Total Hip Arthroplasty
successful modern surgical procedures, eliminating the debilitating
pain associated with arthritis and restoring function to the disabled
patient. It provides a reliable, durable, and predictable excellent
result and is generally regarded as one of the most significant
advances in the management of the end-stage degenerative hip by
rheumatologists, orthopaedic surgeons, the general medical community,
and patients alike. Since its original introduction by Sir John
Charnley at Wrightington Hospital in the United Kingdom in the late
1960s, the annual number of primary total hip arthroplasties performed
has steadily increased. In addition, although originally applied to a
predominantly elderly population, the technology has been extended to
younger and more active patients. An aging population, improved wear
properties and fixation of implants, and techniques designed to provide
more rapid and complete recovery of function all have combined to
increase current and anticipated future demand for total hip
arthroplasty.
hip arthroplasty. It afflicts an estimated 21 million adults in the
United States in at least one joint and may involve the hip in as many
as 1.5% of the American adult population. Osteoarthritis is either
primary, without identifiable cause, or secondary, owing to another
systemic disease, congenital malformation, or structural abnormality of
the hip joint. Joint destruction also can result from inflammatory
arthropathies and rheumatologic disease (Table 12-1).
damage differs among the various causes of the arthritic hip, the final
common pathway is one characterized by destruction of the smooth
articular cartilage, resulting in a high friction articulation. Bone
begins to grind directly on bone, generating debris, joint effusions,
and in some cases frank inflammation and synovitis. The actual source
of pain is unknown but may be capsular distention, synovitis, or
irritated pain receptors within the bone or surrounding tissues. Motion
of the joint becomes painful, especially with weight bearing, and
limits mobility and function of the patient.
of pain located in the groin, buttock, or lateral hip, often radiating
along the anterior thigh toward, but usually not beyond, the knee. The
pain typically is worse with activity, although start-up stiffness
followed by early relief with light activity may occur. Barometric and
weather changes also affect the pain, with damp and cold weather
usually exacerbating the symptoms. Although the pain may wax and wane,
the clinical course is usually progressive. The pace of progression,
however, is unpredictable and multifactorial.
limp, ipsilateral limb shortening, stiffness, and limitation in
mobility and vocational and avocational tasks. Even activities of daily
living, such as toenail care, donning and doffing socks and shoes,
short-distance ambulation, rising from or assuming a seated position,
negotiating stairs, and sleeping, become challenging and impaired. The
impact of the arthritis often becomes overwhelming as each hip cycle,
of which a normal individual experiences roughly a million per year,
causes pain.
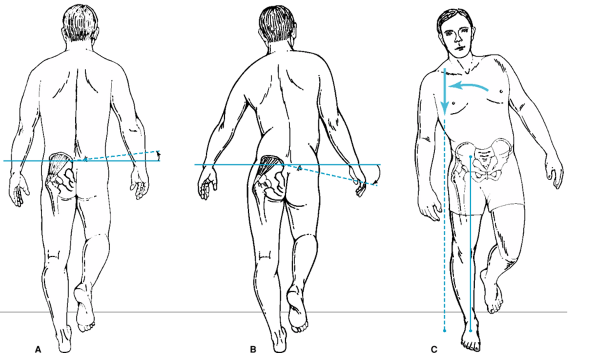
depressed affect, frustration, and anger. Abductor weakness from
involuntary guarding and subsequent atrophy, and from laxity of the
abductor muscles from limb shortening, manifests in several gait
abnormalities. The Trendelenburg gait occurs as the pelvis drops to the
opposite side with ipsilateral single limb stance. The gluteus medius
is unable to pull the body weight over the femoral head. Patients will
compensate for this weakness with an abductor lurch, in which the body
is thrust over the ipsilateral limb during single-limb stance,
positioning the center of body mass
directly
over the femoral head and minimizing the lever arm and resulting torque
imposed by body weight, a so-called Duchenne gait (Fig. 12-1).
The gait will also typically become antalgic, with the patient
minimizing the time spent weight bearing on the involved hip because of
the pain. Finally, shortening of the limb because of loss of the joint
space and bony collapse or penetration may also impact gait, resulting
in a rise and fall of the ipsilateral shoulder with each step.
|
TABLE 12-1 Causes of Degenerative Disease of the HIP
|
|||
|---|---|---|---|
|
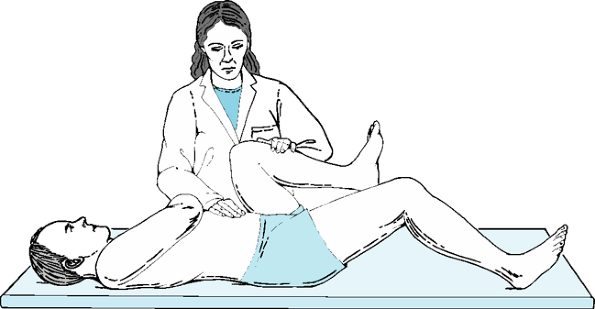
motion, with hip flexion and internal rotation most commonly affected.
