Cystic Lesions
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section
II – Specific Bone Neoplasms and Simulators > 5 – Benign Bone Tumors
> 5.3 – Cystic Lesions
II – Specific Bone Neoplasms and Simulators > 5 – Benign Bone Tumors
> 5.3 – Cystic Lesions
5.3
Cystic Lesions
Sung Wook Seo
Young Lae Moon
Francis Young-In Lee
This chapter includes cystic lesions which may occur in
bone. Epidermal inclusion cysts and ganglions may also occur in
soft-tissues, where the pathophysiology is similar. Simple bone cysts
and aneurysmal bone cysts represent distinct clinical entities which
occur within bone only.
bone. Epidermal inclusion cysts and ganglions may also occur in
soft-tissues, where the pathophysiology is similar. Simple bone cysts
and aneurysmal bone cysts represent distinct clinical entities which
occur within bone only.
Epidermal Inclusion Cyst
Epidermal inclusion cysts are more commonly seen as
soft-tissue lesions, but may involve bone, and present in either case
as painful digital lesions which typically require surgical extirpation.
soft-tissue lesions, but may involve bone, and present in either case
as painful digital lesions which typically require surgical extirpation.
Pathogenesis
Etiology
-
For both soft-tissue and intraosseous
epidermal inclusion cysts, penetrating trauma causes epidermal tissue
to be deposited deep within the tissues
Pathophysiology
-
Epidermal tissue traumatically deposited within the deeper tissue grows and causes cyst formation
-
Cystic lesion filled with keratinous materials lined with flattened squamous epithelium
Diagnosis
Physical Examination and History
Clinical Features
-
Painful, often swollen mass most often within distal digit
-
History of penetrating trauma in many cases
Radiologic Features
-
Intraosseous geographic radiolucent lesion
-
Distal phalanx of fingers common
Pathologic findings
-
Cystic cavity filled with keratinaceous debris
Diagnostic Workup Algorithm
-
Differential diagnosis includes intraosseous synovial or ganglion cyst
-
Diagnosis usually apparent radiographically, confirmed histologically
Treatment
Surgical Indications/Contraindications
-
Painful lesions bone can be curetted and grafted
Degenerative Cyst (GEODES)
Pathogenesis
Etiology
-
Related to underlying degenerative arthritis
Pathophysiology
-
Damage to cartilage theorized to allow fluid intravasation within bone, leading to cyst formation
-
Pathology: Cystic lesion filled with fluid or gelatinous or proteinaceous material, demarcated by fibrocartilaginous tissues (Fig. 5.3-1)
Diagnosis
Physical Examination and History
Clinical Features
-
Dictated by manifestations of underlying arthritis
-
Pain, local tenderness, variable stiffness, loss of motion
Radiologic Features
-
Plain x-ray
-
Cyst: geographic, smooth, often sclerotic
borders with central radiolucency within epiphysis immediately adjacent
to joint, often on both sides of joint -
Variable associated arthritic changes:
-
loss of joint space, osteophytes, subchondral sclerosis, subluxation may be present
-
some joints show few or no degenerative changes
-
-
-
MRI
Pathologic findings
-
Cystic cavity filled with serous fluid,
proteinaceous, or gelatinous material and lined with flattened
fibrocartilaginous tissues
Diagnostic Workup Algorithm
-
Usually evident radiographically without the need for histological confirmation
-
In presence of established arthritis, plain radiographs often suffice
-
In absence of other radiographic signs of arthritis, MRI may be useful
-
-
Differential diagnosis includes other epiphyseal lesions
-
PGCAT: Pigmented villonodular synovitis
(PVNS), giant cell tumor of bone, chondroblastoma, clear cell
chondrosarcoma aneurysmal bone cyst, tuberculosis (and other cause for
Brodie’s abscess)
-
Treatment
Surgical Indications/Contraindications
-
If diagnosis is clear, direct treatment towards underlying joint arthritis
-
If diagnosis not clear, biopsy may be necessary
 |
|
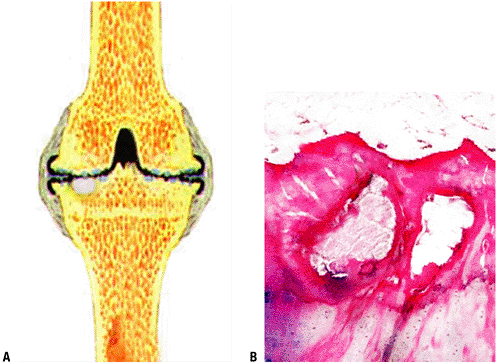
Figure 5.3-1 Histopathology of degenerative cyst. (A) Schematic shows white cyst below thinned (degenerative) cartilage, shown in black. (B) Intraosseous cysts within subchondral bone with overlying fibrillated cartilage.
