Female Athletes
female participation in athletics. This can be partly attributed to the
passage of Title IX in 1972, which prohibited sex discrimination in
sports. In 1971 to 1972, there were 204,015 female high school athletic
participants, compared with 2,675,874 in the 1999 to 2000 school years.
At the collegiate level, there was also a dramatic increase in
participation in sports by women. Excluding football, in 1999 to 2000,
there were 146,618 female and 150,888 male NCAA athletes. The impact of
Title IX has even had an influence at the Olympic level. Field hockey
was added in 1980, and 13 new events were added in 1984. In the 2002
Olympic Games, more women than ever competed, and eight new women’s
events were added, including women’s bobsled, skeleton, and
cross-country ski sprints.
over the past 30 years, sports medicine research focusing on female
athletes is still in its early stages. The unique anatomic,
physiologic, and biomechanical makeup of females deserves separate
attention. Future research needs to focus on injury patterns,
prevention programs, and treatment modalities. Recently, the American
Orthopaedic Society for Sports Medicine published a consensus statement
on female athletic issues for team physicians and other health care
providers. The statement provided an overview of select musculoskeletal
and medical issues important to female athletes.
physiologic differences between the sexes, the female athlete triad,
common stress fractures, anterior cruciate ligament (ACL) injuries, and
exercise in pregnancy.
patterns are seen in both male and female athletes, there are many
anatomic and physiologic variations that explain the differences in
mechanics, performance, and injury rates.
-
In general, females are shorter in stature and have lower body mass.
-
Skeletal differences include shorter
femurs (lower relative leg length with respect to total body height),
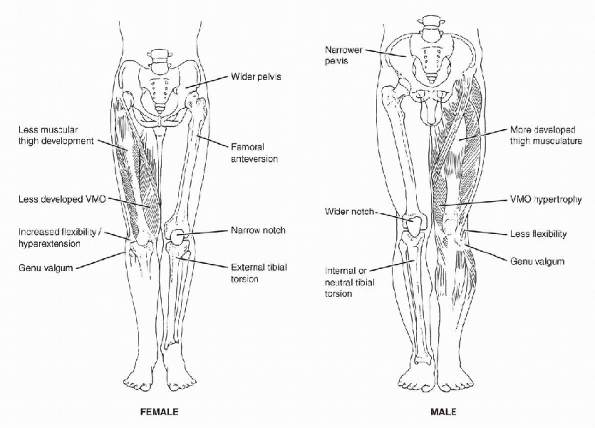
narrower shoulders, a wider pelvis, and larger knee valgus angles. -
Having shorter femurs lowers the center of gravity and improves balance, an advantage in sports such as gymnastics.
-
Narrower shoulders and shorter humeri alter throwing mechanics.
-
A wider pelvis and greater knee valgus
increases the Q-angle (the angle between a line drawn from the anterior
superior iliac spine to the center of the patella and the line from the
center of the patella to the tibial tubercle), predisposing females to
patellofemoral problems.
P.52The Q-angle averages 10 (±5°) degrees in men, compared with 15 (±5°) degrees in women. (See Figure 4-1 for lower extremity alignment differences.)
-
Females reach skeletal maturity earlier than their male counterparts at the average age of 17 to 19 years versus 21 to 22 years.
-
Females can have lower bone density, which may predispose them to fractures.
-
The female body composition tends to be composed of relatively more fat and less muscle mass than equally trained males.
 |
|
Figure 4-1 Lower-extremity alignment differences in females and males that may predispose females to increased risk of injury.
|
stored in and nourishes the body’s organs, is 9% to 12% in females and
approximately 3% in males (the significant difference is largely the
result of the fat in breasts and other gender-specific organs). In the
average nonathlete, normal body fat in females is 18% to 24% and is 12%
to 16% in men. Endurance athletes should maintain body fat of 12% to
18%, but this often drops dangerously to 6% to 8% in elite athletes.
However, dropping below a safe level of body fat directly affects the
ability to menstruate, an important component of the female triad.
Greater body fat leaves females more buoyant, a potential advantage in
water sports.
males are stronger, run faster, and jump higher than equally trained
females. In general, when adjusted for body mass, female strength is
approximately two thirds that of males, with their upper body strength
even less matched than lower body strength compared to males. Women
also tend to have greater ligamentous laxity that may contribute to
increased injury patterns, such as multidirectional instability of the
shoulder, patellofemoral dislocations, and ankle instability.
capacity that are important to consider when implementing training
regimens and in achieving realistic athletic goals. The major
physiologic factors that contribute to aerobic differences in females
include smaller body size, greater body fat, lower muscle mass, and
reduced oxygen-carrying capacity. The VO2max measures the
body’s ability to extract oxygen from the air and deliver it via the
blood to muscle tissue. It is essentially a measure of aerobic
capacity. The average woman has a 15% to 30% lower VO2max because of several factors:
-
Women have lower vital capacity, smaller tidal volumes, and faster respiratory rates due to a smaller thoracic cage.