Flexion contracture may be present. This is measured as an inability to
fully extend the hip while the other hip is flexed, fixing the pelvis
and preventing pelvic hyperextension to achieve hip extension (Thomas
test) (Fig. 12-2). Adduction contracture, which
may require correction at the time of surgery to prevent dislocation of
the total hip arthroplasty, can occur as well, with an inability to
passively abduct the limb. Limb-length discrepancy is common and should
be accurately measured. Actual limb length is measured between two bony
prominences with a fixed relationship to one another, such as the
anterior superior iliac spine and the lateral or medial malleolus.
Measurement between points without such a fixed relationship, such as
the pubic symphysis or umbilicus and a malleolus, will result in
erroneous and unreliable values that vary with pelvic obliquity and
abduction of the hip. Pelvic obliquity causing apparent, accentuated,
or pseudonormalized limb-length inequality should also be recognized to
warn patients about what their perceptions of limb length may be
postsurgery.
extremities and spine should be performed, including an assessment of
the neurologic and circulatory status of the limbs. Other causes of
pain and factors that may compromise the outcome of total hip
arthroplasty should be identified.
the level of the anterior superior iliac spine to distal, will usually
provide adequate visualization of the acetabulum and the length of the
femur in which the prosthesis will sit. A full AP pelvis may be
necessary if significant bone erosion or abnormality exists. A true
lateral hip radiograph (“shoot-through” lateral) allows evaluation of
the anterior and posterior hip joint space. A frog-limb lateral
(Löwenstein) will normally complete the films required for a thorough
evaluation. On occasion, additional pelvic views–inlet, outlet,
obturator and iliac oblique, and false profile–or longer views of the
femur in multiple planes may be useful.
cartilage-containing joint space, with bone articulating directly
against bone. An osteoarthritic hip also may demonstrate subchondral
sclerosis, bony cysts, and marginal osteophytes. Inflammatory
arthropathy tends to be less hypertrophic, with global joint space loss
and in some cases a minimum of periarticular reaction. Avascular
necrosis is characterized by prominent sclerosis and/or cysts of the
femoral head, femoral head collapse, and secondary acetabular arthritic
change. Residual findings from childhood disease may include persistent
uncoverage of the femoral head, acetabular dysplasia, subluxation, coxa
magna, and deformity of the femoral head from slipped capital femoral
epiphysis. Posttraumatic deformity can assume almost any configuration.
arthritic joint and the bony reaction, include bone quality, which may
affect fixation choice, and any anatomic variants that may present
challenges at the time of surgery, such as unusually tall or short
stature, excessive coxa vara or coxa valga, unusually large and
potentially structurally significant cysts, and extremely small or
large femoral or acetabular anatomy.
the anatomic abnormalities either causing or resulting from the
arthritis include Shenton’s line, Klein’s line, Kohler’s line, the
center-edge angle, acetabular index, neck-shaft (CCD) angle, and the
femoral cortical index. Assessment of the radiographs with these tools
may facilitate surgical planning and enhance the surgeon’s appreciation
of the unique reconstructive challenges of each hip.
radiographs will be adequate to establish the diagnosis and cause of
arthritis in most cases, additional studies may be necessary. An MRI
may differentiate intrinsic articular pathology from periarticular soft
tissue irritation and will make the
diagnosis
of early avascular necrosis (AVN) prior to radiographic findings. CT
scans will define complex bony abnormalities, and three-dimensional
reconstructions can improve the surgeon’s three-dimensional
understanding of complex deformities. Nuclear scintigraphy may be
useful to assess metastatic disease when suspected or other sites of
disease that may be primary sources of pain. Laboratory studies
assessing inflammatory disease markers such as rheumatoid factor,
anti–nuclear antibodies, lyme titers, and others can help to define
systemic disease. Complete blood count, sedimentation rate, and
C-reactive protein measurements may be useful to evaluate for local or
systemic infection. Aspiration of the joint, when clinical suspicion
for infection is present, yields fluid that should be analyzed
with
cell count and differential; glucose level; microscopic review with
appropriate stains for bacteria, fungi, and acid-fast bacilli; and
formal culture for these organisms as well.
 |
|
Figure 12-1 A: Normal gluteus medius function. B. Weak gluteus medius causing a positive Trendelenburg sign with the pelvis dropping on the contralateral side. C: Abductor lurch or Duchenne gait.
