Cervical Spine Injuries in Children
examined the autopsied spines of 12 juveniles who had spinal injuries.
All 12 had cartilage endplates that were separated from the vertebral
bodies in the zone of columnar and calcified cartilage, similar to a
Salter-Harris type I fracture, although clinically and
radiographically, a fracture was suggested in only one patient. Only
radiographs at autopsy showed the disruption, represented by a small
gap apparent widening of the intervertebral space.13
age occur in the upper cervical spine, while older children and
adolescents tend to have fractures involving either the upper
or lower cervical spine.163
The upper cervical spine in children is more prone to injury because of
the anatomic and biomechanical properties of the immature spine. The
immature spine is hypermobile because of ligamentous laxity, and the
facet joints are oriented in a more horizontal position; both of these
properties predispose children to more forward translation. Younger
children also have a relatively large head compared to the body, which
changes the fulcrum of motion of the upper cervical spine. All of these
factors predispose younger children to injuries of the upper cervical
spine; with age, the anatomic changes lead to an increased prevalence
of lower cervical spine injuries.
agerelated. Infants with cervical spine injuries should be evaluated
for abuse.81 In children up to 9
years of age, the most common mechanism of injury is related to motor
vehicle accidents; the second most common mechanism in this age group
is falls.33,243
In older children and adolescents, the most common mechanism of
cervical spine injuries is sporting activities, followed by motor
vehicle accidents.13,224
deficits are infrequent in children, and when incomplete there tends to
be a better prognosis for recovery in children than in adults.21,59
Complete neurologic deficits, regardless of patient age, tend to have a
poor prognosis for any recovery and may be indicative of the severity
and magnitude of injury.58,120,155,171
Death from cervical spine injuries tends to be related to the level of
injury and the associated injuries. Higher cervical spine injuries
(i.e., atlanto-occipital dislocation) in younger children are
associated with the highest mortality rate.161,162
Children with significant cervical spine injuries also may have
associated severe head injuries, leading to an increase in mortality.
In a study of 61 pediatric deaths related to spinal cord injuries, 89%
of fatalities occurred at the scene, and most were related to high
cervical cord injuries in patients who had sustained multiple injuries.90
cervical spine is essential when treating a child with a suspected
cervical spine injury. This will allow the physician to differentiate
normal physes or synchondroses from pathologic fractures or ligamentous
disruptions and will alert the physician to any possible congenital
anomalies that may be mistaken for a fracture.
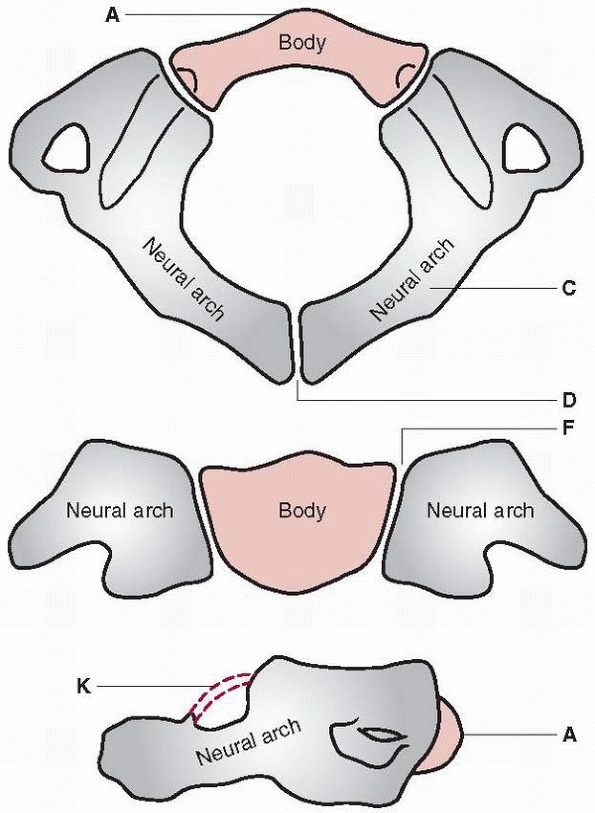
The ossification center for the anterior arch is present in
approximately 20% of individuals at birth, appearing in the remainder
during the first year of life. Occasionally, the anterior arch is
bifid, and the body may be formed from two centers or may fail to
completely appear. The posterior arches usually fuse by the age of 3
years; however, occasionally the posterior synchondrosis between the
two fails to fuse, resulting in a bifid arch. The neurocentral
synchondroses that link the neural arches to the body are best seen on
an open-mouth odontoid view. These synchondroses close by 7 years of
age and should not be mistaken for fractures.41
The canal of the atlas is large to allow for the amount of rotation
that occurs at this joint as well as some forward translation.43
The vertebral arteries are about 2 cm from the midline and run in a
groove on the superior surface of the atlas. This must be remembered
during lateral dissection at the occipital cervical junction. Because
the ring of C1 reaches about normal adult size by 4 years of age,
arthrodesis after this time should not cause spinal canal stenosis.
 |
|
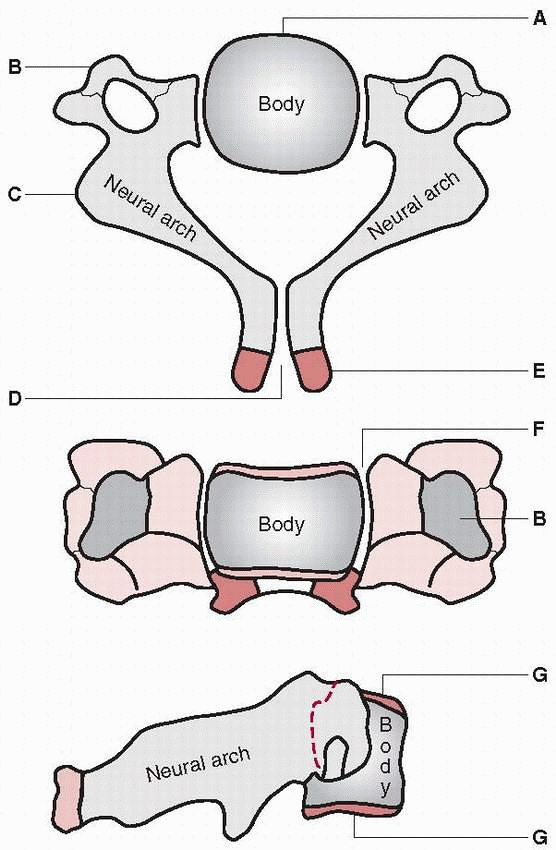
FIGURE 18-1 Diagram of C1 (atlas). The body (A)
is not ossified at birth, and its ossification center appears during the first year of life. The body may fail to develop, and forward extension of neural arches (C) may take its place. Neural arches appear bilaterally about the seventh week (D), and the most anterior portion of the superior articulating surface usually is formed by the body. The synchondrosis of the spinous processes unites by the third year. Union rarely is preceded by the appearance of the secondary center within the synchondrosis. Neurocentral synchondrosis (F) fuses about the seventh year. The ligament surrounding the superior vertebral notch (K) may ossify, especially in later life. (From Bailey DK. Normal cervical spine in infants and children. Radiology 1952;59: 713-714, with permission.) |
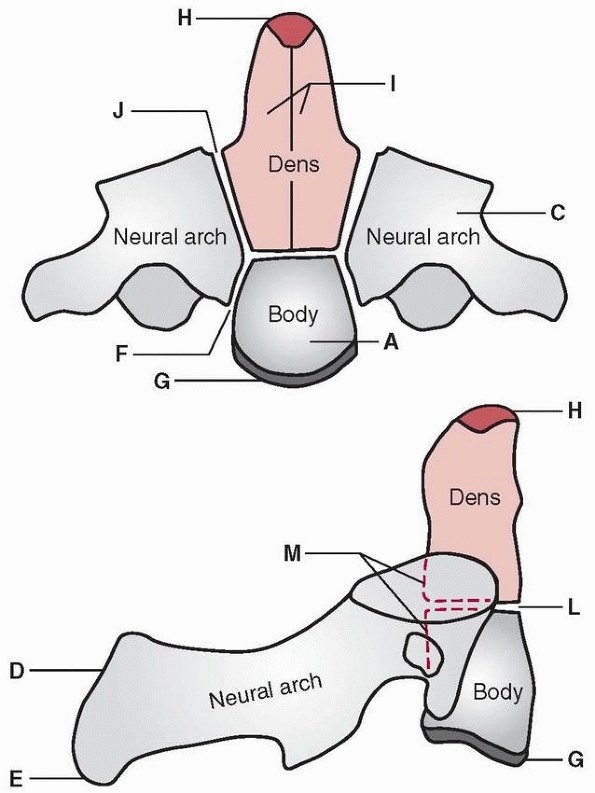
ossification centers: one for the dens, one for the body, and two for
the neural arches (Fig. 18-2). Between the
odontoid and the body of the axis is a synchondrosis or vestigial disk
space that often is mistaken for a fracture line. This synchondrosis
runs well below the level of the articular processes of the axis and
usually fuses at 6 to 7 years of age, although it may persist as a
sclerotic line until 11 years of age.43
The most common odontoid fracture pattern in adults and adolescents is
transverse and at the level of the articular processes. The normal
synchondrosis should not be confused with this fracture; the
synchondrosis is more cup-shaped and below the level of the articular
processes. After 7 years of age, the synchondrosis should not be
present on an open-mouth odontoid view; a fracture should be considered
if a lucent line is present after this age. The neural arches of C2
fuse at 3 to 6 years of age; these are seen as vertical lucent lines on
the open-mouth odontoid view. Occasionally, the tip of the odontoid is
V-shaped (dens bicornum), or a small separate summit
ossification
center may be present at the tip of the odontoid (ossiculum terminale).
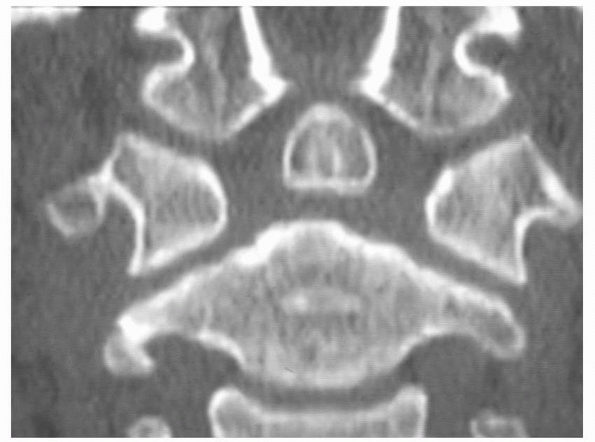
An os odontoideum is believed to result from a history of unrecognized
trauma. The differentiation between an os odontoideum and the
synchondrosis of the body is relatively easy because of their
relationships to the level of the C1-C2 facet (Fig. 18-3).
 |
|
FIGURE 18-2 Diagram of C2 (axis). The body (A) in which one center (occasionally two) appears by the fifth fetal month. Neural arches (C) appear bilaterally by the seventh fetal month. Neural arches fuse (D) posteriorly by the second or third year. Bifid tip (E) of spinous process (occasionally a secondary center is present in each tip). Neurocentral synchondrosis (F) fuses at 3 to 6 years. The inferior epiphyseal ring (G) appears at puberty and fuses at about 25 years of age. The summit ossification center (H) for the odontoid appears at 3 to 6 years and fuses with the odontoid by 12 years. Odontoid (dens) (I).
Two separate centers appear by the fifth fetal month and fuse with each other by the seventh fetal month. The synchondrosis between the odontoid and neural arch (I) fuses at 3 to 6 years. Synchondrosis between the odontoid and body (L) fuses at 3 to 6 years. Posterior surface of the body and odontoid (M). (From: Bailey DK. Normal cervical spine in infants and children. Radiology 1952;59:713-714, with permission.) |
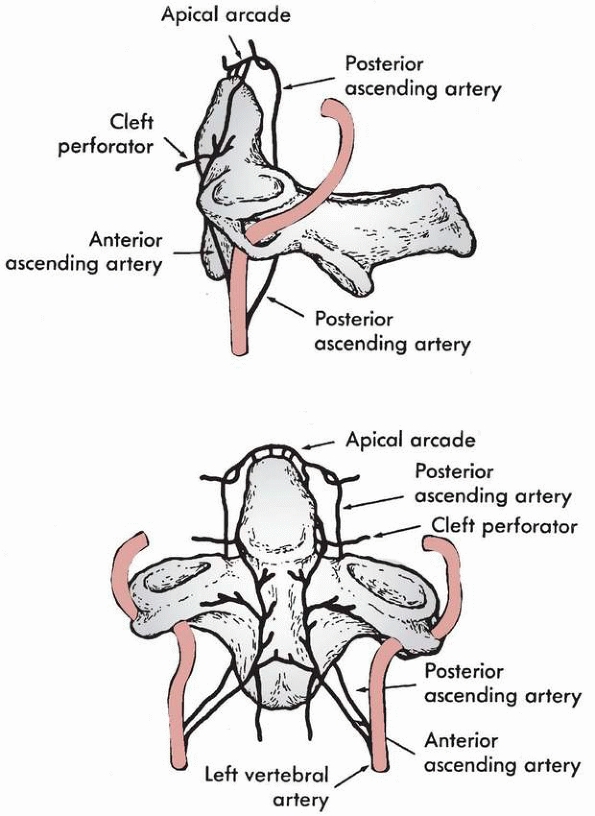
vertebral and carotid arteries. The anterior and posterior ascending
arteries arise from the vertebral artery at the level of C3 and ascend
anterior and posterior to the odontoid, meeting superiorly to form an
apical arcade. These arteries supply small penetrating branches to the
body of the axis and the odontoid process. The internal carotid artery
gives off cleft perforators that supply the superior portion of the
odontoid. This arrangement of arteries and vessels is necessary for
embryologic development and anatomic function of the odontoid. The
synchondrosis prevents direct vascularization of the odontoid from C2,
and vascularization from the blood supply of C1 is not possible because
the synovial joint cavity surrounds the odontoid. The formation of an
os odontoideum after cervical trauma may be related to this peculiar
blood supply (Fig. 18-4).
 |
|
FIGURE 18-3
CT scan showing presence of an os odontoideum. Note the position of the os well above the C1-C2 facets. The scan also shows the vestigial scar of the synchondrosis between the dens and the body below the C1-C2 facet. |
 |
|
FIGURE 18-4
Blood supply to odontoid: posterior and anterior ascending arteries and apical arcade. (From Schiff DC, Parke WW. The arterial supply of the odontoid process. J Bone Joint Surg Am 1973;55:1450-1464, with permission.) |
similar ossification pattern: a single ossification center for the
vertebral body and an ossification center for each neural arch (Fig. 18-5).
The neural arch fuses posteriorly between the second and third years,
and the neurocentral synchondroses between the neural arches and the
vertebral body fuse by 3 to 6 years of age. These vertebrae normally
are wedge-shaped until 7 to 8 years of age.13,131,179
The vertebral bodies, neural arches, and pedicles enlarge by periosteal
appositional growth, similar to that seen in long bones. By 8 to 10
years of age, a child’s spine usually reaches near adult size and
characteristics. There are five secondary ossification centers that can
remain open until 25 years of age.131
These include one each for the spinous processes, transverse processes,
and the ring apophyses about the vertebral endplates. These should not
be confused with fractures.
the adjacent disk. The junction between the vertebral body and the
endplate is similar to a physis of a long bone. The vertebral body is
analogous to the metaphysis and the endplate to the physis, where
longitudinal growth occurs. The junction between the vertebral body and
the endplate has been shown to be weaker than the adjacent vertebral
body or disk, which can result in a fracture at the endplate in the
area of columnar and calcified cartilage of the growth zone, similar to
a Salter-Harris type I fracture of a long bone.13
The inferior end plate may be more susceptible to this injury than the
superior endplate because of the mechanical protection afforded by the
developing uncinate processes.24
 |
|
FIGURE 18-5 Diagram of typical cervical vertebrae, C3 to C7. The body (A) appears by the fifth fetal month. The anterior (costal) portion of the transverse process (B) may develop from a separate center that appears by the sixth fetal month and joins the arch by the sixth year. Neural arches (C) appear by the seventh to ninth fetal week. The synchondrosis between spinous processes (D) usually unites by the second or third year. Secondary centers for bifid spine (E) appear at puberty and unite with spinous process at 25 years. Neurocentral synchondrosis (F) fuses at 3 to 6 years. Superior and inferior epiphyseal rings (G)
appear at puberty and unite with the body at about 25 years. The seventh cervical vertebra differs slightly because of a long, powerful, nonbifid spinous process. (From: Bailey DK. Normal cervical spine in infants and children. Radiology 1952; 59:713-714, with permission.) |
orientation with age. The angle of the C1-C2 facet is 55 degrees in
newborns and increases to 70 degrees at maturity. In the lower cervical
spine, the angle of the facet joints is 30 degrees at birth and 60 to
70 degrees at maturity. This may explain why the pediatric cervical
spine may be more susceptible to injury from the increased motion or
translation allowed by the facet joint orientation.
greater degree of spinal mobility than in adults. Flexion and extension
of the spine at C2-C3 are 50% greater in children between the ages of 3
and 8 years than in adults. The level of the greatest mobility in the
cervical spine descends with increasing age. Between 3 and 8 years of
age, the most mobile segment is C3-C4; from 9 to 11 years, C4-C5 is the
most mobile segment, and from 12 to 15 years, C5-C6 is the most mobile
segment.4,172 This explains the tendency for craniocervical injuries in young children.
treatment recommendations. The atlas can fail to segment from the
skull, a condition called occipitalization of the atlas, and can lead
to narrowing of the foramen magnum, neurologic symptoms, and increased
stresses to the atlantoaxial articulation, which often causes
instability. Failure of fusion of the posterior arch of C1 is not
uncommon and should be sought before any procedure that involves C1.
Wedge-shaped vertebrae, bifid vertebrae, or a combination of these also
can occur. Klippel-Feil syndrome consists of the classic triad of a
short neck, low posterior hairline, and severe restriction of motion of
the neck from fusion of the cervical vertebrae.101,122
Congenital fusion of the cervical spine may predispose a child to
injury from trauma by concentrating stresses in the remaining mobile
segments.
reported congenital anomalies of the odontoid, including aplasia
(complete absence), hypoplasia (partial absence in which there is a
stubby piece at the base of the odontoid located above the C1
articulation), and os odontoideum. Os odontoideum consists of a
separate ossicle of the odontoid with no connection to the body of C2.
The cause may be traumatic. These anomalies also may predispose a child
to injury or instability.
result of motor vehicle accidents, sporting injuries, or pedestrian
injuries.124 Infants are at risk for
cervical spine injuries during the obstetric period as well as during
early development because of their lack of head control; however, most
cervical spine injuries
in infants are spinal cord injury without radiologic abnormality (SCIWORA) and are related to child abuse.13
Younger children may sustain injuries to their neck from seemingly
lowenergy falls of less than 5 feet; however, most of their cervical
spine injuries are sustained as a result of motor vehicle accidents.109,195
As children become adolescents, the prevalence of sporting injuries
increases, as does the prevalence of athleticrelated SCIWORA.33,124
Regardless of the cause, an adequate history may be difficult to obtain
at the initial evaluation, and repeat evaluations may be needed.
cervical spine injuries is pain localized to the cervical region. Other
complaints, such as headache, inability to move the neck, subjective
feelings of instability, and neurologic symptoms, all warrant complete
evaluation. Infants may present with unexplained respiratory distress,
motor weakness, or hypotonia, which warrant further evaluation.
Patients with head and neck trauma, distracting injuries, or altered
levels of consciousness are at high risk for a cervical spine injury
and need to be thoroughly evaluated before obtaining cervical spine
clearance.33 The presence of an
occult cervical spine injury in an uncooperative or obtunded patient
needs to be considered because of the frequency of SCIWORA in the
pediatric population.164,189
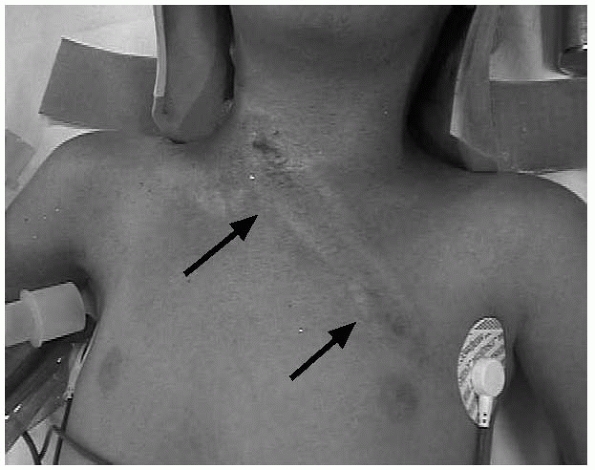
spine injury should begin with inspection. Head and neck trauma is
associated with a high incidence of cervical spine injuries.4,13 Soft tissue abrasions or shoulder-harness marks on the neck from a seatbelt are clues to an underlying cervical spine injury (Fig. 18-6).72,81,107
Unconscious patients should be treated as if they have a cervical spine
injury until further evaluation proves otherwise. The next step in the
evaluation is palpation of the cervical spine for tenderness, muscle
spasm, and overall alignment. The most prominent levels should be the
spinous processes at C2, C3, and C7. Anterior palpation should focus on
the presence of tenderness or swelling. The entire spine should be
palpated and thoroughly examined because 20% of patients with cervical
spine injuries have other spinal fractures.
 |
|
FIGURE 18-6
Clinical photograph of a patient with a cervical spine injury resulting from impact with the shoulder harness of a seat belt. Note location of skin contusions from the seatbelt. |
can be difficult in pediatric patients. Strength, sensation, reflexes,
and proprioception should be documented. In patients who are
uncooperative because of age or altered mental status, repeat
examinations are important; however, the initial neurovascular
examination should be documented even it if entails only gross
movements of the extremities. The evaluation of rectal sphincter tone,
bulbocavernosus reflex, and perianal sensation are important,
especially in obtunded patients and patients with partial or complete
neurologic injuries, regardless of age. Patients who are cooperative
and awake can be asked to perform supervised flexion, extension,
lateral rotation, and lateral tilt. Uncooperative or obtunded patients
should not have any manipulation of the neck.
cervical spine in children. There currently is no consensus regarding
whether or not all pediatric trauma patients require cervical spine
films. The presence of tenderness and a distracting injury are the most
common clinical presentations of a cervical spine injury.230
While some studies have shown that plain radiographs are of low yield
in patients without evidence of specific physical findings, the burden
remains on the treating physician to clear the cervical spine.8,53,130,134
Clearly, patients with tenderness, distracting injuries, neurologic
deficits, head and neck trauma, and altered levels of consciousness
need to have a complete set of cervical spine radiographs. Initial
radiographs should include an anteroposterior view, open-mouth odontoid
view, and lateral view of the cervical spine. Patients who are deemed
unstable in the emergency room and are not able to tolerate multiple
radiographs should have a cross-table lateral view of the cervical
spine until further radiographs can be taken. The false-negative rates
for a single cross-table radiograph have been reported to be 23% to
26%, indicating that complete radiographs are necessary when the
patient is stable.15,200
evaluation of the cervical spine, but these views are unlikely to be
abnormal when standard views show no abnormalities. These views are
helpful, however, in ruling out acute ligamentous injury.180
We recommend flexion and extension views in an alert patient with
midline tenderness who has normal plain films of the cervical spine.
