Pain Relief and Related Concerns in Children’s Fractures
of the management of children’s fractures. In order to be able to
perform satisfactory closed treatment of musculoskeletal injuries,
effective and safe levels of sedation and analgesia are essential in
order to minimize pain and allay apprehensions of the child.127,184
Optimal pain management in the emergency room or other setting is
delivered by the combined efforts of the orthopaedic surgeon and
anesthesiologist or emergency medicine specialist. Numerous techniques,
short of general anesthesia, are available to control pain associated
with fractures in children including local, regional, and intravenous
blocks and moderate or deep sedation. Important factors in choosing a
particular technique include efficacy, safety, ease of administration,
cost, and patient/parent acceptance. The correct use of any of the
available medications for obtaining these goals necessitates an
appropriate understanding of proper dose, desired effects, and untoward
side effects. The purpose of this chapter is to provide a source of
information regarding safe and effective analgesia and sedation for
children with fractures. The definitions of the
various
levels of sedation and the medications used to achieve the desired
sedation state are discussed. Local and regional anesthetic techniques
including intravenous (IV) regional anesthesia (Bier blocks), hematoma
blocks, and femoral nerve blocks (for femoral fractures) are discussed
in depth. The management of postoperative pain and the treatment of the
troublesome side effect of postoperative nausea are discussed. The hope
is that the concepts discussed in this chapter will aid the orthopaedic
surgeon in managing fractures in the emergency room, office, and
hospital setting.
pain and apprehension. Psychologically, their perceptions of the
emergency department, office, or hospital and the impending treatment
of their injury often exacerbate their level of discomfort and anxiety.148
Children with painful injuries about to undergo additionally painful
treatment are entitled to adequate analgesia and sedation. Despite the
rationale of this concept, the problem of undertreatment of pain in
children in the emergency department and postoperative setting has been
documented and is still an all-too-common occurrence.14,80,90,130,151,152,157,158,204
Ignorance of the problem of pain in children, lack of familiarity with
the methods of anesthesia and sedation for children, and apprehension
of complications such as respiratory depression and hypotension are
reasons for the often inadequate management of pain in the pediatric
population.80,90,130,137,147,148,151,153,157,165,204
procedures performed on children in a variety of ambulatory settings,
the American Academy of Pediatrics (AAP) has developed goals for
sedation and analgesia in children. Their purpose is to ensure the
child’s safety and welfare while minimizing the physical discomfort and
negative psychologic impact frequently associated with treatment of
painful injuries, to control the child’s behavior, and to return the
child to a state in which safe discharge is possible.5
From a practical perspective, the method of analgesia/sedation must
also allow for the satisfactory treatment of the primary problem. Thus,
efficacy, safety, ease of administration, patient/parent acceptance,
and cost are important factors to be considered in selecting a
technique.184
anesthesia for the child with a closed fracture requiring manipulation
is to facilitate satisfactory closed treatment of the injury and
obviate the need for a trip to the operating room. The ideal method of
analgesia/sedation would be efficacious and safe in eliminating pain,
promoting patient compliance, and producing amnesia of the procedure.
It would be easy to administer, predictable in its action, and reliable
for a wide range of ages. It would have a rapid onset and short
duration of action, result in no complications or side effects, and be
rapidly reversible. Finally, it would be relatively inexpensive to
administer and completely satisfactory to the child and his or her
parents.13,22,35,37,39,46,50,73,74,75,83,84,93,136,137,147,148,162,184
been used to achieve analgesia and sedation in children with closed
fractures requiring treatment in the ambulatory setting. The techniques
can be grouped into two broad categories: blocks (local, regional, and intravenous) and sedation,
either moderate (formerly referred to as conscious) or deep
(anxiolytics, narcotic analgesics, or dissociative agents alone or in
combination). Each technique incorporates various aspects of the ideal
method described above. It is incumbent upon the person treating
children’s fractures to be aware of the various techniques, their
relative merits, and the potential side effects and complications of
each in order to be able to make an educated decision about which to
use in a particular situation.96,114,115
means a pharmacologically controlled, altered state of consciousness in
which patients maintain their ability to respond purposefully to verbal
commands. For nonverbal patients or young infants, conscious sedation
implies the ability to respond purposefully to physical stimulation,
not simply by reflex withdrawal to pain. Unfortunately, most physicians
and nurses tend to use conscious sedation to mean anything short of a
general anesthetic. For such reasons, the consensus of the 1996 report
by the American Society of Anesthesiologists Task Force on Sedation and
Analgesia by Non-Anesthesiologists is that the term conscious sedation,
although in common use, is imprecise. This report recommends replacing
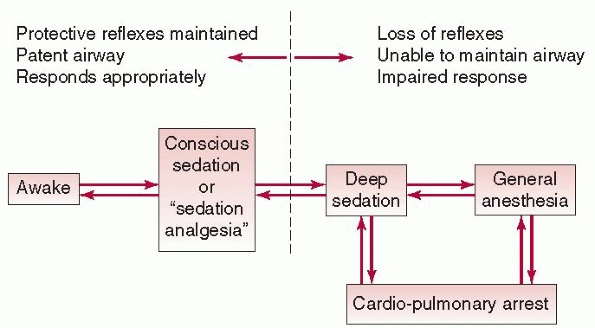
the term with the more descriptive term sedation analgesia (see Fig. 3-1).7
All levels of sedation short of deep are characterized by a state of
depressed consciousness in which a patent airway and protective
reflexes are maintained and from which the individual can be aroused by
physical stimulation or verbal command. Deep sedation
is a more profound state of unconsciousness with loss of protective
airway reflexes. Sedation can be achieved using inhalational agents
such as nitrous oxide or parenteral techniques
including
opioids, benzodiazepines, propofol, or neuroleptic drugs (Ketamine),
alone or in combination. The AAP has established guidelines for
equipment and monitoring for all levels and methods of sedation in an
attempt to guard patients’ welfare during sedation and emergence and
allow safe discharge home afterward (Table 3-1).5,115
The safe and efficacious use of procedural sedation and analgesia
(PSA), specifically by nonanesthesiologists, in a pediatric emergency
department has been demonstrated. In a study performed at Children’s
Hospital of Pittsburgh, PSA was successfully provided in 1177 (98.6%)
of 1194 sedation events, a little more than half of which were for
fracture reduction (643 patients or 52.9%), using parenteral
(intramuscular [IM] or IV) ketamine hydrochloride, fentanyl citrate,
and/or midazolam in various combinations. Complications occurred in
about 18% of patients, but most commonly consisted of hypoxia that was
easily treated.134
 |
|
FIGURE 3-1
Sedation and analgesia for procedures is a continuum. (Reproduced with permission from American Society of Anesthesiologists, from Kaplan RK, Yang CI. Sedation and analgesia in pediatric patients for procedures outside the operating room. Anesthesiol Clin North America. 2002;20(1):181-194, vii.) |
|
TABLE 3-1 Recommended Discharge Criteria after Sedation
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
recognize is that the safest level of sedation is that which permits
purposeful response to verbal or physical stimulation. It is at this
level of sedation that the risk of hypoventilation, apnea, or
cardiovascular instability is minimal. Unfortunately, and realistically
speaking, such relatively light levels of sedation are totally
inadequate for the performance of a painful procedure such as the
reduction of a fracture. Also, the younger and less cooperative the
patient, the less likely it is that so-called conscious sedation can
realistically be achieved at all.113
Therefore, it is very likely that for orthopaedic procedures, children
may have to be sedated to levels at which they are not easily
responsive to verbal stimulation and, as such, are at increased risk
for respiratory and cardiovascular compromise. Even in children in whom
light levels of sedation (true conscious sedation) are possible,
unintended oversedation may occur without warning. Over-sedation may
lead to (a) loss of the airway, (b) impaired protective reflexes,
leading to the possibility of aspiration of gastric contents, and (c)
cardiopulmonary arrest (see Fig. 3-1). It is for these reasons that careful monitoring of sedated patients, as prescribed in the standard guidelines, is imperative.5,7
provide timely detection and correction of abnormalities in respiratory
and cardiovascular function. The monitoring process begins before the
administration of any sedative medications. Monitoring continues
unabated until the patient returns to his or her baseline presedation
level of consciousness and is ready for discharge. Acceptable discharge
criteria are noted later (see Table 3-1).
Vital to the monitoring process is the presence of qualified personnel
who are competent in the use of monitoring devices and capable of
recognizing the clinical signs of airway or hemodynamic instability.
Although skill in at least pediatric basic life support is necessary,
training in pediatric advanced life support is certainly desirable.5,147,148
It is imperative that these skilled health professionals, either
physicians or nurses, are completely dedicated to administering drugs
and observing the patient and monitors during procedures requiring
medications that are known to depress respiratory or cardiovascular
function. Having one person performing both the surgical procedure and
monitoring the patient is a practice that should be strongly
discouraged, as both tasks may be compromised.
parameters that require careful assessment. Monitoring temperature is
usually of minimal importance; the major exceptions are children who
arrive in the hospital either severely hypothermic or febrile.
refers to an assessment taken at frequent, regular intervals. The value
of pulse oximetry as an early detector of impeding hypoxemia has been
well demonstrated.43 The problem
with relying on visual inspection alone to determine the adequacy of
oxygenation is that cyanosis is both a late and variable sign of
hypoxemia. Demonstrable cyanosis requires the presence of at least 5 g
of desaturated hemoglobin per deciliter. Therefore, as an example, a
patient with a hemoglobin level of 10 g/dL would theoretically not even
appear cyanotic until the oxygen saturation level plummets to 50%. For
this same reason, a severely anemic patient may never develop visible
cyanosis even at profound levels of hypoxemia. To add to a potentially
confusing situation, the ambient light (especially fluorescent light)
in many clinical environments may make any patient appear cyanotic.42,43
Therefore, pulse oximetry is essential in all patients with the
potential of becoming heavily sedated to detect abnormalities of
oxygenation rapidly. The pulse oximeter is not perfect, however, and
factors such as patient movement, direct bright light on the probe, and
malposition of the probe can affect the accuracy of pulse oximetry
readings.12,26,42
Simple measures like correct probe placement, shielding the probe site
from bright light, and gentle restraint of the monitoring site can
improve the dependability of this all-important monitor.
oxygenation and requires close observation of the patient and either
intermittent or continuous auscultation of breath sounds. A sedated
child’s head may flex forward easily, producing airway obstruction as
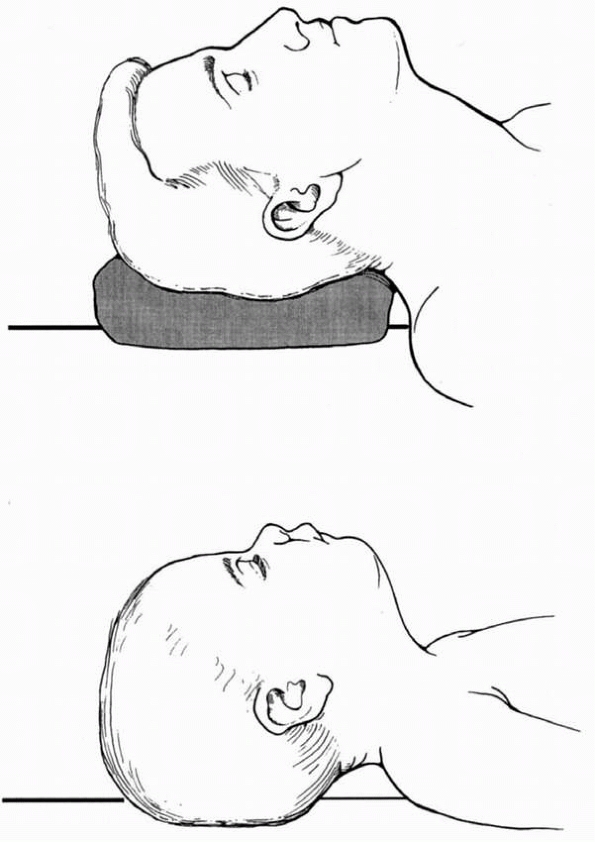
the child begins to fall asleep.42 Maintaining patients in the so-called “sniffing” position helps prevent airway
obstruction (Fig. 3-2).
The sniffing position consists of elevating the patient’s head with
pads under the occiput, keeping the shoulders flat on the table, and
extending the head at the atlanto-occipital junction.169
Children younger than 3 years of age have a relatively large head in
proportion to the size of their trunk and do not require padding under
the occiput.44 Along with continual
assessment of the child’s head position, any restraining devices should
be checked to ensure that they are not contributing to either airway
obstruction or restriction of chest movement.5
Auscultation with the precordial stethoscope is valuable in the
monitoring of both ventilation (breath sounds) and circulation (heart
sounds). Its use is encouraged in the monitoring of deeply sedated
patients.5
 |
|
FIGURE 3-2
The sniffing position. In an adult or in an older child, a folded sheet or towel under the occiput, plus moderate head extension at the atlanto-occipital joint, helps to maintain an open airway. In a child younger than 3 years of age, the relatively large head size in proportion to the trunk makes occipital padding unnecessary.44 |
consists of intermittent determination of heart rate and blood
pressure. In children, normal values for heart rate (Table 3-2) and blood pressure (Table 3-3)
vary with age. A simple formula for calculating the normal systolic
blood pressure and lower limit of normal for systolic blood pressure in
children by age is worth memorizing (Table 3-4).
