Cartilage Lesions
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section
II – Specific Bone Neoplasms and Simulators > 5 – Benign Bone Tumors
> 5.2 – Cartilage Lesions
II – Specific Bone Neoplasms and Simulators > 5 – Benign Bone Tumors
> 5.2 – Cartilage Lesions
5.2
Cartilage Lesions
Felasfa M. Wodajo
Chondromas
Chondromas, according to the latest World Health
Organization classification, include enchondroma, periosteal chondroma,
and enchondromatosis. The common thread is that all chondromas are
tumors of hyaline cartilage. This distinguishes them from other
cartilage tumors such as chondroblastomas and chondromyxoid fibromas,
which are tumors of immature cartilage. The tumors of hyaline cartilage
share a characteristic pattern of cartilage mineralization, evident
radiographically, that allows them to be recognized with a great degree
of certainty (Fig. 5.2-1). Enchondroma is the
intramedullary variety, periosteal chondroma is the surface
counterpart, and enchondromatosis is the congenital chondroma disorder.
The latter is described in more detail in Chapter 7, Congenital and Inherited Bone Conditions.
Organization classification, include enchondroma, periosteal chondroma,
and enchondromatosis. The common thread is that all chondromas are
tumors of hyaline cartilage. This distinguishes them from other
cartilage tumors such as chondroblastomas and chondromyxoid fibromas,
which are tumors of immature cartilage. The tumors of hyaline cartilage
share a characteristic pattern of cartilage mineralization, evident
radiographically, that allows them to be recognized with a great degree
of certainty (Fig. 5.2-1). Enchondroma is the
intramedullary variety, periosteal chondroma is the surface
counterpart, and enchondromatosis is the congenital chondroma disorder.
The latter is described in more detail in Chapter 7, Congenital and Inherited Bone Conditions.
Enchondroma
Enchondromas are benign intramedullary lesions
consisting of mature hyaline cartilage. They are common findings in the
long bones and are generally asymptomatic.
consisting of mature hyaline cartilage. They are common findings in the
long bones and are generally asymptomatic.
 |
|
Figure 5.2-1 Soft tissue chondroma in the foot. Large, deep mass with areas of dark T1 signal on MRI (A) corresponding to mineralized portions well visualized on CT (B).
|
Pathogenesis
Etiology
-
Enchondromas are thought to arise from small rests of physeal cartilage entrapped in the metaphysis of growing bones.
-
Chromosomal abnormalities of 6 and 12 noted in some.
Epidemiology
-
Frequency: second most common benign chondroid lesion, after osteochondroma
-
3% to 17% of all primary bone tumors
-
Autopsy studies: 1.7% of distal femoral metaphyses contain cartilage rests
-
Most lesions are asymptomatic, and therefore the true incidence is probably higher.
-
-
No gender predilection has been noted.
-
Distribution
-
Most common location: small bones of hand (40% to 65% of lesions)
-
Uncommon in distal phalanx
-
Less common in small bones of the feet
-
-
Common in the long bones (25%)
-
Femur >mt humerus
-
-
Solitary enchondromas of flat bones (rib, sternum, scapula): rare
-
Intramedullary cartilage lesion in these bones should lead to concern for chondrosarcoma.
-
In the ribs, chondrosarcoma usually at anterior costochondral junction
P.127 -
-
Solitary enchondromas of the pelvis: very rare
-
Exception: enchondromatosis
-
Converse: Chondrosarcoma is relatively common in the pelvis.
-
-
Solitary enchondromas of spine: rare
-
Converse: Chondrosarcoma is the second most common nonlymphoproliferative primary tumor of the spine after chordoma.
-
Natural History
In general, enchondromas are considered benign, indolent
lesions. Slow or no growth is expected. Pathologic fracture through
enchondromas in the hands and feet is not uncommon.
lesions. Slow or no growth is expected. Pathologic fracture through
enchondromas in the hands and feet is not uncommon.
Malignant Transformation
-
Transformation of enchondromas to malignant chondrosarcoma has been well described (Fig. 5.2-2).
-
The actual incidence is controversial.
Rates of transformation of up to 2% to 3% of large (3 to 7 cm)
enchondromas have been suggested.
 |
|
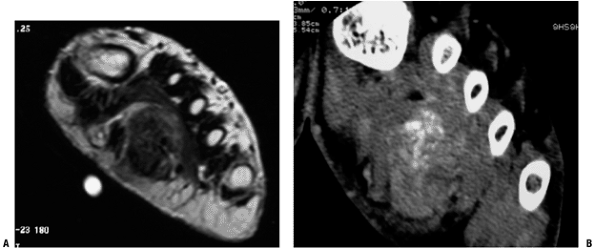
Figure 5.2-2 Transformed enchondroma. (A)
Plain radiograph of a painful lesion in proximal fibula with extensive endosteal scalloping, consistent with an aggressive enchondroma or “carcinoma in situ.” (B) CT scan of distal femur cartilage lesion with periosteal bone reaction indicative of grade 1 or higher chondrosarcoma. |
Atypical or Aggressive Enchondroma (Grade 1/2 Chondrosarcoma; Chondrosarcoma In Situ)
-
More common than malignant transformation
-
Painful intramedullary enchondroma that fills the marrow cavity and demonstrates deep (>mt50%) endosteal scalloping
-
No significant metastatic potential has been described, but they nevertheless should undergo intralesional excision.
Ollier’s Disease (also see Chapter 7.3)
-
Nonhereditary congenital disease with multiple intramedullary foci of cartilage
-
Ranges in presentation from asymptomatic
enchondromatosis to significant growth disturbances, including bone
shortening and deformity -
Often unilateral or even single limb in its distribution
-
Significantly increased risk of secondary transformation to chondrosarcoma, with rates described as high as 5% to 30%
Maffucci’s Syndrome (also see Chapter 7.3)
-
Another disorder with multiple enchondromas but in association with soft tissue lesions, most commonly hemangiomas
-
Predilection for hands and wrist
-
Soft tissue lesions are usually in the same extremity.
