Principles of Nonunion Treatment
One – General Principles: Basics > Complications > 25 –
Principles of Nonunion Treatment
phenomenon. Bone begets bone. Most other tissues in the human body can
only manage to heal with scar, but bone heals by forming new bone. Bone
healing may be affected or interrupted in many ways. Most simply put,
nonunion occurs when a fracture has failed to heal in the expected
time. Delayed union occurs when a fracture has not completely healed in
the time expected. While this may seem self evident, identifying when
healing occurs can be elusive. Establishment of a nonunion can be
defined based on a lack of complete bone healing in a specified time
frame, commonly 6 to 8 months, but this is arbitrary.114 In a more general sense, nonunion occurs when further progress in healing will not occur without intervention.16,18,82
healing is lagging behind what would ordinarily be present in a similar
fracture in the same bone. This, of course, will further depend on not
only the particular bone involved, but also the anatomic region, the
fracture pattern, and the method of treatment. Comparison to healing
times reported in the literature for similar fractures along with
clinical experience is necessary to identify a delayed union. The
potential inconsistencies in this analysis are confounded by the mere
fact that attempts to define a cellular process by reviewing
radiographic data is inherently inaccurate. It has been suggested that
cessation of the periosteal and not the endosteal healing response
prior to fracture bridging may define delayed union at the cellular
level.84
difficult to define and diagnose in real time. The time for distal
radial metaphyseal bone to be considered nonunited will be different
than the hard diaphyseal bone of the tibial shaft. Both clinical and
radiographic findings are necessary for the diagnosis of nonunion, but
these signs may not be evident as primary and secondary healing may be
occurring simultaneously. On a cellular level, nonunion occurs when
there is cessation of a reparative process antecedent to bony union.49,133
and definition due to failure of hardware or the lack thereof in the
presence of nonunion. While hardware failure may make the diagnosis
obvious, with newer implants and techniques (locked nails and plates) a
paucity of clinical symptoms and radiographic
findings may persist long after progress in healing has ceased.
may be caused by a myriad of factors leading to an estimated prevalence
of 2.5%.127 Some of these factors
are and some are not within the surgeon’s control. The risk for
nonunion increases with increasing energy of injury with the incidence
of nonunion approaching 20% in the presence of open fractures and
extensive soft tissue injury.127 The
characteristics of the original injury, the patients ability (or
inability) to generate a normal healing response to the particular
injury, the mechanical and biological environment created by the chosen
treatment method, and the presence or absence of associated infection
are among the factors that can influence the rate and the likelihood of
uncomplicated and timely fracture healing.
fracture influence the innate ability for fracture healing. This is
related, in large part, to the associated vascular supply to the
fracture region. The talar neck, the metaphyseal-diaphyseal junction of
the fifth metatarsal, the femoral neck, and the scaphoid are examples
of anatomic sites that have relatively limited or watershed vascular
supplies that are potentially cut off by fracture. Hence, fractures in
these sites have a propensity for healing complications or the
development of osteonecrosis. On the other hand, the metaphyseal
regions of most other long bones as well as the pelvic bones and
scapula have a robust vascular supply, and in the absence of other
complicating factors, usually heal reliably. The diaphyseal regions of
long bones, especially the tibia, fall between these extremes. The
diaphyseal region of bone has a relatively limited blood supply, and
therefore diaphyseal fractures usually require longer periods of time
to achieve union than metaphyseal fractures.
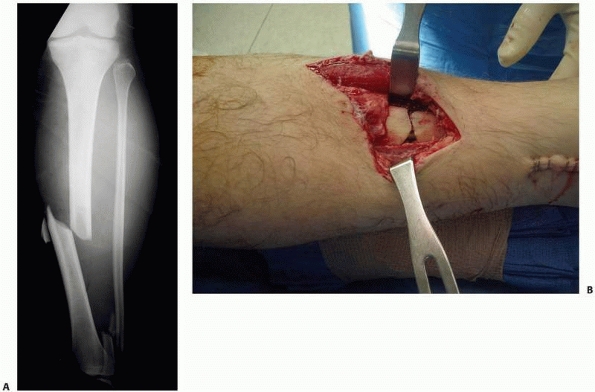 |
|
FIGURE 35-1 Anteroposterior radiograph (A) of an open tibial shaft fracture with associated periosteal stripping seen in the clinical photograph (B).
|
the degree of bone and surrounding soft tissue injury influences the
healing potential. High-energy fractures cause devascularization of the
fractured bone in the form of periosteal stripping or disruption of the
endosteal blood supply or both. This is clearly evident with open
fractures (Fig. 25-1), but internal soft tissue
stripping can occur equally in closed fractures. In addition, severe
high-energy injuries can leave the bone ends nonviable either from
immediate cell death or via the process of apoptosis.15
Bone loss, either traumatically associated with open an fracture or the
result of surgical débridement, is a potential precursor of nonunion.
Another reason nonunion is associated with open fractures is by virtue
of providing a source of bacterial contamination and potential for
infection.
alterations in fracture healing. Specific conditions that are most
notably considered to affect fracture healing are diabetes mellitus and
smoking.46,89
It is postulated that the microvascular disease and perhaps reduced
immunocompetence and neuropathy associated with the diabetic condition
leads to alterations in bone metabolism leading to delayed fracture
healing.104 Diabetes also poses
greater risks for soft tissue healing complications as well as an
increased risk for infection after surgical fracture management.69
Smoking has been associated with altered acute fracture healing as well as failure of nonunion treatment.23,80,89
Evidence suggests that the vasoconstrictive properties of nicotine
inhibit tissue differentiation and the normal angiogenic responses in
the early stages of fracture healing and that nicotine directly
interferes with osteoblast function.31,137,150
Nicotine supplementation as part of a smoking cessation program,
therefore, should also be considered detrimental to bone healing.
shown to negatively impact fracture healing, empirically can lead to
altered healing responses. Any state leading to malnutrition or
immunosuppression, including steroid use, rheumatoid disease, and
malignancy, can negatively impact the body’s healing response,
including fracture healing. Nonsteroidal anti-inflammatory medications,
once used ubiquitously to control postfracture pain, have been
implicated in inducing fracture nonunion through inhibition of
angiogenesis.91 These medications
are now used much more sparingly in the setting of acute fracture or
nonunion repair, especially in the initial weeks after injury, a time
corresponding to the inflammatory phase of fracture healing. Previously
irradiated bone or bone actively infiltrates with tumor also are at
high risk for delayed or nonunion. Although children have higher
healing potential than adults, whether advanced age (once physeal
closure has occurred) is an independent risk factor for nonunion is
unclear.54 Advanced age was found to be an independent risk factor for nonunion in patients with acute clavicle fracture,112 but many other prognostic studies have failed to identify age as a risk factor for nonunion in other anatomic locations.10,30
environment conducive to fracture healing. Unfortunately, the adequacy
of stability is very difficult to define and even more difficult to
quantify. In fact, adequate stability depends, to a great extent, on
the chosen method of stabilization. The natural process of bone
healing, commonly referred to as secondary bone healing, through the
formation of callus accommodates for some motion at the fracture site.
In nature, fractures can heal with no immobilization, but nonunion can
also result. It is clear, however, that fractures heal more reliably
when immobilized. Indeed, most fractures heal with the relatively
limited stability provided by cast immobilization. Rigid internal
fixation, as provided by compression plating techniques, represents the
other end of the stability spectrum associated with fracture care. Such
fractures heal without callus via primary bone healing, a relatively
unnatural, yet often successful, strategy.
 |
|
FIGURE 25-2 A. Postoperative radiograph showing a fibular gap. B. At 3 months postoperative, the gap persists. C. Repair of the atrophic nonunion with bone graft yielded union.
|
on primary or secondary bone healing, improper technique can lead to
inadequate bone healing. A poorly applied cast or one applied to a
severely lipomatous extremity, for instance, may provide inadequate
stability resulting in excessive fracture site motion permitting the
development of a nonunion. Relatively rigid internal fixation
techniques that fail to achieve bone-to-bone contact and compression
(i.e., ones with gaps across the fracture) do not support the primary
bone healing process, which relies upon direct remodeling of bone via
cutting cones that traverse the fracture, and can also lead to nonunion
(Fig. 25-2). Whereas modern surgical techniques
emphasize biologically friendly tissue handling, older techniques that
included anatomic reduction of individual fracture fragments often
required soft tissue stripping from about the fracture site and led to
a suboptimal environment for fracture healing. Whether fracture
reduction is direct or indirect, or the fixation construct is
relatively stable or rigid, minimizing soft tissue disruption is
paramount to maximizing the healing potential and minimizing other
complications that relate to devitalization of bone, namely infection.
controlled infection may slow the healing process and uncontrolled
osteomyelitis can inhibit normal fracture healing altogether. The
inflammatory process in response to infection may inhibit fracture
healing by causing excessive remodeling and osteolysis.45
Tissue necrosis may be accelerated by infection but histologic evidence
indicates that soft tissue disruption caused by the initial trauma and
surgical insult are the primary events leading to bone necrosis in
cases of osteomyelitis associated with fracture.97 Loose dead bone and bone pieces demarcated by osteoclastic activity are eventually transformed into sequestra.97 Infection not only predisposes to nonunion, but makes nonunion repair substantially
more complex, often requiring multistaged treatment protocols.
Septic nonunion implies that there is an infectious process at the site
while aseptic nonunion occurs in the absence of infection. Further
classification of nonunion is an attempt to relate the radiographic
picture to the biologic occurrences, or lack thereof, at the fracture
site. Both septic and aseptic nonunions may be further classified as
atrophic, oligotrophic, and hypertrophic as well as a frank
pseudarthrosis.
nonviable, or avital indicates a poor healing response with little or
no bone forming cells.87,90 This is typically manifested radiographically by absence of any bone reaction (see Fig. 25-2).
This lack of healing response may be due to the injury (e.g., an open
fracture), subsequent surgical treatment (e.g., significant surgical
stripping of soft tissues about the fracture site), or host issues
(e.g., diabetes or smoking).122
Strategies for the treatment of atrophic nonunions generally include a
method to provide a biologic stimulus to the fracture site.
 |
|
FIGURE 25-3 This hypertrophic nonunion resulting after unstable intramedullary nailing of a distal tibial shaft fracture (A,B) was treated simply with plate and screw fixation (C,D) and healed without the need for bone graft (E,F).
|
responses is the hypertrophic nonunion, also referred to as a
hypervascular, viable, or vital nonunion. Associated is an adequate
healing response and good vascularity.87,90
These fractures lack adequate immobilization or stability to progress
to union. The viable healing fibrocartilage cannot mineralize due to
unfavorable mechanical factors (strain/stress) at the fracture site.122
This is manifested radiographically by callus formation, usually
abundant, with an interceding area of fibrocartilage lacking mineral
and thus appearing dark on standard radiographs. Successful treatment
of hypertrophic nonunions utilizes methods to provide the stability
required for the adequate biologic response to come to completion.
Unlike atrophic nonunions, biologic stimulus is not a treatment
necessity (Fig. 25-3).
but usually manifest a minimal radiographic healing reaction (callus)
often due to inadequate approximation of the fracture surfaces. A bone
scan may be necessary to distinguish this type of nonunion from a
frankly atrophic one. The oligotrophic situation will manifest
increased uptake whereas the atrophic nonunion would be relative cold.129
 |
|
FIGURE 25-4 A pseudarthrosis 20 years after shotgun injury and nonoperative treatment.
|
properties of a hypertrophic nonunion due to excessive and chronic
motion, and a synovial lined cavity containing synovial fluid much like
an actual joint may be present. The medullary ends are usually sealed
and an interceding cold cleft can be seen on bone scan.
when ignoring the temporal issues of defining when a fracture should or
should not yet be considered a nonunion. At any given point,
determining if a fracture is united rather than if it is not is a
straightforward but not a simple task. Bone healing is a progressive
process wherein the strength of the reparative process, under usual
circumstances, gradually increases over a long period of time. Clinical
attempts to define union are hampered by utilizing indirect means to
evaluate the strength of the healing process. Furthermore, even if the
integrity of the healing bone could be accurately evaluated, neither
the baseline strength or stiffness of the uninjured bone nor the
fraction of that value required to define union are known. Given these
limitations, indirect means are required in the evaluation of fracture
healing and in determining if union has occurred.
diagnosis of a nonunion may be one of inclusion or exclusion. That is,
when evidence of union exists, nonunion is ruled out. Some diagnostic
modalities such as a bone scan may directly identify nonunion by a
positive test result. In usual clinical practice, the information
gathered from many modalities, such as history, physical exam,
radiographs, and other special tests, is used in concert to determine
the presence or absence of fracture union.
important to both the initial evaluation of the patient being
scrutinized to determine the presence or absence of fracture union
after an acute fracture, as well as in the assessment of a patient with
an established nonunion. The history of the events surrounding the
index injury provides insight into any deviations from the expected
course of fracture healing for the particular fracture being evaluated.
