DUPUYTREN’S DISEASE
been Dupuytren’s contracture in the hands of a stone mason working in a
quarry near Basel, Switzerland (41). He identified the disease in the flexor tendons and in fact could have been describing a rupture of the A1
pulley. Other well-known surgeons, such as Henry Cline and Sir Astley
Cooper, described the condition during the next century and correctly
located the disease in the palmar fascia.
located the disease in the palmar fascia but also speculated that the
etiology might be traumatic. He recommended surgical treatment of the
diseased fascia through transverse incisions and postoperative
splinting with the fingers held in extension. In addition to reporting
the condition, he also lectured extensively on it (8,9). Thus, the choice of “Dupuytren” as an eponym, instead of a descriptive term such as “palmar fibromatosis,” is justifiable.
disease, including the potential role of heredity, trauma,
myofibroblasts, collagen, growth factors, and smooth muscle (which
contributes to the contractile nature of the condition).
rewarding, but careful consideration must be given to patient
selection, history, physical findings, and the patient’s ultimate goals
from both functional and cosmetic perspectives.
disease, the Groupe d’Etude de la Main (GEM) monograph edited by
Hueston and Tubiana (22), an article by Whaley and Elliot in the British Journal of Hand Surgery (52), and Dupuytren’s Disease: Biology and Treatment by McFarlane, McGrouther, and Flint (11) provide the complete history.
believed that microhemorrhages and hemosiderin were part of the
pathophysiology of Dupuytren’s disease (27). He
was able to produce similar histologic lesions in monkeys, but the
animals never developed flexion contractures. Although trauma is often
implicated in the etiology, studies have also shown that the disease
occurs in almost equal frequency in manual and nonmanual workers (17,18).
Low levels of superoxide may stimulate fibroblast growth, which may
also be important in wound healing and the evolution of scar.
regarding the pathophysiology of Dupuytren’s contracture involves the
myofibroblast. Gabbiani and Majno called attention to this cell in 1972
(13). They were able to measure the contractile
forces of myofibroblasts grown in tissue culture and found them
comparable with that of rabbit skeletal muscle. They believed the
myofibroblast was the driving force behind the palmar fascial
contraction.
elaborated on the pathophysiology, believing that the myofibroblasts
contained elements of smooth muscle as well as collagen-producing
fibroblasts. As the disease progressed, the contractile elements
shortened the collagen. Concurrently, intercellular adhesion occurred
in areas known as desmosomes, which contained fibronectin, a gluelike
material that promotes intercellular adherence. Burch believed that
proliferating fibroblasts were the consequence of an autoimmune
response to collagen by “forbidden” lymphocytes (4).
into three stages: stage I, early disease with proliferation of
perivascular fibroblasts; stage II, active disease, with nodular
thickening of the palmar fascia, associated joint contracture, and
hypertrophied fibroblasts as well as orderly deposition of collagen
fibers; and stage III, advanced disease, with progressive joint
contracture and diffuse fibrotic thickening of the palmar fascia.
Histologically, wavy bundles of collagen fibers separating a sparse
number of fibroblasts become apparent (6).
myofibroblasts with their contractile elements, the collagen found in
Dupuytren’s disease is identical to that which predominates in scar
tissue. For example, normal palmar fascia contains almost no type III
collagen. Approximately 30% of the collagen in Dupuytren’s nodules is
type III; in uninvolved regions of the palmar fascia in patients with
Dupuytren’s disease, 10% to 15% of the collagen is type III (14).
a ground substance composed of proteoglycans, proteins, and
hyaluronate. The exact molecular structure of proteoglycans in palmar
fascia has not yet been described, but basic knowledge of proteoglycans
isolated from similar tissue can be extrapolated. Large proteoglycans
primarily contain chondroitin sulfate; smaller proteoglycans contain
dermatan sulfate. They bind to a hyaluronate similar to proteoglycans
in collagen.
sulfate but do not bind to hyaluronate. In the palmar fascia, the
matrix is produced by local cells that synthesize the components from
basic substrates, such as glucose and amino acids. In Dupuytren’s
disease, certain factors may inhibit or activate these metabolic
pathways. Generally, a total increase in the amount of
glycosaminoglycan occurs.
fascia has been the subject of speculation and investigation in the
development of Dupuytren’s disease. Its teleologic role seems to be to
cushion forces transmitted through the skin to the fascia. As a
response to micro- (and macro-) trauma, it becomes involved in
increased vascular proliferation. Rabinowitz et al. (42) have shown that various enzymatic changes may play a role in the etiology of Dupuytren’s disease.
