Principles of Osteoporosis and Fragility Fractures
One – General Principles: Basics > Fracture Types > 18 –
Principles of Osteoporosis and Fragility Fractures
but the definition of osteoporosis has changed. The most commonly used
definition, as defined by the World Health Organization (WHO), is a
bone mineral density (BMD) of 2.5 standard deviations (SDs) or more
below the young normal mean311 (Table 18-1).
But this definition only includes postmenopausal women evaluated by the
total body dual-energy X-ray absorptiometry (DXA) scanning technique.
No similar definition exists for young women or men. Using the WHO
definition, a quarter of all postmenopausal white Americans, a total of
26 million people, are osteoporotic.198
Furthermore, the number of fragility fractures, those of the proximal
humerus, distal forearm, vertebrae, pelvis, hip, and the tibial
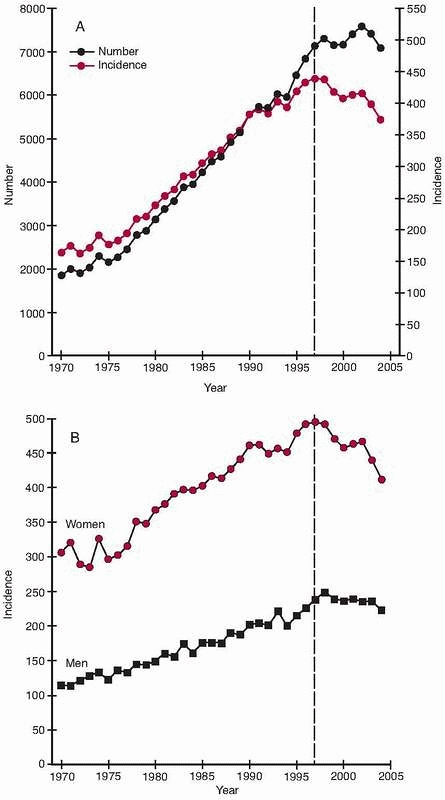
condyles, have risen exponentially during the same period,15,135,220 even if some reports indicate that the increased incidence during the last few years has leveled off or even declined136,199 (Fig. 18-1).
These fractures show a number of common epidemiologic features. The
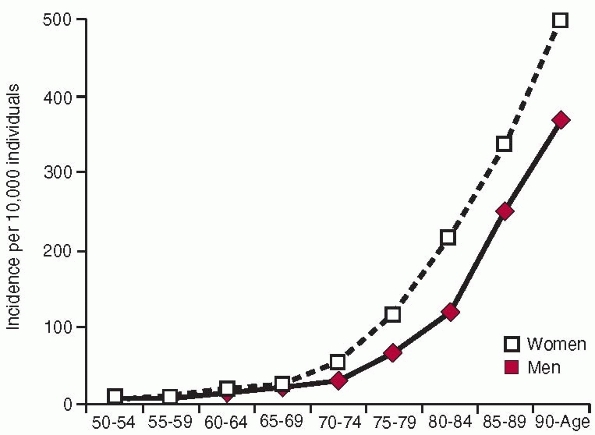
incidence is higher in women than in men and increases exponentially
with age (Fig. 18-2). The fractures also occur at sites where there is a large proportion of trabecular bone.15,201,220 The reason
for the increase in incidence is not fully understood. The changes in
population demographics, particularly the high incidence of elderly in
the population, as well as changes in bone mineral density (BMD) and
other risk factors, have all influenced the incidence of osteoporotic
fractures.
|
TABLE
18-1 World Health Organization Definition of Normal Bone Mineral Density (BMD), Osteopenia, Osteoporosis, and Established Osteoporosis |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
In fact, some researchers have suggested that we should change our
interest from the prevention of osteoporosis to the prevention of falls.127
Approximately one third of community dwellers aged 65 years or older
and 50% to 60% of residents of nursing homes and “old people’s homes”
fall each year, with women falling more often than men.183,263,285,290
Fractures, dislocations, or serious soft tissue injuries result from
about 10% to 15% of falls in patients living in the community213,285,290 and from about 15% to 20% of falls in institutionalized patients.213,285,288 Fractures occur in 3% to 12% of falls in the elderly and are more common in women than in men.286 Hip fractures occur in fewer than 1% of falls.101,183,213,263,285,288,290
The annual prevalence of hip fractures in those with a tendency to fall
is 7% but is 14% among frequent fallers. In the United States, falls
are responsible for the second highest injury-related cost to the
economy.252 Unintentional falling is
an important cause of mortality in the elderly. Twenty-three percent of
injury-related deaths in patients over 65 years of age and 34% in those
over 85 years of age occur as a result of a fall.77 It is therefore obvious that a major goal must be to reduce the frequency of falls.127
Fragility fractures also impose an enormous cost on society. Hip
fracture is a major cause of hospital admission in the elderly, and in
the United States the direct cost of hip fractures was more than $7
billion per year in 1992.238 It was estimated in the United Kingdom to be £750 million per year in 1994.224
In addition, the cost of nursing home care for patients who had hip
fractures in the United States in 1992 was estimated to be $1.5 billion.238
The costs and outcome of hip fractures are often closely monitored
because this fracture is usually regarded as the most significant
osteoporotic fracture. The mortality attributable to osteoporosis is
most obviously associated with hip fractures with the highest incidence
occurring in the first 6 months after fracture.305 Hip fractures are also associated with up to 20% reduction in expected survival54 with the highest mortality occurring in men,271 older patients.146 and nonwhites.146
Also, many patients become permanently disabled after hip fracture,
with the proportion who cannot walk rising from 20% to 50% after the
fracture.119 One third of patients become totally dependent and require institutional care.28
in whites living in northern Europe, followed by whites living in North
America and by Asians, with the lowest incidence being recorded in the
African American population.301 The femaleto-male ratio is 3:1 in whites but is 1:1 in Chinese and the Bantu.301
The incidence is age dependent in both men and women, rising from 2 per
100,000 person-years among white women younger than 35 years to 3032
per 100,000 person-years in women at least 85 years old44,258 (Fig. 18-2). The incidence of hip fracture has also increased during the past 40 years,15,220 even if recent data suggest either a leveling off or a slight downturn in North America and Europe136,199 (Fig. 18-1).
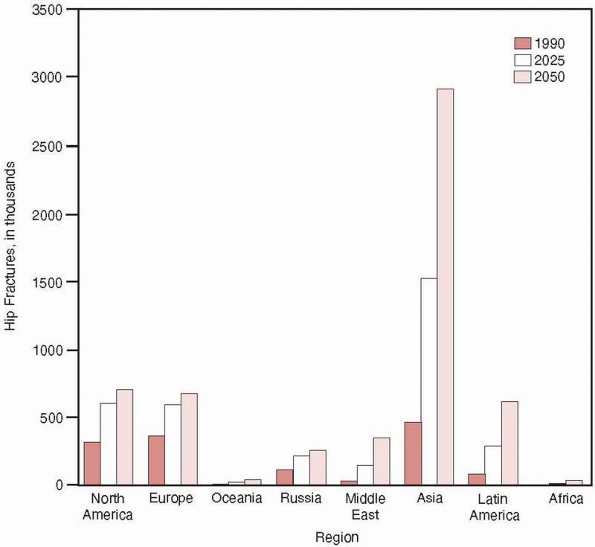
In contrast, the incidence of hip fractures in developing Asian
countries has rapidly increased during the past decades, so that by
2050, it is estimated that 6.3 million hip fractures will occur
globally with more than half of these in occurring in Asia43 (Fig. 18-3).
different ethnic groups, being higher in Scandinavian, American, and
Hong Kong Chinese females than in eastern European females. The rates
in male Hong Kong Chinese and male white Americans are lower than in
male Europeans.112,163,200,219
The femaleto-male ratio is 2:1 in whites, and the incidence is age
dependent in both men and women. In North America, this rises from
fewer than 20 per 100,000 person-years in men and women under 45 years
of age to 1200 per 100,000 person-years in both men and women at least
85 years of age.112,219 According to Swedish data, the incidence of vertebral fracture has increased from 1950 to 1983,15 but this trend has not been confirmed in Denmark106 or in Rochester, New York.212 Mortality following a vertebral fracture is increased in men and women, although it is less than after hip fracture.110,111 Patients with vertebral fractures
also experience a reduction in quality of life, usually as a result of
back pain. In addition, they also have functional limitation, loss of
height, depression, and disability.71,72
 |
|
FIGURE 18-1 Hip fractures in Finland in people 50 years or older between 1970 and 2004. A. Number and crude incidence (per 100,000 persons). B.
Age-adjusted incidence (per 100,000 persons). The latest year of the original report or 1997 is indicated with a dotted vertical line. (Reprinted with permission from Kannus P, Niemi S, Parkkari J, et al. Nationwide decline in incidence of hip fracture. J Bone Miner Res 2006; 21:1836-1838.) |
The BMD at age 18 to 30 years is described as the peak bone mass, being
the highest BMD that the individual will reach in life. It occurs in
different skeletal regions at different ages, possibly as early as 18
years in the hip and as late as 35 years in the distal forearm.4,151,178,281
The factors that determine BMD are poorly understood, but studies in
twins indicate that 60% to 80% of the BMD is determined by heredity.269
Other important factors are environmental factors such as exercise and
nutrition as well as any diseases that interfere with normal growth and
sex and growth hormones.140,179,197,269
It is also likely that both anabolic and catabolic environmental
factors have the greatest impact on bone during skeletal growth. For
example, the skeletal
response
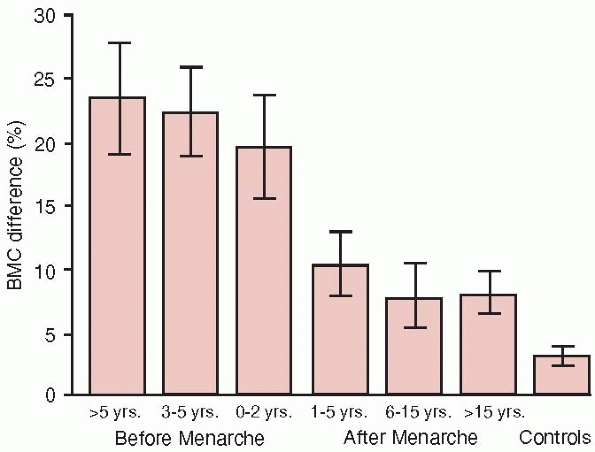
to exercise is most pronounced during the prepubertal and early
pubertal years, this being the period of fastest growth and the highest
accrual of BMD9,133,281 (Fig. 18-4).
 |
|
FIGURE 18-2
Incidence of hip fractures per 10,000 inhabitants in Malmo, Sweden, 1992-1995. (Reprinted with permission from Rogmark C, Sernbo I, Johnell O, et al. Incidence of hip fractures in Malmo, Sweden, 1992-1995. A trend-break. Acta Orthop Scand 1999;70:19-22.) |
stable or shows a slight decrease until menopause. At the menopause,
the levels of estradiol and estrone drop to about 25% and 50% of their
premenopausal values. At this time, they are mainly produced by
extraglandular conversion of androgen precursors in muscles and adipose
tissues. Because the female sex hormones are the most important
hormones regulating BMD in both men and women, an accelerated loss of
BMD naturally occurs during the 5 to 10 years after the menopause.4,174 At this time, the increase in the number of sites undergoing active remodeling leads to BMD loss,4,174 trabecular perforation,227 and an increased risk of fracture.174
The processes that lead to age-related bone loss are probably
multifactorial. With increasing age, calcium absorption is impaired,
which may lead to secondary hyperparathyroidism and accelerated bone
loss. There is also a reduced production of active vitamin D due to
thinning of the skin and reduced exposure to sunlight.254 This process is exacerbated by estrogen deficiency in both elderly men and women.254 The main difference between osteoporotic
individuals and their nonosteoporotic peers seems to be related to
defective bone formation. Bone turnover in osteoporotic individuals may
be elevated, normal, or reduced, but the imbalance between resorption
and formation always seems to be present.69
In addition, with aging, collagen synthesis and the secretion of other
osteotropic factors decline, a fact that also could influence skeletal
strength.144
 |
|
FIGURE 18-3
Estimated numbers of hip fractures in eight geographic regions in 1990, 2025, and 2050. (Reprinted with permission from Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a worldwide projection. Osteoporos Int 1992;2:285-289.) |
 |
|
FIGURE 18-4
The mean (95% confidence interval) playing-to-nonplaying arm difference in bone mineral content of humeral shaft in 105 female tennis and squash players and their 50 controls according to biological age at which training was started (i.e., starting age of playing relative to age at menarche). (Reprinted with permission from Kannus P, Haapasalo H, Sankelo M, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med 1995; 123:27-31.) |
osteoporosis in 80% of women and 50% of men who present with fragility
fractures, a diagnosis of primary involutional osteoporosis is often
made.255 Riggs and Melton255
subdivided primary osteoporosis into type I and type II osteoporosis,
type I being related to the loss of ovarian function after the
menopause and type II being an exaggeration of the normal aging
process. A recent study has emphasized the importance of estrogen in
bone loss in both men and women and proposed a link between type I and
type II osteoporosis.254 Nowadays,
the expression “primary or idiopathic osteoporosis” is more commonly
used than “type I or type II osteoporosis.” Despite this, it is
important to realize that involutional osteoporosis is multifactorial
and that the roles of each specific fracture are still poorly
understood. If there is a cause for osteoporosis such as an endocrine,
metabolic, gastrointestinal, renal, or hematologic disorder in addition
to certain hereditary diseases and drug treatment, the diagnosis is
that of secondary osteoporosis (Table 18-2).
The higher proportion of secondary osteoporosis in men than in women is
usually attributed to alcoholism, malignant disease, long-term
corticosteroid treatment, and hypogonadism295 (Table 18-2).
|
TABLE 18-2 Diseases and Conditions Associated with Secondary Osteoporosis
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
significant advances in the investigation and treatment of osteoporosis
because BMD strongly correlates with bone strength. Variation in the
level of BMD accounts for 60% to 80% of bone strength, but it is
important to realize that bone strength depends not only on the amount
of mineral measured by current techniques but also on the structural
characteristics of the skeleton such as size, shape, and
three-dimensional architecture.4 Up
until now, the prediction of bone strength and risk of fracture has
mainly been based on densitometric measurements, but current research
evaluating the macrogeometric and microgeometric structure of bone will
probably improve the prediction of bone strength and the risk of
fracture in the future.4,13,85
energy radionuclide.4
The technique of photon absorptiometry relies on the relationship
between bone mineral content and the ease with which photons pass
through skeletal tissue (Table 18-3). The
denser the skeleton, the more photons are absorbed by the bone tissue.
This method can only be used in regions with minimal soft tissue,
usually the distal radius or the calcaneus, because the scan cannot
differentiate between absorption in soft tissue and bone.*
Dual-photon absorptiometry (DPA), which uses two different photon
energies, was developed to separately evaluate the absorption in soft
tissue and bone so that skeletal structures surrounded by soft tissues
could be evaluated. However, because of low precision relative to the
rate of change of BMD, DPA is not suitable for monitoring longitudinal
changes.216
|
TABLE 18-3 Methods for Bone Mineral Measurement
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
This method uses x-rays as the photon source, avoiding the problems of
isotope source decay and replacement. The scan time is reduced to
minutes with markedly improved scan image quality and resolution.
However, the most important advance was that precision was markedly
improved compared with the DPA technique, making the technique adequate
for longitudinal monitoring of BMD. DXA is currently the most used
scanning technique for predicting the risk of fractures, establishing
or confirming the diagnosis of osteoporosis, selecting patients for
therapy, and monitoring the effectiveness of therapy192 (Table 18-3).
Fan-beam DXA technology offers semiautomatic vertebral morphometry
(MXA) for screening vertebral deformities with scanning in the lateral
projection.278 Thus far, fan-beam
DXA technology has been used in research to screen for the presence of
fractures, but in the future this technique may be used to improve
fracture prediction. When deciding treatment strategies for
osteoporosis, most clinicians currently use the hip scan, or
occasionally the spine scan in younger patients, as the gold standard.
