Management of the Multiply Injured Child
of 1 year is trauma, not only in the United States, but worldwide.
Estimates of cost to the American public for the care of pediatric
trauma range from over $1 billion112 to $13.8 billion114
annually. A 1997 national pediatric inpatient database reported 84,000
orthopaedic trauma admissions, with a cost of $932.8 million in
hospital charges.54 Hospital charges for treatment of children with femoral fractures in the United States in 2000 was over $222 million.109 In 2003, the mean hospitalization expenditure was $28,137 for injury discharges, with a median of $10,808.136
Although isolated long-bone fractures still comprise the bulk of
orthopaedic injuries in children, a surprising number of these young
patients have multiple system injuries.
reported to occur annually in the United States, resulting in more than
500,000 hospitalizations and 15,000 to 20,000 pediatric deaths.135,140,150 In an urban practice at a level 1 trauma center, 1903 new fractures accounted for 5698 work relative value units.195
Boys are injured twice as often as girls and may account for an even
greater proportion of hospital admissions related to pediatric trauma.151,169
Blunt trauma is the mechanism of injury in most children and
preadolescents, whereas penetrating trauma more often is the source of
multiple injuries in adults. Although blunt trauma in the youngest
children is often due to child abuse, vehicular accidents and falls
from a height account for the more severe multiple injuries in the rest
of childhood.23 The cause of death
from trauma in children is generally severe head injury or severe
combined head, chest, and abdominal trauma.83,156
closely mirror those in adults. In the adolescent age group, alcohol
abuse is now considered a major factor in more than one third of
injuries resulting from accidents.113
Orthopaedists treating teenagers involved in vehicular accidents need
to be aware of the potential alcohol use in this age group and be
prepared to refer adolescents for appropriate counseling to avoid
future accidents and injuries.159
multiple injuries, fractures and other musculoskeletal injuries are
common in multitrauma and contribute significantly to the associated
morbidity.23,37,39,128
In one series from a pediatric trauma center treating children with
polytrauma, femoral shaft fractures accounted for 22% of the fractures;
9% of these fractures were open.23
Although less common, fractures of the spine, pelvis, and scapula and
clavicle were associated with longer stays in the hospital and in the
intensive care unit, in addition to having the highest associated
mortality rates.
diagnostic skill and fracture care. For example, calcaneal fractures
often result from axial loading and most commonly occur after a fall
from a height (40%) or from a motor vehicle accident (MVA) (15%).152,153
Associated fractures have been reported in approximately one third of
children with calcaneal fractures, including spine fractures in 5%.152,153
together. If a pedestrian child has been struck by an automobile, there
are often fractures in the ipsilateral upper and lower extremity.21
In one study, 58% (87/149) of children with femoral fractures due to
MVAs were noted to have associated injuries, including 14% with head
injuries, 6% with chest injuries, 5% with abdominal injuries, and 4%
with genitourinary injuries.77 The
coexistence of a femoral fracture and a head injury indicates
substantial high-energy trauma and has a more guarded prognosis than
does either of these injuries alone.
crosses all socioeconomic and ethnic groups and is the most common
cause of traumatic death in infants and toddlers. Currently, child
abuse is estimated to occur in 15 to 42 of every 1000 children in the
United States annually, resulting in more than 1200 deaths.88 Nonaccidental trauma has higher mortality and morbidity than accidental trauma.141
This diagnosis must be suspected in all cases of multiple injuries in
children younger than 2 years old if there is no obvious and witnessed
plausible explanation of the injuries. Abuse should be considered a
possible cause of injury in all young children with multiple long-bone
fractures in association with head injury. Pediatrician confidence in
identifying these injuries remains low.182
Even a single long-bone fracture associated with a head injury or
abdominal injury should raise suspicion of child abuse. Although the
corner fracture usually is thought of as being most characteristic of
child abuse, the most common extremity fracture caused by abuse is a
single transverse fracture of the femur or humerus, not multiple
fractures.84 There is no fracture
that is absolutely diagnostic of abuse; the entire clinical and social
picture needs to be taken into consideration. Orthopaedic surgeons have
difficulty distinguishing accidental from nonaccidental trauma when
faced with a long-bone fracture.93
Although rib fractures occur in only about 5% of children with multiple
injuries from trauma of other causes, they are more common in child
abuse.55,128
Whereas blunt compressive trauma to the thorax from other causes may
result in lateral rib fractures, the rib fractures seen in child abuse
are often posterolateral and adjacent to the transverse processes of
the thoracic spine.10,88,200
cases of abuse. Some authors have recommended a bone scan in
conjunction with the skeletal survey,115
although this recommendation is controversial since the addition of a
bone scan requires sedation, elevates radiation exposure, and increases
cost.2,84
Falls occur more often in younger children. One unfortunate example is
children who fall out of a second story window that is adjacent to a
bed. Injuries from falls result from direct impact or from deceleration
forces present at the time of landing. Direct impact usually causes
fractures, whereas internal injury more often results from the impact
forces. Although a variety of injuries can result from these falls, the
position of the body at impact and the surface on which the child lands
are important factors that affect the injury severity.59 Injuries associated with falls from heights include head injuries in 39% of children,92 orthopaedic injuries in 34% to 65%,92,132 and mortality in 5%.44
multiplesystem injuries in school-age children and preadolescents.
These injuries occur when a vehicle strikes a child on foot or riding a
bicycle, or when the child is a passenger in a car involved in an
accident. In 2002, more than 300,000 children aged 15 years and younger
were injured and more than 2500 were killed in such MVAs in the United
States.185
For childhood passengers injured or killed in car accidents, the risk
of death is six times greater for those unrestrained than for those
restrained at the time of injury.185
noted that many parents in California with children aged 6 years and
younger were unaware of basic safety information regarding child car
seats and airbags, and that they were also unaware of state laws
regarding child seat restraints. Severe injuries are higher for
children in the front seat.22 In
Arizona, a comparison of injuries sustained in children in MVAs who
were restrained or unrestrained showed higher mortality, longer mean
hospital stays, higher mean hospital charges, more hospital admissions,
and more fractures, intraabdominal injuries, and head injuries in
unrestrained passengers.31
children may be severely injured. Zuckerbraun et al.208
noted a higher incidence of cervical spine injuries in younger
children. Others have noted the importance of padding in child seats in
potentially decreasing the risk of head injury in children restrained
in child safety seats.91
be restrained in car seats when riding in a car, standard adult
shoulder and lap belts do not adequately restrain children who are too
big for car seats and too small for the standard restraints. Age and
size appropriate car seats and restraints are essential for child
occupant safety. Adjustable restraints to better accommodate the size
of the car occupant have been proposed to solve this problem. In
addition, there is increasing public sentiment to require seat belt use
on school buses, a policy that has been in place for physically
disabled student transport for some time.
and on bicycles is a laudable and important goal, the safety of
automobile travel can be dramatically improved with appropriate parent
education regarding child safety seats and airbags and by enforcement
of current laws.
specialized treatment center proved effective in improving survival in
the military setting, trauma centers, using the same principles of
rapid transport and immediate care, have been established throughout
the United States. These trauma centers are supported by the states on
the premise that the first hour after injury is the most critical in
influencing the rates of survival from the injuries. Rapid helicopter
or ambulance transport to an onsite team of trauma surgeons in the
trauma center has led to an improvement in the rates of acute survival
after multiple injuries have occurred.
because more adults than children are severely injured. However,
pediatric trauma centers have been established at numerous medical
centers across the United States with the idea that the care of
pediatric polytrauma patients differs from the care given to adults and
that special treatment centers are important for optimal results.65,67,80
The American College of Surgeons has established specific criteria for
pediatric trauma centers, which include the same principles of rapid
transport and rapid treatment by an in-house surgical team as in adult
trauma centers. A pediatric general surgeon is in the hospital at all
times and heads the pediatric trauma team. This surgeon evaluates the
child first, and the other surgical specialists are immediately
available. General radiographic services and computed tomography (CT)
capability must be available at all times for patient evaluation, and
an operating room must be immediately available.
severely injured children are improved if the children are brought to a
pediatric trauma center rather than a community hospital, 164 the costs
associated with such a center (particularly the on-call costs of
personnel) have limited the number of pediatric trauma centers. Younger
and more seriously injured children have improved outcomes at
children’s hospitals.45 Given the
limited number of pediatric trauma centers, patients frequently are
often either stabilized at other hospitals before transfer to a
pediatric trauma center or treated at an adult trauma center.
reported that there did not appear to be better outcomes for pediatric
trauma patients flown directly to a pediatric trauma center than for
those stabilized at nontrauma centers before transfer to the same
pediatric trauma center. Other centers have documented the need for
improved transfer coordination.147,167
studied the results of pediatric multiple injury care in an adult level
1 trauma center and concluded that the results were comparable to
national standards for pediatric trauma care. Sanchez et al.149
reported that adolescent trauma patients admitted to an adult surgical
intensive care unit (SICU) had similar outcomes to comparable patients
admitted to a pediatric intensive care unit (PICU) in a single
institution. However, those admitted to the SICU were more likely to be
intubated and to have a Swan-Ganz catheter placed and had longer ICU
stays and longer hospital stays.149
The use of a general trauma center for pediatric trauma care may be an
acceptable alternative if it is not feasible to fund a separate
pediatric trauma center.
injuries, the initial medical management focuses on the
life-threatening, nonorthopaedic injuries to stabilize the child’s
condition.114 The responsibility for
initial lifesaving resuscitation is rarely the responsibility of the
orthopaedist; however, such resuscitative efforts by the orthopaedist
may be more commonly required in nontrauma centers and those in rural
settings.
In severe injuries, the establishment of an adequate airway immediately
at the accident site often means the difference between life and death.
The cervical spine needs to be stabilized for transport if the child is
unconscious or if neck pain is present. A special transport board with
a cutout for the occipital area is recommended for children younger
than 6 years of age because the size of the head at this age is larger
in relation to the rest of the body. Because of this larger head size,
if a young child is placed on a normal transport board, the cervical
spine is flexed, a position that is best avoided if a neck injury is
suspected.71
hemorrhage from the injury, either internally or externally, is
assessed. This blood loss is replaced initially with intravenous
crystalloid solution. In younger children, rapid intravenous access may
be difficult. In this situation, the use of intraosseous fluid infusion
should be considered for administration of both fluid and medications.
Guy et al.64 reported successful
intraosseous infusion into the tibias of 15 children between the ages
of 3 months and 10 years. In this series, intraosseous needles were
placed by prehospital and hospital personnel and colloid, crystalloid
solution, and blood were all given by this route; no complications
occurred in the surviving children. Bielski et al.,15
in a rabbit tibia model, likewise demonstrated no adverse effects on
the histology of bone or the adjacent physis with intraosseous
injection of various resuscitation drugs and fluids.
rapidly reversed, the child’s blood pressure must be maintained at an
adequate level for organ perfusion. Most multiply injured children have
sustained blunt trauma rather than penetrating injuries, and most of
the blood loss from visceral injury or from pelvic and femoral
fractures is internal and may be easily underestimated at first. The
“triad of death,” consisting of acidosis, hypothermia, and
coagulopathy, has been described in trauma patients as a result of
hypovolemia and the systemic response to trauma.198 Peterson et al.129 reported that an initial base deficit of 8 portends an increased mortality risk.
pressure, caution needs to be exercised in children with head injuries
so that overhydration is avoided because cerebral edema is better
treated with relative fluid restriction. Excessive fluid replacement
also may lead to further internal fluid shifts, which often produce a
drop in the arterial oxygenation from interstitial pulmonary edema,
especially when there has been direct trauma to the thorax and lungs.
