Initial Management of Open Fractures
One – General Principles: Basics > Principles of Treatment > 10 –
Initial Management of Open Fractures
are those in which there is a breach in the soft tissue envelope over
or near the fracture such that the underlying bone communicates with
the outside environment. Although many closed fractures also may be
associated with significant soft tissue injury, they do not have this
full thickness break in the soft tissues. Fractures may be classified
as either open or closed, with further classification schemes used to
specify the severity of the injury.
Hippocrates (born 460 BCE) recognized that injuries commonly caused
local swelling and admonished against occlusive dressing until after
swelling had abated. Hippocrates favored operative débridement of
purulent material but urged against frequent meddling with wounds that
were progressing appropriately.
origin, considered purulence as necessary “laudable pus” and viewed it
as essential to the healing process. He and his counterparts actively
sought treatments that would lead to a purulent response.152
1510-1590), a French army surgeon, described the need for opening the
wound and the need for the free flow of drainage. He also described the
necessity of débridement of all foreign matter and necrotic local
tissues. In the same era, Brunschwig and Botello also advocated for
operative débridement of necrotic material from nonprogressing wounds.
Desault, in the 18th century, reiterated this belief, advising
extending open wounds to explore and remove dead tissue. It was he who
coined the term débridement.147
Later, the possible effects of the timing of open fracture treatment
were appreciated by his pupil, Larrey, which presaged a topic that
remains controversial even today.
and antibiotics, emergency amputation as a lifesaving measure after
open fracture was not uncommon. In the American Civil War (1861-1865),
the mortality rate for open fractures was 26%. In the Franco-Prussian
war (1870-1871), 13,000 amputations were performed to avoid sepsis or
death. Nevertheless, even
amputation
was no guarantor of favorable outcome, as Billroth (1829-1894) reported
that 36 of 93 (39%) patients with open fractures of the lower
extremities still died.115
débride, stabilize, and allow open fracture wounds to heal by secondary
intent.70 Sulfonamides, applied topically on wounds, became widely used during World War II (1939-1945).70
More proper antibiotics became available during the US-Korean War
(1950-1954). Gustilo and Anderson described the use of antibiotics and
described a classification scheme for open fractures.66
Small beads of polymethylmethacrylate (PMMA), impregnated with
antibiotics, can also be useful in open fracture management. This
technique effectively supplements intravenous antibiotic delivery, and
occupies “dead space” caused by bone loss, minimizing hematoma
formation and bacterial colonization.75,114
with serial wet-to-dry saline gauze dressings. Ideally, uneventful
wound healing by secondary intention ensues. However, this technique
exposes the wound to the external environment, and inevitable
colonization may lead to infection. This is more likely to occur if
internal fixation, particularly surface implants, is present, or if
fascia1 defects exist. Advances in plastic surgery techniques in the
1980s and 1990s, specifically the development of microvascular free
tissue transfer, aided in the coverage of these wounds. This technique
proved useful in both the avoidance and treatment of chronic
osteomyelitis and has become a mainstay of treatment of the most severe
soft tissue injuries. Unfortunately, the availability of plastic
surgeons trained and willing to perform this technique remains limited
in certain areas. Further, not all wounds or hosts are compatible
candidates for free tissue transfer because of local or systemic
vascular disease, injury, or other patient factors.
KCI, San Antonio, TX) has also proved to be a useful adjunct in the
treatment of these complex injuries. Such dressings provide a closed
environment, preventing loss to evaporation, promoting the formulation
of granulation tissue, and preparing the wound site for coverage.
Although a strong adjunct to the treatment of open fracture wounds,
unlike previous thought, it has not decreased the risk of infection
when used in a prolonged manner before microvascular free tissue
transfer.15
open fracture wounds that are used in civilian settings are the results
of lessons learned, forgotten, and repeatedly relearned in the military
setting. These include:
-
Application of a sterile dressing over the open wound in anticipation of more formal débridement in a sterile operative setting
-
Immobilization of the extremity
-
Early administration of antibiotics and tetanus (if required)
-
Urgent operative wound débridement and
irrigation, with stabilization of the skeletal system either
provisionally (e.g., external fixation) or definitively -
Repeated débridements as indicated and
definitive skeletal stabilization and/or soft tissue closure or
coverage as soon as prudent
are the result of higher energy mechanisms than closed fractures, this
is perhaps an oversimplification. Higher energy mechanisms of injury
commonly are associated with the energy required to cause open
injuries. Nevertheless, depending on the anatomic area involved, some
lower energy mechanisms of injury may still result in open fractures.
One example is a motor vehicle collision that leads to a femoral shaft
fracture, in which one of the fragments tents the intact overlying
thigh skin, having traversed the large quadriceps or hamstring
musculature of the thigh remains a closed fracture. This may have
required significantly more energy than a distal tibial fracture
acquired in a soccer game, in which the thin medial soft tissues are
compromised in a simple “inside-to-outside” breach, resulting in an
open fracture.
although if an infection develops later, little correlation has been
shown between the contaminant and the cultures obtained during
treatment of the infection.96 Higher
energy mechanisms typically cause greater soft tissue disruption that
leaves the wound more susceptible to infection by contaminating
bacteria.161
beyond fracture pattern influence prognosis. Local injury variables,
such as the presence of copious foreign debris with highly contaminated
material, and substantial soft tissue and bone devitalization affect
the infection and nonunion rate, and can predispose to a prolonged
recovery and a poor outcome. Systemic variables, such as poor host
quality, medications taken, and nicotine abuse also influence
complication rates. Additional injury and patient specific conditions
influencing outcome include the presence or absence of nerve injury and
remote fractures and injuries can impact functional prognosis.
should be thoroughly evaluated. The patient should be stabilized
according to ATLS protocols.92 Any
life-threatening injuries must be evaluated and treated. Although it is
tempting to immediately address the open wound, one must be assured
that the patient’s airway, breathing, and circulation are in order.
Obviously, many processes may be undertaken in parallel rather than in
series, but it is important to thoroughly work-up what may be a
polytraumatized patient rather than rushing to the operative suite to
address the injured extremity.
history when possible. This should include the site(s) of pain and
instability, the mechanism of injury, and changes in sensation or motor
function. Any pain in the area of the fracture prior to the traumatic
injury may signify a pathologic or stress fracture, as may an unusually
low energy mechanism or a report from the patient that he or she sensed
that the fracture occurred prior to falling down. Any previous
injuries, fractures or surgeries, dermatologic conditions, or radiation
therapy in the area in question may also impact treatment or outcome.
Obtunded, intubated, or otherwise nonverbal patients may be unable to
provide significant history. In these cases witnesses to the accident,
family members, and emergency medical providers in the field may
provide useful information regarding the mechanism of injury and the
patient’s medical condition. This may include specifics regarding the
injury or vehicle collision, the degree of injury to the vehicle(s);
the use or nonuse of seatbelts and other
restraints;
the extent of injuries to others, including passengers in the vehicle;
how far the patient was thrown from the vehicle; the extent of blood
loss in the field; or the patient’s level of responsiveness prior to
arrival in the ED.
Conditions such as nutritional depletion, diabetes mellitus, severe
peripheral vascular disease, rheumatoid arthritis (or any condition
that requires chronic steroid use) may affect wound and/or fracture
healing and may accordingly affect choices for treatment and potential
for an optimal outcome. A history of seizures with long term phenytoin
use may denote impaired bone quality. Level of activity and
weight-bearing status prior to the injury may be a predictor of level
of activity posttreatment. History of surgeries, particularly in terms
of previous incisions and/or indwelling implants in the vicinity of the
open fracture wound, may affect choices for treatment.
constricting clothing about the injury should be removed. Visual
inspection of the limb is undertaken looking for deformity or bone
fragments prominent under the skin or placing the skin at risk, signs
of dysvascularity, previous incisions or other scars, gross debris in
the wound, burns, abrasions, and degloved soft tissues. Any remaining
limb deformity not addressed in the field is corrected. Evaluation for
compartment syndrome should not be overlooked, because this may still
occur in the setting of open fractures.40,149
the fractured end(s) of major fragments often visible in the open
wounds or protruding through the wound. In other cases, however,
whether a fracture is open or not may not be quite as clear. Any wound
in the area of a fracture or even within the same limb segment should
be assumed to represent an open fracture until proved otherwise.
Full-thickness lacerations, even if a simple poke hole, may represent
an open fracture. The wound may be somewhat remote from the fracture if
the fragments have telescoped past each other as the fracture shortens,
a result of severe axial loading. Persistent oozing of blood from the
wound, particularly if fat is noted in the blood, may represent a
decompressing fracture hematoma. This is a common, but not constant
finding. Even open wounds remote from the fracture site may communicate
with the fracture if the skin and subcutaneous tissues have been
degloved from the underlying fascia and deeper tissues (Figure 10-1).
commenting on its location, length, configuration (linear, stellate or
other), orientation (longitudinal, oblique or transverse), condition of
the adjacent skin, and associated abrasions or other injuries. In this
era of increasingly shared responsibilities for caring for these
patients, such documentation cannot be overemphasized. Additionally,
prolonged periods of a joint dislocation or fracture displacement can
place excessive tension on the overlying soft tissues, potentially
leading to full thickness skin breakdown, effectively converting a
closed fracture to an open fracture (Figure 10-2).
This may be significant because surgical incisions tend to be
longitudinal, and the traumatic wound may affect the surgical plan for
approach and access to the fracture. Open fractures in the pelvis may
be contaminated from the external environment or internally by rectal,
vaginal, or urinary flora. An extremely high index of suspicion should
be maintained for an associated open fracture and a thorough
examination is imperative, checking for vaginal, perineal, and rectal
blood, tears in the soft tissue envelope, or protruding bone fragments.
Treatment in these circumstances may include providing fecal diversion
via colostomy. In the best of settings and treated appropriately, these
fractures are associated with severe morbidity and even mortality.
Failure to recognize these injuries as open and contaminated may lead
to even more common adverse outcomes.
the fracture and distally is a vital part of the evaluation.
Circulation is noted by pulse examination, the warmth and color of the
limb, capillary refill, the filling of veins, and ABI testing. Recall
that a malaligned limb due to a displaced fracture or unreduced
dislocation may demonstrate signs of vascular insufficiency and that
realigning the limb to a more appropriate position may provide a return
of blood flow to the limb once the vessel is unkinked. If realigning
the limb does not improve circulation, then a vascular injury should be
suspected and investigated. Never assume that the pulse deficit is
caused by vascular spasm. Doing so may lead to catastrophic
complications and/or legal ramifications. An expanding hematoma or
pulsatile bleeding likely represents an arterial injury.
examined for peripheral nerve function and dermatomal integrity.
Typically this begins with evaluation of light touch and pressure
sensations. Motor strength testing may be difficult because of
splinting, pain, intubation, or chemical sedation or paralysis. It is
important to document what can be examined and whether certain parts of
the examination remain unknown. It is far better to document that
specific portions of the neurological exam could not be determined than
to neglect to comment on this information. Secondary survey and
reexamination should be undertaken when the patient is able to comply,
and any missing portions of the examination documented at that time.
the limb splinted with a noncircumferential splint that stabilizes the
affected bone and the joints above and below if possible. Compressive
dressings may be used sparingly in the setting of a vascular injury.
Repeated evaluations of the wound in the emergency department should
not be undertaken, because this may predispose to an increased rate of
infection. A photograph may be taken of the wound for documentation if
the patient or family is agreeable. This is particularly useful in the
setting of the mangled extremity. Tetanus status is determined and
updated as necessary. Intravenous antibiotics are started as soon as it
is obvious that an open fracture is present.
imaging is indicated to provide the information required to
appropriately treat the patient. Anteroposterior and lateral
radiographs of the entire bone, including adjacent joints, is the
minimum required to properly assess long bone injuries. It is routine
to include the joint above and below the injury in the imaging plan.
