Femoral Neck Fractures: Arthroplasty
locations in the proximal femur. Intertrochanteric (or pertrochanteric)
fractures involve the area of the bone that is mostly or entirely
outside the hip joint capsule, where the soft-tissue attachments
provide good blood supply for healing and for ligamentotaxis. Fractures
of the femoral neck occur through bone inside the capsule, where sparse
soft-tissue attachments and the synovial fluid environment make healing
slower and less reliable. Intertrochanteric fractures are routinely and
successfully treated with reduction and internal fixation, and
nondisplaced or impacted femoral-neck fractures are reliably handled
with fixation in situ. However, the treatment of displaced femoral-neck
fractures involves several controversies.
the fracture or to excise and replace the femoral head. Reduction and
fixation of a displaced femoral-neck fracture is a technically
demanding surgical procedure. It allows the patient to retain his/her
own femoral head, and thereby avoid problems specific to prosthetic
replacement such as dislocation, loosening, wear, or breakage, which
may necessitate revision surgery. This has led some authors to propose
an age-based protocol: younger patients (<50 years) are treated with
reduction and fixation, elderly patients (>70 years) get
arthroplasty, and those in between get individualized decisions based
on general health, activity level, and bone quality (1).
A wide range of implant failure, displacement, and re-operation rates
for internal fixation have been reported in the literature (2,3).
A meta-analysis of 13 studies in the Cochrane database concluded that
internal fixation was associated with less operative trauma than
replacement (decreased operative time, blood loss, transfusion, and
infection) but a higher need for re-operation. No clear differences in
hospital stay, mortality, or functional outcome were found (4).
A more recent meta-analysis of 14 studies showed that those treated
with arthroplasty had a significantly reduced risk of re-operation,
blood loss, long operative times, and infection. However, this study,
in contradistinction to the previous review, found that patients who
had received arthroplasty had a slightly higher early-mortality rate
than those who were treated with fixation (5). A study using cost-analysis methodology
suggested that arthroplasty was the most cost-effective treatment when
complications, mortality, and re-operation rate are evaluated at 2
years postoperation, but the best functional results are achieved with
a healed femoral neck that does not have concomitant osteonecrosis (6).
arthroplasty to be performed for femoral neck fracture. Total hip
replacement (THR) can be performed for patients with acetabular damage,
preexisting degenerative arthritis, or a systemic arthritic disease
such as rheumatoid arthritis. Although some authors (7,8,9)
have suggested that THR has better functional results with lower
complications than hemi-arthroplasty, others have found that THR after
acute fracture has significantly poorer outcomes, in terms of
dislocation and revision rates, than primary THR (5,10,11).
Those reports are retrospective, uncontrolled, and based on few cases.
Two, prospective, randomized studies have compared THR with
hemi-arthroplasty, and showed conflicting results. One study (12) revealed no difference between the two procedures, while the other (13)
suggested that THR had a lower revision rate and higher hip scores. The
study showing differences in acetabular replacement also demonstrated
differences in stem fixation between the two groups.
unipolar or bipolar endoprosthesis. The bipolar design was developed to
reduce metal on cartilage motion and friction, and thereby decrease
acetabular wear and erosion, a postulated cause of postoperative pain.
At the same time as the bipolar head design, modern modular-stem
designs were introduced, complicating analysis. Several studies have
shown good results for bipolar endoprostheses, particularly when they
were cemented in place (14,15,16).
However, cadaveric and radiographic studies revealed that motion occurs
primarily at the outer bearing, particularly when the prosthesis is
loaded (as in walking), reducing the likelihood for protection of the
acetabular cartilage (17,18).
In addition, multiple-prospective comparison studies have failed to
show any significant difference in outcome between the bipolar and the
unipolar designs (2,19,20,21,22).
A prospective study reporting outcomes of 270 patients found
significantly higher hospitalization costs (30%) for patients receiving
a bipolar prosthesis compared to those receiving a unipolar implant (23). The type of implant was chosen by the surgeon, and there were some differences in the two patient populations.
commercially available in the United States, and many offer
hemi-arthroplasty components. Femoral stems come in many designs, made
of a variety of different metals, and with a variety of surfaces and
coating. Many of these stems are substantially more expensive than the
basic Austin-Moore (fenestrated) or Thompson (nonfenestrated) design
that has been in use for decades, and none has been proven superior.
for the stem. While operation without cement is faster, avoids the
cardiovascular morbidity related to methylmethacrylate, and may make
subsequent revision of the stem (if needed) easier, stability is
decreased and the patient is slower in returning to activity. A
retrospective study of 451 cases comparing the Bateman bipolar
prosthesis inserted with or without cement found less thigh pain (13%
vs. 46%) and higher Harris hip scores (86% vs. 79%) in the cemented
group, with no difference in mortality (16). In
a prospective, randomized, trial in which bipolar heads were used, the
group whose hips were not cemented had significantly more pain and need
for walking aids (24). A recent analysis of 18
published studies found that the use of cement resulted in longer
operative times and more blood loss, but better postoperative mobility
and less pain; no differences in mortality or general complications
were found (25). Sixteen of the 18 studies
reviewed concluded that cement should be used routinely, and the
authors of the review agreed. A meta-analysis in the Cochrane Library
found that there was “limited” evidence that cementing the stem of a
hemi-arthroplasty would lead to less pain and improved mobility, but
concluded that the data was insufficient to determine whether that
advantage was offset by other disadvantages of cementing, such as
increased mortality (20). If the stem is
inserted without cement, use of an intramedullary corticocancellous
bone plug, prepared for the head of the femur, may decrease the rate of
subsidence and subsequent thigh pain (26).
 |
|
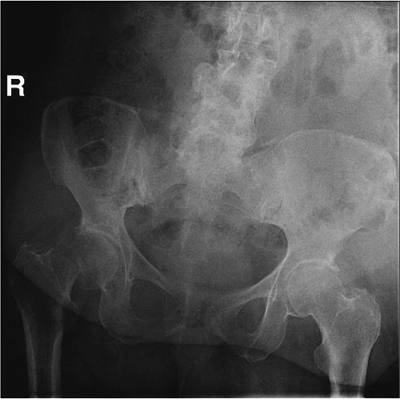
Figure 15.1.