In some instances, in order to accurately assess the appropriate amount
of fluid replacement, a central venous catheter is inserted during
initial resuscitation. A urinary catheter is essential during the
resuscitation to monitor urine output as a means of gauging adequate
organ perfusion.
child’s condition, it is essential to perform a quick but thorough
check for other injuries. A number of injury rating systems have been
proposed, but the Injury Severity Score (ISS) is a valid, reproducible
rating system that can be widely applied in the pediatric polytrauma
setting (Table 4-1).197
Another injury rating system for children that has been shown to be
valid and reproducible is the Pediatric Trauma Score (PTS) (Table 4-2).197
The injury rating system chosen varies among trauma centers, but
whether the ISS or PTS is used, each allows an objective means to
assess mortality risk at the time of initial treatment, as well as
allowing some degree of prediction of future disability.126,169,204
Glasgow Coma Scale (GCS), which evaluates eye opening (1 to 4 points),
motor function (1 to 6 points), and verbal function (1 to 5 points) on
a total scale of 3 to 15 points (Table 4-3).174
There are some limitations in the use of the GCS in children who are
preverbal or who are in the early verbal stages of development, but in
other children this rating system has been a useful guide for
predicting early mortality and later disability. A relative head injury
severity scale (RHISS) is currently being validated40
and is available in trauma registries. As a rough guide in verbal
children, a GCS score of less than 8 points indicates a significantly
worse chance of survival for these children than for those with a GCS
of more than 8. The GCS should be noted on arrival in the trauma center
and again 1 hour after the child arrives at the hospital (Fig. 4-1).
Serial changes in the GCS correlate with improvement or worsening of
the neurologic injury. Repeated GCS assessments over the initial 72
hours after injury may be of prognostic significance. In addition to
the level of oxygenation present at the initial presentation to the
hospital, the 72-hour GCS motor response score has been noted to be
very predictive of later permanent disability as a sequel to the head
injury.117
examination is essential to allow early detection of injuries to the
liver, spleen, pancreas, or kidneys.
noted, and appropriate imaging studies are arranged to evaluate
potential extremity injuries more fully. If extremity deformity is
present, it is important to determine whether the fracture is open or
closed. Sites of external bleeding are examined, and pressure dressings
are applied if necessary to prevent further blood loss. A pelvic
fracture combined with one or more other skeletal injuries has been
suggested to be a marker for the presence of head and abdominal
injuries.190 Major arterial injuries
associated with fractures of the extremity are usually diagnosed early
by the lack of a peripheral pulse. However, abdominal venous injuries
caused by blunt trauma are less common and are less commonly diagnosed
before exploratory laparotomy. About half of abdominal venous injuries
have been reported to be fatal, so the trauma surgeon needs to consider
this diagnosis in children who continue to require substantial blood
volume support after the initial resuscitation has been completed.51
routinely done in the field. However, once the injured child is in the
hospital, the orthopaedist should personally inspect the extremities to
determine the urgency with which definitive treatment is needed. Most
important are whether a vascular injury has occurred and whether the
fracture is open or closed. The back and spine should be carefully
examined. If there is not an open fracture and if the peripheral
vascular function is normal, there is less urgency in treating the
fracture and splinting will suffice until the other organ system
injuries are stabilized.
resuscitated and stabilized and minimizes additional trauma to the soft
tissue envelope surrounding the fracture. Splinting also facilitates
transport of the child within the hospital while the trauma work-up,
including appropriate imaging studies, is completed. If the child is to
be transferred to a trauma center, splints are invaluable for patient
comfort and safety during transfer.
document the extremity function before any treatment. It is important
to remember that a detailed neurologic examination may not be possible
since these are often young and scared children who are in pain and may
have a central nervous system injury. The inability to obtain a
reliable examination should also be documented.
result in some injuries being missed initially. In a series of 149
pediatric polytrauma patients, 13 injuries were diagnosed an average of
15 days following injury, including five fractures (one involving the
spine), four abdominal injuries, two aneurysms, one head injury, and
one facial fracture.101 Given this
9% incidence of delayed diagnosis, it is imperative that polytrauma
patients be reexamined once they are more comfortable to reassess for
potential
sites of injury. In some cases, despite careful inpatient
reevaluations, some pediatric injuries escape detection until later
follow-up visits. In addition, children with head injuries need to be
reassessed once they awaken enough to cooperate with re-examination.
Families and patients need to be informed of the frequency of delayed
diagnosis of some injuries in polytrauma patients so that they can
partner with the medical team in recognizing such injuries (often
evident as previously undetected sites of pain or dysfunction).
|
TABLE 4-1 Injury Severity Score
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
possible after the initial resuscitation and physical examination. Any
extremity suspected of having a significant injury should be examined
on radiograph. If the child has a head injury or if neck pain is noted
on the examination, a lateral cervical spine radiograph is obtained.
Some centers evaluate the cervical spine with a CT scan in children
with polytrauma who have neck pain, a traumatic brain injury (TBI), or
who have been drinking alcohol.148
Further work-up with cervical spine magnetic resonance imaging (MRI) is
necessary before cervical spine clearance in those who have persistent
neck pain or tenderness and should be considered in patients who remain
obtunded (see “Magnetic Resonance Imaging”).
radiograph of this area almost always will detect it. If there is
suspicion of a cervical spine injury on the neutral lateral view, a
lateral flexion
radiograph
of the cervical spine taken in an awake patient will help detect any
cervical instability. The cervical spine of a young child is much more
flexible than the cervical spine in an adult. Under the age of 12
years, the movement of C1 on C2 during flexion of the neck can normally
be up to 5 mm, whereas in adults, this distance should be less than 3
mm. Likewise in this young age group, the distance between C2 and C3 is
up to 3 mm in flexion. No forward movement of C2 on C3 should be
present in a skeletally mature individual when the neck is flexed. This
so-called pseudosubluxation of C2 on C3 in a child should not be
diagnosed as instability that requires treatment because this is a
normal finding in most young children.30
Because it is difficult to detect a fracture of the thoracic or lumbar
spine clinically, radiographs of this area, primarily a lateral view,
should be carefully evaluated, particularly in a comatose child.
|
TABLE 4-2 Pediatric Trauma Score
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||
|
TABLE 4-3 Glasgow Coma Scale
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
injuries. If a head injury is present, CT of the head will detect skull
fractures and intracranial bleeding. With abdominal swelling, pain, or
bruising, CT of the abdomen provides excellent visualization of the
liver and spleen and allows quantification of the amount of hemorrhage
present. Because most hepatic and splenic lacerations are treated
nonoperatively,26,73,143
the CT scan and serial hematocrit levels are used to determine whether
surgical treatment of these visceral lacerations is needed.
CT also is useful for thoroughly evaluating fracture configuration and
determining appropriate treatment options, both surgical and
nonsurgical. If
abdominal
CT is being done to evaluate visceral injury, it is simple to request
that the abdominal CT be extended distally to include the pelvis. CT of
a fractured vertebra will provide the information needed to classify
the fracture as stable or unstable and determine whether operative
treatment is needed.
 |
|
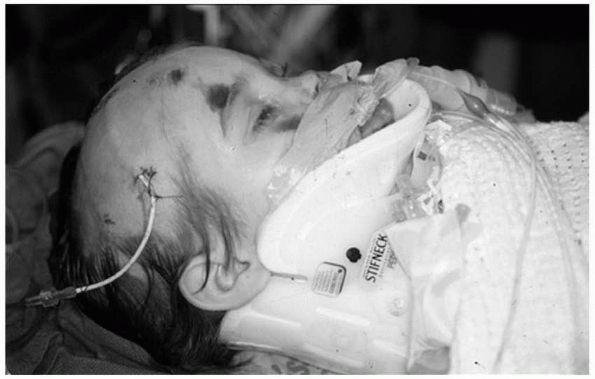
FIGURE 4-1
Temporary cervical spine stabilization is imperative in any child with multitrauma, especially those who are unconscious or complain of neck pain. |
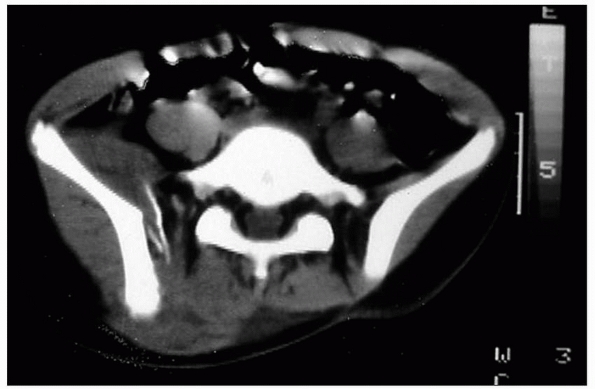 |
|
FIGURE 4-2 CT is an excellent addition to radiographs for evaluation of pelvic fractures.
|
anterior pelvic fractures, as well as with liver and spleen injury.
Although CT and ultrasonography are used to evaluate renal injuries,
the intravenous pyelogram still has a role in helping to diagnose
bladder and urethral injuries.125
Regardless of the methods of imaging, the anatomy of the urethral
disruption often cannot be accurately demonstrated preoperatively.3
of a child with multiple injuries. In conjunction with a skeletal
survey, a technetium-99m bone scan is sometimes used in children with
suspected child abuse to detect previously undetected new or old
fractures.2,84,115
reported that bone scans in 48 children with multiple injuries often
demonstrated an unsuspected injury. Nineteen previously unrecognized
fractures were identified by obtaining radiographs of the areas with
increased isotope uptake. In addition, there were 66 false-positive
areas of increased uptake in the 48 patients. Of their 48 patients, six
had a change in their orthopaedic care as a result of this bone scan,
although this treatment was usually simple cast immobilization of a
nondisplaced fracture. Nonetheless, the bone scan can be a valuable
screening tool in a child with multiple injuries from any cause. In
some instances, the bone scan can be useful to differentiate a normal
variation in skeletal ossification (normal uptake) from a fracture
(increased uptake), particularly in an extremity or a spinal area where
pain is present. Areas of increased uptake require further imaging
studies to determine if orthopaedic treatment is required.
brain or the spine and spinal cord. In young children, the bony spine
is more elastic than the spinal cord. As a result, a spinal cord injury
can occur without an obvious spinal fracture in children with multiple
injuries, particularly in automobile accidents.7,20,49
In the spinal cord injury without radiographic abnormality (SCIWORA)
syndrome, MRI is valuable in demonstrating the site and extent of
spinal cord injury and in defining the level of injury to the disks or
vertebral apophysis. A fracture through the vertebral apophysis is
similar to a fracture through the physis of a long bone and may not be
obvious on planar radiographs. MRI in obtunded and intubated pediatric
trauma patients has been reported to lead to a quicker cervical spine
clearance with a resulting decrease in hospital stay and cost.53
particularly when a hemarthrosis is present. If blood is present on
knee arthrocentesis, MRI can assist in diagnosing an injury to the
cruciate ligaments or menisci. In addition, a chondral fracture that
cannot be seen on routine radiographss may be demonstrated by MRI.
means of detecting hemoperitoneum following injury. Some trauma centers
have replaced peritoneal lavage and laparoscopy with serial ultrasound
evaluations to monitor liver, spleen, pancreas, and kidney injury in
children with multiple injuries.24,73,143
One problem with ultrasonography is the operator-dependent nature of
this imaging study. Another is the fact that, unlike CT,
ultrasonography cannot be used to rule out the frequently concomitant
pelvic fractures. As a result, CT is more often used for assessment and
monitoring of visceral injury in children sustaining multiple injuries.
Comparisons of CT and ultrasonography have demonstrated the superiority
of CT for diagnosing visceral injury in children with polytrauma.36,122,138,170
even more often than orthopaedic injuries. In a review of 494 pediatric
polytrauma patients, Letts et al.101 reported closed head injuries in 17% and skull fractures in 12%, while Schalamon et al.151
reported injuries to the head and neck region in 87% of pediatric
polytrauma patients. It has been clearly demonstrated that a child
recovers more quickly and more fully from a significant head injury
than does an adult.37,104,201
Even children who are in a coma for several hours to several days often
recover full motor function. Mild cognitive or learning deficits may
persist, however, so educational testing needs to be considered for
children who have had head injury and coma. Two factors that have been
linked to poorer functional recovery and more severe permanent
neurologic deficits are a low oxygen saturation level at the time of
presentation to the hospital and a low GCS score 72 hours after the
head injury. Because children with head injuries are often transported
long distances, it is difficult for them to have evacuation of a
cerebral hematoma within 4 hours.172
expected in most children after a head injury, children are often left
with some residual deficits. Many children who sustain TBIs are unaware
of their residual cognitive limitations and tend to overestimate
their mental capacities.66
Children who have had a TBI also often have behavioral problems, the
presence of which may be predictive of behavioral problems in uninjured
siblings as well.171 Greenspan and MacKenzie60
reported that 55% of children in their series had one or more health
problems at 1-year follow-up, many of which were relatively minor.