This information may heighten the index of suspicion not only for
nonunion but also for associated problems such as infection. A
particularly high-energy injury may portend a higher risk for healing
complications. Similarly, the nature of the associated soft tissue
injury maybe prognostic for delayed bone healing. If the fracture was
open, delayed fracture healing is expected and infection becomes more
common.
complete the history of the problem at hand. It is critical to uncover
the type and timing of the initial treatments and any subsequent
interventions. The indication for, the specific details of, and the
result of any additional procedures should be identified. Specifically,
it is critical to know if secondary débridement procedures were done as
planned prophylactic procedures or for the treatment of a documented
infection. Causative organisms, antibiotic susceptibilities, and
details of antibiotic treatments should be elucidated. The clinical
response to such treatments can provide valuable insight into future
responses to similar treatment. The nature of prior surgical procedures
aimed at augmenting fracture healing provides useful information
regarding the diagnosis and helps direct future treatments. It is
important to distinguish prior hardware removal performed for pain from
similar procedures done to promote fracture healing such as nail
dynamization. With a history of prior bone grafting, it should be
clarified if the graft was autologous or from another source. If
autologous, the prior harvest site should be confirmed by physical
examination so that if a future graft harvest is contemplated, a
different site can be prepared. If there was treatment with a bone
growth stimulator, such devices may be incorporated in future
treatments with little or no additional patient expense.
and the potential need for, and utility of, additional operative
intervention is the patient’s individual response to prior treatment,
their current level of disability, time constraints for future
weight-bearing restrictions, and occupational needs. With all other
factors being equal, the patient with a progressive increase in pain
and disability from a nonunited fracture is more likely to benefit from
surgical intervention than a patient with minimal or improving
symptoms. Conversely, the patient with clear radiographic signs of
nonunion but with limited pain and marginal functional disability may
be more suited for less invasive treatment means such as external bone
growth stimulation especially if operative management would provide
untimely loss of employment (Fig. 25-5).
combination of pain, tenderness, and detectible motion at the site of
fracture. It should be noted that symptoms of nonunion can be masked in
patients with relatively stable or rigid fixation such
as
is seen with locked plate constructs. It is not uncommon for such
patients to present with an acute onset of pain and disability after a
long time period associated with full weight bearing with no or a
relative paucity of symptoms (Fig. 25-6). In these circumstances, acute failure of the hardware is often the cause of the new symptoms.
 |
|
FIGURE 25-5 Anteroposterior and lateral radiographs (A,B)
showing a nonunion in a 27-year-old man 6 months after IM nailing of a middiaphyseal femur fracture. At this point, the patient was fully weight bearing with minimal pain and an ultrasound external bone stimulator was applied 20 minutes daily. Six months later and without further surgical intervention, the nonunion is healed (C,D). |
evaluation of fractures as they provide a timely, accurate, and
inexpensive means to diagnose an acute fracture. The utility of plain
radiographs in evaluating fracture union is less clear. Using plain
radiographs, the diagnosis of nonunion is very often arrived at by
excluding union.
 |
|
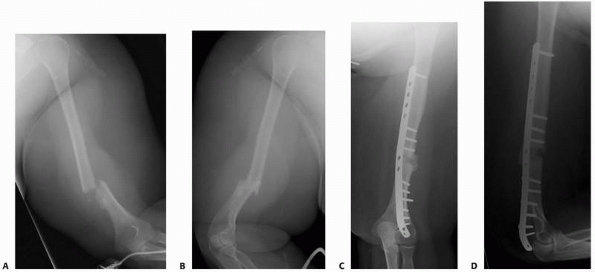
FIGURE 25-6 A patient with an open distal humeral shaft fracture (A,B) was treated with irrigation and débridement and plate fixation (C,D). (continues)
|
typically defined by the presence of bridging callus across the
fracture. Whether circumferential bridging, as evidenced by bridging
across all four cortices seen on orthogonal radiographs, is required to
accurately diagnosis union is unclear. The orthopaedic literature is
conflicted with regard to this requirement. Many studies define union
as healing across only 2 or 3, rather than 4, cortices on the two
orthogonal views. Although identifying the
number
of healed cortices may seem straightforward, in practice this is a very
subjective and imprecise exercise especially in the presence of
implants that obscure visualization. Furthermore, it is often difficult
to know if the radiograph and fracture are coplanar. When not, fracture
gaps may be disguised by overlying bone (Fig. 25-7). Minor variances in the angle of the radiograph can completely disguise a nonunion (Fig. 25-8).
 |
|
FIGURE 25-6 (continued)
Despite having a nonunion at 6 months, she was functioning well and without pain due to the stability provided by the plate. An acute increase in pain occurred as a result of fracture of the plate (E,F). With immobilization, this fracture healed in slight varus without further surgery (G,H). |
stability of the fixation method creates great variations in the
expected biologic healing response and therefore the radiographic
appearance of union. Simple diaphyseal fractures fixed anatomically
with rigid compression plate techniques that promote primary bone
healing without fracture callus may look nearly identical at healing as
they did immediately after fixation (Fig. 25-9).
Under these circumstances, accurate diagnosis of union may be
difficult, but lack of union may be directly or indirectly evident.
Direct evidence is a fracture gap seen on a radiograph taken coplanar
with the fracture (see Figs. 25-7B and 25-8B).
In the absence of direct evidence of nonunion, plain radiographs should
be carefully scrutinized for indirect evidence of a lack
of
healing. Progressively loosened or broken implants indicate persistent
motion at the fracture site. More subtle findings are motion artifacts
seen in bone at or around the margin of seemingly stable implants or
fractured screws without complete loss of fixation (Fig. 25-10).
Judicious utilization of other imaging methods helps to confirm the
diagnosis of nonunion when only indirect evidence is present using
plain radiographs.
 |
|
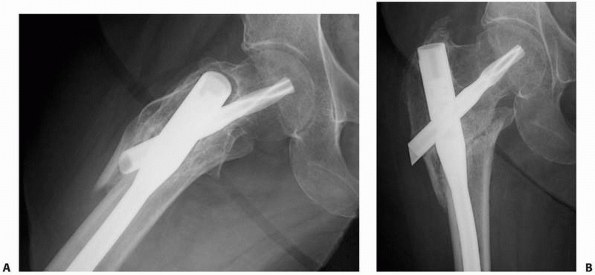
FIGURE 25-7
Radiographs taken out of plane may fail to identify a nonunion. Six months after IM nailing of a reverse obliquity intertrochanteric femur fracture, a lateral radiograph (A) reveals no persistent fracture line because the x-ray beam is not coplanar with the nonunion. The anteroposterior radiograph (B) is coplanar with the fracture and clearly demonstrates the nonunion. Indirect evidence of nonunion is evident on the lateral view (A) in the form of motion artifact around the nail. |
 |
|
FIGURE 25-8 A lateral radiograph (A)
fails to clearly identify a nonunion 8 months after open reduction and internal fixation of a distal humeral shaft fracture, whereas a slightly oblique projection (B) shows the nonunion clearly. |
 |
|
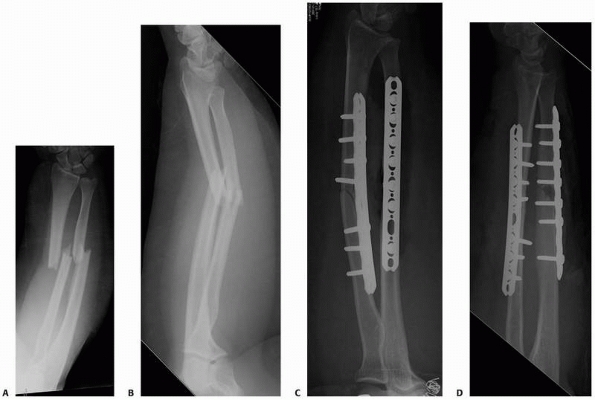
FIGURE 25-9 A patient with open radius and ulna shaft fractures (A,B) was treated with open reduction and internal fixation of both fractures (C,D). (continues)
|
accurately delineate bony anatomy at the site of a suspected nonunion.
Modern CT scans can be reformatted in high quality in any plane. This
allows an image orientation precisely optimized to evaluate the
potential lack of bridging bone, eliminating the major shortcoming of
plain radiography. CT scans have been shown to be highly sensitive
(100%) for detecting tibial nonunion.11
The limitation of CT, however, is the relative lack of specificity
(62%), that can lead to surgery in patients who actually have healed
fractures (Fig. 25-11).11
CT may be useful in providing a quantitative evaluation of fracture
healing and stability. In one study, patients with less than 25%
bridging of the circumference of bone were found to be at high risk
(37.5%) for clinical failure of fracture union, whereas those with
greater than 25% bridging had only 9.7% failure.29
 |
|
FIGURE 25-9 (continued) E,F. The simple ulnar fracture was treated with compression plating technique and healed without callus formation.
|
scintigraphy can be used to help diagnose nonunion, but a positive
result can be relatively nonspecific. On the bone scan, the vast
majority of nonunions show intense tracer uptake at the fracture site,
as do fractures undergoing normal healing.121
Various other types of scans, individually or in combination, have been
used in attempts to differentiate simple nonunion from those that are
complicated by infection. Increased blood flow and blood pool as
demonstrated during the first and second phases of a three-phase bone
scan are consistent with the inflammatory reaction seen with infection
but are not pathognomonic for infection. The combined use of a Tc-99m
and gallium-67 scans has produced inconsistent results for accurately
detecting infection at the site of nonunion.36,121
In contrast to other forms of nonunion, a synovial pseudoarthrosis
correlates with the presence of a cold cleft between two intense areas
of uptake on scintigraphy.37
 |
|
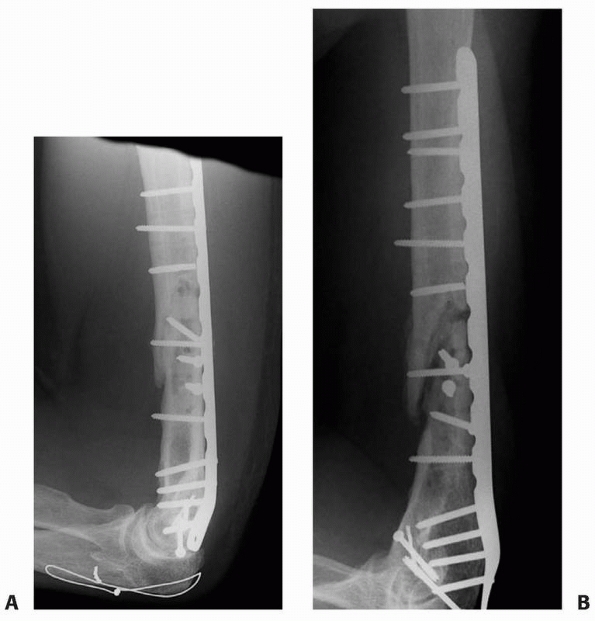
FIGURE 25-10 Fractured or loose screws can be indirect evidence of nonunion. A. Four months after repair of the distal humerus nonunion depicted in Figure 25-8, the lateral radiograph reveals fracture of a distal locking screw. B. Two months later, multiple additional screws are fractured consistent with the diagnosis of a persistent nonunion.
|
healing process or promote additional healing that would otherwise not
have taken place. Such strategies may be most successful for promoting
a delayed union to proceed to union, but healing of established
nonunion has also been reported for some nonoperative treatments. The
attractiveness of nonoperative treatment is essentially the absence of
surgical complications.
indirect intervention. Direct intervention implies application of
treatment directly to the nonunited bone. Examples include electrical
stimulation and ultrasound. Indirect intervention implies institution
of treatment directed more towards the patient as a whole. Examples of
indirect intervention would include maximizing nutrition, alteration of
certain medications, and smoking cessation (Table 25-1).
 |
|
FIGURE 25-11 Lack of specificity of CT in the diagnosis of nonunion. A,B. Anteroposterior and lateral radiographs 6 months after repair of a distal humeral nonunion show equivocal healing. C.