|
 |
|
Figure 12-2 Thomas test for hip flexion contracture.
|
arthritic anatomic locations can confuse the clinical picture. The hip
can be the primary pain generator but can refer pain to the knee or
cause a gait abnormality that exacerbates underlying spinal disease or
ipsilateral or contralateral lower-extremity arthritic joints. Despite
pain in these other areas, the severely arthritic hip should be
addressed primarily. For example, it is generally advisable to replace
the arthritic hip before undertaking spinal surgery, because the
persistent gait abnormality may compromise the results of spine
surgery. Similarly, hip replacement should precede an ipsilateral knee
replacement when both joints are symptomatic and arthritic, because the
referred pain from the hip and hip stiffness can compromise outcome and
rehabilitative efforts after knee surgery. Furthermore, the new center
of rotation of the hip should be used to establish a neutral mechanical
axis (a factor critical in total knee arthroplasty longevity) prior to
embarking on the knee reconstruction. When a true differential
diagnosis dilemma exists as to the actual source of pain, diagnostic
injections of local anesthetic with or without corticosteroid may help
to define the primary source of pain.
arthritis, alternative sources of pain must be sought. An MRI with and
without arthrography along with diagnostic aspiration and injection may
be useful to distinguish intra-articular from extra-articular
pathology. A further workup may include evaluation of other anatomic
locations and the neurologic and metabolic status of the patient (Table 12-2).
Pain that is only presumptively located in the hip is not an indication
for total hip arthroplasty in the absence of proven articular pathology
that warrants such a major intervention.
eliminate pain and, when possible, to restore motion to the joint and
mobility to the patient. Often this requires surgery, but conservative,
nonoperative treatment should be exhausted before proceeding directly
to the operating theatre. The American College of Rheumatology
publishes guidelines for the management of Osteoarthritis. These
guidelines can be used as a paradigm for treating the degenerative hip
of any cause. Simple analgesics, nonsteroidal anti-inflammatory drugs,
and disease modifying agents when available may be offered as a first
line of treatment. These all may be combined with physical therapy and
judicious use of adaptive aids and assistive devices for both
ambulation and other activities of daily living. Intra-articular
injections of corticosteroids may diminish the intensity of acute
inflammatory flares in the joint. Local application of ointments and
compounds are usually not useful around the hip because of the depth of
the joint beneath the often robust soft tissue envelope. Other adjuncts
such as the use of nutraceuticals and intra-articular injections of
viscosupplements may be useful but at present have not been
scientifically proven to be effective. When these modalities fail,
surgical intervention becomes appropriate to consider.
|
TABLE 12-2 Alternative Sources of HIP Pain
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
unresponsive to nonoperative management along with radiographically
proven severe degenerative disease. The patient must also have a
realistic expectation relative to activity level, with a willingness to
minimize impact loading activities and excessive exercise. Active
infection either locally, systemically, or at a distant location is an
absolute contraindication to joint arthroplasty. In addition, pain
about the hip without documented cause or radiographically proven
degenerative disease in the absence of compelling symptoms should not
be treated with hip arthroplasty.
controversial. Younger males with osteoarthritis, for instance, are a
cohort that has a documented higher failure rate after total hip
arthroplasty, presumably because of activity level and intensity.
Counseling with appropriate caution and warning must be given to these
patients contemplating total hip arthroplasty. Other relative
contraindications include the very elderly, those medically at risk for
perioperative
morbidity
or mortality, immunocompromised status increasing risk of infection,
and unwillingness or inability to comply with recommended precautions
or restrictions.
and intuitive. The reconstruction must re-establish normal anatomy, as
closely as possible, with regard to limb length and femoral offset, and
preserve soft tissue tension to ensure stability. Immediate and
long-term fixation of the components along with bearing surfaces that
are optimized to reduce wear are critical to reliable and durable
service. Perioperative complications should be minimized with careful
preoperative planning and vigilant perioperative management. And, most
important, the patient’s pain should be relieved and mobility and
function should be restored.
intraoperative achievement of the surgical goals of total hip
arthroplasty. The process begins with a thorough medical evaluation,
identifying and treating sources of infection, and optimizing the
patients’ cardiovascular, pulmonary, and general health status. The
orthopaedic evaluation includes history, examination with an assessment
of gait disorder, range of motion, limb lengths, neurovascular status
and skin integrity, radiographic imaging, and, if necessary, special
studies. Ideally, with an understanding of the underlying pathology,
the surgeon can use templates on the radiographs to size and place the
components, appreciate the biomechanical alterations of both the
diseased hip and the proposed reconstruction, mentally rehearse the
procedure, and anticipate pitfalls. Patient education regarding
precautions and expectations facilitates postoperative rehabilitation
and discharge planning. Finally, the process culminates in the
operating room with choice of anesthesia and patient preparation
including perioperative antibiotics, urinary bladder management,
careful patient positioning, and meticulous sterile technique.
certain benefits and risks, that enable the hip surgeon to accomplish
the goals of total hip arthroplasty. In addition, modifications of each
of these approaches, some of which have been published and others that
remain technical pearls of master hip surgeons, have evolved over time.