implicated in the pathophysiology of Dupuytren’s disease. For example,
transforming growth factor-β plays a possible role, as does epidermal
growth factor (24). In 1992, Badalamente et al. (1)
focused on the role of platelet-derived growth factor as a cellular
signal for myofibroblast proliferation in the formation of Dupuytren’s
nodules. Finally, Tomasek and Rayan in 1995 (48) correlated the smooth-muscle-like phenotype of fibroblasts in Dupuytren’s disease with increased contractility.
to exist, but the exact genetic pattern is unknown. Controversy
surrounds the theory that the pattern is autosomal dominant with high
penetrance. The disease is 6- to 10-fold more prevalent in men than in
women (51). When Ling interviewed his patients,
they reported that 16% of their relatives had Dupuytren’s disease.
Careful examination of the relatives, however, revealed the incidence
was 68%! Despite this finding, Ling was unable to prove a clear mode of
genetic transmission (28).
a more virulent form of Dupuytren’s disease. For example, in patients
in their teens or 20s, Hueston has referred to the disease as a
“Dupuytren’s diathesis” (21). This may include
plantar fibromatosis (Ledderhose’s disease), penile fibromatosis
(Peyronie’s disease), and knuckle pads. These conditions should be
sought out during the history and physical examination of the patient
because it has been found that they are predictive of a poorer
prognosis.
resembles that of an aggressive fibromatosis. When seen in children,
this condition may resemble Dupuytren’s disease histologically but
predictably will have an extremely virulent clinical course. A similar
condition, juvenile aponeurotic fibroma (31,49),
has been noted to recur as fibrosarcoma with metastatic disease and
death. Certainly, the combination of a young boy with a strong family
history and an early onset of contracture carries a poor prognosis.
Likewise, when the disease appears in young women, it tends to be more
virulent and recurrent. Although this is generally true in younger
women, in my experience older women do not fare much differently from
men in terms of natural history. The presence of osteoarthritis in the
proximal interphalangeal (PIP) or metacarpophalangeal (MP) joints
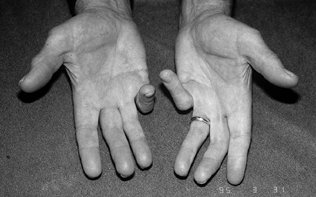
compromises the final result by limiting the eventual range of motion (Fig. 62.1).
 |
|
Figure 62.1.
Persistent PIP flexion contracture in the right small finger with previous amputation of the left small finger in a 27-year-old man with seizure disorder. |
Dupuytren’s disease—acquired immune deficiency syndrome (AIDS), for
example. Free radicals have been linked to the apparent relationship
between Dupuytren’s disease and human immunodeficiency virus
(HIV)–infected patients (3). Other frequently
associated diseases include diabetes mellitus, tuberculosis, epilepsy,
and alcoholism. Dupuytren’s disease has been associated with many other
diseases, with no clear causal relationship. It is particularly
important to document these associated conditions in obtaining a
patient history because some of these conditions may ultimately affect
the prognosis (39).
were concerned that the coexistence of Dupuytren’s disease with carpal
tunnel syndrome might predispose to postoperative complications, such
as increased swelling and complex regional pain syndrome. For this
reason, they suggested minimizing complications by treating the more
significant clinical problem—carpal tunnel syndrome or Dupuytren’s
disease—first. Michon (36) believed that carpal tunnel in Dupuytren’s disease could safely be released concomitantly, as did Gonzalez and Watson (16).
which would affect postoperative management. Hyperextension of the
distal interphalangeal (DIP) joint with associated PIP joint
contracture in Dupuytren’s disease should alert the examiner to the
possibility of boutonniere. If the DIP joint can be passively flexed
following PIP joint release, then a PIP extension splint and a program
of passive flexion should correct the boutonniere (Fig. 62.2).
Postoperative management should focus on maintaining the PIP joint in
full extension as well as concomitant passive flexion of the DIP joint
to pull the lateral bands to their more anatomic position dorsal to the
axis of PIP joint motion.
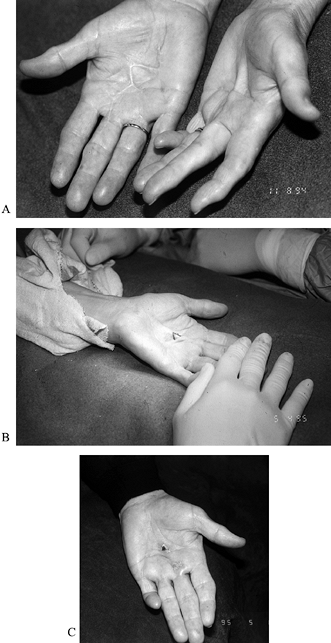 |
|
Figure 62.2. A: Persistent Dupuytren’s contracture in the palm of a 71-year-old woman with severe arteriosclerotic cardiovascular disease. B: Z-plasty of skin contracture with subcutaneous fasciotomy while the patient was under local anesthesia. C: Improved extension postoperatively.
|
surgically, with the Dupuytren’s contracture removed if present.