The coronal CT scan demonstrates a lucent line consistent with the diagnosis of nonunion. Revision nonunion repair was undertaken but solid healing, rather than nonunion, was encountered. Further scrutiny of the CT scan in retrospect reveals healing of both the posterior cortices of the medial (D) and lateral (E) columns. |
necessary ingredient for healing of all tissues, including bone.
Adequate caloric intake, vitamins, and protein are necessary to
optimize healing.55,113,130
Smoking is probably the most commonly studied patient comorbidity.
Therefore, it would seem cessation of smoking would be very important
as a nonoperative intervention to encourage fracture union. Higher
rates of delayed and nonunion have been reported in smokers and the
increased rates are probably related to the number of cigarettes smoked.78,124
The mechanism, although not completely understood, likely relates to
diminished osteoblast function and decreased local vascularity.31,38
healing and increase the risk of nonunion. Diabetic patients with one
or more comorbidities are at increased risk for the development of
nonunion.71 Diet modification and controlled blood sugar levels may minimize the negative effect of diabetes on fracture healing.5
play a role in nonunion in some patients. Although a direct cause or
role is not completely defined, it is certainly suspected. Conditions
like
calcium imbalance, hypogonadism, and thyroid and parathyroid disorders
should be addressed medically by the appropriate specialist.20
|
TABLE 25-1 Nonoperative Treatment of Nonunions
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
including steroids, phenytoin, chemotherapeutic agents, nonsteroidal
anti-inflammatory drugs, and some antibiotics (fluoroquinolones) can
negatively affect bone healing.21,51,102
including human immunodeficiency virus, is desirable in the face of
fracture and probably absolutely necessary in the treatment of an
established nonunion.61
intervention for a nonunion would be application of weight bearing in a
functional brace. This, however, is only reasonably practical for the
tibia. The mechanism for the success of this treatment is said to be
stimulation of osteoblastic activity by mechanical loading.105,120
capacitive coupling, direct current, and inductive coupling have been
used for several decades and are felt to be helpful in bone healing.
The mechanism of action is alteration of the electrical potential at
the fracture site.43,103,126
Requisite conditions of alignment and bone edge proximity are
important. Although results comparable to operative treatment have been
reported,53 some degree of
skepticism probably still exists due to the lack of well-designed
clinical trials. In the only perspective double blind trial of
electrical stimulation, published in 1994, the placebo group which had
no treatment had a 0% healing rate compared to 60% in the treated group.126 The series, however, was small with only 21 patients enrolled.126
Risk factors and relative contraindications for electrical stimulation
are considered to be prolonged nonunion, prior bone graft surgery,
prior electrical stimulation which failed, open fractures, active
osteomyelitis, extensive comminution. and atrophic nonunion.17
Electrical stimulation at this time can probably be considered to be a
generally reasonable, acceptable nonoperative form of treatment for
nonunion. Additional large double blind trials offering Level I
evidence can probably not be expected due to the necessity of the
control group having no treatment for the nonunion for a prolonged
period of time.
the time to union in fresh fractures. Appropriate studies and trials
have been conducted which demonstrate shorter healing times for fresh
fractures using low-intensity ultrasound.62,77
The mechanism is believed to be in part related to the actual
mechanical phenomenon created by the ultrasound. Ultrasound is a form
of low mechanical energy which may be stimulative to bone healing. The
three phases of healing (inflammation, repair, and remodeling) are each
probably influenced as well as angiogenesis, chondrogenesis, and
osteoblastic activity.3,107,116,148
trials for the use of ultrasound in the treatment of nonunions,
however, do not exist and will probably not be done. Once again, there
is an ethical consideration as the control group would essentially need
to have no treatment for their nonunions for a long period of time.
There are, however, studies supporting its use (primarily self-pared
controls for nonunion cases) with healing rates approaching 90% and
healing times ranging from approximately 100 to 180 days.41,48,95
complications with the only downside being the length of time that may
be required to achieve union when compared with operative methods. A
contraindication to nonoperative treatment is malalignment at the
nonunion site which, should the nonunion heal, would still leave the
patient with a functional deficit due to the residual deformity.
is bone healing, there is a wide range of methods available for
achieving this. Whereas a single treatment option is often clearly
superior for an acute fracture (e.g., intramedullary [IM] nailing for a
closed middiaphyseal tibia fracture), several options may be equally
suited for treatment of a nonunion of the same injury (e.g., exchange
nailing, dynamization, plate osteosynthesis, circular external
fixation, and external bone stimulation for a middiaphyseal tibial
nonunion). The vast array of options can usually be refined with
consideration of the integrity of the soft tissue envelope and
coexisting conditions. For instance, nonunion in the face of associated
infection makes repair with plates less and external fixation more
attractive. Malaligned nonunions are not well suited for interventions
that do not address the deformity such as external stimulation or
dynamization. Further refinement of the most desired treatment method
considers surgeon experience and skill with, the relative risks and
benefits of, as well as patient tolerance for, the remaining treatment
methods.
original injury or subsequent surgeries. In nonunion cases where
operative treatment is planned, it may be necessary to acquire soft
tissue coverage by local rotational or free tissue flaps. Of the myriad
of flaps available, only free tissue transfer brings something new to
the local environment in terms of vascularity and oxygen.25,39,40,86 Particular attention should be paid to the soft
tissue on the concave side of the deformity when angular correction of
a malaligned nonunion is planned. A perfect osseous procedure may be
planned and carried out only to have insufficient tissue to allow
tension-free closure to occur at the conclusion of the case. When soft
tissue coverage is lacking and flap coverage is not practical,
deficient soft tissues can be dealt with using an external fixation
technique or through primary shortening by other means. Purposeful
shortening and bone deformation to allow soft tissue closure without
tension followed by gradual correction of alignment and distraction
osteogenesis has been described utilizing the Taylor Spatial Frame
device.94
Another successful strategy is primary shortening during nonunion
repair, followed by secondary lengthening after union has occurred.81,100
repair, there are some common principles. As with most medical
conditions, accurately identifying the diagnosis is a critical first
step to designing a rational treatment plan. This is especially
important when dealing with nonunions. Classifying the nonunion as
hypertrophic, oligotrophic, atrophic, or pseudoarthrosis, identifying
whether it is septic or aseptic, and recognizing associated deformity
are each critical to formulation of the complete diagnosis. The
classification of the nonunion dictates whether a formal takedown is
required and if adjuvant bone grafting is indicated. Hypertrophic
nonunions, by definition, have inherent biologic capacity but lack
sufficient mechanical stability required for completion of union.
Treatment for this diagnosis is therefore focused upon increasing, and
often maximizing, mechanical stability. More rigid forms of fixation
such as plate fixation or a snug fitting nail in a diaphyseal region
are generally preferred to less rigid means such as braces or loose
fitting nails in metaphyseal regions. Because hypertrophic nonunions
have the biologic potential to heal, débridement of the nonunion site
and bone grafting is not an absolute requirement (see Fig. 25-3).
whereas pseudoarthroses are vital, atrophic nonunions and
pseudoarthroses have in common the need for débridement of the nonunion
site. The basic principle of atrophic nonunion management calls for
débridement of the nonviable bone ends back to healthy bleeding bone.
Both of these classes of nonunions also typically require bone
grafting. The relative paucity of healing potential of an atrophic
nonunion calls for a graft with osteoinductive or osteogenic
properties. A pseudoarthrosis, once débrided, has viable vascular ends,
and, technically speaking, may therefore not require a bone graft.
However, in the absence of bone transport or purposeful shortening,
graft is usually used to fill the gap that is invariably left by
débriding the synovial tissue in and around the pseudoarthrosis.
Oligotrophic nonunions are intermediate in their biologic capacity. It
can be difficult to establish whether failure to unite was related to a
primary problem of biology or of mechanics or a combination of both. It
is therefore prudent to aim treatment at improving both.
infection is another general principle of nonunion treatment. Even
severe and complex nonunions can be successfully treated in the absence
of infection while simple nonunions can be recalcitrant in the presence
of infection. If infection is diagnosed prior to nonunion treatment,
then dealing with infection becomes a priority. Removal of associated
implants with serial débridement of necrotic soft tissues and bone
until a stable healthy environment is achieved is a typical first step.
Stabilization by means that are conducive to eradication of infection
often calls for external fixation that spares the zone of infection
from implants. Internal fixation is generally avoided with the notable
exception of antibiotic coated IM nails, which have recently been shown
to be successful in this scenario.99,106,136
Infection treatment continues with organism-specific antibiotics,
usually delivered for 6 weeks parentally. Once clinical and laboratory
data indicate there is infection control, definitive treatment of the
nonunion ensues. If conversion of external to internal fixation is
planned, then a staged protocol consisting of removal of the external
fixator and cast application (when cast application is reasonably
appropriate) allows pin site healing prior to the definitive nonunion
surgery. On occasion, union can be accomplished concurrent with the
antibiotic phase of treatment, but infection treatment should not be
compromised toward this goal.
nonunion repair when preoperative clinical or laboratory evidence of
infection was otherwise absent is occasionally encountered. Internal
fixation and bone grafts in such a clinically noninfected but culture
positive nonunion site can be successfully left in situ, but
appropriate antibiotic therapy should be continued until union occurs.
However, internal fixation and bone grafting in the face of known
infection should be avoided whenever possible.
any associated deformity is paramount to not only the restoration of
normal anatomy, but is also critical to establishing appropriate
mechanics as the site of nonunion to maximally promote healing.
The universality of plate constructs extends from almost any particular
bone to almost any location within a given bone. That is, plates are
applicable to repair of diaphyseal as well as end segment nonunions.7
Whereas IM nailing is almost universally considered the treatment of
choice for acute middiaphyseal fractures of the femur and tibia, and by
some the humerus, plate fixation is applicable and may be preferred for
repair of ununited fractures in these locations.7,65,142
Whether the tibia, femur, or humerus is involved, a pre-existing IM
nail is, in most circumstances, removed at the time of nonunion repair
with plates. However, successful locked plate fixation without nail
removal has been reported using eccentric positioning of the plate that
allows bicortical screw fixation around the nail to augment unicortical
locked screws.92
relative invasiveness, most notably with regard to the potential
compromise of an already marginal soft tissue envelope that is often
encountered. In the absence of soft tissue concerns, where the local
soft tissues can accommodate the bulk of the implant and the dissection
required for insertion, nonunion repair with plate constructs is a very
powerful method that can be used successfully for any class of nonunion
(i.e., atrophic or hypertrophic)
by providing the stability, alignment control, and, when appropriate, the compression required for successful treatment.
