Principles of Complex Regional pain Syndrome
One – General Principles: Basics > Complications > 23 –
Principles of Complex Regional pain Syndrome
described a syndrome that occurred in patients who had suffered gunshot
injuries to major nerves.120 Noting that a leading feature was burning pain, he called the condition causalgia.
At the beginning of the 20th century, Paul Südeck, a clinician in
Hamburg, Germany, used the newly invented technique of roentgenology to
investigate patients with severe pain after injury.145,146
He described a posttraumatic pain syndrome with edema, trophic changes,
and osteoporosis. In 1979, the AO group advocated open reduction and
rigid internal fixation to prevent fracture disease, which was defined
as a combination of circulatory disturbance, inflammation, and pain as
a result of dysfunction of joints and muscles.121 In an intriguing vignette, Channon and Lloyd32
noted that finger stiffness after Colles fracture could be either
simple or associated with swelling and changes in hand temperature. In
the latter case, it did not respond well to physiotherapy. The modern
term for the syndrome described in different circumstances by these
researchers is complex regional pain syndrome, usually abbreviated as CRPS.
sudomotor dysfunction, contracture, and osteoporosis. It used to be
considered a rare, devastating complication of injury, caused by
abnormalities in the sympathetic nervous system (SNS) and seen mainly
in psychologically abnormal patients. Modern research is altering this
view radically. This review will specifically examine CRPS within the
context of orthopaedic trauma surgery. For this reason, the emphasis,
descriptions, and concepts differ slightly from those routinely found
in publications from the International Association for the Study of
Pain (IASP). It is important to appreciate that these apparent
differences are merely counterpoints. The theme is identical.
perception, which are mainly foreign to orthopaedic surgeons. They have
been codified by Merskey and Bogduk119 and because they will be used throughout this text, they are described here.
-
Allodynia
(literally “other pain”) is a painful perception of a stimulus that
should not usually be painful. Thus, for example, a patient will find
gentle stroking of the affected part painful. Allodynia differs from
referred pain, but allodynic pain can occur in areas other than the one
stimulated. There are several forms of allodynia:-
Mechanical (or tactile) allodynia implies pain in response to touch. It may be further subdivided into static mechanical allodynia, implying pain in response to light touch or pressure, and dynamic mechanical allodynia, where the pain occurs as a result of brushing.107
-
In thermal (hot or cold) allodynia, the pain is caused by mild changes in skin temperature in the affected area.
-
-
Hyperalgesia
is an increased sensitivity to pain, which may be caused by damage to
nociceptors or peripheral nerves. Thus, the patient finds gentle
touching with a pin unbearably painful. Hyperalgesia is usually
experienced in focal, discrete areas, typically associated with injury.
Focal hyperalgesia may be divided into two subtypes:-
Primary hyperalgesia describes pain sensitivity that occurs directly in the damaged tissues.
-
Secondary hyperalgesia describes pain sensitivity that occurs in surrounding undamaged tissues.
Rarely, hyperalgesia is seen in a more diffuse, bodywide form. -
-
Hyperpathia
is a temporal and spatial summation of an allodynic or hyperalgesic
response. Thus, the patient finds gentle touching painful, but
repetitive touching either on the same spot or on another part of the
affected limb becomes increasingly unbearable and the pain continues
for a period (up to 30 minutes) after the stimulus has been withdrawn.
In severe cases, the pain may be accentuated by unusual and extraneous
things such as the sudden noise of a door shutting or a draft of cold
air.
that these patients are not malingering or mad. These are absolutely
real perceptions of pain.
much confusion that surrounds this condition. In the past, CRPS was
diagnosed using a variety of nonstandardized and idiosyncratic
diagnostic systems derived solely from the authors’ clinical
experiences, none of which achieved wide acceptance. The condition was
given a number of synonyms (Table 23-1) reflecting site affected, cause and clinical features. During the American Civil War, Mitchell et al.120 noted the burning nature of pain following nerve trauma and described this as causalgia (from the Greek “burning pain”). In contrast, in the 1900s, Südeck145,146 investigated conditions characterized by severe osteoporosis, including some cases of CRPS. The condition was named Südeck’s atrophy by Nonne in 1901.123 Leriche99,100 demonstrated that sympathectomy could alter the clinical features associated with posttraumatic osteoporosis, and De Takats38 first suggested reflex dystrophy in 1937. Evans46 introduced the term reflex sympathetic dystrophy,
based on the theory (following Leriche’s observations) that sympathetic
hyperactivity was involved in the pathophysiology, and this term was
popularized by Bonica.18 In 1940, Homans85 proposed minor causalgia to imply a relationship between Mitchell et al.’s causalgia, renamed major causalgia, and similar conditions arising without direct nerve injury. Causalgic state37 and mimo causalgia126 followed to add to the confusion. Today the term causalgia is reserved for Mitchell et al.’s original use, in which a major nerve injury produces burning pain.141
|
TABLE 23-1 Synonyms for Complex Regional Pain Syndrome
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
consists of pain, hyperpathia, and allodynia, which are relieved by
selective sympathetic blockade. The relationship between CRPS and
sympathetically maintained pain is disputed.141
In CRPS a proportion of the pain is usually sympathetically maintained
and is therefore relieved by sympathetic blockade. However, in CRPS a
process is also taking place that leads to initial tissue edema
followed by severe contracture. This is not an inevitable part of
sympathetically maintained pain.91
Sympathetically maintained pain is not a particularly helpful concept
for the orthopaedic surgeon; however, it will be explored further when
the etiology of CRPS is considered.
interest. The International Association for the Study of Pain (IASP)
has undertaken a major work in analyzing the features of CRPS and
reclassifying the condition.119 A
brief history of this work will help to understand the current
position. The name of the condition was changed to complex regional
pain syndrome (CRPS) at a consensus workshop in Orlando, Florida, in
1994,16,141 and a new set of standardized diagnostic criteria was established119 (Table 23-2). To complement the diagnostic criteria, a broad description of CRPS was offered later22,80:
characterized by a continuing (spontaneous and/or evoked) regional pain
that is seemingly disproportionate in time or degree to the usual
course of any known trauma or other lesion. The pain is regional
(not
in a specific nerve territory or dermatome) and usually has a distal
predominance of abnormal sensory, motor, sudomotor, vasomotor, and/or
trophic findings, including osteoporosis. The syndrome shows variable
progression over time.
|
TABLE
23-2 The Original International Association for the Study of Pain Diagnostic Criteria for Complex Regional Pain Syndrome (CRPS) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||
the cause was believed to be damage to a major nerve, and CRPS type 1,
where it was not.
must be clinical and therefore precise descriptions of symptoms and
signs acquire great importance. Classic descriptions of the condition
describe three stages occurring sequentially.17,38,44,68,137,138 Modern evidence, however, suggests that CRPS does not invariably pass through these stages13,157,173,174
and supports the clinical impression that this evolution is seen in
more severe cases (as might be expected from historic series).