Newer and smaller DXA equipment that measure the radius or the
calcaneus is promising because of lower cost and because the machines
are portable. However, further studies must be undertaken before these
machines can be recommended in general screening programs.
the bone in the range of 100 kHz up to 2 MHz. It started in 1984 with
the introduction of parameter broadband ultrasound attenuation (BUA)161 (Table 18-3). This parameter evaluated the attenuation in the bone, this being mainly caused by scattering but also by absorption.161
Attenuation seems to reflect not only the amount of mineral in the bone
but also the bone structure, elasticity, and strength. Bone
microstructure and material properties have both been shown to affect
QUS parameters, and studies have supported the view that QUS can
predict fractures independent of the BMD value estimated by DXA scan.13,89
It has been suggested that BUA is not only influenced by BMD but also
by the microarchitecture of bone, whereas the speed of sound (SOS) may
vary with the elasticity of bone.13,89 Therefore, QUS approaches may provide a better insight into skeletal status because it relates to mechanical strength (Table 18-3).
The two parameters BUA and SOS are often combined in weighted averages,
most commonly presented as “stiffness,” quantitative ultrasound index
(QUI), or “soundness.” However, none of the indices reflects
biomechanical stiffness, nor do they supply additional information over
those provided by BUA and SOS. They are, however, practical to use
because they summarize BUA and SOS and have a lower precision error,
which probably makes stiffness more suitable for monitoring.
risk. Two large prospective studies with sample sizes of 6500 to 10,000
women showed that QUS measured at the calcaneus can be used to predict
future hip fracture risk equally as well as DXA measurements.13,105
Typically, the risk of fracture increases by approximately a factor of
2 if a QUS value is reduced by 1 SD, but this varies between devices
and QUS parameters as well as between different types of fractures.13,105 The use of QUS in monitoring and diagnosis requires further study.
Standard CT scanners can be adapted to provide qualitative bone density
measurements. QCT is a densitometric technique that measures the actual
volumetric bone density. Other ionizing techniques measure the amount
of mineral within the scanned area.85,216
This is done with QCT by selecting a region in the central portion of
the vertebral body, or any other specified area, and measuring the true
density of trabecular bone. It is also possible to specifically select
cortical bone and estimate bone size and shape. In recent years,
smaller peripheral QCT (pQCT) units have been manufactured that are
capable of measuring BMD in the forearm and the lower leg. The previous
problems of high radiation exposure and poor reproducibility compared
with DXA have been minimized with the new versions of pQCT software.
Although promising, this method has thus far been mainly used for
research purposes, and currently there is no consensus regarding the
role of pQCT for fracture prediction. There are a number of other
techniques using ionizing and nonionizing
sources in the evaluation of BMD, but these methods are not used in clinical practice (Table 18-3).
in the matrix, consisting of about 90% type 1 collagen and 10%
noncollagenous proteins including osteocalcin, the dominant
noncollagenous protein in bone. The basic structure of collagen is a
triple helix consisting of two α-1 chains and one α-2 chain with a high
content of glycine, proline, and hydroxyproline. Procollagen is formed
in the osteoclasts, and after secretion to the extracellular space, the
procollagen I extension peptides are split at the amino (N) terminals
(P1NP) and carboxyl (C) terminals (P1CP) before final fibril formation.
These extension peptides are a marker of bone formation and can be
measured in blood.262 However, type
1 collagen is present in many tissues, particularly the skin, and the
relative contribution from these sites to circulating P1CP and P1NP
influences the estimation of bone formation. In addition, during bone
formation, the bone cells secrete noncollagenous small proteins that
become incorporated into the matrix. One of these, osteocalcin (BGP),
can be measured in blood as a marker of bone formation.189,262
Alkaline phosphatase (ASP) and bone-specific alkaline phosphatase
(BSAP), an enzyme involved in the mineralization of bone, are also used
as markers of bone formation189,262,314 (Table 18-4).
the degradation of bone collagen and as such is a marker of bone
resorption. However, because this marker is present in all types of
collagen and the excretion is largely dependent on collagenrich food,
the clinical interpretation of OHP is difficult.262
The collagen molecules aggregate to fibrils that are stabilized by
covalent cross-links. The pyridinium cross-links comprise pryidinoline
(Pyr) and deoxypryidinoline (D-Pyr), which are present in all mature
collagen except skin. Because D-Pyr is only present in significant
amounts in bone, it is considered to be more bone specific than Pyr.
The pyridinium cross-links are measured as total pryidinolines, free
pryidinolines, and telopeptides, the peptide cross-link fragments at
the N terminals (NTx) and C terminals (CTx), in serum as markers of
bone resorption.154,189,262,314
During bone resorption, osteoclasts also secrete tartrate-resistant
acid phosphatase isoenzymes (TRACP), and the serum concentration of
this enzyme has sometimes been used as a marker of bone resorption.86,206
However, the enzyme is not specific to bone, and it is difficult to
separate from isoenzymes derived from other tissues such as platelets
and erythrocytes. Another collagen degradation product used to estimate
bone resorption is C-terminal cross-linking telopeptide of type 1
collagen, which is found in both serum and urine (Table 18-4).
|
TABLE 18-4 Measurements of Bone Turnover, Evaluating Bone Formation and Resorption, by Bone Metabolic Markers
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
underlying factors such as diurnal rhythm, day-to-day variations,
seasonal variations, menstrual variations, age, sex, diet, alcohol
intake, systemic diseases, medication, and physical activity,98,313
the interpretation of bone markers must be undertaken with care.
Markers have proved to be useful in epidemiologic and interventional
studies in which groups of patients are studied and in patients with
metabolic diseases associated with high bone turnover such as Paget
disease. However, guidelines for classifying and evaluating individual
patients with osteoporosis are less well defined. For example, it is
not possible to separate a large skeleton with a low turnover from a
small skeleton with a high turnover. Nevertheless, some studies have
shown that measurement of a marker of bone turnover or a combination of
markers can identify groups of patients with low BMD or fast bone loss.124 Whether a single measurement can predict low BMD or a future fracture in an isolated patient remains under debate.245 Biochemical markers of bone turnover may also have a role in short-term monitoring of treatment.107 It remains to be proved if measurements of bone markers will add to the predictive values obtained by BMD measurement.
two main types—those related to trauma, such as a tendency to fall, and
those related to bone strength, such as BMD, skeletal architecture, and
bone size4,13,56 (Table 18-5).
However, several risk factors, such as immobility and aging, may
operate through both skeletal and extraskeletal routes. For example,
fracture risk increases with age partly due to increased bone loss and
partly due to the fact that older patients are at greater risk of
fracture than are younger patients, independent of their BMD level.
Clinically, it is important to determine all risk factors, because
women with multiple risk factors and low BMD are at an especially high
risk of fracture56 (Table 18-5, Fig. 18-5).
|
TABLE 18-5 Risk Factors for Osteoporosis, Falls, and Fracture
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
of the breaking strength of bone. Furthermore, the diagnosis of
osteoporosis is only defined by BMD measurement using the DXA technique
and only in women311 (Table 18-1).
BMD was not originally designed to be used as a criterion for
therapeutic intervention but to identify the proportion of the
population at increased risk of fracture. Using this criterion, 30% of
the American postmenopausal female population is now recognized as
having osteoporosis.176 The definition of osteopenia (Table 18-1)
as a BMD between -1 and -2.5 SDs below the young normal mean was meant
to describe a group of individuals at increased risk of developing
osteoporosis but still not having a particularly high risk of fracture.
The classification of established osteoporosis adds the risk factor of
previous fracture to the treatment protocol in an individual patient.259,311 Clinicians must understand that the risk of fracture increases exponentially with decreasing BMD.158
No specific BMD level signifies a “fracture threshold.” The definition
is arbitrary. A decreased BMD of 1 SD is thought to increase the
fracture risk by about 1.5 times but it may be up to 2.5 times
according to the region51,188 (Table 18-6).
One study that followed 8134 non – African American women over 65 years
of age found that the age-adjusted relative risk of hip fracture was
1.6 for each 1-SD decrease in BMD in the lumbar spine and 2.6 for each
1-SD decrease in BMD in the femoral neck51,188 (Table 18-6). It has also been shown that peripheral measurements of the radius and calcaneus can predict future fractures.121,259
independently predict the risk of hip fracture in elderly women as well
as a DXA scan.13,105
In the EPIDOS study, 5662 elderly women with a mean age of 80.4 years
were assessed with calcaneal ultrasound and femoral neck DXA. The
relative risk of hip fracture for a 1-SD reduction was 2.0 for
ultrasound broadband attenuation, 1.7 for SOS, and 1.9 for femoral BMD
measured by the DXA technique.13,105
More prospective validation using studies of perimenopausal and early
postmenopausal women is needed before bone ultrasound can be
recommended for fracture risk assessment in these groups.
It is recommended that patients are selected for bone densitometry on
the basis of significant risk factors. There are several
wellestablished risk factors related to secondary osteoporosis, and
further diagnosis by BMD is indicated in these patients even if they
are asymptomatic (Table 18-5). Similarly, the
diagnosis of osteoporosis may be confirmed with bone densitometry in
patients with previous low-trauma fractures or a vertebral deformity.
important part in the risk of sustaining a hip fracture. The length of
the femoral neck, the hip axis length (HAL), measured between the
external border of the greater trochanter and the inner pelvic rim has
been shown to be an independent predictor of hip fracture.74 A new algorithm was developed by Yoshikawa et al.317 and Beck et al.14
using the principles of single-plane engineering. This estimates
femoral neck mechanical strength from an anteroposterior DXA scan, a
so-called hip strength analysis
(HSA). A similar geometric approach for the prediction of fractures has also been undertaken from forearm bone scans.4
Preliminary data indicate that the inclusion of geometric analyses in
the estimate of risk factors could improve fracture prediction.4,46
 |
|
FIGURE 18-5
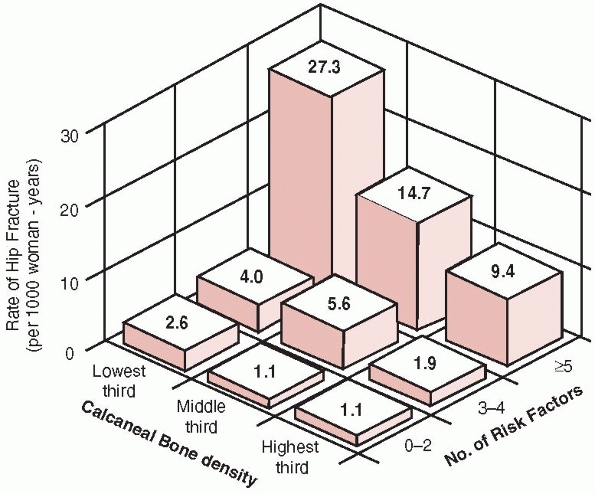
Annual risk of hip fracture according to the number of risk factors and the age-specific calcaneal bone density. (Reprinted with permission from Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332: 767-773.) |
This is probably true during both growth and aging. Daughters of
mothers with osteoporosis have a relatively low BMD compared with
age-matched daughters of mothers without osteoporosis.269
The risk of sustaining a hip fracture in women with a maternal history
of hip fracture is about twice that of women without such a history,
independent of BMD.56 One of the first groups to report a relationship between genetic polymorphism and bone mass was Kelly et al.,147
even though the paper was later withdrawn. Subsequent studies have only
implied a minor association between BMD and a vitamin D receptor.
However, further studies have indicated the importance of genetic
polymorphism in a variety of candidate genes. As an example, it is
likely that there is an association between a specific polymorphism in
the type I collagen,95 transforming growth factor-β (TGFβ),160 the estrogen receptor,153 the type I collagen gene (COLIA1),297 the interleukin 1 and 6 gene,280 low-density lipoprotein receptor – related protein 5,300
and low BMD. It is probable that new associations between genetic
polymorphism and BMD will be made, but because osteoporosis is a
polygenic disease, it is also probable that no single gene will provide
sufficient information to predict the risk of fracture.241,242
However, a combination of a number of genes, perhaps in conjunction
with BMD measurements and other risk factors, could facilitate
prediction of individuals at high risk of fracture.241,242
|
TABLE 18-6 Age-Adjusted Relative Increase in Risk of Fractures
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||
often related to osteoporosis, and it is estimated that osteoporosis
plays a role in up to 75% of fractures in people aged 45 years or older.45
Women who have had either vertebral fractures or nonspine fractures
have an increased risk of sustaining new vertebral fractures,259 and women who have had wrist fractures have an increased risk of sustaining a hip fracture185
independent of the BMD. In a study of osteoporotic fractures (SOS) that
included 9516 white women of 65 years or older, it was found
that a history of fracture increased the risk of hip fracture by 50% independent of the BMD.56
In addition, any type of fracture sustained since the age of 15 years
increases the risk of having subsequent fractures by 70% in
perimenopausal women aged 47 to 56 years independent of BMD.122
Because the risk is independent of BMD, a history of fractures may
indicate an increased tendency to fall, the existence of extraskeletal
risk factors, or a defect in bone strength other than a low BMD.
Frequent falling is also one of the most common risk factors for
fractures, and virtually the same risk factors for falls also account
for fractures99,201,286,287,290 (Table 18-5). Intrinsic risk factors for falls include the following99,101,201,285,286,287,288,290:
-
Old age
-
Female gender
-
Low body mass
-
Medical comorbidities
-
Musculoskeletal diseases
-
Cognitive impairment
-
Gait and balance disorders
-
Sensory impairments
-
Postural hypotension
-
History of previous falls
-
Use of certain medications
-
Benzodiazepines
-
Sedative-hypnotic drugs
-
Antidepressants
-
Antihypertensive medication
-
Antiarrhythmic drugs
-
Diuretics
-
Antiseizure medications
-
slippery and uneven floor surfaces, poor lighting, electrical cords,
foot stools without handrails, slippery top surfaces, and unsuitable
footwear are often classified as extrinsic risk factors.99,101,201,232,285,286,287,288,290
Extrinsic factors play a progressively smaller role in falls as age
advances largely because it is the intrinsic factors that assume a much
more important role as chronic illness becomes a more significant
problem.232
prevalent with advancing age, the risk of sustaining a hip fracture
increasing 1.5 to 2 times every 5 years.225
During the perimenopausal years, the risk of fracture is increased in
perimenopausal women compared with premenopausal women independent of
BMD,158 indicating that risk factors other than BMD account for the increase.13
Lower peak BMD, faster bone loss, smaller bone size, and the higher
prevalence of falls in women may account for this. Mechanical
properties of bone are not only dependent on BMD but also on size,
geometry, and architecture. Gender differences and the recurrence of
fracture may be explained in part by the larger cross-sectional area of
bones in men and differences in subperiosteal bone apposition with
aging.4,261
The Framingham study reported that the relative risk of fracture was
found to be 0.63 in individuals 114% to 123% overweight and 0.33 in
individuals more than 138% overweight.149
Obesity may protect the skeleton in several ways. These are increased
extraglandular production of estrone in the fat tissue, improved
vitamin D status due to storage of vitamin D in fatty tissues, the
provision of a local cushioning effect at the hip when falling, and a
denser and stronger skeleton due to increased skeletal loading in obese
people.
Errors in the measurement of dietary calcium intake and slow changes in
BMD may explain this, but it appears that increased dietary calcium
partially prevents bone loss, although the effect in populations with
high calcium is small.61
A recent meta-analysis supported this view reporting that smoking is a
risk factor for osteoporotic fractures, independent of BMD, in
postmenopausal women.168 This could
be due to the fact that smokers, in comparison with nonsmokers, have an
earlier menopause, are slimmer, have a reduced extraglandular
production of estrogens, and have an increased metabolic clearance rate
of estrogens and that smoking inhibits the function of osteoclasts.
