Pathologic Fractures Associated with Tumors and Unique Conditions of the Musculoskeletal System
One – Basic Principles > 6 – Pathologic Fractures Associated with
Tumors and Unique Conditions of the Musculoskeletal System
musculoskeletal injuries and fractures due to their high activity level
during recreational and sports activities. Whenever the structural
characteristics and the inherited strength of the bone are compromised
by a localized or generalized underlying disorder, the risk of
fractures is increased. The combination of a previous bone abnormality
and a fracture poses special challenges in the decision-making and
management of these injuries. The definition of a pathologic fracture
is one that occurs through abnormal bone. These abnormalities cause the
bone to lack its normal biomechanical and viscoelastic properties.
Pathologic fractures may result from localized or generalized bone
weakness that originates from intrinsic or extrinsic processes.
Examples of localized causes of bone weakness caused by an intrinsic
process are tumors or tumor-like lesions; generalized causes due to an
extrinsic process include osteopenia or osteoporosis of different
etiologies.
features of the most common causes of pediatric pathologic fractures,
including specific patterns of injury and special concerns of
treatment. The goals are to warn and prepare the orthopaedic surgeon
for the correct diagnostic approach and management of these lesions.
should start with a thorough history and physical examination. A
welltaken history may especially help in the development of an accurate
differential diagnosis. Key points that should be investigated include:
|
TABLE 6-1 Common Predisposing Factors for Pathologic Fractures by Peak Age Incidence
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Patient’s age: Some lesions are more
common in specific age groups, including benign and malignant tumors,
as well as other generalized causes of bone weakness (Table 6-1). -
Characterization of the pain, if any:
-
Length: Although most bone tumors present
with increasing pain of several days or weeks of duration, some present
with recent pain. Chronic diseases or processes may also present with a
chronic, intermittent pain. -
Factors that make the pain better or
worse, such as resting, pain medications (e.g., osteoid osteoma is a
classic example of pain that improves rapidly with aspirin or
nonsteroidal anti-inflammatory drugs). -
Inflammatory signs: The presence of a
bony lesion in view of increased temperature, redness, and swelling may
indicate an infection. -
Neurologic signs: Large lesions may present with compressive neurologic changes.
-
-
Radiographic evaluation:
-
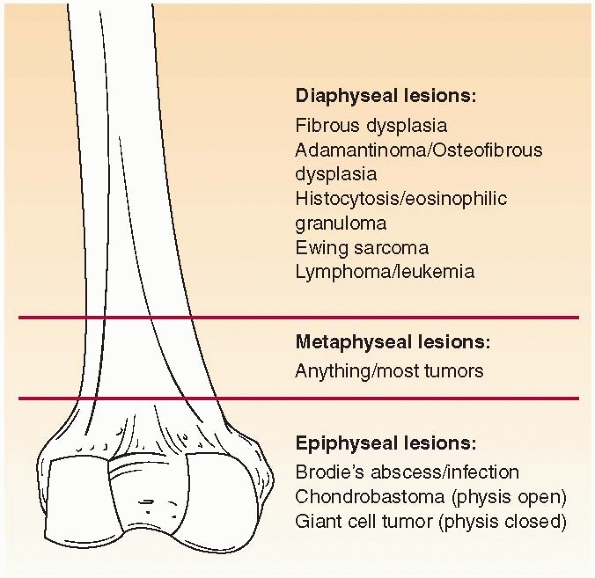
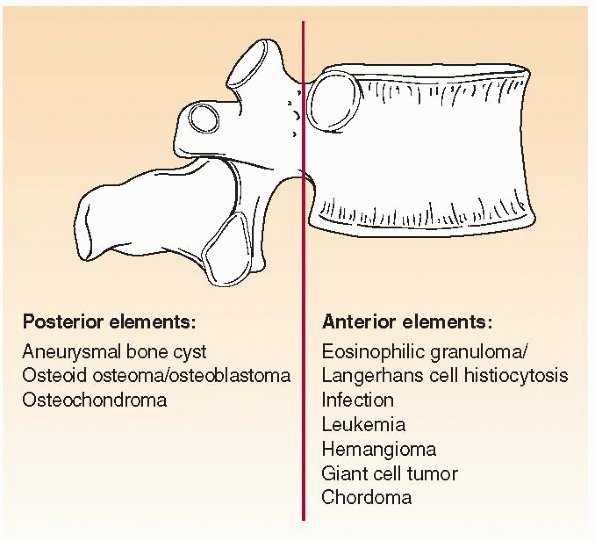
Where is the lesion? Different bone lesions are seen more frequently in specific areas of the body and the bone (Figs. 6-1 and 6-2).
-
What is the lesion’s size and extent?
Aggressive lesions tend to be larger and grow faster. Exceptions
include fibrous dysplasia that may involve not only the entire bone but
also several bones at the same time and nonetheless is a benign
condition. Multiple lesions or generalized bone weakness may pose
another challenge in the prevention and management of pathologic
fractures. -
What is the bone’s response? If the bone
has time to “compensate” for its destruction caused by a lesional
process, new bone formation and cortical thickening may be observed and
will to some point prevent or delay a pathologic fracture. -
Soft tissue mass? The presence of an
associated soft tissue mass may be an indication of a more aggressive,
perhaps malignant process; furthermore, the cortical adjacent to the
associated soft tissue mass will often be severely weakened or
destructed.
-
 |
|
FIGURE 6-1 Schematic distribution of the most common benign and malignant bone lesions seen in the long bones in children.
|
 |
|
FIGURE 6-2 Schematic distribution of the most common benign and malignant bone tumors seen in the spine in children.
|
of a “weakened” to fracture due to a minor trauma. Most studies,
however, are based in the study of metastatic disease in adults.220
More recently, quantitative computerized tomography (CT) has been used
with success to predict the risk of pathologic fractures in children
with cystic lesions of the bone. The combination of bending and
torsional a rigidity measured noninvasively with quantitative CT was
found to be more accurate (97%) for predicting pathologic fracture
through benign bone lesions in children than the standard radiographic
criteria (42% to 61% accuracy).223,479
pathologic fracture is that usually the underlying cause has to be
addressed in order to achieve a successful healing of the fracture. For
that reason, is not uncommon that some of the classic principles of
fracture treatment in children are often altered in order to adapt to
this new situation. In another words, the treatment plan must consider
both the treatment of the fracture and the its underlying cause.
Stage 1, or latent benign lesions, are usually asymptomatic, discovered
incidentally, and seldom associated with pathologic fracture. Stage 2
lesions are intermediate in behavior, and stage 3, or aggressive benign
lesions, are usually symptomatic, grow rapidly, and may be associated
with pathologic fracture.
cyst, is a benign, active or latent, solitary cystic lesion that
usually involves the metaphysis or metadiaphysis of long bones.
Approximately 40% to 80% of these lesions are seen in the proximal
humerus and proximal femur.374,375
In order of decreasing frequency, UBCs are most commonly seen in the
proximal humerus, proximal femur, proximal tibia, distal tibia, distal
femur, calcaneous, distal humerus, radius, fibula, ilium, ulna, and rib.374,375 Although its etilogy is still unknown, theories range
from UBC being a reactive or developmental process caused by
obstruction to the drainage of intersticial fluid, to a true neoplasm.91,96
Isolated cytogenetic analysis have reported on the presence of a
translocation t(16;20)(p11.2;q13) and TP53 mutations in recurrent cases
of UBC.506 It is unclear whether genetic alterations truly play a role in its pathogenesis.
|
TABLE 6-2 Classification of Benign Lesions According to Their Aggressiveness
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||
growth plate: inactive or latent cysts tend to “migrate” away from the
growth plate as longitudinal growth occurs and therefore are far from
the epiphysis; active cysts are in close relationship with the physeal
line and growth arrest may occur prior to treatment.374,375
younger than 20 years old; furthermore, UBCs may regress spontaneously
after skeletal maturity.91,521 The male to female ratio is about 2:1.78,375
UBCs are often asymptomatic and, in more than 70% of cases, the initial
presentation is with a pathologic fracture following minor trauma.82,130,131,521
The fractures are usually incomplete or minimally displaced. The
fractures tend to heal in approximately 6 weeks, and in less than 10%
of the cases the cyst will heal following fracture healing.8,126,129 Lower extremity fractures, particularly around the hip, often need surgical intervention.
diagnosis. UBC is a well defined, centrally located, radiolucent/lytic
cystic lesion, usually surrounded by a sclerotic margin and narrow zone
of transition. Cortical thinning and mild expansion are common. When a
pathologic fracture occurs, there is periosteal reaction and
occasionally the typical “fallen fragment” sign is visualized (fragment
of bone “floating” inside the fluid-filled cystic cavity). CT is useful
for lesions located in areas that are of difficult visualization on
plain films (e.g., spine, pelvis) and to rule out minimally displaced
fractures. Magnetic resonance imaging (MRI) is sometimes used for
differential diagnosis of atypical UBCs. Although the characteristics
are nonspecific, UBCs usually present as low to intermediate signals on
T1-weighted images and a bright and homogeneous signals on T2-weighted
images (Fig. 6-3).330
nonossifying fibroma, fibrous dysplasia, brown tumor of
hyperparathyroidism, bone abscess, and, for calcaneous lesions,
chondroblastoma and giant cell tumor. Diaphyseal tumors may look very
similar to fibrous dysplasia.
away from the growth plate. Although some lesions heal or disappear
spontaneously at puberty,374,375 the majority will persist into adulthood (Table 6-3).
and therefore do not warrant biopsy for diagnostic confirmation,
particularly those lesions in non-weight-bearing bones, can be followed
with serial radiographs.
bone diameter and lesions that are associated with marked cortical
thinning are at high risk of fractures and warrant prophylactic
treatment.215,481 Lesions of weight-bearing bones, especially around the hip, need to be addressed (Fig. 6-4).
Although several attempts have been made to predict the true risk of
pathologic fracture associated with bone cysts, most of the data are
related to other lesions, particularly among adults. More recently, CT
has been shown to be useful predicting the likelihood of fracture. This
method uses a computerized regression system and may help deciding
which cysts warrant intervention.481
pathologic fractures associated with UBCs do not always heal
uneventfully. Malunion, growth arrest, and osteonecrosis (ON) (proximal
femoral fractures) are some of the commonly reported complications.244,321
intramedullary decompression, curettage, and grafting with
medical-grade calcium-sulfate pellets.126,130
used Enders or Rush nails to decompress the cyst. Other methods have
been used including cannulated screws. New grafting materials have also
been used including demineralized bone matrix,445 medical-grade calcium sulfate, and others. Dormans et al.130
described a new minimally invasive technique that combines the
different steps of several techniques and is done percutaneously under
image guidance.
-
Under fluoroscopic guidance, a Jamshidi
trocared needle (CardinalHalth, Dublin, OH) is percutaneously inserted
into the cyst cavity, preferably in the middle of the cyst. -
The cyst is aspirated to confirm the presence of strawcolored fluid.
-
Three to 10 mL of Renografin dye (E.R.
Squibb, Princeton, NJ) are injected to perform a cystogram and confirm
the single fluid-filled cavity. -
A 0.5-cm longitudinal incision is then
made over the site of the aspiration and a 6-mm arthroscopy trocar is
advanced into the cyst cavity through the same cortical hole. The
cortical entry is then enlarged manually. -
Under fluroscopic guidance, percutaneous removal of the cyst lining is done with curved curettes and a pituitary rongeur.
-
An angled curette and/or flexible
intramedullary nail is used to perform the intramedullary decompression
in one direction (toward diaphysis) or in both directions (if the
growth plate is far enough to avoid injury). -
Bone grafting is done with medical-grade
calcium sulfate pellets (Osteoset, Wright Medical Technology,
Arlington, TN) inserted through the same cortical hole and deployed to
completely fill the cavity. The pellets do not offer structural support
but act as a scaffolding for new bone formation and cyst healing.
Angled curettes can be used to
P.124advance pellets into the medullary canal, which also confirms adequate decompression. Tight packing of the cyst is preferred.
-
The wound is closed in a layered fashion.
 |
|
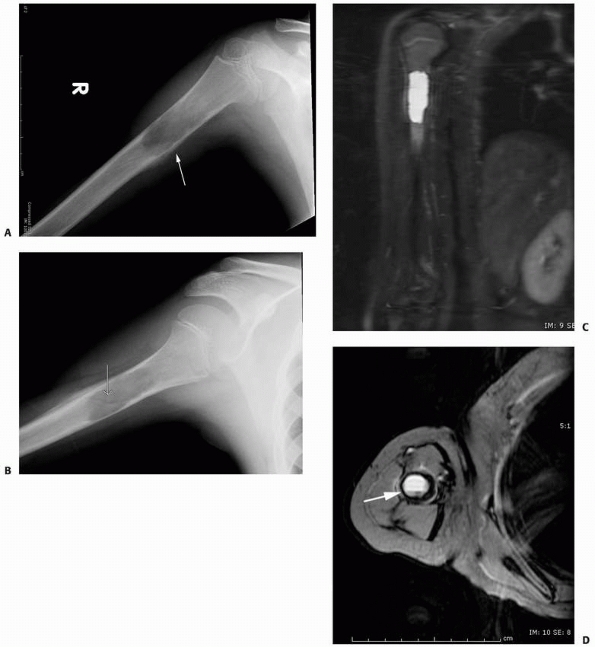
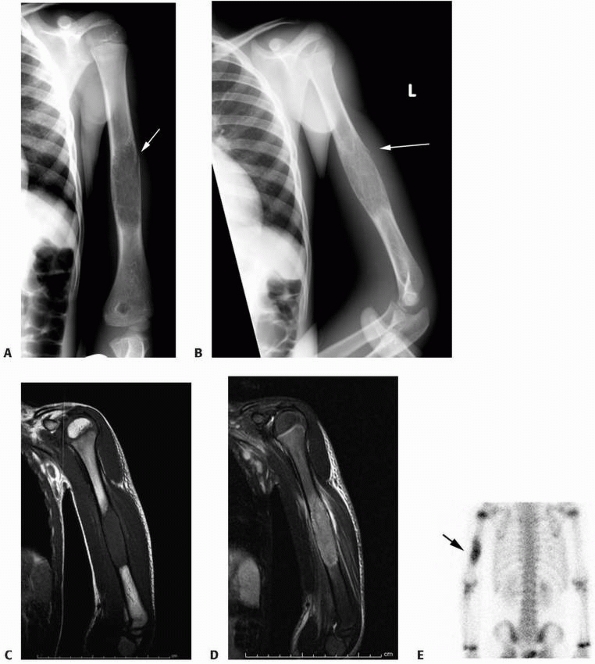
FIGURE 6-3 A 10-year-old boy presented with arm pain after low-energy trauma, 5 days prior. Anteroposterior (A) and lateral (B) radiographs of the right humerus show a nondisplaced pathologic fracture (A–arrow)
through a lytic lesion in the proximal humerus. The lesion is difficult to visualize and the periosteal reaction is also of concern (B–arrow). T2-weighted MRI images show a well-defined, fluid-filled cystic lesion, with fluid-fluid levels (D–arrow) and no soft tissue mass or other worrisome signs in the coronal (C) and axial (D) cuts. The diagnosis was consistent with unicameral bone cyst and conservative treatment was recommended. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
|
TABLE 6-3 Staging of Unicameral Bone Cysts
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
 |
|
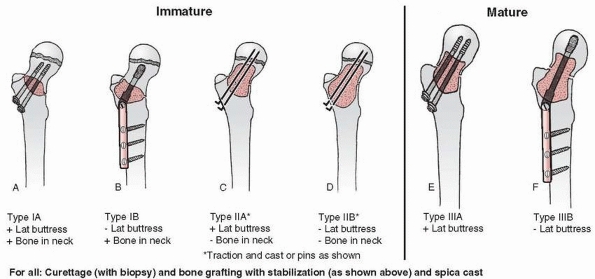
FIGURE 6-4 Classification system for the treatment of pathologic fractures of the proximal femur associated with bone cysts in children. A.
In type IA, a moderately sized cyst is present in the middle of the femoral neck. There is enough bone in the femoral neck and lateral proximal femur (lateral buttress) to allow fixation with cannulated screws, avoiding the physis, after curettage and bone grafting. B. In type IB, a large cyst is present at the base of the femoral neck. There is enough bone proximally in the femoral neck but there is loss of lateral buttress, so a pediatric hip screw and a side plate should be considered rather than cannulated screws after curettage and bone grafting. C,D. In type II A-B, a large lesion is present in the femoral neck, so there is not enough bone beneath the physis to accept screws. There are two options for treatment of these bone cysts: (i) after curettage and bone grafting, parallel smooth pins across the physis can be used in combination with spica cast; (ii) the patient can be treated in traction until the fracture heals (with subsequent spica cast) followed by curettage and bone grafting. E,F. In type IIIA-B, the physis is closing or closed. The lateral buttress is present in type IIIA hips, so cannulated screws can be used to stabilize the fracture after curettage and bone grafting. In type IIIB hips, the loss of lateral buttress makes it necessary to use a pediatric hip screw and a side plate following curettage and bone grafting. In all types, we recommend spica cast immobilization after surgery. |
are benign, locally aggressive bone tumors. They are well-defined,
eccentric or central, expansile, osteolytic, blood-filled lesions
usually seen in the metaphyseal region of long bones or in the
posterior elements of the spine. ABCs have a tendency to expand beyond
the width of the epiphyseal plate. Approximately 75% of ABCs are seen
in patients younger than 20 years old, and 50% are seen in individuals
between 10 and 20 years of age.78,103,297 The estimated incidence ABCs is of approximately 1.4 cases per 100,000, representing around 1.5% of all primary bone tumors.103,297
In order of decreasing frequency, the most commonly involved bones are
the femur (~20%), tibia (~17%), spine (~15%), humerus (~13%), pelvis
(~8%), and fibula (~7%).103,455 The vertebrae are involved in 12% to 27% of patients.78,103,455 The lumbar vertebrae are most commonly affected.63 The primary site of involvement is the posterior elements of the spine with frequent extension into the vertebral body.63,169,394,397
They tend to be destructive lesions, which replace bone and thin the
cortices of the host bone. The elevated viable periosteum usually
maintains a thin osseous shell.
basis of primary ABCs has been in part demonstrated by the chromossomal
translocation t(16;17)(q22;p13) that places the ubiquitin protease USP6
gene under the regulatory influence of the highly active osteoblast
cadherin 11 gene, which is strongly expressed in bones.388
There is a fairly high incidence of ABCs associated with other benign
and malignant tumors such as UBCs, nonossifying fibromas, fibrous
dysplasia, and osteogenic sarcoma.109,303,335
The most common presenting symptom is localized pain and/or swelling of
less than 6 months duration. Spinal lesions may present with radicular
pain.78,115,169,178,275,394
lesion. Although usually the overlying cortex is intact, sometimes a
cortical disruption is identified. When that occurs, a reactive
periosteal reaction is seen.60,275,493
Cystic septation is common, giving rise to the so-called soap bubble or
honeycomb appearance. Lesions in the short tubular bones, such as the
metacarpals and metatarsals, are commonly more central. Spine
involvement is characteristically of the posterior elements; however,
extension
to the anterior body may occur. Pathologic fracture and vertebral
collapse may occur and vertebra plana has been described.60,63,95,178,398
 |
|
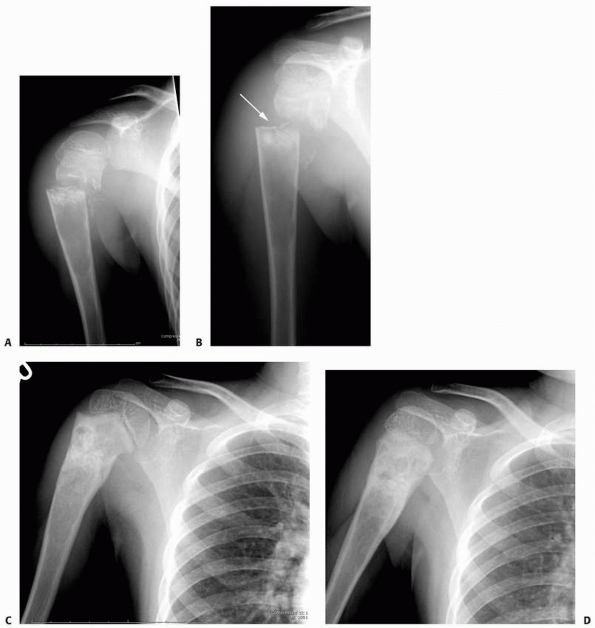
FIGURE 6-5 A 6-year-old boy presented with shoulder pain after a fall. Anteroposterior (A) and lateral (B)
radiographs of the right proximal humerus show a pathologic fracture through a well-defined, lytic lesion in the proximal humeral metaphysis. The fracture presented some comminution that gave the appearance of fallen leaf sign (arrow). This lesion was consistent with unicameral bone cyst and conservative treatment with a fracture brace and sling was initiated. Six weeks after the injury, radiographs (C,D) show consolidation of the fracture and healing of the cyst. The patient was symptom free and returned to full physical activities. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
the lesion is mostly lytic; with growth there is progressive
destruction of bone with poorly demarcated margins. This is followed by
a stabilization phase, with formation of a bone shell with septa.
Later, with further ossification, a bony mass begins to form.109 Characteristically, lesions near the growth plate tend to expand beyond the width of the adjacent epiphysis (Fig. 6-6).
MRI is often helpful in obtaining better definition of axial lesions
and in demonstrating the characteristic double density fluid level,
septation, low signal on T1 images, and high intensity on T2 images;
however, these findings are not pathognomonic for ABC.493
have classified the ABCs into three groups. An aggressive cyst has
signs of reparative osteogenesis with ill-defined margins and no
periosteal shell. An active cyst has an incomplete periosteal shell and
a defined margin between the lesion and the host bone. An inactive cyst
has a complete periosteal shell and a sclerotic margin between the cyst
and the long bone (Fig. 6-7).
Epiphyseal involvement due to extension of metaphyseal/juxtaepiphyseal
lesions may occur, and although it is rare, it may cause growth
disturbance.83 Conservative
treatment with immobilization is inappropriate as a definitive
treatment for pathologic fractures of ABCs. Although the pathologic
fracture will heal, the ABC will persist, if not enlarge, and a
recurrent pathologic fracture will most likely occur.
 |
|
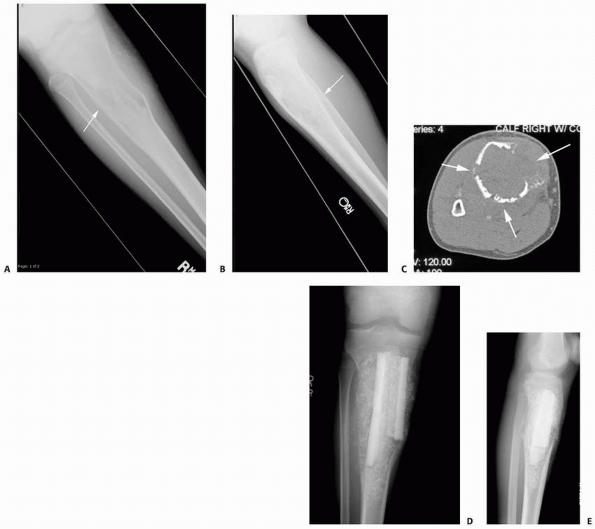
FIGURE 6-6
A 14-year-old boy presented with acute right leg pain following a fall from his bicycle. He reported previous intermittent pain over that same area. On initial plain radiographs (A,B) there is a large, expansile, and destructive lesion in the proximal tibial metaphysis. The bone is mildly expanded and there is a minimally displaced pathologic fracture (arrow). Axial CT image (C) better defines the extensive cortical disruption and soft tissue involvement postfracture anterior-medially. The patient underwent biopsy confirming the diagnosis of aneurysmal bone cyst. After the final pathology report was available, he underwent intralesional excision of the mass with curettage, high-speed burr, electro-cauterization, cryosurgery, and bone grafting with a combination of a strut allograft and crushed cancellous allograft (D,E). (Figures reproduced with permission from the Childrens Orthopaedic Center, Los Angeles, CA.) |
treated ABCs with curettage and bone grafting in seven patients younger
than 10 years of age and noted recurrence in five of the seven patients
at an average of 8 months after the first procedure.
 |
|
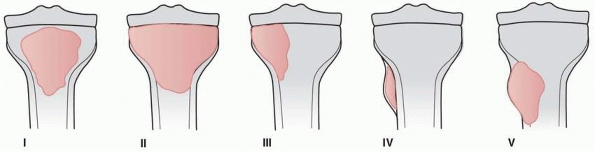
FIGURE 6-7
Classification of morphologic types of aneurysmal bone cyst. (From Campanna R, Bettelli G, Biagini R, et al. Aneurysmal cysts of long bones. Ital J Orthop Traumatol 1985;XI:421-429, with permission.) |
curettage has a recurrence rate of between 8% and 14%.329,455 Polymethylmethacrylate cementation has also been described as an adjuvant to curettage.
based on the notion that there is high recurrence rate associated with
simple intralesional curettage. In the reported series, the recurrence
rate for appendicular lesions was as low as 8%.127
It also takes into account the advent of modern imaging techniques that
help with preoperative planning as well as intraoperative guidance.127,178
We recommend the use of headlamps for enhanced illumination and loupes
for magnification. An image intensifier is available for intraoperative
confirmation of complete tumor excision and appropriate bone grafting.
Diagnostic tissue confirmation is essential part of this technique. For
large spinal tumors, preoperative embolization is recommended (Fig. 6-8).
If instrumentation is needed after spine tumors resection, we recommend
titanium instrumentation that gives a much better visualization of the
spine (less artifact) than stainless steel (Fig. 6-9).
-
Under fluoroscopic guidance, a small
longitudinal incision is made over the cyst. No flaps are created, and
the dissection is carried down to the lesion level. The cyst wall is
usually easily penetrated with curettes. Care should be taken to
control eventual significant bleeding at the time of cyst penetration. -
Lesional tissue is than retrieved and sent for frozen section for diagnostic confirmation.
-
Upon diagnostic confirmation, the
cortical window is enlarged using roungers or a high-speed burr to
allow appropriate visualization and excision. Using angled and straight
curettes of different sizes, the intralesional resection/curettage is
performed (Step 1). -
After the first step, the high speed burr
is used to extend the intralesional margins as well to excise any
residual tumoral cells (Step 2). -
Step 3 entails the use of electrocautery.
This has two goals: first, it helps identify residual tumor pockets and
second, has the theoretical capability of killing residual tumor cells. -
Adjuvant in the form of phenol solution 5% is used for appendicular lesions (Step 4).
-
The lesion is now completely excised and
bone grafting is performed, usually using a combination of allograft
cancellous cubes and demineralized bone matrix paste. Tight packing of
the cyst is preferred. -
Internal fixation is done on case-by-case
basis. Lesions of weight-bearing bones, particularly of the proximal
femur, and some large vertebral lesions may warrant internal
fixation/instrumentation following the 4-step approach. -
The wound is closed in a layered fashion.
 |
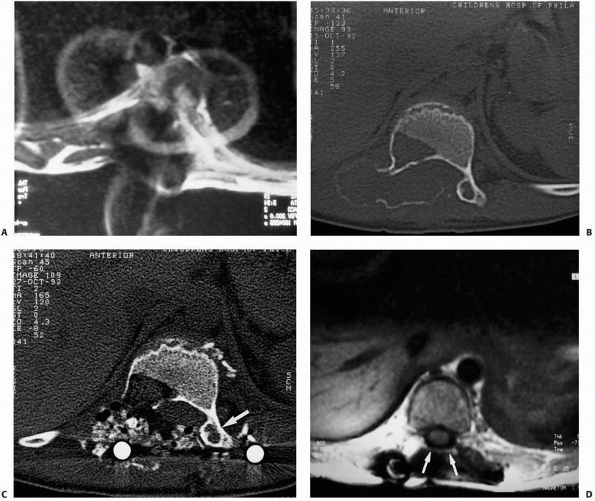
|
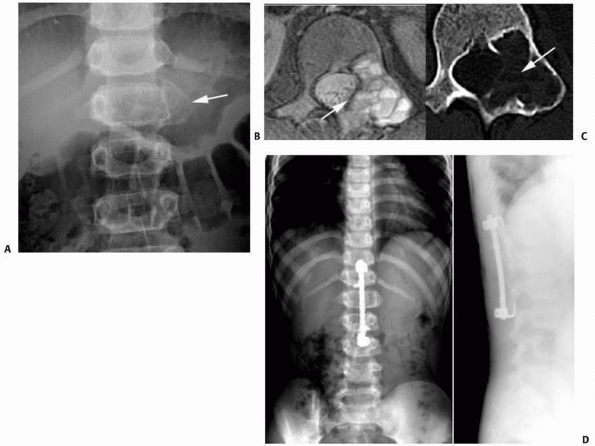
FIGURE 6-8 A 9-year-old boy presented with low back pain and abdominal discomfort. On plain radiographs of the abdomen (A), an expansile lesion involving the left posterior elements of L1 was visualized. Axial T2-weighted MRI (B) and an axial CT scan image (C) show the microfractures at the pedicle and lamina level (arrow)
and the fluid-fluid levels. The patient underwent open biopsy that confirmed the diagnosis of aneurysmal bone cyst, followed by a 4-step approach excision and bone grafting. Limited instrumentation of the spine was performed due to stability compromise (D). (Permission) |
 |
|
FIGURE 6-9
When dealing with pathologic fractures secondary to tumors or tumor-like processes of the spine, if instrumentation is needed, titanium instrumentation allows much better postoperative visualization with both CT and MRI for the detection of tumor recurrence as compared with standard stainless-steel instrumentation. A. Postoperative MRI of the spine with standard stainless-steel instrumentation showing a large degree of artifact that makes interpretation difficult. B. Preoperative CT scan of a patient with an ABC of the spine. C. Postoperative CT scan of the same patient showing an adequate view of the surgical area. D. Postoperative MRI of a patient with a previous spinal tumor again adequately showing the surgical site to monitor for recurrence or persistent tumor. |
tumor or tumor-like condition seen in the growing child. Both FCDs and
the larger variant known as nonossifying fibroma (NOF) may be
associated with pathologic fractures in children. These lesions are
characterized by the presence of fibrous tissue, foam cells, and
multinucleated giant cells.107 Pathologic fractures through these lesions occur more commonly in boys between 6 and 14 years old.80
lesions surrounded by a sclerotic rim with localized cortical thinning,
ranging from 1 to 2 cm in diameter and most commonly found in the
distal femur, proximal tibia, and fibula. FCDs can be seen on
radiographic studies of the lower extremity in approximately 25% of
pediatric patients.80,107,432
In view of their usually asymptomatic nature, it is difficult to
estimate the true incidence. They usually require no treatment other
than observation.
similar distribution of bone involvement; however, multiple lesions are
present in approximately one third of patients.80,133
Radiographically, they present as a well-defined, eccentric radiolucent
cystlike lesion of the metaphysis that may be mostly intracortical or
intramedullary and are usually larger than 4 cm,21 sometimes extending across a substantial portion of the width of the long bone.107 NOFs are usually asymptomatic unless a pathologic fracture is present.21,107
Typically, this tumor remains asymptomatic and is commonly an
incidental radiographic finding. However, lesions with extensive
cortical involvement can cause pathologic fractures.
noted that all pathologic fractures associated with NOFs in the lower
extremity occurred through lesions involving more than 50% of the
transverse cortical diameter. These large lesions were defined as
exhibiting more than 50% cortical involvement on anteroposterior (AP)
and lateral radiographic studies and a height measurement of more than
33 mm.21 In their series, 43% of the
pathologic fractures through NOFs were in the distal tibia. Although
the authors recommended careful observation of these large NOFs, they
suggested that “prophylactic curettage and bone grafting be considered
if there is a reasonable chance of fracture.”21
Their series does not include any large lesions meeting their size
criteria that did not fracture, and their hypothesis has never been
tested in any published series. Drennan et al.133
suggested that large NOFs causing pain might predispose to fracture and
recommended prophylactic curettage and bone grafting for selected
larger lesions.
 |
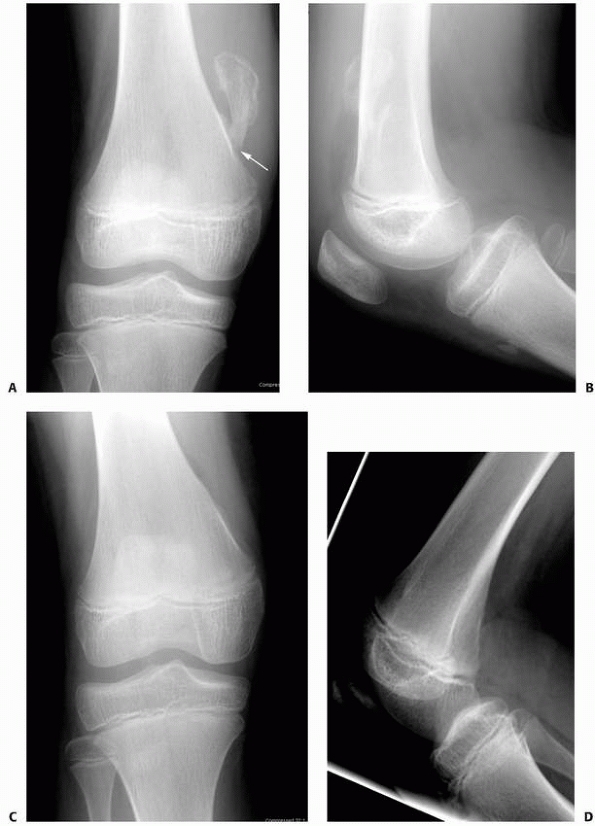
|
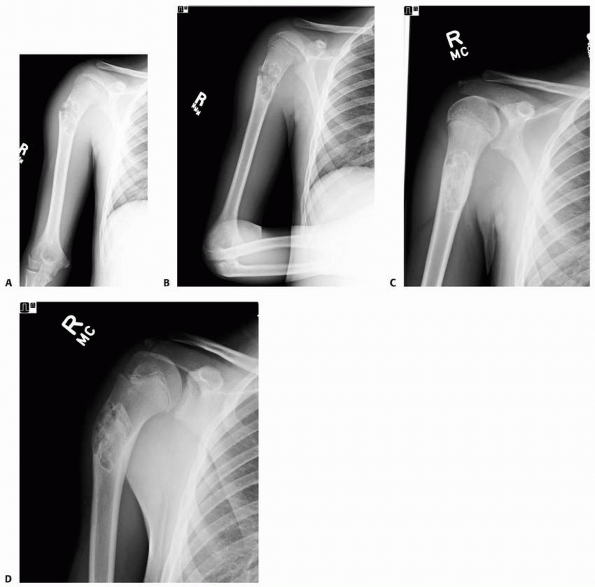
FIGURE 6-10
A 13-year-old girl sustained a fall from her own height and developed pain and deformity around the right shoulder. Anterior-posterior (A) and lateral (B) plain films show a pathologic fracture through a well-defined, eccentric, cortical based lesion in the proximal humerus metaphysis. There is sharp sclerotic rim and the lesion was clinically diagnosed as nonossifying fibroma. After 4 weeks of conservative treatment, the fractured healed (C,D) in a few degrees of varus and the lesion persisted. |
50% of the width of a long bone on both AP and lateral radiographs, are
believed to be prone to pathologic fracture, and most authors have
recommended curettage and bone grafting for these large lesions.21,107,133,139
reported that although absolute size parameters were helpful in
predicting pathologic fracture, they did not imply a requirement for
prophylactic curettage and bone grafting. In their series, 13 (59%)
large NOFs had not had pathologic fracture despite exceeding the
previously established size threshold. In the nine (41%) patients in
whom pathologic fracture occurred, healing was uneventful after closed
reduction and cast immobilization, and no refractures occurred. They
suggested that most patients with large NOFs can be monitored without
intervention, because previous studies support spontaneous resolution
of most of these lesions.21,107,133
All fractured NOFs in their series healed with closed reduction and
immobilization. It may be reasonable to restrict the activity of
patients with large NOFs based on the nine patients in their study with
pathologic fractures caused by trauma.
Surgery is necessary only if the residual lesion of significant size to
predispose the patient to further pathologic fractures or there is
doubt about the nature of the lesion.21,139
Displaced pathologic supracondylar fractures of the distal femur may
require open reduction, bone grafting, and intramedullary fixation.133
lesion and the type of pathologic fracture. Small lesions without
fracture can be observed and may require 1 to 3 years to spontaneously
resolve.80 Substantial lesions of
the lower extremity in active children, even if they are assymptomatic,
should either be followed carefully with serial radiographic studies or
should undergo curettage and bone grafting to avoid pathologic
fracture. Although absolute size parameters may be useful in predicting
pathologic fracture, they do not imply a requirement for prophylactic
curettage and bone grafting. Most patients with large NOFs can be
monitored without surgical intervention, and fractures can be
successfully managed with nonoperative treatment. Our experience is
that a considerable number of incidentally discovered large NOFs do not
fracture. Although we cannot readily identify an accurate denominator,
we infer that many large NOFs remain unindentified and non-problematic.