 |
|
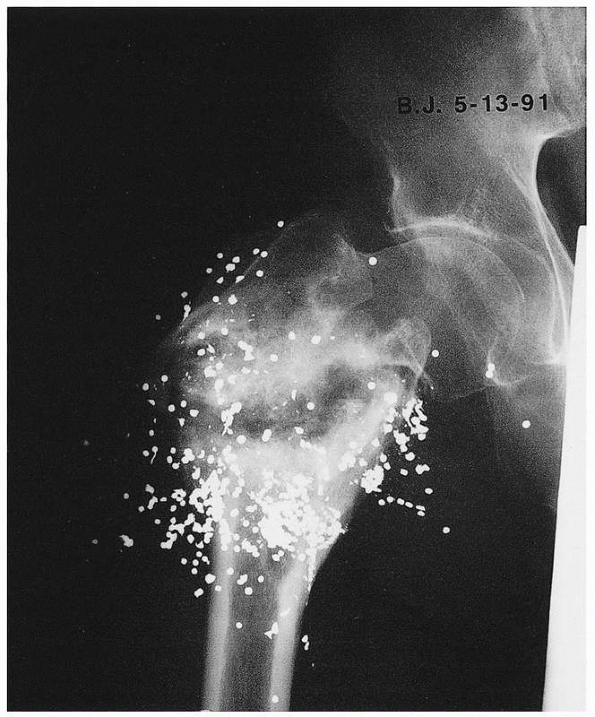
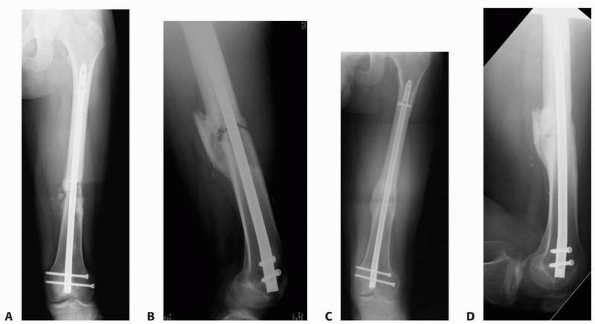
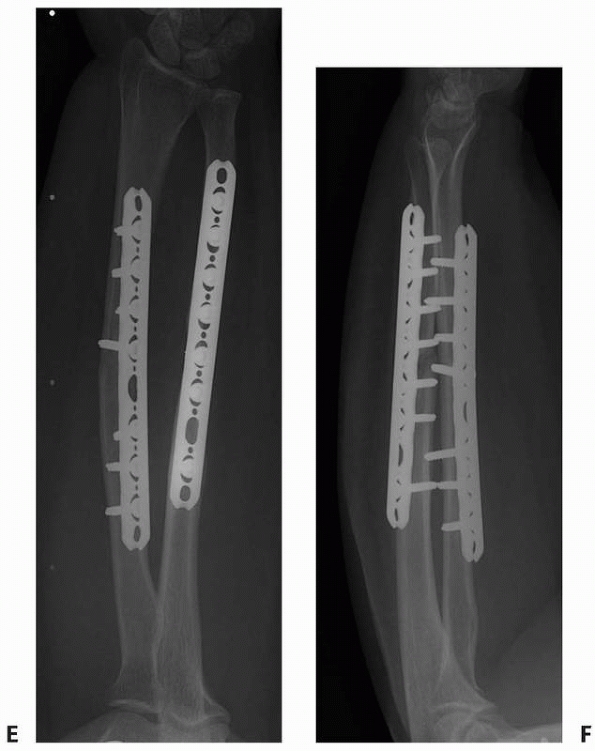
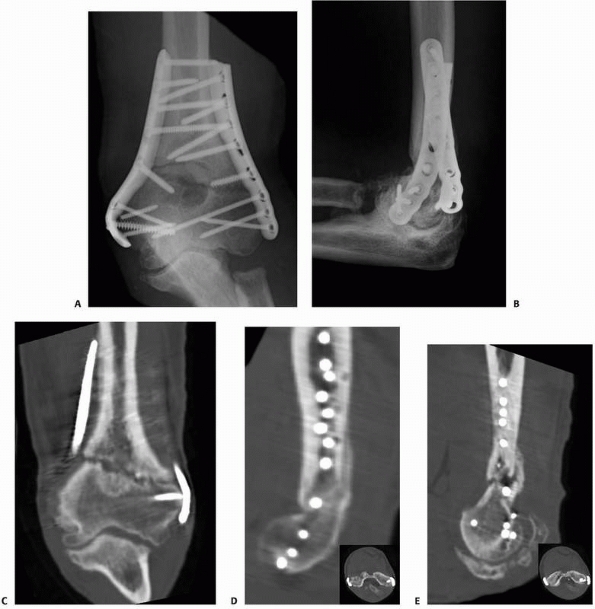
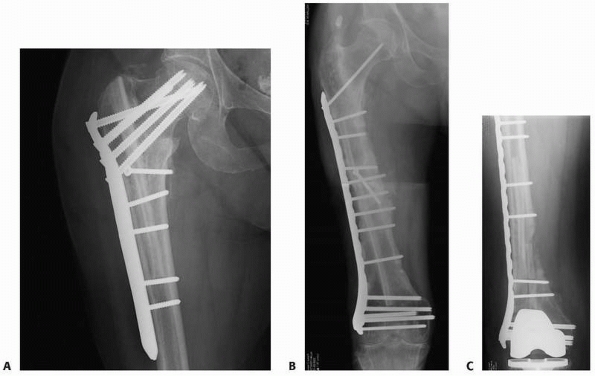
FIGURE 25-12 Plates can be used to treat nonunions at almost any part of any long bone. A. The proximal femur nonunion shown in Figure 25-7
was repaired with a proximal femoral locking plate with iliac crest bone graft and an intramedullary fibular strut. Nonunions of the midshaft and distal ends of the femur can be repaired with a straight lateral plate (B) and a distal femoral locking plate (C), respectively. |
consider the soft tissue envelope. These procedures are often
extensive, and postoperative swelling can be substantial and lead to
blistering, unforeseen wound issues, and even compartment syndrome.
Therefore, efforts to minimizing limb swelling are paramount. A
well-padded splint, one without proximal occlusiveness, is often used
even if not required to protect the mechanical integrity of the repair.
Elevation of the limb above the heart level and cold therapy are
mainstays of the initial postoperative regimen. Careful and timely
observation of wounds is a practice that can identify and potentially
minimize or avoid impending problems.
take three forms: primary nailing of a nonunion in the absence of a
pre-existing nail, exchange nailing, and dynamization. Regardless which
of these three situations is present, nail treatment is most applicable
to diaphyseal nonunion locations. Success is most often reported for
the tibia and femur, with exchange nailing of humeral nonunions being
less consistent unless supplemental bone graft is utilized.19,57,76,139,144
Nailing metaphyseal nonunions has been associated with mixed results
and is dependent upon the specific region being treated with success
most notably being reported for the distal femur and distal tibia.109,145
pre-existing nail in favor of a new nail, is most applicable to
situations where deficiencies of the pre-existing nail can be overcome
with a new, larger reamed nail. Such deficiencies can include lack of
rotational control because of absence or fracture of interlocking
screws and lack of adequate stability caused by an undersized nail. The
reaming associated with an exchange nailing procedure can provide
deposition of small amounts of local bone graft, but these deposits
cannot be expected to fill defects of any substantial size. Therefore,
exchange nailing is most applicable to situations without bone loss,
unless adjuvant open bone grafting accompanies the procedure. Also,
exchange nailing is best considered when angular alignment is
satisfactory. The new nail will tend to follow the pre-existing
intramedullary path of the prior nail and, therefore, angular
malalignments tend to persist after exchange nailing when specific
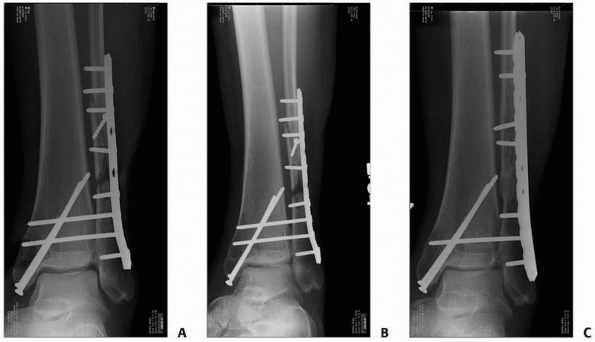
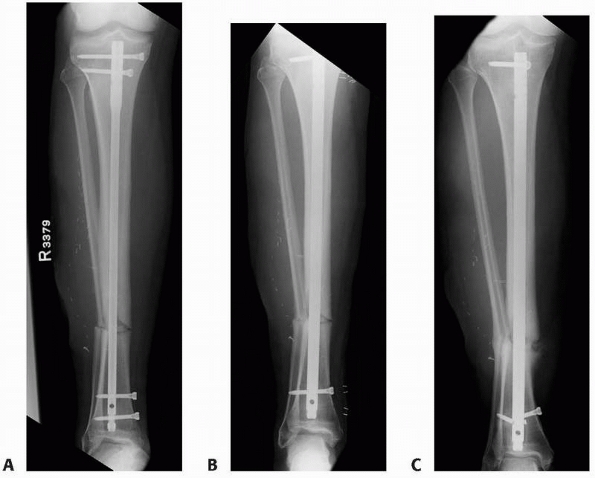
efforts are not taken to correct them (Fig. 25-13).
Alterations of angular alignment can be made during exchange nailing,
but this adds substantial technical challenges to the procedure as
correction of malalignment needs to occur prior to reaming. This
requires mobility of the nonunion, either at baseline or by surgical
means. External devices, such as a femoral distractor, are invaluable
tools to help obtain and maintain alignment during the procedure. When
multiplanar deformities are present, the simultaneous use of two
distractors can be helpful: one in the sagittal plane and one in the
coronal plane. All distractor pins should be placed in locations that
will not interfere with nailing. Union rates for exchange nailing of
femoral and tibial diaphyseal nonunions have ranged substantially, from
less than 50% to over 90%.19,57,98,141,144,146
The degree of overreaming required for effective application of
exchange nailing is somewhat controversial. Newer evidence suggests
that 1 mm of overreaming is sufficient rather than the historic
recommendations for at least 2 mm of overreaming.144
It should be clear that a minimum requirement for exchange nailing is
the ability to insert a large enough nail to provide mechanical
strength to the repair. When considering exchange nailing for the
tibia, an associated fibular osteotomy to allow fracture compression
during repair has been considered an integral part of the procedure,
but recent evidence suggests this is not essential.66
 |
|
FIGURE 25-13 Exchange nailing of a malaligned tibia nonunion. A. An undersized nail was used to treat an open tibial shaft fracture leading to an atrophic nonunion in slight valgus alignment. B. Exchange nailing was performed without specific consideration of the malalignment resulting in almost identical valgus. C. Persistent nonunion, although now oligotrophic, with fractured interlocking screws results.
|
screws at one end of a nail to allow axial shortening with weight
bearing, is a method advocated to promote healing of delayed unions or
nonunions when small gaps are present at the fracture site. Such gaps
may be present due to bone loss, osteoclastic bone resorption, or as a
result of prior static locked nailing with distraction at the fracture
site. Dynamization with modern nails that provide a dynamic
interlocking slot can take two forms. Removal of static screws with
retention or addition of a dynamic screw has the advantage of
maintaining rotational control, but limits the amount of shortening to
the amount of excursion of the dynamic screw within the oval dynamic
slot in the nail, usually just a few millimeters with most nail
designs. This limit may be advantageous to avoid excessive shortening,
or on the other hand, it may be detrimental by preventing sufficient
compression at the fracture to achieve union. The other form of
dynamization is removal of all interlocking screws from one end of the
nail. This allows more freedom for shortening at the expense of a lack
of any axial or rotational control inherent to the nail construct. The
ideal situation for this form of dynamization is when the fracture
pattern itself will result in limited shortening. The compression
allowed by dynamization will also provide increasing rotational
stability.
which end of a nail should be dynamized. Stability is maximized if
screws near the fracture are retained and those on the opposite side of
the isthmus relative to the nonunion site are removed. Another
consideration relates to which end should be allowed to telescope over
the nail. As the bone shortens, the nail will become more prominent on
the side of the nail with removed screws, though predicting the degree
of shortening can be imprecise. Therefore, screws should not be removed
if this result is undesirable. In the case of a retrograde femoral
nail, removal of distal screws, those near the knee, is a notable
example. In this scenario, the driving end of the nail, if devoid of
interlocking screws, can theoretically extend into the knee joint and
cause devastating damage to the patellar articular cartilage.
simplicity and minimal patient morbidity, was at one time commonplace
and even became a routine planned staged procedure prior to
establishment of a healing problem after femoral nailing. This practice
occurred despite a lack of clinical evidence to support its use. Good
results after routine dynamization of acute femur fractures was a
justification for the practice.73,135 Later evidence revealed that high union rates could be expected with static nailing without secondary dynamization.22 In the case of an established nonunion, dynamization is successful in promoting union in only approximately 50% of cases.143,146
and techniques used to treat fractures, circular ring fixators
utilizing thin wires and the concepts of Ilizarov are the mainstays for
treatment of nonunions by external fixation. The general principles of
these techniques are presented in Chapter 8.
