Orthopaedic Infections and Osteomyelitis
One – General Principles: Basics > Complications > 24 –
Orthopaedic Infections and Osteomyelitis
early Sumerian carvings, when the tenets of treatment were irrigation,
immobilization, and bandaging.82 In
these early times, the practice of infection and wound care was
essentially an art and there was very little science applied to it.
Treatment included the use of honey, wine, and donkey feces, and there
were a number of philosophies regarding the value of purulence.
Dominant personalities had a significant influence over medical
practice and the value of purulence persisted because of the writings
of Galen of Pergamum (120-201 A.D.). It was not until the latter third
of the second millennium that the concept of the value of purulence
would be challenged.82
has involved the use of local ointments or salves and the maintenance
of an open wound that permitted purulence to exit the body. Some
important terms were adopted into medical parlance. A sequestrum was defined as “a fragment of dead bone separated from the body.” The word sequestrum is derived from the Latin words sequester meaning “depositary” and sequestrate meaning “to give up for safe keeping.” The word sequestrum is used to describe a detached piece of bone lying within a cavity formed by necrosis. The term involucrum derives from the Latin word involucrum
meaning “enveloping sheath or envelope.” This term describes the
effects of the body’s inflammatory response when trying to envelope and
isolate the sequestrum from the host. The natural history of
osteomyelitis was seen as the process of isolation of the infective
material followed by a slow attempted resorption of the material by the
immune system. However, the term osteomyelitis was not coined until the mid-1800s, when it was adopted by Nelaton.98
anesthesia, antisepsis, and radiography. The first two were important
in all surgical specialties. Anesthesia made surgery tolerable, but
there was still considerable morbidity secondary to infection. It was
not until the mid-1800s that progress with antisepsis permitted
infection control and more effective surgical intervention. As a result
of this, infection issues
became
an integral part of medicine and were studied in a more formal basis.
However, descriptions of the first sequestrectomies of the tibia had
been illustrated as early as 1593 by Scultetus.98
performed using forced immobility and inebriation. Operating rooms were
created because procedures undertaken in the wards horrified patients
who witnessed them and the screams of agony did nothing to encourage
other patients to seek surgical treatment. Thus, the patients were
isolated from the rest of the ward. In the same era, many modern drugs
were developed, including morphine, heroin, nitrous oxide, and ether.
Ether was in fact serendipitously identified as an anesthetic agent
during one of the drug parties that were common at this time. However,
it was first used for anesthesia in Massachusetts General Hospital in
1846 by William T. G. Morton, and its use quickly caught on around the
world. This increased the incentive to undertake surgical procedures.
The ensuing increase in the number of surgical procedures, together
with the lack of antisepsis, meant that the morbidity and mortality of
surgery also increased.98 Pasteur
and Lister are most commonly credited as being the forerunners of
antisepsis, but the most notable achievement in demonstrating the
efficacy of bacterial transmission is the work of Semmelweiss, who, in
1848, demonstrated that hand washing between obstetric deliveries
reduced maternal mortality from 18% to about 1%. Lister read Pasteur’s
work on fermentation and likened tissue putrefaction to the same
process. He subsequently developed carbolic acid, which reduced
mortality from amputation from 43% in an untreated cohort of patients
to 15% in a treated cohort. Despite this significant discovery, his
findings were resisted for decades. Even when his concepts were
adopted, the remaining pieces of the puzzle required for successful
aseptic surgery did not come together for another 100 years.
as the use of anesthesia and antisepsis. Some antibacterial treatments
were introduced, but it was not until the discovery of penicillin by
Alexander Fleming in 1928 that the proven usefulness of antibiotics
became understood. Even Fleming did not vigorously pursue his
discovery. However, when Florey and Chain read Fleming’s initial
report, they pursued and found the true impact of penicillin, which was
effective against streptococci. Since then, many antibiotics have been
developed, but the number of resistant bacteria has also increased.
Hand washing, gloves, hats, enclosed rooms, aseptic techniques, and
early antibiotics all slightly decreased the incidence of surgical
infection. However, the operating theaters in the early 1900s still
admitted observers who coughed, did not use masks, and wore street
clothes. It was not until the mid-20th century that surgeons began to
integrate all the controllable aspects of patient exposure to
infectious agents by attempting to standardize the contributive effects
of the environment, patient, surgeon, wound, antisepsis, antibiotics,
and surgical techniques. It is likely, though, that many of the answers
to the problem of infection remain undiscovered, and it seems likely
that at the moment we do not fully understand the complex symbiosis
between bacteria and humans.
etiology, diagnosis, and management of orthopaedic infections but will
have a specific focus on posttraumatic conditions. Historically, the
treatment of orthopaedic infection was either ablative, when an
amputation was performed, or temporizing with treatment of a chronic
wound or sinus. There was little chance of limb salvage as we know it
today, and infections that were not adequately treated would
occasionally become systemic and fatal.
the femur in the American Civil War and World War I were largely due to
sepsis. In every war, the science of surgery and medicine advances, and
this is particularly true for trauma surgery and extremity injuries,
which still account for approximately 65% of all war-related injuries.83 Thus, many advances in infection treatment and extremity injuries have ironically come about as a result of war.
understand the basics of the interdependence of humans and bacteria.
Bacteria are a necessary part of our existence and normal flora live in
abundance on our bodies. It is worth considering that an individual’s
skin can contain up to 180 different types of bacteria at any given
time.45 There are up to 10
colony-forming units (CFUs) of bacteria in the mouth and perineum.
Nearly 95% of bacteria found in the hands exist under the fingernails.
The average human is composed of 100 trillion cells, but it is thought
that we harbor over a 1000 trillion bacteria in or on our bodies. Our
blood is constantly infiltrated with bacteria from breaks in the skin,
translocation across mucous membranes, and other roots. However, nearly
all of these bacteria are quickly and efficiently eradicated by our
host defense mechanisms. It is the disruption of our own homeostasis
that provides an opportunity for either external contaminant or
opportunistic host bacteria to become pathogenic and cause infection.
While colonization necessarily precedes infection, the presence of
bacteria by itself does not constitute infection. This is highlighted
by the findings of one study of hardware removal in which 50% of
cultures were positive in patients with no signs of symptoms or
infection.80 Thus, there is an
important distinction between colonization and infection. Understanding
the factors that have changed the local or systemic environment with
resultant bacterial infection is the key to effective prophylaxis,
treatment, and improved outcomes in orthopaedic surgery.
acute or chronic depending on the duration of symptoms. Kelly
documented a classification system based on the etiology of the
osteomyelitis.61 There were four
types with type I being hematogenous osteomyelitis. Type II was
osteomyelitis associated with fracture union, while type III was
osteomyelitis without fracture union and type IV was postoperative or
posttraumatic osteomyelitis without a fracture. Weiland et al.123
in 1984 suggested another classification scheme based on the nature of
the bony involvement. In this classification system, there were three
types, with type I being characterized by open exposed bone without
evidence of osseous infection but with evidence of soft tissue
infection. In type II fractures, there was circumferential cortical and
endosteal infection, and in type III fractures, the cortical and
endosteal infection was associated with a segmental defect.
proposed another classification scheme for osteomyelitis focusing on
the tibia. This system was based on the nature of the bone following
soft tissue and bony débridement. They proposed that there were five
different categories.
Type
I posttraumatic tibial osteomyelitis was defined as being present when
the intact tibia and fibula were able to withstand functional loads and
no reconstruction was required. In type II osteomyelitis, the intact
tibia was unable to withstand functional loading and required bone
grafting. In type III osteomyelitis, there was an intact fibula but a
tibial defect that measured no more than 6 cm. The tibial defect
required cancellous bone grafting, tibiofibular synostosis, or
distraction histogenesis. Type IV osteomyelitis was characterized by an
intact fibula but with a defect of more than 6 cm in length, which
required distraction osteogenesis, tibiofibular synostosis, or a
vascularized bone graft. Type V osteomyelitis was characterized by a
tibial defect of more than 6 cm without an intact fibula, which often
required amputation.
categorized osteomyelitis into three primary etiologies—hematogenous,
contiguous (from an adjacent root such as an open fracture or a seeded
implant), or chronic, this being a longstanding osteomyelitis with
mature host reaction.
the beliefs and treatment options of the times, and they have all
become less relevant with current diagnostic and treatment modalities.
However, each classification represented an important effort to
categorize the pathophysiology of bone infection to facilitate the
choice of an effective treatment.
The usefulness of the Cierny-Mader system is its applicability to
clinical practice and the wealth of experience and data gleaned from a
single surgeon’s practice with meticulous records. The hallmark of
Cierny’s approach is the use of oncologic principles for treatment. In
fact, osteomyelitis behaves very similarly to a benign bone tumor in
that it is rarely lethal but has a tendency to return without complete
eradiation. Interestingly, the outcome data reported by Cierny et al.21
indicate that once appropriate surgical treatment is undertaken, the
host may be the most important variable affecting treatment and outcome.
analysis of the physiologic state of the patient or host. The host is
classified by the number of systemic and local comorbidities. An A host
has a healthy physiology and limb with little systemic or local
compromise. The B host is further divided into one with local
compromise (B local), systemic compromise (B systemic), or both (B
systemic/local systemic compromise, which includes any
immunocompromised condition, poor nutrition, diabetes, old age,
multiple trauma, chronic hypoxia, vascular disease, malignancy, or
organ failure such as renal insufficiency or liver failure). Local
compromise includes conditions such as previous surgery or trauma,
cellulitis, radiation fibrosis, scarring from burns or trauma, local
manifestations of vascular disease, lymphoedema, or zone-of-injury
issues. We believe that a new variable of compromise can be identified
in the trauma patient where systemic compromise is due to multiple
organ damage and the consequent systemic response to trauma and local
compromise is defined by the zone-of-injury effects on local tissues.
treatment is greater than the morbidity of disease because of multiple
and severe comorbid conditions that cannot be treated safely. In these
patients, the risks of curative treatment such as extensive surgery, as
might be used with free flaps, or prolonged reconstruction with bone
transport would be greater than that caused by the infective condition
itself. Type C hosts are often better treated with limited nonablative
surgery and suppression or, if appropriate, by an amputation.
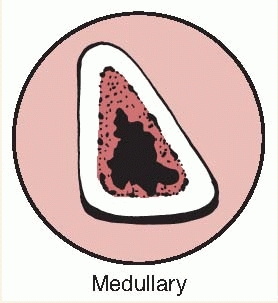
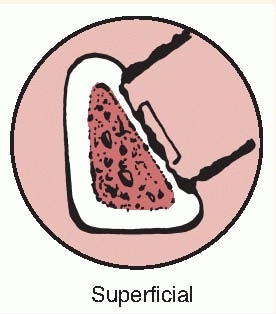
the bone lesion is classified by the extent of involvement and
stability. Type I is a medullary or endosteal infection without
penetration through cortex. This is the type of infection that occurs
after intramedullary nailing. Type II is a superficial osteomyelitis
that involves only the outer cortex and is frequently contiguous with a
pressure ulcer or adjacent abscess. Type III is permeative in that
there is involvement of both cortical and medullary bone but,
importantly, there is no loss of axial stability of the bone. Type IV
also involves cortical and medullary bone but in a segmental fashion
such that axial stability is lost. Types III and IV would be typical
infections related to open fractures. In type IV lesions, the segmental
resection that is required necessitates reconstruction of the bone,
whereas in type III lesions, additional stabilization may not be
required (Tables 24-1 and 24-2).
three host classes allows for the development of practical treatment
strategies. Cierny et al.21 proposed
a detailed treatment regimen defining optimal treatment modalities for
each stage. They achieved an overall clinical 2-year success rate of
91% for all states. As one would expect, when their results were broken
down by class of host and type of lesion. Class A hosts fared the best.
In class A hosts, success rates of 98% were achieved even with type IV
osteomyelitis. The compromised class B host success rates were far
lower, ranging from 79% to 92% depending on anatomic type. In his
series Cierny found that the host class seemed to be more important
than the type of infection. A cumulative success rate of greater than
90% was achieved with most of the failures being in B hosts. C hosts
were recommended for amputation or suppressive treatment.22
The lessons that stem from their findings are that it is important not
just to treat the disease but also the host and that the patient’s
physiologic condition should be optimized. Thus, a B systemic-local
host who has had a previous open fracture but also smokes and has
uncontrolled diabetes, renal insufficiency, and malnutrition should
have all of these problems treated together with the bone disease.
Improving host status would appear to be a fruitful endeavor when one
considers Cierny et al.’s21,22 findings.
results used outcome criteria that were commonly used at that time.
Current outcome studies focus more on subjective patient-based
assessments than on surgeon-based assessments. We do not have much data
on the functional outcomes in the scenarios described by Cierny and
colleagues, and it is possible that some of the patients whom they
salvaged would have fared better with prosthetic replacement and vice
versa. The findings of the LEAP study for acute limb salvage have
raised new questions about the true nature of outcome and success.65
vital to understand the mechanisms by which infections occur. Most
infections encountered in orthopaedics are related to biofilm-forming
bacteria.
Much of our understanding of biofilm bacteria has come from the Centre
for Biofilm Engineering in Bozeman, Montana. Biofilm bacteria are also
important in the oil, food processing, naval, paper manufacturing, and
water processing industries.
|
TABLE 24-1 Cierny-Mader Classification of Bone
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
Planktonic state bacteria are free floating in the extracellular matrix
and are isolated and relatively small in quantity. In this state, the
body host defenses can easily eradicate the organism through the usual
immunologic mechanisms. It is rare for planktonic bacteria to survive
long in the extracellular matrix despite numerous and repeated
occurrences of entry. However, if the bacterial load is large and
sustained, they can overwhelm the host defenses and escape the effects
of antibiotics. They can then invade tissue and blood, leading to
septicemia and death. Planktonic bacteria are also metabolically active
and reproductive. This is an important consideration for antibiotic
treatments that work by either interfering with cell wall or protein
synthesis or with reproduction.
as dead or necrotic tissue, foreign bodies, or any avascular body part
by either direct contamination, contiguous spreading, or hematogenous
seeding, they can attach and begin the process of colonization.
