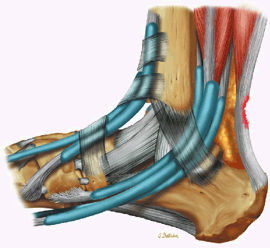
The Ankle and Foot
-
FS PD FSE sequences are performed in all three orthogonal or orthogonal-oblique planes.
-
Decreasing the FOV or increasing the resolution in the coronal plane allows optimal separation of the distal tibial and talar dome chondral surfaces.
or phased-array design), using a 12- to 14-cm field of view (FOV) and a 512 × 256 or 256 × 256 acquisition matrix. Routine protocols for evaluation include:
-
T1- or proton density (PD)-weighted axial, sagittal, and coronal images
-
Fat-suppressed PD-weighted fast spin-echo sequences (FS PD FSE) in all three orthogonal planes (Fig. 5.1)
-
Thin (i.e., 2 to 3 mm) sections
 |
|
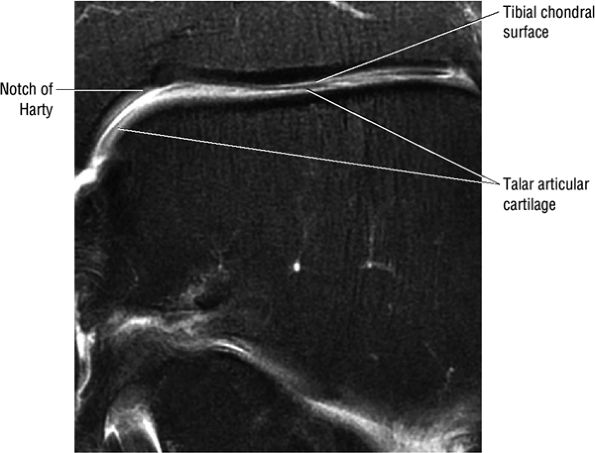
FIGURE 5.1 ● Visualization of tibiotalar articular surfaces using a coronal FS PD FSE sequence. Separation of the tibial and talar chondral surfaces is important in characterizing osteochondral lesions.
|
-
The anterior muscles of the leg are the tibialis anterior (Fig. 5.2), the extensor hallucis longus (Fig. 5.3), the extensor digitorum longus (Fig. 5.4) and the peroneus tertius (Fig. 5.5).
-
The posterior muscles of the leg include a superficial group and deep group. The superficial group is represented by the gastrocnemius (Fig. 5.6), the soleus (Fig. 5.7), and plantaris (Fig. 5.8).
-
The deep group of posterior leg muscles comprises the popliteus (see discussion in Chapter 4 on the knee), the flexor hallucis longus (Fig. 5.9), the flexor digitorum longus (Fig. 5.10), and the tibialis posterior (Fig. 5.11).
-
The lateral muscles of the leg are the peroneus longus (Fig. 5.12) and the peroneus brevis (Fig. 5.13).
-
The muscles of the foot are the extensor digitorum brevis (Fig. 5.14), the abductor hallucis (Fig. 5.15), the flexor digitorum brevis (Fig. 5.16), the abductor digiti minimi (Fig. 5.17), the quadratus plantae (Fig. 5.18), the lumbricals (Fig. 5.19), the flexor hallucis brevis (Fig. 5.20), the adductor hallucis (Fig. 5.21), the flexor digiti minimi brevis (Fig. 5.22), the dorsal interossei (Fig. 5.23), and the plantar interossei muscles (Fig. 5.24).
 |
|
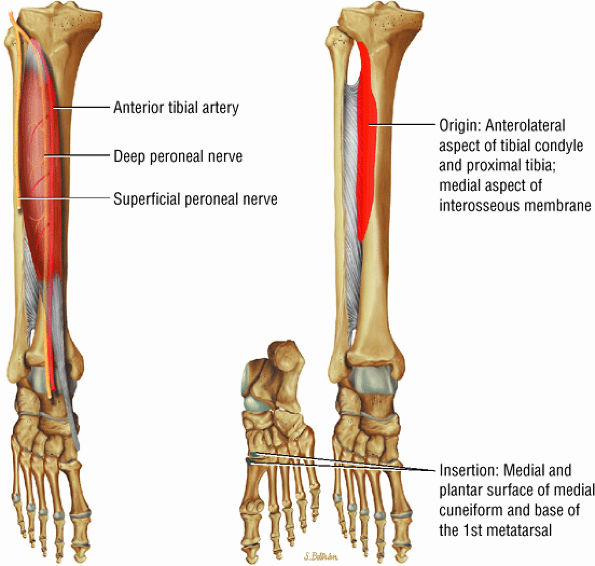
FIGURE 5.2 ● TIBIALIS ANTERIOR The tibialis anterior muscle functions eccentrically after the heel strike to control deceleration of the foot and concentrically after the toe-off in ankle dorsiflexion. In runners and hikers, paratenonitis is associated with the use of excessive eccentric contraction during midfoot and forefoot impact on downhill slopes. Paratenonitis is also associated with direct mechanical irritation from ski boots or hockey skates. The tibialis anterior dorsiflexes and inverts the foot.
|
 |
|
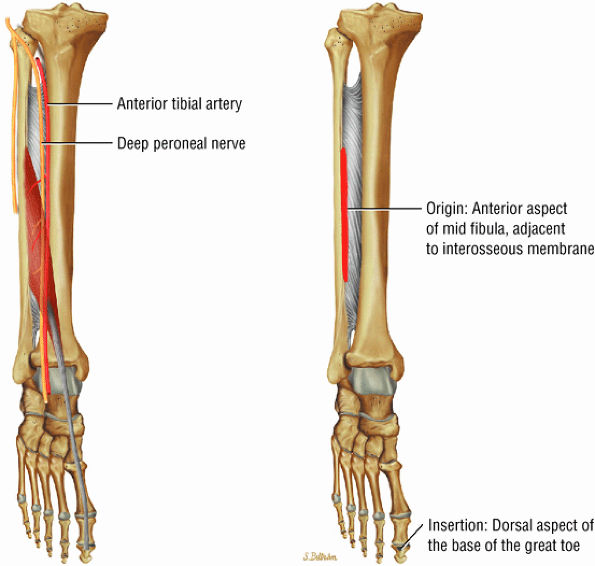
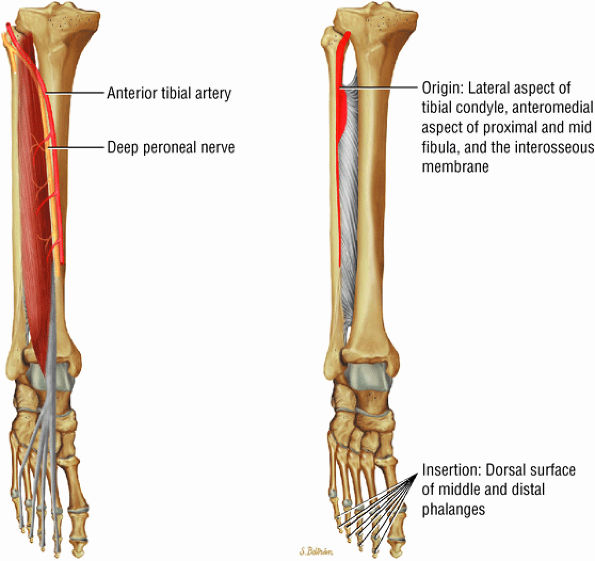
FIGURE 5.3 ● EXTENSOR HALLUCIS LONGUS Extensor hallucis (and extensor digitorum) injuries are similar in origin to injuries of the tibialis anterior tendon. Extensor hallucis longus (EHL) paratenonitis is associated with pain and swelling localized to the ankle joint with painful resisted extension of the hallux. The EHL extends the great toe and dorsiflexes the foot.
|
 |
|
FIGURE 5.4 ● EXTENSOR DIGITORUM LONGUS Extensor digitorum longus (EDL) paratenonitis is associated with pain and swelling over the ankle joint and lateral to the extensor hallucis longus. There is pain with resisted extension of the lesser toes in paratenonitis. The EDL extends the phalanges of the lateral four toes and dorsiflexes the foot.
|
 |
|
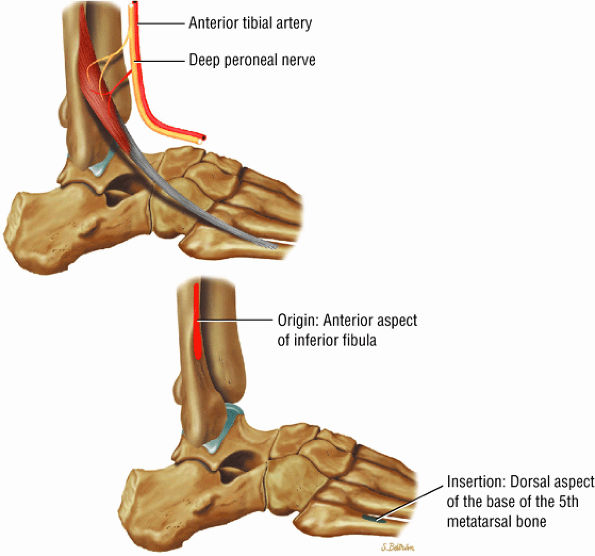
FIGURE 5.5 ● PERONEUS TERTIUS The peroneus tertius represents a lateral slip of the extensor digitorum longus. Isolated ruptures of the peroneus tertius tendons do not occur. The peroneus tertius dorsiflexes and everts the foot.
|
 |
|
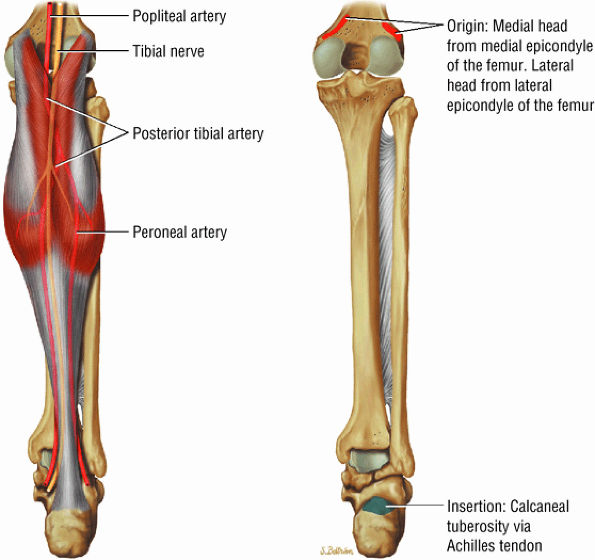
FIGURE 5.6 ● GASTROCNEMIUS The gastrocnemius plantarflexes the foot and also flexes the knee joint, as its origin is on the femoral condyles. In contrast to the soleus muscle (which has a more postural function), the gastrocnemius generates the power for propulsion in walking, running, and jumping.
|
 |
|
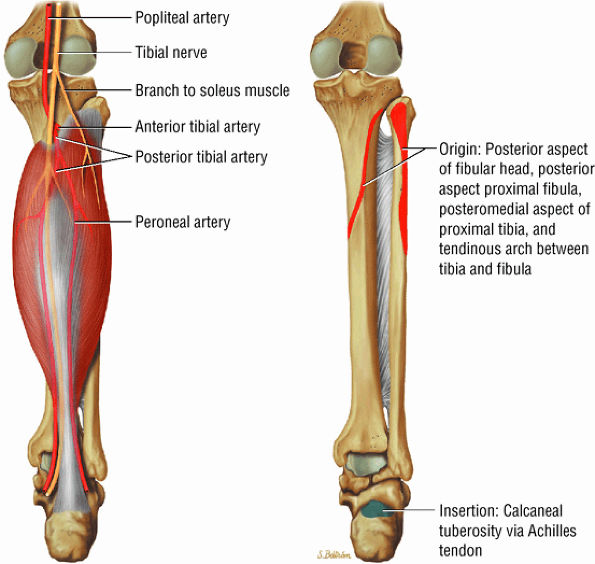
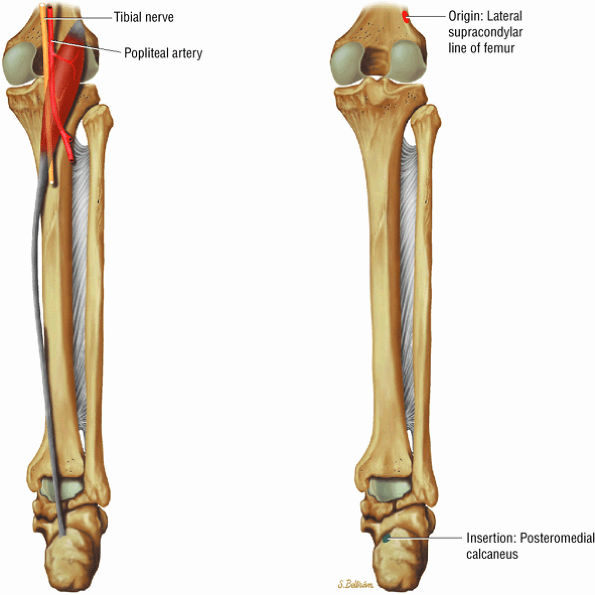
FIGURE 5.7 ● SOLEUS The gastrocnemius and the soleus muscles function in plantarflexion of the foot. The soleus consists primarily of type I or slow-twitch oxidative fibers and rapidly develops disuse atrophy in response to immobilization.
|
 |
|
FIGURE 5.8 ● PLANTARIS The plantaris plantar flexes the foot and is visualized as a 2- to 3-mm hypointense dot-like structure on axial images anteromedial to the Achilles tendon. The plantaris tendon courses obliquely between the gastrocnemius and soleus muscles.
|
 |
|
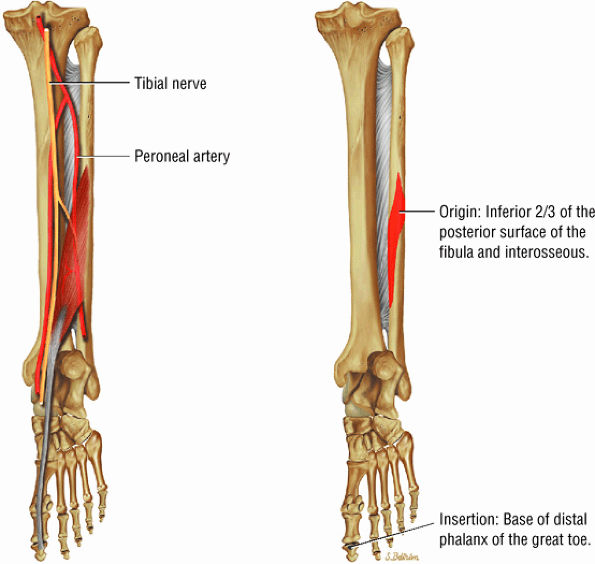
FIGURE 5.9 ● FLEXOR HALLUCIS LONGUS The flexor hallucis longus (FHL) flexes the great toe and plantarflexes the foot. The FHL is susceptible to injury during extremes of ankle plantarflexion and metatarsophalangeal dorsiflexion. The proximal sheath, 10 to 12 cm in length, has no mesotenon and may communicate with both the ankle joint and the sheaths of the flexor digitorum longus and tibialis posterior.
|
 |
|
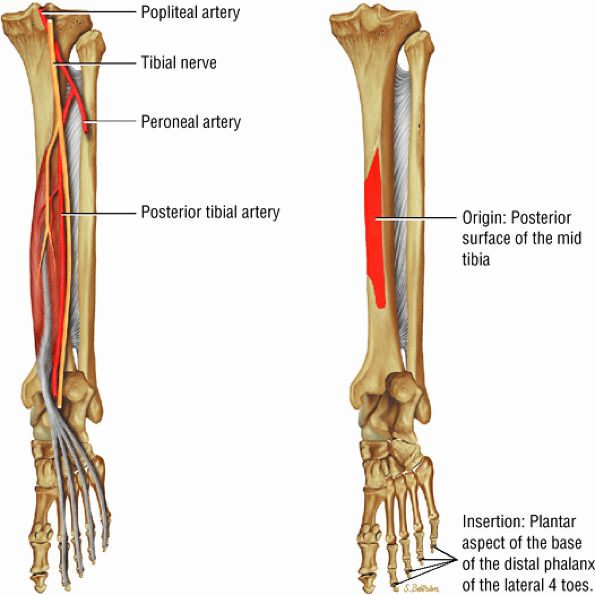
FIGURE 5.10 ● FLEXOR DIGITORUM LONGUS The flexor digitorum longus (FDL) flexes the phalanges of the lateral four toes and plantarflexes the foot. The FDL is superficial to the flexor hallucis in the sole of the foot. Paratenonitis of the FDL is more infrequent than involvement of the flexor hallucis longus.
|
 |
|
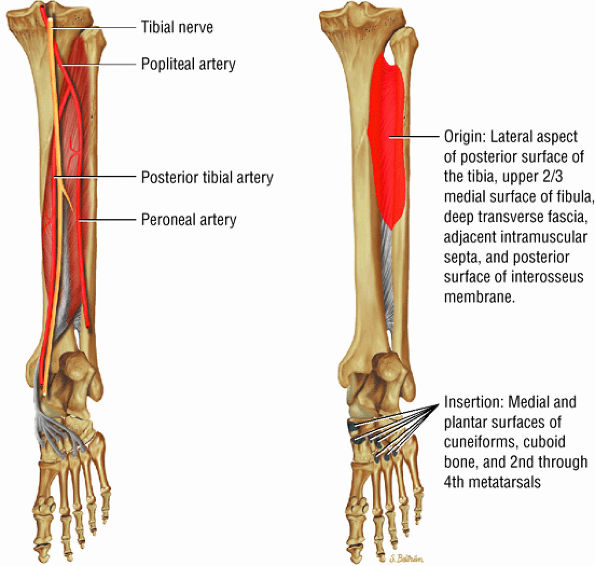
FIGURE 5.11 ● TIBIALIS POSTERIOR The tibialis posterior plantarflexes and inverts the foot. The tibialis posterior tendon passes over (superficial to) the deltoid and changes from a tubular tendon to a flattened structure containing a fibrocartilaginous sesamoid (under the plantar calcaneonavicular ligament).
|
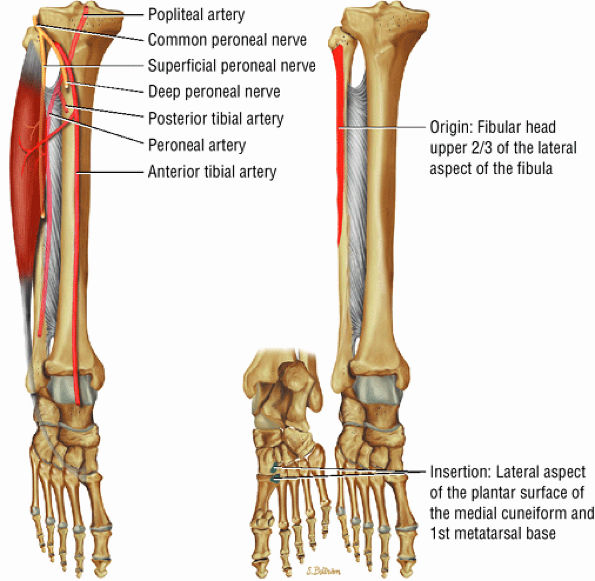 |
|
FIGURE 5.12 ● PERONEUS LONGUS The peroneus longus plantarflexes and everts the foot. The peroneus longus passes underneath the superior peroneal retinaculum, then runs superficial to the calcaneofibular ligament and passes deep to the inferior peroneal retinaculum. The third turn of the peroneus longus occurs as it enters the plantar tunnel between the cuboid and fifth metatarsal base. Ossification of the fibrocartilaginous sesamoid (which protects the tendon as it glides over the cuboid tuberosity) within the peroneus longus occurs in up to 20% of cases.
|
 |
|
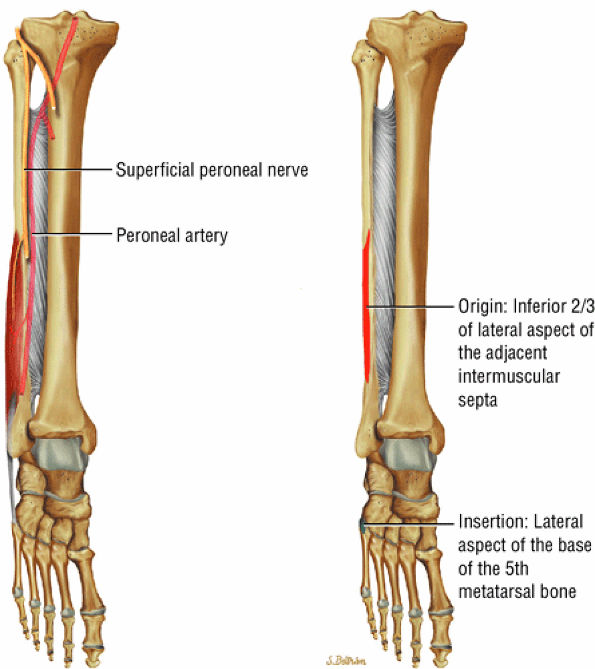
FIGURE 5.13 ● PERONEUS BREVIS The peroneus brevis plantarflexes and everts the foot. The peroneus brevis has a shorter and smaller muscle belly than the peroneus longus and becomes tendinous 2 to 3 cm proximal to the tip of the lateral malleolus.
|
 |
|
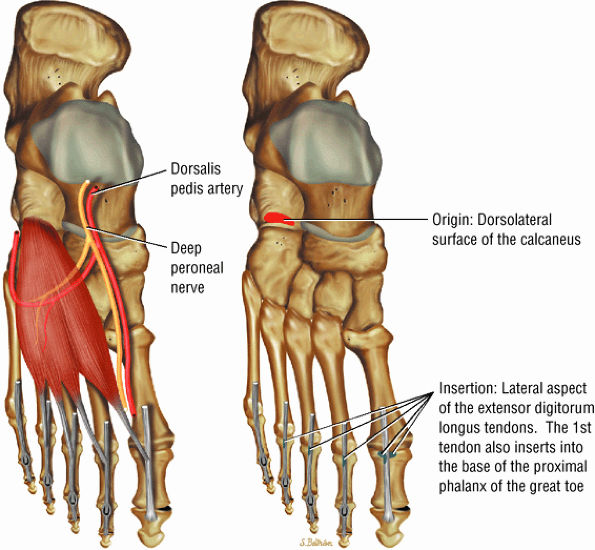
FIGURE 5.14 ● EXTENSOR DIGITORUM BREVIS The extensor digitorum brevis extends the phalanges of the four medial toes. The extensor digitorum brevis and longus tendons contribute to the metatarsophalangeal extensor expansion.
|
 |
|
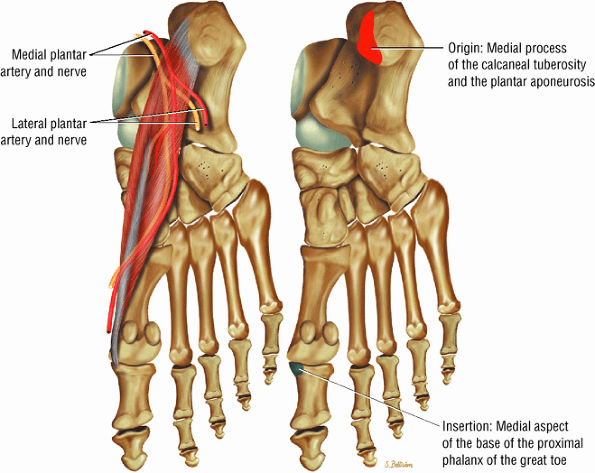
FIGURE 5.15 ● ABDUCTOR HALLUCIS The abductor hallucis functions in abduction of the great toe. In tarsal tunnel syndrome the medial and lateral plantar nerves may be decompressed by releasing the fascia of the abductor hallucis muscle.
|
 |
|
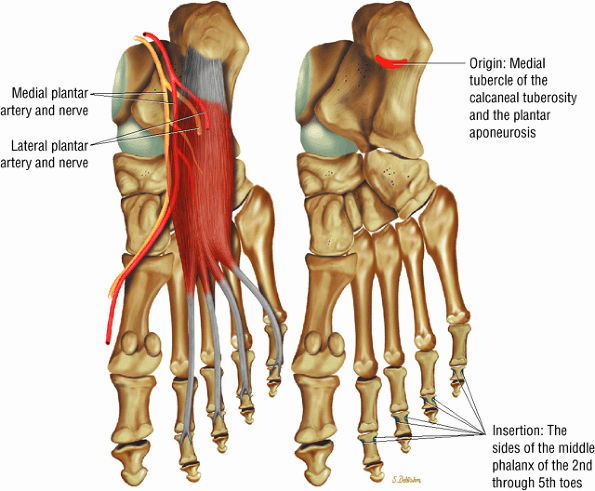
FIGURE 5.16 ● FLEXOR DIGITORUM BREVIS The flexor digitorum brevis (FDB) divides into two slips to allow the passage of the flexor digitorum longus tendon. The FDB flexes the middle phalanges (PIP joints) and is a weak plantarflexor of the MP joint (for the lateral four toes).
|
 |
|
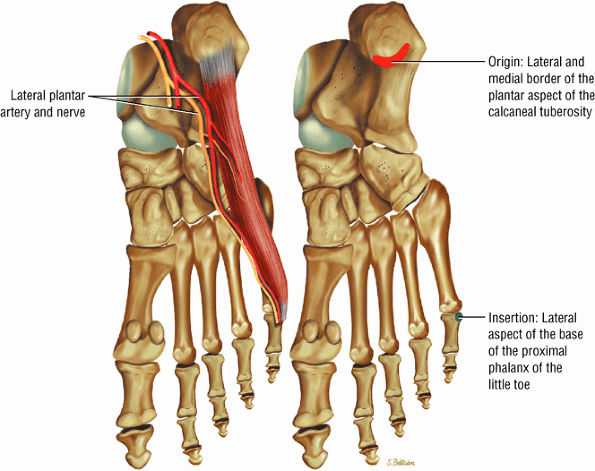
FIGURE 5.17 ● ABDUCTOR DIGITI MINIMI The abductor digiti minimi inserts into the plantar plate and the lateral plantar aspect of the proximal phalanx of the fifth toe. The abductor digiti minimi abducts the fifth toe and assists in flexion.
|
 |
|
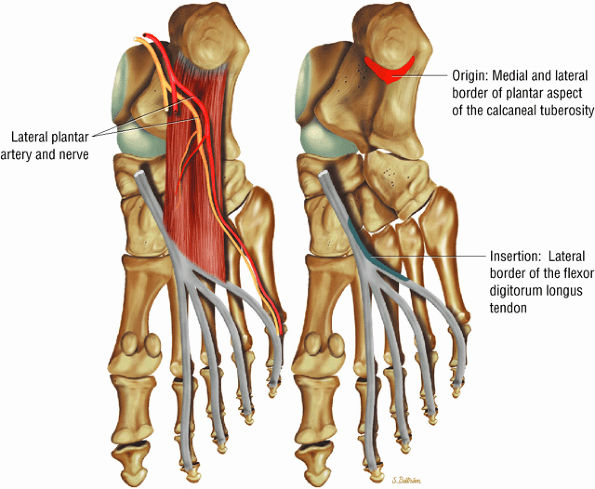
FIGURE 5.18 ● QUADRATUS PLANTAE The quadratus plantae originates from two heads from the medial and lateral aspect of the calcaneus and long plantar ligaments. The quadratus plantae flexes the terminal (distal) phalanges of the lateral four toes.
|
 |
|
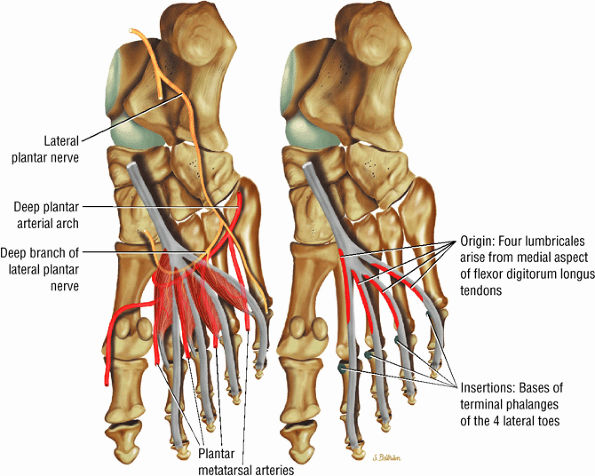
FIGURE 5.19 ● LUMBRICALS The lumbricals are plantarflexors of the metatarsophalangeal joint and extend the toes at the DIP and PIP joints. The lumbrical tendon inserts medially onto the extensor hood.
|
 |
|
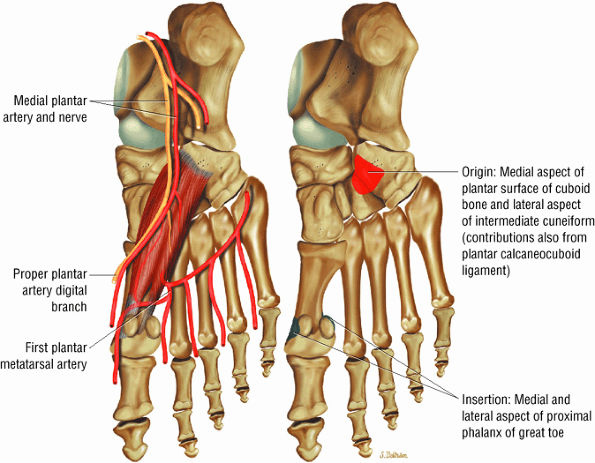
FIGURE 5.20 ● FLEXOR HALLUCIS BREVIS The flexor hallucis brevis functions in flexion of the great toe. Instability of the first metatarsophalangeal joint occurs with loss of flexor hallucis brevis function, as occurs with excision of both tibial and fibula sesamoids.
|
 |
|
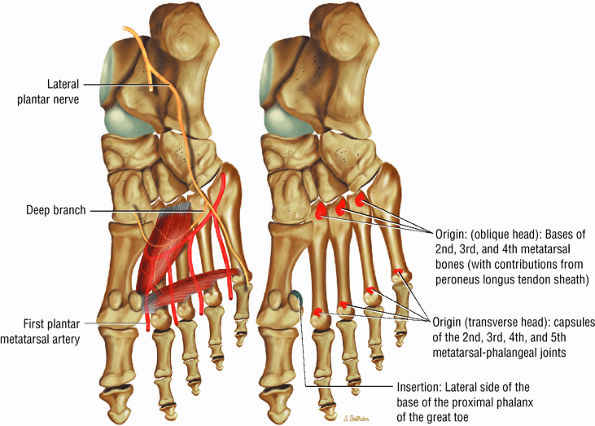
FIGURE 5.21 ● ADDUCTOR HALLUCIS The adductor hallucis has two heads and forms a conjoined tendon. The adductor hallucis adducts the great toe and assists in its flexion.
|
 |
|
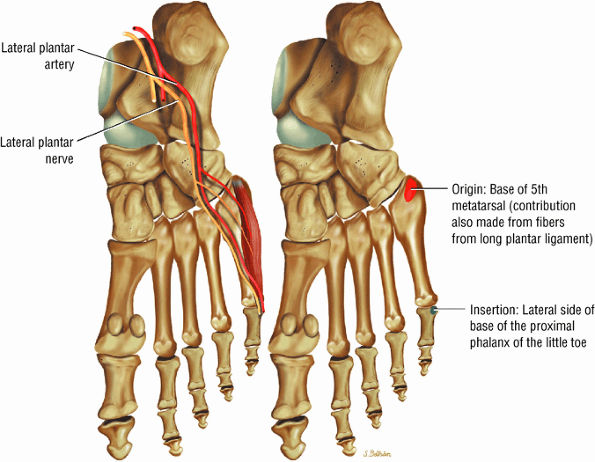
FIGURE 5.22 ● FLEXOR DIGITI MINIMI BREVIS The flexor digiti minimi brevis flexes the little toes.
|
 |
|
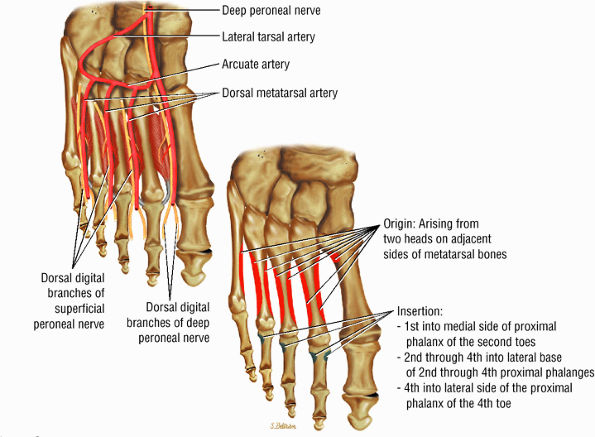
FIGURE 5.23 ● DORSAL INTEROSSEI The four dorsal interosseous muscles stabilize the toes and abduct the second, third, and fourth toes laterally. They also assist in the flexion of the proximal phalanges and extension of the middle and distal phalanges.
|
 |
|
FIGURE 5.24 ● PLANTAR INTEROSSEI There are four dorsal and three plantar interosseous muscles. The plantar interosseous muscles function in adduction of the third, fourth, and fifth toes medially toward the axis of the second toe. They also assist in flexion of the proximal phalanges and extension of the middle and distal phalanges.
|
of the calcaneus and is inferior to the peroneal tubercle, enters the foot at the lateral inferior margin of the cuboid. The extensor hallucis longus tendon is identified along the dorsum of the foot and inserts onto the distal phalanx of the first toe. The interosseous talocalcaneal ligament, with its associated high-signal-intensity fat, is bordered anteriorly by the anterior process of the calcaneus and posteriorly by the lateral process of the talus. The pre-Achilles fat pad (high signal intensity on T1-weighted sequences) is located directly anterior to the Achilles tendon (low spin density).
|
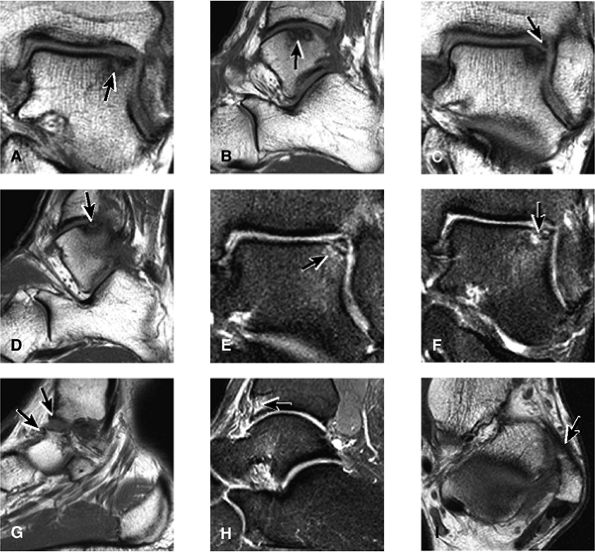
FIGURE 5.25 ● Sagittal anatomy of the ankle and foot. (A) The origins of the anterior talofibular ligament and posterior talofibular ligament are identified arising from the anterior and posterior distal tip of the lateral malleolus. From the origin, the full course of these ligaments can be followed medially on successive sagittal images to their insertions on the anterior and posterior talus. (B) The vertical course of a long segment of the peroneus longus and brevis tendons is often visualized on a single sagittal image through the tendons. This image is useful to further characterize tendinosis and longitudinal tears or splits, and for measuring the gap between completely ruptured tendon fragments. (C) The anterior process of the calcaneus is a common location for fractures that are occult on plain film. They are optimally visualized in the sagittal plane on MR exams. (D) In addition to occurring at the tibiotalar joint, degenerative arthrosis is also commonly found at the posterior subtalar, calcaneocuboid, and talonavicular joints. The cartilage surfaces and subchondral bone at these articulations are optimally visualized in the sagittal plane. (E) The presence of an os trigonum posterior to the talus predisposes certain athletes with a predilection for plantarflexion to the os trigonum syndrome. This is diagnosed on sagittal MR images when edema is visualized within the os trigonum and extends across the synchondrosis into the posterior talus. (F) Abnormal signal in the sinus tarsi manifests as high signal on FS fluid-weighted sequences and low signal on non-FS sequences. This abnormal signal may suggest, but is not specific for, inflammation in the sinus tarsi. Other causes of abnormal signal in the sinus tarsi, which may be incidental and asymptomatic, include extension of joint fluid from the posterior and middle subtalar joints, extension of generalized edema throughout the soft tissues of the ankle from stasis or other causes, enlarged vessels, and ganglion cysts.(G) Two potential causes of an incidental “mass” palpated on physical examination about the Achilles tendon are a low-lying soleus muscle and an accessory soleus muscle, both of which are diagnosed by MR imaging. The normal soleus muscle extends to about the proximal one third or one half of the Achilles tendon. A low-lying soleus will extend to the distal third of the tendon. An accessory soleus is present when there is an extra muscle in the pre-Achilles fat, usually extending to the distal third of the tendon, often near the distal insertion. (H) In the setting of a complete Achilles tendon rupture, the location of the tear may be at the myotendinous junction, mid-tendon, distal tendon, or tendon insertion at the os calcis. In addition, the tear is characterized as transverse or oblique longitudinal. In the case of transverse tears, the distance between the tear and tendinous insertion at the calcaneus is measured. Also, the length of good-quality tendon stump at the calcaneal insertion is measured, since the surgeon often uses the distal stump in the surgical reconstruction or repair. (I) The anteromedial aspect of the tibiotalar joint is a common location for the formation of large osteophytes, which extend anteriorly from the anteromedial tibia and talus. These may cause pain, limit the range of motion, or break off and form loose bodies within the tibiotalar joint. This spectrum of findings is part of the anteromedial impingement syndrome. (J) Ancillary findings at the plantar aponeurosis visualized on sagittal images include bone marrow edema within the inferior calcaneus, inferior calcaneal enthesophyte with marrow edema, and high signal within the flexor digitorum brevis muscle and fat that surround the plantar aponeurosis. These findings suggest active inflammation in the tissues surrounding the plantar aponeurosis. (K) The deltoid ligament is found on sagittal images by finding its origin extending off the bilobed medial malleolus. The medial course of the deltoid ligament components is followed over the next two or three successive sagittal images. (L) The vertical course of the tibialis posterior tendon and the flexor digitorum longus tendon is often visualized on a single image. Triangulating on tendon pathology in both the sagittal and axial planes aids in further characterizing tendon abnormalities.
|
to either the anterior talus or the talar head. The plantar calcaneonavicular ligament, or spring ligament, is located inferior to the lateral malleolus between the lateral talus and tibialis posterior tendon.
|
FIGURE 5.26 ● Coronal anatomy of the ankle and foot. (A) The calcaneofibular ligament (CFL) is identified by finding its origin at the inferior tip of the lateral malleolus. The posterior inferomedial course of the CFL is followed on three or four consecutive coronal images moving posteriorly through the ankle, to its insertion on the posterolateral calcaneus. Optimal evaluation of the CFL involves examining its full course on successive images in both the coronal and sagittal plane for tears, sprain, or scarring. (B) The medial cord of the plantar aponeurosis is normally slightly thicker than the lateral cord, and this mild asymmetry in thickness should not be misinterpreted as plantar aponeurosis scarring or plantar fasciitis. On successive coronal images, the course of the plantar aponeurosis should be followed back to its insertion on the inferior calcaneus and evaluated for the presence of thickening, decreased signal suggestive of scarring, increased signal indicative of plantar fasciitis, and tears. (C) Coronal images are optimal for viewing the lateral process of the talus, which is a frequent site of fractures that are occult on plain films. Fractures of the lateral process of the talus are most common in patients with snowboarding injuries. (D) The talar dome and tibial plafond are optimally visualized on coronal images. They are assessed for the presence of subchondral edema and cystic change with overlying chondral abnormalities. Close attention should be directed to the extreme anterior and posterior margins of the cartilage-bearing articular surfaces of the talar dome and tibial plafond to avoid overlooking osteochondral lesions at these locations. (E) The origin of the anterior talofibular ligament (ATFL) is found at the anterior distal tip of the lateral malleolus, and the ATFL is followed anteriorly on two or three successive coronal images to its insertion at the anterior lateral margin of the talus. (F) The deltoid ligament is optimally visualized in the coronal and axial planes. Tears of the deltoid manifest as loss of fiber striation or diffuse amorphous hyperintensity in the ligament on fluid-weighted sequences. Partial tears are more common than complete tears. (G) Focal fatty atrophy and denervation of the plantar flexor muscles of the foot (abductor digiti minimi, flexor digitorum brevis, and abductor hallucis) may indicate neuropathy involving the tibial nerve or its branches. (H) At the level of the anterior aspect of the talus and calcaneus, the peroneal tendons and flexor tendons turn from their cranial—caudal course to travel an anterior-to-posterior course along the plantar aspect of the foot. The distal portions of the tendons should be examined along the plantar aspect of the foot on successive coronal images for evidence of tendinosis and tears. (I) The base of the fifth metatarsal is a common location for fractures and is often visualized within the FOV on ankle MR exams. (J) At the level of the navicular, the flexor digitorum longus (FDL) and flexor hallucis longus (FHL) tendons run side by side, with the FDL medial to the FHL. Anterior to this level on successive coronal images, the two tendons cross, with the FHL medial to the FDL as the FHL courses to its insertion on the great toe. (K) Stress fractures of the navicular are commonly vertical in the midline of the navicular, an appearance that is well characterized on coronal images. (L) Contusions, stress-related edema, fractures, and degenerative arthritis of the midfoot bones and joints are common causes of midfoot pain and are often optimally identified on fluid-sensitive sequences.
|
|
FIGURE 5.27 ● Axial anatomy of the ankle and foot. (A) The flexor digitorum longus, flexor hallucis longus, peroneus brevis, soleus, and extensor digitorum muscles are examined at this level for strain, tears, or fatty atrophy that may suggest denervation. (B) The tibialis anterior, extensor hallucis longus, and extensor digitorum longus tendons are examined on every ankle MR examination. Extensor tendon pathology is frequently overlooked if these tendons are not included as part of the ankle checklist. (C) Tears and sprains of the anterior syndesmotic ligament are a frequent cause of persistent ankle pain following ankle sprain. The syndesmotic ligaments are thick, tough ligaments that are important ankle stabilizers, and delayed diagnosis of syndesmotic tears may result in significant degenerative arthrosis at the tibiotalar joint due to the resulting ankle instability. The syndesmotic ligaments course obliquely inferiorly from the tibia to the fibula and are not usually visualized in their entirety on a single axial image; rather, their course is followed on at least two or three successive axial images. (D) The peripheral margin of the peroneal tendons and tibialis posterior tendon should normally never extend beyond the peripheral margins of the lateral and medial malleoli, respectively. Tendon subluxation around the posterior corner of either malleolus is indicative of a tear of the overlying flexor retinaculum (medially) or peroneal retinaculum (laterally). When the retinacula are torn, the tendon is free to intermittently sublux or dislocate, leading to tendon degeneration, pain, and tendon dysfunction. (E) Suspected osteochondral lesions of the talar dome are visualized and further characterized on axial images through the top of the talar dome. (F) The peroneus brevis tendon may normally appear somewhat flattened. However, as the tendon degenerates, it becomes U-shaped and drapes around the anterior aspect of the peroneus longus and becomes impinged between the peroneus longus tendon and the lateral malleolus. With further degeneration, the peroneus brevis may split or completely rupture. (G) Evidence of anterior talofibular ligament injury is visualized on the majority of MR ankle examinations and appears as thickening, intermediate signal with ill-defined fibers, or attenuation of the ligament. This is commonly asymptomatic. (H) Because the flexor hallucis longus tendon sheath communicates with the tibiotalar joint, fluid may normally be present within the tendon sheath in proportion to the amount of fluid in the tibiotalar joint. If there is fluid within the tendon sheath out of proportion to that seen in the tibiotalar joint, tenosynovitis is most likely present. The finding of flexor hallucis longus tenosynovitis should prompt a search for an os trigonum, as impingement of the flexor hallucis longus tendon between an os trigonum and the posterior tibial plafond is a common cause for FHL tenosynovitis. (I) The calcaneofibular ligament (CFL) passes anterior and medial to the peroneal tendons. On the image at which the CFL passes directly medial to the peroneus brevis tendon, the appearance of the peroneus brevis and the CFL side by side is occasionally mistaken for a split peroneus brevis tendon. (J) Dilated posterior tibial veins within the tarsal tunnel occasionally compresses the tibial nerve. In the setting of clinical suspicion for tarsal tunnel syndrome or if there is evidence of muscle denervation on MR images, the size of the posterior tibial veins should be described. (K) The spring ligament is identified at this axial image location, extending from the anteromedial calcaneus to the posteromedial navicular. Tears of the spring ligament may result in medial instability and hindfoot valgus. (L) The posterior tibialis tendon (PTT) may normally become thickened and fan-like as it passes posterior to its navicular insertion (prior to also inserting on the cuneiforms and the base of the second through fourth metatarsals). In the absence of other findings, the thickening of the PTT at this level should not be mistaken for focal tendinosis. (M) On inferior images through the ankle, Lisfranc's ligament is occasionally included in the FOV. Lisfranc's ligament extends from the medial cuneiform to the base of the second metatarsal. If Lisfranc's ligament is included in the FOV, the status of the ligament should be described, as undiagnosed Lisfranc ligament tears can lead to debilitating midfoot arthrosis. (N) As the medial and lateral tendons turn from their vertical course to a horizontal course along the plantar aspect of the foot, the tendons may demonstrate a magic-angle artifact, causing the tendons to appear gray on short-TE images, mimicking tendinosis. Correlation with images using longer TE values is advised in such situations.
|
-
Tibiotalar joint
-
Posterior subtalar joint
-
Talocalcaneonavicular joint
-
Fractures occurring at the anterior process of the calcaneus
-
Fractures of the lateral process of the talus
-
Navicular fractures
-
Fractures of the metatarsals
-
Cuboid fractures (particularly stress fractures)
-
High tibiofibular ligaments (the anterior and posterior syndesmosis and the interosseous ligament)
-
Lateral ligaments (the anterior talofibular, calcaneofibular, and posterior talofibular ligaments)
-
Medial ligaments (the components of the deltoid ligament)
-
Sinus tarsi ligaments (the extensor roots, interosseous ligament, and cervical ligament)
-
The lateral tendons (the peroneus longus and brevis tendons)
-
The medial tendons (the posterior tibialis, the flexor digitorum longus, and the flexor hallucis longus tendons)
-
The anterior tendons (the tibialis anterior, the extensor hallucis longus, and the extensor digitorum longus tendons)
-
The posterior tendons (the Achilles and plantaris tendons)
 |
|
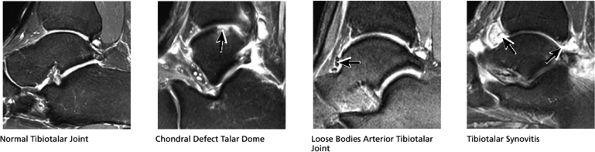
FIGURE 5.28 Tibiotalar Joint.
|
 |
|
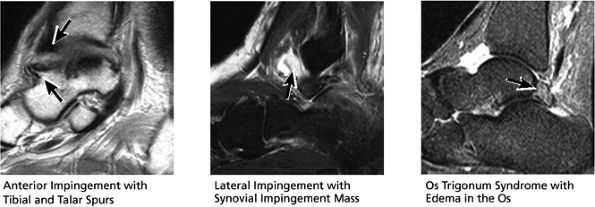
FIGURE 5.29 Impingement.
|
 |
|
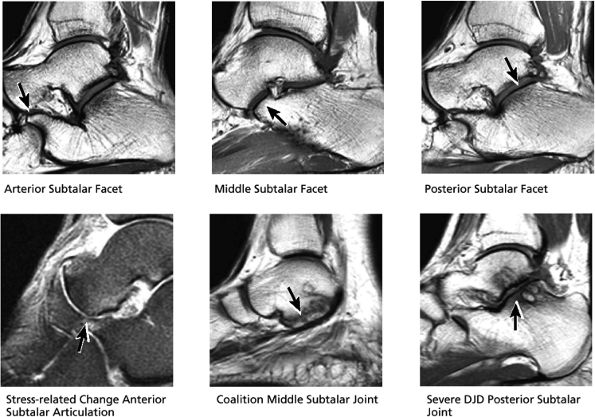
FIGURE 5.30 Subtalar Facets.
|
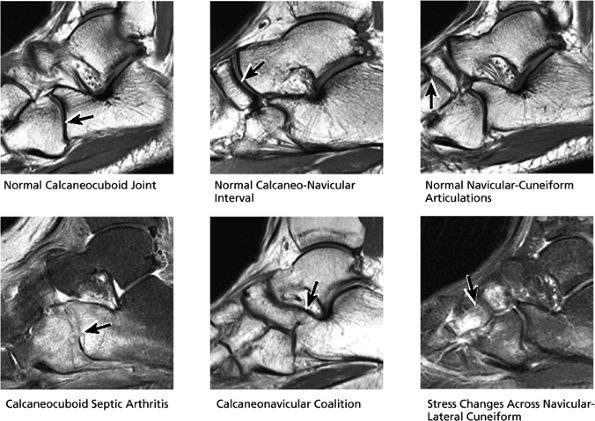 |
|
FIGURE 5.31 Tarsal Joints.
|
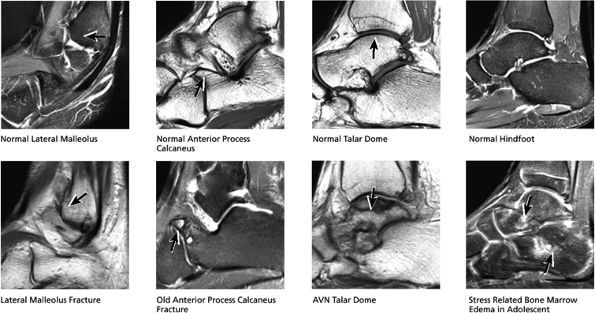 |
|
FIGURE 5.32 Hindfoot.
|
-
The ATFL is found on sagittal images one slice central to the lateral-most sagittal slice that includes the lateral malleolus. The origin of the ATFL is seen at the anterior inferior tip of the lateral malleolus. The anteromedial course of the ATFL can be followed on the next two images moving centrally, to where it inserts on the talus.
-
The posterior talofibular ligament (PTFL) is located in a similar fashion. The origin of the PTFL is at the inferior tip of the lateral malleolus, and the tendon can be followed medially to its insertion on the mid-posterior aspect of the talus. The PTFL is seen in cross-section on sagittal images and has a cord-like appearance. Posterior to the talus, this cord-like appearance should not be mistaken for a loose body in the posterior joint.
-
The calcaneofibular ligament (CFL) also originates from the inferior tip of the lateral malleolus, and courses inferomedially to its attachment on a tubercle on the lateral calcaneus. The course of the CFL is not always well visualized on sagittal images, however, because it may be obscured by overlying peroneal tendons.
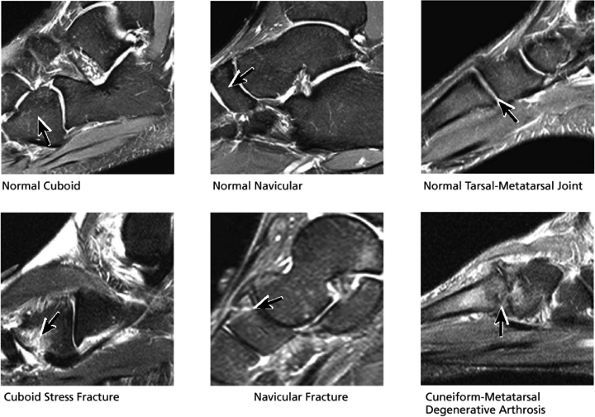 |
|
FIGURE 5.33 Midfoot Forefoot.
|
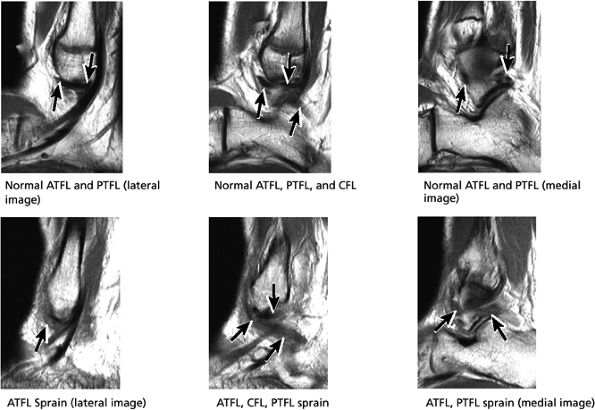 |
|
FIGURE 5.34 Lateral Ligaments.
|
 |
|
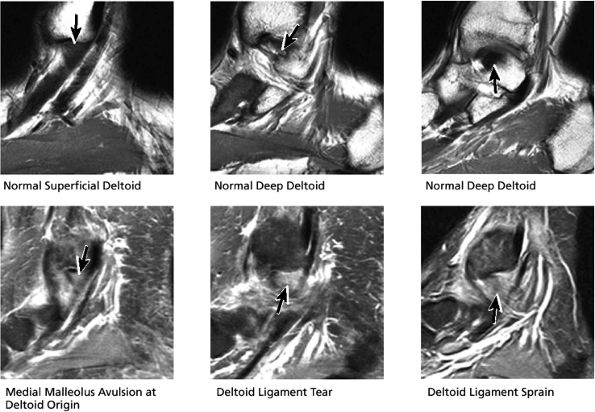
FIGURE 5.35 Deltoid Ligament.
|
 |
|
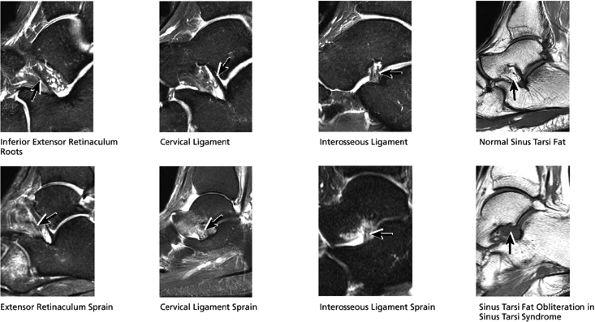
FIGURE 5.36 Sinus Tarsi Ligaments.
|
 |
|
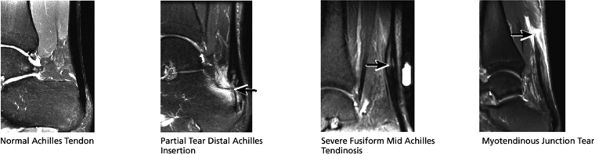
FIGURE 5.37 Achilles Tendon.
|
 |
|
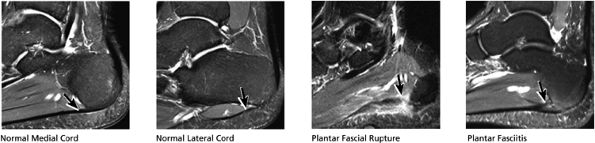
FIGURE 5.38 Plantar Fascia.
|
-
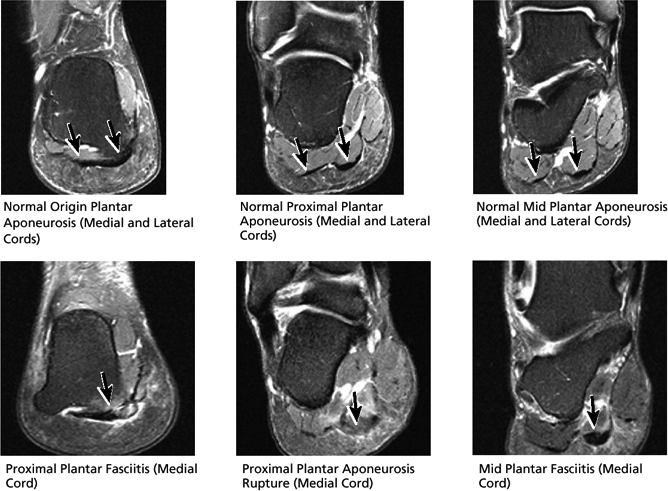
A medial segment inferior to the abductor hallucis muscle
-
A central segment, which originates from the medial process of the calcaneal tuberosity
-
A lateral segment, which originates along the lateral aspect of the calcaneal tuberosity
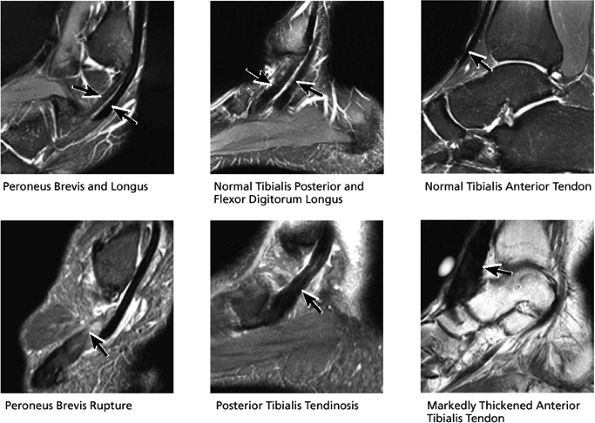 |
|
FIGURE 5.39 Tendons.
|
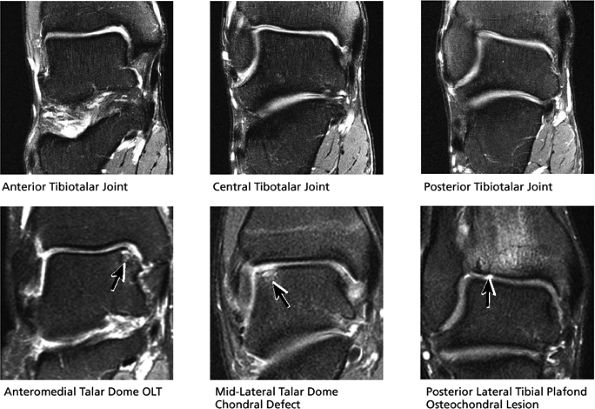 |
|
FIGURE 5.40 Tibiotalar Joint.
|
this image (or possibly one image posterior). On the next one or two posterior images the course of the CFL can be followed from the distal lateral malleolus posteroinferiorly to its insertion on the lateral calcaneus. On the three or four images anterior to the slice through the middle of the lateral malleolus, the full course of the ATFL is seen as a dark bundle of fibers moving directly anteriorly to insert on the lateral aspect of the talus. The lateral ligaments are examined in the coronal plane, and any findings are correlated with those seen in other planes.
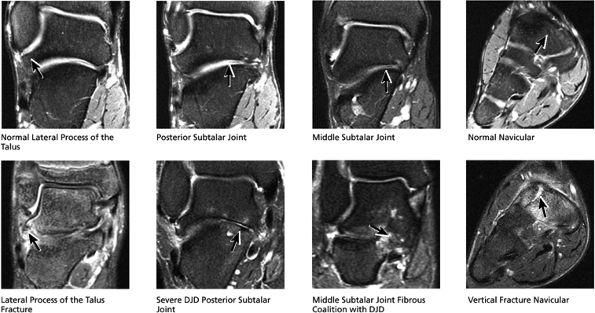 |
|
FIGURE 5.41 Subtalar Joint.
|
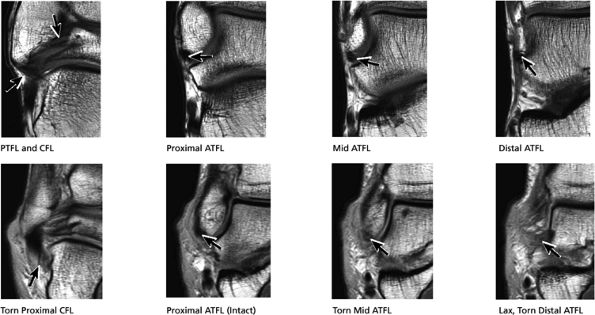 |
|
FIGURE 5.42 Lateral Ligaments.
|
 |
|
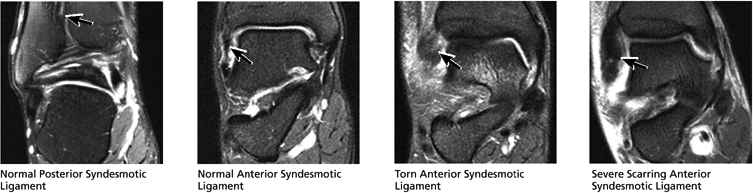
FIGURE 5.43 High Ankle Ligaments.
|
-
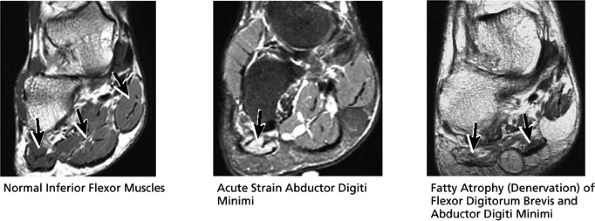
Abductor hallucis
-
Flexor digitorum brevis
-
Abductor digiti minimi
 |
|
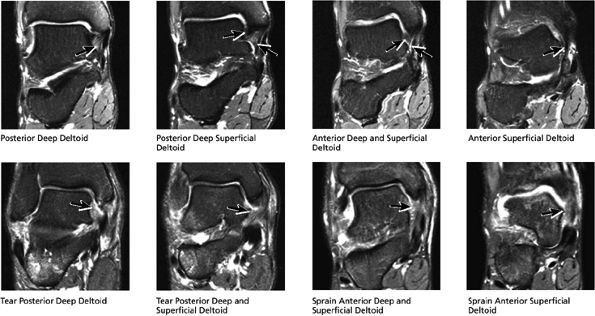
FIGURE 5.44 Deltoid Ligaments.
|
 |
|
FIGURE 5.45 Plantar Aponeurosis.
|
 |
|
FIGURE 5.46 Inferior Flexor Muscles.
|
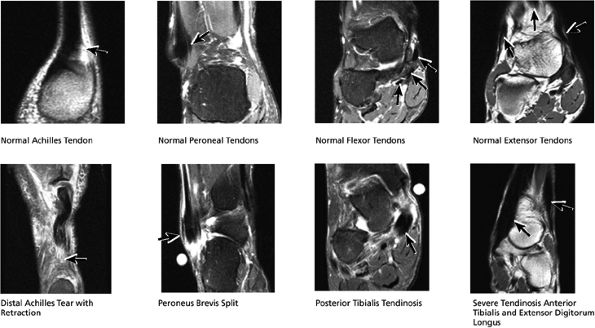 |
|
FIGURE 5.47 Coronal Tendons.
|
arthrosis at the tarsal-metatarsal joints in particular should prompt a careful search for Lisfranc fracture—dislocations in the axial plane.
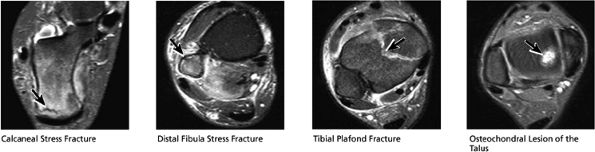 |
|
FIGURE 5.48 ● Hindfoot.
|
-
Posterior tibialis tendon (PTT), the most medial of the three tendons
-
Flexor digitorum longus (FDL), located just posterolateral to and in close apposition with PTT
-
Flexor hallucis longus (FHL), the most lateral and posterior of the three tendons
The peroneal retinaculum prevents the peroneal tendons from subluxing laterally over the lateral malleolus. Tears or stripping of the peroneal retinaculum from the lateral malleolus is inferred when subluxation or dislocation of the peroneal tendons is seen lateral to the lateral malleolus.
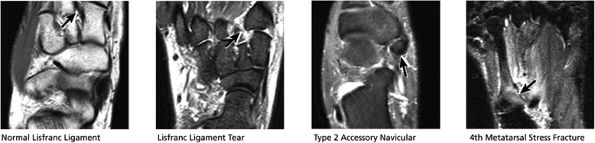 |
|
FIGURE 5.49 Midfoot-Forefoot.
|
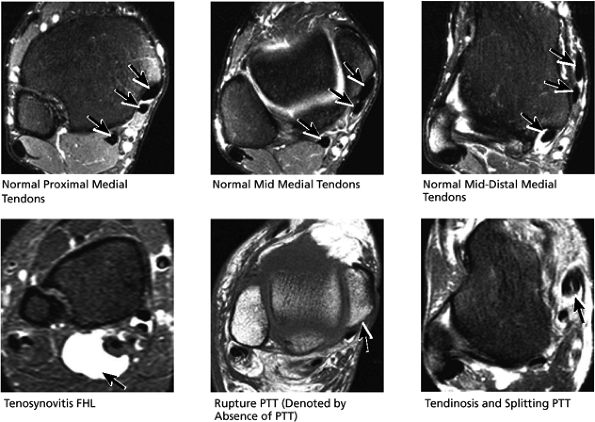 |
|
FIGURE 5.50 Medial Tendons.
|
 |
|
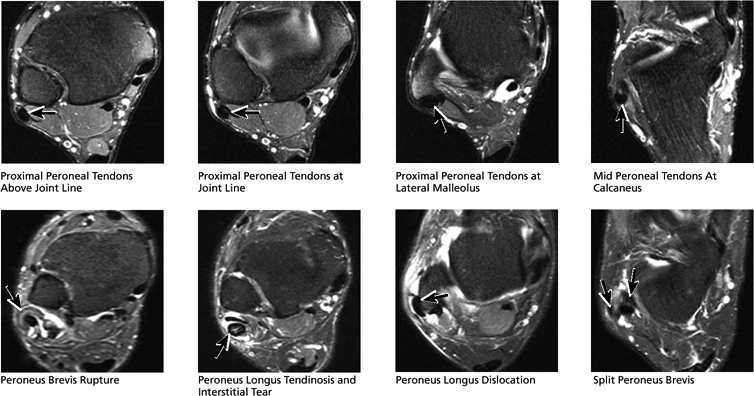
FIGURE 5.51 Lateral Tendons.
|
 |
|
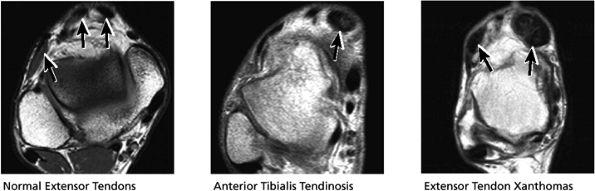
FIGURE 5.52 Anterior Tendons.
|
 |
|
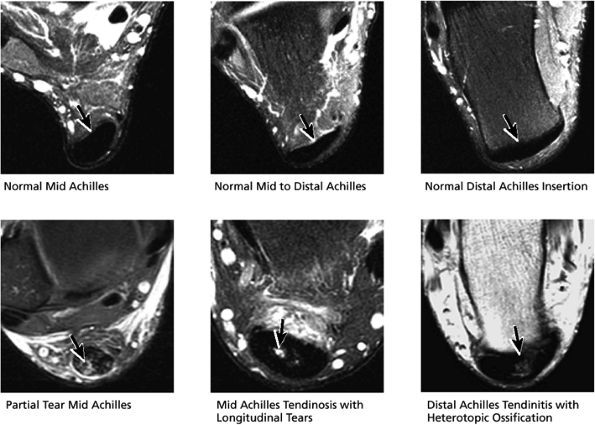
FIGURE 5.53 Posterior Tendons.
|
-
Tibialis anterior (the most likely of the extensor tendons to tear)
-
Extensor hallucis longus
-
Extensor digitorum longus
-
Peroneus tertius
associated with a convex appearance to the tendon anteriorly, as well as tendon thickening and often increased intrasubstance signal. Tears and tendinosis suspected in the sagittal plane are confirmed and further characterized in the axial plane.
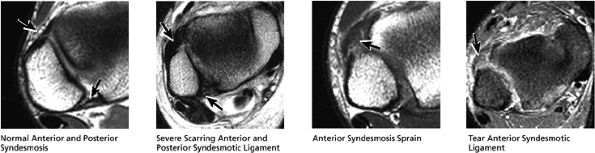 |
|
FIGURE 5.54 High Lateral Ligaments.
|
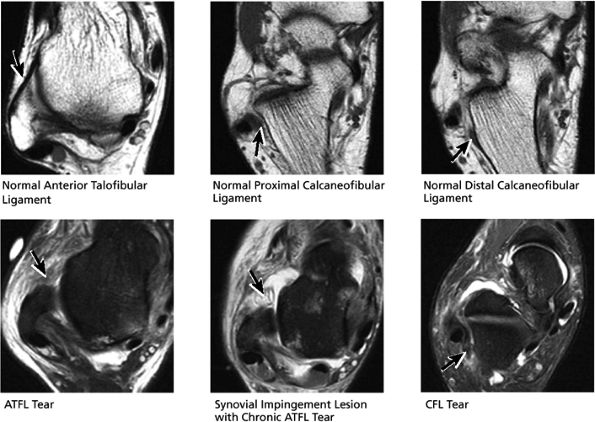 |
|
FIGURE 5.55 Lateral Ligaments.
|
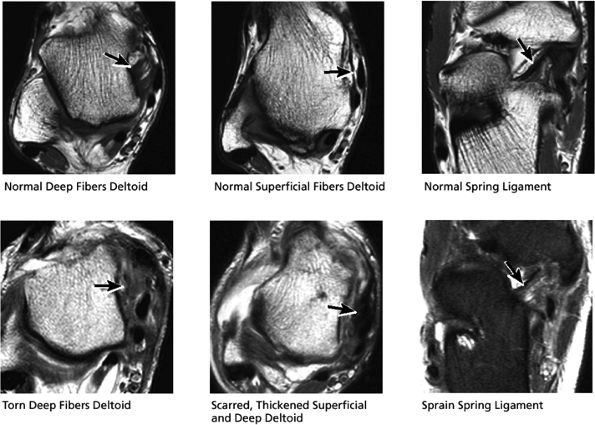 |
|
FIGURE 5.56 Medial Ligaments.
|
lateral) × 8 mm (anterior to posterior) fragment of bone within the osteochondral bed (Fig. 5.57C, D). There is bone marrow edema associated with the osteochondral lesion (Fig. 5.57E). The bone marrow edema is in a 1-cm area. There is interruption of the subchondral plate in the area of the osteochondral lesion (Fig. 5.57F).
 |
|
FIGURE 5.57 Sample Case.
|
-
Medial osteochondral talar dome lesion. There is a fragment of osseous tissue within the osteochondral bed that may be attached by synovium. The fragment measures 8 mm anterior to posterior and 3 mm medial to lateral. This correlates with a stage III osteochondral lesion. There is associated bone marrow edema of 10 mm and subchondral sclerosis. There is irregularity of the overlying subchondral plate. There are also mild cystic changes in the adjacent portion of the talus, although no fluid is directly undermining the osteochondral lesion itself.
-
Anterior osseous impingement of the ankle with spurring of the anterior aspect of the tibiotalar joint and bone marrow edema demonstrated in the anterior distal tibia
-
Chronic thickening of the anterior talofibular ligament without disruption
-
The syndesmotic ligaments consist of the anterior syndesmotic or anterior inferior tibiofibular ligament and the posterior syndesmotic or posterior inferior tibiofibular ligament, the interosseous membrane, and the transverse tibiofibular ligament.
-
The transverse tibiofibular ligament represents the posterior labrum of the ankle and projects inferior to the posterior tibial margin.
-
The deltoid ligament consists of superficial and deep layers.
-
The ATFL is taut in plantarflexion.
-
The tibial slip is the posterior intermalleolar ligament.
-
The tendons of the deep calf and the neurovascular structures of the posterior compartment pass deep to the flexor retinaculum.
-
The anterior compartment of the leg consists of the tibialis anterior, the extensor hallucis longus, the extensor digitorum longus, and the peroneus tertius muscles. The neurovascular bundle contains the deep peroneal nerve and the anterior tibial artery.
-
The posterior compartment is divided into superficial and deep sections by deep transverse fascia. The superficial posterior compartment consists of the gastrocnemius, the plantaris, and the soleus muscles. The deep posterior compartment contains the popliteus, the FDL, the FHL, and the tibialis posterior muscles. The neurovascular supply is provided by the tibial nerve and posterior tibial artery.
-
The anterolateral compartment contains the peroneus longus and peroneus brevis muscles. The neurovascular supply is from the superficial peroneal nerve and branches of the peroneal artery.
fascicle inserts onto the superior border of the calcaneona vicular ligament. The deep part of the deltoid, which is rectangular, consists of a small anterior component (the anterior tibiotalar ligament) and a strong posterior component (the posterior tibiotalar ligament) (Fig. 5.64). The posterior tibiotalar ligament represents the strongest part of the entire medial ligament complex. The deep portion of the deltoid ligament, covered by synovium, is intra-articular.