In contrast, high caffeine consumption does not appear to be associated
with an increased risk of fracture in perimenopausal women.117,122,293 Thus, the adverse effects of caffeine on bone may be only important in the elderly.
sustaining fractures partly due to poor balance, associated illnesses,
frequent falls, and accidents but also due to the adverse effect of
alcohol on bone metabolism.117 Alcohol exerts a direct toxic
effect on bone cells. It affects osteoblast proliferation in vitro and reduces matrix protein synthesis in vivo.214 In contrast, moderate alcohol consumption does not appear to be a risk factor for osteoporosis or fracture.150
reduced loading that occurs in immobile patients leads to increased BMD
loss.90 Immobility also contributes to decreased muscle strength, this being a major risk factor for falls.91,156,159 Decreased muscle strength may also have a direct negative influence on BMD.91,156,159
Increased hip fracture risk has been reported to be linked with poor
quadriceps strength associated with immobility independent of the BMD.213
Several studies have confirmed this showing that mobile women have a
lower risk of hip fracture compared with less mobile women.56,140
However, it is not known whether the adverse effects of physical
inactivity and immobility are mediated by a decreased BMD, coexisting
illnesses, and increased risk of falls or all of these factors.
fractures by impairing BMD, bone quality, and muscle function. They
also tend to decrease physical activity and to increase the likelihood
of falling. Diseases and conditions that have been found to increase
the risk of sustaining fractures include the following56,59,90,101,131:
-
Hyperthyroidism
-
Decreased visual acuity
-
Poor depth perception
-
Mental impairment or dementia
-
Impaired neuromuscular function (e.g., the inability to rise from a chair without using the arms)
-
Hypercorticalism
-
Hypergonadism
-
Hyperparathyroidism
-
Osteomalacia
-
Renal and hepatic diseases
-
Certain malignancies
-
Rheumatoid arthritis
-
Paget disease
-
Gastrectomy and organ transplantations
Studies have reported that treatment with corticosteroids, long-acting
benzodiazepines, anticonvulsant drugs (especially phenytoin),
gonadotrophin-releasing hormone agonists, tamoxifen, long-term
treatment with heparin, cytotoxic drugs, and lithium are associated
with an increased risk of sustaining a fracture.56,131
This association remains after adjusting for BMD, suggesting that the
associated illnesses, impaired health, and increased likelihood of
falls affect the risk of fracture.56,131
but prevention requires a multifaceted strategy including environmental
changes, the provision of adequate calcium intake, supporting physical
activity, improving functional ability, correcting or treating health
disorders, and avoiding polypharmacy.239,289 In individuals with risk factors other than low BMD, these factors should be addressed.
factors, together with the modifiable risk factors, can be used to
identify at-risk groups suitable for bone densitometry and amenable to
different treatment strategies. In the Study of Osteoporotic Fractures,
white women older than 65 years were classified according to their
calcaneal BMD and the number of clinical risk factors for hip fracture.56
The relationship between BMD and fracture risk was least apparent with
fewer clinical risk factors. Thus, women with a higher calcaneal BMD
with more than four clinical risk factors had a higher risk of
sustaining a hip fracture than did women with lower BMDs but few other
risk fractures (Fig. 18-5). The highest risk for hip fractures was found in women with the lowest BMDs who had more than four clinical risk factors.
associated with the rising incidence of osteoporotic fractures make it
imperative to implement prevention strategies in the community.42,236
Hip and vertebral fractures in women are most commonly discussed, but
other fragility fractures are also associated with significant problems.246 In addition, the number of fractures in men and children has increased and we must also discuss these groups.32,133,267
However, general screening to detect low BMD is not considered to be
cost effective because a modest deficit in BMD is associated with a low
absolute risk of sustaining a fracture. The use of drug treatment in
these groups would mean a considerable therapeutic investment to save a
relatively small number of fractures. Furthermore, studies show that it
is only individuals with osteoporosis or osteopenia with fractures in
whom drugs will reduce the incidence of fractures. It is unclear
whether individuals with a more modest deficit in BMD benefit from drug
treatment* (Table 18-6). If the aim
of health care is to reduce the fracture rate in the community widely
accessible, inexpensive intervention programs with no adverse effects
are required.
with an adequate intake of energy, minerals, vitamins, and proteins.
Calcium is the most important nutrient for attaining adequate peak bone
mass, but there is no universal consensus about the
daily
requirement. The 1994 consensus conference discussing the optimum
calcium intake recommended a daily intake of 1200 to 1500 mg for
adolescents, 1000 mg for adults up to 65 years of age, and 1500 mg for
postmenopausal women not receiving estrogen and for elderly individuals.7
Although the results of most studies indicate a beneficial effect from
calcium supplements, especially in individuals with a low intake, the
long-term effect of a high dietary calcium on BMD is unclear. Calcium
also seems to work as a threshold nutritional element with about 400 mg
per day as a limit. Below this level, increasing calcium intake seems
beneficial and necessary.190 The positive correlation between dietary calcium and BMD has been shown in children,169 adolescents,123 and young women,284
indicating that higher calcium intake results in a higher BMD. It has
been calculated that variations in calcium nutrition early in life may
account for as much as 5% to 10% difference in peak adult bone mass,
which would contribute to more than 50% of the difference in the rates
of hip fracture in later life.191
level and serum concentrations of 25-hydroxy vitamin D decline with
age. The current recommendation is that the daily intake of vitamin D
should be about 400 to 800 IU if exposure to sunlight is low,
especially in the elderly, who have decreased ability to activate
precursors in the skin, decreased ability to hydroxylate vitamin D in
the kidney and liver, reduced dietary intake, and diminished absorption
from food. Another problem in frail elderly individuals is achieving an
adequate intake of protein, total energy, and a variety of other
nutritional components such as phosphorus, magnesium, zinc, copper,
iron, fluoride, sodium, and vitamins D, A, C, and K, all of which are
required for normal bone health.
during periods of rapid skeletal change as in the late prepubertal and
early pubertal period. Mechanical loading increases BMD but also
improves bone structure, geometry, architecture, and possibly material
properties such as strength, stiffness, and its energyabsorbing
capacity.133,139,171,172
The biological purpose of this adaptation is to achieve a skeleton that
is more resistant to load but still as light as possible to facilitate
mobility.139 Data have unequivocally shown that physical activity may increase BMD, skeletal geometry, and bone strength by up to 30% to 50%133,139,140 in those individuals in whom training is initiated before puberty133,140,171,172 (Fig. 18-4).
The reason for this can be explained by the fact that the adolescent
growth spurt is the only time in life when bone is added in substantial
amounts to both sides of the bone cortex by endosteal and periosteal
apposition.228 The importance of
regarding exercise during growth as a prevention strategy for fragility
fractures in old age originates from the data that relate exercise to
increased peak bone mass and show that 60% to 70% of the variance in
BMD at 65 years of age is attributed to achieved peak bone mass.269
Bone tissue is also able to respond to exercise in adulthood although
to a lesser extent than during growth. During adulthood physical
activity should be regarded more as bone preserving rather than bone
building because most studies show a 1% to 3% increase in BMD with
exercise.12,115 This has also been shown in Cochrane Database Systematic Reviews.27,272
effect in adulthood may be of great importance in maintaining bone
strength and preventing age-related fractures because only a small
increase in BMD is associated with a significant reduction in the risk
of fracture.51 Furthermore, exercise may cause a reduction in the incidence of fracture through nonskeletal effects.100,128 In the postmenopausal period, physical activity may prevent age-related bone loss.57,116
Brisk walking, climbing up and down stairs, dancing, and callisthenics
are the most suitable activities for older people since they are easily
available and are inexpensive and safe.128
It also appears that exercise should be lifelong if bone strength is to
be maintained because cessation of exercise is followed by a rapid
decline of the exercise-achieved BMD.142,217,299
Regular impact loading activities that create high-magnitude strains
and versatile strain distributions throughout the bone structure best
improve bone strength.115,116,139,140,142,162
Squash, tennis, badminton, aerobics, step exercises, volleyball,
basketball, soccer, gymnastics, weight and power training, and similar
sports may best fulfill these demands.139,140
In contrast, endurance training such as long distance running,
swimming, and cycling has not proved as effective in increasing BMD.140
fractures would be gained from studies that had the incidence of
fracture as their outcome criterion. Unfortunately, no such randomized
controlled trials (RCTs) exist. Instead, we have to rely on prospective
and retrospective observational and case-control studies. These types
of studies consistently show that both past and current physical
activity is associated with a reduced risk of hip fracture in women and
men, the risk reduction being up to 50%.100,128
Several studies also report a dose-response relationship that further
supports the probability of a link. It seems that vigorous activity
during youth followed by more moderate activity during adulthood is the
best combination to prevent hip fracture because vigorous activity in
old age may actually increase the incidence of falls that cause injury.126,276
Studies focusing on physical activity and fractures other than hip
fractures are few and present contradictory results. If anything, these
studies suggest that lifetime physical activity protects against all
types of fractures, although it must be appreciated that vigorous
activity in the elderly may increase the risk of falls and therefore
fractures.57,100,128,184,276
Activity programs for the elderly must therefore be designed
specifically for each individual and be based on the physical abilities
of that person. They should be undertaken with caution and after proper
training.276 It would seem that
promotion of lifelong physical activities is probably one of the most
important goals in public health programs of the new millennium.184,276
Exercise, including balance training, improves balance and decreases
the risk of falling. The greatest effect was seen in those who were
most compliant with the program.177,275,316 In several recent studies, Tai Chi has been shown to be an effective intervention reducing falls by almost 50%.315
The effectiveness of modifying other risk factors has not been
demonstrated in controlled studies. However, it makes sense to modify
the home environment to eliminate as many elements as possible that
could lead to falls. Because previous falls are an independent risk
factor for future falls, it is especially important to evaluate each
elderly person who has fallen for any risk factors in the home
environment.
This
has been successfully used in the PROFET study (Prevention of Falls in
the Elderly Trial), in which intervention decreased the risk of falls
by 70% in patients who had presented to emergency departments with
fall-related injuries.39 The key elements of such programs of risk reduction are as follows:
-
Individual management so that factors relevant to a particular patient are addressed
-
Reduction of environmental hazards
-
Appropriate reduction of medication
-
Education of the individual in behavior strategies
-
Education techniques for getting up after falls
-
Exercise programs to improve strength, balance, and aerobic capacity
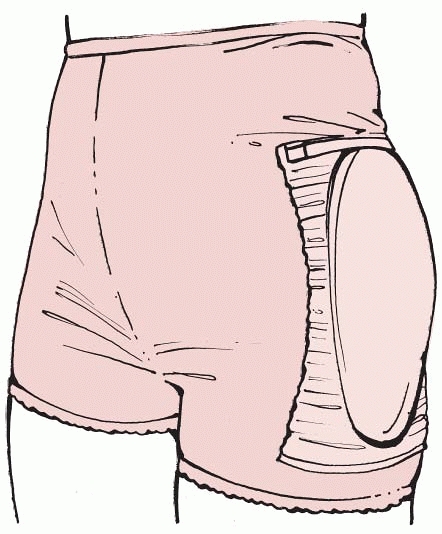
Based on these facts, various hip padding systems have been developed.
There are a number of different types, including an energy-shunting
type (horseshoe) system,113 a crash helmet type,166,230 an energy-absorptive type,270 and an airbag type,36 designed to reduce the impact of the skeleton in a fall55,114,137,138,167 (Fig. 18-6).
Randomized controlled trials, including nursing home residents and
those frail elderly living at home, have shown a protective effect of
34% by hip protectors when using pooled data.67,137,138,167 Based on a subgroup analysis in a previously reported nursing home study, the compliance in the use of hip protectors was 24%.126 In a more recent community-based study, an initial acceptance rate of 57% decreasing to 40% after 2 years118
was found. The effectiveness of hip protectors is verified in one
Cochrane Database Systematic Review including 13 RCTs of a total of
4316 patients.229 So far, no studies
have shown that hip protectors have a general protective effect in
people living at home and the cost-effectiveness remains unclear.229 The most significant problem with this type of prevention strategy appears to be compliance.229
 |
|
FIGURE 18-6 The hip protector underwear.
|
mg daily, are known to slow the rate of bone loss in the elderly and in
individuals with a low calcium intake.123,169,284
There are also studies that suggest that calcium supplements may reduce
the incidence of fractures, but usually calcium supplementation is
regarded as an adjunctive treatment for osteoporosis rather than as a
single treatment.60,247,250 This view is supported in a meta-analysis of 15 trials including 1806 individuals274 and in a Cochrane Database Systematic Review.273
Calcium supplements are safe, although mild gastrointestinal
disturbances such as constipation have been reported. The risk of
kidney stones related to increased urinary calcium excretion does not
appear to be a problem.
treatment of osteoporosis. A French study including 3270 elderly women
who lived in long-term care facilities and who were treated daily for 3
years with 1200 IU calcium and 800 IU vitamin D showed a 29% reduction
in the incidence of hip fracture and a 24% reduction in the incidence
of nonvertebral fracture compared with a placebo group34,35 (Table 18-7).
Another study reported a similar trend with a 50% reduction in
nonvertebral fractures in patients whose daily diet was supplemented
with calcium and vitamin D.61 A
British study, including 2686 men and women living in their own homes,
reported that calcium and vitamin D treatment over a 5-year period
reduced the risk of fracture by 22% and the risk of fractures in the
hip, forearm, or spine by 33%.296
This study implied that calcium and vitamin D treatment might decrease
fracture risk in nursing home residents who did not have a deficient
calcium intake. In contrast, a study of 2578 elderly healthy Dutch
women with a high calcium intake who were treated daily with 400 IU
vitamin D over 3.5 years showed no effect on the risk of hip fracture,175
and a study including 36,282 postmenopausal women aged 50 to 79 years
and followed for an average of 7 years showed no evidence of a reduced
hip fracture risk.125 One published meta-analysis reported that vitamin D treatment alone did not reduce the risk of fractures.88
However, in combination with calcium, the risk of hip fractures was
reduced by 26% in elderly care home residents, although in healthy
individuals living in their own home, there was no reduction in the
incidence of hip fracture, although the risk of sustaining vertebral
fractures was reduced by 54%.88 Similar results were published in another meta-analysis of 8124 individuals.226 Thus, the literature, including a Cochrane Systematic Database Review,8
suggests that calcium and vitamin D should be used routinely in elderly
individuals living in old people’s homes because of a high prevalence
of vitamin D deficiency as a result of low intake, low exposure to
sunlight, and impaired vitamin D synthesis in the skin. In these
cohorts, including seven trials and 10,376 participants, the treatment
led to 21% fewer hip and 13% fewer other nonvertebral fractures.8 In this analysis, there was no effect on vertebral fracture risk.
One meta-analysis including 5292 individuals older than 70 years and a
second meta-analysis including 3324 women older than 70 years concluded
that the fracture reduction effect in the previously mobile elderly is
questionable. However, other reports including 9294 women, aged at
least 60 years, in five different trials suggest that in ambulatory
women the prevalence of hip fracture declines if they are given a dose
of 700 to 800 IU/day.20 Vitamin D in
this dosage is safe and does not require monitoring. When compliance is
low, 150,000 to 300,000 IU can be given intramuscularly twice a year.
Calcium and vitamin D also reduce cortisone-induced bone loss. As has
already been described, there is still controversy regarding whether
calcium and vitamin D supplementation in healthy elderly people with an
adequate intake of dairy products influences the risk of fracture.20,88,94,226,237,296
|
TABLE 18-7 Randomized Controlled Trials with Incidence of Vertebral and Hip Fractures over 3 Yearsa
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
inhibiting bone resorption resulting in, at best, a 5% increase in BMD
over 1 to 3 years.174,231
Additional calcium supplementation seems to further enhance the
beneficial effects of hormone replacement therapy (HRT) treatment.215
Recent data also suggest that smaller doses of HRT than those often
used in early postmenopausal women—in the range of 0.5 to 1 mg of oral
17-estradiol, 25 mg of transdermal 17-estradiol, or 0.3 mg of
conjugated equine estrogens—have a similar beneficial skeletal effect.
Estrogen influences BMD loss for as long as the drug is given.3 When HRT is stopped, bone loss mimics bone loss after the menopause.38,75,173
Fracture data from the Million Women Study, a prospective observational
study including 138,737 postmenopausal women followed for 1.9 to 3.9
years, support this finding.10
risk is reduced by estrogen treatment. Case-control and cohort studies
suggest that HRT decreases the risk of hip fracture by about 30%.149 Two controlled studies of osteoporotic women indicate a 50% reduction in the risk of fractures of the spine.174,182 Another meta-analysis of controlled trials supports this reporting a 33% reduction of vertebral fractures with HRT.292
Another study including 22 randomized trials shows a 27% reduction in
nonvertebral fractures and specifically a 40% reduction in both hip and
wrist fractures.291 The study that
finally supported the theory that estrogen in combination with a
gestagen drug reduces the risk of fracture was the Women’s Health
Initiative Study (WHI). This study included 161,809 healthy
postmenopausal women including 16,608 involved in fracture evaluation
over a 5.2-year period.186,260
After this period, the planned 8-year follow-up study was cancelled
when the adverse negative effects outweighed the positive effects. The
study reported that estrogen reduced hip fracture incidence by 34%,
vertebral fractures by 34%, fragility fractures by 23%, and all
fractures by 24%260 (Table 18-7).
