Bacterial Infection
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section
IV – Basic Science > 27 – Infectious Disorders of Bone and Joint
> 27.1 – Bacterial Infection
IV – Basic Science > 27 – Infectious Disorders of Bone and Joint
> 27.1 – Bacterial Infection
27.1
Bacterial Infection
Acute Osteomyelitis
-
Rapidly progressive pyogenic infection
-
Types
-
Hematogenous: usually in children with local trauma
-
Contiguous: from an open wound, surgical wound, or adjacent area of infection (ear infection, tooth abscess, furuncle)
-
-
Biologic response: a function of the inoculum (number and virulence of the organism) and the host response
-
Spectrum of disease outcomes
-
Control of the infection, with death of the organisms
-
Bacteria live in symbiosis with the host.
-
Bacteria overwhelm the host, causing initially local infection, then sepsis, and possibly death.
-
-
Common age ranges, clinical situations,
organisms, and initial empiric treatment associated with
musculoskeletal infection are listed in Table 27.1-1. -
Bacteria develop an effective protective
environment, an extracellular polysaccharide glycocalyx, within hours
of adherence to bone, biomaterials, and tissue transplants.-
Host defenses and antibiotics are excluded by this biofilm or slime.
-
|
Table 27.1-1 Typical Organisms Associated with Age or Clinical Situation
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hematogenous Osteomyelitis
Pathophysiology
-
Acute hematogenous osteomyelitis usually
presents in the metaphysis of a long bone in a growing child, often
with a history of recent trauma. -
Postcapillary venous sinusoids of the metaphysis are a preferred environment for infection because of:
-
Venous pooling with low pO2, turgid flow, and discontinuity of the endothelial lining of the sinusoidal vascular bed
-
Bacterial adherence to endothelial gaps
-
Recent trauma may alter local environmental factors.
-
Small vessel thrombosis further reduces host defenses.
-
-
Sequence of events induced by the presence of bacteria in bone
-
Deposition of the organism at the postcapillary sinusoidal bed, invoking an acute inflammatory response
-
Increased local capillary permeability, polymorphonuclear (PMN) invasion, and edema
-
-
Opsonization, a precoating of the
bacteria by opsonins, freely circulating serum molecules that are
produced to attach to the surface of microbes, rendering them more
attractive to phagocytes (examples of opsonins include IgG antibody and
the C3b molecule of the complement system) -
Ingestion of bacteria by polymorphs
-
If not contained by the initial immune
response, the infection rapidly produces a compartment-like syndrome:
raised intramedullary tissue pressure (from the edema) exceeds the
arterial inflow pressure at the capillary level; this eliminates the
arteriovenous pressure gradient that drives flow across the capillary,
and flow ceases. -
Ischemic necrosis then follows the spread of infection throughout the medullary space and through the cortex.
-
At the outer margin of the cortex, periosteum is lifted off by pus under pressure, severing the subperiosteal circulation.
-
Cortex is thus isolated from both endosteal and periosteal vascular sources, leading to cortical necrosis.
-
Diagnosis
Radiologic Features
-
Radiography (Fig. 27.1-1)
demonstrates the morphology, with the disease presentation limited to
metaphysis and diaphysis, in the distribution of the circulatory beds
of the nutrient artery and periosteum. -
Infected area creates an irregular zone of osteopenia in the metaphysis, with an external periosteal reaction.
-
Mottled areas of cortical sclerosis in the predominately lytic area represent areas of cortical sequestration.
Clinical Findings
-
Presentation is age dependent.
-
Neonates
-
Difficult diagnosis
-
Must be suspected in a neonate who is septic
-
Neonate may be afebrile.
-
Affected limb may be warm compared to opposite limb.
-
-
Children
-
Incidence higher in males
-
Child will look and feel ill.
-
Refusal to bear weight
-
Associated factors: recent trauma and
infection elsewhere (middle ear infection, tooth abscess, cutaneous
boil, or infected wound)
-
-
Adults
-
Advanced age and a variety of systemic factors predispose to the development of bone infection.
-
Acute vs. chronic infection clinical presentation
-
Typically, a patient with acute bacterial osteomyelitis may be acutely ill, with acute loss of function of an extremity.
-
The presence of chronic illness with an
impaired immune system or partial treatment with oral antibiotics may
mask the presence of infection in bone in adults, especially in the
spine.
-
-
Spine infection often follows infection elsewhere (pneumonia, diverticulitis, cholecystitis, cystitis, pyelonephritis).
-
Pain is the predominant complaint: nonmechanical and localized
-
Lumbar spine 45%
-
Thoracic 35%
-
Cervical 20%
-
-
Half will have no fever or leukocytosis.
-
Spread of infection follows vascular supply with segmental arteries supplying disc and adjacent vertebrae.
-
Staphylococcus aureus, Streptococcus, and Enterobacter predominate.
