Management of the Multiply Injured Patient
One – General Principles: Basics > Principles of Treatment > 9 –
Management of the Multiply Injured Patient
varies among surgeons from different specialties and between different
centers and countries. This has lead to the development of standardized
scoring systems to allow comparable stratification of injuries between
centers and to aid prediction of morbidity and mortality. Polytrauma
patients are the subgroup of injured patients who have sustained
injuries to more than one body region and organ with at least one of
the injuries being life-threatening. The cumulative severity of this
trauma load on the victim’s anatomy and physiology is usually expressed
as an Injury Severity Score (ISS) of 16 or greater or of 18 or greater.7,346
Trentz emphasized the pathophysiologic systemic impact of multiple
trauma when he defined polytrauma as “a syndrome of multiple injuries
exceeding a defined severity (ISS ≥17) with sequential systemic
reactions (systemic inflammatory response syndrome [SIRS] for at least
1 day) that may lead to dysfunction or failure of remote organs and
vital systems, which have not themselves been directly injured.”185,380
respectively. According to the National Trauma Data Bank Annual Report
in 2007, which reported on 1,485,098 cases between 2002 and 2006 in the
United States, 45.2% of patients sustained minor injuries (ISS 1 to 8)
with 32.4% sustaining moderate injuries (ISS 9 to 15), 12.8% severe
injuries (ISS 16 to 24), and 9.6% very severe injuries (ISS >24).368
Motor vehicle-related injuries accounted for 37.9% of all cases,
followed by falls at 30.2%. Blunt trauma accounted for 86.2% of all
cases with penetrating trauma comprising a further 11.1% and burns 1.7%
of the remaining cases. Major peaks occurred in the 16 to 24 years age
group because of motor vehicle- and firearm-related injuries and in the
35 to 44 years age group because of motor vehicle-related injuries.
Males were more prone to trauma, with only 35% of the trauma victims
being female. A review of the mortality rates showed that 3.8% of
females died compared with 4.8% of males. A review of the different
grades of injury severity shows that the mortality rate for patients
with an ISS of 1 to 8 is 0.7%. This compares with 1.9% for an ISS of 9
to 15, 5.3% for an ISS of 16 to 24, and 29.3% for an ISS greater than
24. A review of the mortality rates by organ system shows that patients
with abdominal and thoracic injuries have the highest rates at 10.9%
and 10.1%, respectively, followed by pelvic injuries at 8.4% and brain
and skull injuries at 7.8%. Overall, motor vehicle accidents are
associated with the highest mortality, being responsible for about 40%
of deaths.368
limited and the subject has been given only cursory attention. With an
aging, increasingly active elderly population, it is likely that such
patients will be seen with increasing frequency. The elderly with
diminished physiologic reserve, often in association with significant
comorbidities, require special consideration. The distribution of
injuries and type of injury mechanism are likely to be different in a
population with a high incidence of osteoporosis. Elderly patients can
become multiply injured following low-energy trauma and these injuries
may have worse outcomes. For example, while falls have been reported to
account for only 9% to 11% of injury-related deaths in the general
population, they comprise more than 50% of traumatic deaths in persons
over 65 years of age.9 Patients with
limited mental or physical capacity are also more likely to be involved
in accidents as they are slower to identify and respond to dangerous
situations.181,198
One must also consider the likelihood of a medical emergency such as a
myocardial infarction or stroke precipitating an accident, making it
necessary to treat this pathology together with the patient’s injuries.
admitted to our institution, 3172 (13%) were severely injured having an
ISS equal to or greater than 16. Within this severely injured group,
438 (14%) were over 65 years of age. Therefore, elderly patients with
severe injuries accounted for 1.8% of our overall admissions. The
median age in the elderly group was 75 years (range, 65 to 100 years)
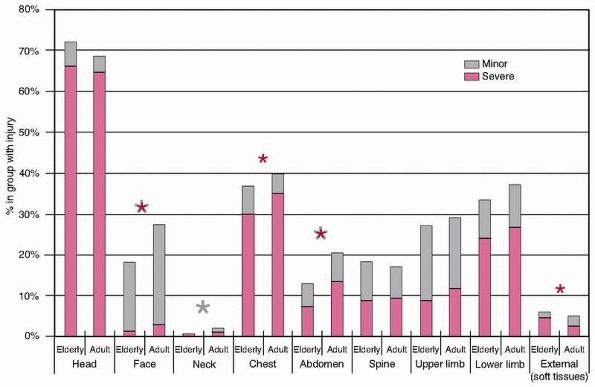
and the median ISS was 25 (range, 16 to 75). Figure 9-1
illustrates the overall distribution of injuries sustained in the
elderly and adult age groups. It can be seen that the overall injury
distribution is similar, although adults sustained more facial, neck,
and abdominal injuries overall and more severe (Abbreviated Injury
Score [AIS] 3 or greater) facial, chest, and abdominal injuries
compared with the elderly patients. Elderly patients sustained more
severe (AIS of 3 or greater) external injuries, all of which were
severe burns. High-energy injuries were responsible for the majority of
these injuries, although relatively minor trauma became increasingly
important in older patients. In patients aged over 65 years who were
operated on in the first 24 hours, 47% underwent neurosurgical, 34%
extremity or spinal, 15% abdominal, and 7% cardiothoracic
interventions. This was not significantly different from the pattern of
interventions undertaken in the adult group with rates of 50%, 36%,
19%, and 12%, respectively. The mortality rate was significantly higher
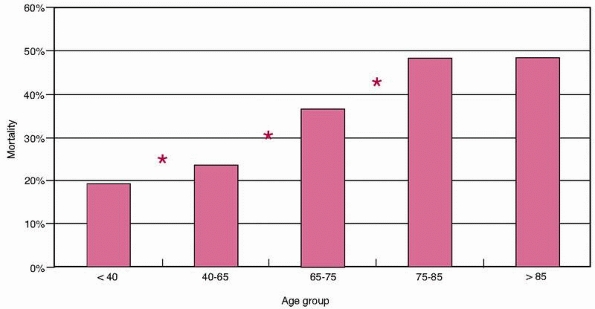
in the elderly patients than the adults (42% versus 20%, P <0.0001). Figure 9-2
shows inpatient mortality by age group. This increased significantly
with age from 19% in patients younger than 40 years to almost 50% in
the over-75 year age group. The difference between consecutive age
groups is statistically significant at each point until the final two
groups. There was no difference observed between the mortality in the
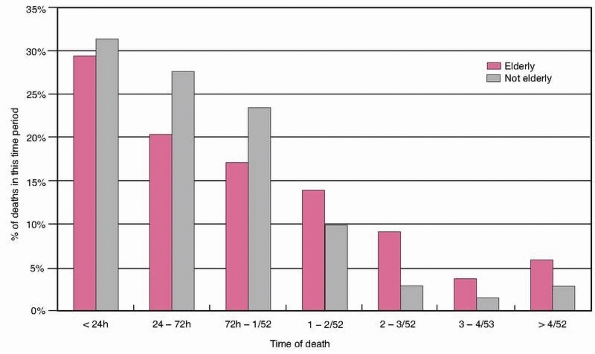
75- to 85-year-old and the over-85 group (48.2% versus 47.4%, P = NS). Figure 9-3
shows the percentage of those who died in each time period, by age
group. It can be seen that in both adult and elderly patients, the
majority died within the first 24 hours, falling off progressively with
time after this. However, the overall trend was for a greater
proportion of the elderly patients to die later compared with the
adults. This difference was only statistically significant for the 24-
to 72-hour and 2- to 3-week periods (P <0.05 and P
<0.001, respectively). Age, ISS, and Glasgow Coma Scale (GCS) score
continued to be predictive of mortality in elderly patients but other
factors relevant in younger adults were not (Table 9-1).
million nonfatal injuries (1270 per 100,000 population/year).256 Motor vehicle-related injuries were the leading cause of death among persons aged 1 to 24 years.256
 |
|
FIGURE 9-1
Distribution of regional injuries by age group (elderly >65 years). Both minor and severe injuries are shown. The asterisks denote statistical significance with the color of the asterisk denoting the severity of the injury. |
deaths in the United States, five risk factors were noted to contribute
to mortality: (a) alcohol use by drivers and pedestrians (43%), (b)
failure to wear a seat belt (30%), (c) failure to have an air bag (4%),
(d) failure to wear a motorcycle helmet (1%), and (e) failure to wear a
bicycle helmet (1%).73 Over these 20
years, the mortality rates attributed to each risk factor declined
because of legislation. There were 153,168 lives saved by decreased
drinking and driving, 129,297 by increased use of seat belts,
4305
by increased use of air bags, 6475 by increased use of motorcycle
helmets, and 239 by increased use of bicycle helmets. Sufficient
evidence was found to prove the effectiveness of lower blood alcohol
concentration laws for young and inexperienced drivers and of
intervention training programs for servers of alcoholic beverages.404
All 50 states and the District of Columbia have laws defining it as a
crime to drive with a blood alcohol concentration (BAC) at or above a
proscribed level, this being 0.08%.
 |
|
FIGURE 9-2 Mortality by age group. *Significant difference between consecutive groups, P <0.05.
|
 |
|
FIGURE 9-3 Percentage of deaths occurring in each time period.
|
prevent the body from being ejected when the car stops. Deceleration
energy is spread over more energy-absorbing parts of the body such as
the pelvis, chest, and shoulders. Safety belts are the single most
effective means of reducing fatal and nonfatal injuries in motor
vehicle accidents, and primary enforcement seat belt laws where police
officers are allowed to stop a driver and issue a ticket for the sole
reason of not wearing a seatbelt are likely to be more effective than
secondary laws that permit nonbelted occupants or drivers to be
ticketed only after being stopped for another moving violation.88,306,342,343 According to the National Highway Traffic Safety Administration (NHTSA).257
seat belt use nationwide was 82% in 2007, ranging from 63.8% in New
Hampshire to 97.6% in Hawaii. Twenty-eight states had primary
enforcement seat belt laws.
However,
almost 70% of the 16- to 34-year-old passenger vehicle occupant
fatalities killed during nighttime hours were unrestrained.257
All states have child passenger protection laws. These vary widely in
age and size requirements and penalties for noncompliance.
Child-restraint use in 1996 was 85% for children younger than 1 year
and 60% for children aged 1 to 4 years. Since 1975, deaths among
children younger than 5 years have decreased by 30% to 3.1 per 100,000
population/year, but rates for age groups of 5 to 15 years have
declined by only 11% to 13%.255 In a
study reviewing accidents involving 4243 children aged 4 to 7 years,
between 1998 and 2002, injuries occurred among 1.81% of all 4- to
7-year-olds, including 1.95% of those in seat belts and 0.77% of those
in belt-positioning booster seats. The odds of injury were 59% lower
for children in belt-positioning boosters than in seat belts. Children
in booster seats sustained no injuries to the abdomen, spine, or lower
extremities, while children in seat belts alone had injuries to all
body regions.92,93
|
TABLE 9-1 Comparison of Continuous Variables between Survivors and Those Who Died, by Age Group
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
8%, whether the driver was belted or not. However, seat belts provide
much greater protection, with seat belt use reducing the risk of death
by 65%, or by 68% if the seat belt is used in combination with an air
bag72 (see Table 3-35).
No differences in the risk of frontal crash deaths were observed
between adult occupants with sled-certified and first-generation air
bags. Consistent with reports of decreases in air bag-related deaths,
significant reductions in frontal deaths among child passengers seated
in the right-front position in sled-certified vehicles were seen.36 Airbags have been reported to be associated with reduced in-hospital mortality and decreased injury severity.402
In a systematic review, helmets have been shown to reduce the risk of
death by 42% and the risk of head injury by around 69% in motorcycle
riders.213
limits, speed-camera networks, and speed-calming measures substantially
reduce the absolute numbers of road deaths. This is apparent in the
United Kingdom, Australia, France, and other countries.302
There is also evidence that speed enforcement detection devices are a
promising intervention for reducing the number of road traffic injuries
and deaths.404 It is of note, however, that in the United States, there are no speed-camera networks.302
pickups, and sport utility vehicles) are responsible for 85.2%
pedestrian deaths in the United States. Heavy trucks, buses, and
motorcycles are responsible for the remainder.288
Buses kill eight times as many pedestrians as cars per mile of vehicle
travel. Vehicle characteristics such as mass, front end design,
visibility,59 and degree of interaction with pedestrians probably determine their risk per mile.288
Therefore, one option to reduce pedestrian fatalities might be the
modification of motor vehicles. However, every type of motor vehicle
has to be evaluated on an individual basis. Thus, lowering the front
end of light trucks, and consequently the point of impact with a
pedestrian’s body, might reduce the likelihood of serious head and
chest injuries.57
design on the mechanism and pattern of injury for vehicular trauma
victims, patients (restrained car occupants, bicyclists, pedestrians)
injured between 1973 and 1978 and between 1994 and 1999 in a specific
region in Germany were compared.303
A lower injury severity (ISS 5.0 versus 12.1), lower incidence of
polytrauma (4.5% versus 15%), and a lower mortality rate (3.4% versus
14%) were measured for all groups during the later period. Given the
same crash severity, these reductions were also related to improvements
in vehicle design rather than just seatbelt use.303
its origins in the late 1960s when it was stated that the quality of
civilian trauma care in the United States was below the standard in
combat zones in Vietnam: “If seriously wounded, the chances of survival
would be better in the zone of combat than on the average city street.”
A trauma system organizes the full range of coordinated care to all
injured patients in a defined geographic area. It includes injury
prevention, prehospital and in-hospital care, and rehabilitation. The
concepts of organized trauma care5 have proved to be one of the most important advances in the care of the injured patient over the last 30 years.157,208 The number of U.S. states with a trauma system increased from 7 in 1981 to 36 in 2002.340 Nevertheless, in 2000 approximately 40% of the U.S. population still lived in states without a trauma system.254
Research and constant reevaluation are necessary for continuous
assessment of the system and improvement of its outcomes and efficiency.209,340 According to a systematic review of published evidence224
of the effectiveness of trauma systems in the United States until 1999,
the implementation of trauma systems decreased hospital mortality of
patients who are severely injured to approximately 15%.49,179,218,224,248
The relative risk of death caused by motor vehicle accidents was 10%
lower in states with organized systems of trauma care than in states
without such systems.254 However, it
took about 10 years to establish an organized system of trauma that was
effective in reducing mortality. Nathens et al.253
concluded that this is consistent with the maturation and development
of trauma triage protocols, interhospital transfer agreements,
organization of trauma centers, and ongoing quality assurance. U.S.
counties with 24-hour availability of surgical specialties, CT
scanners, and operating rooms have a decreased motor vehicle accident
mortality, compared with counties without those resources. Counties
with designated trauma centers have lower motor vehicle-related
mortality rates.232 Recently
published prospectively collected data comparing mortality in trauma
centers to nontrauma centers shows a 25% mortality reduction for
patients younger than 55 years when treated in a trauma center.215
the chain in trauma system as well as on the interplay of these
elements and there is a lack of evidence of the understanding of the
contribution of individual components on the efficiency of the system.
However, prehospital notification protocols and performance improvement
programs appear to be most associated with decreased risk-adjusted odds
of death.208 With regard to prehospital trauma care, there are ongoing national and
international debates and studies as to which system is most favorable35,87 and how prehospital trauma care can be improved.43,61,99,128,293
|
TABLE 9-2 Prehospital Trauma Care Systems
|
||||||
|---|---|---|---|---|---|---|
|
care systems or emergency medical services (EMS) systems can be
differentiated (Table 9-2).
results from the experience in the Vietnam Conflict, where trained
paramedical personnel were responsible for the initial treatment in the
combat zone, whereas physicians were thought to be most effective in a
hospital setting.252 Extensive
medical care at the scene was almost impossible because of combat, so
that “load and go” or “scoop and run” was favored. In contrast,
Franco-German87 EMS systems are
physician directed and in most cases associated with a longer time at
the scene of the accident (“stay and play”) to facilitate stabilization
of the patient before transport to an appropriate hospital.
by using shock rate in the emergency department and early trauma
fatality rate as parameters to assess prehospital outcome, found out
that the emergency department shock rate did not vary significantly
between physician-performed Advanced Life Support (PHYSALS) systems and
paramedic-performed Advanced Life Support (PARAALS) systems. Early
trauma fatality rate was significantly lower in PHYSALS EMS systems
compared to the PARAALS EMS systems. Therefore, a physician at the
scene may be associated with lower early trauma fatality rates.
However, there are a lack of data to allow proper comparison of
outcomes between the EMS systems of different countries.87
prehospital trauma care systems have concluded that there is no
evidence supporting advanced prehospital trauma care. Almost all of
these studies used hospital trauma fatality as the main outcome
parameter and only compared advanced life support (ALS) systems with
basic life support (BLS) systems.47,206,207,315
One further study also compared PARAALS EMS in Montreal to PHYSALS EMS
in Toronto and BLS EMS in Quebec using in-hospital mortality as their
outcome parameter.207 PHYSALS EMS
system was not associated with a reduction in risk of in-hospital
death, and the conclusion was that in urban centers with highly
specialized level I trauma centers, there is no benefit in having
onsite ALS for the prehospital management of trauma patients.207
population. The need for comparative analysis of the injury-,
management-, and outcome-related parameters among the different patient
groups, hospitals, trauma management strategies, and health systems has
stimulated the development of many trauma scoring systems and scales
over the last 40 years.10,34,38,52,191,401
the injuries that have been sustained together with other independent
parameters such as comorbidities, age, and mechanism of injury. They
serve as a common language between clinicians and researchers.
Initially, they were designed for the purpose of field triage and in
that regard they needed to be simple and user friendly. Subsequently,
they have evolved to more complex and research-focused systems. Their
concept is based on converting many independent factors into a
one-dimensional numeric value that ideally represents the patient’s
degree of critical illness. They are often based on complex
mathematical models derived from large data sets and registries such as
the Major Trauma Outcome Study (MTOS) or the Trauma Audit and Research
Network (TARN).54,371
the severity of the anatomic trauma, the level of the physiologic
response, the inherent patient reserves in terms of comorbidities, and
age and, as proved recently, should incorporate immunologic aspects and
genetic predisposition parameters.14,107,118,119,121,122,349
The variety of the potential applications of such scoring systems
ranges from basic prehospital and interhospital triage and mortality
prediction to other prognostic parameters such as length of hospital
stay and risk of disability. They can be used as a tool for comparison
of diagnostic or therapeutic methods and for the auditing of trauma
management.
Some scoring systems are based on a combination of these parameters.
Examples of these are the Trauma and Injury Severity Score (TRISS),38 A Severity Characterisation of Trauma (ASCOT),191 and the Physiologic Trauma Score (PTS).199 Numerous studies have assessed the accuracy, reliability, and specificity of the different trauma scores.53,118
It has been revised a number of times and is continuously monitored and
evolved by a committee of the Association of Advancement of Automotive
Medicine (AAAM).370 Its latest version was published in 2005,106
but the most used versions are the AIS90 and AIS98. In general, the AIS
is an anatomically based, consensus-derived, global severity scoring
system that classifies each injury by body region according to its
relative significance. All different anatomic injuries are matched with
a different seven-digit numbercode. They are classified according to
the affected body region (first digit, with body region 1 = head, 2 = face, 3 = neck, 4 = thorax, 5 = abdomen, 6 = spine, 7 = upper extremities,
8 = pelvis and lower extremities, and 9 = external and thermal injuries), type of anatomic structure (second digit, range 1 to 6), specific anatomic structure (third and fourth digits, range from 02 to 90), and level of the injury
(fifth and sixth digits, range from 00 to 99). The last digit of each
seven-digit AIS code follows a dot and represents the injury severity
of the specific injury on a scale of 1 to 6 (1 = minor, 2 = moderate, 3
= serious, 4 = severe, 5 = critical, and 6 = maximal-currently
untreatable injury). This last severity digit has been developed by a
consensus of many experts and is continuously monitored by the
committee.
Each injury in the patient is allocated an AIS code and the codes are
grouped in six ISS body regions: head and neck, face, chest, abdomen,
extremities and pelvis and external. Only the highest AIS severity
score (post dot digit-seventh digit of the AIS code) in each ISS body
region is used. The ISS is the sum of the squared AIS scores for the
three most severely injured ISS body regions. It can take values from 1
to 75. A value of 75 can be assigned either by the sum of three AIS
severities of 5 in three different ISS body regions, or by the presence
of at least one AIS severity of 6. Any AIS severity 6 is an automatic
ISS 75 independent of any other injuries. The ISS score is virtually
the only anatomic scoring system in widespread use. It has been
validated on numerous occasions and it has been shown to have a linear
correlation with mortality, morbidity, hospital stay, and other
measures of injury severity. Currently, it represents the gold standard
of anatomic trauma scoring systems.32,217,233
However, it has certain weaknesses in that any error in AIS coding or
scoring increases the ISS error. In addition, it is not weighted over
the different body regions and injury patterns, and it often
underestimates the overall anatomic injury, particularly in penetrating
trauma or if there are multiple injuries in one body region. The ISS is
not a useful triage tool as a full description of patient’s injuries is
usually not initially available.
often used in daily clinical practice and research that originates from
the AIS. It is the highest AIS code in a multitrauma patient and is
used by researchers to describe the overall injury in a particular body
region and to compare frequencies of specific injuries and their
relative severity.171,238
described the New ISS (NISS). This is the sum of the squares of the
three highest AIS severity scores regardless of the ISS body regions.
It has been found to be an improvement on the ISS especially for
orthopaedic trauma and penetrating injuries.12,13,146,168
However, it has still not been extensively evaluated and has the
disadvantage of requiring an accurate injury diagnosis before an exact
calculation can be made.
was also introduced to address the weaknesses of the ISS. It was
described as one of the components of the ASCOT and includes all the
serious injuries (AIS severity 3 or greater) in all the body regions.
It is also weighted more toward the head and the torso. All serious
injuries are grouped into four categories (A = head and spine, B =
thorax and anterior neck, C = all remaining serious injuries, D = all
nonserious injuries). The square root of the sum of squares of the AIS
scores of all the injuries in each of the four categories is computed
and by logistic-regression analysis a probability of survival is
calculated. The AP has been proved to be superior to the ISS in
discriminating survivors from nonsurvivors. However, up to now, its
complex computational model has restricted its application and limited
its use.
was subsequently introduced. This is a four-number characterization of
injury. These four numbers are the maximum AIS scores across all body
regions together with the modified A, B, C component scores of the
original AP (mA = head and spine, mB = thorax and neck, mC = all other
serious injuries).68 The mAP
component score values (A, B, C) are equal to the square root of the
sum of the squares of the AIS values for all serious injuries (AIS 3 to
6) in the specified body region groups. This leads to an Anatomic
Profile Score, a single number defined as the weighted sum of the four
mAP components. The coefficients are derived from logistic regression
analysis of 14,392 consecutive admissions to four Level I trauma
centres of the Major Trauma Outcome Study.54
in 1987. It is a scale of anatomic injury within an organ system or
body structure. The OIS offers a common language between trauma
surgeons, but it is not designed to correlate with patient outcomes.