Electrocardiographic (ECG) monitoring is especially important for the
child with an underlying history of a significant cardiac dysrhythmia
or known ECG abnormality such as long QT syndrome or a history of
Wolff-Parkinson-White syndrome. In the absence of monitor artifact, the
pulse oximeter provides a continuous assessment of heart rate. Deeply
sedated children should have blood pressure and heart rate and
respiratory rate measurements determined and recorded at least at
5-minute intervals.5 For children
undergoing more moderate procedural sedation/analgesia, the frequency
of vital sign determination is at the discretion of the physician or
practitioner in responsible for monitoring.5
|
TABLE 3-2 Normal Values for Heart Rate by Age
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
|
TABLE 3-3 Normal Values for Blood Pressure by Age
|
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||
|
TABLE 3-4 Calculation of Normal Blood Pressure by Age
|
|||
|---|---|---|---|
|
It is important to be cognizant of the possibility that, when the
surgical procedure is over and patients are no longer being actively
stimulated, unintentional deep sedation with resulting airway
obstruction and apnea may occur. Therefore, it is essential to remain
vigilant until the patient emerges completely from the sedative
medications. The time to recovery varies depending on the amount and
type of sedative medication given, and this point should be taken into
account when planning a sedation regimen. The durations of action of
particular sedatives and sedative combinations are discussed separately.
determine whether administering sedative medications to a child in an
ambulatory setting, where the airway is uncontrolled and unprotected,
is feasible and safe. It is important first to be aware of the child’s
medical history, previous allergic or adverse drug reactions, current
medications, and presence of coexisting diseases.5
In addition to these basic details, other important factors including
time of last oral (PO) intake, hemodynamic status, presence of other
injuries, and status of the airway must be assessed before considering
sedation for children with musculoskeletal injuries.
Multiple studies support and encourage the liberal intake of clear
liquids up until 2 to 3 hours before the start of a scheduled procedure
in otherwise healthy children.41,120,154,164
Acceptable clear liquids are apple juice, water, sugar water,
sport/electrolyte drinks, and gelatin. Milk (including breast milk),
milk products, and juices with pulp are not considered clear liquids.
For elective procedures in children, most anesthesia and nursing
protocols now adhere to the so-called “2-4-6-8 rule” regarding PO
intake. This rule restricts clear liquids to 2 hours before the start
of an elective procedure requiring anesthesia, breast milk to 4 hours,
baby formula (cow’s milk) to 6 hours, and solid food to 8 hours prior.61
scheduled, elective procedures, those requiring sedation for emergency
procedures are at higher risk for aspiration, so caution is necessary
when considering administration of drugs that may depress protective
airway reflexes.5,176
In trauma patients, the time interval between last PO intake and time
of injury is a critical factor in the retention of gastric contents.123 Children injured within 1 to 2 hours after eating have been shown to have large gastric volumes.24
Gastric emptying may be further slowed in a child with a fracture by
the presence of pain and anxiety and the administration of opioid pain
relievers.69 At present, there is no
reliable method of assessing the volume of gastric contents, although
different methods have been suggested.67 Patient hunger on presentation for surgical treatment has been shown not to be a good indicator of an empty stomach.120
If circumstances of the injury permit and the procedure can wait, a
minimal fasting period of 4 hours is generally recommended before
administering sedative medications. IV fluids should be given to
prevent dehydration, medications to reduce gastric volume
(metoclopramide) or to decrease gastric acidity (histamine-2-receptor
blockers) should be administered intravenously 1 hour before sedative
medications, and sedation should be titrated tightly, utilizing the
minimal levels possible to allow completion of the procedure. The
appropriate dose of metoclopramide is 0.15 mg/kg. Famotidine, a
histamine-2-receptor blocker, may be given in a dose of 0.3 to 0.4
mg/kg IV, with a maximal dose of 20 mg. Pregnancy, morbid obesity,
gastroesophageal reflux, bowel obstruction, and increased intracranial
pressure all magnify the risk of regurgitation and aspiration of
gastric contents. Therefore, additional caution is necessary in
managing patients with any of these conditions. Patients with
coexisting bowel obstruction should not be sedated, and patients with
increased intracranial pressure should not be sedated without
neurosurgical evaluation and input. If treatment cannot wait and the
procedure or the patient is not appropriate for regional anesthesia,
the safest approach is to utilize general anesthesia with a rapid
sequence induction and a protected airway (endotracheal tube).
not always readily apparent. In children, long bone fractures and head
injuries may easily have associated large, concealed hemorrhages.174,191
It is important to assess the patient’s volume status accurately before
administering sedative medications. In a child who is hypovolemic,
sedatives may interfere with catecholamine-mediated compensatory
mechanisms and produce profound hemodynamic instability, leading to
cardiovascular collapse.
does not provide a good indication of the patient’s underlying volume
status.131,194 Children maintain a normal blood pressure for their age in the face of large intravascular volume deficits.194
More reliable signs of ongoing hypovolemia in children include
tachycardia, mottling, cool extremities, poor urine output (less than 1
to 2 mL/kg/hour), and altered level of consciousness. Each of these
signs can imply poor perfusion of different organ systems (skin,
musculoskeletal system, kidneys, and central nervous system,
respectively). Volume replacement, not sedation, should be the initial
goal in the management of hypovolemic children.
Respiratory depression from sedation, with resultant hypercapnia and
hypoxia, may aggravate an underlying closed head injury and worsen its
prognosis.191 In addition, any
pharmacologic change in the patient’s state of consciousness may
confuse the neurologic evaluation. Other injuries to major body
cavities or injuries associated with major blood loss should be
assessed carefully before any sedative medications are administered.
obstructed airway in a sedated child. Several common conditions in
children may predispose to breathing difficulty and airway obstruction
following sedation. For example, children with large tonsils and adenoids may have obstructive sleep apnea.110
Obstructive sleep apnea, which is associated with a history of loud
snoring and daytime sleepiness, may be acutely exacerbated with the
administration of sedative medications.45
Other conditions that may predispose to airway patency following
sedation include micrognathia (short jaw), limited ability to open the
mouth (arthrogryposis), and limited movement of the neck, either
congenital or acquired.17
|
TABLE 3-5 Airway Management Equipment
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
should be appropriately equipped to ensure optimal patient care and
safety. Equipment for resuscitation, airway management (Table 3-5), and vascular access (Table 3-6) must be immediately available for children of all ages and sizes.5
In addition, a positive-pressure oxygen delivery system capable of
delivering at least 90% oxygen for at least 60 minutes must also be
readily available A working suction apparatus must be easily accessible
to handle patient secretions, as well as for unexpected regurgitation
and vomiting.5
assessment, the practitioner must now decide which sedative or
sedatives to use. The ideal sedative should be easy to administer,
quick in onset, devoid of side effects, and rapid in termination of
effects. The abundance of references in the literature extolling the
virtues of different sedative drugs and drug combinations is the best
indicator that there is no one ideal choice. Since each drug has only
some of the properties of an ideal sedative medication and individual
patients may demonstrate considerable variability in response to the
same drugs, it is unwise and perhaps unsafe to try to fit every child
with a fracture into a particular sedation regimen. It is equally
important to remember that for those patients who cannot be adequately
sedated safely, fracture reduction should be performed under general
anesthesia.
|
TABLE 3-6 Vascular Access Equipment
|
|||||||
|---|---|---|---|---|---|---|---|
|
cocktail is a mixture of meperidine (Demerol) and two phenothiazines:
promethazine (Phenergan) and chlorpromazine (Thorazine). For multiple
reasons, this sedative regimen should be avoided.6
Prolonged and profound sedation occurs, often far outlasting the
procedure for which the sedation was intended. One study reported a
mean total recovery time of 19 hours, plus or minus 15 hours, in
children receiving DPT in the emergency department.175 Orthostatic hypotension is possible because promethazine and chlorpromazine are alpha-adrenergic blockers.42
Severe respiratory depression and death, both during and after the
procedure, have occurred in patients sedated with DPT. All three
medications in this mixture lower the seizure threshold, and
phenothiazines can produce dystonic reactions.42 There is no reversal agent for phenothiazine overdose.
be a commonly used sedative for children undergoing painless diagnostic
procedures, such as radiographic studies.21,109
Chloral hydrate may be administered orally or rectally in a dose of 20
to 75 mg/kg. The maximal single dose is 1 g. If more than one dose has
to be given, the upper limit for the total dose is either 100 mg/kg or
2 g, whichever is lower. Children receiving
chloral
hydrate must be observed for at least several hours. Respiratory
depression is unusual, but children with sleep apnea and adenoid and
tonsillar hypertrophy may be particularly vulnerable to airway
obstruction after sedation with chloral hydrate.20 At least one death has been reported following its use.89
These problems emphasize that even sedatives thought to have little
risk of producing respiratory depression must be administered under
properly supervised conditions and with strict adherence to dosage
guidelines.5
sedation of young children (< 6 years old) undergoing therapeutic
procedures, it has several disadvantages for the management of
fractures in children.147 First, the
onset of sedation is slow (40 to 60 minutes). Second, chloral hydrate
has no analgesic properties, and children can become disinhibited and
agitated in response to painful stimuli while under the influence of
the drug. Finally, recovery can be prolonged, taking up to several
hours with residual effects lasting as long as 24 hours. For these
reasons, chloral hydrate is not a preferred technique for sedation in
the management of fractures in children.21,109,136,137,147,148,157,158
treatment of patients with fractures. It provides no analgesia, and it
lacks the rapidity of onset and titratability of IV opioids and
benzodiazepines. The practitioner should be familiar with this
medication, however, because it remains in common use for nonpainful
pediatric procedures. Salient features regarding its administration are
summarized in Table 3-7.
In addition, barbiturates seem to lower the pain threshold and are
therefore a poor choice for producing sedation in the presence of a
painful condition such as a fracture. With these points in mind,
barbiturates should not be used for sedating children with fractures.
|
TABLE 3-7 Chloral Hydrate in Pediatric Sedation
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
1950s to provide anesthesia for patients undergoing office dental
procedures. Its use in the ambulatory setting for fracture management
is more recent.83,84,190
Nitrous oxide is a relatively weak inhalational anesthetic with low
solubility. It has weak sedative and analgesic properties. It acts
quickly on the central nervous system (CNS) and has a fairly short
duration of action, making it a good anesthetic option for fracture
treatment. Other desirable effects of nitrous oxide include a variable
degree of analgesia, sedation, anxiolysis, and amnesia.56,83,84,109,147,148
It does have the advantages of rapid onset, relative ease of use, and
rapid termination of effects. Because it diffuses rapidly into enclosed
air-filled spaces, its use is contraindicated in patients with bowel
obstruction or pneumothorax. Nitrous oxide is also contraindicated in
patients with altered intracranial compliance.109
This mixture of gases is most commonly delivered through a machine
which controls the rate of flow of the gases, regulates the mix, and
scavenges stray nitrous oxide from the surrounding environment. The gas
is self-administered through inspiratory effort with the child holding
the facemask. Once adequate sedation occurs, the child relaxes and
drops the mask. When the mask seal is broken, the flow of nitrous is
stopped. As a safeguard against overdosing, it is important that the
mask not be held by anyone but the patient. Fracture reduction can
begin within a few minutes of administration of the nitrous oxide.147,148
After the fracture has been immobilized, 100% oxygen is administered to
the child for approximately 5 minutes to wash out the nitrous oxide and
prevent diffusion hypoxia.56,84,148,190
administer, works quickly, and does appear to be safe. Nitrous oxide is
quickly eliminated and does not appear to suppress laryngeal reflexes.
The child does not have to have an empty stomach, and intravenous
access is not required.84,190
This method is not region specific and can be used for fractures in all
extremities. On the negative side, administration of nitrous can be a
problem in the child who is uncooperative or anxious with the face mask
and in the child who has difficulty obtaining a tight seal with the
mask.83,84,190
Other potential problems are nausea, vomiting, diffusion hypoxia, and
respiratory depression. Contraindications to the use of nitrous oxide
include the presence of significant cardiac or pulmonary disease, prior
administration of narcotics or sedatives, presence of a pneumothorax or
abdominal distension, middle ear infection, or altered mental status.
effective sedation for the management of fractures but that its
analgesic effects are variable.56,76,84,190 Evans et al.56
found nitrous oxide to provide similar analgesia but also to have a
faster onset of action, a shorter recovery time, and better patient
satisfaction compared to IM meperidine. Gregory and Sullivan76
compared nitrous oxide to IV regional anesthesia (Bier block) in a
prospective study of 28 children with upper extremity fractures and
found
that fracture reduction was completed in less time with nitrous oxide
although the pain response, as measured by a visual analog scale, was
worse. In two separate studies by Hennrikus et al.84 and Wattenmaker et al.190
with a combined total of 76 children, in whom nitrous oxide was used as
the sole anesthetic agent, fracture reduction was successful in 95% and
no complications were encountered. However, “moderate” or “significant”
pain was observed in 41% of the patients during fracture reduction, and
in the Wattenmaker study, analgesia was completely ineffective in 9%.
nitrous oxide alone, some have suggested that it be combined with
regional anesthesia. Hennrikus et al.83
reported on the use of nitrous oxide and hematoma block in 100 children
from 4 to 17 years old with various closed fractures treated in the
emergency department. When the techniques are combined, preliminary
administration of nitrous oxide provides sedation and anxiolysis that
facilitates placement of the regional block as well as an amnestic
response to fracture reduction. The hematoma block provides additional
analgesia both during and after the reduction. The study found a
significant decrease in behavior suggestive of pain with this technique
compared to an earlier study using nitrous oxide alone.83,190
This study illustrates the important point that where possible, the use
of regional anesthesia, in combination with almost any sedation
regimen, is an excellent way to enhance pain relief and minimize the
need for systemic sedative and analgesics.
administered to children with painful injuries to provide analgesia or
to supplement other methods of anesthesia in the emergency department
setting for many years.66,97,98,99,115,119,136,147,184
Narcotics produce analgesia by reversibly binding to opioid receptors.
In higher doses, they also have some sedative properties.