In choosing a surgical approach, the surgeon should carefully consider
familiarity, skill, and the idiosyncrasies and characteristics of each
approach. There is no single best or worst methodology, but a measured
analysis should reveal the right combination of surgeon, patient, and
surgical approach to optimize the outcome.
arthroplasty through a trochanteric osteotomy, which allowed for wide
exposure of the hip and offered the opportunity to adjust abductor
tension when reattaching the trochanter. Unfortunately, nonunion of the
osteotomy occurred in as many as 25% of patients in some series.
Although still useful in complex primary and revision surgery, this
approach has largely been abandoned for exposure of the straightforward
primary total hip arthroplasty.
posteriorly directed incision over the trochanter, incises the
iliotibial band laterally and splits the fibers of the gluteus maximus
muscle. The gluteus medius is elevated, the short external rotators are
detached from their trochanteric insertion, and a posterior capsulotomy
and dislocation are performed. Although this approach provides the most
extensile exposure, higher dislocation rates have been reported.
However, with a more truncated exposure, repair of the soft tissue, and
with the use of larger bearing surfaces, that dislocation rate should
be significantly reduced.
centered over the trochanter. The iliotibial band is split distally,
and the fibers of the tensor fascia lata are split proximally. In the
direct lateral approach, the surgeon detaches the anterior portion of
the gluteus medius and a portion of the vastus lateralis as a soft
tissue sleeve, sometimes with a wafer of bone attached. In the
anterolateral approach, the surgeon detaches the anterior third of the
gluteus medius, often with a wafer of trochanteric bone as well.
Anterior capsulotomy and dislocation are performed. The acetabulum is
well visualized in these approaches, and the dislocation rate has been
reported to be lower. However, these exposures are not as easily
extensile, often require postoperative weight bearing and activity
limitations while the soft tissue/bony abductor sleeve heals, and have
been associated with a higher incidence of gluteus medius weakness and
limp.
along the interval between the tensor fascia lata and the sartorius
muscles. Splitting this interval allows direct visualization of the
anterior capsule, which can be incised, enabling anterior dislocation.
Although this muscle-splitting approach provides good acetabular
exposure and improved hip stability, it is not extensile, and exposure
of the femur can be challenging. Management of intraoperative
complications may necessitate a second, more extensile approach. In
addition, the use of a specialized fracture table for patient
positioning is a prerequisite.
total hip arthroplasty performed through miniaturized incisions using
so-called minimally invasive techniques. Most agree that these
techniques carry with them a steep learning curve, and significant
complications have been reported. Most also agree, though, that more
rapid rehabilitation may be facilitated and that less invasive surgery
has forced hip surgeons to refine and improve surgical technique. The
critical lesson learned from this recent process is that any incision
through which a total hip arthroplasty is performed should be large
enough and at a suitable site to enable proper positioning of the
components with a minimum of soft tissue and bony injury.
understanding of the various options available and their design
features. Both cemented and cementless femoral and acetabular
components are available. Multiple bearing surface options also exist
and can significantly affect the longevity of the hip reconstruction.
with inner polyethylene liners have been used in the past, cemented
acetabular components today consist of all-polyethylene designs of
varying outer and inner diameters. The backside normally is textured to
enhance fixation by promoting cement interdigitation and interlock.
Although these components are generally less expensive, they have
proven to have higher loosening rates than uncemented cups in most
series. This is also probably owing in part to the technical demands of
adequately cementing an acetabular component into a bleeding cancellous
acetabular bed and the difficulty of achieving proper cement
interdigitation into the bone.
of choice in most primary total hip arthroplasties for North American
surgeons. They consist of a metal outer shell of varying diameter,
which is textured on its bone-opposing surface with either sintered
beads, plasma spray, fiber mesh, or tantalum to create pores of optimum
size of 150 to 400 nm to promote bone ingrowth. The shape of this shell
is either less than a hemisphere, hemispheric, or with a peripheral
flare designed to increase the interference fit between the shell and
the bone. Additional features of the metal shell include optional holes
for screw fixation to bone, a locking mechanism for the inner liner,
and the ability to accommodate multiple modular liners with varying
offsets, lips, orientations, inner diameters, and materials, including
in some cases metal, ceramic, and polyethylene. The versatility of
these cups accommodates widespread application and has led to an
outstanding clinical track record of excellent fixation.
successful design can produce excellent long-term results when
implanted with excellent surgical technique.
philosophies, which have influenced their respective stem designs. Both
philosophies rely on proper cement technique to achieve a strong
bone/cement bond. This technique is based on an understanding of bone
cement not as an adhesive, but rather as a grout requiring intrusion
into and interdigitation within the cancellous bone of the inner femur.