Oblique radiographs and comparison views of the contralateral side are
utilized only in select cases. A segmental fracture of the diaphysis or
a noncontiguous additional fracture extending into a joint proximal or
distal to the fracture associated with the open wound is not uncommon
in high energy injuries (e.g., distal third tibial shaft fractures with
extension into ankle joint). Other information, including the thickness
of cortices or the intramedullary
canal,
indwelling implants, prior fractures or deformities that may affect the
surgical plan, or even bone tumors or other abnormalities are noted and
may affect the treatment plan.
 |
|
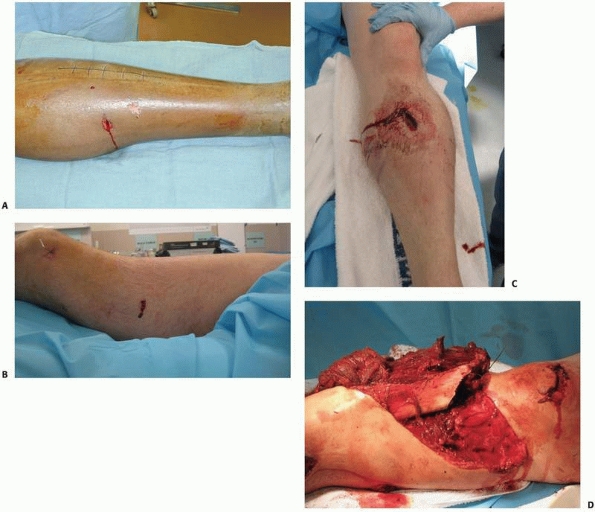
FIGURE 10-1 A. Gustilo type I open tibial shaft fracture with wound on the medial face of the tibia. B.
Gustilo type I open femoral shaft fracture. Note that many surgeons feel that because of the amount of energy required and the amount of soft tissue traversed for this injury to become open, most open femoral shaft fractures should be classified as type III injuries. C. Gustilo type II open proximal tibial shaft fracture. D. Gustilo type III open tibial fracture prior to débridement, note the associated substantial periosteal stripping and severe muscle injury. |
 |
|
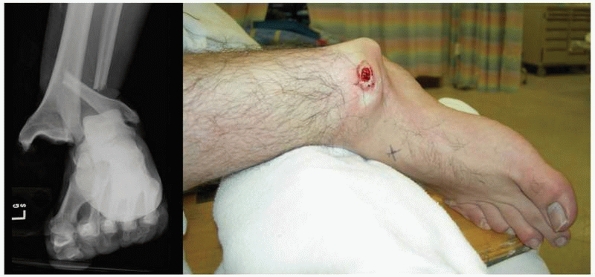
FIGURE 10-2
Closed fracture-dislocation of the ankle with an abrasion and near full thickness skin necrosis. This requires immediate reduction to minimize additional soft tissue injury. |
the soft tissues and bone loss. The presence of air in the subcutaneous
tissues is a common feature of open injuries and may indicate the
extent of degloving and or contamination. Recall that such “air,” in
rare cases may also represent the gas produced by Clostridium perfringens or Escherichia coli.6 In the acute setting, however, this usually represents an open fracture.
of injury, the wound, and the radiographs should all be combined to
give a sense of the injury personality. For periarticular fractures,
particularly those of the distal femur, tibial plateau, tibial pilon,
and distal radius, computerized tomography (CT) scanning is commonly
performed. This gives valuable information regarding the degree of
comminution and or shortening, the orientation of fracture lines, and
facilitates preoperative planning. If temporary spanning external
fixation of the fracture is planned after débridement, the CT scan may
be performed after the fracture has been brought back out to length and
reduced, because the information obtained will be more intelligible.
MRI scans are not commonly obtained in the acute setting after open
fracture.
treated aggressively. Traumatic arthrotomies may occur with or without
fracture. One common traditional diagnostic test used to try to
determine whether the knee joint is open is the saline challenge.
Saline is injected into the knee and the wound is evaluated for
extravasation. A recent study showed that this test is likely
insufficient for diagnosing traumatic knee arthrotomies, even when the
knee is placed through a range of motion after insertion of the saline.88 Maintaining a high level of suspicion may be more prudent.
that falls just short of coming through the skin. In some cases the
fracture fragments may tent the skin. In other cases the skin is so
injured by the injury that it gradually becomes nonviable over the
course of several days. Fractures with skin at risk, by virtue of their
severe displacement and instability, may place their overlying soft
tissue envelope at risk for pressure necrosis. Although certain
specific fractures (e.g., medial ankle skin in lateral or
posterolateral fractures and fracture dislocations of the ankle) may be
predisposed to this issue, almost any fracture that approaches open
status will place tension on the overlying soft tissue structures. In
some respects this creates an urgency equal to that seen with open
fractures, because if the tissues at risk are allowed to die, the
patient is left with an open fracture with an uncloseable wound and
necrotic tissue. The patient may be at risk of requiring complex
microvascular free tissue transfer reconstruction procedures. An
amputation may be required if the patient is not a candidate for such a
procedure or if such an attempt at soft tissue reconstruction were to
fail. As such, emergent fracture reduction, and sometimes fixation, to
resolve soft tissue tension may therefore be required.
fractures with skin at risk in a timely manner cannot be overstated.
This situation commonly occurs in areas of poor or limited soft tissue
coverage. By way of example, certain fractures about the foot and ankle
are particularly prone to placing overlying soft tissues at risk.
Laterally displaced (fracture-) dislocations of the ankle or tibiotalar
joint may place the medial skin overlying the medial malleolus at risk (Figure 10-2). Displaced calcaneal tuberosity fractures may tent or broach the posterior heel skin (Figure 10-3).60
Talar neck fractures, particularly Hawkins III and IV fractures, and
particularly those displaced posteriorly and posteromedially, may cause
vascular or nerve injury or place the overlying skin at risk.
Similarly, high energy tibial plateau fractures with widely displaced
tibial tubercle fragments and highly displaced fractures of the
clavicle are other examples of injuries that may require urgent
reduction and fixation when fracture fragments are placing undue
pressure on their overlying soft tissues.
relevant information, guide treatment, predict outcomes, or assist in
research. Various classifications have been developed for the
description of open fractures, but the most widely used is that
developed by Gustilo and Anderson.66
injury. Skin lacerations are less than one cm, and are clean, without
evidence of deep muscle crushing or foreign debris contamination.
Additionally, the underlying fracture pattern must be consistent with a
low energy injury. Examples include spiral diaphyseal fractures or
rotational periarticular injuries. Higher energy fracture patterns,
such as segmental or bending wedge fractures, should be considered as
higher grade open fractures. Type II open fractures are those with
slightly more soft injury, and with higher energy fracture patterns.
Minimal soft tissue degloving and periosteal stripping is present in
Type II injuries. Muscle crushing and foreign contamination also must
be mild to moderate for Type II designation. Skin lacerations are less
than 10 cm in length. The Type III open fractures include higher energy
injuries. Substantial soft tissue injury, with periosteal stripping,
has occurred, and a crush component is typically present. The fracture
pattern, such as segmental comminution, reflects the higher forces
imparted. If adequate viable soft tissue and skin is present for
coverage, the injury is classified as Type IIIA. If soft tissue
reconstruction is required, such as free or rotational muscle flaps,
then the designation is Type IIIB. When an arterial injury requiring
revascularization is required, Type IIIC classification is invoked (Table 10-1).
fractures. First, the full extent of the underlying soft tissue injury
is often difficult to determine prior to thorough surgical exploration
and débridement. Therefore, the utility of assigning a classification
upon acute presentation is relatively limited. Next, the length of the
skin laceration was emphasized in the original classification
description. However, as experience with open fractures has expanded
over the last several decades, it has become clear that the underlying
muscle and periosteal disruption is more important than the size of the
open wound, and this should be accounted for when typing an open
fracture. This is most often applied with open femoral shaft fractures,
in which
the
skin laceration is small. The high magnitude of force that must be
imparted to cause displacement of a fractured femur through the soft
tissue envelope and skin mandates a higher energy designation,
regardless of the laceration size.
 |
|
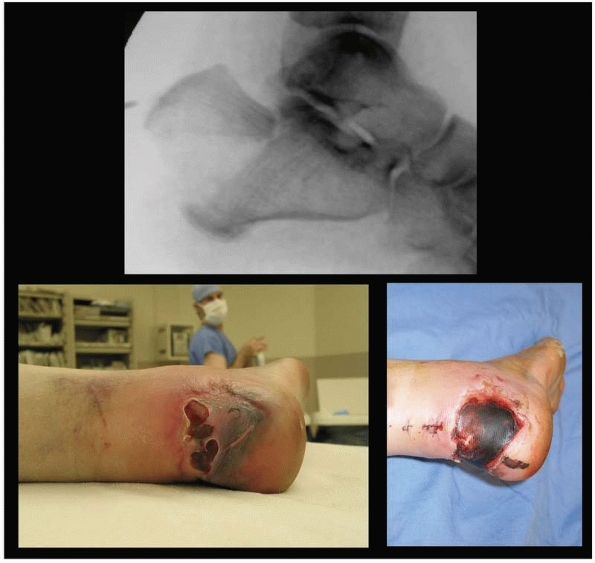
FIGURE 10-3
Deceivingly severe soft tissue injury in this displaced tongue-type calcaneus fracture. Pressure necrosis can convert this closed fracture to an open fracture, leading to significant morbidity. When the thin soft tissue envelope over the posterior hindfoot is at risk, this requires urgent surgical reduction and stabilization. |
an orthopaedic emergency. The rationale has been that it is imperative
to débride and irrigate the wound to minimize the bacterial load to
minimize the risk of infection. There has been much made of the time to
débridement as being critical, with 6 hours after injury to débridement
considered something of an important deadline to meet. Certainly it
makes little sense to needlessly delay surgical débridement of these
wounds in patients who are physiologically ready for the operative
suite. Nonetheless, some polytraumatized patients may be too
physiologically impaired to safely leave the intensive care setting
even for the briefest of operative procedures.
|
TABLE 10-1 Gustilo Classification of Open Fractures
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
obscure, but the rationale for this appears to have some animal-based
evidence. Robson et al. noted that the threshold to sustain an
infection was 105 organisms.127
They found that this threshold was reached in 5.17 hours. Cooney et al.
noted that with 100,000 organisms per gram of tissue, the immune
defenses were overwhelmed and infection ensued.36
risk of infection with delay in treatment after open fracture. Al-Arabi
et al. looked at 248 long bone fractures using a cutoff of 6 hours
postinjury as a marker. There was no statistical difference between the
infection rate of those treated within or beyond the 6 hour cutoff.3 Other authors also have failed to demonstrate a difference.5,10,33,41,144
with an open fracture is physiologically able to go to the operative
suite, preparations for treatment are made. This may include gaining
informed consent from the patient or his or her representative,
finishing all required preoperative resuscitation and other
workup,
consulting with other specialists for both premorbid and concurrent
injuries, early booking of the case with the OR manager, discussing
with other surgeons if “bumping” of elective or less urgent nonelective
cases is required, and arranging with the operating room staff to have
all necessary equipment available. This may include a discussion with
the implant company representative if there are any questions about
availability or other technical issues regarding anticipated implants.
In some cases more than one surgical service may need to work on the
patient in the operative suite and arrangements should be made
regarding the order of events. This is particularly true in the setting
of open fractures with concomitant vascular injury requiring repair.
In the setting of an open fracture, intravenous antibiotics are
typically given immediately upon arrival in the emergency department.
The most common current treatment is for these to be continued for 24
hours and for 24 hours after each subsequent débridement or other
surgery rather than continuously throughout the hospital course. There
has been little evidence to support giving antibiotics any longer than
this in the prophylactic setting.48
and experience when treating each patient with an open fracture. The
individualization of treatment is a cornerstone of treatment.