-
Men have 6% higher hematocrit and 10% to
15% higher hemoglobin concentration, which results in a greater
oxygen-carrying capacity of the blood.
10% lower than males, which affects the ability to lose weight with
training. Because of the aforementioned physiologic differences,
cardiovascular training programs and performance goals for females
should be individualized on the basis of previous athletic experience
and gender.
well as different stages of physiologic maturity, can have an impact on
athletic performance, body composition, bone density, and injuries.
Cyclic endogenous hormones, such as estrogen and progesterone, affect
many physiologic systems in the body such as metabolic,
thermoregulatory, cardiovascular, respiratory, and psychological.
Dysregulation can certainly affect performance and may have an effect
on injury. Several studies have looked at the effect of the menstrual
cycle on performance and injury but the results are varied and mainly
inconclusive. Although there is no definite evidence that a specific
phase of the menstrual cycle increases risk of injury or decreases
performance, one relatively consistent finding is that premenstrual
symptoms can decrease performance and consequently increase risk of
injury. Exogenous hormones, including oral contraceptives, in addition
to decreasing iron deficiency anemia and protecting bone health, are
known to alleviate dysmenorrhea, decrease premenstrual symptoms, and
regulate menses—all of which can be beneficial to the athlete. A few
studies have shown a lower incidence of musculoskeletal injuries with
oral contraceptive use, likely secondary to the alleviation of
premenstrual symptoms and dysmenorrhea.
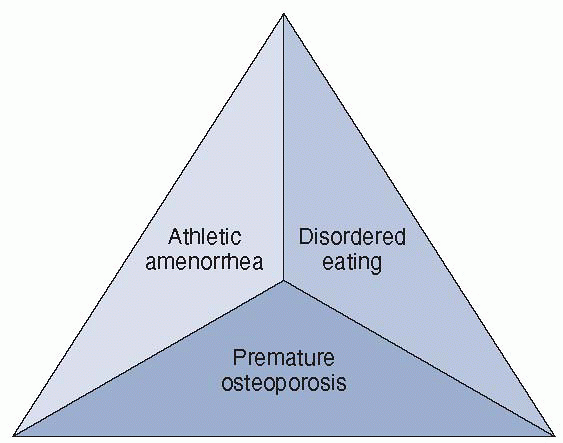
was defined at the Triad Consensus Conference in 1992, led by members
of the American College of Sports Medicine. Each component of the triad
occurs on a spectrum of severity and is often interrelated, which can
lead to serious long-term health consequences. However, not all
components of the triad need to occur simultaneously. Diagnosis of one
component of the triad should alert health care providers to be
suspicious about the other components.
 |
|
Figure 4-2 The female athlete triad.
|
low body weight and body fat both to enhance performance and/or for
appearance. In their quest to maintain low body weight, they may
succumb to disordered eating, which can cause an imbalance of energy
intake versus energy expenditure. Energy depletion can lead to a
dysregulation of the hypothalamic-pituitary-ovarian (HPO) axis,
resulting in hypoestrogenism or athletic amenorrhea. Subsequently, a
lack of exposure to the hormone estrogen can cause premature bone loss
or osteopenia/osteoporosis. Thus, knowledge of the three components and
how they relate is imperative when treating female athletes.
There are three groups of sports that have increased risk for
developing the triad:
-
Sports in which subjective judging is involved—gymnastics, diving, figure skating, and dance
-
Endurance sports—long distance running and swimming
-
Sports with weight classifications—rowing, body building, and martial arts
classic example of being high risk for developing the triad, it can
occur in any physically active female.
range from simple restriction of food intake, to occasional binging and
purging to laxative and/or diuretic abuse, or to frank anorexia nervosa
and/or bulimia nervosa. The majority of females affected with the triad
do not fit the Diagnostic and Statistical Manual of Mental Diseases,
4th edition (DSM IV) criteria of anorexia or bulimia, but may fit into
a more broad diagnosis of eating disorder not otherwise specified
(EDNOS) (Box 4-1). Studies lack consistent and
valid diagnostic instruments for the assessment of disordered eating
and thus prevalence studies may not be accurate. Up to 62% of female
college athletes have some degree of pathologic weight control
behavior. There are several instruments, such as the Eating Attitudes
Tests and Eating Disorders Inventory, that attempt to document the
existence and/or risk of eating disorders, but most instruments are
blatant in what they are searching for and athletes may hide their
signs and symptoms, thus skewing the results.