Headaches were present in 32% and extremity complaints in 13% of
patients. The presence of a lower extremity injury with a head injury
led to a higher risk of residual problems.
head injuries than for adults with similar injuries, orthopaedic care
should be provided in the most timely way possible, and the
orthopaedist should base the orthopaedic care on the assumption of full
neurologic recovery. Waiting for a child to recover from a coma is not
appropriate, and comatose children tolerate general anesthesia well.
The treatment undertaken for the orthopaedic injury is designed to
optimize the orthopaedic outcome from the injury, with the assumption
that the child will make a full neurologic recovery. Unless the
musculoskeletal injuries are treated with the assumption that full
neurologic recovery will take place, long-bone fractures may heal in
angled or shortened positions. Once neurologic recovery occurs, the
primary functional deficit will be from ill-managed orthopaedic
injuries rather than from the neurologic injury.
monitored to prevent excessive pressure, which may lead to further
permanent disability or death. Normally, intracranial pressure does not
exceed 15 mm Hg, and all attempts should be made to keep the pressure
under 30 mm Hg after a head injury. This is accomplished by elevating
the head of the bed to 30 degrees, lowering the PCO2, and restricting
intravenous fluid administration. Ventilator assistance is used to
lower the PCO2, which helps lessen cerebral edema. Fluid restriction
also is recommended if peripheral perfusion can be maintained despite
the polytrauma. Elevation of serum norepinephrine has been shown to
correlate well with the severity of head injury in patients with injury
of multiple organ systems.202
elevation of the intracranial pressure in children with multiple
injuries. Because of this problem, long-bone fractures must be
immobilized to limit fracture motion until definitive fracture care can
be provided. Initial immobilization is usually accomplished by
splinting or casting of the fractures, or by use of traction for
femoral shaft fractures. The use of external or internal fixation of
fractures should be strongly considered to help control elevation of
intracranial pressure. Fracture stabilization also facilitates dressing
changes for the treatment of adjacent soft tissue injury as well as
allowing in-hospital transport for imaging studies and other necessary
treatments.178,179
musculoskeletal injuries, even after the acute phase has passed.
Persistent spasticity, the development of contractures, heterotopic
bone formation in soft tissue, and changes in fracture healing rates
are all sequelae of a head injury in children.
head injury. The early effect of this spasticity is to cause shortening
at the sites of long-bone fractures if traction or splint or cast
immobilization is being used. If fracture displacement or shortening
occurs in a circumferential cast, the bone ends may cause pressure
points between the bone and the cast, leading to skin breakdown at the
fracture site, with a higher risk for deep infection. Even with
skeletal traction for femoral fractures, fracture shortening and
displacement will occur as the spasticity overcomes the traction
forces. Once spasticity develops and long-bone fractures displace,
internal or external fixation is needed to maintain satisfactory
reduction. This operative stabilization should be done as soon as the
spasticity becomes a problem for fracture reduction because fracture
healing is accelerated by a head injury.177,178,179
extremities often leads to subsequent contractures of the joints
spanned by the spastic muscles. Contractures can develop quickly, and
early preventative stretching or splinting should begin while the child
is in the intensive care unit. Nonselective mass action muscle activity
associated with brain injury can be used to help prevent these early
contractures. If the child lies in bed with the hips and knees
extended, there will usually be a strong plantarflexion of the feet at
the ankles. If the hip and knee are flexed, it will be much easier to
dorsiflex the foot at the ankle, so part-time positioning in this way
will prevent early equinus contractures from developing. Stretching and
splinting can often be effective in preventing contractures, and
casting may be needed if contractures develop. If these measures are
not successful and are interfering with rehabilitation, there should be
no hesitation to treat these contractures surgically.
the soft tissues of the extremity as early as a few weeks after a head
injury with persistent coma.86
Although any joint can be affected, the most common sites are the hip
and elbow. There is some evidence that heterotopic bone formation can
be stimulated by surgical incisions. In head-injured teenagers who
undergo antegrade reamed femoral intramedullary nailing of femoral
fractures, heterotopic bone that later restricts hip motion can form at
the nail insertion site.81 A sudden
increase of alkaline phosphatase a few weeks after the onset of coma,
even with fractures coexisting, may mean that heterotopic bone is
starting to form and a more careful examination of the extremities is
indicated.119 Technetium-99 bone
scans show increased isotope uptake in the soft tissue where
heterotopic bone forms, and this imaging study should be considered if
new swelling is noted in the extremity of a comatose child. Other
diagnoses that must be considered in a comatose child with new swelling
of the extremity are a new long-bone fracture and deep venous
thrombosis.166
taken in managing heterotopic bone formation in an injured child. If
the child remains comatose, usually little treatment is administered.
There are no conclusive data to support medical treatment if an early
diagnosis of heterotopic bone formation is made. However, it may be
useful to try to block some of the heterotopic bone formation by use of
salicylates or nonsteroidal antiinflammatory medication once an early
diagnosis is established. If the child has recovered from the head
injury and has heterotopic bone that does not interfere with
rehabilitation, no intervention is required. If there is significant
restriction of joint
motion
from the heterotopic bone, this bone should be excised to facilitate
rehabilitation. The timing of the heterotopic bone excision is somewhat
controversial, but resection should be considered whenever heterotopic
bone significantly interferes with rehabilitation, rather than waiting
for 12 to 18 months until the bone is more mature. After surgical
excision, early postoperative prophylaxis with local low-dose radiation
therapy or medications (salicylates or nonsteroidal antiinflammatory
drugs) is needed to minimize the risk of recurrence. Mital et al.119
reported success in preventing recurrence of heterotopic bone after
excision by use of salicylates at a dosage of 40 mg/kg/day in divided
doses for 6 weeks postoperatively.
It has been demonstrated that polytrauma patients in a coma have a much
higher serum calcitonin level than do conscious patients with similar
long-bone fractures, but how or whether this finding influences
fracture healing is still unclear.43
neurologic deficits in a child with multiple injuries, peripheral nerve
injury should be considered as well during the rehabilitation process.
In one clinical review of brain-injured children, 7% had evidence of an
associated peripheral nerve injury documented by electrodiagnostic
testing.130 For closed injuries, the
peripheral nerve injury is typically associated with an adjacent
fracture or with a stretching injury of the extremity. In most cases,
observation is indicated since these injuries often recover
spontaneously. However, if the nerve injury is at the level of an open
fracture, then exploration of the nerve is indicated. In children being
observed following a nerve injury, if function does not return within 2
to 3 months, then electrodiagnostic testing should be undertaken. It is
important to recognize these injuries because surgical peripheral nerve
repair with nerve grafts offers an excellent chance of nerve function
recovery in young patients.
of pediatric polytrauma patients. Abdominal swelling, tenderness, or
bruising are all signs of injury. CT evaluation has largely replaced
peritoneal lavage or laparoscopy as the initial method of evaluation of
abdominal injury.173 Abdominal
injury is not unusual if a child in an accident has been wearing a lap
seat belt, regardless of whether a contusion is evident.26,184 Bond et al.19
noted that the presence of multiple pelvic fractures strongly
correlated (80%) with the presence of abdominal or genitourinary
injury, whereas the child’s age or mechanism of injury had no
correlation with abdominal injury rates. Although hepatic and splenic
injuries are much more common, 22% of pediatric cases of pancreatitis
have been reported to result from trauma.14
lacerations nonoperatively, by monitoring the hematocrit, by repeating
the abdominal examination frequently, and by serial CT scans or
ultrasound examinations.28,33,34,35,100,173,186
Once the child’s overall condition has stabilized, and the child is
stable to undergo general anesthesia, the presence of nonoperative
abdominal injuries should not delay fracture care.
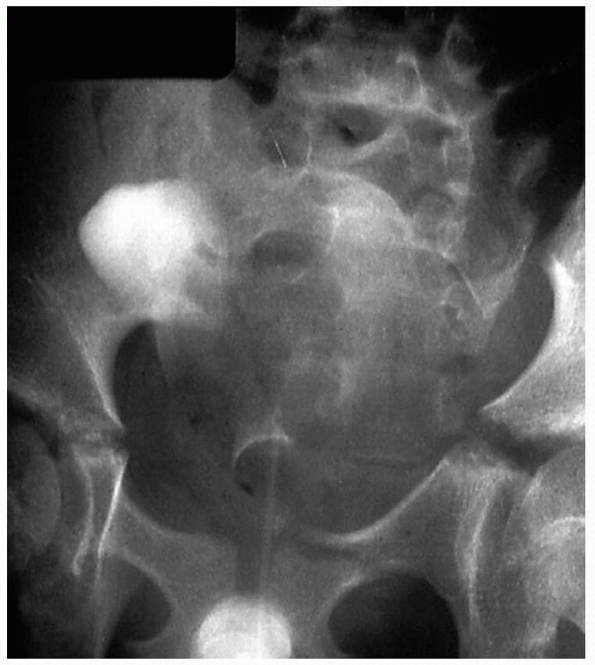
of children with pelvic fractures. Most injuries to the bladder and
urethra are associated with fractures of the anterior pelvic ring (Fig. 4-3).11
Such injuries are more common in males and usually occur at the
bulbourethra, but the bladder, prostate, and other portions of the
urethra can also be injured.11,125
Although less common following pelvic fracture in girls, such injuries
are often associated with severe injuries, including those to the
vagina and rectum, with long-term concerns regarding continence,
stricture formation, and childbearing.133,145
If the iliac wings are displaced or the pelvic ring shape is changed,
it may be necessary to reduce these fractures in order to reconstitute
the birth canal in female patients. There are increased rates of
caesarean section in young women who have had a pelvic fracture.38
Adolescent females with displaced pelvic fractures should be informed
of this potential problem with vaginal delivery. If the injury is
severe, kidney injury may also occur, but most urologic injuries that
occur with pelvic fractures are distal to the ureters.1
syndrome are relatively common in adults with multiple long-bone
fractures, they are rare in young children.106,142
When fat embolism occurs, the signs and symptoms are the same as in
adults: axillary petechiae, hypoxemia, and radiograph changes of
pulmonary infiltrates appearing within several hours of the fractures.
It is likely that some degree of hypoxemia develops in some children
after multiple fractures, but the full clinical picture of fat embolism
seldom develops. If a child with multiple fractures without a head
injury develops a change in sensorium and orientation, hypoxemia is
most likely the cause, and arterial blood gases are essential to
determine the next step in management.
The other primary cause of mental status change after fractures is overmedication with narcotics.
 |
|
FIGURE 4-3
Most injuries to the bladder and urethra are associated with anterior pelvic ring fractures and should be suspected with these injuries. |
oxygenation, the treatment is the same as in adults, generally with
endotracheal intubation, positive pressure ventilation, and hydration
with intravenous fluid. The effect of early fracture stabilization,
intravenous alcohol, or high-dose corticosteroids on fat embolism
syndrome has not been studied well in children with multiple injuries.