-
Increased risk for developing chondrosarcoma, other sarcomas, and even other, nonskeletal malignancies
P.128
Diagnosis (see Algorithm 5.2-1)
Clinical Features
Most common presentation is as an incidental finding,
often in adults undergoing evaluation for other reasons. Often there is
a history of knee or shoulder pain. In one series, magnetic resonance
imaging (MRI) demonstrated that 81% of patients with proximal humerus
enchondromas also had another shoulder disorder potentially causing
pain.
often in adults undergoing evaluation for other reasons. Often there is
a history of knee or shoulder pain. In one series, magnetic resonance
imaging (MRI) demonstrated that 81% of patients with proximal humerus
enchondromas also had another shoulder disorder potentially causing
pain.
Therefore, for patients with vague, nonlocalized pain
and in the presence of an enchondroma, therapeutic measures such as
steroid injections or physical therapy should be administered for both
diagnostic and therapeutic reasons.
and in the presence of an enchondroma, therapeutic measures such as
steroid injections or physical therapy should be administered for both
diagnostic and therapeutic reasons.
Radiologic Features
Radiographs
-
Long bones
-
Mineralized central or eccentric metaphyseal or diaphyseal lesion
-
Punctate rings and arcs mineralization pattern indicative of hyaline cartilage
-
-
Large majority (95%) of lesions demonstrate at least partial mineralization.
-
Typically <6 cm in size, but also sometimes extending for longer lengths (Fig. 5.2-3)
-
-
Short tubular bones of the hands and feet
-
Appearance may be somewhat different
-
Deep endosteal scalloping
-
Minimal radiographic mineralization
-
Well demarcated and sometimes extend to the ends of the bones (see Fig. 5.2-3)
-
Periosteal reaction and cortical disruption are seen in the setting of pathologic fracture.
-
Computed Tomography
-
Computed tomography (CT) is useful for detecting mineralization and extent of endosteal scalloping (see Fig. 5.2-3).
-
The depth and length of endosteal
scalloping have been identified as helpful in distinguishing
enchondroma versus chondrosarcoma (Box 5.2-1 and Fig. 5.2-2).
MRI
-
MRI is useful for determining marrow extent and the presence of soft tissue extension, if any.
-
The lobular nature of enchondromas is demonstrated well on T2-weighted images (see Fig. 5.2-3).
-
The dark areas on T1 and T2 represent
mineralized portions. However, the value of MRI may be more in
diagnosing the source of pain in the patient with a solitary
enchondroma discovered incidentally during evaluation of knee or
shoulder pain.
Bone Scan
-
Variable patterns of uptake can be seen on bone scintigraphy.
-
Some authors have proposed that uptake less than the anterior iliac spine is consistent with the diagnosis of enchondroma.
 |
|
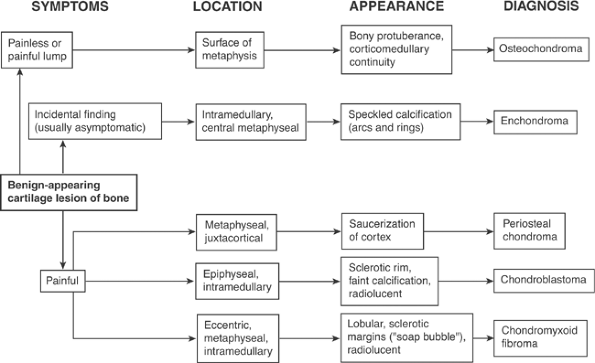
Algorithm 5.2-1. Diagnosis of benign-appearing cartilage lesions of one.
|
P.129
 |
|
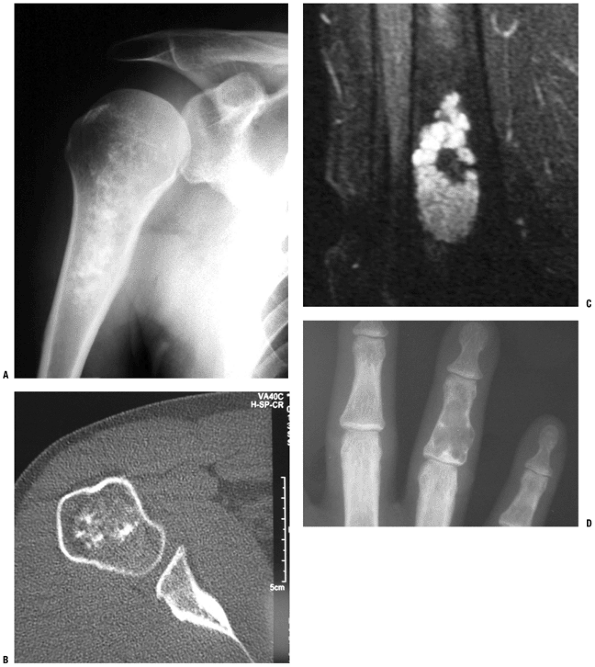
Figure 5.2-3 Enchondroma in various locations. Proximal humerus, radiograph showing typical metaphyseal–diaphyseal location (A) and CT scan showing speckles and whorls of cartilage mineralization (B). (C) T2-weighted MRI of distal femur enchondroma showing the lobular pattern of growth. (D) Plain radiograph of phalangeal enchondroma showing lesion extending to ends of bones and thinning and expanding the cortex.
|
P.130
Box 5.2-1 Objective Signs Worrisome for Malignant Transformation of Enchondromas
-
Periosteal reaction, cortical destruction, and especially soft tissue extension
-
Lysis within a previously mineralized area
-
Deep cortical scalloping, especially greater than two thirds of thickness (see Fig. 5.2-2)
-
Chondrosarcomas have focal areas of greater than two-thirds scalloping in 90% of cases, enchondromas only 10% of cases
-
Longitudinal extent of scalloping is
throughout the length of the lesion in 79% of chondrosarcomas, as
opposed to a shorter extent in enchondromas.