Benzodiazepines are primarily sedatives. They produce hypnosis,
anxiolysis, muscle relaxation, and some amnesia but have no analgesic
properties. When these two different classes of drugs are given in
combination, they act synergistically to induce a deep level of
sedation and analgesia.136
(IM, nasal, PO, and rectal) because it is the most reliable and
manageable.147 The effect following
IV administration is rapid in onset, can be readily titrated, and is
reversible if necessary. IV access should be obtained in a noninjured
extremity and the guidelines for conscious sedation should be adhered
to. The benzodiazepine is ideally administered prior to the narcotic in
order to provide a sedative effect. Low doses of medications should be
given initially and titrated for effect within recommended dosage
levels.115 Supplemental oxygen
should be administered if oxygen saturation falls below 90%. Reversal
agents such as naloxone for the narcotic and flumazenil for the
benzodiazepine should be readily available. Fracture reduction
typically can begin when the patient becomes drowsy.183
As a cautionary note, IV sedation should never be used in children with
history of apnea or airway disease, altered mental status, or
hemodynamic instability or in infants less than 2 months old.
Benzodiazepines provide anxiolysis, hypnosis, centrally mediated
relaxation of muscle tone, antegrade and retrograde amnesia, and
anticonvulsant activity.52,140,170 Benzodiazepines have no analgesic activity and require supplementation for painful procedures.170
used for pediatric sedation. It offers several advantages over other
benzodiazepines.199 It is water soluble and therefore usually relatively painless on injection,140,199
in contradistinction to diazepam, which can be quite painful on
parenteral administration. At physiologic pH, midazolam becomes highly
lipid soluble, facilitating transport into the CNS and onset of
sedative effects.199 Initial
recovery, which is due to redistribution of the drug away from the CNS,
occurs in about 30 minutes. On a milligram-per-milligram basis,
midazolam is at least two to three times as potent as diazepam, and the
elimination half-life of midazolam is significantly shorter than that
of diazepam, which is approximately 24 hours.68
Because of these characteristics, midazolam has supplanted diazepam as
the benzodiazepine of choice for noxious procedural sedation in most
emergency departments.103 However,
despite its relative pharmacokinetic deficiencies, diazepam is an
excellent muscle relaxant and continues to be a good choice for
fracture and/or joint reduction.147,148
Electroencephalographic studies indicate that the blood-brain
equilibration time is 4.8 minutes for midazolam versus 1.6 minutes for
diazepam.29 Therefore, when titrating midazolam for sedation, it is important to wait 5 minutes between doses.
mediated relaxation of skeletal muscle tone are presumed to occur from
a benzodiazepine-induced increase in the availability of glycine
inhibitory neurotransmitters.170 The
sedative effects of benzodiazepines are caused by facilitation of the
action of the inhibitory neurotransmitter gamma-aminobutyric acid.
However, the exact mechanism responsible for causing amnesia is not
known.170
Although generally considered very safe, orally administered midazolam
has been reported to produce airway obstruction in a child with
congenital airway anomalies.105 In general, the incidence of respiratory complications increases with the presence of major vital organ disease.88
However, even in healthy adult volunteers, IV sedation with midazolam
(0.1 mg/kg) can depress the ventilatory response to hypoxia.3 Concomitant administration of opioids greatly increases the risk of respiratory complications.88,170,202 Therefore, extra vigilance
and careful titration of medications to effect are even more important
when using more than one sedative or analgesic medication. As a general
rule, all patients who receive parenteral benzodiazepines (either IV or
IM) must be monitored with pulse oximetry.159
Loss of protective airway reflexes is also unlikely under these
circumstances as long as the physician pays careful attention to the
effects of each incremental dose on the patient’s state of
consciousness.140 Caution is always
urged if the patient’s stomach is full. Slurring of speech is a typical
sign of sedation with benzodiazepines. Children may also exhibit loss
of anxiety, unsolicited smiling, and even laughter.140 In reporting their experience with 2617 children sedated for endoscopic procedures, Massanari et al.112
noted that 36 patients exhibited paradoxical reactions to midazolam,
including inconsolable crying, combativeness, and agitation. These
adverse responses were effectively reversed with flumazenil, a
benzodiazepine antagonist, which is discussed below.
A liquid PO formulation, whose concentration is 2 mg/mL, is available
as Midazolam Hydrochloride Syrup 2 mg/mL. If this formulation is not
available at a particular location, then the practitioner can order the
parenteral form (usually the 5 mg/mL concentration) to be mixed in 5 to
10 mL of a sweet-tasting syrup.132
Acetaminophen and ibuprofen syrup are useful vehicles for the purpose
of concocting an elixir containing parenteral midazolam, keeping in
mind the appropriate pediatric doses of acetaminophen and ibuprofen.
The midazolam can be mixed in 3 to 5 mL of either syrup, and the
mixture is very palatable. Nasal administration of the parenteral
preparation (with no additives) is another option, though one that is
not often used as most children find its administration via this route
to be very unpleasant. In one study, 84% of children given intranasal
midazolam cried in response to administration of the medication.96
Although sublingual administration is a good idea from a pharmacologic
point of view (see discussion under morphine), it requires a degree of
patient cooperation that may be difficult to obtain in children, who
may be unwilling or unable to hold the medication under his or her
tongue.
the anxiety of children undergoing laceration repair in the emergency
department.58,82
The recommended dose of PO midazolam is 0.5 to 0.75 mg/kg, with a
waiting period of 10 to 30 minutes to allow adequate onset of effect.59
The maximal amount of midazolam that should be administered orally has
not been determined, but in practice the total dose is usually limited
to 20 to 25 mg. However, analgesic supplementation in the form of local
anesthetics, opioids, or both is required for painful procedures.
The initial flumazenil dose for children is 10 µg/kg IV, and it may
then be continued at 5 µg/kg/minute until the child awakens, or until a
total dose of 1 mg has been given.92
The elimination half-life of flumazenil is 30 minutes, compared with 1
to 2 hours for midazolam. Therefore, patients who receive flumazenil
should be observed for at least 2 hours before discharge to ensure that
resedation from the original benzodiazepine does not occur. Generally,
the use of flumazenil should be limited to situations of relative or
absolute benzodiazepine overdose leading to respiratory or hemodynamic
compromise. Routinely reversing benzodiazepines is both unnecessary
and, in the absence of persistent monitoring, potentially dangerous.
The anxiolysis and amnesia that midazolam produces make it an excellent
medication for procedural sedation, but supplemental analgesia is
required for painful procedures, such as the reduction of fractures.
For emergency procedures, IV titration is the best and most efficient
way to achieve desirable levels of patient sedation and cooperation. IV
midazolam may be titrated in increments of 0.05 mg/kg in combination
with the administration of opioids (discussed in the next section),
ketamine or a regional anesthetic block (e.g., Bier block, hematoma
block) for pain relief. Oral midazolam, with its mandatory 10- to
30-minute waiting period and limited ability to be titrated
effectively, is probably best reserved for use as a preoperative
medication before elective surgical procedures.
|
TABLE 3-8 Benzodiazepines in Pediatric Sedation
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
synthetic, that bind to specific receptors and produce morphine-like
effects. There are several types and subtypes of opioid receptors.11,172
Opioids vary in their respective affinity for receptor types,
accounting for differences in side effects. Opioids are classified as
pure receptor agonists (e.g., morphine, meperidine, fentanyl),
agonist-antagonists (e.g., nalbuphine), or pure antagonists (e.g.,
naloxone).172
Nausea and vomiting occur because of direct stimulation of the
chemoreceptor trigger zone in the floor of the fourth ventricle of the
medulla oblongata.172 Morphine
sulphate, meperidine, and fentanyl citrate are the most common
narcotics used for intravenous management of acute pain and painful
procedures in the emergency department.147
standard by which other narcotics are compared. It tends to be more
effective for continuous dull pain than the sharp pain typically
associated with fractures.136,137
It is the least lipid soluble of the narcotics listed and, as a
consequence, has a slower onset and longer duration of action (3 to 4
hours). As a result, it is difficult to titrate.136 Although morphine is usually administered parenterally (IV or IM), sublingual and rectal routes have been described.42
Oral morphine is usually used for long-term pain control in patients
with severe, chronic pain. Rectal morphine is unpredictably absorbed,
as is the case with most medications given via this route, and has been
associated with delayed onset of respiratory depression and death,
making it both impractical and unsafe.42,71
0.1 mg/kg. In infants younger than 3 months, the dose should be reduced
by at least one half because of increased susceptibility to respiratory
depression. Morphine should be reserved for painful procedures lasting
at least 30 minutes.42 Morphine is not very lipid soluble so CNS clearance is slow, which accounts for a potential duration of action of 3 to 4 hours.11,42
Hypotension secondary to vasodilation, histamine release, or vagally
mediated bradycardia can occur even with the administration of small
doses of morphine.11 Allergic
reactions are triggered by release of histamines and can cause symptoms
ranging from erythema along the course of the vein in which the
morphine is injected to pruritis to anaphylactic reactions, although
the latter are very rare.172
historically been a commonly used narcotic in the emergency department,
although it has some characteristics that make it less desirable than
other narcotics. It is one tenth as potent as morphine but has better
euphoric properties. It has a slightly faster onset and shorter
duration of action (2 to 3 hours) than morphine. Like morphine,
meperidine is also difficult to titrate. When used for initial pain
management, both drugs, but meperidine in particular, have been shown
to cause a significant increase in sedation recovery times.108
mg/kg. As with morphine, the dose should be reduced by at least one
half in infants younger than 3 months.143
Normeperidine, a metabolic breakdown product of meperidine, has been
associated with seizures, agitation, tremors, and myoclonus.85,94
Meperidine is not recommended for patients with an underlying seizure
disorder. Accumulation of normeperidine is more likely in situations of
prolonged meperidine administration. Therefore, meperidine should be
used cautiously, if at all, in the treatment of chronic pain.42
As with morphine, meperidine may produce hypotension due to various
mechanisms including vasodilation, histamine release, or vagally
mediated bradycardia.11 Like morphine, allergic reactions mediated by histamine release can also occur with meperidine.11
narcotic analgesic that is 100 times more potent than morphine and 1000
times more potent than meperidine on a milligram-to-milligram basis. It
is highly lipid soluble and rapidly penetrates the CNS. It has a rapid
onset of action with peak analgesia in 2 to 3 minutes. When
administered in low doses, its duration of action (approximately 30
minutes) is shorter than the either meperidine or morphine, and it can
be titrated more easily. For sedation, fentanyl is given IV in
increments of 0.5 to 1 µg/kg. The maximal total dose is 4 to 5 µg/kg.42,136,137,147,148
Infants less than 6 months old metabolize fentanyl more slowly than
older children and should be dosed more conservatively (one-third
normal).147,148
Fentanyl is also available in an oral raspberry-flavored lollipop known
as the Fentanyl Oralet, available in 200 µg, 300 µg, and 400 µg amounts.109
The recommended dose ranges from 10 to 20 µg/kg. Troublesome side
effects of this preparation include nausea and vomiting, pruritus, and
oxygen desaturation.153
can occur, especially, although not exclusively, at higher doses.
Respiratory arrest may occur, especially with the coadministration of
other sedatives.202 For these reasons, fentanyl should be titrated slowly to effect.
Nalbuphine and butorphanol are the most commonly used opioid
agonists-antagonists. Nalbuphine and morphine have the same analgesic
potency on a milligram-per-milligram basis.143
Nalbuphine has a shorter elimination half-life. Despite the fact that
this group of drugs has a so-called ceiling effect or limit on the
degree of respiratory depression, opioid agonist-antagonists have no
particular advantage over properly dosed opioids.49
The major problem with opioid agonist-antagonists is that their ceiling
effect on respiratory depression is often accompanied by a ceiling
effect for analgesia.172 A further
disadvantage of agonist-antagonists is that they can reduce the
analgesic effectiveness of pure agonists (e.g., morphine, meperidine,
fentanyl, codeine) if additional analgesia is required.49
Yet another potential problem is that administration of opioid
agonist-antagonists can precipitate acute withdrawal symptoms in
patients receiving opioids on a long-term basis.49
It is indicated as a reversal agent in the event of respiratory
depression associated with narcotic overdose. Rapid reversal of
narcotic effects may precipitate severe hypertension, pulmonary edema,
ventricular or supraventricular irritability, seizures, and cardiac
arrest.10,55
Dysphoria, nausea, and vomiting may also occur. These side effects of
acute narcotic withdrawal are caused by sympathetic nervous system
stimulation from abrupt reversal of analgesia and the sudden perception
of
pain.11
In attempting to reverse narcotic overdose, one must therefore be
cautious to not precipitate acute narcotic withdrawal. Accordingly,
administration of naloxone should be titrated to effect (relief of
respiratory depression) in increments of 1 to 5 µg/kg IV. It is equally
important to remember that naloxone has duration of action of 30 to 45
minutes, which may be shorter than the drug it is being used to
reverse. To prevent resedation, close patient observation is required,
and supplemental dosing of naloxone may be necessary.
a narcotic and a benzodiazepine and reversal is necessary, the narcotic
should be reversed first with naloxone. If respiratory depression
persists after 1 to 2 minutes, the benzodiazepine should be reversed
with flumazenil.134,136,137,184
Both reversal agents have a shorter half-life than the drugs they are
reversing. Therefore, monitoring must continue until all respiratory
effects have dissipated.
of benzodiazepines, the routine use of naloxone to reverse narcotic
sedative medications is unwarranted and, for reasons noted earlier,
potentially dangerous. Naloxone use should be reserved for situations
of airway compromise brought on by relative opioid overdose, and it
should never be used as a way of expediting patient discharge after
procedural sedation.
specific contraindications, including tenuous airway status, unstable
hemodynamic status, history of specific allergic reactions, or age of
less than 2 months old, and with careful monitoring and judicious
administration of drugs, IV sedation (opioids for analgesia and
benzodiazepines for amnesia and anxiolysis) has proven to be safe and
effective for the management of fractures in children of various ages.39,42,95,97,119,134,136,137,147,184,202 In a study by Varela et al.,184
104 children (ages 2 months to 15 years) received IV meperidine
(average dose 1.47 mg/kg) and midazolam (average dose 0.11mg/kg) prior
to fracture manipulation in an ambulatory setting. Physician
satisfaction with the sedation was good or excellent for 94% of the
reductions. Most of the children did display some signs of pain as the
fracture was manipulated; however, 93% had amnesia for the event. Minor
side effects including oversedation, hallucinations, and pruritus, and
emesis occurred in 14% of the patients. There were no episodes of apnea
or cardiorespiratory complications. Eighty-two of 86 parents (98%)
contacted were satisfied with the sedation as well. The authors
stressed the importance of careful patient monitoring, both during and
after the procedure.