Previously, pulmonary embolism was rarely reported in association with
pediatric trauma, but literature reports have increased. The risk of
deep venous thrombosis and pulmonary embolism is increased with older
children, a higher ISS, and central venous catheter placement.42
If an injured child requires ventilator support for several days,
caloric intake through a feeding tube or a central intravenous catheter
is necessary to avoid catabolism, improve healing, and help prevent
complications. The baseline caloric needs of a child can be determined
based on the weight and age of the child. Children on mechanical
ventilation in a PICU have been shown to require 150% of the basal
energy or caloric requirements for age and weight.176 The daily nitrogen requirement for a child in the acute injury phase is 250 mg/kg.
generally suffices as the initial orthopaedic care while the child’s
overall condition is stabilized. Loder107
reported that, in 78 children with multiple injuries, early operative
stabilization of fractures within the first 2 or 3 days after injury
led to a shorter hospital stay, a shorter stay in the intensive care
unit, and a shorter time on ventilator assistance. In addition, there
were fewer complications in those who had surgical treatment of the
fractures less than 72 hours after injury. In a more recent study,
Loder et al.108 reported a trend
toward a higher rate of complications of immobilization (including
pulmonary complications) in fractures treated late (after 72 hours),
but the difference did not reach statistical significance. In this more
recent study, age greater than 7 years and Modified Injury Severity
Score (MISS) ≥140 were predictive of an increased rate of complications
of immobilization. A mixed series of adults and children demonstrated
comparable results for early (within 24 hours) and late (after 24
hours) fixation of fractures in the setting of blunt trauma and severe
head injuries.191
with multiple injuries and have been reported in up to 7% of children
referred to level 1 regional trauma centers.165,193 Survival is related to ISS and type of hospital.193 In two series, 60%-87% of pelvic fractures involved a pedestrian struck by a motor vehicle.158,168 Other common mechanisms include being a passenger in a MVA or falling from a height.158,168 Although many of these pelvic injuries are stable, unstable patterns have been reported in up to 30% of cases.17
associated with the most intense hospital care and higher mortality
rates than other injury combinations.23 In their series of 166 consecutive pelvic fractures, Silber et al.158
reported associated substantial head trauma in 39%, chest trauma in
20%, visceral/abdominal injuries in 19%, and a mortality rate of 3.6% (Fig. 4-4). In this same series,158
12% (20/166) had acetabular fractures, while in another series, 62% of
children (8/13) with pelvic fractures had other orthopaedic injuries.168
near the fracture or from the peritoneum from injured viscera, may
present an immediate threat.76
However, death of children with pelvic fractures appears to be caused
more often by an associated head injury rather than an injury to the
adjacent viscera or vessels.121
Injury to the sciatic nerve or the lumbosacral nerve roots may result
from hemipelvis displacement through a vertical shear fracture.
Nonorthopaedic injuries associated with pelvic fractures led to
long-term morbidity or mortality in 31% (11/36) of patients in one
review of pediatric pelvic fractures.56
Most pelvic fractures in children are treated nonoperatively. However,
in a child or preadolescent, an external fixator can be used to close a
marked pubic diastasis or to control bleeding by stabilizing the pelvis
for transport and other injury care. The external fixator will not
reduce a displaced vertical shear fracture, but the stability provided
is helpful to control the hemorrhage while the child’s condition is
stabilized.137,180 Operative treatment can result in healing by 10 weeks with a low complication rate.79
high-velocity blunt injury involving vehicles. Penetrating injuries are
much less common in children than in adults; however, many low-energy
blunt
injuries can cause puncture wounds in the skin adjacent to fractures,
especially displaced radial, ulnar, and tibial fractures. In children
with multiple injuries, approximately 10% of the fractures are open.23,151
When open fractures are present, 25% to 50% of patients have additional
injuries involving the head, chest, abdomen, and other extremities.151
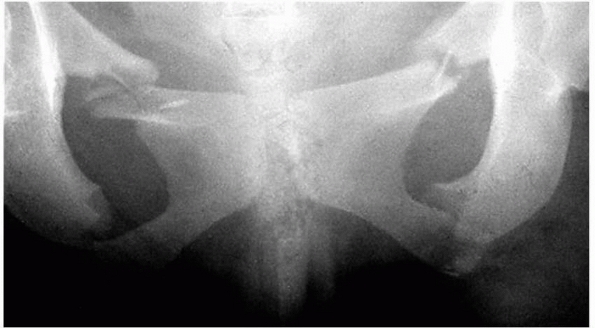 |
|
FIGURE 4-4
Bilateral superior and inferior pubic rami fractures. Genitourinary and abdominal injuries must be ruled out with severe pelvic fractures. |
adjacent to an open fracture is based on the system described by
Gustilo and Anderson62 and Gustilo and colleagues.63
Primary factors that are considered and ranked in this classification
system are the size of the wound, the degree of wound contamination,
and the presence or absence of an associated vascular injury (Table 4-4).
bone puncturing the skin (from the inside to the outside). The wound is
less than 1 cm in size, and there is minimal local soft tissue damage
or contamination.
and is typically associated with a transverse or oblique fracture with
minimal comminution. There is adjacent soft-tissue injury, including
skin flaps or skin avulsion, and a moderate crushing component of
adjacent soft tissue usually is present. Skin grafts or flaps should
not be needed for coverage.
are classified as type III, with associated subgroups A, B, or C; the
letters indicate increasing severity of injury. These fractures
typically result from high-velocity trauma and are associated with
extensive soft tissue injury, a large open wound, and significant wound
contamination. In a type IIIA fracture, there is soft-tissue coverage
over the bone, which is often a segmental fracture. In a type IIIB
fracture, bone is exposed at the fracture site, with treatment
typically requiring skin or muscle flap coverage of the bone. Type IIIC
fractures are defined as those with an injury to a major artery in that
segment of the extremity, regardless of wound size or the other
soft-tissue disruption. Although these injuries are commonly associated
with extensive soft-tissue loss and contamination, a type IIIC injury
may, in fact, be associated with even a small wound in some cases.
|
TABLE 4-4 Classification of Open Fractures
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||
correlate in adults with sequelae of the injury, including the
potential for infection, delayed union, nonunion, amputation, and
residual impairment. The final functional results of type III fractures
in children appear to be superior to results after similar fractures in
adults, likely due to their better peripheral vascular supply.
to that for open fractures in adults. The primary goals are to prevent
infection of the wound and fracture site, while allowing soft tissue
healing, fracture union, and eventual return of optimal function.
Initial emergency care includes the ABCs of resuscitation, application
of a sterile povidone-iodine dressing, and preliminary alignment and
splinting of the fracture. If profuse bleeding is present, a
compression dressing is applied to limit blood loss. In the emergency
department, masks and gloves should be worn as each wound is thoroughly
inspected. Tetanus prophylaxis is provided, and the initial dose of
intravenous antibiotics is given. The dose of tetanus toxoid is 0.5 mL
intramuscularly to be given if the patient’s immunization status is
unknown, or if it is more than 5 years since the last dose. The second
stage of management is the primary surgical treatment, including
initial and (if necessary) repeat débridement of the tissues in the
area of the open fracture until the entire wound appears viable. The
fracture is reduced and stabilized at this time. If the bone ends are
not covered with viable soft tissue, muscle or skin flap coverage is
considered. Vacuum-assisted closure (VAC) Therapy (Kinetic Concepts,
Inc., San Antonio, TX) may be a useful adjunct to facilitate coverage
and obviate the need for flaps in some patients.70,120,196 VAC has been shown to shorten time of healing of wounds associated with open fractures.99
The third and final stage of this management is bony reconstruction as
needed if bone loss has occurred and, ultimately, rehabilitation of the
child.
reported that neither pre- nor postdébridement cultures accurately
predicted the risk of infection in open fractures. He noted that only
20% of wounds (24/119) with positive predébridement cultures and only
28% (9/32) with positive postdébridement cultures became infected.97 Although postdébridement cultures were more predictive of
infection, these cultures identified the causative organism in only 42% (8/19) of infected wounds. Valenziano et al.188
found that cultures at the time of presentation to the trauma center
also were of no value, with only two of 28 patients (7%) with positive
cultures becoming infected, in comparison to five of 89 patients (6%)
with negative initial cultures. Initial cultures were positive in only
two of seven of cases that became infected. Open fractures do not need
to be routinely cultured. Cultures should only be obtained only at the
time of reoperation in patients with clinical evidence of infection.
reported a 13.9% infection rate in 79 patients who received no
antibiotics after open fractures, and a 5.5% rate in 815 patients with
similar injuries who had antibiotic prophylaxis. Bacterial
contamination has been noted in 70% of open fractures in children, with
both Gram-positive and Gram-negative organisms noted, depending on the
degree of wound contamination and adjacent soft tissue injury. We limit
antibiotic administration generally to 48 hours after surgical
treatment of the open fracture.96
firstgeneration cephalosporin (cefazolin 100 mg/kg/day divided q 8 hr,
maximal daily dose 6 g).96 For more
severe type II fractures and for type III fractures, we use a
combination of a cephalosporin and aminoglycoside (gentamicin 5-7.5
mg/kg/day divided q 8 hr).96
penicillin (150,000 units/kg/day divided q 6 hr, maximal daily dose 24
million units) is added to the cephalosporin and aminoglycoside. All
antibiotics are given intravenously for 48 hours. Oral antibiotics are
occasionally used if significant soft tissue erythema at the open
fracture site remains after the intravenous antibiotics have been
completed. Gentamicin levels should be checked after 4 or 5 doses (and
doses adjusted as necessary) during therapy to minimize the risk of
ototoxicity.
surgeries, such as those for repeat irrigation and débridement, delayed
wound closure, open reduction and internal fixation of fractures, and
secondary bone reconstruction procedures.
fracture in the operating room to be the most important step in the
primary management of open fractures in children. Some authors have
reported that significantly higher infection rates occurred if
débridement and irrigation were done more than 6 hours after open
fractures in children.89 A
multicenter report, however, demonstrated an overall infection rate of
1% to 2% after open long-bone fractures, with no difference in
infection rates between groups of patients treated with irrigation and
débridement within 6 hours of injury and those treated between 6 and 24
hours following injury.162 Another study of pediatric type I open fractures reported a 2.5% infection rate with nonoperative treatment.75
One likely reason for the low rates of infection in these two series is
the early administration of intravenous antibiotics in both groups.
Although up to a 24-hour delay does not appear to have adverse
consequences regarding infection rates, it may be necessary to perform
an earlier irrigation and débridement to minimize compromise of the
soft tissue envelope. The débridement needs to be performed carefully
and systematically to remove all foreign and nonviable material from
the wound. The order of débridement typically is (a) excision of the
necrotic tissue from the wound edges; (b) extension of the wound to
adequately explore the fracture ends; (c) débridement of the wound
edges to bleeding tissue; (d) resection of necrotic skin, fat, muscle,
and contaminated fascia; (e) fasciotomies as needed; and (f) thorough
irrigation of the fracture ends and wound.
major problem in wound management and healing, all ischemic muscle is
widely débrided back to muscle that bleeds at the cut edge and
contracts when pinched with the forceps.
fracture, we bring the proximal and distal bone ends into the wound to
allow visual inspection and thorough irrigation and débridement. This
often necessitates extension of the open wound, but is preferable to
leaving the fracture site contaminated. We carefully remove devitalized
bone fragments and contaminated cortical bone with curettes or a small
rongeur. If there is a possible nonviable bone fragment, judgment is
needed as to whether this bone fragment should be removed or left in
place. Small fracture fragments without soft tissue attachments are
removed, whereas very large ones may be retained if they are not
significantly contaminated. Reconstruction of a large segmental bone
loss has a better outcome in children than in adults because children
have a better potential for bone regeneration and a better vascular
supply to their extremities. Nearby major neurovascular structures in
the area of the fracture are identified and protected. Débridement is
complete when all contaminated, dead, and ischemic tissues have been
excised; the bones ends are clean with bleeding edges; and only viable
tissue lines the wound bed.
fracture with sterile normal saline, although lavage using widebore
cystoscopy tubing is a reasonable alternative. We routinely use 9 L of
solution for the lower extremities and 6 L in the upper extremities
because of the smaller compartment size.
soft tissue is used to cover the neurovascular structures, tendons, and
bone ends. If local soft tissue coverage is inadequate, consideration
should be given to local muscle flaps or other coverage methods,
including VAC. The area of the wound that has been incised to extend
the wound for fracture inspection can be primarily closed. The
traumatic wound should either be left open to drain or may be closed
over one or more drains. Wounds that are left open can be dressed with
a moistened povidone-iodine or saline dressing. Types II and III
fractures are routinely reoperated on every 48 to 72 hours for repeat
irrigation and débridement until the wounds appear clean and the tissue
viable. This cycle is repeated until the wound can be sutured closed or
a split-thickness skin graft or local flap is used to cover it. If flap
coverage is necessary, this is optimally accomplished within 1 week of injury.
decreases pain, protects the soft tissue envelope from further injury,
decreases the spread of bacteria, allows stability important for early
soft tissue coverage, and improves the fracture union rate.
children include allowing access to the soft tissue wound and the
extremity for débridement and dressing changes, allowing weight-bearing
when appropriate, and preserving full motion of the adjacent joints to
allow full functional recovery.