multifactorial and often stem from low self-esteem. The athletes often
have body image disturbances. The pressures of adolescence, puberty,
and competition may lead these athletes to find comfort in a false
sense of control with their eating and exercise patterns. Biologic,
psychologic, and social factors all contribute to predisposing risk. A
history of family dysfunction, physical or sexual abuse, perfectionism,
and/or long-term chronic dieting are often found in females who display
signs of eating disorders. Traumatic events—such
as
loss of a family member, friend, or coach—change in competitive
environment (i.e., high school to college and college to professional
world transitions), and acute or chronic injuries can often escalate
the severity of their disordered eating and lead to greater health
consequences. A list of signs and symptoms of disordered eating is
given in Box 4-2, and complications are given in Box 4-3.
Disordered eating can severely affect athletic and academic
performances. The negative energy balance created can decrease
endurance, strength, speed, reaction time, and concentration—all of
which increase the risk of sports-related injuries. Prolonged
insufficient caloric consumption can lead to significant medical and
psychological consequences such as depression and cardiovascular,
endocrine, thermoregulatory, and gastrointestinal complications.
-
Refusal to maintain body weight at or above 85% of normal weight for age and height.
-
Intense fear of gaining weight or becoming fat, even though underweight.
-
Disturbance in the way in which one’s
body weight or shape is experienced, undue influence of body weight or
shape on self-evaluation, or denial of the seriousness of the current
low body weight. -
Amenorrhea—the absence of at least three consecutive menstrual cycles.
-
Recurrent episodes of binge eating:
-
Eating in a discrete period of time an
amount of food that is definitely larger than most people would eat
during a similar period of time. -
A sense of lack of control over eating during the episode.
-
-
Recurrent inappropriate compensatory
behavior to prevent weight gain (i.e., diuretics, enemas, self-induced
vomiting, misuse of laxatives or other medications, fasting, excessive
exercise). -
Binge eating and inappropriate compensatory behaviors occur, on average, at least twice a week for three months.
-
Self-evaluation is unduly influenced by body shape and weight.
-
The disturbance does not occur exclusively during episodes of anorexia nervosa.
-
For females, all of the criteria for anorexia are met except that the individual has regular menses.
-
All the criteria for anorexia are met
except that, despite significant weight loss, the individual’s current
weight is in the normal range. -
All the criteria for bulimia are met
except the binge eating, and inappropriate compensatory mechanisms
occur at a frequency of less than twice a week or for a duration of
less than 3 months. -
The regular use of inappropriate
compensatory behavior by an individual of normal body weight after
eating small amounts of food (i.e., self-induced vomiting after the
consumption of two cookies). -
Repeatedly chewing and spitting out, but not swallowing, large amounts of food.
-
Binge-eating disorder: recurrent episodes
of binge eating in the absence of the regular use of inappropriate
compensatory behaviors characteristic of bulimia nervosa.
balance. Restricting calories, along with intense training schedules,
leads to a severe energy deficit that affects the HPO axis. This
affects the menstrual cycle and can lead to the second component of the
triad, athletic amenorrhea.
is 2% to 5%, but several studies have shown the prevalence in female
athletes may be as high as 66%. The combined effect of poor nutrition
and intense training regimens, leading to significant caloric deficits,
disrupts the reproduction function by suppressing the HPO axis. This is
currently the leading hypothesis in the mechanism of athletic
amenorrhea. There is a decrease in the pulse frequency of
gonadotropin-releasing hormone from the hypothalamus, which leads to
dysfunction in the secretion of luteinizing hormone and
follicle-stimulating hormone from the pituitary gland. Luteinizing
hormone suppression leads to ovarian suppression and anovulation,
resulting in amenorrhea.
has a continuum of disturbances. The dysfunctions range from
luteal-phase deficiency to anovulation, oligomenorrhea, and
hypoestrogenemic amenorrhea. There are two forms of amenorrhea: primary
and secondary. Primary amenorrhea is when menarche has not occurred by age 16. In this form, the reproductive axis has not yet coordinated to produce a
menstrual period. The main contributing factor to primary amenorrhea is
the age at which intense training is begun. The earlier the athlete
begins intense training, the greater the risk of primary amenorrhea. Secondary amenorrhea
is when menses is halted for three or more consecutive months after the
reproductive axis has previously produced at least one menstrual period.