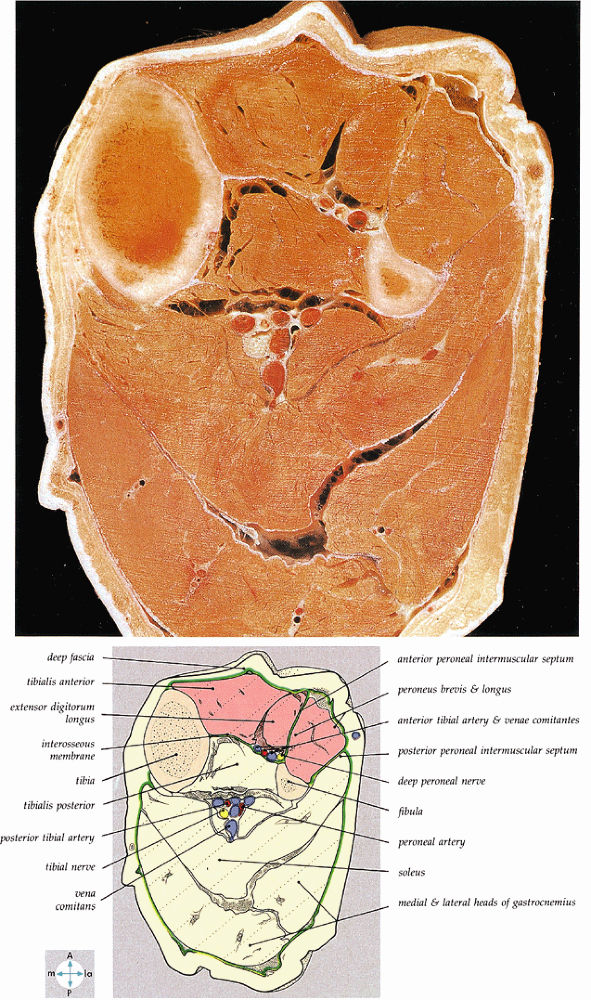 |
|
FIGURE 5.58 ● transverse section through the midcalf shows the anterior and lateral compartments and their contents.
|
 |
|
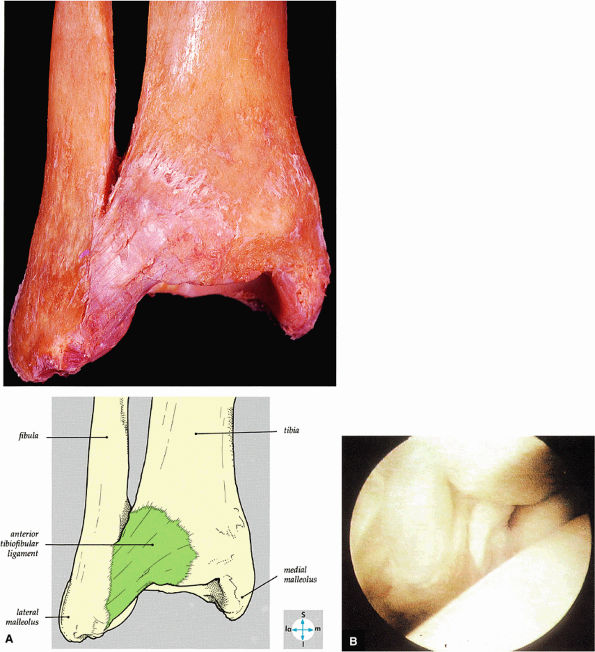
FIGURE 5.59 ● (A) The inferior tibiofibular joint is a fibrous joint. (B) Arthroscopic view of the right ankle demonstrates the syndesmotic ligament and the trifurcation. The trifurcation includes the fibula in the background with the tibia superior and the talus inferior. Approximately 20% of the ligament is intra-articular, and it runs at a 45° angle from the tibia to the fibula.
|
 |
|
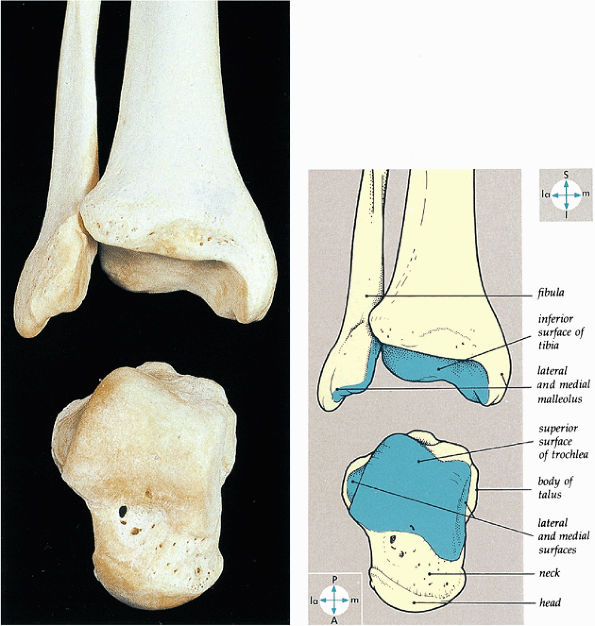
FIGURE 5.60 ● The bones of the ankle joint and their articular surfaces.
|
 |
|
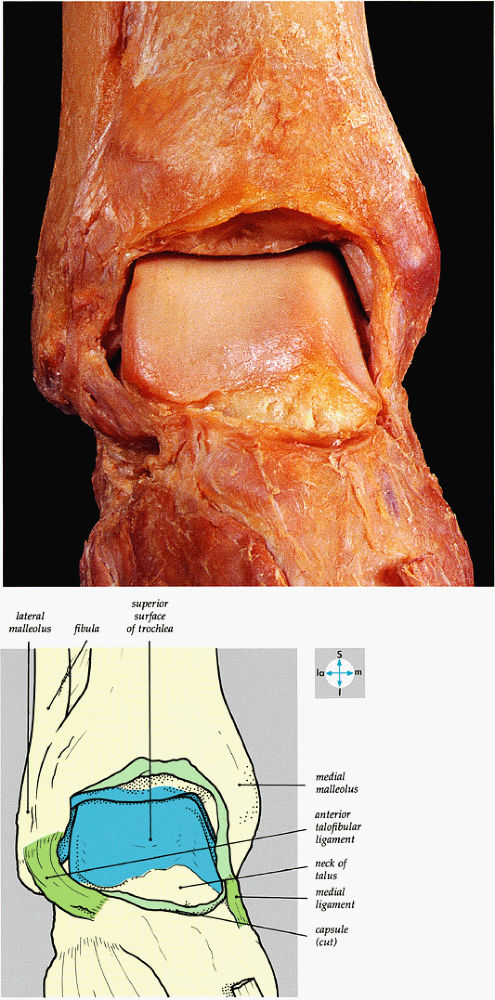
FIGURE 5.61 ● An anterior view of the ankle joint and its articular surfaces is revealed by removal of the capsule.
|
 |
|
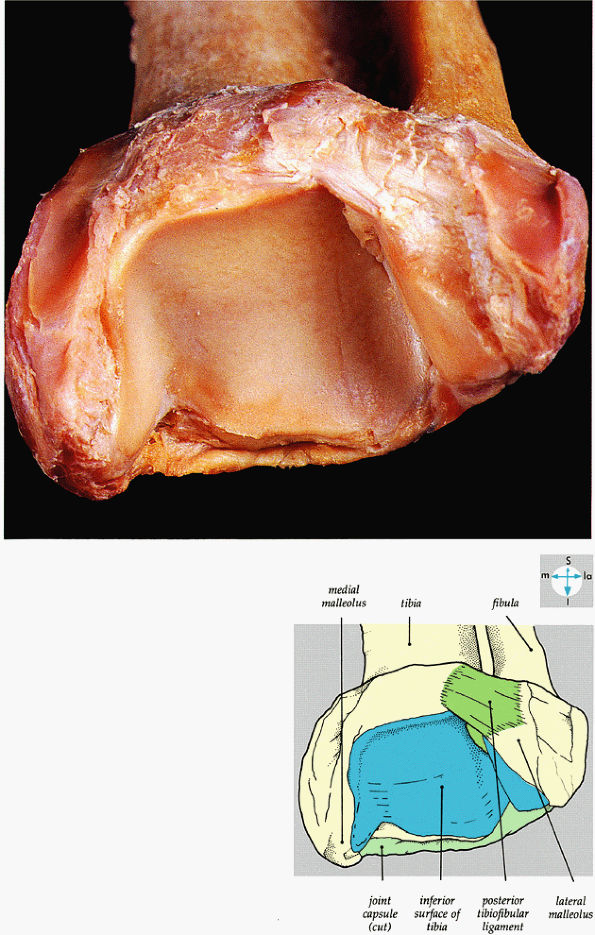
FIGURE 5.62 ● An oblique view of the wedge-shaped articular socket of the ankle joint.
|
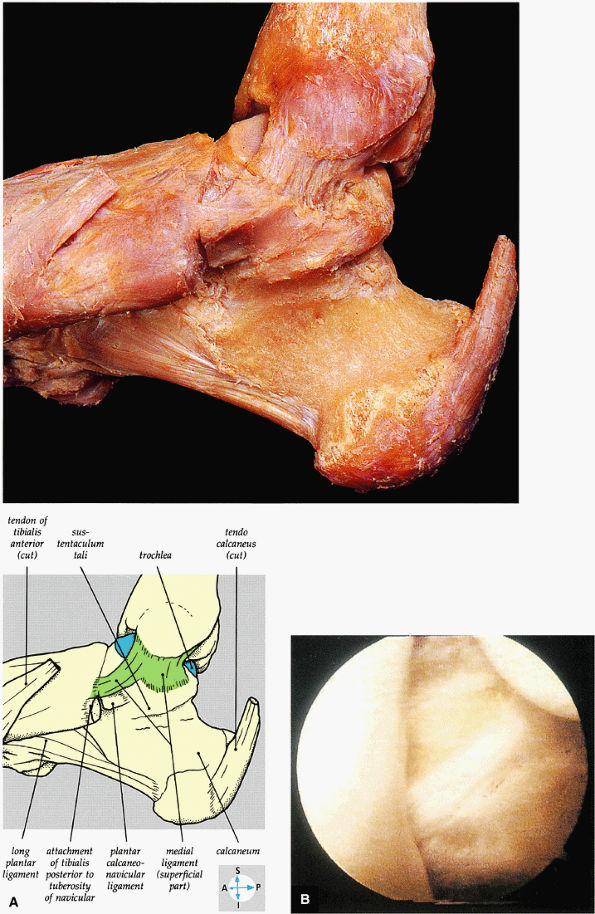 |
|
FIGURE 5.63 ● (A) The medial collateral ligament of the ankle joint can be seen after removal of the capsule. (B) In the right ankle, the deep portion of the deltoid ligament runs from the medial malleolus on the right to the talus on the left.
|
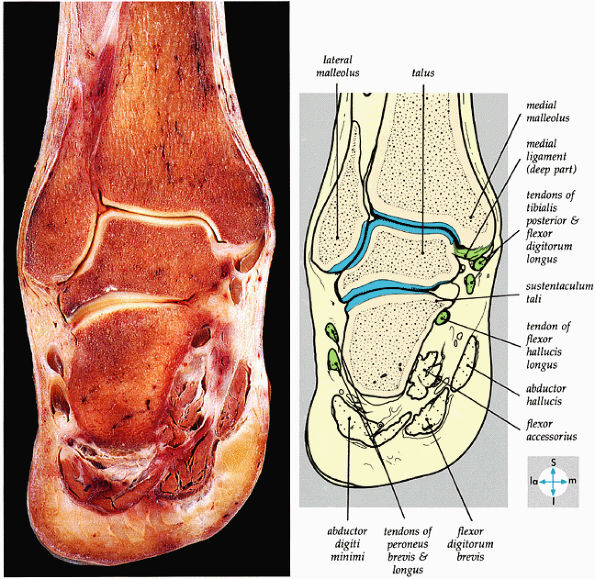 |
|
FIGURE 5.64 ● A coronal section through the ankle (tibiotalar joint) and talocalcaneal joints shows their articular surfaces.
|
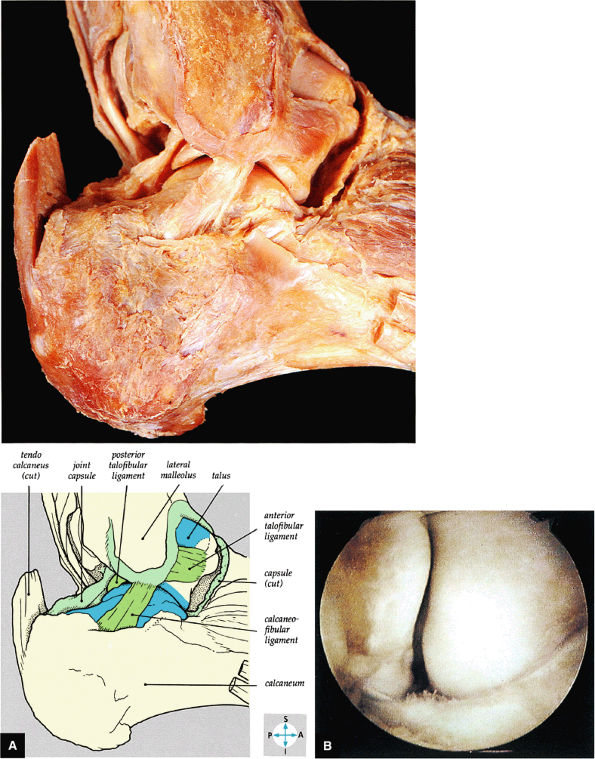 |
|
FIGURE 5.65 ● (A) The lateral collateral ligament of the ankle joint can be seen after removal of the capsule. (B) The anterior talofibular ligament is clearly demonstrated in this right ankle. The fibula is to the left, the talus to the right. It forms the floor of the lateral gutter of the ankle.
|
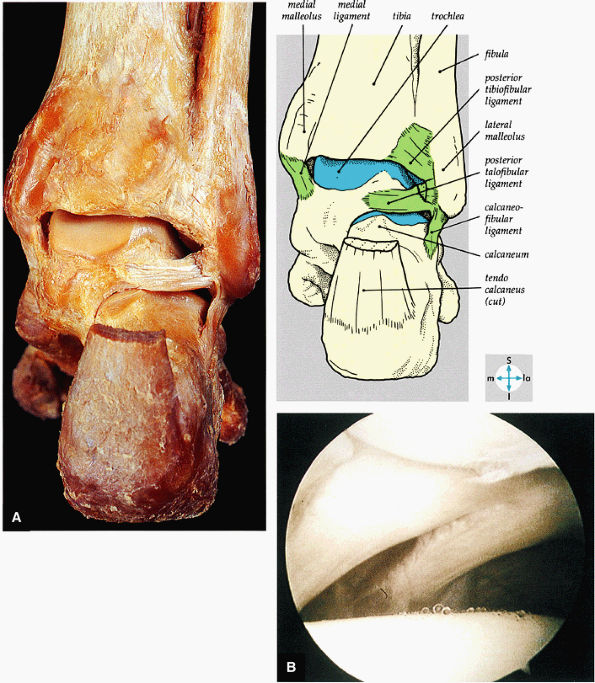 |
|
FIGURE 5.66 ● (A) A posterior view of the ankle joint shows the articular surface of the talus after removal of the capsule. (B) The posterior ankle ligaments in the right ankle. The thick structure to the left is the posterior inferior tibiofibular ligament. The structure to the right is the transverse tibiofibular ligament. In this picture, the tibia is on top and the talus is below.
|
 |
|
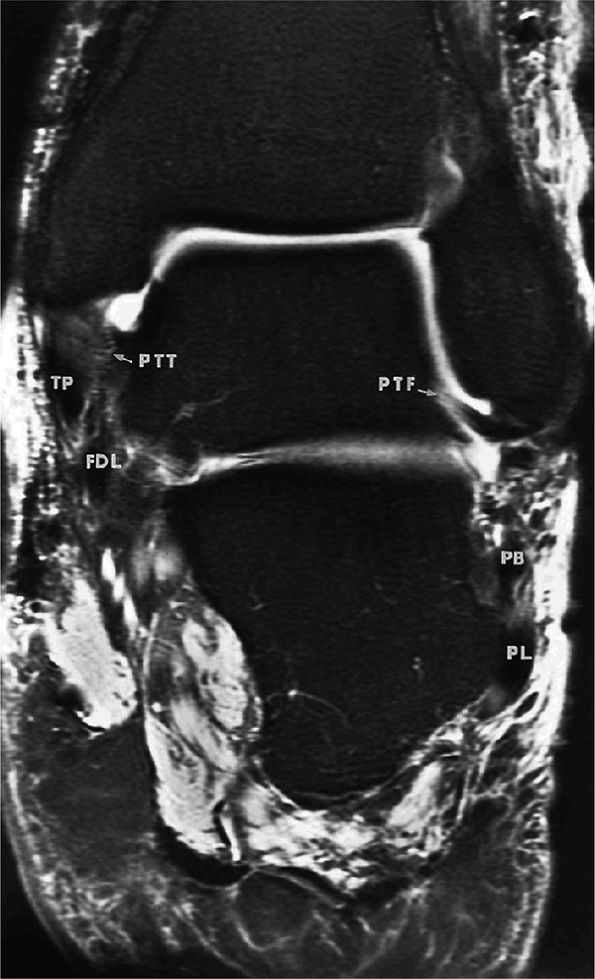
FIGURE 5.67 ● Posterior coronal FS PD FSE image at the level of the posterior talofibular ligament (PTF) and posterior tibiotalar ligament (PTT). TP, tibialis posterior; FDL, flexor digitorum longus; PB, peroneus brevis tendon; PL, peroneus longus tendon.
|
 |
|
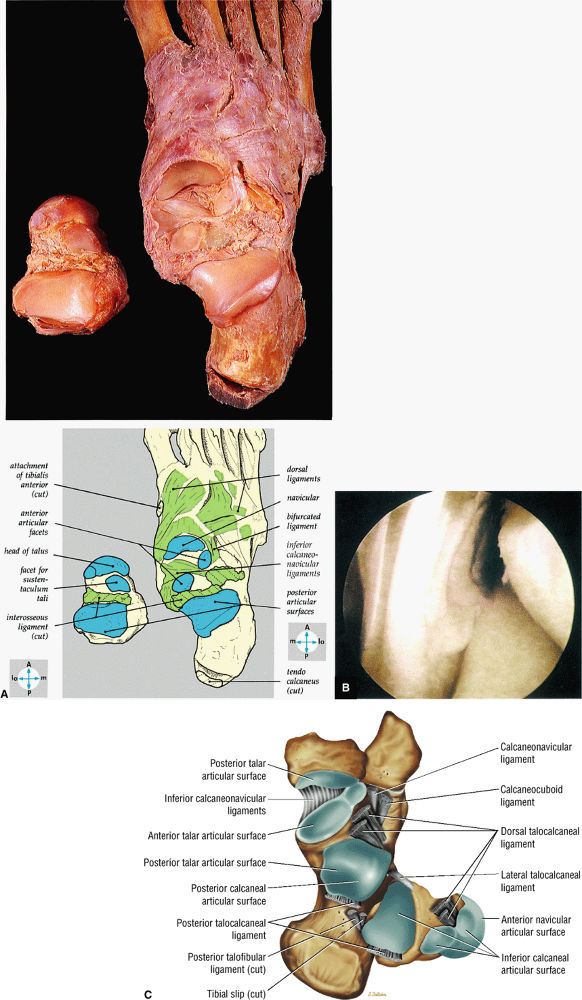
FIGURE 5.68 ● (A) In this gross photograph of the talocalcaneal and talonavicular joints, the talus has been disarticulated and turned over. (B) Arthroscopic picture of the interosseous ligament in the left ankle. The interosseous ligament is very thick and runs in an oblique vertical direction from the talus to the calcaneus. The talocalcaneal articulation is seen to the right of the ligament. (C) Talocal-caneal and talonavicular joints with the talus everted to demonstrate the talar and calcaneal articular surfaces.
|
-
The first layer consists of the abductor hallucis, the flexor digitorum brevis, and the abductor digiti minimi (Fig. 5.75).
-
The second layer consists of the quadratus plantae, the lumbricals, the flexor digitorum longus tendons, and the flexor hallucis longus tendons (Fig. 5.76).
-
The third layer includes the flexor hallucis brevis, the adductor hallucis, and the flexor digiti minimi brevis (Fig. 5.77).
-
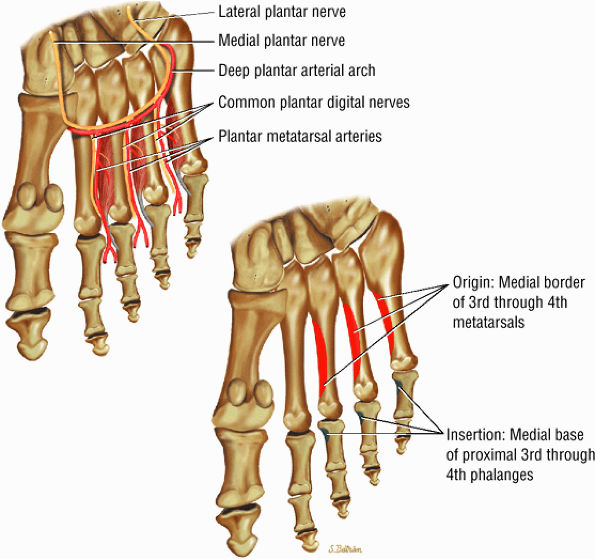
The fourth layer is made up of the interossei plantares (Fig. 5.78), the peroneus longus tendon, and the tibialis posterior tendon (Fig. 5.79).
-
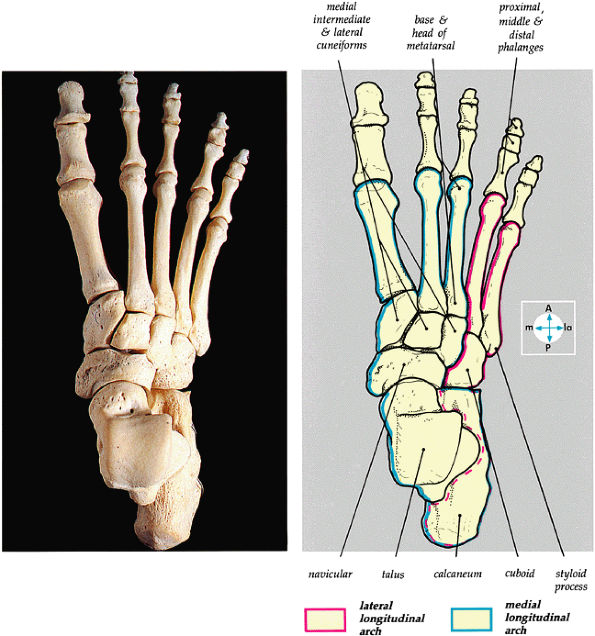
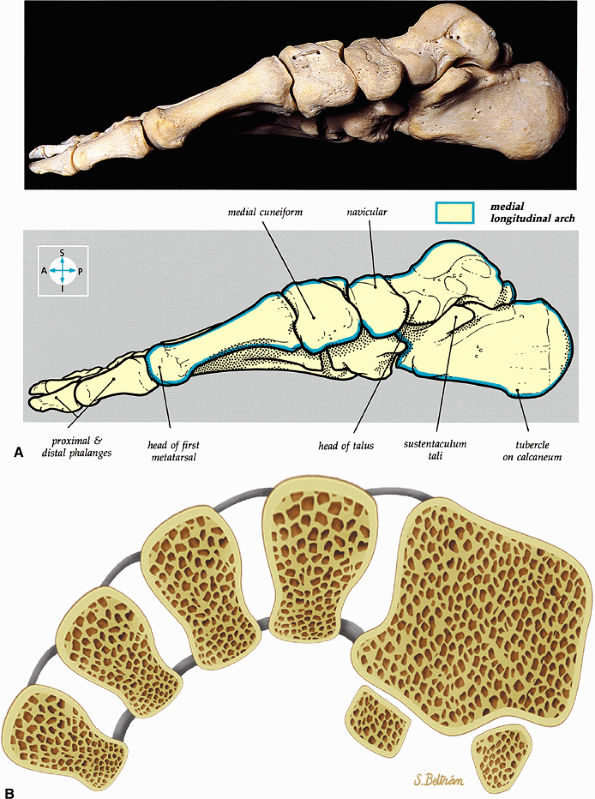
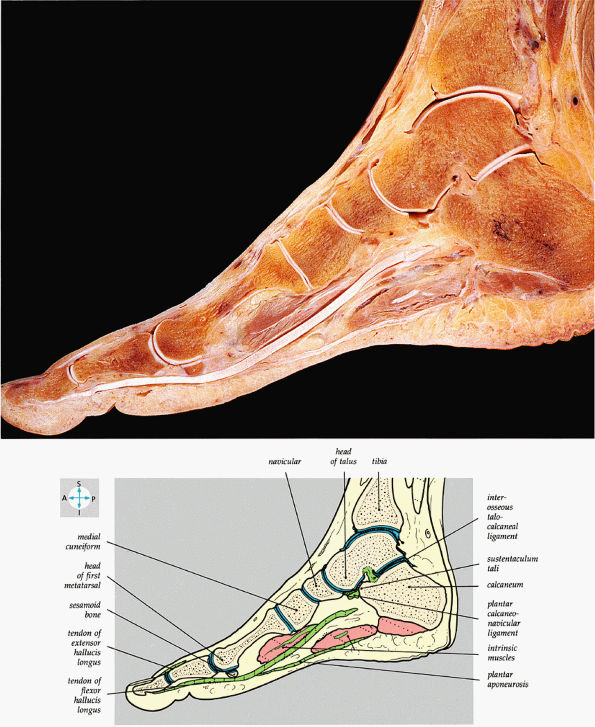
The medial and lateral longitudinal arches are formed by the tarsal and metatarsal bones (Fig. 5.80). The higher medial arch, which forms the instep of the foot, consists of the calcaneus, the talus, the navicular, the three cuneiform bones, and the medial three metatarsals (see Fig. 5.80; Fig. 5.81). The plantar calcaneonavicular (i.e., spring) ligament helps support the head of the talus, which articulates with the navicular anteriorly and the sustentaculum tali posteriorly (Fig. 5.82). The lateral arch consists of the calcaneus, the cuboid, and the lateral two metatarsals.
-
The transverse arch of the foot consists of the five metatarsal bones and the adjacent cuboid and cuneiform bones.
 |
|
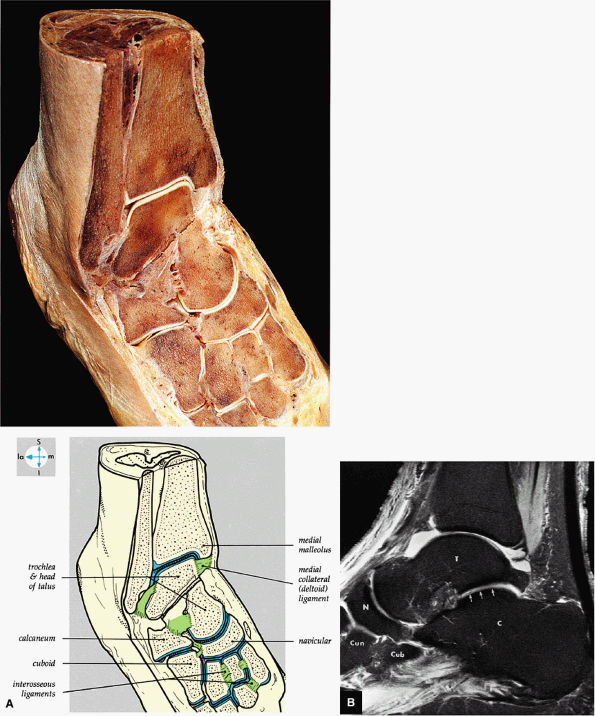
FIGURE 5.69 ● (A) Vertical and horizontal sectioning of the foot and ankle reveals the interrelationships of the tarsal joints. (B) Tibiotalar, subtalar, talonavicular, and navicular cuneiform joints are shown on a FS PD FSE sagittal image. The posterior facet of the subtalar joint is identified (arrows). T, talus; C, calcaneus; N, navicular bone; Cun, cuneiform bone; Cub, cuboid.
|
 |
|
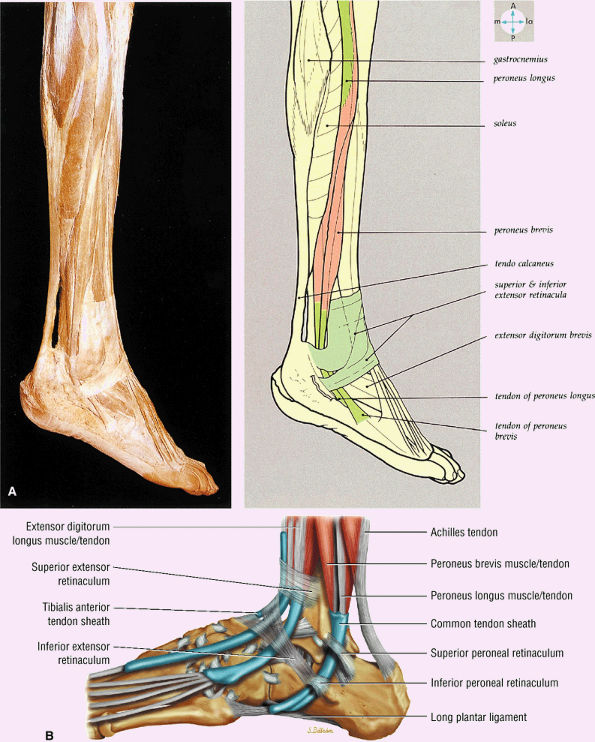
FIGURE 5.70 ● (A) The lateral aspect of the ankle and foot shows the peroneal tendons and the retinacula. (B) Lateral view of the ankle tendons and tendon sheaths.
|
 |
|
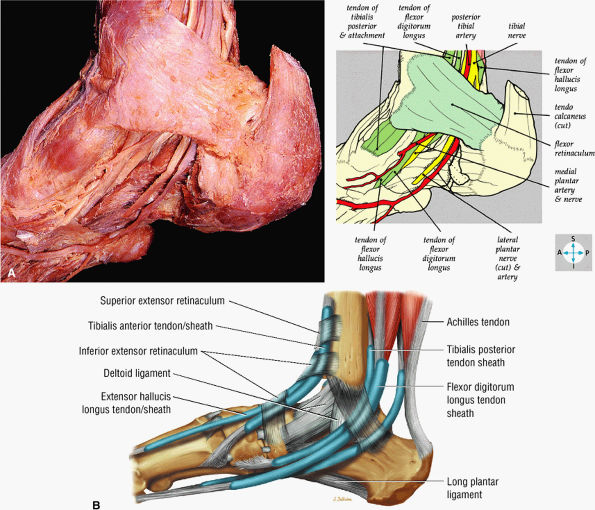
FIGURE 5.71 ● (A) The long tendons and the principal vessels and nerves from the posterior compartment of the leg pass deep to the flexor retinaculum to enter the sole of the foot. (B) A medial view of the ankle tendons and tendon sheaths.
|
 |
|
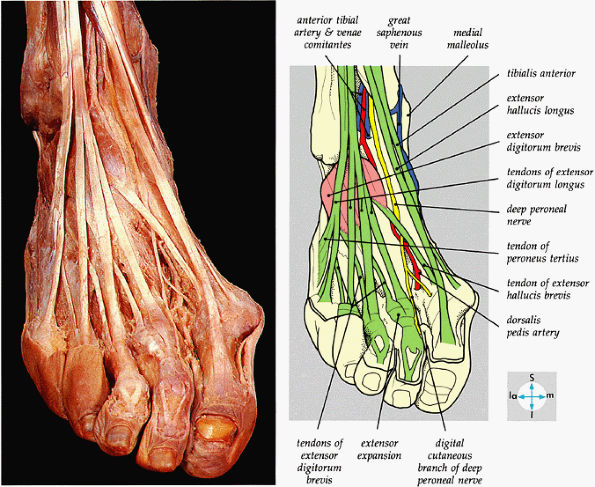
FIGURE 5.72 ● The principal structures of the dorsum of the ankle and foot can be seen after removal of the extensor retinaculum.
|
-
An accessory soleus may present as a mass in the distal calf or medial ankle.
-
The flexor digitorum accessorius is an anomalous muscle posterior to the FHL.
-
The peroneus quartus originates from the peroneus brevis muscle and inserts onto the peroneal tubercle of the calcaneus.
-
In the posterior tibiotalar joint, a low-signal-intensity cortical irregularity may mimic the appearance of osteonecrosis.
-
The posterior inferior tibiofibular ligament may be mistaken for a loose body in the posterior capsule on midsagittal images.
-
Occasionally, the intact PTFL appears as an attenuated structure with signal inhomogeneity.
-
Less frequently, fluid in the peroneal tendon sheath may be confused with a longitudinal tendon tear.
-
In one patient, axial planar images revealed marked asymmetry and hypertrophy of the peroneus brevis muscle and tendon as a normal anatomic variant.
-
An accessory soleus muscle (Fig. 5.86) is an anatomic variant that may present as a mass in the distal calf or medial ankle.17,18
-
The tensor fasciae suralis represents an anomalous muscle and tendon that contributes to the Achilles tendon and originates from the semitendinosus muscle.19 It may be mistaken for a posterior thigh, popliteal, calf, or Achilles tendon mass or lesion. MR imaging demonstrates imaging characteristics of either muscle or tendon.20
-
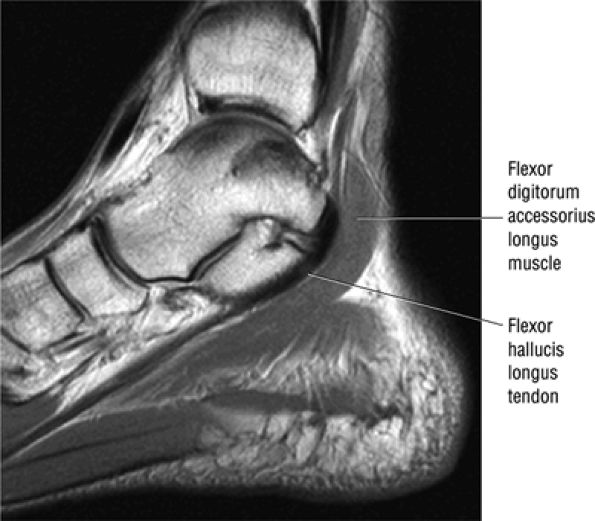
The flexor digitorum accessorius is an anomalous muscle located posterior to the FHL and has been associated with tarsal tunnel syndrome, although it is usually asymptomatic (Fig. 5.87).
-
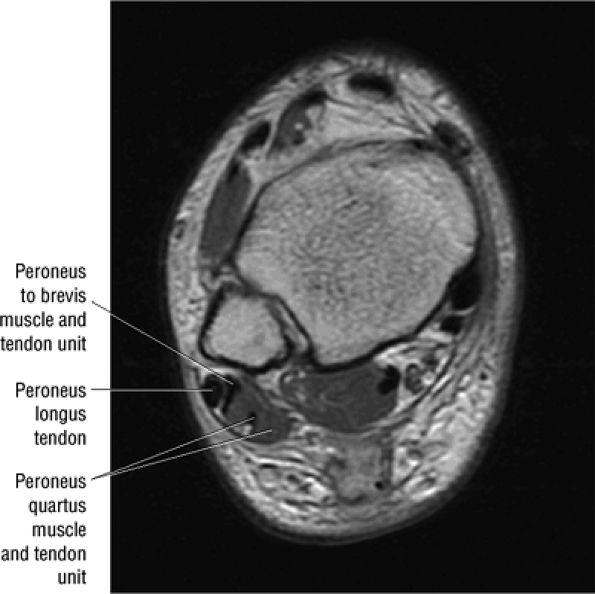
The peroneus quartus muscle is present in 13% to 22% of individuals (Fig. 5.88). This anatomic variant originates from the peroneus brevis muscle and inserts onto the peroneal tubercle of the calcaneus.21
 |
|
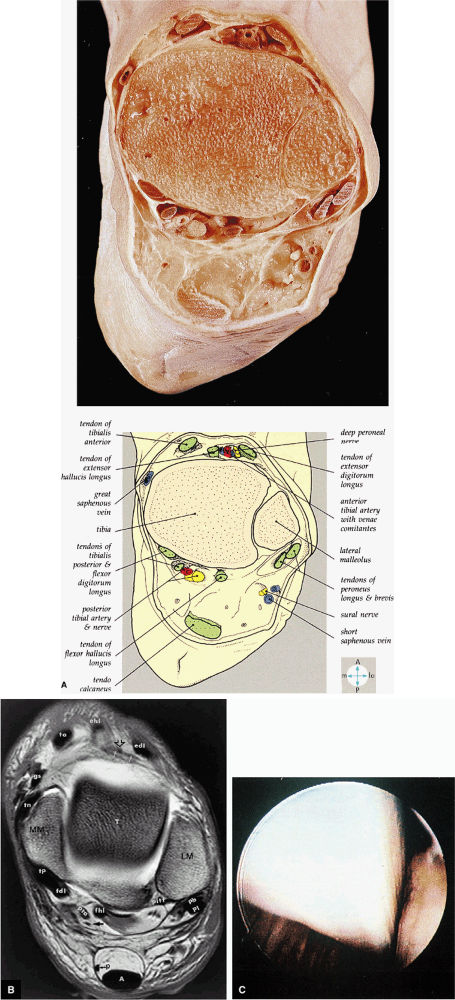
FIGURE 5.73 ● (A) A transverse section through the ankle immediately above the joint cavity shows its anterior and posterior relations. (B) T1 axial MR arthrographic image at the level of the talar dome. Note the position of the distal plantaris tendon (p) medial to the Achilles tendon. MM, medial malleolus; tp, tibialis posterior; fdl, flexor digitorum longus tendon; pta, posterior tibial artery; arrow, tibial nerve; fhl, flexor hallucis longus tendon; pitf, posterior inferior tibiofibular ligament; p, plantaris tendon; A, Achilles tendon; pb, peroneus brevis tendon; pl, peroneus longus tendon; LM, lateral malleolus; T, talus; edl, extensor digitorum longus tendon; open arrow, deep peroneal nerve; white arrows, anterior tibial artery; ehl, extensor hallucis longus tendon; ta, tibialis anterior; gs, greater saphenous vein; tn, tibionavicular ligament. (C) The flexor hallucis longus tendon is an extra-articular structure that cannot usually be seen within the ankle. It normally runs in a sheath just posterior to the ankle capsule and medial to the transverse ligament.
|
hyperintensity on T1-weighted images, secondary to fatty marrow. This finding should not be mistaken for tendon degeneration or a tear. Accessory bones, including the os tibiale externum (an accessory navicular bone medial to the navicular) and the os trigonum (located posterior to the talus and occurring approximately 10% of the time), represent commonly seen secondary ossification centers. These are normal variants that may be misinterpreted as a fracture or loose body.21
 |
|
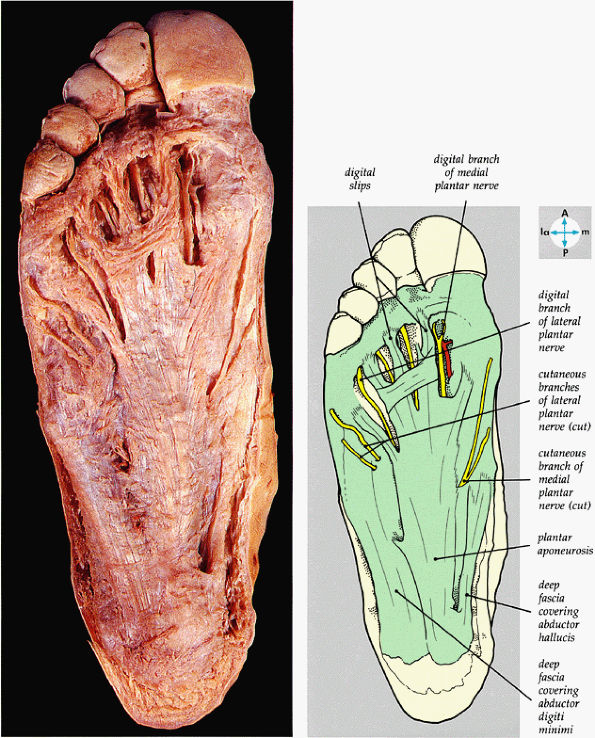
FIGURE 5.74 ● The plantar aponeurosis, deep fascia, and a cutaneous nerve are revealed by removing the skin of the sole of the foot.
|
 |
|
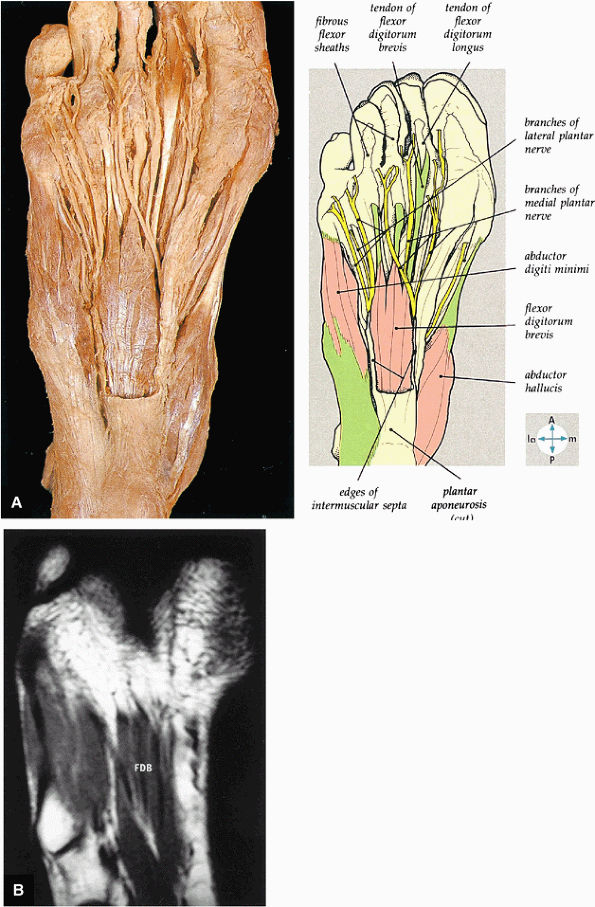
FIGURE 5.75 ● (A) The superficial intrinsic muscles and plantar nerves are shown after removal of the deep fascia, part of the plantar aponeurosis, and the second fibrous tendon sheath. In this specimen, the flexor digitorum brevis has only three tendons. (B) The flexor digitorum brevis (FDB) muscle and the tendons of the first layer of plantar muscles are shown on a T1-weighted axial image.
|
 |
|
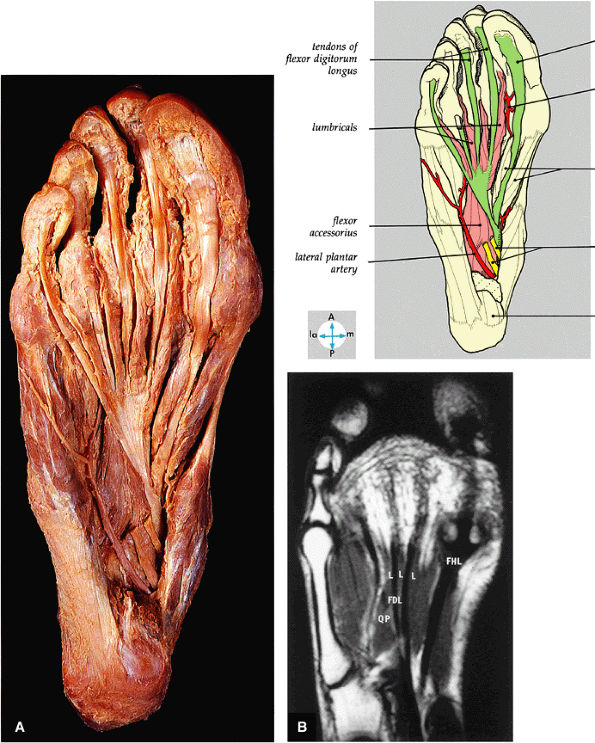
FIGURE 5.76 ● (A) The tendons of the flexor digitorum longus, the flexor hallucis longus, the flexor accessorius, and the lumbricals can be seen after removal of the medial and lateral plantar nerves and the tendons of the flexor digitorum brevis. (B) All of the second layer of plantar muscles—the quadratus plantae muscle (QP), the lumbrical muscles (L), the tendons of the flexor digitorum longus (FDL), and the tendon of the flexor hallucis longus (FHL)—are shown on a T1-weighted axial image.
|
 |
|
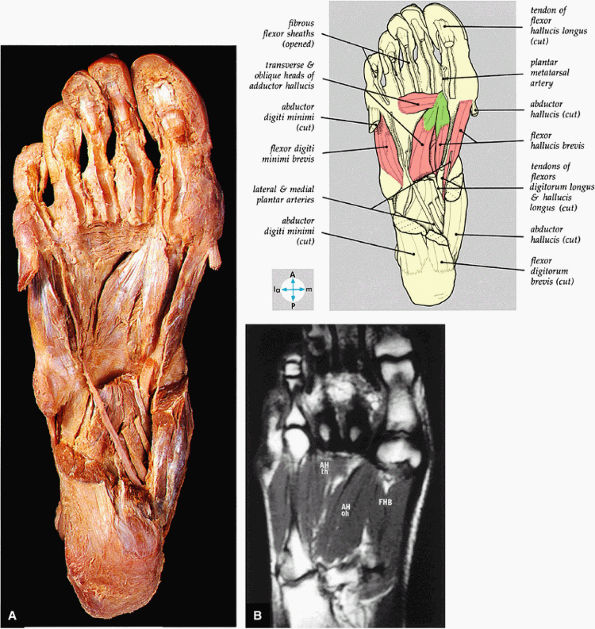
FIGURE 5.77 ● (A) The deep intrinsic muscles are revealed by removal of the long flexor tendon and abductors of the great and little toes. (B) The transverse head (th) and oblique head (oh) of the adductor hallucis muscle (AH) and the flexor hallucis brevis muscle (FHB) of the third layer of plantar muscles are shown on a T1-weighted axial image.
|
 |
|
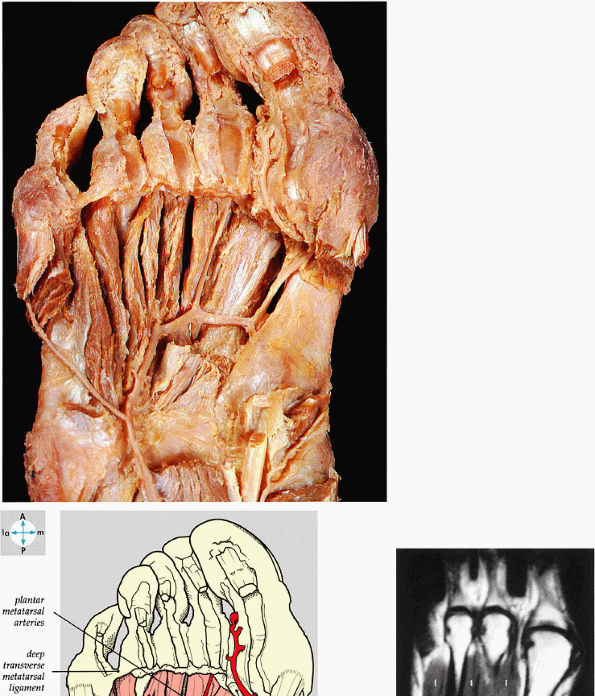
FIGURE 5.78 ● (A) The interosseous muscles and the plantar arterial arch are exposed by removal of the adductor hallucis. (B) The dorsal interosseous muscles (I) of the fourth layer of plantar muscles are shown on a T1-weighted axial image.
|
 |
|
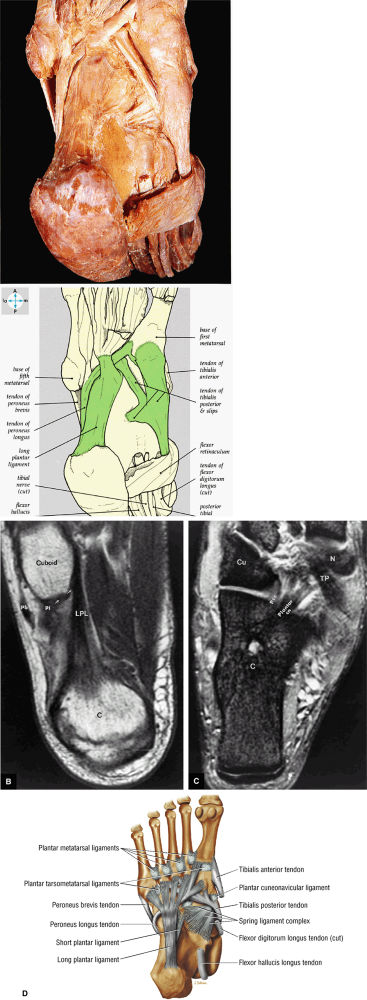
FIGURE 5.79 ● (A) The tendons of the peroneus longus and the tibialis posterior lie deep in the sole of the foot. The long plantar ligament has been preserved. (B) T1-weighted axial image showing the long plantar ligament (LPL) and the course of the peroneus longus (pl) tendon as it passes along the inferior surface of the cuboid within an osseous groove (arrow). An inferior extension of the LPL creates a tunnel for the passage of the pl tendon proximal to its insertion on the base of the first metatarsal and medial cuneiform. pb, peroneus brevis tendon; C, calcaneus. (C) T2* axial image displays the tibialis posterior (TP), the plantar calcaneonavicular ligament (Plantar cn), and the plantar calcaneocuboid ligament (Pcc). Cu, cuboid; C, calcaneus; N, navicular bone. (D) Ligaments and tendons of the plantar surface of the foot (superficial layer).
|
 |
|
FIGURE 5.80 ● The dorsal aspect of the bones of the foot shows the medial and lateral longitudinal arches.
|
 |
|
FIGURE 5.81 ● (A) The medial aspect of the bones of the foot shows the medial longitudinal arch (blue). (B) The transverse arch of the foot in coronal section at the level of the first metatarsal sesamoids.
|
 |
|
FIGURE 5.82 ● A sagittal section of the foot shows the medial longitudinal arch.
|
 |
|
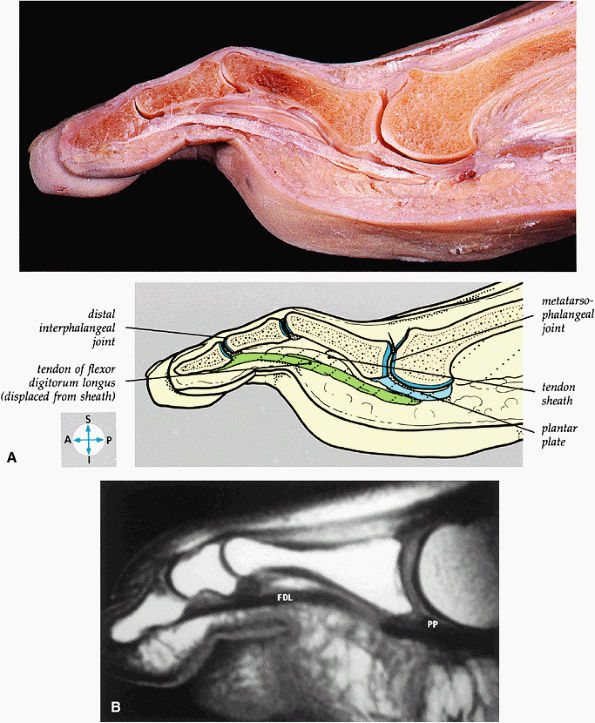
FIGURE 5.83 ● (A) A sagittal section through the third toe shows the metatarsophalangeal and interphalangeal joints. (B) A T1-weighted sagittal image demonstrates the tendon of the flexor digitorum longus (FDL) and the plantar plate (PP).
|
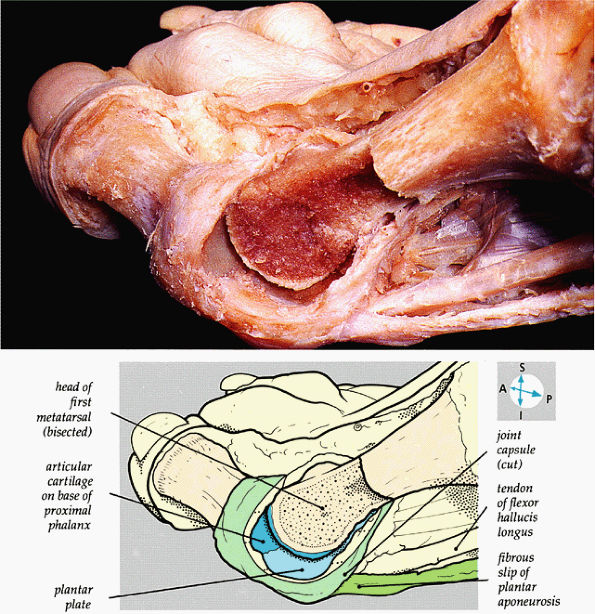 |
|
FIGURE 5.84 ● The internal features of the first metatarsophalangeal joint are revealed when part of the capsule and the distal part of the metatarsal bone are removed.
|
-
Osteochondral lesion of the talus (OLT) is the accepted terminology and should be used in place of transchondral fracture, osteochondral fracture, or osteochondritis dissecans.
-
Osteochondral lesions may involve the posteromedial or anterolateral talus.
-
An equivalent tibial lesion is also referred to as an osteochondral lesion (osteochondral lesion of the tibia).
-
It is important not to overestimate the size of the lesion by including adjacent marrow edema in the dimensions of the lesion proper.
-
Pain: persistent ankle pain after an inversion injury or chronic ankle pain and sprains. If the collateral ligament fails to rupture because articular surfaces are in direct contact, pain may be minimal.
-
Stiffness, swelling, and reduced range of motion
-
Ecchymosis
-
Catching, clicking, locking, or giving way
-
Grade A: The articular cartilage is smooth, intact, and soft.
-
Grade B: The articular cartilage has a rough surface.
-
Grade C: Fibrillations and fissures in the cartilage
-
Grade D: A flap and exposed bone
-
Grade E: A loose, undisplaced fragment
-
Grade F: The fragment is displaced.
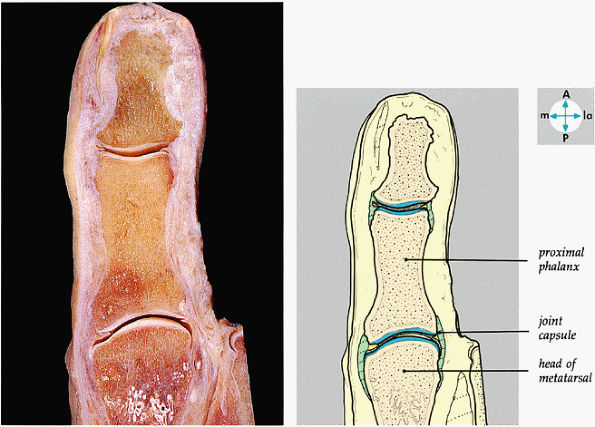 |
|
FIGURE 5.85 ● A longitudinal section through the great toe shows its joints.
|
-
Stage I: A subchondral compression fracture of the talus with no ligamentous sprain. Radiograph results are negative, and lesions may be painless.
-
Stage II: A partially detached osteochondral fragment with a hinge or flap of articular cartilage. A T2- or T2*-weighted image may be necessary to identify the osteochondral fragment.
-
Stage III: A complete nondisplaced fracture remains within the bony crater.
-
Stage IV: Detachment with a loose osteochondral fragment
-
Stage I: A cystic lesion of the talar dome with an intact roof
-
Stage IIA: A cystic lesion with communication to the talar dome surface
-
Stage IIB: An open articular surface lesion with an overlying nondisplaced fragment
-
Stage III: A nondisplaced lesion with lucency
-
Stage IV: A displaced fragment
-
Stage I: Subchondral trabecular compression. Radiograph results are negative, bone scans are posi-tive, and marrow edema is seen on MR imaging (Fig. 5.95).
-
Stage IIA: A subchondral cyst (Fig. 5.96)
-
Stage IIB: Incomplete separation of the fragment (Fig. 5.97)
-
Stage III: Fluid around a nondetached, nondisplaced fragment (Figs. 5.98 and 5.99)
-
Stage IV: A displaced fragment (Fig. 5.100)
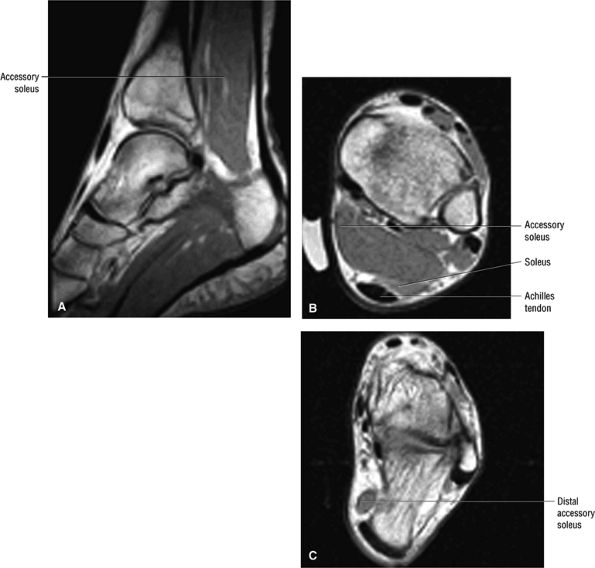 |
|
FIGURE 5.86 ● The accessory soleus muscle (A, sagittal image; B and C, axial images) originates from the anterior surface of the soleus or from the fibula and soleal line of the tibia. The variable insertion of the accessory soleus includes sites along the Achilles tendon, the superior surface of calcaneus, the muscle or fleshy insertion on the superior surface of the calcaneus, the muscular or fleshy insertion on the medial calcaneus, and the tendinous insertion on the medial calcaneus.
|
-
Grade 1: The cartilage is intact, firm, and shiny.
-
Grade 2: The articular cartilage is intact but soft.
-
Grade 3: The cartilage is frayed.
-
A detached cortical fragment that remains low in signal intensity
-
Adherent hyaline articular cartilage, reparative fibrocartilage, and associated fibrous tissue
-
A low- or intermediate-signal-intensity bony defect of the talus on T1-weighted images, depending on the degree of synovial fluid and fibrous tissue, respectively
-
Increased-signal-intensity synovial fluid contents on FS PD FSE images
-
Peripheral areas of low signal intensity within the subchondral bone on T1- and FS PD FSE images, which correlate with reactive bone sclerosis on plain radiographs
-
Abnormalities of the articular surface, including regions of cartilage thinning, bowing, nodularity, or disruption29
-
Accumulation of joint fluid at or undermining the cartilage surface, indicating small fissures or breaks
 |
|
FIGURE 5.87 ● The flexor digitorum accessorius longus muscle is located posterior to the flexor hallucis longus and courses through the tarsal tunnel with the posterior tibial neurovascular bundle.
|
 |
|
FIGURE 5.88 ● The peroneus quartus (PQ) originates from the distal third of the lower leg, including the peroneal muscles, and inserts onto the calcaneus at and proximal to the lateral malleolus. The PQ is located medial or posterior to the peroneal tendons.
|
 |
|
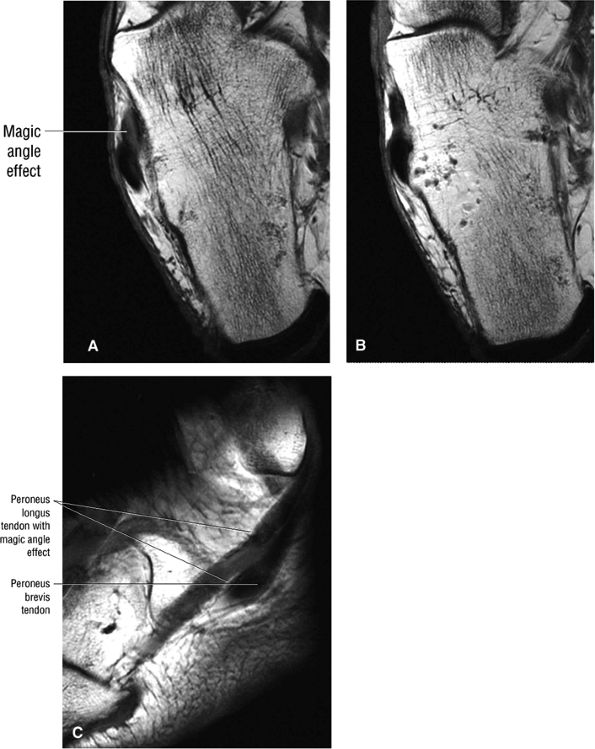
FIGURE 5.89 ● Increased signal intensity involving the peroneus longus tendon just proximal to the peroneal tubercle. This apparent signal intensity increase occurs when the orientation of the collagen fibers in the tendon approximates the magic angle of 55° with the main magnetic vector. T1, PD, and GRE sequences that use a short TE (typically <30 msec, often 10–20 msec) are susceptible to this effect. T2-weighted and FS PD FSE images with longer TE values minimize the magic-angle effect. The normal hypointense peroneus longus is seen at the level of the peroneal tubercle in B. (A, B) Axial T1-weighted images. (C) Sagittal T1-weighted image.
|
activity and limited ankle motion may be sufficient. For acute stage II lesions, casting is usually required.
 |
|
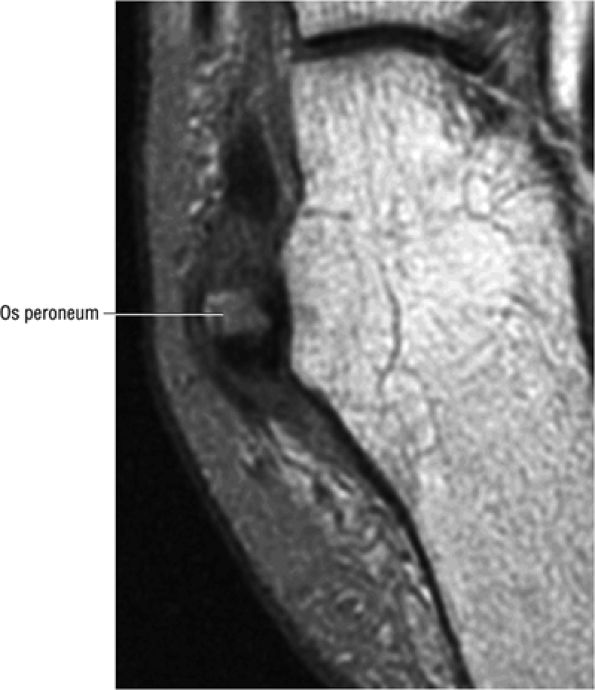
FIGURE 5.90 ● Normal marrow fat signal intensity associated with an asymptomatic os peroneum. The os peroneum is an accessory ossicle within the substance of the distal peroneus longus tendon near the cuboid.
|
-
Free fragment excision
-
Curettage
-
Drilling
-
Abrasion arthroplasty
-
Microfracture (Fig. 5.103).
to determine whether there is enough bone to permit healing, if reattachment with absorbable pins, Kirschner wires, or screws is undertaken. Primary chondral lesions without attached bone are excised, with débridement and drilling or abrading of the base. Loose fragments are either fixed with absorbable pins, Kirschner wires, or screws or are excised with drilling of the base. Displaced lesions are excised, and the base is drilled or abraded if it cannot be reattached.
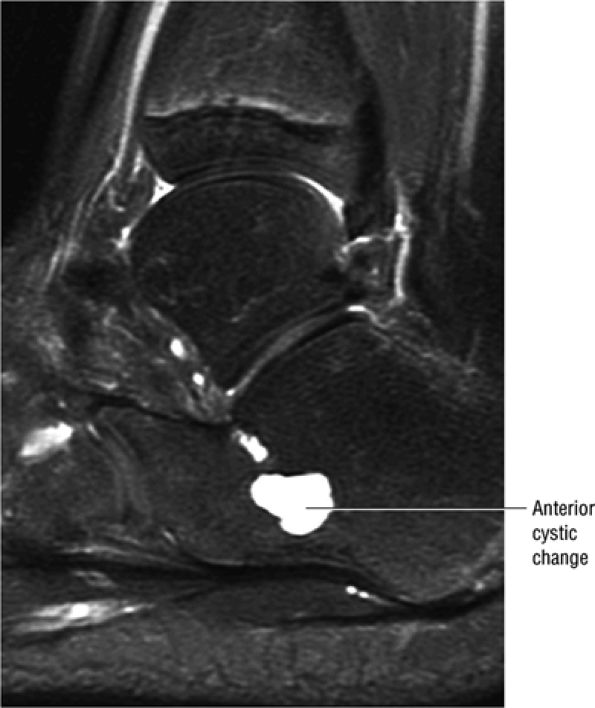 |
|
FIGURE 5.91 ● Anterior calcaneus cystic change. Prominent vascular remnants near the attachment of the cervical and interosseous ligaments are associated with hyperintensity on FS PD FSE images through the lateral aspect of the calcaneus.
|
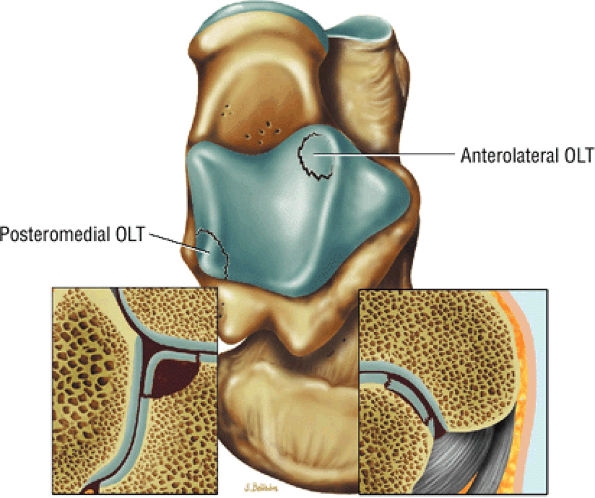 |
|
FIGURE 5.92 ● Anterolateral and posteromedial locations of osteochondral lesions of the talus. The lateral lesions tend to be shallower and wafer-shaped, whereas the medial lesions are deeper and cup-shaped.
|
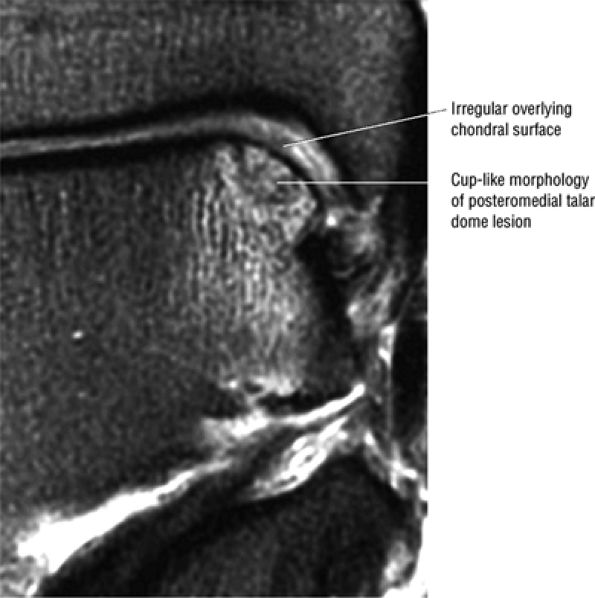 |
|
FIGURE 5.93 ● Medial cup-shaped osteochondral lesion (OLT). Medial OLTs are more common than lateral ones, but lateral lesions are associated with trauma in over 90% of cases. Medial lesions are ascribed to trauma in about 70% of cases. Coronal FS PD FSE image.
|
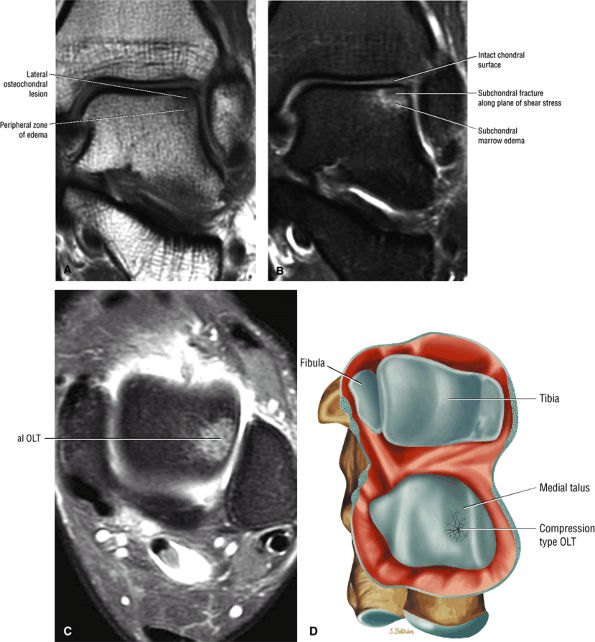 |
|
FIGURE 5.94 ● Lateral OLT with intact overlying chondral surface. Subchondral trabecular fracture and adjacent marrow edema are demonstrated on a coronal T1-weighted image (A). Mid-lateral talar dome location is shown with a cross-sectional area of reactive marrow edema. The edema associated with OLT should not be misinterpreted and result in overestimation of the area of trabecular bone involved. Coronal T1-weighted image. (B) Coronal FS PD FSE image. (C) Axial FS PD FSE image. (D) Color illustration with the capsule cut and the tibia and fibula reflected. Compression-type OLT corresponding with an area of subchondral trabecular compression in a stage I lesion.
|
 |
|
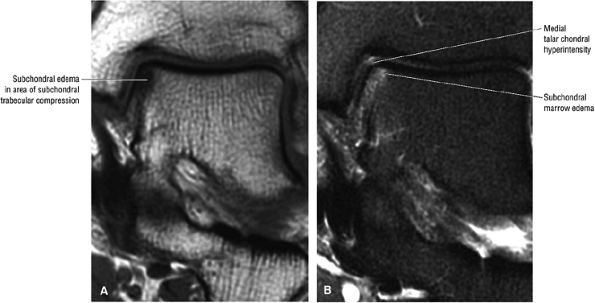
FIGURE 5.95 ● Stage I OLT with subchondral bone marrow edema, which is hypointense on the coronal T1-weighted FSE image (A) and hyperintense on the coronal FS PD FSE image (B). The overlying talar articular cartilage is hyperintense on the FS PD FSE image (B).
|
 |
|
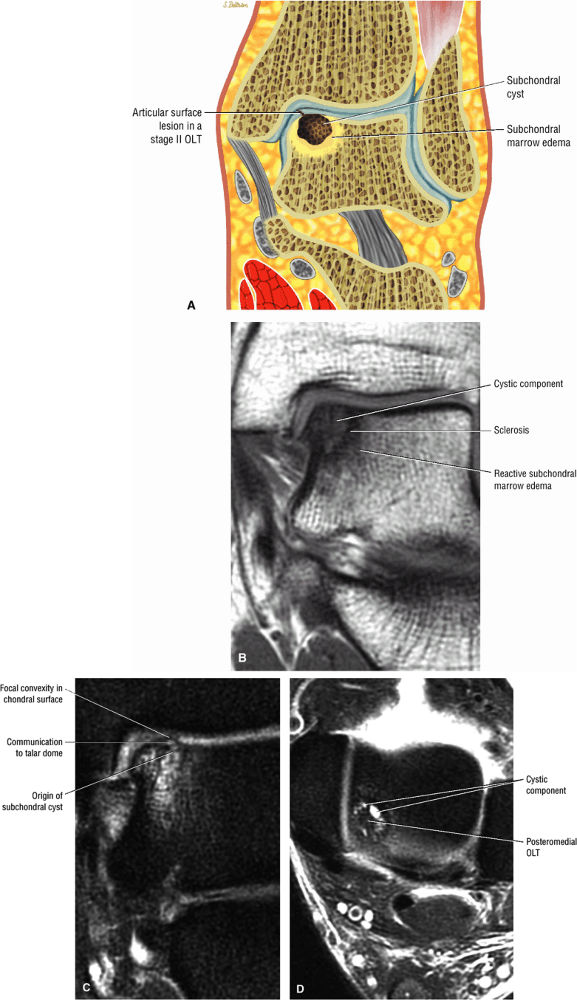
FIGURE 5.96 ● Stage II (IIA) OLT of the medial talar dome with formation of a subchondral cyst and anterior communication with an injured chondral surface. (A) Coronal color graphic of medial OLT. (B) Coronal T1-weighted image. (C) Coronal FS PD FSE image. (D) Axial FS PD FSE image.
|
-
Tendinitis or tendinopathy represents intrasubstance tendon degeneration.
-
Achilles tendinitis is subdivided into non-insertional tendinosis and insertional tendinitis.
-
The Achilles tendon has no synovial sheath but is associated with a paratenon or connective tissue envelope.
-
MR identifies nodular or convex tendon thickening and intratendinous mucoid degeneration.
-
Haglund's deformity represents insertional tendinitis with a posterosuperior calcaneal bony prominence and retrocalcaneal tendo Achilles bursitis.
-
In tendinosis or tendinopathy, there is intrinsic or intrasubstance degeneration of the Achilles tendon.
-
Tendinitis represents the clinical symptoms that develop in association with the degenerative process of tendinosis.
-
In paratendinitis (also known as peritendinitis, since it refers to the peritendinous tissues), there is generalized inflammation of the tissues surrounding the Achilles tendon (pre-Achilles fat).32
-
In paratendinitis (peritendinitis) (Fig. 5.106) with tendinosis, there is inflammation of the surrounding tissues with associated tendon degeneration.
-
The paratenon (also referred to as the peritenon) represents the connective tissue envelope surrounding the Achilles tendon.
-
Paratenonitis is an inflammation of the Achilles tendon connective tissue envelope (usually limited to the posterior paratenon on MR images).
-
An irregular pre-Achilles fat pad may be seen with paratendinitis (peritendinitis) with or without abnormal Achilles tendon morphology.
also be at risk. Although it may occur in both men and women, pronounced lesions are more often seen in males. It affects 11% of runners and 9% of dancers. Overuse of the calf muscles is a primary cause, and several mechanisms contribute to overuse injuries, including:
 |
|
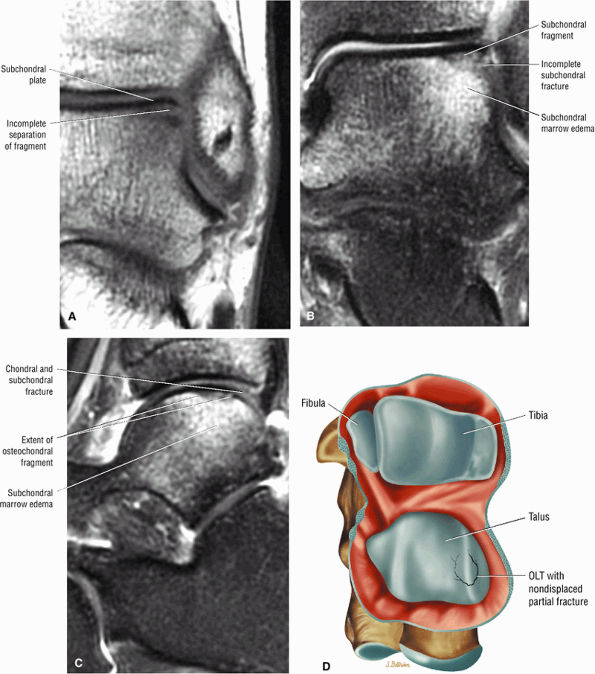
FIGURE 5.97 ● Stage II (IIB) OLT with a shallow and wafer-shaped fragment and incomplete separation from the lateral talar dome. (A) Coronal T1-weighted image. (B) Coronal FS PD FSE image. (C) Sagittal FS PD FSE image. (D) A nondisplaced partial fracture shown in a color graphic corresponding to either a communication with the talar dome or an open articular surface lesion with incomplete separation of the fragment. Subchondral cystic lesions are associated with extension of a fracture to the talar chondral surface.
|
 |
|
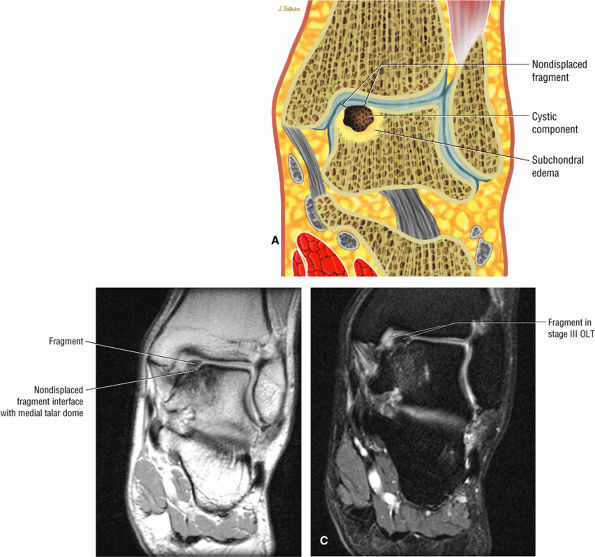
FIGURE 5.98 ● Stage III OLT with fragment adherent to granulation tissue but separated from the overlying chondral surface, (A) Coronal section color graphic. (B) Coronal T1-weighted image. (C) Coronal FS PD FSE image.
|
-
Eccentric loading of a fatigued muscle—tendon unit, seen in overtraining. Runners are susceptible in both acceleration (sprinting) and deceleration (eccentric contraction).
-
Hyperpronation, in which the leg and foot generate opposing forces of rotation: the subtalar joint pronates, the calcaneus everts, and the knee extends.
-
Forefoot varus, particularly in cavus foot
-
Equinus deformity, including triceps surae contracture
-
Insertional changes, such as a calcaneus bony prominence
-
Hypertrophic spurring or enthesophytes
-
Systemic arthropathies, such as HLA-B27 antigen-associated arthritides or rheumatoid arthritis
or nodules may also be identified. New collagen formation and associated fibrillation, nodularity, degeneration, and discoloration contribute to constriction of the peritenon.