The organ injury scaling committee of the American Association for the
Surgery of Trauma (AAST) is responsible for revising and auditing the
OIS tables that can be found on the AAST Web site.367
The severity of each organ injury may be graded from 1 to 6 using the
severity subcategories of the AIS. The injuries can also be divided by
mechanism such as blunt or penetrating or by anatomic descriptions such
as hematoma, laceration, contusion, or vascular.
introduced. It was based on the well-accepted and popular coding system
of the ICD-9 instead of the AIS. The International Classification of Diseases, Ninth Edition (ICD) is a standard taxonomy used by most hospitals and health care providers. The ICD-9 Severity Score (ICISS)319 uses survival risk ratios (SRRs) calculated for each ICD-9 discharge diagnosis. The SRRs are calculated by dividing the number of survivors of each different ICD-9
code by the total number of patients with such an injury. The product
of all the different SRRs of a patient’s injuries produces the ICISS.
Neural networking has been used to further improve ICISS accuracy.
ICISS has been shown to be better than ISS and to outperform TRISS in
identifying outcomes and resource utilization. However, in several
studies, the AP, mAP, and NISS scores appear to outperform ICISS in
predicting hospital mortality.144,243,244,359
physiologic parameters were introduced as field triage tools. The basic
characteristic of these physiology-based scores is that they are
comparatively simple but also time dependent. In 1981, Champion et al.55
hypothesized that early trauma deaths are associated with one of the
three basic systems: the central nervous, cardiovascular, and
respiratory systems. They designed a scoring system, the TS, based on a
large cohort of patients, which focused on five parameters: GCS score,
the unassisted respiratory rate (RR), respiratory expansion, the
systolic blood pressure (SBP), and capillary refill. All contributed
equally in the calculation of this score. It was proved useful in
predicting survival outcomes, with good interrater reliability, but it
was shown to underestimate head injuries and it also incorporated
parameters such as
respiratory expansion and capillary refill, which were difficult to assess in the field.56 Consequently, the same authors developed an RTS 8 years later,56
which was internationally adopted and is still in clinical use as both
a field triage and a clinical research tool. It includes three
variables (GCS, RR, SBP), and a coded value from 0 to 4 can be assigned
to each (Table 9-3). An RTS score may range
from 0 to 12, with lower scores representing a more critical status. In
its initial validation, this physiologic scoring system identified 97%
of the fatally injured as those having an RTS of 11 or less. It also
indicated certain weaknesses, which suggested that it should be used in
combination with an anatomic based score.245,313
Currently, the threshold of 11 is used as a decision-making tool for
transferring an injured patient to a dedicated trauma center.
|
TABLE 9-3 Unweighted Revised Trauma Score as Used in Field Triage
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
prediction, the RTS is used in its weighted form and is called coded
RTS (RTSc). It is calculated with the following mathematical formula
that allows weighting of the three contributing parameters (GCS, RR,
SBP) and their significance:
ranges from 0 to 7.8408, with lower values representing worse
physiologic derangement. The threshold for transfer to dedicated trauma
centers for the RTSc is 4. Besides the obvious calculation difficulties
that this formula may impose, the use of the RTS or the RTSc is
compromised by the fact that the GCS score cannot be estimated in
intubated and mechanically ventilated patients or in intoxicated
patients. Also, the calculated score may vary with the physiologic
parameters, which are often rapidly changing. It may well also
underestimate the severity of trauma in a well-resuscitated patient.
and its latest revision in 2006 (APACHE IV) represents the most modern
scoring system utilized in the demanding environment of intensive care
units (ICUs) and therefore also in intensive trauma units (ITUs). The
evaluated parameters include the age of the injured patient, any
chronic health comorbidities, several physiologicl elements required
for the calculation of the Acute Physiology Score (APS),416
previous length of ITU stay, emergency surgery, admission source, and
diagnosis on admission to ITU. These parameters are responsible for
both the complexity of the APACHE score and its superior prognostic
accuracy.
the physiologic based trauma scores led researchers to develop combined
approaches to more accurately translate the overall injury load of a
trauma victim to a single score or value. The TRISS34
incorporates both the ISS and the RTS, as well as the patient’s age, to
predict survival. The probability for survival (Ps) is expressed via a
specific formula Ps = 1/(1 + e-b), where e is a constant (approximately 2.718282) and b = b0 + b1(RTS) + b2(ISS) + b3(age factor). The b coefficients are derived by regression analysis from the MTOS database.54
The probability of survival according to this model ranges from 0 to
1.000 for a patient with a 100% expectation of survival. TRISS has been
used in numerous studies.* Its value as a predictor of
survival or death has been shown to vary from 75% to 90% depending on
the patient data set used. However, the deficiencies that govern the
ISS and the RTS were also found in their derivative, the TRISS. This is
particularly true of its inability to account for multiple injuries in
the same anatomic region, the variability of the RTS value, the
inability to calculate a value in intubated patients because of the
inaccuracy of the GCS and RR, and the difficulty of assessing
comorbidities and the physiologic reserve of the injured patient.
attempts to incorporate anatomic (AP) and physiology (RTS) parameters,
as well as the patient’s age, in a more efficient way than TRISS. The
ASCOT score is derived from the same formula (Ps = 1/(1 + e-b)
as the TRISS but has different coefficients for blunt and penetrating
injury. The principal claimed advantage of ASCOT was the use of the AP
instead of the ISS, which better reflected the cumulative anatomic
injury load of the patient. However, while the predictive performance
of the ASCOT was marginally better than that of the TRISS, its
complexity is considerably higher.143,225,274
systemic inflammatory response syndrome (SIRS) score on admission
(range, 0 to 4; 1 point for the presence of each of the following:
temperature greater than 38°C or less than 36° C; heart rate greater
than 90 beats/minute; RR greater than 20/ min; neutrophil count greater
than 12,000 or less than 4000/ mm3;
or the presence of 10% bands), the age, and the GCS score into a simple
calculation to predict mortality. This new statistical model appeared
to be accurate and has been shown to be comparable with the TRISS, or
the ICISS, in subsequent studies.199
effort expended in designing these different assessment methodologies,
there are always going to be difficulties in translating the
multifactorial problems inherent in the multiply injured patient into a
number and all scores have advantages and disadvantages. In the future,
it is likely that additional factors such as the immunologic response
to trauma, and possibly genetic predisposition, will be assessed. Until
the development of an “ideal” scoring model, we should be cautious in
our conclusions regarding the existing systems and the prediction of
outcome in the injured patient.
response to injury have delineated three phases. First, there is a
hypodynamic ebb phase (shock) where the body initially attempts to
limit the blood loss and to maintain perfusion to the vital organs.
This is followed by a hyperdynamic flow phase lasting for up to 2
weeks, which is characterized by increased blood flow to remove waste
products and to allow nutrients to reach the site of injury for repair.
The last phase is a recuperation phase that may last for several months
in an attempt to allow the human body to return to its preinjury level.108
However, with more recent knowledge, accumulated mainly during the past
20 years, it has become clear that the physiologic response to injury
is not as simplistic as was initially thought but instead represents a
complex phenomenon involving the immune system, and even today it is
still not fully understood. With the advances made in every field of
medicine and particularly in the disciplines of molecular biology and
molecular medicine, it is now possible not only to characterize, but
also to quantify, the cellular elements and molecular mediators
involved in this dynamic physiologic process.
neuroendocrine system and leads to an adrenocortical response
characterized by the increased release of adrenocorticosteroids and
catecholamines. Subsequently, the work of Hans Selye further
illustrated the importance of this neuroendocrine response to trauma,
pointing out that this was involved in what he named “the general
adaptation syndrome.”337 This is now considered as a forerunner of SIRS.28
This activation of the neuroendocrine system is responsible for the
increase in heart rate, RR, fever, and leukocytosis observed in trauma
patients after major injury. Besides trauma, SIRS can be induced by
other insults such as burns, infection, or major surgery and is defined
as being present when two or more of the criteria shown in Table 9-4 apply.28
traumatic insult is necessary for hemostasis, protection against
invading microorganisms, and the initiation of tissue repair and tissue
healing. Restoration of homeostasis is dependent on the magnitude of
the injury sustained and the vulnerability of the host, who may possess
an abnormal or defective local and systemic immune response and
therefore may fail to control the destructive process. Multiple
alterations in inflammatory and immunologic functions have been
demonstrated in clinical and experimental situations following trauma
and hemorrhage, suggesting that a cascade of abnormalities that
ultimately leads to adult respiratory distress syndrome (ARDS) and
multiple organ dysfunction syndrome (MODS) is initiated in the
immediate postinjury period.116,120,122,123,147
Blood loss and tissue damage caused by fractures and soft tissue crush
injuries induce generalized hypoxemia in the entire vascular bed of the
body. Hypoxemia is the leading cause of damage as it causes all
endothelial membranes to alter their shape. Subsequently, the
circulating immune system, namely the neutrophil and macrophage defense
systems, identify these altered membranes. The damaged endothelial cell
walls, by trying to seal the damaged tissue, induce activation of the
coagulatory system. This explains why these patients develop a drop in
their platelet count. Further cascade mechanisms, such as activation of
the complement system, the prostaglandin system, the specific immune
system, and others, are set in motion.
|
TABLE 9-4 Defined Parameters of the Systemic Inflammatory Response Syndrome (SIRS)
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
is dependent primarily on the severity of the “first-hit phenomenon”
related to the initial trauma and secondarily on the activation of the
various molecular cascades during therapeutic or diagnostic
interventions, surgical procedures, and posttraumatic or postoperative
complications (“second” or “third” hits).109,117
The mediators that are involved in the sequelae of posttraumatic events
are initially released from the local cells at the site of injury and
subsequently systemically. The sequestration and the activation mainly
of polymorphonuclear (PMN) granulocytes, monocytes, and leukocytes
trigger a multifocal molecular and pathophysiologic process. The
mechanism of complement activation, leukostasis, and macrophage
activation has been associated with the concept of the “low flow
syndrome”298 and more recently with endothelial and PMN leukocyte activation.156,205
The cells interact and adhere to the endothelium via adhesion molecules
like L-selectin, ICAM-1, and integrin β2, these being representatives
of the selectin, immunoglobulin, and integrin superfamilies,
respectively.
by losing their autoregulatory mechanisms, can release toxic enzymes,
causing remote organ injury in the form of ARDS or MODS.117,147
the integrity of the host, dysregulation of the immune system will
occur, leading initially to an exaggerated systemic inflammation and,
at a later stage, immune paralysis. The availability of techniques to
measure molecular mediators has allowed different research groups to
search for inflammatory markers that could detect patients in a
borderline condition who are at risk of developing posttraumatic
complications. Alternate treatments may then prevent the onset of
adverse sequelae. Serum markers of immune reactivity can be selectively
grouped into markers of acute phase reactants, mediator activity, and
cellular activity112,231,259,283,360 (Table 9-5).
and widely used of these, partly because of its more consistent pattern
of expression and plasma half-life.286 A measurement of greater than 500 pg/dL in combination with early surgery has been associated with adverse outcome.137 Clinical parameters are also useful in assessing the response to trauma and SIRS was developed for this purpose.222
Although both systems have previously been correlated with injury
severity and early elevation is associated with adverse outcome,309 there has been little
work examining the relationship between these two assessments in
detail. In a recent study, it was found that in the early phase, both
IL-6 and SIRS are closely correlated with the NISS and with each other.
A cutoff value of 200 pg/dL was shown to be significantly diagnostic of
an “SIRS state.” Significant correlations between adverse events and
both the IL-6 level and SIRS state were demonstrated.14
 |
|
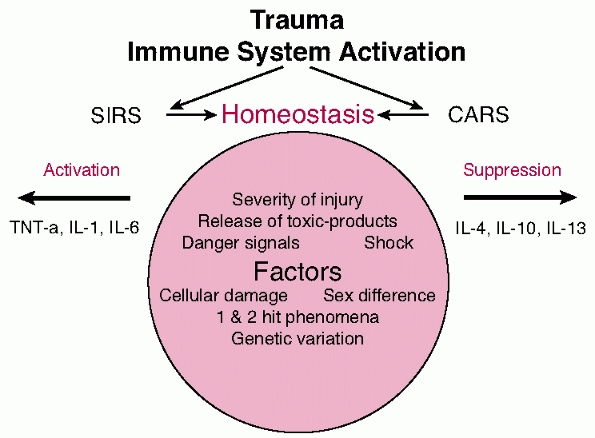
FIGURE 9-4 Diagrammatic representation of the release of mediators.
|
reactivity has led to the discovery of signaling substances termed
alarmins.20 The alarmins are
endogenous molecules capable of activating innate immune responses as a
signal of tissue damage and cell injury. To this group of endogenous
triggers belong such molecules as the high-mobility group box 1
(HMGB1), heat shock proteins (HSPs), defensins, cathelicidin,
eosinophilderived neurotoxin (EDN), and others. These structurally
diverse proteins function as endogenous mediators of innate immunity,
chemoattractants, and activators of antigen presenting cells (APCs).272
HMGB1 is a nuclear protein that influences nuclear transactions and
plays a role in signaling after tissue damage. In contrast to alarmins,
the PAMPs (pathogenassociated molecular patterns) represent
inflammatory molecules of a microbial nature being recognized by the
immune system as foreign because of their peculiar molecular patterns.
Both PAMPs and alarmins are currently considered to belong to the
larger family of damage-associated molecular patterns (DAMPs).20
PAMPs and DAMPs are recognized by our immune system by the expression
of multiligand receptors such as the Toll-like receptors (TLRs).413
Overall, these molecules represent a newly documented superfamily
capable of activating innate immune responses after trauma. The
molecules categorized in this superfamily are expanding but their
pathophysiologic contribution is currently not fully understood.
|
TABLE 9-5 Serum Inflammatory Markers
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
scientists to monitor different variables related to the
endothelial-cell activation and interaction process. We can now achieve
characterization and quantification of the endothelial response to the
initial trauma and to the subsequent stress events, thus monitoring the
clinical course of the patient.101,210
It is now becoming clear that the problem of managing patients with
multiple injuries has shifted from early and effective resuscitation to
the treatment of the host response to injury. The quantification of the
resulting activity of the different circulating mediators may predict a
potential disaster but does not necessarily contribute to the salvage
of the patient at risk. Too much, or too little immune response? Which
one of the two opposites is worse or better? Can we intervene, at what
stage, in which direction, and to which of the affected individuals?
The real question may well be whether all these markers and molecules
are just epiphenomena or related to the outcome. Currently, the effort
is being made to better understand all the processes and the cascade of
events that regulate these responses. Research has been aimed at
describing responses to surgery at the molecular level and in
developing and evaluating techniques to modify surgical stress
responses. The release mechanisms of the surgical stress response as
well as the factors that could amplify the response should be
considered by surgeons. The severity of the injury, type of anesthesia,
administration of adequate pain relief, type of surgical procedure,
timing and length of surgery, preexisting comorbid conditions, any
genetic influences that might lead to an adverse outcome, expertise of
the operating room staff, and expertise of the surgeon are some of the
important factors to be taken into account.
divided into the prehospital and the in-hospital phases. The chance of
survival and the extent of recovery are highly dependent on the medical
care that follows the injury. The speed with which lethal processes are
identified and halted makes the difference between life and death and
between recovery and disability. Time is an independent and cynical
challenger of any physician managing multiply injured patients. Thus,
the adopted approach to this peculiar clinical setting should be based
on getting most things right and very few things wrong. Due to the
inherent imperfections of the human nature of the medical personnel,
this approach should be based on simple and practical principles and be
well organized and standardized.
transfer to the hospital, the initial evaluation and management,
despite its inherent limitations because of lack of time and means,
have been shown to be decisive for the severely injured patient.276 The effect on survival of early extrication,403 the initial management from trained emergency personnel whether they be physicians or paramedics,176,338,374 and, equally important, the fast transfer to the designated trauma centers304,372
have been evaluated and highlighted in numerous studies. The
introduction and the universal acceptance of the Advanced Trauma Life
Support (ATLS)369 and, to a lesser degree, the Prehospital Trauma Life Support (PHTLS)4,408 protocols have contributed immensely toward improved and standardized initial evaluation and management of the trauma patient.
structured initial evaluation of the traumatized patient. The initial
priorities are airway maintenance, breathing, circulation, and
neurologic deficit.
center, protection of the spine, early aggressive prehospital
resuscitation, the implications of telemedicine and informatics, the
advances of the means of transport, and the rationalization of the
location of trauma centers have resulted in minimizing prehospital
mortality and achieving mortality rates that are lower than those
predicted by mathematical models such as TRISS and ASCOT).43,275,323
and sensible decision making regarding the timing of interventions
using a systematic approach.
control of the acute life-threatening conditions. Rapid systematic
assessment is performed to immediately identify potentially
life-threatening conditions. Diagnosis should be followed by
prioritized management of the airway and any breathing disorders
followed by circulatory support as set down in ATLS. This is followed
by the “secondary survey,” this being a complete acute diagnostic
“check-up.” However, this should only be undertaken if there is no
acute life-threatening situation, which would make immediate surgery
necessary. In these cases, this secondary assessment should be delayed
until the patient is properly stabilized.
|
TABLE 9-6 Subjects of Debate in Prehospital Trauma Management
|
|||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||
situation has been treated, and there is complete stability of the
respiratory, hemodynamic, and neurologic systems of the patient. This
is the usual phase where major extremity injuries are managed,
including the acute management of fractures associated with arterial
injuries or the presence of acute compartment syndrome. Fractures can
be temporarily stabilized with external fixation and the compartments
released where required. This primary period may last up to 48 hours.
stabilized and monitored. It is vital to regularly reevaluate the
constantly evolving clinical picture to avoid harmful impact from
intensive
treatment
or the burden of complex operative procedures. Unnecessary surgical
interventions should not be performed during the acute response phase
following trauma. Physiologic and intensive care scoring systems may be
used to monitor clinical progress. In the presence of systemic
inflammation and MODS, appropriate supportive measures should be
undertaken in an intensive care environment.
|
TABLE 9-7 In-Hospital Periods in the Evaluation and Management of the Trauma Patient
|
||||
|---|---|---|---|---|
|
necessary surgical procedures, including final reconstructive measures.
Only when adequate recovery is demonstrated should complex surgical
procedures be contemplated. Such interventions include the definitive
management of complex midface fractures, spinal or pelvic fractures, or
joint reconstruction.
the initial 1 to 3 hours after admission, but because of the
improvement of prehospital trauma care, it is now considered to extend
from the arrival of the emergency services at the scene until control
of the acutely threatening conditions has been achieved. In many
countries, this first period of trauma management is governed by the
principles of the ATLS.372 The
concept of a dedicated trauma team coordinated by a person experienced
in trauma and emergency management has been adopted in most of the
trauma centers.131,268,296
Rapid primary assessment and simultaneous interventions to control the
airway and the cervical spine (a), to facilitate breathing (b), and to
maintain the circulation and the vital blood flow (c) is started
immediately. After establishing a non-life-threatening situation, the
secondary survey follows, where a thorough evaluation aims to identify
all injuries and clinically relevant conditions in the injured patients.
systematic and timely manner, several diagnostic means to assist the
decision making process.150,200,212,299,388,410
The use of standardized diagnostic and therapeutic protocols has been
shown to improve the timing of the acute process, as well as its
quality, and the overall clinical outcome.417
It has been shown that the use of predefined and validated algorithms
effectively guides inexperienced personnel and reduces the mortality,
especially of the moderately severe polytrauma patients (ISS between 20
and 50).23 The primary goal of the
initial management is to diagnose quickly and immediately treat all the
life-threatening conditions, including airway obstruction or injury
causing asphyxia, tension pneumothorax or hemothorax, cardiac
tamponade, open thoracic trauma or flail chest, and massive internal or
external hemorrhage. The acute management of these conditions may
necessitate an urgent transfer to the operating theaters, before
further investigations can be undertaken, thus delaying the diagnostic
algorithms and the secondary survey. A pertinent example would be the
neglect of an intra-abdominal or pelvic hemorrhage, while attempting to
deal with a severe extremity injury. Of particular importance is the
fact that the condition of a polytrauma patient is dynamic and
potentially unstable at any moment. The treating team needs to be
continuously alert, especially at the initial stages of management, as
a previously controlled situation may deteriorate rapidly. The
continuous awareness of the team and the flexibility to change the
current management process are essential.40,63,126,312,354
evaluation of multiple injured patients, which has been reflected in
continuing debate about the standing ATLS protocols. The continuous
monitoring of the blood pressure, electrocardiogram, oxygen saturation
(pulse oximetry), ventilatory rate, the insertion of urine and/or
gastric catheters, and the acquisition of an initial full blood count,
arterial blood gases, and cross-matching of the patient have been
generally accepted as gold standards of the acute phase. There is also
debate about the extent of the initial radiographic evaluation and
imaging that may be useful in the first stages of the patient’s
evaluation and management. The current ATLS course manual recommends
initial anteroposterior (AP) chest, AP pelvic, and lateral cervical
spine radiographs, and the use of diagnostic peritoneal lavage (DPL) or
an abdominal ultrasonogram.
has caused a change in the initial radiographic assessment protocols in
many trauma centers and sometimes there is a degree of confusion
between the trauma and emergency personnel. The necessity for AP pelvic
radiography187,264 and lateral cervical spine radiography183,386 has been disputed by advocates of these new imaging modalities. Existing studies97,100,294,344
demonstrate promising results, and it would seem that despite the
additional cost that these modalities impose, the expected benefits
from their use in time and trauma management effectiveness are
significant. The advantages and pitfalls of these new tools have to be
further evaluated in comparison to current practice, and their use has
to be incorporated in specific protocols. They also need to be
investigated regarding their effect on the overall outcome of the
injured patient and on the rationalization of acute trauma management.
If the obstruction is subglottic emergency cricothyroidotomy or
tracheostomy can be lifesaving. Obstruction of the trachea in the
region of the mediastinum can also cause severe respiratory impairment.
This can lead to severe mediastinal emphysema and tracheal perforation.
The next priority is to maintain respiration, which can be compromised
by thoracic or central nervous dysfunction. Disorders of the
respiratory system can be diagnosed clinically from symptoms and signs
including dyspnea, cyanosis, stridor, depressed conscious level,
abnormal chest expansion, and the presence of major thoracic injuries.