Juxtaposition of the bacteria with a surface or biomaterial is
accomplished by Van der Waals forces, which allow bacteria to develop
irreversible cross-links with the surface (adhesion-receptor
interaction).27
Adhesion is based on time-dependent specific protein adhesion-receptor
interactions, as well as carbohydrate polymer synthesis in addition to
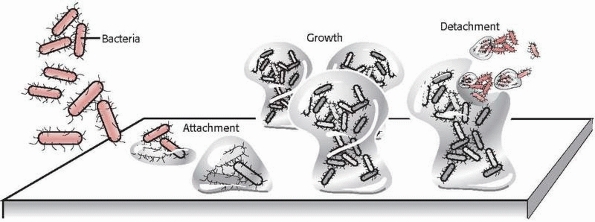
charge and physical forces.61 Following adhesion to a surface, bacteria begin to create a mucopolysaccharide layer called biofilm or slime. They then develop into colonies. These colonies exhibit remarkably resilient behavior. Figure 24-2
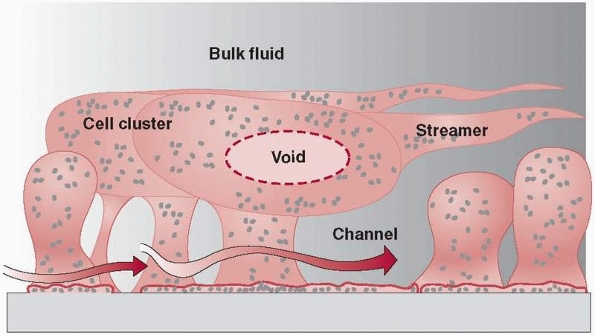
illustrates mature biofilm colonies where pillars of a mature biofilm
are visible distributed on top of a monolayer of surface-associated
cells. In addition to fixed cells, there are motile cells, which
maintain their association with the biofilm for long periods, swimming
between pillars of biofilm-associated bacteria.122
The interaction of the colonies and bacteria demonstrates complex
communication via proteins or markers that can alter bacterial behavior.
|
TABLE 24-2 Cierny-Mader Classification of the Host
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
FIGURE 24-1
Illustration of bacterial attachment to a surface followed by colonization and detachment. (Redrawn with permission after P. Dirckx, MSU Center for Biofilm Engineering, Bozeman, MT.) |
can be killed or neutralized by the host defenses. However, some of
these bacteria may escape destruction and potentially act as a nidus
for future infection. Transition from colonization to infection usually
requires other conditions to exist. This might occur if there was an
inoculum that was larger than threshold levels, impaired host immune
defense mechanisms, traumatized or necrotic tissues, foreign body, or
an acellular or inanimate surface such as dead bone, cartilage, or
biomaterials.
transition from colonization to infection requires bacterial adhesion,
which will usually not occur on viable tissue surfaces. Thus, when
foreign material or dead tissue is found in the body, a “race for the
surface” begins. Host cells will attempt to incorporate nonliving
material or sequester nonviable tissue via encapsulation so that a
well-incorporated biomaterial implant that has such a tissueintegrated
neocapsule will be resistant to bacterial adhesion. Furthermore, the
same tissue integration can often isolate bacteria that have become
sessile on an implant surface by sequestering the bacteria from
necessary nutrients until host mechanisms can act.
mature colonies, tissue integration by the host may be impaired and the
process of infection may proceed. Damaged bone, being relatively
acellular, acts as a suitable surface for bacterial adhesion and
colonization.69 Devitalized bone
devoid of normal periosteum presents a collagen matrix to which
bacteria can bind. Moreover, it has been suggested that bone
sialoprotein can act as a ligand for bacterial binding to bone.69
Biomaterials and other foreign bodies are usually inert and susceptible
to bacterial colonization because they are inanimate. Regardless of how
inert a metal is, it may still modulate molecular events on its
surfaces, these being receptor-ligand interactions, covalent bonding,
and thermodynamic interactions.44,50
The most important feature of any particular method is the interaction
between its outer surface atomic layer and the glycoproteins of
prokarytotic and eukaryotic cells. Stainless steel and cobalt-chromium
and titanium alloys are resistant to corrosion because of several
mechanisms including surface oxide passivates. These surface oxides
form a reactive interface with bacteria that can promote colony
formation. There is therefore a balance between implanting devices with
surface structures that lower corrosion rates but
might
increase the likelihood of surface binding by bacteria. Thus, a large
surface area and bacteria inoculum, combined with local tissue damage
and a compromised or insufficient host response, can collectively
create the necessary conditions for infection.
 |
|
FIGURE 24-2
Mature biofilm colonies showing potential intercolony communication. (Redrawn with permission after P. Dirckx, MSU Center for Biofilm Engineering, Bozeman, MT.) |
This resistance is dependent on the type of surface to which the
organisms are attached. Organisms that adhere to hydrocarbon polymers
are extremely resistant to antibiotics. These same organisms, when
attached to metals, do not resist antibiotic therapy to the same
extent. Bacterial colonies can undergo phenotypic changes and appear to
hibernate. They can survive in a dormant state without causing
infection, and this can explain the recovery of bacteria from
asymptomatic hardware removal.80 So while colonization is a necessary antecedent for infection, colonization alone does not necessarily lead to infection.
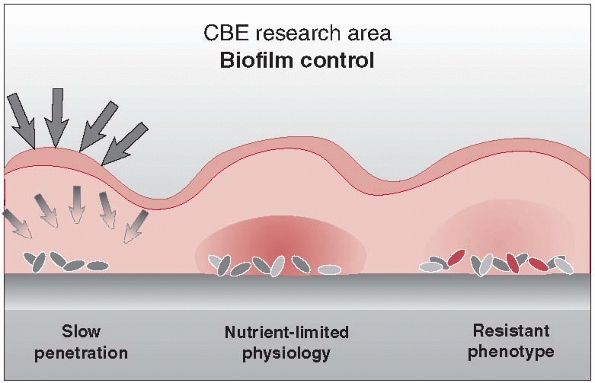
understand and explain this pseudo-resistance. Because the passage of
antibiotics through tissues is based on a diffusion gradient, colonized
bacteria are insulated with a natural barrier of glycocalyx, often
referred to as a slime, through which the circulating antibiotic must diffuse before arriving at the bacterial cell wall (Fig. 24-3).
The antibiotic molecules must then diffuse into the bacterial cell or
be transported by metabolically active bacterial cell membranes.
Because it is theorized that bacteria within biofilms have a decreased
metabolic rate and undergo phenotypic changes, active processes such as
cell membrane formation, which are targeted by antibiotics, would be
similarly decreased (Fig. 24-3).116
Consequently, antibiotic concentrations of 1500 times normal may be
required to penetrate both the biofilm and the bacterial cell wall.
Even then, most antimicrobials work via interference with cell wall
synthesis or cellular reproduction, and they therefore require
metabolically active bacteria to be effective. Thus, bacteria in the
biofilm may be dormant and appear to be pseudoresistant. The more
metabolically inactive the bacteria, the less bactericidal will be the
antibiotic therapy, which is why mature or chronic infections can
rarely be cured with antibiotics alone. Table 24-3
outlines the major antibiotic classes and their mechanisms of action,
all of which may be limited by the bacterial state in biofilm.
 |
|
FIGURE 24-3
Biofilm creates a diffusion barrier that interferes with the ability of antibiotics to reach bacterial organisms. Biofilm bacteria are metabolically inactive and therefore not subject to the mechanism of action of most antibiotics. They appear as “pseudoresistant.” (Redrawn with permission after P. Dirckx, MSU Center for Biofilm Engineering, Bozeman, MT.) |
identify bacteria as foreign. There may be chemotactic mechanisms that
keep immune cells active. The subsequent collection of inflammatory
cells brought in to wall off the bacteria via chemotaxis manifests as purulence,
which is a symptom of the host’s attempt to isolate and destroy the
infection. The acute inflammatory cells will also release a spectrum of
oxidative and enzymatic
products
in an attempt to penetrate the glycocalyx. These mediators and enzymes
are nonspecific and may be toxic to host tissue. Increased host tissue
damage can lead to more surface substrate for local bacteria, creating
a cycle of tissue damage, host response, and exacerbation of infection (Fig. 24-4).
The host tissues will eventually react to limit the spread of infection
macroscopically as well as microscopically. The clinical manifestation
of a sequestered infection is an abscess or involucrum. Alternatively,
if the infection grows and reaches the skin or an internal epithelial
surface, a sinus tract forms as a route to dispel detritus. While the
appearance of a sinus tract is a manifestation of a locally devastating
disease process and indicates severe underlying infection, it should be
remembered that it may also prevent the accumulation of internal
fixation, which can lead to bacteremia and septicemia.
|
TABLE 24-3 Major Antibiotic Classes and Their Mechanism of Action
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||
 |
|
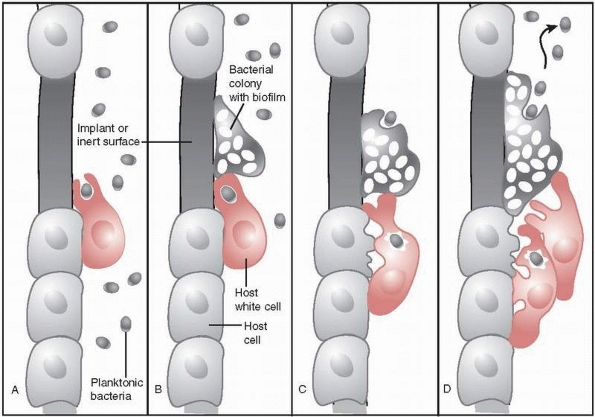
FIGURE 24-4 Autoinjury mechanism of host white cells in response to biofilm bacteria. A. Host white cell engulfs planktonic bacteria and then B. moves to engulf a bacterial colony that has developed but is unable to do so. C.
Host white cell’s next response to engulfed bacteria is to release oxidative enzymes, but those enzymes also cause damage to local host cells. D. Unsuccessful eradication of bacteria and colony growth attracts more host white cells, resulting in increased damage to host tissue. |
infection, which is what many surgeons see in practice. There is
usually a history of intermittent symptoms and drainage that has
responded to some type of antibiotic regimen. What this probably
represents is the inhibition of colony expansion at the borders of the
infectious site. Clinically harmful manifestations of infection are
generally caused by the release of bacteria into the bloodstream that
are metabolically active and release toxins in addition to the release
of oxidative enzymes by the host cell. Although the bacteria remain
susceptible to the body’s host defenses and to antibiotics, their
numbers and continued release into the bloodstream represent a chronic
debilitating disease. Any acute stress on the host environment from
trauma, disease, or immunosuppression can allow the infection to
strengthen and spread. Thus, longstanding infections that were
tolerated by young healthy individuals may suddenly become limb or life
threatening as the individual’s age.
group provide novel opportunities to treat bacterial infection of
orthopaedic implants. These include surface coatings, agents that
inhibit colonization or promote dissolution of colonies, small electric
fields, and low pH and acidic and negatively charged surfaces that are
resistant to biofilms. Surface properties of implants or local or
systemic drugs may help decrease the risk to infection, particularly in
the elderly population, who have decreased immune system activity.17
with open fractures or invasive surgical procedures. Few closed
fractures treated nonoperatively develop osteomyelitis. To improve the
diagnosis of posttraumatic bone infection, it is necessary to
understand the mechanisms of infection, particularly for open fractures.
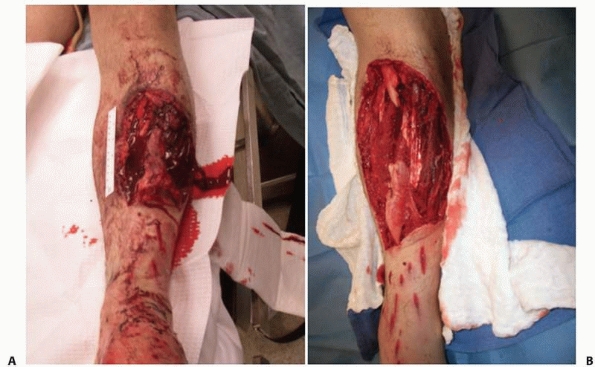 |
|
FIGURE 24-5 Operative photographs of a severe open fracture. A. The appearance before surgical débridement. B.
The appearance after surgical débridement. Note that after débridement, the tissues and wound appear as if they were surgically created. While it is unlikely that all bacteria have been removed, a thorough exploration and débridement leaving behind only viable tissues will minimize the risk of subsequent infection. |
contaminated by bacteria, but a much small percentage develop
infection. The risk of infection correlates significantly with the
degree of soft tissue injury.117 If
one remembers that merely the presence of bacteria in an open wound is
not sufficient to cause infection, it is important to recognize that a
severely contaminated fracture can rarely be débrided to the point of
achieving a sterile or bacteria-free tissue bed. We believe that next
to removing the majority of bacteria from the contaminated tissue bed,
the second major goal of a wide and aggressive débridement is to leave
behind a viable tissue bed with minimal necrotic or inert surfaces for
the remaining bacteria to colonize. By minimizing the bacterial
contamination by eliminating adhesions and nutrition, the host gains an
opportunity to eradicate any remaining contaminants in the zone of
injury. Figure 24-5 demonstrates the concept
of open fracture débridement where a contaminated wound is débrided
until the remaining wound looks as if it is created surgically, with
residual tissue being healthy with little evidence of contamination. It
is important to remember that contamination can penetrate into tissue
planes or locations that are not obvious in the initial wound. The use
of pulsatile irrigation before surgical exploration and débridement may
in fact push the initial contaminants deeper into the tissues and
result in contaminants being left behind in a locally compromised
tissue bed. This will increase the likelihood of both acute and delayed
infection.
bacteria recovered from clinical infections are not necessarily the
bacteria found acutely in the contaminated tissue bed. Several studies
have found that routine cultures of open fractures are not useful
because the predominant organism recovered from acute cultures is
frequently not the organism recovered if and when an infection occurs.
Antibiotic treatment based on the acute culture, whether before or
after débridement, may be detrimental
because
the antibiotic that is chosen may not be specifically indicated and has
the potential to promote changes and overgrowth in the bacterial flora.
In the worst case scenario, routine antibiotic treatment based on
initial wound cultures may promote the development of resistant
bacterial strains.63,92,118
which are not initially present in a traumatic wound. This does not
mean that other bacteria should not be considered and these may depend
on the environment. Clostridium perfringens must be considered if there is soil contamination and Pseudomonas, and Aeromonas hydrophila may be present following a freshwater injury. Vibrio and Erysipelothrix
may be present in saltwater injuries. One possible explanation for the
lack of correlation between acute cultures and the eventual infection
may be that the initial contaminants are of low virulence and easily
neutralized by a combination of débridement and antibiotics but that
the locally and, in polytrauma, the systemically, compromised tissue
bed is susceptible to the more aggressive nosocomial organisms.
that results in traumatic injury that allows pathogenic organisms to
make contact with damaged bone and soft tissues, with a proliferation
and expression of infection.74 In a
patient with traumatic injuries, additional factors that contribute to
the subsequent development of osteomyelitis are the presence of
hypotension, inadequate débridement of the fracture site, malnutrition,
sustained intensive care unit hospitalization, alcoholism, and smoking.42,115
Trauma may lead to interference with the host response to infection.
