Interposition Arthroplasty of the Elbow
interposition arthroplasty, continues to be a viable reconstructive
option in all practice, particularly in the young patient (5,13).
the relatively young adult patient. Although there have been
significant advances in total elbow arthroplasty in recent years, the
ongoing need to address severe arthritis in a patient in the third,
fourth, or fifth decade of life continues to warrant consideration of
this alternative procedure to restore motion and relieve discomfort.
painful or unstable, total elbow replacement may be performed with
minimal technical difficulty, inserting the prosthetic stems into
intact medullary canals of humerus and ulna (3).
Specifically, (a) interposition arthroplasty is especially attractive
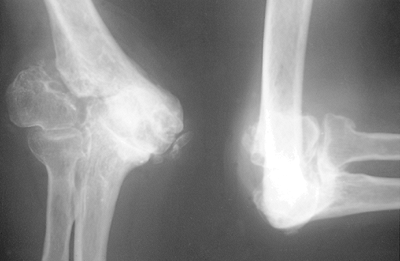
in the management of posttraumatic ankylosis of the elbow (14). The anatomic requisite is that the broad contour of the distal humerus has not been significantly disrupted (Fig. 22-1).
For those in whom the prosthesis is to be avoided, usually due to a
very young patient, less than 40 years of age, reconstruction of the
distal humerus may precede the interposition procedure. (b) In those
less than 40 to 50 years of age with severe pain but with good motion,
interposition may be considered instead of prosthetic replacement (5).
(c) A third indication for this procedure is the young adult with stage
I or II rheumatoid arthritis where the elbow is stiff and/or painful
but the osseous architecture is reasonably intact. Resurfacing this
elbow with an interposed membrane has been found to be successful in
alleviating crepitus and pain, and provides a very satisfactory range
of function (12).
 |
|
Figure 22-1.
The lateral condylar nonunion narrows the distal humeral articulation and thus is a contraindication for interposition arthroplasty. |
 |
|
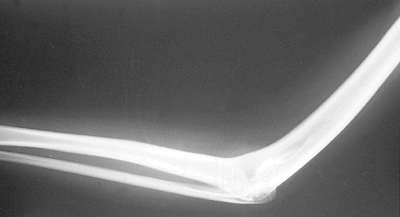
Figure 22-2.
It is important to recognize that conditions such as congenital ankylosis are also associated with a significant deprivation of soft tissue. Absence of collateral ligaments such as occurs in this condition is a contraindication to interposition arthroplasty. |
presence of active infection. The grossly unstable rheumatoid or
posttraumatic elbow cannot be adequately stabilized by a fascial
interposition procedure. Congenital ankylosis of the elbow joint (Fig. 22-2)
lacks the necessary soft-tissue ligamentous and muscular support to
allow soft-tissue interposition. Attempts to use this procedure for
this indication have been abandoned intraoperatively and total elbow
replacement has been substituted, even in the adolescent. As with other
elbow arthroplasty techniques, the patient’s need to use the upper
extremities in ambulation or transfer from bed to chair is a relative
contraindication, since excessive loading of the elbow will destabilize
the joint. The noncompliant patient should be identified and avoided.
assessed before surgery with the intention of addressing this at the
time of the interposition procedure.
films in maximum possible flexion and extension. If there are
associated signs or symptoms of compromise of the ipsilateral shoulder
and wrist, they should also be included in the radiographic studies.
site should be selected by examination of the lower abdomen or lateral
thigh. Hairy donor sites should be avoided, since
the
presence of numerous large hair follicles in the cutis graft may
theoretically predispose to increased incidence of epidermoid inclusion
cysts. Since harvest of the cutis graft will leave a significant
postoperative scar, the patient should participate in the selection of
the most suitable donor site. Because the procedure is done under
tourniquet control, blood loss is not excessive and transfusion is not
anticipated.
for fascial interposition arthroplasty of the elbow. This is the most
commonly used tissue reported in the recent literature (1,4,6,7,8,9).
This is the thick dermal layer of skin that remains after a superficial
epidermal layer of 0.0010 to 0.0012 inch has been peeled back with a
dermatome. Cutis is a very tough and durable but flexible material
that, when attached to the cut surface of the distal humerus, rapidly
adheres to the bone. Cutis has been used by various authors as an
interposing membrane in resection arthroplasties of various joints
since 1913. It has been used in the elbow successfully in many
instances in various centers. One of us prefers the achilles tendon
allograft (BFM). If an achilles tendon allograft is to be used,
availability of this material must, of course, be determine before
surgery.
