CERVICAL DISC DISEASE
Baptist Spine Center, New England Baptist Hospital; and Department of
Orthopaedic Surgery, Tufts University School of Medicine, Boston,
Massachusetts, 02120.
the cervical spine can be considered part of the normal aging process.
The progressive deterioration that develops is commonly found in
asymptomatic individuals but also may lead to neurocompression,
radiculopathy, and myelopathy. The presentation of these syndromes
depends on the specific structures compromised by the degenerative
process. Treatments of axial neck pain, radicular arm pain, and
myelopathy are based on a clear understanding of the natural history of
the disorders and available therapeutic options. This chapter reviews
the pathophysiology of cervical spondylosis and relates it to the
development of clinical manifestations, including the evaluation as
well as the nonoperative and operative management of such problems.
anatomy is essential to appreciate the role of management of the
symptomatic patient. The cervical spine comprises seven vertebrae, each
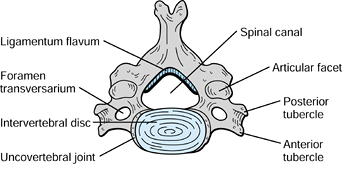
possessing five articulations (Fig. 143.1). The
cranial two vertebrae, the atlas and axis, are anatomically unique,
whereas the third through the seventh (subaxial) vertebrae are more
typical. The first two are rarely involved in the degenerative process
and are
more commonly affected by inflammatory processes such as rheumatoid arthritis (see Chapter 154).
cephalad to caudad and are greater in the transverse than in the
anteroposterior (AP) dimension (46). The
superior endplate surface is concave, whereas the inferior surface is
convex. Uncovertebral joints of Luschka or uncinate processes project
from the superoposterior corner of each vertebral body and form a
synovium-lined articulation with the corresponding vertebra (32).
Short, small pedicles arise from the posterior vertebral body and
extend posterolaterally to the lateral masses. The lateral masses are
unique to the cervical spine and form superior and inferior
articulations via synovium-lined facet joints. The laminae extend
posteromedially from the lateral masses and form into the spinous
process, which in the cervical spine are ordinarily bifid.
 |
|
Figure 143.1.
Cross-sectional diagram of a normal cervical vertebra. Note the articulations of zygapophyseal facet joints and uncovertebral joints of Luschka. |
achieved through the shape and configuration of the intervertebral
discs. These discs make up nearly 22% of the overall length of the
cervical spine (46). They are thicker in height
in the anterior aspect of the intervertebral space supporting the
lordotic curvature. The intervertebral discs increase range of motion
between the vertebral bodies and distribute forces over the length of
the spine (46).
gelatinous nucleus pulposus, the outer annular fibrosis, and the
superior and inferior endplate cartilage. The annulus is composed of
alternating layers of collagen fibers running in oblique directions. A
normal functioning disc will disperse forces by initial expansion of
the nucleus pulposus and stretching of the annular fibers. This process
essentially converts an axial load into horizontal forces absorbed by
the annulus (46).
cervical spine. The neuroforamina are confined zones for the exiting
nerve roots, bordered anteriorly by the lateral aspect of the
intervertebral disc and the uncovertebral joint, superiorly and
inferiorly by the pedicles, and posteriorly by the articular masses,
notably the superior articular facet. Pathologic conditions involving
these structures can lead to critical stenosis of the foramen and nerve
root compression.
cervical spine is subtle and is part of the degenerative cascade. The
initial alterations are suspected to occur within the intervertebral
disc leading to secondary changes in the surrounding facet joints and
soft-tissue structures. Diminished water content along with changes in
the ratio of proteoglycan to collagen, and keratin sulfate to
chondroitin sulfate are early manifestations of degeneration (60).
Because of this, the nucleus pulposus no longer can generate the
hydrostatic intradiscal force required to expand the annular fibers.
This subjects the annular fibers to compression and shear forces,
causing weakening and tearing in the outer layers. Disc protrusion or
frank herniation may ensue, with or without neurocompression.
more prominent in the anterior disc space because the uncovertebral
joints impact on the posterior vertebral bodies as collapse occurs,
preventing further posterior disc height loss. The combined effect
leads to the characteristic loss of cervical lordosis on lateral plain
radiographs (48).
biomechanical forces placed on the uncovertebral joints and articular
facet joints. Osteophytic spurring, often referred to as hard disc, may
develop, leading to encroachment on the neuroforamina. Similarly,
reactive bone forms along the posterior vertebral bodies as the margins
come into greater contact when higher forces are applied. A spondylotic
transverse bar may subsequently form, in combination with bulging of
the posterior disc and stretching of the posterior longitudinal
ligament. Further collapse of the anterior column height leads to
buckling of the ligamentum flavum into the spinal canal, most notably
during neck extension. This combination of events may lead to
spondylosis-induced compromise of the AP diameter of the canal.
potential sources of pain. Distortion of the intervertebral disc may
lead to stretching or compression of the sinuvertebral nerve and finer
nerve endings, with subsequent symptoms (54).
Additionally, distortion or injury of innervated areas such as the
apophyseal facet joints, ligamentous structures, and posterior
musculature may produce pain.
from posterolateral soft disc herniation either contained by the
posterior longitudinal ligament (PLL) or as free material extruded into
and sequestered within the canal (Fig. 143.2).
In addition, foraminal stenosis from the changes previously described
may also lead to impingement on the exiting nerve root (Fig. 143.3).
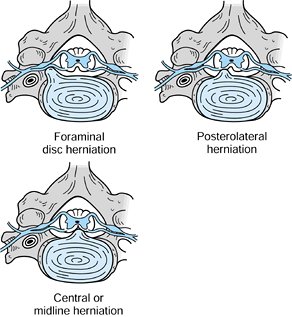 |
|
Figure 143.2. Depiction of types of “soft disc” herniation causing impingement on the exiting nerve root or spinal cord.
|
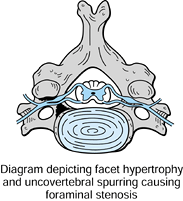 |
|
Figure 143.3. Nerve root impingement secondary to a “hard disc” from spondylosis and foraminal stenosis.
|
The stenotic spinal canal may lead to neurologic dysfunction via
compression of the anterior spinal artery with cord ischemia or
mechanical deformation of the spinal cord from direct pressure, and/or
dynamic compression (Fig. 143.4).
Hyperextension may cause the lax and hypertrophied ligamentum flavum to
buckle, compressing the spinal cord against the anterior spondylitic
bar. Another mechanism of dynamic compression occurs in flexion, where
the spinal cord is stretched over the anterior bony prominences.
Occasionally, a midline soft disc herniation may cause cord compression
and myelopathy combined with a degree of nerve root compression,
leading to a myeloradiculopathy.