Nevertheless, if a surgeon chooses to pursue treatment that may be
considered outside the standard of care, then thorough documentation of
rationale should accompany such treatment. It must be recognized that
fracture location, type, mechanism, severity, operative treatment, and
antibiotic use all play factors in the prevention of infection.
antibiotics as long as drains are in place. There is no evidence to
support routine empiric prolongation of prophylactic antibiotic
treatment past the initial perioperative period, even if the incision
or wound is draining. There is no evidence to support the routine use
of prophylactic antibiotics for patients with external fixators (to
prevent pin tract infections). Antibiotics are not advised as a
substitute for débridement and the aggressive removal of necrotic
and/or contaminated material.
|
TABLE 10-2 Common Types of Antibiotic Given for Open Fractures
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||
cultures at the time of initial débridement, there seems to be little
correlation of these culture results with likelihood of infection or
the specific organism that proves to be the source of the infection.
Valenziano et al. investigated the value of cultures taken during the
initial débridement of an open fracture. Of the initial cultures, 76%
did not demonstrate any growth, and the other 24% only grew skin flora.
Of the isolates that grew from the initial cultures, none were the
organisms that eventually led to wound infections. They concluded that
the use of primary wound cultures in open extremity injuries has no
value in the management of patients sustaining long bone open extremity
fractures.150 Lee looked retrospectively at 245 open fractures.96
He found no difference in the rate of infection in positive or negative
predébridement cultures. In cases that did become infected,
predébridement cultures grew the infecting organism only 22% of the
time and postdébridement cultures grew out the infecting organism only
42% of the time.96 Merritt similarly
showed no correlation between predébridement cultures and the ultimate
infecting organism in infected cases, but did show some correlation
between the organism that ultimately developed and the last piece of
tissue taken in a débridement, so the cultures correlate more with what
the patient left the operative suite with than with what they entered
the operative suite.105
twigs, etc.) in the emergency department should be addressed by removal
immediately. The wound is covered with a saline gauze dressing, rather
than one soaked in iodine or povidone as has been done widely in the
past. There are several reasons for this. Common wound products, such
as blood and fat, inactivate the antibacterial activity of
povidone-iodine.165 Systemic toxicity can occur in large wounds or in the setting of impaired renal function.121
Staining of tissues can make tissue viability during débridement
difficult, and tendon desiccation can occur. The effects of
povidone-iodine on wound healing at the cellular level remain
controversial, but we recommend its avoidance in open wounds due to
these other factors. Once it is established that the patient is
physiologically ready for the operative treatment of the open fracture,
the patient is brought to the operative suite and surrenders to
anesthesia. In patients with fractures at risk for associated
compartment syndrome rather than general anesthesia, regional blocks
that may preclude the ability to examine the patient postoperatively.
of an open fracture is set up separately, often using a separate table
or Mayo stand from the equipment to be used in the subsequent
stabilization of the fracture. This facilitates transforming the
contaminated wound into a clean one and minimizes the chance of
implanting contaminated hardware. During the skin and wound prep, any
ground-in dirt and other debris may be gently removed with a scrub
brush, being mindful of what the soft tissue envelope has already
undergone and realizing
that this may not completely cleanse the skin of any organisms, particularly if the patient is treated in a delayed manner.83
It may be prudent to place a deflated tourniquet on the extremity, if
possible, prior to prepping the extremity. This allows for the
management of the occasional, otherwise uncontrollable hemorrhage that
may ensue when a clot is removed from an unanticipated vascular injury.
Another option is to have a sterile tourniquet ready in the operative
suite. The tourniquet is typically not inflated otherwise, as the local
tissue often has already sustained anoxic significant insult and
because this impairs the ability to evaluate muscle viability.
identification and exploration of the entire zone of injury and to gain
access to the ends of major bone fragments. The zone of injury is often
more extensive than suggested by the open wound. As such, final
classification of the fracture should be made at the time of
débridement. Wounds that appear small or benign and initially
classified as Gustilo type I or II may prove to be better classified as
type III injuries if there is significant periosteal stripping
involved. The mechanism of injury and the amount of displacement and
comminution noted on initial radiographs often provide strong clues to
the degree of injury. Similarly, apparent Gustilo type IIIa injuries
may initially appear to have enough viable skin for soft tissue
coverage. They may need to be reclassified to type IIIb injuries if
swelling precludes closure, if débridement of necrotic skin obviates
closure, or if a flap with tenuous blood supply dies, leaving the soft
tissue envelope uncovered. It is difficult to classify open fractures
of the pelvis and because of the potential of both external and
internal contamination, diverting colostomy has become a mainstay of
treatment in patients with bowel injuries secondary to open pelvic
fractures.
working from outside to inside. Regardless of original orientation,
traumatic wounds are typically transformed to extensile incisions that
facilitate visualization of the underlying deep tissues. Another
advantage of an extensile (longitudinal) incision, is that as the
fracture, which has shortened, is brought back out to length, these
wounds are easier to close than transverse wounds that tend to gap when
stretched. The incisions may be extended until more normal tissue is
encountered, without involving uninjured intact tissues.113
excised. Whenever adequate skin is available, excising 1 to 2 mm of
contused skin back to viable tissue is reasonable. Nevertheless,
excision of skin must be done in a circumspect and prudent manner,
because skin may be a previous commodity in certain anatomic areas
(tibia, hand, foot) and in patients who are not free flap candidates.
Taking care not to leave acute or distally based flaps is essential.74
Detaching skin and underlying subcutaneous tissue from attached fascia
is usually ill advised as this devascularizes the overlying tissues as
well as provides another potential space for fluid to accumulate.
Clearly nonviable skin should be excised, but any skin that is of
marginal viability may be left for later débridement; because skin,
unlike necrotic muscle, is not the major generator of infection.
excised. Unlike with skin, any marginally viable fascia is excised. A
low threshold should be maintained for fasciotomy. In high energy
injuries this is prophylactic against compartment syndrome. In open
fractures with a simple rent in the fascia through which the bone
fragments traversed, a fasciotomy of the related muscular compartment
is advised to gain access to the underlying bone fragments and in
anticipation of muscular swelling in response to the initial injury.
relatively easily assessed, the soft tissue component is not. The
extent of injury to the soft tissue envelope, particularly the muscle
around an open fracture, is often dynamic and evolving. The overlying
wound and even radiographs may underestimate the amount of muscle
damage in a given open fracture. Whereas skin tends to tear or
puncture, and fascia to split or shred, muscle, because of its high
water content, is subject to hydraulic damage by fluid waves when an
injuring object strikes the limb. This is particularly true of high
energy fractures secondary to indirect rapid loading (e.g., a
high-velocity skiing injury resulting in comminution of the tibia or
femur), in which the bone literally explodes into many fragments. These
fragments travel rapidly outward in the muscle and can cause
significant muscle damage even when the outer skin envelope is
seemingly undamaged.27 A small bone
fragment may pierce the skin, producing what appears to be a very minor
type I open fracture, when in fact there may be considerable deep
muscle damage. This occurs because the more rapidly bone is loaded
before fracture, the more energy is required to fracture it, and the
more energy is released from the fracture when it occurs. Because of
this absence of direct physical evidence of trauma, overlooking
nonvital muscle is easy because it may not immediately be evident that
it has been disturbed or damaged. In muscle débridement, the approach
of “when in doubt, take it out” is safest. Necrotic muscle is the major
pabulum for bacterial growth and poses a great danger in anaerobic
infections. Every effort should be made to remove all nonvital muscle
tissue, although this always requires careful judgement.72,113,148
In type I, II, and IIIA open fractures, this may be taken literally,
but in types IIIB and IIIC, débridement of an entire muscle or
compartment may be necessary to meet this axiom. If the major arterial
supply to a severely damaged muscle has been destroyed, the only
recourse is total excision. It has been our experience that if even a
small amount of a muscle belly and its attached tendon can be
preserved, significant function may be retained. For that reason there
may be an indication for leaving marginally viable muscle at the time
of initial débridement in severe open fractures, with a plan for
returning within 24 to 48 hours for redébridement, at which time the
muscle will have better “declared” its viability. Exceptions to this
rule include mass casualties (e.g., wartime injuries in austere areas),
in which case preservation of life takes precedence over the desire to
preserve function.
four tenets of muscle viability are color, consistency, contractility,
and capacity to bleed. Scully et al. studied these, trying to confirm
the reliability of these factors histologically. They found that muscle
consistency and capacity to bleed were the most reliable indicators of
muscle viability.4 We have found
that contractility and consistency are more reliably clinically and
that color of the muscle and the capacity to bleed are easy to
misinterpret. The hypoxemia associated with shock, or the use of a
tourniquet, which is discouraged in most open fractures, may make assessing these variables quite difficult.
for compartment syndrome can be difficult. Muscles in the extremities
receive their blood supply from small arteries that run in the
epimysial layer. Arterioles branch into the central part of the muscle
to supply the deeper muscle. Three zones of postischemic changes that
occur in muscle have been described histologically:143
-
An inner zone of muscle necrosis in which no swelling occurs
-
A zone of partial ischemic injury with viable muscle that swells substantially
-
An outlying zone of normal muscle in which no swelling occurs
one may have superficially viable muscle and a substantial amount of
deeper necrotic muscle, so the surgeon must look beyond the superficial
layers during the débridement. This is done by spreading the muscle in
line with the muscle fibers with a hemostat, allowing the surgeon to
assess the character of the deeper muscle without substantially
injuring the muscle unit.
preserved. In open wounds these are subject to desiccation, which can
be devastating to function. It is essential to provide coverage or at
least to maintain the peritenon. Because maintenance of the peritenon
is important, we tend to copiously irrigate this rather than débride
it. Consideration should be given at the end of the débridement to try
to cover any tendon, and certainly any tendon without peritenon, with
muscle, subcutaneous fat, or skin. If this is not a possible, a moist
dressing is applied and maintained until more definitive coverage can
be procured. Intact arteries and veins and nerves should not be
débrided.
a relatively poor blood supply, the bone is largely without defense
against infection. It is clear that small bits of bone completely
devoid of soft tissue attachments should be removed. Larger cortical
segments with limited soft tissue attachments are a more difficult
problem. Large bony segments with soft tissue attachments should be
retained. In general, cortical fragments without soft tissue attachment
ultimately should be removed. These fragments may be useful in
determining length, rotation and, alignment and may be utilized
provisionally during skeletal stabilization, then removed from the
wound after skeletal stabilization. Even when definitive fixation does
not occur during the same operative setting, fragments deemed to be
important determinants of reduction may be cleansed and retained in a
freezer until they can be used and then discarded.9
Another exception to the strict removal of bone without soft tissue
attachment is the rare case in which a significant portion of the
articular surface is attached to the loose bony fragment. As the bone
in this case is cancellous rather than cortical, and because loss of
the articular surface may cause significant functional and
reconstructive problems, it is preferred in most cases to retain the
fragment, assuming a complete débridement of any gross contaminants can
be obtained.113
delivered into the wound and their ends and the deep tissues explored
for dirt, gravel, grass, clothes, and other foreign debris. The bone
ends are cleaned of hematoma with curettes and saline irrigant.
Occasionally road debris will be embedded in the bone ends. In this
scenario meticulous débridement with dental picks, curettes, and even
motorized burrs may be necessary to eliminate the foreign matter.
debris and necrotic material, irrigation of the wound is performed.