Patient and family wishes and the individual’s activity demands also
influence the decision. Given the historic evidence for spontaneous
resolution and favorable healing characteristics of NOFs, patients with
lesions larger than 50% of the width of the bone should be approached
individually, especially in the presence of clinical symptoms (Fig. 6-11).
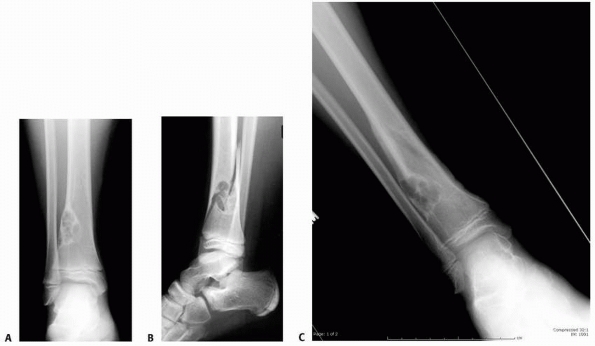 |
|
FIGURE 6-11 An 11-year-old boy fell while playing baseball and developed acute pain over the right distal leg/ankle area. Anteroposterior (A) and lateral (B)
radiographs of the right ankle show a spiral fracture through a well-defined, eccentric lesion in the lateral distal aspect of the tibia metaphysis. There is narrow zone of transition and a sclerotic border. The lesion was thought to be consistent with a nonossifying fibroma, and the fracture was allowed to heal for 5 weeks (C,D). (continues) |
that usually involve the epiphysis of long bones following closure of
the growth plate. Therefore, they are rarely seen in the pediatric
population. In a series of 221 patients with giant cell tumors of bone,344
only 20% of patients were younger than 20 years of age. In decreasing
order of frequency, these tumors most commonly occur in the distal
femur, proximal tibia, proximal humerus, and distal radius. The
incidence of pathologic fractures with giant cell tumors is up to 30%.77,116,373,494,503
epiphyseal lesions that extend into the metaphysis, usually after
physeal closure. Although they start as an eccentric lesion, with
growth, larger lesions can involve the full width of the bone. Little
or no sclerosis is present around the margins and heavy trabeculation
may be present. Periosteal reaction with new bone formation is seen
with pathologic fractures.
tumors in 6% to 30% of patients. The complexity of treatment is
markedly increased if a pathologic fracture is present. Management
depends on the type of fracture and fracture displacement (Table 6-4).
 |
|
FIGURE 6-11 (continued)
The patient then underwent biopsy confirming the diagnosis, followed by curettage and bone grafting. Four months postoperatively (E,F) the lesion is completely healed and the patient resumed normal physical activities. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
treatment, especially if the diagnosis is not certain. Most pathologic
fractures are minimally displaced or nonarticular and require no change
in the treatment plan. For more significant fractures, an attempt at
preserving the joint should be made (Fig. 6-12).
It is still debatable whether the presence of a pathologic fracture
itself directly influences the recurrence rate of giant cell tumors116,503; however, it does influence the reconstruction options and perhaps the overall functional result.
enchondroma is a rare lesion in children. The common presenting symptom
is pain, usually associated with a pathologic fracture. The most common
sites of involvement in decreasing order of frequency are the
phalanges, metacarpals, metatarsals, humerus, and femur. Pathologic
fracture is commonly the presenting symptom for enchondromas located in
the phalanges of the hands or feet, but is rare for enchondromas in
other locations.42,179
lesions with stippled calcification of the cartilage tumor matrix.
Larger lesions may cause cortical thinning and scalloping and
predisposal to pathologic fractures (Fig. 6-13).
|
TABLE 6-4 Treatment of Giant Cell Tumors
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||
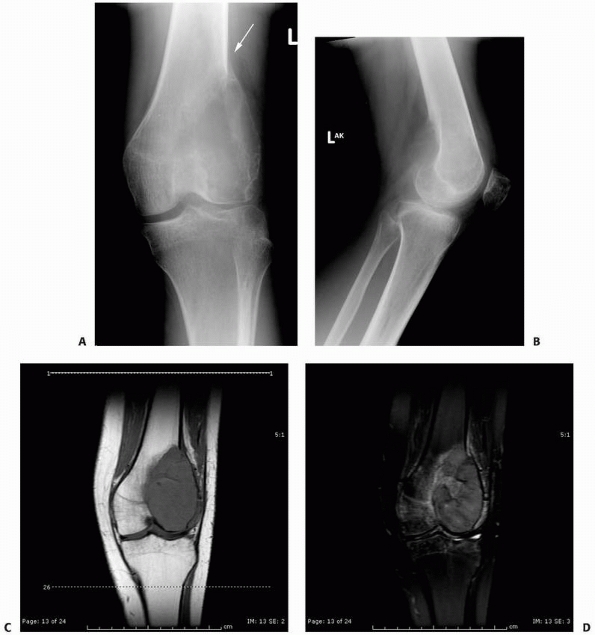 |
|
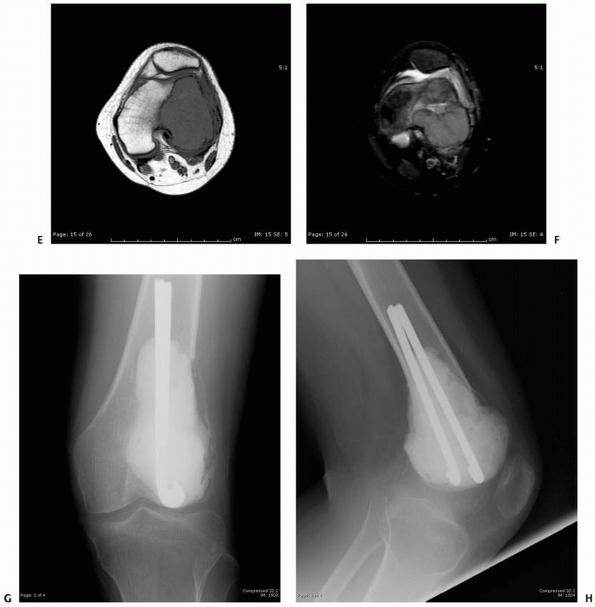
FIGURE 6-12
A 15-year-old girl presented with several months history of progressive left knee pain and recent inability to bear weight. On initial radiographs (A,B) there is a nondisplaced pathologic fracture (arrow) through a well-defined, lytic, eccentric, epiphyseal lesion of the distal femur. There is some bone expansion and metaphyseal extension. MR T1-weighted (C,E) and T2-weighted (D,F), coronal (C,D), |
 |
|
FIGURE 6-12 (continued) and axial (E,F)
images better define the extent of bone and surrounding soft tissue involvement and the presence of cortical disruption. The patient underwent tissue confirmation of giant cell tumor and extended intralesional resection with cryosurgery, cementation, and Rush rod instrumentation (G,H). (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
enchondromatosis (Ollier disease), which is commonly seen between 2 and
10 years of age. Although the lesion itself is similar to solitary
enchondroma, deformity and shortening of the extremity due to growth
disturbance may occur (Fig. 6-14). A unique or
pathognomonic radiographic finding of enchondromatosis is the presence
of linear radiolucencies extending from the metaphysis down the shaft
of the long bone.
necessary when the identity of the lesion is uncertain. Symptomatic
lesions respond well to curettage and bone grafting.42,179,515 Treatment should be individualized for displaced fractures.
the diagnosis. For asymptomatic patients with small lesions with
classic radiographic findings, biopsy is not necessary. Curettage
and
bone grafting are necessary for those lesions with acute or impending
pathologic fracture, or in cases of continued pain. Fixation is not
necessary for those lesions of the short tubular bones but may be
necessary for lesions of the proximal femur or long bone of the lower
extremity. Standard fracture care is adequate to treat most injuries.
 |
|
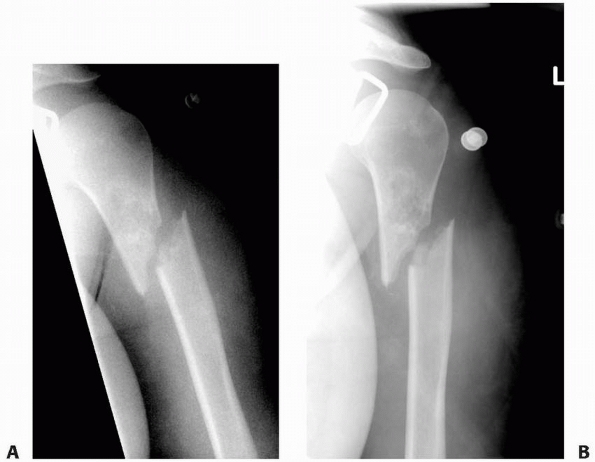
FIGURE 6-13
A 17-year-old girl with developmental delays sustained a fall and developed pain and deformity around the right proximal humerus. Radiographs of the proximal humerus (A,B) demonstrated a pathologic fracture through a right proximal humerus metaphyseal lesion. There is some matrix formation with speckled calcification, some cortical thinning/scalloping, but no soft tissue mass, gross cortical disruption, or other worrisome signs. The lesion was clinically consistent with enchondroma. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
 |
|
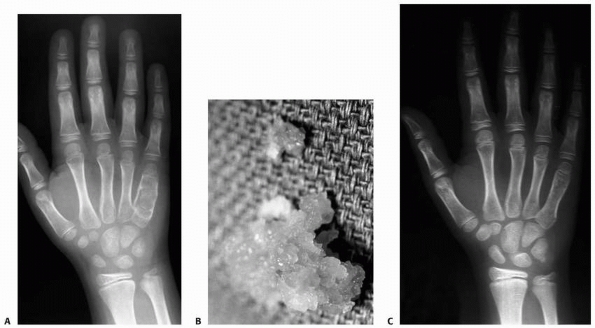
FIGURE 6-14 An 8-year-old boy presented with pain and swelling of the ulnar border of his right hand. A.
Radiographic studies showed an expansile, lucent lesion of the diaphysis of the patient’s right fifth metacarpal with microfractures. The patient had an open incisional biopsy with frozen section, which was consistent with enchondroma with subsequent curettage and bone grafting. B. Gross appearance of material removed at the time of surgery, which is consistent with enchondroma. C. At 6-month follow-up, the fracture is well healed, and there is no sign of recurrent tumor. |
bone in children, and clinical symptoms are usually related to
irritation of the surrounding soft tissue structures. Peroneal nerve
palsy may occur in association with osteochondroma of the proximal
fibula.85 Radiation induced osteochondromas also can occur.318
Fractures through osteochondromas should be observed, and excision
should be reserved for those patients with persistent symptoms after
healing or worsening of symptoms prior to healing (functional
compromise).
disorders with a wide spectrum of clinical presentation, where the
constant
pathologic
finding is the “Langerhans cell.” The present nomenclature defines
solitary osseous lesion as eosinophilic granuloma (EG). EG is a benign
condition with either solitary or multiple lytic bone lesions. The
annual incidence of LCH is at 6 per million children per year.50 Males are affected to a slightly higher degree than females.69,228
It is predominantly a disease of childhood, with more than 50% of cases
diagnosed between the ages of 1 and 15. There is a peak in incidence
between the ages of 1 and 4.50,69
The clinical course of the disease is quite variable, with some forms
undergoing seemingly spontaneous remission. The disease can be
localized to a bone or single system, or multifocal involving multiple
bones and/or systems. Bone pain is the initial symptom in 50% to 90% of
the patients with osseous lesion.101,124
Other reported symptoms in osseous LCH include night pain, soft tissue
swelling, tenderness, pathologic fractures, headaches (skull lesions),
diminished hearing, and otitis media (mastoid lesions) or loose teeth
(mandible lesions). Dull, aching neck or back pain is usually the
presenting symptom of children with spinal LCH.177
Although neurologic symptoms are rarely seen at presentation, several
levels of spinal involvement may occur. Later in the course of the
disease, vertebral collapse may produce pain and spasm, torticollis may
be seen with cervical spine lesions, and kyphosis might be present with
thoracic lesions.165,303,419
Multisystemic LCH has a wide range of manifestations. It includes the
two classic syndromes (Letterer-Siwe disease and
Hand-Schuller-Christian) and also several other symptoms, such as
diabetes insipidus, anterior
pituitary
deficiency (manifested by amenorrhea, hypothyroidism, growth
retardation), skin manifestations (eczema, macular rash, ulcers),
pulmonary compromise (dyspnea, cough, pleural effusion),
lymphadenopathy, hepatosplenomegaly, thrombocytopenia, and anemia.145,186,228,260,261,355
 |
|
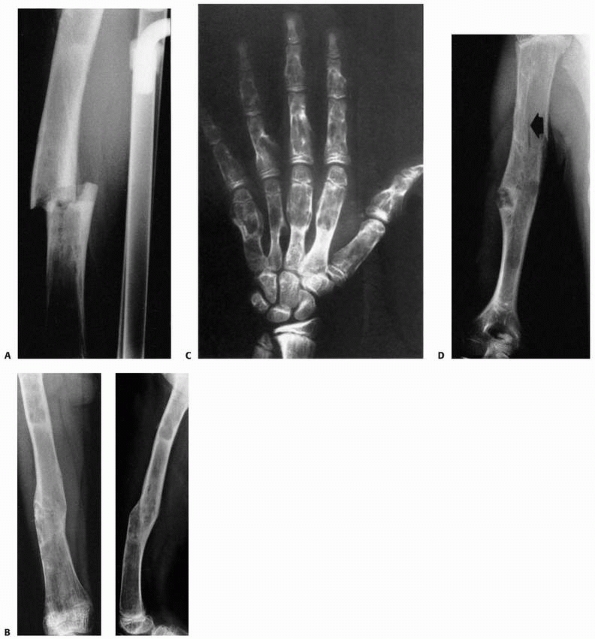
FIGURE 6-15 Multiple enchondromatosis. A.
A 10-year-old girl with multiple enchondromas sustained a spontaneous pathologic fracture of the femur while running. The lateral radiograph shows overriding of the fracture. B. At 3-year follow-up, the fracture is well healed. C. The anteroposterior radiograph of the hand in this patient demonstrated multiple expansile enchondromas of the small bones. D. A radiograph of the humerus shows the streaked-mud appearance of the lateral humerus (arrow). |
 |
|
FIGURE 6-16 A 13-year-old girl presented with right knee pain following direct trauma to that area 10 days prior. On anteroposterior (A) and lateral (B) radiographs, there was a pathologic fracture through the base of a pedunculated osteochondroma (arrow). The patient was very tender around that area and elected surgical excision. Immediately after excision (C,D),
there was improvement of the symptoms. Four weeks later, she returned to full activities. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
on how long the lesion has been present. For this reason, LCH has been
traditionally referred to as the “great imitator.” On plain
radiographs, lesions typically present as radiolucent with well-defined
margins, with or without surrounding sclerosis. Skeletal lesions may be
solitary or multiple. In children, long bone lesions may occur in both
the diaphysis and metaphysis, with destructive osteolysis that erodes
the cortex and overlying expansion by periosteal layering.69,355
Epiphyseal involvement is rare but may occur. The size of the lytic
area may vary from 1 to 4 cm. Vertebral destruction with complete
collapse of the vertebral body is classically referred to as “vertebra
plana.” Adjacent intervertebral disc height is usually maintained (Fig. 6-17).
Spinal lesions can be classified based on the amount and pattern of maximal vertebral collapse177:
grade I (0% to 50% of collapse), grade II (51% to 100%), or grade III
(limited to the posterior elements); and A (symmetric collapse) or B
(asymmetric collapse).
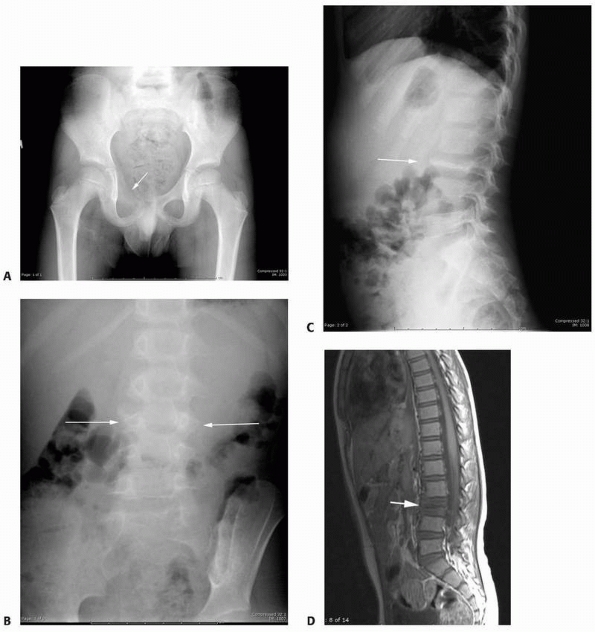 |
|
FIGURE 6-17
A 5-year-old boy presented with a history of several months of intermittent back pain and recent development of right inguinal pain. On pelvic radiographs (A) a lytic lesion of the right superior pubic rami is visualized (arrow). There is no soft-tissue mass, periosteal reaction, or other worrisome signs. The lumbar spine radiographs (B and C) show a classic “vertebra plana” of L3 (arrow). (D) Sagittal T1-weighted MRI shows no soft-tissue mass or other associated lesions, no compromise of the spinal canal and no extension to the posterior elements. The pelvic lesion was biopsied and a diagnosis of polyostotis Langerhans cell histiocytosis was made. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
also to differentiate LCH from malignancies that may present with
similar radiographic appearance.355
Once the diagnosis is established, treatment options include curettage,
curettage and bone grafting, irradiation, chemotherapy, and steroid
injection. All of these forms of treatment have been reported to
promote lesion healing.69,124,182,228
For small lesions with established diagnosis, observation may be the
best option; marginal sclerosis about the lytic area suggests healing.355
Chemotherapy is sometimes indicated, especially in cases of multiple
bone involvement or visceral disease. Various agents have been used
including carboplatin, cyclophosphamide, etoposide, methotrexate,
vinblastine, vincristine, and steroids.22,145
The correct diagnosis should be established with biopsy for most
lesions, and the use of newer diagnostic methods such as
immunohistochemistries (such as CD1A) can be helpful in confirming the
diagnosis of these lesions. The natural history is one of gradual
regression and healing. Standard fracture care is usually sufficient
for pathologic fractures.
in children are osteogenic sarcoma and Ewing sarcoma. Destructive bone
lesions can also be caused by metastatic cancer to bone, being more
common than primary tumors in certain age groups. Careful staging150,337 and subsequent biopsy28,326,405,475
are critical in the evaluation of children with malignant bone tumors.
However, biopsy is not done without risks. One of the main
complications following biopsies is pathologic fracture. To avoid a
pathologic fracture, an oval hole with smooth edges should be used,
preferably in areas of less stress for weight-bearing bones. Sometimes,
the biopsy hole can be filled with bone cement.337
Furthermore, most malignant tumors have a large soft tissue mass that
can be sampled, avoiding the need to make a hole in the bone.
Pathologic fractures can sometimes be the presenting symptom of a
malignant tumor (Fig. 6-18).
of these tumors. Immunohistochemical, molecular genetic, and
cytogenetic tests are often critical in establishing the correct
diagnosis, especially for small round blue cell tumors. The evaluation
and biopsy of these children should preferably be done at a tertiary
center where these techniques are known and available.28,300,326,405
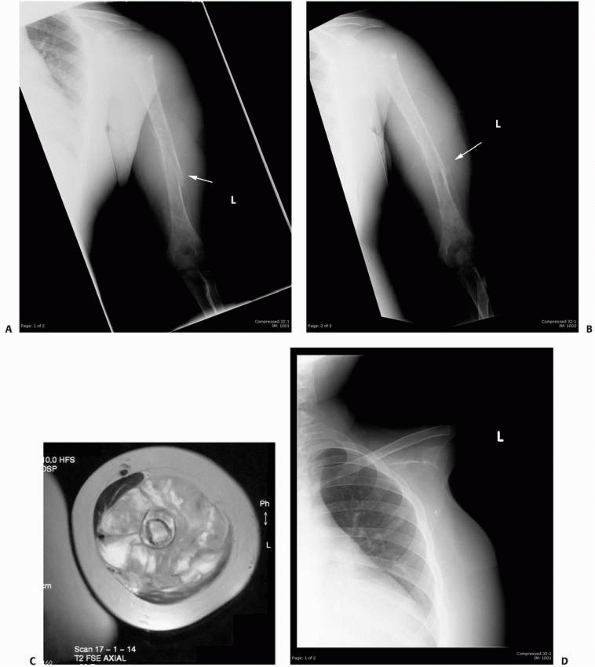 |
|
FIGURE 6-18
A 13-year-old boy presented with several months history of right arm pain and recent increase in pain following minor trauma. Anteroposterior (A) and lateral (B) radiographs show a minimally displaced midshaft humeral pathologic fracture through a poorly defined, permeative, aggressive-looking diaphyseal lesion. (C) T2-weighted axial MRI shows a huge soft tissue mass associated with the bone lesion and involvement of the neurovascular bundle. The patient was diagnosed with Ewing sarcoma, received neoadjuvant chemotherapy, and had a shoulder disarticulation (D), followed by postoperative chemotherapy. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
extremity sarcoma has been the development of limb sparing surgical
techniques for local control of the tumors. Pathologic fracture has
been cited as a contraindication to this procedure because of concerns
about tumor dissemination by fracture hematoma. Several studies have
shown that pathologic fractures eventually heal with neoadjuvant
chemotherapy and may not affect survival rates (Fig. 6-19).146,409,526 Abudu et al.2 reviewed
the surgical treatment and outcome of pathologic fractures in 40
patients with localized osteosarcoma and found that limb-sparing
surgery with adequate margins of excision could be achieved in many
patients without compromising survival, but that 19% of those treated
with limb-sparing surgery had local recurrences. Scully et al.458
reviewed the surgical treatment of 18 patients with osteosarcoma
pathologic fractures. Of the 10 patients who had limb sparing surgery,
three had local recurrences and six had distant recurrences. Although
the distant recurrence rate for patients undergoing amputation was no
different from the rate for those undergoing limb salvage, the
difference in local tumor control approached statistical significance.
All patients who developed local recurrence died. The authors stated
that surgical treatment should be individualized.458 Bacci et al.29
compared the disease free-survival and overall survival of 46 patients
with nonmetastatic osteogenic sarcoma of the extremity and pathologic
fracture to a cohort of 689 patients without pathologic fracture and
found no significant difference. Limb-sparing surgery is possible and
appropriate in carefully selected patients as long as wide margins can
be safely achieved and the function of the child will be better than
that achieved with an amputation and a well-fitting prosthesis.
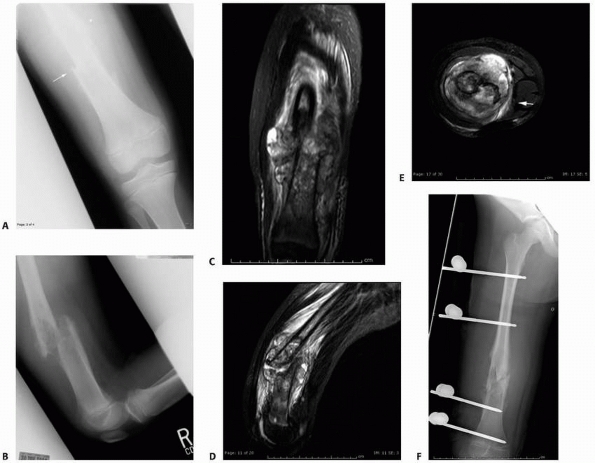 |
|
FIGURE 6-19
An 8-year-old girl sustained a pathologic fracture of the femur after falling off her bicycle. She denied symptoms previous to this injury. The radiographs (A,B) showed a grossly displaced fracture through a poorly defined, mixed lesion in the midshaft of the femur (arrow); there is disorganized periosteal reaction with sunburst sign. T2-weighted coronal (C), sagittal (D), and axial (E) MRI showed extensive soft tissue mass; the neurovascular bundle does not seem do be involved by the tumor mass. The patient underwent biopsy that confirmed osteogenic sarcoma and fracture stabilization with an external fixator at a referring institute. Note that the external fixator pins were inappropriately placed too far from the tumor and fracture site (F), increasing the contaminated area. The surgical treatment options were limited to rotationplasty and amputation. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
another major complication, occurring most commonly after allograft
reconstruction but also associated with endoprosthetic reconstruction.48,451,513 Berrey et al.48
reviewed 43 patients in whom allograft used in reconstruction after
resection of tumors had subsequently fractured. Four fractures healed
with immobilization alone, and the remainder of patients attained
satisfactory results with open reduction and grafting, replacement of
the internal fixation device, or total joint replacement.48 San-Julian and Canadell451
reported on 12 patients with 14 fractures (10.2% of 137 patients with
allograft for limb sparing surgery in their series). They recommended
intramedullary fixation whenever possible to reduce the incidence of
allograft fracture.
disease but are less common in children than in adults. Most are
microfractures and can be managed with conservative fracture management
techniques.
are the appropriate initial approaches by individuals who have
experience in the management of children with musculoskeletal sarcomas.
Furthermore, access to special diagnostic modalities, such as
immunohistochemistry and cytogenetics, will decrease the chances of
misdiagnosis. The decision for or against limb-sparing surgery in
patients with pathologic fracture associated with a bone sarcoma should
be individualized based on factors such as the fracture displacement,
fracture stability, histologic and radiographic response to
chemotherapy, and, most important, the ability to achieve wide margins
for local tumor control. Pathologic fractures that occur after
reconstruction through allograft or endoprosthetic reconstruction often
can be successfully treated with bone grafting or exchange of allograft
or endoprosthesis.
bone characterized by replacement of normal bone and marrow by
fibrous-osseous tissue resulting in decrease of bone strength,
pathologic fracture, and deformity. The disease process may be limited
to a single bone (monostotic FD) or disseminated (poliostotic FD). When
the bone disease is associated with café-au-lait skin hyperpigmentation
and endocrine dysfunction, it is known as McCune-Albright syndrome.
Hyperthyroidism, growth hormone excess, hyperparathyroidism,
hyperprolactinemia, and/or hypercortisolism may be present in any
combination.386,430
The common factor is expansile fibrous tissue lesions of the bone,
which contain woven bone formed by metaplasia with poorly oriented bone
trabeculae.
Often, the lesions are asymptomatic and a pathologic fracture may be
the presenting symptom. Fractures of the long bones are generally not
displaced or incomplete, many being microfractures and presenting with
pain and swelling, usually between ages 6 and 10 with a decline
thereafter.296 The sites of fracture
in decreasing order of frequency are the proximal femur, tibia, ribs,
and bones of the face. The age of first fracture, number of fractures,
and fracture rate are related to the severity of the metabolic
derangement. The endocrinopathies are often associated with
phosphaturia that has a deleterious effect on the normal skeleton and
seems to be related to the incidence of fractures.296 Although the fractures heal rapidly, endosteal callus is poorly formed and periosteal callus is normal.206
With mild deformity, the cortex thickens on the concave side of the
long bone. Nonunion is rare in monostotic FD. Most patients with
polyostotic FD are diagnosed before age 10 years with pain, limp,
deformity, or pathologic fracture.219,306
The bones most commonly affected are the femur, tibia, humerus, radius,
facial bones, pelvis, ribs, and phalanges. Involvement is often
unilateral, usually affecting a single extremity. Spine involvement
occurs with polyostotic FD, and limb-length discrepancy is common.206,219 Some authors believe that polyostotic FD usually does not progress significantly after adulthood,219,514
but others believe that puberty does not affect the bone lesions. In
one series of 37 patients with polyostotic FD, nearly 85% had at least
one fracture and 40% had an average of three fractures.206 Fractures are most common in the femur, humerus, radius, and wrist.305,306
Similar to monostotic FD, the fractures in the polyostotic form are
generally nondisplaced and healing is not delayed; however, nonunion
can occur.206
of approximately 0.5% and that generally occurs approximately 15 years
after the initial diagnosis. Osteogenic sarcoma, fibrosarcoma, and
malignant fibrous histiocytoma are the most common.225 The warning signs for sarcoma in existing lesions of fibrous dysplasia are pain and rapid enlargement of the lesion.225
central lesions often located in the diaphysis. The borders are
commonly sclerotic, and the lesion is mainly lytic with trabeculation.
The metaplastic woven bone comprising the lesion creates the
ground-glass appearance on radiograph (Fig. 6-20).
Bowing and/or angular deformity of tibia and femur are often seen.
Distinguishing polyostotic from monostotic FD may be difficult. Plain
radiograph skeletal surveys usually are done; technetium bone scans are
helpful in identifying multiple lesions that may not be present on
plain radiographic studies.119,216
for most fractures that occur in conjunction with monostotic fibrous
dysplasia. Traction with subsequent casting can be used for femoral
shaft fractures in young children; casts or castbracing for upper and
other lower extremity fractures is often appropriate.190
Operative intervention is indicated for fractures of severely deformed
long bones and those through large cystic areas, especially in the
lower extremities.
very diseased bone and are associated with marked deformity. They often
require more aggressive treatment than fractures seen in the monostotic
form. Conservative immobilization techniques are usually appropriate
for most shaft fractures in children before puberty. Fractures of the
femur can be treated with traction and subsequent casting in young
patients. After adolescence, however, the recurrence of deformity after
surgery is less, and curettage and grafting should be considered for
fractures, especially for large lesions with associated deformity.206,307 Stephenson et al.485
found that in patients younger than 18 years of age, closed treatment
or curettage and bone grafting of lower extremity fractures gave
unsatisfactory results, but internal fixation produced more
satisfactory outcomes.
proximal femur. With recurrent fracture and deformity, a severe coxa
vara resembling a shepherd’s crook develops. Curettage of the lesion
with bone grafting has been recommended for mild deformities,216,307
and fixation is usually needed for large lesions. Femoral neck fracture
or osteotomy for deformity can be stabilized with internal fixation.
For severe shepherd’s crook deformity, medial displacement valgus
osteotomies with plate fixations are needed to restore the
biomechanical stability of the hip.258 For severe lesions, Funk and Wells172 recommended complete excision of the intertrochanteric area and advancement of the psoas and gluteus medius tendons. Breck70
recommended securing the side plate of the femoral nail with bolts and
washers rather than with screws to obtain better stability.
 |
|
FIGURE 6-20 A 6-year-old girl presented with right arm acute pain after hitting the elbow in the bathtub. Radiographs of the humerus (A,B) show a nondisplaced pathologic fracture through a humeral diaphyseal lesion (arrow). The lesion is well defined, mostly lytic but with definite matrix, cortical thinning, no periosteal reaction. MRI T1- (C) and T2-weighted (D)
coronal images demonstrate absence of soft tissue mass or other aggressiveness signs. Bone scan shows increase activity at the lesion and fracture site (arrow) (E). The patient underwent open incisional biopsy that confirmed the diagnostic of fibrous dysplasia. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
required for stabilization. Complete en bloc extraperiosteal excision
with grafting has been shown to be successful for severe lesions but is
seldom needed. Both painful lesions without fracture and impending
pathologic fractures can be treated with bone grafting. Proximal
femoral lesions with pathologic fracture are especially troublesome
because of the propensity for malunion with coxa vara. For fractures
through small lesions, either cast immobilization or curettage with
grafting can be used; osteotomy can be done for residual deformity.172
For larger lesions, internal fixation is necessary. Proximal femoral
pathologic fractures have been stabilized with lag screws, blade plates,190
intramedullary nails, and Enders nails. Cast immobilization and
protected weight bearing are necessary after these procedures to
protect the reduction. Spine fractures are rare but can be treated with
bed rest followed by immobilization with an orthosis.190
The main limitation of bone grafting is the potential resorbtion and
transformation into FD. Autogenous cancellous graft has the higher
likelihood to become FD, and cortical allograft is the least likely to
be transformed.121,151,199
offer hope for a medical treatment for patients with severe FD.
Pamidronate is a second-generation bisphosphonate that has had
documented success in selected patients with the disease. It is a
potent inhibitor of bone resorption and has a lasting effect on bone
turnover. The major effect is decreased bone pain. A few studies have
demonstrated improved bone density with pamidronate therapy in patients
with FD.385
for most fractures in children with monostotic FD. In younger children,
immediate casting or traction and subsequent casting
are
used for most femoral shaft fractures. Because fractures in patients
with polyostotic FD usually occur through very abnormal bone and can
result in marked deformity, they often require more aggressive
treatment (e.g., internal fixation).
surgery is less frequent. Nonoperative treatment of fractures and
curettage and cancellous bone grafting do not generally produce
satisfactory results in children with FD of the lower extremity.
Curettage and grafting are indicated for fractures of severely deformed
long bones and those through large cystic areas, with internal fixation
appropriate for the location and age. Bone graft can be reabsorbed
after placement in extensive lesions, and proximal deformity can occur
after corrective osteotomy. Allograft has a lower likelihood to be
reabsorbed than autograft. Recently, the use of coral as bone
substitute has been shown to be effective.121
is the proximal femur. Proximal femoral lesions with pathologic
fracture are especially difficult to treat because of the tendency for
varus deformity and refracture. Stable fractures through small lesions
can be treated with cast immobilization, but one must be vigilant and
ready to intervene at any sign of varus displacement. Femoral neck
fractures can be stabilized in situ with a cannulated screw or
compression screw and side plate, depending on the extent of
involvement and the nature and location of the fracture. Fixation can
be combined with valgus osteotomy if there is pre-existing deformity or
with curettage and grafting if there is a large area of bone loss.
Postoperative cast immobilization and protected weight bearing usually
are necessary. Varus deformity is best treated with valgus osteotomy of
the subtrochanteric region and internal fixation early in the course of
the disease to restore the normal neck shaft angle and mechanical axis.
Intramedullary load-sharing fixation (such as flexible intramedullary
nails) can be used for juvenile patients with femoral shaft fractures.
For larger lesions with more severe deformity, and in older patients,
rigid fixation often is necessary. Depending on the situation,
intramedullary load-sharing fixation devices that support not only the
femoral neck but also the shaft of the femur (such as second-generation
intramedullary nails) are better and should be used when possible. For
severe shepherd’s crook deformity, medial displacement osteotomies are
needed to restore the biomechanical stability of the hip.
tumor-like fibro-osseous condition. Most patients present before the
age of 5 years, ranging from 5 weeks to 15 years of age.79,401
Clinically, there is usually a painless enlargement of the tibia with
slight to moderate anterior or anterolateral bowing. The disease
process is almost always confined to one tibia, but the ipsilateral
fibula can also be involved. Solitary involvement of the fibula is
infrequent, and bilateral involvement of both tibias is rare. Both
distal and proximal lesions can occur, and with fibular involvement,
the lesion is located distally. Pathologic fractures may be present in
nearly one third of patients79;
however, these fractures are usually incomplete (e.g., stress
fractures, microfractures) or minimally displaced and heal well with
conservative treatment.79 Pseudarthrosis is rare but sometimes delayed union may be a problem.
lytic lesion usually located in the middle third of the tibia,
extending proximally or distally.401
The cortex overlying the lesion is expanded and thinned, and in the
medullary canal, a dense band of sclerosis borders the lesion with
narrowing of the medullary canal. Single areas of radiolucency may be
present and have a ground-glass appearance, but often there are several
areas of involvement with a bubble-like appearance (Fig. 6-21).
cast immobilization in plaster casts. If fractures recur, or if the
lesion is rapidly progressive, wide extraperiosteal resection with
grafting is necessary.79 Open
reduction with bone grafting and internal fixation is recommended for
fractures with angular deformity. Early osteotomy is recommended for
severe bowing deformity, followed by internal fixation (intramedullary
device). Bracing is recommended to prevent fractures and angular
deformity.
disease (NF type-1), is an autosomal dominant condition with variable
penetrance that occurs in 1 in 2500 to 3000 live births.105
It affects neural tissue, vascular structures, skin, and the skeleton.
The diagnosis of NF can be based on the presence of two of the four
following criteria, according to Crawford and Bagamery105:
-
Multiple café-au-lait spots
-
Positive family history for NF
-
Diagnostic biopsy of a neurofibroma
-
Presence of pseudarthrosis of the tibia, hemihypertrophy, or a short, angular scoliosis
pointed out that adult patients with NF usually had more than five
café-au-lait spots with a diameter of more than 1.5 cm. The presence of
café-au-lait spots, however, is not pathognomonic for NF. Whitehouse519
noted that 23% of normal children have one or two café-au-lait spots
with a diameter of more than 0.5 cm, and the presence of five or more
café-au-lait spots is needed to suggest the diagnosis of NF. Although
café-au-lait spots may be present at birth, usually they are not seen
until the patient is 5 or 6 years old.105 Generalized soft tissue hypertrophy of the limbs is present in 37% of adults with NF,342
whereas children have an 11% incidence of limb-length discrepancy and
only a 3% incidence of soft tissue enlargement of the extremities.105
considered a valuable criterion for the diagnosis of NF. These tumors,
however, tend not to be clinically apparent until the child is older
than 12 years of age.105 Plain
radiographic studies are not helpful in identifying these soft tissue
tumors. MRI can be helpful in identifying the soft tissue masses.