One recently published meta-analysis including more than 20,000 women
followed for an average of 4.9 years supported the WHI study results,
reporting that the general fracture risk was reduced by 28%.16
effects including vaginal bleeding, breast tenderness, deep vein
thrombosis and pulmonary embolism, stroke, heart disease, gall bladder
disease, and an increased risk of breast, endometrial, and ovarian
cancer after long-term use.18,41,103,186,260
Women who have had a hysterectomy can be given estrogen alone, but in
others estrogen and a progestogen should be given cyclically or in a
combined continuous regimen to reduce the risk of endometrial cancer.17
Readers should also be aware that the WHI study evaluated younger
postmenopausal women, not only elderly women with osteoporosis, this
being the important group. Whether estrogen influences steroid-induced
bone loss is unclear. In most countries, estrogen is not recommended as
the primary preventative agent for osteoporosis.
leading to a number of adverse effects, selective estrogen receptor
modulators (SERMs) act as estrogen agonists or antagonists depending on
the target tissue. Raloxifene acts as an antagonist of estrogen in the
breast and the endometrium but acts as an agonist on bone and lipid
metabolism. Raloxifene has been shown to prevent menopausal bone loss,
decrease bone turnover to premenopausal levels, and reduce the
incidence of fracture. The evaluation of fracture incidence is based on
a large RCT, the MORE study (Multiple Outcomes of Raloxifene
Evaluation) involving 7705 women with osteoporosis (Table 18-7).
This study reported a 30% reduction of vertebral fractures in women who
did not have a previous vertebral fracture and a 50% reduction in women
who had a previous vertebral fracture.70 No effects were found on nonvertebral fractures.70 Raloxifene also lowers the frequency of breast cancer by 70%31,53 but increases the incidence of venous thrombosis and pulmonary embolism at a similar rate to HRT.11
The RUTH study (Raloxifene Use for The Heart Trial) will provide more
data regarding the effects of raloxifene. Because new SERMs are now in
phase III trials, it is likely that the number of these drugs available
for use will increase in the future.
the prevention of osteoporosis. It acts on estrogen, progesterone, and
androgen receptors either directly or indirectly through metabolites
and has different effects from different target tissues. Tibolone
prevents bone loss in postmenopausal women,21
but so far there are no data regarding fractures. The ongoing Long Term
Intervention on Fractures with Tibolone (LIFT) study will provide data.
characterized by a phosphorous-carbon-phosphorous bond that strongly
binds to the hydroxyapatite crystal with a half-life in bone of several
years. The drug inhibits bone resorption by reducing the recruitment
and activity of osteoclasts and by increasing their apoptosis.78
Because food, calcium, iron, coffee, tea, and orange juice reduce the
absorption of bisphosphonates, the drug should be taken orally while
fasting. However, nowadays the drug can also be administered
intravenously. There are mild adverse effects including dyspepsia,
abdominal pain, and diarrhea in addition to esophagitis that may force
a patient to stop the medication.62 This problem is reduced if the drug is taken in a weekly or monthly dose compared with daily administration.265
If taken intravenously, short-term adverse effects mimicking influenza
are commonly seen for a few days, especially after the first injection.23
treatment of low BMD. A dose of 400 mg per day was given for 2 weeks
and then repeated every 3 months. The increase in BMD was reported to
be about 4% and results showed a reduction
of the rate of vertebral fractures after 2 years of treatment.279,304 A long-term study showed no fracture reduction after 3 years of treatment.109
One meta-analysis, including 13 RCTs of etidronate with more than a
1-year follow-up, reported that the risk of sustaining vertebral
fractures was reduced by 40%, whereas there was no effect on any other
fracture.47 A Cochrane Database
Systematic Review including 1248 patients in 11 controlled trials
verified that a daily dose of 400 mg reduced the risk of vertebral
fractures as a secondary prevention by 41%, while there was no
reduction in hip or nonvertebral fractures.310 There was no prevention of fractures when used as primary prevention.310 Etidronate seems to reduce steroid-induced bone loss, but any effect on fracture incidence is as yet unclear.47
In 2027 osteoporotic women with at least one previous vertebral
fracture, a 5-mg daily dose for 2 years followed by 10 mg daily for a
third year was associated with a reduction of about 47% in vertebral,
wrist, and hip fractures compared with a placebo22 (Table 18-7).
A 4-year study of the use of alendronate in women with low BMD, but
without a pre-existing vertebral fracture, supported these results by
finding a nonsignificant decrease in the frequency of fractures (p = 0.07) and a 45% reduction in new vertebral fractures52 (Table 18-7).
When the data from these two studies were pooled and only women with
osteoporosis were included, it was found that alendronate did reduce
the risk of fracture with 12 to 18 months of treatment.22,25,52
Another placebo-controlled study using 10 mg of alendronate daily in
1908 postmenopausal women with a BMD T-score below -2 SDs reported a
47% reduced risk of nonvertebral fracture after 1 year.235
The incidence of radiologically confirmed vertebral fracture was also
reduced by 89% in men with 2 years of treatment with alendronate223 (Table 18-7).
A meta-analysis including 11 RCTs of the use of alendronate with more
than a 1-year follow-up reported that the risk of sustaining vertebral
fractures was reduced by 48%, whereas in those who were treated with 10
mg of alendronate daily, there was also a 49% risk reduction in
sustaining nonvertebral fractures.50
A Cochrane Database Systematic Review including 12,068 patients in 11
controlled trials verified that a daily dose of 10 mg reduced the risk
of vertebral fractures in secondary prevention by 45%, nonvertebral
fractures by 23%, hip fractures by 53%, and wrist fractures by 50%.309 There was also a reduction of 45% for vertebral fractures when used as primary prevention.309,216
Alendronate seems to reduce steroid-induced bone loss, but whether
there is any effect on fracture incidence has not been fully evaluated.
A study of 2400 women who had had previous vertebral fractures and were
given 5 mg of risedronate per day showed that this reduced the
incidence of new vertebral fractures by 65% after the first year and by
41% over 3 years108 (Table 18-7).
Risedronate treatment over 3 years also reduced the incidence of
vertebral fractures by 49% in another study that included 1226 patients
who had at least two previous vertebral fractures248 (Table 18-7). The overall incidence of nonvertebral fractures in the two studies was reduced by 30% to 40%.108,249
However, the data supporting the reduction in the incidence of hip
fracture by risedronate are less clear. Risedronate treatment in 5445
osteoporotic women aged 70 to 79 years showed a 40% reduction in hip
fracture over 3 years, reaching 60% in those with a previous vertebral
fracture.196 In contrast, the same
treatment in 3896 women older than 80 years who had clinical risk
factors for falls, but without BMD assessment in most cases, had no
effect on the rate of hip fractures.196
A meta-analysis of the effect of risedronate contained five studies
that included vertebral fractures and seven studies with nonvertebral
fractures. This meta-analysis reported that risedronate reduced the
risk of vertebral fractures by 36% and of nonvertebral fractures by 27%.48
A Cochrane Database Systematic Review including 14,049 patients in
seven controlled trials verified that a daily dose of 5 mg reduced the
risk of vertebral fractures in secondary prevention by 39%,
nonvertebral fractures by 20%, and hip fractures by 26%.308 There was no prevention of fractures when used as primary prevention.308
to reduce the incidence of fractures if it is given intravenously once
a year.23 A single infusion of 5 mg
zoledronic acid given to 3889 postmenopausal women in an RCT for 3
years was shown to reduce the risk of sustaining a vertebral fracture
by 70%, a nonvertebral fracture by 25%, a hip fracture by 41%, and a
clinical fracture by 33%.23
reduce the incidence of fractures, but their use has not been as well
documented as the bisphosphonates already discussed. A dose of 800 mg
daily of clodronate seems to reduce the number of vertebral fractures
by 46%.194 This was confirmed in a later publication.193
Ibandronate, in a dose of 2.5 mg daily, seems to reduce the vertebral
fracture risk by 62% and by 50% if given in a dose of 20 mg monthly.240
Tiludronate is used for the treatment of Paget’s disease of bone but
cannot be recommended for the treatment of osteoporosis because of an
absence of relevant data. Orally administered daily pamidronate may be
effective in osteoporosis but has a high incidence of upper
gastrointestinal symptoms, reducing its clinical usefulness.181
In contrast, intravenous infusion of pamidronate is commonly used in
malignant bone disease and in Paget disease of bone with only minor
side effects.251
reduces bone absorption by osteoclast inhibition. The treatment can be
provided by subcutaneous or intramuscular injection. Side effects
include nausea, facial flushes, and diarrhea. This compares unfavorably
with the intranasal administration of salmon calcitonin in which 200 IU
daily provides treatment that has no such side effects. The PROOF study
(Prevent Recurrence Of Osteoporotic Fractures), a 5-year controlled
trial of 1255 postmenopausal women with osteoporosis, reported that 200
IU of intranasal salmon calcitonin per day reduced vertebral fracture
risk by 31% while no effects was found on peripheral fractures37 (Table 18-7).
However, this study must be interpreted with care because 60% of
individuals were lost to follow-up. Doses of 100 and 400 IU had no
effect, and no consistent effect on BMD and bone turnover markers was
noted.37 A meta-analysis of 30 RCTs provided evidence that calcitonin reduces the risk of vertebral fractures by 54%.49 Whether calcitonin influences steroid-induced bone loss is as yet unclear.
results in increased bone resorption and bone loss. By contrast,
intermittent PTH treatment in individuals with osteoporosis stimulates
bone formation, increases BMD, and reduces the risk of fractures.211,268
In one RCT including 1637 postmenopausal women with a previous
vertebral fracture, 20 µg of subcutaneous recombinant human PTH
administered daily for a median of 19 months reduced the incidence of
new vertebral fractures by 65% and 40 µg reduced the incidence by 69%211 (Table 18-7).
The reduction in the incidence of nonvertebral fragility fractures was
53% with both doses during the same period, while BMD increased by 9%
and 13% in the spine and by 3% and 6%, respectively, in the femoral
neck with the two doses after 21 months of treatment. Injection with
PTH has adverse effects, mainly nausea and headache. Another RCT
following 2532 postmenopausal women for 18 months reported that
treatment with 100 mg human parathyroid hormone led to a 58% reduction
of the risk of sustaining a vertebral fracture.97
This study included 1649 postmenopausal women with osteoporosis and at
least one previous vertebral fracture. Two grams of strontium ranelate
per day, administered for 3 years, increased BMD and reduced the risk
of sustaining new vertebral fractures by 49% during the first year and
by 41% during the entire 3-year period (Table 18-7).
In addition, there were no more adverse effects in the treatment group
than in the placebo group. A Cochrane Database Systematic Review
including four controlled trials verified that a daily dose of 2.0
g/day for 3 years reduced the risk of vertebral fractures by 37% and of
nonvertebral fractures by 14%.218
hydroxyapatite crystal of bone. It stimulates osteoblast recruitment
and activity and increases BMD in the spine but less so in the hip.203,253
However, controlled trials have failed to show that fluoride reduces
fractures. If anything, it seems as though the incidence of
nonvertebral fractures might increase. A Cochrane Database Systematic
Review including 1429 individuals in 11 trials verifies this view,
reporting that fluoride does not result in a reduction of fractures.104 Currently, fluoride cannot be recommended for the treatment of osteoporosis.
osteoporosis. Studies report an increase in BMD with their use, but
none provide adequate data about fractures. Alfacalcidol and calcitriol
are vitamin D analogues occasionally used as treatment for
osteoporosis. Studies show a small increase in spine BMD, but because
there are inadequate data regarding fracture treatment with these
drugs, they cannot be regarded as having the potential to reduce
fractures.81,82,222 Treatment with menatetrenone, a vitamin K2 compound, has shown improved BMD.221
osteoporosis, and it has been reported that a low intake of vitamin K
is associated with an increased risk of hip fracture.76
One meta-analysis of menaquinone-4 treatment (oral vitamin K) in
Japanese patients showed a reduction of 60% in vertebral fractures, of
77% in hip fractures, and of 81% in nonvertebral fractures,40
but currently there are no RCTs with an adequate sample size evaluating
the effect of vitamin K on fractures. Growth hormone is another drug
used in the treatment of osteoporosis because it theoretically could
increase muscle strength and BMD. However, there is no proof that it
prevents bone loss and reduces fracture risk in postmenopausal women.
family of isoflavones, may prevent bone loss, but it does not seem to
reduce the incidence of fractures in osteoporotic women.6 Finally, statins have been shown to increase BMD in animal studies,209
but further information is required about their effects in humans
before it can be recommended for the prevention of fragility fractures.
fracture, there are several important age-related factors to consider
when planning treatment. The functional demands in the elderly are
different from those of young healthy people and long-term
immobilization in bed must be avoided. Delaying fracture treatment by
more than 1 day has been reported to increase mortality in the elderly.30,66
Thus, it is probably even more important in the elderly to achieve
stable fracture fixation that will reduce pain and facilitate
mobilization. Reduced bone mass, increased bone brittleness, and
structural changes such as medullary expansion must be taken into
account in the osteoporotic patient when deciding on the type of
surgical method to be used. It must also be understood, when making a
decision regarding treatment, that the osteoporotic patient usually has
low physical demands and a reduced life expectancy. For example,
long-term complications following arthroplasty will not occur in the
majority of elderly patients. Thus, joint replacement surgery is a good
option after displaced femoral neck fractures because the stability
provided by the implant permits immediate weight bearing and
mobilization.257 The major problem
in osteoporotic fracture treatment is fixation of the device to the
bone because bone failure is much more common than implant breakage.
Internal fixation devices such as sliding nail plates, intramedullary
nails, and tension band constructs that permit skeletal loading
minimize stress at the implant-bone interface. Some osteoporotic
fractures are also associated with bone loss. If this occurs, it is
important to achieve bone contact between the two main fragments even
if this results in shortening of the extremity. Good bone contact will
improve the chance of healing, reduce the healing period, and reduce
strains on the fixation device. If plates are used, these should
ideally be used as tension bands, which require cortical contact
opposite the plates. In addition, long plates, where the spacing of the
screws is more important than the number of screws, should be used
because they will distribute the forces over a larger area, reducing
the risk of bone failure.294
of the humerus and distal radius and closed fractures of the tibial
diaphysis, can be mobilized in a sling, cast, or brace.264
Immobilization in casts has the disadvantage of immobilizing the joints
adjacent to the fracture often leading to joint stiffness. Furthermore,
a cast does not control fracture shortening, which is often
seen
in osteoporotic bone, and if the subcutaneous tissue is very mobile, as
it often is in the elderly, cast fixation will not provide adequate
fracture fixation. External fixators can be used, but the main problem
with external fixation in osteoporotic bone is the same as for screw
fixation—namely, loss of fixation. Loosening of the device is often
followed by pin infection and local bone resorption, sometimes leading
to a secondary fracture at the pin site.5
The introduction of hydroxyapatite-coated pins has reduced this
complication because fixation is improved compared with when using
titanium-coated and standard pins.207
Another method used to improve internal fixation and to avoid bone
resorption is to anchor the pins or screws with polymethylmethacrylate
bone cement. This can be inserted into the bone and allowed to harden
before drilling, or it can be inserted into the screw holes just before
the screws are inserted. The screws can then be tightened after the
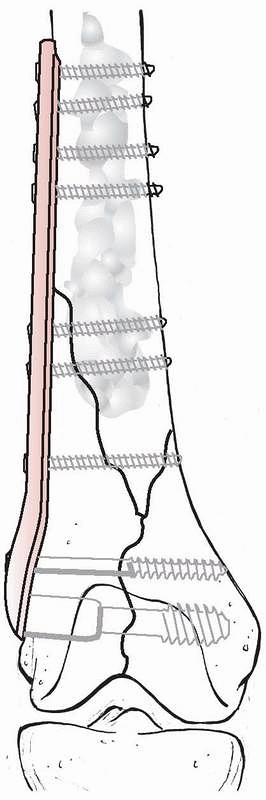
cement hardens (Fig. 18-7). If this method is
used, it is important that the cement does not penetrate the fracture
so as to interfere with fracture healing.
increases the strength of the fixation. Threaded screw holes in the
plates create angular stability between the screws and the plates. For
example, the locking compression plates (LCP) provide 3 times greater
stability than a standard lateral condylar buttress plate and about 2.5
times greater stability than a 95-degree condylar plate in axial
loading.157 This strength is increased if the screws are fixed at multiple angles.266 The use of these multiple screws in fixed angle devices is particularly useful in the metaphyseal region (Fig. 18-8).