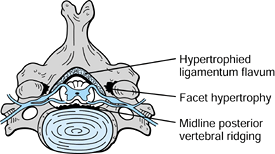 |
|
Figure 143.4. Spondylosis leading to spinal cord compression secondary to hypertrophied ligamentum flavum and spondylotic ridging.
|
secondary to ossification of the PLL (OPLL). The ossified mass arising
from the PLL has been classified as continuous, extending over several vertebral bodies; segmental, ossification at the level of the posterior vertebral bodies only; mixed continuous and segmental; and localized, with ossification at the level of the disc only (13).
symptoms, establishing a prognosis for a patient with axial neck pain
can be difficult. The natural history of neck pain has been evaluated
by Gore et al. (30), who performed a
retrospective review of patients with neck pain followed clinically and
radiographically over a 10-year period. Of these patients, 79% had
diminished pain and 43% had nearly complete relief of symptoms.
However, nearly one third of the study group reported persistent
moderate to severe pain. Outcome could not be correlated with
radiographic or clinical findings, making outcome projections difficult
for patients with neck pain.
well described. Lees and Turner reported on the long-term follow-up of
patients with spondylosis and confirmed that 30% experienced
intermittent radicular symptoms and 25% had persistent pain (45). Progression from radiculopathy to myelopathy is unusual, and most likely these are distinct entities (25).
In general, there is agreement that nonoperative treatment may
alleviate symptoms of cervical spondylitic radiculopathy (CSR) in the
short term, but over a long period of time symptoms frequently recur.
Gore et al. retrospectively reviewed patients with cervical
radiculopathy treated conservatively and noted that 50% had persistent
symptoms at 15-year follow-up (30).
an insidious onset of symptoms and, in general, neurologic function
undergoes episodes of worsening with intervening stable periods (42). However, there are no pathognomonic findings to predict the progression of symptoms (49).
Clarke and Robinson evaluated the natural history of CSM prior to
treatment and concluded that 75% in their cohort experienced episodic
worsening of symptoms; 20% showed slow, steady progression without
intervening stabilizing periods; and 5% experienced rapid onset of the
disease process and progression (18).
Progression to total disability is unusual, although slight incremental
neurologic deterioration may occur with time, resulting in upper and
lower extremity functional deficits. As the neurologic deficit worsens,
improvement of disability becomes more unlikely and complete recovery
even less likely.
degenerative disorders requires interpretation of the patient’s
complaints, meticulous examination, and appropriate selection of
diagnostic tests. To perform a complete evaluation of a patient’s
complaints, first determine if the problem involves neck pain, arm
pain, or a combination of both, or whether there is a myelopathic
component. A detailed history is the initial step in evaluating a
patient with cervical degenerative disc disease. Obtain a complete
description of the symptomatology, including the onset, quality, and
location of pain; inciting and alleviating factors; temporal nature;
degree of impairment; and any associated symptoms.
origin, or it may be related to shoulder, occipitocervical, myofascial,
or visceral pathology. To differentiate the potential multiple sources
of neck pain, establish whether the symptoms are mechanical (increased
with activity and diminished with rest or positioning) or nonmechanical
(no relief with positional changes or rest). Nonmechanical neck pain
may be related to tumor or infection, and such processes should be
carefully sought out. A history of deep-seated aching pain that occurs
only at night and is absent or markedly diminished during the day is
suggestive of neoplasm or infection. Mechanical neck pain is commonly
discogenic in origin and exacerbated with neck extension and rotation
toward the side that is more symptomatic. Patients may describe pain
referred to the shoulder, upper arm region, or interscapular area.
Patients with upper cervical degeneration may also experience occipital
or temporal pain, or retro-ocular headaches. Musculogenic pain, as from
an acute or chronic muscle strain, is more often exacerbated with neck
flexion and rotation, leading to increased symptoms on the opposite
side of head rotation.
herniation, chronic disc degeneration with osteophytic spurring, or
segmental instability. The majority of patients present with a
monoradiculopathy, although several roots can be involved. Symptoms
consist of sharp, lancinating, radiating arm pain associated with
various degrees of dysesthesia, paresthesia, and numbness along a
dermatomal pattern consistent with distribution of the involved nerve
root.
provocative tests. Typically, patients will describe an increase in
pain with Valsalva activities and with neck extension or turning the
head toward the symptomatic side. A Spurling’s sign is indicative of
radiculopathy. This is elicited by neck hyperextension and rotation
toward the symptomatic side, resulting in reproduction of the pain.
This maneuver serves to diminish the available area in an already
compromised neuroforamen, leading to further nerve root compression. A
less reliable provocative sign is the axial compression test, in which
compression on the vertex of the skull may diminish the height of the
foramen and also symptoms. The shoulder abduction sign is a test that
relieves symptoms of compression by lessening nerve root stretch with
placement of the ipsilateral hand on top of the head. Patients may
present with this as the only upper extremity position that provides
relief or comfort.
radiculopathy. Typically, the patterns of pain distribution that
patients describe are imprecise because of anatomic variations,
involvement of multiple levels, or the presence of chronic conditions.
Upper cervical nerve root compression is less common than lower levels;
however, it must be considered in the differential diagnosis of
recalcitrant neck pain.
area of the chest. The symptoms are described as pain with variable
degrees of paresthesia but without a specific motor deficit. A more
classic presentation occurs with compression of the lower cervical
nerve roots. A C-5 radiculopathy produces radiating pain down the
lateral aspect of the shoulder and proximal arm with associated sensory
changes and/or increased fatigue or weakness of shoulder abduction. The
C-5 root solely innervates the deltoid, whereas the biceps has dual
innervation from C-5 and C-6.
the biceps and anterior arm to the radial aspect of the forearm and
index finger and thumb. The biceps and wrist extensors may demonstrate
weakness. The extensor carpi radialis longus and brevis are innervated
by C-6, and the extensor carpi ulnaris is primarily C-7. Therefore,
wrist extensor weakness may reflect compression of either C-6 or C-7.
The brachioradialis reflex is most directly affected with C-6
compression with subtle changes noted in the biceps reflex due to its
dual innervation.
with pain along the posterior shoulder and arm, radiating to the
posterolateral aspect of the forearm to the long finger. Inconsistent
symptoms involving the index and ring digits as well as the first web
space may also be detected. The triceps muscle is affected, resulting
in a diminished reflex and elbow extensor weakness. Triceps weakness is
an infrequent complaint, unless the patient is physically active.
the ulnar aspect of the forearm, small finger, and ulnar half of the
ring finger. The findings are primarily below the elbow, with most
dysfunction noted as numbness along the ulnar digits and weakness in
finger adduction, abduction, and flexion. In chronic C-8 root
compression, intrinsic muscle atrophy may be seen.
complaints may be vague, so myelopathy is not easily picked up on the
initial examination (17,23).