These views must be taken only with a cooperative and alert child; they
should not be used in obtunded or uncooperative patients, nor should
they be done by manually placing the child in a position of flexion and
extension.
with a knowledge of the anatomic ossification centers and variations
that occur in children. Each vertebral level should be systematically
evaluated, as should the overall alignment of the cervical spine with
respect to the anterior and posterior aspects of the vertebral bodies,
the spinolaminar line, and the interspinous distances. The absence of
cervicallordosis, an increase in the
prevertebral soft tissue space, and subluxation of C2 on C3 are all anatomic variations that may be normal in children.41
Ossification centers also may be confused with fractures, most commonly
in evaluation of the dens. The presence of a synchondrosis at the base
of the odontoid can be distinguished from a fracture based on the age
of the patient and the location of synchondrosis well below the facet
joints. Knowledge of these normal variants is useful in evaluating
plain radiographs of the cervical spine in children (Table 18-1).
|
TABLE 18-1 Normal Ossification Centers and Anomalies Frequently Confused with Injury
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
difficult to assess for abnormalities, partly because of the difficulty
in obtaining quality radiographs and partly because of the lack of
discrete and reproducible landmarks. The distance between the occipital
condyles and the facet joints of the atlas should be less than 5 mm;
any distance of more than this suggests an atlanto-occipital disruption.54,172
The foramen magnum and its relationship to the atlas also are useful in
detecting injuries of the atlantooccipital region. The anterior
cortical margin of the foramen magnum is termed the basion, while the
posterior cortical margin of the foramen magnum is termed the
opisthion. The distance between the basion and the tip of the dens
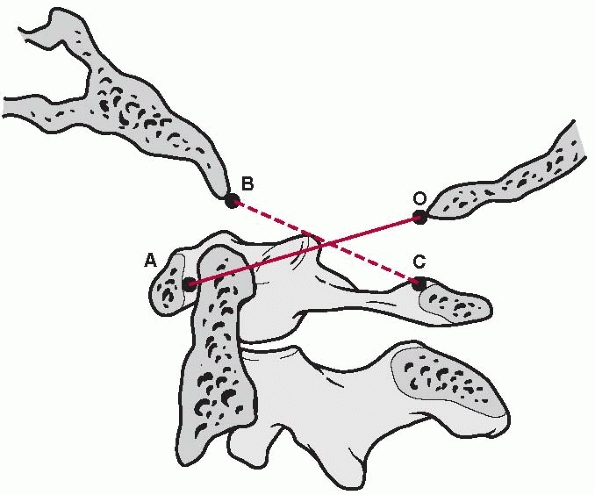
should be less than 12 mm as measured on a lateral radiograph.35 The Powers ratio (Fig. 18-7)
is used to assess the position of the skull base relative to the atlas
and is another way of evaluating the atlanto-occipital region. To
determine this ratio, a line is drawn from the basion to the anterior
cortex of the posterior arch of C1, and this distance is divided by the
distance of a line drawn from the opisthion to the posterior cortex of
the anterior arch of C1. The value should be between 0.7 and 1; a
higher value indicates anterior subluxation of the atlanto-occipital
joint and a lower value indicates a posterior subluxation. The problem
lies in the fact that the basion is not always visible on plain
radiographs. The Wackenheim line, which is drawn along the posterior
aspect of the clivus, probably is the most easily identified line to
determine disruption of the atlanto-occipital joint. If the line does
not intersect the tip of the odontoid tangentially and if this line is
displaced anteriorly or posteriorly, disruption or increased laxity
about the atlanto-occipital joint should be suspected.
 |
|
FIGURE 18-7 The Powers ratio is determined by drawing a line from the basion (B) to the posterior arch of the atlas (C) and a second line from the opisthion (O) to the anterior arch of the atlas (A).
The length of the line BC is divided by the length of the line OA, producing the Powers ratio. (From Lebwohl NH, Eismont FJ. Cervical spine injuries in children. In: Weinstein SL, ed. The Pediatric Spine: Principles and Practice. New York: Raven, 1994,with permission.) |
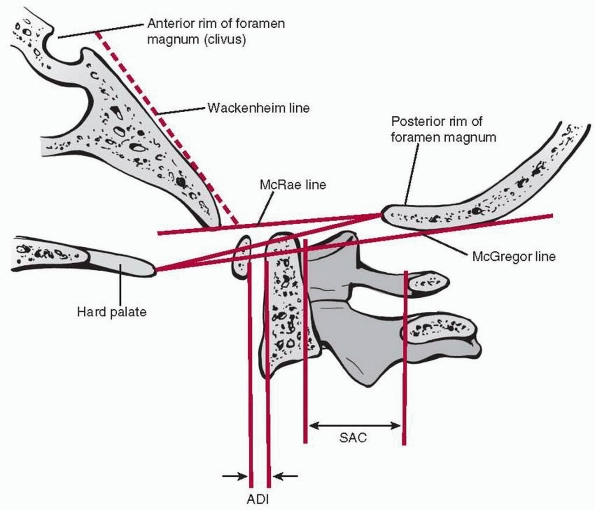
for the spinal canal are two useful measurements for evaluation of the
atlantoaxial joint (Fig. 18-8). The ADI in a
child is considered normal up to 4.5 mm, partly because the unossified
cartilage of the odontoid, which is not seen on plain films, gives an
apparent increase in the interval. At the level of the atlantoaxial
joint, the space taken up is broken into Steel’s rule of thirds: one
third is taken up by the odontoid, one third by the spinal cord, and
one third is space available for the cord. These intervals also are
easily measured on flexion and extension views and are helpful in
determining instability. In children, extension views give the
appearance of subluxation of the anterior portion of the atlas over the
unossified dens, but this represents a pseudosubluxation and not
instability.43,51
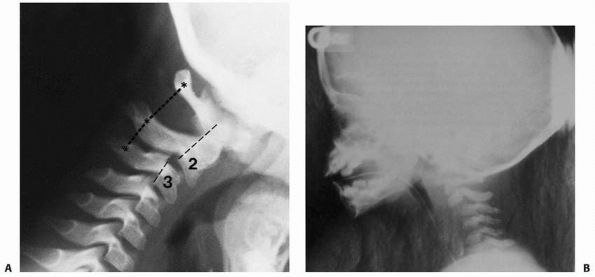
of less than 3 mm at one level is a common anatomic variant in children
at the levels of C2-C3 and C3-C4. This displacement is seen on flexion
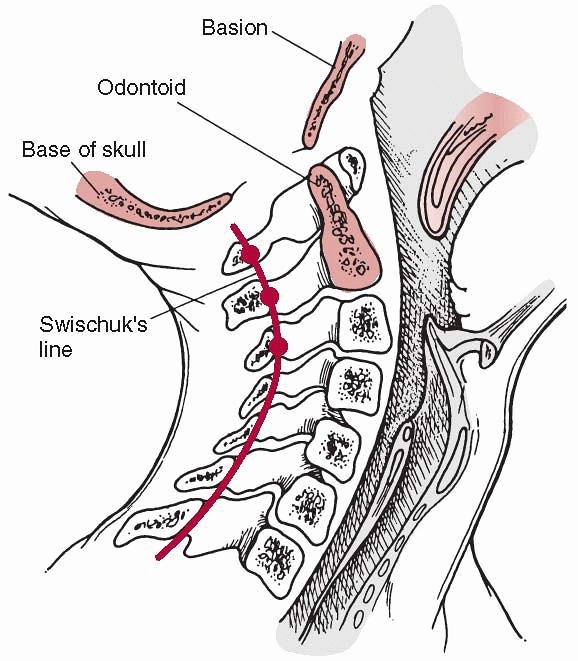
radiographs and reduces in extension. The posterior line of Swischuk218
has been described to differentiate pathologic subluxation from normal
anatomic variation; this line is drawn from the anterior cortex of the
spinous process of C1 to the spinous process of C3 (Fig. 18-9).
The anterior cortex of the spinous process of C2 should lie within 3 mm
of this line; if the distance is more than this, a true subluxation
should be suspected (Fig. 18-10). Widening of
the spinous processes between C1 and C2 of more than 10 mm also is
indicative of a ligamentous injury and should be evaluated by further
imaging studies.3
 |
|
FIGURE 18-8
The ADI and the space available for cord are used in determining atlantoaxial instability. The Wackenheim clivus-canal line is used to determine atlanto-occipital injury, while the McRae and McGregor lines are used in the measurement of basilar impression. (From Copley LA, Dormans JP. Cervical spine disorders in infants and children. J Am Acad Orthop Surg 1998;6:204-214, with permission.) |
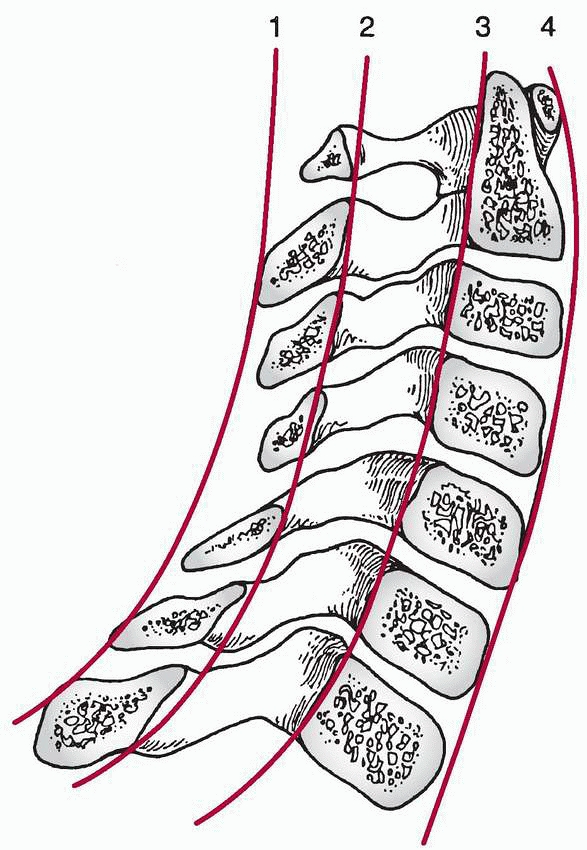
evaluated for overall alignment as well as at each level. The overall
alignment can be evaluated by the continuous lines formed by the line
adjoining the spinous processes, the spinolaminar line, and the lines
adjoining the posterior and anterior vertebral bodies (Fig. 18-11).
These lines should all be smooth and continuous with no evidence of
vertebral translation at any level. Loss of normal cervical lordosis
may be normal in children, but there should be no associated
translation at any level.231 The
interspinous distance at each level should be evaluated and should be
no more than 1.5 times the distance at adjacent levels; if this ratio
is greater, an injury should be suspected. There are calculated norms
for the interspinous distances in children, and any value greater than
two standard deviations above normal is indicative of a ligamentous
injury.129 The measurement of soft
tissue spaces is important in evaluating any evidence of swelling or
hemorrhage, which may be associated with an occult injury. The normal
retropharyngeal soft tissue space should be less than 6 mm at C3 and
less than 14 mm at C6. These spaces may be increased in children
without an injury who are crying at the time of the radiograph, because
the attachment of the pharynx to the hyoid bone results in its forward
displacement
with
crying, producing an apparent increase in the width of these spaces.
These radiographs must be taken with the patient quiet and repeated if
there is any doubt.
 |
|
FIGURE 18-9
The spinolaminar line (Swischuk line) is used to determine the presence of pseudosubluxation of C2 on C3. (From Copley LA, Dormans JP. Cervical spine disorders in infants and children. J Am Acad Orthop Surg 1998;6:201-214, with permission.) |
 |
|
FIGURE 18-10 A.
Pseudosubluxation of C2 on C3. In flexion, the posterior element of C2 should normally align itself with the posterior elements C1 and C3. The relationship of the body of C2 with the body of C3 gives the appearance of subluxation; however, the alignment of the posterior elements of C1-C3 confirms pseudosubluxation. B. True subluxation. |
Most ligamentous injuries can be identified on flexion and extension
views of the cervical spine in a cooperative and alert patient. The
roles of computed tomography (CT) scanning and magnetic resonance
imaging (MRI) continue to evolve in the evaluation of trauma patients.
 |
|
FIGURE 18-11 Normal relationships in the lateral cervical spine: 1, spinous processes; 2, spinolaminar line; 3, posterior vertebral body line; 4,
anterior vertebral body line. (From Copley LA, Dormans JP. Cervical spine disorders in infants and children. J Am Acad Orthop Surg 1998;6: 204-214, with permission.) |
points should be kept in mind. First, the proportion of a child’s head
to his or her body is greater than that of an adult, so care must be
taken not to position the head in flexion to obtain the scan.
Inadvertent flexion may potentiate any occult fracture not seen on
plain films. Second, the radiation doses for CT scanning are
significantly higher than for plain radiographs. CT protocols for
children should be used to limit the amount of radiation that the head
and neck receive during scanning of the cervical spine. While axial
views are standard, coronal and sagittal formatted images and
three-dimensional reconstruction views provide improved anatomic
details of the spine and can be obtained without any additional
radiation to the patient.96 In
patients with head injuries, CT scanning of the cervical spine can be
done at the time of CT scanning of the head to reduce the number of
plain films that may be required to document that there is not a neck
injury.117 However, plain
radiographs remain the standard for initial evaluation of the pediatric
cervical spine; CT scanning as an initial imaging study is associated
with an increase in radiation with no demonstrable benefit over plain
films.2
The advantages of an early MRI are the ability to allow mobilization if
no injury is present and the early detection of an unrecognized spinal
fracture to allow proper treatment. MRI also is useful in evaluating
patients with SCIWORA. MR angiography (MRA) has replaced standard
arteriography for evaluation of the vertebral arteries in patients with
upper cervical spine injuries who have suspected arterial injuries.168
MRI also remains the best imaging modality for evaluating injuries of
the intervertebral disks and is especially useful to detect disk
herniation in adolescent
patients with facet joint injuries that may require operative reduction.
 |
|
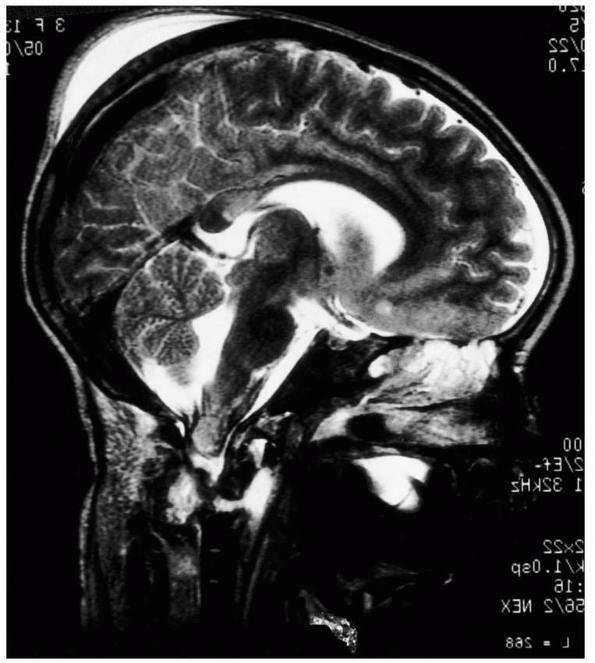
FIGURE 18-12 MRI depicts injury to the cervical cord and upper cervical spine.
|
a cervical spine injury starts with immobilization in the field.
Extraction from an automobile or transport to the hospital may cause
damage to the spinal cord in a child with an unstable cervical spine
injury if care is not taken to properly immobilize the neck. The
immobilization device should allow access to the patient’s oropharynx
and anterior neck if intubation or tracheostomy becomes necessary. The
device should allow splintage of the head and neck to the thorax to
minimize further movement.
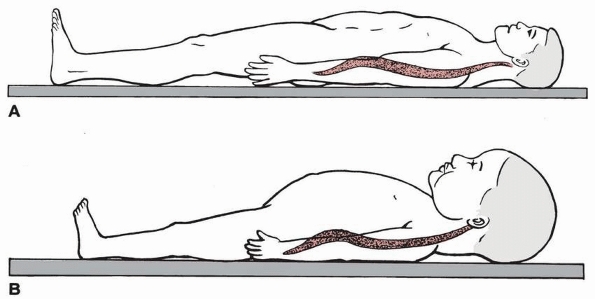
deserves special attention because of the anatomic differences between
children and adults. Compared to adults, children have a
disproportionately larger head with respect to the body. This anatomic
relationship causes a child’s cervical spine to be placed in flexion if
immobilization is done on a standard backboard. Herzenberg et al.103
reported 10 children under the age of 7 years whose cervical spines had
anterior angulation or translation on radiograph when they were placed
on a standard backboard. The use of a backboard with a recess so that
the head can be lowered into it to obtain a neutral position of the
cervical spine is one way to avoid unnecessary flexion. Another is a
split-mattress technique in which the body is supported by two
mattresses and the head is supported by one mattress, allowing the
cervical spine to assume a neutral position. Children younger than 8
years of age should be immobilized on a backboard using one of these
techniques (Figs. 18-13 and 18-14).159
 |
|
FIGURE 18-13 A. Adult immobilized on a standard backboard. B.
Young child on a standard backboard. The relatively large head forces the neck into a kyphotic position. (From Herzenberg JE, Hensinger RN, Dedrick DK, et al. Emergency transport and positioning of young children who have an injury of the cervical spine: the standard backboard may be hazardous. J Bone Joint Surg Am 1989;71:15-22, with permission.) |
immobilization in the trauma setting. While soft collars tend to be
more comfortable and cause less soft tissue irritation, rigid collars
are preferred for patients with acute injuries because they provide
better immobilization. Even rigid collars may allow up to 17 degrees of
flexion, 19 degrees of extension, 4 degrees of rotation, and 6 degrees
of lateral motion48,150
Supplemental sandbags and taping on either side of the head are
recommended in all children and have been shown to limit the amount of
spinal motion to 3 degrees in any plane.109
occur if resuscitation is required. The placement of pediatric patients
on an appropriate board with the neck in a neutral position makes
recognition of some fractures difficult because positional reduction
may have occurred, especially with ligamentous injuries or endplate
fractures. An apparently normal lateral radiograph in a patient with
altered mental status or multiple injuries does not rule out a cervical
spine injury. A study of 4 patients with unstable cervical spine
injuries who had attempted resuscitation in the emergency department
showed that axial traction actually increased the deformity.24
Any manipulation of the cervical spine, even during intubation, must be
done with caution and with the assumption that the patient has an
unstable cervical spine injury until proven otherwise.
the emergency setting if there is an injury that requires treatment.
Specific injuries and their treatment are described later in this
chapter. Further immobilization of some cervical spine injuries
requires a cervical collar. A rigid collar can be used for
immobilization if it is an appropriately fitting device with more
padding than a standard cervical collar placed in the emergency
department.
More
unstable or significant injuries can be treated with a custom orthosis,
a Minerva cast, or a halo device. An advantage of custom devices is the
ability to use lightweight thermoplastic materials that can be molded
better to each patient’s anatomy and can be extended to the thorax (Fig. 18-15).
These devices must be properly applied for effective immobilization,
and skin breakdown, especially over the chin region, needs to be
carefully monitored. Minerva casts tend to provide more immobilization
than thermoplastic devices, but their use is not as common and their
application requires attention to detail.
 |
|
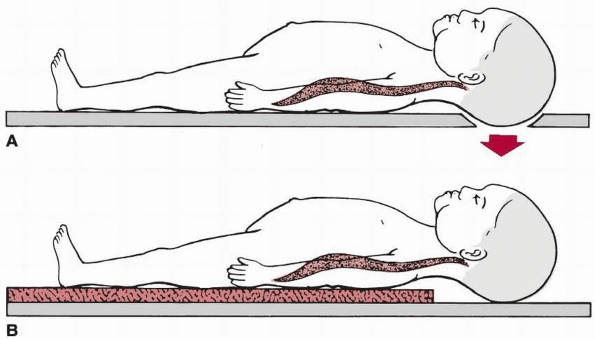
FIGURE 18-14 A.