Nevertheless, the classic descriptions provide the greatest information
concerning the clinical features, and the description that follows
draws on these and will therefore refer to the staging system where it
is helpful to the description.
through the three classic stages, it is essential to grasp the concept
that CRPS is a biphasic condition with early swelling and vasomotor
instability giving way over a variable timescale to late contracture
and joint stiffness.44 The hand and foot are most frequently involved, although involvement of the knee is increasingly recognized.35,36,93 The elbow is rarely affected, whereas shoulder disease is common and some cases of frozen shoulder are probably CRPS.143 The hip is affected in transient osteoporosis of pregnancy.
precipitating trauma, although the delay may be greater. Antecedent
trauma is not essential but within an orthopaedic context it is almost
invariable.44 As the direct effects of injury subside, a new diffuse, unpleasant, neuropathic pain arises.168
Neuropathic pain is pain that occurs without any precipitating noxious
stimulus, and spontaneous or burning pain, hyperalgesia, allodynia, and
hyperpathia are common but not universal features.119
Pain is unremitting (although sleep is often unaffected), worsening and
radiating with time. The pain may be increased by dependency of the
limb, physical contact, emotional upset, or even by extraneous factors
such as a sudden loud noise or a blast of cold air.
although this is less marked with more proximal CRPS. The classic
description of the temporal evolution of the condition divides the
early phase of CRPS into two stages depending on the type of the
vasomotor instability.44 In this
description, initially the limb is dry, hot, and pink (vasodilated,
Stage 1) but after a variable period of days to weeks, it becomes blue,
cold, and sweaty (vasoconstricted, Stage 2). As noted, this classic
evolution is rarely seen. Most commonly, especially in more mild cases,
the vasomotor instability is an increase in temperature sensitivity,
with variable abnormality of sweating. Alternatively, some patients
remain substantially vasodilated,
while others are vasoconstricted with no history of vasodilatation.13,21,157,175
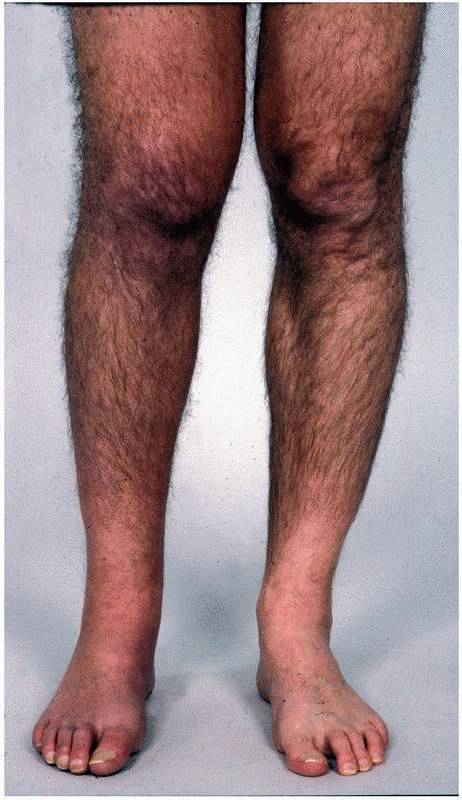 |
|
FIGURE 23-1
A patient with early complex regional pain syndrome type 1 affecting the leg. Note the swelling of the leg and the discoloration of the shin. |
particularly where the distal part of the limb is affected. Initially,
the edema is simple tissue swelling and may be overcome by physical
therapy and elevation, if the patient will permit. With time, however
(in the classic description, passing from stage 1 to stage 2), the
edema becomes more fixed and indurated with coalescence of tissue
planes and structures.
mobility is caused by swelling and pain combined with an apparent
inability to initiate movement or state of neglect or denial with
respect to the limb.27,28,29,61,62 Weakness, dystonia, spasms, tremor, and myoclonus have also been reported15,56,106,137;
however, these are not usually prominent within an orthopaedic context.
As the early phase progresses, loss of joint mobility will increasingly
be the result of the development of contracture. Only if the disease
can be halted in the early phase before fixed contracture has occurred
can complete resolution occur.
which affects every tissue. The skin is thinned and joint creases and
subcutaneous fat disappear. Hairs become fragile, uneven, and curled,
while nails are pitted, ridged, brittle, and discolored brown. Palmar
and plantar fascias thicken and contract simulating Dupuytren disease.106
Tendon sheaths become constricted, causing triggering and increased
resistance to movement. Muscle contracture combined with tendon
adherence leads to reduced tendon excursion. Joint capsules and
collateral ligaments become shortened, thickened, and adherent, causing
joint contracture.
is very variable. Within orthopaedic practice, the large majority of
patients who demonstrate the features of the early phase of CRPS after
trauma will not go on to develop severe late phase contracture,
although a significant proportion will show chronic subclinical
contracture.106
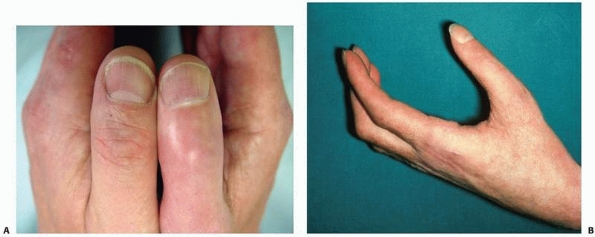 |
|
FIGURE 23-2 The late phase of complex regional pain syndrome (CRPS). A.
Detail of the thumbs of a patient with late CRPS type 1 of the right hand. There is spindling of the digit particularly distally. The nail is excessively ridged and is discolored. B. The hand of a patient with late CRPS type 1. The patient is trying to make a fist. Note the digital spindling and extension contractures with loss of joint creases |
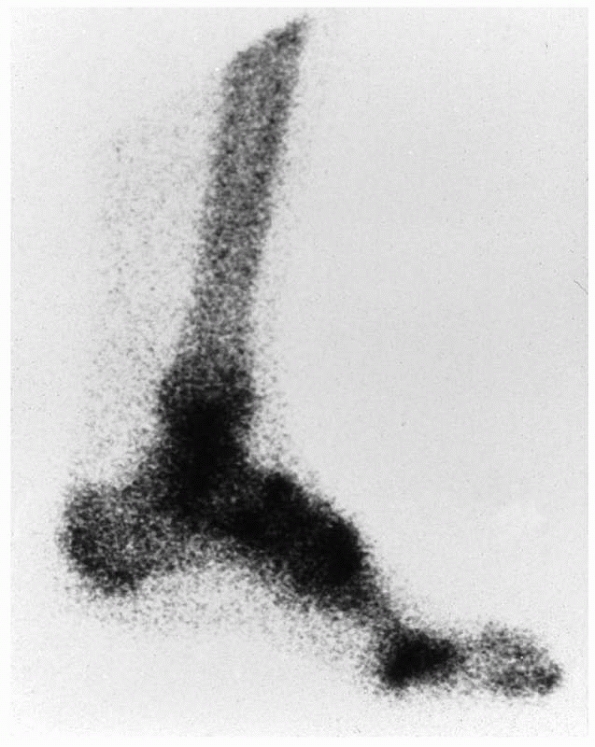 |
|
FIGURE 23-3
Bone scan changes in complex regional pain syndrome (CRPS). The delayed phase of a bone scan of a patient with early CRPS type 1 of the lower leg. There is increased uptake throughout the affected region. The bone scan will usually revert to normal after 6 months. |
Later, the bone scan returns to normal and there are radiographic
features of rapid bone loss: visible demineralization with patchy,
subchondral or subperiosteal osteoporosis, metaphyseal banding, and profound bone loss98 (Fig. 23-4). Despite the osteoporosis, fracture is uncommon, presumably because the patients protect the painful limb very effectively.
 |
|
FIGURE 23-4 Radiographic features of complex regional pain syndrome (CRPS). A.