Technetium 99m-labeled diethylenetriaminepentaacetic acid accumulates
in the soft tissue tumors of NF.323,324 Routine isotopic imaging with this technique can identify lesions as small as 1.5 cm. Lesions as
small as 0.8 cm were seen through a more advanced technique known as
single proton emission computed tomography. Such techniques may be
useful in identifying occult NF and pseudarthrosis of the long bones.
 |
|
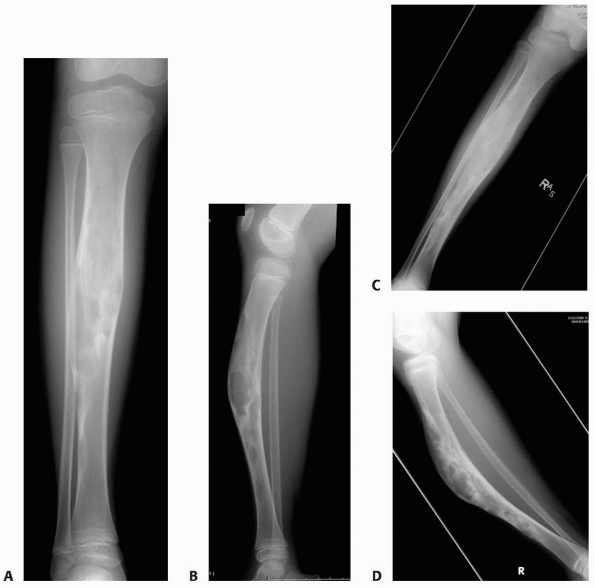
FIGURE 6-21 Anteroposterior (A) and lateral (B)
radiographs of a 4-year-old boy presenting with intermittent leg pain associated with progressive bowing. The images show circumscribed sclerotic and lytic lesion involving the anterior tibial diaphyseal cortex. The patient was thought to have osteofibrous dysplasia and conservative treatment with bracing was elected. Seven years later, the radiographs (C,D) show increased bowing of the tibia measuring approximately 40 degrees. The patient was, however, essentially asymptomatic and was not interested in any surgical treatment. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
be a therapeutic dilemma. The appearance of pseudarthroses and their
resistance to treatment has been postulated to be due to a deficiency
of bone formation secondary to mesodermal dysplasia. The abnormal soft
tissue associated with these pseudarthroses has been postulated to be
the major associated factor in causing pseudarthrosis.525
Approximately 5% of patients with NF eventually develop pseudarthrosis
of the long bones. The tibia is the bone most often affected, but only
55% of the cases of congenital pseudarthroses of the tibia are thought
to be associated with NF.104
the majority of patients do not have a pseudarthrosis at birth but
rather develop it later after a pathologic fracture.369 Brown et al.72
found that in six children with NF, anterior bowing of the leg
developed at an average age of 8 months and then went on to fracture
and pseudarthrosis an average of 4.5 months after the initial clinical
observation of deformity.
pseudarthroses in other locations in children with NF occur and can be
a challenge. Pseudarthroses have been reported in the radius,* ulna,10,11,43,44,309,372,393 both the radius and the ulna,11,43,327,338,429,460 femur, clavicle, and humerus.
osteopenia and osteoporosis, suggesting an abnormal underlying bone
phenotype. This may be another reason as why there is a high incidence
of pseudarthrosis, nonunion, and poor bone healing associated with NF.137
Biopsy specimens of these pseudarthroses invariably reveal fibrous
tissue, but there are reports of found evidence of neural tissue in
biopsy specimens.11,336
None of these findings, however, has been confirmed by electron
microscopy to document the presence of Schwann cells. Radiographic
findings in patients with established pseudarthroses of the tibia
include narrowing or obliteration of the medullary canal at the
pseudarthrosis site, with sclerosis and anterolateral angulation.
Pseudarthrosis of the fibula is associated with valgus deformity of the
ankle.
and the presence of a cystic lesion in the bone. Once a fracture has
occurred, a pseudarthrosis is likely when the fracture line persists
for more than 7 weeks after injury.336
The ends of the fracture gradually become tapered, there is little
callus, and the cortex of the healing bone thickens with a decreased
diameter of the medullary canal. The cause of these radiographic
changes
is unclear. Pseudarthroses have also developed in children with NF
after fracture through normal-appearing forearm bones.245,246
will eventually fracture; corrective osteotomy to correct angular
deformity will only accelerate the progression to pseudarthrosis and
should not be done. Once the fracture occurs, there is little
indication for closed treatment. In conjunction with excision of the
hypotrophic bone ends, methods of treatment of this congenital
pseudarthrosis include intramedullary fixation with iliac bone graft,
fixation with vascularized fibular graft, and Ilizarov compression of
the pseudarthrosis with callotastic lengthening of the proximal tibia.
All of these methods may be complicated by further pathologic fracture
and nonunion.
there is substantial experience with treatment of pseudarthrosis of the
tibia, but results are still disappointing. Bracing has proved
ineffectual in the treatment of an established pseudarthrosis, but may
be useful to prevent fracture and deformity. Surgical procedures have
included bypass grafts,345 onlay grafts,67 grafting with small bone chips, periosteal grafting, intramedullary nailing procedures,14,34 and intramedullary rod fixation after segmental osteotomies.483
The rate of union with these procedures ranges from 7% to 90%, and
eventual amputation has been common. Electrical stimulation has been
used with some success, but most series reporting its use have short
patient follow-up and the electrical stimulation was used in
combination with other surgical techniques. In one series, a 20%
success rate was achieved using direct-current stimulation.71
In another series, union was achieved in 10 of 12 patients with
pseudarthrosis of the tibia through rigid intramedullary rod fixation
and electrical stimulation through implanted electrodes.404 The Farmer procedure, a skin and bone pedicle from the contralateral leg, has a reported union rate approaching 53%.368 Free vascularized fibular grafts also have been used for reconstruction after excision of the involved tibia.368
In one series, 11 of 12 patients with NF and pseudarthrosis of the
tibia were successfully treated with free vascularized fibular grafts.
Union of the pseudarthrosis occurred between 3 to 8 months after
surgery.114,128,516 A free vascularized iliac graft has been used in one patient with NF, resulting in union within 10 weeks.302 Fabry et al.156 obtained union of pseudarthrosis of the tibia in two patients with compression through an Ilizarov fixator. In another series,424
the fractures in three of five patients healed in 4.5 months. The other
two patients needed supplementary iliac grafts, and eventually, the
bone united. It is important to stress that treatment cannot be
considered successful until skeletal maturity has been reached; many of
these series included patients with short follow-up, and few included
follow-up to skeletal maturity.
This modification of the original McFarland bypass procedure, which was
originally done for established pseudarthrosis, was successful in a
series of patients from several centers reviewed by Strong and
Wong-Chung.491 A modified sequential McFarland bypass procedure for prepseudarthrosis of the tibia also has been described.125 More recently, some authors have been reporting on the use recombinant human bone morphogenetic protein with promising results.155,290
family early when previous operative interventions have been
unsuccessful. Amputation usually is at the Syme level, with prosthetic
fitting around the pseudarthrosis. In a gait analysis study, Karol et
al.248 compared 12 patients with
previously operated and healed congenital pseudarthroses of the tibia
with four children with amputations for final treatment of congenital
pseudarthroses of the tibia. They found marked disturbance of gait and
muscle strength in patients with healed congenital pseudarthroses of
the tibia. They concluded that patients with early onset of disease,
early surgery, and transankle fixation had more inefficient gaits than
amputees. Patients with forearm pseudarthroses can be pain free and
function may be satisfactory with observation or splinting. However,
persistence of an ulnar pseudarthrosis in a growing child often leads
to bowing of the radius and posterior lateral subluxation or
dislocation of the radial head.11,12,302,393
Healing after 6 months of casting has been reported in a 2-month-old
infant with a congenital pseudarthrosis of the radius. There was no
clinical evidence of NF at the time of treatment of this patient.191
Union after conventional bone grafting and fixation has been reported
in only a small number of patients with congenital pseudarthrosis of
the forearm.43,44,245,246,327,465
Many of these patients require multiple conventional bone grafting
procedures and often years of immobilization. There are more reports of
patients (and probably many more patients) with pseudarthroses of the
forearm bones who did not respond to multiple grafting procedures.10,44,72,336,393
The results of treatment of congenital pseudarthrosis of the forearm in
NF by free vascularized fibular grafts are encouraging. Allieu et al.12
treated one patient with radial and ulnar pseudarthroses and another
with ulnar pseudarthrosis with free vascularized fibular grafts. They
obtained union in the patient with radial and ulnar pseudarthroses in 6
weeks and in the patient with ulnar pseudarthroses in 3 months. Earlier
conventional grafting techniques had failed in both. Two additional
patients with pseudarthroses of the radius without evidence of NF were
treated with free vascularized fibular grafts, resulting union within 6
weeks.460,525 Mathlin et al.338
reported six pseudarthroses of the forearm bones treated with
vascularized fibular grafting with union in five ranging from 6 to 18
months after surgery. Other surgical options include excision of the
ulnar pseudarthrosis to avoid a later tethering effect on the growing
radius10 and fusion of the distal radius and ulnar joint.393
Creation of a one-bone forearm is often technically successful, but
both length and rotation of the forearm are sacrificed with this
procedure.309,393
abnormality seen in individuals with NF. Although scoliosis was present
in 64% of patients with NF in one series,105 kyphoscoliosis may be the primary contributor to the development of paraplegia.524
Patients younger than 19 years of age may have paraplegia secondary to
vertebral deformity, whereas those patients older than 19 are more
likely to have neurologic deficits secondary to a neurofibroma.
Complete dislocation of the spine with neurologic defect has been
reported in two patients with NF.438 Rib penetration of the enlarged neural foramen with spinal cord
compression in NF has also been reported in four patients.167,319
CT and MRI are useful for evaluating these patients. Resection through
either an anterior or a posterior approach seems satisfactory.319
 |
|
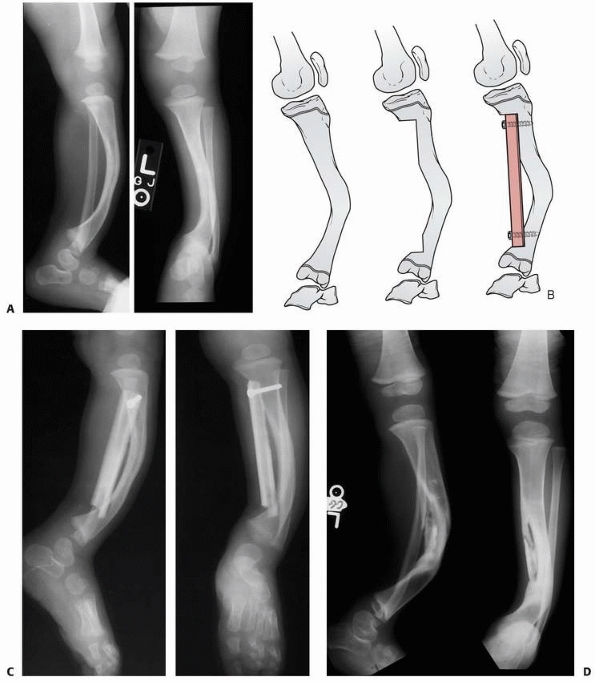
FIGURE 6-22 A.
A 2-year-old boy with neurofibromatosis presented with anterolateral bowing, sclerosis, and partial obliteration of the medullary canal of the tibia without fracture. B. A modified McFarland technique for prophylactic bypass grafting was performed as shown. C. Immediate postsurgical radiographs of the tibia after prophylactic bypass grafting. D. Three years later, radiographs show continued growth of the tibia without fracture but some absorption of the allograft and relative loss of structural support by the allograft related to continued growth. (From Dormans JP. Modified sequential McFarland bypass procedure for prepseudarthrosis of the tibia. J Orthop Tech 1995;3:176-180, with permission.) |
children with NF. Complications are common. The periosteum of the long
bones is less adherent to the bone than normal periosteum, and
extensive subperiosteal hemorrhage may occur resulting from a trauma or
after an osteotomy or other surgical procedure.528
It is important preoperatively to rule out hypertension in children
with NF because 16% of children with NF had hypertension in one series.509
remains controversial. When a child presents with prepseudarthrosis
(angulation without fracture), either bypass grafting with fibular
allograft or bracing are reasonable options. Once pseudarthrosis has
developed, our preference is inserting an intramedullary rod and bone
grafting of both the tibia and fibula when possible (Fig. 6-23). If these procedures fail, free vascularized fibula transfer or resection and bone transport
with circular frame techniques can be considered. Amputation and
prosthetic fitting should be considered early in patients with failure
of the above-mentioned techniques and severe shortening and a stiff
ankle and foot. Conservative options, such as bracing or observation,
for upper extremity pseudarthroses may be justified in a patient with a
non-progressive deformity and a satisfactory functional use of the
extremity. Conventional bone grafting and fixation procedures for
treatment of pseudarthrosis of the upper extremity have very limited
success, and other approaches should be considered. Free vascularized
fibular grafts seem the treatment of choice for upper extremity
pseudarthrosis associated with NF.
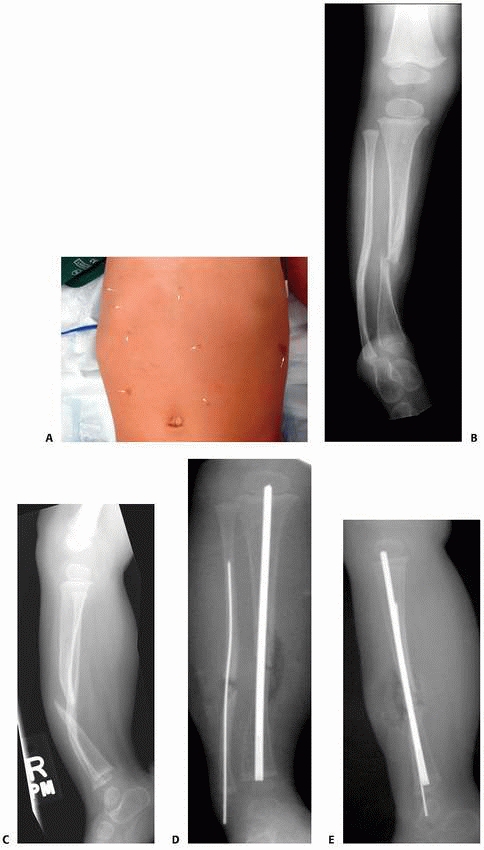 |
|
FIGURE 6-23
A 19-month-old girl presented with right leg bowing and recent inability to bear weight on that extremity. The patient has neurofibromatosis type 1 with associated café-au-lait spots (A). Anteroposterior (B) and lateral (C) radiographs of the tibia and fibula show pseudarthrosis of the tibia diaphysis associated with intramedullary obliteration and bone thinning at the pseudarthrosis level. The fibula presents anterior-lateral deformity and partial obliteration of the medullary canal without fracture. Postoperative images (D,E) following excision of the pseudarthrosis, fibular osteotomy, iliac bonegraft and periosteum grafting, and fixation with a William rod. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
sensory neuropathy disorder characterized by the absence of normal
subjective and objective responses to noxious stimuli in patients with
intact central and peripheral nervous systems. The cause is unknown,
but sporadic reports have appeared in the orthopaedic literature.197,198,279,387
insensitivity to pain include recurrent fractures, osteomyelitis, and
neuropathic joints. Although the lower extremities are most commonly
affected, the spine could also be involved with gross and unstable
spondylolisthesis. Limb-length discrepancy may occur from physeal
damage. Lack of pain perception is associated with the development of
Charcot joints, which may lead to later neuropathic arthropathy. The
weight-bearing joints are usually affected, especially the knees and
ankles. Although fracture healing usually occurs, the arthropathy is
progressive, eventually resulting in gross deformity and instability.
In addition to absence of deep pain, the patients had impaired
temperature sensation.365
closely related sensory disorders including congenital sensory
neuropathy, hereditary sensory radicular neuropathy, familial sensory
neuropathy with anhidrosis, and familial dysautonomia (Riley-Day
syndrome). Acquired conditions with pain insensitivity include
syringomyelia, diabetes mellitus, tabes dorsalis, alcoholism, and
leprosy. Loss of protective sensation promotes selfmutilation, burns,
bruises, and fractures. The disease comes to light when the child
develops teeth and then bites his or her tongue, lips, and fingers.
Joint injury should be recognized and treated early to prevent
progression to gross arthropathy. Early diagnosis and treatment of
fractures is important, usually by conservative manners.197,279
In a severely unstable, degenerated joint, arthrodesis may eventually
be appropriate; however, poor healing, nonunion, and pseudarthrosis are
common in neuropathic joints (Fig. 6-24). Infection rate is also increased, and it is important to make the differentiation between fracture and infection.36 The condition appears to improve with time with the gradual recovery of pain sensation.
metabolism. It is the most common lysosomal storage disease and is
caused by deficient production and activity of the lysosomal enzyme
beta-glucosidase (glucocerebrosidase), resulting in progressive
accumulation of glucosylceramide (glucocerebroside) in macrophages of
the reticuloendothelial system in the spleen, liver, and bone marrow.
The most common sphingolipidosis, it is inherited as an autosomal
recessive trait,251 with most cases noted in Ashkenazic Jews of eastern European origin.280
There are three types of Gaucher disease: type I represents more than
90% of all cases and is the most common type seen by orthopaedic
surgeons. It presents as a chronic nonneuropathic disease with visceral
(spleen and liver) and osseous involvement, also known as the adult
form, although patients present during childhood251;
Type II is an acute, neuropathic disease with central nervous system
involvement and early infantile death; and type III is a subacute
nonneuropathic type with chronic central nervous system involvement.
Types II and III are both characterized as either infantile or
juvenile, and are notable for severe progressive neurologic disease,
usually being fatal.
present with Erlenmeyer flask appearance, osteonecrosis (particularly
of the femoral head), and pathologic fractures, especially of the spine
and femoral neck. Bone lesions are most common in the femur, but they
also occur in the pelvis, vertebra, humerus, and other locations.280
Infiltration of bone by Gaucher cells leads to vessel thrombosis,
compromising the medullary vascular supply and leading to localized
osteonecrosis of the long bones.442 ON of the femoral head occurs in most patients in whom the disease is diagnosed in childhood.
shaft after biopsy, and of the spine, are usually best managed
conservatively. Katz et al.251
reported 23 pathologic fractures in nine children with Gaucher disease;
seven had multiple fractures. In decreasing order of frequency, the
site of involvement included the distal femur, basilar neck of the
femur, spine, and proximal tibia. Fractures also occurred infrequently
in the distal tibia, proximal humerus, rib, and acetabulum. Fractures
of the long bones were transverse and usually in the metaphysis.
Fractures of the spine were either wedge-shaped or centrally depressed
at the end plate. The factors predisposing these children to fracture
included significant medullary space infiltration, cortical bone
erosion, osteonecrosis, and associated disuse osteoporosis.251
11 children had vertebral fractures, usually at two or three sites in
each patient, with either anterior wedging, central vertebral collapse,
or total rectangular collapse. Most patients had relief of their pain
after 1 to 4 months of conservative treatment; two required
decompression laminectomies, and one had a posterior lateral fusion to
stabilize the spine.
that fractures of the upper extremities in Gaucher disease were prone
to occur in areas of prior crisis. Although external callus formed in 6
to 8 weeks in most patients, complete healing with internal callus took
almost 2 years in some. Other authors have found fracture union to be
rapid.187 Both delayed union and nonunion442 have been reported in older patients with Gaucher’s disease.
associated trauma in children with Gaucher disease often heal with a
varus malunion and minimal subsequent remodeling; osteonecrosis of the
femoral head also can be associated with femoral neck fractures.187,280 Goldman and Jacobs187
stated that the presence of a mixed density of bone of the femoral neck
on radiograph with narrowing of the medial cortex was a risk factor for
fracture.
suggested for long bone fractures when appropriate. Stable fractures of
the femoral neck should be treated by immobilization
with
frequent follow-up radiographs. Internal fixation should be used in
unstable femoral neck fractures. Preoperative planning is important,
and the anesthesiologist must recognize that patients with Gaucher
disease may be prone to upper airway obstruction because of
infiltration of the upper airway with glycolipids499 and abnormal clotting function, even when clotting tests are normal.217
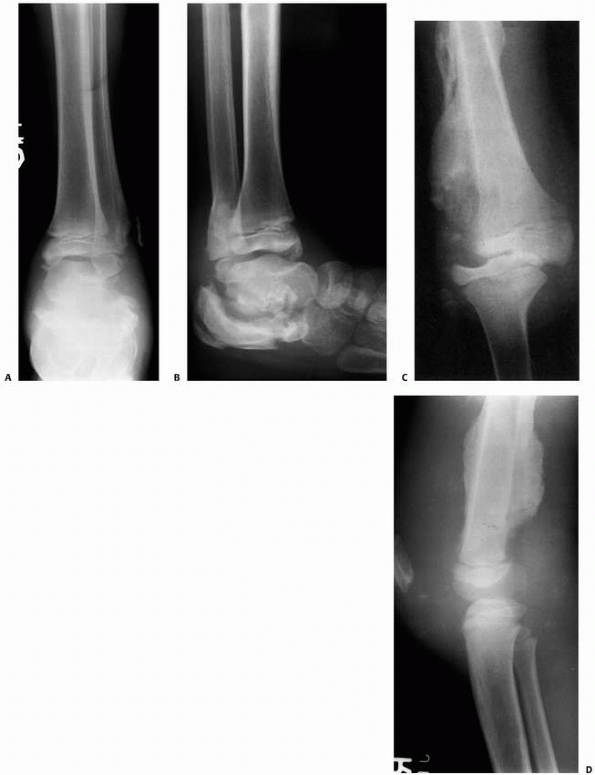 |
|
FIGURE 6-24
This 6-year-old child with anhidrosis, congenital insensitivity to pain, and attention deficit disorder presented with a history of swollen ankles and knees. Anteroposterior (A) and lateral (B) radiographs show Charcot changes in the subtalar joint with calcaneal and distal fibular fractures. Anteroposterior (C) and lateral (D) radiographs of the right knee show large, loose osteochondral fragments, medial subluxation of the femur on the tibia, and extensive periosteal new bone formation in the distal femur. Soft tissue shadows are consistent with her huge knee hemarthrosis. More than 100 mL of sterile serosan guineous fluid was aspirated from the knee at her initial visit. The effusion quickly returned in the days following the aspiration. Because management with casts at another hospital resulted in significant skin breakdown, we stabilized the knees with removable hinged braces. The effusions improved but did not resolve. |
conditions caused by the presence of sickle cell hemoglobin (HbS). The
most common type of SCD, HbS-S, is a homozygous recessive condition in
which individuals inherit the HbS globin gene from each parent. SCD has
systemic effects particularly on splenic function and on the central
nervous, renal, hepatic, and musculoskeletal systems. SCD affects
approximately one in 400 African Americans. Sickle cell trait affects
8% to 10% of the African American population and other groups less
frequently. With sickle cell trait, each individual has inherited a
beta-S globin gene and a beta-A globin gene. Clinical manifestations of
sickle cell trait usually are not apparent. The presence of this
abnormal hemoglobin in red blood cells causes them to be mechanically
fragile, and when they are deoxygenated, the cells assume a sickle
shape, which makes them prone to clumping with blockage of the small
vessels of the spleen, kidneys, and bones.406
Chronic hemolytic anemia is present in most severely affected patients,
and marrow hyperplasia is found in both the long bones and the short
tubular bones. The prevalence of osteopenia and osteoporosis in young
adults with SCD is extremely high and that can be related or predispose
to pathologic fractures.361 These disorders are diagnosed by hemoglobin electrophoresis.92
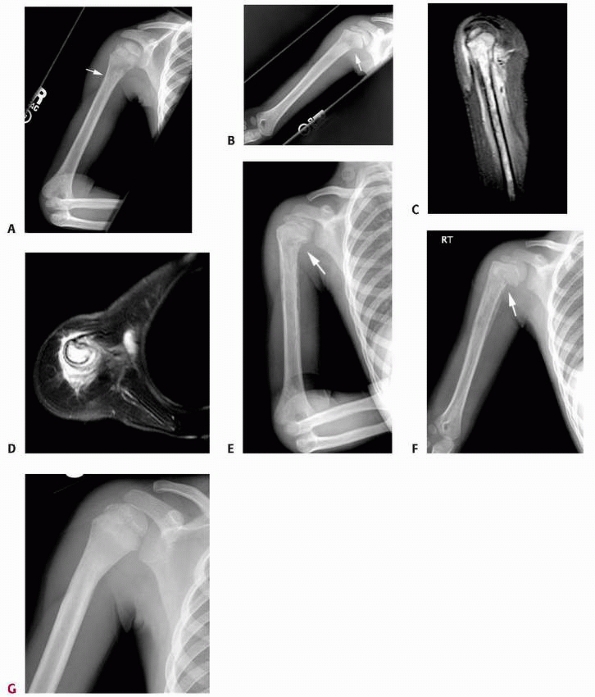 |
|
FIGURE 6-25
A 4-year-old boy with sickle cell disease presented with acute onset of right arm pain, swelling, increased warmth, and low-grade fever. The initial radiographs (A,B) show a poorly defined area of lucency in the proximal humeral metaphysis (arrow). T2-weighted sagittal (C) and axial (D) MRI show intramedullary changes (enhancement) and periosteal reaction/abscess with no soft tissue mass. The clinical diagnosis of osteomyelitis was initially made and the patient was started on intravenous antibiotics. Three weeks later, there was little clinical improvement and new radiographs showed pathologic fracture/insufficiency fracture through the proximal humeral metaphysis (E,F) (arrow). The patient underwent a biopsy that showed that this was an infarct with no superimposed infection. After clinical treatment, the patient’s symptoms improved. At 6-months follow-up, he was completely asymptomatic and the radiographs showed remodeling and continued growth (G). |
Pathologic fractures are often seen in association with osteomyelitis.
In a series of 81 patients with 198 long bone infarcts with occasional
concurrent osteomyelitis,
Bohrer57
found evidence of fracture in 25% of femoral lesions, 20% of humeral
lesions, and a significant percentage also in tibial bone infarcts.
Ebong141 reported pathologic
fractures in 20% of patients with SCD and osteomyelitis. The most
common site of fracture was the femur. The fractures are transverse and
commonly located in the shaft of the long bone,123 and although minimal trauma is needed to cause them,356 they often have significant displacement.57,58
The exact mechanism for pathologic fracture in these patients is
unclear; although it is often associated with bone infarct, the
fracture itself is seldom through the area of infarction. Marrow
hyperplasia may be a major contributing factor; not only does the
hypercellular bone marrow expand the medullary canal with thinning of
both trabecular and cortical bone, but it also extends into widened
Haversian and Volkmann canals.123
This process probably weakens the bone sufficiently so that fractures
occur. Also, children with SCD have significant deficits in the whole
body bone mineral content that persist despite adjustment for poor
growth and decreased lean mass; therefore, these children may be at
increased risk for fragility fractures and suboptimal peak bone mass.73 The healing process seems unaffected, and union usually occurs normally.406
well with conservative treatment. Operative management of fractures in
patients with SCD is potentially hazardous. Extreme care must be taken
to oxygenate the patient’s tissues adequately during the procedure, and
ideally, elective procedures should be preceded by multiple
transfusions to reduce HbS to less than 30% of total hemoglobin levels.
A randomized multicenter study found that a simple conservative
transfusion regimen to raise hemoglobin levels to 10 g/dL was as
effective as an aggressive exchange transfusion regimen (to reduce HbS
to less than 30%) in preventing perioperative complications.
half to two times the daily fluid requirements needed in addition to
routine replacement of fluid losses. The use of a tourniquet in surgery
for patients with SCD is somewhat controversial. Osteonecrosis of the
femoral head is an especially difficult problem in patients with SCD.
Treatment options include conservative measures such as physical
therapy and sometimes core decompression, although some have shown no
difference in the final outcome.378 Patients with total head involvement may require femoral or pelvic osteotomies. Athanassiou-Metaxa et al.27
showed that out of nine children treated with subtrochanteric varus
femoral osteotomy for femoral head osteonecrosis, eight children
experienced improvements in pain, joint motion, and walking. Total
joint replacement is occasionally indicated in young adults. Before
general anesthesia, the patient’s hematocrit should be raised to more
than 30 and hemoglobin to more than 10 g/dL.
cancer. Acute lymphocytic leukemia is one of the most common malignant
diseases in childhood and accounts for 80% of pediatric leukemias.
There is an increased occurrence of lymphoid leukemias in patients with
Down syndrome, immunodeficiencies, and ataxic telangiectasia. The peak
incidence occurs at 4 years of age.
Skeletal lesions occur more frequently in leukemic children than in
adults because leukemic cells can quickly replace the smaller marrow
reserves in children. Approximately 50% to 75% of children with acute
leukemia develop radiographic skeletal manifestations during the course
of their disease.213,351 Pathologic fractures can be seen in up to a third of the patients.268,439,476
Nonspecific juxtaepiphyseal lucent lines are often seen and are a
result of generalized metabolic dysfunction. Sclerotic bands of bone
trabeculae are more typical in older children. Lucencies and
periostitis may mimic osteomyelitis. A characteristic lesion seen
within a month of onset of symptoms is a radiolucent metaphyseal band
adjacent to the physis476; these are
usually bilateral and vary from 2 to 15 mm in width. Osteolytic lesions
with punctate areas of radiolucency are found in the metaphysis and can
either appear moth-eaten or as a confluent radiolucency. Periosteal
reaction often is present with osteolytic lesions and is most common in
the posterior cortex of the distal femoral metaphysis, the medial neck
of the femur, and the diaphysis of the tibia and fibula.476 Most bone lesions in leukemia improve during remission after treatment and tend to progress with worsening of the disease.
treatment. Fracture is most commonly associated with osteoporosis of
the spine, resulting in vertebral collapse (compression fracture). The
thoracic vertebrae are the most commonly involved. Fractures
occasionally occur at other locations and usually after minor trauma.233,378
A bone scan may aid in identifying clinically silent areas but may not
correlate with areas of obvious destruction on radiographs. Spastic
paraparesis has been reported in one patient with vertebral fracture
due to leukemia.233
first step in the treatment of pathologic fractures associated with
leukemia. Most fractures are stable microfractures and can be treated
with conservative immobilization techniques with emphasis on early
ambulation to avoid further problems with disuse osteoporosis. Most
vertebral fractures can be treated nonoperatively with close
observation; however, often a back brace or thoracolumbosacral orthosis
is used to alleviate symptoms.
clotting mechanism that presents most commonly as a functional
deficiency of either factor VIII (hemophilia A) or factor IX
(hemophilia B). Classic hemophilia, or hemophilia A (factor VIII
deficiency),
is an inherited sex-linked recessive disorder. The incidence is one per 10,000 live male births in the United States.7 Christmas disease, or hemophilia B, is a sex-linked recessive factor IX deficiency and occurs in one per 40,000 live births.
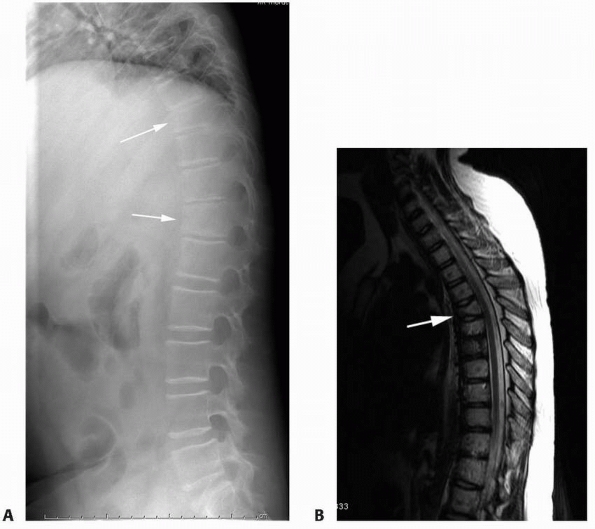 |
|
FIGURE 6-26 This 8-year-old girl presented with back pain, fever, malaise, and weight loss. Lateral radiographs (A) of the spine showed diffuse osteopenia and compression/insufficiency fractures of the vertebral body (arrows). T1-weighted sagittal MRI (B) confirms disease process within the vertebral body (arrow)
and no soft tissue mass or intraspinal involvement. She was diagnosed with acute lymphoblastic leukemia. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
performed, including platelet count, bleeding time, prothrombin time,
and partial thromboplastin time. Deficiency of factor VIII, the most
common form of hemophilia, causes a marked prolongation in the partial
thromboplastin time.467 Once the disease is suspected, specific factor assays can document the type of hemophilia.
include acute hemarthroses (knee, elbow, and ankle, in decreasing order
of frequency), soft tissue and muscle bleeds, acute compartment
syndrome, carpal tunnel syndrome, and femoral nerve neuropraxia (Table 6-5).
The severity of the deficiency often is correlated with circulating
levels of factors VIII or IX. The disease is classified as severe when
clotting activity is less than 1%, moderate when clotting activity is
1% to 5%, and mild when clotting activity is more than 5%. By
definition, each milliliter of normal human plasma contains one unit of
factor activity, and the clinical severity of hemophilia correlates
with the patient’s percentage of normal levels of plasma factor
activity (Table 6-6). Early diagnosis and aggressive management are the keys to lessening complications.
|
TABLE 6-5 Grades of Articular Involvement
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
management of a fracture, the orthopaedist and the hematologist should
work closely together. Preoperatively, the patient should be tested for
the presence of inhibitor and a test dose of factor replacement should
be given to determine the biologic half-life of that factor for that
particular patient.7 Elective
surgery is usually contraindicated in the presence of inhibitor. Most
authors recommend a level of factor activity during surgery ranging
from 70% to 100%,7,283,412 although others believe that approximately 50% is adequate.402,407
Tourniquets are recommended for extremity surgery. The use of routine
drains is not advised, but 24 hours of suction drainage is favored by
some.7,283,412
Factor levels are checked immediately after surgery and then at least
daily. Factor VIII is given every 6 hours, and factor IX is given every
8 hours. It is useful to check a trough level factor activity
immediately before the next dose of factor supplementation. In the
immediate postoperative period, factor levels are maintained at 30% to
40%,7,283
and these levels should be maintained until sutures are removed. During
the rehabilitative period, maintenance levels of factor ranging from
20% to 50% immediately before sessions of physical therapy should be
maintained.7,282,402,412
Intramuscular injections of analgesics should be avoided, as should
aspirin compounds and nonsteroidal anti-inflammatory medications that
affect platelet function. Both acetaminophen and codeine medications
are safe oral analgesics.249 In the
past, hemophiliac patients had an increased risk of operative
infections and delayed wound healing, but aggressive replacement
therapy has minimized those problems.412
(osteopenia and osteoporosis) is frequently diagnosed in hemophiliac patients, especially when there is hepatitis C associated.37,511 Most authors have noted that healing of fractures proceeds primarily with endosteal callus and very little periosteal callus,160,163,257 but Lancourt et al.282
observed significant periosteal calcification in these fractures with a
normal rate of healing. Fractures occur in both the upper and lower
extremities.6,56,160,257,437 Joint dislocations are rare in hemophiliac patients. Floman and Niska166
reported on a 6-year-old boy who sustained a posterior dislocation of
the hip with mild trauma that required a closed reduction under general
anesthesia and immobilization in a hip spica cast. The joint was found
to be enclosed at a 6-year follow-up. Ackroyd and Dinley3
reported on two patients with the patella locked into the intercondylar
notch of the distal femur after sustaining hyperflexion injuries of the
knees, which had limited range of motion owing to arthropathy. These
injuries were treated by flexion of the knee under general anesthesia,
depression of the inferior pole of the patella to unlock it, and then
extension of the knee followed by splinting.
|
TABLE 6-6 Severity of Hemophilia Correlated with Plasma Factor Activity Levels
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Factor replacement is important for about the first week after the
fracture, and levels of factor activity recommended vary from 20% to
50%.6,7,56,160,227,282
Circumferential plaster casts are extremely hazardous in the treatment
of these fractures because of the risk of swelling from bleeding as
well as subsequent compartment syndrome and skin necrosis.485
A Robert Jones dressing may be preferable for fracture immobilization
immediately after injury, and a cast should be applied once active
swelling has stopped.227 All casts
applied should be well padded and split, and the patient should be
monitored carefully for swelling. Fractures of the femur can be treated
with traction and subsequent spica casting.56,294 Some authors consider skeletal traction to be hazardous because of the risk of infection or bleeding,7,227 but Boardman and English56
suggested that with proper replacement therapy, skeletal pins can be
used in the hemophiliac. Replacement therapy is advisable while
fractures are manipulated and casts are changed. Most authors think
that open reduction and internal fixation should be performed in
hemophiliac patients for fractures that would customarily be treated
with such methods.7,56,282 External fixators are not commonly used for patients with hemophilia; however, Lee et al.295
described the use of external fixators (Ilizarov, AO, uni- and biplanar
fixators, and Charnley clamp) in nine patients with hemophilia for
arthrodesis of infected joints, treatment of open fractures, and
osteoclasis. One major complication related to external fixators
occurred in a patient who developed inhibitors. They concluded that
external fixators can be used safely in hemophilic patients without
inhibitors and does not require prolonged factor replacement.295
hematologist is important in providing care for children with
hemophilia. Most fractures in children with hemophilia can be treated
with either traction or cast techniques. Care must be taken to avoid
complications related to compression in these patients, and a
monovalved, well-padded plaster cast provides a safe means of
treatment. A fiberglass cast may not be desirable because a simple
monovalved will fail to expand the cast completely. Operative treatment
should be reserved for fractures that normally require surgery, and the
usual precautions for hemophiliac patients for surgery are observed.
osteomyelitis in North America has changed during the past several
decades. Although the typical clinical picture of acute osteomyelitis
in children is still seen, subtle presentations and more aggressive
pathogens have become more frequent. There are several reasons for this
change, including modification of the clinical course by antibiotics
given before admission162 and,
possibly, increased awareness and an earlier presentation to a medical
facility resulting in earlier diagnosis. Children often present with
acute osteomyelitis. Less common variants include Brodie abscess,
subacute epiphyseal osteomyelitis, viral osteomyelitis,474 and chronic recurrent multifocal osteomyelitis.243 Some patients present with a bone lesion that may be confused with other disease entities, including neoplasm.75
Biopsy is often needed to clarify the diagnosis. Even with appropriate
antibiotic therapy, some patients have recurrent infection, growth
disturbance, and pathologic fractures.
and granulomatous infections), onset (acute, subacute, and chronic),
and routes of infection (hematogenous and direct inoculation). Chronic
osteomyelitis is defined by most authors as osteomyelitis with symptoms
that have been present for longer than 1 month (Fig. 6-27 and Table 6-7).