Conversely, Dupuytren’s nodules need not be excised at the time of A1 pulley release (5).
such as coronary artery disease or history of cerebral vascular
accident, need not cancel surgery but rather should have it done under
local anesthesia, with appropriate sedation. The fasciotomy may be
limited, if necessary, and only the affected digits released. Limited
fasciotomy has occasionally been helpful for such patients (Fig. 62.3) (16,37).
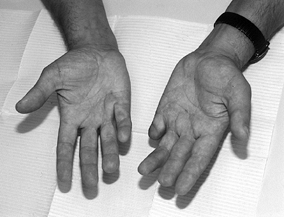 |
|
Figure 62.3.
A 27-year-old man with seizure disorder and recurrent Dupuytren’s disease in both hands. Note previous amputation of the left small finger at the PIP joint level and recurrent flexion contracture of the PIP joint in the right small finger after two previous open fasciectomies. This patient was counseled to continue stretching his right hand and advised to use nonsteroidal medication. No further surgery has yet been required. |
problems of insulin management as well as a slight increased risk of
postoperative wound infection. In the
patient
with diabetes, distinguishing between Dupuytren’s disease and trigger
finger is important. Finally, patients with seizure disorder have a
high likelihood of recurrence and a tendency toward a more aggressive
form of the disease (Fig. 62.4) (45).
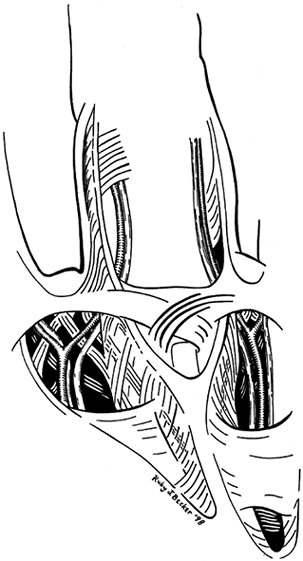 |
|
Figure 62.4.
Normal anatomy of the palm showing lattice-like arrangement of fascial bands surrounding the common digital nerve and artery at the level of bifurcation. |
the volar surface of the hand, with four longitudinal thickened fascial
segments corresponding to the individual finger metacarpals. There is a
separate extension toward the thumb and thenar musculature. The fascia
is attached to the skin of the palm by vertical septae and to the
deeper structures of the hand, the flexor sheath, and intrinsic
musculature through similar longitudinal septae, the ligaments of
Legueu and Juvara.
terminate at the proximal finger crease of the skin. The superficial
transverse fasciculi, as well as a discrete deep transverse fiber
layer, are proximal to the termination and perpendicular to the
longitudinal fasciculi.
distally and the finger flexion crease, the palmar fascia divides into
smaller fascial elements extending onto the finger to join Cleland’s
ligament, Grayson’s ligament, and the lateral digital sheath of Gosset (43) (Fig. 62.5).
The fascia “spirals” around the neurovascular bundle in this region,
and some authors refer to this normal fascial tissue as a “spiral
band.” Located more transversely in the web space, the “natatory
ligament” runs from the deeper palmar fascia across the skin of the web
and becomes adherent to the skin within the web space.
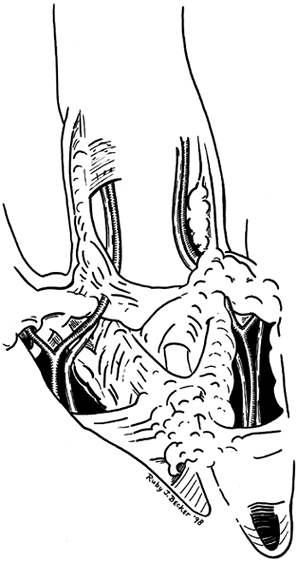 |
|
Figure 62.5.
Pathologic anatomy in which fascial bands have coalesced into fascial cords surrounding the neurovascular bundle. Depending on which bands are involved and to what extent, the neurovascular bundle may be drawn toward the midline in a subcutaneous position, making it vulnerable to surgical dissection. |
(50; D. A. McGrouther, personal communication). Rather, the fascia
reaches the finger through a confluence of attachments to tendon
sheath, intrinsic muscle fascia, and skin. McGrouther has drawn the
analogy between these fascial structures and a wooden lattice
configuration that can flex and extend with the fingers, thereby
becoming longer or shorter with normal function.