-
-
-
-
Talus and proximal radius are special
cases. Hematogenous osteomyelitis can be a difficult diagnosis if bone
is “hidden.” Obtain early magnetic resonance imaging (MRI).-
Proximal radius
-
Enveloped by supinator, acute infection
will produce severe pain and forearm swelling and may present with a
soft tissue compartment syndrome. -
The presence of systemic signs and the
absence of a traumatic cause for the raised tissue pressure should
suggest the correct diagnosis. -
MRI will confirm.
-
-
Talus
-
Infection will mimic cellulitis with pain and local erythema.P.498
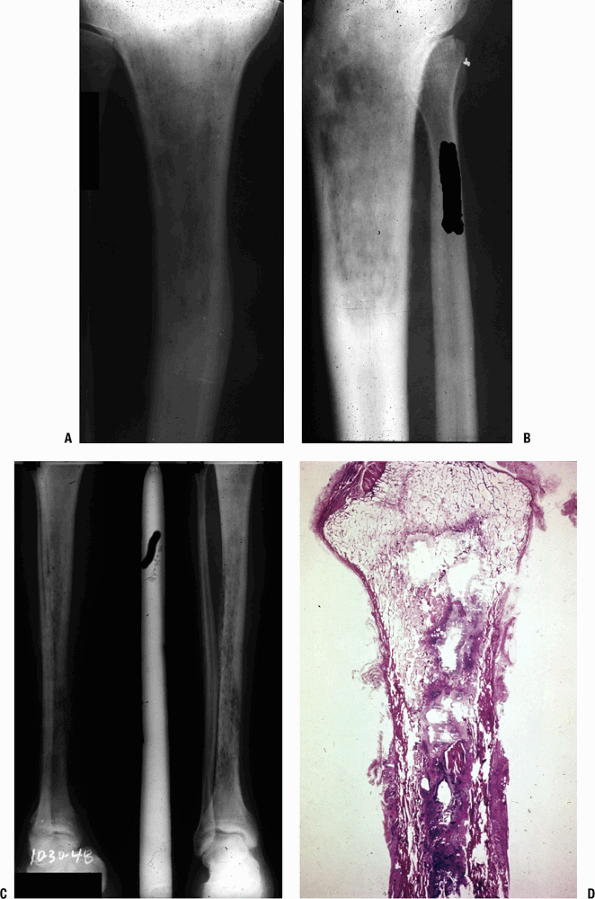 Figure 27.1-1
Figure 27.1-1
(A,B) Acute suppurative osteomyelitis. These radiographs show
permeative changes and periosteal new bone formation that have taken
more than 10 to 14 days to occur. Prior to this the earliest
perceptible change would have been soft tissue swelling adjacent to the
infected bone. (C) Over time, the areas of permeation become mottled as
repair is attempted and the picture becomes that of early chronic
osteomyelitis (here shown in a different case example). (D)
Macrosection of proximal tibia shows the effect of acute osteomyelitis,
with intramedullary accumulations of inflammatory exudates (purulent
exudate). The cortex is permeated, and there is a large cortical
sequestrum. -
Child will not bear weight; an ankle effusion may be sterile.
-
The site of the infection can be confirmed early only by MRI.
P.499 -
-
Differential Diagnosis
-
Osteomyelitis vs. Ewing sarcoma vs. eosinophilic granuloma (Langerhans cell histiocytosis)
-
The linking of clinical, radiographic, and histopathologic findings is essential to establish the diagnosis.
-
Ewing sarcoma can perfectly mimic acute
or subacute osteomyelitis with fever, malaise, leukocytosis, raised
erythrocyte sedimentation rate (ESR), and a radiographic appearance of
lysis with a periosteal reaction. Biopsy is needed to confirm the
diagnosis. -
Eosinophilic granuloma can mimic
infection and tumor. The patient is usually not systemically ill. ESR
may be elevated; the local findings can include pain and erythema;
radiographic picture can be similar to acute osteomyelitis. Biopsy is
necessary.
-
Diagnostic Principles
-
Extent of disease
-
Physical findings
-
Bone tenderness on percussion helps localize infection.
-
Erythema, fluctuance, and, rarely, drainage in the acute setting
-
-
Laboratory parameters: complete blood count (CBC), ESR, C-reactive protein (CRP)
-
White blood cell (WBC) count with increased PMNs indicates infection but is present in only 50% of patients with infection.
-
ESR positive in 92% of pediatric patients with osteomyelitis
-
Time course: rises within 2 days of onset
of infection, continues to rise for 3 to 5 days after onset of
appropriate antibiotics, and returns to normal at 3 weeks
-
-
CRP elevated in 98% of pediatric patients
with osteomyelitis; rises within 6 hours of onset of infection, peaks
at 36 to 50 hours, and returns to normal 1 week after successful therapy-
CRP is preferred for following both acute and chronic infection as it follows the time course of infection more closely.
-
-
Surgery delays normalization of both ESR and CRP.