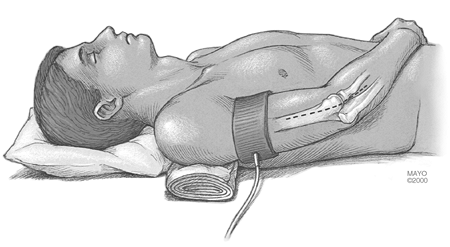
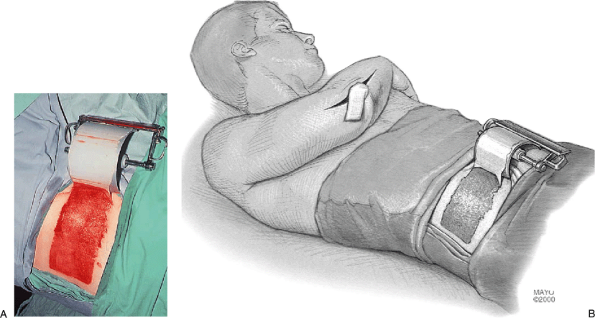
a large pillow or bolster under the ipsilateral shoulder, allowing the
operated extremity to rest across the trunk for the procedure (Fig. 22-3).
For a cutis interposition the skin graft donor site that has been
selected, either abdomen or thigh, is prepared and draped and then
covered until needed. The entire upper extremity is prepared and draped
with stockinette, venous blood is expressed with an elastic bandage,
and a pneumatic tourniquet is applied as proximally as possible on the
upper arm and inflated for the duration of the procedure (Fig. 22-3).
symptomatic, it is exposed and decompressed past the level of
constriction. The extensile Kocher deep lateral approach to the joint
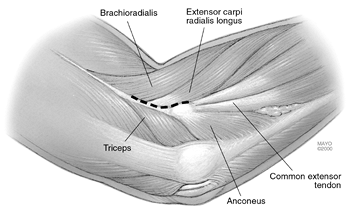
is preferred, and a flap of tissue is elevated laterally to identify
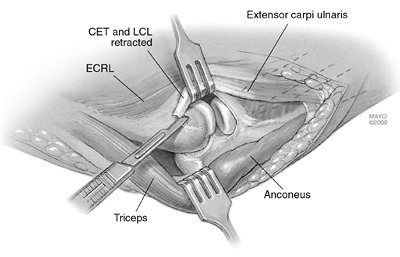
Kocher’s interval (Fig. 22-4). It proceeds down the supracondylar ridge of the humerus between extensor carpi ulnaris
and anconeus muscles. The extensor mass, lateral collateral ligament
origin, and periosteum are dissected off of the lateral condyle and
distal humerus (Fig. 22-5). Further release of the capsule exposes the medial collateral ligaments. If necessary, these are sectioned from within (Fig. 22-6),
although one of us (BFM) preserves the medial ligament. If sectioned,
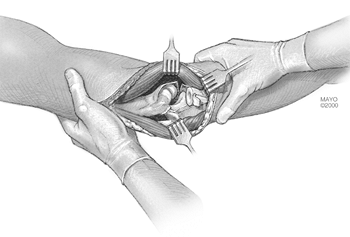
this is most safely performed by release from the ulna. Varus stress
allows the joint to dislocate and exposes the distal humerus and
proximal ulna (Fig. 22-7). We do not
routinely expose or transpose the ulnar nerve unless, as mentioned
earlier, the ulnar nerve has been entrapped in posttraumatic scar on
the medial side of the elbow, in which case a medial approach may be
selected to perform the entire procedure. If the patient has cubital
tunnel syndrome with ulnar nerve compromise, the ulnar nerve is
transposed at this point in the procedure.
 |
|
Figure 22-3. An extensile Kocher approach to the posterolateral aspect of the elbow is preferred.
|
 |
|
Figure 22-4.
Identifying the supracondylar ridge proximally and the interval between the extensor carpi ulnaris and the anconeus muscle distally allows entry into the posterolateral, lateral, and anterolateral aspect of the joint. |
 |
|
Figure 22-5.