-
-
Large unmineralized areas or faint, amorphous calcification
-
Medullary fill of >90%
-
Large lesions: 50% of chondrosarcomas are >10 cm
-
Bone scan radiotracer uptake greater than anterior superior iliac spine
Histologic Features (Fig 7.3-6)
-
Mature lobules of hyaline cartilage, separated by hematopoietic marrow
-
Focal myxoid change may be seen.
-
Long bones: Cartilage should be relatively hypocellular, with only scattered binucleate cells.
-
Lesions from the hands and feet:
Hypercellularity is common, but the diagnosis of chondrosarcoma should
almost never be made in those anatomic locations.
Differential Diagnosis
Intramedullary Infarct
-
Also typically an incidental,
asymptomatic finding, but mineralization appears more cloud-like or
wispy, without typical arcs and rings calcification of cartilage -
It is more common with a history of steroid use or sickle cell disease.
Enostosis
-
Enostoses or “bone islands” are also asymptomatic, incidental findings, but they have distinctive radiographic and CT findings.
-
The lesions have dense, ivory-like
mineralization and are rarely larger than 2 to 3 cm. The periphery
demonstrates a brush-border pattern of trabeculation on CT, and no
uptake is seen on bone scan.
Chondrosarcoma
-
Differentiating between enchondroma and low-grade chondrosarcoma may be difficult (refer to text above and Box 5.2-1).
-
Cortical disruption, periosteal reaction, and deep endosteal scalloping are associated with more aggressive cartilage lesions.
Treatment
Biopsy
-
It is difficult to differentiate grade I chondrosarcoma from benign enchondroma by tissue examination alone.
-
Radiologic and clinical correlation is
essential, and therefore biopsy should be reserved for selected
chondroid lesions with radiologically or clinically worrisome signs.
Surgical Indications/Contraindications
Observation
-
Most lesions need no surgery.
-
On careful search, the pain in the region of the lesion is found to be due to other causes.
-
Simple follow-up of the lesion initially at 3- to 6-month intervals is indicated.
Pathologic Fracture Treatment
-
For enchondromas of the short tubular
bones that present with pathologic fracture, the phalanx is immobilized
until the fracture heals. -
If the cortices are thinned such that
future fractures are predicted, the enchondroma is curetted and the
cavity packed with bone graft or bone cement.
Operative Management
-
Indicated for painful cartilage lesions
that have endosteal scalloping but not periosteal reaction or cortical
disruption (i.e., “aggressive” enchondromas or “chondrosarcoma in
situ”); intralesional excision is recommended. Some authors recommend
the use of physical adjuvants such as phenol or liquid nitrogen to
reduce the risk of recurrence. -
Intramedullary cartilage lesions of the pelvis are considered malignant and should be resected with wide margins.
Results and Outcome
-
Recurrence is rare after intralesional excision of enchondromas in the long bones or short tubular bones.
Periosteal Chondroma
Periosteal or juxtacortical chondromas are small, mildly
painful lesions of the metaphyseal long bones; the etiology is unknown.
They are uncommon and have distinctive radiographic features consisting
of a sclerotic saucerization of the cortex and small size. Cellular
atypia and hypercellularity are common despite the benign nature of the
lesion. Curettage of the lesion is usually curative.
painful lesions of the metaphyseal long bones; the etiology is unknown.
They are uncommon and have distinctive radiographic features consisting
of a sclerotic saucerization of the cortex and small size. Cellular
atypia and hypercellularity are common despite the benign nature of the
lesion. Curettage of the lesion is usually curative.
P.131
Pathogenesis
Epidemiology
-
Mean age: 23 years
-
Gender: no gender predilection
-
Distribution: By definition, the tumor
occurs in a juxta-cortical location, often in the metaphyseal portion
of long bones. The most common site in one series was the proximal
humerus, but other reported cases have varied in location between small
and large long bones.
Natural History
-
Slow growth; no malignant potential
Diagnosis (see Algorithm 5.2-1)
Clinical Features
-
Patients present with mild pain of long duration, often present for 1 or more years.
-
If the lesion is superficial, they may also note slowly growing mass.
-
The lesion can also be discovered incidentally.
Radiologic Features
Radiographs
-
Plain films are often diagnostic.
-
Well-defined “saucerization” of the cortex adjacent to a slightly mineralized soft tissue lesion
-
Thin rim of periosteal bone may extend over lesion (Fig. 5.2-4)
-
No intramedullary extension of the lesion
-
CT
-
CT may demonstrate better the mineralization of the lesion.
-
The mean size of periosteal chondromas is about 2.6 cm, with a round or oval shape, aligned to long axis of bone.
Histologic Features (Fig. 7.3-6)
-
Lobules of mature hyaline cartilage
-
Frequently, hypercellularity, binucleate chondrocytes, and focal, mild cytologic atypia
-
These do not have the same significance as with intramedullary cartilage lesions.
-
Reactive bone formation is seen at the junction of tumor and periosteum.
Differential Diagnosis
Soft Tissue Tumor Pressing on Bone
-
Extraosseous soft tissue tumors can also
present with cortical scalloping and may be difficult to distinguish
from periosteal chondroma. -
The absence of mineralization and size significantly greater than a few centimeters would point toward another diagnosis.