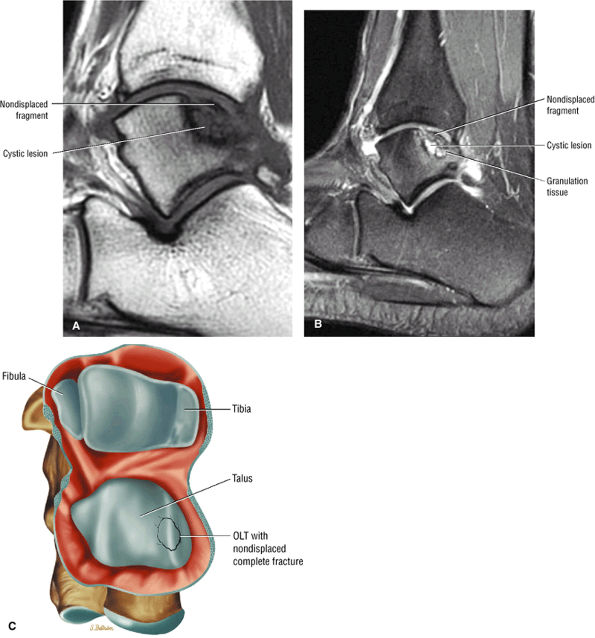 |
|
FIGURE 5.99 ● Nondisplaced fragment in a stage III OLT with intermediate-signal-intensity granulation tissue at the fragment—talus interface. (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image. (C) Color graphic with superior view of talus.
|
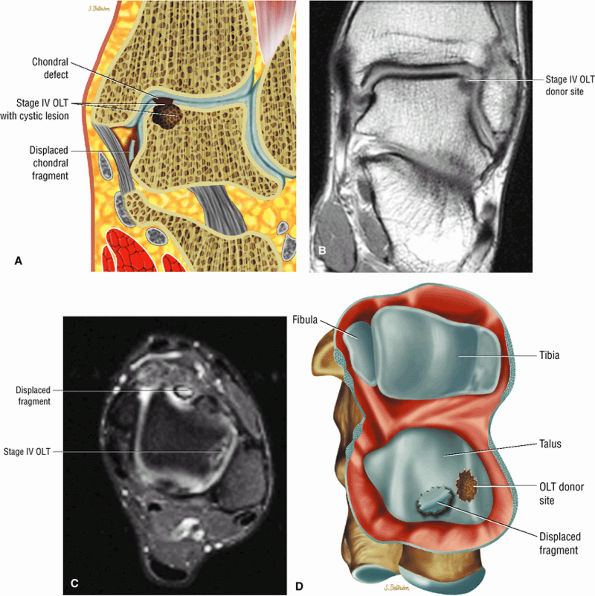 |
|
FIGURE 5.100 ● (A) Stage IV OLT with interruption of the subchondral plate, subchondral cystic change, and medially displaced chondral fragment on a coronal section color illustration. (B, C) Stage IV OLT with the displaced fragment located in the anterior tibiotalar joint capsule. The lateral OLT is the donor site. Arthroscopy is performed on all stage III and IV lesions and stage I and II lesions that fail conservative treatment. (B) Coronal T1-weighted image. (C) Axial FS PD FSE image. (D) Displaced fragment from a medial talar dome donor site in OLT on a color graphic superior view of the talus with the tibia and fibula resected.
|
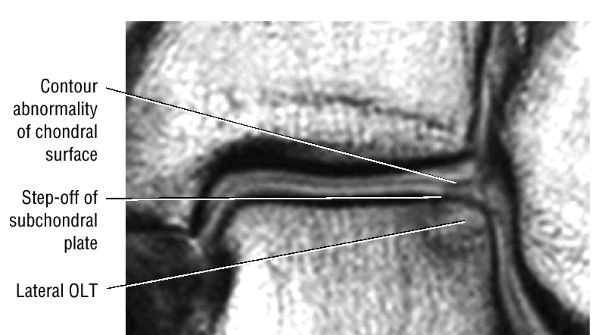 |
|
FIGURE 5.101 ● Contour irregularity of the chondral and subchondral plate and fracture extension to the talar surface in a stage II lesion with incomplete separation of the involved fragment. (A) Coronal T1-weighted image. (B) Coronal FS PD FSE image.
|
-
Increased cross-sectional diameter on axial images
-
Increased anteroposterior dimensions
-
Prominent anterior convexity with focal or diffuse thickening in the sagittal plane
-
Thickening and intermediate signal of peritendinous tissue dorsal, medial, and lateral to the Achilles tendon on T1- or PD-weighted images
-
Intermediate-signal-intensity effacement of peritendinous tissue anterior to the Achilles tendon on T1- or PD-weighted images
-
Hypointense to intermediate signal within an enlarged tendon in hypoxic fibromatosis (Fig. 5.111) on FS PD FSE images
-
Inflammatory fluid anterior to the tendon and proximal extension of fluid in the retrocalcaneal bursa
-
Myxoid degeneration (Fig. 5.112) with increased signal on FS PD FSE or STIR images
-
Calcific (Fig. 5.113) or ossific degeneration with tendon thickening
-
Associated partial tears
-
Enthesopathic insertional tendinitis (Fig. 5.114)
-
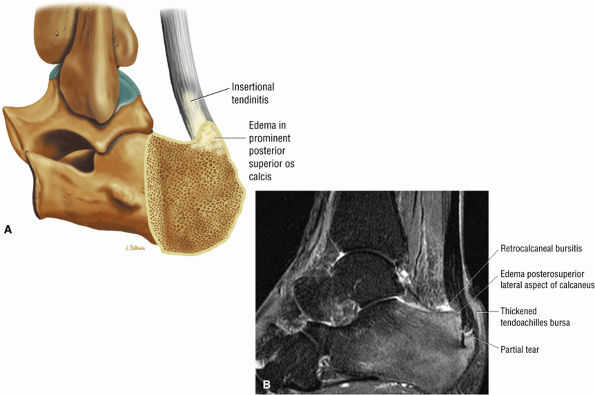
Haglund's deformity (Figs. 5.115, 5.116, and 5.117) (insertional tendinitis with reactive calcaneal marrow edema [see Fig. 5.116], and the constellation of thickened tendon, retrocalcaneal/tendo Achilles bursitis, and a calcaneal bony prominence)
-
Effacement and edema of the pre-Achilles fat body with normal tendon morphology and/or signal
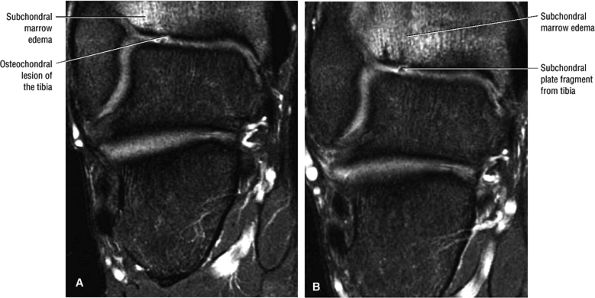 |
|
FIGURE 5.102 ● Coronal FS PD FSE images of an osteochondral lesion of the tibia (A). The subchondral plate fragment is defind on a 2-month follow-up study (B).
|
 |
|
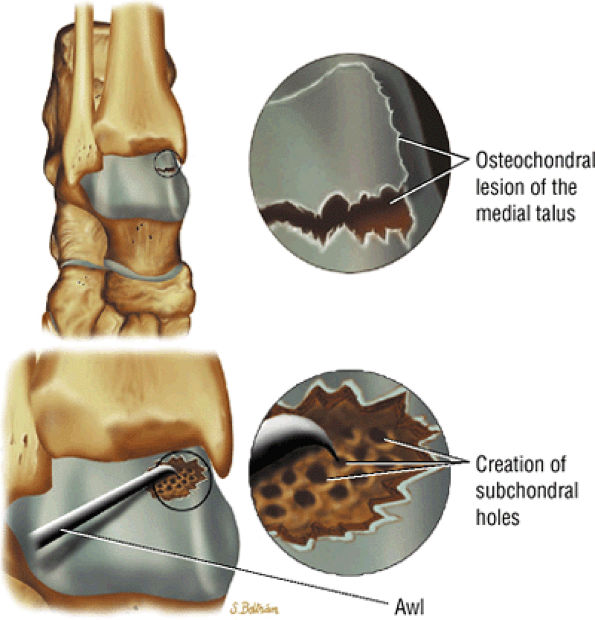
FIGURE 5.103 ● Microfracture treatment of a medial talar lesion. The microfracture awl is typically introduced through the contralateral arthroscopic portal. The awl is used to create subchondral holes in the bed of the lesion.
|
thickening without associated increased signal intensity. It may be difficult to distinguish areas of chronic tendinitis from intrasubstance tendon tears without visualizing either discontinuity in tendon fibers or discrete hyperintense signal intensity on T2-weighted or STIR images with partial tears.
 |
|
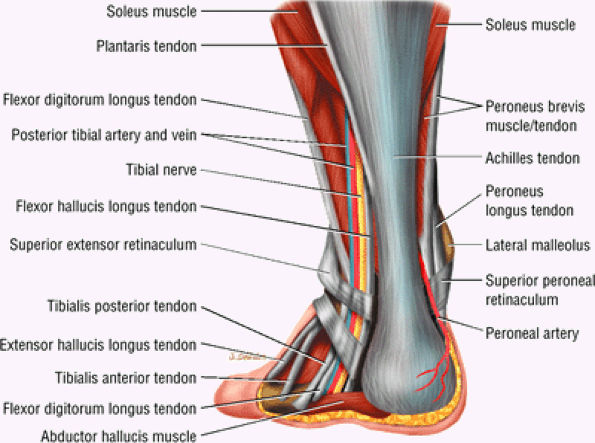
FIGURE 5.104 ● Posterior view of the hindfoot demonstrating the relation of the Achilles tendon to the posterior neurovascular bundle. The musculotendinous junction of the triceps surae (the medial and lateral heads of the gastrocnemius and soleus muscles) marks the superior extent of the Achilles tendon.
|
 |
|
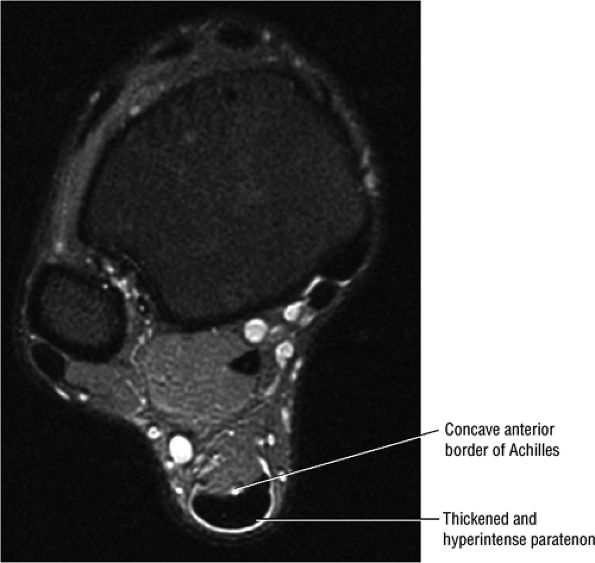
FIGURE 5.105 ● There is no true synovial sheath around the Achilles tendon. It is covered by the paratenon alone. The normal paratenon is not hyperintense on FS PD FSE images. Axial FS PD FSE image.
|
 |
|
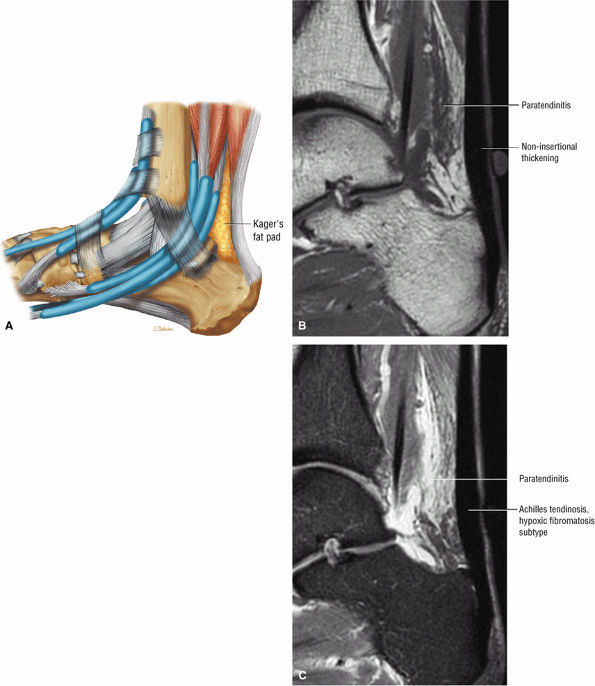
FIGURE 5.106 ● (A) Lateral color graphic of the normal anatomy of Kager's fat pad. Fat deposition deep to the Achilles tendon separates it from the deep compartment of the leg. Paratendinitis (also referred to as peritendinitis) demonstrates hypointensity and effacement of pre-Achilles fat on a sagittal T1-weighted image (B) and hyperintensity of the anterior soft tissue on a sagittal FS PD FSE image (C).
|
 |
|
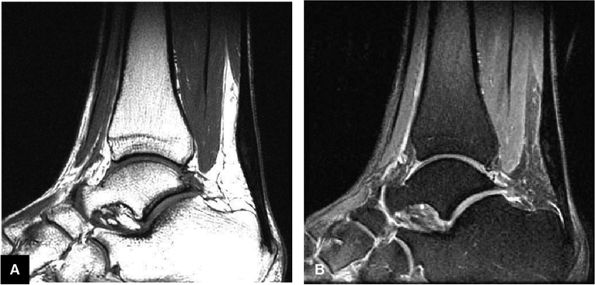
FIGURE 5.107 ● Non-insertional chronic Achilles tendinosis on sagittal T1-weighted (A) and FS PD FSE (B) images. Chemical inflammation is not involved in chronic Achilles tendinosis. Increased levels of the excitatory neurotransmitter glutamate and lactate, however, have been demonstrated in painful midportion tendinosis.
|
 |
|
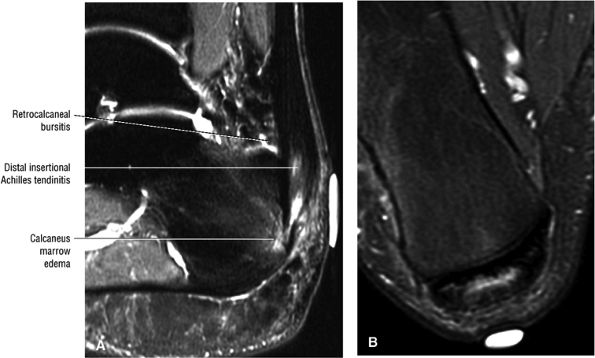
FIGURE 5.108 ● Insertional tendinitis with hyperintensity of the thickened distal Achilles tendon. Retrocalcaneal bursal inflammation and calcaneus marrow edema are shown. In contrast to non-insertional degenerative tendinosis, the process of insertional Achilles tendinitis demonstrates an inflammatory process histologically. Achilles enthesopathy is another term for insertional Achilles tendinitis. FS PD FSE (A) sagittal and (B) axial images.
|
 |
|
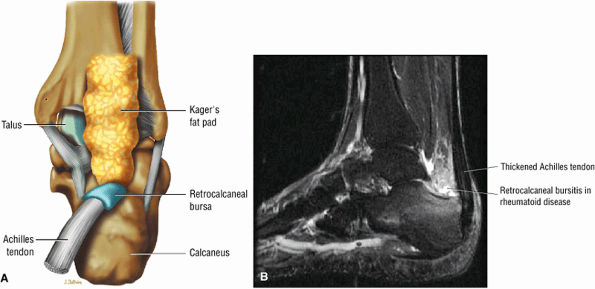
FIGURE 5.109 ● (A) Disk-shaped retrocalcaneal bursa, posterior coronal perspective. (B) Distended retrocalcaneal bursa in rheumatoid arthritis. Sagittal FS PD FSE image.
|
 |
|
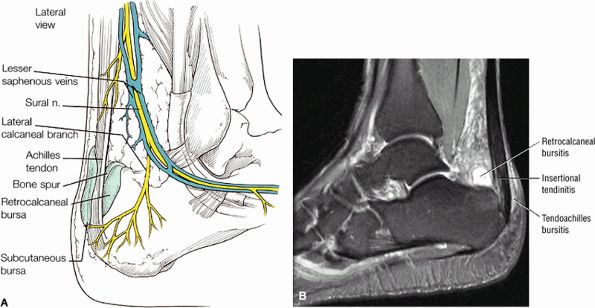
FIGURE 5.110 ● (A) Anatomy of the retrocalcaneal and subcutaneous bursae. (B) Hyperintense retrocalcaneal bursa and more linear tendo-Achilles bursa on a sagittal FS PD FSE image.
|
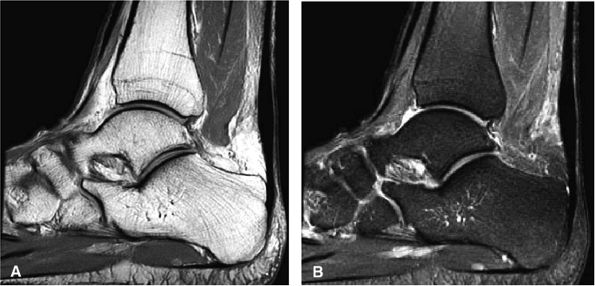 |
|
FIGURE 5.111 ● Painful chronic tendinopathy of the midportion of the Achilles tendon with tendinosis. Tendinosis implies local degenerative changes in the Achilles tendon. (A) Sagittal PD FSE image. (B) Sagittal FS PD FSE image.
|
-
Achilles tendon rupture usually occurs 2 to 6 cm proximal to its os calcis insertion.
-
Sagittal images are used to identify the proximal and distal tendon ends.
-
Axial FS PD FSE images are used to confirm complete rupture (an intact plantaris may simulate an intact tendon in the sagittal plane).
-
Indirect trauma
-
Repetitive microtrauma
-
Overpronation of the foot or heel stress leading to microtears
-
Forced dorsiflexion of the foot against a contracting force (triceps surae group) or eccentric loading in a sudden stretch
-
Direct trauma, causing rupture at the myotendinous junction
-
Soleus muscle atrophy
-
Rheumatoid arthritis, systemic lupus, diabetes mellitus, and gout
-
Chronic tendinitis and partial tears are important predisposing causes in acute rupture.
-
Fluoroquinolone antibiotics (and statin drugs, retinoids, and calcium channel blockers) have been linked to Achilles tendon tears.
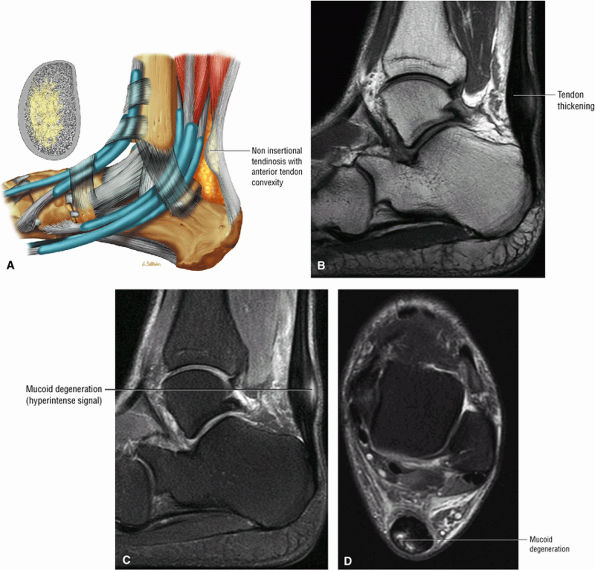 |
|
FIGURE 5.112 ● (A) Non-insertional tendinosis proximal to the os calcis insertion of the Achilles tendon. Intratendinosis degeneration (yellow) is shown on the cross-section through the affected tendon segment. Non-insertional Achilles tendinosis with intratendinous signal intensity is shown on sagittal T1-weighted (B), sagittal FS PD FSE (C), and axial FS PD FSE (D) images. Tendinosis is associated with alterations in the collagen fiber structure and arrangement. An increased amount of interfibrillar glycosaminoglycans (GAGs) occurs in affected areas of tendinosis.
|
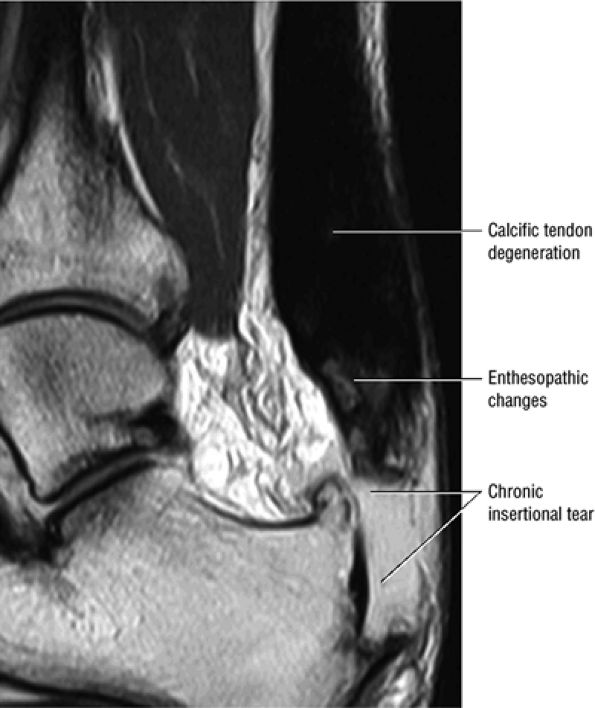 |
|
FIGURE 5.113 ● Thickened calcific tendon degeneration in association with distal tendon rupture. Sagittal T1-weighted image.
|
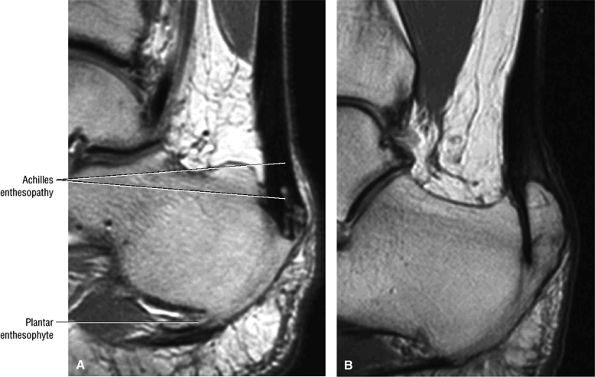 |
|
FIGURE 5.114 ● (A) Achilles enthesopathy or insertional tendinitis with dystrophic changes at the enthesis. A plantar enthesophyte is also demonstrated on this sagittal T1-weighted image. (B) A prominent enthesophyte and os calcis insertion tendinosis. Sagittal T1-weighted image.
|
 |
|
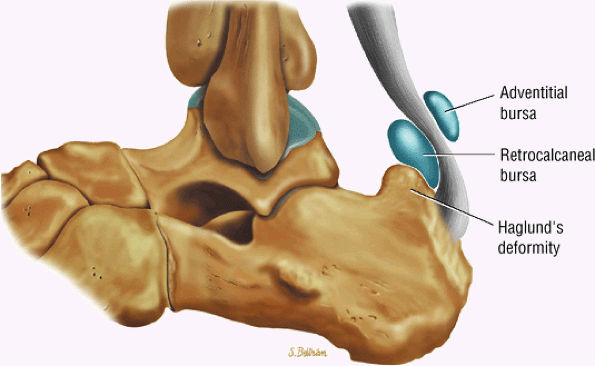
FIGURE 5.115 ● The retrocalcaneal bursa is located between the Achilles tendon and the posterosuperior calcaneal prominence. The adventitial bursa or tendo-Achilles bursa is located between the Achilles tendon and the skin. Lateral color graphic.
|
 |
|
FIGURE 5.116 ● Haglund's deformity with distal Achilles tendinitis, osseous edema of the posterosuperior calcaneus, and visible fluid and/or thickening in the retrocalcaneal bursa and tendo-Achilles bursa. (A) Lateral color illustration. (B) Sagittal FS PD FSE image.
|
 |
|
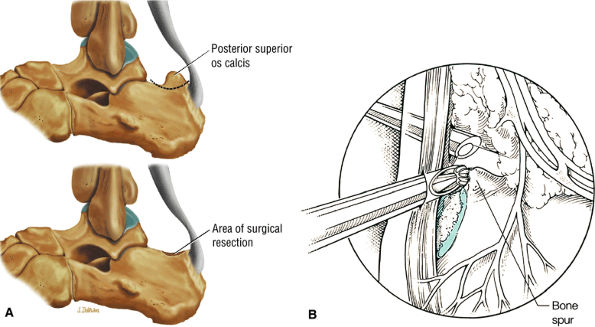
FIGURE 5.117 ● Excision of Haglund deformity. (A) Lateral color illustrations showing excision of the posterior superior os calcis before (top) and after (bottom) surgery. (B) Arthroscopic illustration showing image from the medial portal with lateral placement of a bur to remove the superior angle of the calcaneus.
|
 |
|
FIGURE 5.118 ● A soft-tissue sodium urate deposit (i.e., tophus) located posterior to the Achilles tendon (arrows) demonstrates (A) low signal intensity on a T1-weighted sagittal image and (B) high signal intensity on a STIR sagittal image.
|
to 25% of cases, perhaps because the other tendons of the posterior calf maintain plantarflexion and allow a tendinous gap to be missed on clinical examination. Clinical signs include the hyperdorsiflexion sign, O'Brien's needle test to detect proximal tendon motion as a sign of tendon continuity in acute ruptures, a palpable tendon defect, and Thompsen's sign. The Thompsen test is performed in the prone position and is positive if squeezing the calf does not produce the normal plantarflexion response.
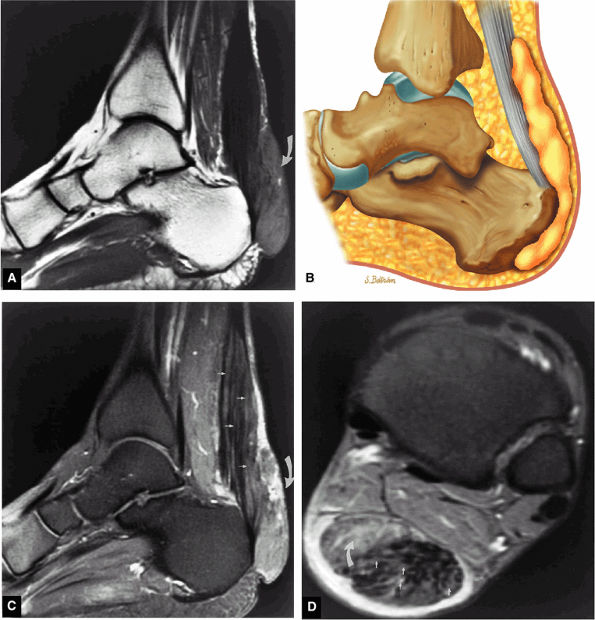 |
|
FIGURE 5.119 ● Xanthomas of the Achilles tendon with a large soft-tissue component (curved arrow) and diffuse infiltration of the Achilles tendon (straight arrow) on (A) a sagittal T1-weighted image, (B) a sagittal color graphic, (C) an FS gadolinium-enhanced sagittal T1-weighted image, and (D) axial FS PD FSE image. Tendinous enhancement is demonstrated with intravenous gadolinium (C, D). The soft-tissue component posterior to the Achilles tendon is hyperintense on the FS PD FSE sequence (B) and demonstrates partial enhancement with intravenous contrast (C).
|
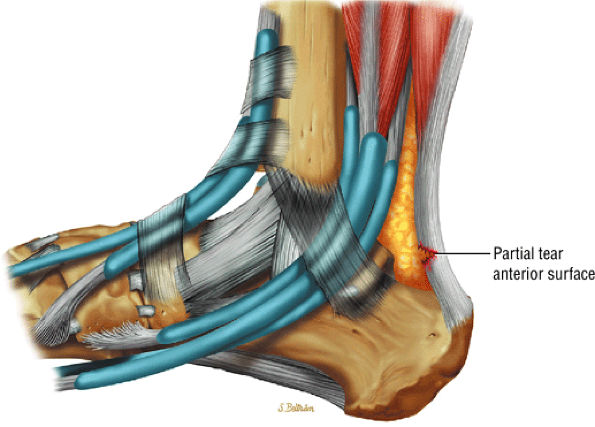 |
|
FIGURE 5.120 ● Anterior surface partial tear of the Achilles tendon on a lateral color graphic.
|
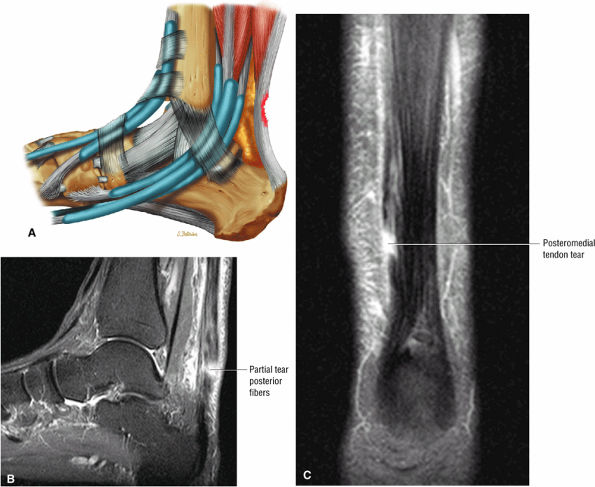 |
|
FIGURE 5.121 ● (A) Posterior surface partial tear of the Achilles tendon proximal to the os calcis. Lateral color lateral illustration. Sagittal (B) and coronal (C) FS PD FSE images of a partial tear of the Achilles tendon. The coronal plane image (C) is useful in demonstrating medial tendon fiber disruption.
|
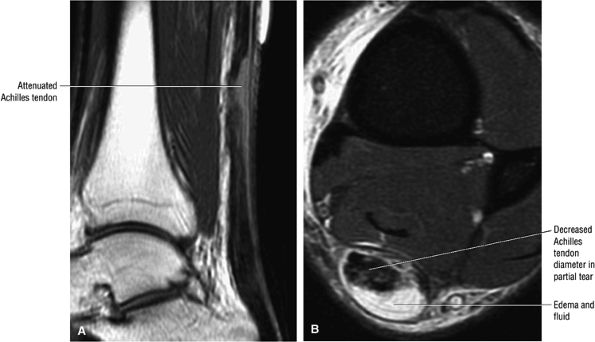 |
|
FIGURE 5.122 ● Attenuated anterior-to-posterior tendon thickness in an extensive partial Achilles tendon tear without a tendinous gap. (A) Sagittal T1-weighted image. (B) Axial FS PD FSE image.
|
-
Type 1: Partial ruptures affecting 50% or less of the tendon (Fig. 5.123)
-
Type 2: Complete ruptures with a tendinous gap of 3 cm or less (Fig. 5.124)
-
Type 3: Complete ruptures with a tendinous gap of 3 to 6 cm (Fig. 5.125)
-
Type 4: Complete tendon ruptures with a defect greater than 6 cm (Fig. 5.126). Type 4 is associated with neglected ruptures.
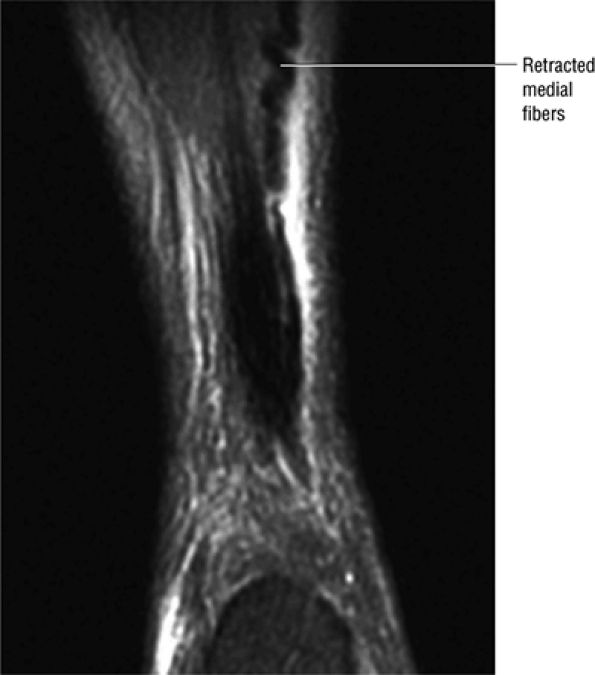 |
|
FIGURE 5.123 ● Partial Achilles tendon tear with the “corkscrew” morphology of partially retracted fibers. Coronal FS PD FSE image.
|
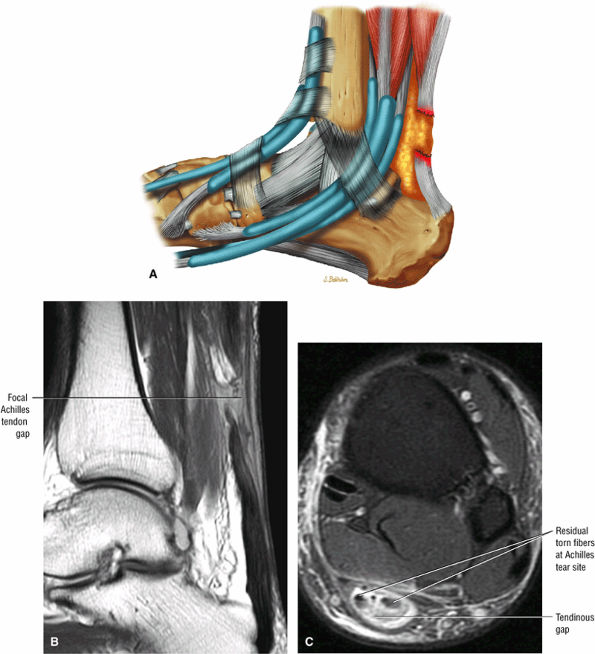 |
|
FIGURE 5.124 ● Focal complete tear of the Achilles tendon with less than 3 cm of retraction. (A) Lateral color graphic. (B) Sagittal T1-weighted image. (C) Axial FS PD FSE image.
|
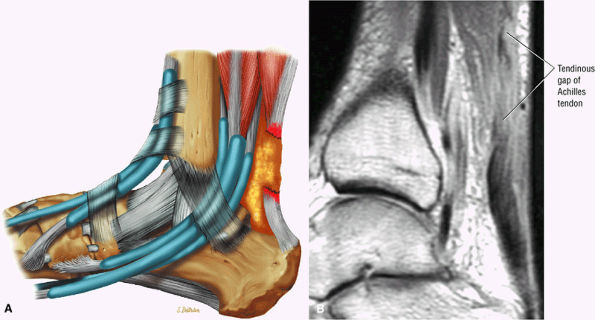 |
|
FIGURE 5.125 ● Type 3 or complete rupture of the Achilles tendon with a tendinous gap of 3 to 6 cm. (A) Lateral color graphic. (B) Sagittal PD FSE image.
|
-
A fluid-filled gap with or without interposed fat at the tear site in complete tendinous disruptions with discontinuity
-
Fraying or corkscrewing (see Fig. 5.123) of the tendon edges associated with proximal tendon retraction
-
In the absence of overlapping tendon edges, no tendon fibers can be seen at the tear site on axial images.
-
Tendon disruption with discontinuity and a wavy retracted tendon
-
Associated hemorrhage or edema in intratendinous or peritendinous soft tissues on axial or sagittal images
-
Disruption of muscle fibers in a musculotendinous junction tear, although on gross examination the tendon appears intact
-
Effacement of Kager's triangle18
-
Intratendinous degeneration
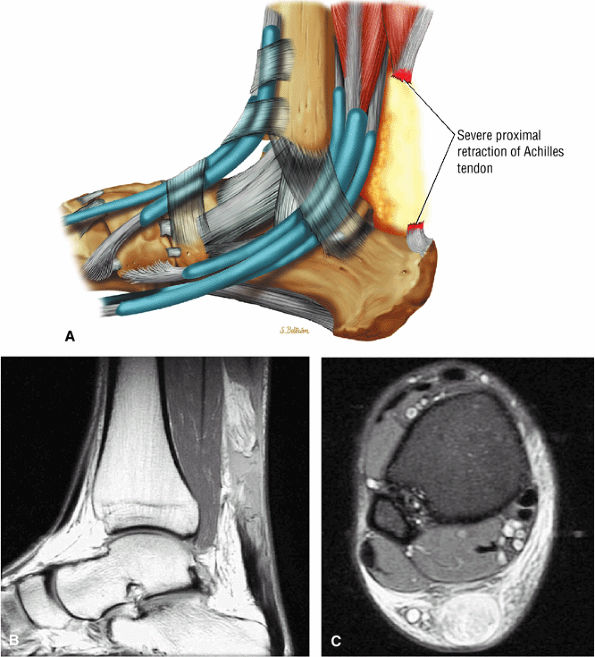 |
|
FIGURE 5.126 ● Complete Achilles rupture with greater than 6 cm of proximal tendon retraction. Fat fills the tendinous gap site proximally. (A) Lateral color graphic. (B) Sagittal PD FSE image. (C) Axial FS PD FSE image.
|
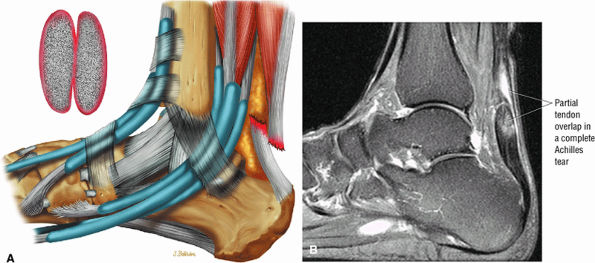 |
|
FIGURE 5.127 ● Partial overlap of torn Achilles tendon ends. (A) Lateral color illustration. (B) Sagittal FS PD FSE image.
|
-
Partial tears demonstrate hyperintense signal with incomplete anterior-to-posterior or posterior-to-anterior extension on FS PD FSE images.
-
Complete tears demonstrate a hyperintense fluid-filled tendinous gap.
-
Tendon rupture usually occurs 2 to 6 cm superior to the os calcis.
-
The size of the rupture varies, based on the degree of tendon retraction.
-
Ruptures demonstrate diffuse convexity of the anterior margin and enlarged tendon ends at the tear site.
spaces, studied at 3 and 6 months by Dillon et al..45 Over time, decreased signal intensity was found in the areas of successful healing. By 12 months, these areas demonstrated low signal intensity within a widened tendon. Caution must be exercised in interpreting MR images obtained in the first 2 months postoperatively; Achilles tendon pathology should not be overread as retear, poor healing, or failed surgical repair.46
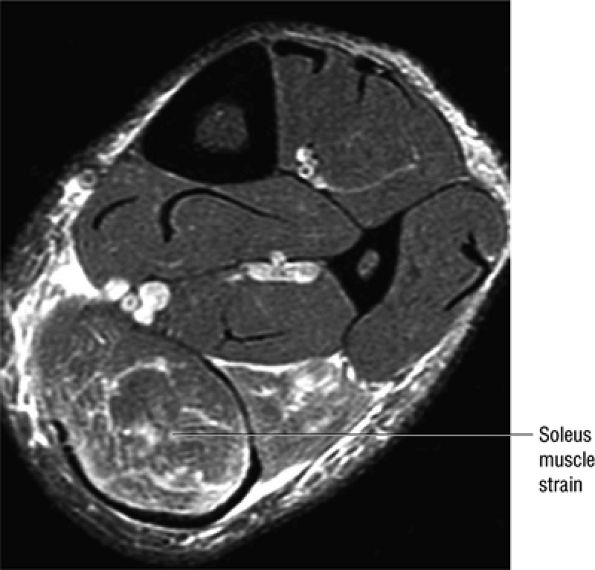 |
|
FIGURE 5.128 ● Grade 1 soleus muscle strain secondary to an Achilles rupture. Axial FS PD FSE image.
|
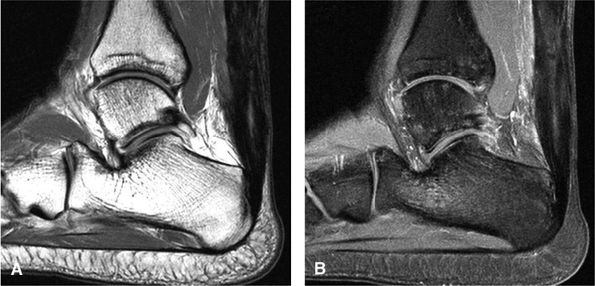 |
|
FIGURE 5.129 ● Residual post-surgical Achilles tendon thickening with an intratendinous scar. (A) Sagittal T1-weighted image. (B) Sagittal FS T1-weighted contrast enhanced image.
|
-
Tendinopathy and progressive tendon tear results in a collapsed pes valgus deformity.
-
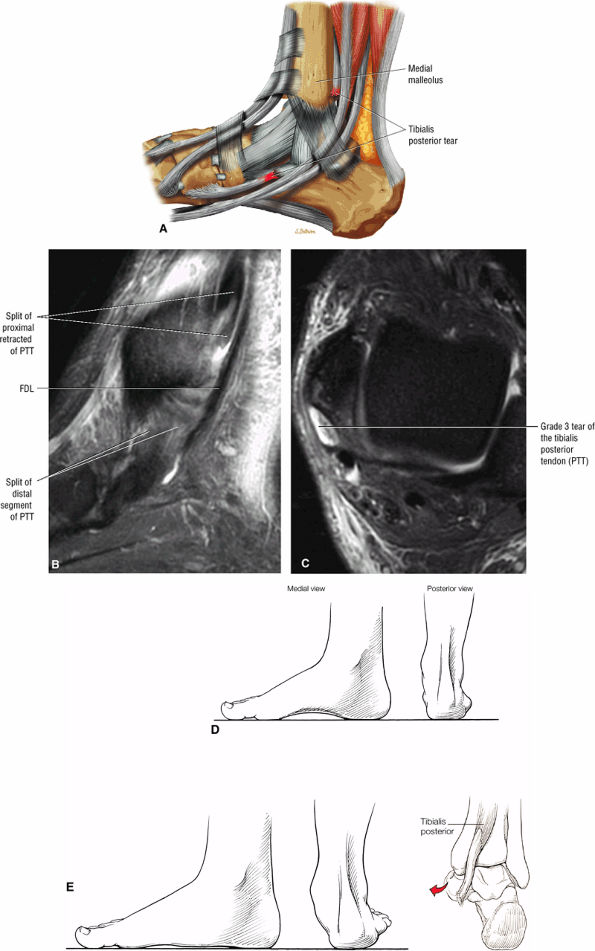
Tibialis posterior tendon tears occur at or just distal to the medial malleolus.
-
Tears are classified as type 1 (enlarged tendon), type 2 (attenuated tendon), or type 3 (complete rupture).
-
Associated medial malleolar spur may contribute to tendon degeneration.
-
Subtendons are evaluated on axial images.
-
Chronic degeneration
-
Trauma
-
Systemic disease
-
Tendon hypovascularity
-
Biomechanical disorders and abnormal insertions
-
Iatrogenic conditions
-
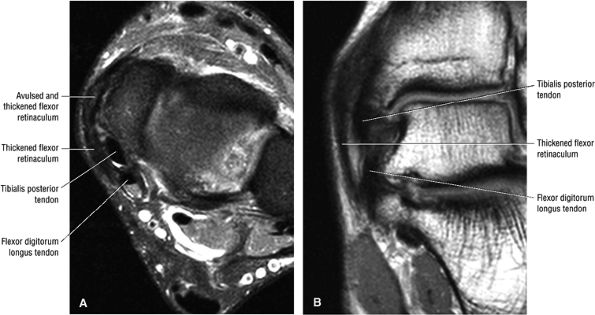
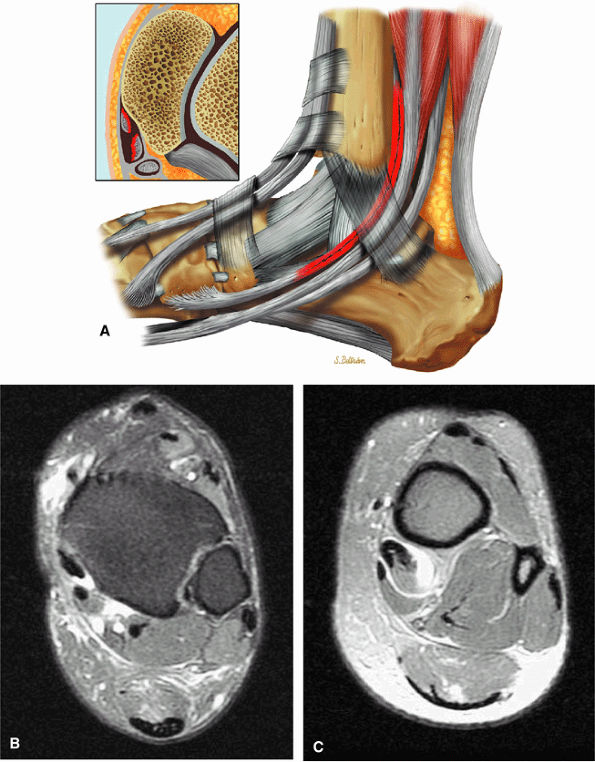
Type 1: There is tendon hypertrophy with heterogeneous signal intensity in intrasubstance vertical splits (Fig. 5.130). Associated findings include increased signal intensity (intrasubstance striations) and girth at the distal tendon insertion to the navicular (a normal variant), osseous spurring with or without fatty marrow signal in the posteromedial aspect medial malleolus (see Fig. 5.130), and tendon dysfunction or dislocation with disruption of the flexor retinaculum (Fig. 5.131).
-
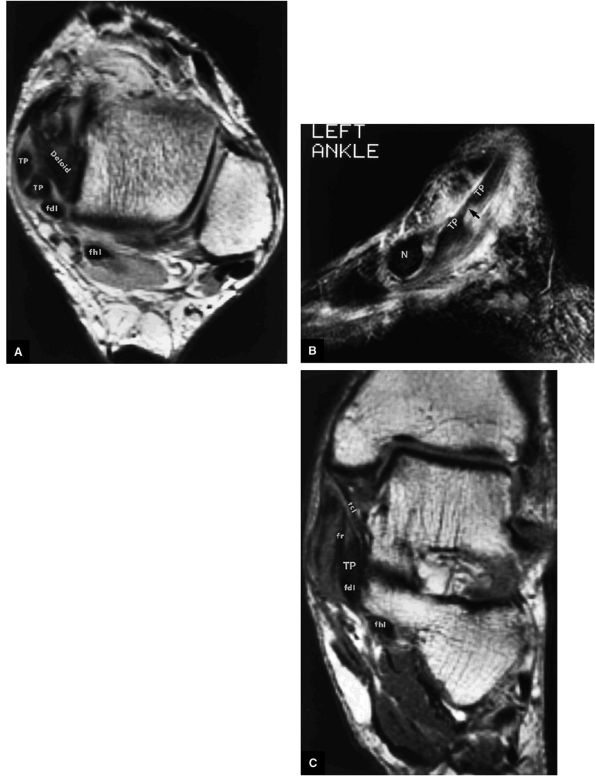
Type 2: There are thin or attenuated sections of the tendon at the level of the medial malleolus53 with variable intratendinous signal change (Fig. 5.132). Subtendons may occur associated with mixed areas of atrophic and hypertrophic tendon segment (Fig. 5.133).
-
Type 3: There is complete tendinous discontinuity with a low- to intermediate-signal-intensity fluid-filled gap (Fig. 5.134). There may be subtendons associated with irregular tendon morphology of the retracted proximal or distal segments on either side of the tendinous gap. Digital avulsion (Fig. 5.135) in the foot at the insertion into the cuneiforms, cuboid, and medial metatarsal bases is uncommon.
-
Chronic dysfunction: In spring ligament laxity or rupture, the superomedial calcaneal navicular component is usually injured. The sinus tarsi syndrome may occur after progression from PTT dysfunction to spring ligament pathology. A more detailed discussion of spring ligament pathology can be found later in the chapter.
of the ends of the tendon.37 Partial or chronic tears or a retracted tendon may present with enlargement. FS PD FSE axial images that extend inferior to the medial malleolus should demonstrate the normal PTT anterolateral to the FDL tendon.
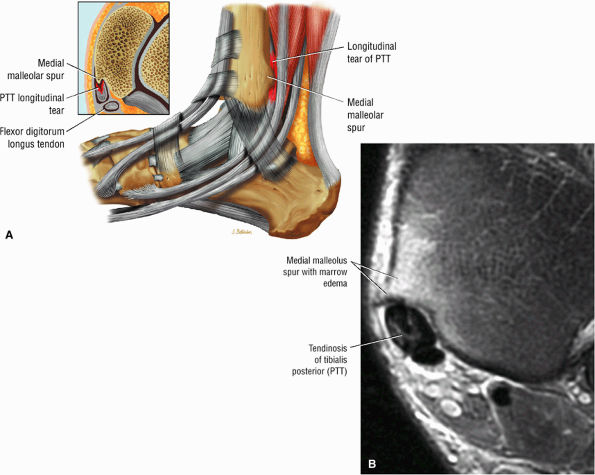 |
|
FIGURE 5.130 ● Type 1 tibialis posterior tendon tear associated with a medial malleolus osseous spur. The normal tibialis posterior tendon is approximately twice the cross-sectional diameter of the flexor digitorum longus. In a type 1 tear the PTT may demonstrate increased thickening, with a cross-sectional diameter up to 5 to 10 times larger than the adjacent flexor digitorum longus tendon. (A) Lateral graphic with an axial insert. (B) Axial FS PD FSE image.
|
-
Prominence or hypertrophy of the medial tubercle of the navicular (sensitivity 89%, specificity 75%)
-
Abnormalities of talonavicular alignment (sensitivity 82%, specificity 100%)
-
Presence of an accessory navicular (sensitivity 20%, specificity 100%)
 |
|
FIGURE 5.131 ● Chronically scarred flexor retinaculum. Dislocation of the tibialis posterior is associated with a torn flexor retinaculum. The retromalleolar groove may also be shallow. (A) Axial FS PD FSE image. (B) Coronal T1-weighted image.
|
-
The spring ligament complex consists of three components: the lateral, intermediate, and superomedial oblique calcaneonavicular ligaments.
-
Degeneration of the spring ligament complex is associated with posterior tibial (tibialis posterior) tendinopathy.
structures are critical static stabilizers of the medial longitudinal arch of the foot, providing support for the head of the talus at the talocalcaneonavicular joint (or acetabulum pedis). The other major stabilizer of the medial longitudinal arch, the PTT, is a dynamic stabilizer. Pathology of the spring ligament complex rarely occurs in isolation and is almost always associated with PTT dysfunction. Attention to the components of the spring ligament on routine MR imaging of the foot is important, since there is often a cascade of failures that can lead to or be seen with acquired pes planus deformity.
 |
|
FIGURE 5.132 ● (A) Type 2 tibialis posterior tear with the generation of two subtendons. Lateral color graphic with axial inset. (B, C) Attenuated tibialis posterior tendon at the level of the medial malleolus with hypertrophy of the more proximal tibialis posterior associated with tendinous fiber retraction. Axial FS PD FSE images.
|
 |
|
FIGURE 5.133 ● (A) A T1-weighted axial image depicting a longitudinal rupture of the tibialis posterior tendon resulting in two subtendons of the tibialis posterior (TP) at the level of the medial malleolus and deltoid ligament. Note that this split creates the appearance of four separate medial tendons. The two anterior medial tendons represent the anterior and posterior half of the tibialis posterior. (B) A T2*-weighted sagittal image identifies the attenuated portion of the tibialis posterior at the longitudinal tear site (arrow). (C) The relationship of the tibialis posterior (TP) to the adjacent medial structures on a corresponding T1-weighted coronal image. fdl, flexor digitorum longus tendon; fhl, flexor hallucis longus tendon; N, navicular bone; tcl, tibiocalcaneal ligament; fr, flexor retinaculum.
|
 |
|
FIGURE 5.134 ● (A) Type 3 tibialis posterior tendon tear with complete tendinous gap. Lateral color graphic. Sagittal (B) and axial (C) FS PD FSE images demonstrate the tendinous gap and frayed or split retracted tendon ends. (D) Normal arch. (E) Pes planus, with loss of the medial longitudinal arch and hindfoot valgus. From the posterior view, the “too many toes” sign can be seen secondary to a rupture of the posterior tibial tendon.
|
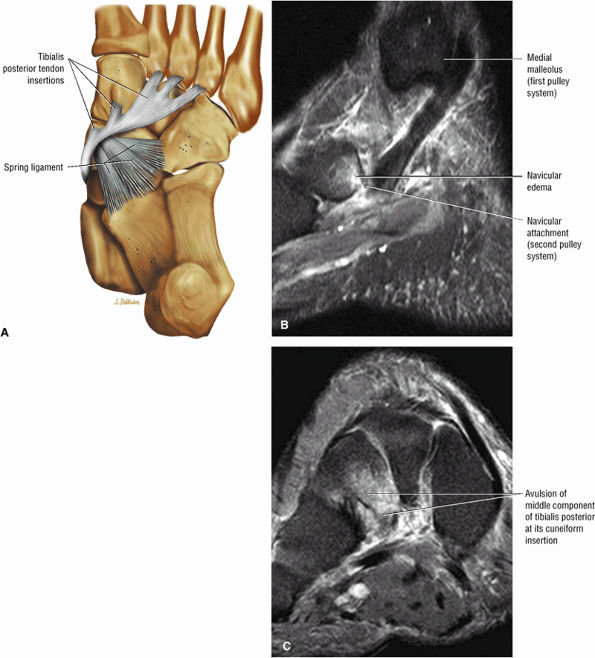 |
|
FIGURE 5.135 ● (A) Plantar view color illustration of the distal insertions of the tibialis posterior tendon to the tuberosity of the navicular plantar surface of all the cuneiform bones, the sustentaculum tali, and the cuboid (not shown). The posterior component of the tibialis posterior inserts on the anterior aspect of the spring ligament. (B) Sagittal FS PD FSE image showing the torn insertion of the anterior component of the tibialis posterior into the navicular tuberosity. (C) A coronal FS PD FSE image shows the avulsed middle component, which normally inserts to the cuneiforms, cuboid, and three metatarsal bones. The middle component has a ligamentous function and provides stability to the plantar arch.
|
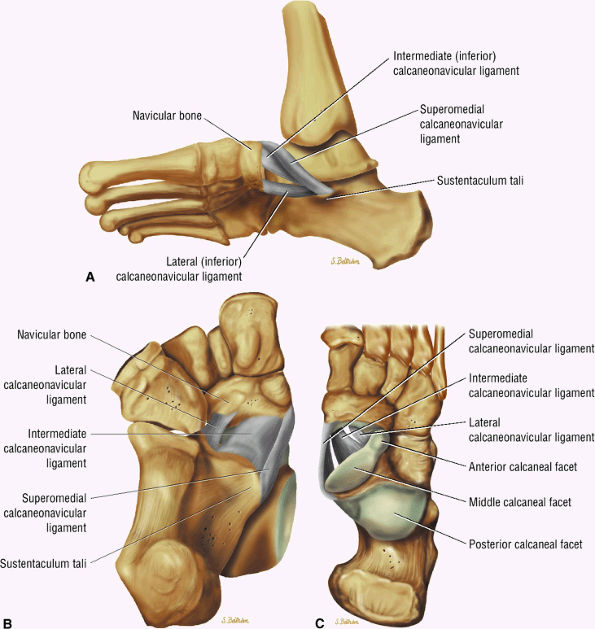 |
|
FIGURE 5.136 ● Spring ligament complex anatomy. Lateral-plantar oblique (A), plantar (B), and superior (C) views.
|
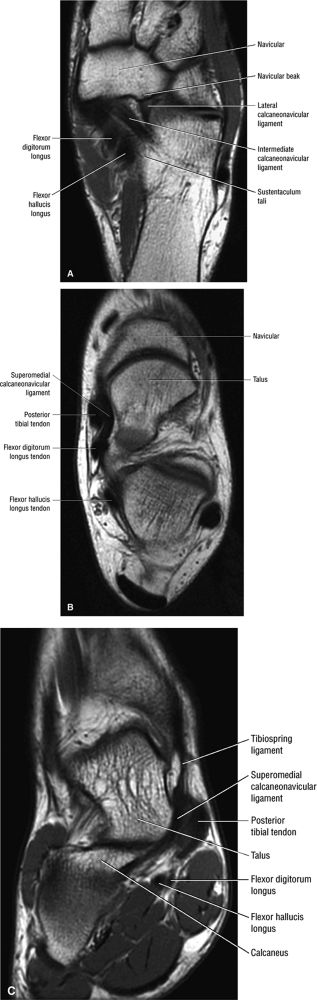 |
|
FIGURE 5.137 ● Normal MR appearance of the spring ligament complex. (A) Axial PD image demonstrates the lateral and intermediate calcaneonavicular ligaments originating from the notch between the anterior and middle articular facets of the calcaneus. The lateral calcaneonavicular ligament inserts on the navicular beak. (B) Axial PD image a few slices superior demonstrates the superomedial calcaneonavicular ligament deep to the posterior tibial tendon as it passes along the lateral aspect of the talar head toward its attachment to the dorsal aspect of the navicular tubercle. (C) Coronal T1-weighted image at the level of the talar head demonstrates the superomedial calcaneonavicular ligament deep to the posterior tibial tendon along the lateral aspect of the talar head.
|
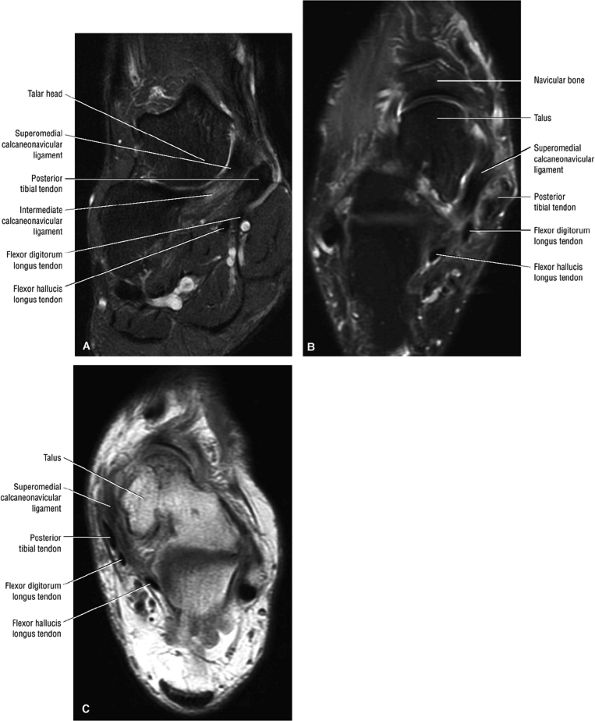 |
|
FIGURE 5.138 ● Pathologic appearances of the superomedial calcaneonavicular ligament. (A) Coronal FS PD image with fat saturation demonstrates a thickened and mildly edematous superomedial calcaneonavicular ligament in a patient who also had posterior tibial tendon strain. (B) Axial FS PD image with fat saturation demonstrates a thickened and edematous superomedial calcaneonavicular ligament in the setting of a partial posterior tibial tendon tear. (C) Axial PD image demonstrates a heterogeneous and irregular superomedial calcaneonavicular ligament in the setting of posterior tibial tendinosis and pes planus.
|
-
Tenosynovitis is associated with hyperintense fluid in the FHL synovial sheath disproportionate to the quantity of tibiotalar joint fluid.
-
Chronic muscle changes and acute tenosynovitis are associated with hyperplantar flexion in ballet dancers.
-
The distal course of the FHL should be examined on coronal images to exclude a distal rupture at its third or lost fibro-osseous tunnel.
-
Fibro-osseous tunnel (first fibro-osseous tunnel) between the medial and lateral tubercles of the posterior talar process
-
Deep to the flexor retinaculum (see Fig. 5.143)
-
The level of the sesamoids within the distal hallux tunnel (third fibro-osseous tunnel) (see Fig. 5.146)
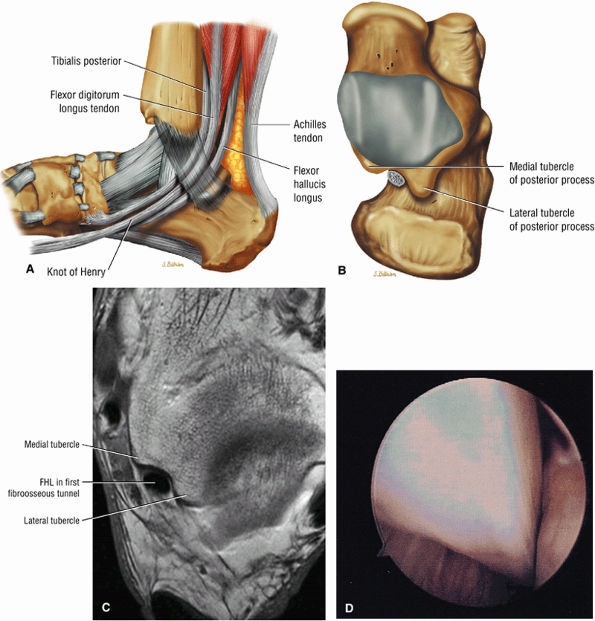 |
|
FIGURE 5.139 ● The flexor hallucis longus (FHL) is associated with three fibroosseous tunnels: a tunnel between the talar tubercles, a second tunnel deep to the sustentaculum tali, and a third tunnel between the hallucal sesamoids. (A) Lateral color illustration showing the course of the FHL and the knot of Henry where the flexor digitorum longus and flexor hallucis longus cross. The FHL is located between the medial and lateral talar tubercles on (B) a color illustration and (C) an axial T1-weighted MR arthrogram. (D) Arthroscopic image showing the FHL running in a sheath just posterior to the ankle capsule.
|
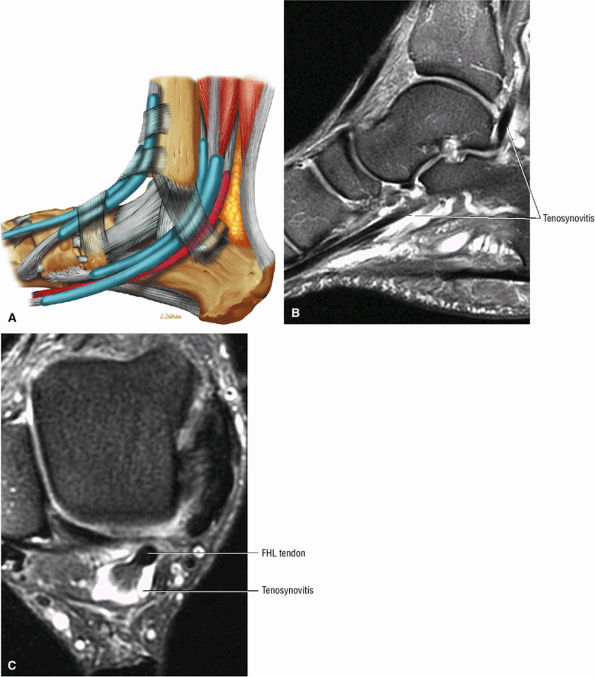 |
|
FIGURE 5.140 ● Flexor hallucis longus (FHL) tenosynovitis. Tendinitis of the FHL posterior to the medial malleolus is known as dancer's tendinitis. (A) Medial perspective lateral color illustration showing injury of the FHL as it passes through the fibro-osseous tunnel from the posterior aspect of the talus to the level of the sustentaculum tali; it acts like a rope through a pulley. Sagittal (B) and axial (C) FS PD FSE images demonstrating the extent of FHL tenosynovitis distal to the medial malleolus.
|
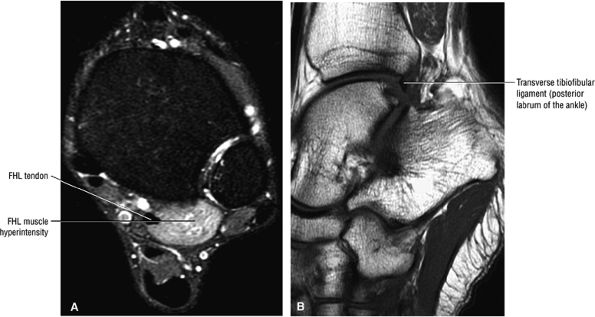 |
|
FIGURE 5.141 ● FHL injuries are associated with extreme plantarflexion and push-off maneuvers, as occur in football players and ballet dancers. There is chronic muscle strain of the FHL just proximal to the sustentaculum tali. (A) Axial FS PD FSE with FHL muscle chronic strain (hyperintense signal). (B) Sagittal T1-weighted image showing extreme plantarflexion in the en pointe position with proximity of the transverse ligament and posterior process.
|
-
Rupture occurs between the extensor retinaculum and the insertion onto the medial first cuneiform and the adjacent base of the first metatarsal.
-
It is important to differentiate between a partial and complete rupture using axial and coronal oblique images.
runners, soccer players, and hikers. Steroid injections also predispose to tendon rupture. The usual mechanisms of injury are eccentric contraction during midfoot and forefoot forced plantarflexion63 and repetitive dorsiflexion and plantarflexion, because plantarflexion places eccentric stress on the tibialis anterior. Rupture may also be related to abrasion from the inferior edge of the extensor retinaculum or from mechanical irritation, such as occurs from ski boots and hockey skates. Traumatic lacerations, related to the superficial and anterior location of this tendon, may also occur. Although the rupture usually occurs proximal to the insertion, no zone of hypovascularity has been documented.
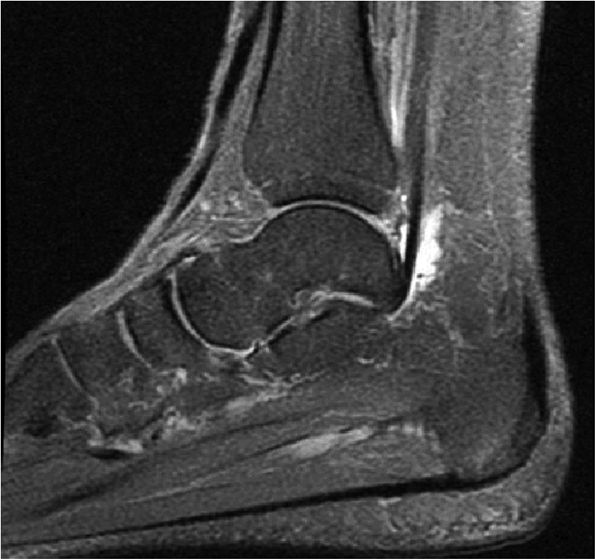 |
|
FIGURE 5.142 ● Tenosynovitis of the FHL between the medial and lateral talar tuberosities.
|
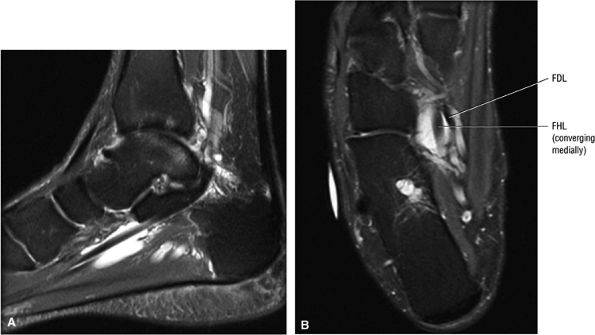 |
|
FIGURE 5.143 ● Tenosynovitis of the FHL distal to the fibro-osseous tunnel between the medial and talar tubercles and underneath the sustentaculum tali. Tenosynovitis is associated with chronic friction and tendon wear between the talar tuberosities, deep to the flexor retinaculum inferior to the sustentaculum tali and distally between the hallucal sesamoids. (A) Sagittal FS PD FSE image. (B) Axial FS PD FSE image.
|
-
A high-grade partial to complete tear (normal width 1 cm) with tendon retraction
-
A mass effect produced by protrusion of the tendon relative to the inferior extensor retinaculum
-
Secondary inflammation of adjacent tendon sheaths
-
Hemorrhage
-
An empty tendon sheath
-
Dorsal exostosis
-
Medial cuneiform morphology with ridge contour associated with distal tendon degeneration of a partial tear (Fig. 5.152)
-
Histologic evidence of peritenonitis
-
Inflammatory changes but no intrinsic tendon degeneration
-
Clinically, patients present with weakness with attempted dorsiflexion, local tenderness and foot drop, and a palpable mass and/or defect. Initial symptoms may be minimal.
-
Enlarged tendon and sheath
-
Fraying of the tendon ends
-
Focal enlargement and partial tendon continuity with preserved tendon fibers in high-grade partial tears
-
Absence of the tendon at the tear site (empty sheath)
-
Associated dorsal osteophytes in ruptures at the medial tarsometatarsal joint
-
Inflammation of the synovial tendon sheath (may be associated with tendon rupture or rheumatoid disease, or may be idiopathic)
-
Fluid within the tendon sheath
-
Associated hemorrhage
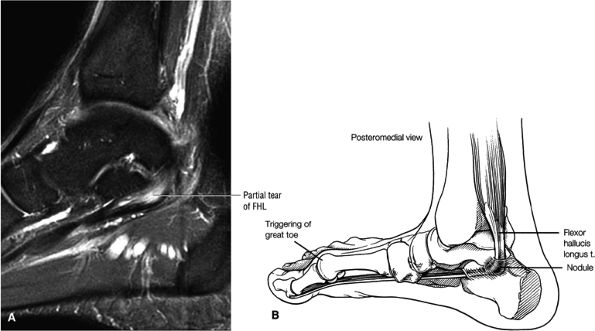 |
|
FIGURE 5.144 ● (A) Sagittal FS PD FSE image showing a nodular partial tear (the partial tear binds in the fibro-osseous tunnel). (B) Hallux saltans may develop as the FHL binds as it passes through the second fibro-osseous tunnel on the medial side of the foot. A nodular partial tear of the FHL may cause triggering of the big toe.
|
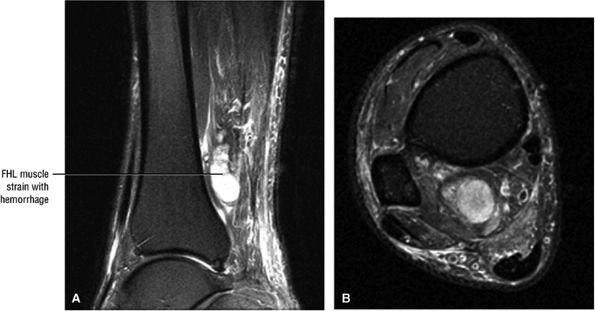 |
|
FIGURE 5.145 ● Stenosing tenosynovitis resulting from chronic inflammation of the FHL peritenon occurs with entrapment of the FHL tendon in its sheath at the level of the ankle joint. A functional hallux rigidus or checkrein deformity (claw hallux) may subsequently develop. (A) Sagittal FS PD FSE image. (B) Axial FS PD FSE image.
|
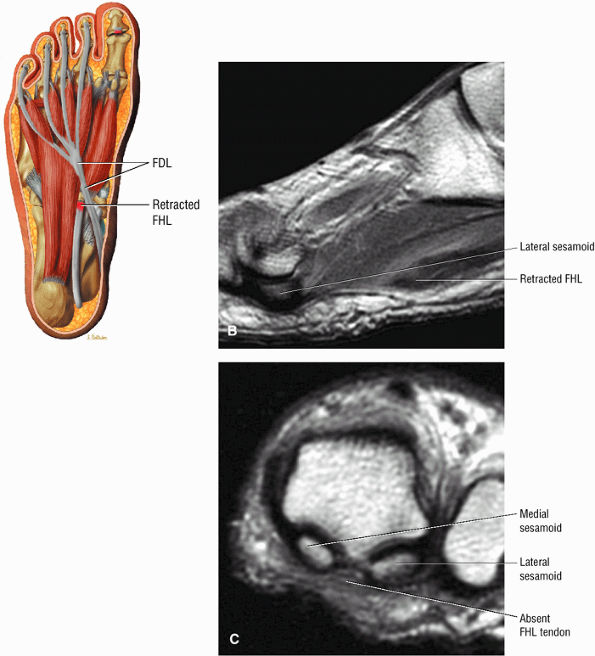 |
|
FIGURE 5.146 ● Distal rupture of the FHL results from acute dorsiflexion or laceration. The fibrous slip connecting the FHL and flexor digitorum longus (FDL) at Henry's knot limits the retraction of FHL proximal to this point. FHL tears proximal to Henry's knot may be associated with tendon recoil into the calf. (A) Plantar view color illustration of FHL distal rupture. (B) Sagittal PD-weighted image with retracted FHL to the plantar aspect of the forefoot. (C) Coronal PD FSE image.
|
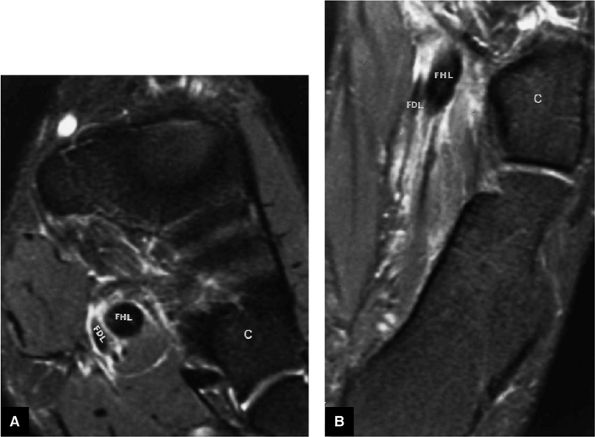 |
|
FIGURE 5.147 ● Distal rupture of the flexor hallucis longus (FHL) tendon with proximal retraction. FS PD FSE coronal (A) and axial (B) images. The retracted FHL tendon is enlarged in cross-sectional area relative to the normal adjacent flexor digitorum longus (FDL) tendon. Associated soft-tissue edema is hyperintense on FS PD FSE images. The FHL tendon normally inserts at the base of the distal phalanx of the first toe. C, cuboid.
|
 |
|
FIGURE 5.148 ● Disproportionate fluid in FHL tendon sheath in a basketball player who had minimal tibiotalar joint fluid. Axial FS PD FSE image.
|
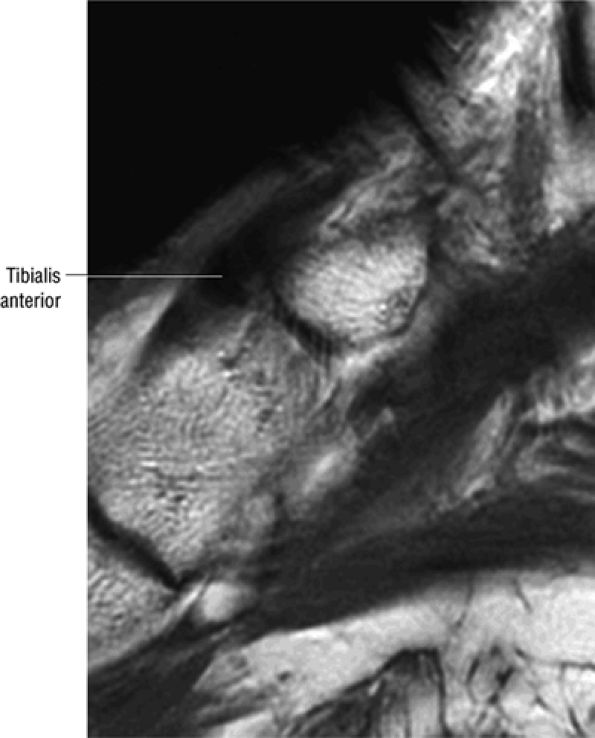 |
|
FIGURE 5.149 ● Distal insertion of the tibialis anterior to the medial cuneiform. The tibialis anterior also attaches to the base of the first metatarsal. Sagittal T1-weighted image.
|
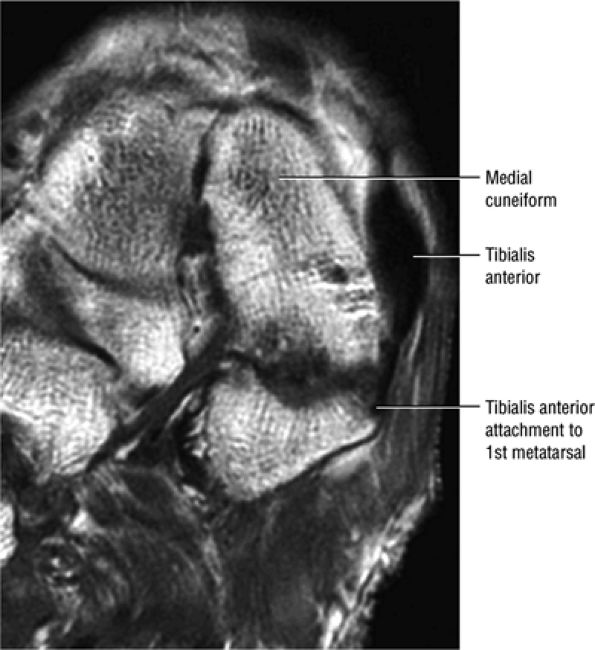 |
|
FIGURE 5.150 ● At its insertion, the most lateral fibers of the tibialis anterior rotate anteriorly and insert on the cuneiform. The medial fibers rotate posteriorly and insert distally on the dorsolateral first metatarsal. Coronal T1-weighted image.
|
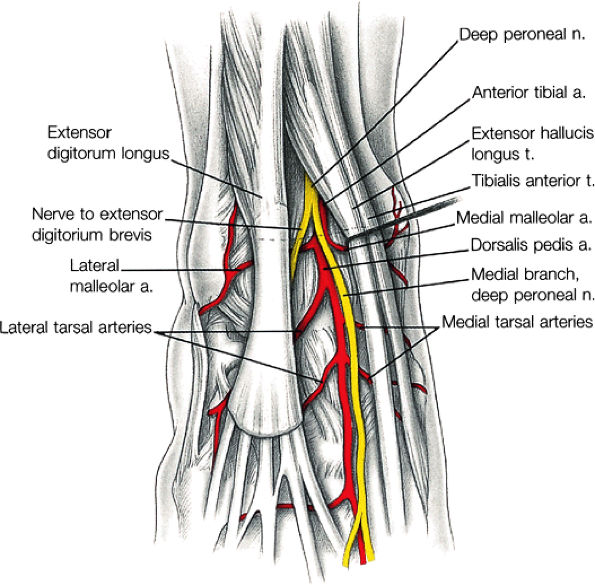 |
|
FIGURE 5.151 ● The tibialis anterior receives its neurovascular supply from the deep peroneal nerve and anterior tibial artery.
|
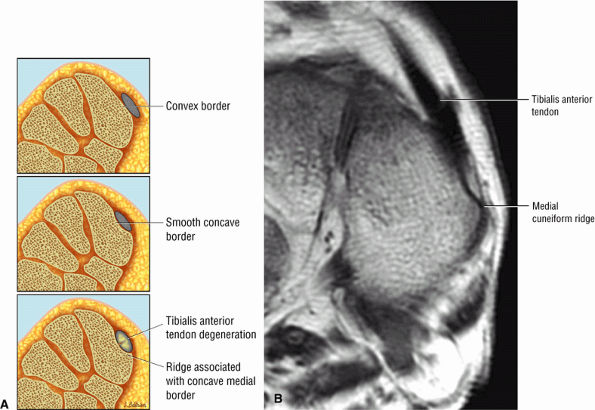 |
|
FIGURE 5.152 ● (A) Medial cuneiform morphology showing convex medial border (top), concave smooth border (middle), and a ridge associated with a concave border and tendon degeneration (bottom). (B) Coronal T1-weighted image showing normal tibialis anterior. However, there is ridge morphology along the medial cuneiform border.
|
sulcus of the distal fibula.63 The peroneal tendons may rupture secondary to trauma or laceration of the lateral aspect of the ankle. MR imaging defines the position of the peroneal tendons as well as the fibular and retinacular anatomy.52 Absence of the low-signal-intensity tendon within the peroneal tendon sheath may be observed on sagittal or axial images. The peroneal tendons are also involved in partial or complete dislocations.