Symptoms and findings can include gait difficulties, spasticity,
decreased manual dexterity, paresthesias in the extremities, urinary
urgency or frequency, and specific extremity or generalized weakness (52).
In contrast to cervical radiculopathy, pain is not a common presenting
finding. Depending on the site of anatomic spinal cord compression, the
symptoms may be quite variable.
complaint. Patients describe insidious and slowly progressive stumbling
or generalized gait disturbances. Patients may initially become aware
of these changes from family members who note a shuffling gait or
frequent falls. The characteristic stooped, wide-based gait of the
elderly is the common end result. Involvement of the upper extremities
may occur concomitantly or follow the gait changes, with complaints of
clumsy or numb hands. Weakness of the hand manifests as decreased grip
strength. Manual dexterity will often suffer and progress until the
patient lacks the ability to complete routine activities such as
buttoning a shirt, counting change, or writing. Several researchers
have noted characteristic hand dysfunction in cervical myelopathy. For
example, Ono et al. reported on the myelopathic hand syndrome,
describing the finger escape sign and grip and release test (53).
The finger escape sign is positive when the patient is asked to hold
all the digits of her hand in an adducted and extended position and the
two ulnar digits fall into abduction and flexion with time. In the grip
and release test, the patient is asked to rapidly form a fist and then
release all digits into extension repeatedly. A patient without
myelopathy should be able to perform this test 20 times in a 10-second
period.
of thorough neurologic and other special testing. The presence of lower
extremity clonus and Babinski extensor plantar responses should be
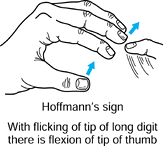
noted. The Hoffmann’s reflex (finger and thumb interphalangeal flexion
with sudden long finger distal interphalangeal joint extension) when
present, and especially when asymmetrical, is strongly suggestive of
cervical myelopathy (Fig. 143.5). Other tests that may be noted include an inverted radial reflex, a scapulohumeral reflex, and Lhermitte’s sign (43).
 |
|
Figure 143.5.
Hoffmann’s reflex is indicative of cervical cord impingement. The reflex is positive if the fingers and thumb react in flexion when the long finger distal interphalangeal joint is flicked into extension. |
understanding of the pathologic process of cervical radiculopathy and
myelopathy. Several techniques are available for the evaluation of the
symptomatic patient. Each modality has its own inherent strengths and
weaknesses, and often combinations of examinations are required.
and oblique views and when instability is suspected, perform dynamic
lateral images in flexion–extension. Evaluate findings such as disc
space narrowing, developmental
canal
stenosis, subluxations and malalignments, and vertebral osteophtye
formation in light of symptoms. Abnormal findings on plain radiographs
may not be the cause of the clinical picture; therefore, further
correlative studies may be necessary prior to recommending specific
treatment. Changes on plain radiographs may also confirm the clinical
suspicion of typical degenerative disease and reassure the clinician
and the patient that appropriate therapy is being followed.
cervical radiculopathy and myelopathy. The AP view demonstrates the
exiting nerve roots to the level of the pedicle. A filling defect is a
typical finding of nerve root compression. The lateral view may detect
spinal cord compression by the disc or posterior vertebral osteophytes
and/or hypertrophied ligamentum flavum. Current practice includes
myelography followed by computed tomography (CT), which permits
visualization of osseous compressive structures, especially in the
neuroforamina (4). However, CT-myelography
infers neural compression by deformity of the dural sac or nerve roots
and cannot directly determine the etiology of contrast blockade.
information about nerve root or spinal cord compression. The advantage
of MRI in detecting direct compression is the intrinsic “contrast”
available from the cerebrospinal fluid (CSF), as seen on T2-weighted
images. This is the most sensitive modality for assessing the
morphology of the spinal cord and its relation to the spinal canal. MRI
also shows intramedullary cord changes, which may relate to disease
prognosis (64). However, MRI is less sensitive
in detecting foraminal stenosis and does not demonstrate cortical
margins as well as CT-myelography.
be utilized to confirm suspected radiculopathy or may be used as an
additional modality to further elucidate the cause of symptoms in a
patient with atypical findings. These tests may be most useful when
attempting to differentiate root compression and a peripheral
neuropathy. Nuclear medicine bone scanning, local diagnostic
injections, discography, and CSF analysis have a limited role in the
diagnostic process.
radiculopathy can be managed nonoperatively. The initial treatment of
moderate to severe symptoms should consist of a soft collar,
nonsteroidal anti-inflammatory agents (NSAIDs), and physical therapy
modalities, including traction, particularly when radicular signs are
present. The limited use of a soft collar may help to decrease the
dynamic compression of an irritated nerve root and permit the pain from
fatigue or spasm in the paraspinal muscles to resolve. Prolonged use of
a collar is not recommended because of the risk of paraspinal muscle
atrophy. Restrict activities to avoid neck extension and heavy lifting
during the acute period. Aspirin, ibuprofen, or NSAIDs may provide pain
relief. Use narcotic pain medications sparingly, especially in elderly
patients. Occasionally, a brief tapered course of oral cortisone may
alleviate symptoms of radiculopathy. Physical therapy modalities such
as heat and ultrasound may improve acute symptoms, but it is unclear
whether they have any effect on natural history. Manual or home
traction may provide relief of nerve root compression through
distraction of the intervertebral foramen.
(ESI) may be recommended for treatment of the inflammatory component of
cervical radiculopathy. The role of ESI is controversial, and the
literature lacks well-designed studies documenting their efficacy.
Before recommending ESI, weigh the short-term relief of symptoms
against possible risks and complications of needle placement (15,65).
in a soft collar to prevent dynamic spinal cord compression. However,
this is a temporizing measure only and is not definitive treatment.
secondary to spondylosis, canal stenosis, or discogenic neck pain are
limited. Whitecloud and Seago reported 70% good-to-excellent results
from anterior interbody fusion for patients with concordant neck pain
on discography (68). However, others have found
that fusion for discogenic neck pain based on provocative testing
yields results that are not much improved from the natural history of
the disorder (22). Conservative management for these individuals remains the treatment of choice.
spondylolisthesis or retrolisthesis is rare in the cervical spine.