Tissue injury or the presence of bacteria triggers activation of the
complement cascade that leads to local vasodilatation, tissue edema,
migration of polymorphonuclear leukocytes (PMNs) to the site of the
injury, and enhanced ability to phagocytes to ingest bacteria.56
Trauma has been reported to delay the inflammatory response to bacteria
as well as to depress cell-mediated immunity and to impair the function
of PMNs, including chemotaxis, superoxide production, and microbial
killing.56 The commonly used system of Cierny-Mader21
has been shown to have a close correlation with the general condition
of the patient rather than the specifics of bone involvement.
osteomyelitis that is inadequately treated. General factors that may
predispose to chronic osteomyelitis include the degree of bone
necrosis, poor nutrition, the infecting organism, the age of the
patient, the presence of comorbidities, and drug abuse.26
The infecting organism generally varies with the cause of the chronic
osteomyelitis. Chronic osteomyelitis results from acute osteomyelitis
and is frequently caused by S. aureus,
although chronic osteomyelitis that occurs after a fracture can be
polymicrobial or gram negative. Intravenous drug users are commonly
found to have Pseudomonas as well as S. aureus
infections. Gram-negative organisms are now seen in up to 50% of all
cases of chronic osteomyelitis, and this may be due to variables such
as surgical intervention, chronic antibiotics, nosocomial causes, or
changes in the bacterial flora of the tissue bed.26
The fundamental problem in chronic osteomyelitis is a slow progressive
revascularization of bone that leaves protected pockets of necrotic
material to support bacterial growth that are relatively protected from
systemic antibiotic therapy. This collection of necrotic tissue, bone,
and bacteria is what becomes termed a sequestrum,
and the body’s attempt to wall off the offending material with reactive
inflammatory tissue, whether this is bone or soft tissue, is termed the
involucrum. The involucrum can be highly
vascular and may be viable and structural, and this should be taken
into consideration during surgical débridement.
cause infections in immunocompromised hosts. Infection is introduced by
direct trauma or injury but may be associated with a penetrating
foreign body or hematogenous spread.
common fungus seen in osteomyelitis. It affects both native and
prosthetic joints, vertebrae, and long bones. Risk factors include loss
of skin integrity, diabetes, malnutrition, immunosuppressive therapy,
intravenous drug use, hyperalimentation, the use of central venous
catheters, intra-articular steroid injections, and the use of
broad-spectrum antibiotics. A combined approach to therapy using
medical and surgical modalities is necessary for optimal results. Azole
antifungals and lipid preparations of Amphotericin B have expanded the
therapeutic options in fungal osteomyelitis as there is reduced
toxicity associated with long-term therapy.74
as of remote surgery or trauma should raise the clinical suspicion of
osteomyelitis. Normal signs of inflammation may be absent and thus the
diagnosis of infection may be difficult. Patients may have a history of
infection at another site, such as the lungs, bladder, or skin in
conjunction with a history of trauma. They usually complain of pain in
the affected area and feel generally unwell. Moreover, reduced
activity, malaise, anorexia, fever, tachycardia, and listlessness may
be present. Local findings include swelling and warmth, occasional
erythema, tenderness to palpation, drainage, and restricted range of
motion in adjacent joints.
surgeon to look for infection include a history of open fracture,
severe soft tissue injury, a history of substance abuse and smoking,
inadequate previous treatment, or an immunocompromised state. These are
all factors that contribute to a B host. Factors affecting treatment
that need to be assessed include the time of onset of the infection,
the status of the soft tissues, the viability of the bone, the status
of fracture healing, implant stability, the condition of the host, and
the neurovascular examination (Fig. 24-6).
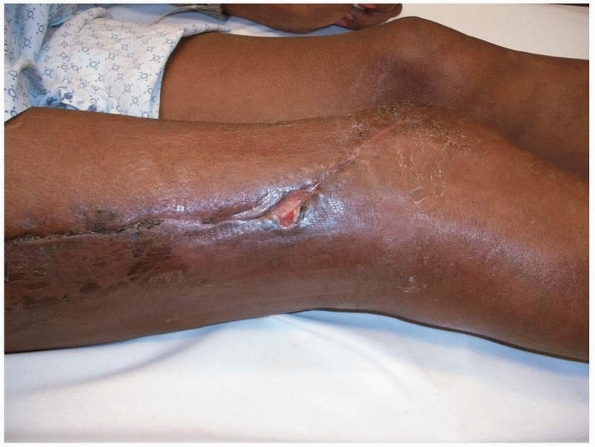 |
|
FIGURE 24-6
Typical appearance of a postoperative wound. The limb looks relatively benign. This patient had an extensive type III infection and had been treated with attempted débridement on several occasions before referral. Poor nutrition and nicotine use together with her previous multiple surgeries made her a B systemic/local host. |
patients show manifestations of systemic disease, but they may be
positive in up to 50% to 75% of cases where there is concomitant
bacteremia or septicemia.124 Blood
cultures that yield coagulasenegative staphylococci, a common
contaminant and pathogen, must be correlated with other clinical
findings before attribution of clinical significance. Blood results
that are suggestive of infection include an elevation of the white
blood cell (WBC) count and elevations in the C-reactive protein (CRP)
and erythrocyte sedimentation rate (ESR) levels. The ESR may be normal
in the first 48 hours but rises to levels about 100 mm/hr and may
remain elevated for several weeks. It is, however, a nonspecific marker.124
Combination of the ESR with the CRP improves specificity such that if
both are negative, the specificity is 90% to 95% for acute
osteomyelitis. In other words, a negative CRP and ESR makes
osteomyelitis unlikely. Their values are also age dependent, and there
is a steady increase in normal values with aging. In one recent study,
the ESR and CRP were found to be useful diagnostic tools for the
detection of an infected arthroplasty. While they had low sensitivities
and positive predictive values and therefore were of little value for
screening, they had high specificity and negative predictive value and
therefore were useful for treatment decisions.49
These studies and other diagnostic studies may not be as useful in
acute postoperative and chronic infections. In the acute setting, the
ESR and CRP are expected to be elevated due to local and systemic
inflammation from the surgical procedure. In chronic infections, the
host has had time to adapt to the offending condition and thus may not
mount the response required to trigger an elevation in these tests.
Once osteomyelitis treatment is initiated, the CRP and ESR are useful
in following the response to treatment. We use the ESR and CRP to
establish a baseline value before débridement and initiation of
antibiotic therapy and to monitor the subsequent response to treatment.
osteomyelitis are often normal. The most common radiographic signs of
bone infection are rarefaction, which represents diffuse
demineralization secondary to inflammatory hyperemia; soft tissue
swelling with obliteration of tissue planes; trabecular destruction;
lysis; cortical permeation; periosteal reaction; and involucrum
formation. Radiologically detectable demineralization may not be seen
for at least 10 days after the onset of acute osteomyelitis.124
When present, mineralization usually signifies trabecular bone
destruction. If the infection spreads to the cortex, usually within 3
to 6 weeks, a periosteal reaction may be seen on radiographs. One study
reported that in cases of proven osteomyelitis, 5% of radiographs were
abnormal initially, 33% were abnormal by 1 week, and 90% were abnormal
by 4 weeks.6 In trauma and fracture
treatment, the nature of callus formation and the obfuscation of bone
by hardware may make radiologic changes difficult to recognize in the
early or middle states of infection. Often it is not until there is a
clear sequestrum, sinus, or involucrum that parallels the clinical
findings that specific radiographic changes are recognized (Fig. 24-7).
useful diagnostic tool. There are numerous types of scintigraphy, but
three scan types are commonly used to diagnose musculoskeletal
infection. These are the bone scan, which uses tagged red cells; the
leukocyte scan, which uses tagged white cells; and the bone marrow
scan, which investigates marrow cell activity. Recently, positron
emission tomography (PET) has shown promise and is undergoing increased
investigation and use.
Technetium is formed as a metastable intermediate during the decay of
molybdenum-99. It has a 6-hour half-life and is relatively inexpensive
and
readily available.28
After intravenous injection, there is a rapid distribution of this
agent throughout the extracellular fluid. Within several hours, more
than half the dose will accumulate in bone, while the remainder is
excreted in the urine. Technetium phosphates bind to both the organic
and inorganic matrix. However, the key characteristic that makes
technetium scanning useful is that there is preferential incorporation
into metabolically active bone. Bone images are usually acquired 2 to 4
hours following intravenous injection of the isotope. A triple-phase
bone scan is one that is useful for examining general inflammation and
related processes. Following the initial injection, dynamic images are
captured over the specified region. These are followed by static images
at later time points. The first phase represents the blood flow phase,
the second phase immediately postinjection represents the bone pooling
phase, and the third phase is a delayed image made at 3 hours when
there is decreased soft tissue activity. Classically, osteomyelitis
presents as a region of increased blood flow, and it should appear
“hot” in all phases with focal uptake in the third phase (Fig. 24-8).
Other processes such as healing fractures, loose prostheses, and
degenerative change do not appear hot in the early phase despite a hot
appearance in the delayed phase. Reported sensitivities of bone
scintigraphy for the detection of osteomyelitis vary considerably from
32% to 100%. Reported specificities have ranged from 0% to 100%.103,120
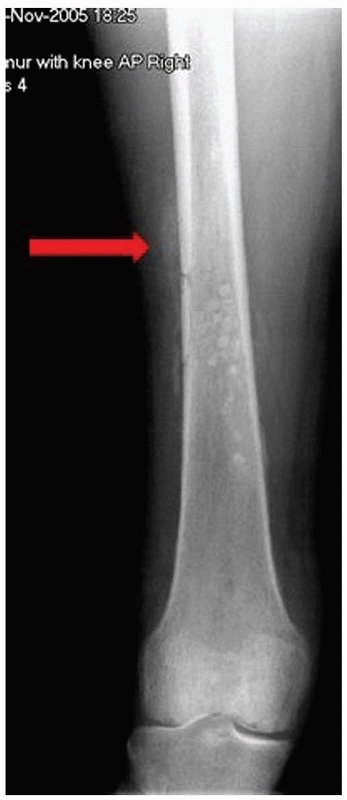 |
|
FIGURE 24-7 Radiograph of patient in Figure 24-6. The arrow points to periosteal reaction.
|
There is also uptake in the blood, especially by leukocytes. Gallium
has been used in conjunction with technetium-99 to increase the
specificity of the bone scanning.40,52
Several mechanisms have been postulated to explain the increased
activity at sites of inflammation. Enhanced blood flow and increased
capillary permeability cause enhanced delivery. Bacteria have high iron
requirements and thus take up gallium. Gallium is strongly bound to
bacterial siderophores and leukocyte lactoferrins. In regions of
inflammation, these proteins are available extracellularly and can bind
with gallium avidly. Chemotaxis also acts to localize gallium-labeled
WBCs at the sites of infection. In a typical study, gallium is injected
intravenously and delayed images are acquired at 48 to 72 hours. The
hallmark of osteomyelitis is the focal increased uptake of gallium.
Unfortunately, gallium’s nonspecific bone uptake can be problematic
because any processes causing reactive new bone formation will appear
hot. In patients with fractures or a prosthesis, osteomyelitis cannot
be easily diagnosed with gallium alone. Gallium images are usually
interpreted in conjunction with a technetium bone scan. Gallium
activity is interpreted as abnormal either if it is incongruous with
the bone scan activity or if there is a matching pattern with gallium
activity. Reported sensitivities and specificities for the diagnosis of
osteomyelitis range from 22% to 100% and 0% to 100%, respectively.2,52,76,103
Despite its lower-than-optimal diagnostic value, gallium still has some
advantages. It is easily administered and it is the agent of choice in
chronic soft tissue injection, although it is less effective in bone
infections. It has also proved useful in following the resolution of an
inflammatory process by showing a progressive decline in activity.
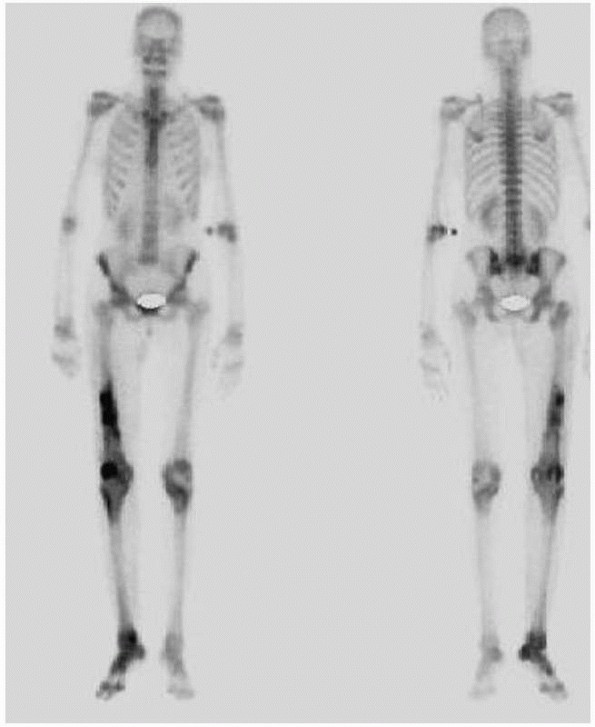 |
|
FIGURE 24-8 Red cell scan of patient in Figure 24-6 demonstrating increased activity in distal femur.
|
(99mTc-HMPAO) (Ceretec; GE Healthcare) -labeled leukocyte scan is the
most common scan used in conjunction with a standard bone scan. The
labeled leukocytes migrate to the region of active infection resulting
in a hot white cell scan over the area of active inflammation. The use
of a combined red cell and white cell scan significantly increases both
the sensitivity and specificity and now represents the gold standard of
radionuclide testing for infection.68 Because of the variable accuracy of both technetium and gallium scans most laboratories routinely use 111In-labeled leukocytes.100,102,106,119
Indium WBC preparations require the withdrawal of approximately 50 mL
of autologous whole blood with a leukocyte count at least 5000 cells/ mm3.