The common extensor tendon (CET) as well as the lateral collateral ligament (LCL) are taken off as a single layer. Occasionally, the collateral ligament can be isolated from the extensor origin and released separately. (Abbreviation: ECRL, extensor carpi radialis longus.) |
 |
|
Figure 22-6.
The anterior and posterior capsules are then released, and the anterior bundle of the medial collateral ligament is sectioned from the ulnar aspect of the medial aspect of the joint. |
 |
|
Figure 22-7. A varus stress allows the elbow to open and dislocate, exposing the distal humerus and proximal ulna.
|
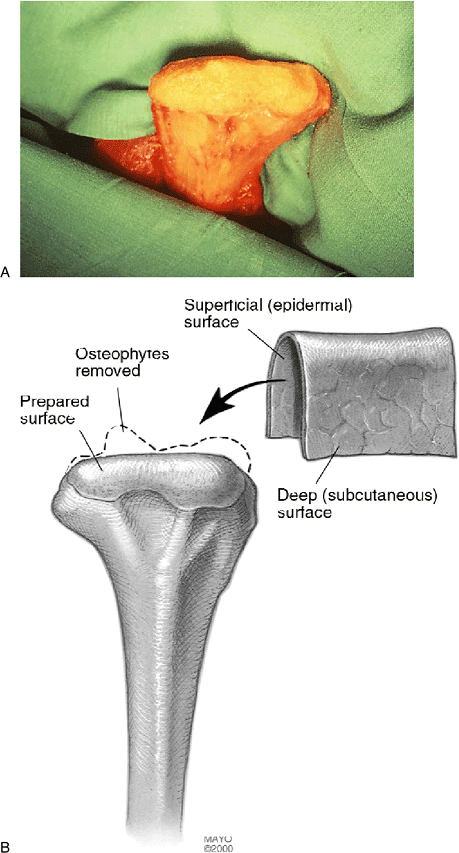
humerus is prepared by excising osteophytes, removing all articular
cartilage and ununited bone fragments and fibrous debris (Fig. 22-8).
That should yield a smooth, rounded surface 4 or 5 cm wide and
approximately 2 cm from anterior to posterior. This prepared cancellous
surface is the tissue to which the
cutis
graft will be attached. On average. only about 1 to 1.5 cm of the
distal humerus is resected. Where possible the articular surfaces of
the proximal radius and ulna are disturbed as little as possible. If
those joint surfaces are irregular, they are simply smoothed down with
bur or rasp before reducing the elbow. We prefer to debride the radial
head if necessary to restore pronation and supination. An intact radial
head provides a much broader distal half of the articulation, resulting
in better postoperative stability.
 |
|
Figure 22-8. A:
The distal humerus is freed of the articular debris and osteophytes, providing a smooth, round surface on which the dermal graft will be applied. B: The dimensions of the distal humerus are typically approximately 4 to 5 cm wide and about 2 cm from anterior to posterior. |
have tended to remove a smaller amount of bone from the distal humerus
than I did earlier in the series. This yields a higher degree of
stability but necessitates that the surgeon, after having reduced the
elbow, ensure that there is a satisfactory range of movement and that
the elbow is not overly tight, since the patient will not regain any
more motion in the postoperative period of rehabilitation than was
present at the completion of the procedure.
procedure, the surgeon determines how large a cutis graft is needed and
then, using a hand-held or motorized dermatome, takes a thin (10 to 12
mils) split-thickness skin graft from the donor site (Fig. 22-9).
The bed of the donor site then has the typical appearance with punctate
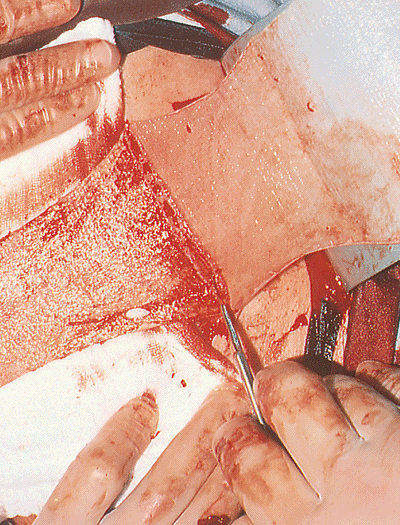
bleeding. The surgeon then excises this deep dermal layer of skin,
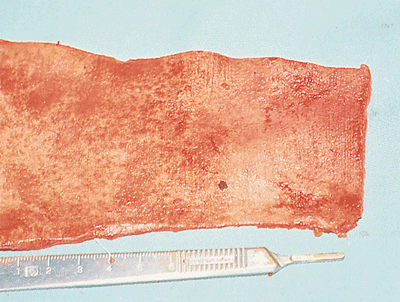
cutting it off of the subcutaneous fat by scalpel dissection (Fig. 22-10), thus harvesting the cutis graft (Fig. 22-11).