Fibrous Cortical Defect
-
This is also a metaphyseal lesion, sometimes contained entirely within the cortex.
-
It is more commonly seen in skeletally immature subjects but is rarely symptomatic.
-
The matrix is fibrous, without the speckled calcification of cartilage, and a surrounding rind of periosteal bone is not seen.
Periosteal Chondrosarcoma
-
Chondrosarcoma arising from the periosteal surface has been described.
-
It is rare and may be difficult to distinguish histologically from benign chondroma.
-
The absence of a well-defined sclerotic rim of cortex and intramedullary extension of the lesion are worrisome signs.
Treatment
Surgical Indications/Contraindications
-
Symptomatic lesions should undergo excision.
-
Most lesions are small, and thus there is
no need for separate biopsy if the radiological and clinical
presentation is typical. Intralesional excision (curettage) is
typically curative, but en bloc excision is better.
Results and Outcome
-
Recurrence after intralesional or en bloc excision is rare.
Osteochondroma
Osteochondromas are common benign bone lesions that
occur as osteocartilaginous bony prominences arising from the
metaphyses of growing bones. Their growth parallels the growth of the
host bone. Asymptomatic, solitary osteochondromas require no treatment.
There is a familial form presenting with many, even dozens of
exostoses. These patients are at increased risk for mechanical and
growth complications and secondary malignant transformation of the
cartilage cap. The familial form is discussed in detail in Chapter 7.3.
occur as osteocartilaginous bony prominences arising from the
metaphyses of growing bones. Their growth parallels the growth of the
host bone. Asymptomatic, solitary osteochondromas require no treatment.
There is a familial form presenting with many, even dozens of
exostoses. These patients are at increased risk for mechanical and
growth complications and secondary malignant transformation of the
cartilage cap. The familial form is discussed in detail in Chapter 7.3.
Pathogenesis
Etiology
-
Most are developmental lesions rather
than true neoplasms, but inactivation of both copies of the EXT1 tumor
suppressor gene is necessary for development.-
Small herniation of physeal tissue into metaphysis,
P.132P.133
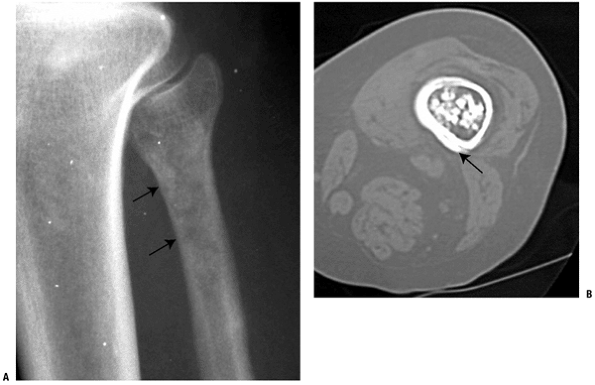
past the periosteal bone cuff (groove of Ranvier) during skeletal growth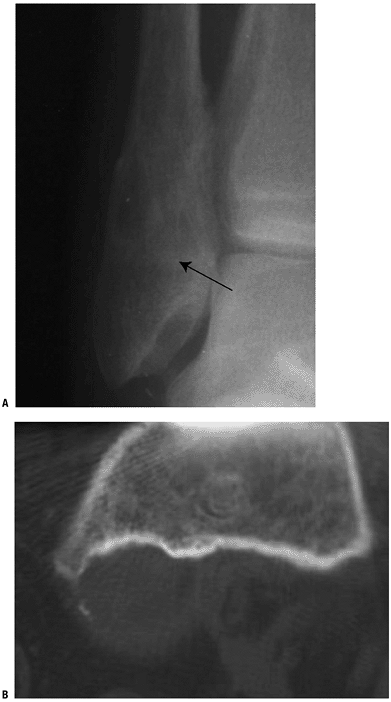 Figure 5.2-4 Periosteal chondroma. Plain radiograph of distal fibula showing a metaphyseal lesion with sclerotic rim (A). CT scan of distal femur showing scalloping of metaphyseal bone and minimally mineralized juxtacortical soft tissue lesion (B).
Figure 5.2-4 Periosteal chondroma. Plain radiograph of distal fibula showing a metaphyseal lesion with sclerotic rim (A). CT scan of distal femur showing scalloping of metaphyseal bone and minimally mineralized juxtacortical soft tissue lesion (B). -
Growth of the exostosis parallels that of the growth plate.
-
Growth ceases with skeletal maturity.
-
-
The minority are the result of iatrogenic injury to the growth plate, such as following surgery or irradiation.
Epidemiology
-
Frequency
-
Most common tumors of bone
-
10% to 15% of all bone tumors
-
20% to 50% of benign bone tumors
-
-
Age: peak within second decade or earlier
-
Male:female 1.6 to 3.4:1
-
Distribution
-
50% occur in lower extremity long bones (especially about the knee).
-
Less commonly in short tubular bones and flat bones (e.g., pelvis, scapula)
-
Natural History
-
Growth continues until the host bone reaches skeletal maturity, then ceases.
-
Secondary chondrosarcoma
-
Very small but documented risk of developing in the cartilage cap
-
Exact incidence unknown (probably <1%)
-
Worrisome signs for secondary malignancy: pain and continued or recurrent growth of exostosis after skeletal maturity
-
Diagnosis (see Algorithm 5.2-1)
Clinical Features
-
Typical clinical history: nontender, painless bony bump
-
Bursal pain
-
Most common locations for large bursa formation: scapula (>mt50% of cases), lesser trochanter, shoulder
-
Bursal contents: inflammatory fluid, hemorrhage, metaplastic cartilage formation (secondary form of synovial chondromatosis)
-
Do not confuse secondary synovial chondromatosis within osteochondroma bursa for malignant degeneration to chondrosarcoma!