The applicability of thin wire fixators to nonunions extends to almost
any location within any long bone as well as to the hand, foot, and
even to the clavicle.24,66,72,75,79,136
Other advantages of Ilizarov’s techniques are the relative paucity of
soft tissue trauma imparted by the repair method and the ability to
slowly correct associated deformities. The latter also protects the
soft tissues from stretching that can accompany acute deformity
correction. Other advantages include the ability to fine tune
correction and the potential for early weight bearing. A limitation to
treatment of end segment nonunions with ring fixators is the proximity
of thin wires to the involved joint.132
Wires that puncture the joint capsule incur the risk of joint sepsis if
pin site infection develops. Computer guided treatment with the Taylor
Spatial Frame is a recent advance that has considerably simplified
Ilizarov type correction of any malalignment, even complex multiplanar
deformities.35,115
The Taylor Spatial Frame differs from traditional Ilizarov fixators by
utilizing adjustable struts that are oriented in a hexapod
configuration. In conjunction with special web-based software programs,
six axes of deformity can be corrected simultaneously and accurately (Fig. 25-14).
fixators, the surgeon must consider if adjuvant open bone grafting is
prudent either at the initial nonunion procedure or in a
planned
staged manner. The characteristics of the nonunion dictate this aspect
of the strategy, with stiff nonunions being differentiated from mobile
nonunions. Radiographic evaluation of the stiff nonunion usually
reveals hypertrophic callus formation, and upon physical examination
stress across the nonunion site is accompanied by pain with resistance
to deformation. In contrast, the mobile nonunion is characterized by
either atrophic features on radiographic examination or by features of
a synovial pseudoarthrosis. On physical examination, the mobile
nonunion moves easily with stress, often without substantial pain. The
stiff nonunions have inherent biologic activity and therefore usually
respond favorably to closed external fixation methods that utilize
compression, distraction, or a combination of both.24,68,72,75
According to the principles of distraction osteogenesis, gradual
distraction of the hypertrophic nonunion stimulates new bone formation
and eventual union. The nonunion acts similarly to the regenerated bone
seen with lengthening or transport procedures. Modest lengthening, of
up to approximately 1.5 cm, can typically be accomplished through a
hypertrophic nonunion. If more length is required, a distant osteotomy
and lengthening at that site can be performed. Before distraction, a
short period of compression, typically 7 to 14 days, may be helpful to
prime the site for the osteogenic process. In certain circumstances,
when there exists a transverse nonunion site where external compression
will result in compression of the fracture fragments, union can be
accomplished with pure compression. Clearly, an advantage of such
gradual treatment, especially when associated with deformity
correction, is the preservation of the often compromised soft tissue
envelope.
 |
|
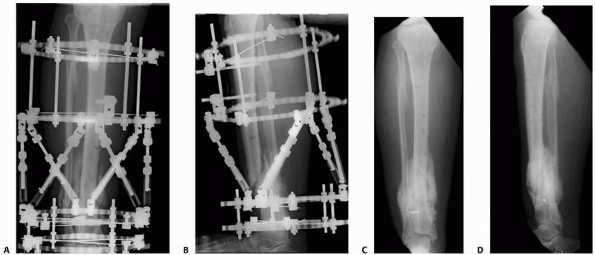
FIGURE 25-14 A,B.
An infected hypertrophic nonunion with varus, shortening, posterior translation, and apex anterior angulation seen immediately after application of the Taylor Spatial Frame. C,D. After gradual correction using computer software guidance, union and substantial correction of the deformity has been achieved. |
requires opening the nonunion site surgically to convert the nonviable
atrophic nonunion to fresh viable bone ends, or in the case of
pseudoarthrosis to resect the synovium, pseudocapsule, and
fibrocartilage covering the bone ends. In either case, the medullary
canal is opened and the site typically bone grafted. Adherents to the
Ilizarov principles may, instead of bone grafting, utilize a
corticotomy of the involved bone at a site with healthy soft tissues
and then transport the intercalary segment with eventual healing being
achieved by compression at the nonunion site and regenerate bone
formation at the corticotomy site, respectively. This technique is
technically much more demanding, potentially more time consuming,
relies on healing at two sites rather than one site, and has the
potential complications inherent to bone transport, but despite this,
it is a powerful strategy in experienced hands especially when
lengthening of more than 2 cm is required.
frames requires management of pin sites. Pin site infection near a
joint has the potential for joint sepsis, and in these cases careful
pin site care and close observation can avoid disastrous consequences.
The accepted strategies for pin site care are many, but at least one
should be chosen and clearly outlined to the patient and caregivers.
Signs and symptoms of infection should prompt more aggressive
treatment, such as initiation of antibiotic therapy or wire removal or
exchange. The potential for safe and early weight bearing is an
advantage of nonunion treatment with ring fixators. Once any associated
deformities are corrected and any soft tissue deficiencies are healed,
some degree of weight bearing in the frame is generally permitted in
all but the most extreme cases.
viable option for the treatment of nonunion. However, when
circumstances are appropriate, arthroplasty can result in rapid and
profound symptomatic and functional improvement. Several factors
determine the appropriateness for arthroplasty. A minimum requirement
is a nonunion located in a periarticular location that has an
associated arthroplasty option that can accommodate the bone resection
required to eliminate the nonunion. Standard hip and shoulder
arthroplasty (either hemi- or total as dictated by other factors such
as the condition of the joint
and
patient demand) are options for nonunions of the femoral neck and
intertrochanteric region and the surgical neck of the humerus,
respectively.56,149
Depending upon other factors, arthroplasty for these indications can
either be an excellent first choice, an option of last resort, or
contraindicated. In the elderly, especially with associated joint
arthritis which may be in the form of pre-existing arthritis,
posttraumatic arthritis, joint destruction from prior implants, or
osteonecrosis, arthroplasty is preferred to other methods of nonunion
treatment. In these circumstances, arthroplasty usually offers the
advantages of immediate weight bearing and concomitant treatment of the
associated arthritis, two things that are not accomplished with
nonunion repair. In physiologically younger patients, arthroplasty
becomes less advantageous due to limited longevity of the implants. In
the absence of substantial and debilitating arthritis in this patient
population, periarticular nonunions are usually best managed with
repair. Regardless of patient age, active infection at the site of
nonunion is a contraindication to total joint arthroplasty. Strategies
for arthroplasty after eradication of infection, often accompanied with
antibiotic spacer placement, are not unreasonable but are associated
with a substantial risk of persistent infection.
that is amenable to arthroplasty is the distal femur. Here, a total
knee arthroplasty that includes distal femoral replacement is
relatively mainstream, technically of moderate but not extreme
complexity, and due to a lack of critical soft tissue attachments on
the distal femur, is generally associated with good functional outcomes.1,13,57,96 Analogous are nonunions of the distal humerus where even standard total elbow replacements are commonly sufficient.119
This is because of a combination of the high frequency of
juxta-articular fractures of the distal humerus, potential problems
with fixation of very distal fractures in this region, and the common
coexistence of osteoporosis. When nonunions are more proximal, a distal
humeral replacing total elbow prosthesis can be used.27
In contrast to the distal metaphyseal ends of the femur and humerus,
metaphyseal nonunions of the proximal ends of these bones are somewhat
less ideal for arthroplasty. The common reason is related to the tendon
insertions onto the greater trochanter of the femur and the greater and
lesser tuberosities of the humerus, respectively. These tendon
attachments must be preserved to maintain normal function and therefore
proximal replacing arthroplasty in these regions should only be
considered in extreme circumstances where other options are of equal or
greater disfavor.108
the critical importance of the integrity of the tibial tubercle, is
typically avoided even in the presence of knee arthritis in favor of
staged knee arthroplasty after nonunion repair. Critical soft tissue
attachments do not limit the applicability of total ankle replacement
for nonunion of the distal tibia but the lack of prostheses that
accommodate bone loss in this location do.
considered, the presence or absence of associated joint arthritis will
increase or decrease the threshold for selecting arthroplasty,
respectively. Similarly, the functional demands and age of the patient
are considered, with physiologically younger patients typically
undergoing nonunion repair rather than arthroplasty. Any documented
history, or even suggestive history, of infection must be identified
and considered. Active infection is a contraindication to arthroplasty.
Arthroplasty can be considered after aggressive treatment of an
infected nonunion. This typically involves relatively radical
débridement of the involved bone, internal implantation of
antibiotic-impregnated cement spacers, and prolonged administration of
organism-specific parental antibiotics. Whether an infection-free
period of time off antibiotics prior to arthroplasty, aimed to
demonstrate eradication of the infection, or whether arthroplasty
should be accompanied by long-term oral suppression is unclear, and
this decision is typically individualized and made in concert with
consultant infectious disease specialists. More distant history of
infection presents a similar quandary. Biopsy or joint aspiration prior
to arthroplasty can be useful to guide decision-making.
often dictated by associated comorbid conditions and by patient
preference rather than a technical inability to eventually achieve
union. Psychologic and psychosocial factors specific to each individual
patient are important to recognize, discuss, and consider before making
a decision for amputation in the setting of nonunion. The invested time
and effort in prior treatments makes some patients reluctant to
consider amputation and eager for fresh ideas and strategies for repair
whereas the same investments in prior failures may leave other patients
frustrated, worn out, and ready to proceed with a definitive procedure
such as amputation. Candid assessments for potential success with
additional attempts at nonunion repair, the required investment of time
and energy of the patient, and the relative functional, cosmetic, and
neurologic (i.e., pain, neuralgia) outcomes of success versus failure
of nonunion repair should be discussed and used to guide treatment
decisions. The chronic pain from nonunion that will dissipate with bone
healing needs to be differentiated from neurogenic pain which is likely
to linger. If such neurogenic pain is chronically disabling, then
efforts at nonunion repair may be misguided and amputation deserves
serious consideration. Also, a contingency plan for what follows if a
future nonunion repair fails is useful. A plan for amputation if
failure occurs with the next intervention may make it much easier for
some patients to select a course of treatment.
graft substance. It has the best and longest documentation and
experience. For instance, autograft from the iliac crest used in the
treatment of tibial and femoral nonunion typically results in union
rates exceeding 90%.6,14,26,42,117
Autogenous bone graft supplies osteogenic and osteoconductive
materials. Osteogenic cells, including stroma cells, are also present
in the graft material. It provides an excellent osteoconductive
scaffold by way of cancellous bone spicules. There is probably little
to no bone morphogenic protein in autogenous graft so that it probably
cannot be considered truly osteoinductive.123
volume grafts, but other sites such as the greater trochanter and the
femoral and tibial condyles can be used for small amounts. It is
estimated that 15% of the osteocytes or osteoblasts survive the bone
graft procedure.34 Autogenous bone grafting leads to concomitant bone formation and resorption.
limited amount which can be harvested. Additionally, the quality of the
autogenous graft will be dependent on host health issues such as
osteoporosis. Donor site morbidity (25% to 40%) includes infection,
pain (acute and chronic), secondary fracture, and hematoma formation.2
Autogenous bone grafting is used in atrophic and some oligotrophic
nonunions and to repair some pseudarthroses. Effective application
requires decortication of the bone in the recipient bed in most
instances for success.
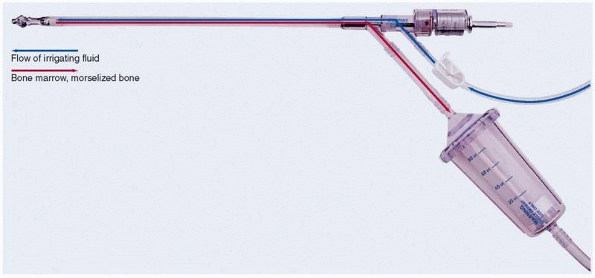
autogenous graft from the femoral canal has been proposed using the
Reamer-Irrigator-Aspirator (Synthes, Paoli, PA) (Fig. 25-15).93
This device was originally designed as a one-pass reamer for IM nailing
to minimize embolic phenomenon. Using this device, the reamings are
evacuated via suction and subsequently can be collected and used as
bone graft. A reamer that is 1 to 4 mm greater than the narrowest part
of the femoral canal as measured on preoperative and/or intraoperative
images is selected. The starting point is the same as for IM nailing
and maybe a piriformis or trochanteric entry site. This is identified
intraoperatively by fluoroscopy. The technique is limited to femoral
canals which are between 10 mm and 16 mm in diameter. This is due to
the currently limited reamer sizes available for the device. Using
standard IM nailing technique, a guidewire is inserted into the canal
of the femur with image control, and a one-time pass reamer is gently
used to ream the femoral canal with an in and out motion so as not to
advance too aggressively. A trap is used to collect the reamings.
Typically, 60 to 80 mL of graft can be harvested with experience. In
the relatively limited experiences reported so far, minimal
complications have occurred (mechanical malfunction, femur fracture,
embolism, excessive blood loss), but the potential certainly exists.93,131
Reamings in general have been shown in in vitro analysis to contain
pluripotential stem cells with the possibility of dedifferentiation
into osteoblasts. Specifically quantitative assessment has demonstrated
the presence of growth factors using the irrigant/aspirate technique.123
An animal study has additionally suggested that a superior quality of
callus may result from reimplantation of graft material harvested in
this way.59 Randomized trials comparing this method of graft harvest to standard iliac crest grafts are needed.
 |
|
FIGURE 25-15 The Reamer-Irrigation-Aspirator.
|
segmental defects. They are advantageous in this situation as they
provide a live bone graft that also has structural properties,
something that is not provided by standard iliac crest cancellous
autograft. The fibula is the most commonly harvested bone, although
other sites such as the iliac crest118 and rib140
have been used. They typically must undergo some degree of hypertrophy
for ultimate success in addition to healing to the host tissue at each
end.67 Double vascularized grafts
(fibula) combined with cancellous grafts have been proposed to gain
additional and more rapid stability.4
It is, however, a technically demanding procedure requiring
microvascular anastomoses. Complications include recurrent graft
fractures and donor site morbidity.60
Alternatives to autologous bone graft, including demineralized bone
matrix, bone marrow aspirate, platelet-rich plasma, allograft, and
ceramics, have been developed and utilized for nonunion treatment with
varying degrees of success. New advances in bioengineering based on an
enhanced understanding of the cellular and molecular aspects of
fracture healing have led to the development and clinical use of growth
factors, such as bone morphogenetic proteins (BMPs), that augment
fracture healing. The details of the basic science and mechanism of
action of these alternatives is presented in Chapter 5.