Irrigation serves to reduce the bacterial count, float out remaining
debris, and cleanse the wound of hematoma to better visualize the
remaining tissues. Both high pressure pulsatile lavage and lower
pressure gravity irrigation are popular.120
Some evidence suggests that higher pressure lavage may injure the
remaining tissue or even send remaining debris deeper into the wound.21
A recent randomized prospective study compared lavage with saline to
lavage with an antibiotic solution and found no advantage in using the
antibiotic solution.2 No consensus
exists regarding the ideal volume of irrigant to be used in open
fractures. Many surgeons choose between 6 and 12 liters, but these
decisions are not based on high level evidence. Volume should be
tailored to the perceived and actual wound contamination with foreign
material, as well as the overall energy of the injury and soft tissue
damage. Alternatively, some surgeons advocate basing irrigation on a
time rather than a specific volume. We generally believe that between 6
and 9 liters is adequate for most open fracture wounds, and use low
pressure without additives to the solution. It must again be stressed
that irrigation should be performed after thorough tissue débridement.
restoration of the bony anatomy and skeletal stabilization. Restoring
the rotational and angular alignment, and particularly axial length, of
diaphyseal and metaphyseal fractures has many benefits for expediting
healing of the soft tissue injury. Fracture reduction restores
appropriate spatial relationships of arteries, veins, and lymphatic
channels, unkinking both large and small caliber conduits, improving
perfusion and circulation to the injury zone. Peripheral motor,
sympathetic, and parasympathetic nerves function optimally when
decompressed, and contribute to initiation of appropriate immune
response and healing. Adequate soft tissue tension also substantially
facilitates later reconstructive procedures and internal fixation if
provisional fixation is selected primarily. Finally, dead space
management is most adequately achieved with anatomic myotendinous and
fascial plane tension. Minimizing motion of fracture fragments is also
important, and decreases persistent soft tissue injury and exaggerated
inflammatory mediator release. Both realignment and stabilization allow
for immediate vascular inflow, delivering mononuclear and
polymorphonuclear cells, as well as antibodies and antibiotics to the
compromised tissues (Figure 10-4). Aside from
the local benefits, fracture stabilization allows for decreased patient
pain, permits mobilization out of recumbency, and minimizes the
difficulty with subsequent diagnostic tests.
has unique implications in the overall treatment plan and outcome. In
two classic studies by Salter et al., the benefits of early joint
mobilization for the health and healing of articular cartilage were
demonstrated.129,130 Immediate articular reconstruction
that allows joint motion may be beneficial for long term patient
outcomes. Acute fracture lines that are visible and accessible directly
in the wound at the initial débridement procedure may represent the
optimal opportunity for anatomic reduction and fixation.60 Often reduction and limited screw placement, coupled with spanning external fixation, is an effective initial treatment plan.
 |
|
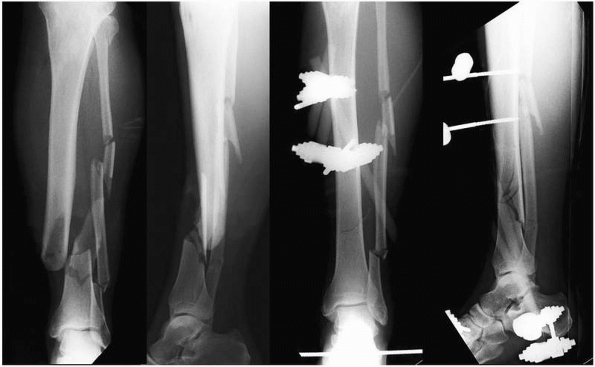
FIGURE 10-4
Example of a high energy Type IIIA open tibial shaft fracture. External fixation and closed reduction was performed acutely following débridement. Appropriate restoration of fracture length and alignment facilitated the early healing response. |
account multiple factors. If additional soft tissue dissection is
necessary to achieve anatomic articular reduction and fixation,
particularly in articular fractures with tenuous soft tissue envelopes
(e.g., pilon and tibial plateau), consideration should be given to
delayed exposure, reduction, and fixation.60
If copious contamination is present within the wound and the joint,
particularly embedded within the cancellous surfaces of the bone, delay
may be prudent. Finally, if the patient’s physiologic status is at all
in question, articular reconstruction clearly takes a much lower
priority than expedient damage control procedures and resuscitation.
its own merits and disadvantages depending on the anatomic region and
the clinical severity and situation. On the least invasive end of the
spectrum, fracture stabilization is sometimes possible with plaster or
fiberglass splints, or skeletal traction. These methods should be
employed in carefully selected situations. Low-grade open wounds
associated with fractures that would otherwise be treated nonsurgically
can be considered for débridement, irrigation, wound closure, and
splint immobilization. For example, humeral shaft fractures associated
with traumatic lacerations are not necessarily indicated for surgical
intervention.132,133 Minimally displaced Grade 1 open tibial fractures may be successfully treated with external immobilization.25
Although plaster splints may provide adequate bony stability, these
rarely allow substantial access to the wound or the extremity. Care
should be taken to ensure that the traumatic wound is thoroughly
débrided and remains benign, and the risks of compartment syndrome are
minimal. Skeletal traction is most frequently employed for open pelvic
or femoral shaft fractures awaiting more definitive surgical
intervention, but is rarely used for definitive treatment.
Circumferential casts, even when windowed for wound access, have a very
limited role in the acute treatment of open extremity fractures.
or definitive stabilization of higher grade open fractures. This
technique typically involves placing transcutaneous half pins remote
from the zone of injury, minimizing additional surgical soft tissue
insult. Excellent access to the wound for dressing changes and
surveillance is usually possible. These frames may be rapidly applied,
and the fracture reduction can be manipulated in multiple planes before
clamp tightening. The clamps may later be loosened and the reduction
revised after the surgical application in a nonsterile setting.
Fracture stabilization following an appropriately placed external
fixator is generally adequate to allow for early limb and patient
mobilization.
open fractures, attention to several technical points is necessary to
avoid complications. When planning pin placement, the definitive
procedure should be carefully considered, and subsequent incisions
marked on the extremity. Even without evidence of gross pin site
infection, pin tract contamination must be anticipated. If pins are
placed within the field of a definitive procedure, the risk of surgical
infection may be increased (Figure 10-5).
A second consideration for pin placement is the capsular reflections of
adjacent joints. Pins placed in the proximal tibia within 7 cm of the
joint line have a higher likelihood of being intraarticular and
therefore may cause pyarthrosis.82
For similar reasons, intra-articular pins, such as in the talus, should
be avoided. Finally, pin sites become stable when motion is minimized
at the soft tissue interface. Thus pins that are placed through a
muscular compartment, such as the anterior compartment of the leg or
lateral compartment of the thigh, may be at higher risk for soft tissue
instability, infection, and loosening (Figure 10-6).97
Aside from meticulous pin site placement, the technique for insertion
should include predrilling, rather than self-drilling pins, to avoid
problems with high temperature generation and bone necrosis.26
 |
|
FIGURE 10-5
In this case of an open pilon fracture, the external fixation pins were placed adjacent to the fibular incision, in the zone of the potential definitive incision. The frame was revised and the pin tracts were allowed to defervesce prior to open reduction and internal fixation. |
Pin site infection is problematic and can lead to pin loosening,
reduction loss, osteomyelitis, and systemic sepsis, frequently
requiring pin removal. Major proposed causes of pin site complications
include instability at the soft tissue-pin interface, inappropriate pin
insertion sites, and technical errors related to insertion.93,140,142
Egol et al. randomized 120 fractures around the wrist treated with
external fixation, and found that hydrogen peroxide and chlorhexidine
pin care regimens did not decrease the infection rate compared with no
pin care.55 Additionally, there was
a relatively low incidence of pin tract infections requiring
intervention. A recent Cochrane review reported on several randomized
trials that evaluated various methods of cleansing, cleansing
solutions, and dressings.97 Based on
the evidence in the data studied, no definitive recommendations could
be made regarding pin site care. We believe that the vital factor for
creation of a stable pin site is immediate immobilization of the
surrounding soft tissues. Typically, rolled gauze (or similar foam pad)
wrapped around the pin occupies the space between the pin and the bar,
allowing for gentle axial compression and stabilization of the skin,
achieving this goal.
 |
|
FIGURE 10-6
Tibial external fixator pins placed from lateral to medial, traversing the anterior compartment, have a high loosening and infectious complication rate. |
external fixation has led to relatively high rates of infection (up to
15%), nonunion (3% to 11%), malunion (up to 36%), and pin tract
infection (up to 50%).7,54,73,78
Because external fixation is currently used much more frequently for
provisional fixation than definitive treatment, conversion from
external fixation to internal fixation is a critical issue. Several
clinical series have indicated that if a tibial pin tract becomes
infected, the pin should be removed and the infection treated until
granulation occurs. Subsequent intramedullary nailing leads to a low
infection rate.101,156
These data confirm animal studies that indicate that treatment of
infected pin sites with débridement and antibiotics can substantially
reduce, but not eliminate, subsequent infections.34
Conversely, other authors have suggested that the history of a pin site
infection is a contraindication to subsequent intramedullary nailing.102 Short periods of provisional external fixation appear to allow safe subsequent conversion to internal fixation.3,16,111
methods involves avoiding infection. The implants and insertion
techniques utilized should be chosen with this in mind, and should
minimize the risk of developing deep infection. For many years the
concept of immediate application of internal fixation implants for open
fracture stabilization was strongly discouraged.31
The presence of metal within a wound is a foreign body that is a
potential substrate for biofilm formation, with a theoretical increased
risk for acute infection.138,146 Additionally, the
potential bone devitalization required to apply an extramedullary
implant was thought to contribute to development of infection.
Nevertheless, following thorough wound débridement and irrigation, the
benefits of internal skeletal stabilization and appropriate wound
closure or coverage may outweigh the inherent infectious risks. Surface
implants, such as plates and screws, and intramedullary implants have
different implications on blood supply and must be considered
separately.
metallurgy, surgical techniques, antibiotic therapy, and understanding
of the pathophysiology of infection have led to the wide-spread use of
immediate internal fixation of open fractures.37,59,89 Several sentinel works have demonstrated acceptable infection rates.11,23,31,32,59,84
Gustilo and Anderson, based on their experience of over 1000 fractures,
suggested that converting an open fracture into a closed fracture
provided the optimal biological environment to prevent contamination
and infection.3 In 1979 Chapman and Mahoney reported on 94 open fractures treated with internal fixation and delayed wound closure.32
Type I wounds had an infection rate of 1.9%, nearly equivalent to the
rate in closed fractures. Type II open fractures had an 8% infection
rate, and Type III open wounds had a 41% infection rate. Subsequent
studies focusing on ankle fractures23,59,84 and tibial plateau fractures11
have also shown low complication rates with protocols that included
immediate plate fracture fixation. More recent clinical and in vitro
data on locking plates indicates that infection rates in open fractures
may be even lower than with standard compression plate devices.58
complications with acute plate fixation of open fractures. First, prior
to operative intervention, intravenous antistaphylococcal antibiotics
should be administered as soon as possible in the emergency department.
The open wound should be covered with a sterile dressing, and the
fracture splinted. In the operating room, meticulous débridement should
be performed, followed by wound irrigation. Surgical approaches for
fracture fixation should be efficient and focused on minimizing
periosteal stripping. Finally, the surgeon must ensure that the
fracture reduction is anatomic, whether rigid compression fixation or
bridge plating techniques are used.
A central principle in open fracture treatment is exposure of the
fracture ends for adequate débridement. After removal of debris, the
fracture can be manipulated and reduced by indirect or direct methods,
and a compression plate can be placed across the fracture for
provisional stabilization. The anteromedial tibial surface is typically
conducive to provisional plating and is exposed by the traumatic
laceration or surgical extensions. Plates used in this fashion should
be placed extraperiosteally to avoid further devascularization of bone (Figure 10-7).