Oblique radiograph of a patient with CRPS type 1 of the foot. There is patchy osteoporosis with accentuation of the osteoporosis beneath the joints. B. Profound osteoporosis in a patient with late severe CRPS type 1 affecting the hand. |
are extremely rare. Thus, severe, chronic CRPS associated with severe
contracture is uncommon with a reported prevalence of less than 2% in
retrospective series.8,75,102,108,129
In contrast, prospective studies designed to look specifically for the
early features of CRPS show that they occur after 30% to 40% of every
fracture and surgical trauma (e.g., total knee replacement),2,3,7,13,14,51,81,135,139 where the features of CRPS have been actively sought. Furthermore, statistically, the features tend to occur together.3 These common early cases of CRPS are usually not specifically diagnosed.139 They resolve substantially either spontaneously or with standard treatment by physical therapy and analgesia within 1 year.13,14,105,139
Some features, particularly stiffness, may remain, suggesting that CRPS
may be responsible for significant long-term morbidity even when mild.5,18 The truly intriguing question is, if CRPS is so common, why is it not a universal finding after trauma or orthopaedic surgery?
identical stimulus in a different limb does not cause it. The incidence
is not changed by treatment method and open anatomic reduction and
rigid internal fixation does not abolish it.135 It is unclear whether injury severity or quality of fracture reduction alters the incidence.3,14 There is, however, an association with excessively tight casts55 and there may be a genetic predilection.41,94,96,111,112 The following etiologies have been proposed:
Most orthopaedic clinicians immediately recognize a “Sudecky”
patient—that is, broadly speaking, a patient who appears to the
clinician to be somebody who is likely to fare poorly after surgical
intervention or trauma, perhaps because of to their inability to
cooperate fully with physical therapy. In fact, the literature fails to
identify this sort of patient and the evidence does not support the
notion that CRPS is primarily psychological.25 Studies of premorbid personality show no consistent abnormality.122,172 Most patients are psychologically normal,158 although emotional lability, low pain threshold,39 hysteria,127 and depression144 have been reported. There is an association with antecedent psychological stress,20,25,63,64,65,156 which probably exacerbates pain in CRPS, as in other diseases.23
It seems likely that the severe chronic pain of CRPS causes depression
and that a “Sudecky” type of patient who develops CRPS is at risk of a
poor outcome because they will not mobilize in the face of pain.
threshold nociceptors, thus preventing tissue damage. Neuropathic pain
in CRPS occurs without appropriate stimulus and has no protective
function. However, injured peripheral nerve fibers undergo cellular
changes, which cause usually innocuous tactile inputs to stimulate the
dorsal horn cells via A-β fibers from low-threshold mechanoreceptors,
causing allodynia in CRPS 2.92,167
Similar C-nociceptor dysfunction explains causalgia. Furthermore,
axonal injury prevents nerve growth factor transport, which is
essential for normal nerve function.104,168 In CRPS 1, covert nerve lesions with artificial synapses have been postulated.43
These “ephases” have not been demonstrated and are unnecessary since
inflammatory mediators released by the initial trauma (and possibly
retained due to a failure of free radical clearance), can sensitize
nociceptors to respond to normally innocuous stimuli.168
include abnormalities in skin blood flow, temperature regulation and
sweating, and edema. However, SNS activity is not usually painful.88,89 In CRPS, however, some pain (termed sympathetically maintained pain [SMP]141)
is SNS dependent. This accounts for spontaneous pain and allodynia,
which may therefore be relieved by stellate ganglion blockade130 and then restored by noradrenalin injection.1,148
Furthermore, there is an abnormal difference in cutaneous sensory
threshold between the limbs, which is reversed by sympathetic blockade,54,57,131,132 while increasing sympathetic activity worsens pain.90
the body’s reaction to injury. After partial nerve division, injured
and uninjured somatic axons express α-adrenergic receptors30 and sympathetic axons come to surround sensory neuron cell bodies in dorsal root ganglia.117,161,168 These changes, which may be temporary,148,159,160
make the somatic sensory nervous system sensitive to circulating
catecholamines and norepinephrine released from postganglionic
sympathetic terminals.
leading to gross scarring. For this reason, the major differential
diagnoses within an orthopaedic context are occult causes of
inflammation such as soft tissue infection or stress fracture. Indeed,
CRPS is associated with inflammatory changes including macromolecule
extravasation125 and reduced oxygen consumption.71,149 In animals, infusion of free radical donors causes a CRPS-like state,150 and amputated human specimens with CRPS show basementmembrane thickening consistent with overexposure to free radicals.151 These considerations suggest that CRPS is an exaggerated local inflammatory response to injury.72,73
In other words, on this hypothesis, CRPS represents a local form of the
systemic free radical disease that causes adult respiratory distress
syndrome and multiple organ failure after severe trauma. This concept
is supported by evidence that the free radical scavenger vitamin C is
effective prophylaxis against post-traumatic CRPS.170,171
changes in early CRPS is a primary capillary imbalance causing stasis,
extravasation, and consequent local tissue anoxia.48,49,114,134
It is a common clinical observation that patients who appear to be at
risk of developing CRPS are unable or unwilling to cooperate with
physical therapy to mobilize their limb after trauma or orthopaedic
surgery. Indeed, undue immobilization has traditionally been believed
to be at least an important contributory factor in the generation of
CRPS or even the sole cause.9,47,121,163
afferent sensory perception but only recently has the possibility of
abnormal efferent motor function been systematically explored.
Classically, it was believed that the “immobile RSD limb” was guarded
by the patient to prevent inadvertent painful movement or sensory
contact.44,60
In fact, CRPS is associated with an abnormality of motor function that
is often overlooked partially because of patient embarrassment and
partly because in the past it has been labeled as “hysterical.”33,152 In 1990, Schwartzman and Kerrigan137
reported a subgroup of CRPS patients with a variety of motor disorders
and a minority of patients with CRPS demonstrate obvious dystonia or
spasms.10,45,110,113
A prospective study of 829 CRPS patients showed that abnormalities of
motor function were reported by 95%, varying from weakness to
incoordination and tremor.30
Objective testing in small numbers of patients shows that CRPS patients
have impaired grip force coordination, target reaching, and grasping.136,164
reasons for the lack of movement in CRPS. Patients demonstrate evidence
of “neglect” of the affected limb, similar to that seen after parietal
lobe stroke. When asked about moving the limb, statements are made such
as “my limb feels disconnected from my body” and “I need to focus all
my mental attention and look at the limb in order for it to move the
way I want… .”59 Another study
revealed bizarre perceptions about a body part including a desperate
desire for amputation. There was a mismatch between limb sensation and
appearance with mental erasure of the affected part. These authors
suggested the term “body perception disturbance” rather than “neglect”
to describe this phenomenon. 101 There appears to be a central sensory
confusion, in that when a nonnoxious stimulus is provided that the
patient finds painful due to allodynia, the patient is unable to
determine whether it is truly painful, and by impairing integration
between sensory input and motor output, movement is impaired.83,115
limb and find it difficult to initiate or accurately direct movement
and there is a mismatch between sensation, perception, and movement.29,60,152
Failure to use the limb appears to relate to this rather than the
traditional view of learned pain avoidance behavior in response to
allodynia. Whatever the exact cause, failure of mobilization may be
central to the etiology of CRPS because all the features of phase 1
CRPS, except pain, are produced in volunteers after a period of cast
immobilization.27,28,29 This may be explained by the fact that activity-dependent gene function is common in the nervous system.168 and normal tactile and proprioreceptive input are necessary for correct central nerve signal processing.103
The rationale for MVF is restoration of the congruence between sensory
and motor information, and it was originally used for the treatment of
phantom limb pain.133 The patients
are instructed to exercise both the unaffected and the affected limb.
However, a mirror is placed so that they cannot see the affected limb,
and when they think they are looking at it, they are actually observing
the mirror image of their normal limb. As might be expected, MVF
resulted in improvement in range of movement; however, in addition in
early CRPS, MVF also abolished or substantially improved pain and
vasomotor instability.150
to understand the recent work from the IASP. In 1994, when the IASP
produced the new diagnostic entity of CRPS, it was descriptive, and
general and based on a consensus.119
Deliberately, it did not imply any etiology or pathology (including any
direct role for the SNS). The intention was to provide an officially
endorsed set of standardized diagnostic criteria to improve clinical
communication and facilitate research.118
In other words, this was intended as a starting point from which
individual researchers could move forward. It was not thought of as a
mature clinical diagnostic device.
criteria have been validated, refined, and developed. The validation
studies suggest that the original criteria are adequately sensitive within the context of a pain clinic
(i.e., they rarely miss a case of actual CRPS); however, the criteria
cause problems of overdiagnosis because of poor specificity.58,80
Comparison of CRPS patients to other proved pain states, such as
chronic diabetic patients with ascending symmetric pain, whose
neuropathy is confirmed by nerve conduction studies, also show that the
criteria are very sensitive but have low specificity, so that a
diagnosis of CRPS may be erroneous in up to 60% of cases.22
assume that any sign or symptom of vasomotor, sudomotor, and
edemarelated change is sufficient to justify the diagnosis and there is
no possibility of providing greater diagnostic or prognostic accuracy
by observing more than one of these features. An additional weakness is
the failure to include motor or trophic signs and symptoms. Numerous
studies have described various signs of motor dysfunction (e.g.,
dystonia, tremor) as important characteristics of this disorder, and
trophic changes have frequently been mentioned in historical clinical
descriptions.26,28 These differentiate CRPS from other pain syndromes.58,138
Finally, the wording of the criteria permits diagnosis based solely on
patientreported historical symptoms. This may be inappropriate in the
context of litigation.