A particular problem that often prevents the use of screws and plates
in osteoporotic bone is the periprosthetic fracture. These can be
treated with plates using wires for fixation around the femoral shaft (Fig. 18-9). Periprosthetic fractures and their treatment are discussed further in Chapter 21.
 |
|
FIGURE 18-7
A displaced distal femur fracture primarily treated by open reduction and open fixation with an angle plate with augmentation of the screw fixation in the bone by polymethylmethacrylate. |
 |
|
FIGURE 18-8 A proximal humerus fracture primarily treated by open reduction and open fixation with a locking plate.
|
osteoporotic long-bone fractures. It is biomechanically more favorable
than plates and screws and will usually permit immediate
weight
bearing. With the introduction of interlocking nails, it is also
possible to nail fractures that are close to the metaphyseal regions in
long bones. The fixation can be improved by the use of several
interlocking screws in different directions or by augmentation of the
screws with bone cement. It is also important to realize that because
osteoporosis causes the diameter of the intramedullary canal to
increase,2,4
larger-diameter nails must often be used in older patients. Even if
osteoporosis does not impair fracture healing, the diminished bone mass
will reduce the amount of callus formation and increase the time taken
to restore adequate bone strength. There are some experimental studies
that show a reduced rate of healing in estrogen-deficient animals.303
Because fracture fixation is reduced in osteoporotic bone, it would be
advantageous to accelerate the healing process. Therefore, autogenous
cancellous bone graft is often recommended to enhance fracture healing
because the osteoconductive bone matrix, osteoinductive growth factors,
and mesenchymal stem cells present in this type of graft are often
thought to stimulate the healing process. However, in patients with
osteoporosis, the amount of cancellous bone available for grafting is
reduced, often necessitating the use of allografts, which are
biologically inferior to autografts and carry the additional risk of
disease transmission. To overcome these problems, growth factors are
available to induce new bone formation. There are also biodegradable
synthetic products such as calcium phosphate cement that fill defects
in osteoporotic bone. These have mainly been used in the treatment of
distal radial fractures33,84,145,155 and proximal tibial fractures307 (Fig. 18-10).
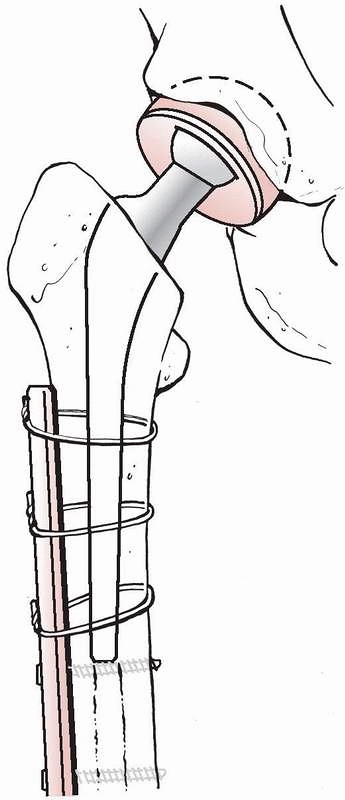 |
|
FIGURE 18-9
A periprosthetic femur fracture primarily treated by open reduction and open fixation with a plate using wires for fixation around the femoral shaft and screws distal to the implant. Excellent screw fixation is achieved through the cement distal to the prosthesis. |
fractures has usually been nonoperative, with the amount of disability
directly relating to the number of fractured vertebrae. However, within
the past few years, vertebroplasty and kyphoplasty have been introduced
as new treatment modalities, although they have not yet been fully
evaluated. So far there are no RCTs published that evaluate these
methods. In both techniques, the crushed vertebrae are filled with
material, usually polymethylmethacrylate bone cement, to avoid further
compression. Kyphoplasty also aims to reduce fracture compression
before the bone cement is injected. The operative techniques for these
procedures have been described elsewhere243 and will therefore only be reviewed briefly in this chapter.
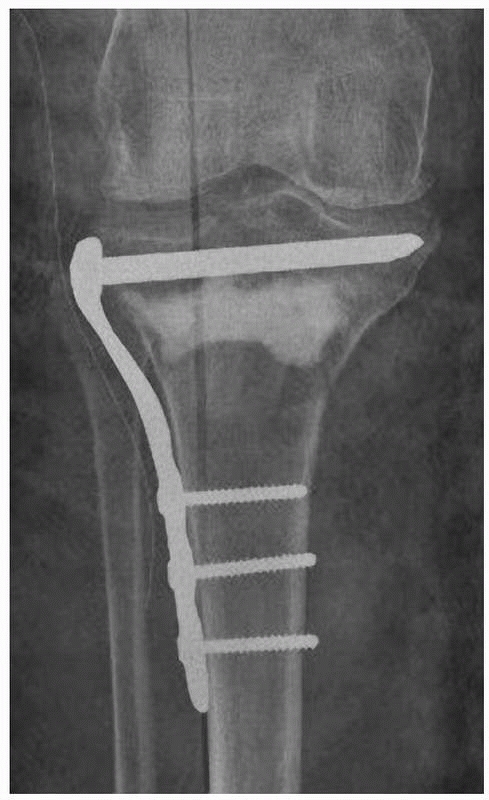 |
|
FIGURE 18-10
A metaphyseal bicondylar proximal tibial fracture primarily treated by open reduction and internal fixation with a locking plate augmented with calcium phosphate cement. |
 |
|
FIGURE 18-11 Vertebroplasty performed in the lumbar spine.
|
Kyphoplasty has evolved from vertebroplasty in the last few years. Both
procedures can usually be undertaken under local anaesthetic. The
patient is positioned prone on bolsters in an attempt to reduce the
Kyphosis, and a trochar and cannula are inserted through the pedicle
into the posterior or central areas of the vertebral body. Radiopaque
bone cement is then injected under fluoroscopic guidance into the
vertebral body (Fig. 18-11). Care must be taken
not to inject the cement outside the vertebral body and particularly
not to inject it into the spinal canal. In kyphoplasty, the patient is
placed in the same position, but once the cannula has been inserted, an
inflated balloon is introduced into the vertebral body under manometric
control. The objective is to partially or fully reduce the compressed
vertebral body. After this has been done, the balloon is removed and
the operation proceeds as for vertebroplasty (Fig. 18-12).
There has been considerable interest in these two techniques, but
unfortunately the methods have not been evaluated in RCTs. Despite
this, the impression is that both procedures are associated with good
pain relief. A retrospective study of 500 patients showed
that
vertebroplasty was associated with significant pain reduction and an
improvement in functional status in 50% of the patients 7 months after
the procedure. The results were obtained regardless of the number of
vertebral fractures that were treated.73
Another study has indicated that there is significant relief from
symptoms and improvement in function within 24 hours of the procedure.65
However, 6 to 12 months after the procedure, there was no evidence of
any difference between the groups of patients. The weakness of this
study is that the controls comprised the individuals who declined
vertebroplasty. One nonrandomized controlled study including 126
patients reported that vertebroplasty was associated with 60%
improvement in pain, 29% improvement in physical functioning, and 43%
reduction in hospital bed-days occupied in the short term.64
However, 12 and 24 months after the intervention, there was no
differences between the surgical and conservatively treated groups.64
One review including 1136 interventions in 793 patients showed that the
procedure was associated with a 60% immediate pain relief.234
One RCT including only 32 patients supported this view reporting that
vertebroplasty was associated with immediate pain relief and
improvement of mobility and function.302
However, these patients were followed for only 2 weeks. Ongoing RCTs
such as the INVEST study (Investigational Vertebroplasty Efficacy and
Safety Trial)96 and the VERTOS II
study (Percutaneous Vertebroplasty Versus Conservative Therapy in
Patients With Osteoporotic Vertebral Compression Fractures)152
will provide us with a higher level of evidence regarding the efficacy
of the procedure. However, it appears that while the technique produces
short-term pain relief, the long-term results are less clear.
 |
|
FIGURE 18-12 Kyphoplasty performed in the lumbar spine.
|
evaluations of the technique. A number of studies report that between
20% and 50% of patients showed no restoration of vertebral body height
after the procedure,170,233 while others report mean reduction in the kyphosis of about 10 degrees.233
Most studies indicate good pain relief immediately after the procedure,
but there is conflicting evidence about the long-term results.170,233
A retrospective controlled study comparing kyphoplasty patients with
nonoperated control subjects showed that the procedure was associated
with immediate pain relief and functional improvement compared with the
patients’ status before surgery and with the control group.306
Hospital stay was also longer in the nonoperatively treated group. One
prospective nonrandomized controlled trial supports this view when
reporting 12% increased vertebral height as well as reduced pain and
improved mobility in patients after kyphoplasty.143
However, the patients selected their own treatment in this study, which
increases the risk of selection bias. The favorable outcome was
maintained after 6 months143 and 12 months.93
Finally, one study including 75 prospective controlled and uncontrolled
studies of both vertebroplasty and kyphoplasty concluded that surgery
was superior to conservative treatment in management of symptomatic
osteoporotic vertebral compression fractures.282,283
minimize cement leakage into the surrounding tissue. However, there are
as yet no studies that directly compare vertebroplasty and kyphoplasty,
although observational studies report an incidence of at least 10%
cement leakage in patients treated with kyphoplasty which is similar to
the incidence noted after vertebroplasty.170,233,306 More serious adverse events have been reported: extravasation into segmental veins,306
leakage into the spinal canal with associated neurologic disturbance,
and perioperative pulmonary edema with myocardial infarction and rib
fractures.170,233
There is also the additional potential problem of a surgical procedure
altering the biomechanical forces in the spine and resulting in new
vertebral fractures. One paper described 12% of patients with new
vertebral fractures within 2 years of a vertebroplasty, most being
adjacent to the operated vertebrae.298
It is likely, however, that the complication rate is higher because
this study only included symptomatic vertebral fractures and the
follow-up was restricted to only 16% of the operated patients.
agents that have been proved to reduce the incidence of osteoporotic
fractures. It can no longer be regarded as acceptable clinical practice
to avoid investigating or treating patients with osteoporosis who
present with low-energy fractures. One fragility fracture indicates
further fractures, and it should be borne in mind that BMD measurement
has a better predictive ability for future fractures than blood
pressure has for a future stroke. The evaluation of the patient must
include a history of risk factors and, in many cases, a BMD scan.
Prevention strategies may involve both pharmacologic and
nonpharmacologic approaches.
we make to virtually all patients with a fragility fracture,
independent of age. There is good evidence in the literature that
physical activity is beneficial for BMD and reduces the incidence of
fracture, especially in postmenopausal women.57,100
We believe that the recommendation to increase physical activity is
advantageous not only from the point of view of reducing the risk of
future fracture but also for other sound biological reasons. There is
good evidence that calcium and vitamin D supplementation can reduce the
risk of sustaining a hip fracture in elderly institutionalized patients.35
At the moment, there is no evidence that any other nutritional
supplement has the same effect. The fact that hip fracture patients are
considerably thinner than age-matched individuals without a fracture141
suggests that increased nutritional intake including calcium, vitamin
D, and protein may also provide protection from future fractures. We
therefore recommend a well-balanced diet in patients presenting with a
low-energy fracture.
Currently, we recommend hip protectors mainly to institutionalized
individuals partly because of the low compliance and lack of data
showing benefit to the elderly living in their own homes. Pharmacologic
prevention is based on several double-blinded prospective RCTs showing
that drug treatment reduces the incidence of fracture. Calcium and
vitamin D given together in sufficient dosage have been shown to reduce
the incidence of fracture particularly in institutionalized patients.35,61,92
We believe that 500 to 1000 mg calcium and 400 to 800 IU of vitamin D
should be provided to all elderly individuals. These doses do not
give rise to significant side effects except for occasional gastrointestinal discomfort.
treatment, we believe that bisphosphonates are usually the drug of
choice. Studies indicate that bisphosphonates have been proved to
reduce the risk of fracture compared with no treatment.22,108
Because of the possibility of gastrointestinal disturbances,
particularly esophagitis, we prescribe a weekly or monthly dose that
has been proves to reduce these effects23,240,265
in preference to an annual bisphosphonate injection. SERMs can also be
recommended for fracture prevention because they have been shown to be
successful in RCTs.70 However, the relative effect of bisphosphonates and the fact that they have increased side effects such as thromboembolism11
mean that SERMs are our second choice for pharmacologic prevention.
Subcutaneous parathyroid hormone (PTH) has been documented to reduce
fractures.24,26,97,211
However, because of its expense, the requirement for daily injections,
and the incidence of potential side effects, we only use this drug in
the most severe cases of osteoporosis or in those patients who have
severe adverse effects with other drugs. We try to avoid giving PTH to
younger patients with osteoporosis because of the lack of long-term
data. There are data indicating that estrogen reduces fracture
incidence in postmenopausal women186,260;
however, because of the serious side effects that were discussed
earlier in this chapter, we currently do not use estrogen as a
first-line treatment option in osteoporosis.
important to assess the functional demands of the patients before we
select a treatment strategy. We must look beyond the simple assessment
of the severity and location of the fracture. In general, we treat
low-energy fractures in the upper and lower limbs slightly differently.
Most fractures of the upper limb are treated nonoperatively because
these fractures usually do not immobilize the patients in bed and
because the results of a nonsurgical approach are generally good. In
addition, the difficulties of internally fixing many of these fractures
and the lower functional demands in the older osteoporotic patient mean
that nonoperative management is often satisfactory. The different
spectrum of surgery in upper and lower limb fractures in the elderly is
discussed further in Chapter 6. Open reduction
and internal fixation, hemiarthroplasty of the shoulder, arthroplasty
of the elbow, and closed reduction and external fixation of the wrist
are usually reserved for more complex fractures.5,79,155,256,318
Some fractures, such as osteoporotic acetabular fractures, can often be
managed nonoperatively with a period of non-weight-bearing
mobilization, although primary arthroplasty is considered for selected
patients. Other major fractures in the lower extremities are treated in
the same way as in patients who do not have osteoporosis, with
operative fixation and mobilization, with full weight bearing being
allowed several weeks after the surgical procedure.145,244
operative treatments for osteoporotic vertebral fractures. However, we
caution surgeons that clinical studies must indicate that these new
techniques are useful before they can be widely recommended.
orthopaedic surgeons. The diagnosis is often made in the orthopaedic
wards or in the outpatient clinic after a low-energy fracture has
occurred. Orthopaedic surgeons must be careful that they do not just
concentrate on the technical aspects of fracture fixation and that they
appreciate the considerable consequences of osteoporosis.
Identification of subjects at high risk of future fracture constitutes
the most rational approach to fracture prevention. We believe that it
is the responsibility of the orthopaedic surgeon to arrange for
patients who present with low-energy fractures to be properly advised
and investigated for osteoporosis. It is also the responsibility of
every orthopaedic surgeon to be aware of the different treatment
modalities that exist and to appropriately advise the patient.
necessarily the province of the orthopaedic surgeon. Referral to an
appropriate physician interested in the investigation and treatment of
osteoporosis is, however, the responsibility of the orthopaedic
surgeon. We believe it likely that the prediction of the risk of future
fractures will determine treatment strategy. Table 18-8 shows the 10-year fracture probability for the common
osteoporotic fractures in Sweden according to the population at risk.132
Similar calculations such as the WHO Fracture Assessment Tool (FRAX)
that provide an assessment of the 10-year probability of fracture are
now available on the Internet as an aid to selecting which patients
require preventative treatment (http://www.shef.ac.uk/FRAX). Similar
tables can help provide country-specific estimates of the risk of
fracture from the relative risk estimates that are acquired from bone
scans and the investigation of risk factors (Table 18-5).