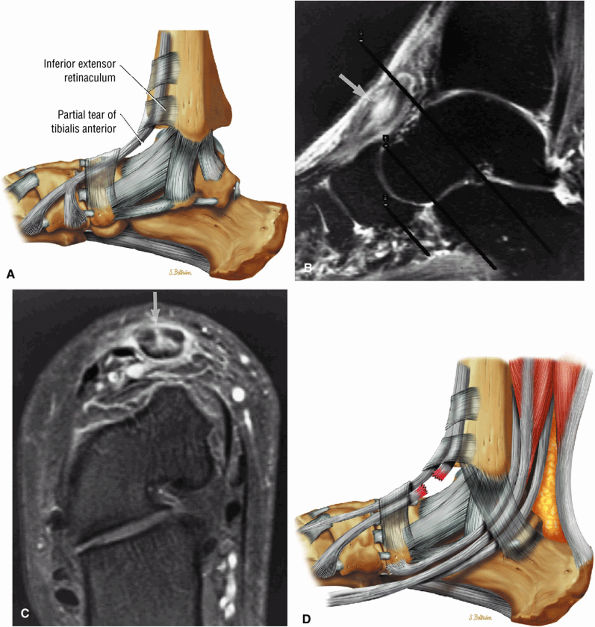 |
|
FIGURE 5.153 ● (A) Partial tear of the tibialis anterior on a lateral color illustration. The tibialis anterior tendon has a relatively straight course deep to the superior extensor retinaculum. There is a potential site of compression and paratenonitis as the tibialis anterior courses between the deep and superficial superior subdivisions of the superomedial band of the inferior extensor retinaculum. The distal tibialis anterior courses deep to the inferior subdivision of the superomedial band of the inferior extensor retinaculum and inferomedial band of the inferior extensor retinaculum. Partial tear of the tibialis anterior tendon (arrow) is shown on (B) fast STIR sagittal and (C) gadolinium-enhanced FS T1-weighted axial oblique images. The axial oblique prescriptions designated from the STIR sagittal image ensure that the axial images are acquired perpendicular to the long axis of the tendon. The gadolinium contrast enhances the partial tear pattern within the tibialis anterior tendon (C, cross-section). (D) Complete discontinuity of the tibialis anterior in younger individuals is usually secondary to tendon laceration. Tendinosis of the tibialis anterior is rare, although paratenonitis may be indirectly caused by eccentric contraction in runners and hikers on downhill surfaces.
|
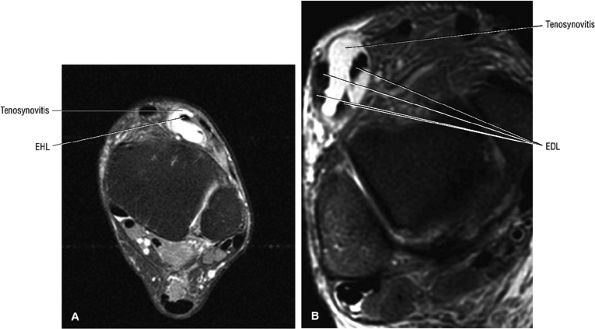 |
|
FIGURE 5.154 ● Tenosynovitis (paratenonitis) of the extensor hallucis longus (EHL) (A) and extensor digitorum longus (EDL) (B) tendons is shown on axial FS PD FSE images. The EHL tendon enlarges distally at its insertion on the dorsal aspect of the distal hallucal phalanx. The EDL bifurcates below the superior extensor retinaculum, and each branch then divides into two tendons under the stem of the inferior extensor retinaculum.
|
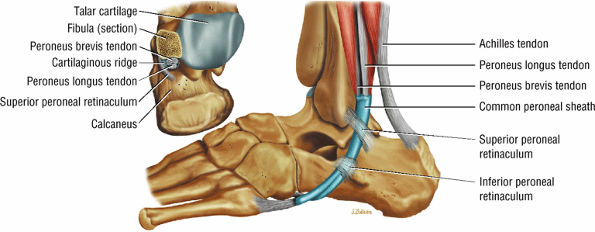 |
|
FIGURE 5.155 ● Normal relationship of the peroneal tendons to the distal fibula and common synovial sheath of the peroneal tendons posterior to the lateral malleolus. The peroneus brevis makes two turns in its course, one at the fibular groove and the other at the peroneal tubercle. The peroneus longus makes three turns, at the fibular groove, the peroneal tubercle, and the cuboid notch (os peroneum).
|
-
The peroneus brevis splits into two subtendons at the level of the retrofibular groove near the distal lateral malleolus.
-
Lateral ligament tears and laxity of the superior peroneal retinaculum lead to peroneus brevis tendon split and tendon subluxation.
-
Longitudinal splits/clefts and fluid are seen within partial tears of the peroneus brevis tendon (Fig. 5.158).
-
There is anteroposterior separation of the split components of the tendon.
-
Subtendons are hypointense on T1- or PD-weighted images with hyperintense fluid between the split subtendons on FS PD FSE images. The peroneus longus tendon may interpose between the two subtendons of the brevis.
-
Partial tears or longitudinal splits occur at the level of the lateral malleolus (Fig. 5.159), and may be associated with a sprain and SPR or lateral ligament complex injury. Identification of peroneus brevis involvement is important in case the peroneus brevis is to be used in a lateral ligament reconstruction.
-
Abrasion of the peroneus brevis from the calcaneofibular ligament or lateral cartilaginous ridge of the fibula is thought to be an initiating factor in the development of longitudinal tears.69 Lateral ligament tears with associated SPR laxity may lead to peroneus brevis tendon splits and anterolateral subluxation of both peroneal tendons.
-
Complete rupture (Fig. 5.160) of a peroneal tendon should be confirmed on images obtained in more than one imaging plane.
-
Reactive lateral calcaneal marrow edema at the attachment site of the inferior peroneal retinaculum (the peroneal tubercle) is associated with acute rupture of the peroneus brevis tendon.
-
Peroneal tendon entrapment or impingement is associated with a fracture of the calcaneus in which lateral fragment displacement narrows the fibulocalcaneal space.63
-
In partial tears the tendon is attenuated and decreased in cross-sectional diameter with an irregular contour.
-
Degenerative changes are intermediate in signal on T1- and PD-weighted images, and fluid associated with tenosynovitis is hyperintense on FS PD FSE images.
-
A split brevis tendon may wrap around the longus tendon (Fig. 5.162).
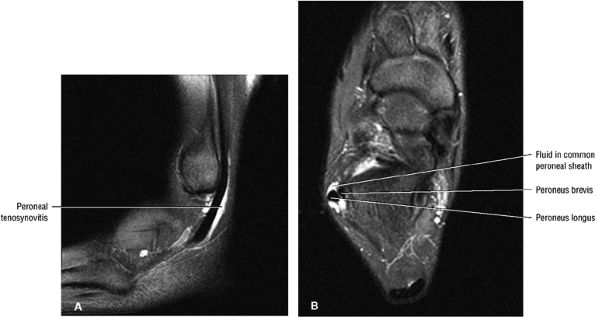 |
|
FIGURE 5.156 ● Tenosynovitis (paratenonitis) of the peroneal tendons on sagittal FS PD FSE (A) and axial FS PD FSE (B) images. Tenosynovitis is associated with overuse injuries. Tendon morphology is intact.
|
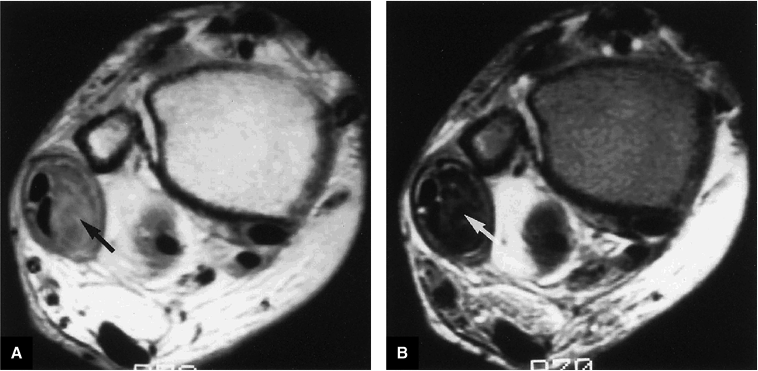 |
|
FIGURE 5.157 ● In chronic peroneal tenosynovitis, thickened low-signal-intensity synovium (arrows) encases the peroneus brevis and longus tendons. There is no increased signal intensity within synovial tissue on (A) intermediate-weighted or (B) T2-weighted axial images.
|
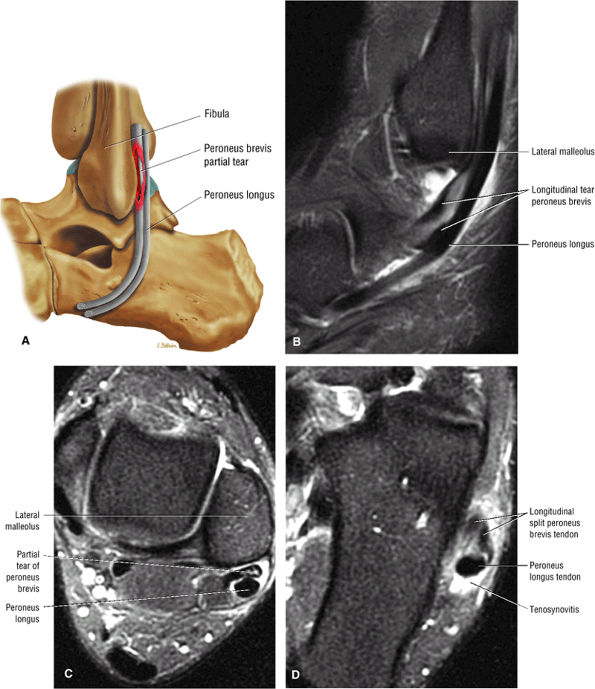 |
|
FIGURE 5.158 ● (A) Color illustration of a peroneus brevis partial tear. Tenosynovitis (paratenonitis) with tendinosis including longitudinal tears and tendinosis of the peroneus brevis usually occurs at the level of the fibular groove at the distal lateral malleolus. Longitudinal tendon tear is shown on sagittal FS PD FSE (B) and axial FS PD FSE (C, D) images. The resultant two subtendons distal to the lateral malleolus are shown in (D).
|
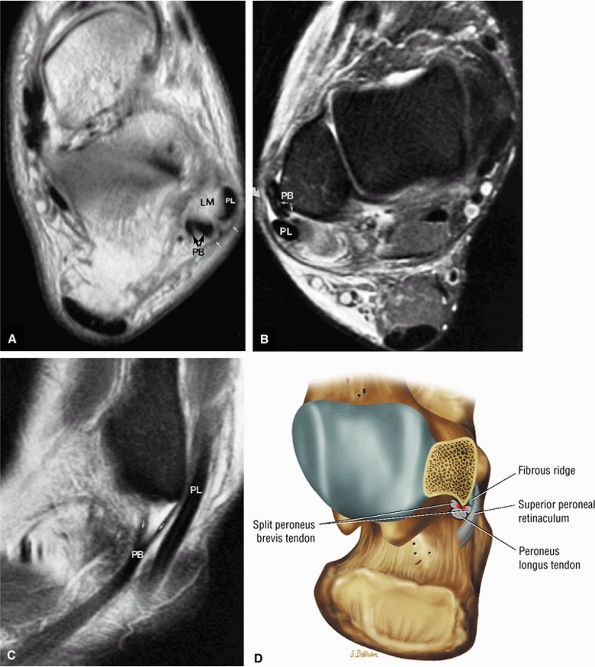 |
|
FIGURE 5.159 ● (A) Intermediate-weighted axial image displaying anterolateral dislocation of the peroneus longus tendon (PL) and split of the peroneus brevis tendon (PB; black arrows), both associated with a torn superior peroneal retinaculum (white arrows). LM, lateral malleolus. (B, C) In a separate case there is anterolateral subluxation of the split (small arrows) peroneus brevis tendon (PB) associated with a torn superior peroneal retinaculum (curved arrow) and partial lateral subluxation of the peroneus longus tendon (PL). (B) FS PD FSE axial image. (C) FS PD FSE sagittal image. (D) Corresponding color illustration of the peroneus brevis split at the level of the lateral malleolus.
|
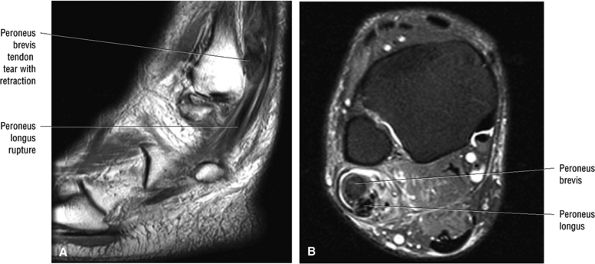 |
|
FIGURE 5.160 ● Acute rupture of both the peroneus brevis and longus tendons. Complete rupture of the peroneus brevis is associated with mechanical trauma and tendon irritation from direct abrasion against the fibular groove in cases of superior peroneal retinacular laxity of the peroneus longus. Tears of the peroneus longus may be associated with peroneus brevis tendon tears at the level of the malleolus. Up to one third of peroneus brevis ruptures are associated with peroneus longus tears. (A) Sagittal T1-weighted image. (B) Axial FS PD FSE image.
|
-
Peroneus longus tendon ruptures are associated with peroneus brevis tendon tears at the level of the malleolus.
-
Isolated peroneus longus tendon tears frequently occur in the mid foot.
-
The painful os peroneum syndrome (POPS) may be associated with a fracture or proximal displacement of the os perineum.
-
Laxity or tear of the SPR may be associated with acute or chronic dislocation of the peroneal tendons.
-
Chronic ankle instability is associated with SPR laxity.
-
Stripping of the SPR produces a pouch or potential space facilitating peroneal tendon dislocation.
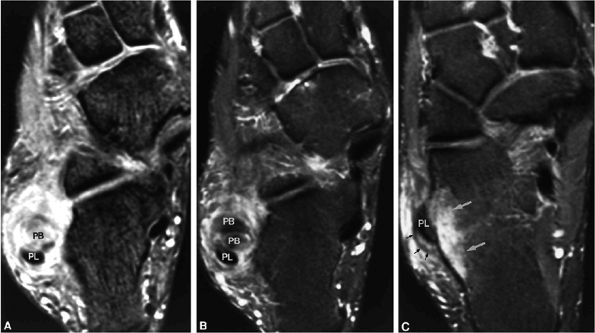 |
|
FIGURE 5.161 ● Rupture of the peroneus brevis tendon on (A) T2*-weighted and (B) FS PD FSE axial images. Note the hypointense torn fibers of the peroneus brevis tendon, which are seen more clearly on the FS PD FSE sequence (B) compared with the T2*-weighted sequence (A), which generates greater hyperintensity at the tear site. (C) Rupture of the peroneus brevis tendon is associated with avulsion of the inferior peroneal retinacular attachment (small black arrows) to the peroneal tubercle of the calcaneus. Adjacent hyperintense subchondral marrow edema is seen in the characteristic location within the peroneal tubercle (large white arrows). PL, peroneus longus tendon.
|
-
Grade I: Elevation of the retinaculum and periosteum from the lateral malleolus with pouch formation
-
Grade II: Elevation of the fibrocartilaginous ridge, the retinaculum, and the periosteum
-
Grade III: Osseous avulsion associated with the fibrocartilaginous lip, retinaculum, and periosteum
-
Type I: A stripped SPR (Figs. 5.136 and 5.137)
-
Type II: A retinacular tear at the distal fibula (Fig. 5.171)
-
Type III: Osseous avulsion of the SPR
-
Type IV: An SPR tear posterior to the distal fibular attachment (Fig. 5.172)
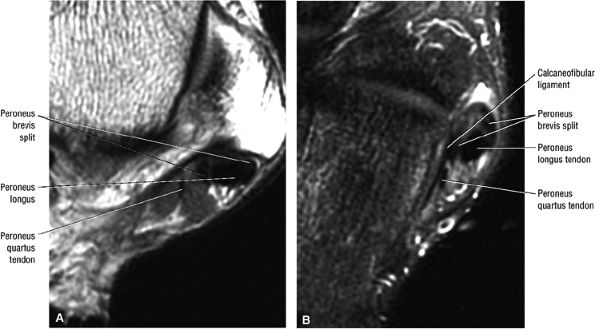 |
|
FIGURE 5.162 ● Peroneus brevis tendon split associated with a peroneus quartus muscle, which may contribute to mechanical crowding in the retromalleolar groove of the distal fibula. Axial PD FSE (A) and FS PD FSE (B) images.
|
-
Grade I: Stretching or partial tearing of ATFL fibers
-
Grade II: A moderate sprain associated with significant edema in which there is partial tearing of the ATFL with stretching of the CFL
-
Grade III: Tearing of the ATFL and CFL, causing ankle instability
useful in assessing the tibial plafond and talar dome. Subchondral and articular damage to the superior talar dome may occur with subluxation of the ankle (i.e., talar displacement in plantarflexion) secondary to ATFL interruption.
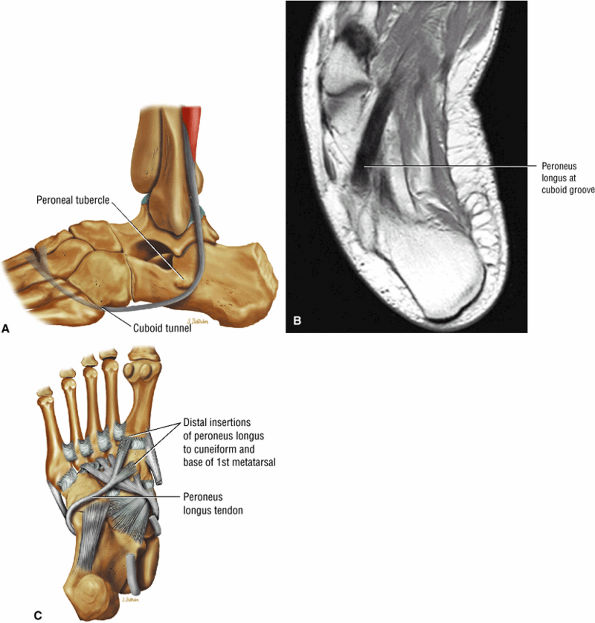 |
|
FIGURE 5.163 ● (A) Normal anatomy of the peroneus longus tendon below the peroneal tubercle and within the cuboid groove. In the plantar aspect (sole) of the foot the peroneus longus receives a second synovial sheath from the groove of the cuboid to its insertion on the base of the first metatarsal and medial cuneiform. Plantar course of the peroneus longus is shown on an axial PD FSE image (B) and a plantar perspective color illustration (C).
|
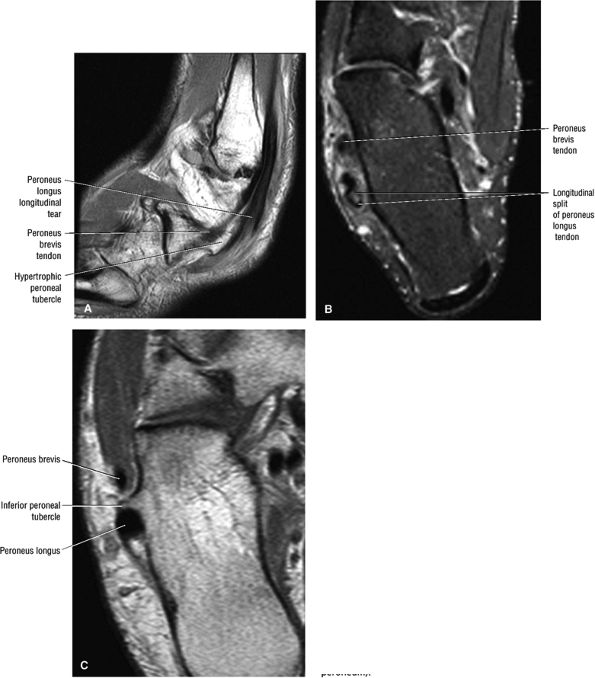 |
|
FIGURE 5.164 ● Longitudinal split of the peroneus longus tendon is visualized adjacent to a prominent peroneal tubercle on a sagittal T1-weighted image (A) and an axial FS PD FSE image (B). Two subtendons are demonstrated at the level of the calcaneus (B). (C) Axial PD FSE image in a separate case with a prominent peroneal tubercle separating the peroneus brevis and longus tendons. Chronic tears of the peroneus longus tendon may occur at the last two turns of the tendon at the level of the peroneal tubercle (seen with a hypertrophied peroneal tubercle) or around the cuboid bone (also associated with an os peroneum).
|
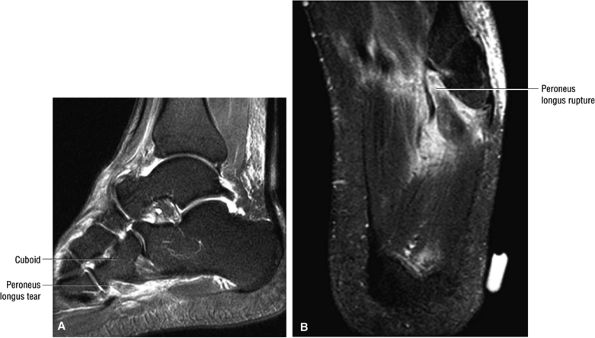 |
|
FIGURE 5.165 ● Acute midfoot tear of the peroneus longus visualized on sagittal FS PD FSE (A) and axial FS PD FSE (B) images. Acute tears of the peroneus longus occur in sports-related injuries or trauma. The midfoot is a common location for tears in older patients secondary to preexisting attritional and mechanical wear.
|
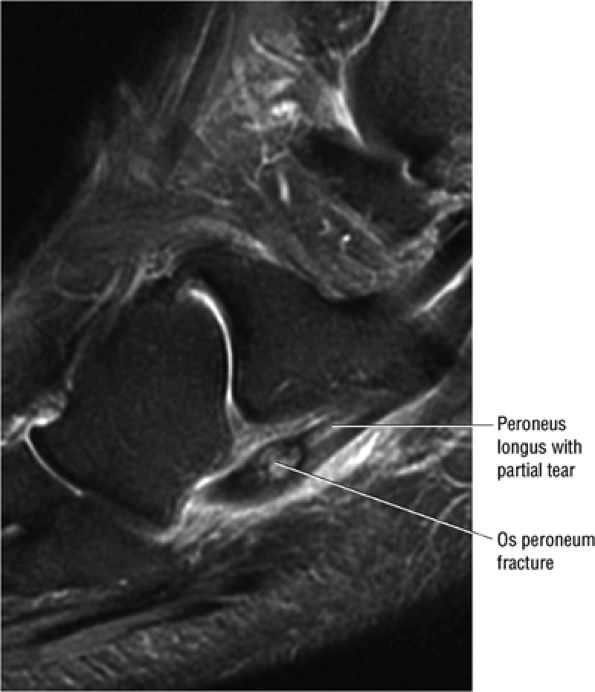 |
|
FIGURE 5.166 ● Sagittal FS PD FSE image of a partial tear of the peroneus longus tendon associated with a fractured os peroneum. Peroneus longus tears are associated with an avulsion fracture in the painful os peroneum syndrome (POPS). In a bipartite os peroneum, separation greater than 6 mm is associated with a fracture and complete tear of the peroneus longus. In a non-bipartite os peroneum, proximal displacement greater than 10 mm is associated with a peroneus longus rupture.
|
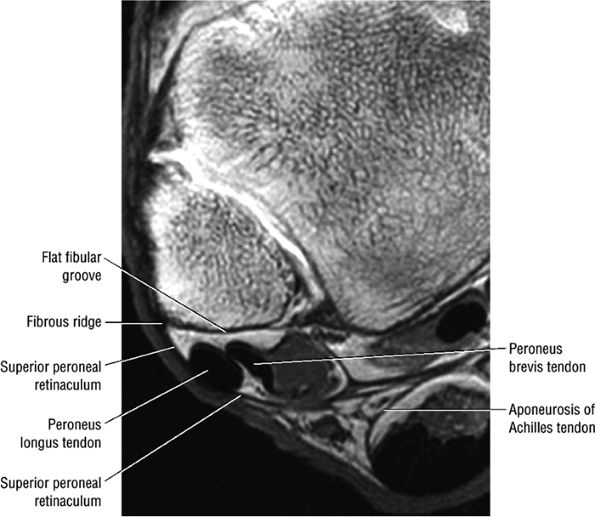 |
|
FIGURE 5.167 ● Normal superior peroneal retinaculum blending with hypointense fibrous ridge along the posterolateral aspect of the lateral malleolus. Axial T1-weighted MR arthrogram.
|
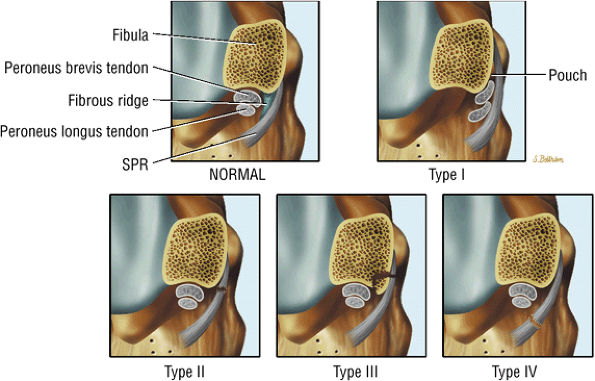 |
|
FIGURE 5.168 ● Spectrum of superior peroneal retinaculum (SPR) injuries based on Oden's surgical classification. In the normal SPR the retinaculum originates from the distal fibula and a small fibrous ridge is identified. A type I injury represents stripping of the SPR from the distal fibula and formation of a potential pouch lateral to the distal fibula. This pouch is associated with subluxation or dislocation of the peroneal tendons. In a type II injury the SPR is avulsed from its fibular insertion. In a type III injury there is an osseous avulsion from the distal fibula. Type IV injuries are associated with SPR disruption at its posterior attachment.
|
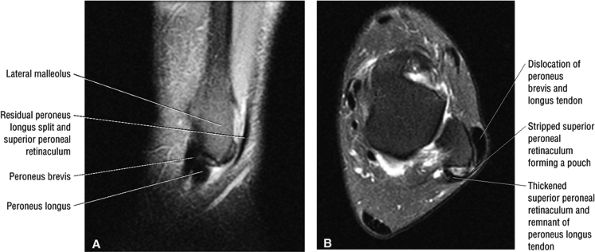 |
|
FIGURE 5.169 ● Dislocation of the peroneal tendons in a type I SPR injury with the peroneus longus and brevis tendons contained within the lateral pouch created by the stripped-off periosteum. The SPR is thickened and is associated with a remnant of the peroneus longus tendon. (A) Sagittal FS PD FSE image. (B) Axial FS PD FSE image.
|
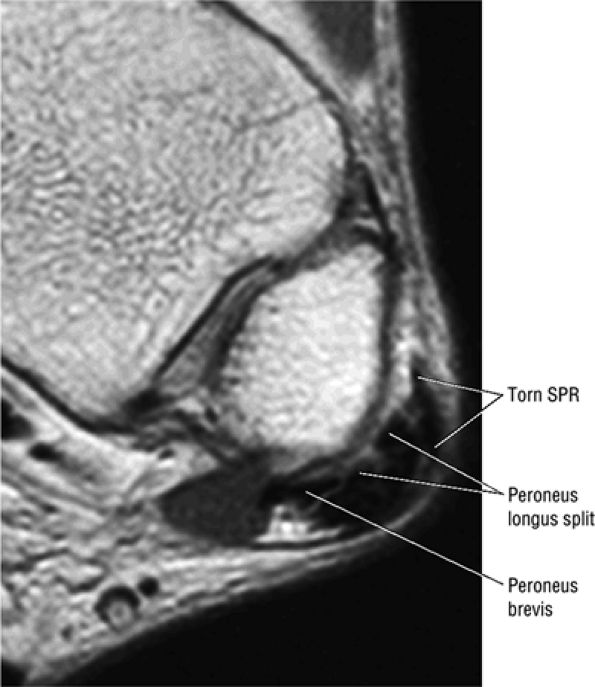 |
|
FIGURE 5.170 ● Longitudinal split of the peroneus longus tendon into two subtendons associated with dislocation of the torn peroneus longus in a type I SPR injury. Axial PD FSE image.
|
-
The ATFL is divided into two distinct bands and is taut in plantarflexion.
-
Injury is associated with inversion, internal rotation, and plantarflexion mechanisms.
-
Use T1- or PD-weighted axial imaging to appreciate increased signal intensity in a sprain with a partial tear and a corresponding FS PD FSE image to document ligament laxity, continuity, or disruption.
35 years of age, and males predominate in this age group. After 40 years of age there is a higher incidence in females. Eighty-five percent of ankle sprains occur laterally, representing 12% of emergency room trauma. A significant number (25% to 50%) of lateral sprains are associated with running and jumping sports.
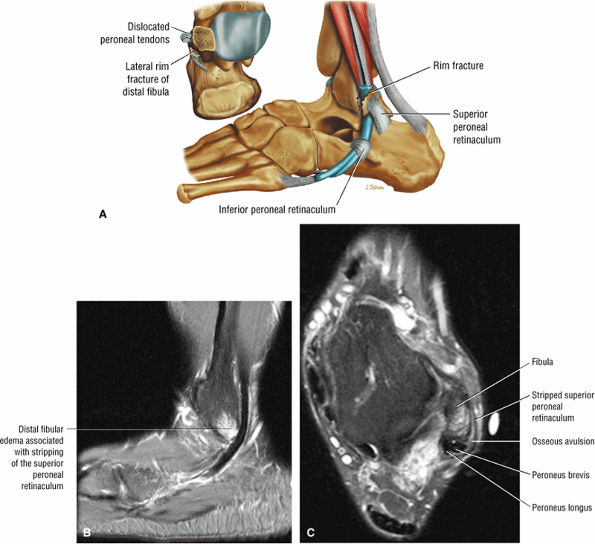 |
|
FIGURE 5.171 ● (A) A rim fracture of the lateral aspect of the lateral malleolus is associated with recurrent subluxation and dislocation of the peroneal tendons, as shown on lateral and superior view illustrations. (B, C) Type III superior peroneal retinacular injury with lateral malleolus fracture or rim sign. Sagittal (B) and axial (C) FS PD FSE images demonstrate hyperintense edema in the lateral malleolus associated with subtle osseous avulsion of the SPR attachment.
|
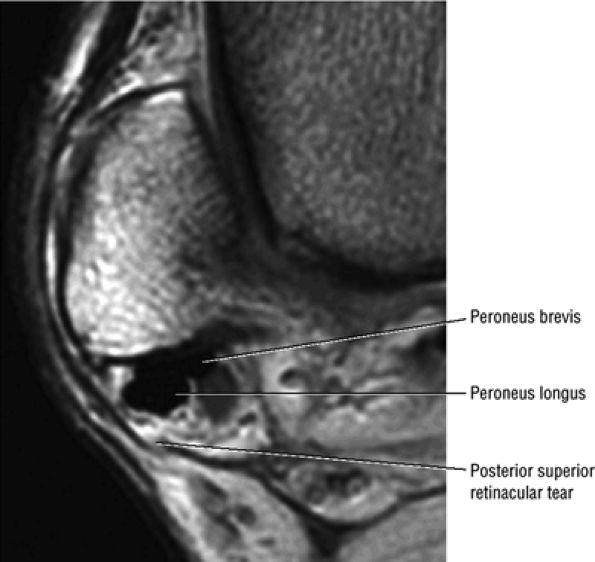 |
|
FIGURE 5.172 ● A superior retinacular tear posterior to the fibular attachment is seen posterior to the peroneal tendons, which are located in a normal position.
|
-
Grade 1: ATFL stretching
-
Grade II: Partial tear of the ATFL, with stretching of the CFL (Fig. 5.177)
-
Grade III: Complete tears of both the ATFL and CFL (Fig. 5.178)
the ATFL is seen as a prominent, low-signal-intensity, 2- to 3-mm band, oriented anteromedially and extending to its talar attachment. Axial oblique images perpendicular to the talonavicular joint may be used to demonstrate ATFL fibers more parallel with the plane of section.
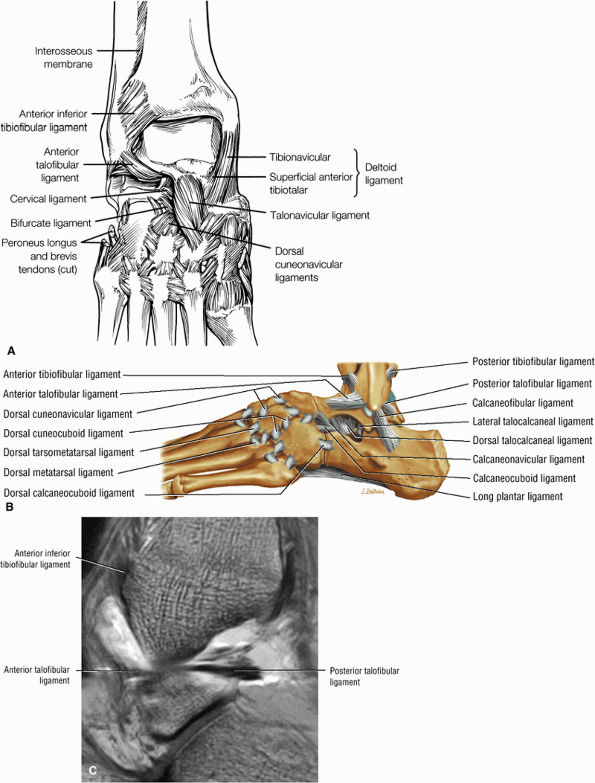 |
|
FIGURE 5.173 ● (A) Anterior and dorsal view of the medial and lateral ligaments of the ankle. (B) Lateral ligaments of the ankle in a lateral view color illustration. (C) Anterior and posterior talofibular ligaments are visualized in the sagittal plane on a sagittal T1-weighted MR arthrogram.
|
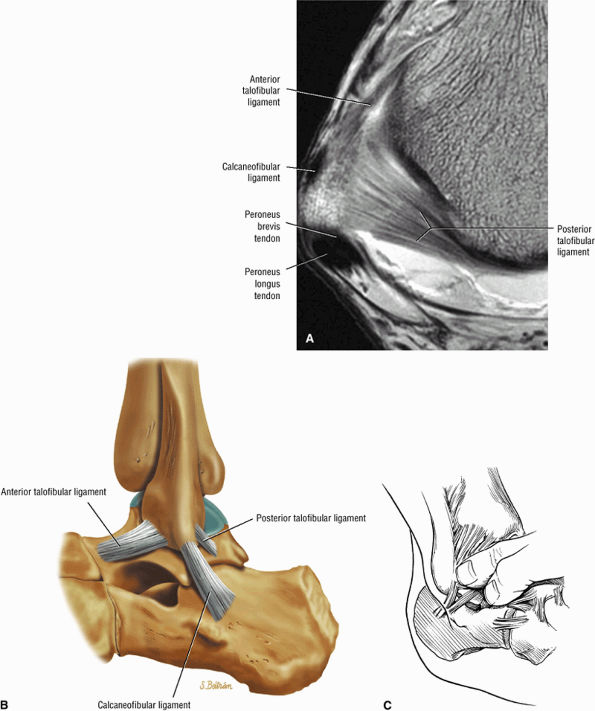 |
|
FIGURE 5.174 ● (A) Axial T1-weighted MR arthrogram showing the lateral gutter between the anterior and posterior talofibular ligaments. The posterior talofibular ligament is the strongest and most deeply seated of the lateral ligaments. It is intracapsular but extrasynovial. On MR axial images, the posterior talofibular ligament fans out at its fibular origin from the depression at the medial and posterior aspect of the lateral malleolus and converges at its insertion onto a prominent tubercle on the posterior surface of the talus. The anterior talofibular ligament may be separated into two distinct bands. (B) The medial and lateral boundaries of the lateral gutter consist of the talus medially and the fibula laterally. (C) Differentiating pain in the lateral gutter from subtalar pain is done by careful palpation. Selective injections can also be helpful in distinguishing the area of maximum pain.
|
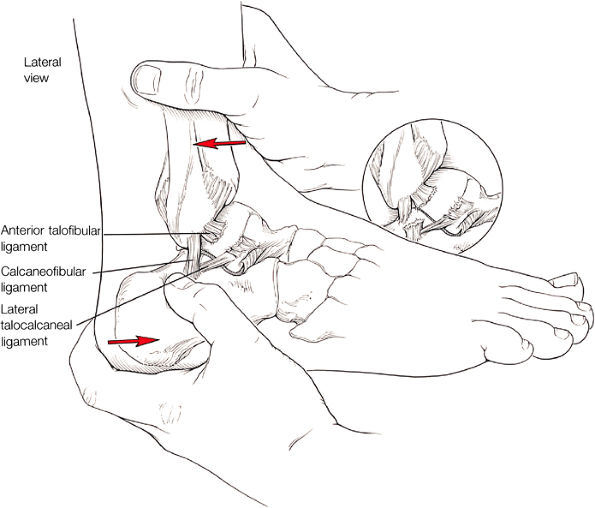 |
|
FIGURE 5.175 ● Anterior drawer test. With the tibia secured, the heel is pulled forward and internally rotated. Note the subluxation of the talus anteriorly on the distal tibia (inset). A double ligament tear leads to increased anterior excursion of the talus on the tibia.
|
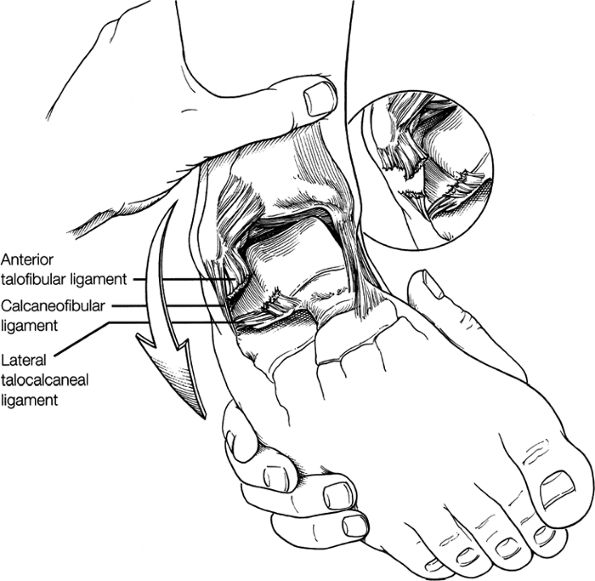 |
|
FIGURE 5.176 ● Inversion stress test. A single ligament tear allows increased talar tilt (inset). A double ligament tear leads to significant talar tilt with inversion stress.
|
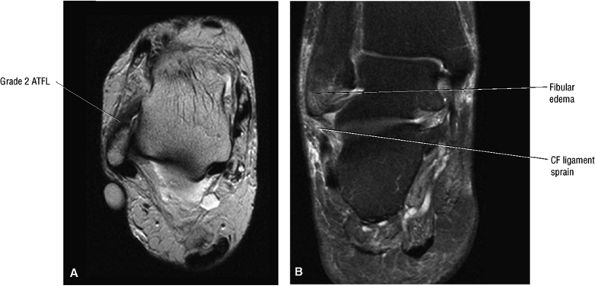 |
|
FIGURE 5.177 ● (A) Grade 2 ATFL sprain with thickened ligament and poorly defined margins on an axial T2-weighted FSE image. (B) Associated lateral malleolus edema and calcaneofibular sprain are demonstrated on a coronal FS PD FSE image.
|
-
A change from hypointense to intermediate signal intensity on T1- or PD-weighted images (Fig. 5.179) and hyperintensity on FS PD FSE images along the course of the tendon at a 45° angle from the lateral malleolus to the talus
-
Localized high-signal-intensity fluid or hemorrhage on FS PD FSE or T2*-weighted images79
-
Extension of hyperintense fluid anterolaterally into soft tissues
-
Blurring of the anterior and posterior ligament margins
-
Thickening (Fig. 5.180) or absence of the ligament in acute injury
-
Subacute to chronic residual thickening in a healed and scarred ligament (Fig. 5.181)
-
Chronic instability associated with attenuated or hypoplastic ligament with sharper, more defined ligament margins
-
Ligament hyperplasia secondary to reinjury with continued stress
-
Avulsed ligament with or without distal fibula avulsion fracture
-
Associated capsular rupture
-
Synovial inflammation
-
Inhomogeneity and hemosiderin associated with hemorrhage
-
Soft-tissue edema adjacent to anterolateral gutter
-
Bone marrow edema at the distal fibular or talar insertion
-
Fluid in tibiotalar effusion and adjacent tendon sheaths (peronea l tendons)
-
The CFL is crossed superficially by the peroneal tendons and stabilizes both the tibiotalar and subtalar joints.
-
CFL sprains and complete tears are seen with ATFL injuries.
-
CFL injuries are associated with subtalar injuries and sinus tarsi ligament pathology.
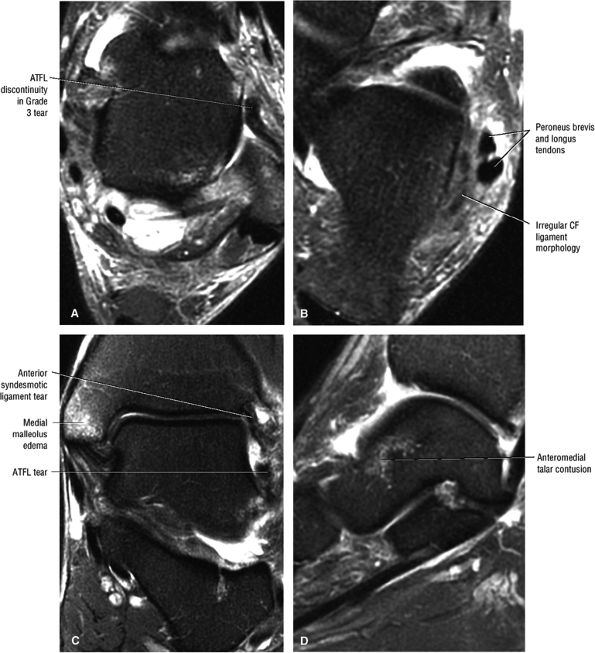 |
|
FIGURE 5.178 ● Grade 3 ATFL tear (A) distally associated with CF ligament disruption (B) on FS PD FSE axial images. Associ-ated medial malleolus edema (C) may occur with a contusion of the anteromedial talus (D) in a combined lateral ligament injury with inversion and dorsiflexion. A posteromedial talar contusion may also be seen in a combined inversion and plantarflexion injury. (C) Coronal FS PD FSE image. (D) Sagittal FS PD FSE image.
|
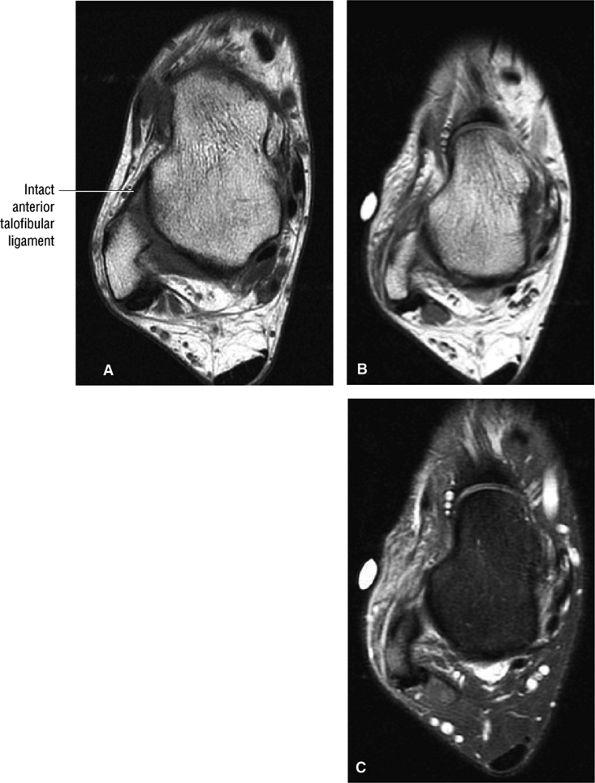 |
|
FIGURE 5.179 ● (A) Normal hypointense anterior talofibular ligament. The anterior talofibular ligament is closely related to the capsule of the talofibular joint and becomes taut in plantarflexion as it braces the body of the talus. Grade 2-3 ATFL sprain with the ATFL is intermediate in signal on the PD-weighted axial image (B) and abnormally thickened and lax on the FS PD FSE image (C).
|
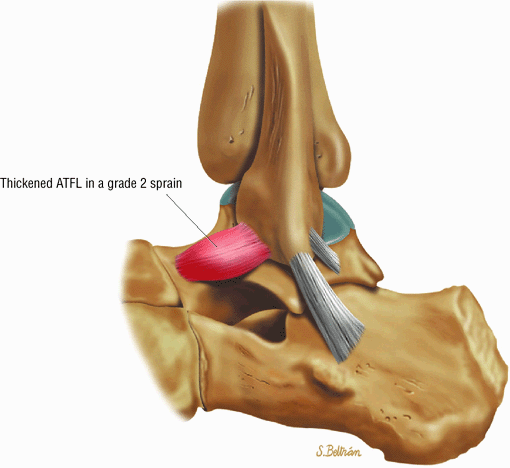 |
|
FIGURE 5.180 ● Thickened ATFL ligament resulting from a grade 2 sprain. Lateral view color illustration.
|
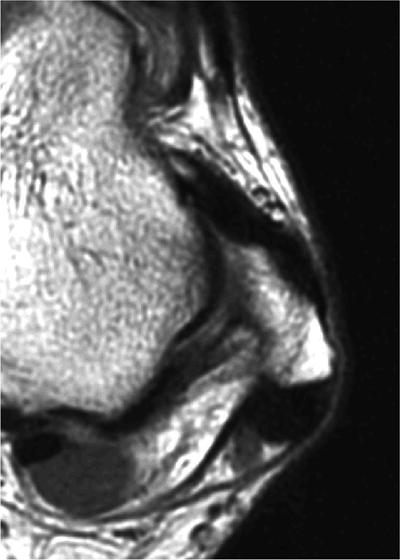 |
|
FIGURE 5.181 ● Chronically thickened ATFL as a sequela of a previous ligament tear.
|
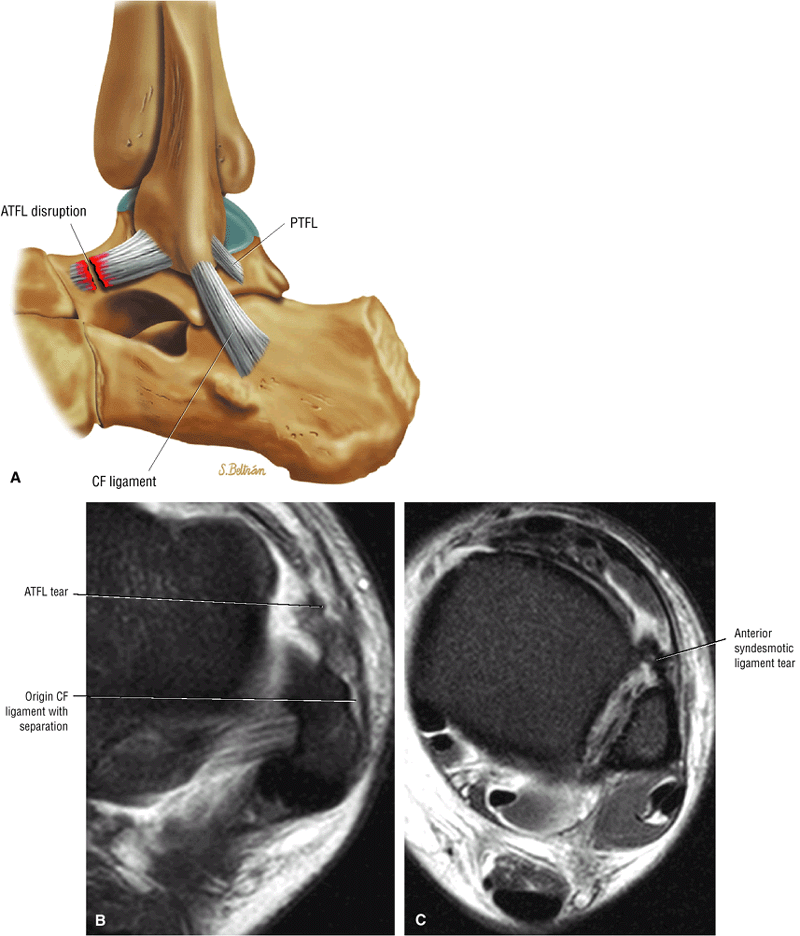 |
|
FIGURE 5.182 ● (A) Acute disruption of the ATFL from its talar insertion. Normally the ATFL inserts anterior to the lateral articular facet of the talus. Axial FS PD FSE images showing a complete distal ATFL tear with wavy ligament morphology associated with a high ankle ligament sprain and CF ligament tear (B) and anterior syndesmotic ligament injury (C).
|
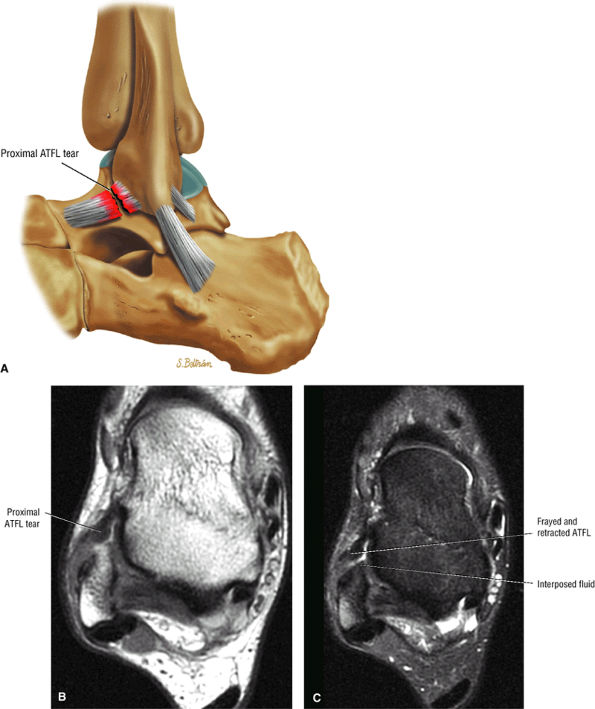 |
|
FIGURE 5.183 ● Acute ATFL disruption from the anterior border of the lateral malleolus. The torn proximal end of the ATFL is wavy and frayed. Fluid is interposed between the ligament and lateral malleolus. (A) Color lateral illustration. (B) Axial PD FSE image. (C) Axial FS PD FSE image.
|
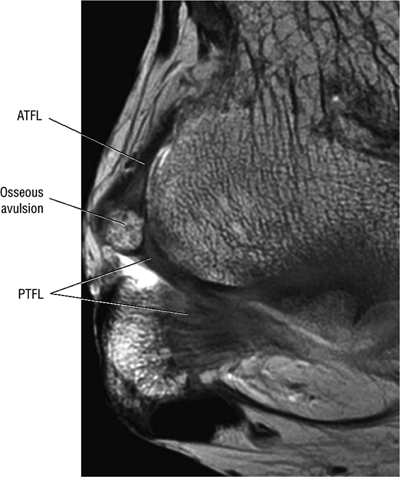 |
|
FIGURE 5.184 ● Chronic osseous avulsion from the lateral malleolus with the ATFL fibers and deep fibers of the PTFL attached to the displaced fragment. These avulsions would be interpreted as a loose body in the lateral gutter on conventional radiography.
|
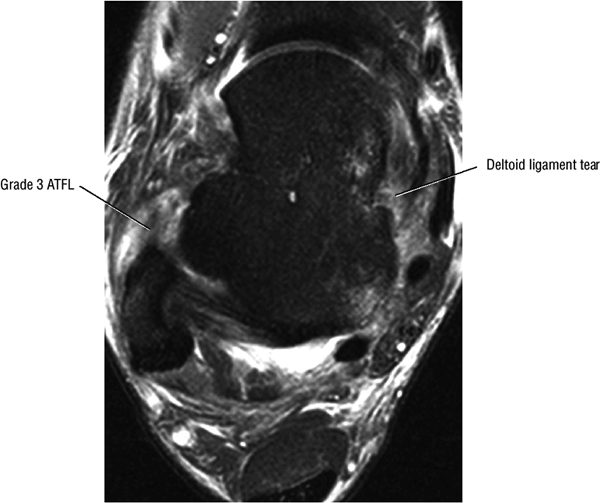 |
|
FIGURE 5.185 ● Association of deltoid ligament sprain with an ATFL tear. Axial FS PD FSE image.
|
-
Localized edema
-
Peroneal retinacular thickening
-
Tenosynovitis
-
Tendon subluxation
-
Segmental interruption or absence of the ligament
-
Edema surrounding disrupted or irregular ligament morphology between the calcaneus and peroneal tendons (Fig. 5.187)
-
Blurring of the medial and lateral margins of the ligament
-
Tenosynovitis
-
An associated tear of the peroneal tendon sheath
-
Attenuation of the ligament with adjacent edema or hemorrhage
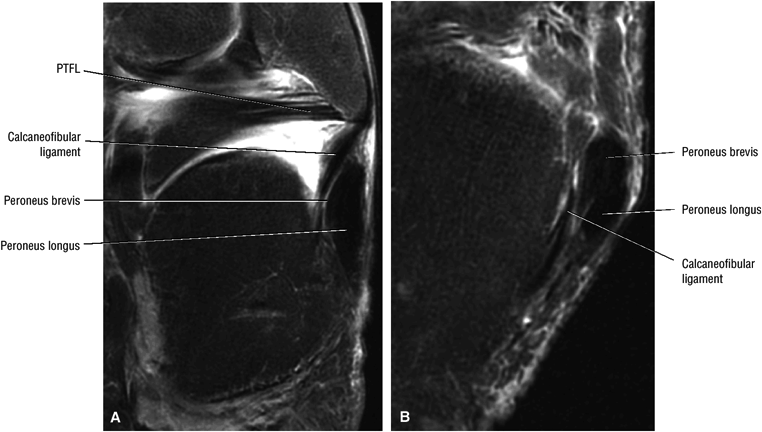 |
|
FIGURE 5.186 ● (A) The calcaneofibular (CF) ligament is a strong cord-like ligament originating from the lower segment of the anterior border of the lateral malleolus and coursing inferiorly and slightly posteriorly to its insertion on the upper part of the lateral surface of the calcaneus. Coronal FS PD FSE image. (B) The CF ligament is crossed superficially by the peroneal tendons and their sheaths, which may leave an imprint on the ligament. Axial FS PD FSE image.
|
-
Evans reconstruction (Fig. 5.189): Transposition of the entire peroneus brevis through the distal fibula
-
Watson-Jones reconstruction (Fig. 5.190): Peroneus brevis tenodesis replaces the ATFL without anatomic replacement of the CFL.
-
Modified Watson-Jones reconstruction: A split peroneus longus or brevis tenodesis, a plantaris tenodesis or graft, and a split Achilles tenodesis
-
Elmslie-fascia lata graft: Used to replace or augment the ATFL and the CFL (more anatomic than modified Watson-Jones)
-
Chrisman and Snook repair (Fig. 5.191): A split peroneus brevis tendon is used instead of fascia lata to replace the ATFL and CFL (more anatomic than modified Watson-Jones).
-
Modified Bröstrom procedure: The modified Bröstrom direct repair with imbrication technique repairs and reinforces the ATFL without sacrificing another musculotendinous unit. Tendon augmentation is achieved using the extensor retinaculum. This technique is particularly useful in athletes and dancers.
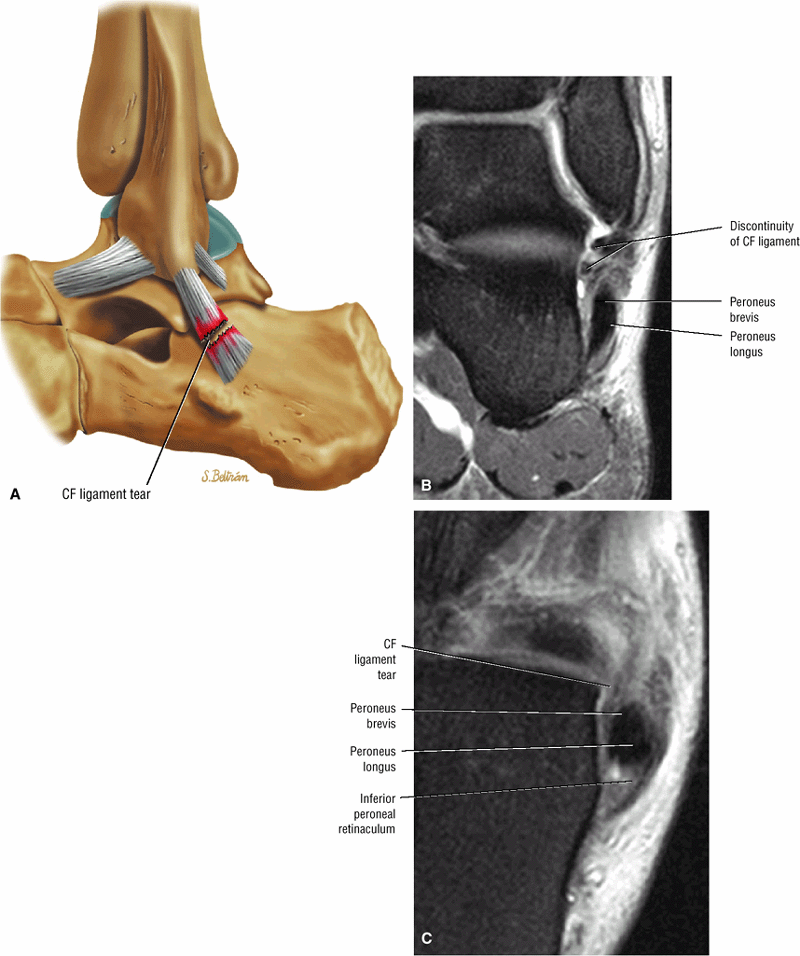 |
|
FIGURE 5.187 ● Calcaneofibular (CF) ligament disruption on a lateral color illustration (A) and coronal (B) and axial (C) FS PD FSE images. CF injuries may be associated with fluid distending the peroneal tendon sheath as a secondary sign. Rupture of the CF ligament does not occur as an isolated finding, and the CF is the second ligament injured in association with the ATFL.
|
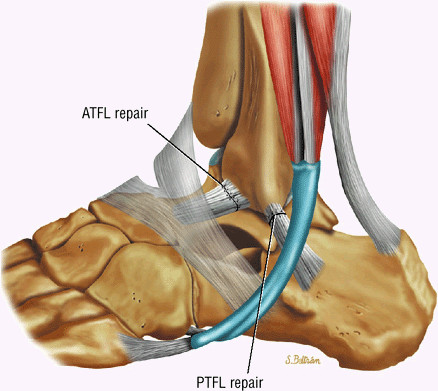 |
|
FIGURE 5.188 ● Primary surgical repair of acutely torn ligaments is usually reserved for high-performance athletes.
|
-
The thick deltoid or medial collateral ligament consists of two sets of fibers, superficial and deep.
-
Up to 10% of deltoid injuries are associated with syndesmotic ligament pathology.
-
Partial tears are more common than grade 3 ligamentous disruptions.
(talotibial) ligament are seen with medial malleolus fractures. Partial tears are more common than complete disruption of fiber morphology with an absent ligament. Hemorrhage frequently accompanies the injury, and there is increased valgus talar tilt. Acute injuries are characterized by a hyperplastic synovial reaction and hemosiderin. Chronic injuries are recognized by fibrous ingrowth or scarring (Fig. 5.196).
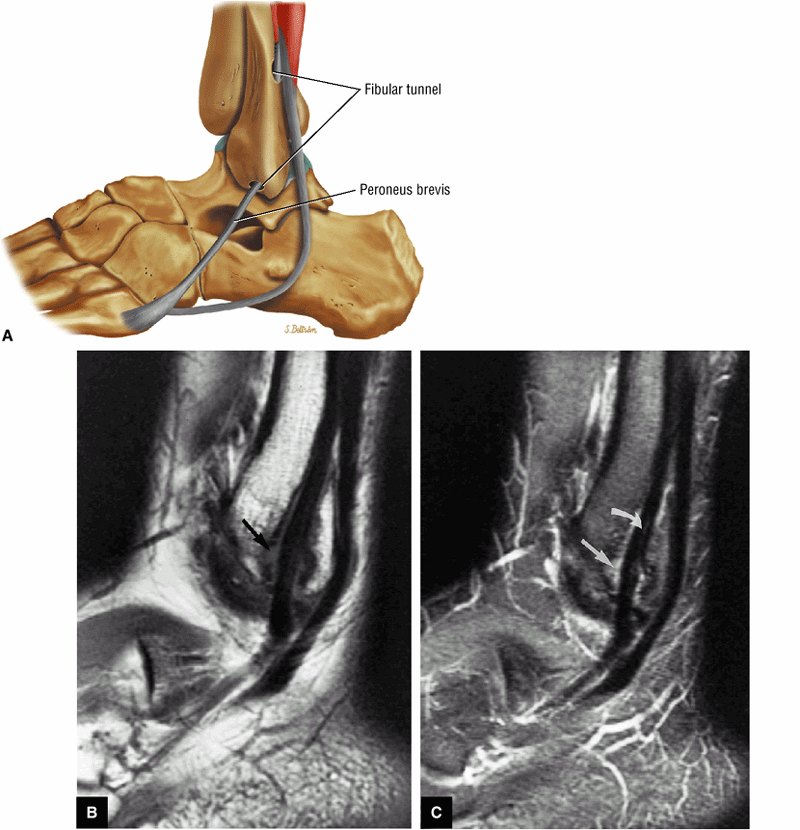 |
|
FIGURE 5.189 ● In the Evans procedure the peroneus brevis tendon is rerouted through a fibular tunnel with proximal reattachment. In a separate case, the Evans technique is used to reconstruct only the calcaneofibular ligament, shown on T1-weighted (B) and FS PD FSE (C) sagittal images. The peroneus brevis tendon is divided, mobilized, and placed in a fibular tunnel. Erosion of the bony tunnel, fluid (straight arrow), and a longitudinal tear of the peroneus brevis tendon within the tunnel (curved arrow) are demonstrated in this symptomatic patient.
|
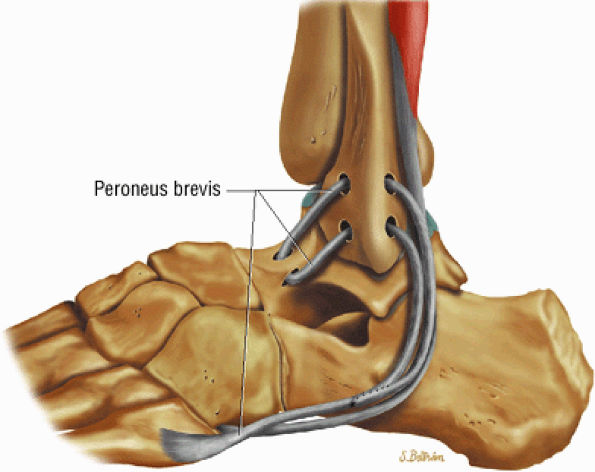 |
|
FIGURE 5.190 ● Watson-Jones procedure with rerouting of the peroneus brevis tendon through fibular and talar tunnels. Reattachment is shown distally (distal to the lateral malleolus).
|
-
Grade I: Stretch injuries (Fig. 5.197)
-
Grade II: Partial tearing (Fig. 5.198)
-
Grade III: Complete ligamentous disruption (Fig. 5.199)
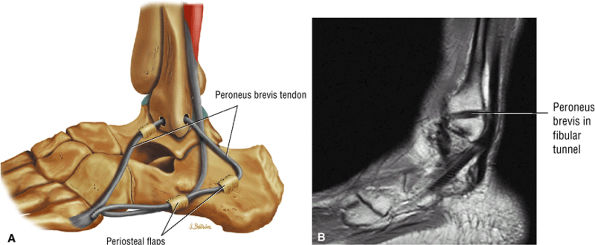 |
|
FIGURE 5.191 ● (A) Lateral color illustration of the Chrisman-Snook procedure with the split peroneus brevis tendon coursing through a fibular tunnel and secured to the talus and calcaneus with the use of periosteal flaps. (B) Corresponding sagittal PD-weighted image demonstrates the mobilized peroneus brevis at the level of the fibular tunnel.
|
stress positioning of the ankle may be effective in showing rupture, thinning, and lengthening of the ligament.
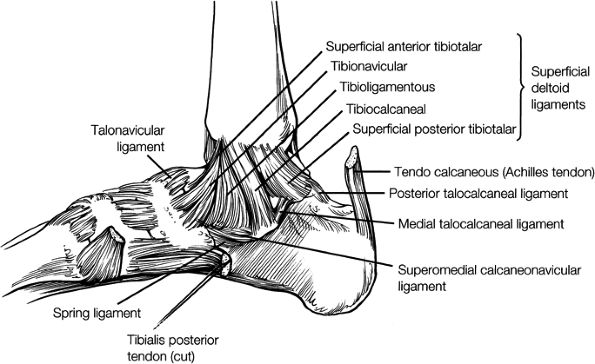 |
|
FIGURE 5.192 ● Medial ligaments of the hindfoot. The fibers of the deltoid ligament provide stability to the medial aspect of the ankle.
|
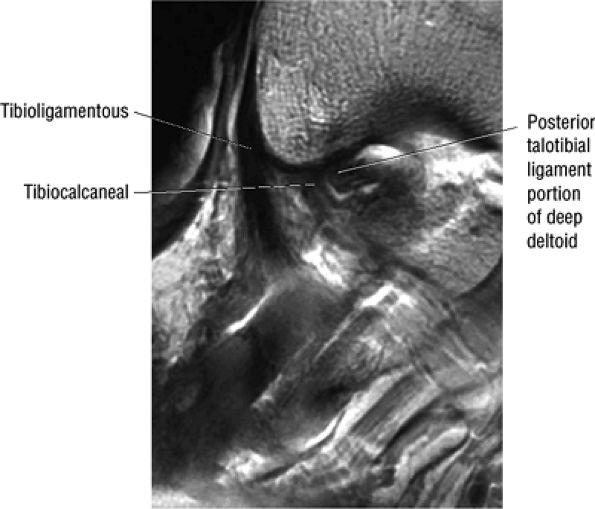 |
|
FIGURE 5.193 ● Superficial deltoid ligament with the tibioligamentous and tibiocalcaneal components identified. The posterior talotibial component of the deep deltoid is also shown. The thick deltoid or medial collateral ligament consists of two sets of fibers, superficial and deep. The superficial fibers are broad and triangular and fan out as an extra-articular sheet from the medial malleolus to the navicular, talus, spring ligament, and calcaneus. Sagittal T1-weighted MR arthrogram.
|
-
Interstitial intermediate signal intensity on T1- or PD-weighted images and diffuse amorphous hyperintensity on FS PD FSE images
-
Associated medial malleolus fractures
-
Medial malleolus subchondral edema
-
Marrow containing ligament ossification
-
Lateral or posterolateral talar displacement
-
Osseous degenerative change in the medial malleolar talar articulation
-
Indistinct margins and loss of fiber striation, especially of talotibial (tibiotalar) fibers of deep layer
-
A mass-like morphology in disruption with associated edema, hemorrhage, and granulation tissue
-
A fluid-filled gap in complete disruption (Fig. 5.200)
-
Fullness in the region of the retromalleolar tendons is secondary to chronic irritation and inflammation.
-
A widened ankle mortise is more common with deltoid injuries with preexisting lateral complex injuries.
-
There may be alignment and translation deformities.
-
Chronic medial ankle pain and instability are usual findings.
-
The tendon tends to heal in the lengthened position.
-
Deltoid ossification is not uncommon.