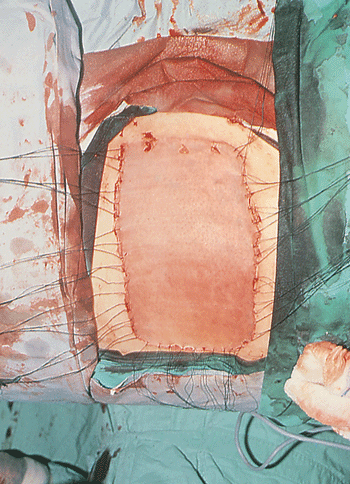
After securing hemostasis with electrocautery, the split-thickness
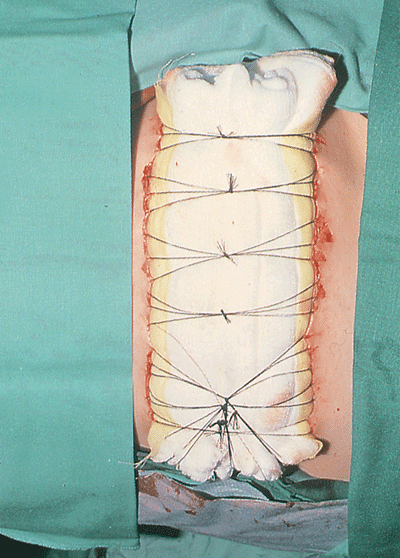
graft is then reapplied over the donor site and held in place with
sutures (Fig. 22-12) and a stent (Fig. 22-13).
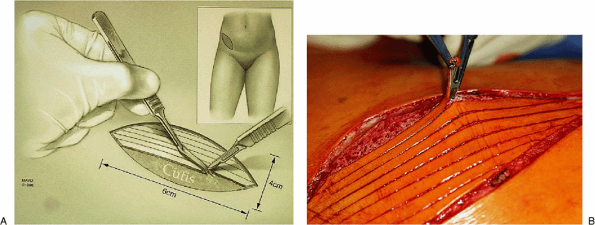
of skin measuring about 4 з 10 cm is outlined in the groin or bikini
line. Strips of dermis 3 to 4 mm wide are sharply removed, leaving the
cutis (Fig. 22-14). The cutis is then sharply excised and the wound is closed with a vertical mattress suture (Fig. 22-15).
This ensures that vascularization of the skin graft and healing to the
cancellous bone surface will be optimized. The surgeon drills
several
small holes through the medial and lateral epicondylar ridges and to
these the dermal graft is sutured, thus covering the distal end of the
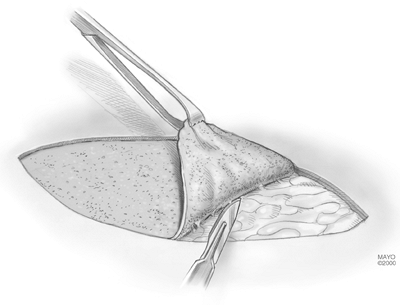
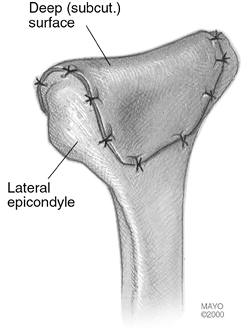
humerus in a manner that resembles a stocking covering a foot (Fig. 22-17). The deep surface of the cutis graft containing some adipose tissue faces the new joint space.
 |
|
Figure 22-9. A,B:
The ipsilateral anterolateral aspect of the abdomen is exposed and a motorized dermatome removes a thin, 10- to 12-mil split-thickness skin graft. |
 |
|
Figure 22-10. The dermis layer is harvested by sharp dissection from the subcutaneous bed immediately below the dermal layer.
|
 |
|
Figure 22-11. Appearance of the harvested cutis graft.
|
 |
|
Figure 22-12. The epidermis is then reapplied as a split-thickness skin graft to the donor site and is held in place with sutures.
|
 |
|
Figure 22-13. A stent stabilizes the reattached dermis.
|
 |
|
Figure 22-14. A,B: Strips of epidermis are removed sharply from an ellipse of skin taken from the groin region.
|
 |
|
Figure 22-15. The cutis is then harvested and the incision is closed.
|
 |
|
Figure 22-16.