An AP pelvis radiograph of a 74-year-old woman with a displaced fracture of the right femoral neck. The position of the proximal femoral shaft explains the shortening and external rotation typically seen on physical examination. |
femoral-neck fractures in physiologically older adult patients (some
have suggested approximately 70 years of age), or in younger patients
with limited life span due to systemic disease, impaired ability to
heal fractures, or irreparably damaged femoral heads (Fig. 15.1).
Nondisplaced or impacted fractures are better treated with fixation in
situ using cannulated screws. Fractures with preexisting arthritic
changes or insufficient acetabular structure should be treated with
total hip arthroplasty. Fractures in moribund, severely demented, or
nonambulatory patients with limited life expectancy may be treated
nonoperatively with analgesia.
physical exam, which may be difficult in many elderly, debilitated
patients. The circumstances surrounding the fall should be elicited,
seeking evidence of a syncopal episode, medication error, or
exacerbation of a chronic medical condition that would require further
workup. Evidence of an unsafe environment or elder abuse may require
social work evaluation. A complete medical history and review of
systems may reveal information that impacts the timing of surgery or
the choice of anesthetic technique. On physical examination, the
injured leg is typically shortened, externally rotated and painful with
motion. The examination should include evaluation for other typical
insufficiency fractures, such as the distal radius, pelvis, or spine
and for fall-associated traumatic conditions such as subdural hematoma.
The involvement of a medical consultant, particularly one who knows the
patient, is useful. Laboratory studies should include a complete blood
count, analysis of serum electrolytes, a blood sample for type and
cross match, chest radiograph, and electrocardiogram.
proximal femur are necessary to plan for the procedure. Lateral films
are difficult to obtain due to patient discomfort and are often of poor
quality. Obesity and osteopenia frequently combine to thwart adequate
visualization,
and repeat films with less penetration may be helpful. Traction and/or
rotation films may be helpful to evaluate the fracture line, although
traction and internal rotation may restore the position of the femur
and make the orientation look normal. Sizing templates for the
prosthetic system can be used to estimate the size of the prosthesis
necessary. Particular attention should be paid to the level of the neck
cut and the size of the femoral head because length discrepancies and
size mismatch are associated with poor outcomes (27).
anesthesia. Regional technique may offer less risk of some anesthetic
complications, but several large retrospective, nonrandomized studies
of hip fracture surgery have failed to show any difference in
mortality, morbidity, or functional outcome (28,29,30,31).
30 minutes, but less than 120 minutes, prior to incision. Usually 2 g
of a broad spectrum cephalosporin (e.g., cefazolin) are given, unless
the patient is allergic or there is another specific reason to give a
different drug.
lateral, or posterior approaches to the hip. One study of relatively
poor methodological quality compared anterior to posterior approaches
and found (for unexplained reasons) significantly increased mortality
in the posterior group (32), and some older studies suggested a higher dislocation rate with posterior approach (33,34).
More recent meta-analysis for comparison of lateral and posterior
approaches for total hip arthroplasty has yielded no major differences
in function or complication rates (35). The surgeon should use the surgical approach with which she/he feels most comfortable.
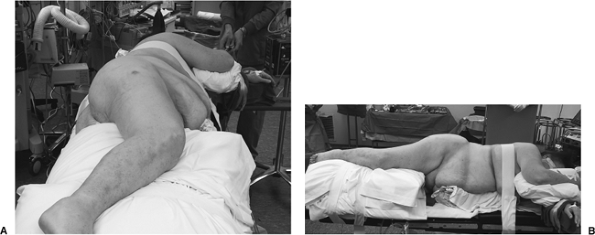
placed in the lateral decubitus position after induction of anesthetic.
Padding is placed on all bony prominences (Fig. 15.2).
A bean bag is used to maintain position, and the down-side arm is
placed out in front of the body. An axillary roll may be used. The
down-side leg is padded and secured to the bed with straps or tape. The
chest may be secured in like fashion, but care should be taken not to
make the chest strap too tight. Efforts are made to orient the pelvis
directly lateral.
circumferentially from above the iliac crest to the toes, and the
draping is applied to allow adequate exposure posteriorly and
proximally. The leg is covered with a stockinette to the mid thigh and
secured with Coban (Fig. 15.3).
The incision is marked on the skin and then adherent
antiseptic-impregnated plastic drapes may be applied to cover the
exposed skin completely, including the medial thigh and groin.
 |
|
Figure 15.2.
The patient is positioned in the lateral decubitis position on a bean bag with care taken to pad all boney prominences. A pillow is placed between the arms and the legs. |
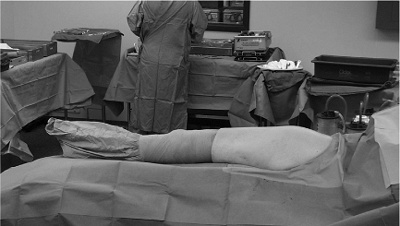 |
|
Figure 15.3. The entire leg and hip are draped free, as shown in this image from a patient other than the one in Figure 15.1. A stockinette is used to the mid thigh.
|
 |
|
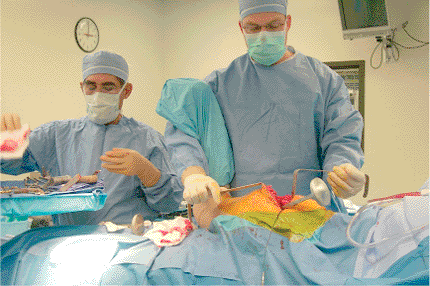
Figure 15.4.
