Primary Malignant Tumors
rare. Most spinal tumors are caused by metastatic spread from another
primary source. Of the 2000 primary bone sarcomas and 6000 soft tissue
sarcomas diagnosed annually in the United States, only about 10% arise
from the spine. Primary tumors of the spinal column are difficult to
classify as one clinical entity. The complex neuromusculoskeletal
development of the spine may account for a spectrum of malignant tumors
with distinct biologic behaviors. Because of biologic heterogeneity,
these tumors have variable sensitivity to radiotherapy and
chemotherapy. Characterization of the tumor type is essential for
devising an appropriate therapeutic strategy.
Approximately 85% of patients with spinal tumors have back pain as the
initial complaint. Diagnosis often is delayed because this presentation
resembles degenerative disc disease. Useful clues to an underlying
neoplasm include slow, gradual onset of pain in the absence of a clear
traumatic event; gradual progression of pain; persistent pain at night;
and symptoms unrelated to mechanical stressors. Although uncommon,
systemic signs may occur, such as weight loss, loss of appetite,
fevers, night sweats, and generalized malaise. Only 10% of patients
have a severe neurologic deficit, but many have radicular pain.
Patients with spinal tumors are more likely to have a specific site of
focal tenderness compared with patients with degenerative disc disease,
particularly if they also have a pathologic compression fracture of the
involved vertebrae. In younger patients, primary malignant tumors
usually are osteosarcoma or Ewing’s sarcoma (Table 11-1).
In older adults, the most common primary malignant spinal tumors are
multiple myeloma (the most common primary tumor of the spine),
chordoma, chondrosarcoma, and malignant fibrous histiocytoma. Sacral
tumors often are diagnosed late in the disease process because the mass
is difficult to appreciate on general musculoskeletal examination. More
than half of all patients with a sacral chordoma have a palpable mass
on rectal examination, however, at the time of presentation.
osteosarcoma, often have an associated soft tissue mass extending
beyond the vertebral body and evident on plain radiographs. Bone
destruction, increased sclerosis, and deformity also may be appreciated
on plain radiographs. Magnetic resonance imaging (MRI) is the study of
choice after plain radiography. With the addition of gadolinium, MRI
can be used to evaluate surrounding soft tissue, neural elements, and
disc involvement and to distinguish between extradural, intradural,
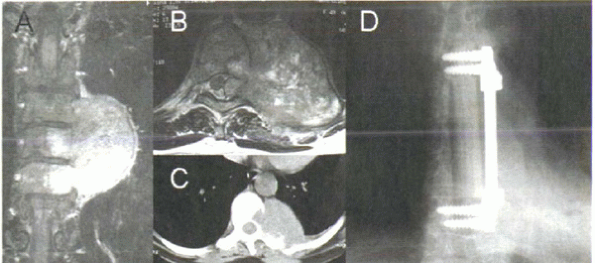
extramedullary, and intramedullary tumors (Fig. 11-1A, B).
Pathologic lesions that involve the posterior elements, especially in
patients younger than age 30 years, are more likely to be benign than
malignant. Diffusion-weighted MRI of the bone marrow helps to
differentiate benign from pathologic vertebral compression fractures.
Malignant pathologic vertebral compression fractures are hyperintense.
In contrast, benign compression fractures are isointense or hypointense
on diffusion-weighted images. Infections usually start in the disc
space and spread to adjacent vertebral bodies above and below the disc
space, whereas neoplasms start in the vertebral body, and the disc
inhibits their spread (see Fig. 11-1A).
further information. Bone scintigraphy allows the entire skeleton to be
screened. Bone scintigraphy can identify lesions 2 to 12 months before
they can be seen on plain radiography. Certain tumors, particularly
multiple myeloma, may not be seen on this study, however. A solitary
lesion in the spine raises the possibility of a primary bone sarcoma.