The cutis graft is then applied to the distal humerus with the superficial aspect of the dermis applied to bone and the deep or subcutaneous surface exposed to the proximal ulna. The graft is held in place with multiple sutures placed through bone. |
 |
|
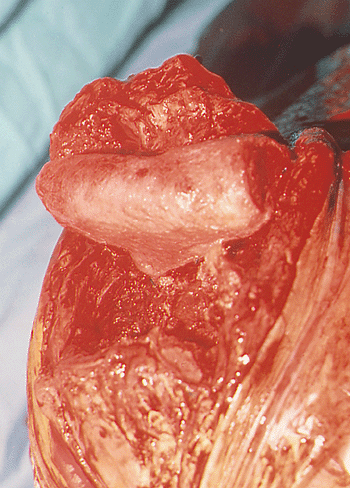
Figure 22-17. The graft completely covers the distal humerus.
|
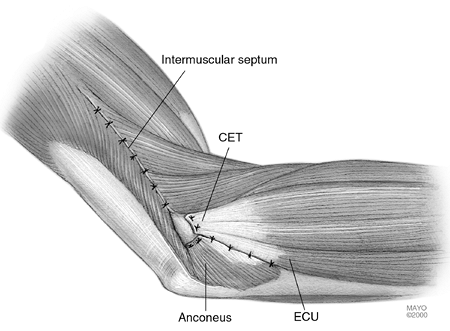 |
|
Figure 22-18.
The elbow is reduced without taking particular care to reattach the collateral ligaments. Nonabsorbable sutures are used to close the extensor mechanism and common extensor intervals. (Abbreviation: ECU, extensor carpi ulnaris.) |
splint and dressing are not disturbed for approximately 2 weeks, at
which time the sutures are removed. During the first 2 weeks the
patient is seen by the upper-extremity therapist for shoulder range of
movement, hand and wrist motion, and instructions in activities of
daily living. When the first postoperative dressing and splint are
removed, many patients are fitted with a hinged cast brace or splint,
allowing freedom of flexion and extension but protecting the
arthroplasty from excessive medial and lateral stresses as the soft
tissues are maturing. By 1 month, resistive flexion exercises are
started, and at 6 weeks extension strengthening exercises are added.
The articulated splint or cast brace is usually discarded at 6 weeks,
but an elastic elbow support may be used for added comfort and
reassurance for another month.
elbow does not provide the same fixed axis of rotation as does a total
elbow replacement device, some degree of medial/lateral laxity must be
accepted. In those elbows without serious bony deficiency in which the
radial head has been preserved, no more than 15 to 20 degrees of
medial/lateral laxity occurs.
ulnar nerve. The elbow is then addressed as described previously with
an extensile Kocher exposure, occasionally releasing a small portion of
the lateral triceps attachment to improve the exposure. The lateral
collateral ligament is released and the anterior and posterior capsules
are excised. The radial head is preserved if at all possible. Pronation
of the articulating surfaces is quite important, removing bone and
deformity to balance the ulnohumeral articulation. Care is taken to
remove the ridge (incisura) of the olecranon to allow a flat
articulation on the humerus. It is important to remove enough bone for
the trochlea and capitellum to accommodate the tendon graft and also to
allow a few millimeters of laxity to ensure adequate motion. We also
attempt to leave a slight ridge of bone medially and laterally on the
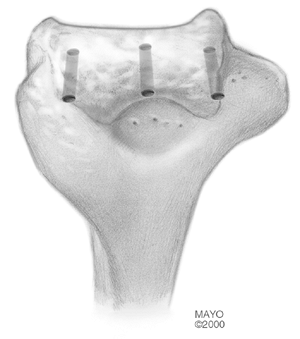
humerus to help stabilize the construction (Fig. 22-19). The distal humerus is prepared by three or four drill holes
from posterior to anterior and covering the full width of the humerus.