The position used by the assistant to support the leg and retract the tissues. It is important to be careful not to contaminate the patient’s leg against the assistant’s face or mask. |
assistant stands on the opposite side. This allows the assistant to
keep his/her hands free while supporting the leg with the hip extended,
knee flexed, and the femur externally rotated (Fig. 15.4).
The incision is centered on the greater trochanter and extends distally
along the lateral aspect of the femur for approximately 10 cm.
Proximally the incision extends approximately 45 degrees toward the
posterior, superior, iliac spine for approximately 10 cm (Fig. 15.5).
With an obese patient, larger incisions will be necessary. The
subcutaneous tissue is divided in line with the incision, and
hemostasis is accomplished with electrocautery. The fascial level is
identified and opened sharply with knife or Bovie, and the incision is
extended distally in the fascia posterior to the iliotibial band and
proximally, splitting the gluteus fascia and muscle bluntly. The
trochanter is exposed (Fig. 15.6).
 |
|
Figure 15.5. The incision is centered on the trochanter and marked on the skin.
|
 |
|
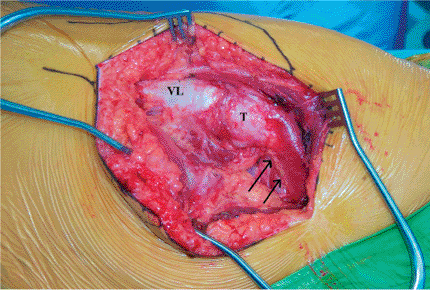
Figure 15.6.
The incision has been made and the fascia divided. As the surgeon would view the field: Distal is to the left, and proximal is to the right; anterior is up and posterior is down. The arrows indicate the posterior edge of the medius tendon inserting onto the trochanter. VL, vastus lateralis; T, trochanter. |
 |
|
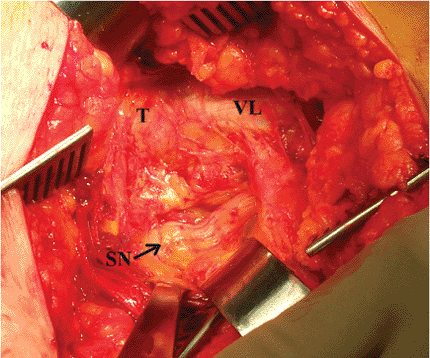
Figure 15.7.
The sciatic nerve is seen passing proximal to distal (left to right) posterior to the quadratus femoris muscle in this image from another patient. T, trochanter; VL, vastus lateralis; SN, sciatic nerve. |
 |
|
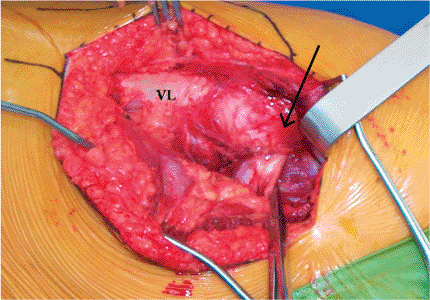
Figure 15.8.
In this picture, distal is to the left, and proximal to the right. The piriformis tendon is indicated by the clamp behind it. The arrow identifies the tip of the trochanter. VL, vastus lateralis. |
Awareness of the nerve position is maintained throughout the operation,
and it is protected from tension by extension of the hip and flexion of
the knee. The piriformis tendon is identified by palpation posterior to
and underneath the edge of the gluteus medius, which is attached to the
trochanter (Figs. 15.8 and 15.9).
The tendon is separated from the underlying capsule by gentle spreading
the right angled clamp and by passing a soft tissue elevator between
the short rotators and the capsule surface (Fig. 15.10).
A tag suture is passed through the tendon approximately 1.0 cm from its
insertion and looped around the tendon to grasp it. The tendon is
divided with the Bovie near the insertion. As an alternative, and to
increase postoperative hip stability, the tendon can be retracted and
attempts made to preserve it throughout the procedure (36). The conjoined tendon of the obturator internus
and the gemelli muscles (see Fig. 15.9)
is identified in like fashion, tagged, and divided. Note that this
tendon is frequently on the undersurface of the musculature and must be
located by palpation rather than by direct vision. When these two
tendons are reflected, the sciatic nerve usually passes behind the
conjoined tendon and attached muscles, and is in front of the
piriformis tendon/muscle. However, there is substantial variation among
patients and one must be on the lookout for a split sciatic nerve on
either side of either tendon.
 |
|
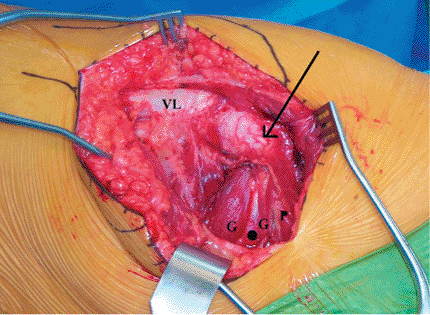
Figure 15.9.