-
-
Radiographs (see Fig. 27.1-1)
-
Initial radiographic sign of infection: soft tissue swelling; always consider tumor or fracture mimicking infection
-
Lucency occurs with active hyperemia, osteoclast action (Fig. 27.1-2).
-
Periosteal reaction begins at 2 weeks.
-
Sclerosis indicates either new bone
formation on dead trabeculae or the presence of dead bone
(sequestration), surrounded and made more apparent by contrast to
living, osteopenic bone.
-
-
Technetium 99m (99mTc) bone scan assesses perfusion and osteoblastic activity; localizes pathology but is not specific.
-
Indium-11-labeled leukocyte scans differentiate infectious from noninfectious causes.
-
83% to 85% sensitivity, 75% to 94% specificity
-
-
MRI can diagnose extent of disease and may show abscess formation.
-
Sensitivity close to 100%
-
Increased fluid content in marrow is a function of edema and hyperemia
-
Decreased marrow signal on T1-weighted images
-
Increased marrow signal on T2-weighted images
-
-
-
-
Identify the organism.
-
Culture the aspirate and obtain cultures at the time of incision and drainage.
-
Treat empirically while awaiting culture results.
-
Positive Gram stain in one third of cases; highly specific, allows focused treatment.
-
Cultures are the gold standard for verifying infection and can be compromised by:
-
Prior antibiotic administration
-
Inadequate tissue sampling
-
Improper handling of specimens
-
-
Polymerase chain reaction (PCR) can amplify and detect bacterial DNA.
-
Allows early diagnosis compared to cultures
-
Results are not affected by concurrent use of antibiotics.
-
False-positive results can arise from contamination.
-
-
Treatment
-
Treatment is based on identifying the
extent of disease and identifying the organism, selecting and
delivering appropriate antibiotic to the site, halting tissue
destruction, and verifying response to treatment.
Nonsurgical Treatment
-
Classical recommendations include empiric treatment after cultures; if no abscess and focal infection
-
Up to 6 weeks of parenteral antibiotic guided by ESR
-
Switch to oral antibiotic only if monitoring of serum bactericidal titers can be obtained.
-
Recent papers have suggested a more simplified approach for hematogenous acute osteomyelitis in a normal host:
-
Shorter course of parenteral antibiotics (5 days)
-
No monitoring of titers
-
Shorter duration of therapy determined by normalization of the CRP and ESR (3 to 4 weeks)
Exceptions would include osteomyelitis in neonates,
trauma, foreign bodies, the immunocompromised host, and gram-negative
organisms.
trauma, foreign bodies, the immunocompromised host, and gram-negative
organisms.
P.500
 |
|
Figure 27.1-2
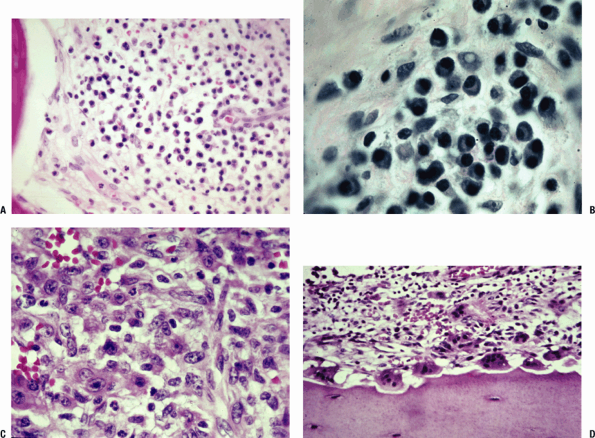
Acute inflammatory exudates. (A) Marrow is replaced by inflammatory cells (PMNs). Adjacent bone shows numerous Howship’s lacunae, indicating brisk clast activity due to the effects of active hyperemia. (B) Higher magnification of acute osteomyelitis. No normal marrow is evident in this field; instead, the medullary space is filled with an acute inflammatory infiltrate. In the upper left corner is marrow that has been altered and shows an edematous reactive appearance to the stroma. (C) High magnification showing the cellular components of the inflammatory infiltrate: segmented neutrophils (PMNs) and extravasated red blood cells dominate the picture. (D) Acute osteomyelitis in bone showing marked effect of clasts on bone, numerous Howship’s lacunae; the cellular infiltrate now shows a few lymphocytes and histiocytes. |
Surgical Treatment
Indications
The classic indication of open drainage in virtually
every case is changing. Given the simplified approach outlined above,
the indications for surgery might include:
every case is changing. Given the simplified approach outlined above,
the indications for surgery might include:
-
Drainage of a large subperiosteal abscess
-
Drainage of a metaphyseal cavity seen on the initial radiograph
-
Failure of response to medical management after 36 to 48 hours
-
Presence of sinus tract or sequestrum
-
Osteomyelitis close to the intracapsular hip or shoulder
Surgical Technique
-
Remove pus and debris.
-
Fenestrate cortex.
-
Remove nonviable bone (sequestrum).