-
A set of signs and symptoms indicating abnormalities in pain processing (e.g., allodynia, hyperalgesia, hyperpathia)
-
Skin color and temperature changes, indicating vasomotor dysfunction
-
Edema and abnormalities of sweating
-
Motor and trophic signs and symptoms
dysfunction from vasomotor instability and the finding of motor and
trophic abnormalities are at variance with the original IASP criteria,
which were therefore modified22,58,80 (Table 23-3).
The important changes are inclusion of clinical signs, their separation
from symptoms, and the inclusion of features of motor abnormalities and
trophic changes. Intriguingly, these subgroups are virtually identical
to those suggested by our group a decade earlier.3
sensitivity and specificity of decision rules for diagnosis of CRPS
compared to neuropathic pain of a proved non-CRPS cause using these
criteria22 (Table 23-4).
These propose different diagnostic criteria depending on the clinical
circumstances. Thus, for purely clinical diagnosis, the criteria
provide a sensitivity of 0.85 and a specificity of 0.69, whereas for
research diagnosis, the criteria provide a sensitivity of 0.70 and
specificity of 0.94, because, in the former circumstance, one wishes to
avoid failing to offer treatment to a possible candidate while in the
latter situation one is more concerned to be investigating a
homogeneous group in whom the diagnosis cannot be in doubt.
and are therefore intended to differentiate CRPS from other causes of
chronic pain within that setting. They do not apply directly to the
diagnosis of CRPS within the context of an orthopaedic practice. The
reason for this apparent conundrum is that the precise nature of CRPS
remains unclear and it is therefore a diagnosis of exclusion.
Conditions from which CRPS must be distinguished in a pain clinic
(e.g., neuropathic pain in association with diabetic neuropathy) are
different from those which apply in an orthopaedic or fracture clinic
(e.g., soft tissue infection or stress fracture). Therefore, the
diagnostic criteria must be slightly different, just as slightly
different criteria are required within a pain clinic for diagnosis of
CRPS depending on whether the diagnosis is being made for clinical or
research purposes.
These were derived empirically from a less formal but similar process
to the IASP consensus approach. The criteria were designed as far as
possible to be objective, but the patient’s veracity was assumed, so no
attempt was made to separate reports of vasomotor or sudomotor
abnormalities from observation of them. A number of the criteria are
quantifiable,2,3,51 which allows their powerful use to investigate treatment.53,54,105
The original criteria were developed in the context of CRPS of the hand
following Colles’ fracture of the wrist, but they have subsequently
been generalized for use in the diagnosis of CRPS in other orthopaedic
scenarios and in the lower limb.13,135
Diagnosis by these criteria, when used after Colles’ fracture, maps
virtually exactly with the Bruehl criteria, suggesting their
reliability.147
of pain perception are examined in comparison with the opposite normal
side. Excessive tenderness is found by squeezing digits in the affected
part between thumb and fingers. This may be quantitated using
dolorimetry but this is usually a research tool.4,6
Allodynia is demonstrated by fine touch and hyperalgesia using a pin.
Hyperpathia is examined by serial fine touch or pin prick.
|
TABLE
23-3 Modified International Association for the Study of Pain Diagnostic Criteria for Complex Regional Pain Syndrome (CRPS) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
not be present at the time of examination. If the patient is reliable,
then a history confirms its presence. Visual inspection is the usual
means of diagnosis.
difference between the limbs. This is greater in CRPS than other pain
syndromes,128,162
and this can be used to distinguish CRPS from other causes of
neuropathic pain. However, thermography has not been validated within
an orthopaedic context and must therefore be used with caution. It is
not usually used in an orthopaedic context.
is not yet clear. As for vasomotor instability, the feature is
inconstant and it may be necessary to rely on history. Excessive
sweating is usually clinically obvious. In a doubtful case, the
resistance to a biro or pencil gently stroked across the limb is
useful. The extent of sweating can be quantified by iontophoresis but
this is rarely undertaken.
may be quantified by hand volume measurement. Similarly, skinfold
thickness and digital circumference may be measured.3,6
clinical examination. The range of finger joint movement may be
accurately quantified.3,6,51 As outlined here, atrophy will affect every tissue within the limb.
earlier. CRPS does not cause arthritis and joint space is preserved.
Sudeck’s technique of assessing bone density by radiographing two
extremities on one plate120,145 remains
useful but densitometry is not usually helpful.156 A normal bone scan without radiographic osteoporosis virtually excludes adult CRPS.
|
TABLE
23-4 Diagnostic Sensitivity and Specificity for the International Association for the Study of Pain Modified Criteria (see Table 23-3) in Distinguishing Patients with Complex Regional Pain Syndrome (CRPS) from Patients with Neuropathic Pain from a Documented Non-CRPS Cause |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
relatively easy clinically but may not as yet be useful. Sensory
neglect can be elucidated either by history or direct sensory
examination with the patient watching or looking away from the affected
limb. Motor neglect is examined by asking the patient to undertake a
simple task initially while looking away and then while watching the
limb. In the upper limb, this can be repetitively opening the closing
the fingers or, in the lower limb, tapping the foot. If there is a
significant improvement when the patient is watching the limb, a degree
of motor neglect is present.61
|
TABLE 23-5 Suggested Criteria for the Diagnosis of Complex Regional Pain Syndrome (CRPS) within an Orthopaedic Setting
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
diagnostic test. The classic case is obvious and direct effects of
trauma, fracture, cellulitis, arthritis, and malignancy are common
alternative diagnoses. The patient is systemically well with normal
general clinical examination, biochemical markers, and infection
indices.
soft tissue edema with late atrophy and fibrosis but is not diagnostic.
However, in CRPS 2, MRI may be useful to demonstrate nerve thinning
with poststenotic dilatation caused by compression and may even
demonstrate a fibrous band causing the compression. It may also
demonstrate neuroma formation, although many neuromas are too small to
be adequately shown.
demonstrating a bony compressing lesion. Electromyographic and nerve
conduction studies are normal in CRPS 1 but may demonstrate a nerve
lesion in CRPS 2.
associations of trauma and orthopaedic surgery. The following are
common differential diagnoses.
-
Soft tissue infection. The clinical features are usually clear. The patient is systemically unwell with raised inflammatory markers.
-
“Mechanical” problems.
Classic examples are incorrect sizing of a total knee replacement
causing pain, swelling, and stiffness; overlong screws impinging on a
joint; or malreduction of an intra-articular fracture (Fig. 23-5).
In accordance with category 4 of the original IASP criteria for CRPS,
all mechanical causes for the symptoms and signs must be excluded
before making a diagnosis of CRPS. However, it must be borne in mind
that the chronic pain of a mechanical problem can itself be the
precipitating cause of CRPS. -
Conscious exaggeration of symptoms.