The short external rotators of the hip. In this picture distal is toward the left and proximal is toward the right. The arrow indicates the tip of the trochanter with gluteus medius attaching. VL, vastus lateralis; G, gemelli (superior and inferior); O, obturator internus; P, piriformis. |
 |
|
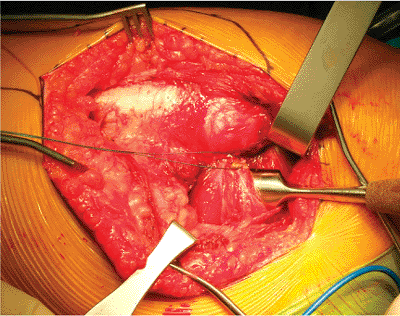
Figure 15.10. Defining the capsular plane with an elevator. Note the tag suture placed in the conjoined tendon of the external rotators.
|
 |
|
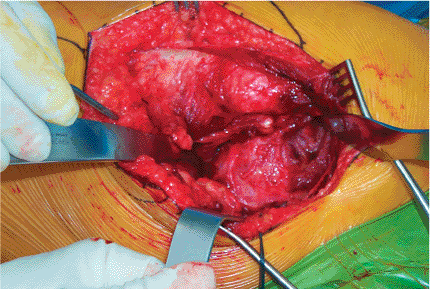
Figure 15.11. The capsule is exposed by retractors placed above and below it after the external rotator muscles are retracted.
|
 |
|
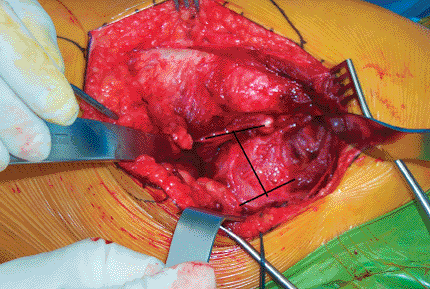
Figure 15.12. The H-shaped capsulotomy.
|
cleaned of attachments by gentle scraping with the soft-tissue
elevator. Homan retractors are used to define the capsular plane
superior and inferior (Fig. 15.11). Internal
rotation of the femur facilitates full exposure of the posterior
capsule. The capsule is opened by incising parallel to the neck of the
femur, and perpendicular at either end, to form a sort of H capsulotomy
(Fig. 15.12). The labrum is protected and
retained. Retraction of the capsular flaps reveals the femoral head and
neck, and the fracture is obvious. Homan retractors can then be placed
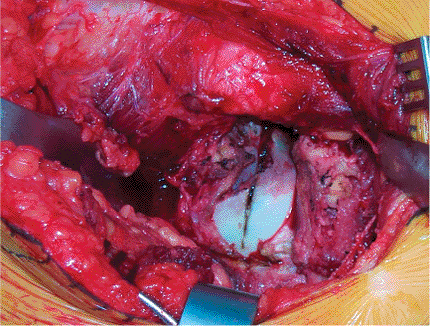
inside the capsule (Fig. 15.13). The labrum is not incised.
directions, of the femur and the head, exposing the broken surface of
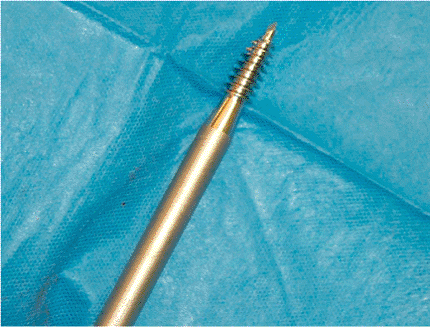
the head. A corkscrew tool (Fig. 15.14) is screwed into the cancellous bone of the head and used to gently lever it out of the acetabulum. The
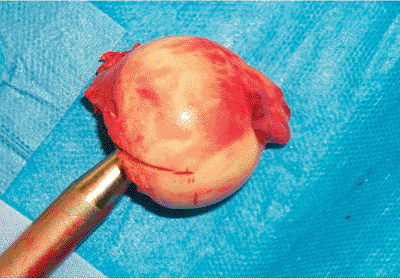
ligamentum teres is cut with curved mayo scissors, and the head is removed (Fig. 15.15).
In difficult cases, the head can be removed in pieces with a rongeur or
by gently cutting it with careful use of osteotomes. If the head can be
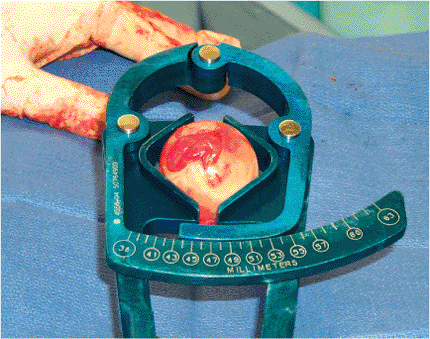
removed intact, the diameter can be measured for guidance in selecting
a prosthetic head (Fig. 15.16).
 |
|
Figure 15.13. Retraction of the capsular flaps reveals the femoral head. The head is marked by the Bovie used to cut the capsule.
|
 |
|
Figure 15.14. The corkscrew tool.
|
 |
|
Figure 15.15. The impaled femoral head is removed by levering the corkscrew tool and cutting the ligamentum teres with heavy scissors.
|
 |
|
Figure 15.16. Measuring the diameter of the removed femoral head to help select the appropriate prosthetic head size.
|
addressed. The hip is internally rotated to bring the neck up and out
of the socket (Fig. 15.17), and the neck
cutting guide is used to mark the angle of the cut approximately 1 to 2
cm (traditionally taught as one fingerbreadth) above the lesser
trochanter (Fig. 15.18). By using preoperative
planning templates, surgeons will have previously indicated the correct
level for the incision; if uncertain, he/she should use fluoroscopy and
a radio-opaque marker or should just cut a little less. Cutting the
neck too short is associated with residual pain and loosening of the
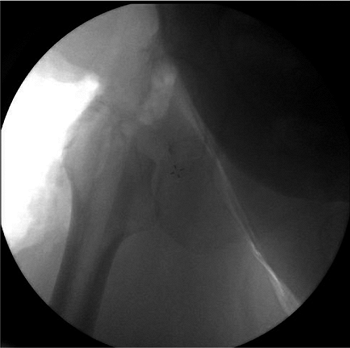
stem (27). Fluoroscopy can be used to verify the level of the neck cut (Fig. 15.19).
