a turnbuckle splint is used to try to resolve
this. This splint is typically used for 8 to 12 weeks.
-
The current design employs a universal
articulation allowing interchange among all three sizes of the humeral
and ulnar components. -
The implant comes in three humeral
lengths: 10, 15, 20 cm; and three cross-sectional
dimensions—extra-small, small, and standard (Fig. 18-39).
Three stem lengths are available: 10 cm for rheumatoid arthritis to
allow shoulder replacement if needed; 15 cm for posttrauma and
revision; and 20 cm for revision and special problems with distal bone
loss. -
The ulnar component is available in three diameters with an extra-long device available for revisions (Fig. 18-39).
-
The polyethylene bushings are removable and interchangeable should these wear over time and require replacement.
 |
|
Figure 18-39. Three lengths and three dimensions of the humeral components and two lengths and three sizes of ulnar components are available.
|
the patient with a grossly unstable elbow due to a distal humeral
nonunion or prior resection.
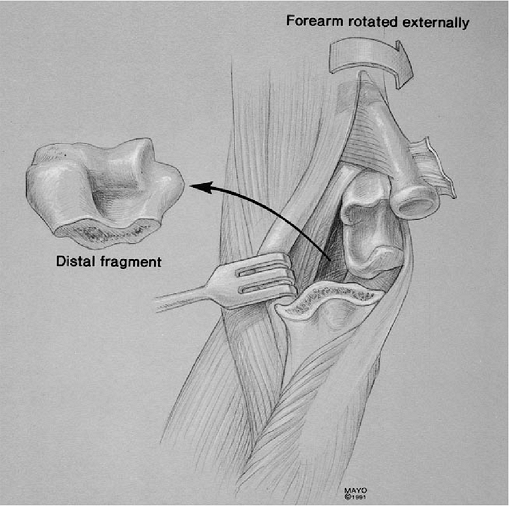
humerus allows ready access to the humeral canal by translating the
distal segment to the lateral margin of the triceps muscle. For acute
fracture or for nonunion, we first excise all fragments while leaving
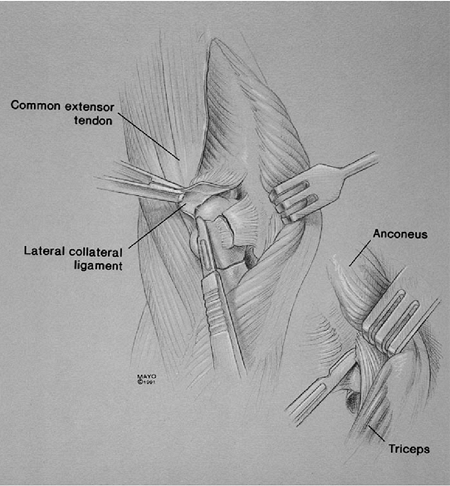
the triceps left attached to the olecranon (Fig. 18-40) (2,7).
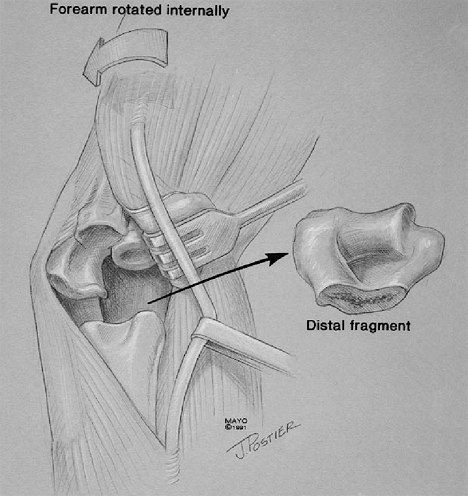
The most difficult technique is exposure of the ulna shaft with the
triceps attached. The ulna is prepared from the medial margin
protecting the ulnar nerve. The essential step is a partial (25% to
50%) Mayo type subperiosteal reflection of the triceps attachment
allowing subluxation of the triceps and greater rotation of the ulna (Fig. 18-42).
The actual execution of the replacement is relatively easy, as the
absent distal humerus eliminates obstruction to coupling the device.
Because of the extent of the usual dissection, we recommend release of
the tourniquet to secure hemostasis before closure. Closure includes
the reattachment of the flexor and extensor muscle mass, as able, to
the margins of the triceps with a No. 0 suture.
concern exists regarding tissue healing, so motion is immediately
allowed, as tolerated.