-
Preserve involucrum.
Subacute Osteomyelitis
Diagnosis
-
Child presents with a painful limp with no or minimal constitutional symptoms.
-
Fever mild or absent
-
WBC often normal, ESR elevated in 50%
-
Radiograph shows lytic lesion with or
without a sclerotic border; in the classic Brodie’s abscess the
sclerotic border fades peripherally; the process can cross the physis,
unlike acute osteomyelitis (Fig. 27.1-3). -
Differential diagnosis includes chondroblastoma (in epiphysis), osteoid osteoma.
-
Normal ESR in both conditions
-
Chondroblastoma rarely has sclerotic borders, may rarely have central mineralization.
-
Osteoid osteoma has more reactive bone
unless close to joint; classic pain pattern worse at night and relieved
dramatically by nonsteroidal anti-inflammatories (NSAIDs) in 70% of
patients.
-
P.501
Treatment
-
Treatment is surgical with débridement and curettage followed by appropriate antibiotics.
Chronic Osteomyelitis
Pathophysiology
-
Involves transition from acute
inflammatory response, with granulation tissue, neutrophils, and
microabscesses, to chronic inflammation, with lymphocytes, plasma
cells, fibrosis, and bony reinforcement (Fig. 27.1-4)
Diagnosis
-
Recurrent drainage from sinuses, fistulae, or ulcers
-
Usually there is a history of:
-
Acute osteomyelitis with inadequate treatment
-
Trauma with open wound or internal fixation
-
Local soft tissue spread in Cierny type C elderly patient (see below) (see Fig. 27.1-4)
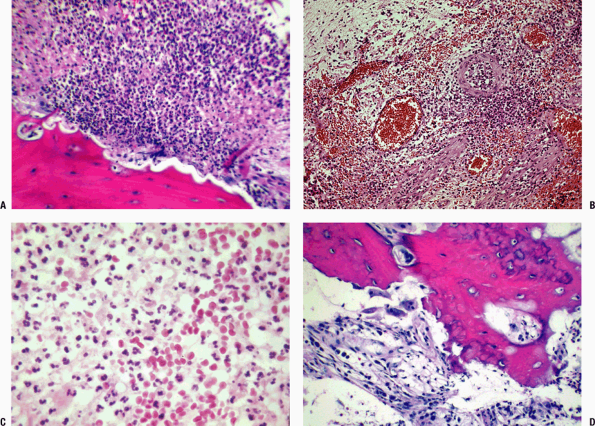
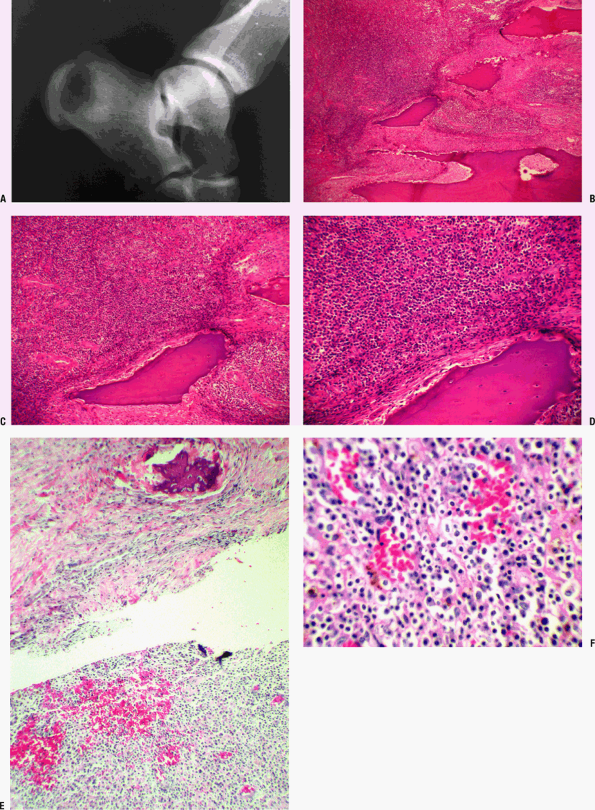 Figure 27.1-3
Figure 27.1-3
Brodie’s abscess. (A) Radiograph showing lesion in calcaneus. A central
zone of lysis is surrounded by a ring of dense reactive bone, with a
fading external border. (B-D) Sequential images of a Brodie’s abscess.
(B) Low-power view reveals shards of bone that show marked evidence of
clast activity; inflammatory infiltrate on the left, reactive zone on
the right. (C) At medium power, numerous vessels are seen as well as
the inflammatory exudate surrounding the trabecular bone. (D) High
power reveals inflammatory exudate, vessels, fibrosis, and bone showing
evidence of reaction. (E) Medium-power photomicrograph of a different
case shows fibrotic wall of a Brodie’s abscess in upper half of field.
A single focus of reactive new bone formation is evident in this wall.