Care is taken to cut the neck in neutral or slight anteversion,
depending upon the design of the stem used. Frequently, posterior
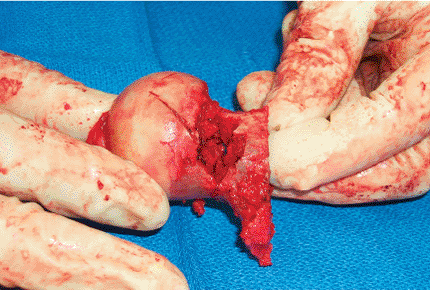
comminution of the neck fracture will be found (Fig. 15.20).
With the head and neck removed, the acetabular sizers can be used to
verify the correct diameter head for the prosthesis. The head should
sit securely within the acetabulum, with contact
all
around the surface and not just on the rim, and should rotate smoothly
without toggle. Prosthetic head size greater than 2 mm smaller than the
contralateral side is associated with pain and loosening (7,27). An oversized head has also been associated with poor postoperative results with regard to pain (7).
 |
|
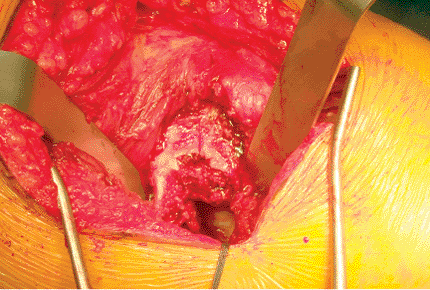
Figure 15.17. The broken surface of the femoral neck.
|
 |
|
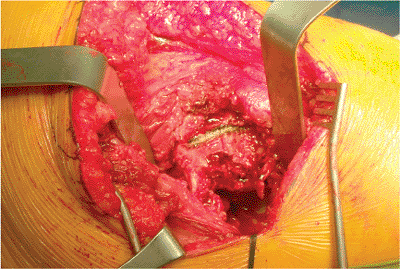
Figure 15.18.
The level and angle of the femoral neck cut is marked with the Bovie based on the use of an angle guide and palpation of the lesser trochanter. |
 |
|
Figure 15.19. Fluoroscopic image taken to confirm the level of the neck cut.
|
 |
|
Figure 15.20.
After the neck cut has been made, the two pieces are held together at the fracture site, showing comminution and bone loss in the neck posteriorly. |
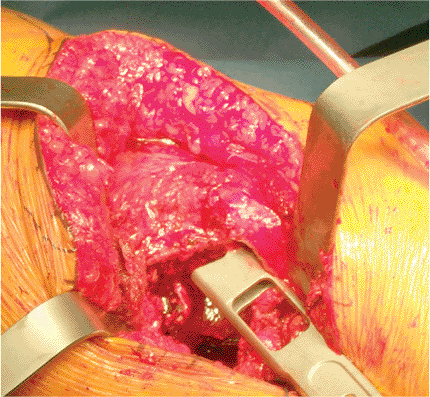
femur. A box osteotome or “cookie cutter” is used to open the
metaphysis, with the surgeon taking care to start the cut well lateral
to avoid varus alignment of the stem, and with awareness of the
rotation to ensure the correct 10 to 15 degrees of anteversion (Fig. 15.21). This can be confusing because of the
posterior position of the surgeon, and it is worth taking time to
ensure correct starting position. Internal rotation of the instrument
will lead to retroversion of the prosthesis, which may increase the
risk of postoperative dislocation. A canal seeker with blunt tip and
side cutting flutes may be used to begin a path down into the
intramedullary canal (Fig. 15.22).
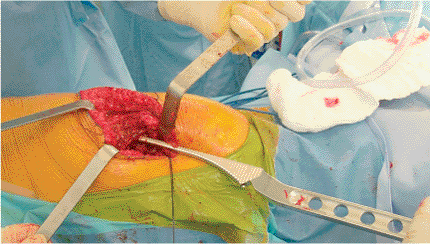
Depending upon the instrumentation for the brand of stem being
utilized, reamers and/or broaches are used to create a space for the
stem (Figs. 15.23 and 15.24).
The broach should be used gently to avoid exploding the metaphysis and
breaking the proximal femur. It is inserted with gentle taps of the
mallet, and after going in a bit, it should be extracted and then
reinserted deeper. The surgeon should pause when the fit is tight to
allow hoop stresses to relax through viscoelasticity. Patience is a
virtue here. The in and out motion will help to avoid incarceration.
The broach is not a file and should not be used as one. It is a device
for shaping the intramedullary canal. The surgeon should avoid varus
alignment. Attention must be paid to the soft tissues to avoid damage
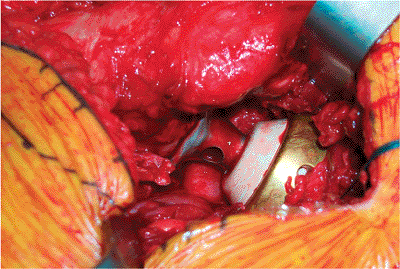
from the broach. A trial stem is inserted, and a trial head component
is attached. The hip is gently reduced by having the assistant apply
traction and slow external rotation, while the surgeon applies pressure
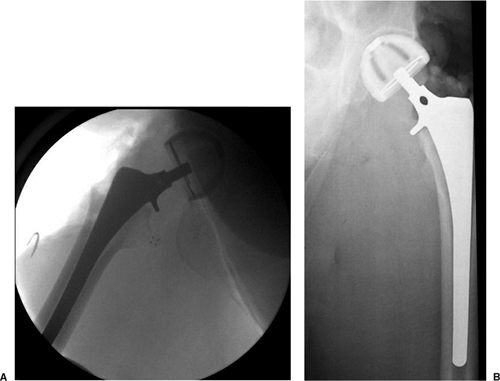
to the head and holds the capsular flaps out of the way (Fig. 15.25). A portable radiograph is taken (Fig. 15.26).