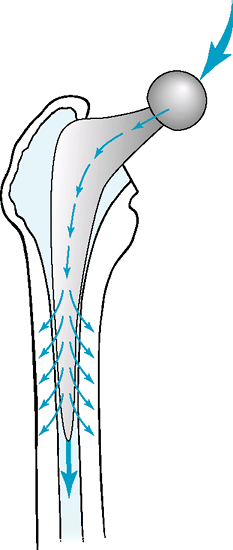
The composite beam philosophy and design strives also to achieve a
perfect bond of the cement to the stem, by texturing, precoating with
methacrylate monomer, or otherwise roughening the surface of the stem.
This bonding of prosthesis to cement and cement to bone can lead to
stress shielding of the proximal bone, with most of the load
transmitted through the stiffer stem, bypassing the periprosthetic bone
(Fig. 12-3). Debonding from the cement or the
bone can occur, which signals loosening and can cause abrasive
production of wear debris and subsequent osteolysis. In contrast, the
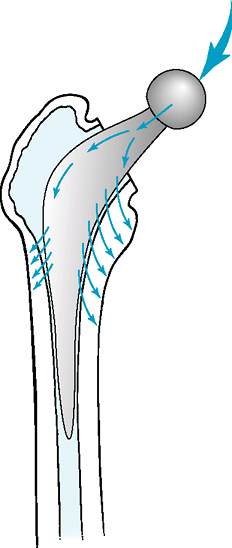
taper-slip philosophy and design strives to engage a multitapered,
polished, collarless stem into the cement mantle, exploiting the
viscoelastic property of cement and its ability to creep. The stem
never achieves a bond with the cement, but rather continues to engage
the cement, often with a small amount of subsidence. The engaging taper
generates hoop stresses that are transmitted radially to the
surrounding bone, favorably loading the periprosthetic bone (Fig. 12-4).
Although the success of some cemented stems from both philosophies has
been outstanding, problems from the loosening of rough surface stems,
leading to extensive periprosthetic bone osteolysis caused by the wear
particles liberated by cement abrasion, dampened enthusiasm for
cemented stems in North America. Nevertheless, the taper-slip
philosophy with its potential for positive bone remodeling and its
proven durability has gained popularity worldwide.
 |
|
Figure 12-3 Force transmission in a composite beam cemented stem reconstruction.
|
acetabular cups, rely on bone ingrowth into a textured surface to
achieve durable fixation. There are a myriad of designs with
variability of material, surface texture and length of coating,
fixation concept (fit and fill versus taper fit), bone preparation
recommendations (broached versus machined), modularity, and
stiffness-reducing features such as coronal slots, and hollowed stems.
Each design feature has potential distinguishing merit, but the
clinical performance of many cementless stems of many designs has been
outstanding. With bone ingrowth, however, comes stress shielding, to
some degree, potential for thigh pain from modulus mismatch and
micromotion concentration at the stem tip, intraoperative femoral
fracture risk, and challenging revisions. Nevertheless, the
straightforward implantation techniques, the potential for permanent
biologic implant fixation, and reliability and predictability of
cementless femoral stems have stimulated enthusiastic use for many
patient demographic groups, particularly in the United States.
 |
|
Figure 12-4 Force transmission in a taper slip cemented stem reconstruction.
|
importance in recent years owing to the pervasive problem of
periprosthetic osteolysis resulting from polyethylene wear in metal
head on conventional high-molecular-weight polyethylene bearing
surfaces. Recently cross-linking of polyethylene, which provides
dramatically improved wear properties in vitro and reduced oxidation
potential, has been introduced. Early critical studies demonstrate
reduced wear compared with conventional polyethylenes. The mechanical
properties of these new polyethylenes are moderately reduced however.
The very low wear rate, the opportunity to use large femoral head
sizes, and improved materials have led to renewed interest in metal
against metal and ceramic against ceramic. In contrast to polyethylene
bearings, where boundary lubrication predominates and where increasing
the head size increases the frictional torque and volumetric wear, with
hard on hard bearing couples, larger-diameter heads favor fluid film
lubrication and reduce the number of wear particles while imparting the
associated benefits of improved stability and range of motion.
some potential problems. Metal on metal couples are associated with
increases in serum cobalt and chromium metal ion levels. The
significance of increased ion levels is unclear, and to date no major
clinical problems have been identified. Carcinogenesis or distant organ
toxicity remain theoretical concerns. It is advisable to avoid the use
of metal-on-metal implants in women of childbearing age, patients with
significant kidney disease, and those with documented metal allergy.