Below, dense mixed inflammatory cells and blood vessels compose the
central zone of the abscess. (F) High-magnification view of another
case shows central zone of a Brodie’s abscess with a mixed inflammatory
infiltrate (lymphocytes, plasma cell, scattered PMNs) and red blood
cells. Brown hemosiderin pigment can also be seen in a background of
pink-staining fibrin. -
-
Systemic symptoms are absent; may be present during flare of increased drainage.
-
Dead bone (microsequestra) is removed by
osteoclasts or is cloaked with new lamellar bone, producing a
radiographic picture of trabecular thickening; the random orientation
of this bony response to the infection produces chaotic cement lines
(so-called mosaic bone) also seen in Paget’s disease (Fig. 27.1-5). -
Cortical sequestration becomes enveloped
by a periosteal response that matures (rapid woven bone deposition,
followed by lamellar bone fill-in of the interstices), forming the
involucrum. -
Without treatment, infection heals by
drainage to the exterior of the body; when treatment fails, pus seeks
to drain through sinus tracts (cloaca). Over time (years), the tracts
epithelialize (pseudo-epitheliomatous hyperplasia; Fig. 27.1-6); after many years, squamous cell carcinoma can occur along the tract.
-
P.502 -
Classification
Cierny and Mader have proposed a practical
classification in which the extent of bone involvement is classified
anatomically to allow appropriate intervention.
classification in which the extent of bone involvement is classified
anatomically to allow appropriate intervention.
-
Clinical situations associated with each anatomic stage
-
Type I: medullary—after intramedullary rod
-
Type II: superficial—open wound perforation from outside
-
Type III: localized—after plate
-
Type IV: diffuse—progression from persistence of medullary, superficial, or localized infection
-
-
The physiologic status of the host
determines the effectiveness of wound healing and thus the prognosis.
The classification of the patient (host) includes:-
A: normal host
-
B: compromised host
-
Local conditions limit perfusion, and
systemic factors affect the immunologic, hematopoietic, and/or
metabolic capabilities of the host. One attempts to optimize the
patient prior to surgery by addressing all reversible factors locally
and systemically.-
Local adverse conditions
-
Chronic lymphedema
-
Venous stasis
-
Major vessel disease
-
Arteritis
-
Extensive scarring
-
Radiation fibrosis
-
-
Systemic factors
-
Malnutrition
-
Immunodeficiency
-
Chronic hypoxia
-
Malignancy
-
Diabetes mellitusP.503
![]() Figure 27.1-4
Figure 27.1-4
Chronic osteomyelitis. (A) The cellular players responding to the
infection change over time. This photomicrograph shows a predominance
of plasma cells (dark cytoplasm, perinuclear clear zone corresponding
to the Golgi apparatus, and eccentric round nucleus) and lymphocytes
(almost no cytoplasm, central nucleus). (B) Slide showing mostly plasma
cells with a few scattered lymphocytes. (C) Plasma cells and
histiocytes are present as part of the chronic inflammatory response
regardless of cause. A histiocyte is a large cell with abundant
cytoplasm (whose nucleus is vesicular; pale, dispersed chromatin
pattern) and a large nucleolus. (D) A team of osteoclasts is seen lined
up on and removing bone. The phenomenon of increased arterial blood
flow brought to a region with associated osteoclastic activity is
termed active hyperemia and was described by Dr. Lent Johnson. -
Advanced age
-
Renal or liver failure
-
Tobacco abuse
-
-
-
-
C: multifactorial problem, not curable with acceptable morbidity; treatment deferred
-
Options are suppression with long-term antibiotics or amputation.
-
-
-
Clinical stage is expressed by anatomic
type and physiologic class, such as stage IVB (diffuse osteomyelitis in
a compromised patient).
Diagnosis
-
Determine extent of disease.
-
CBC, ESR, CRP: Obtain and follow throughout course.
-
Radiographs
-
99mTc bone scan and indium-11-labeled leukocyte scans help determine the activity of the disease.
-
Computed tomography (CT) scan useful for delineation of sequestrum and degree of union.
-
MRI determines the extent of disease.
-
Fistula tracts
-
Extent of edema may exaggerate the zone of actual infection.
-
-
Treatment
-
Determine extent of disease.
-
Optimize host healing potential when possible.