Instability suggested on dynamic flexion–extension radiographs may be
managed by either anterior or posterior segmental fusion.
radiculopathy include (a) failure of a 3-month trial of nonoperative
treatment to relieve persistent or recurrent radicular arm pain with or
without neurologic deficit, and (b) a progressive neurologic deficit (9,27). Neuroradiographic findings must be consistent with the clinical signs and
symptoms, and the duration and magnitude of symptoms must be sufficient to justify surgery.
include anterior decompression with discectomy [anterior cervical
discectomy without fusion (ACD)] with or without interbody fusion
(ACDF/ACD), anterior corpectomy with fusion (ACF), posterior laminotomy
with foraminotomy, or laminectomy or laminoplasty with or without
fusion (Table 143.1).
 |
|
Table 143.1. Indications for the Operative Treatment of Cervical Radiculopathy
|
spine is a relatively safe procedure that takes advantage of normal
anatomic fascial planes during the approach (55,56 and 57).
-
Place the patient in the supine position
with a small roll placed under the shoulder blades to drop the
shoulders from the field and to present the anterior neck favorably.
Strap the shoulders at the side with minimal traction to allow
visualization of the lower cervical spine on lateral radiographs. -
Apply skull traction via a chin halter
device or with Gardner-Wells tongs. Keep head rotation to a minimum
because deep dissection will depend on identifying the vertebral
midline to prevent inadvertent injury to adjacent structures. The
reverse Trendelenburg position facilitates venous drainage and results
in less bleeding during surgery. -
The superficial anatomic landmarks for
incision include the hyoid bone overlying C-3, thyroid cartilage
overlying the C4–5 interspace, and cricoid cartilage overlying the C-6
level. Use transverse incision for exposure in most cases when one or
two discs are to be exposed. When three or more levels are approached,
use a longitudinal incision along the anterior border of the
sternocleidomastoid muscle. The transverse incision is preferred for
its cosmetic appeal and access to the anterior spine, whereas the
longitudinal incision serves to improve visualization of the region
over multiple levels and avoids excessive retraction that may otherwise
be necessary. -
Place the transverse incision along the
anterior neck from the midline to the anterior border of the
sternocleidomastoid in Langer’s lines. -
Divide the superficial fascia and platysma muscle exposing the middle layer of the cervical fascia.
-
Bluntly dissect the pretracheal fascia and palpate the carotid pulse.
structures at risk. The superior and inferior thyroid arteries extend
through the pretracheal fascia from the carotid artery to the midline.
The superior and inferior thyroid arteries travel at the C3–C4 and
C6–C7 levels, respectively. The intervening area provides a relatively
avascular plane for dissection. The recurrent laryngeal nerves are also
at risk during the anterior approach. The right recurrent laryngeal
nerve ascends in the neck after passing around the subclavian vessels
and courses medially and cranially at the C6–C7 level, often along with
the inferior thyroid artery. The left recurrent laryngeal nerve ascends
after curving around the aortic arch along the tracheoesophageal groove
in a more midline and protected position. A left-sided procedure may be
safer, especially when lower cervical segments are approached. However,
the thoracic duct is often visible on the left at the C7–T1 level and
must be protected.
-
Complete blunt dissection through the deeper levels to the prevertebral fascia and vertebral bodies.
-
Once the midline is identified, incise the prevertebral fascia and elevate the medial edges of the longus colli muscles.
-
Place blunt self-retaining retractors
under the leading edges of the muscle. Take care to avoid dissecting
along the longus colli muscle because injury to the cervical
sympathetic plexus is likely. -
Identify the vertebral bodies by their concave appearance and the discs by their more convex contour.
-
Localize the disc space with a radiopaque marker and lateral radiograph.
-
Incise the disc with an annulotomy blade and perform the decompression.
-
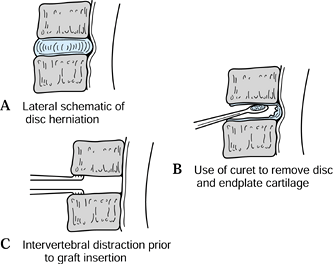
Remove the disc contents and endplate cartilage to the PLL (Fig. 143.6A, Fig. 143.6B and Fig. 143.6C).
The proper technique of discectomy involves removal of disc material in
a posterior to anterior direction and lateral to medial away from the
vertebral arteries. Use thorough evaluative preoperative imaging to
determine the presence of a sequestered disc behind the PLL. Palpate
the PLL for the presence of a rent that may also indicate a sequestered
fragment. In the event that a rent is noted, or if an expected disc
fragment is not identified, remove the PLL with Kerrison rongeurs or
curets. Beware of routine removal of the PLL, because reports of
postoperative epidural hematoma have been associated with this
technique (70).![]() Figure 143.6.
Figure 143.6.
Anterior cervical discectomy with distraction of the interspace and
removal of disc to the posterior longitudinal ligament. See text for
details. -
Removal of endplate and uncovertebral
osteophytes is controversial. The proposed benefits of fusion without
spur resection are that disc space distraction reduces ligamentum
flavum buckling and increases neuroforaminal area. It is believed that
fusion will arrest spur progression, and stability may allow for
resorption over time. However, this is not a consistent phenomenon, and
the location and size of the offending spur must be carefully
considered when performing decompression for spondylitic radiculopathy.
Exposure of the uncinate processes is critical to safely remove
osteophytes. -
Utilize a high-speed burr to excise the
spur from medial to lateral. Judge the adequacy of foraminotomy by the
ability to place the tip of a curet anterior to the exiting nerve root
without significant resistance.
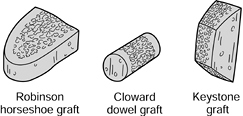 |
|
Figure 143.7. Types of anterior cervical grafts used in interbody arthrodesis.
|
placement of a tricortical iliac crest wedge graft into the disc space
for bony healing.
-
The graft height should be 2 mm greater
than the preexisting disc height, or at least 5 mm, to obtain adequate
compressive strength and to enlarge the neural foramina (1).
Overdistraction of the disc space by greater than 4 mm of the
preexisting height may result in graft collapse and pseudarthrosis (14). Achieve distraction of the disc space with skull traction, laminar spreader, vertebral screws, or combinations of these. -
Burr the endplates to create a flat
surface on both sides of the intervertebral space. Additionally, small
holes may be created in the endplates to promote vascularization of the
graft. -
After measuring the depth and width of
the disc space, harvest the tricortical graft from the anterior iliac
region. Obtain the graft with an oscillating bone saw, because graft
weakening has been associated with the use of osteotomes (37). -
Contour the graft to fit into the disc
space and insert it with the leading cortical edge anteriorly and inset
2 mm beyond the vertebral bodies. The graft may also be inserted in the
reverse position, with the leading cortex directed posteriorly, to
maximize posterior disc space
P.3755
and
foraminal distraction. This has been shown to be an acceptable
alternative to the more traditional graft position. The graft should be
stable with compression after removal of all traction devices.