The Achilles tendon is assessed to determine the width required to
cover the full dimension of the distal humerus (Fig. 22-20).
The tendon portion is generally placed anteriorly, and the broad
fascial portion is directed posteriorly. This provides the maximum
graft thickness anteriorly and makes tissue available for ligament
reconstruction posteriorly. The tendon is then situated over the
humerus and the best portion of the graft needed to cover the humerus
is defined. The anterior excess of the graft is defined and transected.
Using No. 1 nonabsorbable sutures, the anterior graft is sewn to the
anterior humerus, the graft is pulled taut and the posterior flap is
secured (Fig. 22-21). At this juncture the
determination is made of whether the ligament tissue is adequate for
repair. If not, a strip of Achilles graft is taken medially, laterally,
or both, measuring about 0.5 з 6 cm.
 |
|
Figure 22-19. Distal humeral preparation develops bones ridges medially and laterally to help enhance stability.
|
from anterior to posterior, originating at the axis of rotation. The
graft is rotated around the lateral epicondyle and secured with the
suture (Fig. 22-22). A similar technique may be
used medially, but the graft is brought under and secured to the medial
epicondyle. If both ligaments are to be reconstructed, a hole is placed
between the sublime tubercle and the crista supernatoris. Once the
ligaments have been secured, this construct is usually protected with a
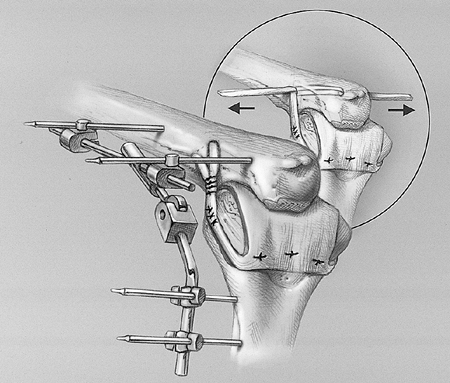
half-frame DJDII™ external fixator (Fig. 22-23).
Before application of the external fixator, the tourniquet is released,
hemostasis is obtained, the triceps is reattached as described in Chapter 1,
and the extensor muscle mass is reattached as well. The ulnar nerve is
again inspected and if it is subluxing or constricted with flexion, it
is translocated into a subcutaneous pocket. Closure is then routine.
 |
|
Figure 22-20. The achilles allograft is of sufficient size to allow easy coverage of the distal humerus.
|
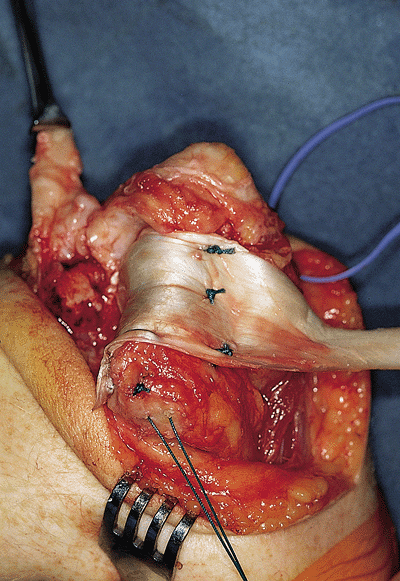 |
|
Figure 22-21. The graft is placed over the distal humerus and secured.
|
 |
|
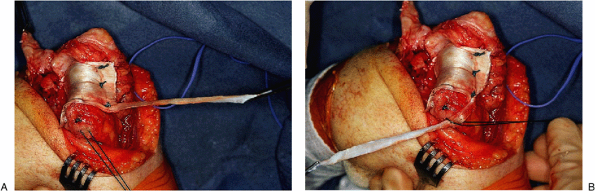
Figure 22-22. A: The posterior proximal aspect of the graft is prepared in 3- to 5-mm strips to use as collateral ligament reconstruction. B: The graft is secured laterally and medially at the anatomic site of ligament origins medially and laterally.
|
 |
|
Figure 22-23. The hinge fixator (DJD II) is applied to protect the reconstruction.
|
motion machine and maintained for 3 to 4 weeks. The fixator is removed
under anesthesia and elbow motion and stability are examined. Activity
is begun and, if the joint is stiff, static splints are prescribed.