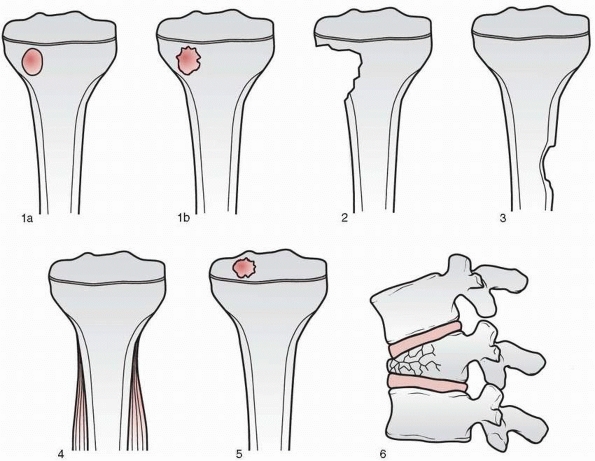 |
|
FIGURE 6-27
Classification of subacute osteomyelitis. Type 1A, punched-out radiolucency suggestive of eosinophilic granuloma. Type 1B, similar but with sclerotic margin; classic Brodie abscess. Type II, metaphyseal lesion with loss of cortical bone. Type III, diaphyseal lesion with excessive cortical reaction. Type IV, lesion with onionskin layering of subperiosteal bone. Type V, concentric epiphyseal radiolucency. Type VI, osteomyelitic lesion of vertebral body. (From Dormans JP, Drummond DS. Pediatric hematogenous osteomyelitis: new trends in presentation, diagnosis, and treatment. J Am Acad Orthop Surg 1994;2:333-341, with permission.) |
osteomyelitis within 10 to 14 days after onset of symptoms; the
earliest finding is loss of defined deep soft tissue planes.236
Because of this early insensitivity of plain radiographic studies,
isotopescanning techniques have been used to aid in diagnosis with
varying rates of success. In proven osteomyelitis, abnormal technetium
scans are seen in 63% to 90% of patients.229
MRI detects increased intramedullary water and decreased fat content,
which occurs when there is inflammatory exudate, edema, hyperemia, and
ischemia, all of which are markers of infection.274 MRI has up to 98% sensitivity and 100% specificity for the detection of osteomyelitis.274
reviewed 1068 patients with osteomyelitis and found only 18 pathologic
fractures, 13 of which occurred in the femur. They thought these
fractures were due to delayed recognition of the infection or
inadequate treatment. Other factors were disuse osteopenia, presence of
a weak involucrum, and excessive surgical removal of involved bone. In
that preantibiotic era, most of the fractures were sustained after
surgical treatment of the osteomyelitis, and the authors believed that
conservation of the involucrum and proper immobilization
could have prevented these injuries. White and Dennison518
noted that before antibiotics, pathologic fractures of osteomyelitis
were common, but union occurred with certainty in the presence of dense
involucrum. Daoud and Saighi-Bouaouina111
reported on 34 patients with hematogenous osteomyelitis complicated by
pathologic fracture, pseudarthrosis, or significant segmental bone
loss. The tibia was affected in 24 cases, the femur was affected in 8
cases, and the humerus was affected in two cases. A pathologic proximal
femoral fracture has been reported in neonatal osteomyelitis.28 Although extremely rare, hematogenous osteomyelitis has also been reported at the site of a closed fracture.81,254,459 Canale et al.81
reported three children with osteomyelitis after closed fracture. They
pointed out that progressive pain and swelling at a fracture site
during healing are suggestive of possible osteomyelitis. Daoud et al.110
reported 35 children with upper femoral osteomyelitis with associated
septic arthritis. The incidence of osteonecrosis (ON) of the femoral
head was approximately 50% both in the group that was treated with
arthroscopy and in the group in which no surgery had been done. They
postulated that ON of the femoral head may be due to compression by
abscess of the vessels lying on the posterior superior femoral neck.
The complications of fracture, dislocation, and displacement of the
capital femoral epiphysis occurred in two thirds of their patients, and
these usually were patients who presented long after an acute phase of
the disease. They recommended surgical drainage of septic hips, and
reduction and stabilization of hips with ON using skin traction and
plaster immobilization for 40 to 60 days. Lewallen and Peterson459
documented osteomyelitis in 40% of 20 nonunions of diaphyseal open
fractures in children. They believed that the presence of infection was
a significant factor in their failure to heal. Long bone lesions also
may be present in congenital rubella,446 and pathologic fractures have been reported in congenital cytomegalic inclusion disease.480
|
TABLE 6-7 Comparison of Acute and Subacute Hematogenous Osteomyelitis
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||
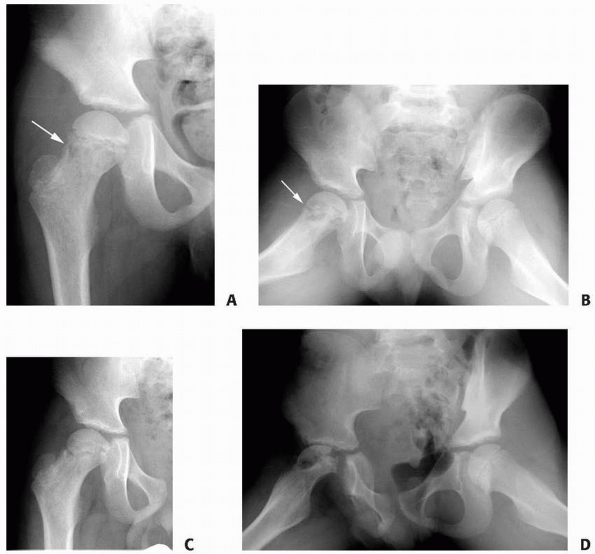 |
|
FIGURE 6-28
A 7-year-old boy presented with classic picture of septic arthritis of the right hip. He underwent promptly irrigation and débridement of the hip. On follow-up just a few weeks later, plain radiographs demonstrated a lytic area in the femoral neck (A,B) (arrow) and blood work showed increased c-reative protein and sedimentation rate. The patient underwent repeated irrigation of the hip and drilling of the lytic area (osteomyelitis) in the femoral neck. Two months later, he developed collapse (pathologic fracture) of the femoral head with gross deformity of the proximal femur (C,D), especially in the lateral views (D) with decreased range of motion. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
to treat and may be associated with complications such as malunion and
growth disturbance (Fig. 6-28).367
Pathologic fractures associated with osteomyelitis are rare in North
America and usually are associated with neglected or chronic
osteomyelitis, neonatal osteomyelitis, or septic arthritis. In children
with chronic osteomyelitis, the purulent material elevates the
periosteum and a supportive involucrum develops. Sequestrectomy of a
portion of the necrotic diaphysis while leaving the supportive
involucrum is often needed to bring the infection under control, but
the timing of this procedure is controversial. Langenskiold286
delayed sequestrectomy of a necrotic femoral shaft for 10 months in a
6-year-old patient to allow the involucrum to develop, but Daoud and
Saighi-Bouaouina111 recommended much
earlier débridement. In patients with active infection, they performed
sequestrectomy with débridement followed by antibiotic therapy for up
to 6 months. Prolonged cast immobilization was necessary. They obtained
healing in 33 of 34 patients with pathologic fractures or
pseudarthroses due to osteomyelitis. The mean healing time of fractures
was 5 months in patients with involucrum. Patients with active
infection without involucrum required débridement, antibiotics, and
subsequent treatment with corticocancellous iliac graft. The mean
healing time was 8.7 months. The patients without active infection and
no involucrum were treated with prolonged immobilization and cancellous
bone graft, supplemented by fixation. Angular deformities were treated
with cast manipulation. Tudisco et al.502
reported on 26 patients with chronic osteomyelitis with average
follow-up of 23 years. Approximately 15% had shortening and angular
deformity of the affected limb. Newer techniques for difficult cases have also been developed.
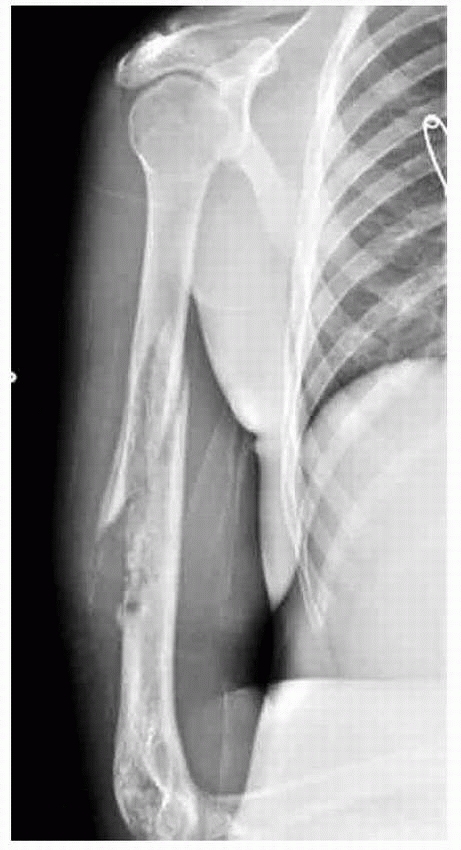 |
|
FIGURE 6-29
This lateral radiograph of the humeral shaft of a 17-year-old boy shows a pathologic fracture through chronic osteomyelitis of the humerus. (Case courtesy of B. David Horn, MD.) |
osteomyelitis leading to pathologic fracture is uncommon. When
osteomyelitis is associated with pathologic fracture (Fig. 6-29),
it usually is neglected chronic osteomyelitis or, rarely, neonatal
osteomyelitis or septic arthritis. The most important step in the
treatment of fracture associated with osteomyelitis is to control the
underlying infection. At a minimum, this requires biopsy for culture
and sensitivities, drainage and débridement of the infection with
immobilization in association with antibiotic therapy (Table 6-8).
In advanced infections, sequestrectomy may be necessary. MRI is useful
in identifying the sequestrum; an attempt should be made to leave as
much supporting involucrum as possible at the time of sequestrectomy.
Bone transport and lengthening may be valuable in certain cases.
Prolonged immobilization with either plaster casts or external fixation
devices may be needed, and segmental bone loss can be treated with bone
transport or grafting.
|
TABLE 6-8 Initial Antibiotic Therapy for Osteomyelitis
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||
several decades. Surgeons experienced with lengthening techniques can
now correct problems that previously had no satisfactory solution. The
very high complication rate that has come with these advances has
decreased with newer techniques and more extensive surgical experience.
Complications with the Wagner method, popular 20 to 30 years ago, were
as high as 92%.224,320
Newer techniques, using gradual lengthening with either monolateral
fixators or fine wire fixators, such as the Ilizarov fixator, have
decreased the complication rate. Fractures that occur in association
with limb lengthening fall into three general categories: (i) fractures
through pin tracks, (ii) fractures through regenerate bone, or (iii)
fractures through bone weakened by disuse osteoporosis. Fractures that
occur through holes left after removal of screws or fine wires
generally occur a few weeks after device removal. The incidence of
these fractures can be minimized by protective weight bearing after
removal of the device and using the smallest possible screw diameter
that is appropriate for the fixation device needed.
fractures. The bone that is formed by distraction callotasis must be
subjected to normal weight-bearing forces over a period of time before
normal bony architecture is established. Fractures that occur through
the lengthening gap can occur either soon after removal of the fixator
or years later (Fig. 6-30). Various reports describe fractures through regenerative bone occurring as late as 2 to 8 years after lengthening.318,320,370 The incidence of fractures has been reported to be as high as 50% for Wagner lengthening but only 3% for newer
techniques.148,152,224,315,370,392,416
At present, most lengthenings are performed through the metaphysis,
which has a larger bone diameter and better blood supply than the
diaphysis (where Wagner lengthening was done).315,395
When fractures occur in regenerate bone, they can be treated with
simple cast immobilization. However, because this method further
promotes osteopenia, many surgeons reapply a fixator, correct any
malalignment caused by the fracture, and compress at the fracture site
until healing. To ensure that the regenerate bone can bear the forces
of normal activity, a variety of imaging methods have been used.53,317
When the regenerate bone attains the density and ultrastructural
appearance (development of the cortex and the medullary canal) of the
adjacent bone, fixator removal is generally safe. Some authors have
reported on decreased incidence of fractures combining lengthening with
internal fixation (intramedullary nail or submuscular plating).235,396
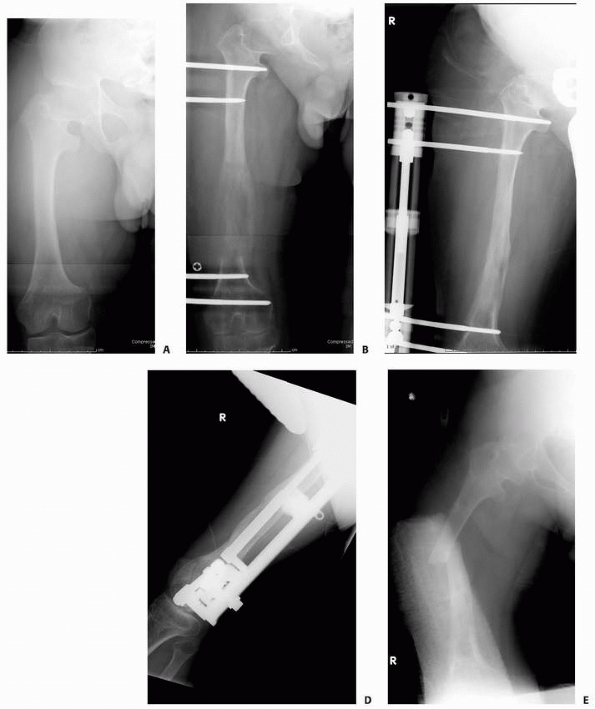 |
|
FIGURE 6-30 Radiograph of a 15-year-old boy with achondroplasia (A) who underwent femoral lengthening with a monolateral external fixator for limb-length discrepancy (B).
The procedure and the lengthening were uneventful and the device was removed after four cortices were visualized on radiographs (C,D). Less than 2 months after external fixator removal, the patient fell and had a pathologic femoral fracture through the regenerate bone (E). (continues) |
and joint contractures that can occur after months in an external
fixation device. Some children, because of pain or anxiety, are
reluctant to bear sufficient weight on their fixator devices, putting
them at risk for disuse osteoporosis. Joint contractures can be related
to either the lengthening itself or insufficient rehabilitation during
and after lengthening. Many of the fractures due to these causes are
avoidable; when they do occur, appropriate immobilization or internal
fixation is used.
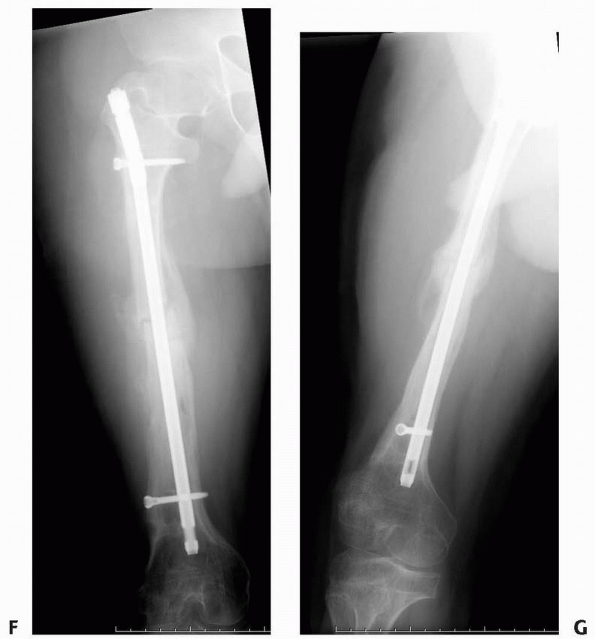 |
|
FIGURE 6-30 (continued)
He underwent open reduction and internal fixation with an intramedullary device and the fracture healed in approximately 3 months (F,G). (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
inherited disorders in which the structure and function of type I
collagen is altered. The fragile bone is susceptible to frequent
fractures and progressive deformity.98,269
OI is identifiable in 1 in 20,000 total births, with an overall
prevalence of approximately 16 cases per million index patients.269,527
The wide spectrum of clinical severity—from perinatal lethal forms to
clinically silent forms—reflects the tremendous genotypic heterogeneity
(more than 150 different mutations of the type 1 procollagen genes
COL1A1 and COL1A2 have been described). Most forms of OI are the result
of mutations in the genes that encode the pro alpha1 and pro alpha2
polypeptide chains of type I collagen.384
As the molecular basis of this continuum of severity is further
elucidated, the phenotypic groupings of the various classifications and
subclassifications may seem arbitrary. However, these classifications
facilitate communication, predict natural history, and help the
clinician plan management strategies.269
From a practical viewpoint of orthopaedic care, patients with OI can be
divided into two groups. One group of patients with severe disease
develops long-bone deformity through repetitive fractures, eventually
requiring open treatment with intramedullary fixation. Another group of
patients has mild disease with frequent fractures, but most of their
injuries respond well to closed methods of treatment and there is less
residual deformity.
marked deformity of the weight-bearing lower extremities, prominence of
the sternum, triangular faces, thin skin, muscle atrophy, and
ligamentous laxity; some develop kyphoscoliosis,46,204,365 basilar impression,349,466 and deafness (due to otosclerosis).205
Despite this multitude of physical problems, children with OI usually
have normal intelligence. Blue sclera, a classic finding in certain
forms of OI, can also be present in normal infants, as well as in
children with hypophosphatasia, osteopetrosis, Marfan syndrome, and
Ehlers-Danlos syndrome.262 Osseous
histologic findings in severe cases reveal a predominance of woven
bone, an absence of lamellar bone, and thinning of the cortical bone
with osteopenia. Children with OI also have a greater incidence of
airway anomalies, thoracic anatomy abnormalities, coagulation
dysfunction, hyperthyroidism, and an increased tendency to develop
perioperative hyperthermia.490
extremity, pain, low-grade fever, and a radiograph showing exuberant,
hyperplastic, callus formation. The callus may occur without fracture
and can have a distinct butterfly shape,262
as opposed to the usual fusiform callus of most healing fractures. The
femur is most commonly involved, but involvement of the tibia and
humerus has been reported.431 The sedimentation rate and serum alkaline phosphatase may be elevated. Because osteosarcoma has been associated with OI,240,265 aggressive-appearing lesions may occasionally require biopsy to confirm their benign nature.
In severe involvement, there is marked osteoporosis, thin cortical
bone, and evidence of past fracture with angular malunion. Both
anterior and lateral bowing of the femur and anterior bowing of the
tibia are common. The long bones may be gracile with multiple cystic
areas. Spinal radiographs may show compression of the vertebrae between
the cartilaginous disc spaces (so-called codfish vertebra). The
presence of wormian bones on a skull radiograph is relatively specific
for OI. Subsequent development of multiple pathologic fractures with
callus and deformity firmly establishes the diagnosis.
radiographic findings. There is no specific laboratory diagnostic test,
although fibroblast cell culture can detect the collagen abnormality in
85% of OI patients.97 In the absence
of multiple fractures, the initial radiographic diagnosis can be
difficult. It is crucial, but often difficult, to distinguish OI from
nonaccidental injury.266,362
Unexplained fractures in mild, undiagnosed OI can drag a family through
unnecessary legal proceedings; conversely, a child with OI may be
abused but not exhibit classic fracture patterns (e.g., corner
fractures) owing to the fragility of their bones. Although no test or
finding is specific, skin biopsy plays an important role.403,486
diaphyseal, and seldom displaced, and they usually heal at a relatively
normal rate in most patients.262,466 Most fractures in patients with OI occur before skeletal maturity. In a series of 31 patients, Moorefield and Miller365
noted 951 fractures, 91% of which occurred before skeletal maturity.
Fractures of the femur and tibia predominate. The humerus is the most
commonly fractured bone in the upper extremity. Multiple long bone
fractures may result in coxa vara, genu valgum, and leg-length
discrepancy. Lateral
dislocation of the radial head has been noted in some patients.262
Olecranon sleeve (apophysis) fractures, which are rare in unaffected
children, are more common in patients with OI, especially the tarda
form (Fig. 6-32).262,490 Zionts and Moon531
reviewed 17 fractures of the olecranon apophysis in 10 children with
mild OI; 15 of these fractures were treated operatively. The same
injury presented in the opposite extremity 1 to 70 months after the
initial fracture in seven of the 10 patients. All fractures had healed
by the time of cast removal; however, two refractured. The authors
concluded that with careful follow-up, cast immobilization can be used
for minimally displaced fractures, but operative management is
suggested for displaced fractures. The high rate of bilateral injury
(70%) suggests that children with OI who sustain this fracture be
counselled about the possible risks of injury to the opposite extremity.531 Also, olecranon fractures in OI children tend to occur at a younger age.200
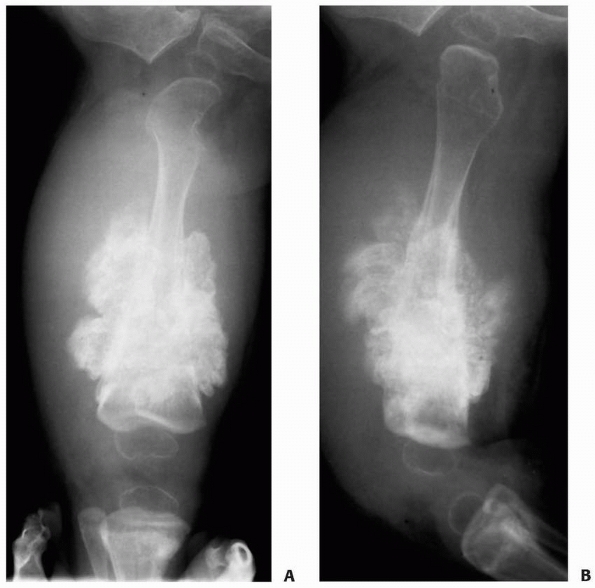 |
|
FIGURE 6-31
This 10-month-old boy with a history of osteogenesis imperfecta presented with a right thigh pain and swelling and refusal to bear weight. Anteroposterior (A) and lateral (B) radiographs of the right femur show the extraordinarily abundant, hyperplastic callus—with the characteristic butterfly shape—that can occur in osteogenesis imperfecta. This appearance may be mistaken for an infection or a neoplastic process. |
 |
|
FIGURE 6-32
A 13-year-old boy with mild osteogenesis imperfecta presented after a fall on an outstretched arm, with inability to move his elbow, pain, and swelling. Radiographs showed a displaced olecranon fracture (A). This fracture pattern is commonly seen in children with osteogenesis imperfecta and is quite uncommon in healthy children. The patient underwent open reduction and internal fixation. The fracture healed after 6 weeks (B). (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
formation is usually adequate and most nonunions seem to be associated
with inadequate fixation and distraction after osteotomies and
fractures.5 Gamble et al.175 emphasized the problem with a report of 12 nonunions in 10 patients. Almost all had type III OI470
and presented with nonpainful clinical deformity and decreased
functional ability. A history of inadequate treatment of the initial
fracture was seen in 50% of these nonunions. One patient eventually
required an amputation for a painful nonunion of a distal femoral
supracondylar fracture.
fractures associated to OI has been well established in the past few
years. Bisphosphonates are a potent inhibitor of bone resorption and
have been used with good results. Among the advantages of using
bisphosphonates are good short-term safety (particularly with regard to
renal function), significant reduction in chronic bone pain, decrease
in the rate of fractures, gain in muscle force, increase in density and
size of vertebral bodies, thickening of bone cortex, and gain in growth
rate.19,184
Some have reported on the negative effects that include decrease in
bone remodeling rate, reduction in growth plate cartilage resorption,
and delay in the healing of osteotomy sites.184
showed that cyclic intravenous administration of pamidronate every 4 to
6 months resulted in a 41.9% increase per year in bone mineral density,
an increase in metacarpal cortical width, and a decrease in fracture
incidence of 1.7 fractures per year. Mobility improved in 16 of the 30
children, and all reported substantial relief of chronic pain. The
results of a study performed by Falk et al.158 supports the findings of Glorieux et al.185
However, they concluded that long-term follow-up is required to
determine whether bisphosphonate therapy will decrease fracture rates
and increase mobility in children with moderate to severe OI.158 Sakkers et al.447
reported a reduction of fracture risk of long bones in children with OI
using oral treatment with olpadronate at a daily dose of 10 mg.
However, the issue of whether bisphosphonates will alter the natural
course of OI remains unresolved.447 Zeitlin et al.530
have also shown that administering cyclical intravenous pamidronate to
children with OI reduces bone pain and fracture incidence and increases
bone density and level of ambulation, with minimal side effects.
Effects on bone include an increase in the size of vertebral bodies as
well as thickening of cortical bone, therefore allowing for more
effective corrective surgery using intramedullary rodding of the long
bones and spinal instrumentation. Specific occupational and
physiotherapy programs are important parts of the treatment procedure.
This multidisciplinary approach will prevail until strategies aiming at
the correction of the basic defect(s) are found.530
most of the mutations in OI are dominant negative, supplying the normal
gene without silencing the abnormal gene may not be beneficial.384
Nonetheless, potential new therapies for OI have been tested in cell
culture systems, animal models, and patients and may offer hope for the
future development of successful therapies.363
balance good, standard fracture care (satisfactory reduction and
casting) with the goal of minimizing immobilization to avoid a vicious
circle: immobilization, weakness, and osteopenia, then refracture.12,269,366 Plaster splints and casts, braces, and air splints have all been used.54,74,173,180,365,466 Protected weight bearing is thought to reduce the incidence of lower extremity fractures.180 Customized splints and braces can add support to limbs weakened by fragile and deformed bone. Letts et al.301
encouraged weight bearing in patients by protecting them with vacuum
pants. The splinting system is a two-layer set of pants with styrofoam
beads between the layers. By evacuating the interval between the
layers, a form-fitting orthosis results, much like the beanbag seating
systems. Both decreased frequency of fracture and increased bone
density were reported after use of this support system.
used for internal fixation of long bone fractures or osteotomies in
children with OI. Plates and screws should be avoided. In patients with
OI, most internal fixation is used for stabilization after corrective
osteotomies. The goals of these osteotomies are to improve function and
reduce fractures in weight-bearing bones by correcting angulation (Fig. 6-33). Porat et al.411
found that the percentage of ambulatory patients in their series went
from 45% to 75% after intramedullary rodding. The amount of bowing that
requires osteotomy has not been defined. In one series,380
the average preoperative bowing was 71 degrees for the femur and 40
degrees for the tibia, but many patients had much less angulation.
Traditionally, multiple osteotomy and rodding procedures (Sofield
technique) involved extensive incisions with significant soft tissue
stripping and blood loss. Sijbrandij469
reported a percutaneous technique in which the deformity is
straightened by closed osteoclasis and Rush pins are inserted along the
proximal axis of the long bones, partially transfixing them to
stabilize them in a new alignment. Most centers now use limited
incisions, thus minimizing blood loss and periosteal stripping, while
ensuring optimally placed osteotomies and efficient, controlled
instrumentation. The choice of fixation device should be based on the
age of the patient and the width of the medullary canal of the bone.
Both fixed-length rods262,410,482 and extensible Bailey-Dubow rods32,33,174,175,380,381,411
are used. Skeletally mature patients and patients with very small
medullary canals are best treated with nonelongating rods, whereas
skeletally immature patients with adequate width of the medullary canal
are best treated with extensible rods.175 Luhmann et al.314
reported a 20-year experience with extensible nails: both overlapping
Rush rods and Bailey-Dubow rods. They first implanted the rods at an
average age of 7 years and averaged more than 5 years before the first
revision. They recommended a posterior position in the canal and using
the stronger overlapping Rush rods technique in the femur whenever the
canal diameter permitted; they advised against using overlapping Rush
rods in the tibia. Malpuri and Joseph370
reported the results of intramedullary rodding of long bones in 16
children with OI over a 10-year period. Sheffield elongating rods or
nonelongating rods were used. The rate of fractures was reduced
drastically after insertion of either type of rod, and the ambulatory
status improved in all patients. With regard to the frequency of
complications requiring reoperations and the longevity of the rods,
Mulpuri and Joseph370 determined results were notably superior after Sheffield rodding.
patients with OI can be treated with open reduction and internal
fixation using two Kirschner wires and tension band technique by
figure-of-eight suture.490 Tibial tubercle avulsion injuries should be treated by surgical stabilization if displaced.
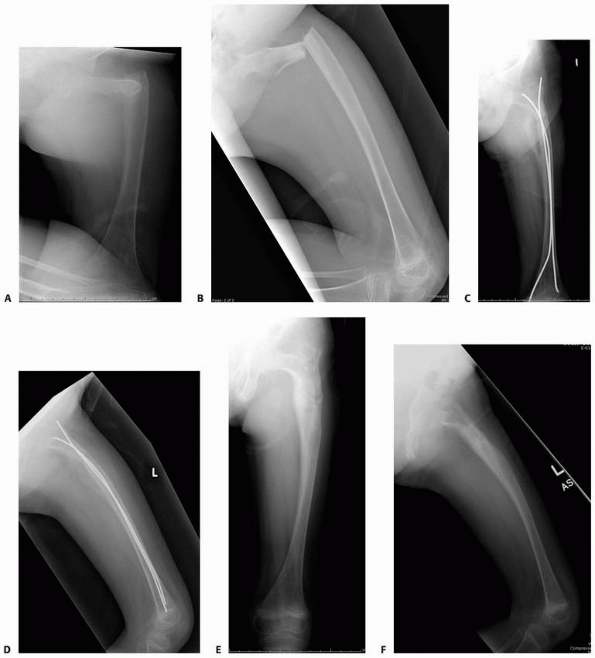 |
|
FIGURE 6-33
This 8-year-old girl presented with pain and deformity around the right hip after minor trauma. The patient had a known history of osteogenesis perfecta. Initial radiographs (A,B) showed grossly displaced fracture of the proximal femur in the subtrochanteric area. The patient underwent closed reduction and internal fixation with titanium elastic nails, and the fracture healed after 5 weeks, with good alignment in both anteroposterior (C) and lateral (D) views. The nails were slightly prominent and the family elected removal of the hardware (E,F). (continues) |
intramedullary fixation in OI. Problems include fracture at the rod
tip, migration of the fixation device, joint penetration, loosening of
components of extensible rods, and fractures through the area of
uncoupled rods. Harrison and Rankin208
compared the complications of 23 extensible rods with those of 27
fixed-length rods. They found that the refracture rate was higher in
the fixed-length rods and fewer surgical interventions were necessary
when extensible rods were used. Gamble et al.174
reported that, although the complication rate approached 69% for
Bailey-Dubow rods (mostly due to loosening of the T-piece), the
fixed-length rods had a complication rate of 55%; however, the
replacement rate for nonelongating rods was 24% but only 12% for the
Bailey-Dubow rods. They recommended crimping the T-piece to the sleeve
and burying it slightly under the bone of the greater trochanter to
prevent displacement. Jerosch239
found a similar 63.5% complication rate for Bailey-Dubow rods but also
thought that they were the best device available. Porat et
al.411
found that the complication rate was 75% for Bailey-Dubow rods and 50%
for nonelongating rods, with a similar percentage requiring reoperation
for both types of nails. Zionts et al.529
reported 40 complications in 40 extensible nailings of 15 children,
finding a much higher complication rate when insertion of the rods was
initiated before 5 years of age. Complications were also higher for
tibial nailing.
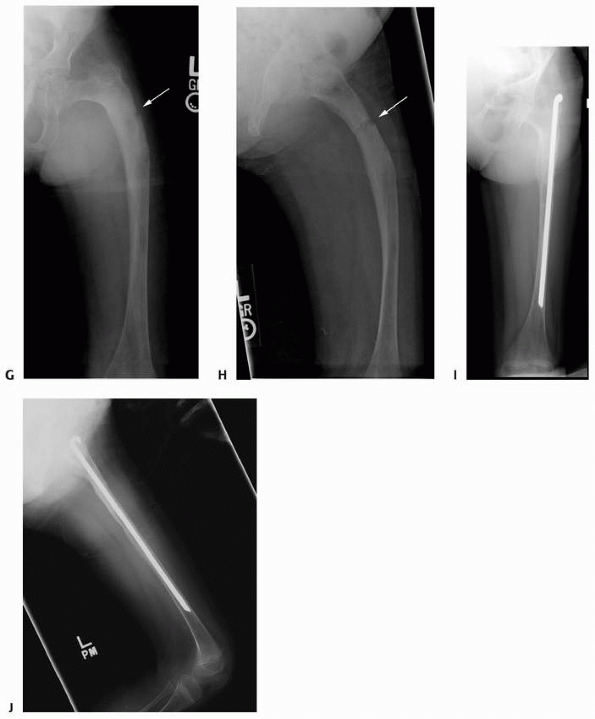 |
|
FIGURE 6-33 (continued)
Three months after removal of the hardware, the patient presented with new trauma to that region followed by pain. Radiographs showed a minimally displaced transverse fracture in the subtrochanteric region (arrow) associated with varus and anterior angulation of the proximal femur (G,H). The patient underwent a Sofield procedure with Rush rods as the internal fixation. Ten weeks after the procedure, there was complete healing at the osteotomy/fracture site and adequate femoral alignment (I,J). (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
Upper extremity fractures should also undergo prolonged splinting after
removal of fracture fixation. Immobilization also may be adequate to
treat stable, minimally displaced fractures just distal or proximal to
the intramedullary rods.175
cause difficulty in both ambulation and transfer. In one series of
nonunions,175 the average age at
diagnosis was close to 10 years and most patients responded to
treatment with intramedullary rods and bone grafting, with healing in
approximately 9 weeks. Nonunion can occur after insertion of rods for
upper extremity fractures. Growth arrest also can follow the use of
intramedullary rods in the lower extremities.
severe OI. Close follow-up is necessary in the first few years of life,
with protective posterior plaster splinting for fractures. Orthoses are
constructed for bracing of the lower extremities to aid in both
standing and ambulation. Standing frames also are used. Once
ambulatory, the child is advanced to the use of a walker or independent
ambulation. Bisphosphonates should be considered at an early age, prior
to fractures and deformity. The length of treatment is still debatable.
Severe bowing of the extremities after recurrent fractures is an
indication for osteotomy and intramedullary rodding. Whenever possible,
surgery is delayed until 6 or 7 years of age. We recommend extensible
rods in skeletally immature patients and nonelongating rods both in
older patients and in younger patients whose canal is not wide enough
for insertion of Bailey-Dubow rods. When possible, we use limited
incisions for insertion of Bailey-Dubow rods to minimize
blood loss and to avoid devascularization of the long bones, which occurs commonly in the so-called open shish-kabob technique.
maximal deformity of the long bones, and a small periosteal incision is
made to permit introduction of a drill bit. After the cortex is drilled
repeatedly, manual osteoclasis completes the osteotomy and the long
bone is straightened. A guide pin is drilled in to the medial edge of
the tip of the greater trochanter, down into the intertrochanteric
femur, and then is tapped distally through the intramedullary canal
through the knee. If callus from an old fracture halts progress, the
guide pin can be drilled through the obstruction and then tapped
farther distally into the middle of the distal flexed knee joint. Next,
the reamer is advanced over the guide pin and the female portion of the
Bailey-Dubow rod is threaded over the guide pin and driven down the
canal. The guide pin is pulled out of the knee joint through a small
arthrotomy incision, and the T-piece is attached to the Bailey-Dubow
sleeve. The T-piece is tapped down into the greater trochanter. The
male portion is then slid into the sleeve to complete assembly of the
expandable rod.
a hip spica cast and then in a brace for 3 to 6 months. When there are
fractures associated with intramedullary rods, these are treated with
either rod revision or immobilization. Every effort should be made to
keep patients ambulatory early after fractures occur to minimize disuse
osteoporosis.