(0.1 mg/kg) combined with a hematoma block is another effective
technique for fracture reduction in an ambulatory setting. With this
particular method of sedation, the midazolam is administered first,
followed by the morphine about 5 minutes later. The hematoma block is
performed, and the fracture is then reduced. As previously outlined,
careful patient monitoring is essential.
opioid and benzodiazepine combinations for pediatric sedation are
summarized in Tables 3-9 and 3-10. Opioid and benzodiazepine
combinations provide amnesia, analgesia, and sedation; the tradeoff is
additive respiratory depression and additive depression of protective
airway reflexes. In both elective and emergent situations, the
practitioner must:
-
Thoroughly evaluate the patient, as discussed earlier in the chapter.
-
Follow standard practice guidelines for deep sedation.5
-
Pay careful attention to dosing limits (see Table 3-10).
-
Be certain that both flumazenil and
naloxone are available. These medications are to be used strictly for
the treatment of absolute or relative overdose of benzodiazepines and
opioids, respectively. They should never be used routinely to expedite
discharge from the emergency room.
|
TABLE 3-9 Opioids in Pediatric Sedation
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TABLE 3-10 Fentanyl and Midazolam in Pediatric Sedation*
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||
phencyclidine, was first synthesized in 1963. It was developed to
produce the “anesthetic state (analgesia, amnesia, loss of
consciousness, and immobility)” without total CNS depression and was
approved for general clinical use in 1970.40,196 The commercial preparation of ketamine is a racemic mixture of two optical isomers with differing activity.196 Ketamine is typically administered via IV or IM routes,72,73,74 although rectal,149 oral,79,178 and intranasal193 routes of administration have been described in the literature (Table 3-11).
titrated and a smaller cumulative dose given to achieve the desired
effect. The onset of action is also quicker and recovery is more rapid.73,116
The IV dose of ketamine is 1 to 2 mg/kg and should be administered
slowly to avoid respiratory depression. The IM route can be used when
IV access is unobtainable. The IM dose is 4 mg/kg. Pain reduction has
actually been shown to be better following IM administration, but
recovery times are significantly longer and nausea and vomiting are
more common, making the IV route more preferable.144
Typically, fracture manipulation may begin within 1 to 2 minutes
following IV administration and 5 minutes after IM administration. A
repeat IM dose can be given after 10 to 15 minutes if the initial
effect is inadequate.73,74,115,116
|
TABLE 3-11 Ketamine in Pediatric Sedation
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
to nor-ketamine. Nor-ketamine has about one third the sedative and
analgesic potency of ketamine. As such, ketamine should be administered
cautiously or in reduced doses to patients with impaired hepatic
function. IV ketamine, given in a dose of 1 to 2 mg/kg, produces
unconsciousness within 30 to 60 seconds. Peak plasma concentrations
occur within 1 minute. Return of consciousness occurs within 10 to 15
minutes, although complete recovery may be delayed.170 Dose requirements and recovery times from ketamine are age related.107
(NMDA) and non-NMDA receptors, nicotinic and muscarinic cholinergic
receptors, and opioid receptors. Agonist actions of ketamine on opioid
receptors play only a minor role in its analgesic effects. The main
site of analgesic action is the NMDA receptor, which explains why
naloxone, a narcotic antagonist, does not reverse the analgesic effect
of ketamine. The psychotomimetic effects of ketamine, however, may
involve interaction with a specific subclass of opioid receptors known
as kappa receptors.101
anesthesia. Dissociative anesthesia refers to a cataleptic state
characterized by functional and electrophysiologic dissociation between
the thalamo-neocortical and limbic systems.196
Patients keep their eyes open and exhibit a slow nystagmic gaze.
Corneal and pupillary reflexes remain intact. Generalized hypertonicity
associated with ketamine are thought to be due primarily to effects on
the CNS and to a lesser extent on nicotinic acetylcholine receptors in
skeletal muscle.101 Patients receiving ketamine may exhibit purposeful movements but not necessarily in response to surgical stimulation.196
In one study of minor surgical procedures with ketamine anesthesia, no
additional analgesics were required for 24 hours postoperatively.86 Amnesia persists for about 1 hour after apparent recovery from ketamine.170
Changes in mood and body image, out-of-body experiences, floating
sensations, and frank delirium are all possible. Emergence phenomena
result from misinterpretation of auditory and visual stimuli at the
neurologic level.196 Although they
usually terminate within 24 hours, prolonged emergence phenomena
lasting as long as 10 to 12 months have been reported in children.126,170 The incidence of emergence reactions is
higher in patients older than 16 years, females, patients who have
received ketamine doses above 2 mg/kg IV, and patients with a history
of abnormal personalities.75,196 There is no evidence that emergence in a quiet environment decreases the incidence of this problem.196
Benzodiazepines (e.g., diazepam and midazolam) are the most effective
treatment for ketamineinduced delirium and hallucinations,115,116,196
and administration of a benzodiazepine 3 to 5 minutes before ketamine
is effective in almost entirely eliminating the possibility of
emergence delirium.188
may occur after ketamine use, and early attempts at ambulation should
be discouraged. Ketamine does not induce seizures and is not
necessarily contraindicated in patients with an underlying seizure
disorder.196 Ketamine is, however,
contraindicated in patients with increased intracranial pressure or
with abnormal intracerebral compliance. Thus, patients with head
injuries should not receive this drug, despite some reports that have
suggested a neuroprotective effect for ketamine.101,177
on the airway. It causes the production of increased salivary and
tracheobronchial secretions, which can lead to coughing, laryngospasm,
and airway obstruction. This problem may be especially treacherous in
patients with an ongoing respiratory infection. Glycopyrrolate, an
antisialogogue, should be administered 3 to 5 minutes before ketamine
(at the same time that the benzodiazepine is given) to ameliorate this
problem.109,115,116
The dose for glycopyrrolate is 5 to 10 µg/kg IV. For large children, a
dose of 0.2 mg (200 µg) of glycopyrrolate given IV is sufficient.
Unless there is some other strong indication for its use, ketamine
should be avoided in patients with ongoing infections of the
respiratory tract.
In addition, ketamine may not protect against aspiration of gastric
contents. In this regard, ketamine is no different from any other
sedative and analgesic except maybe for self-administered 50% nitrous
oxide in oxygen. Ketamine should never be given in an unmonitored
setting, such as a patient’s room on a regular hospital ward, or in a
clinic area that does not have appropriate monitoring and resuscitation
equipment.
leads to the release of endogenous catecholamines. Through such an
effect, ketamine produces a dose-dependent increase in heart rate and
blood pressure; therefore, it is useful in patients with mild
hypovolemia. However, ketamine is also a direct myocardial depressant
and can lead to cardiovascular collapse in patients who are profoundly
hypovolemic, and whose sympathetic nervous system is already maximally
stimulated. Ketamine also causes bronchodilation by the same mechanism
of sympathetic stimulation and, as such, may be used in children with
asthma.109
years. During that time, the safety and efficacy of ketamine sedation
for children undergoing painful procedures in an emergency department
setting has been established.73,74,136,137,141,147,148 In 1990, Green et al.73
performed a meta-analysis of 97 studies of ketamine sedation that
included administration to 11,589 children. Only two children (0.017%)
required intubation for laryngospasm. The incidence of emesis was 8.5%,
but there were no cases of aspiration. Because of its unique properties
and safe track record, ketamine is in many ways an ideal drug for
procedural sedation and analgesia in children in the emergency
department.147
with ketamine for sedation of children during fracture treatment or
management of other musculoskeletal problems. Of the studies reviewed
by Green et al. in their extensive analysis of the literature on
ketamine use in the emergency department, only one mentioned fracture
treatment as one of the indications for sedation by this method.30 A subsequent investigation by Green et al.73,74
on the use of IM ketamine sedation in the emergency department reported
successful utilization in seven children (out of 108 children) with
fractures. Physician satisfaction with the sedation was excellent. Most
of the patients (82.9%) were able to undergo fracture reduction within
5 minutes of ketamine injection. Several minor complications, including
hypersalivation, hypertonicity, rash, and vomiting, did occur but no
major problems were reported. Overall parental satisfaction was high in
this study.
have reported the efficacy and safety of ketamine sedation for fracture
reduction in the emergency department. In the McCarty et al. study,
which included 114 children with a variety of fractures, the time from
administration of the ketamine to manipulation of the fracture averaged
less than 2 minutes following IV dosing and less than 5 minutes
following IM administration.116 Pain
scale scores reflected minimal or no pain during fracture reduction.
Parental satisfaction was high, and 99% of parents responded that they
would allow it to be used again in a similar situation. Airway patency
and independent respiration were maintained. Minor adverse effects
including nausea (13 patients) and vomiting (eight patients) occurred
but only well into the emergence phase of the sedation. No major
problems were encountered.
anesthetic agent. Chemically, propofol is a substituted isopropylphenol
that is virtually insoluble in aqueous solutions and has to be
dissolved in lecithin-containing formulations, gaining it the popular
name of “milk of anesthesia.”170
Although originally used almost exclusively in operating rooms and
intensive care units, it has become increasingly popular in the
ambulatory-care setting for procedural sedation because it has
advantage of rapid induction, owing to its lipid solubility, and short
duration of action. Propofol has comparable amnestic properties to
midazolam with the advantages of more rapid onset (about 40 seconds) of
sedation, faster recovery, smoother emergence, and
antiemetic properties.81,109,161,183 There are several potential disadvantages of propofol, causing some to suggest that only an anesthesiologist use it.95
First, airway patency can be rapidly lost. Second, propofol has
vasodilatory and negative inotropic effects, which can lead to
hypotension.109 Third, as propofol
does not have analgesic properties, the concurrent use of an opioid
analgesic such as fentanyl or morphine is required, further increasing
the risk of respiratory depression and hypotension. Fourth, propofol
may be associated with opisthotonic posturing and myoclonus, neither of
which is desirable in a child with a fracture, and it also may cause
seizures.109 Finally, there is no
reversal agent for propofol, so adverse events must be treated
supportively until the drug is completely metabolized. It is the
author’s opinion that in children with fractures, propofol should be
reserved for administration in the operating room as part of a regimen
of general anesthesia by an anesthesiologist.
compared the safety and efficacy of ketamineversus fentanyl-based
protocols in the emergency management of pediatric fractures. In this
study, patients 5 to 15 years of age needing emergency fracture or
joint reduction were randomized to receive intravenous midazolam plus
either fentanyl or ketamine. During fracture reduction, ketamine
subjects (n = 130) had lower distress scores and parental ratings of
pain and anxiety than did fentanyl subjects (n = 130). Although both
regimens equally facilitated fracture treatment, deep sedation, and
procedural amnesia, orthopaedists favored the ketamine-based technique.
Recovery was 14 minutes longer for ketamine but fewer ketamine subjects
had hypoxia (6% vs. 25%), needed breathing cues (1% vs. 12%), or
required oxygen (10% vs. 20%) than did fentanyl subjects. Two ketamine
subjects did require assisted ventilation briefly and more ketamine
subjects vomited. Adverse emergence reactions were rare but equivalent
between regimens. The authors concluded that ketamine was more
effective for pediatric fracture reduction than fentanyl for pain and
anxiety relief and was associated with fewer respiratory complications,
although vomiting was slightly more frequent and recovery more
prolonged (mean 15 minutes) with ketamine.
compared the frequency and severity of adverse events associated with
four major parenteral drug combinations used for procedural sedation in
the emergency department in 2500 children (mean age 6.7 years old):
ketamine alone (n = 1492; 59.7%), ketamine/midazolam (n = 299; 12.0%),
midazolam/fentanyl (n = 336; 13.4%), and midazolam alone (n = 260;
10.4%). They identified a total of 458 adverse events (respiratory or
nausea/vomiting) in 426 patients (17%), and that patients receiving
ketamine with or without midazolam experienced fewer respiratory
adverse events than those sedated with the combination of midazolam and
fentanyl. Patients who received ketamine did experience more vomiting,
although none aspirated.
safety and efficacy of various forms of analgesia and sedation for
fracture reduction in the emergency department, Migita et al.119
identified eight randomized, controlled trials with a total of 1086
patients that showed, among parenteral drug combinations,
ketamine-midazolam to be associated with less distress during fracture
manipulation than fentanyl citrate-midazolam or propofol-fentanyl and
that patients receiving ketamine-midazolam required significantly fewer
airway interventions than those in whom either fentanyl-midazolam or
propofol-fentanyl were used. In another comparative study of
ketamine-midazolam versus propofol-fentanyl, Godambe et al. found that
propofol-fentanyl was comparable to ketamine in reducing procedural
distress associated with painful orthopaedic procedures in children in
an emergency department setting and that propofol-fentanyl was
associated with a shorter recovery time than ketamine. However,
propofol had a greater potential for respiratory depression and airway
obstruction than ketamine.
combination referred to as “ketofol,” has been shown to be very
effective for procedural sedation.197
This combination of drugs takes advantage of the rapid onset and short
duration of both ketamine and propofol, eliminates the need for
adjunctive benzodiazepine or opioid sedatives, and reduces the
potential adverse synergistic interactions associated with
administration of those drugs.
hematoma, intravenous regional, and regional nerve blocks have been
shown to be variably effective in providing anesthesia for fracture
treatment in children. These methods require the surgeon to be familiar
with regional anatomy, have working knowledge of the pharmocokinetics
and dosing of local anesthetic drugs, and be proficient in the
techniques of administering them. Compared to adults, these techniques
are often technically easier to perform in children because anatomic
landmarks are more readily identifiable,77
and physiologically, the relatively smaller calibers of the peripheral
nerves in children are more susceptible to the pharmacologic actions of
anesthetic agents.192 Local and
regional anesthetic drugs work by blocking the conduction of nerve
impulses. At the cellular level, they depress sodium ion flux across
the nerve cell membrane and, in this way, inhibit the initiation and
propagation of action potentials.173,198
After injection, local anesthetics diffuse toward their intended site
of action and also towards nearby vasculature where uptake is
determined by the number of capillaries, the local blood flow, and the
affinity of the drug for the tissues. Elimination occurs following
vascular uptake by metabolism in the plasma or liver. Vasoconstrictors
such as epinephrine are mixed with local anesthetics to decrease the
vascular uptake and prolong the anesthetic effect.