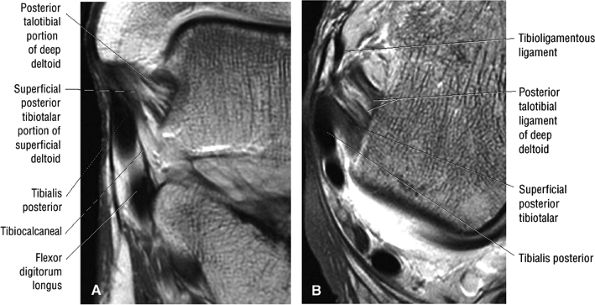 |
|
FIGURE 5.194 ● The posterior talotibial portion of the deep deltoid is shown on a coronal T1-weighted image (A) and an axial T1-weighted MR arthrogram (B). The deep layer of the deltoid ligament consists of a small anterior component, the anterior talotibial ligament, and a strong posterior talotibial ligament.
|
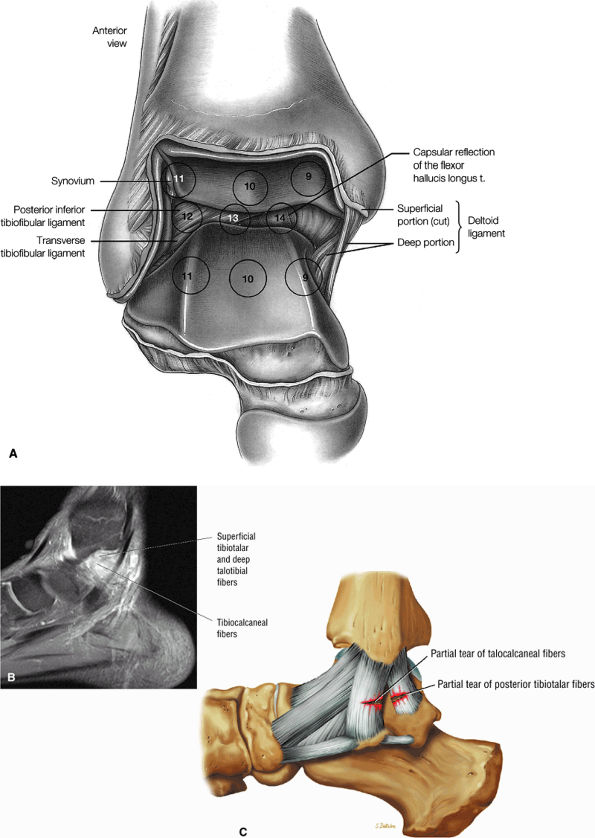 |
|
FIGURE 5.195 ● (A) Superficial (cut) and deep portions of the deltoid as displayed on central arthroscopic examination of the ankle. (9) medial dome talus; (10) central portion of the tibia; (11) articulation of the lateral dome with the distal tibia and fibula and the syndesmotic articulation; (12) posterior inferior tibiofibular ligament; (13) transverse tibiofibular ligament; (14) capsular reflection of the flexor hallucis longus tendon medial to the transverse ligament. (B) Sprain of the deep posterior talotibial deltoid and superficial tibiotalar and tibiocalcaneal fibers on a sagittal FS T1-weighted image. The posterior talotibial ligament is conical, with its base superior and apex posteroinferior. The deep portion of the deltoid ligament is intra-articular and covered only by synovium. (C) Corresponding color illustration of a superficial layer sprain.
|
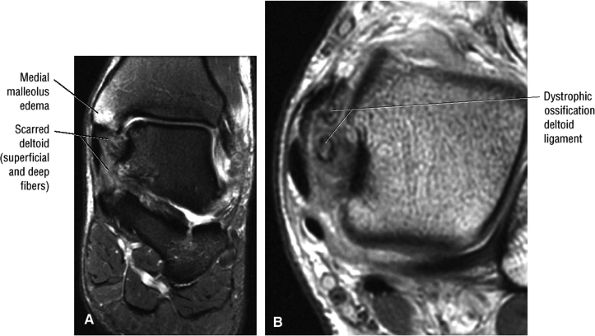 |
|
FIGURE 5.196 ● Chronic deltoid ligament sprain with ossification within the ligamentous fibers. Medial ligament injuries are associated with eversion stress injuries. The weaker tibionavicular and tibiocalcaneal ligaments are injured before the stronger tibioligamentous (tibiospring) and the posterior talotibial ligaments are torn. Avulsion fractures of the medial malleolus are seen in over 50% of eversion injuries. (A) Coronal FS PD FSE image. (B) Axial T1-weighted FSE image.
|
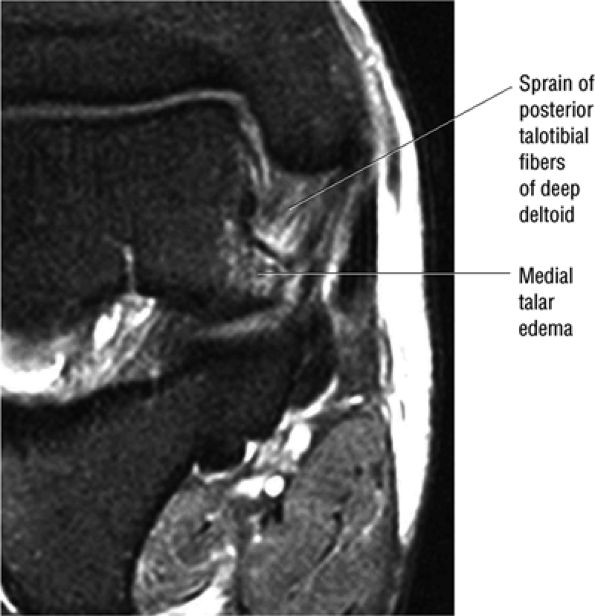 |
|
FIGURE 5.197 ● Deltoid ligament sprain without loss of ligamentous continuity or fiber striations on a coronal FS PD FSE image. The medial talar edema is a secondary sign of acute ligamentous injury.
|
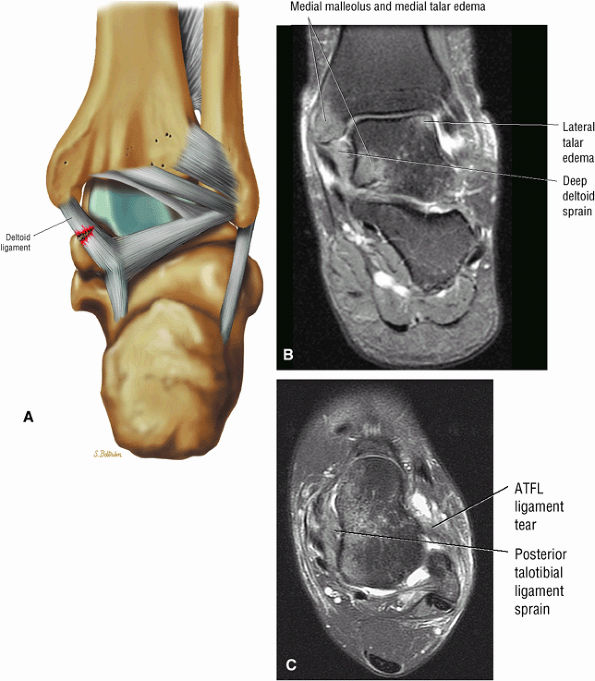 |
|
FIGURE 5.198 ● (A) Posterior view color illustration of a partial tear of the superficial and deep fibers of the deltoid ligament. The deep layer posterior talotibial ligament runs obliquely inferiorly and posteriorly to insert on the medial surface of the talus. Posterior talotibial ligament sprain is usually associated with severe lateral collateral ligament sprain. Coronal (B) and axial (C) FS PD FSE images with contusions indicating combined inversion and ankle eversion. Deltoid ligament injuries are also associated with syndesmotic injuries.
|
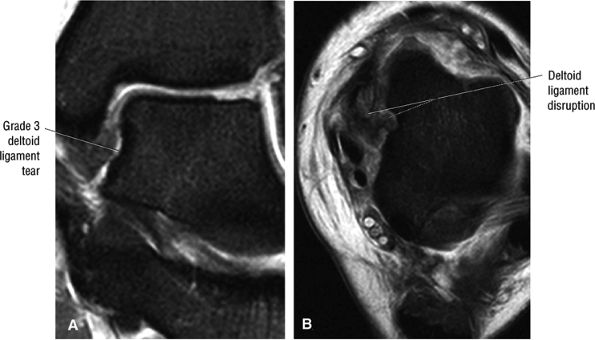 |
|
FIGURE 5.199 ● Complete tear of the deep fibers of the deltoid in a football player sustaining a high ankle sprain with associated syndesmotic and lateral ligamentous injury. (A) Coronal FS PD FSE image. (B) Axial FS PD FSE image.
|
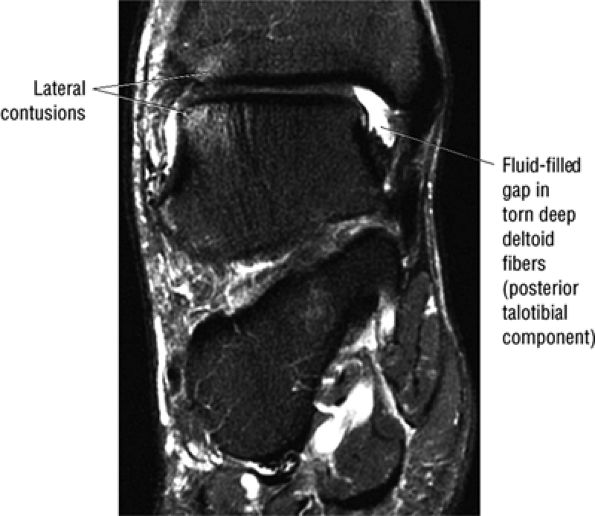 |
|
FIGURE 5.200 ● Fluid-filled gap in a tear of the deep deltoid posterior talotibial fibers. Associated lateral tibiotalar contusions are the result of an eversion mechanism. Coronal FS PD FSE image.
|
-
Syndesmotic sprains or high ankle sprains may involve the anterior inferior tibiofibular (anterior syndesmotic) ligament, the posterior inferior tibiofibular (posterior syndesmotic) ligament, the transverse tibiofibular ligament, and interosseous membrane.
-
Tearing is associated with bi- and tri-malleolar and fibular subluxation and fractures.
-
Severe syndesmosis injuries are seen with superior dislocation of the talus and an increased intermalleolar distance.
-
Axial images at the level of the distal tibial plafond are used to evaluate the anterior and posterior syndesmotic ligaments.
shape. The transverse tibiofibular ligament represents its deep and inferior component and is a true posterior labrum. The PITF also has an interosseous membrane and short fibrous bands.
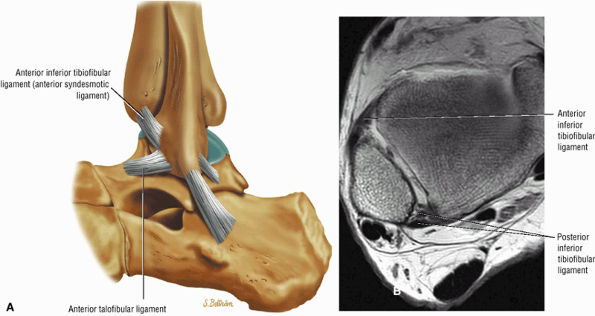 |
|
FIGURE 5.201 ● (A) Lateral color illustration of the anterior inferior tibiofibular ligament (AITF/anterior syndesmotic ligament). The AITF is a flat band of fibers that may be divided into two or three bands or may be multifascicular. It extends obliquely from the anterior inferior border of the lateral malleolus upward and medially to the anterolateral tubercle of the tibia. (B) The AITF is hypointense on this axial T1-weighted image at the level of the tibial plafond.
|
-
The oblique fibers of the AITF run from the anterior inferior lateral malleolus to the anterolateral tubercle tibia and contact the lateral ridge of the trochlear surface of the talus in plantarflexion (Fig. 5.203).
-
The PITF (Fig. 5.204) runs from the posterior lateral malleolus to the posterolateral tibial tubercle.
-
The transverse tibiofibular ligament is deep and inferior to the PITF and runs from the fibular tubercle and digital fossa to the posterior tibial articular surface and the medial border of the medial malleolus. It is a true posterior labrum, projecting below the posterior tibial margin (Fig. 5.205).
-
The tibial slip (Fig. 5.206) extends from the PTFL to the posterior tibia and transverse tibiofibular ligament.
-
The interosseous membrane extends from the medial distal fibular shaft to the lateral distal tibia and forms a vault over the tibiofibular synovial recess.
-
The 1-cm-high synovial recess contains a reddish synovial fringe that descends into the ankle joint between the fibula and the lateral talar surface as the ankle is brought into plantarflexion (Fig. 5.207).
there is palpable tenderness over the syndesmosis and pain on the squeeze test of the fibula and tibia (Fig. 5.208). There may also be mild pain over the ATFL and CFL. The leg is swollen, the ankle is unstable, and the patient cannot bear weight. Muscular splinting and reflex spasms may also be seen.
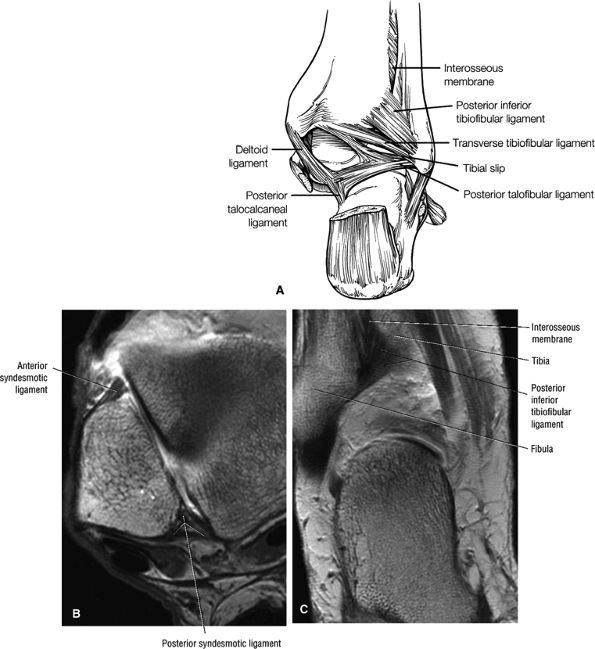 |
|
FIGURE 5.202 ● (A) The posterior ankle ligaments that make up the syndesmosis are the anterior and posterior inferior tibiofibular ligaments, the transverse tibiofibular ligament, and the interosseous ligament. (B) The posterior inferior tibiofibular ligament (PITF/posterior syndesmotic ligament) is quadrilateral in shape and smaller than its anterior counterpart. Axial T1-weighted MR arthrogram. (C) The PITF originates from the posterior border of the lateral malleolus and extends upward and medially onto the posterolateral tibial tubercle. Coronal T1-weighted image.
|
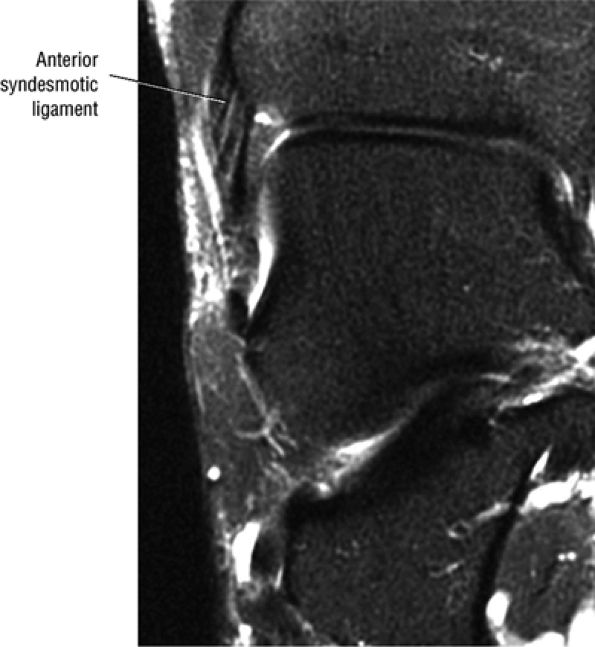 |
|
FIGURE 5.203 ● The anterior inferior tibiofibular ligament fibers increase in length from above downward. During plantarflexion they contact the lateral ridge of the trochlear surface of the talus. Approximately 20% of the ligament is seen intra-articularly via the arthroscope.
|
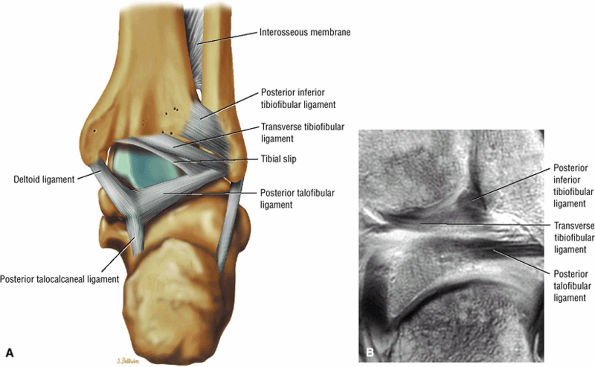 |
|
FIGURE 5.204 ● (A) The transverse tibiofibular ligament lies deep and inferior to the posterior tibiofibular ligament and is sometimes called the deep component of the posterior inferior tibiofibular ligament. It constitutes a true posterior labrum, deepening the tibial articulating surface of the talus. Posterior perspective color illustration. (B) The transverse ligament on a posterior T1-weighted coronal image. The transverse ligament fills the posterior aspect of the medial surface of the lateral malleolus and comes in contact with the articular cartilage of the posterolateral talar surface.
|
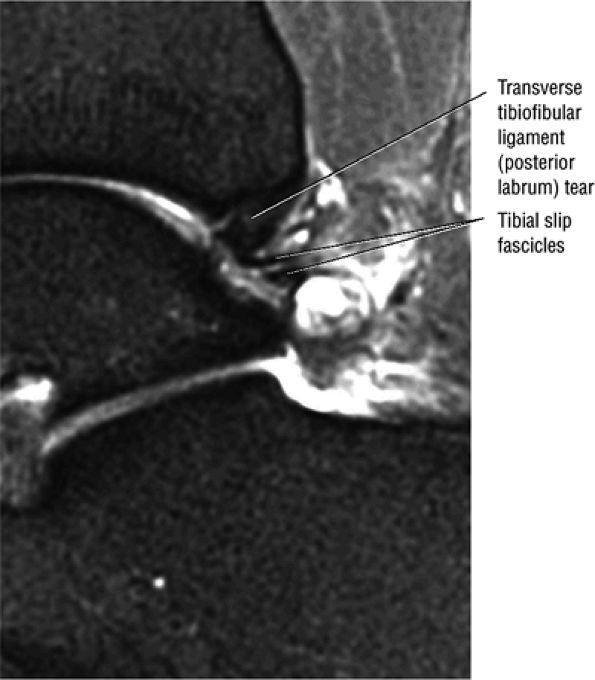 |
|
FIGURE 5.205 ● The transverse tibiofibular ligament or posterior labrum of the ankle demonstrates hypertrophy and degenerative tearing in a ballet dancer. Sagittal FS PD FSE image.
|
-
Grade I: Stretching of the ligament
-
Grade II: Partial tear
-
Grade III: Complete rupture (Fig. 5.209)
-
Blurring of the syndesmotic ligaments
-
Lateral fibular subluxation
-
Fibular shortening
-
A tibiofibular diastasis
-
Thickening and contour irregularity of syndesmotic ligaments (AITF or PITF) (see Fig. 5.209)
-
Frank discontinuity (Fig. 5.210)
-
Fluid hyperintensity and diastasis of AITF and PITF on FS PD FSE images
-
Linear interosseous membrane hyperintensity (Fig. 5.211)
-
Interosseous membrane hemosiderin, fibrosis, or calcification (Fig. 5.212).
-
Involves synovitis and fibrosis of the AITF, the anterior talofibular ligament, and the lateral gutter
-
Synovial thickening, hyalinized scar/meniscoid lesions, and chondral erosion of the lateral talar dome may all be seen.
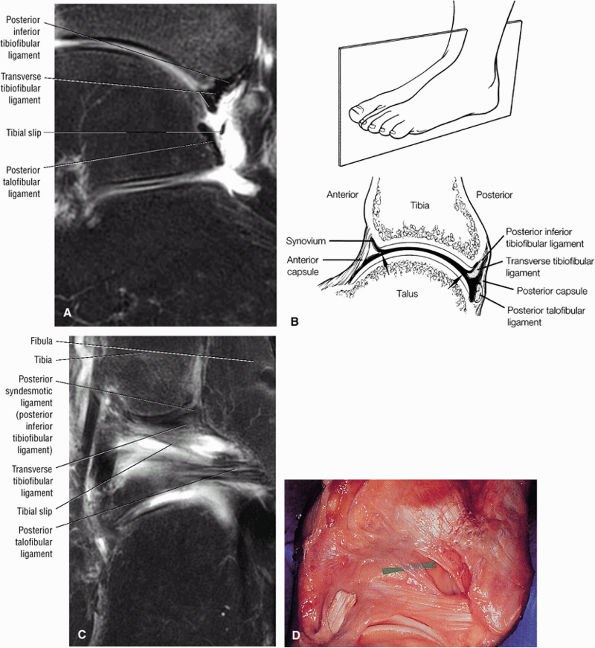 |
|
FIGURE 5.206 ● (A) The tibial slip extends from the superior border of the posterior talofibular ligament and courses medially and superiorly. It inserts on the posterior tibial margin, blending with fibers of the transverse tibiofibular ligament. During plantarflexion, the transverse tibiofibular ligament is tightly squeezed between the posterior tibial margin and the posterior talofibular ligament. During dorsiflexion, however, a synovium-lined cul-de-sac and the tibial slip become apparent. FS PD FSE image. (B) Sagittal illustration showing the relationship of the posterior ligaments. The tibial slip is not illustrated on this section. (C) The tibial slip on a posterior coronal FS PD FSE image. The insertion of the tibial slip may reach to the posterior surface of the medial malleolus and is also referred to as the posterior intermalleolar ligament. (D) The tibial slip (marker) runs from the posterior talofibular ligament and inserts on the posterior tibial margin, blending with the fibers of the transverse tibiofibular ligament.
|
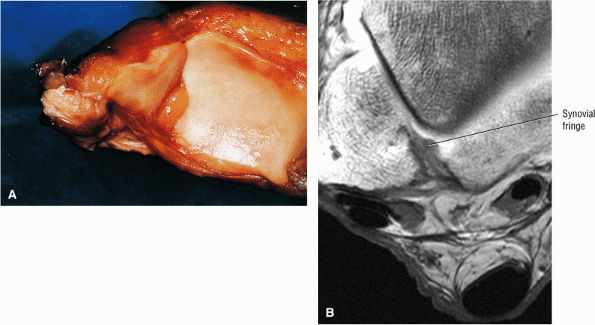 |
|
FIGURE 5.207 ● (A) Synovial fringe seen in the recess between the tibia and fibula. The tissue is usually located posteriorly, adjacent to the posterior inferior tibiofibular ligament. (B) Intermediate-signal-intensity synovial fringe on an axial T1-weighted MR arthrogram.
|
-
Signal-intensity changes in the anterolateral gutter
-
Associated AITF and ATFL injury and effacement of fat signal anterior to the AITF
-
Fluid- to intermediate-signal synovium deep to the AITF and in the anterolateral gutter (Fig. 5.216)
-
Ossicles and/or loose body in soft tissues at the tip of the fibula
-
Hyalinized mass
-
Fibrosis and scarring
-
Talar chondromalacia, indicated by a denuded chondral surface
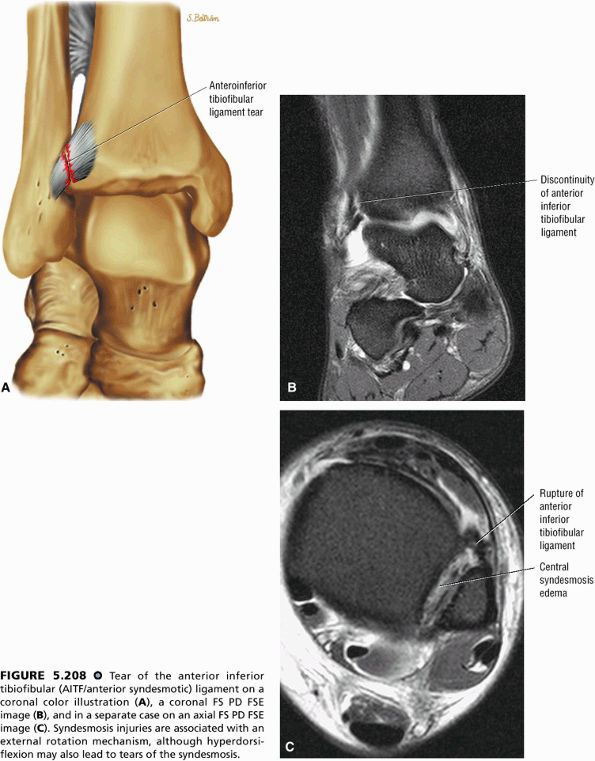 |
|
FIGURE 5.208 ● Tear of the anterior inferior tibiofibular (AITF/anterior syndesmotic) ligament on a coronal color illustration (A), a coronal FS PD FSE image (B), and in a separate case on an axial FS PD FSE image (C). Syndesmosis injuries are associated with an external rotation mechanism, although hyperdorsiflexion may also lead to tears of the syndesmosis.
|
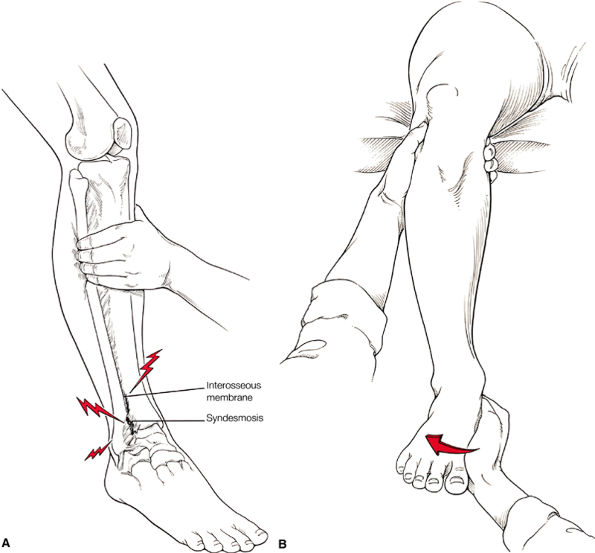 |
|
FIGURE 5.209 ● (A) The “squeeze test” is used clinically to diagnose syndesmosis injuries. By palpating directly over the syndesmosis and more proximally along the interosseous membrane, the fibula is compressed against the tibia above the midpoint of the calf. The test is positive when proximal compression produces distal pain in the area of the torn interosseous membrane and syndesmotic ligament. Anterolateral view illustration. (B) The “external rotation stress test” is also used in diagnosing syndesmotic ankle sprains. It is performed by applying external rotation stress to the foot and ankle with the knee bent in 90° of flexion and the ankle in a neutral position. The test is positive if pain is produced over the anterior or posterior inferior tibiofibular ligaments.
|
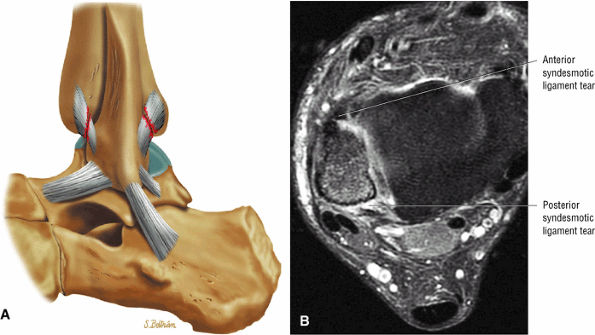 |
|
FIGURE 5.210 ● Tears of both the anterior inferior and posterior inferior tibiofibular ligaments on a lateral color illustration (A) and an axial FS PD FSE image (B). Injuries to the syndesmosis are underestimated and occur in as many as 10% of all ankle injuries.
|
-
Inflamed synovium envelops the AITF and the inferior articulation of the tibia and fibula.
-
Synovitis may involve the anterior and posterior aspects of the syndesmotic ligament.
-
There may be associated loose bodies, chondromalacia, and osteophytes.
-
Bassett's ligament is a separate distal fascicle of the AITF and may be associated with syndesmotic impingement against the lateral talus.
and the lateral malleolus is pulled superiorly when the fibula is rotated medially (Fig. 5.219).
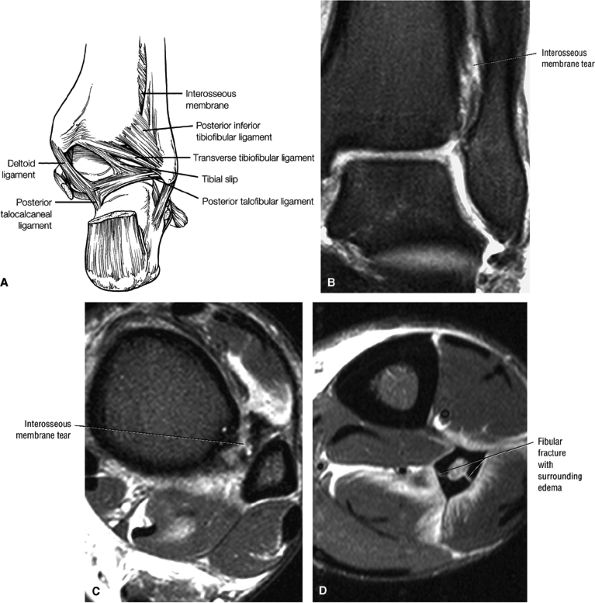 |
|
FIGURE 5.211 ● Interosseous membrane tears as part of a high ankle sprain complex and associated fibular diaphyseal fracture. The interosseous membrane fibers form a vault over the underlying tibiofibular synovial recess. On MR images this recess is approximately 1 cm high. Hyperintense fluid seen in the coronal plane extending above this recess is associated with interosseous membrane injuries in high ankle sprains. (A) Illustration of the relationship of the interosseous membrane to the posterior inferior tibiofibular ligament and other posterior ankle ligaments. (B) Coronal FS PD FSE image. (C, D) Axial FS PD FSE images.
|
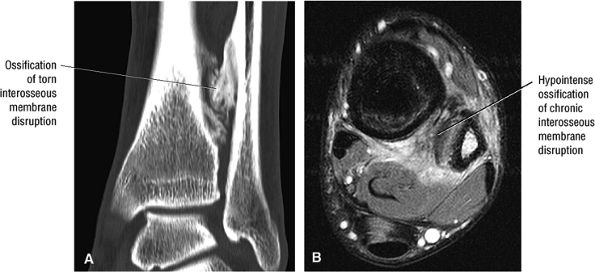 |
|
FIGURE 5.212 ● Chronic interosseous membrane tear (as a complication of a syndesmotic sprain) with ossification. Syndesmotic sprains occur in collision sports such as ice hockey, football, and soccer. (A) Coronal reformatted CT image. (B) Axial FSE PD FSE image.
|
-
Synovial inflammation
-
Synovial nodules
-
Possibly a torn AITF
-
Loose bodies
-
Osteophytes
-
Fluid in the syndesmosis (Fig. 5.221)
-
Chondromalacia of the talar dome with focal fissuring
-
Soft-tissue thickening directly involving or anterior to the AITF (Fig. 5.222)
-
Intermediate signal intensity within the distal fascicle of the AITF on FS PD FSE images
-
Scarring within the inferior articulation of the tibia and fibula (Fig. 5.223)
-
Posterior impingement can occur alone or in combination with anterolateral and syndesmosis impingement.
-
Generalized posterolateral impingement occurs with fibrosis, capsulitis, and synovial swelling involving any or a combination of the posterior ankle ligaments, including the posterior syndesmotic (inferior tibiofibular) ligament, the transverse tibiofibular ligament, the tibial slip, and the PTFL.
-
Normal variations exist in the size of the tibial slip and transverse ligament. These structures should be evaluated for hypertrophy and tears.
proximally to the PTFL distally. Although the PITF and the transverse tibiofibular ligament are primarily involved, any of the posterior ligaments may be affected, including the tibial slip and the PTFL (Fig. 5.225).90 It is seen most often in younger patients who participate in activities involving plantarflexion: female ballet dancers and gymnasts and male football players.
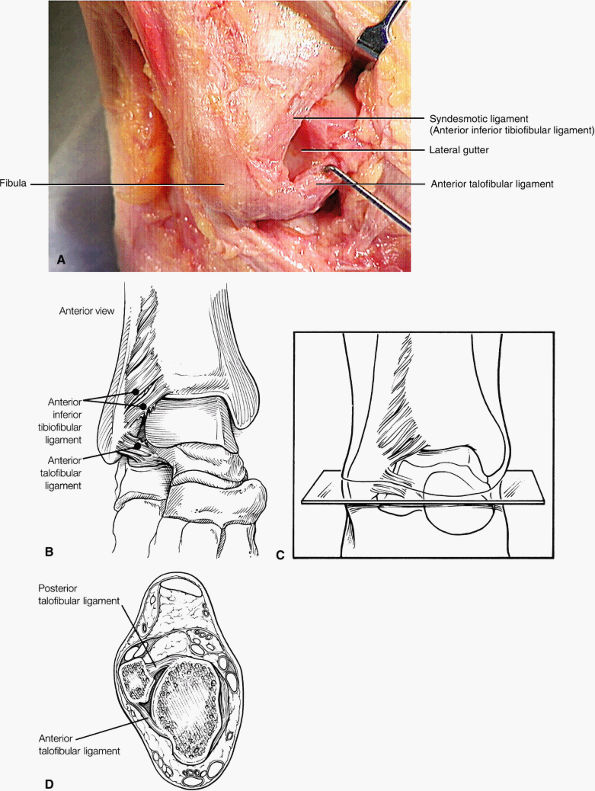 |
|
FIGURE 5.213 ● (A) Dissection with anterolateral exposure of the anterior inferior tibiofibular ligament (anterior syndesmotic ligament), the anterior talofibular ligament, and the lateral gutter. (B) The potential soft-tissue impingement sites. The accessory fascicle of the anterior inferior tibiofibular ligament can impinge across the lateral talar dome. (C) Plane of cross-section through the lateral gutter. (D) Cross-sectional illustration of the lateral gutter. The boundaries of the lateral gutter are the talus medially, the fibula laterally, and the anterior and posterior talofibular ligaments.
|
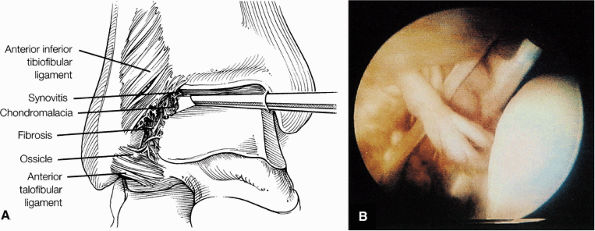 |
|
FIGURE 5.214 ● Soft-tissue impingement. (A) Arthroscopic view through the anteromedial portal shows anterolateral soft-tissue impingement with synovitis and fibrosis and chondromalacia in the anterolateral gutter. (B) Arthroscopic view of the anterolateral impingement lesion. Note the hemosiderin staining of the lateral gutter and associated scar bands and synovitis.
|
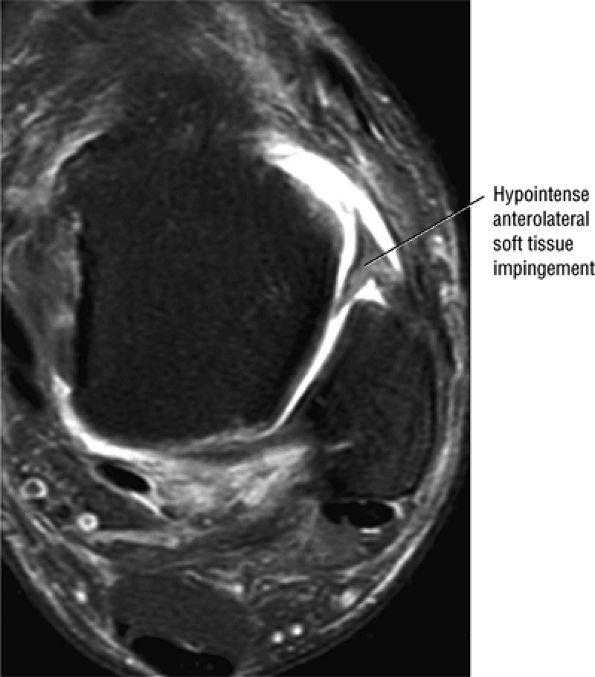 |
|
FIGURE 5.215 ● Anterolateral impingement with hypointense soft-tissue thickening in the anterolateral gutter on an axial FS PD FSE image. Anterolateral impingement of the ankle is the most common type of soft-tissue impingement because of the mechanism of most ankle sprains in inversion. The meniscoid lesion as originally described represented a massive hyalinized connective tissue extending into the joint.
|
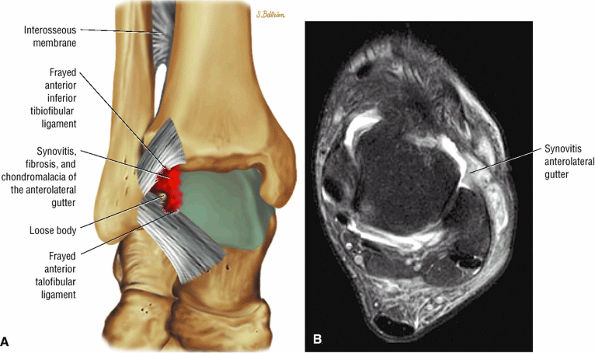 |
|
FIGURE 5.216 ● Soft-tissue anterolateral impingement on a coronal color illustration (A) and an axial FS PD FSE image (B). Pathology is usually limited to the anterior syndesmosis and the lateral gutter. Synovitis may surround the anterior inferior tibiofibular ligament (both anteriorly and posteriorly) and involve the anterior talofibular ligament as well. Fibrosis of the lateral gutter and chondromalacia of the talus and fibula may also be demonstrated.
|
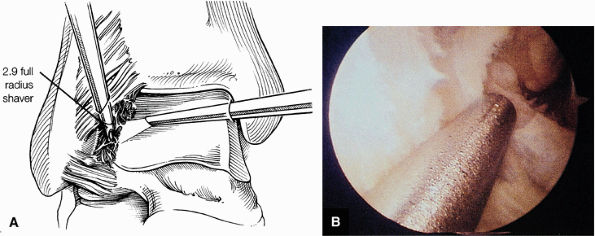 |
|
FIGURE 5.217 ● Débridement of the lateral gutter. (A) Débridement is performed with a small-joint shaver and includes removing inflamed synovium, thickened adhesive bands, osteophytes, and loose bodies. (B) Arthroscopic view through the anteromedial portal. A full-radius shaver is débriding the lateral gutter while avoiding injury to the anterior talofibular ligament.
|
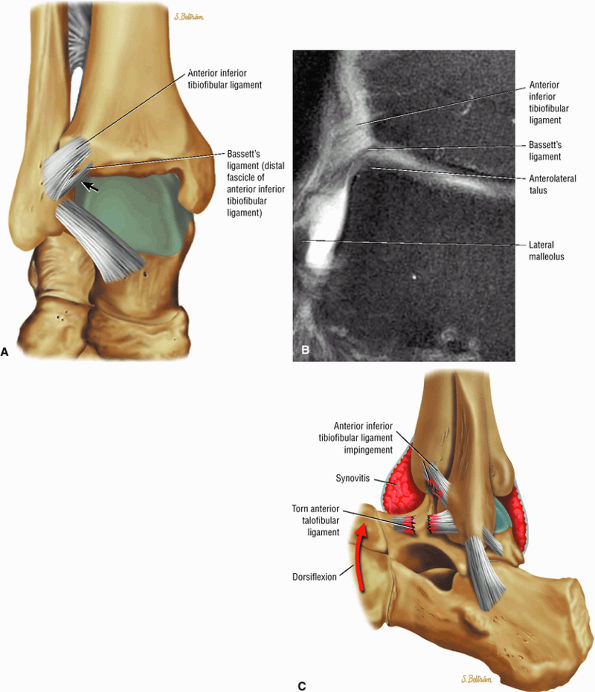 |
|
FIGURE 5.218 ● Syndesmotic impingement may occur from a separate distal fascicle (Bassett's ligament) of the anterior inferior tibiofibular ligament. A tear of the anterior talofibular ligament contributes to laxity in the lateral ankle, and the anterolateral talar dome extrudes anteriorly with dorsiflexion. Bassett's ligament then impinges against the talus. (A) Coronal color illustration. (B) Coronal FS PD FSE image. (C) Lateral view color illustration.
|
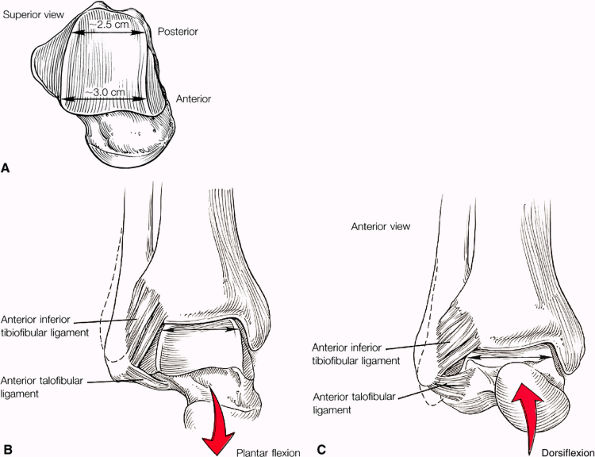 |
|
FIGURE 5.219 ● Tibiofibular joint mechanics. (A) The width of the trochlear surface of the talus is smaller posteriorly than anteriorly. (B) During plantarflexion of the ankle, the malleoli are approximated actively as the lateral malleolus is pulled inferiorly and rotated medially. (C) With dorsiflexion of the ankle, the lateral malleolus moves always from the medial malleolus and is pulled slightly superiorly as the fibula is medially rotated.
|
-
Inhomogeneous fluid and synovium surrounding the posterior ligaments (Fig. 5.227)
-
Capsular thickening
-
Fibrosis
-
Periostitis
-
Ganglion cysts protruding between posterior ligaments
-
Interstitial signal in the posterior ligaments (Fig. 5.228)
-
Gross enlargement of tibial slip or labrum (transverse ligament) (Fig. 5.229)
-
Synovial nodules
-
Anteromedial impingement is associated with pathology of the anterior fibers of the deltoid ligament.
-
Posteromedial impingement occurs as a complication of inversion injuries with injury to the deep layer of the deltoid ligament.
-
Capsular thickening
-
Thickening of the anterior component of the superficial deltoid
-
Anteromedial tibiotalar sclerosis and osteophytes (Fig. 5.231)
-
Associated lateral ligament sprains and osteochondral lesions of the talus
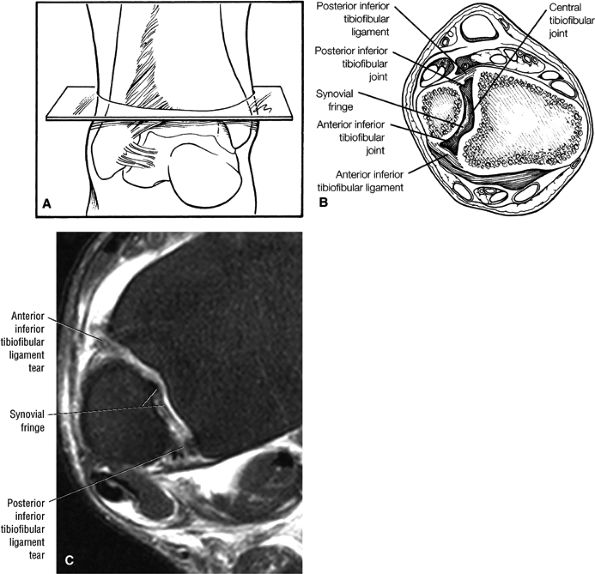 |
|
FIGURE 5.220 ● Syndesmotic impingement. (A) Plane of cross-section through the syndesmosis. (B) Cross-sectional view through the syndesmosis demonstrates that impingement can occur anteriorly, centrally, or posteriorly. (C) Corresponding axial FS PD FSE image shows potential syndesmotic impingement sites.
|
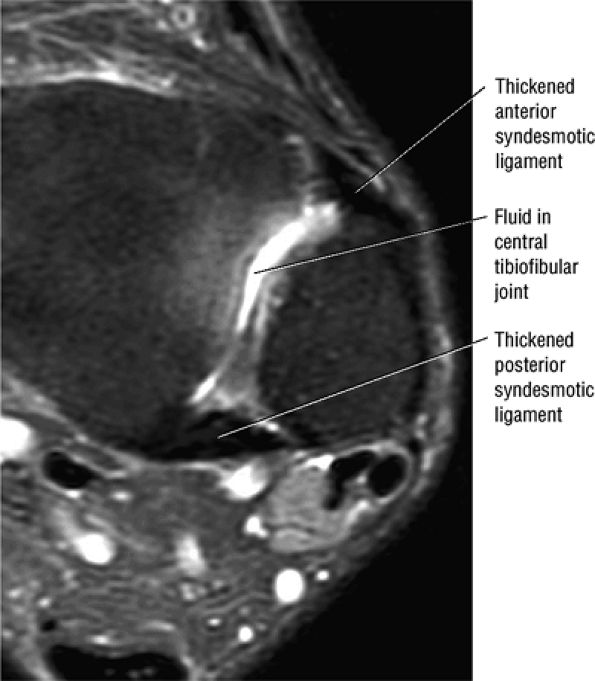 |
|
FIGURE 5.221 ● Syndesmotic impingement with scarred anterior and posterior tibiofibular (syndesmotic) ligaments as a result of a chronic inversion injury with associated ATFL rupture. There is fluid in the tibiofibular synovial recess. Axial FS PD FSE image.
|
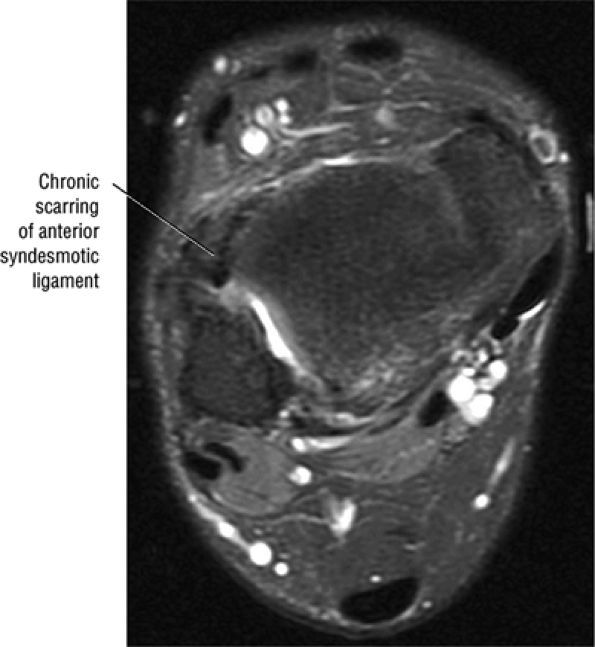 |
|
FIGURE 5.222 ● Nodular thickening in the region of the anterior syndesmotic ligament on an axial FS PD FSE image.
|
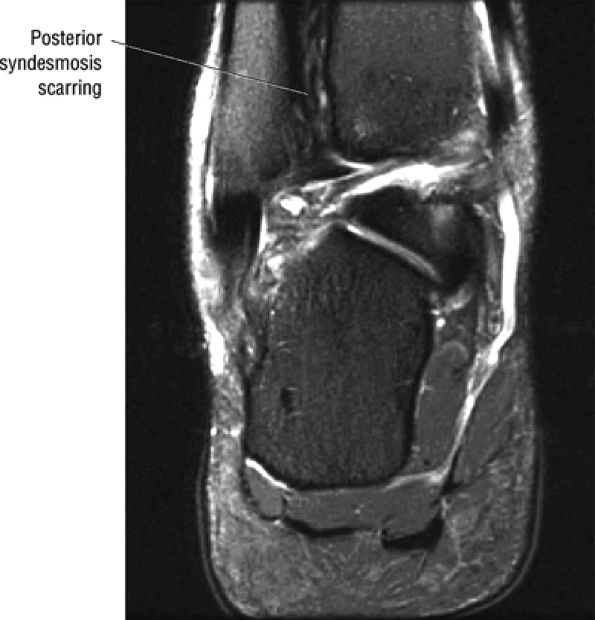 |
|
FIGURE 5.223 ● Syndesmosis scarring in a basketball player with chronic thickening of the anterior and posterior inferior tibiofibular ligaments. Coronal FS PD FSE image.
|
-
Anterior impingement represents spur formation between the anterior inferior tibial plafond and the talar neck.
-
The “door jam” effect is created by loss of dorsiflexion from opposing anterior osteophytes.
-
FS PD FSE sagittal images are used to identify tibiotalar effusion, marrow edema, fragmentation of osteophytes, and associated chondral lesions.
tibial side spur narrower than the talar spur) and, in grade 3 and 4 lesions, greater than 1 cm. Impingement occurs between the anterior surface of the tibia and the superior aspect of the neck of the talus (Fig. 5.234). Typically, the talar sulcus is seen in the superior aspect of the talar neck and the normal tibial—dorsal talar neck angle is at least 60° (Fig. 5.235). Osteophytes on the distal tibia and talar neck, however, decrease the tibiotalar angle to less than 60°.
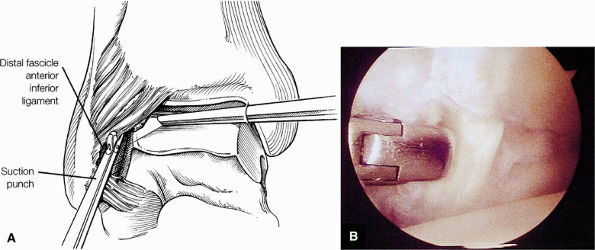 |
|
FIGURE 5.224 ● Excision of torn syndesmotic ligament. (A) Visualization is performed from the anteromedial portal, and a suction punch or small basket is brought into the joint through the anterolateral portal to remove the torn fascicle. (B) Arthroscopic view of excision of the torn syndesmotic ligament using a suction basket.
|
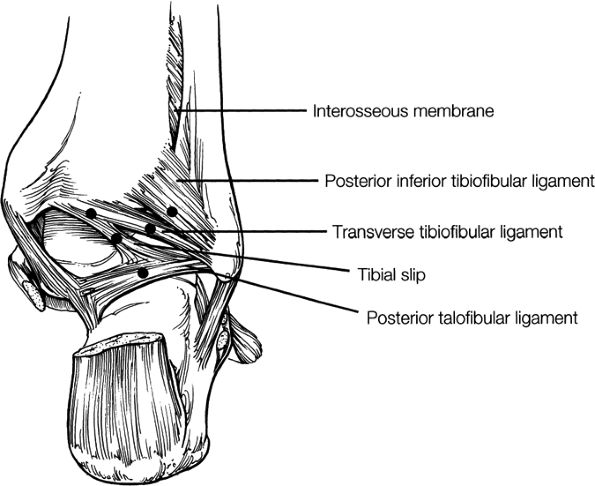 |
|
FIGURE 5.225 ● Posterior impingement sites. Note that the tibial slip can also be an area of soft-tissue impingement.
|
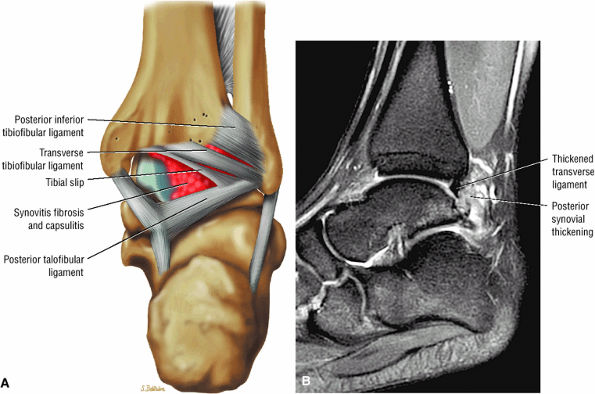 |
|
FIGURE 5.226 ● Posterior impingement in a ballet dancer with synovitis involving the posterior ligaments on color illustration with posterior perspective (A) and a sagittal FS PD FSE image (B). A thickened posterior labrum represents pathology of the transverse ligament. A pseudomeniscus of a hypertrophied tibial slip may also become symptomatic in ballet dancers.
|
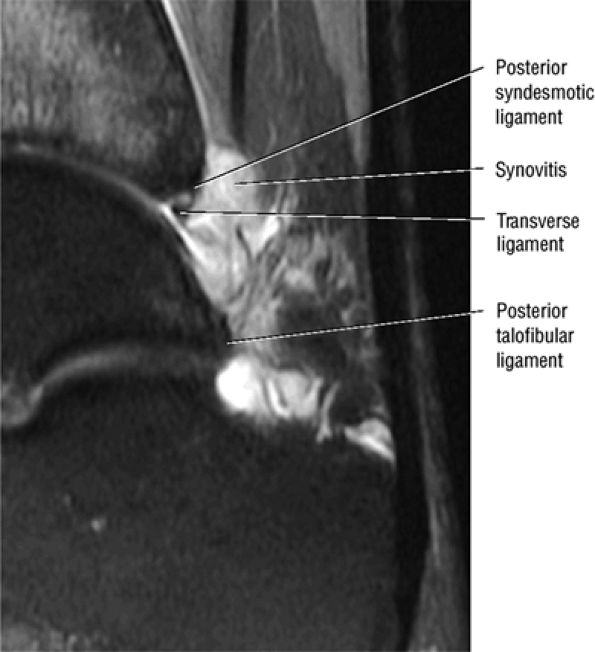 |
|
FIGURE 5.227 ● Generalized posterolateral impingement with fibrosis, capsulitis, and synovial swelling along the posterior aspect of the ankle. Synovial thickening is intermediate on this sagittal FS PD FSE image.
|
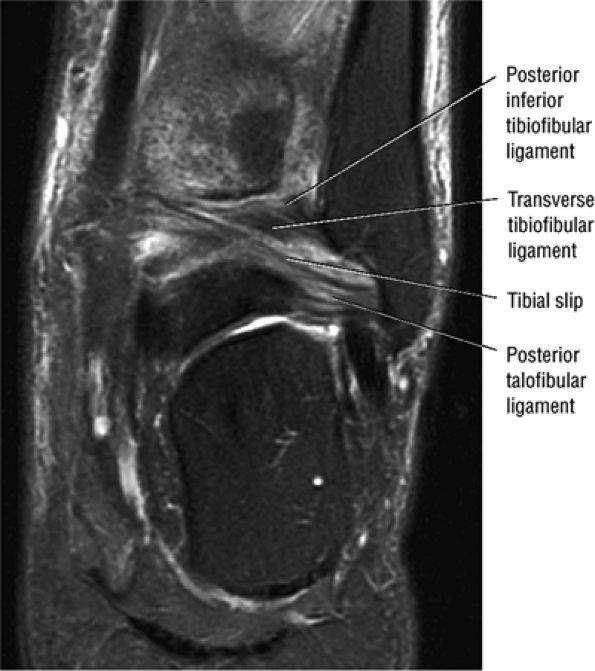 |
|
FIGURE 5.228 ● Hyperintensity of the posterior ligaments as potential sites of soft-tissue impingement on this coronal FS PD FSE image.
|
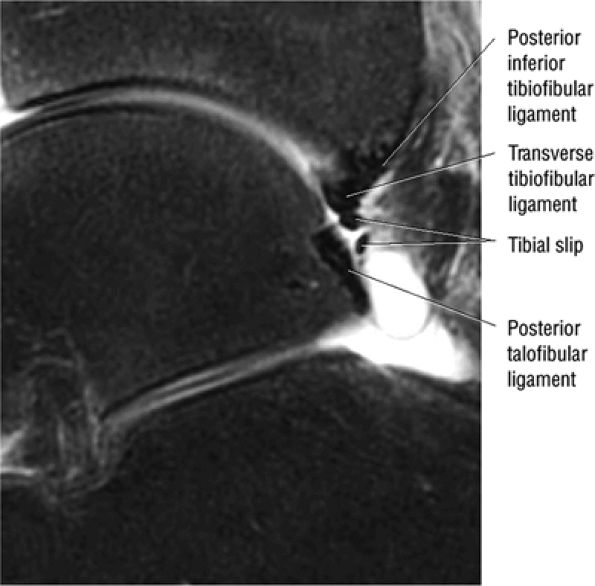 |
|
FIGURE 5.229 ● Enlarged bifid appearance of the tibial slip. The presence of a prominent transverse tibiofibular ligament and large tibial slip may predispose to an increased risk of posterior impingement. Sagittal FS PD FSE image.
|
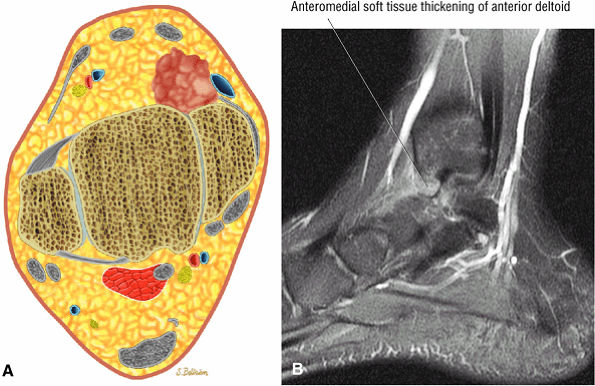 |
|
FIGURE 5.230 ● Anteromedial impingement with soft-tissue thickening associated with a capsular component and a torn anterior component of the deltoid ligament. (A) Axial color illustration. (B) Sagittal FS PD FSE image.
|
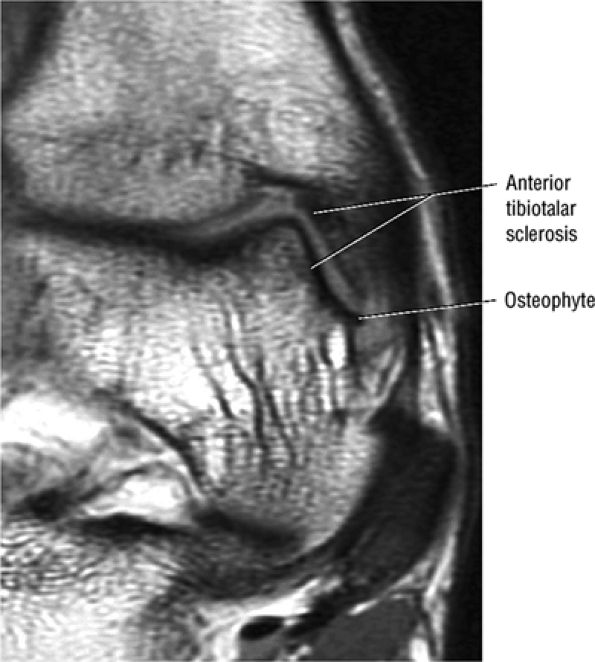 |
|
FIGURE 5.231 ● Osseous sequelae of anteromedial impingement with hypointense tibiotalar sclerosis and osteophyte. Coronal T1-weighted image.
|
-
Grade 1: Abnormal osseous contour of the anteroinferior tibia (Fig. 5.236)
-
Grade 2: A sharp interface between tibial hypertrophic bone and the tibial exostosis (Fig. 5.237). Spurs are greater than 3 mm, but no talar spur is seen.
-
Grade 3: Tibial exostosis with secondary spur formation on the dorsum of the talus (Fig. 5.238)
-
Grade 4: Pantalocrural osteoarthritic destruction (Fig. 5.239)
-
Osteophytes
-
Fluid and synovium adjacent to the anterior talar neck, between the tibial osteophyte and the talus at the capsular attachment
-
Subchondral sclerosis
-
Tibiotalar effusion
-
Talar and anterior distal tibial subchondral edema
-
Discrete cartilaginous defects or a trough in the talus (“tram track” lesion)
-
Fibrous overgrowth
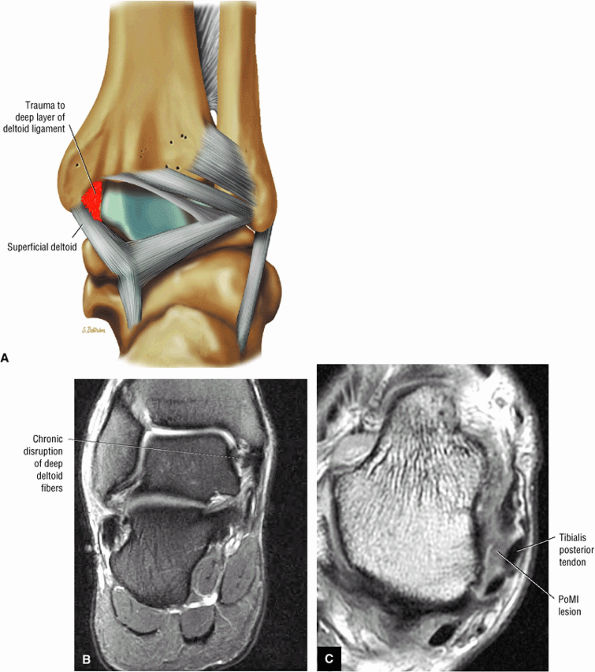 |
|
FIGURE 5.232 ● Posteromedial impingement secondary to disruption of the deep fibers of the deltoid ligament (posterior talotibial component) with associated thickened synovial response deep to the tibialis posterior tendon. (A) Coronal color illustration. (B) Coronal FS PD FSE image. (C) Axial T1-weighted image.
|
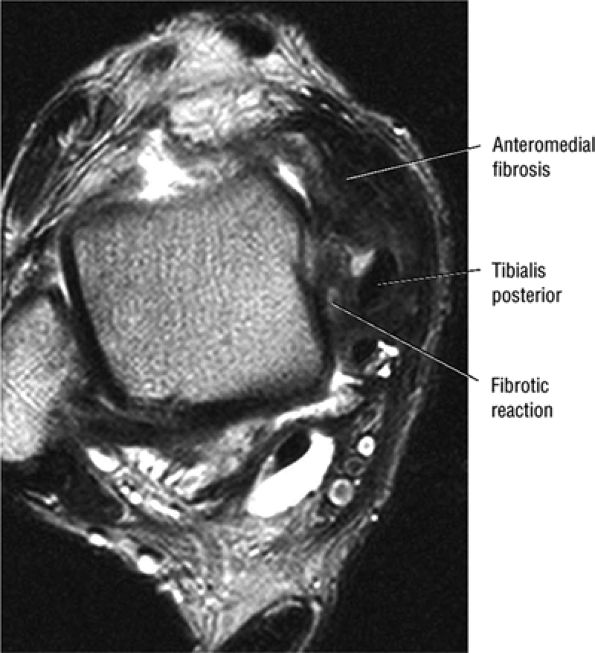 |
|
FIGURE 5.233 ● Gross fibrotic thickening posteromedially deep to the tibialis posterior tendon associated with chronic deltoid tearing. Anteromedial soft tissue fibrosis is also demonstrated. Axial T2-weighted FSE image.
|
-
Sinus tarsi syndrome is associated with sinus tarsi and lateral hindfoot pain and instability with injury to the contents of the tarsal canal and sinus.
-
T1- or PD-weighted sagittal or coronal images demonstrate effacement of sinus tarsi fat.
-
Synovitis, fibrosis, or ligament disruption is identified on FS PD FSE images.
-
Tibialis posterior tendon dysfunction and spring ligament pathology may be associated with sinus tarsi syndrome.
-
The three layers of lateral ligamentous support of the joint are the superficial or peripheral layer (e.g., the calcaneofibular ligament), the intermediate layer (e.g., the cervical ligament), and the deep layer (e.g., the interosseous talocalcaneal ligament).
-
Calcaneofibular ligament
-
Lateral talocalcaneal ligament
-
Interosseous ligament
-
Subtalar joint sprain
however, has not been validated on MR scans of the tarsal sinus and canal, on which the absence of anterior microrecesses of the posterior subtalar joint may be a normal finding.96 Klein and Spreitzer reported that 60% of patients with an abnormal tarsal sinus and canal were clinically diagnosed as having sinus tarsi syndrome.96
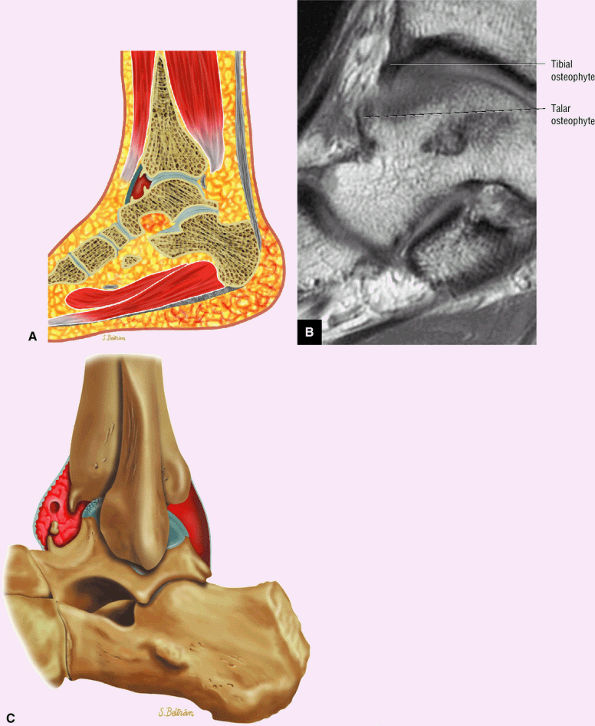 |
|
FIGURE 5.234 ● (A) Lateral color illustration showing potential sites for anterior tibiotalar spurs.(B) Sagittal T1-weighted image with opposing talar and anterior tibial plafond osteophytes. (C) Lateral color illustration demonstrates complications of anterior osseous impingement with anterior osteophytes, synovitis, and a loose body.
|
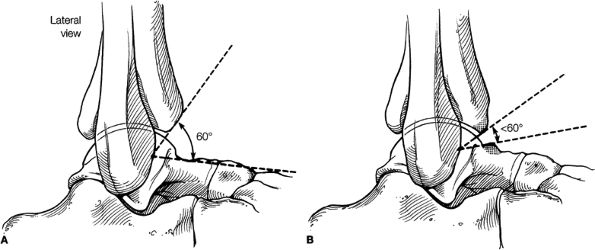 |
|
FIGURE 5.235 ● (A) The normal angle between the distal tibia and the talar neck should be 60° or greater. (B) The presence of osteophytes on the distal tibia or talus can narrow the angle to less than 60°.
|
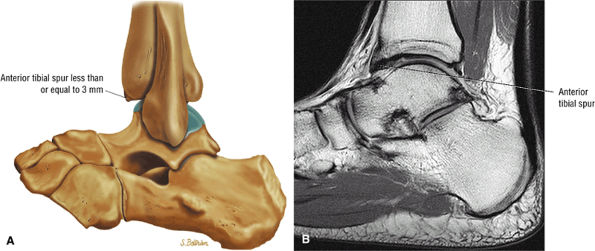 |
|
FIGURE 5.236 ● Anterior impingement with an anterior tibial spur up to 3 mm on a lateral color illustration (A) and a sagittal T1-weighted image (B). Osteophytes may develop as a consequence of degenerative joint disease or be associated with posttraumatic conditions.
|
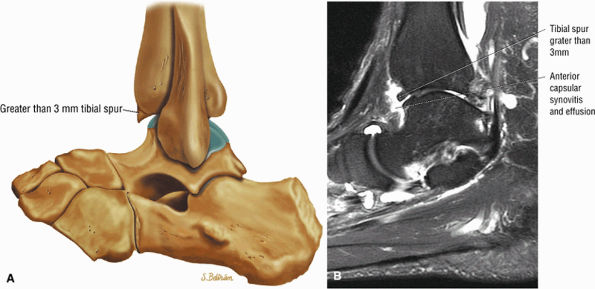 |
|
FIGURE 5.237 ● (A) Osteochondral exostosis with a prominent tibial osteophyte greater than 3 mm. There is no talar spur in a grade 2 ankle spur. Anterior spurs may result from direct trauma following forced dorsiflexion injuries or from forced plantarflexion trauma with capsular avulsion. Osteophytes are seen in anterior impingement associated with athletic trauma, as might occur in football and basketball players and dancers. (B) Prominent anterior tibial spur associated with tibiotalar synovitis and effusion. Sagittal FS PD FSE image.
|
-
Poorly defined margins of interosseous and cervical ligaments (Fig. 5.246)
-
Diffuse infiltration with synovitis and fibrosis (Fig. 5.247)
-
Multiple synovial cystic fluid collections
-
Associated posterior tibial tendon tears97
-
Secondary sclerosis and subchondral cystic change in roof of sinus tarsi and calcaneus
-
Osseous flattening and subchondral sclerosis of the posterior facet of the subtalar joint
-
Subchondral erosions within the critical angle of Gissane
-
Increased signal in the intermediate and medial roots of the inferior extensor retinaculum on FS PD FSE images
-
Synovitis of the anterior capsule of the posterior talocalcaneal joint
-
Lateral ankle ligament sprain (anterior talofibular, calcaneofibular)
-
Subtalar joint dislocation (complete rupture of the interosseous talocalcaneal and cervical ligaments)
-
Os trigonum syndrome pain may be associated with disruption of the cartilaginous synchondrosis between the os trigonum and the lateral tubercle of the posterior process.
-
Stieda's process is a prominent posterior extension of the lateral tubercle.
-
Compression of the os trigonum between the FHL and posterior talofibular ligaments occurs in extreme ankle flexion. Compression of the os trigonum between the calcaneus and talus occurs in the end range of plantarflexion.
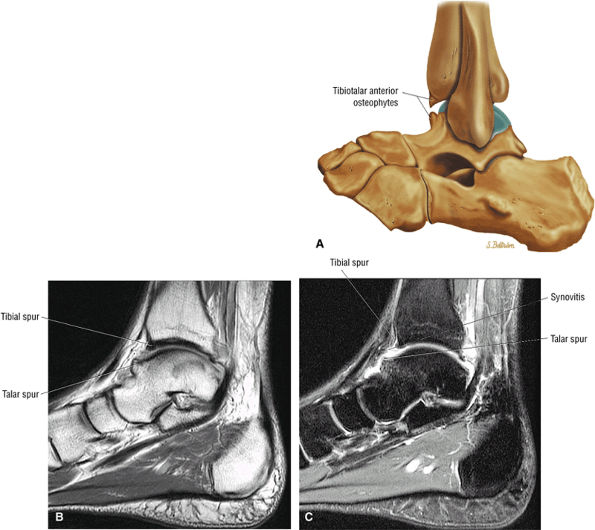 |
|
FIGURE 5.238 ● Opposing tibiotalar spurs on a lateral color illustration (A), a sagittal PD-weighted image (B), and a sagittal FS PD FSE image (C). Osteophytes involving both the tibia and talus can impinge on each other in dorsiflexion and potentially limit motion. Pain, catching, and joint swelling may occur. Osteophytes may be intra-articular, intracapsular, or extra-articular in location.
|
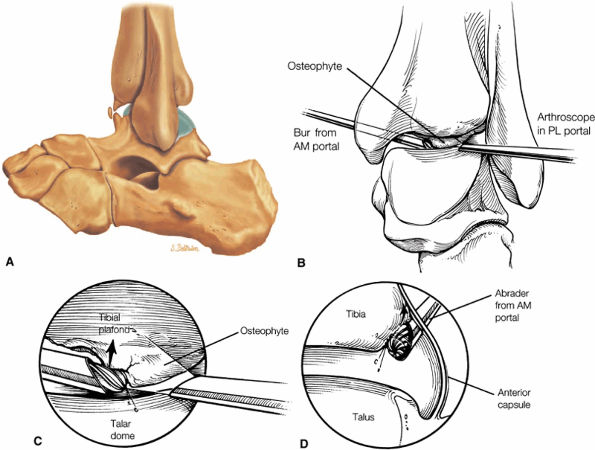 |
|
FIGURE 5.239 ● (A) Lateral color illustration showing superimposed tibial arthrosis with fragmentation. (B –D) Arthroscopic osteophyte removal using the posterolateral portal. (B) Using the posterolateral portal for visualization, the extent of the anterior distal tibial spur can be seen. Posterior view. (C) The osteophyte is removed using a motorized bur. View from the posterolateral portal. (D) The arthroscope is switched to the anterior portals to confirm complete removal of the distal tibial osteophyte. Lateral view.
|
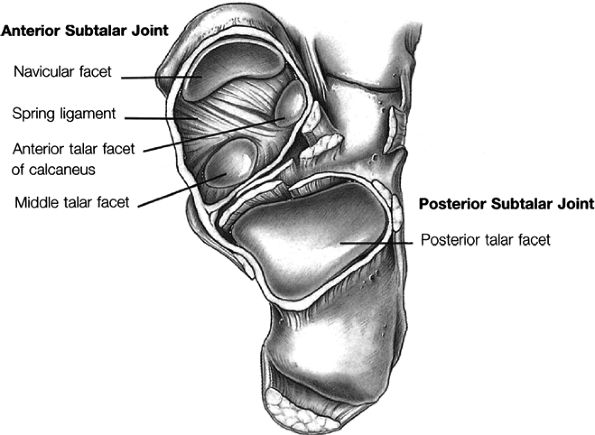 |
|
FIGURE 5.240 ● Anterior subtalar joint with the talus opened away from the calcaneus.
|
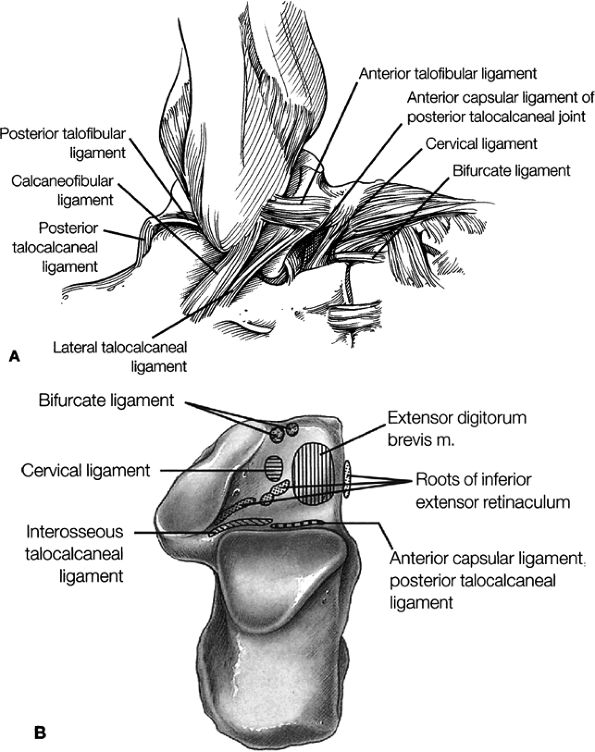 |
|
FIGURE 5.241 ● Ligaments of the subtalar joint. (A) Superficial lateral view of the subtalar joint with bones and ligaments. From this position, the interosseous ligament cannot be seen. (B) Superior view of the insertion sites on the calcaneus with the talus removed.
|
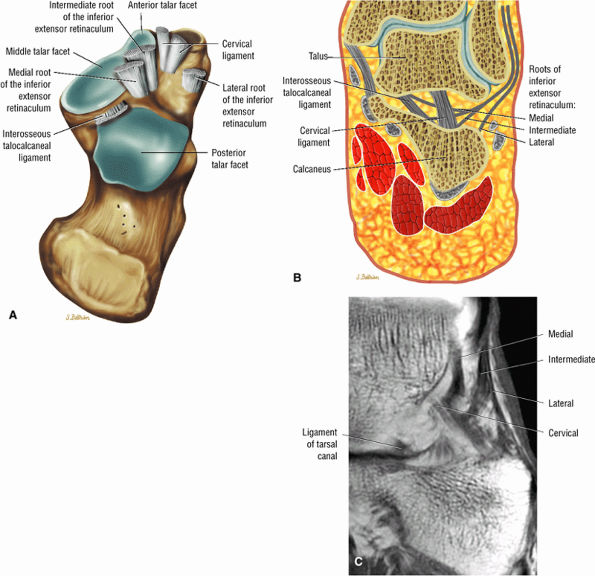 |
|
FIGURE 5.242 ● Tarsal canal and sinus tarsi anatomy on an axial superior view color graphic (A), a color coronal section (B), and a corresponding T1-weighted MR arthrogram (C).
|
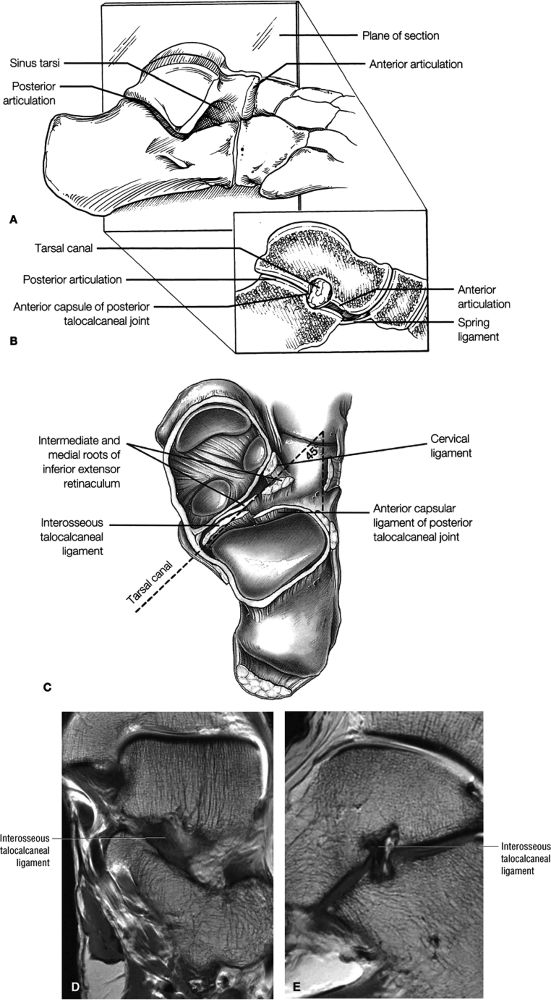 |
|
FIGURE 5.243 ● (A) Lateral view of the sinus tarsi. The sinus tarsi and tarsal canal separate the anterior and posterior articulations of the subtalar joint. The sagittal plane of section helps to demonstrate the anatomy more clearly. (B) After sectioning, the tarsal canal and subtalar articulations are more clearly seen. (C) Axial view of the tarsal canal. Note the location of the interosseous talocalcaneal and cervical ligaments. There is a 45° angle of orientation of the long axis of the sinus tarsi to the lateral aspect of the calcaneus. The interosseous talocalcaneal ligament (part of the deep layer of the subtalar ligaments) is demonstrated on T1-weighted coronal (D) and sagittal (E) images.
|
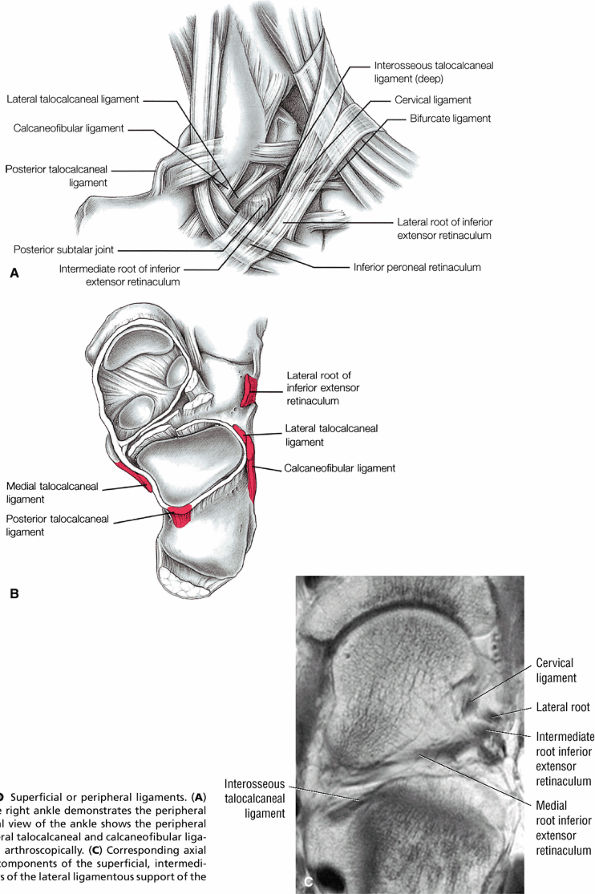 |
|
FIGURE 5.244 ● Superficial or peripheral ligaments. (A) Lateral view of the right ankle demonstrates the peripheral ligaments. (B) Axial view of the ankle shows the peripheral ligaments. The lateral talocalcaneal and calcaneofibular ligaments can be seen arthroscopically. (C) Corresponding axial MR image shows components of the superficial, intermediate, and deep layers of the lateral ligamentous support of the subtalar joint.
|
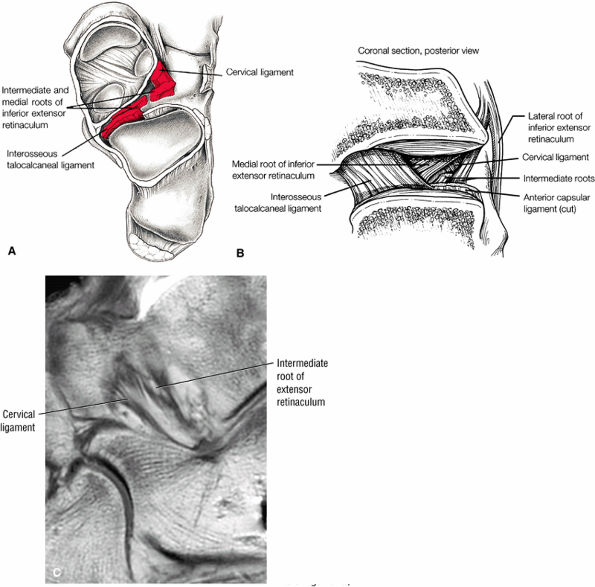 |
|
FIGURE 5.245 ● Intermediate and deep ligaments. (A) Axial view of the subtalar joint demonstrates the deep ligaments. (B) Coronal section of the subtalar joint shows the course of the interosseous talocalcaneal ligament in relation to the cervical ligament and surrounding roots. (C) Corresponding T1-weighted sagittal image of the cervical and intermediate root of the inferior extensor retinaculum (intermediate layer of subtalar ligaments).
|
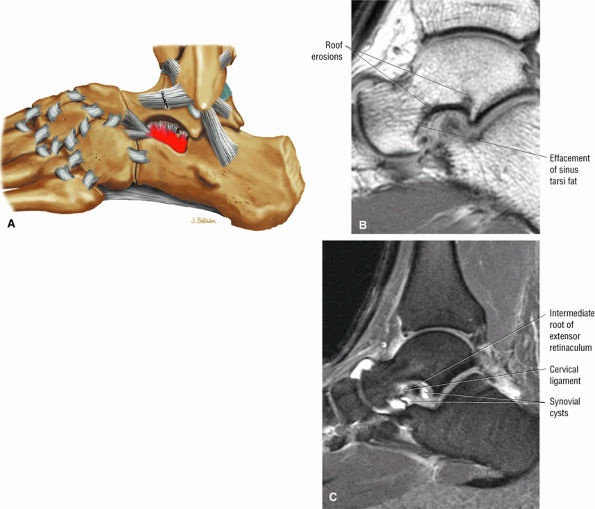 |
|
FIGURE 5.246 ● (A) Sinus tarsi syndrome with synovitis of the synovial recesses and inflammation of the fat contained within the sinus tarsi. Subchondral erosions and synovitis of the sinus tarsi root with hyperintense synovial cysts are shown on sagittal T1-weighted (B) and FS PD FSE (C) images. The majority of cases of sinus tarsi syndrome involve trauma, usually related to a significant inversion sprain. Scarring and degenerative changes to the soft-tissue structures of the sinus tarsi are associated with pain.
|
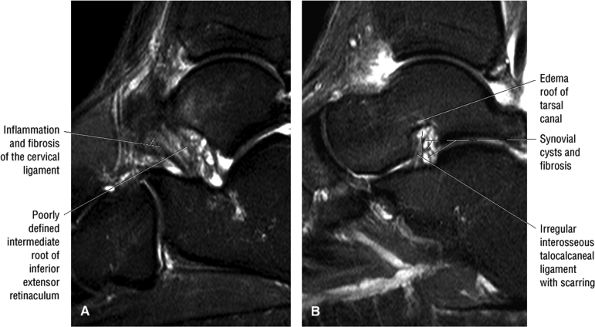 |
|
FIGURE 5.247 ● Poorly defined cervical (A) and interosseous talocalcaneal (B) ligaments associated with synovitis and fibrosis in a patient with lateral foot pain and clinical hindfoot instability. Tibialis posterior tendon dysfunction, spring ligament pathology, and sinus tarsi syndrome may represent a continuum of progressive and related pathologies. Loss of proprioceptive function of the nerve endings in the ligaments in the sinus and tarsal canal secondary to injury may be another causative factor in the progression of sinus tarsi syndrome.
|
|
TABLE 5.1 Lateral Ligamentous Support for the Subtalar Joint
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Type I: Normal tubercle (Fig. 5.249)
-
Type II: Stieda's process (enlarged posterior extension of the lateral tubercle of the talus (Fig. 5.250)
-
Type III: An accessory bone or os trigonum (Fig. 5.251)
-
Type IV: The os trigonum is fused with the talus via the synchondrosis or the syndesmosis (Fig. 5.252).