-
Dry hair
-
Dull pale eyes
-
Dry, flaky skin
-
Lanugo
-
Lack of subcutaneous fat
-
Glossitis
-
Cheilosis
-
Gum disease
-
Brittle nails
-
Swollen parotid glands
-
Sore throat
-
Social withdrawal
-
Depression
-
Secretive behavior
-
Preoccupation with food or eating
-
Psychosomatic complaints
-
Low self-esteem
-
Anxiety
-
Body dissatisfaction
-
Hypotension
-
Bradycardia
-
Arrhythmia
-
Esophagitis, hematemesis
-
Diarrhea, constipation
-
Abnormal liver enzymes
-
Hypokalemia
-
Hyponatremia
-
Hypoglycemia
-
Hypothermia
-
Hypercortisolism
-
Lipid abnormalities
-
Renal calculi
-
Infertility
-
Low-birth-weight infants
-
Peripheral neuropathy
-
Anemia
-
Leukopenia
-
Neutropenia
-
Thrombocytopenia
-
Amenorrhea
-
Osteoporosis
-
The primary treatment of amenorrhea is to treat the energy deficit that is likely contributing to the menstrual dysfunction.
-
The goal is to optimize nutritional status and alter training intensity to maintain a positive energy balance.
-
Observation with the trainer and nutritionist for 3 to 6 months is typical before pharmacologic agents should be initiated.
-
Estrogen replacement therapy and oral
contraceptive pills can restore menses, but low bone mineral density, a
major consequence of hypoestrogenic amenorrhea, may not be restored. -
The Committee on Sports Medicine of the
American Academy of Pediatrics (AAP) recommends that amenorrheic
females under the age of 16 decrease their exercise intensity and
increase their dietary calcium and protein. It is not recommended that
they start hormone replacement therapy. The AAP does, however,
recommend women over the age of 16 with hypothalamic amenorrhea and
hypoestrogenism be started on a low-dose oral contraception.
significant potential precursors to early osteoporosis or osteopenia.
Osteoporosis or osteopenia is the third component of the female athlete
triad. Osteoporosis is defined as bone mineral density (BMD) measured
by a dual-energy x-ray absorptiometry scan (DEXA), greater than 2.5
standard deviations below that of a young, healthy, Caucasian, adult
female (or a T score at or below -2.5). Osteopenia is defined as 1 to
2.5 standard deviations below a normal adult (or a T score of – 1 to –
2.5). Alteration of bone homeostasis (bone formation and bone
resorption) leads to decreased bone mineralization and low bone
density. Estrogen plays a significant role in bone homeostasis.
Estrogen receptors are found in osteoblasts and osteocytes and slow the
resorption of bone by decreasing osteoclastic resorption. Estrogen also
alters the renal handling of and gastrointestinal absorption of
calcium, which is critical for osteoblastic function and bone building.
Young athletes with primary or secondary amenorrhea lack the estrogen
necessary to achieve peak bone mineral density.
end of adolescence. Typically, a young, adolescent, eumenorrheic female
with good nutrition will gain 2% to 4% bone mass a year. A chronically
estrogen-depleted state like athletic amenorrhea can cause a 2% loss of
bone mass a year. Young athletes with intense training patterns and a
negative caloric balance are at increased risk of skeletal fragility.
This is of concern, not only for fractures and stress fractures during
their current sports and training, but leaves them at tremendous risk
for hip, wrist, and spine fractures later in life.
between reproductive function and bone density. Most of these studies
have shown significant increased risk of fracture in those athletes
with menstrual dysfunction. In fact, one study by Barrow et al. found
that almost half of the college female long-distance runners with
irregular menses had at one point reported a history of stress fracture.
resorption in adolescence, it does not reverse the damage that has
already been done. The treatment of amenorrhea with estrogen
replacement therapies may restore bone homeostasis at that point in
time but does not make up for the prior imbalance; thus, these young
athletes will never reach their potential peak BMD. Exogenous estrogen
only normalizes the rate of resorption; it does not have a direct
effect on bone formation.
osteopenia/osteoporosis in female athletes is not well studied.