Computed tomography (CT) with intrathecal contrast is a useful
alternative when MRI is unavailable or cannot be tolerated.
Cerebrospinal fluid can be obtained for additional testing. CT is
better than MRI for determining the degree
of bone involvement (Fig. 11-1C).
Positron emission tomography and single-photon emission computed
tomography are newer techniques that may be used in lieu of bone
scintigraphy, but they are not widely used to date.
 |
|
Figure 11-1 (A) Coronal MRI of thoracic fibrosarcoma. Tumor invades bone readily but is inhibited by the disc space. (B) Axial T2-weighted image shows canal invasion and spinal compression. (C) Axial CT scan shows bone destruction, but contralateral pedicle is free of destruction. (D)
Radiograph after resection and reconstruction. Anterior column support is provided by a strut allograft, fixed with anterior instrumentation. |
diagnosis must be established. Techniques for obtaining tissue for
pathologic evaluation include fine-needle aspiration, percutaneous core
needle biopsy, and open biopsy. The choice of technique depends on the
location and extent of the tumor. Fine-needle aspiration is useful when
the tissue component is large and only a small sample is required. CT
guidance is the most common localization technique. Percutaneous core
needle biopsy is useful when bone tissue is needed. A common approach
is transpedicular with multiplanar fluoroscopy. The diagnostic yield of
needle aspiration and core biopsy of tumors is relatively high (75% to
85%) but lower for infections (50% to 60%). In all cases, the biopsy
tract should be placed to allow its excision during future procedures.
The surgeon who would perform any resection procedure should
participate in the initial patient evaluation and biopsy.
malignant tumors has been used for more than 20 years. Malignant tumors
are staged according to histologic grade, compartmental location, and
presence of metastases. Stage I tumors are low grade, and stage II
tumors are high grade. Stage III tumors are tumors of any grade with
regional or distant metastases. Each stage is subdivided further into A
for intracompartmental tumors and B for
extracompartmental tumors. A high-grade osteosarcoma with extension
into the paravertebral soft tissues is Enneking stage IIB. The Enneking
staging system was designed for pelvic and extremity tumors. In the
spine, differentiating extension of tumor into the spinal canal and the
anterior paraspinal soft tissue is crucial for surgical treatment. This
key characteristic is not reflected, however, in the Enneking staging
system.
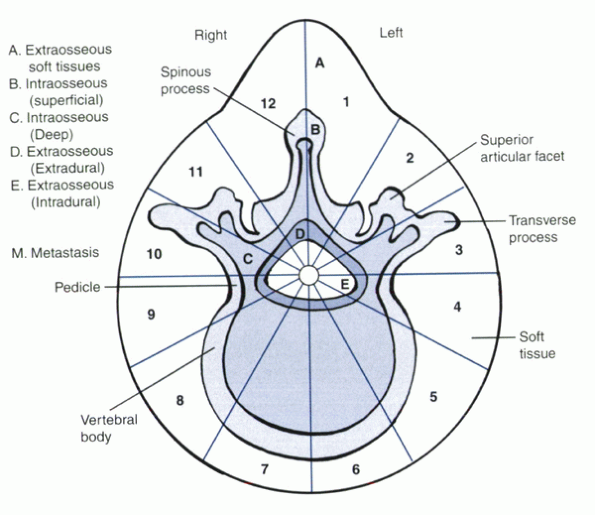
designed specifically for the spine. This system divides the vertebra
into 12 sectors in a clock-face arrangement (Fig. 11-2). Tumor location is defined further by involvement in six areas:
type, location, and size and the patient’s neurologic status and
overall health. Treatment modalities include (see Table 11-1):
-
Chemotherapy
-
Radiotherapy
-
Surgery.