-
-
Occasional associated symptoms:
restricted joint motion, snapping of tendons, subscapular
crepitus/snapping, fracture of pedunculated osteochondroma -
Neurovascular complications: rare
-
Pseudoaneurysm formation and compression neuropathies
-
Popliteal artery and peroneal nerve most commonly affected
-
Radiologic Features
Radiographs
-
Plain film features characteristic and often diagnostic
-
Bony protrusion containing cortical and medullary bone with overlying cartilage cap
-
Both cortical surface and medullary cavity of the host bone contiguous with those of the osteochondroma (Fig. 5.2-5)
-
Mineralization of cartilage cap is not always visualized on plain radiography.
-
-
Two general radiographic forms
-
“Sessile” osteochondromas: broad attachment to host bone
-
“Pedunculated” osteochondromas: narrow stalk connection to host bone
-
CT
-
Very good modality for demonstrating
cortical and medullary continuity, especially in areas of complex
anatomy such as pelvis and spine -
Used to measure thickness of cartilage cap (not as good as MRI)
-
Evaluate for worrisome signs of secondary malignancy
-
Focal regions of radiolucency
-
Destruction of adjacent bone
-
Contiguous soft tissue mass with scattered mineralization
-
MRI
-
Test of choice to evaluate thickness of cartilage cap
-
Normal cartilage cap thickness: 1 to 3 cm in young patients (smaller in adults)
-
Abnormal cap thickness: >mt1.5 cm in adults should be viewed with suspicion
-
-
Useful in demonstrating the presence of corticomedullary continuity, especially of the medullary marrow
-
Contrast enhancement typically restricted to the septa between lobules and to peripheral perichondrium covering cartilage
Bone Scan
-
Uptake on scintigraphy generally
decreases as enchondral ossification slows toward skeletal maturity.
This is, however, not reliable in distinguishing secondary malignancy.
Histologic Features
-
Gross appearance: bony stalk with overlying cartilage cap
-
Cartilage cap of osteochondromas often
irregular, with areas of invagination into the underlying bone
(accounts for heterogeneity of large osteochondromas observed on
imaging studies)
-
-
Histology: Cartilage cap chondrocytes may be arranged in a columnar format, similar to physeal plate (Fig. 7.3-8)
-
Mild hypercellularity and some binucleate cells may be found.
-
Differential Diagnosis
Subungual Exostosis
-
The overlying nail bed is usually deformed.
-
Radiologically, corticomedullary
continuity is not seen; histologically, fibrous proliferation with
cartilage metaplasia is seen.
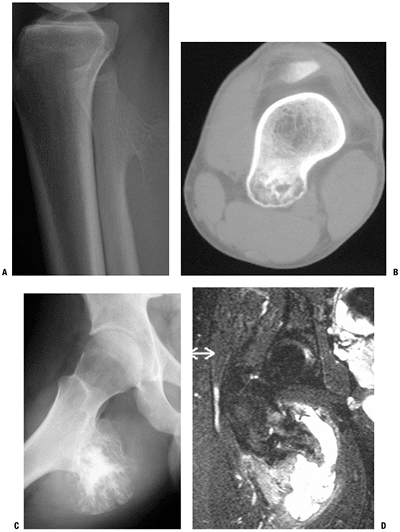 |
|
Figure 5.2-5 Solitary osteochondromas. Plain radiograph of pedunculated osteochondroma in proximal fibula (A) and CT scan of sessile osteochondroma in distal femur (B). Note corticomedullary continuity. Plain radiograph of large proximal femur osteochondroma with large, secondary bursa (C), which, when seen on T2-weighted MRI (D), could be mistaken for overgrown cartilage cap and malignancy.
|
Dysplasia Epiphysealis Hemimelica (Trevor’s Disease)
Periosteal (Juxtacortical) Chondroma
-
This appears as a small soft tissue mass adjacent to bone with smooth scalloping of the underlying cortex.
-
In contrast to an osteochondroma, only a thin shelf of mineralized periosteum covers a portion of the lesion.
Heterotopic Ossification
-
Longstanding peri-articular heterotopic
ossification, especially around the hip, may become confluent with
adjacent bone and radiographically mimic osteochondroma. -
A history that includes known
predisposing factors such as previous hip surgery or paraplegia and the
absence of corticomedullary continuity should allow for distinction.
Parosteal Osteosarcoma
-
This is a low-grade variant of osteosarcoma that grows adjacent to cortical bone, most commonly on the posterior distal femur.
-
In contrast to osteochondroma, it does not recreate a central marrow cavity.
-
The mineralization pattern is ivory-like and dense.
Treatment
-
Observation is the most common treatment for solitary or multiple exostoses.
-
Fractures of osteochondroma almost always heal spontaneously.
-
Surgical excision is indicated for pain or occasionally for cosmesis.
-
More commonly, large pedunculated lesions cause symptoms and undergo excision.
-
Although unusual, repair of pseudoaneurysm and excision of the offending osteochondroma is indicated in those cases.
Results and Outcome
-
Recurrence after resection of
osteochondroma is related to incomplete excision of the cartilage cap
and is estimated to occur 2% of the time.
Chondroblastoma
Chondroblastomas are distinctive benign bone tumors of
immature cartilage cells arising within the epiphyses of long bones and
are most commonly seen in patients with open physes. They commonly
present with several months of periarticular pain. The radiographic
hallmarks include the epiphyseal location, sclerotic margins, and
mineralized matrix, although subtle early findings often delay
diagnosis. Curettage is usually curative. Rare incidences of lung
metastases have been described.
immature cartilage cells arising within the epiphyses of long bones and
are most commonly seen in patients with open physes. They commonly
present with several months of periarticular pain. The radiographic
hallmarks include the epiphyseal location, sclerotic margins, and
mineralized matrix, although subtle early findings often delay
diagnosis. Curettage is usually curative. Rare incidences of lung
metastases have been described.