I fractures and occasionally type II fractures with relatively small
wounds and minimal soft-tissue involvement, difficulties with
soft-tissue management and loss of alignment as swelling subsides are
common with such closed treatment. Most of these injuries involve the
radius or ulna in the upper extremity or the tibia in the lower
extremity. Splint or cast immobilization is generally not satisfactory
for the more unstable type II and most type III injuries.
intramedullary implant in the radius and/or ulna commonly provides
enough stability of the fracture to allow dressing changes through the
cast or splint. For intramedullary fixation, we prefer 2- to
4-mm-diameter flexible titanium implants for stabilizing open fractures
in the forearm when reduction of either the radial or ulnar fracture is
unstable. Since the ulnar canal is straight, the implant chosen is
often at least 80% of the narrowest canal diameter, while the implant
for the radius is generally 50% to 60% of the narrowest canal diameter.
The ulnar implant is inserted antegrade, and the radial implant is
inserted retrograde just proximal to the distal radial physis. One or
both bones can be stabilized, and the implants can be removed easily
after fracture healing.
the radius (and, occasionally, the ulna) generally is appropriate and
provides sufficient stability. A short-arm cast usually is sufficient
to maintain appropriate alignment following such fixation. The pins are
removed in the office at 3 to 4 weeks, but the cast is used for a total
of 6 weeks.
fractures of the femoral shaft. For type III fractures, especially if
there is a large or contaminated soft tissue wound present, external
fixation may be indicated. Trochanteric antegrade nails are gaining
popularity and may be considered in children 10 years old or older or
those who weigh 50 kilograms (110 lbs) or more.
external fixation as our treatment of choice for most open tibial and
femoral fractures in children. Both intramedullary rodding and external
fixation allow access to the wound for débridement and dressing changes
as well as any soft tissue reconstruction needed.123
Wound access, however, may be limited with external fixators,
especially when there are extensive soft tissue wounds. Intramedullary
rods generally are better tolerated by patients and families, do not
require daily care, leave more cosmetic scars, and are load-sharing
devices. With intramedullary rodding, the child is allowed to weight
bear as tolerated following transverse or short oblique fractures, but
weight-bearing is protected for 4 to 6 weeks following comminuted or
spiral fractures.
segmental bone loss, and ring fixators may even be used in such
instances for bone transport. External fixation allows weight bearing
relatively soon after the injury. We find that a uniplanar frame is
best for most fractures and is relatively easy to apply. For some
segmental fractures in the metaphysis and diaphysis, as well as soft
tissue injuries, a ring fixator may be a better choice.
intra-articular fractures. When feasible, fixation should be parallel
to (and avoid) the physis. Cannulated screws often are used in such
instances. Screws or threaded pins should never be placed across the
physis. If fixation across the physis is necessary, smooth pins are
used; they should be removed 3 to 4 weeks after injury to minimize the
risk of growth disturbance.
diaphysis, open reduction and internal fixation can be combined with
external fixation. For diaphyseal fractures in skeletally immature
children, we prefer flexible intramedullary nails to compression plates
for internal fixation of type I, type II, and some type III fractures.
The superiority of intramedullary or external fixation for type IIIB
fractures has not been firmly established. For treatment of a floating
joint, usually the knee or elbow, we almost always stabilize both
fractures operatively.18,103
days until the wounds are clean and all remaining tissue appears
viable. Fracture fixation at the time of initial surgery (as described
previously) facilitates wound management. We prefer to provide soft
tissue coverage of the open fracture and adjacent soft-tissue defect by
5 to 7 days after the injury to limit the risk of later infection. Most
type I wounds heal with local dressing changes. For some type II and
type IIIA fractures, we use delayed wound closure or a split-thickness
skin graft over underlying muscle cover.
types IIIB and IIIC fractures. In the proximal tibia, plastic surgeons
may be needed to provide a gastrocnemius rotational flap, followed by
secondary coverage of the muscle with a skin graft. In the middle third
of the leg, a soleus flap is used with skin graft coverage, and a
vascularized free muscle transfer is necessary if local coverage is
inadequate. Free flaps may be required for coverage of the distal third
of the tibia, especially in adolescents,139
although there is a 60% postoperative complication rate. VAC sometimes
can reduce the need for free tissue transfers. The VAC can convert
wounds that need free tissue to ones that need split-thickness skin
graft or can heal completely.29,120
injuries are either muscle flaps or composite grafts. For a massive
loss of soft tissue and bone, composite grafts of muscle and bone often
are necessary. The younger the child, the better the likelihood that
autogenous graft will fill in a bone defect if there is a
well-vascularized bed from the muscle flap. Free flaps, especially from
the latissimus dorsi, are useful in the
midtibial
and distal tibial regions to decrease infection rates and improve union
rates. Vascularized fibular grafts rarely are used acutely to
reconstruct bone defects, but may be useful after soft tissue healing.
we rely on the healing capacity of young periosteum and bone and the
vascular supply of a child’s extremity. An external fixator is used to
hold the bone shortened about 1 to 2 cm to decrease the size of the
bone loss. In a growing child, 1 to 2 cm of overgrowth can be expected
in the subsequent 2 years after these severe injuries, so the final leg
length will be satisfactory. Autogenous bone graft can be used early,
but if there is surviving periosteum at this site, spontaneous bone
formation often is surprisingly robust and may preclude the need for
bone grafting. In teenagers with bone loss, once the soft tissue has
healed, bone transport using either a uniplanar lengthening device or
an Ilizarov device is our preferred method of reconstruction, although
use of an allograft or vascularized fibular graft may be considered.
preserve all extremities, even with type IIIC open fractures that are
usually treated with primary amputation in adults. Wounds and fractures
that do not heal in adults often heal satisfactorily in children and
preservation of limb length and physes are important in young children.
Although the Mangled Extremity Severity Score (MESS) correlates well
with the need for amputation in adults, the correlation is less in
children.50 In one series,50
the MESS predicted limb amputation or salvage correctly in 86% (31/36)
of children, with 93% accuracy in salvaged limbs but only 63% in
amputated limbs.
possible should be preserved. For example, if the proximal tibial
physis is preserved in a child with a below knee amputation at age 7
years, 3 to 4 inches more growth of the tibial stump can be expected by
skeletal maturity. Thus, even a very short tibial stump in a skeletally
immature child may grow to an appropriate length by skeletal maturity.
As a result, even a short below-knee amputation at the time of injury
would likely be superior to a knee disarticulation in final function.
usually are done through the joint to limit bone spike formation
(overgrowth) at the end of the stump, we prefer to maintain maximal
possible length if amputation becomes necessary as a result of a severe
injury.
operating room for irrigation and débridement of the open fracture, the
orthopaedist may use this opportunity to treat the other fractures as
well, whether operative treatment or closed reduction and casting are
needed. To facilitate patient care and rehabilitation, most long-bone
fractures in these children are treated surgically.
nonorthopaedic benefits to a child with multiple injuries. Among the
potential benefits are ease of patient mobilization, ease of nursing
care, decreased risks of pressure sores, and better access to the
wounds. Pulmonary contusions at the time of injury often lead to
increasing respiratory problems in the first few days after injury.131
If the lungs have been severely contused, protein leaks into the
alveolar spaces, making ventilation more difficult. This may be
exacerbated by the systemic inflammatory response syndrome, which is
commonly seen following severe trauma.142,198 Surfactant dysfunction follows and is most abnormal in patients with the most severe respiratory failure.131
As the time from the injury increases, pulmonary function deteriorates
and general anesthesia becomes more risky. Orthopaedic surgical
treatment before such pulmonary deterioration limits the anesthetic
risks in these patients. In patients with severe pulmonary contusions
and multiple fractures, the use of extracorporeal life support may be
the only treatment available to allow patient survival.155
stabilization of fractures decreases pulmonary and other medical
complications associated with prolonged bed rest that is a part of
nonoperative fracture treatment.13 Most adult trauma centers follow the treatment protocol of early fracture stabilization, even though Poole et al.134
reported that, despite early fracture stabilization simplifying patient
care, pulmonary complications in patients with marked chest trauma were
not prevented and the course of the head injury was not affected. In
children, medical complications are less common, so the recommendations
that mandate early fracture stabilization are somewhat more difficult
to support in young patients. Nonetheless, bruises on the chest or rib
fractures should alert the orthopaedist to potential pulmonary
contusions as a part of the injury complex.127
Initial chest radiographs may not clearly demonstrate the degree of
pulmonary parenchymal injury, and arterial blood gas determinations are
more useful in estimating the anesthetic risk of these patients during
operative care of the fractures.
resuscitation. In a child with multiple closed fractures, definitive
treatment should proceed expeditiously once the child’s condition has
been stabilized. Loder107 reported
that operative stabilization of fractures within the first 2 or 3 days
after injury led to fewer complications, shorter hospital and intensive
care unit stays, and a shorter time on ventilator assistance in
children with multiple injuries. A more recent study by Loder et al.108
reported a trend toward a higher rate of complications in fractures
treated after 72 hours. Although there appear to be other factors
besides the timing of surgery that affect the eventual outcomes of
polytrauma patients, the timing of surgery is a variable that can be
controlled by the surgeon, and it seems prudent to complete fracture
stabilization within 2 to 3 days of injury when possible.
injured children commonly depends on the training, experience, and
personal preference of the orthopaedist. The most common methods
used
are intramedullary rod fixation, external fixation, compression
plating, and locking plating; Kirschner-wires or Steinmann pins may be
used in conjunction with casts.
4-mm-diameter flexible titanium intramedullary rods for stabilization
of long-bone fractures of the upper and lower extremities.
Intramedullary rodding is most commonly used for unstable closed
fractures of the radius and ulna in patients through adolescence and
for femoral shaft fractures in patients between the ages of 5 and
skeletal maturity.179,192
A trochanteric antegrade nails often is a viable option in children 10
years old or older or in those with comminuted femoral fractures. The
tibia also can be fixed with intramedullary rods in children with an
open fracture, polytrauma, a “floating knee” injury (concurrent femur
fracture), or a high-energy, unstable injury (especially during
adolescence). A diaphyseal fracture of the humerus can be treated with
intramedullary fixation in the presence of a “floating” shoulder or
elbow.144
forearm fractures include unstable diaphyseal fractures (especially in
adolescents) and open fractures.58,95,98,111
Forearm fractures can generally be reduced closed, with the
intramedullary implant passed across the fracture site under
fluoroscopy to stabilize the fracture.95 In one study,98
23% (10/43) of closed forearm fractures treated with intramedullary rod
fixation required open reduction. The ulnar implant is placed in
antegrade fashion and can be inserted through the lateral proximal
metaphyseal area or the tip of the olecranon. The radial implant is
inserted retrograde and is contoured to conform to the normal radial
bow before insertion. The insertion point is proximal to the distal
radial physis and the rod can be inserted from the radial aspect of the
distal radius or dorsally (slightly ulnar to Lister’s tubercle).