This is usually seen in the context of litigation, but the secondary
gain from exaggeration may also relate to complex and pathological
interpersonal relationships. This problem has been accidentally made
more acute and severe by the IASP criteria for CRPS
P.611diagnosis. The original criteria (Table 23-2)
are readily mimicked by a patient determined to deceive the examining
clinician. Unfortunately, the modified criteria may also provide a
diagnosis of CRPS in a deceitful patient. Categories 1 and 2 are
simple. The patient merely has to report these problems. Category 3
refers to objective criteria. However, sensory abnormalities rely on
the patient’s subjective response to stimulus. Skin color change can be
caused by deliberate dependency and immobility of the limb. Loss of
joint range of movement can be caused by conscious resistant to
movement, and dystonia, tremor, and weakness can likewise be produced
artifactually. The rise of the Internet means that any reasonably
determined patient can have very great knowledge of the features of
CRPS and the diagnostic criteria. The solution to this problem is to
remember that the IASP criteria are designed to differentiate CRPS from
other chronically painful conditions. They are not intended to deal
with a patient whose veracity is open to question. CRPS is a condition
that inevitably leads to dystrophy,21,44,58,138
and in a patient who has suffered from significant CRPS for any
significant length, objective features of dystrophy, such as nail or
hair dystrophy, skin and subcutaneous tissue atrophy, fixed joint
contracture, and radiographic features of significant osteoporosis with
abnormalities of bone scanning, should be present. If the patient’s
veracity is in doubt, the astute clinician will give only limited or no
credence to those features that can be mimicked and look for
incontrovertible physical signs. -
Psychiatric disease.
Separate from the conscious exaggeration described earlier, psychiatric
disease may cause a patient unconsciously to exaggerate the level or
impact of physical disease. Somatoform disorders describe conditions in
which patients unconsciously exaggerate physical symptoms, and
conversion disorders refer to unconscious exaggeration of physical
signs. These patients are often psychologically fragile, they may have
a history of an unusually severe reaction to multiple minor medical
problems, and they may show a tendency to “catastrophize” life events.
In addition to this direct influence on a diagnosis of CRPS, patients
with CRPS may be depressed because of chronic pain and psychiatric
disease may play an indirect part in the condition. It is often very
useful to obtain formal psychiatric or psychological opinion and
treatment.156 -
Neuropathic pain.
This has been defined and discussed. Neuropathic pain is part of CRPS,
but a patient may have neuropathic pain without having CRPS. However,
neuropathic pain may give rise to CRPS. -
Chronic pain state.
Patients with long-lasting and unremitting pain may become depressed,
particularly when there is a neuropathic element. They learn to avoid
activities that cause pain, and their relatives and carers act to
protect them from perceived injury. This generates a complex
psychosocial situation that may require psychological, psychiatric,
pain therapeutic, and orthopaedic combined management.
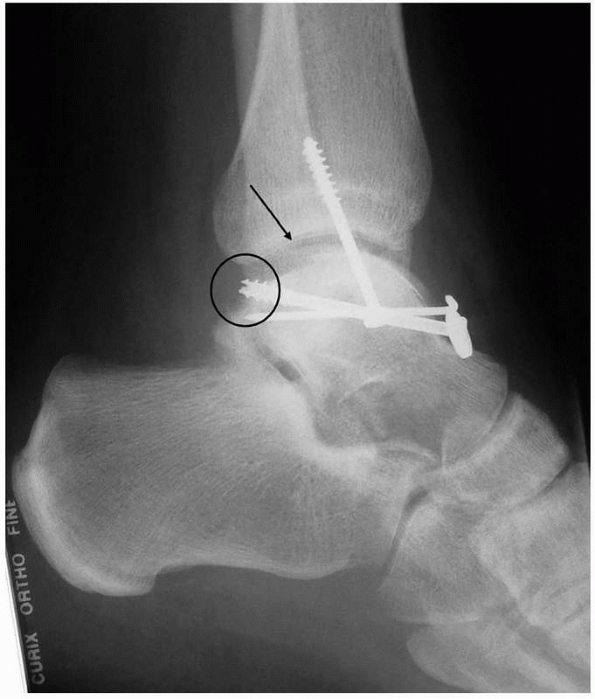 |
|
FIGURE 23-5
A patient referred with a diagnosis of CRPS. This patient with severe pain in his foot was referred some years after internal fixation of a talar body fracture. He has severe pain and dysfunction. The lateral radiograph shows no evidence of significant osteoporosis, which is inconsistent with the diagnosis. The talar body fracture is not reduced (arrow), which renders the ankle and subtalar joints incongruous. Furthermore, the screws are overlong (circle) and impinge on the ankle joint. This patient does not have CRPS; he has a mechanical cause for his severe pain, which was resolved by talar osteotomy, anatomic reduction, and refixation. It is important to exclude mechanical causes for pain before invoking the diagnosis of CRPS. |
but proper scientifically constructed prospectively randomized blinded
studies are few,95 and uncontrolled
investigations are particularly unreliable in CRPS because of the
variety of symptoms and the trend toward self-resolution in the
majority of cases. This is well illustrated by a series of publications
investigating the treatment of early CRPS after Colles’ fracture with
intravenous regional guanethidine blockade (IVRGB). An initial
investigation showed that IVRGB caused improvement in objective
criteria of CRPS severity.54 A
subsequent pilot study appeared to confirm the immediate improvement
induced by IVRGB was associated with sustained symptomatic improvement.53
However, a full prospectively randomized double-blind controlled study
demonstrated that IVRGB actually seemed to worsen the condition.105 The lesson is that these potentially fragile patients must be approached with caution.
common in orthopaedic trauma practice. Most sufferers are sensible
people, concerned about the development of inexplicable pain, but the
occasional “Sudecky” patient fares poorly and should be treated
vigorously. Early treatment, begun before contractures occur, gives
optimal results, so a high index of clinical suspicion must be
maintained. It is not reprehensible to have caused a case of CRPS
through surgery or nonoperative management of injury. However, delay in
diagnosis and treatment may contribute to a poor outcome.
rather than SNS manipulation.26
Initial treatment from the orthopaedic surgeon is with reassurance,
excellent analgesia, and intensive, careful physical therapy avoiding
exacerbation of pain.66 Nonsteroidal
anti-inflammatory drugs may give better pain relief than opiates, and a
centrally acting analgesic such as amitriptyline is often useful even
at this early stage. Immobilization and splintage should generally be
avoided but, if used, joints must be placed in a safe position and
splintage is a temporary adjunct to mobilization. It seems sensible to
give the patients vitamin C in view of the early evidence of its
efficacy.170,171
desensitization. The patient is asked to stroke the area of allodynia,
where stroking is painful. They are reminded that simple stroking
cannot by definition be painful and they are instructed to stroke the
affected part repetitively while looking at it and repeatedly saying
“this does not hurt, it is merely a gentle touch.” The earlier this is
begun, the more effective it is. A similar attitude can be taken with
early loss of joint mobility due to perceived pain rather than
contracture.
specialist should be involved and treatment continued on a shared
basis. Psychological or psychiatric input may be important.25
Secondline treatment is often unsuccessful and many patients are left
with pain and disability. Further treatments include centrally acting
analgesic medications such as amitriptyline, gabapentin, or
carbamazepine; regional anesthesia; calcitonin; the use of
membrane-stabilizing drugs such as mexilitene; sympathetic blockade and
manipulation; desensitization of peripheral nerve receptors with
capsaicin; or transcutaneous nerve stimulation or an implanted dorsal
column stimulator.109,124,142 Behavioral therapy may be necessary in children.165,166,167 Where the knee is affected, epidural anesthesia and continuous passive motion may be appropriate.35,36
there is debate within pain therapy circles as to the utility of
separating CRPS type 1 from type 2 (although there is evidence that
they are symptomatically different21), within orthopaedic practice, it is extremely useful. The wording of the IASP criteria is not surgically precise (Table 23-2).
However, if one substitutes surgically correctable nerve lesion, in
cases of CRPS type 2, treatment should be directed at curing the nerve
lesion. Occult nerve compression should be sought and dealt with. For
example, decompression of a median nerve at the wrist that is causing
CRPS of the hand may abort the CRPS and should be undertaken cautiously
in the presence of active disease.
which usually involve all of the soft tissues. Surgical release must
therefore be radical and expectations limited. Surgery for contracture
should be delayed until the active phase of CRPS has completely passed,
and ideally there should be a gap of at least 1 year since the patient
last experienced pain and swelling.
reported a series of 28 patients who underwent 34 amputations in 31
limbs. Surgery was usually performed for recurrent infection or to
improve residual function. Pain relief was rare and unpredictable, and
neither was infection always cured nor function universally improved.