Amino amide local anesthetics include lidocaine, bupivacaine,
mepivacaine, prilocaine, etidocaine, and the relatively new agent
ropivacaine. Amino ester local anesthetics include procaine,
chloroprocaine, tetracaine, benzocaine, and cocaine. Following
absorption in the blood, esters are broken down by plasma
cholinesterase while amides are bound by plasma proteins and then
metabolized in the liver. Medications within each group have important
intrinsic differences in potency, duration of action, and potential for
toxicity.45,179 For example, lidocaine is significantly less toxic a drug than bupivacaine, but it also has a shorter duration
of action. An important feature of ropivacaine is that even though its
duration of action is similar to bupivacaine, it produces less CNS
toxicity and less cardiac toxicity.155
medications is also determined in part by the type of regional block
performed. For example, single-dose brachial plexus blocks tend to have
a far longer duration than do single-dose epidural or subarachnoid
blocks.45 Local adverse effects
include erythema, swelling, and, rarely, ischemia when injected into
tissues supplied by terminal arteries. Adverse systemic effects are
caused by high blood levels of local anesthetics and include tinnitus,
drowsiness, visual disturbances, muscle twitching, seizures,
respiratory depression, and cardiac arrest. Bupivicaine is particularly
dangerous because it binds with high affinity to myocardial contractile
proteins and can cause cardiac arrest.
local anesthetic agents. Clinically, the most important is systemic
toxicity of the CNS and cardiovascular system from relative overdose
into the circulation (Table 3-12). This type of
reaction is not a medication allergy but simply a function of placing
too much medication into the bloodstream. In the presence of a major
artery, even a few drops of local anesthetic can lead to seizure
activity. In most cases, however, the severity of systemic toxicity is
directly related to the concentration of local anesthetic in the
bloodstream.45 Seizures and cardiac
arrest may be the initial manifestations of systemic toxicity in
patients who rapidly attain a high serum level of medication.54,122,135 Agents with greater intrinsic potency, such as bupivacaine and etidocaine, require lower levels for production of symptoms.45
Dysrhythmias and cardiovascular toxicity may be especially severe with
bupivacaine, and resuscitation of these patients may be prolonged and
difficult.2,45 The prevention and treatment of acute local anesthetic systemic toxicity are outlined in Table 3-13.
Although the potential for CNS toxicity may be diminished with
barbiturates or benzodiazepines, given either as premedications or
during treatment of convulsions, these measures do not alter the
cardiotoxic threshold of local anesthetic agents. With rapid and
appropriate treatment, the fatality rate from local anesthetic
convulsions can be greatly decreased.45 It is essential to stay within accepted dose limits when using any local anesthetic (Table 3-14).
To aid in dose calculations, a simple formula for converting percent
concentration to milligrams per milliliter is provided in Table 3-15.
Although rare, true immune-mediated allergic reactions to local
anesthetics are possible and more likely to occur with amino esters
than with amino amides.27,63 Local nerve damage and reversible skeletal muscle changes have been reported from the use of local anesthetics.45
|
TABLE 3-12 Manifestations of Local Anesthetic Toxicity*
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
|
TABLE 3-13 Prevention and Treatment of Acute Local Anesthetic Systemic Toxicity
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
Although it declined in popularity as brachial plexus blocks were
developed, it was revived in 1963, when its safe and successful use for
the reduction of forearm fractures in adults was reported.87
Subsequently, a number of studies have described the effective use of
this technique of anesthesia for the treatment of upper extremity
fractures in children in an ambulatory setting.13,15,22,32,36,37,57,64,70,93,87,104,128,180 The block has also been described for use in lower extremity fractures but is less commonly utilized.104
upper extremity involves placement of a deflated pneumatic cuff above
the elbow of the injured extremity. Holmes87 introduced the
concept of two cuffs in an effort to minimize tourniquet discomfort
with prolonged inflation, but the practice has not proven to be
necessary for the limited amount of time it takes for fracture
reduction in a child.13,22,37 The tourniquet should be secured with tape to prevent Velcro failure.128
IV access is established in a vein on the dorsum of the hand of the
injured extremity with a 22- or 23-gauge butterfly needle. The arm is
exsanguinated by elevating it for 1 to 2 minutes. Although
exsanguination with a circumferential elastic bandage is described
classically, this method can be more painful and difficult to perform
in an injured extremity and is no more efficacious than the gravity
method.22,37,76,93
The blood pressure cuff is then rapidly inflated to either 100 mm Hg
above systolic blood pressure or between 200 and 250 mm Hg.13,22,37,76,93,128
The arm is lowered after cuff inflation. Lidocaine is administered, the
IV catheter removed, and reduction of the fracture performed. In the
traditional technique, the lidocaine dose is 3 to 5 mg/kg13,37,128 and, in the “mini-dose” technique, 1 to 1.5 mg/kg.22,57,76,93
|
TABLE 3-14 Maximal Recommended Doses of Commonly Used Local Anesthetics in Children
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||
immobilized and radiographs are obtained, in case repeat manipulation
is necessary. In any event, the tourniquet should remain inflated for
at least 20 minutes to permit the lidocaine to diffuse and become
adequately fixed to the tissues, thus minimizing the risk of systemic
toxicity.128,182
The blood pressure cuff may be deflated in either a single stage or
graduated fashion, although single stage release has proven to be
clinically safe and technically easier.57,93,128
During the entire procedure, basic monitoring is required, and cardiac
monitoring is suggested in case toxicity occurs. Routine IV access in
the noninjured extremity may be beneficial but is not required.13,76
Patients should be observed for at least 30 minutes following cuff
deflation for any adverse systemic reactions. Motor and sensory
function typically returns during this period, allowing assessment of
neurovascular status of the injured extremity prior to discharge.182
|
TABLE 3-15 Conversion Formula From % Concentration to Milligrams/Milliliter
|
|||
|---|---|---|---|
|
to the effectiveness of the traditional Bier block, utilizing a
lidocaine dose of 3 to 5 mg/kg, in managing forearm fractures in
children. Four large series with a total of 895 patients undergoing
this technique demonstrated satisfactory anesthesia and successful
fracture reduction in over 90% of cases.13,37,128,180 The most common adverse effect of the procedure in these studies was tourniquet pain in about 6% of patients.37,180 One patient experienced transient dizziness and circumoral parenthesis.180 One patient developed persistent myoclonic twitching following tourniquet deflation and was admitted for observation.128
complications with the “traditional” Bier block (lidocaine, 3 to 5
mg/kg), concerns and anecdotal reports of systemic lidocaine toxicity
(i.e., seizures, hypotension, tachycardia, arrhythmias) have prompted
development of a “mini-dose” (lidocaine, 1 to 1.5 mg/kg) technique of
IV regional anesthesia.22,57,93 Reports by Farrell et al.57 and Bolte et al.22 utilizing a lidocaine dose of 1.5 mg/kg and by Juliano et al.93
using a dose of 1.0 mg/kg in a total of 218 patients have shown the
mini-dose Bier block to be effective in achieving adequate anesthesia
in 94% of children studied. Although the exact mechanism of action is
uncertain, the primary site of action of the IV regional block is
thought to be the small peripheral nerve branches. At this anatomic
level, blockade is better achieved with a larger volume of anesthetic
that can be distributed more completely to the peripheral nerve
receptors. It appears to be the quantity (i.e., volume) and not the
dose of anesthetic that predicates success of the block. For any given
dose of lidocaine, diluting the concentration permits the
administration of a larger volume of fluid (see Table 3-4). This mechanism explains the success of the mini-dose technique. In the series by Juliano et al.,93
forearm fracture reduction was pain free in 43 of 44 patients (98%)
following IV regional block achieved with a very dilute lidocaine
solution (0.125%) and a relatively small total dose (1 mg/kg).
minidose technique, has several advantages. The technique is fairly
easy to administer. The onset of action of the block is relatively fast
(<10 minutes) but also of relatively short duration, allowing for
assessment of neurovascular function in the extremity after fracture
reduction and immobilization. However, rapid recovery may also be
considered a disadvantage as the analgesic effect of the local
anesthetic is lost once the tourniquet is deflated. A recent report in
adults examined the addition of the nonsteroidal anti-inflammatory drug
(NSAID) ketorolac to the local anesthetic solution and found that
patients did obtain prolonged analgesia after the tourniquet was
released.156 However, no pediatric
studies have been performed on this technique. An empty stomach is not
required. Tourniquet discomfort is the
most
common adverse side effect. Inadvertent cuff deflation with loss of
analgesia or systemic toxicity is a potentially significant problem.
Compartment syndrome has also been reported. Technically, placing the
tourniquet and obtaining IV access in the injured extremity can be a
challenge in the uncooperative child, and application of the splint or
cast can be cumbersome with the tourniquet in place. IV regional
anesthesia is unsuitable for lesions above the elbow.87
The technique is contraindicated in patients with underlying heart
block, known hypersensitivity to local anesthetic agents, and seizure
disorders. Although not totally contraindicated, caution is urged when
using this technique in patients with underlying hemoglobinopathies
such as sickle cell disease.
-
Confirm the immediate availability of a
functioning positive-pressure oxygen delivery system, as well as
appropriate airway management equipment (see Table 3-5).5 Also, confirm the immediate availability of medications for the treatment of anesthetic-induced convulsions (see Table 3-13). -
Start an IV infusion in the contralateral
arm. A patent IV line is of paramount importance in treating the
complications of this block. Obtain a baseline set of vital signs,
including systolic and diastolic blood pressure. Monitor pulse oximetry
as well as the ECG continuously. -
Select an appropriate tourniquet. An
orthopaedic tourniquet that can be fastened securely should be used.
Because Velcro may become less adhesive with time, check the tenacity
of the tourniquet before use. As an added safety measure, the
tourniquet may be covered with strong adhesive tape or an elastic
bandage after application. The tourniquet should fully encircle the arm
and overlap back on itself by at least 6 cm. The arm may be minimally
padded with cast padding underneath the tourniquet.22 If a pneumatic tourniquet is used, the physician must be familiar with the location of the tourniquet pressure gauge37
and valves, because these features vary in location from model to
model. Narrow-cuffed double tourniquets may not effectively occlude
arterial flow, and their use has been discouraged.87
Tourniquet discomfort should not be a problem during short procedures,
but if this develops, a second tourniquet can be applied distally over
the anesthetized area of the arm. -
Palpate the radial pulse of the injured limb.
-
Place and secure a short 22-gauge cannula
or 23-gauge butterfly needle in a vein on the dorsum of the hand of the
fractured limb. IV catheters can be secured more readily. If a distal
vein is unavailable, a proximal vein or even an antecubital vein can be
used, but may result in a less effective block.87 -
With the tourniquet deflated, exsanguinate the limb by vertically elevating it above the level of the heart for 60 seconds.
-
Rapidly inflate the tourniquet to a pressure of 225 to 250 mm Hg or 150 mm Hg above the patient’s systolic blood pressure.62
Check for disappearance of the radial pulse. Cross-clamping the tubing
of the cuff after inflation is discouraged because it might prevent
detection of a small leak.87 Constant observation of the cuff pressure gauge is recommended. -
Lower the extremity and slowly inject the
local anesthetic. This injection should be done over a period of 60
seconds. A concentration of 0.125 to 0.5% plain lidocaine (1.25 to 5
mg/mL) is used. (Bupivacaine is contraindicated for this block because
of its cardiotoxicity.) To prevent thrombophlebitis, the local
anesthetic solution must be free of any additives or preservatives.87 In different studies, the recommended dose of lidocaine has varied from 1.5 to 3.0 mg/kg.13,22,37,57,64,93,128,180 A dose of 1.5 mg/kg appears to be safe and effective and may produce a decreased incidence of complications.22 One study has recommended a maximal lidocaine dose of 100 mg for this block.57
The skin of the extremity becomes mottled as the drug is injected. The
patient, unless he or she is very sedated, and the parents, if they are
watching, should be warned that the extremity will look and feel
strange. Analgesia and muscle relaxation develop within 5 minutes of
injection.87 For fractures at the
wrist, placement of a regular Penrose drain tourniquet around the
distal forearm may improve distribution of the local anesthetic
solution at the fracture site (Fig. 3-3). -
To improve analgesia for fracture
reduction, the last 2 mL of local anesthetic solution may be injected
directly into the fracture hematoma (Fig. 3-4). The technique of local infiltration anesthesia, or hematoma block, is discussed later in this chapter. -
Reduce the fracture and apply the cast or splint.