Ceramic-on-ceramic couples carry a risk of fracture despite material
improvements that reduce this risk. Ceramics are also sensitive to
impingement between the femoral head and the prosthetic liner; thus
accurate implant positioning is especially important for these
implants. A squeaking noise may occur in a few patients with
ceramic-on-ceramic bearings.
of implant options, most of which seem to provide at least very good
short-term results. Nuances of differences, which may not emerge until
long-term follow-up is available, may eventually help surgeons
individualize implants to patients based on age, activity level, bone
quality, expectations, longevity, and metabolic status. In the
meantime, an understanding of the design principles of the various
implants, along with their theoretical and proven risks and benefits
must suffice to guide implant choice.
full 360-degree visualization of the acetabulum must be achieved. To
facilitate this, the remaining acetabular labrum is removed along with
the transverse acetabular ligament inferiorly. The fatty remnant of the
pulvinar is resected to identify the fovea and thus the usual limit of
medial reaming. In some cases a large medial osteophyte will need to be
removed to reveal the pulvinar remnant. In addition, capsular resection
or release, based on the preoperative deformities, may be necessary to
enable adequate retraction of the femur for full acetabular exposure.
Retractor placement is entirely dependent on surgical approach and
should be individualized to maximize visualization.
appropriate depth, followed by reaming (in the intended orientation of
the actual implant) to proper size. Reamer size is increased stepwise
until subchondral bleeding bone is identified in a hemispheric shape,
maintaining constant vigilance to central reaming and remaining wall
thicknesses.
strong interference press fit, which should be tested with an
appropriately sized trial. Depending on the appearance of the prepared
acetabulum, a cup size is chosen, with or without fixation holes. Cysts
or defects are bone grafted with autogenous morcellized bone graft as
necessary. The actual shell is impacted into place, with screw holes
positioned superoposterior to avoid the neurovascular structures at
risk in the anterior hemisphere of the acetabulum. Orientation of 10 to
30 degrees of anteversion and 40 to 50 degrees of abduction should be
achieved by use of either a positioner guide or intra-articular
landmarks. A useful pearl is to orient the inferiormost portion of the
cup at the level of the teardrop and the posterior edge of the cup at
the level of the ischium. Stability of the cup is tested with the
inserter in place, and full seating is verified. Screws are placed, if
desired. Overhanging osteophytes that can cause impingement and
dislocation are removed, especially in the
anterosuperior and posteroinferior quadrants. A trial liner can be placed for subsequent trial reduction.
recommended, as the many polyethylene cups provide for a cement mantle
of 1 to 4 mm. Additional cement fixation holes are drilled into the
ilium, ischium, and pubis. The acetabulum is irrigated of debris, which
could compromise cement interdigitation into the cancellous bone.
Cement is introduced in a doughy phase and pressurized. The cup is
inserted, with meticulous attention to positioning. Excess cement is
removed, and the cup is held in place until the cement is fully cured.
head resection, the position of the current center of rotation is
identified and measured relative to other bony landmarks such as the
lesser or greater trochanters. Additional aids to ensure limb-length
equalization and to minimize excessive lengthening may be used at this
point as well. The neck resection level and orientation is established
based on preoperative templating. With the head resected, preparation
of the canal commences. The Pyriformis Fossa, which is lateral and
posterior, must be clearly identified. A box or round osteotome may be
used to remove any retained superolateral femoral neck or any other
obstructing portion of the trochanter preventing access to the
Pyriformis Fossa. Vigilance is necessary to maintain a lateral position
and avoid varus alignment of reamers, broaches, or actual implants.
cemented stem. Indeed, it is useful to conceive of the femoral stem and
the cement as two distinct implants that must optimally interact to
achieve the best result. Therefore, the idiosyncrasies of each
component must be understood. Cement itself is a grout, not an
adhesive, requiring intrusion into and interdigitation with the dense
cancellous bone on the endosteal surface. As it is introduced, it must
be in a viscous enough phase to resist any back bleeding, which creates
laminations and weakened areas in the cement, and to withstand
pressurization without running out of the canal. Of course, the bony
substrate must be prepared properly to accept the cement, occluding the
medullary canal to enable pressurization and retaining the endosteal
adjacent cancellous bony structure.