CRPS often recurred in the stump, especially if the amputation level
was symptomatic at the time of surgery. For this reason, only two
patients wore a prosthesis.
may exacerbate CRPS or precipitate a new attack. This risk must be
balanced carefully against the proposed benefit. The risk of surgically
precipitated recurrence is greatest when the same site is operated on
in a patient with abnormal psychology in the presence of active disease
and lowest when these conditions do not apply. Surgery must be
performed carefully with minimal trauma with excellent and complete
postoperative analgesia. The surgery may be covered by gabapentin.
Ideally, the anesthetist will have a particular interest in the
treatment of CRPS.
mild form, which is often not formally diagnosed, is very common but
not universal in an orthopaedic trauma practice. Although the majority
of cases will resolve with simple management, CRPS is responsible for
significant acute disability and may cause long-term problems.
Z, Raja SN, Wesselmann U, et al. Intradermal injection of
norepinephrine evokes pain in patients with sympathetically maintained
pain. Pain 2000;88:161-168.
RM, Kanis JA. The use of dolorimetry in the assessment of posttraumatic
algodystrophy of the hand. Br J Rheumatol 1989;28:404-409.
RM, Tindale W, Bickerstaff D, et al. Quantitative bone scintigraphy in
reflex sympathetic dystrophy. Br J Rheumatol 1993;32:41-45.
PG. Etude sur le risque algodystrophique. [These pour le doctorat en
medecin diplome d’etat.] Paris: University of Paris, Val de Marne, 1980.
R, Kurtz J. Colles’ fracture: a study of 2000 cases from the New York
State Workmen’s Compensation Board. J Bone Joint Surg Am
1953;35A:643-658.
DR, Charlesworth D, Kanis JA. Changes in cortical and trabecular bone
in algodystrophy. Br J Rheumatol 1993;32:46-51.
F, Riedl B, Sieweke N, et al. Neurological findings in complex regional
pain syndromes—analysis of 145 cases. Acta Neurol Scand
2000;101:262-269.
R. Complex regional pain syndromes: symptoms, signs, and differential
diagnosis. In: Janig W, Stanton-Hicks M, eds. Reflex Sympathetic
Dystrophy: A Deappraisal. Seattle, WA: IASP Press, 1996:79-92.
JJ. Causalgia and other reflex sympathetic dystrophies. In: Bonica JJ,
ed. Management of Pain. Philadelphia: Lea & Febiger, 1990:220-243.
J, Freud S. Studies in Hysteria. Translated and edited by J. Strachey
with the collaboration of A. Freud. New York: Basic Books, 1982.
S. Do psychological factors play a role in the onset and maintenance of
CRPS-1? In: Harden RN, Baron R, et al, eds. Complex Regional Pain
Syndrome. Seattle, WA: IASP Press, 2001.
S. Psychological interventions. In: Wilson P, Stanton-Hicks M, Harden
RN, eds. CRPS: Current Diagnosis and Therapy. Seattle, WA: IASP Press,
2005:201-216.
S, Carlson CR. Predisposing psychological factors in the development of
reflex sympathetic dystrophy. A review of the empirical evidence. Clin
J Pain 1992;8: 287-299.
S, Harden RN, Galer BS, et al. Complex regional pain syndrome: are
there distinct subtypes and sequential stages of the syndrome? Pain
2002;95:119-124.
S, Harden RN, Galer BS, et al. External validation of IASP diagnostic
criteria for complex regional pain syndrome and proposed research
diagnostic criteria. International Association for the Study of Pain.
Pain 1999;81:147-154.
S, Husfeldt B, Lubenow TR, et al. Psychological differences between
reflex sympathetic dystrophy and non-RSD chronic pain patients. Pain
1996;67:107-114.
AW, Lubenow TR, Prithvi Raj P. Traditional interventional therapies.
In: Wilson P, Stanton-Hicks M, Harden RN, eds. CRPS: Current Diagnosis
and Treatment. Seattle, WA: IASP Press, 2005:217-233.
SH. Disuse and CRPS. In: Harden RN, Baron R, Janig W, eds. Complex
Regional Pain Syndrome. Seattle, WA: IASP Press, 2001:141-150.
SH, Galer BS, Benirsche S. Disuse as a cause of signs and symptoms of
CRPS. Abstracts: 8th World Congress on Pain. Seattle, WA: IASP Press;
1996:401.
SH, Nyman M, Gordh T, editors. Immobility in volunteers produces signs
and symptoms of CRPS and a neglect-like state. Abstracts: 9th World
Congress on Pain; 1999. Seattle, WA: IASP Press.
J, Raga S, Meyer R. Painful sequelae of nerve injury. In: Dubner R,
Gebhart G, Bond M, eds. Proceedings of the 5th World Congress on Pain.
Amsterdam: Elsevier Science Publishers; 1988:135-143.
R, Glynn CJ, Buonocore M. Autonomic variations after stellate ganglion
block: are they evidence of an autonomic afference? Funct Neurol
1990;5:245-246.
GN, Lloyd GJ. The investigation of hand stiffness using Doppler
ultrasound, radionuclide scanning and thermography. J Bone Joint Surg
Br 1979;61B:519.
JM. Two cases of hysterical contracture of traumatic origin (Lectures
VII and VIII). Lectures on Diseases of the Nervous System. Nijmegen:
Arts and Boeve; 1889: 84-106.
A, Brunot B, Demangeat JL, et al. Three-phase bone scanning as an aid
to early diagnosis in reflex sympathetic dystrophy of the hand. A study
of 89 cases. Ann Chir Main 1986;5:93-104.
DE, DeLee JC, Ramamurthy S. Reflex sympathetic dystrophy of the knee.
Treatment using continuous epidural anesthesia. J Bone Joint Surg Am
1989;71A:365-369.
JL, Constantinesco A, Brunot B, et al. Three-phase bone scanning in
reflex sympathetic dystrophy of the hand. J Nucl Med 1988;29:26-32.
PW, Claassen AT, Veldman PH, et al. Amputation for reflex sympathetic
dystrophy. J Bone Joint Surg Br 1995;77B:270-273.
P, Dirheimer Y, Pattin S. Algodystrophy: Diagnosis and Therapy of a
Frequent Disease of the Locomotor Apparatus. Berlin: Springer Verlag,
1981.
AG, Stein J. Disappearance of chondrocalcinosis following reflex
sympathetic dystrophy syndrome. Arthritis Rheum 1981;24:747-749.
P, Arlet J, Lartigue G, et al. [Postinjury reflex algodystrophies.
Hemodynamic and anatomopathological study]. Rev Chir Orthop Reparatrice
Appar Mot 1973;59: 401-414.
J, Atkins RM. Algodystrophy is an early complication of Colles’
fracture. What are the implications? J Hand Surg [Br] 1997;22:178-182.
J, Monk C, Atkins RM. Objective improvements in algodystrophy following
regional intravenous guanethidine. J Hand Surg [Br] 1993;18:339-342.
J, Protheroe DL, Atkins RM. Algodystrophy after Colles fractures is
associated with secondary tightness of casts. J Bone Joint Surg Br
1994;76B:901-905.
F, Zoppi M, Maresca M, et al. Skin potential and EMG changes induced by
electrical stimulation. 1. Normal man in arousing and nonarousing
environment. Appl Neurophysiol 1979;42:113-124.
BS, Bruehl S, Harden RN. IASP diagnostic criteria for complex regional
pain syndrome: a preliminary empirical validation study. International
Association for the Study of Pain. Clin J Pain 1998;14:48-54.
BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like
syndrome may be responsible for the motor disturbance in reflex
sympathetic dystrophy (complex regional pain syndrome-1). J Pain
Symptom Manage 1995;10:385-391.