Thoracic injury can cause acute respiratory derangement, including lung
contusion, tension pneumothorax, and hemothorax. Tension pneumothorax
is an acute life-threatening condition. The management of pneumothorax
and hemothorax
should include the insertion of a chest drain to decompress the chest.
|
TABLE 9-8 Conditions That Cause Airway Obstruction
|
|||||
|---|---|---|---|---|---|
|
or secondary myocardial infarction. Alternatively, isolated blunt
thoracic trauma may cause high-pressure edema, which has been observed
following thoracic compression. Management of these two conditions
differs. One requires fluid replacement therapy and the other requires
the use of diuretics. However, the initial management of both types of
edema involves continues suction and the use of PEEP pressures.
impairment, which can be best verified through the use of PCS. Severe
shock may result in severe cerebral hypoxia and subsequent respiratory
impairment. It is important that the emergency physician does not
underestimate the effect of hemorrhagic shock. Continuous observation
of the spontaneously breathing patient with minor injuries can be
justified in these cases. In the severely or multiply injured patient,
immediate intubation and ventilation for adequate oxygenation are
indicated. A tidal volume of 8 to 10 mL/kg of body weight, PEEP of 5
mL, and 50% O2 saturation of the air are prerequisites for adequate ventilation.
immediate management of posttraumatic shock while full evaluation of
respiratory, neurologic, and cardiovascular status is ongoing.
Prolonged shock can lead to further posttraumatic complications and
therefore impact negatively on the patient’s prognosis. Two large-bore
intravenous cannulas should be inserted during the preclinical phase
and rapid fluid replacement therapy should commence as soon as
possible. The cannulas are usually placed in the antecubital fossas and
fastened securely to prevent dislodgement.
intravenous lines can be inserted as appropriate. Single internal
jugular or subclavian vein lines have the disadvantage of being too
long and narrow to allow rapid transfusion of large amounts of fluid.
If lines in the peripheral veins are not feasible, venous cutdown can
be conducted by using the long saphenous vein around the ankle.
Historically, crystalloid solutions were considered unsuitable as they
were rapidly lost from circulation with plasma or serum being
preferred. In the 1960s, it was discovered that resuscitation with
crystalloid solutions was associated with lower rates of renal
impairment and mortality.
space occurred because of edema formation and required additional
replacement. Therefore, infusion of a combination of crystalloid and
blood at a 3:1 ratio was recommended. The application of these
principles, particularly in military conflicts, coincided with the
emergence of “adult respiratory distress syndrome” or “shock lung” as a
clinical entity in survivors of major trauma. Whether this was a
consequence of large-volume crystalloid infusion was unclear. Interest
in the use of colloid products was therefore renewed. However, early
results were conflicting, partly because of shortcomings in trial
design. Meta-analyses of these smaller studies revealed no overall
difference in the rate of pulmonary insufficiency following
resuscitation with either fluid type. Moreover, when final mortality
was considered, particularly in the subgroup of trauma patients, a
significant improvement in the overall survival rate was observed in
the group administered crystalloid.60,327
Crystalloid fluid is, therefore, considered to be the first treatment
choice in most centers and is particularly favored in U.S. trauma
centers. Ringer’s lactate has various theoretical advantages over
isotonic saline, although clinical trials have not shown differences in
outcome. Research into fluid selection for resuscitation is ongoing,
particularly as much early evidence is based on the use of albumin as a
colloid. Since then, newer products with higher molecular weights have
become available that should be more efficient in maintaining fluid in
the intravascular space. There is further evidence, however, that in
cases of severe hemorrhagic shock, increased capillary permeability
allows these molecules to leak into the interstitium, worsening tissue
edema and oxygen delivery.242
administration of hypertonic saline was as effective as large volume
crystalloid have provoked considerable interest in potential clinical
applications.241 This effect was enhanced by combination with dextran.347
Although improvements in microvascular circulation were observed, this
also appeared to increase bleeding. A meta-analysis of early clinical
trials revealed that hypertonic saline offered no advantage over
standard crystalloid resuscitation, although hypertonic saline dextran
might.390 This effect was
particularly striking in those with closed head injury, and further
animal studies have revealed that hypertonic saline can increase
cerebral perfusion while decreasing cerebral edema.339
lost prior to admission is usually unclear. Furthermore, the
identification of external sites of hemorrhage should not distract from
a rigorous search for internal bleeding, the identification of which
can be more problematic. Internal blood loss should be suspected in all
patients, particularly where shock is recalcitrant. This usually occurs
in the thorax, abdomen, or pelvis. Differentiation of the site of
internal bleeding can usually be made by using a combination of
clinical judgment, thoracic and pelvic AP radiographs, and abdominal
ultrasonography. Abdominal ultrasound should be conducted in the first
few minutes of the patient’s arrival to the emergency department where
this is possible. Increasingly, emergency department and trauma
personnel are being trained in ultrasound examination and appropriate
equipment is being made available.
the pulse, blood pressure, capillary refill, and urine output. In the
severely injured or complex patient, invasive techniques including
invasive arterial monitoring and central venous or pulmonary artery
pressure recording should be considered at an early stage. Although
controversy still exists in specific situations, current goals include
normalization of vital signs and maintenance of central venous pressure
between 8 and 15 mm Hg. Serial recording of acid-base parameters, the
base excess and serum lactate in particular, have been shown to be a
particularly useful in assessing response to therapy and detecting the
presence of occult hypoperfusion in apparently stable patients.24,62,234 Ongoing requirement for blood transfusion
should be monitored by regular measurement of the hemoglobin
concentration. This can be rapidly estimated, where necessary, using
the majority of bedside arterial blood gas analyzers. Ongoing excessive
fluid or blood requirement should always prompt a repeated search for
sources of hemorrhage. Shock treatment is a dynamic process, and in
cases where there is ongoing bleeding, surgical intervention is often
indicated.
of cardiovascular status have been introduced including gastric
tonometry, near infrared spectroscopy, transthoracic impedance,
cardiography, central venous oximetry, and skeletal muscle acid-base
estimation. Many of these techniques remain experimental and they are
currently not available on a widespread basis. They may be available in
certain centers and expert advice is essential.
volume but also to preserve the patient’s oxygen carrying capacity. In
cases of massive hemorrhage, this will inevitably require the
replacement of red blood cells. Furthermore, lost, depleted, and
diluted components of the coagulation cascade will also require
replacement. However, it should be noted that it is becoming
increasingly apparent that, particularly in young healthy trauma
victims, much lower hemoglobin concentrations than previously thought
optimal are tolerated and indeed may be beneficial.292
Not only is blood a precious resource, but transfusion also carries the
risk of various complications including the transmission of infective
agents. Traditionally, target hemoglobin concentrations of 10 g/L were
advocated, but it has recently been shown that concentrations as low as
5 g/L are acceptable in normovolemic healthy volunteers.399
Randomized trials in selected normovolemic intensive care patients
showed that the maintenance of hemoglobin concentrations between 7 and
9 g/L resulted in equivalent and perhaps superior outcomes to
maintenance above 10 g/L154 and transfusion requirement has been shown to constitute an independent risk factor for mortality in trauma.221 This may be related to the potential of blood products to cause an inflammatory response in the recipient.3,153
Ideally, fully cross-matched blood should be used but in an emergency
universal donor O-negative blood can be utilized immediately. A sample
should be drawn for cross-match prior to administration as the
transfusion of O-negative blood can interfere with subsequent analysis.
The blood bank should be able to deliver type specific blood within 15
to 20 minutes of the patient’s arrival in the emergency department.
This blood is not fully cross-matched and therefore still carries a
relative risk of transfusion reaction. Cross-matched blood should be
available within 30 to 40 minutes in most cases. Administration of
platelets, fresh-frozen plasma, and other blood products should be
guided by laboratory results and clinical judgment. Expert hematologic
advice is often required.83,145 Procoagulant therapy for severe coagulopathy remains experimental, although early results are promising.
blood transfusion are becoming increasingly relevant, but so far no
convincing evidence has been found that tetrameric polymerized human
hemoglobin can be used on a routine basis.242
Instead, the use of factor VII appears to be a promising alternative in
patients who present with uncontrollable coagulopathy if there is no
surgical source of bleeding. The first randomized prospective trial
documented that no severe side effects appear to occur in trauma
patients. In addition, numerous case reports where the substance was
applied as a bailout demonstrated that it appeared to have an acute,
yet difficult to prove, effect.227,331
causes of shock such as cardiogenic and neurogenic shock. The presence
of flat jugular veins might indicate the presence of hemorrhagic shock.
An elevated jugular venous pressure (JVP) can be diagnostic of
cardiogenic shock, caused by coronary heart disease, myocardial
infarction, cardiac contusion, tension pneumothorax, or cardiac
tamponade. To establish this diagnosis, the insertion of a pulmonary
artery catheter may be necessary.
neurogenic shock, and it is usually because of spinal injury. Loss of
autonomic supply leads to a decrease in vascular tone with blood
pooling in the peripheries. This can occur without significant blood
loss. The resultant increase in skin perfusion leads to warm
peripheries and a decrease in central blood delivery. This type of
shock may be difficult to distinguish from hypovolemia.
attention and often immediate surgical intervention. The heart can be
impaired by cardiac tamponade, tension pneumothorax, and hemothorax or,
in rare cases, by intra-abdominal bleeding. These pathologies may
necessitate immediate surgical intervention including placement of a
chest drain, pericardiocentesis, or emergency thoracotomy. If there is
indirect impairment of cardiac function, medical treatment should be
introduced and normovolemia should be restored. A raised JVP in
cardiogenic shock may be the result of right-sided heart failure. This
should be confirmed by measurement of the central venous pressure.
Impaired right-sided cardiac function may result in blood pooling in
the pulmonary system. This can be difficult to distinguish from
peripheral blood loss. The two can coexist and may impair cardiac
function. Conditions that cause this include cardiac tamponade, tension
pneumothorax, myocardial infarction, and cardiac contusion.
with an elevated central pressure and a decreased peripheral systemic
pressure should alert the treating physician to the possibility of
cardiac tamponade. A normal chest radiograph may not rule out this
possibility, but ultrasound can provide an immediate diagnosis. The
treatment of this condition should include emergency
pericardiocentesis. Following aspiration of 10 mL of fluid from the
pericardial sac, an immediate improvement of the heart stroke volume is
seen with an increase in the peripheral systemic perfusion. Emergency
thoracotomy is rarely indicated. If required, it can be performed
through an incision between the fourth and fifth ribs on the left side,
and then by opening the pericardium in a craniocaudal direction to
avoid injury to the phrenic nerve. One or two transmural stitches allow
temporary cardiac closure and cardiac massage can then be conducted.
and a rapid deterioration of respiratory function, and it can cause
acute right ventricular failure. As the condition progresses, raised
intrathoracic pressure causes reduced right-sided venous return to the
heart. As mediastinal shift occurs, kinking or obstruction of the vena
caval system can lead to complete obstruction resulting in cardiac
arrest. Rapid diagnosis followed by immediate decompression is a
lifesaving measure.
independent from the trauma. This diagnosis should be considered in
elderly people after road traffic accidents. In these patients, MI may
have been caused by hypovolemia, hypoxia, or the acute release of
catecholamines in the bloodstream at the time of the accident or,
alternatively, the MI may have occurred incidentally and actually
caused the accident. A diagnosis of MI can be confirmed from acute
changes on the ECG and an increase of blood markers (CK-MB). The
treatment of MI should include medical therapy to control arrhythmias.
Patients with MI should be treated in the ICU with continuous
monitoring from the medical team.
MI. Contusion is usually seen following a blunt anterior thoracic wall
trauma associated with a fracture of the sternum. Differentiating this
condition from MI in the acute setting is of secondary importance to
the initial management as both diagnoses require similar management,
including control of cardiac arrhythmias and heart failure with
continuous invasive monitoring.
important for the emergency physician to evaluate their neurologic
status fully prior to this. The size and reaction of the pupils are
important indicators of the presence of any central impairment and
abnormal pupillary reaction and size may be seen. The light reflex
reflects the function of the second and third cranial nerves, the
oculocephalic reflex depends on the integrity of the third and fourth
cranial nerves and the corneal reflex represents intact fifth and
seventh cranial nerves. The GCS also provides important information
regarding the neurologic status of patients, particularly where serial
measurements are possible. It can provide a useful aid in clinical
decision making:
|
TABLE
9-9 Use of Preexisting Classification Systems to Assess Whether Patients Are Stable or Can Be Stabilized to Permit Definitive Fracture Fixation* |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
be performed if the GCS score is less than 10, and if the GCS score is
less than 8, continuous intracranial pressure monitoring may be
necessary. This indication represents a rough estimate and the severity
of impact and the clinical condition of the patient should also be used
for evaluation.
complete, patients should be placed into one of four categories in
order to guide the subsequent approach to their care. This is done on
the basis of overall injury severity, the presence of specific
injuries, and current hemodynamic status as detailed in Table 9-9.
Any deterioration in the patient’s clinical state or physiologic
parameters should prompt rapid reassessment and adjustment of the
management approach as appropriate. Achieving end points of
resuscitation is of paramount importance for the stratification of the
patient into the appropriate category. End points of resuscitation
include stable hemodynamics, stable oxygen saturation, lactate level
less than 2 mmol/L, no coagulation disturbances, normal temperature,
urinary output greater than 1 mL/kg/hr, and no requirement for
inotropic support.
injuries, respond to initial therapy, and are hemodynamically stable
without inotropic support. There is no evidence of physiologic
disturbance such as coagulopathy or respiratory distress or ongoing
occult hypoperfusion manifesting as abnormalities of acid-base status.
They are not hypothermic. These patients have
the
physiologic reserve to withstand prolonged operative intervention where
this is appropriate and can be managed using an early total care
approach, with reconstruction of complex injuries.
initial resuscitative attempts but have clinical features, or
combinations of injury, which have been associated with poor outcome
and put them at risk of rapid deterioration. These have been defined as
follows:
-
ISS greater than 40
-
Hypothermia below 35° C
-
Initial mean pulmonary arterial pressure
greater than 24 mm Hg or a greater than 6 mm Hg rise in pulmonary
artery pressure during intramedullary nailing or other operative
intervention -
Multiple injuries (ISS greater than 20) in association with thoracic trauma (AIS greater than 2)
-
Multiple injuries in association with
severe abdominal or pelvic injury and hemorrhagic shock at presentation
(systolic BP less than 90 mm Hg) -
Radiographic evidence of pulmonary contusion
-
Patients with bilateral femoral fracture
-
Patients with moderate or severe head injuries (AIS 3 or greater)
early total care approach, but this should be undertaken with caution
and forethought given to operative strategy should the patient require
a rapid change of treatment rationale. Additional invasive monitoring
should be instituted and provision made for ICU admission. A low
threshold should be used for conversion to a damage control approach to
management, as detailed later.
initial intervention are at greatly increased risk of rapid
deterioration, subsequent multiple organ failure, and death. Treatment
in these cases has evolved to utilize a “damage control” approach. This
entails rapid lifesaving surgery only if absolutely necessary and
timely transfer to the intensive care unit for further stabilization
and monitoring. Temporary stabilization of fractures using external
fixation, hemorrhage control, and exteriorization of gastrointestinal
injuries where possible is advocated. Complex reconstructive procedures
should be delayed until stability is achieved and the acute
immunoinflammatory response to injury has subsided. This rationale is
intended to reduce the magnitude of the “second hit” of operative
intervention or at least delay it until the patient is physiologically
equipped to cope.
severe injuries, and often have ongoing uncontrolled blood loss. They
remain severely unstable despite ongoing resuscitative efforts and are
usually suffering from the effects of a “deadly triad” of hypothermia,
acidosis, and coagulopathy. A damage control approach is certainly
advocated. Only absolutely lifesaving procedures are attempted in order
not to exhaust the biological reserve of these patients. The patients
should then be transferred directly to intensive care for invasive
monitoring and advanced hematologic, pulmonary, and cardiovascular
support. Orthopaedic injuries can be stabilized rapidly in the
emergency department or ICU using external fixation and this should not
delay other therapy. Further reconstructive surgery is delayed and can
be performed if the patient survives.
revolutionized the early radiologic diagnostics in most Level I trauma
centers. Nowadays, the availability of such imaging is standard of care
in these institutions. Nevertheless, many other diagnostic tools are
available to allow a complete picture of all injuries. While clinical
examination and judgment still represent the fundamental basis of
contemporary trauma management, the role of emergency radiology is
continuously expanding and evolving. In the current emergency trauma
setting, the 24/7 availability and immediate proximity of emergency
radiology units (ERUs) to the emergency departments are considered
essential. The architectural design and the infrastructure planning
demand close coordination of the four components of acute trauma
services, these being the resuscitation room, emergency radiology unit,
trauma theaters, and intensive trauma unit.98,227,406
setting in most of the institutions that have adopted the ATLS
concepts. It consists of the standard three radiographs: AP radiographs
of the chest and pelvis and a lateral radiograph of the cervical spine.
These are often taken using portable bedside radiograph machines as
adjuncts of the primary survey. These are followed by abdominal
ultrasound, and in many cases by CT scans and additional plain
radiographs of the extremities. This standard protocol represents the
international consensus of dedicated trauma centers or trauma-treating
general hospitals worldwide.
considered necessary because an urgent intubation may be required and
the patient’s GCS score may not allow for clinical screening. A bedside
lateral C-spine radiograph is considered accurate enough for severe or
unstable fractures and fracture dislocations but has significant
disadvantages in identifying more subtle fractures or clearing the
thoracocervical area.197,278
of the three initial bedside radiographs. Its sensitivity is very high
(greater than 95%) for identifying a large hemothorax, flail chest,
pneumothorax, hemomediastinum, pulmonary contusions, and lacerations.
However, its specificity is quite low and there are injuries that are
likely to be missed, such as diaphragm ruptures or a small hemothorax.48,253,334
phases of trauma evaluation and management has received most criticism.
In general, pelvic trauma occurs in the multiply injured population who
present with potential hemodynamic compromise and, in fact, it can be
used as a paradigm of polytrauma.110
Now that CT scanning is widely used in the secondary survey of
moderately or severely injured trauma patients, it is considered by
many that the routine bedside pelvic radiography for hemodynamically
stable patients may be abandoned. In contrast, in hemodynamically
unstable patients, it is still considered a useful screening tool to
facilitate early notification of the orthopaedic
team
and the interventional radiologist. It also encourages the use of
simple measures of reduction of the disrupted pelvis such as pelvic
binders, plain sheet wrapping, or keeping the lower extremities
adducted in internal rotation.281,301
to offer certain advantages even in the acute setting of the
resuscitation room.48 Recently, the
use of total-body radiographs in the acute evaluation of multiply
injured patients was introduced. Despite the escalating role of modern
CTs, this new technology appears to offer additional vital and quick
information in the resuscitation room setting. This new technology is
based on an enhanced linear slot-scanning device that produces high
quality radiographic two-plane whole body images of any size in
seconds. It has been evaluated in a number of centers,16,247 and its usefulness is expected to be proven in the near future.
acute trauma setting and is considered a vital tool in the hands of the
trained emergency physician or trauma surgeon.317
Although it is operator dependent, it can be carried out at the
patient’s bedside and is quick and noninvasive and can be easily
repeated. The focused assessment with sonography for trauma (FAST)
scan, introduced in 1990, offers a quick, comprehensive, and sensitive
method of detecting free intra-abdominal fluid, or pericardial
effusion. It has a number of useful views, which are detailed in Table 9-10.
The reported sensitivity for intraabdominal free fluid is high (70% to
98%) but is highly dependent on the volume of the free fluid and on the
completion of scanning of all the areas listed in Table 9-10.37,190 As far as the diagnosis of solid organ injuries is concerned, its sensitivity is poor (45% to 85%).262 Its specificity for either free fluid or visceral injuries is high (86% to 100%).37,190 It has been shown to be more sensitive in detecting pneumothorax than plain radiographs,350 and it is excellent in detecting cardiac injuries and pericardial collections (97% to 100%).316
Its limitations are mainly in the identification of solid organ
injuries, which are usually underestimated. The technique is highly
operator dependent,51 and it requires free access to the anatomic areas listed in Table 9-10. Its accuracy is also affected by patient movement.250,326
widespread in the 1980s, its contribution has been immense. When used
in combination with the ATLS secondary survey, it is the best method of
detecting head, spine, chest, and abdominal injury. Its disadvantages
are the length of time it takes to transfer and scan the patient and
then assess the images, its inaccuracy in noncompliant patients, and
its radiation dose.405 Currently, in
certain trauma centers, its use is moving to even earlier phases of
acute trauma management. The use of intravenous contrast enhancement,
the advances in software, and the image reconstruction ability of
modern scanners have shortened the duration and significantly enhanced
the quality of a trauma whole body scan. In particular, the
contemporary MSCT scanners are able to produce high-quality whole body
images in only a few minutes.400
|
TABLE 9-10 Views Used in FAST Scans*
|
||
|---|---|---|
|
diagnostic evaluation have certain disadvantages. This is particularly
true for blunt trauma. The standard clinical examination has a
diagnostic accuracy for abdominal trauma of about 60% to 65%.219,336
Deep peritoneal lavage (DPL), despite its high sensitivity, has a low
specificity and was replaced by FAST; although FAST is limited by the
fact that it provides an overview of intra-abdominal trauma rather than
an accurate diagnosis. Whole body scanning offers additional diagnostic
information regarding the head, spine, pelvis, and chest. Thus, MSCT
minimizes the time to accurate diagnosis, this being particularly true
in hemodynamically stable patients.155
Another advantage of this new CT scanning modality is that it can also
be used after a more traditional approach involving an initial bedside
chest radiograph, a FAST scan, and the initial resuscitation of the
unstable patient, or even after urgent surgery of the patients in
extremis.282,311
The evidence related to the new MSCT-based protocols is encouraging,
especially for intubated, sedated, and hemodynamically stable patients.