The resultant bone of these children is dense, brittle, and highly
susceptible to pathologic fracture. The incidence of osteopetrosis is
approximately 1 per 200,000 births. The inherent problem is a failure
of bone resorption with continuing bone formation and persistent
primary spongiosa. The disorder classically has been divided into a
severe infantile type and a milder form that presents later in life.
Intermediate forms have been identified in which osteopetrosis presents
as renal tubular acidosis.520 Although the number of osteoclasts present in the affected bone is variable,237,349 in the severe form of this disease, the osteoclasts may be increased but function poorly.462
Osteopetrosis is a heterogeneous group of disorders classified into
three main forms: malignant autosomal recessive, intermediate autosomal
recessive, and benign autosomal dominant.
The spinal column may have a sandwich or “rugger jersey” appearance
because of dense, sclerotic bone at each end plate of the vertebrae and
less involvement of the central portion. The long bones tend to have a
dense, marblelike appearance and may have an Erlenmeyer flask shape at
their ends owing to deficient cutback remodeling. Radiolucent
transverse bands may be present in the metaphysis of the long bones,
and these may represent a variable improvement in the resorption defect
during growth of the child.537 There may be bowing of the bones due to multiple fractures,210 spondylolysis,334 or coxa vara.410
The small bones of the hands and feet may show a bone-within-bone
appearance with increased density around the periphery. The unusual
radiographic appearance may initially obscure occult non-displaced
fractures.
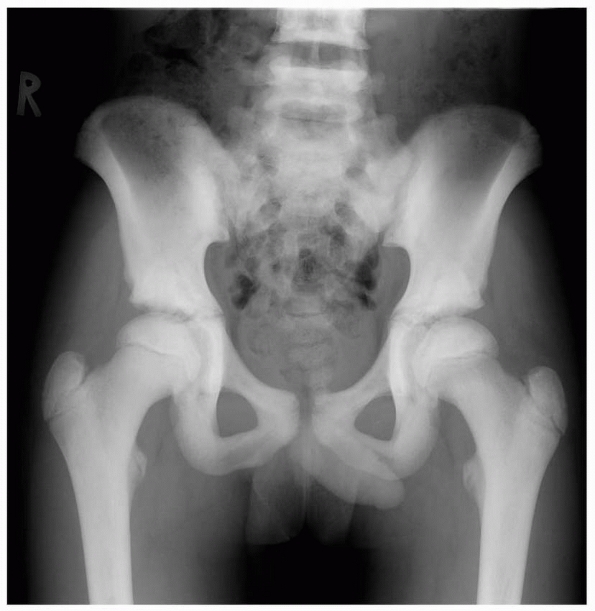 |
|
FIGURE 6-34
Anteroposterior radiograph of the pelvis of an 8-year-old boy with osteopetrosis. Note the typical increased bone density and obliteration of the medullary canal. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
Patients with a severe form of the disease have more fractures than
those with presentation later in childhood. Concurrent blindness can
make patients more susceptible to accidental trauma. Patients with
autosomal dominant osteopetrosis with rugger jersey spine and endobones
of the pelvis (type II) are six times more likely to have fractures
than patients with only sclerosis of the cranial vault (type I).59
transverse or short oblique fractures of the diaphysis, particularly
the femur. Distal physeal fractures with exuberant callus may be
confused with osteomyelitis.357
Common locations for fractures include the inferior neck of the femur,
the proximal third of the femoral shaft, and the proximal tibia.23,357 Although most fractures involve the long bones of the lower extremities, upper extremity fractures also occur frequently.23,210 The onset of callus formation after fracture in osteopetrosis is variable.23,218,237 Although many studies state that fractures in osteopetrosis heal at a normal rate,237,410 others report delayed union and nonunion.23 In a rat model of osteopetrosis, Marks and Schmidt332 found delayed fracture healing and remodeling. Hasenhuttl210
observed that in one patient with recurrent fractures of the forearm,
each succeeding fracture took longer to heal, with the last fracture
taking nearly 5 months to unite.
osteopetrosis should follow the principles of standard pediatric
fracture care, with additional vigilance for possible delayed union and
associated rickets (Fig. 6-36).210,461524 Immobilization is prolonged when delayed union is recognized. Armstrong et al.23 surveyed the membership of the Pediatric Orthopaedic
Society of North America and compiled the combined experience of 58
pediatric orthopaedic surgeons with experience treating pathologic
fractures in osteopetrosis. In this comprehensive review, they
concluded that nonoperative treatment should be strongly considered for
most diaphyseal fractures of the upper and lower limbs in children, but
surgical management is recommended for femoral neck fractures and coxa
vara.
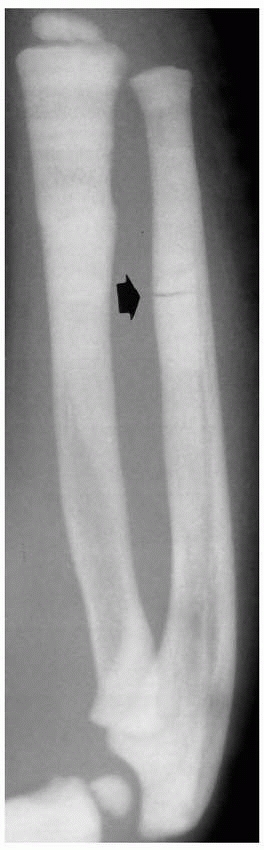 |
|
FIGURE 6-35
This 2-year-old with osteopetrosis presented with forearm pain. An anteroposterior radiograph shows the characteristic increased bone density and absence of a medullary canal, especially in the distal radius and ulna. There is a typical transverse, nondisplaced fracture (arrow) in the distal ulnar diaphysis. |
described insertion of fixation into this bone like “drilling into a
rock.” In intramedullary fixation of femoral fractures, extensive
reaming may be required because the intramedullary canal can be
completely obliterated by sclerotic bone.76 Other authors have found fixation of hip fractures with fixation to be a formidable task,23,357
with damage occurring to the fixation devices on insertion. The bone is
hard enough to break the edges off both chisels and drill bits.
Armstrong et al.23 cautioned, “the surgeon should expect to use several drill bits and possibly more than one power driver.”
with osteopetrosis are at risk for excessive bleeding and infection,
probably related to the hematopoietic dysfunction caused by
obliteration of the marrow cavity.461 Procedures should not be performed unless the platelet count is greater than 50,000 mm3; preoperative platelet transfusions may be necessary.461 Prophylactic antibiotic coverage is advised. Minor procedures should be performed percutaneously whenever possible.461
included transfusions, splenectomy, calcitriol, and adrenal
corticosteroids, but these techniques have proved ineffectual.428,505
Stimulation of host osteoclasts has been attempted with calcium
restriction, calcitriol, steroids, parathyroid hormone, and interferon.
Bone marrow transplantation for severe infantile osteopetrosis has
proved to be an effective means of treatment for some patients;
however, it does not guarantee survival, and it may be complicated by
hypercalcaemia.23,90,94,181,425
is a rare syndrome of short stature and generalized sclerosis of the
entire skeleton. The dense brittle bones of affected children are
highly susceptible to pathologic fractures. Pyknodysostosis is
inherited as an autosomal recessive trait, with an incidence estimated
as 1.7 per 1 million births. Mutations in the gene encoding cathepsin
K, a lysosomal cysteine protease localized exclusively in osteoclasts
is responsible for this disease.171
The long bones are sclerotic with poorly formed medullary canals;
histologic sections show attenuated Haversian canal systems. Patients
with pyknodysostosis have short stature, a hypoplastic face, a nose
with a parrot-like appearance, and both frontal and occipital bossing.
Bulbous distal phalanges of the fingers and toes with spooning of the
nails are common. Coxa vara, coxa valgum, genu valgum, kyphosis, and
scoliosis may be present. Failure of segmentation of the lower lumbar
spine has been reported.431 Results of laboratory studies usually are normal.
that of osteopetrosis. In pyknodysostosis, however, the medullary
canals, although poorly formed, are present and a faint trabecular
pattern is seen. Such sclerotic bone is also seen in Engelmann’s
disease, but clinically those patients are tall and eventually develop
muscle weakness. The distal femur in a patient with pyknodysostosis
usually has an Erlenmeyer flask deformity similar to that found in
patients with Gaucher disease.47
common in pyknodysostosis than in OI, almost all patients with
pyknodysostosis reported in the literature have had pathologic
fractures.40 By age 22 years, one patient had sustained more than 100 fractures.138 The fractures are usually transverse and diaphyseal, and heal with scanty callus.354
The fracture line can persist for nearly 3 years after clinical union,
with an appearance similar to a Looser line. Lower extremity fractures
are the most common,138 and clinical deformity of both the femur and tibia is frequent.
with long-term follow-up suggests that fractures tend to heal readily
in childhood, but nonunion can be a problem in adulthood. Edelson et al.142
reported 14 new cases of pyknodysostosis from a small Arab village.
They described a hangman fracture of C2 in a 2-year-old child that went
on to asymptomatic nonunion. There was 100% incidence of spondylolysis
in their patients aged 9 years or older, with
most
located at L4-L5. None of the spondylolytic lesions showed uptake on
technetium 99m bone scan. Treatment was conservative, with one patient
with symptomatic spondylolysis responding to bed rest.
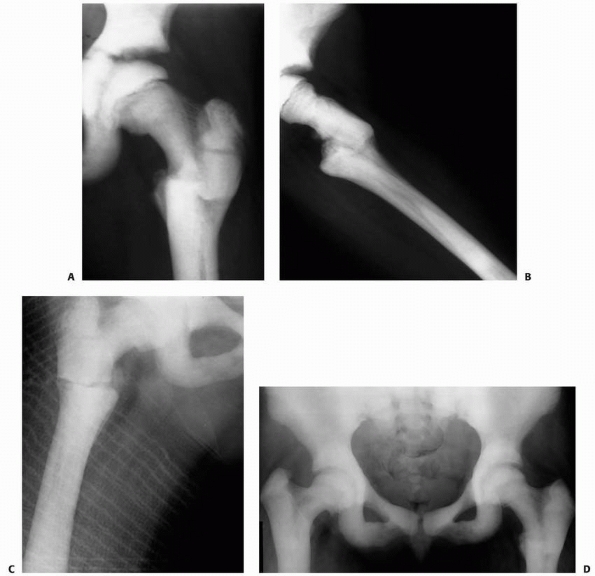 |
|
FIGURE 6-36 A.
This 9-year-old with osteopetrosis sustained similar bilateral subtrochanteric fractures of the femur over a 2-year period. Anteroposterior (A) and lateral (B) femoral radiographs show a healing transverse subtrochanteric fracture of the left femoral. C. One year later, at age 10, she sustained a similar right transverse minimally displaced subtrochanteric femur fracture, which was treated with reduction and a spica cast. D. This anteroposterior radiograph taken at age 14 years shows that both proximal femoral fractures have healed and there is mild residual coxa vara, especially on the right side. |
treated a femoral fracture in an 11-year-old boy with skin traction and
a one-and-a-half hip spica cast. At 6-month follow-up, clinical union
with persistent fracture line was seen. In adults, both plates and
screws and hip screws have been used for proximal femoral fractures.444 Delayed union of tibial fractures has been treated with both compression plating and bone grafting354 and intramedullary nailing with cast immobilization. Roth444
noted that treatment of a hip fracture with fixation was technically
difficult. Cervical immobilization through a Minerva cast and soft
cervical collar has been used for a C2 fracture in a child; the patient
did well, although immobilization was prematurely discontinued.138 Total hip arthroplasty has been performed uneventfully in adults.510
either a deficiency of vitamin D or an abnormality of its metabolism.
The osteoid of the bone is not mineralized, and broad unossified
osteoid seams form on the trabeculae. With failure of physeal
mineralization, the zone of provisional calcification widens and the
ingrowth of blood vessels into the zone is disrupted. In the rickets of
renal failure, the effects of secondary hyperparathyroidism (bone
erosion and cyst formation) are also present. Before
widespread
fortification of common foods, vitamin D deficiency was a common cause
of rickets, but other diseases affecting the metabolism of vitamin D
have become a more common cause. Regardless of the underlying cause,
the various types of rickets share similar clinical and radiographic
features (Fig. 6-37). Although many of the metabolic findings are the same, there are some differences.
 |
|
FIGURE 6-37
Lower extremity radiograph of an 18-month-old boy with rickets. Note the severe bowing, physeal irregularities and widening with flaring of distal tibial metaphysis. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
can occur in rickets. The treatment of rickets depends on
identification of the underlying cause. In addition to nutritional
rickets, many diseases of the various organ systems can affect vitamin
D metabolism, and their treatment is necessary before the clinical
rickets can be resolved (Table 6-9).
|
TABLE 6-9 Rickets: Metabolic Abnormalities
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
sunlight can lead to a vitamin D deficiency. Pathologic fractures from
vitamin D deficiency rickets also occur in children on certain diets:
unsupplemented breast milk,289 diets restricted by religious beliefs,31 and fat diets.143
Fractures are treated with both cast immobilization and correction of
the vitamin deficiency by oral vitamin D supplementation. Oral calcium
supplements also may be necessary, and patients should consume a
vitamin D-fortified milk source.31
affects intestinal absorption of fat-soluble vitamins (such as vitamin
D), resulting in rickets. Biopsy of the small intestine shows
characteristic atrophy of the villi. Treatment is oral vitamin D and a
gluten-free diet. Infants with short gut syndrome may have vitamin
D-deficiency rickets. This syndrome may develop after intestinal
resection in infancy for volvulus or necrotizing enterocolitis, in
intestinal atresia, or after resection of the terminal ileum and the
ileocecal valve.500 Pathologic fractures have been reported, and treatment is immobilization and administration of vitamin D2 with supplemental calcium gluconate.
With congenital biliary atresia, the bile acids, essential for the
intestinal absorption of vitamin D, are inadequate. By age 3 months,
nearly 60% of patients with biliary atresia may have rickets.267
Intravenous vitamin D is often needed for effective treatment of these
patients. After appropriate surgical correction of the hepatic
syndrome, the bone disease gradually improves (Fig. 6-38). The pathologic fractures that develop in these disorders222 can be treated with immobilization.
restriction of physical activity, cerebral palsy, or other coexisting
morbidities. Also, the use of anticonvulsant therapy can interfere with
the hepatic metabolism of vitamin D and result in rickets and
pathologic fractures.450 Fewer fractures occur in institutionalized patients receiving vitamin D prophylaxis.464
of different sarcomas, can cause hypophosphatemic rickets in children.
The onset of rickets may occur anywhere from 2 to 14
months after chemotherapy and can be corrected with the administration of oral phosphates.495 Other mineral deficiencies such as magnesium (a cofactor for parathyroid hormone) can cause rare forms of rickets.
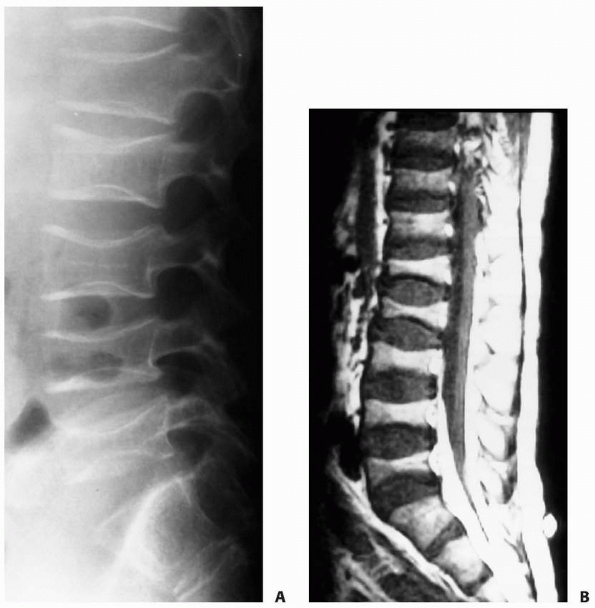 |
|
FIGURE 6-38
This 18-year-old boy with sclerosing cholangitis and a history of steroid use presented with several months of worsening low back pain. A. Lateral radiograph of his lumbar spine shows marked osteopenia, collapsed codfish vertebrae with sclerotic end plates and widened disc spaces and Schmorl nodes. B. This MRI shows flattened concave vertebrae that are smaller in most locations than the adjacent intervertebral discs. He was successfully treated with 3 months in a thoraco-lumbar-sacral-orthosis brace, followed by weaning from the brace and conditioning exercises. |
pathologic fractures. In one study of 12 very-low-birth-weight infants,
the incidence of pathologic fracture was 2.1%, nearly twice the rate of
other premature infants with a birth weight of more than 1500 g.13
The fractures are likely caused by a nutritional osteomalacia that may
evolve into frank rickets in nearly 30% of very-low-birth-weight
infants.13,211,271,272
During the last trimester of pregnancy, the intrauterine growth rate is
exponential—almost two thirds of the birth weight is gained at that
time.434 Eighty percent of both calcium and phosphorus is acquired then.256 Bone loss can be graded by either loss of cortical bone of the humerus413 or loss of bone of the distal radius.271
Other than craniotabes (thinning and softening of the skull bone with
widening of the sutures and fontanelles), the clinical signs of rickets
are generally lacking in these patients.211 The risk factors predisposing these patients to both rickets and fractures include hepatobiliary disease,267,273,495 prolonged total parenteral nutrition,495 chronic lung disease,13 necrotizing enterocolitis,498 patent ductus arteriosus,64 and physical therapy with passive range-of-motion exercises.211,272 In a prospective study of 78 low-birth-weight infants, Koo et al.272
observed a 73% incidence of rickets with associated pathologic
fractures in patients with a birth weight of 800 g or less and only a
15% incidence of rickets with fractures in patients with a birth weight
ranging from 1000 to 1500 g. In most cases, pathologic fractures in
very-low-birth-weight infants are found incidentally on chest
radiograph or gastrointestinal studies. The fractures may be suspected
when physical examination reveals swelling and decreased movement of an
extremity. The differential diagnosis of these fractures is limited but
important: OI, copper deficiency syndrome, child abuse, and pathologic
fracture from overzealous physical therapy.211
Recurrent fractures, physical findings, and a positive family history
are the hallmarks of OI; serum copper levels are useful in establishing
copper deficiency syndrome. Neonatal osteomyelitis may also present a
similar radiographic appearance. If risk factors for infection are
present, the bone lesion should be aspirated and cultured.13
12 (1.2%) of 973 preterm infants had fractures; 11 of 12 had more than
one fracture. Radiographically, osteopenia is first seen at the 4th
week of life. Typically, rib fractures are next seen at 6 to 8 weeks of
life, then fractures of the long bones at 11 to 12 weeks.434
In one study, 54% of fractures were in the upper extremities, 18% in
the lower extremities, 22% in the ribs, and approximately 6% in either
the scapula or the clavicle.272 Most long bone fractures are metaphyseal and may be transverse or greenstick with either angulation or complete displacement.13 Callus is seen at the fracture site in less than a week, and complete remodeling occurs in 6 to 12 months.13,272
Passive range-of-motion exercises for these infants, by both physical
therapists and parents, should be avoided unless it is absolutely
necessary.211 Rib fractures have been associated with vigorous chest physiotherapy.272
Care also should be taken even with routine manipulation of the
extremities during nursing care, and special care should be taken in
restraining the extremities during surgical procedures.272 Splinting is the treatment of choice for pathologic fractures of the long bones in very-low-birth-weight infants.
cardiopulmonary support and hamper nursing care.272
Regardless of the means of immobilization, the prognosis is excellent
for most of these fractures because they go on to complete remodeling
within 12 months; prolonged follow-up is advised. Preventive measures
are important to minimize the risk of fracture in low-birth-weight
infants. Their nutritional need for high levels of calcium, phosphorus,
and vitamin D should be recognized. Alternating high levels of calcium
with low levels of phosphorus in hyperalimentation solutions can help
meet these needs. Because growth arrest is possible after fractures,
follow-up over the first 2 to 3 years of life is advised.
the clinical syndrome is a combination of rickets and secondary
hyperparathyroidism with marked osteoporosis. Affected children present
with short stature, bone pain, muscle weakness, delayed sexual
development, and bowing of the long bones.87 The underlying renal disease may be chronic nephritis, pyelonephritis, congenitally small kidneys, or cystinosis.479
Identification of the renal disorder is important because patients
presenting with rickets due to obstructive uropathy may respond to
surgical treatment of the renal disease.
showed that periods of metabolic instability, characterized as an
alkaline phosphatase of 500 U for at least 10 months, were associated
with progression of deformity. With the adolescent growth spurt,
osseous deformities can accelerate rapidly over a matter of weeks.113
Osteoclastic cysts (brown tumors) may form. Metaphyseal cortical
erosions occur in the lateral clavicle, distal ulna and radius, neck of
the humerus, medial femoral neck, medial proximal tibia, and middle
phalanges of the second and third fingers.230 The proximal femur may become so eroded with tapering and thinning that it has been likened to a rotting fence post.498
In renal osteodystrophy, the Looser zone may represent a true stress
fracture and, with minor trauma, may extend across the full thickness
of the bone with development of a true fracture (Fig. 6-39).
Callus may be scanty in patients with fractures who have untreated
renal disease, but in patients on hemodialysis, abundant callus may
form at the fracture site.399
Phalangeal quantitative ultrasound may be a useful method to assess
bone quality and fracture risk in children and adolescents with bone
and mineral disorders.38
long bones, rib fractures, vertebral compression fractures, and
epiphyseal displacement of the epiphyses occur frequently. Fractures
occur in areas of metaphyseal erosion or through cysts. Immobilization
is used to treat pathologic fractures through both generalized weakened
bone and brown tumors. Once the underlying bone disease is under
control, open procedures such as curettage of cysts with bone grafting
and open reduction of fractures may be considered when appropriate.399 Internal fixation is preferable to external fixation.89
Preoperative tests needed for these patients before surgery include
electrolytes, calcium, phosphorus, and alkaline phosphatase. Before
surgery, these patients may need dialysis, phosphate adjustment, either
medical or surgical correction of hyperparathyroidism, or chelation
therapy for aluminium toxicity. Supplemental vitamin D should be
discontinued or the dose halved 2 to 4 weeks before any procedure that
may require immobilization.87 It is
important to rule out dental abscess, which may be present in as many
as 25% of patients with vitamin D-resistant rickets.350 Postoperative infection may be more common in patients who are on corticosteroid therapy after renal transplantation.376 Prophylactic antibiotics are highly recommended for surgery of all patients with renal osteodystrophy.154
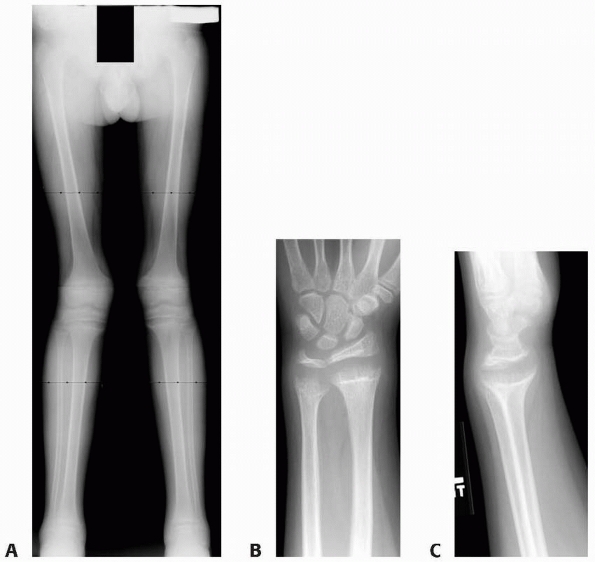 |
|
FIGURE 6-39
This 12-year-old girl with rickets associated with chronic kidney disease presented with complaints of knocked knees and wrist pain. Hip to ankle radiographs (A) showed typical rickets changes with valgus deformity at the knee level. Looser lines around the distal femur, and physeal widening. Wrist images (B,C) demonstrated marked physeal widening and metaphyseal flare of the distal radius and ulna. (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
Sites of involvement include the distal femur, proximal femur, and
proximal humerus, the heads of both the metatarsals and metacarpals,
and the distal radial and ulnar epiphyses, which tend to displace in an
ulnar direction.264 In the proximal femur, both femoral neck fractures188
and slipped capital femoral epiphysis occur. Possible explanations for
displacement of the proximal femoral epiphysis include metaphyseal
erosion with subsequent fracture,188,264
and a layer of fibrous tissue that forms between the physis and the
metaphysis because of the destructive effects of the renal
osteodystrophy.276 The warning signs
and risk factors for slipped capital femoral epiphysis in renal
osteodystrophy include subperiosteal erosion of the medial femoral
neck, increasing width of the physis, bilateral coxa vara, male gender,
and an age between 10 and 20 years (Fig. 6-40).188
With erosion of the cortex of the inferior medial femoral neck, the
femoral head collapses, decreasing the neck shaft angle, and subjecting
the physis to shear forces as it assumes a vertical
orientation. The slip is bilateral in up to 95% of the patients and is usually stable.313,389
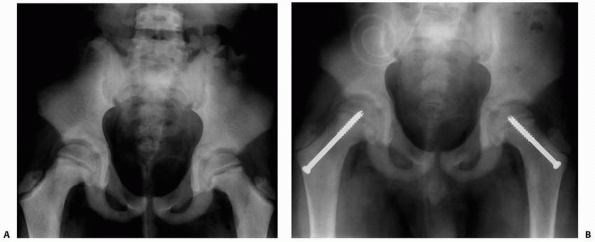 |
|
FIGURE 6-40 This 13-year-old boy with renal osteodystrophy presented with bilateral hip and thigh pain. A.
Anteroposterior pelvic radiograph shows widening of the proximal femoral physes with sclerosis. Slipped capital femoral epiphyses were diagnosed. B. This anteroposterior pelvic radiograph taken 9 months after surgery shows narrowing of the physis and no evidence of further displacement of the capital femoral epiphyses. |
calcitriol, hemodialysis, renal transplantation, and parathyroidectomy,
has improved the long-term survival and quality of life for these
patients. Temporary limitation of weight bearing is recommended if
there is little metaphyseal erosion, minimal coxa vara, and fusion of
the physis is expected within 1 to 2 years.399 If the primary disease is not readily treated, progression of the displacement will occur.313,389 With continuing slippage after medical treatment, most authors recommend in situ fixation.87,188,313,383,388
However, continuing displacement of the proximal femoral epiphysis may
occur even after pinning, because the fixation holds poorly, possibly
because the wide radiolucent zone of the femoral neck in this disorder
is not true physis, but rather poorly mineralized woven bone and
fibrous tissue.350 In a very young
child, threaded pin fixation of the proximal femoral epiphysis may
result in growth abnormality with trochanteric overgrowth.
temporarily until medical treatment resolves the underlying bone
disease and avoids definitive physeal closure.313 For patients younger than 5 years, Hartjen and Koman209
recommended treatment of slipped capital femoral epiphysis with
reduction through Buck traction and fixation with a single specially
fabricated 4.5-mm cortical screw. The distal threads of the screw were
machined off so that only the smooth shank of the screw extended across
the physis. Subtrochanteric osteotomy with fixation or total hip
arthroplasty may be necessary in older patients with severe coxa vara
after slipped capital femoral epiphysis.87,188
noted the contribution of aluminium toxicity to the development of
fractures in renal osteodystrophy. Because phosphorus restriction is
important in children with renal disease, aluminium hydroxide has been
commonly used as a phosphate binder.16
Aluminium intoxication causes defective mineralization. Multiple
pathologic fractures may occur with poor healing. Serum aluminium
levels are not diagnostic, but the use of deferoxamine, a chelation
agent, in an infusion test may provide the diagnosis.362
A bone biopsy is often necessary. After treatment of the renal disease
with correction of the aluminium toxicity by chelation agents, acute
fractures will heal. Severe bowing of the long bones due to fractures
can be treated with multiple osteotomies with intramedullary Rush rod
or plate fixation.389 Recurrence of the syndrome is prevented by use of aluminium-free phosphate-binding agents such as calcium carbonate.449
the most important aspect in the care of all of these injuries. Slipped
capital femoral epiphysis may be the first presenting sign of renal
failure.188 A slipped capital
femoral epiphysis should be stabilized with in situ screw fixation in
older children if progression is noted despite medical treatment.
Multiple screws should be considered because the underlying metaphyseal
bone is quite soft. For treatment of progressive slipped capital
femoral epiphysis in very young children, some form of unthreaded
fixation seems most logical. Most fractures of the long bones respond
readily to cast or splint immobilization, with concurrent aggressive
medical treatment of the underlying metabolic disease. Femoral neck
fractures are treated with anatomic reduction and internal fixation.
The underlying bone disease should be medically treated to ensure
success of open procedures. Significant cysts should be treated with
curettage and bone grafting. Angular deformities of the long bones
should be corrected when the patient is close to maturity.
either congenital disease such as osteogenesis imperfecta or metabolic
disorders such as Cushing syndrome. Rarely, children develop idiopathic
osteoporosis with pathologic fractures. Idiopathic osteoporosis is
characteristically seen 2 years before puberty, but age at presentation
may range from 4 to 16 years.478 Unique metaphyseal impaction fractures are a hallmark of this disorder.226
It usually presents with bone pain, deformities, and fractures. Biopsy
specimens show a quantitative decrease in the amount of bone that has
been linked to both increased resorption241 and primary failure bone formation.478 Osteoblasts in this disorder seem to function normally when stimulated by oral 1,25-hydroxyvitamin D3.49
The etiology in healthy children is likely multifactorial and
incompletely understood. Poor calcium intake during the adolescent
growth spurt may play some role. Symptoms can persist for 1 to 4 years
after diagnosis, with spontaneous resolution in most patients after the
onset of puberty. The only consistent metabolic abnormality is a
negative calcium balance with high rates of fecal excretion of calcium.226 This finding supports the hypothesis that idiopathic juvenile osteoporosis results from intestinal malabsorption of calcium.118
suggested a pathogenetic model for idiopathic osteoporosis in which
impaired osteoblast team performance decreases the ability of
cancellous bone to adapt to the increasing mechanical needs during
growth. The result of this impairment is load failure and fractures.425
only complaint, the most severely affected present with generalized
skeletal pain.117,241,478
Patients have difficulty walking, and their symptoms may be initiated
by mild trauma. In a review of 40 patients with idiopathic
osteoporosis, Smith478 observed that
87% had vertebral fractures and 42% had metaphyseal fractures. Symptoms
of back or extremity pain can predate fractures by 6 months. Generally,
30% of bone mass must be absent before osteoporosis is detected on
radiographs.284 Serum calcium, phosphorus, and alkaline phosphatase levels are usually normal.226
Low plasma calcitriol, a vitamin D metabolite that aids calcium
absorption in the gut, has been observed in juvenile osteoporosis.333
Some authors have noticed that it is mostly a disorder of cancellous
bone, reflecting a decreased modelling activity on the endocortical
surface of the internal cortex.426
osteoporosis in children include the usually difficult interpretation
of bone densitometry and turnover markers and poorly established
guidelines regarding prevention and treatment of bone fragility.
Prospective studies are needed to establish the safety, efficacy, and
optimal drug, duration, and dosage for improving bone quality in
otherwise healthy children. Most authors believe that the bone health
during the first two decades contributes to the lifetime risk of
osteoporosis.30
central areas of the vertebral bodies, and clarity of the dense
vertebral end plates is increased. The long bones lose trabecular
anatomy and show thinning of the cortex.226,473
Once symptoms begin, a mildly lucent area of newly formed bone, a
so-called neo-osseous porosis, is observable in the metaphysis (Fig. 6-41). This is considered weaker than the surrounding bone, which formed before onset of the disease.478
Spinal cord compression has also been reported with vertebral fractures
of osteoporosis in a child. Metaphyseal fractures can start as hairline
cracks that gradually extend across the width of the shaft, and with
further collapse in the femoral shaft, the cracks may telescope into
the distal femur, with later distortion of the femoral condyle.226 Tibial and femoral shaft fractures may heal with bowing. Long bone shaft fractures are either transverse or oblique,226 and the callus formed seems to be normal.226,332 A technetium bone scan may be useful in showing healing fractures that are not obvious on plain radiograph studies.332 No clear-cut effective medical treatment has been found for idiopathic juvenile osteoporosis.189,226,241
Many patients have been treated by both vitamin D and calcium
supplements with equivocal benefit, and usually mineralization of the
skeleton does not improve until puberty, when the disease spontaneously
resolves. Low-dose pamidronate appears promising in the treatment of
childhood osteoporosis.176
summarized the treatment of fractures in juvenile osteoporosis when
they stated that these fractures should undergo anatomic reduction with
immobilization “as little as practical.” They noted both severe
deformity of the long bones and pseudarthrosis when the fractures could
not be immobilized. The bones usually are so soft that they are thought
to be unsuitable for the usual forms of fixation, but femoral neck
fractures in this disorder have been treated with internal fixation.226
undergoing cancer therapy. The cause of reduced bone mineral density is
multifactorial. The disease itself may play a role (e.g., acute
lymphoblastic leukemia, malignant lymphomas, brain tumors, malignant
bone tumors, rhabdomyosarcoma), but specifically the treatment
including corticosteroids, chemotherapy (such as methotrexate,
ifosfomide) and radiation (such as brain radiation that can reduce
growth hormone secretion and cause hypogonadotropic hypogonadism), all
contribute to the development of osteoporosis.448,504 Methotrexate, for example, is believed to inhibit osteogenesis, causing both delayed union and nonunion of fractures.420 The incidence of pathologic fractures after methotrexate use ranges from 19% to 57%.285,420,484
with marked radiolucency of the metaphyseal regions of the long bones.
Radiographic changes in the metaphysis and epiphysis resemble those
seen in scurvy.420 Minimally
displaced transverse fractures occur in the long bones of both the
upper and lower extremities and the small bones of the feet.420,484 Schwartz and Leonidas456
cautioned that stress fractures of the long bones that can occur after
methotrexate therapy can be mistaken for recurrence of leukemia. If
feasible from an oncologic viewpoint, methotrexate should be
discontinued to allow these fractures to heal in a cast. The cast
immobilization itself may result in additional osteopenia and fractures
even though methotrexate is discontinued.456 Persistent nonunion requires open reduction and internal fixation with bone graft.484 Patients
with severe osteoporosis and bone pain without fracture also respond to a halt in methotrexate therapy.420 Prevention is the key and physical activity, adequate vitamin D intake, and sometimes bisphosphonates are some of the options.448,504
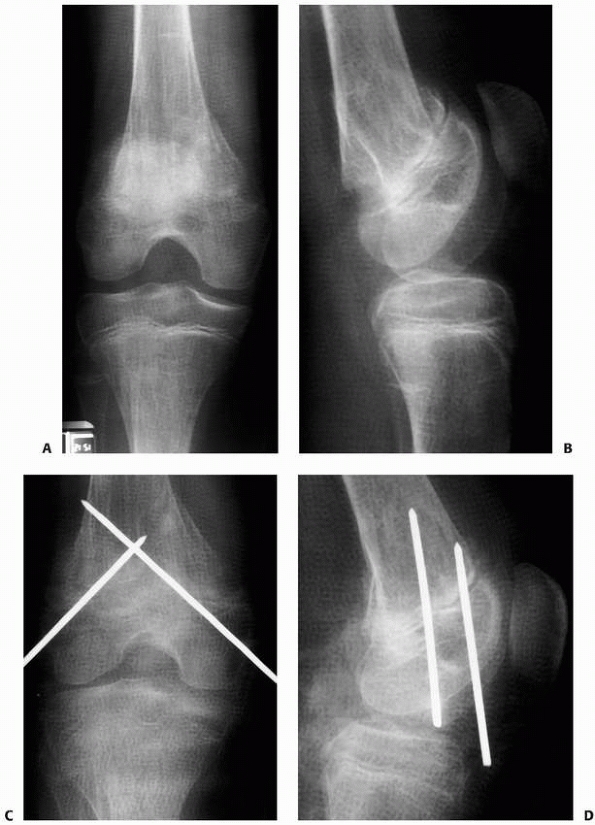 |
|
FIGURE 6-41 A.
Multiple pathologic fractures in a previously healthy teenage boy who developed idiopathic osteoporosis. This anteroposterior radiograph of the right knee and this lateral radiograph (B) demonstrate a displaced distal femoral metaphyseal fracture with apex posterior angulation. C. This was treated with closed reduction and percutaneous pinning and application of a cast. D. This lateral radiograph shows satisfactory alignment with the pins in place. (continues) |
can result in loss of as much as a 44% of mineralization of trabecular
bone. In some patients, osteoporosis may persist for 6 months after
injury.149 Immobilization leads to bone resorption, especially in unstressed areas.285 In one study,149 bone density of the distal radius returned to normal in all patients at 1-year follow-up. Nilsson and Westlin382
found a residual decrease in bone mineralization of the distal femur of
7% at nearly 11 years of follow-up in a study of 30 patients.