The leukocytes are labeled with 1 mCi of indium oxine and then
reinjected. They redistribute in the intravascular space. Immediate
images show activity in the lungs, liver, spleen, and blood pool. The
half-life is about 7 hours. After 24 hours, only the liver, spleen, and
bone marrow show activity. Wounds that heal normally and fully treated
infections show no increase in uptake. Leukocytes that migrate to an
area of active bone infection will show increased uptake (Fig. 24-9). Most results show improved sensitivity (80% to 100%) and specificity (50% to 100%) for the diagnosis of ostemyelitis.29,30,31,54,58 Indium-labeled WBC scans are generally superior to bone scans and gallium scans in the detection of infection. McCarthy et al.72
reported on the use of indium scans in 39 patients who had suspected
osteomyelitis confirmed by bone biopsy. They found indium scans to be
97% sensitive and 82% specific for osteomyelitis. The few
false-positive results occurred in patients who had overlying soft
tissue infections. An accompanying bone scan can help to differentiate
bone infection from soft tissue infection. In these situations, the
indium scan should be performed before the bone scan to avoid
false-positive results. With both tests, the sensitivities and
specificities are in excess of 90%.
imaging and 111In-oxine leukocyte imaging.29
Scientific advances, especially in nuclear medicine, have increased
these choices considerably. Several techniques in nuclear medicine have
significantly aided the diagnosis of infection including imaging with
99mTc-HMPAO and 99mTc-stannous fluoride colloid-labeled leukocytes.58
The principal clinical indications for 99mTc-HMPAO leukocytes include
osteomyelitis and soft tissue sepsis. Chronic osteomyelitis, including
infected joint prostheses, is better diagnosed with 111In-labeled
leukocytes.91 The use of 99mTc-HMPAO
leukocyte scintigraphy in patients with symptomatic total hip or knee
arthroplasty has shown improved diagnostic accuracy through the use of
semiquantitative evaluation.123
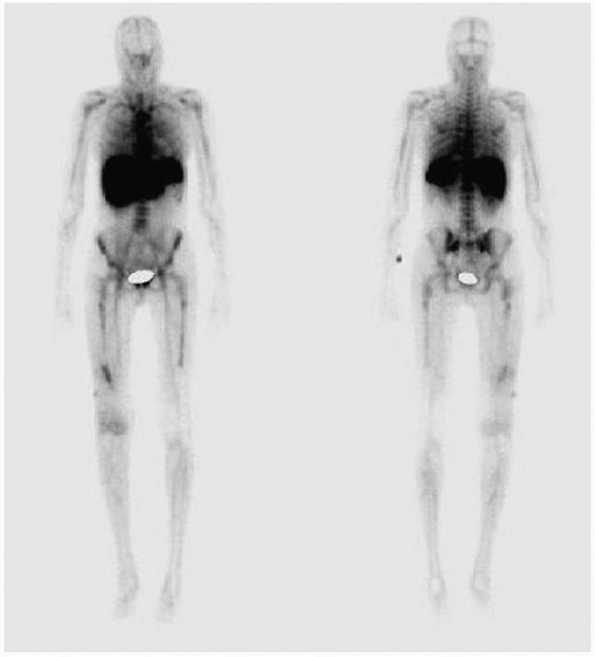 |
|
FIGURE 24-9 White cell scan of patient in Figure 24-6 demonstrating increased accumulation of tracer in distal femur.
|
being increasingly used for diagnosis of infection. The scan evaluates
the bone marrow activity in an area where infection is suspected. The
marrow may be reactive in several conditions that are not infected and
is generally suppressed when infection is present. With the use of
microcolloid bone marrow scans, more information is available to
increase the specificity of diagnosis. There is the possibility of
leukocyte accumulation with certain inflammatory conditions that could
result in a false-positive indium scan. An infection will tend to
suppress marrow activity and thus render the marrow scan cold, while
the white cell scan may still be hot (Fig. 24-10). If the white cell scan is as hot as the marrow scan, it is possible that an infection may not be present. Segura et al.109 examined technetium-labeled white cell scans (Tc-HMPAO) and
technetium microcolloid marrow scans in total joint replacements. They
found that in 77 patients, the white cell scans by themselves had a
sensitivity of 96% and a specificity of 30%. When the colloid scan was
added, the sensitivity decreased to 93% but the specificity increased
to 98%. The addition of a regular red cell scan was not helpful.109 In another study by Palestro et al.,90
an indium-labeled white cell scan was compared with technetium sulfur
colloid scans to differentiate infection from Charcot arthropathy. They
found that white cell scans were positive in 4 of 20 cases, of which 3
were infected. In the 16 negative white cell scans, the marrow scan was
also negative. However, in the 4 positive cases, the marrow scan was
positive in 2 cases that were confirmed to be infected. They concluded
that white cell scans can be positive in hematopoietically active
bones, which can occur in the absence of infection, and that marrow
scans should be used to confirm the diagnosis.
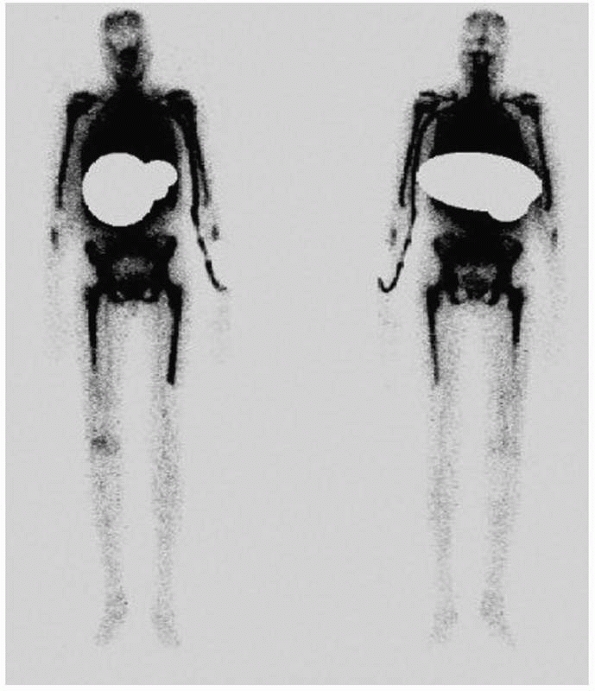 |
|
FIGURE 24-10 Marrow scan of patient in Figure 24-6 demonstrating relative suppression of marrow in distal femur.
|
test remain the gold standard diagnostic method in posttraumatic
infection. This is especially true because the presence of metallic
implants limits the usefulness of magnetic resonance imaging (MRI)
scanning. Classically, a combination of red cell bone scan and a
labeled leukocyte scan has been used. Because standard bone scan agents
and gallium are usually both positive at fracture sites, they have
limited value in the detection of infection following a fracture. With
no discernible uptake in reactive bone, indium-labeled WBC scans are
superior in the detection of infection following fracture. In a
prospective study of 20 patients with suspected osteomyelitis together
with delayed union, Esterhai et al.41 reported 100% accuracy of indium-labeled WBC scans. Seabold et al.107
have shown that the use of indium-labeled WBC scans and bone scans, to
differentiate between soft tissue infections, can be 97% specific for
osteomyelitis. In chronic or recurrent osteomyelitis, bone scans by
themselves are of less value since they show increased uptake for 2
years following the successful treatment and resolution of infection.48 Although gallium scans have historically been shown to be successful in following the resolution of chronic
osteomyelitis, indium-labeled WBC scans appear to be superior. Merkel et al.75
compared indium-labeled WBC and gallium scans in a prospective study of
50 patients. They found that indium-labeled WBC scans had an accuracy
of 83% compared with 57% for gallium scans in the detection of
osteomyelitis. However, it is important to remember that all clinical
data, including a detailed clinical history, a characterization of the
host, appropriate laboratory studies, clinical examination, and
radiographic studies, are important in determining the likelihood and
extent of infection.
marrow scan should be added to the combination of red cell and white
cell scans to increase the accuracy of scintigraphy. We have
investigated the usefulness of these three scans read by a nuclear
medicine specialist in a blinded fashion using receiver operating
curves. We did not find any particular scan combinations that provided
a reliable screening tool (sensitivity), but we did find that certain
combinations provided good treatment decisions (specificity).
Furthermore, the combination of white cell and marrow scans was
equivalent to the combination of all three scans and better than the
combination of red and white cell scans, which implies that the red
cell scan may be of limited value. While the sensitivities of all of
these tests and combinations were low, the specificities remained at
about 90% and the conclusion was that standard red cell scanning may
not be necessary for the diagnosis of posttraumatic infection.
Furthermore, we corroborated the findings of Segura et al.109
and found that the red cell scan added little. Unfortunately, surgeons
often continue to base their suspicion of infection on a simple bone
scan. Table 24-4 illustrates the matrix as a guide to assist the interpretation of bone scan combinations.24
and infectious conditions occurs via several routes. The labeled agents
can bind to activate endothelium (anti-E-selectin). They can also
enhance the influx of leukocytes or related by products (autologous
leukocytes, antigranulocyte antibodies, or cytokines), and they can
enhance glucose uptake by activated leukocytes (18Ffluorodeoxyglucose
[FDG]). In addition, they bind directly to microorganisms (radiolabeled
ciprofloxacin or antimicrobial peptides). Labeling of polyclonal
immunoglobin is a newer technique to investigate infection. It uses
antigranulocyte antibodies, radiolabeled nonspecific human immunoglobin
(IgG), interleukins, and antimicrobial peptides.9
The nonspecific polyclonal IgG prepared from human serum gamma globulin
can be labeled with various agents, including indium, gallium, or
technetium, and can be used for the detection of osteomyelitis.9,95,102
Unlike labeled leukocyte scans, the immunoglobulin agent is easily
prepared with short blood half-lives of about 24 hours. The primary
uptake occurs in the liver with less bone marrow uptake.74
|
TABLE 24-4 Matrix of Scintigraphic Combinations and Potential for Infection
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||
musculoskeletal infection in patients in whom sterile inflammatory
events stimulate infectious processes.78
However, despite its usefulness, this modality has not yet found its
way into common clinical practice. PET with FDG has been shown to
delineate various infective and inflammatory disorders with a high
sensitivity. The FDG-PET scan enables noninvasive detection and
demonstration of the extent of chronic osteomyelitis with 97% accuracy.33 PET scanning is especially accurate in the central skeleton within active bone marrow.32
Although not yet in widespread use, it may well prove to be the single
most useful test in specifically diagnosing bone infection. In one
study, the overall accuracy of FDG-PET in evaluating infection
involving orthopaedic hardware was 96.2% for hip prostheses, 81% for
knee prostheses, and 100% in 15 additional patients with other
orthopaedic implants. In patients with chronic osteomyelitis, the
accuracy is 91%.19 FDG-PET scanning appears to be a sensitive and
specific method for the detection of infective foci due to metallic
implants, which makes it useful in patients with trauma. Sensitivity,
specificity, and accuracy were 100%, 93.3%, and 97%, respectively, for
all PET data. The figures were 100%, 100%, and 100% for the central
skeleton and 100%, 87.5%, and 95%, respectively, for the peripheral
skeleton.104
The sensitivity and specificity of MRI scans for osteomyelitis range
from 60% to 100% and from 50% to 90%, respectively. MRI has the spatial
resolution necessary to accurately evaluate the extent of infection in
preparation for surgical treatment and particularly to localize abscess
cavities. T1- and T2-weighted imaging is usually sufficient; fat
suppression and short tau inversion recovery (STIR) sequences may be
added to improve the imaging of bone marrow and soft tissue
abnormalities. MRI also has the ability to differentiate between
infected bone and involved adjacent soft tissue structures. Images can
be acquired in any orientation and there is no radiation exposure.
Occasionally a sinus tract can be identified (Fig. 24-11).
Gadolinium enhancement should be obtained in the postoperative
population to better differentiate postsurgi-cal artifact from
infection-related bone marrow edema patterns. Gadolinium may
differentiate abscess formation from diffuse inflammatory changes and
noninfectious fluid collections.
decreased signal in T1-weighted images and appears bright in
T2-weighted images. The process presents the replacement of marrow fat
with water from edema, exudates, hyperemia, and ischemia. However, the
MRI signal characteristics that reflect osteomyelitis are intrinsically
nonspecific, and tumors and fractures can also increase the marrow
water content. In patients without prior complications, MRI has been
found to be sensitive, but not specific, for osteomyelitis. When a
fracture or prior surgery is evident, MRI is less specific in the
diagnosis of infection. Furthermore, in the presence of metallic
implants, artifacts make it difficult to comment on areas of interest
that are near the implant. Certain external fixators are not compatible
with
MRI
and thus will preclude its use. We have found that the best use of MRI
is helping to determine the extent of the infection for preoperative
planning. Our experience has been that using MRI to plan the degree of
bone resection or débridement is helpful but that MRI may lead to an
overestimation of the extent of the infection due to the detection of
adjacent edema.
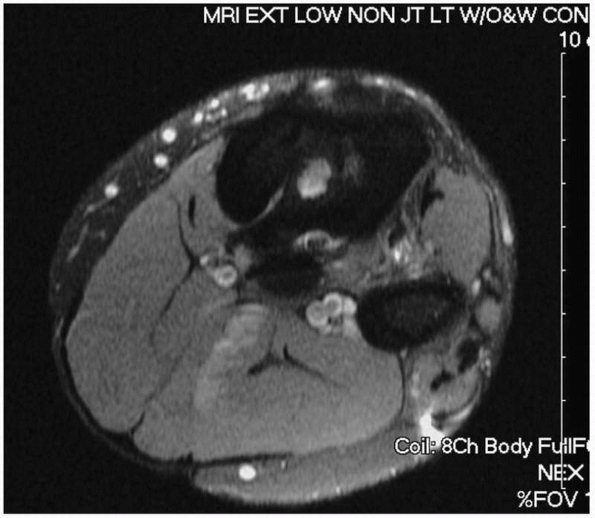 |
|
FIGURE 24-11 Magnetic resonance image of infected tibia with sinus tract leading to central sequestrum (white area in medullary canal).
|
However, CT remains unsurpassed in the imaging of cortical bone. It is
especially useful in delineating the cortical details in chronic
osteomyelitis.112 CT is also useful
in evaluating the adequacy of cortical débridement in the staged
treatment of chronic osteomyelitis. Thus, it can help differentiate
between type III and type IV infections. With modern equipment, CT
scanning around fracture implants has improved and it can help evaluate
both bone pathology and the extent of bone union. CT is also valuable
in the treatment of extensive osteomyelitis in that it can determine
the extent of bony involvement. In chronic cases, with some remodeling
of both host and pathologic bone, it can often differentiate and
identify sequestra or sclerosed diseased bone. It may often demonstrate
useful pathologic findings (Fig. 24-12).
antibiotic resistance patterns are crucial to a successful outcome in
the management of osteomyelitis. With regard to open fractures, the
issue of culturing the predébridement or postdébridement tissue bed is
often discussed among surgeons and infectious disease specialists. In
civilian wounds, gram-positive bacteria usually predominate at the time
of injury but frequently change to gram-negative bacteria, which are
often the cause of late infections. In one study, 119 of 225 open
fractures had positive wound cultures with only 8% of predébridement
cultures correctly identifying the infecting organism, while 7% of
those with negative predébridement cultures also developed infection.