Nevertheless, further proof from well-designed randomized prospective
trials is needed before radically changing the established ATLS
protocols.
central role in the diagnosis and management of injured patients. It
constitutes the gold standard in the detection of traumatic aortic and
vascular injuries. In addition to the detection of these
life-threatening injuries, it offers the possibility of intervening to
halt the hemorrhage, although this requires the presence of a trained
vascular radiologist.103,142
Its inherent disadvantages are the infrastructure that is required, the
low rate of allergic reactions to the contrast, the requirement for an
experienced vascular radiologist given the inconsistent time schedule
of trauma, and, most important, its duration, this being the time taken
to transfer the injured patient to the angiographic suite and perform
the investigation and any intervention that are required.
and more recently, it has been used as the ultimate salvage procedure
in cases where hemodynamic instability persists even after a laparotomy
or thoracotomy has been undertaken.321,375 It is often used successfully in patients with pelvic trauma,387 arterial injury,138,289,379 and abdominal solid organ injuries.19,211,240,330,348
used in acute trauma management are either embolization of moderate to
small vessels and injured solid organs, with gelfoam slurry
or
coils, or percutaneous endovascular stenting of larger vessels. They
are considered to be procedures that are associated with minimal risk,
especially in trauma management where they may be potentially
lifesaving interventions. Currently, the protocols of angiography with
or without radiologic vascular intervention vary significantly between
different designated centers and trauma care systems. Controversies
center on the main issues that have already been discussed these being
the timing of its use, the availability of a vascular radiologist, the
financial implications and its long-term consequences. Further
prospective randomized studies are required to investigate its use.
lifesaving. Examples of conditions requiring emergent operative
treatment that does not permit the use of prolonged diagnostic
procedures are cardiac tamponade, arterial injuries to major vessels,
and head trauma with imminent incarceration. Furthermore, injuries to
cavities associated with severe hemorrhage and shock must be addressed
promptly. Close communication within a cooperative multidisciplinary
approach is, therefore, crucial.
radiograph. However, in the presence of extensive lung contusion or
atelectasis, the diagnosis can be difficult. Ultrasound examination has
shown the potential to identify free thoracic fluid, although CT
remains the gold standard and often reveals the source of any bleeding.1
resultant hemothorax is treated during the primary survey by the
insertion of a chest tube. Usually, the indication for a chest tube
comes from examination of the chest radiograph and only occasionally
are clinical findings the sole basis for chest tube insertion, as a
chest radiograph can usually be performed very rapidly. It is standard
practice to insert the chest tube in the mid-axillary line at the fifth
intercostal space. Lower insertion risks injury to the diaphragm or
intra-abdominal organs. Blunt dissection should be undertaken to
prevent further injury to other organs. This is important, even where
the operator is confident of correctly positioning the chest drain, as
intraabdominal injuries may lead to increased intra-abdominal pressure
and diaphragmatic elevation or even rupture.
should be used to drain a hemothorax. Modern percutaneous drains used
in thoracic medicine are not sufficient for this purpose. The large
diameter reduces the risk of coagulation and allows for rapid blood
evacuation, and the surgeon can be relatively confident that the
drained contents are representative of thoracic blood loss. It is usual
to direct the tube caudally to drain blood and cranially in the
presence of a pneumothorax.
thoracic hemorrhage. In most cases, bleeding is the result of injury to
one intercostal vessel and this will usually arrest spontaneously.
Indications for emergency department thoracotomy remain controversial,
although recognized indications include traumatic arrest and
recalcitrant profound hypotension in penetrating trauma, rapid
exsanguination with more than 1500 mL of blood initially or 250 mL/hr
after chest tube insertion and unresponsive hypotension in blunt
thoracic trauma. As a last resort, catastrophic subdiaphragmatic
hemorrhage may be controlled by cross-clamping the aorta. These
interventions are regarded as useless in patients who present with
blunt thoracic trauma and where there has been no witnessed cardiac
output or in patients with severe head injuries. There is recent
evidence that increased caution should be used before undertaking
emergency thoracotomy in blunt trauma patients for all indications,
particularly in the emergency department, because of a relatively high
rate of nontherapeutic procedures and poor outcome.11,165
aorta is commonly diagnosed erroneously because of poor-quality chest
radiography obtained in emergency situations with a supine position and
insufficient inspiratory effort. The mediastinal enlargement observed
on chest radiographs is often rather nonspecific. Clinically, one
should pay careful attention to the presence of dilated jugular veins,
which helps to differentiate cardiac from aortic injuries. However,
further imaging should be rapidly obtained in the hemodynamically
stable patient, ideally using contrast-enhanced thoracic CT.
Angiography has been regarded as the gold standard in diagnosis, but
although traditional CT scanning sometimes leads to false-positive
results, many centers now prefer contrast-enhanced high-resolution
spiral CT as an investigative modality.50,90,329
surviving long enough to reach the emergency department alive. In most
cases, the adventitia is preserved and further intrathoracic blood loss
is prevented by the parietal pleura. Furthermore, there is increasing
evidence that repair can be delayed in the presence of other
life-threatening injuries, and occasionally conservative management can
be successful.163,202,363 These patients should, however, always be treated in a center with an acute thoracic surgical service.
hemodynamically stable patients consists of permissive hypotension or
active reduction of blood pressure while controlling for a difference
in blood pressure between the upper and lower body parts. Indications
for immediate intervention include the development of hemodynamic
instability without an alternative explanation, hemorrhage via the
chest tubes of more than 500 mL/hr, or a blood pressure gradient
between upper and lower extremities leading to impaired perfusion of
the lower limbs (difference of mean blood pressure greater than 30 mm
Hg). Given the high mortality of emergency repair in cases of traumatic
aortic injury, there is increasing interest in the use of endovascular
stenting in such situations.169,201,322
presence of radiologic mediastinal abnormality, the diagnosis is
generally cardiac tamponade. Pericardiocentesis should be performed. If
there is acute decompensation, an emergent thoracocentesis is
indicated. Further diagnostic tests are too time consuming in this
immediately life-threatening situation. If the patient is still
hemodynamically stable, a very sensitive and readily available test is
the transthoracic echocardiogram.
should be regarded as part of the resuscitative effort and early intervention can be lifesaving.215
Bleeding is more common from multiple small vessels rather than from
injured major vessels and, because of the large volume of the
retroperitoneum, spontaneous arrest of the hemorrhage is unlikely in
severe cases.135 It is also common
for the retroperitoneum to be breached during the injury, further
decreasing the barriers to ongoing hematoma expansion. Treatment with
the pneumatic antishock garment or pelvic belt-straps can give some
temporary stabilization,394 but results are inconclusive and severe complications have been reported in relation to their use.
of selective angiography in these cases to embolize bleeding vessels,
this intervention is often time consuming to organize and perform.
Patients must be relatively stable and careful selection is crucial.
Embolization can be used as an adjunct to other interventions where
continued arterial hemorrhage is suspected. In severe injuries with
profound hemodynamic instability the use of external fixation, a pelvic
C-clamp and open tamponade by packing is recommended.358,366
With the patient supine, the area from the subcostal margin to the
pubic symphysis is prepared with the abdomen and pelvis completely
exposed. If a C-clamp has already been applied for posterior pelvic
instability, it should be rendered mobile. In a C-type injury with
vertical pelvic instability, the lower extremity of the appropriate
side should be accessible to allow reduction where necessary.
fluid, an external fixation device should be applied and a midline
laparotomy should be performed. The intra-abdominal organs should then
be examined for bleeding following standard management protocols for
blunt abdominal trauma (Fig. 9-5). If, however,
initial diagnostic imaging has shown no evidence of intraabdominal
fluid and a major source of pelvic hemorrhage is suspected, a lower
midline laparotomy can be used. Initial attention should be directed to
the retroperitoneum. Following the skin incision, ruptured pelvic soft
tissues are usually readily visible. Any hematoma is evacuated and the
paravesical space is explored for bleeding sources. Large bleeding
vessels should be ligated where possible. In diffuse bleeding,
well-directed packing with external stabilization is recommended.
dorsal source, particularly in cases of posterior pelvic instability,
attempts at further extraperitoneal exploration should be made in the
presacral region. Large bleeding sources can be identified and treated
appropriately. In cases of catastrophic arterial hemorrhage, temporary
control can be achieved by cross-clamping the aorta. Often in venous
hemorrhage, no single bleeding source is identifiable. Usually,
bleeding originates from disruption of the presacral venous plexus or
from the fracture site itself. Again, well-directed packing can often
adequately control hemorrhage. Recent studies have reported mortalities
rates between 25% and 30% following pelvic packing in unstable patients.70,377
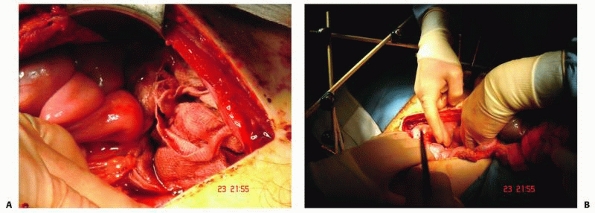 |
|
FIGURE 9-5 A. Midline laparotomy following application of an anterior external fixator. B. Packing of the lower abdominal cavity is shown to address retroperitoneal bleeding.
|
closure is performed and correction of physiologic derangements should
be undertaken without delay, with particular regard being paid to
coagulopathy and hypothermia. Packing is left in situ and changed
routinely at 24 to 48 hours, although in cases of suspected ongoing
hemorrhage and recalcitrant shock, earlier reintervention should be
considered. At planned revision, the cavity should be debrided as
required and any hematoma excised. It should then be thoroughly
examined for sites of ongoing hemorrhage. Further bleeding points can
be dealt with, but if diffuse hemorrhage persists, further packing
should be used and later planned surgical revision undertaken.
situations, with a number of compelling arguments being presented.
There is increasing evidence for conservative management of abdominal
injuries except in the most unstable patients and it should be
remembered that apparent intra-abdominal hemorrhage is often pelvic in
origin. Evacuating an intracranial hematoma, if the patient
exsanguinates, is obviously futile. However, there is equally little,
and some would say less, benefit in saving a patient’s life if the
result is profoundly disabling brain injury or death from tentorial
herniation. Once compensatory autoregulatory mechanisms are
overwhelmed, intracranial pressure rapidly increases. There is evidence
that in people with head injury, mortality from extracranial causes
alone is unusual. In a study
of
almost 50,000 trauma patients, 70% of deaths were attributed to the
head injury alone and only 7% to extracranial trauma, with the rest
caused by a combination of both.105
However, craniotomy should not be undertaken without imaging to confirm
an operable lesion except in very rare circumstances. CT scanning is
time consuming and can cause a significant delay in treatment. This
time might be better spent rapidly attempting hemodynamic
stabilization. There is also evidence that in hypotensive patients
undergoing head CT, emergency laparotomy is required far more
frequently than craniotomy (21% versus 2.5%).407
Furthermore, inferior outcome has been demonstrated in head-injured
patients with shock, suggesting that early correction of hypotension
may protect the patient from secondary brain injury.391
management decisions must be made and clinical experience is essential.
Thankfully, it would appear that such dilemmas seldom occur. In a
review of 800 patients with significant head and abdominal injuries, 52
required craniotomy, 40 required laparotomy, and only 3 required both.373
routinely performed, patients fared badly and the mortality rate
secondary to fat embolism syndrome and organ failure was high. The
major fear of surgeons treating these patients was the development of
fat embolism syndrome. Pulmonary dysfunction is the hallmark of this
syndrome and usually develops several days after trauma. Once the fat
embolism syndrome becomes full blown, treatment is often unsuccessful
and mortality rates of about 50% have been reported.15
intramedullary contents liberated from an unstabilized fracture. It was
therefore concluded that fixation of major fractures could prevent this
complication in addition to being an effective way of minimizing soft
tissue damage and ongoing blood loss. Multiple authors reported
dramatic improvements in the clinical condition when fracture fixation
was performed routinely.175,305,382
A decrease in the incidence of pneumonia and ARDS, a shorter stay in
the ICU, and better survival rates were reported. The first
prospective, randomized trial by Bone et al.27
demonstrated the advantages of early fracture stabilization; this is
now referred to as early total care (ETC). Patients with delayed
fracture stabilization had a prolonged duration of ventilatory therapy
and stayed longer in both intensive care and hospital.27,305
It was therefore accepted that a major aim of the treatment of the
multiple trauma patient with fractures was rapid stabilization of the
pelvic and extremity injuries. An essential prerequisite for ETC was
optimization of retrieval conditions and a reduction of the retrieval
time. Furthermore, the improvements of intensive care medicine with
improved cardiovascular monitoring and facilities for prolonged
ventilatory support facilitated the development of a more aggressive
surgical approach.
high ISS, brain injury, or severe chest trauma limited discussion about
the best management for these polytraumatized patients. As it became
evident that these specific subgroups of polytraumatized patients do
not benefit from ETC, the borderline patient was identified. These
patients were demonstrated to be at particular risk of a poor late
outcome. The clinical and laboratory characteristics of the borderline
patient have been previously described.123
management of these borderline patients together with patients who were
unstable or in an extremis condition. The term damage control
was initially described by the U.S. Navy as “the capacity of the ship
to absorb damage and maintain mission integrity.” In the
polytraumatized patient, this concept of surgical treatment seeks to
control but not to definitively repair the traumainduced injuries early
after trauma. After restoration of normal physiology (core temperature,
coagulation, hemodynamics, respiratory status), the definitive
management of injuries is performed.341
The damage control concept consists of three separate components:
resuscitative surgery for rapid hemorrhage control, restoration of
normal physiologic parameters, and definitive surgical management.
first stage involves early temporary stabilization of unstable
fractures and the control of hemorrhage. The second stage consists of
resuscitation of the patients in the ICU with optimization of their
condition, and the third stage involves delayed definitive fracture
management when the patient’s condition allows. The most popular tool
of the trauma surgeon, to achieve temporary stabilization of a
fractured pelvis or long bone, is the external fixator. External
fixation is a quick and minimally invasive method of providing
stabilization, and it can be very effective in accomplishing early
fracture stabilization but postponing the additional biological
stresses posed by prolonged surgical procedures. The delayed definitive
procedure used for the stabilization of long bone fractures, and in
particular the femur, is most frequently intramedullary nailing, which
is carried out when the condition of the patient allows. Recent studies
have reported that the damage control orthopaedics approach was a safe
treatment method for fractures of the shaft of the femur in selected
multiply injured patients.261,284,325 The application of DCO in multiply injured patients is illustrated in Figure 9-6.
chest, and pelvis who present with life-threatening hemorrhage, an
acute change in the clinical condition may rapidly occur.
Unfortunately, no level I study is available for these patient subsets.
The EAST evidence-based work group conducted a systematic review of the
literature regarding the timing of fracture fixation in different
subsets of patients with multiple trauma.91
They concluded that there is no compelling evidence that early long
bone stabilization either enhances or worsens outcome for patients with
severe head injury or for patients with associated pulmonary trauma.
While the available data suggest that early fracture fixation may
reduce associated morbidity for certain patients with polytrauma, the
work group stopped short of recommending early fixation for all
patients. It is questionable whether a level I study can be obtained in
these variable patient groups.
damage control orthopaedics attempts to reduce the biological load of
surgical trauma in the already traumatized patient. This hypothesis was
assessed in a prospective randomized study by means of measuring
proinflammatory cytokines. Clinically stable patients with an ISS
greater than 16 and a femoral shaft fracture were
randomized
to ETC this being primary intramedullary nailing of the femur within 24
hours and damage control orthopaedics where the femoral fracture was
initially stabilized with an external fixator and subsequently by
intramedullary nailing. A sustained inflammatory response (higher
levels of IL-6) was measured after primary intramedullary femoral
nailing but not after initial external fixation followed by secondary
conversion to an intramedullary implant. The authors concluded that
damage control orthopaedic surgery appears to minimize the additional
surgical impact induced by the acute stabilization of the femur.283
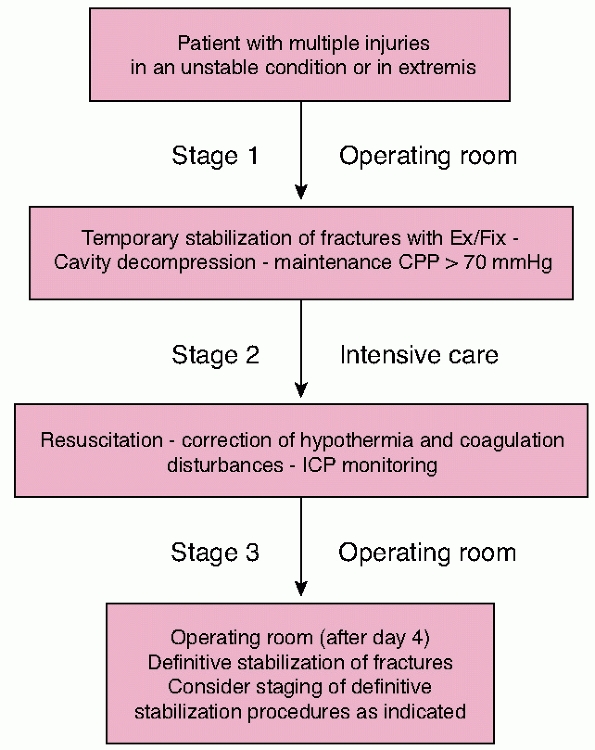 |
|
FIGURE 9-6 The staging of damage control orthopaedics (DCO).
|
DCO concept include the ideal timing of secondary definitive surgery
and whether it is safe to convert an external fixator to an
intramedullary nail or whether this associated with an unacceptably
high infection rate. It has been shown that days 2 to 4 do not offer
optimal conditions for definitive surgery. In general, during this
period, marked immune reactions are ongoing and enhanced generalized
edema is observed.398 Nevertheless,
these patients represent a highly diverse group and individual clinical
judgment is more reliable, especially when combined with information
from the newer laboratory tests. In a retrospective analysis of 4314
patients treated in our clinic, it was found that a secondary procedure
lasting longer than 3 hours was associated with the development of
MODS. Also, the patients who developed complications had their surgery
performed between days 2 and 4, whereas patients who did not go on to
develop MODS were operated between days 6 and 8 (P <0.001).285
can be converted safely to an intramedullary nail, the infection rates
reported in the literature are low ranging from 1.7% to 3%.261,325
According to these reports, conversion of the external fixator to a
nail should be done within the first 2 weeks as this minimizes the risk
of developing deep sepsis.
|
TABLE 9-11 Indications for Early Total Care
|
|
|---|---|
|
mediators has been shown to be sensitive in predicting the clinical
course, morbidity, and mortality in trauma patients.65,170,203
Based on the latest available studies, recommendations can be made in
terms of patient selection for ETC and DCO. These are shown in Tables 9-11 and 9-12.
patients with multifocal injuries to an extremity is a crucial part of
the management concept. Some areas of the body are prone to progressive
soft tissue damage because of their anatomy. Therefore, the recommended
sequence of treatment is tibia, femur, pelvis, spine, and upper
extremity.
extremity injuries should be considered. Initially, trauma surgeons
must undertake the shortest possible interventions in these patients to
minimize the second-hit phenomenon.107,108
The simultaneous employment of different surgical approaches and
different specialties, where this is feasible, will minimize the risks
and facilitate the transfer of the unstable patient to the controlled
environment of the ITU as soon as possible. Even at the later
reconstructive phase of treatment, it may well be possible to undertake
simultaneous operations at different anatomic sites. This is
straightforward if there are contralateral fractures of the upper and
lower extremities or a combination of facial/ thoracic and lower
extremity trauma. These combinations of injuries allow two different
surgical teams, such as orthopaedic and plastic surgeons or orthopaedic
and maxillofacial surgeons, to work together, minimizing the duration
of anesthesia,
the surgical stress on the injured, and at the same time optimizing the operation theatre time and the financial implications.108,113,281
|
TABLE 9-12 Indications for “Damage Control” Surgery
|
||||
|---|---|---|---|---|
|
definitive, specialized care for the injured in the shortest possible
time, the preservation of high standards in acute trauma services
implies complex clinical capabilities, particular infrastructure
logistics, and, even more important, algorithms facilitating
simultaneous activity in diagnosis and treatment. Unfortunately, the
existing evidence regarding the role of simultaneous surgery for
polytraumatized patients is not of adequate quality and quantity to
justify generalized conclusions. However, it is anticipated that
further study will define its role.
surgeon should be aware of the overall distribution of the fractures
rather than merely consider each fracture as an isolated problem. Even
though an early definitive osteosynthesis would be preferred in all
fractures, the general status of the multiply injured patient or the
local fracture conditions may not permit this. In these cases, it is
recommended that careful immobilization of diaphyseal fractures is the
first phase of fracture management.
joints, and urgent open reduction and fixation is impossible,
transarticular external fixation (TEF) should be performed. In any case
with concomitant vascular injury or any evidence of a developing
compartment syndrome, a fasciotomy should be undertaken.
ipsilateral distal femoral and proximal tibial fractures, known as a
floating knee, a similar flexible but nonetheless structured and
priority-oriented management system should be applied. The overall
clinical status of the patients is crucial to the implementation of
this concept. If the floating knee occurs in a stable patient, the
femoral fracture can be treated with a retrograde unreamed nail
inserted through a small incision in the knee joint, which is flexed at
30 degrees. An antegrade tibial nail can then be inserted through the
same incision. If the same fracture pattern occurs in an unstable
patient, the fractures are best treated by the application of a
transarticular external fixator, which spans both fractures. This is
performed as a temporary stabilizing procedure to minimize additional
damage, especially to the soft tissues. A secondary definitive
osteosynthesis can be done, when the patient has safely recovered from
the initial potentially life-threatening injuries. During the whole
procedure, good communication between the anesthesiologist and the
surgeon is very important, because the procedure may have to be adapted
to any change in the patient’s vital parameters.
priorities of treatment are often dictated by the state of the soft
tissues. A high priority is given to femoral head and talar fractures.
Other periarticular fractures have a lower priority unless complicating
factors such as vascular dysfunction, compartment syndrome, or an open
wound are evident. The apparently “minor” injuries to the hand,
fingers, tarsus, and toes are also important. They should also be
considered in the overall management concept and treated appropriately.
Particularly in bilateral tibial fractures, both legs are surgically
cleaned and draped at the same time. However, the operative procedure
is performed sequentially because of the problems of space and handling
inherent in the use of fluoroscopy. If the vital signs of the patient
deteriorate during the operation, the second leg may be temporarily
stabilized using an external fixator. Definitive osteosynthesis can
then be delayed until the general status of the patient is stabilized
again. The priorities in the treatment of bilateral fracture patterns
follow the evaluation of the injury severity with more severe injuries
being stabilized first.
injured patients is usually undertaken secondary to the treatment of
injuries to the head and trunk or of the lower extremity. If there is a
closed fracture of the upper extremity without any associated injury,
such as vascular or nerve damage or compartment syndrome, fractures of
the shoulder girdle, proximal humerus, and humeral shaft are can be
stabilized by a shoulder body bandage known as a Gilchrist bandage. If
definitive osteosynthesis is required, it may be performed during the
secondary management phase, possibly after further imaging. External
fixation is an alternative for the temporary stabilization of humeral
diaphyseal fractures, and transarticular external fixation may be used
to stabilize fractures about the elbow if definitive stabilization has
to be delayed. Primary management of fractures of the forearm wrist and
hand is often with a cast, but, again, temporary external fixator may
be used.
associated with a severe head injury or chest trauma (lung contusions)
require a specially modified strategy. We strongly recommend expanded
monitoring of respiratory function, ventilation (capnography), and
pulmonary hemodynamics. Additionally, intracranial pressure monitoring
is mandatory in patients with severe head injury.44
much easier if a standardized protocol is followed. A thorough clinical
and radiologic examination is essential for the assessment of pelvic
injuries. This examination is usually done during the initial
examination phase. As a consequence of this, the classification of the
pelvic injury may be approximate. However, sophisticated alphanumeric
classifications of pelvic injuries in this context are not of much use.