The hip is moved through a range of motion with the trial components in
place to assess stability. If acceptable, the hip is dislocated and the
trial components are removed.
 |
|
Figure 15.21.
Use of the box osteotome, or “cookie cutter,” to begin the entry into the femoral metaphysic. Internal rotation of the instrument with relation to the femur will place the prosthetic stem in retroversion; external rotation leads to anteversion. |
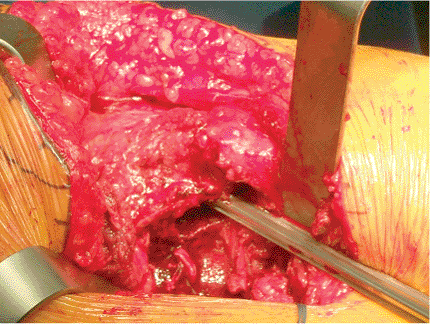 |
|
Figure 15.22. The canal seeker.
|
 |
|
Figure 15.23.
The broach prepares the shape of the intramedullary canal to accept the stem. Take care to avoid varus alignment by holding the handle laterally during insertion. |
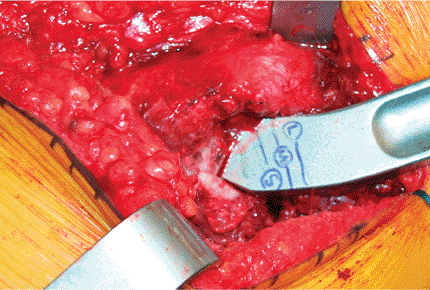 |
|
Figure 15.24. The broach may have marks that indicate the correct depth of insertion, and there may be different options for neck length.
|
 |
|
Figure 15.25. Trial reduction with trial components to assess stability and range of motion as well as obtain intraoperative radiographs.
|
plastic restrictor or a bone plug from the metaphysis at the correct
depth to allow for a few millimeters of cement distal to the tip of the
stem. The acetabulum is protected with sponges. The canal may be
irrigated with jet lavage, brushed to clean marrow contents, and dried
with sponges. Cement is injected from the distal to proximal with a
pressurized cement gun, and the stem is inserted in the correct
version. It is held still while the cement hardens. Excess cement is
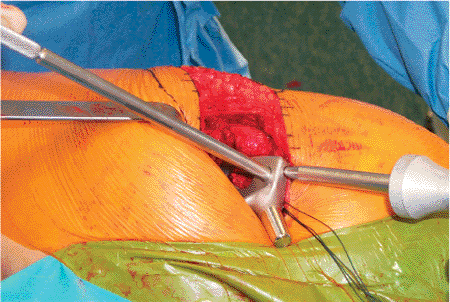
removed with curettes. If cement is not used, a corticocancellous plug,
approximately 2 mm wider than the femoral canal, may be made from the
head of the femur. This plug is inserted into the canal cortical side
distal and advanced with the broach just proximal to the anticipated
tip position for the prosthesis. The stem is inserted and advanced with
the mallet driving the plug distally (Fig. 15.27) (26).
Care should be taken to seat the collar of the prosthesis all the way
down onto the bone of the femoral neck osteotomy (calcar) because
failure to do so is associated with postoperative pain and loosening (27).
seated fully with gentle taps of the mallet. Reduction is performed
gently. Repeat radiographs can be done with the final components in
place (Fig. 15.28). Stability is checked by
placing the hip through a range of motion and gently stressing the
reduction. The hip should flex beyond 90 degrees, adduct beyond
neutral, and externally rotate 15 to 20 degrees without dislocating.
The short external rotators are reattached to their insertions with
nonabsorbable suture placed through bone. Usually, the heavy needle can
be driven through the bone of the trochanter
by
hand or with light taps of the mallet. The fascia lata is closed with
interrupted absorbable sutures and then the incision is closed in
layers. Sterile dressings are applied and a hip abduction pillow is
placed.
 |
|
Figure 15.26. Radiographic evaluation of the trial component fit. A.
A fluoroscopic image with the trial components in place. The stem fills the canal well, there is no varus alignment, and the collar rests on the calcar cut. The trial head sits well in the acetabulum. B. A portable radiograph taken in the operating room to demonstrate fit of the trial components. |
 |
|
Figure 15.27. A cementless insertion of press-fit stem. Care is taken to maintain the correct version during stem insertion.
|
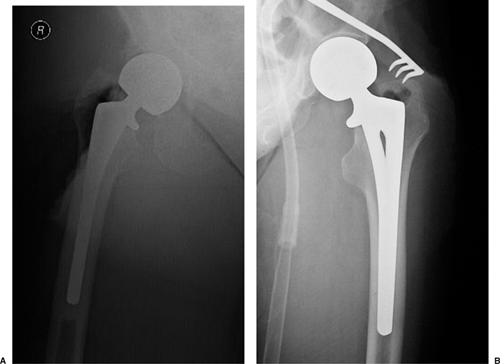 |
|
Figure 15.28. Intraoperative portable radiographs of the final prosthesis for a (A) cemented and (B) cementless prosthesis.
|
 |
|
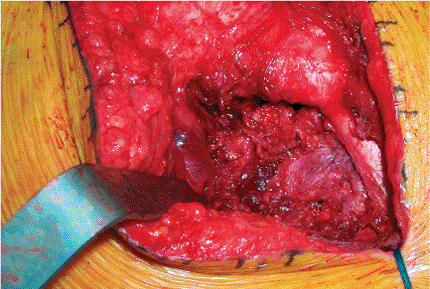
Figure 15.29. The capsule is closed over the components.
|
believed to be appropriate by many physicians, although there is
controversy regarding the method. Mechanical devices such as foot or
calf pumps may be used. Antibiotics are usually continued for 24 hours
after surgery and then stopped. The patient is mobilized from the bed
on postoperative day one and taught appropriate transfer techniques.