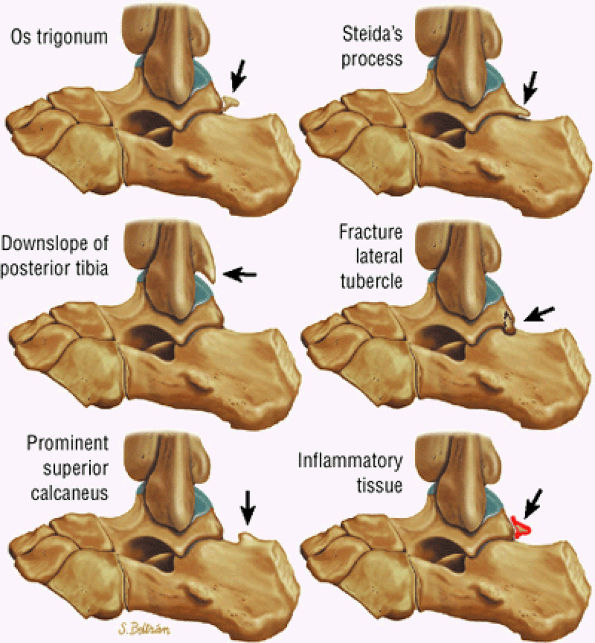 |
|
FIGURE 5.248 ● Lateral perspective color illustrations of the os trigonum and related anatomic structures in the differential diagnosis of posterior impingement.
|
 |
|
FIGURE 5.249 ● Superior view color illustration of the normal medial and lateral tubercles of the posterior process.
|
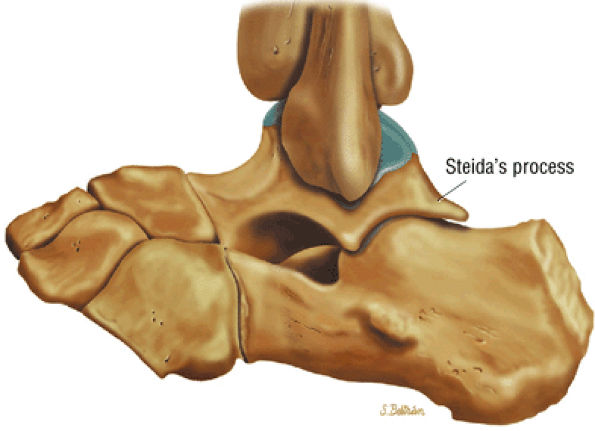 |
|
FIGURE 5.250 ● Lateral color illustration of a prominent lateral talar tubercle of the posterior process (referred to as Stieda's process or a trigonal process).
|
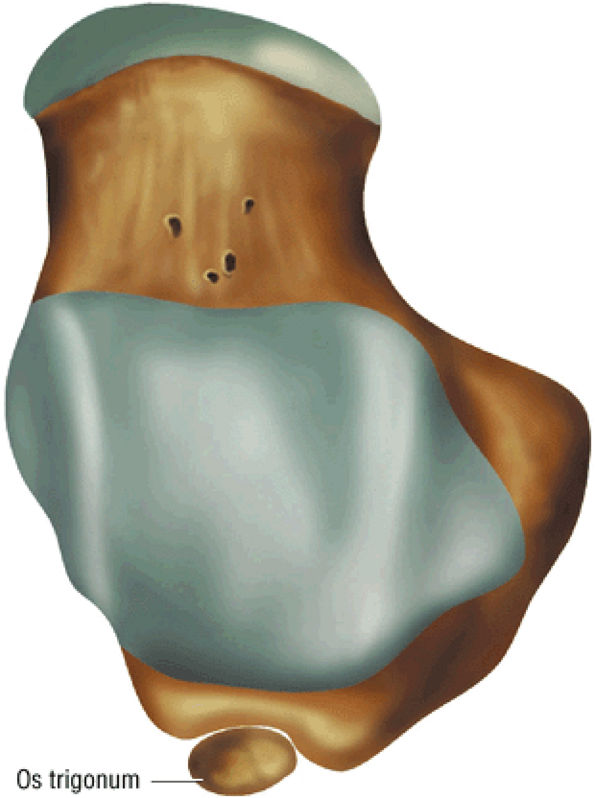 |
|
FIGURE 5.251 ● Os trigonum represents a nonunited secondary ossification center. Less commonly, the ossicle may be attributed to a chronic fracture through the lateral talar tubercle. Superior view color illustration.
|
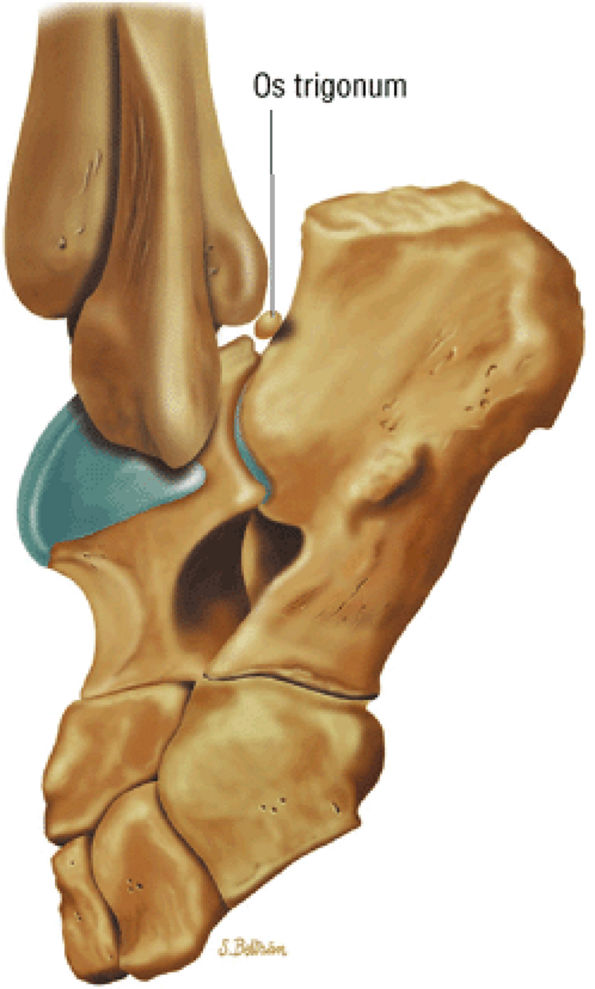 |
|
FIGURE 5.252 ● Intact synchondrosis of os trigonum. Young athletes engaged in sports that involve forced plantarflexion (including ballet dancers, javelin throwers, and soccer players) are prone to os trigonum syndrome. (A) Sagittal T1-weighted image. (B) Sagittal FS PD-weighted image.
|
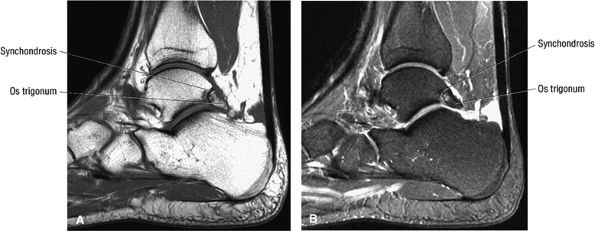 |
|
FIGURE 5.253 ● Nutcracker phenomenon of posterior ankle impingement with the os trigonum situated between the posterior tibial margin and the calcaneus. Acute or chronic disruption of the cartilaginous synchondrosis between the os trigonum and lateral talar tubercle may subsequently develop.
|
-
An enlarged marrow fat-containing os trigonum (Fig. 5.254)
-
Degenerative sclerosis or cystic change between the os trigonum and the talus
-
Sclerosis and edema of the trigonal process
-
Fluid and effacement of surrounding soft tissues
-
Os trigonum or trigonal process hyperintensity on FS PD FSE or STIR images (Fig. 5.255)
-
Hyperintensity in the synchondrosis or posterior talus on FS PD FSE images (Fig. 5.256)
-
Edema and synovitis posterior to the talus and superior to the os trigonum
-
Edema and synovitis posterior to the os trigonum on sagittal images
-
Fracture of the lateral tubercle of the posterior process (Stieda's process) with hyperintense marrow and adjacent hyperintense synovitis on FS PD FSE images (Fig. 5.257)
-
Isolated tenosynovitis of the FHL tendon sheath (partial tethering FHL tendon) (see Fig. 5.254)
-
Possible associated intra-articular loose bodies
-
The accessory navicular, also known as the os tibiale externum, is the development of a congenital navicular tuberosity from a secondary center of ossification.
-
Evaluation includes determining the status of the PTT.
-
FS PD FSE or STIR images in the axial plane are used to appreciate hyperintense edema across the synchondrosis.
-
Type I: A type I accessory navicular is a 4- to 6-mm ossicle. It is a true sesamoid within the PTT and represents 30% of cases.
-
Type II: A type II accessory navicular is triangular or heart-shaped and has a nonossified zone of 1 to 3 mm. There are two subtypes. Type IIa represents more superior placement of the accessory bone, which is subject to avulsion and tension forces. In type IIb, placement of the accessory bone is more inferior and it is subject to shearing forces.
-
Type III: Type III represents a cornuate or enlarged navicular tuberosity.
-
A small (4 to 6 mm), usually marrow fat-containing ossicle proximal to the navicular tuberosity
-
Hypointense to intermediate signal intensity within the distal PTT on T1- or PD-weighted images
-
A 5- to 7-mm separation from the navicular
-
Fat signal in the ossicle suppressed on FS PD FSE images
-
The ossicle may be difficult to differentiate from surrounding hypointense tibialis posterior on FS PD FSE images.
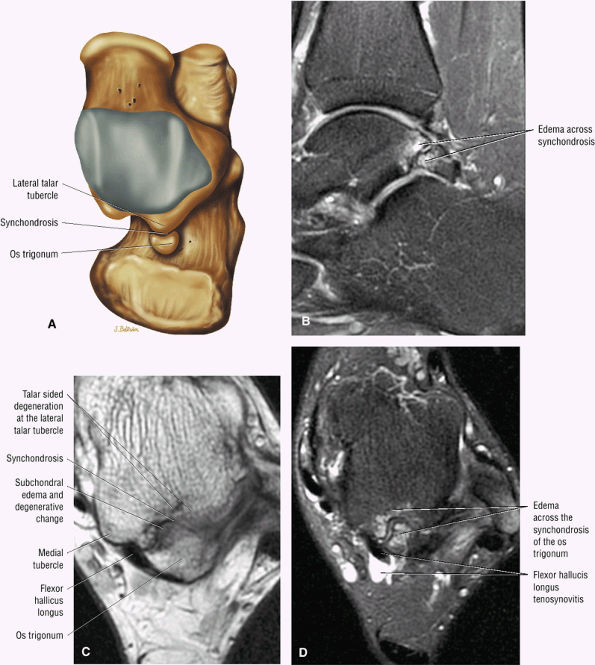 |
|
FIGURE 5.254 ● Edema across the synchondrosis of the os trigonum. Edema associated with degenerative change is hypointense on T1-weighted images and hyperintense on FS PD FSE images. Flexor hallucis longus tenosynovitis is an associated finding. Edema is associated with chronic shearing stress and bone remodeling across the synchondrosis. The os trigonum is also at risk for fragmentation with secondary osteonecrosis. Marrow fat signal is present in the os trigonum on the axial T1-weighted image (C). (A) Axial color illustration, superior view of talus. (B) Sagittal FS PD FSE image. (C) Axial T1-weighted image. (D) Axial FS PD FSE image.
|
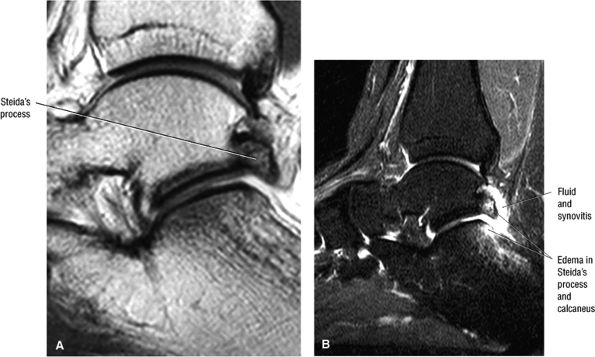 |
|
FIGURE 5.255 ● Degenerative change with edema in a Stieda's process of the talus. There is associated reactive change in the posterior aspect of the posterior facet of the calcaneus. (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image.
|
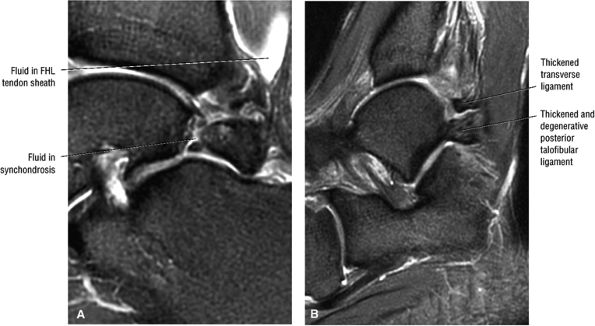 |
|
FIGURE 5.256 ● (A) Hyperintensity within the synchondrosis on a sagittal FS PD FSE image. The synchondrosis interface is irregular. Fluid is seen in the flexor hallucis longus tendon sheath. (B) Associated degeneration of the posterior talofibular ligament and transverse tibiofibular ligament are identified lateral to the os trigonum. Sagittal FS PD FSE image.
|
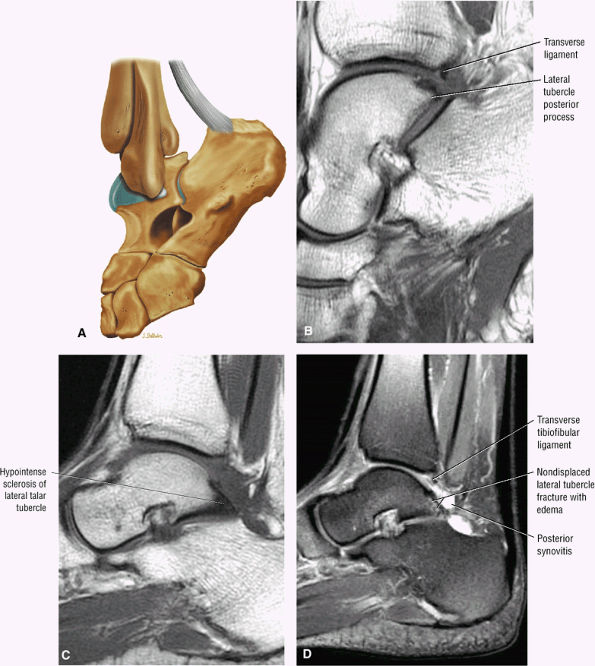 |
|
FIGURE 5.257 ● (A) Normal posterior structures with the foot in demi-pointe position without posterior impingement. Lateral color illustration. (B) Sagittal T1-weighted image showing the proximity of the lateral tubercle of the posterior process and the transverse tibiofibular ligament or posterior labrum of the ankle in demi-pointe position. (C, D) Posterior impingement in a world-class ballet dancer with a fractured lateral talar tubercle representing a pseudoarthrosis. Associated inflammation of the posterior recess of the tibiotalar and subtalar joints is identified. (C) Sagittal T1-weighted image. (D) Sagittal FS PD FSE image.
|
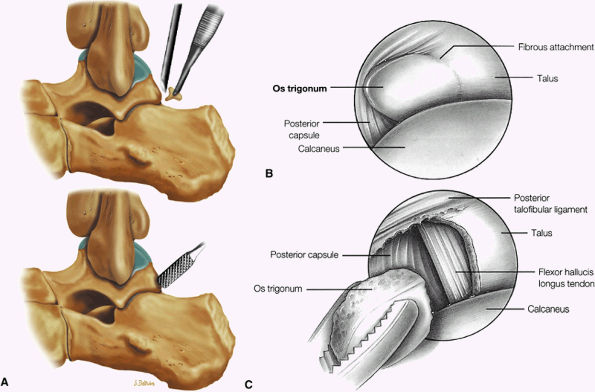 |
|
FIGURE 5.258 ● (A) Excision of an os trigonum. A nonunion of the os trigonum shows significant motion at its fibrous attachment to the talus and irregularity and chondromalacia and fibrosis at its insertion. (B, C) Arthroscopic excision of an os trigonum. (B) Visualization of fibrous attachment of the os trigonum with subtalar arthroscopy. (C) As the os trigonum is removed from the subtalar joint, the flexor hallucis longus is seen.
|
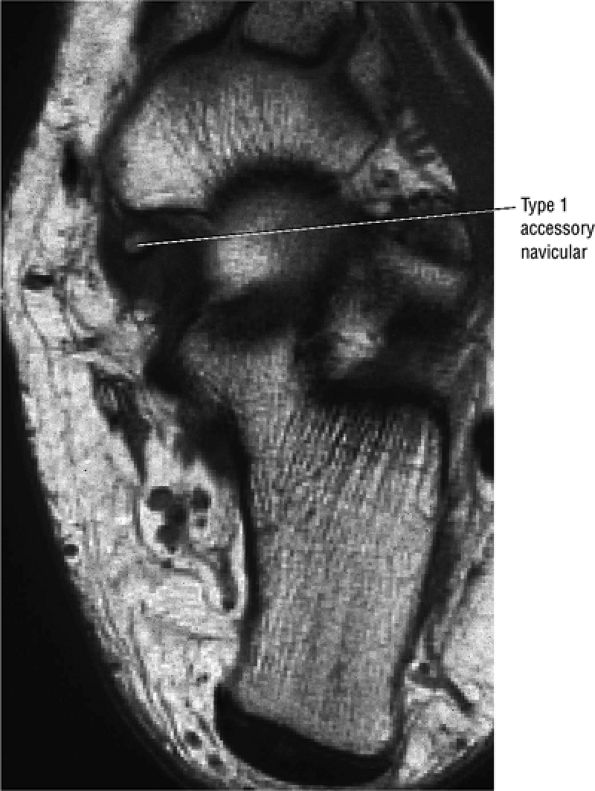 |
|
FIGURE 5.259 ● Type I accessory navicular is seen as a small (marrow fat-containing) ossicle imbedded within the distal tibialis posterior (posterior tibial tendon) on a T1-weighted axial image.
|
-
Marrow fat-containing triangular or heart-shaped ossicle with direct connection to the medial navicular through a synchondrosis
-
Nonossified synchondrosis of low to intermediate signal intensity on T1- or PD-weighted images and hyperintense on FS PD FSE images
-
Associated degenerative sclerosis with or without subchondral cystic degeneration (hypointensity across the synchondrosis on T1- or PD-weighted images)
-
Bursitis
-
Distal thickening in the tibialis posterior tendinosis and tenosynovitis with or without partial to complete PTT tear
-
In asymptomatic patients, there is normal fat-suppressed marrow signal intensity on FS PD FSE images.
-
In symptomatic patients there is usually marrow edema and degenerative hyperemia of the subchondral bone.
-
Osseous edema, which may involve the accessory navicular, the accessory and navicular tuberosity, or the tuberosity alone
-
Enlarged os cornuate marrow fat signal intensity extension of the medial navicular proximally
-
No synchondrosis
-
Normal marrow fat characteristics on all pulse sequences
-
The key ligaments of the capsuloligamentous complex of the first MTP joint are the metatarsosesamoid and sesamoid-phalangeal ligaments.
-
The plantar plate is less substantial in the hallux relative to the lesser metatarsals. It can be visualized between the sesamoid-phalangeal ligaments extending from the sesamoids to the proximal aspect of the proximal phalanx.
-
Turf toe evaluation must include assessment of tearing of the sesamoid-phalangeal ligament, plantar plate, and displacement or fracture of the sesamoids.
Other sesamoid and plantar plate injuries are covered in subsequent discussions.
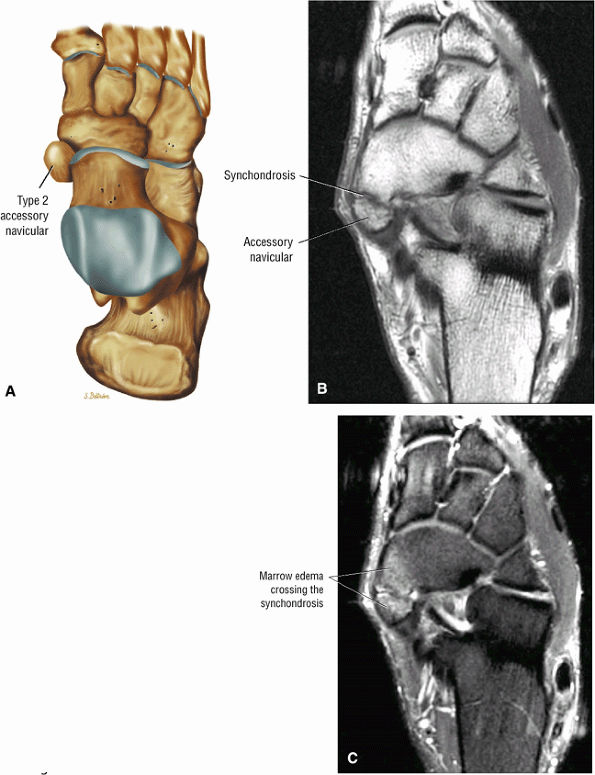 |
|
FIGURE 5.260 ● (A) Type II accessory navicular on a superior view color illustration. (B, C) Symptomatic type II accessory navicular in a professional basketball player with prominent marrow edema on either side of the synchondrosis. The connection of the accessory ossification is through a fibrous or cartilaginous bridge. Repetitive contraction of the tibialis posterior tendon insertion onto the accessory navicular can generate painful shearing forces across the synchondrosis at the level of the medial aspect of the midfoot. (B) Axial T1-weighted image. (C) Axial FS PD FSE image.
|
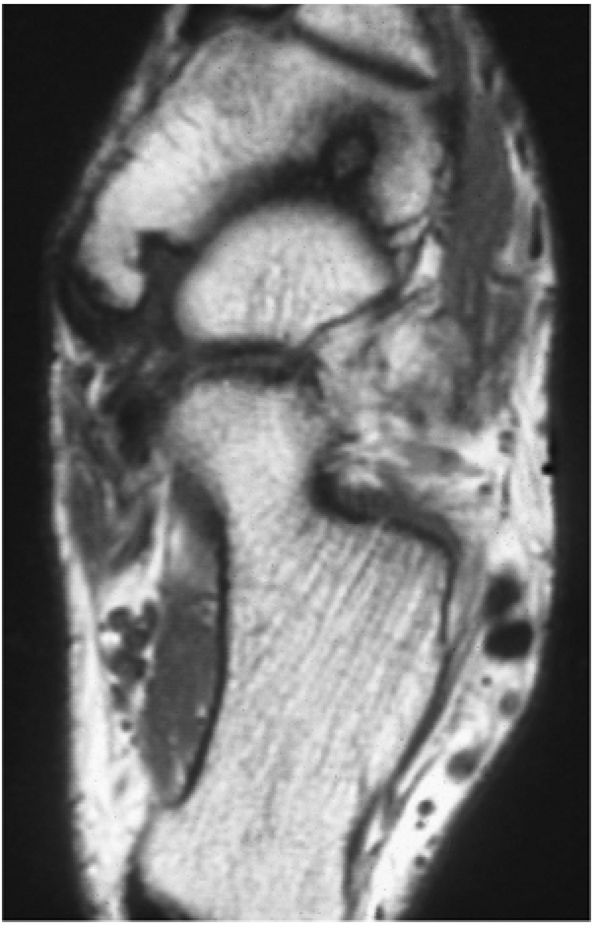 |
|
FIGURE 5.261 ● Type III or cornuate navicular on an axial T1-weighted image. Both the type II and type III navicular are associated with tibialis posterior tendon degeneration and tear and a painful os naviculare syndrome. Degenerative sclerosis may be observed without hyperintensity on FS PD FSE images but may coexist with reactive edema. Edema in the type II and cornuate navicular is associated with more acute clinical symptoms.
|
-
Medial and lateral metatarsosesamoid ligaments
-
Medial and lateral sesamoid-phalangeal ligaments
-
Intersesamoid ligament
-
Medial and lateral collateral MTP ligaments
unrestricted motion of the proximal phalanx and compression of the dorsal articular surface of the metatarsal head. The plantar aspect of the ligamentous complex tears with associated detachment of the plantar plate distal to the sesamoids (Fig. 5.265). An abductor injury may be associated with a turf toe injury (Fig. 5.266). Fracture of the sesamoids or separation of a bipartite sesamoid may result (Fig. 5.267). Other mechanisms of injury may occur with turf toe variants, including:
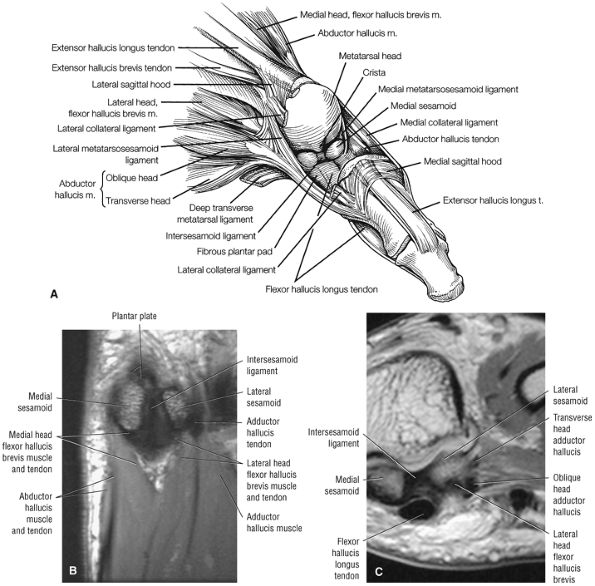 |
|
FIGURE 5.262 ● (A) Superolateral view of the bone-ligament-capsular anatomy of the MTP joint. (B) Axial PD FSE image demonstrating the anatomy of the MTP joint anatomy at the level of the sesamoids. The medial sesamoid is embedded in the tendons of the abductor hallucis and medial head of the flexor hallucis brevis. (C) Coronal PD-weighted image showing the lateral sesamoid embedded in the tendons of the lateral head of the flexor hallucis brevis and transverse and oblique heads of the adductor hallucis.
|
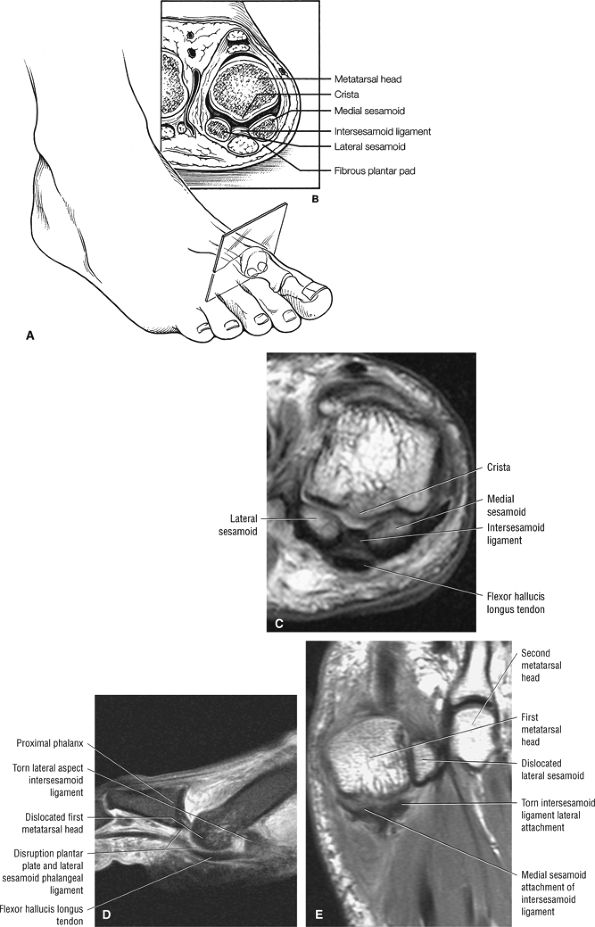 |
|
FIGURE 5.263 ● Sesamoid position. (A) Plane of cross-section to study the sesamoids. (B) Cross-sectional anatomy of the metatarsal head with the medial and lateral sesamoids separated by a small bony ridge, or crista. (C) Coronal T1-weighted image demonstrates the crista of the first metatarsal head and the relationship to the intersesamoid ligament deep to the flexor hallucis longus tendon. (D) Sagittal FS PD FSE and (E) axial PD FSE images of a torn inter-sesmoid ligament visualized in a turf toe with dislocation of the first MTP.
|
-
Valgus injury plus dorsiflexion with injury to the medial ligamentous structures and medial sesamoid
-
Varus injury with lateral capsular tear (Fig. 5.268) and rupture of the adductor hallus tendon
-
Hyperflexion injury secondary to MTP forced plantarflexion (also referred to as sand toe). Hyperflexion occurs in beach volleyball players, football players, dancers, and skimboarders,103 who may develop sprain or tear of the dorsal capsule secondary to exaggerated hyperflexion (Fig. 5.269). Associated injury to the lesser MTP joint may also occur.
-
Grade 1: Stretch injury or minor tearing of first MTP joint capsular-ligamentous complex
-
Grade 2: Partial tear of first MTP capsular-ligamentous complex with an intact articular surface
-
Grade 3: Disruption of the capsular-ligamentous complex with a plantar plate tear from its metatarsal head/neck origin. There is associated impaction of the proximal phalanx into the dorsal aspect of the metatarsal head as well as articular cartilage damage, subchondral marrow edema, and sesamoid fracture or diastasis.
-
Disruption with edema or discontinuity of the sesamoid-phalangeal ligament on sagittal images
-
Tear of the intersesamoid ligament as assessed on axial or coronal images
-
Absence or irregularity of the plantar plate between the sesamoid-phalangeal ligaments (direct visualization of the plantar plate is more difficult in the first MTP compared to the lesser metatarsal MTP joints because of the presence of the sesamoids in the first MTP)
-
Proximal retraction of the sesamoid on sagittal images
-
Edema and/or tearing of the flexor hallucis brevis, abductor, or adductor hallucis tendons
-
Metatarsal head edema
great toe include persistent pain, swelling, stiffness, locking, or grinding. The therapeutic options include treatment of chondromalacia, synovitis, osteochondral lesions, osteophytes, loose bodies, and arthrofibrosis. Arthrodesis may eventually be necessary. The contraindications include the presence of infection, advanced DJD, severe edema, or poor vascular status. Surgical treatment options include ORIF for diastasis of a bipartite sesamoid, a sesamoidectomy, and reattachment of the plantar plate with suture anchor technique.
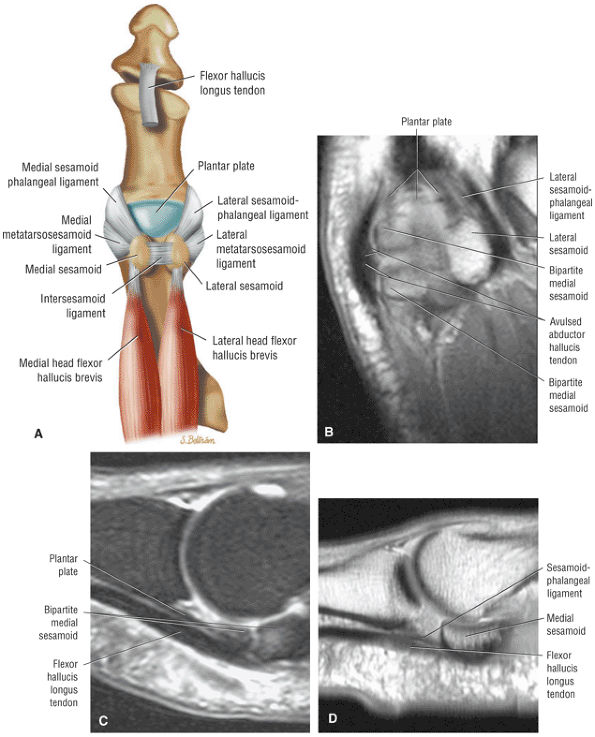 |
|
FIGURE 5.264 ● (A) Hallux MTP capsular-ligamentous-sesamoid complex demonstrates the relationship of the plantar plate distal to the sesamoids and between the sesamoid phalangeal ligaments. The plantar plate is a strong fibrous structure that has a firm attachment to the proximal phalanx and a less substantial attachment to the metatarsal neck. The medial and lateral collateral ligaments consist of an MTP and a metatarsosesamoid ligament. The medial and lateral sesamoid-phalangeal ligaments are directly adjacent to the plantar plate and also bridge and stabilize the sesamoids to the proximal phalanx. (B) Axial PD-weighted image at the level of the plantar plate and sesamoid-phalangeal ligament. (C) Sagittal FS PD FSE image through the plantar plate adjacent to the medial sesamoid and lateral to the sesamoid-phalangeal ligament. (D) Sagittal T1-weighted image of the sesamoid-phalangeal ligament located medial to the plantar plate at the level of the medial sesamoid.
|
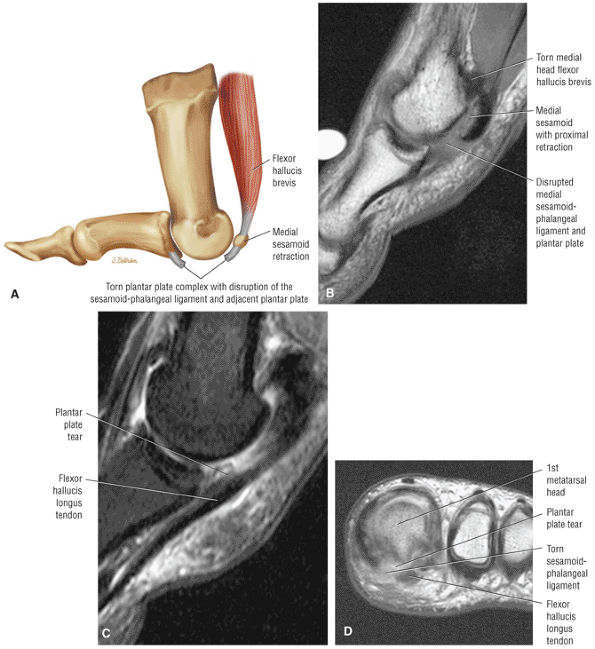 |
|
FIGURE 5.265 ● Turf toe with disruption of the sesamoid-phalangeal ligament and plantar plate of the first (hallux) MTP joint. (A) Lateral color illustration of a retracted sesamoid and disruption of the plantar plate complex. (B) Sagittal PD-weighted image of a retracted medial sesamoid and complete discontinuity of the medial sesamoid-phalangeal ligament and plantar plate. (C) Sagittal FS PD FSE image of the disrupted plantar plate directly lateral to the torn medial sesamoid-phalangeal ligament. (D) Coronal PD-weighted image immediately distal to the sesamoids demonstrates disruption of the plantar plate complex.
|
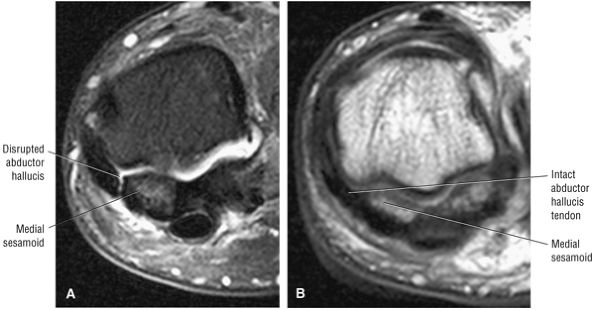 |
|
FIGURE 5.266 ● (A) Turf toe variation with disruption of the abductor hallucis tendon attachment to the medial sesamoid in a professional baseball player. Coronal FS PD FSE image. (B) Normal abductor hallucis tendon attachment shown for comparison in a separate individual. Coronal PD FSE image.
|
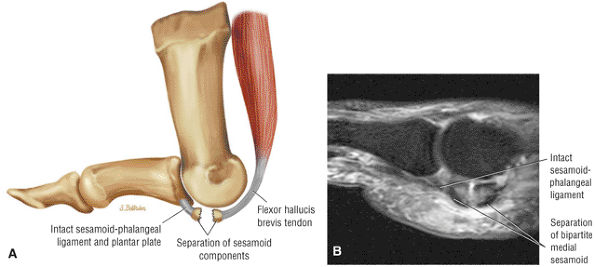 |
|
FIGURE 5.267 ● Turf toe with traumatic separation of a bipartite sesamoid. The sesamoid-phalangeal ligament and plantar plate were intact. (A) Lateral color illustration. (B) Sagittal FS PD FSE image.
|
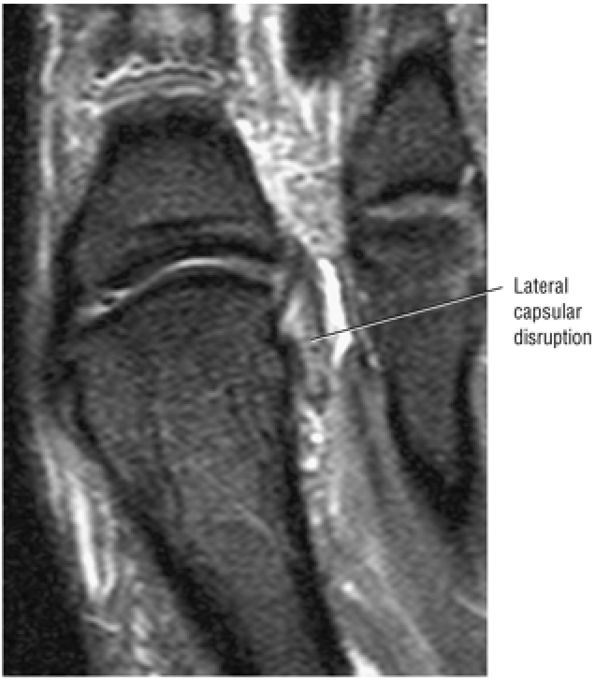 |
|
FIGURE 5.268 ● Lateral collateral ligament disruption at the level of the first metatarsal head. Axial FS PD FSE image.
|
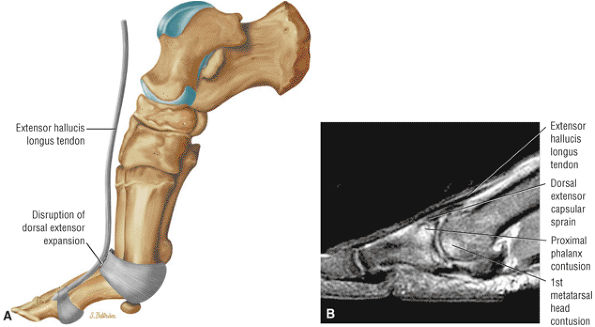 |
|
FIGURE 5.269 ● (A) Color illustration of skimboarder's toe secondary to a hyperdorsiflexion injury with disruption of the dorsal portion of the extensor expansion. (B) Separate case with a dorsal capsular sprain and osseous contusions involving the first metatarsal head and dorsal aspect of the proximal phalanx secondary to a forced plantarflexion injury. Sagittal FS PD FSE image.
|
-
A bipartite sesamoid usually involves the medial sesamoid.
-
The sesamoids may be affected by fracture, osteochondritis, sesamoiditis, and arthritis.
-
FS PD FSE or STIR imaging is required to identify sesamoid edema.
-
CT may be helpful if a fracture cannot be distinguished from bipartite morphology.
is slightly larger than the lateral sesamoid. They are ovoid in shape, separated by an intersesamoidal ridge or crista, and the dorsal surface is cartilaginous and articulates with the concave facets of the first metatarsal head. The sesamoids and the collateral ligaments together stabilize the first MTP joint. Sesamoid disorders include bipartite sesamoids, fractures, turf toe (discussed above), osteochondritis and AVN, sesamoiditis, and arthritis. Ossification of the hallucal sesamoids occurs from 7 to 10 years of age (earlier in females than in males).
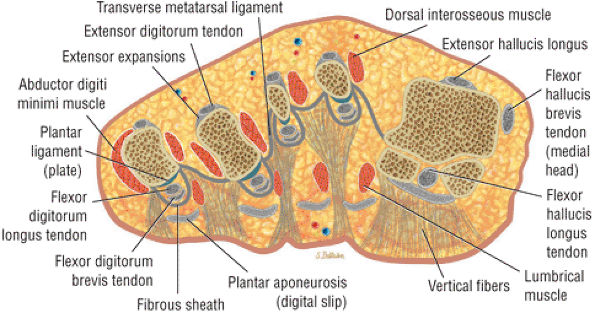 |
|
FIGURE 5.270 ● Coronal color section through the sesamoids. The sesamoids vary in morphology from semiovoid to circular to bean-shaped. The medial sesamoid is usually larger and can be bi-, tri-, or quadripartite.
|
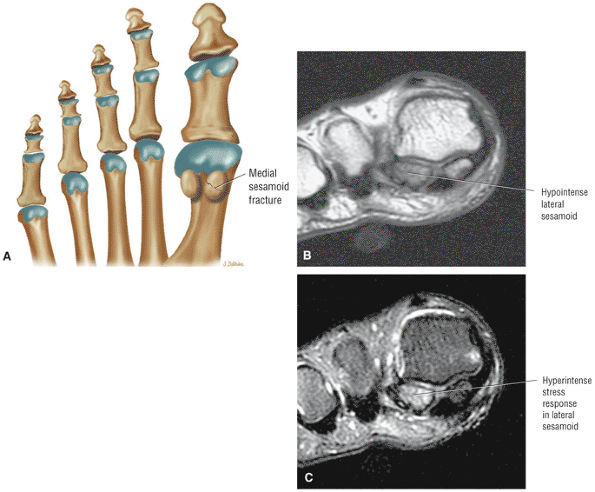 |
|
FIGURE 5.271 ● Stress fracture of the lateral sesamoid in a long-distance runner. (A) Plantar view color graphic of the sesamoids. Sesamoid marrow is hypointense on coronal PD-weighted image (B) and hyperintense on coronal FS PD FSE image (C).
|
-
Bipartite sesamoids, which more frequently involve the medial sesamoid, are characterized by the following:
-
Identical rather than asymmetric division with marrow fat signal intensity on T1- or PD-weighted images
-
The lateral sesamoid is rarely separated into more than two fragments.
-
Smooth margins (rounded edges)
-
No increase in signal intensity on FS PD FSE images
-
Normal surrounding soft tissue and capsular structures
-
-
Typical findings in sesamoid fractures (Fig. 5.271) are:
-
In osteochondritis and AVN (Fig. 5.272) typical MR findings include:
-
Fragmentation of the involved sesamoid
-
Greater involvement of the lateral sesamoid
-
An acute/subacute phase with variable increased signal intensity and a chronic phase with hypointensity on FS PD FSE images
-
-
Sesamoiditis is characterized by:
-
Effusion and synovitis of the first MTP joint
-
Mild subchondral sclerosis between the first metatarsal facets and the dorsal articular surface sesamoids
-
Dorsal sesamoid chondral degeneration with narrowing relative to the metatarsal concave articular facets
-
-
Arthritis signs include:
-
Maintenance of the marrow fat signal by osteophytes
-
Subchondral sclerosis at the articulation with the first metatarsal head
-
Osteophytic spurring
-
MTP joint fluid and effusion
-
Associated hallux valgus (Fig. 5.273) and hallux rigidus (Fig. 5.274)
-
by reduced ability for weight-bearing. Conservative methods are usually successful and include:
 |
|
FIGURE 5.272 ● Bipartite fibular sesamoid with osteochondritis. The bipartite sesamoid is enlarged with smooth edges (curved arrow). Osteochondritis demonstrates hypointensity on both T1-weighted (A) and FS PD FSE (B) axial images.
|
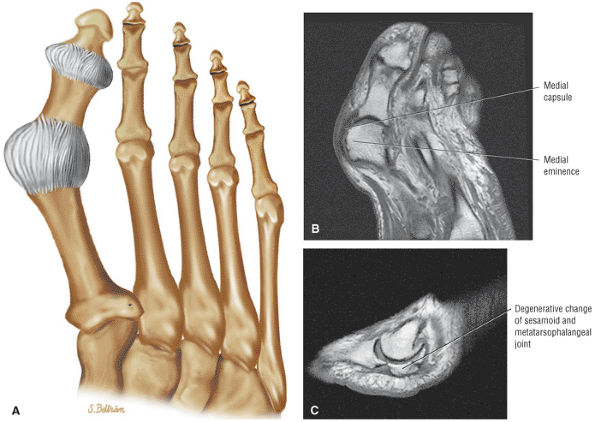 |
|
FIGURE 5.273 ● (A) Hallux valgus deformity on superior view color illustration. There is subluxation of the first MTP base. Axial (B) and sagittal (C) T1-weighted images demonstrate the prominence of the medial eminence of the metatarsal head. With subluxation of the tibial sesamoid there is erosion of the crista of the metatarsal head. The crista normally helps maintain alignment and prevents lateral subluxation of the tibial sesamoid. The first metatarsal may deviate both medially and dorsally. Metatarsus primus varus (medial inclination of the first metatarsal) with dorsal splaying of both the first and fifth metatarsal heads occurs in hallux valgus.
|
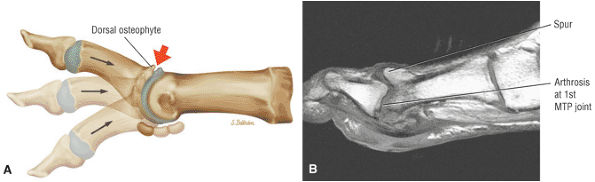 |
|
FIGURE 5.274 ● (A) Hallux rigidus with pain elicited by the axial grind test (axial compression and dorsiflexion). Lateral color illustration. (B) Sagittal T1-weighted image with degenerative first metatarsal osteophytes and joint changes. Hallux rigidus or stiff big toe may be secondary to trauma or increased stress on the joint.
|
-
A below-the-knee walking cast for fractures
-
A shoe with an insert or pad and NSAIDs for osteochondritis and AVN
-
Activity modification and reduced weight-bearing for sesamoiditis
-
Orthotics, a shoe with a stiff insole and padding, and NSAIDs for arthritis
-
Pitfalls in the MR interpretation of the lesser MTP joints include the normal plantar plate recess, the capsular plantar plate attachment, and the distal phalangeal bare area.
-
Intermediate-signal-intensity hyaline articular cartilage may undercut the plantar plate on sagittal images.
-
Plantar plate tears occur most frequently at their distal attachment.
-
MTP joint instability is associated with plantar plate degeneration and rupture.
-
The collateral ligaments, represented by the accessory collateral ligament (ACL) and the phalangeal collateral ligament (PCL) (the PCL is also known as the proper collateral ligament and the main collateral ligament)
-
The flexor tendons, including the flexor digitorum brevis (FDB) and the FDL tendon
-
The extensor head, which, along with the extensor sling, represents the fibroaponeurotic expansion from the extensor digitorum longus (EDL) tendon sheath
-
ACL
-
Extensor tendon and hood
-
Deep transverse intermetatarsal ligament, which blends with the medial and lateral margins of the plantar plate
-
Flexor tendon sheath located centrally within a groove at the undersurface of the plantar plate
MTP joint instability. Capsular distention results in degeneration and weakening of the plantar plate and collateral ligaments. Metatarsalgia may also be associated with stress fractures, Freiberg's infraction, Morton's neuroma, arthritis, and synovial cyst formation.
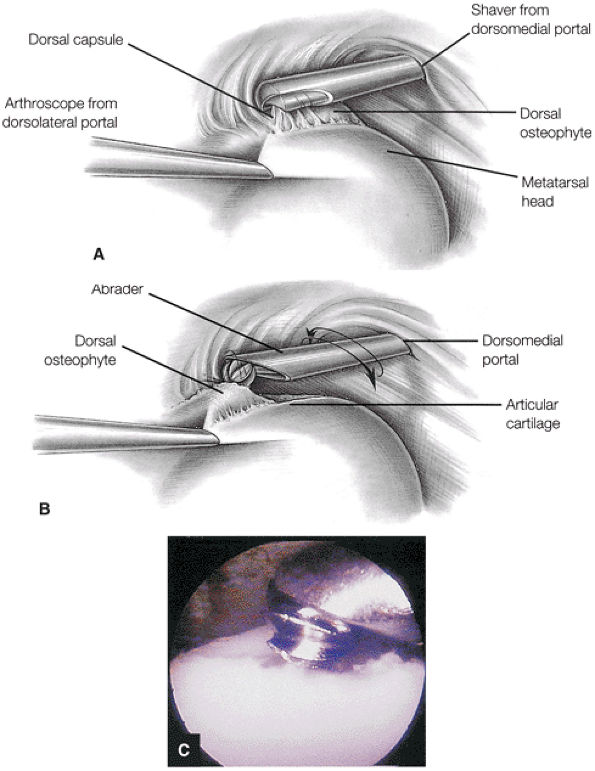 |
|
FIGURE 5.275 ● Excision of a dorsal osteophyte. (A) Visualization from the dorsomedial portal (viewed proximally from within the MTP joint). (B) A small-joint abrader is used to remove enough osteophyte to permit adequate MTP joint dorsiflexion. (C) Arthroscopic view of osteophyte removal with a motorized bur.
|
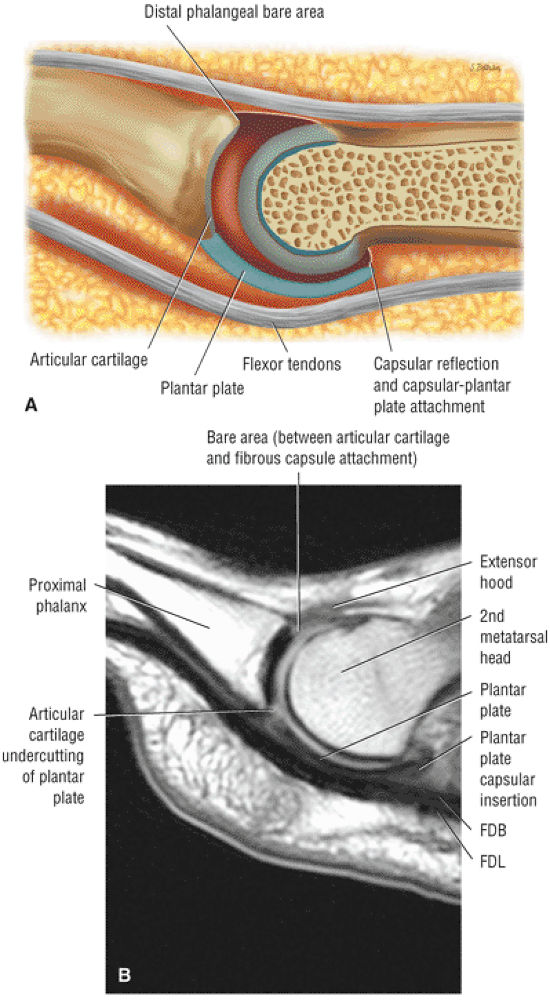 |
|
FIGURE 5.276 ● MTP joint plantar plate anatomy. The hypointense plantar plate is directly deep to the superior border of the flexor tendons. There is a normal undercutting of the plantar plate at the base of the proximal phalanx at the interface with hyaline articular cartilage. (A) Lateral view color illustration of the third MTP joint plantar plate. (B) Sagittal PD FSE image.
|
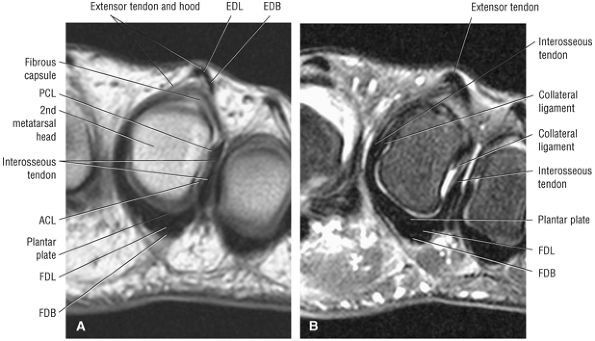 |
|
FIGURE 5.277 ● Coronal plane anatomy of the second MTP joint identifies the plantar plate, the collateral ligaments, the flexor tendons, and the extensor hood. (A) Coronal PD FSE image. (B) Coronal FS PD FSE image.
|
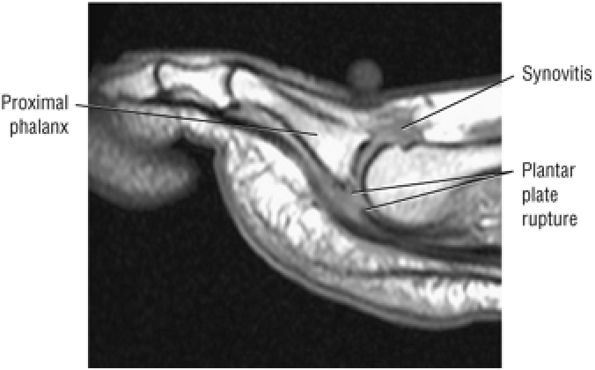 |
|
FIGURE 5.278 ● Distal plantar plate rupture associated with chronic second MTP joint synovitis. Sagittal T1-weighted image.
|
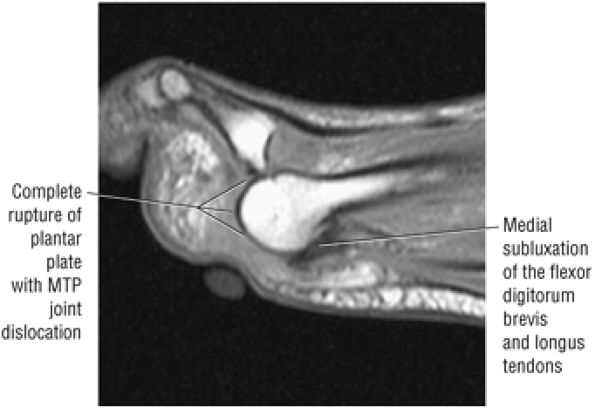 |
|
FIGURE 5.279 ● Third MTP joint instability after aggressive Morton's neuroma resection. There is secondary disruption to the plantar plate complex with associated medial subluxation of the flexor tendons. Sagittal T1-weighted image.
|
-
Fractures of the distal tibia include pilon, Tillaux, juvenile Tillaux, and triplanar (triplane) types.
-
A triplane fracture is a combination of the juvenile Tillaux fracture (Salter-Harris type III fracture) and a Salter-Harris type II fracture and involves a fracture plane in the sagittal, axial, and coronal directions.
-
Fractures of the fibula include Pott, Dupuytren, and Maisonneuve types.
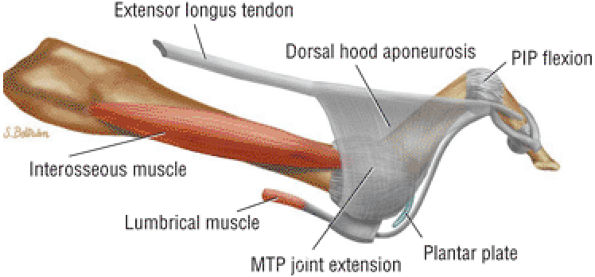 |
|
FIGURE 5.280 ● A claw-toe deformity represents a hammertoe deformity (flexion deformity at the PIP joint with MP dorsiflexion and the DIP in neutral or hyperextension) with the addition of a hyperextension deformity at the MP joint. Both hammertoe and claw toe are examples of sagittal plane deformities. Lateral color illustration.
|
-
Medial malleolus (Fig. 5.282)
-
Lateral and posterior malleoli (Fig. 5.283)
-
A pilon fracture of the distal tibia/load-bearing surface with intra-articular extension
-
A Tillaux fracture of the lateral margin of the distal tibia to the distal articular surface (Fig. 5.284)
-
A juvenile Tillaux fracture of the distal tibial growth plate (Salter-Harris type III) (see Fig. 5.284)
-
A triplane fracture of the lateral distal tibial epiphysis with sagittal, axial, and coronal plane components (a juvenile Tillaux/Salter-Harris type III and Salter-Harris type II combination) (Fig. 5.285)
-
A Pott's fracture of the distal third of the fibula (Fig. 5.286)
-
Le Fort (Wagstaffe-Le Fort) fracture, an avulsion fracture of the anterior syndesmotic ligament from the medial aspect of the distal fibula109
-
A Dupuytren fracture of the fibula, 2 to 7 cm proximal to the distal tibiofibular syndesmosis (Fig. 5.287)
-
A Maisonneuve fracture of the proximal fibula at the proximal to middle third of the diaphysis (Fig. 5.288)
-
Supination-external rotation injuries
-
Supination-adduction injuries
-
Pronation-external rotation injuries
-
Pronation-abduction injuries
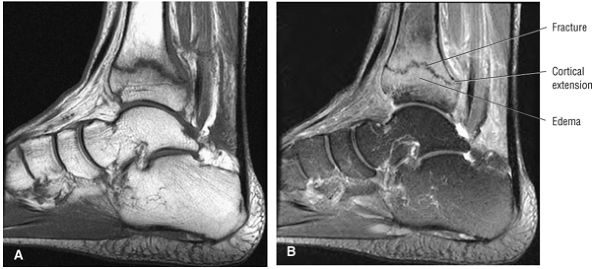 |
|
FIGURE 5.281 ● Distal tibial fracture with hypointense fracture line and extension to cortex. Adjacent edema is hyperintense on FS PD FSE image (B). (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image.
|
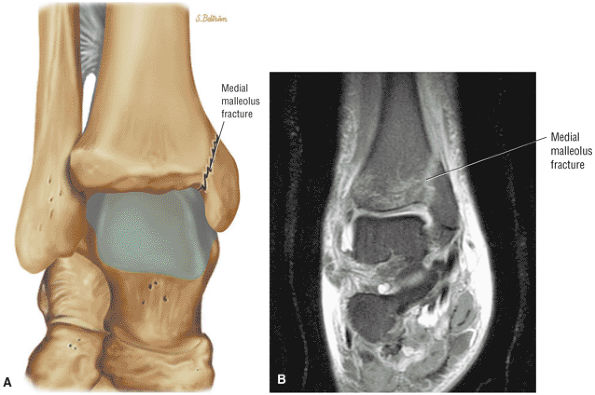 |
|
FIGURE 5.282 ● Medial malleolus fracture on coronal color illustration (A) and coronal FS PD FSE image (B). Isolated malleolar involvement represents a unimalleolar fracture and occurs in eversion injuries. MR is used to evaluate associated ligamentous injury.
|
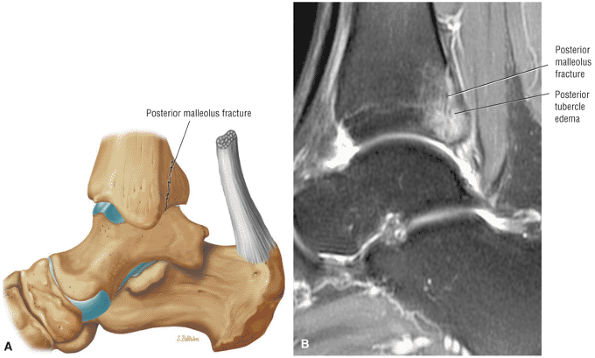 |
|
FIGURE 5.283 ● Posterior malleolus fracture on a lateral color illustration (A) and a sagittal FS PD FSE image (B). Posterior malleolus fractures are associated with trimalleolar fractures also involving the medial and lateral malleoli.
|
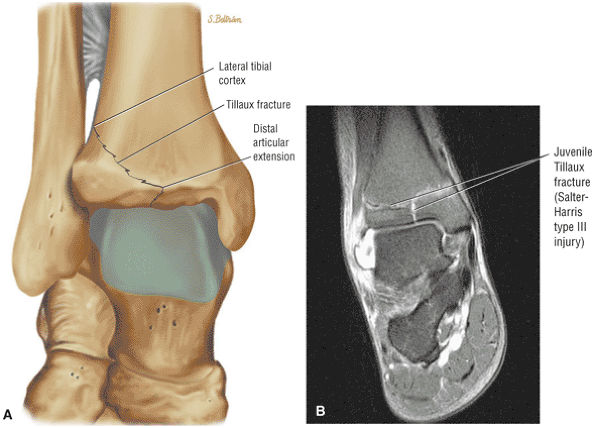 |
|
FIGURE 5.284 ● (A) Coronal color illustration of a Tillaux fracture with avulsion of the lateral margin of the distal tibia. The fracture extends from the distal tibial articular surface (plafond) obliquely and vertically to the lateral tibial cortex. (B) Coronal FS PD FSE image showing a juvenile Tillaux fracture, a Salter-Harris type III injury of the distal tibial physis. The lateral tibial physis is weaker than the medial side of the growth plate since the physis fuses from medial to lateral.
|
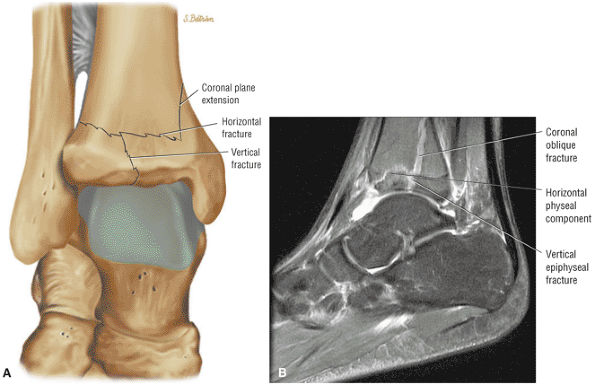 |
|
FIGURE 5.285 ● Triplane fracture with a vertical fracture component of the epiphysis, a horizontal component involving the physis, and a posterior coronal component of the metaphysis. The triplane fracture represents a combination of a juvenile Tillaux fracture and a Salter-Harris type II fracture. (A) Coronal color illustration. (B) Sagittal FS PD FSE image.
|
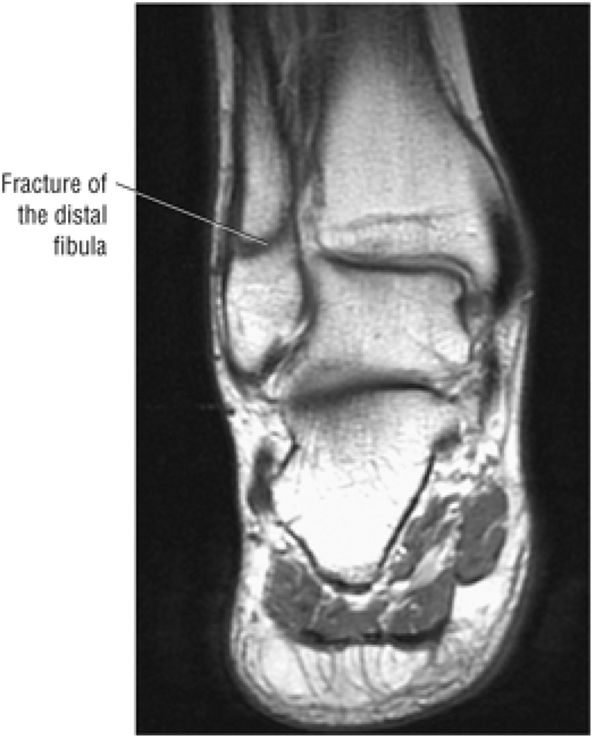 |
|
FIGURE 5.286 ● Coronal T1-weighted image of a large distal fibular fracture associated with a deltoid ligament sprain. The classic Pott's fracture is more proximal at the level of the interosseous membrane. The term “Pott's fracture” usually indicates a distal fibular fracture superior to the tibiofibular syndesmosis. Frequently there is an associated rupture of the deltoid ligament with lateral subluxation of the talus.
|
-
Danis-Weber type A fractures are caused by Lauge-Hansen supination-adduction mechanisms.
-
Danis-Weber type B fractures are caused by Lauge-Hansen supination external rotation or pronation-abduction mechanisms.
-
Danis-Weber type C fractures are caused by Lauge-Hansen pronation-external rotation mechanisms.
-
Stage 1: ATFL rupture
-
Stage 2: Oblique spiral fracture of the lateral malleolus from the plafond extending proximally
-
Stage 3: PITF tear and posterior lip or margin fracture of the tibia
-
Stage 4: Avulsion fracture of the medial malleolus or deltoid ligament rupture.
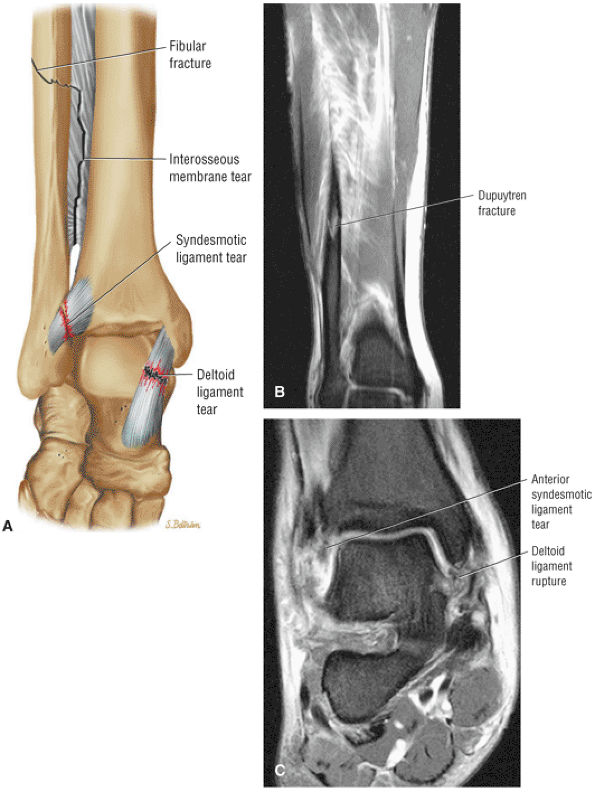 |
|
FIGURE 5.287 ● Dupuytren fracture involving fracture of the fibula 2 to 7 cm superior to the torn tibiofibular syndesmosis. There is associated disruption of the deltoid ligament. (A) Coronal view color illustration. (B, C) Coronal FS PD FSE images.
|
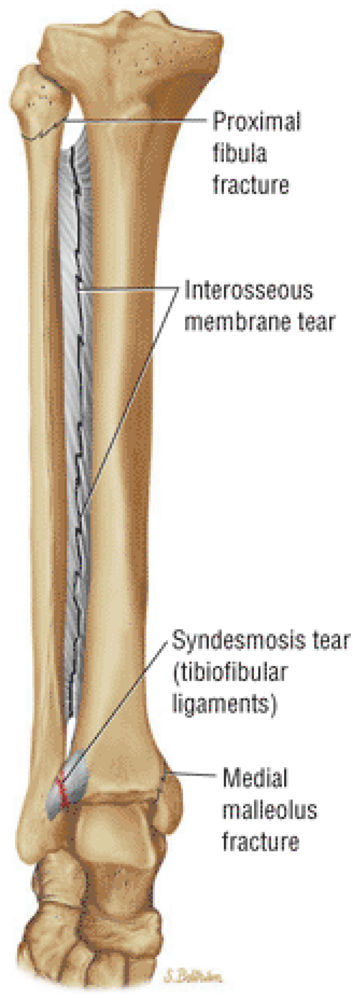 |
|
FIGURE 5.288 ● Maisonneuve eversion fracture involving the proximal fibula with associated disruption of the tibiofibular syndesmosis including the interosseous membrane. A fracture of the medial malleolus is also demonstrated. The more proximal location of the fibular fracture indicates a more extensive injury of the interosseous membrane.
|
-
Stage 1: Lateral ligamentous failure and lateral malleolus avulsion (a transverse fracture at the level of the plafond)
-
Stage 2: A vertical, medially displaced fracture of the medial malleolus
-
Stage 1: Transverse avulsion fracture of the medial malleolus or deltoid ligament rupture
-
Stage 2: Rupture of the anterior and posterior inferior tibiofibular syndesmotic ligaments
-
Stage 3: Horizontal, oblique fibular fracture above the joint (plafond)
-
Type I: Undisplaced fissure fracture
-
Type II: Displaced fracture and articular incongruity
-
Type III: A compression fracture and displacement of weight-bearing segments (crushing subchondral cancellous bone)
-
Malleolar fractures: fracture line, diffuse marrow edema to localized edema adjacent to the fracture site
-
Pilon fractures: separate comminuted distal tibial fragment, fracture extension to the tibial plafond, may or may not be displaced
-
Tillaux fractures: vertical fracture line perpendicular to the epiphysis and a thick lateral physis with lateral fracture extension
-
Triplanar fractures: separate fractures oriented anteroposteriorly in the axial plane, proximal to distal in the coronal plane, and oblique in the sagittal plane
-
Fibular fractures: transverse to oblique fracture line
-
Distal tibial fractures: localized edema adjacent to the fracture site, variable proximal extension of signal, interruption of the distal chondral surface with fluid signal
-
The Salter-Harris classification is divided into five types. Salter-Harris type V represents a compression fracture through the physis.