Young child on a modified backboard that has a cutout to the recess of the occiput, obtaining better supine cervical alignment. B. Young child on modified backboard that has a double-mattress pad to raise the chest, obtaining better supine cervical alignment. (From Herzenberg JE, Hensinger RN, Dedrick DK, et al. Emergency transport and positioning of young children who have an injury of the cervical spine: the standard backboard may be hazardous. J Bone Joint Surg Am 1989;71:15-22, with permission.) |
spine injuries even in children as young as 1 year old. The halo can be
used as either a ring alone to apply traction or with a vest for
definitive immobilization of an unstable cervical spine injury. The
complication rate related to the use of a halo in one series of
patients was 68%; however, all patients were able to wear the halo
until fracture healing occurred or arthrodesis.55
The most common complications in this series were superficial pin track
infection and pin loosening. Other complications that occur less
frequently include dural penetration, supraorbital nerve injury,
unsightly pin scars, and deep infection.17,55
Prefabricated halo vests are used in adults and are easily fitted to
older adolescents. Because of the age and size ranges of children,
however, a custom vest or even a cast vest may be needed. Prefabricated
vests are available in sizes for infants, toddlers, and children, with
measurements based on the circumference of the chest at the xiphoid
process. Improper fitting of a vest may allow unwanted movement of the
neck despite the halo, and any size mismatch requires a custom vest or
cast vest (Fig. 18-16).
 |
|
FIGURE 18-15 Custom-made cervicothoracic brace used to treat a C2 fracture that reduced in extension.
|
consider both the size of the ring and the size of the vest.
Prefabricated rings and prefabricated vests are available for even for
the smallest of patients and are based on circumferential measurements
at the crown and at the xiphoid process. If the size of the patient or
the anatomy of the patient does not fit within these standard sizes,
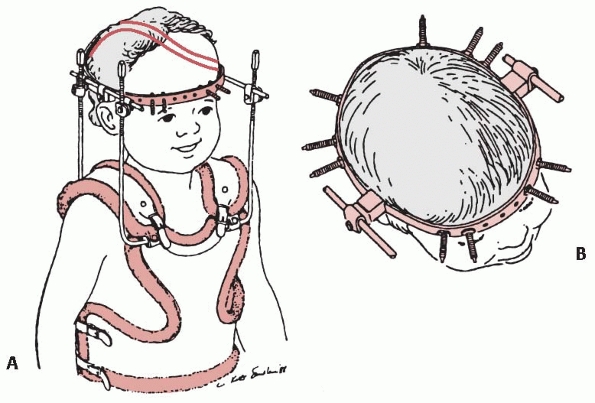
the fabrication of a custom halo may be necessary. Mubarak et al.154 recommended the following steps in the fabrication of a custom halo for a child: (a) the size and configuration
of the head are obtained with the use of the flexible lead wire placed
around the head, (b) the halo ring is fabricated by constructing a ring
2 cm larger in diameter than the wire model, (c) a plaster mold of the
trunk is obtained for the manufacture of a custom bivalved
polypropylene vest, and (d) linear measurements are made to ensure
appropriate length of the superstructure.
 |
|
FIGURE 18-16 A. Custom halo vest and superstructure. B.
In the multiplepin, low-torque technique, 10 pins are used for an infant halo ring attachment. Usually, four pins are placed anteriorly, avoiding the temporal region, and the remaining six pins are placed in the occipital area. (From Mubarak SJ, Camp JF, Fuletich W, et al. Halo application in the infant. J Pediatr Orthop 1989;9:612-613, with permission.) |
special attention because of the dangers of inadvertent skull
penetration with a pin. CT scanning before halo application aids in
determining bone structure and skull thickness. It also aids in
determining whether or not cranial suture interdigitation is complete
and if the fontanels are closed. The thickness of the skull varies
greatly up to 6 years of age and is not similar to that of adults until
the age of 16 years.134 Garfin et al.74
evaluated the pediatric cranium by CT and determined that the skull is
thickest anterolaterally and posterolaterally, making these the optimal
sites for pin placement.
insertion torques used in younger children also deserve special
mention. The placement of pins at the torque pressures used in adults
will lead to penetration during insertion.134
Pins should be inserted at torques of 2- to 4-inch pounds; however, the
variability and reliability of pressures found with various torque
wrenches during cadaver testing are great, and each pin must be
inserted cautiously.46 The use of 8
to 12 pins inserted at lower torque pressures aids in obtaining a
stable ring with less chance of inadvertent penetration (Fig. 18-17).
The insertion of each pin perpendicular to the skull also improves the
pin-bone interface and the overall strength of the construct.47
We have had success using halo vests even in children younger than 2
years of age by using multiple pins inserted to finger-tightness rather
than relying on torque wrenches.
with a local anesthetic; however, in most younger children a general
anesthetic should be used. The patient is positioned on the operating
table in a position that prevents unwanted flexion of the neck and
maintains the proper relationship of the head and neck with the trunk.
The area of skin in the region of pin insertion is cleaned with
antiseptic solution and appropriate areas are shaved as needed for pin
placement posteriorly. The ring is placed while an assistant holds the
patient’s head; it should be placed just below the greatest
circumference of the skull, which corresponds to just above the
eyebrows anteriorly and 1 cm above the tips of the earlobes laterally.
We recommend injection of local anesthetic into the skin and periosteum
through the ring holes in which the pins will be placed. The pins are
placed with sterile technique.
 |
|
FIGURE 18-17
Shaded area represents the “safe zone” for pin placement, avoiding the supraorbital and supratrochlear nerves anteriorly and the temporalis posteriorly. (From Crawford H. Traction. In: Weinstein SL, ed. Pediatric Spine Surgery. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001, with permission.) |
in mind. The thickest area of the skull is anterolaterally and
posterolaterally, and pins inserted at right angles to the bone have
greater force distribution and strength.47,74 Anterior pins should be placed to avoid the anterior position of the supraorbital and supratrochlear nerves (Fig. 18-18).
Placement of the anterior pins too far laterally will lead to
penetration of the temporalis muscle, which can lead to pain with
mastication and talking, as well as early pin loosening. The optimal
position for the anterior pins is in the anterolateral skull, just
above the lateral two thirds of the orbit and just below the greatest
circumference of the skull. The posterior pins are best placed
posterolaterally directly diagonal from the anterior pins. We also
recommend
placing
the pins to finger-tightness originally and tightening two directly
opposing pins simultaneously. During placement of the pins, meticulous
attention should be paid to the position of the ring in order to have a
circumferential fit on the patient’s skull and to avoid any pressure of
the ring on the scalp, especially posteriorly.
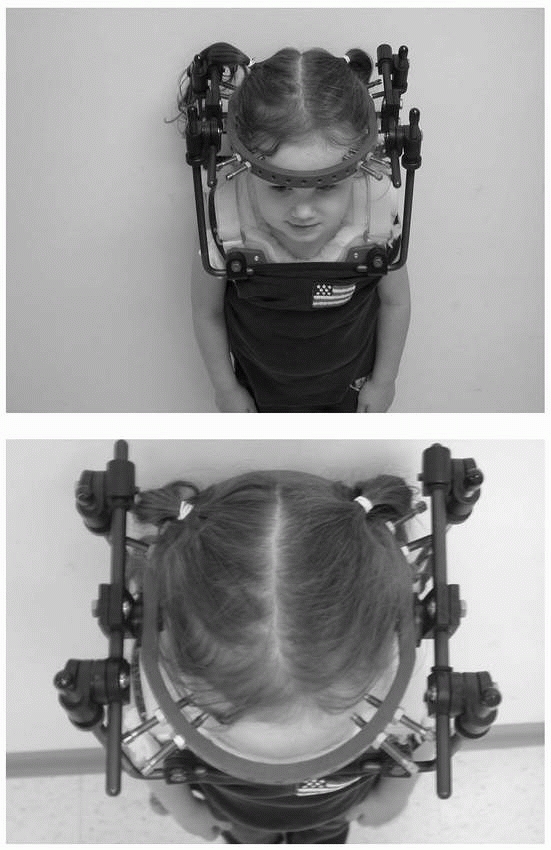 |
|
FIGURE 18-18
Child immobilized in a halo for C1 to C2 rotary subluxation. Note the position of the anterior pins, as well as the placement of the posterior pins at 180 degrees opposite the anterior pins. |
vary according to the age of the patient. In infants and younger
children, we recommend the placement of multiple pins (8 to 12)
tightened to finger-tightness or 2 to 4 inch-pounds to avoid unwanted
skull penetration. In older children, six to eight pins are used and
tightened to 4-inch pounds. In adolescents, four to eight pins can be
tightened with a standard torque wrench to 6 to 8 inch-pounds. Once the
pins are tightened, they must be fastened to the ring by the
appropriate lock nuts or set screws. The halo vest and superstructure
are then applied, with care to maintain the position of the head and
neck. Appropriate positioning of the head and neck can be done by
adjusting the superstructure (see Fig. 18-18).
peroxide/saline cleaning at the pin-skin interface. Retightening of
pins at 48 hours should be avoided in infants and children to prevent
skull penetration; however, in adolescents, the pins can be retightened
at 48 hours with a standard torque wrench. Local erythema or drainage
may occur about the pins and can be managed with oral antibiotics and
continued pin site care. If significant loosening occurs or the
infection is more serious, the pin or pins should be removed.
Occasionally, a dural puncture occurs during pin insertion or during
the course of treatment. This necessitates pin removal and prophylactic
antibiotics until the tear heals, usually at 4 to 5 days.
is unique to children. This condition is defined as a spinal cord
injury in a patient with no visible fracture or dislocation on plain
radiographs, tomograms, or CT scans.
present, and the injury usually results from severe flexion or
distraction of the cervical spine. SCIWORA is believed to occur because
the spinal column (vertebrae and disk space) in children is more
elastic than the spinal cord and can undergo considerable deformation
without being disrupted.37,221
The spinal column can elongate up to 2 inches without disruption,
whereas the spinal cord ruptures with only a quarter-inch of elongation.
patients, although most are believed to be due to a distraction-type
injury in which the spinal cord has not tolerated the degree of
distraction but the bony ligamentous elements have not failed.
Aufdermaur13 suggested another
possibility: a fracture through a pediatric vertebral endplate reduces
spontaneously (much like a Salter-Harris type I fracture), giving a
normal radiograph appearance, although the initial displacement could
have caused spinal cord injury.
perhaps because of predisposing factors such as cervical spine
hypermobility, ligamentous laxity, and an immature vascular supply to
the spinal cord. The reported incidence of this condition varies from
7% to 66% of patients with cervical spine injuries.163,164,242
reported 15 patients who had delayed paralysis after their injuries.
Nine had transient warning signs such as paresthesia or subjective
paralysis. In all patients with delayed onset of paralysis, the spine
had not been immobilized after the initial trauma, and all were
neurologically normal before the second event. This underlines the
importance of diligent immobilization of a suspected spinal cord injury
in a child. Approximately half of the young children with SCIWORA in
reported series had complete spinal cord injuries, whereas the older
children usually had incomplete neurologic deficit injuries that
involved the subaxial cervical spine.10,13,92,148
of these patients, but MRI will show a spinal cord lesion that often is
some distance from the vertebral column injury. As many as 5% to 10% of
children with spinal cord injuries have normal radiographic results.87,99
reviewed spinal injuries at the Toronto Hospital for Sick Children over
15 years and found that children constituted a small percentage of the
patients with acquired quadriplegia or paraplegia. He found that
paraplegia was three times more common than quadriplegia. When a spinal
cord injury is suspected, the neurologic examination must be complete
and meticulous and may take several examinations of sensory and motor
function. If an acute spinal cord injury is documented by examination,
the administration of methylprednisolone within the first 8 hours after
injury has been shown to improve the chances of neurologic recovery.25,26,27,28
Methylprednisolone in the treatment of acute spinal cord injuries has
been shown to improve motor and sensory recovery when evaluated 6 weeks
and 6 months after injury28;
however, this positive effect on neurologic recovery is limited to
those treated within the first 8 hours of injury. The initial loading
dose of methylprednisolone is 30 mg/kg body weight. If the loading dose
is given within 3 hours after injury, then a maintenance infusion of
5.4 mg/kg is given for 24 hours after injury. If the loading dose is
given between 3 and 8 hours after injury, then a maintenance infusion
of 5.4 mg/kg is given for 48 hours after injury. Methylprednisolone
decreases edema, has an anti-inflammatory effect, and protects the cell
membranes from scavenging oxygen-free radicals.25,26,27,28
there was a slight increase in the incidence of wound infections but no
significant increase in gastrointestinal bleeding. All of these studies
involved patients 13 years or older, so no documentation of the
efficacy in young children exists. A combination of methylprednisolone
and GM1-ganglioside (GM1) is being studied for its possible beneficial
effect on an injured spinal cord.76,77,78,79
GM1 is a complex acid-like lipid found at high levels in the cell
membrane of the central nervous system that is thought to have a
neuroprotective and neurofunctional restorative potential. Early
studies have shown that patients given both drugs had improved recovery
over those who had received just methylprednisolone.
includes prophylaxis for stress ulcers, routine skin care to prevent
pressure sores, and initial Foley catheterization followed by
intermittent catheterization and a bowel training program. With
incomplete lesions, children have a better chance than adults for
useful recovery. Hadley et al.87
noted that 89% of pediatric patients with incomplete spinal cord
lesions improved, whereas only 20% of patients with complete injuries
had evidence of significant recovery. Laminectomy has not been shown to
be beneficial and can actually be harmful204,241
because it increases instability in the cervical spine; for example, it
can cause a swanneck deformity or progressive kyphotic deformity.142,207 The risk of spinal deformity after spinal cord injury has been investigated by several researchers.16,19,38,63,120,142 Mayfield et al.142
found that patients who had a spinal cord injury before their growth
spurt all developed spinal deformities, 80% of which were progressive.
Ninety-three percent developed scoliosis, 57% kyphosis, and 18%
lordosis. Sixty-one percent of these patients required spinal
arthrodesis for stabilization of their curves. Orthotic management
usually is unsuccessful, but in some patients it delays the age at
which arthrodesis is necessary. Lower extremity deformities also may
occur, such as subluxations and dislocations about the hip. Pelvic
obliquity can be a significant problem and may result in pressure sores
and difficulty in seating in a wheelchair.
Injuries associated with breech delivery usually are in the lower
cervical spine or upper thoracic spine and are thought to result from
traction, whereas injuries associated with cephalic delivery usually
occur in the upper cervical spine and are thought to result from
rotation. It is unclear whether cesarean section reduces spinal injury
in neonates137; however, Bresnan and Abroms30
noted that neck hyperextension in utero (star-gazing fetus) in breech
presentations is likely to result in an estimated 25% incidence of
spinal cord injury with vaginal delivery and can be prevented by
delivering by cesarean section.
have been reported in infants in forward-facing car seats. Because
infants have poor head control and muscular development, if they are
placed in a forward-facing car seat and a sudden deceleration occurs,
the head continues forward while the remainder of the body is strapped
in the car seat, resulting in a distraction-type injury.45,83
and infants is underdeveloped, and a normal infant cannot adequately
support his or her head until about 3 months of age. Infants,
therefore, cannot protect their spines against excessive forces that
may occur during delivery or during the months after birth. Skeletal
injuries from obstetric trauma are probably underreported because the
infantile spine is largely cartilaginous and difficult to evaluate with
radiographs, especially if the injury is through the cartilage or
cartilage-bone interface.13 A
cervical spine lesion should be considered in an infant who is floppy
at birth, especially after a difficult delivery. Flaccid paralysis with
areflexia usually is followed by a typical pattern of hyperreflexia
once spinal cord shock is over. Brachial plexus palsies also warrant
cervical spine radiographs. MRI can sometimes be helpful in this
diagnosis.
nonoperative and should consist of careful realignment and positioning
of the child on a bed with neck support or a custom cervical thoracic
orthosis. Healing of bony injuries usually is rapid and complete.212
in 1969 described a child abuse syndrome called the shaken infant
syndrome. Children have weak and immature neck musculature and cannot
support their heads when they are subjected to whiplash stresses.
Intercranial and interocular hemorrhages can occur. This injury can
result in death or cerebral injury with retardation and permanent
visual and hearing defects. Fractures of the spinal column and spinal
cord injuries can occur during violent shaking of a child. Swischuk219
reported a spinal cord injury in a 2-year-old that was the result of
violent shaking that produced a cervical fracture-dislocation that
spontaneously reduced.
Plain radiographs often do not clearly show occipital condylar
fractures, and CT with multiplanar reconstruction usually is necessary
to establish the diagnosis.14,42 Tuli et al.225
recommended that a CT scan be obtained in the following circumstances:
presence of lower cranial nerve deficits, associated head injury or
basal skull fracture, or persistent neck pain despite normal
radiographs. Reports of associated cranial nerve deficits vary from 53%
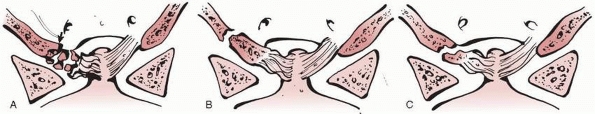
to 31% of patients with occipital condylar fractures.9,92,225 Anderson and Montesano9 described three types of occipital condylar fractures (Table 18-2, Fig. 18-19):
type I, impaction fracture; type II, basilar skull fracture extending
into the condyle; and type III, avulsion fractures. An avulsion
fracture is the only type of occipital condylar fracture that is
unstable. Type I injuries are the result of axial compression with a
component of ipsilateral flexion. Type II injuries are basilar skull
fractures that extend to involve the occipital condyle and usually are
caused by a direct blow. Type III injuries are avulsion fractures of
the inferomedial portion of the condyle that is attached to the alar
ligament. Types I and II occipital condylar fractures usually are
stable and can be treated with a cervical orthosis. Type III or
avulsion fractures can be unstable and may require halo immobilization
or occipitocervical arthrodesis.6
In their classification, type 1 fractures are nondisplaced and type 2
are displaced. They further subdivided type 2 fractures into type 2A,
displaced but stable, and type 2B, displaced and unstable. Most
occipital condylar fractures are stable and can be treated with a
cervical orthosis or halo immobilization. The decision for surgery is
based on cranial cervical instability. Bilateral occipital condylar
fractures usually are unstable and require occipital cervical fusion.92
|
TABLE 18-2 Anderson and Montesano Classification of Occipital Condylar Fractures
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||
reported 11 atlanto-occipital dislocations in 1600 pediatric trauma
patients (a 0.7% prevalence) seen over a 5-year period; six children
died with severe neurologic deficits, but five patients survived with
minimal or no neurologic sequela. This increase in the survival rate
may be due to increased awareness and improved emergency care with
resuscitation and spinal immobilization by emergency personnel.
Atlanto-occipital dislocation occurs in sudden deceleration accidents,
such as motor vehicle or pedestrian-vehicle accidents. The head is
thrown forward, and this can cause sudden craniovertebral separation.
little inherent bony stability. Stability is provided by the ligaments
about the joint. The primary stabilizers are the paired alar ligaments,
the articular capsule, and the tectorial membrane (a continuation of
the posterior longitudinal ligament and the major stabilizer of the
atlanto-occiptal joint). In children, this articulation is not as well
formed as in adults and it is less cup-shaped. Therefore, there is less
resistance to translational forces.13,20,23,34,35,206
 |
|
FIGURE 18-19 Classification of occipital condylar fractures according to Anderson and Monsanto.9 A. Type I fractures can occur with axial loading. B. Type II fractures are extensions of basilar cranial fractures. C.
Type III fractures can result from an avulsion of the condyle during rotation, lateral bending, or a combination of mechanisms. (From Hadley MN. Occipital condyle fractures. Neurosurgery 2002;50[Suppl]:S114-S119, with permission.) |
|
TABLE 18-3 Classification of Occipital Condylar Fractures225
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
dislocation is a ligamentous injury. Although patients with this injury
have a history of trauma, some may have no neurologic findings. Others,
however, may have symptoms such as cranial nerve injury, vomiting,
headache, torticollis, or motor or sensory deficits.34,40,44,93,105,167
Brain stem symptoms, such as ataxia and vertigo, may be caused by
vertebrobasilar vascular insufficiency. There is a high association of
closed head injures that may mask other physical findings. Unexplained
weakness or difficulty in weaning off a ventilator after a closed head
injury may be a sign of this injury.
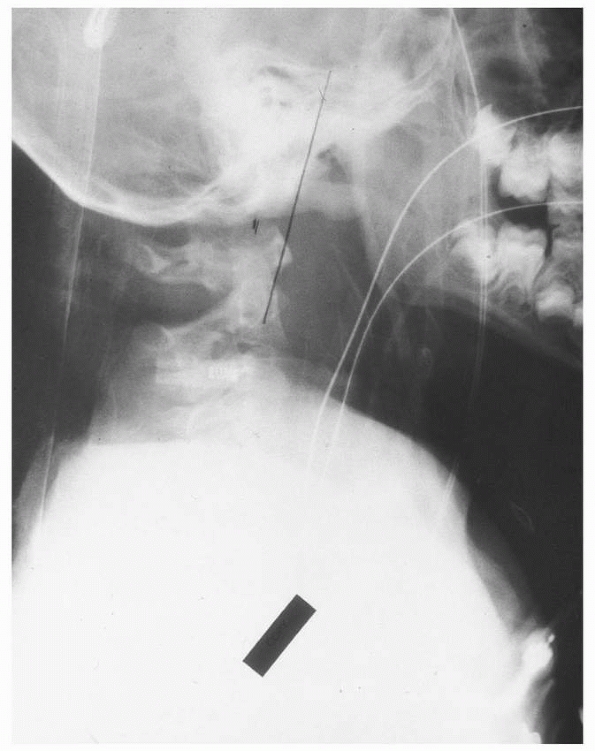
suspicion in children with closed head injuries or associated facial
trauma and must be aware of the radiographic findings associated with
atlanto-occipital dislocation. A significant amount of anterior soft
tissue swelling usually can be seen on a lateral cervical spine
radiograph. This increased anterior soft tissue swelling should be a
warning sign that an atlanto-occipital dislocation may have occurred.
atlantooccipital dislocation are the Wackenheim line, Powers ratio,
dens-basion interval, and occipital condylar distance. The
Wackenheim
line is drawn along the clivus and should intersect tangentially the
tip of the odontoid. A shift anterior or posterior of this line
represents either an anterior or posterior displacement of the occiput
on the atlas (Fig.18-21).