In only 22% did the postdébridement cultures correlate with the
ultimate infecting organism. These data are clinically relevant because
treating the wrong bacteria can promote overgrowth of the true
infecting bacteria or add to the development of resistant organisms. In
recent war experience, military surgeons have noted different bacteria
flora causing infection, but the same principles still apply to their
treatment.15,52,83,118 While cultures of the sinus tract can be helpful, they should not be the sole guide for antibiotic treatment.41 A study by Moussa et al.81
found that 88.7% of sinus tract isolates were identical to operative
specimens in 55 patients with chronic bone infection. However, other
researchers have reported a concordance rate of between 25% and 45%.66
The ability to obtain true deep cultures of the sinus improves
concordance, but it is still not helpful. One study concluded that
nonbone specimens had a worse concordance than bone specimens and were
associated with 52% false negatives and 36% false positives.128
It is important to recognize not only that superficial cultures of
sinus tracts, open wounds, and fractures are unhelpful but also that an
error in bacterial identification may lead to inappropriate antibiotic
selection and ultimately compromise patient outcome. Bone biopsy
remains the preferred diagnostic procedure in chronic osteomyelitis.
Multiple specimens should be obtained if possible, not only to minimize
sampling error but also to increase specificity and sensitivity.
Histologic and microbiological evaluation of percutaneous biopsy
samples should be combined in cases of suspected osteomyelitis. The
sensitivity of culture in the diagnosis of osteomyelitis can be
improved from 42% to 84% by the addition of histologic evaluation.
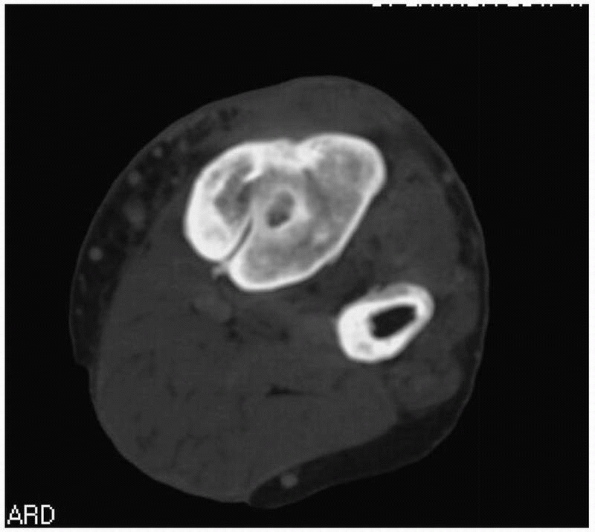 |
|
FIGURE 24-12 Same patient as Figure 24-11 but demonstrating appearance of sinus on computed tomography scan.
|
and RNA or DNA typing are currently in development to facilitate
diagnosis in osteomyelitis. These techniques offer a precise method of
organism identification in cases in which standard techniques do not
identify a pathogen despite the clinical presence of infection. This
scenario is not uncommon in patients who have been treated with
antibiotics shortly before sample collection. These methods target
specific macromolecules unique to the infecting pathogens that are
absent in the host cells.39,116 They have the
potential to provide rapid results with high accuracy.57 The most commonly used method for the diagnosis of orthopaedic infections is the polymerase chain reaction (PCR).57
This has been used to identify microremnants of bacteria by identifying
their nuclear contents. Sequences within bacterial 16S ribosomal RNA
have served as targets for amplification and detection.57
Unfortunately, PCR cannot easily delineate between nuclear materials
from living or dead bacteria. This increases the likelihood of false
positive studies. Further investigations are required before these
techniques can be widely used as currently they lack sufficient
sensitivity and specificity. However, their use remains promising.
There have been recent reports of real-time testing that also appear
more reliable and rapid, which could be very useful when deciding
whether there is ongoing infection before undertaking a procedure
requiring the implantation of orthopaedic hardware.113
of fractures, postoperative infection is an unfortunate reality. The
important questions to be posed are “Is there an infection?” and then
“What do I do now?” The challenge is the difficulty in being certain
whether an infection exists because we do not have an absolutely
reliable method of determining the necessary elements of true
postoperative or posttraumatic infection. As previously discussed, the
establishment of colonization followed by an opportunistic bacteria, in
a compromised host or environment, is necessary to allow an infection
to occur. Furthermore, the accuracy and reliability of available
diagnostic tests are not 100% and therefore experience and clinical
judgment are vital and could be more considered more important than the
tests that are currently available.
principles but also must be tailored to the reality of the individual
clinical scenario. It would be naive to assume that an inflamed and
draining postoperative wound in a polytraumatized patient (B systemic)
with significant fracture treatment (B local) that responds to a short
course of antibiotics has no chance of recurrence. In such a case,
sufficient treatment might have been provided so the balance would tip
to favor the host defense mechanism. However, what probably occurred
was that the threshold level for infection was exceeded and the body
manifested a response in the form of inflammation and drainage. With
the use of antibiotic treatment, the bacterial counts were reduced so
that the host was able to take over and sequester the infection
effectively. Thus, an initial positive response to antibiotic therapy
does not necessarily mean that the infection was eradicated. It simply
means that it was suppressed and possibly sequestered. Using an
oncologic analogy, the infection may have been forced into remission,
possibly for an indefinite period, but many of these patients present
later with signs of infection in the same limb. Unfortunately, by this
time the patient may be older, in poorer health, and less able or
willing to tolerate an aggressive ablative procedure, thus lowering the
potential for cure. In the long run, early suppression may cause the
patient more physical and psychological harm than early aggressive
measures to achieve complete eradication. On the other hand, an
overaggressive approach may require extensive reconstructive efforts
that lead to other problems. Thus, in a world seeking clear
blackand-white answers, osteomyelitis usually presents as a shade of
gray!
multidisciplinary approach that begins with diagnosis and optimization
in the form of medicine and radiology and then combines débridement,
soft tissue coverage, antimicrobial therapy using orthopaedic surgery,
microvascular surgery, and infectious disease. This gives the best
chance of a cure.38,70
Initially, the infection needs to be diagnosed and the host optimized.
This involves treating any comorbidities and optimizing the physiologic
condition of the host. Interventions involving nutrition, the use of
nicotine, diabetes, vascular disease, and improvement of tissue
oxygenation will increase the chances of successful treatment. Second,
the osteomyelitis needs to be classified and staged. It is then
important to identify the organism to determine appropriate
antimicrobial treatment. This can be done independently with a bone
biopsy or deep culture or, more commonly, it is done at the time of
surgery. Identification will also give an idea of the potential
virulence of the causative organism, which may influence decisions
regarding treatment. We recommend that if the risk of sepsis or
amputation is low, then a period of time off all antibiotics may
improve bacterial identification. This may be more important in
longstanding cases where the usual organisms may have been replaced by
other, more exotic species.
host and the infecting organism are understood, determination should be
made regarding one of several general treatment algorithms. Available
treatment options include attempted ablation and cure of the infection,
or, in selective cases such as C hosts who are not suitable for
surgery, some type of suppressive treatment may be undertaken.
Attempted ablation and complete cure have numerous issues and
decision-making steps and will often require the oncologic equivalent
of a wide resection with clean margins. While a surgically clean bed
with extensive resection is desirable, all efforts should be made to
maintain skeletal axial stability where possible. Thus, retention of a
well-vascularized but affected involucrum or a viable segment of bone
adjacent to infection may be preferable to a segmental resection that
would add a level of complexity to the treatment regime. If an adequate
resection would result in an overextensive reconstruction that is
unsuitable for the host’s function or desire, an amputation is the best
option and it should not be considered a failure. In some cases of
life- or limb-threatening infection, debulking of the infection may be
a suitable first step followed by chronic suppression. In these
circumstances, identification of the infecting bacteria is required to
allow use of a specific antibiotic. Otherwise, broad-spectrum
antibiotics are required.
infectious disease colleagues. Modern antimicrobials have become so
numerous and complex that their expert involvement is likely to
increase the chances of successful treatment. Increasingly, in many
hospitals bone infection mandates an infectious disease consult because
of the risk of inadvertently creating resistance pathogens, the
efficacy of combined antibiotic protocols, patient safety issues, and
the cost of new treatment regimens. However, it is still important that
the orthopaedist has an understanding of antimicrobial treatment since
it is the orthopaedist who will initiate the treatment before
consulting his or her infectious disease colleagues. We also recommend
that orthopaedic surgeons recognize that not all infectious disease
practices are evidence
based
and not all specialists have a specific interest and training in bone
infections. Therefore, it is vital that the orthopaedist initiates an
open and collegial partnership with the infectious diseases specialists
in his or her community to work on behalf of the patient. Both the
orthopaedic surgeon and the infectious diseases physicians should work
together to employ a consistent strategy of surgical and
chemotherapeutic treatment predicated on the best evidence and logic
available. Ziran et al.126
showed that a dedicated team approach can enhance the outcomes of
treatment, providing higher cure and successful suppression rates. The
subsequent sections in this chapter will briefly review both systemic
and local antimicrobial agents as well as discuss the techniques and
implants used during treatment. Specific scenarios and algorithms will
then be reviewed.
compounds that promote detachment of stationary colonized bacteria into
the planktonic state, where they are easier to eradicate. The
techniques include surface treatments that inhibit colonization and the
use of a light-activated dye that destroys certain bacteria in wounds.
In this latter technique, indocyanine green, which is a harmless dye,
is placed into the wound and activated with near infrared light. The
light results in the dyereleasing molecules that are toxic to the
methicillin-resistant S. aureus (MRSA)
bacteria. The mechanism of action is so varied and unlike standard
antibiotics that the development of resistance may be unlikely. These
and other methods under development may provide novel adjunctive
treatments to help treat osteomyelitis.
|
TABLE 24-5 Common Bacteria-Specific Antibiotic Regimens
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||
administer, affordable, and based on the in vitro susceptibilities of
the microorganisms. Regimens for the initial treatment of osteomyelitis
by common bacteria are outlined in Table 24-5.
Since bone represents a unique compartment of the body, the
antimicrobial that is used should have reliable bone penetration. Serum
and bone concentrations associated with the use of commonly used
antibiotics are given in Table 24-6. The
duration of antibiotic therapy for osteomyelitis varies. Some
researchers have recommended as little as 2 weeks, whereas others have
suggested longer therapy. There is no general consensus. Short-term
therapy may be used in otherwise healthy patients when total
débridement is combined with healthy host tissue. Long-term antibiotic
therapy is based on the knowledge of how biofilms function and is
usually used in patients with virulent or longstanding infections in
whom the initial débridement will be followed later by staged
reconstruction or in the presence of
retained
implants. If the patient requires grafting or reconstruction following
the successful débridement of osteomyelitis, antibiotics will often be
administered for 6 to 8 weeks. This is followed by a period of
antibiotics with observation of the CRP and ESR levels and possible
repeat biopsy. If there are no ongoing indicators of infection,
reconstruction can safely be performed in possible cases without
additional long-term antibiotic treatment. One caveat of this approach
is that it can only be done if there are no adverse effects from the
use of antibiotics. There are reports of immunosuppression, allergic
reaction, poor tolerance, compliance, and financial hardship that must
also be considered when deciding on long-term antibiotic
administration. To increase patient compliance, the antibiotic agents
that are selected should be the least toxic and the least expensive
possible and preferably require administration once or twice daily.
Antibiotics with excellent oral bioavailability are listed in Table 24-7.
These antibiotics may be substituted for intravenous agents whenever
possible provided that the microorganism is susceptible to these agents
and bone penetration is adequate.
|
TABLE 24-6 Serum and Bone Concentrations after Antibiotic Administration
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
TABLE 24-7 Selected Oral Antimicrobial Agents with Excellent Oral Bioavailability Commonly Used to Treat Osteomyelitis
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
(MRSE). A single dose of vancomycin administered before surgery
followed by two or three doses postoperatively should provide adequate
perioperative prophylaxis in high-risk cases. Vancomycin should only be
used with type I hypersensitivity to cephalosporins in patients with
urticaria, laryngeal edema, and bronchospasm without cardiovascular
shock. Clindamycin is considered the substitute of choice when
cephalosporins are contraindicated.
antibiotic use that merit discussion. Routine prophylaxis is indicated
for most orthopaedic procedures.
prophylactic antibiotics have an important role in the treatment of
closed fractures. The prophylactic use of appropriate antibiotics for
closed fractures and elective cases will reduce the incidence of
postoperative osteomyelitis. Antibiotic administration is not a
substitute for proper aseptic technique, but it is a validated
additional measure to reduce postoperative infection.
a Dutch trauma trial that found, in 2195 cases of closed fracture
surgery, a single preoperative dose of ceftriaxone resulted in an
infection rate of 3.6% in comparison to a placebo group infection rate
of 8.3% (P< .001).12
Furthermore, the trial also found that there was a lower incidence of
nosocomial urinary tract and respiratory tract infections in the first
30 postoperative days (2.3% versus 10.2%, P
<.001). In another retrospective study of 2847 surgical cases, the
timing of antibiotic administration was also found to be an important
factor. If the antibiotics were given more than 2 hours before or 3
hours after the incision,
there was a sixfold increase in the rate of surgical site infection.23
The current recommendation is that antibiotics should be administered
30 to 60 minutes before the incision is made, except when using
vancomycin, where a longer delay permits appropriate infusion rates.46,105
For routine uncomplicated closed fracture surgery, prophylaxis should
not be administered for greater than 24 hours, and many surgeons
believe that only a single dose is necessary. Gillespie and Walenkamp
performed a meta-analysis of 8307 patients undergoing surgical
treatment of hip and long bone fractures, to determine whether
antibiotic prophylaxis reduced the incidence of wound infection. A
total of 22 studies were analyzed. Single doses of antibiotic
prophylaxis were found to significantly reduce the incidence of wound
infection (relative risk, 0.4; 95% confidence interval, 0.24-0.67).46
Controversy exists regarding the appropriate prophylaxis for orthopedic
surgical patients in hospitals with high rates of MRSA. Traditional
first-generation cephalosporins may not provide adequate coverage, and
given the increasing prevalence of MRSA infections in Europe and North
America, further studies are needed to understand the risk/benefit
ratio of using routine vancomycin as a prophylaxis agent. The
disadvantages of potential nephrotoxicity and an increase in the
emergence of VRSA must be weighed against the risk of increased
postoperative infection rates. Currently, neither vancomycin nor
clindamycin is routinely used except in cases of known penicillin or
cephalosporin allergy. In cases of reported penicillin allergy,
patients can be given a small test dose of a cephalosporin after
anesthesia induction to determine if there is any cross-reactivity.
use a first-generation cephalosporin followed by the addition of an
aminoglycoside for more contaminated wounds using supplementary
penicillin if there is any soil contamination. It is perhaps surprising
to realize that this recommendation is more than 30 years old and is
not supported by any newer, high-level evidence-based studies. This
practice was primarily an empirical and theoretical recommendation.