-
Trauma can also occur to the perichondrium, or there may be isolated epiphyseal or metaphyseal injuries or avulsion of the periosteum.
-
Entrapment of the periosteum is associated with Salter-Harris type I injuries.
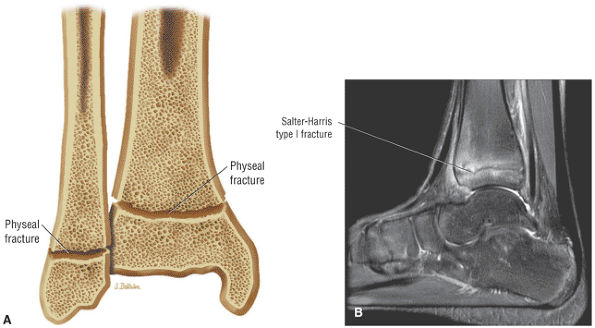 |
|
FIGURE 5.289 ● Salter-Harris type I fracture through the physis. (A) Coronal section color illustration. (B) Sagittal FS PD FSE image.
|
-
Type I: The most common epiphyseal injury is a lateral malleolus Salter-Harris type I fracture (Fig. 5.289), which is through the growth plate.112 A Salter-Harris type I fracture may be complicated by the interposition of periosteum in the growth plate (Fig. 5.290).
-
Type II: Salter-Harris type II fractures (Fig. 5.291) extend through the physis and metaphysis, and displaced type II fractures of the tibial epiphysis are usually associated with a greenstick fracture of the fibula, which occurs with eversion and external rotation injuries.
-
Type III: Salter-Harris type III fractures (Fig. 5.292) extend through the physis and into the epiphysis.
-
Type IV: Salter-Harris type IV fractures (Fig. 5.293) involve the physis, metaphysis, and epiphysis.
-
Type V: In Salter-Harris type V injuries, there is compression across the physis.
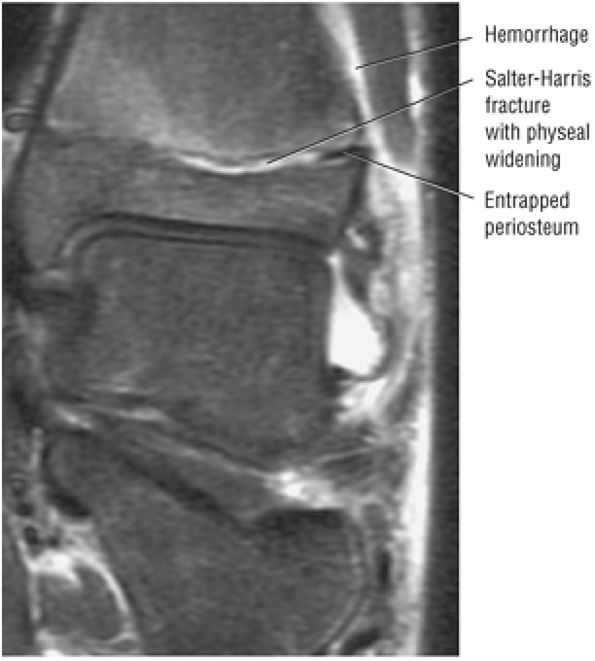 |
|
FIGURE 5.290 ● Enfolded or trapped periosteum in the lateral aspect of a distal tibial Salter-Harris type I physeal fracture. The periosteum typically tears on the tension or distraction side of the fracture site. Entrapped periosteum may result in an irreducible fracture.
|
-
Fractures of the calcaneus are either extra-articular or intra-articular.
-
Intra-articular fractures are divided into Essex-Lopresti type A, or tongue-type, and Essex-Lopresti type B, or joint depression type.
-
75% of calcaneal fractures extend into the subtalar joint.
-
The Rowe classification of calcaneal fractures is based on five types, with type IV and V involving the subtalar joint.
-
Essex-Lopresti type A or tongue-type fractures: The tongue-type fracture is transverse and extends posterior to the posterior facets and the dorsal calcaneal tuberosity.
-
Essex-Lopresti type B or joint depression-type fractures: The depression-type fracture has a secondary fracture line that runs from the body of the calcaneus directly posterior to the fractured articular surface posterior facet.
-
Type I: Fractures of the tuberosity, the sustentaculum tali, and the anterior process; 21% of cases
-
Type II: Beak fractures of the calcaneus and avulsion fractures of the Achilles tendon insertion; 3.8% of cases
-
Type III: Oblique fractures without subtalar involvement; 19.5% of cases
-
Type IV: Involvement of the subtalar joint; 24.7% of cases
-
Type V: Central depression with or without comminution; 31% of cases
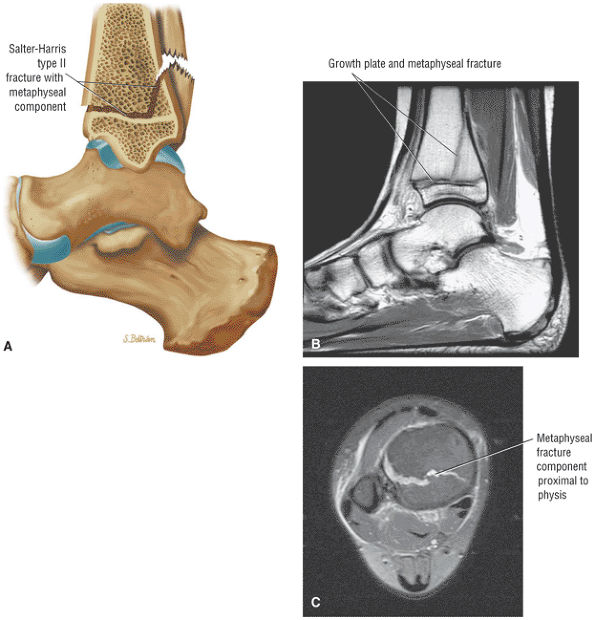 |
|
FIGURE 5.291 ● Salter-Harris type II fracture through the phy-sis and metaphysis of the distal tibia. There is no epiphyseal extension. (A) Lateral color illustration. (B) Sagittal T1-weighted image. (C) Axial FS PD FSE image.
|
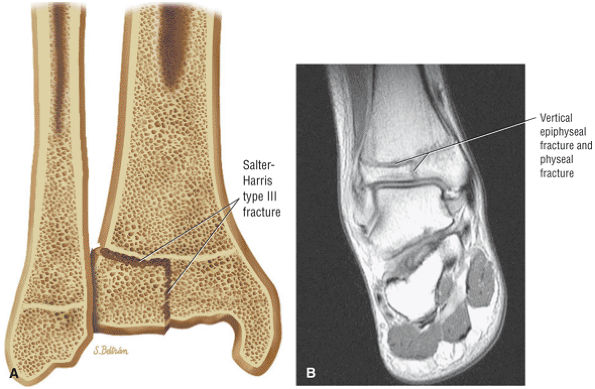 |
|
FIGURE 5.292 ● Salter-Harris type III fracture extending through the physis and epiphysis. (A) Coronal section color illustration. (B) Coronal T1-weighted FSE image.
|
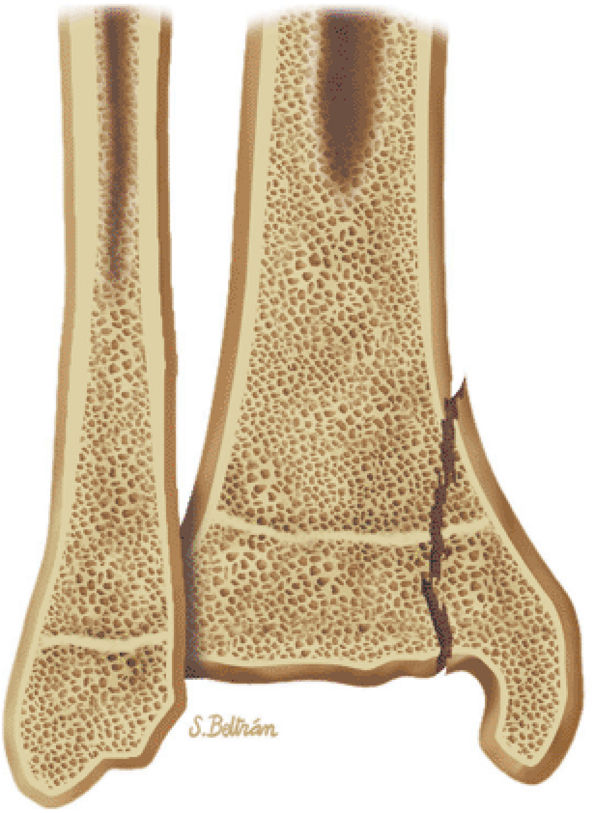 |
|
FIGURE 5.293 ● Salter-Harris type IV with fracture extension through the physis, metaphysis, and epiphysis. Coronal section color illustration.
|
-
For anterior process (Rowe type I) fractures (Fig. 5.295), key characteristics include:
-
Avulsion at the origin of the bifurcate ligament, edema, or chronic hypointense sclerosis
-
Edema on lateral sagittal images
-
Compression fracture with or without avulsion of the navicular tuberosity, edema, and extension to the calcaneocuboid joint
-
Involvement of the extensor digitorum brevis muscle and calcaneocuboid ligament
-
-
For fractures of the sustentaculum tali (Rowe type I), findings include:
-
Possible medial displacement
-
Edema, fluid, or hemorrhage on axial and coronal images
-
-
For beak and avulsion (Rowe type II) fractures, findings are:
-
Edema in the beak or avulsed tuberosity
-
Achilles tendon attached to superior tuberosity
-
A smaller beak fracture and a larger avulsion secondary to sudden contraction of the Achilles tendon
-
Fracture line posterior to the superior calcaneus
-
-
Calcaneal body (Rowe type III) fractures are evaluated on sagittal images to exclude posterior facet involvement and demonstrate:
-
No subtalar joint communication
-
Fracture line posterior to the posterior facet or comminution
-
-
Intra-articular fractures are evaluated on coronal images to display widening of the calcaneus and sagittal images to separate a central depression from a tongue-like fracture. Characteristic findings include:
-
Edema in the joint
-
Depression or tongue-type fracture
-
Chondral extension
-
Free fragments, displayed as hypointense bodies outlined by hyperintense fluid on FS PD FSE images
-
-
Stress fractures (Fig. 5.296) are characterized by:
-
Linear pattern of signal intensity in the posterior calcaneus perpendicular to the long axis
-
More diffuse posterior calcaneus trabecular microfracture edema
-
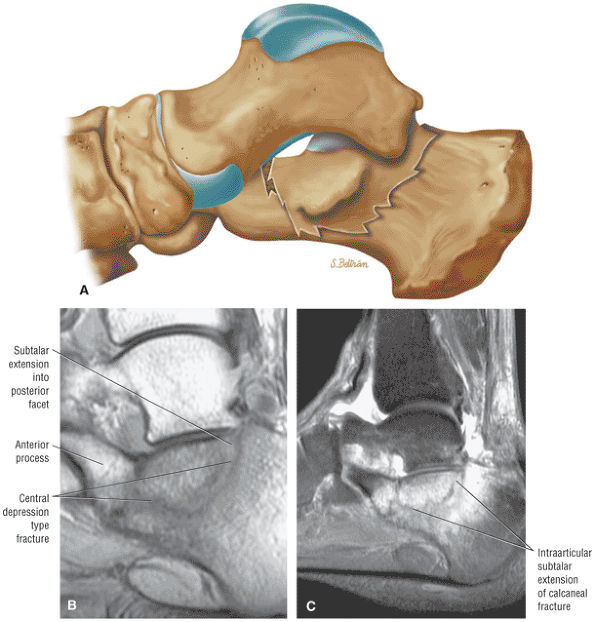 |
|
FIGURE 5.294 ● Essex-Lopresti calcaneal fracture with central depression and subtalar extension. Up to 75% of calcaneal fractures extend into the subtalar joint. Subtalar fracture types are further subdivided into either joint-depression or tongue-type fractures. In the Rowe classification there is subtalar joint involvement in stage IV and stage V (central depression) injuries. (A) Lateral color illustration. (B) Sagittal PD FSE image. (C) Sagittal FS PD FSE image.
|
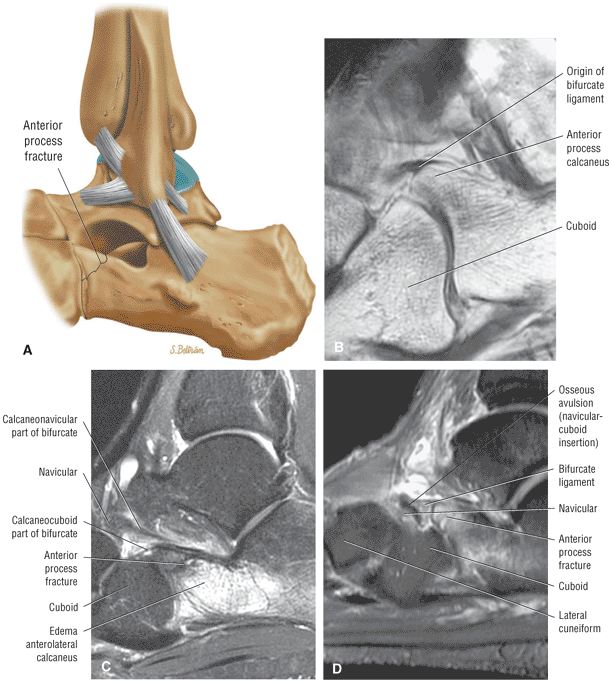 |
|
FIGURE 5.295 ● (A) Fracture of the anterior process of the calcaneus on a lateral perspective color illustration. (B) Calcaneal origin of the bifurcate ligament with an intact anterior process. Sagittal T1-weighted image. (C) Fractured anterior process with extensive reactive anterolateral calcaneal edema. Sagittal FS PD FSE image. (D) Anterior process fracture with osseous avulsion injury of the calcaneonavicular and calcaneocuboid parts of the bifurcate ligament. Inversion and plantarflexion injuries may result in an avulsion injury at the origin of the bifurcate ligament. Associated distal avulsion is less common. Sagittal FS PD FSE image.
|
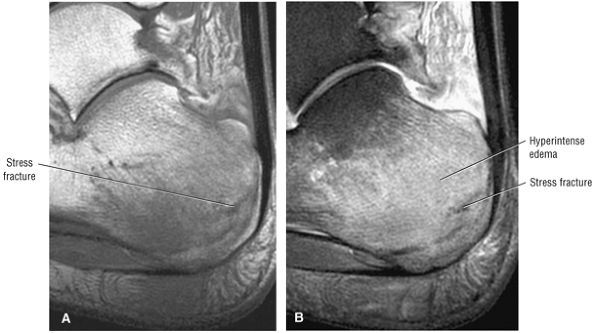 |
|
FIGURE 5.296 ● Posterior calcaneus stress fractures with hypointense fracture segments and adjacent hyperintense marrow edema. (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image. A common location for a calcaneal stress fracture is the upper posterior margin, anterior to the apophyseal plate, perpendicular to the trabecular pattern of the calcaneus.
|
-
Talar fractures may involve the talar head, neck, body, or lateral or posterior process.
-
Talar fractures are associated with high-energy traumatic injuries, including falls and motor vehicle accidents.
-
Lateral process fractures, also known as snowboarder's fractures, are associated with inversion, dorsiflexion, and compressive force.
-
It is important to evaluate for AVN with talar neck fractures.
The talus may be injured at the neck, the body, the head, and the posterior and lateral processes. Since the ankle mortise protects the talus from direct injury, talar fractures usually result from transmitted forces.111 Neck fractures are the most common (50% of cases) and usually occur in males (male-to-female ratio is 3:1) between 30 and 38 years of age. Fractures of the lateral process of the talus are a recently recognized complication of snowboarding, associated with dorsiflexion and inversion injuries.117 Fractures may be nondisplaced, displaced, or open. Open fractures account for 15% of cases. Associated injuries include calcaneus, medial malleolus, and spine fractures.
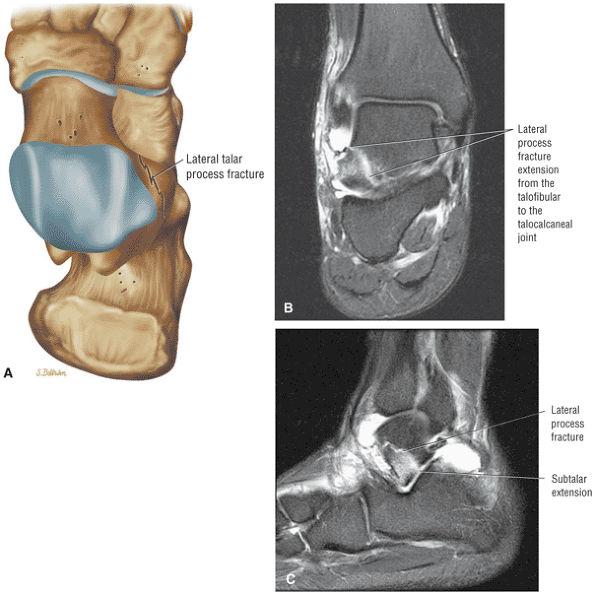 |
|
FIGURE 5.297 ● Snowboarder's or lateral process fracture of the talus. The mechanism of injury is related to eversion of an axial-loaded and dorsiflexed ankle. A lateral process fracture may be further classified as a simple comminuted fracture or as a chip fracture. (A) Superior view color illustration. (B) Coronal FSE PD FSE image. (C) Sagittal FS PD FSE image.
|
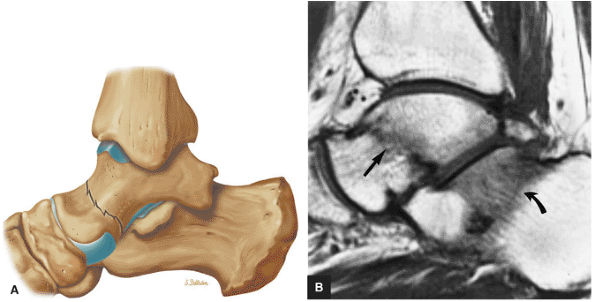 |
|
FIGURE 5.298 ● Talar neck fracture on lateral color illustration (A) and sagittal T1-weighted image (B). The talar fracture line (straight arrow) and calcaneal trabecular trauma (curved arrow) are hypointense on the T1-weighted sequence. Talar neck fractures may be associated with medial malleolus, sustentaculum tali, or metatarsal head fractures.
|
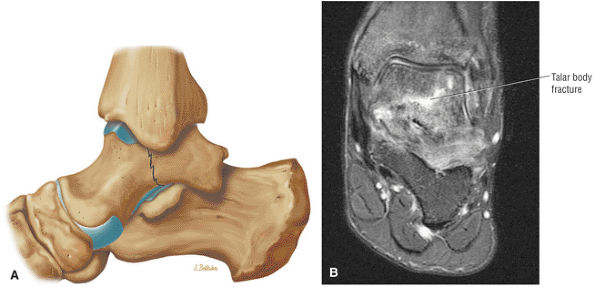 |
|
FIGURE 5.299 ● Talar body fracture on a lateral color illustration (A) and a coronal FS PD FSE image (B). The mechanism of injury is axial loading or a shear force. Associated injuries include fractures of the calcaneus, tibia, or talar neck.
|
-
Type I: No talar displacement
-
Type II: Subluxation and/or dislocation of the talus with respect to the subtalar joint (dislocation of the posterior facet). Type II injuries are the most common type.
-
Type III: Displacement of the talar body (ankle and subtalar dislocation). Type III injuries are also common.
-
Type IV: A rare injury in which there is additional dislocation of the talonavicular joint
-
Fractures of the head of the talus show:
-
Avulsion, usually medially
-
Intra-articular displacement or dislocation of the talonavicular joint
-
Single versus multiple hypointense fracture lines
-
Associated diffuse hypointensity on T1- and PD-weighted images
-
Hyperintense talar head on FS PD FSE images
-
Edema, which may obscure the fracture line
-
Chondral extension to the talonavicular joint
-
-
Lateral process fractures show:
-
Edema in comminuted fracture on coronal and sagittal images
-
Oblique/vertical single fracture and intra-articular displacement
-
Increased subtalar fluid and chondral interruption
-
-
Posterior process fractures, which may be difficult to differentiate from a painful os trigonum on TI-weighted images, display:
-
Irregular separation on sagittal images, which represents the talus and a fragment
-
A complete vertical fluid gap between the talus and the posterior process
-
Adjacent marrow edema
-
Soft-tissue fluid and edema
-
Fluid in the FHL tendon sheath
-
-
Talar neck fractures display:
-
A posterior to transverse sulcus
-
A vertical nondisplaced segment (type I)
-
Subluxation or dislocation of the talus in the subtalar joint (type II)
-
Displacement of the talar body (type III)
-
Fluid and hemorrhage with separation between the head and the neck and body
-
Adjacent marrow fragment
-
Extrusion of the talus from the subtalar joint (Hawkins type III injury) seen on sagittal plane images
-
-
Talar body injuries show:
-
Comminuted fracture
-
Primary fracture in any of the three orthogonal planes
-
Edema across the fracture plane (coronal, sagittal, or axial)
-
Soft-tissue edema and hemorrhage.
-
-
Navicular fractures include fractures of the tuberosity, body avulsion, and stress fractures.
-
The central third of the navicular is avascular and at increased risk for stress fracture and nonunion.
-
Navicular marrow edema visualized on sagittal images should be correlated with axial or coronal images to identify fracture morphology.
a fracture line perpendicular to the long axis of the navicular. They represent 62% of midfoot fractures and 37% of all foot fractures.
-
Avulsion fracture s: Avulsion fractures usually affect the dorsal lip at the insertion of the dorsal tibionavicular ligament (the tibialis posterior insertion). Cortical avulsion fractures involve part of the dorsal cortex, and there may be an elongated cortical fragment. They represent 47% of navicular fractures.
-
Tuberosity fractures: Tuberosity fractures vary in size with the size of the tuberosity but are usually minimally displaced. The fracture edges may be sharp and/or jagged. They represent 24% of navicular fractures.
-
Body fractures: Body fractures are most commonly horizontal and nondisplaced, and may be associated with crush injuries. They account for 29% of navicular fractures.
-
Stress fractures: Stress fractures usually affect the central third of the navicular (in the sagittal plane), which is avascular and therefore at risk for stress fracture and nonunion. They also involve the anteroposterior or short axis length of the navicular. They may be complete or incomplete, but most stress fractures are characterized as partial (96%) and linear. In nonunion, persistence of a fracture gap can be seen. Medullary cysts and cortical notching may persist even after fracture healing.
-
Type 1: Transverse with a dorsal fragment (affecting over 50% of the body)
-
Type 2: A transverse fracture from dorsolateral to plantar medial across the body. The major fragment is dorsomedial; the smaller comminuted fracture is plantar lateral.
-
Type 3: Central or lateral comminution. The major fragment is medial.
-
Avulsion fractures are characterized by:
-
Elongated dorsal cortical fragment
-
Adjacent dorsal soft-tissue and navicular edema
-
Cortical-based fragment that remains hypointense on FS PD FSE images
-
-
Tuberosity fractures (Fig. 5.300) show:
-
Subchondral edema across the fracture
-
Irregular edges at the fracture site
-
Fluid and/or hemorrhage at the fracture site
-
Fluid involving the PTT
-
-
Body fractures display:
-
Vertical or horizontal fracture line and edema
-
Subchondral edema
-
Hyperintensity on FS PD FSE images: epicentered in coronal plane, from dorsolateral to plantar medial within navicular or comminution in sagittal plane
-
-
Stress fractures (Fig. 5.301) are characterized by:
-
Fracture line on direct coronal or axial images
-
Marrow edema, sometimes with a visualized fracture on sagittal images
-
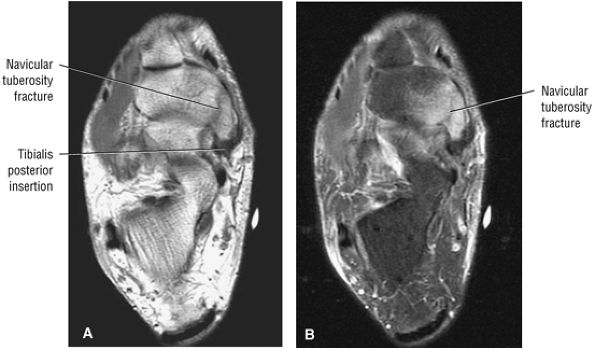 |
|
FIGURE 5.300 ● Navicular tuberosity fracture on axial T1-weighted (A) and FS PD FSE (B) images. A navicular tuberosity fracture involving the tibialis posterior tendon insertion may require operative repair if the proximal displacement is 1 cm or greater.
|
-
Lisfranc fractures may be homolateral or divergent tarsometatarsal fracture dislocations.
-
Lisfranc's ligament extends from the medial cuneiform to the medial aspect of the second metatarsal base.
-
Axial MR images are used to evaluate Lisfranc's ligament and to assess the lateral effect of the first metatarsal base relative to the medial cuneiform and the medial second metatarsal base relative to the intermediate cuneiform in the homolateral type of injury.
-
Fractures are associated with sports-related injuries in athletes and Charcot arthropathy in diabetic patients.
-
Direct coronal images are used to appreciate dorsal/plantar displacement.
-
The base of the second metatarsal, a frequent site of injury, is recessed.
-
The metatarsals are bound by the transverse dorsal and the stronger plantar ligaments.
-
There is no ligament that directly attaches the first metatarsal base to the base of the lesser (second to fifth) metatarsals.
-
The dorsal medial ligament originates from the medial cuneiform and attaches to the first metatarsal.
-
Lisfranc's ligament originates from the medial cuneiform and attaches to the second metatarsal base (the medial aspect).
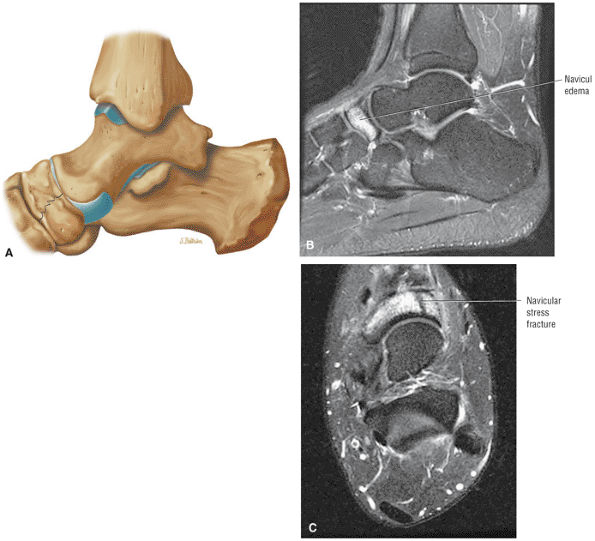 |
|
FIGURE 5.301 ● Navicular stress fracture involving the central to medial aspect of the dorsum of the navicular. Edema is often prominent in the sagittal plane, whereas fracture morphology is visualized in the axial plane. Delayed union and nonunion are potential risks secondary to the relative avascularity and increased shear forces that occur in this region. (A) Lateral color graphic. (B) Sagittal FS PD FSE image. (C) Axial FS PD FSE image.
|
-
Type A: Total incongruity and lateral or dorsoplantar displacement of the first to fifth metatarsals.
-
Type B: Partial incongruity with medial dislocation of the first metatarsal and lateral dislocation of the second to fifth metatarsals
-
Type C: Divergent, with partial or total displacement of the first metatarsal medially and the lesser metatarsals laterally
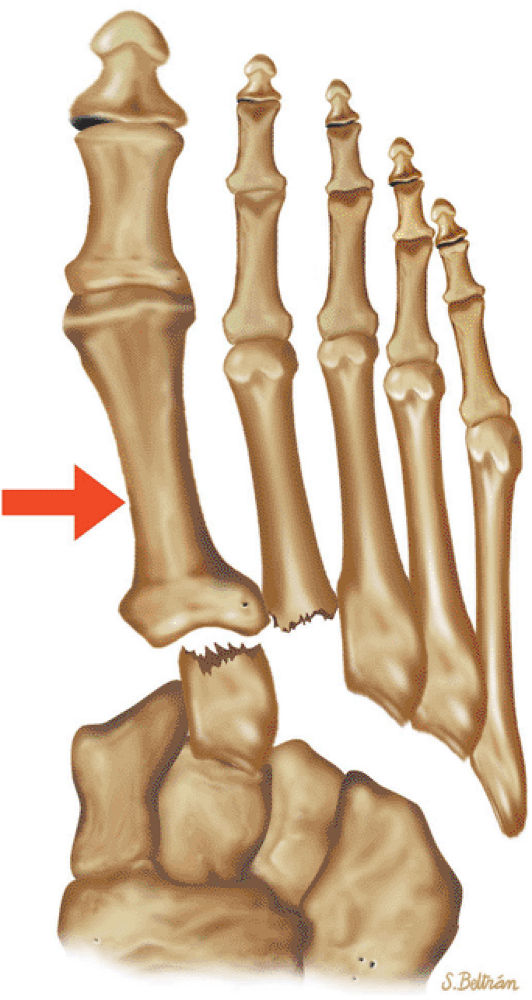 |
|
FIGURE 5.302 ● Homolateral Lisfranc fracture-dislocation with lateral dislocation of the first to fifth metatarsals. Both the homolateral and divergent types of injuries are associated with a fracture of the base of the second metatarsal. Superior view color graphic.
|
or CT examination results are negative. MR imaging has proved to be more sensitive than CT in identifying the extent of posttraumatic marrow hyperemia and the number of bones of the tarsus affected, but thin-section (1.5 mm) CT scans in both the coronal and axial planes are more accurate in identifying small osseous corner or chip fragments in the cuneiforms and base of the metatarsals.
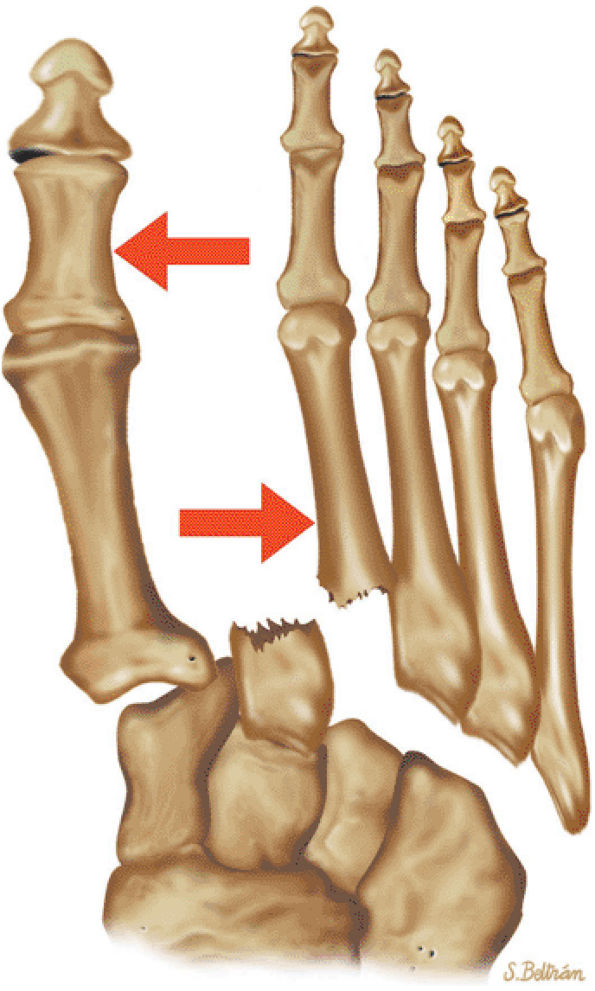 |
|
FIGURE 5.303 ● Divergent Lisfranc fracture-dislocation with medial dislocation of the first metatarsal and lateral dislocation of the second to fifth metatarsals. Superior view color graphic.
|
-
Axial images show lateral displacement of the first to fifth metatarsals in homolateral fractures (Fig. 5.304), medial dislocation of the first metatarsal and lateral dislocation of the second through fifth metatarsals in divergent fractures, and widening of the first-second metatarsal interspace.
-
Fractures may be seen at the base of the second or third metatarsal, the medial/intermediate cuneiform, or the navicular.
-
There may be lateral column shortening caused by an impaction injury of the cuboid with an abduction deformity of the forefoot.
-
Direct coronal images show disruption of the dorsal arch (the second metatarsal is normally found at the apex of the arch).
-
Discontinuity of Lisfranc's ligament may be seen at the medial cuneiform to the medial proximal second metatarsal.
-
Edema is seen at the Lisfranc ligament avulsion fracture, at the proximal medial aspect of the second metatarsal.
-
Subchondral marrow edema of tarsometatarsal joints indicates a chip or trabecular fracture (Fig. 5.305).
-
There may be fraying or tearing of the oblique (Lisfranc) ligament with or without synovitis (Fig. 5.306).
-
Cuboid marrow hyperintensity with trabecular impaction injury (Fig. 5.307)
-
Diabetes-related Lisfranc injuries are chronic and associated with midfoot neuropathic changes, as seen in Charcot arthropathy (Fig. 5.308). On FS PD FSE images superimposed neuropathic hyperintensity can be seen throughout the midfoot and the Lisfranc fracture-dislocation.
-
On direct coronal plane FS PD FSE images, intramedullary hyperintensity in the displaced metatarsals is seen in cross-section.
-
Associated arterial injury is shown on coronal images.
-
Fracture types include stress, head, neck, midshaft, base, first, central (second through fourth), or fifth metatarsal fractures.
-
The Jones fracture occurs through the proximal metatarsal diaphysis and is at risk for delayed or fibrous union.
-
Stress fractures are associated with overuse, including running and dancing.
-
Metatarsal marrow edema may persist after clinical resolution of stress fracture symptoms.
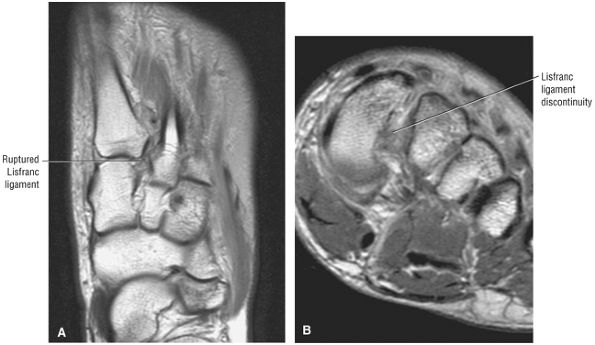 |
|
FIGURE 5.304 ● Ruptured Lisfranc ligament. Lisfranc's ligament is normally composed of a weaker dorsal component and a stronger plantar component. (A) Axial T1-weighted image. (B) Coronal T1-weighted image.
|
-
Fractures of the head are usually caused by direct trauma.
-
Neck fractures are caused by shearing force or direct trauma.
-
Midshaft fractures are caused by direct, blunt, or torsional forces.
-
Fractures of the base are caused by direct trauma, frequently sustained in motor vehicle accidents or a fall from a height.
-
First metatarsal fractures are often caused by crush or twisting injuries.
-
Fractures of the central metatarsals are caused by direct impact or crushing.
-
Fifth metatarsal fractures are caused by plantarflexion and inversion or direct impact.
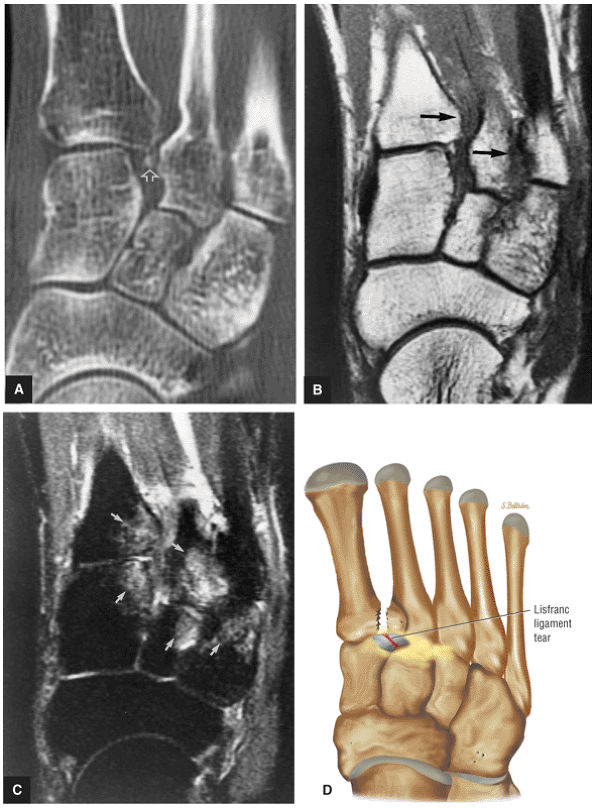 |
|
FIGURE 5.305 ● Homolateral type Lisfranc fracture-dislocation with lateral displacement (large black arrows) shown in metatarsal bases on direct axial CT scan (A), T1-weighted axial image (B), axial STIR image (C), and transverse (superior view) color illustration (D). Although the fracture fragment (open arrow) can be identified between the base of the first and second metatarsals on the CT scan, MR imaging is more sensitive to the extent of traumatic marrow edema (small closed arrows) involving the cuneiforms and metatarsal bases. Associated fractures in a Lisfranc tarsometatarsal dislocation occur at the base of the second or third metatarsals, medial or intermediate cuneiforms, or the navicular bone.
|
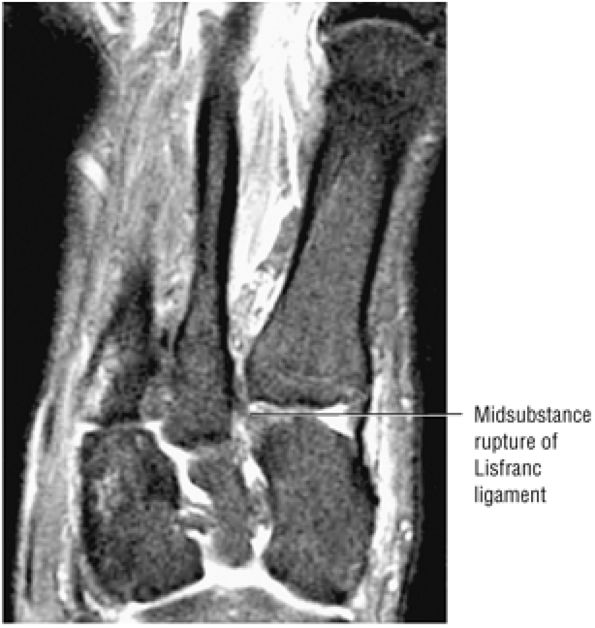 |
|
FIGURE 5.306 ● Complete tear of the obliquely oriented Lisfranc's ligament. Lisfranc's ligament normally connects the lateral aspect of the medial cuneiform to the medial aspect of the second metatarsal base. Complex ligament tears are associated with displacement of the second metatarsal and medial cuneiform on weight-bearing. Axial FS PD FSE image.
|
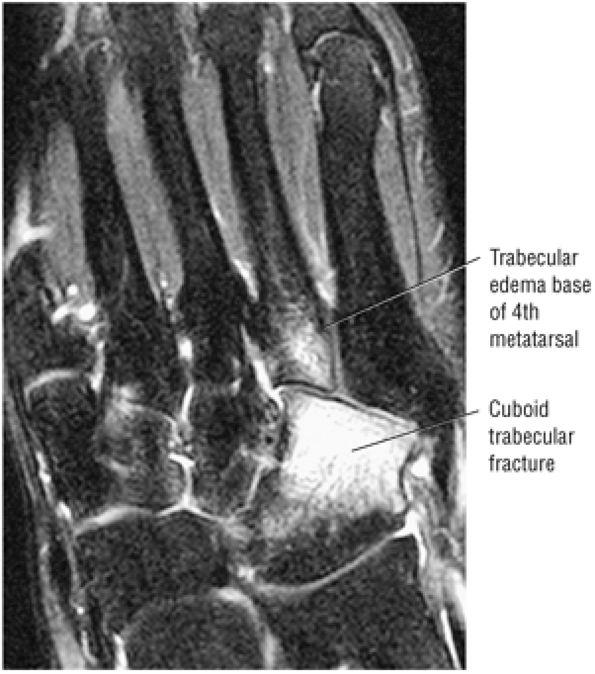 |
|
FIGURE 5.307 ● Cuboid fractures may be associated with Lisfranc injuries. Partial tears of Lisfranc's ligament usually involve the weaker dorsal band. Diastasis of the bases of the first and second metatarsal should always be assessed on MR relative to the medial and intermediate cuneiforms. Axial FS PD FSE image.
|
 |
|
FIGURE 5.308 ● Lisfranc fracture-dislocation as a complication of diabetes. Associated hyperintense neuropathic changes are present in the midfoot. Axial FS PD FSE image.
|
-
Type I: Jones fracture (Fig. 5.312)
-
Type II: Intra-articular fracture
-
Type III: Avulsion fracture (Fig. 5.313)
-
Type IV: Comminuted intra-articular fracture
-
Type V: Involvement of the apophysis
-
Stress fractures (Fig. 5.314) show the following characteristics:P.993P.994P.995
-
Subchondral hypointensity on T1- and PD-weighted images
-
Asymmetric thickening of the cortex
-
Single or multiple fracture lines
-
Location is usually perpendicular to the metatarsal long axis
-
Medullary edema
-
Soft-tissue hyperintensity on FS PD FSE images
-
Edema without visualization of a fracture line in the acute stage
-
-
Fractures of the metatarsal head show:
-
Displacement
-
Edema of the metatarsal head
-
May or may not show dislocation
-
Angulation or rotation of articular surface on sagittal images
-
Chondral fracture with interruption of articular cartilage
-
-
Fractures of the metatarsal neck (Fig. 5.315) are characterized by:
-
Oblique or transverse fracture and edema
-
May or may not be plantar displacement
-
Edema, marrow, and sometimes soft tissue
-
Tendinous interposition
-
Muscle hyperintensity on FS PD FSE images
-
-
Midshaft fractures (Fig. 5.316) show:
-
Oblique or transverse marrow edema
-
Possible shortening, angulation, or displacement
-
Variable proximal-to-distal diaphysis edema
-
-
Fractures of the base of the metatarsal (see Fig. 5.314), including stress fractures of the base of the second metatarsal in ballet dancers (Fig. 5.317), show:
-
Marrow edema
-
Multiple areas of metatarsal involvement
-
Hyperintense base on FS PD FSE images, sometimes with visualization of a fracture fragment in Lisfranc injuries
-
Chip fractures
-
-
Fractures of the first metatarsal show:
-
Soft-tissue effacement with edema/hemorrhage
-
Comminution in direct injuries
-
Avulsion fragment
-
Possible associated Lisfranc injuries
-
Marrow hyperintensity on FS PD FSE images
-
-
Fractures of the central metatarsals show:
-
Displacement as a unit
-
Multiple metatarsal involvement (second, third, and fourth)
-
Displacement with extensive soft tissue and muscle edema
-
-
Fractures of the fifth metatarsal show:
-
Hypointensity of base on T1- or PD-weighted images
-
Extension into the proximal diaphysis
-
Increased fracture gap in nonunion
-
Fibrous tissue or sclerosis.
-
 |
|
FIGURE 5.309 ● Surgical fixation options for Lisfranc joint injuries. Lisfranc injuries have been classified as stage I (Lisfranc ligament sprain), stage II (ruptured Lisfranc ligament with a 2- to 5-mm diaphysis between the first and second metatarsals), and stage III (rupture of Lisfranc's ligament with >5 mm of diastasis and loss of longitudinal arch height). ORIF is used for stage III injuries. Athletes may require early ORIF for all grade 2 and 3 sprains. Screws are placed across the tarsometatarsal joint to create stiffness and support the arch and form a rigid lever.
|
 |
|
FIGURE 5.310 ● Comparison of the morphology of a proximal fifth metatarsal fracture (A), nonunion of the metatarsal apophysis (B), and an os vesalianum (C). Note the irregular margins of a fracture site and the more proximal location of the os vesalianum.
|
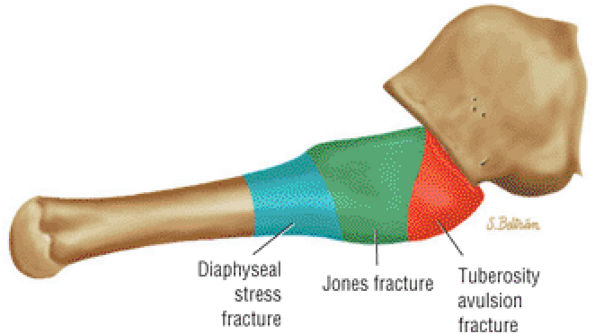 |
|
FIGURE 5.311 ● Lateral color illustration of the three zones of proximal fifth metatarsal fractures: (1) tuberosity avulsion fractures, (2) Jones fractures at the junction of the metaphysis and diaphysis, (3) diaphyseal stress fractures. Avulsion fractures of the tuberosity are the most common type. Contraction of the peroneus brevis tendon secondary to an inversion injury may result in this fracture. The Jones fracture and more distal diaphyseal stress fractures are susceptible to nonunion.
|
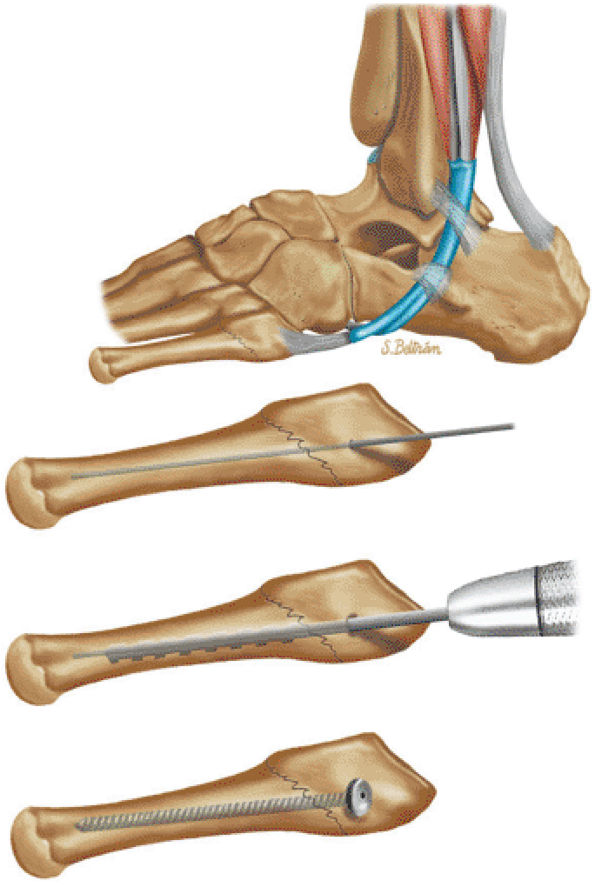 |
|
FIGURE 5.312 ● Open reduction and internal fixation of a Jones fracture. Displaced fractures often require open reduction and internal fixation. Screw fixation and bone grafting are also treatment options.
|
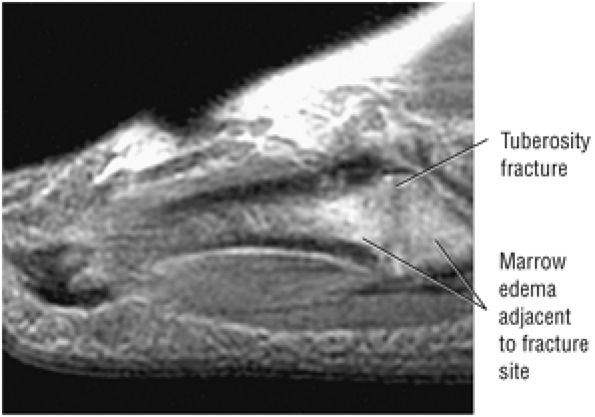 |
|
FIGURE 5.313 ● Simple avulsion fracture of the proximal fifth tuberosity. Contraction of the lateral band of the plantar aponeurosis as well as peroneus brevis tendon pulls may be a causative factor in this injury. Sagittal FS PD FSE image.
|
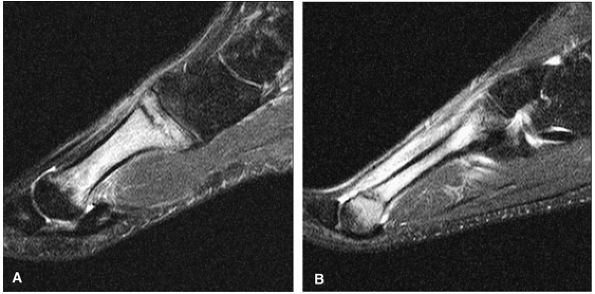 |
|
FIGURE 5.314 ● Stress fractures of the first, third, or fourth metatarsals and the distal aspect of the second metatarsal can be treated conservatively. Stress fracture of the proximal (base) first (A) and distal second (B) metatarsals are shown on these sagittal FS PD FSE images.
|
surgically, usually with ORIF. Complications include nonunion, malunion, and unresolving pain.
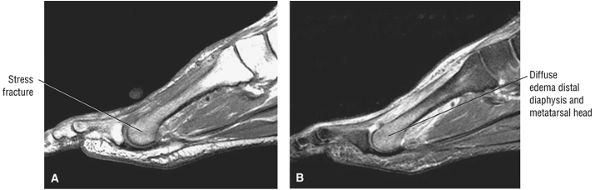 |
|
FIGURE 5.315 ● Distal second metatarsal stress fracture with diffuse trabecular marrow edema on sagittal T1-weighted (A) and FS PD FSE (B) images.
|
-
Compartment syndrome may be acute or chronic (exertional).
-
Anterior, lateral, superficial, and deep posterior compartments may be affected.
-
Axial FS PD FSE images are used to identify diffuse muscle edema and compartmental bulging with convex deep fascial margins.
-
There may be loss of normal muscle striations and muscle herniations in chronic compartment syndrome.
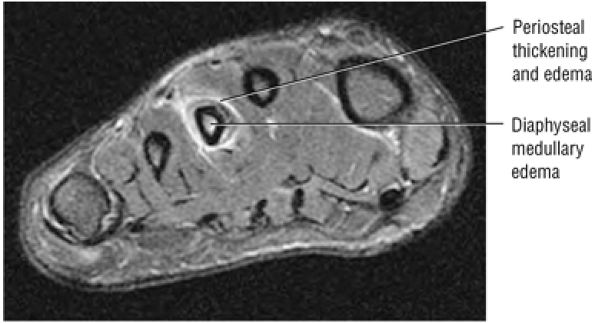 |
|
FIGURE 5.316 ● Persistent diaphyseal marrow edema in a healing third metatarsal stress fracture. Coronal FS PD FSE image.
|
Tibial fractures, especially open fractures involving the proximal and middle thirds, are the most common fractures leading to compartment syndrome. Twenty percent of tibial fractures are associated with this complication. Muscle ruptures, crush injuries, and burns may also precede the development of compartment syndrome.
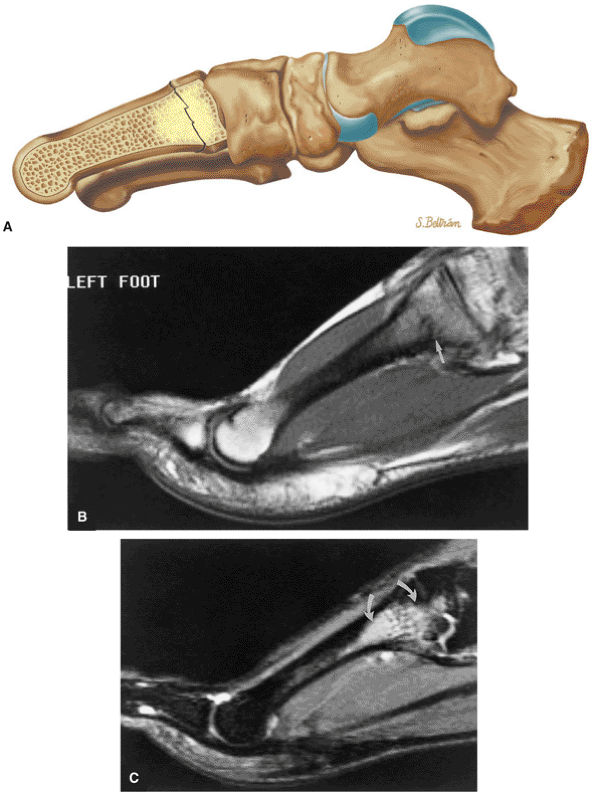 |
|
FIGURE 5.317 ● (A) Second metatarsal base stress fractures are frequently seen in ballet dancers. Cessation of training for up to 6 weeks or until pain with weight-bearing subsides is necessary for healing. MR findings may persist after clinical healing. Lateral color illustration. (B) Second metatarsal base stress fracture in a ballet dancer is shown with hypointense linear stress fracture (straight white arrow) on T1-weighted image. (C) STIR sagittal image displays associated hyperintense bone marrow edema (curved arrows).
|
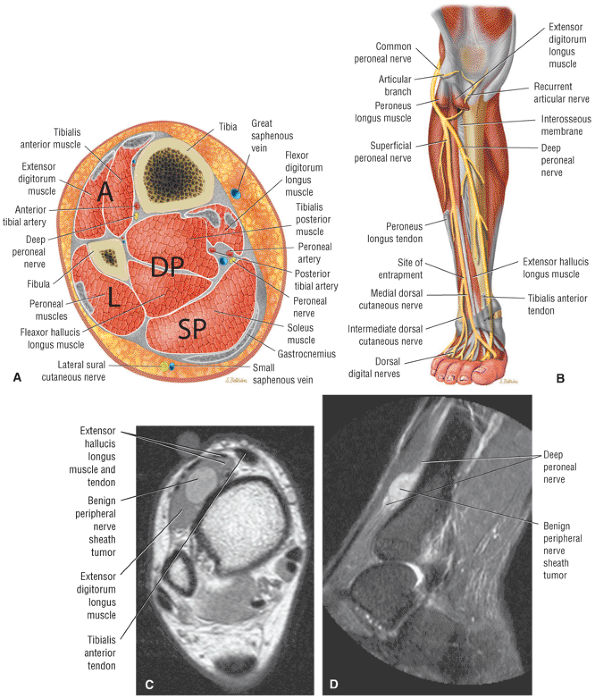 |
|
FIGURE 5.318 ● (A) The lower leg contains four major compartments (A, L, SP, DP): the anterior compartment, the lateral compartment, the superficial posterior compartment, and the deep posterior compartment. The tibialis posterior is sometimes classified as its own separate compartment and not grouped with the deep posterior compartment. (B) Anterior color illustration showing the relationship of the deep peroneal nerve to the anterior compartment. Weakness of dorsiflexion or toe extension, paresthesias of the dorsum of the foot, first web space numbness, and foot drop may be seen in anterior compartment syndrome. The superficial peroneal nerve is contained within the lateral compartment. The sural nerve is within the superficial posterior compartment. The posterior tibial nerve is contained within the deep posterior compartment. (C, D) Benign peripheral nerve sheath tumor (schwannoma) involving the deep peroneal nerve of the anterior compartment. There are no associated findings of compartment syndrome. (C) Axial PD FSE image. (D) Sagittal STIR image.
|
-
Loss of normal muscle striations
-
Subacute hemorrhage
-
Foci of hemosiderin deposition
-
Enlargement and peripheral convex bowing of affected muscle group
-
Calcification (in chronic compartment syndrome)
-
Fat and muscle atrophy (in chronic compartment syndrome)
-
Chronic fibrous replacement
-
Calcific myonecrosis (liquefied necrotic muscle and with a calcific shell)
-
T1 images may be normal in exertional compartment syndrome.
-
Diffuse hyperintensity within the cross-sectional anatomic boundaries of affected muscle and proximal/ distal extension on FS PD FSE images (Fig. 5.319)
-
Fluid, hemorrhage, and edema between muscles in the fascial planes
-
Edema, rhabdomyolysis, and fascial convexity (see Fig. 5.319)
-
Subcutaneous tissue edema
-
Intermediate to increased muscle signal on FS PD FSE or STIR images in exertional compartment syndrome
-
Muscle herniation
double-incision fasciotomy. Reperfusion injury is a potential complication.
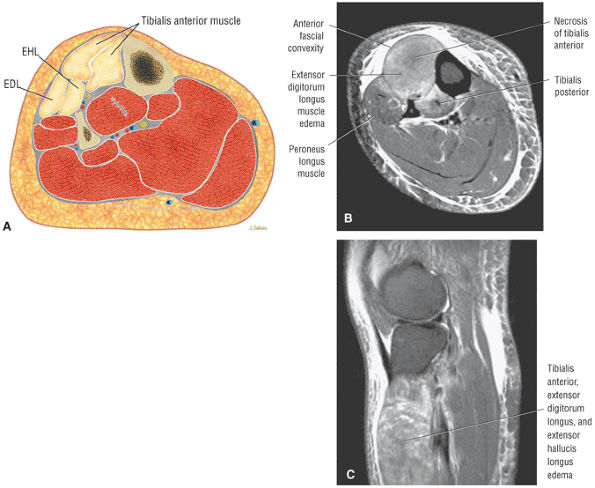 |
|
FIGURE 5.319 ● Anterior compartment syndrome involving the tibialis anterior, extensor digitorum longus, and extensor hallucis longus muscles. The inelastic fascial sheath and increased volume of the involved muscles are associated with edema and relative muscle hypertrophy. Myofiber damage and an increase in osmotic pressure contribute to decreased blood flow. The anterior compartment is the most common of the four compartments involved, followed by the deep posterior compartment, the lateral compartment, and the superficial posterior compartment. (A) Axial color illustration. (B) Axial FS PD FSE image. (C) Sagittal FS PD FSE image.
|
-
Characterized by hyperintense signal along the anterior medial tibial border
-
Related to periosteal avulsion and periostitis at the medial soleus insertional site resulting from hyperpronation
-
Spectrum of findings is classified as grade 1 (periosteal edema) through grade IV (fracture).
-
FS PD FSE or STIR images in the axial plane are used to identify subtle periosteal edema.
tibial stress syndrome. They found that the soleus, the FDL, and the deep crural fascia attached more frequently at the site corresponding to the symptoms of the medial tibial stress syndrome. The tibialis posterior was not found to attach in this area.
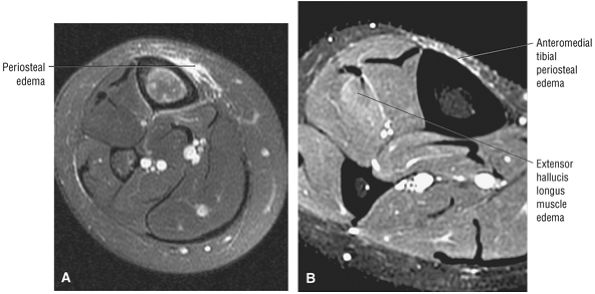 |
|
FIGURE 5.320 ● (A) Hyperintense periosteal edema adjacent to the anteromedial tibial cortex in grade I medial tibial stress syndrome. (B) Grade 1 extensor hallucis longus muscle strain in a long-distance runner with grade 1 medial tibial stress syndrome. Axial FS PD FSE images.
|
-
Grade I: Periosteal edema (Fig. 5.320), which demonstrates mild to moderate increased signal intensity on T2-weighted images, and normal marrow on T1- and T2-weighted images
-
Grade II: Moderate to severe periosteal edema and associated marrow edema, both hyperintense on T2-weighted images
-
Grade III: Marrow edema demonstrated on both T1-weighted and FS PD FSE images (Fig. 5.321)
-
Grade IV: A fracture line is visualized (Fig. 5.322).
tibialis posterior, the FDL, and the soleus muscles, without the predominant involvement of any one particular muscle group.
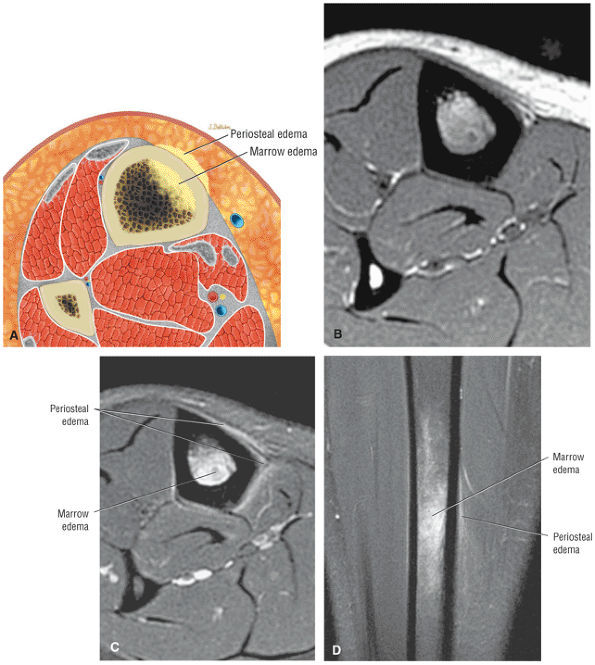 |
|
FIGURE 5.321 ● (A) Axial color cross-section showing marrow and periosteal edema. Grade III medial tibial stress syndrome with marrow and periosteal edema appears hypointense on T1-weighted images (B) and hyperintense on FS PD FSE images (C, D). (B) Axial T1-weighted image. (C) Axial FS PD FSE-weighted image. (D) Coronal FS PD FSE image.
|
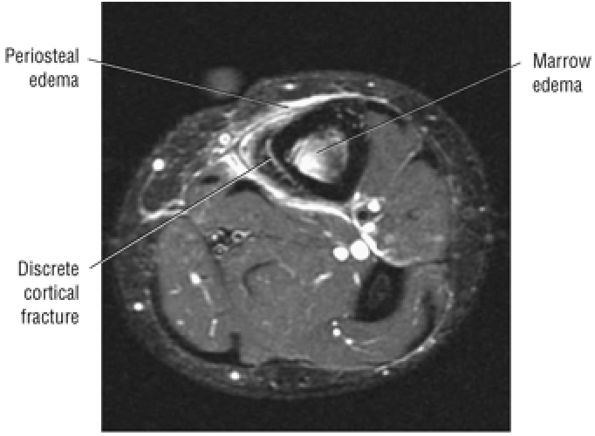 |
|
FIGURE 5.322 ● Grade IV medial tibial stress fracture with a linear hyperintense fracture line within the anteromedial cortex. Axial FS PD FSE image.
|
-
Intermediate signal within deep subcutaneous tissue overlying the medial tibial cortex on T1- or PD-weighted images
-
Intermediate signal within the normally hypointense cortex on T1- or PD-weighted images
-
Eccentric marrow in the medullary bone of the diaphysis
-
Anteromedial to posteromedial extension
-
Extension of edema or fluid to the origin of the soleus posteromedially (soleus bridge)
-
Represents a strain or tear of the medial head of the gastrocnemius muscle
-
There is diffuse hyperintense signal intensity within the medial head on FS PD FSE or STIR images.
-
Soleus muscle strains should be evaluated in association with medial head gastrocnemius strains.
-
Deep venous thrombosis with prominent venous collaterals should not be mistaken for a muscle strain.
-
Axial images are useful in the identification of fascial tearing.
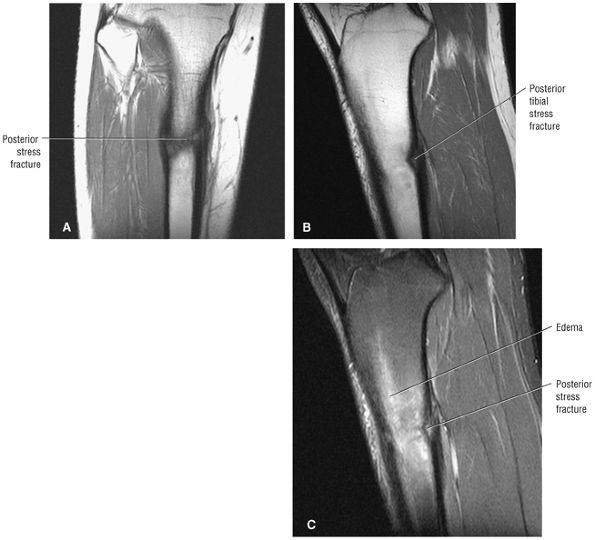 |
|
FIGURE 5.323 ● Intact anterior tibial diaphyseal bone stock with stress fracture restricted to posterior cortex. On coronal plane images (A) it is possible to overestimate the extent of a posterior tibial diaphyseal stress fracture. Stress fractures must always be evaluated in more than one orthogonal plane (B, C). (A) Coronal T1-weighted image. (B) Sagittal T1-weighted image. (C) Sagittal FS PD FSE image.
|
-
Tearing of the medial head of the gastrocnemius at the muscle—tendon junction (the “weak link”)
-
Hemorrhage between the medial head and the soleus without plantaris tearing
-
A partial tear of fascia between the medial head of the gastrocnemius and the soleus
-
A partial tear of the transverse intermuscular septum at the border of the superficial posterior and deep posterior compartments
stance phase. The calf hemorrhage can be associated with aching and cramping pain for several weeks.
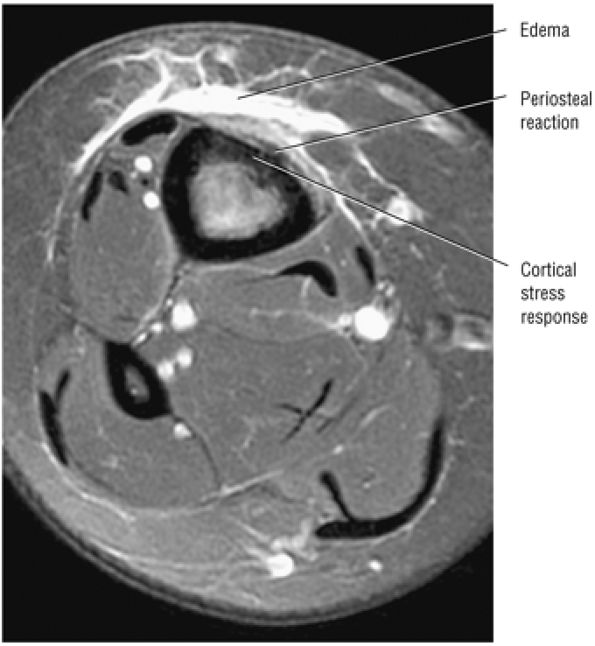 |
|
FIGURE 5.324 ● Insufficiency fracture in a 65-year-old presenting with imaging characteristics similar to those found in medial tibial stress syndrome, which occurs in athletes. Axial FS PD FSE image.
|
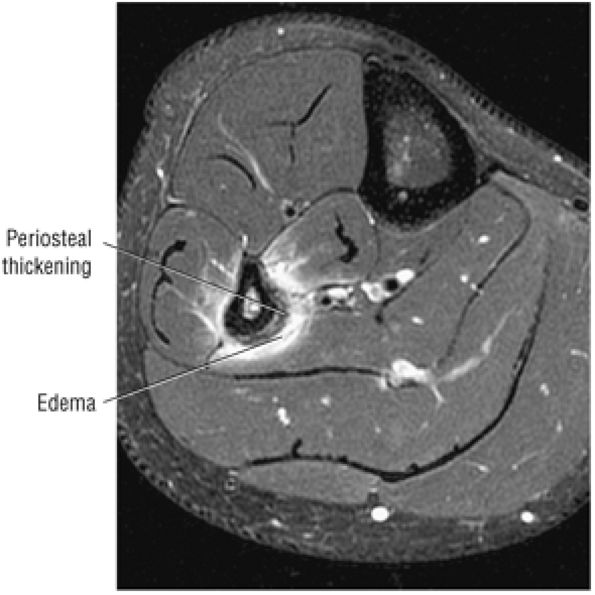 |
|
FIGURE 5.325 ● Fibular stress fracture in a basketball player shows periosteal thickening and periostitis with hyperintense signal adjacent to fibular diaphyseal cortex. Axial FS PD FSE image.
|
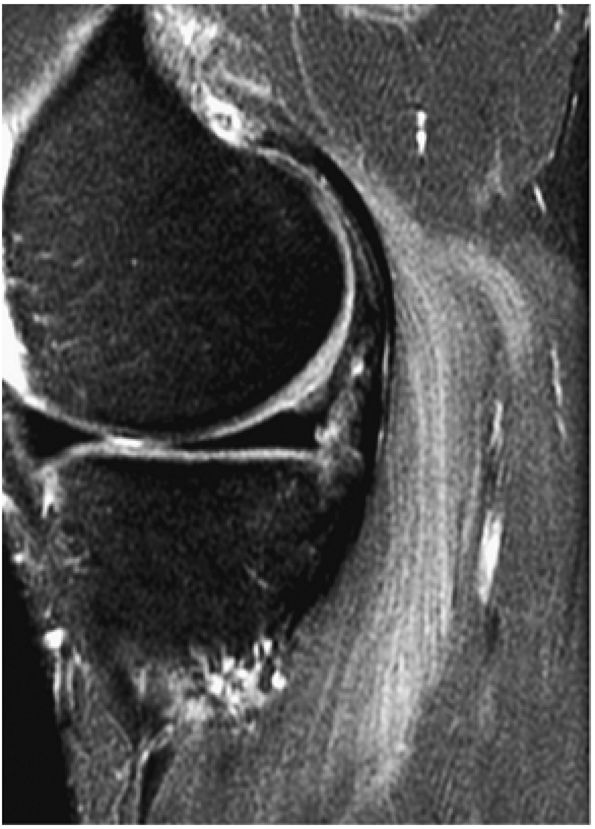 |
|
FIGURE 5.326 ● Hyperintensity in a grade 1 strain of the medial head of the gastrocnemius muscle.
|
-
Grade 1: There is no myofascial disruption, but edema and swelling are evident (Fig. 5.329).
-
Grade 2: There is weakness caused by variable separation of muscle from the tendon or fascia (Fig. 5.330).
-
Grade 3: The myofascial separation is complete and there is loss of muscle function.
-
Edema of the medial head
-
Subacute hemorrhage
-
Laxity of the intermuscular septum between the gastrocnemius and soleus
-
Fluid deep to subcutaneous tissue and between the gastrocnemius and soleus (Fig. 5.331)
-
Fascial discontinuity
-
Fluid-filled gap in myofascial separation
-
Foci of hemosiderin deposition, especially on T2* GRE images.
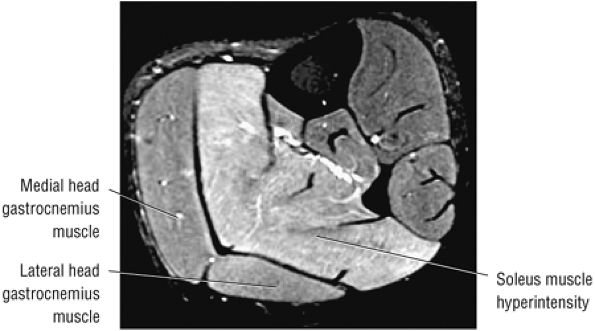 |
|
FIGURE 5.327 ● Delayed-onset muscle soreness in a ballet dancer with hyperintense diffuse edema from soleus muscle strain. Axial FS PD FSE image.
|
 |
|
FIGURE 5.328 ● Grade 2 tear of the medial head of the gastrocnemius with disruption of the fascial layer between the soleus and gastrocnemius and discontinuity of the crural fascia medially. Axial FS PD FSE image.
|
-
The term “tennis leg” has been used for injuries to the medial head of the gastrocnemius (more common presentation of tennis leg) and for plantaris tendon rupture.
-
A myotendinous proximal tear is associated with distal retraction of the plantaris tendon with a hemorrhagic mass effect between the medial head of the gastrocnemius and the soleus muscle.
 |
|
FIGURE 5.329 ● Feathery edema pattern of a grade 1 soleus muscle strain on an axial FS PD FSE image.
|
 |
|
FIGURE 5.330 ● Grade 2 muscle strain characterized by a partial muscle tear of the medial head of the gastrocnemius muscle with hemorrhage and fascial tearing of the muscle—tendon unit. Intramuscular edema and extramuscular fluid are identified. The epimysium of the connective tissue sheath surrounding the muscle is disrupted. Hypointense fascia and hemorrhage with hemosiderin may be mistaken for a retracted plantaris tendon.
|
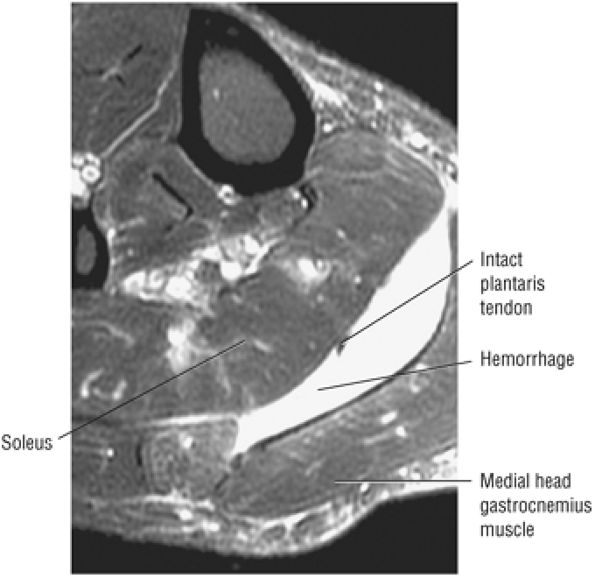 |
|
FIGURE 5.331 ● Hemorrhagic fluid collection between the soleus and the medial head of the gastrocnemius in tennis leg. The plantaris tendon is not disrupted. Axial FS PD FSE image.
|
blood and tissue within the plane of the hematoma. Characteristic MR findings include:
-
A curvilinear fluid collection in the expected location of the plantaris tendon contribution to the medial aspect of the Achilles tendon on distal axial images at the level of the ankle joint
-
Hemorrhage with or without a hemosiderin ring between the medial head of the gastrocnemius and the soleus and inhomogeneity in the retracted tendon (Fig. 5.333)
-
Mass effect from retracted tendon
-
Associated injuries, including partial tear of the gastrocnemius or popliteus muscles, anterior cruciate ligament injuries (with or without anterior translation of the tibia relative to the femur), tibialis posterior strain, and posterolateral corner sprains
-
Identification of the proximal retracted muscle between the popliteus tendon and the lateral head of the gastrocnemius muscle at the level of the knee
-
A distal tear with absence of the distal course of the plantaris (less common)
-
Edema within a portion of or of a complete cross-sectional area at a specified level
-
Edema of the soleus and less commonly of the lateral head of the gastrocnemius
-
Hemorrhagic fluid tracking medially deep to the subcutaneous tissue
-
Possible anterolateral and posteromedial fluid extension between the subcutaneous tissue and the medial head/soleus plane
-
Possible fluid extension anteromedial to the tibia
-
Disruption of the fascia separating the medial head of the gastrocnemius and soleus
-
Curvilinear “comet sign” of fluid extending along the distal course of the plantaris adjacent to the Achilles tendon
-
Absence of the plantaris muscle in proximal tears in the sagittal plane through the knee joint
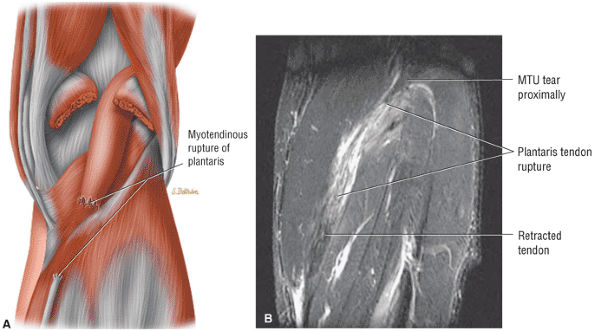 |
|
FIGURE 5.332 ● Plantaris myotendinous rupture with the path of hemorrhage corresponding to the course of the retracted plantaris tendon. (A) Posterior view coronal color graphic. (B) Coronal FS PD FSE image.
|
with complete rupture of the plantaris or tear of the gastrocnemius—soleus muscle complex.