This line is probably the most helpful because it is reproducible and
easy to identify on a lateral radiograph. The Powers ratio (see Fig. 18-7)
is determined by drawing a line from the basion to the posterior arch
of the atlas (BC) and a second line from the opisthion to the anterior
arch of the atlas (OA). The length of line BC is divided by the length
of the line OA, producing the Powers ratio. A ratio of more than 1.0 is
diagnostic of anterior atlantooccipital dislocation. A ratio of less
than 0.7 is diagnostic of posterior atlanto-occipital dislocation.
Values between 1.0 and 0.7 are considered normal.118
The Powers ratio has the advantage of not being affected by
magnification of the radiograph, but the landmarks may be difficult to
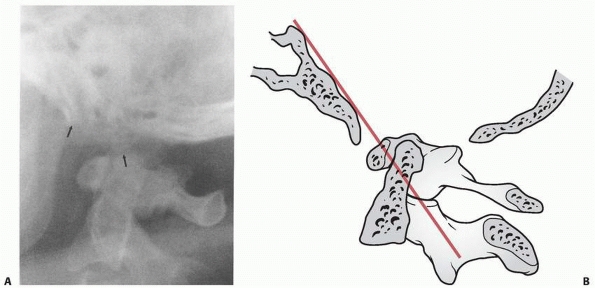
define. Another radiographic measurement is the dens-basion interval.
If the interval measures more than 1.2 cm, then disruption of the
atlantooccipital joint has occurred.35,175 Kaufman et al.115
described an occipital condylar facet distance of more than 5 mm from
the occipital condyle to the C1 facet as indicative of atlantooccipital
injury. They recommended measuring this distance from five reference
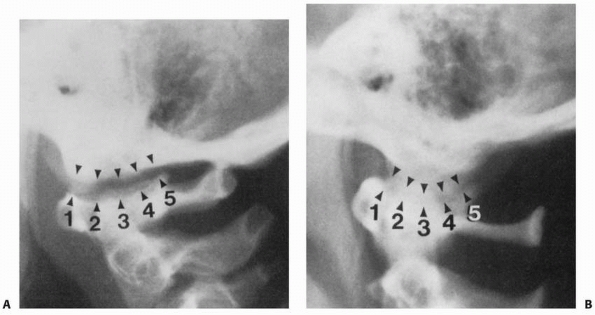
points along the occipital condyle and the C1 facet (Fig. 18-22).
 |
|
FIGURE 18-20
Patient with atlanto-occipital dislocation. Note the forward displacement of the Wackenheim line and the significant anterior soft tissue swelling. |
 |
|
FIGURE 18-21 Craniovertebral dislocation. A. Lateral view shows extensive soft tissue swelling. The distance between the basion and the dens is 2.4 cm (arrows) (normal is <1 cm). B.
Line drawing shows the abnormal relationship between the occiput and the upper cervical spine. (From El-Khoury GY, Kathol MH. Radiographic evaluation of cervical trauma. Semin Spine Surg 1991;3:3-23, with permission.) |
dislocation by showing soft tissue edema around the tectorial membranes
and lateral masses and ligament injury or disruption.36 Steinmetz et al.214 and Sun et al.217
suggested that the disruption of the tectorial membrane is the critical
threshold for instability of the occipitoatlanal joint. Disruption of
the tectorial membrane can best be identified by MRI. Operative Treatment
Because atlanto-occipital dislocation is a ligamentous injury,
nonoperative treatment usually is unsuccessful. Although Farley et al.62 reported successful stabilization in a halo, Georgopoulos et al.80
found persistent atlanto-occipital instability after halo
immobilization. Immobilization in a halo should be used with caution:
if the vest or cast portion is not fitted properly, displacement can
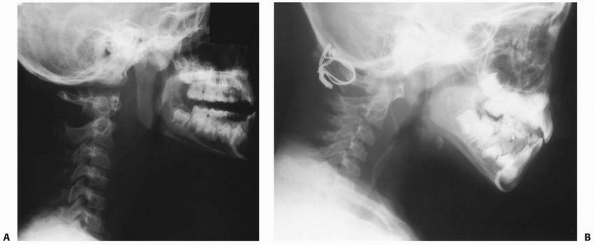
increase (Fig. 18-23) because the head is fixed
in the halo but movement occurs because of inadequate immobilization of
the trunk in the brace or cast. Traction should be avoided because it
can cause distraction of the skull from the atlas. Surgical
stabilization is the recommended treatment.136
Posterior arthrodesis can be performed in situ, with wire fixation or
fixation with a contoured Luque rod and wires or contoured rod and
screw fixation.12,97,147 If the C1-C2 articulation is stable, arthrodesis should be only from the occiput to C1 so that C1-C2 motion is preserved.210
Some researchers have expressed reservations about the chance of
obtaining fusion in the narrow atlanto-occipital interval and have
recommended arthrodesis from the occiput to C2. If stability of the
C1-C2 articulation is questionable, arthrodesis should extend to C2.114
Acute hydrocephalus can occur after this injury or in the early
postoperative period because of changes in cerebrospinal fluid flow at
the cranial cervical junction.
 |
|
FIGURE 18-22
Atlanto-occipital joint measurement points 1 through 5 demonstrated on a normal crosstable lateral skull radiograph in an 8-year-old (A) and a 14-year-old (B). (From: Kaufman RA, Carroll CD, Buncher CR. Atlantooccipital junction: standards for measurement in normal children. AJNR Am J Neuroradiol 1987; 8:995-999, with permission.) |
in patients with Down syndrome as well as in those with a high cervical
arthrodesis below the axis. These patients may be at risk of developing
chronic instability patterns and are at higher risk of having
instability after trauma.
 |
|
FIGURE 18-23 A. Lateral radiograph of a patient with atlanto-occipital dislocation. Note the increase in the facet condylar distance. B. Lateral radiograph after occipital C1 arthrodesis.
|
children in whom the posterior elements are absent at C1 or separation
is extensive in the bifid part of C1 posteriorly, posterior cervical
arthrodesis from the occiput to C2 with iliac crest bone graft is
performed using a periosteal flap from the occiput to provide an
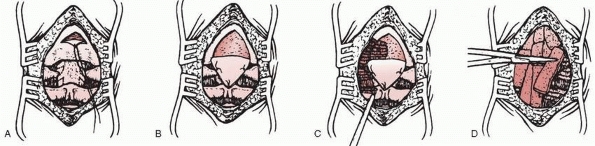
osteogenic tissue layer for the bone graft (Fig. 18-24).125
endotracheal intubation is obtained, and all anesthesia lines are in
place. For younger children, 8 to 12 pins with lower-pressure torque
are used in the halo (see Fig. 18-17); in older children, 4 pins can be used.
 |
|
FIGURE 18-24 Technique of occipitocervical arthrodesis used when the posterior arch of C1 is absent. A. Exposure of the occiput, atlas, and axis. B. Reflection of periosteal flap to cover defect in atlas. C. Decortication of exposed vertebral elements. D.
Placement of autogenous cancellous iliac bone grafts. (From Koop SE, Winter RB, Lonstein JE. The surgical treatment of instability of the upper part of the cervical spine in children and adolescents. J Bone Joint Surg Am 1984;66:403, with permission.) |
head and cervical spine in the prone position with the halo in place.
The radiograph also aids in identifying landmarks and levels; although
once the skin incision is made, the occiput and spinous processes can
be palpated.
to about C3, with care not to expose below C2 to avoid extension of the
fusion to lower levels. An epinephrine and lidocaine solution is
injected into the cutaneous and subcutaneous tissues to help control
local skin and subcutaneous bleeding. The incision is deepened in the
midline to the spinous processes of C2. Once identified, the level of
the posterior elements of C1 or the dura is more easily found.
carried proximally. Extraperiosteal dissection is used to approach the
occiput (see Fig. 18-24A). The dura is not
completely exposed; if possible, any fat or ligamentous tissue present
is left intact. The interspinous ligaments also should be left intact.
triangular incision directly on the posterior skull, with the apex
posteriorly and the broad base over the foramen magnum region. A flap
of 3 or 4 cm at the base can be created. With subperiosteal elevation,
the periosteum can be reflected from the occiput to the spinous
processes of C2 (see Fig. 18-24B). The apex of
the flap is sutured to the spinous process of C2 and is attached
laterally to any posterior elements that are present at C1 or other
lateral soft tissues. After the periosteum is secured to the bone and
any rudimentary C1 ring is exposed subperiosteally, a power burr is
used to decorticate the occiput and any exposed portions of C1 and C2
(see Fig. 18-24C).
No internal fixation is used other than sutures to secure the
periosteum. The wound is closed in a routine fashion, and a body jacket
or cast is applied and attached to the halo. The halo cast is worn
until radiographs show adequate posterior arthrodesis, usually in 8 to
12 weeks.
adolescents in whom the posterior elements of C1 and C2 are intact, a
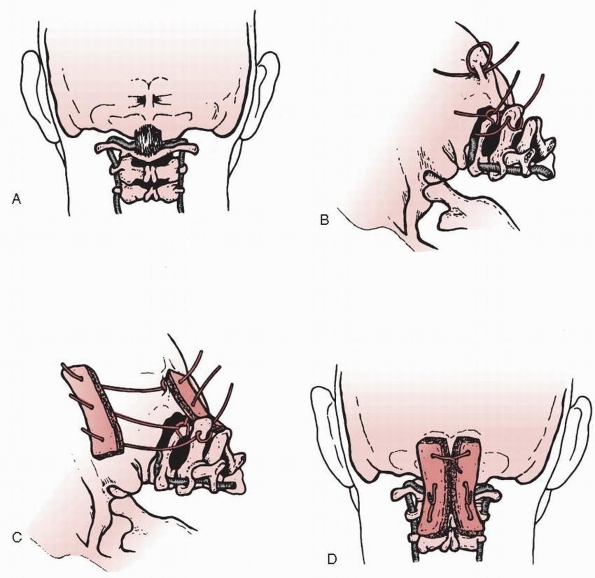
triple-wire technique, as described by Wertheim and Bohlman,233 can be used (Fig. 18-25).
The wires are passed through the outer table of the skull at the
occipital protuberance. Because the transverse and superior sagittal
sinuses are cephalad to the protuberance, they are not endangered by
wire passage.
cranial skeletal traction with the patient on a turning frame or
cerebellar head rest. The patient is placed prone, and a lateral
radiograph is obtained to document proper alignment. The subcutaneous
tissues are injected with an epinephrine solution (1:500,000). A
midline incision is made extending from the external occipital
protuberance to the spine of the third cervical vertebra. The
paraspinous muscles are sharply dissected subperiosteally with a
scalpel, and a periosteal elevator is used to expose the occiput and
cervical laminae, with special care to stay in the midline to avoid the
paramedian venous plexus. At a point 2 cm above the rim of the foramen
magnum, a high-speed diamond burr is used to create a trough on either
side of the protuberance, making a ridge in the center (see Fig. 18-25A).
A towel clip is used to make a hole in this ridge through only the
outer table of bone. A 20-gauge wire is looped through the hole and
around the ridge; then another 20-gauge wire is looped around the arch
of the atlas. A third wire is passed through a hole drilled in the base
of the spinous process of the axis and around this structure, giving
three separate wires to secure the bone grafts on each side of the
spine (see Fig. 18-25B).
of premeasured length and width is removed from the posterior iliac
crest. The graft is divided horizontally into two pieces, and three
holes are drilled into each graft (see Fig. 18-25C). The occiput is decorticated and the grafts are anchored in place with the wires on both sides of the spine (see Fig. 18-25D). Additional cancellous bone is packed around and between the two grafts. The wound is closed in layers over suction drains.
for 6 to 15 weeks, followed by a soft collar that is worn for an
additional 6 weeks.
done with the patient under general anesthesia and with monitoring of
the somatosensory-evoked potentials (Fig. 18-26). A halo ring is
applied initially with the patient supine. Subsequently, the patient is
carefully placed in the prone position, the halo is secured to the
operating table with a halo-positioning device, and the alignment of
the occiput and the cervical spine is confirmed with a lateral
radiograph. The midline is exposed from the occiput to the second or
third cervical vertebra. Particular care is taken to limit the lateral
dissection to avoid damaging the vertebral arteries.85
 |
|
FIGURE 18-25 Technique of occipitocervical arthrodesis used in older adolescents with intact posterior elements of C1 and C2. A. A burr is used to create a ridge in the external occipital protuberance, and then a hole is made in the ridge. B.
Wires are passed through the outer table of the occiput, under the arch of the atlas, and through the spinous process of the axis. C. Corticocancellous bone grafts are placed on the wires. D. Wires are tightened to secure grafts in place. (From Wertheim SB, Bohlman HH. Occipitocervical fusion: indications, technique, and long-term results. J Bone Joint Surg Am 1987;69: 833, with permission.) |
stenosis or for removal of a tumor, the arch of the first or second
cervical vertebra (or both) is removed, with or without removal of a
portion of the occipital bone to enlarge the foramen magnum.
of the midline, are made with a high-speed drill through both cortices
of the occiput, leaving a 1-cm osseous bridge between the two holes of
each pair. The holes are placed caudal to the transverse sinuses. A
trough is fashioned into the base of the occiput to accept the cephalad
end of the bone graft. A corticocancellous graft is obtained from the
iliac crest and is shaped into a rectangle, with a notch created in the
inferior base to fit around the spinous process of the second or third
cervical vertebra. The caudal extent of the intended arthrodesis (the
second or third cervical vertebra) is determined by the presence or
absence of a previous laminectomy, congenital anomalies, or the level
of the instability. On each side, a looped 16- or 18-gauge Luque wire
is passed through the bur holes and looped on itself. Wisconsin button
wires (Zimmer, Warsaw, IN) are passed through the base of the spinous
process of either the second or the third cervical vertebra. The wire
that is going into the left arm of the graft is passed through the
spinous process from right to left. The graft is placed into the
occipital trough superiorly and about the spinous process of the
vertebra that is to be at the caudal level of the arthrodesis (the
second or third cervical vertebrae). The graft is precisely contoured
so that it fits securely into the occipital trough and around the
inferior spinous process before the wires are tightened. The wires are
subsequently crossed, twisted, and cut. An intraoperative radiograph is
made at this point to assess the position of the graft and the wires as
well as the alignment of the occiput and the cephalad cervical
vertebrae. Extension of the cervical spine can be controlled by
positioning of the head with the halo frame, by adjustment of the size
and shape of the graft, and to a lesser extent by appropriate
tightening of the wires.
dislocations are treated with fusion from the occiput to C2 or lower,
Sponseller and Cass210 described
occiput-C1 fusion in two children with atlanto-occipital arthrodesis
who had complete or near-complete neurologic preservation. Their
rationale was that rotation would be preserved by sparing the C1-C2
articulation from fusion and that less stress would be concentrated on
the lower cervical spine by fusing one level instead of two. In both of
their patients, stable fusion was obtained and neurologic status was
maintained.
reviewed to be sure a bifid or hypoplastic C1 arch is not present. The
positioning of the patient and the procedure are done with the patient
under general anesthesia and with monitoring of the
somatosensory-evoked potentials. The procedure is done with the patient
immobilized in a halo vest. The base of the skull to
the
ring of C1 is exposed and the periosteum of the skull is elevated so
that it forms a flap from the foramen magnum located
posteriorly-superiorly. The ring of C1 is carefully exposed, with care
taken not to dissect more than 1 cm to either side of the midline to
protect the vertebral arteries. Care also is taken not to expose any
portion of C2 to prevent bridging of the fusion. The dissection of C1
should be done gently. A trough for the iliac crest bone graft is made
in the occiput at a level directly cranial to the ring of C1. This
trough is unicortical only and extends the width of the exposed portion
of C1. Superior to this, two holes are drilled through the occiput as
close to the trough as possible to avoid an anteriorly translating
vector on the skull when tightening it down to C1. One 22-gauge wire is
passed through the holes and another is placed around the ring of C1.
The periosteal flap is turned down to bridge the occiput-C1 interval. A
small, rectangular, bicortical, iliac crest bone graft approximately
1.5 cm wide and 1 cm high is shaped to fit the trough in the occiput;
the graft is contoured to fit the individual patient’s occiput-C1
interval. The inferior surface of the bone graft is contoured to fit
snugly around the ring of C1 to keep it from migrating anteriorly into
the epidural space. Two holes are drilled directly above the distal end
of the graft, and the wire around C1 is passed through these holes,
forming two distal strands; the wire passed through the occiput forms
two proximal strands. These are twisted together and sequentially
tightened to apply slight compression to the bone graft. This keeps the
graft in the occipital trough and prevents migration into the canal by
the occiput. Additional cancellous bone is added to any available space.
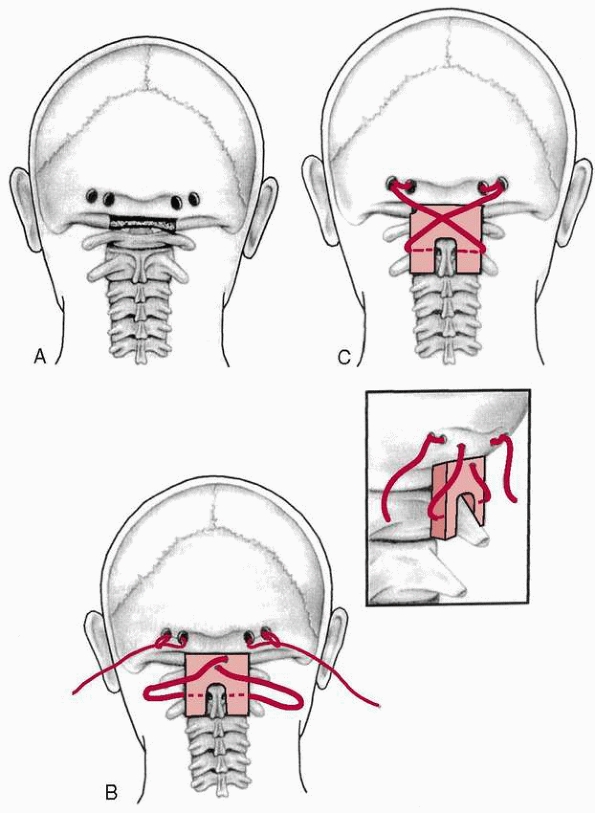 |
|
FIGURE 18-26 Occipitocervical arthrodesis. A.
Four burr holes are placed into the occiput in transverse alignment, with two on each side of the midline, leaving a 1-cm osseous bridge between the two holes of each pair. A trough is fashioned into the base of the occiput. B. Sixteen- or 18-gauge Luque wires are passed through the burr holes and looped on themselves. Wisconsin button wires are passed through the base of the spinous process of either the second or third cervical vertebra. The graft is positioned into the occipital trough and spinous process of the cervical vertebra at the caudal extent of the arthrodesis. The graft is locked into place by the precise contouring of the bone. C. The wires are crossed, twisted, and cut. The extension of the cervical spine can be controlled by positioning of the head with the halo frame, by adjustment of the size and shape of the bone graft, and to a lesser extent by tightening of the wires. (From Dormans JP, Drummond DS, Sutton LN, et al. Occipitocervical arthrodesis in children. J Bone Joint Surg Am 1995;77:1234-1240, with permission.) |
young child and for as long as 12 weeks in an older child or
adolescent. Union is confirmed by a coned, lateral radiograph of the
posterior occiput-C1 interval and by flexion-extension lateral views. A
rigid cervical collar is used for an additional 2 to 4 weeks to protect
the fusion and support the patient’s cervical muscles while motion is
regained.
segmental wire has the advantage of achieving immediate stability of
the occipitocervical junction (Fig. 18-27), which allows the patient to be immobilized in a cervical collar after surgery, avoiding the need for halo immobilization.
upper cervical vertebrae are approached through a longitudinal midline
incision, which extends deep within the relatively avascular
intermuscular septum. The entire field is exposed subperiosteally. A
template of the intended shape of the stainless steel rod is made with
the appropriate length of Luque wire. Two burr holes are made on each
side, about 2 cm lateral to the midline and 2.5 cm above the foramen
magnum. Care should be taken to avoid the transverse and sigmoid sinus
when making these burr holes. At least 10 mm of intact cortical bone
should be left between the burr holes to ensure solid fixation. Luque
wires or Songer cables are passed in an extradural plane through the
two burr holes on each side of the midline. The wires or cables are
passed sublaminar in the upper cervical spine. The rod is bent to match
the template; this usually will have a head-neck angle of about 135
degrees and slight cervical lordosis. A Bend Meister (Sofamor/Danek,
Memphis, TN) may be helpful in bending the rod. The wires or cables are
secured to the rod. The spine and occiput are decorticated, and
autogenous cancellous bone grafting is performed.
caused by an axial load applied to the head and is not a common injury
in children.22,112,114,140,149,184,223 This rare injury accounts for less than 5% of all cervical spine fractures in children.11,21
The force is transmitted through the occipital condyles to the lateral
masses of C1, causing a disruption in the ring of C1, usually in two
places, with fractures occurring in both the anterior and posterior
rings. In children, an isolated single fracture of the ring can occur
with the remaining fracture hinging on a synchondrosis.18
This is an important distinction in children because often fractures
occur through a normal synchondrosis and there can be plastic
deformation of the ring with no evidence of a fracture.114,222
This distinction can be seen on plain radiographs and CT scan, with
fractures appearing through what appears to be normal physes. As the
lateral masses separate, the transverse ligament may be ruptured or
avulsed, resulting in C1 and C2 instability.149
If the two lateral masses are widened more than 7 mm beyond the borders
of the axis on an anteroposterior radiograph, then an injury to the
transverse ligament is presumed. Injury to the transverse ligament may
be from a rupture of the ligament or an avulsion of the ligament
attachment to C1. Jefferson fractures may be evident on plain
radiographs, but CT scans are superior at showing this injury (Fig 18-28).