Advantages of these substitutes in the treatment of nonunion include
reduced or eliminated patient morbidity and increased or unlimited
supply relative to autologous bone graft. The ideal graft substitute
for
nonunion
treatment would be inexpensive, of unlimited supply, easy to prepare
and handle, easy to implant, without adverse reactions, and 100%
efficacious.
these attributes, but none have all. Nonunion healing rates with use of
these substitutes has been reported but there is little in the way of
direct comparison to autologous bone grafting.12,28,50,52,65,76,83,128
Recombinant human osteogenic protein-1 (rhOP-1) was directly compared
to autograft in the treatment of 124 tibial nonunions in a prospective
randomized study.44 At 9 months
after repair using an IM nail, 81% of the rhOP-1 and 85% of the
autograft treated nonunions healed clinically. Radiographic healing in
the rhOP-1 group was 75%, while it remained essentially unchanged from
clinical healing in the autologous group (84%). The main advantage of
rhOP-1 was elimination of the donor site pain, which occurred in 20% of
the patients receiving autograft.
and allograft to autogenous bone graft for reconstruction of diaphyseal
tibial fractures with cortical defects.70
Thirteen patients in the rhBMP-2 group had results comparable to 10
patients in the autograft group. These tibial defects were not
nonunions but with an average of 4 cm in size, they were certainly
unlikely to heal without intervention.
locked compression plating of osteoporotic humeral shaft nonunions
resulted in union in 11 out of 13 patients.110
Both patients with DBM failure united after a secondary iliac crest
bone grafting. By comparison, all 12 treated with autograft from the
same retrospective study healed without further intervention.
Noncomparative data has shown good healing rates, 89% to 92%, with
rhBMP-7 (rhOP-1) in the treatment of various upper and lower extremity
nonunions.33,88
A disadvantage of recombinant BMPs is the cost, although recent data
suggests using BMPs could actually reduce costs when treating complex
or recalcitrant nonunions by reducing the number of procedures and
number of hospital days.32
has been shown to contain osteoprogenitor cells and has both osteogenic
and osteoinductive properties.9 The generally low concentration of such cells (612/cm3) and the variability between patients (12 to 1224/cm3)
has led to the development of improved aspiration techniques with
specialized aspiration needles and cell concentration systems aimed at
increasing both the number and the density of the progenitor cells63
without concentration; some evidence suggests that the number of cells
in marrow aspirates is suboptimal for nonunion treatment.64
Furthermore, some controversy exists as to whether concentrated cells
should be injected directly and percutaneously into nonunion sites or
if application with an osteoconductive carrier after open débridement
of the nonunion is required for optimal results.127
The actual efficiency of direct marrow injection is difficult to
interpret in the face of associated interventions including cast
immobilization and IM nailing that have accompanied the injection in
series reporting 75% to 90% union rates.28,47,52
Platelet-rich plasma (PRP) is harvested as the thin layer between clear
plasma and red blood cells in centrifuged peripheral blood. This fluid
contains concentrated platelets (300%-600%) which are believed to
promote osteoblast proliferation and differentiation.137 However, to date no clinical evidence exits to support the use of PRP in the treatment of nonunions.
(calcium sulfate, calcium phosphates, beta tricalcium phosphate, and
hydroxyapatite) and allograft that lack osteoinductive or osteogenic
properties have little role in promoting bone healing in the setting of
nonunion. These materials are primarily osteoconductive and function
best as graft extenders or carriers for osteoinductive compounds.
persistent inability to achieve smoking cessation even in the face of
potential limb loss. However, hope is not lost and new treatments may
provide some improved results for these patients.
are minimally symptomatic from their nonunion and who are not
candidates for surgery or additional procedures (see Fig. 25-5).
Both electrical and ultrasound stimulation are used in conjunction with
operative treatment in high-risk patients such as smokers and diabetics.
well-aligned tibial diaphyseal nonunions with reasonable success, but
much less favorable results have accompanied exchange nailing for
nonunions of the femur shaft. Nevertheless, in a well aligned isthmal
femoral diaphyseal nonunion which is hypertrophic or oligotrophic,
exchange nailing is still attempted in some cases.7
articular segment, a plate and bone graft technique is used.
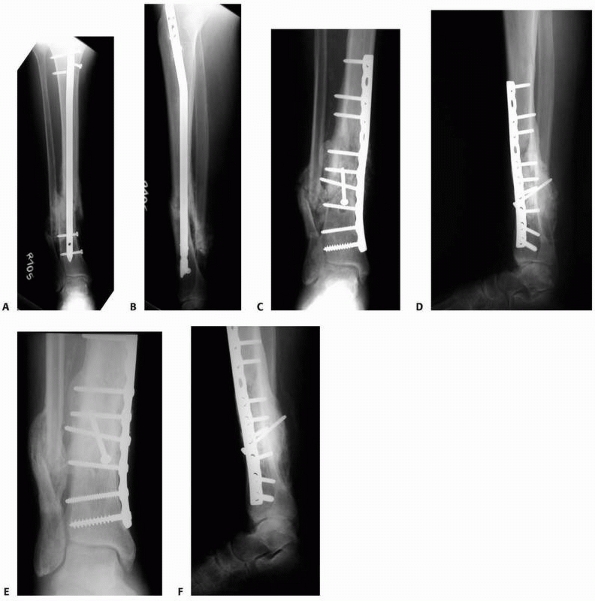
Hypertrophic nonunions are simply stabilized and, when possible,
compressed (Fig. 25-16), while oligotrophic and
atrophic nonunions are also grafted with autogenous cancellous bone. As
experience has been gained with the Reamer-Irrigator-Aspirator
(Synthes, Paoli, PA), the graft volume and quality as well as the
favorable healing reaction with decreased morbidity compared to iliac
crest harvest have been impressive (Fig. 25-17).
primary shortening followed by lengthening is favored. In this
technique, the area of segmental loss is filled with
polymethylmethacrylate cement. At 4 to 6 weeks, when an osteogenic
membrane has formed around the cement, the membrane is surgically
reopened and the cement is removed and generous cancellous bone
grafting is carried out (see Fig. 25-17).
Recorticalization generally occurs slowly but usually by 3 to 6 months.
This, is done in conjunction with internal stabilization most
frequently using a locked intramedullary rod for diaphyseal defects or
locked plates for metaphyseal defects.85
The initial role of the spacer is to maintain limb lengthening and a
space for future grafting by avoiding fibrous tissue in-growth. The
secondary role of the spacer is the induction of a membrane formation.
This membrane is synovial-like with few inflammatory cells.101
The membrane itself serves to contain the graft, prevent fibrous
invasion, and provide growth factors. Immunochemistry has shown that
the membrane produces growth factors and inductive factors including
BMP-2, which is probably maximal at around 4 weeks.101 In his original article, Masquelet85 reported successful use of this two
stage technique in 35 cases with defects ranging from 4 to 25 cm. Other
authors have had similar success with this staged membrane-induced
technique.111,125
The underlying mechanism of the membrane formation is not well
understood, but cases when the membrane itself has generated enough
bone so that secondary grafting is not necessary have been observed in
our practices. It is unclear whether this membrane can form with
substances other than methylmethacrylate, and this technique requires
an excellent soft tissue envelope.85
 |
|
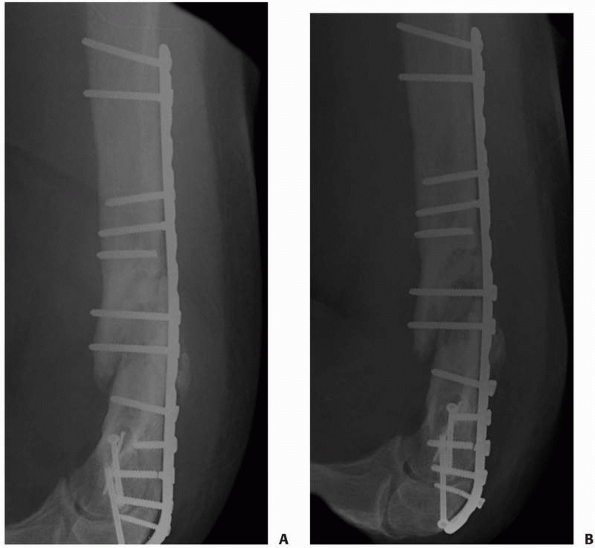
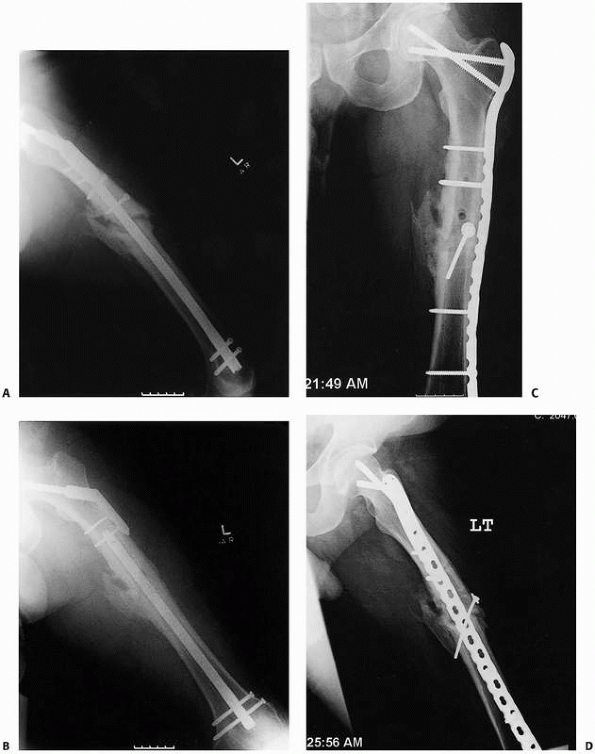
FIGURE 25-16 A,B. Hypertrophic nonunion after retrograde nailing and hip fracture fixation. Treatment with compression plating (C,D) resulted in healing of the diaphyseal nonunion.
|
transfer is not possible, primary shortening over an IM rod followed by
full weight bearing with an elevated shoe is preferred. Once healing
has occurred, the limb is then lengthened with either an internal
skeletal distraction nail (ISKD Orthofix Inc, McKinney, TX) (Fig. 25-18)
or the Ilizarov technique. In some cases with less than 3 or 4 cm of
shortening, patients are often satisfied with the result and do not
desire the lengthening procedure. The internal skeletal distraction
nail seems to be better tolerated than the skinny wire external fixator
techniques. It is, however, no faster. Complications similar to those
with other distraction or transport techniques still exist including
too fast or too slow distraction, failure of or delay in regenerate
formation, adjacent joint problems, need for exchange nailing, and
failure of the distraction device itself.
 |
|
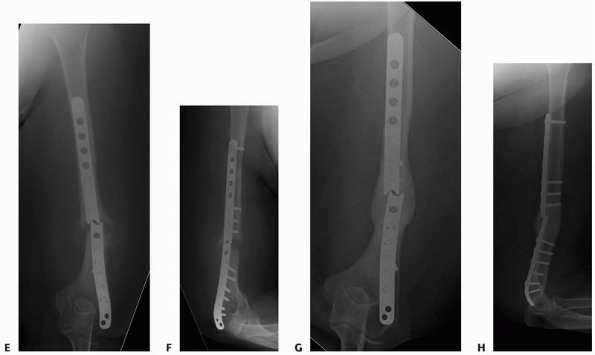
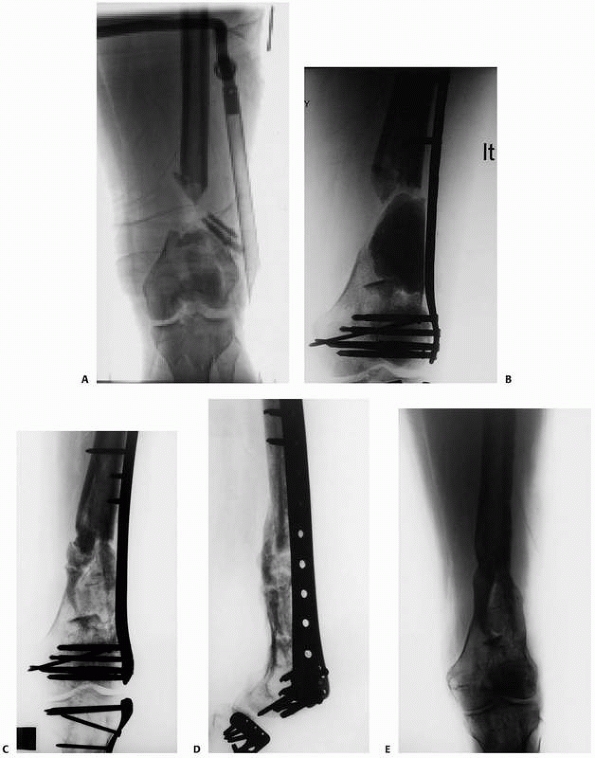
FIGURE 25-17 A. Grade IIIB comminuted open distal femur fracture with bone loss. B. Bridging plate with cement spacer. C,D. Anteroposterior and lateral views after Reamer-Irrigation-Aspirator grafting. E. Anteroposterior view after hardware removal. (Courtesy of Dr. Timothy Weber, Orthoindy Indianapolis, IN.)