Screws should be unicortical (typically 8 to 10 mm in length) to avoid
reamer interference. This can greatly facilitate and expedite the
fixation portion of the procedure.53
amenable to intramedullary nailing are tibial shaft fractures, and
hence they are the most extensively studied. Several decades ago
traditional teaching was that intramedullary nailing, particularly with
associated reaming, significantly damaged the osseous blood supply, and
was ill-advised in open fractures.9,34
The main concern with intramedullary stabilization of open diaphyseal,
and some metaphyseal, fractures is contamination of the medullary
canal. Chapman suggested in 1986 that “the vast majority of open
fractures of the tibia should be treated with external fixation,” based
on the observation that infection rates with nailing were as high as
30%.30 This led Bach and Hansen to randomize 59 high grade open tibial shaft fractures to external fixation or plate fixation.7
Osteomyelitis occurred in 19% of those treated with plates and in 3% of
those treated with external fixation, and these authors concluded that
external fixation should be the treatment of choice in these injuries.
 |
|
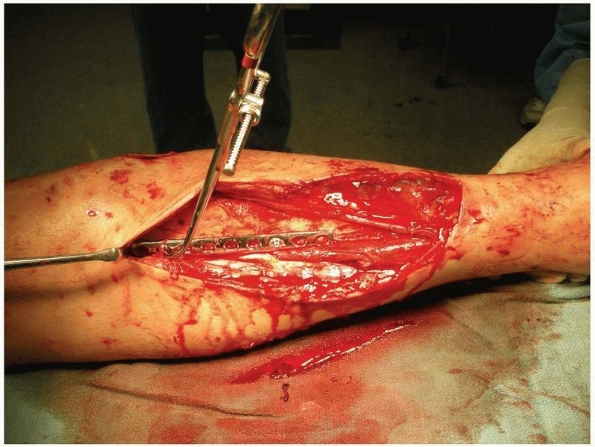
FIGURE 10-7
Example of a small fragment plate placed with unicortical screws for provisional stabilization of an open tibial fracture. The reduction is maintained during reaming and placement of an interlocking intramedullary nail. |
human studies have contributed to changing evidence-based practice
patterns. In 1990 Court-Brown et al. reported a 1.6% infection rate in
a large series of Type I open tibial shaft fractures treated with
locked intramedullary nailing.38 The
same investigators also reported on a series of Type II and III open
fractures treated similarly, and concluded that the infectious
complication rate was similar for nailing and external fixation;
however, less malalignment was noted following nailing.39
Over the last 2 decades additional studies have directly compared
definitive external fixation to locked intramedullary nailing of open
tibial shaft fractures.74,139
In general, external fixation has led to more secondary procedures,
more malalignment, lower union rates, and delayed return to function
compared to intramedullary nails, and no substantial decrease in
infection rates. Currently we favor acute intramedullary nailing with
limited reaming in nearly all open tibial shaft fractures, with the
exception of those with deep, extensive contamination, vascular injury,
or preexisting deformity.
whether reaming is used in the insertion technique has been studied
extensively. Because of similar concerns of disseminating wound
contamination along the entire diaphysis, unreamed nailing has been
favored by some authors.22 At the time of
fracture both the endosteal and periosteal blood supply are injured.126
In animal models reamed nailing has been shown to decrease cortical
perfusion by a significantly greater degree compared with unreamed
nailing.91,137
The endosteal circulation recovers slowly, but a more acute “rebound
effect” is seen with upregulation of the periosteal perfusion.22,65 This effect has not led to healing differences in animal models.136
With substantial traumatic periosteal stripping at the fracture,
however, the endosteal vascular injury with reaming is a theoretical
concern.131 Although early clinical results of unreamed nailing were encouraging,17,18,85,131 other studies have shown high rates of secondary procedures, malunions, and screw breakage rates.141,164
“Limited” reaming, which limits cortical perfusion disruption but still
allows the mechanical benefits of implant use afforded by reaming, may
be an ideal compromise between biology and biomechanics.64,80,81
several. Stimulation of the periosteal blood supply may enhance
fracture healing. A larger diameter nail can be used, with larger
interlocking bolts, improving the stability and fatigue characteristics
during the prolonged healing time.57,186
Moreover, reaming of the isthmus allows for a longer segment of
nail-bone contact, which further augments mechanical stability and
optimizes the ability of the implant to maintain the intraoperative
reduction. In one of the first reports of reamed intramedullary nailing
for open tibial fractures, Williams et al. reported a low complication
rate, with a 2.4% nonunion and infection rate.160
Thirty-three percent of fractures in this series were Type III.
Subsequent prospective studies also showed this treatment method to be
efficacious.86 In a randomized trial
of reamed versus unreamed nailing of open tibial fractures, Keating et
al. demonstrated that reamed nailing allowed for a larger nail, had
fewer hardware failures, and had no difference in union rates,
infections, or functional outcomes.87 These conclusions have been validated by additional data as well.58,164
Patients were randomized to either reamed or unreamed intramedullary
nailing. Of the 406 open fractures, 29% of the reamed group and 24% of
the unreamed group underwent a reoperation or had autodynamization in
the first year, which was not statistically different (P = 0.16).
controversial. In a series of 89 open femur fractures, Brumback et al.
reported no infections in Types I, II, or IIIA fractures.27 Infection rate was 10% in Type IIIB fractures. These results were confirmed by subsequent authors.8,110,112,128
The robust muscular envelope and vascular supply to the femur likely
account for the safety of intramedullary nailing of open femoral shaft
fractures.
stabilization of an open fracture, the method and timing of wound
closure or coverage must be chosen. Because the most critical factor in
achieving a good outcome is a complete mechanical débridement of
contamination and necrotic tissue,51,154
wound treatment should not be determined until after débridement is
complete, lest the surgeon be biased during the tissue resection. Open
fracture wounds comprise a wide spectrum of injury severity, and as
such, the surgeon must be familiar with many different treatment
methods.
This teaching is mainly derived from war settings, in which highly
contaminated fractures led to a high rate of anaerobic infections and
gas gangrene.67,147
More recent support for delayed wound closure is based on a report of
27 patients from four decades ago who developed anaerobic infections.24
These authors closed all open fracture wounds early, some in the
emergency department. Many wounds were severely contaminated, some with
fecal matter, and half with contaminated water. During subsequent
débridements, abundant organic matter was identified within the wound
in several cases because of inadequate initial débridement.
dressing over the wound in the operating room. The wound is then left
covered and sterile on the ward, and the patient is returned to the
operating room 2 to 5 days later for repeat débridement and wound
closure at that time. This prevents immediate sealing of the wound and
potential containment of residual contamination or bacteria. The
drawbacks of this approach are potential colonization of the wound with
nosicomial bacteria skin edge retraction, delay in initiation of the
healing response, and an additional anesthesia.123
In general, the recent trend in the literature indicates that
meticulous débridement by an experienced surgeon followed by primary
wound closure is safe in many circumstances. Delong et al. analyzed a
series of open fractures of varying severity that were treated with
primary closure after thorough débridement.49
Of these, 75% of the Type IIIA fractures were treated with primary
closure, and the overall deep infection rate was 7%, which was similar
to those treated with delayed closure. Another recent comparative study
found no difference in infection rates between open tibial shaft (Types
I to IIIA) treated with either delayed or primary closure.77
Most recently Rajaskeran et al. used strict criteria for primary wound
closure and had minimal infectious or healing complications in Type III
open fractures.124
is absent and closure is tension free, provided no specific
contraindications exist. Reasons to perform delayed wound closure
include the following: delayed presentation (>12 hours), delayed
administration of intravenous antibiotics (>12 hours), deep seated
contamination “ground in” to the bone, high risk of anaerobic
contamination (farmyard injuries, fecal contamination, fresh water
submersion), substantial neurovascular injuries, patient
immunosuppression, or inability to achieve tensionfree closure.
of skin does not need to be resected. Devitalized skin edges should be
excised back to bleeding edges. Skin that is avulsed from its
underlying subcutaneous tissue layers should be assessed for viability.
Nonetheless, the principal risk of inadequate débridement includes
retaining necrotic muscle and foreign debris, and
excessive
skin resection should be avoided. Overzealous skin removal that commits
a wound to a soft tissue coverage procedure may be associated with
greater morbidity and cost than primary closure.100 Nevertheless, open fracture wounds that are not able to be closed primarily occur frequently.
|
TABLE 10-3 Studies Analyzing the Effect of Wound Closure Timing on Complication Rate
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
method is for healing by secondary intention. This requires that no
bone, tendons, or neurovascular structures are present in the wound
bed. When the fascial layer can be closed, the wound can be treated
with wet to dry dressings until granulation tissue forms and the wound
becomes epithelialized. In wounds in which the fascial layer is
incompetent, packing and healing by secondary intention can still be
used. Gauze packings should be in place in all wound recesses to drain
effluent fluid, but should not be packed tightly to avoid organization
of a dead space cavity. As an alternative to wet-to-dry dressings,
negative pressure vacuum-foam dressings are also very effective in
minimizing wound edema and stimulating granulation tissue formation,
which is a necessary first step in secondary intention wound healing.
Although secondary intention generally leads to reliable wound healing,
this requires a significant amount of patient compliance and nursing
assistance, and may not be feasible in large or deep wounds.
This technique involves creating a second linear incision to allow
adequate mobility of the skin and subcutaneous layers to cover the
devitalized and traumatized region. An adequate skin bridge must be
maintained to avoid devascularization of the intervening tissue bridge.
In anatomic regions with less tissue mobility, such as around the leg
and ankle, frequently the “donor” region under the releasing incision
requires skin grafting. When larger defects exist in regions of more
restricted tissues, fasciocutaneous or rotational flaps should be
considered.
closure or relaxing incisions, and a more reliable immediate
postoperative dressing is reliable. Much research has been focused on
the efficacy of antibiotic-eluting polymethyl methacrylate
(PMMA)
cement or calcium salt-based cements. The concept of
antibiotic-impregnated cement beads is that high local tissue
concentrations of antibiotics are achieved (~200 × that of systemic
administration), and systemic levels, and subsequently systemic
complications, are minimized.44,151 Beads may be placed deep in a wound and the skin closed,60
or alternatively a plastic adhesive may be placed over the beads and
the open wound to create a “bead pouch.” Antibiotic elution and local
concentrations are highest during the initial several days,20
and gradually decline over the next 1 to 2 months. During the elution
period the cement substrate also achieves dead space management. In a
defining clinical study, Ostermann et al. reported on 845 open
fractures treated with adjunctive PMMA-antibiotic beads and found the
method superior to open fractures with systemic antibiotics alone.114
A report from the same institution concluded that the cost of
antibiotic beads is justified when considering the costs of chronic
osteomyelitis.162 Moehring et al.106
randomized a cohort of patients with open fractures to systemic versus
local antibiotic treatment. No statistical difference was found, but
the study was likely underpowered to demonstrate a difference in the
low incidence outcome of infection. Calcium sulfate103 and hydroxyapatite163
have also been described as antibiotic carriers, but their use is
mostly applicable in chronic situations with bony defects, with the aim
of avoiding a secondary procedure for removal.
management of open fracture wounds has been the refinement and
availability of vacuum-foam dressing (VFD) systems (e.g., VAC, KCI, San
Antonio, TX). VFDs can be used as wound stabilization until granulation
tissue forms and the wound heals by secondary intention, or more
commonly, as a bridge between the primary procedure and the definitive
soft tissue coverage. When secondary intention is chosen over soft
tissue reconstruction, a VFD can stimulate and expedite granulation
tissue formation.76 Wound healing
still requires multiple dressing changes and high patient compliance,
and this is usually completed using a portable VFD as an outpatient.
coverage have not been fully defined, and continue to evolve as
experience with VFDs expands. DeFranzo et al. described a series of
patients with exposed bone in lower extremity wounds.46
VFD application led to profuse granulation tissue formation over the
bone, and coverage was successful in 71 of 75 cases. VFDs have also
been useful in cases with exposed orthopaedic implants in the wounds.46,119
allow the VFD to produce a stable granulation bed for secondary
intention, and soft tissue coverage procedures are indicated. A VFD is
often ideally suited for temporary wound coverage while awaiting
definitive reconstruction. Advantages of this method include the
ability to minimize dead space within the wound, decrease edema, and
seal the wound from the nosocomial microbiology between débridement
procedures and before definitive treatment. In most wounds with tissue
loss or exposure deep to the fascia1 layers, we prefer definitive soft
tissue reconstruction with free or local muscle coverage. Early plastic
surgery consultation during presentation or initial débridement
procedures is invaluable. We then typically place a VFD initially,
followed by repeat débridement and VFD changes in the operating room
every 48 to 72 hours until wounds coverage is performed.