CT scans also can be used to follow the progress of healing. MRI is
useful in determining the integrity of the transverse atlantal ligament
and detecting fractures through the normal synchondroses of the atlas.
With a fracture through a synchondrosis, associated edema and
hemorrhage are seen on MRI.126 Other
cervical spine fractures may be present with an atlas fractures, and
MRIs should be carefully scrutinized to identify other fractures.143
The classic signs of an atlas fracture in a child are neck pain,
cervical muscle spasm, decreased range of motion, and head tilt.114
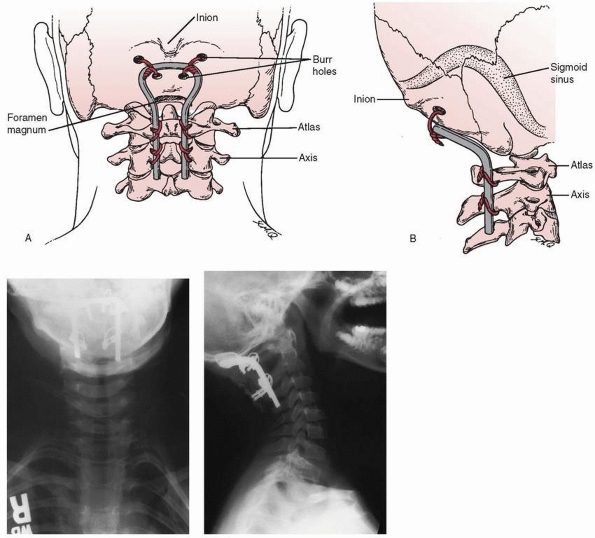 |
|
FIGURE 18-27 Occipitocervical arthrodesis using a contoured rod and segmental wire or cable fixation. (A,B reprinted from Warner WC. Pediatric cervical spine. In: Canale ST, ed. Campbell’s operative orthopaedics. St. Louis: Mosby, 1998; with permission.)
|
(rigid collar or sternal occipital mandibular immobilizer), Minerva
cast, or halo brace. The extent of this immobilization is debatable and
should consider the patients age and cooperation.126
If there is excessive widening (>7 mm), halo traction followed by
halo brace or cast immobilization is recommended. Stablility of C1-C2
must be documented on flexion and extension lateral radiographs once
the fracture is healed. Surgery rarely is necessary to stabilize these
fractures (Fig. 18-29).
This fracture accounts for approximately 10% of all cervical spine
fractures and dislocations in children. The unique feature of odontoid
fractures in children is that the fracture most commonly occurs through
the synchondrosis of C2
distally
at the base of the odontoid. This synchondrosis is a cartilage line at
the base of the odontoid and looks like a physeal or Salter-Harris type
I injury.
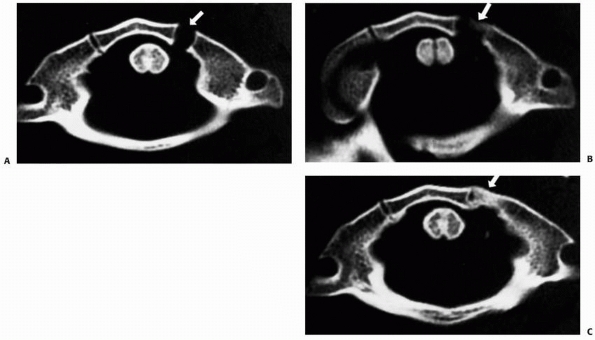 |
|
FIGURE 18-28 A. Initial CT scan through the atlas, demonstrating left anterior synchondrosis diastasis (arrow). B. CT scan 1 month after presentation with callus formation at the synchondrosis, demonstrating healing at the fracture site. C.
CT scan 4 months after presentation, showing bony bridging across the fracture site. (From Judd D, Liem LK, Petermann G. Pediatric atlas fracture: a case of fracture through a synchondrosis and review of the literature. Neurosurgery 2000;46:991-994, with permission.) |
head trauma from a motor vehicle accident or a fall from a height,
although it also can occur after trivial head trauma. Radiographs
should be obtained in any child complaining of neck pain. Clinically,
children with odontoid fractures complain of neck pain and resist
attempts to extend the neck. Odent et al.160
reported that 8 of 15 odontoid fractures in children were the result of
motor vehicle accidents, with the child fastened in a forward-facing
seat. The sudden deceleration of the body as it is strapped into the
car seat while the head continues to travel forward causes this
fracture.
 |
|
FIGURE 18-29 CT scan of an atlas fracture.
|
usually have an intact anterior periosteal sleeve that provides some
stability to the fracture when immobilized in extension and allows
excellent healing of the fracture.11,185,192,201
Growth disturbances are uncommon after this type of fracture. This
synchondrosis normally closes at about 3 to 6 years of age and adds
little to the longitudinal growth of C2.
the plain radiographs. Anteroposterior views usually appear normal, and
the diagnosis must be made from lateral views because displacement of
the odontoid usually occurs anteriorly. Plain radiographs sometimes can
be misleading when the fracture occurs through the synchondrosis and
has spontaneously reduced. When this occurs, the fracture has the
appearance of a nondisplaced Salter-Harris type I fracture. CT scans
with three-dimensional reconstruction views may be needed to fully
delineate the injury.202 MRI also
may be useful in nondisplaced fractures by detecting edema around the
injured area, indicating that a fracture may have occurred. Dynamic
flexion and extension views to demonstrate instability may be obtained
in a cooperative child if a nondisplaced fracture is suspected. These
studies should be done only in a cooperative child and under the direct
supervision of the treating physician.
uneventfully and rarely have complications. Neurologic deficits rarely
have been reported after this injury.160,215 Odent et al.160
described neurologic injuries in 8 of 15 patients, although most were
stretch injuries to the spinal cord at the cervical thoracic junction
and not at the level of the odontoid fracture.
complete reduction of the translation is not necessary. At least 50%
apposition should be obtained to provide adequate cervical alignment,
and then the patient should be immobilized in a Minerva or halo cast or
custom orthosis. This fracture will heal in about 6 to 8 weeks. After
bony healing, stability should be documented by flexion-extension
lateral radiographs. Once the Minerva cast or halo is removed, a soft
collar is worn for 1 to 2 weeks. If an adequate reduction cannot be
obtained by recumbency and hyperextension, then a head halter or halo
traction is needed. Rarely, manipulation under general anesthesia is
needed for irreducible fractures (Fig. 18-30).
Surgery with internal fixation rarely has been reported due to the good
results that are achieved with conservative treatment in children.84,176,193,200,226
separated from the axis by a transverse gap, which leaves the apical
segment without support. Fielding et al.64,65,66,67,68
suggested that this was an unrecognized fracture at the base of the
odontoid. Some studies have documented normal radiographs of the dens
with abnormal radiographs after trivial trauma. This can be explained
by a distraction force being applied by the alar ligaments, which pulls
the tip of the fractured odontoid away from the base and produces a
nonunion.95,110,128,183,194,216,229 Other authors believe this to be of congenital origin because of its association with other congenital anomalies and syndromes.82,203,240. Sankar et al.190
reported that 6 of their 16 patients had associated congenital
anomalies in the cervical spine and only 8 of the 16 reported any
previous trauma.
Signs and symptoms can range from a minor to a frank compressive
myelopathy or vertebral artery compression. Presenting symptoms may be
neck pain, torticollis, or headaches caused by local irritation of the
atlantoaxial joint. Neurologic symptoms can be transient or episodic
after trauma to complete myelopathy caused by cord compression.57
Symptoms may consist of weakness and loss of balance with upper motor
neuron signs, although upper motor neuron signs may be completely
absent. Proprioceptive and sphincter dysfunctions also are common.
Cerebellar infarctions due to vertebrobasilar artery insufficiency
caused by an unstable os odontoideum were described by Sasaki et al.191
 |
|
FIGURE 18-30
Lateral radiograph and CT reconstruction view of odontoid fracture through the synchondrosis of C2. Note the anterior displacement. |
Lateral flexion and extension views should be obtained to determine if
any instability is present. With os odontoideum, there is a space
between the body of the axis and a bony ossicle. The free ossicle of
the os odontoideum usually is half the size of a normal odontoid and is
oval or round, with smooth sclerotic borders. The space differs from
that of an acute fracture, in which the space is thin and irregular
instead of wide and smooth. The amount of instability should be
documented on lateral flexion and extension plain radiographs that
allow measurement of both the anterior and posterior displacement of
the atlas on the axis. Because the ossicle is fixed to the anterior
arch of C1 and moves with the anterior arch of C1 both in flexion and
extension, measurement of the relationship of C1 to the free ossicle is
of little value because they move as a unit. A more meaningful
measurement is made by projecting lines superiorly from the body of the
axis to a line projected inferiorly from the posterior border of the
anterior arch of the atlas. This gives more information as to the
stability of C1-C2. Another measurement that is very helpful is space
available for the cord, which is the distance from the back of the dens
to the anterior border of the posterior arch of C1.
C2. Before arthrodesis is attempted, the integrity of the arch of C1
must be documented by CT scan. Incomplete development of the posterior
arch of C1 is uncommon but has been reported to occur with increased
frequency in patients with os odontoideum. This may necessitate an
occiput to C2 arthrodesis for stability. If a C1-C2 arthrodesis is
done, one must be careful not to overreduce the odontoid and cause
posterior translation.
Care
also must be taken in positioning the neck at the time of arthrodesis
and when tightening the wires if a Gallie or Brooks arthrodesis is
performed to prevent posterior translation (Figs. 18-32 and 18-33). Brockmeyer et al.32 and Wang et al.232 both reported good results with transarticular screw fixation and fusion in the treatment of children with os odontoideum (Fig. 18-34). Wang et al.232
reported the use of this technique in children as young as 3 years of
age. This technique may be preferred depending on the patient’s anatomy
and the surgeon’s experience. Harms et al.94 and Brecknell et al.29 reported that a high-riding vertebral artery may make transarticular screw placement impossible in about 20% of patients.
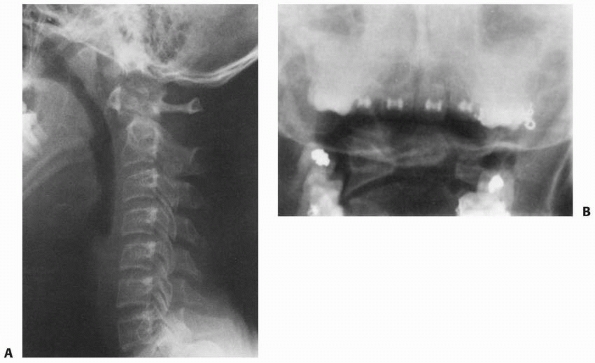 |
|
FIGURE 18-31 Lateral radiograph (A) and open-mouth odontoid radiograph (B)
showing os odontoideum. (From Warner WC. Pediatric cervical spine. In: Canale ST, ed. Campbell’s Operative Orthopaedics. St. Louis: Mosby Year Book, 1999:2817, with permission.) |
intact odontoid against forward displacement. Secondary stabilizers
consist of the apical and alar ligaments, which arise from the tip of
the odontoid and pass to the base of the skull. These also stabilize
the atlanto-occipital joint indirectly.85
The normal distance from the anterior cortex of the dens to the
posterior cortex of the anterior ring of C1 is 3 mm in adults and 4.5
mm in children. In children, if the distance is more than 4.5 mm,
disruption of the transverse ligament is presumed. The spinal canal at
C1 is large compared with other cervical segments and accommodates a
large degree of rotation and some degree of pathologic displacement
without compromising the spinal cord. Steel213
expressed this as a rule of thirds: the spinal canal at C1 is occupied
equally by the spinal cord, odontoid, and a free space, which provides
a buffer zone to prevent neurologic injury. Steel213
found that anterior displacement of the atlas that exceeds a distance
equal to the width of the odontoid may place the spinal cord at risk.
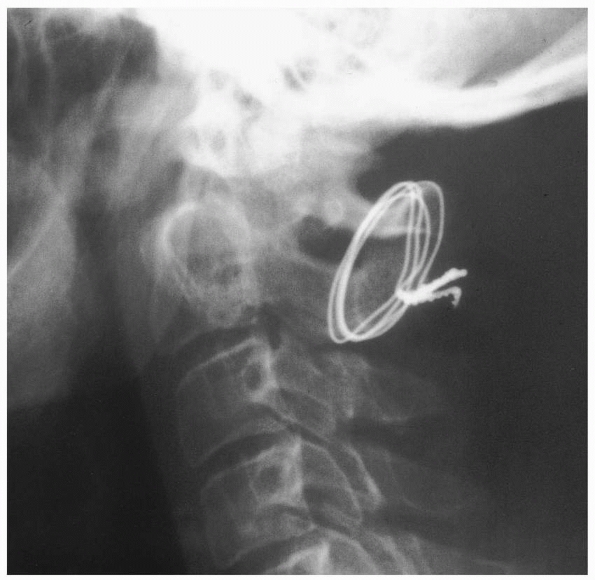 |
|
FIGURE 18-32 Posterior translation of atlas after C1-C2 posterior arthrodesis.
|
usually has a history of cervical spine trauma and complains of neck
pain, often with notable muscle spasms. Diagnosis is confirmed on
lateral radiographs that show an increased atlanto-dens interval. An
active flexion view may be required to show instability in cooperative
patients with unexplained neck pain or neurologic findings. CT scans
are useful to demonstrate avulsion of the transverse ligament from its
origins to the bony ring of C1.
transverse ligament injuries has been reported. For acute injuries,
reduction in extension is recommended, followed by surgical
stabilization of C1 and C2 and immobilization for 8 to 12 weeks in a
Minerva cast, a halo brace, or a cervical orthosis. Flexion and
extension views should be obtained after stabilization to document
stability.
table, with the head supported by traction; the head-thorax relationship is maintained at all times during turning (Fig. 18-35).33
A lateral cervical spine radiograph is obtained to ensure proper
alignment before surgery. The skin is prepared and draped in a sterile
fashion and a solution of epinephrine (1:500,000) is injected
intradermally to aid hemostasis.
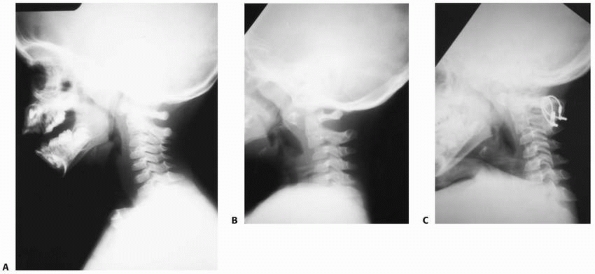 |
|
FIGURE 18-33 A. Lateral radiograph of traumatic C1-C2 instability. B. Note the increase in the atlanto-dens interval. C. Lateral radiograph after C1-C2 posterior arthrodesis.
|
an aneurysm needle, a Mersiline suture is passed from cephalad to
caudad on each side of the midline under the arch of the atlas and then
beneath the lamina of C2. These serve as guides to introduce two
doubled 20-gauge wires. The size of the wire used varies depending on
the size and age of the patient. Two full-thickness bone grafts,
approximately 1.25 × 3.5 cm, are harvested from the iliac crest and
beveled so that the apex of the graft fits in the interval between the
arch of the atlas and the lamina of the axis. Notches are fashioned in
the upper and lower cortical surfaces to hold the circumferential wires
and prevent them from slipping. The doubled wires are tightened over
the graft and twisted on each side. The wound is irrigated and closed
in layers over suction drains.
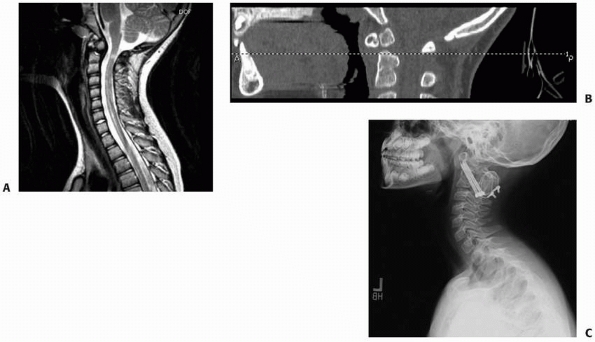 |
|
FIGURE 18-34 MRI (A) and CT scan (B) of 9-year-old girl with os odontodeium. C. After Brooks posterior fusion and transarticular screw fixation.
|
The prone patient then is placed on the operating table with the head
supported by traction, maintaining the head-thorax relationship during
turning. A lateral cervical spine radiograph is obtained to ensure
proper alignment before surgery. The skin is prepared and draped in a
sterile fashion, and a solution of epinephrine (1:500,000) is injected
intradermally to aid hemostasis.
the relatively avascular midline structures, the intermuscular septum,
or ligamentum nuchae. Care should be taken not to expose any more than
the area to be fused to decrease the chance of spontaneous extension of
the fusion. By subperiosteal dissection, the posterior arch of the
atlas and the lamina of C2 are exposed. The muscular and ligamentous
attachments from C2 are removed with a curet. Care should be taken to
dissect laterally along the atlas to prevent injury to the vertebral
arteries and vertebral venous plexus that lie on the superior aspect of
the ring of C1, less than 2 cm lateral to the midline. The upper
surface of C1 is exposed no farther laterally than 1.5 cm from the
midline in adults and 1 cm in children. Decortication of C1 and C2
generally is not necessary. From below, a wire loop of appropriate size
is passed upward under the arch of the atlas either directly or with
the aid of a Mersiline suture. The Mersiline suture can be passed with
an aneurysm needle. The free ends of the wire are passed through the
loop, grasping the arch of C1 in the loop.
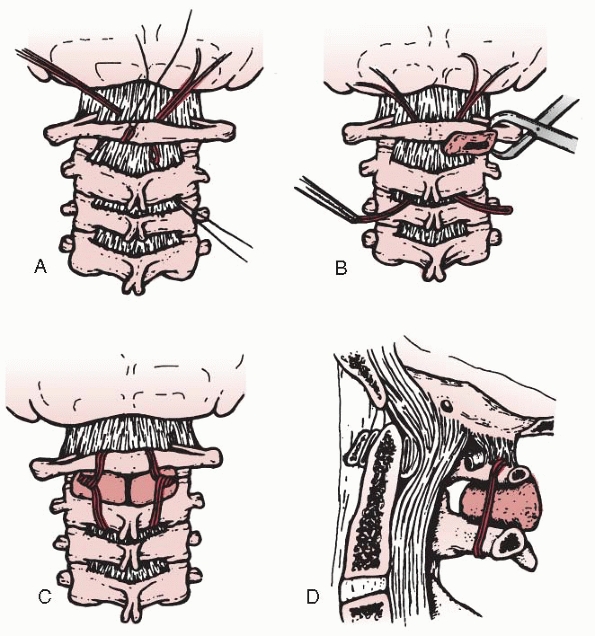 |
|
FIGURE 18-35 Technique of atlantoaxial arthrodesis (Brooks-Jenkins). A. Wires are inserted under the atlas and axis. B. Full-thickness bone grafts from the iliac crest are placed between the arch of the atlas and the lamina of the axis. C,D.
The wires are tightened over the graft and twisted on each side. (From Brooks AL, Jenkins EB. Atlantoaxial arthrodesis by the wedge compression method. J Bone Joint Surg Am 1978;60:279, with permission.) |
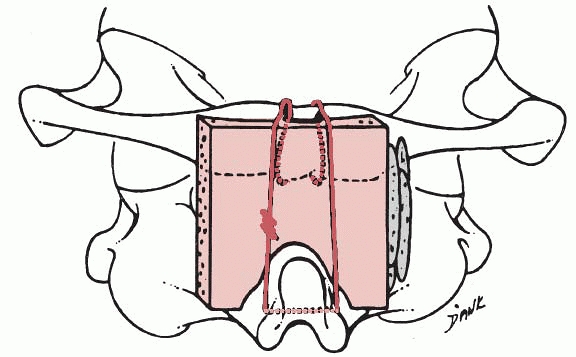 |
|
FIGURE 18-36
Wires are passed under the lamina of the atlas and through the spine of the axis and tied over the graft. This method is used most frequently. (From Fielding JW, Hawkins RJ, Ratzan SA. Spine fusion for atlanto-axial instability. J Bone Joint Surg Am 1976;58:400, with permission.) |
and placed against the lamina of C2 and the arch of C1 beneath the
wire. One end of the wire is passed through the spinous process of C2,
and the wire is twisted on itself to secure the graft in place. The
wound is irrigated and closed in layers with suction drainage tubes.
used to stabilize the atlantoaxial joint. This technique has the
advantage of being biomechanically superior to posterior wiring
techniques,98 and postoperative halo
vest immobilization usually is not required. The disadvantages of this
technique are potential injury to the vertebral artery, its technical
difficulty, and the requirement for sublaminar wire and fusion (Brooks
or Gallie technique). Preoperative imaging should include plain
radiographs, CT scan, MRI, and MRA of the cervical spine. Supervised
dynamic lateral flexion and extension views must determine the
reducibility of the atlantoaxial joint.147
If an anatomic reduction cannot be obtained, transarticular screws
cannot be safely used. MRA can delineate the course of the vertebral
artery through the foramen transversarium and its relationship to the
surrounding bony architecture. Approximately 20% of patients show
anatomic variations in the path of the vertebral artery and osseous
anatomy that would preclude transarticular screw placement.1,29,94
Mayfield skull clamp or with a halo ring attached to the Mayfield
attachment. Under fluoroscopic guidance, proper alignment of the
atlantoaxial joint is confirmed. The spine is prepared and draped from
the occiput to the upper thoracic spine. The upper thoracic spine must
be included in the surgical field to allow percutaneous placement of
the transarticular screw. Percutaneous screw placement may be necessary
because of the cephalad orientation of the C1-C2 transarticular screw.
C3. The C2 inferior facet is used as the landmark for screw entry: the
entry point is 2 mm lateral to the medial edge and 2 mm above the
inferior border of the C2 facet (Fig. 18-37A).