The central part of the finger is not covered by a fascial band but
rather by a fibrofatty tissue that may become involved with Dupuytren’s
disease. Gosset described the continuation of the vertical septae of
Legueu and Juvara and described them blending with the capsule and the
MP joint and extending along the side of the finger to the lateral
digital sheet. Continuations of the natatory ligament are confluent
with this from the web space. Each of these structures can become
involved in the contracture.
the diseased fascia in Dupuytren’s disease does not explain how a
nodule becomes a cord but does quite clearly define the anatomy of the
central cord, which is the diseased longitudinal band of the normal
palmar fascia, known as the pretendinous band. This cord may extend to
the finger, involving the fibrofatty tissue, with the volar surface of
the finger becoming confluent with the central cord. This cord usually
passes
distally in the midline of the finger and may be adherent to the
Grayson’s ligament, tendon sheaths, the lateral digital sheet, or the
skin of the middle phalanx, potentially resulting in MP or PIP flexion
deformity or both (Fig. 62.6).
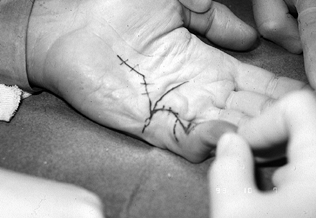 |
|
Figure 62.6.
Severe PIP contracture and MP contracture of the left small finger. A longitudinal incision is directed distally on the finger and broken up into Z-plasty at the level of the PIP joint to facilitate release of the skin contracture. |
contracture of the web space and thus making spreading of the fingers
difficult. This is common in Dupuytren’s disease. On the finger, the
lateral digital sheet and Grayson’s ligament become the lateral cord.
This cord is proximally adherent to the diseased palmar fascia via a spiral cord.
This term describes the path of the diseased fascia as it surrounds the
neurovascular bundle, pulling it toward the midline of the finger and
often leaving it in a vulnerable subcutaneous position. This spiral
cord more often affects the neurovascular bundle when a PIP contracture
greater than 30° exists (33).
complaints may be of the presence of a tender nodule. Later, patients
may have difficulty retrieving objects from their pockets, wearing
gloves, or shaking hands. A tender nodule alone is a relative
contraindication to surgery, as removal may result in recurrence and a
tender scar. Once the patient is no longer able to place her hand flat
on a table top (Hueston’s positive table-top test), consider surgery.
This sign is usually associated with an MP joint contracture of 30° or
more.
for surgery, particularly in young patients. It is quite difficult to
obtain good PIP joint function if the condition is left untreated and
allowed to progress. The patient must understand, however, that surgery
is palliative, and eventual recurrence is probable. In younger patients
with a positive family history, the disease is almost certain to recur.
Careful splinting (particularly at night) for 6 to 12 weeks after
surgery may help prevent recurrent contracture even in the face of
recurrent disease.
to explore the goals and expectations of both the patient and surgeon
as to the eventual outcome of treatment. Although surgical treatment is
available and may have a limited role in some patients, uniform success
has not been reported (12,20,47,53,54).
I have found that cortisone injection in the isolated painful palmar
nodule is somewhat helpful in relieving local symptoms. In the natural
course of the disease, the pain in the nodule disappears. Whether
contracture occurs is not predictable.
general health, and whether previous surgery has been performed, the
choice of anesthesia may vary. Anesthesia may be an axillary block,
general endotracheal, or monitored anesthesia care in which the surgeon
places a wrist block or a digital block plus local anesthesia. Sedation
with Diprovan (propothol) or Versed (midazolam hydrochloride) may be
used. Bier block may be suitable in certain circumstances, but in my
experience the intravenous fluid from the anesthetic often compromises
the surgical field. The patient rarely tolerates the tourniquet for
more than 30 to 60 min, making Bier block comparable to wrist block,
digital block, or local anesthesia with monitored anesthesia care.
Regardless of the choice of anesthetic, if the procedure is not
completed within 2 hours and the tourniquet must be deflated,
dissection becomes increasingly difficult.