-
Obtain operative samples of deep specimens from multiple sites.P.504
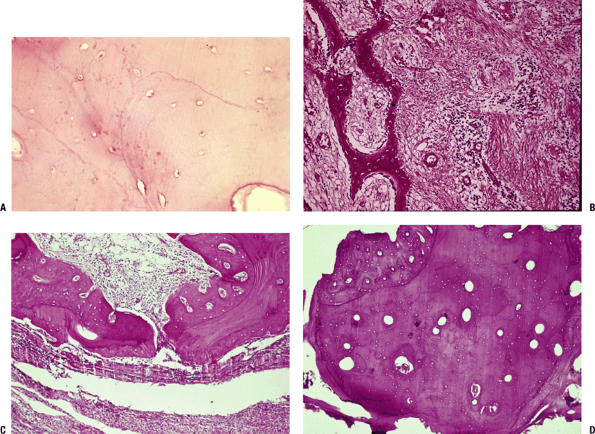 Figure 27.1-5
Figure 27.1-5
Chronic osteomyelitis. (A) High-power view of lamellar bone showing
empty and enlarged lacunae, hallmarks of the enzymatic effect driven by
the agonal osteocyte, termed oncosis. (B)
A walling off of the infected area occurs, marked by fibrosis and
periosteal woven bone formation. (C) Dead bone is either removed by
clasts or cloaked by bone. Early on the appositional bone is woven, as
shown here, or lamellar as the process slows down. Fibrous
encapsulation is shown below. (D) Lamellar bone showing haphazard
cement lines (mosaic bone) and woven bone apposition. This increased
bone formation accounts for the mottled regions of sclerosis seen on
radiographs. -
Eliminate the disease through intravenous antibiotics, surgical débridement.
-
Reconstruct the area using flaps as
necessary for soft tissue coverage, and delayed bone grafting
(autograft, bone morphogenic proteins [BMP], allograft) and
stabilization after infection is controlled.
Surgical Treatment
-
Débridement: Approach as if resecting a locally aggressive benign tumor, erring on the side of resection back to normal tissue.
-
Wound management
-
If compromised soft tissues are present, local or free muscle flaps will promote healing through:
-
Elimination of dead space
-
Soft tissue coverage
-
Improved vascularity
-
-
-
Obtain multiple cultures: Culture results
direct selection of antibiotic coverage, 3 to 6 weeks of intravenous
treatment, plus or minus bone void fillers (polymethylmethacrylate
[PMMA] with antibiotics or biodegradable material with antibiotics). -
Bone stabilization when necessary
-
External fixation: safest, allows distraction osteogenesis and bone transport; pin tract infection incidence is high
-
Repeat internal fixation: problematic given high rate of reactivation of the infection (20% to 50%)
-
Delayed reconstruction of bone voids
-
Autograft/BMP/allograft for cavitary defect
-
Structural autograft or bone transport for segmental defects
-
-
Augment healing with electrical stimulation or ultrasound.
-
-
Amputate when the situation is not reconstructable.
P.505
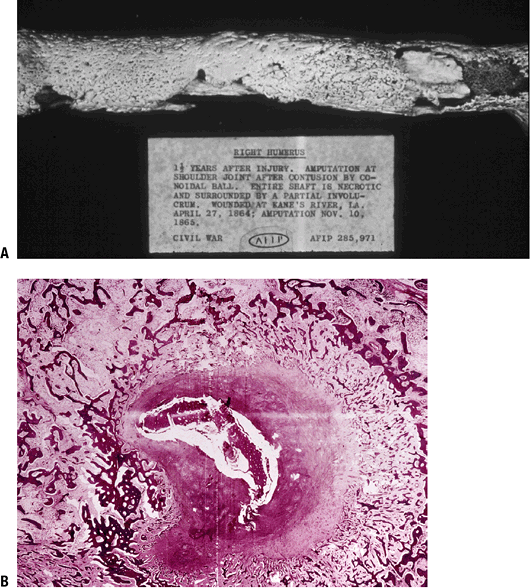 |
|
Figure 27.1-6
Chronic osteomyelitis. (A) Gross specimen of Civil War case from the Armed Forced Institute of Pathology museum. The sequestrated initial cortex (sequestrum) is cloaked by mature woven bone (involucrum); the openings in the involucrum are sites of purulent drainage to the exterior (cloacae). (B) Cross-section of a tibia with chronic osteomyelitis showing involucrum (layers of woven bone) surrounding an inflammatory exudate encompassing the necrotic intitial cortex (sequestrum). |
Complications
-
Long-term complications include sequestra, sinus tract formation, pathologic fracture, squamous cell carcinoma, and amyloidosis.
Squamous Cell Carcinoma
-
Occurs as a result of chronic irritation of draining sinus (1 to 55 years, mean 22 years)
-
Presents as a change in a longstanding
setting of draining sinus: increasing pain, change in drainage or in
appearance of a chronic ulcer; enlarging mass; pathological fracture -
Incidence <2% of patients
-
Anatomic distribution: 85% in lower extremity
-
Radiograph may show new lucency in a longstanding area of mixed lysis and sclerosis.
-
Management is wide resection, often amputation.
-
Prognosis: 14% metastasis; 30% have regional node metastases
Amyloidosis
-
Deposits of a twisted β-pleated
fibrillary protein occur in bone marrow and/or juxta-articular synovial
tissue; rarely recognized clinically.
-
Common endpoint for multiple processes in addition to infection
-
Treatment and cure of the infection allows resolution of the amyloidosis.