Patients may begin weight bearing as tolerated. The abduction pillow
and hip positioning precautions are enforced strictly for 6 weeks.
Transfer techniques and ambulation are taught. Physical therapists and
social workers can help assess the patient’s abilities and needs for
placement decisions. Limited evidence suggests that protein and energy
supplementation may reduce unfavorable outcomes (not including
mortality) in the recovery of hip fracture in elderly patient (37).
patients after cemented hemi-arthroplasty, and by 25% to 70% of
patients after uncemented hemi-arthoplasty (16,24,38). Analgesic use is reported by 26% to 35% of patients (38,39). There does not seem to be any difference in postoperative among those with unipolar and bipolar prostheses (22).
If the size of the prosthetic head is larger or smaller than 2 mm of
the natural head, increased postoperative pain may result (7,27).
endoprosthetic replacement, 83% of those with cemented
hemi-arthroplasties had Harris scores of Good and Excellent, while 50%
of uncemented stems received a good and excellent score (16). A retrospective study of 53 independently mobile patients treated with cemented bipolar prostheses showed a strong
correlation of modified Harris hip score with prefracture levels of
mobility and independence. Seventy-three percent of fully independent
patients who could walk a mile before their fracture had Good and
Excellent hip scores at follow-up, while of those who needed help
shopping or could walk less than 100 yards, 0% to 9% had a good or
excellent outcome (14).
The percentage of patients who achieve unassisted community ambulation
(able to get around outside the home) has been reported as 11% to 30% (7,39,40,41).
One study found that 30% of postsurgery patients with cemented
prostheses needed a higher level of walking aid than before injury; 60%
of patients with uncemented prostheses needed similar assistance (24).
Use of a cane or walking stick was required in 17% to 62% of patients
with uncemented prosthesis and 40% to 50% of patients with cemented
prosthesis; walker or crutch use was required for 15% to 35% and 27% to
40% of patients with cemented and uncemented prostheses, respectively (7,38,39).
The percentages of persons who had ability to ambulate outside the home
dropped from 92% before the injury to 70% to 75% after the operation (21,22).
At an average follow-up of 10 years, only 31% of the patients in one
study retained the ability to ambulate in the community (40). Seventy-five percent of patients with pre-injury senile dementia lost the ability to ambulate at all (42).
However, 11 out of 28 (39%) patients who had been independent in all
activities of daily living (ADLs) and could walk more than 1 mile
before their injury had a strong chance of regaining the ability to
walk a mile after cemented bipolar endoprosthesis (14).
A living situation with a lower level of independence is the result for
11% to 40% of patients with a cemented prosthesis, and is the case for
18% to 56% with an uncemented prosthesis (24,25).
One study found that the percentage of patients who were independent in
basic activities of daily living (dressing, bathing, feeding) dropped
from 80% to 90% pre-injury to 60% postoperatively, and that those
independent in instrumental activities of daily living (shopping,
housework, laundry) dropped from 40% to 60% to approximately 20% (22).
common, occurring in up to 50% of cemented stems and found over 90% in
some series of uncemented stems (16).
Acetabular erosion is also seen, particularly in active patients. The
rate of protrusio acetabuli is 5% to 26% after 5 years (9). The proposed difference between bipolar and unipolar prostheses has not been convincingly demonstrated.
cartilage has been seen on histological examination of biopsy specimens
from patients undergoing revision due to groin pain. The loss of
cartilage correlated with duration of articulation with the prosthesis (43).
or irreducible dislocation is the most common reason for revision.
After 2 years, pain associated with loosening, subsidence, or
erosion is more common. The rate of revision after long-term follow-up varies from 3% to 24% (5,7,9,40,44) with an average of approximately 11% for all types considered. Gebhard et al (7)
found that cemented endoprostheses were revised nearly 8% of the time,
while uncemented prostheses had a 13% revision rate; approximately 2%
of those with total hip arthroplasty also needed revision. With modular
components, the revision from either bipolar or unipolar endoprosthesis
to total hip arthroplasty can be accomplished without removal of the
stem.
after hemi-arthroplasty for femoral neck fracture has been reported
from 5% to 23% (5,38). As reported in most studies, patient mortality at 1 year is between 17% and 34% in most studies (4,5,19,20,23,44,45) but has been reported as higher (5,38) and lower (22). One prospective study of octogenarians found that 30% of postoperative patients had 1-year mortality (19).
At 4 months postoperation, mortality is significantly higher in
patients with dementia than in those without it (33% vs. 12%) and at 1
year the difference is 44% versus 20% (42). Due
to the elderly nature of the patient population, mortality increases
over time. In one study, only 6% of female patients who were over 70
years old at the time of surgery survived 6 years after it (44).
As one would expect from the patient population, medical complications
are common in the postoperative period, including urinary and
respiratory tract infections, DVT and pulmonary embolism, cardiac
failure, arrhythmia or infarction, stroke, gastrointestinal bleeding,
and renal failure. A prospective outcome study of 270 patients revealed
a 16% rate of major medical complication and a 35% rate of minor
medical complication (23).
approximately 5% to 10% of patients; the numbers vary according to the
research question asked. Intra-operative fracture of the femur may
occur from vigorous broaching or forceful reduction and may necessitate
a change to a longer stem and concomitant internal fixation.
Dislocation rates have been reported from 1% to 9% (4,7,20,36,44,46).
However, no differences have been found in dislocation rate between
bipolar and unipolar prostheses. One study has suggested that
dislocation of a bipolar more frequently requires open reduction, but
the numbers from the study are too small for significance to be
calculated (20). Bipolar dislocations do not
necessarily are not always address through open reduction. THR for
fracture may have a higher dislocation rate than hemi-arthroplasty (4,5,11), but this is not a universal finding (7,9). Dislocation after hemi-arthroplasty is a significant event, and according to a retrospective study of 1,000 patients (47),
is associated with a mortality of 65% within 6 months. Dislocation is
more common after posterior than anterior or anterolateral surgical
approaches (7,36), but preservation of the capsule and labrum improves stability and lowers the dislocation rate (36). Incorrect sizing of the prosthetic head has been implicated as a predisposing factor to dislocation (7).