|
Simple Bone Cyst (Unicameral Bone Cyst)
Unicameral bone cyst is a serous or serosanguineous
fluid-filled cavity which typically behaves in an inactive fashion, not
causing symptoms until pathological fracture occurs through the cyst.
The clinical presentation, location, and radiographic features are
classic enough that the diagnosis may usually be readily established.
Treatment continues to evolve, and is site dependent, but often
involves aspiration and injection.
fluid-filled cavity which typically behaves in an inactive fashion, not
causing symptoms until pathological fracture occurs through the cyst.
The clinical presentation, location, and radiographic features are
classic enough that the diagnosis may usually be readily established.
Treatment continues to evolve, and is site dependent, but often
involves aspiration and injection.
Pathogenesis
Etiology
Unknown but theories suggest epiphyseal plate defect or venous outflow obstruction
Epidemiology
-
Ages
-
Peak incidence 4–10 years of age
-
85% within first two decades
-
-
Gender: Males > females 3:1
-
Distribution
-
Proximal humerus most common
-
Proximal femur #2
-
Calcaneus #3
-
Other relatively common sites: proximal tibia, ilium
-
Most common sites in adults: calcaneus and ilium
-
P.142
Pathophysiology
Unknown
Classification
-
Active position: Immediately juxtaposed to growth plate
-
Not to be confused with Enneking stage 2 benign (active) classification
-
-
Inactive position: Growth plate no longer adjacent to cyst
-
Not to be confused with Enneking stage 1 benign (inactive) classification
Diagnosis
Physical Examination and History
Clinical Features
-
Well-defined fluid filled lesion in the metaphysis and diaphysis of the proximal humerus and proximal femur in children
-
Large lesions may cause pathologic fractures, which is the most common clinical presentation
-
Less commonly, they may cause pain and swelling
Radiologic Features
-
Plain radiographs
-
Well-demarcated osteolytic lesion without much marginal sclerosis (Fig. 5.3-2)
-
Central location (occupies entire width of bone)
-
Metadiaphyseal location within long bones
-
Active position: juxtaposed to growth plate on metaphyseal side
-
Inactive position: growth plate no longer adjacent to cyst
-
-
Thinning of cortex
-
Intralesional fracture fragment (fallen leaf or fallen fragment sign)
-
Essentially pathognomonic for simple bone
cyst, as it indicates that the cyst is comprised of fluid (fragment
could not fall to bottom of a solid lesion)
-
-
-
Magnetic resonance imaging
-
Homogenous fluid signal (dark on T1W, bright on T2W)
-
Peripheral rim enhancement only
-
Blood products may be identified if
fracture has occurred (fluid-fluid level) but because the blood is
usually mixed with serous fluid, the hematocrit sign (level of the
horizontal delineation between serum and cells) is lower than that seen
in an ABC
-
Pathologic findings
-
A fibrous membrane-lined cavity containing a clear yellow fluid
-
Fibrous membranes contain fibrous tissues, occasional multinucleated cells and CD 68 (+) foamy histiocytes
Diagnostic Workup Algorithm
-
Plain radiographs
-
If radiographic diagnosis evident on XR, proceed with treatment
-
If diagnosis unclear, obtain MRI
-
-
Magnetic resonance imaging
-
If radiographic diagnosis evident based on XR and MRI, proceed with treatment
-
If diagnosis unclear, aspirate
-
-
Aspiration
-
If aspiration reveals clear fluid, diagnosis confirmed
-