Sclerosing Osteomyelitis of Garré
-
Localized control of an initial infection
is followed by persistent repair with ongoing new bone formation;
clinical presentation insidious with mild ache, local tenderness, no
systemic symptoms or signs (Fig. 27.1-7). -
Radiograph shows dense, progressive sclerosis.
-
Microscopy shows chronic inflammatory infiltrate, fibrovascular marrow, trabecular reinforcement.
Chronic Recurrent Multifocal Osteomyelitis
-
Age: children and adolescents
-
Clinical presentation: appears with
multifocal minimal symptoms of localized pain, swelling, and low-grade
fever; 70% of patients have pustulosis palmoplantarisP.506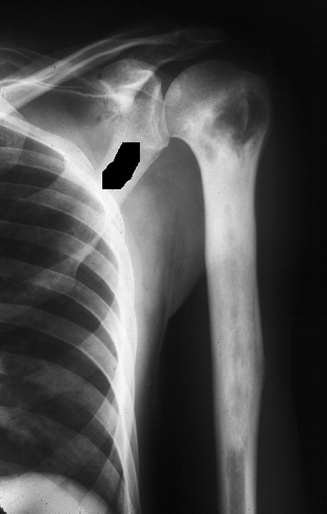 Figure 27.1-7
Figure 27.1-7
Sclerosing osteomyelitis of Garré. Repair predominates in this
condition. The radiograph shows dense sclerosis throughout the humeral
diaphysis. -
Distribution: Clavicle, distal femur, and distal tibia are most common sites.
-
Laboratory evaluation: WBC and CRP are normal; ESR is elevated in all cases.
-
Microbiology: Cultures for bacteria, mycobacteria, fungi are invariably negative.
-
Radiographs: multiple metaphyseal lytic lesions with a thin rim of sclerosis
-
Microscopy shows mixed acute and chronic
inflammatory infiltrate with PMNs forming microabscesses;
microsequestration with osteoclastic resorption is evident; adjacent
marrow fibrovascular with chronic inflammatory infiltrate. -
Usually resolves spontaneously after years
Septic Arthritis
Etiology
Hematogenous Arthritis
-
Most frequent cause of bacterial septic arthritis
-
Infants: hip most common; less commonly knee or ankle
-
Children and adults: knee most common
-
Debilitated older adult with inflammatory arthropathy is at risk.
-
-
Contiguous Arthritis
-
Defined as extension of infection from adjacent acute osteomyelitis
Direct Arthritis
-
Associated with surgery
-
Traumatic introduction of bacteria (open wounds)
-
Hand infection in association with fist fights source of initially missed septic arthritis
-
Any open injury to the soft tissues directly overlying a joint necessitates débridement.
-
Pathophysiology
-
Initiation
-
Inoculation of the organism activates the immune response.
-
Same sequence occurs as outlined in the discussion of the pathophysiology of osteomyelitis.
-
-
Immune response
-
In joints, the profound immune response in the vascular synovium produces pain, swelling, erythema, and loss of motion.
-
-
Cartilage destruction
-
In the swollen, avascular joint space,
enzymes from bacteria, PMNs, and synovial cells cleave the
glycosaminoglycan subunits of cartilage, which are then rapidly
expelled from the cartilage. -
Bacterial and host collagenases then break down the collagen with gross physical breakdown of the cartilage.
-
Loss of joint space on the radiograph
correlates with this; regional osteopenia accompanies the reactive
increased vascular flow to the periarticular region.
-
Diagnosis
Clinical Findings
-
Classic presentation is “sick” patient
with fever, pain, inability to use upper extremity (pseudoparalysis) or
to bear weight on lower extremity.-
Range of motion is markedly reduced and painful.
-
Resting position of maximal joint volume
-
Knee: flexion
-
Hip: flexion, external rotation, abduction
-
-
-
To avoid irreversible joint destruction, timely diagnosis and treatment are mandatory.
Laboratory Findings
-
WBC: elevated with shift to left
-
ESR, CRP elevated in 90% of patients with septic arthritis
Radiologic Features
-
Radiograph: effusion early progressing to joint space loss late; rare to have bony changes except regional osteopenia (Fig. 27.1-8)
-
MRI: evaluates joint effusion, adjacent bone and soft tissue changes
-
Ultrasound: can identify effusion
-
Scintigraphy can help with difficult diagnosis and identify multiple joint problems.
-
Joint aspiration assisted when necessary by fluoroscopic or ultrasound guidance
-
Gram stain, cultures/sensitivity testing, WBC and differential, crystals
-
Thick fluid typically with WBC >50,000/ml (50%) and no crystals
-
P.507
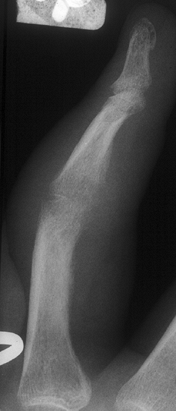 |
|
Figure 27.1-8
Septic arthritis. Loss of joint space occurs as a result of the dissolution of the articular cartilage. The infection extended under the subchondral plate. There is periosteal new bone formation and marked juxta-articular osteopenia. |
|
Table 27.1-2 Clinical Spectrum of Septic Arthritis
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Treatment
-
Confirm diagnosis: joint fluid aspiration
-
Initial surgical management: joint irrigation, open or arthroscopic
-
Drilling of adjacent metaphysis if osteomyelitis is present may be done.