Since the incidence of infection is so low that any treatment will also
have a small statistical effect size, it may not be possible to
undertake a sufficiently powered study to adequate test the success of
this antibiotic regimen. A Cochrane review undertaken in 2004 clearly
identified the usefulness of some antibiotics for the treatment of open
fractures.47 The EAST practice
management guidelines also concluded that antibiotics were useful but
that their use required more scientific study.64
The most recent review by the Surgical Infection Society found that the
current standard antibiotic prophylaxis is based on very limited data.55
true requirement for aminoglycosides at the time of injury. Given that
the initial organism is often a staphylococcus but that the eventual
infective organism is a resistant gram-negative organism, it begs the
question of whether early aminoglycoside administration, which is often
given in adequate doses, will promote the development of a resistant
organism. In two studies, a cephalosporin alone performed as well as
the combination of a cephalosporin and penicillin or the combination of
a cephalosporin and an aminoglycoside.92
Another study examining the use of broad-spectrum antibiotics proposed
that their use could result in the development of resistant bacteria.
are replacing aminoglycosides for the treatment of open fractures,
particularly in patients in the intensive care unit. This view is
supported by an assessment of the U.S. military experience with
high-energy open fractures, which has shown that there is sufficient
level I data to support the use of a cephalosporin but that the use of
aminoglycosides, even for grade III open fractures, may be deleterious.
These recommendations are based on timely and adequate surgical
débridement. If there is a delay to treatment whereby bacterial
colonization may have begun and matured or if the wound is such that
gram-negative or anaerobic conditions exist, then supplementation with
appropriate antibiotics, in addition to a more aggressive initial
débridement, may be useful. Because there is little scientific evidence
about the subject, much current surgical practice is anecdotal. The
authors believe that the initial débridement is the most important
principle of open fracture treatment as well as in the management of
acute or chronic infection. Minimalist approaches to the removal of
devitalized tissue, in the hopes of preventing extensive later
reconstructive surgery, are usually doomed to failure. By the surgeon
taking less initially, the patient is often condemned to lose more
later because of ongoing infection and diffuse tissue destruction.
treatment, there is again little good science and little chance of
undertaking a well-performed scientific study because of the sample
size that would be required. In the classic retrospective study of over
2800 patients, surgical site infections were increased sixfold if
antibiotics were given too early (<2 hours before incision) or too
late (>3 hours after incision).23
A study on over 2000 fracture fixation patients that compared
ceftriaxone to a placebo clearly identified the usefulness of
prophylactic antibiotics.12 The
duration of antibiotic use in open fractures has also been poorly
studied, but the current recommendation is to use antibiotics for 1 to
3 days following wound closure or coverage with ongoing treatment based
on a reassessment of the injury zone. However, the current practice of
continuing the use of antibiotics until definitive wound closure has
occurred has no scientific basis. In fact, the literature suggests that
the long duration of empirical or prophylactic antibiotic use may breed
resistant organisms. The current recommendation is to use antibiotics
for an extended period only if this is supported by the condition of
the wound which will show signs of infection.82
The authors’ institutions summarized the available literature and
concluded that open fractures should be treated with antibiotics for 24
hours following definitive wound closure. These are also the
recommendations being developed in new guidelines by the U.S. Centers
for Disease Control and Prevention (CDC).18
Recently, a journal supplement focusing on military injuries has
examined and summarized the current thinking on extremity infection in
war time injury.82,83
duration of antibiotics to establish infection. The most common
practice is to begin with a 2- to 6-week course of a species-specific,
bioavailable agent.62 Such an agent
may be used until adequate revascularization occurs. With the advent of
many new and expensive oral agents, it may be possible to reduce the
morbidity associated with intravenous use with an early stepdown
program converting to an oral agent. Obviously, this regimen may
be
less successful, with resistant organisms. Thus, to date there have
been few recommendations for the duration of antibiotics in established
osteomyelitis.
infection. Patients who may have been suppressed but incompletely
treated may present with an acutely inflamed, limb-threatening
condition. Our approach has been to continue the use of antibiotics
during the diagnostic and staging period when the necessary tests are
undertaken and the host is optimized. However, we believe that as long
as there are no signs of impending sepsis or limb loss, it is
worthwhile stopping antibiotic treatment 1 to 2 weeks before surgical
intervention so that more precise and reliable bacterial identification
is possible. Begin an empirical course of antibiotics intraoperatively
after all the cultures have been taken and continue until the culture
results are available. In chronic cases, we will cover both
gram-negative and MRSA and collaborate with our infectious diseases
colleagues regarding final antibiotic selection and management.
early studies on infections that recommended that colonized bacteria
are adherent to tissue and needed to be moved from the surface.
Although such mechanical cleansing may work, acutely mature colonies of
bacteria are not easily eradicated with this method. Furthermore, there
is evidence that the velocity of the fluid stream may be deleterious to
both bone and soft tissue cells.3,8,13,53,110
So while high-pressure flow has been shown to remove more bacteria and
debris and to lower the rate of wound infection compared with
low-pressure irrigation, recent in vitro and animal studies suggest
that pressure irrigation may damage bone or push bacteria even deeper
into the wound.8,13,53
Damage to bone and soft tissue may result in necrosis and create a new
iatrogenic surface for bacterial colonization. In short, pulsatile flow
has not been demonstrated to increase efficacy.
The effect of adding bacteriocidal agents to irrigation solutions to
aid with both bacterial removal and destruction has also been studied
in an adherent staphylococci model.8
These studies have shown that while solutions such as Betadine and
hydrogen peroxide are effective in eliminating bacteria, they are also
toxic to osteoblasts. Also, the addition of antibiotics to irrigation
solutions has had mixed results, and overall it appears to have little
benefit but there is a significant increase in cost. Their use as an
adjunct to irrigation solutions is questionable at best.4,35,94
The authors do not add any supplementary chemicals or antibiotics to
irrigation solutions, and we do not recommend the use of full-strength
Betadine placed directly into an open wound.
fluids, detergent-type compounds or surfactant solutions have been
recently investigated as a way of disrupting the hydrophobic or
electrostatic forces that drive the initial stages of bacterial surface
adhesion. A sequential surfactant-irrigation protocol was developed and
shown to be effective in polymicrobial wounds associated with an
established infection.5,36,81
Detergents, or soaps, have been shown to be the only irrigation
solutions that remove additional bacteria beyond the effect of
mechanical irrigation alone.8 Moreover, soap solutions have been found to have minimal effects on bone formation and osteoblast numbers in vitro.7
The proposed mechanism of their effect is based on the formation of
micelles that overcome the strength of the interaction between the
organisms and bone. Castille soap has recently been reported to be
useful in this situation.5,80
antibiotics impregnated in bone cement to reduce infected
arthroplasties was introduced in the 1970s14.
As a result of the success of this work, interest developed in using
antibiotic-impregnated bone cement as treatment for osteomyelitis.99 Keating et al.60 reported a 4% infection rate in 53 open tibial fractures with tobramycinimpregnated beads. Ostermann et al.89
reported a significant difference in infection rates with grade IIIB
fractures treated with aminoglycoside beads together with parenteral
antibiotics compared with patients who only received parenteral
antibiotics. They reported a 6.9% infection rate in 112 patients with
combined therapy compared with a 40.7% infection rate in 27 patients
who only had parenteral antibiotics. The use of antibiotic depots
allows for high local concentrations of antibiotic with little systemic
absorption. Antibiotic release is biphasic with most occurring during
the first few days to weeks after implantation. However, elution of the
antibiotic persists for several weeks. Occasionally, antibiotics can be
recovered after several years, but because the elution is based on a
diffusion gradient, only the outer 1 cm or so of large-volume depots
will elute antibiotic. The core of such large-volume depots will often
contain antibiotic but it will not be useful.62,108
antibiotic depot is the need for a heat-stable antibiotic agent,
because during the cement-hardening process, the exothermic reaction
can render heat-labile antibiotics ineffective. Some of the antibiotics
that have been tried with PMMA include clindamycin, which elutes well
but is not available as a pharmaceutical grade power. Fluoroquinolones
have been reported to have suitable elution, but clinical reports of
their success are lacking.34
Erythromycin was used in some earlier studies, but a subsequent study
showed inadequate elution of erythromycin from Palacos cement.
Mactolides and azalides are unavailable, and tetracycline and polymyxin
E (Colistin) fail to elute from the Palacos cement in clinically useful
quantities. Another issue with PMMA depot systems is that they require
removal. If they are left for a prolonged period, the PMMA spaces can
become encased in scar and may be difficult to remove. After
antibiotics have eluted from the outer surface of the cement mass, the
surface is rendered unprotected and may provide a suitable surface for
secondary colonization. When used acutely for open fractures or for a
short period, removal is not generally problematic and may even be
undertaken percutaneously. There are entire issues of Clinical Orthopaedics and Related Research
(Numbers 295 and 427) dedicated to the use of PMMA antibiotic depot
methods to which the reader is referred for more in-depth information.1,2
The authors’ formulation for PMMA antibiotic-laden beads uses
vancomycin and tobramycin. While up to 4 g of vancomycin and 4.8 g of
tobramycin can be mixed per 40-g bag of cement, the cement may become
difficult to manage. Other PMMA formulations
such
as Cranioplast do not tolerate even small amounts of antibiotic powder.
For routine use, we place between 1 and 2 g of vancomycin and 1.2 to
2.4 g of tobramycin per bag. Depending on the clinical situation, we
have developed a method to create three forms of antibiotic beads. We
first cut a length of 18-gauge steel wire and loop one end. As the bowl
becomes doughy, we roll multiple cement balls of about 1 to 2 cm in
diameter. The wire is then moistened with water and the bead is allowed
to slide and drop on the wire. The adherent cement is cleaned off the
wire to allow subsequent beads to slide down. After the beads are
placed, they can be left to cure as balls or they may be checked into
oblong sausage-shaped beads by rolling them like dough. Alternatively,
they can be shaped into discs by simply pressing each bead flat on the
wire. We prefer the use of discs because they fit better between tissue
planes and are less likely to have local compressive effects on the
tissues (Figs. 24-13 and 24-14).
 |
|
FIGURE 24-13 Antibiotic balls placed on stainless steel wire to form beads. One ball has been flattened to form a disc.
|
been used. These are fashioned by using a large 36 French thoracostomy
tube and placing an 18- to 20-gauge wire inside. After the tube has
been cut to discard the vent holes, bone wax or a Kelly clamp can be
used to obstruct the thin end. The antibiotic-cement mixture is then
injected into the tube in a liquid state. Once the cement cures, the
thoracostomy tube is cut off with a scalpel and the rod can then be
used. It is important to ensure that both canal diameter and rod length
have been measured (Fig. 24-15).
 |
|
FIGURE 24-14
The final appearance of antibiotic discs. We believe that they produce less local tissue pressure and ischemia than antibiotic balls. |
 |
|
FIGURE 24-15 Manufacture of an antibiotic-impregnated cement rod. A. The thoracostomy tube filled with antibiotic cement is cut to allow removal of antibiotic intramedullary rod (bottom). B. The antibiotic-impregnated cement rod.
|
has been off-label use by the surgeon, and despite encouraging results
from several studies, their approval by the U.S. Food and Drug
Administration has been discouragingly slow. There are a number of
commercial antibiotic-impregnated PMMA cements becoming available in
the United States, and of course they have been available for some time
in other parts of the world. We believe that the use of an
aminoglycoside in antibiotic-impregnated cement does not provide the
versatility that we need because vancomycin should be considered when
there is a chance of resistant staphylococcal organisms. Thus,
commercially produced antibiotic-laden cement may best be suited as
prophylaxis during cemented arthroplasty.
local delivery of antibiotics that are resorbable and do not require
removal. Surgical-grade calcium sulfate has been used recently, and its
use is reported in both open fractures and infections.73
Although calcium sulfate products have been promoted as a bone graft
substitute, there are little data in human fractures and infections
demonstrating the efficacy of this dual function of depot and graft.
Calcium sulfates and carbonate will absorb or dissolve independently of
bone formation, whereas calcium phosphates tend to be replaced very
slowly with bone. Furthermore, large volumes of calcium sulfate can
cause an osmotic effect that results in fluid accumulation and the
potential for
seroma
formation and wound drainage. When mixed with fluid and blood, the
calcium drainage looks like bloody pus and may prompt extra surgical
treatments resulting in unacceptable complication rates.11,127
In one study, the use of a calcium sulfate-DBM mixture (Allomatrix;
Wright Medical, Memphis, TN) resulted in an unacceptably high rate of
drainage, infection, and failure. We have heard of numerous anecdotal
reports concerning the drainage problem of calcium sulfate and we do
not recommend its use as a bone graft substitute. However, we have
found it useful as an antibiotic depot. To minimize the drainage
problem, we do not recommend placing a large amount of calcium sulfate
beads into a cavity, but if this is unavoidable, a multiple-level
water-tight closure is essential. We have not found any problems with
drainage when the beads are interspersed in small spaces and tissue
planes. Another feature to note is that the addition of tobramycin to
calcium sulfate greatly prolongs the setting time, which may be 30 to
45 minutes. Vancomycin greatly shortens the setting time, which may be
as fast as 2 minutes, and for this reason the agent may be impractical.
We routinely use both vancomycin and tobramycin for high-risk
infections and find that the effect of tobramycin dominates the
settling profile. Thus, despite a seemingly novel concept and marketing
claims, such products should be used with caution and experience.
simulate a bone graft by serving as an osteoconductive matrix, but they
are resorbed slowly and after elution may behave as a foreign body with
potential reinfection as may occur with PMMA beads.
Gentamicin-impregnated polylactide-polyglycolide copolymer implants are
biodegradable and may not need removal once they have been implanted.