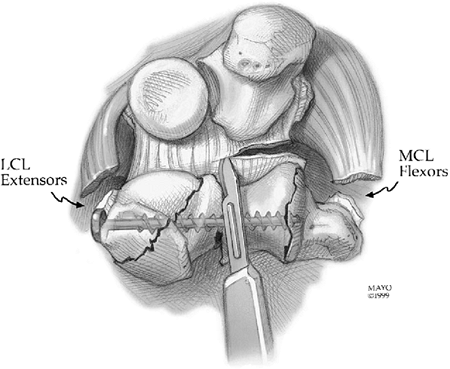
triceps from the olecranon is essential. An aggressive transection of
the fused joint or release of joint adhesions is coupled with a very
aggressive release of the anterior capsule and release of the collateral ligaments and release
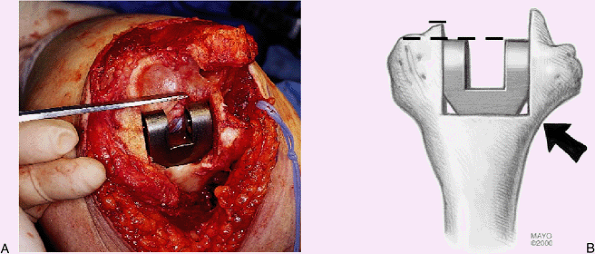
of the flexor/extensor attachments to the humerus (Fig. 18-43). Additional steps to improve extension include the more proximal palcement of the humeral component (Fig. 18-44).
If this is limited by fear of fracture or thinness of the medial
column, the epicondyle may be excised. Increasing the depth of the
insertion of the ulnar component is limited by the coronoid, and too
excessive distal placement results in a prominence of the olecranon.
After the implant has been coupled the last special consideration is
that of the triceps attachment. If
the
triceps is compliant, reattachment may not be a problem and will not
limit flexion. If, however, the anatomic reattachment is not possible
without markedly restricting flexion, we perform an anconeus slide
procedure and reattach the anconeus-triceps complex to the olecranon in
90 to 110 degrees of flexion as appropriate to allow functional flexion
without excessive weakening of the extension force of the mechanism.
 |
|
Figure 18-40. The distal humeral nonunion or acute fracture is excised, leaving the triceps attached.
|
 |
|
Figure 18-41. Exposure of the distal humerus at the lateral margin of the triceps.
|
 |
|
Figure 18-42. Exposure of the distal humerus at the medial aspect of the triceps.
|
 |
|
Figure 18-43. Aggressive soft-tissue release includes the capsule, collateral ligaments, and flexor/extensor muscle origins.
|
 |
|
Figure 18-44. A,B:
In those with fixed contracture, the humerus is placed more proximally. The integrity of the medial column limits the depth of insertion. However, clinical experience indicates that medial epicondyle integrity is not essential for the implant to function in an effective manner. |
latter part of 1981 and has been used almost exclusively by the author
since then with few modifications. This implant, Coonrad-Morrey, has
been used in more than 800 patients in our Mayo practice. The most
common indications have been rheumatoid arthritis (39%), posttraumatic
arthritis (30%), and revision total elbow replacement surgery (24%).
The results for the spectrum of indications are summarized in Table 18-1.
The outcome for rheumatoid arthritis is particularly gratifying as the
survival now rivals that of hip replacement, with 93% survival at 12.5
years (3,9) (Fig. 18-45). Others also report good early results with the semiconstrained concept (4,14).
|
TABLE 18-1. Mayo experience with semiconstrained elbow replacement for a spectrum of conditions
|
|||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||
 |
|
Figure 18-45. Kaplan-Meier survival curve free of revision of a group of 78 patients with rheumatoid arthritis followed a mean of 12.5 years.
|
|
TABLE 18-2. Type and frequency of complications after semiconstrained TEA
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
It is now well known that semiconstrained implants have a lower
incidence of loosening than the earlier, more rigidly constrained
articulated devices (4,9,12,14). Our experience with loosening was almost nonexistent (9)
until recently. In the last 5 years an increased incidence in ulnar
osteolysis has prompted the change from a polymethylacrylate (PMMA)
precoat to a plasma spray surface finish for the ulnar component. The
treatment for loose or unstable implants is treated by
revision/reimplantation, if possible, and is discussed in Chapter 19.
elbow replacements. We have observed 10 fractured components: eight of
the ulna and two of the humeral component over the last 10 years
(1.3%). In all but two instances, the patient had sustained a
significant injury or was using the elbow in an aggressive manner such
as lifting 100-pound bags of feed. Reimplantation has been successful
in all to date (16).
complication of total elbow arthroplasty, cited in the literature as a
complication with a 2% to 26% incidence (8) averaging about 5% (2,11).
Our personal experience is an incidence of 0.5% of patients with motor
deficiency and fewer than 3% with permanent paresthesias. In our
opinion, this complication is lessened by exposing and protecting the
ulnar nerve.