-
After completion of the discectomy,
remove bone from the inferior aspect of the superior vertebral body and
the superior aspect of the inferior vertebral body with specialized
osteotomes. -
The end of the vertebra is beveled to an angle of between 14° and 18°, as recommended by Simmons and Bhalla (59). Maximally distract the neck and measure the graft site.
-
Harvest a rectangular iliac crest graft and contour to match the beveled surfaces of the vertebral bodies.
-
After the graft is impacted in the host bed, release the traction, thus locking the graft in place.
-
Make a trough ½ inch wide and 3/16 inch in depth along the full length of the vertebrae to be fused.
-
Remove the intervening disc and endplate cartilage to a depth of 3/16
inch and impact a unicortical iliac crest graft into place. Insert the
graft with the neck in extension and, after placement, flex the neck to
achieve stability.
the disadvantage of having no direct nerve root decompression, and thus
are seldom used today.
morbidity, the use of allograft bone has become a popular method of
interbody fusion. In one study, although nonunion rates and graft
collapse were more common in ACDF with freeze-dried tricortical iliac
crest allograft, the clinical results were similar to ACDF with
autogenous bone graft (77). Fibular allograft
has also been shown to provide results similar to autograft, with
acceptable single-level fusion rates and the absence of donor site pain
(74). Other studies have found a higher
radiographic nonunion rate with allograft and greater clinical
improvement when autograft is used (44).
Therefore, the results of using allograft bone are difficult to
evaluate. One-level fusion with allograft may be acceptable, but graft
collapse and radiolucencies may persist.
uniformly correlated with a favorable clinical outcome, nor has
nonunion consistently resulted in a clinical failure (16).
The fact that a pseudarthrosis may be associated with a good clinical
result led to the concept of ACD. A major advantage of ACD is the lack
of donor site complications. However, the disadvantage is postoperative
neck pain, which may become severe and is more common than when ACDF is
performed (71). Postdiscectomy collapse and
angular kyphosis may also occur, leading to recurrent nerve root
compression if posterior osteophytes are not widely resected at the
index procedure. Bilateral foraminotomies must be performed to prevent
contralateral radiculopathy due to resultant disc space collapse. If
ACD is to be performed, it most likely should be limited to soft disc
herniations and avoided in patients with evidence of spondylosis who
require disc space distraction.
graft fusion (ACF) may be necessary in situations in which disc
herniation is associated with a sequestered fragment that has migrated
behind the vertebral body. Subtotal anterior corpectomy and fusion may
also be performed when two-level disc disease is present. The
theoretical advantage of ACF over two-level ACDF resides in the number
of sites that must fuse.
-
Accomplish anterior cervical corpectomy
by discectomy above and below the vertebra in question. Then excise the
vertebral body with rongeurs or a high-speed burr to the posterior
cortex. -
Next, remove the posterior shell with angled curets directed away from the dura.
-
Traction or distraction may then be applied to restore sagittal plane alignment at the decompressed level.
-
Harvest an iliac crest strut graft and
insert it into the prepared endplates, and countersink it slightly into
the vertebral bodies. Assess stability of the graft with traction
released and, if necessary, consider application of a rigid external
orthosis, halo, or internal fixation such as an anterior cervical plate.
be performed with laminotomy and foraminotomy, laminectomy, or
laminoplasty (Fig. 143.8, Fig. 143.9).
Careful patient positioning is required to minimize the risk of
neurologic injury and to maximize exposure of the required level.
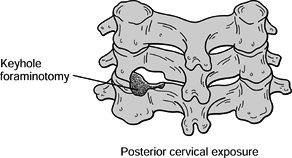 |
|
Figure 143.8. Posterior laminotomy and foraminotomy depicting thinning of the lamina and facet joint with nerve root decompression.
|
 |
|
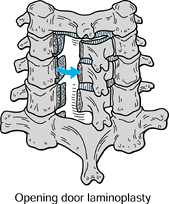
Figure 143.9. Open-door laminoplasty depicting hinged lamina and spinal canal decompression.
|
-
Stabilize the head in the prone position
with Mayfield skull tongs, leaving the face free without sources of
pressure. The reverse Trendelenburg position promotes
P.3756
epidural
venous drainage. The posterior approach to the cervical spine utilizes
an internervous plane in the midline which separates the muscles from
the segmental innervation supplied by the right and left posterior rami
of the cervical nerves. -
Incise the ligamentum nuchae in the
midline and carry the subperiosteal dissection down the spinous
processes and corresponding laminae. In the cervical spine, the laminae
do not override each other as much as in the thoracic spine; therefore,
the interlaminar space may be inadvertently penetrated if caution is
not taken during the exposure. -
Carry dissection out to the lateral edge
of the lateral masses and preserve the facet joint capsule if no fusion
is required or anticipated. -
Remove portions of the inferior and
superior laminae at the level of the specific nerve root compression
and perform partial facetectomy with a high-speed burr. -
To prevent iatrogenic instability, remove no more than 50% of the facet (78). The lamina and thinned bone should be gently lifted off the nerve and spinal cord with small angled curets.
-
Assess foraminotomy by placing a blunt probe or Woodson dental instrument into the neuroforamen to judge its patency.
-
If disc removal is deemed necessary,
expose the nerve root and cauterize the surrounding venous plexus.
Gently retract the nerve root cephalad and remove the disc tissue.
spondylotic radiculopathy with anterior bony ankylosis when cervical
lordosis has been preserved.
-
Perform laminectomy by thinning the cortices at the junction of the laminae and lateral masses bilaterally with a power burr.
-
Use a small Kerrison rongeur to complete the cut and a small angled curet to elevate the laminae.
-
Cauterize the adherent underlying venous plexus to minimize epidural hematoma formation.
-
Loss of the posterior structural support
of the bony elements may increase the risk of subsequent vertebral
subluxation and kyphotic deformity, especially in younger patients, in
whom fusion should be considered at the time of decompression.
spondylotic radiculopathy with predominantly unilateral symptoms. There
are several methods of laminoplasty, which vary by location of the
hinge and means of maintaining the open position (36).
-
As in laminectomy, perform laminoplasty
by thinning the cortex at the lamina and lateral mass junction with a
high-speed burr bilaterally to the inner cortex. -
Thin the hinged side without completing the cut while completing the osteotomy on the opening side.
-
Gently open the lamina either with towel
clips placed through the respective spinous processes or with a
vertebral spreader placed into the defect. Fracture the thinned inner
cortex of the hinged side and hold the posterior elements open.
myelopathy are not as well defined as they are for the treatment of
cervical radiculopathy. A patient with mild, nonprogressive myelopathy
that is long-standing and does not cause significant disability can be
observed closely. Operative intervention is recommended for (a)
progressive myelopathy, (b) moderate or severe myelopathy that is
stable and of short duration (less than 1 year), and (c) mild
myelopathy that affects routine activities of daily living. The age of
the patient or severity of the disease should not serve as a
contraindication for surgery; it must be conveyed to the patient that
the goal of surgery is to prevent neurologic worsening.
decide whether to approach the area of compression with an anterior
technique, a posterior technique, or a combination (Table 143.2).