Bisphosphonates such as alendronate, used in postmenopausal women, are
not recommended for premenopausal women of childbearing age as a result
of their teratogenic effects. The selective estrogen reception
modulator class of agents, such as raloxifene and tamoxifen, are also
indicated for postmenopausal women but are not approved for
premenopausal women. There is currently no pharmacologic treatment for
osteopenia or osteoporosis that is approved by the U.S. Food and Drug
Administration for premenopausal women. Treatment of the athlete with
signs of osteopenia or osteoporosis consists of restoring menses,
improving nutrition, having an intake of 1,500 mg of calcium/day and
performing weight-bearing exercises.
female athletes. The three components are interrelated, and the
presence of one of the components should raise suspicion for the others.
-
Recognition and referral comprise the critical first step in the treatment of these athletes.
-
Ultimately, effective treatment requires
the communication of a multidisciplinary health care team, including
physicians, athletic trainers, coaches, nutritionists, and
psychologists. -
Prevention is a key objective when facing
the female athlete triad. All female athletes, especially those who
present with stress fractures and/or menstrual irregularities, should
be screened for the triad, and preparticipation histories should
include a careful and thorough menstrual and nutritional history. -
Treatment goals involve correcting the
energy deficit, restoring normal menstrual function, increasing dietary
calcium, maintaining and restoring bone mass density, and educating the
athlete on proper nutrition and its effects on performance and health.
architecture of bone. These fractures occur as a result of the
inability of bone to sufficiently remodel after exposure to repetitive
overload. The diagnosis of a stress fracture can often be made on the
basis of history and physical examination. Patients will often report a
recent increase in training regimen (either intensity or duration) over
a short period of time. Running is the most common activity that leads
to stress fractures, but any sport that requires repetitive impact
loading can lead to stress fractures.
-
As part of the history, it is essential
for the physician or treating medical personnel to obtain a detailed
nutritional and menstrual history. -
Physical examination findings can include an antalgic gait, tenderness, and mild swelling over the affected area.
-
If obtained early, initial radiographs
(within 2 to 3 weeks) may not show evidence of callus formation, and
rarely is an actual fracture line seen. -
Within the first 48 to 72 hours, a
triple-phase technetium bone scan will show focal uptake at the
particular site with 100% sensitivity.-
The triple phase bone scan can also help
distinguish the age of the fracture because the angiogram (phase I) and
blood pool images (phase II) normalize over time.
-
-
To help distinguish between stress injuries to bone, infections, and bone tumors, an MRI can be a useful adjunct.
-
After a few weeks, plain radiographs often show evidence of callus formation (Fig. 4-3).
-
A DEXA scan to determine bone mineral density may be warranted for patients who present with multiple stress fractures.
stress fractures are more common in female athletes and military
recruits when compared with their male counterparts. In a
recent
study of college athletes, stress fractures were significantly more
common in women. The fractures occurred most commonly in track and
cross-country athletes. Soccer was the only sport where the incidence
of stress fractures was higher in males. In women, the foot was the
most common anatomic region to be involved, but the tibia and the
femur, respectively, were the most common bones to be involved. In men,
the ankle was the most predominant anatomic site, followed by the foot
and the tibia.
 |
|
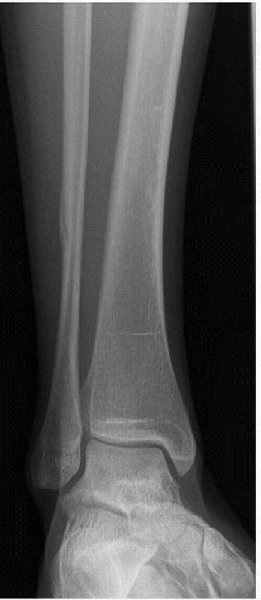
Figure 4-3
A 16-year-old female cross-country athlete presented with 4 weeks of lateral ankle pain while running. Radiograph showed a healing distal fibula stress fracture. |
the female athlete have been studied, such as age, gender, skeletal
alignment, low bone density, hormonal factors, training parameters, and
footwear. Risk factors for stress factors in female track-and-field
athletes include significantly older age at menarche, a history of
irregular menses, and restrictive eating patterns and dieting—all
factors that reduce bone density. A high longitudinal arch, leg length
discrepancy, and excessive forefoot varus have all been associated with
increased risk of recurrent stress fractures, with the tibia being the
most common location. There is an increased risk of pubic ramus
fractures in integrated military training, presumably because of
increased stride length set by males during marching.
-
The treatment of stress fractures should
not only focus on the fracture, but also identify predisposing risk
factors for which intervention may be warranted. -
A multidisciplinary approach to treatment is often necessary.
-
For example, those who report disordered eating habits should be referred to a nutritionist and possibly a sports psychologist.