BS, Harden N. Motor abnormalities in CRPS: a neglected but key
component. In: Harden N, Baron R, et al, eds. Complex Regional Pain
Syndrome. Seattle, WA: IASP Press, 2001.
BS, Harden RN. Motor abnormalities in CRPS: a neglected but key
component. In: Harden RN, Baron R, Janig W, editors. Complex Regional
Pain Syndrome. Seattle, WA: IASP Press, 2001:135-140.
BS, Jensen M. Neglect-like symptoms in Complex Regional Pain Syndrome:
results of a self-administered survey. J Pain Symptom Manage
1999;18:213-217.
JH, de Bruijn H, de Bruijn-Kofman AT, et al. Reflex sympathetic
dystrophy: early treatment and psychological aspects. Arch Phys Med
Rehabil 1994;75:442-446.
JH, de Bruijn-Kofman AT, de Bruijn HP, et al. Stressful life events and
psychological dysfunction in complex regional pain syndrome type I.
Clin J Pain 1998;14: 143-147.
JH, Dijkstra PU, Groothoff JW, et al. Reflex sympathetic dystrophy of
the upper extremity—a 5.5-year follow-up. Part II. Social life events,
general health, and changes in occupation. Acta Orthop Scand Suppl
1998;279:19-23.
JH, Harden RN. Physical and occupational therapies. In: Wilson P,
Stanton-Hicks M, Harden RN, eds. CRPS: Current Diagnosis and Therapy.
Seattle, WA: IASP Press, 2005:173-179.
JJ, Wilson PR. RSD score: criteria for the diagnosis of reflex
sympathetic dystrophy and causalgia. Clin J Pain 1992;8:260-263.
CJ, Basedow RW, Walsh JA. Pain relief following postganglionic
sympathetic blockade with I.V. guanethidine. Br J Anaesth
1981;53:1297-1302.
RJ. Conditions associated with impaired oxygen extraction. In:
Gutierrez G, Vincent JL, eds. Tissue Oxygen Utilisation. Berlin:
Springer Verlag, 1991:350-369.
RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the
pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun
1987;3:13-18.
JG. Hyperadrenergic-effected limb causalgia: relief by IV pharmacologic
norepinephrine blockade. Am Heart J 1982;103:152-153.
RN. The rationale for integrated functional restoration. In: Wilson P,
Stanton-Hicks M, Harden RN, eds. CRPS: Current Diagnosis and Therapy.
Seattle, WA: IASP Press, 2005:163-171.
RN, Bruehl S, Galer BS, et al. Complex regional pain syndrome: are the
IASP diagnostic criteria valid and sufficiently comprehensive? Pain
1999;83:211-219.
RN, Bruehl S, Stanos S, et al. Prospective examination of pain-related
and psychological predictors of CRPS-like phenomena following total
knee arthroplasty: a preliminary study. Pain 2003;106:393-400.
RN, Swan M, King A, et al. Treatment of complex regional pain syndrome:
functional restoration. Clin J Pain 2006;22:420-424.
LE, Mackinnon SE. Reflex sympathetic dystrophy in the hands: clinical
and scintigraphic criteria. Radiology 1984;152:517-522.
AR, Carroll D, Glynn CJ, et al. Intravenous regional sympathetic
blockade for pain relief in reflex sympathetic dystrophy: a systematic
review and a randomized, double-blind crossover study. J Pain Symptom
Manage 1995;10:13-20.
W, Koltzenburg M. Possible ways of sympathetic afferent interaction.
In: Janig W, Schmidt RF, eds. Reflex Sympathetic Dystrophy:
Pathophysiological Mechanisms and Clinical Implications. New York: VCH
Verlagsgesellschaft, 1992:213-243.
W, Koltzenburg M. What is the interaction between the sympathetic
terminal and the primary afferent fibre? In: Basbaum AI, et al, eds.
Towards a new pharmacology of pain. Chichester: John Wiley and Sons;
1991:331-352.
W. CRPS 1 and CRPS 2: A strategic view. In: Harden RN, Baron R, Janig
W, eds. Complex Regional Pain Syndrome. Seattle, WA: IASP Press, 2p.
3-15.
W. The sympathetic nervous system in pain: physiology and
pathophysiology. In: Stanton-Hicks M, editor. Pain in the Sympathetic
Nervous System. Massachussetts: Kluwer Academic Publishers, 1990:17-89.
T, Komatsu T, Hosoda R, et al. Angiotensin-converting enzyme gene
polymorphism in patients with neuropathic pain. In: Devor M, Rowbotham
M, Wiesenfeld-Hallin D, eds. Proceedings of the 9th World Conference on
Pain. Seattle, WA: IASP Press; 2000:471-476.
WS. A critical review of controlled clinical trials for peripheral
neuropathic pain and complex regional pain syndromes. Pain
1997;73:123-139.
F, Genant HK, Bekerman C, et al. The reflex sympathetic dystrophy
syndrome. II. Roentgenographic and scintigraphic evidence of
bilaterality and of periarticular accentuation. Am J Med
1976;60:332-338.
F, McCarty DJ, Sims J, et al. The reflex sympathetic dystrophy
syndrome. I. Clinical and histologic studies: evidence for
bilaterality, response to corticosteroids, and articular involvement.
Am J Med 1976;60:321-331.
R. Oedeme dur aigu post-traumatique de la main avec impotence
fonctionelle complete. Transformation soudaine cinq heures apres
sympathectomie humerale. Lyon Chir 1923;20:814-818.
R. Traitement par la sympathectomie periarterielle des osteoporoses
traumatiques. Bull Mem Soc Chir Paris 1926;52:247-251.
JS, Kersten P, McCabe CS, et al. Body perception disturbance: a
contribution to pain in complex regional pain syndrome (CRPS). Pain
2007;133:111-119.
A. Fractures of the distal end of radius. A clinical and statistical
study of end results. Acta Orthop Scand 1959;suppl 41.
J, Tegenthoff M, Malin JP. Changes of cortical motor area size during
immobilization. Electroencephalogr Clin Neurophysiol 1995;97:382-386.
RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide
genes in adult sensory neurons. Nature 1989;337:362-364.
JA, Atkins RM. Intravenous regional guanethidine blockade in the
treatment of posttraumatic complex regional pain syndrome type 1
(algodystrophy) of the hand. J Bone Joint Surg Br 2002;84B:380-386.
C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static
(pressure) mechanical allodynia in migraine. Cephalalgia
2006;26:852-856.
TR, Buvanendran A, Stanton-Hicks M. Implanted Therapies. In: Wilson P,
Stanton-Hicks M, Harden RN, eds. CRPS: Current Diagnosis and Therapy.
Seattle, WA: IASP Press, 2005:235-253.
SE, Holder LE. The use of three-phase radionuclide bone scanning in the
diagnosis of reflex sympathetic dystrophy. J Hand Surg [Am]
1984;9:556-563.
A, Wade J. Profile of Caucasian women with possible genetic
predisposition to reflex sympathetic dystrophy: a pilot study. Clin J
Pain 1994;10:210-217.
A, Wade JA. Genetic considerations in CRPS. In: Harden RN, Baron R,
Janig W, eds. Complex Regional Pain Syndrome. IASP Press; 2001:227-238.
CD, Obeso JA, Traub MM, et al. Muscle spasms associated with Sudeck’s
atrophy after injury. Br Med J (Clin Res Ed) 1984;288:173-176.
H, Jimbo Y, Watanabe K. Haemodynamic changes in early phase reflex
sympathetic dystrophy. Scand J Plast Reconstr Surg Hand Surg
1996;30:133-138.
CS, Haigh RC, Halligan PW, et al. Referred sensations in patients with
complex regional pain syndrome type 1. Rheumatology (Oxford)
2003;42:1067-1073.
CS, Haigh RC, Ring EF, et al. A controlled pilot study of the utility
of mirror visual feedback in the treatment of complex regional pain
syndrome (type 1). Rheumatology (Oxford) 2003;42:97-101.