The factors that are critical in this decision process are the site of
compression, presence or absence of spinal stability, sagittal
alignment of the cervical spine, and extent of the disease process (41).
 |
|
Table 143.2. Indications for the Operative Treatment of Cervical Myelopathy
|
spinal cord limited to the intervertebral disc space without
intervening stenosis of the canal at the vertebral body level indicates
an anterior decompression. Anterior decompression can be performed with
ACDF using the techniques described. Remove the spondylotic ridge, as
well as any other areas of spur formation deemed clinically significant
that may require hemicorpectomy. If multilevel disease is present,
consider anterior corpectomy and strut graft fusion. When compressive
pathology is present at the disc level as well as posterior to the
vertebral body, as with OPLL, then an ACDF will increase the AP
diameter at the disc level only and not the remainder of the spinal
canal. Anterior corpectomy allows more complete decompression.
alignment, an anterior decompression is also indicated. This is best
performed with ACF to realign and decompress the spine. When
accompanied by significant subluxations, kyphosis may need to be
approached from a combined anterior and posterior direction. In the
presence of spondylotic spurring and a congenitally tight spinal canal
over several segments, consider the posterior approach. A compression
ratio of less than 0.5 (sagittal diameter divided by the transverse
diameter of the spinal cord) may also indicate a posterior
decompression (43). This can be performed with laminectomy or laminoplasty, as previously described.
longitudinal incision, based on the number of levels involved.
Decompression of the spinal cord is accomplished by removal of the
middle third of the vertebral body. Recommendations for safe
decompression to within 5 mm of the transverse foramen allow removal of
disc or bone 7.5 mm lateral to the midline at C-3 and 9.5 mm at C-6,
which can be estimated intraoperatively (63).
Because patient anatomy varies, evaluate this on an individual basis
based on the preoperative imaging studies. In anterior decompression
for OPLL, a “floating procedure” may be performed (72).
The anterior ossified mass is not actually removed but rather is
released from its surrounding attachments. The localized OPLL segment
mass migrates anteriorly and allows decompression of the spinal cord.
Because it is possible to encounter absence of the dura or coalescence
of dura and PLL in the anterior approach to OPLL, this form of
decompression is often recommended (61).
to two vertebral bodies are removed, but because of its curvature this
graft is less useful when longer struts are needed. In corpectomies of
three or more levels, a fibular graft is indicated. The iliac crest
graft has more exposed cancellous sites for incorporation, but the
fibula is mechanically stronger.
of cervical radiculopathy and myelopathy is less clear than in
traumatic conditions (Fig. 143.10). In
degenerative disc disease, various studies suggest that nonunion rates
and graft dislodgement increase with the number of levels operated on (9,76).
The goals of applying instrumentation are to provide immediate
stability, increase fusion rate, prevent loss of fixation of the bone
graft, improve postoperative rehabilitation, and possibly avoid the
requirements for an external orthosis (34).
 |
|
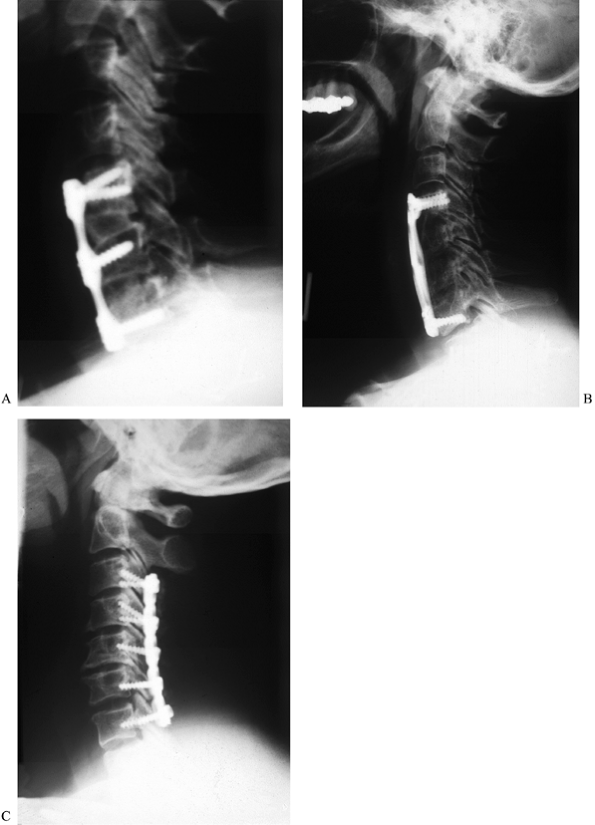
Figure 143.10. A:
Lateral radiograph shows interbody fusion at C5-6 and C6-7 with segmental plating from C-5 to C-7 (the Peak Polyaxial Anterior Cervical Plate, Depuy-Acromed, Cleveland, OH). This patient, who had a history of cigarette smoking, underwent anterior fusion with plating for cervical radiculopathy with neck pain. The plate was used to improve fusion. B: Lateral radiograph shows strut grafting and anterior plating from C-4 to C-7 (the Orion Plate, Sofamor-Danek, Inc., Memphis, TN). This patient underwent anterior corpectomy and fusion for cervical spondylotic myelopathy. C: Lateral radiograph shows laminectomy and lateral mass plating from C-3 to C-7 (Axis Plate, Sofamor-Danek, Inc., Memphis, TN). This patient underwent the procedure for myelopathy due to multilevel cervical spondylosis. |
Avascularity beneath the plate has also been detected, although its
significance is unclear. It is doubtful that anterior plating for
single-level ACDF increases fusion rate (21).