revealed that 26 patients (70%) had excellent or good results. There is
only a single report regarding the outcome for painful, nonankylosis
posttraumatic arthritis. When used to treat the stiff joint with
intrinsic pathology, a satisfactory rate of 80% was reported. This
reflected an improvement-of-motion
arc from 30 to 100 degrees and minimal pain in 88% (16). Of 13 patients with traumatic arthritis, Cheng and Morrey (5)
documented 75% satisfactory results using fascia lata, a mean of 5
(range, 2 to 12) years after surgery. It is of note that those without
preexisting instability revealed 80% satisfactory outcomes.
resorption, heterotopic bone formation, triceps rupture, medial and
lateral subluxation, infection, and seroma formation in the fascial
graft donor site, and long-term failure.
humeral condyles and often causes no difficulty. If significant
instability develops as a late sequela, ligamentous reconstruction may
be beneficial. I (BFM) have successfully avoided instability by
repairing or reconstructing the collateral ligaments and applying a
distraction device as mentioned earlier (14).
related to the surgical exposure rather than to the procedure itself.
This can be minimized by using the exposure described subsequently or
by elevating the triceps in continuity.
managed promptly and aggressively. For superficial infections and
cellulitis, the part should be placed at rest, elevated, and
immobilized in a long-arm posterior splint while appropriate
antibiotics are administered. If the infection involves the deep
structures, open drainage and excision of the fascial graft may be
required. If bony infection occurs, removal of the implant and osseous
debridement is required. Although this will leave the elbow more
unstable, a useful limb often can be salvaged. Salvage with prosthetic
replacement is out of the question.
will usually resolve over a period of weeks. These collections rarely
require drainage. If such an accumulation persists or is unusually
large, drainage, if undertaken, must be done with strict aseptic
technique. Needle aspiration should be attempted first.
instability may occur. The result can deteriorate with time, especially
in the active individual. Typically, prosthetic replacement is the
salvage procedure of choice and is readily performed and has recently
been shown to be more than 90% successful in a series of 13 patients so
treated at the Mayo Clinic (3).
comminuted fracture of the left olecranon process in a motor vehicle
accident and was treated elsewhere with screw fixation of the olecranon
and long-arm cast immobilization for 1 month. He presented 6 months
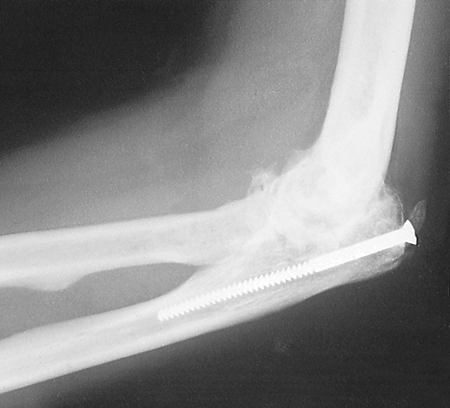
later with ankylosis of the left elbow at 75 degrees of flexion with
the radiograph showing
marked narrowing of the elbow joint (Fig. 22-24). Cutis arthroplasty was performed with a lower abdominal donor site (Fig. 22-25).
Postoperative management was routine, with a rigid postoperative
plaster splint worn for 2 weeks, followed by a cast brace for 4 more
weeks, followed by a vigorous resistive exercise therapy program.
 |
|
Figure 22-24. Posttraumatic arthrosis in a 43-year-old right-handed electrician.
|
 |
|
Figure 22-25. A cutis arthroplasty was performed with a lower abdominal donor site.
|
following surgery and, as shown here, 2 years later has range of elbow
motion of from 15 to 115 degrees with full pronation and supination (Fig. 22-26).