EM, Janig W, Devor M, et al. Peripheral nerve injury triggers
noradrenergic sprouting within dorsal root ganglia. Nature
1993;363:543-546.
H. Essence, investigation, and management of “neuropathic” pains: hopes
from acknowledgment of chaos. Muscle Nerve 1995;18:455-456; author
reply 8-62.
H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic
Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP
Press, 1994.
ME, Allgower M, Schneider R, et al. Manual of internal fixation.
Techniques recommended by the AO group. 2nd ed. London/New York:
Springer Verlag; 1979.
DV, Novy DM. Psychological characteristics of reflex sympathetic
dystrophy versus myofascial pain syndromes. Reg Anesth 1996;21:202-208.
N. Über die Radiolographische nachweisbare akute und kronische
“Knochenatrophie” (Südeck bie Nerven-Erkrankungen). Fortschr Geb
Röntgenstr 1901;5: 293-297.
AL. Evidence-based pharmacotherapy for CRPS and related conditions. In:
Wilson P, Stanton-Hicks M, Harden RN, eds. CRPS: Current Diagnosis and
Therapy. Seattle, WA: IASP Press, 2005:181-200.
WJ, Arntz IE, Claessens RM, et al. Reflex sympathetic dystrophy of the
hand: an excessive inflammatory response? Pain 1993;55:151-157.
J, Touchon J, Besset A, et al. La personnalite du sujet souvrant
d’algodystrophie sympathique reflexe. Etudes Psychometrques par le test
MMPI. Rheumatologie 1981; 23:351-354.
RB, Adler D, Humphreys M. Reflex sympathetic dystrophy: electronic
thermography as an aid in diagnosis. Orthop Rev 1987;16:561-566.
DD, Long S, Wilsey B, Rafii A. Analysis of peak magnitude and duration
of analgesia produced by local anesthetics injected into sympathetic
ganglia of complex regional pain syndrome patients. Clin J Pain
1998;14:216-226.
P, Francini F, Maresca M, et al. Skin potential and EMG changes induced
by cutaneous electrical stimulation. II. Subjects with reflex
sympathetic dystrophies. Appl Neurophysiol 1979;42:125-134.
P, Francini F, Zoppi M, et al. Cutaneous pain threshold changes after
sympathetic block in reflex dystrophies. Pain 1975;1:167-175.
VS, Roger-Ramachandran D. Synaesthesia in phantom limbs induced with
mirrors. Proc R Soc Lond B Biol Sci 1996;263:377-386.
JC, Moreau R, Bernat M, et al. [Contribution of dynamic isotopic tests
in the study of algodystrophies]. Rev Rhum Mal Osteoartic
1979;46:235-241.
PP, Ward AJ, Smith EJ, et al. Algodystrophy and osteoporosis after
tibial fractures. J Bone Joint Surg Br 1993;75B:450-452.
J, Wenzelburger R, Deuschl G, et al. Kinematic analysis of the upper
extremity in CRPS. In: Harden RN, Baron R, Janig W, eds. Complex
Regional Pain Syndrome. Seattle, WA: IASP Press, 2001:119-128.
SP, Harden RN, Wagner-Raphael L, et al. A prospective clinical model
for investigating the development of CRPS. In: Harden RN, Baron R,
Janig W, eds. Complex Regional Pain Syndrome. Seattle, WA: IASP Press,
2001:151-164.
M, Janig W, Hassenbusch S, et al. Reflex sympathetic dystrophy:
changing concepts and taxonomy. Pain 1995;63:127-133.
M, Rauck R, Hendrickson M, et al. Miscellaneous and experimental
therapies. In: Wilson P, Stanton-Hicks M, Harden RN, eds. CRPS: Current
Diagnosis and Therapy. Seattle, WA: IASP Press, 2005:255-274.
J, Stillwell GK. Reflex sympathetic dystrophy syndrome of the upper
extremity: analysis of total outcome of management of 125 cases. Arch
Phys Med Rehabil 1981;62:549-554.
P. Über die akute (reflektorische) Knochenatrophie nach Entzündungen
und Verletzungen in den Extremitäten und ihre klinischen Erscheinungen.
Fortschr Geb Rontgenstr 1901;5:227-293.
McBride AR, Barnett AJ, Livingstone JA, et al. Complex regional pain
syndrome (type 1): a comparison of 2 diagnostic criteria methods. Clin
J Pain 2008;24: 637-640.
der Laan L, Goris RJ. Reflex sympathetic dystrophy. An exaggerated
regional inflammatory response? Hand Clin 1997;13:373-385.
der Laan L, Kapitein P, Verhofstad A, et al. Clinical signs and
symptoms of acute reflex sympathetic dystrophy in one hindlimb of the
rat, induced by infusion of a freeradical donor. Acta Orthop Belg
1998;64:210-217.
der Laan L, ter Laak HJ, Gabreels-Festen A, et al. Complex regional
pain syndrome type I (RSD): pathology of skeletal muscle and peripheral
nerve. Neurology 1998;51: 20-25.
Hilten JJ, Blumberg H, Schwartzman R. Factor IV: movement disorders and
dystrophy. pathophysiology and measurement. In: Wilson P, Stanton-Hicks
M, Harden RN, eds. CRPS: Current Diagnosis and Therapy. Seattle, WA:
IASP Press, 2005:119-137.
Hilten JJ, van de Beek WJ, Vein AA, et al. Clinical aspects of
multifocal or generalized tonic dystonia in reflex sympathetic
dystrophy. Neurology 200126;56:1762-1765.
Houdenhove B, Vasquez G, Onghena P, et al. Etiopathogenesis of reflex
sympathetic dystrophy: a review and biopsychosocial hypothesis. Clin J
Pain 1992;8: 300-306.
Houdenhove B, Vasquez G. Is there a relationship between reflex
sympathetic dystrophy and helplessness? Case reports and a hypothesis.
Gen Hosp Psychiatry 1993; 15:325-329
PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex
sympathetic dystrophy: prospective study of 829 patients. Lancet
1993;342:1012-1016.
G, Ernst J, Henniaux M, et al. [Attempt at a psychological approach in
algoneurodystrophy]. Rev Rhum Mal Osteoartic 1982;49:767-769.
LK, Gordh TJ, Torebjork E. Effects of regional intravenous guanethidine
in patients with neuralgia in the hand; a follow-up study over a
decade. Pain 1995;62: 379-385.
PD, Devor M. Sensory afferent impulses originate from dorsal root
ganglia as well as from the periphery in normal and nerve injured rats.
Pain 1983;17:321-339.
R, Schattschneider J, Wasner G, et al. Grip force coordination in CRPS.
In: Harden RN, Baron R, Janig W, eds. Complex Regional Pain Syndrome.
Seattle, WA: IASP Press, 2001:129-134.
R, Olsson GL. Management of pediatric patients with CRPS. In: Wilson P,
Stanton-Hicks M, Harden RN, eds. CRPS: Current Diagnosis and Therapy.
Seattle, WA: IASP Press, 2005:275-289.
RT, Berde CB, Wolohan M, et al. Reflex sympathetic dystrophy in
children. Clinical characteristics and follow-up of 70 patients. J Bone
Joint Surg Am 1992;74A: 910-919.
PE, Tuinebreijer WE, Breederveld RS, et al. Can vitamin C prevent
complex regional pain syndrome in patients with wrist fractures? A
randomized, controlled, multicenter dose-response study. J Bone Joint
Surg Am 2007;89A:1424-1431.
PE, Tuinebreijer WE, Kreis RW, et al. Effect of vitamin C on frequency
of reflex sympathetic dystrophy in wrist fractures: a randomised trial.
Lancet 1999;354: 2025-2028.
A. Results of the treatment of posttraumatic reflex sympathetic
dystrophy of the upper extremity with regional intravenous blocks of
methylprednisolone and lidocaine. Acta Orthop Belg 1998;64:452-456.