-
Leave the cuff inflated for at least 15
minutes, even if the surgical procedure takes less time to prevent
significant entry of local anesthetic into the general circulation.87 -
Monitor the patient closely for at least
15 minutes for any complications related to the block. The treatment of
local anesthetic-induced systemic toxicity has been discussed (see Table 3-12). -
Depending on whatever sedation has been administered, the patient should be monitored until discharge criteria are met (see Table 3-1). An assistant must be present to watch the patient, the tourniquet, and the monitors at all times.
 |
|
FIGURE 3-3 Penrose drain tourniquet on the forearm to improve distribution of local anesthetic at the fracture site.
|
 |
|
FIGURE 3-4
Hematoma block performed by barbotage. Half the anesthetic is injected into the hematoma and then withdrawn from the fracture site until original volume is regained. The mixed material is then repeatedly aspirated and injected until the anesthetic is dispersed in the hematoma about the fracture site. |
anesthesia for the reduction of fractures, particularly in the distal
radius but also about the ankle.1,4,33,50,91
In this technique, a local anesthetic agent is injected directly into
the hematoma surrounding the fracture, the location of which is
confirmed by aspirating blood into the syringe (see Fig. 3-4).
This block is quick and relatively simple to administer. The skin is
prepped with a bactericidal agent and draped at the site of
infiltration. The fracture hematoma is aspirated with a 20- or 22-gauge
needle and then injected with plain lidocaine. The typical dose of
lidocaine is 1 to 3 mg/kg, which should be concentrated so as to limit
the total amount of fluid injected to less than 10 mL in order to avoid
elevating soft-tissue compartment pressures and minimize the risk of
creating a compartment syndrome or other neurovascular problem.203
Although the medication is rapidly absorbed into the circulation, the
resulting systemic blood levels of local anesthetic have been shown to
be well below those required for CNS toxicity.118
The anesthetic inhibits the generation and conduction of painful
impulses primarily in small nonmyelinated nerve fibers in the
periosteum and local tissues.136
Although direct injection of the hematoma theoretically converts a
closed fracture into an open one, there have been no reports of
infection with this technique.33 Reported complications with hematoma blocks in the upper extremity include compartment syndrome,203 temporary paralysis of the anterior interosseous nerve,203 and acute carpal tunnel syndrome.102
included in reports of this method, there are no studies of hematoma
block anesthesia administered exclusively to a pediatric population. In
three separate studies authored by Dinley and Michelinakis,50 Case,33 and Johnson and Noffsinger91 with a combined total of 491 adult and
pediatric patients, hematoma block was shown to be effective for the
reduction of a variety of fractures of the distal upper extremity in
patients of all ages. Despite the generally favorable experience with
hematoma block anesthesia, other methods of regional anesthesia have
been shown to be more effective for the management of upper extremity
fractures. A study by Abbaszadegan and Johnson1
found that analgesia during fracture reduction was superior with IV
regional (Bier block) anesthesia compared to hematoma block and that
fracture alignment following reduction was better as well. The authors
concluded that the more favorable outcomes achieved with Bier block
were related to better analgesia and muscle relaxation.1 Alioto et al.4
described the use of an intra-articular hematoma block in the lower
extremity for the manipulative reduction of ankle fractures in a
population that included both children and adults. The youngest patient
in their study group was 12 years old. The authors recommended that the
block be administered via a slow direct injection with careful ECG
monitoring for any evidence of dysrhythmias, and with this method,
found the technique to be safe, effective, and well tolerated by
patients.4
The hematoma is localized by aspirating blood into the syringe. The
local anesthetic solution is given gradually by barbotage —the
alternate injection of a small amount of medication and withdrawal of a
small amount of hematoma—until all of the medication has been given.
forearm and hand. Initial use of the technique is attributed to Halsted
and Hall, who first utilized axillary block for outpatient procedures
in 1884. The technique has since proven to be a safe and reliable
method of anesthesia for a variety of outpatient surgical procedures in
the upper extremity in both adults and children.192
It is an excellent choice of anesthesia for treatment of fractures
below the elbow because it provides muscle relaxation in addition to
analgesia. Cramer et al.46 reported
on the successful use of axillary anesthesia by orthopaedic surgeons in
the emergency department for the reduction of forearm fractures in
children. In this study, effective anesthesia was achieved in 105 of
111 (95%) children with no complications.
child in a supine position with the injured arm abducted and externally
rotated 90 degrees. IV access is usually established in the uninjured
extremity. Mild sedation may be helpful prior to the procedure. The
axilla is prepped with a bactericidal solution and draped with sterile
towels. The block is performed using a 1.0 % lidocaine solution at a
dose of 3 to 5 mg/kg. As with the Bier block, a larger volume of local
anesthetic is preferable and can be achieved by using a more dilute
concentration of drug. The target for delivery of the anesthetic agent
is the axillary sheath, which contains the axillary artery and vein
surrounded by the radial nerve (behind), median nerve (above), and
ulnar
nerve
(below). The musculocutaneous nerve courses outside of this sheath
through the coracobrachialis muscle and, for this reason, may escape
blockade, explaining the unreliability of this technique for anesthesia
above the elbow.
accurate delivery of the anesthetic into the axillary sheath including
blind injection into the neurovascular sheath, patient-reported
paresthesia, use of a nerve stimulator, and transarterial puncture.
Elicitation of paresthesias provides reliable evidence of position
within the neurovascular sheath but may be uncomfortable and requires a
conscious and cooperative patient. For these reasons, it cannot be used
in most children. The use of a nerve stimulator and insulated needle to
elicit a motor response is another effective method to determine
accurate location within the sheath. However, this technique requires
special equipment (nerve stimulator and insulated needles), which may
not be readily available in an ambulatory setting, and threshold
stimulation of the nerves may be distressful to the conscious patient.
With this method, the axillary artery is palpated, and a 23-gauge
butterfly needle, connected via extension tubing to a syringe
containing lidocaine, is inserted perpendicular to the artery. The
needle is advanced while being continuously aspirated until a flash of
arterial blood is seen and then advanced through the artery.
Approximately two thirds of the lidocaine is injected into the sheath
deep to the artery, checking by aspiration after every 5 cc to ensure
extravascular positioning. The needle is withdrawn to the superficial
side of the artery and the remaining lidocaine is injected. Pressure is
held over the puncture site for 5 minutes, and fracture manipulation
can usually begin shortly thereafter.
because of the dearth of subcutaneous fat making for a technically
easier procedure in a child than an adult. Of course, this advantage
can be offset if the child is obese or uncooperative. From a
pharmacokinetic standpoint, the local anesthetic diffuses more rapidly
and with enhanced blockade of the nerves, which are smaller in diameter
in children compared to adults.192
The duration of the block is usually prolonged enough to allow repeat
manipulation of the fracture in the event of an unsatisfactory
reduction.
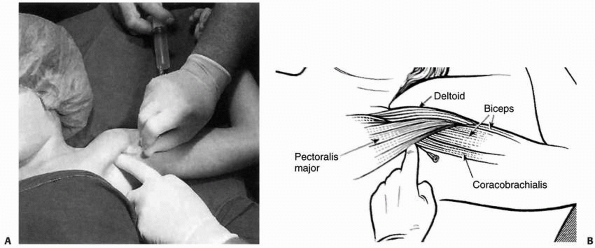 |
|
FIGURE 3-5
Technique of needle insertion for axillary block. The axillary artery is palpated and the needle inserted at the lateral edge of the pectoralis major and parallel to the coracobrachialis. (Adapted from McCarty EC, Mencio GA. Anesthesia and analgesia for the ambulatory management of children’s fractures. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 3rd ed. Philadelphia: Saunders, 2003.) |
include systemic lidocaine toxicity, hematoma formation, and persistent
neurologic symptoms. Horner’s syndrome has also been reported. In
actuality, complications of axillary block anesthesia are rare.192 None were encountered in the series reported by Cramer et al.46
of 111 children with displaced forearm fractures treated in an
emergency department setting. Contraindications to axillary block
anesthesia are the presence of a coagulopathy of any type, a
pre-existing neurologic or vascular abnormality of the extremity,
axillary lymphadenitis, or an uncooperative or combative patient.
any fracture of the upper extremity below the elbow, more-distal upper
extremity blocks at the wrist or of the digital nerves in the hand may
be useful for treatment of fractures or minor surgical procedures of
the hand. Anesthesia to the digits can be achieved by block of the
common digital nerves near the point of bifurcation at the level of the
metacarpal heads or by block of the radial and ulnar digital nerves at
the base of each finger. This technique is most useful for treatment of
phalangeal fracture(s) of a single digit. For injuries involving
multiple digits or the metacarpals, anesthesia of the hand can be
achieved by blocking the three major nerves of the upper extremity at
the wrist (wrist block). The median nerve is located on the radial side
of the palmaris longus tendon approximately 2 cm proximal to the wrist
crease and can be blocked with 3 to 5 mL of local anesthetic (Fig. 3-6A).
The ulnar nerve is blocked on the radial side of the flexor carpi
ulnaris about 2 cm proximal to the volar wrist crease with 3 to 5 mL of
local anesthetic, and the dorsal and volar cutaneous branches of the
nerve are blocked by subcutaneous
injection of an additional 2 to 3 mL of anesthetic (Fig. 3-6A).
Alternatively, the ulnar nerve may be approached from the ulnar side of
the wrist, just dorsal to the flexor carpi ulnaris tendon (Fig. 3-6B).
The terminal branches of the radial nerve are blocked by injection of 1
to 2 mL of anesthetic along the extensor pollicis longus tendon as it
crosses the base of the first metacarpal and across the “snuff box” to
the radial side of the extensor pollicis brevis tendon (Fig. 3-6C).
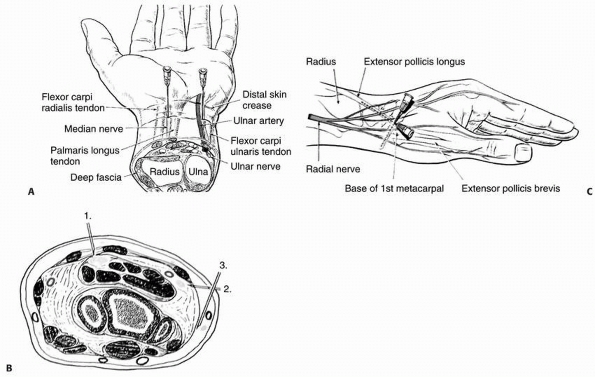 |
|
FIGURE 3-6 A.
Technique for median and ulnar nerve blockade at the wrist. The median nerve is approached from the palmar side of the wrist between the palmaris longus and flexor carpi radialis. The ulnar nerve can be approached between the flexor carpi ulnaris tendon and the ulnar artery. B. Alternative method for ulnar nerve blockade. The ulnar can also be approached from the ulnar side of the wrist just dorsal to the flexor carpi ulnaris tendon. C. Technique for radial nerve block at the wrist. Needle is inserted where the extensor pollicis longus tendon crosses the base of the first metacarpal and approximately 2 to 3 mm of local anesthetic injected as the needle is advanced along the tendon to the radial tubercle. The needle is then redirected at a right angle across the anatomical “snuff box” and an additional 1 to 2 mm injected to the radial border of the extensor pollicis longus tendon. (From McCarty EC, Mencio GA. Anesthesia and analgesia for the ambulatory management of children’s fractures. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children, 3rd ed. Philadelphia: Saunders, Philadelphia, 2003.) |
Although the majority of children with femoral fractures are not
managed on an outpatient basis, femoral nerve blockade can provide
excellent anesthesia and analgesia for the initial management of this
injury including manipulation of the fracture, application of an
immediate spica cast, or placement of a traction pin. It is a good
option for children unable to undergo general anesthesia or for those
who cannot be sedated for any reason. This technique is most effective
for fractures of the middle third of the femur but less so for
fractures of the proximal and distal thirds of the bone, as these areas
also receive sensory innervation from branches of the obturator and
sciatic nerves, respectively.
 |
|
FIGURE 3-7
Section of right thigh immediately below the inguinal ligament, showing femoral nerve under cover of fascia iliaca and its block by a barrage technique. (Reproduced with permission from Berry FR. Analgesia in patients with fractured shaft of femur. Anesthesia 1977;32:577.) |
and draping the inguinal area and palpating the femoral artery. A 22-
or 23-gauge needle on a syringe containing an appropriately dosed local
anesthetic agent (typically either 1% to 1.5% lidocaine with 1:200,000
epinephrine, dosed up to 7 mg/kg18 or 0.5% bupivicaine, dosed at 1 to 1.5 mg/kg145) is inserted one fingerbreadth lateral to the artery and 1 to 2 centimeters below the inguinal ligament (Fig. 3-7).
The needle is advanced at a 30 to 45-degree angle to the skin and the
syringe aspirated as the needle passes through the deep fascia into the
femoral triangle. If no blood is aspirated, the anesthetic agent is
injected
around
the femoral nerve. Alternatively, the nerve can be blocked more
proximally within the fascia iliaca compartment by entering just above
the inguinal ligament with the advantage of accessing all branches of
the nerve before it starts to arborize. As with axillary block, the
volume of the anesthetic is the key to achieving anesthesia with this
technique. The onset of analgesia occurs within 10 minutes and, with
the use of long-acting agents such as bupivicaine, may last up to 8
hours.48
In one randomized control study, regional blockade of the femoral nerve
was shown to provide clinically superior pain relief compared with IV
morphine sulfate throughout the initial 6 hours of management in
children aged 16 months to 15 years with isolated femoral shaft
fractures.188
inadvertent arterial punctures with no long-term sequelae and no
neurologic complications.48,78,145
Other potential complications include systemic toxicity from
intravascular injection, infection, and injury to the nerve. As with
the axillary block, this method may be difficult in obese children as
well as the young and/or uncooperative child. Contraindications include
any pre-existing neurologic abnormality of the injured lower extremity
and the inability to manage complications of systemic toxicity.
with musculoskeletal injuries can be accomplished with opioids, NSAIDs,
or local anesthetic agents. Simultaneous use of more than one modality
may be beneficial to minimize the side effects from any one particular
approach (e.g., the use of NSAIDs to decrease the incidence of nausea,
vomiting, or even respiratory depression from opioids). The end point
is to make patients comfortable while minimizing adverse reactions.
analgesia. It is important for the practitioner to understand the
rationale behind different dosage regimens to maximize pain relief for
the patient.