Many authors argued that definitive soft tissue coverage within 72
hours yielded the most reliable results and fewest complications. With
the introduction of VFDs, which provided excellent wound management
while awaiting definitive coverage, the next critical question was how
interim VFD use affected the time between injury and definitive soft
tissue coverage. Dedmond et al. used a temporary VFD in a series of 24
Type IIIB open tibia fractures for an average of 14 days.45
Infection rate requiring surgery was 29% in this group, and the overall
infection rate was similar to historical controls. Nonetheless, the
authors did note a substantially lower rate of free tissue transfer
than predicted following VFD use. Bhattacharyya et al. reviewed 38 Type
IIIB open tibial shaft fractures, all treated with temporary VFD, and
found a significantly higher infection rate in patients who had
definitive coverage greater than 7 days from injury (57%) compared with
those with earlier coverage (13%).15
effects of VFDs on wounds continue to be elucidated. Several theories
have been proposed that may not be mutually exclusive. First, the
subatmospheric pressure creates an environment of soft tissue
microdeformation and strain in the range of 5% to 20%.135
This mechanical milieu is stimulatory for cellular chemotaxis and
proliferation, as well as neoangiogenesis, and the subsequent formation
of granulation tissue.94,99,104,107,155
Second, the suction effect is able to actively clear undesirable
substances from the local injury zone, including interstitial edema,
bacteria, and inflammatory mediators.90,107,154 By reducing edema, oxygen and nutrient inflow is stimulated, and venous drainage is less impaired.145
Decreasing the spike of local inflammatory mediator concentration may
be a critical mechanism of VFDs. Mediators such as histamine and
substance P increase capillary permeability and establish a vicious
cycle of persistent edema, impaired microcirculatory perfusion, and
ongoing necrosis and potential for infection. This has been termed
wound “compartment syndrome,” and a VFD may interrupt this sequence and
allow for resuscitation of the zone of stasis (Figure 10-8).108,153
appears to be integral to the VFD’s success. If standard gauze is
placed in a wound and sealed and connected to continuous subatmospheric
pressure, cell apoptosis is greater and migration and proliferation are
significantly less than with a reticulated open cell foam dressing.104
Computer simulation models have determined that reticulated open cell
foam leads to a repeated mosaic pattern of strain and microdeformation
at the dressing-tissue interface, which may be a particularly important
component of VFD systems.158 As a
result, the differences in the structural scaffold through which the
suction is applied leads to activation of a divergent pathways of genes.50
successful clinical reports of VFDs. The surgeon must not forget that
despite the potential of new VFD technology, the cornerstone of open
fracture wound management is still thorough wound exposure,
exploration, débridement, and irrigation. Over the last few decades
free tissue transfer for open fracture wounds has decreased
consistently.116 This may be due in part to improved understanding of wound care, advancing technology and techniques,
or a decreasing interest level of microvascular reconstructive surgeons.98
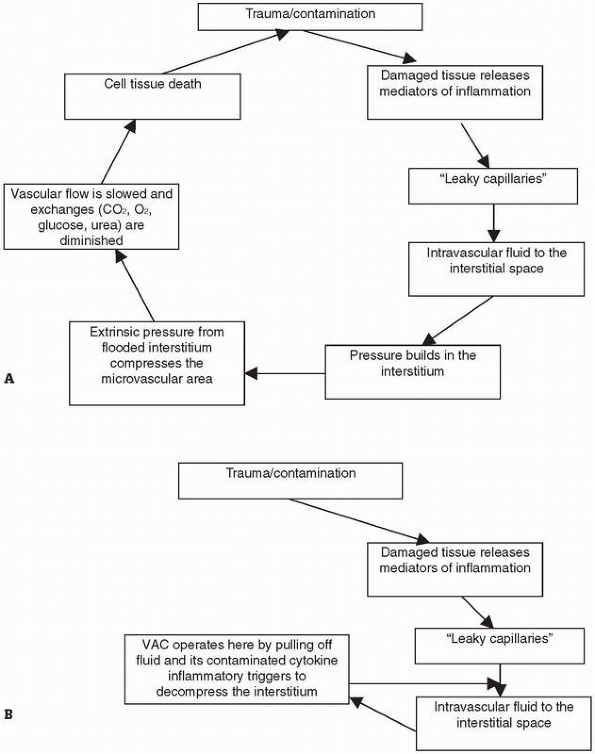 |
|
FIGURE 10-8 A.
When cells/tissues are damaged, they release cytokines/inflammatory mediators that set in motion a cascade of events which may result in impaired exchange at the microcirculatory level resulting in further tissue necrosis/cellular death. B. NPWT/ROCF pulls the fluid from the interstitial space, actively evacuating the edema and its contained cytokines and mediators of inflammation. This decompresses the otherwise compressed interstitial space and thereby decompresses the extrinsic “push” on the microvascular space. The diminution of flow is thereby averted as is the diminution of exchange of 02, C02, glucose, and urea. Cellular distress and secondary necrosis are thereby minimized. (Adapted from Webb LX, Pape HC. J Orthop Trauma 2008;22:S135-S137, with permission.) |
Advanced Trauma Life Support recommendations. Antibiotics and tetanus
are given as indicated by the injury and the patient’s tetanus status.
Awake patients with isolated open fractures undergo a thorough history,
physical, and imaging to guide initial management. Documentation of the
examination and what portions could and could not be completed are
essential. Limbs not already realigned in the field are realigned in
the emergency department. Open wounds are covered and protected in
anticipation of an imminent sterile débridement in the operative suite (Table 10-4).
Obtunded patients, particularly those in extremis who may not be able
to undergo emergent débridement, may undergo wound irrigation under
clean conditions, with removal of gross debris, in the emergency
department. This is performed only if there is an
anticipated
significant (>24 hour) delay until formal débridement. The surgeon
must use his or her best judgment in this setting. The limb is splinted
in a noncircumferential manner. If patient transfer prior to definitive
treatment in anticipated, the importance of communication with the
receiving surgeon cannot be overemphasized. Specific details of
intraoperative findings, the disruption or integrity of specific
anatomic structures, and what exactly was done can have significant
subsequent treatment implications. Ideally, any additional or more
invasive procedures should be discussed with the surgeon who will be
devising the definitive treatment plan, to ensure initial procedures
are not counterproductive.
|
TABLE 10-4 Tenets of Débridement
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
tissue injury is the major cause of complications and poor outcomes for
patients with open fractures. Open fractures, because of their exposure
to the environment and periosteal stripping, are predisposed to a
variety of complications. The management of these complications is
complex because there are a multitude of factors that influence acute
treatment. Failures of omission such as not recognizing a fracture as
open may cause the fracture to be treated or débrided less
aggressively, which may favor infection. Although the optimal timing
for the treatment of open fractures remains a matter of controversy,
timely débridement is still favored by most surgeons. Local factors
such as bone or soft issue loss, vascular or nerve injury, or
compartment syndrome may all influence the potential to complications.
after open fractures. Open fractures are commonly seen in high energy
mechanism and in polytraumatized patients. Such patients may have
concomitant head, chest, or abdomen injuries in addition to multiple
fractures. Such polytrauma puts the patient as risk for a systemic
inflammatory response syndrome (SIRS), which may lead to acute
respiratory distress syndrome (ARDS) and/or multisystem organ failure,
which may lead to death. Lengthy initial procedures done on such
polytraumatized patients may exacerbate this systemic inflammatory
response. As such, the concept of damage control orthopaedics has
arisen, in which acute interventions are limited to only necessary
procedures (fasciotomies, irrigation and débridement, provisional
stabilization) to minimize the surgical insult while stabilizing the
patient and achieving skeletal stability. Complex and lengthy
reconstructive procedures are reserved for days later, when the patient
has been resuscitated.
with open fractures. A contamination rate of 65% has been reported,
though the infection rate ranges from 0% to 2% for type I fractures,
through 10% to 50% for type III fractures.117
Any acute infection puts the patient at risk for systemic sepsis and/or
chronic osteomyelitis. Immunocompromised patients (e.g., diabetic
patients, organ transplant patients, those on systemic corticosteroids
or with HIV) are also at increased risk for complications, particularly
infection, after open fracture.19 Malnourished patients may also be at risk.
but edema and a draining wound is a common but not constant finding.
White blood cell count, sedimentation rate and C-reactive protein
levels are commonly elevated. Frankly infected wounds are treated with
irrigation and débridement. It is generally prudent to obtain
radiographs as part of the work up of an infected limb after open
fracture. Radiographs may
lend
insight regarding whether the fixation construct remains stable or
whether revision may be necessary. Also, foreign bodies, air, or
radiolucencies in the bone may be visible. Fulminant infections that do
not respond to irrigation and débridement and lead to sepsis may
require amputation as a lifesaving measure. Controversy remains about
whether to maintain stability and the existing fracture fixation
construct in the face of infection or to remove any hardware that may
harbor organisms in hopes of eradicating the infection.52,95
fractures. In this setting the patient may or may not have an elevated
WBC and the ESR and C-reactive protein may not be as elevated as in an
acute infection. The patient may have vague discomfort and more obvious
signs of infection (edema, draining wounds) are commonly not present.
Chronic osteomyelitis is typically treated with débridement of any and
all infected and necrotic material and may require revision of
previously reconstructed fractures. The metallic surfaces of fracture
fixation implants may require removal as these offer a site for
persistent microorganism colonization.
there are instances when immediate treatment may not be indicated.
Overly aggressively surgery, treating sick polytraumatized patients and
those with poor soft tissues locally may predispose to poor outcomes.
Remember that many of these high energy injuries are seen in
polytraumatized patients who are not physiologically able to withstand
extensive blood loss or time in the operative suite. The concept of
damage control orthopaedics has emerged as a strategy for the treating
the patient as a whole. Brief procedures that can aid in the patient’s
resuscitation through minimizing additional insult and decreasing the
potential for a devastating systemic inflammatory response are
indicated. These include débridements to decrease the load of organisms
and necrotic material; external fixation of long bones, periarticular
fractures, and unstable joints; and fasciotomies for tight muscle
compartments. Performing only brief, safe procedures in the acute phase
allows for better resuscitation of the patient as well as time for
better preoperative planning of complex injuries.
While the open fracture may have failed to unite due to an underlying
infection, nonunion also occurs in the absence of infection and may be
due to devascularization at the fracture site or failure to provide
adequate mechanical stability at the fracture site. Nevertheless,
occult infection should be suspected and investigated as a part of the
workup in the treatment of nonunions. Nonunions may be characterized by
pain at the fracture site and continued decreased function compared
with preinjury.
for residual decrease in function after open fracture, which may be
considered akin to complications. Soft tissue injury to muscle or
tendons may lead to extensive scarring, loss of muscle excursion, and
loss of joint motion. Tendon loss may lead to loss of specific joint
motion and may be amenable to reconstructive tendon transfers.
Similarly, nerve injuries that occur at the time of open fracture—which
may be the direct result of a sharp fracture fragment lacerating the
nerve or are more often the result of a tension or traction injury—may
cause significant morbidity. Loss of sensation, decrease in sensation,
change in sensation, or an area of hyperacute sensation may all occur.
Nerve injuries may also lead to significant motor deficits. Loss of
protective sensation in the setting of a severe open fracture may
influence the decision to amputate or attempt limb salvage in the
setting of massive trauma.
segments are the goal before arrival in the operative suite, in certain
cases this may be deferred. Patients with injuries to the abdomen,
chest, head, or with dysvascular limbs who require emergent surgical
treatment should not have such treatment delayed for extremity
radiographs. These may be obtained in the operative suite using either
fluoroscopy or portable x-ray machines.
the ideal timing for open fracture surgical treatment. We continue to
treat open fractures as emergencies. Bacterial contamination and
proliferation continues until surgical débridement is performed. In
general, infection risk increases with time, although the development
of infection is contingent upon multiple variables. It is left to the
surgeon’s judgment to determine whether to insist that a
polytraumatized patient go to the operating room for emergent
débridement, or whether it is more prudent for the patient to undergo
further resuscitation prior to such treatment. The energy of the
injury, the nature of the open wound, the type of contamination, the
overall condition of the patient, underlying medical conditions, and
the anticipated length of surgery and blood loss are all factors in
this decision.