If aspiration reveals blood, may be from fracture hematoma, but consider cytological examination
-
If aspiration fails due to solid tissue, proceed with open biopsy
-
Treatment
Surgical Indications/Contraindications
Surgical and Nonoperative Options
-
Various treatment methods have been
described. Treatment options include aspiration/injection therapies
(steroid, bone marrow, demineralized bone matrix, or combinations),
elastic intramedullary nailing, multiple drilling, curettage & bone
grafting alone or with supplementary internal fixation (femoral neck
lesion)-
Upper extremity cysts: aspiration/injection preferred
-
Low morbidity of procedure (outpatient, minimal recovery)
-
Low morbidity of repeat fracture if treatment fails (usually extra-articular and most go on to heal with non-operative care)
-
-
Proximal femoral cysts: lower threshold
for considering curettage & bone grafting alone or with
supplementary internal fixation-
Higher risk of fracture in proximal femur warrants consideration of open procedure
-
Proximal femoral fractures generally require ORIF
-
-
-
Observation may be elected, particularly for humeral lesions and calcaneal lesions discovered incidentally
-
Calcaneal simple cysts
-
Often discovered due to pain
-
Need to distinguish source of pain (may be unrelated plantar fasciitis)
-
-
-
Following pathological fracture through simple cyst, approximately 1/7 will show healing of cyst with healing of the fracture
-
For proximal humeral pathological
fracture, allow fracture to heal and re-evaluate radiographically
before considering operative treatment -
For pathological proximal femoral fracture, ORIF should be combined with curettage and grafting of lesion
-
Results and Outcome
-
Progressive lesional healing and resolution in 60% to 70% of lesions
-
Partial lesional healing in 20% to 30%
-
No lesional healing and/or cyst recurrence in 10% to 20%
-
Deteriorating results with extended follow-up should dampen enthusiasm for short-term follow-up of newer techniques
P.143
 |
|
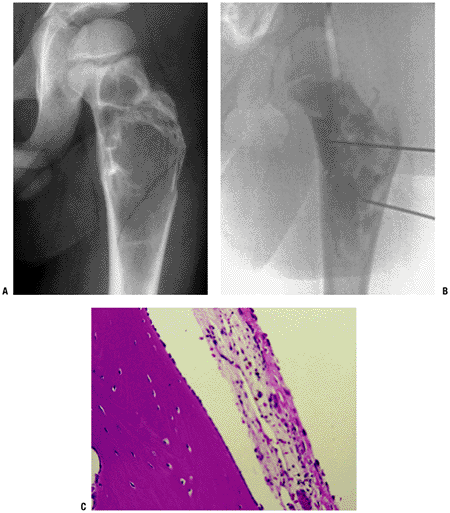
Figure 5.3-2 Unicameral bone cyst in the proximal femur. (B) Cystography shows radioopaque dye filling throughout the lesion. (C) Photomicrography demonstrates cystic lesion lined with a fibrocellular membrane.
|
Aneurysmal Bone Cyst
Aneurysmal bone cysts differ from other cystic bone
lesions in having the potential for aggressive behavior and hence need
to be distinguished from the other types of bone cysts. Furthermore,
these benign lesions must be distinguished histologically from
telangiectatic osteosarcoma. Treatment for aneurismal bone cyst is
typically more aggressive than for other bone cysts.
lesions in having the potential for aggressive behavior and hence need
to be distinguished from the other types of bone cysts. Furthermore,
these benign lesions must be distinguished histologically from
telangiectatic osteosarcoma. Treatment for aneurismal bone cyst is
typically more aggressive than for other bone cysts.
Pathogenesis
Etiology
-
Primary ABC likely neoplastic
-
Rearrangements of either USP6 or CDH11 in high percentage of primary ABCs
-
Secondary ABC (associated with other primary bone lesions) likely reactive
-
Chromosomal rearrangements not seen
-
Epidemiology
-
Ages: Mean 11 years (peak range 1 to 20)
-
Frequency: 0.3 per 100,000
-
Gender: No predilection
Diagnosis
Physical Examination and History
Clinical Features
-
May cause pain, swelling and pathologic fracture.