The drill trajectory is angled medially 5 to 10 degrees. On the lateral
fluoroscopic radiograph, the drill trajectory is adjusted toward the
posterior cortex of the anterior arch of C1. Percutaneous placement of
the C1-C2 facet screws may be necessary if the intraoperative
atlantoaxial alignment precludes drilling or placement of screws
through the operative incision. After tapping, a 3.5-mm lag screw is
placed across the C1-C2 joint (Fig. 18-37B).
Another screw is then placed in exactly the same way on the other side.
After placement of the C1-C2 transarticular screw, a bone graft is
harvested from the posterior iliac crest. A traditional posterior C1-C2
fusion is done using either the Gallie or the Brooks technique (Fig 18-38).
with polyaxial screws and rods (Fig. 18-39).
This technique has the advantages of minimizing the risk of vertebral
artery injury, does not require the use of sublaminar wires, and does
not require an intact posterior arch of C1. Disadvantages are the
anatomic limitations of the C1 lateral mass, which may prevent the use
of a 3.5-mm screw, and the potential risk of irritation or injury of
the C2 ganglion.
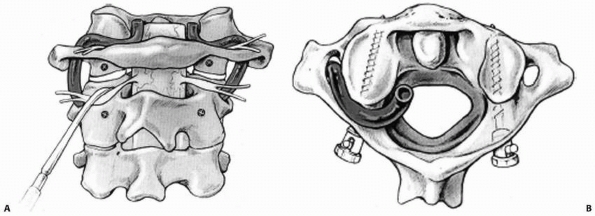 |
|
FIGURE 18-37 Posterior C1-C2 transarticular screw fixation. A. Location of entry points in C1 and C2 for screw placement. B.
Polyaxial screws placed bicortically into the lateral mass. (From Harms J, Melcher RP. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine 2001;26:2467-2471, with permission.) |
Mayfield skull clamp or with a halo ring attached to the Mayfield
attachment. Under fluoroscopic guidance, proper alignment of the
atlantoaxial joint is confirmed. The cervical spine is exposed from the
occiput to C3. The C1-C2 complex is exposed to the lateral border of
the C1-C2 articulation. The C1-C2 joint is exposed and opened by
dissection over the superior surface of the C2 pars interarticularis.
The dorsal root ganglion of C2 is retracted in a caudal direction to
expose the entry point for the C1 screw. This entry point is at the
midpoint of the C1 lateral mass at its junction with the posterior arch
of C1. A 2-mm high-speed burr is used to mark the starting point for
the drill. The drill bit is directed in a straight to slightly
convergent trajectory in the anteroposterior plane, and parallel to the
posterior arch of C1 in the sagittal plane. After determining the
appropriate screw length, the drill hole is tapped and a 3.5-mm
polyaxial screw is inserted. A number 4 Penfield elevator is used to
define the medial border of the C2 isthmus or pedicle. The starting
point for the C2 pedicle screw is in the superior and medial quadrant
of the C2 lateral mass. A C2 pedicle pilot hole is drilled with a 2-mm
drill in a 20 to 30 degree convergent and cephalad trajectory, using
the superior and medial surface of the C2 pedicle as a guide. The hole
is tapped, and a 3.5-mm polyaxial screw of appropriate length is
inserted. Fixation of the rods to the polyaxial screws is obtained with
locking nuts (Fig. 18-40). C1 and C2 are
decorticated posteriorly and cancellous bone from the posterior iliac
crest is used for bone graft. Rigid cervical collar immobilization is
used postoperatively.
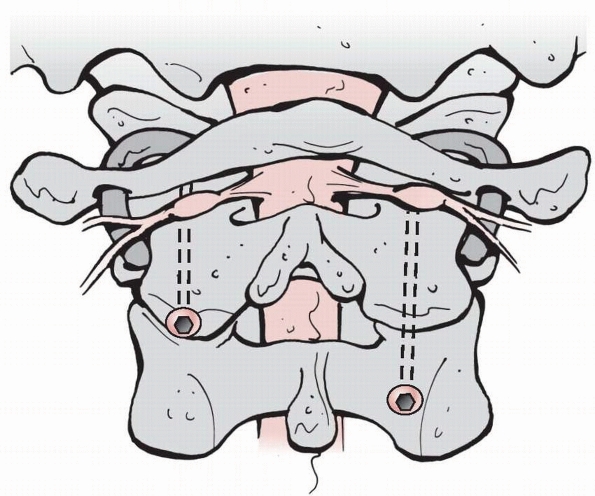 |
|
FIGURE 18-38
Position of vertebral arteries and position of screws across atlantoaxial joint. (From Menezes AH. Surgical approaches to the craniocervical junction. In: Weinstein SL, ed. Pediatric Spine Surgery. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001.) |
rare, chronic atlantoaxial instability occurs in certain conditions
such as juvenile rheumatoid arthritis, Reiter syndrome, Down syndrome,
and Larsen syndrome. Bone dysplasia—such as Morquio polysaccharidosis,
spondyloepiphyseal dysplasia, and Kniest syndrome—also may be
associated with atlantoaxial instability, as well as os odontoideum,
Klippel-Feil syndrome, and occipitalization of the atlas.39,50,91,99,123,127,151
incidences of associated anomalies of the cervical spine, such as Apert
syndrome, hemifacial microsomy, and Goldenhar syndrome.205
Treatment recommendations are individualized based on the natural
history of the disorder and future risk to the patient. Although there
is little literature on cervical spine instability in each of these
syndromes, there has been considerable interest in the incidence and
treatment of atlantoaxial instability in children with Down syndrome.5,49,177,178,227,238
more than 5 mm. The Committee on Sports Medicine of the American Academy of Pediatrics (AAP) issued a policy statement in 19845
asserting that Down syndrome patients with 5 to 6 mm of instability
should be restricted from participating in sports that carry a risk of
stress to the head and neck. In 1995, the AAP retired this
recommendation and issued the following statement: “From the available
scientific evidence, it is reasonable to conclude that lateral plain
x-rays of the cervical spine are of potential but unproven value in
detecting patients at risk for developing spinal cord injury during
sports participation.”6 Current
opinion is that in asymptomatic children, yearly examinations to detect
any neurologic symptoms or signs of myelopathy are more predictive of
progressive myelopathy or neurologic injury than are screening
radiographs.49 Evaluation of lateral
cervical spine radiographs in full flexion and full extension is still
required before participation in sports considered by the Special
Olympics to have potential risk: certain activities that axially load
the head in flexion, such as gymnastics, diving, and soccer.209 Davidson49
found that neurologic signs were more predictive of impending
dislocation than the radiograph criteria. Studies have shown that by
adolescence, the frequency of atlantoaxial instability approaches 10%
to 30%.6,39,178,197,239 It also appears that 12%39 to 16%177 of children with Down syndrome who have instability develop neurologic signs and symptoms.
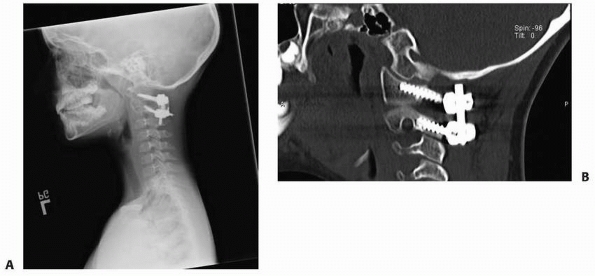 |
|
FIGURE 18-39 Radiograph (A) and MRI (B) after fixation with polyaxial screws and rods.
|
translation of more than 10 mm. In patients with less than 10 mm of
translation and a neurologic deficit or history of neurologic symptoms,
surgical stabilization also may be indicated. Once surgical
stabilization is needed, the treating physician must understand the
increased risk of complications (i.e., pseudarthrosis) in this patient
population. Segal et al.197 reported
a high complication rate after posterior arthrodesis of the cervical
spine in patients with Down syndrome. Six of 10 patients developed
resorption of the bone graft and associated pseudarthrosis. Other
complications in this patient population after attempted posterior
arthrodesis were wound infection, dehiscence of the operative site,
instability of adjacent motion segments, and neurologic sequelae.205
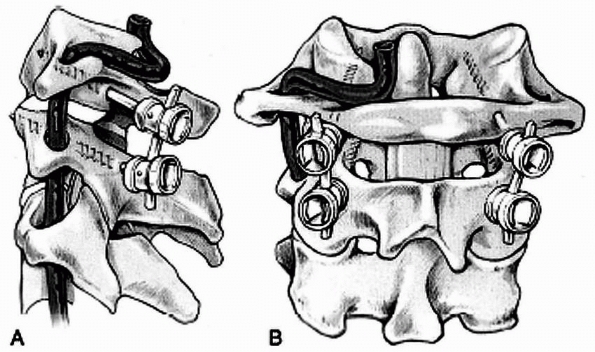 |
|
FIGURE 18-40 Lateral (A) and posterior (B)
views after C1-C2 fixation by the polyaxial screw and rod technique. (From Harms J, Melcher RP. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine 2001;26:2467-2471, with permission.) |
childhood torticollis. This condition is known by several names, such
as rotatory dislocation, rotatory displacement, rotatory subluxation,
and rotatory fixation. Atlantoaxial rotatory subluxation probably is
the most accepted term used, except for long-standing cases (3 months),
which are called rotatory fixation.
atlantoaxial joint; half of the rotation of the cervical spine occurs
there. Through this range of motion at the C1-C2 articulation, some
children develop atlantoaxial rotatory subluxation. The two most common
causes are trauma and infection; the most common cause is an upper
respiratory infection (Grisel syndrome).234
Subluxation also can occur after a retropharyngeal abscess,
tonsillectomy, pharyngoplasty, or trivial trauma. There is free blood
flow between the veins and lymphatics draining the pharynx and the
periodontoid plexus.170 Any inflammation
of these structures can lead to attenuation of the synovial capsule or
transverse ligament or both, with resulting instability. Another
potential etiologic factor is the shape of the superior facets of the
axis in children. Kawabe et al.116
showed that the facets are smaller and more steeply inclined in
children than in adults. A meniscus-like synovial fold was found
between C1 and C2 that could prohibit reduction after displacement has
occurred. Although atlantoaxial rotatory subluxation is most commonly
seen from inflammatory syndromes, it also can occur after trauma.
Type 1 is a unilateral facet subluxation with an intact transverse
ligament. This is the most common and benign type. Type 2 is a
unilateral facet subluxation with anterior displacement of 3 to 5 mm.
The unilateral anterior displacement of one of the lateral masses may
indicate an incompetent transverse ligament with potential instability.
Type 3 is bilateral anterior facet displacement with more than 5 mm of
anterior displacement. This type is associated with deficiencies of the
transverse and secondary ligaments, which can result in significant
narrowing of the space available for the cord at the atlantoaxial
level. Type 4 is an unusual type in which the atlas is displaced
posteriorly. This usually is associated with a deficient dens. Although
types 3 and 4 are rare, neurologic involvement may be present or
instantaneous death can occur. Both types must be managed with great
care.
cock-robin position of rotating to one side, as well as lateral flexion
to the other (Fig. 18-42). When rotatory
subluxation is acute, the child resists attempts to move the head and
has pain with any at-tempts at correction. Usually, the child is able
to make the deformity worse but cannot correct it. Associated muscle
spasms of the sternocleidomastoid muscle occur predominantly on the
side of the long sternocleidomastoid muscle in an attempt to correct
the deformity. If the deformity becomes fixed, the pain subsides but
the torticollis and the decreased range of motion will persist.66
If rotatory fixation has been present for a long time in a small child,
plagiocephaly is sometimes noted. Neurologic abnormalities are
extremely rare, although a few cases have been reported.
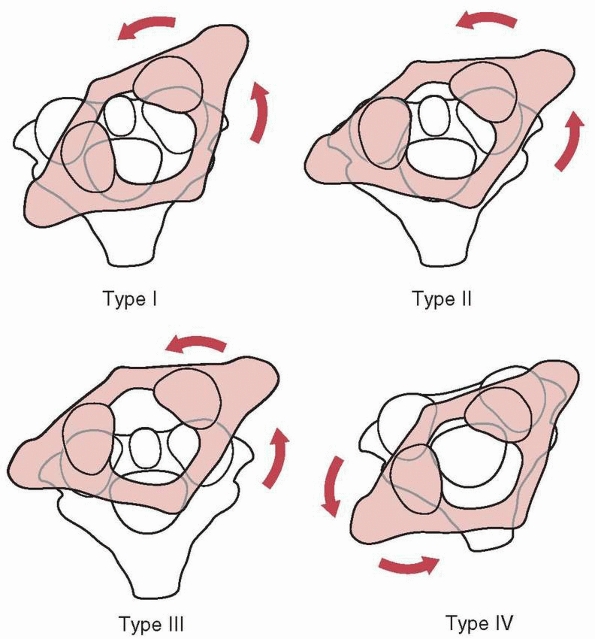 |
|
FIGURE 18-41
Classification of rotary displacement. (From Fielding JW, Hawkins RJ. Atlantoaxial rotary fixation. J Bone Joint Surg Am 1977;59: 37, with permission.) |
 |
|
FIGURE 18-42 Child with rotary subluxation of C1 on C2. Note the direction of head tilt and rotation of the neck.
|
of the associated torticollis and difficulty in positioning the head
and neck. Anteroposterior and open-mouth odontoid views should be taken
with the shoulders flat and the head in as neutral a position as
possible.139 Lateral masses that
have rotated forward appear wider and closer to the midline, whereas
the opposite lateral mass appears narrower and farther away from the
midline on this view. One of the facet joints may be obscured because
of apparent overlapping. The distance between the lateral mass and the
dens also will be asymmetric. On the lateral view, the lateral facet
appears anterior and usually appears wedge-shaped instead of the normal
oval shape. The posterior arches of the atlas may fail to superimpose
because of head tilt, giving the appearance of fusion of C1 to the
occiput (occipitalization). Flexion and extension lateral views are
recommended to exclude instability.
This technique is limited in the acute stage because pain restricts the
motion necessary for a satisfactory study. With atlantoaxial rotatory
fixation, cineradiography
may
be helpful in confirming the diagnosis by showing that the atlas and
axis are rotating as a unit. However, this technique requires high
radiation exposure and generally has been replaced by CT scanning.11,56,68,75,174
CT should be performed with the head and body positioned as close to
neutral as possible. This will show a superimposition of C1 on C2 in a
rotated position and will allow the degree and amount of malrotation to
be quantified. Some researchers have recommended dynamic CT scans taken
with the patient looking to the right and the left to diagnose rotatory
fixation.173 McGuire et al.146
classified findings on dynamic CT scans into three stages: stage 0,
torticollis but a normal dynamic CT scan; stage 1, limitation of motion
with less than 15 degrees difference between C1 and C2, but with C1
crossing the midline; and stage 2, fixed with C1 not crossing the
midline. Duration of treatment and intensity of treatment were greater
the higher the stage. Three-dimensional CT scans also are helpful in
identifying rotatory subluxation.192 Ishii et al.111
reported the use of the lateral inclination angle to grade the severity
of subluxation: grade I, no lateral inclination; grade 2, less than 20
degrees; and grade 3, more 20 degrees (Fig.18-43).
They also noted adaptive changes in the superior facet joint of C2 in
grade 3 subluxations and reported that grade 3 subluxations were more
commonly irreducible. MRI demonstrates more soft tissue detail, such as
associated spinal cord compression and underlying vertebral or soft
tissue infections (Fig. 18-44).186
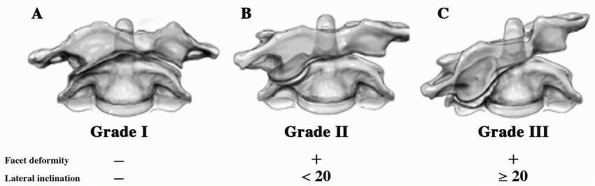 |
|
FIGURE 18-43
Classification of chronic atlantoaxial rotatory fixation: grade I, no lateral inclination; grade II, <20 degrees; grade III, >20 degrees. (From Ishii K, Chiba K, Maruiwa H, et al. Pathognomonic radiological signs for predicting prognosis in patients with chronic atlantoaxial rotatory fixation. J Neurosurg Spine 2006; 5:385-391, with permission.) |
ophthalmologic problems, sternocleidomastoid tightness from muscular
torticollis, brain stem or posterior fossa tumors or abnormalities,
congenital vertebral anomalies, and infections of the vertebral column.
Many patients probably never receive medical treatment because symptoms
may be mild and the subluxation may reduce spontaneously over a few
days before medical attention is sought. If rotatory subluxation has
been present for a week or less, a soft collar, anti-inflammatory
medication, and an exercise program are indicated. If this fails to
produce improvement and the symptoms persist for more than a week, head
halter traction should be initiated. This can be done either at home or
in the hospital, depending on the social situation and the severity of
symptoms. Muscle relaxants and analgesics also may be needed. Phillips
and Hensinger173 found that if
rotatory subluxation was present for less than 1 month, head halter
traction and bedrest were usually sufficient to relieve symptoms. If
the subluxation has been present for longer than a month, successful
reduction is not very likely.40
However, halo traction can still be used to try to reduce the
subluxation. The halo allows increased traction weight to be applied
without interfering with opening of the jaw or causing skin pressure on
the mandible. While the traction is being applied, active rotation to
the right and left should be encouraged. Once the atlantoaxial rotatory
subluxation has been reduced, motion has been restored, and the
reduction is documented by CT scan, the patient is maintained in a halo
vest for 6 weeks. If reduction cannot be maintained, posterior
atlantoaxial arthrodesis is recommended. Even though internal rotation
and alignment of the atlas and axis may not be restored, successful
fusion should result in the appearance of normal head alignment by
relieving the muscle spasms that occurred in response to the
malrotation. Posterior arthrodesis also is recommended if any signs of
instability or neurologic deficits secondary to the subluxation are
present, if the deformity has been present for more than 3 months, or
if conservative treatment of 6 weeks of immobilization has failed.
The mechanism of injury is forced hyperextension and axial loading.
Most reports of this injury have been in children under the age of 2
years.61,71,106,121,169,174,182,188
This injury probably occurs more frequently in this age group because
of the disproportionately large head, poor muscle control, and
hypermobility. The possibility of child abuse also must be considered.121,181,228 Patients present with neck pain and resist any movement of the head and neck. There should be a positive history of trauma (Fig. 18-45).
the axis, usually with some forward subluxation of C2 on C3. One must
be sure this is a fracture and not a persistent synchondrosis of the
axis.141,157,208,228,237 Differentiating a persistent synchondrosis from a fracture may be difficult. Several radiographic
findings can help distinguish congenital spondylolysis from a hangman’s
fracture. With congenital spondylolysis, there should be a symmetrical
osseous gap with smooth, clearly defined cortical margins; no
prevertebral soft tissue swelling should be observed; and there should
be no signs of instability. Often, small foci of ossification are seen
in the defect. CT scans show the defect to be at the level of the
neurocentral chondrosis. MRI does not show any edema or soft tissue
swelling that typically is present with a fracture.153,228
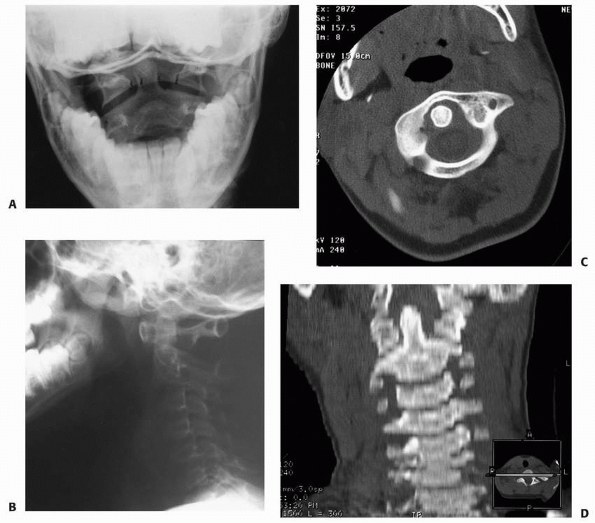 |
|
FIGURE 18-44 A,B. Odontoid view and lateral cervical spine radiograph of rotary subluxation of C1 on C2. C. Note the asymmetry on the open-mouth odontoid view. D. CT and CT reconstruction documenting rotary subluxation.
|
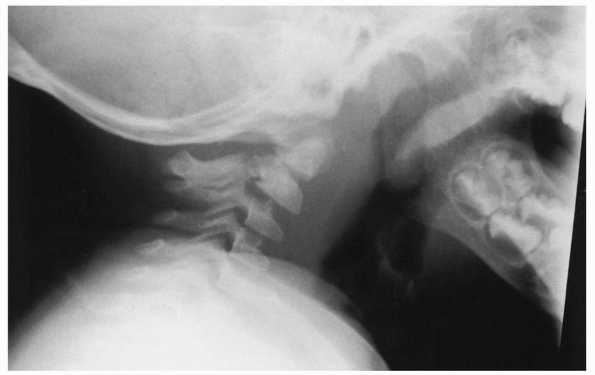 |
|
FIGURE 18-45 Lateral radiograph of patient with traumatic C2 spondylolisthesis (hangman’s fracture).
|
immobilization in a Minerva cast, halo, or cervical orthosis for 8 to
12 weeks. Pizzutillo et al.174
reported that 4 of 5 patients healed with immobilization. If union does
not occur or there is documented instability, a posterior or anterior
arthrodesis can be done to stabilize this fracture.
and usually occur in teenagers or older children. Lower cervical spine
injuries in children as opposed to those in adults can occur through
the cartilaginous endplate.52 The
endplate may break completely through the cartilaginous portion
(Salter-Harris type I) or may exit through the bony edge (Salter-Harris
type II). Usually, the inferior endplate fractures because of the
protective effect of the uncinate processes of the superior endplate.13
flexion or distraction injury to the cervical spine. The patient
usually has point tenderness at the injury site and complains of neck
pain. Initial radiographs may be normal except for loss of normal
cervical lordosis. This may be a normal finding in young children but
should be evaluated for possible ligamentous injury in an adolescent.