Pathogenesis
-
Etiology is unknown.
-
Chromosomal abnormalities on 5 and 8 have been identified.
Epidemiology
-
Uncommon lesion
-
Only about 500 cases have been reported in the literature.
-
Represents 1% of bone tumors
-
-
Peak age: Majority are discovered in the second decade, and most patients are under 20 years, but any age may be involved.
-
Gender: slight male preponderance
-
Distribution
-
Most common in the long bones (proximal humerus >mt distal femur >mt proximal tibia >mt proximal femur)
-
Almost exclusively within the epiphysis (may extend into epimetaphysis)
-
Flat bone sites: acetabulum, ilium
-
Other distinctive but less common locations
-
Talus, calcaneus, patella, craniofacial bones
-
Typically in older patients
-
-
Pathophysiology
-
Tumor of immature cartilage cells (chondroblasts) rather than mature cartilage cells (chondrocytes)
-
Without mature cells, does not produce
hyaline cartilage, so lacks typical radiographic features of hyaline
cartilage mineralization (arcs and rings)
Natural History
Typical course involves slow growth, with untreated
lesions eventually eroding into the articular space. Invasion of
adjacent joint, when it does occur, travels via the cruciate ligaments
or ligamentum teres. Synovitis is especially seen when the tumor
involves the joint.
lesions eventually eroding into the articular space. Invasion of
adjacent joint, when it does occur, travels via the cruciate ligaments
or ligamentum teres. Synovitis is especially seen when the tumor
involves the joint.
The tumor has occasional aggressive behavior. Although
considered a benign tumor, there are well-documented incidences of lung
metastases. Only a handful of cases are documented, and in half of
these cases, the tumor was originally in flat bones and most had
experienced local recurrence. Unfortunately, no clear radiologic or
histologic findings are known to be predictive of metastasis. The
histological appearance of the metastases was similar to that of the
original lesion, showing no malignant features. There appears to be a
long interval to metastasis and a long survival time after metastasis,
corresponding to the slow growth of the lesion in general.
considered a benign tumor, there are well-documented incidences of lung
metastases. Only a handful of cases are documented, and in half of
these cases, the tumor was originally in flat bones and most had
experienced local recurrence. Unfortunately, no clear radiologic or
histologic findings are known to be predictive of metastasis. The
histological appearance of the metastases was similar to that of the
original lesion, showing no malignant features. There appears to be a
long interval to metastasis and a long survival time after metastasis,
corresponding to the slow growth of the lesion in general.
Diagnosis (see Algorithm 5.2-1)
Clinical Features
Radiologic Features
Radiographs
-
Plain radiographs are often diagnostic, showing lytic, epiphyseal lesions with minimally sclerotic borders (Fig. 5.2-7).
-
Sometimes faint speckled mineralization is seen.
-
Not the characteristic arcs and rings of hyaline cartilage
CT
-
Matrix calcification is best seen on CT, as is the otherwise faint sclerotic border.
-
Secondary aneurysmal bone cyst formation is common.
-
Presence of mineralization on CT scan may be helpful in predicting the presence of underlying chondroblastoma (see Fig. 5.2-7).
MRI
-
Marrow edema, sometimes dramatic and extending into the metaphysis, is a hallmark of chondroblastoma (see Fig. 5.2-7).
-
In some lesions, an adjacent joint effusion or synovitis can be seen on MRI (see Fig. 5.2-6).
Bone Scan
-
Increased radiotracer uptake is seen.
Histologic Features
-
Preponderance of round or polygonal cells, with indented or folded “coffee bean” nuclei
-
Cells packed into “cobblestone” pseudolobulated pattern
-
Foci of eosinophilic chondroid matrix and reactive multinucleate giant cells
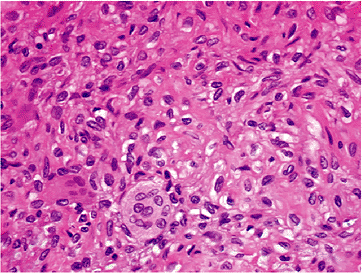 Figure 5.2-6
Figure 5.2-6
Tissue section from chondroblastoma shows plump polygonal cells with
coffee-bean nuclei in cobblestone pattern separated by chicken-wire
calcification. Scattered giant cells are also seen. -
Peripheral intercellular “chicken-wire calcification”
-
Mitoses should be occasional and not atypical.
Differential Diagnosis
-
A frequently employed mnemonic for the
differential diagnosis of epiphyseal lesions is PGCAT (pigmented
villonodular synovitis, giant cell tumor, chondroblastoma, clear cell
chondrosarcoma, aneurysmal bone cyst, and tuberculosis).
Subchondral Cyst/Intraosseous Ganglion
-
Because of the proximity to the
subchondral surface of some chondroblastomas, the possibility of a
subchondral cyst should be entertained. -
Both lesions may have a sclerotic rim and
marrow edema on MRI, but subchondral cysts should not demonstrate any
mineralized matrix and are more common in older patients with
associated degenerative joint changes. -
Similar cysts may occur with pigmented villonodular synovitis in younger individuals.
-
Marrow edema is usually less extensive with cysts.
Giant Cell Tumor
-
Although most giant cell tumors occur in adults, they may occur in patients with an open growth plate.
-
The epicenter of the rare pediatric giant
cell tumor of bone is usually in the metaphysis, however, with
occasional erosion into the epiphysis. -
In a typical chondroblastoma, the epicenter is within the epiphysis, with occasional erosion into the metaphysis.
-
Giant cell tumor lacks any mineralization.