Persistent osteoporosis after cast immobilization for fracture can
contribute to refracture.
rare. Although the exact incidence remains unknown, it results from
hyperplasia of the parathyroid gland. Symptoms are associated with high
serum calcium and inappropriate parathyroid hormone level, causing
increased osteoclastic activity, leading to general demineralization of
the skeleton and hypercalcemia. In severely affected patients, osteitis
fibrosa cystica may develop with fibrous tissue replacement of bone and
formation of cysts. In a large retrospective study, among the 44
children and adolescents, ranging in age from 6 to 18 (mean 13) years,
83% were
symptomatic and 43% had nephrolithiasis. Two had multiple endocrine neoplasias.322
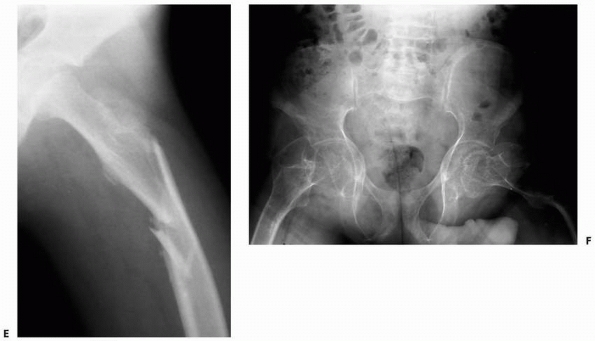 |
|
FIGURE 6-41 (continued) E. A few months later, he sustained a left proximal femoral fracture, which was treated with a spica cast. F.
This anteroposterior pelvic radiograph taken 3 years later shows healed proximal femoral fractures with varus angulation and severe osteopenia of the pelvis and femora with profusion of both acetabuli. |
hyperparathyroidism seen in infants is congenital primary
hyperparathyroidism, which results from an autosomal recessive trait140
and is lethal without parathyroidectomy. These patients present with
respiratory difficulty, hypotonia, poor feeding with constipation, and
failure to thrive.423 Serum calcium
is markedly increased in most patients, but a gradual rise above normal
serum levels may occur in some infants with serial measurements.421
Radiographs reveal demineralization of the skeleton. Marked resorption
is present in the femoral necks and distal tibiae, with decreased
trabeculae and poorly defined cortices.140 Periosteal elevation is common, and when it is severe, the long bones may actually look cloaked with new bone (Fig. 6-42).
Periosteal resorption of the bone of the middle phalanges is believed
to be characteristic of this disease. Brown tumors are rare in infancy.
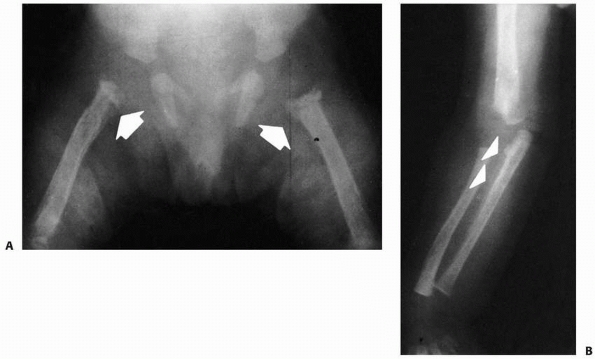 |
|
FIGURE 6-42 A.
Newborn with hyperparathyroidism. There is marked demineralization of bone, and marked resorption is present in the proximal femurs (arrows). B. Periosteal elevation is present along the ulna (arrows). (Courtesy of Bruce Mewborne, MD.) |
presentation is subtler. Weakness, anorexia, and irritability are
present in 50% of patients, and constipation is present in 28%.52
Renal calculi also are present in 25% of patients, and polyuria,
excessive thirst, bone pain, abdominal distension, pancreatitis, and
swelling of the knees are occasionally present.18,52,112 Approximately 50% of older patients have osteopenia and other osseous
signs of hyperparathyroidism.52 The serum calcium is only moderately elevated in many patients, but 24-hour urine calcium excretion is abnormally high.52,400
If the diagnosis is uncertain, selective venous catheterization for
parathyroid hormone can be done, localizing the gland by either
ultrasound, CT, or MRI.55
are rare in infancy. Increased levels of parathyroid hormone results in
decreased function and numbers of osteoblasts, and hence delayed union
of pathologic fractures,283 but this problem has only been reported in adults; healing occurred after parathyroidectomy.237
Most fractures are successfully treated with simple immobilization.
Occasionally, a fracture through a cyst or brown tumor requires
curettage and bone grafting after a period of initial healing.522
disorder that is most frequently caused by pituitary or adrenocortical
tumors, resulting in excessive production of cortisol and its related
compounds. If the hyperactivity of the adrenal cortex is due to
pituitary gland stimulation, the syndrome is most precisely known as
Cushing disease.108 In children, hypercortisolism is most often caused by carcinoma, adenoma, hyperplasia of the adrenal cortex,340 Ewing sarcoma,414 or exogenous corticosteroid therapy. The elevated adrenal corticosteroids inhibit the formation of osteoblasts,207 resulting in increased resorption of the bone matrix and decreased bone formation.242
stature with excessive weight gain, moon facies, presence of a buffalo
hump, hirsutism, weakness, and hypertension.340 Cutaneous striae are rare, and the genitalia are of normal size. Mortality is well over 50%.340
In older children, the clinical picture is somewhat different: truncal
obesity, short stature, a lowered hairline, acne, weakness, emotional
lability, hirsutism, cutaneous striae, hypertension, and ecchymosis.
Radiographic findings may include severe osteopenia and a retarded bone
age. Fractures of the ribs, vertebrae, and long bones have been
reported in children with Cushing syndrome.341
In terms of diagnostic studies, it has been shown that a single
cortisol value at midnight followed by overnight high-dosage
dexamethasone test led to rapid and accurate confirmation and
diagnostic differentiation, respectively, of hypercortisolemia caused
by pituitary and adrenal tumors.41
The associated fractures usually can be treated with standard
immobilization techniques, but care should be taken not to increase the
extent of osteopenia through excessive immobilization. In patients on
corticosteroid therapy, the dose should be reduced, converted to an
alternate-day schedule, or discontinued, if possible.414
Also, children and adolescents who have Cushing syndrome may have
significant alterations in body composition that result in a small but
significant decrease in bone mass and increase in visceral adiposity.
Long-term monitoring of body fat and bone mass should be mandatory
after treatment.299
amounts of fresh fruit or vegetables leading to depletion in vitamin C.
It takes up to 6 to 12 months before symptoms arise, including
asthenia, vascular purpura, bleeding, and gum abnormalities. In 80% of
cases, the manifestations of scurvy include musculoskeletal symptoms
consisting of arthralgia, myalgia, hemarthrosis, and muscular hematomas.157
Because vitamin C is essential for normal collagen formation,
deficiency of the vitamin results in defective osteogenesis, vascular
breakdown, delayed healing, and wound dehiscence.197
Children experience severe lower limb pain related to subperiosteal
bleeding. Although scurvy is often due to a dietary deficiency of
vitamin C,197,377,391
both aspirin and phenytoin are associated with decreased plasma levels
of ascorbic acid. Vitamin C deficiency also is present in patients with
myelomeningocele,346 although its
contribution to fracture in that population is unclear. Infants with
scurvy may present with irritability, lower extremities tenderness,
weakness, pseudoparalysis, and possibly bleeding gums (if teeth have
erupted). Subperiosteal hemorrhages may exist as well as hemorrhage
into the subcutaneous tissues, muscles, urinary system, and
gastrointestinal tract.293 Anemia is
also a common finding. In developing countries, older children with
scurvy presenting with inability to walk may be misdiagnosed as having
poliomyelitis.422
osteonecrosis, osteopenia, and/or periosteal proliferation. Trabecular
and cortical osteoporosis is common.157
Profound demineralization is evident. In advanced disease, the long
bones become almost transparent with a ground-glass appearance and
extreme thinning of the cortex. Calcium accumulates in the zone of
provisional calcification adjacent to the physis and becomes densely
white (Fränkel line). Fractures generally occur in the scurvy line
(Trummerfeld zone)—the radiolucent juxtaepiphyseal area above Fränkel
line where the matrix is not converted to bone. Dense lateral spurs,
known as the Pelken sign, may be seen.195
A characteristic finding of scurvy is the corner sign in which a
peripheral metaphyseal defect exists where fibrous tissue replaces
absorbed cortex and cartilage.35
Cupping of the metaphysis is common in both scurvy and rickets; in
rickets, the metaphysis is ragged, whereas in scurvy, the metaphysis is
sharply outlined.195 The epiphysis
becomes ringed with a thin, dense line (Wimberger sign). The periosteal
elevation caused by hemorrhage calcifies within 10 days of treatment
with vitamin C (Fig. 6-43).
The most common sites of fracture, in order of frequency, are the
distal femur, proximal humerus, costochondral junction of the ribs, and
distal tibia.195 Fractures of the
long bones generally are nondisplaced metaphyseal buckle fractures with
mild angulation. In contrast, marked epiphyseal displacement occurs
with a moderate amount of callus present even in untreated patients.
Exuberant callus forms once vitamin C is administered. Standard
immobilization, with administration of vitamin C, is adequate for most
fractures. Remodeling potential is high in these patients.457
Even healed fractures that appear to have undergone growth arrest
should be observed, because the potential for continued growth with
medical treatment of the vitamin C deficiency can be nearly normal.471 For infants who are older than 12 months
of age and have begun weight bearing, spine films are recommended to rule out vertebral fractures.316
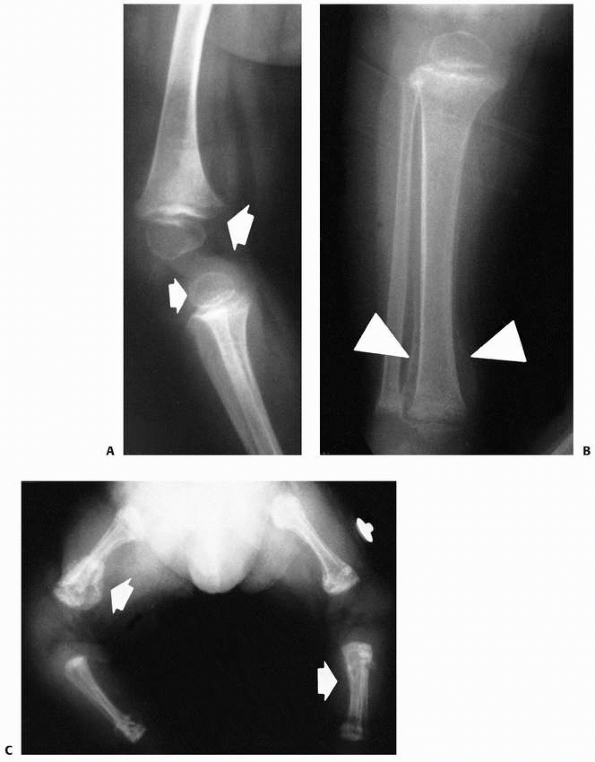 |
|
FIGURE 6-43 Scurvy. A.
A 10-month-old boy presented with a 2-week history of refusal to walk with tenderness of the lower extremities. He had a history of milk and cereal intake only. There are signs of scurvy in the metaphysis (large arrow). The dense white line in the zone of the provisional calcification of the distal femur is known as the Fränkel line. The radiolucent juxtaepiphyseal line above the white line is known as the scurvy line. The peripheral metaphyseal defect, where fibrous tissue replaces absorbed cortex in cartilage, is known as the corner sign. Wimberger sign is a thin, dense line surrounding the epiphysis (small arrow). B. This is a child with healing scurvy. There is marked periosteal calcification around the distal tibia (arrows). C. A newborn with scurvy. Periosteal hemorrhage has become calcified in the bones of the lower extremity (arrows). (Courtesy of Bruce Mewborne, MD.) |
reported on a 14-month-old girl with scurvy with bilateral distal
femoral epiphysis displacement. This condition resolved after treatment
with vitamin C, but limb-length discrepancy developed on one side.415
In two patients with distal femoral fractures, healing went on to
cupping of the metaphysis with an appearance similar to that in central
growth arrest.347,472
of collagen. Copper deficiency results in a decreased number of
collagen crosslinks, with adverse effects on both bone and blood
vessels.196 Copper deficiency can occur by 3 months in low-birth-weight infants214
and after prolonged total parenteral nutrition. Copper deficiency can
also develop as a result of excessive supplemental zinc ingestion.65
Another cause of copper deficiency is disruption of one gene on the X
chromosome causing a defect in the process of copper absorption, with
consequent deficiency of available copper at the cellular level,
resulting in abnormalities of collagen formation and brain maturation,
leading to early death.512
those who are primarily milk fed and are on semistarvation diets with
concurrent vomiting and diarrhea.102 Both rib and wrist enlargement are frequent,196 and neutropenia is common.214 The diagnosis is commonly based on clinical presentation and decreased levels of serum copper.
chromosome 13 is associated with accumulation of excess copper in the
body, initially in the liver and brain. With time, copper
accumulates
in the kidneys, causing renal damage and osteoarticular changes (e.g.,
osteoporosis, osteomalacia, and pathologic fractures).512
very similar to those in rickets, including metaphyseal cupping,
flaring, demineralization of the skeleton, and subperiosteal elevation
with calcification.488 There are some radiographic differences between scurvy and copper deficiency syndrome.196
The corner sign is frequently absent in copper deficiency, the
metaphyseal spurs are not strictly lateral but sickle shaped, and
radiolucent bands of the metaphysis are absent. Bone age also is
frequently retarded. Pathologic fractures have been reported in copper
deficiency syndrome. Cordano et al.123
noted prompt healing of a distal femoral fracture in an infant, but the
fracture recurred before treatment of the copper deficiency. Such
injuries can be treated like those in scurvy, with simple
immobilization and concurrent correction of the copper deficiency.
cerebral palsy (CP) may develop osteoporosis. The main causes of low
bone density and osteoporosis in children and adolescents with CP are
lack of activity, nutritional issues, and pharmacologic treatments
(e.g., anticonvulsivants drugs).408
documented 134 extremity fractures, primarily in quadriplegics. When
the mechanism of injury was known, most of these fractures were the
consequence of a fall, often associated with seizure activity.
Approximately 46% of these fractures involved the femoral shaft, 6%
were fractures of the head or neck of the femur, 15% involved the tibia
and fibula, and 13% were humeral fractures. These authors believed that
contracture or paralytic dislocation of the hip joint predisposed these
patients to femoral fractures.
reported on 156 children with CP who were treated for fractures. The
mean age at the time of the first fracture was 10 years; 66% of
patients had spastic quadriplegia, of those 83% were nonambulatory.
Most fractures (82%) occurred in the lower limbs. The risk factors for
fracture were nonambulatory CP child on anticonvulsant therapy,
resulting in a high incidence of low-energy fractures.415
children with moderate-to-severe motor impairment, the rate of fracture
was 4% per year. Children with greater body fat, feeding gastrostomy,
and history of fracture were at highest risk of fractures.489 Leet et al.297
reported on 418 children with CP: 243 (58%) had quadriplegia, 120 (29%)
had diplegia, and 55 (13%) hemiplegia. Of these, 366 were spastic, 23
mixed tone, 13 athetoid, and 16 classified as others. Pathologic
fractures were seen in 50 children (12%). Older age at first fracture
and use of valproic acid were predictive of fractures and defined a
group of children who may benefit from treatment interventions to
increase bone density.297
emphasized that spontaneous fractures can occur in patients with CP
without episodes of trauma, and factors such as disuse atrophy,
nutritional deficiencies, and pre-existing joint contractures
contributed to these injuries. Nearly 50% of the full-time bedridden
patients they studied developed spontaneous fractures. The diagnosis
usually was delayed because the patients were noncommunicative.
Anticonvulsant therapy may contribute to osteoporosis in patients with
multiple fractures—low levels of serum vitamin D were seen in 42% of
patients in one series.291 One study
concluded that unless sunlight exposure can be guaranteed, vitamin D
supplementation should be considered for children and adults in
residential care, especially if they are on anticonvulsant therapy,
even in areas with year-round sunshine.51 Fractures through osteoporotic bone can occur both above and below fixation devices.
their treatment through either closed or open methods can be quite
difficult. In a large series of patients, McIvor and Samilson348
recommended closed treatment through skeletal traction, hip spica cast,
or long-leg cast. Approximately 65% of the femoral shaft fractures and
86% of distal femoral fractures went on to malunion. Despite malunion,
most patients regained their prefracture function. Nearly 21% of their
patients had refractures, and the authors believed that this was due to
disuse osteopenia, inadequate reduction, or joint contractures. Closed
treatment of these fractures can be complicated by the development of
decubitus ulcers. Closed fractures, especially those of the femur, can
become open injuries during treatment, owing to spasticity or
inadequate immobilization.348,360
Hip spica casts are difficult to use in patients with severe flexion
contractures or dislocation of the hip. The healing time of femoral
fractures treated through immobilization varies from 1 to 3.5 months.348,359 Fractures of the humerus have been treated with light hanging-arm casts or sling-andswath bandages.360
Hip nails with side plates, compression plates, and intramedullary
fixations also have been used for femoral shaft fractures in patients
with CP. The mean healing time has been 5.3 months.348
treated four femoral fractures in young patients with CP with flexible
intramedullary nails with good outcomes. Femoral neck fractures may
require in situ pinning, but observation may be adequate in
asymptomatic bedridden patients. Although he advocated open fixation of
some lower extremity fractures in patients with mental retardation,
Sherk463 cautioned that some
patients may have inadequate motivation to resume ambulation even with
successful healing of their injuries. Medical management of these
patients must also be emphasized. In patients with cerebral palsy and
multiple fractures, Lee and Lyne291 recommended metabolic supplementation, along with traditional fracture care.
impact on bone mineral density in nonambulant children with CP,
participation in 50% longer periods of standing (in either upright or
semiprone standing frames) improved vertebral but not proximal tibial
volumetric trabecular bone mineral density. The authors concluded that
such intervention might reduce the risk of vertebral fractures but is
unlikely to reduce the risk of lower limb fractures in children with CP.88
reported in children with CP due to spasticity of the extensor
mechanism of the knee in the presence of established knee flexion
contracture.255,310,443 Lloyd-Roberts et al.310
reported on eight patients with this injury who presented with
deterioration in walking and decreased endurance. All had knee flexion
contractures. Seven of the eight patients complained of pain and local
tenderness at the distal pole of the patella. In a series of 88
patients, fragmentation was seen in only 8%.443 Patella alta and elongation of the patella are frequent in affected patients.255,443 Children
predisposed to distal pole patellar fractures are spastic ambulators
with flexion contractures of the knees, patella alta, and a history of
falls.255 Extension casting may be helpful in symptomatic patients.443
If conservative treatment is unsatisfactory, then hamstring lengthening
with correction of the knee flexion contracture can result in both
healing of the fracture and relief of symptoms.310,443 Some authors255,310 also have excised the avulsed distal pole of the patella to relieve chronic symptoms.
fractures, epiphyseal separations may occur. In a report of nine
epiphyseal separations involving the distal femur and proximal humerus
in four severely affected children with spastic quadriplegic CP, the
clinical-radiologic features confirmed the cause to be scurvy. The
fractures healed nicely with treatment with vitamin C and splintage.24
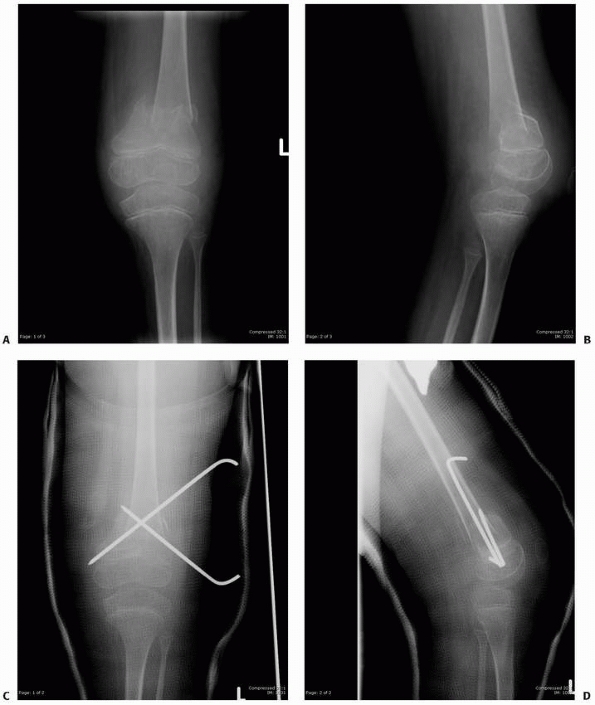 |
|
FIGURE 6-44
An 11-year-old boy with total body involvement cerebral palsy was receiving physical therapy when he developed pain and swelling around the left knee. Radiographs showed displaced femoral supracondylar fracture (A,B). In order to be able to fit to the brace adequately, closed reduction and percutaneous pinning was performed (C,D). (continues) |
child to his or her prefracture level of function. If the patient is
ambulatory, conventional forms of fracture treatment should be used (Fig. 6-44). In nonambulatory children with CP, a goal
of fracture care should be to preserve the ability to transfer. Special
precautions should be used in closed treatment of fractures in these
patients. The patients’ spasticity and inability to communicate make
them prone to skin problems, so casts should be properly applied and
well padded, usually with felt and polyurethane foam. Extra padding
should be placed over the patella, anterior ankle, and heel, and a snug
cast mould should be placed above the calcaneus to prevent proximal
migration of the heel. When indicated, operative fracture fixation
should be used in ambulatory patients. Elastic intramedullary nails can
be a very effective way to treat femoral fractures in children with
spasticity (Fig. 6-45).
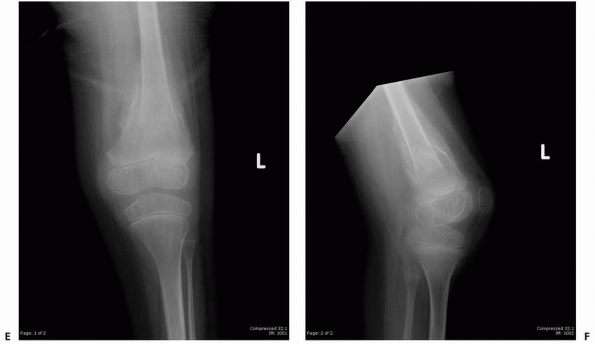 |
|
FIGURE 6-44 (continued) The fracture healed in good alignment and the pins were removed after 6 weeks (E,F). (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.)
|
children with CP. Traditionally, long-leg casts or spica casts were
used after multiple muscle lengthening or hip osteotomies, then after
several weeks, the cast was removed and therapy begun. After cast
treatment, however, the osteopenia was worse, the joints were stiff,
and fractures—especially in the distal femoral metaphysis—occurred
during therapy or transfers. We and others358,359
use foam abduction pillows and knee immobilizers and an intensive
therapy program in the immediate postoperative period to avoid the
deconditioning, osteopenia, and joint stiffness that develop after
prolonged cast immobilization. In ambulatory children who need hip
osteotomies, use of rigid internal fixation allows standing and gait
training within 2 weeks, preventing not only osteopenia but also the
risk that the child may never regain the full level of preoperative
function after a prolonged period of cast immobilization.
both malunion and shortening may be accepted. Well-padded splints or
casts are adequate treatment for many displaced fractures. Acute
femoral shaft fractures can be treated with a heavily padded hip spica
cast and distal fractures of the femur by a long-leg cast. Distal
femoral buckle fractures in nonambulatory children are safely treated
with a knee immobilizer. If a long-leg cast is used for a fracture of
the lower extremity and the joint of the involved side is dislocated,
the rigid cast may function as a lever arm, with the posterior fracture
of the proximal femur beyond the cast (Fig. 6-46).
pathologic fractures of the lower extremities. The etiology is
multifactorial but results from decreased bone mineral density due to
disuse (nonambulators), immobilization after reconstructive surgical
procedures, and increased urinary calcium loss.311 The incidence of fractures in children with myelomeningocele ranges from 12% to 31%.134,135,161,238,358,359,443,517
The locations of these fractures, in order of decreasing frequency, are
midshaft of the femur, distal femur, midshaft of the tibia, proximal
femur, femoral neck, distal femoral physis, and proximal tibia.134 Fractures of the distal tibia also have been reported in numerous series.144,238,278,501,517
Both metaphyseal and diaphyseal fractures, usually resulting from minor
trauma, are often either incomplete or impacted with intact periosteum.312 They tend to heal rapidly—nonunion is rare.134 Physeal fractures, however, may take 3 to 33 months to heal.517
Children with flail limbs tend to pick up one leg and drop it out of
the way when they roll over in bed or twist around while in a sitting
position, and this may be enough force to cause a fracture.238
Because protective sensation is absent, the child can neither
anticipate impending injury nor be aware of injury once it has
occurred. The level of neurologic involvement also affects the
incidence of fractures. In a series of 76 fractures, Lock and Aronson312 found that 41% occurred with neurologic deficit at
the thoracic level, 36% occurred with deficit at the upper lumbar
level, and only 13% occurred in patients with lower lumbar or sacral
deficits. Nearly 86% of these fractures occurred before 9 years of age,
and 76% were associated with cast immobilization. Most fractures after
immobilization occur within 4 weeks of cast removal,135 and in one series,312
30% of patients with casts had multiple cluster fractures of either the
casted extremity or the partially casted contralateral extremity. In
addition to the inherent disuse osteoporosis from immobilization,
casting causes stiffness of joints with concentration of force on the
osteoporotic bone adjacent to the joints.312 Boytim et al.68
reported neonatal fractures in six infants with myelomeningocele and
concluded that the risk of fracture was 17% for patients with thoracic
or high lumbar level deficits with significant contracture of the lower
extremities. The authors cautioned that particular care must be used to
avoid fractures in these patients during physical therapy, positioning
for radiographs, or surgical procedures. Fractures associated with
spina bifida are, however, most commonly seen in early adolescence.26
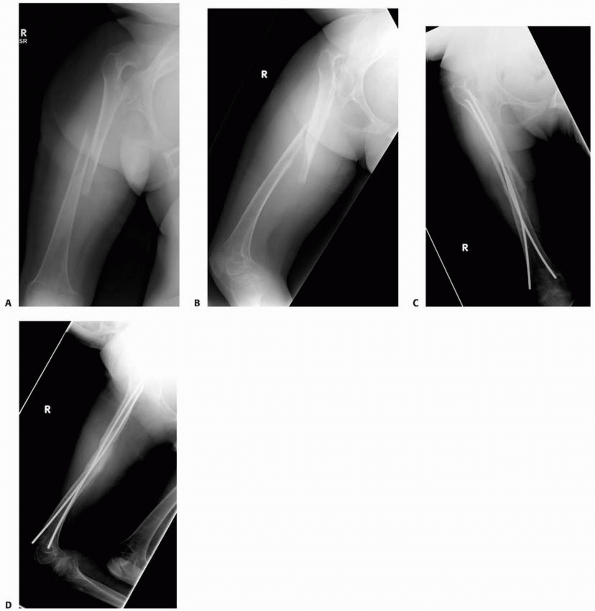 |
|
FIGURE 6-45
A 12-year-old girl with cerebral palsy and in-house-walking capabilities had an unwitnessed trauma to the right thigh, developing pain and deformity. Radiographs showed a displaced fracture of the femoral shaft (A,B). The patient underwent closed reduction followed by titanium elastic nail fixation. At 6 weeks follow-up, there was abundant callus formation (C,D). (Figures reproduced with permission from The Childrens Orthopaedic Center, Los Angeles, CA.) |
quality of bone developed by activity appears to be the best protection
against pathologic fractures,” and the orthopaedist should assist these
patients in maintaining the highest activity level possible.
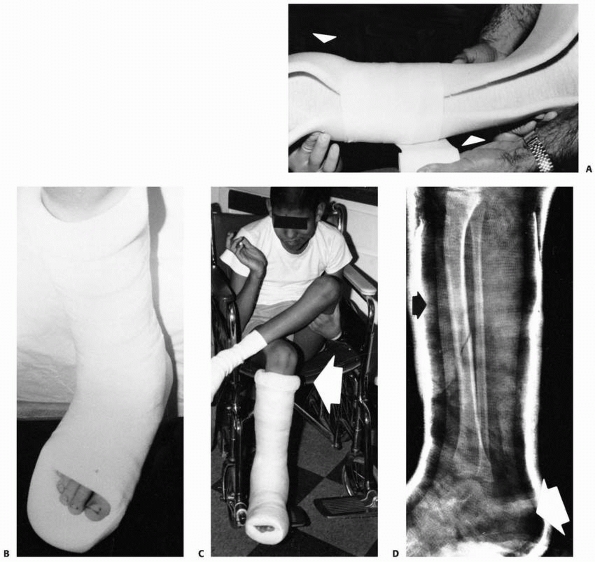 |
|
FIGURE 6-46 Casting in neuromuscular fractures. A. A polyurethane foam short-leg cast is being placed on a patient. Two long rectangular sheets of foam (arrows) are placed anteriorly and posteriorly over the stockinette, and Webril padding is wrapped around the foam. B. A long toe plate is needed to prevent injury to the foot of the patient. C. A thick, protective cuff of foam is formed by folding the polyurethane toward the center of the cast with the stockinette (arrow). D. The Webril must be wrapped quite snugly to compress the foam against the underlying extremity evenly (black arrow).
Extra foam is placed over the anterior ankle and over the Achilles tendon to prevent proximal migration of the foot in the cast. A plaster cast is usually applied and covered with a layer of fiberglass for strength. A lateral radiograph verifies the position of the heel in the cast (white arrow). |
The clinical presentation may mimic infection, with elevated
temperature and swelling, redness, and local warmth at the fracture
site.439,501
Fractures of the proximal tibia may be confused with septic arthritis
of the knee, with swelling up to the midthigh and limited knee flexion.
Both the white blood cell count and erythrocyte sedimentation rate are
often elevated. Immobilization of these injuries usually results in a
dramatic decrease in swelling and redness of the extremity within 2 to
3 days of casting. With healing, the radiographic picture can be
alarming, with epiphyseal plate widening, metaphyseal fracture, and
periosteal elevation.201 The radiographic differential diagnoses should include osteomyelitis, sarcoma, leukemia, and Charcot joint.144
walking or passive joint motion after injury, results in an exuberant
healing reaction (Fig. 6-47).144
Repetitive trauma delays resumption of normal endochondral
ossification, resulting in abnormal thickening of the cartilage in the
zone of hypertrophy and the physeal widening seen on radiographs.517 In a study of 19 chronic physeal fractures, Rodgers et al.144
compared MRI with histology and found that adjacent to this thickened,
disorganized zone of hypertrophy is juxtametaphyseal fibrovascular
tissue that enhances gadolinium on MRI. Delayed union is common, and
premature growth arrest occurs in 29% to 55% of patients.312,516 Anschuetz et al.17
reported a unique syndrome in three patients with myelomeningocele and
fracture. These children sustained fractures of the lower extremities
during long-term immobilization and with cast removal went on to
dramatic cardiopulmonary distress with increased pulse rate,
hypotension, and increased respiratory rate. Fever also developed with
decreased hematocrit levels. They suggested that the
etiology
of this problem was loss of intravascular volume into the fracture
sites and recommended intravenous replacement of fluid losses, along
with careful splinting of associated fractures.
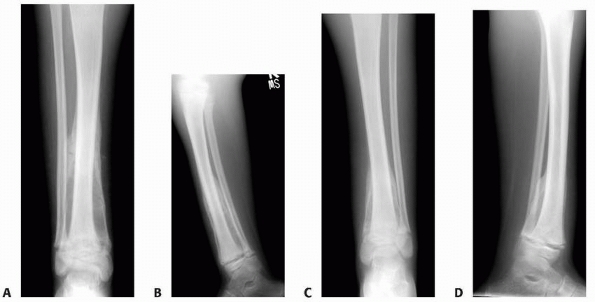 |
|
FIGURE 6-47
A 10-year-old boy with low-lumbar spina bifida and community ambulation (with braces) presented with chronic bilateral leg/ankle pain. Anteroposterior (A,C) and lateral (B,D) radiographs of both tibia and fibula show stress/insufficiency fracture of the distal tibial physis associated with extensive periosteal bone formation, characteristic of myelomeningocele. |
more difficult to treat than metaphyseal or diaphyseal long bone
fractures and require lengthy immobilization with strict avoidance of
weight bearing to avoid destructive repetitive trauma to the physis.144
Either a plaster cast or a snug-fitting total-contact orthosis is
suggested for immobilization, and union can be determined by return of
the physis to normal width on radiographs.517 Kumar et al.278
emphasized that application of a long-leg cast for 8 to 12 weeks is
necessary to obtain satisfactory healing of physeal fractures of the
tibia, and weight bearing is to be avoided until union occurs.
have been used for long-bone fractures in children with
myelomeningocele. A bilateral hip spica cast is suggested for
supracondylar fractures of the femur, because use of a one-and-a-half
hip spica cast may predispose the uninjured side to fracture.134
A bulky Webril dressing approximately 1.5 cm thick wrapped with an
elastic bandage can be used instead of a plaster or fiberglass cast.
Lock and Aronson312 used Webril
immobilization for an average of 1 to 3 weeks in their patients with
fractures and discontinued immobilization when callus was visible. They
found similar outcomes in patients treated with Webril dressings and
those treated with casts; however, there was much less difficulty with
pressure sores in the group treated with Webril dressings. Kumar et al.278
used a polyurethane padded long-leg posterior plaster splint for
metaphyseal and diaphyseal fractures for 3 weeks, followed by bracing.
Drennan and Freehafer134 recommend a
well-padded cast for 2 to 3 weeks for infants with fracture and braces
or Webril immobilization for incomplete fractures that followed
surgery. Injuries with deformity were placed in a cast. Mobilization
was begun as soon as practical to prevent further osteopenia—patients
with shaft fractures began ambulation 2 weeks after injury. Shortening
was not a problem in their series. Lock and Aronson312 cautioned that brace treatment of acute fractures may cause pressure sores. Drummond et al.135
reported on 18 fractures treated by closed techniques that resulted in
three malunions, two shortenings, and two episodes of pressure sores;
one patient had four refractures. Drabu and Walker132
noted a mean loss of knee movement of 58 degrees in 67% of fractures
about the knee. The stiffness began 2 months after fracture and was
well established by 6 months but resolved almost completely in all
patients 3 years after injury. They suggested that aggressive physical
therapy to restore knee motion is probably not necessary in these
injuries.
reported that most patients with proximal femoral epiphyseal
displacement can be treated with hip spica casts. Reduction and pinning
with subtrochanteric osteotomy may be necessary in certain patients.
Bailey-Dubow rods may be valuable in multiple recurrent pathologic
fractures of the femoral or tibial shaft.281
If operative treatment is necessary, it should be noted that the
incidence of malignant hyperthermia is higher in patients with
myelomeningocele than in other children.15
allergy in children with myelomeningocele have been reported with
increasing frequency.131,312
Minor allergic reactions, such as rash, edema, hives, and respiratory
symptoms, are common when children with myelodysplasia are exposed to
latex products such as gloves, catheters, and balloons. Between 18% and
40% of children with myelodysplasia are allergic to latex.159 Meeropol et al.352
emphasized that every child with myelomeningocele should be screened
for latex allergy, and those with a positive history should be
evaluated individually by the anesthesiologist for preoperative
prophylaxis. Current preoperative prophylaxis begins 24 hours before
surgery and is continued for 24 hours after surgery. Medications used
include diphenhydramine 1 mg/kg every 6 hours (maximum 50 mg),
methylprednisolone 1 mg/kg every 6 hours (maximum 125 mg), and
cimetidine 5 mg/kg every 6 hours (maximum 300 mg). A latex-free
environment must also be provided throughout the hospitalization.
can be tolerated, and stable or minimally angulated fractures can be
treated with either polyurethane splints or Webril dressings. Fractures
with significant deformity may require reduction and immobilization in
a cast heavily padded with polyurethane foam. In children who walk,
fractures should be carefully aligned with heavily padded casts that
allow continued protective weight bearing, if possible. Hip spica casts
may be necessary for femoral shaft fractures. Fractures of the proximal
femur should be treated by immobilization and any later deformity
corrected by osteotomy. Any patient considered for operative
intervention should be treated prophylactly with latex-free gloves and
equipment. Physeal fractures are treated with padded long leg casts and
nonweight bearing. Long-term follow-up is encouraged for physeal
injuries because of the risk of growth arrest. Significant
discrepancies can be addressed through contralateral epiphysiodesis or
bridge resection.