Widening of the posterior interspinous distance
is suggestive of this injury. MRI may be helpful in documenting ligamentous damage.
displacement of one segment on the other can occur, and secondary
adaptive changes in the growing spine may make reduction difficult.
Posterior ligamentous injuries should be protected with an extension
orthosis, and patients should be followed closely for the development
of instability. If signs of instability are present, then a posterior
arthrodesis should be performed. Guidelines for instability in children
have not been fully developed. Instability in adults has been defined
as angulation between adjacent vertebrae in the sagittal plane of 22
degrees more than the adjacent normal segment or translation in the
sagittal plane of 3.5 mm or more.165,166,235,236
subaxial spine in children, are caused by flexion and axial loading
that results in loss of vertebral body height. This can be detected on
a lateral radiograph. Because the vertebral disks in children are more
resilient than the vertebral bodies, the bone is more likely to be
injured. Compression fractures are stable injuries and heal in children
in 3 to 6 weeks. Many compression fractures may be overlooked because
of the normal wedge shape of the vertebral bodies in young children.
Immobilization in a cervical collar is recommended for 3 to 6 weeks.
Flexion and extension films to confirm stability should be obtained 2
to 4 weeks after injury. In children under 8 years of age, the
vertebral body may reconstitute itself with growth, although Schwarz et
al.196 reported that kyphosis of
more than 20 degrees may not correct with growth. Associated injuries
can include anterior teardrop, laminar, and spinous process fractures.
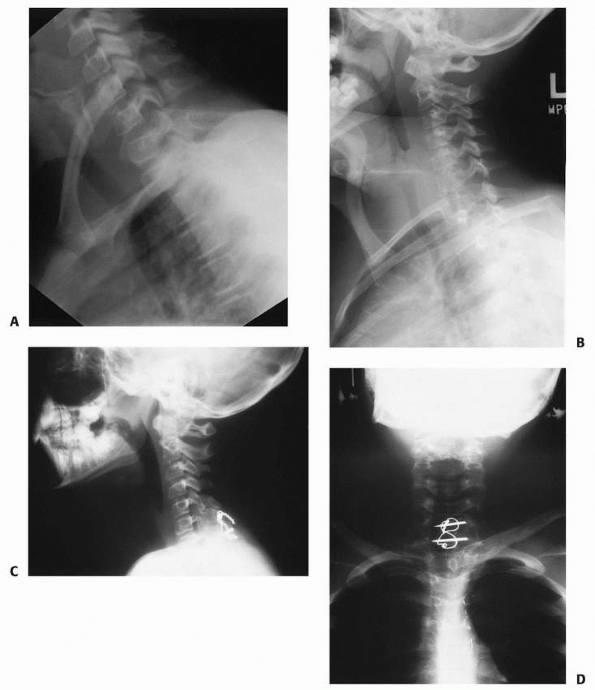 |
|
FIGURE 18-46 A,B. Lateral radiograph of a patient with so-called perched facets, demonstrating a facet dislocation. C,D. Lateral and anteroposterior radiographs after reduction and posterior arthrodesis.
|
dislocations are the second most common injuries in the subaxial spine
in children. Most occur in adolescents and are similar to adult
injuries. The diagnosis usually can be made on anteroposterior and
lateral radiographs. In children, the so-called perched facet is a true
dislocation. The cartilaginous components are overlapped and locked. On
the radiograph, the facet appears perched because the overlapped
cartilage cannot be seen. Unilateral facet dislocation is treated with
traction and reduction. If reduction cannot be easily obtained, open
reduction and arthrodesis are indicated. Complete bilateral facet
dislocation, although rare, is more unstable and has a higher incidence
of neurologic deficit (Fig. 18-46). Treatment consists of reduction and stabilization with a posterior arthrodesis.
These injuries are caused by an axial load. Radiographic evaluation
should consist of anteroposterior and lateral views. CT scans aid in
detecting any spinal canal compromise from retropulsed fracture
fragments and occult laminar fractures. The posterior aspect of the
vertebral body can displace posteriorly, causing canal compromise and
neurologic deficit. If no neurologic deficit or significant canal
compromise is present, then treatment consists of traction followed by
halo immobilization. Anterior arthrodesis rarely is recommended in
pediatric patients, except in a patient with a burst fracture and
significant canal compromise.199 Anterior arthrodesis destroys the anterior growth potential; as posterior growth continues, a kyphotic deformity may occur (Fig. 18-47). In older children and adolescents, anterior instrumentation can be used for stabilization (Fig. 18-48).
have been reported. These injuries can occur from either a
hyperextension or flexion axial loading injury. Associated
anterosuperior avulsion or compression fractures of the vertebral body
may occur. The diagnosis usually is made on plain radiographs that show
a fracture line through the pedicles. Oblique views may be necessary to
better identify the fracture line. CT scanning may be useful in
differentiating an acute fracture from a normal synchondrosis.
Treatment consists of immobilization in a cervical orthosis or halo
brace. Surgical stabilization is recommended only for truly unstable
fractures or nonunions. Neurologic involvement is rare.
The patient is turned prone on the operating table, with care taken to
maintain traction and proper alignment of the head and neck. The head
may be positioned in a head rest or maintained in skeletal traction.
Radiographs are obtained to confirm adequate alignment of the vertebrae
and to localize the vertebrae to be exposed. Extension of the fusion
mass can occur when extra vertebrae or spinous processes are exposed in
the cervical spine. A midline incision is made over the chosen spinous
processes, and the spinous process and lamina are exposed
subperiosteally to the facet joints. If the spinous process is large
enough, a hole is made in the base of the spinous process with a towel
clip or Lewin clamp. An 18-gauge wire is passed through this hole,
looped over the spinous process, and passed through the hole again. A
similar hole is made in the base of the spinous process of the inferior
vertebra to be fused, and the wire is passed through this vertebra. The
wire is then passed through this hole, looped under the inferior aspect
of the spinous process, and then passed back through the same hole. The
wire is tightened and corticocancellous bone grafts are placed along
the exposed lamina and spinous processes. The wound is closed in
layers. If the spinous process is too small to pass wires, then an in
situ arthrodesis can be performed and external immobilization used.
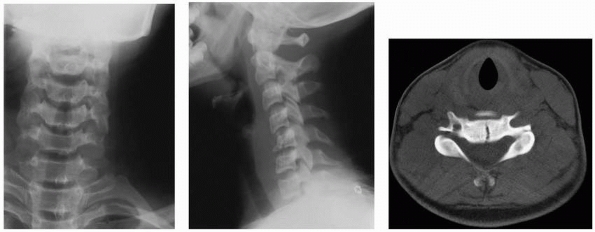 |
|
FIGURE 18-47 Anteroposterior and lateral radiographs and CT scan of patient with a minimally displaced burst fracture of C5.
|
16-gauge wire and threaded Kirschner wires. The threaded Kirschner
wires are passed through the bases of the spinous processes of the
vertebrae to be fused. This is followed by a figure-of-eight wiring
with a 16-gauge wire (Fig. 18-50). After
tightening the wire about the Kirschner wires, strips of
corticocancellous and cancellous bone are packed over the posterior
arches of the vertebrae to be fused.
or screw-and-rod systems can be used in the lower cervical spine. The
instrumentation should be of appropriate size to match the size of the
child’s cervical spine.
the lower cervical spine have been described. They differ primarily in
the entry points for the screws and in the trajectory of screw
placement, which yield different exit points.138,187
at the center of the rectangular posterior face of the lateral mass or
can be measured 5 mm medial to the lateral edge and midway between the
facet joints (Fig. 18-51A). The drill is directed perpendicular to the posterior wall of the vertebral body with a 10-degree lateral angle (Fig. 18-51B).
This trajectory establishes an exit point slightly lateral to the
vertebral artery and below the exiting nerve root. The lateral mass
depth from C3
to
C6 ranges from 6 to 14 mm in men (average 8.7 mm) and 6 to 11 mm in
women (average 7.9 mm). An adjustable drill guide set to a depth of 10
to 12 mm is used to prevent penetration beyond the anterior cortex. The
depth can be gradually and safely increased if local anatomy permits.
If the additional 20% of pullout strength with bicortical fixation is
desired, the exit point should be at the junction of the lateral mass
and the transverse process. Lateral fluoroscopic imaging makes it
easier to choose the optimal trajectory and avoid penetration of the
subjacent facet joint (Fig. 18-51C), which is especially important at the caudal level of fixation because this joint should be included in the fusion.
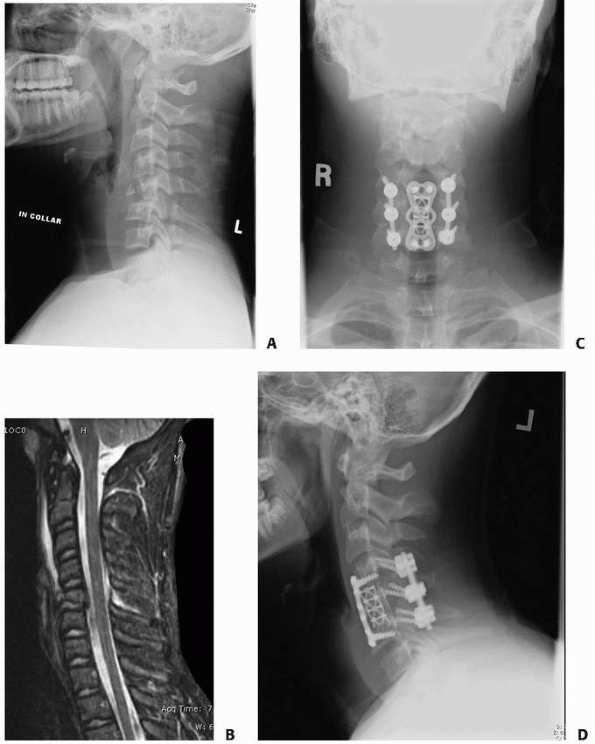 |
|
FIGURE 18-48 Radiograph (A) and MRI (B) of 12-year-old boy with three-column injury sustained during football game. C,D. After anterior and posterior fusion and fixation with anterior plate and screws and posterior instrumentation.
|
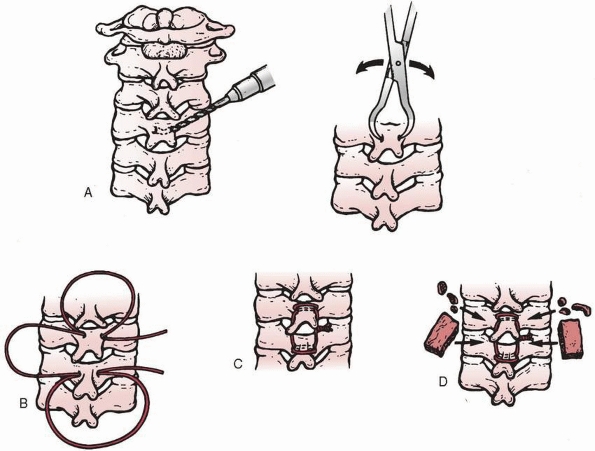 |
|
FIGURE 18-49 Technique of posterior arthrodesis in subaxial spine levels C3-C7. A. A hole is made in the spinous process of the vertebrae to be fused. B. An 18-gauge wire is passed through both holes and around the spinous processes. C. The wire is tightened. D.
Corticocancellous bone grafts are placed. (From Murphy MJ, Southwick WO. Posterior approaches and fusions. In: Cervical Spine Research Society. The Cervical Spine. Philadelphia: JB Lippincott, 1983:506-507, with permission.) |
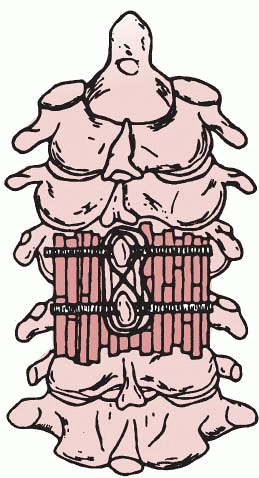 |
|
FIGURE 18-50
Alternative fixation method for posterior arthrodesis of C3-C7. A 16-gauge wire is placed in a figure-of-eight pattern around two threaded Kirschner wires passed through the bases of the spinous processes of the vertebrae to be fused. (From Hall JE, Simmons ED, Danylchuk K, et al. Instability of the cervical spine and neurological involvement in Klippel-Feil syndrome: a case report. J Bone Joint Surg Am 1990; 72:460, with permission.) |
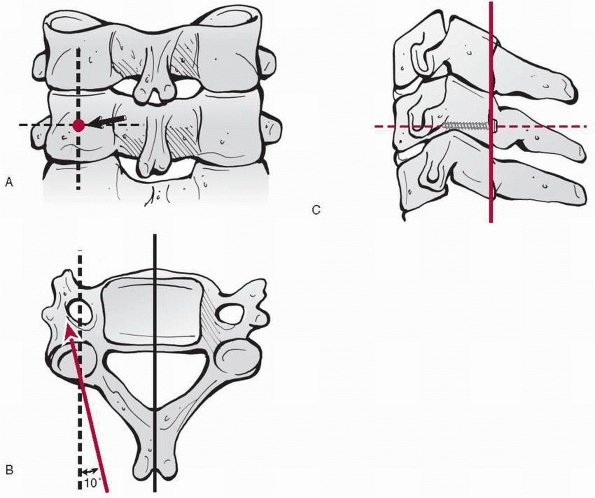 |
|
FIGURE 18-51 Roy-Camille technique of lateral mass screw insertion. A. Entry point for screw insertion. B. Drill is directed perpendicular to posterior wall of vertebral body with a 10-degree lateral angle. C.
Final screw position. (From Heller JG, Jeffords P. Internal fixation of the cervical spine. Posterior instrumentation of the lower cervical spine. In: Frymoyer JW, Wiesel SW, eds. The Adult and Pediatric Spine. 3rd ed. Phila-delphia: Lippincott Williams & Wilkins, 2004, with permission.) |
medial and rostral (proximal) to the center point of the posterior
surface of the lateral mass (Fig. 18-52A). It
is oriented at a 45- to 60-degree rostral angle, parallel to the
adjacent facet joint articular surface, and at a 25-degree lateral
angle (Fig. 18-52B). This trajectory
establishes an exit point lateral to the vertebral artery and above the
exiting nerve root while engaging the lateral portion of the ventral
cortex of the superior articular facet (Fig. 18-52C).
The proper trajectory for this technique is more difficult to achieve
that in the Roy-Camille technique. The prominence of the thorax can
impede proper alignment of the drill and guide, risking injury to the
nerve root if the second cortex is penetrated. The depth of penetration
at this angle is approximately 18 mm, compared to 14 mm with the
Roy-Camille technique, which has some implications for purchase
strength and mode of screw failure.
the neutral position in a Mayfield head holder. The posterior arch of
C1 and the spinous process, laminae, and medial-lateral masses of C2
are exposed. A high-speed drill is used to open a small cortical window
at the junction of the C2 spinous process
and the lamina on the left, close to the rostral margin of the C2 lamina (Fig. 18-53).
With a hand drill, the contralateral (right) lamina is carefully
drilled along its length, with the drill visually aligned along the
angle of the exposed contralateral laminar surface. A small ball probe
is used to palpate the length of the drill hole and verify that no
cortical breakthrough into the spinal canal has occurred. A 4-mm
diameter polyaxial screw is inserted along the same trajectory. In the
final position, the screw head remains at the junction of the spinous
process and lamina on the left, with the length of the screw within the
right lamina. Next, a small cortical window is made at the junction of
the spinous process and lamina of C2 on the right, close to the caudal
aspect of the lamina. Using the same technique, a 4-mm diameter screw
is placed into the left lamina, with the screw head remaining on the
right side of the spinous process (Fig. 18-54). Appropriate rods are then placed into the screw heads and attached to C1 screws or lateral mass screws below C2 (Fig. 18-55).133
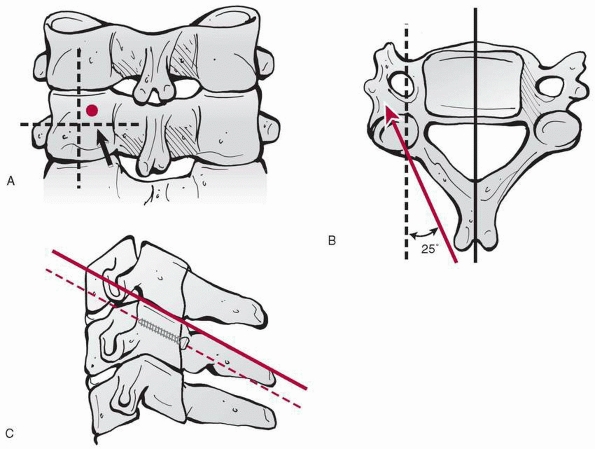 |
|
FIGURE 18-52 Magerl technique of lateral mass screw insertion. A. Entry point for screw insertion. B. Drill is directed at a 25-degree lateral angle. C.
Final screw position. (From Heller JG, Jeffords P. Internal fixation of the cervical spine. Posterior instrumentation of the lower cervical spine. In: Frymoyer JW, Wiesel SW, eds. The Adult and Pediatric Spine. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2004:??, with permission.) |
 |
|
FIGURE 18-53
C2 translaminar screw placement (see text). (From Leonard JR, Wright NM. Pediatric atlantoaxial fixation with bilateral, crossing C2 translaminar screws. Technical note. J Neurosurg: Pediatrics 2006;104:59-63, with permission.) |
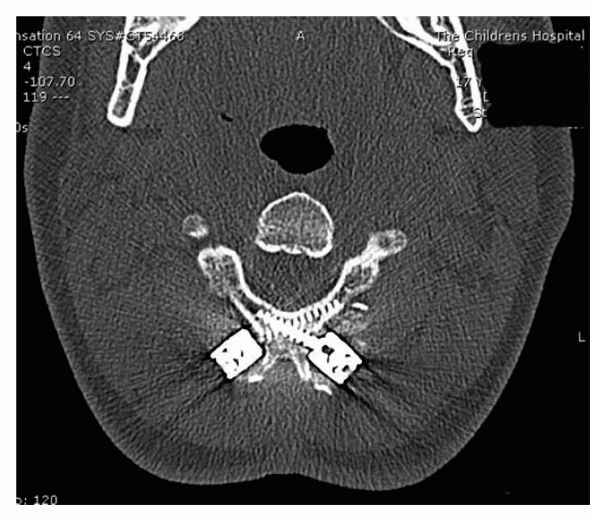 |
|
FIGURE 18-54 CT shows placement of screws.
|
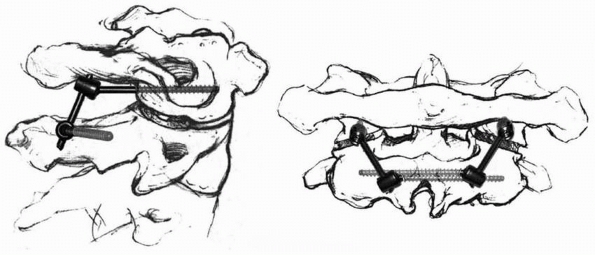 |
|
FIGURE 18-55 Lateral (left) and anteroposterior (right) views of completed C1-C2 fixation with C1 lateral mass screws connected to C2 laminar screws (lateral view).
(From Leonard JR, Wright NM. Pediatric atlantoaxial fixation with bilateral, crossing C2 translaminar screws. Technical note. J Neurosurg: Pediatrics 2006;104:59-63, with permission.) |
Madawi A, Solanki G, Casey AT, et al. Variation of the groove in the
axis vertebra for the vertebral artery. Implications for
instrumentation. J Bone Joint Surg Br 1997; 79:820-823.
KM, Grossman DC, Langer SC, et al. Use of helical computed tomography
for imaging the pediatric cervical spine. Acad Emerg Med
2004;11:228-236.
JJ, Zembo M, Nadell J, et al. C1-C2 posterior soft tissue injuries with
neurologic impairment in children. J Pediatr Orthop 1990;10:596-601.
Academy of Orthopaedic Surgeons, Committee on Pediatric Orthopaedics.
Trauma of the Cervical Spine. Position Statement. Rosemont, IL: Author;
1990.
Academy of Pediatrics. Committee on Sports Medicine. Atlantoaxial
instability in Down syndrome. Pediatrics 1984;74:152-154.
Academy of Pediatrics Committee on Sports Medicine and Fitness.
Atlantoaxial instability in Down syndrome: subject review. Pediatrics
1995;96(1 Pt 1): 151-154.
JM, Schutt AH. Spinal injury in children: a review of 156 cases seen
from 1950 through 1978. Mayo Clin Proc 1980;55:499-504.
LD, Smith BL Jr, DeTorre J, et al. The role of polytomography in the
diagnosis and treatment of cervical spine injuries. Clin Orthop Relat
Res 1982;165:64-68.
JA, Finlay DB, Allen MJ, et al. A review of cervical-spine radiographs
in casualty patients. Br J Radiol 1987;60:1059-1061.
V, Aebi M. Anterior and posterior cervical spine fusion and
instrumentation. In: Weinstein SL, ed. Pediatric Spine Surgery. 2nd ed.
Philadelphia: Lippincott Williams & Wilkins, 2001:209-226.
JM, Tutt LK, Kaye JJ, et al. Occipital condyle fractures: clinical
presentation and imaging findings in 76 patients. Emerg Radiol
2005;11:342-347.
BL, Long WB, Hynes GD, et al. Clinical indications for cervical spine
radiographs in the traumatized patient. Am J Surg 1987;153:473-477.
EP, Elefante R, Smaltino F, et al. Angiographic study on the vertebral
artery in cases of deformities of the occipitocervical joint. AJR Am J
Roentgenol 1969;107: 526-529.