YB, Nader M, Hamidian-Jahromi AR, Woods DA. The effect of the timing of
antibiotics and surgical treatment on infection rates in open long-bone
fractures: a 9-year prospective study from a district general hospital.
Injury 2007;38(8):900-905.
JO. Comparison of soap and antibiotic solutions for irrigation of
lower-limb open fracture wounds. A prospective, randomized study. J
Bone Joint Surg Am 2005l; 87(7):1415-1422.
P, Marti-Garin D, Murias-Alvarez J, Puente-Alonso C. External fixation
and secondary intramedullary nailing of open tibial fractures. A
randomised, prospective trial. J Bone Joint Surg Br 1997;79(3):433-437.
CP, Sako Y, Scully RE. An evaluation of the surgeon’s criteria for
determining the viability of muscle during débridement. AMA Arch Surg.
1956;73(6):1031-1035.
RU, Mehta JA, Cripps R. Delayed presentation is no barrier to
satisfactory outcome in the management of open tibial fractures. Injury
2004;35(4):411-416.
AW, Hansen ST, Jr. Plates versus external fixation in severe open
tibial shaft fractures. A randomized trial. Clin Orthop Relat Res 1989
Apr(241):89-94.
F, Sr., Baixauli EJ, Sanchez-Alepuz E, Baixauli F, Jr. Interlocked
intramedullary nailing for treatment of open femoral shaft fractures.
Clin Orthop Relat Res 1998 May(350):67-73.
DP, Taitsman LA, Beingessner D, et al. Open diaphyseal long bone
fractures: a reduction method using devitalized or extruded osseous
fragments. J Orthop Trauma 2007;21(8):574-578.
DA, Parikh J. Effect of time delay from injury to primary management on
the incidence of deep infection after open fractures of the lower
extremities caused by blunt trauma in adults. J Orthop Trauma
1993;7(6):532-535.
SK, Agnew SG, Mayo KA, et al. Immediate internal fixation of open,
complex tibial plateau fractures: treatment by a standard protocol. J
Orthop Trauma 1992;6(1): 78-86.
M, Guyatt G, Tornetta P, 3rd, et al. Randomized trial of reamed and
unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint
Surg Am 2008;90(12): 2567-2578.
M, Tornetta P, 3rd, Sprague S, et al. Predictors of reoperation
following operative management of fractures of the tibial shaft. J
Orthop Trauma 2003;17(5): 353-361.
T, Mehta P, Smith M, Pomahac B. Routine use of wound vacuum-assisted
closure does not allow coverage delay for open tibia fractures. Plast
Reconstr Surg 2008;121(4):1263-1266.
PA, Meek RN, O’Brien PJ. External fixation and delayed intramedullary
nailing of open fractures of the tibial shaft. A sequential protocol. J
Bone Joint Surg Am 1990; 72(5):729-735.
T, Olson SA, Lee S, Chapman MW. Nonreamed locking intramedullary
nailing for open fractures of the tibia. Clin Orthop Relat Res 1997
Jun(339):58-64.
LB, Kassman S, Stegemann P, France J. Prospective study of union rate
of open tibial fractures treated with locked, unreamed intramedullary
nails. J Orthop Trauma 1994;8(1):45-49.
JI, 3rd, Wongworawat MD. High-pressure pulsatile lavage causes soft
tissue damage. Clin Orthop Relat Res 2004 Oct(427):13-17.
TJ, Endicott M, Capra SE. Treatment of open ankle fractures. Immediate
internal fixation versus closed immobilization and delayed fixation.
Clin Orthop Relat Res 1989 Mar(240):47-52.
PW, Urban JG. Early weight-bearing treatment of open fractures of the
tibia. An end-result study of 63 cases. J Bone Joint Surg Am
1969;51(1):59-75.
RJ, Ellison PS, Jr., Poka A, et al. Intramedullary nailing of open
fractures of the femoral shaft. J Bone Joint Surg Am
1989;71(9):1324-1331.
RC, Bosse MJ, MacKenzie EJ, Patterson BM. Impact of smoking on fracture
healing and risk of complications in limb-threatening open tibia
fractures. J Orthop Trauma 2005;19(3):151-157.
MW, Mahoney M. The role of early internal fixation in the management of
open fractures. Clin Orthop Relat Res 1979 Jan-Feb(138):120-131.
CP, Siddique I, Zenios M, et al. Early versus delayed surgical
treatment of open tibial fractures: effect on the rates of infection
and need of secondary surgical procedures to promote bone union. Injury
2005;36(5):656-661.
JC, Stapley SA, Bowley DM, et al. Spread of infection, in an animal
model, after intramedullary nailing of an infected external fixator pin
track. J Orthop Res 2001; 19(1):155-159.
WP, 3rd, Fitzgerald RH, Jr., Dobyns JH, Washington JA, 2nd.
Quantitative wound cultures in upper extremity trauma. J Trauma
1982;22(2):112-117.
CM, Christie J, McQueen MM. Closed intramedullary tibial nailing. Its
use in closed and type I open fractures. J Bone Joint Surg Br
1990;72(4):605-611.
CM, McQueen MM, Quaba AA, Christie J. Locked intramedullary nailing of
open tibial fractures. J Bone Joint Surg Br 1991;73(6):959-964.
KE, Limbird TJ, Green NE. Open fractures of the diaphysis of the lower
extremity in children. Treatment, results, and complications. J Bone
Joint Surg Am 1992; 74(2):218-232.
DJ, Kanakaris NK, Giannoudis PV. Débridement and wound closure of open
fractures: the impact of the time factor on infection rates. Injury
2007;38(8):879-889.
BT, Kortesis B, Punger K, et al. The use of negative-pressure wound
therapy (NPWT) in the temporary treatment of soft-tissue injuries
associated with high-energy open tibial shaft fractures. J Orthop
Trauma 2007;21(1):11-17.
AJ, Argenta LC, Marks MW, et al. The use of vacuum-assisted closure
therapy for the treatment of lower-extremity wounds with exposed bone.
Plast Reconstr Surg 2001;108(5):1184-1191.
ES, DeBerardino TM, Brooks DE, et al. Antimicrobial efficacy of
external fixator pins coated with a lipid stabilized
hydroxyapatite/chlorhexidine complex to prevent pin tract infection in
a goat model. J Trauma 2001;50(6):1008-1014.
EP, Caplan ES, Weaver LD, et al. Duration of preventive antibiotic
administration for open extremity fractures. Arch Surg
1988;123(3):333-339.
KL, Norbury K, Kieswetter K, et al. Comparative analysis of global gene
expression profiles between diabetic rat wounds treated with
vacuum-assisted closure therapy, moist wound healing or gauze under
suction. Int Wound J 2008;5(5):615-624.
KF, Hoffman WY, Delgado ED, Contreras DM. Unreamed rod with early wound
closure for grade IIIA and IIIB open tibial fractures: analysis of 40
consecutive patients. Orthopedics 1998;21(5):531-535.
RP, Jr. Treatment of infection after fracture fixation. Opinion: retain
stable implant and suppress infection until union. J Orthop Trauma
2007;21(7):503-505.
RP, Nork SE, Barei DP, Mills WJ. Provisional plating of Type III open
tibia fractures prior to intramedullary nailing. J Orthop Trauma
2005;19(6):412-414.
CC, Simmons SC, Browner BD, Weigel MC. Severe open tibial fractures.
Results treating 202 injuries with external fixation. Clin Orthop Relat
Res 1988 May(230):98-115.
KA, Paksima N, Puopolo S, et al. Treatment of external fixation pins
about the wrist: a prospective, randomized trial. J Bone Joint Surg Am
2006;88(2):349-354.
H, Hauke C, Arens S, et al. PC-Fix and local infection
resistance—influence of implant design on postoperative infection
development, clinical and experimental results. Injury 2001;32(Suppl
2):B38-43.
AC, Thomas D, Cunningham B, et al. Stability of reamed and unreamed
intramedullary tibial nails: a biomechanical study. Injury
1995;26(7):483-485.
CG, Schmidt AH, Kyle RF, et al. A prospective, randomized study of
intramedullary nails inserted with and without reaming for the
treatment of open and closed fractures of the tibial shaft. J Orthop
Trauma 2000;14(3):187-193.
JL, Johnson KD, Hansen ST, Jr. Immediate internal fixation of open
ankle fractures. Report of 38 cases treated with a standard protocol. J
Bone Joint Surg Am 1984;66(9):1349-1356.
MJ, Mehta S, Barei DP, Nork SE. Treatment protocol for open AO/OTA type
C3 pilon fractures with segmental bone loss. J Orthop Trauma
2008;22(7):451-457.
S, Majumder S, Batchelor AG, et al. Fix and flap: the radical
orthopaedic and plastic treatment of severe open fractures of the
tibia. J Bone Joint Surg Br 2000;82(7): 959-966.
O, Utvag SE, Reikeras O. Effects of graded reaming on fracture healing.
Blood flow and healing studied in rat femurs. Acta Orthop Scand
1994;65(1):32-36.
O, Utvag SE, Reikeras O. Restoration of bone flow following fracture
and reaming in rat femora. Acta Orthop Scand 1994;65(2):185-190.
RB, Anderson JT. Prevention of infection in the treatment of 1025 open
fractures of long bones: retrospective and prospective analyses. J Bone
Joint Surg Am 1976; 58(4):453-458.
EJ, Agel J, Selznick HS, et al. Deleterious effect of smoking on
healing of open tibia-shaft fractures. Am J Orthop 2002;31(9):518-521.
PJ, Giannoudis PV, Probst C, et al. The risk of local infective
complications after damage control procedures for femoral shaft
fracture. J Orthop Trauma 2006; 20(3):181-189.
CJ, Adams CA, Jr., Eachempati SR. Surgical Infection Society guideline:
prophylactic antibiotic use in open fractures: an evidence-based
guideline. Surg Infect (Larchmt) 2006;7(4):379-405.
KA, Infante AF, Walling AK, Sanders RW. Open fractures of the
calcaneus: soft-tissue injury determines outcome. J Bone Joint Surg Am
2003;85-A(12):2276-2282.
C, Patzakis MJ, Tetsworth KD, Levin LS. Musculoskeletal sepsis:
principles of treatment. Instr Course Lect 2003;52:733-743.
MB, Chapman JR, Agel J, et al. Treatment of type II, IIIA, and IIIB
open fractures of the tibial shaft: a prospective comparison of
unreamed interlocking intramedullary nails and half-pin external
fixators. J Orthop Trauma 1998;12(1):1-7.
SL, Ostermann PA, Seligson D. The antibiotic bead pouch technique. The
management of severe compound fractures. Clin Orthop Relat Res 1993
Oct(295):54-62.
D, Jr., Sanders RW, Scaduto JM, et al. Vacuum-assisted wound closure
(VAC therapy) for the management of patients with high-energy soft
tissue injuries. J Orthop Trauma 2003;17(10):683-638.
E, Tetsworth K, Radziejowski MJ, Wiesniewski TF. Comparison of delayed
and primary wound closure in the treatment of open tibial fractures.
Arch Orthop Trauma Surg 2007;127(2):131-136.
JL, Swiontkowski MF, Sanders R. Treatment of open fractures of the
tibial shaft: ender nailing versus external fixation. A randomized,
prospective comparison. J Bone Joint Surg Am 1989;71(8):1231-1238.
TM, Aksenov SA, Schemitsch EH. Effect of limited and standard reaming
on cortical bone blood flow and early strength of union following
segmental fracture. J Orthop Trauma 1998;12(6):400-406.
TM, Weinberg JA, Aksenov SA, Schemitsch EH. Effect of unreamed, limited
reamed, and standard reamed intramedullary nailing on cortical bone
porosity and new bone formation. J Orthop Trauma 2001;15(1):18-27.