Clear Cell Chondrosarcoma
-
This is a rare variant of chondrosarcoma with an epiphyseal predilection.
-
It has been hypothesized that it is a malignant variant of chondroblastoma.
-
The radiological differences usually include loss of geographic, sclerotic margins, and metaphyseal or extraosseous extension.
-
Most occur in adults.
Aneurysmal Bone Cyst
-
The epiphysis is an unusual location for
an aneurysmal bone cyst, which is typified on plain radiographs by
septations and absence of mineralization. -
On MRI, often numerous fluid–fluid levels are seen.
-
Since one third of chondroblastomas have secondary aneurysmal bone cyst components, both may be present within a single lesion.
Osteomyelitis
-
Infections are the most frequent cause of radiolucent lesions within the pediatric epiphysis.
-
The clinical history is often shorter and may be accompanied by other clinical signs or laboratory evidence of infection.
-
Similar to chondroblastoma, osteomyelitis usually shows extensive edema on MRI.
-
Tuberculous osteomyelitis of the long bones is an infection that has a predilection for the epiphysis.
P.137
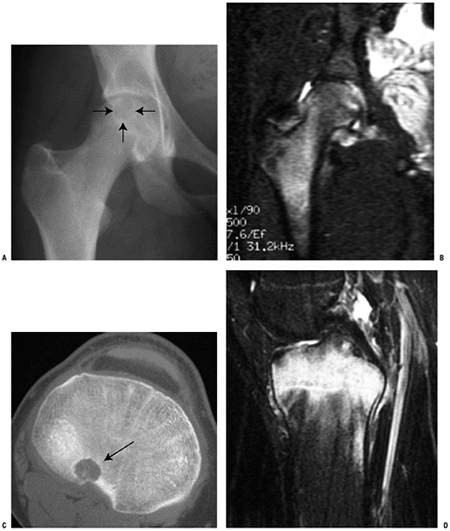 |
|
Figure 5.2-7 Chondroblastoma. (A) Plain radiograph of proximal femoral lesion showing epiphyseal location and sclerotic margins. (B) T2-weighted MRI demonstrates perilesional edema, and synovitis and effusion of adjacent joint. (C) CT scan is best modality for visualizing chondroid mineralization. (D) Often intense marrow edema is seen on MRI.
|
P.138
Treatment
Surgical Indications/Contraindications
-
Primary tumor treatment
-
Because of the progressive nature of the lesion, curettage is indicated in all chondroblastomas.
-
Some authors have advocated the use of a physical adjuvant such as liquid nitrogen or phenol.
-
The rate of recurrence with simple curettage appears relatively low, and the role of physical adjuvant is not yet clear.
-
-
Pulmonary metastases are typically nonprogressive lesions and may be observed or excised.
Results and Outcome
-
Local recurrence rates: 14% to 18%, usually within 2 years
-
Adjacent joint synovitis usually resolves with tumor excision.
Chondromyxoid Fibroma
Chondromyxoid fibroma is a rare but distinctive bone
tumor of immature cartilage origin that presents as an eccentric lytic
lesion, usually immediately on the opposite side of the growth plate as
its other immature cartilage tumor counterpart, chondroblastoma.
Despite a high recorded incidence of local recurrence after curettage,
no metastases have been reported.
tumor of immature cartilage origin that presents as an eccentric lytic
lesion, usually immediately on the opposite side of the growth plate as
its other immature cartilage tumor counterpart, chondroblastoma.
Despite a high recorded incidence of local recurrence after curettage,
no metastases have been reported.
Pathogenesis
-
Etiology is unknown.
-
Rearrangements of long arm of chromosome 6 described
-
Distinctive matrix composition in
chondroblastoma differs from that of other benign cartilage tumors
(chondroblastoma, enchondroma, osteochondroma, chondrosarcoma): high
expression of hydrated proteoglycans (myxoid component), type II
cartilage (chondrocytic component), and types I, III, and VI cartilage
Epidemiology
-
Exceedingly rare tumor
-
<1% of bone tumors
-
Age: mean 31 years (72% younger than 40)
-
Slight male >mt female preponderance
-
Distribution
-
Half of lesions occur in long bones.
-
Most commonly proximal tibia
-
Metaphyseal or metadiaphyseal region, sometimes eroding into epiphysis
-
One third occur in flat bones.
-
Most commonly ilium (half)
-
Bones of feet next most common (metatarsals usually)
-
-
Natural History
-
Although the tumor appears to cause
sometimes extensive bone destruction and may recur repeatedly after
treatment, there are no reported cases of metastasis.
Diagnosis (see Algorithm 5.2-1)
Clinical Features
-
Pain, sometimes of long duration, is typically seen.
-
Pathologic fracture is unusual.
Radiologic Features
-
Radiolucent, sharply marginated eccentric metaphyseal lesion
-
Lobulated, septated, or “soap bubble” appearance
-
Usually elongated along the long axis of the bones
-
Cortical thinning common
-
Cortical destruction and soft tissue extension occasionally seen
-
Periosteal reaction uncommon
Histologic Features
-
Low-power histology: prominent lobular or zonation pattern
-
Lobules consist of a central, hypocellular myxoid area rimmed with more cellular regions.
-
-
High-power histology: spindle or stellate-shaped cells with eosinophilic cytoplasm embedded within myxoid matrixP.139
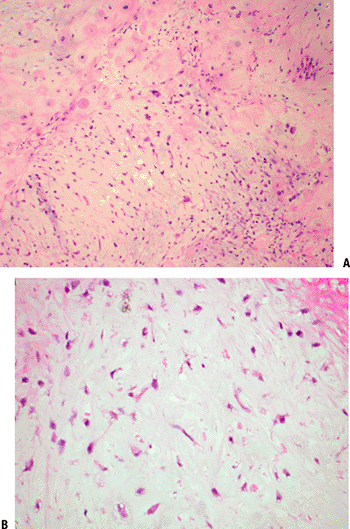 Figure 5.2-8 Chondromyxoid fibroma histopathology. (A) Low power view shows lobules of low cellularity myxoid areas separated by more cellular regions. (B) Higher power view shows characteristic stellate cells.