The potential benefits of instrumentation may not outweigh the risks in
these situations. Whether multiple-level ACDF fusion is improved by
instrumentation remains to be determined,
and
presently no guidelines are available for their use. Two-level ACDF has
a higher pseudarthrosis rate than single levels, and instrumentation is
often used in certain situations such as patients who are actively
smoking. Although rare, three-level ACDF may be accompanied by anterior
plating; however, no scientific data support its use. The main use of
anterior plating in ACF is to prevent graft dislodgement. When long
graft constructs are used, a plate may be inserted at the inferior
vertebra as a buttress where graft displacement most commonly occurs (75).
conditions is also controversial. Posterior decompressive laminectomy
may require concomitant fusion in patients with preexisting instability
based on imaging studies. Whether the addition of instrumentation such
as lateral mass plating or facet joint wiring increases fusion rate
while improving the postoperative course is unknown.
allowed to gradually increase their postoperative activities and are
encouraged out of bed with directed therapy as needed. Liquids are
started with a gradual advance to solids as tolerated. Brace management
is controversial and dealt with on an individualized basis. Patients
recovering from one- or two-level ACDF may be treated in a rigid or
soft orthosis based on their surgeon’s preference. We recommend rigid
external orthoses in ACDF, especially when multilevel decompression and
fusion are performed in the absence of anterior plating. Such devices
include Philadelphia collars or, in severely osteoporotic individuals,
halo-vest immobilization. External wear may continue for 6–12 weeks
based on radiographic progression of the fusion and the patient’s
comfort level. A gradual weaning process may follow from a rigid to a
soft collar to no immobilization. A soft collar is all that is needed
for posterior laminotomy, laminectomy, or laminoplasty when performed
in the absence of fusion.
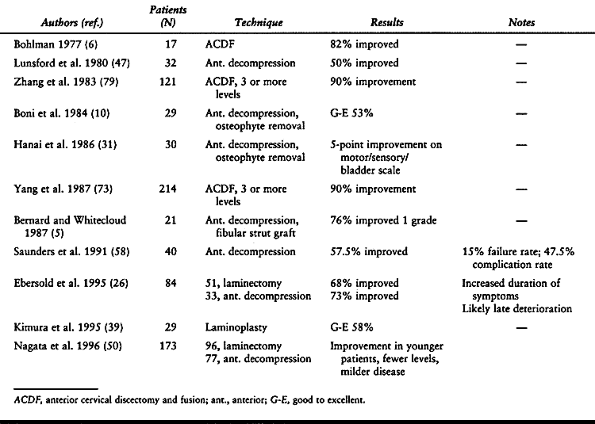
radiculopathy and myelopathy vary, depending on the type of approach
utilized and the severity of the disease. Literature review is depicted
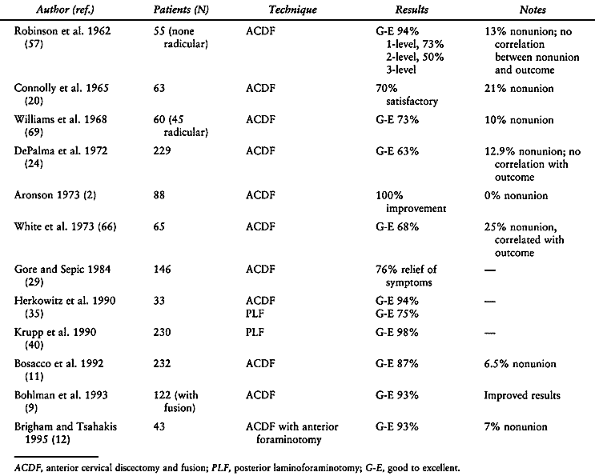
in Table 143.3 and Table 143.4.
Limitations in drawing firm conclusions from previous reports stem from
the lack of uniform patient population, inclusion of different disease
processes in the same analysis (soft versus hard disc), and
inconsistency of establishing successful results. Overall, the surgical
treatment of radiculopathy yields satisfactory results in greater than
90% of patients. Although controversial, it appears that patients who
attain a solid fusion do have better outcomes than those with a
pseudarthrosis. The results of treatment of myelopathy also are
variable, as depicted by the numerous
techniques
utilized to decompress the spinal cord. Overall, patients with greater
neurologic deficits tend to experience less improvement in symptoms
following surgery than those with more acute and less severe neurologic
findings.
 |
|
Table 143.3. Results of Surgical Treatment of Cervical Radiculopathy
|
 |
|
Table 143.4. Results of Surgical Treatment of Cervical Myelopathy
|
involves dissection and retraction of numerous vital vascular,
respiratory, neural, and intestinal structures. An overall 0.2%
incidence of neck site complications based on an extensive review of
published series has been reported in one series (67).
It is with an understanding of the potential complications that may
occur that improvements in techniques and results may follow.
The possible causes are traumatic division, stretch injury, compression
from postoperative swelling, and injury from thermal necrosis. Injury
is much more likely during a right-sided approach because the right
subclavian is occasionally anomalous. In these cases, the right
recurrent laryngeal nerve, having no vessel to follow, may cross the
surgical field directly and may be easily injured during exposure to
the mid-cervical spine. The injury is manifested as a hoarse, weak
voice with a risk of aspiration due to the inability to completely
close the larynx. When symptoms persist for longer than 6 weeks,
referral to an otolaryngologist is recommended for evaluation and
possible vocal cord injection.
following anterior cervical surgery is common and is estimated to occur
transiently in 8% of patients. When persistent symptoms develop,
evaluation should include a lateral radiograph to check bone graft
position. Esophageal lacerations occur in 0.25% to 0.7% of patients (38).
When identified, immediate primary repair should be performed, the
wound appropriately drained, and the patient started on broad-spectrum
antibiotics.
surgical approach or decompression are rare but can have devastating
sequelae. The structures that may potentially be injured include the
carotid sheath contents, superior and inferior thyroid arteries, and
vertebral artery. Avoid overzealous retraction and use blunt-edged
retractors to reduce the risk of injury to these vessels. Knowledge of
the anatomy of the vertebral artery and its relationship to the lateral
disc space and vertebral body, as well as maintaining midline
orientation during decompression, all serve to minimize the risk of
injury estimated to occur in 0.3% to 0.5% of patients (62).
The thoracic duct is at some risk during left-sided approaches to the
cervicothoracic junction. If a chylous effusion is encountered, injury
to the duct must be suspected.
perhaps the most devastating complication that can occur in anterior
cervical surgery. An incidence of 0.1% to 0.64% has been reported in
the literature (28). The literature indicates
that the drill and dowel technique and the presence of myelopathy are
the major risk factors for neurologic injury. In addition, neck
manipulation during intubation, cervical malalignment following
decompression and grafting, and postoperative epidural hematoma must
all be considered in the evaluation of the patient with postoperative
neurologic deterioration. Management should include maintenance of
normotensive blood pressure, administration of steroids, and imaging
studies to assess for possible graft dislodgement (28). If compressive pathology is identified, rapid reexploration and decompression are indicated.
Even though bony union may not occur, a stable fibrous union can
develop and account for the lack of symptoms in some patients with
pseudarthrosis. However, several reports have found better clinical
results when solid fusion is attained (9,55,66).