-
Make a transverse incision over the
diseased ray or rays in or immediately adjacent to the distal palmar
crease. Extensions of the incision toward the involved digit and
proximally in the palm may be through either zigzag or straight-line
incisions, with the straight-line incisions designed for later Z-plasty (Fig. 62.7).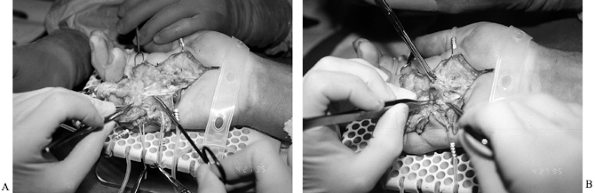 Figure 62.7. A: Dissection from proximal to distal, with the diseased fascia held with an Allis clamp. B:
Figure 62.7. A: Dissection from proximal to distal, with the diseased fascia held with an Allis clamp. B:
Underlying preserved common digital nerve at the level of bifurcation.
Note intimate involvement between diseased fascia still held by Kocher
and underlying digital nerve. A relatively large pacinian corpuscle is
located just proximal to the bifurcation. Pacinian corpuscles, usually
visible to the naked eye and under 3.5- or 2.5-power loupe
magnification, are ideal landmarks to alert the surgeon to the near
proximity of the digital nerve. -
Bruner-type incisions may be used when
the PIP joint is not involved. When the incision must be extended
distally on the finger to the PIP joint or further, use a
P.1741
straight, longitudinal incision broken into the appropriate number of Z-plasties. In the palm the Bruner-type incision lends itself well to the V-Y closure (23). -
When the longitudinal incision is used, and a PIP contracture is involved in Dupuytren’s disease, employ a classic 60° Z-plasty
at the level of the PIP joint. This technique theoretically allows a
75% gain in length when the joint is mobilized. Once the joint has been
mobilized, the transverse line of the Z-plasty should lie at or close to the level of the PIP joint. -
Leave the wound open, like the distal
palmar wound, to facilitate drainage and avoid tension on the wound
closure. Normal skin has the potential for plastic deformation, but
diseased skin of Dupuytren’s disease rarely does. -
Begin exposing the diseased fascia
through the proximal incision at the base of the palm, which should be
extended to include normal fascia. Using the basic, general surgical
principle of dissecting from normal toward abnormal tissue, identify
the distal margin of the transverse carpal ligament, superficial palmar
arch, and associated common digital arteries and nerves and trace them
distally through the lumbrical canals. -
As you encounter diseased fascia,
carefully separate it from the skin and underlying tissues, dividing
the diseased superficial fibrous sepae as well as the diseased tissue
deep within the palm. -
It is frequently possible to identify the
ligaments of Legueu and Juvara. Divide them close to intrinsic muscle
or tendon sheath, removing a small segment of normal tissue,
particularly when dense adherence exists between the diseased tissue
and the flexor sheath. -
Perform the majority of the dissection
with a #15 blade, beaver blade, or sharp tenotomy scissors. Maintain
tension on the diseased fascia, holding it superficially with a
single-tooth forceps, a Kocher clamp, or an Allis clamp (Fig. 62.7). -
Maintain traction on the skin flaps using
single- or double-hook retraction and with the aid of a specialized
surgical hand table if an assistant is unavailable. -
Place sterile rubber bands or vessel
loops around the neurovascular bundles to protect them and mark their
location. At the level of the transverse incision and approaching the
MP joint, be particularly alert for the possibility of a “spiral cord”
that displaces the common digital nerve to the involved digits in a
superficial position. Clinically, a “soft pulpy mass” as described by
Short and Watson should alert the surgeon to the presence of this
structure (44). Often, the neurovascular bundle
will appear to pass directly through the diseased tissue; in fact, in a
recurrence, it often does. -
Use magnification, a 3.5-power loupe, or
an operating microscope to better visualize the neurovascular bundle.
Carefully protect the nerve and sharply dissect it longitudinally from
the surrounding tissue. The nerve frequently turns acutely 45° or 90°
as it is deformed by the diseased collagen, making dissection tedious. -
As the dissection is carried onto the finger, mobilize the Z-plasty incisions, which should have been outlined before the surgical dissection. In general, cuts between
P.1742
5 mm and 10 mm in length are sufficient and designed such that with closure of the Z-plasty the transverse limb lies at or near the PIP flexion crease. -
Ideally, the entire segment of diseased
fascia with the involved ray is removed in one segment from proximal to
distal. The distal diseased fascia may adhere to the flexor sheath,
collateral ligaments of the PIP joint, lateral digital sheet of Gosset,
or Grayson’s ligaments. It rarely extends dorsally and should not
involve Cleland’s ligaments. -
Depending on the length of the procedure
and your confidence in having maintained adequate hemostasis with
bipolar electrocautery through the course of the procedure, you may
elect to close a portion of the wound before deflating the tourniquet. -
With the open-palm technique, leave the
entire transverse incision open. With this technique, hematoma
accumulation in the palm is unlikely; likewise with the fingers in the
case of distal Z-plasty, where the
transverse limb is left open. With a tighter closure, meticulous
hemostasis following tourniquet deflation and before wound closure is
more important. -
I prefer to close the wound with 5-0 nylon sutures placed in a horizontal or vertical mattress fashion.