Instead, the simple AO classification, the ABC system (Fig. 9-7), can assist in the decision-making process.246
In this classification, type A injuries include stable fractures such
as fractures of the pelvic rim, avulsion fractures, and undisplaced
anterior pelvic ring fractures. The posterior rim is not injured at
all. Type B injuries comprise fractures with only partially intact
posterior structures and rotational dislocations may be possible.
Sometimes, this injury may initially be an internal rotational
dislocation resulting in excellent bony compression and stabilization
of the pelvis. On the other hand, they still carry a high risk of
intra-abdominal injuries. If the injury results in the open book type
of fracture with both alae being externally rotated, urogenital lesions
and hemorrhagic complications are much more common.
 |
|
FIGURE 9-7 Classification of pelvic ring fractures in A, B, and C type fracture similar to the AO classification.
|
be difficult, a CT scan of the pelvis is strongly recommended. If there
is no CT available, diagonal inlet and outlet radiographs may serve as
an alternative. In C type injuries, the pelvis shows translational
instability of the dorsal pelvic ring, such that the stabilizing
structures are all divided (Fig. 9-8). One or
both hemipelves are separated from the trunk. This injury is associated
with an extremely high rate of hemorrhagic complications and other
pelvic injuries.
implications. In type A injuries, operative treatment is generally not
required, whereas in type B injuries, adequate stabilization is
obtained by osteosynthesis of the anterior pelvic ring only. Type C
injuries require anterior and posterior osteosynthesis for adequate
stability.
injury has proved useful. Here, transsymphyseal, transpubic,
transacetabular, and transiliacal fractures are differentiated from the
transiliosacral and transsacral fractures. This process is easy to
memorize and requires a structured analysis of the radiographs. For
each of the injured regions, we have standardized the recommendations
for the osteosynthesis. Thus, an adequate management plan is available
for the small numbers of unstable pelvic fractures that will be seen.
Because more than 80% of unstable pelvic injuries are associated with
multiple injuries, stabilization in the supine position is preferred
during the primary period. Additionally, the supine position offers the
advantage of facilitating repair of both parts of the common combined
symphyseal and iliosacral rupture. Generally speaking, we recommend the
earliest stabilization possible for fractures of the pelvic ring to
avoid ongoing blood loss and to simplify ICU care and early ambulation.288
 |
|
FIGURE 9-8 Type C pelvic fracture. Three-dimensional computed tomography scan.
|
These injuries comprise about 10% of pelvic injuries and they are
associated with a significantly higher mortality of between 30% and
60%, in comparison with simple pelvic injuries. During the early phase,
hemorrhage is the most common cause of death. Later on, ARDS and MODS
occur as sequelae of blood loss, and initially persistent shock
determines the further course and eventual outcome of the patient.
priorityguided management concepts save the lives of these severely
injured patients and improve their prognosis. A variety of methods for
hemorrhage control in pelvic injuries are discussed in the literature.
With these techniques, several complex therapeutic protocols have been
developed. Our experience has resulted in a rather simple algorithm
requiring three decisions to be made within the first 30 minutes after
admission. The therapeutic goal is based on a combined strategy of
intensive shock treatment, early stabilization of the pelvic ring, and
potential operative hemorrhage control and packing rather than a single
treatment option. Once hemorrhage control is satisfactory, the
associated urogenital and intestinal injuries should be treated
expeditiously to avoid septic complications.
is the primary goal. During the first laparotomy, intraperitoneal
ruptures of the bladder are repaired. In injuries of the urethra, it is
recommended that the urethra be splinted with a transurethral catheter
in the acute phase and the definitive reconstructive procedure be
undertaken during the secondary period to reduce the rate of late
strictures. If early realignment is not possible, then a suprapubic
catheter should be inserted.
anus, a temporary colostomy of the transverse colon generally
guarantees proper excretion and safeguards the healing process in the
pelvis. At the end of the procedure, an extensive antegrade washout of
the distal part is assumed to reduce the microbial load. Any potential
muscular or skin necrosis is radically debrided to reduce the risk of
infection.
injuries in multiply injured patients is mandatory, if only for
intensive care nursing purposes. Nonoperative treatment using a plaster
jacket or a halo-body fixator is unsuitable for multiply injured
patients, because the immobilization of the patients carries a high
risk. Not only are the intensive care nursing procedures much easier
after internal stabilization, but also the period of immobilization and
the period of intensive care stay are significantly reduced. Spinal
fractures associated with neurologic dysfunction are usually stabilized
at the same time as the spinal cord is decompressed. However, in recent
years, there has been a move toward stabilizing more unstable injuries
of the spine in patients who present without neurologic symptoms for
the same reasons. It is our experience that after diagnosing an
unstable injury of the spine in a patient who does not have neurologic
symptoms, a closed reduction should be undertaken if there is a
fracture of the cervical spine or an AO type C rotational injury of the
lower thoracic or lumbar spines.
operating room just before the actual procedure. It is important to
realize that even if there is a slight suspicion that a fracture
fragment or a protruding intervertebral disc may narrow the spinal
canal after closed reduction, further diagnostic imaging with CT or MRI
should be carried out preoperatively.
reduction may be difficult because of coexisting extremity injuries. In
these cases, the proper correction of rotation and axis should be
obtained intraoperatively.
intervertebral disc, open reduction is always indicated to avoid spinal
cord compression.
management of the upper (C1-3) and lower (C4-7) cervical spine. The
patient’s head is fixed to a special reduction apparatus using the rim
of the halo fixator. In thoracic or lumbar spine injuries, associated
injuries to the chest and abdomen have to be considered. Nonetheless,
in our experience, injuries requiring dorsal and ventral stabilization
may usually be fixed with a dorsal internal fixator in the acute
management period. Depending on the general status of the patient, the
ventral stabilization may be performed during the secondary period.
Even intrathoracic or intra-abdominal injuries are not necessarily a
contraindication to the use of the prone position, which is required
for dorsal instrumentation. The prone position can even be used
successfully in patients with severe lung injury.
ETC or the DCO approach must be stabilized before being admitted to the
ITU. Stabilized fractures not only reduce pain but also minimize the
release of intramedullary contents into the circulation and secondary
damage to the soft tissues. Furthermore, nursing is easier and early
functional treatment can be initiated.
fractures is often difficult. A skin contusion over an otherwise closed
fracture may present more therapeutic and prognostic problems than an
inside-out puncture wound in an open fracture. Although the skin wound
may not be particularly impressive, this type of blunt injury can lead
to a significant weakening of the natural skin barrier. As necrosis is
the main complication of a skin contusion infection can occur,
particularly in the ICU environment. This issue has been addressed by
the development of a classification system that allows the clinician to
decide the appropriate therapeutic approach that would be beneficial to
the patient’s overall condition.267 The classification of soft tissue injuries is detailed in Table 9-13.
of open fractures are of paramount importance. It involves careful
assessment of the damage to the soft tissues, radical débridement,
extensive irrigation, and finally stable fracture fixation. Careful
assessment of the injury severity is the first step in the development
of a treatment strategy. The time and mechanism of injury, the energy
of the causative force, and the severity of the fracture should be
considered. The extent of any coexisting vascular and nerve damage and
the general condition of the patient are also of great importance. In
high-energy trauma, the soft tissues may be severely damaged and may
require careful evaluation and extensive débridement during the initial
assessment.
|
TABLE 9-13 Classification of Soft Tissue Injury in Closed Fractures267
|
|
|---|---|
|
usually associated with less soft tissue damage and may almost be
treated like closed injuries. After the initial débridement, the
fracture may be appropriately stabilized.
have the particular problem of extensive soft tissue damage combined
with significant bone destruction. This injury requires a graded
concept of care. The treatment plan considers an adequate debridement,
initial temporary stabilization followed by definitive secondary
stabilization, as well as the closure of the wound. Our experience with
this type of injury indicates that each fracture has almost unique
characteristics, which require individual management. In multiply
injured patients, the overall injury severity has to be considered, as
do the extent of the shock and the initial blood loss. Once these
factors have been taken into account, a clear therapeutic plan should
be established for each patient. Open fractures are discussed further
in Chapters 10 and 12.
years for the grading of open fractures, but the standard system of
classifying the soft tissue component of a fracture remains that of
Gustilo and Anderson.139 Despite the
doubts that have been raised over its reliability, it seems likely to
remain in common use as it is fairly simple to remember and to apply.
soft tissue damage is even more crucial. In this group, the prognosis
for the soft tissue damage depends on a multitude of parameters,
including tissue hypoxia, acidosis, and hypoperfusion of the
extremities caused by secondary to hemorrhagic shock. All these factors
should be taken into account in clinical decision making and planning.
techniques and a better appreciation of the usefulness of the Ilizarov
technique, limb preservation, especially in Grade IIIb and IIIc
fractures of the lower extremity, is more commonly attempted nowadays.
Reconstructive bone and soft tissue surgery usually requires repeated
operations, long-term hospital stays, and prolonged periods of
treatment. The surgeon must appreciate that this is very difficult for
the patient and his or her family and there are often significant
social and economic consequences. Several authors have therefore looked
into criteria to help guide surgeons in their decision between
reconstruction and amputation of a severely injured extremity. From the
surgical point of view, an attempt to preserve the limb often seems to
be the best decision for the patient. However, from a socioeconomic
point, multiple prolonged hospital stays may have severe effects on the
patient. The financial loss for the patient from prolonged hospital
stays and time off work may prove to be higher than that associated
with a primary amputation, and not infrequently multiple attempts at
reconstruction leave patients incapable of earning their living for
more than 2 years.335 Additionally,
it should be remembered that patients with reconstructed limbs often
find it difficult to return to their occupations at all.
amputation, the question that arises is whether the amputation was
unavoidable or whether reconstruction was possible. If the patient
dies, the question is whether the severity of the injuries was
underestimated initially and would an early amputation have saved the
patient’s life. Last, if the patient survives after primary
reconstruction but suffers from complications requiring prolonged
treatment, the question is whether the bad outcome justified the
resources that were expended.
evaluated the Mangled Extremity Severity Score (MESS) by
retrospectively studying 24 patients with Gustilo III-C fractures. The
results confirmed high predictability. To improve the predictive value,
nerve damage and a detailed assessment of the bone and soft tissue
damage were included. The new score that resulted from this is called
the Nerve Injury, Ischemia, Soft Tissue Injury, Skeletal Injury, Shock
and Age (NISSSA) score. It has been shown to have a sensitivity of
81.8% and a specificity of 92.3%.229 Amputations are discussed further in Chapter 13.
debridement is the first step in the operative treatment plan. All soft
tissues have to be considered. If the debridement is overcautious, this
may lead to a deterioration of the patient’s condition and even organ
failure. Adequate surgical exposure of the injury is essential to both
assess and treat the soft tissue damage. In multiply injured patients,
there is a high risk of late soft tissue necrosis secondary to impaired
soft tissue perfusion, which may occur with posttraumatic edema,
increased capillary permeability, massive volume resuscitation, and an
unstable circulation. Therefore, in many patients, regular operative
explorations need to be scheduled. These second-look surgeries allow
for continuous assessment of the soft tissues. This strategy enables
the surgeon to undertake redébridement procedures every 48 hours if
required.
of the patient and the extent of the coexisting injuries. Any lengthy
reconstruction or reimplantation procedure may potentially harm the
patient and induce a life-threatening situation. Attention also has to
be paid to the long-term prognosis of an open injury in the multiply
injured patient. All these parameters need to be considered when
constructing a therapeutic plan.
Points and Grade IIIa, b, and c Soft Tissue Injuries. In this subgroup
of multiply injured patients, reconstruction is indicated. The surgical
process is now largely standardized. After a radical débridement, the
second step consists of vascular repair, if this is required. This may
necessitate the use of an interposition vein graft. Following this, the
fractures should be stabilized, ideally using intramedullary
osteosynthesis. Intramedullary implants are much less damaging to the
soft tissues than direct osteosynthesis. There is less soft tissue
stripping and only minimal impairment of the circulation of the bone.196
on the extent of the injury. In most cases, the wound will be
temporarily covered with synthetic skin grafts or vacuum systems,
before final closure using plastic reconstructive surgery techniques.
This is discussed further in Chapter 14. In general, the expected result of reconstructive limb-saving strategy should be better than that of amputation.
Points with Complete or Incomplete Amputations. The surgical management
of these injuries is very similar. The option of reimplantation has to
be considered and this may require referral to a specialized center. If
reimplantation is anticipated, appropriate preparations must be made.
Hemorrhage should be stopped by elevation and application of a pressure
bandage. The treatment of the amputated limb follows clear emergency
medicine guidelines.362
considered for replantation. Children have an improved tissue
regenerative ability and have better functional outcome results than
similarly injured adults.
Points. In recent years, Level 1 trauma centers have improved their
critical care and fracture management techniques and they now succeed
in saving most severely traumatized extremities. Unfortunately, these
limbs still sometimes require secondary amputation because of
insufficient planning.174 In this
subgroup of most severely injured patients with extremity injuries, the
preservation of the extremity should not be attempted at all. The
principle “life before limb” should hold true and the indications for
amputation are generous. If the decision to amputate a limb is made,
the actual procedure should ideally be performed quickly through
healthy tissue using a guillotine method. Under these circumstances,
primary closure is associated with an extremely high rate of
complications because the overall extent of the soft tissue damage and
posttraumatic edema cannot be adequately estimated.
management of open intra-articular fractures. First, the injury is
débrided and the joint surface is reconstructed using a minimal
invasive osteosynthesis technique (MIO). The joint is then immobilized
by bridging, or transarticular, external fixation. The definitive
osteosynthesis is carried out secondarily following soft tissue
healing. In this procedure, the previously reconstructed articular
segment is attached to the metaphysis. Sometimes bone shortening has to
be accepted, at least temporarily, to close potential bony or soft
tissue defects. The Ilizarov frame is often used under these
circumstances. This is discussed further in Chapter 14.
represents bad practice. The relative hypoxia of the tissues may lead
to impaired and delayed wound healing associated with a higher risk of
wound infection. In small soft tissue injuries, we recommend secondary
closure of the wound after covering the wound with artificial skin
until the swelling decreases. An absolute prerequisite for wound
closure is to completely cover implants with well-perfused soft
tissues. In these defects, artificial skin replacements are used
primarily and the wound is secondarily closed later over a period of
several days. In some selected cases, continuous wound closure may also
be an option.
is often achieved by local soft tissue transpositions following
appropriate mobilization of the soft tissues. In extensive soft tissue
defects associated with exposure of bone with significant periosteal
damage, the soft tissues used to cover the defect require to be very
well perfused. Soft tissue reconstruction should be undertaken within
72 hours of the trauma or there is danger of further damage.
challenging for the surgeon and require a well-defined therapeutic
strategy. The overall concept of soft tissue coverage depends on the
extent of uncovered bone, tendons, and nerves. For bone associated with
significant periosteal stripping, damaged neurovascular structures, and
injuries involving open joints, soft tissue cover with well-perfused
tissues is essential. To achieve satisfactory results, timely
communication and continuous cooperation between trauma and plastic
surgeons are essential.
medium-sized soft tissue defects. These flaps consist of different
combinations of muscle, fascia, and skin, which are usually well
perfused. They are very adaptable but are associated with a number of
disadvantages. In multiply injured patients, it may be difficult to use
local flaps because of coexisting injuries to the adjacent soft
tissues. Meticulous preoperative planning is mandatory. Local flaps are
discussed in more detail in Chapter 14.
commonly used in multiply injured patients. However, the choice of flap
is often difficult. On the one hand, the patient may need urgent soft
tissue closure, but on the other hand, a prolonged procedure may be
contraindicated. Carefully planning is essential; see Chapter 14.
thoracic trauma and suffer from a variable degree of respiratory
insufficiency. Management strategies for these patients should begin on
arrival at the trauma center. The objective is to initiate treatment
early to minimize the risk of development of atelectasis and/or
parenchymal damage. Mechanical ventilation should facilitate alveolar
recruitment and enhance intrapulmonary gas distribution. Modern
ventilation strategies with low tidal volume (4 to 8 mL/kg), best
positive end-expiratory pressure (PEEP), low airway pressures (less
than 35 cm H2O), and an inspiratory oxygen concentration of
55% to 60% are often ideal. Hypercapnia may be allowed up to a certain
degree. This is known as permissive hypercapnia (PHC).384 It is well tolerated in patients with ARDS and a PCO2
of 60 to 120 mm Hg. Clinical experience shows that the pressure
controlled ventilation with inversed ratio ventilation (I:E [1:1 to
4:1]), low tidal volumes (4 to 8 mL/kg), frequencies of 10 to 15/min,
PHC (PCO2 about 70 mm Hg), and an individual PEEP (5 to 12 cm H2O), a high oxygen concentration (FIO2 less than 0.5) and a high airway pressure can prevent the lung from further ventilation damage.409
Early experiences using other ventilation strategies, such as bilevel
positive airway pressure (BiPAP), demonstrate that they are also
feasible, although there may be problems with BiPAP in cases where
long-term sedation is required. One of the most recent concepts
developed for the prevention of pulmonary failure is the recruitment of
alveoli by a temporary increase in PEEP (open lung concept).2
It does not cause sustained cardiovascular side effects and also does
not lead to the development of bronchopleural fistulas. However, the
clinical relevance of this new concept remains to be proved in larger
series.239
low-tidalvolume ventilation strategy with an experimental strategy
based on the original “open-lung approach,” combining low tidal volume,
lung recruitment maneuvers, and high PEEP. The authors concluded that
for patients with acute lung injury and ARDS, a multifaceted
ventilation strategy designed to recruit and open the lung resulted in
no significant difference in all-cause hospital mortality or barotrauma
compared with an established lowtidal-volume ventilation strategy. This
“open-lung” strategy did appear to improve secondary end points related
to hypoxemia and the use of rescue therapies.230
trauma, and ARDS is its most critical form. In ARDS, the lungs become
swollen with water and protein, and breathing becomes impossible,
leading to death in 3% to 40% of cases. Activated blood cells,
cytokines, toxins, cell debris, and local tissue damage facilitate
endothelial cell damage leading to decompensation of lymph drainage and
pulmonary interstitial edema. Patients with ARDS have higher hospital
mortality rates and reduced long-term pulmonary function and quality of
life. ARDS is treated with mechanical ventilation, which can provide
life support but often at the expense of further lung injury.
Ventilation that employs a low tidal volume inhaled in each breath
reduces the risk of death in patients who are critically ill with ARDS.
The use of steroids has been controversial despite the fact that
published trails support the administration of low- to moderatedose
corticosteroids in the treatment of early- and late-phase ARDS.80
The impact of clinical risk factors in the conversion from acute lung
injury to acute ARDS in severe multiple trauma patients has also been
evaluated. It has been shown that the impact of pulmonary contusion,
the APACHE II score and disseminated intravascular coagulation may help
to predict the conversion of acute lung injury to ARDS in severe
multiple trauma.412 Historically,
three phases of ARDS have been differentiated, the third leading to a
state of scarring of pulmonary tissue and often irreversible loss of
organ function. Currently, we believe that the formation of scar tissue
is often the result of high intra-alveolar pressures because of
inadequate ventilation techniques. Because of the improved ventilation
strategies described earlier, the late form is usually no longer seen.290
inflammatory response of the host to a variety of insults. Currently,
it is believed that in the early phase of MODS, circulating cytokines
cause universal endothelium injury in organs. In the later phase of
MODS, overexpression of inflammatory mediators in the interstitial
space of various organs is considered a main mechanism of parenchymal
injury. The difference in constitutive expression and the upregulation
of adhesion molecules in vascular beds and the density and potency of
intrinsic inflammatory cells in different organs are the key factors
determining the sequence and severity of organ dysfunction.393
commonly reported sequence is pulmonary failure followed by the liver
and intestine.82,95
conform to the roles set by the predictive parameters following trauma.
Some patients do worse while some others fare better than predicted. In
the early 1990s, it was recognized that these differences in the
clinical course of the patients and their outcomes are subject to
biological variation in the context of trauma or surgery.137
This biological variation is highly dependent on the genetic
constitution of the patient, and the importance of genes as the cause
of diseases or as predisposing factors has become indisputable. The
observed polymorphisms are of different natures. Some of them are
mutations located within endonuclease restriction sites, whereas others
are single nucleotide polymorphisms or consist of insertions or
deletions of larger fragments, as detected by polymerase chain reaction
techniques.121 The polymorphism can
be located within the gene or in the promoter region. Polymorphisms are
different alleles, none of which is predominant in the population. A
specific polymorphism variation can be associated with a genetic
disease. The polymorphism can also interact with the environment and
then exert detrimental actions.
techniques, there has been increased interest in conducting
disease-gene association studies determining the role of genetic
variations in the inflammatory response to injury and infection.
sepsis is no longer a myth. A growing body of evidence suggests that
genetic susceptibility influences the development of surgical
sepsis
and its sequelae, ARDS and MODS. The identification of functional
polymorphisms in several cytokine genes and other important molecules
provides a potential mechanism whereby these variations may exist.
Several studies have reported the relationship between different
polymorphic variants and the risk of developing posttraumatic
complications.127,129,180,300
not enough just to determine the presence of a polymorphism. One has to
take several criteria into account. Patients have different genetic
constitutions. Investigating polymorphisms linked to disease can be
blurred by the existing genetic variation. Therefore, it is necessary
to determine the overall genetic constellation of the population under
investigation. Furthermore, the power of the study has to be sufficient
to achieve specific results. One has to consider whether other genes
may be involved. These genes might be the actual cause for the
differences investigated. The gene investigated is then just an
epiphenomenon. It is therefore important to study family genetics at
the same time. The family constitution can tell more about the
underlying genes that are involved in the disease process. If
differences in disease outcome are linked to one or more genetic
polymorphisms, one has to perform a subsequent study in another cohort.