However, there is little clinical experience, and it is possible that
they elicit an inflammatory response that may mimic acute infection.
treatment is débridement. All nonviable and inert structures should be
débrided to remove infected material and debris without destabilizing
the bony structure. The goal is to convert a necrotic, hypoxic,
infected wound to a viable wound. The critical judgment for the surgeon
occurs when there is potentially infected bone, which might be
partially vascularized, which is needed to maintain the structural
stability of the bone. Sinus tracts that are present for longer than 1
year should be excised and sent for pathologic examination to rule out
an occult carcinoma.101 Soft tissue
retraction should be minimal and flaps should not be created. There is
a balance between leaving behind infection, which may result in
recurrence, and resection and subsequent destabilization, which might
necessitate extensive surgical reconstruction with its associated
risks. The risk/benefit ratio must be evaluated in each case and should
form the basis of thorough informed consent. Meticulous débridement is
one of the most important initial steps in the treatment of infected
bone and soft tissue. The limits of débridement have classically been
determined by the “paprika sign,” which is characterized by punctuate
cortical or cancellous bleeding (Fig. 24-16).
Efforts should be made to limit any periosteal stripping that may
further devitalize the bone. Reactive new bone surrounding an area of
chronic inflammation is living and sometimes does not require
débridement.20 Any sequestrated dead bone needs to be identified and removed, whereas live bone may be preserved (Fig. 24-17).
Rapid débridement can be achieved with a high speed burr but used with
continuous irrigation to limit thermal necrosis. Laser Doppler
flowmetry may facilitate accurate assessment of the microvascular
status of bone, thereby identifying it for removal.37
However, we have found this technique to have little benefit compared
with visual inspection. Laser Doppler flowmetry is the only
nondestructive in vivo method of blood flow determination that provides
instantaneous determination of perfusion.
 |
|
FIGURE 24-16 A close up of the paprika sign demonstrating bone vascularity.
|
reaming is an effective method of débridement that preserves cortical
stability. In general, one should overream the medullary canal by 2 mm.
Excessive reaming may cause cortical necrosis and exacerbate infection
by increasing the surface area of dead bone. Lavage can be performed
from the reaming entry portal with the canal irrigator tips used in
arthroplasty, with egress being provided through a vent or previous
locking screw holes. Dull reamers and the generation of head should be
avoided to prevent further cortical necrosis.
débridement technique has shown favorable results in the treatment of
medullary osteomyelitis. In a cohort of 32 patients who had
had an average of 3.2 surgical operations for osteomyelitis, Pape et al.91
found that reaming of the medullary canal was successful in that 84% of
patients were able to return to their previous profession and 97% were
pain free. Evidence for the treatment of an infected intramedullary
nail has been largely derived from observational data. Pommer et al.96
found that reaming of an infected intramedullary canal resulted in
eradication of infection in all patients when the infection occurred
after primary intramedullary nailing compared with 62% of those with
multiple operations before reaming and nailing. In another series, 25
patients with posttraumatic osteomyelitis, of whom 22 were treated with
intramedullary reaming, were followed for at least 6 months.87
Twenty-one of the 22 patients were free of any recurrent infection
after an average period of 26 months. In a more recent study, 40
patients with chronic osteomyelitis were treated with intramedullary
reaming, with only 4 patients having a recurrent infection.88
If the medullary infection is too proximal or distal for a tight reamer
fit, saucerization must be undertaken with the trough being created to
débride the canal directly. Biomechanically, the most desirable shape
for the trough is an oval with this shape resulting in minimal
diminution of the bone’s torsional strength. If segmental resection is
undertaken or there is more than 30% loss of circumferential cortical
contact, stabilization is required.
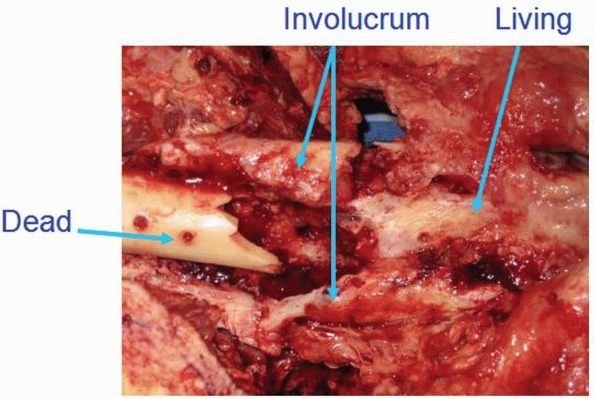 |
|
FIGURE 24-17
The appearance of bone at débridement. Note that the living bone has a pinkish hue and a petechial appearance indicating vascularity. The surrounding bone is involucrum and is also vascular. Resection of the involucrum should be at the judgment of the surgeon. The dead bone is clearly avascular and requires resection. |
surgical fracture treatment, subsequent drainage, wound dehiscence,
antibiotic treatment, or unplanned surgery may potentially have
osteomyelitis. Many of these patients present with a paucity of medical
records and other details, and often their own recollection of events
is poor. Therefore, we presume infection until we have strong evidence
to the contrary, particularly if symptoms occur in conjunction with
fracture nonunion.
optimize the host status. Improving nutrition and tissue oxygenation is
important before embarking on surgical treatment. Occasionally,
hyperbaric oxygen is helpful if it is available and can be tolerated by
the patient. Ideally, the patient should stop smoking, but this is
generally difficult to accomplish. However, we encourage patients to
limit nicotine use because it is thought that nicotine causes local
microvascular effects. Therefore, nicotine patches and gum, while
useful for smoking cessation programs, may not be useful for local
tissue optimization. Consultation with a primary care physician will
help stabilize chronic medical conditions in many patients.
surgical intervention. Multiply operated limbs are B local by
definition and heavily scarred and immobilized tissues may present
risks for subsequent wound healing. Some patients may not be candidates
or may not tolerate the extensive surgery that is required, and
therefore compromises in treatment and patient expectations may be
necessary. Many surgeons are now opting to treat exposed bone with a
vacuum-assisted closure device. While this can often produce healthy
granulating tissue bed, it should be remembered that the underlying
bone is compromised and has a higher risk of nonunion and infection.
Another problem is that the soft tissue envelope tends to be adherent
layer of scar over the bone, which may not tolerate secondary surgery
well. Secondary surgery under these circumstances may well result in
further infection. For this reason, we still advocate the use of
healthy muscle or fascial flaps that not only help being an external
blood supply to the surface of the bone but can better tolerate
subsequent surgical procedures.
the WBC, ESR, and CRP is undertaken. If these tests are negative and
there is no further reason to suspect infection, then we treat the
patient as having an aseptic nonunion but we will pay attention to our
intraoperative findings. If there is any indication of infection
despite normal laboratory findings, we obtain intraoperative cultures
or undertake a biopsy and await results. One common scenario is a
presumed aseptic case where routine cultures end up growing a few
colonies of bacteria. The issue is whether the culture results
represent a real infection or contamination. In these cases, we discuss
the surgical findings with the infectious diseases specialist who
explains their assessment of the validity of the cultures. If we
believe that the risk of infection is low, we may cover the patient
with a short course of culture-specific oral antibiotics. However, if
we believe that the risk is high, we use a longer course of antibiotic
treatment. We have found that even when there is little diagnostic and
operative evidence of infection, patients may still develop later
infection. We have no way of knowing if the subsequent infection was a
resurgence of an old occult infection or a secondary infection from the
recent surgical intervention.
the organism, we will often remove the initial hardware and then take
numerous cultures and biopsy samples of bone and soft tissue. We will
then temporarily stabilize the bone in the least invasive manner using
a cast, brace, or monolateral external fixator. Sometimes it is helpful
to obtain two different sets of cultures. One set of cultures is
obtained from the most suspicious areas during the initial part of the
surgical procedure. The second set of cultures is obtained after
débridement from the margins of the tissue bed. If this methodology is
used, one can assess whether the débridement was adequate, especially
if the initial cultures were positive. If we remain uncertain about the
extent of the infection, we will attempt to use MRI with contrast to
determine the intramedullary and soft tissue extent of the infection
and then plan our treatment accordingly. Unfortunately, in a majority
of cases, there is implanted metal, which makes the use of MRI less
applicable. When possible, we use scintigraphic studies preoperatively
despite their limitations. We have anecdotally noted significant
variations in the accuracy of the readings between various radiologists
and encourage working with a few radiologists who are interested and
experienced with musculoskeletal infection. Because of our own findings
and those in the literature, we now depend less on scintigraphy than on
other signs and generally no longer use the red cell scan. The initial
findings of PET scanning are encouraging.
identified, and the extent of the infection is delineated, we decide
whether the goal is to cure the infection, suppress the infection, or
recommend amputation. In compromised hosts, cure can usually only be
achieved by complete excision of all the infected tissue, which often
means that amputation is required. In healthier hosts, marginal
resection leaving some bacteria behind or even intra-lesional
resections when the infection is periarticular can lead to cure with
appropriate antibiotic therapy. Generally speaking, our preference is
to advise the patient that resection with a clean margin has the best
chance of cure.
the general principles that bone defects of 6 cm or greater require
bone transport with distraction osteogenesis, whereas smaller defects
can often undergo bone grafting. In the tibia, we prefer the
posterolateral tibia-pro-fibula technique, but in some cases we will
undertake central bone grafting. We try to avoid the exclusive use of
demineralized bone matrix (DBM) and allograft because we have found a
relatively low incidence of bone union. The low success rates using DBM
products may be due to the poor vascularity found in such tissue beds
and patients. Anecdotally, we have noted cases where patients present
years after failed DBM grafting of the distal tibia, and during
reoperation the original DBM material appears to be unchanged. This
indicates failure of angiogenesis.
promise. We have found that appropriate use of BMP as an inductor
together with a common void filler such as allograft, tricalcium
phosphate (TCP), or autograft has greatly enhanced the success of limb
salvage in complex cases and is associated with little morbidity. The
newer technique of graft harvest with the reamer-irrigator-aspirator
(RIA) also holds promise for the treatment of large defects and our
initial experience has been positive.85 Further study on these techniques is required.
 |
|
FIGURE 24-18 Conversion of external to internal fixation. A.
A ring fixator has been used to stabilize the tibia after excision of infected bone. Antibiotic beads have been placed in the defect and a proximal corticotomy has been undertaken. B. Bone transport was successful but the regenerate was slow to mature. C. A locking plate was applied as an “internal fixator” using proximally and distally locked screws. |
infection remains the bone transport techniques described by Ilizarov.
These are described in detail in Chapter 14.
While this technique is not simple for either patient or surgeon, there
are notable advantages compared with grafting techniques. These include
the early development of regenerate bone soon after bone resection. If
bone grafting is used, one must wait 8 weeks or longer as part of a
staged reconstruction plan. Also, the effects of corticotomy include
increasing blood flow in the limb for 4 to 6 weeks, and this may have a
positive effect in infection cases. Most important, function is
improved. The patient is encouraged to weight bear and to resume most
activities of daily living. In many cases, patients can return to work,
participate in recreational activities, and even swim. In cases where
transport takes a considerable period or where transport has finished
but one has to wait for maturation of regenerate, we have used long
percutaneously inserted locked plates as an internal fixator that spans
and protects maturing regenerate bone. This allows for early removal of
the ring fixator (Fig. 24-18). Alternatively, some cases are amenable to transport over a nail (Fig. 24-19).
We encourage surgeons who wish to treat bone infection to be familiar
with Ilizarov’s method and to seek out appropriate training.
functional rehabilitation. Often the patients are extremely debilitated
from years of disability due to their condition. Treatments that permit
early weight bearing and encourage range
of
motion in adjacent joints promote physical and psychological
well-being. It is unusual for treatment to fail because patients have
been walking on their limb. Usually failure is due to inadequate
resection, inadequate stability, and inadequate biology.
 |
|
FIGURE 24-19 Bone transport over an intramedullary nail. A. A nail has been used to stabilize a tibia after resection of infected bone. A proximal corticotomy has been undertaken. B. The tibia/nail construct has been stabilized with an adjustable, dynamic monolateral external fixator. C.
After successful bone transport, bone graft was used at the docking site to facilitate bone union. The nail supports the regenerate bone during maturation. |
collaboration with colleagues. We depend heavily on the expertise of
our colleagues in infectious disease, psychiatry, medicine,
microvascular surgery, social work, and physical and occupational
therapy. However, even with access to these experts and appropriate
surgery, the success rate for the treatment of osteomyelitis is still
significantly less than 100%. There is no doubt that prevention remains
the best overall strategy.
JO. Comparison of soap and antibiotic solutions for irrigation of
lower-limb open fracture wounds. A prospective, randomized study. J
Bone Joint Surg Am 2005; 87A:1415-1422.
JB, Volz RG. Efficacy of a topical antibiotic irrigant in decreasing or
eliminating bacterial contamination in surgical wounds. Clin Orthop
Relat Res 1984:114-117.
M, Schemitsch EH, Adili A, et al. High- and low-pressure pulsatile
lavage of contaminated tibial fractures: an in vitro study of bacterial
adherence and bone damage. J Orthop Trauma 1999;13:526-533.
TR, Johnson RA, Sherman FC. Gallium scintigraphy for diagnosis of
septic arthritis and osteomyelitis in children. J Pediatr Orthop
1986;6:317-325.
JJ, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects
with bone graft and calcium sulfate. Clin Orthop Relat Res
2003;411:245-254.
H, Broekhuizen T, Patka P, et al. Randomized controlled trial of
single-dose antibiotic prophylaxis in surgical treatment of closed
fractures: the Dutch Trauma Trial. Lancet 1996;347:1133-1137.
H, Doyon F, Desplaces N, et al. Epidemiology of bacterial infection
during management of open leg fractures. Eur J Clin Microbiol Infect
Dis 1999;18: 315-323.
DF, Nach RJ, Spinzia J. An experimental and clinical study of the
effectiveness of antibiotic wound irrigation in preventing infection.
Surg Gynecol Obstet 1964;118: 783-787.
AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical
site infection, 1999. Hospital Infection Control Practices Advisory
Committee. Infect Control Hosp Epidemiol 1999;20:250-278.
TK, Zhuang H, Nakhoda KZ, et al. Applications of fluorodeoxyglucose
positron emission tomography in the diagnosis of infection. Nucl Med
Commun 2003;24: 615-624.
G. Treating chronic osteomyelitis: evolving our antibiotic protocols.
Paper presented at Musculoskeletal Infection Society, August 2000.
DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic
administration of antibiotics and the risk of surgical-wound infection.
N Engl J Med 1992;326:281-286.