J, Moore T. Anatomy of the distal knee joint and pyarthrosis following
external fixation. J Orthop Trauma 1999;13(4):241-246.
KJ, Banks DM, Phieffer LS, et al. Evaluation of standard surgical
preparation performed on superficial dermal abrasions. J Orthop Trauma
2000;14(3):206-211.
EE, Davlin LB. Open ankle fractures. The indications for immediate open
reduction and internal fixation. Clin Orthop Relat Res 1993
Jul(292):118-127.
S, Tornetta P, 3rd. Open fractures of the tibia treated by immediate
intramedullary tibial nail insertion without reaming: a prospective
study. J Orthop Trauma 2007; 21(3):153-157.
JF, O’Brien PI, Blachut PA, et al. Reamed interlocking intramedullary
nailing of open fractures of the tibia. Clin Orthop Relat Res 1997
May(338):182-191.
JF, O’Brien PJ, Blachut PA, et al. Locking intramedullary nailing with
and without reaming for open fractures of the tibial shaft. A
prospective, randomized study. J Bone Joint Surg Am 1997;79(3):334-341.
GR, Boody AR, Wongworawat MD, Jobe CM. The accuracy of the saline load
test in the diagnosis of traumatic knee arthrotomies. J Orthop Trauma
2007;21(7): 442-443.
AY, Shelton ML. Primary internal fixation of open fractures: a
retrospective study of the use of metallic internal fixation in fresh
open fractures. J Trauma 1972; 12(9):756-763.
DV, Bower CE, Reade CC, et al. Effect of vacuum-assisted closure
therapy on early systemic cytokine levels in a swine model. Wound
Repair Regen 2006;14(2): 210-215.
MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus nonreaming
in medullary nailing: interference with cortical circulation of the
canine tibia. Arch Orthop Trauma Surg 1990;109(6):314-316.
L, Rancan M, Mica L, et al. Vacuum-assisted closure therapy increases
local interleukin-8 and vascular endothelial growth factor levels in
traumatic wounds. J Trauma 2009;66(3):749-757.
S, Ricci WM. Treatment of infection after fracture fixation. Opinion:
two-stage protocol: treatment of nonunion after treatment of infection.
J Orthop Trauma 2007; 21(7):505-506.
A, Temple J, Santy J. Pin site care for preventing infections
associated with external bone fixators and pins. Cochrane Database Syst
Rev 2008(4):CD004551.
XY, Li WZ, Li YJ, et al. [The influence of vacuum-assisted drainage on
the growth of capillaries in the wound produced by explosion in pig].
Zhonghua Shao Shang Za Zhi 2007;23(4):292-295.
DJ, Copeland GE, Zlowodzki M, Cole PA. The application of
dermatotraction for primary skin closure. Am J Surg 2005;190(1):123-126.
PD, Saleh M, Douglas DL. Risk of deep infection with intramedullary
nailing following the use of external fixators. J R Coll Surg Edinb
1991;36(4):268-271.
DJ, Merkow RL, Gustilo RB. Infection after intramedullary nailing of
severe open tibial fractures initially treated with external fixation.
J Bone Joint Surg Am 1989; 71(6):835-838.
MD, Wild LM, Schemitsch EH, Waddell JP. The use of an
antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute
in the treatment of infected long bone defects: early results of a
prospective trial. J Orthop Trauma 2002;16(9):622-627.
AK, Schmidt M, Feeley T, Kieswetter K. Effects of negative pressure
wound therapy on fibroblast viability, chemotactic signaling, and
proliferation in a provisional wound (fibrin) matrix. Wound Repair
Regen 2007;15(6):838-846.
HD, Gravel C, Chapman MW, Olson SA. Comparison of antibiotic beads and
intravenous antibiotics in open fractures. Clin Orthop Relat Res 2000
Mar(372): 254-261.
MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a
new method for wound control and treatment: animal studies and basic
foundation. Ann Plast Surg 1997;38(6):553-562.
MJ, David LR, Schneider AM, et al. Use of subatmospheric pressure to
prevent progression of partial-thickness burns in a swine model. J Burn
Care Rehabil 1999;20(1 Pt 1):15-21.
D, Thompson RE, Frauchiger VM, et al. Anodic plasma chemical treatment
of titanium Schanz screws reduces pin loosening. J Orthop Trauma
2005;19(8):543-550.
T, Yokoyama K, Ohtsuka H, et al. Intramedullary nailing for open
fractures of the femoral shaft: evaluation of contributing factors on
deep infection and nonunion using multivariate analysis. Injury
2005;36(9):1085-1093.
PJ, Turen CH, Brumback RJ, Scarboro JM. Conversion of external fixation
to intramedullary nailing for fractures of the shaft of the femur in
multiply injured patients. J Bone Joint Surg Am 2000;82(6):781-788.
PJ, Meek RN, Powell JN, Blachut PA. Primary intramedullary nailing of
open femoral shaft fractures. J Trauma 1991;31(1):113-116.
PA, Seligson D, Henry SL. Local antibiotic therapy for severe open
fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br
1995;77(1):93-97.
BM, Matros E, Pribaz JJ, Orgill DP. Lower extremity trauma: trends in
the management of soft-tissue reconstruction of open tibia-fibula
fractures. Plast Reconstr Surg 2006;117(4):1315-1322; discussion 23-24.
MJ, Harvey JP, Jr., Ivler D. The role of antibiotics in the management
of open fractures. J Bone Joint Surg Am 1974;56(3):532-541.
FR, Kubiak EN, Sathappan SS, Di Cesare PE. Topical negative pressure in
the treatment of infected wounds with exposed orthopaedic implants. J
Wound Care 2006; 15(3):111-116.
B, Jeray K, Schemitsch E, et al. Fluid lavage in patients with open
fracture wounds (FLOW): an international survey of 984 surgeons. BMC
Musculoskelet Disord 2008;9:7.
S, Dheenadhayalan J, Babu JN, et al. Immediate primary skin closure in
type-III A and B open fractures: results after a minimum of 5 years. J
Bone Joint Surg Br 2009;91(2):217-224.
FW, Roberts MD. Primary closure of open fractures of the tibia and
fibula by fibular fixation and relaxing incisions. J Trauma
1970;10(10):853-866.
RB, Field P. The effects of continuous compression on living articular
cartilage: an experimental investigation. J Bone Joint Surg Am
1960;42:31-90.
RB, Simmonds DF, Malcolm BW, et al. The biological effect of continuous
passive motion on the healing of full-thickness defects in articular
cartilage. An experimental investigation in the rabbit. J Bone Joint
Surg Am 1980;62(8):1232-1251.
R, Jersinovich I, Anglen J, et al. The treatment of open tibial shaft
fractures using an interlocked intramedullary nail without reaming. J
Orthop Trauma 1994; 8(6):504-510.
A, Horowitch A, Aboulafia A, et al. Functional bracing for comminuted
extra-articular fractures of the distal third of the humerus. J Bone
Joint Surg Br 1990; 72(2):283-287.
A, Chan CK, Penafort R, Sengupta S. A simple practical protocol for
care of metalskin interface of external fixation. Med J Malaysia
2006;61(Suppl A):62-65.
V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations
of wounds and cell proliferation. Plast Reconstr Surg
2004;114(5):1086-1096; discussion 97-98.
EH, Kowalski MJ, Swiontkowski MF, et al. Comparison of the effect of
reamed and unreamed locked intramedullary nailing on blood flow in the
callus and strength of union following fracture of the sheep tibia. J
Orthop Res 1995;13(3): 382-389.
EH, Kowalski MJ, Swiontkowski MF, et al. Cortical bone blood flow in
reamed and unreamed locked intramedullary nailing: a fractured tibia
model in sheep. J Orthop Trauma 1994;8(5):373-382.
AH, Swiontkowski MF. Pathophysiology of infections after internal
fixation of fractures. J Am Acad Orthop Surg 2000;8(5):285-291.
FJ, Mullett H, O’Rourke K. Unreamed intramedullary nail versus external
fixation in grade III open tibial fractures. J Trauma
2002;52(4):650-654.
RW, Kellam JF. Open tibial diaphyseal fractures. Results of unreamed
locked intramedullary nailing. Clin Orthop Relat Res 1995
Jun(315):114-118.
S, Stromsoe K, Alho A, et al. Acute compartment syndrome: for how long
can muscle tolerate increased tissue pressure? Eur J Surg
1992;158(8):437-438.
J, Smith A, Woods D. The effect of time delay on infection in open
long-bone fractures: a 5-year prospective audit from a district general
hospital. Ann R Coll Surg Engl 2004;86(2):108-112.
IS. The versatility of negative pressure wound therapy with reticulated
open cell foam for soft tissue management after severe musculoskeletal
trauma. J Orthop Trauma 2008;22(10 Suppl):S146-151.
A, Zimmerli W. Diagnosis and treatment of infections associated with
fracture-fixation devices. Injury 2006;37(Suppl 2):S59-66.
CH, Burgess AR, Vanco B. Skeletal stabilization for tibial fractures
associated with acute compartment syndrome. Clin Orthop Relat Res 1995
Jun(315):163-168.
CP, Chattar-Cora D, O’Neill A, et al. Efficacy of primary wound
cultures in long bone open extremity fractures: are they of any value?
Arch Orthop Trauma Surg 2002;122(5):259-261.
H, Dingeldein E, Bergmann R, Reuss K. The release of gentamicin from
polymethylmethacrylate beads. An experimental and pharmacokinetic
study. J Bone Joint Surg Br 1978;60-B(2):270-275.
OH, Wangensteen SD. The Rise of Surgery from Empiric Craft to
Scientific Discipline. Minneapolis: University of Minnesota Press, 1978.
A, Toksvig-Larsen S. Pin site care in external fixation sodium chloride
or chlorhexidine solution as a cleansing agent. Arch Orthop Trauma Surg
2004;124(8): 555-558.
A, Toksvig-Larsen S, Lindstrand A. No difference between daily and
weekly pin site care: a randomized study of 50 patients with external
fixation. Acta Orthop Scand 2003;74(6):704-708.
LX, Dedmond B, Schlatterer D, et al. The contaminated high-energy open
fracture: a protocol to prevent and treat inflammatory mediator
storm-induced soft-tissue compartment syndrome (IMSICS). J Am Acad
Orthop Surg 2006;14(10 Spec No.): S82-86.
LX, Pape HC. Current thought regarding the mechanism of action of
negative pressure wound therapy with reticulated open cell foam. J
Orthop Trauma 2008;22(10 Suppl):S135-137.
EF, Court-Brown CM. Primary external fixation and secondary
intramedullary nailing in the treatment of tibial fractures. Injury
1992;23(6):373-376.
R, Zhao Y, Kieswetter K, et al. Effects of dressing type on 3D tissue
microdeformations during negative pressure wound therapy: a
computational study. J Biomech Eng 2009;131(3):031012.
MM, Askins V, Hinkes EW, et al. Primary reamed intramedullary nailing
of open femoral shaft fractures. Clin Orthop Relat Res 1995
Sep(318):182-190.
P. The prevention of infection in open fractures. In: Bunker TD, Colton
CL, Webb JK, eds. Frontiers in Fracture Management. Cambridge:
Cambridge University Press, 1989.
BA, Roberts CS, Seligson D, et al. Cost of antibiotic beads is
justified: a study of open fracture wounds and chronic osteomyelitis. J
Long Term Eff Med Implants 2007;17(3):181-185.
JL, Price MF, Chuang PI, et al. Inhibition of povidone-iodine’s
bactericidal activity by common organic substances: an experimental
study. Surgery 1985;98: 25-29.
J, Wanich T, Gardner M, et al. PMMA is superior to hydroxyapatite for
colony reduction in induced osteomyelitis. Clin Orthop Relat Res
2007;462:190-194.
BH, Darowish M, Klatt BA, et al. Intramedullary nailing in open tibia
fractures: a comparison of two techniques. Int Orthop
2004;28(4):235-238.