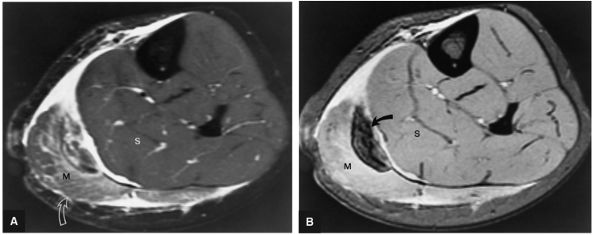 |
|
FIGURE 5.333 ● Acute rupture of the plantaris with intermuscular hemorrhage (solid curved arrow) identified in the plane between the soleus (S) and medial head of the gastrocnemius muscle (M) groups. (A) Hemorrhage is isointense to hyperintense on an FS PD FSE axial image. (B) On a T2*-weighted axial image the hemorrhage demonstrates hypointensity (magnetic susceptibility effect). Edema of the medial head of the gastrocnemius muscle (open curved arrow) is best demonstrated on the FS PD FSE sequence (A).
|
-
Coalitions can be osseous, cartilaginous, or a fibrous union of two or more bones involving the hindfoot or midfoot.
-
A synostosis is a complete coalition, a synchondrosis is cartilaginous, and a syndesmosis is fibrous.
-
An osseous bar is extra-articular, whereas a bridge is intra-articular.
-
Talocalcaneal coalitions are best visualized on coronal images; calcaneonavicular coalitions require sagittal images.
-
Synostosis (a complete coalition)
-
Synchondrosis (cartilaginous coalition)
-
Syndesmosis (fibrous coalition)
-
Extra-articular bars (e.g., calcaneonavicular)
-
Intra-articular bridges (e.g., talocalcaneal)
there are no neural elements in nonosseous coalitions, microfractures and osseous remodeling act as pain generators. Histologic examination also reveals vascular proliferation, osteoblastic and osteoclastic activity, fibrous connective tissue, and cellular components.
 |
|
FIGURE 5.334 ● Talocalcaneal coalition. (A) Coronal color section of a solid osseous talocalcaneal coalition. (B) T1-weighted coronal image with continuity (arrow) of marrow fat from the tali (T) through the sustentaculum tali (ST). (C) T1-weighted sagittal images show secondary signs of talocalcaneal coalition with talar beaking. N, navicular; T, talus.
|
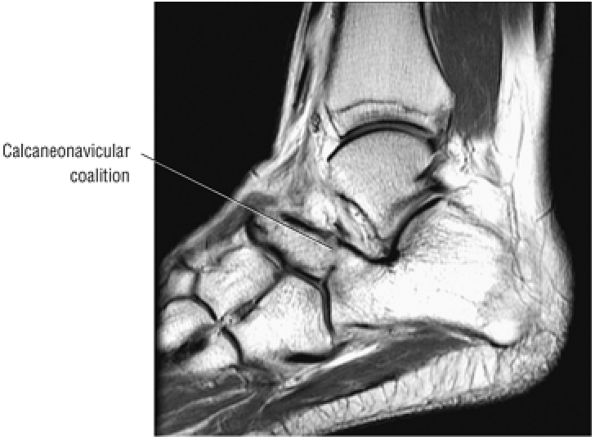 |
|
FIGURE 5.335 ● Solid calcaneonavicular coalition visualized laterally on a T1-weighted sagittal image.
|
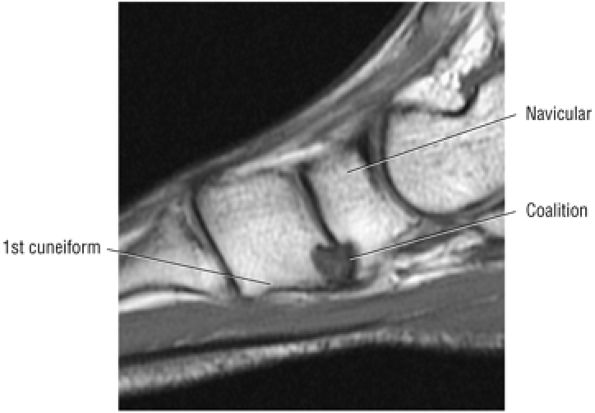 |
|
FIGURE 5.336 ● Fibrous coalition between the navicular first cuneiform articulations on a sagittal T1-weighted image.
|
-
Hypoplasia of the head of the talus
-
Abnormal close approximation of the navicular bone and the calcaneus, with irregularity of opposing cortical surfaces
-
Subchondral sclerosis at a synchondrosis or syndesmosis
-
A linear hyperintense (on FS PD FSE images) calcaneonavicular interface in nonosseous coalitions
-
Reactive subchondral marrow edema
-
Solid marrow continuity between the calcaneus and navicular on lateral sagittal images
Wechsler et al.133 have also demonstrated subchondral hyperintensity, thought to be secondary to altered biomechanics, in calcaneonavicular coalitions. A potential pitfall of MR imaging is mistaking proliferative low-signal-intensity synovitis for fibrous coalition.
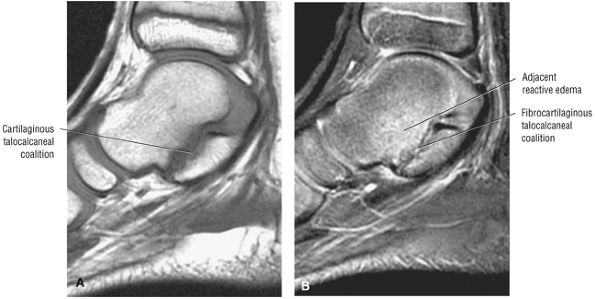 |
|
FIGURE 5.337 ● (A) Sagittal T1-weighted image showing a symptomatic talocalcaneal cartilaginous coalition. (B) The adjacent marrow edema can be appreciated on this sagittal FS PD FSE image.
|
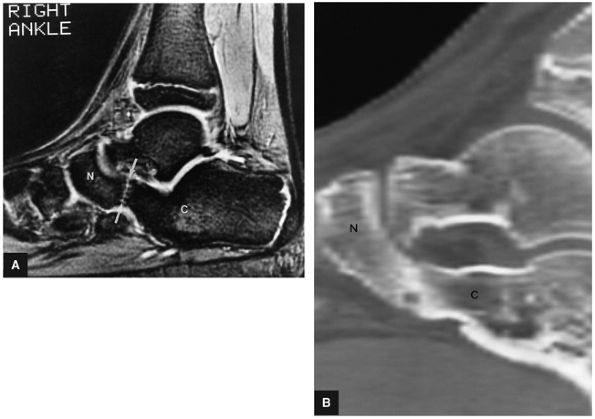 |
|
FIGURE 5.338 ● Fibrous calcaneonavicular coalition (arrows) displayed on a T2*-weighted sagittal image (A) and a corresponding 2D reformatted CT sagittal image (B). The MR image demonstrates the lack of complete solid bony continuity between the calcaneus and navicular bone more accurately than the CT scan. N, navicular bone; C, calcaneus.
|
-
Talar beaking adjacent to the talonavicular articulation (not to be confused with the normal talar ridge)
-
Degenerative dorsal changes in the calcaneocuboid joint on lateral sagittal images
-
A broadening lateral talar process
-
A ball-and-socket ankle joint
-
An osseous connection with fat signal bone marrow continuity between the talus and the calcaneus
-
Narrowing with an irregular articular interface and sclerosis across the middle facet in cartilaginous, fibrous, or fibrocartilaginous unions
-
Possible hypoplastic sustentaculum tali
-
Talar neck concavity on sagittal views
-
Intermediate-signal fibrocartilaginous interface on FS PD FSE images
-
Low to intermediate signal in fibrous tissue syndesmosis on FS PD FSE images
-
Tarsal tunnel syndrome is an entrapment or compression neuropathy of the posterior tibial nerve.
-
A space-occupying lesion may be identified, as well as denervation or atrophy of the muscles supplied by the posterior tibial nerve or one of its branches.
-
Causative factors include fracture, varicosities, ganglions, lipomas, nerve sheath tumors, and a thickened flexor retinaculum and accessory muscles.
-
Trauma (a fractured sustentaculum tali or medial tu-bercle posterior talar process), identified in 17% of cases
-
Varicosities (Fig. 5.342), identified in 13% of cases (Perthes' tourniquet test for varicosities tests the deep venous system and the posterior tibial venae comitantes by occluding the superficial venous system)
-
Ganglia (Fig. 5.343)
-
Lipomas (see Fig. 5.342)
-
Neurilemomas
-
A thickened flexor retinaculum
-
Accessory muscle (FDL)
-
Synovitis (inflammatory arthropathies)
-
Tarsal coalition
-
Diabetes or other systemic disease, identified in 34.4% of cases
-
Valgus heel and forefoot pronation, identified in 8% of cases
-
Fibrosis, identified in 9% of cases
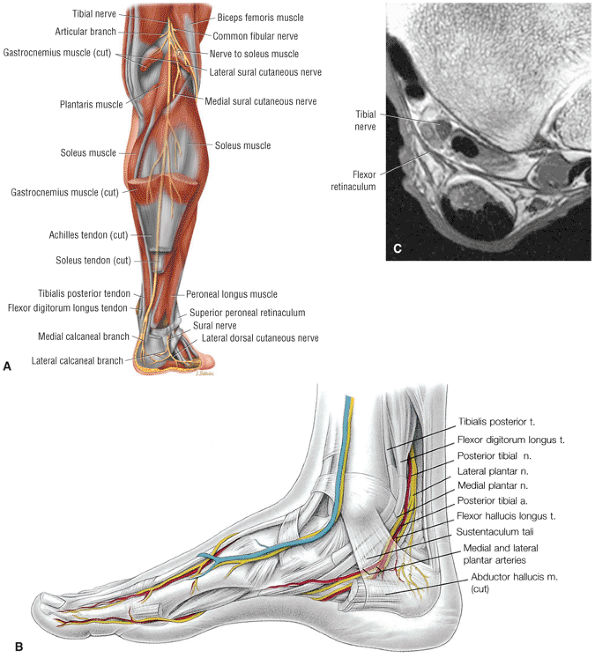 |
|
FIGURE 5.339 ● (A) Course of the tibial nerve in the lower leg. (B) The posterior tibial nerve lies between the flexor digitorum longus and flexor hallucis longus, divides into the medial and lateral plantar nerves, and gives off calcaneal branches. (C) Axial T1-weighted image of the distal tibia at the level of the tarsal tunnel. The tibial nerve can be seen proximal to its division into the medial and lateral plantar nerves. The tarsal tunnel is bordered by the flexor retinaculum (medially), the calcaneus, and the talus (laterally). The longitudinal course of the tarsal tunnel extends from about the medial malleolus to the level of the abductor hallucis muscle distally.
|
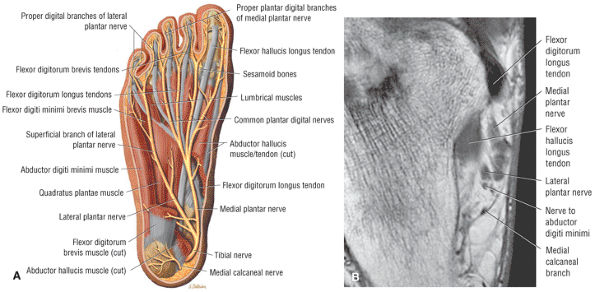 |
|
FIGURE 5.340 ● The tibial nerve divides into its two terminal branches (the lateral and medial plantar nerves) as it passes through the tarsal tunnel. These terminal branches supply the muscles on the plantar aspect of the foot. (A) Axial plantar view of the plantar nerves. (B) Axial T1-weighted image with branching of the medial and lateral plantar nerves at the level of the sustentaculum tali.
|
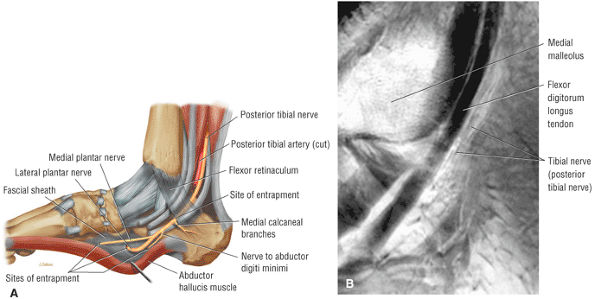 |
|
FIGURE 5.341 ● (A) Potential sites of entrapment of the tibial nerve. Compression of the tibial nerve or its lateral and medial plantar nerve branches may result in tarsal tunnel syndrome. Pain or sensory disturbances of the sole of the foot and palsies of the intrinsic foot muscles may result. (B) The course of the tibial nerve as it passes deep to the flexor retinaculum and posterior to the flexor digitorum longus tendon. Sagittal T1-weighted image.
|
 |
|
FIGURE 5.342 ● (A) Tarsal tunnel syndrome with fatty atrophy of the abductor hallucis, flexor digitorum brevis, and abductor digiti minimi. Coronal T1-weighted image. (B) Prominent veins in association with denervation hyperintensity of the abductor hallucis and flexor digitorum brevis. Coronal FS PD FSE image. (C) In comparison, an example of a lipoma as a true space-occupying lesion resulting in atrophy of the abductor digiti minimi muscle. Axial FS PD FSE image.
|
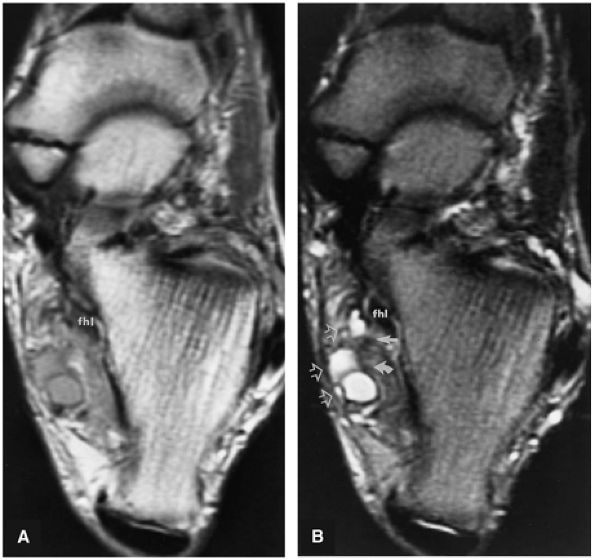 |
|
FIGURE 5.343 ● (A) Intermediate-weighted and (B) T2-weighted axial images display the tarsal tunnel syndrome secondary to neurovascular compression by a septated ganglion (open arrows) at the level of the medial (straight arrow) and lateral plantar (curved arrow) nerves. The ganglion is hyperintense on the T2-weighted image (B). fhl, flexor hallucis longus tendon.
|
-
Fractures (usually of the sustentaculum tali or medial tubercle posterior process of the talus)
-
Serpiginous venous signal varicosities, intermediate signal on T1- or PD-weighted images and hyperintense on FS PD FSE images
-
Ganglia, hypointense to intermediate on T1- or PD-weighted images and hyperintense on FS PD FSE images
-
Lipomas, hyperintense on T1- or PD-weighted images and hypointense on FS PD FSE or STIR images
-
Neurilemomas, intermediate signal on T1- or PD-weighted images and hyperintense on FS PD FSE images
-
Thickened flexor retinaculum (lacinate ligament), hypointense on T1- or PD-weighted images
-
Muscle signal in the presence of accessory muscle
-
Proliferative synovitis, intermediate signal on T1- or PD-weighted images and intermediate to hyperintense on FS PD FSE images
-
Fat marrow signal in bony coalition or low to intermediate signal in a fibrous coalition on T1- or PD-weighted images
-
Fat marrow signal in talar exostosis
-
Diffuse muscle edema in muscle groups supplied by the posterior tibial nerve and its branches (e.g., the abductor hallucis by the medial plantar nerve and the abductor digiti quinti by the lateral plantar nerve)
-
Tenosynovitis with hyperintense fluid in the flexor hallucis sheath on FS PD FSE images
lesion is identified near or within the tarsal tunnel preoperatively, subsequent surgical decompression of the posterior tibial nerve may not be successful. They found that surgical decompression in patients with a history of previous surgical treatment for pain in the foot, plantar fasciitis, or systemic inflammatory disease did not show favorable results compared with patients with space-occupying lesions and coalitions.139 Complications include recurrent tarsal tunnel syndrome.
-
Represents a metatarsalgia with localized enlargement of the interdigital nerve between the third and fourth metatarsal heads
-
FS PD FSE or STIR images in the coronal plane are used to distinguish between intermetatarsal bursitis and the teardrop-shaped soft-tissue plantar mass seen in Morton's neuroma.
 |
|
FIGURE 5.344 ● Morton's neuroma with localized fusiform enlargement of the common digital nerve between the third and fourth metatarsal heads.
|
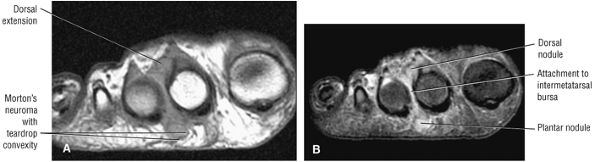 |
|
FIGURE 5.345 ● Teardrop-shaped Morton's neuroma involving the second intermetatarsal space. There is both dorsal and plantar extension with direct adherence of the perineural fibroma to the intermetatarsal bursa. (A) Coronal T1-weighted image. (B) Coronal FS PD FSE image.
|
-
A teardrop-shaped mass arising between the plantar aspects of the involved metatarsal heads, hypointense to intermediate on T1- and PD-weighted images and intermediate to hyperintense on FS PD FSE and STIR images (Fig. 5.346)
-
Fibrosis of the epineurium and perineurium modifying the degree of signal inhomogeneity
-
Increased signal intensity on T2*-weighted, STIR, and FS PD FSE images (in contrast to a conventional neuroma or neurofibroma, which displays hyperintensity on both FS PD FSE and T2*-weighted images)
-
Fat signal in the plantar subcutaneous tissue superficial to the mass
-
Thickened and inflamed intermetatarsal bursa
-
Effacement of the plantar subcutaneous fat by the convex border of the mass
-
Intermetatarsal bursal fluid (Fig. 5.347)
-
Intermediate to hyperintense mass on T2* GRE images
-
Diffuse enhancement after contrast administration (Fig. 5.348)
-
Enhanced lining of the inflamed intermetatarsal bursa
of the deep transverse intermetatarsal ligament, a painful stump neuroma, and continued pain. Steroid injections may cause subcutaneous fat pad atrophy.
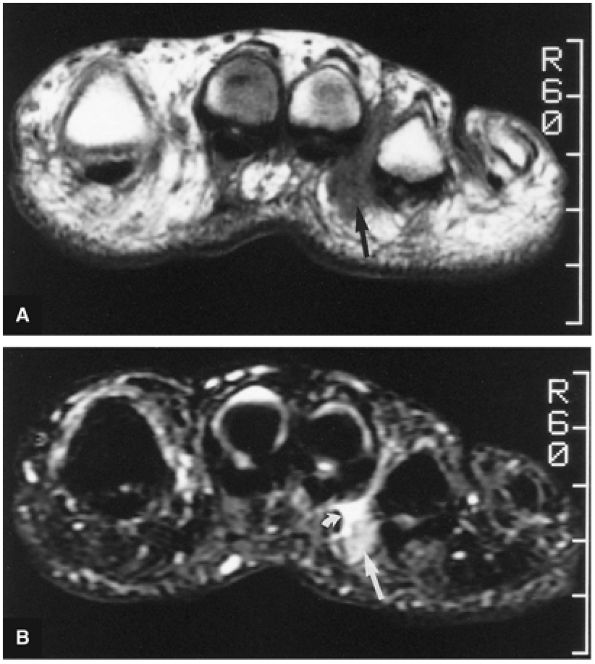 |
|
FIGURE 5.346 ● Morton's neuroma (straight arrow), located between the third and fourth metatarsal heads, is isointense with adjacent inflammation on a T1-weighted coronal image (A) and is mildly hyperintense on a STIR coronal image (B). Inflammatory soft-tissue edema demonstrates greater hyperintensity (curved arrow) than reactive connective-tissue proliferation and associated nerve degeneration. STIR protocols are more sensitive for Morton's neuroma than either T1- or T2-weighted images.
|
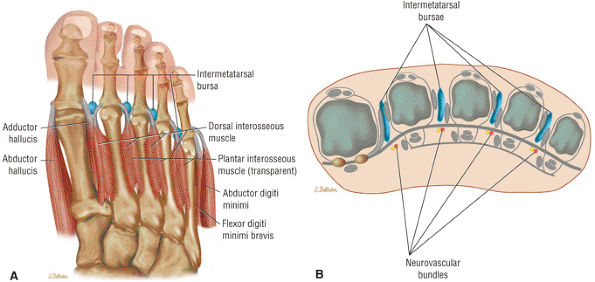 |
|
FIGURE 5.347 ● Intermetatarsal bursa sites for potential inflammation in association with Morton's neuroma. Axial (A) and coronal (B) color illustrations.
|
 |
|
FIGURE 5.348 ● Recurrence of a Morton's neuroma (straight arrow) identified on an intravenous gadolinium-enhanced FS T1-weighted coronal image. Irregularly marginated hyperintensity can be identified between the plantar aspects of the third and fourth metatarsals. Reactive inflammatory soft-tissue changes also demonstrate hyperintensity on gadolinium-enhanced image (curved arrow).
|
-
Represents fibrous and collagenous nodules in the plantar aponeurosis
-
May present as single or multiple nodular lesions
-
Occurs in a more distal location than plantar fasciitis
-
The upper or deep border may be infiltrative.
-
FS PD FSE or STIR images are used to appreciate central hyperintensity in single nodules.
-
A proliferative phase, in which there is fibroblastic activity and cellular proliferation
-
An involutional phase, which is an active phase with nodule formation
-
A residual phase, in which there is reduced fibroblastic activity and collagen maturation
plantar fibromas may explain their relative hypointensity on FS PD FSE sequences. Adjacent inflammatory edema is usually not present. The differential diagnosis includes a ganglion, neurofibroma, or fibrosarcoma and in most cases can be made by the presence of increased signal intensity on FS PD FSE images. Characteristic MR findings include:
-
Hypointense nodule (see Fig. 5.349)
-
A central region of low to intermediate signal intensity
-
Effacement of plantar subcutaneous tissue
-
The long axis of the nodule is seen along the long axis of the plantar fascia.
-
Adjacent plantar fascia is unaffected and of normal thickness.
-
Mild hyperintensity of the plantar subcutaneous tissue on FS PD FSE images
-
Convex dorsal (deep) and plantar (superficial) margins (see Fig. 5.350)
-
Infiltrative upper margins and lesions deep to the plantar aponeurosis146
-
The plantar margin is better defined than the infiltrative upper margin.
-
Inhomogeneous signal intensity in lesions consisting of multiple nodules
-
Inhomogeneous enhancement after contrast administration
-
Central areas of intermediate to increased signal intensity on FS PD FSE, T2* GRE, or STIR images146
 |
|
FIGURE 5.349 ● Single, less than 1 cm, nodular lesion of plantar fibromatosis distal to the os calcis attachment of the plantar fascia. Note the well-defined plantar margin and mild central hyperintensity on the FS PD FSE sequence. (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image.
|
-
Plantar fasciitis is also known as heel pain syndrome or subcalcaneal pain syndrome.
-
The origin of the plantar fascia from the calcaneal tuberosity is most commonly affected.
-
Microtrauma of the plantar fascia or repetitive tensile overload may lead to an inflammatory response.
-
There may be plantar fascial tearing, or involvement may be localized to the medial, central, or lateral portions of the aponeurosis.
-
Effacement of subcutaneous fat by fibrosis and edema seen in the sagittal plane may assist in early diagnosis.
with a calcaneal spur, inflammatory changes, or thickening of the plantar aponeurosis adjacent to the calcaneus. Subcutaneous edema may be seen both superficial and deep to the plantar fascia. A heel spur (a plantar calcaneal enthesophyte, actually located in the short toe flexor origins and not the aponeurosis) is identified in 50% of cases of adult plantar heel pain. It usually occurs in middle-aged (average 45 years of age) females (twice as common in women as in men).
 |
|
FIGURE 5.350 ● Large lesion of plantar fibromatosis with capsular margins demonstrates heterogeneous areas of hyperintensity on the FS PD FSE sequence and enhancement with intravenous contrast. The long axis is parallel to the plantar aponeurosis in the sagittal plane. (A) Axial plantar view color illustration. (B) Coronal FS PD FSE image. (C) Sagittal FS T1-weighted contrast-enhanced image.
|
-
Repetitive tensile overload affecting the central band
-
A reparative inflammatory response to traumatic overload
-
Heel cord contractures
-
Pes planus or cavus
-
A calcaneal spur within the flexor brevis origin
-
Initial prefascial thickening and effacement of subcutaneous tissue superficial (plantar) to the os calcis attachment (Fig. 5.353 and 5.354)
-
Enthesophyte, with or without marrow fat signal (Fig. 5.355)
-
Partial detachment of the medial or lateral cord (Fig. 5.356)
-
Adjacent calcaneal erosions (tuberosity of the calcaneus)
-
Thickening of the proximal plantar fascia (Fig. 5.357)
-
Subcutaneous tissue edema deep to the plantar fascia (see Fig. 5.357)
-
Reactive calcaneal marrow edema
-
Fluid-filled gap in fascial rupture (Fig. 5.358)
-
In acute and subacute stages, AVN of the talus is indicated by a localized subchondral ischemic focus with surrounding diffuse edema or by a more diffuse infarct pattern throughout the entire talar body.
-
Posttraumatic etiologies include a talar neck fracture and talar dislocation.
-
Follow-up MR studies are used to document resolution of bone marrow edema and to help define the AVN focus.
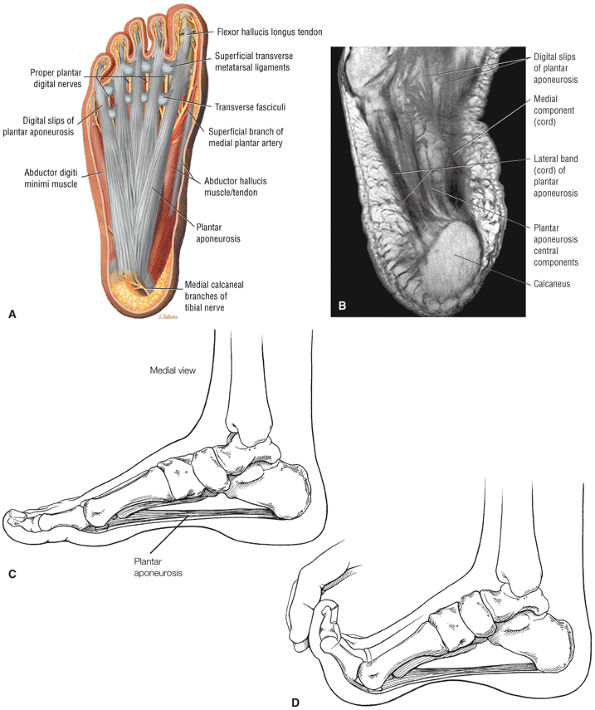 |
|
FIGURE 5.351 ● (A) The plantar fascia consists of a central aponeurosis and medial and lateral components. Plantar view axial plane color illustration. (B) On MR studies the plantar aponeurosis is usually described in terms of a medial and lateral cord or component. Axial T1-weighted image. (C) Normal position of plantar aponeurosis. (D) Dorsiflexion with the toes increases the tension on the plantar aponeurosis, which causes the longitudinal arch to rise (windlass mechanism). Failure of the longitudinal arch to rise is associated with pes planus and abnormal stretching and elongation of the plantar aponeurosis.
|
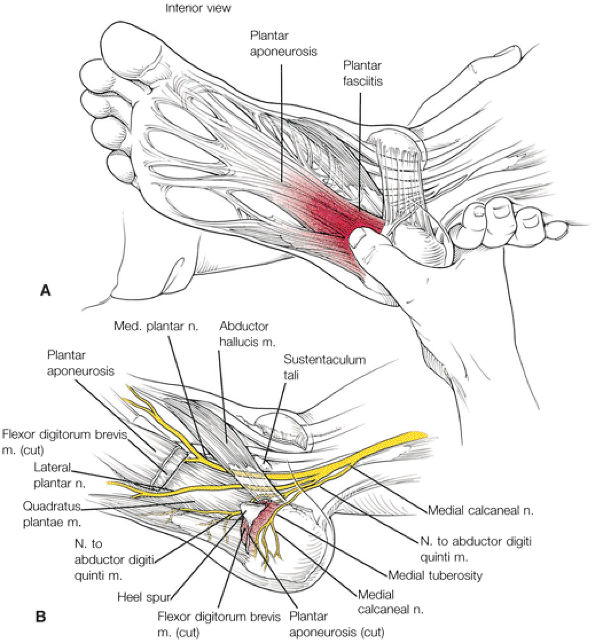 |
|
FIGURE 5.352 ● Plantar fasciitis. (A) Palpation of the plantar fascia at the medial calcaneal tuberosity often elicits pain. (B) There are numerous causes for plantar heel pain, including entrapment of the medial or lateral plantar nerves.
|
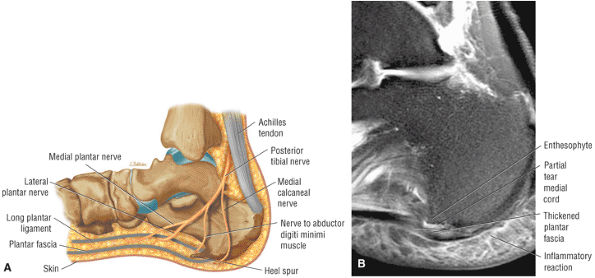 |
|
FIGURE 5.353 ● (A) Structures associated with the presence of heel pain. Lateral color illustration. (B) Plantar fasciitis with partial tearing of the central to medial component of the plantar aponeurosis. Perifascial soft-tissue edema is hyperintense. Sagittal FS PD FSE image.
|
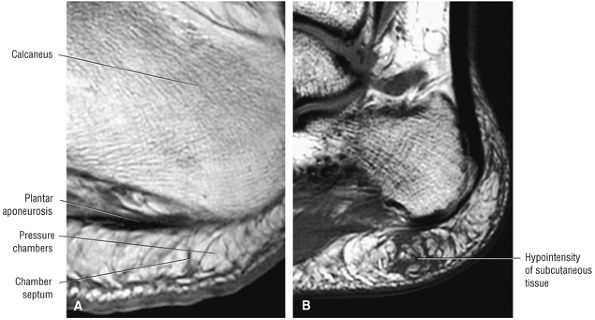 |
|
FIGURE 5.354 ● (A) The subcutaneous tissue is up to 2 cm thick at the sole of the foot. It has a pressure chamber system that functions as a shock absorber and stabilizer for the sole of the foot. (B) Signal-intensity changes of fibrosis and edema frequently precede plantar aponeurosis inflammatory changes and thickening.
|
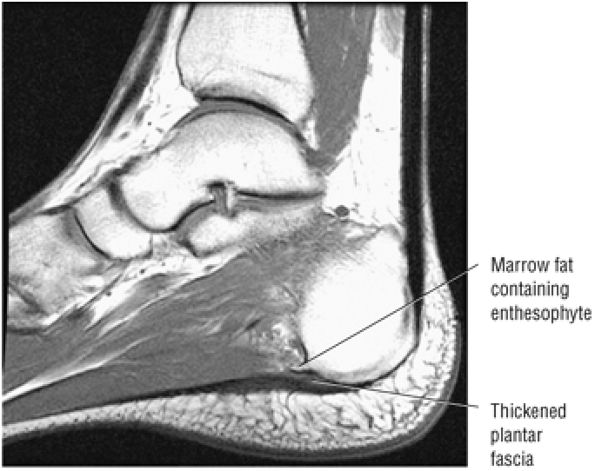 |
|
FIGURE 5.355 ● Plantar calcaneal enthesophyte associated with a thickened plantar fascia. A spur or enthesophyte on the leading edge of the inferior calcaneal surface is not pathognomonic of plantar fasciitis and may be seen in as few as 10% of symptomatic patients. Sagittal T1-weighted image.
|
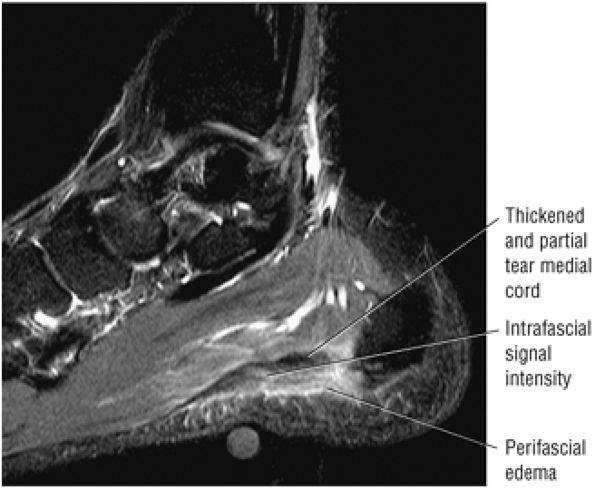 |
|
FIGURE 5.356 ● Thickened segment of the medial cord in plantar fasciitis on a sagittal FS PD FSE image. Plantar fasciitis may have a traumatic, vascular, neurologic, arthritic, infectious, autoimmune, or mechanical cause. Plantar fasciitis is the result of chronic overload of the plantar fascia with inadequate healing, chronic inflammation, and fatigue failure.
|
-
Stage 0: Ischemia
-
Stage I: A necrotic focus with edema
-
Stage II: A large well-defined necrotic area
-
Stage III: A subchondral fracture (analogous to Ficat III lesion in the hip)
-
Stage IV: Subchondral collapse and secondary osteoarthrosis
bone marrow edema, or it may demonstrate a diffuse pattern of involvement, similar in morphology to bone infarcts seen in other locations. Nontraumatic AVN of the talus is similar in appearance to AVN of the distal femoral condyle of the knee. Early in the disease, there is a small hypointense focus that corresponds to the necrotic lesion seen in the superior talar dome. This, however, is frequently overshadowed by an extreme pattern of diffuse bone marrow edema throughout the entire talus. This hyperemia or bone marrow edema is hypointense on T1-weighted images and hyperintense on FS PD FSE or STIR images. Caution should be taken not to mistake this pattern of bone marrow edema for infection, tumor, or reflex sympathetic dystrophy. Bone biopsy is not required. Within 12 months, the bone marrow edema shows partial resolution, leaving behind a persistent, well-defined focus of osteonecrosis. In contrast to the bone marrow edema pattern in the hip, which may precede the development of AVN, it is less common to see diffuse marrow talar edema without an associated focus of AVN. The morphology of this early AVN, however, may be less well demarcated and smaller prior to the resolution of associated marrow edema. Bilaterality and asynchrony of involvement is not uncommon in the talus with AVN. Follow-up MR examination within 12 months can be used to document resolution of bone marrow edema.
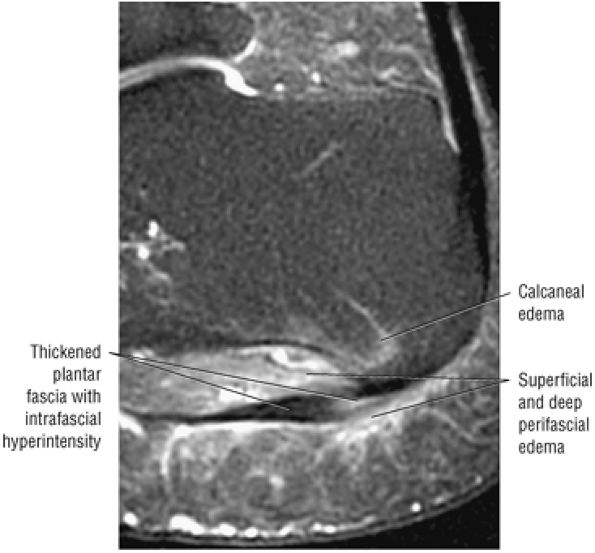 |
|
FIGURE 5.357 ● Inflammation of the lateral cord of the plantar fascia with perifascial edema deep and plantar to the aponeurosis. There is reactive calcaneal marrow hyperintensity adjacent to the insertion of the plantar fascia. Sagittal FS PD FSE image.
|
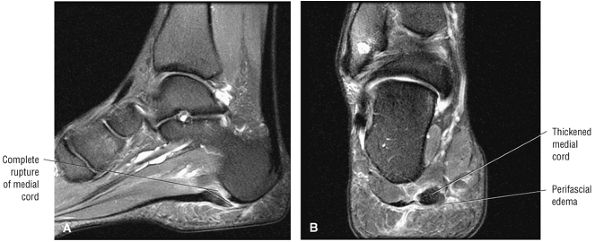 |
|
FIGURE 5.358 ● Complete rupture of the medial cord of the plantar fascia. Steroid injections may be associated with delayed rupture of the plantar fascia. Surgical plantar fasciectomy has fewer associated complications (longitudinal arch strain, lateral plantar nerve dysfunction, stress fracture) than are seen in patients who sustain plantar fascia rupture after steroid injection. (A) Sagittal FS PD FSE image. (B) Coronal FS PD FSE image.
|
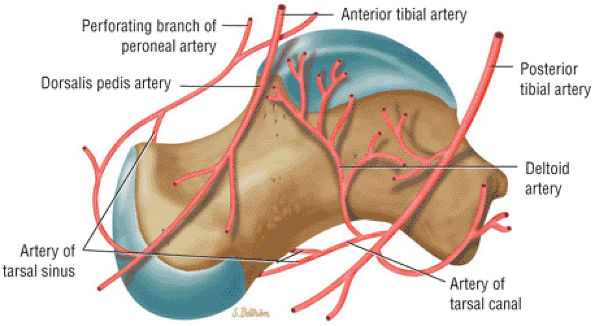 |
|
FIGURE 5.359 ● Talar arterial blood supply. The main artery to the body of the talus is supplied by the artery of the tarsal canal. Lateral color graphic.
|
-
A small hypointense necrotic focus in the superior talar dome (Fig. 5.360)
-
A sclerotic line directly adjacent to the subchondral plate
-
A sclerotic line marginating the central marrow fat signal
-
Diffuse osteonecrosis with a serpiginous bone infarct pattern (Fig. 5.361)
-
Associated talar neck fracture
-
Disproportionate diffuse marrow edema
-
Marrow edema adjacent to an ischemic focus
-
A central signal void secondary to necrosis with a hyperintense rim (on FS sequences)
-
Resolution of bone marrow edema in chronic cases with persistence of a well-demarcated focus of osteonecrosis
-
Double line sign (a hypointense outer line caused by sclerosis and fibrosis and a hyperintense inner line caused by granulation tissue) on FS PD FSE images
-
Sagittal images are the most sensitive in demonstrating the initial flattening of the second metatarsal head.
-
Trauma either from a single episode or from repeated injury of a metatarsal at risk (e.g., the long second metatarsal) is the primary etiology of Freiberg's infraction.
compression of the chondral surface, cortical thickening, and necrosis of the trabecular marrow. Deformity of the metatarsal head, shaft hypertrophy, and secondary osteoarthritis of the MTP joint result from repeated weight-bearing trauma.
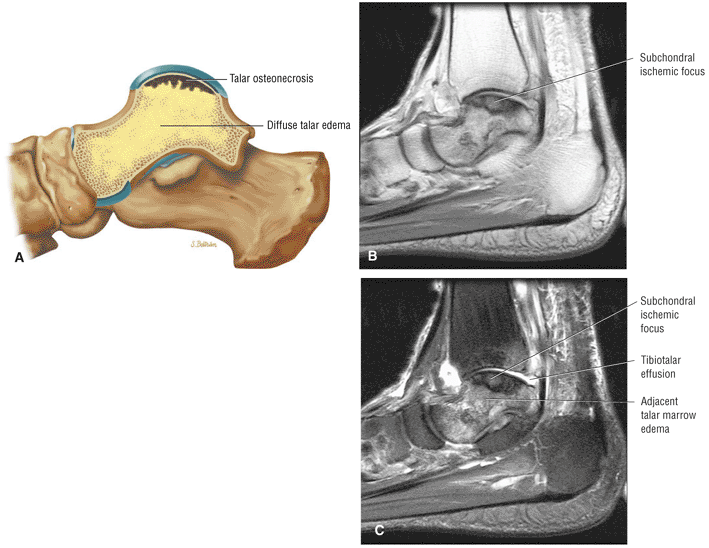 |
|
FIGURE 5.360 ● Focal subchondral talar ischemic focus characteristic of AVN (osteonecrosis) of the talus. The ischemic zone is well demarcated. The associated talar edema may extend diffusely in the acute stages of ischemia. (A) Lateral color illustration. (B) Sagittal T1-weighted image. (C) Sagittal FS PD FSE-weighted image.
|
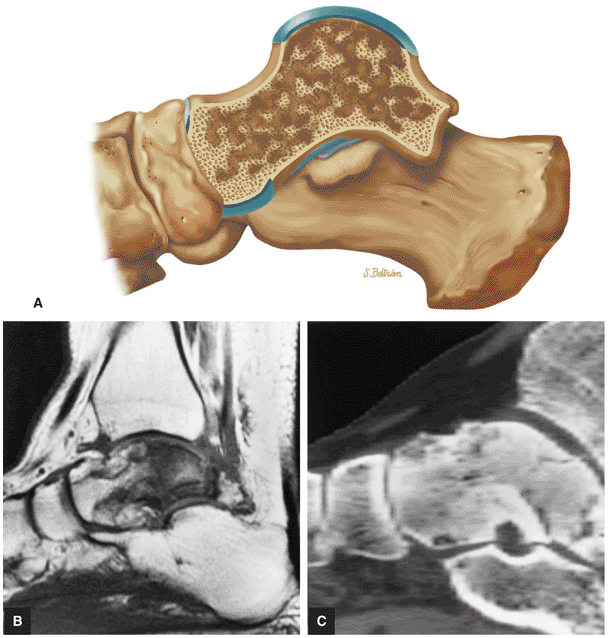 |
|
FIGURE 5.361 ● Diffuse form of talar ischemia with an infarction pattern involving the entire talar body. (A) Lateral color illustration. (B) Sagittal T1-weighted image. (C) Lateral reformatted CT image.
|
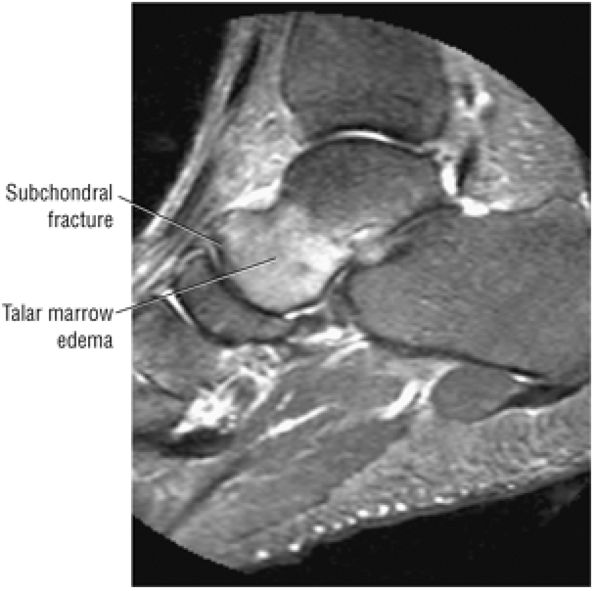 |
|
FIGURE 5.362 ● Talar head subchondral fracture, which may be mistaken for regional migratory osteoporosis or ischemia. Sagittal FS PD FSE image.
|
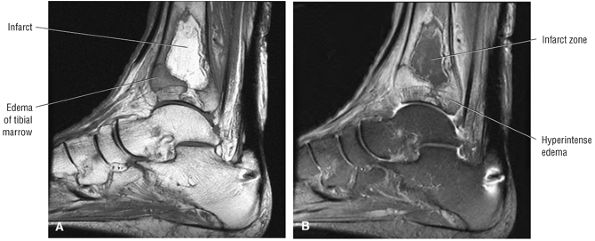 |
|
FIGURE 5.363 ● Serpiginous distal tibial infarct pattern with the ischemic zone demonstrating marrow fat signal on a sagittal T1-weighted image (A) and hypointensity on FS PD FSE image (B).
|
-
Stage I: Decreased subchondral density plus metatarsal head flattening
-
Stage II: Sclerotic and radiolucent areas leading to fragmentation, metatarsal head deformity and enlargement, cortical thickening, and physeal fusion
-
Stage III: Degenerative osteoarthritis
images. The increased signal intensity of the articular cartilage surface is best shown on FS PD FSE images. Other changes include:
-
Subchondral marrow hyperemia on STIR and FS PD FSE sequences
-
Irregularity and flattening of the epiphysis with spurring (in more advanced disease)
-
Loss of joint space and metatarsal shaft hypertrophy (in more advanced disease)
-
Sclerosis (see Figs. 5.364 and 5.365)
-
Fragmentation and flattening of the subchondral plate on sagittal images
-
Variable degenerative subchondral marrow changes and edema (see Figs. 5.364 and 5.365)
-
Joint effusion
-
Loose bodies
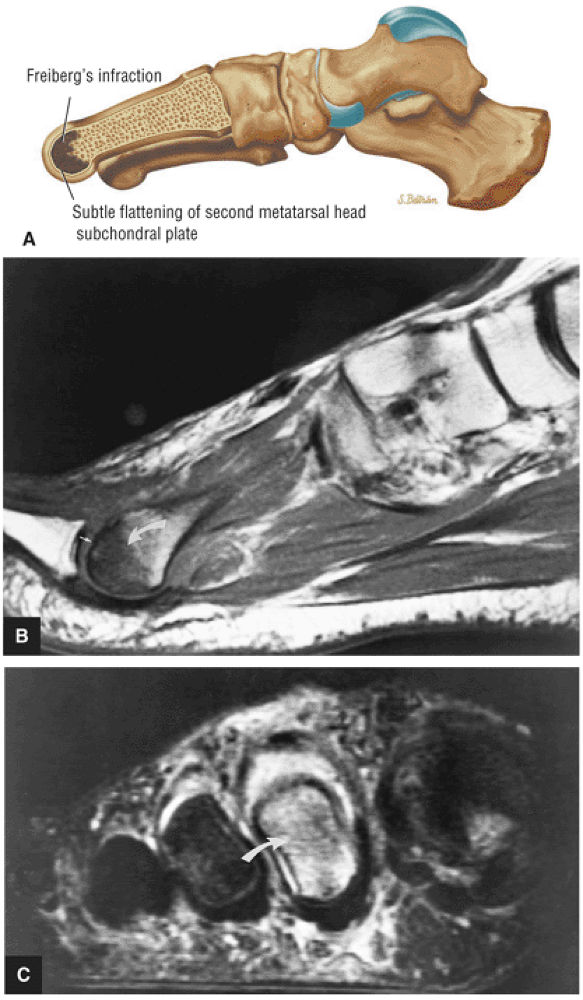 |
|
FIGURE 5.364 ● (A) Lateral color illustration of Freiberg's infraction, which represents an osteochondrosis most commonly involving the second metatarsal head. Freiberg's infraction (curved arrow) with osteonecrosis of the second metatarsal head is hypointense on a T1-weighted sagittal image (B) and hyperintense on STIR coronal image (C). Early flattening of the metatarsal head is appreciated on the T1-weighted sagittal image (small arrows).
|
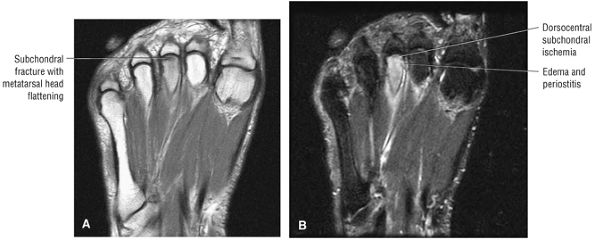 |
|
FIGURE 5.365 ● Less common third metatarsal head involvement in Freiberg's infraction. Degenerative arthritis and synovitis with distal metatarsal head fragmentation of articular cartilage and subchondral bone may occur as complications. (A) Axial T1-weighted image. (B) Axial FS PD FSE image.
|
-
Osteomyelitis is associated with pressure point ulcers and marrow changes that are hypointense on T1-weighted images and hyperintense on FS PD FSE or STIR images.
-
Neuropathic joint involvement is usually independent of the presence of soft-tissue ulcers and typically involves multiple joints.
-
Neuropathic marrow changes are not hyperintense on T2* GRE images. Hyperintense marrow on T2* contrast indicates increased free water and thus has a high association with osteomyelitis.
involved in diabetic neuropathy without overlying ulcers. Common sites for pressure lesions and infection include the metatarsal heads, the calcaneal tuberosity, the tendo-Achilles bursa, the soft tissue medial to the first metatarsal head, the malleoli, the distal toes, and the tarsometatarsal joints. Ulcers often are found at the curve of the first and fifth metatarsal heads. Approximately one third of diabetic foot ulcers are neuropathic, one third are ischemic, and one third are mixed. Neuropathic ulcers are more likely to be under the metatarsal head with thick hyperkeratosis, whereas ischemic ulcers have a fibrotic base without hyperkeratosis. “Charcot foot” is a term indicating foot collapse, with flattening of the arch, osseous destruction, bone formation, and subluxation/ dislocation. Osteomyelitis is an important consequence of foot ulcers and skin or soft-tissue infection. A tract from ulcer to bone is sometimes found.
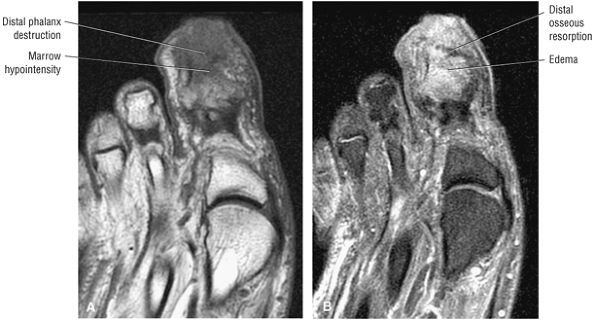 |
|
FIGURE 5.366 ● Osteomyelitis of the distal phalanx of the hallus in a diabetic. There is involvement of the periosteum, cortex, marrow, and cancellous bone. (A) Axial T1-weighted image. (B) Axial FS PD FSE image.
|
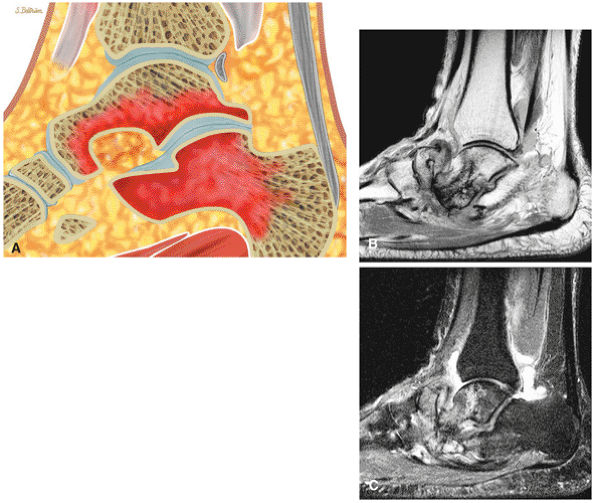 |
|
FIGURE 5.367 ● (A) Acute neuropathic osteoarthropathy with edema of multiple regional joints without contiguous soft-tissue signal intensity changes. Lateral color illustration. Sagittal T1-weighted (B) and FS PD FSE (C) images show chronic neuropathic disease with deformity and fragmentation of the navicular and advanced talonavicular arthritis. The hyperintense changes of the talus and navicular are not associated with infection.
|
-
Stage I: An acute inflammatory process
-
Stage II: A reparative process
-
Stage III: Consolidation stage of healing
Collapse of the midmetatarsus along the weight-bearing axis of the foot is commonly seen as a deformity associated with neuroarthropathy. A more localized region or focus of increased subchondral or medullary signal intensity may be associated with osteomyelitis.
-
Hypointense to intermediate-signal pressure lesion or ulcer
-
The ulcer may have a necrotic cystic center.
-
Effacement of the subcutaneous fat or fat plane
-
Replacement of fat marrow in neuropathic arthropathy
-
Neuropathic changes in the MTP, tarsometatarsal, and intertarsal joints
-
A periosteal reaction and cortical destruction in osteomyelitis or neuropathic foot
-
Muscle atrophy with fat signal intensity in neuropathic foot
-
Cellulitis
-
Thick-walled abscess with central necrosis
-
Neuropathic and osteomyelitis changes are hyperintense on FS PD FSE or STIR images.
-
Osteomyelitis superimposed over neuropathic change produces a focal area of greatest hyperintensity.
-
Soft-tissue tract from skin ulcer to bone and osseous erosion and marrow edema in osteomyelitis (Fig. 5.368)
-
Joint abnormalities including fragmentation and resorption associated with neuropathic changes
-
Reactive marrow edema in area adjacent to soft-tissue inflammation
-
Associated diabetic muscle infarction (Fig. 5.369)
-
Hyperintensity on T2* GRE images in osteomyelitis versus neuropathic marrow, which is not hyperintense
-
Abscess enhances along peripheral wall after contrast administration.
-
Enhancement may be present in both neuropathy and osteomyelitis.
-
A larger area of diffuse enhancement in cellulitis
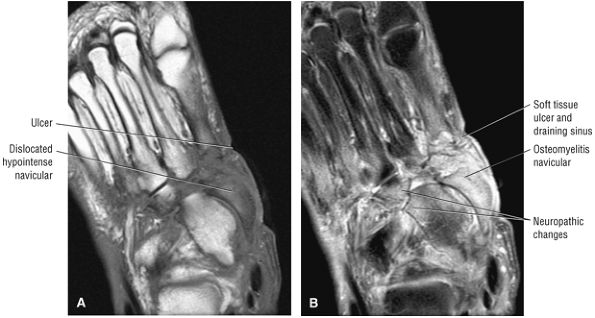 |
|
FIGURE 5.368 ● Osteomyelitis of the navicular is hypointense on axial T1-weighted image (A) and hyperintense on FS PD FSE image (B). The osteomyelitis coexists with adjacent neuropathic changes. A draining ulcer is located adjacent to the navicular. The navicular marrow demonstrates greater hyperintensity than surrounding neuropathic change.
|
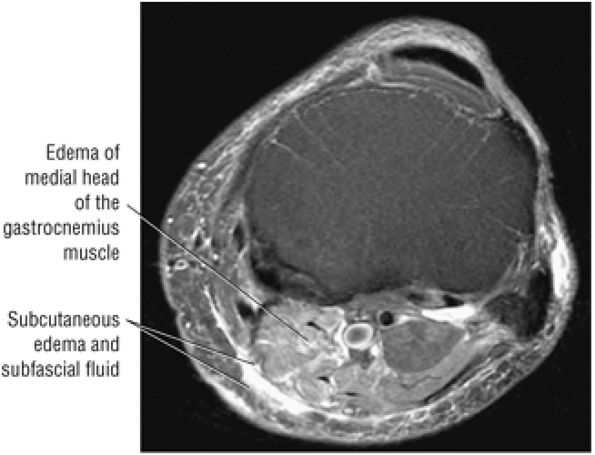 |
|
FIGURE 5.369 ● Diabetic muscle infarction with hyperintensity of the medial head of the gastrocnemius muscle on axial FS PD FSE image.
|
-
Primary signs include osseous erosion, adjacent soft-tissue mass (abscess), and a sinus tract.
-
Marrow is hypointense on T1-weighted images and hyperintense on FS PD FSE or STIR images.
-
Early osteomyelitis (see also the discussion on the neuropathic foot in diabetes) is hypointense on T1-weighted images and hyperintense on T2, FS PD FSE, or STIR images.
-
On FS PD FSE sequences in the acute or subacute phase, a diffuse or patchy increase of signal intensity in the medullary bone indicates marrow involvement.
-
Primary signs of osteomyelitis include osseous erosion with a soft-tissue mass (Fig. 5.371) and a sinus tract or adjacent abscess.
-
A soft-tissue hemangioma may mimic soft-tissue infection; however, adjacent osseous marrow fat signal is preserved.
-
A peripheral rim of low signal intensity, representing reactive bone, may demarcate the focus over time.
-
Alterations in signal intensity may also be seen at sites of cortical transgression, periosteal reaction, soft-tissue masses, and sequestra.
-
Staphylococcal osteomyelitis of the distal tibial metaphysis may present with a stellate pattern of signal change that mimics the MR appearance of a stress fracture. On FS PD FSE images, infected material in serpiginous tracts is seen as linear segments of high signal intensity.
-
MR imaging defines the metaphyseal-equivalent locations of the talus at risk for infection both proximally and distally (Fig. 5.372). FS PD FSE or STIR techniques are required to increase sensitivity for areas that are adjacent to cartilage and that border the physeal plates, areas commonly involved in hematogenous osteomyelitis.
-
New terminology is complex regional pain syndrome (CRPS).
-
Type I CRPS replaces the term sympathic dystrophy (pain related to a noxious event or immobilization).
-
Type II CRPS replaces the term causalgia (pain related to nerve injury, but not necessarily limited to the distribution of the injured nerve).
-
Osteoporosis and CRPS may demonstrate similar patchy marrow patterns of hypointensity on T1-weighted images and hyperintensity on FS PD FSE images.
the inciting event or expected healing response. The incidence of RSD following trauma is difficult to estimate.
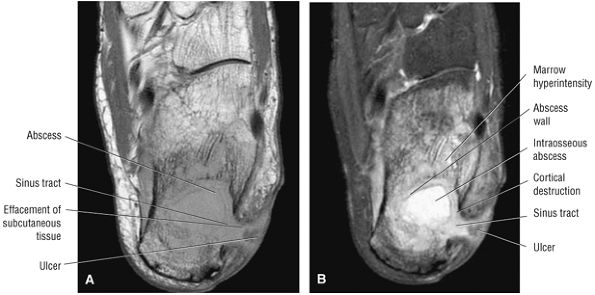 |
|
FIGURE 5.370 ● Calcaneal osteomyelitis with an interosseous abscess, cortical erosion, and direct communication with a sinus tract communicating to the skin. (A) Axial T1-weighted image. (B) Axial FS PD FSE image.
|
-
Stage 1: Stage 1 has a duration of weeks to months. The limb has nonfocal pain, swelling with associated joint stiffness and decreased range of motion, and increased skin temperature.
-
Stage 2: Stage 2 has a duration of 3 to 6 months. Pain continues but decreases over time. Swelling evolves into thickening of the dermis and fascia. Early signs of atrophy and osteoporosis become evident, and the extremity becomes cooler.
-
Stage 3: Stage 3 is the atrophic stage. Pain continues and atrophy is exacerbated by continued decreased range of motion and increased joint stiffness. The extremity is cooler, with decreased vascularity.
-
Skin thickening and the presence of soft-tissue edema in stage I RSD
-
Skin thickening and thinning without soft-tissue edema in stage II RSD
-
Muscle atrophy in stage III RSD
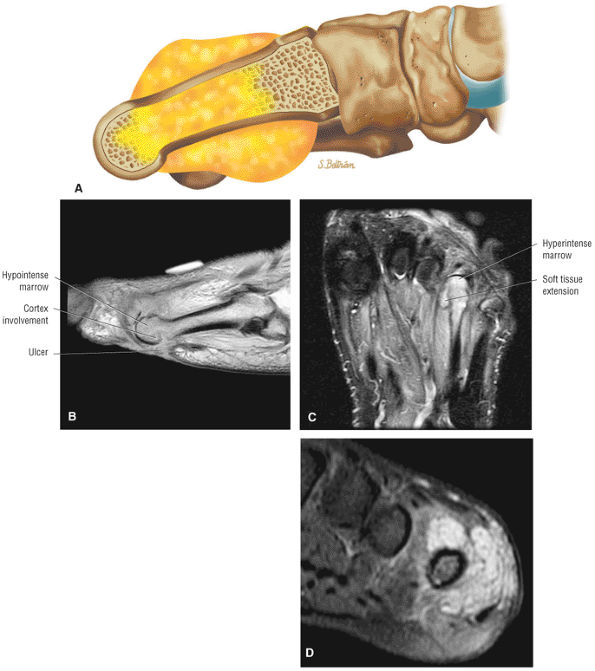 |
|
FIGURE 5.371 ● (A) Metatarsal osteomyelitis with medullary and soft-tissue extension in a diabetic foot. Lateral color illustration. (B –D) Fourth metatarsal osteomyelitis with plantar surface forefoot ulcer. Soft-tissue hyperintense hemangioma with associated marrow signal changes may be mistaken for infection. (B) Sagittal T1-weighted image. (C) Axial FS PD FSE image. (D) Coronal FS PD FSE image.
|
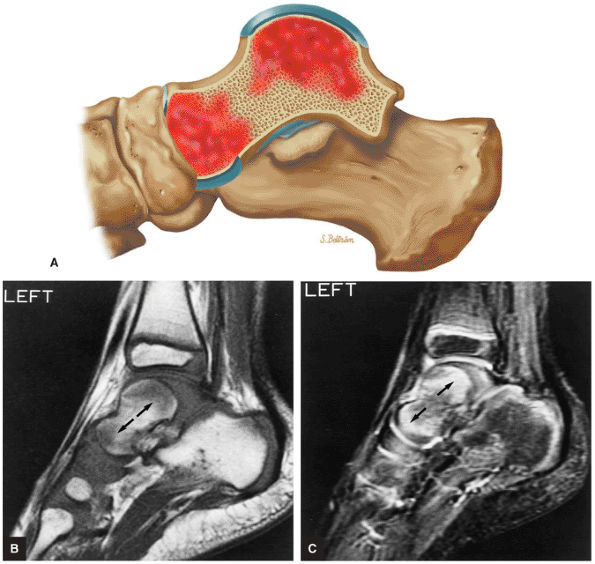 |
|
FIGURE 5.372 ● (A) Osteomyelitis with hematogenous spread to the talus. Lateral color illustration. (B, C) Osteomyelitis of the left talus with involved metaphyseal-equivalent locations (arrows) of the talus. There is hypointense signal intensity on T1-weighted sagittal images (B) and hyperintense signal intensity on STIR sagittal images (C).
|
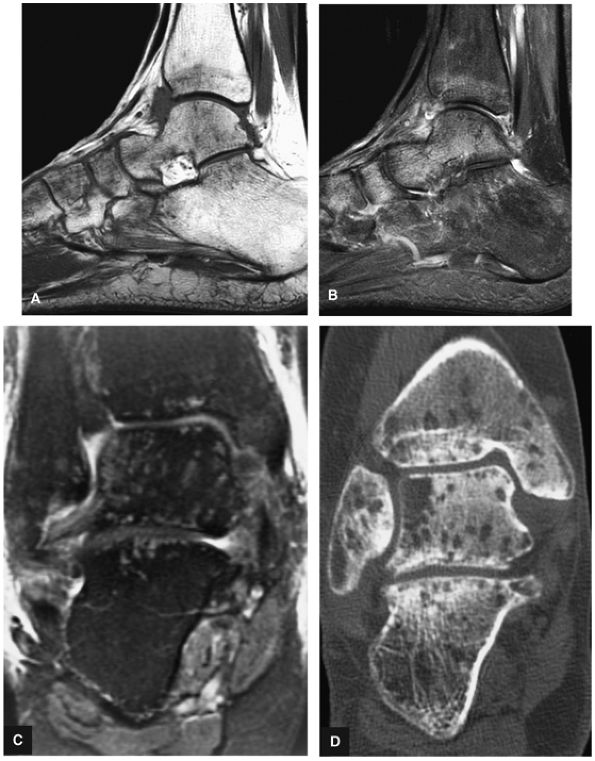 |
|
FIGURE 5.373 ● Complex regional pain syndrome (reflex sympathetic dystrophy) with patchy marrow hypointensity in subarticular areas on T1-weighted image (A) and patchy hyperintensity on FS PD FSE image (B). Disuse osteopenia (osteoporosis) may mimic MR findings of complex regional pain syndrome. Diffuse hyperemia is also common in the pediatric age group. Follow-up MR studies are helpful in documenting the return of normal marrow homogeneity. (C) Coronal FS PD FSE image. (D) Coronal CT image.
|
-
Degenerative arthritis targets both the tibiotalar joint and the hindfoot.
-
Rheumatoid arthritis frequently involves the forefoot and talonavicular joint.
-
There may be associated synovitis and subchondral marrow edema in rheumatoid disease.
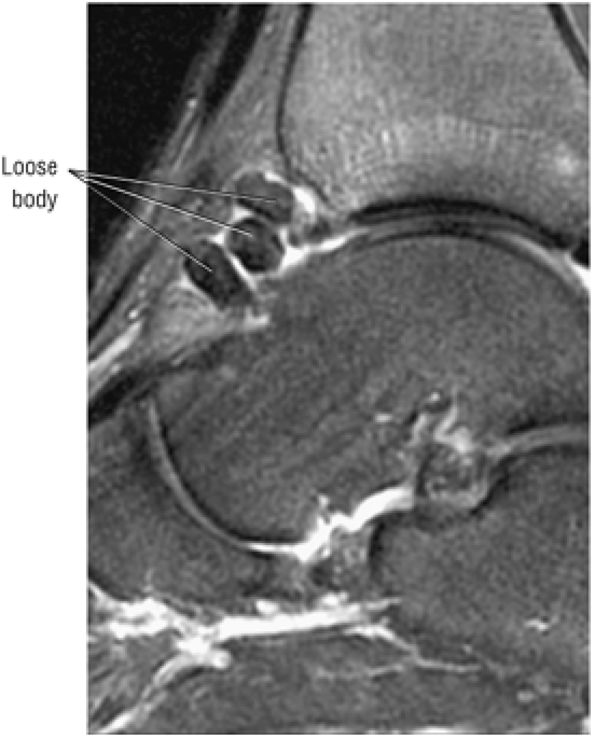 |
|
FIGURE 5.374 ● Anterior tibiotalar joint loose bodies on sagittal FS PD FSE image.
|
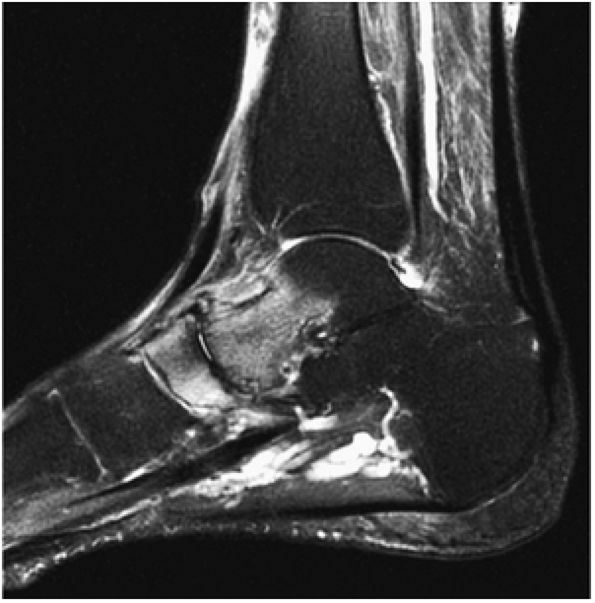 |
|
FIGURE 5.375 ● Talonavicular hyperemia with diffuse joint space narrowing in midfoot involvement of rheumatoid arthritis. Sagittal FS PD FSE image.
|
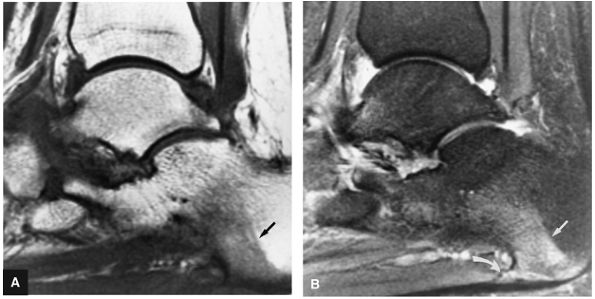 |
|
FIGURE 5.376 ● Seronegative arthritis with a painful heel correlated with a calcaneal enthesophyte (straight arrow). The band-like region of marrow edema (curved arrow) is hypointense on the T1-weighted sagittal image (A) and hyperintense on the FS PD FSE sagittal image (B).
|
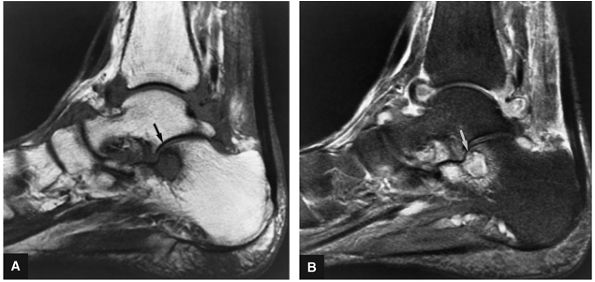 |
|
FIGURE 5.377 ● Degenerative subchondral cyst involving the anterior aspect of the posterior facet of the subtalar joint on (A) T1-weighted sagittal image and (B) FS PD FSE sagittal image. The cyst and adjacent marrow are hyperintense on the FS PD FSE (B). Patient also has a tibiotalar joint effusion with capsular distention and synovitis.
|
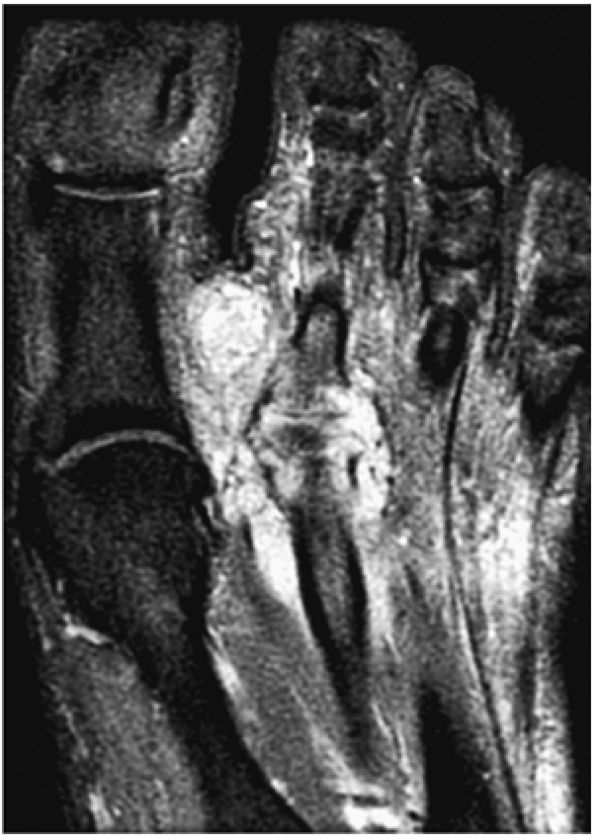 |
|
FIGURE 5.378 ● Distal metatarsal erosion and hypointense soft-tissue mass in sarcoid arthropathy of the second MTP joint. Axial FS PD FSE image.
|
-
Ganglia are commonly located in the dorsolateral foot, including the talonavicular joint.
-
Intravenous contrast shows peripheral enhancement without central hyperintensity.
-
Cyst-like structures with central intermediate signal intensity should be further evaluated with intravenous contrast to exclude a soft-tissue sarcoma.
-
A medial malleolus bursa may have imaging characteristics similar to a ganglion.
Giant cell tumor of the tendon sheath about the ankle appears as a heterogeneous mass on GRE images, with low-signal-intensity hemosiderin deposits. The intense vascularity of these lesions may demonstrate enhancement on dynamic perfusion imaging with a MR paramagnetic contrast agent.172 A medial malleolus adventitial bursa may mimic the morphology and signal intensity of a ganglion (Fig. 5.381). These bursa are associated with figure skating and skiing, which produce direct irritation over the malleolar prominences.
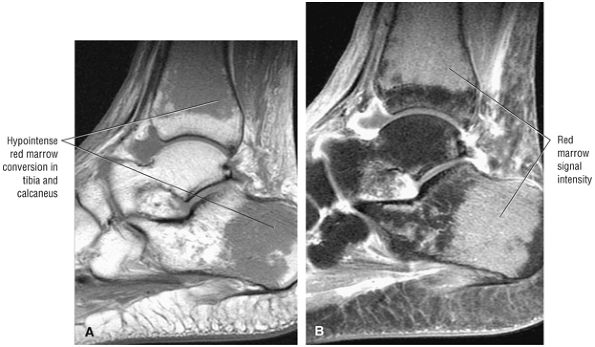 |
|
FIGURE 5.379 ● Red marrow conversion in sickle cell anemia. Note that the red marrow of the tibia does not extend to the physeal line. (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image.
|
 |
|
FIGURE 5.380 ● Fluid-filled ganglion originating from the calcaneocuboid articulation is hypointense on a coronal T1-weighted image (A) and hyperintense on corresponding FS PD FSE image (B).
|
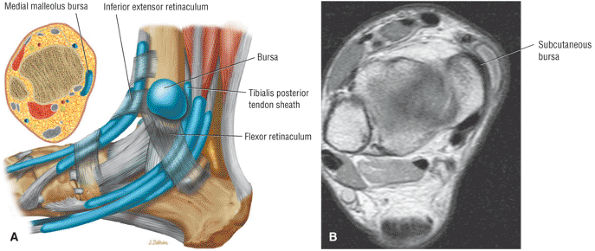 |
|
FIGURE 5.381 ● (A) The medial malleolar subcutaneous bursa superficial to the tibialis posterior and deep to the skin. This bursa is located posterior to the inferior extensor retinaculum and superior to the flexor retinaculum. Lateral color illustration with axial insert. (B) Discrete medial malleolar subcutaneous bursa intermediate in signal intensity on axial PD FSE image.
|