Stability of both fractures may be achieved by instrumenting only the
radius or the ulna in younger children, but both bones are more
commonly fixed in adolescents. Intramedullary fixation of open forearm
fractures appears to decrease the rate of loss of reduction.58,111 In one series,98
reduction was maintained in all 27 patients treated with rodding of
both bones or of only the radius, compared with loss of reduction in
32% (7/22) of patients in whom only the ulna was rodded. The high rate
of failure may be due to the small diameter pins (1.6 or 2.0 mm) used
to fix the ulna in this series.98 A cast is used for further immobilization.
the elbow region 6 to 12 months after insertion. Despite the utility of
flexible intramedullary implants for stabilizing forearm fractures in
children, the radius and ulna in young patients have significant
remodeling capacity and not all fractures require anatomic reduction. A
closed reduction and cast immobilization may suffice. Displaced distal
forearm fractures in polytrauma patients are often well treated with
closed reduction and percutaneous pinning, thus affording sufficient
stability for use of a short-arm cast in these polytrauma patients.
intramedullary rodding of forearm fractures, 50% of patients had
complications including loss of reduction, infection, hardware
migration, nerve injury, and delayed union, although 95% (19/20) of
patients had excellent or good results at follow-up.41 In another series,205
compartment syndromes occurred in six of 80 (7.5%) patients with
forearm fractures treated with intramedullary fixation; risk factors in
this study were reported to be increased operative time and increased
intraoperative use of fluoroscopy.
the most common technique is retrograde insertion from the medial and
lateral metaphyseal region of the distal femur, 2 to 3 cm proximal to
the physis. Two rods are used to cross the fracture site and obtain
purchase in the proximal femur, usually with one at the base of the
femoral neck and the other at the base of the greater trochanter. Rod
diameter is generally 40% of the intramedullary diameter of the femoral
isthmus, up to a maximum rod size of 4 to 4.5 mm (depending on
manufacturer). A cast is not necessary postoperatively, although a
fracture brace can be used to help control rotation at the fracture
site and provide some patient comfort during early walking, especially
for proximal third fractures or those with significant comminution. The
implants usually are removed within 1 year of the fracture.68,74
One study showed that intramedullary nailing of the femur had more
complications in comminuted fractures and children weighing over 100
pounds,52 while another noted higher complication rates in children 10 years old or older at the time of surgery.72
femoral shaft fractures in the pediatric population should be reserved
for those with a closed proximal femoral physis. In younger children,
rod insertion at the piriformis fossa may interfere with the vascular
supply to the femoral epiphysis, may cause growth arrest of the greater
trochanter (i.e., apophysis with resultant coxa valga), or may
interfere with the appositional bone growth at the base of the femoral
neck, thereby thinning this region and potentially predisposing the
child to a femoral neck fracture.12,27,102,118,124 Some authors have advocated rigid intramedullary rodding using an entrance point at the tip of the greater trochanter.57,181
Although the use of trochanteric antegrade nails is increasingly
common, there are not yet sufficient data to confirm the safety and
efficacy of such an approach. The specific indications for
intramedullary fixation of the femur are discussed in more detail in Chapter 22.
increasingly common for diaphyseal tibial fractures. The most common
indications currently are open fractures, “floating knee” injuries, and
unstable diaphyseal fractures in adolescents. The rods are inserted in
antegrade fashion, with medial and lateral entrance points distal to
the physis and avoiding the tibial tubercle. As with femoral fractures,
rod diameter is 40% of the narrowest intramedullary diameter, with a
maximum rod size of 4 to 4.5 mm (depending on implant manufacturer). A
short-leg walking cast or fracture boot often is used for comfort for
the first 4 to 6 weeks postoperatively, although a splint may be used
initially to allow access to wounds associated with an open fracture or
degloving injury.
plates to stabilize long-bone fractures, especially in the femoral
shaft, in children with multiple injuries.25,90 Kregor et al.90 reported an average overgrowth of the femur of 9 mm, and all fractures healed in a near anatomic position. Caird et al.25
noted that 3% of patients (2/60) had a limb length discrepancy of
greater than 2.5 cm following femoral plating, including a 5-cm
discrepancy in one child. The disadvantages of compression plating
include the need for more extensive operative exposure at the site of
the
fracture, the fact that they are not load-sharing devices, and the
usual need to remove the plate through a relatively long incision once
healing is complete. Newer minimally invasive percutaneous submuscular
plating techniques have eliminated some of the problems associated with
traditional plating (Fig. 4-5).78,160 Refracture may occur through the screw holes left after plate removal if physical activity is resumed too quickly.78
Stiffness of adjacent joints is rarely a problem in children unless
there has been an associated severe soft tissue injury. The number of
cortices the screws cross on each side of the fracture may be fewer in
children than in adults, because a cast or splint is routinely used in
young patients. Kanlic et al.78 reported an 8% incidence of leg length discrepancy after submuscular bridge plating.
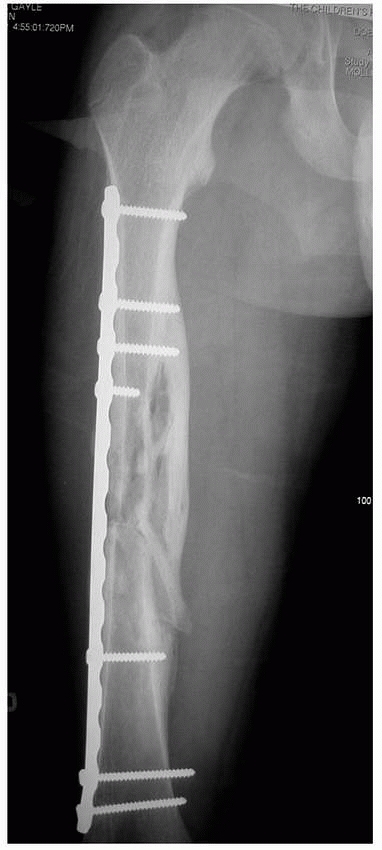 |
|
FIGURE 4-5
Stabilization of femoral shaft fractures in children with multitrauma can be obtained with several methods. Minimally invasive percutaneous submuscular plating techniques can occasionally be used. (Courtesy of Steven T. Morgan, MD, Denver, CO.) |
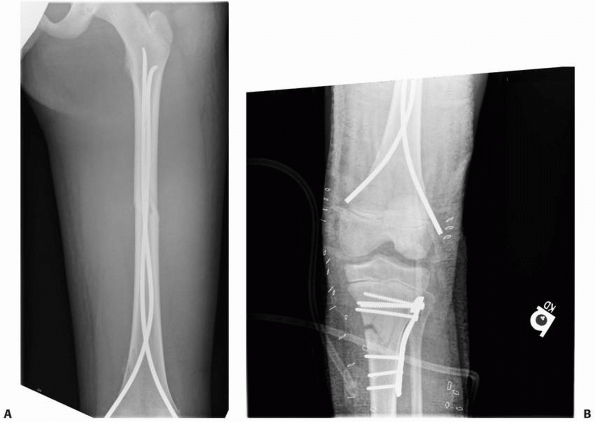 |
|
FIGURE 4-6 “Floating knee” injury in a 12-year-old child included (A) a femoral shaft fracture, the femoral physeal fracture was reduced with flexible IM nails. B.
Open fractures of the tibia were treated with débridement and irrigation and stabilization with internal fixation. (Courtesy of Michelle Caird, MD, Ann Arbor, MI.) |
we prefer flexible intramedullary nails, as noted earlier. The use of
compression plates in the forearm requires a larger operative incision
with a resultant scar, a second extensive procedure for plate removal,
and a significant risk of refracture following hardware removal. We do
not believe that the healing capability of the young child requires the
rigid fixation of compression plating to obtain fracture union.
with multiple injuries are open fractures with significant soft tissue
injury, fractures in children with a head injury and coma, and
“floating knee” fractures of the femur and tibia (Fig. 4-6).5,6,16,18,85,103,144,154,178,206 With advances in intramedullary rod techniques, external fixation is now uncommon. A unilateral
fixator generally is sufficient to hold the fracture reduced in this age group.
predrilled and the pins placed in the operating room under fluoroscopic
control. The caliber of the pin should be less than 30% of the diameter
of the bone into which it is to be inserted to minimize the risk of
fracture through a pin site. The distal and proximal pins must be
inserted at a level to avoid the physis, and we recommend leaving at
least 1 to 2 cm between the pin and physis, partly to avoid any adverse
effect on the physis should a pin track infection occur. The proximal
tibial physis is more distal anteriorly below the tibial tubercle, and
this area must be avoided or a recurvatum deformity of the proximal
tibia and knee will result. The external fixator is usually left in
place until fracture healing is complete, but it can be removed once
the reason for placement has resolved (such as waking from coma or
healing of a skin wound).48,201
If the fixator is removed early, a walking cast is applied. Transverse
open fractures reduced out to length take longer to heal than do
oblique fractures reduced with slight overlap. Refracture is a
well-described risk following fixator removal. However, refracture
rates have been variable, with a 21% rate noted in a series in which a
rigid transfixion type of fixator was used177 and a 1.4% rate in a series with more flexible unilateral frames.16
One report indicated that if 3 of the 4 cortices at the fracture site
appear to be healing on anteroposterior and lateral radiographs of the
fracture, the refracture rate after frame removal should be low.163
We prefer to dynamize the fixator early to stimulate callus formation,
although the effect of dynamization on refracture rates is unclear.47,82
(80%) survived, but after 1 year, 22% were disabled, mainly from a
brain injury.189 At 9 years after
the injuries, 12% had significant physical disability, whereas 42% had
cognitive impairment. In this group, however, the SF-36 or functional
outcome survey did not differ from the control population. The best
predictor of long-term disability was the Glasgow Outcome Scale from 6
weeks after injury and later.189 Letts et al.101
reported that 71.6% of multiply injured children made a full recovery,
with a mean of 28 weeks until full recovery. Of the 53 residual
deficits in 48 patients, the common deficits were neurologic (38%),
psychosocial (34%), and musculoskeletal (24%).101 Outcomes of children with pelvic fractures were near normal status at 6 months.157
chosen for a child with multiple injuries, it is important that an
orthopaedist be involved in the care of the child from the start. While
recognizing the need to care for the other organ system injuries the
child has sustained, it is important to advocate for the expeditious
and appropriate treatment of the fractures that are present. Failure to
do so will leave the multiply injured child with musculoskeletal
disability once healing of the other injuries occurs.
problems relate to either sequelae of the head injury or of the
orthopaedic injuries.
Vernon T. Tolo, MD, for his past contributions to this chapter. We
thank Donna M. Zink and Kristi A. Overgaard for their assistance during
preparation of this chapter.
WA, Sugarman JM, Fallat ME, et al. Indicators of genitourinary tract
injury or anomaly in cases of pediatric blunt trauma. J Pediatr Surg
1996;31(1): 86-89,discussion 90.
DE, O’Malley KJ, Summerton DJ, et al. The type of urethroplasty for a
pelvic fracture urethral distraction defect cannot be predicted
preoperatively. J Urol 2003; 170(2 Pt 1):464-467.
MC, McCormack JE, Scriven RJ, et al. Venous thromboembolic events in
pediatric trauma patients: is prophylaxis necessary? J Trauma
2005;59(6):1345-1349.
KA, Cha ES, Bensard DD, et al. The positive predictive value of rib
fractures as an indicator of nonaccidental trauma in children. J Trauma
2003;54(6):1107-1110.
E, Ates Y, Germiyanoglu C, et al. Role of Tile classification in
predicting urethral injuries in pediatric pelvic fractures. J Trauma
1997;42(2):285-287.
JH, Austin SM, Warner WC, et al. Interlocking intramedullary nailing of
femoralshaft fractures in adolescents: preliminary results and
complications. J Pediatr Orthop 1994;14(2):178-183.
SB, Scholten DJ, Bonnell BW, et al. Long-bone fractures in the
polytrauma patient. The role of early operative fixation. Am Surg
1989;55(6):356-358.
RJ, Bassett GS, Fideler B, et al. Intraosseous infusions: effects on
the immature physis—an experimental model in rabbits. J Pediatr Orthop
1993;13(4):511-515.
WW, Durbin RA. Ipsilateral fractures of the femur and tibia in children
and adolescents. J Bone Joint Surg Am 1991;73(3):429-439.
SJ, Gotschall CS, Eichelberger MR. Predictors of abdominal injury in
children with pelvic fracture. J Trauma 1991;31(8):1169-1173.
PP, Vogt MT, Ward WT. Pediatric spinal cord injury without radiographic
abnormality (SCIWORA): the absence of occult instability and lack of
indication for bracing. Spine 2002;27(24):2788-2800.