-
Female athletes should be educated about
the inherent risks of irregular menstrual periods. Those with irregular
menses should be referred to an obstetrician/gynecologist. In several
studies, the use of oral contraceptive medication to help normalize
menstrual irregularities seems to show a protective effect against
future stress fractures. Biomechanical factors should also be addressed
when appropriate.
-
-
Treating the fracture requires a period of relative rest.
-
The goal is to heal the stress fractures without allowing the athlete to become deconditioned.
-
Treatment entails avoiding the offending
activity and switching to nonimpact activity, such as swimming,
low-resistance cycling, or elliptical training. -
The rate of activity progression should
be determined by the athlete’s symptoms. If pain occurs during an
activity, stop for a few days and then gradually resume activity.
-
-
Certain stress factors are considered to
be “high-risk fractures” and warrant greater attention because of their
high incidence of delayed union or complete fracture.-
Bones commonly involved are the femoral
neck, patella, anterior tibial cortex, medial malleolus, talus, tarsal
navicular, fifth metatarsal, and great toe sesamoids. -
The femoral neck stress fracture has been shown to be four times as common in female runners as in male runners.
-
-
If the fracture is on the tensile side
(superior side of the femoral neck), pinning in situ is recommended to
avoid the devastating complication of a displaced femoral neck
fracture. The potential complications include avascular necrosis, varus
deformity, delayed union, and decreased return to play. -
If the fracture is on the compression
side of the femoral neck, immediate discontinuation of the offending
activity and either non-weight-bearing or partial weight-bearing should
be instituted. Once pain free, a gradual return to activity should
begin. If pain occurs at any point during return to activity,
progression should be halted.-
Fractures present in the proximal or
distal one third of the tibia are usually on the compression or
posteromedial side of the bone, and healing is generally not
problematic. Casts or braces are rarely necessary unless pain persists
but a quicker return to play may be possible if a brace is used. -
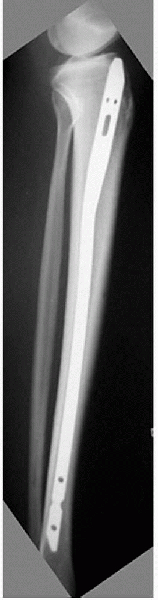
Another “at-risk” fracture is on the
anterior cortex of the tibia, described radiographically as the
“dreaded black line.” Constant tension from posterior muscle forces and
the relative hypovascularity of this area predispose the site to
nonunion or delayed union. Fractures at this anatomic site are common
in athletes who leap or jump. Because of this fracture’s unpredictable
healing pattern and prolonged treatment time (average 12.5 months),
intramedullary nailing has been advocated for the high-level athlete
for a quicker return to sport (Fig. 4-4).
-
-
Focus on preventive measures and
recognition of stress injuries by athletes, coaches, and medical
personnel should help decrease the incidence and improve treatment.
common than in males. The mechanism of injury is most commonly
noncontact during deceleration, landing, or cutting. The “at-risk”
position of the leg is with knee extended, hip adducted and internally
rotated, and leg externaly rotated. Once a valgus moment is produced
with the leg in the aforementioned position, the ACL is at significant
risk. Soccer, basketball, field hockey, lacrosse, and skiing appear to
be the sports with the greatest risk.
-
Intrinsic factors—limb alignment, joint laxity, notch width, hormonal, and ligament size
-
Extrinsic factors—muscular strength and
balances, neuromuscular control, body movements, shoe surface friction,
and skill development
pronation have been implicated as contributing factors. The femoral
notch size has also been implicated as a risk factor for noncontact ACL
injury. Currently, anatomic factors identified in the literature
include a notch width in patients with bilateral ACL tears that is less
than in patients with unilateral tears. Notch width is smaller in
females than males (see Fig. 4-1),
and the notch width index (condylar width to notch width) in females is
less than males. Some researchers have suggested that increased laxity,
especially in patients with recurvatum, may contribute to the increased
incidence. The increased laxity may contribute to diminished joint
proprioception, causing the knee to be less sensitive to potential
damaging forces.
 |
|
Figure 4-4
A college basketball player underwent intramedullary nailing for an anterior tibia stress fracture. This fracture was refractory to healing for 6 months prior to treatment. (Courtesy of Dr. Glen Ross.) |
as a cause of ACL injury are somewhat controversial. Some studies have
suggested an increased incidence of ACL injuries during the estrogen
surge at midcycle, whereas others report an increase around the time of
menses. The use of oral contraceptives has also been investigated to
try to understand hormonal influences but no definitive conclusions can
be made at the present time.
prospective study looking at risk factors associated with noncontact
ACL injuries. In women, narrow notch width, generalized joint laxity,
and increased body mass index were all significant risk factors. There
was a trend toward significance for knee laxity on KT-2000 testing. In
these military recruits, the presence of one of these factors led to a
relative risk 2.7 to 4 times those without risk factors. Using a
regression model, including femoral notch width, body mass index, and
generalized joint laxity, the authors were able to predict 75% of the
ACL injuries.
intrinsic risk factors, changes can be made to influence potential
extrinsic causes. Knee joint position during landing, landing forces,
and cutting maneuvers have been implicated as potential risk factors (Fig. 4-5).