|
 |
|
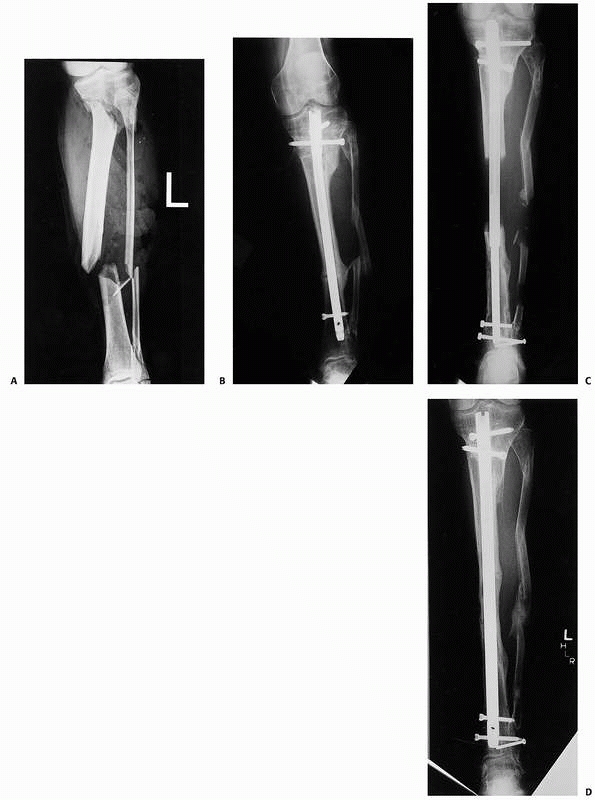
FIGURE 25-18 A.
A 40-year-old woman with a grade IIIB open tibia. The central fragment was completely stripped of soft tissue. She was not a candidate for free tissue transfer. B. After resection of significant bone, the leg was shortened and the fracture was treated with a locked rod. The fracture healed with full weight-bearing ambulation in a built up shoe. Note the overlapping fibula. Only her local tissues, which were adequate in volume after shortening, were used for coverage. C. Subsequent lengthening was then achieved with an internal skeletal distraction nail. D. After exchange nailing, the regenerate was mature at about 6 months. |
P, Moran M, Houshian S, et al. Distal femoral fractures treated by
hinged total knee replacement in elderly patients. J Bone Joint Surg Br
2006;88:1065-1070.
ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone
graft harvesting. Clin Orthop Relat Res 1996;329:300-309.
Y, Ito M, Harada Y, et al. Low-intensity pulsed ultrasound accelerates
rat femoral fracture healing by acting on the various cellular
reactions in the fracture callus. J Bone Miner Res 2001;16:671-680.
HA, Parsons JR, Lin SS. The effects of blood glucose control upon
fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop
Res 2002;20:1210-1216.
C, Ricci WM, Bolhofner BR. Indirect reduction and plating of distal
femoral nonunions. J Orthop Trauma 2002;16:287-296.
C, Ricci WM, Bolhofner BR. Results of indirect reduction and plating of
femoral shaft nonunions after intramedullary nailing. J Orthop Trauma
2001;15: 254-263.
MH, Stanford R, Turner R. Hyperbaric oxygen therapy for promoting
fracture healing and treating fracture nonunion. Cochrane Database Syst
Rev 2005;1: CD004712.
M, Tornetta P III, Sprague S, et al. Predictors of reoperation
following operative management of fractures of the tibial shaft. J
Orthop Trauma 2003;17:353-361.
T, Bouchard KA, Phadke A, et al. The accuracy of computed tomography
for the diagnosis of tibial nonunion. J Bone Joint Surg Am
2006;88:692-697.
T, Gazdzik TS, Szczepanski T. Benefit of percutaneous injection of
autologous platelet-leukocyte-rich gel in patients with delayed union
and nonunion. Eur Surg Res 2008;40:289-296.
P, Trojani C, Walch G, et al. Shoulder arthroplasty for the treatment
of the sequelae of fractures of the proximal humerus. J Shoulder Elbow
Surg 2001;10: 299-308.
J Jr, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects
with bone graft and calcium sulfate. Clin Orthop Relat Res
2003;411:245-254.
J Jr, Tinsley K, Ricci WM, et al. Induction of chondrocyte apoptosis
following impact load. J Orthop Trauma 2003;17:635-641.
HR. Treatment of ununited fractures of the long bones; diagnosis and
prevention of nonunion. J Bone Joint Surg Am 1965;47:174-178.
CT, Shaman P, Heppenstall RB, et al. Tibial nonunion treated with
direct current, capacitive coupling, or bone graft. Clin Orthop Relat
Res 1995;321;223-234.
MR, O’Connor DP, Monla YT, et al. Metabolic and endocrine abnormalities
in patients with nonunions. J Orthop Trauma 2007;21:557-570.
KM, Saunders MM, Kirsch T, et al. Effect of COX-2-specific inhibition
on fracture-healing in the rat femur. J Bone Joint Surg Am
2004;86-A:116-123.
RJ. Intramedullary nailing of femoral shaft fractures. Part II:
fracture-healing with static interlocking fixation. J Bone Joint Surg
Am 1988;70:1453-1462.
RC, Bosse MJ, MacKenzie EJ, et al. Impact of smoking on fracture
healing and risk of complications in limb-threatening open tibia
fractures. J Orthop Trauma 2005; 19:151-157.
MA, Guerreschi F, Holman JA, et al. Distraction osteogenesis in the
treatment of stiff hypertrophic nonunions using the Ilizarov apparatus.
Clin Orthop Relat Res 1994;301:159-163.
N, Mathes SJ. Comparison of the effect of bacterial inoculation in
musculocutaneous and random-pattern flaps. Plast Reconstr Surg
1982;70:1-10.
MW, Finkemeier CG. Treatment of supracondylar nonunions of the femur
with plate fixation and bone graft. J Bone Joint Surg Am
1999;81:1217-1228.
A, Veillette CJ, Sanchez-Sotelo J, et al. Linked elbow replacement: a
salvage procedure for distal humeral nonunion. J Bone Joint Surg Am
2008;90:1939-1950.
JF, Guse R, Tiedeman J, et al. Autologous marrow injection as a
substitute for operative grafting of tibial nonunions. Clin Orthop
Relat Res 1991;266:259-270.
CM, Dickson K, Cody DD, et al. Computed tomography reformation in
evaluation of fracture healing with metallic fixation: correlation with
clinical outcome. J Trauma 2008;65:1421-1424.
CM, McQueen MM. Nonunions of the proximal humerus: their prevalence and
functional outcome. J Trauma 2008;64:1517-1521.
TK, Whitesides TE Jr, Heller JG, et al. Nicotine on the
revascularization of bone graft. An experimental study in rabbits.
Spine 1994;19:904-911.
Z, Dimitriou R, Giannoudis PV. Health economics: a cost analysis of
treatment of persistent fracture nonunions using bone morphogenetic
protein-7. Injury 2007;38:371-377.
R, Dahabreh Z, Katsoulis E, et al. Application of recombinant BMP-7 on
persistent upper and lower limb non-unions. Injury 2005;36(Suppl
4):S51-S59.
NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor
sites: techniques and complications. J Am Acad Orthop Surg
2001;9:210-218.
J, Alavi A, Mandell GA, et al. Sequential technetium-99m/gallium-67
scintigraphic evaluation of subclinical osteomyelitis complicating
fracture nonunion. J Orthop Res 1985;3:219-225.
JL Jr, Brighton CT, Heppenstall RB, et al. Detection of synovial
pseudarthrosis by 99mTc scintigraphy: application to treatment of
traumatic nonunion with constant direct current. Clin Orthop Relat Res
1981;161:15-23.
MA, Frost PJ, Iida-Klein A, et al. Effects of nicotine on cellular
function in UMR 106-01 osteoblast-like cells. Bone 1991;12:283-286.
LJ, Price DC, Mathes SJ, et al. Dynamic properties of blood flow and
leukocyte mobilization in infected flaps. World J Surg 1990;14:796-803.
J, Wood MB. Experimental comparison of bone revascularization by
musculocutaneous and cutaneous flaps. Plast Reconstr Surg 1987;79:81-90.
GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic
protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am
2001;83-A(Suppl 1):S151-S158.
B, Klaue P. Mechanical stability and posttraumatic osteitis: an
experimental evaluation of the relation between infection of bone and
internal fixation. Injury 1977; 9:23-29.
NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in
20 cases of ununited fracture. Acta Orthop Scand 1993;64:671-672.
LC, Cullinane DM, Barnes GL, et al. Fracture healing as a postnatal
developmental process: molecular, spatial, and temporal aspects of its
regulation. J Cell Biochem 2003;88:873-884.
PV, Kanakaris NK, Dimitriou R, et al. The synergistic effect of
autograft and BMP-7 in the treatment of atrophic nonunions. Clin Orthop
Relat Res 2009 (epub ahead of print).
MacDonald DA, Matthews SJ, et al. Nonunion of the femoral diaphysis.
The influence of reaming and nonsteroidal anti-inflammatory drugs. J
Bone Joint Surg Br 2000;82:655-658.
A, Sangwan SS, Siwach RC, et al. Percutaneous bone marrow grafting for
the treatment of tibial nonunion. Injury 2005;36:203-206.
HR, Bernstein RA, Abbott J. Treatment of ununited tibial fractures: a
comparison of surgery and pulsed electromagnetic fields (PEMF).
Orthopedics 1992;15: 711-719.
GJ, Springer BD, Jacofsky DJ, et al. Total knee arthroplasty for
salvage of failed internal fixation or nonunion of the distal femur. J
Arthroplasty 2005;20: 344-349.
DJ, Lee SS, Goulet JA. Success of exchange reamed intramedullary
nailing for femoral shaft nonunion or delayed union. J Orthop Trauma
2000;14:178-182.
TO, Wieling R, Green JM, et al. Effect of reimplanted particles from
intramedullary reaming on mechanical properties and callus formation. A
laboratory study. J Bone Joint Surg Br 2007;89:1534-1538.
WJ, Lewis CP, Lavy CB. Open fractures of the tibia in HIV positive
patients: a prospective controlled single-blind study. Injury
2004;35:852-856.
JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing
by noninvasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am
1994;76:26-34.
P, Mathieu G, Poignard A, et al. Percutaneous autologous bone-marrow
grafting for nonunions. Surgical technique. J Bone Joint Surg Am
2006;88(Suppl 1 Pt 2):322-327.
P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow
grafting for nonunions. Influence of the number and concentration of
progenitor cells. J Bone Joint Surg Am 2005;87:1430-1437.
C, Sama D, Toro JB, et al. Plate fixation of ununited humeral shaft
fractures: effect of type of bone graft on healing. J Bone Joint Surg
Am 2006;88:1442-1447.
CW, Wu CC, Su CY, et al. Exchange nailing for aseptic tibial shaft
nonunion: emphasis on the influence of a concomitant fibulotomy. Chang
Gung Med J 2006;29: 283-290.
K, Tomita K, Hashimoto F, et al. Long-term follow-up of vascularized
bone grafts for the reconstruction of tibial nonunion: evaluation with
computed tomographic scanning. J Trauma 1992;32:693-697.