B, Klein N. Bone and Joint Infections. In: Dee R, Hurst LC, Gruber MA,
Kottmeier SA, eds. Principles of Orthopaedic Practice. 2nd ed. New
York: McGraw-Hill, 1997:317-344.
J, Hogt AH, Feijen J. Biomedical polymers: bacterial adhesion,
colonization, and infection. CRC Crit Rev Biocompat 1986;2:219-301.
FL. Abdominal abscess detection: gallium, 111In-, and 99mTc-labeled
leukocytes, and polyclonal and monoclonal antibodies. Semin Nucl Med
1996;26:51-64.
FL, Seabold JE, Brown ML, et al. Procedure guideline for
technetium-99m-HMPAO-labeled leukocyte scintigraphy for suspected
infection/inflammation. Society of Nuclear Medicine. J Nucl Med
1997;38:987-990.
VQ, Nelson JD, Haltalin KC. Osteomyelitis in infants and children. A
review of 163 cases. Am J Dis Child 1975;129:1273-1278.
FR, O’Halloran JJ, Quale JM. In vitro elution of ciprofloxacin from
polymethylmethacrylate cement beads. J Orthop Res 1994;12:79-82.
DR, Duff GP, Dahners LE, et al. High-pressure pulsatile lavage
irrigation of intraarticular fractures: effects on fracture healing. J
Orthop Trauma 1998;12:460-463.
DR, Wilson FC. Topical antibiotic irrigation in the prophylaxis of
operative wound infections in orthopedic surgery. Orthop Clin North Am
1991;22:419-426.
PJ, Schmidt AH. Assessment of bone viability in patients with
osteomyelitis: preliminary clinical experience with laser Doppler
flowmetry. J Orthop Trauma 1992; 6:327-332.
JJ, Wirganowicz PZ, Mar T. An aggressive surgical approach to the
management of chronic osteomyelitis. Clin Orthop Relat Res 1994:229-239.
BI. The polymerase chain reaction. A new method of using molecular
genetics for medical diagnosis. N Engl J Med 1990;322:178-183.
J, Alavi A, Mandell GA, et al. Sequential technetium-99m/gallium-67
scintigraphic evaluation of subclinical osteomyelitis complicating
fracture nonunion. J Orthop Res 1985;3:219-225.
JL Jr, Goll SR, McCarthy KE, et al. Indium-111 leukocyte scintigraphic
detection of subclinical osteomyelitis complicating delayed and
nonunion long bone fractures: a prospective study. J Orthop Res
1987;5:1-6.
RP, Nelson CL, Harrison BH. The effect of wound environment on the
incidence of acute osteomyelitis. Clin Orthop Relat Res 1993:289-297.
D, Balon HR, Irwin R. Sequential technetium-99m sulfur
colloid/indium-111 white blood cell imaging in macroglobulinemia of
Waldenstrom. Clin Nucl Med 1990;15:389-391.
B, Vaudaux P, Magnin M, et al. Novel animal model for studying the
molecular mechanisms of bacterial adhesion to bone-implanted metallic
devices: role of fibronectin in Staphylococcus aureus adhesion. J Orthop Res 1996;14:914-920.
Z, Tseng CH, Pei Z, et al. Molecular analysis of human forearm
superficial skin bacterial biota. Proc Natl Acad Sci U S A
2007;104:2927-2932.
WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal
femoral and other closed long bone fractures. Cochrane Database Syst
Rev 2001;1(CD000244).
RA, Roberts I, Gillespie WJ. Antibiotics for preventing infection in
open limb fractures. Cochrane Database Syst Rev 2004(CD003764).
GD, Lundy MM, Frederick RJ, et al. Predicting the cure of osteomyelitis
under treatment: concise communication. J Nucl Med 1983;24:110-113.
NV, Masri BA, Garbuz DS, et al. Use of erythrocyte sedimentation rate
and C-reactive protein level to diagnose infection before revision
total knee arthroplasty. A prospective evaluation. J Bone Joint Surg Am
2007;89A:1409-1416.
AG, Barth E, Webb LX. Microbial adhesion and the pathogenesis of
biomaterial-centered infections. In: Gustilo RB, Tsukayama DT, eds. Orthopaedic Infection. Philadelphia: WB Saunders, 1989:3-25.
DW, McAndrew MP. Bacterial osteomyelitis in adults: evolving
considerations in diagnosis and treatment. Am J Med 1996;101:550-561.
MF, Graham G, Lancaster J, et al. Gallium-67/technetium-99m methylene
diphosphonate ratio imaging: early rabbit osteomyelitis and fracture. J
Nucl Med 1985; 26:272-277.
SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage
propagates bacteria into soft tissue. Clin Orthop Relat Res
2005;439:27-31.
JR, Barge ML, Fajon O, et al. Sternal infection and retrosternal
abscess shown on Tc-99m HMPAO-labeled leukocyte scintigraphy. Clin Nucl
Med 2004;29:194-195.
CJ, Adams CA Jr, Eachempati SR. Surgical Infection Society guideline:
prophylactic antibiotic use in open fractures: an evidence-based
guideline. Surg Infect (Larchmt 2006;7:379-405.
RC, Rodriguez R, Manning T, et al. Effects of accidental trauma on
cytokine and endotoxin production. Crit Care Med 1993;21:839-845.
DP, Hinrichs SH, Garvin KL. Molecular diagnostics for the detection of
musculoskeletal infection. Clin Orthop Relat Res 1999:37-46.
DK. Nuclear medicine and infection detection: the relative
effectiveness of imaging with 111In-oxine-, 99mTc-HMPAO-, and
99mTc-stannous fluoride colloidlabeled leukocytes and with
67Ga-citrate. J Nucl Med Technol 2003;31:196-201; quiz 203-194.
JF, Blachut PA, O’Brien PJ, et al. Reamed nailing of open tibial
fractures: does the antibiotic bead pouch reduce the deep infection
rate? J Orthop Trauma 1996;10: 298-303.
HT, Flemming RH, Gilmore MF, et al. In vitro measurement and computer
modelling of the diffusion of antibiotic in bone cement. J Biomed Eng
1986;8:149-155.
FA, Bone LB, Born CT, et al. EAST Practice Management Guidelines Work
Group: practice management guidelines for prophylactic antibiotic use
in open fractures. 2000.
EJ, Bosse MJ. Factors influencing outcome following limb-threatening
lower limb trauma: lessons learned from the Lower Extremity Assessment
Project (LEAP). J Am Acad Orthop Surg 2006;14(10 suppl):S205-S210.
JE, Brown ML, Hauser MF, et al. In-111-labeled leukocyte scintigraphy
in suspected orthopedic prosthesis infection: comparison with other
imaging modalities. Radiology 1988;168:235-239.
JL, Prokuski L, Biermann JS. Chronic infected tibial nonunions with
bone loss. Conventional techniques versus bone transport. Clin Orthop
Relat Res 1994:139-146.
JW Jr, Jupiter JB, Weiland AJ, et al. Clinical classification of
posttraumatic tibial osteomyelitis. J Bone Joint Surg Am
1989;71A:1422-1428.
K, Velchik MG, Alavi A, et al. Indium-111-labeled white blood cells in
the detection of osteomyelitis complicated by a pre-existing condition.
J Nucl Med 1988; 29:1015-1021.
MD, Wild LM, Schemitsch EH, et al. The use of an
antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute
in the treatment of infected long bone defects: early results of a
prospective trial. J Orthop Trauma 2002;16:622-627.
SE, Zuckerman JD, Koval KJ. Posttraumatic tibial osteomyelitis:
diagnosis, classification, and treatment. Bull Hosp Jt Dis
1993;52:11-16.
KD, Brown ML, Dewanjee MK, et al. Comparison of
indium-labeled-leukocyte imaging with sequential technetium-gallium
scanning in the diagnosis of low-grade musculoskeletal sepsis. A
prospective study. J Bone Joint Surg Am 1985;67A:465-476.
KD, Brown ML, Fitzgerald RH Jr. Sequential technetium-99m
HMDP-gallium-67 citrate imaging for the evaluation of infection in the
painful prosthesis. J Nucl Med 1986;27:1413-1417.
IL, Palmer EL, Scott JA, et al. Polyclonal, nonspecific 111In-IgG
scintigraphy in the evaluation of complicated osteomyelitis and septic
arthritis. Q J Nucl Med 1999;43:29-37.
RM, Mayrose J, Reardon RF, et al. A multicenter comparison of tap water
versus sterile saline for wound irrigation. Acad Emerg Med
2007;14:404-409.
FW, Anglen JO, Gehrke JC, et al. The significance of positive cultures
from orthopedic fixation devices in the absence of clinical infection.
Am J Orthop 1997;26: 617-620.
FW, Gainor BJ, Anglen JO, et al. Disinfecting agents for removing
adherent bacteria from orthopaedic hardware. Clin Orthop Relat Res
1996:255-262.
CK, Hsu JR, Solomkin JS, et al. Prevention and management of infections
associated with combat-related extremity injuries. J Trauma 2008;64(3
suppl):S239-251.
JT, Stahel PF, Smith WR, et al. A new minimally invasive technique for
large volume bone graft harvest for treatment of fracture nonunions.
Orthopedics 2008;31: 257-261.
WW, Dorrington SM, Slack MP, et al. Inhibition of tobramycin diffusion
by binding to alginate. Antimicrob Agents Chemother 1988;32:518-523.
PE, Gösele A, Buess P. The value of intramedulary reaming in the
treatment of chronic osteomyelitis of long bones. Arch Orthop Trauma
1990;190:341-347.
PE, Brunazzi MG. Intramedullary reaming and soft tissue procedures in
treatment of chronic osteomyelitis of long bones. Orthopedics
1994;17:433-440.
PA, Henry SL, Seligson D. [Value of adjuvant local antibiotic
administration in therapy of open fractures. A comparative analysis of
704 consecutive cases.] Langenbecks Arch Chir 1993;378:32-36.
CJ, Mehta HH, Patel M, et al. Marrow versus infection in the Charcot
joint: indium-111 leukocyte and technetium-99m sulfur colloid
scintigraphy. J Nucl Med 1998;39:346-350.
HC, Zwipp H, Regel G, et al. [Chronic treatment refractory
osteomyelitis of long tubular bones—possibilities and risks of
intramedullary boring.] Unfallchirurg 1995; 98:139-144.
B, Jeray K, Schemitsch E, et al. Fluid lavage in patients with open
fracture wounds (FLOW): an international survey of 984 surgeons. BMC
Musculoskelet Disord 2008;23:7.
JY, Garin E, Derrien C, et al. Diagnosis of osteomyelitis in the
diabetic foot with a 99mTc-HMPAO leucocyte scintigraphy combined with a
99mTc-MDP bone scintigraphy. Diabetes Metab 2002;28(6 Pt 1):485-490.
A, David A, Richter J, et al. [Intramedullary boring in infected
intramedullary nail osteosyntheses of the tibia and femur.]
Unfallchirurg 1998;101:628-633.
BD, Wilson FC, Funderburk CH. The use of bacitracin irrigation to
prevent infection in postoperative skeletal wounds. An experimental
study. J Bone Joint Surg Am 1989;71A:427-430.
RH, Fischman AJ, Needleman M, et al. Radio-labeled, nonspecific,
polyclonal human immunoglobulin in the detection of focal inflammation
by scintigraphy: comparison with gallium-67 citrate and
technetium-99m-labeled albumin. J Nucl Med 1989;30:385-389.
M, Corea JR, Ali MS, et al. Squamous cell carcinoma in chronic
osteomyelitis. Report of a case and review of the literature. Clin
Orthop Relat Res 1985: 264-267.
M, Stumpe KD, Trentz O, et al. Detection of metallic implant-associated
infections with FDG PET in patients with trauma: correlation with
microbiologic results. Radiology 2003;226:391-398.
AH, Swiontkowski MF. Pathophysiology of infections after internal
fixation of fractures. J Am Acad Orthop Surg 2000;8:285-291.
JE, Flickinger FW, Kao SC, et al.
Indium-111-leukocyte/technetium-99m-MDP bone and magnetic resonance
imaging: difficulty of diagnosing osteomyelitis in patients with
neuropathic osteoarthropathy. J Nucl Med 1990;31:549-556.
JE, Nepola JV, Conrad GR, et al. Detection of osteomyelitis at fracture
nonunion sites: comparison of two scintigraphic methods. AJR Am J
Roentgenol 1989;152: 1021-1027.
SK, Seeley JV, Telehowski P, et al. Volume and surface area study of
tobramycinpolymethylmethacrylate beads. Clin Orthop Relat Res
2004:298-303.
AB, Munoz A, Brulles YR, et al. What is the role of bone scintigraphy
in the diagnosis of infected joint prostheses? Nucl Med Commun
2004;25:527-532.
SJ, Bice TG, Gooden HA, et al. Comparison of bulb syringe and pulsed
lavage irrigation with use of a bioluminescent musculoskletal wound
model. J Bone Joint Surg Am 2006;88A:2167-2174.
JS, Gold RH, Bassett LW, et al. Musculoskeletal infection of the
extremities: evaluation with MR imaging. Radiology 1988;166(1 Pt
1):205-209.
CP, Chattar-Cora D, O’Neill A, et al. Efficacy of primary wound
cultures in long bone open extremity fractures: are they of any value?
Arch Orthop Trauma Surg 2002;122:259-261.
Nostrand D, Abreu SH, Callaghan JJ, et al. In-111-labeled white blood
cell uptake in noninfected closed fracture in humans: prospective
study. Radiology 1988;167: 495-498.
ER, Mirro R, Gartner JC. Pitfalls on the diagnosis of acute
osteomyelitis by bone scan. Clin Pediatr (Phila) 1980;19:597-601.
FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features,
therapeutic considerations, and unusual aspects. N Engl J Med
1970;282:198-206.
AJ, Moore JR, Daniel RK. The efficacy of free tissue transfer in the
treatment of osteomyelitis. J Bone Joint Surg Am 1984;66A:181-193.
G, Aigner RM, Schwarz T. Diagnosis of bone infection using 99m Tc-HMPAO
labelled leukocytes. Nucl Med Commun 2001;22:1201-1206.
BH, Rao N, Hall RA. A dedicated team approach enhances outcomes of
osteomyelitis treatment. Clin Orthop Relat Res 2003;414:31-36.
BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone
matrix/ allograft for nonunions and posttraumatic reconstruction of the
appendicular skeleton: preliminary results and complications. J Trauma
2007;63:1324-1328.
AF, Galvis W, Jaimes F, et al. Lack of microbiological concordance
between bone and nonbone specimens in chronic osteomyelitis: an
observational study. BMC Infect Dis 2002;2:8.