1.3%. Reattachment is successful in 50% either with the same technique
as used for the primary procedure or with an anconeus rotational
reconstruction (see Chapter 11). Notable weakness of the triceps was observed in fewer than 10% of patients.
experience with total elbow arthroplasty, in our personal experience
the incidence is currently just over 1%. In the last 10 years there
have been five significant wound problems from among 300 procedures.
rheumatoid arthritis, fracture of the medial supracondylar column is
not uncommon and is not considered a significant event. This has
occurred in approximately 5% of our patients. If the column is
extremely thin, we simply excise the fragment. Otherwise, the fragment
is secured to the implant with a No. 5 Mersilene suture. We do not
alter our postoperative course as a result of this occurrence, as no
adverse effects have been appreciated as a result of this to date.
was treated with semiconstrained total elbow replacement. The Mayo
Modified Coonrad implant was selected for stability and because of the
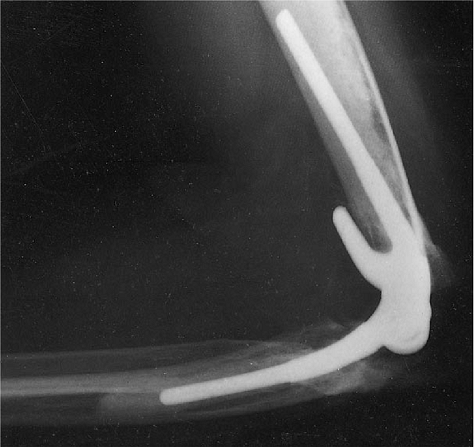
favorable long-term results (Fig. 18-47). At 5 years the patient is pain free, has an arc of motion of 25 to 135 degrees, and shows no evidence of loosening (Fig. 18-48).
 |
|
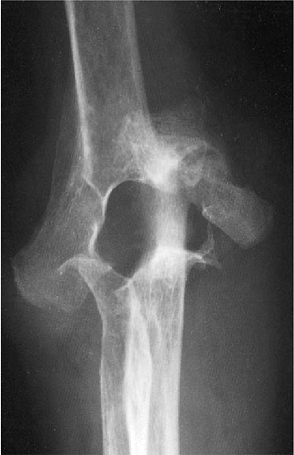
Figure 18-46. Severe rheumatoid arthritis with grade IV radiographic changes (severe architectural distribution).
|
 |
|
Figure 18-47. After replacement with a Mayo modified Coonrad implant (A), a stable cement interface is observed (B).
|
 |
|
Figure 18-48. At 5 years no loosening has occurred, the bone graft has matured, and virtually normal function has been restored.
|
 |
|
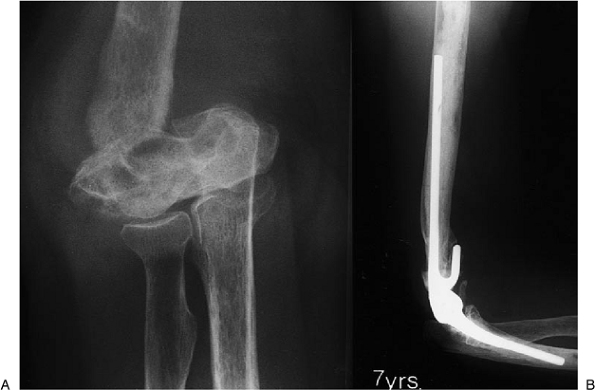
Figure 18-49. Patient with distal humeral nonunion of 2 years’ duration (A). Excellent function persists 4 years after replacement (B).
|
 |
|
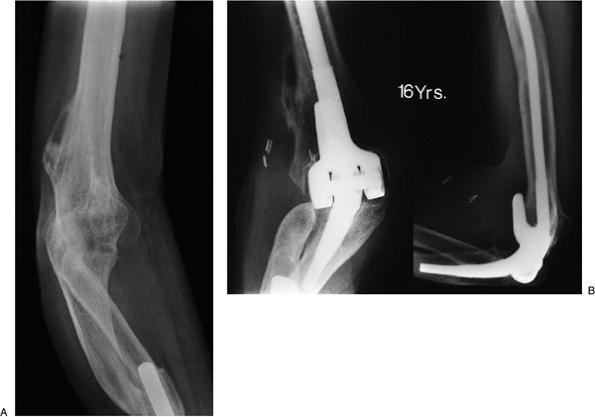
Figure 18-50. Severe deformity, spontaneously fused elbow, and one-bone forearm (A). Patient continues to function well 16 years after replacement (B).
|
DRJ, Morrey BF. The Coonrad-Morrey total elbow arthroplasty in patients
who have rheumatoid arthritis: a ten to fifteen year follow-up study. J Bone Joint Surg 1998;80A:1327–1335.
N, Loehr J, Ivosevic-Radovanovic D, et al. Semiconstrained elbow
prostheses with special reference to the GSB III prosthesis. Clin Orthop 1988;232:104.
AG, Adams R, Morrey BF. Semiconstrained total elbow replacement for the
treatment of posttraumatic arthritis and dysfunction. J Bone Joint Surg 1997;79A:1211–1222.