BJ, Slauterbeck J, Benjamin JB. Fracture patterns and mechanisms in
pedestrian motor-vehicle trauma: the ipsilateral dyad. J Orthop Trauma
1992;6(3):279-282.
JK, Jing Y, Wang S, et al. Patterns of severe injury in pediatric car
crash victims: Crash Injury Research Engineering Network database. J
Pediatr Surg 2006;41(2): 362-367.
SL, Gotschall C, Robertson W Jr, et al. The relationships of skeletal
injuries with trauma score, injury severity score, length of hospital
stay, hospital charges, and mortality in children admitted to a
regional pediatric trauma center. J Pediatr Orthop 1994;14(4):449-453.
E, Illi OE, Soder C, et al. Ruptured spleen in children—15-year
evolution in therapeutic concepts. Eur J Pediatr Surg 1992;2(3):157-161.
MS, Mueller KA, Puryear A, et al. Compression plating of pediatric
femoral shaft fractures. J Pediatr Orthop 2003;23(4):448-452.
DJ, Sprouse LR 2nd, Smith LA, et al. Injuries in pediatric patients
with seatbelt contusions. Am Surg 2003;69(12):1095-1099.
JP, Boboyono JM, Ricard J, et al. Management of abdominal contusion in
polytraumatized children. Int Surg 1991;76(2):119-121.
DA, Ruth B, Teich S. Wound management with vacuum-assisted closure:
experience in 51 pediatric patients. J Pediatr Surg 2005;40(1):128-132.
HS, Filtzer DL. Pseudosubluxation and other normal variations in the
cervical spine in children. A study of 160 children. J Bone Joint Surg
Am 1965;47(7): 1295-1309.
L, Reilly KM, Telfer J. Odds of critical injuries in unrestrained
pediatric victims of motor vehicle collision. Pediatr Emerg Care
2006;22(9):626-629.
LE, Wilke HJ, Augat P, et al. Effect of dynamization on gap healing of
diaphyseal fractures under external fixation. Clin Biomech (Bristol,
Avon) 1995;10(5):227-234.
DR, Baird TB, Gormley P, et al. Pediatric splenic injuries with a
contrast blush: successful nonoperative management without angiography
and embolization. J Pediatr Surg 2004;39(6):969-971.
MC, Pfeifer J, DeLuca FG. Nonoperative management of splenic and
hepatic trauma in the multiply injured pediatric and adolescent
patient. Arch Surg 1995;130(3): 332-338.
BD, Mutabagani KH, Martin LC, et al. Focused abdominal sonography for
trauma (FAST) in children with blunt abdominal trauma. J Trauma
2000;48(5):902-906.
PM, Buck JR, Dudgeon DL, et al. One-year experience in a regional
pediatric trauma center. J Pediatr Surg 1985;20(1):8-13.
CE, Bosse MJ, McCarthy ML, et al. Effect of trauma and pelvic fracture
on female genitourinary, sexual, and reproductive function. J Orthop
Trauma 1997;11(2): 73-81.
S, DiRusso S, Sullivan T, et al. Validation of a relative head injury
severity scale for pediatric trauma. J Trauma
2007;63(1):172-177,discussion 177-178.
MC, Roy DR, Giza E, et al. Complications of intramedullary fixation of
pediatric forearm fractures. J Pediatr Orthop 1998;18(1):14-21.
C, Michon B, Pettersen G, et al. Venous thromboembolism after severe
injury in children. Acta Haematol 2006;115(3-4):198-200.
Bastiani G, Mosconi F, Spagnol G, et al. High calcitonin levels in
unconscious polytrauma patients. J Bone Joint Surg Br
1992;74(1):101-104.
D, Karaiskakis M, Velmahos GC, et al. Pelvic fractures in pediatric and
adult trauma patients: are they different injuries? J Trauma
2003;54(6):1146-1151,discussion 1151.
E, Ciardelli R, Vincent JL. Fatal outcome after polytrauma:
multiple-organ failure or cerebral damage? Resuscitation
1998;36(1):15-18.
BG, Sponseller PD, Ain M, et al. Comparison of dynamic versus static
external fixation for pediatric femur fractures. J Pediatr Orthop
2002;22(4):428-430.
M, Strong ML, MacIntosh R. External fixation maintained until fracture
consolidation in the skeletally immature. J Pediatr Orthop
1993;13(1):98-101.
YJ, Valentine RJ, Myers SI, et al. Blunt pediatric vascular trauma:
analysis of 41 consecutive patients undergoing operative intervention.
J Vasc Surg 1994;20(3): 419-424,discussion 424-425.
JM, Luedtke L, Ganley TJ, et al. Titanium elastic nails for pediatric
femur fractures: lessons from the learning curve. Am J Orthop
2002;31(2):71-74.
JB, Lim CK, Flynn JM, et al. The efficacy of magnetic resonance imaging
in pediatric cervical spine clearance. Spine 2002;27(11):1176-1179.
GJ, Vitale MA, Kessler MW, et al. The most frequent traumatic
orthopaedic injuries from a national pediatric inpatient population. J
Pediatr Orthop 2005;25(1): 39-44.
VF, Gotschall CS, Eichelberger MR, et al. Rib fractures in children: a
marker of severe trauma. J Trauma 1990;30(6):695-700.
JE, Swenning TA, Burd TA, et al. Proximal femoral radiographic changes
after lateral transtrochanteric intramedullary nail placement in
children. J Bone Joint Surg Am 2003;85-A(7):1295-1301.
OD, Mahboubi S, Stafford PW, et al. The utility of the pelvic
radiograph in the assessment of pediatric pelvic fractures. J Trauma
2003;55(2):236-239, discussion 239-240.
RB, Anderson JT. Prevention of infection in the treatment of 1025 open
fractures of long bones: retrospective and prospective analyses. J Bone
Joint Surg Am 1976; 58(4):453-458.
RB, Mendoza RM, Williams DN. Problems in the management of type III
(severe) open fractures: a new classification of type III open
fractures. J Trauma 1984; 24(8):742-746.
JA Jr, Shorter N, Miller D, et al. Organization and function of a
regional pediatric trauma center: does a system of management improve
outcome? J Trauma 1983;23(8): 691-696.
G, Dennis M, Zhang L, et al. Childhood head injury and metacognitive
processes in language and memory. Dev Neuropsychol 2004;25(1-2):85-106.
SD, Drvaric DM, Darr K, et al. The operative stabilization of pediatric
diaphyseal femur fractures with flexible intramedullary nails: a
prospective analysis. J Pediatr Orthop 1994;14(4):501-507.
SD, Gallagher D, Harris M, et al. Undiagnosed fractures in severely
injured children and young adults. Identification with technetium
imaging. J Bone Joint Surg Am 1994;76(4):561-572.
D Jr, Sanders RW, Scaduto JM, et al. Vacuum-assisted wound closure (VAC
therapy) for the management of patients with high-energy soft tissue
injuries. J Orthop Trauma 2003;17(10):683-688.
JE, Hensinger RN, Dedrick DK, et al. Emergency transport and
positioning of young children who have an injury of the cervical spine.
The standard backboard may be hazardous. J Bone Joint Surg Am
1989;71(1):15-22.
CA, Skaggs DL, Tang CW, et al. Use of flexible intramedullary nails in
pediatric femur fractures. J Pediatr Orthop 2006;26(4):497-504.
R, Nerlich M, Muggia-Sullam M, et al. Blunt abdominal trauma in cases
of multiple trauma evaluated by ultrasonography: a prospective analysis
of 291 patients. J Trauma 1992;32(4):452-458.
RI, Keller HW, Huber PM, et al. Flexible intramedullary nailing as
fracture treatment in children. J Pediatr Orthop 1996;16(5):602-605.
AH, Letts M. Injuries associated with fracture of the femur secondary
to motor vehicle accidents in children. Am J Orthop
2003;32(9):459-462,discussion 462.
EM, Anglen JO, Smith DG, et al. Advantages of submuscular bridge
plating for complex pediatric femur fractures. Clin Orthop Relat Res
2004;426:244-251.
MA, Goulet JA, Mueller KL, et al. Operative treatment of unstable
pediatric pelvis and acetabular fractures. J Pediatr Orthop
2005;25(1):34-38.
D, Harcke HT, Mendez AA, et al. Heterotopic ossification in central
nervous system-injured patients following closed nailing of femoral
fractures. Clin Orthop Relat Res 1990;256:254-259.
CC, Subasi M, Arslan H, et al. Is external fixation in pediatric
femoral fractures a risk factor for refracture? J Pediatr Orthop
2004;24(1):17-20.
D, Albert MC, Robertson WW Jr, et al. Complex femur fractures in
children: treatment with external fixation. J Pediatr Orthop
1990;10(5):588-591.
MM, Shagoury C, Lewis FR. Can adult trauma surgeons care for injured
children? J Trauma 1992;32(6):729-737,discussion 737-739.
PJ, Song KM, Routt ML, Jr., et al. Plate fixation of femoral shaft
fractures in multiply injured children. J Bone Joint Surg Am
1993;75(12):1774-1780.
S, Sances A Jr, Carlin F. Biomechanical analysis of padding in child
seats and head injury. Biomed Sci Instrum 2002;38:453-458.
M, Bouchard S, St-Vil D, et al. Falls from heights among children: a
retrospective review. J Pediatr Surg 1999;34(7):1060-1063.
WG, Dubowitz H. What factors affect the identification and reporting of
child abuse-related fractures? Clin Orthop Relat Res 2007;461:219-225.
JT, Dietrich AM, Abdessalam SF, et al. Effective use of the air
ambulance for pediatric trauma. J Trauma 2004;56(1):89-93.
P, Prevot J, Ligier JN, et al. Elastic stable intramedullary nailing in
forearm shaft fractures in children: 85 cases. J Pediatr Orthop
1990;10(2):167-171.
WF, Uhl R, Krieves M, et al. Management of open fractures in pediatric
patients: current teaching in Accreditation Council for Graduate
Medical Education (ACGME) accredited residency programs. J Pediatr
Orthop B 2008;17(1):1-6.
S, Nicol RO, Stott NS. Intramedullary fixation for pediatric unstable
forearm fractures. Clin Orthop Relat Res 2002;402:245-250.
BE, Rasmussen TE, Smith DL, et al. Experience with wound VAC and
delayed primary closure of contaminated soft tissue injuries in Iraq. J
Trauma 2006;61(5): 1207-1211.
MJ, Atkinson CC, Mooney DP. Application of the APSA evidence-based
guidelines for isolated liver or spleen injuries: a single institution
experience. J Pediatr Surg 2004;39(3):487-490.
M, Davidson D, Lapner P. Multiple trauma in children: predicting
outcome and long-term results. Can J Surg 2002;45(2):126-131.
M, Jarvis J, Lawton L, et al. Complications of rigid intramedullary
rodding of femoral shaft fractures in children. J Trauma
2002;52(3):504-516.
HS, High WM Jr, Ewing-Cobbs L, et al. Memory functioning during the
first year after closed head injury in children and adolescents.
Neurosurgery 1988;22(6 Pt 1):1043-1052.
RT, Gullahorn LJ, Yian EH, et al. Factors predictive of immobilization
complications in pediatric polytrauma. J Orthop Trauma
2001;15(5):338-341.
RT, O’Donnell PW, Feinberg JR. Epidemiology and mechanisms of femur
fractures in children. J Pediatr Orthop 2006;26(5):561-566.
SJ, Schootman M, Gordon JE, et al. Magnetic resonance imaging of the
knee in children and adolescents. Its role in clinical decision-making.
J Bone Joint Surg Am 2005;87(3):497-502.
SJ, Schootman M, Schoenecker PL, et al. Complications and outcomes of
open pediatric forearm fractures. J Pediatr Orthop 2004;24(1):1-6.
EJ, Morris JA Jr, de Lissovoy GV, et al. Acute hospital costs of
pediatric trauma in the United States: how much and who pays? J Pediatr
Surg 1990;25(9): 970-976.
RF, Portnoy J, Blow FC, et al. Injury type, injury severity, and repeat
occurrence of alcohol-related trauma in adolescents. Alcohol Clin Exp
Res 1994;18(2):261-264.