-
Apply a bulky dressing loosely with a
dorsal splint and encourage the patient to flex and extend the fingers
when he awakens in the recovery room or when the axillary block wears
off. -
Change the dressing within 24 to 48
hours. Provide the patient detailed instructions for wound care and for
a home exercise program. Remove sutures in 7 to 10 days. An outpatient
hand therapy program is crucial in obtaining good results.
the dense fascial attachments between the skin and the palm that may be
involved in Dupuytren’s disease, make rotation flaps difficult if not
impossible. The central palmar skin has been shown by microangiography
to have limited circulation (7). Any surgical
procedure, therefore, should limit the amount of undermining of the
skin in this region and avoid straight longitudinal incisions that may
predispose to later contracture.
incisions to treat Dupuytren’s disease are described than are detailed
here. In a recent textbook, McGrouther lists more than 40 surgical
techniques that have been published since Dupuytren’s original
description (34). All involve variations on zig-zag incisions, Z-plasties, local rotation flaps, and V-Y–plasty. This latter procedure, originally described by Palmen (40), has been more recently popularized by King et al. (23).
The goal of this and any wound closure in Dupuytren’s disease should be
to avoid tension while maintaining circulation to and viability of the
flap.
-
Outline an incision from an area just
proximal to the involved fascia in the involved finger and zig-zag
distally at angles between 90° and 110° onto the finger and distal to
the diseased fascia. Transverse incisions extend off the apex of the
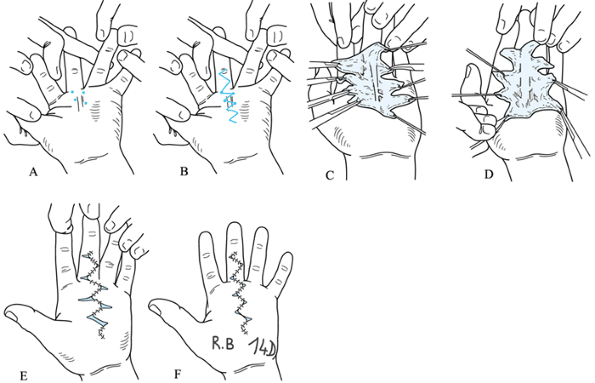
zig-zag incision (Fig. 62.8).![]() Figure 62.8. “Honeycomb incision” allowing skin closure with V-Y-plasty. A: First four basic markers for the top angles of the zig-zag incision at the palmodigital level. B: The incision design. C,D: The wide exposure allowed by this technique enables extensive and safe palmar and digital fasciectomy. E:
Figure 62.8. “Honeycomb incision” allowing skin closure with V-Y-plasty. A: First four basic markers for the top angles of the zig-zag incision at the palmodigital level. B: The incision design. C,D: The wide exposure allowed by this technique enables extensive and safe palmar and digital fasciectomy. E:
At the end of the operation, note the complete correction of the
contracture; only the zig-zag incision is sutured. Transverse incisions
become small open areas, avoiding tension on wounds and preventing
hematomas. F: The same patient after 14
days. (Redrawn from Bedeschi P. Various Views and Techniques.
Management of the Skin. Part 1. Honeycomb Technique. In. McFarlane RM,
McGrouther DA, Flint MH, eds. Dupuytren’s Disease. Biology and Treatment. The Hand and Upper Limb Series, Vol 5. Edinburgh: Churchill Livingstone, 1990:311.) -
Protect the neurovascular structures throughout and remove all the diseased fascia.
-
Suture the zig-zag incisions and leave the transverse incisions open.
-
Do not use postoperative splinting; initiate therapy immediately.
believed that the only form of surgical treatment that could
potentially cure Dupuytren’s disease was radical fasciectomy. It
initially appeared to yield satisfactory results in more than 200
cases; however, Skoog (46) noted that,
following radical fasciectomy, some patients had pain when gripping
objects and a general feeling of the palm’s being “unprotected.” Skoog
proposed a selective aponeurosectomy. He also reported that the
transverse elements of the palmar aponeurosis seemed to be spared the
changes of Dupuytren’s disease. In the selective aponeurosectomy
(commonly referred to as partial fasciectomy), only the diseased palmar fascia is removed, and the transverse elements are left intact.