HG, Ford S, Bezmalnovic Z, et al. The effect of axial traction during
orotracheal intubation of the trauma victim with an unstable cervical
spine. Ann Emerg Med 1988; 17:25-29.
MB. Pharmacological treatment of acute spinal cord injury: current
status and future projects. J Emerg Med 1993;11(Suppl 1):43-48.
MB. Treatment of acute spinal cord injury with methylprednisolone:
results of a multicenter randomized clinical trial. J Neurotrauma
1991;8(Suppl 1):47-50.
MB, Shepard MJ, Collins WF Jr, et al. Methylprednisolone or naloxone
treatment after acute spinal cord injury: 1-year follow-up data.
Results of the Second National Acute Spinal Cord Injury Study. J
Neurosurg 1992;76:23-31.
MB, Shepard MJ, Collins WF Jr, et al. A randomized controlled trial of
methylprednisolone or naloxone in the treatment of acute spinal cord
injury: results of the Second National Spinal Cord Injury Study. N Engl
J Med 1990;322:1405-1411.
MJ, Abroms IF. Neonatal spinal cord transection secondary to
intrauterine hyperextension of neck in breech presentation. J Pediatr
1974;84:734-737.
DL, Apfelbaum RI. A new occipitocervical fusion constuct in pediatric
patients with occipitocervical instability. Technical note. J Neurosurg
1999;90(Suppl 2):271-275.
DL, York JE, Apfelbaum RI. Anatomic suitability of C1-C2 transarticular
screw placement in pediatric patients. J Neurosurg 2000;92(Suppl
1):7-11.
CV, Alley JB, Ross M, et al. Magnetic resonance imaging of suspected
atlanto-occipital dislocation. Spine 1992;17:245-248.
SW, French HG, Roberts JM, et al. Chronic atlanto-axial instability in
Down syndrome. J Bone Joint Surg Am 1985;67:1356-1360.
JK, Deponte RJ. Chronic atlantoaxial rotatory fixation: correction by
cervical traction, manipulation, and branching. J Pediatr Orthop
1986;6:631-635.
C, Costagliola C, Shamsaldin M, et al. Occipital condyle fractures: a
hidden nosological entity. An experience with 10 cases. Acta Neurochir
(Wien) 2004;146: 779-784.
HS, Filtzer DL. Pseudosubluxation and other normal variations in the
cervical spine in children. J Bone Joint Surg Am 1965;47:1295-1309.
LA, Dormans JP, Pepe MD, et al. Accuracy and reliability of torque
wrenches used for halo application in children. J Bone Joint Surg Am
2003;85:2199-2204.
LA, Pepe MD, Tan V, et al. A comparison of various angles of halo pin
insertion in an immature skull model. Spine 1999;24:1777-1780.
C, Dietrich AM, Bowman MJ, et al. Pediatric cervical-spine
immobilization: achieving neutral position? J J Trauma 1995;39:729-732.
RG. Atlantoaxial instability in individuals with Down syndrome: a fresh
look at the evidence. Pediatrics 1988;81:857-865.
T, Lee CK. Traumatic atlanto-occipital instability: a case report with
follow-up and a new diagnostic technique. Spine 1990;15:595-597.
AM, Ginn-Pease ME, Bartkowski HM, et al. Pediatric cervical spine
fractures: predominately subtle presentation. J Pediatr Surg
1991;26:995-1000.
D, Maulbauer MS, Kaufman RA, et al. Childhood survival of
atlanto-occipital dislocation: underdiagnosis, recognition, treatment,
and review of the literature. Pediatr Neurosurg 1994;21:105-111.
JP, Criscitiello AA, Drummond DS, et al. Complications in children
managed with immobilization in a halo vest. J Bone Joint Surg Am
1995;77:1370-1373.
J, Panjabi M, Gerber M, et al. CT-functional diagnostics of the
rotatory instability of the cervical spine: 1. An experimental study on
cadavers. Spine 1987;12: 197-205.
MA, Theodore N, Adams M, et al. Pediatric cervical spine injuries:
report of 102 cases and review of the literature. J Neurosurg 2000;92(1
Suppl):12-17.
DF, Fielding JW. Defects of the pedicle and spondylolisthesis of the
second cervical vertebra. J Bone Joint Surg Br 1981;63:526-528.
JW, Hawkins RJ. Atlanto-axial rotary fixation (fixed rotary subluxation
of the atlanto-axial joint). J Bone Joint Surg Am 1977;59:37-44.
JW, Stillwell WT, Chynn KY, et al. Use of computed tomography for the
diagnosis of atlanto-axial rotatory fixation. A case report. J Bone
Joint Surg Am 1978; 60:1102-1104.
JM, Closkey RF, Mahboubi S, et al. Role of magnetic resonance imaging
in the assessment of pediatric cervical spine injuries. J Pediatr
Orthop 2002;22:573-577.
S, Barthel MJ, Flannery AM, et al. Cervical spine fractures sustained
by young children in forward-facing car seats. Pediatrics
1989;84:348-354.
SR, Roux R, Botte MJ, et al. Skull osteology as it affects halo pin
placement in children. J Pediatr Orthop 1986;6:434-436.
RB, Rothman SLG, Kier EL. The role of computed tomography in the
evaluation of upper cervical spine pathology. Comput Tomogr
1978;2:79-97.
FH, Dorsey FC, Coleman WP. Past and current clinical studies with GM-1
ganglioside in acute spinal cord injury. Rev Ann Emerg Med
1993;22:1041-1047.
FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal cord
injury—a randomized, placebo-controlled trial with GM-1 ganglioside. N
Engl J Med 1991;324:1829-1838.
FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal cord
injury—a randomized, placebo-controlled trial with GM-1 ganglioside
[erratum]. N Engl J Med 1991;325:1669-1670.
G, Pizzutillo PD, Lee MS. Occipito-atlanto instability in children. A
report of five cases and review of the literature. J Bone Joint Surg Am
1987;69:429-436.
J, Hadji M, Raul JS. Odontoid fractures in the child with neurologic
injury. Direct osteosynthesis with a cortico-spongious screw and
literature review. Childs Nerv Syst 1997;13:105-107.
SA, Dick HM, Thompson RC, et al. Occipitocervical arthrodesis:
indications, technique, and results. Clin Orthop Relat Res
1969;65:118-129.
MN, Zabramski JM, Browner CM, et al. Pediatric spinal trauma: review of
122 cases of spinal cord vertebral column injuries. J Neurosurg
1988;68:18-24.
JE, Denis F, Murray J. Exposure of the upper cervical spine for spinal
decompression by a mandible and tongue-splitting approach. Case report.
J Bone Joint Surg Am 1977;59:121-125.
JA, Deliganis AV, Baxter AB, et al. Radiologic and clinical spectrum of
occipital condyle fractures: retrospective review of 107 consecutive
fractures in 95 patients. AJR Am J Roentgenol 2002;178:1261-1268.
DJ, Emans JB. The correlation of preoperative three-dimensional
computed tomography reconstructions with operative findings in
congenital scoliosis. Spine 2003; 28:2531-2534.
JG, Jeffords P. Internal fixation of the cervical spine. C. Posterior
instrumentation of the lower cervical spine. In: Frymoyer JW, Wiesel
SW, eds. The Adult and Pediatric Spine. Philadelphia: Lippincott
Williams & Wilkins, 2004:803-816.
T, Cunningham BW, Olerud C, et al. Biomechanical comparison of five
different atlantoaxial posterior fixation techniques. Spine
2000;25:2877-2883.
RN, DeVito PD, Ragsdale CG. Changes in the cervical spine in juvenile
rheumatoid arthritis. J Bone Joint Surg Am 1986;68:189-198.
RN, Lang JE, MacEwen GD. Klippel-Feil syndrome: a constellation of
associated anomalies. J Bone Joint Surg Am 1974;56:1246-1252.
JE, Hensinger RN, Dedrick DK, et al. Emergency transport and
positioning of young children who have an injury of the cervical spine:
the standard backboard may be hazardous. J Bone Joint Surg Am
1989;71:15-22.
M, Baker HR. The atlanto-axial joint: roentgenographic and anatomical
study of normal and abnormal motion. J Bone Joint Surg Am
1964;46:1739-1752.
C, Griffith R, Joyce SM. Cervical spine stabilization in pediatric
patients. Evaluation of current techniques. Ann Emerg Med
1987;16:1121-1126.
K, Chiba K, Maruiwa H, et al. Pathognomonic radiological signs for
predicting prognosis in patients with chronic atlantoaxial rotatory
fixation. J Neurosurg Spine 2006;5:385-391.
G. Fracture of the atlas vertebra: report of four cases and a review of
those previously recorded. Br J Surg 1920;7:407-422.
DB, Liem LK, Petermann G. Pediatric atlas fracture: a case of fracture
through a synchondrosis and review of the literature. Neurosurgery
2000;46:991-995.
RA, Carroll CD, Buncher CR. Atlanto-occipital junction: standards for
measurement in normal children. AJNR Am J Neuroradiol 1987;8:995-999.
HT, Hollingshead MC, Chung CJ, et al. Using CT of the cervical spine
for early evaluation of pediatric patients with head trauma. AJR Am J
Roentgenol 2001;177: 1405-1409.
K, Worley G, Griffin T, et al. Pediatric traumatic atlanto-occipital
dislocation: five cases and a review. J Pediatr Orthop 2001;21:585-589.
LS, Kraus JF, Sterling HM. Acute spinal-cord lesions in a pediatric
population: epidemiological and clinical features. Paraplegia
1980;18:206-219.
RM, Foley JJ, Norton PL. Spine and pelvic deformity in childhood and
adolescent paraplegia. J Bone Joint Surg Am 1965;47:659-682.
M, Feil A. Anomalies de la collone vertebrale par absence des vertebres
cervicales; avec cage thoraque remontant jusqu’ala bas du crane. Bull
Soc Anat Paris 1912; 87:185.
M, Takahashi H, Mikawa Y. Atlanto-axial dislocation in Down syndrome:
report of two cases requiring surgical correction. Spine
1986;11:195-200.
SE, Winter RB, Lonstein JE. The surgical treatment of instability of
the upper part of the cervical spine in children and adolescents. J
Bone Joint Surg Am 1984;66: 403-411.
LR, Loder RT, Farley FA, et al. Nuchal cord changes in children with os
odontoideum: evidence for associated trauma. J Pediatr Orthop
1998;18:815-819.
LR, Strouse PJ. Cervical spine standards for flexion radiograph
interspinous distance ratios in children. Acta Radiol 2000;7:615-619.
KP, Senac M, Hardin WD Jr, et al. Utility of the cervical spine
radiograph in pediatric trauma. Am J Surg 1989;158:540-542.
NH, Eismont FJ. Cervical spine injuries in children. In: Weinstein SL,
ed. The Pediatric Spine: Principles and Practice. Philadelphia:
Lippincott Williams & Wilkins, 2001:553-566.
JR, Wright NM. Pediatric atlantoaxial fixation with bilateral, crossing
C2 translaminar screws. Technical note. J Neurosurg:Pediatrics
2006;104:59-63.
JM, Meza MP, Pollack IF, et al. Direct injury to the cervical spine of
a child by a lap-shoulder belt resulting in quadriplegia: case report.
J Trauma 1996;41:747-749.
K, Masaki T, Kokubun Y. Fetal spinal cord injury secondary to
hyperextension of the neck: no effect of caesarean section. Dev Med
Child Neurol 1976;18: 228-232.
F, Seeman P. Stable posterior fusion of the atlas and axis by
transarticular screw fixation. In: Kehr P, Weidner A, eds. Cervical
Spine. Vienna: Springer-Verlag, 1985: 322-327.
S, Sgouros S, Jeyapalan K, et al. Imaging of childhood torticollis due
to atlanto-axial rotatory fixation. Childs Nerv Syst 1995;11:667-671.
LS, Vetter LW, Tolo VT. Cervical anomaly stimulating hangman’s fracture
in a child. J Bone Joint Surg Am 1982;64:299-300.
JK, Erkkila JC, Winter RB. Spine deformities subsequent to acquired
childhood spinal cord injury. Orthop Trans 1979;3:281-282.
BJ, Klassen RA, Chao EY, et al. Acute fracture and dislocations of the
cervical spine in children and adolescents. J Bone Joint Surg Am
1993;75:988-995.
KJ, Silber J, Flynn JM, et al. Torticollis in children: can dynamic
computed tomography help determine severity and treatment? J Pediatr
Orthop 2002;22: 766-770.
AH. Surgical approaches to the craniocervical junction. In: Weinstein
SL, ed. Pediatric Spine Surgery. 2nd ed. Philadelphia: Lippincott
Williams & Wilkins, 2001: 127-148.
AH, Ryken JC. Craniovertebral junction abnormalities. In: Weinsten SL,
ed. The Pediatric Spine: Principles and Practice. 2nd ed. Philadelphia:
Lippincott Williams & Wilkins, 2001:219-238.
Y, Watanabe R, Yamano Y, et al. Fractures through a synchondrosis of
the anterior arch of the atlas. J Bone Joint Surg Br 1987;69:483.
PJ, Ellingsen JM, Hauswirth BE, et al. Thermoplastic Minerva body
jacket—a practical alternative to current methods of cervical spine
stabilization. Phys Ther 1987; 67:223-225.
S, Dehdashti AR, Kehrli P, et al. Occipital condyle fractures in
children: case report and review of the literature. Pediatr Neurosurg
2003;38:265-270.
J, Karasick D. Spondylolysis of the axis vertebra: a rare anomaly
simulating hangman’s fracture. AJR Am J Roentgenol 1999;172:556-557.
S, Moir CR. Predictive factors of the outcome of traumatic cervical
spine fracture in children. J Pediatr Surg 1994;29:1409-1411.
ER, Smoker WRK. The forgotten condyle: the appearance, morphology, and
classification of occipital condyle fractures. AJNR Am J Neuroradiol
1996;17:507-513.
RE, Lahrendanta TV, Kaitila II, et al. Familial spondylolisthesis of
the axis is vertebra. J Bone Joint Surg Br 1986;68:704-706.
T, Langlais J, Glorion C, et al. Fractures of the odontoid process: a
report of 15 cases in children younger than 6 years. J Pediatr Orthop
1999;19:51-54.
JB, Klein BL, Gotschall CS, et al. Age and outcome in pediatric
cervical spine injury: 11-year experience. Pediatr Emerg Care
1994;10:132-137.
D, Pollack IF. Spinal cord injury without radiologic abnormality in
children: the SCIWORA syndrome. J Trauma 1989;29:654-664.
MM, White AA III, Johnson RM. Cervical spine mechanics as a function of
transection of components. J Biomech 1975;8(5):327-336.
SM, Dickman CA, Sonntag VK, et al. Traumatic atlanto-occipital
dislocation with survival. Neurosurgery 1991;28:574-579.
M, Lieberson R, Shatsky S. Hangman’s fracture or primary spondylolysis:
a patient and a brief review. Pediatr Radiol 1991;21:367-368.
WW, Rothman RH, Brown MD. The pharyngovertebral veins: an anatomical
rationale for Grisel syndrome. J Bone Joint Surg Am 1984;66:568-574.
JC, Tepas JJ 3rd, Mollitt DL, et al. Pediatric cervical spine injuries:
defining the disease. J Pediatr Surg 2001;36:373-376.
GF, Gourard D, Hardy JR, et al. Roentgenographical study of the
stability of the cervical spine in children. J Pediatr Orthop
1984;4:346-352.
WA, Hensinger RN. The management of rotatory atlantoaxial subluxation
in children. J Bone Joint Surg Am 1989;71:664-668.
PD, Rocha EF, D’Astous J, et al. Bilateral fractures of the pedicle of
the second cervical vertebra in the young child. J Bone Joint Surg Am
1986;68:892-896.
SM, Scolia FH. Atlantoaxial instability in individuals with Down
syndrome: epidemiologic, radiographic, and clinical studies. Pediatrics
1987;4:555-560.
I, Boyce WT, Duncan B, et al. Clinical prediction of cervical spine
injuries in children: radiographic abnormalities. Am J Dis Child
1987;141:199-201.
ME, Chung K, Barnes PD, et al. Role of flexion-extension radiographs in
blunt pediatric cervical spine injury. Acad Emerg Med 2001;8:237-245.
RK, Mullett JH, Burke TE. Hangman’s fracture cause by suspected child
abuse. A case report. J Pediatr Orthop B 2002;11:329-332.
MH, Mayfrank L, Rohde V, et al. Surgically treated traumatic
synchondrotic disruption of the odontoid process in a 15-month-old
girl. Childs Nerv Syst 1998;14: 85-87.
CJ, O’Malley M, Dorgan JC, et al. A pictorial review of atlantoaxial
rotatory fixation: key points for the radiology. Clin Radiol
2001;56:947-958.
R, Saillant G, Mazel C. Internal fixation of the unstable cervical
spine by posterior osteosynthesis with plates and screws. In: Sherk HH,
ed. The Cervical Spine. 2nd ed. Philadelphia: JB Lippincott,
1989:390-412.
WN, Wills BPD, Dormans JP, et al. Os odontoideum revisited: the case
for a multifactorial etiology. Spine 2006;31:979-984.
R. Three-dimensional computed tomography in infantile atlantoaxial
rotatory fixation. J Bone Joint Surg Br 1994;76:367-370.
N, Könings P, Hassler W, et al. Typical and atypical fractures of the
odontoid process in young children. Report of two cases and a review of
the literature. Acta Neurochir (Wien) 1996;138:524-530.
GR, Wright SW, Fein JA, et al. Pediatric cervical spine injury
sustained in falls from low heights. Ann Emerg Med 1997;30:249-252.
N, Genelin F, Schwarz AF. Posttraumatic cervical kyphosis in children
cannot be prevented by nonoperative methods. Injury 1994;25:173-175.
LS, Drummond DS, Zanotti RM, et al. Complications of posterior
arthrodesis of the cervical spine in patients who have Down syndrome. J
Bone Joint Surg Am 1991; 73:1547-1560.
I, Ram Z, Hadani M. The anterior cervical approach for traumatic
injuries to the cervical spine. Clin Orthop Relat Res 1993;292:144-150.
MA, Doris PE. Limitation of the cross-table lateral view in detecting
cervical spine injuries: a retrospective review. Ann Emerg Med
1981;10:508-513.
EW, Day RA, Kaufman BA, et al. Subdental synchondrosis fracture in
children: the value of three-dimensional computerized tomography.
Pediatr Neurosurg 1996;25:256-259.
HH, Whitaker LA, Pasquariello PS. Fascial malformations and spinal
anomalies: a predictable relationship. Spine 1982;7:526-531.
ST, Madden JD, Esterly JR, et al. Transection of the spinal cord. A
rare obstetrical complication of cephalic delivery. Arch Dis Child
1971;46:291-294.
F, Svien HJ, Bickel WH, et al. Swan neck deformity following extensive
cervical laminectomy. J Bone Joint Surg Am 1974;56:564-580.
T, Skinner SR, Shonnard NH. Persistent synchondrosis of the second
cervical vertebra simulating a hangman’s fracture in a child. J Bone
Joint Surg Am 1993;75: 1228-1230.
Olympics, Inc. Participation by individuals with DS who suffer from
atlantoaxial dislocation. Washington, DC: Author; 1983.
PD, Herzenberg JE. Cervical spine injuries in children. In: Clark CR,
Dvorak J, Ducker TB, et al, eds. The Cervical Spine. Philadelphia:
Lippincott-Raven, 1998: 357-371.
MP, Lechner RM, Anderson JS. Atlantooccipital dislocation in children:
presentation, diagnosis, and management. Neurosurg Focus 2003;14:1-7.
JM, Chong WK, Barber C, et al. A new appraisal of abnormalities of the
odontoid process associated with atlantoaxial subluxation and
neurological disability. Brain 1994;117:133-148.
PP, Poffenbarger GJ, Durham S, et al. Spectrum of occipitoatlantoaxial
injury in young children. J Neurosurg 2000;93(1 Suppl):28-39.
AR, The mechanism of injury to the spinal cord in the neck without
damage to the vertebral column. J Bone Joint Surg Br 1951;33:453-547.
C, Harish S, Saifuddin A, et al. Displaced fracture through the
anterior atlantal synchondrosis. Skeletal Radiol 2005;34:547-549.
VT, Weiland AJ. Unsuspected atlas fractures and instability associated
with oropharyngeal injury: case report. J Trauma 1979;19:278-280.
T, Kawaji Y, Moriya K, et al. Two cases of odontoid fracture in
preschool children. J Spinal Disord Tech 2006;19:204-207.
Rijn RR, Kool DR, de Witt Hamer PC, et al. An abused 5-month-old girl:
hangman’s fracture or congenital arch defect? J Emerg Med 2005;29:61-65.
P, Simon H, Pressman BD, et al. A prospective multicenter study of
cervical spine injury in children. Pediatrics 2001;108:E20.
JW, Stevens DB, Young AB. Traumatic paraplegia in children without
contiguous spinal fracture or dislocation. Neurosurgery 1983;12:439-445.
J, Vokshoor A, Kim S, et al. Pediatric atlantoaxial instability:
management with screw fixation. Pediatr Neurosurg 1999;30:70-78.
SB, Bohlman HH. Occipitocervical fusion: indications, technique, and
long-term results. J Bone Joint Surg Am 1987;69:833-836.
AA III, Johnson RM, Panjabi MM, et al. Biomechanical analysis of
clinical stability in the cervical spine. Clin Orthop Relat Res
1975;109:85-96.
JP III, Baker DH, Miller WA. CT appearance of congenital defect
resembling the hangman’s fracture. Pediatr Radiol 1999;29:549-550.
F, Peterson H, MacCarty C. Incidence of spinal column deformity after
multiple level laminectomy in children and adults. J Neurosurg
1982;57:441-445.
BS, Morrison K, Gaines B, et al. Effect of age on cervical spine
injuries in children after motor vehicle collisions: effectiveness of
restraint devices. J Pediatr Surg 2004;39:483-486.