This cohort has to show similar linkage of genes to outcome. A number
of studies214,300,307,332,397
have indicated the influence of specific polymorphic variants of
important genes in the development of posttraumatic sepsis. However,
most of the studies have been undertaken on small populations, which
are not necessarily geographically or ethnically distinct, and for this
reason the results may be difficult to interpret. Because of these
problems, multicenter, international studies will be required to
investigate the application of genetic information to patients.
Single nucleotide polymorphism genotyping assays using microarray
techniques are suitable for this. Early identification of patients at
risk would permit direct interventions with biological response
modifiers in an attempt to improve morbidity and mortality rates. Early
positive results have been achieved in septic patients and
goal-directed therapeutic low-dose steroid supplementation, blood
glucose control, and activated protein C therapy appears to be
associated with an improved outcome after sepsis.75,307,308 Hopefully, similar achievements can be made for patients with acute trauma in the future.
the immediate postoperative period. This requires mobilization of the
extremities during the course of the intensive care treatment. Passive
continuous motion may be used, but mobilization of all major joints
must be performed and should be part of a standardized rehabilitation
program.
these measures must be maintained and they may be accompanied by active
exercises by the patient. These should be performed under the
supervision of a trained physiotherapist. The modes of mobilization and
the degree of weight bearing should be carefully discussed between the
treating surgeon and the physical therapist. Patients tend to be
cautious about mobilization and there is often a particular fear about
weight bearing. This can often be explained by the severe psychological
impact induced by the traumatic insult. Reassurance of the patient is
an important additional factor if adequate mobilization is going to be
achieved. These factors are important not only with regard to the
maintenance of joint mobility but also to prevent osteoporosis induced
by immobility. It is crucial that patients realize the importance of
muscular activity, joint mobility, and weight bearing with reference to
neuromuscular function and the maintenance of an optimal osseous
microstructure.
trauma, special care must be taken to avoid the development of
secondary brain damage. These patients also benefit from early
rehabilitation measures. An appropriate transfer to a rehabilitation
center is advisable. Although it may be considered appropriate to
commence treatment in the primary center, the patients are often still
under the influence of sedative drugs or undergoing withdrawal symptoms
from these drugs. In this situation, a thorough workup cannot be
performed and cognitive training is useless. In an ideal situation,
transfer to a specialized facility may overlap with the normalization
of the withdrawal symptoms and thus forms the basis of a timely
beginning of the rehabilitation program.
traditionally focused on mortality rates, the incidence of preventable
deaths, complication rates, in-hospital morbidity, and length of
hospital stay.34,319
However, because of the advances of acute trauma management and the
increased survival rates over the last few decades, long-term
functional recovery, health-related quality-of-life, return to work,
and patient’s satisfaction have been added to the classic trauma
evaluation endpoints.
result of multiple phases and factors, including diagnostic procedures,
therapeutic interventions, inherent characteristics of the patient, and
effectiveness of the trauma services over a long period of time, from
the time of the accident until rehabilitation or even later. It is the
end result of this multifactorial and complicated system that is of
most concern to the patient. In the last two decades, the importance of
the patient’s point of view and his or her perception of health
outcomes have been acknowledged and have led to the development of a
large number of functional and patient-related outcome scores.33
long-term functional outcome of trauma is not only based on patients’
concerns as proper assessment facilitates the development and
improvement of management guidelines, discharge and rehabilitation
planning, and the optimal allocation of resources. Additionally, the
long-term outcome of trauma management has significant social and
economic implications.33
thus the appropriate time interval for assessment of long-term outcomes
is usually longer than the customary time-frame of 2 years. In
particular, social rehabilitation, including return-towork or hobbies,
change of occupation or retirement, appears to be a long-term process.
This suggests that the evaluation of the functional outcome following
polytrauma should be based
on
a lengthened follow-up period by which time surgical outpatient review
is sporadic, if it is still occurring at all. This fact together with
the complexity of parameters associated with the outcome of polytrauma
explains the difficulty of long-term assessment and the scarcity of
comprehensive clinical studies and adequate data.
care encompasses parameters related to quality-of-life, return to work
or sports, persistent physical or psychological complaints, and
restrictions and acquired disabilities. The term “quality of life”
entered Index Medicus in 1977.134,415
To apply its concept in the specific clinical setting of trauma, four
basic areas should be considered: physical function, psychological
function, social function, and symptoms.381
The unique character of this outcome assessment is that it relies
largely on subjective variables judged by the patients themselves.
quantify outcome after mainly isolated injuries in almost all anatomic
areas. In the case of polytrauma, they are often used to objectively
quantify the anatomic and physical components of the final outcome.
Examples are the Lysholm and Merle d’Aubigne scores. For patients with
multiple musculoskeletal disorders, patient-assessed scales have been
developed that describe self-reported complaints and subjective
parameters. In addition, numerous scoring systems have been developed
to determine psychological outcome after trauma.
According to most published series, they seem to determine a major
proportion of the patient’s quality of life with respect to functional
status and pain.
gender, injury severity, sociodemographic status, social support, and
psychological sequelae, have also been reported.46,160,164
Clinically relevant psychological impairments such as anxiety,
depression, and posttraumatic stress disorder have been reported,
especially within the first year after injury, when they have a
prevalence of 30% to 60%. In succeeding years, the prevalence drops and
has been reported to be 7% to 22%.235 The importance of psychological outcomes, particularly posttraumatic stress disorder, has been highlighted in many series.324,357
It is described as an anxiety disorder that can develop after exposure
to a terrifying event or ordeal in which grave physical harm occurred
or was threatened.
severity of injury, trauma management practices, and rehabilitation,
there is strong evidence that the quality of life and the overall
outcome are significantly impaired after major trauma. In the past, the
principal aim of treatment was the prevention of late organ failure and
death. In contrast, today, the ultimate goal of trauma care is to
restore patients to the previous functional status and role in society.
A number of studies reporting on the functional outcome after multiple
trauma are shown in Table 9-14,* whereas in Table 9-15124,173,355,396
the commonly used functional outcome scores are presented. The
measurement of outcome still lacks accuracy, but we anticipate
significant improvement in this important aspect of trauma.
health system around the world. In patients with multiple injuries, the
issue of reimbursement is still unresolved and has been the subject of
much recent debate.
result of their disproportionate funding and inadequacy of
reimbursement policies.** Before, however, one decides to
evaluate the real cost of a specific procedure to a national health
system, one must be familiar with the factors that a thorough economic
analysis should include. A thorough economic analysis of any medical
condition measures direct, indirect, and intangible costs.182,365
It incorporates both fixed and variable costs, direct expenses and
indirect expenses associated with the duration of therapy, final
functional outcome, disability payments, and quality of life.76,186,295
it is these costs that the clinician has least control over. The
variable costs are mostly related to clinical practice and they have
been more extensively studied. Direct medical and nonmedical costs are
easier to record compared with the indirect ones, and most of the
literature focuses on them. The indirect and intangible costs are more
difficult to estimate and they require longer patient follow-up.
However, they can be significantly larger than the direct costs. A
major deficiency of the existing health economics studies is their lack
of an all-inclusive cost analysis.
is complicated as it has to encompass the entire trauma system,
including prehospital, in-hospital, and posthospital care. Because of
this, the financial implications are difficult to assess, and they are
especially difficult in polytrauma patients, where there are many
aspects to evaluate. Examples of these are the prehospital and
emergency services; the intensity of the medical and nursing staff
workload, which varies with each patient; and the element of “trauma
readiness.”96 “Trauma readiness” is
related to the expertise of the personnel involved in patient care, the
effectiveness of the infrastructure, and the efficiency of coordination
of the trauma team. The expense of maintaining a dedicated trauma team
on a 24/7 schedule has proved to be the most difficult economic
parameter to assess, rate, and reimburse.364 Over the years, several authors have attempted to address the issue of trauma and polytrauma costs.†
In all of these studies, it was evident that conventional cost
accounting methods were inadequate to assess the costs despite recent
advances in “operations management” and health economics. The necessary
components of an all-inclusive economic analysis of a trauma system
were first outlined in the Model Trauma Care Systems Plan published by
the U.S. Bureau of Health Resources Development in 1992.94 Table 9-16 presents a description of the different aspects of trauma-related health economics.
spending on injuries in the United States in 1987 was $64.7 billion. In
2000, it accounted for 10.3% of the total medical expenditure and had
reached $117.2 billion. In the United Kingdom, in 1994 the cost of
treating trauma was £20 billion, and in 2003 it was reported to be £34
billion.236,269,389 Major increases in the socioeconomic costs of trauma and polytrauma
can be seen in studies from Germany, Switzerland, and the rest of Europe.*
|
TABLE 9-14 Fifteen Representative Studies Focused on Long-term Outcome of Major Trauma Patients
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
one can easily understand why providing trauma services is not really
sustainable. The issue of reimbursement is still unresolved and has
been the focus of much debate recently. Most health care organizations
and trauma systems apply a predetermined charge for their trauma
services, which does not relate to each patient’s direct or indirect
medical and nonmedical costs. It is therefore no surprise that,
especially for complex cases and polytrauma patients, such cost
estimation has proved to be inaccurate. It has been estimated that a
comparison of the actual direct costs of polytrauma and its relative
reimbursement has
resulted in a negative balance of 80% to 900% in different health systems.346
In England, the cost of treatment of any multiple injured patient is
estimated using the “polytrauma tariff.” This tariff is used in all
patients with multiple injuries instead of calculating the costs of the
different interventions in each individual polytrauma case. The concept
of Payment by Results was introduced across the National Health Service
(NHS) in England in 2005 and is based on the average cost of providing
a particular treatment or health service. The dominant diagnosis or
procedural code determines the tariff, as well as the length of
hospital stay.85 In 2000, the NHS fiscal services calculated this tariff to be £1500.00.346 According to the website of the Department of Health86
for the financial year 2006 to 2007, the polytrauma tariff is a
standard sum of £3004 for those younger than 69 years and £5716 for
older than 70 years. Additional costs are calculated depending on the
length of hospital stay for these patients if their hospitalization is
prolonged for longer than 1 week for the older-than-69-years group or
longer than 4 weeks for the older-than-70-years group. However, poor
documentation, inefficient coding, and the inherent weaknesses of using
average
costs in complex and multisystem diseases like polytrauma have resulted
in incorrect tariffs and therefore significant loss of revenue for the
hospitals.260
|
TABLE 9-15 Commonly Used Functional Outcome Scores in the Clinical Setting of Polytrauma
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
TABLE
9-16 Different Aspects of Health Economics That Needs to Be Evaluated in an All-Inclusive Financial Profile of Trauma and Polytrauma Care |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||
factors that are contributing to this inadequate system of cost
evaluation of medical treatment. The absence of a single formal trauma
network in the United Kingdom and the existence of many small informal
networks centered on teaching or large general hospitals has almost
certainly hindered efforts to achieve a complete financial assessment
of the trauma services. Without an accurate assessment of the overall
services, it is difficult to quantify full reimbursement and less easy
to justify adequate resources.
networks varies significantly. It is often unrecognized and underfunded
by the authorities. These units often function under significant
pressure. The continuous use of their resources for trauma and
polytrauma patients prolongs the elective waiting lists and limits the
level of service that can provided to local patients for the more
financially rewarding routine elective treatments.74,81,172,287
Thus, these centers often present a worse profile according to strict
financial and managerial criteria in comparison to smaller hospitals
with a smaller trauma workload. Moreover, NHS revenues from elective
orthopaedic cases have been recorded to be more than those of acute
trauma cases, emphasizing the problem of the insufficiency of the
“trauma tariffs.”22 Unfortunately,
as the numbers of trauma patients increases, the administrative focus
is directed more toward problems with elective waiting lists, thus
reducing the resources of the specialist trauma services even further.22
The authors identified one of the main problems as the establishment of
the out-of-area transfer system (OATS) for pelvic trauma in 1999.
Analysis showed that only 60% of treated cases were reimbursed because
of the retrospective manner of the calculation.
centers demands a more accurate and up-to-date estimation of the actual
volume of the trauma care services and their direct medical burdens.
complete evaluation of the financial implications of polytrauma does
not exist. The assessment of the cost-effectiveness of any trauma
system must be correlated with the return of trauma victims to a
productive life. The complexity and multiplicity of the different
aspects of these patients and their treatment represent the main reason
for this deficiency. However, its necessity cannot be overstressed to
facilitate the development and monitoring of the required services and
to demonstrate the deficiency of the associated financial frameworks.
Trauma centers must identify and understand their cost structure not
only to improve their efficiency but also to survive. In this context,
medical and financial researchers must focus on all the different
aspects of polytrauma expenses. More specifically, the following
recommendations can be made:
-
The direct medical costs should include
all the diagnostic and therapeutic procedures and interventions
incurred by these patients and avoid the limitations of the “polytrauma
tariff.” The target should be to achieve an accurate assessment of all
the expenses of the trauma hospital services to claim satisfactory
reimbursement. -
The concept of “trauma readiness” is of
particular importance for the hospital personnel and services. The
variability and intensity of the trauma workload cannot be compared
with those of any other medical service. The 24/7 availability of a
trauma team and the financial implications of this must be included at
any economic analysis and should be reimbursed. -
At the same time, the costs of
prehospital services related to trauma and polytrauma should also be
assessed on a prospective and all-inclusive basis taking into account
the aspects of “readiness” and also the secondary transportation of
individual polytrauma patients to tertiary specialized centers with
established pelvic and spine treatment units. -
The health authorities should evaluate
the tertiary referral centers of expert trauma services using different
criteria and financial algorithms than those applied to the referring
hospital centers. The criteria defining success in these hospitals
should be compared with those of similar trauma centers with similar
workloads and multidisciplinary readiness, and not with those of
hospitals that provide services of a more elective nature. -
The difficulties of evaluating the
quality of life and psychosocial costs of trauma and polytrauma should
not discourage the researchers. A prospective study following these
patients until their final outcome should be initiated as soon as
possible. It would provide all the necessary information about the real
socioeconomic burden of contemporary trauma. -
The fragmentation of health services and
trauma networks dealing with the assessment of the economics of
polytrauma must be avoided. The conclusions of a prospective study
estimating the socioeconomic burden of trauma should include all the
health care providers that are involved with polytrauma care.
J, Moore EE, Ciesla DJ, et al. Blood transfusion and the two-insult
model of post-injury multiple organ failure. Shock 2001;15:302-306.
J, Adam RU, Gana TJ, et al. Trauma patient outcome after the
Prehospital Trauma Life Support program. J Trauma 1997;42:1018-1021.
College of Emergency Physicians. Trauma care systems development,
evaluation, and funding. Ann Emerg Med Clin North Am 1999;34:308.
College of Surgeons/Committee of Trauma. National Trauma Data Bank
annual report 2005, dataset version 5.0. Chicago: American College of
Surgeons Committee on Trauma 2005.
AG, Stanghelle JK, Finset A, et al. Long-term prevalence of impairments
and disabilities after multiple trauma. J Trauma 1997;42:54-61.
SP, O’Neill B, Haddon W Jr, et al. The Injury Severity Score: a method
for describing patients with multiple injuries and evaluating emergency
care. J Trauma 1974;14:187-196.
ME, Oktar GL, Kayi-Cangir A, et al. Emergency thoracotomy for blunt
thoracic trauma. Ann Thorac Cardiovasc Surg 2002;8:78-82.
Z, Offner PJ, Moore EE, et al. NISS predicts post injury multiple organ
failure better than the ISS. J Trauma 2000;48:624-627.
ZJ, Varga E, Tomka J, et al. The New Injury Severity Score is a better
predictor of extended hospitalization and intensive care unit admission
than the Injury Severity Score in patients with multiple orthopaedic
injuries. J Orthop Trauma 2003;17: 508-512.
RC, Chang LY, Purdue GF, et al. Detecting genetic predisposition for
complicated clinical outcomes after burn injury. Burns 2006;32:821-827.
JP, Colins JA. Theoretical and clinical aspects of post traumatic fat
embolism syndrome. AAOS Instr Course Lett 1973;22:38-44.
S, Potgieter H, Nicol A, et al. Report on a new type of trauma
full-body digital x-ray machine. Emerg Radiol 2003;10:23-29.
B, Duchosal MA, Siegrist CA, et al. Proximal splenic artery
embolization for blunt splenic injury: clinical, immunologic, and
ultrasound-Doppler follow-up. J Trauma 2007;62:1481-1486.
WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid
resuscitation for hypotensive patients with penetrating torso injuries.
N Engl J Med 1994;331: 1105-1109.
M, Shoemaker WC, Avakian S, et al. Evaluation of a comprehensive
algorithm for blunt and penetrating thoracic and abdominal trauma. Am
Surg 1991;57:737-746.
O, Magliore L, Claridge JA, et al. The golden hour and the silver day:
detection and correction of occult hypoperfusion within 24 hours
improves outcome from major trauma. J Trauma 1999;47-5:964-969.
T, Alkadhi H, Schertler T, et al. [Application of multislice spiral CT
(MSCT) in multiple injured patients and its effect on diagnostic and
therapeutic algorithms]. Rofo 2004;176:1734-1742.
KD, Goosen J, Plani F, et al. The use of low dosage x-ray
(Lodox/Statscan) in major trauma: comparison between low dose x-ray and
conventional x-ray techniques. J Trauma 2006;60:1175-1181; discussion
1181-1183.
L, Johnson K, Weigelt J, et al. Early versus delayed stabilization of
femoral fractures. A prospective randomized study. J Bone Joint Surg Am
1989;71A:336-40.
RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure
and guidelines for the use of innovative therapies in sepsis. The
ACCP/SCCM Consensus Conference Committee. American College of Chest
Physicians/Society of Critical Care Medicine, Chest 1992;101:1644-1655.
O, Wrotchford A, Hollis S, et al. A new approach to outcome prediction
in trauma: a comparison with the TRISS model. J Trauma 2006;61:701-710.
CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method.
Trauma Score and the Injury Severity Score. J Trauma 1987;27:370-378.
ER, Kyrychenko SY, Ferguson SA. Driver mortality in frontal crashes:
comparison of newer and older airbag designs. Traffic Inj Prev
2005;6:24-30.
ER, Scerbo M, Kufera JA, et al. Deaths among drivers and right-front
passengers in frontal collisions: redesigned air bags relative to
first-generation air bags. Traffic Inj Prev 2008;9:48-58.
J, Walker A, Sloan JP, et al. Evaluation of focused assessment with
sonography in trauma (FAST) by UK emergency physicians. Emerg Med J
2006;23:446-448.
B, Lindsey M, Rowe K, et al. Hemoglobin drops within minutes of
injuries and predicts need for an intervention to stop hemorrhage. J
Trauma 2007;63:312-315.
EM, Jurkovich GJ, Nathens AB, et al. Hypertonic resuscitation of
hypovolemic shock after blunt trauma: a randomized controlled trial.
Arch Surg 2008;143:139-148; discussion 149.
EM, Nathens AB, Rivara FP, et al. Brain Trauma Foundation. Management
of severe head injury: institutional variations in care and effect on
outcome. Crit Care Med 2002;30:1870-1876.
JP. The Injury Severity Score of road traffic casualties in relation to
mortality, time of death, hospital treatment time, and disability.
Accid Anal Prev 1975;7:249-255.
C, Zwingmann J, Kabir K, et al. [Faster diagnostics by digital x-ray
imaging in the emergency room: a prospective study in multiple trauma
patients]. Z Orthop Unfall 2007;145:772-777.
RH. Trauma mortality in Orange County: the effect of implementation of
a regional trauma system. Ann Emerg Med 1984;13:1-10.
MG, McLaughlin JS, Downing SW, et al. Management of traumatic aortic
rupture: a 30-year experience. Ann Surg 2002;236:465-469.
O, Siani A. Focused assessment with sonography for trauma (FAST): what
it is, how it is carried out, and why we disagree. Radiol Med (Torino)
2004;108:443-453.
HR, Copes WS, Sacco WJ, et al. Improved predictions from a Severity
Characterization Of Trauma (ASCOT) over Trauma and Injury Severity
Score (TRISS): results of an independent evaluation. J Trauma
1996;40:42-48.
HR, Copes WS, Sacco WJ, et al. The Major Trauma Outcome Study:
establishing national norms for trauma care. J Trauma 1990;30:1356-1365.
RM, Marshall SB, Piek J, et al. Early and late systemic hypotension as
a frequent and fundamental source of cerebral ischemia following severe
brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl
(Wien) 1993;59:121-125.
CB, Park P, Kim YH, et al. Comparison of visibility measurement
techniques for forklift truck design factors. Appl Ergon
2009;40:280-285.
P-L, Yip G, Quinonez LG. Crystalloids vs. colloids in fluid
resuscitation: a systematic review. Crit Care Med 1999;27:200-210.
DJ, Sava JA, Street JH 3rd, Jordan MH. Secondary overtriage: a
consequence of an immature trauma system. J Am Coll Surg
2008;206:131-137.
JA, Crabtree TD, Pelletier SJ, et al. Persistent occult hypoperfusion
is associated with a significant increase in infection rate and
mortality in major trauma patients. J Trauma 2000;48:8-14; discussion
15.
MN, Kimlin E, Walsh M, et al. Identification and resuscitation of the
trauma patient in shock. Emerg Med Clin North Am 2007;25:623-642.
JP, Minhas PS, Fryer TD, et al. Effect of hyperventilation on cerebral
blood flow in traumatic head injury: clinical relevance and monitoring
correlates. Crit Care Med 2002;30:1950-1959.
N, Giannoudis PV, Kourgeraki O, et al. Interleukin 13 and inflammatory
markers in human sepsis. Br J Surg 2004;91:762-768.
WH, Salinas J, Convertino VA, et al. Heart rate variability and its
association with mortality in prehospital trauma patients. J Trauma
2006;60:363-370; discussion 370.
DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline
resuscitation of patients with hypotension and severe traumatic brain
injury: a randomized controlled trial. JAMA 2004;291:1350-1357.
CC, Osborn PM, Moore EE, et al. Preperitoneal pelvic packing for
hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma
2007;62:834-839.
G, O’Keefe G. Scene disposition and mode of transport following rural
trauma: a prospective cohort study comparing patient costs. J Emerg Med
2000;18: 349-354.
P, McKnight B, Rivara FP, Grossman DC. Association of driver air bags
with driver fatality: a matched cohort study. BMJ 2002;324:1119-1122.
P, Rivara FP, Olson CM, Smith KM. Changes in traffic crash mortality
rates attributed to use of alcohol, or lack of a seat belt, air bag,
motorcycle helmet, or bicycle helmet, United States, 1982-2001. Inj
Prev 2006;12:148-154.