When a hip fracture alone is considered, a 10-year probability of 10%
or greater provides a cost-effective threshold for treating women.130
Because the aim of the assessment of fracture risk is to target
cost-effective treatment interventions to those at the highest risk, we
hope it can be decided more easily who to treat.
|
TABLE 18-8 Ten-Year Probability of Fracture (%) According to Age and Risk Relative to the Average Populationa
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CI, Robinson CM, Court-Brown CM, et al. Prospective randomized
controlled trial of an intramedullary nail versus dynamic screw and
plate for intertrochanteric fractures of the femur. J Orthop Trauma
2001;15:394-400.
HG, Johnell O, Karlsson MK. An age-related medullary expansion can have
implications for the long-term fixation of hip prostheses. Acta Orthop
Scand 2004;75: 154-159.
HG, Johnell O, Karlsson MK. Long-term effects of oestrogen therapy on
bone loss in postmenopausal women: a 23-year prospective study. BJOG
2004;111: 335-339.
HG, Josefsson PO. Pin-tract complications in external fixation of
fractures of the distal radius. Acta Orthop Scand 1999;70:116-118.
P, Toussaint A, Christiansen C, et al. Ipriflavone in the treatment of
postmenopausal osteoporosis: a randomized controlled trial. JAMA
2001;285:1482-1488.
A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues
for preventing fractures associated with involutional and
postmenopausal osteoporosis. Cochrane Database Syst Rev 2005:CD000227.
DA, McKay HA, Mirwald RL, et al. A 6-year longitudinal study of the
relationship of physical activity to bone mineral accrual in growing
children: the University of Saskatchewan Bone Mineral Accrual Study. J
Bone Miner Res 1999;14:1672-1679.
E, Beral V, Reeves G, et al. Fracture incidence in relation to the
pattern of use of hormone therapy in postmenopausal women. JAMA
2004;291:2212-2220.
E, Grady D, et al. Raloxifene and cardiovascular events in osteoporotic
postmenopausal women: 4-year results from the MORE (Multiple Outcomes
of Raloxifene Evaluation) randomized trial. JAMA 2002;287:847-857.
EJ, Ramsdale SJ. Increase in femoral bone density in young women
following high-impact exercise. Osteoporos Int 1994;4:72-75.
DC, Gluer CC, Cauley JA, et al. Broadband ultrasound attenuation
predicts fractures strongly and independently of densitometry in older
women. A prospective study. Study of Osteoporotic Fractures Research
Group. Arch Intern Med 1997;157: 629-634.
TJ, Ruff CB, Warden KE, et al. Predicting femoral neck strength from
bone mineral data. A structural approach. Invest Radiol 1990;25:6-18.
V, Banks E, Reeves G. Evidence from randomised trials on the long-term
effects of hormone replacement therapy. Lancet 2002;360:942-944.
SA, Weiss NS, Voigt LF, et al. Risk of endometrial cancer in relation
to use of oestrogen combined with cyclic progestagen therapy in
postmenopausal women. Lancet 1997;349:458-461.
M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared
with arthroplasty for displaced fractures of the femoral neck. A
meta-analysis. J Bone Joint Surg Am 2003;85A:1673-1681.
HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D
supplementation: a meta-analysis of randomized controlled trials. JAMA
2005;293: 2257-2264.
NH, Bjarnason K, Haarbo J, et al. Tibolone: prevention of bone loss in
late postmenopausal women. J Clin Endocrinol Metab 1996;81:2419-2422.
DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of
alendronate on risk of fracture in women with existing vertebral
fractures. Fracture Intervention Trial Research Group. Lancet
1996;348:1535-1541.
DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for
treatment of postmenopausal osteoporosis. N Engl J Med
2007;356:1809-1822.
DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone
and alendronate alone or in combination in postmenopausal osteoporosis.
N Engl J Med 2003;349:1207-1215.
DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with
alendronate in women with osteoporosis: the Fracture Intervention
Trial. FIT Research Group. J Clin Endocrinol Metab 2000;85:4118-4124.
JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to
compare the efficacy of teriparatide [recombinant human parathyroid
hormone (1-34)] with alendronate in postmenopausal women with
osteoporosis. J Clin Endocrinol Metab 2002;87:4528-4535.
D, Shea B, Iovine R, et al. Exercise for preventing and treating
osteoporosis in postmenopausal women. Cochrane Database Syst Rev
2002:CD000333.
SK, Tinetti ME, Speechley M, et al. Factors associated with short-
versus long-term skilled nursing facility placement among
community-living hip fracture patients. J Am Geriatr Soc
1990;38:1139-1144.
AJ, Robertson MC, Gardner MM, et al. Falls prevention over 2 years: a
randomized controlled trial in women 80 years and older. Age Ageing
1999;28: 513-518.
JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction
in postmenopausal women treated with raloxifene: 4-year results from
the MORE trial. Multiple outcomes of raloxifene evaluation. Breast
Cancer Res Treat 2001;65:125-134.
JR, Nguyen TV, Schneider D, et al. Mortality after all major types of
osteoporotic fracture in men and women: an observational study. Lancet
1999;353:878-882.
MW, Bucholz R, Cornell C. Treatment of acute fractures with a
collagencalcium phosphate graft material. A randomized clinical trial.
J Bone Joint Surg Am 1997;79:495-502.
MC, Arlot ME, Delmas PD, et al. Effect of calcium and cholecalciferol
treatment for three years on hip fractures in elderly women. BMJ (Clin
Res) 1994;308:1081-1082.
MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip
fractures in the elderly women. N Engl J Med 1992;327:1637-1642.
CH 3rd, Silverman S, Andriano K, et al. A randomized trial of nasal
spray salmon calcitonin in postmenopausal women with established
osteoporosis: the prevent recurrence of osteoporotic fractures study.
PROOF Study Group. Am J Med 2000;109: 267-276.
C, Christensen MS, Transbol I. Bone mass in postmenopausal women after
withdrawal of oestrogen/gestagen replacement therapy. Lancet
1981;1:459-461.
J, Ellis M, Hooper R, et al. Prevention of falls in the elderly trial
(PROFET): a randomised controlled trial. Lancet 1999;353:93-97.
S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of
fractures: systematic review and meta-analysis of randomized controlled
trials. Arch Intern Med 2006;166:1256-1261.
GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins
and the risk of breast cancer in postmenopausal women. N Engl J Med
1995;332: 1589-1593.
C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of survival
after osteoporotic fractures. Am J Epidemiol 1993;137:1001-1005.
C, Melton LJ 3rd. Magnitude and impact of osteoporosis and fractures.
In: Press A, ed. Osteoporosis. San Diego, CA; 1996:419-434.
N, Adams J, Pols H, et al. Age, gender, and geographical effects on hip
geometry and bone mineral distribution: The EPOS study. Osteoporos Int
1997;7:291.
A, Guyatt G, Krolicki N, et al. A meta-analysis of etidronate for the
treatment of postmenopausal osteoporosis. Osteoporos Int
2001;12:140-151.
A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for
postmenopausal osteoporosis. III. Meta-analysis of risedronate for the
treatment of postmenopausal osteoporosis. Endocrine Rev 2002;23:517-523.
A, Tugwell P, Zytaruk N, et al. Meta-analyses of therapies for
postmenopausal osteoporosis. VI. Meta-analysis of calcitonin for the
treatment of postmenopausal osteoporosis. Endocrine Rev 2002;23:540-551.
A, Wells G, Willan A, et al. Meta-analyses of therapies for
postmenopausal osteoporosis. II. Meta-analysis of alendronate for the
treatment of postmenopausal women. Endocrine Rev 2002;23:508-516.
SR, Black DM, Nevitt MC, et al. Bone density at various sites for
prediction of hip fractures. The Study of Osteoporotic Fractures
Research Group. Lancet 1993; 341:72-75.
SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of
fracture in women with low bone density but without vertebral
fractures: results from the Fracture Intervention Trial. JAMA
1998;280:2077-2082.
SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of
breast cancer in postmenopausal women: results from the MORE randomized
trial. Multiple Outcomes of Raloxifene Evaluation. JAMA
1999;281:2189-2197.
SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in
white women. Study of Osteoporotic Fractures Research Group. N Engl J
Med 1995;332: 767-73.
GP, Stocke KS, Ehsani AA, et al. Weight-bearing exercise training and
lumbar bone mineral content in postmenopausal women. Ann Intern Med
1988;108:824-828.
HW. Osteoporosis of the slender smoker. Vertebral compression fractures
and loss of metacarpal cortex in relation to postmenopausal cigarette
smoking and lack of obesity. Arch Intern Med 1976;136:298-304.
P, Favier F, Grandjean H, et al. Fall-related factors and risk of hip
fracture: the EPIDOS prospective study. Lancet 1996;348:145-149.
B, Dallal GE, Krall EA, et al. A controlled trial of the effect of
calcium supplementation on bone density in postmenopausal women. N Engl
J Med 1990;323: 878-883.
B, Harris SS, Krall EA, et al. Effect of calcium and vitamin D
supplementation on bone density in men and women 65 years of age or
older. N Engl J Med 1997;337:670-676.
J, Ortner DJ, Stix AI, et al. Hip fracture and osteoporosis in a XIIth
Dynasty female skeleton from Lisht, upper Egypt. J Bone Miner Res
1997;12:881-888.
TH, Bryant C, Browne L, et al. Clinical outcomes after acute
osteoporotic vertebral fractures: a 2-year nonrandomized trial
comparing percutaneous vertebroplasty with conservative therapy. Med J
Austral 2006;184:113-117.
TH, Champion B, Clark WA. Management of acute osteoporotic vertebral
fractures: a nonrandomized trial comparing percutaneous vertebroplasty
with conservative therapy. Am J Med 2003;114:257-265.
R, Schoechtner H, Buchinger W. The influence of immediate surgical
treatment of proximal femoral fractures on mortality and quality of
life. Operation within 6 hours of the fracture versus later than 6
hours. J Bone Joint Surg Br 2003;85:1107-1113.
A, Mallmin H, Michaelsson K, et al. External hip protectors to prevent
osteoporotic hip fractures. Lancet 1997;350:563-564.
KE, Cauley J, Lipschutz R, et al. Weight change and fractures in older
women. Study of Osteoporotic Fractures Research Group. Arch Intern Med
1997;157:857-863.
EF, Hodgson SF, Eastell R, et al. Cancellous bone remodeling in type
I.postmenopausal) osteoporosis: quantitative assessment of rates of
formation, resorption, and bone loss at tissue and cellular levels. J
Bone Miner Res 1990;5:311-319.
B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in
postmenopausal women with osteoporosis treated with raloxifene: results
from a 3-year randomized clinical trial. Multiple Outcomes of
Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637-645.
B, Black DM, Nevitt MC, et al. Contribution of vertebral deformities to
chronic back pain and disability. The Study of Osteoporotic Fractures
Research Group. J Bone Miner Res 1992;7:449-456.
B, Block JE, Smith R, et al. An examination of the association between
vertebral deformities, physical disabilities, and psychosocial
problems. Maturitas 1988;10: 283-296.
AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain
reduction and improvement in functional mobility after percutaneous
polymethylmethacrylate vertebroplasty retrospective report of 245
cases. Radiology 2003;226:366-372.
KG, Cummings SR, Black D, et al. Simple measurement of femoral geometry
predicts hip fracture: the study of osteoporotic fractures. J Bone
Miner Res 1993;8: 1211-1217.
DT, Zhang Y, Hannan MT, et al. The effect of postmenopausal estrogen
therapy on bone density in elderly women. N Engl J Med
1993;329:1141-1146.
D, Weber P, Willett WC, et al. Vitamin K intake and hip fractures in
women: a prospective study. Am J Clin Nutr 1999;69:74-79.
L, Warner M. Injury Chartbook, Health, United States, 1996-1997,
Statistics. Hyattsville, MD: National Centre for Health and Statistics,
1997.
MA, Herscovici D Jr, DiPasquale TG, et al. A comparison of open
reduction and internal fixation and primary total elbow arthroplasty in
the treatment of intraarticular distal humerus fractures in women older
than age 65. J Orthop Trauma 2003;17: 473-480.
P, Deramond H, Rosat P, et al. [Preliminary note on the treatment of
vertebral angioma by percutaneous acrylic vertebroplasty].
Neurochirurgie 1987;33:166-168.
JC, Goldgar D. Treatment of postmenopausal osteoporosis with high doses
of synthetic calcitriol. A randomized controlled study. Ann Intern Med
1990;113: 649-655.
JC, Riggs BL, Recker RR, et al. The effect of calcitriol on patients
with postmenopausal osteoporosis with special reference to fracture
frequency. Proc Soc Exp Biol Med 1989;191:287-292.
MM, Robertson MC, Campbell AJ. Exercise in preventing falls and
fall-related injuries in older people: a review of randomised
controlled trials. Br J Sports Med 2000;34:7-17.
AR, Lane JM, Glaser D, et al. Alternatives to autogenous bone graft:
efficacy and indications. J Am Acad Orthop Surg 1995;3:1-8.
HK, Cann CE, Ettinger B, et al. Quantitative computed tomography of
vertebral spongiosa: a sensitive method for detecting early bone loss
after oophorectomy. Ann Intern Med 1982;97:699-705.
P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism
and prediction of fracture in elderly women. J Bone Miner Res
2004;19:386-393. Epub 2003 Dec 22.
LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing
falls in elderly people. Cochrane Database Syst Rev 2003:CD000340.
WJ, Avenell A, Henry DA, et al. Vitamin D and vitamin D analogues for
preventing fractures associated with involutional and postmenopausal
osteoporosis. Cochrane Database Syst Rev 2001:CD000227.
CC. Quantitative ultrasound techniques for the assessment of
osteoporosis: expert agreement on current status. The International
Quantitative Ultrasound Consensus Group. J Bone Miner Res
1997;12:1280-1288.
WC, Ooms ME, Bezemer PD, et al. Different risk profiles for hip
fractures and distal forearm fractures: a prospective study. Osteoporos
Int 1996;6:427-431.
WC, Ooms ME, Hofstee HM, et al. Falls in the elderly: a prospective
study of risk factors and risk profiles. Am J Epidemiol
1996;143:1129-1136.
F, Brazier M, Kamel S, et al. Effects on bone mineral density of
calcium and vitamin D supplementation in elderly women with vitamin D
deficiency. Joint Bone Spine 2003;70:203-208.
IA, Da Fonseca K, Hillmeier J, et al. Reduction of pain and fracture
incidence after kyphoplasty: 1-year outcomes of a prospective
controlled trial of patients with primary osteoporosis. Osteoporos Int
2005;16:2005-2012.
AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for
secondary prevention of low-trauma fractures in elderly people
(Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised
placebo-controlled trial. Lancet 2005; 365:1621-1628.
SF, Reid DM, Blake G, et al. Reduced bone density and osteoporosis
associated with a polymorphic Sp1 binding site in the collagen type I
alpha 1 gene. Nat Genet 1996;14:203-205.
LA, Jarvik JG, Heagerty PJ, et al. INvestigational Vertebroplasty
Efficacy and Safety Trial (INVEST): a randomized controlled trial of
percutaneous vertebroplasty. BMC musculoskeletal disorders 2007;8:126.
SL, Bone HG, Ettinger MP, et al. Effect of recombinant human
parathyroid hormone(1-84) on vertebral fracture and bone mineral
density in postmenopausal women with osteoporosis: a randomized trial.
Ann Intern Med 2007;146:326-339.
SL, Dresner-Pollak R, Parker RA, et al. Diurnal variation of bone
mineral turnover in elderly men and women. Calcif Tissue Int
1997;60:419-423.
SL, Myers ER, Maitland LA, et al. Fall severity and bone mineral
density as risk factors for hip fracture in ambulatory elderly. JAMA
1994;271:128-133.
EW, Cauley JA, Seeley DG, et al. Physical activity and osteoporotic
fracture risk in older women. Study of Osteoporotic Fractures Research
Group. Ann Intern Med 1998;129:81-88.
JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of
hip fracture in women. The Northeast Hip Fracture Study Group. N Engl J
Med 1991;324:1326-1331.
JA, Schwarz DF, Wolfson V, et al. The impact of falls in an inner-city
elderly African American population. J Am Geriatr Soc 1992;40:673-678.
F, Stampfer MJ, Goldhaber SZ, et al. Prospective study of exogenous
hormones and risk of pulmonary embolism in women. Lancet
1996;348:983-987.
D, Welch V, Shea B, et al. Fluoride for treating postmenopausal
osteoporosis. Cochrane Database Syst Rev 2000:CD002825.
D, Dargent-Molina P, Schott AM, et al. Ultrasonographic heel
measurements to predict hip fracture in elderly women: the EPIDOS
prospective study. Lancet 1996; 348:511-514.
M, Overgaard K, Christiansen C. Does the Prevalence of Vertebral
Fractures Increase? Osteoporosis 1990. Copenhagen; 1990. p. 95.
ST, Gertz BJ, Genant HK, et al. The effect of short-term treatment with
alendronate on vertebral density and biochemical markers of bone
remodeling in early postmenopausal women. J Clin Endocrinol Metab
1993;76:1399-1406.
ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on
vertebral and nonvertebral fractures in women with postmenopausal
osteoporosis: a randomized controlled trial. Vertebral Efficacy With
Risedronate Therapy (VERT) Study Group. JAMA 1999;282:1344-1352.
ST, Watts NB, Jackson RD, et al. Four-year study of intermittent cyclic
etidronate treatment of postmenopausal osteoporosis: 3 years of blinded
therapy followed by one year of open therapy. Am J Med 1993;95:557-567.
R, Karlsson MK, Jonsson B, et al. Long-term morbidity and mortality
after a clinically diagnosed vertebral fracture in the elderly: a 12-
and 22-year follow-up of 257 patients. Calcif Tissue Int
2005;76:235-242.
R, Karlsson MK, Nilsson BE, et al. Prevalent vertebral deformities
predict increased mortality and increased fracture rate in both men and
women: a 10-year population-based study of 598 individuals from the
Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos
Int 2003;14:61-68.
R, Redlund-Johnell I, Mellstrom D, et al. Vertebral deformation in
urban Swedish men and women: prevalence based on 797 subjects. Acta
Orthop Scand 2001; 72:273-278.
WC, Myers ER, Morris JN, et al. Impact near the hip dominates fracture
risk in elderly nursing home residents who fall. Calcif Tissue Int
1993;52:192-198.
A, Kannus P, Sievanen H, et al. Randomised controlled trial of effect
of high-impact exercise on selected risk factors for osteoporotic
fractures. Lancet 1996;348: 1343-1347.
A, Oja P, Sievanen H, et al. Effect of two training regimens on bone
mineral density in healthy perimenopausal women: a randomized
controlled trial. J Bone Miner Res 1998;13:483-490.
M, Colditz GA, Stampfer MJ, et al. Caffeine, moderate alcohol intake,
and risk of fractures of the hip and forearm in middle-aged women. Am J
Clin Nutr 1991;54:157-163.
T, Grazier K, Kelsey J, Stauffer R. The Frequency of Occurrence,
Impact, and Cost of Selected Musculoskeletal Conditions in the United
States. Chicago: American Academy of Orthopaedic Surgeons, 1984.
D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with
alendronate in postmenopausal women under 60 years of age. Early
Postmenopausal Intervention Cohort Study Group. N Engl J Med
1998;338:485-492.
SL, Slemenda CW, Johnston CC Jr. Baseline measurement of bone mass
predicts fracture in white women. Ann Intern Med 1989;111:355-361.
J, Kröger H, Honkanen R, et al. Risk factors for perimenopausal
fractures. A prospective population-based study. Osteoporosis
International 1998;8(suppl 3):6.
JZ, Skugor M, Hangartner T, et al. Relation of nutrition, body
composition, and physical activity to skeletal development: a
cross-sectional study in preadolescent females. J Am Coll Nutr
1998;17:136-147.
KK, Lenora J, Gerdhem P, et al. Serial assessment of serum bone
metabolism markers identifies women with the highest rate of bone loss
and osteoporosis risk. J Clin Endocrinol Metab 2008;May 6.
RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation
and the risk of fractures. N Engl J Med 2006;354:669-683.
TL, Sievanen H, Khan KM, et al. Shifting the focus in fracture
prevention from osteoporosis to falls. BMJ.Clinical research ed
2008;336:124-126.
RM, Magnus JH, Fonnebo V. Physical activity and predisposition for hip
fractures: a review. Osteoporos Int 1997;7:503-513.
O, Gullberg B, Kanis JA, et al. Risk factors for hip fracture in
European women: the MEDOS Study. Mediterranean Osteoporosis Study. J
Bone Miner Res 1995;10: 1802-1815.
JA, Dawson A, Oden A, et al. Cost-effectiveness of preventing hip
fracture in the general female population. Osteoporos Int
2001;12:356-361.
JA, Delmas P, Burckhardt P, et al. Guidelines for diagnosis and
management of osteoporosis. The European Foundation for Osteoporosis
and Bone Disease. Osteoporos Int 1997;7:390-406.
JA, Oden A, Johnell O, et al. The burden of osteoporotic fractures: a
method for setting intervention thresholds. Osteoporos Int
2001;12:417-427.
P, Haapasalo H, Sankelo M, et al. Effect of starting age of physical
activity on bone mass in the dominant arm of tennis and squash players.
Ann Intern Med 1995; 123:27-31.
P, Niemi S, Parkkari J, et al. Hip fractures in Finland between 1970
and 1997 and predictions for the future. Lancet 1999;353:802-805.
P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly
people with use of a hip protector. N Engl J Med 2000;343:1506-1513.
M, Bass S, Seeman E. The evidence that exercise during growth or
adulthood reduces the risk of fragility fractures is weak. Best Pract
Res Clin Rheumatol 2001;15: 429-450.
MK, Linden C, Karlsson C, et al. Exercise during growth and bone
mineral density and fractures in old age. Lancet 2000;355:469-470.
C, Hillmeier J, Noldge G, et al. Treatment of painful vertebral
fractures by kyphoplasty in patients with primary osteoporosis: a
prospective nonrandomized controlled study. J Bone Miner Res
2005;20:604-612.
M, Ankersen L, Eriksen EF, et al. Demonstration of cellular aging and
senescence in serially passaged long-term cultures of human trabecular
osteoblasts. Osteoporos Int 1997;7:514-524.
PJ, Morrison N, Sambrook PN, et al. Genetics and osteoporosis: role of
the vitamin D receptor gene. Agents Actions 1994;42:i-ii.
TL, Slovik DM, Schoenfeld DA, et al. Quantitative digital radiography
versus dual photon absorptiometry of the lumbar spine. J Clin
Endocrinol Metab 1988;67: 839-844.
DP, Felson DT, Anderson JJ, et al. Hip fracture and the use of
estrogens in postmenopausal women. The Framingham Study. N Engl J Med
1987;317:1169-1174.
DP, Felson DT, Hannan MT, et al. Caffeine and the risk of hip fracture:
the Framingham Study. Am J Epidemiol 1990;132:675-684.
JM, Lorentzon M, Norjavaara E, et al. Pubertal timing predicts previous
fractures and BMD in young adult men: the GOOD study. J Bone Miner Res
2006;21: 790-795.
C, Verhaar H, Lampmann L, et al. VERTOS II: Percutaneous vertebroplasty
versus conservative therapy in patients with painful osteoporotic
vertebral compression fractures; rationale, objectives and design of a
multicenter randomized controlled trial. Trials 2007;8:33.
S, Inoue S, Hosoi T, et al. Association of bone mineral density with
polymorphism of the estrogen receptor gene. J Bone Miner Res
1996;11:306-311.
G, Thamsborg G, Bhatia H, et al. Quantitation of urinary
hydroxypyridinium cross-links from collagen by high-performance liquid
chromatography. Scand J Clin Lab Invest 1992;52:657-662.
P, Runnqvist K, Jonsson K, et al. Norian SRS versus external fixation
in redisplaced distal radial fractures. A randomized study in 40
patients. Acta Orthop Scand 1999;70:1-5.
K, Luukinen H, Laippala P, et al. Physiological factors and medications
as predictors of injurious falls by elderly people: a prospective
population-based study. Age Ageing 1996;25:29-38.
KJ, Hoehl JJ, Kummer FJ, et al. Distal femoral fixation: a
biomechanical comparison of the standard condylar buttress plate, a
locked buttress plate, and the 95-degree blade plate. J Orthop Trauma
1997;11:521-524.
H, Huopio J, Honkanen R, et al. Prediction of fracture risk using axial
bone mineral density in a perimenopausal population: a prospective
study. J Bone Miner Res 1995;10:302-306.
H, Tuppurainen M, Honkanen R, et al. Bone mineral density and risk
factors for osteoporosis: a population-based study of 1600
perimenopausal women. Calcif Tissue Int 1994;55:1-7.
BL, Knudsen JY, Jensen HK, et al. A sequence variation: 713-8delC in
the transforming growth factor-beta 1 gene has higher prevalence in
osteoporotic women than in normal women and is associated with very low
bone mass in osteoporotic women and increased bone turnover in both
osteoporotic and normal women. Bone 1997;20:289-294.
LE. Using functional loading to influence bone mass and architecture:
objectives, mechanisms, and relationship with estrogen of the
mechanically adaptive process in bone. Bone 1996;18(1 suppl):37S-43S.
EM, Chan HH, Woo J, et al. Normal ranges for vertebral height ratios
and prevalence of vertebral fracture in Hong Kong Chinese: a comparison
with American Caucasians. J Bone Miner Res 1996;11:1364-1368.
JB. Impacts in patients with hip fractures and in vitro study of the
padding effect: introduction of a hip protector. Acta Orthop Scand
1990;61:S239.
MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral
density, and risk of hip fracture: recognition of a major effect. BMJ
(Clin Res) 1997;315: 841-846.
WT, Leung SS, Ng MY, et al. Bone mineral content of two populations of
Chinese children with different calcium intakes. Bone Miner
1993;23:195-206.
IH, Dudeney S, Reinhardt MK, et al. Initial outcome and efficacy of
“kyphoplasty” in the treatment of painful osteoporotic vertebral
compression fractures. Spine 2001;26:1631-1638.
C, Ahlborg HG, Besjakov J, et al. A school curriculum-based exercise
program increases bone mineral accrual and bone size in prepubertal
girls: two-year data from the Pediatric Osteoporosis Prevention (POP)
study. J Bone Miner Res 2006;21: 829-835.
C, Alwis G, Ahlborg H, et al. Exercise, bone mass, and bone size in
prepubertal boys: one-year data from the pediatric osteoporosis
prevention study. Scand J Med Sci Sports 2007;17:340-347.
P, Graafmans WC, Ooms ME, et al. Vitamin D supplementation and fracture
incidence in elderly persons. A randomized, placebo-controlled clinical
trial. Ann Intern Med 1996;124:400-406.
SR, Ward JA, Williams P, et al. The effect of a 12-month exercise trial
on balance, strength, and falls in older women: a randomized controlled
trial. J Am Geriatr Soc 1995;43:1198-1206.
M, Mellstrom D, Ohlsson C. Age of attainment of peak bone mass is site
specific in Swedish men: the GOOD study. J Bone Miner Res
2005;20:1223-1227.
M, Swanson C, Andersson N, et al. Free testosterone is a positive,
whereas free estradiol is a negative, predictor of cortical bone size
in young Swedish men: the GOOD study. J Bone Miner Res
2005;20:1334-1341.
JC, Hayes WC. The use of quantitative computed tomography to estimate
risk of fracture of the hip from falls. J Bone Joint Surg Am
1990;72:689-700.
EG, Argueta R, Whitaker MD, et al. Pamidronate: an unrecognized problem
in gastrointestinal tolerability. Osteoporos Int 1994;4:320-322.
EG, Wahner HW, O’Fallon WM, et al. Treatment of postmenopausal
osteoporosis with transdermal estrogen. Ann Intern Med 1992;117:1-9.
H, Koski K, Honkanen R, et al. Incidence of injury-causing falls among
older adults by place of residence: a population-based study. J Am
Geriatr Soc 1995;43: 871-876.
H, Ljunghall S, Persson I, et al. Risk factors for fractures of the
distal forearm: a population-based case-control study. Osteoporos Int
1994;4:298-304.
H, Ljunghall S, Persson I, et al. Fracture of the distal forearm as a
forecaster of subsequent hip fracture: a population-based cohort study
with 24 years of follow-up. Calcif Tissue Int 1993;52:269-272.
JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of
coronary heart disease. N Engl J Med 2003;349:523-534.
PJ, Hott M, Modrowski D, et al. An uncoupling agent containing
strontium prevents bone loss by depressing bone resorption and
maintaining bone formation in estrogen-deficient rats. J Bone Miner Res
1993;8:607-615.
D, Johnell O, Wedel H. Meta-analysis of how well measures of bone
mineral density predict occurrence of osteoporotic fractures.
BMJ.Clinical research ed 1996; 312:1254-1259.
PW, Jones RG, Purves DA, et al. Commercial assays for serum osteocalcin
give clinically discordant results. Clin Chem 1994;40:358-363.
V, Kostial K, Simonovic I, et al. Bone status and fracture rates in two
regions of Yugoslavia. Am J Clin Nutr 1979;32:540-549.
RB, Barden HS, Bisek JP, et al. Dual-energy x-ray absorptiometry for
totalbody and regional bone-mineral and soft-tissue composition. Am J
Clin Nutr 1990; 51:1106-1112.
E, Selby P, Davies M, et al. Clodronate reduces vertebral fracture risk
in women with postmenopausal or secondary osteoporosis: results of a
double-blind, placebo-controlled 3-year study. J Bone Miner Res
2004;19:728-736.
E, Selby P, de Takats D, et al. Effects of clodronate on vertebral
fracture risk in osteoporosis: a 1-year interim analysis. Bone
2001;28:310-315.
M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal
bone loss in women without osteoporosis. A double-blind, randomized,
controlled trial. Alendronate Osteoporosis Prevention Study Group. Ann
Intern Med 1998;128: 253-261.
MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of
hip fracture in elderly women. Hip Intervention Program Study Group. N
Engl J Med 2001; 344:333-340.
D, Johnell O, Ljunggren O, et al. Free testosterone is an independent
predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J
Bone Miner Res 2006;21:529-535.
PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the
risk of vertebral fracture in women with postmenopausal osteoporosis. N
Engl J Med 2004; 350:459-468.
PJ, Sebert JL, Reginster JY, et al. Fluoride salts are no better at
preventing new vertebral fractures than calcium-vitamin D in
postmenopausal osteoporosis: the FAVO Study. Osteoporos Int 1998;8:4-12.
PJ, Slosman DO, Delmas PD, et al. Strontium ranelate: dose-dependent
effects in established postmenopausal vertebral osteoporosis: a 2-year
randomized placebo controlled trial. J Clin Endocrinol Metab
2002;87:2060-2066.
HE, Tverdal A, Falch JA. Risk factors for hip fracture in middle-aged
Norwegian women and men. Am J Epidemiol 1993;137:1203-1211.
C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a
marker of osteoclast function. Calcif Tissue Int 1982;34:285-290.
A, Toksvig-Larsen S, Maltarello MC, et al. A comparison of
hydroxyapatite-coated, titanium-coated, and uncoated tapered
external-fixation pins. An in vivo study in sheep. J Bone Joint Surg Am
1998;80:547-554.
L, Charles P, Bekker PJ, et al. Risedronate increases bone mass in an
early postmenopausal population: two years of treatment plus one year
of follow-up. J Clin Endocrinol Metab 1998;83:396-402.
G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro
and in rodents by statins. Science 1999;286:1946-1949.
AH, Baker SP, Van Natta ML, et al. Risk factors associated with falls
and injuries among elderly institutionalized persons. Am J Epidemiol
1991;133:1179-1190.
RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone
(1-34) on fractures and bone mineral density in postmenopausal women
with osteoporosis. N Engl J Med 2001;344:1434-1441.
MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the
study of osteoporotic fractures. The Study of Osteoporotic Fractures
Research Group. J Am Geriatr Soc 1993;41:1226-1234.
T, Sambrook P, Kelly P, et al. Prediction of osteoporotic fractures by
postural instability and bone density. BMJ (Clin Res)
1993;307:1111-1115.
HK, Lundby L, Rasmussen K, et al. Alcohol decreases serum osteocalcin
in a dose-dependent way in normal subjects. Calcif Tissue Int
1990;46:173-178.
JW, Komar L, Cosman F, et al. Calcium potentiates the effect of
estrogen and calcitonin on bone mass: review and analysis. Am J Clin
Nutr 1998;67:18-24.
A, Karlsson C, Nyquist F, et al. Bone loss and fracture risk after
reduced physical activity. J Bone Miner Res 2005;20:202-207.
S, Cranney A, Wells GA, et al. Strontium ranelate for preventing and
treating postmenopausal osteoporosis. Cochrane Database Syst Rev
2006:CD005326.
TW, Felsenberg D, Varlow J, et al. The prevalence of vertebral
deformity in european men and women: the European Vertebral
Osteoporosis Study. J Bone Miner Res 1996;11:1010-1018.
KJ, Bengner U, Johnell O, et al. Increasing age-adjusted risk of
fragility fractures: a sign of increasing osteoporosis in successive
generations? Calcif Tissue Int 1989;44: 157-167.
H, Shiraki M. Clinical evaluation of menatetrenone in the treatment of
involutional osteoporosis. IVth International Symposium on
Osteoporosis, Hong Kong, 1993.
H, Shiraki M, Hayashi T, Nakamura T. Reduced occurrence of vertebral
crush fractures in senile osteoporosis treated with 1 alpha
(OH)-vitamin D3. Bone Miner 1987;3:47-52.
A, Chao A, Ross RK, et al. Exercise and other factors in the prevention
of hip fracture: the Leisure World study. Epidemiology 1991;2:16-25.
E, Wells G, Shea B, et al. Meta-analyses of therapies for
postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of
vitamin D treatment in preventing osteoporosis in postmenopausal women.
Endocrine reviews 2002;23:560-569.
AM. Age-related structural changes in trabecular and cortical bone:
cellular mechanisms and biomechanical consequences. Calcif Tissue Int
1984;36(suppl 1): S123-S128.
MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip
fractures in the elderly. Cochrane Database Syst Rev 2004:CD001255.
J, Kannus P, Heikkila J, et al. Energy-shunting external hip protector
attenuates the peak femoral impact force below the theoretical fracture
threshold: an in vitro biomechanical study under falling conditions of
the elderly. J Bone Miner Res 1995; 10:1437-1442.
Writing Group. Effects of hormone therapy on bone mineral density:
results from the Postmenopausal Estrogen/Progestin Interventions (PEPI)
trial. The Writing Group for the PEPI. JAMA 1996;276:1389-1396.
BC. Falls among the elderly: a review of the methods and conclusions of
epidemiologic studies. J Am Geriatr Soc 1982;30:367-371.
FM, Ho E, Campbell-Hupp M, et al. Early radiographic and clinical
results of balloon kyphoplasty for the treatment of osteoporotic
vertebral compression fractures. Spine 2003;28:2260-2267.
WT, Veldhuizen AG, The B, et al. Percutaneous vertebroplasty as a
treatment for osteoporotic vertebral compression fractures: a
systematic review. Eur Spine J 2006; 15:1749-1758.
HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled,
randomized trial of the effects of alendronate on bone density and
fracture risk in postmenopausal women with low bone mass: results of
the FOSIT study. Foxamax International Trial Study Group. Osteoporos
Int 1999;9:461-468.
G, Atkinson EJ, O’Fallon WM, et al. Determinants of reduced survival
following hip fractures in men. Clin Orthop 1995 Oct:260-265.
J, Cockayne S, King C, et al. Randomised controlled trial of calcium
and supplementation with cholecalciferol (vitamin D3) for prevention of
fractures in primary care. BMJ (Clin Res) 2005;330:1003.
A, Furner S, Rice D. Musculoskeletal Condition in the United States.
Park Ridge, IL: American Academy of Orthopaedic Surgeons, 1992.
MA, Hadley EC, Hornbrook MC, et al. The effects of exercise on falls in
elderly patients. A preplanned meta-analysis of the FICSIT Trials.
Frailty and Injuries: Cooperative Studies of Intervention Techniques.
JAMA 1995;273:1341-1347.
EY. Once-monthly ibandronate for postmenopausal osteoporosis: review of
a new dosing regimen. Clinical therapeutics 2006;28:475-490.
RD, Singrakhia MD. Painful osteoporotic vertebral fracture.
Pathogenesis, evaluation, and roles of vertebroplasty and kyphoplasty
in its management. J Bone Joint Surg Am 2003;85A:2010-2022.
PS. Tibial condylar fractures. Impairment of knee joint stability as an
indication for surgical treatment. J Bone Joint Surg Am
1973;55:1331-1350.
P, Fledelius C, Rosenquist C, et al. High bone turnover is associated
with low bone mass in both pre- and postmenopausal women. Bone
1996;19:291-298.
NF, Chan JK, Thamer M, et al. Medical expenditures for the treatment of
osteoporotic fractures in the United States in 1995: report from the
National Osteoporosis Foundation. J Bone Miner Res 1997;12:24-35.
RR, Hinders S, Davies KM, et al. Correcting calcium nutritional
deficiency prevents spine fractures in elderly women. J Bone Miner Res
1996;11:1961-1966.
J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of
risedronate on vertebral fractures in women with established
postmenopausal osteoporosis. Vertebral Efficacy with Risedronate
Therapy (VERT) Study Group. Osteoporos Int 2000;11:83-91.
JY, Wilson KM, Dumont E, et al. Monthly oral ibandronate is well
tolerated and efficacious in postmenopausal women: results from the
monthly oral pilot study. J Clin Endocrinol Metab 2005;90:5018-5024.
IR, Ames RW, Evans MC, et al. Long-term effects of calcium
supplementation on bone loss and fractures in postmenopausal women: a
randomized controlled trial. Am J Med 1995;98:331-335.
IR, Wattie DJ, Evans MC, et al. Continuous therapy with pamidronate, a
potent bisphosphonate, in postmenopausal osteoporosis. J Clin
Endocrinol Metab 1994;79: 1595-1599.
D, MacKenzie E, Associates. Cost of Injury in the United States: A
report to Congress. San Francisco: University of California, 1989.
BL, Hodgson SF, O’Fallon WM, et al. Effect of fluoride treatment on the
fracture rate in postmenopausal women with osteoporosis. N Engl J Med
1990;322:802-809.
BL, Khosla S, Melton LJ 3rd. A unitary model for involutional
osteoporosis: estrogen deficiency causes both type I and type II
osteoporosis in postmenopausal women and contributes to bone loss in
aging men. J Bone Miner Res 1998;13:763-773.
BL, Melton LJ 3rd. Clinical review 8: Clinical heterogeneity of
involutional osteoporosis: implications for preventive therapy. J Clin
Endocrinol Metab 1990;70: 1229-1232.
CM, Page RS, Hill RM, et al. Primary hemiarthroplasty for treatment of
proximal humeral fractures. J Bone Joint Surg Am 2003;85A:1215-1223.
C, Carlsson A, Johnell O, et al. A prospective randomised trial of
internal fixation versus arthroplasty for displaced fractures of the
neck of the femur. Functional outcome for 450 patients at 2 years. J
Bone Joint Surg Br 2002;84B:183-188.
C, Sernbo I, Johnell O, et al. Incidence of hip fractures in Malmo,
Sweden, 1992-1995. A trend-break. Acta Orthop Scand 1999;70:19-22.
PD, Davis JW, Epstein RS, et al. Pre-existing fractures and bone mass
predict vertebral fracture incidence in women. Ann Intern Med
1991;114:919-923.
JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen
plus progestin in healthy postmenopausal women: principal results From
the Women’s Health Initiative randomized controlled trial. JAMA
2002;288:321-333.
OP, Kivela SL, Honkanen R, et al. Incidence of falling injuries leading
to medical treatment in the elderly. Public Health 1991;105:373-386.
A, Sharpe FE, Ebramzadeh E, et al. Factors influencing the outcome of
closed tibial fractures treated with functional bracing. Clin Orthop
1995;8-24.
T, Bone HG, Crepaldi G, et al. Therapeutic equivalence of alendronate
70 mg once-weekly and alendronate 10 mg daily in the treatment of
osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano)
2000;12:1-12.
E, Tsalamandris C, Formica C, et al. Reduced femoral neck bone density
in the daughters of women with hip fractures: the role of low-peak bone
density in the pathogenesis of osteoporosis. J Bone Miner Res
1994;9:739-743.
M. The development of a passive protective device for the elderly to
prevent hip fractures from accidental falls. Adv Bioeng 1992;22:505-208.
B, Bonaiuti D, Iovine R, et al. Cochrane Review on exercise for
preventing and treating osteoporosis in postmenopausal women. Eur
Medicophys 2004;40:199-209.
B, Wells G, Cranney A, et al. Calcium supplementation on bone loss in
postmenopausal women. Cochrane Database Syst Rev 2004:CD004526.
B, Wells G, Cranney A, et al. Meta-analyses of therapies for
postmenopausal osteoporosis. VII. Meta-analysis of calcium
supplementation for the prevention of postmenopausal osteoporosis.
Endocrine Rev 2002;23:552-559.
A, Gruber W, Baldwin M, et al. The effect of multidimensional exercises
on balance, mobility, and fall risk in community-dwelling older adults.
Phys Ther 1997;77:46-57.
CD. Consensus Development Statement. Who are candidates for prevention
and treatment for osteoporosis? Osteoporos Int 1997;7:1-6.
P, Cummings SR, Genant HK, et al. Morphometric X-ray absorptiometry of
the spine: correlation in vivo with morphometric radiography. Study of
Osteoporotic Fractures Research Group. Osteoporos Int 1994;4:238-244.
T, Thamsborg G, Steiniche T, et al. Effect of intermittent cyclical
etidronate therapy on bone mass and fracture rate in women with
postmenopausal osteoporosis. N Engl J Med 1990;322:1265-1271.
L, Mellstrom D, Ljunggren O, et al. IL6 and IL1B polymorphisms are
associated with fat mass in older men: the MrOS Study Sweden. Obesity
(Silver Spring) 2008;16:710-713.
RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of
vertebral compression fractures: an updated systematic review and
meta-analysis. Eur Spine J 2007;16:1085-1100.
RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for
vertebral compression fractures: a comparative systematic review of
efficacy and safety. Spine 2006;31:2747-2755.
D, Lyle RM, McCabe GP, et al. Dietary calcium, protein, and phosphorus
are related to bone mineral density and content in young women. Am J
Clin Nutr 1998;68:749-754.
ME. Factors associated with serious injury during falls by ambulatory
nursing home residents. J Am Geriatr Soc 1987;35:644-648.
ME, Doucette J, Claus E, et al. Risk factors for serious injury during
falls by older persons in the community. J Am Geriatr Soc
1995;43:1214-1221.
ME, Doucette JT, Claus EB. The contribution of predisposing and
situational risk factors to serious fall injuries. J Am Geriatr Soc
1995;43:1207-1213.
ME, Liu WL, Ginter SF. Mechanical restraint use and fall-related
injuries among residents of skilled nursing facilities. Ann Intern Med
1992;116:369-374.
ME, McAvay G, Claus E. Does multiple risk factor reduction explain the
reduction in fall rate in the Yale FICSIT Trial? Frailty and Injuries
Cooperative Studies of Intervention Techniques. Am J Epidemiol
1996;144:389-399.
ME, Speechley M, Ginter SF. Risk factors for falls among elderly
persons living in the community. N Engl J Med 1988;319:1701-1707.
DJ, Bell-Syer SE. Hormone replacement therapy and prevention of
nonvertebral fractures: a meta-analysis of randomized trials. JAMA
2001;285:2891-2897.
DJ, Bell-Syer SE. Hormone replacement therapy and prevention of
vertebral fractures: a meta-analysis of randomised trials. BMC
musculoskeletal disorders 2001; 2:7.
DJ, Campbell MK, Thomas RE, et al. Prediction of perimenopausal
fractures by bone mineral density and other risk factors. J Bone Miner
Res 1996;11:293-297.
H, Hearn TC, Schatzker J. The strength of plate fixation in relation to
the number and spacing of bone screws. J Orthop Trauma 1996;10:204-208.
E, Royet O, Wendling D. [Aetiologic features of osteoporosis in male
patients aged less than 50 years: study of 28 cases with a comparative
series of 30 patients over the age of 50.] Rev Med Interne
1998;19:479-485.
DP, Doll R, Khaw KT. Effect of four monthly oral vitamin
D3.cholecalciferol supplementation on fractures and mortality in men
and women living in the community: randomised double blind controlled
trial. BMJ (Clin Res) 2003;326:469.
AG, Burger H, Huang Q, et al. Relation of alleles of the collagen type
Ialpha1 gene to bone density and the risk of osteoporotic fractures in
postmenopausal women. N Engl J Med 1998;338:1016-1021.
AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body
fracture after percutaneous vertebroplasty in patients with
osteoporosis. Radiology 2003;226: 119-124.
O, Alborg HG, Duppe H, et al. Reduced training is associated with
increased loss of BMD. J Bone Miner Res 2005;20:906-912.
Meurs JB, Trikalinos TA, Ralston SH, et al. Large-scale analysis of
association between LRP5 and LRP6 variants and osteoporosis. JAMA
2008;299:1277-1290.
MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with
optimal pain medication treatment: short-term clinical outcome of
patients with subacute or chronic painful osteoporotic vertebral
compression fractures. The VERTOS study. AJNR 2007;28:555-560.
NB, Harris ST, Genant HK, et al. Intermittent cyclical etidronate
treatment of postmenopausal osteoporosis. N Engl J Med 1990;323:73-79.
M, Herlein S, Birnbaum K, et al. [Kyphoplasty—a new minimally invasive
treatment for repositioning and stabilising vertebral bodies.] Z Orthop
Ihre Grenzgeb 2003;141:406-411.
RD, Zhang H, Bronson DG. Experimental tibial plateau fractures
augmented with calcium phosphate cement or autologous bone graft. J
Bone Joint Surg Am 2003; 85A:222-231.
G, Cranney A, Peterson J, et al. Risedronate for the primary and
secondary prevention of osteoporotic fractures in postmenopausal women.
Cochrane Database Syst Rev 2008:CD004523.
GA, Cranney A, Peterson J, et al. Alendronate for the primary and
secondary prevention of osteoporotic fractures in postmenopausal women.
Cochrane Database Syst Rev 2008:CD001155.
GA, Cranney A, Peterson J, et al. Etidronate for the primary and
secondary prevention of osteoporotic fractures in postmenopausal women.
Cochrane Database Syst Rev 2008:CD003376.
Assessment of fracture risk and its application to screening for
postmenopausal osteoporosis. Report of a WHO Study Group. World Health
Organ Tech Rep Ser 1994; 843:1-129.
CA, Walsh K, Cooper C, et al. Dietary calcium, physical activity, and
risk of hip fracture: a prospective study. BMJ (Clin Res)
1989;299:889-892.
HW, Scheidt-Nave C, Kissling C, et al. Seasonal variation of
biochemical indexes of bone turnover: results of a population-based
study. J Clin Endocrinol Metab 1998; 83:68-75.
HW, Seibel MJ, Ziegler R. Comparison of total and bone-specific
alkaline phosphatase in patients with nonskeletal disorder or metabolic
bone diseases. Clin Chem 1996;42:1796-804.
S1L, Barnhart HX, Ellison GL, et al. The effect of Tai Chi Quan and
computerized balance training on postural stability in older subjects.
Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies on
Intervention Techniques. Phys Ther 1997;77: 371-381; discussion 82-84.
L, Whipple R, Derby C, et al. Balance and strength training in older
adults: intervention gains and Tai Chi maintenance. J Am Geriatr Soc
1996;44:498-506.
T, Turner CH, Peacock M, et al. Geometric structure of the femoral neck
measured using dual-energy x-ray absorptiometry. J Bone Miner Res
1994;9: 1053-1064.