Injury to superficial nerves may result in numbness or pain with
neuroma formation. Superior gluteal artery injury has also been
reported in iliac crest bone harvest as well as iliac crest fracture.
can be diminished with strict attention to dissection within the
ligamentum nuchae and subperiosteally along the laminae. Reattachment
of the paraspinal muscles, especially to the C-2 spinous process, may
prevent loss of cervical lordosis following posterior decompression (51).
Avoidance of placement of instruments into the spinal canal and
thinning of the cortex with a high-speed burr followed by the use of
curets during decompression may diminish the risk of neurologic injury.
and when indicated, surgery must be directed at the specific pathology leading to symptoms (Table 143.1, Table 143.2).
We manage cervical radiculopathy based on the number of levels involved
and the degree of neck pain present. Significant neck pain and up to
two levels of radicular symptoms are managed with anterior cervical
discectomy and fusion. Radiculopathy without significant neck pain is
managed with posterior laminoforaminotomy. In the uncommon situation in
which a migrated disc fragment is located behind the vertebral body, a
subtotal corpectomy followed by strut graft fusion is the preferred
treatment. Radiculopathy involving three levels or greater is managed
by laminoforaminotomy or laminoplasty for unilateral and laminectomy
for bilateral symptoms. Laminectomy is frequently accompanied by
lateral mass plating if there is associated instability. Another option
is multilevel anterior discectomy and fusion with plating. For these
multilevel cases, the anterior approach is preferred if there is loss
of cervical lordosis, and the posterior approach is preferred if there
is maintenance of lordosis.
the site of spinal cord compression and the sagittal alignment of the
cervical spine. Single- or two-level disease is treated with anterior
decompression and fusion either with ACDF, if impingement is at the
disc level, or with subtotal corpectomy if compression is behind the
vertebral body. Multiple-level compression at greater than two levels
is managed with anterior decompression and fusion or posterior
decompression, depending on the pathology identified. Myelopathy with
cervical kyphosis is approached with anterior decompression and strut
graft fusion. Laminoplasty or laminectomy plus lateral mass plating and
fusion is recommended for three- (or more) level disease with
maintenance of cervical lordosis. If there is significant neck pain in
addition to myelopathy, fusion is preferred over laminoplasty. Combined
anterior and posterior decompression and fusion may be performed when
severe circumferential cord compression is present.
conditions, the physician must be attentive to symptoms and signs of
radiculopathy and myelopathy. Appropriate selection of imaging and
other diagnostic tests is important for making the correct diagnosis
and for cost-effectiveness. The treatment of patients with cervical
disc disease is largely nonoperative. Only those patients who failed
conservative treatment should undergo surgery for symptomatic relief of
radicular arm pain or improvement of neurologic deficits. Patients with
cervical spondylotic myelopathy should be treated more aggressively to
prevent permanent loss of neurologic function. The choice of anterior
versus posterior approach depends on the patient’s symptoms, the
location of neural compression, the sagittal alignment, the number of
levels involved, and the surgeon’s preference.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
G, Ross J. The Accuracy of Imaging Studies of the Degenerative Cervical
Spine: Myelography, Myelo-Computed Tomography, and Magnetic Resonance
Imaging. In: Weisel S, ed.Seminars in Spine Surgery— Cervical Disc Disease. Philadelphia: WB Saunders, 1995:9.
T, Whitecloud T. Cervical Spondylotic Myelopathy and
Myeloradiculopathy—Anterior Decompression and Stabilization with
Autogenous Fibula Strut Graft. Clin Orthop 1987;221:149.
H. Cervical Spondylosis with Moderate to Severe Myelopathy: A Report of
17 Cases Treated by Robinson Anterior Cervical Discectomy and Fusion. Spine 1977;2:151.
D, Berman A, Levenberg R, Dosacco S. Surgical Results in Anterior
Cervical Discectomy and Fusion Using a Countersunk Interlocking
Autogenous Iliac Crest Bone Graft. Orthopedics 1992;15:923.
R. Ossification of the Posterior Longitudinal Ligament: Clinical
Manifestations and Surgical Treatment. In: Weisel S, ed. Seminars in Spine Surgery—Cervical Disc Disease. Philadelphia: WB Saunders, 1995:33.
R, Herkowitz H, Kurz L. Effect of Distraction on the Union Rate of
Smith-Robinson Type Anterior Cervical Discectomy and Fusion. Presented
at the Cervical Spine Research Society Annual Meeting, Palm Desert, CA,
1992.
L, Maurette P, Pointillart V, et al. Long Term Results of Cervical
Epidural Steroid Injection With and Without Morphine in Chronic
Cervical Radicular Pain. Pain 1994;58:239.
P, Esses S, Kostuik J. Anterior Cervical Fusion Outcome. Analysis of
Patients Fused With and Without Anterior Cervical Plates. J Spinal Disord 1996;9:202.
H, Kurz L, Overholt D. Surgical Management of Cervical Soft Disc
Herniation: A Comparison between the Anterior and Posterior Approach. Spine 1990;15:1026.
W, Schatke H, Muke R. Clinical Results of the Foraminotomy as Described
by Frykholm for the Treatment of Lateral Cervical Disc Herniation. Acta Neurochir 1990;107:22.
S, Connolly K, Incorvania B, et al. Anterior Cervical Fusion Using
Allograft Versus Autograft Bone. Presented at the Cervical Spine
Research Society Annual Meeting, Baltimore, MD, 1994.
L, Bissoneete D, Zorub D. Anterior Surgery for Cervical Disc Disease.
Part 2: Treatment of Cervical Spondylotic Myelopathy in 32 Cases. J Neurosurg 1980;53:12.
K, Ohashi T, Abe J, et al. Cervical Myelopathy in Elderly Patients:
Clinical Results and MRI Findings Before and After Decompression
Surgery. Spinal Cord 1996;34:220.
R, Bernini P, Shireffs T, Reeves A. Central Corpectomy for Cervical
Spondylotic Myelopathy: A Consecutive Series with Long Term Follow-up
Evaluation. J Neurosurg 1991;74:163.
M, Emery S, Dudley A, et al. Vertebral Artery Injury during Anterior
Decompression of the Cervical Spine—A Retrospective Review of Ten
Patients. J Bone Joint Surg Br 1993;75:410.
A, Ring D, Scuderi G, Garfin S. Vertebral Artery Location in Relation
to Vertebral Body as Determined by Two-Dimensional Computed Analysis
Evaluation. Spine 1994;19:2637.
I, Ikeda A, Shibuya R, et al. Clinical Long Term Results of Anterior
Cervical Discectomy without Interbody Fusion for Cervical Disc Disease.
Spine 1991;16:272.
W, Rosenwasser R. An Early Comparative Analysis of the Use of Fibular
Allograft versus Autograft Iliac Crest Graft for Interbody Fusion after
Anterior Cervical Discectomy. Spine 1993;18:1123.