Most studies report that females tend to land with the knee and hip in
a more extended position. When landing with the knee in a position of
extension, females tend to recruit the quadriceps eccentrically to a
greater degree than the hamstrings. Research has also shown with low
knee flexion angles, the maximal force generated by the quadriceps
exceeds the tensile strength of the ACL. Females have also been shown
to have less gluteus medius activation than in males. This is important
because hip motion influences knee motion. A recent study in female
athletes showed that the knee abduction angle at landing was 8 degrees
greater, and the knee flexion angle at
landing
was 10 degrees lower in the ACL injured than uninjured athletes. They
concluded from their study that decreased neuromuscular control
(increased dynamic valgus and knee abduction moments) were risk factors
for ACL injury, with 73% specificity and 78% sensitivity.
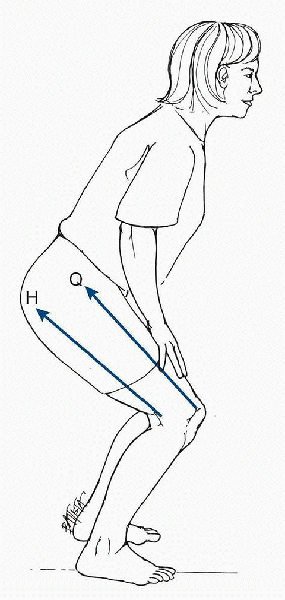 |
|
Figure 4-5
Co-contraction of the quadriceps and hamstring muscles. During co-contraction of the quadriceps and hamstrings, the pull of the hamstrings (H) applies a posterior shear force that protects the anterior cruciate ligament from the shear force of the quadriceps (Q). (From Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore: Lippincott Williams & Wilkins, 2004.) |
the gym during isolated exercises, many cannot translate isolated
muscle strength into coordinated skilled movement. As a result, there
have been many neuromuscular training prevention programs developed to
decrease the incidence of ACL injury and all with great success. For
example, Sportsmetrics is a three-part prevention program focusing on
flexibility, strengthening, and plyometrics. During phase I, proper
jumping techniques are taught. Phase II concentrates on building
strength and agility, and phase III focuses on achieving maximal
vertical height. Data from their studies have shown a decrease
incidence of ACL injuries. The program itself was found to decrease
peak landing forces, decrease varus and valgus motion with landing, and
increase hamstring strength, thus improving hamstring-toquadriceps peak
torque ratio. Another program is the California ACL Prevention Project:
Prevent Injury and Enhance Performance PEP Program. The program has
five components (avoidance, flexibility, strengthening, plyometrics,
and agilities) that are performed 2 to 3 times weekly. Randomized
controlled trials using this program have shown significant decreases
in ACL injuries, noncontact ACL injuries, and practice ACL injuries.
-
Regular exercise (at least three times
per week) is preferable to intermittent activity. Competitive
activities should be discouraged. -
Vigorous exercise should not be performed in hot, humid weather or during a period of febrile illness.
-
Ballistic movements (jerky, bouncy
motions) should be avoided. Exercise should be done on a wooden floor
or a tightly carpeted surface to reduce shock and provide a sure
footing. -
Deep flexion or extension of joints
should be avoided because of connective tissue laxity. Activities that
require jumping, jarring motions, or rapid changes in direction should
be avoided because of joint instability. -
Vigorous exercise should be preceded by a
5-minute period of muscle warm-up. This can be accomplished by slow
walking or stationary cycling with low resistance. -
Vigorous exercise should be followed by a
period of gradually declining activity that includes gentle stationary
stretching. Because connective tissue laxity increases the risk of
joint injury, stretches should not be taken to the point of maximum
resistance. -
Heart rate should be measured at times of
peak activity. Target heart rates and limits established in
consultation with the physician should not be exceeded. -
Care should be taken to gradually rise
from the floor to avoid orthostatic hypotension. Some form of activity
involving the legs should be continued for a brief period. -
Liquids should be taken liberally before
and after exercise to prevent dehydration. If necessary, activity
should be interrupted to replenish fluids. -
Women who have led sedentary lifestyles
should begin with physical activity of very low intensity and advance
levels very gradually. -
Activity should be stopped and a physician should be consulted if any unusual symptoms appear.