have popularized skin grafting in Dupuytren’s disease. In addition to
providing wound coverage, the technique prevents recurrent disease from
forming beneath the graft. A short period of immobilization is
required, and there may be morbidity associated with a donor site. As
with open-palm technique, full-thickness skin grafting at the site of
Dupuytren’s resection allows the fingers to be brought into full
extension with no tension, particularly at the level of the proximal
interphalangeal joint. A skin graft here not only prevents recurrent
disease but may also minimize tension on the incisional scar and
further prevent contracture of the scar.
the foot or the groin. The instep of the foot better resembles palmar
skin, but local size constraints permit only small amounts of graft to
be harvested. The groin has the added risk that the graft may bear
hair, an equally undesirable outome. The constraints have led me to use
the upper inner portion of the arm or the lateral side of the leg (Fig. 62.9). Although it controls local recurrence
in the area immediately beneath the flap, skin grafting does not
prevent Dupuytren’s disease from occurring in other areas of the palm.
Always consider skin grafting in recurrent disease where the skin is
scarred from previous surgery in addition to being diseased.
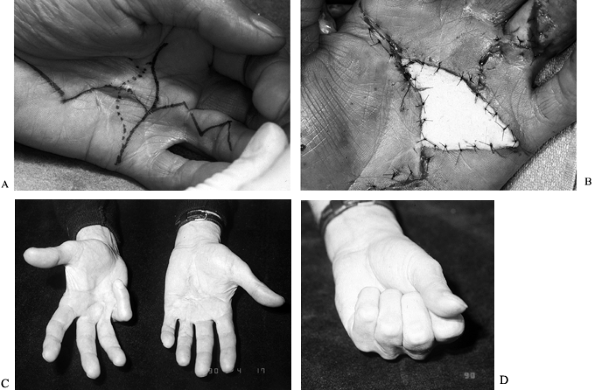 |
|
Figure 62.9. A:
Recurrent contracture (third time) in the palm of a 67-year-old retired avid golfer. Severe contralateral disease was present as well. This longitudinal skin and fascial contracture caused pain when the patient was gripping a club. B: The site of excised skin and diseased fascia was covered by a full-thickness skin graft harvested from the lateral thigh with the donor defect closed primarily. C: Six months postoperatively, the hand in full extension. D: Full flexion. Note severe PIP flexion contracture in the contralateral hand in C. This hand was asymptomatic and, in fact, enabled the patient to grip a golf club better. |
skin grafting requires a 7- to 10-day period of splint immobilization
to ensure graft adherence. During this time, the patient may remove the
splint four or five times per day to gently flex and extend the
involved fingers. Patients must be watched closely for signs of
increasing edema, disproportionate pain, stiffness, infection, and
signs of complex regional pain syndrome. Once the wound has healed,
sutures are removed, and the skin graft has taken, have the patient
begin a more active range-of-motion program. Some patients progress
rapidly on their own but require the services of a trained hand
physiotherapist. Many scars are erythematous, thick, hard, and painful.
Elastomer supported by Coban helps to minimize the amount of redness
and swelling that develops. Cortisone injection of the scar and regular
use of nonsteroidal antiinflammatory drugs have also been beneficial in
reducing edema and scar formation.
disease, the most important complications to include are digital nerve
injury, infection, and recurrence. The patient may be at greater risk
for each of these complications, depending on certain preexisting
conditions. For example, severe contractures or dimpling of the skin
may increase the risk of digital nerve injury or digital nerve
dysesthesia following fasciectomy. A strong family history of
Dupuytren’s disease, surgery at an early age in men or women, or
surgery in women may predispose to recurrence. Tender scars, chronic
edema, reflex sympathetic dystrophy (more appropriately now called
complex regional pain syndrome), and limited range of motion are less
common with the open-palm technique (30).
is more common when the palm is closed. The surgeon needs to remember
that the central portion of the palm is a relatively disvascular area (7).
The creation of large flaps in the palm of the hand or significant
dissection that undermines the wound margins may compromise the
circulation temporarily or permanently, leading to skin slough and
secondary contracture.
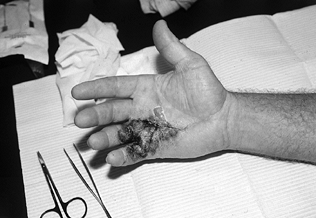 |
|
Figure 62.10.