-
Locations:
-
Femur, tibia, spine, humerus, pelvis and fibula most common
-
2/3 in long bones
-
-
Primary or secondary lesion associated
with giant cell tumor, unicameral bone cyst, chondroblastoma, fibrous
dysplasia, giant cell tumor, osteoblastoma and osteosarcoma -
Spontaneous regression
-
May occur in active primary ABC
-
Very uncommon in aggressive or secondary lesions
-
Radiologic Features
-
Plain x-ray (Figs. 5.3-3 and 5.3-4)
-
Expansile, eccentric radiolucent lesion with septations
-
Classically, blown-out cortex with eggshell-thin rim of reactive bone
-
-
Common sites: metaphysis of long bones and posterior elements of vertebrae, distal phalanx of fingers
-
Fluid-fluid (blood/serum) levels can be seen on MRI or CT scans.
-
Not pathognomonic for primary ABC
-
Also seen in telangiectactic osteosarcoma and secondary ABCs
-
UBC fluid-fluid levels may be seen after fracture (usually solitary)
-
Pathologic Features
-
Expansile, eccentric osteolytic blood-filled lesion containing multiple cysts separated by fibrous sept.
-
Sponge like cavernous spaces filled with blood
-
Cavities surrounded by gray or brownish
tissue with an osseous component (reactive osteoblast-lined bone) and
multinucleated cells -
Solid variant of ABC consists of fibrous or granular tissue with local hemorrhages and a layer of reactive bone
Diagnostic Workup Algorithm
-
Differential diagnosis: giant cell tumor, telangiectatic osteosarcoma, osteoblastoma
-
Workup
-
Radiographic evaluation with x-rays, MRI, BS suggests consideration of diagnosis
-
Biopsy confirms diagnosis
-
Treatment
Surgical Indications/Contraindications
-
Inactive lesions (unusual): Intralesional excision (curettage)
-
Active and aggressive lesions (usual): Intralesional excision (curettage) and bone grafting
-
Arterial embolization (Ethibloc), and
injection with demineralized bone and/or bone marrow have also been
described as alternatives to curettage with some success and may play a
role in difficult locations such as the pelvis but they do not allow
thorough histological examination of contents
-
-
Pathologic fractures through ABCs
-
Usually require curettage and grafting +/- stabilization to allow fracture healing and control of lesion
-
-
Pelvic ABCs
-
Spontaneous regression has been observed after biopsy
-
Consider observation after biopsy
-
If regression occurs, no need for further surgery
-
If progression occurs, proceed to curettage
-
-
-
Spinal ABCs
-
Extended intralesional curettage with grafting (+/- limited fusion when necessary)
-
Embolization poses risks of vascular complications related to cord and of cerebral embolic phenomenon
-
-
Incompletely resectable, aggressive, and/or recurrent ABCs:
-
Radiotherapy may cause secondary sarcoma
and therefore should be avoided as primary treatment for this benign
process but may play a role in isolated cases-
Use low-dose (26 to 30 Gy) radiotherapy (RT)
-
Successful in 90% of cases
-
-
Results and Outcome
-
Intralesional excision (curettage) and bone grafting
-
Recurrence is common: approximately 30% (20% to 70% in reported series)
-
Usually responds to repeat curettage
-
Some lesions stabilize and/or regress
-
Recurrences no more common in younger children
-
-
P.145
 |
|
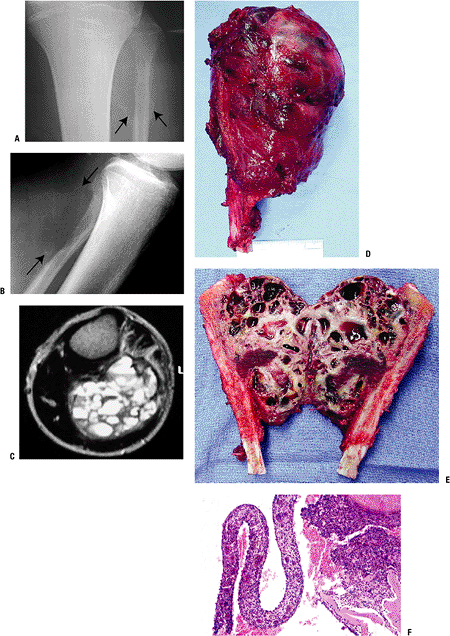
Figure 5.3-3 A case of aneurysmal bone cyst in the proximal fibula in a 15-year-old boy. (A,B) Radiographs show an expansile lesion. (C) MRI shows multiple cysts with fluid-fluid levels. Gross (D,E) and pathologic (F) specimens demonstrate multiloculated cysts filled with bloody fluid.