Duchenne muscular dystrophy must be managed so as not to cause
premature loss of the ability to walk343,508 or transfer.232
In patients 9 to 10 years old, increasing muscle weakness and joint
contractures contribute to falls, and a loss of normal muscle bulk and
fat limit the cushioning on impact.468 Patients in lower extremity braces seem to sustain few fractures in falls,508 probably because the overlying orthoses provide some protection.468
Patients confined to a wheelchair can fall because they have poor
sitting balance, and fractures are frequent because these patients are
more osteoporotic than ambulatory individuals.468
In a chart review of 143 boys with genetically confirmed
dystrophinopathies, boys treated with steroids ambulated independently
3.3 years longer than the untreated group and had a lower prevalence of
scoliosis. However, vertebral compression fractures occurred in 32% of
the treated group, whereas no vertebral fractures were seen in the
nontreatment group; long-bone fractures were 2.6 times greater in
steroid-treated patients.263
Osteoporosis is most profound in the lower extremities and begins to
develop early while still ambulating. Consequently, frequent fractures
may result in loss of ambulation.287 Larson and Henderson287
reported that bone density in the proximal femur was profoundly
diminished even when gait was minimally affected, and then
progressively decreased to nearly four standard deviations below
age-matched normal.287 Fractures are seldom displaced and are frequently minimally painful because there is minimal muscle spasm.468 Fractures tend to heal rapidly. The most commonly fractured bone is the femur231,232,467 followed by the proximal humerus.467
muscular dystrophy to prolong mobility has been shown to increase the
rate of osteoporosis and consequently, increase the risk of fracture. A
study of 33 boys with Duchenne muscular dystrophy demonstrated the
incidence of vertebral fractures in these patients after the initiation
of corticosteroid treatment; 40 months after commencement of steroids
the first vertebral fracture emerged. By 100 months of treatment,
approximately 75% of patients had sustained a vertebral fracture.66
muscular dystrophy: limb stability and maintenance of maximal function
during fracture healing. In ambulatory patients, treatment methods
should allow children to maintain the ability to walk as the fracture
heals. When ambulatory ability is tenuous, even minor bruises or ankle
sprains508 may end walking ability. As little as 1 week in a wheelchair can prematurely end ambulation508; patients at bed rest for more than 2 weeks will likely lose the ability to ambulate.343 Hsu231
reported that 25% of ambulatory patients with muscular dystrophy lost
the ability to walk after sustaining fractures. In one of these
patients, the ankle was casted in 20 degrees of plantarflexion, and the
resulting contracture prevented ambulation at the end of treatment.
reported on 20 femoral fractures in 16 patients with muscular
dystrophy. Six of the seven ambulatory patients were able to walk after
treatment. In the nonambulatory patients in this series, most had
supracondylar femoral fractures which were splinted for 2 to 3 weeks,
with emphasis on physical therapy to maintain functional abilities.
Although union was achieved rapidly, hip and knee flexion contractures
often increased in these patients and up to 20 degrees of angulation of
the fracture was routinely accepted. One patient with slipped capital
femoral epiphysis was treated successfully with pinning in situ.
muscular dystrophy is to avoid making matters worse. The patient should
be mobilized as soon as possible in a lightweight cast or orthosis.
Aggressive physical therapy should be used to maintain functional
status. In a very young child, midshaft femoral fractures can be
treated by traction and hip spica techniques, but in an older patient,
ambulatory cast bracing might be a better choice.
disorders affecting children in whom there are at least two or more
joint contractures in multiple body areas. There are at least a few
hundred arthrogrypotic syndromes. Arthrogryposis has an incidence of 3
in 10,000 live births.523 Although
the etiology is unknown and likely multifactorial, there is a lack of
fetal joint movement after initially normal development, leading to
collagen proliferation, fibrotic replacement of muscle, a marked
thickening of joint capsules, taut ligaments, and capsular tightness
resulting in joint stiffness.203 Dislocations can occur with severe shortening of the involved muscles.
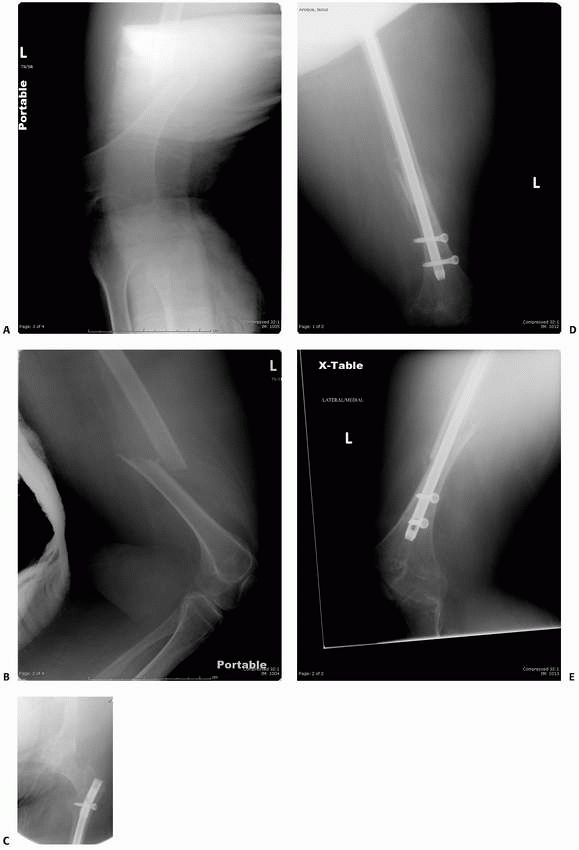 |
|
FIGURE 6-48
This 15-year-old domiciliary-ambulatory boy with Duchenne muscular dystrophy who sustained a fall at home had this displaced femoral shaft fracture (A,B). Due to his prefracture ambulatory status, he underwent closed reduction and intramedullary fixation of his fracture (C,D,E). |
reported 16 fractures in nine infants with arthrogryposis; an
ipsilateral dislocated hip was present in 35% of patients. Most
fractures involved the femur, with the remainder mostly tibial
fractures, one humeral fracture, and one clavicle fracture. Epiphyseal
separations occurred in the proximal tibia, distal femur, and proximal
humerus. Clinical symptoms included poor feeding, irritability, and
fussiness when handled. The involved extremity was thickened, and there
was often an increased white blood cell count. Plain radiographs after
acute injury, especially with epiphyseal separations, were not helpful,
and arthrogram was used in one patient to evaluate a distal femoral
epiphyseal separation. With healing, these fractures develop exuberant
callus with rapid union and ready remodeling of angulated midshaft
fractures.
Postnatal fractures are most common in patients with either knee
contracture or dislocation of the hip, and postnatal injury could
possibly be reduced by avoidance of forceful manipulation of these
extremities. Older patients with lower extremity contractures do not
seem to have difficulty with pathologic fractures.482
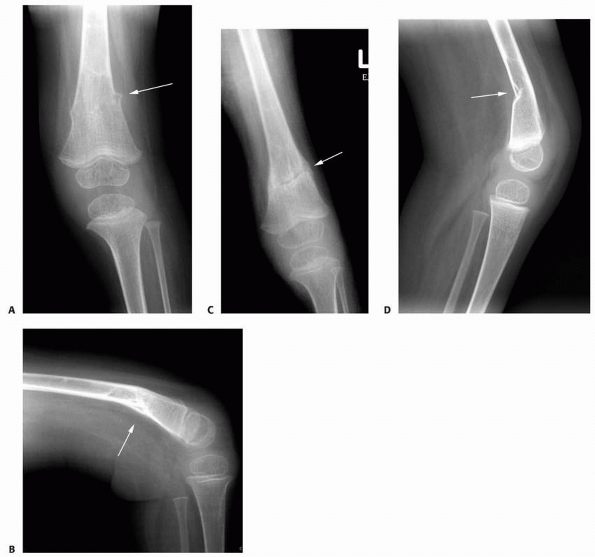 |
|
FIGURE 6-49
A 4-year-old boy with arthrogryposis and bilateral knee extension contracture presented with swelling and pain around the knee. Initial films show minimally displaced transverse fracture through the distal femoral metaphysis (arrow). After 4 weeks in a long-leg cast, radiographs show new bone formation (arrow) and good alignment of the fracture in both views. |
in most Western countries but occasionally occurs in children who live
in less developed countries. There are few reports in the literature
concerning fractures in patients with poliomyelitis. Robin436
reported 62 fractures in patients with poliomyelitis. More than half
were fractures of the femur, and 90% of those injuries were
supracondylar fractures. More than half of the fractures occurred after
cast immobilization, and joint stiffness also was associated with a
significant number of fractures. There were no epiphyseal injuries in
this series.
Because most fractures have very little displacement, reduction is
seldom necessary; if there is pre-existing deformity, manual
osteoclasis through the fracture site can be used to correct
deformities. Robin436 stressed that
joint mobility must be obtained before general mobilization of the
patient to reduce the incidence of fracture after plaster
immobilization. He also emphasized that fractures in these patients
heal rapidly, and immobilization
times should be reduced accordingly, with walking or standing in casts to decrease osteoporosis.
reports of pathologic fractures usually are included in larger series
of patients with fractures and myelomeningocele because of the clinical
similarities in presentation.250,435 Fractures of the femur, especially supracondylar fractures, are most common,168 but tibial fractures also are common.168
In most patients, these are pathologic fractures through osteoporotic
bone. Children with a spinal cord injury have a substantially lower
bone mineral density at the hip and knee in comparison with children
without disability, placing them at least at the same risk for lower
extremity fractures as adults with spinal cord injury. Children may
actually be much more susceptible to fractures than adults since they
lack of activity in a period of their life where exercise is essential
for optimal bone health.288 Although fractures in paraplegics appear to heal rapidly with abundant callus,168 animal experiments suggest that in denervated limbs, the quality of the callus in fractures is compromised.234
Children with traumatic peripheral nerve lesions may have distal tibial
physeal lesions similar to those in patients with myelomeningocele,
neuropathic arthropathy of the small joints of the foot, and soft
tissue ulcers.454
spinal cord injury is most commonly recommended. Skin traction is
contraindicated because of the possibility of skin necrosis.147 Comarr et al.100
also used pillow sandbag splints and, for certain fractures, treated
patients with a turning frame. Open reduction and internal fixation of
these fractures is controversial. The conservative techniques used for
the treatment of similar fractures in children with myelomeningocele
might best be applied to fractures in children with traumatic
paraplegia.134,250,435
suggested that continuous splinting will worsen the disuse
osteoporosis. He recommended careful manipulation of the lower
extremities when they are out of ambulatory braces to reduce the
incidence of fracture. Robin435
emphasized that immobilization should be discontinued as soon as
possible after fracture healing. Patient activity should be limited
until knee motion is restored.
lower extremity fractures in children with traumatic paraplegia.
Returning to ambulation with protection by walking braces is permitted
once callus and joint motion are adequate. Every effort should be made
to restore the child’s prefracture function. Moderate malunion and
shortening are acceptable in patients who are nonambulatory. Operative
treatment of such fractures should be reserved for selected patients
whose function would be significantly compromised by less than anatomic
reduction.
A. Bone disturbances in injuries to the spinal cord and cauda equina
(paraplegia): their prevention by ambulation. J Bone Joint Surg Am
1948;30:982-987.
A, Sferopoulos NK, Tillman RM, et al. The surgical treatment and
outcome of pathological fractures in localised osteosarcoma. J Bone
Joint Surg Br 1996;78(5): 694-698.
A, Ritchie D. Hyperparathyroidism with increased bone density in the
areas of growth. J Bone Joint Surg Br 1954;36-B(2):257-260.
AK, Nilsson IM. Fractures in haemophiliacs with special reference to
complications and treatment. Acta Chir Scand 1967;133(4):293-302.
Y, Gomis R, Yoshimura M, et al. Congenital pseudarthrosis of the
forearm—two cases treated by free vascularized fibular graft. J Hand
Surg Am 1981;6(5):475-481.
DJ, Schoenecker PL, Sheridan JJ, et al. Use of an intramedullary rod
for the treatment of congenital pseudarthrosis of the tibia. J Bone
Joint Surg Am 1992;74(2): 161-168.
TE, Drummond DS, Breed AL, et al. Malignant hyperthermia in
myelomeningocele: a previously unreported association. J Pediatr Orthop
1981;1(4):401-403.
SP, Bergstein JM, Sherrard DJ. Aluminum intoxication from aluminum
containing phosphate binders in children with azotemia not undergoing
dialysis. N Engl J Med 1984;310(17):1079-1084.
RH, Freehafer AA, Shaffer JW, et al. Severe fracture complications in
myelodysplasia. J Pediatr Orthop 1984;4(1):22-24.
F, Zamboni G, Lauriola S, et al. Early bisphosphonate treatment in
infants with severe osteogenesis imperfecta. J Pediatr
2006;149(2):174-179.
MA, Peterson HA, Dahlin DC. Pathological fractures through nonossifying
fibromas. Review of the Mayo Clinic experience. J Bone Joint Surg Am
1981;63(6):980-988.
RJ, Brenner MK, Pritchard J. Controversies and new approaches to
treatment of Langerhans cell histiocytosis. Hematol Oncol Clin North Am
1998;12(2):339-357.
DG, Newfield JT, Gillespie R. Orthopedic management of osteopetrosis:
results of a survey and review of the literature. J Pediatr Orthop
1999;19(1):122-132.
JM, Gilday DL. The futility of bone scanning in neonatal osteomyelitis:
concise communication. J Nucl Med 1980;21(5):417-420.
M, Kirkos J, Koussi A, et al. Avascular necrosis of the femoral head
among children and adolescents with sickle cell disease in Greece.
Haematologica 2002;87(7):771-772.
AG, Ro JY, Fanning CV, et al. Core needle biopsy and fine-needle
aspiration in the diagnosis of bone and soft-tissue lesions. Hematol
Oncol Clin North Am 1995; 9(3):633-651.
G, Ferrari S, Longhi A, et al. Nonmetastatic osteosarcoma of the
extremity with pathologic fracture at presentation: local and systemic
control by amputation or limb salvage after preoperative chemotherapy.
Acta Orthop Scand 2003;74(4):449-454.
S, Fisher J, Parks JS. An outbreak of vitamin D deficiency rickets in a
susceptible population. Pediatrics 1979;64(6):871-877.
JK, Cain TE, Tullos HS. Intramedullary fixation for congenital
pseudarthrosis of the tibia. J Bone Joint Surg Am 1992;74(2):169-178.
E, Weigl D, Parvari R, et al. Congenital insensitivity to pain.
Orthopaedic manifestations. J Bone Joint Surg Br 2002;84(2):252-257.
GI, Federico G, Bertelloni S, et al. Assessment of bone quality by
quantitative ultrasound of proximal phalanges of the hand and fracture
rate in children and adolescents with bone and mineral disorders.
Pediatr Res 2003;54(1):125-136.
K, Piskin A, Guclu B, et al. Aneurysmal bone cyst recurrence in
children: a review of 56 patients. J Pediatr Orthop 2007;27(8):938-943.
RJ, Masur VN. Pyknodysostosis—a report of two cases with a brief review
of the literature. Int J Oral Maxillofac Surg 2000;29(6):439-442.
DL, Riar J, Keil M, et al. Diagnostic tests for children who are
referred for the investigation of Cushing syndrome. Pediatrics
2007;120(3):e575-e586.
DF. Congenital forearm pseudarthrosis: report of six cases and review
of the literature. J Pediatr Orthop 1989;9(4):438-443.
BH Jr, Lord CF, Gebhardt MC, et al. Fractures of allografts. Frequency,
treatment, and end-results. J Bone Joint Surg Am 1990;72(6):825-833.
S, Baroncelli GI, Di Nero G, et al. Idiopathic juvenile osteoporosis:
evidence of normal osteoblast function by 1,25-dihydroxyvitamin D3
stimulation test. Calcif Tissue Int 1992;51(1):20-23.
S, Nesbit ME Jr, Egeler RM, et al. Epidemiologic study of Langerhans
cell histiocytosis in children. J Pediatr 1997;130(5):774-784.
F, Basu D, Pettifor JM. Pathological long-bone fractures in residents
with cerebral palsy in a long-term care facility in South Africa. Dev
Med Child Neurol 2002; 44(2):119-122.
A, Hall K, Sjogren L, et al. Primary hyperparathyroidism in children.
Brief review of the literature and a case report. Acta Paediatr Scand
1970;59(3):249-258.
CE, Herzenberg JE, Dipietro MA. Radiographic imaging for Ilizarov limb
lengthening in children. Pediatr Radiol 1991;21(2):117-120.
J, Andersen PE Jr. Fracture patterns in two types of autosomal-dominant
osteopetrosis. Acta Orthop Scand 1989;60(1):110-112.
SP. Growth disturbances of the distal femur following sickle cell bone
infarcts and-or osteomyelitis. Clin Radiol 1974;25(2):221-235.
A, Levy WM, Aegerter E. Primary and secondary aneurysmal bone cyst: a
radiological study of 75 cases. Radiology 1978;126(1):75-83.
JE, Gordon KE, Doley JM, et al. Vertebral fractures in boys with
Duchenne muscular dystrophy. Clin Pediatr (Phila) 2003;42(4):353-356.
J, Chantada G, Rosso D, et al. Langerhans cell histiocytosis:
retrospective evaluation of 123 patients at a single institution.
Pediatr Hematol Oncol 1999;16(5):377-385.
LW. Treatment of fibrous dysplasia of bone by total femoral plating and
hip nailing. A case report. Clin Orthop 1972;82:82-83.
CT, Friedenberg ZB, Zemsky LM, et al. Direct-current stimulation of
nonunion and congenital pseudarthrosis. Exploration of its clinical
application. J Bone Joint Surg Am 1975;57(3):368-377.
GA, Osebold WR, Ponseti IV. Congenital pseudarthrosis of long bones: a
clinical, radiographic, histologic and ultrastructural study. Clin
Orthop 1977;(128):228-242.
AM, Kawchak DA, Schall JI, et al. Bone area and bone mineral content
deficits in children with sickle cell disease. Pediatrics
2005;116(4):943-949.
ST, Puhl J, Watson FM, et al. Acute osteomyelitis following closed
fractures. Report of three cases. J Bone Joint Surg Am
1975;57(3):415-418.
R, Dal Monte A, Gitelis S, et al. The natural history of unicameral
bone cyst after steroid injection. Clin Orthop Relat Res
1982;166:204-211.
JM, Dormans JP, Drummond DS, et al. Proximal fibular osteochondroma
with associated peroneal nerve palsy: a review of six cases. J Pediatr
Orthop 1995;15(5): 574-577.
P, Leon F, Zafra M, et al. Fractures of osteochondroma during physical
exercise. Am J Sports Med 2003;31(6):1003-1006.
HS, Levin S, Kopits S, et al. Reconstructive surgery in children with
azotemic osteodystrophy. J Bone Joint Surg Am 1971;53(2):216-228.
JM, Ward KA, Alsop CW, et al. A randomized controlled trial of standing
programme on bone mineral density in nonambulant children with cerebral
palsy. Arch Dis Child 2004;89(2):131-135.
CJ, Chao TY, Chu DM, et al. Osteoblast and osteoclast activity in a
malignant infantile osteopetrosis patient following bone marrow
transplantation. J Pediatr Hematol Oncol 2004;26(1):5-8.
SM, Alavi A, Russell MO. Management of osteonecrosis in sickle-cell
anemia and its genetic variants. Clin Orthop 1978;130:158-174.
PF, Krivit W, Cervenka J, et al. Successful bone-marrow transplantation
for infantile malignant osteopetrosis. N Engl J Med
1980;302(13):701-708.
PJ, Riesenburger RI, Klimo P Jr, et al. Vertebra plana due to an
aneurysmal bone cyst of the lumbar spine. Case report and review of the
literature. J Neurosurgc 2006; 105(6 Suppl):490-495.
J. Simple bone cysts. Studies of cyst fluid in six cases with a theory
of pathogenesis. J Bone Joint Surg Am 1960;42-A:609-616.
J, Kohler R, Sales De Gauzy J, et al. Epidemiology of aneurysmal bone
cyst in children: a multicenter study and literature review. J Pediatr
Orthop B 2004;13(6): 389-394.
H. The basophil adenomas of the pituitary body and their clinical
manifestations. Bull Johns Hopkins Hosp 1932;50:137-195.
M, Buraczewski J. Aneurysmal bone cyst. Pathology, clinical course, and
radiologic appearances. Cancer 1969;23(2):371-389.
A, Saighi-Bouaouina A. Treatment of sequestra, pseudarthroses, and
defects in the long bones of children who have chronic hematogenous
osteomyelitis. J Bone Joint Surg Am 1989;71(10):1448-1468.
RT, Riggs BL, Scholz DA. Back pain and vertebral crush fractures: an
unemphasized mode of presentation for primary hyperparathyroidism. Ann
Intern Med 1975;83(3):365-367.
JR, Fisher R, Lum G, et al. Angular deformity of the lower extremity in
children with renal osteodystrophy. J Pediatr Orthop 1992;12(3):291-299.
Boer HH, Verbout AJ Nielsen HK, et all. Free vascularized fibular graft
for tibial pseudarthrosis in neurofibromatosis. Acta Orthop Scand
1988;59(4):425-429.
Kleuver M, Van Der Heul RO, Veraart BE. Aneurysmal bone cyst of the
spine: 31 cases and the importance of the surgical approach. J Pediatr
Orthop B 1998;7(4): 286-292.
BM, Jaffer SN, Griffin AM, et al. Joint salvage for pathologic fracture
of giant cell tumor of the lower extremity. Clin Orthop Relat Res
2007;459:96-104.
MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and
treatment. J Bone Joint Surg Am 2005;87(8):1848-1864.
PE, Sew-Hoy A, Krom W. Bilateral isolated olecranon fractures in an
infant as presentation of osteogenesis imperfecta. Orthopedics
1992;15(6):741-743.
J, Piguet C, Bernard F, et al. A new clinical score for disease
activity in Langerhans cell histiocytosis. Pediatr Blood Cancer
2004;43(7):770-776.
JP, Dormans NJ. Use of percutaneous intramedullary decompression and
medical-grade calcium sulfate pellets for treatment of unicameral bone
cysts of the calcaneus in children. Orthopedics 2004;27(1
Suppl):s137-s139.
JP, Hanna BG, Johnston DR, et al. Surgical treatment and recurrence
rate of aneurysmal bone cysts in children. Clin Orthop Relat Res
2004(421):205-211.
JP, Krajbich JI, Zuker R, et al. Congenital pseudarthrosis of the
tibia: treatment with free vascularized fibular grafts. J Pediatr
Orthop 1990;10(5):623-628.
JP, Pill SG. Fractures through bone cysts: unicameral bone cysts,
aneurysmal bone cysts, fibrous cortical defects, and nonossifying
fibromas. Instr Course Lect 2002; 51:457-467.
JP, Sankar WN, Moroz L, et al. Percutaneous intramedullary
decompression, curettage, and grafting with medical-grade calcium
sulfate pellets for unicameral bone cysts in children: a new minimally
invasive technique. J Pediatr Orthop 2005;25(6): 804-811.
JP, Templeton J, Schreiner MS, et al. Intraoperative latex anaphylaxis
in children: classification and prophylaxis of patients at risk. J
Pediatr Orthop 1997;17(5): 622-625.
DS, Moreau M, Cruess RL. Postoperative neuropathic fractures in
patients with myelomeningocele. Dev Med Child Neurol 1981;23(2):147-150.
DS, Moreau M, Cruess RL. The results and complications of surgery for
the paralytic hip and spine in myelomeningocele. J Bone Joint Surg Br
1980;62-B(1): 49-53.
S, Briody J, Schindeler A, et al. Decreased bone mineral density in
neurofibromatosis type 1: results from a pediatric cohort. J Pediatr
Orthop 2007;27(4):472-475.
ME, Kneisl JS. Pathologic fractures through nonossifying fibromas: is
prophylactic treatment warranted? J Pediatr Orthop 1997;17(6):808-813.
WW. Pathological fracture complicating long bone osteomyelitis in
patients with sickle cell disease. J Pediatr Orthop 1986;6(2):177-181.
JG, Obad S, Geiger R, et al. Pycnodysostosis. Orthopedic aspects with a
description of 14 new cases. Clin Orthop 1992;80:263-276.
DV, Levitsky LL Schey W, et al. Resurgence of nutritional rickets
associated with breast-feeding and special dietary practices.
Pediatrics 1980;65(2):232-235.
S, Kattapuram SV, Egglin TK. Ewing sarcoma. Radiographic pattern of
healing and bony complications in patients with long-term survival.
Cancer 1991;68(7): 1531-1535.
U, Ruegsegger P, Anliker M, et al. Loss and recovery of trabecular bone
in the distal radius following fracture-immobilization of the upper
limb in children. Klin Wochenschr 1979;57(15):763-767.
WF, Gearen PF. Fibrous dysplasia of the femoral neck. Treatment by
cortical bone-grafting. J Bone Joint Surg Am 1986;68(9):1415-1422.
BA. Roentgenological changes in the bones in cases of
pseudohypertrophic muscular dystrophy. Arch Neurol Psychiatry
1941;46:868-876.
KS, Brown J, Douglas DL. Osteotomy and intramedullary nailing for the
correction of progressive deformity in vitamin D-resistant
hypophosphatasemic rickets. JR Coll Surg Edinb 1993;38(1):50-54.
L, Ghafil D, Gerroudj M, et al. Bone morphogenetic protein 7 in the
treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg
Br 2006;88(1):116-118.
G, Lammens J, Van M, et al. Treatment of congenital pseudarthrosis with
the Ilizarov technique. J Pediatr Orthop 1988;8(1):67-70.
MJ, Heeger S, Lynch KA, et al. Intravenous bisphosphonate therapy in
children with osteogenesis imperfecta. Pediatrics 2003;111(3):573-578.
E, Sakai D, Blatt T. The effect of hip reduction on function in
patients with myelomeningocele. Potential gains and hazards of surgical
treatment. J Bone Joint Surg Am 1978;60(2):169-173.
V, Spain M, Matthews JM. The haemophilic pseudotumour or haemophilic
subperiosteal haematoma. J Bone Joint Surg Br 1965;47:256-265.
B, Walker C, Jackson A, et al. The orthopaedic management of
hypophosphataemic rickets. J Pediatr Orthop 1991;11(3):367-373.
Y, Niska M. Dislocation of the hip joint complicating repeated
hemarthrosis in hemophilia. J Pediatr Orthop 1983;3(1):99-100.
Y, Nakata K, Yasui N, et al. Novel mutations of the cathepsin K gene in
patients with pycnodysostosis and their characterization. J Clin
Endocrinol Metab 2000;85(1): 425-431.
JG, Rinsky LA, Strudwick J, et all. Non-union of fractures in children
who have osteogenesis imperfecta. J Bone Joint Surg Am
1988;70(3):439-443.
JG, Strudwick WJ, Rinsky LA, et al. Complications of intramedullary
rods in osteogenesis imperfecta: Bailey-Dubow rods versus nonelongating
rods. J Pediatr Orthop 1988;8(6):645-649.
LM, Cheung JC, Daniels MW, et al. Low-dose intravenous pamidronate
reduces fractures in childhood osteoporosis. J Pediatr Endocrinol Metab
2003;16(6): 887-892.
S, Mehta S, Dormans JP. Langerhans cell histiocytosis of the spine in
children. Long-term follow-up. J Bone Joint Surg Am
2004;86-A(8):1740-1750.
S, Mehta S, Dormans JP. Modern surgical treatment of primary aneurysmal
bone cyst of the spine in children and adolescents. J Pediatr Orthop
2005;25(3):387-392.
R, Suppelna G. Solitary enchondroma at the hand. Long-term follow-up
study after operative treatment. J Hand Surg Br 2004;29(1):64-66.
LH, Binder H, Weintrob J, et al. Rehabilitation of children and infants
with osteogenesis imperfecta. A program for ambulation. Clin Orthop
1990;(51):254-262.
EJ, Vossen JM, Van Loo IH, et al. Autosomal recessive osteopetrosis:
variability of findings at diagnosis and during the natural course.
Pediatrics 1994;93(2):247-253.
I, Tolo VT, D’Ambra P, et al. Langerhans cell histiocytosis of bone in
children and adolescents. J Pediatr Orthop 2003;23(1):124-130.
CP Jr, Hefele MC, Peabody TD, et al. Aneurysmal bone cyst of the
extremities. Factors related to local recurrence after curettage with a
high-speed burr. J Bone Joint Surg Am 1999;81(12):1671-1678.
FH, Bishop NJ, Plotkin H, et al. Cyclic administration of pamidronate
in children with severe osteogenesis imperfecta. N Engl J Med
1998;339(14):947-952.
MP, Carpentieri DF, Dormans JP. Langerhans cell histiocytosis: clinical
presentation, pathogenesis, and treatment from the LCH etiology
research group at The Children’s Hospital of Philadelphia. UPOJ
2002;15:67-73.
AB, Lane JM, Salvati E. Slipped capital femoral epiphyses complicating
renal osteodystrophy: a report of three cases. Radiology
1978;126(2):333-339.
WB, Yankaskas BC, Guilford WB. Roentgenographic classifications of
hemophilic arthropathy. Comparison of three systems and correlation
with clinical parameters. J Bone Joint Surg Am 1989;71(2):237-244.
PJ, Price BA, Ellis HA, et al. Pseudarthrosis of the radius associated
with neurofibromatosis. A case report. Clin Orthop 1982;(171):175-179.
M, Horodniceanu C, Steinherz R. The radiographic manifestations of bone
changes in copper deficiency. Pediatr Radiol 1980;9(2):101-104.
KJ, Multhopp H, Ganey T, et al. Orthopaedic manifestations in
congenitally insensate patients. J Pediatr Orthop 1990;10(4):514-521.
JT, Forlin E, Bowen JR. Charcot joint disease of the shoulders in a
patient who had familial sensory neuropathy with anhidrosis. A case
report. J Bone Joint Surg Am 1992;74(9):1415-1417.
JT, Kumar SJ, Macewen GD. Fibrous dysplasia of the proximal part of the
femur. Long-term results of curettage and bone-grafting and mechanical
realignment. J Bone Joint Surg Am 1998;80(5):648-658.
DP. Displaced olecranon apophyseal fractures in children with
osteogenesis imperfecta. J Pediatr Orthop 2005;25(2):154-157.
MT, Newbern DH, Neuhauser EB. Metaphyseal and physeal injuries in
children with spina bifida and meningomyeloceles. Am J Roentgenol
Radium Ther Nucl Med 1965;95:168-177.
JG. Arthrogryposis (multiple congenital contractures). In: Rimoin DL,
Connor JM, Pyeritz RE, et al., eds. Emery and Rimion’s Principles and
Practice of Medical Genetics. Vol. 168. 5th ed. Philadelphia: Churchill
Livingstone, 2007:3785-3856.
DA, Winter RB, Lutter L, et al. Osteogenesis imperfecta. Radiographic
classification, natural history, and treatment of spinal deformities. J
Bone Joint Surg Am 1992; 74(4):598-616.
HL, Crockard HA, Stevens JM, et al. The operative management of basilar
impression in osteogenesis imperfecta. Neurosurgery 1990;27(5):782-786.
WH, Dudley HR Jr, Barry RJ. The natural history of fibrous dysplasia.
An orthopaedic, pathological, and roentgenographic study. Am J Orthop
1962;44-A:207-233.
WJ, Rankin KC. Osteogenesis imperfecta in Zimbabwe: a comparison
between treatment with intramedullary rods of fixed-length and
self-expanding rods. J R Coll Surg Edinb 1998;43(5):328-332.
CA, Koman LA. Treatment of slipped capital femoral epiphysis resulting
from juvenile renal osteodystrophy. J Pediatr Orthop 1990;10(4):551-554.
K. Osteopetrosis. Review of the literature and comparative studies on a
case with a 24-year follow-up. Am J Orthop 1962;44-A:359-370.
RE, Scheurer SL, Alexander R, et al. Trauma to the bones of small
infants from passive exercise: a factor in the etiology of child abuse.
J Pediatr 1984;104(1):47-50.
SD, Drvaric DM, Darr K, et al. The operative stabilization of pediatric
diaphyseal femur fractures with flexible intramedullary nails: a
prospective analysis. J Pediatr Orthop 1994;14(4):501-507.
SD, Gallagher D, Warrior R, et al. The prognostic significance of the
skeletal manifestations of acute lymphoblastic leukemia of childhood. J
Pediatr Orthop 1994; 14(1):105-111.
RM, Kirchner SG, O’Neill JA Jr, et al. Skeletal changes of copper
deficiency in infants receiving prolonged total parenteral nutrition. J
Pediatr 1978;92(6):947-949.
T, Iguchi M, Shimura A, et al. Computed tomography and bone
scintigraphy is polyostotic fibrous dysplasia. Report of a case. Oral
Surg Oral Med Oral Pathol 1980; 50(6):580-583.
SC, Parker CC, Brady RO, et al. MRI of multiple platyspondyly in
Gaucher disease: response to enzyme replacement therapy. J Comput
Assist Tomogr 1993;17(5): 806-809.
JA, Springfield DS, Hayes WC. Predicting pathologic fracture risk in
the management of metastatic bone defects. Clin Orthop Relat Res
1995(312):120-135.
J, Cabe GD, Tedrow JR, et al. Failure of trabecular bone with simulated
lytic defects can be predicted non-invasively by structural analysis. J
Orthop Res 2004; 22(3):479-486.
RW, Riseborough EJ. Lengthening of the lower extremity by the Wagner
method. A review of the Boston Children’s Hospital Experience. J Bone
Joint Surg Am 1981; 63(7):1122-1131.
DM, Gilchrist GS, Mullan BP, et al. Langerhans cell histiocytosis:
diagnosis, natural history, management, and outcome. Cancer
1999;85(10):2278-2290.
DW, Savage JP, Wilson TG, et al. The technetium phosphate bone scan in
the diagnosis of osteomyelitis in childhood. J Bone Joint Surg Am
1983;65(4):431-437.
AC, Kooh SW, Fraser D, et al. Renal osteodystrophy in children with
chronic renal failure: an unexpectedly common and incapacitating
complication. Pediatrics 1982;70(5):742-750.
A, Olerud S. The healing of fractures in denervated limbs. An
experimental study using sensory and motor rhizotomy and peripheral
denervation. J Trauma 1965;5(5): 571-579.
CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a
case series and description of the technique. J Pediatr Orthop
2007;27(5):504-509.
MA, Nelson JD. Etiology and medical management of acute suppurative
bone and joint infections in pediatric patients. J Pediatr Orthop
1982;2(3):313-323.
CC. Fractures of the lower limbs in spina bifida cystica: a survey of
44 fractures in 122 children. Dev Med Child Neurol 1970;Suppl 22:88.
J, Mazzotti I, Tomasevic M. Complications after treatment of patients
with osteogenesis imperfecta with a Bailey-Dubow rod. Arch Orthop
Trauma Surg 1998; 117(4-5):240-245.
AG, Helmig O, Ternowitz T, et al. Chronic recurrent multifocal
osteomyelitis: a follow-up study. J Pediatr Orthop 1988;8(1):49-58.
FA, Gillespie R. Pseudarthrosis of the radius after fracture through
normal bone in a child who had neurofibromatosis. A case report. J Bone
Joint Surg Am 1989; 71(9):1419-1421.
O, Ogawa R. Pseudarthrosis of the radius associated with
neurofibromatosis: report of a case and review of the literature. J
Pediatr Orthop 1990;10(1):128-131.
FS, August CS, Fallon MD, et al. Osteopetrorickets. The paradox of
plenty. Pathophysiology and treatment. Clin Orthop 1993;94:64-78.
LA, Haideri NF, Halliday SE, et al. Gait analysis and muscle strength
in children with congenital pseudarthrosis of the tibia: the effect of
treatment. J Pediatr Orthop 1998;18(3):381-386.
CK, Rapaport SI. Bleeding times and platelet aggregation after
analgesics in hemophilia. Ann Intern Med 1972;77(2):189-193.
JJ, Freiberger RH. Fragmentation of the lower pole of the patella in
spastic lower extremities. Radiology 1971;101(1):97-100.
HJ, Sloan RE, Hoffman W, et al. Accumulation of nitrogen and six
minerals in the human fetus during gestation. Hum Biol 1951;23(1):61-74.
DR, Guille JT, Horn BD, et al. The stubbed great toe: importance of
early recognition and treatment of open fractures of the distal
phalanx. J Pediatr Orthop 2001;21(1):31-34.
JG, Morcuende JA. Dramatic subperiosteal bone formation following
physeal injury in patients with myelomeningocele. Iowa Orthop J
2002;22:94-98.
TN, Teh J, Goodman TR. Paediatric manifestations of Langerhans cell
histiocytosis: a review of the clinical and radiological findings. Clin
Radiol 2003;58(4): 269-278.
SE, Wenger DE, Gilchrist GS, et al. Langerhans cell histiocytosis
(histiocytosis X) of bone. A clinicopathologic analysis of 263
pediatric and adult cases. Cancer 1995; 76(12):2471-2484.
JB, Bobenchko WP. Osteogenesis imperfecta: an orthopaedic discussion
and surgical review. J Bone Joint Surg Br 1971;53:72-89.
WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of
long-term daily corticosteroid treatment in Duchenne muscular
dystrophy. Neurology 2007;68(19): 1607-1613.
JR, Ozonoff MB, Steinbach HL. Epiphyseal displacement after metaphyseal
fracture in renal osteodystrophy. Am J Roentgenol Radium Ther Nucl Med
1972; 115(3):547-554.
L, Ockenden BG, Townsend AC. Osteosarcoma occurring in osteogenesis
imperfecta. Report of two cases. J Bone Joint Surg Br
1967;49(2):314-323.