Postoperative hematoma in the hand of a 42-year-old man whose fasciotomy was performed using the closed-palm technique. Although his ultimate result was satisfactory, additional therapy was required for PIP and MP joint stiffness secondary to hematoma resolution. |
range of motion may occasionally occur after open palmar fasciectomy
and have been referred to as a flare reaction. It occurs more often
when a fairly acute presentation of palmar fibromatosis with a painful
nodule initially exists. Should it develop, I recommend treatment with
nonsteroidal antiinflammatory medication. In combination with
compressive elastomer (otoform and Coban) to the wound, elevation, and
an occasional steroid injection in the latter stages of the disease, it
may calm the process. Six to 12 months later, if a contracture
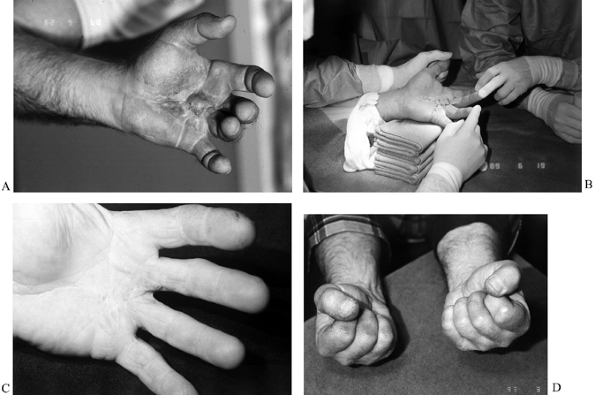
persists, surgical release may once again be considered (Fig. 62.11). Treatment of severe recurrence includes the possibility of skin grafting (Fig. 62.9).
 |
|
Figure 62.11. A:
Inflamed palm of a 57-year-old man who was seen 3 months following fasciectomy for Dupuytren’s disease in which the palmar skin was closed. He developed a local wound dehiscence and secondary infection. He also developed a secondary “flare” reaction and had been scheduled for revision surgery. Instead, he was treated with nonsteroidal antiinflammatory medication and therapy in the form of elastomer and edema control, intermittent protective splinting, and a general range-of-motion program. B: One year later, he underwent revision fasciectomy using the open-palm technique. C,D: Three months postoperatively, full extension and flexion. (Amputation of the contralateral small finger was from an industrial accident.) |
occasionally indicated. It is usually performed at the request of the
patient who, for some reason, cannot tolerate an additional procedure
on the involved finger. Amputation should be done with a caveat: Spread
of the disease to other digits should not involve amputation of each of
the fingers. Instead, resection of the contracture should be
performed
along with PIP joint arthrodesis, with shortening of the bone to
correct the deformity and allow extension of the finger without severe
damage to the neurovascular bundle. This is an excellent salvage
procedure. In certain individuals, for whom motion is extremely
important (a musician, for example), PIP joint replacement
arthro-plasty, with a silicone-type prosthesis, may afford enough
improved motion to alter the functional course.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
P. Various Views and Techniques. Management of the Skin. Part 1:
Honeycomb Technique. In: McFarlane RM, McGrouther DA, Flint MH, eds. Dupuytren’s Disease: Biology and Treatment. The Hand and Upper Limb Series, Vol 5. Edinburgh: Churchill Livingstone, 1990;311.
RI. Open Fasciectomy in Full Thickness Skin Graft in a Correction of
Digital Flexion Deformity. In: Hueston JT, Tubiana R, eds. Dupuytren’s Disease, 2nd ed. Edinburgh: Churchill Livingstone, GEM Monograph, 1985:158.
JD, Lister GD, Wolfe T. Fasciectomy and Dupuytren’s Disease: A
Comparison between the Open-Palm Technique and Wound Closure. J Hand Surg 1984;9A:53.
C, Balmes P, Mary H, et al. Juvenile Fibromatosis Resembling
Aponeurotic Fibroma and Congenital Multiple Fibromatosis. One Case with
Pleuropulmonary Involvement. Cancer 1988;61:146.
RM. Patterns of the Diseased Fascia in the Fingers in Dupuytren’s
Contracture. Displacement of the Neurovascular Bundle. Plast Reconstr Surg 1974;51:31.
T. Dupuytren’s Contracture with Special Reference to Aetiology and
Improved Surgical Treatment. Its Occurrence in Epileptics. Notes on
Knuckle-Pads. Acta Chir Scand [Suppl] 1948;139:27.
T. The Transverse Elements of the Palmar Aponeurosis in Dupuytren’s
Contracture. Their Pathological and Surgical Significance. Scand J Reconstruct Surg 1967;1:51.
L, Clemmensen T, Oleson E, Ulfeldt M. The Effect of a Diuretic (Centyl,
Leo) on the Oedema of the Hand Following Surgical Treatment of
duPuytren’s Contracture. Acta Orthop Scand 1970;41:411.