Figure 5.2-8 Chondromyxoid fibroma histopathology. (A) Low power view shows lobules of low cellularity myxoid areas separated by more cellular regions. (B) Higher power view shows characteristic stellate cells.-
Areas of hyaline cartilage in 20%
-
Aneurysmal bone cyst components in 10%
-
Chondroblastoma-like cells as well as multinucleated giant cells occasionally seen
-
Differential Diagnosis
Chondrosarcoma
-
Myxoid chondrosarcoma may have some of the same radiologic features of chondromyxoid fibroma.
-
Chondrosarcoma will not have the typical sclerotic, well-defined margins of chondromyxoid fibroma.
-
The presence of large,
pleomorphic-appearing cells in chondromyxoid fibroma may also
erroneously suggest a diagnosis of chondrosarcoma.-
Mitoses are infrequent and atypical mitoses are not seen in chondromyxoid fibroma.
-
The center of the myxoid lobules in chondrosarcoma is hypercellular instead of hypocellular, as in chondromyxoid fibroma.
-
Giant Cell Tumor
-
This also occurs in the metaphyses of young adults and presents as a lytic mass.
-
The radiographic appearance, however, is not one of lobulation, and the margins are less sclerotic on plain radiographs.
-
Histologically, the lesions are quite distinct.
Nonossifying Fibroma
-
This also can appear as an eccentric, metaphyseal lesion.
-
Typically, these are painless, incidental lesions and are often already partially ossified.
Treatment
-
Because of the rarity of the tumor, no large study is available to compare different types of treatment.
-
Most lesions have been treated with intralesional excision (curettage).
Results and Outcome
-
Local recurrence rate: 15% to 25% recurrence (higher in younger patients in some series)
Acknowledgments
I gratefully acknowledge research assistance by Brittany
L. Rice, MLS, AHIP, and Smita Jhaveri of the Suburban Hospital Medical
Library.
L. Rice, MLS, AHIP, and Smita Jhaveri of the Suburban Hospital Medical
Library.
Suggested Reading
Bauer TW, Dorfman HD, Latham JT. Periosteal chondroma. A clinicopathologic study of 23 cases. Am J Surg Pathol 1982;6:631–637.
Flemming DJ, Murphey MD. Enchondroma and chondrosarcoma. Semin Musculoskelet Radiol 2000;4:59–71.
Kransdorf M, Meis J. From the archives of the AFIP. Extraskeletal osseous and cartilaginous tumors of the extremities. Radiographics 1993;13:853–884.
Kunze
E, Graewe T, Peitsch E. Histology and biology of metastatic
chondroblastoma. Report of a case with a review of the literature. Pathol Res Pract 1987;182:113–123.
E, Graewe T, Peitsch E. Histology and biology of metastatic
chondroblastoma. Report of a case with a review of the literature. Pathol Res Pract 1987;182:113–123.
Lee KCY, Davies AM, Cassar-Pullicino VN. Imaging the complications of osteochondromas. Clin Radiol 2002;57:18–28.
Levy JC, Temple HT, Mollabashy A, et al. The causes of pain in benign solitary enchondromas of the proximal humerus. Clin Orthop Relat Res 2005:181–186.
Marcial-Seoane R, Marcial-Seoane M, Ramos E, et al. Extraskeletal chondromas. Bol Asoc Med P R 1990;82:394–402.
Marco RA, Gitelis S, Brebach GT, et al. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg 2000;8:292–304.
Murphey
MD, Choi JJ, Kransdorf MJ, et al. Imaging of osteochondroma: variants
and complications with radiologic-pathologic correlation. Radiographics 2000;20:1407–1434.
MD, Choi JJ, Kransdorf MJ, et al. Imaging of osteochondroma: variants
and complications with radiologic-pathologic correlation. Radiographics 2000;20:1407–1434.
Murphey
MD, Walker E, Wilson A, et al. From the archives of the AFIP: imaging
of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics 2003;23:1245–1278.
MD, Walker E, Wilson A, et al. From the archives of the AFIP: imaging
of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics 2003;23:1245–1278.
Nojima T, Unni KK, McLeod RA, et al. Periosteal chondroma and periosteal chondrosarcoma. Am J Surg Pathol 1985;9:666–677.
Pierz KA, Stieber JR, Kusumi K, et al. Hereditary multiple exostoses: one center’s experience and review of etiology. Clin Orthop Relat Res 2002:49–59.
Sandberg AA. Genetics of chondrosarcoma and related tumors. Curr Opin Oncol 2004;16:342–354.
Springfield DS, Capanna R, Gherlinzoni F, et al. Chondroblastoma. A review of seventy cases. J Bone Joint Surg [Am] 1985;67:748–755.
Vasseur MA, Fabre O. Vascular complications of osteochondromas. J Vasc Surg 2000;31:532–538.
Wilson
AJ, Kyriakos M, Ackerman LV. Chondromyxoid fibroma: radiographic
appearance in 38 cases and in a review of the literature. Radiology 1991;179:513–528.
AJ, Kyriakos M, Ackerman LV. Chondromyxoid fibroma: radiographic
appearance in 38 cases and in a review of the literature. Radiology 1991;179:513–528.
Wu CT, Inwards CY, O’Laughlin S, et al. Chondromyxoid fibroma of bone: a clinicopathologic review of 278 cases. Hum Pathol 1998;29:438–446.