osteosarcoma, Ewing’s sarcoma, and multiple myeloma. For Ewing’s
sarcoma and osteosarcoma, multiagent chemotherapy achieves 90% tumor
kill; subsequent surgical resection leads to 75% to 85% 5-year
survival. For osteosarcoma, tumor kill of less than 90% with
preoperative chemotherapy has a 25% 5-year survival. Postoperative
radiotherapy is instituted if margins are positive after surgical
resection. Chordomas and chondrosarcomas are not well treated with
chemotherapy or radiotherapy. Treatment is mainly marginal or wide en
bloc resection. Multiple myeloma is treated best initially with
radiotherapy. If systemic involvement is widespread, chemotherapy may
be added. Solitary plasmacytoma is an isolated form of multiple
myeloma. Although it occurs rarely as a true solitary lesion, the
5-year survival is significantly better than for disseminated multiple
myeloma (see Table 11-1).
-
The neural elements are protected from further injury caused by compression.
-
Spinal stability is maintained.
-
Resection is performed to achieve a cure.
 |
|
Figure 11-2
Weinstein-Boriani-Biagini staging system for spine tumors. On the transverse plane, the vertebra is divided into 12 radiating zones (numbered clockwise 1 to 12) and into five layers (A to E from prevertebral to dural involvement). The longitudinal extent of the tumor is recorded. Each tumor is identified by the numbers of the sectors occupied, the letters of the layers involved, and the vertebrae of occurrence. (From Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum: treatment and outcome in 21 cases. Spine 1996;21:1569-1577. |
|
TABLE 11-1 PRIMARY MALIGNANT TUMORS OF THE SPINE
|
||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
uniform system to describe surgical margins. Surgical procedures are
defined as intralesional, marginal, wide, or radical.
the resection. The tumor can be entered intentionally, when a curettage
technique is used, or inadvertently, when access to normal tissue
around the tumor mass is limited. In a marginal resection, the plane of
dissection is through the reactive zone of the tumor. A wide resection
requires that a cuff of normal tissue completely surround the tumor
mass. At no place should the tumor be encountered in either marginal or
wide resection. Within the canal, the dura must be excised in most
cases to achieve a wide tumor margin. A radical resection is not
possible in the spine because this requires that the entire compartment
be resected. Even if the patient were willing to sacrifice the spinal
cord or cauda equina, the spinal canal causes the compartment to extend
the entire length of the spine, making radical resection impossible. En
bloc resections in the spine have marginal or wide margins.
Most patients maintain relatively normal bowel and bladder function if
the resection spares one S3 nerve root and both S1 and S2 nerve roots (Table 11-2).
Preserving all the nerve roots on one side (unilateral S1-5), while
resecting the entire contralateral side, also enables most patients to
maintain normal bowel and bladder function.
marginal or wide resections can be performed by anterior and posterior
approaches (Fig. 11-4). To remain outside the
tumor, a portion of the canal ring, such as one of the pedicles, must
be free of tumor. Remaining outside the tumor is important because
local recurrence correlates highly with poor clinical outcome. The
likelihood of recurrence is two to five times higher if the surgical
margin contains tumor cells, whether it is microscopically or
macroscopically evident. The method of tumor resection also may be
important. A piecemeal resection, even though negative margins in the
tumor bed are achieved, leads to a higher recurrence rate than en bloc
resection.
|
TABLE 11-2 BOWEL AND BLADDER FUNCTION AFTER SACRAL RESECTION
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
 |
|
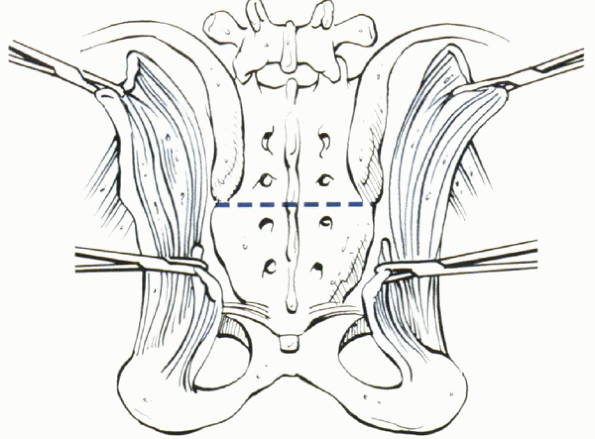
Figure 11-3
Technique of low sacral resection of a chordoma or other primary tumor distal to S3. (From Levine AM, Crandall DG. The textbook of spinal surgery, 2nd ed. New York: Lippincott-Raven, 1997:1983-2006. |
stabilization. In most cases, anterior column support is established
with a strut graft or cage. Instrumentation provides fixation until
arthrodesis is achieved (see Fig. 11-1D). Stable fixation, which is imperative after resection, may require anterior and posterior fixation with long constructs.