with a canal finder but should not be reamed vigorously, which could
remove the cancellous bone, burnish the endosteum, and significantly
compromise the shear strength at the bone/cement interface by
eliminating the cancellous structure into which cement must intrude for
strength. Serial broaching establishes the size of the stem that
achieves stable fixation. Calcar reaming is performed for a collared
stem and may be performed for a collarless stem. Trial reduction should
be performed with trial neck segments and trial heads of varying neck
lengths. Range of motion, limb length, and soft tissue tension
assessment should be carried out at this point, along with a careful
evaluation of stability in the at-risk positions, determined by the
surgical approach. Biomechanical parameters can be modified by choosing
different cup liner options, such as lipped, face-changing, or
extra-offset liners, or stem options including offset, neck length, and
head size. Proper component orientation should be verified. A useful
technique to ensure a combined cup and stem anteversion of 30 to 60
degrees is to rotate the fully extended femur until the transverse
plane of the head matches the face of the acetabulum. The degree of
femoral internal rotation establishes the combined anteversion angle.
Limb length should also be assessed at this point, using the
methodology of measuring the femoral center of rotation to a fixed
anatomic landmark and comparing that with the value obtained prior to
head resection as described above, or using any system or device that
is reproducible for the individual surgeon.
broach is removed. The canal is brushed and cleared of any loose
cancellous bone. The endosteal bone covering the entrance to the lesser
trochanter may be removed with a large curette without excavating the
lesser trochanter to allow for cement interdigitation in that area. The
canal diameter is sized for a cement restrictor, and this is placed 1
to 2 cm distal to the intended tip of the stem. The stem is assembled
with any centralizers on the back table, avoiding contact of the
surface of the stem with blood or other contaminants that may
compromise the cement/implant interface. The canal is irrigated with
pulsatile lavage and dried to provide the optimum interface for cement
application. Cement is mixed, and once a doughy viscosity has been
reached, is introduced in a retrograde fashion using a cement gun.
Pressurization with a proximal canal occluder is held for a sustained
period of time, depending on the behavior characteristics of the
cement, but long enough to allow steady flow of the cement into the
cancellous structure. Some advocate venting of the canal at this point
to prevent the rare cardiovascular collapse reported in the literature,
although most surgeons eliminate this step except in the most high risk
individuals. Immediate insertion of the stem should follow release of
pressurization to prevent any backflow of cement out of the interstices
of the cancellous bone. Meticulous attention to alignment in the AP and
medial-lateral (ML) dimensions and to proper anteversion of 10 to 20
degrees is critical. The stem, once fully seated, should be held firmly
until the cement hardens, but excess cement should be removed prior to
final curing. The technique for cementing a femoral stem is demanding,
and each detail contributes to an ideal result.
much more idiosyncratic. The alignment issues and the methodology for
lateralization and opening the medullary canal pertain to cementless
stems, just as described above for cemented stems. For stems designed
for fit and fill, a distal reaming process followed by a proximal
reaming or broaching step establishes stable fixation. Alternatively,
tapered stems may require only serial broaching, exploiting the richly
vascularized cancellous bony structure, which can be compacted to
support a stem in three-point fixation. Trial reduction is performed
exactly as described for a cemented stem. The actual cementless stem is
slightly larger than the broaches or trials; therefore, it should be
inserted firmly but carefully, particularly as it begins to seat. One
must resist the urge to pound harder on the implant as resistance is
met. Rather, multiple lighter taps gently seat the implant while
avoiding an intraoperative fracture. Circumferential inspection around
the visible proximal femur is advisable.
If
a fracture is identified, the prosthesis should be removed enough to
effect complete reduction. Cerclage of the intertrochanteric and, if
necessary, the subtrochanteric region can restore the integrity of the
proximal femur and its ability to resist the hoop stresses imparted by
the implant. The implant is reinserted and stability is verified.
reduction may be performed. Minor adjustments in the neck length can
correct any soft tissue laxity or tightness resulting from the final
implant seating at a location slightly different from the broach or
trial. Any areas of bony or soft tissue impingement should be relieved.
The actual head is then impacted over the clean and dry neck. The hip
is articulated, soft tissue or bony repair is completed, depending on
the chosen surgical approach, and the fascia, subcutaneous tissues, and
skin are closed in a routine fashion.
usually as tolerated, unless an intraoperative complication requiring
protection has occurred. Walking assistive devices such as walker,
crutches, or cane are recommended for support and to avoid falls. They
may be gradually discontinued over 3 to 6 weeks, and sometimes even
sooner. Although some controversy exists about the utility of
instructing patients in dislocation precautions, conventional wisdom
suggests that patients should be warned to avoid the at-risk positions,
determined by the surgical approach.
mobilization. There is a general shift away from parenteral narcotics
because of their associated complications, especially sedation,
confusion, and postoperative nausea and vomiting. A multimodal approach
is preferred by many, including elements such as regional anesthetic
and block techniques, preoperative and postoperative long-acting oral
analgesics, intraoperative wound infiltration, and all supplemented
with immediate-release narcotics and or intramuscular or subcutaneous
narcotics for breakthrough pain.