SA, Cook D, Fitzgerald M, et al. Complementary use of radiological
skeletal survey and bone scintigraphy in detection of bony injuries in
suspected child abuse. Arch Dis Child 2003;88(5):387-390.
RC Jr, Bensard DD, Moore EE, et al. Pelvic fracture geometry predicts
risk of life-threatening hemorrhage in children. J Trauma
1993;35(3):423-429.
LJ, Rivara FP, Grady MS, et al. Predictors of survival and severity of
disability after severe brain injury in children. Neurosurgery
1992;31(2):254-264.
RA, Garvin KL, Crosby LA. Avascular necrosis of the femoral head in an
adolescent following intramedullary nailing of the femur. A case
report. J Bone Joint Surg Am 1994;76(11):1706-1708.
MA, Garber JE, Stinson JT. Ectopic bone formation in children and
adolescents with head injuries: its management. J Pediatr Orthop
1987;7(1):83-90.
JF 3rd, Argenta LC, Marks MW, et al. Treatment of soft tissue defects
in pediatric patients using the V.A.C. system. Clin Orthop Relat Res
2000;376:26-31.
CA, Fischer RP, Cotler HB, et al. Selective management of pediatric
pelvic fractures: a conservative approach. J Pediatr Surg
1987;22(6):538-540.
KH, Coley BD, Zumberge N, et al. Preliminary experience with focused
abdominal sonography for trauma (FAST) in children: is it useful? J
Pediatr Surg 1999; 34(1):48-52,discussion 52-54.
DE, Mazur JM, Cummings RJ. Femoral head avascular necrosis associated
with intramedullary nailing in an adolescent. J Pediatr Orthop
1995;15(1):21-23.
R, Kramer R, Martus P, et al. Prognostic value of trauma scores in
pediatric patients with multiple injuries. J Trauma 2000;49(4):729-736.
MH, Newman KD, Eichelberger MR, et al. Thoracic trauma in children: an
indicator of increased mortality. J Pediatr Surg
1990;25(9):961-965,discussion 965-966.
DL, Schinco MA, Kerwin AJ, et al. Evaluation of initial base deficit as
a prognosticator of outcome in the pediatric trauma population. Am Surg
2004;70(4): 326-328.
U, Seeger W, Buchhorn R, et al. Surfactant abnormalities in patients
with respiratory failure after multiple trauma. Am Rev Respir Dis
1989;140(4):1033-1039.
DA, Schall LC, Gardner MJ, et al. Impact of pediatric trauma centers on
mortality in a statewide system. J Trauma 2000;49(2):237-245.
JC, Trieu L, Kendig T, et al. National injury-related hospitalizations
in children: public versus private expenditures across preventable
injury mechanisms. J Trauma 2007;63(3 Suppl):S10-S19.
RB. The use of external fixation devices in the management of severe
lower-extremity trauma and pelvic injuries in children. Clin Orthop
Relat Res 1984;188:21-33.
MC, Hollman AS, Davis CF. Comparison of computed tomography and
ultrasonographic imaging in the assessment of blunt abdominal trauma in
children. Br J Surg 1997;84(8):1144-1146.
B, Valerio IL, Stewart DH, et al. Microvascular free flap
reconstruction in pediatric lower extremity trauma: a 10-year review.
Plast Reconstr Surg 2005;115(6): 1618-1624.
JB, Partrick DA, Nydam TL, et al. Nonaccidental trauma is a major cause
of morbidity and mortality among patients at a regional level 1
pediatric trauma center. J Pediatr Surg 2006;41(12):2013-2015.
BG, Bugmann P, Le Coultre C. Blunt injuries to liver, spleen, kidney,
and pancreas in pediatric patients. Eur J Pediatr Surg
1992;2(3):154-156.
A, Reis M, Molina M, et al. Supracondylar fractures of the humerus
associated with ipsilateral forearm fractures in children: a report of
47 cases. J Pediatr Orthop 2001;21(3):307-312.
KF, McCammon KA, Sumfest JM, et al. Open reconstruction of pediatric
and adolescent urethral strictures: long-term follow-up. J Urol
2003;169(5):1818-1821, discussion 1821.
S, Zhao C, McClemens E, et al. Pediatric orthopaedic patients
presenting to a university emergency department after visiting another
emergency department: demographics and health insurance status. J
Pediatr Orthop 2007;27(6):690-694.
B, Waxman K, Jones T, et al. Cervical spine clearance in blunt trauma:
evaluation of a computed tomography-based protocol. J Trauma
2005;59(1):179-183.
JL, Lucas J, Feustel PJ. Outcome of adolescent trauma admitted to an
adult surgical intensive care unit versus a pediatric intensive care
unit. J Trauma 2001;51(3): 478-480.
TL, Weiner DS. Calcaneal fractures in children. An evaluation of the
nature of the injury in 56 children. Clin Orthop Relat Res
1982;171:150-155.
LE, Goulet JA, Greenfield ML, et al. Extracorporeal life support for
patients with significant orthopaedic trauma. Clin Orthop Relat Res
1997;339:32-40.
OP, Oswanski MF, Stringfellow KC, et al. Pediatric blunt trauma: a
retrospective analysis in a Level I trauma center. Am Surg
2006;72(6):538-543.
PR, Densmore J, Werner M, et al. Pediatric pelvic injury: functional
outcome at 6-month follow-up. J Pediatr Surg
2005;40(1):107-112,discussion 112-113.
JS, Flynn JM, Koffler KM, et al. Analysis of the cause, classification,
and associated injuries of 166 consecutive pediatric pelvic fractures.
J Pediatr Orthop 2001;21(4): 446-450.
HA, Barnett NP, Spirito A. Adolescent alcohol use and injury. A summary
and critical review of the literature. Minerva Pediatr
2004;56(3):291-309.
EL, Hedequist D, Morgan SJ, et al. Results and technique of unstable
pediatric femoral fractures treated with submuscular bridge plating. J
Pediatr Orthop 2006; 26(2):177-181.
CJ, Taylor GA, Newman KD, et al. Safety-belt injuries in children with
lap-belt ecchymosis: CT findings in 61 patients. AJR Am J Roentgenol
1991;157(1):111-114.
DL, Kautz SM, Kay RM, et al. Effect of delay of surgical treatment on
rate of infection in open fractures in children. J Pediatr Orthop
2000;20(1):19-22.
DL, Leet AI, Money MD, et al. Secondary fractures associated with
external fixation in pediatric femur fractures. J Pediatr Orthop
1999;19(5):582-586.
JS Jr, Martin LF, Young WW, et al. Do trauma centers improve outcome
over non-trauma centers: the evaluation of regional trauma care using
discharge abstract data and patient management categories. J Trauma
1990;30(12):1533-1538.
KM, Sherman N, Alexander MA. Coexistence of deep venous thrombosis and
heterotopic ossification in the pediatric patient. Arch Phys Med
Rehabil 1993;74(5): 547-551.
SV, Holland AJ, Fahy F, et al. Transfer of pediatric trauma patients to
a tertiary pediatric trauma centre: appropriateness and timeliness. J
Trauma 2007;62(5): 1229-1233.
T, Haider A, DiRusso SM, et al. Prediction of mortality in pediatric
trauma patients: new injury severity score outperforms injury severity
score in the severely injured. J Trauma 2003;55(6):1083-1087,discussion
1087-1088.
SE, Albrecht R, Foley D, et al. Surgeon-directed ultrasound for trauma
is a predictor of intra-abdominal injury in children. Am Surg
2004;70(2):164-167,discussion 167-168.
EE, Taylor HG, Kaugars AS, et al. Sibling relationships and behavior
after pediatric traumatic brain injury. J Dev Behav Pediatr
2003;24(1):24-31.
RC, Gupta S, White DK. Severe head injury in children: geographical
range of an emergency neurosurgical practice. Emerg Med J
2004;21(4):433-437.
M, Nance ML, Holmes JHT, et al. Pediatric blunt abdominal injury: age
is irrelevant and delayed operation is not detrimental. J Trauma
2007;63(3):608-614.
EC, Perkowski P, Villarreal D, et al. Morbidity and mortality of
children following motor vehicle crashes. Arch Surg 2003;138(2):142-145.
SJ, Watkins S, Tong TK, et al. Measured energy expenditure in pediatric
intensive care patients. Am J Dis Child 1989;143(4):490-492.
VT. Orthopaedic treatment of fractures of the long bones and pelvis in
children who have multiple injuries. Instr Course Lect 2000;49:415-423.
DR, Hoffinger S. Intramedullary nailing of femoral shaft fractures in
children via the trochanter tip. Clin Orthop Relat Res 2000;376:113-118.
MJ, Sege RD, Olson L, et al. Intentional injury management and
prevention in pediatric practice: results from 1998 and 2003 American
Academy of Pediatrics Periodic Surveys. Pediatrics 2005;116(4):996-1000.
AK, Sorrells DL, Halvorson E, et al. Pulmonary embolism: which
pediatric trauma patients are at risk? J Pediatr Surg
2005;40(1):124-127.
Department of Transportation NHTSA. Traffic safety facts 2002. DOT HS
809 620. 2004;1-202. Available at:
http://www.nrd.nhsta.dot.gov/Pubs/TSF2004.PDF. Accessed June 29,2009.
F, Anderson CL, Agran P, et al. Child safety seat knowledge among
parents utilizing emergency services in a level I trauma center in
Southern California. Pediatrics 2002;110(5):e61.
CP, Chattar-Cora D, O’Neill A, et al. Efficacy of primary wound
cultures in long bone open extremity fractures: are they of any value?
Arch Orthop Trauma Surg 2002;122(5):259-261.
der Sluis CK, Kingma J, Eisma WH, et al. Pediatric polytrauma:
short-term and long-term outcomes. J Trauma 1997;43(3):501-506.
WD, Garcia VF. Pediatric pelvic fractures combined with an additional
skeletal injury is an indicator of significant injury. Surg Gynecol
Obstet 1993;177(5):468-472.
GC, Arroyo H, Ramicone E, et al. Timing of fracture fixation in blunt
trauma patients with severe head injuries. Am J Surg
1998;176(4):324-329, discussion 329-330.
L, Delronge G, Lamoureux J. Orthopaedic treatment of paediatric
multiple trauma patients. A new technique. Int Surg 1988;73(3):177-179.
MG, Kessler MW, Choe JC, et al. Pelvic fractures in children: an
exploration of practice patterns and patient outcomes. J Pediatr Orthop
2005;25(5):581-587.
MY, Kim KA, Griffith PM, et al. Injuries from falls in the pediatric
population: an analysis of 729 cases. J Pediatr Surg
2001;36(10):1528-1534.
RL, Connolly PT. In children undergoing chest radiography what is the
specificity of rib fractures for nonaccidental injury? Arch Dis Child
2004;89(5):490-492.
HW, Knights RM, Bawden HN. Neuropsychological deficits following head
injury in children. J Clin Neuropsychol 1984;6(3):267-286.
B, Mencio GA, Green NE. Open reduction and internal fixation of
pediatric forearm fractures. J Pediatr Orthop 1996;16(5):644-650.
EH, Gullahorn LJ, Loder RT. Scoring of pediatric orthopaedic
polytrauma: correlations of different injury scoring systems and
prognosis for hospital course. J Pediatr Orthop 2000;20(2):203-209.
PS, Pring ME, Gaynor TP, et al. Compartment syndrome following
intramedullary fixation of pediatric forearm fractures. J Pediatr
Orthop 2004;24(4):370-375.
JJ, Churchill RS, Cooperman DR, et al. The floating knee in the
pediatric patient. Nonoperative versus operative stabilization. Clin
Orthop 2000;376:124-136.
XG, Zhao GF, Ma YF, et al. Research progress in mechanism of traumatic
brain injury affecting speed of fracture healing. Chin J Traumatol
2007;10(6):376-380.
BS, Morrison K, Gaines B, et al. Effect of age on cervical spine
injuries in children after motor vehicle collisions: effectiveness of
restraint devices. J Pediatr Surg 2004;39(3):483-486.