|
P.146
 |
|
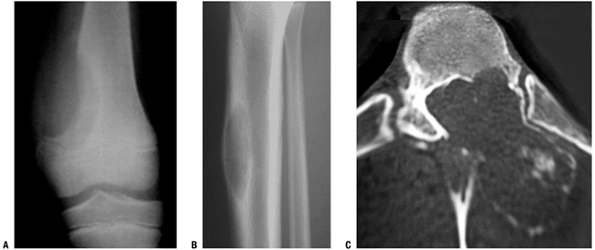
Figure 5.3-4 Radiographs of other cases of aneurysmal bone cyst in the distal femur (A), tibia (B), and vertebra (C).
|
Suggested Reading
Aho HJ, Aho AJ, and Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol 1982;395(2):169–179.
Biesecker JL, Marcove RC, Huvos AG, et al. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer 1970;26(3):615–625.
Chang CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection of bone marrow or methylprednisolone. J Bone Joint Surg [Br] 2002;84(3):407–412.
Chigira M, Shimizu T, Arita S, et al. Radiological evidence of healing of a simple bone cyst after hole drilling. Arch Orthop Trauma Surg 1986;105(3):150–153.
Cole WG. Treatment of aneurysmal bone cysts in childhood. J Pediatr Orthop 1986;6(3):326–329.
Gokturk E, Kose N. Simple bone cysts treated by percutaneous autologous marrow grafting. J Bone Joint Surg [Br] 1997;79(4):695.
Green JA, Bellemore MC, Marsden FW. Embolization in the treatment of aneurysmal bone cysts. J Pediatr Orthop 1997;17(4):440–443.
Kyriakos, M., and Hardy, D.: Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer, 68(8): 1770–80, 1991.
Lichtenstein L. Aneurysmal bone cyst; observations on fifty cases. J Bone Joint Surg [Am] 1957;39(4):873–882.
Maclin TB, Jr. Posttraumatic epidermal inclusion cyst. A case report. J Am Podiatry Assoc 1965;55:209–210.
Oliveira
AM, Perez-Atayde AR, Dal Cin P et al. Aneurysmal bone cyst variant
translocations upregulate USP6 transcription by promoter swapping with
the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene. 2005 May 12;24(21):3419–26.
AM, Perez-Atayde AR, Dal Cin P et al. Aneurysmal bone cyst variant
translocations upregulate USP6 transcription by promoter swapping with
the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene. 2005 May 12;24(21):3419–26.
Oliveira
AM, Perez-Atayde AR, Inwards CY, et al. USP6 and CDH11 oncogenes
identify the neoplastic cell in primary aneurysmal bone cysts and are
absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004 Nov;165(5):1773–8011.
AM, Perez-Atayde AR, Inwards CY, et al. USP6 and CDH11 oncogenes
identify the neoplastic cell in primary aneurysmal bone cysts and are
absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004 Nov;165(5):1773–8011.
Papagelopoulos PJ, Choudhury SN, Frassica FJ, et al. Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Joint Surg [Am] 2001;83(11):1674–1681.
Ramirez AR, Stanton RP. Aneurysmal bone cyst in 29 children. J Pediatr Orthop 2002;22(4):533–539.
Rougraff
BT, Kling TJ. Treatment of active unicameral bone cysts with
percutaneous injection of demineralized bone matrix and autogenous bone
marrow. J Bone Joint Surg [Am] 2002;84(6):921–929.
BT, Kling TJ. Treatment of active unicameral bone cysts with
percutaneous injection of demineralized bone matrix and autogenous bone
marrow. J Bone Joint Surg [Am] 2002;84(6):921–929.
Shinozaki T, Arita S, Watanabe H, et al. Simple bone cysts treated by multiple drill-holes. 23 cysts followed 2–10 years. Acta Orthop Scand 1996;67(3):288–290.
Yu CL, D’Astous J, Finnegan M. Simple bone cysts. The effects of methylprednisolone on synovial cells in culture. Clin Orthop Relat Res 1991;(262):34–41.