Z, Dimitriou R, Chalidis B, Giannoudis PV. Coagulopathy and the role of
recombinant human activated protein C in sepsis and following
polytrauma. Expert Opin Drug Saf 2006;5:67-82.
Z, Dimitriou R, Giannoudis PV. Health economics: a cost analysis of
treatment of persistent fracture nonunions using bone morphogenetic
protein-7. Injury 2007;38:371-377.
DP, Ochs M, Hoyt DB, et al. Paramedic-administered neuromuscular
blockade improves prehospital intubation success in severely
head-injured patients. J Trauma 2003;55:713-719.
DP, Stern J, Sise MJ, et al. A follow-up analysis of factors associated
with headinjury mortality after paramedic rapid sequence intubation. J
Trauma 2005;59:486-90.
DP, Valentine C, Ochs M, et al. The Combitube as a salvage airway
device for paramedic rapid sequence intubation. Ann Emerg Med
2003;42:697-704.
EN, Hollands JM, Schramm GE, Micek ST. Role of corticosteroids in the
management of acute respiratory distress syndrome. Clin Ther
2008;30:787-799.
of Health. Chief executive’s report to the NHS. Retrieved April 15,
2009, from
http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/AnnualReports/
DH_4134613.
TB, Sleet DA, Shults RA, et al. Reviews of evidence regarding
interventions to increase the use of safety belts. Am J Prev Med
2001;21(suppl 4):48-65.
JV, Tortella BJ, Drivet WJ, et al. Factors influencing successful
intubation in the prehospital setting. Prehosp Disaster Med
1995;10:259-264.
SW, Sperling JS, Mirvis SE, et al. Experience with spiral computed
tomography as the sole diagnostic method for traumatic aortic rupture.
Ann Thorac Surg 2001; 72:495-501.
CM, Bosse MJ, Clancy TV, et al. EAST Practice Management Guidelines
Work Group. Practice management guidelines for the optimal timing of
long-bone fracture stabilization in polytrauma patients: the EAST
Practice Management Guidelines Work Group. J Trauma 2001;50:958-967.
DR, Elliott MR, Winston FK. Belt-positioning booster seats and
reduction in risk of injury among children in vehicle crashes. JAMA
2003;289:2835-40.
DR, Runge J, Mackay M, et al. Booster seats for children: closing the
gap between science and public policy in the United States. Traffic Inj
Prev 2003;4:5-8.
AB, Bishop GS, Walsh JC, et al. The economic status of trauma centers
on the eve of health care reform. J Trauma 1994;36:835-844.
AK, Benneker LM, Jeger V, et al. Total-body digital x-ray in trauma. An
experience report on the first operational full body scanner in Europe
and its possible role in ATLS. Injury 2008;39:525-529.
SC. Designing and building a new emergency department: the experience
of one chest pain, stroke, and trauma center in Columbus, Ohio. J Emerg
Nurs 2006; 32:144-148.
M, Probst C, Hildebrand F, et al. [The influence of transportation mode
on mortality in polytraumatized patients. An analysis based on the
German Trauma Registry]. Unfallchirurg 2007;110:334-340.
Kon Jin PH, Goslings JC, Ponsen KJ, et al. Assessment of a new trauma
workflow concept implementing a sliding CT scanner in the trauma room:
the effect on workup times. J Trauma 2008;64:1320-1326.
DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on
biological agents, specifically tumour necrosis factor α (TNFα)
blocking agents and interleukin-1 receptor antagonist (IL-1ra), for the
treatment of rheumatic diseases. Ann Rheum Dis 2005;64(suppl
4):iv2-iv14.
M, Pasquier C, Guerrini P, et al. [Short- and long-term outcome of 250
patients admitted in surgical intensive care units after multiple
injuries]. Agressologie 1990;31: 633-636.
TA, Champion HR, Sacco WJ, et al. Mortality of patients with head
injury and extracranial injury treated in trauma centers. J Trauma
1989;29-9:1193-1201.
PV, Abbott C, Stone M, et al. Fatal systemic inflammatory response
syndrome following early bilateral femoral nailing. Intensive Care Med
1998;24:641-642.
PV, Fogerty S. Initial care of the severely injured patient: predicting
morbidity from subclinical findings and clinical proteomics. Injury
2007;38:261-262.
PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures,
associated injuries, and mortality: the United Kingdom perspective. J
Trauma 2007;63:875-883.
PV, Harwood PJ, van Griensven M, et al. Correlation between IL-6 levels
and the SIRS score: can an IL-6 cut off predict a SIRS state? J Trauma
2008;65:646-652.
PV, Hildebrand F, Pape HC. Inflammatory serum markers in patients with
multiple trauma. Can they predict outcome? J Bone Joint Surg Br
2004;86B:313-323.
PV, Kanakaris NK. The unresolved issue of health economics and
polytrauma: the UK perspective. Injury 2008;39:705-709.
PV, Smith RM, Perry SL, et al. Immediate IL-10 expression following
major orthopaedic trauma: relationship to anti-inflammatory response
and subsequent development of sepsis. Intens Care Med 2000;26:1076-1081.
PV, Smith RM, Windsor AC, et al. Monocyte human leukocyte antigen-DR
expression correlates with intrapulmonary shunting after major trauma.
Am J Surg 1999;177:454-459.
PV, Tosounidis TI, Kanakaris NK, Kontakis G. Quantification and
characterisation of endothelial injury after trauma. Injury
2007;38:1373-1381.
PV, van Griensven M, Tsiridis E, et al. The genetic predisposition to
adverse outcome after trauma. J Bone Joint Surg Br 2007;89B:1273-1279.
BS, Gilson JS, Bergner M, et al. The Sickness Impact Profile.
Development of an outcome measure of health care. Am J Public Health
1975;65:1304-1310.
T, Rosenfeld JV, Winter CD. “Talk and die” patients presenting to a
major trauma centre over a 10-year period: a critical review. J Clin
Neurosci 2007;14: 618-623.
MN, Zhou W, Williams PL, et al. -308GA and TNFB polymorphisms in acute
respiratory distress syndrome. Eur Respir J 2005;26:382-389.
RP, Cummings GR, Phelan HA, et al. Does increased emergency medical
services prehospital time affect patient mortality in rural motor
vehicle crashes? A statewide analysis. Am J Surg 2009;197:30-34.
AC, Lagan AL, Aganna E, et al. TNF and TNFR polymorphisms in severe
sepsis and septic shock: a prospective multicentre study. Genes Immun
2004;5:631-640.
SA, Moore EE, Hoyt DB, et al. The life-sustaining capacity of human
polymerized hemoglobin when red cells might be unavailable. J Am Coll
Surg 2002;195:445-452.
RT, Gould RJ, Peclet M, et al. The mangled extremity syndrome (MES): a
severity grading system for multisystem injury of the extremity. J
Trauma 1985;25: 1147-1150.
A, Long CJ. Investigation of the Karnofsky Performance Status as a
measure of quality of life. Health Psychol 1984;3:129-142.
MR, Vrahas MS, Thomas KA. Pressure-volume characteristics of the intact
and disrupted pelvic retroperitoneum. J Trauma 1998;44-3:454-459.
M, Schwermann T, Lefering R, et al. [DRG reimbursement for multiple
trauma patients: a comparison with the comprehensive hospital costs
using the German trauma registry]. Unfallchirurg 2004;107:68-75.
M, Campbell M, Hoffer EK. Traumatic abdominal aortic injury treated by
endovascular stent placement. Emerg Radiol 2007;13:329-331.
RB, Mendoza RM, Williams DN. Problems in the management of type III
(severe) open fractures: a new classification of type III open
fractures. J Trauma 1984; 24:742-746.
J, Scott J, Boyd-Kranis RL, et al. Admission angiography for blunt
splenic injury: advantages and pitfalls. J Trauma 2001;51:1161-1165.
A, Murata A, Matsuda T, et al. The efficacy and limitations of
transarterial embolization for severe hepatic injury. J Trauma
2002;52:1091-1096.
A, Murata A, Matsuda T, et al. The usefulness of transcatheter arterial
embolization for patients with blunt polytrauma showing transient
response to fluid resuscitation. J Trauma 2004;57:271-276.
EL, Mendeloff J, Farrell LS, et al. Validation of TRISS and ASCOT using
a non-MTOS trauma registry. J Trauma 1995;38:83-88.
EL, Waller CH, Farrell LS, et al. A comparison among the abilities of
various injury severity measures to predict mortality with and without
accompanying physiologic information. J Trauma 2005;58:244-251.
JD, de Moerloose P, Samma M. Perioperatoire GdieH. Massive transfusion
and coagulopathy: pathophysiology and implications for clinical
management. Can J Anaesth 2004;51-4:293-310.
PJ, Giannoudis PV, Probst C, et al. Which AIS based scoring system is
the best predictor of outcome in orthopaedic blunt trauma patients? J
Trauma 2006;60: 334-340.
PJ, Giannoudis PV, van Griensven M, et al. Alterations in the systemic
inflammatory response after early total care and damage control
procedures for femoral shaft fracture in severely injured patients. J
Trauma 2005;58:446-452.
M, Ong G, Tandberg D, et al. Out-of-hospital spinal immobilization: its
effect on neurologic injury. Acad Emerg Med 1998;5:214-219.
JS, Burnham RS. The effect of polytrauma in persons with traumatic
spine injury. A prospective database of spine fractures. Spine
2000;25:55-60.
M, Imhof HG, Trentz O. [Shock trauma room management of the
multiple-traumatized patient with skull-brain injuries. A systematic
review of the literature]. Unfallchirurg 2004;107:871-880.
DL, Howey T, Sanders R, Johansen K. Limb salvage versus amputation.
Preliminary results of the Mangled Extremity Severity Score. Clin
Orthop 1990;256:80-86.
T, Heinemann B, Sauerland S, et al. Immunologic alterations associated
with high blood transfusion volume after multiple injury: effects on
plasmatic cytokine and cytokine receptor concentrations. Shock
2003;20:497-502.
PC, Wells G, Blajchmann MA. A multicentre randomized controlled
clinical trial of transfusion requirements in critical care. N Engl J
Med 1999;54:898-905.
C, Ahle H, Mack MG, et al. Traumatic injuries of the pelvis and
thoracic and lumbar spine: does thin-slice multidetector-row CT
increase diagnostic accuracy? Eur Radiol 2004;14:1751-1760.
F, Pape HC, Krettek C. [The importance of cytokines in the
posttraumatic inflammatory reaction]. Unfallchirurg 2005;108:793-803.
TL, Anderson JP, Sieber WJ, et al. Outcome after major trauma: 12-month
and 18-month follow-up results from the Trauma Recovery Project. J
Trauma 1999; 46:765-771.
TL, Hoyt DB, Anderson JP. The importance of gender on outcome after
major trauma: functional and psychologic outcomes in women versus men.
J Trauma 2001;50:270-273.
TL, Hoyt DB, Stein MB, et al. Gender differences in long-term
posttraumatic stress disorder outcomes after major trauma: women are at
higher risk of adverse outcomes than men. J Trauma 2002;53:882-888.
JB, Niles SE, Miller CC, et al. Prehospital physiologic data and
lifesaving interventions in trauma patients. Milit Med 2005;170:7-13.
JB, Salinas J, McManus JM, et al. Manual vital signs reliably predict
need for life-saving interventions in trauma patients. J Trauma
2005;59:821-828; discussion 828-829.
JHt, Bloch RD, Hall RA, et al. Natural history of traumatic rupture of
the thoracic aorta managed nonoperatively: a longitudinal analysis. Ann
Thorac Surg 2002; 73-4:1149-1154.
JJ, Scott MJ, Bullock TK, et al. Thoracotomy for blunt trauma:
traditional indications may not apply. Am Surg 2003;69-12:1108-1111.
HR Jr, Poole GV Jr, Hansen KJ, et al. Salvage of lower extremities
following combined orthopedic and vascular trauma. A predictive salvage
index. Am Surg 1987; 53:205-208.
H, Strada G. Injury Severity Score versus New Injury Severity Score for
penetrating injuries. Prehosp Disaster Med 2002;17:27-32.
G, Piscione F, Di Tommaso L, et al. Thoracic aortic emergencies: impact
of endovascular surgery. Ann Thorac Surg 2004;77-2:591-596.
T, Gando S, Murata A, et al. Predicting the severity of systemic
inflammatory response (SIRS)-associated coagulopathy with hemostatic
molecular markers and vascular endothelial injury markers. J Trauma
2007;63:1093-1098.
BJ, Crandall JR, Okamoto M. Influence of age-related stature on the
frequency of body region injury and overall injury severity in child
pedestrian casualties. Traffic Inj Prev 2006;7:290-298.
S, Reed MR. Payment by results and coding practice in the National
Health Service: the importance for orthopaedic surgeons. J Bone Joint
Surg Br 2007;89B: 1427-1430.
B, Snoek J, Bond MR, et al. Disability after severe head injury:
observations on the use of the Glasgow Outcome Scale. J Neurol
Neurosurg Psychiatry 1981;44: 285-293.
K, Daines M, Howey T, et al. Objective criteria accurately predict
amputation following lower extremity trauma. J Trauma 1990;30:568-572.
K, Cadami A, Seibert G. Incidence of ARDS in patients with multiple
musculoskeletal injuries: effect of early operative stabilization of
fractures. J Trauma 1985;25: 375-384.
JH, Murphy MP, Dickson RL, et al. Emergency physician-verified
out-of-hospital intubation: miss rates by paramedics. Acad Emerg Med
2004;11:707-709.
SA, Lichtig LK, Knauf RA, et al. Identification and categorization of
and cost for care of trauma patients: a study of 12 trauma centers and
43,219 state-wide patients. J Trauma 1994;37:303-308.
G, Mock C, MacKenzie E, et al. The Sickness Impact Profile as a tool to
evaluate functional outcome in trauma patients. J Trauma
1995;39:625-631.
GJ, Mock C. Systematic review of trauma system effectiveness based on
registry comparisons. J Trauma 1999;47(suppl 3):S46-S55.
V, Schafmayer C, Schniewind B, et al. Are postoperative complications
genetically determined by TNF-beta NcoI gene polymorphism? Surgery
2004;135:365-373.
K, Jensen J, Olsson LL, et al. Why the elderly fall in residential care
facilities, and suggested remedies. J Fam Pract 2004;53:41-52.
KG, Korner M, Linsenmaier U, et al. [Priority-oriented shock trauma
room management with the integration of multiple-view spiral computed
tomography]. Unfallchirurg 2004;107:937-944.
JL, White AA 3rd, Pastides H, Bisbee GE Jr. The impact of
musculoskeletal disorders on the population of the United States. J
Bone Joint Surg Am 1979;61A: 959-964.
B, Sevi R, Jeroukhimov I, et al. Is routine portable pelvic X-ray in
stable multiple trauma patients always justified in a high technology
era? Injury 2007;38:559-563.
Y, Jung KY, Kim CY, et al. Validation of the International
Classification of Diseases 10th Edition-based Injury Severity Score
(ICISS). J Trauma 2000;48:280-285.
AW, Sirois M, Laupland KB, et al. Prospective evaluation of hand-held
focused abdominal sonography for trauma (FAST) in blunt abdominal
trauma. Can J Surg 2005;48:453-460.
KW, Vassar MJ, Harry RL, Layton KD. Hospitalization charges, costs, and
income for firearm-related injuries at a university trauma center. JAMA
1995;273:1768-1773.
WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic
health evaluation: a physiologically based classification system. Crit
Care Med 1981; 9:591-597.
M, Krotz MM, Degenhart C, et al. Current role of emergency US in
patients with major trauma. Radiographics 2008;28:225-242.
Jagodic H, Jagodic K, Podbregar M. Long-term outcome and quality of
life of patients treated in surgical intensive care: a comparison
between sepsis and trauma. Crit Care 2006;10:R134.
C, Schandelmaier P, Rudolf J, Tscherne H. [Current status of surgical
technique for unreamed nailing of tibial shaft fractures with the UTN
(unreamed tibia nail)]. Unfallchirurg 1994;97:575-599.
PD, Brazil K, Lohfeld LH. Risk factors for falls and injuries in a
long-term care facility in Ontario. Can J Public Health 2001;92:117-120.
DA, Malone DL, McCarter RJ, et al. Predictors of mortality in adult
trauma patients: the physiologic trauma score is equivalent to the
Trauma and Injury Severity Score. J Am Coll Surg 2002;194:695-704.
CA, Ruchholtz S, Sauerland S, et al. [Personnel and structural
requirements for the shock trauma room management of multiple trauma. A
systematic review of the literature]. Unfallchirurg 2004;107:851-861.
PC, Ho KK, Chung TK, et al. Emergency aortic stent grafting for
traumatic rupture of the thoracic aorta. Hong Kong Med J 2003;9:435-440.
T, Verhoye JP, Corbineau H, et al. Surgical treatment of acute
traumatic rupture of the thoracic aorta: a timing reappraisal? Eur J
Cardiothorac Surg 2002;212: 282-287.
Z, Lausevic M, Trbojevic-Stankovic J, et al. Predicting multiple organ
failure in patients with severe trauma. Can J Surg 2008;51:97-102.
U, Gobiet W, Regel G, et al. [Functional, neuropsychological, and
social outcome of polytrauma patients with severe craniocerebral
trauma]. Unfallchirurg 1997;100:552-560.
M, Boutiere B, Camoin-Jau L, et al. Systemic endothelial activation is
greater in septic than in traumatic-hemorrhagic shock but does not
correlate with endothelial activation in skin biopsies. Crit Care Med
2002;30:808-814.
M, Branas C, Mulder D. Advanced versus basic life support in the
prehospital setting: the controversy between the scoop and run and the
stay-and-play approach to the care of the injured patient. Int J
Disaster Med 2004;2:1-9.
M, Mulder DS, Jurkovich GJ, Sampalis JS. The association between trauma
system and trauma center components and outcome in a mature
regionalized trauma system. Surgery 2005;137:647-658.
M, Mulder DS, Lavoie A, Sampalis JS. Implementation of a trauma care
system: evolution through evaluation. J Trauma 2004;56:1330-1335.
WC, Chen YF, Lin CH, et al. Emergent transcatheter arterial
embolization in hemodynamically unstable patients with blunt splenic
injury. Acad Radiol 2008;15: 201-208.
T, Bail HJ, Manegold S, et al. [Shock trauma room diagnosis: initial
diagnosis after blunt abdominal trauma. A review of the literature].
Unfallchirurg 2004;107: 892-902.
BC, Ivers R, Norton R, et al. Helmets for preventing injury in
motorcycle riders. Cochrane Database Syst Rev 2008;1:CD004333.
PR, Galley HF, Abdel-Fattah A, Webster NR. Influence of interleukin-10
polymorphisms on interleukin-10 expression and survival in critically
ill patients. Crit Care Med 2003;31:34-38.
EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect
of trauma-center care on mortality. N Engl J Med 2006;354:366-378.
EJ, Shapiro S, Moody M, et al. Predicting posttrauma functional
disability for individuals without severe brain injury. Med Care
1986;24:377-387.
EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on
hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85
conversion table. Med Care 1989;27:412-422.
EJ. Review of evidence regarding trauma system effectiveness resulting
from panel studies. J Trauma 1999;47(suppl 3):S34-S41.
RC, Tiwary AD, Shackford SR, et al. Intra-abdominal injury following
blunt trauma. Identifying the high-risk patient using objective risk
factors. Arch Surg 1989; 124:809-813.
DL, Dunne J, Tracy JK, et al. Blood transfusion, independent of shock
severity, is associated with worse outcome in trauma. J Trauma
2003;545:898-905.
DL, Kuhls D, Napolitano LM, et al. Back to basics: validation of the
admission systemic inflammatory response syndrome score in predicting
outcome in trauma. J Trauma 2001;51:458-463.
GT, Hemphill JC, Morabito D, et al. Cerebral oxygenation during
hemorrhagic shock: perils of hyperventilation and the therapeutic
potential of hypoventilation. J Trauma 2000;48:1025-1032; discussion
1032-1033.
NC, Mullins RJ, MacKenzie EJ, et al. Systematic review of published
evidence regarding trauma system effectiveness. J Trauma 1999;47(suppl
3):S25-S33.
J, Cayten CG, Byrne DW, et al. Comparison between TRISS and ASCOT
methods in controlling for injury severity. J Trauma 1992;33:326-332.
VJ, Moore EE. Optimal trauma outcome: trauma system design and the
trauma team. Emerg Med Clin North Am 2007;25:643-54.
U, Halcomb JB, Pusateri AE. Intravenous rFVIIa administered for
hemorrhage control in hypothermic coagulopathic swine with grade V
liver injuries. J Trauma 2001;50:721-729.
MG, Heckman JD, Corley FG. Severe open fractures of the lower
extremity: a retrospective evaluation of the Mangled Extremity Severity
Score (MESS). J Orthop Trauma 1994;8:81-87.
MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal
volumes, recruitment maneuvers, and high-positive end-expiratory
pressure for acute lung injury and acute respiratory distress syndrome:
a randomized controlled trial. JAMA 2008; 299:637-645.
M, Adina H, Schmidt J. Correlation of procalcitonin and C-reactive
protein to inflammation, complications, and outcome during the
intensive care unit course of multiple-trauma patients. Crit Care
2005;10:R1.
SM, McGwin G Jr, Abernathy JH 3rd, et al. Motor vehicle crash-related
mortality is associated with prehospital and hospital-based resource
availability. J Trauma 2003; 54:273-279.
JW, Evans G, Kilgo PD, et al. A comparison of the abilities of nine
scoring algorithms in predicting mortality. J Trauma 2002;53:621-628;
discussion 628-629.
A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with
increased mortality in hemodynamically stable, high-risk, surgical
patients. Crit Care 2004;8:R60-R65.
E, Wiedmann HP, Leutwein B, et al. Technical parameters influencing the
severity of injury of front-seat, belt-protected car passengers on the
impact side in car-to-car side collisions with the main impact between
the front and rear seats (B-pillars). Int J Legal Med 1992;105:11-15.
DR, Gommers D, Papadakos PJ, Lachmann B. Mechanical ventilation affects
pulmonary inflammation in cardiac surgery patients: the role of the
open-lung concept. J Cardiothorac Vasc Anesth 2007;21:279-284.
AM, Lavery RF, Barone A, et al. Angiographic embolization for liver
injuries: low mortality, high morbidity. J Trauma 2003;55:1077-1081;
discussion 1081-1082.
EE. Hypertonic saline dextran for postinjury resuscitation:
experimental background and clinical experience. Aust N Z J Surg
1991;61:732-736.