Wide variations in plasma opioid levels occur, leading to periods of
sedation alternating with prolonged periods of no pain relief at all.60
In addition, for pediatric care, IM dosing is a particularly poor
choice because children often choose to hide their pain rather than
risk having to undergo an injection. The end result with intermittent
dosing, especially with IM narcotics, is undertreatment of pain.
With PCA, intravenous self-titration of small doses of opioids at
frequent intervals eliminates the wide variations in plasma drug levels
seen with intermittent dosing. It also allows patients to gain control
over their pain management, which may be of psychologic importance to
the patient’s well-being. PCA was first evaluated in adolescents in
1987, after several years of successful use in adults.28 Since then, this modality has been used for children as young as 6 years of age.16
Depending on the intelligence and cooperative ability of the child, it
is conceivable that PCA could be used for younger individuals, although
careful assessment of each individual situation is required.
|
TABLE 3-16 Parenteral Opioid Dosing Schedule for Analgesia in Children*
|
||||
|---|---|---|---|---|
|
improved pain control and greater patient satisfaction have been
demonstrated.16 Further improvement
in pain relief may be achieved with the addition of a continuous
background infusion of opioids to maintain the plasma concentrations of
the analgesic during sleep. However, adding a background infusion may
increase the risk of opioid-associated nausea, sedation, and hypoxemia.51,200
Conceivably, for younger children or for children incapable of reliably
pushing the button on the PCA cord, “parent-controlled analgesia” may
be useful. This approach has led to many instances of oversedation. In
general, PCA is safest when only the patient is operating the device.
the maintenance dose, the lockout interval (the period during which no
further administration of medication will occur despite attempts to do
so by the patient), and the 4-hour maximum dose (Table 3-17). For PCA, morphine is more effective than meperidine.186
Opioids other than morphine should be used only for patients allergic
to morphine, or in whom morphine produces intolerable side effects.
Whenever possible, the persistent use of one medication helps avoid
dosing errors.25 The use of the PCA
pump should be explained to patients preoperatively. Effective use of a
loading dose will avoid the problem of having to “catch up” with
soaring levels of pain. Mishaps have occurred with PCA pumps due to
programming errors, so staff must be well-trained in safe use of the
equipment.195 Treatment of opioid-related side effects is outlined in Table 3-17.
continued management of diminishing postoperative pain, as soon as oral
intake is tolerated. Several oral analgesics are available, and their
appropriate use is summarized in Table 3-18.
All of these drugs have side effects including mood changes, nausea,
vomiting, constipation, dizziness, and pruritus. The occurrence and
degree of side effects vary from patient to patient, so the physician
should be prepared to change dosing regimens based on patient
response.
The use of NSAIDs (see the following section) as part of the analgesic
regimen may be helpful in reducing or eliminating troublesome
opioid-related side effects.
|
TABLE 3-17 Patient-Controlled Analgesia in Children
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
TABLE 3-18 Dosing Schedules and Formulations for Oral Opioids in Children
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||
surgery with excellent results. Communication and cooperation between
surgeons and anesthesiologists is critical to identify appropriate
candidates for this modality of analgesia whenever feasible.139
Unlike opioids, which produce analgesia by effects on CNS receptors,
NSAIDs act peripherally by inhibiting prostaglandin synthesis and
decreasing inflammation. Inflammatory mechanisms play an important part
in the pathogenesis of postoperative pain18;
therefore, the use of NSAIDs makes good sense in the postoperative
setting. Also, although NSAIDs have some troubling side effects of
their own, they do not produce respiratory depression, nausea, and
vomiting, which are some of the bothersome features of opioids. Thus,
using NSAIDs either as an adjunct or as a substitute for opioids when
feasible should help lessen opioidinduced nausea, vomiting, and
respiratory depression in the surgical patient.18
A history of sensitivity to aspirin or a history of nasal polyps may be
associated with potentially fatal cross-sensitivity to other NSAIDs.171 In children with asthma, the prevalence of aspirin sensitivity may be as high as 28%.138
Therefore, asthmatic children should probably receive only those NSAIDs
that do not cross-react with aspirin. These medications include
acetaminophen, salsalate, and choline magnesium trisalicylate (see Table 3-18).166
In a child with a chronic underlying bleeding disorder, NSAIDs are not
necessarily contraindicated. Consultation with the child’s hematologist
is advised regarding the use of specific medications in this class.
|
TABLE 3-19 Dosing Schedules and Formulations for Nonsteroidal Anti-inflammatory Drugs in Children
|
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||
only orally but also IV and IM. A loading dose of 1.0 mg/kg may provide
similar analgesia as 0.1 mg/kg of morphine.111
The pharmacology of ketorolac has been extensively reviewed and both
its mode of action and adverse reactions are generally typical of
NSAIDs.106 The major controversy with this drug remains its effect on hemostasis and bleeding. Rusy et al.146
found that ketorolac contributed to increased blood loss and more
difficulty in achieving surgical hemostasis in pediatric tonsillectomy
patients. Caution is advised in administering ketorolac or any other
NSAID in a perioperative situation in which bleeding has been or can be
significant. Ketorolac has been associated with an increased incidence
of nonunion in patients undergoing spine fusion. Suggested dosing
schedules for some of the more common NSAIDs are listed in Table 3-19.
Acetaminophen is considered a member of this class of medications,
although its mechanism of action is central and its effects on
prostaglandin synthesis and the inflammatory response are comparatively
very weak.187
physiologic alterations.201
Both central (epidural) and peripheral (e.g., brachial plexus) nerve
blocks may be used for this purpose. The physician must ensure that the
pain relief achieved does not mask the signs and symptoms of developing
vascular or neurologic compromise.53,124
Additional helpful measures include not forcing intake of oral fluids
until the child is hungry and minimizing early postoperative
ambulation, especially when opioids have been given.17
H, Jonsson U. Regional anesthesia preferable for Colles’ fracture:
controlled comparison with local anesthesia. Acta Orthop Scand
1990;61:348-349.
G. Cardiac arrest following regional anesthesia with etidocaine or
bupivacaine [editorial]. Anesthesiology 1979;51:285-287.
R, Furia J, Marquardt J. Hematoma block for ankle fractures: a safe and
efficacious technique for manipulations. J Orthop Trauma 1995;9:113-116.
Academy of Pediatrics Committee of Drugs. Guidelines for monitoring and
management of pediatric patients during and after sedation for
diagnostic and therapeutic procedures. Pediatrics 1992;6:1110-1115.
Academy of Pediatrics Committee of Drugs. Reappraisal of lytic
cocktail/demerol, phenergen, and thorazine (DPT) for the sedation of
children. Pediatrics 1995; 95:598-602.
Society of Anesthesiologists Task Force on Sedation and Analgesia by
Non-Anesthesiologists. Practice guidelines for sedation and analgesia
by non-anesthesiologists. Anesthesiology 1996;84:459-471.
B, Nilsson T, Ibler M, et al. Muscle tone under fentanyl-nitrous oxide
anaesthesia measured with a transducer apparatus in cholecystectomy
incisions. Acta Anaesthesiol Scand 1977;21(1):1-4.
I, Turndorf H. Severe hypertension and multiple atrial premature
contractions following naloxone administration. Anesth Anal
1979;58:524-525.
P, Stanley T. Pharmacology of intravenous narcotic anesthetics. In:
Miller R, ed. Anesthesia. 2nd ed. New York: Churchill-Livingstone, 1986.
S, Hyatt J, Shah N, et al. The effect of sensor malpositioning of pulse
oximeter accuracy during hypoxemia. Anesthesiology 1993;79:248-254.
C, Blasier R, Dodge B. Intravenous regional anesthesia: a safe and
cost-effective outpatient anaesthetic for upper extremity fracture
treatment in children. J Pediatr Orthop 1991;11:717-720.
J, Kean J, Lennox-Holt P. The child’s perception of the diseases and
experience of pain in juvenile chronic arthritis. J Rheumatol
1983;10(1):61-65.
C, Lehn B, Yee J, et al. Patient-controlled analgesia in children and
adolescents: a prospective comparison with intramuscular administration
of morphine for postoperative analgesia. J Pediatr 1991;118:461-466.
F. Anesthesia for the child with a difficult airway. In: Berry F, ed.
Anesthetic management of difficult and routine pediatric patients. New
York: Churchill-Livingstone, 1990.
P, Baraldi E, Pattenazzo A, et al. Adverse effect of chloral hydrate in
two young children with obstructive sleep apnea. Pediatrics
1993;92:461-463.
L, Leake L. Chloral hydrate for emergent pediatric procedural sedation:
a new look at an old drug. Am J Emerg Med 1991;9:530-534.
R, Stevens P, Scott S, et al. Mini-dose Bier block intravenous regional
anesthesia in the emergency department treatment of pediatric
upper-extremity injuries. J Pediatr Orthop 1994;14:534-537.
L. Patient-controlled analgesia in children and adolescents. In:
Ferrante FM, Ostheimer GW, Covino BG, eds. Patient-Controlled
Analgesia. Boston: Blackwell Scientific Publishing, 1990:129-138.
R Jr, Broadman L. Patient-controlled analgesia for postoperative pain
control in adolescents [abstract]. Anesth Anal 1987;66:S22.
M, Maitre P, Crevoisier C, et al. EEG effects of benzodiazepines. II.
Pharmacodynamic modeling of the EEG effects of midazolam and diazepam.
Clin Pharmacol Ther 1990;48:555-567.
G, Lari S, Serra G. La ketamina in ortopedia e traumatologia:
indicazioni e limiti. Chir Degli Organi Movimento 1972;61:99-104.
RS, Browner GJ, Cheng NG, et al. Femoral nerve block for femoral shaft
fractures in a paediatric Emergency department: can it be done better?
Eur J Emerg Med 2003; 10:258-263.
W, Said E. Intravenous regional anesthesia in the treatment of forearm
and wrist fractures and dislocations in children. Can J Surg
1993;36:225-228.
D. Trauma in children. In: Levin D, Morris F, eds. Essentials of
Pediatric Intensive Care. St. Louis: Quality Medical Publishing,
1990:671-676.
G. Dissociative anesthesia. In: Corssen G, Reves J, Stanley T, eds.
Intravenous anesthesia and analgesia. Philadelphia: Lea & Febiger,
1988.
CJ, Todres ID. The pediatric airway. In: Cote CJ, Todres ID, Ryan JF,
et al., eds. Practice of Anesthesia for Infants and Children. New York:
Elsevier Health Services, 1986:31-51.
B. Clinical pharmacology of local anesthetic agents. In: Cousins M,
Bridenbaugh P, eds. Neural Blockade in Clinical Anesthesia and
Management of Pain. 2nd ed. Philadelphia: J.B. Lippincott, 1988:111-144.
K, Glasson S, Mencio GA, et al. Reduction of forearm fractures in
children using axillary block anesthesia. J Orthop Trauma
1995;9(5):407-410.
RJ, Innes GM. Intravenous ketamine sedation of pediatric patients in
the emergency department. Ann Emerg Med 1997;29:146-150.
J, Anand K. Basic aspects of acute pediatric pain and sedation. In:
Deshpande J, Tobias, JD, eds. The Pediatric Pain Handbook. St. Louis:
Mosby, 1996:48.
E, Robinson D, Morton N. Comparison of patient-controlled analgesia
with and without a background infusion after lower abdominal surgery in
children. Br J Anaesth 1993;71:670-673.
J, Reichert C, Brown K. Compartment syndrome associated with
bupivacaine and fentanyl analgesia in pediatric orthopaedics.
1997;17:285-288.
J, Buckley S, Alexander A, et al. Analgesia for the reduction of
fractures in children: a comparison of nitrous oxide with intramuscular
sedation. J Pediatr Orthop 1995;15:73-77.
R, Swanson S, Walter J. Safe and effective iv regional anesthesia for
use in the emergency department. Ann Emerg Med 1984;14:239-241.
D, Jacobs I. A randomized, controlled trial of oral midazolam and
buffered lidocaine for suturing lacerations in children (the SLIC
trial). Ann Emerg Med 1995; 25:209-214.
F. Patient Characteristics Influencing Effective Use of
Patient-Controlled Analgesia. Boston: Blackwell Scientific
Publications, 1990.
L, Kulick R. Emergency department analgesic use in pediatric trauma
victims with fractures. Ann Emerg Med 1994;23:203-207.
T, Fukusaki M, Nakamura H, et al. Quantitative evaluation of gastric
contents using ultrasound. J Clin Anesth 1993;5:451-455.
D, Forrest P, Purdie G. Comparison of the recovery characteristics of
diazepam and midazolam. Br J Anesthe 1988;60:520-524.
G, Boas R. Fatal outcome with use of rectal morphine for postoperative
pain control in an infant. Br Med J 1992;304:766-767.
SM, Johnson N. Ketamine sedation for pediatric procedures: part 2,
review and implications. Ann Emerg Med 1990;19:1033-1046.
SM, Nakamura R, Johnson NE. Ketamine sedation for pediatric procedures:
part 1, a prospective series. Ann Emerg Med 1990;19:1024-1032.
SM, Sherwin TS. Incidence and severity of recovery agitation after
ketamine sedation in young adults. Am J Emerg Med 2005;23:142-144.
P, Sullivan J. Nitrous oxide compared with intravenous regional
anesthesia in pediatric forearm fracture management. J Pediar Orthop
1996;16:187-191.
G, Love B. Femoral nerve block: a simple and safe method of instant
analgesia for femoral shaft fractures in children. Aust NZ J Surg
1979;49:592-594.
CJ, Strait RT, Hennes H. A clinical trial of propofol vs midazolam for
procedural sedation in a pediatric emergency department. Acad Emerg Med
1999;6:989-997.