A, Kawai S, Utsunomiya T, et al. Bone disease in infants and children
with hepatobiliary disease. Arch Dis Child 1974;49(8):641-646.
D, Satsuma S, Kamegaya M, et al. Musculoskeletal conditions of acute
leukemia and malignant lymphoma in children. J Pediatr Orthop B
2005;14(3):156-161.
S, Inoue A. Aggressive bone tumorous lesion in infancy: osteofibrous
dysplasia of the tibia and fibula. J Pediatr Orthop 1993;13(5):577-581.
WW, Oestreich AE, Sherman R, et al. Radiological case of the month.
Osteopenia, rickets, and fractures in preterm infants. Am J Dis Child
1985;139(10):1045-1046.
SW, Jones G, Reilly BJ, et al. Pathogenesis of rickets in chronic
hepatobiliary disease in children. J Pediatr 1979;94(6):870-874.
MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical
presentation, and imaging. AJR Am J Roentgenol 1995;164(3):573-580.
B, Mehls O, Ritz E. Morphological studies on pathogenesis of epiphyseal
slipping in uremic children. Virchows Arch A Pathol Anat Histol
1974;362(2): 129-143.
SJ, Cowell HR, Townsend P. Physeal, metaphyseal, and diaphyseal
injuries of the lower extremities in children with myelomeningocele. J
Pediatr Orthop 1984;4(1): 25-27.
AT, Loder RT, Hensinger R. Telescoping intramedullary rodding with
Bailey-Dubow nails for recurrent pathologic fractures in children
without osteogenesis imperfecta. J Pediatr Orthop 1998;18(1):4-8.
JE, Gilbert MS, Posner MA. Management of bleeding and associated
complications of hemophilia in the hand and forearm. J Bone Joint Surg
Am 1977;59(4): 451-460.
A. Femur remodelled during growth after osteomyelitis causing coxa vara
and shaft necrosis. J Pediatr Orthop 1982;2(3):289-294.
CM, Henderson RC. Bone mineral density and fractures in boys with
Duchenne muscular dystrophy. J Pediatr Orthop 2000;20(1):71-74.
R, Johnston TE, Smith BT, et al. Bone mineral density of the hip and
knee in children with spinal cord injury. J Spinal Cord Med
2007;30(Suppl 1):S10-S14.
FY, Sinicropi SM, Lee FS, et al. Treatment of congenital pseudarthrosis
of the tibia with recombinant human bone morphogenetic protein-7
(rhBMP-7). A report of five cases. J Bone Joint Surg Am
2006;88(3):627-633.
RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia.
Is there a need for a radical surgical approach? J Bone Joint Surg Br
2006;88(5):658-664.
VN, Srivastava A, Nithyananth M, et al. Fracture neck of femur in
haemophilia A—experience from a cohort of 11 patients from a tertiary
centre in India. Haemophilia 2007;13(4):391-394.
AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic
fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res
2004;19(4):571-577.
A, Windhager R, Lang S, et al. Aneurysmal bone cyst. A population-based
epidemiologic study and literature review. Clin Orthop Relat Res
1999;363:176-179.
GM, Abad V, Charmandari E, et al. Effects of child- and
adolescent-onset endogenous Cushing syndrome on bone mass, body
composition, and growth: a 7-year prospective study into young
adulthood. J Bone Miner Res 2007;22(1):110-118.
GD, Greenfield GB, Heinrich SD. Evaluation of the child with a bone or
soft tissue neoplasm. Orthop Clin North Am 1996;27(3):431-451.
M, Monson R, Weber K. The prevention of recurrent fractures of the
lower extremities in severe osteogenesis imperfecta using vacuum pants:
a preliminary report in four patients. J Pediatr Orthop
1988;8(4):454-457.
PC. Congenital pseudarthrosis of the tibia. Three cases treated by free
vascularized iliac crest graft. Clin Orthop 1983;(175):45-50.
WM, Miller AA, Bonakdarpour A, et al. Aneurysmal bone cyst secondary to
other osseous lesions. Report of 57 cases. Am J Clin Pathol
1975;63(1):1-8.
RJ, Ketcham AS. Maffucci syndrome: functional and neoplastic
significance. Case report and review of the literature. J Bone Joint
Surg Am 1973;55(7):1465-1479.
L, Jaffee HL. Fibrous dysplasia of bone: a condition affecting one,
several or many bones, the graver cases of which may present abnormal
pigmentation of skin, premature sexual development, hyperthyroidism or
still other extra skeletal abnormalities. Arch Pathol 1942;33:777-815.
F, Nikakhtar B. Current advances in the therapy of secondary
hyperparathyroidism and osteitis fibrosa. Miner Electrolyte Metab
1991;17(4):250-255.
GC. Treatment of defects of the ulna in children by establishing
cross-union with the radius. J Bone Joint Surg Br 1973;55(2):327-330.
GC, Jackson AM, Albert JS. Avulsion of the distal pole of the patella
in cerebral palsy. A cause of deteriorating gait. J Bone Joint Surg Br
1985;67(2):252-254.
RT, Hensinger RN. Slipped capital femoral epiphysis associated with
renal failure osteodystrophy. J Pediatr Orthop 1997;17(2):205-211.
SJ, Sheridan JJ, Capelli AM, et al. Management of lower-extremity
deformities in osteogenesis imperfecta with extensible intramedullary
rod technique: a 20-year experience. J Pediatr Orthop 1998;18(1):88-94.
S, Dormans JP, D’Angio G. Malignant degeneration of radiation-induced
osteochondroma. Skeletal Radiol 1997;26(3):195-198.
MR, Huizenga BA. Spinal cord compression by displaced ribs in
neurofibromatosis. A report of three cases. J Bone Joint Surg Am
1988;70(7):1100-1102.
H, Shannak A, Amr S. Surgical treatment of pathological subtrochanteric
fractures due to benign lesions in children and adolescents. J Pediatr
Orthop 1984; 4(1):63-69.
GA, Harcke HT, Harkey C, et al. SPECT imaging of para-axial
neurofibromatosis with technetium-99m DTPA. J Nucl Med
1987;28(11):1688-1694.
GA, Herrick WC, Harcke HT, et al. Neurofibromas: location by scanning
with Tc-99m DTPA. Work in progress. Radiology 1985;157(3):803-806.
HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with
malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am
1982;64(8):1121-1127.
SC Jr, Schmidt CJ. Bone remodeling as an expression of altered
phenotype: studies of fracture healing in untreated and cured
osteopetrotic rats. Clin Orthop 1978; 37:259-264.
HK, Tsang RC, Hug G, Et Al. Calcitriol deficiency in idiopathic
juvenile osteoporosis. Am J Dis Child 1982;136(10):914-917.
V, Sissons HA. Aneurysmal bone cyst. A review of 123 cases including
primary lesions and those secondary to other bone pathology. Cancer
1988;61(11):2291-2304.
Z. Pseudarthrosis of the radius associated with neurofibromatosis. A
case report. J Bone Joint Surg Am 1977;59(7):977-978.
AD, Seeger LL, Eckardt JJ. The role of plain radiography, computed
tomography, and magnetic resonance imaging in sarcoma evaluation.
Hematol Oncol Clin North Am 1995;9(3):571-604.
C, Gilbert A, Azze RG. Congenital pseudarthrosis of the forearm:
treatment of six cases with vascularized fibular graft and a review of
the literature. Microsurgery 1993;14(4):252-259.
RG, Bahn RC, Hayles AB. Primary adrenocortical nodular dysplasia as a
cause of Cushing syndrome in infants and children. Mayo Clin Proc
1982;57(1):58-63.
DG, Kinali M, Gallagher AC, et al. Fracture prevalence in Duchenne
muscular dystrophy. Dev Med Child Neurol 2002;44(10):695-698.
AG, Seale NS. Prevalence of dental abscess in a population of children
with vitamin D-resistant rickets. Pediatr Dent 1991;13(2):91-96.
E, Frost J, Pugh L, et al. Latex allergy in children with
myelodysplasia: a survey of Shriners hospitals. J Pediatr Orthop
1993;13(1):1-4.
SC, Simon MA, Laros GS, et al. Pycnodysostosis. A clinical,
pathological, and ultramicroscopic study of a case. J Bone Joint Surg
Am 1978;60(8):1122-1127.
JS, Harty MP, Mahboubi S, et al. Langerhans cell histiocytosis:
presentation and evolution of radiologic findings with clinical
correlation. Radiographics 1995;15(5): 1135-1146.
F, Cardoso Dias R, Dabney KW, et al. Soft-tissue release for spastic
hip subluxation in cerebral palsy. J Pediatr Orthop 1997;17(5):571-584.
F, Girardi H, Lipton G, et al. Reconstruction of the dysplastic spastic
hip with peri-ilial pelvic and femoral osteotomy followed by immediate
mobilization. J Pediatr Orthop 1997;17(5):592-602.
RG, Segal JB, Ashar BH, et al. High prevalence and correlates of low
bone mineral density in young adults with sickle cell disease. Am J
Hematol 2006;81(4):236-241.
DS, Nebeker HG, Ott SM, et all. Use of the deferoxamine infusion test
in the diagnosis of aluminum-related osteodystrophy. Ann Intern Med
1984;101(6): 775-779.
S, Mcmahon HP, Farrar GJ. Emerging therapeutic approaches for
osteogenesis imperfecta. Trends Mol Med 2005;11(6):299-305.
J, Svensson O, Holmberg M, et al. Orthopedic aspects of familial
insensitivity to pain due to a novel nerve growth factor beta mutation.
Acta Orthop 2006;77(2): 198-202.
WG Jr, Miller GR. Aftermath of osteogenesis imperfecta: the disease in
adulthood. J Bone Joint Surg Am 1980;62(1):113-119.
DL, Ayerza MA, Calabrese ME, et al. The use of a bone allograft for
reconstruction after resection of giant-cell tumor close to the knee. J
Bone Joint Surg Am 1993; 75(11):1656-1662.
CS 2nd, Francis KC, Johnston AS, et al. Current concepts on the
treatment of solitary unicameral bone cyst. Clin Orthop Relat Res
1973;97:40-51.
CS 2nd, Francis KC, Marcove RC, et al. Treatment of unicameral bone
cyst. A follow-up study of one hundred seventy-five cases. J Bone Joint
Surg Am 1966;48(4): 731-745.
CL, Evarts CM, Popowniak K. Musculoskeletal complications of renal
transplantation. Surg Clin North Am 1971;51(5):1205-1209.
LD, Aguilar C, Earles AN, et al. Physical therapy alone compared with
core decompression and physical therapy for femoral head osteonecrosis
in sickle cell disease. Results of a multicenter study at a mean of
three years after treatment. J Bone Joint Surg Am 2006;88(12):2573-2582.
RW, James P. Telescoping intramedullary stabilization of the lower
extremities for severe osteogenesis imperfecta. J Pediatr Orthop
1990;10(2):219-223.
BE, Westlin NE. Restoration of bone mass after fracture of the lower
limb in children. Acta Orthop Scand 1971;42(1):78-81.
JR, Douglas JF. Bilateral slipping of the upper femoral epiphysis in
end-stage renal failure. A report of two cases. J Bone Joint Surg Br
1980;62-B(1):18-21.
C, Smith P, Mi Z, et al. Potential of gene therapy for treating
osteogenesis imperfecta. Clin Orthop Relat Res 2000(379
Suppl):S126-S133.
M, Zacharin M. Intramedullary rodding and bisphosphonate treatment of
polyostotic fibrous dysplasia associated with the McCune-Albright
syndrome. J Pediatr Orthop 2002;22(2):255-260.
T, Inoue A, Izumo S. Congenital insensitivity to pain with anhidrosis.
A case report. J Bone Joint Surg Am 1990;72(2):279-282.
AM, Perez-Atayde AR, Inwards CY, et al. USP6 and CDH11 oncogenes
identify the neoplastic cell in primary aneurysmal bone cysts and are
absent in so-called secondary aneurysmal bone cysts. Am J Pathol
2004;165(5):1773-1780.
WL, Bowen RE, McDonough PW, et al. Outcome of slipped capital femoral
epiphysis in renal osteodystrophy. J Pediatr Orthop 2003;23(2):169-174.
WL, Namba R, Goodman WG, et al. Aluminum toxicity complicating renal
osteodystrophy. A case report. J Bone Joint Surg Am 1989;71(3):446-452.
K, Merikanto J. Diaphyseal bone lengthening in children using Wagner
device: long-term results. J Pediatr Orthop 1991;11(4):449-451.
DM, Eilert RE, Waldstein G. Congenital pseudarthrosis of the ulna: a
report of two cases and a review of the literature. J Pediatr Orthop
1985;5(4):463-467.
D, Herzenberg JE, Paremain G, et al. Femoral lengthening over an
intramedullary nail. A matched-case comparison with Ilizarov femoral
lengthening. J Bone Joint Surg Am 1997;79(10):1464-1480.
PJ, Choudhury SN, Frassica FJ, et al. Treatment of aneurysmal bone
cysts of the pelvis and sacrum. J Bone Joint Surg Am
2001;83-A(11):1674-1681.
PJ, Currier BL, Galanis EC, et al. Vertebra plana of the lumbar spine
caused by an aneurysmal bone cyst: a case report. Am J Orthop
1999;28(2):119-124.
AM. The actions of parathyroid hormone on bone: relation to bone
remodeling and turnover. Calcium homeostasis and metabolic bone
disease. Part III of IV parts; PTH and osteoblasts, the relationship
between bone turnover and bone loss, and the state of the bones in
primary hyperparathyroidism. Metabolism 1976;25(9):1033-1069.
YK, Unni KK, Mcleod RA, et al. Osteofibrous dysplasia:
clinicopathologic study of 80 cases. Hum Pathol 1993;24(12):1339-1347.
CR, Burns J, McAllion SJ. Osteogenesis imperfecta: the distinction from
child abuse and the recognition of a variant form. Am J Med Genet
1993;45(2):187-192.
DC, Simonis RV. Electrical stimulation in the treatment of congenital
pseudarthrosis of the tibia. J Bone Joint Surg Br 1985;67(3):454-462.
TD, Simon MA. Making the diagnosis: keys to a successful biopsy in
children with bone and soft-tissue tumors. Orthop Clin North Am
1996;27(3):453-459.
H, Sueiro R. Osteoporosis in children with neuromuscular diseases and
inborn errors of metabolism. Minerva Pediatr 2007;59(2):129-135.
L, Subhadharaphandou T, Dhanachaim, et al. Prognostic factors among 130
patients with osteosarcoma. Clin Orthop 1997;(345):206-214.
SN, Marks SC Jr. The heterogeneity of the osteopetroses reflects the
diversity of cellular influences during skeletal development. Bone
1995;17(5):437-445.
S, Heller E, Seidman DS, et al. Functional results of operation in
osteogenesis imperfecta: elongating and nonelongating rods. J Pediatr
Orthop 1991;11(2):200-203.
AK, Kuhns LR, Guire KE. New standards of cortical mass in the humerus
of neonates: a means of evaluating bone loss in the premature infant.
Radiology 1980; 134(3):639-644.
C, Sirikulchayanonta V, Mahachokelertwattana P, et al. Cushing syndrome
caused by Ewing’s sarcoma secreting corticotropin releasing factor-like
peptide. Am J Dis Child 1992;146(9):1103-1105.
P, Hohmann F, Kuhl J, et al. Vertebral remodeling in eosinophilic
granuloma of the spine. A long-term follow-up. Spine
1998;23(12):1351-1354.
AH, Frech RS, Vietti TJ. Osteoporotic fractures secondary to
methotrexate therapy of acute leukemia in remission. Cancer
1970;25(3):580-585.
F, Travers R, Norman ME, et al. Deficient bone formation in idiopathic
juvenile osteoporosis: a histomorphometric study of cancellous iliac
bone. J Bone Miner Res 2000;15(5):957-963.
F, Travers R, Norman ME, et al. The bone formation defect in idiopathic
juvenile osteoporosis is surface-specific. Bone 2002;31(1):85-89.
PG, Green RH, Coggins AM, et al. Malignant osteoporosis: hypercalcaemia
after bone marrow transplantation. Arch Dis Child 1995;66:638-639.
JD, Huffer WE, August CS, et al. The hematopoietic effects of
prednisone therapy in four infants with osteopetrosis. J Pediatr
1979;94(2):210-214.
PF, Kranik A, Van Herpe L, et al. Congenital pseudarthrosis of both
bones of the forearm. A case report. J Bone Joint Surg Am
1976;58(7):1032-1023.
M, Fisher LW, Shenker A, et al. Fibrous dysplasia of bone in the
McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol
1997;151(6): 1587-1600.
P, Karnel F, Hajek P. Fibrous metaphyseal defects—determination of
their origin and natural history using a radiomorphological study.
Skeletal Radiol 1988; 17(1):8-15.
RH, Murrell JS. Traumatic ischaemia in a haemophiliac. Report of a case
of prolonged haemostasis with cryoprecipitate during decompression and
skin grafting. J Bone Joint Surg Br 1971;53(1):113-117.
S, Mckay D, Nason S. Dislocation of the spine in neurofibromatosis. A
report of two cases. J Bone Joint Surg Am 1982;64(8):1240-1242.
WB, Schwend RM, Jaramillo D, et al. Chronic physeal fractures in
myelodysplasia: magnetic resonance analysis, histologic description,
treatment, and outcome. J Pediatr Orthop 1997;17(5):615-621.
DI, Scott JA, Barranger J, et al. Evaluation of Gaucher disease using
magnetic resonance imaging. J Bone Joint Surg Am 1986;68(6):802-808.
RK, Levine DB. Fragmentation of the distal pole of the patella in
spastic cerebral palsy. J Bone Joint Surg Am 1977;59(7):934-939.
BT, Kling TJ. Treatment of active unicameral bone cysts with
percutaneous injection of demineralized bone matrix and autogenous bone
marrow. J Bone Joint Surg Am 2002;84-A(6):921-929.
R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome
with olpadronate in children with osteogenesis imperfecta: a 2-year
randomized placebo controlled study. Lancet 2004;363(9419):1427-1431.
IB, Coburn JW, Foley J, et al. Effects of oral calcium carbonate on
control of serum phosphorus and changes in plasma aluminum levels after
discontinuation of aluminum-containing gels in children receiving
dialysis. J Pediatr 1986;108(5 Pt 1): 767-770.
HW, Veth RP, Pruszczynski M, et al. Aneurysmal bone cysts treated by
curettage, cryotherapy and bone grafting. J Bone Joint Surg Br
1997;79(1):20-25.
SP, Temple HT, O’Keefe RJ, et al. The surgical treatment of patients
with osteosarcoma who sustain a pathologic fracture. Clin Orthop
1996;(324):227-232.
F, Glimcher MJ, Holtrop ME, et al. Human osteopetrosis: a histological,
ultrastructural, and biochemical study. J Bone Joint Surg Am
1980;62(3):384-399.
HH, Cruz M, Stambaugh J. Vitamin D prophylaxis and the lowered
incidence of fractures in anticonvulsant rickets and osteomalacia. Clin
Orthop 1977;29:251-257.
Y. Osteogenesis imperfecta. Review of the literature with presentation
of 29 cases. Am J Dis Child 1975;129(6):679-687.
FN. Recovery from epiphyseal invagination: sequel to an unusual
complication of scurvy. J Bone Joint Surg Am 1970;52(2):384-390.
M, Nagrath AR, Maini PS. Changes in trabecular pattern of the upper end
of the femur as an index of osteoporosis. J Bone Joint Surg Am
1970;52(3):457-467.
R, Gigante C, Bisinella G, et al. Musculoskeletal manifestations in
pediatric acute leukemia. J Pediatr Orthop 2008;28(1):20-28.
R, Specht EE. Osseous lesions and pathologic fractures in congenital
cytomegalic inclusion disease: report of a case. Clin Orthop
1979;(144):280-283.
BD, Hauser-Kara DA, Hipp JA, et al. Predicting fracture through benign
skeletal lesions with quantitative computed tomography. J Bone Joint
Surg Am 2006;88(1): 55-70.
H, Millar EA. Fragmentation, realignment, and intramedullary rod
fixation of deformities of the long bones in children. J Bone Joint
Surg Am 1959;41:1371-1391.
H, Dickson RA. Reversed dynamic slings for knee-flexion contractures in
the hemophiliac. J Bone Joint Surg Am 1975;57(2):282-283.
RE, Urbaniak J. Use of the tourniquet during surgery in patients with
sickle cell hemoglobinopathies. Clin Orthop 1980;(151):231-233.
RD, Pepin M, Byers PH. Studies of collagen synthesis and structure in
the differentiation of child abuse from osteogenesis imperfecta. J
Pediatr 1996;128(4): 542-547.
RB, London MD, Hankin FM, et al. Fibrous dysplasia. An analysis of
options for treatment. J Bone Joint Surg Am 1987;69(3):400-409.
NS, Zionts LE. Displaced fractures of the apophysis of the olecranon in
children who have osteogenesis imperfecta. J Bone Joint Surg Am
1993;75(7):1026-1033.
ML, Wong-Chung J. Prophylactic bypass grafting of the prepseudarthrotic
tibia in neurofibromatosis. J Pediatr Orthop 1991;11(6):757-764.
RJ, Meyer JS, Dormans JP, et al. Diagnosing aneurysmal and unicameral
bone cysts with magnetic resonance imaging. Clin Orthop Relat Res
1999(366):186-190.
HW, Kuo DP, Shu WP, et al. Giant-cell tumor of bone: analysis of 208
cases in Chinese patients. J Bone Joint Surg Am 1982;64(5):755-761.
JD, Atwood R, Lowe S, et al. Anesthetic considerations in the child
with Gaucher disease. J Clin Anesth 1993;5(2):150-153.
RJ, Gertner JM. Vitamin D deficiency rickets as a late complication of
the short gut syndrome during infancy. J Pediatr Surg
1981;16(3):230-235.
PF, Cowell HR, Steg NL. Lower extremity fractures simulating infection
in myelomeningocele. Clin Orthop 1979;44:255-259.
C, Farsetti P, Gatti S, et al. Influence of chronic osteomyelitis on
skeletal growth: analysis at maturity of 26 cases affected during
childhood. J Pediatr Orthop 1991;11(3):358-363.
RE, Wunder JS, Isler MH, et al. Giant cell tumor of long bone: a
Canadian Sarcoma Group study. Clin Orthop Relat Res 2002;397:248-258.
Der Sluis IM, Van Den Heuvel-Eibrink MM. Osteoporosis in children with
cancer. Pediatr Blood Cancer 2008;50(2 Suppl):474-478; discussion 486.
Lie Peters EM, Aronson DC, Everts V, et al. Failure of calcitriol
treatment in a patient with malignant osteopetrosis. Eur J Pediatr
1993;152(10):818-821.
De Dios AM, Bond JR, Shives TC, et al. Aneurysmal bone cyst. A
clinicopathologic study of 238 cases. Cancer 1992;69(12):2921-2931.
F, Shah N, Porter M. Bilateral charnley low-friction arthroplasty with
cement in a patient with pyknodysostosis. A case report. J Bone Joint
Surg Am 2006;88(8): 1846-1848.
TA, Scholz DT, Oldenburg J, et al. Osteoporosis in haemophilia—an
underestimated comorbidity? Haemophilia 2007;13(1):79-84.
JM. Copper: not too little, not too much, but just right. Based on the
triennial Pewterers Lecture delivered at the National Hospital for
Neurology, London, on 23 March 1995. J R Coll Physicians Lond
1995;29(4):280-288.
J, Temple HT, Pitcher JD, et al. Salvage of failed massive allograft
reconstruction with endoprosthesis. Clin Orthop Relat Res
2006;443:296-301.
C. Polystotic fibrous dysplasia—Albright syndrome. A review of the
literature and report of four male cases, two of which were associated
with precocious puberty. J Bone Joint Surg Am 1949;31:175-183.
K, Tsuchiya H, Sakurakichi K, et al. Treatment of lower limb
deformities and limb-length discrepancies with the external fixator in
Ollier’s disease. J Orthop Sci 2007;12(5):471-475.
AJ, Daniel RK. Congenital pseudarthrosis of the tibia: treatment with
vascularized autogenous fibular grafts. A preliminary report. Johns
Hopkins Med J 1980; 147(3):89-95.
DR, Jeffcoat BT, Herring JA. The guarded prognosis of physeal injury in
paraplegic children. J Bone Joint Surg Am 1980;62(2):241-246.
H, James J. Self-limiting neonatal primary hyperparathyroidism
associated with familial hypocalciuric hypercalcaemia. Arch Dis Child
1993;69(3 Spec No): 319-321.
RB, Moe JH, Bradford DS, et al. Spine deformity in neurofibromatosis. A
review of 102 patients. J Bone Joint Surg Am 1979;61(5):677-694.
J, Dormans J, Rang M. Pseudarthrosis of the rabbit tibia: a model for
congenital pseudarthrosis? J Pediatr Orthop 1991;11(3):277-283.
JS, Paulian G, Huvos AG, et al. The histological response to
chemotherapy as a predictor of the oncological outcome of operative
treatment of Ewing sarcoma. J Bone Joint Surg Am 1998;80(7):1020-1033.
L, Fassier F, Glorieux FH. Modern approach to children with
osteogenesis imperfecta. J Pediatr Orthop B 2003;12(2):77-87.
LE, Moon CN. Olecranon apophysis fractures in children with
osteogenesis imperfecta revisited. J Pediatr Orthop 2002;22(6):745-750.
AH, Ahmed HA, Qaisruddin S, et al. Osteomyelitis and septic arthritis
in sickle cell disease in the eastern province of Saudi Arabia. Int
Orthop 1992;16(4):398-402.
WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of
pathogenesis and management. J Bone Joint Surg Am 1977;59(3):287-305.
A, Browne RS, Wassef M, et al. The clinical features of early bleeding
into the muscles of the lower limb in severe haemophiliacs. J Bone
Joint Surg Br 1983;65(1):19-23.
RJ, Westbrook HW, Riggs W Jr. Childhood acute lymphocytic leukemia.
Initial radiological bone involvement and prognosis. Am J Dis Child
1972;124(5):653-654.
PK, Drummond DS, Dormans J, et al. Orthopaedic manifestations of
chronic graft-versus-host disease. J Pediatr Orthop 1998;18(5):572-575.
TH, Brown ML, Fitzgerald RH Jr, et al. Magnetic resonance imaging:
application in musculoskeletal infection. Magn Reson Imaging
1985;3(3):219-230.
TR, Heyman S. Skeletal scintigraphy of pseudo-osteomyelitis in Gaucher
disease. Two case reports and a review of the literature. Clin Nucl Med
1992;17(4):279-282.
P, Witvoet J, Sedel L. Avascular necrosis of the femoral head after
allogenic bone marrow transplantation. A retrospective study of 27
consecutive THAs with a minimal 2-year follow-up. J Bone Joint Surg Br
1996;78(6):878-883.
EE, Jordan HH. Radiologic aspects of hemophilic pseudotumors in bone.
Am J Roentgenol Radium Ther Nucl Med 1972;115(3):525-539.
OW, Cornelius LJ, Edwards LR, et al. NIH consensus development
statement on hydroxyurea treatment for sickle cell disease. NIH Consens
State Sci Statements 2008; 25(1):1-30.
VL, Parmley RT, Bozzini M, et al. Radiotherapy of pseudotumors of bone
in hemophiliacs with circulating inhibitors to factor VIII. Am J
Hematol 1991;36(1):55-59.
CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection
of bone marrow or methylprednisolone. J Bone Joint Surg Br
2002;84(3):407-412.
HS, Oh JH, Kim HS, et al. Unicameral bone cysts: a comparison of
injection of steroid and grafting with autologous bone marrow. J Bone
Joint Surg Br 2007;89(2):222-226.
JT, Amato D, Deber RB. Managing public payment for high-cost,
high-benefit treatment: enzyme replacement therapy for Gaucher disease
in Ontario. CMAJ 2001;165(5): 595-596.
N, Gotze H, Pedersen A, et al. Skeletal scintigraphy and radiography at
onset of acute lymphocytic leukemia in children. Med Pediatr Oncol
1983;11(4):291-296.
GP, Drummond DS, Davidson RS, et al. Bone infarction versus infection
in sickle cell disease in children. J Pediatr Orthop 1996;16(4):540-544.
JK, Tsakiris D, Briggs JD, et al. Osteonecrosis and fractures following
renal transplantation. Clin Radiol 1985;36(1):27-35.
AM, James CD, King DR, et al. Head trauma in children with congenital
coagulation disorders. J Pediatr Surg 1994;29(1):28-32.
JP, Drummond DS. Pediatric hematogenous osteomyelitis: new trends in
presentation, diagnosis, and treatment. J Am Acad Orthop Surg
1994;2(6):333-341.
CH, Bryant DD Jr, Coles MJ 3rd, et al. Osteomyelitis in patients who
have sickle cell disease. Diagnosis and management. J Bone Joint Surg
Am 1991;73(9):1281-1294.
EH. Radiocolloids in the management of hemophilic arthropathy in
children and adolescents. Clin Orthop 1991;264:129-135191 476. Ferris
B, Walker C, Jackson A, et al. The orthopaedic management of
hypophosphataemic rickets. J Pediatr Orthop 1991; 11(3):367-373.
ML, Rosenbloom BE, Kay AC, et al. A less costly regimen of alglucerase
to treat Gaucher disease. N Engl J Med 1992;327(23):1632-1636.
DJ, Phillips DJ, Heinrich SD. Orthopedic manifestations of acute
pediatric leukemia. Orthop Clin North Am 1996;27(3):635-644.
JS, Maciver JE, Went LN. The bone changes in sickle cell anaemia and
its genetic variants. J Bone Joint Surg Br 1959;41-B:711-718.
A, Wosko I, Kandzierski G. Intra-articular bleeding in children with
hemophilia: the prevention of arthropathy. J Pediatr Orthop
1989;9(2):182-185.
IM, Gupta S, Palmer MK, et al. The prognostic significance of
radiological and symptomatic bone involvement in childhood acute
lymphoblastic leukaemia. Med Pediatr Oncol 1979;6(1):51-55.
I, Le Balc’h T, Yvart J, et al. MR imaging of hemophilic arthropathy of
the knee: classification and evolution of the subchondral cysts. Magn
Reson Imaging 1992;10(1): 67-75.
GI, Mathews JA, Bennett AE. Controlled trial of joint aspiration in
acute haemophilic haemarthrosis. Ann Rheum Dis 1972;31(5):423.
M, Lebel E, Dweck A, et al. Orthopedic considerations in Gaucher
disease since the advent of enzyme replacement therapy. Acta Orthop
Scand 2004;75(6):641-653.
JM, Miller KL, Anderson AM, et al. Arthroscopic synovectomy in children
and adolescents with hemophilia. J Pediatr Hematol Oncol
2003;25(9):726-731.
CT, Burke C. Double-blind studies on the use of steroids in the
treatment of acute hemarthrosis in patients with hemophilia. N Engl J
Med 1970;282(12):639-642.
A, Gumruk F, Gurgey A. The effect of hydroxyurea on the coagulation
system in sickle cell anemia and beta-thalassemia intermedia patients:
a preliminary study. Pediatr Hematol Oncol 2003;20(6):429-434.
A, Garty I, Katzuni E. Bone infarction in children with sickle cell
disease: early diagnosis and differentiation from osteomyelitis. Eur J
Pediatr 1984;142(2):93-97.
A, Segal-Kupershmit D, Zalman L, et al. Effect of hydroxyurea in sickle
cell anemia: a clinical trial in children and teenagers with severe
sickle cell anemia and sickle cell beta-thalassemia. Pediatr Hematol
Oncol 1999;16(3):221-232.
S, Fulco JD, Karayalcin G, et al. Gray scale ultrasound: evaluation of
iliopsoas hematomas in hemophiliacs. AJR Am J Roentgenol
1979;133(1):103-105.
F, Ezra E, Khermosh O, et al. Simple bone cysts treated by percutaneous
autologous marrow grafting. A preliminary report. J Bone Joint Surg Br
1996;78(6):934-937.
MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic
treatment to prevent joint disease in boys with severe hemophilia. N
Engl J Med 2007;357(6): 535-544.
G, Carnelli V, Ferrari M, et al. Prognostic significance of
radiological bone involvement in childhood acute lymphoblastic
leukaemia. Arch Dis Child 1977;52(7):530-533.
PJ, Fietz MJ, Hopwood JJ. Diagnosis of lysosomal storage disorders:
current techniques and future directions. Expert Rev Mol Diagn
2004;4(5):677-691.
EH, Flessa HC, Glueck HI. The management of deep soft tissue bleeding
and hemarthrosis in hemophilia. Clin Orthop 1972;82:92-107.
RM, Mankin H. Use of plain radiography to optimize skeletal outcomes in
children with type 1 Gaucher disease in Brazil. J Pediatr Orthop
2007;27(3):347-350.
CF, Smith-Whitley K, Mcgowan KL. Positive blood cultures in sickle cell
disease: time to positivity and clinical outcome. J Pediatr Hematol
Oncol 2003;25(5):390-395.
R, Kilcoyne RF, Geraghty S, et al. Utility of magnetic resonance
imaging for management of hemophilic arthropathy in children. J Pediatr
1993;123(3):388-392.
WL, Galleno H. Operative treatment versus steroid injection in the
management of unicameral bone cysts. J Pediatr Orthop 1984;4(1):1-7.
JS, Ryu KN. Hemophilic pseudotumor involving the musculoskeletal
system: spectrum of radiologic findings. AJR Am J Roentgenol
2004;183(1):55-61.
DR, Chan LS, Hiti A, et al. Outcome of sickle cell anemia: a 4-decade
observational study of 1056 patients. Medicine (Baltimore)
2005;84(6):363-376.
EC. Pathogenesis, early diagnosis, and prophylaxis for chronic
hemophilic synovitis. Clin Orthop 1997;343:6-11.
RL, Graham JJ, Selirio E. Excision of pseudotumor with repair by bone
graft of pathological fracture of femur in hemophilia. J Bone Joint
Surg Am 1973;55(4):827-832.
RJ, Poehling GG, Gray R, et al. Orthopedic complications of renal
transplantation in children. Transplant Proc 1979;11(1):104-106.
GM, Cheng MY, Yeung CY. Back pain and vertebral compression: an
uncommon presentation of childhood acute lymphoblastic leukemia. J
Pediatr Orthop 1987;7(2): 175-178.
O, Marchetti PG, Bartolozzi P. Final results obtained in the treatment
of bone cysts with methylprednisolone acetate (depo-medrol) and a
discussion of results achieved in other bone lesions. Clin Orthop Relat
Res 1982(165):33-42.
CM, Beelen DW. Avascular osteonecrosis after allogeneic hematopoietic
stem-cell transplantation: diagnosis and gender matter. Transplantation
2004;78(7):1055-1063.
R, Huurman WW, Lippiello L, et al. Prostaglandin levels in unicameral
bone cysts treated by intralesional steroid injection. J Pediatr Orthop
1989;9(5):516-519.
A, Mauro MA, Staab EV, et al. Soft-tissue hemorrhage in hemophiliac
patients. Computed tomography and ultrasound study. Radiology
1983;147(3):811-814.
AL, Berdon WE, Wolff JA, et al. Acute lymphocytic leukemia masquerading
as acute osteomyelitis. A report of two cases. Pediatr Radiol
1980;9(1):33-35.
DL, Kim SK, Greene NW, et al. Differentiation between bone infarction
and acute osteomyelitis in children with sickle-cell disease with use
of sequential radionuclide bone-marrow and bone scans. J Bone Joint
Surg Am 2001;83-A(12):1810-1813.
JE, Glasier CM, Blaisier RD, et al. Osteomyelitis in children with
sickle cell disease: early diagnosis with contrast-enhanced CT.
Radiology 1991;179(3):731-733.
P, Lipton JM, Sahdev I, et al. Allogenic bone marrow transplantation in
severe Gaucher disease. Pediatr Res 1992;31(5):503-507.
H, Haramati N, Flusser G. The diagnostic role of gadolinium enhanced
MRI in distinguishing between acute medullary bone infarct and
osteomyelitis. Magn Reson Imaging 2000;18(3):255-262.
L, Kallio MJ, Eskola J, et al. Serum C-reactive protein, erythrocyte
sedimentation rate, and white blood cell count in acute hematogenous
osteomyelitis of children. Pediatrics 1994;93(1):59-62.
EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and
aggressive transfusion regimens in the perioperative management of
sickle cell disease. The Preoperative Transfusion in Sickle Cell
Disease Study Group. N Engl J Med 1995;333(4):206-213
SY, Esmail AN, Bunin N, et al. Avascular necrosis in children with
acute lymphoblastic leukemia. J Pediatr Orthop 2000;20(3):331-335.
SM, Lundeen GA, Scott SM, et al. Autogenic bone marrow injections as a
treatment for simple bone cyst. J Pediatr Orthop 1998;18(5):616-620.
A, Elstein D, Kannai R, et al. Low-dose enzyme replacement therapy for
Gaucher disease: effects of age, sex, genotype, and clinical features
on response to treatment. Am J Med 1994;97(1):3-13.