treatment varies, depending on tumor type, size, location, and overall
patient health (see Table 11-1). Most authors
agree that persons with life expectancies greater than 3 months should
be offered surgical treatment, even if palliative, to treat pain caused
by spinal instability and neurologic dysfunction attributable to neural
compression.
 |
|
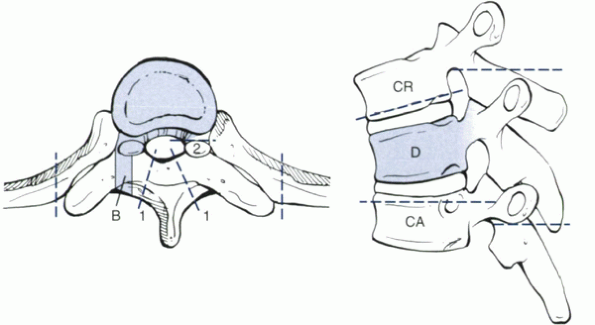
Figure 11-4
Method of resection of tumor involving almost the entire vertebral body. When one portion of the canal ring is intact, such as a pedicle, a two-stage resection can be performed with negative margins. The laminae initially are removed along dashed lines 1. The uninvolved transverse process and posterior part of the pedicle are removed up to dashed line 2. B is the site of a previous transpedicular biopsy. D is the involved vertebra. If more than one vertebra is involved, D is the most cranial involved vertebra. CR is the adjacent uninvolved cranial vertebra. CA is the adjacent caudal uninvolved vertebra. |
the spine are challenging. A unique feature of musculoskeletal tumors
of the spine, compared with tumors in the extremities and pelvis, is
the proximity of the neural elements. En bloc resection with negative
margins is difficult to achieve without injuring the spinal cord and
nerve roots. Successful treatment relies on prompt diagnosis based on
clinical history and physical examination findings, advanced imaging,
and carefully planned biopsy. Multidisciplinary treatment regimens are
applied according to tumor cell type, location, and size and overall
patient health. Surgical treatment must consider not only the tumor
itself, but also the status of the surrounding neural elements and the
overall biomechanical stability of the spine.
P, Gunterberg B, Meis-Kindblom JM, et al. Prognostic factors and
outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based
study of 69 cases. Cancer 2001;91:1201-1212.
S, Biagini R, De Iure F, et al. Lumbar vertebrectomy for the treatment
of bone tumors: surgical technique. Chir Organi Mov 1994;79:163-173.
S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the
sacrum: treatment and outcome in 21 cases. Spine 1996;21:1569-1577.
S, Weinstein JN, Biagnini R. Primary bone tumors of the spine:
terminology and surgical staging. Spine 1997;22:1036-1044.
MW. Radical resection of vertebral body tumours: a surgical technique
used in ten cases. J Bone Joint Surg 1994;76B:765-772.
N, Steinberger AA, Moore F, et al. Indications and results of combined
anterior-posterior approaches for spine tumor surgery. J Neurosurg
1996;85:438-446.
R, Yaszemski MJ, Currier BL, et al. Relationship between surgical
margins and local recurrence in sarcomas of the spine. Clin Orthop
2002;127-132.