Medial/lateral stability was excellent, with 15 degrees of deviation on
stress testing. He has been followed at intervals and has recently
retired from his work at age 61; a recent radiograph 18 years after
surgery shows preservation of excellent flexion and extension (Fig. 22-27).
 |
|
Figure 22-26. A: After surgery the patient has an arc of motion of 15 to 115 degrees. B: Normal pronation and supination.
|
 |
|
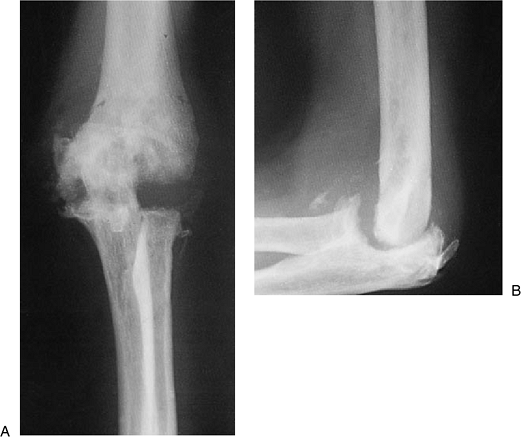
Figure 22-27. A,B: Radiographs 18 years after surgery show preservation of excellent joint surface, and the patient is pleased with the result.
|
elbow and after radial head excision developed arthrodesis and an
unstable joint (Fig. 22-28). At surgery the
lateral ligament was dysfunctional scar tissue, so an interposition
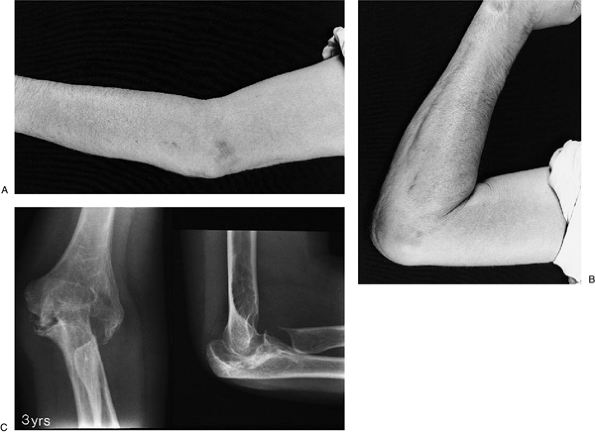
arthroplasty with ligament reconstruction was carried out (Fig. 22-29). The reconstruction was protected with a hinged external fixator (Fig. 22-30A,B). A satisfactory outcome, including adequate motion of 25 to 120 degrees (Fig. 22-31A,B) and minimal pain, was attained at 3 years (Fig. 22-31C).
 |
|
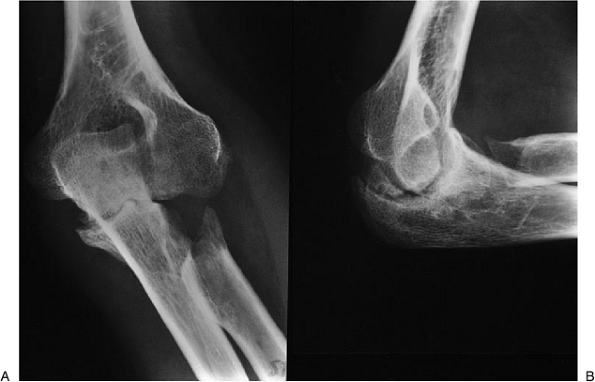
Figure 22-28. A,B: Extensive posttrauma arthrosis with instability and radial head excision.
|
 |
|
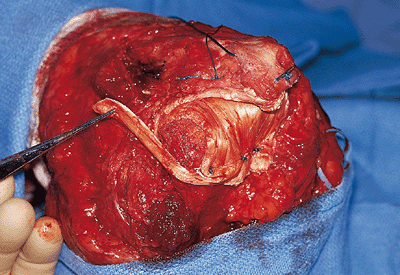
Figure 22-29. An achilles tendon allograft interposition ligament reconstruction was performed.
|
 |
|
Figure 22-30. A,B: The reconstruction was protected with the external fixator.
|
 |
|
Figure 22-31. Satisfactory results at 3 years, both clinically (A,B) and radiographically (C).
|
IG, Goncharenko IV, Kozhin NP, et al. Restoration of the function of
the cubital joint in extensive defects of bones and soft tissues using
endoprosthesis and free skin grafts. Acta Chir Plast 1989;31:143–147.
MMJ, Kataoka Y. Late radiographic results after resection skin
interposition arthroplasty of the elbow in rheumatoid arthritis. Rheumatology 1991;15:42–46.
V. Anatomical interposition arthroplasty with dermal graft. A study of
51 elbow arthroplasties on 48 rheumatoid patients. Z Rheumatol 1987;46:132–135.