sequelae can be eliminated by vigilance. Preoperative medical
evaluation and clearance along with expert anesthesia will
substantially reduce the risks associated with anesthesia. Careful
surgical technique along with a thorough knowledge of the anatomy
reduces risk of neurologic or vascular injury. Intraoperative fractures
occurring during implant insertion should be identified and fixed as
described in the technique section above. Infection is perhaps the most
dreaded complication for both surgeon and patient alike. Antibiotics,
usually from the first-generation cephalosporin family, administered
preoperatively and continued for 24 hours postoperatively are the
single most effective prophylaxis against infection. Additional
interventions that may further reduce the incidence of infection
include meticulous sterile technique, operating in a laminar flow
environment or under ultraviolet lights, the use of body exhaust
systems, Betadine-impregnated adhesive skin drapes, antibiotic
irrigation, gentle handling of the soft tissues, and careful wound
management.
dramatically impair early rehabilitation and recovery; therefore,
prophylaxis is appropriate. There is a risk of deep vein thrombosis
following total hip arthroplasty. Pharmacologic prophylaxis using a
low-molecular-weight heparin, pentasaccharide, or Coumadin is
appropriate for most patients, and usually is continued for 10 days to
3 months, depending on the chosen agent and the individual patient risk
factors. Mechanical adjuncts include pneumatic compression devices,
compression stockings, and rapid mobilization. Rapid mobilization
enhances return of pulmonary, bowel, and bladder function and reduces
complications such as pneumonia, urinary tract infection, severe
constipation, and skin breakdown. Additional aids such as urinary
bladder catheterization, stool softeners, pulmonary toilet, and
cushioned mattresses or pressure-point protectors can be helpful. Total
hip arthroplasty can be associated with two to three units of blood
loss from intraoperative and postoperative bleeding. Routine blood
count monitoring should continue during hospitalization, and sometimes
even after discharge, to avoid anemia-related complications. In
addition, perioperative use of marrow stimulants such as Erythropoietin
may minimize overall exposure to blood transfusions, and autologous
predonation may reduce exposure to allogeneic blood.
period. Any bacteremia can potentially cause infection in a prosthetic
joint. Because nonsurgical treatment of infected prostheses is
notoriously unsuccessful, and because the operative treatment is often
associated with morbidity and even mortality, vigilant prophylaxis is
mandated. Any systemic or distant infection should be treated
aggressively. The choice of prophylaxis against bacteremia induced by
other surgery should be guided by the organisms most likely present at
the surgical site. Controversy exists regarding prophylaxis before
routine dental care and other less invasive procedures such as
endoscopy. Some would argue that the risk of antibiotic resistance and
adverse reactions increases with prophylaxis, and therefore it should
not be routine, at least after 2 years from surgery except in the
immunocompromised host. However, it is the opinion of the author that
the benefits of any reduction in the likelihood of infection following
even these minor procedures more than outweigh the minimal risks of
resistance or adverse reaction to antibiotic use, particularly in the
elderly population in whom joint replacement is most common and who are
more likely to be immunocompromised from chronic disease. The risk of
dislocation reduces dramatically after 3 months; however, there is a
lifelong cumulative risk. Wear-induced periprosthetic osteolysis is a
significant long-term challenge. Modern bearing surfaces, which reduce
particulate wear and its sequelae, should reduce the incidence of this
periprosthetic osteolysis. Aseptic loosening is primarily related to
the service life of the prosthesis. The cumulative risk of loosening
increases over time, but even at 25 to 30 years of follow-up remains
low at or about 1% per year total. Unfortunately, the only definite
solution is surgical revision. Catastrophic failure of the implant
itself is rare, because metallurgic modifications
stimulated by fracture of early-generation prostheses have been implemented.
DJ, Von Knoch M, Schleck CD, et al. The cumulative long-term risk of
dislocation after primary Charnley total hip arthroplasty. J Bone Joint Surg Am. 2004;86-A:9–14.
of the hip: a compendium of evidence-based information and resources.
http://www.aaos.org/Research/documents/ oainfo_hip.asp. Accessed
December 2006.
CB, Barrett JA, Losina E, et al. Incidence rates of dislocation,
pulmonary embolism, and deep infection during the first six months
after elective total hip replacement. J Bone Joint Surg Am. 2003;85-A:20–26.
for the medical management of osteoarthritis of the hip and knee: 2000
update. American College of Rheumatology Subcommittee on Osteoarthritis
Guidelines. Arthritis Rheum. 2000;43:1905–1915.