In most series, wound hematoma occurs in 3 to 4% and infection is seen
in approximately 2% of patients. Deep infection, which has been
reported to occur in 0 to 18% of patients (5), requires further surgery and often results in Girdlestone hip resection.
JN, Calder SJ, Anderson GH, et al. Treatment for displaced
intracapsular fractures of the proximal femur: a prospective randomized
trial in patients aged 65 to 79 years. J Bone Joint Surg 2001;83(21): 206–212.
EM, Sahni V, Acharya A, et al. Management of intracapsular femoral neck
fractures in the elderly; is it time to rethink our strategy? Injury 2004;35:125–129.
M, Parker MJ, Fleischer S. Internal fixation versus arthroplasty for
intracapsular proximal femoral fractures in adults (Cochrane Review).
In: The Cochrane Library. Issue 3. Chichester, UK: John Wiley & Sons, Ltd; 2005.
M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared
with arthroplasty for displaced fractures of the femoral neck: a
meta-analysis. J Bone Joint Surg Am 2003;85(9):1673–1681.
JS, Amstutz HC, Zinar DM, et al. A comparison of total hip arthroplasty
and hemi-arthroplasty for treatment of acute fracture of the femoral
neck. Clin Orthop 1992;282:123–131.
RJ, Gibson MJ, Moran CG. Dislocation after primary arthroplasty for
subcapital fracture of the hip: wide range of movement is a risk
factor. J Bone Joint Surg Br 1991;73:11–12.
LD, Glousman R, Hoy AL, et al. Treatment of femoral neck fractures with
total hip replacement versus cemented and noncemented hemiarthroplasty.
J Arthroplasty 1986;1:21–28.
P, Riley D, Ellery J, et al. Displaced subcapital fractures of the
femur: a prospective randomized comparison of internal fixation,
hemiarthroplasty and total hip replacement. Injury 1989;20(5):291–293.
S, Bannister G. Cemented bipolar hemiarthroplasty for displaced
intracapsular fracture in the mobile active elderly patient. Injury 2004;35:152–156.
WH, Chen WM, Huang CK, et al. Bateman bipolar hemiarthroplasty for
displaced intracapsular femoral neck fractures: cemented versus
uncemented. Clin Orthop 1994;302:75–82.
SJ, Anderson GH, Jagger C, et al. Unipolar or bipolar prosthesis for
displaced intracapsular hip fracture in octogenarians: a randomized
prospective study. J Bone Joint Surg Br 1996;78(3):391–394.
MJ, Gurusamy K. Arthroplasties (with and without bone cement) for
proximal femoral fractures in adults (Cochrane Review). In: The Cochrane Library. Issue 2. Chichester, UK: John Wiley & Sons, Ltd; 2004.
RA, Koval KJ, Aharenoff GB, et al. Modular unipolar vs. bipolar
prosthesis: a prospective evaluation of functional outcomes after
femoral neck fracture. J Ortho Trauma 1995;9(4):298–302.
RJH, Broughton NS, Desai K, et al. Bipolar hemiarthroplasty for
subcapital fracture of the femoral neck: a prospective randomized trial
of cemented Thompson and uncemented Moore stems. J Bone Joint Surg Br 1991;73(2):322–324.
RJK, MacDowell A, Crossman P, et al. Cemented or uncemented
hemiarthroplasty for displaced intracapsular fractures of the hip: a
systematic review. Injury 2002;33:13–17.
KM, Parker MJ. Austin Moore hemiarthroplasty: technical aspects and
their effects on outcome, in patients with fractures of the neck of
femur. Injury June 2002;33(5):419–422.
TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general
anesthesia for hip fracture repair: a longitudinal observation of 741
elderly patients during 2-year follow-up. Am J Orthop 2000;29:25–35.
KJ, Aharonoff GB, Rosenberg AD, et al. Functional outcome after hip
fracture: effect of general versus regional anesthesia. Clin Orthop 1998;348:37–41.
RM, Pellicci PM, Lyden JP. Bipolar hemiarthroplasty for fracture of the
femoral neck: Clinical review with special emphasis on prosthetic
motion. J Bone Joint Surg Am 1988;70:1001–1010.
BM, Bogoch ER. Posterior versus lateral surgical approach for total hip
arthroplasty in adults with osteoarthritis (Cochrane Review). In: The Cochrane Library. Issue 3. Chichester, UK: John Wiley & Sons, Ltd; 2004.
J, Jalovaara P. Functional comparison between uncemented Austin-Moore
hemiarthroplasty and osteosynthesis with three screws in displaced
femoral neck fractures: a matched pair study of 168 patients. Int Orthop 2004;28:28–31.
T, Wingstrand H, Partanen J, et al. Hemiarthroplasty or osteosynthesis
in cervical hip fractures: matched pair analysis in 892 patients. Arch Orthop Trauma Surg 2003;122(3):143–147.
Dortmont LMC, Douw CM, van Breukelen AMA, et al. Outcome after
hemi-arthroplasty for displaced intracapsular femoral neck fracture
related to mental state. Injury 2000;31:327–331.
SW, Jakob RP, Gautier E. Ten-year patient and prosthesis survival after
unipolar hip hemiarthroplasty in female patients over 70 years old. J Arthroplasty 2003;18(5):587–591.
GL, Keller RB, Littenberg B, et al. Outcomes after displaced fractures
of the femoral neck: a meta-analysis of one hundred and six published
reports. J Bone Joint Surg Am 1994;76(1):15–25.