H, Wagner V, Bonadio W, et al. The effect of oral midazolam on anxiety
of preschool children during laceration repair. Ann Emerg Med
1990;19:1006-1009.
W, Shin A, Klingelberger C. Self-administered nitrous oxide and a
hematoma block for analgesia in the outpatient reduction of fractures
in children. J Bone Joint Surg 1995;77-A:335-339.
W, Simpson R, Klingelberger C, et al. Self-administered nitrous oxide
analgesia for pediatric reductions. J Pediatr Orthop 1994;14:538-542g.
S, Ozolins M, Elliott C, et al. Assessment of children’s distress
during painful medical procedures. Health Psych 1983;2:133-147.
P, Mazur J, Cummings R, et al. Low-dose lidocaine intravenous regional
anesthesia for forearm fractures in children. J Pediatr Orthop
1992;12:633-635.
R, Foley K, Gabrinsky P, et al. Central nervous system excitatory
effects of meperidine in cancer patients. Ann Neurol 1983;13:180-185.
R, Yang CI. Sedation and analgesia in pediatric patients for procedures
outside the operating room. Anesthesiol Clin North America.
2002;20(1):181-194, vii.
H, Keifer A, Rosenberger J, et al. Comparison of the safety and
efficacy of intranasal midazolam or sufentanil for preinduction of
anesthesia in pediatric patients. Anesthesiology 1989;76:209-215.
RM, Luhmann JD, Luhmann SJ. Emergency department management of painand
anxiety-related to orthopedic fracture care: a guide to analgesic
techniques and procedural sedation in children. Paediatr Drugs
2004;6:11-31.
RM, Porter FL, Miller JP, et al. Comparison of fentanyl/midazolam with
ketamine/midazolam for pediatric orthopedic emergencies. Pediatrics
1998;102: 956-963.
RM, Porter FL, Miller JP, et al. Comparison of fentanyl/midazolam with
ketamine/midazolam for pediatric orthopedic emergencies (comment).
Pediatrics 1999; 104:1167-1168.
J, Olerud C. Neurological complications of dynamic reduction of Colles’
fractures without anesthesia compared with traditional manipulation
after local infiltration anesthesia. J Orthop Trauma 1987;1:43-47.
B, Zurakowski D. Sedation patterns in pediatric and general community
hospital emergency departments. Pediatr Emerg Care 1998;14:99-103.
W, Jones W. Intravenous lidocaine for anesthesia in the lower
extremity. A prospective study. J Bone J Surg 1984;66:1056-1060.
JD, Reid S. Effects of initial pain treatment on sedation recovery time
in pediatric emergency care. Pediatr Emerg Care 2006;22:100-103.
S, Hershey S. Sedation for imaging and invasive procedures. In:
Deshpande J, Tobias J, eds. The pediatric pain handbook. St. Louis:
Mosby, 1996:263-317.
D, Orr W, Smith R. Sleep apnea, hypersomnolence, and upper airway
obstruction secondary to adenotonsillar enlargement. Arch Otolaryngol
Head Neck Surg 1977; 103:383-386.
M, Novitsky J, Reinstein L. Paradoxical reaction in children associated
with midazolam use during endoscopy. Clin Pediatr 1997;36:681-684.
EC, Mencio GA. Anesthesia and analgesia for the ambulatory management
of children’s fractures. Philadelphia: Saunders, 2003:606-617.
EC, Mencio GA, Green NE. Anesthesia and analgesia for the ambulatory
management of fractures in children. J Am Acad Orthop Surg 1999;7:81-91.
EC, Mencio GA, Walker LA, et al. Ketamine sedation for the reduction of
children’s fractures in the emergency department. J Bone Joint Surg
2000;82:912-918.
S, Rubenstein R, Gartner J, et al. Acute flank pain and reversible
renal dysfunction associated with nonsteroidal anti-inflammatory drug
use. Pediatrics 1993;92: 459-460.
R, Quick A, Lobmeyer L. Plasma lidocaine levels following hematoma
block for distal radius fractures. J Orthop Trauma 1989;3:187-189.
RT, Klein EJ, Garrison MM. Sedation and analgesia for pediatric
fracture reduction in the emergency department: a systematic review.
Arch Pediatr Adolesc Med 2006;160:46-51.
M, Wishar HY, Nummo WS. Gastric contents at induction of anesthesia—is
a 4-hour fast necessary. Br J Anesthe 1983;55:1185-1187.
R, Koury S. Respiratory arrest after intramuscular ketamine injection
in a 2-year-old child. Am J Emerg Med 1996;14:580-581.
D, Crawford R, Scurlock J. Severe hypoxia and acidosis following local
anesthetic-induced convulsions. Anesthesiology 1983;53:1185-1187.
B, Lugg P, Turner P, et al. Outpatient treatment of upper extremity
injuries in childhood using intravenous regional anaesthesia. J Pediatr
Orthop 1988;8:576-579.
I, Ramaioli F, Mapelli A. Prospettive sull’impiego clinico della
Ketamina cloridrato in ortopedia e traumatologia pediatrica. Minerva
Anes 1974;40:159-162.
R, Levin D. Shock. In: Levin D, Morris F, eds. Essentials of Pediatric
Intensive Care. St. Louis: Quality Medical Publishing, 1990.
B, Simpson T, Hauch MA, et al. Flumazenil reverses sedation after
midazolaminduced general anesthesia in ambulatory surgery patients.
Anesth Analg 1990;71(4): 371-376.
RD, Singh S, Pierce MC. Safe and efficacious use of procedural sedation
and analgesia by nonanesthesiologists in a pediatric emergency
department. Arch Pediatr Adolesc Med 2003;157:1090-1096.
J, Roberts M. Providing safe and effective sedation and analgesia for
pediatric patients. Emerg Med Reports 1993;14:207-217.
G, Coulson A, Siegel SC, et al. Aspirin intolerance in chronic
childhood asthma: detected by oral challenge. Pediatrics
1975;56:443-448.
L, Seleny F, Goodarzi M. Comparison of the calming and sedative effects
of nalbuphine and pentazocine for paediatric premedication. Can Anaesth
Soc J 1980;27: 546-549.
M, Wathen J, Bajaj L, et al. Adverse events associated with procedural
sedation and analgesia in a pediatric emergency department: a
comparison of common parenteral drugs. Acad Emerg Med 2005;12:508-513.
L, Houck C, Sullivan L, et al. A double-blind evaluation of ketorolac
tromethamine versus acetaminophen in pediatric tonsillectomy patients,
effects on analgesia, and bleeding. 1995;80:226-229.
N, Weisman S, Rosenblum M, et al. The use of oral transmucosal fentanyl
citrate for painful procedures in children. Pediatrics 1995;95:335-339.
M, Triebwasser A, Keon T. Ingestion of liquids compared to preoperative
fasting in paediatric outpatients. Anesthesiology 1990;72:593-597.
R, Steinberg R, Kreitzer J, et al. Intravenous regional anesthesia
using lidocaine and ketorolac. Anesth Anal 1995;81:110-113.
T, Yee J, Foley M, et al. Midazolam for conscious sedation during
pediatric oncology procedures: safety and recovery parameters.
1990;88:1172-1179.
J, Santer L. Respiratory arrest following intramuscular ketamine
injection in a 4-year-old child. Ann Emerg Med 1993;22:613-615.
EG, Pribble C, Bassett F, et al. Use of propofol sedation in a
pediatric emergency department: A prospective study. Clin Pediatr
2001;40:663-671.
M, Hoyt J, Gergis S. Studies in muscle rigidity, nitrous oxide, and
narcotic analgesic agents. Anesth Analg 1972;51:16-20.
W, Stewart J, Muir J. The effect of preoperative apple juice on gastric
contents, thirst, and hunger in children. Can J Anaesth 1989;36:55-58.
D, Simon R. Aspirin sensitivity: respiratory and cutaneous
manifestations. In: Middleton E Jr, ed. Allergy: Principles and
Practice. 3rd ed. St. Louis: Elsevier, 1988:1537-1554.
H, Hengstmann J, Shuttler J. Pharmacokinetics of fentanyl as a possible
explanation for recurrent respiratory depression. Br J Anaesth
1979;51:741-745.
RK. Nonopioid and Nonsteroidal Analgesic, Antipyretic, and
Anti-inflammatory Drugs. Philadelphia: J.B. Lippincott, 1987:240-250.
G. Neural physiology and local anesthetic action. In: Cousins MB,
Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and
Management of Pain. Philadelphia: J.B. Lippincott, 1987.
T. Anesthesia for trauma in the pediatric patient. In: Gregory G, ed.
Pediatric Anesthesia. New York: Churchill-Livingstone, 1989.
T. Pain control, analgesia, and sedation. In: Barkin R, Asch S, Caputo
G, et al., eds. Pediatric Emergency Medicine: Concepts and Clinical
Practice. St. Louis: Mosby Year-Book, 1992.
L. Complications related to anaesthesia in infants and children. A
prospective survey of 40,240 anaesthetics. Br J Anesth 1988;61:263-269.
J. Sedation in the pediatric intensive care unit. In: Deshpande J,
Tobias J, eds. The Pediatric Pain Handbook. St. Louis: Mosby,
1996:255-261.
J, Phipps S, Smith B, et al. Oral ketamine premedication to alleviate
the distress of invasive procedures in pediatric oncology patients.
Pediatrics 1992;90:537-541.
G, Mather L. Properties, absorption, and disposition of local
anesthetic agents. In: Cousins MB, Bridenbaugh PO, eds. Neural Blockade
in Clinical Anesthesia and Management of Pain. Philadelphia: J.B.
Lippincott, 1987.
P, Batten J, Hjorth D, et al. Intravenous regional anaesthesia for the
treatment of upper limb injuries in childhood. Aust NZ J Surg
1986;56:153-155.
A, Salem Y, Padeh S, et al. Is propofol safe for procedural sedation in
children. A prospective evaluation of propofol vs ketaminein pediatric
critical care. Crit Care Med 2002;30:1231-1236.
C, Lorfing K, Schmidt T. Intravenous sedation for the closed reduction
of fractures in children. J Bone J Surg 1995;77(3):340-345.
L. The safety of ketamine sedation in the treatment of traumatic
fractures in children. J Bone Joint Surg Am 2001;83-A:1593-1594.
P, Mortensen M. Pharmacokinetics of common analgesics,
anti-inflammatories and antipyretics in children. Clin Pharmacokniet
1989;17(Suppl 1):116-137.
JE, Gao D, Merritt G, et al. A randomized controlled trial comparing a
fascia iliaca compartment nerve block to a traditional systemic
analgesic for femur fractures in a pediatric emergency department. Ann
Emerg Med 2007;50:162-171.
JE, Roback MG, Mackenzie T, et al. Does midazolam alter the clinical
effects of intravenous ketamine sedation in children? A double-blind,
randomized, controlled, emergency department trial. Ann Emerg Med
2000;36:579-588.
I, Kasser J, McGravey A. Self-administered nitrous oxide for fracture
reduction in children in an emergency room setting. J Orthop Trauma
1990;4:35-38.
D. The pediatric trauma patient. In: Rasch D, Webster D, eds. Clinical
Manual of Pediatric Anesthesia. New York: McGraw-Hill, 1994.
R. Anesthesia for pediatric trauma. In: Stene JK, Grande CM, eds.
Trauma Anesthesia. Baltimore: Williams & Wilkins, 1991.
EV, Andolfatto G. A prospective evaluation of “ketofol”
(ketamine/propofol combination) for procedural sedation and analgesia
in the emergency department. Ann Emerg Med 2007;49:23-30.
S, Chudnofsky C, Dronen S. Comparison of midazolam and diazepam for
conscious sedation in the emergency department. Ann Emerg Med
1993;22:201-205.
M, Purcell G. Patient-controlled analgesia—the value of a background
infusion [letter]. Anaesthesia Intensive Care 1990;18:575-576.
M, Nichols D, Deshpande J, et al. Midazolam-fentanyl intravenous
sedation in children: case report of respiratory arrest. Pediatrics
1990;86:463-467.
KE, Anderson JL, Pribble CG, et al. Propofol for procedural sedation in
children in the emergency department. Ann Emerg Med 2003;42:773-782.
P, Joly A, Cluzel Field B, et al. Axillary block in children: single or
multiple injection? Paediatr Anaesth 2000;10:35-39.
AJ, Eyres RL, Cole WG. A comparison of prilocaine and lidocaine for
intravenous regional anaesthesia for forearm fracture reduction in
children. Paediatr Anaesth 2002; 12:146-150.
S, Silver P, Bock K, et al. Pediatric sedation for procedures titrated
to a desired degree of immobility results in unpredictable depth of
sedation. Pediatr Emerg Care 2001;17: 414-420.
E, Marhofer P, Greher M, et al. Brachial plexus anaesthesia in
children: lateral infraclavicular vs. axillary approach. Paediatr
Anaesth 2003;13:103-108.
JA, Wall EJ, Foad SL. Hematoma block reduces narcotic pain medication
after femoral elastic nailing in children. J Pediatr Orthop
2004;24:254-256.
GM, Nowakowski R, Troshynski TJ, et al. Risk reduction in pediatric
procedural sedation by application of an American Academy of
Pediatrics/American Society of Anesthesiologists process model.
Pediatrics 2002;109:236-243.
RM, Luhmann JD, Luhmann SJ. Emergency department management of pain and
anxiety related to orthopedic fracture care: a guide to analgesic
techniques and procedural sedation in children. Paediatr Drugs
2004;6:11-31.
RJ, Thompson JP. Anaesthesia for manipulation of forearm fractures in
children: a survey of current practice. Paediatr Anaesth
2000;10:273-277.
EC, Mencio GA, Green NE. Anesthesia and analgesia for the ambulatory
management of fractures in children. J Am Acad Orthop Surg 1999;7:81-91.