-
-
Intravenous antibiotics for 3 to 4 weeks (Table 27.1-2)
-
Follow-up: clinical examination and ESR/CRP
-
Follow closely for prompt return of clinical findings to normal.
-
Persistent pain, swelling, and fever
suggests return to operating room for second washout and débridement;
do not hesitate to do this if minimal clinical response over 24 to 48
hours.
-
-
Late diagnosis: If diagnosis is missed
and joint destruction has occurred, initial surgical goal is to
eliminate the infection; the long-term options are arthrodesis vs.
arthroplasty, using precautions to lower the risk of recurrent
infection.
P.508
Suggested Reading
Belli E, Matteini C, Andreano T. Sclerosing osteomyelitis of Garré periostitis ossificans. J Craniofac Surg 2002;13:765–768.
Berendt T, Byren I. Bone and joint infection. Clin Med 2004;4:510–518.
Chambers
JB, Forsythe DA, Bertrand S, et al. Retrospective review of
osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682–685.
JB, Forsythe DA, Bertrand S, et al. Retrospective review of
osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682–685.
Chen CE, Ko JY, Wang JW, et al. Infection after intramedullary nailing of the femur. J Trauma 2003;55:338–344.
Cierny G III. Musculoskeletal sepsis chronic osteomyelitis: Results of treatment. AAOS Instr Course Lect 1990;39:495.
Cierny G III. A clinical staging system for adult osteomyelitis. Contemp Orthop 1985:10–17.
Darville T, Jacobs RF. Management of acute hematogenous osteomyelitis in children. Pediatr Infect Dis J 2004;23:255–257.
Duffy
CM, Lam P, Ditchfield M, et al. Chronic recurrent multifocal
osteomyelitis: review of orthopaedic complications at maturity. J Pediatr Orthop 2002;22(4):501–505.
CM, Lam P, Ditchfield M, et al. Chronic recurrent multifocal
osteomyelitis: review of orthopaedic complications at maturity. J Pediatr Orthop 2002;22(4):501–505.
Kocher
MS, Mandiga R, Murphy JM, et al. A clinical practice guideline for
treatment of septic arthritis in children:efficacy in improving process
of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg [Am] 2003;85(6):994–999.
MS, Mandiga R, Murphy JM, et al. A clinical practice guideline for
treatment of septic arthritis in children:efficacy in improving process
of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg [Am] 2003;85(6):994–999.
McGrory JE, Pritchard DJ, Unni KK, et al. Malignant lesions arising in chronic osteomyelitis. Clin Orthop Rel Res 1999;362:181–189.
Parsons B, Strauss E. Surgical management of chronic osteomyelitis. Am J Surg 2004;188(1A Suppl):57–66.
Patzakis
MJ, Zalavrad C. Systemic disorders: Infection. In Vaccar AR, ed.
Orthopaedic Knowledge Update 8. Rosemont, IL: American Academy of
Orthopaedic Surgeons, 2005.
MJ, Zalavrad C. Systemic disorders: Infection. In Vaccar AR, ed.
Orthopaedic Knowledge Update 8. Rosemont, IL: American Academy of
Orthopaedic Surgeons, 2005.
Rasool MN. Primary subacute haematogenous osteomyelitis in children. J Bone Joint Surg [Br]2001;83(1):93–98.
Tetsworth K, Cierny GIII. Osteomyelitis debridement techniques. Clin Orthop Rel Res 1999;360:87–96.
Vaccaro
AR, Patzakis MJ, Zalavras C, eds. Systemic disorders, Chapter 20:
Infection. In Orthopaedic Knowledge Update 8. Rosemont, IL: American
Academy of Orthopaedic Surgeons, 2005:217–228.
AR, Patzakis MJ, Zalavras C, eds. Systemic disorders, Chapter 20:
Infection. In Orthopaedic Knowledge Update 8. Rosemont, IL: American
Academy of Orthopaedic Surgeons, 2005:217–228.
Vinh
TN, Sweet DE. Infectious diseases of bone and joint. In Connor DH, ed.
Pathology of Infectious Diseases (2 volumes), 5th ed. Stamford, CT:
Appleton & Lange, 1997, Vol. II, Ch. 182, pp. 1601–1631.
TN, Sweet DE. Infectious diseases of bone and joint. In Connor DH, ed.
Pathology of Infectious Diseases (2 volumes), 5th ed. Stamford, CT:
Appleton & Lange, 1997, Vol. II, Ch. 182, pp. 1601–1631.