-
Maternal heart rate should not exceed 140 beats per minute.
-
Strenuous activities should not exceed 15 minutes in duration.
-
No exercise should be performed in the supine position after the fourth month of gestation is completed.
-
Exercises that use Valsalva’s maneuver should be avoided.
-
Caloric intake should be adequate to meet not only the extra energy needs of pregnancy, but also the exercise performed.
female ACL reconstructed athletes. Controversy exists in the literature
regarding outcomes of ACL reconstruction when comparing men versus
women. Although some studies have suggested higher clinical failure
rates in women, other researchers have not found significant
differences. Currently, most authors agree that gender alone should not
be used as selection criteria for ACL reconstruction. In terms of graft
selection, many surgeons have shown a trend in using hamstring grafts
for women as a result of improved cosmesis and minimizing graft site
morbidity. However, reduced peak torque of the hamstring muscles has
been reported, and concern exists over a tendency toward
postreconstruction residual laxity and tunnel widening. Barrett et al.
performed a prospective review comparing hamstring and patellar tendon
ACL reconstruction in female patients. Although not statistically
significant, there was a trend toward a greater failure rate and
increased laxity on physical examination and KT-1000 arthrometer
differences. In the hamstring group, there was a significant increase
in pain compared with the
patellar
tendon group. In a case-control comparison of hamstring versus bone
patellar tendon bone in female athletes, no functional differences were
seen between the two groups. However, there was significantly greater
kneeling avoidance, numbness/dysesthesia, and loss of passive extension
in the bone-patellar tendon-bone group. The authors concluded that
hamstrings were an acceptable graft alternative in the female athlete.
women and there are increasing numbers of female athletic participants,
future research needs to focus on appropriate prevention and treatment.
suggests that it is beneficial, but physiological parameters must be
monitored, and limitations must be applied individually. The goals
throughout pregnancy should be to maintain or improve preexisting
levels of fitness without risk to the mother or the developing fetus.
Exercise in the supine position should be avoided because of potential
risk to the great vessels from gravity acting on the uterus.
image, avoidance of excessive weight gain, decreases in musculoskeletal
complaints (back pain), improved labor symptoms, and facilitation of
postpartum recovery. Potential risks include environmental exposure,
dehydration, hypoxia, and uterine trauma. Hot and humid environments
should be avoided for risk of dehydration. A meta-analysis was
performed in 1992 to help determine safe exercise recommendations
during pregnancy. After analyzing the 18 studies involved, the authors
concluded that pregnant women can exercise safely three times a week
for 43 minutes at a heart rate of 144 beats per minute. In 1994, the
American College of Obstetrics and Gynecology published revised
guidelines for exercise pregnancy (Box 4-4).
Thus, exercise during pregnancy can have many potential benefits, if
appropriate caution is observed and certain restrictions are used.
KL, Malcolm SA, Thomas SA. Risk factors for stress fractures in female
track-and-field athletes: a retrospective analysis. Clin J Sport Med
1995;5:229-235.
SA, Berfeld JA, Boyajian-O’Neil LA, et al. Female athlete issues for
the team physician: a consensus statement. Med Sci Sports Exerc
2003;35:1785-1793.
TE, Lindenfeld TN, Riccobene JV, et al. The effects of neuromuscular
training on the incidence of knee injury in female athletes: a
prospective study. Am J Sports Med 1999;27:699-706.
ML, Ott SM. Special concerns of the female athlete. In: Fu FH, Stone
DA, eds. Sports Injuries. Philadelphia: Lippincott Williams &
Wilkins, 2001:215-264.
SM, Ferris CM, Fu FU. Risk factors associated with noncontact anterior
ligament injuries in female athletes. Instr Course Lect 2002;51:307-310.
EA, Tran EV, Wells CL. Effects of physical exercise on pregnancy
outcomes, a meta-analytic review. Med Sci Sports Exerc
1991;23:1234-1239.
A. The female athlete triad. In: Garrett WE, Lester GE, McGowan J, et
al., eds. Women’s Health in Sports and Exercise. Rosemont, IL: American
Academy of Orthopaedic Surgeons, 2001: 451-465.
JM, Scoville CR, Williams GN. Risk factors associated with noncontact
injury of the anterior cruciate ligament. Am J Sports Med
2003;31:831-842.