L, Lavoie A, Bergeron E, et al. Modeling probability-based injury
severity scores in logistic regression models: the logit transformation
should be used. J Trauma 2007; 62:601-605.
ME, Flye CW. Initial experience with Lodox Statscan imaging system for
detecting injuries of the pelvis and appendicular skeleton. Emerg
Radiol 2006;13: 129-133.
D, Ruchholtz S, Waydhas C, Schweiberer L. [Is maximum management of
polytrauma patients financially assured?] Langenbecks Arch Chir Suppl
Kongressbd 1996;113:323-325.
AB, Jurkovich GJ, Cummings P, et al. The effect of organized systems of
trauma care on motor vehicle crash mortality. JAMA 2000;283:1990-1994.
AB, Jurkovich GJ, Rivara FP, et al. Effectiveness of state trauma
systems in reducing injury-related mortality: a national evaluation. J
Trauma 2000;48:25-30.
Highway Traffic Safety Administration. National occupant protection use
survey—1996: Controlled intersection study. Research Note. Washington,
DC: US Department of Transportation, 1997.
Highway Traffic Safety Administration. Traffic safety facts. Seat belt
use in 2007: Use rates in the states and territories. Washington, DC:
US Department of Transportation, 2008:1-3.
M, Scherer MA. C-reactive protein levels for early detection of
postoperative infection after fracture surgery in 787 patients. Acta
Orthop 2008;79:428-432.
PJ, Turen CH, Brumback RJ, Scarboro JM. Conversion of external fixation
to intramedullary nailing for fractures of the shaft of the femur in
multiply injured patients. J Bone Joint Surg Am 2000;82A:781-788.
MS, Yardan T, Guven H, et al. Diagnostic value of ultrasonography in
the evaluation of blunt abdominal trauma. Diagn Interv Radiol
2005;11:41-44.
AK, Barleben A, Porral D, et al. Utility of plain film pelvic
radiographs in blunt trauma patients in the emergency department. Am
Surg 2006;72:951-954.
U, Neudeck F, Wihs HJ, Schmit-Neuerburg KP. [Emergency care and
treatment costs of polytrauma patients]. Langenbecks Arch Chir Suppl
Kongressbd 1996; 113:641-645.
HJ, Tscherne H. Pathophysiology and classification of soft tissue
injuries associated with fractures. In Tscherne H, Gotzen L, eds.
Fractures with Soft Tissue Injuries. Berlin: Springer Verlag, 1984:1-9.
of National Statistics. Personal Social Services Expenditure and Unit
Costs: England: 2003-2004. Retrieved April 15, 2009, from
http://www.statistics.gov.uk/.
S, de Charro FT. The effect of medical care by a helicopter trauma team
on the probability of survival and the quality of life of hospitalized
victims. Accid Anal Prev 2001;33:129-138.
T, Baker SP, Long W. A modification of the injury severity score that
both improves accuracy and simplifies scoring. J Trauma 1997;43:922-925.
JJ, Riederer M. [Quality assessment of multiple trauma management bu
ISS, TRISS or ASCOT?]. Schweiz Med Wochenschr 2000;130:499-504.
JJ. Can the “golden hour of shock” safely be extended in blunt
polytrauma patients? Prospective cohort study at a level I hospital in
eastern Switzerland. Prehosp Disaster Med 2002;17:75-80.
JJ. Mortality of blunt polytrauma: a comparison between emergency
physicians and emergency medical technicians—prospective cohort study
at a level I hospital in eastern Switzerland. J Trauma 2003;55:355-361.
L, Cooper DJ, Irons S, et al. Cervical spine clearance in unconscious
traumatic brain injury patients: dynamic flexion-extension fluoroscopy
versus computed tomography with three-dimensional reconstruction. J
Trauma 2006;60:341-345.
IN, Kanakaris N, Triantafillidis A, et al. Autopsy findings from 111
deaths in the 1999 Athens earthquake as a basis for auditing the
emergency response. Br J Surg 2004;91:1633-1640.
C, Kanakaris NK, Kontakis G, Giannoudis P. Pelvic ring disruptions:
treatment modalities and analysis of outcomes. Int Orthop
2009;33:329-338.
HC, Giannoudis PV, Krettek C, et al. Timing of fixation of major
fractures in blunt polytrauma: role of conventional indicators in
clinical decision making. J Orthop Trauma 2005;19:551-562.
HC, Giannoudis PV, Krettek C. The timing of fracture treatment in
polytrauma patients: relevance of damage control orthopaedic surgery.
Am J Surg 2002;183: 622-629.
HC, Grimme K, van Griensven M, et al. Impact of intramedullary
instrumentation versus damage control for femoral fractures on
immunoinflammatory parameters: prospective randomized analysis by the
EPOFF Study Group. J Trauma 2003;55:7-13.
HC, Rixen D, Morley J, et al. Impact of the method of initial
stabilization for femoral shaft fractures in patients with multiple
injuries at risk for complications (borderline patients). Ann Surg
2007;246:491-499.
HC, Stalp M, van Griensven M, et al. [Optimal timing for secondary
surgery in polytrauma patients: an evaluation of 4314 serious-injury
cases]. Chirurg 1999;70: 1287-1293.
HC, van Griensven M, Rice J, et al. Major secondary surgery in blunt
trauma patients and perioperative cytokine liberation: determination of
the clinical relevance of biochemical markers. J Trauma
2001;50:989-1000.
HC, Zelle B, Lohse R, et al. Evaluation and outcome of patients after
polytrauma: can patients be recruited for long-term follow-up? Injury
2006;37:1197-1203.
MB, Herber S, Schmiedt W, et al. Long-term follow-up after endovascular
treatment of acute aortic emergencies. Cardiovasc Intervent Radiol
2008;31:23-35.
FB, Slutsky AS, van Vught AJ, Heijnen CJ. Ventilator-induced lung
injury and multiple system organ failure: a critical review of facts
and hypotheses. Intensive Care Med 2004;30:1865-1872.
S, Meerding WJ, van Baar ME, et al. Cost estimation of injury-related
hospital admissions in 10 European countries. J Trauma
2005;59:1283-1290; discussion 1290-1291.
guidelines for blood component therapy: a report by the American
Society of Anaesthesiologists Task Force on Blood Component Therapy.
Anaesthesiology 1996; 84:732-747.
C, Hildebrand F, Frink M, et al. [Prehospital treatment of severely
injured patients in the field: an update]. Chirurg 2007;78:875-884.
TH, Cheung NK, Yeung JH, et al. Do trauma teams make a difference? A
single centre registry study. Resuscitation 2007;73:374-381.
H, Schlag G, Hammerschmidt DE. Quantitative assessment of leukostasis
in experimental hypovolemic-traumatic shock, Acta Chir Scand
1984;150:113-117.
G, Bayeff-Filloff M. [Diagnosis and immediate therapeutic management of
limb injuries. A systematic review of the literature]. Unfallchirurg
2004;107:919-926.
CL, Perrey C, Pravica V, et al. Genetic variation in proinflammatory
and anti-inflammatory cytokine production in multiple organ dysfunction
syndrome. Crit Care Med 2002;30:2216-2221.
M, Pape HC, Otte D, Krettek C. Improvements in passive car safety led
to decreased injury severity: a comparison between the 1970s and 1990s.
Injury 2005; 36:484-488.
AN, Spanjersberg WR, Frankema SP, et al. Helicopter emergency medical
services (HEMS): impact on on-scene times. J Trauma 2007;63:258-262.
EB, von Bonsdorff H, Hakkinen S, et al. Primary operative fixation of
long bone fractures in patients with multiple injuries. J Trauma
1977;17:111-121.
FP, Thompson DC, Cummings P. Effectiveness of primary and secondary
enforced seat belt laws. Am J Prev Med 1999;16(suppl 1):30-39.
D, Siegel JH, Friedman HP. Sepsis/SIRS, physiologic classification,
severity stratification, relation to cytokine elaboration, and outcome
prediction in posttrauma critical illness. J Trauma 2001;51:458-463.
CS, Pape HC, Jones AL, et al. Damage control orthopaedics: evolving
concepts in the treatment of patients who have sustained orthopaedic
trauma. Instr Course Lect 2005;54:447-462.
GL, Leonard PR, Nagib MG. Analysis of management in 33 closed head
injury patients who “talked and deteriorated.” Neurosurgery
1987;21:51-55.
H. Effect of aeromedical aircraft on care of trauma patients:
evaluation using the Revised Trauma Score. South Med J
1992;85:1065-1071.
GS, Feliciano DV, Schmidt JA, et al. The role of surgeon-performed
ultrasound in patients with possible cardiac wounds. Ann Surg
1996;223:737-744; discussion 744-746.
S, Nast-Kolb D, Waydhas C, et al. [Cost analysis of clinical treatment
of polytrauma patients]. Chirurg 1995;66:684-692.
R, Osler T, Emery S, et al. The end of the Injury Severity Score (ISS)
and the Trauma and Injury Severity Score (TRISS): ICISS, an
International Classification of Diseases, ninth revision-based
prediction tool, outperforms both ISS and TRISS as predictors of trauma
patient survival, hospital charges, and hospital length of stay. J
Trauma 1998;44:41-49.
WJ, MacKenzie EJ, Champion HR, et al. Comparison of alternative methods
for assessing injury severity based on anatomic descriptors. J Trauma
1999;47:441-446; discussion 446-447.
H, Nguyen-Tang T, Stern R, et al. Control of severe hemorrhage using
C-clamp and arterial embolization in hemodynamically unstable patients
with pelvic ring disruption. Arch Orthop Trauma Surg 2005;125:443-447.
A 2nd, Kibbe M, Matsumura J, Eskandari MK. Blunt traumatic aortic
transection: endoluminal repair with commercially available aortic
cuffs. J Vasc Surg 2003;38: 1132-1135.
G, Di Bartolomeo S, Nardi G, et al. Road traffic accidents with
vehicular entrapment: incidence of major injuries and need for advanced
life support. Eur J Emerg Med 1999;6:285-291.
NA, Chiros CE, Sigford B, et al. Characteristics and rehabilitation
outcomes among patients with blast and other injuries sustained during
the Global War on Terror. Arch Phys Med Rehabil 2008;89:163-170.
TM, Boswell SA, Scott JD, et al. External fixation as a bridge to
intramedullary nailing for patients with multiple injuries and with
femur fractures: damage control orthopaedics. J Trauma 2000;48:613-621.
TM, Rodriguez A, Chiu WC, et al. Focused assessment with sonography for
trauma (FAST): results from an international consensus conference. J
Trauma 1999; 46:466-472.
G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions
in critically ill patients: a systematic review of randomised trials.
BMJ 1998;69:961-964.
A, Ziegler D, Beck A, et al. [Costs for acute, stationary treatment of
polytrauma patients]. Unfallchirurg 2002;105:1043-1048.
M, Prokop M, Lammer J. Traumatic injuries: imaging and intervention of
large arterial trauma. Eur Radiol 2002;12:1617-1631.
CJ, Uflacker R, de Gregorio MA, et al. Stent-graft treatment of trauma
to the supra-aortic arteries. A review. J Cardiovasc Surg (Torino)
2007;48:537-549.
MA, Halcomb JB, Hedner U. The effect of recombinant factor VIIa on
coagulopathic pigs with grade V liver injuries. J Trauma
2002;53:252-259.
O, Laun RA, Held B, et al. Association of interleukin-10 promoter
polymorphism with the incidence of multiple organ dysfunction following
major trauma: results of a prospective pilot study. Shock
2004;21:306-310.
SJ, Weisberg A, Scalea TM, et al. Blunt splenic injuries: nonsurgical
treatment with CT, arteriography, and transcatheter arterial
embolization of the splenic artery. Radiology 1991;181:189-196.
BW, Luchette FA, Esposito TJ, et al. Old fashion clinical judgment in
the era of protocols: is mandatory chest x-ray necessary in injured
patients? J Trauma 2005;59: 324-330; discussion 330-332.
A, Regel G, Bauch S, et al. [Long-term results of therapy of polytrauma
patients with special reference to serial fractures of the lower
extremity]. Unfallchirurg 1994; 97:57-63.
ML, Blake AM, Whitley M, et al. The benefit of routine thoracic,
abdominal, and pelvic computed tomography to evaluate trauma patients
with closed head injuries. Am J Surg 2003;186:609-613; discussion
613-614.
D, Kwan I, Kelly AM, et al. Advanced trauma life support training for
ambulance crews. Cochrane Database Syst Rev 2001;2:CD003109.
SR. Effects of small-volume resuscitation on intracranial pressure and
related cerebral variables. J Trauma 1997;42:S48-S53.
S, Nathens AB, Elliott AC, et al. Effect of trauma systems on motor
vehicle occupant mortality: a comparison between states with and
without a formal system. J Trauma 2006;61:1374-1378.
RA, Elder RW, Sleet DA, et al. Primary enforcement seat belt laws are
effective even in the face of rising belt use rates. Accid Anal Prev
2004;36:491-493.
RA, Nichols JL, Dinh-Zarr TB, et al. Effectiveness of primary
enforcement safety belt laws and enhanced enforcement of safety belt
laws: a summary of the Guide to Community Preventive Services
systematic reviews. J Safety Res 2004;35:189-196.
C, Stegmaier J, Kirchhoff C, et al. [Analysis of failure modes in
multislice computed tomography during primary trauma survey]. Rofo
2008;180:733-739.
JH, Mason-Gonzalez S, Dischinger PC, et al. Causes and costs of
injuries in multiple trauma patients requiring extrication from motor
vehicle crashes. J Trauma 1993;35:920-931.
M, Williams K, White C, Moran CG. The financial cost of treating
polytrauma: implications for tertiary referral centres in the United
Kingdom. Injury 2005;36: 733-737.
GJ, Kramer GC, Perron P. A comparison of several hypertonic solutions
for resuscitation of bled sheep. J Surg Res 1985;39:517-528.
G, Testa A, Sher S, et al. Occult traumatic pneumothorax: diagnostic
accuracy of lung ultrasonography in the emergency department. Chest
2008;133:204-11.
S, Luengtaviboon K, Benjacholamas V, et al. Significance of a widened
mediastinum in blunt chest trauma patients. J Med Assoc Thai
2000;83:1296-1301.
M, Koch C, Regel G, et al. [Development of a standardized instrument
for quantitative and reproducible rehabilitation data assessment after
polytrauma (HASPOC)]. Chirurg 2001;72:312-318.
M, Koch C, Ruchholtz S, et al. Standardized outcome evaluation after
blunt multiple injuries by scoring systems: a clinical follow-up
investigation 2 years after injury. J Trauma 2002;52:1160-1168.
DM, O’Toole R, Scalea TM. Multidisciplinary approach for patients with
pelvic fractures and hemodynamic instability. Scand J Surg
2007;96:272-280.
SC, Langley JD, Civil ID. Comparing measures of injury severity for use
with large databases. J Trauma 2002;53:326-332.
W, Gebhard F, Perl M, et al. Biochemical characterization of individual
injury pattern and injury severity. Injury 2003;34-12:879-887.
N, Haas N, Flory PJ, et al. Criteria for amputation, reconstruction and
replantation of extremities in multiple trauma patients. Chirurg
1989;60:774-781.
PN, Sherman AJ, Silver JM, et al. Traumatic rupture of the aorta:
immediate or delayed repair? Ann Surg 2002;235-6:796-802.
Force on Principles for Economic Analysis of Health Care Technology.
Economic analysis of health care technology. A report on principles.
Ann Intern Med 1995;123: 61-70.
American College of Surgeons Committee on Trauma Leadership. In: Clark
DE, Fantus RJ, eds. National Trauma Data Bank (NTDB) annual report.
Chicago: 2007: 1-64.
American College of Surgeons. Advanced Trauma Life Support (ATLS)
students manual. 6th edition. Chicago, IL: American College of Surgeons
1997.
Association for the Advancement of Automotive Medicine (AAAM).
Retrieved April 15, 2009, from http://www.carcrash.org/index.html.
SH. Helicopter emergency medical services transport outcomes
literature: annotated review of articles published 2000-2003. Prehosp
Emerg Care 2004;8: 322-333.
M, Messick J, Rutledge R, et al. Head CT scanning versus urgent
exploration in the hypotensive blunt trauma patient. J Trauma
1993;34-1:40-44.
A, Russo SG, Hollmann MW. Paramedic versus emergency physician
emergency medical service: role of the anaesthesiologist and the
European versus the Anglo-American concept. Curr Opin Anaesthesiol
2008;21:222-227.
A, Dormagen JB, Madsen JE, et al. A protocol for angiographic
embolization in exsanguinating pelvic trauma: a report on 31 patients.
Acta Orthop 2006;77: 462-468.
A, Glott T, Soberg HL, et al. Pelvic trauma with displaced sacral
fractures: functional outcome at 1 year. Spine 2007;32:1437-1443.
A, Madsen JE, Skaga NO, Røise O. Extraperitoneal pelvic packing: a
salvage procedure to control massive traumatic pelvic hemorrhage. J
Trauma 2007;62: 843-852.
T, Thordarson D. Functional outcome of multiply injured patients with
associated foot injury. Foot Ankle Int 2002;23:340-343.
T, Monsky WL, London J, et al. Evaluation of short-term and long-term
complications after emergent internal iliac artery embolization in
patients with pelvic trauma. J Vasc Interv Radiol 2008;19:840-847.
OL. Polytrauma: pathophysiology, priorities, and management. In Rüedi
TP, Buckley RE, Moran CG. AO Principles of Fracture Management.
Stuttgart/New York: Thieme, 2007;337-347.
A, Andreade A, Zacharogiannis C, et al. Myocardial contusion presented
as acute myocardial infarction after chest trauma. Echocardiography
2001;18:167-170.
SN, Knudsen NW, Neligan PJ, Sebastian MW. Massive transfusion exceeding
50 units of blood products in trauma patients. J Trauma
2002;53-2:291-295.
GC, Theodorou D, Tatevossian R, et al. Radiographic cervical spine
evaluation in the alert asymptomatic blunt trauma victim: much ado
about nothing. J Trauma 1996;40:768-774.
GC, Toutouzas KG, Vassiliu P, et al. A prospective study on the safety
and efficacy of angiographic embolization for pelvic and visceral
injuries. J Trauma 2002; 53:303-308; discussion 308.
G, Eisold C, Sauerland S, et al. [Diagnosis and immediate therapeutic
management of chest trauma. A systematic review of the literature].
Unfallchirurg 2004; 107:881-891.
SB, Annest JL, Ryan GW. Surveillance for fatal and nonfatal injuries:
United States, 2001. MMWR Surveill Summ 2004;53:1-57.
CE, Kramer GC, Grady JJ, et al. Efficacy of hypertonic 7.5% saline and
6% dextran-70 in treating trauma: a meta-analysis of controlled
clinical studies. Surgery 1997;122:609-616.
SL, Shackford SR, Fenwick J. The effect of secondary insults on
mortality and long-term disability after severe head injury in a rural
region without a trauma system. J Trauma 1993;34-3:377-381.
H, Ma S. The cytokine storm and factors determining the sequence and
severity of organ dysfunction in multiple organ dysfunction syndrome.
Am J Emerg Med 2008; 26:711-715.
LD, Morandi MM, Pearse M, et al. The immediate treatment of pelvic ring
disruption with the pelvic stabilizer. Bull Hosp Jt Dis 1997;56:104-106.
J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey:
construction of scales and preliminary tests of reliability and
validity. Med Care 1996;34:220-233.
E, Hirasawa H, Oda S, et al. Cytokine-related genotypic differences in
peak interleukin-6 blood levels of patients with SIRS and septic
complications. J Trauma 2005;59:1181-1189.
C, Nast-Kolb D, Trupka A, et al. Posttraumatic inflammatory response,
secondary operations, and late multiple organ failure. J Trauma
1996;40:624-631.
RB, Viele MK, Feiner J. Human cardiovascular and metabolic response to
acute, severe isovolaemic haemorrhage. JAMA 1998;279:217-221.
P, Mauritz W, Fridrich P, et al. Emergency room management of patients
with blunt major trauma: evaluation of the multislice computed
tomography protocol exemplified by an urban trauma center. J Trauma
2007;62:584-591.
TA, Rivara FP, Cummings P, et al. Harborview assessment for risk of
mortality: an improved measure of injury severity on the basis of
ICD-9-CM. J Trauma 2000;49: 530-540.
RF, Fabian TC, Fischer PE, et al. Impact of airbags on a Level I trauma
center: injury patterns, infectious morbidity, and hospital costs. J Am
Coll Surg 2008;206: 962-968.
C, Willis C, Hendrikz JK, Bellamy N. Speed enforcement detection
devices for preventing road traffic injuries. Cochrane Database Syst
Rev 2006;2:CD004607.
JE, Hinshaw JW, Hughes MJ, et al. Quantitative assessment of diagnostic
radiation doses in adult blunt trauma patients. Ann Emerg Med
2008;52:93-97.
M, Poletti PA, Becker CD, et al. Traumatic injuries: organization and
ergonomics of imaging in the emergency environment. Eur Radiol
2002;12:959-968.
DH, Victor NS, Holcroft JW. Priorities in the management of multiple
trauma: intracranial versus intra-abdominal injury. J Trauma
1993;35:271-276.
CG, Bouillon B, Lackner CK, et al. [Prehospital Trauma Life Support
(PHTLS): an interdisciplinary training in preclinical trauma care].
Unfallchirurg 2008;111:688-694.
TP, Fuchs PC, Horvat N, Pallua N. Is high PEEP low volume ventilation
in burn patients beneficial? A retrospective study of 61 patients.
Burns 2004;30:368-373.
A, Buhren V. [Shock trauma room management of spinal injuries in the
framework of multiple trauma. A systematic review of the literature].
Unfallchirurg 2004;107:911-918.
JS, Sheng L, Wang SH, et al. The impact of clinical risk factors in the
conversion from acute lung injury to acute respiratory distress
syndrome in severe multiple trauma patients. J Int Med Res
2008;36:579-586.
BA, Brown SR, Panzica M, et al. The impact of injuries below the knee
joint on the long-term functional outcome following polytrauma. Injury
2005;36:169-177.
BA, Panzica M, Vogt MT, et al. Influence of workers’ compensation
eligibility upon functional recovery 10 to 28 years after polytrauma.
Am J Surg 2005;190:30-36.
JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health
Evaluation (APACHE) IV: hospital mortality assessment for today’s
critically ill patients. Crit Care Med 2006;34:1297-1310.
B, Ruchholtz S, Nast-Kolb D, et al. [Quality management in early
clinical multiple trauma care. Documentation of treatment and
evaluation of critical care quality]. Unfallchirurg 1997;100:811-819.