M, Karaoglu S, Cilli F, et al. Treatment of femoral nonunions by using
cyclic compression and distraction. Clin Orthop Relat Res
2005;436:222-228.
MM, Ricci WM, Borrelli J Jr, et al. A protocol for treatment of
unstable ankle fractures using transarticular fixation in patients with
diabetes mellitus and loss of protective sensibility. Foot Ankle Int
2003;24:838-844.
AL, Bucholz RW, Bosse MJ, et al. Recombinant human BMP-2 and allograft
compared with autogenous bone graft for reconstruction of diaphyseal
tibial fractures with cortical defects. A randomized, controlled trial.
J Bone Joint Surg Am 2006;88: 1431-1441.
KB, Maiers-Yelden KA, Marsh JL, et al. Ankle fractures in patients with
diabetes mellitus. J Bone Joint Surg Br 2005;87:489-495.
T, Tsuchiya H, Sakurakichi K, et al. Reconstruction with distraction
osteogenesis for juxta-articular nonunions with bone loss. J Trauma
2005;58:1213-1222.
J, Makela EA, Turunen V, et al. Percutaneous bone grafting in the
treatment of the delayed union and non-union of tibial fractures.
Injury 2002;33:239-245.
M, Eralp L, Sen C, et al. Management of stiff hypertrophic nonunions by
distraction osteogenesis: a report of 16 cases. J Orthop Trauma
2003;17:543-548.
GM, Papadokostakis GM, Alpantaki K, et al. Intramedullary nailing for
nonunion of the humeral diaphysis: a review. Injury 2006;37:953-960.
TK, Ryaby JP, McCabe J, et al. Accelerated healing of distal radial
fractures with the use of specific, low-intensity ultrasound. A
multicenter, prospective, randomized, double-blind, placebo-controlled
study. J Bone Joint Surg Am 1997;79:961-973.
A, Usenius JP, Aarnio M, et al. Are smokers a risk group for delayed
healing of tibial shaft fractures? Ann Chir Gynaecol 1993;82:254-262.
J, Bauduin G, Driesen R, et al. Treatment of nonunion of the humerus
using the Ilizarov external fixator. Clin Orthop Relat Res
1998;353:223-230.
JR, Taitsman LA, Barei DP, et al. Femoral nonunion: risk factors and
treatment options. J Am Acad Orthop Surg 2008;16:88-97.
J, Nadarajah R, Allen PW, et al. Ilizarov external fixator: acute
shortening and lengthening versus bone transport in the management of
tibial nonunions. Injury 2005;36:662-668.
PR, Gershuni DH. Treatment of nonunion of fractures in the
epiphyseal-metaphyseal region of long bones. J Orthop Trauma
1987;1:141-151.
M, Cozzolino F, Cozzolino A, et al. Platelet gel supplementation in
long bone nonunions treated by external fixation. J Orthop Trauma
2008;22:342-345.
AC, Fitoussi F, Begue T, et al. [Reconstruction of the long bones by
the induced membrane and spongy autograft]. Ann Chir Plast Esthet
2000;45:346-353.
SJ, Alpert BS, Chang N. Use of the muscle flap in chronic
osteomyelitis: experimental and clinical correlation. Plast Reconstr
Surg 1982;69:815-829.
M. Aseptic nonunion. In: Ruedi TP, Murphy W, eds. AO Principles of
Fracture Management. Stuttgart: Thieme Vercal, 2000:748-762.
MD, DiPasquale DJ, Wild LM, et al. The effect of smoking on clinical
outcome and complication rates following Ilizarov reconstruction. J
Orthop Trauma 2003;17: 663-667.
M, Li G, Marsh DR. Nonsteroidal anti-inflammatory drug-induced fracture
nonunion: an inhibition of angiogenesis? J Bone Joint Surg Am
2006;88(Suppl 3): 140-147.
B, Srivastav S, Mittal V, et al. Use of locking compression plates for
long bone nonunions without removing existing intramedullary nail:
review of literature and our experience. J Trauma 2008;65:482-486.
JT, Stahel PF, Smith WR, et al. A new minimally invasive technique for
large volume bone graft harvest for treatment of fracture nonunions.
Orthopedics 2008;31: 257.
SJ, Helfet DL, Rozbruch SR. Temporary intentional leg shortening and
deformation to facilitate wound closure using the Ilizarov/Taylor
spatial frame. J Orthop Trauma 2006;20:419-424.
PA, van der Krans A, Patka P, et al. Low-intensity pulsed ultrasound in
the treatment of nonunions. J Trauma 2001;51:693-702.
TR, Green A, McGuigan FX. Late prosthetic shoulder arthroplasty for
displaced proximal humerus fractures. J Shoulder Elbow Surg
1995;4:271-280.
JK, Bae JH, Oh CW, et al. Treatment of femoral and tibial diaphyseal
nonunions using reamed intramedullary nailing without bone graft.
Injury 2008;39:952-959
H, Yokoyama K, Higashi K, et al. Use of antibiotic-impregnated bone
cement nail to treat septic nonunion after open tibial fracture. J
Trauma 2002;52:364-366.
D, Catagni MA, Argnani F, et al. Ilizarov treatment of tibial nonunions
with bone loss. Clin Orthop Relat Res 1989;241:146-165.
P, Masquelet AC, Bareille R, et al. Induced membranes secrete growth
factors including vascular and osteoinductive factors and could
stimulate bone regeneration. J Orthop Res 2004;22:73-79.
AC, Prpa B, Rouse MS, et al. Levofloxacin and trovafloxacin inhibition
of experimental fracture-healing. Clin Orthop Relat Res 2003;414:95-100.
B, Kann P, Forst T, et al. Bone mineral density and bone metabolism in
diabetes mellitus. Horm Metab Res 1997;29:584-591.
VD, Papakostas I, Stamatis ED, et al. Current concepts in delayed bone
union and nonunion. Clin Podiatr Med Surg 2006;23:445-453, viii.
Z, Jun PZ, Jie XJ, et al. Use of antibiotic cement rod to treat
intramedullary infection after nailing: preliminary study in 19
patients. Arch Orthop Trauma Surg 2007;127:945-951.
NM, Goldberg BB, Forsberg F, et al. Power Doppler assessment of
vascular changes during fracture treatment with low-intensity
ultrasound. J Ultrasound Med 2003;22:145-153.
J, Colleran K, Borens O, et al. Nonunions of the distal tibia treated
by reamed intramedullary nailing. J Orthop Trauma 2004;18:603-610.
D, Kloen P, Kadzielski J, et al. Locking compression plates for
osteoporotic nonunions of the diaphyseal humerus. Clin Orthop Relat Res
2004;425:50-54.
J, Lakovaara M, Flinkkila T, et al. Staged method using antibiotic
beads and subsequent autografting for large traumatic tibial bone loss:
22 of 23 fractures healed after 5 to 20 months. Acta Orthop
2007;78:520-527.
CM, Court-Brown CM, McQueen MM, et al. Estimating the risk of nonunion
following nonoperative treatment of a clavicular fracture. J Bone Joint
Surg Am 2004; 86-A:1359-1365.
H. Nonunion and malunion. In Browner BD, Levine AM, Jupiter JB, eds.
Skeletal Trauma. Philadelphia: WB Saunders, 1998:501-541.
SR, Pugsley JS, Fragomen AT, et al. Repair of tibial nonunions and bone
defects with the Taylor Spatial Frame. J Orthop Trauma 2008;22:88-95.
JJ, Bachner EJ, Bendo JA, et al. Low-intensity pulsed ultrasound
increases calcium incorporation in both differentiating cartilage and
bone cell cultures. Trans Orthop Res Soc 1989;14:15.
M, Morgan SJ, Linford E, et al. Central bone grafting for nonunion of
fractures of the tibia: a retrospective series. J Bone Joint Surg Br
2009;91:522-529.
AH, Anzel SH, Salyer WA. Transfer of vascularized grafts of iliac bone
to the extremities. J Bone Joint Surg Am 1987;69:1319-1327.
A, Burkhalter WE, Latta LL. Functional bracing in the treatment of
delayed union and nonunion of the tibia. Int Orthop 2003;27:26-29.
K, Daneels F, Obrie E. Technetium-99m-diphosphonate, gallium-67 and
labeled leukocyte scanning techniques in tibial nonunion. Acta Orthop
Belg 1992; 58(Suppl 1):168-172.
RK. Histology of Fracture Repair and Nonunion. Bulletin of the Swiss
Association for Study of Internal Fixation. Bern, Switzerland:
Association for Study of Internal Fixation, 1978.
G, Herrmann S, Green J, et al. Quantitative assessment of growth
factors in reaming aspirate, iliac crest, and platelet preparation.
Bone 2006;39:1156-1163.
MA, Finnegan M, Natarajan R, et al. Effect of smoking on tibial shaft
fracture healing. Clin Orthop Relat Res 1999;365:184-200.
PB, Werner CM, Dumont CE. Two-stage reconstruction with free
vascularized soft tissue transfer and conventional bone graft for
infected nonunions of the tibia: 6 patients followed for 1.5 to 5
years. Acta Orthop 2005;76:878-883.
G, King JB. A prospective, double-blind trial of electrical capacitive
coupling in the treatment of nonunion of long bones. J Bone Joint Surg
Am 1994;76:820-826.
MK, Miclau T. Autologous iliac crest bone graft: should it still be the
gold standard for treating nonunions? Injury 2007;38(Suppl 1):S75-S80.
R, Liang TS, Tay BK. Autologous marrow injection in the treatment of
delayed and non-union in long bones. Singapore Med J 1993;34:412-417.
MA, Jones EA, Strachan RK, et al. Prediction of fracture healing in the
tibia by quantitative radionuclide imaging. J Bone Joint Surg Br
1987;69:441-447.
TK. Prevention of complications in orthopedic surgery secondary to
nutritional depletion. Clin Orthop Relat Res 1987;222;91-97.
P, Norris B. Reamer-Irrigator-Aspirator as a bone graft harvester.
Techniques in Foot & Ankle Surgery 2007;6:100-107.
P, Polyzois D. Septic arthritis of the major joints of the lower limb
after periarticular external fixation application: are conventional
safe corridors enough to prevent it? Injury 2005;36:239-247.
J. Delayed union and nonunion of fractures. In: Crenshaw A, ed.
Campbell’s Operative Orthopedics. St. Louis: Mosby, 1992:1287-1345.
R, Conway J. Antibiotic cement-coated interlocking nail for the
treatment of infected nonunions and segmental bone defects. J Orthop
Trauma 2007;21:258-268.
BO, Alho A, Ekeland A, et al. Interlocking intramedullary nailing in
femoral shaft fractures. A report of 48 cases. J Bone Joint Surg Am
1985;67:1313-1320.
S, Bumbasirevic M, Lesic A, et al. Modification of the Ilizarov
external fixator for aseptic hypertrophic nonunion of the clavicle: an
option for treatment. J Orthop Trauma 2006;20:122-128.
SW, Lin SS, Wang CR, et al.Bone healing of tibial lengthening is
delayed by cigarette smoking: study of bone mineral density and
torsional strength on rabbits. J Trauma 1999;46:110-115.
CJ, McKee MD. Growth factors—BMPs, DBMs, and buffy coat products: are
there any proven differences amongst them? Injury 2007;38(Suppl
1):S38-S48.
MJ, Hakanson R, Stover MD, et al. Failure of exchange reamed
intramedullary nails for ununited femoral shaft fractures. J Orthop
Trauma 2000;14:335-338.
DA, Johnson DL, Miao M. Compression plating for nonunion after failed
external fixation of open tibial fractures. J Bone Joint Surg Am
1992;74:1279-1285.
CC. The effect of dynamization on slowing the healing of femur shaft
fractures after interlocking nailing. J Trauma 1997;43:263-267.
CC. Exchange nailing for aseptic nonunion of femoral shaft: a
retrospective cohort study for effect of reaming size. J Trauma
2007;63:859-865.
CC, Shih CH, Chen WJ, et al. High success rate with exchange nailing to
treat a tibial shaft aseptic nonunion. J Orthop Trauma 1999;13:33-38.
KH, Parvizi J, Wang SJ, et al. Exposure to low-intensity ultrasound
increases aggrecan gene expression in a rat femur fracture model. J
Orthop Res 1996;14:802-809.
B, Chiu KY, Wang M. Hip arthroplasty for failed internal fixation of
intertrochanteric fractures. J Arthroplasty 2004;19:329-333.
LW, Ma L, Cheung LK. Changes in blood perfusion and bone healing
induced by nicotine during distraction osteogenesis. Bone
2008;43:355-361.
